Math Is Fun Forum
You are not logged in.
- Topics: Active | Unanswered
#526 2019-03-29 00:09:51
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,479
Re: crème de la crème
493) Jacques Cousteau
Jacques Cousteau, in full Jacques-Yves Cousteau, (born June 11, 1910, Saint-André-de-Cubzac, France—died June 25, 1997, Paris), French naval officer, ocean explorer, and coinventor of the Aqua-Lung, known for his extensive underseas investigations.
After graduating from France’s naval academy in 1933, he was commissioned a second lieutenant. However, his plans to become a navy pilot were undermined by an almost fatal automobile accident in which both his arms were broken. Cousteau, not formally trained as a scientist, was drawn to undersea exploration by his love both of the ocean and of underwater diving. In 1943 Cousteau and French engineer Émile Gagnan developed the first fully automatic compressed-air Aqua-Lung (scuba apparatus), which allowed divers to swim freely underwater for extended periods of time. Cousteau helped to invent many other tools useful to oceanographers, including the diving saucer (an easily maneuverable small submarine for seafloor exploration), in 1959, and a number of underwater cameras.
Cousteau served in World War II as a gunnery officer in France and later was a member of the French Resistance against the German occupation of the country. He subsequently was awarded the Legion of Honour for his espionage work. Cousteau’s experiments with underwater filmmaking began during the war. He also was involved in conducting oceanographic research at a centre in Marseille with French naval officer Philippe Tailliez. When the war ended, he continued working for the French navy, heading the Undersea Research Group at Toulon.
To expand his work in marine exploration, Cousteau founded numerous marketing, manufacturing, engineering, and research organizations, which were incorporated in 1973 as the Cousteau Group. In 1950 he converted a British minesweeper into the Calypso, an oceanographic research ship, aboard which he and his crew carried out numerous expeditions. Cousteau eventually popularized oceanographic research and the sport of scuba diving in the book 'Le Monde du silence' (1953; The Silent World), written with Frédéric Dumas. In 1956 he adapted the book into a documentary film, codirected with French motion-picture director Louis Malle, that won both the Palme d’Or at that year’s Cannes international film festival and an Academy Award in 1957, one of three Oscars his films received. Also in 1957, Cousteau became director of the Oceanographic Museum of Monaco. He led the Conshelf Saturation Dive Program, conducting experiments in which men lived and worked for extended periods of time at considerable depths along the continental shelves. The undersea laboratories, called Conshelf I, II, and III, sat at depths of 10 metres (about 30 feet), 30 metres (about 100 feet), and 102.4 metres (about 336 feet), respectively, in the Mediterranean Sea near Marseilles. In 1974 he formed the Cousteau Society, a nonprofit environmental group dedicated to marine conservation.
Cousteau produced and starred in many television programs, including the American series 'The Undersea World of Jacques Cousteau' (1968–76). Several documentaries were coproduced with his son Philippe, until Philippe’s untimely death in a plane crash in 1979. He was awarded the U.S. Presidential Medal of Freedom in 1985. In addition to The Silent World, Cousteau also wrote Par 18 mètres de fond (1946; Through 18 Metres of Water), 'The Living Sea' (1963), 'Three Adventures: Galápagos, Titicaca, the Blue Holes' (1973), 'Dolphins' (1975), and 'Jacques Cousteau: The Ocean World'(1985). His last book, 'The Human, the Orchid, and the 'Octopus: Exploring and Conserving Our Natural World' (2007)', was published posthumously.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#527 2019-03-31 00:12:48
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,479
Re: crème de la crème
494) Antonio de Ulloa
Antonio de Ulloa y de la Torre-Giral, (12 January 1716 - 3 July 1795) was a Spanish general of the navy, explorer, scientist, author, astronomer, colonial administrator and the first Spanish governor of Louisiana. He was appointed to that office after France ceded the territory to Spain in 1763, following its defeat by Great Britain in the Seven Years' War. Ulloa's rule was resisted by the French Creole colonists in New Orleans, who expelled him in 1768 from West Louisiana.
Ulloa had already established an international reputation in science, having been part of the French Geodesic Mission in present-day Ecuador. He published an extensive record of his observations and findings on the South American trip, which was published in French in 1848 and in English as ‘A Voyage to South America’ (1806). He was a Fellow of the Royal Society and a foreign member of the Royal Swedish Academy of Sciences.
Life
Ulloa was born in Seville, Spain. His father was an economist. Ulloa entered the navy in 1733. In 1735, he, along with fellow Spaniard Jorge Juan, was appointed to the French Geodesic Mission. The French Academy of Sciences was sending this scientific expedition to present-day Ecuador to measure a degree of meridian arc at the equator.
Ulloa worked in Ecuador from 1736 to 1744, during which time the two Spaniards discovered the element platinum in the area. Ulloa was the first person to write a scientific description of the metal. Ulloa is sometimes incorrectly credited with discovering platinum, because of this. In 1745, having finished their scientific labours, Ulloa and Jorge Juan prepared to return to Spain, agreeing to travel on different ships in order to minimize the danger of losing their important samples and records.
The ship upon which Ulloa was travelling was captured by the British, and he was taken to England as a prisoner. In that country, through his scientific attainments, Ulloa gained the friendship of the men of science, and was made a Fellow of the Royal Society of London. In a short time, through the influence of the president of this society, he was released and able to return to Spain. He published an account of the people and countries he had encountered during the French Geodesic Mission (1748), which was translated into English and published as ‘A Voyage to South America’ (1806).
Ulloa became prominent as a scientist and was appointed to serve on various important scientific commissions. He is credited with the establishment of the first museum of natural history, the first metallurgical laboratory in Spain, and the observatory of Cadiz. In 1751, de Ulloa was elected a foreign member of the Royal Swedish Academy of Sciences.
In 1758 he returned to South America as governor of Huancavelica in Peru and the general manager of the quicksilver mines there. He held this position until 1764.
After France was defeated by the English in the Seven Years' War, it ceded its territories west of the Mississippi River to Spain. Ulloa was appointed by the Spanish Crown to serve as the first Spanish governor of West Louisiana, and reached New Orleans, the major city and port, on 5 March 1766. The French colonists refused to recognize Spanish rule, and expelled Ulloa from Louisiana by a Creoleuprising during the Louisiana Rebellion of 1768. On 28 October, as riots broke out in New Orleans, the governor and his pregnant wife were taken to a Spanish vessel. The Superior Council voted that the governor leave within three days. He complied, leaving on 1 November. The revolt was ultimately crushed by forces under Alejandro O'Reilly in 1769, establishing Spanish dominance in the colony once and for all.
For the remainder of his life, Ulloa served as a naval officer. In 1779 he became lieutenant-general of the naval forces. Ulloa died at Isla de Leon, Cádiz, in 1795.
Legacy
As a result of his scientific work in Peru, Ulloa published ‘Relación histórica del viaje á la América Meridional’ (Madrid, 1784), which contains a full, accurate, and clear description of the greater part of South America geographically, and of its inhabitants and natural history. (It was published in English in 1806.)
In collaboration with Jorge Juan mentioned above, he also wrote ‘Noticias secretas de América’, giving valuable information regarding the early religious orders in Spanish America. This work was published by David Barry in London, 1826.
Ulloa is the namesake for the meteorological term "Ulloa's halo" (also known as "Bouguer's halo"), which an observer may see infrequently in fog when the sun breaks through (for example, on a mountain) - effectively a "fog-bow" (as opposed to a "rain-bow"). A fog-bow is defined as "an infrequently observed meteorological phenomenon; a faint white, circular arc or complete ring of light that has a radius of 39 degrees and is centered on the antisolar point. When observed, it is usually in the form of a separate outer ring around an anticorona."

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#528 2019-04-02 00:24:21
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,479
Re: crème de la crème
495) King Camp Gilette
King Camp Gillette, (born January 5, 1855, Fond du Lac, Wisconsin, U.S. - died July 9, 1932, Los Angeles, California), American inventor and first manufacturer of a razor with disposable blades.
Gillette, reared in Chicago, was forced by his family’s loss of possessions in the fire of 1871 to go to work, so he became a traveling salesman of hardware. An employer noted his predilection for mechanical tinkering, which sometimes resulted in commercially profitable inventions, and advised him to invent “something that would be used and thrown away” so that the customer would keep coming back.
While honing a permanent straight-edge razor in 1895, Gillette had the idea of substituting a thin double-edged steel blade placed between two plates and held in place by a Τ handle. Instead of being sharpened, the removable blade would simply be thrown away once it became dull. Gillette had no background in metallurgy, and manufacturing such a blade proved a challenge. It was some six years before William Nickerson developed a way to mass-produce the blades from sheet metal. The Gillette Safety Razor Company’s first sale, in 1903, consisted of a lot of 51 razors and 168 blades; by the end of 1904, it had produced 90,000 razors and 12,400,000 blades. Gillette’s innovative sales strategy—he sold the razors for a loss and made his profits on the blades—helped make the product a success.
Gillette then turned his intellectual energies to publicizing a view of utopian socialism in a series of books and other writings. He found competition wasteful and envisaged a planned society in which economic effort would be rationally organized by engineers. In 1910 he vainly offered former president Theodore Roosevelt a million dollars to act as president of an experimental “World Corporation” in the Arizona Territory. Gillette remained president of his company until 1931 but retired from active management in 1913.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#529 2019-04-04 01:11:41
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,479
Re: crème de la crème
496) Alexandre-Edmond Becquerel
Alexandre-Edmond Becquerel (24 March 1820 – 11 May 1891), known as Edmond Becquerel, was a French physicist who studied the solar spectrum, magnetism, electricity and optics. He is credited with the discovery of the photovoltaic effect, the operating principle of the solar cell, in 1839. He is also known for his work in luminescence and phosphorescence. He was the son of Antoine César Becquerel and the father of Henri Becquerel, one of the discoverers of radioactivity.
Biography
Becquerel was born in Paris and was in turn the pupil, assistant and successor of his father at the Muséum national d'Histoire naturelle. He was also appointed professor at the short-lived Agronomic Institute at Versailles in 1849, and in 1853 received the chair of physics at the Conservatoire des Arts et Métiers. He was associated with his father in much of his work.
The first photovoltaic device
In 1839, at age 19, experimenting in his father's laboratory, Becquerel created the world's first photovoltaic cell. In this experiment, silver chloride or silver bromide was used to coat the platinum electrodes; once the electrodes were illuminated, voltage and current were generated. Because of this work, the photovoltaic effect has also been known as the "Becquerel effect".
Photographic discoveries
Becquerel was an early experimenter in photography. In 1840, he discovered that the silver halides, natively insensitive to red and yellow light, became sensitive to that part of the spectrum in proportion to their exposure to blue, violet and ultraviolet light, allowing daguerreotypes and other photographic materials to be developed by bathing in strong red or yellow light rather than by chemical treatment. In practice this technique was rarely used. In 1848 he produced color photographs of the spectrum, and also of camera images, by a technique later found to be akin to the Lippmann interference method, but the camera exposures required were impractically long and the images could not be stabilized, their colors persisting only if kept in total darkness, however this work is based on the discoveries of J. T. Seebeck prior to 1810.
Other studies
Becquerel paid special attention to the study of light, investigating the photochemical effects and spectroscopic characters of solar radiation and the electric arc light, and the phenomena of phosphorescence, particularly as displayed by the sulfides and by compounds of uranium. It was in connection with these latter inquiries that he devised his phosphoroscope, an apparatus which enabled the interval between exposure to the source of light and observation of the resulting effects to be varied at will and accurately measured.
He investigated the diamagnetic and paramagnetic properties of substances and was keenly interested in the phenomena of electrochemical decomposition, accumulating much evidence in favor of Faraday's law of electrolysis and proposing a modified statement of it which was intended to cover certain apparent exceptions. In 1853, Becquerel discovered thermionic emission.
Publications
In 1867 and 1868 Becquerel published 'La lumière, ses causes et ses effets' (Light, its Causes and Effects), a two-volume treatise which became a standard text. His many papers and commentaries appeared in French scientific journals, mainly the French Academy of Science's widely distributed Comptes Rendus, from 1839 until shortly before his death in 1891.
Honors and awards
Becquerel was elected a member of the Royal Swedish Academy of Sciences in 1886.
The Becquerel Prize for "outstanding merit in photovoltaics" is awarded annually at the European Photovoltaic Solar Energy Conference and Exhibition (EU PVSEC).

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#530 2019-04-06 01:09:40
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,479
Re: crème de la crème
497) Swarts, Frédéric Jean Edmond
(B. Ixelles, Belgium, 2 September 1866; d. Ghent, Belgium, 6 September 1940)
Frédéric Swarts entered the University of Ghent in 1883 and received doctorates in chemistry (1889) and medicine (1891). His father, Théodore Swarts had succeeded Kekulé as professor of chemistry at the university in 1871. The younger Swarts spent his entire professional career at Ghent, first as répétiteur and then, on his father’s retirement in 1903, as professor of chemistry. He was a member of the Académie Royale des Sciences des Lettres et des Beaux-Arts de Belgique, which awarded him its Gold Medal, corresponding member of the Institut de France, president of the Institut International de Chimie Solvay, and charter member and vice-president of the 'International Union of Pure and Applied Chemistry'.
After the discovery of fluorine, few of its compounds had been prepared because of the reactivity and toxicity of the element. Swarts was among the first to study organic fluorine compounds. Unable to use methods of direct fluorination because of the violence of the reactions, he developed a double decomposition process using inorganic fluorides, especially antimony trifluoride and mercurous fluoride, and organic polyhalides, where the halogen atoms are on the same carbon atom (the Swarts reaction, 1892). The first synthesis of an organic fluorine compound was trichlorofluoromethane (1891). Swarts synthesized many aliphatic chlorofluoro and bromofluoro derivatives of hydrocarbons, alcohols, and acids. In 1922 he prepared trifluoroacetic acid, the strongest organic acid known.
Swarts made the first extensive investigations of organic-fluorine compounds. He coupled his syntheses of organic fluorine compounds with physicochemical studies and determined their heats of combustion, molecular refractions, and viscosities, proving that fluorinated organic compounds have weaker intermolecular forces than the corresponding nonfluorinated compounds.
The aliphatic chlorofluoro compounds became the first fluorochemicals to be used commercially after Thomas Midgley and A. L. Henne in 1930, using a modified Swarts reaction, prepared the group of fluorinated methanes and ethanes known as the Freons.
Swarts made the first extensive investigations of organic-fluorine compounds. He coupled his syntheses of organic fluorine compounds with physicochemical studies and determined their heats of combustion, molecular refractions, and viscosities, proving that fluorinated organic compounds have weaker intermolecular forces than the corresponding nonfluorinated compounds.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#531 2019-04-08 00:13:20
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,479
Re: crème de la crème
498) Sir Rowland Hill
Sir Rowland Hill, (born December 3, 1795, Kidderminster, Worcestershire, England—died August 27, 1879, Hampstead, London), British administrator and educator, originator of the penny postage system, principally known for his development of the modern postal service, which was subsequently adopted throughout the world.
The son of an English schoolmaster, Hill was interested in problems of teaching; for about 15 years he operated schools in which he emphasized student democracy, rigid self-discipline, and intensive teaching. His wide-ranging interests included printing, astronomy, mathematics, and transportation.
Hill’s proposals for postal reform, formulated between 1835 and 1837, were based on the notion that revenue derived from taxes should increase with the growth of the population and national prosperity. He therefore suggested a lower levy on letters, since high taxes reduced the volume of mail and thus diminished the revenue derived therefrom; a uniform postage rate irrespective of distance, since excessive numbers of rates for letters traveling different distances greatly increased accounting expenses; and that all mail should be prepaid. To effect the last, he proposed a device that subsequently became known as the postage stamp. Hill managed to put his program into effect in 1840, despite bureaucratic hostility. He was knighted in 1860.
Perhaps one of the most famous people from Tottenham's' history is that of Sir Rowland Hill who is world famous for the development of the Penny Post System and the introduction of the postage stamp.
Born in Kidderminster in Worcestershire on the 3rd December 1795, Rowland was the third son of Thomas Wright Hill. At the age of seven his father took charge of a boarding school in Birmingham and this is where Rowland was educated. Even at a very young age Rowland would help in the work of the school, and In 1807 Rowland Hill became a student-teacher at his father's school. As a sideline Rowland was also an inventor, although most of his ideas were never used. He was to take a very early interest in mechanical engineering and ,following lengthy experimentation, he was responsible for improved methods of machine printing.
Bruce Castle School, perhaps the most famous of the many private schools in Tottenham, was opened in 1827 and began as a branch establishment of the Hill family school at Hazelwood near Birmingham. Rowland Hill was the first headmaster of Bruce Castle School and, on leaving the school in 1833, handed over to his brother Arthur Hill who retired in 1866.
Among two innovative features of schooling at Bruce Castle was the use of tokens as a reward for good work. These were forfeited in the event of misdemeanours and served as a medium of exchange. They formed a transferable currency system within the school and were considered to introduce the boys to economics and to facilitate mental arithmetic. The second innovation was the introduction of a school magazine which provided the pupils with information on the running of the school and its leisure time activities.
Rowland Hill decided he needed a new challenge and became interested in a project for the colonisation of South Australia. From 1834 till 1839 he was Secretary for the South Australian Commission, but he kept his interest in science and mechanics.
In the mid 1800's there was increasing public concern about the shortcomings of the Post-Office, which prompted both parliamentary inquiries and royal commissions to address the matter but they failed to suggest an effective method of resolving the difficulties. After carefully studying the matter himself, Rowland Hill issued his now famous booklet entitled 'Post Office Reform: It's importance and Practicability'. In his paper he suggested changes that were seen to be so simple and effective that people wondered why nobody had ever thought of them before.
The enormous amount of time and labour involved in the collection, making out the charges, delivery and obtaining payment for letters under the old system of paying according to distance was bureaucratic and inefficient. So Rowland Hill's answer was that a uniform charge be introduced that would sweep away the bureaocracy and vast amounts of useless labour thus cheapening the whole operation, which would enable the Post Office to fix the postage rate as low as 'One Penny'
But no doubt the most innovative of his proposals was the introduction of prepayment of the postage charge by means of an affixed stamp, which of course has now been adopted by every country in the world and a method that is still used to this day.
Despite initial opposition to the scheme and obstructive measures from official quarters, the General Public were fully in favour of the scheme and poured in so many petitions of support that in 1840 the Penny Post was introduced under the supervison of Rowland Hill himself.
In an article published in 'The British Workman' it was reported "In his walks in the beautiful grounds of Bruce Castle,Tottenham, which may be called the family home of the Hill's, Sir Rowland pondered over and matured some of his most important postal plans"
After a few years service in the management of the Brighton Railway Company, in 1846 he was appointed secretary to the Postmaster General. Then in 1854 he became Chief Secretary and practical Director of the Post Office.
Queen Victoria knighted Rowland Hill for his service to the Empire in 1860 and he received the honour K.C.B .
Following 4 more years of successful service he was to retire from the Post Office. Sir Rowland Hill retired in 1864, in poor health.
Sir Rowland Hill died at his home in Hampstead at the age of 84 on the 27th August 1879. His remains were laid in Westminster Abbey, London on the 4th September 1880 following a service in his honour which was attended by a number of distinguished people of the day. Among the many compliments paid to him was as follows
" Our country may be grateful for a man of such keen intellect and desire to be useful. The only matter for regret is that his invaluable services and administrative powers were not more extensively employed by Government for the benefit of the nation"

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#532 2019-04-10 01:33:35
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,479
Re: crème de la crème
499) Marcian Hoff
Marcian Edward "Ted" Hoff Jr. (born October 28, 1937 in Rochester, New York) is one of the inventors of the microprocessor.
Education and work history
Hoff received a bachelor's degree in electrical engineering from the Rensselaer Polytechnic Institute in 1958. He applied for his first two patents based on work done for the General Railway Signal Corp. of Rochester, New York during the summers of his undergraduate study. He received a National Science Foundation Fellowship to enroll in Stanford University, where he received his master's degree in 1959 and his Ph.D. in 1962. As part of his Ph.D. dissertation, Hoff co-invented the least mean squares filter with Bernard Widrow.
Hoff joined Intel in 1968 as employee number 12, and is credited with coming up with the idea of using a "universal processor" rather than a variety of custom-designed circuits in the architectural idea and an instruction set formulated with Stanley Mazor in 1969 for the Intel 4004 - the chip that started the microprocessor revolution in the early 1970s. Development of the silicon-gate design methodology and the actual chip design was done by Federico Faggin, who also led the project during 1970-1971.
In 1980, Hoff was named the first Intel Fellow, which is the highest technical position in the company. He stayed in that position until 1983 when he left for Atari. He remained with Atari until March 1995.
In 1996 he became executive vice president of sales and marketing for Sega of America.
Popular culture
Hoff was featured in an Intel advertisement, calling him the "rock star" of Intel and comparing him to the rock stars of American culture.
Awards
In 1954, he was one of the Westinghouse Science Talent Search (now Intel STS) finalists. He was awarded the Stuart Ballantine Medal in 1979, the IEEE Cledo Brunetti Award in 1980, and the Franklin Institute Certificate of Merit in 1996. He was inducted into the National Inventors Hall of Fame in 1996 and received the National Medal of Technology and Innovation in 2009 from President Barack Obama. He was made a Fellow of the Computer History Museum in 2009 "for his work as part of the team that developed the Intel 4004, the world's first commercial microprocessor." He received the 2011 IEEE/RSE Wolfson James Clerk Maxwell Award.
Biography
Marcian "Ted" Hoff was born in Rochester, New York, in 1937. He received his B.S. in electrical engineering from Rensselaer Polytechnic Institute (1958) and an M.S. (1959) and Ph.D. (1962) from Stanford University.
Hoff joined Intel in 1968 and is credited with the idea of using a universal processor to replace custom-designed circuits. This arose from a contract Intel had with Japanese company Busicom to build a set of integrated circuits for their new electronic calculator. Working with Stan Mazor, Hoff defined the instruction set and architectural specifications of the new chip, known as the Intel 4004. Fellow team members Masatoshi Shima and Federico Faggin implemented the design in silicon, creating the world's first commercial microprocessor. The microprocessor is now the core technology of all modern electronics systems.
In 1980, Hoff was named the first Intel Fellow and stayed in that position until 1983, when he went to Atari as vice president of technology. Hoff was most recently chief technologist at Teklicon, an intellectual property consulting firm, at which he served from 1990 to 2007.
Hoff shares the U.S. National Medal of Technology (2009) with Faggin and Mazor and the Kyoto Prize (1997) with Faggin, Mazor, and Shima.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#533 2019-04-12 01:29:13
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,479
Re: crème de la crème
500) Moritz Hermann von Jacobi
(B. Potsdam, Germany, 21 September 1801; d. St. Peterburg, Russia, 27 February 1874)
Physics.
At the urging of his parents Jacobi studied architecture at Göttingen and in 1833 set up practice in Königsberg, where his younger brother Carl was a professor of mathematics. He also began to turn his attention to physics and chemistry. In 1835 he went to the University of Dorpat as a professor of civil engineering, and in 1837 he moved to St. Petersburg. There he became a member of the Imperial Academy of sciences (adjunct in 1839, extraordinary in 1842, and ordinary in 1847) and devoted his energies to research on electricity and its various practical applications, his interest in this subject having developed since his days in Göttingen.
Jacobi engaged in a number of studies of great interest in the fast-developing subject of electricity, dealing especially with its possible technical applications. Although most of the results of his work were published and were generally available, their impact was minimal. One reason for this certainly lies in his physical isolation from the centers of development in electricity in France and England. Another can probably be found in that most of his practical applications proved to be premature; that is, the technology had not developed enough to sustain them.
Jacobi’s most interesting work, reported to the St. Petersburg Academy in 1838 and to the British Association two years later, was his investigation of the power of an electromagnet as a function of various parameters; electric current, thickness of wire, number of turns on the helix, diameter of the helix, and thickness of the iron core. Of great practical value in the design of motors and generators, this work was pursued in greater detail by Henry Rowland and John Hopkinson almost half a century later.
In May 1834 Jacobi built one of the first practical electric motors. He performed a variety of tests on it, for instance measuring its output by determining the amount of zinc consumed by the battery. In 1838 his motor drove a twenty-eight-foot boat carrying a dozen Russian officials on the Neva River at a speed of one and one-half miles per hour. His hopes of covering the Neva with a fleet of magnetic boats were doomed from the beginning, however, by the cost of battery-powered operation and by the fumes that such batteries emitted.
In a separate enterprise Jacobi was asked to continue the work of Baron Pavel Schilling, who had demonstrated the needle (electromagnetic) telegraph to the Russian government in 1837 but who had died that year before an experimental line could be set up. Jacobi improved on Schilling’s design and by 1839 had constructed an instrument quite similar to Morse’s first, and earlier, receiver. Various experimental lines were run in succeeding years, but practical telegraphy did not come to Russia until the 1850’s, with the introduction of the Siemens and Halske system.
In 1838 Jacobi announced his discovery of the process he called“galvanoplasty” (now called electrotyping), the reproduction of forms by electrodeposition. In subsequent publications he described his techniques in great detail.
The inventor of the first electric motor, Moritz von Jacobi came from an Ashkenazi Jewish family in Potsdam. His father, Simon Jakobi, was personal banker to King Friedrich Wilhelm III of Prussia, and his younger brother was the renowned mathematician, Carl Gustav Jacobi.
Jacobi studied at Berlin and Gottingen Universities, and began his career as a government architect. In 1833, he moved to Konigsberg (Kaliningrad) and began to experiment with electromagnets. In May of the following year, he developed the first working electric motor. He described his motor in precise detail in a memorandum the following year, and was awarded an honorary doctorate by Konigsberg University. He also moved to the Duchy of Livonia, where he taught civil architecture at the University of Dorpat. Two years later, he was invited by Nicholas I to continue developing his electrical motor at the Imperial Academy of Sciences in St. Petersburg.
His investigation delved into the power of electromagnetism in motors and generators. During these studies he deduced the maximum power theorem (Jacobi's law). With the financial support of Tsar Nicholas, Jacobi built a 28-foot paddle boat powered with an improved version of his motor, which carried up to 14 passengers on the Neva River, as demonstrated in September 1838.
Also in 1838, Jacobi discovered the ability to make printing plates through the process of electrotyping, the chemical reproduction of exact forms in metal. By the following year, electrotyping was already being used to print government documents. This technology was also used to create metal statues without having to cast them. Jacobi was also involved in the development of the electric telegraph. From 1842-1845 he built a telegraph line between St. Petersburg and Tsarskoe Selo using underground cables, and produced the first letter-typing telegraph machine.
In 1840, Jacobi was awarded the recently instigated Demidov Prize for his achievements. He became a naturalized Russian citizen, and spent the last years of his life as the head of the Physics Office at the Russian Academy of Sciences. He died of a heart attack at the age of 72, and was buried in the Smolenskoye Lutheran Cemetery.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#534 2019-04-14 00:31:46
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,479
Re: crème de la crème
501) Audrey Hepburn
Actress Audrey Hepburn, star of Breakfast at Tiffany's, remains one of Hollywood's greatest style icons and one of the world's most successful actresses.
Synopsis
Actress, fashion icon, and philanthropist Audrey Hepburn was born on May 4, 1929, in Brussels, Belgium. At age 22, she starred in the Broadway production of Gigi. Two years later, she starred in the film ‘Roman Holiday’ (1953) with Gregory Peck. In 1961, she set new fashion standards as Holly Golightly in ‘Breakfast at Tiffany's’. Hepburn is one of the few actresses to win an Emmy, Tony, Grammy, and Academy Award. In her later years, acting took a back seat to her work on behalf of children.
Audrey Hepburn (born Audrey Kathleen Ruston; 4 May 1929 – 20 January 1993) was a British actress, model, dancer, and humanitarian. Recognised as a film and fashion icon, Hepburn was active during Hollywood's Golden Age. She was ranked by the American Film Institute as the third-greatest female screen legend in Golden Age Hollywood, and was inducted into the International Best Dressed List Hall of Fame.
She shot to stardom after playing the lead role in ‘Roman Holiday’ (1953), for which she was the first actress to win an Academy Award, a Golden Globe Award, and a BAFTA Award for a single performance. That same year, Hepburn won a Tony Award for Best Lead Actress in a Play for her performance in Ondine. She went on to star in a number of successful films, such as ‘Sabrina (1954), The Nun's Story (1959), Breakfast at Tiffany's (1961), Charade (1963), My Fair Lady (1964),’ and ‘Wait Until Dark’ (1967), for which she received an Academy Award, Golden Globe, and BAFTA nominations. Hepburn won three BAFTA Awards for Best British Actress in a Leading Role. In recognition of her film career, she was awarded the Lifetime Achievement Award from BAFTA, the Golden Globe Cecil B. DeMille Award, the Screen Actors Guild Life Achievement Award, and the Special Tony Award. She remains one of only 15 people who have won Academy, Emmy, Grammy, and Tony Awards.
Background
Born on May 4, 1929, in Brussels, Belgium, Audrey Hepburn was a talented performer known for her beauty, elegance and grace. Often imitated, she remains one of Hollywood's greatest style icons. A native of Brussels, Hepburn spent part of her youth in England at a boarding school there. During much of World War II, she studied at the Arnhem Conservatory in The Netherlands. After the Nazis invaded the country, Hepburn and her mother struggled to survive. She reportedly helped the resistance movement by delivering messages, according to an article in ‘The New York Times’.
After the war, Hepburn continued to pursue an interest in dance. She studied ballet in Amsterdam and later in London. In 1948, Hepburn made her stage debut as a chorus girl in the musical ‘High Button Shoes’ in London. More small parts on the British stage followed. She was a chorus girl in ‘Sauce Tartare’ (1949), but was moved to a featured player in ‘Sauce Piquante’ (1950).
That same year, Hepburn made her feature film debut in 1951's ‘One Wild Oat’, in an uncredited role. She went on to parts in such films as ‘Young Wives' Tales’ (1951) and ‘The Lavender Hill Mob’ (1951), starring Alec Guiness. Her next project on the New York stage introduced her to American audiences.
On Broadway
At the age of 22, Audrey Hepburn went to New York to star in the Broadway production of ‘Gigi’, based on the book by the French writer Colette. Set in Paris around 1900, the comedy focuses on the title character, a young teenage girl on the brink of adulthood. Her relatives try to teach her ways of being a courtesan, to enjoy the benefits of being with a wealthy man without having to marry. They try to get a friend of the family, Gaston, to become her patron, but the young couple has other ideas.
Only a few weeks after the play premiered, news reports indicated that Hepburn was being wooed by Hollywood. Only two years later, she took the world by storm in the film ‘Roman Holiday’ (1953) with Gregory Peck. Audiences and critics alike were wowed by her portrayal of Princess Ann, the royal who escapes the constrictions of her title for a short time. She won an Academy Award for Best Actress for this performance.
The next year Hepburn returned to the Broadway stage to star in ‘Ondine’ with Mel Ferrer. A fantasy, the play told the story of a water nymph who falls in love with a human played by Ferrer. With her lithe and lean frame, Hepburn made a convincing sprite in this sad story about love found and lost. She won the 1954 Tony Award for Best Actress in a Play for her performance. While the leading characters in the play grew apart, the actors found themselves becoming closer. The two also made a dynamic pair off stage and Hepburn and Ferrer got married on September 25, 1954, in Switzerland.
Film Star
Back on the big screen, Hepburn made another award worthy performance in ‘Sabrina’ (1954) as the title character, the daughter of a wealthy family's driver. Sabrina returned home after spending time in Paris as a beautiful and sophisticated woman. The family's two sons, Linus and David, played by Humphrey Bogart and William Holden, never paid her much mind until her transformation. Pursuing her onetime crush David, Sabrina unexpectedly found happiness with his older brother Linus. Hepburn earned an Academy Award nomination for her work on this bittersweet romantic comedy.
Showcasing her dancing abilities, Hepburn starred opposite Fred Astaire in the musical ‘Funny Face’ (1957). This film featured Hepburn undergoing another transformation. This time, she played a beatnik bookstore clerk who gets discovered by a fashion photographer played by Astaire. Lured by a free trip to Paris, the clerk becomes a beautiful model. Hepburn’s clothes for the film were designed by Hubert de Givenchy, one of her close friends.
Stepping away from lighthearted fare, Hepburn co-starred in the film adaptation of Leo Tolstoy's ‘War and Peace’ with her husband, Mel Ferrer, and Henry Fonda in 1956. Three years later, she played Sister Luke in ‘The Nun's Story’ (1959), which earned her an Academy Award nomination. The film focused on her character's struggle to succeed as a nun. A review in Variety said "Audrey Hepburn has her most demanding film role, and she gives her finest performance." Following that stellar performance, she went on to star in the John Huston-directed western ‘The Unforgiven’ (1960) with Burt Lancaster. That same year, her first child, a son named Sean, was born.
Returning to her glamorous roots, Hepburn set new fashion standards as Holly Golightly in ‘Breakfast at Tiffany'’s (1961), which was based on a novella by Truman Capote. She played a seemingly lighthearted, but ultimately troubled New York City party girl who gets involved with a struggling writer played by George Peppard. Hepburn received her fourth Academy Award nomination for her work on the film.
Later Work
For the rest of the 1960s, Hepburn took on a variety of roles. She starred with Cary Grant in the romantic thriller ‘Charade’ (1963). Playing the lead in the film version of the popular musical ‘My Fair Lady’ (1964), she went through one of the most famous metamorphoses of all time. As Eliza Doolittle, she played an English flower girl who becomes a high society lady. Taking on more dramatic fare, she starred a blind woman in the suspenseful tale ‘Wait Until Dark’ (1967) opposite Alan Arkin. Her character used her wits to overcome the criminals that were harassing her. This film brought her a fifth Academy Award nomination. That same year, Hepburn and her husband separated and later divorced. She married Italian psychiatrist Andrea Dotti in 1969, and the couple had a son, Luca, in 1970.
In the 1970s and 1980s, Hepburn worked sporadically. She starred opposite Sean Connery in ‘Robin and Marian’ (1976), a look at the central figures of the Robin Hood saga in their later years. In 1979, Hepburn co-starred with Ben Gazzara in the crime thriller ‘Bloodline’. Hepburn and Gazzara teamed up again for the 1981 comedy ‘They All Laughed’, directed by Peter Bogdanovich. Her last screen role was in ‘Always’ (1989) directed by Steven Spielberg.
Legacy
In her later years, acting took a back seat to her work on behalf of children. She became a goodwill ambassador for UNICEF in the late 1980s. Traveling the world, Hepburn tried to raise awareness about children in need. She understood too well what it was like to go hungry from her days in The Netherlands during the German Occupation. Making more than 50 trips, Hepburn visited UNICEF projects in Asia, Africa, and Central and South America. She won a special Academy Award for her humanitarian work in 1993, but she did not live long enough to receive it. Hepburn died on January 20, 1993, at her home in Tolochenaz, Switzerland after a battle with colon cancer.
Her work to help children around the world continues. Her sons, Sean Ferrer and Luca Dotti, along with her companion Robert Wolders, established the Audrey Hepburn Memorial Fund at UNICEF to continue Hepburn's humanitarian work in 1994. It is now known as the Audrey Hepburn Society at the US Fund for UNICEF.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#535 2019-04-16 00:35:44
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,479
Re: crème de la crème
502) Har Gobind Khorana
Har Gobind Khorana, (born January 9, 1922?, Raipur, India—died November 9, 2011, Concord, Massachusetts, U.S.), Indian-born American biochemist who shared the 1968 Nobel Prize for Physiology or Medicine with Marshall W. Nirenberg and Robert W. Holley for research that helped to show how the nucleotides in nucleic acids, which carry the genetic code of the cell, control the cell’s synthesis of proteins.
Khorana was born into a poor family and attended the University of the Punjab at Lahore, India (now in Pakistan), and the University of Liverpool, England, on government scholarships. He obtained his Ph.D. at Liverpool in 1948. He began research on nucleic acids during a fellowship at the University of Cambridge (1951) under Sir Alexander Todd. He held fellowships and professorships in Switzerland at the Swiss Federal Institute of Technology, in Canada at the University of British Columbia (1952–59), and in the United States at the University of Wisconsin (1960–70). In 1966 Khorana became a naturalized citizen of the United States, and in 1971 he joined the faculty of the Massachusetts Institute of Technology, where he remained until he retired in 2007. In addition to the Nobel Prize, Khorana received the Albert Lasker Basic Medical Research Award (1968) and the National Medal of Science (1987).
In the 1960s Khorana confirmed Nirenberg’s findings that the way the four different types of nucleotides are arranged on the spiral “staircase” of the DNA molecule determines the chemical composition and function of a new cell. The 64 possible combinations of the nucleotides are read off along a strand of DNA as required to produce the desired amino acids, which are the building blocks of proteins. Khorana added details about which serial combinations of nucleotides form which specific amino acids. He also proved that the nucleotide code is always transmitted to the cell in groups of three, called codons. Khorana also determined that some of the codons prompt the cell to start or stop the manufacture of proteins. Khorana made another contribution to genetics in 1970, when he and his research team were able to synthesize the first artificial copy of a yeast gene. His later research explored the molecular mechanisms underlying the cell signaling pathways of vision in vertebrates. His studies were concerned primarily with the structure and function of rhodopsin, a light-sensitive protein found in the retina of the vertebrate eye. Khorana also investigated mutations in rhodopsin that are associated with retinitis pigmentosa, which causes night blindness.
Har Gobind Khorana (9 January 1922 – 9 November 2011) was an Indian-American biochemist. While on the faculty of the University of Wisconsin–Madison, he shared the 1968 Nobel Prize for Physiology or Medicine with Marshall W. Nirenberg and Robert W. Holley for research that showed the order of nucleotides in nucleic acids, which carry the genetic code of the cell and control the cell's synthesis of proteins. Khorana and Nirenberg were also awarded the Louisa Gross Horwitz Prize from Columbia University in the same year.
Born in British India, Khorana served on the faculties of three universities in North America. He became a naturalized citizen of the United States in 1966, and received the National Medal of Science in 1987.
Biography
Khorana was born to Krishna Devi Khorana and Ganpat Rai Khorana, in Raipur, a village in Punjab, British India (now in present-day Pakistan) in a Hindu family. The exact date of his birth is not certain but he believed that it might have been 9 January 1922; this date was later shown in some documents, and has been widely accepted. He was the youngest of five children. His father was a patwari, a village agricultural taxation clerk in the British Indian government. In his autobiography, Khorana wrote this summary: "Although poor, my father was dedicated to educating his children and we were practically the only literate family in the village inhabited by about 100 people." The first four years of his education were provided under a tree, a spot that was, in effect, the only school in the village.
He attended D.A.V. High School in Multan, in West Punjab. Later, he studied at the Punjab University in Lahore, with the assistance of scholarships, where he obtained a bachelor's degree in 1943 and a Master of Science degree in 1945.
Khorana lived in British India until 1945, when he moved to England to study organic chemistry at the University of Liverpool on a Government of India Fellowship. He received his PhD in 1948 advised by Roger J. S. Beer. The following year, he pursued postdoctoral studies with Professor Vladimir Prelog at ETH Zurich in Switzerland. He worked for nearly a year on alkaloid chemistry in an unpaid position.
During a brief period in 1949, he was unable to find a job in his original home area in the Punjab. He returned to England on a fellowship to work with George Wallace Kenner and Alexander R. Todd on peptides and nucleotides. He stayed in Cambridge from 1950 until 1952.
He moved to Vancouver, British Columbia, with his family in 1952 after accepting a position with the British Columbia Research Council at University of British Columbia. Khorana was excited by the prospect of starting his own lab, a colleague later recalled. His mentor later said that the Council had few facilities at the time but gave the researcher "all the freedom in the world". His work in British Columbia was on "nucleic acids and synthesis of many important biomolecules" according to the American Chemical Society.
In 1960 Khorana accepted a position as co-director of the Institute for Enzyme research at the Institute for Enzyme Research at the University of Wisconsin at Madison. He became a professor of biochemistry in 1962 and was named Conrad A. Elvehjem Professor of Life Sciences at Wisconsin–Madison. While at Wisconsin, "he helped decipher the mechanisms by which RNA codes for the synthesis of proteins" and "began to work on synthesizing functional genes" according to the American Chemical Society. During his tenure at this University, he completed the work that led to sharing the Nobel prize. The Nobel web site states that it was "for their interpretation of the genetic code and its function in protein synthesis". Har Gobind Khorana's role is stated as follows: he "made important contributions to this field by building different RNA chains with the help of enzymes. Using these enzymes, he was able to produce proteins. The amino acid sequences of these proteins then solved the rest of the puzzle."
He became a US citizen in 1966. Beginning in 1970, Khorana was the Alfred P. Sloan Professor of Biology and Chemistry at the Massachusetts Institute of Technology and later, a member of the Board of Scientific Governors at The Scripps Research Institute. He retired from MIT in 2007.
Har Gobind Khorana married Esther Elizabeth Sibler in 1952. They had met in Switzerland and had three children, Julia Elizabeth, Emily Anne, and Dave Roy.
Research
Ribonucleic acid (RNA) with two repeating units (UCUCUCU → UCU CUC UCU) produced two alternating amino acids. This, combined with the Nirenberg and Leder experiment, showed that UCU genetically codes for serine and CUC codes for leucine. RNAs with three repeating units (UACUACUA → UAC UAC UAC, or ACU ACU ACU, or CUA CUA CUA) produced three different strings of amino acids. RNAs with four repeating units including UAG, UAA, or UGA, produced only dipeptides and tripeptides thus revealing that UAG, UAA and UGA are stop codons.
Their Nobel lecture was delivered on 12 December 1968. Khorana was the first scientist to chemically synthesize oligonucleotides. This achievement, in the 1970s, was also the world's first synthetic gene; in later years, the process has become widespread.] Subsequent scientists referred to his research while advancing genome editing with the CRISPR/Cas9 system.
Subsequent research
He extended the above to long DNA polymers using non-aqueous chemistry and assembled these into the first synthetic gene, using polymerase and ligase enzymes that link pieces of DNA together, as well as methods that anticipated the invention of polymerase chain reaction (PCR). These custom-designed pieces of artificial genes are widely used in biology labs for sequencing, cloning and engineering new plants and animals, and are integral to the expanding use of DNA analysis to understand gene-based human disease as well as human evolution. Khorana's invention(s) have become automated and commercialized so that anyone now can order a synthetic oligonucleotide or a gene from any of a number of companies. One merely needs to send the genetic sequence to one of the companies to receive an oligonucleotide with the desired sequence.
After the middle of the 1970s, his lab studied the biochemistry of bacteriorhodopsin, a membrane protein that converts light energy into chemical energy by creating a proton gradient. Later, his lab went on to study the structurally related visual pigment known as rhodopsin.
A summary of his work was provided by a former colleague at the University of Wisconsin: "Khorana was an early practitioner, and perhaps a founding father, of the field of chemical biology. He brought the power of chemical synthesis to bear on deciphering the genetic code, relying on different combinations of trinucleotides."
Awards and honors
In addition to sharing the Nobel prize (while he was working at the University of Wisconsin–Madison in the U.S.), Khorana was elected as Foreign Member of the Royal Society (ForMemRS) in 1978. In 2007, the University of Wisconsin–Madison, the Government of India (DBT Department of Biotechnology), and the Indo-US Science and Technology Forum jointly created the Khorana Program, jointly. The mission of the Khorana Program is to build a seamless community of scientists, industrialists, and social entrepreneurs in the United States and India.
The program is focused on three objectives: Providing graduate and undergraduate students with a transformative research experience, engaging partners in rural development and food security, and facilitating public-private partnerships between the U.S. and India. The Wisconsin–India Science and Technology Exchange Program (WINStep Forward, WSF) adopted administration responsibilities for the Khorana program in 2007. WINStep Forward was jointly created by Drs. Aseem Ansari and Ken Shapiro at the University of Wisconsin–Madison. WINStep Forward also administers the nationally competitive S.N. Bose Programs for Indian and American students, respectively, to promote both fundamental and applied research not only in biotechnology but broadly across all STEM (science, technology, engineering, and mathematics) fields, including medicine, pharmacy, agriculture, wildlife and climate change.
In 2009, Khorana was hosted by the Khorana Program and honored at the 33rd Steenbock Symposium in Madison, Wisconsin.
Other honours included the Louisa Gross Horwitz Prize from Columbia University and the Lasker Foundation Award for Basic Medical Research, both in 1969, the Willard Gibbs Medal of the Chicago section of the American Chemical Society, in 1974, the Gairdner Foundation Annual Award, in 1980 and the Paul Kayser International Award of Merit in Retina Research, in 1987.
On 9 January 2018, a Google Doodle celebrated the achievements of Har Gobind Khorana on what would have been his 96th birthday.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#536 2019-04-17 01:27:45
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,479
Re: crème de la crème
503) Ányos Jedlik
Ányos István Jedlik (11 January 1800 – 13 December 1895) was a Hungarian] inventor, engineer, physicist, and Benedictine priest. He was also a member of the Hungarian Academy of Sciences, and author of several books. He is considered by Hungarians and Slovaks to be the unsung father of the dynamo and electric motor.
Career
He was born in Szimő, Kingdom of Hungary (today Zemné, Slovakia). His mother was a member of a Hungarian noble family, while his father's family was of Slovak origin moving in 1720 from Liptó County to Szimő.
Jedlik's education began at high schools in Nagyszombat (today Trnava) and Pressburg (today Bratislava). In 1817 he became a Benedictine, and from that time continued his studies at the schools of that order, where he was known by his Latin name Stephanus Anianus. He lectured at Benedictine schools up to 1839, then for 40 years at the Budapest University of Sciences department of physics-mechanics. Few guessed at that time that his activities would play an important part in bringing up a new generation of physicists. He became the dean of the Faculty of Arts in 1848, and by 1863 he was rector of the University. From 1858 he was a corresponding member of the Hungarian Academy of Sciences and from 1873 was an honorary member. After his retirement he continued working and spent his last years in complete seclusion at the priory in Győr, where he died.
Scientific work
In 1827, Jedlik started experimenting with electromagnetic rotating devices which he called lightning-magnetic self-rotors, and in 1828 he demonstrated the first device which contained the three main components of practical direct current motors: the stator, rotor, and commutator. In the prototype both the stationary and the revolving parts were electromagnetic. The first electromotor, built in 1828, and Jedlik's operating instructions are kept at the Museum of Applied Arts in Budapest. The motor still works perfectly today. However, Jedlik only reported his invention decades later and the true date of it is uncertain.
He was a prolific author. In 1845, Jedlik was the first university professor in the Kingdom of Hungary who began teaching his students in Hungarian instead of Latin. His cousin Gergely Czuczor, a Hungarian linguist, asked him to create a Hungarian technical vocabulary in physics, the first of its kind, by which he became one of its founders.
In the 1850s he conducted optical and wave-mechanical experiments, and at the beginning of the 1860s he constructed an excellent optical grate.
He was ahead of his contemporaries in his scientific work, but he did not speak about his most important invention, his prototype dynamo, until 1856; it was not until 1861 that he mentioned it in writing in a list of inventory of the university. Although that document might serve as evidence of Jedlik's being the first dynamo, the invention of the dynamo is linked to Siemens's name because Jedlik's invention did not rise to notice at that time.
In 1863 he discovered the possibility of voltage multiplication and in 1868 demonstrated it with a "tubular voltage generator", which was successfully displayed at the Vienna World Exposition in 1873. It was an early form of the impulse generators now applied in nuclear research. The jury of the World Exhibition of 1873 in Vienna awarded his voltage multiplying condenser of cascade connection with a prize "For Development". Through this condenser, Jedlik framed the principle of surge generation by cascaded connection. (The cascade connection was an other important invention of Ányos Jedlik).
Dynamo invention
Jedlik's best known invention is the principle of dynamo self-excitation.
In 1827, Jedlik started experimenting with electromagnetic rotating devices which he called electromagnetic self-rotors.
In the prototype of the single-pole electric starter, both the stationary and the revolving parts were electromagnetic. In essence, the concept is that instead of permanent magnets, two opposed electromagnets induce the magnetic field around the rotor. He formulated the concept of the self-excited dynamo about 1861, six years before Siemens and Wheatstone.
As one side of the coil passes in front of the north pole, crossing the line of force, current is induced. As the frame rotates further the current diminishes, then arriving at the front of the south pole it rises again but flows in the opposite direction. The frame is connected to a commutator, thus the current always flows in the same direction in the external circuit.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#537 2019-04-19 00:04:37
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,479
Re: crème de la crème
504) Johann Wolfgang Döbereiner
Johann Wolfgang Döbereiner, (born Dec. 13, 1780, Hof an der Saale [Germany]—died March 24, 1849, Jena), German chemist whose observation of similarities among certain elements anticipated the development of the periodic system of elements.
As a coachman’s son, Döbereiner had little opportunity for formal schooling, but he was apprenticed to an apothecary, read widely, and attended learned science lectures. Eventually he was able to attend the University of Jena, where he became an assistant professor (1810) and later was supervisor of science instruction. He was a lifelong friend of Johann Wolfgang von Goethe.
During the 1820s Döbereiner’s experiments with the ignition of hydrogen on contact with powdered platinum led the Swedish chemist J.J. Berzelius to develop the concept of catalysis. Toward the end of the decade Döbereiner found that the properties of bromine, a liquid, seem halfway between those of chlorine gas and the solid iodine. He recalled a comparable graduation of properties in two other sequences—calcium, strontium, barium; and sulfur, selenium, tellurium. He showed that in each triad the mean of the lightest and heaviest atomic weights approximated the atomic weight of the middle element. But he could not substantiate his hypothesis with a sufficient number of triads, and his findings were regarded in his time as merely interesting curiosities. Döbereiner also discovered the organic compound furfural and developed the separation of calcium and magnesium.
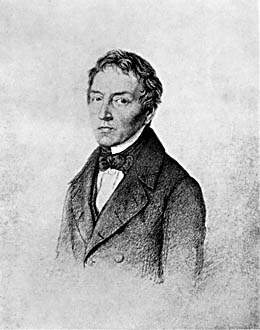
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#538 2019-04-21 00:11:06
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,479
Re: crème de la crème
505) James Beaumont Neilson
James Beaumont Neilson, (born June 22, 1792, Shettleston, Lanark, Scot.—died Jan. 18, 1865, Queenshill, Kirkcudbright), Scottish inventor who introduced the use of a hot-air blast instead of a cold-air blast for the smelting of iron, thus greatly advancing the technology of iron production.
In 1817 Neilson was appointed foreman of the Glasgow Gasworks. Soon afterward he became manager and engineer, and he remained with the firm for 30 years.
During the early 19th century, ironworkers in Great Britain believed that a blast of cold air was the most efficient method for smelting iron. Neilson demonstrated that the opposite was true. His idea, first tested at the Clyde Ironworks, Glasgow, was patented in 1828. Use of the hot blast tripled iron output per ton of coal and permitted the profitable recovery of iron from lower-grade ores. It also made possible the efficient use of raw coal and lower grades of coal instead of coke and permitted the construction of larger smelting furnaces.
James Beaumont Neilson (22 June 1792 – 18 January 1865) was a Scottish inventor whose hot-blast process greatly increased the efficiency of smelting iron.
Life
He was the son of the engineer Walter Neilson, a millwright and later engine wright, who had been a partner of David Mushet in Calder Ironworks, Glasgow. He was born in Shettleston and was trained as an engine wright. After the failure of a colliery at Irvine he was appointed foreman of the Glasgow Gasworks in 1817 at the age of 25. Five years later he became the manager and engineer there, a position he held for 40 years.
While trying to solve a problem with a blast furnace at Wilsontown Ironworks, Neilson realized that the fuel efficiency of the furnace could be increased by blowing it with hot air, rather than cold air, by passing it through a red-hot vessel. Experiments were continued at Clyde Iron Works, leading to his forming a partnership with Charles Macintosh and others to exploit it. Patents were obtained for the system in 1828.
Experimentation showed that a temperature of 600° Fahrenheit reduced consumption to a third of that with cold blast, and enabled raw coal to be used instead of coke, with a further cost saving. It also enabled the exploitation of black band ironstone, the use of which had previously proved unprofitable.
In the early 1830s litigation was successfully conducted against those who adopted his methods without licence. After that, Neilson and his partners licensed it widely at one shilling per ton iron made, a level low enough to discourage evasion. The royalties were initially low, but by 1840 were producing £30,000 per year from 58 ironmaste.
Certain infringers were intransigent. Between 1839 and the expiry of the patent in 1842 a considerable number of proceedings were brought. Neilson v Baird was heard in the Court of Session in 1843, in a trial lasting 10 days and costing £40,000. Further proceedings against Baird ended in the award of damages of £160,000.
Neilson retired from Glasgow Gasworks in 1847. He bought an estate on Bute. Later he bought an estate at Queenshill, near Kirkcudbright. There he died. He is buried in the family mausoleum at Tongland Kirkyard. His son, Walter Montgomerie Neilson, erected Neilson's Monument to his memory on the hill at Queenshill in 1883.
Both in Glasgow and near Kirkcubright, he founded institutions for the education of working men.
He was a grandfather of High Excellency Nikolai Sultan Giray Crimea Khan 1836—1920, a russian Active Privy Councillor and senator.
William Neilson, James's brother, founded the Glasgow engineers and locomotive manufacturers Neilson and Company, in 1836, partly financed by James. James's son Walter took over the running of the firm in 1843.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#539 2019-04-23 01:31:44
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,479
Re: crème de la crème
506) Isaac Charles Johnson
Isaac Charles Johnson (28 January 1811 – 29 November 1911) was a British cement manufacturer, and a pioneer of the Portland cement industry.
Born in London, his father was a charge-hand at Francis & White's "Roman Cement" plant in Nine Elms. He himself worked there as a labourer from age 16 while studying chemistry. In 1833 he became manager of John Bazeley White's cement plant at Swanscombe on the Thames Estuary which at that time was producing "Artificial Cement" and "Roman Cement". Joseph Aspdin's product was successful but very expensive,and was later improved independently by his son William. Johnson set to work trying to discover its composition but because Aspdin's product was protected by explicit patents and extreme secrecy it was impossible to market a copy. After nearly two years' work, he succeeded and started marketing his own considerably improved version.
Johnson, a highly moral man, Mayor of Gateshead and a JP, was able to claim that he was the inventor of "true" Portland cement and is generally recognised as such. Aspdin, however, was driven out of business by financial problems caused by the success of Johnson's superior and cheaper product and this led to Johnson taking over Aspdins Cement works at Gateshead. Unfortunately this resulted in an embittered Aspdin making wild and vitriolic charges of how his product had been copied. Johnson left J.B. White's shortly afterwards, and, setting up his own company, established a succession of cement plants at Frindsbury, Cliffe and Greenhithe in Kent, and acquired William Aspdin's plant at Gateshead, County Durham. He pioneered several innovations, including the production of low-water rawmix slurries, and new designs for kilns and industrial chimneys. His company remained a relatively large and successful player in the British cement industry for the next 60 years. The Greenhithe plant was uprated with rotary kilns in 1901. In 1911 I C Johnson & Co became a part of the Blue Circle Group, and his Greenhithe plant remained in operation until 1971. In 1910 on his 100th birthday Johnson was presented with a silver tea service by representatives of the cement industry in Britain and several European Countries.
Johnson wrote the Autobiography of Isaac Charles Johnson Esq, JP (published by Farncombe & Sons, London, 1912), which was published after his death.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#540 2019-04-25 00:06:13
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,479
Re: crème de la crème
507) Martin van Marum
Martin(us) van Marum (20 March 1750, Delft – 26 December 1837, Haarlem) was a Dutch physician, inventor, scientist and teacher, who studied medicine and philosophy in Groningen. Van Marum introduced modern chemistry in the Netherlands after the theories of Lavoisier, and several scientific applications for general use. He became famous for his demonstrations with instruments, most notable the ‘Large electricity machine’, to show statical electricity and chemical experiments while curator for the Teylers Museum.
Biography
Early career
Born in Delft, Van Marum moved to Haarlem in 1776 ‘because the Haarlemmers had more taste in the sciences than anywhere else in the Netherlands’. After his arrival in Haarlem he began to practise medicine, but devoted himself mainly to lecturing on physical subjects and creating instruments to demonstrate physical theory. He must have made a big impression on Haarlem society, because he became a member of the Dutch Society of Science in the same year, but was named director and curator of their cabinet of curiosities in the next year.
Van Marum received at first no salary, but by scaring off the former cabinet concierge Nicolaus Linder, he was able to collect Linder's' salary of 100 guilders, and when the cabinet moved later in 1777 to new quarters in the Grote Houtstraat 51, van Marum lived there as concierge. He managed to scare off Linder by obtaining permission from the society to allow his servants to keep tips they received from cabinet visitors; a source of income that Linder had come to rely on. Then van Marum increased this salary to 300 from 100 by adding responsibilities to his list of duties, such as a summer garden in the Rozenprieel and eliminating other expenses.
Curator of two museums
In 1779 he was entrusted with the care of the ‘Second society’ left to Haarlem by Pieter Teyler van der Hulst (1702–1778), which led under his direction to the foundation of the Teylers Museum. The Teyler legacy was split into three societies, one for religion, one for science, and one for the arts, known as the first, second, and third societies. The caretakers had to meet in Teyler's home weekly, and each society had 5 caretakers, so all of the gentlemen involved lived in Haarlem.
In 1794 van Marum became secretary as well as director of the Dutch Society of Science. Under his management, both societies were advanced to the position of the most noted in Europe. Period travelogues mention both Museums.
Besides being involved with the ‘Hollandsche Maatschappij der Wetenschappen’, he was an ordinary member (5 December 1776) and a corresponding member (from 25 December 1776) of the 'Provinciaal Utrechtsch Genootschap van Kunsten en Wetenschappen', a member of the 'Bataafsch Genootschap der Proefondervindelijke wijsbegeerte' from 1784, a member of the 'Zeeuwsch Genootschap der Wetenschappenfrom' 27 August 1782, corresponding member of the 'Académie des Sciences' from 1783, and a member of the ‘Vergadering van Notabelen voor het departement Zuiderzee’ from 29 March 1814. In 1808 he was asked by Louis Bonaparte to be a member of the committee for the formation of the Koninklijk Instituut along with Jeronimo de Bosch, Jean Henri van Swinden, and Martinus Stuart. He became member of the institute the same year.
Merging societies, separate collections
Under his guidance the two societies slowly merged. His name is associated with the ‘Electriseermachine’, the largest electricity demonstration machine with Leiden jars built in the 18th century and at the time a crowd pleaser for the young Teylers museum. The demonstration model is still on display, as is a smaller version in the Museum Boerhaave of Leiden. Van Marum's researches (especially in connection with electricity) were remarkable for their number and variety.
The Teyler's Museum kept its role as a museum of scientific research (some of the periodical subscriptions he started are still running) and is a repository of important scientific demonstration models from the period. Not only items regarding electricity, but also weather stations, industrial models, steam engines, and other examples of the budding industrial revolution were collected and lovingly displayed. The collection of the Teyler's was mostly based on scientific theory, while the collection of the Dutch Society of Science was mostly based on scientific practise. The rooms in the Grote Houtstraat were filled with stuffed animals and other "naturalia", while the summer garden was a modern continuation of Linder's old Linnaeus hortus once located behind the original city hall quarters in the Prinsenhof. Since Linder had not known any Latin, it was easier for Van Marum to entertain foreign visitors with stories of Linnaean trivia and of course, the Haarlem story of tulip mania.
Teylers Museum
In 1784, when the Teylers Museum opened its new 'Oval Room', the artist Vincent Jansz van der Vinne was hired as curator of the art collection and lived in Pieter Teyler's former residence that was called the "Fundatiehuis" as concierge and caretaker of the art collection. He left the next year because of continuous disagreements with van Marum over art and the opening hours of the museum.
Van der Vinne was an artist born into an important Haarlem artist family – he was the great-grandson of Vincent van der Vinne. The Teyler's museum replaced him with another local artist, Wybrand Hendricks, who painted the famous oval room and many other Haarlem scenes. Hendricks is largely responsible for the Teyler's collection of Old Master prints, most notably the purchase in Rome 1790 of a print & drawing collection formerly owned by Christina of Sweden. Apparently he got along under van Marum, but when he left in 1819 at the age of 75, the Teyler's decided to discontinue the purchase of art "for the decline in art enthusiasts in this city".
During the tenure of Hendriks, van Marum himself was busy giving public demonstrations of electricity in the Oval room, but was also collecting in this period (this is why he was so involved with the lifestyle of the concierge of the Fundatiehuis, since he was there every day). He concentrated on scientific publications for the Teyler library. He concentrated his efforts on three aspects: 1) Greek and Latin authors, among them the church fathers, 2) Works of natural history including travelogues, and 3) natural history periodicals, including all publications of the Royal Society of London and all publications of the Dutch Society of Science, which Teyler had been a member of, but could not be on the board of, due to religious differences with the board. The criteria for purchase was always expense. If a Society member could afford to purchase it himself it was not worth adding to the collection. Any member could suggest purchases, however, which explains why the collection is filled with richly illustrated examples of contemporary publications. The most impressive of these are the large illustrated books of travellers. To view the collection, van Marum organised ‘gentleman evenings’ in Pieter Teyler's library, a tradition that still exists.
Legacy
Though the public is allowed access during the day to the museum rooms, the private rooms of Pieter Teyler in the "Fundatiehuis" are only open one day a year, on Monument Day. Because of its rich scientific tradition, many noted scholars of Physics moved to Haarlem to work at Teyler's, including Nobel prize winners Pieter Zeeman and Hendrik Lorentz.
Of van Marum's three categories, only the first was discontinued at the close of the 19th century. The collection of periodicals which has been expanded through exchange networks, contains uninterrupted series that are among the oldest in the world.
The Teyler's museum created a new wing in 1996 to house a rotational display of van Marum's library collection, such as the works of John James Audubon in combination with contemporary stuffed birds of Naturalis.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#541 2019-04-27 01:01:53
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,479
Re: crème de la crème
508) Alois Senefelder
Alois Senefelder, Alois also spelled Aloys, (born Nov. 6, 1771, Prague—died Feb. 26, 1834, Munich), German inventor of lithography.
The son of an actor at the Theatre Royal in Prague, Senefelder was unable to continue his studies at the University of Ingolstadt after his father’s death and thus tried to support himself as a performer and author, but without success. He learned printing in a printing office, purchased a small press, and sought to do his own printing.
Desiring to publish plays that he had written but unable to afford the expensive engraving of printing plates, Senefelder tried to engrave them himself. His work on copper plates was not proving very successful when an accident led to his discovery of the possibilities of stone (1796).
Senefelder records that one day he jotted down a laundry list with grease pencil on a piece of Bavarian limestone. It occurred to him that if he etched away the rest of the surface, the markings would be left in relief. Two years of experimentation eventually led to the discovery of flat-surface printing (modern lithography). In 1818 he documented his discovery in ‘Vollständiges Lehrbuch der Steindruckerey’ (1818; A Complete Course of Lithography).
Senefelder later accepted an offer from a music publisher, Johann Anton André, to set himself up at Offenbach and train others in his lithographic process. In later years the king of Bavaria settled a handsome pension on Senefelder.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#542 2019-04-29 00:23:48
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,479
Re: crème de la crème
509) Waldemar Jungner
Ernst Waldemar Jungner (June 19, 1869 – August 30, 1924) was a Swedish inventor and engineer. In 1899 he invented the nickel-iron electric storage battery (NiFe), the nickel-cadmium battery (NiCd) and the rechargeable alkaline silver-cadmium battery (AgCd). As an inventor he also fabricated a fire alarm based on different dilutions of metals. He worked on the electrolytic production of sodium carbonate, and patented a rock drilling device.
Early life
Ernst Waldemar Jungner was born in 1869 in Västra Götaland County, Sweden.[1] His parents were ministers, and his father died when Waldemar was 13 years old. In 1869, the year he was born, failed harvests caused famine throughout Sweden, which affected Jungner's health. He also contracted measles and scarlet fever.
Education
He attended Skara upper secondary school, and studied chemistry, mathematics, astronomy, botany, geology and Latin at Uppsala University. He went on to carry out further studies at the Royal Institute of Technology (KTH) in Stockholm.
Business
In 1900 he started the firm "Ackumulator Aktiebolaget Jungner". There was a long patent dispute with Edison which was won by Edison in the end because he had larger financial resources. This caused serious problems to Jungner's firm. The company managed to survive by using a slightly different name "Nya Ackumulator Aktiebolaget Jungner" in 1904. Jungner left the management of the company at this time, but remained a consultant to the new firm. This company was wound up in 1910, and a new company "Ackumulator Aktiebolaget Jungner" was created, which profitably used new technology developments. A descedant company "NiFe Junger" in 1991 became part of Saft Groupe S.A..
Battery use
Nickel-cadmium batteries were commonly used in the power systems of rockets and artificial satellites through t the 1960s and 1970s, as well as in terrestrial portable electrical devices.
On the rescue mission to Umbreto Nobile and his companions on the North pole expedition in 1928, several batteries were dropped from an airplane to supply electricity to the radio of the expedition. Only the Jungner NiFe battery worked.
Later life
Jungner patented designs for a fuel cell in 1907. He carried out investigations into the production of cement, and the extraction of radium from ores. Jungner joined the Royal Swedish Academy of Engineering Sciences in 1922 and in 1924 he received the Swedish Chemical Society´s Oscar Carlson Medal. Jungner died in 1924 of pneumonia at the age of 55.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#543 2019-05-01 00:35:40
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,479
Re: crème de la crème
510) Gaston Planté
Gaston Planté, (born April 22, 1834, Orthez, France—died May 21, 1889, Paris), French physicist who produced the first electric storage battery, or accumulator, in 1859; in improved form, his invention is widely used in automobiles.
Planté followed an academic career, beginning in Paris as a lecture assistant in physics at the Conservatory of Arts and Crafts in 1854 and, six years later, rising to the post of professor of physics at the Polytechnic Association for the Development of Popular Instruction.
In 1859 Planté began experiments that resulted in construction of a battery for the storage of electrical energy; his first model contained two sheets of lead, separated by rubber strips, rolled into a spiral, and immersed in a solution containing about 10 percent sulfuric acid. A year later he presented a battery to the Academy of Sciences consisting of nine of the elements described above, housed in a protective box with the terminals connected in parallel. His battery could deliver remarkably large currents.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#544 2019-05-03 01:11:33
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,479
Re: crème de la crème
511) Sir William Robert Grove
Sir William Robert Grove, (born July 11, 1811, Swansea, Glamorgan, Wales—died August 1, 1896, London), British physicist and a justice of Britain’s High Court (from 1880), who built the first fuel cell in 1842 and first offered proof of the thermal dissociation of atoms within a molecule.
Grove was educated by private tutors and then at Brasenose College, Oxford, and also studied law at Lincoln’s Inn and was called to the bar in 1835. Ill health interrupted his law career, and he turned to science. In 1839 he developed the two-fluid electric cell, known as the Grove battery, consisting of amalgamated zinc in dilute sulfuric acid and a platinum cathode in concentrated nitric acid, the liquids being separated by a porous container. At the London Institution, where he was professor of experimental philosophy (1840–47), he used his platinum-zinc batteries to produce electric light for one of his lectures. In 1842 he developed the “gas battery,” the first fuel cell, in which the formation of water from hydrogen and oxygen gas generated an electric current.
His classic On the Correlation of Physical Forces (1846) enunciated the principle of conservation of energy a year before the German physicist Hermann von Helmholtz did so in his famous paper Über die Erhaltung der Kraft (“On the Conservation of Force”).
His scientific career led to the practice of patent and other law after 1853. He was appointed to the Court of Common Pleas in 1871 and was knighted in 1872. After retirement from the bench in 1887, he resumed his scientific studies.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#545 2019-05-05 00:58:28
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,479
Re: crème de la crème
512) Gerardus Mercator
Gerardus Mercator, original name Gerard De Cremer, or Kremer?, (born March 5, 1512, Rupelmonde, Flanders [now in Belgium]—died December 2, 1594, Duisburg, Duchy of Cleve [Germany]), Flemish cartographer whose most important innovation was a map, embodying what was later known as the Mercator projection, on which parallels and meridians are rendered as straight lines spaced so as to produce at any point an accurate ratio of latitude to longitude. He also introduced the term atlas for a collection of maps.
Mercator’s family had moved from Germany to Flanders shortly before he was born. He was educated in Hertogenbosch (Netherlands), receiving training in Christian doctrine, dialectics, and Latin. In 1530 he entered the Catholic University of Leuven (Louvain [Belgium]) to study the humanities and philosophy and graduated with a master’s degree in 1532.
Religious doubts assailed him about this time, for he could not reconcile the biblical account of the origin of the universe with that of Aristotle. After two years of study which led him to Antwerp and Mechelen he emerged from his personal crisis, fortified in his faith, with less enthusiasm for philosophical speculation. Moreover, he brought back to Leuven a freshly acquired taste for geography.
Under the guidance of Gemma Frisius, the leading theoretical mathematician in the Low Countries, who was also a physician and astronomer, Mercator mastered the essentials of mathematics, geography, and astronomy. Frisius and Mercator also frequented the workshop of Gaspar à Myrica, an engraver and goldsmith. The combined work of these three men soon made Leuven an important centre for the construction of globes, maps, and astronomical instruments. In 1534 Mercator married Barbara Schellekens, by whom he had six children.
By the time he was age 24, Mercator was a superb engraver, an outstanding calligrapher, and a highly skilled scientific-instrument maker. In 1535–36 he cooperated with Myrica and Frisius in constructing a terrestrial globe and in 1537 its celestial counterpart. These globes demonstrate the free and graceful italic lettering with which Mercator was to change the face of 16th-century maps. During that period he also began to build his reputation as the foremost geographer of the century with a series of printed cartographic works: in 1537 a map of Palestine, in 1538 a map of the world on a double heart-shaped projection, and about 1540 a map of Flanders. In 1540 he also published a concise manual on italic lettering, the Literarum Latinarum quas Italicas cursoriasque vocant scribende ratio, for which he engraved the wood blocks himself.
In 1544 he was arrested and imprisoned on a charge of heresy. His inclination to Protestantism, and frequent absences from Leuven to gather information for his maps, had aroused suspicions; he was one of 43 citizens so charged. But the university authorities stood behind him. He was released after seven months and resumed his former way of life. He obtained a privilege to print and publish books and was free to continue his scientific studies.
In 1552 Mercator moved permanently to Duisburg in the Duchy of Cleve. Once there, he became a well-known figure. He assisted the duke in establishing a grammar school by helping to design its curriculum. After establishing a cartographic workshop and engaging his own engravers, he returned to his main interest.
In 1554 he published a map of Europe that he had begun at Leuven, and between 1559 and 1562 he taught mathematics in the grammar school. During these busy years he also undertook genealogical research for Duke Wilhelm, drew up a Concordance of the Gospels, and composed a detailed commentary on the first part of the Letter of Paul to the Romans. In 1564 he completed a map of Lorraine (now lost) and another of the British Isles. Public recognition of his accomplishments came in 1564 with his appointment as court “cosmographer” to Duke Wilhelm of Cleve. During these years he perfected his projection, which enabled mariners to steer a course over long distances by plotting straight lines without continual adjustment of compass readings. This technique immortalized his name in the “Mercator projection,” which he used on his map of the world in 1569.
Mercator then began to execute a series of publications intended to describe the creation of the world and its subsequent history. This Atlas—the term still used to indicate a collection of maps—was never fully realized.
In 1569, as the first section, he published a chronology of the world from the Creation to 1568. He then published 27 of the maps originally prepared by the Greek geographer Ptolemy, with corrections and commentary in 1578, under the title 'Tabulae Geographicae C. Ptolemei ad mentem autoris restitutae et emendatae'. The next part of the Atlas, consisting of a set of new maps covering France, Germany, and the Netherlands, came out in 1585, with maps of Italy, “Sclavonia” (now the Balkan countries), and Greece following in 1589. A last section, on the British Isles, was included in an edition with the previous sections, which was seen through the press after his death by his son in 1595. Another printing followed in 1602, and further maps were added in a later edition of 1606, usually called the “Mercator–Hondius Atlas.”

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#546 2019-05-07 00:11:03
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,479
Re: crème de la crème
513) Alexander Bain (inventor)
Alexander Bain (12 October 1810 – 2 January 1877) was a Scottish inventor and engineer who was first to invent and patent the electric clock. He installed the railway telegraph lines between Edinburgh and Glasgow.
(In 1840, Alexander Bain, a Scottish clock and instrument maker was the first to invent and patent a clock powered by electric current. His original electric clock patent is dated October 10, 1840. On January 11, 1841, Alexander Bain along with John Barwise, a chronometer maker, took out another important patent describing a clock in which an electromagnetic pendulum and an electric current is employed to keep the clock going instead of springs or weights. Later patents expanded on his original ideas.)
Early life
Bain was born in Houstry, Caithness, Scotland. He was baptised in the local kirk on 22 November 1810. His father was a crofter. He had a twin sister, Margaret, and, in total, he had six sisters and six brothers. Bain did not excel in school and was apprenticed to a clockmaker in Wick.
Career
Having learned the art of clockmaking, he went to Edinburgh, and in 1837 to London, where he obtained work as a journeyman in Clerkenwell. Bain frequented the lectures at the Polytechnic Institution and the Adelaide Gallery and later constructed his own workshop in Hanover Street.
Electric clocks
In 1840, desperate for money to develop his inventions, Bain mentioned his financial problems to the editor of the Mechanics Magazine, who introduced him to Sir Charles Wheatstone. Bain demonstrated his models to Wheatstone, who, when asked for his opinion, said "Oh, I shouldn't bother to develop these things any further! There's no future in them." Three months later Wheatstone demonstrated an electric clock to the Royal Society, claiming it was his own invention. However, Bain had already applied for a patent for it. Wheatstone tried to block Bain's patents, but failed. When Wheatstone organised an Act of Parliament to set up the Electric Telegraph Company, the House of Lords summoned Bain to give evidence, and eventually compelled the company to pay Bain £10,000 and give him a job as manager, causing Wheatstone to resign.
Bain's first patent was dated 11 January 1841, and was in the names of John Barwise, chronometer maker, and Alexander Bain, mechanist. It describes his electric clock which uses a pendulum kept moving by electromagnetic impulses. He improved on this in later patents, including a proposal to derive the required electricity from an "earth battery", which consisted of plates of zinc and copper buried in the ground.
In December 1841, Bain in conjunction with Lieutenant Thomas Wright RN, patented a method for using electricity to control railway engines by turning off steam, marking time, giving signals, and printing information at different locations. The most significant idea incorporated in the patent was his plan for inverting the needle telegraph earlier developed by Ampere, Wheatstone and others: instead of making signals by a pivoted magnetic needle under the influence of an electromagnet, he made them by suspending a movable coil between the poles of a fixed magnet. A similar concept appears in Sir William Thomson's siphon recorder. Bain also proposed to make the coil record messages by printing them, an idea he developed further in a subsequent patent. His telegraph was also successful abroad: "The initial Austrian Bain instruments were made by Johann Michael Ekling in Vienna, and later by the k.k. Telegraphenwerkstätte Wein, the Imperial Royal Telegraph Workshops Vienna."
"For many years I have devoted myself to rendering electricity practically useful, and have been extensively engaged, not only in this country, but in America and on the Continent, in the construction and working of the Electric Telegraph; while at the same time, the employment of electricity in the measurement of time has also engaged my attention."
Alexander Bain, ‘A Short History of the Electric Clocks’
Surviving examples
Bain's extant electric clocks come from two stages of development between the 1840s and the 1860s. Examples can be seen in the National Museum of Scotland, the National Maritime Museum of London, the London Science Museum and the Deutsches Uhrenmuseum. The most rare and interesting mantel clocks are in private hands. One featured in the "Electrifying Time" exhibition in 1977 at the London Science Museum. Bain sometimes found complex and ornate solutions to relatively simple, although not easy to solve problems. The most complex, his mantel clock, worked on an electro-magnetic pull push, pull push for each complete period of swing employing a pendulum with opposing magnetic fields. At a very similar time, Matthäus Hipp constructed a more simple and reliable system which used a simple toggle switch to impulse the pendulum every so often.
Facsimile machine
Bain worked on an experimental fax machine in 1843 to 1846. He used a clock to synchronise the movement of two pendulums for line-by-line scanning of a message. For transmission, Bain applied metal pins arranged on a cylinder made of insulating material. An electric probe that transmitted on-off pulses then scanned the pins. The message was reproduced at the receiving station on electrochemically sensitive paper impregnated with a chemical solution similar to that developed for his chemical telegraph. In his patent description dated 27 May 1843 for "improvements in producing and regulating electric currents and improvements in timepieces, and in electric printing, and signal telegraphs," he claimed that "a copy of any other surface composed of conducting and non-conducting materials can be taken by these means". The transmitter and receiver were connected by five wires. In 1850 he applied for an improved version but was too late, as Frederick Bakewell had obtained a patent for his superior "image telegraph" two years earlier in 1848.
Bain's and Bakewell's laboratory mechanisms reproduced poor quality images and were not viable systems because the transmitter and receiver were never truly synchronized. In 1861, the first practical operating electro-mechanical commercially exploited telefax machine, the Pantelegraph, was invented by the Italian physicist Giovanni Caselli. He introduced the first commercial telefax service between Paris and Lyon at least 11 years before the invention of workable telephones.
Chemical telegraph
On 12 December 1846, Bain, who was then living in Edinburgh, patented a chemical telegraph. He had seen that the Morse and other telegraphs then in use were comparatively slow, due to the mechanical inertia of their moving parts, and realized that the signal current could be used to make a readable mark on a moving paper tape soaked in a mixture of ammonium nitrate and potassium ferrocyanide, which gave a blue mark when a current was passed through it.
The speed at which marks could be made on the paper was so high that hand signalling could not keep up with it, and so Bain devised a method of automatic signalling using punched paper tape. The concept was later used by Wheatstone in his automatic sender. Bain's chemical telegraph was tried between Paris and Lille, and attained a speed of 282 words in 52 seconds, a great advance on Morse's telegraph which could only give about 40 words per minute.
In England Bain's telegraph was used on the wires of the Electric Telegraph Company to a limited extent, and in 1850 it was used in America by Henry O'Reilly. However, it incurred the hostility of Samuel Morse, who obtained an injunction against it on the grounds that the paper tape and alphabet used fell under his patent. Consequently, by 1859 Bain's telegraph was in use on only one line and never really entered general usage.
Later life
Initially Bain made a considerable sum from his inventions but lost his wealth in poor investments. In 1873, Sir William Thomson, Sir William Siemens, Latimer Clark and others obtained a Civil List pension for Bain from Prime Minister William Ewart Gladstone of £80 per year.
Death and legacy
Bain was buried in the Auld Aisle Cemetery, Kirkintilloch. It was restored in 1959. The headstone had a fallacious date of death (1876) which was later corrected to 1877. JD Wetherspoons pub in Wick, close to where Alexander Bain served his apprenticeship, is now named 'The Alexander Bain' after the inventor. Also, as a tribute to his inventions, the main BT building in Glasgow is named Alexander Bain House. One of the earliest examples of an electrically impulsed pendulum clock is on display at the Deutsches Uhrenmuseum.
In 2016 he was awarded the Technology & Engineering Emmy Award "for his pioneering work in the transmission of images"

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#547 2019-05-09 00:06:44
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,479
Re: crème de la crème
514) William Reid Clanny
William Reid Clanny, (born 1776, Bangor, County Down, Ire.—died Jan. 10, 1850, near Sunderland, Durham, Eng.), physician who invented one of the first safety lamps (1813) for use in coal mines; some of its features were incorporated in Sir Humphry Davy’s safety lamp, which was the precursor of modern safety lamps.
Educated at the University of Edinburgh (M.D.), Clanny served with the navy before becoming a private practitioner. In Clanny’s time, a serious hazard of coal mining was ignition by miners’ lamps of firedamp, an explosive mixture of air and methane, a gas commonly present in deposits of coal. Clanny developed a miner’s lamp that would not ignite firedamp but was unwieldy; he later reduced its bulk and adapted several improvements devised by Davy, one of which was a shield of metal gauze.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#548 2019-05-11 02:56:08
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,479
Re: crème de la crème
515) François Isaac de Rivaz
François Isaac de Rivaz (Paris, December 19, 1752 – Sion, July 30, 1828) was an inventor and a politician. He invented a hydrogen-powered internal combustion engine with electric ignition and described it in a French patent published in 1807. In 1808 he fitted it into a primitive working vehicle – "the world's first internal combustion powered automobile".
Biography
Isaac was born in Paris to a family from Valais. In 1763 the family settled in Moûtiers in Savoy (Kingdom of Sardinia). The last two boys, Anne Joseph and Isaac, came to settle in St. Gingolph in Valais. It is not known at which schools he studied, but he became fluent in Latin plus mastering mathematics and geometry, whilst continuing his study of mechanics throughout his life. He qualified as both a surveyor and notary and worked for the state of Valais.
Isaac had many interests plus an intuitive and extremely curious mind which was driven by the demon of discovery. His experimental work was overflowing. He experimented with steam-powered vehicles in the late 18th century. He also studied the ignition of combustible gases.
Internal combustion engine
After retirement from the Army, living in Switzerland, he invented a primitive internal combustion engine which he constructed in 1807. It was powered by a mixture of hydrogen and oxygen manually ignited by electric spark, but the engine neither involved the in-cylinder compression, the crank, nor the connecting rod. A year later, Isaac built an early automobile for his new engine to power.
Alternative claims for internal combustion engines
Coincidentally, in 1807 Nicéphore Niépce installed his 'moss, coal-dust and resin'-fueled Pyréolophore internal combustion engine in a boat and powered up the river Saone in France to be granted a patent by the Emperor Napoleon Bonaparte. The discrete, virtually simultaneous, implementations of these two designs of internal combustion in different modes of transport means that the de Rivaz engine can be correctly described as "the worlds first use of an internal combustion engine in an auto-mobile (in 1808)", whilst the "Pyréolophore (in 1807) was the world's first use of an internal combustion engine in a ship".
Although de Rivaz's early work is credited as the first use of the internal combustion engine in an automobile, the further development and mass production of the invention never truly began until the late nineteenth century.
In 1824, the French physicist Nicolas Léonard Sadi Carnot scientifically established the thermodynamic theory of idealized heat engines. This highlighted the shortcoming of these pioneering designs, whereby they needed a compression mechanism to increase the difference between the upper and lower working temperatures and potentially unlock sufficient power and efficiency. Gasoline was not used for internal combustion engines until 1870 when carburetors were invented to convert non-combustible liquid fuels into a combustible gaseous mixture form.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#549 2019-05-13 00:15:51
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,479
Re: crème de la crème
516) Sir John Bennet Lawes, 1st Baronet
Sir John Bennet Lawes, 1st Baronet, (born Dec. 28, 1814, Rothamsted, Harpenden, Hertfordshire, Eng.—died Aug. 31, 1900, Rothamsted), English agronomist who founded the artificial fertilizer industry and Rothamsted Experimental Station, the oldest agricultural research station in the world.
Lawes inherited his father’s estate, Rothamsted, in 1822. In 1842, after long experimentation with the effects of manures on potted plants and field crops on his estate, he patented a process for treating phosphate rock with sulfuric acid to produce superphosphate. That year he opened the first fertilizer factory, thus initiating the artificial fertilizer industry. The following year, the chemist J.H. (later Sir Henry) Gilbert joined him, and they began a collaboration lasting more than a half century; Lawes considered 1843 the year of the station’s foundation. Together, the pair studied the effects of different fertilizers on crops. They also researched animal nutrition, including the value of different fodders and the sources of animal fat.
In 1867 the Royal Society awarded Lawes and Gilbert jointly a Royal Medal. In 1882 Lawes was created a baronet. Seven years later he ensured the continuation of the Rothamsted experiments by setting up the Lawes Agricultural trust.
(Phosphorite, also called phosphate rock, rock with a high concentration of phosphates in nodular or compact masses. The phosphates may be derived from a variety of sources, including marine invertebrates that secrete shells of calcium phosphate, and the bones and excrement of vertebrates.
The thickest deposits of phosphorite form in areas characterized by carbonaceous shale and chert. The phosphorite is usually carbonaceous and pelletal, and it is mixed with skeletal matter and phosphatic shells. Deposits may be up to one metre (about 3 feet) thick. Phosphorites also form on stable areas associated with sandstone or shale. These deposits are not carbonaceous but do contain nodules and phosphatized shells. Typical phosphorite beds contain about 30 percent phosphorous pentoxide (P2O5) and constitute the primary source of raw materials for most of world’s production of phosphate fertilizers. Significant deposits of phosphorites in the United States include the Phosphoria Formation in Idaho and the Monterey Formation in California. Major deposits also occur in the Sechura Desert in Peru. Alteration of phosphorites tends to leach carbonates and sulfides and increase the percentage of phosphorus pentoxide. The Phosphoria Formation, for example, contains about 34 percent phosphorus pentoxide near the surface compared to only about 28 percent at depth.)
(Sulfuric acid, sulfuric also spelled sulphuric (H2SO4), also called oil of vitriol, or hydrogen sulfate, dense, colourless, oily, corrosive liquid; one of the most important of all chemicals, prepared industrially by the reaction of water with sulfur trioxide (see sulfur oxide), which in turn is made by chemical combination of sulfur dioxide and oxygen either by the contact process or the chamber process. In various concentrations the acid is used in the manufacture of fertilizers, pigments, dyes, drugs, explosives, detergents, and inorganic salts and acids, as well as in petroleum refining and metallurgical processes. In one of its most familiar applications, sulfuric acid serves as the electrolytein lead–acid storage batteries.
Sulfuric acid, sulfuric also spelled sulphuric (H2SO4), also called oil of vitriol, or hydrogen sulfate, dense, colourless, oily, corrosive liquid; one of the most important of all chemicals, prepared industrially by the reaction of water with sulfur trioxide (see sulfur oxide), which in turn is made by chemical combination of sulfur dioxide and oxygen either by the contact process or the chamber process. In various concentrations the acid is used in the manufacture of fertilizers, pigments, dyes, drugs, explosives, detergents, and inorganic salts and acids, as well as in petroleum refining and metallurgical processes. In one of its most familiar applications, sulfuric acid serves as the electrolytein lead–acid storage batteries.
Pure sulfuric acid has a specific gravity of 1.830 at 25 °C (77 °F); it freezes at 10.37 °C (50.7 °F). When heated, the pure acid partially decomposes into water and sulfur trioxide; the latter escapes as a vapour until the concentration of the acid falls to 98.3 percent. This mixture of sulfuric acid and water boils at a constant temperature of 338 °C (640 °F) at one atmosphere pressure. Sulfuric acid is commonly supplied at concentrations of 78, 93, or 98 percent.
Sulfuric acid is a very strong acid; in aqueous solutions it ionizes completely to form hydronium ions and hydrogen sulfate ions. In dilute solutions the hydrogen sulfate ions also dissociate, forming more hydronium ions and sulfate ions. In addition to being an oxidizing agent, reacting readily at high temperatures with many metals, carbon, sulfur, and other substances, concentrated sulfuric acid is also a strong dehydrating agent, combining violently with water; in this capacity, it chars many organic materials, such as wood, paper, or sugar, leaving a carbonaceous residue.
The term fuming sulfuric acid, or oleum, is applied to solutions of sulfur trioxide in 100 percent sulfuric acid; these solutions, commonly containing 20, 40, or 65 percent sulfur trioxide, are used for the preparation of organic chemicals.)

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#550 2019-05-15 00:38:47
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,479
Re: crème de la crème
517) James Nasmyth
James Hall Nasmyth (sometimes spelled Naesmyth, Nasmith, or Nesmyth) (19 August 1808 – 7 May 1890) was a Scottish engineer, philosopher, artist and inventor famous for his development of the steam hammer. He was the co-founder of Nasmyth, Gaskell and Company manufacturers of machine tools. He retired at the age of 48, and moved to Penshurst, Kent where he developed his hobbies of astronomy and photography.
James Nasmyth, (born August 19, 1808, Edinburgh, Scotland—died May 7, 1890, London, England), British engineer known primarily for his invention of the steam hammer.
Nasmyth showed an extraordinary mechanical inclination while still a schoolboy in Edinburgh, building successful model steam engines. For two years he worked in Henry Maudslay’s machine shop in London and subsequently moved to Manchester, where rapid industrialization was in progress. In 1836 he began to build his own foundry near the junction of the Bridgewater Canal with the newly opened Liverpool and Manchester Railway. He made machine tools of all kinds along with a variety of steam-powered machines. Isambard Kingdom Brunel, when designing his steamship Great Britain, originally made plans for paddle wheels of exceptional size. Nasmyth solved the challenging problem of forging the drive shaft by designing and fabricating a powerful steam hammer, which he patented in 1842. Although the Great Britain was eventually furnished with screw propellers instead of paddle wheels, the steam hammer immediately became an important part of the metallurgical math of the Industrial Revolution.
Besides steam hammers, Nasmyth manufactured more than 100 steam locomotives, many small high-pressure steam engines, and a variety of pumps, hydraulic presses, and other machines. At the age of 48 he retired from the foundry in order to devote himself to his hobby, astronomy. He wrote The Moon: Considered as a Planet, a World, and a Satellite (1874).

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline