Math Is Fun Forum
You are not logged in.
- Topics: Active | Unanswered
#1 Re: Dark Discussions at Cafe Infinity » crème de la crème » Yesterday 18:33:05
2374) Alfred Hershey
Gist:
Work
Bacteriophages are viruses that attach themselves to bacteria, emptying their genetic material into them, which leads to the rapid spawning of new phage inside the bacteria. By applying genetic concept and developing statistical approaches in their studies of bacteriophages, Max Delbrück, Salvador Luria, and Alfred Hershey were able to shed new light on a range of unanswered questions within genetics. For example, in 1952 Hershey and Martha Chase were able to demonstrate that DNA was transferred from bacteriophages to bacteria, a discovery that confirmed DNA as the bearer of genetic information.
Summary
A.D. Hershey (born Dec. 4, 1908, Owosso, Mich., U.S.—died May 22, 1997, Syosset, N.Y.) was an American biologist who, along with Max Delbrück and Salvador Luria, won the Nobel Prize for Physiology or Medicine in 1969. The prize was given for research done on bacteriophages (viruses that infect bacteria).
Hershey earned a doctorate in chemistry from Michigan State College (now Michigan State University) in 1934 and then took a position at Washington University School of Medicine in St. Louis, Mo. He joined the staff of the Genetics Research Unit of the Carnegie Institution of Washington in 1950 after giving up his position as professor at Washington University. In 1963 he became director of the Genetics Research Unit.
Hershey, Delbrück, and Luria began exchanging information on phage research in the early 1940s. In 1945 Hershey and Luria, working independently, demonstrated the occurrence of spontaneous mutation in both the bacteriophages and the host. The next year, Hershey and Delbrück independently discovered the occurrence of genetic recombination in phages—i.e., that different strains of phages inhabiting the same bacterial cell can exchange or combine genetic material. Delbrück incorrectly interpreted his results as specifically induced mutations, but Hershey and one of his students proved that the results they had obtained were recombinations by showing that the genetic processes in question correspond with the crossing-over of parts of similar chromosomes observed in cells of higher organisms.
Hershey is most noted for the so-called blender experiment that he performed with Martha Chase in 1952. By showing that phage DNA is the principal component entering the host cell during infection, Hershey proved that DNA, rather than protein, is the genetic material of the phage.
Details
Alfred Day Hershey (December 4, 1908 – May 22, 1997) was an American Nobel Prize–winning bacteriologist and geneticist.
Early years
Hershey was born in Owosso, Michigan to Robert Day and Alma Wilbur Hershey. He earned a B.S. in chemistry in 1930, and Ph.D. in bacteriology in 1934 from Michigan State University. Shortly after, Hershey accepted a faculty position at Washington University in St. Louis, serving as an instructor of bacteriology and immunology from 1934 to 1950.
Bacteriophage research
At Washington University, Hershey worked closely with department head Jacques Bronfenbrenner to investigate bacteriophages, or phages—viruses that infect and replicate inside bacteria. Hershey's work on the factors impacting the virus' ability to infect its targets brought him to the attention of fellow phage researchers Max Delbrück and Salvador Luria.
The Phage Group
In 1943, Delbrück invited Hershey to Vanderbilt University to discuss his phage research. Together, with Luria, they would form the core of an informal network of researchers called "the Phage group". Three years later, Hershey and Delbrück would independently discover that different strains of bacteriophage can both exchange genetic material when infecting the same bacterial cell. This process results in hybrid phages containing genetic material from both sources, which Hershey referred to as "genetic recombination".
Hershey left Washington University in 1950 for the Department of Genetics of the Carnegie Institution of Washington, a predecessor of Cold Spring Harbor Laboratory. Two years later, he and Martha Chase would conduct the famous Hershey–Chase, or "Waring Blender" experiment. Their work confirmed that DNA, not protein, was the genetic material of life.
Later years and death
In 1962, Hershey was named director of the Department of Genetics, a position he held until his retirement in 1970. He would live on the grounds of Cold Spring Harbor Laboratory (CSHL) for the rest of his life.
Hershey's work with bacteriophage would earn him a share of the 1969 Nobel Prize in Physiology or Medicine with Delbrück and Luria, "for their discoveries concerning the replication mechanism and the genetic structure of viruses."
Although officially retired from scientific research, Hershey would continue to pursue new projects. In 1971, he edited The Bacteriophage λ, an extensive volume on the subject, published by CSHL Press that same year. In 1981, Hershey became a founding member of the World Cultural Council.
Hershey died from congestive heart failure on May 22, 1997 at his home in Laurel Hollow, New York. He was 88 years old. At the time, he was survived by his wife Harriet Davidson (1918–2000) and their only child, Peter Manning Hershey (1956–1999).
Following his death, Frank Stahl, a member of The Phage Group, wrote: "The Phage Church, as we were sometimes called Phage group, was led by the Trinity of Delbrück, Luria, and Hershey. Delbrück's status as founder and his ex cathedra manner made him the pope, of course, and Luria was the hard-working, socially sensitive priest-confessor. And Al (Hershey) was the saint."

#2 Re: This is Cool » Miscellany » Yesterday 18:03:08
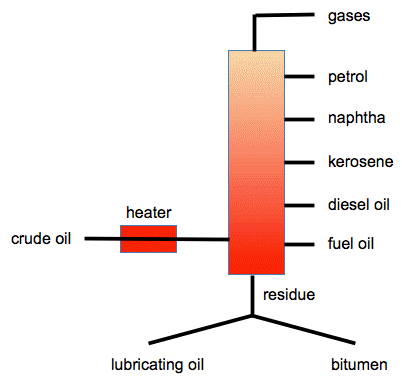
2426) Naphtha
Gist
Naphtha is a flammable liquid mixture of hydrocarbons, primarily derived from crude oil distillation, that serves as a crucial feedstock and fuel in various industries. It is used to produce high-octane gasoline, petrochemicals for plastics, fertilizers, and is also used as a solvent and for fuels like lighter fluid or camp stoves. Due to its flammability, it must be handled with care, and its vapor is heavier than air and can travel to an ignition source.
The main uses of crude oil naphtha fall into the general areas of (i) precursor to gasoline and other liquid fuels, (ii) solvents or diluents for paints, (iii) dry-cleaning solvents, (iv) solvents for cutback asphalt, (v) solvents in rubber industry, and (vi) solvents for industrial extraction processes.
Summary
Naphtha is any of various volatile, highly flammable liquid hydrocarbon mixtures used chiefly as solvents and diluents and as raw materials for conversion to gasoline. Naphtha was the name originally applied to the more volatile kinds of petroleum issuing from the ground in the Baku district of Azerbaijan and Iran. As early as the 1st century ad, naphtha was mentioned by the Greek writer Dioscorides and the Roman writer Pliny the Elder. Alchemists used the word principally to distinguish various mobile liquids of low boiling point, including certain ethers and esters.
In modern usage the word naphtha is usually accompanied by a distinctive prefix. Coal-tar naphtha is a volatile commercial product obtained by the distillation of coal tar. Shale naphtha is obtained by the distillation of oil produced from bituminous shale by destructive distillation. Petroleum naphtha is a name used primarily in the United States for petroleum distillate containing principally aliphatic hydrocarbons and boiling higher than gasoline and lower than kerosene.
Details
Naphtha is a flammable liquid hydrocarbon mixture. Generally, it is a fraction of crude oil, but it can also be produced from natural-gas condensates, petroleum distillates, and the fractional distillation of coal tar and peat. In some industries and regions, the name naphtha refers to crude oil or refined petroleum products such as kerosene or diesel fuel.
Naphtha is also known as Shellite in Australia.
Modern period
Since the 19th century, solvent naphtha has denoted a product (xylene or trimethylbenzenes) derived by fractional distillation from petroleum; these mineral spirits, also known as "Stoddard Solvent," were originally the main active ingredient in Fels Naptha laundry soap. The naphtha in Fels Naptha was later removed as a cancer risk.
The usage of the term "naphtha" during this time typically implies petroleum naphtha, a colorless liquid with a similar odor to gasoline. However, "coal tar naphtha," a reddish brown liquid that is a mixture of hydrocarbons (toluene, xylene, and cumene, etc.), could also be intended in some contexts.
Petroleum
In older usage, "naphtha" simply meant crude oil, but this usage is now obsolete in English. There are a number of cognates to the word in different modern languages, typically signifying "petroleum" or "crude oil."
The Ukrainian & Belarusian word (nafta), Lithuanian, Latvian, & Estonian "nafta," and the Persian naft mean "crude oil." The Russian word (neft') means "crude oil," but (nafta) is a synonym of ligroin. Also, in Albania, Bosnia and Herzegovina, Bulgaria, Croatia, Finland, Italy, Serbia, Slovenia, and Macedonia nafta (нафта in Cyrillic) is colloquially used to indicate diesel fuel and crude oil. In the Czech Republic and Slovakia, nafta was historically used for both diesel fuel and crude oil, but its use for crude oil is now obsolete and it generally indicates diesel fuel. In Bulgarian, nafta means diesel fuel, while neft, as well as petrol (петрол in Cyrillic), means crude oil. Nafta is also used in everyday parlance in Argentina, Uruguay and Paraguay to refer to gasoline/petrol. Similarly, in Flemish, the word naft(e) is used colloquially for gasoline. In Poland, the word nafta means kerosene, and colloquially crude oil (the technical name for crude oil is ropa naftowa, also colloquially used for diesel fuel as ropa).
Types
Naphtha has been divided into two types by many sources in order to differentiate between common grades more clearly:
One source distinguishes by boiling point as well as carbon atom count per molecule:
* Light naphtha is the fraction boiling between 30 and 90 °C (86 and 194 °F) and consists of molecules with 5–6 carbon atoms.
* Heavy naphtha boils between 90 and 200 °C (194 and 392 °F) and consists of molecules with 6–12 carbon atoms.
Chemistry of Hazardous Materials differentiates light and heavy based on the carbon atom count and hydrocarbon structure:
* Light [is] a mixture consisting mainly of straight-chained and cyclic aliphatic hydrocarbons having from five to six carbon atoms per molecule.
* Heavy [is] a mixture consisting mainly of straight-chained and cyclic aliphatic hydrocarbons having from seven to nine carbon atoms per molecule.
Some sources also define petroleum naphtha, which contains both heavy and light naphtha, and typically consists of 15-30% of crude oil by weight.
Uses:
Heavy crude oil dilution
Naphtha is used to dilute heavy crude oil to reduce its viscosity and enable/facilitate transport; undiluted heavy crude cannot normally be transported by pipeline, and may also be difficult to pump onto oil tankers. Other common dilutants include natural-gas condensate and light crude. However, naphtha is a particularly efficient dilutant and can be recycled from diluted heavy crude after transport and processing. The importance of oil dilutants has increased as global production of lighter crude oils has fallen and shifted to exploitation of heavier reserves.
Fuel
Light naphtha is used as a fuel in some commercial applications. One notable example is wick-based cigarette lighters, such as the Zippo, which draw "lighter fluid"—naphtha—into a wick from a reservoir to be ignited using the flint and wheel.
It is also a fuel for camping stoves and oil lanterns, known as "white gas", where naphtha's low boiling point makes it easy to ignite. Naphtha is sometimes preferred over kerosene because it clogs fuel lines less. The outdoor equipment manufacturer MSR published a list of trade names and translations to help outdoor enthusiasts obtain the correct products in various countries.
Naphtha was also historically used as both a fuel and a working fluid in some small boats where steam technology was impractical; most were built to circumvent safety laws relating to traditional steam launches.
As an internal combustion engine fuel, petroleum naphtha has seen very little use and suffers from lower efficiency and low octane ratings, typically 40 to 70 RON. It can be used to run unmodified diesel engines, though it has a longer ignition-delay than diesel. Naphtha tends to be noisy in combustion due to the high pressure rise rate. There is a possibility of using naphtha as a low-octane base fuel in an octane-on-demand concept, with the engine drawing a high-octane mix only when needed. Naptha benefits from lesser emissions in refinement: fuel energy losses from "well-to-tank" are 13%; lower than the 22% losses for petroleum.
Plastics
Naphtha is a crucial component in the production of plastics.
Additional Information
Naphtha is a term used to refer to a group of volatile, flammable mixtures of liquid hydrocarbons that are used mainly as solvents, diluents, or raw materials for gasoline conversion. It is a lightweight petrochemical feedstock that is separated from crude oil in the fractional distillation process along with kerosene and jet fuel.
There are many specific types of naphtha that vary in the amounts and types of hydrocarbons contained in their unique blend. Refineries can produce various forms of naphtha, and each has specific guidelines in how it should be handled and stored. Generally speaking, the flammability and volatility of naphtha should be taken into consideration as they are significant safety hazards.
Uses and Safety
As mentioned above, naphtha is commonly used as a solvent. It is used in hydrocarbon cracking, laundry soaps, and cleaning fluids. Naphtha is also used to make varnishes, and sometimes is used as a fuel for camp stoves and as a solvent (diluent) for paint. Although naphtha has many uses, some forms of it can be dangerous. Many kinds of naphtha can cause skin irritation, upset stomachs, and other health problems if people are exposed to them. Some forms are also carcinogens, and thus inhalation or ingestion of the chemical should be avoided.
#3 Dark Discussions at Cafe Infinity » Coach Quotes - IV » Yesterday 16:57:08
- Jai Ganesh
- Replies: 0
Coach Quotes - IV
1. While the coach is entitled to celebrate the team's victories, there is a manner and a way of doing so without aggravating the opponent. - Diego Maradona
2. The strategy of my coach and me was that we looked at pictures of all the best pole vaulters from around the world, and we took the best parts from them, and we created a person that had never existed. We then started to work toward being such a person. - Sergei Bubka
3. I was in the tennis bubble. I wasn't thinking about the big picture. I didn't notice what they said on television, I wasn't reading any papers. I had a coach and a manager, and they kept me in the bubble. - Boris Becker
4. I coach a few guys and they work very, very hard, but in our day we did it because we just loved it. - Linford Christie
5. Puffy produced four of the tracks on the album. Those are the four songs that are collaborations between Puffy and me. And he gives me my space to work even when we work together, like with my producer and my vocal coach. - Jennifer Lopez
6. I don't feel I'm qualified to be a coach outside the high school level. I think I would need to do more education to really be a good coach. - Carl Lewis
7. My former coach, Simen Agdestein, used to be the best player in Norway. - Magnus Carlsen
8. Many tennis coaches are enablers. They need the job more than the player needs the coach, and if the coach needs the job more than the player needs the coach, he can't effect change. - Ivan Lendl.
#4 Jokes » Banana Jokes - II » Yesterday 16:33:38
- Jai Ganesh
- Replies: 0
Q: Why did the banana go out with the prune?
A: Because he couldn't find a date.
* * *
Q: What is the easiest way to make a banana split?
A: Cut it in half.
* * *
Broccoli: I look like a tree.
Walnut: I look like a brain.
Mushroom: I look like an umbrella.
Banana: Dude! Change the topic.
* * *
Q: If a crocodile makes shoes, what does a banana make ?
A: Slippers !
* * *
Q: What do you call solid gold bananas?
A: A bunch of money.
* * *
#5 Science HQ » Bohrium » Yesterday 15:31:04
- Jai Ganesh
- Replies: 0
Bohrium
Gist
Bohrium is a synthetic element, meaning it is not a naturally-occurring element. Several isotopes of Bohrium have been discovered, the most stable of which is bohrium-270, with a half-life of just 61 seconds.
Bohrium is an artificially produced radioactive element. It is probably silvery or metallic gray. It's most stable isotope, Bh-262 has an half life of 17 seconds.
Summary
Bohrium is a synthetic chemical element; it has symbol Bh and atomic number 107. It is named after Danish physicist Niels Bohr. As a synthetic element, it can be created in particle accelerators but is not found in nature. All known isotopes of bohrium are highly radioactive; the most stable known isotope is 270Bh with a half-life of approximately 2.4 minutes, though the unconfirmed 278Bh may have a longer half-life of about 11.5 minutes.
In the periodic table, it is a d-block transactinide element. It is a member of the 7th period and belongs to the group 7 elements as the fifth member of the 6d series of transition metals. Chemistry experiments have confirmed that bohrium behaves as the heavier homologue to rhenium in group 7. The chemical properties of bohrium are characterized only partly, but they compare well with the chemistry of the other group 7 elements.
Details
Bohrium (Bh) is a synthetic element in Group VIIb of the periodic table. It is thought to be chemically similar to the rare metal rhenium.
In 1976 Soviet scientists at the Joint Institute for Nuclear Research in Dubna, Russia, U.S.S.R., announced that they had synthesized element 107, later given the official name bohrium, by bombarding a target of bismuth-209 with ions of chromium-54. The resultant collisions were reported to have produced an isotope of the element with a mass number of 261 and a half-life of 1–2 milliseconds. The existence of the element was confirmed by West German physicists at the Institute for Heavy Ion Research (Gesellschaft für Schwerionenforschung [GSI]) in Darmstadt.
Additional Information:
Appearance
Bohrium is a highly radioactive metal.
Uses
At present, bohrium is of research interest only.
Biological role
Bohrium has no known biological role.
Natural abundance
Bohrium does not occur naturally and only a few atoms have ever been made. It will probably never be isolated in observable quantities. It was created by the so-called ‘cold fusion’ method. This involved the bombardment of bismuth with atoms of chromium.

#6 Re: Jai Ganesh's Puzzles » General Quiz » Yesterday 15:00:48
Hi,
#10629. What does the term in Geography Central business district mean?
#10630. What does the term in Geography Centroid mean?
#7 Re: Jai Ganesh's Puzzles » English language puzzles » Yesterday 14:41:40
Hi,
#5825. What does the adjective lekker mean?
#5826. What does the noun lemming mean?
#8 Re: Jai Ganesh's Puzzles » Doc, Doc! » Yesterday 14:26:15
Hi,
#2506. What does the medical term Ileocecal valve mean?
#9 Re: Jai Ganesh's Puzzles » 10 second questions » Yesterday 14:13:17
Hi,
#9775.
#10 Re: Jai Ganesh's Puzzles » Oral puzzles » Yesterday 13:55:14
Hi,
#6280.
#11 Re: Exercises » Compute the solution: » Yesterday 13:20:15
Hi,
2626.
#12 This is Cool » Fossil » 2025-10-24 21:48:59
- Jai Ganesh
- Replies: 0
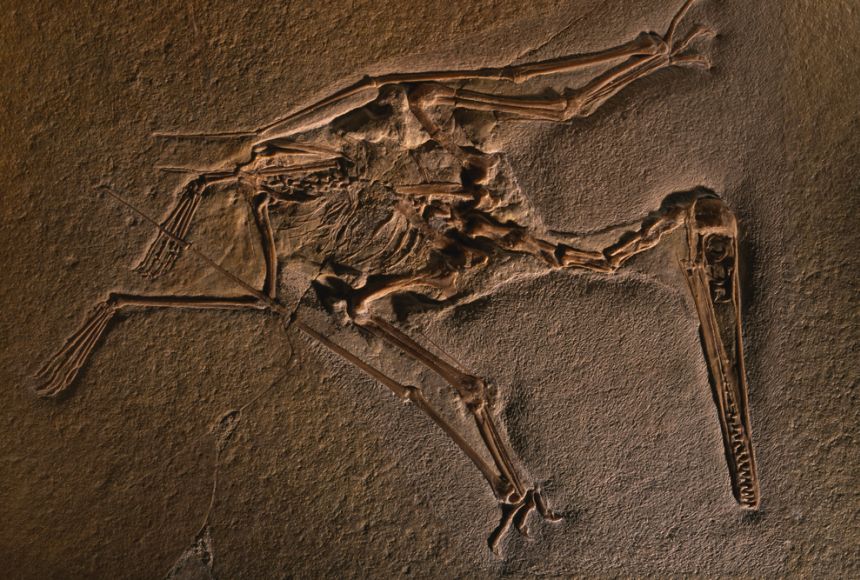
Fossil
Gist
Fossils are the preserved remains of plants and animals whose bodies were buried in sediments, such as sand and mud, under ancient seas, lakes and rivers. Fossils also include any preserved trace of life that is typically more than 10 000 years old.
Fossils are the preserved remains or traces of once-living organisms, typically from more than 10,000 years ago, such as a dinosaur bone or an ammonite shell. They can include hard parts like bones and shells, or even evidence of an organism's activity, like footprints, and are formed through natural processes like being buried in sediment and mineralized over time.
Body fossils are the remains of past animals, plants, and microorganisms. Body fossils include bones, teeth, skin, shells, wood, and leaves. Molds, casts, steinkerns, and impressions are also body fossils since they result from marks made by the remains of organizations.
Summary
A fossil (from Classical Latin fossilis, lit. 'obtained by digging') is any preserved remains, impression, or trace of any once-living thing from a past geological age. Examples include bones, shells, exoskeletons, stone imprints of animals or microbes, objects preserved in amber, hair, petrified wood and DNA remnants. The totality of fossils is known as the fossil record. Though the fossil record is incomplete, numerous studies have demonstrated that there is enough information available to give a good understanding of the pattern of diversification of life on Earth. In addition, the record can predict and fill gaps such as the discovery of Tiktaalik in the arctic of Canada.
Paleontology includes the study of fossils: their age, method of formation, and evolutionary significance. Specimens are sometimes considered to be fossils if they are over 10,000 years old. The oldest fossils are around 3.48 billion years to 4.1 billion years old. The observation in the 19th century that certain fossils were associated with certain rock strata led to the recognition of a geological timescale and the relative ages of different fossils. The development of radiometric dating techniques in the early 20th century allowed scientists to quantitatively measure the absolute ages of rocks and the fossils they host.
There are many processes that lead to fossilization, including permineralization, casts and molds, authigenic mineralization, replacement and recrystallization, adpression, carbonization, and bioimmuration.
Fossils vary in size from one-micrometre (1 μm) bacteria to dinosaurs and trees, many meters long and weighing many tons. The largest presently known is a Sequoia sp. measuring 88 m (289 ft) in length at Coaldale, Nevada. A fossil normally preserves only a portion of the deceased organism, usually that portion that was partially mineralized during life, such as the bones and teeth of vertebrates, or the chitinous or calcareous exoskeletons of invertebrates. Fossils may also consist of the marks left behind by the organism while it was alive, such as animal tracks or feces (coprolites). These types of fossil are called trace fossils or ichnofossils, as opposed to body fossils. Some fossils are biochemical and are called chemofossils or biosignatures.
Details
A fossil is any remnant, impression, or trace of an animal or plant of a past geologic age that has been preserved in Earth’s crust. The complex of data recorded in fossils worldwide—known as the fossil record—is the primary source of information about the history of life on Earth.
Only a small fraction of ancient organisms are preserved as fossils, and usually only organisms that have a solid and resistant skeleton are readily preserved. Most major groups of invertebrate animals have a calcareous skeleton or shell (e.g., corals, mollusks, brachiopods, bryozoans). Other forms have shells of calcium phosphate (which also occurs in the bones of vertebrates), or silicon dioxide. A shell or bone that is buried quickly after deposition may retain these organic tissues, though they become petrified (converted to a stony substance) over time. Unaltered hard parts, such as the shells of clams or brachiopods, are relatively common in sedimentary rocks, some of great age.
The hard parts of organisms that become buried in sediment may be subject to a variety of other changes during their conversion to solid rock, however. Solutions may fill the interstices, or pores, of the shell or bone with calcium carbonate or other mineral salts and thus fossilize the remains, in a process known as permineralization. In other cases there may be a total replacement of the original skeletal material by other mineral matter, a process known as mineralization, or replacement. In still other cases, circulating acid solutions may dissolve the original shell but leave a cavity corresponding to it, and circulating calcareous or siliceous solutions may then deposit a new matrix in the cavity, thus creating a new impression of the original shell.
By contrast, the soft parts of animals or plants are very rarely preserved. The embedding of insects in amber (a process called resin fossilization) and the preservation of the carcasses of Pleistocene mammoths in ice are rare but striking examples of the fossil preservation of soft tissues. Leaves, stems, and other vegetable matter may be preserved through the process of carbonization, where such parts are flattened between two layers of rock. The chemical reduction of the part produces a carbon film that occurs on one layer of rock, while an impression of that part occurs on the other layer of the rock.
Fossils of hard and soft parts that are too small to be observed by the naked eye are called microfossils. Some fossils are completely devoid of plant and animal parts but show evidence of an organism’s activities. Such traces of organisms, which are appropriately known as “trace fossils,” include tracks or trails, preserved waste products, and borings.
The great majority of fossils are preserved in a water environment because land remains are more easily destroyed. Anaerobic conditions at the bottom of the seas or other bodies of water are especially favourable for preserving fine details, since no bottom faunas, except for anaerobic bacteria, are present to destroy the remains. In general, for an organism to be preserved two conditions must be met: rapid burial to retard decomposition and to prevent the ravaging of scavengers; and possession of hard parts capable of being fossilized.
In some places, such as the Grand Canyon in northern Arizona, one can observe a great thickness of nearly horizontal strata representing the deposition of sediment on the seafloor over many hundreds of millions of years. It is often apparent that each layer in such a sequence contains fossils that are distinct from those of the layers that are above and below it. In such sequences of layers in different geographic locations, the same, or similar, fossil floras or faunas occur in the identical order. By comparing overlapping sequences, it is possible to build up a continuous record of faunas and floras that have progressively more in common with present-day life forms as the top of the sequence is approached.
The study of the fossil record has provided important information for at least four different purposes. The progressive changes observed within an animal group are used to describe the evolution of that group. Fossils also provide the geologist a quick and easy way of assigning a relative age to the strata in which they occur. The precision with which this may be done in any particular case depends on the nature and abundance of the fauna: some fossil groups were deposited during much longer time intervals than others. Fossils used to identify geologic relationships are known as index fossils.
Fossil organisms may provide information about the climate and environment of the site where they were deposited and preserved (e.g., certain species of coral require warm, shallow water, or certain forms of deciduous angiosperms can only grow in colder climatic conditions).
Fossils are useful in the exploration for minerals and mineral fuels. For example, they serve to indicate the stratigraphic position of coal seams. In recent years, geologists have been able to study the subsurface stratigraphy of oil and natural gas deposits by analyzing microfossils obtained from core samples of deep borings.
Fossil collection as performed by paleontologists, geologists, and other scientists typically involves a rigorous excavation and documentation process. Unearthing the specimen from the rock is often painstaking work that includes labeling each part of the specimen and cataloging the location of each part within the rock. Those fossils slated for removal from the rock are slowly and carefully excavated using techniques designed to prevent or minimize damage to the specimen. Such fossils often become part of museum or university collections.
Many other fossils, however, are collected by hobbyists and commercial entities. Often such specimens are not carefully documented or excavated, resulting in a loss of data from the site and risking potential damage to the specimen. For these reasons and the fact that it stimulates nonscientific collecting, the commercial exploitation of fossils is controversial among academic paleontologists.
Additional Information
Fossils are the preserved remains, or traces of remains, of ancient organisms.
A fossil can preserve an entire organism, just part, or traces of one (for example, footprints). Bones, shells, fur, skin, footprints, feathers and leaves can all become fossils.
Fossils can be very large or very small. The smallest fossils are called microfossils and are only visible with a microscope. Pollen fossils are microfossils. Fossils you can see with your eyes are called macrofossils and can be several meters long and weigh several tons. An example of a macrofossil could be a petrified tree or a dinosaur bone.
Preserved remains are defined as fossils if they are older than 10,000 years old. The oldest fossils are almost 4 billion years old and are traces of ocean-dwelling bacteria. Some of the youngest fossils (10 000 years old) are, for example, the fossilized teeth of woolly mammoths.
Fossilization
The word fossil comes from the Latin word fossus, meaning "having been dug up." Fossils are often found in rock formations, which although formed deep underground, have been moved to the surface (by plate tectonics) and/or been eroded to the Earth’s surface by wind, ice, rain and water.
Fossilization is the process of remains becoming fossils. Fossilization is rare. Most organisms decompose fairly quickly after they die.
For an organism to be fossilized, the remains usually need to be covered by sediment soon after death. Sediment can include the sandy or muddy seafloor, volcanic ash, and even sticky tar.
Over time, minerals in the sediment seep into the remains. The remains become fossilized. Fossilization more commonly occurs in organisms with hard, bony body parts, such as skeletons, teeth, or shells. Soft-bodied organisms, such as worms, are rarely fossilized, because soft parts quickly decompose or are eaten.
Sometimes, however, the sticky resin of a tree can become fossilized. This is called fossilized resin or amber. Amber can preserve the bodies of many delicate, soft-bodied organisms, such as ants, flies, and mosquitoes. While fossils found in rocks made of a soft sea floor are usually compressed, amber preserves the fossils in three dimensions.
Body Fossils and Trace Fossils
The fossils of bones, teeth, and shells are called body fossils. Most dinosaur fossils are collections of body fossils.
Trace fossils are rocks that have preserved evidence of biological activity. They are not fossilized remains, just the traces of organisms. The imprint of an ancient leaf or footprint is a trace fossil. Burrows can also create impressions in soft rocks or mud, leaving a trace fossil.
Paleontologists
Paleontologists are people who study fossils. Paleontologists find and study fossils all over the world, in almost every environment, from the hot desert to the humid jungle. Studying fossils helps them learn about when and how different species lived millions of years ago. Sometimes, fossils tell scientists how the Earth has changed. Fossils also help scientists study how species have evolved over the course of millions of years.
Fossils of ancient marine animals called ammonites have been unearthed in the highest mountain range in the world, the Himalayas in Nepal. This tells scientists that millions of years ago, the rocks that became the Himalayas were at the bottom of the ocean.
Fossils of an ancient giant shark, a megalodon (Carcharocles megalodon), have been found in the landlocked U.S. state of Utah. This tells scientists that millions of years ago, the middle of North America was probably entirely underwater when this animal lived.
#13 Re: Dark Discussions at Cafe Infinity » crème de la crème » 2025-10-24 17:20:34
2373) Max Delbrück
Gist:
Work
Bacteriophages are viruses that attach themselves to bacteria, emptying their genetic material into them, which leads to the rapid spawning of new phage inside the bacteria. By applying genetic concept and developing statistical approaches in their studies of bacteriophages, Max Delbrück, Salvador Luria, and Alfred Hershey were able to shed new light on a range of unanswered questions within genetics. For example, in 1943 Delbrück and Luria proved through statistical investigations that bacteria, like more complex organisms, develop via mutations.
Summary
Max Ludwig Henning Delbrück (September 4, 1906 – March 9, 1981) was a German–American biophysicist who participated in launching the molecular biology research program in the late 1930s. He stimulated physical scientists' interest into biology, especially as to basic research to physically explain genes, mysterious at the time. Formed in 1945 and led by Delbrück along with Salvador Luria and Alfred Hershey, the Phage Group made substantial headway unraveling important aspects of genetics. The three shared the 1969 Nobel Prize in Physiology or Medicine "for their discoveries concerning the replication mechanism and the genetic structure of viruses". He was the first physicist to predict what is now called Delbrück scattering.
Details
Max Delbrück (born Sept. 4, 1906, Berlin, Ger.—died March 9, 1981, Pasadena, Calif., U.S.) was a German-born U.S. biologist, a pioneer in the study of molecular genetics. With Alfred Day Hershey and Salvador Luria, he was awarded the 1969 Nobel Prize for Physiology or Medicine for work on bacteriophages—viruses that infect bacteria.
Delbrück received a Ph.D. in physics (1930) from the University of Göttingen. His interest in bacteriophages was aroused while he was a research assistant at the Kaiser Wilhelm Institute for Chemistry in Berlin (1932–37). A refugee from Nazi Germany, Delbrück went to the United States in 1937, serving as a faculty member of the California Institute of Technology (1937–39; 1947–81) and of Vanderbilt University (1940–47). He became a U.S. citizen in 1945.
In 1939 Delbrück discovered a one-step process for growing bacteriophages that, after a one-hour latent period, would multiply to produce several hundred thousands of progeny. Delbrück soon began to collaborate with Luria, and in 1943 they announced their discovery that a bacterium that has been infected by a bacteriophage can undergo spontaneous mutations so that it becomes immune to the phage. In 1946 Delbrück and Hershey independently discovered that the genetic material of different kinds of viruses can combine to create new types of viruses. This process was previously believed to be limited to higher, sexually reproducing forms of life.

#14 Re: This is Cool » Miscellany » 2025-10-24 16:52:19
2425) Gemology
Gist
Gemology is the science of studying and identifying natural and artificial gemstones. A gemologist examines gemstones for their beauty, durability, and rarity by studying their composition, physical and optical properties, origin, and treatments. This field involves techniques like microscopic inspection, hardness tests, and the use of specialized tools like refractometers to identify and grade gems for purposes in the jewelry, astrological, and industrial sectors.
Summary
Gemology or gemmology is the science dealing with natural and artificial gemstone materials. It is a specific interdisciplinary branch of mineralogy. Some jewelers (and many non-jewelers) are academically trained gemologists and are qualified to identify and evaluate gems.
Background
It is often difficult to obtain an expert judgement from a neutral laboratory. Analysis and estimation in the gemstone trade usually have to take place on site. Professional gemologists and gemstone buyers use mobile laboratories, which pool all necessary instruments in a travel case. Such so-called travel labs even have their own current supply, which makes them independent from infrastructure. They are also suitable for gemological expeditions.
Gemstones are basically categorized based on their crystal structure, specific gravity, refractive index, and other optical properties, such as pleochroism. The physical property of "hardness" is defined by the irregular Mohs scale of mineral hardness.
Gemologists study these factors while valuing or appraising cut and polished gemstones. Gemological microscopic study of the internal structure is used to determine whether a gem is synthetic or natural by revealing natural fluid inclusions or partially melted exogenous crystals that are evidence of heat treatment to enhance color.
The spectroscopic analysis of cut gemstones also allows a gemologist to understand the atomic structure and identify its origin, which is a major factor in valuing a gemstone. For example, a ruby from Myanmar (Burma) will have definite internal and optical activity variance from a Thai ruby.
When the gemstones are in a rough state, the gemologist studies the external structure; the host rock and mineral association; and natural and polished color. Initially, the stone is identified by its color, refractive index, optical character, specific gravity, and examination of internal characteristics under magnification.
Details
Gemology is the science of studying, cutting, and valuing precious stones, but the essence of gemology is in identifying the gemstones. One who works in the field of gemology is called a gemologist, and jewelers and goldsmiths also may be gemologists.
Some collectors and investors may be interested only in gems' monetary value, but to distinguish one gemstone from another, they will need to seek out a gemologist. Gemologists examine gemstones—both discovered raw and synthesized in the laboratory—using microscopes, computerized tools, and other grading instruments.
Key Takeaways
* Gemology is the science of identifying gemstones.
* Gemologists examine gemstones—both discovered raw and synthesized in the laboratory—using microscopes, computerized tools, and other grading instruments.
* The field of gemology contains professionals such as appraisers, goldsmiths, jewelers, lapidaries, and scientists.
* Investing in gemstones may be risky, but the precious metals sector can be less speculative for inexperienced investors.
* Unlike other types of investments, gemstones may not be as easily liquidated if you have an urgent need for cash.
Understanding Gemology
At its heart, gemology is about identifying gems. Gemologists identify a gemstone by its specific characteristics and properties, such as cut, color, quality, and clarity. Some rubies and garnets, for example, are impossible to distinguish by their appearance, but their underlying physical properties differ considerably. Many people are familiar with a group of criteria that is used in gemology to identify diamonds—the 4Cs of color, clarity, cut, and carat.
Gemology and Its Professionals
In addition to gemologists, the field of gemology contains numerous other professionals, including appraisers, jewelers, lapidaries, metalworkers, and scientists.
Gemologists may become certified as professional appraisers, whose expertise is useful in many other industries, including jewelry sales and investing. Jewelers need to understand gemology to answer their customers’ questions and identify any gems brought to them. Goldsmiths and other metalworkers need specific knowledge about the physical characteristics of gems to create appropriate settings. For example, a setting that would be ideal for a diamond could damage an opal, and the amount of pressure used to set the prongs on a garnet could break a stone of tanzanite.
Lapidaries, or gem cutters, also need special knowledge, as appropriate cutting and polishing techniques vary from gem to gem. What would work well for one gemstone would be a waste of time or even disastrous for another gem. Scientists with degrees in geology, chemistry, and even physics make up the smallest group of gemologists, although they are very influential. Scientists add to gemology's knowledge base by developing new testing techniques and researching new gemstones.
Tip
The International Gem Society offers an online Professional Gemologist certification course while The Gemological Institute of America offers a Graduate Gemologist program.
Gemstones as Investments
When returns in the stock market decline, aggressive investors often seek out alternatives that may hold more promise of increasing returns on invested capital (ROIC) than traditional investment types. Or, some investors might want to consider tangible assets simply as a way to diversify their holdings even during good market conditions. Investing in gemstones—in particular, those that are rare or of exceptional quality—likely would at least retain, and probably increase in value.
However, unlike other types of investments, gemstones may not be as easily liquidated if you have an urgent need for cash. This drawback is especially founded for rare, precious stones and jewelry that would appeal to elite buyers only. Gemstone investing can seem exciting to those who want to make quick returns, but it is highly speculative and should only be undertaken by experienced professionals. Investing in the precious metals sector, however, is different because there are standards as well as specific investment vehicles for them in the financial markets.
The term "investment-grade" is often tossed around by those who want to sell gems or try to convince other people to invest in them. However, this practice is frowned upon in financial services because there are no formal standards for what constitutes investment-grade gemstones, as there are for investment-grade bonds, for example.
Careers in Gemology
With advances in gemstone synthesis, gemology has become an important field of study. A credential in gemology can offer numerous career paths:
* Appraiser. Evaluate gemstones, antique and contemporary jewelry, and fine watches. Write detailed descriptions and determine valuation.
* Auction Specialist. Oversee buying and selling during the lively process of auctioning privately owned one-of-a-kind jewelry.
* Bench Jeweler. Manufacture and repair fine jewelry using craftsmanship skills and expert techniques.
* Buyer. Monitor industry and consumer trends and seek out gems and finished jewelry pieces to sell profitably.
* Designer. Create unique jewelry designs using precious gemstones.
* Lab and Research Professional. Investigate new gem finds, treatment processes, and detection methods in the field and laboratory.
* Retailer. A career in the fast-paced environment of retail jewelry sales can be rewarding, exciting, and lucrative.
* Wholesaler. Import and sell diamonds, colored stones, cultured pearls, finished jewelry, and watches from locations around the world.
How to Become a Gemologist
In the field of gemology, there are many career opportunities. Some of the most common include appraisers, retail associates, lab gemologists, and jewelry designers. However, it can be tough to break into these fields; most require at least some formal training. In general, here are the steps you should follow if you want to break into a career in the field of gemology:
Determine What Area of Gemology You Want to Work In
As a professional gemologist, there are a number of different careers you can pursue. Before you can figure out the right educational path, you must first decide what area of gemology you want to pursue. You might consider reading about different career paths or speaking to people that already work in these professions.
Assess Your Skills
Any profession should utilize or enhance your current skills. The most general job requirements for a gemologist are being detail-oriented and having good interpersonal skills, hand-eye coordination, and finger dexterity. In addition, being a good salesperson may make you particularly successful in a retail outlet or as a gemstone wholesaler. If you are creative or have a special interest in fashion, pursuing a career as a gemstone designer might be a good fit. Finally, if you are very meticulous or pay close attention to detail, working as an appraiser or as someone who repairs jewelry might be a good fit for you.
Additional Information
Gemology is the scientific study of gemstones. Although there may be investors and collectors who are only interested in the monetary value of gems, they'll need a scientific approach when the time comes to distinguish one gemstone from another. Whom will they seek out? Gemologists.
Goldsmiths (and other metalworkers) need specific knowledge about the physical characteristics of gems in order to create appropriate settings. For example, a setting that would be ideal for a diamond could damage an opal, and the amount of pressure used to set the prongs on a garnet could break a tanzanite. Some gems can withstand the heat of repair work involving high temperature soldering. If metalworkers take precautions, they can leave them in their settings. Other gemstones are so heat sensitive they would need to remove them.
Lapidaries, or gem cutters, also need special knowledge. Appropriate cutting and polishing techniques vary from gem to gem. What would work well for one gemstone would be a waste of time or even disastrous on another gem. Faceting and gemstone color management go hand in hand. How cutters orient the rough can greatly impact the appearance of the finished gem. Cutting style is also a part of color management.
The choice of cut can lighten or darken a gem, which will considerably affect both the appearance and the value of the stone. The shape, number, and location of facets influence the brilliance of the gem. Lapidaries much choose angles for facet cutting carefully. They must consider all these factors to minimize the amount of gemstone rough sacrificed to create a beautiful faceted gem.
The Scientists
Although scientists with degrees in geology, chemistry, and even physics make up the smallest group of gemologists, they're influential. The systematic measurement and recording of the physical and optical properties used to identify gemstones is a well-established but ongoing scientific process.
For centuries, the lapidary was in the best position to recognize the differences in gems with similar appearances. The faceting process offered a perspective on gemstones no other gemologist had. Many inclusions, materials trapped inside gemstones, and physical characteristics, such as hardness, were readily apparent when cutting and polishing a gem.
Scientists continue to add to this knowledge by developing new testing techniques and researching new gemstones discovered in nature and synthesized in the laboratory.
Gemstone Identification
Gem identification is the heart of gemology. For example, some rubies and garnets are impossible to distinguish by their appearance, but their physical properties differ considerably. Ruby and garnet crystallography varies greatly. While the visible shapes of individual stones may vary, the crystal structures of these gems at the atomic level are distinctive. Garnets form in the isometric or cubic system, while rubies form in the hexagonal system.
Mineralogical techniques are also used to help identify gemstones. Scratch tests, in which various substances are used to scratch an unknown gem, determine hardness. A gem's reaction to acid and even heat can yield important clues to its identity. Of course, these destructive tests aren't appropriate for cut gems.
Scientists have also devised non-destructive tests to identify gemstones. They have designed instruments to measure the physical and optical properties of gems — like specific gravity and refractive index — without damaging them. Today, even people without extensive scientific training or expensive laboratory equipment can use these methods for gem identification.
Getting Started in Gemology
If you're interested in learning about gems, first, learn how they're categorized and the terms used to describe them. Next, study their physical and optical properties. With this background, you can start learning how to identify gemstones.
Of course, there are many side roads to travel as you study gemology. Perhaps you'll become fascinated with phenomenal gems or with inclusions found in natural gems. People interested in gemstone collecting may also become interested in learning how to cut gems and make jewelry.

#15 Dark Discussions at Cafe Infinity » Coach Quotes - III » 2025-10-24 15:47:35
- Jai Ganesh
- Replies: 0
Coach Quotes - III
1. My paternal poppa, Alec, was a taxi driver and swimming coach. He taught all his grandchildren how to swim and loved all kinds of sport. - Ellyse Perry
2. I believe in Coach Louis Wong. He is so much more than just a football coach. - Stephen Covey
3. My coach told me if I broke the national record for the 200, I could run a 100. - Usain Bolt
4. I didn't really care if I had a coach that much, me personally, because I was brought up to think for myself. - Billie Jean King
5. While the coach is entitled to celebrate the team's victories, there is a manner and a way of doing so without aggravating the opponent. - Diego Maradona
6. I was playing cricket first and my cricket coach was the one that introduced me to track and field. - Usain Bolt.
#16 Re: Jai Ganesh's Puzzles » General Quiz » 2025-10-24 15:24:54
Hi,
#10627. What does the term in Biology Ecological efficiency mean?
#10628. What does the term in Biology Ecological pyramid mean?
#17 Re: Jai Ganesh's Puzzles » English language puzzles » 2025-10-24 15:09:56
Hi,
#5823. What does the adjective holistic mean?
#5824. What does the noun homburg mean?
#18 Jokes » Banana Jokes - I » 2025-10-24 14:58:03
- Jai Ganesh
- Replies: 0
Q: Why do bananas wear suntan lotion?
A: Because they peel!
* * *
Q: What is Beethoven's favorite fruit?
A: (sing to the tune of 5th symphony): Banana..na....! Banana..na....!
* * *
Q: What do you call two banana skins?
A: A pair of slippers.
* * *
Q: When banana growers are heart broken, what do they sing?
A: What else but Peelings?
* * *
Q: Why did the banana go to see the doctor?
A: The banana was not peeling very well.
* * *
#19 Re: Jai Ganesh's Puzzles » Doc, Doc! » 2025-10-24 14:30:35
Hi,
#2505. What does the medical term Endoscopic endonasal surgery mean?
#20 Re: Jai Ganesh's Puzzles » 10 second questions » 2025-10-24 14:20:18
Hi,
#9774.
#21 Re: Jai Ganesh's Puzzles » Oral puzzles » 2025-10-24 14:10:50
Hi,
#6279.
#22 Re: Exercises » Compute the solution: » 2025-10-24 13:51:56
Hi,
2625.
#23 This is Cool » Radiography » 2025-10-23 18:05:19
- Jai Ganesh
- Replies: 0
Radiography
Gist
Radiography is a medical imaging technique that uses X-rays to create static images of the inside of the body to diagnose fractures, infections, or locate foreign objects. During the procedure, an X-ray beam is passed through the body, and the remaining radiation is captured on film or a digital detector to form an image. These images are used by doctors to visualize internal structures and aid in diagnosis and treatment planning.
It is used to diagnose or treat patients by recording images of the internal structure of the body to assess the presence or absence of disease, foreign objects, and structural damage or anomaly. During a radiographic procedure, an x-ray beam is passed through the body.
Summary
X-ray or radiography uses a very small dose of ionizing radiation to produce pictures of the body's internal structures. X-rays are the oldest and most frequently used form of medical imaging. They are often used to help diagnosed fractured bones, look for injury or infection and to locate foreign objects in soft tissue. Some x-ray exams may use an iodine-based contrast material or barium to help improve the visibility of specific organs, blood vessels, tissues or bone.
Details
Radiography is an imaging technique using X-rays, gamma rays, or similar ionizing radiation and non-ionizing radiation to view the internal form of an object. Applications of radiography include medical ("diagnostic" radiography and "therapeutic radiography") and industrial radiography. Similar techniques are used in airport security, (where "body scanners" generally use backscatter X-ray). To create an image in conventional radiography, a beam of X-rays is produced by an X-ray generator and it is projected towards the object. A certain amount of the X-rays or other radiation are absorbed by the object, dependent on the object's density and structural composition. The X-rays that pass through the object are captured behind the object by a detector (either photographic film or a digital detector). The generation of flat two-dimensional images by this technique is called projectional radiography. In computed tomography (CT scanning), an X-ray source and its associated detectors rotate around the subject, which itself moves through the conical X-ray beam produced. Any given point within the subject is crossed from many directions by many different beams at different times. Information regarding the attenuation of these beams is collated and subjected to computation to generate two-dimensional images on three planes (axial, coronal, and sagittal) which can be further processed to produce a three-dimensional image.
Industrial radiography
Industrial radiography is a method of non-destructive testing where many types of manufactured components can be examined to verify the internal structure and integrity of the specimen. Industrial Radiography can be performed utilizing either X-rays or gamma rays. Both are forms of electromagnetic radiation. The difference between various forms of electromagnetic energy is related to the wavelength. X and gamma rays have the shortest wavelength and this property leads to the ability to penetrate, travel through, and exit various materials such as carbon steel and other metals. Specific methods include industrial computed tomography.
Image quality
Image quality will depend on resolution and density. Resolution is the ability of an image to show closely spaced structure in the object as separate entities in the image while density is the blackening power of the image. Sharpness of a radiographic image is strongly determined by the size of the X-ray source. This is determined by the area of the electron beam hitting the anode. A large photon source results in more blurring in the final image and is worsened by an increase in image formation distance. This blurring can be measured as a contribution to the modulation transfer function of the imaging system.
Additional Information
X-ray, electromagnetic radiation of extremely short wavelength and high frequency, with wavelengths ranging from about {10}^{-8} to {10}^{-12} metre and corresponding frequencies from about {10}^{16} to {10}^{20} hertz (Hz).
X-rays are commonly produced by accelerating (or decelerating) charged particles; examples include a beam of electrons striking a metal plate in an X-ray tube and a circulating beam of electrons in a synchrotron particle accelerator or storage ring. In addition, highly excited atoms can emit X-rays with discrete wavelengths characteristic of the energy level spacings in the atoms. The X-ray region of the electromagnetic spectrum falls far outside the range of visible wavelengths. However, the passage of X-rays through materials, including biological tissue, can be recorded with photographic films and other detectors. The analysis of X-ray images of the body is an extremely valuable medical diagnostic tool.
X-rays are a form of ionizing radiation—when interacting with matter, they are energetic enough to cause neutral atoms to eject electrons. Through this ionization process the energy of the X-rays is deposited in the matter. When passing through living tissue, X-rays can cause harmful biochemical changes in genes, chromosomes, and other cell components. The biological effects of ionizing radiation, which are complex and highly dependent on the length and intensity of exposure, are still under active study (see radiation injury). X-ray radiation therapies take advantage of these effects to combat the growth of malignant tumours.
X-rays were discovered in 1895 by German physicist Wilhelm Konrad Röntgen while investigating the effects of electron beams (then called cathode rays) in electrical discharges through low-pressure gases. Röntgen uncovered a startling effect—namely, that a screen coated with a fluorescent material placed outside a discharge tube would glow even when it was shielded from the direct visible and ultraviolet light of the gaseous discharge. He deduced that an invisible radiation from the tube passed through the air and caused the screen to fluoresce. Röntgen was able to show that the radiation responsible for the fluorescence originated from the point where the electron beam struck the glass wall of the discharge tube. Opaque objects placed between the tube and the screen proved to be transparent to the new form of radiation; Röntgen dramatically demonstrated this by producing a photographic image of the bones of the human hand. His discovery of so-called Röntgen rays was met with worldwide scientific and popular excitement, and, along with the discoveries of radioactivity (1896) and the electron (1897), it ushered in the study of the atomic world and the era of modern physics.
Fundamental characteristics:
Wave nature
X-rays are a form of electromagnetic radiation; their basic physical properties are identical to those of the more familiar components of the electromagnetic spectrum—visible light, infrared radiation, and ultraviolet radiation. As with other forms of electromagnetic radiation, X-rays can be described as coupled waves of electric and magnetic fields traveling at the speed of light (about 300,000 km, or 186,000 miles, per second). Their characteristic wavelengths and frequencies can be demonstrated and measured through the interference effects that result from the overlap of two or more waves in space. X-rays also exhibit particle-like properties; they can be described as a flow of photons carrying discrete amounts of energy and momentum. This dual nature is a property of all forms of radiation and matter and is comprehensively described by the theory of quantum mechanics.
Though it was immediately suspected, following Röntgen’s discovery, that X-rays were a form of electromagnetic radiation, this proved very difficult to establish. X-rays are distinguished by their very short wavelengths, typically 1,000 times shorter than the wavelengths of visible light. Because of this, and because of the practical difficulties of producing and detecting the new form of radiation, the nature of X-rays was only gradually unraveled in the early decades of the 20th century.
In 1906 the British physicist Charles Glover Barkla first demonstrated the wave nature of X-rays by showing that they can be “polarized” by scattering from a solid. Polarization refers to the orientation of the oscillations in a transverse wave; all electromagnetic waves are transverse oscillations of electric and magnetic fields. The very short wavelengths of X-rays, hinted at in early diffraction studies in which the rays were passed through narrow slits, was firmly established in 1912 by the pioneering work of the German physicist Max von Laue and his students Walter Friedrich and Paul Knipping. Laue suggested that the ordered arrangements of atoms in crystals could serve as natural three-dimensional diffraction gratings. Typical atomic spacings in crystals are approximately 1 angstrom (1 × {10}^{-10} metre), ideal for producing diffraction effects in electromagnetic radiation of comparable wavelength. Friedrich and Knipping verified Laue’s predictions by photographing diffraction patterns produced by the passage of X-rays through a crystal of zinc sulfide. These experiments demonstrated that X-rays have wavelengths of about 1 angstrom and confirmed that the atoms in crystals are arranged in regular structures.
In the following year, the British physicist William Lawrence Bragg devised a particularly simple model of the scattering of X-rays from the parallel layers of atoms in a crystal. The Bragg law shows how the angles at which X-rays are most efficiently diffracted from a crystal are related to the X-ray wavelength and the distance between the layers of atoms. Bragg’s physicist father, William Henry Bragg, based his design of the first X-ray spectrometer on his son’s analysis. The pair used their X-ray spectrometer in making seminal studies of both the distribution of wavelengths in X-ray beams and the crystal structures of many common solids—an achievement for which they shared the Nobel Prize for Physics in 1915.
Particle nature
In the early 1920s, experimental studies of the scattering of X-rays from solids played a key role in establishing the particle nature of electromagnetic radiation. In 1905 German physicist Albert Einstein had proposed that electromagnetic radiation is granular, consisting of quanta (later called photons) each with an energy hf, where h is Planck’s constant (about 6.6 × {10}^{-34} joule∙second) and f is the frequency of the radiation. Einstein’s hypothesis was strongly supported in subsequent studies of the photoelectric effect and by the successes of Danish physicist Niels Bohr’s model of the hydrogen atom and its characteristic emission and absorption spectra (see Bohr atomic model). Further verification came in 1922 when American physicist Arthur Compton successfully treated the scattering of X-rays from the atoms in a solid as a set of collisions between X-ray photons and the loosely bound outer electrons of the atoms.
Adapting the relation between momentum and energy for a classical electromagnetic wave to an individual photon, Compton used conservation of energy and conservation of momentum arguments to derive an expression for the wavelength shift of scattered X-rays as a function of their scattering angle. In the so-called Compton effect, a colliding photon transfers some of its energy and momentum to an electron, which recoils. The scattered photon must thus have less energy and momentum than the incoming photon, resulting in scattered X-rays of slightly lower frequency and longer wavelength. Compton’s careful measurements of this small effect, coupled with his successful theoretical treatment (independently derived by the Dutch scientist Peter Debye), provided convincing evidence for the existence of photons. The approximate wavelength range of the X-ray portion of the electromagnetic spectrum, 10−8 to 10−12 metre, corresponds to a range of photon energies from about 100 eV (electron volts) to 1 MeV (million electron volts).

#24 Re: Dark Discussions at Cafe Infinity » crème de la crème » 2025-10-23 17:07:43
2372) Odd Hassel
Gist:
Work
In nature organisms are composed of an enormously varied number of chemical compounds, with the element carbon as a common component. The binding energy between atoms in carbon compounds determines their structure, but the structures are not completely rigid. They are flexible to a certain degree. Consequently, molecules can assume different conformations, which has ramifications for their way of reacting with other substances. At the end of the 1940s, Odd Hassel published pioneering works about different conformations for ring-shaped molecules with six carbon atoms.
Summary
Odd Hassel (born May 17, 1897, Kristiania [now Oslo], Nor.—died May 11, 1981, Oslo) was a Norwegian physical chemist and corecipient, with Derek H.R. Barton of Great Britain, of the 1969 Nobel Prize for Chemistry for his work in establishing conformational analysis (the study of the three-dimensional geometric structure of molecules).
Hassel studied at the University of Oslo and received his doctorate at the University of Berlin in 1924. He joined the faculty of the University of Oslo in 1925 and from 1934 to 1964 was a professor of physical chemistry and director of the physical chemistry department. He began intensive research on the structure of cyclohexane (a 6-carbon hydrocarbon molecule) and its derivatives in 1930 and discovered the existence of two forms of cyclohexane. At this time he set forth the basic tenets of conformational analysis and wrote Kristallchemie (1934; Crystal Chemistry). After the mid-1950s Hassel’s research dealt mainly with the structure of organic halogen compounds.
Details
Odd Hassel (17 May 1897 – 11 May 1981) was a Norwegian physical chemist and Nobel Laureate.
Biography
Hassel was born in Kristiania (now Oslo), Norway. His parents were Ernst Hassel (1848–1905), a gynaecologist, and Mathilde Klaveness (1860–1955). In 1915, he entered the University of Oslo where he studied mathematics, physics and chemistry, and graduated in 1920. Victor Goldschmidt was Hassel's tutor when he began studies in Oslo, while Heinrich Jacob Goldschmidt, Victor's father, was Hassel's thesis advisor. Father and son were important figures in Hassel's life and they remained friends. After taking a year off from studying, he went to Munich, Germany to work in the laboratory of Professor Kasimir Fajans.
His work there led to the detection of absorption indicators. After moving to Berlin, he worked at the Kaiser Wilhelm Institute, where he began to do research on X-ray crystallography.[5] He furthered his research with a Rockefeller Fellowship, obtained with the help of Fritz Haber. In 1924, he obtained his PhD from Humboldt University of Berlin, before moving to his alma mater, the University of Oslo, where he worked from 1925 through 1964. He became a professor in 1934.
His work was interrupted in October, 1943 when he and other university staff members were arrested by the Nasjonal Samling and handed over to the occupation authorities. He spent time in several detention camps, until he was released in November, 1944.
Work
Heinrich Jacob Goldschmidt was Hassel's thesis advisor and father of Victor Goldschmidt.
Hassel originally focused on inorganic chemistry, but beginning in 1930 his work concentrated on problems connected with molecular structure, particularly the structure of cyclohexane and its derivatives. He introduced the Norwegian scientific community to the concepts of the electric dipole moments and electron diffraction. The work for which he is best known established the three-dimensionality of molecular geometry. He focused his research on ring-shaped carbon molecules, which he suspected filled three dimensions instead of two, the common belief of the time. By using the number of bonds between the carbon and hydrogen atoms, Hassel demonstrated the impossibility of the molecules existing on only one plane. This discovery led to him being awarded the Nobel Prize in Chemistry for 1969.
Honors
Hassel was awarded the Nobel Prize in Chemistry in 1969, shared with English chemist Derek Barton.
He received the Guldberg-Waage Medal (Guldberg-Waage Medal) from the Norwegian Chemical Society and the Gunnerus Medal from the Royal Norwegian Society of Science and Letters, both in 1964.
Hassel held honorary degrees from the University of Copenhagen (1950) and Stockholm University (1960). An annual lecture named in his honor is given at the University of Oslo.
He was an honorary Fellow of the Norwegian Chemical Society, Chemical Society of London, Norwegian Academy of Science and Letters, Royal Danish Academy of Sciences and Letters and Royal Swedish Academy of Sciences.
He was made a Knight of the Order of St. Olav in 1960.

#25 Dark Discussions at Cafe Infinity » Coach Quotes - II » 2025-10-23 16:36:27
- Jai Ganesh
- Replies: 0
Coach Quotes - II
1. I'd give my life to be the national team coach. - Diego Maradona
2. My coach is pushing me harder than ever to make sure I stay at a good level. - Usain Bolt
3. My brother Gary, who was my coach, five years my elder, studied human movements at Queensland University in Brisbane. We used to train together every day, and we'd train for so long that at the end of a session, we would physically almost collapse. - Matthew Hayden
4. I'm very fortunate to have a coach that I got to stay with all this time. Every year the bond gets stronger and better, and we understand each other more. And it's like she can tell if I walk into the gym what kind of mood I'm in, what she has to fix for the practice I need, or how I'm feeling. - Simone Biles
5. My coach keeps telling me to say I'm not going to retire. I should just go through the motions and see what I feel every year and see if I really want to do it, but personally, I want to do it, but my coach says just take your time, don't rush. - Usain Bolt
6. There are times I might coach one or two workouts a year when the regular coach gets caught in traffic. - Mark Spitz
7. I tell you, it was kind of two-fold. I fortunately had a lot of support. My coach was amazing - he told me to focus on being prepared and that is what I did. Every athlete is nervous - any athlete who tells you they're not nervous isn't telling you the truth. I was as prepared as I could be. - Carl Lewis
8. I was born in a very poor family. I used to sell tea in a railway coach as a child. My mother used to wash utensils and do lowly household work in the houses of others to earn a livelihood. I have seen poverty very closely. I have lived in poverty. As a child, my entire childhood was steeped in poverty. - Narendra Modi.