Math Is Fun Forum
You are not logged in.
- Topics: Active | Unanswered
#451 Re: Jai Ganesh's Puzzles » English language puzzles » 2025-10-12 15:01:43
Hi,
#5803. What does the adjective livid mean?
#5804. What does the noun llama mean?
#452 Re: Jai Ganesh's Puzzles » Doc, Doc! » 2025-10-12 14:49:56
Hi,
#2494. What does the medical term Bypass surgery mean?
#453 Re: Jai Ganesh's Puzzles » 10 second questions » 2025-10-12 14:41:13
Hi,
#9762.
#454 Re: Jai Ganesh's Puzzles » Oral puzzles » 2025-10-12 14:27:55
Hi,
#6268.
#455 Re: Exercises » Compute the solution: » 2025-10-12 14:11:19
Hi,
2611.
#456 Re: Dark Discussions at Cafe Infinity » crème de la crème » 2025-10-11 16:49:30
2362) Gerhard Herzberg
Gist:
Work
Our world consists of atoms that are assembled in molecules. During many chemical reactions, molecules are broken down into smaller parts, free radicals, that are quickly combined with other parts and form new molecules. Molecules absorb light of fixed wavelengths, and these light spectrums can be used with quantum mechanical calculations to figure out how different molecules are constructed. Gerhard Herzberg developed these methods, and during the 1950s and 1960s he mapped out the chemical structure of a great many free radicals.
Summary
Gerhard Herzberg (born Dec. 25, 1904, Hamburg, Ger.—died March 3, 1999, Ottawa, Ont., Can.) was a Canadian physicist and winner of the 1971 Nobel Prize for Chemistry for his work in determining the electronic structure and geometry of molecules, especially free radicals—groups of atoms that contain odd numbers of electrons. His work provided the foundation for molecular spectroscopy.
Herzberg became Privatdozent (unsalaried lecturer) at the Darmstadt Institute of Technology in 1930 but fled Nazi Germany in 1935 and obtained a position with the University of Saskatchewan. From 1945 to 1948 he worked at the University of Chicago’s Yerkes Observatory in Williams Bay, Wisconsin, after which he returned to Canada, where he joined the National Research Council, Ottawa.
Herzberg’s spectroscopic studies not only provided experimental results of prime importance to physical chemistry and quantum mechanics but also helped stimulate a resurgence of investigations into the chemical reactions of gases. He devoted much of his research to diatomic molecules, in particular the most common ones—hydrogen, oxygen, nitrogen, and carbon monoxide. He discovered the spectra of certain free radicals that are intermediate stages in numerous chemical reactions, and he was the first to identify the spectra of certain radicals in interstellar gas. Herzberg also contributed much spectrographic information on the atmospheres of the outer planets and the stars. His most important works are Atomspektren und Atomstruktur (1936; Atomic Spectra and Atomic Structure) and a long-standing reference work, the four-volume Molecular Spectra and Molecular Structure (1939–79).
Details
Gerhard Heinrich Friedrich Otto Julius Herzberg (December 25, 1904 – March 3, 1999) was a German-Canadian pioneering physicist and physical chemist, who won the Nobel Prize for Chemistry in 1971, "for his contributions to the knowledge of electronic structure and geometry of molecules, particularly free radicals". Herzberg's main work concerned atomic and molecular spectroscopy. He is well known for using these techniques that determine the structures of diatomic and polyatomic molecules, including free radicals which are difficult to investigate in any other way, and for the chemical analysis of astronomical objects. Herzberg served as Chancellor of Carleton University in Ottawa, Canada from 1973 to 1980.
Early life and family
Herzberg was born in Hamburg, Germany on December 25, 1904 to Albin H. Herzberg and Ella Biber. He had an older brother, Walter, who was born in January 1904. Herzberg started Vorschule (pre-school) late, after contracting measles. Gerhard and his family were atheists and kept this fact hidden. His father died in 1914, at 43 years of age, after having suffered from dropsy and complications due to an earlier heart condition. Herzberg graduated Vorschule shortly after his father's death. He married Luise Herzberg (née Oettinger), a spectroscopist and fellow researcher in 1929. (Luise Herzberg, died in 1971.)
Honours and awards
Herzberg's most significant award was the 1971 Nobel Prize in Chemistry, which he was awarded "for his contributions to the knowledge of electronic structure and geometry of molecules, particularly free radicals". During the presentation speech, it was noted that at the time of the award, Herzberg was "generally considered to be the world's foremost molecular spectroscopist."
Herzberg was honoured with memberships or fellowships by a very large number of scientific societies, received many awards and honorary degrees in different countries. The NSERC Gerhard Herzberg Canada Gold Medal for Science and Engineering, Canada's highest research award, was named in his honour in 2000. The Canadian Association of Physicists also has an annual award named in his honour. The Herzberg Institute of Astrophysics is named for him. He was made a member of the International Academy of Quantum Molecular Science. Asteroid 3316 Herzberg is named after him. In 1964 he was awarded the Frederic Ives Medal by the OSA. He was later named an Honorary Member of the Society. At Carleton University, there is a building named after him that belongs to the Physics and Mathematics/Statistics Departments, Herzberg Laboratories. Herzberg was elected a Fellow of the Royal Society (FRS) in 1951.
The main building of John Abbott College in Montreal is named after him. Carleton University named the Herzberg Laboratories building after him. A public park in the College Park neighbourhood of Saskatoon also bears his name.

#457 Re: This is Cool » Miscellany » 2025-10-11 16:29:31
2414) Benzaldehyde
Gist
Benzaldehyde (C6H5CHO) is the simplest aromatic aldehyde, known for its strong bitter almond-like odor. It is a colorless liquid used as a flavoring and fragrance agent, a solvent, and an intermediate in the production of dyes, pharmaceuticals, and other organic compounds. Benzaldehyde can be synthesized by oxidizing benzyl alcohol or through the hydrolysis of benzal chloride, and it has a melting point of -26 degrees Centigrade and a boiling point of 178.1 degrees Centigrade.
Benzaldehyde has numerous uses, most notably as a flavoring in foods to mimic almond, and as a fragrance in perfumes and other scented products. It is also a crucial intermediate in the synthesis of various organic compounds, including pharmaceuticals like ephedrine, as well as dyes, plastics, and other chemicals. Other applications include its use as a bee repellent and as a preservative in cosmetics and personal care products.
Summary
Benzaldehyde (C6H5CHO) is an organic compound consisting of a benzene ring with a formyl substituent. It is among the simplest aromatic aldehydes and one of the most industrially useful.
It is a colorless liquid with a characteristic odor similar to that of bitter almonds and cherry, and is commonly used in cherry-flavored sodas. A component of bitter almond oil, benzaldehyde can be extracted from a number of other natural sources. Synthetic benzaldehyde is the flavoring agent in imitation almond extract, which is used to flavor cakes and other baked goods.
Production
Benzaldehyde can be produced from both petroleum-based chemicals or plant-derived chemicals. Synthetic benzaldehyde is primarily produced using liquid phase chlorination and oxidation of toluene. Numerous other methods have been developed, such as the partial oxidation of benzyl alcohol, alkali hydrolysis of benzal chloride, and the carbonylation of benzene (the Gatterman-Koch reaction).
Natural benzaldehyde is produced from cinnamaldehyde obtained from cassia oil by the retro-aldol reaction: the cinnamaldehyde is heated in an aqueous/alcoholic solution between 90 °C and 150 °C with a base (most commonly sodium carbonate or bicarbonate) for 5 to 80 hours, followed by distillation of the formed benzaldehyde. This reaction also yields acetaldehyde. The natural status of benzaldehyde obtained in this way is controversial.
Occurrence
Benzaldehyde and similar chemicals occur naturally in many foods. Most of the benzaldehyde that people eat is from natural plant foods, such as almonds.
Almonds, apricot seeds, apple seeds, and cherry seed contain significant amounts of amygdalin. This glycoside breaks up under enzyme catalysis into benzaldehyde, hydrogen cyanide and two equivalents of glucose.
Details
Benzaldehyde (C6H5CHO) is the simplest representative of the aromatic aldehydes, occurring naturally as the glycoside amygdalin. Prepared synthetically, it is used chiefly in the manufacture of dyes, cinnamic acid, and other organic compounds, and to some extent in perfumes and flavouring agents.
Benzaldehyde was first isolated in 1803, and in the 1830s the German chemists Justus von Liebig and Friedrich Wöhler investigated the compound in studies that laid the foundation for the structural theory of organic chemistry. Industrially, benzaldehyde is made by a process in which toluene is treated with chlorine to form benzal chloride, followed by treatment of benzal chloride with water.
Benzaldehyde is readily oxidized to benzoic acid and is converted to addition products by hydrocyanic acid or sodium bisulfite. It undergoes simultaneous oxidation and reduction with alcoholic potassium hydroxide (a Cannizzaro reaction), giving potassium benzoate and benzyl alcohol; with alcoholic potassium cyanide, it is converted to benzoin; with anhydrous sodium acetate and acetic anhydride, it gives cinnamic acid.
Benzaldehyde is a colourless liquid with an odour of almond oil. It has a melting point of −26 °C (−14.8 °F) and a boiling point of 179 °C (354.2 °F). It is only slightly soluble in water and is completely soluble in ethanol and diethyl ether.
Additional Information
Benzaldehyde is an aromatic aldehyde in which the -CHO group is directly bonded to the aromatic ring. It is a compound with a molecular formula C7H6O that has several industrial applications, including the preparation of dyes, cosmetic products, and flavoring agents. It is also known as the oil of bitter almonds, as it is found in the glucoside amygdalin, which occurs in bitter almonds.
Benzoic acid, the simplest benzene-based carboxylic acid, has been known since the 16th century. One of its discoverers was the legendary clairvoyant Nostradamus. Its most common natural source is gum benzoin, a resin found in the bark of trees of the genus Styrax.
Most benzoic acid produced today is synthetic. Its first industrial synthesis was the hydrolysis of benzotrichloride to calcium benzoate, followed by acidification. This method has been completely displaced by the air oxidation of toluene, which avoids the problem of product contamination with chlorinated byproducts.
Many processed foods contain benzoic acid or one of its salts as a preservative. The acid inhibits the growth of bacteria, molds, and yeasts; it works best when the food has an acidic pH value. Benzoic acid also is often found in topical antifungal preparations.

#458 Re: Jai Ganesh's Puzzles » General Quiz » 2025-10-11 15:54:40
Hi,
#10605. What does the term in Biology Dendrite mean?
#10606. What does the term in Biology Denitrification mean?
#459 Re: Jai Ganesh's Puzzles » English language puzzles » 2025-10-11 15:33:09
Hi,
#5801. What does the adjective earthbound mean?
#5802. What does the adjective earthly mean?
#460 Re: Jai Ganesh's Puzzles » Doc, Doc! » 2025-10-11 15:17:45
Hi,
#2493. What does the medical term Butterfly rash mean?
#461 Dark Discussions at Cafe Infinity » Clown Quotes » 2025-10-11 14:54:40
- Jai Ganesh
- Replies: 0
Clown Quotes
1. But the fact that some geniuses were laughed at does not imply that all who are laughed at are geniuses. They laughed at Columbus, they laughed at Fulton, they laughed at the Wright Brothers. But they also laughed at Bozo the Clown. - Carl Sagan
2. I remain just one thing, and one thing only, and that is a clown. It places me on a far higher plane than any politician. - Charlie Chaplin
3. By blood a king, in heart a clown. - Alfred Lord Tennyson
4. The drummer in my first band was killed in Vietnam. He kind of signed up and joined the marines. Bart Hanes was his name. He was one of those guys that was jokin' all the time, always playin' the clown. - Bruce Springsteen
5. I was always the kid in school who tried to get attention, not necessarily the class clown, but I'd do little unexpected performances. - Leonardo DiCaprio
6. I grew up in the Bronx where you would stay up late with your girlfriends, just being silly in our bedrooms, whatever. And I was always the clown. - Jennifer Lopez
7. My school was really small, but I was called the Class Clown! - Serena Williams
8. I've never been naturally fashion conscious. I'm the kind of person who sees a whole outfit in a magazine, runs out and buys it but looks like a clown. - Brooke Shields.
#462 Jokes » Photographer Jokes - II » 2025-10-11 14:30:40
- Jai Ganesh
- Replies: 0
Q: What kind of photos does a turtle take?
Shellfies.
* * *
Q: Did you hear about the guy who stole all those photos?
A: I think he was framed.
* * *
Q: Why did the photographer get into an argument with the curator at the art gallery?
A: He wasn't in the right frame of mind.
* * *
Q: What do you call a photo taken by a cat?
A: A paw-trait.
* * *
Q: What did snow white say when her photos weren't ready yet?
A: Some Day My Prints Will come!
* * *
#463 Re: Jai Ganesh's Puzzles » 10 second questions » 2025-10-11 14:19:32
Hi,
#9761.
#464 Re: Jai Ganesh's Puzzles » Oral puzzles » 2025-10-11 14:00:04
Hi,
#6267.
#465 Re: Exercises » Compute the solution: » 2025-10-11 13:46:57
Hi,
2610.
#466 Re: Dark Discussions at Cafe Infinity » crème de la crème » 2025-10-10 18:13:30
2361) Dennis Gabor
Gist:
Work
During the 19th century photographic methods were developed that resulted in two-dimensional images. In 1951 Dennis Gabor discovered a way of producing images with the illusion of depth. The method was based on interference—interaction between light waves—and coherence—light waves aligned in phase with one another. Light falling on an object is captured on photographic film along with a reference beam that did not fall on the object. When only the reference beam falls on the developed film, the light is bent so that a reproduction with depth is produced.
Summary
Dennis Gabor (born June 5, 1900, Budapest, Hung.—died Feb. 8, 1979, London, Eng.) was a Hungarian-born electrical engineer who won the Nobel Prize for Physics in 1971 for his invention of holography, a system of lensless, three-dimensional photography that has many applications.
A research engineer for the firm of Siemens and Halske in Berlin from 1927, Gabor fled Nazi Germany in 1933 and worked with the Thomson-Houston Company in England, later becoming a British subject. In 1947 he conceived the idea of holography and, by employing conventional filtered-light sources, developed the basic technique. Because conventional light sources generally provided either too little light or light that was too diffuse, holography did not become commercially feasible until the demonstration, in 1960, of the laser, which amplifies the intensity of light waves.
In 1949 Gabor joined the faculty of the Imperial College of Science and Technology, London, where in 1958 he became professor of applied electron physics. His other work included research on high-speed oscilloscopes, communication theory, physical optics, and television. Gabor was awarded more than 100 patents.
Details
Dennis Gabor (5 June 1900 – 9 February 1979) was a Hungarian-British physicist who received the Nobel Prize in Physics in 1971 for his invention of holography. He obtained British citizenship in 1946 and spent most of his life in England.
Life and career
Gabor was born as Günszberg Dénes, into a Jewish family in Budapest, Austria-Hungary. In 1900, his family converted to Lutheranism. Dennis was the first-born son of Günszberg Bernát and Jakobovits Adél. Despite having a religious background, religion played a minor role in his later life and he considered himself agnostic. In 1902, the family received permission to change their surname from Günszberg to Gábor. He served with the Hungarian artillery in northern Italy during World War I.
He began his studies in engineering at the Budapest University of Technology and Economics in 1918, later in Germany, at the Technische Hochschule Charlottenburg in Berlin, now known as Technische Universität Berlin. At the start of his career, he analysed the properties of high voltage electric transmission lines by using cathode-beam oscillographs, which led to his interest in electron optics. Studying the fundamental processes of the oscillograph, Gabor was led to other electron-beam devices such as electron microscopes and TV tubes. He eventually wrote his PhD thesis on Recording of Transients in Electric Circuits with the Cathode Ray Oscillograph in 1927, and worked on plasma lamps.
In 1933 Gabor fled from Nazi Germany, where he was considered Jewish, and was invited to Britain to work at the development department of the British Thomson-Houston company in Rugby, Warwickshire. During his time in Rugby, he met Marjorie Louise Butler, and they married in 1936. He became a British citizen in 1946, and it was while working at British Thomson-Houston in 1947 that he invented holography, based on an electron microscope, and thus electrons instead of visible light. He experimented with a heavily filtered mercury arc light source. The earliest visual hologram was only realised in 1964 following the 1960 invention of the laser, the first coherent light source. After this, holography became commercially available.
Gabor's research focused on electron inputs and outputs, which led him to the invention of holography. The basic idea was that for perfect optical imaging, the total of all the information has to be used; not only the amplitude, as in usual optical imaging, but also the phase. In this manner, a complete holo-spatial picture can be obtained. Gabor published his theories of holography in a series of papers between 1946 and 1951.
Gabor also researched how human beings communicate and hear; the result of his investigations was the theory of granular synthesis, although Greek composer Iannis Xenakis claimed that he was actually the first inventor of this synthesis technique. Gabor's work in this and related areas was foundational in the development of time–frequency analysis.
In 1948 Gabor moved from Rugby to Imperial College London, and in 1958 became professor of Applied Physics until his retirement in 1967. His inaugural lecture on 3 March 1959, 'Electronic Inventions and their Impact on Civilisation' provided inspiration for Norbert Wiener's treatment of self-reproducing machines in the penultimate chapter in the 1961 edition of his book Cybernetics.
As part of his many developments related to CRTs, in 1958 Gabor patented a new flat screen television concept. This used an electron gun aimed perpendicular to the screen, rather than straight at it. The beam was then directed forward to the screen using a series of fine metal wires on either side of the beam path. The concept was significantly similar to the Aiken tube, introduced in the US the same year. This led to a many-years patent battle which resulted in Aiken keeping the US rights and Gabor the UK. Gabor's version was later picked up by Clive Sinclair in the 1970s, and became a decades-long quest to introduce the concept commercially. Its difficult manufacturing, due to the many wires within the vacuum tube, meant this was never successful. While looking for a company willing to try to manufacture it, Sinclair began negotiations with Timex, who instead took over production of the ZX81.
In 1963 Gabor published Inventing the Future which discussed the three major threats Gabor saw to modern society: war, overpopulation and the Age of Leisure. The book contained the now well-known expression that "the future cannot be predicted, but futures can be invented." Reviewer Nigel Calder described his concept as, "His basic approach is that we cannot predict the future, but we can invent it..." Others such as Alan Kay, Peter Drucker, and Forrest Shaklee have used various forms of similar quotes. His next book, Innovations: scientific, technological, and social which was published in 1970, expanded on some of the topics he had already earlier touched upon, and also pointed to his interest in technological innovation as mechanism of both liberation and destruction.
In 1971 he was the single recipient of the Nobel Prize in Physics with the motivation "for his invention and development of the holographic method" and presented the history of the development of holography from 1948 in his Nobel lecture.
While spending much of his retirement in Italy at Lavinio Rome, he remained connected with Imperial College as a senior research fellow and also became staff scientist of CBS Laboratories, in Stamford, Connecticut; there, he collaborated with his lifelong friend, CBS Labs' president Dr. Peter C. Goldmark in many new schemes of communication and display. One of Imperial College's new halls of residence in Prince's Gardens, Knightsbridge is named Gabor Hall in honour of Gabor's contribution to Imperial College. He developed an interest in social analysis and published The Mature Society: a view of the future in 1972. He also joined the Club of Rome and supervised a working group studying energy sources and technical change. The findings of this group were published in the report Beyond the Age of Waste in 1978, a report which was an early warning of several issues that only later received widespread attention.
Following the rapid development of lasers and a wide variety of holographic applications (e.g., art, information storage, and the recognition of patterns), Gabor achieved acknowledged success and worldwide attention during his lifetime. He received numerous awards besides the Nobel Prize.
Gabor died in a nursing home in South Kensington, London, on 9 February 1979. In 2006 a blue plaque was put up on No. 79 Queen's Gate in Kensington, where he lived from 1949 until the early 1960s.
Personal life
On 8 August 1936, he married Marjorie Louise Butler. They did not have any children.

#467 Re: This is Cool » Miscellany » 2025-10-10 17:52:31
2413) Glycerol
Gist
Glycerin is used for its moisturizing properties in skincare products, acting as a humectant to draw moisture to the skin. It also functions as an emollient in cosmetics, a laxative for constipation, a component of e-liquids for e-cigarettes, and a solvent in biological labs. Additionally, it is used in the food industry as an artificial sweetener, in antifreeze to prevent freezing in engines and on runways, and to reduce pressure in the eyes and skull.
Glycerin is a type of carbohydrate known as a sugar alcohol or a polyol. This odorless liquid has a sweet taste and a syrupy consistency. While glycerin occurs naturally in plants through the fermentation of sugars, most of the glycerin nowadays is produced from the hydrolysis of fats and oils.
Summary
Glycerol is a simple triol compound. It is a colorless, odorless, sweet-tasting, viscous liquid. The glycerol backbone is found in lipids known as glycerides. It is also widely used as a sweetener in the food industry and as a humectant in pharmaceutical formulations. Because of its three hydroxyl groups, glycerol is miscible with water and is hygroscopic in nature.
Modern use of the word glycerine (alternatively spelled glycerin) refers to commercial preparations of less than 100% purity, typically 95% glycerol.
Structure
Although achiral, glycerol is prochiral with respect to reactions of one of the two primary alcohols. Thus, in substituted derivatives, the stereospecific numbering labels the molecule with a sn- prefix before the stem name of the molecule.
Details
Glycerol, is a clear, colourless, viscous, sweet-tasting liquid belonging to the alcohol family of organic compounds; molecular formula HOCH2CHOHCH2OH. Until 1948 all glycerol was obtained as a by-product in making soaps from animal and vegetable fats and oils, but industrial syntheses based on propylene or sugar has accounted for an increasingly large percentage of production since that time. The term glycerin (or glycerine), introduced in 1811 by French chemist Michel-Eugène Chevreul, is ordinarily applied to commercial materials containing more than 95 percent glycerol. Though Chevreul gave glycerin its name, the substance was first isolated in 1783 by German Swedish chemist Carl Wilhelm Scheele, who described it as the “sweet principle of fat.”
Glycerol has numerous uses. It is a basic ingredient in the gums and resins used to make many modern protective coatings such as automotive enamels and exterior house paints. Glycerin reacted with nitric and sulfuric acid forms the explosive nitroglycerin (or nitroglycerine).
Glycerol is also a component of mono- and diglyceride emulsifiers, which are used as softening agents in baked goods, plasticizers in shortening, and stabilizers in ice cream. Its varied uses in the pharmaceutical and toilet goods fields include skin lotions, mouthwashes, cough medicines, drug solvents, serums, vaccines, and suppositories. Another significant use is as a protective medium for freezing red blood cells, sperm cells, eye corneas, and other living tissues. At one time, its largest single use was as automotive antifreeze; methanol and ethylene glycol have replaced it for this purpose.
Fats and oils are valued chiefly as sources of the carboxylic acids that are present, combined in the form of esters with glycerol. When the acids are set free from these compounds, glycerol remains as a solution in water and is purified by coagulating and settling extraneous matter, evaporating the water, and distilling.
Additional Information
Glycerol is a naturally occurring alcohol. It is an odorless liquid that is used as a solvent, sweetening agent, and also as medicine.
When glycerol is in the intestines, it attracts water into the gut, softening stools and relieving constipation. When glycerol is in the blood, it attracts water so that the water stays in the body longer. This might help an athlete exercise for longer.
People use glycerol for constipation, improving athletic performance, and for certain skin conditions. It is also used for stroke, obesity, ear infections, and many other conditions, but there is no good scientific evidence to support these uses.
Glycerol is banned by the World Anti-Doping Agency (WADA).
Uses & Effectiveness
Likely Effective for
Constipation. Using glycerol as a suppository or as an enema in the rectum decreases constipation in adults and children at least 2 years of age. It is US FDA approved for this use.
Possibly Effective for
Athletic performance. Taking glycerol by mouth along with water helps to keep the body hydrated for longer during exercise. But it's not clear if this helps improve athletic performance.
An inherited skin disorder that causes dry, scaly skin (ichthyosis). Applying a specific product (Dexeryl, Pierre Fabre Laboratoires) containing glycerol and paraffin to the skin reduces symptoms like itching and scales in children with ichthyosis. It's not clear if applying glycerol alone helps.
Possibly Ineffective for
Swelling (inflammation) of membranes that protect the brain and spinal cord (meningitis). Taking glycerol by mouth doesn't reduce the risk of death or seizures in people with bacterial meningitis. But it might reduce the risk of deafness in children who survive the infection.
Growth and development in premature infants. Giving glycerol into the rectum, as a suppository or as an enema, doesn't seem to help premature infants start to take food by mouth sooner.
Likely Ineffective for
Stroke. Giving glycerol by IV doesn't improve symptoms after a stroke. IV products can only be given by a healthcare provider.
There is interest in using glycerol for a number of other purposes, but there isn't enough reliable information to say whether it might be helpful.
Note: Glycerol is a naturally occurring alcohol. It is an odorless liquid that is used as a solvent, sweetening agent, and also as medicine.

#468 This is Cool » Calcium Hydroxide » 2025-10-10 17:11:48
- Jai Ganesh
- Replies: 0
Calcium Hydroxide
Gist
Calcium hydroxide is a versatile chemical used for many applications, including as a key component in mortars and plasters for construction, a clarifying agent in water and sewage treatment, a neutralizing agent in agriculture to adjust soil pH, a processing aid in the paper industry, and an ingredient in some food products like masa for tortillas. It's also used to remove hair from animal hides in the tanning process, as a disinfectant, and in various chemical manufacturing processes.
The most common name for the chemical compound Ca(OH)2 is slaked lime. It is also known by several other names, including hydrated lime, caustic lime, builder's lime, pickling lime, and slack lime.
Summary
Calcium hydroxide (traditionally called slaked lime) is an inorganic compound with the chemical formula Ca(OH)2. It is a colorless crystal or white powder and is produced when quicklime (calcium oxide) is mixed with water. Annually, approximately 125 million tons of calcium hydroxide are produced worldwide.
Calcium hydroxide has many names including hydrated lime, caustic lime, builders' lime, slaked lime, cal, and pickling lime. Calcium hydroxide is used in many applications, including food preparation, where it has been identified as E number E526. Limewater, also called milk of lime, is the common name for a saturated solution of calcium hydroxide.
Uses
Calcium hydroxide is commonly used to prepare lime mortar.
One significant application of calcium hydroxide is as a flocculant, in water and sewage treatment. It forms a fluffy charged solid that aids in the removal of smaller particles from water, resulting in a clearer product. This application is enabled by the low cost and low toxicity of calcium hydroxide. It is also used in fresh-water treatment for raising the pH of the water so that pipes will not corrode where the base water is acidic, because it is self-regulating and does not raise the pH too much.
Another large application is in the paper industry, where it is an intermediate in the reaction in the production of sodium hydroxide. This conversion is part of the causticizing step in the Kraft process for making pulp. In the causticizing operation, burned lime is added to green liquor, which is a solution primarily of sodium carbonate and sodium sulfate produced by dissolving smelt, which is the molten form of these chemicals from the recovery furnace.
In orchard crops, calcium hydroxide is used as a fungicide. Applications of 'lime water' prevent the development of cankers caused by the fungal pathogen Neonectria galligena. The trees are sprayed when they are dormant in winter to prevent toxic burns from the highly reactive calcium hydroxide. This use is authorised in the European Union and the United Kingdom under Basic Substance regulations.
Calcium hydroxide is used in dentistry, primarily in the specialty of endodontics due to its antibacterial properties and induction of hard-tissue deposition.
Details
Calcium hydroxide (Ca(OH)2) is a soft white powder that is widely used as a raw material in the chemical industry. It forms when calcium oxide is mixed with water. The compound has two hydroxide ions (OH−) for each ion of calcium (Ca2+). The chemical is ionic, with aqueous and electrolytic dissociations both producing calcium ions and hydroxide ions.
Production and uses
Calcium hydroxide is usually produced through the reaction of calcium oxide (CaO) with water (H2O). This reaction is highly exothermic, which means that a great deal of heat is produced.
This reaction occurs when water is added to dry portland cement to make concrete, and the mixture becomes warm, releasing heat. An alternate method of production involves reacting sodium hydroxide with calcium chloride in an aqueous double displacement reaction. In this reaction, sodium chloride also forms.
In the construction industry, calcium hydroxide is a main ingredient in mortar for bricks and stone, plaster, and cement. It is mixed with sand and water to create a pastelike slurry. Once the water evaporates, the sand and calcium hydroxide left behind form a strong adhesive material that holds the bricks or stones together.
Calcium hydroxide is used as a neutralizing chemical, because it reacts with acids to create water and a salt in a double displacement reaction. This neutralization can occur in farm soils to help with plant growth or in waterways to reduce the effects of acid rain. It is also added to foods to prevent the food from becoming too acidic.
Calcium hydroxide is one of many chemicals added to industrial scrubbers that remove nitrogen and sulfur oxides from the exhaust gases that are a byproduct of many industrial processes. Removing these oxides helps to reduce the amount of acid rain that forms due to industrial pollutants.
Calcium hydroxide is also used in the manufacturing of some pesticides, in the production of paints and waterproofing materials, and as an additive to oils and lubricants to improve the ability for these fluids to flow. Calcium hydroxide acts as an accelerant in the production of rubber and plastics. It is also used in the paper industry to treat wood so that converting the wood to pulp is easier. When animal hides that will be tanned to create leather need to be chemically treated to remove hair and fats, a solution of calcium hydroxide is used.
In 1920 calcium hydroxide was first used in root canal fillings. Any exposed dental pulp was treated with calcium hydroxide, and in the mid-20th century capping dental pulp with calcium hydroxide became standard practice.
Chemical and physical properties
Calcium hydroxide is soluble in glycerol and in acids but only slightly soluble in water. A saturated solution of calcium hydroxide, called limewater, reacts with acids to form salts. Calcium hydroxide reacts with carbon dioxide, forming calcium carbonate in the process.
Calcium hydroxide has a molar mass of 74.09 grams per mole. It has a melting point of 580 °C (1,076 °F) and a density of 2.24 grams per cubic centimetre.
If the skin is not properly protected, contact with calcium hydroxide can lead to chemical burns. Long-term exposure can cause lung damage.
Additional Information
Calcium Hydroxide, often known by its common names slaked lime or hydrated lime, is a versatile chemical compound with a wide range of applications. From its critical role in construction and water treatment to its use in the food industry and medical field, Calcium Hydroxide is an unsung hero in many essential processes.
What is Calcium Hydroxide?
Calcium Hydroxide, with the chemical formula Ca(OH)2, is an inorganic compound that is widely utilized in various industries due to its reactive properties. This compound is created through the process of combining calcium oxide (quicklime) with water, resulting in an exothermic reaction that produces calcium hydroxide and heat.
Definition and Chemical Composition
Calcium Hydroxide is defined as a white, powdery substance composed of calcium, hydrogen, and oxygen atoms. Its chemical composition is represented by the formula Ca(OH)₂, indicating that each molecule contains one calcium atom bonded to two hydroxide groups. This structure makes it a strong base, capable of neutralizing acids and participating in various chemical reactions.
Common Names
Calcium Hydroxide is known by several common names, reflecting its different uses and forms:
* Slaked Lime: This name emphasizes the hydration process that converts calcium oxide to calcium hydroxide.
* Hydrated Lime: A term used to denote the water content in the compound.
* Pickling Lime: Refers to its use in the food industry, particularly in the pickling process.
* Builders’ Lime: Commonly used in construction and masonry.
Natural Occurrence
In nature, Calcium Hydroxide is found in its mineral form known as portlandite. However, it is more commonly produced synthetically for industrial and commercial applications. The natural occurrence of calcium hydroxide in portlandite is relatively rare, and it is usually encountered in association with volcanic and metamorphic rocks. This mineral can be found in regions with significant geological activity, where high temperatures and pressures facilitate its formation.
In summary, Calcium Hydroxide is a versatile and essential compound with a straightforward yet powerful chemical composition. Its various names and forms reflect its widespread utility across different sectors, and while it does occur naturally, it is predominantly manufactured to meet the demands of modern industry.
Physical and Chemical Properties
Calcium Hydroxide, Ca(OH)2, possesses a range of physical and chemical properties that make it a valuable substance in various applications. Understanding these properties is essential for effectively utilizing and handling this compound.
Appearance and Physical State
Calcium Hydroxide typically appears as a white, odorless powder or crystalline substance. In its solid state, it can be fine and soft to the touch, or it may form larger crystalline aggregates. When mixed with water, it forms a slurry or a viscous paste, which is often referred to as lime putty.
Solubility and pH
Calcium Hydroxide is sparingly soluble in water. At 20°C, about 1.73 grams of Calcium Hydroxide dissolve in one liter of water, forming a solution known as limewater. This solution is mildly alkaline, with a high pH value typically around 12.4. This high pH indicates that Calcium Hydroxide is a strong base, capable of neutralizing acids effectively.

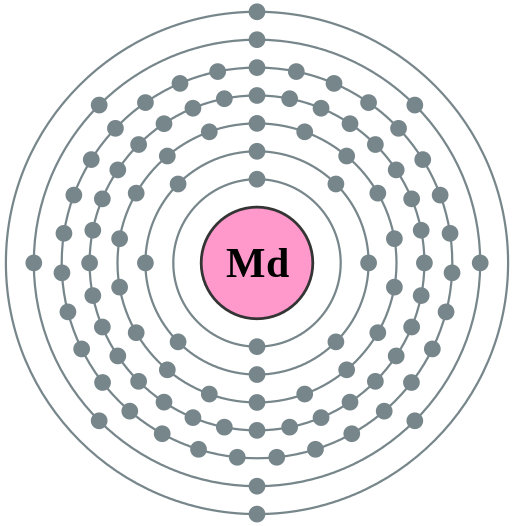
#469 Science HQ » Mendelevium » 2025-10-10 16:32:09
- Jai Ganesh
- Replies: 0
Mendelevium
Gist
Mendelevium (Md) is a highly radioactive, synthetic element with atomic number 101, discovered in 1955. It is a transuranic element in the actinide series and is named after Dmitri Mendeleev, the father of the periodic table. Since it can only be produced in microscopic amounts by bombarding lighter elements like einsteinium with alpha particles, it has no practical uses and is used solely for scientific research.
Element 101 is Mendelevium (Md), a synthetic, radioactive metallic transuranic element in the actinide series, named after Dmitri Mendeleev, the creator of the periodic table. Discovered in 1955 by American scientists, it is produced in extremely small quantities in laboratories by bombarding einsteinium-253 with alpha particles.
Summary
Mendelevium is a synthetic chemical element; it has symbol Md (formerly Mv) and atomic number 101. A metallic radioactive transuranium element in the actinide series, it is the first element by atomic number that currently cannot be produced in macroscopic quantities by neutron bombardment of lighter elements. It is the thirteenth actinide, the ninth transuranic element, and the first transfermium; it is named after Dmitri Mendeleev, the father of the periodic table.
Like all the transfermiums, it can only be produced in particle accelerators by bombarding lighter elements with charged particles. The element was first produced in 1955 by bombarding einsteinium with alpha particles, the method still used today. Using commonly-available microgram quantities of einsteinium-253, over a million mendelevium atoms may be made each hour. The chemistry of mendelevium is typical for the late actinides, with a dominant +3 oxidation state but also a +2 oxidation state accessible in solution. All known isotopes of mendelevium have short half-lives; there are currently no uses for it outside basic scientific research, and only small amounts are produced.
Details
Mendelevium (Md) is a synthetic chemical element of the actinoid series of the periodic table, atomic number 101. It was the first element to be synthesized and discovered a few atoms at a time. Not occurring in nature, mendelevium (as the isotope mendelevium-256) was discovered (1955) by American chemists Albert Ghiorso, Bernard G. Harvey, Gregory R. Choppin, Stanley G. Thompson, and Glenn T. Seaborg at the University of California, Berkeley, as a product resulting from the helium-ion (alpha-particle) bombardment of a minute quantity (about a billion atoms) of einsteinium-253 (atomic number 99). The element was named after Russian chemist Dmitry Mendeleyev.
In about a dozen repetitions of the experiment, the team of scientists produced 17 atoms of mendelevium, which were identified by the ion-exchange adsorption-elution method (mendelevium behaved like its rare-earth homologue thulium) and by the electron-capture decay of its daughter isotope fermium-256. Fifteen other isotopes of mendelevium, all radioactive, have been discovered. The stablest is mendelevium-258 (51.5-day half-life). Studied by means of radioactive tracer techniques, mendelevium exhibits a predominant +3 oxidation state, as would be expected by its position in the actinoid series; a slightly stable +2 oxidation state is also known.
Additional Information:
Appearance
A radioactive metal, of which only a few atoms have ever been created.
Uses
Mendelevium is used only for research.
Biological role
Mendelevium has no known biological role.
Natural abundance
Mendelevium does not occur naturally. It is made by bombarding einsteinium with alpha particles (helium ions).
#470 Dark Discussions at Cafe Infinity » Cloudy Quotes » 2025-10-10 15:36:39
- Jai Ganesh
- Replies: 0
Cloudy Quotes
1. There is a sort of jealousy which needs very little fire; it is hardly a passion, but a blight bred in the cloudy, damp despondency of uneasy egoism. - George Eliot
2. The mental body, like the astral, varies much in different people; it is composed of coarser or of finer matter, according to the needs of the more or less unfolded consciousness connected with it. In the educated it is active and well-defined; in the undeveloped it is cloudy and inchoate. - Annie Besant
3. Morality without religion is only a kind of dead reckoning - an endeavor to find our place on a cloudy sea by measuring the distance we have run, but without any observation of the heavenly bodies. - Henry Wadsworth Longfellow
4. I'd advise people to never step out without wearing an SPF, not even on a cloudy day. - Anushka Sharma (Sun Protection Factor)
5. A cloudy day or a little sunshine have as great an influence on many constitutions as the most recent blessings or misfortunes. - Joseph Addison
6. In October, a maple tree before your window lights up your room like a great lamp. Even on cloudy days, its presence helps to dispel the gloom. - John Burroughs.
#471 Jokes » Photographer Jokes - I » 2025-10-10 15:15:09
- Jai Ganesh
- Replies: 0
Q: Did you hear about how the photographer died?
A: It makes me shutter.
* * *
Q: Where does a cow hang his photos?
A: In a mooooseum.
* * *
Two girls: "A tray of sushi, please."
Waiter: "To eat or to post photos of on Instagram?
* * *
Q: What do you call a mixed media artist without a girlfriend?
A: Homeless.
* * *
Q: Why shouldn't you steal from someone holding a camera?
A: Because they have a photographic memory.
* * *
#472 Re: Jai Ganesh's Puzzles » General Quiz » 2025-10-10 15:04:03
Hi,
#10603. What does the term in Geography Cardinal directions mean?
#10604. What does the term in Geography Carrying capacity mean?
#473 Re: Jai Ganesh's Puzzles » English language puzzles » 2025-10-10 14:37:20
Hi,
#5799. What does the verb (used with object) deceive mean?
#5800. What does the adjective deceptive mean?
#474 Re: Jai Ganesh's Puzzles » Doc, Doc! » 2025-10-10 14:26:13
Hi,
#2492. What does the medical term Bogota bag mean?
#475 Re: Jai Ganesh's Puzzles » 10 second questions » 2025-10-10 14:16:32
Hi,
#9760.