Math Is Fun Forum
You are not logged in.
- Topics: Active | Unanswered
Pages: 1
#1 Yesterday 18:19:50
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,979
Chemical Equilibrium
Chemical Equilibrium
Gist
Chemical equilibrium is the state in a reversible reaction where the rates of the forward and backward reactions are equal, resulting in constant concentrations of reactants and products over time. It is a dynamic process in a closed system where no net change occurs. The equilibrium constant quantifies the ratio of products to reactants.
Chemical equilibrium is the state in a reversible reaction where the rate of the forward reaction (reactants to products) equals the rate of the reverse reaction (products to reactants), resulting in constant, unchanging concentrations of both reactants and products over time, even though individual molecules are still reacting. It's a dynamic process, meaning reactions haven't stopped but are balanced, and is typically observed in a closed system, often shown with a double arrow in chemical equations.
Summary
In a chemical reaction, chemical equilibrium is the state in which both the reactants and products are present in concentrations which have no further tendency to change with time, so that there is no observable change in the properties of the system. This state results when the forward reaction proceeds at the same rate as the reverse reaction. The reaction rates of the forward and backward reactions are generally not zero, but they are equal. Thus, there are no net changes in the concentrations of the reactants and products. Such a state is known as dynamic equilibrium. It is the subject of study of equilibrium chemistry.
Historical introduction
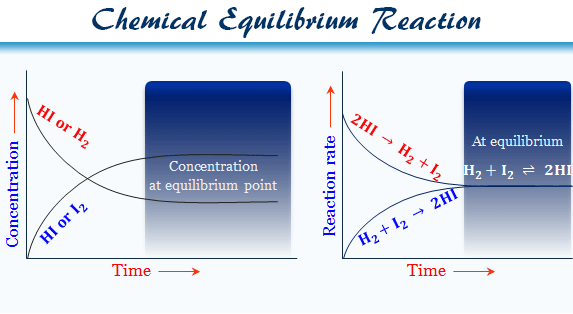
The concept of chemical equilibrium was developed in 1803, after Berthollet found that some chemical reactions are reversible. For any reaction mixture to exist at equilibrium, the rates of the forward and backward (reverse) reactions must be equal. In the following chemical equation, arrows point both ways to indicate equilibrium.
Details
Chemical equilibrium is the condition in the course of a reversible chemical reaction in which no net change in the amounts of reactants and products occurs. A reversible chemical reaction is one in which the products, as soon as they are formed, react to produce the original reactants. At equilibrium, the two opposing reactions go on at equal rates, or velocities, and hence there is no net change in the amounts of substances involved. At this point the reaction may be considered to be completed; i.e., for some specified reaction condition, the maximum conversion of reactants to products has been attained.
Chemical equilibrium can be considered analogous to equilibrium in physical systems. For example, water in an insulated container with a temperature at the freezing point exists in both liquid and solid forms. The mass of the water does not change, but nevertheless there is physical activity with liquid water freezing onto the ice and ice melting back into liquid, with both processes occuring at the same time and at the same rate so the proportion of ice to water does not change.
The value of the equilibrium constant varies with the molar concentration, temperature, and pressure according to the principle of Le Chatelier, which states that any change in those conditions will change the equilibrium in such a way to counteract the effect of the change. For example, if the concentration of a reactant is increased, the reaction will proceed to convert the extra reactant into product until equilibrium is reached. If pressure increases, the forward reaction happens faster until a new equilibrium is reached. For increases in temperature, for an exothermic reaction (which releases heat), the equilibrium constant decreases, and the equilibrium constant increases for a endothermic reaction (which absorbs heat).
When the product and reactants are in the same phase (e.g, gas, liquid), the equilibrium is a homogeneous equilibrium. When the reactants are in different phase, such as salt (solid) dissolving in water (liquid), the equilibrium is a heterogenous equilibrium.
Solubility and equilibrium
The solubility product constant, Ksp, specifically describes the equilibrium between a solid ionic compound and its dissociated ions in a solution. For example for salt dissolving in water, the equilibrium constant Ksp does not include the concentration of the water, since water is not a product or a reactant, and thus the equilibrium constant is the concentrations of the sodium and chlorine ions divided by the concentration of the salt.
The solubility of an ionic compound is determined by various factors, including the common ion effect, which is a direct application of Le Chatelier’s principle. This principle relates to ionic equilibrium by explaining how changes in concentration, temperature, or pressure affect the equilibrium of reactions involving ions. The common ion effect occurs when an ion that is already present in a solution is added to the solution; the effect reduces the solubility of a weak electrolyte or suppresses the ionization of a weak acid or base.
For systems that are not in equilibrium, one can use the reaction quotient Q, which is calculated in the same way as the equilibrium constant K. If Q is greater than K, the reverse reaction happens, favoring the reactants until equilibrium is reached. If Q is greater than K, the forward reaction happens, favoring the products until equilibrium is reached.
Additional Information
Chemical equilibrium refers to the final mixture of a chemical reaction, where the reactants and products are done changing. In a chemical reaction, reactants are converted into products. A general belief is that all chemical reactions proceed to completion (where all reactants are converted into products). But this is not true in all cases. A lot of chemical reactions proceed only to a certain extent, i.e. the reactants are not fully converted into products and the resulting mixture contains both reactants and products. After some time, the concentration of reactants or products becomes constant and we get a state of equilibrium for the system.
Irreversible reaction
A chemical reaction which proceeds only in the forward direction so that the reactants are converted into products and products do not react with each other to reform reactants is called an irreversible reaction.
Reversible reaction
A chemical reaction in which reactants react together to form products and products formed react with each other directly to reform the original reactant back is known as a reversible reaction.
Characteristics of Chemical Equilibrium
These are some of the characteristics of chemical equilibrium:
* The chemical reaction should be reversible.
* The equilibrium can be attained only if the system is closed, i.e. the reaction should be carried out in a closed vessel.
* The opposing processes (i.e. forward and backward reactions) occur at the same rate and there is a dynamic but stable condition.
* The observable properties of the system such as concentration, pressure, color, etc. becomes constant at equilibrium and remains unchanged thereafter.
* By bringing a change in conditions such as temperature, pressure or concentration, the equilibrium point can be shifted to the right or left hand side as required. Thus the reaction can be controlled to get more yield of products.
* The equilibrium can be approached from either direction.
* A catalyst does not alter the equilibrium point. It only increases the rate of reaction. The equilibrium is attained however.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
Pages: 1