Math Is Fun Forum
You are not logged in.
- Topics: Active | Unanswered
- Index
- » Science HQ
- » Sodium
Pages: 1
#1 2025-06-16 17:57:10
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,052
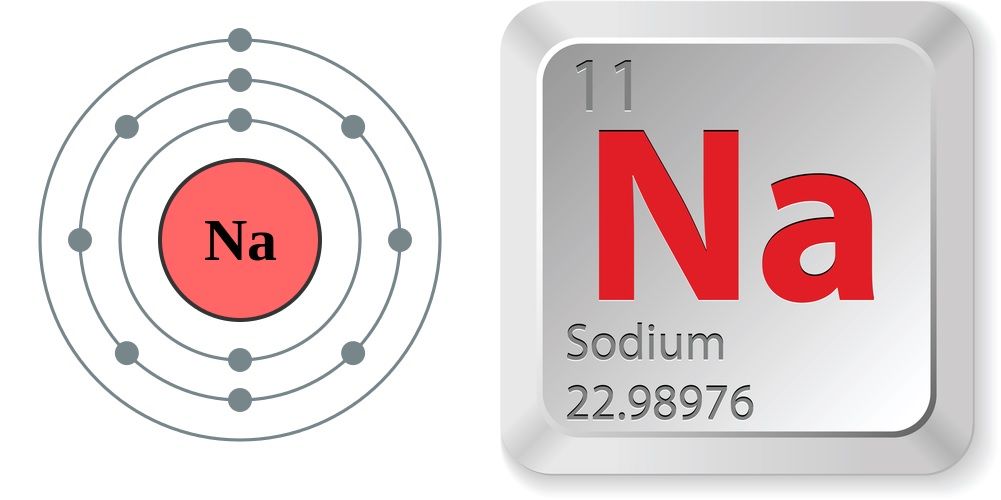
Sodium
Sodium
Gist
Sodium, with the symbol Na, is a chemical element, specifically a soft, silvery-white, highly reactive alkali metal. It's atomic number is 11 and its atomic mass is approximately 23. Sodium is essential for maintaining fluid balance, nerve and muscle function, and blood pressure regulation in the body. It is found abundantly in nature, primarily as sodium chloride (common table salt).
Summary
Sodium is a chemical element; it has symbol Na (from Neo-Latin natrium) and atomic number 11. It is a soft, silvery-white, highly reactive metal. Sodium is an alkali metal, being in group 1 of the periodic table. Its only stable isotope is 23Na. The free metal does not occur in nature and must be prepared from compounds. Sodium is the sixth most abundant element in the Earth's crust and exists in numerous minerals such as feldspars, sodalite, and halite (NaCl). Many salts of sodium are highly water-soluble: sodium ions have been leached by the action of water from the Earth's minerals over eons, and thus sodium and chlorine are the most common dissolved elements by weight in the oceans.
Sodium was first isolated by Humphry Davy in 1807 by the electrolysis of sodium hydroxide. Among many other useful sodium compounds, sodium hydroxide (lye) is used in soap manufacture, and sodium chloride (edible salt) is a de-icing agent and a nutrient for animals including humans.
Sodium is an essential element for all animals and some plants. Sodium ions are the major cation in the extracellular fluid (ECF) and as such are the major contributor to the ECF osmotic pressure. Animal cells actively pump sodium ions out of the cells by means of the sodium–potassium pump, an enzyme complex embedded in the cell membrane, in order to maintain a roughly ten-times higher concentration of sodium ions outside the cell than inside. In nerve cells, the sudden flow of sodium ions into the cell through voltage-gated sodium channels enables transmission of a nerve impulse in a process called the action potential.
Details
Sodium (Na) is a chemical element of the alkali metal group (Group 1 [Ia]) of the periodic table. Sodium is a very soft silvery-white metal. Sodium is the most common alkali metal and the sixth most abundant element on Earth, comprising 2.8 percent of Earth’s crust. It occurs abundantly in nature in compounds, especially common salt—sodium chloride (NaCl)—which forms the mineral halite and constitutes about 80 percent of the dissolved constituents of seawater.
Element Properties
atomic number : 11
atomic weight : 22.9898
melting point : 97.81 °C (208 °F)
boiling poin : 882.9 °C (1,621 °F)
specific gravity : 0.971 (20 °C)
oxidation states : +1, −1 (rare)
Properties and production
Because sodium is extremely reactive, it never occurs in the free state in Earth’s crust. In 1807 Sir Humphry Davy became the first to prepare sodium in its elemental form, applying electrolysis to fused sodium hydroxide (NaOH). Sodium is an important constituent of a number of silicate materials, such as feldspars and micas. There are huge deposits of rock salt in various parts of the world, and sodium nitrate deposits exist in Chile and Peru. The sodium content of the sea is approximately 1.05 percent, corresponding to a concentration of approximately 3 percent of sodium halides. Sodium has been identified in both the atomic and ionic forms in the spectra of stars, including the Sun, and the interstellar medium. Analysis of meteorites indicates that the silicate material present has an average content of approximately 4.6 atoms of sodium for every 100 atoms of silicon.
Lighter than water, sodium can be cut with a knife at room temperature but is brittle at low temperatures. It conducts heat and electricity easily and exhibits the photoelectric effect (emission of electrons when exposed to light) to a marked degree.
Sodium is by far the most commercially important alkali metal. Most processes for the production of sodium involve the electrolysis of molten sodium chloride. Inexpensive and available in tank-car quantities, the element is used to produce gasoline additives, polymers such as nylon and synthetic rubber, pharmaceuticals, and a number of metals such as tantalum, titanium, and silicon. It is also widely used as a heat exchanger and in sodium-vapour lamps. The yellow colour of the sodium-vapour lamp and the sodium flame (the basis of an analytical test for sodium) is identified with two prominent lines in the yellow portion of the light spectrum.
Significant uses
Two of the earliest uses of metallic sodium were in the manufacture of sodium cyanide and sodium peroxide. Significant quantities were used in the manufacture of tetraethyl lead as a gasoline additive, a market that disappeared with the advent of unleaded gasoline. Substantial amounts of sodium are used in the manufacture of sodium alkyl sulfates as the principal ingredient in synthetic detergents.
Sodium also is used as a starting material in the manufacture of sodium hydride (NaH) and sodium borohydride (NaBH4). In addition, sodium is employed in the production of dyes and dye intermediates, in the synthesis of perfumes, and in a wide variety of organic reductions. It is used in the purification of hydrocarbons and in the polymerization of unsaturated hydrocarbons. In many organic applications, sodium is used in the form of dispersions in hydrocarbon liquid media.
Molten sodium is an excellent heat-transfer fluid, and, because of this property, it has found use as coolant in liquid-metal fast breeder reactors. Sodium is used extensively in metallurgy as a deoxidant and as a reducing agent for the preparation of calcium, zirconium, titanium, and other transition metals. Commercial production of titanium involves reduction of titanium tetrachloride (TiCl4) with sodium. The products are metallic Ti and NaCl.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
Pages: 1
- Index
- » Science HQ
- » Sodium