Math Is Fun Forum
You are not logged in.
- Topics: Active | Unanswered
Pages: 1
#1 2025-09-06 21:32:34
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,728
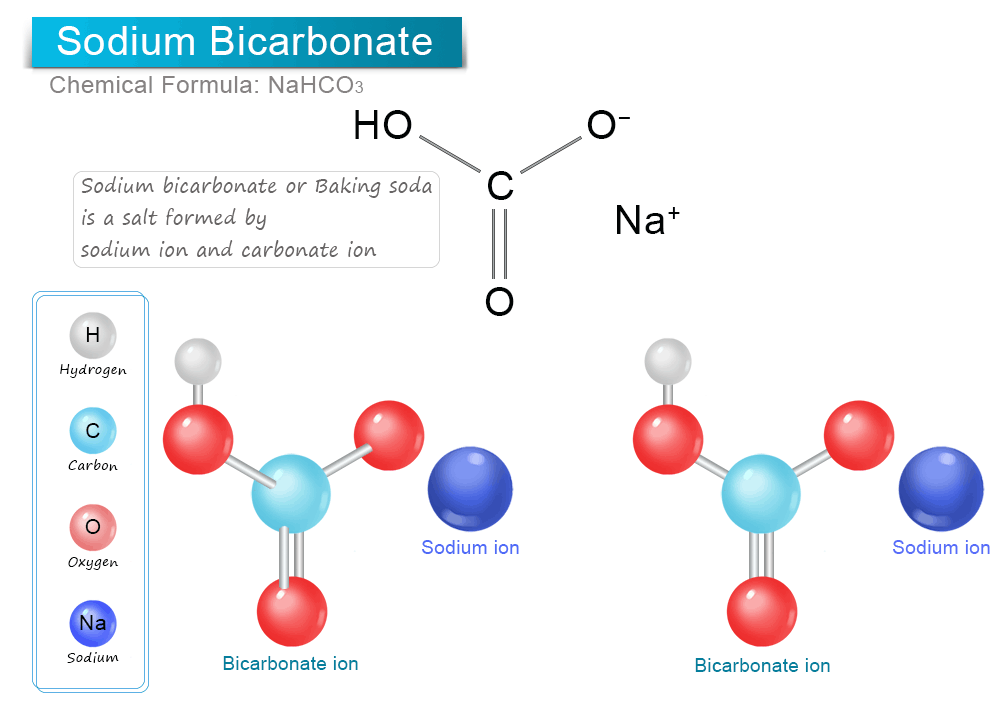
Sodium Bicarbonate
Sodium Bicarbonate
Gist
Sodium bicarbonate , also known as baking soda, is used to relieve heartburn, sour stomach, or acid indigestion by neutralizing excess stomach acid. When used for this purpose, it is said to belong to the group of medicines called antacids. It may be used to treat the symptoms of stomach or duodenal ulcers.
People commonly use sodium bicarbonate for indigestion. It is also used for stomach ulcers, athletic performance, kidney damage, dental plaque, tooth discoloration, and many other conditions, but there is no good scientific evidence to support many of these uses.
Summary
Sodium bicarbonate (NaHCO3) is awhite crystalline or powdery solid that is a source of carbon dioxide and so is used as an ingredient in baking powders, in effervescent salts and beverages, and as a constituent of dry-chemical fire extinguishers. Its slight alkalinity makes it useful in treating gastric or urinary hyperacidity and acidosis. It is also employed in certain industrial processes, as in tanning and the preparation of wool.
Many bakery products are leavened by carbon dioxide from added baking soda or sodium bicarbonate in baking powder. When added without the offsetting amounts of dry acids or acid salts present in baking powder, sodium bicarbonate tends to make dough or batter alkaline, causing flavour deterioration and discoloration and slowing carbon dioxide release. Addition of an acid-reacting substance promotes vigorous gas evolution and maintains acidity within a favourable range. The rate of gas release affects the size of the bubbles produced in the dough or batter, consequently influencing the grain, volume, and texture of the finished product.
Details
Sodium bicarbonate (IUPAC name: sodium hydrogencarbonate), commonly known as baking soda or bicarbonate of soda (or simply "bicarb" especially in the UK) is a chemical compound with the formula NaHCO3. It is a salt composed of a sodium cation (Na+) and a bicarbonate anion. Sodium bicarbonate is a white solid that is crystalline but often appears as a fine powder. It has a slightly salty, alkaline taste resembling that of washing soda (sodium carbonate). The natural mineral form is nahcolite, although it is more commonly found as a component of the mineral trona.
As it has long been known and widely used, the salt has many different names such as baking soda, bread soda, cooking soda, brewing soda and bicarbonate of soda and can often be found near baking powder in stores. The term baking soda is more common in the United States, while bicarbonate of soda is more common in Australia, the United Kingdom, and New Zealand. Abbreviated colloquial forms such as sodium bicarb, bicarb soda, bicarbonate, and bicarb are common.
The prefix bi- in "bicarbonate" comes from an outdated naming system predating molecular knowledge. It is based on the observation that there is twice as much carbonate per sodium in sodium bicarbonate (NaHCO3) as there is in sodium carbonate (Na2CO3). The modern chemical formulas of these compounds now express their precise chemical compositions which were unknown when the name bi-carbonate of potash was coined.
Uses:
Cooking:
In cooking, baking soda is primarily used in baking as a leavening agent. When it reacts with acid or is heated, carbon dioxide is released, which causes expansion of the batter and forms the characteristic texture and grain in cakes, quick breads, soda bread, and other baked and fried foods. When an acid is used, the acid–base reaction can be generically represented as follows:
NaHCO3 + H+ → Na+ + CO2 + H2O
Acidic materials that induce this reaction include hydrogen phosphates, cream of tartar, lemon juice, yogurt, buttermilk, cocoa, and vinegar. Baking soda may be used together with sourdough, which is acidic, making a lighter product with a less acidic taste. Since the reaction occurs slowly at room temperature, mixtures (cake batter, etc.) can be allowed to stand without rising until they are heated in the oven.
Heat can also by itself cause sodium bicarbonate to act as a raising agent in baking because of thermal decomposition, releasing carbon dioxide at temperatures above 80 °C (180 °F), as follows:
2 NaHCO3 → Na2CO3 + H2O + CO2
When used this way on its own, without the presence of an acidic component (whether in the batter or by the use of a baking powder containing acid), only half the available CO2 is released (one CO2 molecule is formed for every two equivalents of NaHCO3). Additionally, in the absence of acid, thermal decomposition of sodium bicarbonate also produces sodium carbonate, which is strongly alkaline and gives the baked product a bitter, soapy taste and a yellow color.
Baking powder
Baking powder, also sold for cooking, contains around 30% of bicarbonate, and various acidic ingredients that are activated by the addition of water, without the need for additional acids in the cooking medium. Many forms of baking powder contain sodium bicarbonate combined with calcium acid phosphate, sodium aluminium phosphate, or cream of tartar. Baking soda is alkaline; the acid used in baking powder avoids a metallic taste when the chemical change during baking creates sodium carbonate.
Food additive
It is often used in conjunction with other bottled water food additives to add taste. Its European Union E number is E500.
Pyrotechnics
Sodium bicarbonate is one of the main components of the common "black snake" firework. The effect is caused by the thermal decomposition, which produces carbon dioxide gas to produce a long snake-like ash as a combustion product of the other main component, sucrose. Sodium bicarbonate also delays combustion reactions through the release of carbon dioxide and water, both of which are flame retardants, when heated.
Mild disinfectant
It has weak disinfectant properties and it may be an effective fungicide against some organisms.
Fire extinguisher
Sodium bicarbonate can be used to extinguish small grease or electrical fires by being thrown over the fire, as heating of sodium bicarbonate releases carbon dioxide. However, it should not be applied to fires in deep fryers; the sudden release of gas may cause the grease to splatter. Sodium bicarbonate is used in BC dry chemical fire extinguishers as an alternative to the more corrosive monoammonium phosphate in ABC extinguishers. The alkaline nature of sodium bicarbonate makes it the only dry chemical agent, besides Purple-K, that was used in large-scale fire suppression systems installed in commercial kitchens.
Sodium bicarbonate has several fire-extinguishing mechanisms that act simultaneously. It decomposes into water and carbon dioxide when heated, an endothermic reaction that deprives the fire of heat. In addition, it forms intermediates that can scavenge the free radicals which are responsible for the propagation of fire. With grease fires specifically, it also has a mild saponification effect, producing a soapy foam that can help smother the fire.
Neutralization of acids
Sodium bicarbonate reacts spontaneously with acids, releasing CO2 gas as a reaction product. It is commonly used to neutralize unwanted acid solutions or acid spills in chemical laboratories. It is not appropriate to use sodium bicarbonate to neutralize base even though it is amphoteric, reacting with both acids and bases.
Sports supplement
Sodium bicarbonate is taken as a sports supplement to improve muscular endurance. Studies conducted mostly in males have shown that sodium bicarbonate is most effective in enhancing performance in short-term, high-intensity activities.
Agriculture
Sodium bicarbonate can prevent the growth of fungi when applied on leaves, although it will not kill the fungus. Excessive amounts of sodium bicarbonate can cause discolouration of fruits (two percent solution) and chlorosis (one percent solution). Sodium bicarbonate is also commonly used as a free choice dietary supplement in sheep to help prevent bloat.
Medical uses and health
Sodium bicarbonate mixed with water can be used as an antacid to treat acid indigestion and heartburn. Its reaction with stomach acid produces salt, water, and carbon dioxide:
NaHCO3 + HCl → NaCl + H2O + CO2(g)
A mixture of sodium bicarbonate and polyethylene glycol dissolved in water and taken orally, is an effective gastrointestinal lavage preparation and laxative prior to gastrointestinal surgery, gastroscopy, etc.
Intravenous sodium bicarbonate in an aqueous solution is sometimes used for cases of acidosis, or when insufficient sodium or bicarbonate ions are in the blood. In cases of respiratory acidosis, the infused bicarbonate ion drives the carbonic acid/bicarbonate buffer of plasma to the left, and thus raises the pH. For this reason, sodium bicarbonate is used in medically supervised cardiopulmonary resuscitation. Infusion of bicarbonate is indicated only when the blood pH is markedly low (< 7.1–7.0).
HCO3− is used for treatment of hyperkalemia, as it will drive K+ back into cells during periods of acidosis. Since sodium bicarbonate can cause alkalosis, it is sometimes used to treat aspirin overdoses. Aspirin requires an acidic environment for proper absorption, and a basic environment will diminish aspirin absorption in cases of overdose. Sodium bicarbonate has also been used in the treatment of tricyclic antidepressant overdose. It can also be applied topically as a paste, with three parts baking soda to one part water, to relieve some kinds of insect bites and stings (as well as accompanying swelling).
Some alternative practitioners, such as Tullio Simoncini, have promoted baking soda as a cancer cure, which the American Cancer Society has warned against due to both its unproven effectiveness and potential danger in use. Edzard Ernst has called the promotion of sodium bicarbonate as a cancer cure "one of the more sickening alternative cancer scams I have seen for a long time".
Sodium bicarbonate can be added to local anaesthetics, to speed up the onset of their effects and make their injection less painful. It is also a component of Moffett's solution, used in nasal surgery.
It has been proposed that acidic diets weaken bones. One systematic meta-analysis of the research shows no such effect. Another also finds that there is no evidence that alkaline diets improve bone health, but suggests that there "may be some value" to alkaline diets for other reasons.
Antacid (such as baking soda) solutions have been prepared and used by protesters to alleviate the effects of exposure to tear gas during protests.
Similarly to its use in baking, sodium bicarbonate is used together with a mild acid such as tartaric acid as the excipient in effervescent tablets: when such a tablet is dropped in a glass of water, the carbonate leaves the reaction medium as carbon dioxide gas (HCO3− + H+ → H2O + CO2↑ or, more precisely, HCO3− + H3O+ → 2 H2O + CO2↑). This makes the tablet disintegrate, leaving the medication suspended and/or dissolved in the water together with the resulting salt (in this example, sodium tartrate).
Personal hygiene
Sodium bicarbonate is also used as an ingredient in some mouthwashes. It has anticaries and abrasive properties. It works as a mechanical cleanser on the teeth and gums, neutralizes the production of acid in the mouth, and also acts as an antiseptic to help prevent infections. Sodium bicarbonate in combination with other ingredients can be used to make a dry or wet deodorant. Sodium bicarbonate may be used as a buffering agent, combined with table salt, when creating a solution for nasal irrigation.
It is used in eye hygiene to treat blepharitis. This is done by adding a teaspoon of sodium bicarbonate to cool water that was recently boiled followed by gentle scrubbing of the eyelash base with a cotton swab dipped in the solution.
Veterinary uses
Sodium bicarbonate is used as a cattle feed supplement, in particular as a buffering agent for the rumen.
Cleaning agent
Sodium bicarbonate is used in a process to remove paint and corrosion called sodablasting. As a blasting medium, sodium bicarbonate is used to remove surface contamination from softer and less resilient substrates such as aluminium, copper, or timber that could be damaged by silica sand abrasive media.
A manufacturer recommends a paste made from baking soda with minimal water as a gentle scouring powder. Such a paste can be useful in removing surface rust because the rust forms a water-soluble compound when in a concentrated alkaline solution. Cold water should be used since hot-water solutions can corrode steel. Sodium bicarbonate attacks the thin protective oxide layer that forms on aluminium, making it unsuitable for cleaning this metal.
A solution of baking soda in warm water will remove the tarnish from silver when the silver is in contact with a piece of aluminium foil.
Baking soda is commonly added to washing machines as a replacement for water softener and to remove odors from clothes. When diluted with warm water, it is also almost as effective in removing heavy tea and coffee stains from cups as sodium hydroxide.
During the Manhattan Project to develop the nuclear bomb in the early 1940s, the chemical toxicity of uranium was an issue. Uranium oxides were found to stick very well to cotton cloth and did not wash out with soap or laundry detergent. However, the uranium would wash out with a 2% solution of sodium bicarbonate. Clothing can become contaminated with toxic dust of depleted uranium (DU), which is very dense, hence it is used for counterweights in a civilian context and in armour-piercing projectiles. DU is not removed by normal laundering; washing with about 6 ounces (170 g) of baking soda in 2 gallons (7.5 L) of water will help wash it out.
Additional Information:
Key Points/Overview
Sodium bicarbonate is also called baking soda and is actually a type of salt.
Some popular uses for baking soda are to help baked goods rise, as an antacid to treat indigestion, and as a general household cleaner.
The FDA regards sodium bicarbonate as “Generally Recognized as Safe” (GRAS) for use as a food additive, active ingredient in toothpaste, and antacid.
Uses & Benefits:
Baking & Food Preparation
Baking soda available in the grocery store is pure, food-grade sodium bicarbonate. Bakers add a small amount of baking soda to the mixture of flour, sugar, eggs, butter and other ingredients in cakes, cookies and other baked goods. The resulting chemical reaction then helps batter expand or rise inside a hot oven. Without baking soda and this chemical reaction, muffins, cakes and breads would fall flat.
Personal Care Products & Medicines
In skin care and personal care products like lotions and bath salts, sodium bicarbonate helps control a product’s acid-base balance to keep it from spoiling. In toothpaste, sodium bicarbonate helps to remove stains from teeth by dislodging tiny particles of food or beverages that can blemish tooth enamel. It is also a common ingredient in deodorant because it can help neutralize smelly, acidic scents.
Sodium bicarbonate also is an active ingredient in antacid products used to relieve heartburn and treat acid indigestion. It works by quickly neutralizing stomach acid and temporarily relieving symptoms of acid reflux.
Cleaning Products & Solvents
Sodium bicarbonate is a common ingredient in cleaning, detergent and degreasing products. In cleaning products, sodium bicarbonate can react with vinegar to create a solution that helps unclog drains or remove grime in ovens. Its slight abrasiveness is extremely efficient for eliminating burnt residues or grease.
The chemical properties of sodium bicarbonate can also help enhance the efficiency of laundry detergent by increasing the water’s pH level, which helps to repel dirt from fibers, leading to fresher laundry.
Safety Information
People have been using sodium bicarbonate for thousands of years. Ancient Egyptians used natural deposits of the mineral to clean their teeth and make paints for writing. In the 1830s, New York bakers began adding sodium bicarbonate and sour milk to dough to make bread.
The U.S. Food and Drug Administration (FDA) states that sodium bicarbonate is GRAS (Generally Recognized as Safe) as a direct food additive. It also states that sodium bicarbonate is generally recognized as safe as an antacid and as an anticaries (tooth decay fighting) active ingredient for over-the-counter use within certain conditions.
The Cosmetic Ingredient Review (CIR), an independent expert panel made up of academic researchers and industry scientists, also has evaluated the scientific data and concluded that sodium bicarbonate is safe as a cosmetic ingredient at current levels of use. In 2005, the CIR Expert Panel considered available new data on sodium bicarbonate and reaffirmed its safety conclusion.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
Pages: 1