Math Is Fun Forum
You are not logged in.
- Topics: Active | Unanswered
- Index
- » Science HQ
- » Rubidium
Pages: 1
#1 2025-06-24 15:22:27
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,052
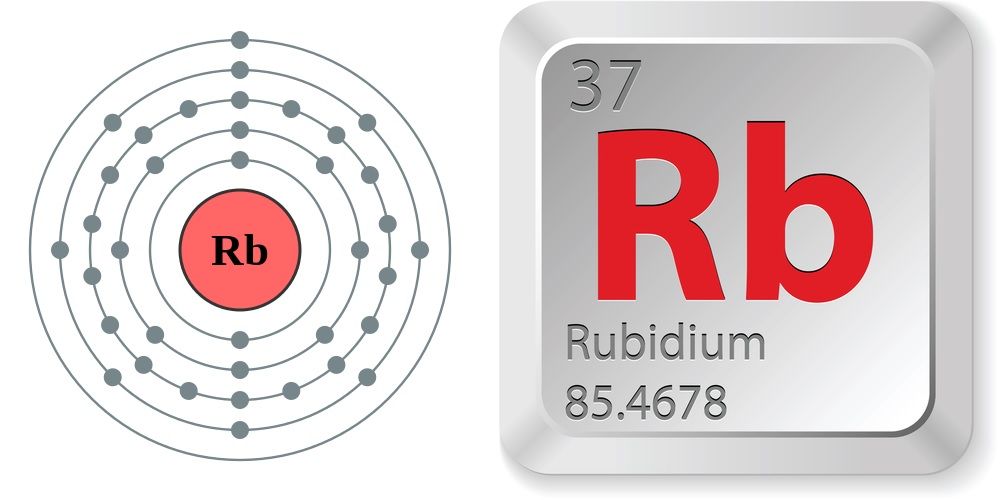
Rubidium
Rubidium
Gist
Rubidium is a soft, silvery-white alkali metal with the atomic number 37. It's known for its high reactivity, especially with air and water, and its ability to produce a violet-yellow flame color. Rubidium is relatively rare, but it has several applications, including use in specialty glasses for fiber optics, as a component in photocells, and in fireworks. It's also used in atomic clocks and in experimental quantum computing.
Rubidium and its salts have few commercial uses. The metal is used in the manufacture of photocells and in the removal of residual gases from vacuum tubes. Rubidium salts are used in glasses and ceramics and in fireworks to give them a purple colour.
Summary
Rubidium is a chemical element; it has symbol Rb and atomic number 37. It is a very soft, whitish-grey solid in the alkali metal group, similar to potassium and caesium. Rubidium is the first alkali metal in the group to have a density higher than water. On Earth, natural rubidium comprises two isotopes: 72% is a stable isotope 85Rb, and 28% is slightly radioactive 87Rb, with a half-life of 48.8 billion years – more than three times as long as the estimated age of the universe.
German chemists Robert Bunsen and Gustav Kirchhoff discovered rubidium in 1861 by the newly developed technique, flame spectroscopy. The name comes from the Latin word rubidus, meaning deep red, the color of its emission spectrum. Rubidium's compounds have various chemical and electronic applications. Rubidium metal is easily vaporized and has a convenient spectral absorption range, making it a frequent target for laser manipulation of atoms. Rubidium is not a known nutrient for any living organisms. However, rubidium ions have similar properties and the same charge as potassium ions, and are actively taken up and treated by animal cells in similar ways.
Characteristics:
Physical properties
Rubidium is a very soft, ductile, silvery-white metal. It has a melting point of 39.3 °C (102.7 °F) and a boiling point of 688 °C (1,270 °F). It forms amalgams with mercury and alloys with gold, iron, caesium, sodium, and potassium, but not lithium (despite rubidium and lithium being in the same periodic group). Rubidium and potassium show a very similar purple color in the flame test, and distinguishing the two elements requires more sophisticated analysis, such as spectroscopy.
Chemical properties
Rubidium is the second most electropositive of the stable alkali metals and has a very low first ionization energy of only 403 kJ/mol. It has an electron configuration of [Kr]5s1 and is photosensitive. Due to its strong electropositive nature, rubidium reacts explosively with water[16] to produce rubidium hydroxide and hydrogen gas. As with all the alkali metals, the reaction is usually vigorous enough to ignite metal or the hydrogen gas produced by the reaction, potentially causing an explosion. Rubidium, being denser than potassium, sinks in water, reacting violently; caesium explodes on contact with water. However, the reaction rates of all alkali metals depend upon surface area of metal in contact with water, with small metal droplets giving explosive rates. Rubidium has also been reported to ignite spontaneously in air.
Details
Rubidium (Rb) is a chemical element of Group 1 (Ia) in the periodic table, the alkali metal group. Rubidium is the second most reactive metal and is very soft, with a silvery-white luster.
Rubidium was discovered (1861) spectroscopically by German scientists Robert Bunsen and Gustav Kirchhoff and named after the two prominent red lines of its spectrum. Rubidium and cesium often occur together in nature. Rubidium, however, is more widely scattered and seldom forms a natural mineral; it is found only as an impurity in other minerals, ranging in content up to 5 percent in such minerals as lepidolite, pollucite, and carnallite. Brine samples have also been analyzed that contain up to 6 parts per million of rubidium.
In the principal commercial process of rubidium production, small amounts of rubidium are obtained from the mixture of alkali metal carbonates remaining after lithium salts are extracted from lepidolite. Primarily a potassium carbonate, this by-product also contains approximately 23 percent rubidium and 3 percent cesium carbonates.
The primary difficulty associated with the production of pure rubidium is that it is always found together with cesium in nature and is also mixed with other alkali metals. Because these elements are very similar chemically, their separation presented numerous problems before the advent of ion-exchange methods and ion-specific complexing agents such as crown ethers. Once pure salts have been prepared, it is a straightforward task to convert them to the free metal. This can be done by electrolysis of the fused cyanide or by reduction with calcium or sodium followed by fractional distillation.
Rubidium is difficult to handle because it ignites spontaneously in air, and it reacts violently with water to yield a solution of rubidium hydroxide (RbOH) and hydrogen, which bursts into flames; rubidium is therefore kept in dry mineral oil or an atmosphere of hydrogen. If a metal sample has a large enough surface area, it can burn to form superoxides. Rubidium superoxide (RbO2) is a yellow powder. Rubidium peroxides (Rb2O2) can be formed by oxidation of the metal with the required amount of oxygen. Rubidium forms two other oxides (Rb2O and Rb2O3).
It is used in photoelectric cells and as a “getter” in electron tubes to scavenge the traces of sealed-in gases. Rubidium atomic clocks, or frequency standards, have been constructed, but they are not as precise as cesium atomic clocks. However, aside from these applications, rubidium metal has few commercial uses and is of very minor economic significance. High prices and an uncertain and limited supply discourage the development of commercial uses.
Natural rubidium makes up about 0.01 percent of Earth’s crust; it exists as a mixture of two isotopes: rubidium-85 (72.15 percent) and the radioactive rubidium-87 (27.85 percent), which emits beta rays with a half-life of about 6 × {10}^{11} years. A large number of radioactive isotopes have been artificially prepared, from rubidium-79 to rubidium-95. One estimate of the age of the solar system as 4.6 billion years is based on the ratio of rubidium-87 to strontium-87 in a stony meteorite. Rubidium easily loses its single valence electron but no others, accounting for its oxidation number of +1, although several compounds that contain the anion, Rb-, have been synthesized.
Rubidium and cesium are miscible in all proportions and have complete solid solubility; a melting-point minimum of 9 °C (48 °F) is reached. Rubidium forms a number of mercury amalgams. Because of the increased specific volume of rubidium, as compared with the lighter alkali metals, there is a lesser tendency for it to form alloy systems with other metals.
Element Properties
atomic number : 37
atomic weight : 85.47
melting point : 38.9 °C (102 °F)
boiling point : 688 °C (1,270 °F)
specific gravity : 1.53 (at 20 °C, or 68 °F)
oxidation states : +1, -1 (rare).
Additional Information:
Appearance
A soft metal that ignites in the air and reacts violently with water.
Uses
Rubidium is little used outside research. It has been used as a component of photocells, to remove traces of oxygen from vacuum tubes and to make special types of glass.
It is easily ionised so was considered for use in ion engines, but was found to be less effective than caesium. It has also been proposed for use as a working fluid for vapour turbines and in thermoelectric generators.
Rubidium nitrate is sometimes used in fireworks to give them a purple colour.
Biological role
Rubidium has no known biological role and is non-toxic. However, because of its chemical similarity to potassium we absorb it from our food, and the average person has stores of about half a gram.
It is slightly radioactive and so has been used to locate brain tumours, as it collects in tumours but not in normal tissue.
Natural abundance
Rubidium occurs in the minerals pollucite, carnallite, leucite and lepidolite. It is recovered commercially from lepidolite as a by-product of lithium extraction. Potassium minerals and brines also contain rubidium and are another commercial source.
Facts
* Atomic number (number of protons in the nucleus): 37
* Atomic symbol (on the periodic table of elements): Rb
* Atomic weight (average mass of the atom): 85.4678
* Density: 0.886 ounces per cubic inch (1.532 grams per cubic cm)
* Phase at room temperature: solid
* Melting point: 102.7 degrees Fahrenheit (39.3 degrees Celsius)
* Boiling point: 1,270.4 F (688 C)
* Number of natural isotopes (atoms of the same element with a different number of neutrons): 2. There are also 29 artificial isotopes created in a lab.
* Most common isotopes: Rb-85 (72.2 percent of natural abundance), Rb-87 (27.8 percent of natural abundance).
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
Pages: 1
- Index
- » Science HQ
- » Rubidium