Math Is Fun Forum
You are not logged in.
- Topics: Active | Unanswered
Pages: 1
#1 2024-02-06 18:13:03
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,247
Electron
Electron
Gist
An electron is an elementary particle consisting of a charge of negative electricity equal to about 1.602 × {10}^{-19} coulomb and having a mass when at rest of about 9.109 × {10}^{-31} kilogram or about ¹/₁₈₃₆ that of a proton.
Summary
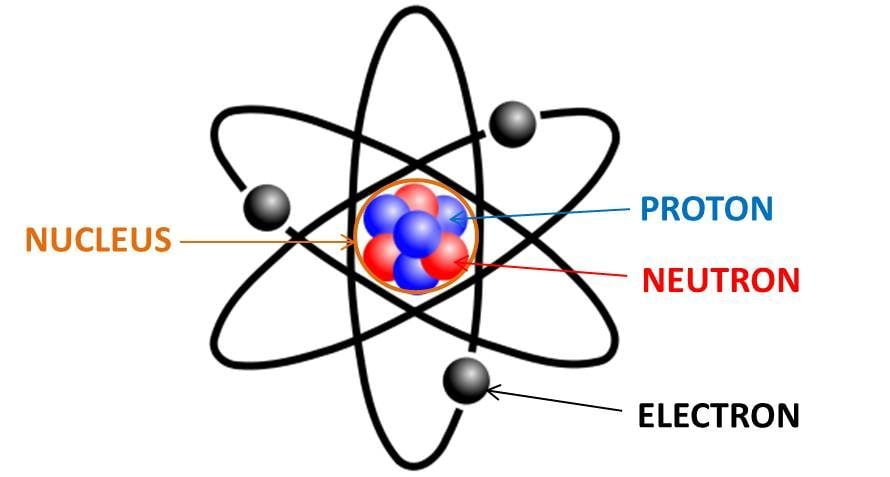
Electron, one of the three basic subatomic particles—along with protons and neutrons—that make up atoms, the basic building blocks of all matter and chemistry. The negatively charged electrons circle an atom’s central nucleus, which is formed by positively charged protons and the electrically neutral particles called neutrons. (The nucleus of the ordinary hydrogen atom is an exception, containing only one proton and no neutrons.) Like opposite ends of a magnet that attract one another, the negative electrons are attracted to a positive force, which binds them to the nucleus. The nucleus is small and dense compared with the electrons, which are the lightest charged particles in nature. The electrons circle the nucleus in orbital paths called shells, each of which holds only a certain number of electrons.
The electron was discovered in 1897 by the English physicist J.J. Thomson during investigations of cathode rays. His discovery of electrons, which he initially called corpuscles, played a pivotal role in revolutionizing knowledge of atomic structure. Under ordinary conditions electrons are bound to the positively charged nuclei of atoms by the attraction between opposite electric charges. In a neutral atom the number of electrons is identical to the number of positive charges on the nucleus. Any atom, however, may have more or fewer electrons than positive charges and thus be negatively or positively charged as a whole; these charged atoms are known as ions. Not all electrons are associated with atoms; some occur in a free state with ions in the form of matter known as plasma.
Within any given atom, electrons move about the nucleus in an orderly arrangement of orbitals, the attraction between electrons and nucleus overcoming repulsion among the electrons that would otherwise cause them to fly apart. These orbitals are organized in concentric shells proceeding outward from the nucleus with an increasing number of subshells. The electrons in orbitals closest to the nucleus are held most tightly; those in the outermost orbitals are shielded by intervening electrons and are the most loosely held by the nucleus. As the electrons move about within this structure, they form a diffuse cloud of negative charge that occupies nearly the entire volume of the atom. The arrangement of electrons in orbitals and shells around the nucleus is referred to as the electronic configuration of the atom. This electronic configuration determines not only the size of an individual atom but also the chemical activity of the atom. The classification of elements within groups of similar elements in the periodic table, for example, is based on the similarity in their electron structures.
Within the field of particle physics, there are two ways of classifying electrons. The electron is a fermion, a type of particle named after the Fermi-Dirac statistics that describe its behaviour. All fermions are characterized by half-integer values of their spin, where spin corresponds to the intrinsic angular momentum of the particle. The concept of spin is embodied in the wave equation for the electron formulated by P.A.M. Dirac. The Dirac wave equation also predicts the existence of the antimatter counterpart of the electron, the positron. Within the fermion group of subatomic particles, the electron can be further classified as a lepton. A lepton is a subatomic particle that reacts only by the electromagnetic, weak, and gravitational forces; it does not respond to the short-range strong force that acts between quarks and binds protons and neutrons in the atomic nucleus.
The lightest stable subatomic particle known, the electron carries a negative charge of 1.602176634 × {10}^{-19} coulomb, which is considered the basic unit of electric charge. The rest mass of the electron is 9.1093837015 × {10}^{-31} kg, which is only 1/1,836 the mass of a proton. An electron is therefore considered nearly massless in comparison with a proton or a neutron, and the electron mass is not included in calculating the mass number of an atom.
Details
The electron is a subatomic particle with a negative one elementary electric charge. Electrons belong to the first generation of the lepton particle family, and are generally thought to be elementary particles because they have no known components or substructure. The electron's mass is approximately 1/1836 that of the proton. Quantum mechanical properties of the electron include an intrinsic angular momentum (spin) of a half-integer value, expressed in units of the reduced Planck constant, ħ. Being fermions, no two electrons can occupy the same quantum state, per the Pauli exclusion principle. Like all elementary particles, electrons exhibit properties of both particles and waves: They can collide with other particles and can be diffracted like light. The wave properties of electrons are easier to observe with experiments than those of other particles like neutrons and protons because electrons have a lower mass and hence a longer de Broglie wavelength for a given energy.
Electrons play an essential role in numerous physical phenomena, such as electricity, magnetism, chemistry, and thermal conductivity; they also participate in gravitational, electromagnetic, and weak interactions. Since an electron has charge, it has a surrounding electric field; if that electron is moving relative to an observer, the observer will observe it to generate a magnetic field. Electromagnetic fields produced from other sources will affect the motion of an electron according to the Lorentz force law. Electrons radiate or absorb energy in the form of photons when they are accelerated.
Laboratory instruments are capable of trapping individual electrons as well as electron plasma by the use of electromagnetic fields. Special telescopes can detect electron plasma in outer space. Electrons are involved in many applications, such as tribology or frictional charging, electrolysis, electrochemistry, battery technologies, electronics, welding, cathode-ray tubes, photoelectricity, photovoltaic solar panels, electron microscopes, radiation therapy, lasers, gaseous ionization detectors, and particle accelerators.
Interactions involving electrons with other subatomic particles are of interest in fields such as chemistry and nuclear physics. The Coulomb force interaction between the positive protons within atomic nuclei and the negative electrons without allows the composition of the two known as atoms. Ionization or differences in the proportions of negative electrons versus positive nuclei changes the binding energy of an atomic system. The exchange or sharing of the electrons between two or more atoms is the main cause of chemical bonding.
In 1838, British natural philosopher Richard Laming first hypothesized the concept of an indivisible quantity of electric charge to explain the chemical properties of atoms. Irish physicist George Johnstone Stoney named this charge 'electron' in 1891, and J. J. Thomson and his team of British physicists identified it as a particle in 1897 during the cathode-ray tube experiment.
Electrons participate in nuclear reactions, such as nucleosynthesis in stars, where they are known as beta particles. Electrons can be created through beta decay of radioactive isotopes and in high-energy collisions, for instance, when cosmic rays enter the atmosphere. The antiparticle of the electron is called the positron; it is identical to the electron, except that it carries electrical charge of the opposite sign. When an electron collides with a positron, both particles can be annihilated, producing gamma ray photons.
Additional Information
An electron is a negatively charged subatomic particle that can be either bound to an atom or free (not bound). An electron that is bound to an atom is one of the three primary types of particles within the atom -- the other two are protons and neutrons.
Together, protons and electrons form an atom's nucleus. A proton has a positive charge that counters the electron's negative charge. When an atom has the same number of protons and electrons, it is in a neutral state.
Electrons are unique from the other particles in multiple ways. They exist outside of the nucleus, are significantly smaller in mass and exhibit both wave-like and particle-like characteristics. An electron is also an elementary particle, which means that it is not made up of smaller components. Protons and neutrons are thought to be made up of quarks, so they are not elementary particles.
Shells, subshells and orbitals
In the early days of atomic study, scientists believed that an atom's electrons circled the nucleus in spherical orbits at specific distances, much like planets circle a sun. In this model -- referred to as the Bohr model -- the orbits furthest from the nucleus contain the greatest amount of energy. When an electron jumps from a higher energy orbit to a lower energy orbit, the atom releases electromagnetic radiation.
The Bohr model is no longer thought to be accurate, particularly as it pertains to how the electrons orbit the nucleus. While the model can still be useful in understanding the basics of electron distribution and different energy levels, it fails to consider the complexity of that distribution and how electrons inhabit the space around the nucleus, according to current quantum theory.
Electron movement is determined by calculating the probability of finding electrons in specific regions within the space that surrounds the atom's nucleus -- rather than by assuming fixed trajectories. The mathematically defined regions are based on three structural patterns:
* Shells. The concept of a shell originates with the Bohr model, although the theory around shells has evolved. Physicists now believe that a shell is a region of probability surrounding the nucleus. An atom can contain up to seven electron shells, depending on the type of atom. The shells exist at different levels around the nucleus. Shells furthest from the nucleus have the highest amounts of energy and those nearest have the lowest. Each shell is limited to a specific number of electrons, depending on its level and the configuration. A shell can contain one or more subshells, and a subshell can contain one or more orbitals.
* Subshells. A subshell is a collection of one or more orbitals of a specific type. There are four types of orbitals and subsequently four types of subshells -- designated as s, p, d and f, depending on their orbitals. An s subshell contains one s orbital, a p subshell contains three p orbitals, a d subshell contains five d orbitals, and an f subshell contains seven f orbitals. It has also been theorized that an atom can support a g subshell that contains nine g orbitals.
* Orbitals. An orbital is a specifically shaped region of space around the nucleus where an electron is most likely found. In other words, it is the region with the highest probability (over 90%) of containing the electron as it travels around the nucleus. An orbital might be shaped like a sphere (s orbital), a dumbbell (p orbital) or a more complex shape (d and f orbitals). Whatever its shape, an orbital can include a maximum of two electrons.
An atom's shells are numbered consecutively, starting at the nucleus and working out. A shell's number is often referred to as its n value. For example, the third shell might be referred to as n=3 or 3n. Letters are also sometimes used to refer to the shells. These include K, L, M, N, O, P and Q, again starting from the nucleus and working out. For instance, the third shell might be referred to as the M shell or 3m.
Each shell contains one or more specific types of subshells, which determine the maximum number of electrons that the shell can contain. For example, the first shell (K) contains a single s subshell that includes only one s orbital. As a result, the maximum number electrons that the shell can contain is two. This means that an atom that has only a K shell is limited to two electrons. Only two elements, hydrogen and helium, have a single shell. Hydrogen contains only one electron and helium contains two.
The subshell/orbital configuration varies from one shell to the next, growing more complex until the fifth shell, at which point the complexity starts to taper off. For instance, the second shell (L) includes an s subshell and a p subshell. The s subshell contains one s orbital, and the p subshell contains three p orbitals. This means the shell can support up to eight electrons.
However, an atom with an L shell also contains a K shell. In fact, the L shell will start filling up after the K shell is filled. This means that an atom with an L shell can support up to 10 electrons because of the presence of both the K and L shells. For example, lithium and neon contain both K and L shells. A lithium atom has only three electrons, two in the K shell and one in the L shell, but a neon atom has 10 electrons, two in the K shell and eight in the L shell.
In general, this same pattern continues for all seven shells, with the inner shells filling up with electrons before the outer shells. However, this is only a tendency. Electrons gravitate toward the most stable configuration, which is usually the inner shells, but it's also possible for an outer shell to start filling up with electrons before the lower shell is completely full.
Regardless of the order in which shells fill with electrons, the shells themselves determine the maximum number of electrons they can support based on their subshells and orbitals. All but the first shell includes a p subshell, only the third through sixth shells contain d subshells, and only the fourth and fifth contain f subshells. All seven shells include an s subshell.
Electrons and electricity
In electrical conductors, current flows as a result of electrons jumping from atom to atom as they move from negative to positive electric poles. In semiconductor materials, current also results from electron movement, however, the movement is based on electron deficiencies in atoms. An electron-deficient atom in a semiconductor is called a hole. In this case, the current moves from positive to negative electric poles.
The charge of a single electron is referred to as the unit electrical charge. It carries a negative charge that is equal to but opposite the positive charge on a proton or hole. However, the amount of electrical charge is usually not measured on a single electron because that amount is so small.
Instead, the standard unit of electrical charge is the coulomb (symbolized by C). A coulomb contains about 6.24 x {10}^{18} electrons. An electron's charge (symbolized by e) is about 1.60 x {10}^{-19} C. The mass of an electron at rest (symbolized by me) is approximately 9.11 x {10}^{-31} kilograms (kg). If electrons are accelerated to nearly the speed of light, as in a particle accelerator, they will have greater mass because of relativistic effects.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
Pages: 1