Math Is Fun Forum
You are not logged in.
- Topics: Active | Unanswered
#1401 2022-06-05 13:59:23
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,396
Re: Miscellany
1375) Scurvy
Summary
Scurvy is a disease resulting from a lack of vitamin C (ascorbic acid). Early symptoms of deficiency include weakness, feeling tired and sore arms and legs. Without treatment, decreased red blood cells, gum disease, changes to hair, and bleeding from the skin may occur. As scurvy worsens there can be poor wound healing, personality changes, and finally death from infection or bleeding.
It takes at least a month of little to no vitamin C in the diet before symptoms occur. In modern times, scurvy occurs most commonly in people with mental disorders, unusual eating habits, alcoholism, and older people who live alone. Other risk factors include intestinal malabsorption and dialysis. While many animals produce their own vitamin C, humans and a few others do not. Vitamin C is required to make the building blocks for collagen. Diagnosis is typically based on physical signs, X-rays, and improvement after treatment.
Treatment is with vitamin C supplements taken by mouth. Improvement often begins in a few days with complete recovery in a few weeks. Sources of vitamin C in the diet include citrus fruit and a number of vegetables, including red peppers, broccoli, and tomatoes. Cooking often decreases the residual amount of vitamin C in foods.
Scurvy is rare compared to other nutritional deficiencies. It occurs more often in the developing world in association with malnutrition. Rates among refugees are reported at 5 to 45 percent. Scurvy was described as early as the time of ancient Egypt. It was a limiting factor in long-distance sea travel, often killing large numbers of people. During the Age of Sail, it was assumed that 50 percent of the sailors would die of scurvy on a major trip. A Scottish surgeon in the Royal Navy, James Lind, is generally credited with proving that scurvy can be successfully treated with citrus fruit in 1753. Nevertheless, it was not until 1795 that health reformers such as Gilbert Blane persuaded the Royal Navy to routinely give lemon juice to its sailors.
Details
Scurvy, also called vitamin C deficiency, one of the oldest-known nutritional disorders of humankind, is caused by a dietary lack of vitamin C (ascorbic acid), a nutrient found in many fresh fruits and vegetables, particularly the citrus fruits. Vitamin C is important in the formation of collagen (an element of normal tissues), and any deficiency of the vitamin interferes with normal tissue synthesis, a problem that underlies the clinical manifestations of the disorder.
Symptoms of scurvy usually become apparent within several months of vitamin C being absent from the diet, by which time lingering pools of vitamin C in fat, muscle, and other tissues have been depleted. Initial symptoms of scurvy include fatigue and soreness and stiffness of the joints and lower extremities. As the condition progresses, the gums swell and bleed, and teeth may loosen. Bleeding under the skin and in deep tissues, slow wound healing, anemia, and changes in personality are other indications of advanced disease. Left untreated, death ensues, typically as a result of bleeding or of complications from infection.
Some of the earliest evidence for a disorder suggesting scurvy dates to 3800–3600 BCE, captured in characteristic bone changes in the skeleton of a roughly one-year-old child in Egypt. Another early probable case of scurvy, described from the skeletal remains of a child in England, dates to 2200–1970 BCE. In addition, accounts of what was probably scurvy are found in ancient writings. The first clear-cut descriptions of the disorder, however, appear in the records of the medieval Crusades. Later, toward the end of the 15th century, scurvy became the major cause of disability and mortality among sailors on long sea voyages. In 1753 Scottish naval surgeon James Lind showed that scurvy could be cured and prevented by ingestion of the juice of oranges and lemons. Soon citrus fruits became so common aboard ship that British sailors were referred to as “limeys.”
In modern times, full-blown cases of vitamin C deficiency are relatively rare, being limited primarily to situations involving general malnutrition, such as in impoverished parts of the world. In developed regions, scurvy may still be seen, however, in elderly adults and in individuals who follow restrictive diets (e.g., because of a food allergy) or lack basic access to fruits and vegetables and in alcoholic individuals who consume a severely imbalanced diet. Smokers, pregnant or lactating women, and persons with AIDS (acquired immunodeficiency syndrome), inflammatory bowel disease, or type 1 diabetes often require increased amounts of vitamin C in their diets because of decreased absorption by the body.
Infants fed reconstituted milk or milk substitutes without a vitamin C or orange juice supplement are also at increased risk. Symptoms peculiar to infantile scurvy (Barlow disease) include swelling and pain of the lower extremities and lesions of the growing bones.
Administration of vitamin C is the specific therapy for scurvy. Even in cases of severe deficiency, a daily dose of 100 mg (1 mg = 0.001 gram) for adults or 10 to 25 mg for infants and children, accompanied by a normal diet, commonly produces a cure within several days.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1402 2022-06-06 16:03:33
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,396
Re: Miscellany
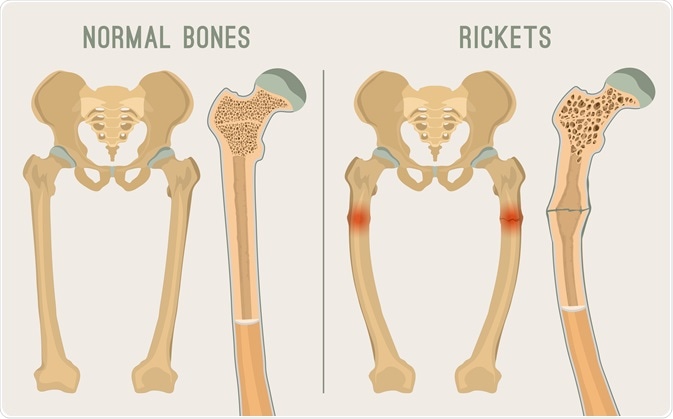
1376) Rickets
Summary
Rickets is a condition that results in weak or soft bones in children, and is caused by either dietary deficiency or genetic causes. Symptoms include bowed legs, stunted growth, bone pain, large forehead, and trouble sleeping. Complications may include bone deformities, bone pseudofractures and fractures, muscle spasms, or an abnormally curved spine.
The most common cause of rickets is a vitamin D deficiency, although hereditary genetic forms also exist. This can result from eating a diet without enough vitamin D, dark skin, too little sun exposure, exclusive breastfeeding without vitamin D supplementation, celiac disease, and certain genetic conditions. Other factors may include not enough calcium or phosphorus. The underlying mechanism involves insufficient calcification of the growth plate. Diagnosis is generally based on blood tests finding a low calcium, low phosphorus, and a high alkaline phosphatase together with X-rays.
Prevention for exclusively breastfed babies is vitamin D supplements. Otherwise, treatment depends on the underlying cause. If due to a lack of vitamin D, treatment is usually with vitamin D and calcium. This generally results in improvements within a few weeks. Bone deformities may also improve over time. Occasionally surgery may be performed to correct bone deformities. Genetic forms of the disease typically require specialized treatment.
Rickets occurs relatively commonly in the Middle East, Africa, and Asia. It is generally uncommon in the United States and Europe, except among certain minority groups. It begins in childhood, typically between the ages of 3 and 18 months old. Rates of disease are equal in males and females. Cases of what is believed to have been rickets have been described since the 1st century, and the condition was widespread in the Roman Empire. The disease was common into the 20th century. Early treatments included the use of cod liver oil.
Details
Rickets is a disease of infancy and childhood characterized by softening of the bones, leading to abnormal bone growth and caused by a lack of vitamin D in the body. When the disorder occurs in adults, it is known as osteomalacia.
The relationship between vitamin D and bone rigidity
Vitamin D (or, more specifically, calcitriol) is a steroid hormone that is produced in the skin by the action of sunlight’s ultraviolet rays on its precursor, 7-dehydrocholesterol (provitamin D3). Vitamin D is also absorbed from the diet, especially from fortified milk and from liver and fish oils.
Following its production in the skin or absorption in the gastrointestinal tract, vitamin D is transported through the blood to the liver, where it is converted to calcidiol (25-hydroxyvitamin D). Calcidiol is then transported through the blood to the kidneys, where it is metabolized to calcitriol (1,25-dihydroxyvitamin D), the most active form of vitamin D. Calcitriol stimulates the small intestine, bone, and kidney to absorb calcium, as well as the minerals phosphate and magnesium; in bone, the absorption process leads to the deposition of the inorganic salt calcium phosphate, which is responsible for bone rigidity.
In the absence of calcitriol, the calcium absorption process does not proceed normally. Low serum calcium concentrations prompt the secretion of a substance known as parathormone from the parathyroid glands; parathormone liberates calcium from bone in order to restore serum calcium concentrations. Hence, although the production of osteoid, the protein matrix on which calcium is deposited, is normal or increased in vitamin D deficiency, the matrix is poorly calcified. This results in soft bones, the literal meaning of the term osteomalacia.
Causes of rickets
While rickets is said to arise generally from a lack of vitamin D in the body, specific causes have been described. For example, vitamin D deficiency can result from a lack of the vitamin in the diet, insufficient conversion in the skin by ultraviolet light, inefficient dietary absorption, or the abnormal conversion of vitamin D to its metabolites. Contributing factors to the development of rickets in children include having been breast-fed exclusively for a prolonged period of time (human breast milk contains low amounts of vitamin D), living in temperate regions where sunlight exposure is limited in winter, and having dark-pigmented skin. Certain underlying conditions, such as liver, kidney, or gastrointestinal disease, can interfere with the normal metabolism or absorption of vitamin D. In chronic kidney disease, for example, the conversion of calcidiol to calcitriol is decreased or absent, resulting in an inability to absorb calcium.
In other instances, rickets and rickets-type disorders may be caused by inherited defects in genes whose products are involved in vitamin D or phosphate metabolism. In hereditary hypophosphatemic rickets, for example, an increased rate of phosphate clearance from the body by the renal tubules of the kidneys results in loss of bone mineral and, in severe cases, in rickets-type deformities and dwarfism. The disease, which is rare and is most commonly inherited as an X-linked dominant disorder (one copy of the mutated gene on the X chromosome is sufficient to produce the disease), tends to start in early childhood.
Another inherited form of rickets is vitamin D-dependent rickets type I (VDDRI), in which a defect in the enzyme that converts calcidiol to calcitriol produces vitamin D deficiency and causes the loss of calcium from bone. Vitamin D-dependent rickets type II (VDDRII) involves loss-of-function mutations in a gene for the vitamin D receptor, with the result that tissues are unable to absorb calcitriol. VDDRII is associated with rickets, hypocalcemia (decreased serum calcium), and in some cases alopecia (baldness). Both VDDRI and VDDRII are autosomal recessive (two copies of the mutated gene, one from each parent, are required to cause disease) and manifest in infancy or early childhood.
A variety of similar syndromes exist. For example, de Toni–Fanconi syndrome is characterized by rickets deformities and renal tubule defects. In addition, tumours that produce substances capable of inhibiting the reabsorption of phosphate by the kidneys (oncogenic osteomalacia) may lead to rickets-type deformities. Tumours that cause hypophosphatemia (decreased serum phosphate) are often hard to locate because they are small and occur in fibrous or mesenchymal tissue, including bone.
Symptoms of rickets
Softened bones are readily curved, and their growth is stunted. In rickets there also is an overgrowth of cartilage, resulting in the enlargement of the ends of long bones and in the junction of the ribs with the rib cage in the chest (rachitic rosary). Common early symptoms of rickets include restlessness, profuse sweating, lack of muscle tone in the limbs and abdomen, softening of the bones of the skull, delay in learning to sit, crawl, and walk, and delay in the eruption of the teeth. Tetany (spasms of the hands and feet as well as cramps and twitching of the muscles) may also occur. Unless treatment is begun early, rickets may produce conditions such as bowlegs, knock-knees, a bulging forehead, and short stature. A narrowed chest and pelvis may be responsible later in life for increased susceptibility to lung diseases and difficulties in childbearing, respectively.
Diagnosis and treatment of rickets
Rickets is diagnosed through an assessment of family medical history, X-rays, and blood and urine tests. A combination of X-rays, which reveal bone deformities characteristic of rickets, and knowledge of calcium, phosphate, calcidiol, and calcitriol levels typically leads to a definitive diagnosis.
Rickets is usually effectively treated with large supplemental doses of vitamin D concentrates (often in the form of calcitriol), with exposure to sunlight, and with a well-balanced diet. Vitamin D supplementation, usually in fortified milk, has been important in preventing the incidence of rickets in northern and temperate climates. Inherited forms of rickets often are treated with massive doses of vitamin D and supplementary phosphate and calcium.
The first treatment found to be effective for rickets was cod liver oil. Cod liver oil and exposure to sunlight were recognized as preventive and curative therapies for nutritional rickets in humans in the 18th and 19th centuries, respectively; however, these treatments were not generally accepted until the early 20th century. The existence of a vitamin able to mimic the effects of cod liver oil was indicated in experimental animals in 1918. In 1924 it was demonstrated that the curative effects of ultraviolet light resulted from the formation of vitamin D by such irradiation. Up until that time, vitamin D deficiency was a worldwide problem, particularly in the temperate zones. With the isolation of vitamin D2 (ergocalciferol, the form of vitamin D found in plants and fungi) in 1930–31 in England and Germany and of 7-dehydrocholesterol (the precursor of vitamin D) from hog skin in 1937 in Germany, the fortification of foods with the vitamin became possible.
Epidemiology of rickets
As a result of therapeutic developments in the 20th century, the prevalence of rickets decreased, particularly in developed countries such as the United States, the United Kingdom, and Australia, where it eventually became rare. Today the distribution and prevalence of rickets are aligned primarily with risk factors. Hence, it is most prevalent in peoples who are dark-skinned and in developing countries where access to vitamin D-fortified foods is lacking. Africa, the Middle East, and parts of Asia rank among the world’s most heavily affected regions.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1403 2022-06-07 14:07:23
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,396
Re: Miscellany
1377) Vitamin A Deficiency
Summary
Vitamin A deficiency (VAD) or hypovitaminosis A is a lack of vitamin A in blood and tissues. It is common in poorer countries, especially among children and women of reproductive age, but is rarely seen in more developed countries. Nyctalopia (night blindness) is one of the first signs of VAD, as the vitamin has a major role in phototransduction. Xerophthalmia, keratomalacia, and complete blindness can follow if the deficiency is more severe.
Vitamin A deficiency is the world's leading cause of preventable childhood blindness, and is critical to achieving Millennium Development Goal 4 to reduce child mortality. About 250,000 to 500,000 malnourished children in the developing world go blind each year from a deficiency of vitamin A, around half of whom die within a year of becoming blind. The United Nations Special Session on Children in 2002 set a goal of the elimination of VAD by 2010.
The prevalence of night blindness due to VAD is also high among pregnant women in many developing countries. VAD also contributes to maternal mortality and other poor outcomes in pregnancy and lactation.
VAD also diminishes the ability to fight infections. In countries where children are not immunized, infectious diseases such as measles have higher fatality rates. As elucidated by Alfred Sommer, even mild, subclinical deficiency can also be a problem, as it may increase children's risk of developing respiratory and diarrheal infections, decrease growth rate, slow bone development, and decrease likelihood of survival from serious illness.
VAD is estimated to affect about one-third of children under the age of five around the world. It is estimated to claim the lives of 670,000 children under five annually. Around 250,000–500,000 children in developing countries become blind each year owing to VAD, with the highest prevalence in Southeast Asia and Africa. According to the World Health Organization (WHO), VAD is under control in the United States, but in developing countries, VAD is a significant concern. Globally, 65% of all children aged 6 to 59 months received two doses of vitamin A in 2013, fully protecting them against VAD (80% in the least developed countries).
Details
Vitamin A deficiency is a nutritional disorder caused by a deficiency of vitamin A (also called retinol), a fat-soluble compound that is essential for various biological functions, especially vision. Retinaldehydes and retinoic acids are biologically active derivatives from retinol, and 11-cis retinaldehyde is an essential form of vitamin A that is required for normal vision. Retinoic acid is essential for normal cell morphogenesis, growth, and differentiation. Vitamin A is also needed for iron utilization and normal immunity. Deficiency of vitamin A can lead to visual impairment and skin lesions.
In developing countries, vitamin A deficiency is one of the common causes of blindness, affecting more than a quarter of a million children each year with a 50 percent mortality rate within the year. Increased mortality in children is primarily from infectious diseases, measles, respiratory diseases, and diarrhea. In the United States, vitamin A deficiency is typically due to diseases associated with malabsorption of fat, such as celiac disease. Because 90 percent of absorbed vitamin A is stored in the liver, zinc deficiency can also interfere with the release of vitamin A from storage. Chronic alcoholism can also cause deficiency because alcohol competes for alcohol dehydrogenase, a key enzyme required for the conversion of retinol to retinaldehyde in the eye. Other causes of deficiency include interference from drugs such as neomycin and mineral-oil laxative abuse.
Upon seeking medical help, patients often complain of difficulty seeing in the dark (night blindness), which results from low levels of 11-cis retinaldehyde, required for dark adaptation of vision. Other symptoms of deficiency include dryness of the conjunctiva (xerosis), corneal ulcers and necrosis (keratomalacia), hyperkeratotic skin lesions (increased keratinization of the epithelium), and development of small white patches on the conjunctiva (Bitot’s spots). Serum levels below the norm are commonly seen in advanced stages of deficiency.
Night blindness, poor wound healing, and other signs of deficiency can be effectively treated with appropriate levels of vitamin A. Excessive levels of vitamin A should be avoided as the vitamin is fat soluble and can accumulate in the body, leading to symptoms of vitamin A toxicity and congenital malformations in women who are pregnant.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1404 2022-06-08 14:14:53
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,396
Re: Miscellany
1378) Vitamin K
Summary
Vitamin K refers to structurally similar, fat-soluble vitamers found in foods and marketed as dietary supplements. The human body requires vitamin K for post-synthesis modification of certain proteins that are required for blood coagulation (K from koagulation, German for "coagulation") or for controlling binding of calcium in bones and other tissues. The complete synthesis involves final modification of these so-called "Gla proteins" by the enzyme gamma-glutamyl carboxylase that uses vitamin K as a cofactor.
Vitamin K is used in the liver as the intermediate VKH2 to deprotonate a glutamate residue and then is reprocessed into vitamin K through a vitamin K oxide intermediate. The presence of uncarboxylated proteins indicates a vitamin K deficiency. Carboxylation allows them to bind (chelate) calcium ions, which they cannot do otherwise. Without vitamin K, blood coagulation is seriously impaired, and uncontrolled bleeding occurs. Research suggests that deficiency of vitamin K may also weaken bones, potentially contributing to osteoporosis, and may promote calcification of arteries and other soft tissues.
Chemically, the vitamin K family comprises 2-methyl-1,4-naphthoquinone (3-) derivatives. Vitamin K includes two natural vitamers: vitamin K1 (phylloquinone) and vitamin K2 (menaquinone). Vitamin K2, in turn, consists of a number of related chemical subtypes, with differing lengths of carbon side chains made of isoprenoid groups of atoms. The two most studied ones are menaquinone-4 (MK-4) and menaquinone-7 (MK-7).
Vitamin K1 is made by plants, and is found in highest amounts in green leafy vegetables, because it is directly involved in photosynthesis. It is active as a vitamin in animals and performs the classic functions of vitamin K, including its activity in the production of blood-clotting proteins. Animals may also convert it to vitamin K2, variant MK-4. Bacteria in the gut flora can also convert K1 into MK-4. All forms of K2 other than MK-4 can only be produced by bacteria, which use these during anaerobic respiration. Vitamin K3 (menadione), a synthetic form of vitamin K, was used to treat vitamin K deficiency, but because it interferes with the function of glutathione, it is no longer used this way in human nutrition.
Details
Vitamin K plays a key role in helping the blood clot, preventing excessive bleeding. Unlike many other vitamins, vitamin K is not typically used as a dietary supplement.
Vitamin K is actually a group of compounds. The most important of these compounds appears to be vitamin K1 and vitamin K2. Vitamin K1 is obtained from leafy greens and some other vegetables. Vitamin K2 is a group of compounds largely obtained from meats, cheeses, and eggs, and synthesized by bacteria.
Vitamin K1 is the main form of vitamin K supplement available in the U.S.
Recently, some people have looked to vitamin K2 to treat osteoporosis and steroid-induced bone loss, but the research is conflicting. At this point there is not enough data to recommend using vitamin K2 for osteoporosis.
Why do people take vitamin K?
Low levels of vitamin K can raise the risk of uncontrolled bleeding. While vitamin K deficiencies are rare in adults, they are very common in newborn infants. A single injection of vitamin K for newborns is standard. Vitamin K is also used to counteract an overdose of the blood thinner Coumadin.
While vitamin K deficiencies are uncommon, you may be at higher risk if you:
* Have a disease that affects absorption in the digestive tract, such as Crohn's disease or active celiac disease
* Take drugs that interfere with vitamin K absorption
* Are severely malnourished
* Drink alcohol heavily
In these cases, a health care provider might suggest vitamin K supplements.
Uses of vitamin K for cancer, for the symptoms of morning sickness, for the removal of spider veins, and for other conditions are unproven. Learn more about vitamins k2 and d3 as well as which foods pack the highest amount.
How much vitamin K should you take?
The recommended adequate intake of vitamin K you take in, both from food. Most people get enough vitamin K from their diets.
There have been no adverse effects of vitamin K seen with the levels found in food or supplements. However, this does not rule out danger with high dose. Researchers have not set a maximum safe dose.
Can you get vitamin K naturally from foods?
Good natural food sources of vitamin K include:
* Vegetables like spinach, asparagus, and broccoli
* Legumes like soybeans
You can also meet your daily requirement with foods that have lesser amounts of vitamin K:
* Eggs
* Strawberries
* Meat like liver
What are the risks of taking vitamin K?
Side effects of oral vitamin K at recommended doses are rare.
Interactions. Many drugs can interfere with the effects of vitamin K. They include antacids, blood thinners, antibiotics, aspirin, and drugs for cancer, seizures, high cholesterol, and other conditions.
Risks. You should not use vitamin K supplements unless your health care provider tells you to. People using Coumadin for heart problems, clotting disorders, or other conditions may need to watch their diets closely to control the amount of vitamin K they take in. They should not use vitamin K supplements unless advised to do so by their health care provider.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1405 2022-06-09 14:22:25
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,396
Re: Miscellany
1379) Helium
Summary
Helium (He) is a chemical element, inert gas of Group 18 (noble gases) of the periodic table. The second lightest element (only hydrogen is lighter), helium is a colourless, odourless, and tasteless gas that becomes liquid at −268.9 °C (−452 °F). The boiling and freezing points of helium are lower than those of any other known substance. Helium is the only element that cannot be solidified by sufficient cooling at normal atmospheric pressure; it is necessary to apply pressure of 25 atmospheres at a temperature of 1 K (−272 °C, or −458 °F) to convert it to its solid form.
Abundance and isotopes
Helium constitutes about 23 percent of the mass of the universe and is thus second in abundance to hydrogen in the cosmos. Helium is concentrated in stars, where it is synthesized from hydrogen by nuclear fusion. Although helium occurs in Earth’s atmosphere only to the extent of 1 part in 200,000 (0.0005 percent) and small amounts occur in radioactive minerals, meteoric iron, and mineral springs, great volumes of helium are found as a component (up to 7.6 percent) in natural gases in the United States (especially in Texas, New Mexico, Kansas, Oklahoma, Arizona, and Utah). Smaller supplies have been discovered in Algeria, Australia, Poland, Qatar, and Russia. Ordinary air contains about 5 parts per million of helium, and Earth’s crust is only about 8 parts per billion.
The nucleus of every helium atom contains two protons, but, as is the case with all elements, isotopes of helium exist. The known isotopes of helium contain from one to six neutrons, so their mass numbers range from three to eight. Of these six isotopes, only those with mass numbers of three (helium-3, or 3He) and four (helium-4, or 4He) are stable; all the others are radioactive, decaying very rapidly into other substances. The helium that is present on Earth is not a primordial component but has been generated by radioactive decay. Alpha particles, ejected from the nuclei of heavier radioactive substances, are nuclei of the isotope helium-4. Helium does not accumulate in large quantities in the atmosphere because Earth’s gravity is not sufficient to prevent its gradual escape into space. The trace of the isotope helium-3 on Earth is attributable to the negative beta decay of the rare hydrogen-3 isotope (tritium). Helium-4 is by far the most plentiful of the stable isotopes: helium-4 atoms outnumber those of helium-3 about 700,000:1 in atmospheric helium and about 7,000,000:1 in certain helium-bearing minerals.
Properties
Helium-4 is unique in having two liquid forms. The normal liquid form is called helium I and exists at temperatures from its boiling point of 4.21 K (−268.9 °C) down to about 2.18 K (−271 °C). Below 2.18 K, thermal conductivity of helium-4 becomes more than 1,000 times greater than that of copper. This liquid form is called helium II to distinguish it from normal liquid helium I. Helium II exhibits the property called superfluidity: its viscosity, or resistance to flow, is so low that it has not been measured. This liquid spreads in a thin film over the surface of any substance it touches, and this film flows without friction even against the force of gravity. By contrast, the less plentiful helium-3 forms three distinguishable liquid phases of which two are superfluids. Superfluidity in helium-4 was discovered by the Russian physicist Pyotr Leonidovich Kapitsa in the mid-1930s, and the same phenomenon in helium-3 was first observed by Douglas D. Osheroff, David M. Lee, and Robert C. Richardson of the United States in 1972.
A liquid mixture of the two isotopes helium-3 and helium-4 separates at temperatures below about 0.8 K (−272.4 °C, or −458.2 °F) into two layers. One layer is practically pure helium-3; the other is mostly helium-4 but retains about 6 percent helium-3 even at the lowest temperatures achieved. The dissolution of helium-3 in helium-4 is accompanied by a cooling effect that has been used in the construction of cryostats (devices for production of very low temperatures) that can attain—and maintain for days—temperatures as low as 0.01 K (−273.14 °C, or −459.65 °F).
Production and uses
Helium gas (98.2 percent pure) is isolated from natural gas by liquefying the other components at low temperatures and under high pressures. Adsorption of other gases on cooled, activated charcoal yields 99.995 percent pure helium. Some helium is supplied from liquefaction of air on a large scale; the amount of helium obtainable from 1,000 tons (900 metric tons) of air is about 112 cubic feet (3.17 cubic metres), as measured at room temperature and at normal atmospheric pressure.
Helium is used as an inert-gas atmosphere for welding metals such as aluminum; in rocket propulsion (to pressurize fuel tanks, especially those for liquid hydrogen, because only helium is still a gas at liquid-hydrogen temperature); in meteorology (as a lifting gas for instrument-carrying balloons); in cryogenics (as a coolant because liquid helium is the coldest substance); and in high-pressure breathing operations (mixed with oxygen, as in scuba diving and caisson work, especially because of its low solubility in the bloodstream). Meteorites and rocks have been analyzed for helium content as a means of dating.
Details
Helium (from Greek, romanized: helios, lit. 'sun') is a chemical element with the symbol He and atomic number 2. It is a colorless, odorless, tasteless, non-toxic, inert, monatomic gas and the first in the noble gas group in the periodic table. Its boiling and melting point are the lowest among all the elements. It is the second lightest and second most abundant element in the observable universe (hydrogen is the lightest and most abundant). It is present at about 24% of the total elemental mass, which is more than 12 times the mass of all the heavier elements combined. Its abundance is similar to this in both the Sun and in Jupiter, due to the very high nuclear binding energy (per nucleon) of helium-4, with respect to the next three elements after helium. This helium-4 binding energy also accounts for why it is a product of both nuclear fusion and radioactive decay. Most helium in the universe is helium-4, the vast majority of which was formed during the Big Bang. Large amounts of new helium are created by nuclear fusion of hydrogen in stars.
Helium was first detected as an unknown, yellow spectral line signature in sunlight during a solar eclipse in 1868 by Georges Rayet, Captain C. T. Haig, Norman R. Pogson, and Lieutenant John Herschel, and was subsequently confirmed by French astronomer Jules Janssen. Janssen is often jointly credited with detecting the element, along with Norman Lockyer. Janssen recorded the helium spectral line during the solar eclipse of 1868, while Lockyer observed it from Britain. Lockyer was the first to propose that the line was due to a new element, which he named. The formal discovery of the element was made in 1895 by chemists Sir William Ramsay, Per Teodor Cleve, and Nils Abraham Langlet, who found helium emanating from the uranium ore, cleveite, which is now not regarded as a separate mineral species, but as a variety of uraninite. In 1903, large reserves of helium were found in natural gas fields in parts of the United States, by far the largest supplier of the gas today.
Liquid helium is used in cryogenics (its largest single use, absorbing about a quarter of production), and in the cooling of superconducting magnets, with its main commercial application in MRI scanners. Helium's other industrial uses—as a pressurizing and purge gas, as a protective atmosphere for arc welding, and in processes such as growing crystals to make silicon wafers—account for half of the gas produced. A well-known but minor use is as a lifting gas in balloons and airships. As with any gas whose density differs from that of air, inhaling a small volume of helium temporarily changes the timbre and quality of the human voice. In scientific research, the behavior of the two fluid phases of helium-4 (helium I and helium II) is important to researchers studying quantum mechanics (in particular the property of superfluidity) and to those looking at the phenomena, such as superconductivity, produced in matter near absolute zero.
On Earth, it is relatively rare—5.2 ppm by volume in the atmosphere. Most terrestrial helium present today is created by the natural radioactive decay of heavy radioactive elements (thorium and uranium, although there are other examples), as the alpha particles emitted by such decays consist of helium-4 nuclei. This radiogenic helium is trapped with natural gas in concentrations as great as 7% by volume, from which it is extracted commercially by a low-temperature separation process called fractional distillation. Terrestrial helium is a non-renewable resource because once released into the atmosphere, it promptly escapes into space. Its supply is thought to be rapidly diminishing. However, some studies suggest that helium produced deep in the earth by radioactive decay can collect in natural gas reserves in larger than expected quantities, in some cases, having been released by volcanic activity.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1406 2022-06-10 13:58:10
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,396
Re: Miscellany
1380) Pellagra
Summary
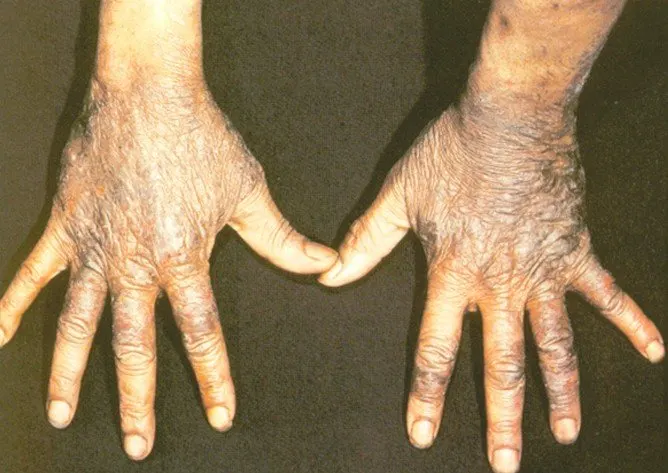
Pellagra is a disease caused by a lack of the vitamin niacin (Vitamin B3). Symptoms include inflamed skin, diarrhea, dementia, and sores in the mouth. Areas of the skin exposed to either sunlight or friction are typically affected first. Over time affected skin may become darker, stiffen, peel, or bleed.
There are two main types of pellagra, primary and secondary. Primary pellagra is due to a diet that does not contain enough niacin and tryptophan. Secondary pellagra is due to a poor ability to use the niacin within the diet. This can occur as a result of alcoholism, long-term diarrhea, carcinoid syndrome, Hartnup disease, and a number of medications such as isoniazid. Diagnosis is typically based on symptoms and may be assisted by urine testing.
Treatment is with either niacin or nicotinamide supplementation. Improvements typically begin within a couple of days. General improvements in diet are also frequently recommended. Decreasing sun exposure via sunscreen and proper clothing is important while the skin heals. Without treatment death may occur.
The disease occurs most commonly in the developing world, specifically sub-Saharan Africa.
Details
What is pellagra?
Pellagra is a disease caused by low levels of niacin, also known as vitamin B-3. It’s marked by dementia, diarrhea, and dermatitis, also known as “the three Ds”. If left untreated, pellagra can be fatal.
While it’s much less common than it used to be, thanks to advancements in food production, it’s still a problem in many developing countries. It can also affect people whose bodies don’t properly absorb niacin.
What are the symptoms?
The main symptoms of pellagra are dermatitis, dementia, and diarrhea. This is because niacin deficiency is most noticeable in body parts with high rates of cell turnover, such as your skin or gastrointestinal tract.
Dermatitis related to pellagra usually causes a rash on the face, lips, feet, or hands. In some people, dermatitis forms around the neck, a symptom known as Casal necklace.
Additional dermatitis symptoms include:
* red, flaky skin
* areas of discoloration, ranging from red to brown
* thick, crusty, scaly, or cracked skin
* itchy, burning patches of skin
In some cases, the neurological signs of pellagra appear early on, but they’re often hard to identify. As the disease progresses, possible dementia symptoms include:
* apathy
* depression
* confusion, irritability, or mood changes
* headaches
* restlessness or anxiety
* disorientation or delusions
Other possible pellagra symptoms include:
* sores on the lips, tongue, or gums
* decreased appetite
* trouble eating and drinking
* nausea and vomiting
What causes it?
There are two types of pellagra, known as primary pellagra and secondary pellagra.
Primary pellagra is caused by diets low in niacin or tryptophan. Tryptophan can be converted to niacin in the body, so not getting enough can cause niacin deficiency.
Primary pellagra is most common in developing countries that depend on corn as a staple food. Corn contains niacytin, a form of niacin that humans can’t digest and absorb unless prepared properly.
Secondary pellagra occurs when your body can’t absorb niacin. Things that can prevent your body from absorbing niacin include:
* alcoholism
* eating disorders
* certain medications, including anti-convulsants and immunosuppressive drugs
* gastrointestinal diseases, such as Crohn’s disease and ulcerative colitis
* cirrhosis of the liver
* carcinoid tumors
* Hartnup disease
How is it diagnosed?
Pellagra can be difficult to diagnose because it causes a range of symptoms. There’s also no specific test for diagnosing niacin deficiency.
Instead, your doctor will start by checking for any gastrointestinal problems, rashes, or changes in your mental state. They may also test your urine.
In many cases, diagnosing pellagra involves seeing if your symptoms respond to niacin supplements.
How is it treated?
Primary pellagra is treated with dietary changes and a niacin or nicotinamide supplement. It may also need to be given intravenously. Nicotinamide is another form of vitamin B-3. With early treatment, many people make a full recovery and start feeling better within a few days of starting treatment. Skin improvement may take several months. However, if left untreated, primary pellagra usually causes death after four or five years.
Treating secondary pellagra usually focuses on treating the underlying cause. However, some cases of secondary pellagra also respond well to taking niacin or nicotinamide either orally or intravenously.
While recovering from either primary or secondary pellagra, it’s important to keep any rashes moisturized and protected with sunscreen.
Living with pellagra
Pellagra is a serious condition that’s caused by low levels of niacin, due to either malnutrition or an absorption problem. If left untreated, it can cause death. While primary pellagra responds well to niacin supplementation, secondary pellagra can be harder to treat, depending on the underlying cause.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1407 2022-06-11 13:56:34
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,396
Re: Miscellany
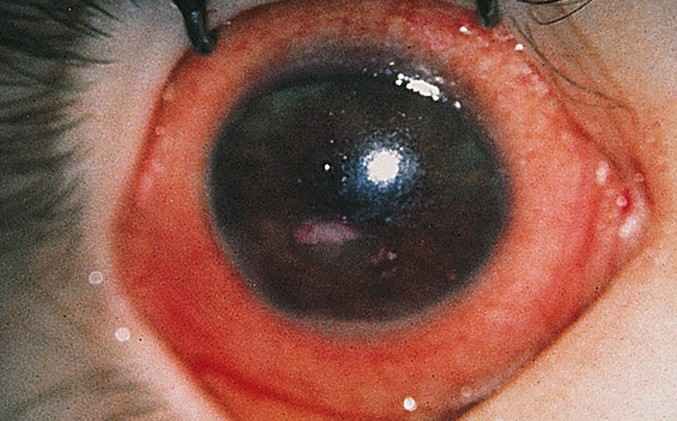
1381) Xerophthalmia
Summary
Xerophthalmia (from Ancient Greek meaning "dry" and "ophthalmos" meaning "eye") is a medical condition in which the eye fails to produce tears. It may be caused by vitamin A deficiency, which is sometimes used to describe that condition, although there may be other causes.
Xerophthalmia caused by a severe vitamin A deficiency is described by pathologic dryness of the conjunctiva and cornea. The conjunctiva becomes dry, thick and wrinkled. The first symptom is poor vision at night. If untreated, xerophthalmia can lead to dry eye syndrome, corneal ulceration, and ultimately to blindness as a result of corneal and retinal damage.
Xerophthalmia usually implies a destructive dryness of the conjunctival epithelium due to dietary vitamin A deficiency—a rare condition in developed countries, but still causing much damage in developing countries. Other forms of dry eye are associated with aging, poor lid closure, scarring from a previous injury, or autoimmune diseases such as rheumatoid arthritis and Sjögren's syndrome, and these can all cause chronic conjunctivitis. Radioiodine therapy can also induce xerophthalmia, often transiently, although in some patients late onset or persistent xerophthalmia has been observed.
The damage to the cornea in vitamin A associated xerophthalmia is quite different from damage to the retina at the back of the globe, a type of damage which can also be due to lack of vitamin A, but which is caused by lack of other forms of vitamin A which work in the visual system. Xerophthalmia from hypovitaminosis A is specifically due to lack of the hormone-like vitamin A metabolite retinoic acid, since (along with certain growth-stunting effects) the condition can be reversed in vitamin A deficient rats by retinoic acid supplementation (however the retinal damage continues). Since retinoic acid cannot be reduced to retinal or retinol, these effects on the cornea must be specific to retinoic acid. This is in keeping with retinoic acid's known requirement for good health in epithelial cells, such as those in the cornea.
Details
Xerophthalmia is a disease that causes dry eyes due to vitamin A deficiency. If it goes untreated, it can progress into night blindness or spots on your eyes. It can even damage the cornea of your eye and cause blindness.
This disease is rare in the U.S. It’s more commonly seen in developing countries, where people are more likely to have nutrient deficiencies. It can be treated with vitamin A supplements.
Causes of Xerophthalmia
Vitamin A, or retinol, is an essential nutrient. Its main function is to help maintain your eye health and vision. It also protects your vital organs, including your lungs and heart, and supports your immune system.
Your body can’t make its own vitamin A. You need to eat foods — like carrots and meat — or take supplements that are rich in vitamin A. Getting enough vitamin A is important for your health.
A lack of vitamin A in your diet may cause dry eyes, which is called xerophthalmia.
Risk Factors of Xerophthalmia
Xerophthalmia can happen because of underlying conditions that cause vitamin A deficiency. It has the following risk factors:
* Young age. Vitamin A deficiency is more common in infants and children. It can hinder a child’s growth, have negative effects on vital organs, and complicate other diseases or infections. If a child doesn’t get enough vitamin A as they are growing, it may cause xerophthalmia or childhood blindness.
Vitamin A deficiency can also occur in children due to diseases like measles and respiratory infections. Such infections increase the chances of xerophthalmia in children.
Severe xerophthalmia affects infants far more than adults. Children 3 to 6 years of age are at a higher risk of developing night blindness due to xerophthalmia.
Other factors. These are some other risk factors of xerophthalmia in children and adults, including:
* Poverty. People who live in poverty or can't afford proper meals are more likely to develop diseases like xeropthalmia.
* Lack of nutrition education. Those who don’t receive proper education about nutrition are usually unaware of the benefits of vitamin A. This can lead to a lower intake of vitamin A in their diet.
* Malnutrition. Lack of proper nutrition may lead to vitamin A deficiency. Severe malnutrition can result in dry eyes and may cause night blindness.
* Other diseases. Diseases like pancreatitis or inflammatory bowel disease can cause vitamin A deficiency..
* Liver problems. Chronic liver disease or liver cirrhosis can prevent vitamin A from being absorbed into the body. The resulting vitamin A deficiency may lead to xeropthalmia.
* Chronic diarrhea. People who have diarrhea repeatedly are at an increased risk of xeropthalmia due to vitamin A depletion.
* Alcoholism. Drinking excess alcohol may decrease the levels of vitamin A in your body.
Symptoms of Xerophthalmia
Xerophthalmia is a progressive disease that begins with dry eyes and may keep getting worse. These are the typical symptoms of xerophthalmia:
* Drying and wrinkling of the outer layer of your eye, or conjunctiva
* Night blindness, an eye disease in which you can’t see in dim light
* Ulcers or scars on your cornea
* Bitot’s spots, or white spots on your conjunctiva
* Softening of your cornea
Xerophthalmia is a preventable disease that can be easily treated. But in extreme cases, it can cause permanent blindness.
Diagnosis of Xerophthalmia
If you have obvious symptoms like dry eyes, talk to your doctor. They may physically examine you and check your symptoms and history. They may ask you questions about your diet.
Your doctor may also order a blood test to check the vitamin A levels in your body. If you have severe xerophthalmia or night blindness, your doctor may start your treatment right away.
Treatment of Xerophthalmia
The main treatment for xerophthalmia is vitamin A therapy or supplementation. It can be given orally or by injection. Your doctor may also give you other medications like antibiotics to prevent eye infections.
Vitamin A therapy is used to treat xerophthalmia in adults and children. The dose depends on your age and the severity of your xerophthalmia.
Your doctor may ask you to eat more yellow-colored fruits and vegetables rich in beta-carotene. They may also suggest that you add green leafy vegetables, meat, and dairy to your diet. If your xerophthalmia is due to other factors, your doctor can work with you to treat the underlying cause.
Prevention of Xerophthalmia
Xerophthalmia can be prevented with vitamin A supplements. Increasing the levels of vitamin A in your diet can also help prevent this disease. Some foods that are rich in vitamin A that you can easily add to your meals include:
* Fish liver or fish oil
* Chicken
* Meat like beef
* Eggs
* Carrots
* Lemons
* Mangos
* Yams
* Dairy or milk products
* Green vegetables
Adding these vitamin A-rich foods to your diet will not only help to prevent xerophthalmia but may also help you to maintain your overall health.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1408 2022-06-12 13:53:30
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,396
Re: Miscellany
1382) Electrolytic cell
Summary
An electrolytic cell is an electrochemical cell that requires an external source of electrical energy (voltage applied between two electrodes) to drive a chemical reaction that would not otherwise occur. This is in contrast to a galvanic cell, which itself is a source of electrical energy and the foundation of a battery. The net reaction taking place at a galvanic cell operate is a spontaneous reaction, while the net reaction taking place at an electrolytic cell is the opposite of this spontaneous reaction.
Principles
In an electrolytic cell, a current is passed through the cell by an external voltage, causing an otherwise non-spontaneous chemical reaction to proceed. In a galvanic cell, the progress of a spontaneous chemical reaction causes an electric current to flow. An equilibrium electrochemical cell is at the state between an electrolytic cell and a galvanic cell. The tendency of a spontaneous reaction to push a current through the external circuit is exactly balanced by an external voltage that is called a counter electromotive force or counter e.m.f. so that no current flows. If this counter voltage is increased the cell becomes an electrolytic cell and if it is decreased the cell becomes a galvanic cell.
An electrolytic cell has three components: an electrolyte and two electrodes (a cathode and an anode). The electrolyte is usually a solution of water or other solvents in which ions are dissolved. Molten salts such as sodium chloride are also electrolytes. When driven by an external voltage applied to the electrodes, the ions in the electrolyte are attracted to an electrode with the opposite charge, where charge-transferring (also called faradaic or redox) reactions can take place. Only with an external electrical potential (i.e., voltage) of correct polarity and sufficient magnitude can an electrolytic cell decompose a normally stable, or inert chemical compound in the solution. The electrical energy provided can produce a chemical reaction which would not occur spontaneously otherwise.
Details
Electrolytic cell is any device in which electrical energy is converted to chemical energy, or vice versa. Such a cell typically consists of two metallic or electronic conductors (electrodes) held apart from each other and in contact with an electrolyte (q.v.), usually a dissolved or fused ionic compound. Connection of the electrodes to a source of direct electric current renders one of them negatively charged and the other positively charged. Positive ions in the electrolyte migrate to the negative electrode (cathode) and there combine with one or more electrons, losing part or all of their charge and becoming new ions having lower charge or neutral atoms or molecules; at the same time, negative ions migrate to the positive electrode (anode) and transfer one or more electrons to it, also becoming new ions or neutral particles. The overall effect of the two processes is the transfer of electrons from the negative ions to the positive ions, a chemical reaction. An example is the electrolysis of sodium chloride (common salt), forming sodium metal and chlorine gas; the energy required to make the reaction proceed is supplied by the electric current. Other common applications of electrolysis include electrodeposition for refining or plating of metals and the production of caustic soda.
In the case of substances that generate energy, rather than consume it, when they react with each other, some or all of this energy can be converted to electricity if the reaction can be divided into an oxidation and a reduction that can be made to occur at separate electrodes. In the lead-acid storage battery, for example, lead dioxide, lead metal, and sulfuric acid react to form lead sulfate and water; the separate processes are the oxidation of lead to lead sulfate at one electrode and the reduction of lead dioxide to lead sulfate at the other while electric charge is transported through the electrolyte by the migration of hydrogen ions. These processes create a driving force (a voltage or electrical potential) that causes electricity to flow through an external circuit joining the two electrodes. Many other chemical combinations have been utilized in cells and batteries.
Other cells for generating electricity by means other than motion of a conductor in a magnetic field include solar cells, in which electron flow between semiconductors results from absorption of light, and fuel cells, in which a continuous supply of liquid or gaseous oxidizing agent, such as oxygen, removes electrons from the cathode as a reducing agent, such as hydrogen, supplies electrons to the anode.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1409 2022-06-13 17:52:51
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,396
Re: Miscellany
1383) Electrolysis
Summary
Electrolysis is the process by which electric current is passed through a substance to effect a chemical change. The chemical change is one in which the substance loses or gains an electron (oxidation or reduction). The process is carried out in an electrolytic cell, an apparatus consisting of positive and negative electrodes held apart and dipped into a solution containing positively and negatively charged ions. The substance to be transformed may form the electrode, may constitute the solution, or may be dissolved in the solution. Electric current (i.e., electrons) enters through the negatively charged electrode (cathode); components of the solution travel to this electrode, combine with the electrons, and are transformed (reduced). The products can be neutral elements or new molecules. Components of the solution also travel to the other electrode (anode), give up their electrons, and are transformed (oxidized) to neutral elements or new molecules. If the substance to be transformed is the electrode, the reaction is often one in which the electrode dissolves by giving up electrons.
Electrolysis is used extensively in metallurgical processes, such as in extraction (electrowinning) or purification (electrorefining) of metals from ores or compounds and in deposition of metals from solution (electroplating). Metallic sodium and chlorine gas are produced by the electrolysis of molten sodium chloride; electrolysis of an aqueous solution of sodium chloride yields sodium hydroxide and chlorine gas. Hydrogen and oxygen are produced by the electrolysis of water.
Details
In chemistry and manufacturing, electrolysis is a technique that uses direct electric current (DC) to drive an otherwise non-spontaneous chemical reaction. Electrolysis is commercially important as a stage in the separation of elements from naturally occurring sources such as ores using an electrolytic cell. The voltage that is needed for electrolysis to occur is called the decomposition potential. The word "lysis" means to separate or break, so in terms, electrolysis would mean "breakdown via electricity".
Overview
Electrolysis is the passing of a direct electric current through an electrolyte producing chemical reactions at the electrodes and decomposition of the materials.
The main components required to achieve electrolysis are an electrolyte, electrodes, and an external power source. A partition (e.g. an ion-exchange membrane or a salt bridge) is optional to keep the products from diffusing to the vicinity of the opposite electrode.
The electrolyte is a chemical substance which contains free ions and carries electric current (e.g. an ion-conducting polymer, solution, or a ionic liquid compound). If the ions are not mobile, as in most solid salts, then electrolysis cannot occur. A liquid electrolyte is produced by:
* Solvation or reaction of an ionic compound with a solvent (such as water) to produce mobile ions
* An ionic compound melted by heating
The electrodes are immersed separated by a distance such that a current flows between them through the electrolyte and are connected to the power source which completes the electrical circuit. A direct current supplied by the power source drives the reaction causing ions in the electrolyte to be attracted toward the respective oppositely charged electrode.
Electrodes of metal, graphite and semiconductor material are widely used. Choice of suitable electrode depends on chemical reactivity between the electrode and electrolyte and manufacturing cost. Historically, when non-reactive anodes were desired for electrolysis, graphite (called plumbago in Faraday's time) or platinum were chosen. They were found to be some of the least reactive materials for anodes. Platinum erodes very slowly compared to other materials, and graphite crumbles and can produce carbon dioxide in aqueous solutions but otherwise does not participate in the reaction. Cathodes may be made of the same material, or they may be made from a more reactive one since anode wear is greater due to oxidation at the anode.
Process of electrolysis
The key process of electrolysis is the interchange of atoms and ions by the removal or addition of electrons due to the applied current. The desired products of electrolysis are often in a different physical state from the electrolyte and can be removed by physical processes (e.g. by collecting gas above an electrode or precipitating a product out of the electrolyte).
The quantity of the products is proportional to the current, and when two or more electrolytic cells are connected in series to the same power source, the products produced in the cells are proportional to their equivalent weight. These are known as Faraday's laws of electrolysis.
Each electrode attracts ions that are of the opposite charge. Positively charged ions (cations) move towards the electron-providing (negative) cathode. Negatively charged ions (anions) move towards the electron-extracting (positive) anode. In this process electrons are effectively introduced at the cathode as a reactant and removed at the anode as a product. In chemistry, the loss of electrons is called oxidation, while electron gain is called reduction.
When neutral atoms or molecules, such as those on the surface of an electrode, gain or lose electrons they become ions and may dissolve in the electrolyte and react with other ions.
Industrial uses
* Electrometallurgy of aluminium, lithium, sodium, potassium, magnesium, calcium, and in some cases copper.
* Production of chlorine and sodium hydroxide, called the Chloralkali process.
* Production of sodium chlorate and potassium chlorate.
* Production of perfluorinated organic compounds such as trifluoroacetic acid by the process of electrofluorination.
* Purifying copper from refined copper.
* Production of fuels such as hydrogen for spacecraft, nuclear submarines and vehicles.
* Rust removal and cleaning of old coins and other metallic objects.
Manufacturing processes
In manufacturing, electrolysis can be used for:
* Electroplating, where a thin film of metal is deposited over a substrate material. Electroplating is used in many industries for either functional or decorative purposes, as in-vehicle bodies and nickel coins.
* Electrochemical machining (ECM), where an electrolytic cathode is used as a shaped tool for removing material by anodic oxidation from a workpiece. ECM is often used as a technique for deburring or for etching metal surfaces like tools or knives with a permanent mark or logo.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1410 2022-06-14 14:26:33
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,396
Re: Miscellany
1384) Electrical resistance and conductance
The electrical resistance of an object is a measure of its opposition to the flow of electric current. Its reciprocal quantity is electrical conductance, measuring the ease with which an electric current passes. Electrical resistance shares some conceptual parallels with mechanical friction. The SI unit of electrical resistance is the ohm (Ω), while electrical conductance is measured in siemens (S) (formerly called 'mhos' and then represented by ℧).
The resistance of an object depends in large part on the material it is made of. Objects made of electrical insulators like rubber tend to have very high resistance and low conductance, while objects made of electrical conductors like metals tend to have very low resistance and high conductance. This relationship is quantified by resistivity or conductivity. The nature of a material is not the only factor in resistance and conductance, however; it also depends on the size and shape of an object because these properties are extensive rather than intensive. For example, a wire's resistance is higher if it is long and thin, and lower if it is short and thick. All objects resist electrical current, except for superconductors, which have a resistance of zero.
The resistance R of an object is defined as the ratio of voltage V across it to current I through it, while the conductance G is the reciprocal:
For a wide variety of materials and conditions, V and I are directly proportional to each other, and therefore R and G are constants (although they will depend on the size and shape of the object, the material it is made of, and other factors like temperature or strain). This proportionality is called Ohm's law, and materials that satisfy it are called ohmic materials.
In other cases, such as a transformer, diode or battery, V and I are not directly proportional. The ratio V/I is sometimes still useful, and is referred to as a chordal resistance or static resistance, since it corresponds to the inverse slope of a chord between the origin and an I–V curve. In other situations, the derivative
may be most useful; this is called the differential resistance.How Resistance Works
In our voltage tutorial we discussed how voltage seems to behave like a pushing force, that pushes electrons from atom to atom through a wire creating an electric circuit. It turns out that this process is not 100% efficient! The atoms in a copper wire are always vibrating around just a small amount due to the heat energy in them and when electrons try to move through the wire bouncing from atom to atom, sometimes they’ll bump into an atom that’s in the way. The movement of that electron gets “resisted”.
How Current Works
That resistance is turned into heat, and in fact is the basic principle behind the electric heaters you find at most big box stores, as well as the filaments in tried and true incandescent light bulbs that hopefully you’ve swapped out for LEDs by now! Instead of copper wire they use a filament wire that has a higher resistance to the flow of electrons!
Metals are generally considered to be very low resistance materials and due to this they make great conductors of electricity. There are many materials however that do not. Most resistors are made out of a metal-oxide material that is designed to resist the flow of current at a specific value or “Ohm”.
* Voltage is the difference in charge (or potential energy) between two points
* Current is the rate at which that charge flows
* Resistance is a material’s tendency to resist the flow of that charge (or current).
What Is Ohm's Law?
Resistance is measured in a unit called Ohms, which is represented by the Greek letter Omega (Ω).
Ohm’s law states that “the current through a conductor between two points is directly proportional to the voltage across the two points.”
We talk about resistors in Ohms. A resistor might be a 220 Ohm resistor, or it might be 6.8K Ohm resistor. To give you a frame of reference, something with an Ohm value of 1 or less is considered to be a very low resistance. Most wire you use in your projects will fit in this category. A 1 megaohm resistor (or 1 million Ohms) is a very high level of resistance and almost no current will pass through.
Sometimes you need to find the correct resistance value to limit current. The formula for calculating resistance is: R = E ÷ A
In this algebraic expression, Resistance (R) is equal to current (A) divided by voltage (I). As with any algebraic expression, you can find the answer to any one of these variables as long as you have the other two.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1411 2022-06-15 14:22:06
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,396
Re: Miscellany
1385) Rheostat
Summary
Rheostat is an adjustable resistor used in applications that require the adjustment of current or the varying of resistance in an electric circuit. The rheostat can adjust generator characteristics, dim lights, and start or control the speed of motors. Its resistance element can be a metal wire or ribbon, carbon, or a conducting liquid, depending on the application. For average currents, the metallic type is most common; for very small currents, the carbon type is used; and for large currents, the electrolytic type, in which electrodes are placed in a conducting fluid, is most suitable. A special type of rheostat is the potentiometer, an instrument that measures an unknown voltage or potential difference by balancing it, wholly or in part, by a known potential difference. A more-common potentiometer is simply a resistor with two fixed terminals and a third terminal connected to a variable contact arm; it is used for such purposes as a volume control in audio equipment.
Details
The most common way to vary the resistance in a circuit continuously is to use a rheostat. It is basically used to adjust magnitude of current in a circuit by changing length. The word rheostat was coined about 1845 by Sir Charles Wheatstone, from the Greek rheos meaning "stream", and -states (from " to set, to cause to stand") meaning "setter, regulating device", which is a two-terminal variable resistor. The term "rheostat" is becoming obsolete, with the general term "potentiometer" replacing it. For low-power applications (less than about 1 watt) a three-terminal potentiometer is often used, with one terminal unconnected or connected to the wiper.
Where the rheostat must be rated for higher power (more than about 1 watt), it may be built with a resistance wire wound around a semicircular insulator, with the wiper sliding from one turn of the wire to the next. Sometimes a rheostat is made from resistance wire wound on a heat-resisting cylinder, with the slider made from a number of metal fingers that grip lightly onto a small portion of the turns of resistance wire. The "fingers" can be moved along the coil of resistance wire by a sliding knob thus changing the "tapping" point. Wire-wound rheostats made with ratings up to several thousand watts are used in applications such as DC motor drives, electric welding controls, or in the controls for generators. The rating of the rheostat is given with the full resistance value and the allowable power dissipation is proportional to the fraction of the total device resistance in circuit. Carbon-pile rheostats are used as load banks for testing automobile batteries and power supplies.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1412 2022-06-16 14:20:22
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,396
Re: Miscellany
1386) Ceilometer
Summary
A ceilometer is a device that uses a laser or other light source to determine the height of a cloud ceiling or cloud base. Ceilometers can also be used to measure the aerosol concentration within the atmosphere. A ceilometer that uses laser light is a type of atmospheric lidar (light detection and ranging) instrument.
Details
Ceilometer is a device for measuring the height of cloud bases and overall cloud thickness. One important use of the ceilometer is to determine cloud ceilings at airports. The device works day or night by shining an intense beam of light (often produced by an infrared or ultraviolet transmitter or a laser), modulated at an audio frequency, at overhead clouds. Reflections of this light from the base of the clouds are detected by a photocell in the receiver of the ceilometer. There are two basic types of ceilometers: the scanning receiver and the rotating transmitter.
The scanning-receiver ceilometer has its separate light transmitter fixed to direct its beam vertically. The receiver is stationed a known distance away. The parabolic collector of the receiver continuously scans up and down the vertical beam, searching for the point where the light intersects a cloud base. When a reflection is detected, the ceilometer measures the vertical angle to the spot; a simple trigonometric calculation then yields the height of the cloud ceiling. Many modern scanning-receiver ceilometers use a laser pulse to identify the height of a cloud’s base and top and various points in between to create a vertical profile of the cloud.
The rotating-transmitter ceilometer has its separate receiver fixed to direct reflections only from directly overhead while the transmitter sweeps the sky. When the modulated beam intersects a cloud base directly over the receiver, light is reflected downward and detected.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1413 2022-06-17 14:18:29
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,396
Re: Miscellany
1387) Ammeter
Summary
An ammeter (abbreviation of Ampere meter) is a measuring instrument used to measure the current in a circuit. Electric currents are measured in Amperes (A), hence the name. The ammeter is usually connected in series with the circuit in which the current is to be measured. An ammeter usually has low resistance so that it does not cause a significant voltage drop in the circuit being measured.
Instruments used to measure smaller currents, in the milliampere or microampere range, are designated as milliammeters or microammeters. Early ammeters were laboratory instruments that relied on the Earth's magnetic field for operation. By the late 19th century, improved instruments were designed which could be mounted in any position and allowed accurate measurements in electric power systems. It is generally represented by letter 'A' in a circuit.
Details
Ammeter is an instrument for measuring either direct or alternating electric current, in amperes. An ammeter can measure a wide range of current values because at high values only a small portion of the current is directed through the meter mechanism; a shunt in parallel with the meter carries the major portion.
Ammeters vary in their operating principles and accuracies. The D’Arsonval-movement ammeter measures direct current with accuracies of from 0.1 to 2.0 percent. The electrodynamic ammeter uses a moving coil rotating in the field produced by a fixed coil. It measures direct and alternating current with accuracies of from 0.1 to 0.25 percent. In the thermal ammeter, used primarily to measure alternating current with accuracies of from 0.5 to 3 percent, the measured current heats a thermoconverter (thermocouple); the small voltage thus generated is used to power a millivoltmeter. Digital ammeters, with no moving parts, use a circuit such as the dual slope integrator to convert a measured analogue (continuous) current to its digital equivalent. Many digital ammeters have accuracies better than 0.1 percent.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1414 2022-06-18 13:52:04
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,396
Re: Miscellany
1388) Industrial truck
Summary
Industrial trucks are trucks that are not licensed to travel on public roads (commercial trucks are licensed to travel on public roads). Industrial trucks are used to move materials over variable paths and when there is insufficient (or intermittent) flow volume such that the use of a conveyor cannot be justified. They provide more flexibility in movement than conveyors and cranes because there are no restrictions on the area covered, and they provide vertical movement if the truck has lifting capabilities. Different types of industrial trucks can be characterized by whether or not they have forks for handling pallets, provide powered or require manual lifting and travel capabilities, allow the operator to ride on the truck or require that the operator walk with the truck during travel, provide load stacking capability, and whether or not they can operate in narrow aisles.
Hand trucks (including carts and dollies), the simplest type of industrial truck, cannot transport or stack pallets, is non-powered, and requires the operator to walk. A pallet jack, which cannot stack a pallet, uses front wheels mounted inside the end of forks that extend to the floor as the pallet is only lifted enough to clear the floor for subsequent travel. A counterbalanced lift truck (sometimes referred to as a forklift truck, but other attachments besides forks can be used) can transport and stack pallets and allows the operator to ride on the truck. The weight of the vehicle (and operator) behind the front wheels of truck counterbalances weight of the load (and weight of vehicle beyond front wheels); the front wheels act as a fulcrum or pivot point. Narrow-aisle trucks usually require that the operator stand-up while riding in order to reduce the truck's turning radius. Reach mechanisms and outrigger arms that straddle and support a load can be used in addition to the just the counterbalance of the truck. On a turret truck, the forks rotate during stacking, eliminating the need for the truck itself to turn in narrow aisles. An order picker allows the operator to be lifted with the load to allow for less-than-pallet-load picking. Automated guided vehicles (AGVs) are industrial trucks that can transport loads without requiring a human operator.
An electric tug is a small battery powered and pedestrian operated machine capable of either pushing or pulling a significantly heavier load than itself.
Details
Industrial truck is a carrier designed to transport materials within a factory area with maximum flexibility in making moves. Most industrial trucks permit mechanized pickup and deposit of the loads, eliminating manual work in lifting as well as transporting. Depending on their means of locomotion, industrial trucks may be classified as hand trucks or power trucks.
Hand trucks with two wheels permit most of the load to be carried on the wheels, but some of the load must be assumed by the operator to balance the truck during movement. Common two-wheel hand trucks include the barrel, box, drum, hopper, refrigerator, paper-roll, and tote-box trucks. Four-wheel hand trucks are found in many more varieties, including dollies, high- and low-bed flat trucks, carts, rack carriers, wagons, and various hand-lift trucks having mechanical or hydraulic lifting mechanisms for raising and lowering a load.
Power trucks are propelled by batteries and an electric-motor drive or by an internal-combustion engine with either a mechanical drive or a generator and electric-motor drive. Propane and diesel engines are used in place of gasoline engines on some types. The non-lift platform truck is used simply for hauling, but other power trucks are provided with mechanisms, usually hydraulic, for lifting the loads. Forklift trucks are equipped with a forklike mechanism on the front end designed to pick up loads on specially designed platforms, called pallets, elevate the load to the desired height, transport it, and deposit it at the desired location and height. Ram trucks have a single protruding ram for handling coiled material. The crane truck is a portable boom crane mounted on an industrial truck; it may be used with hooks, grabs, and slings for bundled or coiled material. The straddle truck resembles a gantry crane on four pneumatic-tired wheels; the operator rides above the inverted U-frame, within which the load—lumber, bar steel, or pipe—is carried on elevating bolsters. Other common types include high- and low-lift platform trucks, motorized pedestrian-led, side-clamp, tractor, and side-loading trucks.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1415 2022-06-19 14:23:14
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,396
Re: Miscellany
1389) Electroplating
Summary
Electroplating is a general name for processes that produce a metal coating on a solid substrate through the reduction of cations of that metal by means of a direct electric current. The part to be coated acts as the cathode (negative electrode) of an electrolytic cell; the electrolyte is a solution of a salt of the metal to be coated; and the anode (positive electrode) is usually either a block of that metal, or of some inert conductive material. The current is provided by an external power supply.
Electroplating is widely used in industry and decorative arts to improve the surface qualities of objects—such as resistance to abrasion and corrosion, lubricity, reflectivity, electrical conductivity, or appearance. It may also be used to build up thickness on undersized or worn-out parts, or to manufacture metal plates with complex shape, a process called electroforming. It is also used to purify metals such as copper.
The term "electroplating" may also be used occasionally for processes that use an electric current to achieve oxidation of anions on to a solid substrate, as in the formation of silver chloride on silver wire to make silver/silver-chloride electrodes.
Electropolishing, a process that uses an electric current to remove metal cations from the surface of a metal object, may be thought of as the opposite of electroplating.
Details
Electroplating is a process of coating with metal by means of an electric current. Plating metal may be transferred to conductive surfaces (metals) or to nonconductive surfaces (plastics, wood, leather) after the latter have been rendered conductive by such processes as coating with graphite, conductive lacquer, electroless plate, or a vaporized coating.
Figure 1 shows a typical plating tank containing copper sulfate (CuSO4) solution. A dynamo supplies electric current, which is controlled by a rheostat. When the switch is closed, the cathode bar, which holds the work to be plated, is charged negatively. Some of the electrons from the cathode bar transfer to the positively charged copper ions, setting them free as atoms of copper metal. These copper atoms take their place on the cathode surface, copperplating it. Concurrently, as shown in the drawing, the same number of sulfate ions are discharged on the copper anodes, thereby completing the electrical circuit. In so doing, they form a new quantity of copper sulfate that dissolves in the solution and restores it to its original composition. This procedure is typical of nearly all ordinary electroplating processes; the current deposits a given amount of metal on the cathode and the anode dissolves to the same extent, maintaining the solution more or less uniformly. If this balance is perfect and there are no side reactions or losses, a 100 percent cathode efficiency and 100 percent anode efficiency could possibly be realized.
If the metal surface of the cathode is chemically and physically clean, the discharged atoms of copper are deposited within normal interatomic spacing of the atoms of the basis metal and attempt to become an integral part of it. In fact, if the basis metal is copper, the new copper atoms will frequently arrange themselves to continue the crystal structure of the basis metal, the plate becoming more or less indistinguishable from and inseparable from the basis metal.
If suitable solutions of different metals are mixed, it is possible to plate a wide variety of alloys of metals. By this means plated brass can be made more or less indistinguishable from cast brass. It is also possible, however, to deposit alloys or compounds of metals that cannot be produced by melting and casting them together. For example, tin-nickel alloy plate has been used commercially for its hardness and corrosion resistance, which are superior to that of either metal alone. The deposit consists of a tin-nickel compound (Sn-Ni) that cannot be produced in any other way.
Other common alloy plates include bronze and gold, with varying properties, such as different colours or hardnesses. Magnetic alloy plates of such metals as iron, cobalt, and nickel are used for memory drums in computers. Solder plate (Sn-Pb) is used in printed circuit work.
Development of electroplating.
While some metal coating procedures date back to ancient times, modern electroplating started in 1800 with Alessandro Volta’s discovery of the voltaic pile, or battery, which made noteworthy quantities of direct current electricity available. At about the same time, the battery was employed to deposit lead, copper, and silver. After a nodule of copper had been deposited on a silver cathode, the copper could not be removed. In the same year, zinc, copper, and silver were deposited on themselves and on a variety of basis metals (the metals on which the plating is applied), such as gold and iron.
Electroplating on a commercial scale was begun about 1840–41 and was accelerated by the discovery of cyanide solutions for plating silver, gold, copper, and brass. A cyanide-copper solution, for example, gave adherent deposits of copper directly on iron and steel. A cyanide-copper solution is still used for this purpose and also for the initial plating on zinc die castings. The copper sulfate solution described above corrodes these metals, giving nonadherent deposits.
Electroplating has become a large and growing industry with sophisticated engineering and equipment requirements. It shows these metals in a single rectangle in their proper relationship to each other. The only metal shown outside the rectangle that is in common use is chromium, which is usually plated at low-current efficiencies of about 10–20 percent. Iron, cobalt, nickel, copper, zinc, ruthenium, rhodium, palladium, silver, cadmium, tin, iridium, platinum, gold, and lead are more or less commonly used for plating. The others can be deposited easily but have not found much use in this way either owing to cost or availability or lack of useful properties.
The introduction of chromium plating in 1925 stimulated repercussions all through the plating industry. Chromium was essentially a bright plate and retained its brightness indefinitely. Chromium plate found a ready market in the automotive and appliance fields, in which the merits of the combination plate nickel-chromium or copper-nickel-chromium were soon proven. The requirements for closer control procedures in bath composition, temperature, and current density were reflected in better control and development of other processes.
So-called hard-chromium plating likewise created a new way of improving the wear resistance of machine parts and improving their operation owing to good frictional and heat resistance properties. Worn or undersized parts were built up with chromium plate.
While nonmetallic materials have been plated since the mid-19th century, a period of rapid growth in the utilization of electroplated plastics began in 1963 with the introduction of ABS plastic (acrylonitrile-butadiene-styrene), which was readily plated. The plastic part is first etched chemically by a suitable process, such as dipping in a hot chromic acid–sulfuric acid mixture. It is next sensitized and activated by first dipping in stannous chloride solution and then in palladium chloride solution. It is then coated with electroless copper or nickel before further plating. A useful degree of adhesion is obtained (about 1 to 6 kg per cm [5 to 30 pounds per inch]) but is in no way comparable to the adhesion of metals to metals.
Principal applications.
Copperplating is used extensively to prevent case hardening of steel on specified parts. The entire article may be copperplated and the plate ground off on the areas to be hardened. Silver plating is used on tableware and electrical contacts; it has also been used on engine bearings. The most extensive use of gold plating is on jewelry and watch cases. Zinc coatings prevent the corrosion of steel articles, while nickel and chromium plate are used on automobiles and household appliances.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1416 2022-06-20 14:28:30
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,396
Re: Miscellany
1390) Evaporation
Summary
Evaporation is a process by which an element or compound transitions from its liquid state to its gaseous state below the temperature at which it boils; in particular, the process by which liquid water enters the atmosphere as water vapour in the water cycle.
Evaporation, mostly from the oceans and from vegetation, replenishes the humidity of the air. It is an important part of the exchange of energy in the Earth-atmosphere system that produces atmospheric motion and therefore weather and climate. This transfer of water between Earth’s surface and the atmosphere occurs when some molecules in a water mass have attained sufficient kinetic energy to eject themselves from the water surface. The main factors affecting evaporation are temperature (specifically, the temperature difference between the evaporating surface and the air), relative humidity, wind speed, and solar radiation.
Details
Evaporation is a type of vaporization that occurs on the surface of a liquid as it changes into the gas phase. The surrounding gas must not be saturated with the evaporating substance. When the molecules of the liquid collide, they transfer energy to each other based on how they collide with each other. When a molecule near the surface absorbs enough energy to overcome the vapor pressure, it will escape and enter the surrounding air as a gas. When evaporation occurs, the energy removed from the vaporized liquid will reduce the temperature of the liquid, resulting in evaporative cooling.
On average, only a fraction of the molecules in a liquid have enough heat energy to escape from the liquid. The evaporation will continue until an equilibrium is reached when the evaporation of the liquid is equal to its condensation. In an enclosed environment, a liquid will evaporate until the surrounding air is saturated.
Evaporation is an essential part of the water cycle. The sun (solar energy) drives evaporation of water from oceans, lakes, moisture in the soil, and other sources of water. In hydrology, evaporation and transpiration (which involves evaporation within plant stomata) are collectively termed evapotranspiration. Evaporation of water occurs when the surface of the liquid is exposed, allowing molecules to escape and form water vapor; this vapor can then rise up and form clouds. With sufficient energy, the liquid will turn into vapor.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1417 2022-06-21 15:54:47
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,396
Re: Miscellany
1391) Vaporization
Summary
Vaporization is a conversion of a substance from the liquid or solid phase into the gaseous (vapour) phase. If conditions allow the formation of vapour bubbles within a liquid, the vaporization process is called boiling. Direct conversion from solid to vapour is called sublimation.
Heat must be supplied to a solid or liquid to effect vaporization. If the surroundings do not supply enough heat, it may come from the system itself as a reduction in temperature. The atoms or molecules of a liquid or solid are held together by cohesive forces, and these forces must be overcome in separating the atoms or molecules to form the vapour; the heat of vaporization is a direct measure of these cohesive forces.
Condensation of a vapour to form a liquid or a solid is the reverse of vaporization, and in the process heat must be transferred from the condensing vapour to the surroundings. The amount of this heat is characteristic of the substance, and it is numerically the same as the heat of vaporization.
Details
Vaporization (or vaporisation) of an element or compound is a phase transition from the liquid phase to vapor. There are two types of vaporization: evaporation and boiling. Evaporation is a surface phenomenon, whereas boiling is a bulk phenomenon.
Evaporation is a phase transition from the liquid phase to vapour (a state of substance below critical temperature) that occurs at temperatures below the boiling temperature at a given pressure. Evaporation occurs on the surface. Evaporation only occurs when the partial pressure of vapor of a substance is less than the equilibrium vapor pressure. For example, due to constantly decreasing pressures, vapor pumped out of a solution will eventually leave behind a cryogenic liquid.
Boiling is also a phase transition from the liquid phase to gas phase, but boiling is the formation of vapor as bubbles of vapor below the surface of the liquid. Boiling occurs when the equilibrium vapor pressure of the substance is greater than or equal to the environmental pressure. The temperature at which boiling occurs is the boiling temperature, or boiling point. The boiling point varies with the pressure of the environment.
Sublimation is a direct phase transition from the solid phase to the gas phase, skipping the intermediate liquid phase. Because it does not involve the liquid phase, it is not a form of vaporization.
The term vaporization has also been used in a colloquial or hyperbolic way to refer to the physical destruction of an object that is exposed to intense heat or explosive force, where the object is actually blasted into small pieces rather than literally converted to gaseous form. Examples of this usage include the "vaporization" of the uninhabited Marshall Island of Elugelab in the 1952 Ivy Mike thermonuclear test.
At the moment of a large enough meteor or comet impact, bolide detonation, a nuclear fission, thermonuclear fusion, or theoretical antimatter weapon detonation, a flux of so many gamma ray, x-ray, ultraviolet, visual light and heat photons strikes matter in a such brief amount of time (a great number of high-energy photons, many overlapping in the same physical space) that all molecules lose their atomic bonds and "fly apart". All atoms lose their electron shells and become positively charged ions, in turn emitting photons of a slightly lower energy than they had absorbed. All such matter becomes a gas of nuclei and electrons which rise into the air due to the extremely high temperature or bond to each other as they cool. The matter vaporized this way is immediately a plasma in a state of maximum entropy and this state steadily reduces via the factor of passing time due to natural processes in the biosphere and the effects of physics at normal temperatures and pressures.
A similar process occurs during ultrashort pulse laser ablation, where the high flux of incoming electromagnetic radiation strips the target material's surface of electrons, leaving positively charged atoms which undergo a coulomb explosion
![]()
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1418 2022-06-22 13:51:58
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,396
Re: Miscellany
1392) Condensation
Summary
Condensation is the change of the state of matter from the gas phase into the liquid phase, and is the reverse of vaporization. The word most often refers to the water cycle. It can also be defined as the change in the state of water vapor to liquid water when in contact with a liquid or solid surface or cloud condensation nuclei within the atmosphere. When the transition happens from the gaseous phase into the solid phase directly, the change is called deposition.
Details
Condensation is a deposition of a liquid or a solid from its vapour, generally upon a surface that is cooler than the adjacent gas. A substance condenses when the pressure exerted by its vapour exceeds the vapour pressure of the liquid or solid phase of the substance at the temperature of the surface where condensation occurs. Heat is released when a vapour condenses. Unless this heat is removed, the surface temperature will increase until it is equal to that of the surrounding vapour.
If air were free of tiny particles, called aerosols, condensation would only occur when the air was extremely supersaturated with water vapour. In the atmosphere, however, there is an abundant supply of aerosols, which serve as nuclei, called condensation nuclei, on which water vapour may condense. Some are hygroscopic (moisture-attracting), and condensation begins on them when the relative humidity is less than 100 percent, but other nuclei require some supersaturation before condensation begins.
In the atmosphere the relative humidity of the air is increased, and condensation results when air temperature is reduced to the dew point or when sufficient water vapour is added to saturate the air. Condensation accounts for the formation of dew, fog, and clouds. For rain to occur, other physical processes are required.
Applications of condensation
In cloud chambers a liquid (sometimes water, but usually isopropanol) condenses upon contact with a particle of radiation thus producing an effect similar to contrails
Condensation is a crucial component of distillation, an important laboratory and industrial chemistry application.
Because condensation is a naturally occurring phenomenon, it can often be used to generate water in large quantities for human use. Many structures are made solely for the purpose of collecting water from condensation, such as air wells and fog fences. Such systems can often be used to retain soil moisture in areas where active desertification is occurring—so much so that some organizations educate people living in affected areas about water condensers to help them deal effectively with the situation.
It is also a crucial process in forming particle tracks in a cloud chamber. In this case, ions produced by an incident particle act as nucleation centers for the condensation of the vapor producing the visible "cloud" trails.
Commercial applications of condensation, by consumers as well as industry, include power generation, water desalination, thermal management, refrigeration, and air conditioning.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1419 2022-06-23 13:50:01
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,396
Re: Miscellany
1393) Sublimation
Summary
Sublimation, in physics, is a conversion of a substance from the solid to the gaseous state without its becoming liquid. An example is the vaporization of frozen carbon dioxide (dry ice) at ordinary atmospheric pressure and temperature. The phenomenon is the result of vapour pressure and temperature relationships. Freeze-drying of food to preserve it involves sublimation of water from the food in a frozen state under high vacuum.
Details
Sublimation is the transition of a substance directly from the solid to the gas state, without passing through the liquid state. Sublimation is an endothermic process that occurs at temperatures and pressures below a substance's triple point in its phase diagram, which corresponds to the lowest pressure at which the substance can exist as a liquid. The reverse process of sublimation is deposition or desublimation, in which a substance passes directly from a gas to a solid phase. Sublimation has also been used as a generic term to describe a solid-to-gas transition (sublimation) followed by a gas-to-solid transition (deposition). While vaporization from liquid to gas occurs as evaporation from the surface if it occurs below the boiling point of the liquid, and as boiling with formation of bubbles in the interior of the liquid if it occurs at the boiling point, there is no such distinction for the solid-to-gas transition which always occurs as sublimation from the surface.
At normal pressures, most chemical compounds and elements possess three different states at different temperatures. In these cases, the transition from the solid to the gaseous state requires an intermediate liquid state. The pressure referred to is the partial pressure of the substance, not the total (e.g. atmospheric) pressure of the entire system. So, all solids that possess an appreciable vapour pressure at a certain temperature usually can sublime in air (e.g. water ice just below 0 °C). For some substances, such as carbon and math, sublimation is much easier than evaporation from the melt, because the pressure of their triple point is very high, and it is difficult to obtain them as liquids.
The term sublimation refers to a physical change of state and is not used to describe the transformation of a solid to a gas in a chemical reaction. For example, the dissociation on heating of solid ammonium chloride into hydrogen chloride and ammonia is not sublimation but a chemical reaction. Similarly the combustion of candles, containing paraffin wax, to carbon dioxide and water vapor is not sublimation but a chemical reaction with oxygen.
Sublimation is caused by the absorption of heat which provides enough energy for some molecules to overcome the attractive forces of their neighbors and escape into the vapor phase. Since the process requires additional energy, it is an endothermic change. The enthalpy of sublimation (also called heat of sublimation) can be calculated by adding the enthalpy of fusion and the enthalpy of vaporization.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1420 2022-06-24 14:11:54
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,396
Re: Miscellany
1394) Millstone
Summary
Millstone is one of a pair of flat, round stones used for grinding grain. One millstone is stationary; the other rotates above it in a horizontal plane. Grain is poured through a hole in the centre of the rotating millstone, flowing into shallow grooves, called channels, which radiate from the centre of the stationary millstone. The channels lead the grain onto the flat grinding section, called the land, and to the edge, where it emerges as flour. Sharpening a millstone consists of recarving worn channels and checking and smoothing the land.
Details
Millstones or mill stones are stones used in gristmills, for grinding wheat or other grains. They are sometimes referred to as grindstones or grinding stones.
Millstones come in pairs: a convex stationary base known as the bedstone and a concave runner stone that rotates. The movement of the runner on top of the bedstone creates a "scissoring" action that grinds grain trapped between the stones. Millstones are constructed so that their shape and configuration help to channel ground flour to the outer edges of the mechanism for collection.
The runner stone is supported by a cross-shaped metal piece (millrind or rynd) fixed to a "mace head" topping the main shaft or spindle leading to the driving mechanism of the mill (wind, water (including tide) or other means).
History
The earliest evidence for stones used to grind food is found in northern Australia, at the Madjedbebe rock shelter in Arnhem Land, dating back around 60,000 years. Grinding stones or grindstones, as they were called, were used by the Aboriginal peoples across the continent and islands, and they were traded in areas where suitable sandstone was not available in abundance. Different stones were adapted for grinding different things and varied according to location. One important use was for foods, in particular to grind seeds to make bread, but stones were also adapted for grinding specific types of starchy nuts, ochres for artwork, plant fibres for string, or plants for use in bush medicine, and are still used today. The Australian grindstones usually comprise a large flat sandstone rock (for its abrasive qualities), used with a top stone, known as a "muller", "pounder", or pestle. The Aboriginal peoples of the present state of Victoria used grinding stones to crush roots, bulbs, tubers and berries, as well as insects, small mammals and reptiles before cooking them.
Neolithic and Upper Paleolithic people used millstones to grind grains, nuts, rhizomes and other vegetable food products for consumption. These implements are often called grinding stones, and used either saddle stones or rotary querns turned by hand. Such devices were also used to grind pigments and metal ores prior to smelting.
In India, grinding stones (Chakki) were used to grind grains and spices. These consist of a stationary stone cylinder upon which a smaller stone cylinder rotates. Smaller ones, for household use, were operated by two people. Larger ones, for community or commercial use, used livestock to rotate the upper cylinder. Today a majority of the stone flour mills (Atta Chakki) are equipped with lower stone rotating and upper stone stationary millstones also called Shikhar Emery Stones which are made from abrasive emery grains and grits, with a binding agent similar to Sorel Cement. These stones are made from two types of emery abrasives - Natural Jaspar Red Emery or Synthetic Calcined Bauxite Black Emery.
Millstones were introduced to Britain by the Romans during the 1st century AD and were widely used there from the 3rd century AD onwards.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1421 2022-06-25 13:52:11
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,396
Re: Miscellany
1395) Flour
Summary
Flour is a powder made by grinding raw grains, roots, beans, nuts, or seeds. Flours are used to make many different foods. Cereal flour, particularly wheat flour, is the main ingredient of bread, which is a staple food for many cultures. Corn flour has been important in Mesoamerican cuisine since ancient times and remains a staple in the Americas. Rye flour is a constituent of bread in central and northern Europe.
Cereal flour consists either of the endosperm, germ, and bran together (whole-grain flour) or of the endosperm alone (refined flour). Meal is either differentiable from flour as having slightly coarser particle size (degree of comminution) or is synonymous with flour; the word is used both ways. For example, the word cornmeal often connotes a grittier texture whereas corn flour connotes fine powder, although there is no codified dividing line.
An American health agency has cautioned not to eat raw flour doughs or batters. Raw flour can contain bacteria like E. coli and needs to be cooked like other foods.
Details
Flour is finely ground cereal grains or other starchy portions of plants, used in various food products and as a basic ingredient of baked goods. Flour made from wheat grains is the most satisfactory type for baked products that require spongy structure. In modern usage, the word flour alone usually refers to wheat flour, the major type in Western countries.
A brief treatment of flour and flour milling follows.
Wheat grains, or kernels, are composed of the starchy endosperm, or food-storage portion, constituting about 85 percent; several outer layers that make up the bran, constituting about 13 percent; and the oily germ, or embryo plant, approximately 2 percent. In the production of refined flour, the purpose of the milling process is to separate the endosperm from the other kernel portions. In the production of whole wheat flour, all parts of the kernel are used.
In modern milling of refined flours the wheat kernels are cleaned and tempered by the addition or removal of moisture and then split open by a pair of rolls. The finest particles, called break flour, are sieved out and bagged. Coarser particles of endosperm (called semolina) and pieces of bran with endosperm attached are then subjected to a series of rolls in which semolina of steadily reducing size is gradually ground to flour and the bran separated out. The flour is usually bleached and treated to obtain the improved bread-making qualities formerly achieved by natural aging. Flour grades are based on the residual amount of branny particles.
When flour is mixed with water to make dough, its protein content is converted to gluten, an elastic substance that forms a continuous network throughout the dough and is capable of retaining gas, thus causing the baked product to expand, or rise. The strength of the gluten depends upon the protein content of the flour. Soft wheats, containing approximately 8–12 percent protein, produce flours that are suitable for products requiring minimal structure, such as cakes, cookies (sweet biscuits), piecrusts, and crackers. Hard wheats, which are high in protein (approximately 12–15 percent), produce flours that are suitable for products requiring stronger structure, such as breads, buns, hard rolls, and yeast-raised sweet rolls.
The wide variety of wheat flours generally available includes whole wheat, or graham, flour, made from the entire wheat kernel and often unbleached; gluten flour, a starch-free, high-protein, whole wheat flour; all-purpose flour, refined (separated from bran and germ), bleached or unbleached, and suitable for any recipe not requiring a special flour; cake flour, refined and bleached, with very fine texture; self-rising flour, refined and bleached, with added leavening and salt; and enriched flour, refined and bleached, with added nutrients.
Flours are also made from other starchy plant materials including barley, buckwheat, chickpeas, lima beans, oats, peanuts, potatoes, soybeans, rice, and rye.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1422 2022-06-26 13:53:58
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,396
Re: Miscellany
1396) Mortal and pestle
Summary
Mortar and pestle is an ancient device for milling by pounding. The mortar is a durable bowl commonly made of stone, ceramic, or wood. The pestle is a rounded grinding club often made of the same material as the mortar. Together with the saddle quern (a round stone rolled or rubbed on a flat stone bed), the mortar and pestle was the first means known for grinding grain; the grain was placed in a shallow depression in a stone, the mortar, and pounded with a rodlike stone, the pestle. Smaller refined versions of the mortar and pestle have continued to find use in kitchens for preparing pastes and other finely ground elements of cuisine, in pharmacy for preparing medicines, and in chemical laboratories.
Details
Mortar and pestle is a set of two simple tools used from the Stone Age to the present day to prepare ingredients or substances by crushing and grinding them into a fine paste or powder in the kitchen, laboratory, and pharmacy. The mortar is characteristically a bowl, typically made of hard wood, metal, ceramic, or hard stone such as granite. The pestle is a blunt, club-shaped object. The substance to be ground, which may be wet or dry, is placed in the mortar where the pestle is pounded, pressed, and rotated into the substance until the desired texture is achieved.
Mortars and pestles have been used in cooking since prehistory; today they are typically associated with the profession of pharmacy due to their historical use in preparing medicines. They are used in chemistry settings for pulverizing small amounts of chemicals; in arts and cosmetics for pulverizing pigments, binders, and other substances; in ceramics for making grog; in masonry and in other types of construction requiring pulverized materials. In cooking, they are typically used to crush spices, to make pesto and certain math such as the mojito, which requires the gentle crushing of sugar, ice, and mint leaves in the glass with a pestle.
The invention of mortars and pestles seems related to that of quern-stones, which use a similar principle of naturally indented, durable, hard stone bases and mallets of stone or wood to process food and plant materials, clay, or minerals by stamping, crushing, pulverizing and grinding.
A key advantage of the mortar is that it presents a deeper bowl for confining the material to be ground without the waste and spillage that occurs with flat grinding stones. Another advantage is that the mortar can be made large enough for a person to stand upright and adjacent to it and use the combined strength of their upper body and the force of gravity for better stamping. Large mortars allow for several people with several pestles to stamp the material faster and more efficiently. Working over a large mortar that a person can stand next to is physically easier and more ergonomical (by ensuring a better posture of the whole body) than for a small quern, where a person has to crouch and use the uncomfortable, repetitive motion of hand grinding by sliding.
Mortars and pestles anticipate modern blenders and grinders and can be described as having the function of small, mobile, hand-operated mills that don't require electricity or fuel to operate.
Large wooden mortars and wooden pestles would predate and lead to the invention of butterchurns, as domestication of livestock and use of dairy (during the Neolithic) came well after the mortar and pestle. Butter would be churned from cream or milk in a wooden container with a long wooden stick, very like the use of wooden mortars and pestles.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1423 2022-06-27 14:12:45
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,396
Re: Miscellany
1397) Grinding machine
Summary
A grinding machine, often shortened to grinder, is a power tool (or machine tool) used for grinding. It is a type of machining using an abrasive wheel as the cutting tool. Each grain of abrasive on the wheel's surface cuts a small chip from the workpiece via shear deformation.
Grinding is used to finish workpieces that must show high surface quality (e.g., low surface roughness) and high accuracy of shape and dimension. As the accuracy in dimensions in grinding is of the order of 0.000025 mm, in most applications it tends to be a finishing operation and removes comparatively little metal, about 0.25 to 0.50 mm depth. However, there are some roughing applications in which grinding removes high volumes of metal quite rapidly. Thus, grinding is a diverse field.
Details
A grinding machine, often shortened to grinder, is one of the power tools or machine tools used for grinding, it is a type of machining using an abrasive wheel as the cutting tool. Each grain of abrasive on the wheel’s surface cuts a small chip from the workpiece via shear deformation.
Grinding is used to finish workpieces that must show high surface quality (e.g., low surface roughness) and high accuracy of shape and dimension. As the accuracy in dimensions in grinding is of the order of 0.000025 mm, in most applications, it tends to be a finishing operation and removes comparatively little metal, about 0.25 to 0.50 mm depth.
However, there are some roughing applications in which grinding removes high volumes of metal quite rapidly. Thus, grinding is a diverse field.
Overview
The grinding machine consists of a bed with a fixture to guide and hold the workpiece, and a power-driven grinding wheel spinning at the required speed. The speed is determined by the wheel’s diameter and the manufacturer’s rating. The grinding head can travel across a fixed workpiece, or the workpiece can be moved while the grinding head stays in a fixed position.
Fine control of the grinding head or table position is possible using a vernier calibrated hand wheel or using the features of numerical controls.
Grinding machines remove material from the workpiece by abrasion, which can generate substantial amounts of heat. To cool the workpiece so that it does not overheat and go outside its tolerance, grinding machines incorporate a coolant.
The coolant also benefits the machinist as the heat generated may cause burns. In high-precision grinding machines (most cylindrical and surface grinders), the final grinding stages are usually set up so that they remove about 200 nm (less than 1/10000 in) per pass – this generates so little heat that even with no coolant, the temperature rise is negligible.
Types Of Grinding Machines
These machines include the:
* Belt grinder, which is usually used as a machining method to process metals and other materials, with the aid of coated abrasives. Analogous to a belt sander (which itself is often used for wood but sometimes metal). Belt grinding is a versatile process suitable for all kind of applications, including finishing, deburring, and stock removal.
* Bench grinder, which usually has two wheels of different grain sizes for roughing and finishing operations and is secured to a workbench or floor stand. Its uses include shaping tool bits or various tools that need to be made or repaired. Bench grinders are manually operated.
* Cylindrical Grinders: A cylindrical grinder is used for shaping the outside of a workpiece. These machines accept workpieces in a variety of shapes as long as they can be rotated through a central axis. In a cylindrical grinder, both the workpiece and grinding wheel are simultaneously rotated. Outside diameter grinders, internal diameter grinders, and centerless grinders are all types of cylindrical grinders.
* Surface Grinders: A surface grinder consists of an abrasive wheel, a chuck (a workpiece holding device), and a rotary table. The chuck is used to hold the material in place while the wheel and object are rotated to produce a smooth finish.
* Centerless Grinders: A centerless grinder is a type of cylindrical grinder which uses two rotary wheels to secure the workpiece in place. Unlike a centered grinder, a centerless grinder does not make use of a spindle. The speed of the rotation of the wheels determines at what rate the material is removed.
* Tool & Cutter Grinders: A tool and cutter grinder makes use of a CNC machine tool with up to 5 axes and multiple grinding wheels. These devices are used for sharpening and producing milling cutters such as drills, endmills, and step tools. It is also widely used for producing the tools needed in the woodworking and metal cutting industries.
* Jig grinder, which as the name implies, has a variety of uses when finishing jigs, dies, and fixtures. Its primary function is in the realm of grinding holes for drill bushings and grinding pins. It can also be used for complex surface grinding to finish work started on a mill.
* Gear grinder, which is usually employed as the final machining process when manufacturing a high-precision gear. The primary function of these machines is to remove the remaining few thousandths of an inch of material left by other manufacturing methods (such as gashing or hobbling).
* Centre grinder, which is usually employed as a machining process when manufacturing all kinds of high-precision shafts. The primary function of these machines is to grind the centers of a shaft very precisely. Accurate round center holes on both sides ensure a position with high repeat accuracy on the live centers.
* Die grinder, which is a high-speed hand-held rotary tool with a small diameter grinding bit. They are typically air driven (using compressed air), but can be driven with a small electric motor directly or via a flexible shaft.
* Angle grinder, another handheld power tool, often used in fabrication and construction work.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1424 2022-06-28 14:27:32
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,396
Re: Miscellany
1397) Otoscope
Summary
An otoscope is a tool which shines a beam of light to help visualize and examine the condition of the ear canal and eardrum. Examining the ear can reveal the cause of symptoms such as an earache, the ear feeling full, or hearing loss.
Details
An otoscope or auriscope is a medical device which is used to look into the ears. Health care providers use otoscopes to screen for illness during regular check-ups and also to investigate ear symptoms. An otoscope potentially gives a view of the ear canal and tympanic membrane or eardrum. Because the eardrum is the border separating the external ear canal from the middle ear, its characteristics can be indicative of various diseases of the middle ear space. The presence of earwax (cerumen), shed skin, pus, canal skin edema, foreign body, and various ear diseases can obscure any view of the eardrum and thus severely compromise the value of otoscopy done with a common otoscope, but confirm the presence of obstructing symptoms.
The most commonly used otoscopes consist of a handle and a head. The head contains a light source and a simple low-power magnifying lens, typically around 8 diopters (3.00x Mag). The distal (front) end of the otoscope has an attachment for disposable plastic ear specula. The examiner first straightens the ear canal by pulling on the pinna (usually the earlobe, side or top) and then inserts the ear speculum side of the otoscope into the external ear. It is important to brace the hand holding the otoscope against the patient's head to avoid injury to the ear canal by placing the index finger or little finger against the head. The examiner can then look through a lens on the rear of the instrument and see inside the ear canal. In many models, the lens can be removed, which allows the examiner to insert instruments through the otoscope into the ear canal, such as for removing earwax. Most models also have an insertion point for a bulb capable of pushing air through the speculum which is called pneumatic otoscope. This puff of air allows an examiner to test the mobility of the tympanic membrane.
Many otoscopes used in doctors offices are wall-mounted while others are portable. Wall-mounted otoscopes are attached by a flexible power cord to a base, which serves to hold the otoscope when it's not in use and also serves as a source of electric power, being plugged into an electric outlet. Portable models are powered by batteries in the handle; these batteries are usually rechargeable and can be recharged from a base unit. Otoscopes are often sold with ophthalmoscopes as a diagnostic set.
Diseases which may be diagnosed by an otoscope include otitis media and otitis externa, infection of the middle and outer parts of the ear, respectively.
Otoscopes are also frequently used for examining patients' noses (avoiding the need for a separate nasal speculum) and (with the speculum removed) upper throats.
The most commonly used otoscopes—those used in emergency rooms, pediatric offices, general practice, and by internists- are monocular devices. They provide only a two-dimensional view of the ear canal, its contents, and usually at least a portion of the eardrum, depending on what is within the ear canal and its status. Another method of performing otoscopy (visualization of the ear) is use of a binocular microscope, in conjunction with a larger plastic or metal ear speculum, with the patient supine and the head tilted, which provides a much larger field of view and the added advantages of a stable head, far superior lighting, and most importantly, depth perception. A binocular (two-eyed) view is required in order to judge depth. If wax or another material obstructs the canal and/or a view of the entire eardrum, it can easily and confidently be removed with specialized suction tips and other microscopic ear instruments, whereas the absence of depth perception with the one-eyed view of a common otoscope makes removal of anything more laborious and hazardous. Another major advantage of the binocular microscope is that both of the examiner's hands are free, since the microscope is suspended from a stand. The microscope has up to 40x power magnification, which allows much more detailed viewing of the entire ear canal, and of the entire eardrum unless edema of the canal skin prevents it. Subtle changes in the anatomy are much more easily detected and interpreted than with a monocular view otoscope. Traditionally only ENT specialists (otolaryngologists) and otologists (subspecialty ear doctors) acquire binocular microscopes and the necessary skills and training to use them, and incorporate their routine use in evaluating patient's ear complaints. Studies have shown that reliance on a monocular otoscope to diagnose ear disease results in a more than 50% chance of misdiagnosis, as compared to binocular microscopic otoscopy. The expense of acquiring a binocular microscope is only one obstacle to its being more widely adapted to general medicine. The low level of familiarity with binocular otoscopy among pediatric and general medicine professors in physician training programs is probably a more difficult obstacle to overcome. Thus, the standard of general otologic diagnosis and ear care remains, for the most part, the largely antiquated monocular otoscope.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1425 2022-06-29 14:15:36
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,396
Re: Miscellany
1398) Fetoscopy
Summary
Fetoscopy is an endoscopic procedure during pregnancy to allow surgical access to the fetus, the amniotic cavity, the umbilical cord, and the fetal side of the placenta. A small (3–4 mm) incision is made in the abdomen, and an endoscope is inserted through the abdominal wall and uterus into the amniotic cavity. Fetoscopy allows for medical interventions such as a biopsy (tissue sample) or a laser occlusion of abnormal blood vessels (such as chorioangioma) or the treatment of spina bifida.[1]
Fetoscopy is usually performed in the second or third trimester of pregnancy. The procedure can place the fetus at increased risk of adverse outcomes, including fetal loss or preterm delivery, so the risks and benefits must be carefully weighed in order to protect the health of the mother and fetus(es). The procedure is typically performed in an operating room by an obstetrician-gynecologist.
Details
Fetoscopy is a technique that utilizes a small camera or scope to examine and perform procedures on the fetus during pregnancy. The scope is introduced through a small incision on the mother's abdomen and placed into the amniotic sac through the uterus. This allows a visual assessment of any abnormalities during pregnancy. Fetoscopy is used for several different diseases and has been used to perform surgical procedures and collecting biopsies.
Which conditions are treated with Fetoscopy?
Historically, fetoscopy has been utilized for many different fetal conditions. However, only three disease conditions routinely utilize this technique.
* Twin-Twin Transfusion Syndrome - A fetoscope is utilized to provide a visualization of the abnormal blood vessels between twins that cause this disease. The scope also allows the placement of the laser device which is utilized to disconnect abnormal blood vessel connections
* Amniotic Band Syndrome - Fetoscopy allows visualization of the abnormal bands of tissue from the amniotic membrane which are stuck to the fetus. These bands can cause strictures or amputations of vital organs and limbs. The scope allows the ability to release the bands.
* Congenital Diaphragmatic Hernia - A potential fetal therapy for CDH is the placement of a balloon in the trachea that promotes lung growth. This technique requires fetoscopy to visualize the fetal airway (trachea). Balloon occlusion for CDH remains experimental and is under clinical investigation at this point. This therapy is currently undergoing clinical trials.
Please keep in mind that fetoscopy is a rarely utilized procedure and for each patient diagnosed with any of the above conditions, only a few will need fetal intervention. The recommendation to perform fetal intervention and fetoscopy will be determined by your team of doctors at the Center and discussed with you.
What special considerations should be made at time of delivery?
* Type of delivery - If all goes well with the fetoscopic intervention, your pregnancy will be allowed to progress to term and depending on the condition, delivery usually does not require a Cesarean delivery. The need for this fetal intervention should not impact your type of delivery. The delivery plan should be carefully discussed between the mother and the obstetrician.
* Place of delivery - Depending on the condition, your baby may or may not need special medical care after birth. If all the prenatal monitoring suggests that your baby is doing well, the baby can be delivered at the hospital of your choice. However, the hospital should be prepared to handle any intensive care of your newborn and have a neonatal intensive care unit with the capability to provide specialized care.
* Time of delivery - There is no reason to intentionally induce early delivery. After the fetoscopic procedure, your pregnancy will be closely monitored. The team at the Center may recommend early delivery for pregnancies that appear to be in danger.
What will happen at the Fetoscopy procedure?
The entire team will carefully plan for the fetoscopy procedure with preparation to handle all potential complications. Generally, the procedure is performed under sedation and local anesthesia. Your doctors will repeat a detailed ultrasound to confirm the problem and identify the abnormalities. A small skin incision is made to allow the placement of the scope. Once inside the amniotic sac, your doctors will perform the necessary procedure. On occasion, the fetoscopy procedure cannot be performed with a small skin incision due to the location of the fetuses and placenta in the uterus. In these situations, the procedure requires a larger incision to expose the uterus in order to provide a safe window for the scope.
The fetoscopy procedure is performed in the operating room with all the special equipment necessary to ensure the safety of you and your baby. Afterwards, the mother will be admitted to the Women's Center to monitor for preterm labor and complications at Children's Memorial Hermann Hospital.
What are the risks and considerations?
The major risk of fetoscopy is injuring and losing the fetus during the procedure. The risks and benefits of the procedure will be carefully explained. If all goes well with the procedure, your pregnancy will be carefully monitored for preterm labor and premature delivery.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline