Math Is Fun Forum
You are not logged in.
- Topics: Active | Unanswered
Pages: 1
#1 2025-04-22 17:36:06
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 50,582
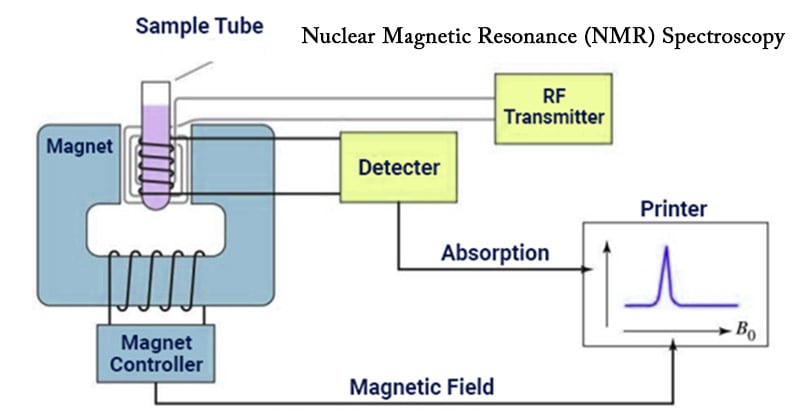
Nuclear Magnetic Resonance
Nuclear Magnetic Resonance
Gist
Nuclear Magnetic Resonance (NMR) is a physical phenomenon and a spectroscopic technique that examines the magnetic properties of atomic nuclei. It's widely used in chemistry, biology, and materials science to study molecular structures, chemical reactions, and physical properties of materials.
Summary
Nuclear magnetic resonance (NMR) is a powerful technique which allows the study of the magnetic properties of an atom's nucleus It involves placing nuclei within an external magnetic field enabling thus them to undergo precession The 'resonance' part of the names implies the fact that a second (usually perpendicular) radiofrequency pulse tuned to the precessional frequency of target nuclei allows them to absorb and then emit electromagnetic energy
Nuclear magnetic resonance is exploited in a number disciplines and fields, including magnetic resonance imaging, molecular physics and chemistry An offshoot of this technique, nuclear magnetic resonance spectroscopy allows precise analysis of molecular constitution of a chemical microenvironment.
Details
Nuclear magnetic resonance (NMR) is a physical phenomenon in which nuclei in a strong constant magnetic field are disturbed by a weak oscillating magnetic field (in the near field[1]) and respond by producing an electromagnetic signal with a frequency characteristic of the magnetic field at the nucleus. This process occurs near resonance, when the oscillation frequency matches the intrinsic frequency of the nuclei, which depends on the strength of the static magnetic field, the chemical environment, and the magnetic properties of the isotope involved; in practical applications with static magnetic fields up to ca. 20 tesla, the frequency is similar to VHF and UHF television broadcasts (60–1000 MHz). NMR results from specific magnetic properties of certain atomic nuclei. High-resolution nuclear magnetic resonance spectroscopy is widely used to determine the structure of organic molecules in solution and study molecular physics and crystals as well as non-crystalline materials. NMR is also routinely used in advanced medical imaging techniques, such as in magnetic resonance imaging (MRI). The original application of NMR to condensed matter physics is nowadays mostly devoted to strongly correlated electron systems. It reveals large many-body couplings by fast broadband detection and should not be confused with solid state NMR, which aims at removing the effect of the same couplings by Magic Angle Spinning techniques.
The most commonly used nuclei are 1H and 13C , although isotopes of many other elements, such as 19F, 31P , and 29Si , can be studied by high-field NMR spectroscopy as well. In order to interact with the magnetic field in the spectrometer, the nucleus must have an intrinsic angular momentum and nuclear magnetic dipole moment. This occurs when an isotope has a nonzero nuclear spin, meaning an odd number of protons and/or neutrons (see Isotope). Nuclides with even numbers of both have a total spin of zero and are therefore not NMR-active.
In its application to molecules the NMR effect can be observed only in the presence of a static magnetic field. However, in the ordered phases of magnetic materials, very large internal fields are produced at the nuclei of magnetic ions (and of close ligands), which allow NMR to be performed in zero applied field. Additionally, radio-frequency transitions of nuclear spin I > 1/2
with large enough electric quadrupolar coupling to the electric field gradient at the nucleus may also be excited in zero applied magnetic field (nuclear quadrupole resonance).
In the dominant chemistry application, the use of higher fields improves the sensitivity of the method (signal-to-noise ratio scales approximately as the power of 3/2
with the magnetic field strength) and the spectral resolution. Commercial NMR spectrometers employing liquid helium cooled superconducting magnets with fields of up to 28 Tesla have been developed and are widely used.
It is a key feature of NMR that the resonance frequency of nuclei in a particular sample substance is usually directly proportional to the strength of the applied magnetic field. It is this feature that is exploited in imaging techniques; if a sample is placed in a non-uniform magnetic field then the resonance frequencies of the sample's nuclei depend on where in the field they are located. This effect serves as the basis of magnetic resonance imaging.
The principle of NMR usually involves three sequential steps:
* The alignment (polarization) of the magnetic nuclear spins in an applied, constant magnetic field B0.
* The perturbation of this alignment of the nuclear spins by a weak oscillating magnetic field, usually referred to as a radio frequency (RF) pulse. The oscillation frequency required for significant perturbation is dependent upon the static magnetic field (B0) and the nuclei of observation.
* The detection of the NMR signal during or after the RF pulse, due to the voltage induced in a detection coil by precession of the nuclear spins around B0. After an RF pulse, precession usually occurs with the nuclei's Larmor frequency and, in itself, does not involve transitions between spin states or energy levels.
The two magnetic fields are usually chosen to be perpendicular to each other as this maximizes the NMR signal strength. The frequencies of the time-signal response by the total magnetization (M) of the nuclear spins are analyzed in NMR spectroscopy and magnetic resonance imaging. Both use applied magnetic fields (B0) of great strength, usually produced by large currents in superconducting coils, in order to achieve dispersion of response frequencies and of very high homogeneity and stability in order to deliver spectral resolution, the details of which are described by chemical shifts, the Zeeman effect, and Knight shifts (in metals). The information provided by NMR can also be increased using hyperpolarization, and/or using two-dimensional, three-dimensional and higher-dimensional techniques.
NMR phenomena are also utilized in low-field NMR, NMR spectroscopy and MRI in the Earth's magnetic field (referred to as Earth's field NMR), and in several types of magnetometers.
Additional Information
Nuclear magnetic resonance (NMR), selective absorption of very high-frequency radio waves by certain atomic nuclei that are subjected to an appropriately strong stationary magnetic field. This phenomenon was first observed in 1946 by the physicists Felix Bloch and Edward M. Purcell independently of each other. Nuclei in which at least one proton or one neutron is unpaired act like tiny magnets, and a strong magnetic field exerts a force that causes them to precess in somewhat the same way that the axes of spinning tops trace out cone-shaped surfaces while they precess in the Earth’s gravitational field. When the natural frequency of the precessing nuclear magnets corresponds to the frequency of a weak external radio wave striking the material, energy is absorbed from the radio wave. This selective absorption, called resonance, may be produced either by tuning the natural frequency of the nuclear magnets to that of a weak radio wave of fixed frequency or by tuning the frequency of the weak radio wave to that of the nuclear magnets (determined by the strong constant external magnetic field).
Nuclear magnetic resonance is used to measure nuclear magnetic moments, the characteristic magnetic behaviour of specific nuclei. Because these values are significantly modified by the immediate chemical environment, however, NMR measurements provide information about the molecular structure of various solids and liquids.
By the early 1980s nuclear magnetic resonance techniques had begun to be used in medicine to visualize soft tissues of the body. This application of NMR, called magnetic resonance imaging (MRI), presented a hazard-free, noninvasive way to generate visual images of thin slices of the body by measuring the nuclear magnetic moments of ordinary hydrogen nuclei in the body’s water and lipids (fats). NMR images show great sensitivity in differentiating between normal tissues and diseased or damaged ones. By the late 1980s MRI had proved superior to most other imaging techniques in providing images of the brain, heart, liver, kidneys, spleen, pancreas, breast, and other organs. MRI provides relatively high-contrast, variable-toned images that can show tumours, blood-starved tissues, and neural plaques resulting from multiple sclerosis. The technique presents no known health hazards, but it cannot be used on individuals who have cardiac pacemakers or certain other metal-containing devices implanted in their bodies.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
Pages: 1