Math Is Fun Forum
You are not logged in.
- Topics: Active | Unanswered
Pages: 1
#1 2025-02-16 16:38:53
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 50,584
Latent Heat
Latent Heat
Gist
Latent heat can be understood as hidden energy which is supplied or extracted to change the state of a substance without changing its temperature or pressure. This includes the latent heat of fusion (solid to liquid), the latent heat of vaporization (liquid to gas) and the latent heat of sublimation (solid to gas).
Summary
Latent heat is the energy absorbed or released by a substance during a change in its physical state (phase) that occurs without changing its temperature. The latent heat associated with melting a solid or freezing a liquid is called the heat of fusion; that associated with vaporizing a liquid or a solid or condensing a vapour is called the heat of vaporization. The latent heat is normally expressed as the amount of heat (in units of joules or calories) per mole or unit mass of the substance undergoing a change of state.
For example, when a pot of water is kept boiling, the temperature remains at 100 °C (212 °F) until the last drop evaporates, because all the heat being added to the liquid is absorbed as latent heat of vaporization and carried away by the escaping vapour molecules. Similarly, while ice melts, it remains at 0 °C (32 °F), and the liquid water that is formed with the latent heat of fusion is also at 0 °C. The heat of fusion for water at 0 °C is approximately 334 joules (79.7 calories) per gram, and the heat of vaporization at 100 °C is about 2,230 joules (533 calories) per gram. Because the heat of vaporization is so large, steam carries a great deal of thermal energy that is released when it condenses, making water an excellent working fluid for heat engines.
Latent heat arises from the work required to overcome the forces that hold together atoms or molecules in a material. The regular structure of a crystalline solid is maintained by forces of attraction among its individual atoms, which oscillate slightly about their average positions in the crystal lattice. As the temperature increases, these motions become increasingly violent until, at the melting point, the attractive forces are no longer sufficient to maintain the stability of the crystal lattice. However, additional heat (the latent heat of fusion) must be added (at constant temperature) in order to accomplish the transition to the even more-disordered liquid state, in which the individual particles are no longer held in fixed lattice positions but are free to move about through the liquid. A liquid differs from a gas in that the forces of attraction between the particles are still sufficient to maintain a long-range order that endows the liquid with a degree of cohesion. As the temperature further increases, a second transition point (the boiling point) is reached where the long-range order becomes unstable relative to the largely independent motions of the particles in the much larger volume occupied by a vapour or gas. Once again, additional heat (the latent heat of vaporization) must be added to break the long-range order of the liquid and accomplish the transition to the largely disordered gaseous state.
Latent heat is associated with processes other than changes among the solid, liquid, and vapour phases of a single substance. Many solids exist in different crystalline modifications, and the transitions between these generally involve absorption or evolution of latent heat. The process of dissolving one substance in another often involves heat; if the solution process is a strictly physical change, the heat is a latent heat. Sometimes, however, the process is accompanied by a chemical change, and part of the heat is that associated with the chemical reaction.
Details
Latent heat (also known as latent energy or heat of transformation) is energy released or absorbed, by a body or a thermodynamic system, during a constant-temperature process—usually a first-order phase transition, like melting or condensation.
Latent heat can be understood as hidden energy which is supplied or extracted to change the state of a substance without changing its temperature or pressure. This includes the latent heat of fusion (solid to liquid), the latent heat of vaporization (liquid to gas) and the latent heat of sublimation (solid to gas).
The term was introduced around 1762 by Scottish chemist Joseph Black. Black used the term in the context of calorimetry where a heat transfer caused a volume change in a body while its temperature was constant.
In contrast to latent heat, sensible heat is energy transferred as heat, with a resultant temperature change in a body.
Usage
Graph of temperature of phases of water heated from −100 °C to 200 °C – the dashed line example shows that melting and heating 1 kg of ice at −50 °C to water at 40 °C needs 600 kJ
The terms sensible heat and latent heat refer to energy transferred between a body and its surroundings, defined by the occurrence or non-occurrence of temperature change; they depend on the properties of the body. Sensible heat is sensed or felt in a process as a change in the body's temperature. Latent heat is energy transferred in a process without change of the body's temperature, for example, in a phase change (solid/liquid/gas).
Both sensible and latent heats are observed in many processes of transfer of energy in nature. Latent heat is associated with the change of phase of atmospheric or ocean water, vaporization, condensation, freezing or melting, whereas sensible heat is energy transferred that is evident in change of the temperature of the atmosphere or ocean, or ice, without those phase changes, though it is associated with changes of pressure and volume.
The original usage of the term, as introduced by Black, was applied to systems that were intentionally held at constant temperature. Such usage referred to latent heat of expansion and several other related latent heats. These latent heats are defined independently of the conceptual framework of thermodynamics.
When a body is heated at constant temperature by thermal radiation in a microwave field for example, it may expand by an amount described by its latent heat with respect to volume or latent heat of expansion, or increase its pressure by an amount described by its latent heat with respect to pressure.
Latent heat is energy released or absorbed by a body or a thermodynamic system during a constant-temperature process. Two common forms of latent heat are latent heat of fusion (melting) and latent heat of vaporization (boiling). These names describe the direction of energy flow when changing from one phase to the next: from solid to liquid, and liquid to gas.
In both cases the change is endothermic, meaning that the system absorbs energy. For example, when water evaporates, an input of energy is required for the water molecules to overcome the forces of attraction between them and make the transition from water to vapor.
If the vapor then condenses to a liquid on a surface, then the vapor's latent energy absorbed during evaporation is released as the liquid's sensible heat onto the surface.
The large value of the enthalpy of condensation of water vapor is the reason that steam is a far more effective heating medium than boiling water, and is more hazardous.
Meteorology
In meteorology, latent heat flux is the flux of energy from the Earth's surface to the atmosphere that is associated with evaporation or transpiration of water at the surface and subsequent condensation of water vapor in the troposphere. It is an important component of Earth's surface energy budget. Latent heat flux has been commonly measured with the Bowen ratio technique, or more recently since the mid-1900s by the eddy covariance method.
Additional Information
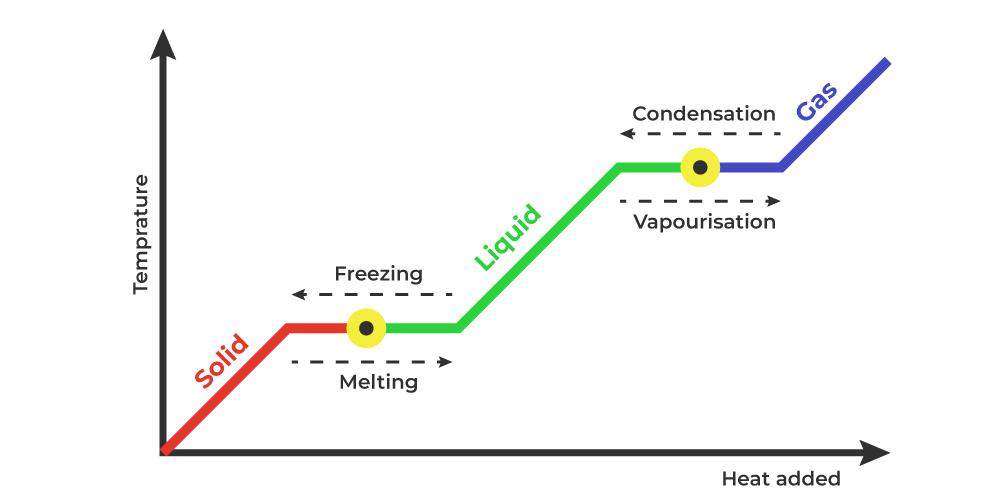
The heat energy given to any solid substance increases its temperature until it reaches its melting point. At the melting point, the heat energy supplied does not change the temperature of the substance but the energy absorbed is required to change the phase of the substance. It changes from solid to liquid and the heat supplied is called the latent heat of fusion.
Similarly, the heat energy given to the liquid substance increases its temperature until it reaches its boiling point. At boiling point, the heat energy supplied does not change the temperature of the liquid but the energy absorbed is required to change the phase of the substance. It changes liquid to gas and the heat supplied is called the latent heat of vaporization.
Latent heat is called by various names depending upon the phase it changes.
Solid-to-Liquid: Latent Heat of Fusion
Liquid-to-Gas: Latent Heat of Vaporization
Solid-to-Gas: Latent Heat of Sublimation
Formula for Latent Heat
The formula for calculating the latent heat is given:
Q = ML
where,
L is Latent Heat
Q is Amount of Heat Released or Absorbed
M is Mass of Substance.
The heat Q that must be supplied or withdrawn for an object of mass M to change phases is stated in this equation. Latent heat is denoted by the letter L.
Unit of Latent Heat
Latent heat is nothing but the heat required per kg to change the phase of any substance. Its unit is J⁄Kg.
Types of Latent Heat
Depending on the state of change in the matter, latent heat is generally categorized into three categories, which include
* Latent Heat of Fusion
* Latent Heat of Vaporization
* Latent Heat of Sublimation
Latent Heat of Fusion
The heat consumed or emitted when matter melts, changing state from solid to fluid-structure at a constant temperature, is known as latent heat of fusion.
The heat energy required to transform a solid into a fluid at atmospheric pressure is the latent heat of fusion while the temperature remains constant during the operation. The enthalpy shift of any solid as it melts is known as the latent heat of fusion.
When the heat of fusion is expressed in terms of a unit of mass, it is referred to as the specific heat of fusion, whereas the molar heat of fusion refers to the enthalpy change per mole of material.
The inward energy of the fluid state is greater than that of the solid state. This means that energy must be delivered to the solid in order to dissolve it, and energy must be removed from a fluid when it solidifies because the particles in the fluid have a more fragile intermolecular force and so have larger potential energy (a sort of bond-separation energy for intermolecular powers).
Latent Heat of Vaporization
The heat consumed or expelled as matter disintegrates, changing phase from fluid to gas at a constant temperature, is known as latent heat of vaporization.
The heat of water vaporization is the most well-known. The heat of vaporization is defined as the amount of heat required to convert 1 g of a fluid into a fume without changing the fluid’s temperature. After a substance’s temperature has reached a breaking point, latent heat is necessary to change the state of the substance from fluid to gas at this point.
It’s worth noting that latent heat is associated with no change in temperature but a change in form. The disappearance of water has an obvious cooling effect, whereas the accumulation has a warming effect, due to the high heat of vaporization.
Latent Heat of Sublimation
When exposed to the open air, some chemicals, such as naphthalene, convert straight from solid to gas. The latent heat of sublimation is the amount of heat required for a substance to change from a solid to a gaseous state or the amount of heat required to remove heat from a gaseous material to turn into its solid state.
Specific Latent Heat
Specific latent heat is defined as the heat required to change the phase of one kg of any substance. It is similar to Latent Heat but the amount of substance is fixed to one kg.
The formula for specific latent heat is:
L = Q/m
where,
L is specific latent heat
Q is heat absorbed or released
m is mass of a substance.
Sensible Heat
The heat transferred by a body or thermodynamic system that affects the temperature of the body or system, as well as some macroscopic variables of the body or system except pressure or volume, is called sensible heat.
Reasonable Heat and Meteorology
Meteorologists use reasonable heat to study the various parameters of the climate and predict the various natural events. When latent heat is released into the atmosphere it affects the climate. The reasonable heat absorbed and released into the atmosphere interacts with the hot or cold air and changes the climatic condition. conceivably delivering an extreme climate.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
Pages: 1