Math Is Fun Forum
You are not logged in.
- Topics: Active | Unanswered
#1 This is Cool » International Date Line » Today 17:11:07
- Jai Ganesh
- Replies: 0
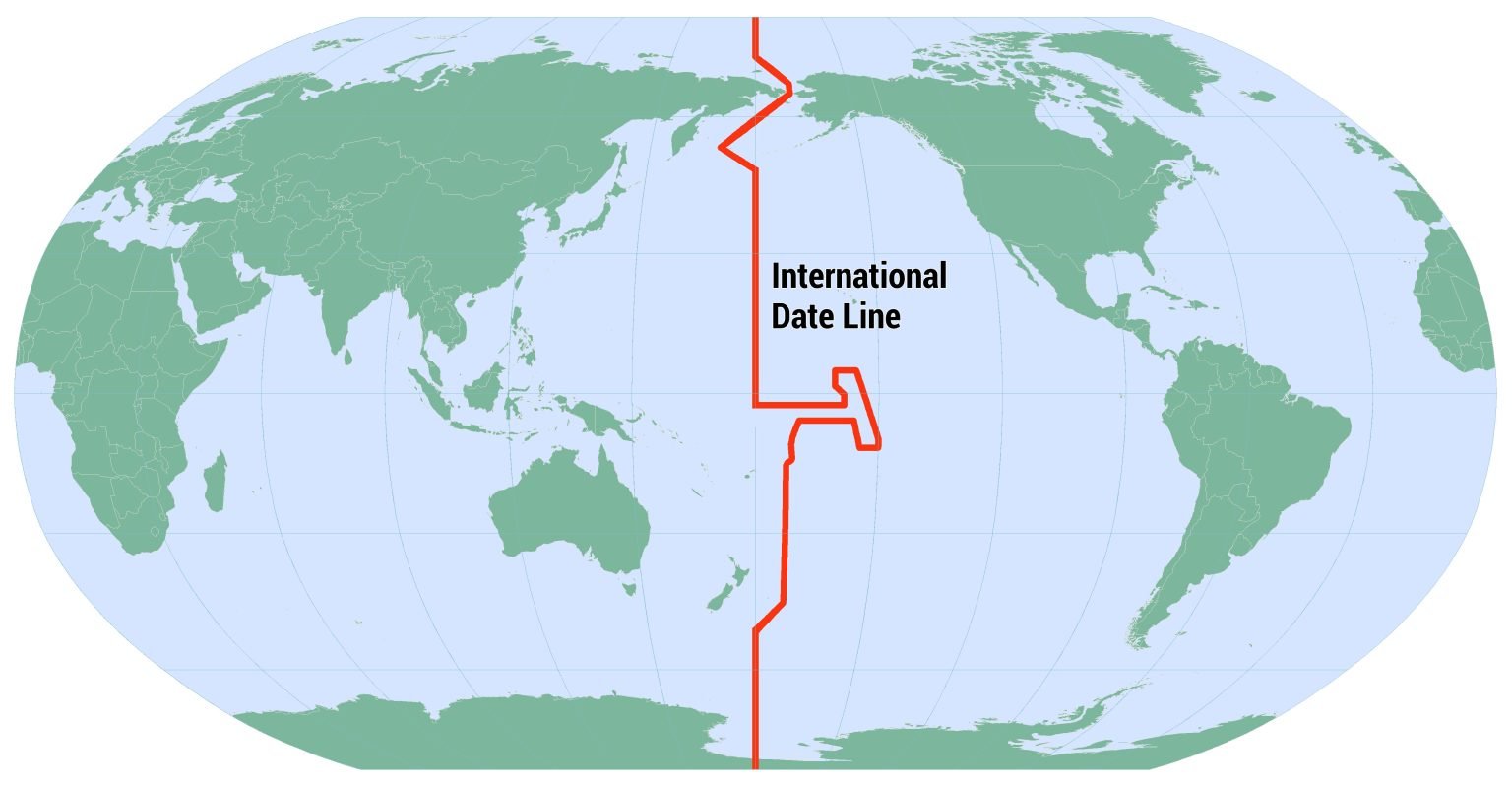
International Date Line
Gist
The international date line, established in 1884, passes through the mid-Pacific Ocean and roughly follows a 180 degrees longitude north-south line on the Earth. It is located halfway around the world from the prime meridian — the 0 degrees longitude line in Greenwich, England.
The International Date Line (IDL) is the line extending between the South and North Poles that is the boundary between one calendar day and the next. It passes through the Pacific Ocean, roughly following the 180.0° line of longitude and deviating to pass around some territories and island groups.
Summary
International Date Line, imaginary line extending between the North Pole and the South Pole and arbitrarily demarcating each calendar day from the next. It corresponds along most of its length to the 180th meridian of longitude but deviates eastward through the Bering Strait to avoid dividing Siberia and then deviates westward to include the Aleutian Islands with Alaska. South of the Equator, another eastward deviation allows certain island groups to have the same day as New Zealand.
The International Date Line is a consequence of the worldwide use of timekeeping systems arranged so that local noon corresponds approximately to the time at which the sun crosses the local meridian of longitude (see Standard Time). A traveler going completely around the world while carrying a clock that he advanced or set back by one hour whenever he entered a new time zone and a calendar that he advanced by one day whenever his clock indicated midnight would find on returning to his starting point that the date according to his own experience was different by one day from that kept by persons who had remained at the starting point. The International Date Line provides a standard means of making the needed readjustment: travelers moving eastward across the line set their calendars back one day, and those traveling westward set theirs a day ahead.
Details
The International Date Line (IDL) is the line extending between the South and North Poles that is the boundary between one calendar day and the next. It passes through the Pacific Ocean, roughly following the 180.0° line of longitude and deviating to pass around some territories and island groups. Crossing the date line eastbound decreases the date by one day, while crossing the date line westbound increases the date.
The line is a cartographic convention and is not defined by international law. This has made it difficult for cartographers to agree on its precise course and has allowed countries through whose waters it passes to move it at times for their convenience.
Geography
A simplified illustration of the relation between the International Date Line, the date, and the time of day. Each color represents a different date.
Example depicting situation at 04:00 GMT Tuesday. (Times are approximate, since time zone boundaries generally do not exactly coincide with meridians. Night and day is illustrative only; daylight hours depend on latitude and time of year.)
Circumnavigating the globe
People traveling westward around the world must set their clocks:
* Back by one hour for every 15° of longitude crossed, and
* Forward by 24 hours upon crossing the International Date Line.
People traveling eastward must set their clocks:
* Forward by one hour for every 15° of longitude crossed, and
* Back by 24 hours upon crossing the International Date Line.
Moving forward or back 24 hours generally also implies a one day date change.
The 14th-century Arab geographer Abulfeda predicted that circumnavigators would accumulate a one-day offset to the local date. This phenomenon was confirmed in 1522 at the end of the Magellan–Elcano expedition, the first successful circumnavigation. After sailing westward around the world from Spain, the expedition called at Cape Verde for provisions on Wednesday, 9 July 1522 (ship's time). However, the locals told them that it was actually Thursday, 10 July 1522. The crew was surprised, as they had recorded each day of the three-year journey without omission. Cardinal Gasparo Contarini, the Venetian ambassador to Spain, was the first European to give a correct explanation of the discrepancy.
Description
This description is based on the most common understanding of the de facto International Date Line.
The IDL is roughly based on the meridian of 180° longitude, roughly down the middle of the Pacific Ocean, and halfway around the world from the IERS Reference Meridian, the successor to the historic Greenwich prime meridian running through the Royal Greenwich Observatory. In many places, the IDL follows the 180° meridian exactly. In other places, however, the IDL deviates east or west away from that meridian. These various deviations generally accommodate the political and economic affiliations of the affected areas.
Proceeding from north to south, the first deviation of the IDL from 180° is to pass to the east of Wrangel Island and the Chukchi Peninsula, the easternmost part of Russian Siberia. (Wrangel Island lies directly on the meridian at 71°32′N 180°0′E, also noted as 71°32′N 180°0′W.) It then passes through the Bering Strait between the Diomede Islands at a distance of 1.5 kilometres (0.93 mi) from each island at 168°58′37″ W. It then bends considerably west of 180°, passing west of St. Lawrence Island and St. Matthew Island.
The IDL crosses between the U.S. Aleutian Islands (Attu Island being the westernmost) and the Commander Islands, which belong to Russia. It then bends southeast again to return to 180°. Thus, all of Russia is to the west of the IDL, and all of the United States is to the east except for the insular areas of Guam, the Northern Mariana Islands, and Wake Island, reaching the hypothetical, but not used UTC–13:00 time zone.
The IDL remains on the 180° meridian until passing the equator. Two U.S.-owned uninhabited atolls, Howland Island and Baker Island, just north of the equator in the central Pacific Ocean (and ships at sea between 172.5°W and 180°), have the earliest time on Earth (UTC−12:00 hours).
The IDL circumscribes Kiribati by swinging far to the east, almost reaching the 150°W meridian. Kiribati's easternmost islands, the southern Line Islands south of Hawaii, have the latest time on Earth (UTC+14:00 hours).
South of Kiribati, the IDL returns westward but remains east of 180°, passing between Samoa and American Samoa. Accordingly, Samoa, Tokelau, Wallis and Futuna, Fiji, Tonga, Tuvalu, and New Zealand's Kermadec Islands and Chatham Islands are all west of the IDL and have the same date. American Samoa, the Cook Islands, Niue, and French Polynesia are east of the IDL and one day behind.
The IDL then bends southwest to return to 180°. It follows that meridian until reaching Antarctica, which has multiple time zones. Conventionally, the IDL is not drawn into Antarctica on most maps.
Facts dependent on the IDL
According to the clock, the first areas to experience a new day and a New Year are islands that use UTC+14:00. These include portions of the Republic of Kiribati, including Millennium Island and Kiritimati in the Line Islands. The first major cities to experience a new day are Auckland and Wellington, New Zealand (UTC+12:00 or UTC+13:00 during daylight saving time).
A 1994 realignment of the IDL made Caroline Island one of the first points of land on Earth to reach January 1, 2000, on the calendar (UTC+14:00). As a result, this atoll was renamed Millennium Island.
Every day for 2 hours from 10:00 to 12:00 UTC there are 3 different days on earth. Example: On Tuesday 10:33 UTC it is Monday 22:33 on Baker Island (US), 23:33 on Midway (US), Pago Pago (American Samoa) and Alofi (Niue), Tuesday almost everywhere else on earth and Wednesday 00:33 in Kiritimati (Kiribati) in the Line Islands. Then 1 hour 11 minutes later at 11:44 UTC it is Monday 23:44 on Baker Island, Tuesday almost everywhere else on earth, Wednesday 01:44 in Kiritimati and 00:44 in Canton Island (Kiribati) in the Phoenix Islands, Apia (Samoa), Atafu (Tokelau) and Nukuʻalofa (Tonga) (also in Auckland during summer when NZDT is observed). Chatham Islands (NZ) are also nominally 2 days ahead of Baker Island for 45 minutes in the winter (CHAST) and 1 hour 45 minutes in the summer (CHADT).
The areas that are the first to see the daylight of a new day vary by the season. Around the June solstice, the first area would be any place within the Kamchatka Time Zone (UTC+12:00) that is far enough north to experience midnight sun on the given date. At the equinoxes, the first place to see daylight would be the uninhabited Millennium Island in Kiribati, which is the easternmost land located west of the IDL.
Near the December solstice, the first places would be Antarctic research stations using New Zealand Time (UTC+13:00) during summer that experience midnight sun. These include Amundsen-Scott South Pole Station, McMurdo Station, Scott Base and Zucchelli Station.
Additional Information
The date line, also called the International Date Line, is a boundary from which each calendar day starts. Areas to the west of the date line are one calendar day ahead of areas to the east.
The date line runs from the North Pole to the South Pole through the Pacific Ocean. It is not a straight line, however. The date line curves around several landmasses. For example, it curves around the islands that make up the nation of Kiribati, so that all regions of the country remain on the same day. The date line makes a big detour between Asia and North America in the Bering Strait. Cape Dezhnev, Russia, is always a day ahead of Cape Prince of Wales, Alaska, even though the landmasses are less than 80 kilometers (50 miles) apart.
The date line, which roughly follows the 180-degree meridian, is about halfway around the globe from the prime meridian, which measures 0-degrees longitude.
#2 Re: This is Cool » Miscellany » Today 16:33:49
2390) Anaconda
Gist
Is anaconda a real snake?
Yes, an anaconda is a very real, giant, non-venomous snake, but it's not a single species. Anacondas are large, heavy constrictors from tropical South America that are excellent swimmers. The largest species, the green anaconda, is the world's heaviest snake and can grow to impressive lengths.
Anacondas are semi aquatic snakes found in tropical South America, notable in the Amazon and different to pythons. They are some of the largest snakes in the world and are known for their swimming ability. “Anaconda” is the common name for the genus Eunectes, a genus of boa.
Summary
Anaconda, (genus Eunectes), any of three to five species of large, constricting, water-loving snakes found in tropical South America classified in the family Boidae (see also boa). Green anacondas (Eunectes akayima, the northern green anaconda, and E. murinus, the southern green anaconda) are among the largest snakes in the world, growing up to 9 meters (29.5 feet) long—rivaling the reticulated python (Python reticulatus) in length—and weighing up to 250 kg (550 pounds). The yellow, or southern, anaconda (E. notaeus), however, is much smaller, adult females reaching a maximum length of about 4.4–4.6 meters (roughly 14.4–15.1 feet) long. Historically, two additional forms, the beni (E. beniensis) and the dark-spotted anaconda (E. deschauenseei), which are closely related to E. notaeus, have been classified as separate species; however, growing morphological and genetic evidence suggests that both should be classified as yellow anacondas.
Natural history
Green anacondas—also called giant anacondas and common anacondas—are olive-colored snakes with alternating oval-shaped black spots. Despite much attention paid to a handful of colossal individuals, most adults do not exceed 5 meters (16 feet) in length. This group was divided formally into two species in 2024 based on the discovery of genetic differences between northern and southern populations. Northern green anacondas inhabit lowland basins, swamps, and even some drier grassy areas east of the Andes Mountains, extending from eastern Ecuador, northern Peru, and northern Brazil northward to Colombia, Venezuela, Guyana, Suriname, French Guiana, and Trinidad and Tobago. Southern green anacondas, which prefer marshes and other wet areas to drier habitats, are found from eastern Peru and northern Brazil south through northern Bolivia, extreme eastern Paraguay, and southern Brazil. Yellow anacondas inhabit swamps, marshes, and other aquatic habitats from the Paraguay River basin southward to northeastern Argentina; the species is distinguished from others by its yellowish coloration, which is broken up by pairs of overlapping black spots. Dark-spotted anacondas inhabit the seasonally flooded lower reaches of the Amazon River and coastal watersheds, extending between the Marowijne (or Maroni) River, separating Suriname and French Guiana southeast to the Tocantins River in Brazil. Benis are found primarily in seasonally flooded areas of north-central Bolivia.
Anacondas exhibit sexual dimorphism, females being larger and heavier than males. In general, they are carnivorous apex predators that consume fish and other vertebrates (including caimans and other reptiles, mammals, and waterfowl). These snakes can seize a large animal by the neck and throw their coils around it, killing it by constriction. They also kill smaller prey, such as small turtles and diving birds, with the mouth and sharp backward-pointing teeth alone. Kills made onshore are often dragged into the water, perhaps to avoid attracting jaguars, which prey on anacondas, and to ward off biting ants attracted to the carcass. Yellow anacondas target smaller vertebrate prey while also feeding on carrion, whereas green anacondas lie in the water (generally at night) to ambush larger prey, such as large caimans and mammals (including capybaras, deer, tapirs, and peccaries) that come to drink near the shoreline. Although anacondas occasionally attack people, encounters between them are rare.
Very little is known about the reproductive habits of the beni and the dark-spotted anaconda. Green and yellow anacondas, however, are polyandrous, a mating system in which single females mate with multiple males. In green and yellow anacondas, this can occur in a writhing mass of individuals, called a breeding ball, that can last several weeks. This behavior is not universal. Many females spend the breeding season, which spans the months April and May, with only one male. After mating concludes, some female green anacondas may cannibalize one or more of their male suitors (see also cannibalism, animal). Anacondas do not lay eggs. Rather, females incubate the eggs within their bodies for up to seven months before giving birth to live young. On average, female green anacondas produce about 30 live young, and female yellow anacondas give birth to about 40. The young snakes receive no parental investment, and they become sexually mature between the ages of three and four years. Anacondas live for about 10–20 years in the wild and up to 30 years in captivity.
Conservation status
Four species of anacondas are classified as species of least concern by the International Union for Conservation of Nature and Natural Resources (IUCN), and the conservation status of the northern green anaconda has yet to be assessed. The population trends of each species are not well known, but herpetologists note that these snakes are likely threatened in at least some parts of their ranges by habitat loss—due to the draining of wetland habitats for agriculture—and hunting. The yellow anaconda, the beni, and the dark-spotted anaconda are hunted for their meat and hide, and the yellow anaconda, the beni, and both species of green anaconda are targeted by people out of fear or in retaliation for attacks on livestock. Green anacondas appear to bear the brunt of this persecution because of their menacing size.
Details
Anacondas or water boas are a group of large boas of the genus Eunectes. They are a semiaquatic group of snakes found in tropical South America. Three to five extant and one extinct species are currently recognized, including one of the largest snakes in the world, E. murinus, the green anaconda.
Description
Although the name applies to a group of snakes, it is often used to refer only to one species, in particular, the common or green anaconda (Eunectes murinus), which is the largest snake in the world by weight, and the second longest after the reticulated python.
Distribution and habitat
Found in tropical South America from Ecuador, Brazil, Colombia and Venezuela south to Argentina.
Feeding
All five species are aquatic snakes that prey on other aquatic animals, including fish, river fowl, and caiman. Videos exist of anacondas preying on domestic animals such as goats and sometimes even young jaguars that venture too close to the water.
Relationship with humans
While encounters between people and anacondas may be dangerous, they do not regularly hunt humans. Nevertheless, threat from anacondas is a familiar trope in comics, movies, and adventure stories (often published in pulp magazines or adventure magazines) set in the Amazon jungle. Local communities and some European explorers have given accounts of giant anacondas, legendary snakes of much greater proportion than any confirmed specimen.
Although charismatic, there is little known on the biology of wild anacondas. Most of our knowledge comes from the work of Dr. Jesús A. Rivas and his team working in the Venezuelan Llanos.
Indigenous mythology
According to the founding myth of the Huni Kuin, a man named Yube fell in love with an anaconda woman and was turned into an anaconda as well. He began to live with her in the deep world of waters. In this world, Yube discovered a hallucinogenic drink with healing powers and access to knowledge. One day, without telling his anaconda wife, Yube decided to return to the land of men and resume his old human form. The myth also explains the origin of cipó or ayahuasca—a hallucinogenic drink taken ritualistically by the Huni Kuin.
Additional Information
Some of the largest snakes in the world, Anacondas are known for their swimming ability and there are many types.
Anacondas are semi aquatic snakes found in tropical South America, notable in the Amazon and different to pythons. They are some of the largest snakes in the world and are known for their swimming ability. “Anaconda” is the common name for the genus Eunectes, a genus of boa. Eunectes means “good swimmer” in Greek, according to SeaWorld.
There are four recognized species of anaconda, according to Bill Heyborne, a herpetologist and professor of biology at Southern Utah University. They are the green anaconda, the yellow or Paraguayan anaconda, the dark-spotted anaconda and the Beni or Bolivian anaconda. "They can be differentiated from one another genetically, but also based on their size and geographic range," Heyborne said.
Heyborne said that when most people say anaconda, they are actually referring to the green anaconda, the largest of the four species. The green anaconda is the heaviest snake in the world and one of the longest.
According to the Mythology.net , anacondas feature prominently in South American myths, sometimes appearing as shapeshifters , as the creator of the water, as vicious human-eaters, or as magical, spiritual beings with healing properties.
There have also been reports of anacondas reaching lengths of 40, 50 and 100 feet (12, 15 and 30 meters) — far longer than anything scientifically verified. These reports have given rise to the Giant Anaconda myth, popularized in the early 20th century by explorers and colonists, according to Loren Coleman and Jerome Clark's "Cryptozoology A to Z".
Anacondas are stocky, muscular snakes that are thicker than other boas, according to Reptiles Cove. They have thick necks and narrow but large heads. All anacondas have nostrils and eyes on the tops of their heads, which allow them to see above the water while remaining mostly submerged. They have a thick black stripe that runs from the eye to the jaw.
Anacondas have small, smooth scales that grow larger toward the posterior of their bodies. They have loose, soft skin that can handle a great deal of water absorption, according to the University of Michigan’s Animal Diversity Web (ADW).
Near their cloacal region, anacondas have spurs on their scales. Males have larger spurs than females, though females are overall larger and longer snakes. In fact, anacondas exhibit the largest sexual dimorphism (with the female being larger) of any tetrapod species, according to Jesus Rivas, a herpetologist and founder of the Anaconda Project.
Anacondas’ coloring and size depends on the species. Their spotted, green, yellow and brown color palettes allow them to blend in with tropical rivers and rainforests, according to Sciencing.
True to their name, these are greenish-brown, olive, or greenish-gray. They have black or brown egg-shaped spots on the mid-to posterior back of their bodies. Their sides are sometimes more yellow than green with egg-shaped spots with yellow centers, according to the Jacksonville Zoo.
Green anacondas’ length is still a matter of debate, said Heyborne. They are quite difficult to measure. It is hard to stretch out a captive anaconda, not to mention potentially dangerous for the snake, according to Rivas. People who see anacondas in the wild are likely to overestimate their length due to fear. Also, an anaconda that has recently eaten will look much larger than one that hasn’t, causing exaggerated estimations of size. Furthermore, the skins of dead snakes can be stretched, meaning that it is hard to scientifically verify the length of those, too, especially historical samples, according to Wonderpolis.
While many publications, including National Geographic and the San Diego Zoo list anacondas’ maximum verified length as 29 or 30 feet (9 m), Rivas, who has captured and measured more than 1,000 anacondas, believes they don’t grow much longer than 20 feet (6 m). The Guinness Book of World Records lists the longest snake ever recorded as a 25-foot (7.6 m) reticulated python.
The average size of female anacondas is around 15 feet (4.5 m), and the average size of males is around 9 feet (2.7 m), according to Boas and Pythons of the World.
Weights are also not known for sure. Estimates top out at about 550 lbs. (250 kilograms), according to National Geographic, but the average is probably somewhere between 100 and 150 lbs. (45 and 68 kg), according to "The Lives of Amphibians and Reptiles in an Amazonian Rainforest" (Cornell, 2005) by William E. Duellman.
Yellow anacondas have yellow, golden-tan, or yellow-green coloring with black or dark brown blotches, spots, streaks, and dorsal bands. Each snake has a unique pattern of yellow and black scales on the bottom of its tail. The average length is about 9 feet (2.7 m), according to the World Land Trust.
Not much is known about these anacondas, which were long considered a hybrid of yellow and green anacondas until scientists determined they were their own species. Their coloring is similar to the green anaconda, according to World Atlas

#3 Science HQ » Astatine » Today 15:39:49
- Jai Ganesh
- Replies: 0
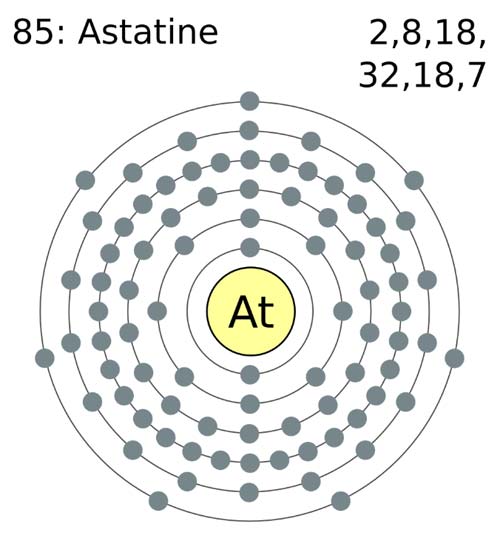
Astatine
Gist
Astatine (At) is the heaviest naturally occurring halogen, a rare and highly radioactive element with atomic number 85. It is unstable, with all isotopes having short half-lives, and occurs only in trace amounts in nature as a decay product of other elements. Astatine is primarily synthesized for use in research and medicine, particularly in radiotherapy and the treatment of thyroid diseases, as it tends to accumulate in the thyroid gland like iodine.
Astatine's primary use is in scientific research and targeted cancer therapy, specifically with its isotope astatine-211, which delivers alpha particles to destroy cancer cells, including for thyroid and ovarian cancers. Due to its extreme rarity and intense radioactivity, astatine is not used in everyday applications but is studied in advanced labs for understanding radioactive decay, halogen chemistry, and to develop new medical treatments for hard-to-target cancers.
Summary
Astatine is a chemical element; it has symbol At and atomic number 85. It is the rarest naturally occurring element in the Earth's crust, occurring only as the decay product of various heavier elements. All of astatine's isotopes are short-lived; the most stable is astatine-210, with a half-life of 8.1 hours. Consequently, a solid sample of the element has never been seen, because any macroscopic specimen would be immediately vaporized by the heat of its radioactivity.
The bulk properties of astatine are not known with certainty. Many of them have been estimated from its position on the periodic table as a heavier analog of fluorine, chlorine, bromine, and iodine, the four stable halogens. However, astatine also falls roughly along the dividing line between metals and nonmetals, and some metallic behavior has also been observed and predicted for it. Astatine is likely to have a dark or lustrous appearance and may be a semiconductor or possibly a metal. Chemically, several anionic species of astatine are known and most of its compounds resemble those of iodine, but it also sometimes displays metallic characteristics and shows some similarities to silver.
The first synthesis of astatine was in 1940 by Dale R. Corson, Kenneth Ross MacKenzie, and Emilio G. Segrè at the University of California, Berkeley. They named it from the Ancient Greek ástatos 'unstable'. Four isotopes of astatine were subsequently found to be naturally occurring, although much less than one gram is present at any given time in the Earth's crust. Neither the most stable isotope, astatine-210, nor the medically useful astatine-211 occur naturally; they are usually produced by bombarding bismuth-209 with alpha particles.
Details
Astatine (At) is a radioactive chemical element and the heaviest member of the halogen elements, or Group 17 (VIIa) of the periodic table. Astatine, which has no stable isotopes, was first synthetically produced (1940) at the University of California by American physicists Dale R. Corson, Kenneth R. MacKenzie, and Emilio Segrè, who bombarded bismuth with accelerated alpha particles (helium nuclei) to yield astatine-211 and neutrons. Naturally occurring astatine isotopes have subsequently been found in minute amounts in the three natural radioactive decay series, in which they occur by minor branching (astatine-218 in the uranium series, astatine-216 in the thorium series, and astatine-215 and astatine-219 in the actinium series). Thirty-two isotopes are known; astatine-210, with a half-life of 8.1 hours, is the longest lived. Because astatine has no stable or long-lived isotopes, it was given its name from the Greek word astatos, meaning “unstable.”
Element Properties
atomic number : 85
stablest isotope : 210
oxidation states : −1, +1, +3(?), +5, +7(?)
Production and use
Metallic bismuth may be used as a target material. From this, astatine may readily be removed by distillation in air from a stainless-steel tube. The free element begins to distill at 271 °C (520 °F, or the melting point of bismuth), but the operation is best carried out at 800 °C (1,500 °F) with subsequent redistillation. If an aqueous solution of astatine is desired, the element may be separated by washing with an appropriate aqueous solution. Alternatively, the halogen may be removed from the target by chemical methods, such as dissolving in nitric acid, with the latter being removed by boiling.
Another procedure involves the use of a metallic thorium target, which—after bombardment—is dissolved in concentrated hydrochloric acid containing hydrogen fluoride and chlorine.
Analysis
Because of the short half-lives of astatine isotopes, only very small quantities have been available for study. With the exception of a few spectrometric and mass-spectrometric studies, most investigations of astatine chemistry have used tracer techniques, which involve using chemical reactions in a solution with similarly reacting elements as carriers. The amount of astatine is then calculated from the measured radioactivity of the reaction products. However, the rarity of astatine means that these solutions are extremely dilute, with concentrations around or below {10}^{-10} molarity (the number of moles per litre of solution). At such concentrations, the effects of impurities can be very serious, especially for a halogen such as astatine, which exists in several oxidation states and can form many organic compounds. Iodine has been used as a carrier in most experiments. Techniques applied include coprecipitation, solvent extraction, ion exchange, and other forms of chromatography (separation by adsorption differences), electrodeposition (deposition by an electric current), electromigration (movement in an electric field), and diffusion. A direct identification of some astatine compounds has been made by mass spectrometry.
Except for nuclear properties, the only physical property of astatine to be measured directly is the spectrum of atomic astatine. Other physical properties have been predicted from theory and by extrapolation from the properties of other elements.
Chemical properties
Some of the chemical properties of the element have been established. It generally resembles iodine. Thus, like iodine, it concentrates in the thyroid gland of higher animals. A substantial portion, however, is distributed throughout the body and acts as an internal radiation source.
The astatide ion, At−, is quantitatively coprecipitated with insoluble iodides, such as silver iodide or thallium iodide. The diffusion coefficient of the iodide ion is 1.42 times that of the astatide ion, which moves more slowly toward the anode than the former under given conditions. The ion is formed by reduction of the element, using zinc or sulfur dioxide. It is oxidized to the zero valence state by the ferric ion, Fe3+, iodine (I2), and dilute nitric acid. Thus, the astatide ion is a stronger reducing agent than the iodide ion, and free iodine is a stronger oxidizing agent than astatine.
Free astatine is characterized by volatility from solution and by extractability into organic solvents. It undergoes disproportionation in alkaline media. Astatine is coprecipitated with cesium iodide and thus appears to form polyhalide anions. Astatine extracted into chloroform has been shown to coprecipitate homogeneously with iodine when a portion of the latter is crystallized. Astatine seems to be present as the iodide, which appears to be more polar (i.e., showing separation of electric charge) in character than iodine bromide. It is somewhat soluble in water and much more soluble in benzene and carbon tetrachloride.
Astatine is known to occur in positive oxidation numbers. The astatate ion, (AtO3)−, is coprecipitated with insoluble iodates, such as silver iodate (AgIO3), and is obtained by the oxidation of lower oxidation states with hypochlorite, periodate, or persulfate. So far no evidence for perastatate has been found, but this may be because the ion, (AtO6)5−, may show little tendency to coprecipitate with potassium iodate (KIO4).
Astatine in the +1 state is stabilized by complexation, and complexes formulated as dipyridine astatine perchlorate [At(py)2] [ClO4] and dipyridine astatine nitrate [At(py)2] [NO3] have been prepared. Compounds with the formulas (C6H5)AtCl2, (C6H5)2AtCl, and (C6H5)AtO2 have also been obtained. A variety of methods may be used to synthesize astatobenzene, C6H5At.
Additional Information:
Appearance
Astatine is a dangerously radioactive element.
Uses
There are currently no uses for astatine outside of research. The half-life of the most stable isotope is only 8 hours, and only tiny amounts have ever been produced.
A mass spectrometer has been used to confirm that astatine behaves chemically like other halogens, particularly iodine.
Biological role
Astatine has no known biological role. It is toxic due to its radioactivity.
Natural abundance
Astatine can be obtained in a variety of ways, but not in weighable amounts. Astatine-211 is made in nuclear reactors by the neutron bombardment of bismuth-200.
#4 Re: Jai Ganesh's Puzzles » General Quiz » Today 14:53:15
Hi,
#10559. What does the term in Biology Cilium mean?
#10560. What does the term in Biology Circadian rhythm mean?
#5 Re: Jai Ganesh's Puzzles » English language puzzles » Today 14:35:50
Hi,
#5753. What does the verb (used with object) dedicate mean?
#5754. What does the noun deduction mean?
#6 Re: Jai Ganesh's Puzzles » Doc, Doc! » Today 14:22:46
Hi,
#2470. What does the medical term Tracheostomy mean?
#7 Re: Jai Ganesh's Puzzles » 10 second questions » Today 14:12:47
Hi,
#9736.
#8 Re: Jai Ganesh's Puzzles » Oral puzzles » Today 14:02:33
Hi,
#6243.
#9 Jokes » Love Jokes - IV » Today 13:50:40
- Jai Ganesh
- Replies: 0
Knock Knock.
Who's there?
Olive!
Olive who?
Olive you!
* * *
Knock Knock.
Who's there?
Owl.
Owl(s) who?
"Owl always love you".
* * *
The gods gave man fire and he invented fire engines.
They gave him love and he invented marriage.
* * *
True love is when your pet comes to your room on its own.
* * *
Roses are red, violets are blue, if you love Star Wars, may the force be with you.
* * *
#10 Re: Exercises » Compute the solution: » Today 13:34:15
Hi,
Good!
2576.
#11 Re: Dark Discussions at Cafe Infinity » crème de la crème » Yesterday 21:58:17
2337) Victor Ambrose
Gist:
Work
Our genome can be likened to an instruction manual for all cells in our body. Every cell contains the same set of instructions. Yet, different cell types have very distinct characteristics. These differences arise from gene regulation, which allows each cell to select only the relevant instructions. In 1993, Victor Ambros and Gary Ruvkun discovered microRNA, a new class of tiny RNA molecules that play a crucial role in gene regulation. This new dimension to gene regulation is fundamentally important for how organisms develop and function.
Summary
Victor Ambros (born December 1, 1953, Hanover, New Hampshire, U.S.) is an American developmental biologist and molecular geneticist best known for his pioneering work in the discovery of microRNA (miRNA), a type of small RNA molecule that serves essential functions in regulating gene expression. Ambros’s contributions to the discovery of miRNA had a profound impact on scientific understanding of cell function and mechanisms underlying gene activity and disease and was particularly important for the fields of molecular biology and developmental biology. For his discoveries, he was awarded the 2024 Nobel Prize for Physiology or Medicine (shared with American molecular biologist and geneticist Gary Ruvkun).
Education and early research
Ambros spent his youth in Vermont, where his parents encouraged his interest in science. After graduating from high school, he attended the Massachusetts Institute of Technology (MIT), where he earned a bachelor’s degree in biology in 1975. He remained at MIT to pursue a Ph.D. in genetics, working under the guidance of American virologist and Nobelist David Baltimore while carrying out research aimed at better understanding the genomic structure and replication of poliovirus. In 1979 Ambros completed a Ph.D. and continued on at MIT as a postdoctoral researcher in the laboratory of biologist H. Robert Horvitz. There he later worked with Ruvkun, who was also a postdoctoral student with Horvitz, to investigate genetic factors dictating the timing of events in the development of the nematode Caenorhabditis elegans.
Lin-4 and miRNA
In the late 1980s, after joining the faculty at Harvard University, Ambros studied more deeply a strain of C. elegans that carries a mutation in a gene known as lin-4. Lin-4 exerts temporal control over developmental events in C. elegans larvae by negatively regulating LIN-14 protein. In the course of their investigations, Ambros and his team realized that lin-4 produces only a very short strand of RNA, which is not translated into protein, and that lin-4 interacts with a gene known as lin-14. Although it was apparent to Ambros and others that lin-4 somehow regulates lin-14 activity, the mechanism was a mystery.
In 1992, while still trying to elucidate lin-4 regulatory mechanisms, Ambros moved his laboratory to Dartmouth College. The following year his laboratory published its findings on the short RNA produced by lin-4. He and Ruvkun then compared their insights on lin-4 and lin-14, which Ruvkun’s laboratory had been investigating, and found that the short lin-4 RNA sequence was complementary to a segment of lin-14 messenger RNA (mRNA). They also showed that binding of lin-4 RNA to lin-14 mRNA blocks LIN-14 protein production. Following the publication of their work, it was recognized that they had discovered not only a novel RNA molecule—miRNA—but also a previously unknown mechanism of gene regulation. In 2008 Ambros joined the faculty at the University of Massachusetts Medical School, where his research continues to center on characterizing the role of miRNA in development.
Awards and honors
Ambros has received numerous awards and honors throughout his career, including the 2008 Lasker Award (shared with Ruvkun and David C. Baulcombe), the 2008 Gairdner International Award (shared with Ruvkun), and the 2014 Wolf Prize (shared with Ruvkun and Nahum Sonenberg). Ambros is a fellow of the American Academy of Arts and Sciences (2011) and the American Association for the Advancement of Science (2018).
Details
Victor R. Ambros (born December 1, 1953) is an American developmental biologist who discovered the first known microRNA (miRNA). He is a professor at the University of Massachusetts Medical School. He completed both his undergraduate and doctoral studies at the Massachusetts Institute of Technology. Ambros received the Nobel Prize in Physiology or Medicine in 2024 for his research on microRNA.
Biography:
Early life and education
Ambros was born in New Hampshire. His father, Longin Ambros, attended Sigismund Augustus Gymnasium in Vilnius 1937-1939 and was a Polish World War II refugee. Victor grew up on a small dairy farm in Hartland, Vermont, in a family of eight children and attended Woodstock Union High School.
From the Massachusetts Institute of Technology, Ambros received a Bachelor of Science with a major in biology in 1975 and a Doctor of Philosophy in biology in 1979. His doctoral supervisor was David Baltimore, a 1975 Nobel laureate in Physiology or Medicine. Ambros continued his research at MIT as the first postdoctoral fellow in the lab of future Nobel laureate H. Robert Horvitz.
Career
Ambros became a faculty member at Harvard University in 1984. However, Harvard denied tenure to Ambros shortly after he discovered what is now known as microRNA. About this, Baltimore later said in 2008: "They lost a potential Nobel laureate because they simply didn’t see in him the potential that he had ... It’s the nature of a seminal discovery that it’s seminal in retrospect. You can’t know ahead of time."
Ambros joined the faculty of Dartmouth College in 1992. He joined the faculty at the University of Massachusetts Medical School in 2008, and currently holds the title of Silverman Professor of Natural Sciences in the program in Molecular Medicine, endowed by his former Dartmouth student, Howard Scott Silverman.
Research
In 1993, Ambros and his co-workers Rosalind Lee and Rhonda Feinbaum reported in the journal Cell that they had discovered single-stranded non-protein-coding regulatory RNA molecules in the organism C. elegans. Previous research, including work by Ambros and Horvitz, had revealed that a gene known as lin-4 was important for normal larval development of C. elegans, a nematode often studied as a model organism. Specifically, lin-4 was responsible for the progressive repression of the protein LIN-14 during larval development of the worm; mutant worms deficient in lin-4 function had persistently high levels of LIN-14 and displayed developmental timing defects.
Ambros and colleagues found that lin-4, unexpectedly, did not encode a regulatory protein. Instead, it gave rise to some small RNA molecules, 22 and 61 nucleotides in length, which Ambros called lin-4S (short) and lin-4L (long). Sequence analysis showed that lin-4S was part of lin-4L: lin-4L was predicted to form a stem-loop structure, with lin-4S contained in one of the arms, the 5' arm. Furthermore, Ambros, together with Gary Ruvkun (Harvard), discovered that lin-4S was partially complementary to several sequences in the 3' untranslated region of the messenger RNA encoding the LIN-14 protein. Ambros and colleagues hypothesized and later determined that lin-4 could regulate LIN-14 through binding of lin-4S to these sequences in the lin-14 transcript in a type of antisense RNA mechanism.
In 2000, another C. elegans small RNA regulatory molecule, let-7, was characterized by the Ruvkun lab and found to be conserved in many species, including vertebrates. These discoveries, among others, confirmed that Ambros had in fact discovered a class of small RNAs with conserved functions, now known as microRNA.
Ambros was elected to the United States National Academy of Sciences in 2007. He was elected a Fellow of the American Academy of Arts and Sciences in 2011. In 2024 he shared the Nobel Prize in Physiology and Medicine with Gary Ruvkun "for the discovery of microRNA and its role in post-transcriptional gene regulation".

#12 This is Cool » Magnet » Yesterday 17:06:38
- Jai Ganesh
- Replies: 0
Magnet
Gist
A magnet is a material or object that produces a magnetic field. This magnetic field is invisible but is responsible for the most notable property of a magnet: a force that pulls on other ferromagnetic materials, such as iron, steel, nickel, cobalt, etc. and attracts or repels other magnets.
A magnet is any object that produces its own magnetic field that interacts with other magnetic fields. Magnets have two poles, a north pole and a south pole. The magnetic field is represented by field lines that start at a magnet's north pole and end at the south pole.
Summary
A magnet is a material or object that produces a magnetic field. This magnetic field is invisible but is responsible for the most notable property of a magnet: a force that pulls on other ferromagnetic materials, such as iron, steel, nickel, cobalt, etc. and attracts or repels other magnets.
A permanent magnet is an object made from a material that is magnetized and creates its own persistent magnetic field. An everyday example is a refrigerator magnet used to hold notes on a refrigerator door. Materials that can be magnetized, which are also the ones that are strongly attracted to a magnet, are called ferromagnetic (or ferrimagnetic). These include the elements iron, nickel and cobalt and their alloys, some alloys of rare-earth metals, and some naturally occurring minerals such as lodestone. Although ferromagnetic (and ferrimagnetic) materials are the only ones attracted to a magnet strongly enough to be commonly considered magnetic, all other substances respond weakly to a magnetic field, by one of several other types of magnetism.
Ferromagnetic materials can be divided into magnetically "soft" materials like annealed iron, which can be magnetized but do not tend to stay magnetized, and magnetically "hard" materials, which do. Permanent magnets are made from "hard" ferromagnetic materials such as alnico and ferrite that are subjected to special processing in a strong magnetic field during manufacture to align their internal microcrystalline structure, making them very hard to demagnetize. To demagnetize a saturated magnet, a certain magnetic field must be applied, and this threshold depends on coercivity of the respective material. "Hard" materials have high coercivity, whereas "soft" materials have low coercivity. The overall strength of a magnet is measured by its magnetic moment or, alternatively, the total magnetic flux it produces. The local strength of magnetism in a material is measured by its magnetization.
An electromagnet is made from a coil of wire that acts as a magnet when an electric current passes through it but stops being a magnet when the current stops. Often, the coil is wrapped around a core of "soft" ferromagnetic material such as mild steel, which greatly enhances the magnetic field produced by the coil.
Details
A magnet is a special kind of metal which is made out of (Iron Nickel and Cobalt) When a magnet goes near a special kind of metal or another magnet, and the poles (sides) touching are opposite, it will pull, or attract the other object closer. If the two poles are the same, the magnet and the other object will push away, or repel, from each other. This attraction and repulsion is called magnetism. All magnets have north and south poles. Opposite poles are attracted to each other, while the same poles repel each other like south and south and north and north. When you rub a piece of iron along a magnet, the north-seeking poles of the atoms in the iron line up in the same direction. The force generated by the aligned atoms creates a magnetic field.
Types of magnet
Soft magnets (meaning impermanent magnets) are often used in electromagnets some of the magnets are (bar magnet,wand and ball magnet). These increase (often hundreds or thousands of times) the magnetic field of a wire that carries an electrical current and is wrapped around the magnet. The field also increases with the current. Magnetic Materials: Soft Magnets. Soft magnetic materials are those materials that are easily magnetised and demagnetised.
Permanent magnets have ferromagnetism. They occur naturally in some rocks, particularly lodestone, but are now commonly manufactured. A magnet's magnetism decreases when it is heated and increases when it is cooled. It has to be heated at around 1,000 degrees Celsius (1,830 °F). Like poles (S-pole and S-pole/N-pole and N-pole) will repel each other while unlike poles (N-pole and S-pole) will attract each other.
Magnets are only attracted to special metals. Iron, cobalt and nickel are magnetic. Metals that have iron in them attract magnets well. Steel is one. Metals like brass, copper, zinc and aluminium, silver are not attracted to magnets. Non-magnetic materials such as wood and glass are not attracted to magnets as they do not have magnetic materials in them.
Rare earth magnets
Lanthanum elements can make strong magnets. The spin of their electrons can be aligned, resulting in very strong magnetic fields. So these elements are used for high-strength magnets when their high price is not a concern. The most common types of rare-earth magnets are samarium–cobalt and neodymium–iron–boron (NIB) magnets.
Natural magnets
Natural/permanent magnets are not artificial. They are a kind of rock called lodestone or magnetite.
The compass
A compass uses the Earth's magnetic field, and points to the North magnetic pole. A north side of the magnet is attracted to the south side of another magnet. However, the north side of the compass points to the north pole, this can only mean that the "north pole" is really the magnetic south, and the "South magnetic pole" is really the magnetic north.
Discovery
Ancient people discovered magnetism from lodestones (or magnetite) which are naturally magnetized pieces of iron ore. Lodestones, suspended so they could turn, were the first magnetic compasses.
The earliest known surviving descriptions of magnets and their properties are from Anatolia, India, and China about 2500 years ago. The properties of lodestones and their affinity for iron were written of by Pliny the Elder in his encyclopedia Naturalis Historia.
Additional Information
A magnet is any material capable of attracting iron and producing a magnetic field outside itself. By the end of the 19th century all the known elements and many compounds had been tested for magnetism, and all were found to have some magnetic property. The most common was the property of diamagnetism, the name given to materials exhibiting a weak repulsion by both poles of a magnet. Some materials, such as chromium, showed paramagnetism, being capable of weak induced magnetization when brought near a magnet. This magnetization disappears when the magnet is removed. Only three elements, iron, nickel, and cobalt, showed the property of ferromagnetism (i.e., the capability of remaining permanently magnetized).
You probably know that magnets attract specific metals and they have north and south poles. Opposite poles attract each other while like poles repel each other. Magnetic and electrical fields are related, and magnetism, along with gravity and strong and weak atomic forces, is one of the four fundamental forces in the universe.
But none of those facts answers the most basic question: What exactly makes a magnet stick to certain metals? Or why don't they stick to other metals? Why do they attract or repel each other, depending on their positioning? And what makes neodymium magnets so much stronger than the ceramic magnets we played with as children?
To understand the answers to these questions, it helps to have a basic definition of a magnet. Magnets are objects that produce magnetic fields and attract metals like iron, nickel and cobalt. The magnetic field's lines of force exit the magnet from its north pole and enter its south pole. Permanent or hard magnets create their own magnetic field all the time. Temporary or soft magnets produce magnetic fields while in the presence of a magnetic field and for a short while after exiting the field. Electromagnets produce magnetic fields only when electricity travels through their wire coils.
Because electrons and protons are tiny magnets, all materials have some sort of magnetic property. In most materials, however, the way electrons spin in opposite directions cancels out an atom's magnetic properties. Metals are the most common choices to manufacture magnets. Although some are made from simple metals, combinations of metals — called alloys — produce magnets of different strengths. For example:
Ferrites or ceramic magnets: These are like those used in refrigerator magnets and elementary-school science experiments. They contain iron oxide and other metals in a ceramic composite. A ceramic magnet known as lodestone, or magnetite, was the first magnetic material discovered and occurs naturally. Even though the ceramic magnet has been around for so long, they weren't commercially produced until 1952. Although they're common and keep their magnetism, they tend to have a weaker magnetic field (known as the energy product) than other types of magnets.
Alnico magnets: These were developed in the 1930s and are made from aluminum, nickel and cobalt. They're stronger than ceramic magnets, but not as strong as the ones that incorporate a class of elements known as rare-earth metals.
Neodymium magnets: These contain iron, boron and the rare-earth element neodymium, and as of this writing, they are the strongest commercially available magnets. They first appeared in the 1980s after scientists at the General Motors Research Laboratories and the Sumitomo Special Metals Company published their research.
Samarium cobalt magnets: These were developed by scientists at the Dayton University Research University in the 1960s, and combine cobalt with the rare-earth element samarium. In the past few years, scientists have also discovered magnetic polymers, or plastic magnets. Some of these are flexible and moldable. However, some work only at extremely low temperatures, and others pick up only very lightweight materials, like iron filings.
The Basics
Many of today's electronic devices require magnets to function. This reliance on magnets is relatively recent, primarily because most modern devices require magnets that are stronger than the ones found in nature. Lodestone, a form of magnetite, is the strongest naturally occurring magnet. It can attract small objects, like paper clips and staples.
By the 12th century, people had discovered that they could use lodestone to magnetize pieces of iron, creating a compass. Repeatedly rubbing lodestone along an iron needle in one direction magnetized the needle. It would then align itself in a north-south direction when suspended. Eventually, scientist William Gilbert explained that this north-south alignment of magnetized needles was due to Earth behaving like an enormous magnet with north and south poles.
A compass needle isn't nearly as strong as many of the permanent magnets used today. But the physical process that magnetizes compass needles and chunks of neodymium alloy is essentially the same. It relies on microscopic regions known as magnetic domains, which are part of the physical structure of ferromagnetic materials, like iron, cobalt and nickel. Each domain is essentially a tiny, self-contained magnet with a north and south pole. In an unmagnetized ferromagnetic material, each domain's north pole points in a random direction. Magnetic domains that are oriented in opposite directions cancel one another out, so the material does not produce a net magnetic field.
In magnets, on the other hand, most or all the magnetic domains point in the same direction. Rather than canceling one another out, the microscopic magnetic fields combine to create one large magnetic field. The more domains point in the same direction, the stronger the overall field. Each domain's magnetic field extends from its north pole into the south pole of the domain ahead of it.
This explains why breaking a magnet in half creates two smaller magnets with north and south poles. It also explains why opposite poles attract — the field lines leave the north pole of one magnet and naturally enter the south pole of another, essentially creating one larger magnet. Like poles repel each other because their lines of force are traveling in opposite directions, clashing with each other rather than moving together.

#13 Re: This is Cool » Miscellany » Yesterday 16:38:30
2389) Vatican City
Gist
Vatican City is the world's smallest sovereign state, an independent city-state and the administrative center of the Roman Catholic Church, located as an enclave within Rome, Italy. Ruled by the Pope as the head of the Holy See, it is a site of immense religious and cultural importance, home to landmarks like St. Peter's Basilica, the Vatican Museums, and the Sistine Chapel. Established by the Lateran Treaty of 1929, it serves as the global spiritual headquarters for the Catholic Church and is a pilgrimage site for Christians worldwide.
Vatican City is famous for being the sovereign spiritual and administrative center of the Roman Catholic Church, the world's smallest independent state, and a major pilgrimage site. It is home to significant religious and cultural landmarks like St. Peter's Basilica and the Sistine Chapel with Michelangelo's frescoes, as well as the extensive Vatican Museums with its vast collection of art, all of which draw millions of tourists and pilgrims annually.
Summary
Vatican City, officially the Vatican City State, often shortened as the Vatican, is a landlocked sovereign state and city-state. Ruled by the pope, it is an enclave within Rome and serves as the administrative centre of the Catholic Church. Vatican City is governed by the See of Rome, commonly known as the Holy See, itself a sovereign entity under international law, which maintains its temporal power, governance, diplomacy, and spiritual independence. Vatican is also used as a metonym for the Holy See, which is the central governing body of the Catholic Church and Vatican City, comprising the pope and the Roman Curia. The independent state of Vatican City came into existence in 1929 via the Lateran Treaty between the Holy See and the Kingdom of Italy, which spoke of it as a new creation, not as a vestige of the much larger Papal States (756–1870), which had previously encompassed much of Central Italy.
With an area of 49 hectares (121 acres)[g] and a population of about 882 in 2024, it is the smallest sovereign state in the world both by area and by population. It is among the least populated capitals in the world. As governed by the Holy See, Vatican City State is an ecclesiastical or sacerdotal-monarchical state ruled by the pope, who is the bishop of Rome and head of the Catholic Church; the highest state functionaries are all Catholic clergy of various origins. The Holy See dates to early Christianity and is the principal episcopal see of the Catholic Church, which in 2018 had about 1.329 billion baptized Catholics in the world, in the Latin Church and 23 Eastern Catholic Churches. After the Avignon Papacy (1309–1377) the popes have mainly resided at the Apostolic Palace within what is now Vatican City, although at times residing instead in the Quirinal Palace in Rome or elsewhere.
Vatican City contains religious and cultural sites such as St Peter's Basilica, the Sistine Chapel, the Vatican Apostolic Library, and the Vatican Museums. They feature some of the world's most famous paintings and sculptures. The economy of Vatican City is supported financially by donations from Catholic believers, by the sale of postage stamps and souvenirs, fees for admission to museums, and sales of publications. Vatican City has no taxes, and items are duty-free.
Details
Vatican City is a landlocked ecclesiastical state, seat of the Roman Catholic Church, and an enclave surrounded by Rome, situated on the west bank of the Tiber River. Vatican City is the world’s smallest fully independent nation-state.
Layout of the city
Vatican City’s medieval and Renaissance walls form its boundaries, except on the southeast at St. Peter’s Square (Piazza San Pietro). Of the six entrances, only three—the piazza, the Arco delle Campane (Arch of the Bells) in the facade of St. Peter’s Basilica, and the entrance to the Vatican Museums and Galleries in the north wall—are open to the public. The most imposing building is St. Peter’s Basilica, built during the 4th century and rebuilt during the 16th century. Erected over the tomb of St. Peter the Apostle, it is the second largest religious building (after Yamoussoukro Basilica) in Christendom.
The Vatican Palace is the residence of the pope within the city walls. The Holy See is the name given to the government of the Roman Catholic Church, which is led by the pope as the bishop of Rome. As such, the Holy See’s authority extends over Catholics throughout the world. Since 1929 it has resided in Vatican City, which was established as an independent state to enable the pope to exercise his universal authority.
Vatican City has its own telephone system, post office, gardens, astronomical observatory, radio station, banking system, and pharmacy, as well as a contingent of Swiss Guards responsible for the personal safety of the pope since 1506. Almost all supplies—including food, water, electricity, and gas—must be imported. There is no income tax and no restriction on the import or export of funds. As the Holy See, it derives its income from the voluntary contributions of more than one billion Roman Catholics worldwide, as well as interest on investments and the sale of stamps, coins, and publications. Banking operations and expenditures have been reported publicly since the early 1980s.
History and governance
The city of Rome has been an important center of Christianity since the early days of the church. St. Peter, considered the first pope, is thought to have lived and died in Rome. In 313 Emperor Constantine I issued the Edict of Milan, which ended official persecution of Christians and opened the door to the growth of the church in both spiritual and material terms. By the 4th century the church had gained control of a great deal of territory, called the Patrimony of St. Peter, in and around Rome. Papal influence in central Italy began to increase in the 5th century, as the Roman Empire fell apart and the people of the area began to rely on the pope for protection from invading armies. By about the year 600 the church was one of the largest landowners in the world.
The legal basis for the foundation of the Papal States was provided by the Donation of Pippin, which granted the pope the rights to large parts of central Italy in 754. In the 9th century the first city walls (Leonine Walls) were completed under Pope Leo IV. Between the 12th and 14th centuries the Vatican underwent something of a building boom as a new palace was built and the Leonine Walls were restored. The Vatican fell into decay after 1309, when the office of the papacy was moved to Avignon in France. The pope’s return to Rome in 1377 marked the beginning of a revitalization.
Italy became a unified country in the 19th century, which led to major changes in the Vatican’s political status. Most immediately, the church lost its land to the new country. Some of the papal territories voted to join the Kingdom of Sardinia in 1859. Italy annexed the rest of the Papal States by 1870 and made Rome the Italian capital. To protest the incorporation into a unified Italy, each pope thereafter remained a voluntary “prisoner of the Vatican,” never leaving the small territory of the papal grounds. This situation lasted nearly 60 years.
In 1929 a solution to this ongoing problem was found. Vatican City’s independent sovereignty was recognized by the Fascist Italian government in the Lateran Treaty. Sovereignty is exercised by the pope upon his election as the head of the Roman Catholic Church. He has absolute executive, legislative, and judicial powers within the city. While most of the inhabitants of Vatican City are priests or nuns, they also include several hundred laypersons engaged in secretarial, domestic, trade, and service occupations.
Institutions and attractions
Special extraterritorial privileges are extended to more than 10 other buildings in Rome and to Castel Gandolfo, the pope’s summer residence in the Alban Hills. In addition, Vatican City maintains embassies in numerous foreign nations.
The Vatican enjoyed a cultural golden age during the Renaissance, when the popes were among Italy’s foremost patrons of the arts. The Vatican Museums and Galleries, the frescoes by Michelangelo in the Sistine Chapel, the frescoes by Pinturicchio in the Borgia Apartment, and Raphael’s Stanze (“Rooms”) attract critics, artists, and flocks of tourists from throughout the world. Years of restoration work on the Sistine Chapel frescoes were completed in 1994, making it possible to view Michelangelo’s work in full vibrant colors. In 2000 the millennial Jubilee focused world attention on Vatican City.
The Vatican Apostolic Library contains a priceless collection of some 150,000 manuscripts and 1.6 million printed books, many from pre-Christian and early Christian times. The Vatican publishes its own influential daily newspaper, L’Osservatore Romano, and its press can print books and pamphlets in any of 30 languages, from old Ecclesiastical Georgian to Tamil. Since 1983 the Vatican has produced its own television programming. Its radio broadcasts are heard in some 40 languages in many parts of the world. Vatican City was designated a UNESCO World Heritage site in 1984. Pop. (2019 est.) 453.
Additional Information
The Vatican City, one of the most sacred places in Christendom, attests to a great history and a formidable spiritual venture. A unique collection of artistic and architectural masterpieces lie within the boundaries of this small state. At its centre is St Peter's Basilica, with its double colonnade and a circular piazza in front and bordered by palaces and gardens. The basilica, erected over the tomb of St Peter the Apostle, is the largest religious building in the world, the fruit of the combined genius of Bramante, Raphael, Michelangelo, Bernini and Maderno.
Outstanding Universal Value:
Brief synthesis
One of the most sacred places in Christendom, Vatican City stands as a testimony to a history of about two millennia and to a formidable spiritual venture. Site of the tomb of the Apostle Saint Peter, first of the uninterrupted succession of Roman Pontiffs, and therefore a main pilgrimage centre, the Vatican is directly and tangibly linked with the history of Christianity. Furthermore, it is both an ideal and an exemplary creation of the Renaissance and of Baroque art. It exerted an enduring influence on the development of the arts from the 16th century.
The independent State, defined by the Lateran Treaty of 11 February 1929, extends its territorial sovereignty over an area of 44 ha in the centre of Rome: Vatican City enclosed by its walls and open toward the city through Bernini’s colonnade of Saint Peter’s. The boundaries of the city-state contain masterpieces and living institutions that are a witness to the unique continuity of the crucial role played by this place in the history of mankind. The Centre of Christianity since the foundation of Saint Peter’s Basilica by Constantine (4th century), and at a later stage the permanent seat of the Popes, the Vatican is at once the pre-eminently holy city for Catholics, an important archaeological site of the Roman world and one of the major cultural reference points of both Christians and non-Christians.
Its prestigious history explains the development of an architectural and artistic ensemble of exceptional value. Beneath the basilica of Saint Peter, reconstructed in the 16th century under the guidance of the most brilliant architects of the Renaissance, remains of the first basilica founded by Constantine still exist, as well as ruins of the circus of Caligula and Nero, and a Roman necropolis of the 1st century AD, where Saint Peter’s tomb is located. Under Julius II’s patronage in 1506, an extraordinary artistic era was inaugurated, leading to the decoration of Raphael’s Stanze and of the Sistine Chapel with frescoes by Michelangelo, along with the building of the new basilica, completed in 1626, fruit of the combined genius of Bramante, Raphael, Michelangelo, Bernini, Maderno and Della Porta.
The Vatican Palace is the result of a long series of additions and modifications by which, from the Middle Ages, the Popes rivalled each other in magnificence. The original building of Nicholas III (1277-1280) was enlarged in the 15th, 16th and 17th centuries: the history of the arts of the Renaissance and Baroque periods finds here iconic models.
In 1475, Sixtus IV founded the Vatican Library, which is the first open to the public in Europe; the collections of manuscripts and books, prints, drawings, coins and decorative arts, constantly increased through the centuries, making it an invaluable repository of human culture.
From the mid-18th century, the popes’ efforts were also directed towards expanding the private collections of antiquities dating back to the Renaissance: their transformation into public museums accessible to scholars and connoisseurs marks the origin of the Vatican Museums. New buildings were built specifically to house the classical sculptures, such as the Pio-Clementine Museum, which represents a milestone in the history of European culture. The 19th- and 20th-century additions of new and diverse collections and buildings accord with the tradition of papal patronage.
Criterion (i): The Vatican, a continuous artistic creation whose progress spreads over centuries, represents a unique masterpiece of the modelling of a space, integrating creations which are among the most renowned of mankind: not only the world famous icon of sacred architecture, the basilica of Saint Peter, but also the chapel of Nicholas V decorated by Fra Angelico, the Borgia apartment with frescoes by Pinturicchio, the Stanze of Raphael and his students, the Sistine Chapel, whose mural decoration, begun by Perugino, Botticelli and other painters, was completed in the 16th century with the frescoes of the ceiling and the monumental Last Judgement by Michelangelo, who left his last murals in the Pauline Chapel.
Criterion (ii): The Vatican exerted a deep influence on the development of art from the 16th century. Architects have visited it to study the constructions of Bramante (the Basilica of Saint Peter, the Belvedere Court), of Michelangelo (the cupola of Saint Peter), of Bernini (Saint Peter's colonnade, the Baldacchino of the Basilica). Both within and outside Europe, the Vatican buildings have been abundantly copied and imitated, the paintings (the frescoes of Raphael and Michelangelo) and the antiquities of the Museums no less so.
Criterion (iv): The Vatican is both an ideal and exemplary religious and palatial creation of the Renaissance and of Baroque art.
Criterion (vi): Site of the tomb of Saint Peter and pilgrimage centre, the Vatican is directly and materially linked with the history of Christianity. For more than a thousand years, mankind has accumulated, in this privileged site, the treasures of its collective memory (manuscripts and books of the Library) and of its universal genius.
Integrity
The boundaries of the property, which coincide with the entire territory of the Vatican City State, have preserved their original integrity and characteristics. The exceptional urban, architectural and aesthetic values, even through successive additions and changes in form and design, invariably maintain the highest standards of artistic quality and workmanship, building an organic ensemble of unparalleled harmony. Civil and sacred buildings, which have been in use for centuries, maintain their religious, cultural, institutional and diplomatic functions unaltered.
Authenticity
The property meets the required conditions of authenticity, since most of its features are still preserved and maintained in their initial form, perform their primary functions and truthfully convey their original spiritual and cultural values. The extensive restoration campaigns conducted on some of the most significant monuments of the site since the date of the inscription ensure the material conservation of the heritage and strengthen its capacity for expressing its values.
Protection and management requirements
The property is safeguarded by the law for the protection of the cultural heritage (no. 355, 25/07/2001) and by several rules of procedure issued by the various institutions of the Holy See in charge of heritage. For instance, the body responsible for the preservation and maintenance of Saint Peter’s Basilica, the Fabbrica di S.Pietro, was founded in 1506 and is still active. The legal protective mechanism and traditional management system are adequate and ensure the effective protection of the site. The state of conservation of the property is constantly and carefully monitored, with special attention paid to the impact of the huge number of pilgrims and visitors.

#14 Dark Discussions at Cafe Infinity » Close Quotes - VII » Yesterday 15:38:47
- Jai Ganesh
- Replies: 0
Close Quotes - VII
1. I can't do with mountains at close quarters - they are always in the way, and they are so stupid, never moving and never doing anything but obtrude themselves. - D. H. Lawrence
2. There's no doubt who was a leader in space after the Apollo Program. Nobody came close to us. And our education system, in science, technology, engineering and math, was at the top of the world. It's no longer there. We're descending rather rapidly. - Buzz Aldrin
3. One of the best ways to see tree flowers is to climb one of the tallest trees and to get into close, tingling touch with them, and then look broad. - John Muir
4. Close by the Rights of Man, at the least set beside them, are the Rights of the Spirit. - Victor Hugo
5. It's a brilliant surface in that sunlight. The horizon seems quite close to you because the curvature is so much more pronounced than here on earth. It's an interesting place to be. I recommend it. - Neil Armstrong
6. No evidence compels the conclusion that the minimum required intake of any vitamin comes close to the optimum intake that sustains good health. - Linus Pauling
7. Before I lost my voice, it was slurred, so only those close to me could understand, but with the computer voice, I found I could give popular lectures. I enjoy communicating science. It is important that the public understands basic science, if they are not to leave vital decisions to others. - Stephen Hawking
8. It's interesting that I had such a close relationship with my grandfather. Because your parents always judge you: they say, 'You shouldn't do this, you shouldn't do that.' But with your grandparents you have a feeling that you can say anything or you can do anything, and they will support you. That's why you have this kind of connection. - Novak Djokovic.
#15 Science HQ » Polonium » Yesterday 15:18:51
- Jai Ganesh
- Replies: 0
Polonium
Gist
Polonium (Po, atomic number 84) is a rare, highly radioactive metal discovered by Marie and Pierre Curie in 1898. It has no stable isotopes, with Polonium-210 (210Po) being the most common, formed as a decay product of uranium-238. Although Po-210 poses no external hazard, internal contamination from inhalation or ingestion can cause significant internal radiation doses, leading to severe health effects or death. Its industrial applications include the removal of static electricity in industries like paper and metal rolling.
Polonium has limited industrial uses, including antistatic devices, heat sources for space equipment, and neutron sources for research and nuclear applications. However, its extreme radioactivity and short half-life make it dangerous and challenging to handle, with potential applications for poisoning and in the development of atomic weapons.
Summary
Polonium is a chemical element; it has symbol Po and atomic number 84. A rare and highly radioactive metal (although sometimes classified as a metalloid) with no stable isotopes, polonium is a chalcogen and chemically similar to selenium and tellurium, though its metallic character resembles that of its horizontal neighbors in the periodic table: thallium, lead, and bismuth. Due to the short half-life of all its isotopes, its natural occurrence is limited to tiny traces of the fleeting polonium-210 (with a half-life of 138 days) in uranium ores, as it is the penultimate daughter of natural uranium-238. Though two longer-lived isotopes exist (polonium-209 with a half-life of 124 years and polonium-208 with a half-life of 2.898 years), they are much more difficult to produce. Today, polonium is usually produced in milligram quantities by the neutron irradiation of bismuth. Due to its intense radioactivity, which results in the radiolysis of chemical bonds and radioactive self-heating, its chemistry has mostly been investigated on the trace scale only.
Polonium was discovered on 18 July 1898 by Marie Skłodowska-Curie and Pierre Curie, when it was extracted from the uranium ore pitchblende and identified solely by its strong radioactivity: it was the first element to be discovered in this way. Polonium was named after Marie Skłodowska-Curie's homeland of Poland, which at the time was partitioned between three countries. Polonium has few applications, and those are related to its radioactivity: heaters in space probes, antistatic devices, sources of neutrons and alpha particles, and poison (e.g., poisoning of Alexander Litvinenko). It is extremely dangerous to humans.
Details
Polonium (Po) is a radioactive, silvery-gray or black metallic element of the oxygen group (Group 16 [VIa] in the periodic table). The first element to be discovered by radiochemical analysis, polonium was discovered in 1898 by Pierre and Marie Curie, who were investigating the radioactivity of a certain pitchblende, a uranium ore. The very intense radioactivity not attributable to uranium was ascribed to a new element, named by them after Marie Curie’s homeland, Poland. The discovery was announced in July 1898. Polonium is extremely rare, even in pitchblende: 1,000 tons of the ore must be processed to obtain 40 milligrams of polonium. Its abundance in the Earth’s crust is about one part in {10}^{15}. It occurs in nature as a radioactive decay product of uranium, thorium, and actinium. The half-lives of its isotopes range from a fraction of a second up to 103 years; the most common natural isotope of polonium, polonium-210, has a half-life of 138.4 days.
Polonium usually is isolated from by-products of the extraction of radium from uranium minerals. In the chemical isolation, pitchblende ore is treated with hydrochloric acid, and the resulting solution is heated with hydrogen sulfide to precipitate polonium monosulfide, PoS, along with other metal sulfides, such as that of bismuth, Bi2S3, which resembles polonium monosulfide closely in chemical behaviour, though it is less soluble. Because of the difference in solubility, repeated partial precipitation of the mixture of sulfides concentrates the polonium in the more soluble fraction, while the bismuth accumulates in the less soluble portions. The difference in solubility is small, however, and the process must be repeated many times to achieve a complete separation. Purification is accomplished by electrolytic deposition. It can be produced artificially by bombarding bismuth or lead with neutrons or with accelerated charged particles.
Chemically, polonium resembles the elements tellurium and bismuth. Two modifications of polonium are known, an α- and a β-form, both of which are stable at room temperature and possess metallic characteristics. The fact that its electrical conductivity decreases as the temperature increases places polonium among the metals rather than the metalloids or nonmetals.
Because polonium is highly radioactive—it disintegrates to a stable isotope of lead by emitting alpha rays, which are streams of positively charged particles—it must be handled with extreme care. When contained in such substances as gold foil, which prevent the alpha radiation from escaping, polonium is used industrially to eliminate static electricity generated by such processes as paper rolling, the manufacture of sheet plastics, and the spinning of synthetic fibres. It is also used on brushes for removing dust from photographic film and in nuclear physics as a source of alpha radiation. Mixtures of polonium with beryllium or other light elements are used as sources of neutrons.
Element Properties
atomic number : 84
atomic weight : 210
melting point : 254 °C (489 °F)
boiling point : 962 °C (1,764 °F)
density : 9.4 g/{cm}^{3}
oxidation states : −2, +2, +3(?), +4, +6.
Additional Information:
Appearance
A silvery-grey, radioactive semi-metal.
Uses
Polonium is an alpha-emitter, and is used as an alpha-particle source in the form of a thin film on a stainless steel disc. These are used in antistatic devices and for research purposes.
A single gram of polonium will reach a temperature of 500°C as a result of the alpha radiation emitted. This makes it useful as a source of heat for space equipment.
It can be mixed or alloyed with beryllium to provide a source of neutrons.
Biological role
Polonium has no known biological role. It is highly toxic due to its radioactivity.
Natural abundance
Polonium is a very rare natural element. It is found in uranium ores but it is uneconomical to extract it. It is obtained by bombarding bismuth-209 with neutrons to give bismuth-210, which then decays to form polonium. All the commercially produced polonium in the world is made in Russia.
#16 Re: Jai Ganesh's Puzzles » General Quiz » Yesterday 14:53:48
Hi,
#10557. What does the term in Geography Bourne (stream) mean?
#10558. What does the term in Geography Box canyon mean?
#17 Re: Jai Ganesh's Puzzles » English language puzzles » Yesterday 14:41:03
Hi,
#5751. What does the verb (used with object) despise mean?
#5752. What does the noun despite mean?
#18 Re: Jai Ganesh's Puzzles » Doc, Doc! » Yesterday 14:13:37
Hi,
#2469. What does the medical term Macrophage signify?
#19 Re: Jai Ganesh's Puzzles » 10 second questions » Yesterday 14:04:30
Hi,
#9735.
#20 Re: Jai Ganesh's Puzzles » Oral puzzles » Yesterday 13:52:31
Hi,
#6242.
#21 Jokes » Love Jokes - III » Yesterday 13:44:12
- Jai Ganesh
- Replies: 0
Q: Did you hear about the love affair between sugar and cream?
A: It was icing on the cake.
* * *
Q: How do you transfer funds even faster than electronic banking?
By getting Married!
* * *
Q: What happened when two vampires went on a blind date?
A: It was love at first bite!
* * *
Q: What's the difference between love and herpes?
A: Love doesn't last forever.
* * *
After a quarrel, a husband said to his wife, "You know, I was a fool when I married you."
She replied, "Yes, dear, but I was in love and didn't notice."
* * *
#22 Re: Exercises » Compute the solution: » Yesterday 13:28:11
Hi,
2575.
#23 Re: Dark Discussions at Cafe Infinity » crème de la crème » 2025-09-15 21:50:24
2336) John M. Jumper
Gist:
Work
Proteins control and drive all the chemical reactions that together are the basis of life. Proteins generally consist of 20 different amino acids. These are linked together in long strings that fold up to make a three-dimensional structure. In 2020, John Jumper and Demis Hassabis presented an AI model called AlphaFold2. With its help, they have been able to predict the structure of virtually all known proteins. AlphaFold2 has been widely used in many areas, including research into pharmaceuticals and environmental technology.
Summary
John M. Jumper (born 1985, Little Rock, Arkansas, U.S.) is an American computer scientist who was awarded the 2024 Nobel Prize in Chemistry for his work using artificial intelligence (AI) to find the three-dimensional structure of proteins. He shared half the prize with his colleague, English computer scientist Demis Hassabis, and the other half of the prize was awarded to American biochemist David Baker.
Early life and career
Jumper spent his childhood and teenage years in Little Rock, Arkansas. He majored in mathematics and physics at Vanderbilt University and graduated with a bachelor’s degree in 2007. He went on to complete a master’s degree in physics at the University of Cambridge in 2008.
Jumper spent the next three years at D.E. Shaw Research, a computational laboratory in New York City, where he developed simulations to examine the dynamics of proteins and other molecules. Starting in 2011 Jumper shifted his study of computational biology to the University of Chicago, where he applied machine learning to explore the physics of protein folding. He graduated from the University of Chicago with a master’s degree (2012) and a Ph.D. (2017) in chemistry. He then joined Google’s DeepMind, an AI development company, in 2017 as a research scientist working on AlphaFold, which predicts the three-dimensional structure of proteins using machine-learning algorithms.
Solving protein folding
Proteins are large molecules that are directly involved in the chemical processes essential for life and are built up from 20 amino acids that can be combined in many different ways. The function of a protein is determined by its three-dimensional structure, which can be quite complex based on how the string of amino acids is folded.
How a protein is folded is determined by its amino acid sequence. However, even a small protein of only 100 amino acids can have 1047 possible three-dimensional structures. Predicting a protein’s structure from its amino acid sequence became a key problem in molecular biology.
In 1994 biologists John Moult and Krzysztof Fidelis founded the Critical Assessment of protein Structure Prediction (CASP) challenge to test methods for predicting protein structures. Every two years, contestants were given the amino acid sequences for proteins whose structure had been determined but not published and were challenged to predict the protein structures.
Progress was slow. By the mid-2010s the best models in the CASP challenge were about 40 percent accurate. DeepMind entered its protein structure program AlphaFold in CASP13 in 2018 and delivered an astonishing accuracy of about 60 percent, far ahead of any competitors. However, improvement beyond that was difficult, but by then Jumper had joined DeepMind and used his experience with protein simulation to help develop AlphaFold2.
AlphaFold2 was trained on databases of amino acid sequences and protein structures and used a neural network called a transformer to find a likely protein structure. At CASP14 in 2020 AlphaFold2 reached an accuracy of 90 percent, which is comparable with experimental results. The problem of finding a protein structure given an amino acid sequence had been solved.
Jumper, DeepMind CEO Hassabis, and their collaborators used AlphaFold2 to calculate the structure of almost all of the more than 50,000 human proteins in 2021. They then went even further and calculated the structures of almost all of the 200 million known proteins, which come from about 1 million different species, or as Hassabis called it, “the entire protein universe.” By predicting how proteins organize themselves, researchers can develop more effective drugs that target specific proteins whose structures contribute to diseases. Since 2023 Jumper has been the director of Google DeepMind.
Jumper is also the recipient of the Wiley Prize in Biomedical Sciences (2022) and the BBVA Foundation Frontiers of Knowledge Award in Biology and Biomedicine (2023, shared with Hassabis and Baker). Jumper also received the VinFuture Prize (2022), the Canada Gairdner International Award (2023), and the Albert Lasker Basic Medical Research Award (2023, shared with Hassabis).
Details
John Michael Jumper (born 1985) is an American chemist and computer scientist. Jumper and Demis Hassabis were awarded with the 2024 Nobel Prize in Chemistry for protein structure prediction.
He currently serves as director at Google DeepMind. Jumper and his colleagues created AlphaFold, an artificial intelligence (AI) model to predict protein structures from their amino acid sequence with high accuracy. Jumper stated that the AlphaFold team plans to release 100 million protein structures.
The scientific journal Nature included Jumper as one of the ten "people who mattered" in science in their annual listing of Nature's 10 in 2021.
Education
Jumper received a Bachelor of Science with majors in physics and mathematics from Vanderbilt University in 2007, a Master of Philosophy in theoretical condensed matter physics from the University of Cambridge in 2010 on a Marshall Scholarship, a Master of Science in theoretical chemistry from the University of Chicago in 2012, and a Doctor of Philosophy in theoretical chemistry from the University of Chicago in 2017. His doctoral advisors at the University of Chicago were Tobin R. Sosnick and Karl Freed.
Career
Jumper's research investigates algorithms for protein structure prediction.
AlphaFold
AlphaFold is a deep learning algorithm developed by Jumper and his team at DeepMind, a research lab acquired by Google's parent company Alphabet Inc. It is an artificial intelligence program which performs predictions of protein structure.
Awards and honors
In November 2020, AlphaFold was named the winner of the 14th Critical Assessment of Structure Prediction (CASP) competition. This international competition benchmarks algorithms to determine which one can best predict the 3D structure of proteins. AlphaFold won the competition, outperforming other algorithms scoring above 90 for around two-thirds of the proteins in CASP's global distance test (GDT), a test that measures the degree to which a computational program predicted structure is similar to the lab experiment determined structure, with 100 being a complete match, within the distance cutoff used for calculating GDT.
In 2021, Jumper was awarded the BBVA Foundation Frontiers of Knowledge Award in the category "Biology and Biomedicine". In 2022 Jumper received the Wiley Prize in Biomedical Sciences and for 2023 the Breakthrough Prize in Life Sciences for developing AlphaFold, which accurately predicts the structure of a protein. In 2023 he was awarded the Canada Gairdner International Award and the Albert Lasker Award for Basic Medical Research.
In 2024, Jumper and Demis Hassabis shared half of the Nobel Prize in Chemistry for their protein folding predictions, the other half went to David Baker for computational protein design.
In 2025, Jumper received the Golden Plate Award of the American Academy of Achievement and the Marshall Medal of the Marshall Aid Commemoration Commission. He was elected a Fellow of the Royal Society in 2025.

#24 Re: Dark Discussions at Cafe Infinity » Greatest Mathematicians from 1 CE ... » 2025-09-15 21:26:51
22) Niccolò Fontana Tartaglia
Nicolo, known as Tartaglia (1499/1500 – 13 December 1557), was an Italian mathematician, engineer (designing fortifications), a surveyor (of topography, seeking the best means of defense or offense) and a bookkeeper from the then Republic of Venice. He published many books, including the first Italian translations of Archimedes and Euclid, and an acclaimed compilation of mathematics. Tartaglia was the first to apply mathematics to the investigation of the paths of cannonballs, known as ballistics, in his Nova Scientia (A New Science, 1537); his work was later partially validated and partially superseded by Galileo's studies on falling bodies. He also published a treatise on retrieving sunken ships.
Personal life
Nicolo was born in Brescia, the son of Michele, a dispatch rider who travelled to neighbouring towns to deliver mail. In 1506, Michele was murdered by robbers, and Nicolo, his two siblings, and his mother were left impoverished. Nicolo experienced further tragedy in 1512 when King Louis XII's troops invaded Brescia during the War of the League of Cambrai against Venice. The militia of Brescia defended their city for seven days. When the French finally broke through, they took their revenge by massacring the inhabitants of Brescia. By the end of battle, over 45,000 residents were killed. During the massacre, Nicolo and his family sought sanctuary in the local cathedral. But the French entered and a soldier sliced Nicolo's jaw and palate with a saber and left him for dead. His mother nursed him back to health but the young boy was left with a speech impediment, prompting the nickname "Tartaglia" ("stammerer"). After this he would never shave, and grew a beard to camouflage his scars.
His surname at birth, if any, is disputed. Some sources have him as "Niccolò Fontana", but others claim that the only support for this is a will in which he named a brother, Zuampiero Fontana, as heir, and point out that this does not imply he had the same surname.
#25 This is Cool » Aniline » 2025-09-15 18:38:35
- Jai Ganesh
- Replies: 0
Aniline
Gist
Aniline, or benzenamine (C6H5NH2), is the simplest aromatic amine, an industrially important organic compound used to make dyes, rubber, plastics, and other chemicals. It is a colorless to brown, oily liquid that darkens on exposure to air and light and has a characteristic odor. Aniline is a weak base, easily absorbed by the skin, and is a combustible liquid that can form flammable vapor/air mixtures and produce toxic fumes when heated or burned.
Aniline was first obtained in 1826 by the destructive distillation of indigo. Its name is taken from the specific name of the indigo-yielding plant Indigofera anil (Indigofera suffruticosa); its chemical formula is C6H5NH2.
Aniline is a chemical intermediate used to make a wide range of products, including dyes, pharmaceuticals (like paracetamol and Tylenol), polyurethane plastics, and rubber products like tires. It is also used in the agricultural industry to produce pesticides and fungicides.
Summary
Aniline (From Portuguese: anil, meaning 'indigo shrub', and -ine indicating a derived substance) is an organic compound with the formula C6H5NH2. Consisting of a phenyl group (−C6H5) attached to an amino group (−NH2), aniline is the simplest aromatic amine. It is an industrially significant commodity chemical, as well as a versatile starting material for fine chemical synthesis. Its main use is in the manufacture of precursors to polyurethane, dyes, and other industrial chemicals. Like most volatile amines, it has the odor of rotten fish. It ignites readily, burning with a smoky flame characteristic of aromatic compounds. It is toxic to humans.
Relative to benzene, aniline is "electron-rich". It thus participates more rapidly in electrophilic aromatic substitution reactions. Likewise, it is also prone to oxidation: while freshly purified aniline is an almost colorless oil, exposure to air results in gradual darkening to yellow or red, due to the formation of strongly colored, oxidized impurities. Aniline can be diazotized to give a diazonium salt, which can then undergo various nucleophilic substitution reactions.
Like other amines, aniline is both a base (pKaH = 4.6) and a nucleophile, although less so than structurally similar aliphatic amines.
Because an early source of the benzene from which they are derived was coal tar, aniline dyes are also called coal tar dyes.
Details
Aniline is an organic base used to make dyes, drugs, explosives, plastics, and photographic and rubber chemicals.
Aniline was first obtained in 1826 by the destructive distillation of indigo. Its name is taken from the specific name of the indigo-yielding plant Indigofera anil (Indigofera suffruticosa); its chemical formula is C6H5NH2.
Aniline is prepared commercially by the catalytic hydrogenation of nitrobenzene or by the action of ammonia on chlorobenzene. The reduction of nitrobenzene can also be carried out with iron borings in aqueous acid.
A primary aromatic amine, aniline is a weak base and forms salts with mineral acids. In acidic solution, nitrous acid converts aniline into a diazonium salt that is an intermediate in the preparation of a great number of dyes and other organic compounds of commercial interest. When aniline is heated with organic acids, it gives amides, called anilides, such as acetanilide from aniline and acetic acid. Monomethylaniline and dimethylaniline can be prepared from aniline and methyl alcohol. Catalytic reduction of aniline yields cyclohexylamine. Various oxidizing agents convert aniline to quinone, azobenzene, nitrosobenzene, p-aminophenol, and the phenazine dye aniline black.
Pure aniline is a highly poisonous, oily, colourless substance with a pleasant odour.
Additional Information
Aniline is the simplest member of the primary aromatic amines, in which one or more hydrogen atoms of the benzene ring are replaced by amino (-NH2) group.
Derivatives of aniline include a wide variety of different substances. Some of these (like benzidine and MOCA) are composed of two combined aromatic rings.
Many aromatic amines may cause methemoglobinemia in humans. Aniline and many of its derivatives are known or suspected human carcinogens. Several aniline derivatives can also cause skin sensitization. Classical members of this family are bladder carcinogens 2-naphtylamine and benzidine, both of which have been restricted in the European Union (EU) implying that there is no exposure to these compounds.
A large number of substances in the aniline group are on the market in the EU. Several aniline derivatives can be found also from the list of substances restricted under REACH. Aniline compounds are also formed as degradation products from azo-colourants, pharmaceuticals and from aromatic isocyanates used for polyurethane polymers, lacquers, foams and adhesives.
When looking at those aniline substances that are produced or imported in the EU at amounts above 1,000 tonnes per year (tpa) according to the European Chemical Agency’s (ECHA) registration database and that have significant health hazards, (other than only irritation/corrosion).
