Math Is Fun Forum
You are not logged in.
- Topics: Active | Unanswered
- Index
- » Science HQ
- » Europium
Pages: 1
#1 2025-08-20 16:33:10
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,159
Europium
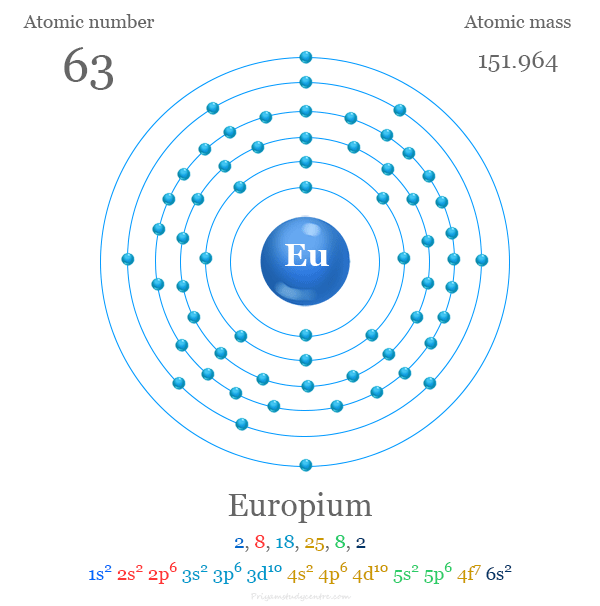
Europium
Gist
Europium (Eu) is a silvery-white, rare-earth metal known for its reactivity and unique properties. It's a lanthanide element, the most reactive of its group, and is characterized by its silvery shine. It's not found freely in nature, but primarily in minerals like monazite and bastnasite. Europium has several notable applications, including use in control rods for nuclear reactors, as a phosphor activator, and in some anti-forgery measures.
Summary
Euopium is a chemical element; it has symbol Eu and atomic number 63. It is a silvery-white metal of the lanthanide series that reacts readily with air to form a dark oxide coating. Europium is the most chemically reactive, least dense, and softest of the lanthanides. It is soft enough to be cut with a knife. Europium was discovered in 1896, provisionally designated as Σ; in 1901, it was named after the continent of Europe.[9] Europium usually assumes the oxidation state +3, like other members of the lanthanide series, but compounds having oxidation state +2 are also common. All europium compounds with oxidation state +2 are slightly reducing. Europium has no significant biological role but is relatively non-toxic compared to other heavy metals. Most applications of europium exploit the phosphorescence of europium compounds. Europium is one of the rarest of the rare-earth elements on Earth.
Europium, a rare earth element, is used in a variety of applications due to its unique properties. It's particularly known for its luminescent properties, making it useful in lighting, displays, and as a phosphor activator. It also acts as a neutron absorber, which is used in nuclear reactors, and is used in currency for anti-forgery measures.
Details
Europium (Eu) is a chemical element, a rare-earth metal of the lanthanide series of the periodic table. Europium is the least dense, the softest, and the most volatile member of the lanthanide series.
The pure metal is silvery, but after even a short exposure to air it becomes dull, because it readily oxidizes in air to form Eu(OH)2 ∙ H2O. Europium quickly reacts with water and diluted acids—except hydrofluoric acid (HF), in which it is protected by a layer of EuF3. Europium is a very strong paramagnet above about 90 K (−183 °C, or −298 °F); below that temperature the metal orders antiferromagnetically, forming a spiral structure.
The element was discovered in 1901 by French chemist Eugène-Anatole Demarçay and named for Europe. One of the least abundant rare earths (its concentration in Earth’s crust is nearly the same as bromine’s), it occurs in minute amounts in many rare-earth minerals such as monazite and bastnasite and also in the products of nuclear fission.
Both of its naturally occurring isotopes are stable: europium-151 (47.81 percent) and europium-153 (52.19 percent). A total of 34 (excluding nuclear isomers) radioactive isotopes, varying in mass from 130 to 165 and having half-lives as short as 0.9 millisecond (europium-130) and as long as 36.9 years (europium-150), have been characterized.
Europium is usually separated from the other rare earths by reducing it to the +2 oxidation state and precipitating it with sulfate ions. The metal has been prepared by electrolysis of the fused halides and by reduction of its oxide by lanthanum metal followed by distillation of the europium metal. Europium exists in a single allotropic (structural) form. It is body-centred cubic with a = 4.5827 Å at room temperature. The primary use of europium is in red phosphors in optical displays and TV screens that use cathode-ray tubes and in glass for fluorescent lamps. It is also used in scintillators for X-ray tomography and as a source of blue color in light-emitting diodes (LEDs).
In its predominant oxidation state of +3, europium behaves as a typical rare earth, forming a series of generally pale pink salts. The Eu3+ ion is paramagnetic because of the presence of unpaired electrons. Europium possesses the most easily produced and stablest +2 oxidation state of the rare earths. Europium(+3) solutions can be reduced by zinc metal and hydrochloric acid to give Eu2+ in solution; the ion is stable in dilute hydrochloric acid if oxygen from the air is excluded. A series of white to pale yellow or green europium(+2) salts are known, such as europium(II) sulfate, chloride, hydroxide, and carbonate. The halides may be prepared by hydrogen reduction of the anhydrous trivalent halides.
Element Properties
atomic number : 63
atomic weight : 151.965
melting point : 822 °C (1,512 °F)
boiling point : 1,527 °C (2,781 °F)
specific gravity : 5.244 (25 °C)
oxidation states : +2, +3.
Additional Information:
Appearance
A soft, silvery metal that tarnishes quickly and reacts with water.
Uses
Europium is used in the printing of euro banknotes. It glows red under UV light, and forgeries can be detected by the lack of this red glow.
Low-energy light bulbs contain a little europium to give a more natural light, by balancing the blue (cold) light with a little red (warm) light.
Europium is excellent at absorbing neutrons, making it valuable in control rods for nuclear reactors.
Europium-doped plastic has been used as a laser material. It is also used in making thin super-conducting alloys.
Biological role
Europium has no known biological role. It has low toxicity.
Natural abundance
In common with other lanthanides, europium is mainly found in the minerals monazite and bastnaesite. It can be prepared from these minerals. However, the usual method of preparation is by heating europium(Ill) oxide with an excess of lanthanum under vacuum.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
Pages: 1
- Index
- » Science HQ
- » Europium