Math Is Fun Forum
You are not logged in.
- Topics: Active | Unanswered
#76 2018-01-28 15:42:43
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,728
Re: Miscellany
63) Indus Valley Civilization
The Indus Valley Civilization (the Harappan Civilization or the Indus Civilization or the Indus River Valley Civilization) is one of the earliest civilizations in the world. The civilization flourished around the Indus River basin and its tributaries consisting of present day Pakistan and northwest India. In India the largest Harappan site is located in Haryana Rakhigarhi, and the second largest is in Dholavira, Gujarat. Construction of well-planned cities by using bricks, and proper drainage system are the main features of this civilization. At the time of its height, the Indus Valley Civilization geographically extended to Egypt or Mesopotamia. On the basis of archaeological research and radio carbon dating, origin of the Indus Valley Civilization lies between 3300 – 1300 BCE. Though people used to live on the banks of the Indus River before this period but it was only during a Bronze Age when they actually started living as civilized and urban societies. Harappa and Mohenjo-daro were the two prominent cities of Indus Valley Civilization.
Facts about Indus Valley Civilization
Mohenjo-daro is the largest site and Allahdino is the smallest site of the Indus Valley Civilization.
The Indus Valley Civilization had a total population of over five million. Most of its people were artisans and traders. Merchants were very wealthy and given extra political powers.
Towns were laid down in a rectangular pattern. Most of the houses were two-storey and very spacious. Town planning is a unique feature of the Indus Valley Civilization. There were well built granaries, citadels, burial grounds and bathing platforms.
The towns used to have great baths. Though the exact purpose of baths is not clear but it is believed that these might be used for religious bathing.
Brick lined sewers is another very prominent feature of the Indus Valley Civilization. Mohenjo-daro is the most prominent of all cities of the civilization with respect to sewerage system. Bricks used in construction were built in the ratio of 4:2:1 having 11 inches length, 5.5 inches width and 2.75 inches depth.
Length, mass and time was accurately measured by the people of the Indus Valley Civilization. Also the system of uniform weights and measures were developed by them.
People were familiar with certain new techniques in metallurgy. They used these techniques to produce lead, copper, tin and bronze.
Art was in its full form during the Indus Valley Civilization. At the time of excavation many bronze, copper and pottery products along with terra-cotta toys have been excavated. Steatite seals engraved with animal figures is however the most notable among all these. The most common of all animals that was used on seals was Humpless bull or unicorn.
There are three stages of Harappan Civilization which are Pre-Harappan, Harappan and Post-Harappan known as Rojde, Desalpur and Surkotada respectively
Lothal, Balakot, Suktagendor and Allahdin (Pakistan) are the cities in Harappan civilization that were the major ports of that time.
Engineering skills were at its heights and it is clear from the construction of docks especially at Lothal.
Wheels used in Haprappa were axeless.
Roads especially in Mohenjo-daro were as wide as 10.5 mt. Harappa also had wider roads having width of 30 foot.
Similarities between the Indus Valley Civilization and the Egyptian River Valley Civilization have been noticed by the scholars. First of all both the civilizations were dependent upon river system and flourished around the Indus and the Nile rivers respectively. It has also been found out that both of these civilizations had almost similar lifestyle and customs.
Limestone and steatite were the most common materials used to make stone sculpture in Harappa.
Information about rituals and beliefs of the Indus Valley Civilization is limited at present. But according to studies it is clear that people living in this civilization used to worship deities especially fertility deities. Religious activities were performed by priests. Ritualistic baths were part of Harappan Civilization. On some of the seals there are pictures of people doing meditation and sitting in a crossed legged posture.
Also there is very less information about the language used in the Indus Valley Civilization but some scholars believe its closeness to Vedic scripts.
Reason of Indus Valley Civilization decline
Major reason given by scholars regarding the decline of the Indus Valley Civilization was a shift in the course of river and natural disasters such as drought, flood etc. Also there was decline in the trade with Egypt and Mesopotamia. Some of the scholars also believe that wars with the Aryan Civilization may also be the reason of their decline.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#77 2018-01-30 00:25:28
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,728
Re: Miscellany
64) Clinometer
A clinometer or inclinometer is an instrument for measuring angles of slope (or tilt), elevation or depression of an object with respect to gravity. It is also known as a tilt indicator, tilt sensor, tilt meter, slope alert, slope gauge, gradient meter, gradiometer, level gauge, level meter, decimeter, and pitch & roll indicator. Clinometers measure both inclines (positive slopes, as seen by an observer looking upwards) and declines (negative slopes, as seen by an observer looking downward) using three different units of measure: degrees, percent, and topo. Astrolabes are inclinometers that were used for navigation and locating astronomical objects from ancient times to the Renaissance.
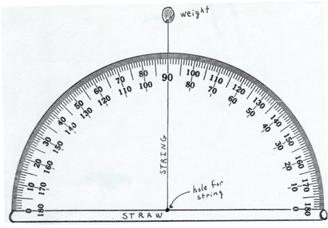
A clinometer is a tool that is used to measure the angle of elevation, or angle from the ground, in a right - angled triangle. You can use a clinometer to measure the height of tall things that you can't possibly reach to the top of, flag poles, buildings, trees. Follow the directions below to create your own clinometer.
You will need:
A protractor with a small hole on the center spot or
Print out of paper protractor (see below)
Poster board or card board (can be from a box) to back the protractor
20 cm or about 8 inches of string or strong cotton
Weight - such as a metal nut, paper clips or a small piece of clay
Glue and Scissors
A straw
Clear Tape
Directions:
If you are making a protractor, cut out the copy of the protractor.
Get the piece of poster board or an empty box. Stick the paper protractor on top of the card and cut the joined pieces.
Get the straw and tape it to the straight edge of your protractor that you made above.
With your pen or pencil, poke a hole through the center of the protractor where it meets the straw. Push the string through the hole and tie a large knot on the other side so it won't pull through.
Tie your weight to the other end of the string.
To use the clinometer:
The diagram shows what the assembled clinometer will look like when laying an a flat surface. When using it, the straw will be on the top.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#78 2018-01-31 00:34:07
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,728
Re: Miscellany
65) Wernicke-Korsakoff Syndrome (WKS)
What is Wernicke-Korsakoff syndrome (WKS)?
Wernicke-Korsakoff syndrome (WKS) is a type of brain disorder caused by a lack of vitamin B-1, or thiamine. The syndrome is actually two separate conditions that can occur at the same time, Wernicke’s disease (WD) and Korsakoff syndrome. Usually, people get the symptoms of WD first. WD is also known as Wernicke’s encephalopathy.
Symptoms of WKS may include confusion, changes to the eyes and vision, or exaggerated storytelling, among others.
Alcoholism, or chronic alcohol misuse, is the most common cause of WKS. WKS can also be linked to diet deficiencies or other medical conditions that impair the absorption of vitamin B-1.
Risk Factors
What are the risk factors for WKS?
Risk factors for WKS are related to your diet and lifestyle.
The major risk factors for developing WKS are malnourishment and chronic alcohol misuse. Other risk factors for WKS include:
inability to afford medical care and proper food
kidney dialysis, which reduces vitamin B-1 absorption
AIDS, which makes you more likely to develop conditions that lead to vitamin B-1 deficiency
Causes
What causes WKS?
The no. 1 cause of WKS is alcoholism.
The less common causes of WKS are conditions that limit nutritional absorption. Eating and nutrient absorption can be restricted by:
gastric bypass surgery, which makes it difficult to meet nutritional needs due to limited food portions
gastric cancer, which may limit the absorption of essential nutrients
colon cancer, which can result in pain that causes you to put off eating
eating disorders
Alcoholism is the no. 1 cause of WKS because people with the condition generally have a poor diet. Alcohol also prevents vitamin B-1 absorption and storage.
Symptoms
What are the symptoms of WKS?
Lesions on the brain cause WD. These lesions are the result of a vitamin B-1 deficiency.
Prominent symptoms of WD are:
double vision
a drooping upper eyelid, also known as ptosis
up-and-down or side-to-side eye movements
loss of muscle coordination, or ataxia, which may interfere with walking
a confused mental state, which frequently leads to combativeness or violent behavior
WD can later develop into Korsakoff’s syndrome. People who have WKS have a variety of issues relating to memory. You may experience memory loss or be unable to form new memories.
You may also have the following symptoms if you have WKS:
amnesia for events that happen after the onset of the disorder
difficulty understanding the meaning of information
difficulty putting words into context
hallucinations
exaggerated storytelling, or confabulation
Diagnosis
How is WKS diagnosed?
Diagnosing WKS isn’t always easy.
An individual with WKS is often mentally confused. This can make communication with the doctor difficult. Your doctor may overlook the possibility of a physical disorder if you’re confused.
Signs of alcoholism
Your doctor may first check for signs of alcoholism. They may check your blood alcohol levels. Sometimes, they’ll take a liver function test to check for liver damage. Liver damage is a common sign of alcoholism.
Liver damage caused by chronic alcoholism can elevate your liver enzymes. Diagnosis of chronic alcoholism includes a physical examination to assess your:
heart rate
eye movements
reflexes
blood pressure
body temperature
Signs of nutritional deficiency
Your doctor will look for clinical signs that point to a vitamin B-1 deficiency. This can involve blood tests that measure thiamine levels and your general nutritional health.
Nutritional tests your doctor may order to make sure you aren’t malnourished include:
Serum albumin test. This test measures the levels of albumin, a protein in the blood. Low levels of albumin may signal nutritional deficiencies as well as kidney or liver problems.
Serum vitamin B-1 test. This test checks vitamin B-1 levels in the blood. Enzyme activity in the red blood cells (RBCs) can be tested. Low enzyme activity in the RBCs signals a vitamin B-1 deficiency.
Other tests
You may also need imaging tests, which can help your doctor find any damage that’s characteristic of WKS. Diagnostic imaging tests for WKS include:
an electrocardiogram (ECG or (EKG) before and after taking vitamin B-1, which can help your doctor find abnormalities
a CT scan to check for brain lesions related to WD
an MRI scan to look for brain changes related to WD
Your doctor may also use a neuropsychological test to determine the severity of any mental deficiencies.
Treatments
How is WKS treated?
WKS treatment should begin immediately. Prompt treatment may delay or stop disease progression. Treatments are also able to reverse nonpermanent brain abnormalities.
Treatment may first involve hospitalization. At the hospital, you’ll be monitored to ensure your digestive system is absorbing food properly.
The treatment for WKS may include:
vitamin B-1 given through an intravenous line (IV) in the arm or hand
vitamin B-1 given by mouth
a balanced diet to keep vitamin B-1 levels up
treatment for alcoholism
After diagnosis, your doctor will most likely give you vitamin B-1 intravenously. Fast treatment may reverse many of the neurological symptoms of WKS.
In a small number of cases, treatment of vitamin B-1 deficiency produces a negative reaction. This is more common in people with alcoholism.
Negative reactions to receiving vitamin B-1 may vary, and can include alcohol withdrawal symptoms such as insomnia, sweating, or mood swings. You may also experience hallucinations, confusion, or agitation.
Outlook
What’s the outlook for people with WKS?
The outlook for WKS is based on how far the disease has advanced.
Receiving early treatment before irreversible damage has occurred will dramatically improve your outlook.
Mortality rates are high if WKS is left untreated. Most deaths are the result of a lung infection, blood poisoning, or irreversible brain damage.
Those who receive fast treatment can see progress in:
eye problems
muscle coordination
confusion
Abstaining from alcohol allows continued recovery of memory and mental function.
Pevention
How can you prevent WKS?
You can prevent WKS by avoiding alcohol and eating a balanced diet rich in vitamin B-1.
Foods rich in vitamin B-1 include:
rice
peas
whole wheat bread
spinach
oranges
milk
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#79 2018-02-02 00:48:44
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,728
Re: Miscellany
66) Discoverer of vitamins A, B, and D
Elmer Verner McCollum
Born: March 03, 1879 in Fort Scott, Kansas, United States
Died: November 15, 1967 in Baltimore, Maryland, United States
Other Names: McCollum, Elmer Verner; McCollum, Elmer V.
Nationality: American
Occupation: Biochemist
Elmer Verner McCollum was a distinguished biochemist, organic chemist, and nutritionist who studied nutrition and its effects on health and metabolism. The discoverer of vitamins A, B and D, McCollum also found the nutritional cause of rickets, a bone disease. His twenty-six years of research at the School of Hygiene and Public Health at the Johns Hopkins Medical School in Baltimore continued his earlier research and made him an authority on how diets lacking certain vitamins or trace minerals can cause diseases. He developed a biological method to analyze foods and popularized the use of rats as an extremely valuable experimental model. By modifying the rats' diets, McCollum produced animals with rickets. Because of these studies, rickets, a disease in which bones become deformed, is virtually nonexistent in developed countries. In a field of research that had few findings of importance for decades, he was instrumental in ushering in a new era.
McCollum, the fourth of five children and the first son, was born on March 3, 1879, near Fort Scott, Kansas, to Cornelius Armstrong McCollum, a farmer believed to be of Scottish descent, and Martha Catherine Kidwell McCollum, the daughter of mountain people also originally from Scotland. McCollum grew up on a farm, and as a child he was very inquisitive, a trait he had all of his life. Oddly enough for a man who would devote his life to researching nutrition and the role vitamins play in having good health, as an infant he suffered from scurvy, a nutritional disease later determined to be caused by the lack of vitamin C. He was cured when his mother fed him apple peels, which contain this vitamin. His parents understood the value of advanced education, and his mother in particular was determined that her children should receive higher education. He attended the University of Kansas in Lawrence, where as a sophomore he became interested in organic chemistry. At the end of his junior year, he was elected to membership in Sigma Xi, the honorary scientific society. He graduated with a baccalaureate degree in 1903 and a master's of arts degree a year later. McCollum continued his education at Yale University in New Haven, Connecticut, and graduated in 1906 with a doctorate in organic chemistry.
After an unsuccessful search for a position in the field of organic chemistry, McCollum began to study biochemistry. In 1907 he became an instructor in agricultural chemistry at the Wisconsin Agricultural Experimental Station and quickly rose through the academic ranks, becoming a full professor in 1913. In his early work he performed chemical analyses on the diets of dairy cattle and studied how different diets affected the cows' health and reproductive capacities. However, he thought the procedures were long and tedious in such large animals. Fortuitously, references in scientific journals about using mice in other experiments led him to develop a rat colony for his nutritional research. This was the first rat colony used in the study of nutritional aspects of disease. The rats were fed various diets, each one lacking certain substances. Their short life span quickly enabled McCollum to determine what effect the diets had. This work helped to popularize the use of rats in other experimental situations.
In 1912 McCollum noticed that rats fed a diet deficient in certain fats grew normally again when the fats were added back in. This discovery was the first of a fat-soluble nutrient, which McCollum named vitamin A. Subsequent studies have shown that vitamin A helps makes teeth and bones strong and is necessary for normal vision and healthy skin. McCollum later demonstrated that a water-soluble substance, which he called vitamin B, was also necessary for normal health. He had created the alphabetical nomenclature for vitamins.
Although pleased with his rapid rise at the Wisconsin Agricultural Experimental Station, McCollum felt somewhat restricted in his research. At this time, funds from the Rockefeller Foundation established a School of Hygiene and Public Health at Johns Hopkins Medical School in Baltimore. Highly respected among his peers, McCollum was offered a position as the first faculty member--professor of biochemistry--which he assumed in 1917. He was made emeritus professor in 1945.
A coincidence led to the discovery for which McCollum is best known: producing rickets in rats. The rats had bending, fractures, and swellings of the ends of the long bones in their legs. But McCollum did not know recognize it as rickets until John Howland, professor of pediatrics at Johns Hopkins Medical School, stopped by McCollum's office to inquire whether anyone had ever produced rickets in rats. After McCollum described his rats, he and Howland concluded the rats had rickets. The discovery threw new light on the disease. McCollum identified the missing factor in the rats' diets that resulted in rickets, which he named vitamin D. (Another researcher had discovered a different vitamin, so C was taken.) Vitamin D aids in the absorption of calcium, which is used in bones and teeth, and is found in fortified milk, fish such as tuna and sardines, dairy products, and egg yolks. It also is formed in the body when a person is exposed to sunlight. Because of this discovery, people changed their diets to include vitamin D, and now rickets is rare in most developed countries. Some of McCollum's research efforts focused on trace elements, simple substances essential to life in very small quantities. Through his research he started to isolate these substances. McCollum showed that a deficiency of calcium eventually would produce muscular spasms. Other trace elements he focused on include fluorine, zinc, and manganese.
In addition to his laboratory research, McCollum lectured widely about progress and problems in nutritional studies. In 1923 he started writing articles for the popular press in McCall's magazine. In honor of his outstanding contributions in the field of nutrition, he received many awards. He was a member of numerous national and international organizations devoted to public health, including the international committee on vitamin standards of the League of Nations in 1931, the food and nutrition board of the National Research Council starting in 1942, and the World Health Organization. In 1948 the McCollum-Pratt Institute was created at Johns Hopkins for the study of trace elements. Years after his retirement in 1946, he remained actively interested in nutrition and related health fields and published the comprehensive book A History of Nutrition in 1957. He died in Baltimore, Maryland, November 15, 1967.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#80 2018-02-04 02:58:06
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,728
Re: Miscellany
67) Hypermnesia
Hypermnesia defines great memory capacity. This capacity is often judged as a gift by others, yet considered a pathology by the medical field. This case occurs in the context of a great improvement in episodic memory. In cases of emotional hypermnesia, memories of traumatic events of life such as war or deportation continually resurface (Targowla's syndrome). It is advisable, however, not to classify an exceptional photographic or eidetic memory as hypermnesia, since these are not pathologies but rare abilities to memorize stimuli.
Hypermnesia is an improvement in memory that occurs with related attempts to retrieve previously encoded material. Hypermnesia is the net result of reminiscence and forgetting, such that the amount of reminiscence must exceed the amount of forgetting resulting in a net improvement overall. Ballard was the first to observe hypermnesia using a multiple recall task in 1913, the same task with which he discovered reminiscence. After asking young school children to memorize poetry, he initially retested their recall abilities 2 days later only to discover that the class improved by 10 percent over the initial recall level.
Hypermnesia was not considered a phenomenon until the mid-1970s when Erdelyi and his associates completed further testing. Two suggestions have been offered to explain the increases in net recall over testing. One suggestion is that pictures and high imagery words enhance hypermnesia, speculating that this nature of stimuli is more recognizable and hence less susceptible to forgetting. The second suggestion is Roediger's level of recall hypothesis claiming that any variable that produces greater levels of depth processing lead to greater hypermnesia.
A 1991 study by Otani and Hodge suggests that hypermnesia does not occur with recognition but is found in cued recall experiments, showing that the improvement in memory performance is due to an increased rate of item recovery facilitated by relational processing. Relational processing can be made easier with well-categorized stimuli and helps to increase the availability or retrieval cues that in turn help to generate the to-be-remembered items. The phenomenon of hypermnesia is one that continues to be examined, particularly in terms of its generalization.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#81 2018-02-06 01:13:47
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,728
Re: Miscellany
68) Virus
Virus, an infectious agent of small size and simple composition that can multiply only in living cells of animals, plants, or bacteria. The name is from a Latin word meaning “slimy liquid” or “poison.”
The earliest indications of the biological nature of viruses came from studies in 1892 by the Russian scientist Dmitry I. Ivanovsky and in 1898 by the Dutch scientist Martinus W. Beijerinck. Beijerinck first surmised that the virus under study was a new kind of infectious agent, which he designated contagium vivum fluidum, meaning that it was a live, reproducing organism that differed from other organisms. Both of these investigators found that a disease of tobacco plants could be transmitted by an agent, later called tobacco mosaic virus, passing through a minute filter that would not allow the passage of bacteria. This virus and those subsequently isolated would not grow on an artificial medium and were not visible under the light microscope. In independent studies in 1915 by the British investigator Frederick W. Twort and in 1917 by the French Canadian scientist Félix H. d’Hérelle, lesions in cultures of bacteria were discovered and attributed to an agent called bacteriophage (“eater of bacteria”), now known to be viruses that specifically infect bacteria.
The unique nature of these organisms meant that new methods and alternative models had to be developed to study and classify them. The study of viruses confined exclusively or largely to humans, however, posed the formidable problem of finding a susceptible animal host. In 1933 the British investigators Wilson Smith, Christopher H. Andrewes, and Patrick P. Laidlaw were able to transmit influenza to ferrets, and the influenza virus was subsequently adapted to mice. In 1941 the American scientist George K. Hirst found that influenza virus grown in tissues of the chicken embryo could be detected by its capacity to agglutinate (draw together) red blood cells.
A significant advance was made by the American scientists John Enders, Thomas Weller, and Frederick Robbins, who in 1949 developed the technique of culturing cells on glass surfaces; cells could then be infected with the viruses that cause polio (poliovirus) and other diseases. (Until this time, the poliovirus could be grown only in the brains of chimpanzees or the spinal cords of monkeys.) Culturing cells on glass surfaces opened the way for diseases caused by viruses to be identified by their effects on cells (cytopathogenic effect) and by the presence of antibodies to them in the blood. Cell culture then led to the development and production of vaccines (preparations used to elicit immunity against a disease) such as the poliovirus vaccine.
Scientists were soon able to detect the number of bacterial viruses in a culture vessel by measuring their ability to break apart (lyse) adjoining bacteria in an area of bacteria (lawn) overlaid with an inert gelatinous substance called agar—viral action that resulted in a clearing, or “plaque.” The American scientist Renato Dulbecco in 1952 applied this technique to measuring the number of animal viruses that could produce plaques in layers of adjoining animal cells overlaid with agar. In the 1940s the development of the electron microscope permitted individual virus particles to be seen for the first time, leading to the classification of viruses and giving insight into their structure.
Advancements that have been made in chemistry, physics, and molecular biology since the 1960s have revolutionized the study of viruses. For example, electrophoresis on gel substrates gave a deeper understanding of the protein and nucleic acid composition of viruses. More-sophisticated immunologic procedures, including the use of monoclonal antibodies directed to specific antigenic sites on proteins, gave a better insight into the structure and function of viral proteins. The progress made in the physics of crystals that could be studied by X-ray diffraction provided the high resolution required to discover the basic structure of minute viruses. Applications of new knowledge about cell biology and biochemistry helped to determine how viruses use their host cells for synthesizing viral nucleic acids and proteins.
The revolution that took place in the field of molecular biology allowed the genetic information encoded in nucleic acids of viruses—which enables viruses to reproduce, synthesize unique proteins, and alter cellular functions—to be studied. In fact, the chemical and physical simplicity of viruses has made them an incisive experimental tool for probing the molecular events involved in certain life processes. Their potential ecological significance was realized in the early 21st century, following the discovery of giant viruses in aquatic environments in different parts of the world.
Definition
Viruses occupy a special taxonomic position: they are not plants, animals, or prokaryotic bacteria (single-cell organisms without defined nuclei), and they are generally placed in their own kingdom. In fact, viruses should not even be considered organisms, in the strictest sense, because they are not free-living; i.e., they cannot reproduce and carry on metabolic processes without a host cell.
All true viruses contain nucleic acid—either DNA (deoxyribonucleic acid) or RNA (ribonucleic acid)—and protein. The nucleic acid encodes the genetic information unique for each virus. The infective, extracellular (outside the cell) form of a virus is called the virion. It contains at least one unique protein synthesized by specific genes in the nucleic acid of that virus. In virtually all viruses, at least one of these proteins forms a shell (called a capsid) around the nucleic acid. Certain viruses also have other proteins internal to the capsid; some of these proteins act as enzymes, often during the synthesis of viral nucleic acids. Viroids (meaning “viruslike”) are disease-causing organisms that contain only nucleic acid and have no structural proteins. Other viruslike particles called prions are composed primarily of a protein tightly complexed with a small nucleic acid molecule. Prions are very resistant to inactivation and appear to cause degenerative brain disease in mammals, including humans.
Viruses are quintessential parasites; they depend on the host cell for almost all of their life-sustaining functions. Unlike true organisms, viruses cannot synthesize proteins, because they lack ribosomes (cell organelles) for the translation of viral messenger RNA (mRNA; a complementary copy of the nucleic acid of the nucleus that associates with ribosomes and directs protein synthesis) into proteins. Viruses must use the ribosomes of their host cells to translate viral mRNA into viral proteins.
Viruses are also energy parasites; unlike cells, they cannot generate or store energy in the form of adenosine triphosphate (ATP). The virus derives energy, as well as all other metabolic functions, from the host cell. The invading virus uses the nucleotides and amino acids of the host cell to synthesize its nucleic acids and proteins, respectively. Some viruses use the lipids and sugar chains of the host cell to form their membranes and glycoproteins (proteins linked to short polymers consisting of several sugars).
The true infectious part of any virus is its nucleic acid, either DNA or RNA but never both. In many viruses, but not all, the nucleic acid alone, stripped of its capsid, can infect (transfect) cells, although considerably less efficiently than can the intact virions.
The virion capsid has three functions: (1) to protect the viral nucleic acid from digestion by certain enzymes (nucleases), (2) to furnish sites on its surface that recognize and attach (adsorb) the virion to receptors on the surface of the host cell, and, in some viruses, (3) to provide proteins that form part of a specialized component that enables the virion to penetrate through the cell surface membrane or, in special cases, to inject the infectious nucleic acid into the interior of the host cell.
Host range and distribution
Logic originally dictated that viruses be identified on the basis of the host they infect. This is justified in many cases but not in others, and the host range and distribution of viruses are only one criterion for their classification. It is still traditional to divide viruses into three categories: those that infect animals, plants, or bacteria.
Virtually all plant viruses are transmitted by insects or other organisms (vectors) that feed on plants. The hosts of animal viruses vary from protozoans (single-celled animal organisms) to humans. Many viruses infect either invertebrate animals or vertebrates, and some infect both. Certain viruses that cause serious diseases of animals and humans are carried by arthropods. These vector-borne viruses multiply in both the invertebrate vector and the vertebrate host.
Certain viruses are limited in their host range to the various orders of vertebrates. Some viruses appear to be adapted for growth only in ectothermic vertebrates (animals commonly referred to as cold-blooded, such as fishes and reptiles), possibly because they can reproduce only at low temperatures. Other viruses are limited in their host range to endothermic vertebrates (animals commonly referred to as warm-blooded, such as mammals).
Size and shape
The amount and arrangement of the proteins and nucleic acid of viruses determine their size and shape. The nucleic acid and proteins of each class of viruses assemble themselves into a structure called a nucleoprotein, or nucleocapsid. Some viruses have more than one layer of protein surrounding the nucleic acid; still others have a lipoprotein membrane (called an envelope), derived from the membrane of the host cell, that surrounds the nucleocapsid core. Penetrating the membrane are additional proteins that determine the specificity of the virus to host cells. The protein and nucleic acid constituents have properties unique for each class of virus; when assembled, they determine the size and shape of the virus for that specific class. The genomes of Mimiviruses and Pandoraviruses, which are some of the largest known viruses, range from 1 to 2.5 Mb (1 Mb = 1,000,000 base pairs of DNA).
Most viruses vary in diameter from 20 nanometres (nm; 0.0000008 inch) to 250–400 nm; the largest, however, measure about 500 nm in diameter and are about 700–1,000 nm in length. Only the largest and most complex viruses can be seen under the light microscope at the highest resolution. Any determination of the size of a virus also must take into account its shape, since different classes of viruses have distinctive shapes.
Shapes of viruses are predominantly of two kinds: rods, or filaments, so called because of the linear array of the nucleic acid and the protein subunits; and spheres, which are actually 20-sided (icosahedral) polygons. Most plant viruses are small and are either filaments or polygons, as are many bacterial viruses. The larger and more-complex bacteriophages, however, contain as their genetic information double-stranded DNA and combine both filamentous and polygonal shapes. The classic T4 bacteriophage is composed of a polygonal head, which contains the DNA genome and a special-function rod-shaped tail of long fibres. Structures such as these are unique to the bacteriophages.
Animal viruses exhibit extreme variation in size and shape. The smallest animal viruses belong to the families Parvoviridae and Picornaviridae and measure about 20 nm and about 30 nm in diameter, respectively. Viruses of these two families are icosahedrons and contain nucleic acids with limited genetic information. Viruses of the family Poxviridae are about 250 to 400 nm in their longest dimension, and they are neither polygons nor filaments. Poxviruses are structurally more complex than simple bacteria, despite their close resemblance. Animal viruses that have rod-shaped (helical) nucleocapsids are those enclosed in an envelope; these viruses are found in the families Paramyxoviridae, Orthomyxoviridae, Coronaviridae, and Rhabdoviridae. Not all enveloped viruses contain helical nucleocapsids, however; those of the families Herpesviridae, Retroviridae, and Togaviridae have polygonal nucleocapsids. Most enveloped viruses appear to be spherical, although the rhabdoviruses are elongated cylinders.
The criteria used for classifying viruses into families and genera are primarily based on three structural considerations: (1) the type and size of their nucleic acid, (2) the shape and size of the capsids, and (3) the presence of a lipid envelope, derived from the host cell, surrounding the viral nucleocapsid.
The nucleic acid
As is true in all forms of life, the nucleic acid of each virus encodes the genetic information for the synthesis of all proteins. In almost all free-living organisms, the genetic information is in the form of double-stranded DNA arranged as a spiral lattice joined at the bases along the length of the molecule (a double helix). In viruses, however, genetic information can come in a variety of forms, including single-stranded or double-stranded DNA or RNA.
The nucleic acids of virions are arranged into genomes. All double-stranded DNA viruses consist of a single large molecule, whereas most double-stranded RNA viruses have segmented genomes, with each segment usually representing a single gene that encodes the information for synthesizing a single protein. Viruses with single-stranded genomic DNA are usually small, with limited genetic information. Some single-stranded DNA viruses are composed of two populations of virions, each consisting of complementary single-stranded DNA of polarity opposite to that of the other.
The virions of most plant viruses and many animal and bacterial viruses are composed of single-stranded RNA. In most of these viruses, the genomic RNA is termed a positive strand because the genomic RNA acts as mRNA for direct synthesis (translation) of viral protein. Several large families of animal viruses, and one that includes both plant and animal viruses (the Rhabdoviridae), however, contain genomic single-stranded RNA, termed a negative strand, which is complementary to mRNA. All of these negative-strand RNA viruses have an enzyme, called an RNA-dependent RNA polymerase (transcriptase), which must first catalyze the synthesis of complementary mRNA from the virion genomic RNA before viral protein synthesis can occur. These variations in the nucleic acids of viruses form one central criterion for classification of all viruses.
A distinctive large family of single-stranded RNA viruses is called Retroviridae; the RNA of these viruses is positive, but the viruses are equipped with an enzyme, called a reverse transcriptase, that copies the single-stranded RNA to form double-stranded DNA.
The protein capsid
The protein capsid provides the second major criterion for the classification of viruses. The capsid surrounds the virus and is composed of a finite number of protein subunits known as capsomeres, which usually associate with, or are found close to, the virion nucleic acid.
There are two major classes of viruses based on the protein capsid: (1) those in which a single (or segmented) linear nucleic acid molecule with two free ends is essentially completely extended or somewhat coiled (a helix) and (2) those in which the nucleic acid, which may or may not be a covalently closed circle, is wound tightly into a condensed configuration, like a ball of yarn. These two classes of virus assume in the first case a long, extended rodlike structure and in the second case a symmetrical polygon.
By far the best-studied example of a helical rod-shaped virus is the tobacco mosaic virus, which was crystallized by Wendell Stanley in 1935. The tobacco mosaic virus contains a genome of single-stranded RNA encased by 2,130 molecules of a single protein; there are 161/3 protein molecules for each turn of the RNA helix in the ratio of three nucleotides for each protein molecule.
Under the right environmental conditions, viral RNA and protein molecules in liquid suspension will assemble themselves into a perfectly formed and fully infectious virus. The length of the helical virus capsid is determined by the length of the nucleic acid molecule, which is the framework for the assembly of the capsid protein. The various helical viruses have different lengths and widths, depending on the size of the nucleic acid as well as on the mass and shape of the protein molecule. Some of these helical viruses form rigid rods, while others form flexible rods, depending on the properties of the assembled proteins.
Polygonal viruses vary greatly in size, from 20 to 150 nm in diameter, essentially proportional to the size of the nucleic acid molecule coiled up inside the virion. Most, if not all, of the polygonal viruses are icosahedral; like a geodesic dome, they are formed by equilateral triangles, in this case 20. Each triangle is composed of protein subunits (capsomeres), often in the form of hexons (multiples of six) that are the building blocks of the capsid. There are 12 vertices (the apical junctions of these 20 triangles), each comprising a penton (five subunits). These icosahedral virions have three axes of fivefold, threefold, and twofold rotational symmetry. The number of capsomeres is a basis for taxonomic classification of these virus families. Certain icosahedral viruses, usually those that are more complex, contain internal proteins adhering to the nucleic acid that are not accessible at the surface of the virions.
The lipoprotein envelope
Surrounding viruses of either helical or icosahedral symmetry are lipoprotein envelopes, unit membranes of two lipid layers interspersed with protein molecules (lipoprotein bilayer). These viral membranes are composed of phospholipids and neutral lipids (largely cholesterol) derived from cell membranes during the process known as budding. Virtually all proteins of the cell membrane, however, are replaced by proteins of viral origin during budding. Although all the viral envelope lipids originate from the cell, their relative proportions vary from those in the cell membrane because the viral proteins select only certain lipids during budding.
Associated with the virion membrane are “integral” glycoproteins, which completely traverse the lipid bilayer, and “peripheral” matrix proteins, which line the inner surface. The glycoproteins contain regions of amino acids that, in the first step of viral infection, recognize host-cell receptors. Matrix proteins appear to function in the selection of regions of the cell membrane to be used for the viral membrane, as well as to aid the virion in entering cells.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#82 2018-02-08 00:15:41
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,728
Re: Miscellany
69) Triple point
One of the key actions in freeze drying is triple point. People are a bit confused with how this happens and why, and there is also a misconception that this is sublimation. The fact is that triple point is a stage you must achieve for the sublimation phase to start. Sublimation is the transition of a substance directly from the solid to the gas phase without passing through the intermediate liquid phase.
Triple Point Explained:
In physics and chemistry, the triple point of a substance is the temperature and pressure at which three phases (gas, liquid, and solid) of that substance may coexist in thermodynamic equilibrium.
Triple point of water:
Scientific explanation: The single combination of pressure and temperature at which pure water, pure ice, and pure water vapour can coexist in a stable equilibrium occurs at exactly 273.16 kelvins (0.01 °C) and a pressure of 611.73 pascals (ca. 6.1173 millibars, 0.0060373057 atm).
Simply put, the triple point of water is the only temperature at which water can exist in all three states of matter; solid (ice), liquid (water), and gas (water vapour). This temperature is 0.01°C.
At that point, it is possible to change all of the substance to ice, water, or vapour by making infinitesimally small changes in pressure and temperature.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#83 2018-02-17 18:34:43
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,728
Re: Miscellany
70) The Land of Midnight Sun
The day that never ends
Like a prolonged sunset and sunrise all at once, the midnight sun colours heaven and earth in a reddish yellow light.
It’s tempting to wonder about all the sights and experiences that have been made under the midnight sun through the ages – by people living off the sea at the Lofoten and Vesterålen archipelagos, or the Sami reindeer herders of the far north.
The phenomena has at least made a lasting impression on several Norwegian artists and writers. This excerpt is from Knut Hamsuns Pan (1894): “Night was coming on again; the sun just dipped into the sea and rose again, red, refreshed, as if it had been down to drink. I could feel more strangely on those nights than anyone would believe” ...
The earth is rotating at a tilted axis relative to the sun, and during the summer months the North Pole is angled towards our star. That’s why, for several weeks, the sun never sets above the Arctic Circle.
Going there, you can live these moments yourself: Doing a whale safari, or exploring the wilderness inland, takes on a new dimension at night in the summer months, when you literally get to see the nature and wildlife in a different light.
If you’re not afraid of the sometimes chilly summer nights in the north, you could try a midnight swim – or you can pitch your tent in the wild and stay up while the sun doesn’t go down. Many sights and activities are open at night during these weeks, so you can do midnight golfing, cycling, river paddling or sea kayaking, or maybe just find a quiet spot to fish.
If you travel to the arctic islands of Svalbard, the sun doesn’t set between April and late August. Here you can do a midnight walk on a glacier or look at the reddish sky from a moving dogsled, experiencing the unique climate and nature near the North Pole.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#84 2018-02-18 22:18:38
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,728
Re: Miscellany
71) Einstein's riddle:
The situation
a) There are 5 houses in five different colors.
b) In each house lives a person with a different nationality.
c) These five owners drink a certain type of beverage, smoke a certain brand of cigar and keep a certain pet.
d) No owners have the same pet, smoke the same brand of cigar or drink the same beverage.
The question is: Who owns the fish?
Hints
i) the Brit lives in the red house
ii) the Swede keeps dogs as pets
iii) the Dane drinks tea
iv) the green house is on the left of the white house
v) the green house's owner drinks coffee
vi) the person who smokes Pall Mall rears birds
vii) the owner of the yellow house smokes Dunhill
viii) the man living in the center house drinks milk
ix) the Norwegian lives in the first house
x) the man who smokes blends lives next to the one who keeps cats
xi) the man who keeps horses lives next to the man who smokes Dunhill
xii) the owner who smokes BlueMaster drinks beer
xiii) the German smokes Prince
xiv) the Norwegian lives next to the blue house
xv) the man who smokes blend has a neighbor who drinks water
Einstein wrote this riddle last century. He said that 98% of the world could not solve it.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#85 2018-02-20 15:27:30
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,728
Re: Miscellany
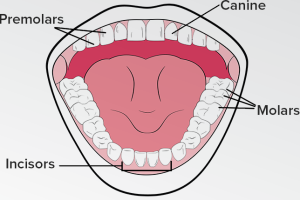
72) Four Different Types Of Teeth & Their Function
Four Different Types Of Teeth & Their Function
Teeth are perhaps the most important element found in our mouth. It plays a major role in our ability to eat as well as presentation and various other bodily tasks. In fact, if we say that healthy teeth are vital to oral health, it would not be a false statement to make. As the holder of this great gift of nature, Dr. Shervin Louie, the renowned dentist of Los Angeles, says that everyone should know and understand the what are the types of teeth human’s evolve throughout the age, their functionality, and how to take care of them. This knowledge is essential to every person who takes a keen interest in maintaining their oral health. So, let us look at the four types of main teeth and how important their functionality is to us.
Four Main Types Of Teeth
Humans have two sets of teeth from which we develop four different types of teeth throughout our life span. These two sets of teeth are:
Primary
Permanent
Primary teeth set contains the infant or baby teeth. These teeth do not change their form until the time we grow to a more mature age in the early teens. Starting from early teens, the first of the permanent teeth starts to appear in our mouth. Once we start to develop permanent teeth, the four different types of them start to evolve as we age through the life. “These four types of teeth are about to play a major role in your life,” states Dr. Shervin Louie, a dentist in Los Angeles. Let’s learn about them now.
Incisors:
Incisors are the pair of eight teeth directly situated in the front and center of our mouth. The format includes four on top and four on the bottom. These teeth are immensely important since we use them to take the first bite of food or anything that you break with them (latter is a dangerous activity though). These teeth come in the first 6-months and then develop in a lifelong formidable shape between the ages of 6 – 8 years. Regular brushing keeps these teeth healthier and strong as well as good calcium diet.
Canines:
There are four canine teeth in our mouth and these are the sharpest of all. These teeth first appear between 11 and 20 months of age and take their lifelong formidable shape between 11 to 12 years. These teeth should stay in healthy form because they are used to tear and wear your food and plays an important part in a proper digestion process.
Like the Incisors, two are located above and two below. These teeth along with Incisors play a vital role in digestion and presentation in the form of our smile. So bad habits like smoking and chewing betel nuts severely damages them and you may end up going to a dentist in Los Angeles for a treatment. Therefore, brush daily, do flossing between the gaps of these teeth, and avoid bad habits to escape severe disease or damage.
Premolars:
Premolar teeth are situated at each side of your mouth in deep settings. These teeth are used for chewing and grinding food so that it becomes totally in a semi-liquid form helping food particles to gulp down the throat and digest smoothly. These teeth first premolar in the upper jaw appears at the age of 10 while the second in the bottom appears at 11.
Since these teeth are situated deep into the mouth and rarely gets exposure to the outside air, Dr. Shervin Louie, the renowned dentist in Los Angeles, recommends a thorough brushing and the use of mouth rinsing agents in order to protect them from bacterial attack. Also, these teeth have the most higher chances of developing plaque, tarter, and germs because of their remote location so the methods mentioned above will give them good protection.
Molars:
The final type of our teeth are the molars, a formidable replacement of permanent premolar ones. In the premolar form, there were only two teeth above and two below. However, when molars appear at the age 11 – 13 years, they add two more teeth to their pair – four above and four below –.
These molars will remain in their current form your entire life, given that you keep a good care of your dental health. As described above, molars are more prone to germ attacks and other deficiencies because of their remote location in your mouth so follow the methods of cleaning described by Dr. Shervin Louie in the paragraph above for premolars.
So, these are the major four types of teeth humans grow throughout their lifespan and their precious functionality which gives them an edge in our bodily system. As a great tip, apart from the daily care, you take off your dental health at home, it is recommended that you should at least visit twice a dentist in the 6-months period for a thorough checkup of your oral health.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#86 2018-02-22 14:36:11
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,728
Re: Miscellany
73) Port of Antwerp
The Port of Antwerp in Flanders, Belgium, is a port in the heart of Europe accessible to capesize ships. It is Europe’s second-largest seaport, after Rotterdam. Antwerp stands at the upper end of the tidal estuary of the Scheldt. The estuary is navigable by ships of more than 100,000 Gross Tons as far as 80 km inland. Like Hamburg, the Port of Antwerp's inland location provides a more central location in Europe than the majority of North Sea ports. Antwerp's docks are connected to the hinterland by rail, road, and river and canal waterways. As a result, the port of Antwerp has become one of Europe's largest seaports, ranking second behind Rotterdam by total freight shipped. Its international rankings vary from 11th to 20th (AAPA).
In 2012, the Port of Antwerp handled 14,220 sea trade ships (190.8 million tons of cargo, 53.6% in containers), 57,044 inland barges (123.2 million tons of cargo),[4] and offered liner services to 800 different maritime destinations.
Recent history
Antwerp's potential was recognized by Napoleon Bonaparte and he ordered the construction of Antwerp's first lock and dock in 1811. Called the Bonaparte Dock, it was joined by a second dock - called the Willem Dock after the Dutch King - in 1813. When the Belgian revolution broke out in 1830, there was a well-founded fear that the Dutch would blockade the Scheldt again but, in the event, they contented themselves with levying a stiff toll. Fortunately, the young Belgium had friends in Britain and particularly in the person of Lord Palmerston, who believed the existence of Belgium would be beneficial to Britain, and that, in consequence, it was important to make sure that the newly born state was economically viable. With his support, the Belgian government was able to redeem the Dutch Toll in 1863. By that time, the Kattendijk Dock had been completed in 1860 and the all important Iron Rhine Railway to the Ruhr had been finished in 1879. Antwerp then experienced a second golden age and by 1908 eight docks had been constructed. The opening of the Royers Lock, commenced in 1905, meant that ships drawing up to 31 feet (9.4 m) of water were able to enter the existing docks and access the new Lefèbvre and America docks.
Such was the situation at the outbreak of the First World War in 1914. The British, and Winston Churchill, then First Lord of the Admiralty, in particular were well aware of the Port of Antwerp's strategic importance, so much so that Churchill arrived in Antwerp on 4 October 1914 to take charge of the defence of the city and its port.
In 1944 during the Second World War Allied forces liberated Antwerp on 4 September. The port and facilities were relatively undamaged and no major reconstruction work was required. However the port could not be used until 28 November, after the estuary approaches were cleared by the Battle of the Scheldt. Walcheren was the key that allowed use of the port, located further upstream on the right bank of the southern estuary of the river. Walcheren was attacked by Canadian and British forces and on 8 November all German resistance on the island had been overrun. An agreement assigned a large portion of the northern section of the port to the Americans and the southern section and the city of Antwerp to the British forces. The first US cargo vessel James B. Weaver arrived on 28 November 1944 with men of the 268th Port Company and their equipment on board. By mid-December the port was operating in high gear and, on average, some 9,000 civilians were employed by the Americans. Despite enemy air attacks, rockets and buzz bombs, operations were never entirely halted, although they were interrupted. In the first half year of 1945, the average amount of cargo discharged was around 0.5 million tons per month. After the close of the hostilities in Europe, the port was used for shipments of ammunition, vehicles, tanks and personnel to the Pacific. After the capitulation of Japan, shipments were directed to the United States. As from November 1945 the activities declined and by October 1946 all US Army operations ceased.
When peace returned work started on the Grote Doorsteek, an ambitious plan which ultimately resulted in the extension of the docklands on the right bank of the Scheldt to the Dutch border. The construction of the Berendrecht Lock was the crowning element of this plan. It was the world's largest shipping lock when inaugurated in 1989. Since 1989, development has been concentrated on the creation of fast turnround tidal berths, both on the Right Bank (Europa Terminal and the North Sea Terminal) and on the Left Bank (Deurganck Dock).
Port lay-out
The Right Bank
With the opening of the Berendrecht Lock (1989), a crowning achievement in developing the right bank dock complex was obtained. With a length of 500 m between the lock gates and a width of 68 m, the Berendrecht lock is the largest lock in the world. This lock has a depth of 13.50 m, which makes the sill depth at mean high water equal to 17.75 m. Apart from the Lock, still further development of the right bank has been undertaken on the banks of the Scheldt outside the dock complex. Two large container terminals have been opened here. In 1990, the Europe terminal was operative, while secondly, the North Sea terminal became operative in 1997. The older areas of the port, such as the Bonaparte dock,[9] are being modernized as needs dictate to make them suitable for modern cargo handling operations. Among this modernisation, an upgrade of the Amerika dock, the Albert dock and the third harbour dock are being done to make them accessible to Panamax ships, which have a draught of 42 feet (13 m). Other modernisation projects being undertaken is the Delwaide dock, which will soon be able to serve the latest generation of container vessels. The Southern part of the Delwaide dock, the MSC Home Terminal is a partnership between PSA Hesse-Noord Natie and Mediterranean Shipping Company (MSC). Due to a total quay length of more than 2 km, several ships can be handled at the same time. The MSC Home Terminal has an annual capacity of more than 3.6 million twenty-foot equivalent units (TEU).
The Left Bank
The first plans for the development of the Waaslandhaven on Antwerp’s left bank were prepared in the boom years of the 1960s. At that time, it was hoped that agreement could be reached with the Dutch on the construction of the Baalhoek Canal, which would have run from Kallo in Belgium through the Drowned Land of Saefthinge (on Dutch territory) into the Western Scheldt. This grand concept had the advantage that it would cut off the difficult bend known as the Bocht van Bath and facilitate access to deep draught ships.
Work started on the Kallo Lock in 1979, and by the end of the 1980s the basic outlines of the Waaslandhaven were by and large complete. The main constituents are the Waasland Canal, the Verrebroek Dock, and the Vrasene Dock. The abandonment of the Baalhoek Canal project meant that an additional dock, known as the Doel Dock, would never be fitted out for shipping.
The development of the sites in the new docklands got off to a slow start, but took off in the 1990s. Nowadays, the trades handled in the Vrasene Dock include forest products, fruit juice, cars, plastic granulates, scrap and bulk gas. The equipping of the Verrebroek Dock started in 1996 and saw the arrival of its first seagoing ship in 2000. When finalized, this dock will offer a total of 5 km of berths with a draught of 14.5 m.
The Deurganck Dock
Since the existing container terminals on the right bank of the Scheldt have reached their maximum capacity and the container freight volume keeps increasing (in 2007 it expanded by 8.2% to 8 million TEU), a new dock complex was constructed: the tidal Deurganck Dock, which is open to the river and which does not require vessels to pass through any lock. The first terminal in this dock was opened on July 6, 2005. The full capacity of the dock is estimated at more than 8 to 9 million TEU. The Deurganck dock has a wharf length of 5.5 km and consists of a total of 1,200,000 cubic metres of concrete. The Kieldrecht Lock, a new lock at the end of the Deurganckdock, giving access to the docks in the port area on the left bank opened in June 2016 and is the largest dock in the world. The lock is deeper than the Berendrecht Lock, the previous largest, in response to the trend towards ever-larger ships. The lock, which represents an investment of 340 million euros, is the second lock into the enclosed harbours and represents a failsafe feature; had the sole lock failed, any vessels inside would have been trapped, whereas it is highly improbable that both locks might simultaneously fail. On the landward side, facing the dock complex, the lock leads into the Waasland canal. From there the ships have easy access to all the other docks on the left bank: the Doel dock, the Verrebroek dock, the Vrasene dock and the North and South mooring docks.
Future
In October 2010, the port approved a long-term investment plan, worth 1.6 billion Euros over the next 15 years. The port would improve existing facilities, and acquire land from General Motors which is closing its Antwerp factory.
Unlike the Port of Rotterdam, which has been able to expand westwards along the river Maas to Europoort and extend into the North Sea with Maasvlakte, Antwerp has little scope for further westward expansion. The northern (right bank) docks already reach the Dutch border, and on the left bank Belgium has a nuclear power plant downstream of the Deurganck dock. The Netherlands has territory on the south bank of the Scheldt, so the Port of Antwerp does not control the outer estuary of the river as it reaches the sea.
Lillo Port Centre
On the east bank of the river Scheldt, but to the west of the main port area lies the old village of Lillo, where the Port of Antwerp has built a new visitor centre. Coach parties can arrive here, and (after a brief introduction and the donning of hard hats and hi-viz jackets) an official guide boards the coach and directs the party to visit places that would otherwise be prohibited under ISPS. (International Ship and Port Facility Security).

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#87 2018-02-24 19:59:02
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,728
Re: Miscellany
74) Statue of Liberty
Statue of Liberty, formally Liberty Enlightening the World, colossal statue on Liberty Island in the Upper New York Bay, U.S., commemorating the friendship of the peoples of the United States and France. Standing 305 feet (93 metres) high including its pedestal, it represents a woman holding a torch in her raised right hand and a tablet bearing the adoption date of the Declaration of Independence (July 4, 1776) in her left. The torch, which measures 29 feet (8.8 metres) from the flame tip to the bottom of the handle, is accessible via a 42-foot (12.8-metre) service ladder inside the arm (this ascent was open to the public from 1886 to 1916). An elevator carries visitors to the observation deck in the pedestal, which may also be reached by stairway, and a spiral staircase leads to an observation platform in the figure’s crown. A plaque at the pedestal’s entrance is inscribed with a sonnet, “The New Colossus” (1883) by Emma Lazarus. It was written to help raise money for the pedestal, and it reads:
Not like the brazen giant of Greek fame,
With conquering limbs astride from land to land;
Here at our sea-washed, sunset gates shall stand
A mighty woman with a torch, whose flame
Is the imprisoned lightning, and her name
Mother of Exiles. From her beacon-hand
Glows world-wide welcome; her mild eyes command
The air-bridged harbor that twin cities frame.
“Keep, ancient lands, your storied pomp!” cries she
With silent lips. “Give me your tired, your poor,
Your huddled masses yearning to breathe free,
The wretched refuse of your teeming shore.
Send these, the homeless, tempest-tost to me,
I lift my lamp beside the golden door!”
A French historian, Edouard de Laboulaye, made the proposal for the statue. Funds were contributed by the French people, and work began in France in 1875 under sculptor Frédéric-Auguste Bartholdi. The statue was constructed of copper sheets, hammered into shape by hand and assembled over a framework of four gigantic steel supports, designed by Eugène-Emmanuel Viollet-le-Duc and Alexandre-Gustave Eiffel. The colossus was presented to the American minister to France Levi Morton (later vice president) in a ceremony in Paris on July 4, 1884. In 1885 the completed statue, 151 feet 1 inch (46 metres) high and weighing 225 tons, was disassembled and shipped to New York City. The pedestal, designed by American architect Richard Morris Hunt and built within the walls of Fort Wood on Bedloe’s Island, was completed later. The statue, mounted on its pedestal, was dedicated by President Grover Cleveland on October 28, 1886. Over the years the torch underwent several modifications, including its conversion to electric power in 1916 and its redesign (with repoussé copper sheathed in gold leaf) in the mid-1980s, when the statue was repaired and restored by both American and French workers for a centennial celebration held in July 1986. The site was added to UNESCO’s World Heritage List in 1984.
The statue was at first administered by the U.S. Lighthouse Board, as the illuminated torch was considered a navigational aid. Because Fort Wood was still an operational Army post, responsibility for the maintenance and operation of the statue was transferred in 1901 to the War Department. It was declared a national monument in 1924, and in 1933 the administration of the statue was placed under the National Park Service. Fort Wood was deactivated in 1937, and the rest of the island was incorporated into the monument. In 1956 Bedloe’s Island was renamed Liberty Island, and in 1965 nearby Ellis Island, once the country’s major immigration station, was added to the monument’s jurisdiction, bringing its total area to about 58 acres (about 24 hectares). Exhibits on the history of the Statue of Liberty, including the statue’s original 1886 torch, are contained in the statue’s base.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#88 2018-02-27 00:53:17
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,728
Re: Miscellany
75) Tachometer
Tachometer, device for indicating the angular (rotary) speed of a rotating shaft. The term is usually restricted to mechanical or electrical instruments that indicate instantaneous values of speed in revolutions per minute, rather than devices that count the number of revolutions in a measured time interval and indicate only average values for the interval.
Mechanical tachometers utilize the fact that the centrifugal force on a rotating mass depends on the speed of rotation and can be used to stretch or compress a mechanical spring. A resonance, or vibrating-reed, tachometer uses a series of consecutively tuned reeds to determine engine speed by indicating the vibration frequency of the machine.
Electrical tachometers are of several types. The eddy-current, or drag, type is widely used in automobile speedometers; a magnet rotated with the shaft being measured produces eddy currents that are proportional to angular speed. Electric-generator tachometers work by generating either an alternating or a direct current. The stroboscope, an instrument that illuminates rotating objects so that they appear to have stopped moving, can be used as a tachometer.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#89 2018-03-01 00:27:31
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,728
Re: Miscellany
76) The Life of Dolly
Making Dolly
Dolly was part of a series of experiments at The Roslin Institute that were trying to develop a better method for producing genetically modified livestock. If successful, this would mean fewer animals would need to be used in future experiments. Scientists at Roslin also wanted to learn more about how cells change during development and whether a specialised cell, such as a skin or brain cell, could be used to make a whole new animal.
These experiments were carried out at The Roslin Institute by a team led by Professor Sir Ian Wilmut. Because of the nature of the research, the team was made up of many different people, including scientists, embryologists, surgeons, vets and farm staff.
Dolly was cloned from a cell taken from the mammary gland of a six-year-old Finn Dorset sheep and an egg cell taken from a Scottish Blackface sheep. She was born to her Scottish Blackface surrogate mother on 5th July 1996. Dolly’s white face was one of the first signs that she was a clone because if she was genetically related to her surrogate mother, she would have had a black face.
Because Dolly’s DNA came from a mammary gland cell, she was named after the country singer Dolly Parton.
Why was Dolly so important?
Dolly was important because she was the first mammal to be cloned from an adult cell. Her birth proved that specialised cells could be used to create an exact copy of the animal they came from. This knowledge changed what scientists thought was possible and opened up a lot of possibilities in biology and medicine, including the development of personalised stem cells known as iPS cells.
However, Dolly was not the first ever cloned mammal. That honour belongs to another sheep which was cloned from an embryo cell and born in 1984 in Cambridge, UK. Two other sheep, Megan and Morag, had also been cloned from embryonic cells grown in the lab at The Roslin Institute in 1995 and six other sheep, cloned from embryonic and foetal cells, were born at Roslin at the same time as Dolly. What made Dolly so special was that she had been made from an adult cell, which no-one at the time thought was possible.
Dolly’s life
Dolly was announced to the world on 22nd February 1997 to a frenzy of media attention. The Roslin team chose to make the announcement at this time to coincide with the publication of the scientific paper which describes the experiments that produced her. Dolly captured the public’s imagination – no small feat for a sheep – and sparked a public debate about the possible benefits and dangers of cloning.
In the week following the announcement, The Roslin Institute received 3,000 phone calls from around the world.
When Dolly was one year old, analysis of her DNA showed that her telomeres were shorter than would be expected for a normal sheep of the same age. Telomeres are ‘caps’ on the ends of DNA molecules that protect the DNA from damage. As an animal or person ages, their telomeres become progressively shorter, exposing the DNA to more damage.
It’s thought that Dolly had shorter telomeres were because her DNA came from an adult sheep and the telomeres had not been fully renewed during her development. This could have meant that Dolly was ‘older’ than her actual age. However, extensive health screens on Dolly at the time did not find any conditions which could be directly related to premature or accelerated ageing.
Dolly spent her life at The Roslin Institute and, apart from the occasional media appearance, led a normal life with the other sheep at the Institute. Over the years Dolly had a total of six lambs with a Welsh Mountain ram called David. Their first lamb, Bonnie, was born in April 1998, twins Sally and Rosie were born the following year and triplets Lucy, Darcy and Cotton the year after.
After Dolly gave birth to her last lambs in September 2000, it was discovered that she had become infected by a virus called Jaagsiekte sheep retrovirus (JSRV), which causes lung cancer in sheep. Other sheep at The Roslin Institute had also been infected with JSRV in the same outbreak.
In 2001, Dolly was diagnosed with arthritis after farm staff noticed her walking stiffly. This was successfully treated with anti-inflammatory medication, although the cause of the arthritis was never discovered.
Dolly continued to have a normal quality of life until February 2003, when she developed a cough. A CT scan showed tumours growing in her lungs and the decision was made to euthanise Dolly rather than risk her suffering. Dolly was put to sleep on 14th February 2003, at the age of six.
Where is Dolly now?
After her death The Roslin Institute donated Dolly’s body to the National Museum of Scotland in Edinburgh, where she has become one of the museum’s most popular exhibits. Dolly is back on display in the museum after an extensive gallery refurbishment, alongside an interactive exhibit on the ethics of creating transgenic animals featuring current research from The Roslin Institute.
Dolly’s life
Dolly was announced to the world on 22nd February 1997 to a frenzy of media attention. The Roslin team chose to make the announcement at this time to coincide with the publication of the scientific paper which describes the experiments that produced her. Dolly captured the public’s imagination – no small feat for a sheep – and sparked a public debate about the possible benefits and dangers of cloning.
Photograph: Dolly and her lamb, Bonnie

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#90 2018-03-02 00:44:28
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,728
Re: Miscellany
77) Electric Iron
How Does Temperature Regulation In An Electric Iron Work?
The working of an electric iron is very simple – it takes current from the mains and heats up a coil inside it. This heat is then transferred to the base plate which is pressed against clothes to remove creases.
Back when I was learning how to iron my clothes, I was rather annoyed by the whole process. For no reason whatsoever, it kept switching on and off of its own accord. As much as I was irritated by this, I was also intrigued by the strange phenomenon. Thankfully, I soon came to know that it was the ‘automatic power cut’ feature that prompted this action in the iron.
You’ve almost certainly observed this automatic power on/off function in electric irons, but do you know how it works? How does the iron know when to cut off the power? More importantly, how does an iron go about actually doing that?
What does the thermostat do in an electric iron?
The most important component that helps to regulate temperature in an electric iron is the ‘thermostat’. Everyone has heard of thermostats in reference to air conditioners, water coolers, maintaining temperature balance in the home, and a number of other appliances that deal in temperature control.
The basic function of a thermostat can be deduced from the name alone; the word is formed from two Greek words: ‘thermo’ (heat) and ‘statis’ (status quo or constant). As the name implies, a thermostat’s basic function is to keep the heat constant in a given setting.
Working of electric iron
The electric iron that we use to press the creases out of our garments also contains a thermostat, which makes sure that the iron doesn’t get too hot if it’s kept switched on or left unattended for an extended period of time. Let’s take a look at exactly how the mechanism works.
An electric iron relies on a basic combination of heat and pressure to remove creases from clothes. When an electric current is passed through a coil (or any other heating element present in the iron), it gets very hot. This heat is then transferred to the base plate (the smooth, flat surface that you place against clothes while ironing) through conduction, which elegantly and precisely irons your clothes.
However, if the iron is continuously drawing electricity from the power supply, the heating element continues getting hotter. This causes a lot of energy wastage (as an iron consumes a lot of electricity even in a few minutes), ruins your clothes, and in the worst cases, causes nasty (and potentially dangerous!) accidents.
Therefore, it’s essential that the iron doesn’t heat up to hazardous temperatures.
Bimetallic strip
The thermostat in an iron uses a bimetallic strip, and as the name implies, a bimetallic strip is made up of two different types of metal – with dissimilar coefficients of expansion – that are bonded together. This means that in the presence of heat, they expand differently. This bimetallic strip is connected to a contact spring through small pins.
At moderate temperatures, the contact point remains in physical contact with the bimetallic strip. However, when the temperature of the iron exceeds a certain limit, the strip begins to bend towards the metal with a lower coefficient of expansion. As a result, the strip ceases to be physically connected to the contact point, the circuit opens and current ceases to flow.
Given that the circuit remains open for some time, the temperature of the iron drops, the strip acquires its original shape, and the current flows again. This cycle is repeated until you switch off its power supply from the main electricity source. This is the reason why your iron seems to power on and off of its own accord.
Now that we’ve cleared that up, if you thought that your iron was dysfunctional and were thinking of taking it to a electrician, you should feel proud of yourself – you just saved some money!

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#91 2018-03-03 15:20:56
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,728
Re: Miscellany
78) Sulfuric Acid
Sulfuric acid, sulfuric also spelled sulphuric (H2SO4), also called oil of vitriol, or hydrogen sulfate, dense, colourless, oily, corrosive liquid; one of the most important of all chemicals, prepared industrially by the reaction of water with sulfur trioxide, which in turn is made by chemical combination of sulfur dioxide and oxygen either by the contact process or the chamber process. In various concentrations the acid is used in the manufacture of fertilizers, pigments, dyes, drugs, explosives, detergents, and inorganic salts and acids, as well as in petroleum refining and metallurgical processes. In one of its most familiar applications, sulfuric acid serves as the electrolyte in lead–acid storage batteries.
Pure sulfuric acid has a specific gravity of 1.830 at 25 °C (77 °F); it freezes at 10.37 °C (50.7 °F). When heated, the pure acid partially decomposes into water and sulfur trioxide; the latter escapes as a vapour until the concentration of the acid falls to 98.3 percent. This mixture of sulfuric acid and water boils at a constant temperature of 338 °C (640 °F) at one atmosphere pressure. Sulfuric acid is commonly supplied at concentrations of 78, 93, or 98 percent.
Sulfuric acid is a very strong acid; in aqueous solutions it ionizes completely to form hydronium ions and hydrogen sulfate ions. In dilute solutions the hydrogen sulfate ions also dissociate, forming more hydronium ions and sulfate ions. In addition to being an oxidizing agent, reacting readily at high temperatures with many metals, carbon, sulfur, and other substances, concentrated sulfuric acid is also a strong dehydrating agent, combining violently with water; in this capacity, it chars many organic materials, such as wood, paper, or sugar, leaving a carbonaceous residue.
The term fuming sulfuric acid, or oleum, is applied to solutions of sulfur trioxide in 100 percent sulfuric acid; these solutions, commonly containing 20, 40, or 65 percent sulfur trioxide, are used for the preparation of organic chemicals.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#92 2018-03-05 02:05:06
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,728
Re: Miscellany
79) The Bee Hummingbird
Renowned among birdwatchers everywhere as the world’s smallest bird, although the Vervain Hummingbird (Mellisuga minima), of Jamaica and Hispaniola, is virtually as tiny. The Bee Hummingbird is endemic to the main island of Cuba, although it formerly also occurred on the Isle of Youth, and is currently considered to be Near Threatened under IUCN criteria. It is easily found only in three regions of Cuba, namely the extreme west, on the Guanahacabibes Peninsula, further east in the vicinity of the Zapata Swamp, and more widely in the far east of the island. The species feeds on nectar and insects. While females are distinguished from the much more widespread and ubiquitous Cuban Emerald (Chlorostilbon ricordii) by their smaller size, much shorter tail, turquoise-blue upperparts, and cleaner, whiter underparts, among other features, males, which sing from high perches and in flight, are even more easily identified, and highly sought by visiting birdwatchers, because of the brilliant iridescent red head and throat, with elongated neck plumes.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#93 2018-03-06 15:59:40
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,728
Re: Miscellany
80) Adrenaline
Adrenaline is one of the most known hormones in a human body with a very powerful effect on the organs of a body. Evolutionary developed as a response to the extreme situations, adrenaline helps to exercise the organism to the maximum of its capacities.
History of Research
The history of discovery of adrenaline was rocky and most of it was a result of incorrect experiments, but fortuitously it led to great discoveries. In contrast to the majority of endocrine glands, some of which were already discovered by Galen in 2nd century, the adrenal glands have been overlooked for centuries and were only discovered in the 16th century. Even then, the function of the adrenal glands was entirely unknown until the middle of the 19th century when some clues emerged. For example, in 1716, the Académie des sciences de Bordeaux in France had a competition asking: Quel est l’usage des glandes surrénales? (What is the use of the adrenal glands?). The later famous Charles de Montesquieu (1689-1755) judged the competition. After reading the prize submissions, Montesquieu decided that none of the submissions merited a prize and expressed the hope that one day the problem of the adrenals would be solved.
The first hint that the adrenal glands were important came from the clinical observations of the British physician Thomas Addison in 1855. He had consulted on patients who were severely fatigued, had weight loss, vomiting, and a strange darkening of the skin. He found at the autopsy of the patients that they all had damaged adrenal glands. He proposed that destruction of the adrenal glands — organs with no known function — caused their deaths. About a year later Charles-Édouard Brown-Séquard in France surgically removed the adrenal glands from laboratory animals. All the animals died, supporting the hypothesis that the adrenals were essential for life.
Neither Addison nor Brown-Séquard knew the actual function of the adrenal glands. Developing evidence that glands like the adrenals secrete active chemicals into the blood was a major intellectual hurdle. Also is was hard to demonstrate with available methods in the latter half of the 19th century. In 1889, Brown-Séquard, being already 72 years old and a very famous senior scientist announced that he had been rejuvenated by injections of extract of particles. This was an incorrect experiment as there were not enough male hormone testosterone used to do anything. But Brown-Séquard’s claims created a world-wide sensation. He stimulated interest in the possibility that extracts of organs might have major physiological effects.
A few years later in England, George Oliver in collaboration with Edward Sharpey-Schafer discovered that extracts of the adrenaline glands raised the blood pressure of dogs. George Oliver was a physician commissioned in a small resort town, where he had spare time to do research. One of his experiments was to feed his son with adrenal glands that he got from a local butcher and try to measure the effect with a device that he had invented himself. Oliver used the device to check on possible changes in the radial artery. This was again an incorrect experiment, as we now know that adrenaline is not absorbed into the body orally. Besides, Oliver’s measuring device was probably not accurate. However, this was an act of serendipity, which prompted physician to take his research further.
In London Oliver met a famous physiologist professor Edward Sharpey-Schafer, who, as a matter of pure interest, injected an extract of the adrenal glands into dogs and was astonished to find that the blood pressure in dogs rose tremendously. This was the first and clear example, that the substance produced in the inner gland had tremendous physiological effect. Immediately after that a race was on to find what was the substance in the adrenal gland that caused the blood pressure to rise. There were labs throughout the world, particularly in Germany, England and US that tried to purify that substance. A number of people claimed to have found it, but actual discovery happened in 1901. The active substance in the adrenal glands responsible for raising blood pressure was isolated by Jokichi Takamine, a Japanese immigrant in the United States. He named it ‘adrenaline’.
Fight or Flight
Adrenaline represents a small molecule synthesized in the center of the adrenal gland — the medulla. The body takes an amino acid called the tyrosine as a starting point to add specific chemical groups to make adrenaline. After being synthesized adrenaline is stored in the gland until there is a need for it. At that moment, the hormone is secreted into the blood to act on distant organs. The idea of what it does was first formulated by Walter Cannon, a famous physiologist at Harvard medical school in 1910-1940. By then it was known that Adrenaline affects almost every organ in the body, but Walter Cannon summed it all together, introducing the notion of “fight or flight response”.
Our ancient ancestors living in a hostile world had to be prepared to react to threats and to be able to mobilize all resources in a short period of time. A rabbit when approached by a wolf has to run away, so as a wolf in order to catch a rabbit has to run at a maximal speed. Adrenaline that the adrenal glands of the rabbit and wolf will release, will integrate all the organs in the body to respond to the need for maximal exertion. The “Fight of flight response” refers to the primary instinct of predatory or victim, to exercise which the help of adrenaline will be needed. The hormone will increase output of blood from the heart to the exercising muscles — bringing more oxygen and nutrients to keep muscles going vigorously. It will cause a heart to beat faster and harder, push a liver to release glucose into blood, make fat to release fatty acids and glycerol, which are nutrients for muscles. In the end, adrenaline will cause the airways in lungs to dilate allowing faster and easier breathing.
Adrenaline glands are located near kidneys and they were missed for thousands of years because they are usually covered up in fat. There are two names of this hormone, which are synonyms: adrenaline and epinephrine. The world health organization name is epinephrine and it comes from the Greek “Epi” (near) and “Nephron” (kidney). Adrenaline is Latin – “ad” (side) and “renal” (kidney).
Amusing Ourselves to Run
Does Adrenaline have anything to do with the feeling of excitement? Many people think that. In English, there are common expressions with adrenaline, for example: “getting someone’s adrenaline going” or “adrenaline junkie”. However, adrenaline has no direct connection with how we feel. Getting on a roller-coaster ride in an amusement park, you might feel excited or frightened. That will trigger adrenaline secretion, but a “feeling” largely comes from a brain. Adrenaline cannot go from a blood into a brain. There are blood-brain barriers that keep it out. Likewise, when adrenaline is injected to people into laboratory they will feel their heart-rate raising, they may feel a little strange, but they will not experience fear or excitement.
It is possible, that stimulate from a body affect the emotional experiences we have, as there are many receptors in tissues carrying nerve signals back to the brain. However, it is an important notice that an experience stimulates secretion of adrenaline, but not vice versa. Emotion first, then an adrenaline secretion second.
Adrenaline is not only released under fear. There is some amount of it released constantly. The level of secretion goes up in response to things that require greater activity of a body. The downside of that, in our modern world, is that things like fear or emotions that do not trigger a physical activity can also stimulate the release of adrenaline. Anything that is exciting can activate the release of adrenaline including video games, watching a movie thriller, attending a soccer match, and getting into an argument. Typical responses are what you would expect: heart beating harder and faster, perspiration under the arms, and slight tremor of the hands if very agitated. The health consequences are generally not important. However, in some people, especially people over 50 or those with existing heart disease, stress responses to sudden excitement can precipitate a heart attack. How emotions can trigger sudden death or heart attacks is an active area of medical investigation.
People can live a normal life without any adrenaline. People that had their adrenal glands surgically removed get pills to replace the cortisol and aldosterone (the two hormones produced in adrenal glands that are essential for life), but they do not need any treatment with adrenaline. Life is possible without it. However, those people would most probably not be able to exert themselves to a maximum that they could when they still had their adrenal glands.
Adrenaline and Medicine
Short after discovery of adrenaline, it was found that it could help in cardiac resuscitation. Adrenaline was used in treatment of many problems such as asthma, anaphylactic shock, croup in infancy to give a few. Dentists use adrenaline by injecting it into the gums, together with the local anesthetic, as adrenaline enables the blood vessels to let a local anesthetic stay longer in the teeth.
Anaphylactic shock is still treated by adrenaline in its pure substance. However, most of the drugs on the base of adrenaline are medical improvements. The hormone cannot be given orally, because it gets metabolized in the liver before it ever reaches the blood. People have developed chemical analogue of adrenaline, that is available orally or that can be inhaled for asthmatics. Adrenaline for injections for cardiac resuscitation was initially produced from tons of adrenal glands from beef or sheep, but now it is chemically synthesized.
The use of adrenaline in a treatment of cardiac arrest — to start the heart going again — is probably the most known. In the US, cardiac arrest kills several hundred thousand people each year. Many of these deaths are due to ventricular fibrillation, often caused by a heart attack. For more than 100 years, adrenaline has been used as a drug in cardiopulmonary resuscitation in an effort to revive these people. Although not conclusive, several recent studies have suggested that adrenaline may not be desirable in cardiac arrests. Just a few years ago, an editorial in the Journal of the American Medical Association suggested that it would be useful and ethical to conduct a serious clinical trial to find out if adrenaline was either beneficial or harmful during cardiac arrests. Despite modern emphasis on ‘evidence-based medicine’, a substantial fraction of current diagnostic and therapeutic clinical practices lack a strong foundation.
Natural Doping
If a sportsman wants to take a doping, he or she will not use adrenaline directly, but will use related drugs. One of the most important is clenbuterol, which is an adrenaline-like drug. In Europe, it was heavily used in the cattle feed to increase the size of muscles and to lower the body fat. Many Olympic athletes have been banned from competition for taking clenbuterol. This used to be a problem for those athletes who have asthma, but recently, the Olympic officials allowed certain adrenaline-like drugs to be used by asthmatic people. As adrenaline substitute is inhaled through lungs the concentrations are still too low to affect muscles much.
Contrariwise, there are drugs that block the effect of adrenaline — beta-blocking drugs like propranolol. Those are also banned in some Olympic sports, particularly in shooting. Adrenaline, when you get excited, can make your hands shake. A shooter taking a drug that blocks the effect of adrenaline can shoot straighter. There have been precedents of athletes banned from the competition for taking these drugs. However, not only athletes use them, it is quite common when professional musicians take such drug to block the effect of adrenaline in order to hold their hands more steadily.
The sympathetic nervous system — nerves that go to all the organs in the body — innervates major organs in the body and modulates their activity. For example, neurons in sympathetic nervous system release noradrenaline near cells in the heart, causing them to beat harder and faster. Adrenaline has many effects similar to those of the sympathetic nervous system but reaches target tissues via the blood. The two systems add together. Noradrenaline is the main transmitter in the sympathetic nervous system. Its structure is very similar to adrenaline, missing just one small chemical group (methyl group) in comparison. If you take out the adrenal glands the effect is probably relatively modest, but if you damage the sympathetic nervous system then you are very sick.
Latest Research
The most exciting question is how exactly adrenaline works. The whole signaling pathway of adrenaline is a model for many other drugs and hormones. Most drugs work by interacting with specific chemicals in the body and adrenaline as well interacts with specific proteins called adrenaline receptors. These are execute genes family, of self-surface receptors called the G-protein-coupled receptors.
Someone has estimated that 10-20 % of the available drugs work through G-protein-coupled receptors. Adrenaline (or any other drug or hormone) fits into them like a key into a lock. In 2012 Robert Lefkowitz at Duke University and Brian Kobilka at Stanford University shared a Nobel prize in chemistry for their work on structure of these receptors. Kobilka has continued to do work using x-ray crystallography to see exactly how adrenaline sits into these proteins and how the conformation of the protein changes.
That is an enormous part of medicine. Deeper understanding of how this signaling pathways work is of fundamental biological interest and could have therapeutic implications. It could lead to new drugs, with novel capacities to activate the adrenaline receptors.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#94 2018-03-06 18:26:50
- Monox D. I-Fly
- Member

- From: Indonesia
- Registered: 2015-12-02
- Posts: 2,000
Re: Miscellany
There are two names of this hormone, which are synonyms: adrenaline and epinephrine. The world health organization name is epinephrine and it comes from the Greek “Epi” (near) and “Nephron” (kidney). Adrenaline is Latin – “ad” (side) and “renal” (kidney).
So that's why in the Elements Collectible Card Game the name of the upgraded Adrenaline card is Epinephrine:
http://elementscommunity.org/forum/life-63/adrenaline-epinephrine/
Actually I never watch Star Wars and not interested in it anyway, but I choose a Yoda card as my avatar in honor of our great friend bobbym who has passed away.
May his adventurous soul rest in peace at heaven.
Offline
#95 2018-03-08 00:03:07
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,728
Re: Miscellany
ganesh wrote:There are two names of this hormone, which are synonyms: adrenaline and epinephrine. The world health organization name is epinephrine and it comes from the Greek “Epi” (near) and “Nephron” (kidney). Adrenaline is Latin – “ad” (side) and “renal” (kidney).
So that's why in the Elements Collectible Card Game the name of the upgraded Adrenaline card is Epinephrine:
http://elementscommunity.org/forum/life-63/adrenaline-epinephrine/
Yes, Monox D. I-Fly!
81) Brain coral
Brain coral is a common name given to various corals in the families Mussidae and Merulinidae, so called due to their generally spheroid shape and grooved surface which resembles a brain. Each head of coral is formed by a colony of genetically identical polyps which secrete a hard skeleton of calcium carbonate; this makes them important coral reef builders like other stony corals in the order Scleractinia. Brain corals are found in shallow warm-water coral reefs in all the world's oceans. They are part of the phylum Cnidaria, in a class called Anthozoa or "flower animals". The lifespan of the largest brain corals is 900 years. Colonies can grow as large as 1.8 m (6 ft) or more in height.
Brain corals extend their tentacles to catch food at night. During the day, they use their tentacles for protection by wrapping them over the grooves on their surface. The surface is hard and offers good protection against fish or hurricanes. Branching corals, such as staghorn corals, grow more rapidly, but are more vulnerable to storm damage. Like other genera of corals, brain corals feed on small drifting animals, and also receive nutrients provided by the algae which live within their tissues. The behavior of one of the most common genera, Favia, is semiaggressive; it will sting other corals with its extended sweeper tentacles during the night.
Favia is a genus of reef-building stony corals in the family Mussidae. Members of the genus are massive or thickly encrusting colonial corals, either dome-shaped or flat, and a few are foliaceous. There is a great diversity of form even among individuals of the same species. The corallites project slightly above the surface of the coral and each has its own wall. In most species, the corallites are plocoid and in some, monocentric. The septa and costae linked to the corallite wall are well developed and covered by fine teeth. The polyps only extend and feed during the night. Each one has a small number of tapering tentacles which often have a darker coloured tip; these are called stinger tentacles, or sweeper tentacles. They use these to sweep the water to see if any other coral is in its area; if so, then they begin to sting the other coral. This is commonly known as coral war. Each coral is trying to make sure it has enough room around it so they can continue to grow and have more surface area for their offspring. The columella is parietal and spongy, and there are vesicles on both the endotheca and exotheca. Members of this genus are widespread in both the Atlantic Ocean and the Indo-Pacific.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#96 2018-03-10 00:12:20
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,728
Re: Miscellany
82) Astigmatism
Astigmatism is a common and generally treatable imperfection in the curvature of your eye that causes blurred distance and near vision.
Astigmatism occurs when either the front surface of your eye (cornea) or the lens, inside your eye, has mismatched curves. Instead of having one curve like a round ball, the surface is egg shaped. This causes blurred vision at all distances.
Astigmatism is often present at birth and may occur in combination with nearsightedness or farsightedness. Often it's not pronounced enough to require corrective action. When it is, your treatment options are corrective lenses or surgery.
Symptoms
Signs and symptoms of astigmatism may include:
i) Blurred or distorted vision
ii) Eyestrain or discomfort
iii) Headaches
iv) Difficulty with night vision
v) Squinting
When to see a doctor
See an eye doctor if your eye symptoms detract from your enjoyment of activities or interfere with your ability to perform everyday tasks. An eye doctor can determine whether you have astigmatism and, if so, to what degree. He or she can then advise you of your options to correct your vision.
Children and adolescents
Children may not realize their vision is blurry, so they need to be screened for eye disease and have their vision tested by a pediatrician, an ophthalmologist, an optometrist or another trained screener at the following ages and intervals.
a) During the newborn period
b) At well-child visits until school age
c) During school years, every one to two years at well-child visits, at the eye doctor, or through school or public screenings
d) Request an Appointment
Causes
Your eye has two structures with curved surfaces that bend (refract) light onto the retina, which makes the images:
(i) The cornea, the clear front surface of your eye along with the tear film
(ii) The lens, a clear structure inside your eye that changes shape to help focus on near objects
In a perfectly shaped eye, each of these elements has a round curvature, like the surface of a smooth ball. A cornea and lens with such curvature bend (refract) all incoming light to make a sharply focused image directly on the retina, at the back of your eye.
A refractive error
If either your cornea or lens is egg shaped with two mismatched curves, light rays aren't bent properly, causing a refractive error. This makes a blurry image. Astigmatism is a type of refractive error.
Astigmatism occurs when your cornea or lens is curved more steeply in one direction than in another. You have corneal astigmatism if your cornea has mismatched curves. You have lenticular astigmatism if your lens has mismatched curves.
Either type of astigmatism can cause blurred vision. Blurred vision may occur more in one direction, either horizontally, vertically or diagonally.
Astigmatism may be present from birth, or it may develop after an eye injury, disease or surgery. Astigmatism isn't caused or made worse by reading in poor light, sitting too close to the television or squinting.
Other refractive errors
Astigmatism may occur in combination with other refractive errors, which include:
Nearsightedness (myopia). This occurs when your cornea is curved too much or your eye is longer than normal. Instead of being focused precisely on your retina, light is focused in front of your retina, making distant objects seem blurry.
Farsightedness (hyperopia). This occurs when your cornea is curved too little or your eye is shorter than normal. The effect is the opposite of nearsightedness. When your eye is in a relaxed state, light never comes to a focus on the back of your eye, making nearby objects seem blurry.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#97 2018-03-11 15:32:50
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,728
Re: Miscellany
83) Altimeter
Altimeter, instrument that measures the altitude of the land surface or any object such as an airplane. The two main types are the pressure altimeter, or aneroid barometer, which approximates altitude above sea level by measuring atmospheric pressure, and the radio altimeter, which measures absolute altitude (distance above land or water) based on the time required for a radio wave signal to travel from an airplane, a weather balloon, or a spacecraft to the ground and back.
The pressure altimeter operates on the principle that average atmospheric pressure decreases linearly with altitude. A typical pressure altimeter is illustrated in the figure. The instrument is enclosed in a case that is connected to the outside of the aircraft by an air pressure inlet at the rear of the housing. Two or more aneroid capsules—i.e., thin corrugated metallic bellows from which air has been exhausted—are positioned near the inlet. These capsules expand when the outside air pressure falls (as in climbing) and contract when the outside air pressure rises (as in descending). By a mechanical arrangement of sector gears, pinion, backlash spring, and crankshaft, the expansion or contraction of the aneroid capsules is converted to the movement of pointers on a dial. The graduated scale dial is marked off in metres or feet, and a series of gear-driven pointers similar to the hands of a clock may be used to indicate the altitude in units of hundreds, thousands, or tens of thousands. The barometric scale dial records the air pressure in millibars (mb). Because atmospheric pressure is measured relative to sea level, a pressure altimeter must be adjusted with a barosetting knob in order to compensate for small variations in barometric pressure caused by changes in local weather.
The radio altimeter measures the distance of an aircraft above the ground rather than above sea level. The altitude is equal to one-half the time that it takes a pulse of radio energy to travel from the aircraft to the ground and back multiplied by the speed of the pulse (equivalent to the speed of light). The measured altitude is displayed on a video screen. Radio altimeters are used in automatic navigation and blind-landing systems.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#98 2018-03-13 00:19:01
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,728
Re: Miscellany
84) Dextrose
What is dextrose?
Dextrose is the name of a simple sugar that is made from corn and is chemically identical to glucose, or blood sugar. Dextrose is often used in baking products as a sweetener, and can be commonly found in items such as processed foods and corn syrup.
Dextrose also has medical purposes. It is dissolved in solutions that are given intravenously, which can be combined with other drugs, or used to increase a person’s blood sugar.
Because dextrose is a “simple” sugar, the body can quickly use it for energy.
Simple sugars can raise blood sugar levels very quickly, and they often lack nutritional value. Examples of other simple sugars include glucose, fructose, and galactose. Products that are typically made of simple sugars include refined sugar, white pasta, and honey.
What are common dextrose preparations?
Dextrose is used to make several intravenous (IV) preparations or mixtures, which are available only at a hospital or medical facility.
Dextrose is also available as an oral gel or in oral tablet form over the counter from pharmacies.
Each dextrose concentration has its own unique uses. Higher concentrations are typically used as “rescue” doses when someone has a very low blood sugar reading.
How is dextrose used?
Dextrose is used in various concentrations for different purposes. For example, a doctor may prescribe dextrose in an IV solution when someone is dehydrated and has low blood sugar. Dextrose IV solutions can also be combined with many drugs, for IV administration.
Dextrose is a carbohydrate, which is one part of nutrition in a normal diet. Solutions containing dextrose provide calories and may be given intravenously in combination with amino acids and fats. This is called total parenteral nutrition (TPN) and is used to provide nutrition to those who cannot absorb or get carbohydrates, amino acids, and fats through their gut.
High-concentration dextrose injections are only given by professionals. These injections are administered to people whose blood sugar may be very low and who cannot swallow dextrose tablets, foods, or drinks.
If a person’s potassium levels are too high (hyperkalemia), sometimes doctors also give dextrose injections of 50 percent, followed by insulin intravenously. This may be done in the hospital setting. When the cells take in the extra glucose, they also take in potassium. This helps to lower a person’s blood potassium levels. The dextrose is given to prevent the person from being hypoglycemic. The insulin is treating the elevated potassium.
People with diabetes or hypoglycemia (chronically low blood sugar) may carry dextrose gel or tablets in case their blood sugar gets too low. The gel or tablets dissolve in a person’s mouth and quickly boost blood sugar levels. If a person’s blood sugar is less than 70 mg/dL and they are having low blood sugar symptoms, they may need to take the dextrose tablets. Examples of low blood sugar symptoms include weakness, confusion, sweating, and too-fast heart rate.
What precautions should I take when using dextrose?
A medical provider should not give dextrose to people with certain kinds of medical conditions. This is because the dextrose could potentially cause too-high blood sugar or fluid shifts in the body that lead to swelling or fluid buildup in the lungs.
Avoid dextrose
if you have hyperglycemia, or high blood sugar
if you have hypokalemia, or low potassium levels in the blood
if you have peripheral edema, or swelling in the arms, feet, or legs
if you have pulmonary edema, when fluids build up in the lungs
If you are diabetic and your doctor prescribes dextrose oral gel or tablets for you, these should only be used when you have a low blood sugar reaction. Your doctor or diabetes educator should teach you how to spot the signs of low blood sugar and when to use the tablets. If you need to have the gel or tablets on hand, you should keep them with you at all times and you should keep some at home. Your doctor should also explain to other family members when to use the gel or tablets, in case others need to give them to you.
If you have an allergy to corn, you could have an allergic reaction to dextrose. Talk to your doctor before using it.
Monitoring your blood sugar while on dextrose
Even if you don’t have certain conditions, it is important to continually check your blood sugar if they are receiving dextrose. This can ensure that the dextrose does not dangerously increase blood sugar. You can check your blood sugar with home tests. They involve testing blood from a finger prick on a blood strip. For those who are physically unable to test their blood at home, urine glucose tests are available, though they’re not as reliable.
If you do find that you or someone else is having a negative reaction due to low blood sugar, the dextrose tablets should be taken immediately. According to the Joslin Diabetes Center, four glucose tablets are equal to 15 grams of carbs and can be taken in the case of low blood sugar levels (unless otherwise advised by your doctor). Chew the tablets thoroughly before swallowing. No water is needed. Your symptoms should improve within 20 minutes. If they don’t, consult your doctor.
The dextrose gel often comes in single-serving tubes, which are poured directly into the mouth and swallowed. If you haven’t felt any positive changes after 10 minutes, repeat with another tube. If your blood sugar is still too low after an additional 10 minutes, contact your doctor.
Dextrose in children
Dextrose can be used in children similarly to how it is used in adults, as a medical intervention for hypoglycemia.
In cases of severe pediatric hypoglycemia, children will often be given dextrose intravenously. Prompt and early treatment in children and infants with hypoglycemia is essential, as untreated hypoglycemia can result in neurological damage. If they’re able to take it, dextrose may be given to children orally.
In the case of neonatal hypoglycemia, which can be caused by several disorders such as metabolism defects or hyperinsulinism, infants can have small amounts of dextrose gel added to their diet to help them maintain healthy blood sugar levels. Consult your doctor for how much dextrose to add to their diet. Infants that were born prematurely are at risk for hypoglycemia, and may be given dextrose via an IV.
Dextrose powder and bodybuilding
Dextrose is naturally calorie-dense and easy for the body to break down for energy. Because of this, dextrose powder is available and sometimes used as a nutritional supplement by bodybuilders who are looking to increase weight and muscle.
While the boost in calories and easy to break down nature of dextrose can benefit bodybuilders or those looking to increase muscle mass, it’s important to note that dextrose lacks other essential nutrients that are needed to accomplish this goal. Those nutrients include protein and fat. Dextrose powder’s simple sugars also make it easier to break down, while complex sugars and carbohydrates may benefit bodybuilders more, as they are more successful at helping fat to burn.
What are the side effects of dextrose?
Dextrose should be carefully given to people who have diabetes, because they might not be able to process dextrose as quickly as would someone without the condition. Dextrose can increase the blood sugar too much, which is known as hyperglycemia.
Symptoms include:
i) fruity odor on the breath
ii) increasing thirst with no known causes
iii) dry skin
iv) dehydration
v) nausea
vi) shortness of breath
vii) stomach upset
viii) unexplained fatigue
ix) urinating frequently
x) vomiting
xi) confusion
Effect on blood sugar
If you need to use dextrose, your blood sugar could increase too much afterward. You should test your blood sugar after using dextrose tablets, as directed by your doctor or diabetes educator. You may need to adjust your insulin to lower your blood sugar.
If you are given IV fluids with dextrose in the hospital, your nurse will check your blood sugar. If the blood sugar tests too high, the dose of your IV fluids may be adjusted or even stopped, until your blood sugar reaches a safer level. You could also be given insulin, to help reduce your blood sugar.
Outlook
Dextrose’s simple sugar composition makes it useful as a treatment for hypoglycemia and low blood sugar for patients of all ages, with some treatment options being convenient and portable. It is safe to use long-term on an as-needed basis. Dextrose does not come without risks, however, and even those without diabetes should carefully monitor their blood sugar when taking it.
Always consult a doctor before stopping treatment for diabetes, or if you test your blood sugar and it is high. If you have glucose gel or tablets in your home, keep them away from children. Large amounts taken by small children could be especially dangerous.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#99 2018-03-14 23:08:12
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,728
Re: Miscellany
85) Osmosis
Osmosis, the spontaneous passage or diffusion of water or other solvents through a semipermeable membrane (one that blocks the passage of dissolved substances—i.e., solutes). The process, important in biology, was first thoroughly studied in 1877 by a German plant physiologist, Wilhelm Pfeffer. Earlier workers had made less accurate studies of leaky membranes (e.g., animal bladders) and the passage through them in opposite directions of water and escaping substances. The general term osmose (now osmosis) was introduced in 1854 by a British chemist, Thomas Graham.
If a solution is separated from the pure solvent by a membrane that is permeable to the solvent but not the solute, the solution will tend to become more dilute by absorbing solvent through the membrane. This process can be stopped by increasing the pressure on the solution by a specific amount, called the osmotic pressure. The Dutch-born chemist Jacobus Henricus van’t Hoff showed in 1886 that, if the solute is so dilute that its partial vapour pressure above the solution obeys Henry’s law (i.e., is proportional to its concentration in the solution), then osmotic pressure varies with concentration and temperature approximately as it would if the solute were a gas occupying the same volume. This relation led to equations for determining molecular weights of solutes in dilute solutions through effects on the freezing point, boiling point, or vapour pressure of the solvent.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#100 2018-03-16 01:07:32
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,728
Re: Miscellany
86) Nitrous oxide
Nitrous oxide (N2O), also called dinitrogen monoxide, laughing gas, or nitrous, one of several oxides of nitrogen, a colourless gas with pleasant, sweetish odour and taste, which when inhaled produces insensibility to pain preceded by mild hysteria, sometimes laughter. (Because inhalation of small amounts provides a brief euphoric effect and nitrous oxide is not illegal to possess, the substance has been used as a recreational drug.) Nitrous oxide was discovered by the English chemist Joseph Priestley in 1772; another English chemist, Humphry Davy, later named it and showed its physiological effect. A principal use of nitrous oxide is as an anesthetic in surgical operations of short duration; prolonged inhalation causes death. The gas is also used as a propellant in food aerosols. In automobile racing, nitrous oxide is injected into an engine’s air intake; the extra oxygen allows the engine to burn more fuel per stroke. It is prepared by the action of zinc on dilute nitric acid, by the action of hydroxylamine hydrochloride (NH2OH·HCl) on sodium nitrite (NaNO2), and, most commonly, by the decomposition of ammonium nitrate (NH4NO3).

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline