Math Is Fun Forum
You are not logged in.
- Topics: Active | Unanswered
#26 Re: Jai Ganesh's Puzzles » 10 second questions » 2025-09-13 14:34:35
Hi,
#9732.
#27 Re: Jai Ganesh's Puzzles » Oral puzzles » 2025-09-13 14:17:26
Hi,
#6239.
#28 Re: Exercises » Compute the solution: » 2025-09-13 13:59:10
Hi,
2582.
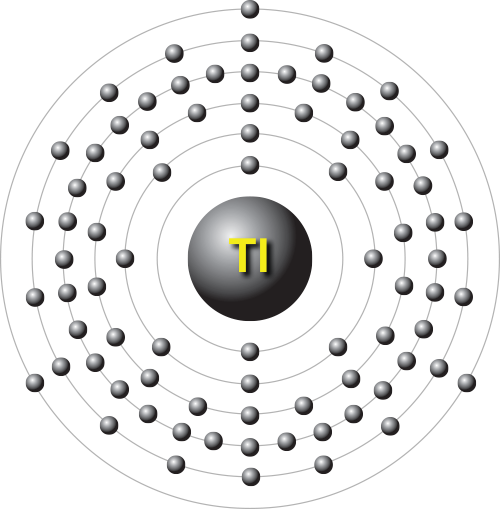
#29 Science HQ » Thallium » 2025-09-13 13:41:24
- Jai Ganesh
- Replies: 0
Thallium
Gist
Thallium (Tl) is a highly toxic, silvery-white post-transition metal, atomic number 81, found in trace amounts in the Earth's crust and sulfide ores. Although once used in rodenticides and some medical applications, its use has been largely banned due to severe poisoning risks, mimicking symptoms of other diseases, and potential for criminal misuse. Thallium poisoning can be treated with Prussian Blue, and the element is still used in some specialized electronic and optical applications.
Thallium blood concentration levels are normal below 2 µg/L, and toxic at concentrations greater than 200 µg/L. Long-term effects of thallium exposure can include difficulty walking, various involuntary movement disorders, and impairment of thought and mood. Neurological damage resolves slowly and may be permanent.
Summary
Thallium is a chemical element; it has symbol Tl and atomic number 81. It is a silvery-white post-transition metal that is not found free in nature. When isolated, thallium resembles tin, but discolors when exposed to air. Chemists William Crookes and Claude-Auguste Lamy discovered thallium independently, in 1861, in residues of sulfuric acid production. Both used the newly developed method of flame spectroscopy, in which thallium produces a notable green spectral line. Thallium, from Greek thallós, meaning "green shoot" or "twig", was named by Crookes. It was isolated by both Lamy and Crookes in 1862, Lamy by electrolysis and Crookes by precipitation and melting of the resultant powder. Crookes exhibited it as a powder precipitated by zinc at the International Exhibition, which opened on 1 May that year.
Thallium tends to form the +3 and +1 oxidation states. The +3 state resembles that of the other elements in group 13 (boron, aluminium, gallium, indium). However, the +1 state, which is far more prominent in thallium than the elements above it, recalls the chemistry of alkali metals and thallium(I) ions are found geologically mostly in potassium-based ores and (when ingested) are handled in many ways like potassium ions (K+) by ion pumps in living cells.
Commercially, thallium is produced not from potassium ores, but as a byproduct from refining of heavy-metal sulfide ores. Approximately 65% of thallium production is used in the electronics industry and the remainder is used in the pharmaceutical industry and in glass manufacturing. It is also used in infrared detectors. The radioisotope thallium-201 (as the soluble chloride TlCl) is used in small amounts as an agent in a nuclear medicine scan, during one type of nuclear cardiac stress test.
Soluble thallium salts (many of which are nearly tasteless) are highly toxic and they were historically used in rat poisons and insecticides. Because of their nonselective toxicity, use of these compounds has been restricted or banned in many countries. Thallium poisoning usually results in hair loss.
Details
Thallium (Tl) is a chemical element, metal of main Group 13 (IIIa, or boron group) of the periodic table, poisonous and of limited commercial value. Like lead, thallium is a soft, low-melting element of low tensile strength. Freshly cut thallium has a metallic lustre that dulls to bluish gray upon exposure to air. The metal continues to oxidize upon prolonged contact with air, generating a heavy nonprotective oxide crust. Thallium dissolves slowly in hydrochloric acid and dilute sulfuric acid and rapidly in nitric acid.
Rarer than tin, thallium is concentrated in only a few minerals that have no commercial value. Trace amounts of thallium are present in sulfide ores of zinc and lead; in the roasting of these ores, the thallium becomes concentrated in the flue dusts, from which it is recovered.
British chemist Sir William Crookes discovered (1861) thallium by observing the prominent green spectral line generated by selenium-bearing pyrites that had been used in the manufacture of sulfuric acid. Crookes and French chemist Claude-Auguste Lamy independently isolated (1862) thallium, showing it to be a metal.
Two crystalline forms of the element are known: close-packed hexagonal below about 230 °C (450 °F) and body-centred cubic above. Natural thallium, the heaviest of the boron group elements, consists almost entirely of a mixture of two stable isotopes: thallium-203 (29.5 percent) and thallium-205 (70.5 percent). Traces of several short-lived isotopes occur as decay products in the three natural radioactive disintegration series: thallium-206 and thallium-210 (uranium series), thallium-208 (thorium series), and thallium-207 (actinium series).
Thallium metal has no commercial use, and thallium compounds have no major commercial application, since thallous sulfate was largely replaced in the 1960s as a rodenticide and insecticide. Thallous compounds have a few limited uses. For example, mixed bromide-iodide crystals (TlBr and TlI) that transmit infrared light have been fabricated into lenses, windows, and prisms for infrared optical systems. The sulfide (Tl2S) has been employed as the essential component in a highly sensitive photoelectric cell and the oxysulfide in an infrared-sensitive photocell (thallofide cell). Thallium forms its oxides in two different oxidation states, +1 (Tl2O) and +3 (Tl2O3). Tl2O has been used as an ingredient in highly refractive optical glasses and as a colouring agent in artificial gems; Tl2O3 is an n-type semiconductor. Alkali halide crystals, such as sodium iodide, have been doped or activated by thallium compounds to produce inorganic phosphors for use in scintillation counters to detect radiation.
Thallium imparts a brilliant green coloration to a bunsen flame. Thallous chromate, formula Tl2CrO4, is best used in the quantitative analysis of thallium, after any thallic ion, Tl3+, present in the sample has been reduced to the thallous state, Tl+.
Thallium is typical of the Group 13 elements in having an s2p1 outer electron configuration. Promoting an electron from an s to a p orbital allows the element to be three or four covalent. With thallium, however, the energy required for s → p promotion is high relative to the Tl–X covalent bond energy that is regained on formation of TlX3; hence, a derivative with a +3 oxidation state is not a very energetically favoured reaction product. Thus, thallium, unlike the other boron group elements, predominantly forms singly charged thallium salts having thallium in the +1 rather than the +3 oxidation state (the 6s2 electrons remain unused). It is the only element to form a stable singly charged cation with the outer electron configuration (n-1)d10ns2, which is, unusually enough, not an inert gas configuration. In water the colourless, more stable thallous ion, Tl+, resembles the heavier alkali metal ions and silver; the compounds of thallium in its +3 state are easily reduced to compounds of the metal in its +1 state.
In its oxidation state of +3, thallium resembles aluminum, although the ion Tl3+ appears to be too large to form alums. The very close similarity in size of the singly charged thallium ion, Tl+, and the rubidium ion, Rb+, makes many Tl+ salts, such as the chromate, sulfate, nitrate, and halides, isomorphous (i.e., have an identical crystal structure) to the corresponding rubidium salts; also, the ion Tl+ is able to replace the ion Rb+ in the alums. Thus, thallium does form an alum, but in doing so it replaces the M+ ion, rather than the expected metal atom M3+, in M+M3+(SO4)2∙12H2O.
Soluble thallium compounds are toxic. The metal itself is changed to such compounds by contact with moist air or skin. Thallium poisoning, which may be fatal, causes nervous and gastrointestinal disorders and rapid loss of hair.
Element Properties
atomic number : 81
atomic weight : 204.37
melting point : 303.5 °C (578.3 °F)
boiling point : 1,457 °C (2,655 °F)
specific gravity : 11.85 (at 20 °C [68 °F])
oxidation states : +1, +3.
Additional Information:
Appearance
A soft, silvery-white metal that tarnishes easily.
Uses
The use of thallium is limited as it is a toxic element. Thallium sulfate was employed as a rodent killer – it is odourless and tasteless – but household use of this poison has been prohibited in most developed countries.
Most thallium is used by the electronics industry in photoelectric cells. Thallium oxide is used to produce special glass with a high index of refraction, and also low melting glass that becomes fluid at about 125K.
An alloy of mercury containing 8% thallium has a melting point 20°C lower than mercury alone. This can be used in low temperature thermometers and switches.
Biological role
Thallium has no known biological role. It is very toxic and there is evidence that the vapour is both teratogenic (disturbs the development of an embryo or foetus) and carcinogenic. It can displace potassium around the body affecting the central nervous system.
Natural abundance
Thallium is found in several ores. One of these is pyrites, which is used to produce sulfuric acid. Some thallium is obtained from pyrites, but it is mainly obtained as a by-product of copper, zinc and lead refining.
Thallium is also present in manganese nodules found on the ocean floor.
#30 Dark Discussions at Cafe Infinity » Close Quotes - IV » 2025-09-13 00:41:38
- Jai Ganesh
- Replies: 0
Close Quotes - IV
1. Members of India's diaspora, living in distant lands of the world, my good wishes to all of you. You may be far away from India, but you are always close to our hearts. - Atal Bihari Vajpayee
2. Thankfully, I have my mom and a small group of close friends who are there for me 24/7 and whom I can trust and depend on. - Christina Aguilera
3. China and India are close neighbours linked by mountains and rivers and the Chinese and Indian peoples have enjoyed friendly exchanges for thousands of years. - Li Peng
4. Every country should conduct its own reforms, should develop its own model, taking into account the experience of other countries, whether close neighbours or far away countries. - Mikhail Gorbachev
5. My inner strength comes from my friends. I have a very close group of friends and family, and we all help each other through our dark times. - Kathy Bates
6. Viggo Mortensen had the biggest impact on me in terms of approach, dedication, intention, and artistic outlook, and I'm nowhere close to how good he is as an artist, and I wouldn't even put myself in the same category as an actor. - Orlando Bloom
7. Avoid having your ego so close to your position that when your position falls, your ego goes with it. - Colin Powell
8. We are not even close to finishing the basic dream of what the PC can be. - Bill Gates.
#31 Jokes » Librarian Jokes - VI » 2025-09-13 00:20:22
- Jai Ganesh
- Replies: 0
Q: Did you know that even big tough guys read?
A: Yeah, just ask Conan the Librarian.
* * *
Q: Did you hear about the librarian who couldn't understand sarcasm?
A: Apparently, she couldn't read between the lines.
* * *
Q: Have you read the book Raising Dogs? No?
A: You should, it's a pup-up book.
* * *
Q: Did you hear about the power outage at the Arizona State University library?
A: Thirty students were stuck on the escalator for three hours.
* * *
Q: What do you get when you cross a librarian and a lawyer?
A: All the information in the world, but you can't understand a word of it.
* * *
#32 Re: This is Cool » Miscellany » 2025-09-13 00:04:57
2386) Bat (Mammal)
Gist
Mammals are a group of vertebrate animals. Examples of mammals include rats, cats, dogs, deer, monkeys, apes, bats, whales, dolphins, and humans.
Bats are mammals, and are the only mammals capable of true, sustained flight. As mammals, they are warm-blooded, covered in fur, and nourish their young with milk. Their wings are unique extensions of their forelimbs, consisting of long, spread-out fingers with a thin membrane of skin stretching between them. Bats belong to the order Chiroptera, and there are over 1,300 species worldwide.
A bat is a type of mammal from the order Chiroptera, characterized by its ability to fly using wings formed by a membrane stretched between its long fingers and body. Like other mammals, bats are warm-blooded, have fur, and nourish their young with milk. They are the only mammals capable of true, sustained flight and play vital roles in ecosystems by controlling insect pests, pollinating plants, and spreading seeds.
Summary
A bat, (order Chiroptera) is any member of the only group of mammals capable of flight. This ability, coupled with the ability to navigate at night by using a system of acoustic orientation (echolocation), has made the bats a highly diverse and populous order. More than 1,200 species are currently recognized, and many are enormously abundant. Observers have concluded, for example, that some 100 million female Mexican free-tailed bats (Tadarida brasiliensis mexicana) form summer nursery colonies in Texas, where they produce about 100 million young in five large caves. The adult males are equal in number to the females, though they do not all range as far north as Texas. Furthermore, this species is found throughout tropical America. Thus, one species alone numbers, at the very least, in the hundreds of millions.
General features
All bats have a generally similar appearance in flight, dominated by the expanse of the wings, but they vary considerably in size. The order is usually divided into two well-defined suborders: the Megachiroptera (the large Old World fruit bats) and the Microchiroptera (small bats found worldwide). Among members of the Megachiroptera, flying foxes (Pteropus) have a wingspan of 1.5 metres (about 5 feet) and a weight of 1 kg (2.2 pounds). The largest insectivorous bat is probably the naked, or hairless, bat (Cheiromeles torquatus); it weighs about 250 grams (about 9 ounces). The largest of the carnivorous bats (and the largest bat in the New World) is the spectral bat (Vampyrum spectrum), also known as the tropical American false vampire bat, with a wingspan of over 60 cm (24 inches). The tiny hog-nosed, or bumblebee, bat (Craseonycteris thonglongyai) of Thailand is one of the smallest mammals. It has a wingspan of barely 15 cm (6 inches) and weighs about 2 grams (about 0.07 ounce).
Bats vary in colour and in fur texture. Facial appearance, dominated by the muzzle and ears, varies strikingly between families and often between genera. In several families a complex fleshy adornment called the nose leaf surrounds the nostrils. Although the exact function of these facial appurtenances has yet to be determined, scientists believe they may help to direct outgoing echolocation calls (see below Orientation). Wing proportions are modified according to mode of flight. The tail and the membrane between the legs also differ, perhaps as adaptations to feeding, flight, and roosting habits. Finally, bats vary in the postures they assume when roosting, particularly in whether they hang suspended or cling to a wall and in the manner in which the wings are folded and used.
Distribution
Bats are particularly abundant in the tropics. In West Africa, for example, more than 30 genera embracing nearly 100 species have been cataloged; in the United States 19 genera, totaling about 45 species, are known. Of the 18 bat families, 3—the vesper bats (family Vespertilionidae), free-tailed bats (family Molossidae), and horseshoe bats (family Rhinolophidae)—are well represented in the temperate zones. A few American leaf-nosed bats (family Phyllostomidae) range into mild temperate regions. Several vesper bats range well into Canada.
The Vespertilionidae are found worldwide except in the polar regions and on isolated islands. The brown bats of genus Myotis have a range almost equal to that of the entire order. The free-tailed bats and sheath-tailed bats (family Emballonuridae) also encircle the Earth but are restricted to the tropics and subtropics. The horseshoe bats extend throughout the Old World, the roundleaf bats (family Hipposideridae) and Old World fruit bats (family Pteropodidae) throughout the Old World tropics, and the leaf-nosed bats throughout the New World tropics and slightly beyond. The other families have more restricted ranges.
Details
For centuries, bats have been called sinister and spooky, likely because of their beady eyes and razor-sharp fangs. But there’s more to these nocturnal creatures than meets the eyes. There are more than 1,300 species of bats in the world, making them the second most common group of mammals after rodents. Some weigh less than a penny, while others have a wingspan of six feet, but all are impressive and vital members of their ecosystems.
Winging it
The scientific name for bats is Chiroptera, which is Greek for “hand wing.” That’s because bats have four long fingers and a thumb, each connected to the next by a thin layer of skin. They are the only mammals in the world that can fly, and they are remarkably good at it. Their flexible skin membrane and movable joints allow them to change direction quickly and catch mosquitoes in midair.
Classification
There are two main types of bats: microbats and megabats. Most bats are microbats, which eat insects like moths, that come out at night. Vampire bats are the only species of microbats that feed on blood rather than insects. But not to worry—they prefer to drink from cattle and horses, not humans.
To navigate dark caves and hunt after dark, microbats rely on echolocation, a system that allows them to locate objects using sound waves. They echolocate by making a high-pitched sound that travels until it hits an object and bounces back to them. This echo tells them an object’s size and how far away it is.
In contrast, megabats live in the tropics and eat fruit, nectar, and pollen. They have larger eyes and a stronger sense of smell than microbats but have smaller ears because they don’t echolocate. There are more than 150 species of megabats, which are usually, but not always, larger than microbats.
Roosting
Bats can be found nearly everywhere, except in polar regions, extreme deserts, and a few isolated islands. They spend their daylight hours hiding in roosts around the tropics, dense forests, and wetlands. Roosts are where bats go to rest, usually in cracks and crevices that keep them hidden and protected. The most common roosts are existing structures such as caves, tree hollows, and old buildings.
Seasons often dictate where any bats choose their homes. depending on the time of year because they hibernate during the winter. For example, in the winter, some may hibernate in caves, and in the summer, they’ll return to an attic. Because good roosts can be hard to find, many live in giant colonies with millions of other bats.
No matter where they spend their seasons, all bats roost upside down. They can hang from their hind feet and legs while resting. Scientists still aren’t sure why bats do this, but here’s one theory: Bats have to fall into flight, which makes hanging upside down the best way to escape quickly.
Nature’s conservationists
Despite all the misconceptions surrounding bats, they are very important to humans and the environment. Insect-eating microbats consume millions of bugs a night, acting as a natural pest control for plants. Thanks to bats, farmers might rely less on toxic pesticides, which costs them millions of dollars each year. Nectar-drinking bats pollinate plants so they can produce fruit. In fact, more than 500 plant species, including mangoes, bananas, and avocados, depend on bats for pollination. Finally, fruit-eating bats help disperse seeds so rainforests can grow, helping to mitigate the effects of widespread deforestation.
Additional Information
Bats are flying mammals of the order Chiroptera. With their forelimbs adapted as wings, they are the only mammals capable of true and sustained flight. Bats are more agile in flight than most birds, flying with their very long spread-out digits covered with a thin membrane or patagium. The smallest bat, and arguably the smallest extant mammal, is Kitti's hog-nosed bat, which is 29–34 mm (1.1–1.3 in) in length, 150 mm (5.9 in) across the wings and 2–2.6 g (0.071–0.092 oz) in mass. The largest bats are the flying foxes, with the giant golden-crowned flying fox (Acerodon jubatus) reaching a weight of 1.6 kg (3.5 lb) and having a wingspan of 1.7 m (5 ft 7 in).
The second largest order of mammals after rodents, bats comprise about 20% of all classified mammal species worldwide, with over 1,400 species. These were traditionally divided into two suborders: the largely fruit-eating megabats, and the echolocating microbats. But more recent evidence has supported dividing the order into Yinpterochiroptera and Yangochiroptera, with megabats as members of the former along with several species of microbats. Many bats are insectivores, and most of the rest are frugivores (fruit-eaters) or nectarivores (nectar-eaters). A few species feed on animals other than insects; for example, the vampire bats are haematophagous (feeding on blood). Most bats are nocturnal, and many roost in caves or other refuges; it is uncertain whether bats have these behaviours to escape predators. Bats are distributed globally in all except the coldest regions. They are important in their ecosystems for pollinating flowers and dispersing seeds; many tropical plants depend entirely on bats for these services. Globally, they transfer organic matter into cave ecosystems and arthropod suppression. Insectivory by bats in farmland constitutes an ecosystem service that has paramount value to humans: even in today’s pesticide era, natural enemies account for almost all pest suppression in farmed ecosystems.
Bats provide humans with some direct benefits, at the cost of some disadvantages. Bat dung has been mined as guano from caves and used as fertiliser. Bats consume insect pests, reducing the need for pesticides and other insect management measures. Some bats are also predators of mosquitoes, suppressing the transmission of mosquito-borne diseases. Bats are sometimes numerous enough and close enough to human settlements to serve as tourist attractions, and they are used as food across Asia and the Pacific Rim. However, fruit bats are frequently considered pests by fruit growers. Due to their physiology, bats are one type of animal that acts as a natural reservoir of many pathogens, such as rabies; and since they are highly mobile, social, and long-lived, they can readily spread disease among themselves. If humans interact with bats, these traits become potentially dangerous to humans.
Depending on the culture, bats may be symbolically associated with positive traits, such as protection from certain diseases or risks, rebirth, or long life, but in the West, bats are popularly associated with darkness, malevolence, witchcraft, vampires, and death.

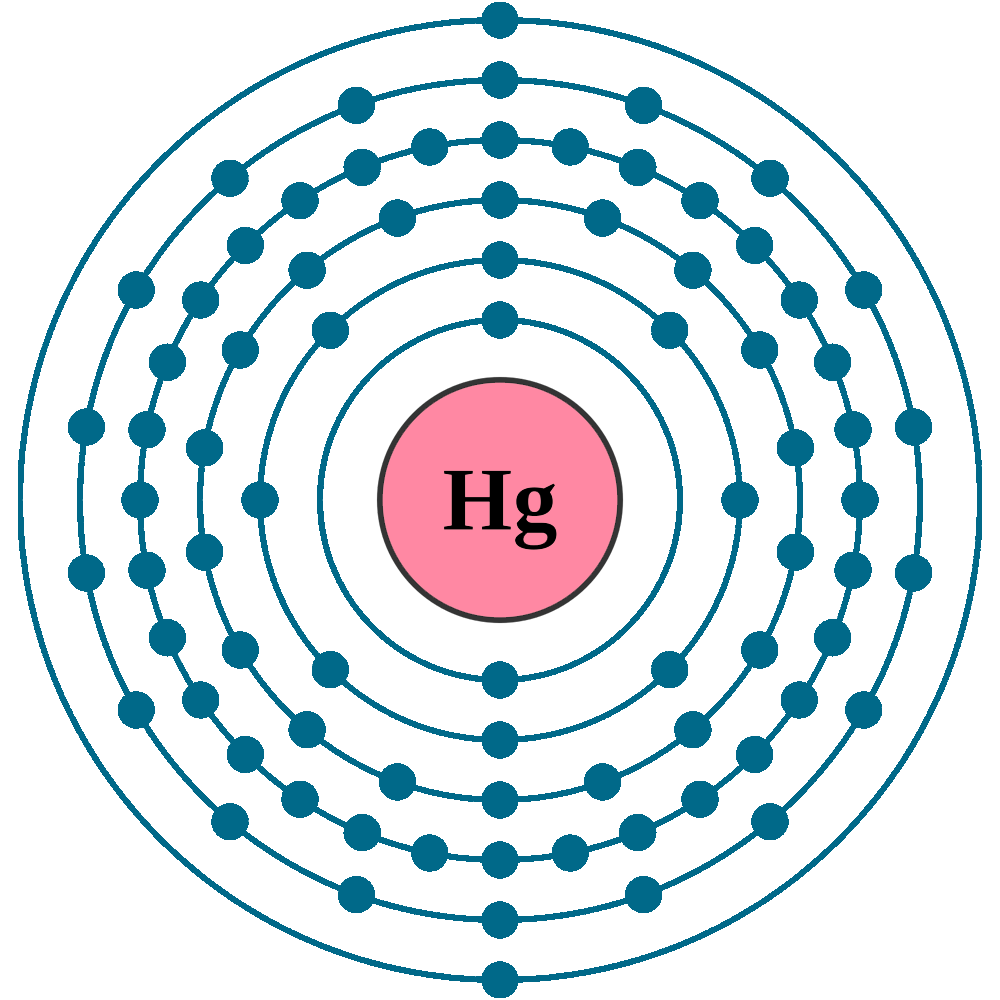
#33 Science HQ » Mercury » 2025-09-12 15:35:00
- Jai Ganesh
- Replies: 0
Mercury (Element)
Gist
Mercury is a chemical element with the symbol Hg and atomic number 80. It is a heavy, silvery-white metal that is unique for being the only metallic element that remains liquid at standard temperature and pressure, though other metals like gallium melt just above room temperature. Also known as quicksilver, it forms alloys called amalgams with other metals and is found in nature in deposits of cinnabar. Mercury is toxic and can cause serious health problems, particularly to the nervous system, and bioaccumulates in the environment and food chains.
Mercury has historically been used in thermometers, barometers, electrical switches, fluorescent lamps, dental amalgams, and some batteries, pharmaceuticals, and cosmetics. However, due to its toxicity, many of these uses are being phased out in favor of alternatives, with the Minamata Convention on Mercury working to eliminate its use in products and industries globally.
Summary
Mercury is a chemical element; it has symbol Hg and atomic number 80. It is commonly known as quicksilver. A heavy, silvery d-block element, mercury is the only metallic element that is known to be liquid at standard temperature and pressure; the only other element that is liquid under these conditions is the halogen bromine, though metals such as caesium, gallium, and rubidium melt just above room temperature.
Mercury occurs in deposits throughout the world mostly as cinnabar (mercuric sulfide). The red pigment vermilion is obtained by grinding natural cinnabar or synthetic mercuric sulfide. Exposure to mercury and mercury-containing organic compounds is toxic to the nervous system, immune system and kidneys of humans and other animals; mercury poisoning can result from exposure to water-soluble forms of mercury (such as mercuric chloride or methylmercury) either directly or through mechanisms of biomagnification.
Mercury is used in thermometers, barometers, manometers, sphygmomanometers, float valves, mercury switches, mercury relays, fluorescent lamps and other devices, although concerns about the element's toxicity have led to the phasing out of such mercury-containing instruments. It remains in use in scientific research applications and in amalgam for dental restoration in some locales. It is also used in fluorescent lighting. Electricity passed through mercury vapor in a fluorescent lamp produces short-wave ultraviolet light, which then causes the phosphor in the tube to fluoresce, making visible light.
Details
Mercury (Hg) is a chemical element, liquid metal of Group 12 (IIb, or zinc group) of the periodic table.
Element Properties
atomic number : 80
atomic weight : 200.592
melting point : -38.83 °C (-37.89 °F)
boiling point : 356.62 °C (673.91 °F)
specific gravity : 13.5 at 20 °C (68 °F)
valence : 1, 2.
Properties, uses, and occurrence
Mercury was known in Egypt and also probably in the East as early as 1500 bce. The name mercury originated in 6th-century alchemy, in which the symbol of the planet was used to represent the metal; the chemical symbol Hg derives from the Latin hydrargyrum, “liquid silver.” Although its toxicity was recognized at an early date, its main application was for medical purposes.
Mercury is the only elemental metal that is liquid at room temperature. (Cesium melts at about 28.5 °C [83 °F], gallium at about 30 °C [86 °F], and rubidium at about 39 °C [102 °F].) Mercury is silvery white, slowly tarnishes in moist air, and freezes into a soft solid like tin or lead at −38.83 °C (−37.89 °F). It boils at 356.62 °C (673.91 °F).
It alloys with copper, tin, and zinc to form amalgams, or liquid alloys. An amalgam with silver is used as a filling in dentistry. Mercury does not wet glass or cling to it, and this property, coupled with its rapid and uniform volume expansion throughout its liquid range, made it useful in thermometers. (Mercury thermometers were supplanted by more accurate electronic digital thermometers in the early 21st century.) Barometers and manometers also used its high density and low vapour pressure. However, mercury’s toxicity has led to its replacement in these instruments. Gold and silver dissolve readily in mercury, and in the past this property was used in the extraction of these metals from their ores.
The good electrical conductivity of mercury makes it exceptionally useful in sealed electrical switches and relays. An electrical discharge through mercury vapour contained in a fused silica tube or bulb produces a bluish glow rich in ultraviolet light, a phenomenon exploited in ultraviolet, fluorescent, and high-pressure mercury-vapour lamps. Some mercury is used in the preparation of pharmaceuticals and agricultural and industrial fungicides.
In the 20th century the use of mercury in the manufacture of chlorine and sodium hydroxide by electrolysis of brine depended upon the fact that mercury employed as the negative pole, or cathode, dissolves the sodium liberated to form a liquid amalgam. In the early 21st century, however, mercury-cell plants for manufacturing chlorine and sodium hydroxide have mostly been phased out.
Mercury occurs in Earth’s crust on the average of about 0.08 gram (0.003 ounce) per ton of rock. The principal ore is the red sulfide, cinnabar. Native mercury occurs in isolated drops and occasionally in larger fluid masses, usually with cinnabar, near volcanoes or hot springs. Extremely rare natural alloys of mercury have also been found: moschellandsbergite (with silver), potarite (with palladium), and gold amalgam. Over 90 percent of the world’s supply of mercury comes from China; it is often a by-product of gold mining.
Cinnabar is mined in shaft or open-pit operations and refined by flotation. Most of the methods of extraction of mercury rely on the volatility of the metal and the fact that cinnabar is readily decomposed by air or by lime to yield the free metal. Mercury is extracted from cinnabar by roasting it in air, followed by condensation of the mercury vapour. Because of the toxicity of mercury and the threat of rigid pollution control, attention is being directed toward safer methods of extracting mercury. These generally rely on the fact that cinnabar is readily soluble in solutions of sodium hypochlorite or sulfide, from which the mercury can be recovered by precipitation with zinc or aluminum or by electrolysis.
Mercury is toxic. Poisoning may result from inhalation of the vapour, ingestion of soluble compounds, or absorption of mercury through the skin.
Natural mercury is a mixture of seven stable isotopes: 196Hg (0.15 percent), 198Hg (9.97 percent), 199Hg (16.87 percent), 200Hg (23.10 percent), 201Hg (13.18 percent), 202Hg (29.86 percent), and 204Hg (6.87 percent). Isotopically pure mercury consisting of only mercury-198 prepared by neutron bombardment of natural gold, gold-197, has been used as a wavelength standard and for other precise work.
Principal compounds
The compounds of mercury are either of +1 or +2 oxidation state. Mercury(II) or mercuric compounds predominate. Mercury does not combine with oxygen to produce mercury(II) oxide, HgO, at a useful rate until heated to the range of 300 to 350 °C (572 to 662 °F). At temperatures of about 400 °C (752 °F) and above, the reaction reverses with the compound decomposing into its elements. Antoine-Laurent Lavoisier and Joseph Priestley used this reaction in their study of oxygen.
There are relatively few mercury(I) or mercurous compounds. The mercury(I) ion, Hg22+, is diatomic and stable. Mercury(I) chloride, Hg2Cl2 (commonly known as calomel), is probably the most important univalent compound. It was used in antiseptic salves. Mercury(II) chloride, HgCl2 (also called bichloride of mercury or corrosive sublimate), is perhaps the commonest bivalent compound. Although extremely toxic, this odourless, colourless substance has a wide variety of applications. In agriculture it is used as a fungicide, in medicine it was sometimes employed as a topical antiseptic in concentrations of one part per 2,000 parts of water, and in the chemical industry it serves as a catalyst in the manufacture of vinyl chloride and as a starting material in the production of other mercury compounds. Mercury(II) oxide, HgO, provides elemental mercury for the preparation of various organic mercury compounds and certain inorganic mercury salts. This red or yellow crystalline solid is also used as an electrode (mixed with graphite) in zinc-mercuric oxide electric cells and in mercury batteries. Mercury(II) sulfide, HgS, is a black or red crystalline solid used chiefly as a pigment in paints, rubber, and plastics.
Additional Information:
Appearance
A liquid, silvery metal.
Uses
Mercury has fascinated people for millennia, as a heavy liquid metal. However, because of its toxicity, many uses of mercury are being phased out or are under review.
It is now mainly used in the chemical industry as catalysts. It is also used in some electrical switches and rectifiers.
Previously its major use was in the manufacture of sodium hydroxide and chlorine by electrolysis of brine. These plants will all be phased out by 2020. It was also commonly used in batteries, fluorescent lights, felt production, thermometers and barometers. Again, these uses have been phased out.
Mercury easily forms alloys, called amalgams, with other metals such as gold, silver and tin. The ease with which it amalgamates with gold made it useful in recovering gold from its ores. Mercury amalgams were also used in dental fillings.
Mercuric sulfide (vermilion) is a high-grade, bright-red paint pigment, but is very toxic so is now only used with great care.
Biological role
Mercury has no known biological role, but is present in every living thing and widespread in the environment. Every mouthful of food we eat contains a little mercury.
Our daily intake is less than 0.01 milligrams (about 0.3 grams in a lifetime), and this we can cope with easily. However, in much higher doses it is toxic and one form of mercury – methylmercury – is particularly dangerous. It can accumulate in the flesh of fish and be eaten by people, making them ill.
Natural abundance
Mercury rarely occurs uncombined in nature, but can be found as droplets in cinnabar (mercury sulfide) ores. China and Kyrgyzstan are the main producers of mercury. The metal is obtained by heating cinnabar in a current of air and condensing the vapour.
#34 Re: Exercises » Compute the solution: » 2025-09-12 14:46:03
Hi,
2581.
#35 Re: Jai Ganesh's Puzzles » Oral puzzles » 2025-09-12 14:24:55
Hi,
#6238.
#36 Re: Jai Ganesh's Puzzles » Doc, Doc! » 2025-09-12 13:53:03
Hi,
#2465. What does the medical term Heterochromia iridum mean?
#37 Re: Jai Ganesh's Puzzles » Doc, Doc! » 2025-09-11 23:38:57
Hi,
#2464. What does the medical term Heart failure signify?
#38 Re: Jai Ganesh's Puzzles » Oral puzzles » 2025-09-11 23:13:24
Hi,
#6237.
#39 Re: Jai Ganesh's Puzzles » 10 second questions » 2025-09-11 22:51:14
Hi,
#9731.
#40 Dark Discussions at Cafe Infinity » Close Quotes - III » 2025-09-11 22:24:31
- Jai Ganesh
- Replies: 0
Close Quotes - III
1. If I get stuck, I look at a book that tells me how someone else did it. I turn the pages, and then I say, 'Oh, I forgot that bit,' then close the book and carry on. Finally, after you've figured out how to do it, you read how they did it and find out how dumb your solution is and how much more clever and efficient theirs is! - Richard P. Feynman
2. Success is different for everyone; everybody defines it in their own way, and that's part of what we do in 'Close Up', finding what it was each person wanted to achieve and what their willingness to sacrifice for that was. - William Shatner
3. From my close observation of writers... they fall into two groups: 1) those who bleed copiously and visibly at any bad review, and 2) those who bleed copiously and secretly at any bad review. - Isaac Asimov
4. Never let your ego get so close to your position that when your position goes, your ego goes with it. - Colin Powell
5. If you're not comfortable with public speaking - and nobody starts out comfortable; you have to learn how to be comfortable - practice. I cannot overstate the importance of practicing. Get some close friends or family members to help evaluate you, or somebody at work that you trust. - Hillary Clinton
6. Happiness, for me, has to be real - life that is made of real conversations, of spending quality time with close friends, walks in nature and woods, praying, feeling real gratitude, reading good books, being able to be in the moment and hearing the sounds of nature. - Bhumika Chawla
7. Politics is just like show business. You have a hell of an opening, coast for a while, and then have a hell of a close. - Ronald Reagan
8. China and India are close neighbours linked by mountains and rivers and the Chinese and Indian peoples have enjoyed friendly exchanges for thousands of years. - Li Peng.
#41 Re: This is Cool » Miscellany » 2025-09-11 21:03:40
2385) 49th Parallel
Gist
The 49th parallel most commonly refers to the 49th parallel north, a line of latitude that forms the international border between the United States and Canada for a significant portion of their boundary, a result of treaties in 1818 and 1846. This parallel also serves as the southern border for several Canadian provinces and the northern border for various U.S. states.
Summary
The 49th parallel north is a circle of latitude that is 49° north of Earth's equator. It crosses Europe, Asia, the Pacific Ocean, North America, and the Atlantic Ocean.
The city of Paris is about 15 kilometres (9 miles) south of the 49th parallel and is the largest city between the 48th and 49th parallels. Its main airport, Charles de Gaulle Airport, lies on the 49th parallel.
Roughly 2,030 kilometres (1,260 miles) of the Canada–United States border was designated to follow the 49th parallel from British Columbia to Manitoba on the Canada side, and from Washington to Minnesota on the U.S. side, more specifically from the Strait of Georgia to the Lake of the Woods. This international border was specified in the Anglo-American Convention of 1818 and the Oregon Treaty of 1846, though survey markers placed in the 19th century cause the border to deviate from the 49th parallel by up to 810 metres (0.503 miles).
From a point on the ground at this latitude, the sun is above the horizon for 16 hours, 12 minutes during the summer solstice and 8 hours, 14 minutes during the winter solstice.
This latitude also roughly corresponds to the minimum latitude in which astronomical twilight can last all night near the summer solstice. All-night astronomical twilight lasts about from June 9 to July 2. At midnight on the summer solstice, the altitude of the sun is about −17.56°.
Slightly less than one-eighth of the Earth's surface is north of the 49th parallel.
Details
When there’s a continent at stake, arguments over borders can make or break the peace across the whole region.
What happens when a continent has only two major nations? In the case of Canada and the United States of America, the 5,525 mile long line delineating the two nations is currently the longest undefended international boundary in the world, often cited as an example to the rest of the world of how two nations can cooperate.
Today, the countries are seen as model neighbours, but it took centuries of arguing, skirmishes, and outright wars to settle the matter of who got what in North America. Even after a century and a half of relative peace, today there are still a few areas that are still in dispute, and it doesn't show signs of being settled any time soon.
If you're interested in how this comparatively simple division of land occurred, here’s how it went down:
1700s
1775 - During the Revolutionary War, the American colonists hoped Quebec and Nova Scotia would be their allies against the British. The soon-to-be U.S. got impatient, though, and took matters into its own hands, invading its neighbor to the north and taking Montreal in the first big military maneuver of the war. A month later, they were pushed back in a major defeat known as the Battle of Quebec.
1783 - After the war, a victorious U.S. demanded that Great Britain give them Canada. Unsurprisingly, the British refused. The parties settled the boundaries, or so they thought, by using the 45th parallel as the northern border between New York and New Brunswick, and creating an imaginary line through the Great Lakes. No one knew much about what existed beyond the Mississippi River to the west, so those boundaries were left murky, to say the least.
1800s
1803 - The young United States made a very good deal with France, known as the Louisiana Purchase. For 15 million dollars, they bought a vast swath of territory that reached from Gulf of Mexico to the Rockies (or Stony Mountains, as they were called then). The British and the U.S. used the watershed between the Hudson Bay and the Mississippi/Missouri rivers to establish the northern border for the newly purchased lands. That turned out to be a bad idea, because the watershed was too flat to be measured accurately.
1812 - During the War of 1812, the United States invaded Canada again, not once, but twice, as a way of attacking British interests. Some Americans thought taking Canada would be a walk in the park, but they suffered a humiliating defeat in the Siege of Detroit, aided by the Native American Tecumseh. As well, British forces land near Washington D.C., burning the US Capitol and the Whitehouse.
In 1814, a treaty restored the original boundaries. Many Canadians fought during the war, which created a new sense of a national identity.
1818 - American settlers had streamed further west, encouraged by a belief in Manifest Destiny, which held that America was meant to stretch from coast to coast. That pressured Britain and the US to return to the border-negotiating table.
Surveyors were struggling against great odds to map exactly what existed in the largely rugged terrain. The powers-that-were decided to use a straight line - the 49th parallel - to demarcate the border up to the Rockies. Left unresolved was the mysterious land beyond, called Columbia in Canada and Oregon in the U.S.
For now, Britain and the U.S allowed it to remain open to whoever could survive out there. Surveying mistakes led to an anomaly that still exists, the Northwest Angle. It’s part of Minnesota, but if you want to go there by land, you have to travel through Canada, twice.
1846 - The agreement to keep the Oregon and Columbia territories neutral was falling apart. Thousands of Americans had stakes in the region, and the U.S. was pushing hard against Britain’s Hudson Bay Company, which controlled Canadian interests. President James K. Polk demanded that U.S. territory be extended northward, past the 49th parallel.
The slogan “54° 40’ or Fight” became a rallying cry for his supporters. In the end, the U.S. blinked, and Polk agreed to let the 49th parallel become the official dividing line for the westernmost areas of the two countries, resolving (almost) the last major piece of the Canadian-U.S. puzzle. Spats over small areas would continue for decades.
1867 - Canada is granted its independence from Great Britain. Based upon a drive for self-determination from the Canadian provinces, and a desire from Britain for Canada to defend itself against American encroachment, this process was peaceful and did not lead to a military dispute as seen in America's war of Independence.
The first Canadian prime minister Sir John A. MacDonald drives the creation of a transcontinental railroad in order to allow quick movements of troops to the western territories of Canada in case of American expansion.
1900s
1908 - A treaty between the two nations is signed, establishing a joint commission tasked with surveying and delineating the border between the two neighbours.
1925 - Canada and America agree to make the International Boundary Commission permanent, in order to maintain the land and monuments along the border.
One of its responsibilities is maintenance of the Peace Arch, which was built on the exact line between the two nations, in Washington State in the U.S. and British Columbia in Canada. On the U.S side, the monument reads "Children Of a Common Mother," and on the Canadian side "Brethren Dwelling together in Unity." And for the most part, both are true.
Current
The U.S. and Canada have agreed to disagree over a few remaining bits and bobs, including Machias Seal Island in the east, which has a Canadian lighthouse but is claimed by the U.S.
There is also the matter of the Northwest Passage, which Canada says is hers, but the U.S. says is international shipping waters. A small piece off territorial waters off the coast of the Yukon (a Canadian Territory) is claimed by the U.S. as a special economic zone.
As far as we know, there are no more plans to invade, however. It was a long road, but in the end, Canada and the United States reached a harmonious relationship that stretches from the Atlantic to the Pacific.
Additional Information
The 49th parallel serves as the border between the United States and Canada due to a series of historical agreements and treaties that shaped its establishment. The boundary is drawn along the 49th parallel from Lake of the Woods in the east to the Strait of Georgia in the west. Several key events led to this decision, making it a straightforward and agreed-upon solution for both nations.
The choice of the 49th parallel as a border was both practical and symbolic. It provided a simple, horizontal line that was straightforward to draw and maintain.
This choice allowed me and others involved to easily demarcate the land and limit disputes. The decision also meant a fair division of territory between the nations, which allowed for westward expansion without constant conflict.
Despite its simplicity, drawing a border along the 49th parallel wasn’t easy. The land includes plains, mountains, and everything in between, complicating the task. Surveyors, including those on my team, faced many obstacles, like harsh weather, as they worked over several decades to accurately map and mark this boundary.

#42 Jokes » Librarian Jokes - V » 2025-09-11 15:47:10
- Jai Ganesh
- Replies: 0
Q: On a scale from one to ten, how obsessed with Harry Potter are you?
A: About nine and three quarters.
* * *
Q: Why did the student throw a book at the Librarian?
A: He wanted to Face-Book her.
* * *
Q: What do Turkish librarians eat for lunch?
A: Shhhh Kebabs.
* * *
Q: What did the frog say when he landed on a book?
Reddit! Reddit! Reddit!
* * *
Q: What kind of writing pays the most?
A: Ransom notes.
* * *
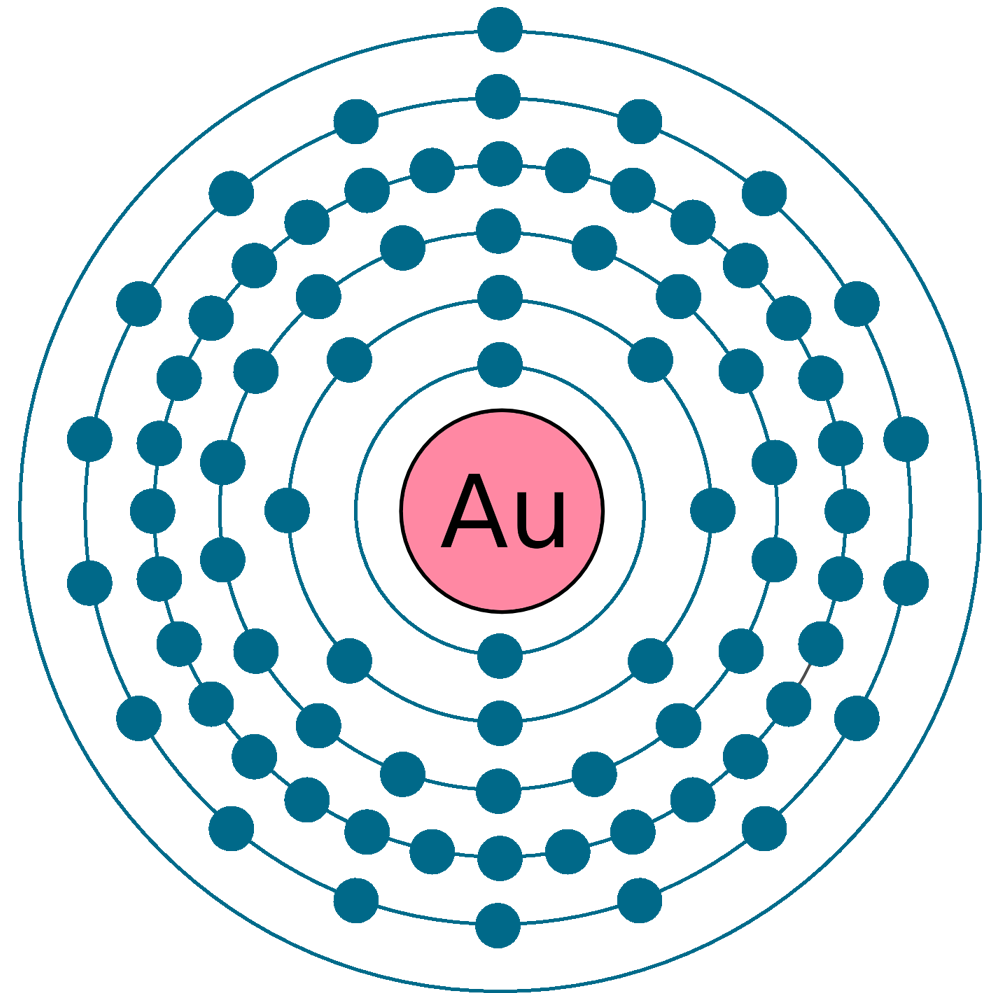
#43 Science HQ » Gold » 2025-09-11 15:23:09
- Jai Ganesh
- Replies: 0
Gold
Gist
Gold is a chemical element with the symbol Au (from the Latin aurum) and atomic number 79, known for its bright yellow color and high density. It's a transition metal, belonging to the noble metals, which means it is highly unreactive, resistant to corrosion, and a good conductor of heat and electricity. Gold is extremely malleable and ductile, allowing it to be hammered into thin sheets or drawn into wires, and it is widely used in jewelry, electronics, finance, and dentistry.
Gold is called "Au" because the symbol comes from its Latin name, aurum, which means "shining dawn" or "glow of sunrise". The chemical symbol is a short, two-letter abbreviation derived from this Latin word, which was a common practice for elements known since antiquity, as Latin was a widely known language among educated people.
Summary
Gold is a chemical element; it has chemical symbol Au (from Latin aurum) and atomic number 79. In its pure form, it is a bright, slightly orange-yellow, dense, soft, malleable, and ductile metal. Chemically, gold is a transition metal, a group 11 element, and one of the noble metals. It is one of the least reactive chemical elements, being the second lowest in the reactivity series, with only platinum ranked as less reactive. Gold is solid under standard conditions.
Gold often occurs in free elemental (native state), as nuggets or grains, in rocks, veins, and alluvial deposits. It occurs in a solid solution series with the native element silver (as in electrum), naturally alloyed with other metals like copper and palladium, and mineral inclusions such as within pyrite. Less commonly, it occurs in minerals as gold compounds, often with tellurium (gold tellurides).
Gold is resistant to most acids, though it does dissolve in aqua regia (a mixture of nitric acid and hydrochloric acid), forming a soluble tetrachloroaurate anion. Gold is insoluble in nitric acid alone, which dissolves silver and base metals, a property long used to refine gold and confirm the presence of gold in metallic substances, giving rise to the term "acid test". Gold dissolves in alkaline solutions of cyanide, which are used in mining and electroplating. Gold also dissolves in mercury, forming amalgam alloys, and as the gold acts simply as a solute, this is not a chemical reaction.
A relatively rare element when compared to silver (though thirty times more common than platinum), gold is a precious metal that has been used for coinage, jewelry, and other works of art throughout recorded history. In the past, a gold standard was often implemented as a monetary policy. Gold coins ceased to be minted as a circulating currency in the 1930s, and the world gold standard was abandoned for a fiat currency system after the Nixon shock measures of 1971.
In 2023, the world's largest gold producer was China, followed by Russia and Australia. As of 2020, a total of around 201,296 tonnes of gold exist above ground. If all of this gold were put together into a cube shape, each of its sides would measure 21.7 meters (71 ft). The world's consumption of new gold produced is about 50% in jewelry, 40% in investments, and 10% in industry. Gold's high malleability, ductility, resistance to corrosion and most other chemical reactions, as well as conductivity of electricity have led to its continued use in corrosion-resistant electrical connectors in all types of computerized devices (its chief industrial use). Gold is also used in infrared shielding, the production of colored glass, gold leafing, and tooth restoration. Certain gold salts are still used as anti-inflammatory agents in medicine.
Details
Gold (Au), chemical element, is a dense lustrous yellow precious metal of Group 11 (Ib), Period 6, of the periodic table of the elements. Gold has several qualities that have made it exceptionally valuable throughout history. It is attractive in colour and brightness, durable to the point of virtual indestructibility, highly malleable, and usually found in nature in a comparatively pure form. The history of gold is unequaled by that of any other metal because of its perceived value from earliest times.
Element Properties
atomic number : 79
atomic weight : 196.96657
melting point : 1,063 °C (1,945 °F)
boiling point : 2,966 °C (5,371 °F)
specific gravity : 19.3 at 20 °C (68 °F)
oxidation states : +1, +3.
Properties, occurrences, and uses
Gold is one of the densest of all metals. It is a good conductor of heat and electricity. It is also soft and the most malleable and ductile of the elements; an ounce (31.1 grams; gold is weighed in troy ounces) can be beaten out to 187 square feet (about 17 square metres) in extremely thin sheets called gold leaf.
Because gold is visually pleasing and workable and does not tarnish or corrode, it was one of the first metals to attract human attention. Examples of elaborate gold workmanship, many in nearly perfect condition, survive from ancient Egyptian, Minoan, Assyrian, and Etruscan artisans, and gold continues to be a highly favoured material out of which to craft jewelry and other decorative objects.
Because of its unique qualities, gold has been the one material that is universally accepted in exchange for goods and services. In the form of coins or bullion, gold has occasionally played a major role as a high-denomination currency, although silver was generally the standard medium of payments in the world’s trading systems. Gold began to serve as backing for paper-currency systems when they became widespread in the 19th century, and from the 1870s until World War I the gold standard was the basis for the world’s currencies. Although gold’s official role in the international monetary system had come to an end by the 1970s, the metal remains a highly regarded reserve asset, and approximately 45 percent of all the world’s gold is held by governments and central banks for this purpose. Gold is still accepted by all nations as a medium of international payment.
Gold is widespread in low concentrations in all igneous rocks. Its abundance in Earth’s crust is estimated at about 0.005 part per million. It occurs mostly in the native state, remaining chemically uncombined except with tellurium, selenium, and bismuth. The element’s only naturally occurring isotope is gold-197. Gold often occurs in association with copper and lead deposits, and, though the quantity present is often extremely small, it is readily recovered as a by-product in the refining of those base metals. Large masses of gold-bearing rock rich enough to be called ores are unusual. Two types of deposits containing significant amounts of gold are known: hydrothermal veins, where it is associated with quartz and pyrite (fool’s gold); and placer deposits, both consolidated and unconsolidated, that are derived from the weathering of gold-bearing rocks.
Veins enriched in gold form when the gold was carried up from great depths with other minerals, in an aqueous solution, and later precipitated. The gold in rocks usually occurs as invisible disseminated grains, more rarely as flakes large enough to be seen, and even more rarely as masses or veinlets. Crystals about 2.5 cm (1 inch) or more across have been found in California. Masses, some on the order of 90 kg (200 pounds), have been reported from Australia.
Alluvial deposits of gold found in or along streams were the principal sources of the metal for ancient Egypt and Mesopotamia. Other deposits were found in Lydia (now in Turkey) and the lands of the Aegean and in Persia (Iran), India, China, and other lands. During the Middle Ages the chief sources of gold in Europe were the mines of Saxony and Austria. The era of gold production that followed the Spanish discovery of the Americas in the 1490s was probably the greatest the world had witnessed to that time. The exploitation of mines by slave labour and the looting of palaces, temples, and graves in Central and South America resulted in an unprecedented influx of gold that literally unbalanced the economic structure of Europe. From Christopher Columbus’s discovery of the New World in 1492 to 1600, more than 225,000 kg (8,000,000 ounces) of gold, or 35 percent of world production, came from South America. The New World’s mines—especially those in Colombia—continued into the 17th and 18th centuries to account for 61 and 80 percent, respectively, of world production; 1,350,000 kg (48,000,000 ounces) were mined in the 18th century.
Russia became the world’s leading producer of gold in 1823, and for 14 years it contributed the bulk of the world supply. During the second era of expanding production (1850–75), more gold was produced in the world than in all the years since 1492, primarily because of discoveries in California and Australia. A third marked increase (1890–1915) stemmed from discoveries in Alaska, Yukon Territory (now Yukon), and South Africa. A major factor in the increase of the world’s supply of gold was the introduction in 1890 of the cyanide process for the recovery of gold from low-grade ores and ores containing minute, particle-sized gold. Gold production continued to rise throughout the 20th century, partly because of the improvement in recovery methods and partly because of the continual growth and expansion of South Africa’s gold-mining operations.
In the late 20th century, four countries—South Africa, Russia, the United States, and Australia—accounted for two-thirds of the gold produced annually throughout the world. In the early 21st century, China was the world leader in gold production. During this period, Australia, the United States, Russia, Canada, and South Africa also continued to supply large amounts of the precious metal.
Because pure gold is too soft to resist prolonged handling, it is usually alloyed with other metals to increase its hardness for use in jewelry, goldware, or coinage. Most gold used in jewelry is alloyed with silver, copper, and a little zinc to produce various shades of yellow gold or with nickel, copper, and zinc to produce white gold. The colour of these gold alloys goes from yellow to white as the proportion of silver in them increases; more than 70 percent silver results in alloys that are white. Alloys of gold with silver or copper are used to make gold coins and goldware, and alloys with platinum or palladium are also used in jewelry. The content of gold alloys is expressed in 24ths, called karats; a 12-karat gold alloy is 50 percent gold, and 24-karat gold is gold that is more than 99 percent pure.
Because of its high electrical conductivity (71 percent that of copper) and inertness, the largest industrial use of gold is in the electric and electronics industry for plating contacts, terminals, printed circuits, and semiconductor systems. Thin films of gold that reflect up to 98 percent of incident infrared radiation have been employed on satellites to control temperature and on space-suit visors to afford protection. Used in a similar way on the windows of large office buildings, gold reduces the air-conditioning requirement and adds to the beauty. Gold has also long been used for fillings and other repairs to teeth.
Gold is one of the noblest—that is, least chemically reactive—of the transition elements. It is not attacked by oxygen or sulfur, although it will react readily with halogens or with solutions containing or generating chlorine, such as aqua regia. It also will dissolve in cyanide solutions in the presence of air or hydrogen peroxide. Dissolution in cyanide solutions is attributable to the formation of the very stable dicyanoaurate ion, [Au(CN)2]−.
Like copper, gold has a single s electron outside a completed d shell, but, in spite of the similarity in electronic structures and ionization energies, there are few close resemblances between gold on the one hand and copper on the other.
Compounds
The characteristic oxidation states of gold are +1 (aurous compounds) and +3 (auric compounds). The state +1 is generally quite unstable, and most of the chemistry of gold involves the state +3. Gold is more easily displaced from solution by reduction than any other metal. Even platinum will reduce Au3+ ions to metallic gold.
Among the relatively few gold compounds of practical importance are gold(I) chloride, AuCl; gold(III) chloride, AuCl3; and chlorauric acid, HAuCl4. In the first compound, gold is in the +1 oxidation state, and in the latter two, the +3 state. All three compounds are involved in the electrolytic refining of gold. Potassium cyanoaurate, K[Au(CN)2], is the basis for most gold-plating baths (the solution employed when gold is plated). Several organic compounds of gold have industrial applications. For example, gold mercaptides, which are obtained from sulfurized terpenes, are dissolved in certain organic solutions and used for decorating china and glass articles.
Additional Information:
Appearance
A soft metal with a characteristic yellow colour. It is chemically unreactive, although it will dissolve in aqua regia (a mixture of nitric and hydrochloric acids).
Uses
Most mined gold is stored as bullion. It is also, however, used extensively in jewellery, either in its pure form or as an alloy. The term ‘carat’ indicates the amount of gold present in an alloy. 24-carat is pure gold, but it is very soft. 18- and 9-carat gold alloys are commonly used because they are more durable.
The metal is also used for coinage, and has been used as standard for monetary systems in some countries.
Gold can be beaten into very thin sheets (gold leaf) to be used in art, for decoration and as architectural ornament. Electroplating can be used to cover another metal with a very thin layer of gold. This is used in gears for watches, artificial limb joints, cheap jewellery and electrical connectors. It is ideal for protecting electrical copper components because it conducts electricity well and does not corrode (which would break the contact). Thin gold wires are used inside computer chips to produce circuits.
Dentists sometimes use gold alloys in fillings, and a gold compound is used to treat some cases of arthritis.
Gold nanoparticles are increasingly being used as industrial catalysts. Vinyl acetate, which is used to make PVA (for glue, paint and resin), is made using a gold catalyst.
Biological role
Gold has no known biological role, and is non-toxic.
Natural abundance
Gold is one of the few elements to occur in a natural state. It is found in veins and alluvial deposits. About 1500 tonnes of gold are mined each year. About two-thirds of this comes from South Africa and most of the rest from Russia.
Seawater contains about 4 grams of gold in 1,000,000 tonnes of water. Overall this is a huge amount of gold stored in the oceans but, because the concentration is so low, attempts to reclaim this gold have always failed.
#44 Re: Exercises » Compute the solution: » 2025-09-11 14:15:54
Hi,
2580.
#45 Dark Discussions at Cafe Infinity » Close Quotes - II » 2025-09-10 20:37:17
- Jai Ganesh
- Replies: 0
Close Quotes - II
1. To draw you must close your eyes and sing. - Pablo Picasso
2. Each morning sees some task begun, each evening sees it close; Something attempted, something done, has earned a night's repose. - Henry Wadsworth Longfellow
3. It is vain for the coward to flee; death follows close behind; it is only by defying it that the brave escape. - Voltaire
4. I usually tried to stay in the net for 45 minutes, half an hour longer than most batsmen would stick at the county nets. There was a reason for this so-called gluttony of practice: it was a conscious effort to make myself concentrate for long periods of time in circumstances as close to the real thing as I could make them. Geoffrey Boycott
5. Boys used to call me Soda in school days. Soda means 'serving officers daughters association.' I miss those days when I had a very protected life: one could get close and bond with other army people that they gradually would become your extended family. - Anushka Sharma
6. If I get stuck, I look at a book that tells me how someone else did it. I turn the pages, and then I say, 'Oh, I forgot that bit,' then close the book and carry on. Finally, after you've figured out how to do it, you read how they did it and find out how dumb your solution is and how much more clever and efficient theirs is! - Richard P. Feynman
7. I don't have many friends; I'm very much a loner. As a child I was very isolated, and I've never been really close to anyone. - Anthony Hopkins
8. The just is close to the people's heart, but the merciful is close to the heart of God. - Khalil Gibran.
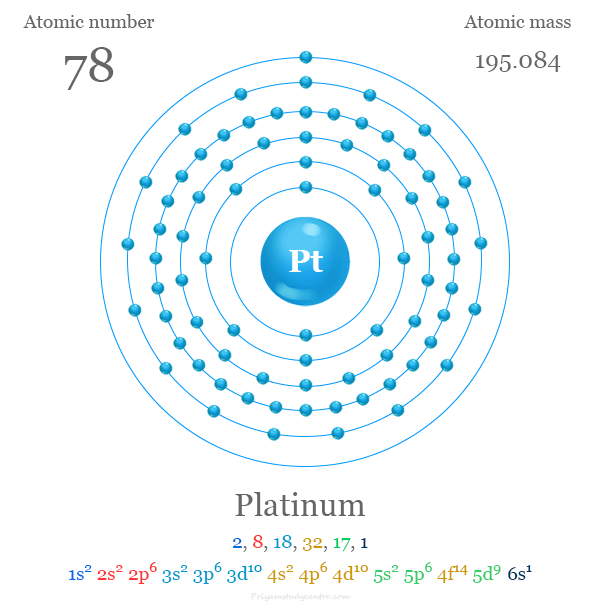
#46 Science HQ » Platinum » 2025-09-10 18:44:16
- Jai Ganesh
- Replies: 0
Platinum
Gist
Platinum is known for its superior durability, resisting scratches and tarnish better than gold. It has a higher density and doesn't wear down as quickly as gold, making it ideal for rings and bracelets. Gold, particularly in higher purity forms like 24-carat, is softer and more prone to bending or scratching.
The auto industry uses platinum for catalytic converters, which can help reduce the toxicity of gases and pollutants in the exhaust that an internal combustion engine creates. Platinum and other platinum-grade metals in catalytic converters have led to a secondary market for scrap converters, which scrap businesses will buy in order to extract the metal for resale. The metal is also used in thermometers, laboratory equipment, electrodes, and dentistry equipment.
Summary
Platinum is a chemical element; it has symbol Pt and atomic number 78. It is a dense, malleable, ductile, highly unreactive, precious, silverish-white transition metal. Its name originates from Spanish platina, a diminutive of plata "silver".
Platinum is a member of the platinum group of elements and group 10 of the periodic table of elements. It has six naturally occurring isotopes. It is one of the rarer elements in Earth's crust, with an average abundance of approximately 5 μg/kg. It occurs in some nickel and copper ores along with some native deposits, with 90% of current production from deposits across Russia's Ural Mountains, Colombia, the Sudbury basin of Canada, and a large reserve in South Africa. Because of its scarcity in Earth's crust, only a few hundred tonnes are produced annually, and given its important uses, it is highly valuable as well as a major precious metal commodity.
Platinum has remarkable resistance to corrosion, even at high temperatures, and is therefore considered a noble metal. Consequently, platinum is often found chemically uncombined as native platinum. Because it occurs naturally in the alluvial sands of various rivers, it was first used by pre-Columbian South American natives to produce artifacts. It was referenced in European writings as early as the 16th century, but it was not until Antonio de Ulloa published a report on a new metal of Colombian origin in 1748 that it began to be investigated by scientists.
Platinum is used in catalytic converters, laboratory equipment, electrical contacts and electrodes, platinum resistance thermometers, dentistry equipment, and jewelry. Platinum is used in the glass industry to manipulate molten glass, which does not "wet" platinum. Elemental platinum has not been linked to adverse health effects. Compounds containing platinum, such as cisplatin, oxaliplatin and carboplatin, are applied in chemotherapy against certain types of cancer.
Details
Platinum (Pt) is a chemical element, the best known and most widely used of the six platinum metals of Groups 8–10, Periods 5 and 6, of the periodic table. A very heavy, precious, silver-white metal, platinum is soft and ductile and has a high melting point and good resistance to corrosion and chemical attack. For example, its surface remains bright after being brought to white heat in air, and, though it readily dissolves in aqua regia, it is scarcely attacked by simple acids. (It does dissolve slowly in hydrochloric acid in the presence of air.) Small amounts of iridium are commonly added to give a harder, stronger alloy that retains the advantages of pure platinum.
Platinum, one of the most abundant platinum metals, and its alloys are indispensable in the chemical laboratory for electrodes and for crucibles and dishes in which materials can be heated to high temperatures. Platinum is used for electrical contacts and sparking points because it resists both the high temperatures and chemical attack of electric arcs. Jewelry and dental alloys account for much of its use; platinum-iridium is used for surgical pins. The prototype international standard kilogram of mass was made from an alloy of 90 percent platinum and 10 percent iridium. The electrical resistivity of platinum is relatively high and depends markedly upon the temperature; the International Temperature Scale from −259.35 to 961.78 °C (−434.83 to 1,763.2 °F)is defined in terms of a resistance thermometer made with platinum wire. As a catalyst, platinum has many applications, notably in automotive catalytic converters and in petroleum refining.
The Italian-French physician Julius Caesar Scaliger alluded (1557) to a refractory metal, probably platinum, found between Darién and Mexico. The first certain discovery was in the alluvial deposits of the Río Pinto, Colombia. The Spaniards called the new metal platina del Pinto for its resemblance to silver. The world’s most important deposits occur in the Transvaal of South Africa. Other deposits are found in Russia, Finland, Ireland, Borneo, New South Wales, New Zealand, Brazil, Peru, and Madagascar. In North America native platinum is found in Alaska, California, and Oregon, in British Columbia, and in Alberta. Placer deposits are the most productive sources of the native element. The ordinary variety of native platinum is called polyxene; it is 80 percent to 90 percent platinum, with 3 percent to 11 percent iron, plus the other platinum metals, and gold, copper, and nickel. For mineralogical properties, see native element (table). Platinum is also found in the very rare native alloy platiniridium. Platinum occurs combined with math as sperrylite (PtAs2) in the copper–nickel mining district near Sudbury, Ontario, and with sulfur as cooperite (PtS) in the Transvaal. (For information about the mining, recovery, and production of platinum, see platinum processing.)
Platinum is rapidly attacked by fused alkali oxides and peroxides and also by fluorine and chlorine at about 500 °C. It is capable of absorbing large volumes of hydrogen, and, with palladium, it is one of the most reactive platinum metals.
Platinum forms an important series of compounds with the oxidation states of +2 and +4. Many of these compounds contain coordination complexes in which chloride ion (Cl−), ammonia (NH3), or other groups are bonded to a central platinum atom. Among the transition metals, platinum has one of the greatest tendencies to form bonds directly with carbon. Platinum also combines with a number of nonmetallic elements on heating, such as phosphorus, math, antimony, silicon, sulfur, and selenium.
Natural platinum is a mixture of six isotopes: platinum-190 (0.012 percent), platinum-192 (0.782 percent), platinum-194 (32.86 percent), platinum-195 (33.78 percent), platinum-196 (25.21 percent), and platinum-198 (7.36 percent). All are stable except platinum-190, which has been reported as a long-lived alpha emitter.
Element Properties
atomic number : 78
atomic weight : 195.09
melting point : 1,769 °C (3,216 °F)
boiling point : 3,827 °C (6,920 °F)
specific gravity : 21.45 (20 °C)
oxidation states : +2, +4.
Additional Information
Appearance:
A shiny, silvery-white metal as resistant to corrosion as gold.
Uses
Platinum is used extensively for jewellery. Its main use, however, is in catalytic converters for cars, trucks and buses. This accounts for about 50% of demand each year. Platinum is very effective at converting emissions from the vehicle’s engine into less harmful waste products.
Platinum is used in the chemicals industry as a catalyst for the production of nitric acid, silicone and benzene. It is also used as a catalyst to improve the efficiency of fuel cells.
The electronics industry uses platinum for computer hard disks and thermocouples.
Platinum is also used to make optical fibres and LCDs, turbine blades, spark plugs, pacemakers and dental fillings.
Platinum compounds are important chemotherapy drugs used to treat cancers.
Biological role
Platinum has no known biological role. It is non-toxic.
Natural abundance
Platinum is found uncombined in alluvial deposits. Most commercially produced platinum comes from South Africa, from the mineral cooperite (platinum sulfide). Some platinum is prepared as a by-product of copper and nickel refining.
#47 Jokes » Librarian Jokes - IV » 2025-09-10 14:28:30
- Jai Ganesh
- Replies: 0
Q: Why is a math book always unhappy?
A: Because it always has lots of problems.
* * *
Q: What do dogs and story tellers have in common?
A: They both have tails!
* * *
Q: What is it called when someone gets suffocated by a book?
A: Literally murder!
* * *
Q: Have you seen the Bruce Willis movie where an entire library gets destroyed?
It's called "Die Hardcover".
* * *
Q: Why can't lawyers trick a librarian?
A: Because librarians can read the fine print.
* * *
Q: What did the surfer say to the librarian?
A: Is my book over dude?
* * *
#48 Re: Exercises » Compute the solution: » 2025-09-10 14:09:20
Hi,
You are correct!
2579.
#49 Jokes » Librarian Jokes - III » 2025-09-09 15:42:18
- Jai Ganesh
- Replies: 0
Q: Why should you be careful about what you say around a librarian?
A: Because they can read lips.
* * *
Q: What has a spine but, no bones?
A: A book.
* * *
Q: Who's the biggest liar in school?
A: The Lie-brarian.
* * *
Q: What did the librarian say to John Cusack?
A: Shhhhh! Don't Say Anything.
* * *
Q: What did the librarian say to the astronaut?
A: Find space for a book.
* * *
#50 Re: Introductions » Hello Math Is Fun! » 2025-09-09 14:45:03
Hi GretaJKelly,
Welcome to the forum!
See the links:
and
Systems of Linear and Quadratic Equations.
Links In the MathsIsFun website!