Math Is Fun Forum
You are not logged in.
- Topics: Active | Unanswered
#26 This is Cool » Silica » 2025-10-20 17:17:11
- Jai Ganesh
- Replies: 0
Silica
Gist
Silica is the common name for silicon dioxide (SiO2), a naturally occurring mineral composed of one silicon and two oxygen atoms. It is the most abundant mineral in the Earth's crust, found in sand, granite, and rocks, and is used in a wide array of products including glass, concrete, and electronics. Silica is generally safe but can be a choking hazard, and inhaling fine airborne dust from certain industrial activities can lead to serious lung diseases like silicosis.
Silica is used in a wide range of applications, including glass manufacturing, construction (cement, concrete), electronics (semiconductors, microchips), and industrial processes like water filtration and chemical manufacturing. It also functions as a common food additive, a desiccant in moisture-absorbing packets, and a component in products like pottery, ceramics, and even some cosmetics and supplements.
Summary
Silicon dioxide, also known as silica, is an oxide of silicon with the chemical formula SiO2, commonly found in nature as quartz. In many parts of the world, silica is the major constituent of sand. Silica is one of the most complex and abundant families of materials, existing as a compound of several minerals and as a synthetic product. Examples include fused quartz, fumed silica, opal, and aerogels. It is used in structural materials, microelectronics, and as components in the food and pharmaceutical industries. All forms are white or colorless, although impure samples can be colored.
Silicon dioxide is a common fundamental constituent of glass.
Natural occurrence:
Geology
SiO2 is most commonly encountered in nature as quartz, which comprises more than 10% by mass of the Earth's crust. Quartz is the only polymorph of silica stable at the Earth's surface. Metastable occurrences of the high-pressure forms coesite and stishovite have been found around impact structures and associated with eclogites formed during ultra-high-pressure metamorphism. The high-temperature forms of tridymite and cristobalite are known from silica-rich volcanic rocks. In many parts of the world, silica is the major constituent of sand.
Biology
Even though it is poorly soluble, silica occurs in many plants such as rice. Plant materials with high silica phytolith content appear to be of importance to grazing animals, from chewing insects to ungulates. Silica accelerates tooth wear, and high levels of silica in plants frequently eaten by insects may have developed as a defense mechanism against predation.
Silica is also the primary component of rice husk ash, which is used, for example, in filtration and as supplementary cementitious material (SCM) in cement and concrete manufacturing.
Silicification in and by cells has been common in the biological world and it occurs in bacteria, protists, plants, and animals (invertebrates and vertebrates).
Prominent examples include:
* Tests or frustules (i.e. shells) of diatoms, Radiolaria, and testate amoebae.
* Silica phytoliths in the cells of many plants including Equisetaceae, many grasses, and a wide range of dicotyledons.
* The spicules forming the skeleton of many sponges.
Details
Also called silica sand or quartz sand, silica is silicon dioxide (SiO2). Silicon compounds are the most significant component of the Earth’s crust. Since sand is plentiful, easy to mine and relatively easy to process, it is the primary ore source of silicon. The metamorphic rock, quartzite, is another source.
Silicon (Si) is a semi-metallic or metalloid, because it has several of the metallic characteristics. Silicon is never found in its natural state, but rather in combination with oxygen as the silicate ion in silica-rich rocks such as obsidian, granite, diorite, and sandstone. Feldspar and quartz are the most significant silicate minerals. Silicon alloys include a variety of metals, including iron, aluminum, copper, nickel, manganese and ferrochromium.
Description
Also called silica sand or quartz sand, silica is made of silicon dioxide (SiO2). Silicon compounds are the most significant component of the Earth’s crust. Since sand is plentiful, easy to mine and relatively easy to process, it is the primary ore source of silicon. The metamorphic rock, quartzite, is another source.
Silicon (Si) is a semi-metallic or metalloid, because it has several of the metallic characteristics. Silicon is never found in its natural state, but rather in combination with oxygen as the silicate ion - in silica-rich rocks such as obsidian, granite, diorite, and sandstone. Feldspar and quartz are the most significant silicate minerals. Silicon alloys include a variety of metals, including iron, aluminum, copper, nickel, manganese and ferrochromium.
Relation to Mining
In almost all cases, silica mining uses open pit or dredging mining methods with standard mining equipment. Except for temporarily disturbing the immediate area while mining operations are active, sand and gravel mining usually has limited environmental impact.
Uses
Ferrosilicon alloys are used to improve the strength and quality of iron and steel products. Tools, for instance, are made of steel and ferrosilicon.
In addition to tool steels, an example of “alloy steels,” ferrosilicon is used in the manufacture of stainless steels, carbon steels, and other alloy steels. An alloy steel refers to all finished steels other than stainless and carbon steels. Stainless steels are used when superior corrosion resistance, hygiene, aesthetic, and wear-resistance qualities are needed.
Carbon steels are used extensively in suspension bridges and other structural support material, and in automotive bodies, to name a few.
Silicon is used in the aluminum industry to improve castability and weldability. Silicon-aluminum alloys tend to have relatively low strength and ductility, so other metals, especially magnesium and copper, are often added to improve strength.
In the chemicals industry, silicon metal is the starting point for the production of silianes, silicones, fumed silica, and semiconductor-grade silicon. Silanes are the used to make silicone resins, lubricants, anti-foaming agents, and water-repellent compounds. Silicones are used as lubricants, hydraulic fluids, electrical insulators, and moisture-proof treatments.
Semiconductor-grade silicon is used in the manufacture of silicon chips and solar cells. Fumed silica is used as a filler in the cement and refractory materials industries, as well as in heat insulation and filling material for synthetic rubbers, polymers and grouts.
Silica is used in ceramics and in making glass.
Silicon is considered a semiconductor. This means that it conducts electricity, but not as well as a metal such as copper or silver. This physical property makes silicon an important commodity in the computer manufacturing business.
Additional Information
Silica is a compound of the two most abundant elements in Earth’s crust, silicon and oxygen, SiO2. The mass of Earth’s crust is 59 percent silica, the main constituent of more than 95 percent of the known rocks. Silica has three main crystalline varieties: quartz (by far the most abundant), tridymite, and cristobalite. Other varieties include coesite, keatite, and lechatelierite. Silica sand is used in buildings and roads in the form of portland cement, concrete, and mortar, as well as sandstone. Silica also is used in grinding and polishing glass and stone; in foundry molds; in the manufacture of glass, ceramics, silicon carbide, ferrosilicon, and silicones; as a refractory material; and as gemstones. Silica gel is often used as a desiccant to remove moisture.

#27 Re: Dark Discussions at Cafe Infinity » crème de la crème » 2025-10-20 16:41:20
2369) Bernard Katz
Gist:
Work
The nervous systems of people and animals consist of many nerve cells with long extensions, or nerve fibers. Signals are conveyed between cells by small electrical currents and by special substances known as signal substances. The transfers occur via contacts, or synapses. In the 1950s Bernard Katz studied how impulses in motor neurons activate muscular activity by measuring variations in electrical charges. For example, he showed how the signal substance acetylcholine in synapses is released in certain amounts.
Summary
Sir Bernard Katz (born March 26, 1911, Leipzig, Germany—died April 20, 2003, London, England) was a German-born British physiologist who investigated the functioning of nerves and muscles. His studies on the release of the neurotransmitter acetylcholine—which carries impulses from nerve fibre to muscle fibre or from one nerve ending to another—won him a share (with Julius Axelrod and Ulf von Euler) of the 1970 Nobel Prize for Physiology or Medicine.
After receiving a medical degree from the University of Leipzig in 1934, Katz immigrated to England, where he pursued advanced studies at University College in London, taking a Ph.D. in 1938. Upon receiving a Carnegie fellowship, he studied in Australia (1939–42) and then served in the Royal Australian Air Force during World War II. He returned to University College in 1946 and from 1952 to 1978 was professor and head of the biophysics department. Katz was knighted in 1969.
Katz wrote Electric Excitation of Nerve (1939), Nerve, Muscle and Synapse (1966), and The Release of Neural Transmitter Substances (1969). He and his associates made numerous discoveries concerning the chemistry of nerve transmission, including the role of calcium ions in promoting the release of neurotransmitter substances and the fact that quanta of these substances are being released constantly at random intervals.
Details
Sir Bernard Katz (26 March 1911 – 20 April 2003)[2] was a German-born British physician and biophysicist, noted for his work on nerve physiology; specifically, for his work on synaptic transmission at the nerve-muscle junction. He shared the Nobel Prize in physiology or medicine in 1970 with Julius Axelrod and Ulf von Euler. He was made a Knight Bachelor in 1969.
Life and career
Katz was born in Leipzig, Germany, to a Jewish family originally from Russia, the son of Eugenie (Rabinowitz) and Max Katz, a fur merchant. He was educated at the Albert Gymnasium in that city from 1921 to 1929 and went on to study medicine at the University of Leipzig. He graduated in 1934 and fled to Britain in February 1935.
Katz went to work at University College London, initially under the tutelage of Archibald Vivian Hill. He finished his PhD in 1938 and won a Carnegie Fellowship to study with John Carew Eccles at the Kanematsu Institute of Sydney Medical School. During this time, both he and Eccles gave research lectures at the University of Sydney. He obtained British nationality in 1941and joined the Royal Australian Air Force in 1942. He spent the war in the Pacific as a radar officer and in 1946 was invited back to UCL as an assistant director by Hill. For three years until 1949, the Katz family lived with Hill and his wife Margaret in the top flat of their house in Highgate.
Back in England he also worked with the 1963 Nobel prize winners Alan Hodgkin and Andrew Huxley. Katz was made a professor at UCL in 1952 and head of the Biophysics Department; he was elected a Fellow of the Royal Society (FRS) in 1952. He stayed as head of Biophysics until 1978 when he became emeritus professor.
Katz married Marguerite Penly in 1945. He died in London on 20 April 2003, at the age of 92. His son Jonathan is Public Orator of the University of Oxford.
Research
His research uncovered fundamental properties of synapses, the junctions across which nerve cells signal to each other and to other types of cells. By the 1950s, he was studying the biochemistry and action of acetylcholine, a signalling molecule found in synapses linking motor neurons to muscles, used to stimulate contraction. Katz won the Nobel for his discovery with Paul Fatt that neurotransmitter release at synapses is "quantal", meaning that at any particular synapse, the amount of neurotransmitter released is never less than a certain amount, and if more is always an integral number times this amount. Scientists now understand that this circumstance arises because, prior to their release into the synaptic gap, transmitter molecules reside in like-sized subcellular packages known as synaptic vesicles, released in a similar way to any other vesicle during exocytosis.
Katz's work had immediate influence on the study of organophosphates and organochlorines, the basis of new post-war study for nerve agents and pesticides, as he determined that the complex enzyme cycle was easily disrupted.
Collections
Katz's son Jonathan presented the personal archive of his father to University College London in 2003. The collection includes biographical documents, correspondence, notes on lectures, publications, and research material.

#28 Dark Discussions at Cafe Infinity » Co-operation Quotes » 2025-10-20 16:16:10
- Jai Ganesh
- Replies: 0
Co-operation Quotes
1. If you will work in co-operation, forgetting the past, burying the hatchet, you are bound to succeed. - Muhammad Ali Jinnah
2. Stop-and-search has the potential to cause immense resentment and honesty to the police, with all the implications that has for generating distrust and ending co-operation from the public, if it is not used fairly. - Theresa May
3. A sincere and steadfast co-operation in promoting such a reconstruction of our political system as would provide for the permanent liberty and happiness of the United States. - James Madison
4. There is no doubt that this government and this country are benefiting from the reforms that we brought in the 1980s, and that couldn't have been done without the co-operation of the trade union movement. - Bob Hawke
5. I want Infosys to be a company which is globally respected and in where people belonging to different nationalities, races and religious beliefs will work with intense competition but utmost courtesy, dignity and co-operation in adding greater value to our stakeholders day after day. - N. R. Narayana Murthy
6. We are ready to engage in international co-operation against terrorism with a view to safeguarding national interests and regional security and stability. - Li Peng
7. Europe has found itself confronted with fresh challenges - challenges of a global character, the nature of which is directly connected with changes in the international climate and the difficulties of seeking new models for co-operation. - Boris Yeltsin
8. We can realise a lasting peace and transform the East-West relationship to one of enduring co-operation. - George H. W. Bush
9. If co-operation is a duty, I hold that non-co-operation also under certain conditions is equally a duty. - Mahatma Gandhi.
#29 Re: Jai Ganesh's Puzzles » General Quiz » 2025-10-20 15:51:30
Hi,
#10619. What does the term in Geography Cay mean?
#10620. What does the term in Geography Celestial pole mean?
#30 Re: Jai Ganesh's Puzzles » English language puzzles » 2025-10-20 15:39:39
Hi,
#5815. What does the adverb de jure mean?
#5816. What does the noun deliberation mean?
#31 Re: Jai Ganesh's Puzzles » Doc, Doc! » 2025-10-20 15:07:21
Hi,
#2501. What does the medical term Fatty liver disease (FLD) mean?
#32 Jokes » Apple Jokes - V » 2025-10-20 14:33:51
- Jai Ganesh
- Replies: 0
Q: What kind of apple has a short temper?
A: A crab apple.
* * *
Q: What do you get when you cross an apple with a Christmas tree?
A: Pineapple.
* * *
Q: What is the left side of an apple?
A: The part that you don't eat.
* * *
Q: How do you make an apple puff?
Chase it round the garden.
* * *
Q: What do you get if you cross a jogger with an apple pie ?
A: Puff pastry !
* * *
#33 Re: Jai Ganesh's Puzzles » 10 second questions » 2025-10-20 14:23:19
Hi,
#9770.
#34 Re: Jai Ganesh's Puzzles » Oral puzzles » 2025-10-20 14:09:46
Hi,
#6276.
#35 Re: Exercises » Compute the solution: » 2025-10-20 13:54:12
Hi,
2621.
#36 Re: This is Cool » Miscellany » 2025-10-20 00:01:25
2421) Elevator phobia
Gist
Elevator phobia, or elevatophobia, is an intense, irrational fear of elevators that can stem from claustrophobia (fear of enclosed spaces), acrophobia (fear of heights), or fear of malfunctions. Symptoms include intense anxiety and panic attacks, and it can lead to avoiding elevators by taking the stairs, even in high-rise buildings. Overcoming it often involves techniques like cognitive behavioral therapy (CBT) or exposure therapy, along with relaxation methods and breathing exercises.
Summary:
Where Elevator Phobias Might Originate
It’s different for each person, but there are some common elevator phobia origins. They include the following.
Agoraphobia – This is when someone fears being stuck somewhere that is nearly impossible to escape from. Individuals with agoraphobia could fear the elevator because it closes them in and they begin to panic that they won’t be able to get out again.
Claustrophobia – This is when someone fears being in enclosed spaces. Elevators are typically small in size, making people feel tightly enclosed when they are in them.
Previous Experiences – Whether someone had a previous experience with an actual elevator, or the enclosed space of the elevator makes him or her relive a past trauma, previous experiences could cause fear in someone when riding an elevator.
Hollywood – Even though everyone understands a movie is just a movie, those that include frightening elevator scenes can tell the subconscious that elevators are scary, leading someone to avoid riding in them.
How to Overcome an Elevator Phobia
One of the best ways to overcome many phobias is to understand the thing or situation one is afraid of. Someone might speak with an elevator maintenance worker to learn how the elevator works, then observe it for a time or two before trying to take a ride. If you have tenants who have anxiety surrounding elevators, it may be helpful to call in your elevator maintenance company.
If the phobia is quite extreme, a psychologist may be able to provide more insight on getting over a fear of elevators. If someone lives or works somewhere that an elevator is necessary on a regular basis, this might be a great option.
Details
If you get anxious or panicky while on an elevator, or you go out of your way to avoid taking elevators altogether, you are not alone! Many people have a fear of elevators. If this is you, read on to learn more about this problem and how you can overcome it.
Different Phobias Related to Elevators
A fear of elevators can stem from what’s known technically as a “specific phobia.” About 12.5% of adults in the United States will have a specific phobia in their lifetime. There are different types of specific phobias that can be associated with elevators, as you’ll see described below.
If you have a specific phobia, you’ll experience anxiety symptoms when in the presence of the feared object or situation. These include increased heart rate, shortness of breath, sweating, and nausea.
The phobias related to elevators are:
Claustrophobia
Claustrophobia is the fear of small, confined spaces. It can be triggered by many different places ranging from trains to tunnels to elevators. For many people, being in a small elevator, particularly if it is crowded, might trigger feelings of panic related to claustrophobia.
Cleithrophobia
Cleithrophobia is the fear of being trapped. It is a fear of a situation, rather than a specific place. This phobia is not specific to elevators but is commonly triggered by them. In an elevator, you might feel trapped if you think you might not be able to open the doors or get out. The lack of control over escaping is the defining feature of this phobia.
Acrophobia
Acrophobia is the fear of heights. This might be activated by elevators in tall buildings or skyscrapers. People with acrophobia might have trouble working or living in tall buildings with elevators because of the fear of being high off the ground. If you have acrophobia and live in an area like New York City, where you frequently encounter tall buildings with elevators, you might find it difficult to navigate your daily life.
Basophobia
Basophobia is the fear of falling. While an aversion to falling is a natural human instinct, people with basophobia have an intense fear of falling that is out of proportion to the situation. You might have trouble riding in elevators if you are afraid of the elevator suddenly falling, despite the safety features built into elevators.
Agoraphobia
Agoraphobia is a fear of being in places or situations that might cause feelings of helplessness, anxiety, or panic. You might have trouble leaving your home or other places that feel safe if you have agoraphobia. Going into public or riding an elevator might trigger feelings of panic for someone with this phobia.
Causes of a Fear of Elevators
Previous negative experiences:
You might have a fear of elevators because of a previous scary experience riding in one. For example, you might have been stuck in an elevator for a prolonged period of time. Or maybe you were in an elevator that stalled between floors or felt like it suddenly dropped. Any of these types of experiences could potentially trigger a fear of elevators.
Misconceptions about elevator safety:
Many myths or misunderstandings about elevators and their safety can worsen a fear of elevators. Here are some common misconceptions about elevators:
Running out of air:
A common myth is that elevators only hold a certain amount of air. You might therefore fear that if you get stuck you will have trouble breathing. However, elevators are not air-tight and do not have a limited supply of oxygen! Feelings of breathlessness on elevators are more likely related to panic attack symptoms rather than actually running out of air.
Elevator cable safety:
Some people believe that elevators are suspended by a single rope. This is false. Elevators are constructed with multiple steel cables that allow for safe movement up and down. Learn more about elevator cables here.
Elevators can free-fall:
Many people with a fear of elevators might believe that elevators can free-fall if something goes wrong. This is not the case. Elevators have safety features, both electronic and mechanical, that prevent this type of occurrence.
Safety Tips for Riding in Elevators
When riding in an elevator, it is important to take general safety precautions to ensure a smooth ride. Here are some tips to follow:
* Never hold a door open with your hand or an object. If you need to keep a door open, press and hold the “Door Open” button.
* If you are trying to catch the elevator and the door is closing, don’t stop it. Keeping the doors from closing could result in you getting injured.
* Don’t step onto an elevator if it seems overcrowded. Wait for the next one!
* If the door doesn’t open when the elevator stops at your floor, press the “Door Open” button. If the door doesn’t open after a few seconds, press the “Help” or “Emergency” button and wait for assistance.
* If a problem arises, try not to panic! Take a few deep breaths and press the “Emergency” button.
Additional Information
As a more modern invention, it has no official Greek "phobia" name; however, the fear of elevators is relatively common. According to the National Elevator Industry, Inc., elevators provide 18 billion passenger trips in the U.S. each year with millions of passengers repeatedly arriving safely at their destination. Yet, many people feel at least a slight nervousness when contemplating an elevator ride.
In some people, the fear of elevators is triggered by an existing phobia, but the fear often appears alone. Like any phobia, the fear of elevators ranges from mild to severe.
Fear of Elevators: Are Elevators Safe?
For those of us who work in the elevator industry, it’s hard to imagine anyone having a fear of elevators. Seeing these machines at work and knowing the evolution of elevators, we wouldn’t think twice when stepping into the cab. Yet, we know that elevator phobias are very real, and media is partially to blame for that. Films will often portray elevators in a way that is not accurate in the slightest. Thankfully, we are here to assure you that none of the extreme scenarios you see on the big screen are possible.
In movies, hoistway doors will open when they shouldn’t, characters will get stuck in elevators for days and a superhero will need to save the cab from free falling down the shaft. It may seem like a harmless way to move the plot along, but myths like these contribute to the fear of elevators that a number of commuters suffer from. Elevators are a part of daily life, so being afraid of them can inhibit many from experiencing the ease that they bring to tenants.
So how do you overcome your fear? Perhaps learning more about the many safety features that elevators have is a good place to start.
Are Elevators Safe?
Over the course of one day, elevators will globally carry 325 million people to their destinations. The worldwide popularity of the passenger elevator can be traced directly to its roots in safety. Before the 1800s, elevators were seen as a convenient way to move heavy items or as an attraction.
This all changed at the World’s Fair in 1853 when an inventor showcased his newest idea in front of a crowd. He stood in an elevator and had his assistant cut the cable that was holding him up. To their amazement, he only dropped a couple of feet — his new safety device caught the cab. This revolutionary invention is what we now call a “safety” activated by a governor. It is a component in every elevator you’ve ever ridden in.
Since the debut of the mechanical safety and overspeed governor, elevators have constantly evolved to become the safest form of vertical transportation — even better than stairs. Next time you are debating whether or not to take the stairs, take these safety features into account!

#37 Dark Discussions at Cafe Infinity » Clue Quotes » 2025-10-19 15:20:06
- Jai Ganesh
- Replies: 0
Clue Quotes
1. My theory is that if you look confident you can pull off anything - even if you have no clue what you're doing. - Jessica Alba
2. My parents were simpletons. Everyday living was a big thing in that small village where I was born. They had no clue about music. - Ilaiyaraaja
3. There's too many people in seats of power who just haven't got a clue what they're doing. They're bean counters, and it just pisses me off because consequently our kids go to see math movies. - Pierce Brosnan
4. I have no clue why, but maybe sometimes when there's someone you don't hear from, it's the person you want to hear from the most. - Janet Jackson
5. That idea of URL was the basic clue to the universality of the Web. That was the only thing I insisted upon. - Tim Berners-Lee
6. I wasn't trained as an actor at all. I had studied painting in America and had no clue about acting when I came back. - Deepti Naval.
#38 Re: Jai Ganesh's Puzzles » General Quiz » 2025-10-19 14:55:23
Hi,
#10617. What does the term in Geography Causeway mean?
#10618. What does the term in Geography Cave mean?
#39 Re: Jai Ganesh's Puzzles » English language puzzles » 2025-10-19 14:30:43
Hi,
#5813. What does the noun delegate mean?
#5814. What does the noun discretion mean?
#40 Re: Jai Ganesh's Puzzles » Doc, Doc! » 2025-10-19 14:09:23
Hi,
#2500. What does the medical term Metabolic dysfunction–associated steatotic liver disease mean?
#41 Re: Jai Ganesh's Puzzles » 10 second questions » 2025-10-19 14:00:07
Hi,
#9769.
#42 Re: Jai Ganesh's Puzzles » Oral puzzles » 2025-10-19 13:50:27
Hi,
#6275.
#43 Re: Exercises » Compute the solution: » 2025-10-19 13:37:39
Hi,
2620.
#44 Jokes » Apple Jokes - IV » 2025-10-19 13:27:11
- Jai Ganesh
- Replies: 0
Q: Why did the apple stop in the middle of the road?
A: Because he ran out of juice.
* * *
Q: What did the worm want to do when he grew up?
A: He wanted to join the Apple Core (Corps).
* * *
Q: What do you call a fruit that is rough around the edges?
A: A bad apple.
* * *
Q: What do you say when a fruit wins the talent show?
A: How about them apples?
* * *
Q: Dad, do you like baked apples?
A: Yes son, why?
Q: The orchard's on fire.
* * *
#45 Re: Exercises » Compute the solution: » 2025-10-18 23:36:30
Hi,
2619.
#46 Science HQ » Rutherfordium » 2025-10-18 17:32:14
- Jai Ganesh
- Replies: 0
Rutherfordium
Gist
Rutherfordium (Rf) is a synthetic, highly radioactive chemical element with atomic number 104, found in group 4 of the periodic table. It does not occur naturally and must be created in a laboratory through nuclear reactions in particle accelerators. Named after physicist Ernest Rutherford, its properties are difficult to study because it has a very short half-life, with the most stable known isotope 267Rf lasting about 48 minutes.
Because rutherfordium is made within the lab, there are not very many uses for this element commercially. On the other hand, rutherfordium has been used within the laboratory setting to conduct research. Most elements that are highly radioactive are used for nuclear power and medicinal purposes.
Summary
Rutherfordium is a synthetic chemical element; it has symbol Rf and atomic number 104. It is named after physicist Ernest Rutherford. As a synthetic element, it is not found in nature and can only be made in a particle accelerator. It is radioactive; the most stable known isotope, 267Rf, has a half-life of about 48 minutes.
In the periodic table, it is a d-block element and the second of the fourth-row transition elements. It is in period 7 and is a group 4 element. Chemistry experiments have confirmed that rutherfordium behaves as the heavier homolog to hafnium in group 4. The chemical properties of rutherfordium are characterized only partly. They compare well with the other group 4 elements, even though some calculations had indicated that the element might show significantly different properties due to relativistic effects.
In the 1960s, small amounts of rutherfordium were produced at Joint Institute for Nuclear Research in the Soviet Union and at Lawrence Berkeley National Laboratory in California. Priority of discovery and hence the name of the element was disputed between Soviet and American scientists, and it was not until 1997 that the International Union of Pure and Applied Chemistry (IUPAC) established rutherfordium as the official name of the element.
Details
Rutherfordium (Rf) is an artificially produced radioactive transuranium element in Group IVb of the periodic table, atomic number 104. Soviet scientists at the Joint Institute for Nuclear Research at Dubna, Russia, U.S.S.R., announced in 1964 the discovery of element 104, which they named kurchatovium, symbol Ku (for Igor Kurchatov, a Soviet nuclear physicist). In 1969, a group of American researchers at the Lawrence Radiation Laboratory of the University of California at Berkeley announced that they had identified isotopes of the element, different from the one identified by the Soviets; the Americans then proposed the name rutherfordium, in honour of the British physicist Ernest Rutherford.
In their experiment, the Soviets bombarded plutonium-242 with ions of neon-22, claiming to have obtained an isotope of element 104 that had a mass number of 260 and a half-life of 0.3 second. The Soviets then performed a series of chemical experiments with the isotope to demonstrate that it behaved in a manner that had been predicted for the element. When the workers at Dubna later used a more refined measuring technique, however, they found that the half-life of the isotope was 0.1 second, not 0.3 second as originally reported. This finding cast doubt on the chemical experiments with the element, because the results of those experiments could not have been obtained with atoms having a half-life of 0.1 second.
The American investigators did not follow the same procedure as the Dubna group, because the American equipment could not accelerate neon-22 ions to the necessary energies. Instead, they bombarded a target of californium-249 with ions of carbon-12 and carbon-13. Although unable to obtain the same isotope as the Soviet scientists, the Berkeley team did report positive identification of two, possibly three, isotopes of element 104. The bombardment of californium-249 with carbon-12 produced an isotope with a mass number of 257 and a half-life of 4–5 seconds; the carbon-13 bombardment produced an isotope with a mass number of 259 and a half-life of three to four seconds. The investigators at Berkeley subsequently, by bombarding curium-248 with oxygen-18, synthesized an isotope of element 104 that has a mass number of 261 and a half-life of 70 seconds.
Although the Soviets could make only a few atoms of their mass-260 isotope, the Berkeley group obtained thousands of the atoms having mass numbers of 257 and 259. Moreover, because the latter isotopes have longer half-lives, the Berkeley team was able to measure the energies of their emissions (alpha particles) and to detect their decay products (nobelium isotopes), thereby providing more extensive evidence of their discovery. The International Union of Pure and Applied Chemistry eventually ruled that element 104 be named rutherfordium.
Element Properties
atomic number : 104
mass of most stable isotope : 261.
Additional Information:
Appearance
A radioactive metal that does not occur naturally. Relatively few atoms have ever been made.
Uses
At present, it is only used in research.
Biological role
Rutherfordium has no known biological role.
Natural abundance
Rutherfordium is a transuranium element. It is created by bombarding californium-249 with carbon-12 nuclei.

#47 Re: Dark Discussions at Cafe Infinity » crème de la crème » 2025-10-18 17:02:23
2368) Ulf von Euler
Gist:
Work
Human and animal nervous systems consist of a large variety of cells with long nerve fibers. Small electrical currents and special chemical substances (neurotransmitters) are passed between cells through contacts (synapses). In 1947 Ulf von Euler discovered the neurotransmitter norepinephrine, which plays an important role in producing fight-or-flight signals. He subsequently showed that norepinephrine is formed and stored in packages, or vesicles, sent between neurons via synapses.
Summary
Ulf von Euler (born Feb. 7, 1905, Stockholm, Sweden—died March 9, 1983, Stockholm) was a Swedish physiologist who, with British biophysicist Sir Bernard Katz and American biochemist Julius Axelrod, received the 1970 Nobel Prize for Physiology or Medicine. All three were honoured for their independent study of the mechanics of nerve impulses.
Euler was the son of 1929 Nobel laureate Hans von Euler-Chelpin. After his graduation from the Karolinska Institute in Stockholm, Euler served on the faculty of the institute from 1930 to 1971. He joined the Nobel Committee for Physiology and Medicine in 1953 and was president of the Nobel Foundation for 10 years (1966–75).
Euler’s outstanding achievement was his identification of noradrenaline (norepinephrine), the key neurotransmitter (or impulse carrier) in the sympathetic nervous system. He also found that norepinephrine is stored within nerve fibres themselves. These discoveries laid the foundation for Axelrod’s determination of the role of the enzyme that inhibits its action, and the method of norepinephrine’s reabsorption by nerve tissues. Euler also discovered the hormones known as prostaglandins, which play active roles in stimulating human muscle contraction and in the regulation of the cardiovascular and nervous systems.
Details
Ulf Svante von Euler (7 February 1905 – 9 March 1983) was a Swedish physiologist and pharmacologist. He shared the Nobel Prize in Physiology or Medicine in 1970 for his work on neurotransmitters.
Life
Ulf Svante von Euler-Chelpin was born in Stockholm, the son of two noted scientists, Hans von Euler-Chelpin, a professor of chemistry, and Astrid Cleve, a professor of botany and geology. His father was German and the recipient of Nobel Prize for Chemistry in 1929, and his maternal grandfather was Per Teodor Cleve, Professor of Chemistry at the Uppsala University, and the discoverer of the chemical elements thulium and holmium. Von Euler-Chelpin studied medicine at the Karolinska Institute in 1922. At Karolinska, he worked under Robin Fåhraeus in blood sedimentation and rheology and did research work on the pathophysiology of vasoconstriction. He presented his doctoral thesis in 1930, and was appointed as assistant professor in pharmacology in the same year, with the support of G. Liljestrand. From 1930 to 1931, von Euler-Chelpin got a Rochester Fellowship to do his post-doctoral studies abroad. He studied in England with Sir Henry Dale in London and with I. de Burgh Daly in Birmingham, and then proceeded to the continent, studying with Corneille Heymans in Ghent, Belgium and with Gustav Embden in Frankfurt, Germany. Von Euler liked to travel, so he also worked and learned biophysics with Archibald Vivian Hill, again in London in 1934, and neuromuscular transmission with G. L. Brown in 1938. From 1946 to 1947, he worked with Eduardo Braun-Menéndez in the Instituto de Biología y Medicina Experimental in Buenos Aires, which was founded by Bernardo Houssay. His unerring instinct to work with important scientific leaders and fields was to be proved by the fact that Dale, Heymans, Hill and Houssay went to receive the Nobel prize in physiology or medicine.
In 1981, von Euler became a founding member of the World Cultural Council.
From 1930 to 1957, von Euler was married to Jane Anna Margarethe Sodenstierna (1905–2004). They had four children: Hans Leo, scientist administrator at the National Institutes of Health, Bethesda, Maryland, U.S.A.; Johan Christopher, anesthesiologist, Serafimer Hospital, Stockholm; Ursula Katarina, Ph.D., curator at The Royal Collections, The Royal Court, Stockholm, Sweden; and Marie Jane, Chemical Engineer, Melbourne, Australia. In 1958, von Euler married countess Dagmar Cronstedt, a radio broadcaster who had during the Second World War worked at Radio Königsberg, broadcasting German propaganda to neutral Sweden.
Research
His short stay as a postdoctoral student in Dale's laboratory was very fruitful: in 1931 he discovered with John H. Gaddum an important autopharmacological principle, substance P. After returning to Stockholm, von Euler pursued further this line of research, and successively discovered four other important endogenous active substances, prostaglandin, vesiglandin (1935), piperidine (1942) and noradrenaline (1946).
In 1939 von Euler was appointed full professor of physiology at the Karolinska Institute, where he remained until 1971. His early collaboration with Liljestrand had led to an important discovery, which was named the Euler–Liljestrand mechanism (a physiological arterial shunt in response to the decrease in local oxygenation of the lungs).
From 1946 on, however, when noradrenaline (abbreviated NA or NAd) was discovered, von Euler devoted most of his research work to this area. He and his group studied thoroughly its distribution and fate in biological tissues and in the nervous system in physiological and pathological conditions, and found that noradrenaline was produced and stored in nerve synaptic terminals in intracellular vesicles, a key discovery which changed dramatically the course of many researches in the field. In 1970 he was distinguished with the Nobel Prize for his work, jointly with Sir Bernard Katz and Julius Axelrod. Since 1953 he was very active in the Nobel Foundation, being a member of the Nobel Committee for Physiology or Medicine and chairman of the board since 1965. He also served as vice-president of the International Union of Physiological Sciences from 1965 to 1971. Among the many honorary titles and prizes he received in addition to the Nobel, were the Gairdner Prize (1961), the Jahre Prize (1965), the Stouffer Prize (1967), the Carl Ludwig Medaille (1953), the Schmiedeberg Plaquette (1969), La Madonnina (1970), many honorary doctorates from universities around the world, and the membership to several erudite, medical and scientific societies. He was elected a member of the American Philosophical Society in 1970, a member of both the American Academy of Arts and Sciences and the United States National Academy of Sciences in 1972, and a Foreign Member of the Royal Society (ForMemRS) in 1973.

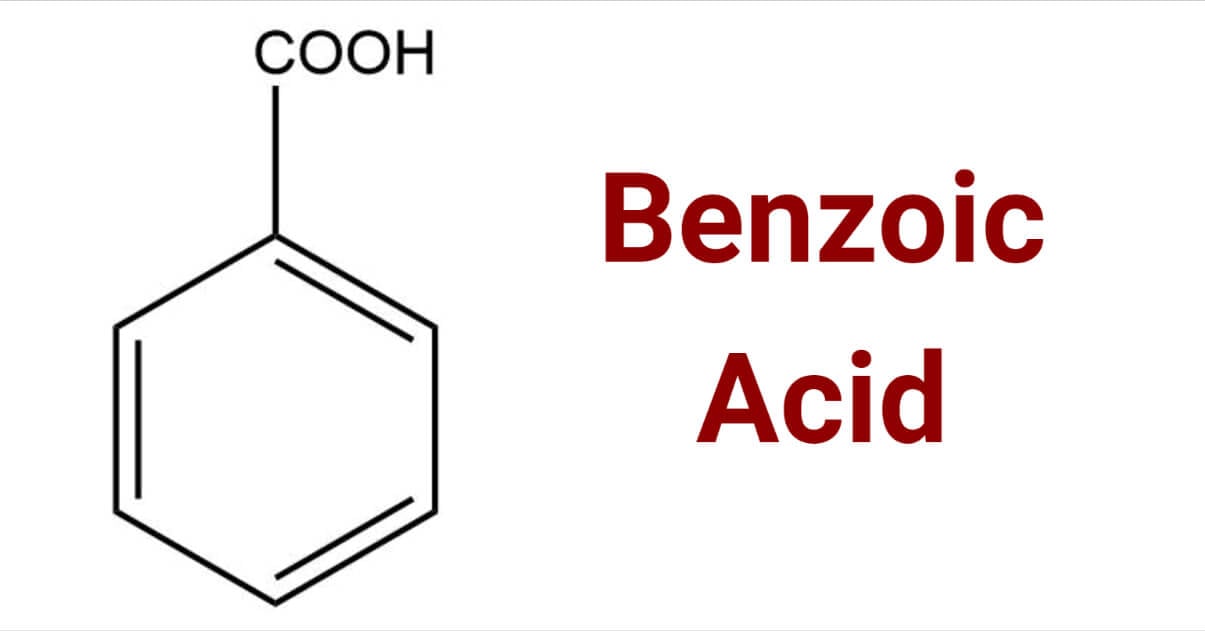
#48 This is Cool » Benzoic Acid » 2025-10-18 16:26:13
- Jai Ganesh
- Replies: 0
Benzoic Acid
Gist
Benzoic acid is a white crystalline organic compound with the formula C7H6O2 that has various uses, including as a food and cosmetic preservative and in the production of ointments, insect repellents, and dyes. It is a simple aromatic carboxylic acid, naturally found in some plants, and is known for its antiseptic properties. While useful, benzoic acid is a skin and eye irritant, and its combination with vitamin C can form benzene, a known carcinogen.
Benzoic acid is used as a preservative in food and drinks, an antifungal and antibacterial agent in medications and cosmetics, and a precursor for producing plastics and other chemicals. It's also found in products like toothpaste, insect repellents, and some cleaning supplies.
Summary
Benzoic acid is a white, crystalline organic compound belonging to the family of carboxylic acids, widely used as a food preservative and in the manufacture of various cosmetics, dyes, plastics, and insect repellents.
First described in the 16th century, benzoic acid exists in many plants; it makes up about 20 percent of gum benzoin, a vegetable resin. It was first prepared synthetically about 1860 from compounds derived from coal tar. It is commercially manufactured by the chemical reaction of toluene (a hydrocarbon obtained from petroleum) with oxygen at temperatures around 200° C (about 400° F) in the presence of cobalt and manganese salts as catalysts. Pure benzoic acid melts at 122° C (252° F) and is very slightly soluble in water.
Among the derivatives of benzoic acid are sodium benzoate, a salt used as a food preservative; benzyl benzoate, an ester used as a miticide; and benzoyl peroxide, used in bleaching flour and in initiating chemical reactions for preparing certain plastics.
Details
Benzoic acid is a white or colorless crystalline organic compound with the formula C6H5COOH, whose structure consists of a benzene ring (C6H6) with a carboxyl (−C(=O)OH) substituent. The benzoyl group is often abbreviated "Bz" (not to be confused with "Bn," which is used for benzyl), thus benzoic acid is also denoted as BzOH, since the benzoyl group has the formula –C6H5CO. It is the simplest aromatic carboxylic acid. The name is derived from gum benzoin, which was for a long time its only source.
Benzoic acid occurs naturally in many plants and serves as an intermediate in the biosynthesis of many secondary metabolites. Salts of benzoic acid are used as food preservatives. Benzoic acid is an important precursor for the industrial synthesis of many other organic substances. The salts and esters of benzoic acid are known as benzoates.
Production:
Industrial Preparation
Benzoic acid is produced commercially by partial oxidation of toluene with oxygen. The process is catalyzed by cobalt or manganese naphthenates. The process uses abundant materials, and proceeds in high yield.
The first industrial process involved the reaction of benzotrichloride (trichloromethyl benzene) with calcium hydroxide in water, using iron or iron salts as catalyst. The resulting calcium benzoate is converted to benzoic acid with hydrochloric acid. The product contains significant amounts of chlorinated benzoic acid derivatives. For this reason, benzoic acid for human consumption was obtained by dry distillation of gum benzoin. Food-grade benzoic acid is now produced synthetically.
Laboratory synthesis
Benzoic acid is cheap and readily available, so the laboratory synthesis of benzoic acid is mainly practiced for its pedagogical value. It is a common undergraduate preparation.
Benzoic acid can be purified by recrystallization from water because of its high solubility in hot water and poor solubility in cold water. The avoidance of organic solvents for the recrystallization makes this experiment particularly safe. This process usually gives a yield of around 65%.
By hydrolysis
Like other nitriles and amides, benzonitrile and benzamide can be hydrolyzed to benzoic acid or its conjugate base in acid or basic conditions.
From Grignard reagent
Bromobenzene can be converted to benzoic acid by "carboxylation" of the intermediate phenylmagnesium bromide. This synthesis offers a convenient exercise for students to carry out a Grignard reaction, an important class of carbon–carbon bond forming reaction in organic chemistry.
Oxidation of benzyl compounds
Benzyl alcohol and benzyl chloride and virtually all benzyl derivatives are readily oxidized to benzoic acid.
Additional Information
Benzoic acid, the simplest benzene-based carboxylic acid, has been known since the 16th century. One of its discoverers was the legendary clairvoyant Nostradamus. Its most common natural source is gum benzoin, a resin found in the bark of trees of the genus Styrax.
Most benzoic acid produced today is synthetic. Its first industrial synthesis was the hydrolysis of benzotrichloride to calcium benzoate, followed by acidification. This method has been completely displaced by the air oxidation of toluene, which avoids the problem of product contamination with chlorinated byproducts.
Many processed foods contain benzoic acid or one of its salts as a preservative. The acid inhibits the growth of bacteria, molds, and yeasts; it works best when the food has an acidic pH value. Benzoic acid also is often found in topical antifungal preparations.
#49 Dark Discussions at Cafe Infinity » Clubs Quotes - II » 2025-10-18 15:24:21
- Jai Ganesh
- Replies: 0
Clubs Quotes - II
1. There was a time when I was - after my very first record from Nashville, I thought I might not be one of those who actually really makes it, and I may end up back in Canada, just playing clubs. And that might - this might have just been it. - Shania Twain
2. I don't really go to clubs anymore. I'm actually quite settled. Living in Highgate with my dog and my husband and my daughter! I'm not a hell-raiser. But don't burst the bubble. Behind closed doors, for sure, I'm a hell-raiser. - Kate Moss
3. I have lived my dream and played at the finest of cricket grounds across the globe, and I want to thank the groundsmen, clubs, associations, and everyone who painstakingly prepare the arena for our performances. - Virender Sehwag
4. I was the first person from my family to enter films. So, everything connected with films was new to me, including fans and fan clubs. - Ajith Kumar
5. Myself and Cameron Lickle travel around North America in an RV and we conduct tennis clinics in clubs. - Mats Wilander.
#50 Jokes » Apple Jokes - III » 2025-10-18 14:57:18
- Jai Ganesh
- Replies: 0
I made an account just to tell this joke.
Aren't you going to give me an apple-ause?
* * *
Q: What do you call an apple that's been around the world?
A: Johnny Appleseed.
* * *
Q: What did the apple say to the almond?
A: You're Nuts!
* * *
Q: What did one maggot say to the other who was stuck in an apple?
A: Worm your way out of that one, then!
* * *
Q: What lives in apples and is an avid reader?
A: A bookworm !
* * *