Math Is Fun Forum
You are not logged in.
- Topics: Active | Unanswered
#1 Re: This is Cool » Miscellany » Yesterday 18:11:50
2388) Gulf
Gulf
"Gulf" refers to a large area of sea that is partially enclosed by land, such as the Gulf of Mexico, or a wide, deep chasm or separation between things, like the "gulf between rich and poor". The term can also refer to the famous Gulf Oil company, known for its lubricants and lubricants for vehicles.
A gulf is a portion of the ocean that penetrates land. Gulfs vary greatly in size, shape, and depth. They are generally larger and more deeply indented than bays. Like bays, they often make excellent harbors.
Summary
A gulf is a large inlet from an ocean or their seas into a landmass, larger and typically (though not always) with a narrower opening than a bay. The term was used traditionally for large, highly indented navigable bodies of salt water that are enclosed by the coastline. Many gulfs are major shipping areas, such as the Persian Gulf, Gulf of Mexico, Gulf of Finland, and Gulf of Aden.
Geographical Meaning
A gulf is a coastal indentation or a portion of the ocean or sea that penetrates the land.
It is typically larger and more deeply indented than a bay.
Examples include the Persian Gulf, the Gulf of Aden, and the Gulf of Finland.
Figurative Meaning
The term "gulf" can describe a wide or impassable separation, or a significant difference between two things or groups.
For instance, there is a widening gulf between different social classes.
Details
A gulf is a portion of the ocean that penetrates land. Gulfs vary greatly in size, shape, and depth. They are generally larger and more deeply indented than bays. Like bays, they often make excellent harbors. Many important trading centers are located on gulfs.
Gulfs may be formed by movements in Earth's crust. The planet's tectonic plates may rift, or break apart, creating a gulf. Or one plate may fold under another, a process called subduction. Subduction may create a gulf by making downfolds, or troughs, in the rock under the ocean.
Gulfs are sometimes connected to the ocean by narrow passages of water called straits. Gulfs can also have wide openings and are sometimes indistinguishable from larger bodies of water.
Major Gulfs
The Gulf of Mexico, bordered by the United States, Mexico, and the island nation of Cuba, is the world's largest gulf. It has a coastline of about 5,000 kilometers (3,100 miles). The Gulf of Mexico is connected to the Atlantic Ocean by the Straits of Florida, between Cuba and the U.S. state of Florida. It is connected to the Caribbean Sea by the Yucatán Channel, between Cuba and the Mexican peninsula of Yucatán.
The Gulf of Mexico is an important economic site for all three countries. The process of upwelling occurs near the Florida coast of the gulf, creating a rich variety of sea life. Upwelling is the process in which cold, nutrient-rich water from the bottom of the gulf is brought to the surface.
Fish and other organisms thrive in areas of upwelling. Commercial, sport, and recreational fishing thrive in the Gulf of Mexico. Oil deposits sit beneath the western Gulf of Mexico. Both Mexico (in the Bay of Campeche) and the U.S. (mainly around the coasts of Texas and Louisiana) have oil wells in the Gulf of Mexico.
The Gulf Stream, one of the most powerful ocean currents in the world, originates in the Gulf of Mexico. Gulf ports, including Houston, Texas; New Orleans, Louisiana; Veracruz, Mexico; and Havana, Cuba, continue to be important cities where goods are imported and exported by sea.
The Gulf of Mexico is also the site of strong storms. Hurricanes and other storms need warm water to develop. The Gulf of Mexico is a very warm body of water, so storms can often increase their strength. Cuba and Florida are regularly hit by hurricanes on their Atlantic and Gulf coasts.
Pollution also threatens life in the Gulf of Mexico. Oil shipping and drilling can spill tons of petroleum into the ecosystem. Two huge rivers, the Mississippi in the U.S. and the Grijalva in Mexico, empty into the gulf. Chemicals used for agriculture and industry have seeped into the water, helping to create one of the largest dead zones in the world. (A dead zone is a region where there is little oxygen or life beneath the surface of the ocean.)
River management has redirected the flow of the Mississippi River. Canals, dams, and drainage systems for agriculture and industry have provided power and irrigated land. They have also reduced the wetlands at the rivers mouth and delta. The Gulfs wetlands slow storms as they move toward land. The loss of these wetlands may have contributed to the destruction brought by Hurricane Katrina to the Gulf Coast between central Florida and Texas in 2005.
The Gulf of Carpentaria, on Australia's northeast coast, is an inlet of the Arafura Sea. Because the sea and the gulf are shallow, the exchange of water between the two is reduced. Sediment collects at the mouth of the gulf, forming underwater barriers. The low shore is bordered in some areas by wetlands and swamps.
This shallow gulf with a wide mouth creates the conditions for a yearly spectacle called the Morning Glory Cloud. In September and October, sea breezes from the Gulf of Carpentaria and the Arafura Sea meet and create an enormous, fast-moving cloud over the gulf. The Morning Glory Cloud can be 1,000 kilometers (621 miles) long and move at a rate of 60 kilometers per hour (37 miles per hour).
The Persian Gulf is an arm of the Arabian Sea bordered by Iran, Iraq, Kuwait, Saudi Arabia, Qatar, Bahrain, the United Arab Emirates, and Oman. Vast deposits of petroleum in this region make the Persian Gulf strategically important. Middle Eastern countries depend on the gulf for trade and for access to the Indian Ocean. All countries that consume oil from the region, including the U.S., have a vital interest in keeping the gulf open to shipping.
Additional Information
A gulf is any large coastal indentation. More specifically, such a feature is the reentrant of an ocean, regardless of size, depth, configuration, and geologic structure. The nomenclature for gulfs is far from uniform; names that may refer to sizable gulfs in various places include bay, bight, firth, sound, and fjord. In addition, a number of pronounced concavities of oceanic margins have no proper name at all. As such, many of the characteristics of gulfs may also apply to bays and other similar geographies.
This problem of nomenclature extends to the difference between gulfs and seas. There are many small seas, such as the Sea of Marmara (11,000 square km [about 4,200 square miles]) and the Sea of Azov (38,000 square km [about 14,700 square miles]), which, strictly speaking, are really gulfs of the ocean or other seas (the Sea of Azov is a gulf of the Black Sea). The Gulf of Aden (about 270,000 square km [about 104,000 square miles]), another example, is part of the Arabian Sea, and these water bodies have a common regime (similar tides, precipitation, evaporation, and so forth). The narrow sound of Bab el-Mandeb connects the gulf with the vast Red Sea (438,100 square km [about 169,000 square miles]) and exhibits a number of specific geomorphic features. The Red Sea in turn has two small gulfs to the north—namely, those of Suez and Aqaba.
The Bay of Bengal and the Arabian Sea are both gulfs, approximately the same size and having the same monsoonal water circulation. The Bay of Bengal is, however, the largest of the gulfs, with a surface area of 2,172,000 square km (838,600 square miles), a length of 1,850 km (1,150 miles), and a width of about 1,600 km (1,000 miles).
In some cases, the width of a gulf may exceed its length. The Great Australian Bight has the widest mouth (2,800 km [1,740 miles]). The Gulf of Guinea is the deepest; its maximum depth (6,363 metres [20,876 feet]) exceeds that of the Bay of Bengal by more than 1,000 metres (about 3,300 feet).
Topographic characteristics
Single gulfs usually are formed along linear shores of the continents. If the shoreline is irregular and has a complex geologic structure, groups of gulfs of a similar nature may occur. Most shorelines have small reentrants of various size that are called bays.
The shape and bottom topography of gulfs are amazingly diverse. They are determined by the geologic structure and development of the region. Homogeneous bedrock of low strength or resistance results in simple shapes and shallow depths. The Gulf of Riga (of the Baltic Sea) is a possible example of the type. Long, narrow arms with approximately parallel shores of the south Kara Sea extend inland for about 800 km (about 500 miles). They occupy troughs that originated by erosion during a period of lower sea level (Baidaratskaya Bay, Obskaya Bay with Tazovskaya Bay tributary, Yenisey Bay, Gydanskaya Bay). Deep, angular gulfs, on the other hand, are created along fractures, faults, and rifts (e.g., Varanger Fjord); they usually have irregular bottom topography. Parallel fractures form extremely deep, narrow gulfs with parallel shores, such as the Gulf of California. Genuine fjord-gulfs are notable for their very high length-to-width ratios (up to 50:1). In regions that have undergone nonuniform deformation and uplift, gulfs of complicated and irregular shape and bottom topography are consequently formed; the Gulf of St. Lawrence is an example.
Gulfs are connected with the sea by means of one or more straits. Sometimes there may be an archipelago in the mouth of the gulf, as in the Gulf of Bothnia. There are some gulfs that open into the sea or into another gulf on opposite sides (Baffin Bay, the Gulf of Aden, and the Gulf of Oman).
Factors that affect the characteristics of gulfs
Gulfs may differ from the adjacent ocean (or sea) by virtue of water properties and dynamics and processes of sedimentation. Such differences are determined by the size and the shape of a given gulf, by the depth and bottom topography, and, to a considerable extent, by the degree of isolation from the ocean. Climatic conditions also are important. Isolation from an adjacent ocean depends on the ratio of width of mouth to total surface area of a gulf or on the cross section of the mouth to total water volume. If there is a sill (a submarine ridge or rise), the ratio of depth above the sill to the depth of the gulf is of great importance. No extensive comparisons of these ratios have been made to date; hence, any analysis of controlling variables must remain somewhat qualitative.
A high sill hampers the water exchange between an ocean and gulf and may lead to stagnation (oxygen deficiency), as is found in some fjords of Norway, in the Red Sea, and, particularly, in the Black Sea. Also, the presence of a sill causes independent circulation of gulf waters, generated by local winds and the runoff of rivers. Sills are not indispensable for the formation of an independent circulation, however. A narrow mouth, as in the Gulf of Bothnia, leads to the same result.
In humid climates, the waters of gulfs are freshened by river runoff. Salinity is particularly low in the gulfs of the Baltic Sea and along the southern coast of the Kara Sea. Water becomes almost fresh in their heads, especially in the spring when snow begins to thaw. Gulfs of the arid zone suffer from intensive evaporation and receive little river runoff. Thus, salinity increases markedly in this climatic regime—up to 60 parts per thousand in the Persian Gulf and up to 350 parts per thousand in the Kara-Bogaz-Gol (a gulf of the Caspian Sea). In addition to its effect on salinity, river runoff delivers organic matter and nutrient salts that may determine the specific features of life in the gulfs. The number of genera and species of organisms is small, but the organisms present tend to develop in quantities. That is why shrimp, oyster, and other fisheries are concentrated in many gulfs.
Funnel-shaped gulfs, in which the depth gradually decreases headward, usually have resonant tides. The tidal range at the head of such gulfs is several times greater than that in the open ocean (e.g., Bristol Channel, Río de la Plata, Mezenskaya Bay, Shelikhova Gulf). The world maximum tidal range has been registered in the Bay of Fundy (18 metres [59 feet]). The regularity (magnitude and frequency) of the flood tide may be distorted in such instances, and the duration of the flood tide may become much shorter than that of the ebb tide. This may cause the phenomenon of tidal bore, in which a steep wave will move rapidly upstream for dozens of kilometres.
Gulfs of simple shape with a narrow mouth and a high degree of isolation from the ocean are often subject to seiches. These free oscillations can result from rapid changes of atmospheric pressure and, of course, from tectonic movements such as earthquakes. Seiches gradually decrease, but some oscillation continues long after their cause disappears. A high rise of the water (storm surge) occurs in long and shallow gulfs if winds from the sea are protracted. Such phenomena are difficult to predict, and the high water levels may cause floods. Seiches commonly occur at the heads of Helgoländer Bay in the North Sea and in the Gulf of Finland.
Certain aspects of sedimentation are affected by the isolation of gulfs from the ocean and river runoff. The rate of sediment accumulation in gulfs of limited area may be very high. This, of course, is a function of river discharge; sediment composition is usually similar to that of the load transported by entering rivers. Deposition of calcium carbonate often occurs in shallow gulfs in the arid zones where few if any perennial streams exist. The bottoms of long gulfs (or gulfs having sills) are usually covered with silt even at the shallowest depths (e.g., Hudson Bay, the Bo Hai [Gulf of Chihli], the inlets or gubas of the Kara Sea, the Gulf of Riga). Only strong tidal currents can prevent this siltation and, in some cases, cause the opposite phenomenon of bottom erosion. Currents maintain the existence of or actively deepen bottom troughs in narrow-mouthed gulfs whose depths are more than 200 metres (about 660 feet), whereas depths of adjacent parts of the open ocean are only on the order of some dozens of metres.
Waves of the open ocean either do not penetrate into comparatively isolated gulfs or—if they do—they become greatly reduced after entry. Small local waves that are related to gulf size prevail there. This tends to make gulfs quite navigable, and seaports and harbours have generally been situated on them.
Classification of gulfs
The geologic structure and developmental history of gulfs are as varied as are those of the continents or oceans proper. The factors discussed above influence the morphological peculiarities of gulfs, and the latter in turn permit some general division or classification of these features to be made. The several groups in one possible scheme are discussed here using typical gulfs of each group as examples.
Areas situated in open concavities of the continental coast (Gulf of Alaska, Bay of Biscay, Gulf of Guinea, Great Australian Bight, Bay of Bengal, Gulf of Tehuantepec, for example) are classified as the A1 group. The depth of these gulfs in the region of the mouth usually is on the order of kilometres. The continental shelf and continental slope are generally pronounced. The general shape of such gulfs is simple; width of mouth usually exceeds its length. Water circulation and its physical properties are similar to those of the oceans. The character of the marine faunas does not differ from that of oceanic areas.
Large areas considerably isolated from oceans, such as the Gulf of Mexico and Baffin Bay, are designated as group A2. The former includes a geosynclinal hollow, founded in the Mesozoic Era (251 million to 65.5 million years ago) and finally shaped during the Paleogene and Neogene periods (65.5 million to 2.6 million years ago). It is connected with the ocean by the narrow and relatively shallow Straits of Florida and the Yucatán Channel. Baffin Bay is a rift hollow that is connected by straits with the Atlantic.
Ocean gulfs, such as the Gulfs of Oman, California, Aden, and some others, have smaller areas and are isolated to a lesser degree. These features, in group A3, have shapes that are determined by young faults and fractures. Depths in these gulfs generally exceed 1 kilometre (0.6 mile). Unlike the previous group, in which gulfs might be of composite geologic structure, these occupy areas that have undergone only a single episode of deformation.
Gulfs situated on the continental shelf, such as the Bay of Fundy, Hudson Bay, Río de la Plata, San Matías Gulf (off Argentina), and others, are in group B. The depth of such gulfs is up to 200 metres (about 660 feet) or more, and their configuration is determined by geologic conditions. Because shelf areas repeatedly became dry land when the sea level fell during the ice ages, these gulfs received their final shape during the Pleistocene Epoch. The Gulf of St. Lawrence is included in this group, though it is really intermediate between groups A3 and B. It contains both a pronounced shelf and a long trough up to 530 metres (1,740 feet) deep.
Gulfs of intercontinental and marginal seas are considered to be a third category. These may be divided into group C1, which consists of gulfs of basin seas, including the deepwater part only (Gulf of Aqaba) or both the deepwater and the shelf parts (Gulf of Honduras), and group C2, the shelf gulfs of the same seas (e.g., the Persian Gulf, the Gulf of Suez, Anadyrsky Gulf, the Bristol and Norton channels, and Shelikhova Gulf).
Finally, there are the gulfs of the shelf seas (gubas of the Arctic seas of Russia, gulfs of the Baltic and the White Seas, the Gulf of Carpentaria, the Bo Hai, and many others), which are placed in group D. The shallow character of the shelf seas influences the water dynamics of the gulfs. Water exchange is weakened, and sediments may accumulate in the gulf mouths, thus forming submarine barriers and further reducing exchange.

#2 Dark Discussions at Cafe Infinity » Close Quotes - VI » Yesterday 17:21:09
- Jai Ganesh
- Replies: 0
Close Quotes - VI
1. There's a gap between what I want to do, what I do on camera, and what gets edited. Right? So the goal is to try and close the gaps. What's the biggest compliment is if I read a review and it's exactly what I wrote down in my diary before ever filming it. That's really cool. That's the biggest signifier of closing the gaps. - Matthew McConaughey
2. I have very few friends. I have a handful of close friends, and I have my family, and I haven't known life to be any happier. - Brad Pitt
3. Eradications are special. Zero is a magic number. You either do what it takes to get to zero and you're glad you did it; or you get close, give up and it goes back to where it was before, in which case you wasted all that credibility, activity, money that could have been applied to other things. - Bill Gates
4. Happiness, for me, has to be real - life that is made of real conversations, of spending quality time with close friends, walks in nature and woods, praying, feeling real gratitude, reading good books, being able to be in the moment and hearing the sounds of nature. - Bhumika Chawla
5. We are not even close to finishing the basic dream of what the PC can be. - Bill Gates
6. I barely need to reiterate what you already know: the close links that exist between our people and the people of Venezuela and Hugo Chavez, the promoter of the Bolivarian Revolution and the United Socialist Party he founded. - Fidel Castro
7. One sees qualities at a distance and defects at close range. - Victor Hugo
8. I don't think there is a perfect athlete. But if I had to come close to picking someone who demonstrates all the traits that I feel an athlete should have, I would say the perfect athlete would be Tiger Woods. He has the ability, he's humble and he's very good at what he does. - Jackie Joyner-Kersee.
#3 Jokes » Love Jokes - II » Yesterday 16:57:58
- Jai Ganesh
- Replies: 0
Q: What is love?
A: The delusion that one woman differs from another.
* * *
Q: How did the girl get a prince to fall in love with her?
A: She wore a raspberry beret.
* * *
Q: Why shouldn't you marry a tennis player?
A: Because love means nothing to them!
* * *
Q: How do you turn a fox into an elephant?
A: Marry it.
* * *
Q: What did the toaster say to the slice of bread?
A: I want you inside me!
* * *
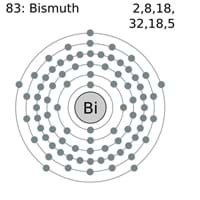
#4 Science HQ » Bismuth » Yesterday 16:22:46
- Jai Ganesh
- Replies: 0
Bismuth
Gist
Bismuth (symbol Bi, atomic number 83) is a brittle, silvery-white post-transition metal with low toxicity that is used in alloys, electronics, pharmaceuticals (like Pepto-Bismol), and paints. Recovered as a by-product of other metals, bismuth's unique bismuth crystal structure and chemical properties lead to its common use in various industrial and medical applications.
Bismuth finds its main uses in pharmaceuticals, atomic fire alarms and sprinkler systems, solders and other alloys and pigments for cosmetics, glass and ceramics.
Summary
Bismuth is a chemical element; it has symbol Bi and atomic number 83. It is a post-transition metal and one of the pnictogens, with chemical properties resembling its lighter group 15 siblings antimony. Elemental bismuth occurs naturally, and its sulfide and oxide forms are important commercial ores. The free element is 86% as dense as lead. It is a brittle metal with a silvery-white color when freshly produced. Surface oxidation generally gives samples of the metal a somewhat rosy cast. Further oxidation under heat can give bismuth a vividly iridescent appearance due to thin-film interference. Bismuth is both the most diamagnetic element and one of the least thermally conductive metals known.
Bismuth was formerly understood to be the element with the highest atomic mass whose nuclei do not spontaneously decay. However, in 2003 it was found to be very slightly radioactive. The metal's only primordial isotope, bismuth-209, undergoes alpha decay with a half-life roughly a billion times longer than the estimated age of the universe.
Bismuth metal has been known since ancient times. Before modern analytical methods bismuth's metallurgical similarities to lead and tin often led it to be confused with those metals. The etymology of "bismuth" is uncertain. The name may come from mid-sixteenth-century Neo-Latin translations of the German words weiße Masse or Wismuth, meaning 'white mass', which were rendered as bisemutum or bisemutium.
Bismuth compounds account for about half the global production of bismuth. They are used in cosmetics; pigments; and a few pharmaceuticals, notably bismuth subsalicylate, used to treat diarrhea. Bismuth's unusual propensity to expand as it solidifies is responsible for some of its uses, as in the casting of printing type.[ Bismuth, when in its elemental form, has unusually low toxicity for a heavy metal. As the toxicity of lead and the cost of its environmental remediation became more apparent during the 20th century, suitable bismuth alloys have gained popularity as replacements for lead. Presently, around a third of global bismuth production is dedicated to needs formerly met by lead.
Details
Bismuth (Bi) is the most metallic and the least abundant of the elements in the nitrogen group (Group 15 [Va] of the periodic table). Bismuth is hard, brittle, lustrous, and coarsely crystalline. It can be distinguished from all other metals by its colour—gray-white with a reddish tinge.
Element Properties:
atomic number : 83
atomic weight : 208.98040
melting point : 271.3 °C (520.3 °F)
boiling point : 1,560 °C (2,840 °F)
density : 9.747 gram/{cm}^{3} at 20 °C (68 °F)
oxidation states : +3, +5
History
Bismuth evidently was known in very early times, since it occurs in the native state as well as in compounds. For a long period, however, it was not clearly recognized as a separate metal, having been confused with such metals as lead, antimony, and tin. Miners during the Middle Ages apparently believed bismuth to be a stage in the development of silver from baser metals and were dismayed when they uncovered a vein of the metal thinking they had interrupted the process. In the 15th-century writings of the German monk Basil Valentine this element is referred to as Wismut, a term that may have been derived from a German phrase meaning “white mass.” In any case it was Latinized to bisemutum by the mineralogist Georgius Agricola, who recognized its distinctive qualities and described how to obtain it from its ores. Bismuth was accepted as a specific metal by the middle of the 18th century, and works on its chemistry were published in 1739 by the German chemist Johann Heinrich Pott and in 1753 by the Frenchman Claude-François Geoffroy.
Occurrence and distribution
Bismuth is about as abundant as silver, contributing about 2 × {10}^{-5} weight percent of Earth’s crust. Its cosmic abundance is estimated as about one atom to every 7,000,000 atoms of silicon. It occurs both native and in compounds. In the native state, it is found in veins associated with lead, zinc, tin, and silver ores in Bolivia, Canada, England, and Germany. Its naturally occurring compounds are chiefly the oxide (bismite or bismuth ochre, Bi2O3), the sulfide (bismuthinite or bismuth glance, Bi2S3), and two carbonates (bismutite, (BiO)2CO3, and bismutosphaerite). Commercial bismuth, however, is produced largely as a by-product in the smelting and refining of lead, tin, copper, silver, and gold ores. Thus, it comes—for example—from tungsten ores in South Korea, lead ores in Mexico, copper ores in Bolivia, and both lead and copper ores in Japan. By the early 21st century, however, China was leading the world in both the mining and the refining of bismuth. Pure bismuth can also be obtained by reducing the oxide with carbon or by roasting the sulfide in the presence of charcoal and metallic iron to remove the sulfur.
Bismuth forms only one stable isotope, that of mass 209. A large number of radioactive isotopes are known, most of them very unstable.
Commercial production and uses
Bismuth is volatile at high temperature, but it usually remains with the other metals after smelting operations. Electrolytic refining of copper leaves bismuth behind as one component of the anode sludge. Separation of bismuth from lead by the Betterton–Kroll process involves the formation of high-melting calcium or magnesium bismuthide (Ca3Bi2 or Mg3Bi2), which separates and can be skimmed off as dross. The dross may be chlorinated to remove the magnesium or calcium, and finally the entrained lead. Treatment with sodium hydroxide then produces highly pure bismuth. An alternative separation, the Betts process, involves electrolytic refining of lead bullion (containing bismuth and other impurities) in a solution of lead fluosilicate and free fluosilicic acid, bismuth being recovered from the anode sludge. Separation of bismuth from its oxide or carbonate ores can be effected by leaching with concentrated hydrochloric acid. Dilution then precipitates the oxychloride, BiOCl. This, on heating with lime and charcoal, produces metallic bismuth.
Metallic bismuth is used principally in alloys, to many of which it imparts its own special properties of low melting point and expansion on solidification (like water and antimony). Bismuth is thus a useful component of type-metal alloys, which make neat, clean castings; and it is an important ingredient of low-melting alloys, called fusible alloys, which have a large variety of applications, especially in fire-detection equipment. A bismuth–manganese alloy has been found effective as a permanent magnet. Small concentrations of bismuth improve the machinability of aluminum, steel, stainless steels, and other alloys and suppress the separation of graphite from malleable cast iron. Thermoelectric devices for refrigeration make use of bismuth telluride, Bi2Te3, and bismuth selenide, Bi2Se3. Liquid bismuth has been used as a fuel carrier and coolant in the generation of nuclear energy.
The principal chemical application of bismuth is in the form of bismuth phosphomolybdate (BiPMo12O40), which is an effective catalyst for the air oxidation of propylene and ammonia to acrylonitrile. The latter is used to make acrylic fibres, paints, and plastics. Pharmaceutical uses of bismuth have been practiced for centuries. It is effective in indigestion remedies and antisyphilitic drugs. Slightly soluble or insoluble salts are utilized in the treatment of wounds and gastric disorders and in outlining the alimentary tract during X-ray examination, and bismuth is sometimes injected in the form of finely divided metal, or as suspensions of its insoluble salts. Substantial quantities of the oxychloride, BiOCl, have been used to impart a pearlescent quality to lipstick, nail polish, and eye shadow.
Properties and reactions
Bismuth is a rather brittle metal with a somewhat pinkish, silvery metallic lustre. Bismuth is the most diamagnetic of all metals (i.e., it exhibits the greatest opposition to being magnetized). It is hard and coarsely crystalline. It undergoes a 3.3 percent expansion when it solidifies from the molten state. Its electrical conductivity is very poor, but somewhat better in the liquid state than in the solid. With respect to thermal conductivity, it is the poorest of all metals except mercury.
Although it does not tarnish in air at ordinary temperatures, bismuth forms an oxide coating when heated and is oxidized rapidly at its boiling point of 1,560 °C. The yellow colour of this oxide distinguishes it from those formed by other metals. At red heat, bismuth reacts with steam, but it is not affected by cold, air-free water; it combines directly with sulfur and with the halogens (fluorine, chlorine, bromine, iodine). The element is not attacked by hydrochloric acid, and only slightly by hot sulfuric acid, but it is rapidly dissolved by either dilute or concentrated nitric acid.
Bismuth atoms have the same electronic structure in their outermost shell as do the other elements of the nitrogen group. They can, therefore, form three single covalent bonds, exhibiting either a +3 or −3 oxidation state. The element has a somewhat lower electronegativity than the others, and its lone pair of electrons is evidently quite inert, causing the +5 state of bismuth to be rare and unstable.
Analytical and physiological chemistry
Bismuth is usually determined gravimetrically, being precipitated and weighed as the phosphate or the oxychloride, BiOCl. To produce the latter, a suitable amount of hydrochloric acid is added to a nitric acid solution containing the bismuth, and the resulting solution is poured into a large volume of water, causing the oxychloride to precipitate. Volumetric and colorimetric methods of determination are also available.
Bismuth is relatively nontoxic, the least so of the heavy metals. It is generally not an industrial hazard. Although bismuth and certain of its compounds find considerable therapeutic use, some authorities recommend that other remedies be substituted. Soluble inorganic bismuth compounds are toxic.
Additional Information:
Appearance
Bismuth is a high-density, silvery, pink-tinged metal.
Uses
Bismuth metal is brittle and so it is usually mixed with other metals to make it useful. Its alloys with tin or cadmium have low melting points and are used in fire detectors and extinguishers, electric fuses and solders.
Bismuth oxide is used as a yellow pigment for cosmetics and paints, while bismuth(III) chloride oxide (BiClO) gives a pearly effect to cosmetics. Basic bismuth carbonate is taken in tablet or liquid form for indigestion as ‘bismuth mixture’.
Biological role
Bismuth has no known biological role, and is non-toxic.
Natural abundance
Bismuth occurs as the native metal, and in ores such as bismuthinite and bismite. The major commercial source of bismuth is as a by-product of refining lead, copper, tin, silver and gold ores.
#5 Re: Jai Ganesh's Puzzles » General Quiz » Yesterday 15:40:25
Hi,
#10555. What does the term in Geography Borough mean?
#10556. What does the term in Geography Boundary mean?
#6 Re: Jai Ganesh's Puzzles » English language puzzles » Yesterday 15:23:34
Hi,
#5749. What does the noun inlet mean?
#5750. What does the adjective innate mean?
#7 Re: Jai Ganesh's Puzzles » Doc, Doc! » Yesterday 15:11:18
Hi,
#2468. What does the medical term Axonotmesis mean?
#8 Re: Jai Ganesh's Puzzles » 10 second questions » Yesterday 14:40:36
Hi,
#9734.
#9 Re: Jai Ganesh's Puzzles » Oral puzzles » Yesterday 14:32:41
Hi,
#6241.
#10 Re: Exercises » Compute the solution: » Yesterday 13:48:00
Hi,
Good work!
2574.
#11 Re: This is Cool » Miscellany » 2025-09-14 22:29:02
2387) Blue Whale
Gist
Blue whales are the largest animals ever to live on our planet. They feed almost exclusively on krill, straining huge volumes of ocean water through their baleen plates (which hang from the roof of the mouth and work like a sieve). Some of the biggest individuals may eat up to 6 tons of krill a day.
There are an estimated 10,000 to 25,000 blue whales left in the world, with recent research indicating that the population may be increasing. The species is still classified as endangered by the IUCN Red List of Threatened Species due to historical whaling, but current threats include entanglement in fishing gear and vessel strikes.
Summary
The blue whale (Balaenoptera musculus) is a marine mammal and a baleen whale. Reaching a maximum confirmed length of 29.9–30.5 m (98–100 ft) and weighing up to 190–200 t (190–200 long tons; 210–220 short tons), it is the largest animal known to have ever existed. The blue whale's long and slender body can be of various shades of greyish-blue on its upper surface and somewhat lighter underneath. Four subspecies are recognized: B. m. musculus in the North Atlantic and North Pacific, B. m. intermedia in the Southern Ocean, B. m. brevicauda (the pygmy blue whale) in the Indian Ocean and South Pacific Ocean, and B. m. indica in the Northern Indian Ocean. There is a population in the waters off Chile that may constitute a fifth subspecies.
In general, blue whale populations migrate between their summer feeding areas near the poles and their winter breeding grounds near the tropics. There is also evidence of year-round residencies, and partial or age- and gender-based migration. Blue whales are filter feeders; their diet consists almost exclusively of krill. They are generally solitary or gather in small groups, and have no well-defined social structure other than mother–calf bonds. Blue whales vocalize, with a fundamental frequency ranging from 8 to 25 Hz; their vocalizations may vary by region, season, behavior, and time of day. Orcas are their only natural predators.
The blue whale was abundant in nearly all the Earth's oceans until the end of the 19th century. It was hunted almost to the point of extinction by whalers until the International Whaling Commission banned all blue whale hunting in 1966. The International Union for Conservation of Nature has listed blue whales as Endangered as of 2018. Blue whales continue to face numerous man-made threats such as ship strikes, pollution, ocean noise, and climate change.
Details
A blue whale (Balaenoptera musculus) is the most massive animal ever to have lived, a species of baleen whale that weighs approximately 150 tons and may attain a length of more than 30 metres (98 feet). The largest accurately measured blue whale was a 29.5-metre female that weighed 180 metric tons (nearly 200 short [U.S.] tons), but there are reports of 33-metre catches that may have reached 200 metric tons. Their hearts, which typically weigh about 180 kg (roughly 400 pounds), are the largest of any animal.
The blue whale is a cetacean and is classified scientifically within the order Cetacea as a rorqual (family Balaenopteridae) related to the gray whale (family Eschrichtiidae) and the right whales (Balaenidae and Neobalaenidae) of the baleen whale suborder, Mysticeti.
Blue whales are blue-gray in colour with lighter gray mottling in the form of large spots, which appear as if they were dabbed on with a huge paintbrush. The lower surfaces of the flippers are lighter gray or white in some instances. The blue whale has been called the sulfur-bottom whale because of the yellowish underside of some individuals that is reminiscent of the pale yellow colour of that chemical element; this coloration is imparted by certain algae (diatoms) living on the whale’s body. The blue whale has a wide head, a small dorsal fin located near the fluke, and 80–100 long grooves running lengthwise down the throat and chest. Its mouth contains up to 800 plates of short, wide, black baleen, or “whalebone,” with thick, coarse bristles used for catching food. Females are generally larger than males, and the largest animals live in the Southern Ocean around Antarctica.
The blue whale is found alone or in small groups in all oceans, but populations in the Southern Hemisphere are much larger. In the Northern Hemisphere, blue whales can be seen regularly in the Gulf of St. Lawrence and off the coasts of Monterey, California, and Baja California, Mexico. They spend the summer in polar waters, feeding on shrimplike crustaceans called krill. During a dive, the blue whale may engage in a series of turns and 360° rolls to locate prey and rapidly reorient its body to sweep up large concentrations of krill in a single open-mouthed lunge. A single adult blue may consume as much as eight tons of krill per day. In the winter blue whales move toward the Equator to breed. After a gestation of about 12 months, one calf about 8 metres (about 26 feet) long is born in temperate waters. While nursing, calves gain up to 90 kg (about 198 pounds) per day on the rich milk of their mothers. Young are weaned after seven to eight months, when they have reached a length of about 15 metres (about 49 feet).
Once the most important of the commercially hunted baleen whales, the blue whale was greatly reduced in numbers during the first half of the 20th century. In the 1930–31 season alone the worldwide kill of blue whales exceeded 29,000. The species has been protected from commercial whaling since the mid-1960s. Populations of blue whales appear to be recovering and are estimated worldwide at between 10,000 and 25,000 animals. However, the International Union for Conservation of Nature still lists the blue whale as an endangered species.
Additional Information
The blue whale is the largest animal on the planet, weighing as much as 200 tons (approximately 33 elephants). The blue whale has a heart the size of a Volkswagen Beetle. Its stomach can hold one ton of krill and it needs to eat about four tons of krill each day. They are the loudest animals on Earth and are even louder than a jet engine. Their calls reach 188 decibels, while a jet reaches 140 decibels. Their low frequency whistle can be heard for hundreds of miles and is probably used to attract other blue whales.
Whales are at the top of the food chain and have an important role in the overall health of the marine environment. During the 20th century, the blue whale was an important whaling target and even after it was protected and commercial whaling stopped in 1966, exploitation efforts by the former Soviet Union persisted.
Like other large whales, blue whales are threatened by environmental change including habitat loss and toxics. Blue whales can also be harmed by ship strikes and by becoming entangled in fishing gear. Although commercial whaling no longer represents a threat, climate change and its impact on krill (shrimp-like crustaceans), blue whales' major prey, makes this cetacean particularly vulnerable.

#12 Jokes » Love Jokes - I » 2025-09-14 21:37:56
- Jai Ganesh
- Replies: 0
Q: What's the difference between love and marriage?
A: Love is one long sweet dream, and marriage is the alarm clock.
* * *
Boy: "I love you so much, I could never live without you."
Girl: "Is that you or the beer talking?"
Boy: "It's me talking to the beer."
* * *
What's the definition of a happy marriage?
One where the husband gives and the wife takes.
* * *
Q: What's the difference between love and marriage?
A: Love is blind and marriage is an eye-opener!
* * *
Q: Who is the perfect husband?
A: One who keeps his mouth shut and his checkbook open!
* * *
#13 Dark Discussions at Cafe Infinity » Close Quotes - V » 2025-09-14 21:27:07
- Jai Ganesh
- Replies: 0
Close Quotes - V
1. We have a close, unshakable bond between the United States and Israel, and between the American and Israeli people. We share common values and a commitment to a democratic future for the world, and we are both committed to a two-state solution. But that doesn't mean that we're going to agree. - Hillary Clinton
2. When I was climbing, I built up a close relationship with the Sherpa people. - Edmund Hillary
3. My inner strength comes from my friends. I have a very close group of friends and family, and we all help each other through our dark times. - Kathy Bates
4. Every country should conduct its own reforms, should develop its own model, taking into account the experience of other countries, whether close neighbours or far away countries. - Mikhail Gorbachev
5. When I'm down or maybe when it's close in the match, I feel like I'm still in it. I don't feel like I'm letting down. Mentally, I'm still really, really tough. - Maria Sharapova
6. Avoid having your ego so close to your position that when your position falls, your ego goes with it. - Colin Powell
7. You can get too close as a team. You need time away from each other. You change in the same dressing room, you play on the same cricket field, you stay in the same hotel, you travel in the same planes and buses. C'mon - this business of everyone holding hands and being pally is nonsense. - Glenn Turner
8. I don't like to leave my children for long periods of time. It's made me more picky about roles that are close, especially on television. - Brooke Shields.
#14 Science HQ » Lead » 2025-09-14 17:30:11
- Jai Ganesh
- Replies: 0
Lead
Gist
Lead is a chemical element with the symbol Pb and atomic number 82. It is a heavy, dense, and soft metal known for being malleable, corrosion-resistant, and having a low melting point. Historically, lead was used in water pipes, leading to the terms "plumbing" and "plumber" from its Latin name, plumbum. While it is a stable element, it is also highly toxic and should be handled and disposed of with care.
Lead and lead compounds have been used in a wide variety of products found in and around our homes, including paint, ceramics, pipes and plumbing materials, solders, gasoline, batteries, ammunition and cosmetics
Summary
Lead is a chemical element with the symbol Pb (from the Latin plumbum) and atomic number 82. It is a heavy metal denser than most common materials. Lead is soft, malleable, and has a relatively low melting point. When freshly cut, it appears shiny gray with a bluish tint, but it tarnishes to dull gray on exposure to air. Lead has the highest atomic number of any stable element, and three of its isotopes are endpoints of major nuclear decay chains of heavier elements.
Lead is a relatively unreactive post-transition metal. Its weak metallic character is shown by its amphoteric behavior: lead and lead oxides react with both acids and bases, and it tends to form covalent bonds. Lead compounds usually occur in the +2 oxidation state rather than the +4 state common in lighter members of the carbon group, with exceptions mostly limited to organolead compounds. Like the lighter members of the group, lead can bond with itself, forming chains and polyhedral structures.
Easily extracted from its ores, lead was known to prehistoric peoples in the Near East. Galena is its principal ore and often contains silver, encouraging its widespread extraction and use in ancient Rome. Production declined after the fall of Rome and did not reach similar levels until the Industrial Revolution. Lead played a role in developing the printing press, as movable type could be readily cast from lead alloys. In 2014, annual global production was about ten million tonnes, over half from recycling. Lead's high density, low melting point, ductility, and resistance to oxidation, together with its abundance and low cost, supported its extensive use in construction, plumbing, batteries, ammunition, weights, solders, pewter, fusible alloys, lead paints, leaded gasoline, and radiation shielding.
Lead is a neurotoxin that accumulates in soft tissues and bones. It damages the nervous system, interferes with biological enzymes, and can cause neurological disorders ranging from behavioral problems to brain damage. It also affects cardiovascular and renal systems. Lead's toxicity was noted by ancient Greek and Roman writers, but became widely recognized in Europe in the late 19th century.
Details
Lead is a chemical element. Its chemical symbol is Pb, which comes from plumbum, the Latin word for lead. Its atomic number is 82, atomic mass is 207.2 and has a melting point of 327.8°C. It is a very poisonous and heavy metal, and is also the ending element to the stable elements, although the next element, bismuth, is so weakly radioactive that it can be considered stable for practical purposes.
Properties:
Physical properties
Lead is a shiny, gray-blue poor metal. It gets tarnished easily to a dull gray color. It is soft and malleable. It is very shiny when it is melted. It is very heavy. It is very corrosion-resistant. It is made stronger by adding antimony or calcium. It can form an alloy with sodium. It is toxic to people and animals when swallowed.
Chemical properties
Lead burns in air with a grayish-white flame, making toxic fumes of lead(II) oxide. Only the surface is corroded by air. It dissolves in nitric acid to make lead(II) nitrate. It does not dissolve in sulfuric or hydrochloric acid. It reacts with sodium nitrate to make lead(II) oxide and sodium nitrite. It reacts with chlorine to make lead(II) chloride. Lead(II) oxide reacts with lead sulfide to make lead metal and sulfur dioxide.
Chemical compounds
Lead makes chemical compounds in two main oxidation states: +2 and +4. +2 compounds, also known as lead(II) compounds or plumbous compounds, are weak oxidizing agents. +4 compounds, also known as lead(IV) compounds or plumbic compounds, are strong oxidizing agents. Lead compounds are toxic just like the element. The lead halides do not dissolve in water. Lead(IV) oxide is the most common lead(IV) compound. It is a black solid. The lead oxides are all colored, while the other salts are white or colorless. Lead nitrate and lead(II) acetate are the soluble compounds of lead.
Mixed oxidation state compounds
Mixed oxidation state compounds contain lead in the +2 and +4 oxidation state.
Occurrence
Lead is found very rarely in the earth's crust as a metal. Normally, lead is in the mineral galena. Galena is lead sulfide. Galena is the main lead ore.
History
Lead was used for thousands of years because it is easy to get from the ground and easy to shape and work with. The Romans used lead very commonly. They used it for pipes, drinking vessels, and fasteners.
Preparation
Lead is made from galena. Galena is made pure by froth flotation to get all the impurities out. Then the lead sulfide is roasted in a furnace to make lead(II) oxide. The lead(II) oxide is heated with coke to make liquid lead metal.
Uses:
As an element
Lead is used in the ballast of sailboats. It is also used in weight belts for scuba diving. It is also used to make shotgun pellets and bullets for small arms. Some printing presses use lead type because it can be easily shaped. It can be used outside because it does not corrode in water.
Lead has been used to make pipes and water ducts, as it is easy to form and cast, and the parts made from lead are easy to fit, and clean water does not corrode lead. The names "plumbing" for waterworks, and "plumber" for a pipe fitter, come from the Latin name of lead, plumbum.
Most lead is used in lead acid batteries, though. The lead is oxidized, making electricity. Sheets of lead are used to block sound in some places. Lead is used in radiation shielding. Molten lead can be used as a coolant in nuclear reactors. It used to be mixed with tin to make the pipes in pipe organs. Different amounts of lead make different sounds. In addition, lead has found its usage in solder.
It is used in some solder. It is used in covering for wires that carry high voltage. Some tennis rackets have lead in them to make them heavier. It is used to balance wheels of cars, to make statues, and to make decorative looks in buildings.
As chemical compounds
Many lead compounds are used to make colored glazes in ceramics. Lead can be used in PVC pipes. Lead compounds are added to candles to make them burn better. Lead glass has lead(II) oxide in it. Lead compounds are still used as pigments in some places. Lead compounds were added to gasoline, but are now outlawed. Some lead compounds are semiconductors and are used in photodetectors.
Old uses
Lead was used in many red, yellow, and white pigments in paints. Lead was also used in pesticides. Lead used to be used in pipes carrying water, but now it is not because lead can leach into the water.
Safety
Although it can be safely touched, exposure to lead should be avoided – it is very toxic to humans and other animals when swallowed, and its use is restricted in many countries.
If someone is exposed to lead for a long time, it ruins their kidneys and gives them abdominal pains. Lead also ruins the nervous system. Lead paint was being eaten by children and they were getting lead poisoning.
The best way to understand lead and its properties is to read its MSDS.
Additional Information
Lead (Pb) is a soft, silvery white or grayish metal in Group 14 (IVa) of the periodic table. Lead is very malleable, ductile, and dense and is a poor conductor of electricity. Known in antiquity and believed by the alchemists to be the oldest of metals, lead is highly durable and resistant to corrosion, as is indicated by the continuing use of lead water pipes installed by the ancient Romans. The symbol Pb for lead is an abbreviation of the Latin word for lead, plumbum.
Element Properties
atomic number : 82
atomic weight : 207.19
melting point : 327.5 °C (621.5 °F)
boiling point : 1,744 °C (3,171.2 °F)
density : 11.29 gram/{cm}^{3} at 20 °C (68 °F)
oxidation states : +2, +4
Occurrence and distribution
Lead is mentioned often in early biblical accounts. The Babylonians used the metal as plates on which to record inscriptions. The Romans used it for tablets, water pipes, coins, and even cooking utensils; indeed, as a result of the last use, lead poisoning was recognized in the time of Augustus Caesar. The compound known as white lead was apparently prepared as a decorative pigment at least as early as 200 bce. Modern developments date to the exploitation in the late 1700s of deposits in the Missouri-Kansas-Oklahoma area in the United States.
On a weight basis, lead has nearly the same abundance in Earth’s crust as tin. Cosmically, there is 0.47 lead atom per 106 silicon atoms. The cosmic abundance is comparable to those of cesium, praseodymium, hafnium, and tungsten, each of which is regarded as a reasonably scarce element.
Although lead is not abundant, natural concentration processes have resulted in substantial deposits of commercial significance, particularly in the United States but also in Canada, Australia, Spain, Germany, Africa, and South America. Significant deposits are found in the United States in the western states and the Mississippi valley. Rarely found free in nature, lead is present in several minerals, but all are of minor significance except the sulfide, PbS (galena, or lead glance), which is the major source of lead production throughout the world. Lead is also found in anglesite (PbSO4) and cerussite (PbCO3). By the early 21st century, China, Australia, the United States, Peru, Mexico, and India were the world’s top producers of lead in concentrate.
Lead may be extracted by roasting the ore and then smelting it in a blast furnace or by direct smelting without roasting. Additional refining removes impurities present in the lead bullion produced by either process. Almost half of all refined lead is recovered from recycled scrap.
Uses of the metal
Only a single crystalline modification, with a close-packed metallic lattice, is known. Properties that are responsible for the many uses of elemental lead include its ductility, ease of welding, low melting point, high density, and ability to absorb gamma radiation and X-radiation. Molten lead is an excellent solvent and collector for elemental silver and gold. The structural applications of lead are limited by its low tensile and fatigue strengths and its tendency to flow even when only lightly loaded.
When freshly cut, lead oxidizes quickly, forming a dull gray coating, formerly thought to be lead suboxide, Pb2O, but now recognized as a mixture of lead and lead monoxide, PbO, which protects the metal from further corrosion. Similarly, although lead is soluble in dilute nitric acid, it is only superficially attacked by hydrochloric or sulfuric acids because the insoluble chloride (PbCl2) or sulfate (PbSO4) coatings that are formed prevent continued reaction. Because of this general chemical resistance, considerable amounts of lead are used in roofing, as coverings for electric cables placed in the ground or underwater, and as linings for water pipes and conduits and structures for the transportation and processing of corrosive substances.
Elemental lead can also be oxidized to the Pb2+ ion by hydrogen ions, but the insolubility of most salts of Pb2+ makes lead resistant to attack by many acids. Oxidation under alkaline conditions is easier to effect and is favoured by the formation of the soluble species of lead in the +2 oxidation state. Lead oxide (PbO2, with lead as the Pb4+ ion) is among the stronger oxidizing agents in acidic solution, but it is comparatively weak in alkaline solution. The ease of oxidation of lead is enhanced by complex formation. The electrodeposition of lead is best effected from aqueous solutions containing lead hexafluorosilicate and hexafluorosilicic acid.
Lead has many other applications, the largest of which is in the manufacture of storage batteries. It is used in ammunition (shot and bullets) and as a constituent of solder, type metal, bearing alloys, fusible alloys, and pewter. In heavy and industrial machinery, sheets and other parts made from lead compounds may be used to dampen noise and vibration. Because lead effectively absorbs electromagnetic radiation of short wavelengths, it is used as a protective shielding around nuclear reactors, particle accelerators, X-ray equipment, and containers used for transporting and storing radioactive materials. Together with the compound lead oxide (PbO2) and with lead-antimony or lead-calcium alloys, it is employed in common storage batteries.
Properties of the element
Lead and its compounds are toxic and are retained by the body, accumulating over a long period of time—a phenomenon known as cumulative poisoning—until a lethal quantity is reached. The toxicity of lead compounds increases as their solubility increases. In children the accumulation of lead may result in cognitive deficits; in adults it may produce progressive renal disease. Symptoms of lead poisoning include abdominal pain and diarrhea followed by constipation, nausea, vomiting, dizziness, headache, and general weakness. Elimination of contact with a lead source is normally sufficient to effect a cure. The elimination of lead from insecticides and paint pigments and the use of respirators and other protective devices in areas of exposure have reduced lead poisoning materially. The recognition that the use of tetraethyl lead, Pb(C2H5)4, as an antiknock additive in gasoline was polluting the air and water led to the compound’s elimination as a gasoline constituent in the 1980s. (For full treatment of lead and lead mining and refining, see also lead poisoning.)
Nuclear properties
Lead is formed both by neutron-absorption processes and the decay of radionuclides of heavier elements. Lead has four stable isotopes; their relative abundances are lead-204, 1.48 percent; lead-206, 23.6 percent; lead-207, 22.6 percent; and lead-208, 52.3 percent. Three stable lead nuclides are the end products of radioactive decay in the three natural decay series: uranium (decays to lead-206), thorium (decays to lead-208), and actinium (decays to lead-207). More than 30 radioactive isotopes have been reported. Of the radioactive isotopes of lead, the following appear as members of the three natural decay series: (1) thorium series: lead-212; (2) uranium series: lead-214 and lead-210; (3) actinium series: lead-211. The atomic weight of natural lead varies from source to source, depending on its origin by heavier element decay.
Compounds
Lead shows oxidation states of +2 and +4 in its compounds. Among the many important lead compounds are the oxides: lead monoxide, PbO, in which lead is in the +2 state; lead dioxide, PbO2, in which lead is in the +4 state; and trilead tetroxide, Pb3O4. Lead monoxide exists in two modifications, litharge and massicot. Litharge, or alpha lead monoxide, is a red or reddish yellow solid, has a tetragonal crystal structure, and is the stable form at temperatures below 488 °C (910 °F). Massicot, or beta lead monoxide, is a yellow solid and has an orthorhombic crystal structure; it is the stable form above 488 °C. Both forms are insoluble in water but dissolve in acids to form salts containing the Pb2+ ion or in alkalies to form plumbites, which have the PbO22− ion. Litharge, which is produced by air oxidation of lead, is the most important commercial compound of lead; it is used in large amounts directly and as the starting material for the preparation of other lead compounds. Considerable quantities of PbO are consumed in manufacturing the plates of lead-acid storage batteries. High-quality glassware (lead crystal) contains as much as 30 percent litharge, which increases the refractive index of the glass and makes it brilliant, strong, and resonant. Litharge is also employed as a drier in varnishes and in making sodium plumbite, which is used for removing malodorous thiols (a family of organic compounds containing sulfur) from gasoline.
PbO2, found in nature as the brown-to-black mineral plattnerite, is commercially produced from trilead tetroxide by oxidation with chlorine. It decomposes upon heating and yields oxygen and lower oxides of lead. PbO2 is used as an oxidizing agent in the production of dyestuffs, chemicals, pyrotechnics, and matches and as a curing agent for polysulfide rubbers. Trilead tetroxide (known as red lead, or minium) is produced by further oxidation of PbO. It is the orange-red to brick-red pigment commonly used in corrosion-resistant paints for exposed iron and steel. It also reacts with ferric oxide to form a ferrite used in making permanent magnets.
Another economically significant compound of lead in the +2 oxidation state is lead acetate, Pb(C2H3O2)2, a water-soluble salt made by dissolving litharge in concentrated acetic acid. The common form, the trihydrate, Pb(C2H3O2)2·3H2O, called sugar of lead, is used as a mordant in dyeing and as a drier in certain paints. In addition, it is utilized in the production of other lead compounds and in gold cyanidation plants, where it primarily serves to precipitate soluble sulfides from solution as PbS.
Various other salts, most notably basic lead carbonate, basic lead sulfate, and basic lead silicate, were once widely employed as pigments for white exterior paints. Since the mid-20th century, however, the use of such so-called white lead pigments has decreased substantially because of a concern over their toxicity and attendant hazard to human health. The use of lead math in insecticides has virtually been eliminated for the same reason.
More Information:
Appearance
A dull, silvery-grey metal. It is soft and easily worked into sheets.
Uses
This easily worked and corrosion-resistant metal has been used for pipes, pewter and paint since Roman times. It has also been used in lead glazes for pottery and, in this century, insecticides, hair dyes and as an anti-knocking additive for petrol. All these uses have now been banned, replaced or discouraged as lead is known to be detrimental to health, particularly that of children.
Lead is still widely used for car batteries, pigments, ammunition, cable sheathing, weights for lifting, weight belts for diving, lead crystal glass, radiation protection and in some solders.
It is often used to store corrosive liquids. It is also sometimes used in architecture, for roofing and in stained glass windows.
Biological role
Lead has no known biological role. It can accumulate in the body and cause serious health problems. It is toxic, teratogenic (disturbs the development of an embryo or foetus) and carcinogenic.
Daily intake of lead from all sources is about 0.1 milligrams. The average human body stores about 120 milligrams of lead in the bones.
Natural abundance
Lead is chiefly obtained from the mineral galena by a roasting process. At least 40% of lead in the UK is recycled from secondary sources such as scrap batteries and pipes.

#15 Re: Jai Ganesh's Puzzles » General Quiz » 2025-09-14 16:40:07
Hi,
#10553. What does the term in Geography Border mean?
#10554. What does the term in Geography Bornhardt mean?
#16 Re: Jai Ganesh's Puzzles » English language puzzles » 2025-09-14 16:22:22
Hi,
#5747. What does the noun matador mean?
#5748. What does the noun matchwood mean?
#17 Re: Jai Ganesh's Puzzles » Doc, Doc! » 2025-09-14 15:58:50
Hi,
#2467. What does the medical term Doppler ultrasonography mean?
#18 Re: Jai Ganesh's Puzzles » 10 second questions » 2025-09-14 15:02:39
Hi,
#9733.
#19 Re: Jai Ganesh's Puzzles » Oral puzzles » 2025-09-14 14:30:02
Hi,
#6240.
#20 Re: Exercises » Compute the solution: » 2025-09-14 14:10:41
Hi,
2583.
#21 Re: Dark Discussions at Cafe Infinity » crème de la crème » 2025-09-13 18:32:39
2335) Demis Hassabis
Gist
Work:
Proteins control and drive all the chemical reactions that together are the basis of life. Proteins generally consist of 20 different amino acids. These are linked together in long strings that fold up to make a three-dimensional structure. In 2020, Demis Hassabis and John Jumper presented an AI model called AlphaFold2. With its help, they have been able to predict the structure of virtually all known proteins. AlphaFold2 has been widely used in many areas, including research into pharmaceuticals and environmental technology.
Summary
Demis Hassabis (born July 27, 1976, London, England) is an English computer scientist who was awarded the 2024 Nobel Prize in Chemistry for his work using artificial intelligence (AI) to predict protein structures. He shared half the prize with his colleague, American computer scientist John M. Jumper, and the other half of the prize was awarded to American biochemist David Baker.
Early life and career
Hassabis spent his early life in north London. The eldest son of a Greek Cypriot father and a Chinese Singaporean mother, Hassabis developed a keen interest in chess and computer programming. A chess player from age four, Hassabis obtained the rank of chess master at age 13. His affection for computer games inspired him to code his first game, a version of Othello, and teach himself computer programming from books.
Hassabis would go on to write code for the games company Bullfrog Productions, where by age 17 he served as the lead developer of the AI-generated video game Theme Park (1994). During this period he attended grammar school at Queen Elizabeth’s School and secondary school at Christ’s College, Finchley. At Finchley he completed his examinations at age 16, two years early.
During the 1990s Hassabis studied computer science at the University of Cambridge, where he captained the college’s chess team. After graduating from Cambridge in 1997, Hassabis became the lead AI programmer at Lionhead Studios, where he assisted in the development of the game Black & White (2001). He left Lionhead Studios in 1998 to found Elixir Studios, where he went on to produce a number of award-winning AI-based video games, including Republic: The Revolution (2003) and Evil Genius (2004).
After selling his holdings in Elixir Studios in 2005, Hassabis took his passion for AI algorithms in a different direction. He pursued a Ph.D. in cognitive neuroscience at the University College London (UCL), in large part to understand how the human brain managed imagination and memory. After graduating from UCL in 2009, Hassabis did his postdoctoral work at Harvard University and the Massachusetts Institute of Technology (MIT) and became a Henry Wellcome fellow at UCL.
DeepMind
Hassabis founded DeepMind, an AI-based start-up company, along with New Zealand computer scientist Shane Legg and English entrepreneur Mustafa Suleyman in 2011. Over the next four years he developed a series of AI models and trained them using deep learning to play and master video games without teaching the AI the rules. Hassabis and his colleagues sold DeepMind to Google in 2014, with Hassabis remaining to serve as CEO.
The DeepMind AI became the platform for AlphaGo, which would defeat top go player Lee Sedol four games to one in 2016. AlphaGo’s neural networks had learned to play go from human players and by playing itself. AlphaGo was in turn surpassed by AlphaGo Zero, which, starting from only the rules of go, shut out AlphaGo 100 games to 0. A more general neural network, Alpha Zero, used the same techniques to quickly master chess and shogi.
AI solves protein folding
Despite the media interest generated by the victories of DeepMind’s game-playing AIs, Hassabis was actually interested in applying what he learned from those AI successes to scientific problems, specifically that of protein folding. Proteins are large molecules that are directly involved in the chemical processes essential for life and are built up from 20 amino acids that can be combined in many different ways. The function of a protein is determined by its three-dimensional structure, which can be quite complex, based on how the string of amino acids is folded.
How a protein is folded is determined by its amino acid sequence. However, even a small protein of only 100 amino acids can have 1047 possible three-dimensional structures. Predicting a protein’s structure from its amino acid sequence became a key problem in molecular biology.
In 1994 biologists John Moult and Krzysztof Fidelis founded the Critical Assessment of protein Structure Prediction (CASP) challenge to test methods for predicting protein structures. Every two years, contestants were given the amino acid sequences for proteins whose structure had been determined but not published and were challenged to predict the protein structures.
Progress was slow. By the mid-2010s the best models in the CASP challenge were about 40 percent accurate. DeepMind entered its protein structure program AlphaFold in CASP13 in 2018 and delivered an astonishing accuracy of about 60 percent, far ahead of any competitors. However, improvement beyond that was difficult, until Jumper joined DeepMind and used his experience with protein simulation to help develop AlphaFold2.
AlphaFold2 was trained on databases of amino acid sequences and protein structures and used a neural network called a transformer to find a likely protein structure. At CASP14 in 2020, AlphaFold2 reached an accuracy of 90 percent, which is comparable with experimental results. The problem of finding a protein structure given an amino acid sequence had been solved.
Hassabis, Jumper, and their collaborators used AlphaFold2 to calculate the structure of almost all of the more than 50,000 human proteins in 2021. They then went even further and calculated the structures of almost all of the 200 million known proteins, which come from about 1 million different species, or as Hassabis called it, “the entire protein universe.”
Hassabis has garnered a number of accolades, including the Royal Society’s Mullard Award (2014), the Pius XI Medal from the Pontifical Academy of Sciences (2020), and the Wiley Prize in Biomedical Sciences (2022). In 2023 he was awarded the Breakthrough Prize in Life Sciences, the Canada Gairdner International Award, and the Albert Lasker Basic Medical Research Award.
Details
Sir Demis Hassabis (born 27 July 1976) is a British artificial intelligence (AI) researcher and entrepreneur. He is the chief executive officer and co-founder of Google DeepMind and Isomorphic Labs, and a UK Government AI Adviser. In 2024, Hassabis and John M. Jumper were jointly awarded the Nobel Prize in Chemistry for their AI research contributions for protein structure prediction.
Hassabis is a Fellow of the Royal Society and has won many prestigious awards for his research efforts, including the Breakthrough Prize, the Canada Gairdner International Award and the Lasker Award. In 2017 he was appointed a CBE and was included in the Time 100, a list of the most influential people in the world. In 2024 Hassabis was knighted for his work on AI. He was listed in the Time 100 again in 2025, this time featured in one of the five covers of the printed version.
Early life and education
Hassabis was born to Costas and Angela Hassabis. His father is Greek Cypriot and his mother is from Singapore. Demis grew up in North London. In his early career he was a video game AI programmer and designer and an expert board games player. A child prodigy in chess from the age of four, Hassabis reached master standard at the age of 13 with an Elo rating of 2300 (at the time the second-highest rated player in the world for his age group after Judit Polgar) and captained many of the England junior chess teams. He represented the University of Cambridge in the Oxford–Cambridge varsity chess matches of 1995, 1996 and 1997, winning a half blue.
He first got interested in technology after buying his first computer in 1984, a ZX Spectrum 48K, funded from chess winnings. He taught himself how to program from books. He subsequently wrote his first AI program on a Commodore Amiga to play the reversi board game.
Between 1988 and 1990 Hassabis was educated at Queen Elizabeth's School, Barnet, a boys' grammar school in North London. He was subsequently home-schooled by his parents for a year, before studying at the comprehensive school Christ's College, Finchley. He completed his A-level exams a year early at 16.
Bullfrog Productions
Asked by Cambridge University to take a gap year owing to his young age, Hassabis began his computer games career at Bullfrog Productions after entering an Amiga Power "Win-a-job-at-Bullfrog" competition. He began by level designing on Syndicate and then at 17 co-designing and lead-programming on the 1994 game Theme Park, with the game's designer Peter Molyneux. Theme Park, a simulation video game, sold several million copies and inspired a whole genre of simulation sandbox games. He earned enough from his gap year to pay his own way through university.
University of Cambridge
Hassabis left Bullfrog to study at Queens' College, Cambridge, where he completed the Computer Science Tripos and graduated in 1997 with a double first.
Career and research:
Lionhead
After graduating from Cambridge, Hassabis worked at Lionhead Studios. Games designer Peter Molyneux, with whom Hassabis had worked at Bullfrog Productions, had recently founded the company. At Lionhead, Hassabis worked as lead AI programmer on the 2001 god game Black & White.
Elixir Studios
Hassabis left Lionhead in 1998 to found Elixir Studios, a London-based independent games developer, signing publishing deals with Eidos Interactive, Vivendi Universal and Microsoft. In addition to managing the company, Hassabis served as executive designer of the games Republic: The Revolution and Evil Genius. Each received BAFTA nominations for their interactive music scores, created by James Hannigan.
The release of Elixir's first game, Republic: The Revolution, a highly ambitious and unusual political simulation game, was delayed due to its huge scope, which involved an AI simulation of the workings of an entire fictional country. The final game was reduced from its original vision and greeted with lukewarm reviews, receiving a Metacritic score of 62/100. Evil Genius, a tongue-in-cheek Austin Powers parody, fared much better with a score of 75/100. In April 2005 the intellectual property and technology rights were sold to various publishers and the studio was closed.
Neuroscience research
Following Elixir Studios, Hassabis returned to academia to obtain his PhD in cognitive neuroscience from UCL Queen Square Institute of Neurology in 2009 supervised by Eleanor Maguire. He sought to find inspiration in the human brain for new AI algorithms.
He continued his neuroscience and artificial intelligence research as a visiting scientist jointly at Massachusetts Institute of Technology (MIT), in the lab of Tomaso Poggio, and Harvard University, before earning a Henry Wellcome postdoctoral research fellowship to the Gatsby Computational Neuroscience Unit at UCL in 2009 working with Peter Dayan.
Working in the field of imagination, memory, and amnesia, he co-authored several influential papers published in Nature, Science, Neuron, and PNAS. His very first academic work, published in PNAS, was a landmark paper that showed systematically for the first time that patients with damage to their hippocampus, known to cause amnesia, were also unable to imagine themselves in new experiences. The finding established a link between the constructive process of imagination and the reconstructive process of episodic memory recall. Based on this work and a follow-up functional magnetic resonance imaging (fMRI) study, Hassabis developed a new theoretical account of the episodic memory system identifying scene construction, the generation and online maintenance of a complex and coherent scene, as a key process underlying both memory recall and imagination. This work received widespread coverage in the mainstream media and was listed in the top 10 scientific breakthroughs of the year by the journal Science. He later generalised these ideas to advance the notion of a 'simulation engine of the mind' whose role it was to imagine events and scenarios to aid with better planning.
DeepMind
Hassabis is the CEO and co-founder of DeepMind, a machine learning AI startup, founded in London in 2010 with Shane Legg and Mustafa Suleyman. Hassabis met Legg when both were postdocs at the Gatsby Computational Neuroscience Unit, and he and Suleyman had been friends through family. Hassabis also recruited his university friend and Elixir partner David Silver.
DeepMind's mission is to "solve intelligence" and then use intelligence "to solve everything else". More concretely, DeepMind aims to combine insights from systems neuroscience with new developments in machine learning and computing hardware to unlock increasingly powerful general-purpose learning algorithms that will work towards the creation of an artificial general intelligence (AGI). The company has focused on training learning algorithms to master games, and in December 2013 it announced that it had made a pioneering breakthrough by training an algorithm called a Deep Q-Network (DQN) to play Atari games at a superhuman level by using only the raw pixels on the screen as inputs.
DeepMind's early investors included several high-profile tech entrepreneurs. In 2014, Google purchased DeepMind for £400 million. Although most of the company has remained an independent entity based in London, DeepMind Health has since been directly incorporated into Google Health.
Since the Google acquisition, the company has notched up a number of significant achievements, perhaps the most notable being the creation of AlphaGo, a program that defeated world champion Lee Sedol at the complex game of Go. Go had been considered a holy grail of AI, for its high number of possible board positions and resistance to existing programming techniques. However, AlphaGo beat European champion Fan Hui 5–0 in October 2015 before winning 4–1 against former world champion Lee Sedol in March 2016 and winning 3–0 against the world's top-ranked player Ke Jie in 2017. Additional DeepMind accomplishments include creating a neural Turing machine, reducing the energy used by the cooling systems in Google's data centers by 40%, advancing research on AI safety, and the creation of a partnership with the National Health Service (NHS) of the United Kingdom and Moorfields Eye Hospital to improve medical services and identify the onset of degenerative eye conditions.
DeepMind has also been responsible for technical advances in machine learning, having produced a number of award-winning papers. In particular, the company has made significant advances in deep learning and reinforcement learning, and pioneered the field of deep reinforcement learning which combines these two methods. Hassabis has predicted that artificial intelligence will be "one of the most beneficial technologies of mankind ever" but that significant ethical issues remain.
Hassabis has also warned of the potential dangers and risks of AI if misused, and has been a strong advocate of further AI safety research being needed. In 2023, he signed the statement that "Mitigating the risk of extinction from AI should be a global priority alongside other societal-scale risks such as pandemics and nuclear war". He considers however that a pause on AI progress would be very hard to enforce worldwide, and that the potential benefits (e.g. for health and against climate change) make it worth continuing. He said that there is an urgent need for research on evaluation tests that measure how capable and controllable new AI models are.
AlphaFold
In 2016, DeepMind turned its artificial intelligence to protein structure prediction, a 50-year grand challenge in science, to predict the 3D structure of a protein from its 1D amino acid sequence. This is an important problem in biology, as proteins are essential to life, almost every biological function depends on them, and the function of a protein is thought to be related to its structure. Knowing the structure of a protein can be very helpful in drug discovery and disease understanding. In December 2018, DeepMind's tool AlphaFold won the 13th Critical Assessment of Techniques for Protein Structure Prediction (CASP) by successfully predicting the most accurate structure for 25 out of 43 proteins. "This is a lighthouse project, our first major investment in terms of people and resources into a fundamental, very important, real-world scientific problem", Hassabis said to The Guardian.
In November 2020, DeepMind again announced world-beating results in the CASP14 edition of the competition with AlphaFold 2, a new version of the system. It achieved a median global distance test (GDT) score of 87.0 across protein targets in the challenging free-modeling category, much higher than the same 2018 results with a median GDT < 60, and an overall error of less than the width of an atom (<1 Angstrom), making it competitive with experimental methods, and leading the organisers of CASP to declare the problem essentially solved. Over the next year DeepMind used AlphaFold2 to fold all 200 million proteins known to science, and made the system and these structures openly and freely available for anyone to use via the AlphaFold Protein Structure Database developed in collaboration with EMBL-EBI.
Personal life
Hassabis resides in North London with his family. He is also a lifelong fan of Liverpool FC. Hassabis is the main subject of the documentary called The Thinking Game, which premiered at the 2024's Tribeca Festival, from the same filmmaker as the award-winning documentary AlphaGo (2017) which chronicles the famous 2016 $1M challenge match in Seoul, South Korea, between Lee Sedol and AlphaGo.

#22 Re: Jai Ganesh's Puzzles » General Quiz » 2025-09-13 16:35:20
Hi,
#10551. What does the term in Geography Body of water mean?
#10552. What does the term in Geography Bog mean?
#23 Re: Jai Ganesh's Puzzles » English language puzzles » 2025-09-13 16:16:07
Hi,
#5745. What does the adjective gregarious mean?
#5746. What does the noun gremlin mean?
#24 This is Cool » Glucose » 2025-09-13 15:52:10
- Jai Ganesh
- Replies: 0
Glucose
Gist
Glucose is a simple sugar (a monosaccharide) with the chemical formula C₆H₁₂O₆ that serves as the body's primary energy source. Your body derives glucose from the food you eat, and it is transported through the bloodstream to cells, where hormones like insulin help regulate its uptake for energy production (ATP) and storage as glycogen. Plants produce glucose through photosynthesis, and it is central to the metabolism of all living organisms, fueling virtually all energy-requiring processes.
Glucose is a simple sugar and the main type of carbohydrate that serves as the primary source of energy for the body's cells, fueling functions from muscle contraction to nerve impulse conduction. It is carried in the bloodstream as blood sugar or blood glucose and enters cells with the help of the hormone insulin. Your body obtains glucose from the foods you eat, particularly carbohydrates like fruits, bread, and pasta, which are broken down into this fundamental fuel, or it can produce glucose from other substances through processes like gluconeogenesis.
Summary
Glucose is a sugar with the molecular formula C6H12O6. It is the most abundant monosaccharide, a subcategory of carbohydrates. It is made from water and carbon dioxide during photosynthesis by plants and most algae. It is used by plants to make cellulose, the most abundant carbohydrate in the world, for use in cell walls, and by all living organisms to make adenosine triphosphate (ATP), which is used by the cell as energy. Glucose is often abbreviated as Glc.
In energy metabolism, glucose is the most important source of energy in all organisms. Glucose for metabolism is stored as a polymer, in plants mainly as amylose and amylopectin, and in animals as glycogen. Glucose circulates in the blood of animals as blood sugar. The naturally occurring form is d-glucose, while its stereoisomer l-glucose is produced synthetically in comparatively small amounts and is less biologically active. Glucose is a monosaccharide containing six carbon atoms and an aldehyde group, and is therefore an aldohexose. The glucose molecule can exist in an open-chain (acyclic) as well as ring (cyclic) form. Glucose is naturally occurring and is found in its free state in fruits and other parts of plants. In animals, it is released from the breakdown of glycogen in a process known as glycogenolysis.
Glucose, as intravenous sugar solution, is on the World Health Organization's List of Essential Medicines. It is also on the list in combination with sodium chloride (table salt).
Details
Dietary glucose is a monosaccharide (simple sugar), making it the simplest type of carbohydrate (carb).
When you consume dietary glucose, your body converts it into blood glucose. This is one of your body’s primary fuel sources, along with fat and protein.
According to the American Heart Association, the body digests complex carbs more slowly than simple carbs, making them a healthier and steadier energy source.
If you’re living with diabetes, perhaps more important is that complex carbs release glucose into the bloodstream gradually rather than immediately. This makes them less likely to cause blood glucose spikes.
Unmanaged glucose levels may have permanent and severe effects.
How does the body process glucose?
Your body ideally uses glucose multiple times per day.
When you eat, your body quickly starts processing glucose and other carbohydrates. Then, enzymes begin to break them down with help from the pancreas.
The pancreas plays a key roleTrusted Source in the way your body metabolizes glucose.
When blood glucose levels increase, the pancreas releases a hormone called insulin. This manages the rising blood sugar level by getting glucose into your cells.
Then, muscle, fat, and other cells use glucose for energy or store it as fat for later use.
If your pancreas doesn’t produce insulin the way it should, you may develop diabetes. In this case, you may need medical treatment to help process and regulate glucose in the body.
Insulin resistance
A 2018 review suggests that diabetes may also occur from insulin resistance. This is when the body’s cells do not sense insulin, and too much sugar remains in the bloodstream.
When the body doesn’t respond to insulin the way it should, it stops glucose from entering your cells and being used for energy. Your cells respond by signaling the creation of ketones, which occurs at night and during fasting or dieting.
Over time, insulin resistance may lead to low insulin levels, according to the American Diabetes Association (ADA). Your body may also release fat from fat cells, and the liver will keep releasing ketones, lowering your blood pH to an acidic level.
This typically occurs in type 1 diabetes, where there’s little to no insulin production.
In type 2 diabetes, insulin levels do eventually decrease, but typically not to a level that raises ketones high enough to cause the blood to be acidic.
When your body cannot use glucose properly, the buildup of ketones and changes in blood pH may lead to ketoacidosis. This is a severe, life threatening complication of diabetes that requires immediate medical treatment.
Ketogenic diet and diabetes
The keto diet has gained popularity, but it’s a medical diet with risks.
According to a 2019 study, a low carb or keto diet may reduce body weight, but people with diabetes and taking certain medications may have an increased risk of developing ketoacidosis.
Everyone may experience other adverse effects, such as high cholesterol, which is associated with cardiovascular disease.
It’s best to speak with your doctor before starting any diet plan to help prevent complications.
How do you test your glucose?
Monitoring glucose levels is important for people with diabetes.
A simple blood test called a blood glucose meter is one of the most common ways to test glucose at home when living with diabetes, according to the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)Trusted Source.
Here’s how to use a blood glucose meter:
* Using a small lancet needle, prick the side of your fingertip to produce a drop of blood.
* Apply the blood to a testing strip.
* Place the strip into a meter.
* The meter shows how much glucose is in your blood at that moment.
* Working with a doctor to help set your glucose goals is important, as these depend on factors like your condition, age, and health history.
How often should you check your blood sugar levels?
Your needs, goals, and treatment plan may dictate how often and when to check your blood sugar level.
To stay on top of your glucose levels, speak with a doctor about when and how frequently you should check your levels. They may suggest checking your levels at the following times:
* before and after meals
* before and after exercise
* during long or intense exercise
* before bedtime
* when starting new medications or a new insulin schedule
* when starting a new work schedule
* when traveling across time zones
Continuous glucose monitor
When managing diabetes, you may consider using a continuous glucose monitoring (CGM) system. The device automatically tracks your glucose 24 hours per day and alerts you when it gets too high or low.
According to the NIDDK, the benefits of a CGM include:
* needing fewer finger pricks
* helping better manage glucose
* leading to fewer emergencies.
Additional Information
Glucose is a one of a group of carbohydrates known as simple sugars (monosaccharides). Glucose (from Greek glykys; “sweet”) has the molecular formula C6H12O6. It is found in fruits and honey and is the major free sugar circulating in the blood of higher animals.
Glucose is the source of energy in cell function. The regulation of its metabolism is of great importance and is relevant in various metabolic processes, examples being fermentation and gluconeogenesis. Molecules of starch, the major energy-reserve carbohydrate of plants, consist of thousands of linear glucose units. Another major compound composed of glucose is cellulose, which is also linear. Dextrose is the molecule d-glucose.
The maintenance of the glucose content of vertebrate blood requires glucose 6-phosphate to be converted to glucose. This process occurs in the kidney, in the lining of the intestine, and most importantly in the liver. The liver stores excess glucose as glycogen, a reserve carbohydrate, and releases it when blood glucose levels drop, thereby preventing hypoglycemia. In addition, the liver can produce glucose from non-carbohydrate sources through gluconeogenesis, which helps ensure a steady supply of glucose for the body.

#25 Re: Jai Ganesh's Puzzles » Doc, Doc! » 2025-09-13 14:45:13
Hi,
#2466. What does the medical term Brachiocephalic artery mean?