Math Is Fun Forum
You are not logged in.
- Topics: Active | Unanswered
#101 2018-03-18 00:36:09
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,505
Re: Miscellany
87) Calcium Oxide
Calcium oxide, chemical compound, CaO, a colorless, cubic crystalline or white amorphous substance. It is also called lime, quicklime, or caustic lime, but commercial lime often contains impurities, e.g., silica, iron, alumina, and magnesia. It is prepared by heating calcium carbonate (e.g., limestone) in a special lime kiln to about 500°C to 600°C, decomposing it into the oxide and carbon dioxide. Calcium oxide is widely used in industry, e.g., in making porcelain and glass; in purifying sugar; in preparing bleaching powder, calcium carbide, and calcium cyanamide; in water softeners; and in mortars and cements. In agriculture it is used for treating acidic soils (liming). It is incandescent when heated to high temperatures; the Drummond light, or limelight, provides a brilliant white light by heating a cylinder of lime with the flame of an oxyhydrogen torch. Calcium oxide is a basic anhydride, reacting with water to form calcium hydroxide; during the reaction (slaking) much heat is given off and the solid nearly doubles its volume.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#102 2018-03-20 00:48:11
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,505
Re: Miscellany
88) Integrated Circuits
An integrated circuit (IC), sometimes called a chip or microchip, is a semiconductor wafer on which thousands or millions of tiny resistors, capacitors, and transistors are fabricated. An IC can function as an amplifier, oscillator, timer, counter, computer memory, or microprocessor. A particular IC is categorized as either linear (analog) or digital, depending on its intended application.
Linear ICs have continuously variable output (theoretically capable of attaining an infinite number of states) that depends on the input signal level. As the term implies, the output signal level is a linear function of the input signal level. Ideally, when the instantaneous output is graphed against the instantaneous input, the plot appears as a straight line. Linear ICs are used as audio-frequency (AF) and radio-frequency (RF) amplifiers. The operational amplifier(op amp) is a common device in these applications.
Digital ICs operate at only a few defined levels or states, rather than over a continuous range of signal amplitudes. These devices are used in computers, computer networks, modems, and frequency counters. The fundamental building blocks of digital ICs are logic gates, which work with binary data, that is, signals that have only two different states, called low (logic 0) and high (logic 1).
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#103 2018-03-22 14:14:56
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,505
Re: Miscellany
89) Refrigerator
In the refrigeration cycle, there are five basic components: fluid refrigerant; a compressor, which controls the flow of refrigerant; the condenser coils (on the outside of the fridge); the evaporator coils (on the inside of the fridge); and something called an expansion device. Here’s how they interact to cool your food.
1. The compressor constricts the refrigerant vapor, raising its pressure, and pushes it into the coils on the outside of the refrigerator.
2. When the hot gas in the coils meets the cooler air temperature of the kitchen, it becomes a liquid.
3. Now in liquid form at high pressure, the refrigerant cools down as it flows into the coils inside the freezer and the fridge.
4. The refrigerant absorbs the heat inside the fridge, cooling down the air.
5. Last, the refrigerant evaporates to a gas, then flows back to the compressor, where the cycle starts all over.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#104 2018-03-25 00:07:18
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,505
Re: Miscellany
90) Cerebrum
Cerebrum, the largest and uppermost portion of the brain. The cerebrum consists of the cerebralhemispheres and accounts for two-thirds of the total weight of the brain. One hemisphere, usually the left, is functionally dominant, controlling language and speech. The other hemisphere interprets visual and spatial information.
The cerebral hemispheres consist of an inner core of myelinated nerve fibres, the white matter, and an outer cortex of gray matter. The cerebral cortex is responsible for integrating sensory impulses, directing motor activity, and controlling higher intellectual functions. The human cortex is several centimetres thick and has a surface area of about 2,000 square cm (310 square inches), largely because of an elaborate series of convolutions; the extensive development of this cortex in humans is believed to distinguish the human brain from those of other animals. Nerve fibres in the white matter primarily connect functional areas of the cerebral cortex. The gray matter of the cerebral cortex usually is divided into four lobes, roughly defined by major surface folds. The frontal lobe contains control centres for motor activity and speech, the parietal for somatic senses (touch and position), the temporal for auditory reception and memory, and the occipital for visual reception. Sometimes the limbic lobe, involved with smell, taste, and emotions, is considered to be a fifth lobe.
Numerous deep grooves in the cerebral cortex, called cerebral fissures, originate in the extensive folding of the brain’s surface. The main cerebral fissures are the lateral fissure, or fissure of Sylvius, between the frontal and temporal lobes; the central fissure, or fissure of Rolando, between the frontal and parietal lobes, which separates the chief motor and sensory regions of the brain; the calcarine fissure on the occipital lobe, which contains the visual cortex; the parieto-occipital fissure, which separates the parietal and occipital lobes; the transverse fissure, which divides the cerebrum from the cerebellum; and the longitudinal fissure, which divides the cerebrum into two hemispheres.
A thick band of white matter that connects the two hemispheres, called the corpus callosum, allows the integration of sensory input and functional responses from both sides of the body. Other cerebral structures include the hypothalamus, which controls metabolism and maintains homeostasis, and the thalamus, a principal sensory relay centre. These structures surround spaces (ventricles) filled with cerebrospinal fluid, which helps to supply the brain cells with nutrients and provides the brain with shock-absorbing mechanical support.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#105 2018-03-27 00:16:03
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,505
Re: Miscellany
91) Diamond
Diamond, a mineral composed of pure carbon. It is the hardest naturally occurring substance known; it is also the most popular gemstone. Because of their extreme hardness, diamonds have a number of important industrial applications.
The hardness, brilliance, and sparkle of diamonds make them unsurpassed as gems. In the symbolism of gemstones, the diamond represents steadfast love and is the birthstone for April. Diamond stones are weighed in carats (1 carat = 200 milligrams) and in points (1 point = 0.01 carat). In addition to gem-quality stones, several varieties of industrial diamonds occur, and synthetic diamonds have been produced on a commercial scale since 1960.
Diamonds are found in three types of deposits: alluvial gravels, glacial tills, and kimberlite pipes. The kimberlite pipes (such as those at Kimberley, South Africa) form from intrusions of magma into the Earth’s crust and deliver diamonds and other rocks and minerals from the mantle. The pipes themselves are often less than 100 million years old. However, the diamonds they carry were formed 1 to 3.3 billion years ago at depths of more than about 75 miles (120 km). Diamonds found in alluvial and glacial gravels must have been released by fluvial or glacial erosion of the kimberlite matrix and then redeposited in rivers or in glacial till.
Diamonds vary from colourless to black, and they may be transparent, translucent, or opaque. Most diamonds used as gems are transparent and colourless or nearly so. Colourless or pale blue stones are most valued, but these are rare; most gem diamonds are tinged with yellow. A “fancy” diamond has a distinct body colour; red, blue, and green are rarest, and orange, violet, yellow, and yellowish green more common. Most industrial diamonds are gray or brown and are translucent or opaque, but better-quality industrial stones grade imperceptibly into poor quality gems. The colour of diamonds may be changed by exposure to intense radiation (as released in a nuclear reactor or by a particle accelerator) or by heat treatment.
A very high refractive power gives the diamond its extraordinary brilliance. A properly cut diamond will return a greater amount of light to the eye of the observer than will a gem of lesser refractive power and will thus appear more brilliant. The high dispersion gives diamonds their fire, which is caused by the separation of white light into the colours of the spectrum as it passes through the stone.
The scratch hardness of diamond is assigned the value of 10 on the Mohs scale of hardness; corundum, the mineral next to diamond in hardness, is rated as 9. Actually, diamond is very much harder than corundum; if the Mohs scale were linear, diamond’s value would be about 42. The hardness of a diamond varies significantly in different directions, causing cutting and polishing of some faces to be easier than others.
In the atomic structure of diamond, as determined by X-ray diffraction techniques, each carbon atom is linked to four equidistant neighbours throughout the crystal. This close-knit, dense, strongly bonded crystal structure yields diamond properties that differ greatly from those of graphite, native carbon’s other form.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#106 2018-03-29 00:33:59
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,505
Re: Miscellany
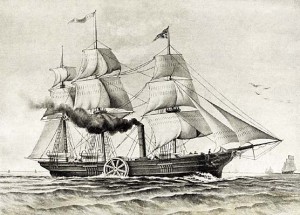
92) Savannah
Savannah, either of two historic U.S. ships, each representing a landmark in navigation. In 1819 the first Savannah, named for its home port in Georgia (although built in New York) became the first ship to cross the Atlantic Ocean employing steam power. Its small steam engine and pinewood fuel supply were good for only a part of the 24-day crossing. For most of the voyage the Savannah relied on a full spread of sail, but the voyage demonstrated the practicability of steam navigation on the ocean. The sight of the 300-ton vessel off the Irish coast brought a cutter hastening to the ship’s assistance, because its plume of black smoke had been mistaken for evidence of a fire on board.
The second Savannah, launched at Camden, N.J., in 1959, was the world’s first nuclear-powered cargo ship, built experimentally by the U.S. government to demonstrate the potential of nuclear power for nonmilitary shipping. Displacing 22,000 tons, the Savannah was 181.5 m (595.5 feet) long and had accommodations for 60 passengers as well as 9,400 tons of cargo. Its cruising speed was about 20 knots, and in the 1960s it carried out a large number of demonstration cruises in the Atlantic and elsewhere. Despite its success, high costs discouraged early imitation by commercial shippers.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#107 2018-03-31 00:20:31
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,505
Re: Miscellany
93) Ostrich
Ostrich, (Struthio camelus), large flightless bird found only in open country in Africa. The largest living bird, an adult male may be 2.75 metres (about 9 feet) tall—almost half of its height is neck—and weigh more than 150 kg (330 pounds); the female is somewhat smaller. The ostrich’s egg, averaging about 150 mm (6 inches) in length by 125 mm (5 inches) in diameter and about 1.35 kg (3 pounds), is also the world’s largest. The male is mostly black but has white plumes in the wings and tail; females are mostly brown. The head and most of the neck, reddish to bluish in colour, is lightly downed; the legs, including the powerful thighs, are bare. The head is small, the bill short and rather wide; the big brown eyes have thick black lashes.
Ostriches are seen individually, in pairs, in small flocks, or in large aggregations, depending on the season. The ostrich relies on its strong legs—uniquely two-toed, with the main toe developed almost as a hoof—to escape its enemies, chiefly humans and the larger carnivores. A frightened ostrich can achieve a speed of 72.5 km (45 miles) per hour. If cornered, it can deliver dangerous kicks.
Ostriches live mainly on vegetation but also take some animal food, mainly insects; they can go without water for long periods. Breeding males emit lionlike roars and hisses as they fight for a harem of three to five hens. A communal nest scraped in the ground contains more than a dozen shiny, whitish eggs. The major hen of the harem may get rid of some of the eggs to make incubation more manageable. The male sits on the eggs by night; the females take turns during the day. The chicks hatch in about 40 days and when a month old can keep up with running adults. To escape detection, chicks as well as adults may lie on the ground with neck outstretched, a habit that may have given rise to the mistaken belief that the ostrich buries its head in the sand when danger threatens. Ostrich plumes adorned the helmets of medieval European knights, and in the 19th century such plumes were sold for women’s finery. This demand led to the establishment of ostrich farms in South Africa, the southern United States, Australia, and elsewhere, but the trade collapsed after World War I. Ostriches are now raised for their meat and hide, which provides a soft, fine-grained leather. The birds have been trained for saddle and sulky racing, but they tire easily and are not well suited for training. They do well in captivity and may live 50 years.
The ostrich is typical of a group of flightless birds called ratites. Ostrich populations differing slightly in skin colour, size, and egg features formerly were considered separate species, but now they are considered to be merely races of Struthio camelus. Most familiar is the North African ostrich, S. camelus camelus, ranging, in much-reduced numbers, from Morocco to Sudan. Ostriches also live in eastern and southern Africa. The Syrian ostrich (S. camelus syriacus) of Syria and Arabia became extinct in 1941. The ostrich is the only living species in the genus Struthio. Ostriches are the only members of the family Struthionidae in the order Struthioniformes—a group that also contains kiwis, emus, cassowaries, and rheas. The oldest fossil relatives of ostriches belong to the species Calciavis grandei, which were excavated from the Green River Formation in Wyoming and date to the Eocene Epoch, some 56 million to 34 million years ago.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#108 2018-04-02 00:14:29
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,505
Re: Miscellany
94) Polymorphism (Crystallography)
Polymorphism, in crystallography, the condition in which a solid chemical compound exists in more than one crystalline form; the forms differ somewhat in physical and, sometimes, chemical properties, although their solutions and vapours are identical. The existence of different crystalline or molecular forms of elements is called allotropy, although it has been suggested that the meaning of allotropy should be restricted to different molecular forms of an element, such as oxygen (O2) and ozone (O3), and that polymorphism be applied to different crystalline forms of the same species, whether a compound or an element. Differences in the crystalline forms of many elements and compounds were discovered during the 1820s by Eilhardt Mitscherlich, a German chemist.
Among polymorphs of certain compounds, one is more stable than the others under all conditions; in the cases of other compounds, one polymorph is stable within a particular range of temperature and pressure while another is stable under a different set of conditions. In either circumstance, the rate at which a less stable polymorph becomes more stable often is so low that an intrinsically unstable form may persist indefinitely. As an example of the first class, calcium carbonate has an orthorhombic form (i.e., having three unequal crystalline axes at right angles to each other) called aragonite and a hexagonal form (having three equal axes intersecting at angles of 60 degrees and a fourth axis at right angles to these three) called calcite. Calcite is the stabler form; aragonite changes into calcite rapidly at temperatures around 470° C (about 880° F) but very slowly at room temperatures. The second class is represented by silica, which has three forms—quartz, tridymite, and cristobalite—each of which is stable only in its particular range of temperature and pressure, the others slowly changing into the stable modification.
The conditions under which synthetic crystalline substances are prepared often dictate the formation of one or another polymorph; in the manufacture of pigments, particular care is required because the colour, reflectivity, and opacity frequently vary among the polymorphic modifications of a single substance.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#109 2018-04-04 01:00:18
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,505
Re: Miscellany
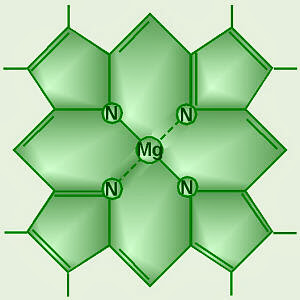
95) Chlorophyll
Chlorophyll, any member of the most important class of pigments involved in photosynthesis, the process by which light energy is converted to chemical energy through the synthesis of organic compounds. Chlorophyll is found in virtually all photosynthetic organisms, including green plants, prokaryotic blue-green algae (cyanobacteria), and eukaryotic algae. It absorbs energy from light; this energy is then used to convert carbon dioxide to carbohydrates.
Chlorophyll occurs in several distinct forms: chlorophylls a and b are the major types found in higher plants and green algae; chlorophylls c and d are found, often with a, in different algae; chlorophyll e is a rare type found in some golden algae; and bacterio-chlorophyll occurs in certain bacteria. In green plants chlorophyll occurs in membranous disklike units (thylakoids) in organelles called chloroplasts. The chlorophyll molecule consists of a central magnesium atom surrounded by a nitrogen-containing structure called a porphyrin ring; attached to the ring is a long carbon–hydrogen side chain, known as a phytol chain. Variations are due to minor modifications of certain side groups. Chlorophyll is remarkably similar in structure to hemoglobin, the oxygen-carrying pigment found in the red blood cells of mammals and other vertebrates.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#110 2018-04-04 18:55:51
- Monox D. I-Fly
- Member

- From: Indonesia
- Registered: 2015-12-02
- Posts: 2,000
Re: Miscellany
Chlorophyll occurs in several distinct forms: chlorophylls a and b are the major types found in higher plants and green algae; chlorophylls c and d are found, often with a, in different algae; chlorophyll e is a rare type found in some golden algae; and bacterio-chlorophyll occurs in certain bacteria.
Do you know a species of sea slug who can do photosynthesis? Which clorophyll do they use?
Actually I never watch Star Wars and not interested in it anyway, but I choose a Yoda card as my avatar in honor of our great friend bobbym who has passed away.
May his adventurous soul rest in peace at heaven.
Offline
#111 2018-04-04 20:52:19
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,505
Re: Miscellany
After decades of searching, scientists have finally found direct evidence to show that the emerald green sea slug (Elysia chlorotica) takes genes from the algae it eats to perform photosynthetic processes, just like a plant. This means it can get all the energy it needs from sunlight, allowing it to survive without food for months.
Scientists have known for over 40 years that the emerald green sea slug takes chloroplasts - organelles found in plant and algal cells that facilitate photosynthesis - from the yellow-green algae it eats, called Vaucheria litorea. Referred to as ‘kleptoplasty’, this process allows the chloroplasts to continue photosynthesising in their new sea slug home for up to nine months after transferring from the algae. By photosynthesising, the sea slug produces lipids when the energy from the sunlight is combined with water and carbon dioxide, which gives it all the nourishment it needs, no additional food required.
But exactly how the emerald green sea slug manages to maintain these organelles in working order for so long has proven to be a frustratingly complex puzzle - one that was not made easier by an experiment completed by researchers at the University of Dusseldorf in Germany in 2013. The team gave their emerald green sea slugs a drug that completely halted any photosynthetic activity in their cells, but the slugs still managed to survive for 55 days, without any food. They ended up a little smaller and paler, so food wouldn’t have gone astray if they were offered it, but it was proof that the organelles they 'stole' from their last algae meal were somehow still working for them.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#112 2018-04-06 00:44:10
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,505
Re: Miscellany
96) Tungsten
Tungsten is known as one of the toughest things found in nature. It is super dense and almost impossible to melt. Pure tungsten is a silver-white metal and when made into a fine powder can be combustible and can spontaneously ignite. Natural tungsten contains five stable isotopes and 21 other unstable isotopes.
Tungsten is used in many different ways because it is very strong and durable. It is very resistant to corrosion and has the highest melting point and highest tensile strength of any element. Its strength comes when it is made into compounds, though. Pure tungsten is very soft.
Atomic number: 74
Atomic symbol: W
Atomic weight: 183.84
Melting point: 6,191.6 F (3,422 C)
Boiling point: 3,068.3 F (5,555 C)
History
The first use of tungsten was more than 350 years ago. Chinese porcelain makers used a tungsten pigment that was a unique peach color, according to the Royal Society of Chemistry.
Much later, in 1779, Peter Woulfe examined a mineral from Sweden and realized it contained a new type of metal, but that’s about as far as the research went. In 1781, Wilhelm Scheele continued the research on this new metal and isolated an acidic white oxide. Neither one of these men are credited with the element’s discovery, though.
Juan and Fausto Elhuyar get that honor. At the Seminary at Vergara in Spain, they researched this mysterious metal. In 1783 they isolated the metal oxide from wolframite and then, unlike the others, reduced it to tungsten metal by heating it with carbon.
Sources
Most tungsten resources are found in China, South Korea, Bolivia, Great Britain, Russia and Portugal, as well as in California and Colorado. Though it is found in these many places, 80 percent of world’s supply is controlled by China, according to the BBC.
The element naturally occurs in the minerals scheelite, wolframite, huebnertie and ferberite. It is harvested from the minerals by reducing tungsten oxide with hydrogen or carbon.
Once it is sourced, tungsten is often mixed into alloys. The hardest alloys are shaped using diamonds. Diamonds are the only things harder than some tungsten alloys.
Uses
One of the most common, and hardest, tungsten compounds is tungsten carbide. Because of its strength when made into compounds, tungsten is used to harden saw blades and make drill bits. It can take around 10 minutes to cut just one drill bit from tungsten using a diamond cutting system, according to the BBC. Some jewelers also use tungsten carbide to make wedding bands and other rings.
Another tungsten compound that is particularly useful is tungsten disulfide. It is used as a dry lubricant in temperatures as high as 932 degrees Fahrenheit (500 degrees Celsius), according to the Jefferson Lab.
Some other uses of tungsten include metal evaporation work, the manufacturing of paints, making glass-to-metal seals and creating electron and television tubes.
The military uses tungsten to make bullets and missiles used in “kinetic bombardment.” This type of attack uses a super dense material to breach armor instead of explosives.
Its resistance to heat is helpful when using it in the heating elements for electrical furnaces, spacecraft applications, welding and other high-temperature applications. It was also used in making different types of lighting for this reason. The hotter a filament can get without melting, the brighter the bulb. In 1908 inventor William D. Coolidge discovered that tungsten was an ideal filament material. Today, though, most bulbs use more energy efficient materials. It is still used in X-ray filaments and in electrical contacts of various electronics, however.
Biologically, some bacteria use tungsten to reduce carboxylic acids to aldehydes.
Who knew?
This element is used for trickery. “Tungsten may not have gold’s luster, but it does have its density (within 0.36 percent) which means that if you cover a brick of tungsten with a coating of gold – and you test the brick to see if it weighs as much as gold – it will be almost correct,” Amanda Simson, an assistant professor of chemical engineering at the University of New Haven. “Thus, tungsten has been found in counterfeit gold bricks.”
Tungsten comes from a Swedish term, tung sten, that means "heavy stone.”
Tungsten's chemical symbol is a W, which may seem weird since there isn’t a W in the word. The W actually comes from the element’s other name, wolfram. The name wolfram comes from the mineral the element was discovered in, wolframite. Wolframite means "the devourer of tin," which is appropriate since the mineral interferes with the smelting of tin.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#113 2018-04-08 01:00:23
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,505
Re: Miscellany
97) Complex Number
A complex number is a quantity of the form v + iw, where v and w are real numbers, and i represents the unit imaginary numbers equal to the positive square root of -1. The set C of all complex numbers corresponds one-to-one with the set R R of all ordered pairs of real numbers.The set C also corresponds one-to-one with the points on a geometric plane.
The set of complex numbers is two-dimensional, and a coordinate plane is required to illustrate them graphically.This is in contrast to the real numbers, which are one-dimensional, and can be illustrated by a simple number line.The rectangular complex number plane is constructed by arranging the real numbers along the horizontal axis, and the imaginary numbers along the vertical axis.Each point in this plane can be assigned to a unique complex number, and each complex number can be assigned to a unique point in the plane.
Complex numbers are used in engineering, particularly in electronics. Real numbers are used to denote electrical resistance, imaginary numbers are used to denote reactance, and complex numbers are used to represent impedance.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#114 2018-04-10 00:51:44
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,505
Re: Miscellany
98) Corundum
Corundum, naturally occurring aluminum oxide mineral (Al2O3) that is, after diamond, the hardest known natural substance. Its finer varieties are the gemstones sapphire and ruby (qq.v.), and its mixtures with iron oxides and other minerals are called emery (q.v.).
Corundum in its pure state is colourless, but the presence of small amounts of impurities can impart a broad range of hues to the mineral. Ruby owes its red colour to chromium, sapphire its blue shades to the presence of iron and titanium; most corundum contains nearly 1 percent iron oxide. The mineral readily weathers to other aluminous minerals—e.g., margarite, zoisite, sillimanite, and kyanite.
Corundum crystallizes in the hexagonal system, forming pyramidal or rounded barrel shapes. It is widespread in nature, being found in igneous, metamorphic, and sedimentary rocks. Large deposits are rare, however. Some of the richest deposits occur in India, Myanmar (Burma), Russia, Zimbabwe, and South Africa. The largest corundum, found in Transvaal, S.Af., is 0.65 m (about 2 feet) long and 40 cm (about 1 foot) in diameter.
In addition to its use as a precious gem, corundum finds some use as an abrasive, owing to the extreme hardness of the material (9 on the Mohs hardness scale). It is used for grinding optical glass and for polishing metals and has also been made into sandpapers and grinding wheels. Because of its high melting point (2,040° C, or 3,700° F), it has also been used in refractories.
In most industrial applications corundum has been replaced by synthetic materials such as alumina, an aluminum oxide made from bauxite. Artificial corundum may be produced as a specialty product, as for gem use, by slow accretion and controlled growth on a boule in an oxyhydrogen flame. This procedure is known as the Verneuil process (q.v.).

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#115 2018-04-12 00:17:40
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,505
Re: Miscellany
99) Electrocardiogram
What Is an Electrocardiogram (ECG, EKG)?
The electrocardiogram (ECG or EKG) is a diagnostic tool that is routinely used to assess the electrical and muscular functions of the heart. While it is a relatively simple test to perform, the interpretation of the ECG tracing requires significant amounts of training. Numerous textbooks are devoted to the subject.
The heart is a two stage electrical pump and the heart's electrical activity can be measured by electrodes placed on the skin. The electrocardiogram can measure the rate and rhythm of the heartbeat, as well as provide indirect evidence of blood flow to the heart muscle.
A standardized system has been developed for the electrode placement for a routine ECG. Ten electrodes are needed to produce 12 electrical views of the heart. An electrode lead, or patch, is placed on each arm and leg and six are placed across the chest wall. The signals received from each electrode are recorded. The printed view of these recordings is the electrocardiogram.
By comparison, a heart monitor requires only three electrode leads – one each on the right arm, left arm, and left chest. It only measures the rate and rhythm of the heartbeat. This kind of monitoring does not constitute a complete ECG.
Basic Anatomy of the Heart
The heart has four chambers – the right and left atrium and the right and left ventricle.
The right side of the heart collects blood from the body and pumps it to the lungs while the left side of the heart receives blood from the lungs and pumps it to the body.
Blood flows through the body in the following way:
Oxygen-rich blood from the lungs enters the left atrium through the pulmonary veins.
Blood then flows into the left ventricle where it is pumped into the aorta and is distributed to the rest of the body. This blood supplies organs and cells with oxygen and nutrients necessary for metabolism.
Blood that returns to the heart is depleted of oxygen and carries carbon dioxide, the waste product of metabolism. The blood enters the right atrium though the vena cava, where it is collected and pumped to the right ventricle.
The right ventricle then pumps blood through the pulmonary artery to the lungs where carbon dioxide is stripped off, oxygen is replaced, and the cycle begins again.
Like any muscle, the heart requires oxygen and nutrients to function. Oxygen and nutrients are supplied by arteries that originate from the aorta. These vessels branch out to supply all the regions of the heart with oxygen rich blood.
Electrically, the heart can be divided into upper and lower chambers. An electrical impulse is generated in the upper chambers of the heart that causes the atria to squeeze and push blood into the ventricles. There is a short delay to allow the ventricles to fill. The ventricles then contract to pump blood to the body and the lungs.
Conducting system of the heart: SA means sinoatrial node. AV means atrioventricular node. RB and LB mean right and left bundle, respectively, and are the nerves that spread the electric impulse from the AV node into the ventricles.
The heart has its own automatic pacemaker called the sinaoatrial, or SA node, located in the right atrium. The SA node acts independently of the brain to generate electricity for the heart to beat.
Normally, the impulse generated by the SA node runs through the heart's electrical grid and signals the muscle cells in the atria to beat simultaneously, allowing for a coordinated squeeze of the heart. Contraction of the atria pushes blood into the ventricles.
The electrical signal that was generated in the SA node travels to a junction box between the atria and ventricles (the AV node) where it is delayed for a few milliseconds to allow the ventricles to fill.
The electrical signal then travels through the ventricles, stimulating those heart muscle cells to contract. Ventricular contraction pumps blood to the body (from the left ventricle) and the lungs (from the right ventricle).
There is a short pause to allow blood to return to the heart and fill before the electrical cycle repeats itself for the next heartbeat.
Heart Function and the ECG
Electrode leads on the chest wall are able to detect electrical impulses that are generated by the heart. Multiple leads provide many electrical views of the heart. By interpreting the tracing, the physician can learn about the heart rate and rhythm as well as blood flow to the ventricles (indirectly).
Rate refers to how fast the heart beats. Normally, the SA node generates an electrical impulse 50-100 times per minute. Bradycardia (brady=slow+cardia=heart) describes a heart rate less than 50 beats per minute. Tachycardia (tachy=fast+cardia=heart) describes a heart rate faster than 100 beats per minute.
Rhythm refers to the type of heartbeat. Normally, the heart beats in a sinus rhythm with each electrical impulse generated by the SA node resulting in a ventricular contraction, or heartbeat. There are a variety of abnormal electrical rhythms, some are normal variants and some are potentially dangerous. Some electrical rhythms do not generate a heartbeat and are the cause of sudden death.
Examples of heart rhythms include:
Normal sinus rhythm
Sinus tachycardia
Sinus bradycardia
Atrial fibrillation
Atrial flutter
Ventricular tachycardia
Ventricular fibrillation
There can also be delays in transmission of the electrical impulse anywhere in the system, including the SA node, the atria, the AV node, or in the ventricles. Some aberrant impulses cause normal variants of the heart rhythm and others can be potentially life threatening. Some examples include:
1st degree AV block
2nd degree AV block, type I (Wenckebach)
2nd degree AV block, type II
3rd degree AV block or complete heart block
Right bundle branch block
Left bundle branch block
There can also be short circuits that can lead to abnormal electrical pathways in the heart causing abnormalities of rate and rhythm. Wolfe-Parkinson-White (WPW) syndrome is a condition where an abnormal accessory pathway at the AV node can cause tachycardia.
The ECG tracing can also provide information about whether the heart muscle cells are conducting electricity appropriately. By analyzing the shape of the electrical waves, the physician may be able to determine if there is decreased blood flow to parts of the heart muscle. The presence of an acute blockage associated with a myocardial infarction or heart attack can be determined as well. That's one of the reasons that an ECG is done as soon as possible when a patient presents with chest pain.
What Happens During an ECG?
The ECG is a relatively simple test to perform. It is non-invasive and does not hurt. Patches are placed on the skin to detect electrical impulses that the heart generates. These impulses are recorded by an ECG machine. Four patches are placed on the limbs. One is placed on each shoulder or upper arm and one on each leg. These are called the limb leads. There are six patches that are placed on the chest wall beginning just to the right of the breast bone. Patches are placed in the shape of a semi-circle ending near the left axilla (underarm). These are called the chest leads. These patches are connected to an ECG machine that records the tracings and prints them onto paper.
Newer machines also have video screens that help the technician, nurse, or doctor decide whether the quality of the tracing is adequate or whether the test should be repeated. ECG machines are also equipped with computer programs that can help interpret the ECG, although they are not completely accurate.
In certain situations, the physician may want to look at the heart from different angles after the initial ECG is done. The chest leads may then be placed across the right chest wall or on the back.
The skin should be clean and dry to prevent electrical interference to get an acceptable tracing for interpretation. Sometimes that means shaving chest hair or aggressively toweling off the skin. Shivering or tremors can interfere with the tracing and cause interference that affects the quality of the ECG tracing. Usually, the patient has to hold still for 5-10 seconds without moving to get an accurate ECG.
Reasons to Have an ECG
The ECG is used to assess heart function. Patients who complain of chest pain or shortness of breath will often have an ECG as one of the first tests to help determine if there is an acute myocardial infarction or heart attack present. Even if there is no heart attack, the ECG can help decide whether the pain is due to angina or narrowing of blood vessels to the heart muscle (atherosclerosis). It is important to realize that an initial ECG may be normal even if there is heart disease present. Serial EKGs may be needed over time to find an abnormality.
ECGs are often performed when a patient complains of lightheadedness, palpitations, or syncope (passing out) since abnormal heart rate and rhythms may affect the heart's ability to pump blood and provide the body with oxygen.
ECG Interpretation
Interpreting an ECG requires a fair amount of education and experience. Numerous textbooks are devoted to ECG interpretation. The ECG is just one test to assess the heart. History and physical examination remain the cornerstones for diagnosing heart disease. The doctor-patient discussion may uncover the potential for heart problems even if the ECG is normal.
Most often, the ECG assessment includes the following:
i) determination of the rate,
ii) assessment of the rhythm,
iii) evaluation of the electrical conduction patterns. Heart muscle that is irritated conducts electricity differently than heart muscle that is normal. Abnormal conduction may be apparent during ventricular contraction and during ventricular recovery.
The ECG records the heart tracing in12 leads: Six limb leads (I, II, III, AVR, AVL, AVF) and six chest leads (V1-V6).
The P wave looks at the atria. The QRS complex looks at the ventricles and the T wave evaluates the recovery stage of the ventricles while they are refilling with blood.
The time it takes for electricity to travel from the SA node to the AV node is measured by the PR interval. The QRS interval measures electrical travel time through the ventricles and the QT interval measures how long it takes for the ventricles to recover and prepare to beat again.
The computers imbedded in most ECG machines are able to measure the time it takes for the electrical impulse to travel from the SA node to the ventricles. These measurements can help the doctor assess heart rate and some types of heart block.
Computer programs may also try to interpret the ECG. And as artificial intelligence and programming improves, they are often correct. However, there are enough subtleties in interpretation that the human element is still a very important part of the assessment. The ECG machine is not always correct.
The decision to act upon the results of an ECG depends not only upon the ECG tracing, but also upon the clinical situation. A normal ECG does not exclude heart disease and an abnormal ECG may be the "normal" baseline for that patient.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#116 2018-04-13 00:35:42
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,505
Re: Miscellany
100) Heavy water
Heavy water (D2O), also called deuterium oxide, water composed of deuterium, the hydrogen isotope with a mass double that of ordinary hydrogen, and oxygen. (Ordinary water has a composition represented by H2O.) Thus, heavy water has a molecular weight of about 20 (the sum of twice the atomic weight of deuterium, which is 2, plus the atomic weight of oxygen, which is 16), whereas ordinary water has a molecular weight of about 18 (twice the atomic weight of ordinary hydrogen, which is 1, plus oxygen, which is 16).
Ordinary water as obtained from most natural sources contains about one deuterium atom for every 6,760 ordinary hydrogen atoms. and the residual water is thus enriched in deuterium content. Continued electrolysis of hundreds of litres of water until only a few millilitres remain yields practically pure deuterium oxide. This operation, until 1943 the only large-scale method used, has been superseded by less expensive processes, such as fractional distillation (D2O becomes concentrated in the liquid residue because it is less volatile than H2O). The heavy water produced is used as a moderator of neutrons in nuclear power plants. In the laboratory heavy water is employed as an isotopic tracer in studies of chemical and biochemical processes.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#117 2018-04-14 22:10:20
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,505
Re: Miscellany
101) Hafnium Carbide
Hafnium carbide (HfC) is a chemical compound of hafnium and carbon. With a melting point of about 3900 °C it is one of the most refractory binary compounds known. However, it has a low oxidation resistance, with the oxidation starting at temperatures as low as 430 °C.
Hafnium carbide is usually carbon deficient and therefore its composition is often expressed as
(x = 0.5 to 1.0). It has a cubic (rock-salt) crystal structure at any value of x.Hafnium carbide powder is obtained by the reduction of hafnium(IV) oxide with carbon at 1800 to 2000 °C. A long processing time is required to remove all oxygen. Alternatively, high-purity HfC coatings can be obtained by chemical vapor deposition from a gas mixture of methane, hydrogen, and vaporized hafnium(IV) chloride. Because of the technical complexity and high cost of the synthesis, HfC has a very limited use, despite its favorable properties such as high hardness (>9 Mohs) and melting point.
The magnetic properties of
change from paramagnetic for x ≤ 0.8 to diamagnetic at larger x. An inverse behavior (dia-paramagnetic transition with increasing x) is observed for , despite its having the same crystal structure as .
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#118 2018-04-15 22:51:13
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,505
Re: Miscellany
102) Sartorius Muscle
Long and thin, the sartorius muscle spans the distance of the thigh. It originates at anterior superior iliac spine (a bony projection on the uppermost part of the pelvis) and travels to the upper shaft of the tibia, or shinbone. As such, the sartorius is the longest muscle in the human body.
The muscle helps flex, adduct, and rotate the hip. In addition, it helps with the knee's flexion. The femoral artery supplies oxygen-rich blood to the muscle. It is innervated by the femoral nerve as well as the intermediate cutaneous nerve of the thigh.
The sartorius muscle may be susceptible to pes anserine bursitis, which also involves inflammation within the knee's medial (middle) portion. Typically, this condition results from overworking the muscle, and it is an occupational hazard for most athletes. Symptoms often include swelling, tenderness, and pain. Since the muscle covers a range of motion, severe injury such as a tear or rupture can be debilitating.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#119 2018-04-16 17:37:45
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,505
Re: Miscellany
103) Electronic Organ
The electronic organ resembles a spinet, or upright, piano in size and general shape. Most instruments of this general type rely upon electronic oscillators (circuits carrying an alternating current at a specific frequency) to produce their sound. Each oscillator is capable of frequency variation for different pitches and is capable of reproducing a single melodic line. The instrument’s multiple oscillators make it capable of reproducing music having multiple parts, such as a fugue by Johann Sebastian Bach.
The 200-ton, keyboard-operated telharmonium, which used rotating electromagnetic tone-wheels to generate sound, was an important precursor to the electronic organ. Made in 1904 by the American inventor Thaddeus Cahill, it was exhibited in Massachusetts and New York in 1906 but lapsed into obscurity by World War I. The first successful electronic organ was developed in 1928 in France by Edouard Coupleux and Armand Givelet. It used electronic oscillators in place of the pipes of a conventional organ and was operated with keyboards and a pedal board. Another notable early electronic organ was the Rangertone (1931), invented by Richard H. Ranger of the United States. In 1934 the Orgatron was introduced by Frederick Albert Hoschke; in this organ, tone was generated by reeds that vibrated by electrically fan-blown air, with the vibrations picked up electrostatically and amplified.
One of the most important and well known of the electronic organs is the Hammond organ, a sophisticated instrument having two manuals, or keyboards, and a set of pedals operated by the feet. It was patented by its American inventor Laurens Hammond in 1934. Unlike most other instruments of its type, it produces its sound through a complex set of rotary, motor-driven generators. By means of a series of controls affecting the harmonics, or component tones, of the sound, a great variety of timbres (tone colours) can be reproduced that to some degree imitate the sound of other instruments, such as the violin, the flute, the oboe, and the orchestral percussion instruments.
By the 1960s organ manufacturers had expanded their technology, supplanting vacuum tubes with transistors and solid-state circuitry. Circuits and components designed to operate television and radio receivers and high-fidelity phonographs were adapted to produce music. In the 1970s digital microcircuitry was used to operate a computer organ. In this device, sounds are not created internally but have been prerecorded (sampled) and stored in the computer from which they can later be retrieved. Musical tones or shapes—recorded from conventional windblown pipe organs—are coded into digital form and may be re-created by a special computer at the touch of the keys and stops. Other devices have been used to control reverberation, pitch, and the attack or delay of a note.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#120 2018-04-18 00:04:12
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,505
Re: Miscellany
104) Tartaric Acid
Tartaric acid, also called dihydroxybutanedioic acid, a dicarboxylic acid, one of the most widely distributed of plant acids, with a number of food and industrial uses. Along with several of its salts, cream of tartar (potassium hydrogen tartrate) and Rochelle salt (potassium sodium tartrate), it is obtained from by-products of wine fermentation. In a partially purified form, tartar was known to the ancient Greeks and Romans; the free acid was first isolated in 1769 by Swedish chemist Carl Wilhelm Scheele. The lees, or sediments, and other waste products from fermentation are heated and neutralized with calcium hydroxide; the precipitated calcium tartrate is then treated with sulfuric acid to produce free tartaric acid. Rochelle salt is prepared from the crude crystalline potassium acid salt, called argol, by neutralization with sodium carbonate. Purified cream of tartar comes chiefly from the filtrates from production of the acid and Rochelle salt. A third salt, tartar emetic (antimony potassium tartrate), is made from the potassium acid salt and antimony oxide.
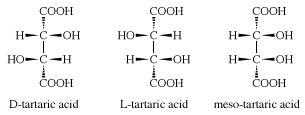
Three stereoisomeric forms of tartaric acid exist: (1) dextrorotatory tartaric acid (D-tartaric acid) found in grapes and several other fruits, (2) levorotatory tartaric acid (L-tartaric acid) obtained chiefly by resolution of racemic tartaric acid, and (3) a meso or achiral form. Racemic tartaric acid (an equal mixture of D- and L-tartaric acid) is prepared commercially by the molybdenum- or tungsten-catalyzed oxidation of maleic anhydride with hydrogen peroxide.
Study of the crystallographic, chemical, and optical properties of the tartaric acids by French chemist and microbiologist Louis Pasteur laid the basis for modern ideas of stereoisomerism.
The various tartaric acids and the common tartrate salts are all colourless, crystalline solids readily soluble in water. Tartaric acid is widely used as an acidulant in carbonated drinks, effervescent tablets, gelatin desserts, and fruit jellies. It has many industrial applications—e.g., in cleaning and polishing metals, in calico printing, in wool dyeing, and in certain photographic printing and development processes. Rochelle salt is used in silvering mirrors, in processing cheese, and in compounding mild cathartics. Cream of tartar is incorporated into baking powders, hard candies, and taffies; and it is employed in the cleaning of brass, the electrolytic tinning of iron and steel, and the coating of other metals with gold and silver. Tartar emetic is used as an insecticide and a dyeing mordant.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#121 2018-04-20 01:07:53
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,505
Re: Miscellany
105) Capacitor
Capacitor, device for storing electrical energy, consisting of two conductors in close proximity and insulated from each other. A simple example of such a storage device is the parallel-plate capacitor. If positive charges with total charge +Q are deposited on one of the conductors and an equal amount of negative charge −Q is deposited on the second conductor, the capacitor is said to have a charge Q.
Capacitors have many important applications. They are used, for example, in digital circuits so that information stored in large computer memories is not lost during a momentary electric power failure; the electric energy stored in such capacitors maintains the information during the temporary loss of power. Capacitors play an even more important role as filters to divert spurious electric signals and thereby prevent damage to sensitive components and circuits caused by electric surges.
Principle of the capacitor
To understand how a charged capacitor stores energy, consider the following charging process. With both plates of the capacitor initially uncharged, a small amount of negative charge is removed from the lower plate and placed on the upper plate. Thus, little work is required to make the lower plate slightly positive and the upper plate slightly negative. As the process is repeated, however, it becomes increasingly difficult to transport the same amount of negative charge, since the charge is being moved toward a plate that is already negatively charged and away from a plate that is positively charged. The negative charge on the upper plate repels the negative charge moving toward it, and the positive charge on the lower plate exerts an attractive force on the negative charge being moved away. Therefore, work has to be done to charge the capacitor.
Where and how is this energy stored? The negative charges on the upper plate are attracted toward the positive charges on the lower plate and could do work if they could leave the plate. Because they cannot leave the plate, however, the energy is stored. A mechanical analogy is the potential energy of a stretched spring. Another way to understand the energy stored in a capacitor is to compare an uncharged capacitor with a charged capacitor. In the uncharged capacitor, there is no electric field between the plates; in the charged capacitor, because of the positive and negative charges on the inside surfaces of the plates, there is an electric field between the plates with the field lines pointing from the positively charged plate to the negatively charged one. The energy stored is the energy that was required to establish the field.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#122 2018-04-22 02:09:41
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,505
Re: Miscellany
106) Plytypus
Platypus, (Ornithorhynchus anatinus), also called duckbill, a small amphibious Australian mammal noted for its odd combination of primitive features and special adaptations, especially the flat, almost comical bill that early observers thought was that of a duck sewn onto the body of a mammal. Adding to its distinctive appearance are conspicuous white patches of fur under the eyes. The fur on the rest of the body is dark to light brown above, with lighter fur on the underside.
The platypus is common in waterways of eastern Australia, where it generally feeds on bottom-dwelling invertebrates but also takes an occasional frog, fish, or insect at the water’s surface. This shy creature forages most actively from dusk to dawn, sheltering during the day in burrows dug into stream banks. It is exquisitely adapted for its aquatic lifestyle, having a flattened torpedo-like body, dense waterproof fur, and strong front limbs used for swimming as well as digging. Even the head is streamlined, each ear being housed in a groove together with a small eye. The senses of sight, smell, and hearing are essentially shut down while the platypus is submerged to feed, but it possesses a unique electromechanical system of electroreceptors and touch receptors that allow it to navigate perfectly underwater (see also electroreception). Similar electroreceptors are also present in echidnas, which, together with the platypus, make up the mammalian order Monotremata, a unique group with an exceptionally ancient history.
Natural History
Platypuses are generally solitary, spending their lives either feeding along the bottoms of rivers, streams, and lakes or resting in burrows dug into the banks. They are extremely energetic, feeding almost continuously while in the water, shoveling through streambed debris with their flat bills as they hunt for larval insects and freshwater crustaceans (a favourite food). The platypus uses its sophisticated electromechanical system to detect minute electrical signals given off by the muscles of its prey. After feeding, it retires to its burrow, the entrance of which is large enough to admit only the platypus and serves to squeeze excess moisture from the fur.
The platypus is found in terrain ranging from the high country of Tasmania and the Australian Alps to lowland areas close to the sea. Although it has on occasion been seen swimming in salt water, the platypus must feed in fresh water, where its electrical navigation system is operative. The platypus is present in all eastern Australian states in both eastward- and westward-flowing river systems, but it is absent from far northern Queensland and, unlike its relatives, the echidnas, does not appear to have colonized the island of New Guinea.
Generally most active around dawn and dusk (crepuscular), platypuses can also be active during the day depending on the season, cloud cover, stream productivity, and even individual preference. Platypuses are not known to hibernate. However, they have an unusually low body temperature for mammals (about 32 °C [90 °F]). Studies have shown that they can maintain a constant body temperature even after extended periods in water with temperatures as low as 4 °C (39 °F), a fact that puts to rest the belief that monotremes cannot regulate their body temperature.
Form And Function
Platypuses range in length from 38 to 60 cm (15 to 24 inches); males are generally larger than females. Aquatic adaptations include the flat streamlined body, dorsally placed eyes and nostrils, and dense waterproof fur that keeps the platypus well insulated. Long guard hairs protect the soft underfur, which remains dry even after hours in the water. The extensive webbing on the front feet extends well past the claws and is essential in propelling the animal through the water. The paddlelike tail acts as a stabilizer during swimming, while the back feet act as rudders and brakes.
Odd skeletal features of platypuses include an archaic robust shoulder girdle and a short, wide humerus providing extensive muscle attachment areas for the exceptionally strong front limbs. The outside of the bill is covered by soft, sensitive skin. Inside the bill, adult platypuses do not have true teeth but instead have developed flat pads of hardened gum tissue. Male platypuses have a spur on the inner side of each ankle that is connected to a venom gland located over the thighs. The spurs can be wielded in defense, and the venom is potent enough to kill small animals and cause intense pain in humans if the spur penetrates the skin.
Evolution, Paleontology, And Classification
Aquatically adapted platypus-like monotremes probably evolved from a more-generalized terrestrial monotreme. The first occurrence in the fossil record of a platypus-like monotreme is from about 110 million years ago, in the early Cretaceous Period, when Australia was still connected to South America by Antarctica. Until recently this Cretaceous monotreme (Steropodon galmani, known by a stunning opalized jaw) was placed within the platypus family, but, partly on the basis of molecular studies and partly on dental structure, it is now classified in its own family, Steropodontidae.
The living platypus family (Ornithorhynchidae) includes the extinct genera Monotrematum (which dates to the Paleocene Epoch some 61 million years ago) and Obdurodon (which may have first emerged near the boundary of the Oligocene and Miocene epochs some 23 million years ago) and the living Ornithorhychus. The discovery of M. sudamericanum in 62-million-year-old Patagonian sediments confirmed that platypuses were once distributed through the southern continents that were once linked geographically (Gondwana). Species of Monotrematum and Obdurodon retained functional teeth and were more robust than the living platypus, Obdurodon measuring up to 60 cm (24 inches) long.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#123 2018-04-25 01:21:08
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,505
Re: Miscellany
107) Electric generator
Electric generator, also called dynamo, any machine that converts mechanical energy to electricity for transmission and distribution over power lines to domestic, commercial, and industrial customers. Generators also produce the electrical power required for automobiles, aircraft, ships, and trains.
The mechanical power for an electric generator is usually obtained from a rotating shaft and is equal to the shaft torque multiplied by the rotational, or angular, velocity. The mechanical power may come from a number of sources: hydraulic turbines at dams or waterfalls; wind turbines; steam turbines using steam produced with heat from the combustion of fossil fuels or from nuclear fission; gas turbines burning gas directly in the turbine; or gasoline and diesel engines. The construction and the speed of the generator may vary considerably depending on the characteristics of the mechanical prime mover.
Nearly all generators used to supply electric power networks generate alternating current, which reverses polarity at a fixed frequency (usually 50 or 60 cycles, or double reversals, per second). Since a number of generators are connected into a power network, they must operate at the same frequency for simultaneous generation. They are therefore known as synchronous generators or, in some contexts, alternators.
Synchronous Generators
A major reason for selecting alternating current for power networks is that its continual variation with time allows the use of transformers. These devices convert electrical power at whatever voltage and current it is generated to high voltage and low current for long-distance transmission and then transform it down to a low voltage suitable for each individual consumer (typically 120 or 240 volts for domestic service). The particular form of alternating current used is a sine wave. This has been chosen because it is the only repetitive shape for which two waves displaced from each other in time can be added or subtracted and have the same shape occur as the result. The ideal is then to have all voltages and currents of sine shape. The synchronous generator is designed to produce this shape as accurately as is practical. This will become apparent as the major components and characteristics of such a generator.
Rotor
The central shaft of the rotor is coupled to the mechanical prime mover. The magnetic field is produced by conductors, or coils, wound into slots cut in the surface of the cylindrical iron rotor. This set of coils, connected in series, is thus known as the field winding. The position of the field coils is such that the outwardly directed or radial component of the magnetic field produced in the air gap to the stator is approximately sinusoidally distributed around the periphery of the rotor. he field density in the air gap is maximum outward at the top, maximum inward at the bottom, and zero at the two sides, approximating a sinusoidal distribution.
Stator
The stator of the elementary generator in Figure 2 consists of a cylindrical ring made of iron to provide an easy path for the magnetic flux. In this case, the stator contains only one coil, the two sides being accommodated in slots in the iron and the ends being connected together by curved conductors around the stator periphery. The coil normally consists of a number of turns.
When the rotor is rotated, a voltage is induced in the stator coil. At any instant, the magnitude of the voltage is proportional to the rate at which the magnetic field encircled by the coil is changing with time—i.e.,the rate at which the magnetic field is passing the two sides of the coil. The voltage will therefore be maximum in one direction when the rotor has turned 90° from the position shown in Figure 2 and will be maximum in the opposite direction 180° later. The waveform of the voltage will be approximately of the sine form.
Frequency
The rotor structure of the generator in Figure 2 has two poles, one for magnetic flux directed outward and a corresponding one for flux directed inward. One complete sine wave is induced in the stator coil for each revolution of the rotor. The frequency of the electrical output, measured in hertz (cycles per second) is therefore equal to the rotor speed in revolutions per second. To provide a supply of electricity at 60 hertz, for example, the prime mover and rotor speed must be 60 revolutions per second, or 3,600 revolutions per minute. This is a convenient speed for many steam and gas turbines. For very large turbines, such a speed may be excessive for reasons of mechanical stress. In this case, the generator rotor is designed with four poles spaced at intervals of 90°. The voltage induced in a stator coil, which spans a similar angle of 90°, will consist of two complete sine waves per revolution. The required rotor speed for a frequency of 60 hertz is then 1,800 revolutions per minute. For lower speeds, such as are employed by most water turbines, a larger number of pole pairs can be used. The possible values of rotor speed, in revolutions per minute, are equal to 120 f/p, where f is the frequency and p the number of poles.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#124 2018-04-27 00:17:38
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,505
Re: Miscellany
108) Pyrometer
Pyrometer, device for measuring relatively high temperatures, such as are encountered in furnaces. Most pyrometers work by measuring radiation from the body whose temperature is to be measured. Radiation devices have the advantage of not having to touch the material being measured. Optical pyrometers, for example, measure the temperature of incandescent bodies by comparing them visually with a calibrated incandescent filament that can be adjusted in temperature. In an elementary radiation pyrometer, the radiation from the hot object is focused onto a thermopile, a collection of thermocouples, which generates an electrical voltage that depends on the intercepted radiation. Proper calibration permits this electrical voltage to be converted to the temperature of the hot object.
In resistance pyrometers a fine wire is put in contact with the object. The instrument converts the change in electrical resistance caused by heat to a reading of the temperature of the object. Thermocouple pyrometers measure the output of a thermocouple (q.v.) placed in contact with the hot body; by proper calibration, this output yields temperature. Pyrometers are closely akin to the bolometer and the thermistor and are used in thermometry.
(qv in British : abbreviation for (denoting a cross reference) : quod vide)

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#125 2018-04-29 00:49:10
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,505
Re: Miscellany
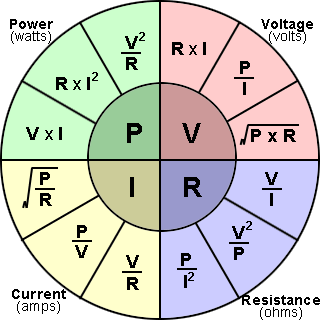
109) Ohm's Law
Ohm’s law, description of the relationship between current, voltage, and resistance. The amount of steady current through a large number of materials is directly proportional to the potential difference, or voltage, across the materials. Thus, if the voltage V (in units of volts) between two ends of a wire made from one of these materials is tripled, the current I (amperes) also triples; and the quotient V/I remains constant. The quotient V/I for a given piece of material is called its resistance, R, measured in units named ohms. The resistance of materials for which Ohm’s law is valid does not change over enormous ranges of voltage and current. Ohm’s law may be expressed mathematically as V/I = R. That the resistance, or the ratio of voltage to current, for all or part of an electric circuit at a fixed temperature is generally constant had been established by 1827 as a result of the investigations of the German physicist Georg Simon Ohm.
Alternate statements of Ohm’s law are that the current I in a conductor equals the potential difference V across the conductor divided by the resistance of the conductor, or simply I = V/R, and that the potential difference across a conductor equals the product of the current in the conductor and its resistance, V = IR. In a circuit in which the potential difference, or voltage, is constant, the current may be decreased by adding more resistance or increased by removing some resistance. Ohm’s law may also be expressed in terms of the electromotive force, or voltage, E, of the source of electric energy, such as a battery. For example, I = E/R.
With modifications, Ohm’s law also applies to alternating-current circuits, in which the relation between the voltage and the current is more complicated than for direct currents. Precisely because the current is varying, besides resistance, other forms of opposition to the current arise, called reactance. The combination of resistance and reactance is called impedance, Z. When the impedance, equivalent to the ratio of voltage to current, in an alternating current circuit is constant, a common occurrence, Ohm’s law is applicable. For example, V/I = Z.
With further modifications Ohm’s law has been extended to the constant ratio of the magnetomotive force to the magnetic flux in a magnetic circuit.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline