Math Is Fun Forum
You are not logged in.
- Topics: Active | Unanswered
#926 2021-02-04 00:13:58
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,354
Re: Miscellany
904) Citadelle of Quebec
The Citadelle of Quebec (French: Citadelle de Québec), also known as La Citadelle, is an active military installation and the secondary official residence of both the Canadian monarch and the Governor General of Canada. It is located atop Cap Diamant, adjoining the Plains of Abraham in Quebec City, Quebec. The citadel contains the oldest military building in Canada, and forms part of the fortifications of Quebec City, which is one of only two cities in North America still surrounded by fortifications, the other being Campeche, Mexico.
The strategic importance of Cap Diamant was recognized by the French as early as 1608. Several defensive fortification were built on the site by the French, and the British after their conquest of New France. The modern citadel was built from 1820 to 1850, in effort to secure Quebec City against a potential American attack. The British used the citadel until 1871, when they formally handed the property over to the Canadian government. Following the handover, the citadel was used as a military installation by the Canadian Armed Forces, and as an royal and viceregal residence.
The Citadelle is a National Historic Site of Canada and forms part of the Fortifications of Québec National Historic Site of Canada. The fortress is located within the Historic District of Old Québec, which was designated a World Heritage Site in 1985. The site receives some 200,000 visitors annually.
History
Cap Diamant's strategic value was identified by Samuel de Champlain in 1608 and led him to found Quebec City at the base of the escarpment. The promontory was practically insurmountable and thus the only side of the settlement ever to be heavily fortified was the west, the only one not naturally protected by the hill.
Early fortifications
French-rule
The first protective wall (enceinte)—Major Provost's palisade—was built by command of Governor General of New France Louis de Buade, sieur de Frontenac and completed just in time for the Battle of Quebec in 1690. Three years later, a plan by engineer Josué Boisberthelot de Beaucours for new, 75-metre (246 ft) wide enceinte was developed by the French military engineer Jacques Levasseur de Néré and approved in 1701 by King Louis XIV's Commissary General of Fortifications, Sébastien Le Prestre de Vauban.
The proposal to build a full fort was deemed by the government in France to be too costly, despite both the importance and vulnerability of Quebec City. After the fall of Louisbourg in 1745, considerable work on the battlements took place under the direction of military engineer Gaspard-Joseph Chaussegros de Léry.
British-rule
The first British Lieutenant Governor of Quebec, General James Murray, saw the weakness of Quebec City's defences (indeed, Murray's post existed precisely because the British had conquered Quebec City four years before Murray's appointment as governor in 1763). He urged the construction of a citadel, but the imperial government at Westminster, like the French before, deemed a large fort to be of little value; a smaller, wooden citadel was built.
During the American Revolutionary War, after seizing Montreal in the autumn of 1775, American rebels, led by General Richard Montgomery and Benedict Arnold, attempted to take Quebec on 31 December. There, Montgomery was killed and Arnold wounded and forced to retreat. The Americans attempted to keep Quebec under siege, but withdrew after the arrival of British reinforcements in the spring of 1776.
Present fortification (1820–present)
As tensions between the United Kingdom and the United States, as well as fears of further rebellion in British North America,[6] grew in the late 18th century, the British reinforced the defences of their colonies according to a plan drawn up in the 1790s by Gother Mann. The ramparts around the Upper Town cliff and four martello towers (still extant) on the Plains of Abraham were completed before 1812. A citadel was a key part of Mann's design, but no fort was built because the cost was deemed prohibitive.
That opinion finally shifted following the War of 1812; as part of a wider improvement of Canada's defences coordinated by the Duke of Richmond, then Governor-in-Chief of British North America, the existing star fort was built between 1820 and 1850 under the direction of Lieutenant Colonel Elias Walker Durnford of the Royal Engineers. Intended to secure Quebec City against the Americans and serve as a refuge for the British garrison in the event of attack or rebellion, the Citadelle incorporated a section of the French enceinte of 1745 and the layout was based on Sébastien Le Prestre de Vauban's design for an arms, munitions, and supplies depot, as well as a barracks. That, though, was somewhat of an anachronism by the time of the fort's completion, in comparison to other contemporary European military architecture. Additional buildings were completed in 1850.
After Canadian Confederation in 1867, Canada became responsible for its own defence; the British departed the Citadelle in 1871. Two batteries of the Royal Canadian Artillery were established at the Citadelle and an artillery school was opened in 1871, followed by a cavalry school. From the late 19th century, living conditions for soldiers at the fort gradually improved; canteens were opened and the casemates were made more comfortable. The preservation of much of the fortifications and defences of Quebec is due to the intervention of Governor General of Canada the Marquess of Dufferin and Ava, who also established the Citadelle as a viceregal residence in 1872, reviving a tradition dating to the founding of New France. Since 1920, the Citadelle has been the home station of the Royal 22e Régiment of the Canadian Forces.
The Quebec Conferences of 1943 and 1944, in which Governor General of Canada the Earl of Athlone, Prime Minister of Canada William Lyon Mackenzie King, British Prime Minister Winston Churchill, and US President Franklin D. Roosevelt discussed strategy for World War II, were held at the Citadelle of Quebec.
The Historic Sites and Monuments Board of Canada designated the Citadel as a national historic site in 1946.[9] The fortress was designated as a National Historic Site of Canada in 1980 and, five years later, the Historic District of Old Québec, of which the Citadelle is a part, was placed on UNESCO's list of World Heritage Sites.
Function
The Citadelle is a functioning military installation for the Canadian Armed Forces, as well as an official residence of both Canada's monarch and its governor general. The latter, by tradition, resides there for several weeks during the summer as well as other shorter periods throughout the year. As is done at the other federal royal residence, Rideau Hall in Ottawa, Canadian award presentations and investitures and ceremonies for both incoming and outgoing ambassadors and high commissioners to Canada are held at the Citadelle. The residence is also open to the public, running a visitors' program and free tours of the state rooms throughout the year as well as educational tours for students. The Citadelle attracts approximately 200,000 visitors each year.
A number of military ceremonies related to the Royal 22e Régiment take place at the Citadelle's parade ground, such as the changing of the guard and of battalion command and the consecration of each successive Batisse the Goat as regimental mascot. Additionally, daily at noon, a cannon is fired from the fort, the sound of which can be heard throughout Quebec City. Originally, two guns were fired each day, at 12:00 pm to alert Quebec City residents of the lunch hour and Angelus, or noon-day prayer and at 9:30 pm, marking the curfew for gunners and soldiers in the city. The tradition has continued since 1871, save for between 1994 and 2004.
Buildings
The fort is an uneven star shaped citadel and comprises four bastions and three straight curtain walls, all constructed with locally quarried sandstone. Within its walls are 24 buildings constructed mostly of grey cut stone.
Royal and viceregal residence
The Officer's Barracks, a neo-Norman structure built in 1831 by the British Army, has been a residence of the Governor General of Canada since 1872. The residence today has a total of 153 rooms over 4,459 square metres (48,000 sq ft), including offices for the governor general's secretary.
The entrance into the original area of the residence is through a set of double doors beneath a neo-classical porch bearing the words GOUVERNEUR GÉNÉRAL on the frieze and the crest of the Royal Arms of Canada in the tympanum. Within is a foyer with marble tile floor and a stair descending to the basement and, through another set of doors in a screen with translucent leaded glass sidelights and fanlight, is a hall; both rooms are in the Georgian style, in beige, cream, and gold. The Small Dining Room is similarly Georgian in decor, with robin's egg blue walls and white-painted trim. From the ceiling hang two crystal chandeliers.
Soon after the building became a royal residence, additions to accommodate the viceregal party and household were required, including a ballroom and sunroom. These, however, were on 2 February 1976 destroyed by fire and the other rooms of the residence suffered smoke and water damage. The Department of Public Works and Government Services Canada (which oversees the infrastructure of the Citadelle) restored the original wing and new state rooms were built in place of the lost post-1872 additions, the work being completed in 1984.
This new wing was built at the east end of and at a slight angle to the 1831 structure, the roof being copper and the exterior walls of the same masonry as the adjacent buildings, but, using more regulated block sizes and a flatter relief of pilasters and windows, as well as less detailing overall. The wing contains a separate entrance and ceremonial foyer with twin spiral staircases ascending to a piano nobile; the stairs have wood handrails with aluminum pickets and between them is a niche for sculpture. On the upper level is an event space, lounge, and sunroom with a terrace overlooking the St. Lawrence River. The former two areas are fully barrel vaulted and linked together by a continuous, narrow skylight in the roof under which crystal pendants of different lengths hang and transfer the natural light into the rooms. Interiors of the modern addition were designed by Quebec artist Madeleine Arbour, who was inspired by the colours of winter in Quebec, and use Canadian materials, including granite, walnut, and aluminum. The residence is furnished with pieces from the Crown Collection, mostly in New France style, antique furniture mixed with contemporary Canadian art.
Royal 22e Régiment Museum
Building 15, constructed in 1750, also known as the powder magazine, houses the Museum of the Royal 22e Régiment and Canadian Forces Museum, which collects, preserves, and displays artifacts of Canadian military historical significance, as well as the Museum, which features weapons, uniforms, and other military artifacts of the Royal 22e Régiment. The Museum is affiliated with: CMA, CHIN, OMMC and Virtual Museum of Canada.
Other buildings
Building 1, the Former Hospital Administration Building, is the principal structure in Mann's Bastion. It is a two-story, rectangular, symmetrical stone structure with a hipped-roof pierced by two brick chimneys. The west-facing facade features restrained decorative detailing of pilasters supporting a flat cornice along the length of the five-bay façade. Loopholes are visible on the second floor on both sides of the building. The rear elevation facing into the bastion is functional and plain in appearance.
Building 2, also known as the Men's Barracks, was formerly the armory and powder magazine. It faces the parade ground near the throat of the Prince of Wales Bastion. It is a long, rectangular, two story masonry building built of smooth limestone with a hipped roof clad in sheet copper. The main façade of the building has little ornamentation and is regulated and orderly, with small, regularly placed, multi-paned windows.
Building 5, the Former Powder Magazine, is a low, rectangular stone structure with a gabled roof and a surrounding protective blast wall. Two doors at ground level and three openings at gable level pierce the symmetrical façade. Along the plain side elevations are three half-barrel-vaulted passages, or traverses, two on the west and one on the east.
Building 7, also known as the Memorial Building, forms part of a complex of three structures, which includes a chapel and a workshop, situated near the parade ground. They are attached by a 19th-century protective wall. It is a small, rectangular, one-story masonry edifice with a pyramidal stone roof and a row of windows on all four elevations. Its principal façade is distinguished by a modern porchway. Governor General of Canada Georges Vanier and his wife Pauline Vanier are interred here at the commemorative chapel.
Located within the King's Bastion and adjacent to the governor general's residence, Building 10 is the former military prison and presently the museum annex. Constructed in 1842, it is a two-story rectangular stone structure with a hipped roof pierced by three brick chimneys. The west elevation facing the citadel is restrained but decorated by pilasters. Loopholes pierce the walls on both stories. There are several irregularly placed windows with iron bars.
Building 14, the former ordnance store, stands along the parade ground. The long, rectangular two story structure is constructed of stone with a hipped roof clad in copper sheet. The symmetrical façade features small, evenly spaced windows.
Building 16 is the museum office and former cooperage.
Building 17, also known as the Men's Barracks, stands on the south side of the parade ground near the throat of the Dalhousie Bastion. Two stories high for most of its length, the rectangular building has exterior walls of stone and features symmetrical elevations, a hipped, copper clad roof, and a projecting course of stone above the ground floor.
Clearly seen from the river and aligned on the meridian for observation purposes, Building 20, also known as the Ball House, is the former observatory and time ball tower. It is a compact, tall, two story stone structure of several architectural shapes. The rectangular section with a gabled roof was the former observatory and the square section formerly housed the time ball installation topped with an antenna. The building is entered from the ground floor of its small porch, which joins the two buildings.
Building 32, formerly the Defensive Guard House, is located at the eastern end of the north main ditch below the King's Bastion. It is a small, squat, one story masonry edifice with a gable roof supported by wood rafters. A chimney rises through the roof, which is covered in painted tin sheeting.
The former caponier, Building 46, is located at the south end of the ditch separating the counterscarp from the Men's Barracks. Its two walls and small turret in the middle of its gabled roof are the only visible elements of this small stone building. It is integrated with the ramparts and pierced with loopholes.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#927 2021-02-05 00:14:01
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,354
Re: Miscellany
905) Lubrication
Lubrication, introduction of any of various substances between sliding surfaces to reduce wear and friction. Nature has been applying lubrication since the evolution of synovial fluid, which lubricates the joints and bursas of vertebrate animals. Prehistoric people used mud and reeds to lubricate sledges for dragging game or timbers and rocks for construction. Animal fat lubricated the axles of the first wagons and continued in wide use until the petroleum industry arose in the 19th century, after which crude oil became the chief source of lubricants. The natural lubricating capacity of crude oil has been steadily improved through the development of a wide variety of products designed for the specific lubricating needs of the automobile, the airplane, the diesel locomotive, the turbojet, and power machinery of every description. The improvements in petroleum lubricants have in turn made possible the increase in speed and capacity of industrial and other machinery.
There are three basic varieties of lubrication: fluid-film, boundary, and solid.
Fluid-Film Lubrication.
Interposing a fluid film that completely separates sliding surfaces results in this type of lubrication. The fluid may be introduced intentionally, as the oil in the main bearings of an automobile, or unintentionally, as in the case of water between a smooth rubber tire and a wet pavement. Although the fluid is usually a liquid, it may also be a gas. The gas most commonly employed is air.
To keep the parts separated, it is necessary that the pressure within the lubricating film balance the load on the sliding surfaces. If the lubricating film’s pressure is supplied by an external source, the system is said to be lubricated hydrostatically. If the pressure between the surfaces is generated as a result of the shape and motion of the surfaces themselves, however, the system is hydrodynamically lubricated. This second type of lubrication depends upon the viscous properties of the lubricant.
Boundary Lubrication.
A condition that lies between unlubricated sliding and fluid-film lubrication is referred to as boundary lubrication, also defined as that condition of lubrication in which the friction between surfaces is determined by the properties of the surfaces and properties of the lubricant other than viscosity. Boundary lubrication encompasses a significant portion of lubrication phenomena and commonly occurs during the starting and stopping of machines.
Solid Lubrication.
Solids such as graphite and molybdenum disulfide are widely used when normal lubricants do not possess sufficient resistance to load or temperature extremes. But lubricants need not take only such familiar forms as fats, powders, and gases; even some metals commonly serve as sliding surfaces in some sophisticated machines.
A lubricant primarily controls friction and wear, but it can and ordinarily does perform numerous other functions, which vary with the application and usually are interrelated.
Control Functions.
The amount and character of the lubricant made available to sliding surfaces have a profound effect upon the friction that is encountered. For example, disregarding such related factors as heat and wear but considering friction alone between two oil-film lubricated surfaces, the friction can be 200 times less than that between the same surfaces with no lubricant. Under fluid-film conditions, friction is directly proportional to the viscosity of the fluid. Some lubricants, such as petroleum derivatives, are available in a great range of viscosities and thus can satisfy a broad spectrum of functional requirements. Under boundary lubrication conditions, the effect of viscosity on friction becomes less significant than the chemical nature of the lubricant. Delicate instruments, for example, must not be lubricated with fluids that would attack and corrode the finer metals.
Wear occurs on lubricated surfaces by abrasion, corrosion, and solid-to-solid contact. Proper lubricants will help combat each type. They reduce abrasive and solid-to-solid contact wear by providing a film that increases the distance between the sliding surfaces, thereby lessening the damage by abrasive contaminants and surface asperities. The role of a lubricant in controlling corrosion of surfaces is twofold. When machinery is idle, the lubricant acts as a preservative. When machinery is in use, the lubricant controls corrosion by coating lubricated parts with a protective film that may contain additives to neutralize corrosive materials. The ability of a lubricant to control corrosion is directly related to the thickness of the lubricant film remaining on the metal surfaces and the chemical composition of the lubricant.
Lubricants also can assist in controlling temperature by reducing friction and carrying off the heat that is generated. Effectiveness depends upon the amount of lubricant supplied, the ambient temperature, and the provision for external cooling. To a lesser extent, the type of lubricant also affects surface temperature.
Other Functions.
Various lubricants are employed as hydraulic fluids in fluid transmission devices. Others can be used to remove contaminants in mechanical systems. Detergent-dispersant additives, for instance, suspend sludges and remove them from the sliding surfaces of internal-combustion engines.
In specialized applications such as transformers and switchgear, lubricants with high dielectric constants act as electrical insulators. For maximum insulating properties, a lubricant must be kept free of contaminants and water. Lubricants also act as shock-damping fluids in energy-transferring devices (e.g., shock absorbers) and around such machine parts as gears that are subjected to high intermittent loads.
A wide variety of lubricants are available. The principal types are reviewed here.
Liquid, Oily Lubricants.
Animal and vegetable products were certainly man’s first lubricants and were used in large quantities. But, because they lack chemical inertness and because lubrication requirements have become more demanding, they have been largely superseded by petroleum products and by synthetic materials. Some organic substances such as lard oil and other are still in use as additives because of their special lubricating properties.
Petroleum lubricants are predominantly hydrocarbon products extracted from fluids that occur naturally within the Earth. They are used widely as lubricants because they possess a combination of the following desirable properties: (1) availability in suitable viscosities, (2) low volatility, (3) inertness (resistance to deterioration of the lubricant), (4) corrosion protection (resistance to deterioration of the sliding surfaces), and (5) low cost.
Synthetic lubricants generally can be characterized as oily, neutral liquid materials not usually obtained directly from petroleum but having some properties similar to petroleum lubricants. In certain ways they are superior to hydrocarbon products. Synthetics exhibit greater stability of viscosity with temperature changes, resistance to scuffing and oxidation, and fire resistance. Since the properties of synthetics vary considerably, each synthetic lubricant tends to find a special application.
Another form of oily lubricant is grease, a solid or semisolid substance consisting of a thickening agent in a liquid lubricant. Soaps of aluminum, barium, calcium, lithium, sodium, and strontium are the major thickening agents. Nonsoap thickeners consist of such inorganic compounds as modified clays or fine silicas, or such organic materials as arylureas or phthalocyanine pigments. Lubrication by grease may prove more desirable than lubrication by oil under conditions when (1) less frequent lubricant application is necessary, (2) grease acts as a seal against loss of lubricant and ingress of contaminants, (3) less dripping or splattering of lubricant is called for, or (4) less sensitivity to inaccuracies in the mating parts is needed.
Solid Lubricants.
A solid lubricant is a film of solid material composed of inorganic or organic compounds or of metal.
There are three general kinds of inorganic compounds that serve as solid lubricants:
1. Layer-lattice solids: materials such as graphite and molybdenum disulfide, commonly called molysulfide, have a crystal lattice structure arranged in layers. Strong bonds between atoms within a layer and relatively weak bonds between atoms of different layers allow the lamina to slide on one another. Other such materials are tungsten disulfide, mica, boron nitride, borax, silver sulfate, cadmium iodide, and lead iodide. Graphite’s low friction is due largely to adsorbed films; in the absence of water vapour, graphite loses its lubricating properties and becomes abrasive. Both graphite and molysulfide are chemically inert and have high thermal stability.
2. Miscellaneous soft solids: a variety of inorganic solids such as white lead, lime, talc, bentonite, silver iodide, and lead monoxide are used as lubricants.
3. Chemical conversion coatings: many inorganic compounds can be formed on a metallic surface by chemical reaction. The best known such lubricating coatings are sulfide, chloride, oxide, phosphate, and oxalate films.
Solid organic lubricants are usually divided into two broad classes:
1. Soaps, waxes, and fats: this class includes metallic soaps of calcium, sodium, lithium; animal waxes (e.g., beeswax and spermaceti wax); fatty acids (e.g., stearic and palmitic acids); and fatty esters (e.g., lard and tallow).
2. Polymeric films: these are synthetic substances such as polytetrafluoroethylene and polychlorofluoroethylene. One major advantage of such film-type lubricants is their resistance to deterioration during exposure to the elements. Thus, 1/2-inch- (1.3-centimetre-) thick plates of polymeric film are used in modern prestressed concrete construction to permit thermal movement of beams resting atop columns. Such expansion and contraction of the structural members is facilitated by the long-lived polymeric film plate.
Thin films of soft metal on a hard substrate can act as effective lubricants, if the adhesion to the substrate is good. Such metals include lead, tin, and indium.
Gaseous Lubricants.
Lubrication with a gas is analogous in many respects to lubrication with a liquid, since the same principles of fluid-film lubrication apply. Although both gases and liquids are viscous fluids, they differ in two important particulars. The viscosity of gases is much lower and the compressibility much greater than for liquids. Film thicknesses and load capacities therefore are much lower with a gas such as air (see Table 1). In equipment that handles gases of various kinds, it is often desirable to lubricate the sliding surfaces with gas in order to simplify the apparatus and reduce contamination to and from the lubricant. The list of gases used in this manner is extensive and includes air, steam, industrial gases, and liquid-metal vapours.
With so many types of materials capable of acting as lubricants under certain conditions, coverage of the properties of all of them is impractical. Mention is made only of those properties usually considered characteristic of commercially significant fluid lubricants.
Viscosity.
Of all the properties of fluid lubricants, viscosity is the most important, since it determines the amount of friction that will be encountered between sliding surfaces and whether a thick enough film can be built up to avoid wear from solid-to-solid contact. Viscosity customarily is measured by a viscometer, which determines the flow rate of the lubricant under standard conditions; the higher the flow rate, the lower the viscosity. The rate is expressed in centipoises, reyns, or seconds Saybolt universal (SSU) depending, respectively, upon whether metric, English, or commercial units are used. In most liquids, viscosity drops appreciably as the temperature is raised. Since little change of viscosity with fluctuations in temperature is desirable to keep variations in friction at a minimum, fluids often are rated in terms of viscosity index. The less the viscosity is changed by temperature, the higher the viscosity index.
Pour Point.
The pour point, or the temperature at which a lubricant ceases to flow, is important in appraising flow properties at low temperature. As such, it can become the determining factor in selecting one lubricant from among a group with otherwise identical properties.
Flash Point.
The flash point, or the temperature at which a lubricant momentarily flashes in the pressure of a test flame, aids in evaluating fire-resistance properties. Like the pour-point factor, the flash point may in some instances become the major consideration in selecting the proper lubricant, especially in lubricating machinery handling highly flammable material.
Oiliness.
Oiliness generally connotes relative ability to operate under boundary lubrication conditions. The term relates to a lubricant’s tendency to wet and adhere to a surface. There is no formal test for the measurement of oiliness; determination of this factor is chiefly through subjective judgment and experience. The most desirable lubricant for a specific use need not necessarily be the oiliest; e.g., long-fibre grease, which is low in oiliness as compared with machine oils, is usually preferable for packing rolling bearings.
Neutralization Number.
The neutralization number is a measure of the acid or alkaline content of new oils and an indicator of the degree of oxidation degradation of used oils. This value is ascertained by titration, a standard analytical chemical technique, and is defined as the number of milligrams of potassium hydroxide required to neutralize one gram of the lubricant.
Penetration Number.
The penetration number, applied to grease, is a measure of the film characteristics of the grease. The test consists of dropping a standard cone into the sample of grease being tested. Gradations indicate the depth of penetration: the higher the number, the more fluid the grease.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#928 2021-02-06 00:39:18
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,354
Re: Miscellany
906) Refraction
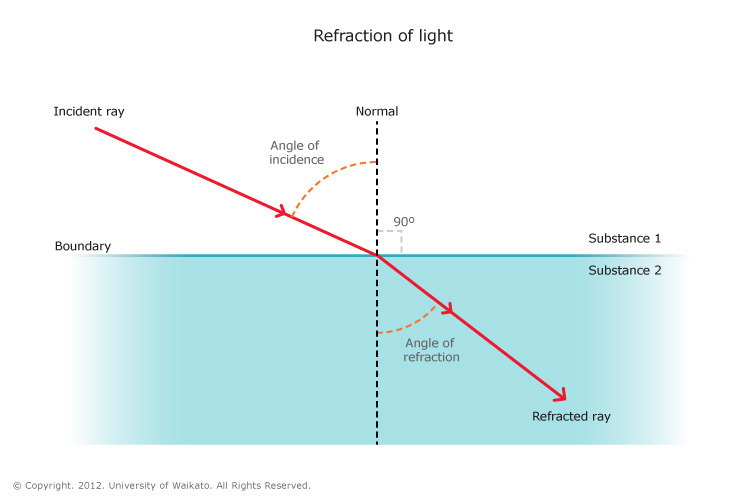
Refraction, in physics, the change in direction of a wave passing from one medium to another caused by its change in speed. For example, waves in deep water travel faster than in shallow. If an ocean wave approaches a beach obliquely, the part of the wave farther from the beach will move faster than that closer in, and so the wave will swing around until it moves in a direction perpendicular to the shoreline. The speed of sound waves is greater in warm air than in cold. At night, air is cooled at the surface of a lake, and any sound that travels upward is refracted down by the higher layers of air that still remain warm. Thus, sounds, such as voices and music, can be heard much farther across water at night than in the daytime.
The electromagnetic waves constituting light are refracted when crossing the boundary from one transparent medium to another because of their change in speed. A straight stick appears bent when partly immersed in water and viewed at an angle to the surface other than 90°. A ray of light of one wavelength, or colour (different wavelengths appear as different colours to the human eye), in passing from air to glass is refracted, or bent, by an amount that depends on its speed in air and glass, the two speeds depending on the wavelength. A ray of sunlight is composed of many wavelengths that in combination appear to be colourless. Upon entering a glass prism, the different refractions of the various wavelengths spread them apart as in a rainbow.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#929 2021-02-07 00:13:21
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,354
Re: Miscellany
907) Newton's laws of motion
Newton’s laws of motion, relations between the forces acting on a body and the motion of the body, first formulated by English physicist and mathematician Sir Isaac Newton.
Newton’s first law states that, if a body is at rest or moving at a constant speed in a straight line, it will remain at rest or keep moving in a straight line at constant speed unless it is acted upon by a force. This postulate is known as the law of inertia. The law of inertia was first formulated by Galileo Galilei for horizontal motion on Earth and was later generalized by René Descartes. Before Galileo it had been thought that all horizontal motion required a direct cause, but Galileo deduced from his experiments that a body in motion would remain in motion unless a force (such as friction) caused it to come to rest.
Newton’s second law is a quantitative description of the changes that a force can produce on the motion of a body. It states that the time rate of change of the momentum of a body is equal in both magnitude and direction to the force imposed on it. The momentum of a body is equal to the product of its mass and its velocity. Momentum, like velocity, is a vector quantity, having both magnitude and direction. A force applied to a body can change the magnitude of the momentum, or its direction, or both. Newton’s second law is one of the most important in all of physics. For a body whose mass m is constant, it can be written in the form F = ma, where F (force) and a (acceleration) are both vector quantities. If a body has a net force acting on it, it is accelerated in accordance with the equation. Conversely, if a body is not accelerated, there is no net force acting on it.
Newton’s third law states that when two bodies interact, they apply forces to one another that are equal in magnitude and opposite in direction. The third law is also known as the law of action and reaction. This law is important in analyzing problems of static equilibrium, where all forces are balanced, but it also applies to bodies in uniform or accelerated motion. The forces it describes are real ones, not mere bookkeeping devices. For example, a book resting on a table applies a downward force equal to its weight on the table. According to the third law, the table applies an equal and opposite force to the book. This force occurs because the weight of the book causes the table to deform slightly so that it pushes back on the book like a coiled spring.
Newton’s laws first appeared in his masterpiece, ‘Philosophiae Naturalis Principia Mathematica’ (1687), commonly known as the Principia. In 1543 Nicolaus Copernicus suggested that the Sun, rather than Earth, might be at the centre of the universe. In the intervening years Galileo, Johannes Kepler, and Descartes laid the foundations of a new science that would both replace the Aristotelian worldview, inherited from the ancient Greeks, and explain the workings of a heliocentric universe. In the Principia Newton created that new science. He developed his three laws in order to explain why the orbits of the planets are ellipses rather than circles, at which he succeeded, but it turned out that he explained much more. The series of events from Copernicus to Newton is known collectively as the Scientific Revolution.
In the 20th century Newton’s laws were replaced by quantum mechanics and relativity as the most fundamental laws of physics. Nevertheless, Newton’s laws continue to give an accurate account of nature, except for very small bodies such as electrons or for bodies moving close to the speed of light. Quantum mechanics and relativity reduce to Newton’s laws for larger bodies or for bodies moving more slowly.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#930 2021-02-08 00:54:22
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,354
Re: Miscellany
908) Kepler's laws of planetary motion
Kepler’s laws of planetary motion, in astronomy and classical physics, laws describing the motions of the planets in the solar system. They were derived by the German astronomer Johannes Kepler, whose analysis of the observations of the 16th-century Danish astronomer Tycho Brahe enabled him to announce his first two laws in the year 1609 and a third law nearly a decade later, in 1618. Kepler himself never numbered these laws or specially distinguished them from his other discoveries.
Kepler’s three laws of planetary motion can be stated as follows: (1) All planets move about the Sun in elliptical orbits, having the Sun as one of the foci. (2) A radius vector joining any planet to the Sun sweeps out equal areas in equal lengths of time. (3) The squares of the sidereal periods (of revolution) of the planets are directly proportional to the cubes of their mean distances from the Sun. Knowledge of these laws, especially the second (the law of areas), proved crucial to Sir Isaac Newton in 1684–85, when he formulated his famous law of gravitation between Earth and the Moon and between the Sun and the planets, postulated by him to have validity for all objects anywhere in the universe. Newton showed that the motion of bodies subject to central gravitational force need not always follow the elliptical orbits specified by the first law of Kepler but can take paths defined by other, open conic curves; the motion can be in parabolic or hyperbolic orbits, depending on the total energy of the body. Thus, an object of sufficient energy—e.g., a comet—can enter the solar system and leave again without returning. From Kepler’s second law, it may be observed further that the angular momentum of any planet about an axis through the Sun and perpendicular to the orbital plane is also unchanging.
The usefulness of Kepler’s laws extends to the motions of natural and artificial satellites, as well as to stellar systems and extrasolar planets. As formulated by Kepler, the laws do not, of course, take into account the gravitational interactions (as perturbing effects) of the various planets on each other. The general problem of accurately predicting the motions of more than two bodies under their mutual attractions is quite complicated; analytical solutions of the three-body problem are unobtainable except for some special cases. It may be noted that Kepler’s laws apply not only to gravitational but also to all other inverse-square-law forces and, if due allowance is made for relativistic and quantum effects, to the electromagnetic forces within the atom.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#931 2021-02-09 00:03:56
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,354
Re: Miscellany
909) Refrigeration
Refrigeration, the process of removing heat from an enclosed space or from a substance for the purpose of lowering the temperature.
In the industrialized nations and affluent regions in the developing world, refrigeration is chiefly used to store foodstuffs at low temperatures, thus inhibiting the destructive action of bacteria, yeast, and mold. Many perishable products can be frozen, permitting them to be kept for months and even years with little loss in nutrition or flavour or change in appearance. Air-conditioning, the use of refrigeration for comfort cooling, has also become widespread in more developed nations.
Before mechanical refrigeration systems were introduced, ancient peoples, including the Greeks and Romans, cooled their food with ice transported from the mountains. Wealthy families made use of snow cellars, pits that were dug into the ground and insulated with wood and straw, to store the ice. In this manner, packed snow and ice could be preserved for months. Stored ice was the principal means of refrigeration until the beginning of the 20th century, and it is still used in some areas.
In India and Egypt evaporative cooling was employed. If a liquid is rapidly vaporized, it expands quickly. The rising molecules of vapour abruptly increase their kinetic energy. Much of this increase is drawn from the immediate surroundings of the vapour, which are therefore cooled. Thus, if water is placed in shallow trays during the cool tropical nights, its rapid evaporation can cause ice to form in the trays, even if the air does not fall below freezing temperatures. By controlling the conditions of evaporation, it is possible to form even large blocks of ice in this manner.
Cooling caused by the rapid expansion of gases is the primary means of refrigeration today. The technique of evaporative cooling, as described heretofore, has been known for centuries, but the fundamental methods of mechanical refrigeration were only discovered in the middle of the 19th century. The first known artificial refrigeration was demonstrated by William Cullen at the University of Glasgow in 1748. Cullen let ethyl ether boil into a partial vacuum; he did not, however, use the result to any practical purpose. In 1805 an American inventor, Oliver Evans, designed the first refrigeration machine that used vapour instead of liquid. Evans never constructed his machine, but one similar to it was built by an American physician, John Gorrie, in 1844.
Commercial refrigeration is believed to have been initiated by an American businessman, Alexander C. Twinning, in 1856. Shortly afterward, an Australian, James Harrison, examined the refrigerators used by Gorrie and Twinning and introduced vapour-compression refrigeration to the brewing and meat-packing industries. A somewhat more complex system was developed by Ferdinand Carré of France in 1859. Unlike earlier vapour-compression machines, which used air as a coolant, Carré’s equipment contained rapidly expanding ammonia. (Ammonia liquefies at a much lower temperature than water and is thus able to absorb more heat.) Carré’s refrigerators were widely used, and vapour-compression refrigeration became, and still is, the most widely used method of cooling.
In spite of the successful use of ammonia, that substance had a severe disadvantage: if it leaked, it was unpleasant as well as toxic. Refrigeration engineers searched for acceptable substitutes until the 1920s, when a number of synthetic refrigerants were developed. The best known of these substances was patented under the brand name of Freon. Chemically, Freon was created by the substitution of two chlorine and two fluorine atoms for the four hydrogen atoms in methane (CH4); the result, dichlorofluoromethane (CCl2F2), is odourless and is toxic only in extremely large doses.
The basic components of a modern vapour-compression refrigeration system are a compressor; a condenser; an expansion device, which can be a valve, a capillary tube, an engine, or a turbine; and an evaporator. The gas coolant is first compressed, usually by a piston, and then pushed through a tube into the condenser. In the condenser, the winding tube containing the vapour is passed through either circulating air or a bath of water, which removes some of the heat energy of the compressed gas. The cooled vapour is passed through an expansion valve to an area of much lower pressure; as the vapour expands, it draws the energy of its expansion from its surroundings or the medium in contact with it. Evaporators may directly cool a space by letting the vapour come into contact with the area to be chilled, or they may act indirectly—i.e., by cooling a secondary medium such as water. In most domestic refrigerators, the coil containing the evaporator directly contacts the air in the food compartment. At the end of the process, the hot gas is drawn toward the compressor.
In the 1960s certain characteristics of semiconductors began to be utilized for commercial refrigeration. Chief among these was the Peltier effect, named after the French chemist Jean Peltier, who observed in 1834 that electric currents passing through the junction of two different metals sometimes caused the junction to cool. When the junction is made from semiconductors such as bismuth telluride, the Peltier effect is of magnitude sufficient to permit its commercial use.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#932 2021-02-10 00:04:47
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,354
Re: Miscellany
910) Manganese processing
Manganese processing, preparation of the ore for use in various products.
Manganese (Mn) is a hard, silvery white metal with a melting point of 1,244 °C (2,271 °F). Ordinarily too brittle to be of structural value itself, it is an essential agent in steelmaking, in which it removes impurities such as sulfur and oxygen and adds important physical properties to the metal. For these purposes it is most often employed as a ferromanganese or silicomanganese alloy; as a pure metal it is added to certain nonferrous alloys.
Manganese is an allotropic metal—that is, its crystal structure changes with temperature. While cooling from the molten state down to 1,138 °C (2,080 °F), it solidifies into a body-centred cubic structure called the delta (δ) phase; from that point down to 1,100 °C (2,000 °F) it is in the face-centred cubic gamma (γ) phase, and from this point down to room temperature it goes through the beta (β) and alpha (α) phases. These last two phases, characterized by complex cubic structures, are extremely hard and brittle, while the simpler gamma phase is more ductile.
Manganese metal oxidizes superficially in air, rusts in moist air, and burns in air or oxygen at elevated temperatures. It decomposes water slowly when cool and rapidly when heated, forming hydrogen gas and manganous hydroxide, and it dissolves readily in dilute mineral acids, generating hydrogen and various manganous salts. The chemical reactivity of the metal accounts for its utility in metallurgy and in various chemical compounds.
History
Metallic manganese was first isolated in 1774 by Johan Gottlieb Gahn, a Swedish mineralogist who reduced pyrolusite, a manganese dioxide ore, with carbon. In 1856 Robert Forester Mushet, a British steelmaker, used manganese to improve the ability of steel produced by the Bessemer process to withstand rolling and forging at elevated temperatures. A tough wear-resistant steel containing approximately 12 percent manganese was developed in Sheffield, England, by Robert Abbott Hadfield in 1882. Ferromanganese was first smelted commercially in a blast furnace in 1875; electric-furnace production began in 1890. Pure manganese was not available commercially until 1941, following work on electrolysis conducted in the 1930s under S.M. Shelton at the U.S. Bureau of Mines. By the early 21st century, manganese production had expanded to several locations throughout the world, and Australia, South Africa, China, Gabon, and Brazil became the largest producers.
Ores
The most important manganese ores are the oxides pyrolusite, romanechite, manganite, and hausmannite and the carbonate ore rhodochrosite. Rhodonite and braunite, both silicate ores, are frequently found with the oxides. Only ores containing greater than 35 percent manganese are considered commercially exploitable. Impurities include oxides of other metals, such as iron, that are reduced along with manganese upon smelting; nonmetallic elements such as phosphorus, sulfur, and math; and metallic “gangue oxides” such as silica, alumina, lime, and magnesia, which, with the exception of silica, generally remain in the slag upon smelting.
A relatively abundant metal, manganese is widely distributed throughout Earth’s crust. In addition to terrestrial sources, manganese is present in nodules that are distributed widely over the seafloor. Higher-grade nodules contain 10 to 20 percent manganese along with significant amounts of cobalt, copper, and nickel.
Mining And Concentrating
The mining of manganese ores is usually done in open pits. Some ores are upgraded by washing, and undersized ores can be agglomerated by sintering. Several processes have been developed for mining seafloor nodules, but they cannot compete economically with the ready exploitation of high-grade terrestrial deposits.
Extraction And Refining
Pure manganese is produced by hydrometallurgical and electrolytic processes, while ferromanganese and silicomanganese are produced by the smelting of ores in a blast furnace or, more commonly, in an electric furnace. The latter process, involving the reduction of manganese oxides by carbon, is actually a complex thermodynamic problem. The higher oxides (MnO2, Mn2O3, and Mn3O4) can all be reduced to manganous oxide (MnO) by carbon monoxide, but this lower oxide can be reduced to the metal only at elevated temperatures by carbon. Smelting is further complicated by the action of the gangue oxides. For example, silica, an acidic compound, can combine with MnO and prevent it from being reduced—a problem that can be corrected by the use of ores high in such basic constituents as lime and magnesia or by the addition of basic fluxes such as roasted limestone. However, this generates more slag, which tends to dissolve manganese and lower the amount of metal recovered in the melt. In addition, depending on the smelting temperature and the acidity or basicity of the slag, silica can be reduced to silicon and enter the molten metal.
High-carbon ferromanganese
The primary product of the smelting process outlined above is a carbon-saturated ferroalloy containing 76 to 80 percent manganese, 12 to 15 percent iron, up to 7.5 percent carbon, and up to 1.2 percent silicon. It can be produced by two methods. In the first, ores are selected on the basis of their acidity so that, on smelting, 70 to 80 percent of the manganese is recovered in the melt while a slag containing 30 to 42 percent manganese is also obtained. (This slag can be resmelted to produce silicomanganese). The second method, which employs basic ores or fluxes, recovers 85 to 90 percent of the metal and generates a slag low enough in manganese to be discarded. The first method consumes 2,400 to 2,800 kilowatt-hours of electric power per ton of product, while the second, reflecting the higher energy needed to calcine the fluxes and continue smelting to a higher recovery of metal, consumes 2,600 to 3,100 kilowatt-hours per ton.
Silicomanganese
This alloy, containing 65 to 68 percent manganese, 16 to 21 percent silicon, and 1.5 to 2 percent carbon, is produced by the smelting of slag from high-carbon ferromanganese or of manganese ore with coke and a quartz flux. Smelting temperatures are higher than in ferromanganese production, and greater energy is needed to reduce the quartz to silicon, so that power consumption is 3,800 to 4,800 kilowatt-hours per ton.
The carbon content of the alloy is significantly lowered by the presence of silicon. Further removal of carbon can occur on cooling, owing to the formation of silicon carbide, which floats to the top of the metal and is collected in the slag. A silicomanganese of even lower carbon content (less than 0.1 percent) can be obtained by resmelting silicomanganese with more coke and quartz. This product is used as a reducing agent in the manufacture of low-carbon ferromanganese.
Medium- and low-carbon ferromanganese
To obtain a product of low carbon and silicon content, manganese ore, lime flux, and coal are fused in a furnace, forming a melt rich in MnO. This is then contacted with silicomanganese or low-carbon silicomanganese. The silicon in these alloys reduces the MnO to manganese metal and is itself oxidized into the slag. The carbon content of the particular silicomanganese reducing agent carries over to the final ferromanganese product—about 1 percent when silicomanganese is used and 0.1 percent with low-carbon silicomanganese.
Another method of producing medium-carbon silicomanganese involves refining molten high-carbon ferromanganese by blowing it with oxygen. This oxidizes the carbon until its content in the metal is less than 1.5 percent.
Electrolytic manganese
For applications in which pure manganese is preferred, manganese ores are roasted to obtain an MnO calcine, and this is dissolved in sulfuric acid to form a manganous sulfate solution. The addition of ammonia precipitates iron and aluminum, and the addition of hydrogen sulfide precipitates math, copper, zinc, lead, cobalt, and molybdenum. The purified solution is then fed into the cathode portion of an electrolytic cell, and, with the passage of electric current, manganese is deposited in layers a few millimetres thick on a stainless-steel cathode sheet. Cathodes are extracted periodically, and the manganese deposits are removed by hammering. The flakes are heated to 500 °C (925 °F) to remove hydrogen, resulting in a powdered manganese of greater than 99.9 percent purity.
The Metal And Its Alloys
More than 90 percent of the manganese produced goes into metallurgical applications, the pure metal being used in copper and aluminum alloys and ferromanganese and silicomanganese employed in steel and cast iron.
Steel
Most manganese is consumed as high-carbon ferromanganese for addition to carbon steels. In steels of lower carbon content, the medium- and low-carbon ferroalloy and even electrolytic manganese are employed.
As a desulfurizer, manganese forms stable, high-melting sulfide particles, thereby removing sulfur from the crystalline grain boundaries of the metal, where it can cause “hot shortness” (the inability to stand up to hot working). As a deoxidizer, silicomanganese is more effective than either silicon or manganese alone, since the simultaneous reaction of the two elements with oxygen produces a manganous silicate, which is low-melting and readily separates from the steel.
Most important, as an alloying agent, manganese improves strength, hardness, hardenability, and abrasion resistance. For example, Hadfield steel, containing 10 to 14 percent manganese, is a wear-resistant steel noted for its capacity to be work-hardened. In low-alloy steels, additions of up to 1.2 percent manganese, in combination with other elements, increases yield and tensile strength.
Nonferrous alloys
Powdered electrolytic manganese is briquetted with aluminum shot and added to aluminum in concentrations up to 2 percent. These alloys have greater strength, wear resistance, and corrosion resistance than can be provided by pure aluminum.
Aside from its standard alloying agents (aluminum, nickel, and zinc) copper can be improved by the addition of manganese. Manganese serves as a deoxidizer of the molten alloy, lowers the liquidus and solidus (thereby improving castability), and has a general strengthening effect. Special alloys, such as those with high electrical resistivity or high thermal expansion, can also be produced.
Chemical Compounds
Manganous oxide is made by the reduction of manganous dioxide (MnO2) by carbon, hydrogen, carbon monoxide, or hydrocarbons at temperatures between 400 and 800 °C (750 and 1,450 °F). Manganese is readily assimilated by plants in this form, so that MnO is used as a fertilizer supplement in manganese-deficient regions. For use in fertilizer, MnO is obtained by the reduction of ores; high-purity MnO is used in specialty ceramics.
Although it occurs naturally in manganese ores, manganous dioxide, which finds its principal application as a depolarizer in dry-cell batteries, is usually produced synthetically. Manganese ore is reduced and then leached with sulfuric acid. The solution is purified, and manganous dioxide is obtained by electrolysis. It can also be obtained by the oxidation of manganese compounds of lower valance or by the thermal decomposition of manganese nitrate.
Potassium permanganate (KMnO4) finds wide use as an oxidizing agent. It is produced by alkaline fusion under oxidizing conditions, followed by electrolysis.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#933 2021-02-11 00:19:48
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,354
Re: Miscellany
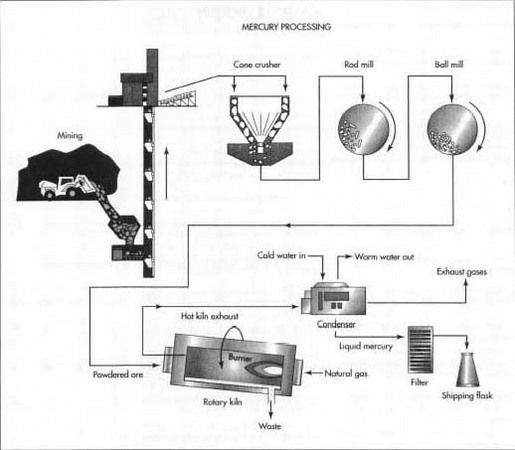
911) Mercury processing
Mercury processing, preparation of the ore for use in various products.
Mercury (Hg) has a unique combination of physical properties. Its low melting point (−38.87 °C [−38 °F]) and boiling point (356.9 °C [674 °F]), high specific gravity (13.5 grams per cubic centimetre), uniform volume expansion over the entire range of temperatures in its liquid state, and high surface tension (so that it does not wet glass) make it useful for the measurement of temperature in thermometers and of pressure in barometers and manometers. In addition, the high electrical conductivity of liquid mercury has led to its use in sealed electric switches and relays, industrial power rectifiers, fluorescent and mercury-vapour lamps, mercury cell batteries, and as moving cathodes in the large-scale production of chlorine and caustic soda.
Because mercury is highly toxic, care must be exercised in its handling and transport. By limiting exposure to mercury metal, vapours, and compounds through such preventive measures as proper ventilation, plant cleanliness and personal hygiene, industrial plants can be made relatively safe from the dangers of mercury poisoning.
History
The recovery and uses of mercury, also known as quicksilver, have been described since antiquity. Its use in the early 2nd millennium BCE in Egypt has been implied but not authenticated, as the use of synonyms in ancient writings obscures the meaning of some writers, but the mining and concentrating of cinnabar, the most common ore of mercury, were certainly described in the 4th century BCE. Alchemists in China were believed to have used mercury in trying to convert base metals to gold as early as the 2nd century BCE, and the Roman writer Pliny the Elder wrote in the 1st century about the recovery of quicksilver by distillation and condensation, the forerunner of modern methods of metallurgical treatment. Pliny also described the trade in mercury and cinnabar between Spain and Rome.
Because mercury was credited in folklore with the power to ward off evil spirits and cure various ailments, it acquired various therapeutic and agricultural uses. By the 16th century, crude furnaces for treating cinnabar by distillation and condensation were meeting the growing demand for quicksilver in medicine and in the amalgamation of gold and silver ores. Beginning in the 17th century, advancing science and technology brought a continuous increase in demand for mercury for use in thermometers, barometers, and electrical and chemical applications.
In the early mining and furnacing of cinnabar and mercury, workers showed the symptoms of mercury poisoning, but little was known of the cause and treatment. As operators learned to reduce the escape of gases by improving furnaces and condensers and to promote personal hygiene, the incidence of poisoning declined. Throughout history, cinnabar has been used as a pigment or colouring because of its attractive red colour, and in the 19th century some American Indians in California complained of illness that was diagnosed as mercury poisoning caused by cinnabar in war paint. Little was known about the release of mercury into the environment by the chemical, electrical, and battery industries until the 20th century, when the medical profession and government agencies began to evaluate plants and operations. Thereafter, regulations reducing plant emissions improved the environment in and around these operations.
Ores
There are more than 25 known minerals containing mercury, but the principal ore mineral is cinnabar, a soft, red to reddish brown mercury sulfide. Some cinnabar deposits may also contain elemental mercury. The mineral has been found in all continents except Antarctica. It occurs in all types and ages of rock, usually alone but sometimes in association with antimony, gold, iron, and zinc.
Large commercial deposits of mercury have been mined at Almadén, Spain; Idrija, Slovenia; Monte Amiata, Italy; Santa Barbara, Peru; and New Almaden, California, U.S. The world’s leading producers of mercury are China, Kyrgyzstan, and Chile.
Mining And Concentrating
Mercury deposits are small and irregular, occurring sometimes as disseminated deposits but usually as veinlets. This precludes large-scale, highly mechanized mining methods. The most common method of ore recovery is underground mining, with conventional drilling and blasting followed by scraping or mechanical loading into ore cars.
Because most cinnabar as mined contains less than 1 percent mercury, various mineral-processing methods, such as jigging, shaking, screening, elutriation, and flotation, have been practiced to concentrate the ore. Flotation separation by the usual procedures for sulfide ores has had some success in the United States, but, because cinnabar is soft and friable, crushing and grinding the ore to reduce it to a size small enough to liberate the mineral may cause significant losses as slimes in the flotation tank. Although various methods are effective in producing higher-grade concentrates of cinnabar for furnacing, they cannot compete economically with the direct furnacing of the ore either as mined or after preliminary sorting by hand. A growing source of mercury in the United States is as a by-product of large, low-grade gold mining operations.
Extraction And Refining
Pyrometallurgy
The pyrometallurgical extraction of mercury from its ore is essentially a distillation process. When heat is applied to the sulfide ore in the presence of air, oxygen combines with the sulfur to form sulfur dioxide, and the metal is liberated at a temperature above its boiling point. The gases are then passed through a series of U-shaped tubes to condense the mercury vapour to the liquid phase.
Various vertical furnaces have been used to extract quicksilver since the earliest known crude furnaces were used at the Almadén Mine in Spain in the 12th century. The most common furnace in use in Europe is the Cermak-Spirek shaft furnace, which can treat either coarse feed (at least 4 centimetres, or 1.5 inches) or (with modification) finer material. The furnace can also accept different grades of ore. The ore is mixed with charcoal or coke fuel and charged to the top of the furnace. Combustion of fuel by a blast of hot air at the bottom produces hot gases, which, at about 300 °C (570 °F), pass upward through the falling ore and vaporize the liberated mercury. The heat generated by this oxidation-reduction reaction raises the temperature of the incoming air for yet more efficient combustion, and hot gases at the top of the furnace, where the temperature reaches 700 °C (1,300 °F), dry the freshly charged rock and coke.
Retorts are used for mercury extraction in small mining operations or to burn soot collected in the condensing tubes of large furnaces. Retorts are cheap to install, but they are more costly to operate than furnaces because the material in such batch operations must be manually charged and removed.
In the United States, rotary and multiple-hearth furnaces have been widely used, offering the advantage over other furnaces of higher capacity and continuous operation. Mechanical feeding and discharge reduces exposure to mercury vapours, sulfur dioxide fumes, and dust and lowers labour costs as well.
Hydrometallurgy
Mercury can be leached from ores and concentrates with a solution of sodium hydroxide and sodium sulfide. It can then be recovered by precipitation with aluminum or by electrolysis. Leaching is more costly than furnacing and is not effective on ores of irregular composition.
Secondary mercury
Significant quantities of mercury have been reclaimed from dental amalgams, oxide and acetate sludges, and battery scrap. Virtually all the metal can be recovered from scrapped mercury cells, mercury boilers, electrical apparatuses, and control instruments.
Refining
Metal produced by furnacing is known as prime virgin mercury (having a purity of more than 99.9 percent) and is bright and clean in appearance. This grade is suitable for most uses. When required, impurities can be removed by multiple distillation, usually in retort-type furnaces.
The Metal And Its Alloys
Mercury is packaged in cast-iron, wrought-iron, or spun-steel bottles or flasks 10 to 18 centimetres (4 to 7 inches) in diameter and about 30 centimetres high. The net weight of one flask of mercury is 34.5 kilogram (76 pounds), the commercial unit of world trade.
Electrical uses
One of the greatest uses of mercury has been as a moving cathode that settles at the bottom of electrolytic cells in the production of chlorine and caustic soda. During the electrolysis of brine, liberated sodium amalgamates with the mercury cathode and then reacts with water to form sodium hydroxide. (Chlorine is generated at the anode.) Losses of mercury in the brine sludge, wash water, and caustic soda have caused a decline of this application in favour of other processes that do not use mercury.
Dry-cell batteries are a large consumer of mercury. Mercury batteries can be operated at high temperatures and humidity, have long life spans, and deliver the same ampere-hours of service at their rated current ranges whether operated continuously or intermittently. The major applications of these batteries have been for hearing aids, photography, and military equipment. Other electrical applications included rectifier bulbs, oscillators, and power control switches. Mercury-vapour lamps have been used in industrial floodlighting, street lighting, motion-picture projection, photography, and heat therapy.
Metallurgical uses
Frozen mercury has been used for the precision casting of complex or intricate parts. After casting, the mercury mold can easily be removed by melting without damaging the cast product.
Mercury amalgamates, or mixes, readily with many metals. Amalgams of mercury, silver, and tin have been the most successful material for repairing dental cavities. Gold and silver have long been recovered by the amalgamation process, and amalgams of sodium and potassium have been used as reducing agents.
Chemical Compounds
Compounds of mercury have many uses in pharmacology, in chemical-process industries, and in agriculture.
Bichloride of mercury, mercurochrome, and ointments of metallic mercury, yellow mercuric oxide, and ammoniated mercuric chloride have served as skin antiseptics. Mercurous chloride, or calomel, is employed as a diuretic and cathartic.
Organic mercury compounds, particularly phenylmercury acetate, are used as agricultural fungicides for treating seeds, spraying fruit trees, and controlling weed growth. In paint manufacture, these compounds are used in the mildew proofing of paints. Mercuric chloride or mercuric sulfate is utilized as a catalyst in converting acetylene into vinyl chloride, vinyl acetate, and acetaldehyde. Other uses as catalysts are in the production of methyl styrene and glacial acetic acid.
Mercury fulminate, resulting from a reaction of alcohol and mercuric nitrate, explodes on impact and is used in percussion caps and detonators for other explosives.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#934 2021-02-12 00:08:25
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,354
Re: Miscellany
912) Radio-frequency heating
Radio-frequency heating, process of heating materials through the application of radio waves of high frequency—i.e., above 70,000 hertz (cycles per second). Two methods of radio-frequency heating have been developed. One of these, induction heating, has proved highly effective for heating metals and other materials that are relatively good electric conductors. The other method, called dielectric heating, is used with materials that are poor conductors of electricity.
Induction Heating.
In this method the material to be heated is placed in a high-frequency electromagnetic field produced by a conductor or coil called an inductor, which is connected to a radio-frequency generator. The electromagnetic field causes electrical currents to be excited in regions of the material that lie within the field of the inductor. These currents heat the object. The precise amount of heat generated is dependent on three factors: (1) the magnitude of the induced currents, (2) the resistance of the material to the flow of the currents, and (3) the length of time the material is exposed to the field.
Induction heating is used extensively in the metalworking industry to heat metals for hardening, soldering, brazing, and tempering and annealing. The induction-heating process is also employed in the fusion of metals and the production of high-quality alloys. Since the late 1970s American physicists have applied this type of radio-frequency heating to some types of experimental fusion reactors. Their objective is to use the technique to heat plasmas in fusion reactors known as tokamaks. During one series of experiments, researchers found that radio waves will heat plasma provided that their frequency equals the cyclotron frequency of the plasma ions—i.e., the rate at which the ions travel around the doughnut-shaped magnetic field of a cyclotron (q.v.). Approximately 600 kilowatts of radio-frequency energy were utilized to heat the plasma to roughly 23,000,000 K.
Dielectric Heating.
This method is designed to make use of the heat generated in poor electrical conductors, including insulators (e.g., rubber, plastics, and wood), when such materials are placed in a varying, high-frequency electromagnetic field. The heat results from electrical losses that occur in a material located between two metal plates (electrodes) which form a kind of capacitor connected to a radio-frequency oscillator. Unlike induction heating, in which nonuniform heating may occur, dielectric heating makes it possible to heat an object evenly throughout.
Dielectric heating has many varied applications, particularly in industry. For example, it is used for drying lumber and gypsum wallboard, for the rapid heating of special glues in furniture making, and for preheating in molding plastics and glasslike materials. In addition, dielectric heating provides the basis for microwave ovens, which are widely used for cooking food.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#935 2021-02-13 00:57:42
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,354
Re: Miscellany
913) Furnace
Furnace, structure in which useful heat is produced by combustion or other means. Historically, the furnace grew out of the fireplace and stove, following the availability of coal for heating. A coal furnace is made up of several elements: a chamber containing a grate on which combustion takes place and through which ashes drop for disposal; a chimney to carry away smoke and provide a draft of air; another source of air supply to help burn volatile gases and hydrocarbons; and a metal surface over which the hot gases pass and which transfers heat to circulating water or air. Coal furnaces are still widely used in industry, where they are usually equipped with mechanical stokers.
Chemical energy is transformed into heat by burning fuels such as coal, wood, oil, and hydrocarbon gases. Electrical energy is transformed into heat in an electric furnace or an electric burner. Solar radiation energy is used in the solar furnace, a device for concentrating large amounts of solar energy into a small area. Nuclear energy is transformed into heat energy in atomic reactors, so that these function as furnaces in nuclear power stations. Furnaces may apply their heat to other devices, as boilers, ovens, and kilns, or they may apply it directly to material in the course of being processed, as in steel production.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#936 2021-02-15 00:06:48
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,354
Re: Miscellany
914) Dysentery
Dysentery, infectious disease characterized by inflammation of the intestine, abdominal pain, and diarrhea with stools that often contain blood and mucus. Dysentery is a significant cause of illness and death in young children, particularly those who live in less-developed countries. There are two major types: bacillary dysentery and amebic dysentery, caused respectively by bacteria and by amoebas.
Bacillary dysentery, or shigellosis, is caused by bacilli of the genus Shigella. Symptomatically, the disease ranges from a mild attack to a severe course that commences suddenly and ends in death caused by dehydration and poisoning by bacterial toxins. After an incubation period of one to six days, the disease has an abrupt onset with fever and the frequent production of watery stools that may contain blood. Vomiting may also occur, and dehydration soon becomes obvious owing to the copious loss of bodily fluids. In advanced stages of the disease, chronic ulceration of the large intestine causes the production of bloody stools.
The most severe bacillary infections are caused by Shigella dysenteriae type 1 (formerly Shigella shigae), which is found chiefly in tropical and subtropical regions. S. flexneri, S. sonnei, and S. boydii are other Shigella bacilli that cause dysentery. Other types of bacterial infections, including salmonellosis (caused by Salmonella) and campylobacteriosis (caused by Campylobacter), can produce bloody stools and are sometimes also described as forms of bacillary dysentery. The treatment of bacillary dysentery is based on the use of antibiotics. The administration of fluids and, in some cases, blood transfusions may be necessary.
Amebic dysentery, or intestinal amebiasis, is caused by the protozoan Entamoeba histolytica. This form of dysentery, which traditionally occurs in the tropics, is usually much more chronic and insidious than the bacillary disease and is more difficult to treat because the causative organism occurs in two forms, a motile one and a cyst, each of which produces a different disease course. The motile form causes an acute dysentery, the symptoms of which resemble those of bacillary dysentery. The cyst form produces a chronic illness marked by intermittent episodes of diarrhea and abdominal pain. Bloody stools occur in some patients. The chronic type is the more common of the two and is marked by frequent remissions and exacerbations of symptoms. The chronic form may also produce ulcerations of the large intestine and pockets of infection in the liver. Both forms of amebic dysentery are treated with drugs that specifically kill the amebic parasites that thrive in the intestines.
Dysentery is transmitted through the ingestion of food or water that has been contaminated by the feces of a human carrier of the infective organism. The transmission is often by infected individuals who handle food with unwashed hands. The spread of amebic dysentery is often accomplished by people who are carriers of the disease but who at the time show no symptoms. Dysentery is commonly found when people are crowded together and have access only to primitive sanitary facilities. Spread of the disease can be controlled by boiling drinking water and by adequately disposing of human waste to avoid the contamination of food.
![]()
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#937 2021-02-17 00:18:20
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,354
Re: Miscellany
915) Croup
Croup, acute respiratory illness of young children characterized by a harsh cough, hoarseness, and difficult breathing. The illness is caused by infection of the upper airway in the region of the larynx (voice box), with infection sometimes spreading into the lower airway to the trachea (windpipe). Some cases result from allergy or physical irritation of these tissues. The symptoms are caused by inflammation of the laryngeal membranes, by spasms of the laryngeal muscles, or by inflammation around the trachea. In some cases, inflammation occurs around the bronchial tree as well.
Viral infections are the most common cause of croup, the most frequent being those with the parainfluenza and influenza viruses. Such infections are most prevalent among children under the age of three years, and they strike most frequently in late fall and winter. Generally, the onset of viral croup is preceded by the symptoms of the common cold for several days. Most children with viral croup can be treated at home with the inhalation of mist from an appropriate vaporizer. Epinephrine and corticosteroids have also been used to reduce swelling of the airway. In cases of severe airway obstruction, hospitalization may be necessary.
Bacterial croup, also called epiglottitis, is a more serious condition that is often caused by Haemophilus influenzae type B. It is characterized by marked swelling of the epiglottis, a flap of tissue that covers the air passage to the lungs and that channels food to the esophagus. The onset is usually abrupt, with high fever and breathing difficulties. Because of the marked swelling of the epiglottis, there is obstruction at the opening of the trachea, making it necessary for the patient to sit and lean forward to maximize the airflow. Epiglottitis generally strikes children between the ages of three and seven years. Children with epiglottitis require prompt medical attention. An artificial airway must be opened, preferably by inserting a tube down the windpipe. Patients are given antibiotics, which generally relieve the inflammation within 24 to 72 hours. The occurrence of epiglottitis is decreasing in the Western world owing to an effective vaccine against H. influenzae.
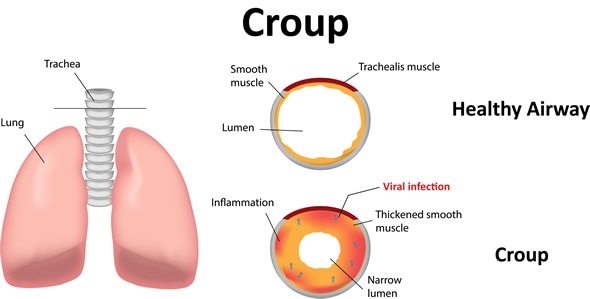
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#938 2021-02-19 00:15:11
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,354
Re: Miscellany
916) Nylon
Nylon, any synthetic plastic material composed of polyamides of high molecular weight and usually, but not always, manufactured as a fibre. Nylons were developed in the 1930s by a research team headed by an American chemist, Wallace H. Carothers, working for E.I. du Pont de Nemours & Company. The successful production of a useful fibre by chemical synthesis from compounds readily available from air, water, and coal or petroleum stimulated expansion of research on polymers, leading to a rapidly proliferating family of synthetics.
Nylon can be drawn, cast, or extruded through spinnerets from a melt or solution to form fibres, filaments, bristles, or sheets to be manufactured into yarn, fabric, and cordage; and it can be formed into molded products. It has high resistance to wear, heat, and chemicals.
When cold-drawn, it is tough, elastic, and strong. Most generally known in the form of fine and coarse filaments in such articles as hosiery, parachutes, and bristles, nylon is also used in the molding trade, particularly in injection molding, where its toughness and ability to flow around complicated inserts are prime advantages.
Polyamides may be made from a dicarboxylic acid and a diamine or from an amino acid that is able to undergo self-condensation, or its lactam, characterized by the functional group ―CONH― in a ring, such as ε-caprolactam. By varying the acid and the amine, it is possible to make products that are hard and tough or soft and rubbery. Whether made as filaments or as moldings, polyamides are characterized by a high degree of crystallinity, particularly those derived from primary amines. Under tension, orientation of molecules continues until the specimen is drawn to about four times its initial length, a property of particular importance in filaments.
Two of the ingredients that are used to synthesize the most common nylon, adipic acid and hexamethylenediamine, each contain six carbon atoms, and the product has been named nylon-6,6. When caprolactam is the starting material, nylon-6 is obtained, so named because it has six carbon atoms in the basic unit.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#939 2021-02-21 00:10:11
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,354
Re: Miscellany
917) Rayon
Rayon, artificial textile material composed of regenerated and purified cellulose derived from plant sources. Developed in the late 19th century as a substitute for silk, rayon was the first man-made fibre.
Rayon is described as a regenerated fibre because the cellulose, obtained from soft woods or from the short fibres (linters) that adhere to cottonseeds, is converted to a liquid compound, squeezed through tiny holes in a device called a spinnerette, and then converted back to cellulose in the form of fibre. The first practical steps toward producing such a fibre were represented by attempts to work with the highly flammable compound nitrocellulose, produced by treating cotton cellulose with nitric acid. In 1884 and 1885 in London, British chemist Sir Joseph Wilson Swan exhibited fibres made of nitrocellulose that had been treated with chemicals in order to change the material back to nonflammable cellulose. Swan did not follow up the demonstrations of his invention; thus, the development of rayon as a practical fibre really began in France, with the work of industrial chemist Hilaire Bernigaud, comte de Chardonnet, who is frequently called the father of the rayon industry. In 1889 Chardonnet exhibited fibres made by squeezing a nitrocellulose solution through spinnerettes, hardening the emerging jets in warm air, and then reconverting them to cellulose by chemical treatment. Manufacture of “Chardonnet silk,” an early type of rayon and the first commercially produced man-made fibre, began in 1891 at a factory in Besançon.
Although Chardonnet’s process was simple and involved a minimum of waste, it was slow, expensive, and potentially dangerous. In 1890 another French chemist, Louis-Henri Despeissis, patented a process for making fibres from cuprammonium rayon. This material was based on the Swiss chemist Matthias Eduard Schweizer’s discovery in 1857 that cellulose could be dissolved in a solution of copper salts and ammonia and, after extrusion, be regenerated in a coagulating bath. In 1908 the German textile firm J.-P. Bemberg began to produce cuprammonium rayon as Bemberg silk.
A third type of cellulose—and the type most commonly made today—was produced in 1891 from a syrupy, yellow, sulfurous-smelling liquid that three British chemists—Charles F. Cross, Edward J. Bevan, and Clayton Beadle—discovered by dissolving cellulose xanthate in dilute sodium hydroxide. By 1905 the British silk firm Samuel Courtauld & Company was producing this fibre, which became known as viscose rayon (or simply viscose). In 1911 the American Viscose Corporation began production in the United States.
Modern manufacture of viscose rayon has not changed in its essentials. Purified cellulose is first treated with caustic soda (sodium hydroxide). After the alkali cellulose has aged, carbon disulfide is added to form cellulose xanthate, which is dissolved in sodium hydroxide. This viscous solution (viscose) is forced through spinnerettes. Emerging from the holes, the jets enter a coagulating bath of acids and salts in which they are reconverted to cellulose and coagulated to form a solid filament. The filament may be manipulated and modified during the manufacturing process to control lustre, strength, elongation, filament size, and cross section as demanded.
Rayon has many properties similar to cotton and can also be made to resemble silk. Readily penetrated by water, the fibre swells and loses strength when wet. It can be washed in mild alkaline solutions but loses strength if subjected to harsh alkalies. Common dry-cleaning solvents are not harmful. In apparel, rayon is used alone or in blends with other fibres in applications where cotton is normally used. High-strength rayon, produced by drawing (stretching) the filaments during manufacture to induce crystallization of the cellulose polymers, is made into tire cord for use in automobile tires. Rayon is also blended with wood pulp in papermaking.
Rayon remains an important fibre, although production has declined in industrial countries because of environmental concerns connected with the release of carbon disulfide into the air and salt by-products into streams. Such concerns have led to the development of new types of rayon such as lyocell. Lyocell is produced by dissolving wood cellulose in a nontoxic amine oxide solvent, which is washed from the regenerated fibres and recovered for reuse.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#940 2021-02-23 00:02:17
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,354
Re: Miscellany
918) Polyester
Polyester, a class of synthetic polymers built up from multiple chemical repeating units linked together by ester (CO-O) groups. Polyesters display a wide array of properties and practical applications. Permanent-press fabrics, disposable soft-drink bottles, compact discs, rubber tires, and enamel paints represent only a few of the products made from this group.
Polyesters most commonly are prepared from a condensation reaction between an organic alcohol (containing hydroxyl [OH] groups) and a carboxylic acid (containing carboxyl [COOH] groups). These two functional groups react to form the characteristic ester linkage.
R and R′ represent the linked units that, repeated thousands of times within a single molecule, make up the long polymeric chain. The precise composition and structure of these repeating units vary widely, but roughly speaking they can be grouped into chains that are aliphatic (i.e., have an open structure) and those that contain ring-shaped molecular groups—particularly the large hydrocarbon aromatic groups.
Among the aliphatic group are the unsaturated polyesters, a class of resins that are molded into fibreglass-reinforced structures such as pleasure-boat hulls. Another aliphatic polyester is polyglycolic acid, a special type of degradable polymer that is made into bioabsorbable surgical sutures.
Ring-containing polyesters are the larger and commercially more important group. By far the most prominent member of this class is polyethylene terephthalate (PET), a stiff, strong polymer that is spun into fibres known by such trademarks as Dacron and Terylene. PET is also extruded into the film known as Mylar and is blow-molded into disposable beverage bottles. A related polyester is polybutylene terephthalate (PBT). PBT is used in applications similar to those of PET, and it is also employed in a synthetic rubber known as copolyester elastomer.
In general, the more aromatic groups included in the repeating units, the stiffer and higher-melting the polymer. This rule can be illustrated by polycarbonate, a rigid, tough, crystal-clear resin from which compact discs are made, and the polyarylates, a class of engineering plastics that often take the place of metals in machine parts.
Alkyd resins are oil-modified polyesters used in paints, varnishes, and other kinds of coating materials.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#941 2021-02-24 00:57:00
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,354
Re: Miscellany
919) Terrycloth
Terrycloth, terry cloth, terry cotton, terry towelling, terry, terry towel or simply towelling is a fabric woven with many protruding loops of thread which can absorb large amounts of water. It can be manufactured by weaving or knitting. Terrycloth is woven on special looms that have two beams of longitudinal warp through which the filler or weft is fired laterally. The first industrial production of terrycloth towels was initiated by the English manufacturer Christy in 1850. According to the Oxford English Dictionary, the word may derive from French tiré 'drawn', past participle of tirer 'draw out'.
About
There are two types of terry fabrics:
Towel terry : This is a woven fabric with long loops that can absorb large amounts of water. Its content is usually 100% cotton, but may sometimes contain polyester.
French terry : This is a fabric, used in men's, women's and children's clothes. One of its sides is flat, while the other side is with cross loops. It can be 100% cotton or be made from a variety of fibres, sometimes with spandex (also known as elastane or lycra). It is often warp-knitted, and the term French terry is colloquially used for all warp-knitted terry.
It is the length of loops that determines how much fluid is absorbed by the cloth as longer loops provide more surface area to absorb and come in contact with the fluid.
Items that may be made from terrycloth include babies' reusable nappies (UK English) or diapers (US English), towels, bathrobes, bedlinen, and sweatbands for the wrist or head. Terrycloth is also sometimes used to make sweat jackets. Terry towelling hats with a shallow brim were once popular with cricketers (like English wicketkeeper Jack Russell), but are no longer in fashion.
An alternative fabric used for towels is waffle fabric. A modern synthetic alternative is microfiber.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#942 2021-02-25 00:17:11
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,354
Re: Miscellany
920) Flax
Flax, (Linum usitatissimum), plant of the family Linaceae, cultivated both for its fibre, from which linen yarn and fabric are made, and for its nutritious seeds, called flaxseed or linseed, from which linseed oil is obtained. Though flax has lost some of its value as a commercial fibre crop owing to the availability of synthetic fibres, flaxseed has grown in popularity as a health food, and flax remains economically significant in a number of countries around the world, including China, Russia, and Canada.
Physical Description
Flax is a herbaceous annual. When densely planted for fibre, plants average 0.9 to 1.2 metres (3 to 4 feet) in height, with slender stalks 2.5 to 4 mm (about 0.10 to 0.15 inch) in diameter and with branches concentrated at the top. Plants cultivated for seed are shorter and many-branched. The leaves, alternating on the stalk, are small and lance-shaped. The flowers, borne on stems growing from the branch tips, have five petals, usually blue in colour but sometimes white or pink. The fruits are small dry capsules composed of five lobes.
The plant is adaptable to a variety of soils and climates but grows best in well-drained sandy loam and in temperate climates. In most areas planting of the same land with flax is limited to once in six years to avoid soil exhaustion. Cool moist growing seasons produce the most-desirable fibre.
Fibre
A bast fibre, flax is one of the oldest textile fibres. Evidence of its use has been found in the prehistoric lake dwellings of Switzerland. Fine linen fabrics, indicating a high degree of skill, have been discovered in ancient Egyptian tombs. Phoenician traders apparently brought linen from the Mediterranean area to Gaul and Britain, and the Romans introduced linen manufacture throughout their empire. In the 17th century the German states and Russia were major sources of raw material, and the linen industry was established in the Netherlands, Ireland, England, and Scotland. In North America the expansion of the cotton industry reduced the importance of linen.
Harvesting the fibre usually takes place after the lower portion of the stalk has turned yellow but before the fruit is fully mature. The fibre is obtained by subjecting the stalks to a series of operations, including retting (the use of moisture and microorganisms to dissolve the tissues surrounding the fibres), drying, crushing, and beating.
Fibre colour ranges from buff to gray, with the best qualities creamy white. The fibre strands, which measure about 30 to 75 cm (12 to 30 inches) long, are made up of individual cylindrically shaped cells with fairly smooth surfaces. Fine grades of flax fibres are made into woven fabrics and laces for apparel and household furnishings. Lower grades are used for products requiring strength and the ability to withstand moisture—such as canvas, twine, fire hose, bagging, industrial sewing thread, and fishnet.
Linen is valued for its strength, lustre, durability, and moisture absorbency. It is resistant to attack by microorganisms, and its smooth surface repels dirt. It is stronger than cotton, dries more quickly, and is more slowly affected by exposure to sunlight. It can be bleached to a pure white but dyeing is somewhat difficult because the fibres are not readily penetrated. Although linen increases in strength when wet, the excessive use of alkalies in laundering can weaken the fibres. Low elasticity, imparting hard, smooth texture, also makes linen subject to wrinkling, which can be reduced by chemical treatment. Because linen absorbs and releases moisture quickly and is a good conductor of heat, linen garments have a cooling effect on the wearer.
Flaxseed
Flaxseed is a rich source of the omega-3 fatty acid alpha-linolenic acid (ALA) and is high in a class of phytoestrogens known as lignans. It is also high in dietary fibre, protein, iron, calcium, manganese, thiamin, magnesium, phosphorus, and copper. The seeds can be eaten raw or toasted; added ground or whole to salads, morning cereal, and smoothies; or incorporated into baked goods.
Linseed oil, derived from the seeds, is used in the production of paints, printing inks, linoleum, varnish, and oilcloth. It has the highest level of ALA of any vegetable oil, and food-grade linseed oil is sometimes taken as a nutritional supplement. It also can be used in cooking, though it is somewhat unstable and goes rancid quickly.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#943 2021-02-26 00:11:09
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,354
Re: Miscellany
921) Wool
Wool, animal fibre forming the protective covering, or fleece, of sheep or of other hairy mammals, such as goats and camels. Prehistoric man, clothing himself with sheepskins, eventually learned to make yarn and fabric from their fibre covering. Selective sheep breeding eliminated most of the long, coarse hairs forming a protective outer coat, leaving the insulating fleecy undercoat of soft, fine fibre.
Wool is mainly obtained by shearing fleece from living animals, but pelts of slaughtered sheep are sometimes treated to loosen the fibre, yielding an inferior type called pulled wool. Cleaning the fleece removes “wool grease,” the fatty substance purified to make lanolin (q.v.), a by-product employed in cosmetics and ointments.
Wool fibre is chiefly composed of the animal protein keratin. Protein substances are more vulnerable to chemical damage and unfavourable environmental conditions than the cellulose material forming the plant fibres. Coarser than such textile fibres as cotton, linen, silk, and rayon, wool has diameters ranging from about 16 to 40 microns (a micron is about 0.00004 inch). Length is greatest for the coarsest fibres. Fine wools are about 1.5 to 3 inches (4 to 7.5 centimetres) long; extremely coarse fibres may be as much as 14 inches in length. Wool is characterized by waviness with up to 30 waves per inch (12 per centimetre) in fine fibres and 5 per inch (2 per centimetre) or less in coarser fibres. Colour, usually whitish, may be brown or black, especially in coarse types, and coarse wools have higher lustre than fine types.
Single wool fibres can resist breakage when subjected to weights of 0.5 to 1 ounce (15 to 30 grams) and when stretched as much as 25 to 30 percent of their length. Unlike vegetable fibres, wool has a lower breaking strength when wet. The resilient fibre can return to its original length after limited stretching or compression, thus imparting to fabrics and garments the ability to retain shape, drape well, and resist wrinkling. Because crimp encourages fibres to cling together, even loosely twisted yarns are strong, and both crimp and resilience allow manufacture of open-structured yarns and fabrics that trap and retain heat-insulating air. The low density of wool allows manufacture of lightweight fabrics.
Wool fibre has good to excellent affinity for dyestuffs. Highly absorbent, retaining as much as 16 to 18 percent of its weight in moisture, wool becomes warmer to the wearer as it absorbs moisture from the air, thus adjusting its moisture content and, consequently, its weight, in response to atmospheric conditions. Because moisture absorption and release are gradual, wool is slow to feel damp and does not chill the wearer by too-rapid drying.
Wool that has been stretched during yarn or fabric manufacture may undergo relaxation shrinkage in washing, with fibres resuming their normal shape. Felting shrinkage occurs when wet fibres, subjected to mechanical action, become matted into packed masses. Wool has good resistance to dry-cleaning solvents, but strong alkalies and high temperatures are harmful. Washing requires the use of mild reagents at temperatures below 20° C (68° F), with minimum mechanical action. The performance of wool has been improved by development of finishes imparting insect and mildew resistance, shrinkage control, improved fire resistance, and water repellency.
Woolen yarns, usually made from shorter fibres, are thick and full and are used for such full-bodied items as tweed fabrics and blankets. Worsteds, usually made from longer fibre, are fine, smooth, firm, and durable. They are used for fine dress fabrics and suitings. Wool that has had no previous use is described as new wool, or, in the United States, as virgin wool. The limited world supply results in the use of recovered wools. In the United States, wool recovered from fabric never used by the consumer is called reprocessed wool; wool recovered from material that has had use is called reused wool. Recovered wools, employed mainly in woolens and blends, are often of inferior quality because of damage suffered during the recovery process.
Australia, Russia, New Zealand, and Kazakhstan lead in fine-wool production, and India leads in the production of the coarser wools known as carpet wools. Leading consumers include the United Kingdom, the United States, and Japan.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#944 2021-02-27 00:01:51
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,354
Re: Miscellany
922) Valve
Valve, in mechanical engineering, device for controlling the flow of fluids (liquids, gases, slurries) in a pipe or other enclosure. Control is by means of a movable element that opens, shuts, or partially obstructs an opening in a passageway. Valves are of seven main types: globe, gate, needle, plug, butterfly, poppet, and spool.
In the globe valve, the movable element may be a tapered plug or a disk that fits a seat on the valve body; the disk may carry a replaceable rubber or leather washer, as in a household water faucet. In a gate valve, the movable element is a wedge-shaped disk that seats against two tapered faces in the valve body. A needle valve has a long tapered needle fitting in a tapered seat.
A plug valve is a conical plug with a hole perpendicular to its axis fitting in a conical seat in the valve body at right angles to the pipe. By turning the plug the hole is either lined up with the pipe to permit flow or set at right angles to block the passage.
A butterfly valve is a circular disk pivoted along one diameter. In the fully open position, the disk is parallel to the direction of flow. The damper in a stovepipe or a warm-air heating system is of this type, which is also used in the intake passage to carburetors on gasoline engines. On hydraulic turbines such valves may be 20 feet or more in diameter.
Some valves operate automatically; check (or nonreturn) valves, for example, are self-acting and permit flow in one direction only. They are made in several types. If the movable element in the globe valve were kept on its seat by gravity or a spring, it would permit flow from left to right but not from right to left.
Safety valves, which are usually of the poppet type, open at a predetermined pressure. The movable element may be kept on its seat by a weighted lever or a spring strong enough to hold the valve closed until the pressure is reached at which safe operation requires opening.
On gasoline engines, poppet valves are used to control the admission and rejection of the intake and exhaust gases to the cylinders. The valve, which consists of a disk with a tapered edge attached to a shank, is held against the tapered seat by a compressed spring. The valve is raised from its seat by the action of a rotating cam that pushes on the bottom of the shank, permitting gas flow between region, which leads to the intake or exhaust pipes, and region, which leads to the cylinder.
In hydrostatic fluid-power systems, in which the working medium is usually pressurized oil, spool valves are employed to regulate the oil flow. The valve provides two flow paths for the output from a pump. In the extreme upper position, as shown, active flow is from the pump port to the working, or load, port; discarded fluid from the load passes from port to the tank or sump port. In the extreme lower position, the functions of ports are reversed. In the mid or neutral position of the spool, ports are blocked. The movement of the spool may be manually or electrically controlled.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#945 2021-02-28 00:12:38
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,354
Re: Miscellany
923) Enteritis
Enteritis, inflammation of the intestines (especially of the small intestine), caused by irritants, poisons, viral or bacterial infections, or unknown factors. The symptoms are extremely variable but usually include continuous or intermittent diarrhea, occasionally bloody, accompanied by painful abdominal cramps. Fever is common and sometimes overshadows the digestive symptoms; serious complications may occur, especially in infants and the elderly. Enterocolitis involves the colon as well as the small intestine, and gastroenteritis includes stomach inflammation. Regional enteritis (ileitis, or Crohn disease) is a chronic inflammation that, in its classic form, is confined to the terminal portion of the ileum, the portion of the small intestine farthest from the stomach. In all forms of enteritis, treatment is usually directed toward relief of symptoms, with anti-inflammatory agents playing an important role.
Enteritis occurs as a result of inflammation of the small intestine, in particular, but this can extend to the stomach and large intestine in some people suffering from this illness.
A number of different types of enteritis exist. The most frequently occurring causes include over medication, alcohol or drugs, radiation, impaired blood flow, bacterial or viral infection as well as inflammation, for example, ulcerative colitis or Crohn’s disease.
The symptoms typically associated with enteritis are diarrhoea, abdominal plan, nausea, vomiting and fever.
Viral enteritis generally abates after a few days, without the need to access to medical treatment. However, it is very important to seek medical advice if symptoms persist for four days or more.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#946 2021-03-01 00:21:59
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,354
Re: Miscellany
924) Mica (mineral)
Mica is a mineral name given to a group of minerals that are physically and chemically similar. They are all silicate minerals, known as sheet silicates because they form in distinct layers. Micas are fairly light and relatively soft, and the sheets and flakes of mica are flexible. Mica is heat-resistant and does not conduct electricity. There are 37 different mica minerals. The most common include: purple lepidolite, black biotite, brown phlogopite and clear muscovite.
Type : Mineral
Mineral Classification : Silicate
Mohs Hardness : 2.5-4 (lepidolite); 2.5-3 biotite; 2.5-3 phlogopite; 2-2.5 muscovite
Crystal System : Monoclinic
Color : purple, rosy, silver, gray (lepidolite); dark green, brown, black (biotite); yellowish-brown, green white (phlogopite); colorless, transparent (muscovite)
Luster : pearly to vitreous
Description
Mica is a mineral name given to a group of minerals that are physically and chemically similar. They are all silicate minerals, known as sheet silicates because they form in distinct layers. Micas are fairly light and relatively soft, and the sheets and flakes of mica are flexible. Mica is heat-resistant and does not conduct electricity. There are 37 different mica minerals. The most common include: purple lepidolite, black biotite, brown phlogopite and clear muscovite.
Relation to Mining
The mica industry can be divided into two distinct but interdependent industries: those that produce sheet mica, and those that produce flake mica. Each industry, although somewhat dependent on the other, produces different end products.
Sheet Mica Mining:
Sheet mica is recovered by either sinking a shaft along the strike and dip of a pegmatite or by open-pit surface mining of semi-hard pegmatite ore. In either case, it is a very economically risky mining procedure because of the cost involved in locating the vein and the unpredictability of the quality and quantity of the mica that might be recovered once the vein is located and worked.
In underground mining, the main shaft is driven through the pegmatite at suitable angles to the dip and strike using air drills, hoists and explosives. Crosscuts and raises are developed to follow promising exposures of mica. When a pocket of mica is found, extreme care is exercised in the removal to minimize damage to the crystals. Small explosive charges of 40% to 60% strength are carefully placed around the pocket and care is exercised with the drilling procedure so the mica will not be penetrated. The charge is just sufficient to shake the mica free from the host rock. After blasting, the mica is hand-picked and placed in boxes or bags for transporting to the trimming shed where it is graded, split, and cut to various specified sizes for sale.
Sheet mica is no longer mined in the U.S. because of the high cost of mining, the small market, and the high capital risk. Most sheet mica is mined in India, where labor costs are comparatively low.
Flake Mica Mining:
The flake mica produced in the U.S. comes from several sources: the metamorphic rock called schist as a by-product of processing feldspar and kaolin resources, from placer deposits, and from pegmatites. It is mined by conventional open-pit methods. In soft residual material, dozers, shovels, scrapers and front-end loaders are used in the mining process. North Carolina’s production accounts for half of total U.S. mica production. Hard-rock mining of mica-bearing ore requires drilling and blasting. After blasting, the ore is reduced in size with drop balls and loaded on the trucks with shovels for transport to the processing plant, where mica, quartz and feldspar are extracted.
Uses
The principal use of ground mica is in gypsum wallboard joint compound, where it acts as a filler and extender, provides a smoother consistency, improves workability, and prevents cracking. In the paint industry, ground mica is used as a pigment extender that also facilitates suspension due to its light weight and platy morphology. The ground mica also reduces checking and chalking, prevents shrinkage and shearing of the paint film, provides increased resistance to water penetration and weathering,
and brightens the tone of colored pigments. Ground mica also is used in the well-drilling industry as an additive to drilling “muds.”
The plastic industry used ground mica as an extender and filler and also as a reinforcing agent. The rubber industry uses ground mica as an inert filler and as a mold lubricant in the manufacture of molded rubber products, including tires.
Sheet mica is used principally in the electronic and electrical industries. The major uses of sheet and block mica are as electrical insulators in electronic equipment, thermal insulation, gauge “glass”, windows in stove and kerosene heaters, dielectrics in capacitors, decorative panels in lamps and windows, insulation in electric motors and generator armatures, field coil insulation, and magnet and commutator core insulation.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#947 2021-03-02 00:21:26
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,354
Re: Miscellany
925) Gastroenteritis
Gastroenteritis, acute infectious syndrome of the stomach lining and the intestine. It is characterized by diarrhea, vomiting, and abdominal cramps. Other symptoms can include nausea, fever, and chills. The severity of gastroenteritis varies from a sudden but transient attack of diarrhea to severe dehydration.
Numerous viruses, bacteria, and parasites can cause gastroenteritis. Microorganisms cause gastroenteritis by secreting toxins that stimulate excessive water and electrolyte loss, thereby causing watery diarrhea, or by directly invading the walls of the gut, triggering inflammation that upsets the balance between the absorption of nutrients and the secretion of wastes.
Viral gastroenteritis, or viral diarrhea, is perhaps the most common type of diarrhea worldwide; rotaviruses, caliciviruses, Norwalk viruses, and adenoviruses are the most common causes. Other forms of gastroenteritis include food poisoning, cholera, and traveler’s diarrhea, which develops within a few days after traveling to a country or region that has unsanitary water or food. Traveler’s diarrhea is caused by exposure to enterotoxin-producing strains of the common intestinal bacterium Escherichia coli.
The treatment of gastroenteritis depends on the cause and the severity of symptoms and may include antibiotics or simply supportive care. Adults tend to have milder cases of the illness than do children and the very old, who run the risk of dehydration due to diarrhea and vomiting.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#948 2021-03-03 00:35:45
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,354
Re: Miscellany
926) Bacterial disease
Bacterial disease, any of a variety of illnesses caused by bacteria. Until the mid-20th century, bacterial pneumonia was probably the leading cause of death among the elderly. Improved sanitation, vaccines, and antibiotics have all decreased the mortality rates from bacterial infections, though antibiotic-resistant strains have caused a resurgence in some illnesses. In the early 21st century, tuberculosis, which is caused by Mycobacterium tuberculosis—several strains of which had developed resistance to one or more drugs widely used to treat the infection—was among the deadliest infectious diseases worldwide.
Bacteria cause disease by secreting or excreting toxins (as in botulism), by producing toxins internally, which are released when the bacteria disintegrate (as in typhoid), or by inducing sensitivity to their antigenic properties (as in tuberculosis). Other serious bacterial diseases include cholera, diphtheria, bacterial meningitis, tetanus, Lyme disease, gonorrhea, and syphilis.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#949 2021-03-04 00:37:23
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,354
Re: Miscellany
927) White blood cell
White blood cell, also called leukocyte or white corpuscle, a cellular component of the blood that lacks hemoglobin, has a nucleus, is capable of motility, and defends the body against infection and disease by ingesting foreign materials and cellular debris, by destroying infectious agents and cancer cells, or by producing antibodies.
A healthy adult human has between 4,500 and 11,000 white blood cells per cubic millimetre of blood. Fluctuations in white cell number occur during the day; lower values are obtained during rest and higher values during exercise. An abnormal increase in white cell number is known as leukocytosis, whereas an abnormal decrease in number is known as leukopenia. White cell count may increase in response to intense physical exertion, convulsions, acute emotional reactions, pain, pregnancy, labour, and certain disease states, such as infections and intoxications. The count may decrease in response to certain types of infections or drugs or in association with certain conditions, such as chronic anemia, malnutrition, or anaphylaxis.
Although white cells are found in the circulation, most occur outside the circulation, within tissues, where they fight infections; the few in the bloodstream are in transit from one site to another. As living cells, their survival depends on their continuous production of energy. The chemical pathways utilized are more complex than those of red blood cells and are similar to those of other tissue cells. White cells, containing a nucleus and able to produce ribonucleic acid (RNA), can synthesize protein. White cells are highly differentiated for their specialized functions, and they do not undergo cell division (mitosis) in the bloodstream; however, some retain the capability of mitosis. On the basis of their appearance under a light microscope, white cells are grouped into three major classes—lymphocytes, granulocytes, and monocytes—each of which carries out somewhat different functions.
Lymphocytes, which are further divided into B cells and T cells, are responsible for the specific recognition of foreign agents and their subsequent removal from the host. B lymphocytes secrete antibodies, which are proteins that bind to foreign microorganisms in body tissues and mediate their destruction. Typically, T cells recognize virally infected or cancerous cells and destroy them, or they serve as helper cells to assist the production of antibody by B cells. Also included in this group are natural killer (NK) cells, so named for their inherent ability to kill a variety of target cells. In a healthy person, about 25 to 33 percent of white blood cells are lymphocytes.
Granulocytes, the most numerous of the white cells, rid the body of large pathogenic organisms such as protozoans or helminths and are also key mediators of allergy and other forms of inflammation. These cells contain many cytoplasmic granules, or secretory vesicles, that harbour potent chemicals important in immune responses. They also have multilobed nuclei, and because of this they are often called polymorphonuclear cells. On the basis of how their granules take up dye in the laboratory, granulocytes are subdivided into three categories: neutrophils, eosinophils, and basophils. The most numerous of the granulocytes—making up 50 to 80 percent of all white cells—are neutrophils. They are often one of the first cell types to arrive at a site of infection, where they engulf and destroy the infectious microorganisms through a process called phagocytosis. Eosinophils and basophils, as well as the tissue cells called mast cells, typically arrive later. The granules of basophils and of the closely related mast cells contain a number of chemicals, including histamine and leukotrienes, that are important in inducing allergic inflammatory responses. Eosinophils destroy parasites and also help to modulate inflammatory responses.
Monocytes, which constitute between 4 and 8 percent of the total number of white blood cells in the blood, move from the blood to sites of infection, where they differentiate further into macrophages. These cells are scavengers that phagocytose whole or killed microorganisms and are therefore effective at direct destruction of pathogens and cleanup of cellular debris from sites of infection. Neutrophils and macrophages are the main phagocytic cells of the body, but macrophages are much larger and longer-lived than neutrophils. Some macrophages are important as antigen-presenting cells, cells that phagocytose and degrade microbes and present portions of these organisms to T lymphocytes, thereby activating the specific acquired immune response.
Specific types of cells are associated with different illnesses and reflect the special function of that cell type in body defense. In general, newborns have a high white blood cell count that gradually falls to the adult level during childhood. An exception is the lymphocyte count, which is low at birth, reaches its highest levels in the first four years of life, and thereafter falls gradually to a stable adult level.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#950 2021-03-05 00:31:13
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,354
Re: Miscellany
928) Red blood cell
Red blood cell, also called erythrocyte, cellular component of blood, millions of which in the circulation of vertebrates give the blood its characteristic colour and carry oxygen from the lungs to the tissues. The mature human red blood cell is small, round, and biconcave; it appears dumbbell-shaped in profile. The cell is flexible and assumes a bell shape as it passes through extremely small blood vessels. It is covered with a membrane composed of lipids and proteins, lacks a nucleus, and contains hemoglobin—a red iron-rich protein that binds oxygen.
The function of the red cell and its hemoglobin is to carry oxygen from the lungs or gills to all the body tissues and to carry carbon dioxide, a waste product of metabolism, to the lungs, where it is excreted. In invertebrates, oxygen-carrying pigment is carried free in the plasma; its concentration in red cells in vertebrates, so that oxygen and carbon dioxide are exchanged as gases, is more efficient and represents an important evolutionary development. The mammalian red cell is further adapted by lacking a nucleus—the amount of oxygen required by the cell for its own metabolism is thus very low, and most oxygen carried can be freed into the tissues. The biconcave shape of the cell allows oxygen exchange at a constant rate over the largest possible area.
The red cell develops in bone marrow in several stages: from a hemocytoblast, a multipotential cell in the mesenchyme, it becomes an erythroblast (normoblast); during two to five days of development, the erythroblast gradually fills with hemoglobin, and its nucleus and mitochondria (particles in the cytoplasm that provide energy for the cell) disappear. In a late stage the cell is called a reticulocyte, which ultimately becomes a fully mature red cell. The average red cell in humans lives 100–120 days; there are some 5.2 million red cells per cubic millimetre of blood in the adult human.
Though red cells are usually round, a small proportion are oval in the normal person, and in certain hereditary states a higher proportion may be oval. Some diseases also display red cells of abnormal shape—e.g., oval in pernicious anemia, crescent-shaped in sickle cell anemia, and with projections giving a thorny appearance in the hereditary disorder acanthocytosis. The number of red cells and the amount of hemoglobin vary among different individuals and under different conditions; the number is higher, for example, in persons who live at high altitudes and in the disease polycythemia. At birth the red cell count is high; it falls shortly after birth and gradually rises to the adult level at puberty.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline