Math Is Fun Forum
You are not logged in.
- Topics: Active | Unanswered
#2526 2025-06-03 20:36:21
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 51,132
Re: Miscellany
2326) Plastics/Plastic Tecnology
Gist
Plastics are polymers, which means they are made by linking chains of the molecules (called monomers) together to create a large molecule (a polymer). An example of this is polystyrene. These links make polymers strong and durable. That's why poly- appears in common names for plastic, like polyethylene.
Plastic technology encompasses the science, engineering, and practices involved in creating, processing, and utilizing plastic materials. It involves understanding the properties of polymers, developing new materials, and designing and manufacturing plastic products for various applications.
Plastic is defined as a material that contains an essential ingredient an organic substance of large molecular weight. It is also defined as polymers of long carbon chains. Carbon atoms are linked in chains and are produced in long-chain molecules
Summary
Plastics are a wide range of synthetic or semisynthetic materials composed primarily of polymers. Their defining characteristic, plasticity, allows them to be molded, extruded, or pressed into a diverse range of solid forms. This adaptability, combined with a wide range of other properties such as low weight, durability, flexibility, chemical resistance, low toxicity, and low-cost production, has led to their widespread use around the world. While most plastics are produced from natural gas and petroleum, a growing minority are produced from renewable resources like polylactic acid.
Between 1950 and 2017, 9.2 billion metric tons of plastic are estimated to have been made, with more than half of this amount being produced since 2004. In 2023 alone, preliminary figures indicate that over 400 million metric tons of plastic were produced worldwide. If global trends in plastic demand continue, it is projected that annual global plastic production will exceed 1.3 billion tons by 2060. The primary uses for plastic include packaging, which makes up about 40% of its usage, and building and construction, which makes up about 20% of its usage.
The success and dominance of plastics since the early 20th century has had major benefits for mankind, ranging from medical devices to light-weight construction materials. The sewage systems in many countries relies on the resiliency and adaptability of polyvinyl chloride. It is also true that plastics are the basis of widespread environmental concerns, due to their slow decomposition rate in natural ecosystems. Most plastic produced has not been reused. Some is unsuitable for reuse. Much is captured in landfills or as plastic pollution. Particular concern focuses on microplastics. Marine plastic pollution, for example, creates garbage patches. Of all the plastic discarded so far, some 14% has been incinerated and less than 10% has been recycled.
In developed economies, about a third of plastic is used in packaging and roughly the same in buildings in applications such as piping, plumbing or vinyl siding. Other uses include automobiles (up to 20% plastics), furniture, and toys. In the developing world, the applications of plastic may differ; 42% of India's consumption is used in packaging. Worldwide, about 50 kg of plastic is produced annually per person, with production doubling every ten years.
The world's first fully synthetic plastic was Bakelite, invented in New York in 1907, by Leo Baekeland, who coined the term "plastics". Dozens of different types of plastics are produced today, such as polyethylene, which is widely used in product packaging, and polyvinyl chloride (PVC), used in construction and pipes because of its strength and durability. Many chemists have contributed to the materials science of plastics, including Nobel laureate Hermann Staudinger, who has been called "the father of polymer chemistry", and Herman Mark, known as "the father of polymer physics".
Structure
Most plastics contain organic polymers. The vast majority of these polymers are formed from chains of carbon atoms, with or without the attachment of oxygen, nitrogen or sulfur atoms. These chains comprise many repeating units formed from monomers. Each polymer chain consists of several thousand repeating units. The backbone is the part of the chain that is on the main path, linking together a large number of repeat units. To customize the properties of a plastic, different molecular groups called side chains hang from this backbone; they are usually attached to the monomers before the monomers themselves are linked together to form the polymer chain. The structure of these side chains influences the properties of the polymer.
Details
A plastic is a polymeric material that has the capability of being molded or shaped, usually by the application of heat and pressure. This property of plasticity, often found in combination with other special properties such as low density, low electrical conductivity, transparency, and toughness, allows plastics to be made into a great variety of products. These include tough and lightweight beverage bottles made of polyethylene terephthalate (PET), flexible garden hoses made of polyvinyl chloride (PVC), insulating food containers made of foamed polystyrene, and shatterproof windows made of polymethyl methacrylate.
The composition, structure, and properties of plastics
Many of the chemical names of the polymers employed as plastics have become familiar to consumers, although some are better known by their abbreviations or trade names. Thus, polyethylene terephthalate and polyvinyl chloride are commonly referred to as PET and PVC, while foamed polystyrene and polymethyl methacrylate are known by their trademarked names, Styrofoam and Plexiglas (or Perspex).
Industrial fabricators of plastic products tend to think of plastics as either “commodity” resins or “specialty” resins. (The term resin dates from the early years of the plastics industry; it originally referred to naturally occurring amorphous solids such as shellac and rosin.) Commodity resins are plastics that are produced at high volume and low cost for the most common disposable items and durable goods. They are represented chiefly by polyethylene, polypropylene, polyvinyl chloride, and polystyrene. Specialty resins are plastics whose properties are tailored to specific applications and that are produced at low volume and higher cost. Among this group are the so-called engineering plastics, or engineering resins, which are plastics that can compete with die-cast metals in plumbing, hardware, and automotive applications. Important engineering plastics, less familiar to consumers than the commodity plastics listed above, are polyacetal, polyamide (particularly those known by the trade name nylon), polytetrafluoroethylene (trademark Teflon), polycarbonate, polyphenylene sulfide, epoxy, and polyetheretherketone. Another member of the specialty resins is thermoplastic elastomers, polymers that have the elastic properties of rubber yet can be molded repeatedly upon heating. Thermoplastic elastomers are described in the article elastomer.
Plastics also can be divided into two distinct categories on the basis of their chemical composition. One category is plastics that are made up of polymers having only aliphatic (linear) carbon atoms in their backbone chains. All the commodity plastics listed above fall into this category. The structure of polypropylene can serve as an example; here attached to every other carbon atom is a pendant methyl group (CH3):
The other category of plastics is made up of heterochain polymers. These compounds contain atoms such as oxygen, nitrogen, or sulfur in their backbone chains, in addition to carbon. Most of the engineering plastics listed above are composed of heterochain polymers. An example would be polycarbonate, whose molecules contain two aromatic (benzene) rings.
Additional Information:
Introduction
A plastic is a kind of material that is made by people and can be formed into almost any shape. Most plastics are strong, long-lasting, and lightweight. They resist damage by water, heat, chemicals, and electricity. In addition, plastics can be made in many colors.
There are about 50 main types of plastic. They have countless uses. Manufacturers often use plastics in place of more expensive materials. In nylon stockings, for example, plastic takes the place of silk. In vinyl house siding, plastic takes the place of wood. In many automobile body parts, plastic takes the place of metal.
Making Plastics
Most plastics are made from chemicals that come from petroleum (oil), natural gas, or coal. Heating these chemicals causes them to break down into molecules. (Molecules are groups of two or more atoms, which are the tiny building blocks of everything.) Scientists then join these molecules into chains. These chains make up plastics. Different combinations of molecules form different kinds of plastic.
Plastics can be made into almost any shape by heating them at a high temperature. The heat softens the plastic, which can then be poured into a mold. As the softened plastic cools, it hardens. When reheated, some types of plastic will soften again. The plastic can then be made into new shapes. Other types of plastic will stay hard even when reheated.
History
In 1869 John Wesley Hyatt, a U.S. inventor, made the first plastic. He called it celluloid because he made it from a plant material called cellulose. In 1909 a U.S. chemist named Leo H. Baekeland developed the first plastic made completely from synthetic (human-made) materials. Baekeland named the new material Bakelite. Scientists developed many more plastics from the 1920s through the 1940s. Later scientists invented stronger plastics and blended plastics with other materials.
Plastics and the Environment
Plastics are very useful, but they can also cause many problems for the environment. Items made out of plastic do not break down. When they are thrown out they take up room in landfills. A great deal of plastic waste winds up in the oceans, where it can hurt animals. Because they do not break down, things like plastic bags, bottles, and fishing lines collect in large areas of the ocean. Sea turtles and other animals may eat the plastic. The animals can also be hurt when plastic fishing lines get wrapped around their bodies. People who are concerned about the environment try to encourage people to recycle plastics instead of throwing them away. Recycled plastic can be turned into clothing, outdoor furniture, playground equipment, and more bottles.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2527 2025-06-30 23:15:52
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 51,132
Re: Miscellany
2327) Bimetallic Strip
Gist
Bimetal strips are used in miniature circuit breakers to protect circuits from excess current. A coil of wire is used to heat a bimetal strip, which bends and operates a linkage that unlatches a spring-operated contact. This interrupts the circuit and can be reset when the bimetal strip has cooled down.
Summary:
Bimetallic Strip Method
Bimetallic strips are used for temperature measurement and control. They operate based on the differential expansion of two metals with different coefficients of thermal expansion. While not highly precise, their durability and cost-effectiveness make them ideal for ON/OFF temperature control applications.
How Do Bimetallic Strips Work?
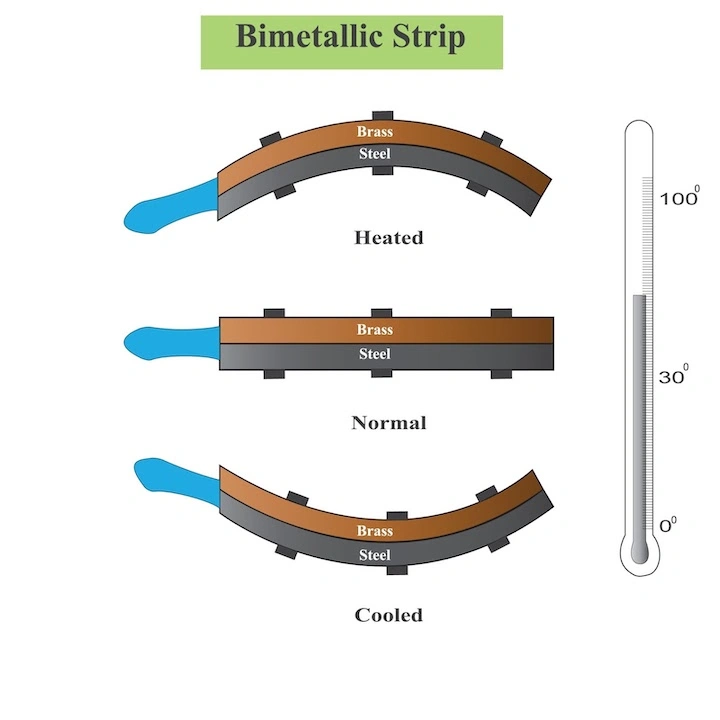
* A bimetallic strip consists of two bonded metal strips, each expanding at different rates when exposed to heat
* As temperature changes, the strip bends due to the difference in expansion rates.
* This bending movement is used to activate a switch or move a pointer in thermometers.
Temperature Range and Utility
* Bimetallic strips can operate in a temperature range from -180°C to 430°C.
* They find usage in various applications, from oven thermometers to home and industrial control thermostats.
These features make bimetallic strips suitable for specific temperature measurement applications where ruggedness and low cost are more important than high accuracy.
Details
What is a Bimetallic Strip?
A bimetallic strip is composed of two dissimilar metals joined together, usually in the form of two strips or two ribbons. The two metals are specifically dissimilar in terms of their electrical conductivity, thermal conductivity, and mechanical properties. When the strip is exposed to heat, the two dissimilar metals expand at different rates, and the resulting bending is utilized to determine the value of the temperature change. This simplicity makes the bimetallic strip an ideal component for a wide variety of applications.
How is a Bimetallic Strip Constructed?
A bimetallic strip consists of two thin strips of metal that are typically brass, copper, or steel. The strips are layered on top of each other, with one end joined and the other end free, allowing the assembly to bend and respond to temperature changes. Typically the two strips or ribbons that compose a bimetallic strip are secured together by welding or soldering.
How Does a Bimetallic Strip Work?
The two dissimilar metals in bimetallic strips are chosen based on the application and the desired properties. The operating mechanism that allows for movement of the bimetallic strip is from Thermodynamics, and the equation for linear thermal expansion helps to understand it:
Now, if you have two metal strips exposed to the same change in temperature and the same original length, but they have different coefficients of linear thermal expansion, you will see varying changes in length.
For an increase in temperature, the metal with the higher coefficient of thermal expansion will grow more than the other strip, causing the strip to bend towards the metal with the lower thermal coefficient.
For a decrease in temperature, the strip with the higher coefficient of linear thermal expansion decreases in length more, causing the strip to bend towards the metal with the higher thermal coefficient.
Now, the only operating mechanism for the movement of a bimetallic strip is the application of heat — the two metal strips are joined together, and the bending is caused by the difference in thermal expansion coefficients of the two metals. Basically, the thermal energy is converted to mechanical energy and a resulting displacement. In other words, when one metal expands more than the other due to a change in temperature, the strip bends or flexes to the contact point. That contact point is a bimetallic strip sensor that activates a switch and sends a signal to a control device or circuit.
Important Physical Properties of Bimetallic Strip Materials
When designing a bimetallic strip for any application, it is important to evaluate certain physical properties of the proposed materials:
* Coefficient of thermal expansion (how the material responds to changes in temperature)
* Modulus of elasticity (the ratio of stress to strain for a material undergoing elastic deformation)
* Maximum operating temperature (the maximum temperature the material can operate at without losing significant mechanical properties or permanently deforming)
* Electrical conductivity (especially for electrical applications)
* Stiffness and ductility
Common Bimetallic Strip Designs
Evaluating material properties is an important starting point for bimetallic strip design. Understanding the most prevalent designs is also a benefit at the outset because best practice is to have knowledge of previous designs in your area before creating something new — knowing what others have explored before you explore it yourself can save a lot of time and struggle.
The most common bimetallic strip designs are disc, ribbon, and coil designs. Of those, the disc design is the most prevalent, which consists of two metal discs stacked together. Ribbon designs consist of two metal strips joined together, and coils consist of two metal strips that are wound together into a coil. Each of these designs has its advantages and disadvantages, so the best design for a particular application should be chosen based on your requirements.
Common Applications for a Bimetallic Strip
The most common use for a bimetallic strip is as a temperature-sensitive switch. This type of switch is used in a wide range of applications, including thermostats, refrigerators, and other household appliances. But bimetallic strips are also used in a variety of mechanical applications. They can be used as a spring to provide tension or adjust the tension on a part. They can also be used to make a variety of mechanical linkages, such as a bell crank, which changes the direction of a force.
Bimetallic strips are also used in electrical applications, often as relays, which are electrical switches that control circuits. They can also be used as a current-limiting device, a type of resistor that limits the amount of current flowing through a circuit. Bimetallic strips are also used in a variety of other applications, including heat-sensitive switches, thermal fuses, and temperature-sensitive resistance elements.
In all of these applications, the two metals are carefully chosen based on their properties in order to achieve the desired result.
Additional Information
A bimetallic strip or bimetal strip is a strip that consists of two strips of different metals which expand at different rates as they are heated. The different expansion rates cause the strip to bend one way if heated, and in the opposite direction if cooled below its initial temperature. Thus, a bimetal strip converts a temperature change into mechanical displacement. The metal with the higher coefficient of thermal expansion is on the outer side of the curve when the strip is heated and on the inner side when cooled. Common applications include temperature sensing (thermometer) and regulation (thermostat).
The invention of the bimetallic strip is generally credited to John Harrison, an eighteenth-century clockmaker who made it for his third marine chronometer (H3) of 1759 to compensate for temperature-induced changes in the balance spring. Harrison's invention is recognized in the memorial to him in Westminster Abbey, England.
Characteristics
The strip consists of two strips of different metals which expand at different rates as they are heated, usually steel and copper, or in some cases steel and brass. The strips are joined together throughout their length by riveting, brazing or welding. The different expansions force the flat strip to bend one way if heated, and in the opposite direction if cooled below its initial temperature. The metal with the higher coefficient of thermal expansion is on the outer side of the curve when the strip is heated and on the inner side when cooled. The sideways displacement of the strip is much larger than the small lengthways expansion in either of the two metals.
In some applications, the bimetal strip is used in the flat form. In others, it is wrapped into a coil for compactness. The greater length of the coiled version gives improved sensitivity.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2528 2025-07-01 21:20:57
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 51,132
Re: Miscellany
2328) Geothermal Energy
Gist
Geothermal energy is heat derived from the Earth's interior. It's a renewable energy source, continuously replenished by the Earth's core, and can be harnessed for electricity generation, heating, and cooling.
Geothermal energy refers to the heat within the Earth's interior, which can be harnessed for various purposes. This heat originates from the planet's formation and the radioactive decay of elements within the Earth's core and mantle. It's a renewable energy source that can be used for heating buildings, generating electricity, and other applications.
Geothermal energy is generated by harnessing the heat from the Earth's interior. This heat, primarily from the slow decay of radioactive particles in the Earth's core, is used to produce steam, which then drives turbines connected to electricity generators. The process involves drilling into geothermal reservoirs to access steam or hot water, which is then used to generate power.
Summary
Geothermal energy, a natural resource of heat energy from within Earth that can be captured and harnessed for cooking, bathing, space heating, electrical power generation, and other uses. The total amount of geothermal energy incident on Earth is vastly in excess of the world’s current energy requirements, but it can be difficult to harness for electricity production. Despite its challenges, geothermal energy stands in stark contrast to the combustion of greenhouse gas-emitting fossil fuels (namely coal, petroleum, and natural gas) driving much of the climate crisis, and it has become increasingly attractive as a renewable energy source.
Mechanism and potential
Temperatures increase below Earth’s surface at a rate of about 30 °C per km in the first 10 km (roughly 90 °F per mile in the first 6 miles) below the surface. This internal heat of Earth is an immense store of energy and can manifest aboveground in phenomena such as volcanoes, lava flows, geysers, fumaroles, hot springs, and mud pots. The heat is produced mainly by the radioactive decay of potassium, thorium, and uranium in Earth’s crust and mantle and also by friction generated along the margins of continental plates.
Worldwide, the annual low-grade heat flow to the surface of Earth averages between 50 and 70 milliwatts (mW) per square meter. In contrast, incoming solar radiation striking Earth’s surface provides 342 watts per square meter annually (see solar energy). In the upper 10 km of rock beneath the contiguous United States alone, geothermal energy amounts to 3.3 × {10}^{25} joules, or about 6,000 times the energy contained in the world’s oil reserves. The estimated energy that can be recovered and utilized on the surface is 4.5 × {10}^{6} exajoules, or about 1.4 × {10}^{6} terawatt-years, which equates to roughly three times the world’s annual consumption of all types of energy.
Although geothermal energy is plentiful, geothermal power is not. The amount of usable energy from geothermal sources varies with depth and by extraction method. Normally, heat extraction requires a fluid (or steam) to bring the energy to the surface. Locating and developing geothermal resources can be challenging. This is especially true for the high-temperature resources needed for generating electricity. Such resources are typically limited to parts of the world characterized by recent volcanic activity or located along plate boundaries (such as along the Pacific Ring of Fire) or within crustal hot spots (such as Yellowstone National Park and the Hawaiian Islands). Geothermal reservoirs associated with those regions must have a heat source, adequate water recharge, adequate permeability or faults that allow fluids to rise close to the surface, and an impermeable caprock to prevent the escape of the heat. In addition, such reservoirs must be economically accessible (that is, within the range of drills). The most economically efficient facilities are located close to the geothermal resource to minimize the expense of constructing long pipelines. In the case of electric power generation, costs can be kept down by locating the facility near electrical transmission lines to transmit the electricity to market. Even though there is a continuous source of heat within Earth, the extraction rate of the heated fluids and steam can exceed the replenishment rate, and, thus, use of the resource must be managed sustainably.
Uses and history
Geothermal energy use can be divided into three categories: direct-use applications, geothermal heat pumps (GHPs), and electric power generation.
Details
Geothermal energy is heat that is generated within Earth. (Geo means “earth,” and thermal means “heat” in Greek.) It is a renewable resource that can be harvested for human use.
About 2,900 kilometers (1,800 miles) below Earth’s crust, or surface, is the hottest part of our planet: the core. A small portion of the core’s heat comes from the friction and gravitational pull formed when Earth was created more than four billion years ago. However, the vast majority of Earth’s heat is constantly generated by the decay of radioactive isotopes, such as potassium-40 and thorium-232.
Isotopes are forms of an element that have a different number of neutrons than the most common versions of the element’s atom.
Potassium, for instance, has 20 neutrons in its nucleus. Potassium-40, however, has 21 neutrons. As potassium-40 decays, its nucleus changes, emitting enormous amounts of energy (radiation). Potassium-40 most often decays to isotopes of calcium (calcium-40) and argon (argon-40).
Radioactive decay is a continual process in the core. Temperatures there rise to more than 5,000° Celsius (about 9,000° Fahrenheit). Heat from the core is constantly radiating outward and warming rocks, water, gas, and other geological material.
Earth’s temperature rises with depth from the surface to the core. This gradual change in temperature is known as the geothermal gradient. In most parts of the world, the geothermal gradient is about 25° C per 1 kilometer of depth (1° F per 77 feet of depth).
If underground rock formations are heated to about 700-1,300° C (1,300-2,400° F), they can become magma. Magma is molten (partly melted) rock permeated by gas and gas bubbles. Magma exists in the mantle and lower crust, and sometimes bubbles to the surface as lava.
Magma heats nearby rocks and underground aquifers. Hot water can be released through geysers, hot springs, steam vents, underwater hydrothermal vents, and mud pots.
These are all sources of geothermal energy. Their heat can be captured and used directly for heat, or their steam can be used to generate electricity. Geothermal energy can be used to heat structures such as buildings, parking lots, and sidewalks.
Most of the Earth’s geothermal energy does not bubble out as magma, water, or steam. It remains in the mantle, emanating outward at a slow pace and collecting as pockets of high heat. This dry geothermal heat can be accessed by drilling, and enhanced with injected water to create steam.
Many countries have developed methods of tapping into geothermal energy. Different types of geothermal energy are available in different parts of the world. In Iceland, abundant sources of hot, easily accessible underground water make it possible for most people to rely on geothermal sources as a safe, dependable, and inexpensive source of energy. Other countries, such as the U.S., must drill for geothermal energy at greater cost.
Harvesting Geothermal Energy: Heating and Cooling:
Low-Temperature Geothermal Energy
Almost anywhere in the world, geothermal heat can be accessed and used immediately as a source of heat. This heat energy is called low-temperature geothermal energy. Low-temperature geothermal energy is obtained from pockets of heat about 150° C (302° F). Most pockets of low-temperature geothermal energy are found just a few meters below ground.
Low-temperature geothermal energy can be used for heating greenhouses, homes, fisheries, and industrial processes. Low-temperature energy is most efficient when used for heating, although it can sometimes be used to generate electricity.
People have long used this type of geothermal energy for engineering, comfort, healing, and cooking. Archaeological evidence shows that 10,000 years ago, groups of Native Americans gathered around naturally occurring hot springs to recuperate or take refuge from conflict. In the third century BCE, scholars and leaders warmed themselves in a hot spring fed by a stone pool near Lishan, a mountain in central China. One of the most famous hot spring spas is in the appropriately named town of Bath, England. Starting construction in about 60 CE, Roman conquerors built an elaborate system of steam rooms and pools using heat from the region’s shallow pockets of low-temperature geothermal energy.
The hot springs of Chaudes Aigues, France, have provided a source of income and energy for the town since the 1300s. Tourists flock to the town for its elite spas. The low-temperature geothermal energy also supplies heat to homes and businesses.
The United States opened its first geothermal district heating system in 1892 in Boise, Idaho. This system still provides heat to about 450 homes.
Co-Produced Geothermal Energy
Co-produced geothermal energy technology relies on other energy sources. This form of geothermal energy uses water that has been heated as a byproduct in oil and gas wells.
In the United States, about 25 billion barrels of hot water are produced every year as a byproduct. In the past, this hot water was simply discarded. Recently, it has been recognized as a potential source of even more energy: Its steam can be used to generate electricity to be used immediately or sold to the grid.
One of the first co-produced geothermal energy projects was initiated at the Rocky Mountain Oilfield Testing Center in the U.S. state of Wyoming.
Newer technology has allowed co-produced geothermal energy facilities to be portable. Although still in experimental stages, mobile power plants hold tremendous potential for isolated or impoverished communities.
Geothermal Heat Pumps
Geothermal heat pumps (GHPs) take advantage of Earth’s heat, and can be used almost anywhere in the world. GHPs are drilled about three to 90 meters (10 to 300 feet) deep, much shallower than most oil and natural gas wells. GHPs do not require fracturing bedrock to reach their energy source.
A pipe connected to a GHP is arranged in a continuous loop—called a "slinky loop"—that circles underground and above ground, usually throughout a building. The loop can also be contained entirely underground, to heat a parking lot or landscaped area.
In this system, water or other liquids (such as glycerol, similar to a car’s antifreeze) move through the pipe. During the cold season, the liquid absorbs underground geothermal heat. It carries the heat upward through the building and gives off warmth through a duct system. These heated pipes can also run through hot water tanks and offset water-heating costs.
During the summer, the GHP system works the opposite way: The liquid in the pipes is warmed from the heat in the building or parking lot, and carries the heat to be cooled underground.
The U.S. Environmental Protection Agency has called geothermal heating the most energy-efficient and environmentally safe heating and cooling system. The largest GHP system was completed in 2012 at Ball State University in Indiana. The system replaced a coal-fired boiler system, and experts estimate the university will save about two million dollars a year in heating costs.
Harvesting Geothermal Energy: Electricity
In order to obtain enough energy to generate electricity, geothermal power plants rely on heat that exists a few kilometers below the surface of Earth. In some areas, the heat can naturally exist underground as pockets steam or hot water. However, most areas need to be “enhanced” with injected water to create steam.
Dry-Steam Power Plants
Dry-steam power plants take advantage of natural underground sources of steam. The steam is piped directly to a power plant, where it is used to fuel turbines and generate electricity.
Dry steam is the oldest type of power plant to generate electricity using geothermal energy. The first dry-steam power plant was constructed in Larderello, Italy, in 1911. Today, the dry-steam power plants at Larderello continue to supply electricity to more than a million residents of the area.
There are only two known sources of underground steam in the United States: Yellowstone National Park in Wyoming and The Geysers in California. Since Yellowstone is a protected area, The Geysers is the only place where a dry-steam power plant is in use. It is one of the largest geothermal energy complexes in the world, and provides about a fifth of all renewable energy in the U.S. state of California.
Flash-Steam Power Plant
Flash-steam power plants use naturally occurring sources of underground hot water and steam. Water that is hotter than 182° C (360° F) is pumped into a low-pressure area. Some of the water “flashes,” or evaporates rapidly into steam, and is funneled out to power a turbine and generate electricity. Any remaining water can be flashed in a separate tank to extract more energy.
Flash-steam power plants are the most common type of geothermal power plants. The volcanically active island nation of Iceland supplies nearly all its electrical needs through a series of flash-steam geothermal power plants. The steam and excess warm water produced by the flash-steam process heat icy sidewalks and parking lots in the frigid Arctic winter.
The islands of the Philippines also sit over a tectonically active area, the "Ring of Fire" that rims the Pacific Ocean. Government and industry in the Philippines have invested in flash-steam power plants, and today the nation is second only to the United States in its use of geothermal energy. In fact, the largest single geothermal power plant is a flash-steam facility in Malitbog, Philippines.
Binary Cycle Power Plants
Binary cycle power plants use a unique process to conserve water and generate heat. Water is heated underground to about 107°-182° C (225°-360° F). The hot water is contained in a pipe, which cycles above ground. The hot water heats a liquid organic compound that has a lower boiling point than water. The organic liquid creates steam, which flows through a turbine and powers a generator to create electricity. The only emission in this process is steam. The water in the pipe is recycled back to the ground, to be reheated by Earth and provide heat for the organic compound again.
The Beowawe Geothermal Facility in the U.S. state of Nevada uses the binary cycle to generate electricity. The organic compound used at the facility is an industrial refrigerant (tetrafluoroethane, a greenhouse gas). This refrigerant has a much lower boiling point than water, meaning it is converted into gas at low temperatures. The gas fuels the turbines, which are connected to electrical generators.
Enhanced Geothermal Systems
Earth has virtually endless amounts of energy and heat beneath its surface. However, it is not possible to use it as energy unless the underground areas are "hydrothermal." This means the underground areas are not only hot, but also contain liquid and are permeable. Many areas do not have all three of these components. An enhanced geothermal system (EGS) uses drilling, fracturing, and injection to provide fluid and permeability in areas that have hot—but dry—underground rock.
To develop an EGS, an “injection well” is drilled vertically into the ground. Depending on the type of rock, this can be as shallow as one kilometer (0.6 mile) to as deep as 4.5 kilometers (2.8 miles). High-pressure cold water is injected into the drilled space, which forces the rock to create new fractures, expand existing fractures, or dissolve. This creates a reservoir of underground fluid.
Water is pumped through the injection well and absorbs the rocks’ heat as it flows through the reservoir. This hot water, called brine, is then piped back up to Earth’s surface through a “production well.” The heated brine is contained in a pipe. It warms a secondary fluid that has a low boiling point, which evaporates to steam and powers a turbine. The brine cools off, and cycles back down through the injection well to absorb underground heat again. There are no gaseous emissions besides the water vapor from the evaporated liquid.
Pumping water into the ground for EGSs can cause seismic activity, or small earthquakes. In Basel, Switzerland, the injection process caused hundreds of tiny earthquakes that grew to more significant seismic activity even after the water injection was halted. This led to the geothermal project being canceled in 2009.
Geothermal Energy and the Environment
Geothermal energy is a renewable resource. Earth has been emitting heat for about 4.5 billion years, and will continue to emit heat for billions of years into the future because of the ongoing radioactive decay in Earth’s core.
However, most wells that extract the heat will eventually cool, especially if heat is extracted more quickly than it is given time to replenish. Larderello, Italy, site of the world’s first electrical plant supplied by geothermal energy, has seen its steam pressure fall by more than 25 percent since the 1950s.
Reinjecting water can sometimes help a cooling geothermal site last longer. However, this process can cause “micro-earthquakes.” Although most of these are too small to be felt by people or register on a scale of magnitude, sometimes the ground can quake at more threatening levels and cause the geothermal project to shut down, as it did in Basel, Switzerland.
Geothermal systems do not require enormous amounts of freshwater. In binary systems, water is only used as a heating agent, and is not exposed or evaporated. It can be recycled, used for other purposes, or released into the atmosphere as nontoxic steam. However, if the geothermal fluid is not contained and recycled in a pipe, it can absorb harmful substances such as math, boron, and fluoride. These toxic substances can be carried to the surface and released when the water evaporates. In addition, if the fluid leaks to other underground water systems, it can contaminate clean sources of drinking water and aquatic habitats.
Advantages
There are many advantages to using geothermal energy either directly or indirectly:
* Geothermal energy is renewable; it is not a fossil fuel that will be eventually used up. Earth is continuously radiating heat out from its core, and will continue to do so for billions of years.
* Some form of geothermal energy can be accessed and harvested anywhere in the world.
* Using geothermal energy is relatively clean. Most systems only emit water vapor, although some emit very small amounts of sulfur dioxide, nitrous oxides, and particulates.
* Geothermal power plants can last for decades and possibly centuries. If a reservoir is managed properly, the amount of extracted energy can be balanced with the rock’s rate of renewing its heat.
* Unlike other renewable energy sources, geothermal systems are “baseload.” This means they can work in the summer or winter, and are not dependent on changing factors such as the presence of wind or sun. Geothermal power plants produce electricity or heat 24 hours a day, seven days a week.
The space it takes to build a geothermal facility is much more compact than other power plants. To produce a GWh (a gigawatt hour, or one million kilowatts of energy for one hour, an enormous amount of energy), a geothermal plant uses the equivalent of about 1,046 square kilometers (404 square miles) of land. To produce the same GWh, wind energy requires 3,458 square kilometers (1,335 square miles), a solar photovoltaic center requires 8,384 square kilometers (3,237 square miles), and coal plants use about 9,433 square kilometers (3,642 square miles).
Geothermal energy systems are adaptable to many different conditions.
They can be used to heat, cool, or power individual homes, whole districts, or industrial processes.
Disadvantages
Harvesting geothermal energy still poses many challenges:
* The process of injecting high-pressure streams of water into the planet can result in minor seismic activity, or small earthquakes.
* Geothermal plants have been linked to subsidence, or the slow sinking of land. This happens as the underground fractures collapse upon themselves. This can lead to damaged pipelines, roadways, buildings, and natural drainage systems.
* Geothermal plants can release small amounts of greenhouse gases such as hydrogen sulfide and carbon dioxide.
* Water that flows through underground reservoirs can pick up trace amounts of toxic elements such as math, mercury, and selenium. These harmful substances can be leaked to water sources if the geothermal system is not properly insulated.
Although the process requires almost no fuel to run, the initial cost of installing geothermal technology is expensive. Developing countries may not have the sophisticated infrastructure or start-up costs to invest in a geothermal power plant. Several facilities in the Philippines, for example, were made possible by investments from U.S. industry and government agencies. Today, the plants are Philippine-owned and operated.
Geothermal Energy and People
Geothermal energy exists in different forms all over Earth (by steam vents, lava, geysers, or simply dry heat), and there are different possibilities for extracting and using this heat.
In New Zealand, natural geysers and steam vents heat swimming pools, homes, greenhouses, and prawn farms. New Zealanders also use dry geothermal heat to dry timber and feedstock.
Other countries, such as Iceland, have taken advantage of molten rock and magma resources from volcanic activity to provide heat for homes and buildings. In Iceland, almost 90 percent of the country’s people use geothermal heating resources. Iceland also relies on its natural geysers to melt snow, warm fisheries, and heat greenhouses.
The United States generates the most amount of geothermal energy of any other country. Every year, the U.S. generates at least 15 billion kilowatt-hours, or the equivalent of burning about 25 million barrels of oil. Industrial geothermal technologies have been concentrated in the western U.S. In 2012, Nevada had 59 geothermal projects either operational or in development, followed by California with 31 projects, and Oregon with 16 projects.
The cost of geothermal energy technology has gone down in the last decade, and is becoming more economically possible for individuals and companies.
Additional Information
Geothermal energy is thermal energy extracted from the crust. It combines energy from the formation of the planet and from radioactive decay. Geothermal energy has been exploited as a source of heat and/or electric power for millennia.
Geothermal heating, using water from hot springs, for example, has been used for bathing since Paleolithic times and for space heating since Roman times. Geothermal power (generation of electricity from geothermal energy), has been used since the 20th century. Unlike wind and solar energy, geothermal plants produce power at a constant rate, without regard to weather conditions. Geothermal resources are theoretically more than adequate to supply humanity's energy needs. Most extraction occurs in areas near tectonic plate boundaries.
The cost of generating geothermal power decreased by 25% during the 1980s and 1990s. Technological advances continued to reduce costs and thereby expand the amount of viable resources. In 2021, the US Department of Energy estimated that power from a plant "built today" costs about $0.05/kWh.
In 2019, 13,900 megawatts (MW) of geothermal power was available worldwide. An additional 28 gigawatts provided heat for district heating, space heating, spas, industrial processes, desalination, and agricultural applications as of 2010. As of 2019 the industry employed about one hundred thousand people.
The adjective geothermal originates from the Greek roots γῆ (gê), meaning Earth, and θερμός (thermós), meaning hot.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2529 2025-07-02 22:56:15
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 51,132
Re: Miscellany
2329) Quasar
Gist
A quasar is an extremely luminous active galactic nucleus (AGN). It is sometimes known as a quasi-stellar object, abbreviated QSO.
A quasar is a very luminous galactic core, or active galactic nucleus (AGN), powered by a supermassive black hole actively accreting matter. As gas and dust spiral into the black hole, they form a swirling accretion disk that heats up and emits intense radiation across the electromagnetic spectrum. This makes quasars exceptionally bright, sometimes outshining the entire galaxy they reside in.
Summary
A quasar is an extremely luminous active galactic nucleus (AGN). It is sometimes known as a quasi-stellar object, abbreviated QSO. The emission from an AGN is powered by accretion onto a supermassive black hole with a mass ranging from millions to tens of billions of solar masses, surrounded by a gaseous accretion disc. Gas in the disc falling towards the black hole heats up and releases energy in the form of electromagnetic radiation. The radiant energy of quasars is enormous; the most powerful quasars have luminosities thousands of times greater than that of a galaxy such as the Milky Way. Quasars are usually categorized as a subclass of the more general category of AGN. The redshifts of quasars are of cosmological origin.
The term quasar originated as a contraction of "quasi-stellar [star-like] radio source"—because they were first identified during the 1950s as sources of radio-wave emission of unknown physical origin—and when identified in photographic images at visible wavelengths, they resembled faint, star-like points of light. High-resolution images of quasars, particularly from the Hubble Space Telescope, have shown that quasars occur in the centers of galaxies, and that some host galaxies are strongly interacting or merging galaxies. As with other categories of AGN, the observed properties of a quasar depend on many factors, including the mass of the black hole, the rate of gas accretion, the orientation of the accretion disc relative to the observer, the presence or absence of a jet, and the degree of obscuration by gas and dust within the host galaxy.
About a million quasars have been identified with reliable spectroscopic redshifts, and between 2-3 million identified in photometric catalogs. The nearest known quasar is about 600 million light-years from Earth, while the record for the most distant known AGN is at a redshift of 10.1, corresponding to a comoving distance of 31.6 billion light-years, or a look-back time of 13.2 billion years.
Quasar discovery surveys have shown that quasar activity was more common in the distant past; the peak epoch was approximately 10 billion years ago. Concentrations of multiple quasars are known as large quasar groups and may constitute some of the largest known structures in the universe if the observed groups are good tracers of mass distribution.
Details
Quasar, an astronomical object of very high luminosity found in the centres of some galaxies and powered by gas spiraling at high velocity into an extremely large black hole. The brightest quasars can outshine all of the stars in the galaxies in which they reside, which makes them visible even at distances of billions of light-years. Quasars are among the most distant and luminous objects known.
Discovery of quasars
The term quasar derives from how these objects were originally discovered in the earliest radio surveys of the sky in the 1950s. Away from the plane of the Milky Way Galaxy, most radio sources were identified with otherwise normal-looking galaxies. Some radio sources, however, coincided with objects that appeared to be unusually blue stars, although photographs of some of these objects showed them to be embedded in faint, fuzzy halos. Because of their almost starlike appearance, they were dubbed “quasi-stellar radio sources,” which by 1964 had been shortened to “quasar.”
The optical spectra of the quasars presented a new mystery. Photographs taken of their spectra showed locations for emission lines at wavelengths that were at odds with all celestial sources then familiar to astronomers. The puzzle was solved by the Dutch American astronomer Maarten Schmidt, who in 1963 recognized that the pattern of emission lines in 3C 273, the brightest known quasar, could be understood as coming from hydrogen atoms that had a redshift (i.e., had their emission lines shifted toward longer, redder wavelengths by the expansion of the universe) of 0.158. In other words, the wavelength of each line was 1.158 times longer than the wavelength measured in the laboratory, where the source is at rest with respect to the observer. At a redshift of this magnitude, 3C 273 was placed by Hubble’s law at a distance of slightly more than two billion light-years. This was a large, though not unprecedented, distance (bright clusters of galaxies had been identified at similar distances), but 3C 273 is about 100 times more luminous than the brightest individual galaxies in those clusters, and nothing so bright had been seen so far away.
An even bigger surprise was that continuing observations of quasars revealed that their brightness can vary significantly on timescales as short as a few days, meaning that the total size of the quasar cannot be more than a few light-days across. Since the quasar is so compact and so luminous, the radiation pressure inside the quasar must be huge; indeed, the only way a quasar can keep from blowing itself up with its own radiation is if it is very massive, at least a million solar masses if it is not to exceed the Eddington limit—the minimum mass at which the outward radiation pressure is balanced by the inward pull of gravity (named after English astronomer Arthur Eddington). Astronomers were faced with a conundrum: how could an object about the size of the solar system have a mass of about a million stars and outshine by 100 times a galaxy of a hundred billion stars?
The right answer—accretion by gravity onto supermassive black holes—was proposed shortly after Schmidt’s discovery independently by Russian astronomers Yakov Zel’dovich and Igor Novikov and Austrian American astronomer Edwin Salpeter. The combination of high luminosities and small sizes was sufficiently unpalatable to some astronomers that alternative explanations were posited that did not require the quasars to be at the large distances implied by their redshifts. These alternative interpretations have been discredited, although a few adherents remain. For most astronomers, the redshift controversy was settled definitively in the early 1980s when American astronomer Todd Boroson and Canadian American astronomer John Beverly Oke showed that the fuzzy halos surrounding some quasars are actually starlight from the galaxy hosting the quasar and that these galaxies are at high redshifts.
By 1965 it was recognized that quasars are part of a much larger population of unusually blue sources and that most of these are much weaker radio sources too faint to have been detected in the early radio surveys. This larger population, sharing all quasar properties except extreme radio luminosity, became known as “quasi-stellar objects” or simply QSOs. Since the early 1980s most astronomers have regarded QSOs as the high-luminosity variety of an even larger population of “active galactic nuclei,” or AGNs. (The lower-luminosity AGNs are known as “Seyfert galaxies,” named after the American astronomer Carl K. Seyfert, who first identified them in 1943.)
Finding quasars
Although the first quasars known were discovered as radio sources, it was quickly realized that quasars could be found more efficiently by looking for objects bluer than normal stars. This can be done with relatively high efficiency by photographing large areas of the sky through two or three different-coloured filters. The photographs are then compared to locate the unusually blue objects, whose nature is verified through subsequent spectroscopy. This remains the primary technique for finding quasars, although it has evolved over the years with the replacement of film by electronic charge-coupled devices (CCDs), the extension of the surveys to longer wavelengths in the infrared, and the addition of multiple filters that, in various combinations, are effective at isolating quasars at different redshifts. Quasars have also been discovered through other techniques, including searches for starlike sources whose brightness varies irregularly and X-ray surveys from space; indeed, a high level of X-ray emission is regarded by astronomers as a sure indicator of an accreting black-hole system.
Physical structure of quasars
Quasars and other AGNs are apparently powered by gravitational accretion onto supermassive black holes, where “supermassive” means from roughly a million to a few billion times the mass of the Sun. Supermassive black holes reside at the centres of many large galaxies. In about 5–10 percent of these galaxies, gas tumbles into the deep gravitational well of the black hole and is heated to incandescence as the gas particles pick up speed and pile up in a rapidly rotating “accretion disk” close to the horizon of the black hole. There is a maximum rate set by the Eddington limit at which a black hole can accrete matter before the heating of the infalling gas results in so much outward pressure from radiation that the accretion stops. What distinguishes an “active” galactic nucleus from other galactic nuclei (the 90–95 percent of large galaxies that are currently not quasars) is that the black hole in an active nucleus accretes a few solar masses of matter per year, which, if it is accreting at around 1 percent or more of the Eddington rate, is sufficient to account for a typical quasar with a total luminosity of about {10}^{39} watts. (The Sun’s luminosity is about 4 × {10}^{26} watts.)
In addition to black holes and accretion disks, quasars have other remarkable features. Just beyond the accretion disk are clouds of gas that move at high velocities around the inner structure, absorbing high-energy radiation from the accretion disk and reprocessing it into the broad emission lines of hydrogen and ions of other atoms that are the signatures of quasar spectra. Farther from the black hole but still largely in the accretion disk plane are dust-laden gas clouds that can obscure the quasar itself. Some quasars are also observed to have radio jets, which are highly collimated beams of plasma propelled out along the rotation axis of the accretion disk at speeds often approaching that of light. These jets emit beams of radiation that can be observed at X-ray and radio wavelengths (and less often at optical wavelengths).
Because of this complex structure, the appearance of a quasar depends on the orientation of the rotation axis of the accretion disk relative to the observer’s line of sight. Depending on this angle, different quasar components—the accretion disk, emission-line clouds, jets—appear to be more or less prominent. This results in a wide variety of observed phenomena from what are, in reality, physically similar sources.
Evolution of quasars
The number density of quasars increases dramatically with redshift, which translates through Hubble’s law to more quasars at larger distances. Because of the finite speed of light, when quasars are observed at great distances, they are observed as they were in the distant past. Thus, the increasing density of quasars with distance means that they were more common in the past than they are now. This trend increases until “look-back times” that correspond to around three billion years after the big bang, which occurred approximately 13.5 billion years ago. At earlier ages, the number density of quasars decreases sharply, corresponding to an era when the quasar population was still building up. The most distant, and thus earliest, quasars known were formed less than a billion years after the big bang.
Individual quasars appear as their central black holes begin to accrete gas at a high rate, possibly triggered by a merger with another galaxy, building up the mass of the central black hole. The current best estimate is that quasar activity is episodic, with individual episodes lasting around a million years and the total quasar lifetime lasting around 10 million years. At some point, quasar activity ceases completely, leaving behind the dormant massive black holes found in most massive galaxies. This “life cycle” appears to proceed most rapidly with the most-massive black holes, which become dormant earlier than less-massive black holes. Indeed, in the current universe the remaining AGN population is made up predominantly of lower-luminosity Seyfert galaxies with relatively small supermassive black holes.
In the present-day universe there is a close relationship between the mass of a black hole and the mass of its host galaxy. This is quite remarkable, since the central black hole accounts for only about 0.1 percent of the mass of the galaxy. It is believed that the intense radiation, mass outflows, and jets from the black hole during its active quasar phase are responsible. The radiation, outflows, and jets heat up and can even remove entirely the interstellar medium from the host galaxy. This loss of gas in the galaxy simultaneously shuts down star formation and chokes off the quasar’s fuel supply, thus freezing both the mass in stars and the mass of the black hole.
Additional Information
A quasar is an extremely active and luminous type of active galactic nucleus (AGN). All quasars are AGNs, but not all AGNs are quasars.
Quasars are a subclass of active galactic nuclei (AGNs), extremely luminous galactic cores where gas and dust falling into a supermassive black hole emit electromagnetic radiation across the entire electromagnetic spectrum. The gas and dust become luminous as a result of the extreme gravitational and frictional forces exerted on them as they fall into the black hole. Quasars are amongst the most luminous objects in the known Universe, typically emitting thousands of times more light than the entire Milky Way. They are distinguished from other AGNs by their enormous luminosity, and their enormous distances from Earth. As the speed of light is finite, objects observed from Earth are seen as they were when the light we see left them. The nearest quasars to Earth are still several hundred million light-years away, meaning that they are observed now as they were 600 million years ago. The absence of quasars closer to Earth does not mean that there were never quasars in our region of the Universe, but instead means that quasars existed when the universe was younger. The study of quasars provides fascinating insights into the evolution of the Universe.
In 1996 Hubble’s 100 000th exposure was celebrated by capturing an image of a quasar located 9 billion light-years from Earth.
In 2019 it was announced that Hubble had observed the brightest quasar in the early Universe. After 20 years of searching, astronomers identified the ancient quasar with the help of strong gravitational lensing. A dim galaxy is located right between the quasar and Earth, bending the light from the quasar and making it appear three times as large and 50 times as bright as it would be without the effect of gravitational lensing. Even still, the lensed quasar is extremely compact and unresolved in images from optical ground-based telescopes. Only Hubble’s sharp vision allowed it to resolve the system, and this unique object provides an insight into the birth of galaxies when the Universe was less than a billion years old. Hubble’s study of gravitationally lensed quasars has also contributed to our understanding of the rate of expansion of the Universe.
Hubble has also imaged quasar ghosts — ethereal green objects which mark the graves of these objects that flickered to life and then faded. These unusual structures orbit their host galaxies and glow in a bright and eerie green hue, and offer insights into the pasts of these galaxies.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2530 2025-07-06 23:36:34
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 51,132
Re: Miscellany
2330) Continent
Gist
A continent is one of the Earth's seven main landmasses. They are, in approximate order of size: Asia, Africa, North America, South America, Antarctica, Europe, and Australia. These are conventionally defined by convention rather than strict criteria.
The seven continents in order from largest to smallest by land area are: Asia, Africa, North America, South America, Antarctica, Europe, and Australia (sometimes called Oceania or Australasia).
What defines a Continent?
By convention, continents "are understood to be large, continuous, discrete masses of land, ideally separated by expanses of water". By this definition, all continents have to be an island of some metric.
Summary
A continent is any of several large geographical regions. Continents are generally identified by convention rather than any strict criteria. A continent could be a single large landmass, a part of a very large landmass, as in the case of Asia or Europe within Eurasia, or a landmass and nearby islands within its continental shelf. Due to these varying definitions, the number of continents varies; up to seven or as few as four geographical regions are commonly regarded as continents. Most English-speaking countries recognize seven regions as continents. In order from largest to smallest in area, these seven regions are Asia, Africa, North America, South America, Antarctica, Europe, and Australia (sometimes called Oceania or Australasia). Different variations with fewer continents merge some of these regions; examples of this are merging Asia and Europe into Eurasia, North America and South America into the Americas (or simply America), and Africa, Asia, and Europe into Afro-Eurasia.
Oceanic islands are occasionally grouped with a nearby continent to divide all the world's land into geographical regions. Under this scheme, most of the island countries and territories in the Pacific Ocean are grouped together with the continent of Australia to form the geographical region of Oceania.
In geology, a continent is defined as "one of Earth's major landmasses, including both dry land and continental shelves". The geological continents correspond to seven large areas of continental crust that are found on the tectonic plates, but exclude small continental fragments such as Madagascar that are generally referred to as microcontinents. Continental crust is only known to exist on Earth.
The idea of continental drift gained recognition in the 20th century. It postulates that the current continents formed from the breaking up of a supercontinent (Pangaea) that formed hundreds of millions of years ago.
Details
When geographers identify a continent, they usually include all the islands associated with it. Japan, for instance, is part of the continent of Asia. Likewise, Greenland and all the islands in the Caribbean Sea are usually considered part of North America.
Together, the continents add up to about 148 million square kilometers (57 million square miles) of land. Continents make up most—but not all—of Earth’s land surface. A very small portion of the total land area is made of islands that are not considered physical parts of continents. The ocean covers almost three-fourths of Earth. The area of the ocean is more than double the area of all the continents combined. All continents border at least one ocean. Asia, the largest continent, has the longest series of coastlines.
Coastlines, however, do not indicate the actual boundaries of the continents. Continents are defined by their continental shelves. A continental shelf is a gently sloping area that extends outward from the beach far into the ocean. A continental shelf is part of the ocean, but also part of the continent.
To human geographers, continents are also culturally distinct. The continents of Europe and Asia, for example, are actually part of a single, enormous piece of land called Eurasia. But historically, the areas of Asia and Europe have been separated because of people’s perceptions about their different cultures. Because of this, most geographers continue to divide Eurasia into Europe and Asia. An imaginary line, running from the northern Ural Mountains in Russia south to the Caspian and Black Seas, separates Europe, to the west, from Asia, to the east.
Building the Continents
Earth formed 4.6 billion years ago from a great, swirling cloud of cosmic dust and gas. The continuous smashing of space debris and the pull of gravity made the inside of Earth heat up. As the heat increased, some of Earth’s rocky materials melted and rose to the surface, where they cooled and formed a crust. Heavier material sank toward Earth’s center. Eventually, Earth came to have three main layers: the core, the mantle and the crust.
The crust and the top portion of the mantle form a rigid shell around Earth that is broken into huge sections called tectonic plates. The heat from inside Earth causes the plates to slide around on the molten mantle. Today, tectonic plates continue to slowly slide around the surface, just as they have for hundreds of millions of years. Geologists believe the interaction of the plates, a process called plate tectonics, contributed to the creation of continents.
Studies of rocks found in ancient areas of North America have revealed that the oldest known pieces of the continents began to form nearly 4 billion years ago, soon after Earth formed. At that time, a primitive ocean covered Earth. Only a small fraction of the crust was made of continental material. Scientists theorize that this material built up along the boundaries of tectonic plates during a process called subduction. During subduction, plates collide and the edge of one plate slides beneath the edge of another.
When heavy oceanic crust subducted toward the mantle, it melted in the mantle’s intense heat. Once it melted, the rock became lighter. Now in the form of magma, it rose through the overlying plate and burst out as lava. When the lava cooled, it hardened into igneous rock.
Gradually, the igneous rock built up into small volcanic islands above the surface of the ocean. Over time, these islands grew bigger, partly as the result of more lava flows and partly from the buildup of material scraped off descending plates. When plates carrying islands subducted, the islands themselves did not descend into the mantle. Their material fused with that of islands on the neighboring plate. This made even larger landmasses in the form of the first continents.
The building of volcanic islands and continental material through plate tectonics is a process that continues today. Continental crust is much lighter than oceanic crust. In subduction zones, where tectonic plates interact with each other, oceanic crust always subducts beneath continental crust. Oceanic crust is constantly being recycled in the mantle. For this reason, continental crust is much, much older than oceanic crust.
Wandering Continents
If you could visit Earth as it was millions of years ago, it would look very different. The continents have not always been where they are today. About 480 million years ago, most continents were scattered chunks of land lying along or below the Equator. Millions of years of continuous tectonic activity changed their positions, and by 240 million years ago, almost all of the world’s land was joined in a single, huge continent.
By about 200 million years ago, the forces that helped form the supercontinent caused it to begin to break apart. The pieces of the supercontinent that began to move apart were the beginnings of the continents that we know today.
A giant landmass that would become Europe, Asia, and North America separated from another mass that would split up into other continents. In time, Antarctica and Australia, still joined together, broke away and drifted south. The small piece of land that would become the peninsula of India broke away and for millions of years moved north as a large island. It eventually collided with Asia. Gradually, the different landmasses moved to their present locations.
The positions of the continents are always changing. North America and Europe are moving away from each other at the rate of about 2.5 centimeters (1 inch) per year. If you could visit the planet in the future, you might find that part of the U.S. state of California had separated from North America and become an island. Africa might have split in two along the Great Rift Valley. It is even possible that another supercontinent may form someday.
Continental Features
The surface of the continents has changed many times because of mountain building, weathering, erosion and buildup of sediment. Continuous, slow movement of tectonic plates also changes surface features.
The rocks that form the continents have been shaped and reshaped many times. Great mountain ranges have risen and then have been worn away. Ocean waters have flooded huge areas and then gradually dried up. Massive ice sheets have come and gone, sculpting the landscape in the process.
Today, all continents have great mountain ranges, vast plains, extensive plateaus, and complex river systems. The landmasses’ average elevation above sea level is about 838 meters (2,750 feet).
Although each is unique, all the continents share two basic features: old, geologically stable regions, and younger, somewhat more active regions. In the younger regions, the process of mountain building has happened recently and often continues to happen.
The power for mountain building, or orogeny, comes from plate tectonics. One way mountains form is through the collision of two tectonic plates. The impact creates wrinkles in the crust, just as a rug wrinkles when you push against one end of it. Such a collision created Asia’s Himalaya mountain range several million years ago. The plate carrying India slowly and forcefully shoved the landmass of India into Asia, which was riding on another plate. The collision continues today, causing the Himalayas to continually grow taller.
Recently formed mountains, called coastal ranges, rise near the western coasts of North and South America. Older, more stable mountain ranges are found in the interior of continents. The Appalachians of North America and the Urals, on the border between Europe and Asia, are older mountain ranges that are not geologically active.
Even older than these ancient, eroded mountain ranges are flatter, more stable areas of the continents called cratons. A craton is an area of ancient crust that formed during the Earth’s early history. Every continent has a craton. Microcontinents, like New Zealand, lack cratons.
Cratons have two forms: shields and platforms. Shields are bare rocks that may be the roots or cores of ancient mountain ranges that have completely eroded away. Platforms are cratons with sediment and sedimentary rock lying on top.
The Canadian Shield makes up about a quarter of North America. For hundreds of thousands of years, sheets of ice up to 3.2 kilometers (2 miles) thick coated the Canadian Shield. The moving ice wore away material on top of ancient rock layers, exposing some of the oldest formations on Earth. When you stand on the oldest part of the Canadian Shield, you stand directly on rocks that formed more than 3.5 billion years ago.
North America
North America, the third-largest continent, extends from the tiny Aleutian Islands in the northwest to the Isthmus of Panama in the south. The continent includes the enormous island of Greenland (an autonomous territory of Denmark) in the northeast. In the far north, the continent stretches halfway around the world, from Greenland to the Aleutians. But at Panama’s narrowest part, the continent is just 50 kilometers (31 miles) across.
Young mountains—including the Rockies, North America’s largest chain—rise in the West. Some of Earth’s youngest mountains are found in the Cascade Range of the U.S. states of Washington, Oregon and California. Some peaks there began to form only about a million years ago—a wink of an eye in Earth’s long history. North America’s older mountain ranges rise near the East Coast of the United States and Canada.
In between the mountain systems lie wide plains that contain deep, rich soil. Much of the soil was formed from material deposited during the most recent glacial period. This ice age reached its peak about 18,000 years ago. As glaciers retreated, streams of melted ice dropped sediment on the land, building layers of fertile soil in the plains region. Grain grown in this region, called the “breadbasket of North America,” feeds a large part of the world.
North America contains a variety of natural wonders. Landforms and all types of vegetation can be found within its boundaries. North America has deep canyons, such as Copper Canyon in the Mexican state of Chihuahua. Yellowstone National Park, in the U.S. state of Wyoming, has some of the world’s most active geysers. Canada’s Bay of Fundy has the greatest variation of tide levels in the world. The Great Lakes form the planet’s largest area of fresh water. In the U.S. state of California, giant sequoias—the largest tree species in the world—grow more than 76 meters (250 feet) tall and nearly 31 meters (100 feet) around.
Greenland, off the east coast of Canada, is the world’s largest island. Despite its name, Greenland is mostly covered with ice. Its ice is a remnant of the great ice sheets that once blanketed much of the North American continent. Greenland is the only place besides Antarctica that still has an ice sheet.
From the freezing Arctic to the tropical jungles of Central America, North America has more climate variation than any other continent. Almost every type of ecosystem is represented somewhere on the continent, from coral reefs in the Caribbean to Greenland’s ice sheet to the Great Plains in the United States and Canada. North America has two overarching types of ecology, both of which support a wide variety of flora and fauna. One is the Nearctic region, which spans Canada, most of the United States and northern Mexico. Animals native to this region include bison (Bison bison), moose (Alces alces) and the California condor (Gymnogyps californianus). The other is the Neotropical region, which covers southern Mexico and extends south. Animals native to this region include llamas (Lama glama), tapirs and vipers.
Today, North America is made of Canada, the United States, Greenland, Mexico, Belize, Costa Rica, El Salvador, Guatemala, Honduras, Nicaragua, Panama, and the island countries and territories that dot the Caribbean Sea and the western North Atlantic. People have migrated to North America for thousands of years and continue to immigrate to North America today. Since the United States became a country in 1783, more than 86 million people have immigrated to the country, adding to the Indigenous people, colonizers and formerly enslaved people (mostly Africans) already in the country. Despite their lives, cultures and customs being threatened or destroyed by European colonizers, more than 500 Indigenous nations continue to live in North America, including the Inuit of Arctic Canada and Alaska, the Iroquois of the United States and the Nahua of Mexico.
Most of North America sits on the North American Plate. Parts of the Canadian province of British Columbia and the U.S. states of Washington, Oregon, and California sit on the tiny Juan de Fuca Plate. Parts of California and the Mexican state of Baja California sit on the enormous Pacific Plate. Parts of Baja California and the Mexican states of Baja California Sur, Sonora, Sinaloa, and Jalisco sit on the Cocos Plate. The Caribbean Plate carries most of the small islands of the Caribbean Sea (south of the island of Cuba) as well as Central America from Honduras to Panama. The Hawaiian Islands, in the middle of the Pacific Ocean on the Pacific Plate, are usually considered part of North America.
South America
South America is connected to North America by the narrow Isthmus of Panama. These two continents were no’t always connected; they came together only 3 million years ago. South America is the fourth-largest continent and extends from the sunny beaches of the Caribbean Sea to the frigid waters near the Antarctic Circle.
South America’s southernmost islands, called Tierra del Fuego, are less than 1,120 kilometers (700 miles) from Antarctica. These islands even host some Antarctic birds, such as penguins, albatrosses and terns. Though its name comes from Spanish colonizers, the islands were home to many Indigenous groups, including the Yaghan. During colonization, many Yaghan died from European diseases and colonial violence, but there remains a sizable population on the islands, which now belong to Argentina and Chile.
The Andes, Earth’s longest terrestrial mountain range, stretch the entire length of South America. Many active volcanoes dot the range. These volcanic areas are fueled by heat generated as a large oceanic plate, called the Nazca Plate, grinds beneath the plate carrying South America.
The central-southern area of South America has pampas, or plains. These rich areas are ideal for agriculture. Growing wheat is a major industry in the pampas. Grazing animals, such as cattle and sheep, are also raised in the pampas region.
In northern South America, the Amazon River and its tributaries flow through the world’s largest tropical rainforest. In volume, the Amazon is the largest river in the world. More water flows from it than from the next six largest rivers combined.
South America is also home to the world’s tallest waterfall, Angel Falls, in the country of Venezuela. Water flows more than 979 meters (3,212 feet)—almost a mile. The falls are so high that most of the water evaporates into mist or is blown away by wind before it reaches the ground.
South American rainforests contain an enormous wealth of animal and plant life. More than 15,000 species of plants and animals are found only in the Amazon Basin, including the Amazon River dolphin (Inia geoffrensis) and the blue-throated macaw (Ara glaucogularis). Many Amazonian plant species are sources of food and medicine for the rest of the world. Scientists are trying to find ways to preserve this precious and fragile environment as people move into the Amazon Basin and clear land for settlements and agriculture.
Twelve independent countries make up South America: Brazil, Colombia, Argentina, Peru, Venezuela, Chile, Ecuador, Bolivia, Paraguay, Uruguay, Guyana, and Suriname. The territories of French Guiana, once colonized by and now an incorporated part of France, and the Falkland Islands, part of the United Kingdom, are also part of South America. The colonization of South America by Spain and Portugal destroyed many Indigenous cultures, but others, such as the Guarani in Brazil, have successfully fought against their oppressors to maintain their ways of life.
Almost all of South America sits on top of the South American Plate.
Europe
Europe, the sixth-largest continent, contains just 7% of the world’s land. In total area, the continent of Europe is only slightly larger than the country of Canada. However, the population of Europe is more than twice that of South America. Europe has more than 40 countries and many well-known cities, including London, England; Paris, France; Berlin, Germany; Rome, Italy; Madrid, Spain; and Moscow, Russia.
Most European countries have access to the ocean. The continent is bordered by the Arctic Ocean in the north, the Atlantic Ocean in the west, the Caspian Sea in the southeast, and the Mediterranean and Black Seas in the south. The nearness of these bodies of water and the navigation of many of Europe’s rivers played a major role in the continent’s history. Early Europeans learned the river systems of the Volga, Danube, Don, Rhine and Po, and could successfully travel the length and width of the small continent for trade, communication or conquest.
Navigation and colonization outside of Europe was an important part of the development of the continent’s economic, social, linguistic and political legacy. Europeans colonized land on every continent except Antarctica, leading to huge, and often catastrophic, changes for the Indigenous people. European countries extracted natural resources from the countries they colonized, leading to increased wealth for the colonizers at the expense of the people in their colonies, who continue to face wealth disparities and political instability to this day.
In the east, the Ural Mountains separate Europe from Asia. The nations of Russia and Kazakhstan straddle both continents. Another range, the Kjølen Mountains, extends along the northern part of the border between Sweden and Norway. To the south, the Alps form an arc stretching from Albania to Austria, then across Switzerland and northern Italy into France. As the youngest and steepest of Europe’s mountains, the Alps geologically resemble the Rockies of North America, another young range.
A large area of gently rolling plains extends from northern France eastward to the Urals. A climate of warm summers, cold winters and plentiful rain helps make much of this European farmland very productive.
Human development in Europe led to the extinction or near extinction of many wild animals indigenous to the continent. Some indigenous animals that have survived include the Eurasian lynx (Lynx lynx), the Golden eagle (Aquila chrysaetos) and Mediterranean tortoises (Testudo graeca).
Almost all of Europe sits on the massive Eurasian Plate.
Africa
Africa, the second-largest continent, covers an area more than three times that of the United States. From north to south, Africa stretches about 8,000 kilometers (5,000 miles). It is connected to Asia by the Isthmus of Suez in Egypt.
The Sahara, which covers much of North Africa, is the world’s largest hot desert. The world’s longest river, the Nile, flows more than 6,560 kilometers (4,100 miles) from its most remote headwaters in Lake Victoria to the Mediterranean Sea in the north. A series of falls and rapids along the southern part of the river makes navigation difficult. The Nile has played an important role in the history of Africa. In ancient Egyptian civilization, it was a source of life for food, water, and transportation.
The top half of Africa is mostly dry, hot desert. The middle area has savannas, or flat, grassy plains. This region is home to wild animals such as lions (Panthera leo), giraffes, elephants, hyenas, cheetahs (Acinonyx jubatus) and wildebeests. The central and southern areas of Africa are dominated by rainforests. Many of these forests thrive around Africa’s other great rivers, the Zambezi, the Congo and the Niger. These rivers were also home to Great Zimbabwe, the Kingdom of Kongo and the Ghana Empire, respectively. However, trees from the rainforests fed by these rivers are being cut down for many of the same reasons deforestation is taking place in the rainforests of South America and Asia: development for businesses, homes and agriculture.
Much of Africa is a high plateau surrounded by narrow strips of coastal lowlands. Hilly uplands and mountains rise in some areas of the interior. Glaciers on Mount Kilimanjaro in Tanzania sit just miles from the tropical jungles below. Even though Kilimanjaro is not far from the Equator, snow covers its summit all year long.
In eastern Africa, a giant depression called the Great Rift Valley runs from the Red Sea to the country of Mozambique. (The rift valley actually starts in southwestern Asia.) The Great Rift Valley is a site of major tectonic activity, where the continent of Africa is splitting into two. Geologists have already named the two parts of the African Plate. The Nubian Plate carries most of the continent to the west of the rift, while the Somali Plate carries the far eastern part of the continent, including the so-called “Horn of Africa.” The Horn of Africa is a peninsula that resembles the upturned horn of a rhinoceros. The countries of Eritrea, Ethiopia, Djibouti and Somalia sit on the Horn of Africa and the Somali Plate.
Africa is home to 56 countries but only 18.3% of the world’s total population. The area of central-eastern Africa is important to scientists who study evolution and the earliest origins of humanity. This area is thought to be the place where hominins began to evolve. During the era of colonization, more than 12.5 million Africans were kidnapped from Africa and enslaved as part of the transatlantic slave trade. Later, nearly all of Africa was colonized by Europe, until the beginning in the mid-20th century when African leaders began to break free. Today, many indigenous groups in Africa continue to fight for their autonomy. The Maasai in Tanzania, for example, who raise cattle as part of their traditional culture, are fighting for land that is being taken away to use for other agricultural purposes or game reserves.
The entire continent of Africa sits on the African Plate.
Asia
Asia, the largest continent, stretches from the eastern Mediterranean Sea to the western Pacific Ocean. There are more than 40 countries in Asia. Some are among the most-populated countries in the world, including China, India and Indonesia. About 60 percent of Earth’s population lives in Asia. More than a third of the world’s people live in China and India alone. Asia has the world’s highest population of Indigenous people of any continent, but some, like the Hmong of Southeast Asia, have faced systemic persecution. Asia includes many islands, some of them countries. The Philippines, Indonesia, Japan and Taiwan are major island nations in Asia.
Most of Asia’s people live in cities or fertile farming areas near river valleys, plains and coasts. The plateaus in Central Asia are largely unsuitable for farming and are thinly populated.
Asia has some of the world’s most desired natural resources, which European countries sought to exploit through colonization. Through the years, European colonizers have gained wealth through the control of spices, opium, rubber and oil. Today, foreign powers compete for political influence and access to natural resources, such as rare earth materials, lithium, timber, oil and natural gas.
Asia accounts for almost a third of the world’s land. The continent has a wide range of climate regions, from polar in the Siberian Arctic to tropical in equatorial Indonesia. Parts of Central Asia, including the Gobi Desert in China and Mongolia, are dry year-round. Southeast Asia, on the other hand, depends on the annual monsoons, which bring rain and make agriculture possible. Asia’s climate and topography vary greatly, providing ecosystems for a wide variety of animals, including King cobras, Asian elephants (Elephas maximus) and Oriental scops owls (Otus sunia).Monsoon rains and snowmelt feed Asian rivers, such as the Ganges, the Yellow, the Mekong, the Indus and the Yangtze. The rich valley between the Tigris and Euphrates Rivers in western Asia is called the “Fertile Crescent” for its place in the development of agriculture and human civilization.Asia is the most mountainous of all the continents. More than 50 of the highest peaks in the world are in Asia. Mount Everest, which reaches more than 8,700 meters (29,000 feet) high in the Himalayan range, is the highest point on Earth. These mountains have become major destination spots for adventurous travelers.Plate tectonics continuously push the mountains higher. As the landmass of India pushes northward into the landmass of Eurasia, parts of the Himalayas rise at a rate of about 2.5 centimeters (1 inch) every five years. Asia contains not only Earth’s highest elevation, but also its lowest place on land: the shores of the Dead Sea in Israel, Jordan and the Palestinian West Bank (under Israeli control). The land there lies more than 390 meters (1,300 feet) below sea level.
Although the Eurasian Plate carries most of Asia, it is not the only one supporting major parts of the large continent. The Arabian Peninsula, in the continent’s southwest, is carried by the Arabian Plate. The Indian Plate supports the Indian peninsula, sometimes called the South Asian subcontinent. The Australian Plate carries some islands in Indonesia. The North American Plate carries eastern Siberia and the northern islands of Japan.
Australia
In addition to being the smallest continent, Australia is the flattest and the second-driest, after Antarctica. The continent is sometimes called Oceania, to include the thousands of tiny islands of the Central and South Pacific, most notably Melanesia, Micronesia and Polynesia (including the U.S. state of Hawai‘i). However, the continent of Australia itself includes only the nation of Australia, the eastern portion of the island of New Guinea (part of the nation of Papua New Guinea) and the island nation of New Zealand.Australia covers just fewer than 8.5 million square kilometers (about 3.5 million square miles). Its population is about 31 million. It is the most sparsely populated continent, after Antarctica.A plateau in the middle of mainland Australia makes up most of the continent’s total area. Rainfall is light on the plateau, and not many people have settled there. The Great Dividing Range, a long mountain range, rises near the east coast and extends from the northern part of the territory of Queensland through the territories of New South Wales and Victoria. Mainland Australia is known for the Outback, a desert area in the interior. This area is so dry, hot, and barren that few people live there.In addition to the hot plateaus and deserts in mainland Australia, the continent also features lush equatorial rain forests on the island of New Guinea, tropical beaches, and high mountain peaks and glaciers in New Zealand. Most of Australia’s people live in cities along the southern and eastern coasts of the mainland. Major cities include Perth, Sydney, Brisbane, Melbourne and Adelaide, all in Australia. The country of Australia has two main Indigenous groups: Aboriginal and Torres Strait Islander. European colonization of Australia threatened and harmed the culture and health of both Indigenous groups, but they were able to resist and protect their way of life, which they continue to do to this day.
Biologists who study animals consider Australia a living laboratory. When the continent began to break away from Antarctica more than 60 million years ago, it carried a cargo of animals with it. Isolated from life on other continents, the animals developed into creatures unique to Australia, such as the koala (Phascolarctos cinereus), the platypus (Ornithorhynchus anatinus) and the Tasmanian devil (Sarcophilus harrisii).The Great Barrier Reef, off mainland Australia’s northeast coast, is another living laboratory. The world’s largest coral reef ecosystem, it is home to thousands of species of fish, sponges, marine mammals, corals, and crustaceans. The reef itself is 1,920 kilometers (1,200 miles) of living coral communities. By some estimates, it is the world’s largest living organism. Most of Australia sits on the Australian Plate. The southern part of the South Island of New Zealand sits on the Pacific Plate.
Antarctica
Antarctica is the windiest, driest and iciest place on Earth. Antarctica is larger than Europe or Australia, but unlike those continents, it has no permanent human population. People who live and work there are scientific researchers and support staff, such as pilots and cooks. The large animals that live in Antarctica, such as penguins, albatrosses and seals, generally rely on the sea to survive. The climate of Antarctica makes it impossible to support agriculture or a permanent civilization. Temperatures in Antarctica, much lower than Arctic temperatures, plunge lower than -73 degrees Celsius (-100 degrees Fahrenheit).Scientific bases and laboratories have been established in Antarctica for studies in fields like geology, oceanography and meteorology. The freezing temperatures of Antarctica make it an excellent place to study the history of Earth’s atmosphere and climate. Ice cores from the massive Antarctic ice sheet have recorded changes in Earth’s temperature and atmospheric gases for thousands of years. Antarctica is also an ideal place for discovering meteorites, or stony objects that have impacted Earth from outer space. The dark meteorites, often made of metals like iron, stand out from the white landscape of most of the continent.Antarctica is almost completely covered with ice, sometimes as thick as 3.2 kilometers (2 miles). In winter, Antarctica’s surface area may double as pack ice builds up in the ocean around the continent. Like all other continents, Antarctica has volcanic activity. The most active volcano is Mount Erebus, which is less than 1,392 kilometers (870 miles) from the South Pole. Evidence of its frequent eruptions can be found in the hot, molten rock beneath the continent’s icy surface.Antarctica does not have any countries. However, scientific groups from different countries inhabit the research stations. A multinational treaty negotiated in 1959 and reviewed in 1991 states that research in Antarctica can only be used for peaceful purposes. McMurdo Station, the largest community in Antarctica, is operated by the United States. Vostok Station, where the coldest temperature on Earth was recorded, is operated by Russia. All of Antarctica sits on the Antarctic Plate.
Additional Information
A continent is one of the larger continuous masses of land, namely, Asia, Africa, North America, South America, Antarctica, Europe, and Australia, listed in order of size. (Europe and Asia are sometimes considered a single continent, Eurasia.)
There is great variation in the sizes of continents; Asia is more than five times as large as Australia. The largest island in the world, Greenland, is only about one-fourth the size of Australia. The continents differ sharply in their degree of compactness. Africa has the most regular coastline and, consequently, the lowest ratio of coastline to total area. Europe is the most irregular and indented and has by far the highest ratio of coastline to total area.
The continents are not distributed evenly over the surface of the globe. If a hemisphere map centred in northwestern Europe is drawn, most of the world’s land area can be seen to lie within that hemisphere. More than two-thirds of the Earth’s land surface lies north of the Equator, and all the continents except Antarctica are wedge shaped, wider in the north than they are in the south.
The distribution of the continental platforms and ocean basins on the surface of the globe and the distribution of the major landform features have long been among the most intriguing problems for scientific investigation and theorizing. Among the many hypotheses that have been offered as explanation are: (1) the tetrahedral (four-faced) theory, in which a cooling earth assumes the shape of a tetrahedron by spherical collapse; (2) the accretion theory, in which younger rocks attached to older shield areas became buckled to form the landforms; (3) the continental-drift theory, in which an ancient floating continent drifted apart; and (4) the convection-current theory, in which convection currents in the Earth’s interior dragged the crust to cause folding and mountain making.
Geological and seismological evidence accumulated in the 20th century indicates that the continental platforms do “float” on a crust of heavier material that forms a layer completely enveloping the Earth. Each continent has one of the so-called shield areas that formed 2 billion to 4 billion years ago and is the core of the continent to which the remainder (most of the continent) has been added. Even the rocks of the extremely old shield areas are older in the centre and younger toward the margins, indicating that this process of accumulation started early. In North America the whole northeast quarter of the continent, called the Canadian, or Laurentian, Shield, is characterized by the ancient rocks of what might be called the original continent. In Europe the shield area underlies the eastern Scandinavian peninsula and Finland. The Guiana Highlands of South America are the core of that continent. Much of eastern Siberia is underlain by the ancient rocks, as are western Australia and southern Africa.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2531 2025-07-08 23:30:59
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 51,132
Re: Miscellany
2331) Hydroelectricity
Gist
Hydroelectricity is the electricity generated by hydropower, which uses the power of moving water to produce energy. This is typically achieved by harnessing the force of falling water, often through dams that create reservoirs and control water flow. The water then passes through turbines, which are connected to generators that produce electricity.
Summary
Hydroelectricity is electricity made by generators that are turned by the movement of water. It is usually made with dams that partly block a river to make a reservoir of water. Water is released, and the pressure of the dam (potential energy stored in the dam) forces the water down pipes that lead to a turbine. This causes the turbine to turn, to turn a generator which makes electricity.
This renewable energy method makes about one sixth of the world's electricity. It produces less pollution than the fires of steam engines do. Some places such as Norway and Quebec get most of their electricity this way.
Because all methods have advantages and disadvantages, most countries have several ways to generate electricity. For example, hydroelectric methods have certain advantages, and atomic energy has quite different advantage.For most countries today, hydroelectric energy is the preferred, or one of the preferred methods. Mainly because it is a renewable energy which means that you can reuse it and it will never run out.
Advantages
The way the electricity is produced does not harm the environment as much as fossil fuels like oil or coal do. Hydroelectricity is very powerful and safe, and produces no waste.
An important advantage of hydroelectric dams is their ability to be used as a peaking power plant. When the electricity demand declines, the dam simply stores more water. Water that has been stored in a reservoir can be released (let go) when needed, so the energy can be made quickly. Some hydroelectricity generators use pumped storage to store excess energy (often during the night), by using the electricity to pump water up into a basin. Electricity can be generated when demand increases. This flexibility also makes hydroelectricity a good match for less controllable intermittent energy sources. When the wind is not blowing or the sun is not shining, hydroelectricity can be created.
Using stored water in river dams is sometimes complicated by irrigation needs which may happen out of phase with peak electrical demands.
Another advantage is that hydroelectricity cannot run out as long as there is a good water supply. Once the dam is built, the electricity costs very little, no waste or pollution is produced, and electricity can be generated whenever it is needed.
A few hydro turbines do not have a dam but instead use the current of the "run of the river". They produce less electricity and cannot store energy for later use.
Disadvantages
The building of large dams to hold water can damage the environment. In 1983, the Australian government stopped the Tasmanian state government from building a dam on the Gordon River in Tasmania after a huge public protest. The dam would have flooded the Franklin River. The Three Gorges Dam in China is the world's largest hydroelectricity project, and the world's largest power plant of any kind. The dam has flooded a huge area, meaning that 1.2 million people had to be moved. Scientists are concerned about many problems with the dam, such as pollution, silt, and the danger of the dam wall breaking. Also it doesn’t provide many jobs for people, is expensive to run and set up and is a real danger to marine life.
Details
Hydroelectricity, or hydroelectric power, is electricity generated from hydropower (water power). Hydropower supplies 15% of the world's electricity, almost 4,210 TWh in 2023, which is more than all other renewable sources combined and also more than nuclear power. Hydropower can provide large amounts of low-carbon electricity on demand, making it a key element for creating secure and clean electricity supply systems. A hydroelectric power station that has a dam and reservoir is a flexible source, since the amount of electricity produced can be increased or decreased in seconds or minutes in response to varying electricity demand. Once a hydroelectric complex is constructed, it produces no direct waste, and almost always emits considerably less greenhouse gas than fossil fuel-powered energy plants. However, when constructed in lowland rainforest areas, where part of the forest is inundated, substantial amounts of greenhouse gases may be emitted.
Construction of a hydroelectric complex can have significant environmental impact, principally in loss of arable land and population displacement. They also disrupt the natural ecology of the river involved, affecting habitats and ecosystems, and siltation and erosion patterns. While dams can ameliorate the risks of flooding, dam failure can be catastrophic.
In 2021, global installed hydropower electrical capacity reached almost 1,400 GW, the highest among all renewable energy technologies. Hydroelectricity plays a leading role in countries like Brazil, Norway and China. but there are geographical limits and environmental issues. Tidal power can be used in coastal regions.
China added 24 GW in 2022, accounting for nearly three-quarters of global hydropower capacity additions. Europe added 2 GW, the largest amount for the region since 1990. Meanwhile, globally, hydropower generation increased by 70 TWh (up 2%) in 2022 and remains the largest renewable energy source, surpassing all other technologies combined.
History
Hydropower has been used since ancient times to grind flour and perform other tasks. In the late 18th century hydraulic power provided the energy source needed for the start of the Industrial Revolution. In the mid-1700s, French engineer Bernard Forest de Bélidor published Architecture Hydraulique, which described vertical- and horizontal-axis hydraulic machines, and in 1771 Richard Arkwright's combination of water power, the water frame, and continuous production played a significant part in the development of the factory system, with modern employment practices. In the 1840s, hydraulic power networks were developed to generate and transmit hydro power to end users.
By the late 19th century, the electrical generator was developed and could now be coupled with hydraulics. The growing demand arising from the Industrial Revolution would drive development as well. In 1878, the world's first hydroelectric power scheme was developed at Cragside in Northumberland, England, by William Armstrong. It was used to power a single arc lamp in his art gallery. The old Schoelkopf Power Station No. 1, US, near Niagara Falls, began to produce electricity in 1881. The first Edison hydroelectric power station, the Vulcan Street Plant, began operating September 30, 1882, in Appleton, Wisconsin, with an output of about 12.5 kilowatts. By 1886 there were 45 hydroelectric power stations in the United States and Canada; and by 1889 there were 200 in the United States alone.
At the beginning of the 20th century, many small hydroelectric power stations were being constructed by commercial companies in mountains near metropolitan areas. Grenoble, France held the International Exhibition of Hydropower and Tourism, with over one million visitors 1925. By 1920, when 40% of the power produced in the United States was hydroelectric, the Federal Power Act was enacted into law. The Act created the Federal Power Commission to regulate hydroelectric power stations on federal land and water. As the power stations became larger, their associated dams developed additional purposes, including flood control, irrigation and navigation. Federal funding became necessary for large-scale development, and federally owned corporations, such as the Tennessee Valley Authority (1933) and the Bonneville Power Administration (1937) were created. Additionally, the Bureau of Reclamation which had begun a series of western US irrigation projects in the early 20th century, was now constructing large hydroelectric projects such as the 1928 Hoover Dam. The United States Army Corps of Engineers was also involved in hydroelectric development, completing the Bonneville Dam in 1937 and being recognized by the Flood Control Act of 1936 as the premier federal flood control agency.
Hydroelectric power stations continued to become larger throughout the 20th century. Hydropower was referred to as "white coal". Hoover Dam's initial 1,345 MW power station was the world's largest hydroelectric power station in 1936; it was eclipsed by the 6,809 MW Grand Coulee Dam in 1942. The Itaipu Dam opened in 1984 in South America as the largest, producing 14 GW, but was surpassed in 2008 by the Three Gorges Dam in China at 22.5 GW. Hydroelectricity would eventually supply some countries, including Norway, Democratic Republic of the Congo, Paraguay and Brazil, with over 85% of their electricity.
Future potential
In 2021 the International Energy Agency (IEA) said that more efforts are needed to help limit climate change. Some countries have highly developed their hydropower potential and have very little room for growth: Switzerland produces 88% of its potential and Mexico 80%. In 2022, the IEA released a main-case forecast of 141 GW generated by hydropower over 2022–2027, which is slightly lower than deployment achieved from 2017–2022. Because environmental permitting and construction times are long, they estimate hydropower potential will remain limited, with only an additional 40 GW deemed possible in the accelerated case.
Modernization of existing infrastructure
In 2021 the IEA said that major modernisation refurbishments are required.
Additional Information
Hydroelectric energy, also called hydroelectric power or hydroelectricity, is a form of energy that harnesses the power of water in motion—such as water flowing over a waterfall—to generate electricity. People have used this force for millennia. Over 2,000 years ago, people in Greece used flowing water to turn the wheel of their mill to ground wheat into flour.
How Does Hydroelectric Energy Work?
Most hydroelectric power plants have a reservoir of water, a gate or valve to control how much water flows out of the reservoir, and an outlet or place where the water ends up after flowing downward. Water gains potential energy just before it spills over the top of a dam or flows down a hill. The potential energy is converted into kinetic energy as water flows downhill. The water can be used to turn the blades of a turbine to generate electricity, which is distributed to the power plant’s customers.
Types of Hydroelectric Energy Plants
There are three different types of hydroelectric energy plants, the most common being an impoundment facility. In an impoundment facility, a dam is used to control the flow of water stored in a pool or reservoir. When more energy is needed, water is released from the dam. Once water is released, gravity takes over and the water flows downward through a turbine. As the blades of the turbine spin, they power a generator.
Another type of hydroelectric energy plant is a diversion facility. This type of plant is unique because it does not use a dam. Instead, it uses a series of canals to channel flowing river water toward the generator-powering turbines.
The third type of plant is called a pumped-storage facility. This plant collects the energy produced from solar, wind, and nuclear power and stores it for future use. The plant stores energy by pumping water uphill from a pool at a lower elevation to a reservoir located at a higher elevation. When there is high demand for electricity, water located in the higher pool is released. As this water flows back down to the lower reservoir, it turns a turbine to generate more electricity.
How Widely Is Hydroelectric Energy Used Around the World?
Hydroelectric energy is the most commonly-used renewable source of electricity. China is the largest producer of hydroelectricity. Other top producers of hydropower around the world include the United States, Brazil, Canada, India, and Russia. Approximately 71 percent of all of the renewable electricity generated on Earth is from hydropower.
What Is the Largest Hydroelectric Power Plant in the World?
The Three Gorges Dam in China, which holds back the Yangtze River, is the largest hydroelectric dam in the world, in terms of electricity production. The dam is 2,335 meters (7,660 feet) long and 185 meters (607 feet) tall, and has enough generators to produce 22,500 megawatts of power.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2532 2025-07-09 21:19:10
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 51,132
Re: Miscellany
2332) Ocean
Gist
The ocean is a vast body of saltwater that covers approximately 70.8% of Earth's surface. It is a single, interconnected body of water, but for geographical and historical reasons, it's often divided into five main oceans: the Pacific, Atlantic, Indian, Southern (or Antarctic), and Arctic Oceans. The ocean plays a vital role in regulating the planet's climate, supporting a vast array of marine life, and providing resources for human populations.
Summary
The ocean is the body of salt water that covers approximately 70.8% of Earth. The ocean is conventionally divided into large bodies of water, which are also referred to as oceans (the Pacific, Atlantic, Indian, Antarctic/Southern, and Arctic Ocean), and are themselves mostly divided into seas, gulfs and subsequent bodies of water. The ocean contains 97% of Earth's water and is the primary component of Earth's hydrosphere, acting as a huge reservoir of heat for Earth's energy budget, as well as for its carbon cycle and water cycle, forming the basis for climate and weather patterns worldwide. The ocean is essential to life on Earth, harbouring most of Earth's animals and protist life, originating photosynthesis and therefore Earth's atmospheric oxygen, still supplying half of it.
Ocean scientists split the ocean into vertical and horizontal zones based on physical and biological conditions. Horizontally the ocean covers the oceanic crust, which it shapes. Where the ocean meets dry land it covers relatively shallow continental shelfs, which are part of Earth's continental crust. Human activity is mostly coastal with high negative impacts on marine life. Vertically the pelagic zone is the open ocean's water column from the surface to the ocean floor. The water column is further divided into zones based on depth and the amount of light present. The photic zone starts at the surface and is defined to be "the depth at which light intensity is only 1% of the surface value" (approximately 200 m in the open ocean). This is the zone where photosynthesis can occur. In this process plants and microscopic algae (free-floating phytoplankton) use light, water, carbon dioxide, and nutrients to produce organic matter. As a result, the photic zone is the most biodiverse and the source of the food supply which sustains most of the ocean ecosystem. Light can only penetrate a few hundred more meters; the rest of the deeper ocean is cold and dark (these zones are called mesopelagic and aphotic zones).
Ocean temperatures depend on the amount of solar radiation reaching the ocean surface. In the tropics, surface temperatures can rise to over 30 °C (86 °F). Near the poles where sea ice forms, the temperature in equilibrium is about −2 °C (28 °F). In all parts of the ocean, deep ocean temperatures range between −2 °C (28 °F) and 5 °C (41 °F). Constant circulation of water in the ocean creates ocean currents. Those currents are caused by forces operating on the water, such as temperature and salinity differences, atmospheric circulation (wind), and the Coriolis effect. Tides create tidal currents, while wind and waves cause surface currents. The Gulf Stream, Kuroshio Current, Agulhas Current and Antarctic Circumpolar Current are all major ocean currents. Such currents transport massive amounts of water, gases, pollutants and heat to different parts of the world, and from the surface into the deep ocean. All this has impacts on the global climate system.
Ocean water contains dissolved gases, including oxygen, carbon dioxide and nitrogen. An exchange of these gases occurs at the ocean's surface. The solubility of these gases depends on the temperature and salinity of the water. The carbon dioxide concentration in the atmosphere is rising due to CO2 emissions, mainly from fossil fuel combustion. As the oceans absorb CO2 from the atmosphere, a higher concentration leads to ocean acidification (a drop in pH value).
The ocean provides many benefits to humans such as ecosystem services, access to seafood and other marine resources, and a means of transport. The ocean is known to be the habitat of over 230,000 species, but may hold considerably more – perhaps over two million species. Yet, the ocean faces many environmental threats, such as marine pollution, overfishing, and the effects of climate change. Those effects include ocean warming, ocean acidification and sea level rise. The continental shelf and coastal waters are most affected by human activity.
Details
The ocean is a huge body of saltwater that covers about 71 percent of Earth’s surface. The planet has one global ocean, though oceanographers and the countries of the world have traditionally divided it into five distinct regions: the Pacific, Atlantic, Indian, and Arctic oceans. Beginning in the 20th century, some oceanographers labeled the seas around Antarctica the Southern Ocean, and in 2021 National Geographic officially recognized this fifth ocean.
An estimated 97 percent of the world’s water is found in the ocean. Because of this, the ocean has considerable impact on weather, temperature, and the food supply of humans and other organisms. Despite its size and impact on the lives of every organism on Earth, the ocean remains a mystery. More than 80 percent of the ocean has not been mapped, explored, or even seen by humans. A far greater percentage of the surfaces of the moon and the planet Mars has been mapped and studied than of our own ocean floor.
Although there is much more to learn, oceanographers have already made some amazing discoveries. For example, we know that the ocean contains towering mountain ranges and deep canyons, known as trenches, just like those on land. The peak of the world’s highest mountain—Mount Everest in the Himalaya, measuring 8.85 kilometers (5.49 miles) high—would not even break the surface of the water if it was placed in the Pacific Ocean’s Mariana Trench or Philippine Trench, two of the deepest parts of the ocean.
On the other hand, the Atlantic Ocean is relatively shallow because large parts of its seafloor are made up of continental shelves—parts of the continents that extend far out into the ocean. The average depth of the entire ocean is 3,720 meters (12,200 feet).
It is unknown how many different species call the ocean their home. With many marine ecosystems suffering from rising sea temperatures, pollution, and other problems, some oceanographers believe the number of species is dropping. Still, there may be many positive surprises waiting for oceanographers in the years ahead. It could be that more than 90 percent of the ocean’s species are still undiscovered, with some scientists estimating there are anywhere between a few hundred thousand and a few million more to be discovered. Currently, scientists know of around 226,000 ocean species.
Learning more about the seafloor and the rest of the ocean is the passion of National Geographic Explorer Marcello Calisti. He is a biorobotics expert who is developing an undersea exploration vehicle that uses “legged locomotion,” inspired by the way an octopus moves under water. His long-range goal is to design robots that can explore the depths that are difficult for humans to reach.
Since the ocean is so vast, there is plenty for future oceanographers from all corners of the globe to explore and discover.
Additional Information:
Introduction
An ocean is a huge body of salt water. Oceans cover nearly 71 percent of Earth’s surface. They contain almost 98 percent of all the water on Earth.
There is one world ocean, but it is divided into five main areas: the Pacific, the Atlantic, the Indian, the Arctic, and the Southern, or Antarctic. Together, they can be seen as one world ocean because they have no real borders, and water flows freely between them. Smaller parts of these oceans are called seas, gulfs, and bays.
Ocean Water
Ocean water is salty. The saltiness comes from a chemical substance called sodium chloride, which is dissolved in the water. (The salt that people eat is sodium chloride in the form of crystals.)
Winds and other forces cause ocean water to be constantly in motion. Large amounts of ocean water move around Earth in patterns called currents. Ocean currents may be warm or cold. Warm currents tend to bring warm weather and rain to nearby land. Cold currents tend to cause a dry climate. The Gulf Stream is a warm current that runs north along the eastern coast of the United States.
Winds also cause ocean water to move in waves. Steady, powerful winds cause big waves. Gentle breezes create ripples. Large swells in ocean water usually come from stormy weather.
Tides are another way that ocean water moves. Tides are the rise and fall of ocean levels. That happens throughout the day. On a beach, for example, the ocean covers more sand at high tide than at low tide. The pull of a force called gravity between Earth and the Moon and the Sun causes tides.
Ocean Floor
The ocean floor has many levels. The shallowest part of the oceans, called the continental shelf, lies along the edges of the continents. The edges of the continental shelf slope down toward the deep parts of the oceans, called the basins. At the bottom of the basins are large, flat plains.
In some places, deep cracks called trenches cut into the ocean floor. In other places, underwater mountain chains, called oceanic ridges, rise up from the floor. Earthquakes sometimes occur along the trenches and ridges. Parts of the ridges contain active volcanoes.
Ocean Life
Living things inhabit all levels of Earth’s oceans. Ocean plants grow fairly close to the water’s surface because they need sunlight to stay alive. Sunlight penetrates the water to only about 656 feet (200 meters). The most numerous ocean plants are called phytoplankton. Those tiny, one-celled plants drift with the ocean currents. Various kinds of sea grass and other plants also grow in the world’s oceans. Seaweeds, which are plantlike forms of algae, are plentiful as well.
Like ocean plants, most ocean animals live in shallower water. This is because there are more plants and animals to eat near the water’s surface. But animals also can be found in deep water, including within the oceans’ deepest, darkest trenches.
The largest ocean animal is the blue whale. No larger animal has ever lived on Earth. The tiniest animals are a form of plankton called zooplankton. Hundreds of thousands of other types of animal also live in the ocean. Those include clams, crabs, squid, dolphins, and many different kinds of fish. Corals and sea anemones look like plants, but they are animals, too.
Importance of the Oceans
The world’s oceans are important to life on Earth. Oceans are a great source of food for people around the world. They also provide minerals, oil, and natural gas. Phytoplankton and algae create much of the world’s oxygen. Oceans also help to keep climates stable by storing heat from the Sun.
Today, many dangers threaten the health of the oceans. People pollute oceans by dumping poisonous waste and garbage into them. Ocean pollution reduces oxygen in the water and harms ocean life. Overfishing and oil spills harm ocean life as well.
People called oceanographers study the oceans to try to keep them healthy. Some examine the quality of the water and the way the water moves. Others look at the structures of the seafloors and basins. Another group of oceanographers is interested in the plants and animals that live in oceans.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2533 2025-07-14 22:53:11
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 51,132
Re: Miscellany
2333) Otoscope
Gist
An otoscope is a tool which shines a beam of light to help visualize and examine the condition of the ear canal and eardrum. Examining the ear can reveal the cause of symptoms such as an earache, the ear feeling full, or hearing loss.
During an ear exam, a tool called an otoscope is used to look at the outer ear canal and eardrum. The otoscope has a light, a magnifying lens, and a funnel-shaped viewing piece with a narrow, pointed end called a speculum.
Summary
An otoscope is a medical device used to examine the ear canal and eardrum. It consists of a light source, a magnifying lens, and a speculum (a cone-shaped viewing piece). The otoscope allows healthcare providers to visualize the ear canal and identify potential issues such as infections, blockages, or other abnormalities.
Otoscopy is the formal name for an ear examination. Although the ear is a small part of the body, it consists of many parts.1
* The external ear contains the external auditory canal, a tube connecting the outer part of the ear to the middle ear.
* The eardrum, or tympanic membrane, separates the outer ear from the middle ear.
* The middle ear, or tympanic cavity, contains the eustachian tube.
* The eustachian tube connects the middle of the ear to the ear to the back of the nose.
* The inner ear has small parts that are responsible for hearing and balance.
There are several reasons you may get an otoscopy:
* For a routine physical
* To check for an ear infection
* To find out the cause of ear symptoms, like an earache or feeling of fullness
* To screen for hearing loss
* To examine for excess wax in the ear canal
* To check for an object blocking the ear canal
Details
An otoscope or auriscope is a medical device used by healthcare professionals to examine the ear canal and eardrum. This may be done as part of routine physical examinations, or for evaluating specific ear complaints, such as earaches, sense of fullness in the ear, or hearing loss.
Usage:
Function
An otoscope enables viewing and examination of the ear canal and tympanic membrane (eardrum). As the eardrum is the border between the external ear canal and the middle ear, its characteristics can indicate various diseases of the middle ear space. Otoscopic examination can help diagnose conditions such as acute otitis media (infection of the middle ear), otitis externa (infection of the outer ear), traumatic perforation of the eardrum, and cholesteatoma.
The presence of cerumen (earwax), shed skin, pus, canal skin edema, foreign bodies, and various ear diseases, can obscure the view of the eardrum and thus compromise the value of otoscopy done with a common otoscope, but can confirm the presence of obstructing symptoms.
Otoscopes can also be used to examine patients' noses (avoiding the need for a separate nasal speculum) and upper throats (by removing the speculum).
Method of use
The most common otoscopes consist of a handle and a head. The head contains a light source and a magnifying lens, typically around 8 diopters (3× magnification), to help illuminate and enlarge ear structures. The distal (front) end of the otoscope has an attachment for disposable plastic ear specula.
The examiner first pulls on the pinna (usually the earlobe, side or top) to straighten the ear canal, and then inserts the ear speculum side of the otoscope into the outer ear. It is important to brace the index or little finger of the hand holding the otoscope against the patient's head to avoid injuring the ear canal. The examiner then looks through the lens on the rear of the instrument to see inside the ear canal.
In many models, the examiner can remove the lens and insert instruments like specialized suction tips through the otoscope into the ear canal, such as for removing earwax. Most models also have an insertion point for a bulb that pushes air through the speculum (pneumatic otoscopy) for testing eardrum mobility.
Types:
Many otoscopes for doctors' offices are wall-mounted, with an electrical cord providing power from an electric outlet. Portable otoscopes powered by batteries (usually rechargeable) in the handle are also available.
Otoscopes are often sold with ophthalmoscopes as a diagnostic set.
Monocular and binocular
Most otoscopes used in emergency rooms, pediatric offices, general practice, and by internists are monocular devices. These provide a two-dimensional view of the ear canal and its contents, and usually at least a portion of the eardrum.
Another method of performing otoscopy (visualization of the ear) is by using a binocular (two-eyed) microscope in conjunction with a larger plastic or metal ear speculum, which provides a much larger field of view. The microscope is suspended from a stand, which frees up both of the examiner's hands; the patient is placed in a supine position and their head is tilted, which keeps the head stable and enables better lighting. The binocular view enables depth perception, which makes removal of earwax or other obstructing materials easier and less hazardous. The microscope also has up to 40× magnification, allowing more detailed viewing of the entire ear canal, and of the entire eardrum (unless prevented by edema of the canal skin). Subtle changes in the anatomy can also be more easily detected and interpreted.
Traditionally, binocular microscopes are only used by otolaryngologists (ear, nose, and throat specialists) and otologists (subspecialty ear doctors). Their widespread adoption in general medicine is hindered by cost and lack of familiarity among pediatric and general medicine professors in physician training programs. Studies have shown that reliance on a monocular otoscope to diagnose ear disease results in a more than 50% chance of misdiagnosis, as compared to binocular microscopic otoscopy.
Pneumatic otoscope
The pneumatic otoscope is used to examine the eardrum for assessing the health of the middle ear. This is done by assessing the eardrum's contour (normal, retracted, full, or bulging), its color (gray, yellow, pink, amber, white, red, or blue), its translucency (translucent, semi-opaque, opaque), and its mobility (normal, increased, decreased, or absent). The pneumatic otoscope is the standard tool used in diagnosing otitis media (infection of the middle ear).
The pneumatic otoscope has a pneumatic (diagnostic) head, which contains a lens, an enclosed light source, and a nipple for attaching a rubber bulb and tubing. By gently squeezing and releasing the bulb in rapid succession, the degree of eardrum mobility in response to positive and negative pressure can be observed. The head is designed so that an airtight chamber is produced when a speculum is attached and fitted snugly into the patient's ear canal. Using a rubber-tipped speculum or adding a small sleeve of rubber tubing at the end of a plastic speculum, can help improve the airtight seal and also help avoid injuring the patient.
By replacing the pneumatic head with a surgical head, the pneumatic otoscope can also be used to clear earwax from the ear canal, and to perform diagnostic tympanocentesis (drainage of fluid from the middle ear) or myringotomy (creation of incision in the eardrum). The surgical head consists of an unenclosed light source and a lens that can swivel over a wide arc.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2534 2025-07-16 20:20:01
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 51,132
Re: Miscellany
2334) Sea
Gist
A sea is a large body of salt water. There are particular seas and the sea. The sea commonly refers to the ocean, the interconnected body of seawaters that spans most of Earth.
Summary
A sea is a large body of salt water. It may be an ocean, or may be a large saltwater lake which like the Caspian Sea, lacks a natural outlet.
Characteristics:
Seawater
Seawater is salty. The open ocean has about 35 grams (1.2 oz) solids per litre, a salinity of 35 part per thousand. The Mediterranean Sea is a little higher at 37part per thousand and the Dead Sea has as much as 300 grams (11 oz) dissolved solids per litre. Sodium chloride is the main salt present, making up about 85% of the solids in solution. There are also 5 grams (0.18 oz) per litre of the chlorides of other metals such as potassium and magnesium and 3 grams (0.11 oz) of sulphates, carbonates, bromides and other salts. A kilogram (2.2 lb) of salt can be found in 28 litres or one cubic foot of typical ocean water. Despite differences in the levels of salinity in different seas, the relative composition of the dissolved salts is very stable throughout the world's oceans.
Temperature
The temperature of the sea depends on the amount of sunlight falling on the surface. In the tropics, with the sun nearly overhead, the temperature of the surface layers can rise to over 30 °C (86 °F). Near the poles the temperature in balance with the sea ice is about −2 °C (28 °F). Cold water is denser than warm water and tends to sink. There is a continuous circulation of water in the oceans. Warm surface currents cool as they move away from the tropics, the water becomes denser and sinks. The cold water moves back towards the equator as a deep sea current, driven by changes in the temperature and density of the water, eventually welling up again towards the surface. Deep sea water has a temperature between −2 °C (28 °F) and 5 °C (41 °F) in all parts of the globe.
Oxygen
The amount of oxygen found in seawater depends mostly on the plants growing in it. These are mainly algae, including phytoplankton, but also include some vascular plants such as seagrasses. In daylight the photosynthetic activity of these plants produces oxygen which dissolves in the seawater where it is used by marine animals. At night, photosynthesis stops, and the amount of dissolved oxygen declines. In the deep sea where not enough light penetrates for plants to grow, there is very little dissolved oxygen.
Seawater is a little alkaline and during historic times has had a pH of about 8.2. The pH is expected to reach 7.7 by the year 2100, an increase of 320% in acidity in a century. One important element for the formation of skeletal material in marine animals is calcium but it is easily precipitated out in the form of calcium carbonate as the sea becomes more acid. This is likely to have profound effects on certain planktonic marine organisms because their ability to form shells will be reduced. These include single-celled algae called coccolithophorids and foraminifera. These are important parts of the food chain. Reducing their numbers will have significant results. In tropical areas, corals will be affected by a lack of calcium, with knock-on effects for other reef residents.
Waves
Wind blowing over the surface of a body of water forms waves. The friction between air and water caused by a gentle breeze on a pond causes ripples to form. A strong blow over the ocean causes larger waves as the moving air pushes against the raised ridges of water. The waves reach their greatest height when the rate at which they travel nearly matches the speed of the wind. The waves form at right angles to the direction from which the wind blows. In open water, if the wind continues to blow, as happens in the Roaring Forties in the southern hemisphere, long, organized masses of water called swell roll across the ocean.
Details
The phrase “the Seven Seas” has been around for centuries, but that term really refers to different parts of the ocean and several other large bodies of water. There are actually more than seven seas in the world. But what makes a sea different from other bodies of water?
That is not an easy question to answer, because the definition of a sea leaves some room for interpretation. In general, a sea is defined as a portion of the ocean that is partly surrounded by land. Given that definition, there are about 50 seas around the world. But that number includes water bodies not always thought of as seas, such as the Gulf of Mexico and the Hudson Bay.
Moreover, in some cases, a sea is completely landlocked. The Caspian Sea is the most famous example, though this sea, which lies between Russia and Iran, is also referred to as the world’s largest lake. Other seas surrounded by land include the Aral Sea and the Dead Sea. They contain saltwater and have been called seas for many years, but many oceanographers and geographers are more inclined to call them lakes.
Still, that leaves dozens of water bodies that fit the traditional definition of a sea, even though they can be quite different from one another. A sea can be more than 2.6 million square kilometers (1 million square miles) in area, such as the Caribbean Sea. Or, it can be as tiny as the Sea of Marmara, which is less than 12,950 square kilometers (5,000 square miles) in area. This tiny Turkish sea connects the Aegean Sea and the Black Sea.
A sea can also be very warm for most of the year. The Red Sea, for instance, has an average temperature of around 30 degrees Celsius (86 degrees Fahrenheit). It is also the saltiest sea, containing 41 parts of salt per 1,000 parts of seawater. Seas can be quite cold, too. The Greenland Sea, for instance, has surface water that hovers near the freezing mark most of the year.
The variety of the sizes, temperatures, and locations of the Earth’s seas also means that the marine ecosystems within each sea can vary greatly from one to the other. The Baltic Sea in Scandinavia is the world’s youngest sea having formed between 10 thousand and 15 thousand years ago from glacial erosion. It contains a unique mixture of saltwater and freshwater, making it the largest brackish water body on the planet. As a result, the Baltic Sea contains a small, but rare, variety of freshwater and saltwater plants and animals that have been able to adapt to their brackish environment.
Not surprisingly, the diversity of the world’s seas also draws National Geographic explorers, such as oceanographer Katy Croff Bell. She was part of the crew aboard the exploration vessel Nautilus, a ship that shared its scientific discoveries in the Mediterranean Sea, the Black Sea, and elsewhere with students around the world in online lessons and chats. She says the seas—big and small, cold and warm—can teach scientists about the rest of the world. “We’re going to places that have never been explored to see what’s there,” Bell told MIT Technology Review in 2015. “There are things we can’t even conceive of out there, and it will take a long, long time to fully understand our own planet.”
Additional Information
A sea is a large body of salt water. There are particular seas and the sea. The sea commonly refers to the ocean, the interconnected body of seawaters that spans most of Earth. Particular seas are either marginal seas, second-order sections of the oceanic sea (e.g. the Mediterranean Sea), or certain large, nearly landlocked bodies of water.
The salinity of water bodies varies widely, being lower near the surface and the mouths of large rivers and higher in the depths of the ocean; however, the relative proportions of dissolved salts vary little across the oceans. The most abundant solid dissolved in seawater is sodium chloride. The water also contains salts of magnesium, calcium, potassium, and mercury, among other elements, some in minute concentrations. A wide variety of organisms, including bacteria, protists, algae, plants, fungi, and animals live in various marine habitats and ecosystems throughout the seas. These range vertically from the sunlit surface and shoreline to the great depths and pressures of the cold, dark abyssal zone, and in latitude from the cold waters under polar ice caps to the warm waters of coral reefs in tropical regions. Many of the major groups of organisms evolved in the sea and life may have started there.
The ocean moderates Earth's climate and has important roles in the water, carbon, and nitrogen cycles. The surface of water interacts with the atmosphere, exchanging properties such as particles and temperature, as well as currents. Surface currents are the water currents that are produced by the atmosphere's currents and its winds blowing over the surface of the water, producing wind waves, setting up through drag slow but stable circulations of water, as in the case of the ocean sustaining deep-sea ocean currents. Deep-sea currents, known together as the global conveyor belt, carry cold water from near the poles to every ocean and significantly influence Earth's climate. Tides, the generally twice-daily rise and fall of sea levels, are caused by Earth's rotation and the gravitational effects of the Moon and, to a lesser extent, of the Sun. Tides may have a very high range in bays or estuaries. Submarine earthquakes arising from tectonic plate movements under the oceans can lead to destructive tsunamis, as can volcanoes, huge landslides, or the impact of large meteorites.
The seas have been an integral element for humans throughout history and culture. Humans harnessing and studying the seas have been recorded since ancient times and evidenced well into prehistory, while its modern scientific study is called oceanography and maritime space is governed by the law of the sea, with admiralty law regulating human interactions at sea. The seas provide substantial supplies of food for humans, mainly fish, but also shellfish, mammals and seaweed, whether caught by fishermen or farmed underwater. Other human uses of the seas include trade, travel, mineral extraction, power generation, warfare, and leisure activities such as swimming, sailing, and scuba diving. Many of these activities create marine pollution.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2535 2025-07-17 20:12:28
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 51,132
Re: Miscellany
2335) Valley
Gist
A valley is a low-lying area of land between hills or mountains, often with a river or stream running through it. Valleys are typically formed by erosion from rivers, glaciers, or other geological processes. They can vary in size and shape, from narrow canyons to wide, flat plains.
Summary
A valley is an elongated low area often running between hills or mountains and typically containing a river or stream running from one end to the other. Most valleys are formed by erosion of the land surface by rivers or streams over a very long period. Some valleys are formed through erosion by glacial ice. These glaciers may remain present in valleys in high mountains or polar areas.
At lower latitudes and altitudes, these glacially formed valleys may have been created or enlarged during ice ages but now are ice-free and occupied by streams or rivers. In desert areas, valleys may be entirely dry or carry a watercourse only rarely. In areas of limestone bedrock, dry valleys may also result from drainage now taking place underground rather than at the surface. Rift valleys arise principally from earth movements, rather than erosion. Many different types of valleys are described by geographers, using terms that may be global in use or else applied only locally.
Details
Valleys are depressed areas of land–scoured and washed out by the conspiring forces of gravity, water, and ice. Some hang; others are hollow. They all take the form of a "U" or "V."
Rivers and streams make most primary valley cuts, carving steep-walled sides and a narrow floor that from afar looks like the letter "V." The gradient of the river—how quickly it drops—helps define the steepness of the sides and the width of the floor. Mountain valleys, for example, tend to have near-vertical walls and a narrow channel, but out on the plains, the slopes are shallow and the channel is wide.
As waters wind toward the sea, they add to natural twists in the land by stripping sediment from the outsides of bends and dumping it on the insides. The bulk of the rock and dirt is dredged from the bottom of the channel, a process called down cutting that can ultimately lead to deep, slender chasms like Black Canyon in Colorado's Gunnison National Park.
Types of Valleys
Some river and stream valleys, especially those in the mountains or located near the North and South Poles, are transformed by glaciers.
The massive blocks of snow and ice slowly creep downhill where they will meet the least resistance: valleys already cut by rivers and streams. As the glaciers ooze, they pick up rocks and grind away at the valley floor and sides, pressing the "V" into a "U." When the glacier melts, a U-shaped valley marks the spot where the snow and ice once flowed.
Side valleys are formed by tributaries to streams and rivers and feed the main stem. Where the main channel is carved deeper than the tributary, as commonly occurs during glaciations, the side valleys are left hanging. Waterfalls often cascade from the outlet of the upper valley into the drainage below.
Hollows, like those in Appalachia, are small valleys nestled between mountains or hills.
Giant valleys, called rifts, are found where two pieces of Earth's crust are separated or split apart. One such example is the Great Rift Valley, a rift system stretching from the Middle East to southern Africa.
Additional Information
A valley is elongate depression of the Earth’s surface. Valleys are most commonly drained by rivers and may occur in a relatively flat plain or between ranges of hills or mountains. Those valleys produced by tectonic action are called rift valleys. Very narrow, deep valleys of similar appearance are called gorges. Both of these latter types are commonly cut in flat-lying strata but may occur in other geological situations.
Wherever sufficient rainfall occurs, opportunity exists for the land surface to evolve to the familiar patterns of hills and valleys. There are, of course, hyperarid environments where fluvial activity is minimal. There also are geomorphological settings where the permeability of rocks or sediments induce so much infiltration that water is unable to concentrate on the land surface. Moreover, some landscapes may be so young that insufficient time has elapsed for modification by fluvial action. The role of fluvial action on landscape, including long-term evolutionary processes, is considered here in detail. For additional information on fluvial and hillslope processes relating to valley formation, see river.
Probably the world’s deepest subaerial valley is that of the Kāli Gandaki River in Nepal. Lying between two 8,000-metre (26,000-foot) Himalayan peaks, Dhaulāgiri and Annapūrna, the valley has a total relief of six kilometres (four miles). Because the Himalayas are one of the Earth’s most active areas of tectonic uplift, this valley well illustrates the principle that the most rapid downcutting occurs in areas of the most rapid uplift. The reason for this seeming paradox lies in the energetics of the processes of degradation that characterize valley formation. As will be discussed below, the steeper the gradient or slope of a stream, the greater its expenditure of power on the streambed. Thus, as uplift creates higher relief and steeper slopes, rivers achieve greater power for erosion. As a consequence, the most rapid processes of relief reduction can occur in areas of most rapid relief production.
Perhaps the most famous example of a canyon is the Grand Canyon of the Colorado River in northern Arizona. The Grand Canyon is about 1.6 km (1 mile) deep and 180 metres (590 feet) to 30 km (19 miles) wide and occurs along a 443-km- (275-mile-) long reach where the Colorado River incised into a broad upwarp of sedimentary rocks.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2536 2025-07-18 22:12:44
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 51,132
Re: Miscellany
2336) Archipelago
Gist
An archipelago is an area that contains a chain or group of islands scattered in lakes, rivers, or the ocean.
The largest archipelago in the world is the Malay Archipelago, also known as Insulindia or the Indo-Australian Archipelago. It's a vast group of islands located between mainland Southeast Asia and Australia, and it includes over 25,000 islands, with Indonesia and the Philippines being its largest components.
Summary
The word “archipelago” comes from the medieval Italian word archi, meaning chief or principal, and the Greek word pelagus, meaning gulf, pool, or pond.
Most archipelagos are formed when volcanoes erupt from the ocean floor; these are called oceanic islands. The islands of the Hawaiian archipelago, for example, were formed by a series of volcanic eruptions that began more than 80 million years ago and are still active today.
Archipelagos can also form as a result of erosion, sedimentary deposits, rising sea level, and other geographic processes. The Florida Keys are an example of a coral cay archipelago, which form when ocean currents transport sediments that gradually build up on the reef surface.
Continental fragments are archipelagos that have separated from a continental land mass due to the Earth’s tectonic movements. The Farallon Islands off the coast of California are an example of continental fragments.
Continental archipelagos, such as British Columbia’s Inside Passage, are islands that form close to the coast of a continent.
Details
An archipelago is a group of islands closely scattered in a body of water. Usually, this body of water is the ocean, but it can also be a lake or river.
Most archipelagoes are made of oceanic islands. This means the islands were formed by volcanoes erupting from the ocean floor. An archipelago made up of oceanic islands is called an island arc.
Many island arcs were formed over a single “hot spot.” The Earth’s crust shifted while the hot spot stayed put, creating a line of islands that show exactly the direction the crust moved.
The Hawaiian Islands continue to form this way, with a hot spot remaining relatively stable while the Pacific tectonic plate moves northwest. There are 137 Hawaiian islands, reefs and atolls, stretching from Kure and Midway in the west to the "Big Island" of Hawaii in the east. The Big Island is still being formed by the active volcanoes Mauna Loa and Kilauea. The island arc will grow as Loihi, a seamount southeast of the Big Island, eventually punctures the ocean surface as Hawaii's youngest island.
Japan is another island arc. The Japanese archipelago consists of four large islands, from Hokkaido, in the far north, through Honshu, Shikoku, and Kyushu in the far south. Japan also includes more than 3,000 smaller islands. In several places in the Japanese archipelago, volcanoes are still active.
Volcanoes do not form all archipelagoes. Many archipelagoes are continental islands formed only after the last ice age. As glaciers retreated, sea levels rose and low-lying valleys were flooded. Coastal mountain ranges became archipelagoes just off the mainland.
The largest archipelago in the world was formed by glacial retreat. The Malay Archipelago, between the Pacific and Indian Oceans, contains more than 25,000 islands in Southeast Asia. The thousands of islands of Indonesia and Malaysia are a part of the Malay Archipelago. At least some of these islands—and the straits that separate them—were part of mainland Asia during the last ice age.
Finland’s Archipelago Sea, part of the Baltic Sea, also emerged after the last ice age. There are more than 50,000 islands in the Archipelago Sea, although many of them do not measure half a hectare (one acre). Some of the islands are close enough to be connected by bridges.
Islands of the archipelago sea were never coastal mountaintops, however. They were formed by post-glacial rebound. In this process, land that was squashed by the weight of heavy glaciers during the Ice Age slowly regains its shape, like a sponge. Because post-glacial rebound is still occurring, islands continue to rise from the Archipelago Sea.
Additional Information
An archipelago, sometimes called an island group or island chain, is a chain, cluster, or collection of islands. An archipelago may be in an ocean, a sea, or a smaller body of water. Example archipelagos include the Aegean Islands (the origin of the term), the Canadian Arctic Archipelago, the Stockholm Archipelago, the Malay Archipelago (which includes the Indonesian and Philippine Archipelagos), the Lucayan (Bahamian) Archipelago, the Japanese archipelago, and the Hawaiian Archipelago.
Geographic types:
Archipelagos may be found isolated in large amounts of water or neighboring a large land mass. For example, Scotland has more than 700 islands surrounding its mainland, which form an archipelago.
Depending on their geological origin, islands forming archipelagos can be referred to as oceanic islands, continental fragments, or continental islands.
Oceanic islands
Oceanic islands are formed by volcanoes erupting from the ocean floor. The Hawaiian Islands and Galapagos Islands in the Pacific, and Mascarene Islands in the south Indian Ocean are examples.
Continental fragments
Continental fragments are islands that were once part of a continent, and became separated due to natural disasters. The fragments may also be formed by moving glaciers which cut out land, which then fills with water. The Farallon Islands off the coast of California are examples of continental islands.
Continental Islands
Continental islands are islands that were once part of a continent and still sit on the continental shelf, which is the edge of a continent that lies under the ocean. The islands of the Inside Passage off the coast of British Columbia and the Canadian Arctic Archipelago are examples.
Artificial archipelagos
Artificial archipelagos have been created in various countries for different purposes. Palm Islands and The World Islands in Dubai were or are being created for leisure and tourism purposes. Marker Wadden in the Netherlands is being built as a conservation area for birds and other wildlife.
Superlatives
The largest archipelago in the world by number of islands is the Archipelago Sea, which is part of Finland. There are approximately 40,000 islands, mostly uninhabited.
The largest archipelagic state in the world by area, and by population, is Indonesia.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2537 2025-07-19 18:13:55
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 51,132
Re: Miscellany
2337) Peninsula
Gist
A peninsula is a landmass that is surrounded by water on three sides and connected to a larger landmass on the fourth side. It's like a piece of land that juts out into the water, almost an island but still attached to the mainland.
A peninsula is generally defined as a piece of land surrounded on most sides by water. A peninsula may be bordered by more than one body of water, and the body of water does not have to be an ocean or a sea.
Summary
A peninsula, in physical geography, is a piece of land that is nearly surrounded by water. The word is derived from the Latin paene (“almost”) and insula (“island”). In its original sense it connotes attachment to a larger landmass by a neck of land (isthmus) narrower than the peninsula itself, but it is often extended to apply to any long promontory, the coastline of which is markedly longer than the landward boundary.
The world’s largest peninsula is Arabia, covering about 1 million square miles (2.6 million square km) in Southwest Asia. It is bounded on the west by the Red Sea, on the south by the Gulf of Aden and the Arabian Sea, and on the east by the Persian Gulf and the Gulf of Oman. Other prominent peninsulas in Asia include the Malay Peninsula in Southeast Asia; the Kamchatka Peninsula in far eastern Russia; the Korean Peninsula; Anatolia (modern Asian Turkey); and Sinai, a triangular peninsula in northeastern Egypt that links Asia and Africa. Peninsulas elsewhere in Africa include Cape Verde, a peninsula in west-central Senegal that is the westernmost point of the African continent, and the Horn of Africa, the easternmost point of the continent, which comprises the countries of Djibouti, Eritrea, Ethiopia, and Somalia.
Conspicuous peninsulas in North America include Florida in the United States, the Ungava Peninsula in Canada, and Baja California in western Mexico. The Yucatán is bordered by the Gulf of Mexico and the Caribbean Sea and includes three Mexican states as well as large parts of Belize and Guatemala. The U.S. state of Alaska also fits the definition, though it is very large and has a number of its own peninsulas (among them are the Alaska, Kenai, and Seward peninsulas). South America’s most notable peninsula is La Guajira on the continent’s northwestern coast.
Europe has several significant peninsulas. In Scandinavia, Norway and Sweden form one peninsula, and Denmark forms another. To the south are Italy, the Iberian Peninsula (Spain and Portugal), the Balkans, and the Peloponnese (part of Greece). In Ukraine, the Crimean Peninsula extends into the Black Sea.
Peninsulas in Australia include Cape York (the continent’s northernmost extremity), Eyre (in South Australia), and Cobourg (in Arnhem Land). The Coromandel Peninsula is a prominent feature on New Zealand’s North Island. The Antarctic Peninsula is an 800-mile (1,300-km) northward extension of Antarctica toward the southern tip of South America.
Details
A peninsula is a landform that extends from a mainland, is connected to the mainland on only one side, and is mostly surrounded by water. Peninsulas exist on each continent. The largest peninsula in the world is the Arabian Peninsula.
Etymology
The word peninsula derives from Latin paeninsula, from paene 'almost' and insula 'island'. The word entered English in the 16th century.
Definitions
A peninsula is generally defined as a piece of land surrounded on most sides by water.
A peninsula may be bordered by more than one body of water, and the body of water does not have to be an ocean or a sea. A piece of land on a very tight river bend or one between two rivers is sometimes said to form a peninsula, for example in the New Barbadoes Neck in New Jersey, United States. A peninsula may be connected to the mainland via an isthmus; for example, the Isthmus of Corinth connects to the Peloponnese peninsula.
Formation and types
Peninsulas can be formed by continental drift, glacial erosion, glacial meltwater, glacial deposition, marine sediment, marine transgressions, volcanoes, divergent boundaries or river sedimentation. More than one factor may contribute to the formation of a peninsula. For example, in the case of Florida, continental drift, marine sediment, and marine transgressions all contributed to its shape.
Peninsulas can also be man-made. Typically, they are built as protection from ocean or sea waves by building a Breakwater, which sometimes connects back to land. They can also be built to expand areas of a city; for example, Copenhagen is planning to create a peninsula that houses 35,000 residents by 2070.
Glaciers
In the case of formation from glaciers (for example, the Antarctic Peninsula or Cape Cod), peninsulas can be created due to glacial erosion, meltwater or deposition. If erosion formed the peninsula, softer and harder rocks were present, and since the glacier only erodes softer rock, it formed a basin. This may create peninsulas, and occurred for example in the Keweenaw Peninsula.
In the case of formation from meltwater, melting glaciers deposit sediment and form moraines, which act as dams for the meltwater. This may create bodies of water that surround the land, forming peninsulas.
If deposition formed the peninsula, the peninsula was composed of sedimentary rock, which was created from a large deposit of glacial drift. The hill of drift becomes a peninsula if the hill formed near water but was still connected to the mainland, for example during the formation of Cape Cod about 23,000 years ago.
Others
In the case of formation from volcanoes, when a volcano erupts magma near water, it may form a peninsula (such as the Alaskan Peninsula). Peninsulas formed from volcanoes are especially common when the volcano erupts near shallow water. Marine sediment may form peninsulas by the creation of limestone. A rift peninsula may form as a result of a divergent boundary in plate tectonics (such as the Arabian Peninsula), while a convergent boundary may also form peninsulas (for example, Gibraltar or the Indian subcontinent). Peninsulas can also form due to sedimentation in rivers. When a river carrying sediment flows into an ocean, the sediment is deposited, forming a delta peninsula.
Marine transgressions (changes in sea level) may form peninsulas, but may also affect existing peninsulas. For example, the water level may change, which causes a peninsula to become an island during high water levels. Similarly, wet weather causing higher water levels make peninsulas appear smaller, while dry weather make them appear larger. Sea level rise from global warming will permanently reduce the size of some peninsulas over time.
Uses
Peninsulas are noted for having acted as shelters for prehistoric humans and Neanderthals. The landform is advantageous because it gives hunting access to both land and sea animals. They can also serve as markers of a nation's borders. In history, peninsulas have played a vital role in trade and commerce because of their access to water through an isthmus. The Malay Peninsula, located at the convergence of the Indian Ocean and the China Seas, played an important role in east-west trade.
Additional Information
A peninsula is a piece of land that is almost entirely surrounded by water but is connected to the mainland on one side.
Peninsulas can be very small, sometimes only large enough for a single lighthouse, for instance. Lighthouses often sit on peninsulas near rocky coastlines to warn sailors that they are getting close to land.
Peninsulas can also be very large. Most of the U.S. state of Florida is a peninsula that separates the Gulf of Mexico and the Atlantic Ocean.
Peninsulas are found on every continent. In North America, the narrow peninsula of Baja California, in Mexico, separates the Pacific Ocean and the Sea of Cortez, also called the Gulf of California. In Europe, the nations of Portugal and Spain make up the Iberian Peninsula. The so-called Horn of Africa, which juts into the Arabian Sea on central Africas east coast, is a huge peninsula. The nations of North Korea and South Korea make up the Korean Peninsula in eastern Asia. In Australia, the Cape York Peninsula is only 160 kilometers (99 miles) from the island of New Guinea. The Antarctic Peninsula seems to point to the tip of South America, several hundred kilometers (miles) away.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2538 2025-07-21 21:05:10
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 51,132
Re: Miscellany
2338) Seabed
Gist
The seabed (also known as the seafloor, sea floor, ocean floor, and ocean bottom) is the bottom of the ocean. All floors of the ocean are known as seabeds.
The average depth of the ocean is about 3,682 meters (12,080 feet). The deepest part of the ocean is called the Challenger Deep and is located beneath the western Pacific Ocean in the southern end of the Mariana Trench, which runs several hundred kilometers southwest of the U.S. territorial island of Guam.
Summary
The seabed is the most extensive habitat on the planet. It occupies at least 75% of the Earth's surface, far more if the planar areas of the shelves and slopes are taken into account. It follows that fluxes of materials across the sediment–water interface, and the mechanisms that mediate and constrain those fluxes, are likely to have global significance. Just how important the seabed is globally has been costed for nutrient cycling, which for marine shallow water systems is >US$ 40,000 {ha}^{-1} {year}^{-1}, 86% of the value of this service for all terrestrial and aquatic systems (Constanza et al., 1997). To this must be added the smaller areas (<1%) occupied by the sediment–water interface of wetlands, lakes and rivers, where local fluxes can be high and their significance belies their lesser size. However, what must also be taken into account, but is often overlooked, is the contribution of living benthic particles to pelagic systems, particularly planktonic, a process which is likely to profoundly influence the dynamics of water column populations and communities (see below). Benthic organisms are often also key drivers of biogeochemical fluxes through their bioturbatory activities in sediments. An understanding of the linkages between the fluxes of inorganic materials, the fluxes of living particles and benthic patterns, and processes within the sediment will be central to a proper evaluation of the significance of the benthic environment in marine ecosystems.
Details
The seabed (also known as the seafloor, sea floor, ocean floor, and ocean bottom) is the bottom of the ocean. All floors of the ocean are known as seabeds.
The structure of the seabed of the global ocean is governed by plate tectonics. Most of the ocean is very deep, where the seabed is known as the abyssal plain. Seafloor spreading creates mid-ocean ridges along the center line of major ocean basins, where the seabed is slightly shallower than the surrounding abyssal plain. From the abyssal plain, the seabed slopes upward toward the continents and becomes, in order from deep to shallow, the continental rise, slope, and shelf. The depth within the seabed itself, such as the depth down through a sediment core, is known as the "depth below seafloor". The ecological environment of the seabed and the deepest waters are collectively known, as a habitat for creatures, as the "benthos".
Most of the seabed throughout the world's oceans is covered in layers of marine sediments. Categorized by where the materials come from or composition, these sediments are classified as either: from land (terrigenous), from biological organisms (biogenous), from chemical reactions (hydrogenous), and from space (cosmogenous). Categorized by size, these sediments range from very small particles called clays and silts, known as mud, to larger particles from sand to boulders.
Features of the seabed are governed by the physics of sediment transport and by the biology of the creatures living in the seabed and in the ocean waters above. Physically, seabed sediments often come from the erosion of material on land and from other rarer sources, such as volcanic ash. Sea currents transport sediments, especially in shallow waters where tidal energy and wave energy cause resuspension of seabed sediments. Biologically, microorganisms living within the seabed sediments change seabed chemistry. Marine organisms create sediments, both within the seabed and in the water above. For example, phytoplankton with silicate or calcium carbonate shells grow in abundance in the upper ocean, and when they die, their shells sink to the seafloor to become seabed sediments.
Human impacts on the seabed are diverse. Examples of human effects on the seabed include exploration, plastic pollution, and exploitation by mining and dredging operations. To map the seabed, ships use acoustic technology to map water depths throughout the world. Submersible vehicles help researchers study unique seabed ecosystems such as hydrothermal vents. Plastic pollution is a global phenomenon, and because the ocean is the ultimate destination for global waterways, much of the world's plastic ends up in the ocean and some sinks to the seabed. Exploitation of the seabed involves extracting valuable minerals from sulfide deposits via deep sea mining, as well as dredging sand from shallow environments for construction and beach nourishment.
Structure
Most of the oceans have a common structure, created by common physical phenomena, mainly from tectonic movement, and sediment from various sources. The structure of the oceans, starting with the continents, begins usually with a continental shelf, continues to the continental slope – which is a steep descent into the ocean, until reaching the abyssal plain – a topographic plain, the beginning of the seabed, and its main area. The border between the continental slope and the abyssal plain usually has a more gradual descent, and is called the continental rise, which is caused by sediment cascading down the continental slope.
The mid-ocean ridge, as its name implies, is a mountainous rise through the middle of all the oceans, between the continents. Typically a rift runs along the edge of this ridge. Along tectonic plate edges there are typically oceanic trenches – deep valleys, created by the mantle circulation movement from the mid-ocean mountain ridge to the oceanic trench.
Hotspot volcanic island ridges are created by volcanic activity, erupting periodically, as the tectonic plates pass over a hotspot. In areas with volcanic activity and in the oceanic trenches there are hydrothermal vents – releasing high pressure and extremely hot water and chemicals into the typically freezing water around it.
Deep ocean water is divided into layers or zones, each with typical features of salinity, pressure, temperature and marine life, according to their depth. Lying along the top of the abyssal plain is the abyssal zone, whose lower boundary lies at about 6,000 m (20,000 ft). The hadal zone – which includes the oceanic trenches, lies between 6,000 and 11,000 metres (20,000–36,000 ft) and is the deepest oceanic zone.
Depth below seafloor
Depth below seafloor is a vertical coordinate used in geology, paleontology, oceanography, and petrology (see ocean drilling). The acronym "mbsf" (meaning "meters below the seafloor") is a common convention used for depths below the seafloor.
Sediments
Sediments in the seabed vary in origin, from eroded land materials carried into the ocean by rivers or wind flow, waste and decompositions of sea creatures, and precipitation of chemicals within the sea water itself, including some from outer space. There are four basic types of sediment of the sea floor:
* Terrigenous (also lithogenous) describes the sediment from continents eroded by rain, rivers, and glaciers, as well as sediment blown into the ocean by the wind, such as dust and volcanic ash.
* Biogenous material is the sediment made up of the hard parts of sea creatures, mainly phytoplankton, that accumulate on the bottom of the ocean.
* Hydrogenous sediment is material that precipitates in the ocean when oceanic conditions change, or material created in hydrothermal vent systems.
* Cosmogenous sediment comes from extraterrestrial sources.
Terrigenous and biogenous
Terrigenous sediment is the most abundant sediment found on the seafloor. Terrigenous sediments come from the continents. These materials are eroded from continents and transported by wind and water to the ocean. Fluvial sediments are transported from land by rivers and glaciers, such as clay, silt, mud, and glacial flour. Aeolian sediments are transported by wind, such as dust and volcanic ash.
Biogenous sediment is the next most abundant material on the seafloor. Biogenous sediments are biologically produced by living creatures. Sediments made up of at least 30% biogenous material are called "oozes." There are two types of oozes: Calcareous oozes and Siliceous oozes. Plankton grow in ocean waters and create the materials that become oozes on the seabed. Calcareous oozes are predominantly composed of calcium shells found in phytoplankton such as coccolithophores and zooplankton like the foraminiferans. These calcareous oozes are never found deeper than about 4,000 to 5,000 meters because at further depths the calcium dissolves. Similarly, Siliceous oozes are dominated by the siliceous shells of phytoplankton like diatoms and zooplankton such as radiolarians. Depending on the productivity of these planktonic organisms, the shell material that collects when these organisms die may build up at a rate anywhere from 1 mm to 1 cm every 1000 years.
Hydrogenous and cosmogenous
Hydrogenous sediments are uncommon. They only occur with changes in oceanic conditions such as temperature and pressure. Rarer still are cosmogenous sediments. Hydrogenous sediments are formed from dissolved chemicals that precipitate from the ocean water, or along the mid-ocean ridges, they can form by metallic elements binding onto rocks that have water of more than 300 °C circulating around them. When these elements mix with the cold sea water they precipitate from the cooling water. Known as manganese nodules, they are composed of layers of different metals like manganese, iron, nickel, cobalt, and copper, and they are always found on the surface of the ocean floor.
Cosmogenous sediments are the remains of space debris such as comets and asteroids, made up of silicates and various metals that have impacted the Earth.
Size classification
Another way that sediments are described is through their descriptive classification. These sediments vary in size, anywhere from 1/4096 of a mm to greater than 256 mm. The different types are: boulder, cobble, pebble, granule, sand, silt, and clay, each type becoming finer in grain. The grain size indicates the type of sediment and the environment in which it was created. Larger grains sink faster and can only be pushed by rapid flowing water (high energy environment) whereas small grains sink very slowly and can be suspended by slight water movement, accumulating in conditions where water is not moving so quickly. This means that larger grains of sediment may come together in higher energy conditions and smaller grains in lower energy conditions.
Benthos
Benthos, from Ancient Greek (bénthos) 'the depths [of the sea]'), also known as benthon, is the community of organisms that live on, in, or near the bottom of a sea, river, lake, or stream, also known as the benthic zone. This community lives in or near marine or freshwater sedimentary environments, from tidal pools along the foreshore, out to the continental shelf, and then down to the abyssal depths.
Many organisms adapted to deep-water pressure cannot survive in the upper parts of the water column. The pressure difference can be very significant (approximately one atmosphere for every 10 metres of water depth).
Because light is absorbed before it can reach deep ocean water, the energy source for deep benthic ecosystems is often organic matter from higher up in the water column that drifts down to the depths. This dead and decaying matter sustains the benthic food chain; most organisms in the benthic zone are scavengers or detritivores.
The term benthos, coined by Haeckel in 1891, comes from the Greek noun βένθος 'depth of the sea'. Benthos is used in freshwater biology to refer to organisms at the bottom of freshwater bodies of water, such as lakes, rivers, and streams. There is also a redundant synonym, benthon.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2539 2025-07-22 17:57:54
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 51,132
Re: Miscellany
2339) Atoll
Gist
An atoll is a ring-shaped island, or series of islets, formed by coral reefs surrounding a lagoon. These reefs typically encircle a body of water, which can be a shallow lagoon or a deeper basin. Most atolls are found in warm, tropical or subtropical oceans and seas where coral can thrive.
Summary
Atoll, coral reef enclosing a lagoon. Atolls consist of ribbons of reef that may not always be circular but whose broad configuration is a closed shape up to dozens of kilometres across, enclosing a lagoon that may be approximately 50 metres (160 feet) deep or more.
Most of the reef itself is a submarine feature, rising from the abyssal floors of the ocean to just beneath high-tide level. Around the rim along the top of the reef there are usually low, flat islands or more continuous strips of low, flat land. Some of these islands have been settled by oceanic peoples like the Maldivians, Polynesians, and Micronesians for many centuries.
The origin of atolls has always fascinated sailors and naturalists, who early appreciated that, although reef-building organisms inhabit only the shallowest depths of the sea (about 100 metres [330 feet]), the reefs rose from much deeper. The modern explanation of atolls incorporates the theory of Charles Darwin, who suggested that atolls represented the final stage of a continuing upgrowth of reef around a sinking extinct volcanic island that had long since disappeared from view.
Reefs tend to grow outward from a fringing-reef stage toward the better conditions of open water and also grow upward if the foundations beneath are sinking. After thousands of years the actively growing reef structure becomes separated from the volcanic shoreline by an intervening stretch of lagoon water. This is the barrier-reef stage. The volcanic island eventually subsides from view, leaving a reef whose uppermost part is like a saucer whose rim reaches up to sea level and whose deeper central area is a lagoon.
Different kinds of reefs and volcanic islands are found together in the tropical oceans, related to each other in such a way that they can be interpreted as representing the progressive stages postulated by the subsidence theory. Stronger direct evidence for subsidence has come from geologic drilling of atolls (first at Enewetak atoll in 1952), which revealed the presence of volcanic rock about 1,400 metres (4,600 feet) below the modern reef top. Changes in sea level complicate the subsidence model. These have been relatively frequent during the last 2,000,000 years or more and mostly result from cycles of glaciation.
Details
An atoll is a ring-shaped coral reef, island, or series of islets. The atoll surrounds a body of water called a lagoon.
An atoll is a ring-shaped coral reef, island, or series of islets. An atoll surrounds a body of water called a lagoon. Sometimes, atolls and lagoons protect a central island. Channels between islets connect a lagoon to the open ocean or sea.
Atolls develop with underwater volcanoes, called seamounts. First, the volcano erupts, piling up lava on the seafloor. As the volcano continues to erupt, the seamount's elevation grows higher, eventually breaking the surface of the water. The top of the volcano becomes an oceanic island.
In the next stage, tiny sea animals called corals begin to build a reef around the island. The type of corals that build reefs are called hermatypic corals, or hard corals. Hermatypic corals create a hard exoskeleton of limestone (calcium carbonate). Billions of these limestone exoskeletons are the reef.
This coral reef, called a fringing reef, surrounds the island just below the ocean surface. The thin, shallow strip of water between the fringing reef and the island is the lagoon.
Over millions of years, the volcanic island erodes and sinks to the seafloor. This process is called subsidence. The seamount erodes into the sea, its top made flat by the constant pounding of powerful ocean waves. As it subsides, the flat-topped seamount is called a guyot.
As the island subsides to become a guyot, its ring-shaped fringing reef turns into a barrier reef. A barrier reef is farther from shore, and has a deeper lagoon. The barrier reef protects the lagoon from the harsh winds and waves of the open ocean.
Subsidence brings slight differences in ocean chemistry that change the reef radically. The outer, ocean-facing side of the reef remains a healthy marine ecosystem. Corals on the inner, lagoon-facing side, however, begin to slowly decay. The algae that corals need to survive face much more competition for fewer nutrient resources. The limestone decays, changing the color of the lagoon from deep ocean blue to bright teal.
In the final stage of an atoll’s formation, ocean waves break apart pieces of the limestone reef. They pound, break, and erode the coral into tiny grains of sand. This sand and other material deposited by waves or wind pile up on the reef. This material, including organic matter such as plant seeds, form a ring-shaped island or islets. This is an atoll.
Hermatypic corals only live in warm water. An island that is located where ocean temperatures are just warm enough to support hermatypic corals is said to be at the “Darwin point," named after Charles Darwin. The famous naturalist was the first to outline how atolls form.
Atolls and People
The rocky or sandy shores of atolls have been important sites throughout human history. Often, their low-lying elevation has proved perilous.
Atolls are often hidden by ocean waves. Thousands of ships, from ancient Polynesian canoes to sophisticated American warships, have been stranded and wrecked on hidden atolls.
The Kon-Tiki, probably the most famous raft in history, became one of these atoll casualties. The Kon-Tiki was a large balsa raft built and sailed by explorer Thor Heyerdahl and his crew in 1947. The Kon-Tiki successfully sailed 6,980 kilometers (4,340 miles) from Peru to the South Pacific. The most difficult challenge of the journey was not the waves, currents, or trade winds of the open ocean. It was the atolls of Polynesia, the final part of their journey.
The quick-moving currents around atolls prevented the Kon-Tiki from docking at the first Polynesian island it encountered. It wrecked on the shallow coral of the second, Raroia atoll. Raroia was uninhabited, but nearby Native islanders in canoes eventually rescued the Europeans on the washed-up wreck.
The Kon-Tiki was eventually hauled out of Raroia, but atoll wrecks are popular dive sites throughout the Pacific. Shipwrecks from the 18th century through World War II lie at the bottom of atolls such as Kwajalein, part of the Marshall Islands.
Atolls are often uninhabited "desert" islands. (Desert does not refer to the islands' climate, but their "deserted" or uninhabited status.) Many are remote and difficult to reach. In the 20th century, this isolation made them attractive as testing sites for nuclear weapons from the United States, Britain, and France.
The first hydrogen bomb, for instance, was tested by the United States at Bikini Atoll, part of the Marshall Islands in the Pacific Ocean. The Pacific Proving Grounds, a series of 2,000 atolls and other islands under U.S. jurisdiction, were the site to more than a hundred massive nuclear explosions between 1947 and 1962. France continued nuclear testing on the atoll of Moruroa until 1995.
Nations throughout Polynesia, including the "nuclear-free zone" of New Zealand, protested extended nuclear testing. Reefs were being destroyed, and some tests dropped toxic fallout onto nearby inhabited islands. After Castle Bravo, the first hydrogen bomb test, the U.S. evacuated residents of the Rongelap and Rongerik atolls, and later compensated them for medical conditions associated with radiation poisoning.
The same elements that make atolls popular for nuclear testing also make them attractive to tourists. Atolls are sparsely populated, low-lying islands whose white, sandy beaches and placid lagoons are ideally suited to the tourism industry.
Many atolls, however, have few tourists and are among the world's underdeveloped countries. Atolls have few natural resources. Soil quality on atolls is very poor, and erosion is a constant threat. Most native residents on atolls practice subsistence agriculture and fishing. Almost all food and fuel is imported, often at great cost.
Fisheries and support for the shipping industry help support communities on remote atolls. Some atoll communities have taken advantage of their equatorial location and established launch sites for low-orbit satellites. Others have found other sources of revenue. The nation of Tuvalu, for instance, is a series of isolated atolls in the Pacific. Every year, it receives millions of dollars for use of its ".tv" Internet domain name.
Atolls, along with sandbars, are among islands with the lowest elevation. They are constantly, naturally at risk from erosion due to wind and waves. Atolls are also at risk from sea-level rise. As the the ocean level rises, atolls—and any infrastructure on them—are flooded and may drown altogether.
Island nations such as Maldives and Kiribati are fortifying their atolls by dredging the seafloor. Sand elevates certain areas and widens others, creating a more stable landmass.
Maldives and Kiribati have also taken political measures to protect their citizens from the possibility of atolls sinking beneath the sea. Maldives often leads international conferences on the impacts of global warming, which is associated with sea level rise. Maldives and Kiribati have also taken steps to outline a permanent evacuation process should sea level rise threaten to drown inhabited atolls.
Additional Information
An atoll is a ring-shaped island, including a coral rim that encircles a lagoon. There may be coral islands or cays on the rim. Atolls are located in warm tropical or subtropical parts of the oceans and seas where corals can develop. Most of the approximately 440 atolls in the world are in the Pacific Ocean.
Two different, well-cited models, the subsidence model and the antecedent karst model, have been used to explain the development of atolls. According to Charles Darwin's subsidence model, the formation of an atoll is explained by the sinking of a volcanic island around which a coral fringing reef has formed. Over geologic time, the volcanic island becomes extinct and eroded as it subsides completely beneath the surface of the ocean. As the volcanic island subsides, the coral fringing reef becomes a barrier reef that is detached from the island. Eventually, reef and the small coral islets on top of it are all that is left of the original island, and a lagoon has taken the place of the former volcano. The lagoon is not the former volcanic crater. For the atoll to persist, the coral reef must be maintained at the sea surface, with coral growth matching any relative change in sea level (sinking of the island or rising oceans).
An alternative model for the origin of atolls is called the antecedent karst model. In the antecedent karst model, the first step in the formation of an atoll is the development of a flat top, mound-like coral reef during the subsidence of an oceanic island of either volcanic or nonvolcanic origin below sea level. Then, when relative sea level drops below the level of the flat surface of coral reef, it is exposed to the atmosphere as a flat topped island which is dissolved by rainfall to form limestone karst. Because of hydrologic properties of this karst, the rate of dissolution of the exposed coral is lowest along its rim and the rate of dissolution increases inward to its maximum at the center of the island. As a result, a saucer shaped island with a raised rim forms. When relative sea level submerges the island again, the rim provides a rocky core on which coral grow again to form the islands of an atoll and the flooded bottom of the saucer forms the lagoon within them.
Island nations made of atolls include Maldives, in the Indian Ocean, and Kiribati, in the Pacific. Tourism is a key factor in both the Maldivian and Kiribati economies.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2540 2025-07-23 16:59:11
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 51,132
Re: Miscellany
2340) Volcano
Gist
Volcanoes are openings, or vents where lava, tephra (small rocks), and steam erupt onto the Earth's surface. Volcanic eruptions can last days, months, or even years.
A volcano is an opening in a planet or moon's crust through which molten rock, hot gases, and other materials erupt. Volcanoes often form a hill or mountain as layers of rock and ash build up from repeated eruptions.
Summary
A volcano is commonly defined as a vent or fissure in the crust of a planetary-mass object, such as Earth, that allows hot lava, volcanic ash, and gases to escape from a magma chamber below the surface.
On Earth, volcanoes are most often found where tectonic plates are diverging or converging, and because most of Earth's plate boundaries are underwater, most volcanoes are found underwater. For example, a mid-ocean ridge, such as the Mid-Atlantic Ridge, has volcanoes caused by divergent tectonic plates whereas the Pacific Ring of Fire has volcanoes caused by convergent tectonic plates. Volcanoes resulting from divergent tectonic activity are usually non-explosive whereas those resulting from convergent tectonic activity cause violent eruptions. Volcanoes can also form where there is stretching and thinning of the crust's plates, such as in the East African Rift, the Wells Gray-Clearwater volcanic field, and the Rio Grande rift in North America. Volcanism away from plate boundaries most likely arises from upwelling diapirs from the core–mantle boundary called mantle plumes, 3,000 kilometres (1,900 mi) deep within Earth. This results in hotspot volcanism or intraplate volcanism, in which the plume may cause thinning of the crust and result in a volcanic island chain due to the continuous movement of the tectonic plate, of which the Hawaiian hotspot is an example. Volcanoes are usually not created at transform tectonic boundaries where two tectonic plates slide past one another.
Volcanoes, based on their frequency of eruption or volcanism, are referred to as either active or extinct. Active volcanoes have a history of volcanism and are likely to erupt again while extinct ones are not capable of eruption at all as they have no magma source. "Dormant" volcanoes have not erupted in a long time- generally accepted as since the start of the Holocene, about 12000 years ago- but may erupt again. These categories aren't entirely uniform; they may overlap for certain examples.
Large eruptions can affect atmospheric temperature as ash and droplets of sulfuric acid obscure the Sun and cool Earth's troposphere. Historically, large volcanic eruptions have been followed by volcanic winters which have caused catastrophic famines.
Other planets besides Earth have volcanoes. For example, volcanoes are very numerous on Venus. Mars has significant volcanoes. In 2009, a paper was published suggesting a new definition for the word 'volcano' that includes processes such as cryovolcanism. It suggested that a volcano be defined as 'an opening on a planet or moon's surface from which magma, as defined for that body, and/or magmatic gas is erupted.'
Details
A volcano is a vent in the crust of Earth or another planet or satellite, from which issue eruptions of molten rock, hot rock fragments, and hot gases. A volcanic eruption is an awesome display of Earth’s power. Yet, while eruptions are spectacular to watch, they can cause disastrous loss of life and property, especially in densely populated regions of the world. Sometimes beginning with an accumulation of gas-rich magma (molten underground rock) in reservoirs near Earth’s surface, they can be preceded by emissions of steam and gas from small vents in the ground. Swarms of small earthquakes, which may be caused by a rising plug of dense, viscous magma oscillating against a sheath of more-permeable magma, may also signal volcanic eruptions, especially explosive ones. In some cases, magma rises in conduits to the surface as a thin and fluid lava, either flowing out continuously or shooting straight up in glowing fountains or curtains. In other cases, entrapped gases tear the magma into shreds and hurl viscous clots of lava into the air. In more violent eruptions, the magma conduit is cored out by an explosive blast, and solid fragments are ejected in a great cloud of ash-laden gas that rises tens of thousands of metres into the air. One feared phenomenon accompanying some explosive eruptions is the nuée ardente, or pyroclastic flow, a fluidized mixture of hot gas and incandescent particles that sweeps down a volcano’s flanks, incinerating everything in its path. Great destruction also can result when ash collects on a high snowfield or glacier, melting large quantities of ice into a flood that can rush down a volcano’s slopes as an unstoppable mudflow.
Strictly speaking, the term volcano means the vent from which magma and other substances erupt to the surface, but it can also refer to the landform created by the accumulation of solidified lava and volcanic debris near the vent. One can say, for example, that large lava flows erupt from Mauna Loa volcano in Hawaii, referring here to the vent; but one can also say that Mauna Loa is a gently sloping volcano of great size, the reference in this case being to the landform. Volcanic landforms have evolved over time as a result of repeated volcanic activity. Mauna Loa typifies a shield volcano, which is a huge, gently sloping landform built up of many eruptions of fluid lava. Mount Fuji in Japan is an entirely different formation. With its striking steep slopes built up of layers of ash and lava, Mount Fuji is a classic stratovolcano. Iceland provides fine examples of volcanic plateaus, while the seafloor around Iceland provides excellent examples of submarine volcanic structures.
Volcanoes figure prominently in the mythology of many peoples who have learned to live with eruptions, but science was late in recognizing the important role of volcanism in the evolution of Earth. As late as 1768, the first edition of the Encyclopædia Britannica gave voice to a common misconception by defining volcanoes as “burning mountains, which probably are made up of sulphur and some other matter proper to ferment with it, and take fire.” Today geologists agree that volcanism is a profound process resulting from the thermal evolution of planetary bodies. Heat does not easily escape from large bodies such as Earth by the processes of conduction or radiation. Instead, heat is transferred from Earth’s interior largely by convection—that is, the partial melting of Earth’s crust and mantle and the buoyant rise of magma to the surface. Volcanoes are the surface sign of this thermal process. Their roots reach deep inside Earth, and their fruits are hurled high into the atmosphere.
Volcanoes are closely associated with plate tectonic activity. Most volcanoes, such as those of Japan and Iceland, occur on the margins of the enormous solid rocky plates that make up Earth’s surface. Other volcanoes, such as those of the Hawaiian Islands, occur in the middle of a plate, providing important evidence as to the direction and rate of plate motion.
The study of volcanoes and their products is known as volcanology, but these phenomena are not the realm of any single scientific discipline. Rather, they are studied by many scientists from several specialties: geophysicists and geochemists, who probe the deep roots of volcanoes and monitor signs of future eruptions; geologists, who decipher prehistoric volcanic activity and infer the likely nature of future eruptions; biologists, who learn how plants and animals colonize recently erupted volcanic rocks; and meteorologists, who determine the effects of volcanic dust and gases on the atmosphere, weather, and climate.
Clearly the destructive potential of volcanoes is tremendous. But the risk to people living nearby can be reduced significantly by assessing volcanic hazards, monitoring volcanic activity and forecasting eruptions, and instituting procedures for evacuating populations. In addition, volcanism affects humankind in beneficial ways. Volcanism provides beautiful scenery, fertile soils, valuable mineral deposits, and geothermal energy. Over geologic time, volcanoes recycle Earth’s hydrosphere and atmosphere.
Additional Information
A volcano is an opening in a planet or moon’s crust through which molten rock, hot gases, and other materials erupt. Volcanoes often form a hill or mountain as layers of rock and ash build up from repeated eruptions.
Volcanoes are classified as active, dormant, or extinct. Active volcanoes have a recent history of eruptions; they are likely to erupt again. Dormant volcanoes have not erupted for a very long time but may erupt at a future time. Extinct volcanoes are not expected to erupt in the future.
Inside an active volcano is a chamber in which molten rock, called magma, collects. Pressure builds up inside the magma chamber, causing the magma to move through channels in the rock and escape onto the planet’s surface. Once it flows onto the surface the magma is known as lava.
Some volcanic eruptions are explosive, while others occur as a slow lava flow. Eruptions can occur through a main opening at the top of the volcano or through vents that form on the sides. The rate and intensity of eruptions, as well as the composition of the magma, determine the shape of the volcano.
Volcanoes are found on both land and the ocean floor. When volcanoes erupt on the ocean floor, they often create underwater mountains and mountain ranges as the released lava cools and hardens. Volcanoes on the ocean floor become islands when the mountains become so large they rise above the surface of the ocean.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2541 2025-07-24 17:12:31
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 51,132
Re: Miscellany
2341) Bay of Bengal
Gist
The Bay of Bengal is named after the Bengal region, which encompasses present-day Bangladesh and the Indian states of West Bengal, Tripura, and the Barak Valley in Southern Assam. It's the largest bay in the world and a major feature of the northeastern Indian Ocean.
Summary
The Bay of Bengal is the northeastern part of the Indian Ocean. Geographically it is positioned between the Indian subcontinent and the Indochinese peninsula, located south of the Bengal region.
Many South Asian and Southeast Asian countries are dependent on the Bay of Bengal. Geopolitically, the bay is bounded on the west and northwest by India, on the north by Bangladesh, and on the east by Myanmar and the Andaman and Nicobar Islands of India. Its southern limit is a line between Sangaman Kanda, Sri Lanka, and the northwesternmost point of Sumatra, Indonesia. Cox's Bazar, the longest sea beach in the world and Sundarbans, the largest mangrove forest and the natural habitat of the Bengal tiger, are located along the bay.
The Bay of Bengal occupies an area of 2,600,000 square kilometres (1,000,000 sq mi). A number of large rivers flow into the Bay of Bengal: the Ganges–Hooghly, the Padma, the Brahmaputra–Jamuna, the Barak–Surma–Meghna, the Irrawaddy, the Godavari, the Mahanadi, the Brahmani, the Baitarani, the Krishna, the Kaveri and the Penna River.
Details
Bay of Bengal, large but relatively shallow embayment of the northeastern Indian Ocean, occupying an area of about 839,000 square miles (2,173,000 square km). It lies roughly between latitudes 5° and 22° N and longitudes 80° and 90° E. It is bordered by Sri Lanka and India to the west, Bangladesh to the north, and Myanmar (Burma) and the northern part of the Malay Peninsula to the east. According to the definition of the International Hydrographic Bureau, the southern boundary extends from Dondra Head at the southern end of Sri Lanka in the west to the northern tip of the Indonesian island of Sumatra in the east. The bay is about 1,000 miles (1,600 km) wide, with an average depth of more than 8,500 feet (2,600 meters). The maximum depth is 15,400 feet (4,694 meters). A number of large rivers—the Mahanadi, Godavari, Krishna, and Kaveri (Cauvery) on the west and the Ganges (Ganga) and Brahmaputra on the north—flow into the Bay of Bengal. The Andaman and Nicobar groups, which are the only islands, separate the bay from the Andaman Sea.
Physical features:
Physiography
The Bay of Bengal is bordered to the north by a wide continental shelf that narrows to the south and by slopes of varying gradient on the northwest, north, and northeast, all cut by canyons from the rivers. Most important are the Ganges-Brahmaputra, Andhra, Mahadevan, Krishna, and Godavari canyons. These were former estuaries when the shoreline was at the margin of the continental shelf during the Pleistocene Epoch (about 2,600,000 to 11,700 years ago). The deep floor of the bay is occupied by a vast abyssal (deep-sea) plain that slopes to the south. The main submarine features include the beginning of the long, seismically active Java Trench near the Nicobar-Sumatra mainland and of the aseismic Ninetyeast Ridge. The fan of sediments of the Ganges River is the widest—5 to 7 miles (8 to 11 km)—and thickest in the world. The bay itself was formed as the Indian subcontinent collided with Asia within roughly the past 50 million years.
Climate
The climate of the Bay of Bengal is dominated by the monsoons. From November through April a continental high-pressure system north of the bay produces northeast winds (the northeast monsoon) characteristic of the winter season. During the northern summer (June–September) the rain-bearing southwest monsoon prevails, as intense heat produces a low-pressure system over the continent and a subsequent air flow from the ocean.
Cyclones—intense tropical storms of high winds and torrential rains—occur in spring (April–May) and fall (October–November); these are the weeks preceding the onset of monsoon rains and the weeks following their retreat. A cyclone in November 1970 in the Ganges River delta resulted in the deaths of an enormous number of people and livestock. A storm of comparable magnitude in April 1991 devastated the eastern shore of Bangladesh, and another powerful cyclone devastated the coastal Indian state of Orissa (now Odisha) in October 1999. Water spouts occur frequently in the bay during the summer months.
Hydrology
A unique feature of the bay is the extreme variability of its physical properties. Temperature in the offshore areas, however, is warm and markedly uniform at all seasons, decreasing somewhat toward the north. Surface densities are considerably greater in spring than in fall, when river discharge is highest. Surface salinity, normally measuring 33 to 34 parts per thousand, can fall to nearly half that level and can extend well south of the bay during the fall. Below the surface layer is an oxygen-poor intermediate layer that has high salinity and undergoes only weak circulation. Weak upwelling occurs in the northeast during the northeast monsoon. The sea presents alternately slick and ruffled surfaces over shallow internal waves all along the east-coast shelf. Surface movements of the waters change direction with the season, the northeast monsoon giving them a clockwise circulation, the southeast monsoon a counterclockwise circulation. Severe storms occur at the change of monsoon, particularly to the south in October.
In addition to water-level changes resulting from waves and tide, the average sea level varies throughout the year. Because rainfall and riverine input exceed evaporation, the bay exhibits a net water gain annually. The bay is also subject to occasional tsunamis; one such event, caused by an undersea earthquake near the Indonesian island of Sumatra in December 2004, devastated extensive coastal areas of the bay, particularly in Sri Lanka and the Andaman and Nicobar Islands.
Bottom deposits
Sediments in the Bay of Bengal are dominated by terrigenous deposits from the rivers, derived mainly from the Indian subcontinent and from the Himalayas. Calcareous clays and oozes are found near the Andaman and Nicobar Islands and atop the Ninetyeast Ridge. The amount of organic matter present in the continental-shelf sediment of the northern part of the east coast is poor compared with the world’s average for nearshore sediments.
Economic aspects:
Resource exploitation
The Bay of Bengal has a distinct tropical marine ecosystem, and copious river drainage into the northern part of the bay and the profusion of wetlands, marshes, and mangroves increase productivity of nearshore fish species. The exploitation of these resources is carried out by small-scale fisheries; commercial fishing in deeper waters is done largely by countries bordering the bay and by Japan. The annual catch of prawns, the major export crop, has remained stable despite intensified harvesting. Several species of tuna found in the bay also are important. The tuna fishery is confined to the true oceanic sector of the bay, south of latitude 15° N, since fresh water runoff from the large rivers greatly influences the nearshore waters.
Petroleum and natural-gas discoveries have been made in the Bay of Bengal, notably offshore of the Godavari and Manandi deltas. The bay has a geologic setting similar to that of the Indus River basin and the western margin of the Indian Peninsula. Hydrocarbon resources in the Bay of Bengal generally are located in deep areas, as compared to those in the Arabian Sea. There are placer deposits of titanium off northeastern Sri Lanka and rare earths off northeastern India. Heavy mineral sands occur around Nagapatnam (in Tamil Nadu state) on the southeastern Indian coast, near Chennai (Madras), and in coastal areas around Vishakhapatnam. They consist of ilmenite, garnet, sillimanite, zircon, rutile, and manganite.
Transportation
The principal trade routes for large tankers en route from the Persian Gulf to the Strait of Malacca pass south of the Bay of Bengal. Hence, oceanic transportation is limited to carriage of cargoes to and from Sri Lanka, Bangladesh, and the east coast of India. Principal ports in India are Kolkata (Calcutta), Haldia, Vishakhapatnam, Chennai, Cuddalore, and Paradeep. Sri Lankan ports of importance are Colombo and Trincomalee. Dhaka and Chittagong are noteworthy in Bangladesh, and Akyab (Sittwe) is Myanmar’s chief port on the Bay of Bengal. Haldia, Vishakhapatnam, and Paradeep are well developed as iron ore terminals, reflecting India’s profitable exportation of raw materials.
Study and exploration
The Periplus Maris Erythraei, an early Greek manual of sailing directions written in the 1st century ce, described sailing routes from the Red Sea (Maris Erythraei) to coastal areas along the Arabian Sea and Bay of Bengal to eastern India north of the Ganges delta. During the 2nd century ce, Ptolemy described voyages from the Ganges across the Bay of Bengal to the Strait of Malacca. Based on these descriptions, it is presumed that Indian and Malayan navigators had been crossing the Bay of Bengal on trading voyages for some time. Colonizing voyages began in the 1st century ce and can be divided into two periods: gradual colonization between the 1st and 6th centuries and development journeys between the 7th and 10th centuries.
Chinese maritime dominance of the Bay of Bengal dates from the Nan (Southern) Song dynasty (1127–1279). In 1405–33 the renowned admiral Zheng He led voyages for the purpose of exacting tribute and extending Chinese political influence in the Indian Ocean. He crossed the bay and visited ports in Sri Lanka. The Portuguese explorer Vasco da Gama led the first European voyage into the bay, which—after circumnavigating Africa—reached Calicut (now Kozhikode, India) in 1498. By 1511 the Portuguese had reached and occupied Malacca (now Melaka, Malaysia). The other major European voyages of the 16th to 19th century passed well to the south of the bay.
Interest in the scientific study of the bay grew in the 20th century, especially after World War II. The postwar voyages of the research vessels Galathea of Denmark, Vityaz of the U.S.S.R., and Pioneer and Anton Bruun of the United States undertook significant work in the region, and a notable effort was the International Indian Ocean Expedition (1960–65), during which a considerable amount of information was gathered. The National Institute of Oceanography, established in India in 1966, furthered this research, utilizing the vessels Sagar Kanya and Gaveshani. Indian Ocean fishery research and development programs have been carried out on a coordinated basis by Bangladesh, India, Myanmar, Sri Lanka, and several countries on the periphery of the Bay of Bengal, including Indonesia, Malaysia, Thailand, and Maldives.
Additional Information
A bay is defined as a coastal water body that connects to the main water body, such as a lake, ocean, or a larger bay. Although they form in different ways, the world’s largest bays like the Gulf of Guinea, Bay of Bengal, and the Gulf of Mexico formed through plate tectonics. Bays are of different sizes, with the larger ones referred to as sea, gulfs, bight, or sound.
The Bay of Bengal is the Indian Ocean’s second-largest subdivision after the Arabian Sea. It has a surface area of 2,600,000 {km}^{2} and stretches over a maximum length of 2,090 km and a maximum width of 1,610 km. This bay has an average depth of 2,600 m and a maximum depth of 4,694 m. The Bay of Bengal is the world’s largest water body referred to as a bay.
The Bay of Bengal forms the northeastern portion of the Indian Ocean. It is shared by the Southeast and South Asia countries and separated from the Burma Sea (Andaman Sea) to its east by the Nicobar Islands and the Andaman Islands. The bay’s southern limit is between Sangaman Kanda in Sri Lanka and Sumatra, Indonesia.
The Bay of Bengal is bound by several Asian countries, including India to the northwest and west, Myanmar to the east, Bangladesh to the north, Sri Lanka to the southwest, and Indonesia to the southeast. India’s Nicobar and Andaman Islands also border the bay on the east. The bay hosts the world’s largest mangrove forest (Sundarbans) and longest beach (Cox’s Bazar).
The Climate Of The Bay Of Bengal
The climate of the Bay of Bengal can be defined as a monsoon climate. Between November and April (winter season), a continental high-pressure system on the bay’s north causes the northeast monsoons to form. The rain-bearing southwest winds prevail during summer (June to September). Cyclones occur between April and May (spring) and October and November (fall). The Bay of Bengal's monsoon moves northwestward, striking the Andaman and Nicobar Islands in May and mainland India before the end of June.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2542 2025-07-25 16:57:43
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 51,132
Re: Miscellany
2342) Arabian Sea
Gist
The Arabian Sea is the northwest part of the Indian Ocean. To its west are the Guardafui Channel, Somali Sea and the Arabian Peninsula. To its east is the Indian Peninsula. It covers around 4,600,000 kilometres (2,900,000 miles). The Arabian Sea is one of the warmest seas.
Summary:
Introduction
The Arabian Sea is located between the Indian and Arabian peninsulas. In Roman times its name was the Erythraean Sea, while the medieval Arabs called it the Sea of India. It formed a part of the principal sea route between India and Europe for centuries.
Physical Features
The Arabian Sea covers a total area of about 1,491,000 square miles (3,862,000 square kilometers). To the north, the Gulf of Oman connects the Arabian Sea with the Persian Gulf via the Strait of Hormuz. To the west, the Gulf of Aden connects it with the Red Sea via the Strait of Bab el-Mandeb. The countries bordering the sea include India, Pakistan, Iran, Oman, Yemen, and Somalia. Most of the Arabian Sea has depths that exceed 9,800 feet (3,000 meters), and there are no islands in the middle.
Deep water reaches close to the bordering lands except in the northeast, off Pakistan and India. The average depth of the sea is approximately 8,970 feet (2,734 meters). Wheatley Deep is the deepest known point of the sea at 19,038 feet (5,803 meters). The chief islands in the sea are India’s Lakshadweep islands, Socotra (Yemen), and Masira and the Kuria Muria Islands (Oman).
Economy
Small-scale fishing activity is carried out in the Arabian Sea, especially off the east coast of Africa and the Arabian Peninsula. India, Pakistan, Sri Lanka, Iran, Oman, Yemen, France, the United Arab Emirates, South Korea, and the Maldives are the principal fishing nations.
The Arabian Sea has some of the biggest shipping lanes in the world. There are a number of ports serving the countries bordering the sea. Among the largest are Muhammad Bin Qasim and Karachi in Pakistan, and Mumbai for general cargo and Marmagao for iron ore in India.
Study and Exploration
The Arabian Sea was formed within the past 150 million years when the landmass of the Indian subcontinent is said to have moved north and collided with Asia. From about the 700s or 800s ce onward, Arab and Persian seafarers learned to use the monsoon winds to their advantage. ceLater, between the 800s and 1400s, navigators compiled instructions for sailing between various ports on the sea. These works are called rahmangs, or books of routes in Persian. Some of them contain useful information on navigating by the stars, and on winds, currents, soundings, coastlines, approaches, and the islands of the Arabian Sea.
Environmental Issues
There are high levels of phosphates and other nutrients in the western part of the sea. Due to this, fish life in this part of the Arabian Sea is rich. A periodic occurrence in the Arabian Sea, however, is the death of the fish in large numbers. Phosphate levels are high because of the coastal upswelling, or the upward movement of the subsurface waters. This movement brings nutrients settled on the seafloor up towards the surface. Fishes living near the sea surface include tuna, sardine, billfish, wahoo, sharks, lancet fish, and moonfish.
A periodic occurrence in the Arabian Sea, however, is the death of the fish in large numbers. This happens because the subsurface water that lacks oxygen floats up to the surface, where the fish die because they cannot get the oxygen they need to breathe.
Details
The Arabian Sea is located in the northwestern part of the Indian Ocean, situated between the Arabian Peninsula and the Indian subcontinent. It merges with the Gulf of Oman to the northwest and the Gulf of Aden in the southwest, and spans a total area of 1,491,000 square miles. The depth of the sea varies as it joins the Indian Ocean to the south, but it is generally approximated at 8,970 feet.
The Sea provides an important link between the neighboring areas of Iran, the Arabian Peninsula (including Yemen, Oman, United Arab Emirates), Pakistan, the Horn of Africa nations, and India. For this reason it has gained eminence as a vitally important historical trade route. Smaller political areas also fringe the Arabian Sea, including Socotra off the coast of Yemen, the Khuriyya Muriyya islands and the Lakshadweep islands.
Geography
The Arabian Sea is connected to surrounding bodies of water by a series of gulfs and straits that provide a steady avenue into the sea. The largest and most notable of the gulfs which feed into the sea are the Gulf of Oman, which connects the Arabian Sea to the Persian Gulf and the Gulf of Aden that connects the Arabian Sea to the Red Sea. However, gulfs are not the only access point into the Arabian Sea, as two significant rivers drain into its borders. The Arabian is augmented by water flowing down the Indus and Narmada rivers, which are the principal means of access to the Arabian Sea.
The Arabian Sea is distinguished by its remarkably deep water level that is often maintained close to land masses. The deep water level is hypothesized to be one of the reasons that there are no significant island developments in the center of the Arabian Sea, despite the fact that islands are quite proliferate on the outer borders. The Arabian Sea floor, while far below the surface, exhibits a startling complexity akin to standard land formations. Perhaps most notable among the submarine features is the Maldive Ridge, which runs along the ocean floor from the Arabian Sea into the Indian Ocean. This ridge, which is essentially similar to a mountain range on land, eventually rises above the water level to become the Maldive Islands.
The Maldive Ridge, along with other sea floor formations in the Arabian Sea, are hypothesized to be the result of seismic activity roughly 50 million years ago. According to geologists, during this time period Asia collided with the subcontinent of India, forming the Arabian Sea and its unique underground ridges. After the initial collision of the two land masses, the Arabian Sea has been shaped by a variety of highly influential factors, including erosion from water currents. One of the deepest areas of the sea floor is where the Indus River meets the Arabian Ocean. In the place where the two bodies of water meet, a sharp canyon has been formed on the sea floor, as the forces of the incoming water sweep away the sandy bottom. Also as a result of this erosion, the Indus River carries the sediment further into the sea, eventually depositing the excess into ridges or other formations.
Climate
The Arabian Sea contributes to a monsoon climate in the surrounding region by providing the water necessary for the wet storms. During the monsoon season, winds on the sea generally blow from the southwest and are particularly cold. Winds during that season are actually so strong that they succeed in sweeping away some of the salt content of the upper levels of sea water. During the monsoon season, the upper waters are less then 35 parts per thousand salinity while in the non-monsoon season salinity is over 36 parts per thousand. The change in salinity is attributed to the flow of the wind, which shifts to a northwestern flow when the monsoons are finished.
Wildlife and Natural Resources
A rich and varied aquatic habitat is supported in the Arabian Sea by high levels of inorganic nutrients. These nutrients, particularly phosphate, occur in the sea as a result of a water currents along the continental shelf that circulate nutrients from the sea floor. The effect of aquatic recirculation is particularly pronounced in the shallower areas of the sea, where light can penetrate the water. Other important natural resources in the region include petroleum and natural gas.
The Arabian Sea is also notable for its large population of pelagic fish, or those fish which live near the surface of the water. Many varieties are harvested in small scale fishing operations in the area, including tuna, sardines, bilfish, wahoo, and a variety of sharks. However, while the Arabian Sea hosts large fish populations at the moment, the marine habitat is constantly under threat from periodic mass mortality. Occasionally in the Arabian Sea the fish population falls victim to a particularly strong upsurge of phosphate. When phosphate rises in great quantities, the level of oxygen is dramatically reduced, resulting in mass aquatic mortality.
History
Water transport along the Arabian Sea had been established prior to Roman times, and only grew as time went on. Early trade routes that combined land travel with sections of marine travel were later eschewed to only encompass water transport as the trading lanes became more accessible. The early trade routes that crossed the sea quickly became established in the international trading community, leading many leaders to construct canals in an effort to further trade. Early examples of canal building can be found in the region now covered by the Suez Canal. In their manifestations, however, the shallow canals were particularly unstable and would often be swallowed in sand storms.
The Arabian Sea reached a historical heyday in the ninth century C.E., when Arab and Persian seamen began to use the sea as a means of communication with neighboring communities. By mastering the wind currents of the sea, seafarers were able to accurately navigate to southern Arabia, East Africa, and a variety of Red Sea ports. Written documentation exists that gave detailed instructions for sailing on the Arabian Sea, and the routes that had to be followed to arrive successfully at a desired location. These instructions, called rahmangs "book of routes," are a valuable source for analyzing marine knowledge prior to modern times.
In more recent history, the John Murray/Mabahiss Expedition of 1933-1934 formed the bulk of modern–day information regarding the sea. Its intricate studies of sea currents, sea floor land formations, and sediment, published over a 30-year period, helped to awaken the modern academic world to the scientific importance of the Arabian Sea.
Economy
While the Arabian Sea played an historic role in trade, it continues to be a vital area for international shipping today. The Arabian Sea became a major player in the international shipping scene with the construction of the Suez Canal in 1869. While the Suez Canal does not directly enter into the Arabian Sea, its construction allowed for greater marine trade in the area.
The Arabian Sea is considered to be one of the world's busiest shipping lanes, primarily due to its proximity to the Red Sea and the Persian Gulf. Most of the ships that use the Arabian Sea for transportation purposes are large tankers, whose travels often conclude in East Asia, Europe or The Americas.
Environmental Concerns and Conservation Efforts
The Arabian Sea, due its seasonal weather fluctuations, offers excellent examples of biological adaptation to environment. However, the diverse aquatic habitat is currently under threat from the oil industry, which uses the sea as a shipping lane. Oil spills, anchor damage, and sedimentation are constant threats in the region. The sea environment is also harmed by mechanized fishing practices, including the use of dynamite, that cause the ecosystem to become unstable.
Currently, plans are being considered that would protect the delicate wildlife of the Arabian Sea, particularly the turtle and coral populations. However, in order to implement a conservation program in the region, a wide variety of nations must multilaterally agree to take steps towards preservation.
Additional Information
The Arabian Sea is a region of sea in the northern Indian Ocean, bounded on the west by the Arabian Peninsula, Gulf of Aden and Guardafui Channel, on the northwest by Gulf of Oman and Iran, on the north by Pakistan, on the east by India, and on the southeast by the Laccadive Sea and the Maldives, on the southwest by Somalia. Its total area is 3,862,000 sq km (1,491,000 sq mi) and its maximum depth is 5,395 meters (17,700 feet). The Gulf of Aden in the west connects the Arabian Sea to the Red Sea through the strait of Bab-el-Mandeb, and the Gulf of Oman is in the northwest, connecting it to the Persian Gulf.
Geography
The Arabian Sea's surface area is about 3,862,000 sq km (1,491,130 sq mi). The maximum width of the sea is approximately 2,400 km (1,490 mi), and its maximum depth is 5,395 metres (17,700 ft). The biggest river flowing into the sea is the Indus River.
The Arabian Sea has two important branches: the Gulf of Aden in the southwest, connecting with the Red Sea through the strait of Bab-el-Mandeb; and the Gulf of Oman to the northwest, connecting with the Persian Gulf. There are also the gulfs of Khambhat and Kutch on the Indian Coast. The Arabian Sea has been crossed by many important marine trade routes since the 3rd or 2nd millennium BCE. Major seaports include Kandla Port, Mundra Port, Pipavav Port, Dahej Port, Hazira Port, Mumbai Port, Nhava Sheva Port (Navi Mumbai), Mormugão Port (Goa), New Mangalore Port and Kochi Port in India, the Port of Karachi, Port Qasim, and the Gwadar Port in Pakistan, Chabahar Port in Iran and the Port of Salalah in Salalah, Oman. The largest islands in the Arabian Sea include Socotra (Yemen), Masirah Island (Oman), Lakshadweep (India) and Astola Island (Pakistan). The countries with coastlines on the Arabian Sea are Yemen, Oman, Pakistan, Iran, India and the Maldives.
Limits
The International Hydrographic Organization defines the limits of the Arabian Sea as follows:
* On the west: the eastern limit of the Gulf of Aden.
* On the north: a line joining Ràs al Hadd, east point of the Arabian Peninsula (22°32'N) and Ràs Jiyùni (61°43'E) on the coast of Pakistan.
* On the south: a line running from the southern extremity of Addu Atoll in the Maldives, to the eastern extremity of Ràs Hafun (the easternmost point of Africa, 10°26'N).
* On the east: the western limit of the Laccadive Sea a line running from Sadashivgad on the west coast of India (14°48′N 74°07′E) to Cora Divh (13°42′N 72°10′E) and thence down the west side of the Laccadive and Maldive archipelagos to the most southerly point of Addu Atoll in the Maldives.
Hydrography
The International Indian Ocean Expedition in 1959 was among the first to perform hydrographic surveys of the Arabian Sea. Significant bathymetric surveys were also conducted by the Soviet Union during the 1960s.
Hydrographic features
Significant features in the northern Arabian Sea include the Indus Fan, the second largest fan system in the world. The De Covilhao Trough, named after the 15th century Portuguese explorer Pero de Covilhăo, reaches depths of 4,400 metres (14,436 ft) and separates the Indus Fan region from the Oman Abyssal Plain, which eventually leads to the Gulf of Oman.
The southern limits are dominated by the Arabian Basin, a deep basin reaching depths over 4,200 metres (13,780 ft). The northern sections of the Carlsberg Ridge flank the southern edge of the Arabian Basin.
The deepest parts of the Arabian Sea are in the Alula-Fartak Trough on the western edge of the Arabian Sea off the Gulf of Aden. The trough, reaching depths over 5,360 metres (17,585 ft), traverses the Gulf of Aden and the Arabian Sea. The deepest known point is in the Arabian Sea limits at a depth of 5,395 metres (17,700 ft). Other significant deep points are part of the Arabian Basin, which include a 5,358 metres (17,579 ft) deep point off the northern limit of Calrsberg Ridge.
Seamounts
Prominent sea mounts off the Indian west coast include Raman Seamount named after C. V. Raman, Panikkar Seamount, named after N. K. Panikkar, and the Wadia Guyot, named after D. N. Wadia.
Sind'Bad Seamount, named after the fictional explorer Sinbad the Sailor, Zheng He Seamount, and the Mount Error Guyot are some notable sea mounts in western Arabian Sea.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2543 2025-07-26 17:07:25
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 51,132
Re: Miscellany
2343) Lake Titicaca
Gist
Lake Titicaca is the largest freshwater lake in South America and the highest of the world's large lakes. Titicaca is one of less than twenty ancient lakes on earth, and is thought to be three million years old. Lake Titicaca sits 3810 m above sea level and is situated between Peru to the west and Bolivia to the east.
Lake Titicaca, at an elevation of 12,507 feet (3,812 meters) in the Andean Altiplano, is the highest large lake in the world. More than 120 miles long and 50 miles wide, it was the center of the Incan civilization, and today straddles the boundary between Peru and Bolivia.
Summary
Lake Titicaca is a large freshwater lake in the Andes mountains on the border of Bolivia and Peru. It is often called the highest navigable lake in the world. Titicaca is the largest lake in South America, both in terms of the volume of water and surface area. It has a surface elevation of 3,812 m (12,507 ft).
Overview
The lake is located at the northern end of the endorheic Altiplano basin high in the Andes on the border of Peru and Bolivia. The western part of the lake lies within the Puno Region of Peru, and the eastern side is located in the Bolivian La Paz Department.
The lake consists of two nearly separate subbasins connected by the Strait of Tiquina, which is 800 m (2,620 ft) across at the narrowest point. The larger subbasin, Lago Grande (also called Lago Chucuito), has a mean depth of 135 m (443 ft) and a maximum depth of 284 m (932 ft). The smaller subbasin, Wiñaymarka (also called Lago Pequeño, "little lake"), has an average depth of 9 m (30 ft) and a maximum depth of 40 m (131 ft). The overall average depth of the lake is 107 m (351 ft).
Five major river systems feed into Lake Titicaca. In order of their relative flow volumes, these are Ramis, Coata, Ilave, Huancané, and Suchez. More than 20 other smaller streams empty into Titicaca. The lake has 41 islands, some of which are densely populated.
Having only a single season of free circulation, the lake is monomictic, and water passes through Lago Huiñaimarca and flows out the single outlet at the Río Desaguadero, which then flows south through Bolivia to Lake Poopó. This only accounts for about 10% of the lake's water balance. Evapotranspiration, caused by strong winds and intense sunlight at high altitude, balances the remaining 90% of the water loss. It is nearly a closed lake.
Since 2000, Lake Titicaca has experienced constantly receding water levels. Between April and November 2009 alone, the water level dropped by 81 cm (32 in), reaching the lowest level since 1949. This drop is caused by shortened rainy seasons and the melting of glaciers feeding the tributaries of the lake. Water pollution is also an increasing concern because cities in the Titicaca watershed grow, sometimes outpacing solid waste and sewage treatment infrastructure. According to the Global Nature Fund (GNF), Titicaca's biodiversity is threatened by water pollution and the introduction of new species by humans. A 2011 United Nations report found alarming concentrations of Cd, As, and lead in various parts of the lake. In 2012, the GNF nominated the lake "Threatened Lake of the Year".
Details
Lake Titicaca, the world’s highest lake navigable to large vessels, lying at 12,500 feet (3,810 metres) above sea level in the Andes Mountains of South America, astride the border between Peru to the west and Bolivia to the east. Titicaca is the second largest lake of South America (after Maracaibo). It covers some 3,200 square miles (8,300 square km) and extends in a northwest-to-southeast direction for a distance of 120 miles (190 km). It is 50 miles (80 km) across at its widest point. A narrow strait, Tiquina, separates the lake into two bodies of water. The smaller, in the southeast, is called Lake Huiñaymarca in Bolivia and Lake Pequeño in Peru; the larger, in the northwest, is called Lake Chucuito in Bolivia and Lake Grande in Peru.
The meaning of the name Titicaca is uncertain, but it has been variously translated as Rock of the Puma or Crag of Lead. Titicaca lies between Andean ranges in a vast basin (about 22,400 square miles [58,000 square km] in area) that comprises most of the Altiplano (High Plateau) of the central Andes. In the snow-covered Cordillera Real on the northeastern (Bolivian) shore of the lake, some of the highest peaks in the Andes rise to heights of more than 21,000 feet (6,400 metres).
The lake averages between 460 and 600 feet (140 and 180 metres) in depth, but the bottom tilts sharply toward the Bolivian shore, reaching its greatest recorded depth of 920 feet (280 metres) off Isla Soto in the lake’s northeast corner.
More than 25 rivers empty their waters into Titicaca; the largest, the Ramis, draining about two-fifths of the entire Titicaca Basin, enters the northwestern corner of the lake. One small river, the Desaguadero, drains the lake at its southern end. This single outlet empties only 5 percent of the lake’s excess water; the rest is lost by evaporation under the fierce sun and strong winds of the dry Altiplano.
Titicaca’s level fluctuates seasonally and over a cycle of years. During the rainy season (summer, from December to March) the level of the lake rises, normally to recede during the dry winter months. It was formerly believed that Titicaca was slowly drying up, but modern studies have seemed to refute this, indicating a more or less regular cycle of rise and fall.
Titicaca’s waters are limpid and only slightly brackish, with salinity ranging from 5.2 to 5.5 parts per 1,000. Surface temperatures average 56 °F (14 °C); from a thermocline at 66 feet (20 m) temperatures drop to 52 °F (11 °C) at the bottom. Analyses show measurable quantities of sodium chloride, sodium sulfate, calcium sulfate, and magnesium sulfate in the water.
Lake Titicaca’s fish life consists principally of two species of killifish (Orestias)—a small fish, usually striped or barred with black—and a catfish (Trichomycterus). In 1939, and subsequently, trout were introduced into Titicaca. A large frog (Telmatobius), which may reach a length of nearly a foot, inhabits the shallower regions of the lake.
Forty-one islands, some of them densely populated, rise from Titicaca’s waters. The largest, Titicaca Island (Spanish: Isla de Titicaca, also called Isla del Sol), lies just off the tip of the Copacabana Peninsula in Bolivia.
Ruins on the lake’s bottom (where the remains of a temple were discovered in 2000), on its shore, and on the islands attest to the previous existence of one of the oldest civilizations known in the Americas. The chief site is at Tiwanaku, Bolivia, at the southern end of the lake. On Titicaca Island ruins of a temple mark the spot where, according to the tradition of the Incas (a Quechuan people of Peru who established an empire about 1100 ce), the legendary founders of the Inca dynasty, Manco Capac and Mama Ocllo, were sent down to Earth by the Sun.
The Aymara people living in the Titicaca Basin still practice their ancient methods of agriculture on stepped terraces that predate Inca times. They grow barley, quinoa (a type of pigweed that produces a small grain), and the potato, which originated on the Altiplano. The highest cultivated plot in the world was found near Titicaca—a field of barley growing at a height of 15,420 feet (4,700 metres) above sea level. At this elevation the grain never ripens, but the stalks furnish forage for llamas and alpacas, the American relatives of the camel that serve the Indians as beasts of burden and provide meat and wool. The lake plain is covered with vast numbers of pre-Columbian raised platform fields and ditches, now abandoned, which were constructed to improve drainage and enhance the region’s agricultural potential. This ancient system of reclamation has been revived in some areas in both Peru and Bolivia.
The remnants of an ancient people, the Uru, still live on floating mats of dried totora (a reedlike papyrus that grows in dense brakes in the marshy shallows). From the totora, the Uru and other lake dwellers make their famed balsas—boats fashioned of bundles of dried reeds lashed together that resemble the crescent-shaped papyrus craft pictured on ancient Egyptian monuments.
In 1862 the first steamer to ply the lake was prefabricated in England and carried in pieces on muleback up to the lake. Today vessels make regular crossings from Puno, on the Peruvian shore, to the small Bolivian port of Guaqui. A narrow-gauge railway connects Guaqui with La Paz, capital of Bolivia. One of the world’s highest railways runs from Puno down to Arequipa and the Pacific, completing for land-bound Bolivia, an important link with the sea, and also to Cuzco.
Additional Information
Lake Titicaca (Quechua: Titiqaqa Qucha, "Titiqaqa Lake") is a large, deep lake in the Andes mountains. The eastern part is in Bolivia and the western part of it is in Peru. It is the largest lake in South America. Lake Titicaca is at 3,812 m (12,507 ft) above sea level. It is often called the "highest navigable lake" in the world. It means that it is the highest lake that boats use for trade. There are many other lakes in the world that are higher. The lake has 41 islands. Some of the islands are home to many people.
Ecology
Lake Titicaca is home to more than 530 species of water animals. Several threatened species such as the huge Titicaca water frog and the Titicaca grebe, a bird which cannot fly, only live in or near the lake.
Since 2000, the water level of Lake Titicaca has gone down. This is because of shorter rainy seasons and the melting of glaciers. The Global Nature Fund (GNF) says that the natural life in and around Lake Titicaca is under threat from water pollution and the introduction of new species by humans.
Islands:
Uros
The "Floating Islands" are small islands made by the Uros (or Uru) people. They use layers of cut totora, a thick reed that grows in Lake Titicaca. The Uros make the islands by continuously bending over the reeds that grow in the lake.
Legend says that the Uru people came from the Amazon river area, and moved to Lake Titicaca. The local people did not allow them to have their own land. They then built the reed islands, which could be moved into deep water or to different parts of the lake for safety.
The islands are a golden colour. Many are about 15 by 15 m (50 by 50 ft) big. The largest are about half the size of a football field. Each island has a few houses. The people living together on an island are usually all related. Some of the islands have watchtowers and other buildings, also made out of reeds.
As of 2011, about 1,200 Uros lived on 60 islands. They are mostly in the west corner of the lake near Puno, a large port town in Peru. The islands have become one of Peru's tourist attractions. This means that the Uros can earn money by bringing visitors to the islands by motorboat and selling crafts.
Amantani
Amantani is another small island on Lake Titicaca. The people living here speak the Quechua language. About 4,000 people live in 10 communities on the nearly circular 15 sq km (6 sq mi) island. It has two mountain peaks, called Pachatata (Father Earth) and Pachamama (Mother Earth). Both peaks have ancient ruins on the top. The hillsides planted with wheat, potatoes, and vegetables. Most of the small fields are worked by hand. Long stone fences divide the fields, and cattle and sheep also graze on the hillsides.
Taquile
Taquile is a hilly island located 45 km (28 mi) east of Puno. About 2,200 people live here. It is narrow and long and was used as a prison during the Spanish Colony and into the 20th century. In 1970, it became property of the Taquile people, who have inhabited the island since then. There are pre-Inca ruins on the highest part of the island. Taquile is famous for its weaving and knitting. "Taquile and Its Textile Art" were honoured by UNESCO with the label "Masterpieces of the Oral and Intangible Heritage of Humanity".
Isla del Sol
Isla del Sol (Spanish for "island of the sun") is one of the largest islands of the lake. It is a rocky, hilly island. There are no cars or paved roads on the island. About 800 families live here. There are over 180 ruins on the island. Most of these are from the Inca period around the 15th century AD. Among the ruins on the island are the Sacred Rock, a labyrinth-like building called Chicana, Kasa Pata, and Pilco Kaima. The island is also mentioned in Inca mythology.
Isla de la Luna
Isla de la Luna (Spanish for “island of the moon”) lies east of the bigger Isla del Sol. Legends say that this is where Viracocha told the moon to rise. Archaeological excavations show that the Tiwanaku peoples (around 650–1000 AD) built a large temple on the Island of the Moon. The buildings on the island today were built by the Inca on top of the earlier Tiwanaku ones.
Suriki
Suriki lies in the Bolivian part of Lake Titicaca. Suriki is the last place where they still make reed boats.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2544 2025-07-27 16:24:00
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 51,132
Re: Miscellany
2344) Angel Falls
Gist
Angel Falls is the world's highest uninterrupted waterfall. Located in Canaima National Park, the second largest national park in Venezuela, the waterfall tumbles from a cleft near the summit of table top mountain Auyán-tepu into what is known as Devil's Canyon, 3212 feet (979m) below.
Angel Falls is located on the Churun River, which is a tributary of the Caroni River in Venezuela. While the Caroni is a larger river that the Churun flows into, the waterfall itself is directly on the Churun. The Churun is a part of the larger Orinoco River basin.
Summary
Angel Falls (Spanish: Salto Ángel; Pemon: Kerepakupai Merú or Parakupá Vená) is a waterfall in Venezuela. It is the world's tallest uninterrupted waterfall, with a height of 979 metres (3,212 ft), and a plunge of 807 m (2,648 ft). The waterfall drops over the edge of the Auyán-tepui mountain in the Canaima National Park (Spanish: Parque Nacional Canaima), a UNESCO World Heritage Site in the Gran Sabana region of Bolívar State. The height figure, 979 m (3,212 ft), mostly consists of the main plunge but also includes about 400 metres (1,300 ft) of sloped cascade and rapids below the drop and then a 30-metre-high (100 ft) plunge downstream of these talus rapids.
The falls are along a fork of the Río Kerepacupai Merú which flows into the Churún River, a tributary of the Carrao River, itself a tributary of the Orinoco River.
With regard to overall height, a revisited validation of waterfall measurements is not available, and there is still uncertainty whether Angel Falls or South Africa's Tugela Falls is the tallest (both measurements were taken at considerable distance from the two waterfalls).
Details
Angel Falls is the tallest waterfall in the world. It fell 3,212 feet (979 m) but recently dropped to 3,196 feet (974 m) mountain called Auyantepui. It is in Venezuela. The drop is so far that the water turns into mist when it reaches the bottom.
The falls are named for Jimmy Angel. Jimmy Angel was an American airplane pilot who crashed at the top of the falls in 1937.
Angel Falls is also called Salto Ángel or indigenous Kerepakupai-merú. The indigenous name derived from the Pemón natives means "falls from the deepest place". Ironically, the more famous name of the falls had nothing to do with the connotation that its water fell from the heavens.
Tourism
Angel Falls is one of Venezuela's top tourist attractions, though a trip to the falls is a complicated affair. The falls are located in an isolated jungle. A flight from Maiquetia Airport, Puerto Ordaz, or Ciudad Bolívar is required to reach Canaima camp, the starting point for river trips to the base of the falls. River trips generally take place from June to December, when the rivers are deep enough for use by the Pemon guides. During the dry season (December to March), the volume of water is less than in other months.
Media:
Cinema
Angel Falls was the main inspiration for the fictional Paradise Falls in Pixar's animated film, Up.
The American fantasy-romance film What Dreams May Come (1998), starring Robin Williams, Cuba Gooding Jr, and Annabella Sciorra, is set in Venezuela and shows Angel Falls.
Angel Falls was used as a setting for a scene in the action film Point Break (2015); actors Edgar Ramirez and Luke Bracey free-climb the Falls.
In the film narrated by Lowell Thomas, Seven Wonders of the World (1956), Angel Falls was included as one of the seven wonders.
The 1990 film Arachnophobia was partly set at Angel Falls.
Books
In 1997, Folco Quilici wrote Cielo verde a novel – long present in the bestseller list in Italy. Spanish writer Alberto Vázquez-Figueroa covered Jimmie Angel's adventures in his 1998 novel Ícaro— ISBN 9788408025023, later translated into several foreign languages. There is also another book that details how Angel Falls got its name: "Truth or Dare: The Jimmie Angel Story" written by Jan-Willem de Vries ISBN 9781419673665 Angel Falls by Kathryn Casey was published in September 2023; it is a historical fiction story inspired by the life of the photographer Ruth Robertson.
Additional Information
Angel Falls, waterfall in the Guiana Highlands in Bolívar state, southeastern Venezuela, on the Churún River, a tributary of the Caroní, 160 miles (260 km) southeast of Ciudad Bolívar. The highest waterfall in the world, the cataract drops 3,212 feet (979 metres) and is 500 feet (150 metres) wide at the base. It leaps from a flat-topped plateau, Auyán-Tepuí (“Devils Mountain”), barely making contact with the sheer face. The falls are located in Canaima National Park, and, because of the dense jungle surrounding the falls, they are best seen from the air.
The falls, first sighted by outsiders in the 1930s, were named for James Angel, an American adventurer who crash-landed his plane on a nearby mesa in 1937. In late 2009 Venezuelan Pres. Hugo Chávez declared that the falls should be referred to as Kerepakupai Merú, an Indigenous name.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2545 Yesterday 17:06:01
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 51,132
Re: Miscellany
2345) Lake Baikal
Gist
Lake Baikal is so deep because it sits within a rift valley formed by the Baikal Rift Zone, where the Earth's crust is slowly pulling apart. This tectonic activity has created a deep basin over millions of years, making it the deepest continental rift on Earth. The lake is also very old, which has allowed it to accumulate significant sediment, further contributing to its depth.
Situated in south-east Siberia, the 3.15-million-ha Lake Baikal is the oldest (25 million years) and deepest (1,700 m) lake in the world. It contains 20% of the world's total unfrozen freshwater reserve.
Lake Baikal is often considered the oldest, as clear evidence shows that it is 25–30 million years old. Lake Zaysan may be even older, of Cretaceous origin and at least 66 million years old (most likely around 70 million years), but its exact age is controversial and labeled with some uncertainty.
Summary
Lake Baikal is a rift lake and the deepest lake in the world. It is situated in southern Siberia, Russia between the federal subjects of Irkutsk Oblast to the northwest and the Republic of Buryatia to the southeast.
At 31,722 sq km (12,248 sq mi)—slightly larger than Belgium—Lake Baikal is the world's seventh-largest lake by surface area, as well as the second largest lake in Eurasia after the Caspian Sea. However, because it is also the deepest lake, with a maximum depth of 1,642 metres (5,387 feet), Lake Baikal is the world's largest freshwater lake by volume, containing 23,615.39 cu km (5,670 cu mi) of water or 22–23% of the world's fresh surface water, more than all of the North American Great Lakes combined. It is also the world's oldest lake at 25–30 million years, and among the clearest. It is estimated that the lake contains around 19% of the unfrozen fresh water on the planet.
Lake Baikal is home to thousands of species of plants and animals, many of them endemic to the region. It is also home to Buryat tribes, who raise goats, camels, cattle, sheep, and horses, on the eastern side of the lake within Buryatia, where the mean temperature varies from a winter minimum of −19 °C (−2 °F) to a summer maximum of 14 °C (57 °F). The region to the east of Lake Baikal is referred to as Transbaikalia or as the Transbaikal, and the loosely defined region around the lake itself is sometimes known as Baikalia. UNESCO declared Baikal a World Heritage Site in 1996.
Details
Lake Baikal, lake located in the southern part of eastern Siberia within the republic of Buryatia and Irkutsk oblast (province) of Russia. It is the oldest existing freshwater lake on Earth (20 million–25 million years old), as well as the deepest continental body of water, having a maximum depth of 5,315 feet (1,620 metres). Its area is some 12,200 square miles (31,500 square km), with a length of 395 miles (636 km) and an average width of 30 miles (48 km). It is also the world’s largest freshwater lake by volume, containing about one-fifth of the fresh water on Earth’s surface, some 5,500 cubic miles (23,000 cubic km). Into Lake Baikal flow more than 330 rivers and streams, the largest of which include the Selenga, Barguzin, Upper (Verkhnyaya) Angara, Chikoy, and Uda.
Baikal lies in a deep structural hollow surrounded by mountains, some of which rise more than 6,600 feet (2,000 metres) above the lake’s surface. The sedimentary strata on the floor of the lake may be as much as 20,000 feet (6,100 metres) thick. Breaks in Earth’s crust produce hot mineral springs in the area. There are occasional severe earthquakes; in 1862 a quake inundated about 77 square miles (200 square km) in the northern Selenga delta, creating a new bay in Baikal known as Proval Bay.
The lake hollow is not symmetrical, having steep slopes on the western shores and gentler slopes on the eastern. The meandering shoreline runs for some 1,300 miles (2,100 km), with large indentations at the bays of Barguzin, Chivyrkuysky, and Proval and at Ayaya and Frolikha inlets; the Svyatoy Nos Peninsula juts out into the lake from the eastern shore. Baikal contains some 45 islets and islands, the largest of which are Olkhon (about 270 square miles [700 square km]) and Bolshoy (Great) Ushkany (3.6 square miles [9.4 square km]). The influx of water into the lake is primarily from rivers, chiefly the Selenga. The only outflow is through the Angara River, a tributary of the Yenisey.
Baikal’s climate is much milder than that of the surrounding territory. Winter air temperatures average −6 °F (−21 °C), and August temperatures average 52 °F (11 °C). The lake surface freezes in January and thaws in May or June. The water temperature at the surface in August is between 50 and 54 °F (10 and 12 °C) and reaches 68 °F (20 °C) in the offshore shallows. Waves can be as high as 15 feet (4.6 metres). The water is very clear; from the surface one can see to 130 feet (40 metres). Its salinity is low, and it contains few minerals.
Plant and animal life in the lake is rich and various. There are between 1,500 and 1,800 animal species at different depths, and hundreds of plant species live on or near the surface. The majority of the species are endemic to Baikal. There are some 50 species of fish, belonging to seven families; the most numerous of these are the 25 species of gobies. The omul salmon is heavily fished; also important are the grayling, lake whitefish, and sturgeon. Unique to the lake is a fish called the golomyanka, of the family Comephoridae, which gives birth to live young. The one mammal species is the Baikal seal, or nerpa (Phoca sibirica). There are more than 320 bird species in the Baikal area.
Industries along the shores of Baikal include mining (mica and marble), the manufacture of cellulose and paper, shipbuilding, fisheries, and timber. There are many mineral springs, and visitors come to Goryachinsk for the curative properties of the waters. A pulp and paper mill built on Lake Baikal’s southern shore in 1966 drew strong environmental protests from Soviet scientists and writers because its wastes were polluting the water, and in 1971 the Soviet government adopted a decree to protect the lake from polluting emissions. Further pollution controls were resisted, however, and industrial waste at the site remained a concern in the late 1990s.
The Limnological Institute of the Siberian Division of the Russian Academy of Sciences is located in the town of Listvyanka, as is the Baikal Sanatorium, and the hydrobiological station of Irkutsk State University is in Bolshiye Koty (Bolshoy Koti). The protection of natural resources in the area began with the establishment of the Barguzinsky Nature Reserve in 1916; subsequently there were added the Baikalsky (1969) and Baikalo-Lenskiy (1986) nature reserves, the Frolikhinskiy (1976) and Kabansky (1974) wildlife reserves, and the Zabaikalsky and Pribaikalsky national parks (both 1986). The Lake Baikal Coastal Protection Zone, covering the lake and its environs (a total of 34,000 square miles [88,000 square km]), was created in 1987, and the same area was designated a UNESCO World Heritage site in 1996.
Additional Information
Situated in south-east Siberia, the 3.15-million-ha Lake Baikal is the oldest (25 million years) and deepest (1,700 m) lake in the world. It contains 20% of the world's total unfrozen freshwater reserve. Known as the 'Galapagos of Russia', its age and isolation have produced one of the world's richest and most unusual freshwater faunas, which is of exceptional value to evolutionary science.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2546 Today 17:13:33
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 51,132
Re: Miscellany
2346) Caspian Sea
Gist
The Caspian Sea is considered a sea, rather than a lake, primarily due to its size, salinity, and historical and political considerations. While it is landlocked and has some characteristics of a lake, its massive size and the fact that it contains saltwater, though less salty than the ocean, are key factors in its classification as a sea.
The coastlines of the Caspian are shared by Azerbaijan, Iran, Kazakhstan, Russia, and Turkmenistan. The Caspian is divided into three distinct physical regions: the Northern, Middle, and Southern Caspian.
Perhaps no geographical feature is more important to the future of Central Asia than the Caspian Sea, rich as it is in natural resources and central to transport routes that link littoral states to each other and to regions beyond. It is also crucial as a focal point to bring together the states of Central Asia.
Summary
The Caspian Sea is the world's largest inland body of water, described as the world's largest lake and usually referred to as a full-fledged sea. An endorheic basin, it lies between Europe and Asia: east of the Caucasus, west of the broad steppe of Central Asia, south of the fertile plains of Southern Russia in Eastern Europe, and north of the mountainous Iranian Plateau. It covers a surface area of 371,000 sq km (143,000 sq mi) (excluding the highly saline lagoon of Garabogazköl to its east), an area approximately equal to that of Japan, with a volume of 78,200 cu km (19,000 cu mi). It has a salinity of approximately 1.2% (12 g/L), about a third of the salinity of average seawater. It is bounded by Kazakhstan to the northeast, Russia to the northwest, Azerbaijan to the southwest, Iran to the south, and Turkmenistan to the southeast. The name of the Caspian Sea is derived from the ancient Iranic Caspi people.
The lake stretches 1,200 km (750 mi) from north to south, with an average width of 320 km (200 mi). Its gross coverage is 386,400 sq km (149,200 sq mi) and the surface is about 27 m (89 ft) below sea level. Its main freshwater inflow, Europe's longest river, the Volga, enters at the shallow north end. Two deep basins form its central and southern zones. These lead to horizontal differences in temperature, salinity, and ecology. The seabed in the south reaches 1,023 m (3,356 ft) below sea level, which is the third-lowest natural non-oceanic depression on Earth after Baikal and Tanganyika lakes.
With a surface area of 371,000 square kilometres (143,000 sq mi), the Caspian Sea is nearly five times as big as Lake Superior (82,000 square kilometres (32,000 sq mi)). The Caspian Sea is home to a wide range of species and is famous for its caviar and oil industries. Pollution from the oil industry and dams on rivers that drain into it have harmed its ecology. It is predicted that during the 21st century, the depth of the sea will decrease by 9–18 m (30–60 ft) due to global warming and the process of desertification, leading to an ecocide.
Details
Caspian Sea, world’s largest inland body of water. It lies to the east of the Caucasus Mountains and to the west of the vast steppe of Central Asia. The sea’s name derives from the ancient Kaspi peoples, who once lived in Transcaucasia to the west. Among its other historical names, Khazarsk and Khvalynsk derive from former peoples of the region, while Girkansk stems from Girkanos, “Country of the Wolves.”
The elongated sea sprawls for nearly 750 miles (1,200 km) from north to south, although its average width is only 200 miles (320 km). It covers an area of about 149,200 square miles (386,400 square km)—larger than the area of Japan—and its surface lies some 90 feet (27 metres) below sea level. The maximum depth, toward the south, is 3,360 feet (1,025 metres) below the sea’s surface. The drainage basin of the sea covers some 1,400,000 square miles (3,625,000 square km). The sea contains some 63,400,000,000 acre-feet or 18,800 cubic miles (78,200 cubic km) of water—about one-third of Earth’s inland surface water. The sea is bordered in the northeast by Kazakhstan, in the southeast by Turkmenistan, in the south by Iran, in the southwest by Azerbaijan, and in the northwest by Russia.
The Caspian is the largest salt lake in the world, but that has not always been true. Scientific studies have shown that until relatively recent geologic times, approximately 11 million years ago, it was linked, via the Sea of Azov, the Black Sea, and the Mediterranean Sea, to the world ocean. The Caspian is of exceptional scientific interest, because its history—particularly former fluctuations in both area and depth—offers clues to the complex geologic and climatic evolution of the region. Human-made changes, notably those resulting from the construction of dams, reservoirs, and canals on the immense Volga River system (which drains into the Caspian from the north), have affected the contemporary hydrologic balance. Caspian shipping and fisheries play an important role in the region’s economy, as does the production of petroleum and natural gas in the Caspian basin. The sea’s splendid sandy beaches also serve as health and recreation resorts.
Physical features
The Caspian basin, as a whole, is usually divided into the northern, middle, and southern Caspian, based partly on underwater relief and partly on hydrologic characteristics. The sea contains as many as 50 islands, most of them small. The largest are Chechen, Tyuleny, Morskoy, Kulaly, Zhiloy, and Ogurchin.
Shoreline features
The shores of the northern Caspian are low and reflect the great accumulation of alluvial material washed down by the Ural, Terek, and, above all, Volga rivers, whose deltas are extensive. The western shore of the middle Caspian is hilly. The foothills of the Greater Caucasus Mountains loom close but are separated from the coast by a narrow marine plain. The Abşeron Peninsula, on which the city of Baku is sited, thrusts out into the sea there, while just to its south the floodplain of the Kura and Aras rivers forms the Kura-Aras Lowland along the western shore of the southern Caspian. The southwestern and southern Caspian shores are formed of the sediments of the Länkäran and Gīlān-Māzanderān lowlands, with the high peaks of the Talish and Elburz ranges rearing up close inland. The eastern shore of the southern Caspian is low, formed partly by sediments derived from the erosion of the cliffs along the sea. The shoreline there is broken sharply by the low, hilly Cheleken and Türkmenbashi peninsulas. Just to the north, behind the east shore of the middle Caspian, is the Kara-Bogaz-Gol (Garabogazköl), formerly a shallow gulf of the Caspian but now a large lagoonlike embayment that is separated from the sea by a man-made embankment. For the most part, the eastern shore of the middle Caspian is precipitous, with the sea destroying the margin of the limestone plateaus of Tüpqaraghan and Kendyrli-Kayasansk.
The major rivers—the Volga, Ural, and Terek—empty into the northern Caspian, with their combined annual flow accounting for about 88 percent of all river water entering the sea. The Sulak, Samur, Kura, and a number of smaller rivers flow in on the western shore of the middle and southern Caspian, contributing about 7 percent of the total flow into the sea. The remainder comes in from the rivers of the southern, Iranian shore. Apart from the Atrak (Atrek) River of southern Turkmenistan, the sea’s arid eastern shore is notable for a complete lack of permanent streams.
Submarine features
The northern Caspian, with an area of 38,380 square miles (99,404 square km), is the shallowest portion of the sea, with an average depth of 13 to 26 feet (4 to 8 metres), reaching a maximum of 66 feet (20 metres) along the boundary with the middle Caspian. The bottom is formed of a monotonously rippling sedimentary plain, broken only by a line of southern bars and shoals—some of which constitute the foundations for Tyuleny and Kulaly islands and the Zhemchuzhny shoals—reflecting underlying structural rises. Beyond that belt, known as the Mangyshlak Bank, the middle Caspian, 53,250 square miles (137,917 square km) in area, forms an irregular depression with an abrupt western slope and a gentler eastern gradient. The shallowest portion—a shelf with depths reaching 330 to 460 feet (100 to 140 metres)—extends along both shores, with the western slope furrowed by submerged landslides and canyons. The remains of ancient river valleys have been discovered on the gentler eastern slope; the bottom of the depression comprises a plain that deepens to the west. The Abşeron Bank, a belt of shoals and islands rising from submerged elevations of older rocks, marks the transition to the southern Caspian, a depression covering about 57,570 square miles (149,106 square km). That depression is fringed by a shelf that is narrow to the west and south but widens to the east. A series of submerged ridges breaks up the relief to the north, but otherwise the bottom of the depression is a flat plain and contains the Caspian’s greatest depths.
Geology of the Caspian Sea
The relief of the Caspian Sea reflects its complex geologic structure. The northern Caspian Sea bottom is extremely old, dating to Precambrian times, or at least about 541 million years ago. The bottom of the northern and middle Caspian has a continental-type crustal structure. The northern portion is a section of the northern Caspian tectonic depression, a vast downwarp in the great ancient structural block known as the Russian Platform. The Mangyshlak Bank links the mountainous Tüpqaraghan Peninsula to the east with underlying western shore structures; those are the remnants of an outlying structural uplift of the Hercynian mountain-building movement, which occurred some 300 million years ago. It has been suggested that the middle Caspian depression resulted from a sagging at the edge of those ancient structures that occurred in late Paleozoic times, before about 252 million years ago. The bottom of the middle Caspian is highly complex. To the west the submarine shelf is part of the sagging edge of the Greater Caucasus Geosyncline (a downwarp of Earth’s crust), while the submerged Turan Platform in the east swells up in the feature known as the Kara-Bogaz (Garabogaz) Swell. The features of the Abşeron Peninsula region, along with the folded structures on the western side of the southern Caspian depression, derive from the Alpine mountain-building and folding processes (dating from some 26 to 10 million years ago) that created the Caucasus ranges. The border between the middle and southern Caspian is, in fact, still experiencing folding activity. The entire southern Caspian rests on a very ancient suboceanic-type basalt crustal structure, although that rock is covered in the south by huge accumulations of sedimentary layers many miles thick.
Until the beginning of the late Miocene Epoch (about 13.8 million years ago), the sea basin of the Caspian was connected to the Black Sea through the structural depression known as the Manych Trench (or Kuma-Manych Depression). After a late Miocene uplift, the Caspian became an enclosed body, with oceanic submarine characteristics preserved today only in the southern Caspian. The ocean connection was temporarily reestablished in the early Pleistocene Epoch (about 2.6 million years ago), and it is possible that there also was a link north across the Russian Plain to the Barents Sea of the Arctic Ocean.
Since about 2 million years ago, glaciers have advanced and retreated across the Russian Plain, and the Caspian Sea itself—in successive phases known as Baku, Khazar, and Khvalyn—alternately shrank and expanded. That process left a legacy in the form of peripheral terraces that mark old shorelines and can also be traced in the geologically recent underlying sedimentary layers.
The Caspian Sea bottom is now coated with recent sediments, finely grained in the shallow north but with shell deposits and oolitic sand—reflecting the high lime content of the Caspian waters—widespread in other coastal areas. Calcium carbonate also affects the composition of the much deeper bottom layers.
Climate
The northern Caspian lies in a moderately continental climate zone, while the middle (and most of the southern) Caspian lies in the warm continental belt. The southwest is touched by subtropical influences, and that remarkable variety is completed by the desert climate prevailing on the eastern shore. Atmospheric circulation is dominated in winter by the cold, clear air of the Asiatic anticyclone, while in summer spurs of the Azores high-pressure and the South Asian low-pressure centres are influential. Complicating factors are the cyclonic disturbances rippling in from the west and the tendency of the Caucasus Mountains to block them. As a result of those factors, northerlies and northwesterlies (nearly one-third of occurrences) and southeasterlies (more than one-third) dominate circulation patterns. Savage storms are associated with northerly and southeasterly winds.
Summer air temperatures are fairly evenly distributed—average July to August figures range between 75 and 79 °F (24 and 26 °C), with a maximum of 111 °F (44 °C) on the sunbaked eastern shore—but winter monthly average temperatures range from 14 °F (−10 °C) in the north to 50 °F (10 °C) in the south. Average annual precipitation, falling mainly in winter and spring, varies from 8 to 67 inches (200 to 1,700 mm) over the sea, with the least falling in the east and the most in the southwestern region. Evaporation from the sea surface is high, reaching 40 inches (1,015 mm) per year. Ice formation affects the northern Caspian, which usually freezes completely by January, and in exceptionally cold years ice that floats along the western shore comes as far south as the Abşeron Peninsula.
Hydrology
Short-term wind-induced fluctuations in the sea level can measure up to 7 feet (2 metres), though their average is about 2 feet (60 cm). Seiches (free or standing-wave oscillation of the sea surface mainly caused by winds and local changes in atmospheric pressure) are typically less pronounced. Tidal changes are but a few inches (or centimetres), and seasonal rises induced by high spring water in the rivers are not much greater.
One of the more-fascinating aspects of study of the Caspian has been the reconstruction of long-term fluctuations over the centuries from archaeological, geological, and historical evidence. It seems that since the 1st century bce the Caspian’s water level has fluctuated by at least 23 feet (7 metres). The main reasons for the long-term fluctuations are climatic changes that determine a balance between water gains (river influx and precipitation) and losses (evaporation). During the first three decades of the 20th century, the level of the Caspian was close to 86 feet (26 metres) below sea level, but in 1977 it dropped to 96 feet (29 metres), the lowest level noted during the past 400 to 500 years. A rapid rise in water level began in 1978—in the mid-1990s the sea was at 87 feet (26.5 metres) below sea level—but after 1995 the sea’s water level fell slightly, only to rebound again in the early 21st century. The lowering that took place between 1929 and 1977 was attributed to climatic changes that increased evaporation and reduced river influx—amplified by reservoir construction on the Volga to supply river water for irrigation and industry. The rise in water level after 1978 also resulted from climate change causing an increase in the inflow from the Volga, which during some years was considerably greater than average. An increase in precipitation over the sea itself and decrease of evaporation also contributed to the phenomenon. In 1980 Soviet hydrologists stemmed the outflow into the Kara-Bogaz-Gol by constructing sand barricades between the Caspian and the lagoon. Planners have given serious attention to the feasibility of other measures for stabilizing the Caspian’s water level.
In summer the average surface temperature of the Caspian ranges from 75 to 79 °F (24 to 26 °C), with the south a little warmer. There are, however, significant winter contrasts, from 32 to 45 °F (0 to 7 °C) in the north to 46 to 50 °F (8 to 10 °C) in the south. Upwellings of deep water at the eastern shore—a result of prevailing-wind activity—can also bring a marked drop in summer temperature.
Salinity in the Caspian is about 12.8 parts per thousand on average, but that figure conceals a variation from a mere 1 part per thousand near the Volga outlet to a high of 200 parts per thousand in the Kara-Bogaz-Gol, where intense evaporation occurs. In the open sea, distribution of salinity is markedly uniform; from the surface to the bottom it increases by only 0.1 to 0.2 part per thousand. Caspian waters differ from those of the ocean in their high sulfate, calcium, and magnesium carbonate content and—as a result of river inflow—lower chloride content.
Circulation of water masses occurs, basically, in a counterclockwise movement (north-to-south along the western shore), with a complex pattern developing farther south, where there are several subsidiary movements. Currents can be speeded up where they coincide with strong winds, and the sea surface is often ruffled by wave action. The maximum storm waves, occurring near the Abşeron Peninsula, have been measured at more than 30 feet (9 metres).
Marine life
About 850 animal and more than 500 plant species live in the Caspian. Although the number of species is relatively low for a body of water of that size, many of them are endemic (i.e., found only there). Blue-green algae (cyanobacteria) and diatoms constitute the greatest biomass concentrations, and there are several species of red and brown algae. Animal life—which has been affected greatly by changes in salinity—includes fish species such as sturgeon, herring, pike, perch, and sprat; several species of mollusks; and a variety of other organisms including sponges. Some 15 species of Arctic (such as the Caspian seal) and Mediterranean types complement the basic fauna. Some organisms have migrated to the Caspian relatively recently: barnacles, crabs, and clams, for example, have been transported by sea vessels, while gray mullets have been deliberately introduced by humans.
Additional Information
The Caspian Sea is the world’s largest water body that is enclosed or bordered by land on all sides. This massive lake is located between Asia and Europe.
The Caspian Sea may have derived its name from the Caspi people who lived in South Caucasus. However, the Persians and Greeks referred to the sea as the Hyrcanian Ocean.
The Caspian Sea is best known for its oil industry and caviar. It is also home to numerous plant and animal species. However, pollution, particularly from the oil industry, is a primary threat to the sea’s ecology, with environmental bodies calling for controlled industrial activities in the region.
Where Is The Caspian Sea?
The Caspian Sea is an endorheic basin (drainage basin without an outflow). It covers a total surface area of about 386,400 sq km and is about 1,200 km long and 320 km wide. The sea’s surface is approximately 27 m below sea level and has a volume of 78,200 cu km. The Caspian Sea accounts for between 40-44% of the world’s lacustrine waters.
Five countries in Asia and Europe share the Caspian Sea’s coastline. It is bordered to the north and east by Kazakhstan and from north to the west by Russia. Azerbaijan borders the sea to the southwest, while Iran is in the south. Turkmenistan lies along the southern portion of the eastern coast. Besides the five countries bordering the sea, the Caspian Sea drains Armenia, the eastern part of Georgia, the western portion of Uzbekistan, and the northeastern part of Turkey.
The Caspian Sea consists of three separate regions, namely Southern, Northern, and Middle Caspian. The Mangyshlak Threshold forms the Middle-Northern boundary, while the Apsheron Threshold separates the Southern from the Middle Caspian. The Northern Caspian is a shallow region with an average depth of between 5 and 6 meters. It carries less than 1% of the sea’s volume. The Middle Caspian is about 190 meters deep, while the Southern Caspian is the deepest region, about 1,000 meters deep. The Southern region accounts for 66%, while the Middle region accounts for 33% of the total water volume.
Is The Caspian Sea A Lake?
Going by the definition of a sea, the Caspian Sea is a lake and not a sea as it is an enclosed water body without any direct outlet to the ocean. However, 5.5 million years ago, it was part of the ancient Parathethys Sea and got landlocked as a result of tectonic uplift and sea-level fall. Hence, the seafloor of the Caspian Sea is composed of oceanic basalt and not continental granite. The Caspian Sea is also massive and forms waves like the sea along its shores. The composition of the water of the Caspian Sea also varies from almost fresh in the northern parts of the lake to saltier southwards. The mean salinity of the Caspian Sea is now about one-third that of the oceans.
Is The Caspian Sea A Freshwater Or Saline Water Body?
Although most sources consider the Caspian Sea the world’s largest lake, it is not the largest freshwater lake. It can be classified as a brackish water body. The water in the Caspian Sea is neither completely freshwater nor completely saline water. The water is 1.2% saline. The Caspian’s mean salinity is about one-third the salinity of the oceans.
About 5.5 million years ago, the Caspian Sea was part of the Tethys Ocean but was completely cut off from the ocean due to plate tectonic. The sea became landlocked, without any outflow. Therefore, any water that gets into the sea, is either absorbed into the ground or evaporates, leading to the water becoming saline.
The Caspian Sea is not uniformly saline. The Iranian shore is more saline than the sea’s northern portion. The Caspian’s northern portion contains considerably freshwater because of the inflow of freshwater. Over 100 rivers empty into the Caspian Sea, including the Volga River, Europe’s largest river. Other rivers that flow into the Caspian are Amu Darya, Kura, and Ural Rivers. Most of these rivers contain fresh water and flow into Caspian from the north, making the sea’s northern portion less saline.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline