Math Is Fun Forum
You are not logged in.
- Topics: Active | Unanswered
#876 2021-03-17 00:47:18
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,161
Re: crème de la crème
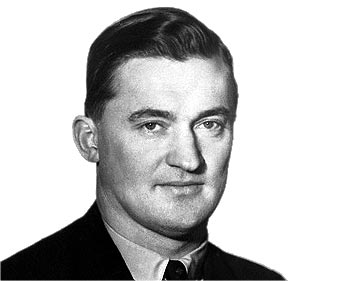
842) Axel Hugo Teodor Theorell
Axel Hugo Teodor Theorell, (born July 6, 1903, Linköping, Sweden—died Aug. 15, 1982, Stockholm), Swedish biochemist whose study of enzymes that facilitate oxidation reactions in living cells contributed to the understanding of enzyme action and led to the discovery of the ways in which nutrients are used by organisms in the presence of oxygen to produce usable energy. Theorell won the Nobel Prize for Physiology or Medicine in 1955.
While serving as an assistant professor of biochemistry at Uppsala University (1932–33; 1935–36), Theorell was the first to isolate crystalline myoglobin, an oxygen-carrying protein found in red muscle (1932). At the Kaiser Wilhelm Institute (now Max Planck Institute), Berlin (1933–35), he worked with Otto Warburg in isolating from yeast a pure sample of the “old yellow enzyme,” which is instrumental in the oxidative interconversion of sugars by the cell. Theorell found that the enzyme is composed of two parts: a nonprotein coenzyme—the yellow riboflavine (vitamin B2) phosphate—and a protein apoenzyme. His discovery (1934) that the coenzyme actively facilitates oxidation of the sugar glucose by binding a hydrogen atom at a specific site on the riboflavin molecule marked the first time that the effect of an enzyme was attributed to the chemical activity of specific atoms.
As director of the biochemical department of the Nobel Medical Institute, Stockholm (1937–70), Theorell studied the oxidative enzyme cytochrome c, determining the precise nature of the chemical linkage between the iron-bearing, nonprotein porphyrin portion and the apoenzyme. His investigation of the hydrogen-transfer enzyme, alcohol dehydrogenase, led to the development of sensitive blood tests that have found wide application in the determination of legal definitions of intoxication. Besides the Nobel Prize, Theorell received a number of awards and honours. He also served as president of the Swedish Royal Academy of Science and International Union of Biochemistry.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#877 2021-03-19 00:30:49
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,161
Re: crème de la crème
843) Johann Wilhelm Ritter
Johann Wilhelm Ritter, (born December 16, 1776, Samitz bei Haynau, Silesia [now Zamienice, Poland]—died January 23, 1810, Munich), German physicist who discovered the ultraviolet region of the spectrum and thus helped broaden humanity’s view beyond the narrow region of visible light to encompass the entire electromagnetic spectrum from the shortest gamma rays to the longest radio waves.
A pharmacist in Liegnitz, Silesia, from 1791 to 1795, Ritter studied medicine at the University of Jena, where he taught until he gained the patronage of the duke of Saxe–Gotha. In 1800, only months after the English chemist William Nicholson succeeded in decomposing water into hydrogen and oxygen by electrolysis, Ritter duplicated the experiment but arranged the electrodes so that he could collect the two gases separately. Soon thereafter he discovered the process of electroplating.
In 1801 Ritter made the startling discovery that silver chloride, which decomposes in the presence of light, is more rapidly decomposed when exposed to the invisible, theretofore unknown radiation beyond the violet end of the spectrum.
Ritter devoted most of his efforts to the study of electricity and electrochemistry. In 1801 he observed thermoelectric currents and anticipated the discovery of thermoelectricity by Thomas Johann Seebeck. Ritter invented the dry voltaic cell in 1802 and an electrical storage battery the following year.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#878 2021-03-21 00:06:17
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,161
Re: crème de la crème
844) Sir Cyril Norman Hinshelwood
Sir Cyril Norman Hinshelwood, (born June 19, 1897, London, Eng.—died Oct. 9, 1967, London), British chemist who worked on reaction rates and reaction mechanisms, particularly that of the combination of hydrogen and oxygen to form water, one of the most fundamental combining reactions in chemistry. For this work he shared the 1956 Nobel Prize for Chemistry with the Soviet scientist Nikolay Semyonov.
Hinshelwood obtained his doctorate at the University of Oxford in 1924 and became professor of chemistry there in 1937. After retiring from Oxford in 1964 he became a senior research fellow at Imperial College, London.
About 1930 Hinshelwood began investigating the complex reaction in which hydrogen and oxygen atoms combine to form water. He showed that the products of this reaction help to spread the reaction further in what is essentially a chain reaction.
He next sought to explore molecular kinetics within the bacterial cell. Upon observing the biological responses of bacteria to changes in environment, he concluded that more or less permanent changes in a cell’s resistance to a drug could be induced. This finding was important in regard to bacterial resistance to antibiotic and other chemotherapeutic agents. Hinshelwood was knighted in 1948. His publications include ‘The Kinetics of Chemical Change in Gaseous Systems’ (1926) and ‘The Chemical Kinetics of the Bacterial Cell’ (1946).
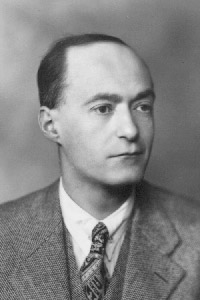
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#879 2021-03-23 00:04:50
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,161
Re: crème de la crème
845) Wolfgang Köhler
Wolfgang Köhler, (born January 21 [January 9, Old Style], 1887, Revel, Estonia, Russian Empire [now Tallinn, Estonia]—died June 11, 1967, Enfield, New Hampshire, U.S.), German psychologist and a key figure in the development of Gestalt psychology, which seeks to understand learning, perception, and other components of mental life as structured wholes.
Köhler’s doctoral thesis with Carl Stumpf at the University of Berlin (1909) was an investigation of hearing. As assistant and lecturer at the University of Frankfurt (1911), he continued his auditory research. In 1912 he and Kurt Koffka were subjects for experiments on perception conducted by Max Wertheimer, whose report on the experiments launched the Gestalt movement. Thereafter Köhler was associated with Wertheimer and Koffka as the three endeavoured to gain acceptance for the new theory.
As director of the anthropoid research station of the Prussian Academy of Sciences at Tenerife, Canary Islands (1913–20), Köhler conducted experiments on problem-solving by chimpanzees, revealing their ability to devise and use simple tools and build simple structures. His findings appeared in the classic Intelligenzprüfungen an Menschenaffen (1917; The Mentality of Apes), a work that emphasized insight and led to a radical revision of learning theory. Another major work, Die physischen Gestalten in Ruhe und im stationären Zustand (1920; “Physical Gestalt in Rest and Stationary States”), was based on an attempt to determine the relation of physical processes in nervous tissue to perception.
In 1921 Köhler became head of the psychological institute and professor of philosophy at the University of Berlin, directing a series of investigations that explored many aspects of Gestalt theory and publishing Gestalt Psychology (1929). Outspoken in his criticism of Adolf Hitler’s government, Köhler went to the United States in 1935 and was professor of psychology at Swarthmore College in Pennsylvania until 1955.
(Gestalt psychology, school of psychology founded in the 20th century that provided the foundation for the modern study of perception. Gestalt theory emphasizes that the whole of anything is greater than its parts. That is, the attributes of the whole are not deducible from analysis of the parts in isolation. The word Gestalt is used in modern German to mean the way a thing has been “placed,” or “put together.” There is no exact equivalent in English. “Form” and “shape” are the usual translations; in psychology the word is often interpreted as “pattern” or “configuration.”
Gestalt theory originated in Austria and Germany as a reaction against the associationist and structural schools’ atomistic orientation (an approach which fragmented experience into distinct and unrelated elements). Gestalt studies made use instead of phenomenology. This method, with a tradition going back to Johann Wolfgang von Goethe, involves nothing more than the description of direct psychological experience, with no restrictions on what is permissible in the description. Gestalt psychology was in part an attempt to add a humanistic dimension to what was considered a sterile approach to the scientific study of mental life. Gestalt psychology further sought to encompass the qualities of form, meaning, and value that prevailing psychologists had either ignored or presumed to fall outside the boundaries of science.
The publication of Czech-born psychologist Max Wertheimer’s “Experimentelle Studien über das Sehen von Bewegung” (“Experimental Studies of the Perception of Movement”) in 1912 marks the founding of the Gestalt school. In it Wertheimer reported the result of a study on apparent movement conducted in Frankfurt am Main, Germany, with psychologists Wolfgang Köhler and Kurt Koffka. Together, these three formed the core of the Gestalt school for the next few decades. (By the mid-1930s all had become professors in the United States.)
The earliest Gestalt work concerned perception, with particular emphasis on visual perceptual organization as explained by the phenomenon of illusion. In 1912 Wertheimer discovered the phi phenomenon, an optical illusion in which stationary objects shown in rapid succession, transcending the threshold at which they can be perceived separately, appear to move. The explanation of this phenomenon—also known as persistence of vision and experienced when viewing motion pictures—provided strong support for Gestalt principles.
Under the old assumption that sensations of perceptual experience stand in one-to-one relation to physical stimuli, the effect of the phi phenomenon was apparently inexplicable. However, Wertheimer understood that the perceived motion is an emergent experience, not present in the stimuli in isolation but dependent upon the relational characteristics of the stimuli. As the motion is perceived, the observer’s nervous system and experience do not passively register the physical input in a piecemeal way. Rather, the neural organization as well as the perceptual experience springs immediately into existence as an entire field with differentiated parts. In later writings this principle was stated as the law of Prägnanz, meaning that the neural and perceptual organization of any set of stimuli will form as good a Gestalt, or whole, as the prevailing conditions will allow.
Major elaborations of the new formulation occurred within the next decades. Wertheimer, Köhler, Koffka, and their students extended the Gestalt approach to problems in other areas of perception, problem solving, learning, and thinking. The Gestalt principles were later applied to motivation, social psychology, and personality (particularly by Kurt Lewin) and to aesthetics and economic behaviour. Wertheimer demonstrated that Gestalt concepts could also be used to shed light on problems in ethics, political behaviour, and the nature of truth. Gestalt psychology’s traditions continued in the perceptual investigations undertaken by Rudolf Arnheim and Hans Wallach in the United States.)

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#880 2021-03-25 00:03:28
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,161
Re: crème de la crème
846) Nikolay Nikolayevich Semyonov
Nikolay Nikolayevich Semyonov, Semyonov also spelled Semënov, (born April 15 [April 3, Old Style], 1896, Saratov, Russia—died Sept. 25, 1986, Moscow, U.S.S.R.), Soviet physical chemist who shared the 1956 Nobel Prize for Chemistry with Sir Cyril Hinshelwood for research in chemical kinetics. He was the second Soviet citizen (after the émigré writer Ivan Bunin) to receive a Nobel Prize.
Semyonov was educated in St. Petersburg, graduating from the city’s university in 1917, the year of the Russian Revolution, and taught for a time at the University of Tomsk in western Siberia. Associated with the Leningrad A.F. Ioffe Physicotechnical Institute from 1920 to 1931, he became a professor at the Leningrad (St. Petersburg) Polytechnic Institute in 1928. He was director of the Institute of Chemical Physics at the Academy of Sciences of the U.S.S.R. after 1931 and became a professor at Moscow State University in 1944.
Like Hinshelwood, Semyonov conducted research on the mechanism of chemical chain reactions and their significance in relation to explosions. Semyonov was the first to show that chain reactions are the norm in chemical transformations of matter. He published the influential book O nekotorykh problemakh khimicheskoy kinetiki i reaktsionnoy sposobnosti (1954; Some Problems in Chemical Kinetics and Reactivity).

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#881 2021-03-28 00:15:54
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,161
Re: crème de la crème
847) Larisa Semyonovna Latynina
Larisa Semyonovna Latynina, (born December 27, 1934, Kherson, Ukraine, U.S.S.R.), Soviet gymnast who was the first woman athlete to win nine Olympic gold medals and was one of the most decorated competitors in the history of the Games.
At the 1956 Games in Melbourne, Australia, Latynina, who was educated at the Kiev State Institute of Physical Culture, won the women’s competition in the combined exercises, the vault, and the floor exercise (in which she tied for first place). At the 1960 Olympics in Rome she again placed first in the combined and the floor exercise, and in Tokyo in 1964 she captured her third consecutive gold medal in the floor exercise.
Latynina also won gold medals as a member of the Soviet Union’s six-member women’s gymnastics team in 1956, 1960, and 1964. In addition, she was awarded five silver and four bronze medals in those three Games. Her record of 18 career Olympic medals stood until 2012, when it was surpassed by American swimmer Michael Phelps. After she retired from competition, Latynina was a teacher and national senior coach and was active in the planning of the 1980 Olympics in Moscow.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#882 2021-03-30 00:01:41
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,161
Re: crème de la crème
848) Werner Forssmann
Werner Theodor Otto Forssmann was born in Berlin on August 29, 1904, the son of Julius Forssmann and Emmy Hindenberg. He was educated at the Askanische Gymnasium (secondary grammar school) in Berlin. Leaving school in 1922, he went to the University of Berlin to study medicine, passing his State Examination in 1929. For his clinical training he went to the University Medical Clinic, working under Professor Georg Klemperer, and he studied anatomy under Professor Rudolph Fick. For clinical instruction in surgery he went, in 1929, to the August Victoria Home at Eberswalde near Berlin.
It was here that he was the first to develop a technique for the catheterization of the heart. This he did by inserting a cannula into his own antecubital vein, through which he passed a catheter for 65 cm and then walked to the X-ray department, where a photograph was taken of the catheter lying in his right auricle.
Subsequently he worked at the Charité, Berlin, and the City Hospital at Mainz, and then went to the Rudolf Virchow Hospital in Berlin for specialist training in urology under Dr. Karl Heusch. He was appointed Chief of the Surgical Clinic of the City Hospital at Dresden-Friedrichstadt and at the Robert Koch Hospital, Berlin. At the beginning of the Second World War, Forssmann served as a Sanitary Officer, reaching the rank of Surgeon-Major; but he became a prisoner of war until his release in 1945, when he went into practice with his wife, in the Schwarzwald.
From 1950 onwards he practised as a urological specialist at Bad Kreuznach, and since 1958 he has been Chief of the Surgical Division of the Evangelical Hospital at Düsseldorf, where he now lives.
In 1956 he was awarded, together with André Cournand and Dickinson W. Richards, the Nobel Prize for Physiology or Medicine and he was, in the same year, appointed Honorary Professor of Surgery and Urology at the Johannes Gutenberg University, Mainz.
In 1954 he was awarded the Leibniz Medal of the German Academy of Sciences; in the same year he was Guest of Honour at the National University of Cordoba, Argentina, where he was appointed Honorary Professor in 1961.
Since 1962 he is a Member of the Executive Board of the German Surgical Society. He is also a Member of the American College of Chest Physicians, and Honorary Member of the Swedish Society of Cardiology, the German Society of Urology, and the German Child Welfare Association.
In 1933 Forssmann married Dr. Elsbet Engel, who is also a specialist in urology. They have six children: Klaus (b. 1934), Knut (b. 1936), Jörg (b. 1938), Wolf (b. 1939), Bernd (b. 1940), and Renate (b. 1943).
Werner Forssmann died on June 1, 1979.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#883 2021-04-01 00:06:43
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,161
Re: crème de la crème
849) Daniel Bovet
Daniel Bovet, (born March 23, 1907, Neuchâtel, Switz.—died April 8, 1992, Rome, Italy), Swiss-born Italian pharmacologist who received the 1957 Nobel Prize for Physiology or Medicine for his discoveries of certain chemotherapeutic agents—namely, sulfa drugs, antihistamines, and muscle relaxants.
Bovet studied at the University of Geneva, graduating with a doctorate in science in 1929. That same year, he went on to the Pasteur Institute in Paris and became head of the therapeutic chemistry laboratory there in 1939. In 1937 Bovet discovered the first antihistamine substance, which (in counteracting the effect of histamine) is effective in treating allergic reactions. This discovery led to development of the first antihistamine drug for humans in 1942, and in 1944 one of Bovet’s own discoveries, pyrilamine, was produced as a drug.
In 1947 Bovet was invited to establish a laboratory of chemotherapeutics at the Superior Institute of Health in Rome, and eventually he took Italian citizenship. There he turned his attention to curare, a drug used to relax muscles during surgery. Because the drug was expensive and somewhat unpredictable in its effects, a low-cost dependable synthetic alternative was desired. Bovet produced hundreds of synthetic alternatives, of which gallamine and succinylcholine came into widespread use.
In 1964 Bovet became professor of pharmacology at the University of Sassari, on the Italian island of Sardinia. He served as the head of the psychobiology and psychopharmacology laboratory of the National Research Council (Rome) from 1969 until 1971, when he became professor of psychobiology at the University of Rome (1971–82).

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#884 2021-04-03 00:03:39
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,161
Re: crème de la crème
850) Pavel Alekseyevich Cherenkov
Pavel Alekseyevich Cherenkov, Cherenkov also spelled Čerenkov, (born July 15 [July 28, New Style], 1904, Novaya Chigla, Russia—died Jan. 6, 1990, U.S.S.R.), Soviet physicist who shared the 1958 Nobel Prize for Physics with fellow Soviet scientists Igor Y. Tamm and Ilya M. Frank for the discovery and theoretical interpretation of the phenomenon of Cherenkov radiation.
A peasant’s son, Cherenkov graduated from Voronezh State University in 1928; he later became a research student at the P.N. Lebedev Physical Institute. In 1934, working on his dissertation under the guidance of and in collaboration with Sergei Ivanovich Vavilov, he observed that electrons produce a faint blue glow when passing through a transparent liquid at high velocity. This Cherenkov radiation, which was correctly explained by Tamm and Frank in 1937, led to the development of the Cherenkov counter, or Cherenkov detector, that later was used extensively in experimental nuclear and particle physics. Cherenkov continued to do research in nuclear and cosmic-ray physics at the P.N. Lebedev Physical Institute. Cherenkov was elected to the U.S.S.R. Academy of Sciences as a corresponding (1964) and subsequently full (1970) member.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#885 2021-04-05 00:02:30
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,161
Re: crème de la crème
851) Jack Nicklaus
Jack Nicklaus, in full Jack William Nicklaus, byname the Golden Bear, (born January 21, 1940, Columbus, Ohio, U.S.), American professional golfer, a dominating figure in world golf from the 1960s to the ’80s.
While a student at Ohio State University, Nicklaus won the U.S. Amateur Championship in 1959 and again in 1961. Also in 1961 Nicklaus set a scoring record of 282 for an amateur in the U.S. Open. (For Nicklaus’s account of his career at the U.S. Open, see U.S. Open: Jack Nicklaus’s personal reflections.) After he turned professional in 1962, Nicklaus won the Masters Tournament six times (1963, 1965, 1966, 1972, 1975, 1986), the U.S. Open four times (1962, 1967, 1972, 1980), the Professional Golfers’ Association of America (PGA) Championship five times (1963, 1971, 1973, 1975, 1980), and the British Open (Open Championship) three times (1966, 1970, 1978). His victories overseas included six Australian Open titles. Nicklaus was a member of the winning U.S. World Cup team six times (1963, 1964, 1966, 1967, 1971, 1973) and was a record three-time individual World Cup winner (1963, 1964, 1971). By 1986 he had played in 100 major championships, finishing in the top three 45 times. Nicklaus was named PGA Player of the Year five times (1967, 1972, 1973, 1975, 1976), and he was elected to the World Golf Hall of Fame in 1974. He joined the Senior PGA Tour (later renamed the Champions Tour) in 1990 and retired from tournament golf in 2005. His career totals include 73 PGA victories—a number exceeded only by Sam Snead and Tiger Woods—and a record 18 victories in the four major professional championships. In addition to possessing great natural ability and power, Nicklaus showed remarkable composure under the severest competitive pressure.
Nicklaus designed several golf courses, including Muirfield Village Golf Course in Ohio, site of the Nicklaus-sponsored Memorial Tournament beginning in 1976. He also wrote several books, including ‘Golf My Way’ (1974; cowritten with Ken Bowden), ‘Nicklaus by Design’ (2002; cowritten with Chris Millard), and ‘Jack Nicklaus: Memories and Mementos from Golf’s Golden Bear’ (2007; cowritten with David Shedloski). In 2005 Nicklaus was awarded the Presidential Medal of Freedom.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#886 2021-04-07 00:02:41
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,161
Re: crème de la crème
852) Ilya Mikhaylovich Frank
Ilya Mikhaylovich Frank, (born October 10 [October 23, New Style], 1908, St. Petersburg, Russia—died June 22, 1990, Moscow, Russia, U.S.S.R.), Soviet winner of the Nobel Prize for Physics in 1958 jointly with Pavel A. Cherenkov and Igor Y. Tamm, also of the Soviet Union. He received the award for explaining the phenomenon of Cherenkov radiation.
After graduating from Moscow State University in 1930, Frank worked at the Leningrad Optical Institute. He returned to Moscow to work at the P.N. Lebedev Physical Institute (1934–70) and from 1940 was a professor at Moscow State University.
In 1937 Frank and Tamm provided the theoretical explanation of Cherenkov radiation, an effect discovered by Cherenkov in 1934 in which light is emitted when charged particles travel through an optically transparent medium at speeds greater than the speed of light in that medium. The effect led to the development of Cherenkov counters for detecting and measuring the velocity of high-speed particles, allowing discoveries of new elementary particles such as the antiproton.
Frank later worked on theoretical and experimental nuclear physics and the design of reactors, and from 1957 he headed the neutron laboratory at the Joint Institute for Nuclear Research in Dubna. In 1946 Frank was elected a corresponding member, and in 1968 a full member, of the U.S.S.R. Academy of Sciences.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#887 2021-04-09 00:07:41
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,161
Re: crème de la crème
853) Kristin Otto
Kristin Otto, (born Feb. 7, 1966, Leipzig, E.Ger.), German swimmer, the first female athlete to win six gold medals at a single Olympic Games.
Otto entered a special sports school at age 11 after East Germany’s comprehensive scouting program identified her as a swimming prospect. In 1982 she set her first world record as a member of her country’s 4 × 100-metre medley relay team. A favourite to win a medal at the 1984 Olympics in Los Angeles, Otto was unable to compete because of East Germany’s boycott. Later that year she cracked a vertebra and spent nine months in a neck brace; although physicians advised her to give up her sport, she returned to compete at the 1986 world championships in Madrid, winning four gold and two silver medals.
At the 1988 Olympic Games in Seoul, South Korea, Otto entered six events and won gold medals in all of them. Her individual victories included the 100-metre butterfly, 50-metre freestyle, 100-metre freestyle, and 100-metre backstroke. She also swam the lead leg in East Germany’s 4 × 100-metre freestyle relay victory and the backstroke leg in the 4 × 100-metre individual medley relay. Prior to Otto’s achievement, no woman had won more than four gold medals at a single Olympics.
Otto was considered one of the most versatile female swimmers, winning world or Olympic championships in the backstroke, butterfly, freestyle, and individual medley. She retired after the 1988 Olympics.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#888 2021-04-10 00:10:41
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,161
Re: crème de la crème
854) Norman Joseph Woodland
Norman Joseph Woodland (September 6, 1921 – December 9, 2012) was an American inventor, best known as one of the inventors of the barcode, for which he received a patent in October 1952. Later, employed by IBM, he developed the format which became the ubiquitous Universal Product Code (UPC) of product labeling and check-out stands.
Biography
Woodland was born in Atlantic City, New Jersey, on September 6, 1921, to Jewish parents, the elder of two boys.
After graduating from Atlantic City High School, Woodland did military service in World War II as a technical assistant with the Manhattan Project in Oak Ridge, Tennessee. Woodland went on to earn his Bachelor of Science in Mechanical Engineering (BSME) from Drexel University (then called Drexel Institute of Technology) in 1947. From 1948 to 1949, he worked as a lecturer in mechanical engineering at Drexel.
In 1948, Bernard Silver, a fellow Drexel Institute graduate student with Woodland, overheard a supermarket executive asking the dean of engineering if the Institute could determine how to capture product information automatically at checkout. The dean turned down the request, but Silver was interested enough to mention the problem to Woodland. After working on some preliminary ideas, Woodland was persuaded that they could create a viable product.
Woodland took some stock market earnings, quit his teaching job and moved to his grandfather's Florida apartment. While at the beach, Woodland again considered the problem, recalling, from his Boy Scout training, how Morse code dots and dashes are used to send information electronically. He drew dots and dashes in the sand similar to the shapes used in Morse code. After pulling them downward with his fingers, producing thin lines resulting from the dots and thick lines from the dashes, he came up with the concept of a two-dimensional, linear Morse code, and after sharing it with Silver and adapting optical sound film technology, they applied for a patent on October 20, 1949, receiving U.S. Patent 2,612,994 ‘Classifying Apparatus and Method’ on October 7, 1952, covering both linear barcode and circular bulls-eye printing designs.
Woodland was employed by IBM in 1951, and although Woodland and Silver wanted IBM to develop the technology, it wasn't commercially feasible, so they sold the patent in 1952 for $15,000 to Philco, which sold it to RCA later in 1952. RCA (The RCA Corporation was a major American electronics company, which was founded as the Radio Corporation of America in 1919) went on to attempt to develop commercial applications through the 1960s until the patent expired in 1969.
After RCA interested the National Association of Food Chains in 1969 in the idea, and they formed the U.S. Supermarket Ad Hoc Committee on a Uniform Grocery Product Code, rival IBM became involved in 1971, finding out about Woodland's work and transferring him to their North Carolina facilities, where he played a key role in developing the most important version of the technology, the Universal Product Code (UPC), beating RCA in a competition.
The first item scanned was a packet of chewing gum in an Ohio supermarket in 1974.
Woodland died from the effects of Alzheimer's disease on December 9, 2012, in Edgewater, New Jersey.
Awards
• In 1973, IBM presented Woodland with their Outstanding Contribution Award.
• In 1992, he was awarded the National Medal of Technology from President George H. W. Bush for his contribution to barcode technology.
• In 1998 Woodland received an honorary degree from his alma mater, Drexel University.
• In 2011, Woodland was inducted into the National Inventors Hall of Fame.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#889 2021-04-12 00:23:06
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,161
Re: crème de la crème
855) Igor Yevgenyevich Tamm
Igor Yevgenyevich Tamm, (born July 8 [June 26, Old Style], 1895, Vladivostok, Siberia, Russia—died April 12, 1971, Moscow, Russia, Soviet Union), Soviet physicist who shared the 1958 Nobel Prize for Physics with Pavel A. Cherenkov and Ilya M. Frank for his efforts in explaining Cherenkov radiation. Tamm was one of the theoretical physicists who contributed to the construction of the first Soviet thermonuclear bomb.
Tamm’s father was an engineer in the city of Yelizavetgrad (now Kirovohrad, Ukr.), where he was responsible for building and managing electric power stations and water systems. Tamm graduated from the gymnasium there in 1913 and went abroad to study at the University of Edinburgh. The following year he returned to Moscow State University, and he graduated in 1918. In 1924 he became a lecturer in the physics department, and in 1930 he succeeded his mentor, Leonid I. Mandelstam, to the chair of theoretical physics. In 1933 Tamm was elected a corresponding member of the Soviet Academy of Sciences. The following year, he joined the P.N. Lebedev Physics Institute of the Soviet Academy of Sciences (FIAN), where he organized and headed the theoretical division, a position he occupied until his death.
Tamm’s early studies of unique forms of electron bonding (“Tamm surface levels”) on the surfaces of crystalline solids had important applications in the later development of solid-state semiconductor devices. In 1934 Cherenkov had discovered that light is emitted when gamma rays pass through a liquid medium. In 1937 Tamm and Frank explained this phenomenon as the emission of light waves by electrically charged particles moving faster than the speed of light in a medium. Tamm developed this theory more fully in a paper published in 1939. For these discoveries Tamm, Frank, and Cherenkov received the 1958 Nobel Prize for Physics.
Immediately after World War II, Tamm, though a major theoretician, was not assigned to work on the atomic bomb project, possibly for political reasons. In particular, he was branded a “bourgeois idealist” and his brother an “enemy of the state.” Nevertheless, in June 1948, when physicist Igor V. Kurchatov needed a strong team to investigate the feasibility of creating a thermonuclear bomb, Tamm was recruited to organize the theoretical division of FIAN in Moscow. The Tamm group came to include physicists Yakov B. Zeldovich, Vitaly L. Ginzburg, Semyon Z. Belenky, Andrey D. Sakharov, Efim S. Fradkin, Yuri A. Romanov, and Vladimir Y. Fainberg. Between March and April 1950, Tamm and several members of his group were sent to the secret installation known as Arzamas-16 (near the present-day village of Sarov) to work under physicist Yuly Khariton’s direction on a thermonuclear bomb project. One bomb design, known as the Sloika (“Layer Cake”), was successfully tested on Aug. 12, 1953. Tamm was elected a full member of the Academy of Sciences in October 1953 and the same year was awarded a Hero of Socialist Labour. On Nov. 22, 1955, the Soviet Union successfully tested a more modern thermonuclear bomb that was analogous to the design of the American physicists Edward Teller and Stanislaw Ulam.
Tamm spent the latter decades of his career at the Lebedev Institute, where he worked on building a fusion reactor to control fusion, using a powerful magnetic field in a donut-shaped device known as a Tokamak reactor.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#890 2021-04-14 00:04:52
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,161
Re: crème de la crème
856) Frederick Sanger
Frederick Sanger, (born August 13, 1918, Rendcombe, Gloucestershire, England—died November 19, 2013, Cambridge), English biochemist who was twice the recipient of the Nobel Prize for Chemistry. He was awarded the prize in 1958 for his determination of the structure of the insulin molecule. He shared the prize (with Paul Berg and Walter Gilbert) in 1980 for his determination of base sequences in nucleic acids. Sanger was the fourth two-time recipient of the Nobel Prize.
Education
Sanger was the middle child of Frederick Sanger, a medical practitioner, and Cicely Crewsdon Sanger, the daughter of a wealthy cotton manufacturer. The family expected him to follow in his father’s footsteps and become a medical doctor. After much thought, he decided to become a scientist. In 1936 Sanger entered St. John’s College, Cambridge. He initially concentrated on chemistry and physics, but he was later attracted to the new field of biochemistry. He received a bachelor’s degree in 1939 and stayed at Cambridge an additional year to take an advanced course in biochemistry. He and Joan Howe married in 1940 and subsequently had three children.
Because of his Quaker upbringing, Sanger was a conscientious objector and was assigned as an orderly to a hospital near Bristol when World War II began. He soon decided to visit Cambridge to see if he could enter the doctoral program in biochemistry. Several researchers there were interested in having a student, especially one who did not need money. He studied lysine metabolism with biochemist Albert Neuberger. They also had a project in support of the war effort, analyzing nitrogen from potatoes. Sanger received a doctorate in 1943.
Insulin Research
Biochemist Albert C. Chibnall and his protein research group moved from Imperial College in London to the safer wartime environment of the biochemistry department at Cambridge. Two schools of thought existed among protein researchers at the time. One group thought proteins were complex mixtures that would not readily lend themselves to chemical analysis. Chibnall was in the other group, which considered a given protein to be a distinct chemical compound.
Chibnall was studying insulin when Sanger joined the group. At Chibnall’s suggestion, Sanger set out to identify and quantify the free-amino groups of insulin. Sanger developed a method using dinitrofluorobenzene to produce yellow-coloured derivatives of amino groups. Information about a new separation technique, partition chromatography, had recently been published. In a pattern that typified Sanger’s career, he immediately recognized the utility of the new technique in separating the hydrolysis products of the treated protein. He identified two terminal amino groups for insulin, phenylalanine and glycine, suggesting that insulin is composed of two types of chains. Working with his first graduate student, Rodney Porter, Sanger used the method to study the amino terminal groups of several other proteins. (Porter later shared the 1972 Nobel Prize for Physiology or Medicine for his work in determining the chemical structure of antibodies.)
On the assumption that insulin chains are held together by disulphide linkages, Sanger oxidized the chains and separated two fractions. One fraction had phenylalanine at its amino terminus; the other had glycine. Whereas complete acid hydrolysis degraded insulin to its constituent amino acids, partial acid hydrolysis generated insulin peptides composed of several amino acids. Using another recently introduced technique, paper chromatography, Sanger was able to sequence the amino-terminal peptides of each chain, demonstrating for the first time that a protein has a specific sequence at a specific site. A combination of partial acid hydrolysis and enzymatic hydrolysis allowed Sanger and the Austrian biochemist Hans Tuppy to determine the complete sequence of amino acids in the phenylalanine chain of insulin. Similarly, Sanger and the Australian biochemist E.O.P. Thompson determined the sequence of the glycine chain.
Two problems remained: the distribution of the amide groups and the location of the disulphide linkages. With the completion of those two puzzles in 1954, Sanger had deduced the structure of insulin. For being the first person to sequence a protein, Sanger was awarded the 1958 Nobel Prize for Chemistry.
Sanger and his coworkers continued their studies of insulin, sequencing insulin from several other species and comparing the results. Utilizing newly introduced radiolabeling techniques, Sanger mapped the amino acid sequences of the active centres from several enzymes. One of these studies was conducted with another graduate student, Argentine-born immunologist César Milstein. (Milstein later shared the 1984 Nobel Prize for Physiology or Medicine for discovering the principle for the production of monoclonal antibodies.)
RNA Research
In 1962 the Medical Research Council opened its new laboratory of molecular biology in Cambridge. The Austrian-born British biochemist Max Perutz, British biochemist John Kendrew, and British biophysicist Francis Crick moved to the new laboratory. Sanger joined them as head of the protein division. It was a banner year for the group, as Perutz and Kendrew shared the 1962 Nobel Prize for Chemistry and Crick shared the 1962 Nobel Prize for Physiology or Medicine with the American geneticist James D. Watson and the New Zealand-born British biophysicist Maurice Wilkins for the discovery of DNA (deoxyribonucleic acid).
Sanger’s interaction with nucleic acid groups at the new laboratory led to his pursuing studies on ribonucleic acid (RNA). RNA molecules are much larger than proteins, so obtaining molecules small enough for technique development was difficult. The American biochemist Robert W. Holley and his coworkers were the first to sequence RNA when they sequenced alanine-transfer RNA. They used partial hydrolysis methods somewhat like those Sanger had used for insulin. Unlike other RNA types, transfer RNAs have many unusual nucleotides. This partial hydrolysis method would not work well with other RNA molecules, which contain only four types of nucleotides, so a new strategy was needed.
The goal of Sanger’s lab was to sequence a messenger RNA and determine the genetic code, thereby solving the puzzle of how groups of nucleotides code for amino acids. Working with British biochemists George G. Brownlee and Bart G. Barrell, Sanger developed a two-dimensional electrophoresis method for sequencing RNA. By the time the sequence methods were worked out, the code had been broken by other researchers, mainly the American biochemist Marshall Nirenberg and the Indian-born American biochemist Har Gobind Khorana, using in vitro protein synthesis techniques. The RNA sequence work of Sanger’s group did confirm the genetic code.
DNA Research
By the early 1970s Sanger was interested in deoxyribonucleic acid (DNA). DNA sequence studies had not developed because of the immense size of DNA molecules and the lack of suitable enzymes to cleave DNA into smaller pieces. Building on the enzyme copying approach used by the Swiss chemist Charles Weissmann in his studies on bacteriophage RNA, Sanger began using the enzyme DNA polymerase to make new strands of DNA from single-strand templates, introducing radioactive nucleotides into the new DNA. DNA polymerase requires a primer that can bind to a known region of the template strand. Early success was limited by the lack of suitable primers. Sanger and British colleague Alan R. Coulson developed the “plus and minus” method for rapid DNA sequencing. It represented a radical departure from earlier methods in that it did not utilize partial hydrolysis. Instead, it generated a series of DNA molecules of varying lengths that could be separated by using polyacrylamide gel electrophoresis. For both plus and minus systems, DNA was synthesized from templates to generate random sets of DNA molecules from very short to very long. When both plus and minus sets were separated on the same gel, the sequence could be read from either system, one confirming the other. In 1977 Sanger’s group used this system to deduce most of the DNA sequence of bacteriophage ΦX174, the first complete genome to be sequenced.
A few problems remained with the plus and minus system. Sanger, Coulson, and British colleague Steve Nicklen developed a similar procedure using dideoxynucleotide chain-terminating inhibitors. DNA was synthesized until an inhibitor molecule was incorporated into the growing DNA chain. Using four reactions, each with a different inhibitor, sets of DNA fragments were generated ending in every nucleotide. For example, in the A reaction, a series of DNA fragments ending in A (adenine) was generated. In the C reaction, a series of DNA fragments ending in C (cytosine) was generated, and so on for G (guanine) and T (thymine). When the four reactions were separated side by side on a gel and an autoradiograph developed, the sequence was read from the film. Sanger and his coworkers used the dideoxy method to sequence human mitochondrial DNA. For his contributions to DNA sequencing methods, Sanger shared the 1980 Nobel Prize for Chemistry. He retired in 1983.
Additional Honours
Sanger’s additional honours included election as a fellow of the Royal Society (1954), being named a Commander of the Order of the British Empire (CBE; 1963), receiving the Royal Society’s Royal Medal (1969) and Copley Medal (1977), and election to the Order of the Companions of Honour (CH; 1981) and the Order of Merit (OM; 1986). In 1993 the Wellcome Trust and the British Medical Research Council established a genome research centre, honouring Sanger by naming it the Wellcome Trust Sanger Institute.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#891 2021-04-16 00:13:17
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,161
Re: crème de la crème
857) Henryk Sienkiewicz
Henryk Sienkiewicz, in full Henryk Adam Alexander Pius Sienkiewicz, pseudonym Litwos, (born May 5, 1846, Wola Okrzejska, Poland—died November 15, 1916, Vevey, Switzerland), Polish novelist, winner of the Nobel Prize for Literature in 1905.
Sienkiewicz’s family owned a small estate but lost everything and moved to Warsaw, where Sienkiewicz studied literature, history, and philology at Warsaw University. He left the university in 1871 without taking a degree. He had begun to publish critical articles in 1869 that showed the influence of Positivism, a system of philosophy—popular in Poland and elsewhere at the time—emphasizing in particular the achievements of science. His first novel, Na marne (In Vain), was published in 1872, and his first short story, “Stary sługa” (“An Old Retainer”), in 1875. Sienkiewicz traveled in the United States (1876–78) and, upon his return to Poland after a prolonged stay in Paris, published a number of successful short stories, among them “Janko muzykant” (1879; “Yanko the Musician”), “Latarnik” (1882; “The Lighthouse Keeper”), and “Bartek zwyciezca” (1882; “Bartek the Conqueror”). The last story appears in a volume of his stories entitled Charcoal Sketches and Other Tales (1990), and there is also a volume of his stories entitled Selected Tales (1976).
From 1882 to 1887 Sienkiewicz was coeditor of the daily Słowo (“The Word”). In 1900, to celebrate the 30th year of his career as a writer, the Polish people presented him with the small estate of Oblęgorek, near Kielce in south-central Poland, where he lived until 1914. At the outbreak of World War I he went to Switzerland, where, together with the famous politician and pianist Ignacy Paderewski, he promoted the cause of Polish independence and organized relief for Polish war victims.
Sienkiewicz’s great trilogy of historical novels began to appear in Słowo in 1883. It comprises Ogniem i mieczem (1884; With Fire and Sword; filmed 1999), Potop (1886; The Deluge; filmed 1974), and Pan Wołodyjowski (1887–88; Pan Michael, also published as Fire in the Steppe; filmed 1969). Set in the later 17th century, the trilogy describes Poland’s struggles against Cossacks, Tatars, Swedes, and Turks, stressing Polish heroism with epic range and with clarity and simplicity. The finest of the three works, With Fire and Sword, describes the Poles’ attempts to halt the rebellion of the Zaporozhian Cossacks led by Bohdan Khmelnytsky.
Sienkiewicz’s other novels include the widely translated Quo vadis? (1896; Eng. trans. Quo vadis; filmed 1909, 1913, 1951, 2001), a historical novel set in Rome under Nero, which established Sienkiewicz’s international reputation. Although Sienkiewicz’s major novels have been criticized for their theatricality and lack of historical accuracy, they display great narrative power and contain vivid characterizations.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#892 2021-04-18 00:04:00
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,161
Re: crème de la crème
858) Petrus Jacobus Kipp
Petrus Jacobus Kipp, (Utrecht, 5 March 1808 – Delft, 3 February 1864) was a Dutch apothecary, chemist and instrument maker. He became known as the inventor of the Kipp apparatus, chemistry equipment for the development of gases.
Biography
Kipp passed his pharmacist exam in 1829 in Utrecht with a thesis on seven substances.[1] When he found that, contrary to the academic medicine education with national value, his exam had mere local value, to start a pharmacy in faraway Delft, he had to pass a further test at the provincial medical council. At the beginning of the 19th century, in Delft as in other cities, there was a surplus of pharmacists. Many decided to combine their work with other activities. So too Kipp started in 1830 in Delft a trade in scientific instruments and chemicals. At first his chemicals business made most profit, but after publishing a catalogue in 1850 with instruments imported from Germany and France, his sales of instruments grew in importance.
In 1840, Kipp was elected into the medical council of the city of Delft. In this responsibility, he conducted various chemical investigations assigned by the authorities, e.g., of drinking water and of lamp oil used in street lighting. Many pharmacists in those times were working on professionalisation of the profession. Kipp was one of the founders in 1842 of the Nederlandsche Maatschappij ter Bevordering der Pharmacy (Dutch Company for the advancement of Pharmacy). Also in 1842, in Delft the Polytechnische Hogeschool was founded by King Willem II. The following year, Kipp's friend Carel Frederik Donnadieu was appointed Professor of Chemistry there. Between 1844 and 1850, Kipp earned extra money by translation of German chemistry books to Dutch for use at the university.
In 1842, Kipp published the results of research into the presence of math in livers and kidneys in rabbits. For this research, he used an apparatus to generate hydrogen, which had been developed in 1836 by the English scientist James Marsh. Because the hydrogen production could not easily be stopped during the experiments, Kipp was dissatisfied with the design, and decided to develop his own gas generator. His first version was created by the German glassblower Heinrich Geißler; but was very fragile. The same year, Kipp make a new design, created again by Geißler. This design would be the model for all future versions of the Kipp's apparatus. In 1844, Kipp published two descriptions in the Tijdschrift voor Handel and Nijverheid (Journal for trade and industry). The oldest known copy of the apparatus is owned by the Boerhaave Museum in Leiden. It is 62 cm high and was made between 1845 and 1875.
Kipp was married with Hanna Petronella Regina Heijligers. Together they had ten children. After his death in 1864, his activities were continued by his wife and their sons Willem and Anton under the name of 'P.J. Kipp en Zonen.' Willem ran the pharmacy, later under his own name 'W.A. Kipp,' while Anton did the trade of chemicals and instruments. At the end of the 19th century, the own production of scientific and medical instruments grew in importance more than the import of foreign products. In 2008, the three companies still are active: the Delft pharmacy W.A. Kipp, the Delft instrument maker Kipp & Zonen, and the trade company Salm and Kipp.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#893 2021-04-20 00:12:20
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,161
Re: crème de la crème
859) Andrew Kay
Andrew F. Kay (January 22, 1919 – August 28, 2014) was a businessman and innovator. He was President and CEO of Kaypro, a personal computer company, which at one time was the world's fourth largest manufacturer of computers, and the largest in the world in sales of portable computers.
Kay, a 1940 graduate of MIT, started his career with Bendix followed by two years at Jet Propulsion Laboratory. In 1952, Kay founded Non-Linear Systems, a manufacturer of digital instrumentation. NLS developed a reputation for providing rugged durability in critical applications for everything from submarines to spacecraft. At NLS he invented the digital voltmeter, in 1954.
Kaypro began selling computers in the early 1980s. In 1985, it had more than $120 million in revenues, dwarfing what had been its parent, NLS. But the company's success was relatively short-lived; in 1990 it filed for Chapter 11 bankruptcy protection, and it was liquidated in 1992.
In the late 1990s, Kay founded Kay Computers, which similarly manufactured and sold personal computers. The company lasted for less than ten years.
Kay later was a Senior Business Advisor to Accelerated Composites, LLC.
He co-founded the Rotary Club of Del Mar, California.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#894 2021-04-22 00:09:12
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,161
Re: crème de la crème
860) John Howard Kyan
John Howard Kyan (27 November 1774 – 5 January 1850) was the inventor of the 'kyanising' process for preserving wood. He was the son of John Howard Kyan of Mount Howard and Ballymurtagh, County Wexford, and was born in Dublin on 27 November 1774. His father owned valuable copper mines in Wicklow (now worked by the Wicklow Copper Mines Company) and, for some time worked them himself. The son was educated to take part in the management of the mines, but soon after he entered the company its fortunes declined, and in 1804 his father died almost penniless.
History of kyanizing
Development
For a time Kyan was employed at some vinegar works at Newcastle upon Tyne, but subsequently removed to London, to Greaves's vinegar brewery in Old Street Road. The decay of the timber supports in his father's copper mines had already directed his attention to the question of preserving wood, and as early as 1812 he began experiments with a view to discovering a method of preventing the decay. Eventually he found that bichloride of mercury or corrosive sublimate, as it was commonly called, gave the best results and, without revealing the nature of the process, he submitted a block of oak impregnated with that substance to the Admiralty in 1828. It was placed in the 'fungus pit' at Woolwich, where it remained for three years exposed to all the conditions favourable to decay. When taken out in 1831, it was found to be perfectly sound, and after further trials it still remained unaffected.
Patents
Kyan patented his discovery in 1832 (Nos. 8263 and 6309), extending the application of the invention to the preservation of paper, canvas, cloth, cordage, etc. A further patent was granted in 1836 (No. 7001).
The preservative action of a solution of bichloride of mercury was previously well known, and Kyan's process merely consisted in the submersion of timber or other materials in a tank containing a solution of corrosive sublimate in water. It was maintained by the inventor that permanent chemical combination took place between the mercurial salt and the woody fibre, but this was contested.
Publicity
The process attracted great attention. Faraday chose it as the subject of his inaugural lecture at the Royal Institution on 22 February 1833, on his appointment as Fullerian professor of chemistry. Dr. Birkbeck gave a lecture upon it at the Society of Arts on 9 December 1834, and in 1835 the Admiralty published the report of a committee appointed by the board to inquire into the value of the new method. In 1836, Kyan sold his rights to the Anti-Dry Rot Company, an Act of Parliament being passed which authorised the raising of a capital of £250,000. Tanks were constructed at Grosvenor Basin, Pimlico, at the Grand Surrey Canal Dock, Rotherhide, and at the City Road Basin. Great things were predicted of 'kyanising,' as the process then began to be called. A witty writer in 'Bently's Miscellany' for January 1837 told how the muses had adopted Kyan's improvement to preserve their favourite trees. At a dinner given to celebrate the success which attended the experiment, a song, which became popular, was first sung. The opening verse runs:
Have you heard, have you heard
Anti-dry Rot's the word?
Wood will never wear out, thanks to Kyan, to Kyan!
He dips in a tank any rafter or plank,
And makes it immortal as Dian, as Dian!
Applications
Among the early applications of the process was the kyanising of the palings round the Inner Circle, Regent's Park, which was carried out in 1835 as an advertisement, small brass plates being attached to the palings at intervals stating that the wood had been submitted to the new process. The plates soon disappeared, but the original palings still remain in good condition.
The timber used in building the Oxford and Cambridge Club, British Museum, Royal College of Surgeons, Westminster Bridewell, the new roof of the Temple Church, and the Ramsgate harbour works was also prepared by Kyan's process. When wooden railway sleepers ("ties" in the USA) became general (in place of the stone blocks used on the early lines), a very profitable business for Kyan's company was anticipated, and for a time these hopes were realised.
The process was also applied to ships' timbers and sails. The whaler ‘John Palmer’ received some treated wood as she underwent repairs in 1833 and on her return to England from the Pacific in 1837 her master, Captain Elisha Clark, wrote a testimonial for the process.
Decline
It became evident that iron fastenings could not be used in wood treated with corrosive sublimate, on account of the corrosive action, and it was said that the wood became brittle. The salt was somewhat expensive and Sir William Burnett's method of preserving timber by chloride of zinc, and afterwards the application of creosote for that purpose, proved severe competitors. Doubts began to be expressed as to the real efficiency of kyanising, and the process gradually ceased to be employed.
Other inventions and interests
Besides the invention with which his name is associated, Kyan took out patents in 1833 (No. 6534) for propelling ships by a jet of water ejected at the stern, and in 1837 (No. 7460) for a method of obtaining ammoniacal salts from gas liquor. He was a member of the London Electrical Society and also the author of 'The Elements of Light and their Identity with those of Matter radiant or fixed,' 1838. He died on 5 January 1850 at New York City, where he was engaged on a plan for filtering the water supplied to that city by the Croton aqueduct.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#895 2021-04-24 00:04:03
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,161
Re: crème de la crème
861) George Wells Beadle
George Wells Beadle, (born Oct. 22, 1903, Wahoo, Neb., U.S.—died June 9, 1989, Pomona, Calif.), American geneticist who helped found biochemical genetics when he showed that genes affect heredity by determining enzyme structure. He shared the 1958 Nobel Prize for Physiology or Medicine with Edward Tatum and Joshua Lederberg.
After earning his doctorate in genetics from Cornell University (1931), Beadle went to the laboratory of Thomas Hunt Morgan at the California Institute of Technology, where he did work on the fruit fly, Drosophila melanogaster. Beadle soon realized that genes must influence heredity chemically.
In 1935, with Boris Ephrussi at the Institut de Biologie Physico-Chimique in Paris, he designed a complex technique to determine the nature of these chemical effects in Drosophila. Their results indicated that something as apparently simple as eye colour is the product of a long series of chemical reactions and that genes somehow affect these reactions.
After a year at Harvard University, Beadle pursued gene action in detail at Stanford University in 1937. Working there with Tatum, he found that the total environment of a red bread mold, Neurospora, could be varied in such a way that the researchers could locate and identify genetic changes, or mutants, with comparative ease. They exposed the mold to X rays and studied the altered nutritional requirements of the mutants thus produced. These experiments enabled them to conclude that each gene determined the structure of a specific enzyme that, in turn, allowed a single chemical reaction to proceed. This “one gene–one enzyme” concept won Beadle and Tatum (with Lederberg) the Nobel Prize in 1958.
In addition, the use of genetics to study the biochemistry of microorganisms, outlined in the landmark paper “Genetic Control of Biochemical Reactions in Neurospora” (1941), by Beadle and Tatum, opened up a new field of research with far-reaching implications. Their methods immediately revolutionized the manufacture of penicillin and provided insights into many biochemical processes.
In 1946 Beadle became professor and chairman of the biology division at the California Institute of Technology and served there until 1960, when he was invited to succeed R. Wendel Harrison as chancellor of the University of Chicago; the title of president was reassigned to the position a year later. He retired from the university to direct (1968–70) the American Medical Association’s Institute for Biomedical Research.
His major works include ‘An Introduction to Genetics’ (1939; with A.H. Sturtevant), ‘Genetics and Modern Biology’ (1963), and ‘The Language of Life’ (1966; with Muriel M. Beadle).

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#896 2021-04-26 00:03:44
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,161
Re: crème de la crème
862) Edward L. Tatum
Edward L. Tatum, in full Edward Lawrie Tatum, (born Dec. 14, 1909, Boulder, Colo., U.S.—died Nov. 5, 1975, New York, N.Y.), American biochemist who helped demonstrate that genes determine the structure of particular enzymes or otherwise act by regulating specific chemical processes in living things. His research helped create the field of molecular genetics and earned him (with George Beadle and Joshua Lederberg) the Nobel Prize for Physiology or Medicine in 1958.
Tatum earned his doctorate from the University of Wisconsin in 1934. As a research associate at Stanford University (1937–41), Tatum collaborated with Beadle in an attempt to confirm the following concepts: all biochemical processes in all organisms are ultimately controlled by genes; all these processes are resolvable into series of individual sequential chemical reactions (pathways); each reaction is in some way controlled by a single gene; and the mutation of a single gene results only in an alteration in the ability of the cell to carry out a single chemical reaction.
At Stanford, Tatum and Beadle used X rays to induce mutations in strains of the pink bread mold Neurospora crassa. They found that some of the mutants lost the ability to produce an essential amino acid or vitamin. Tatum and Beadle then crossed these strains with normal strains of the mold and found that their offspring inherited the metabolic defect as a recessive trait, thereby proving that the mutations were in fact genetic defects. Their research showed that when a genetic mutation can be shown to affect a specific chemical reaction, the enzyme catalyzing that reaction will be altered or missing. Thus, they showed that each gene in some way determines the structure of a specific enzyme (the one-gene–one-enzyme hypothesis).
As a professor at Yale University (1945–48), Tatum successfully applied his methods of inducing mutations and studying biochemical processes in Neurospora to bacteria. With Lederberg, he discovered the occurrence of genetic recombination, or “gender,” between Escherichia coli bacteria of the K-12 strain. Largely because of their efforts, bacteria became the primary source of information concerning the genetic control of biochemical processes in the cell.
Tatum returned to Stanford in 1948 and joined the staff of the Rockefeller Institute for Medical Research (now Rockefeller University), New York City, in 1957.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#897 2021-04-28 00:09:20
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,161
Re: crème de la crème
863) Joshua Lederberg
Joshua Lederberg, (born May 23, 1925, Montclair, N.J., U.S.—died Feb. 2, 2008, New York, N.Y.), American geneticist, pioneer in the field of bacterial genetics, who shared the 1958 Nobel Prize for Physiology or Medicine (with George W. Beadle and Edward L. Tatum) for discovering the mechanisms of genetic recombination in bacteria.
Lederberg studied under Tatum at Yale (Ph.D., 1948) and taught at the University of Wisconsin (1947–59), where he established a department of medical genetics. In 1959 he joined the faculty of the Stanford Medical School, serving as director of the Kennedy Laboratories of Molecular Medicine there from 1962 to 1978, when he moved to New York City to become president of Rockefeller University. He held that post until 1990.
With Tatum he published “Gene Recombination in Escherichia coli” (1946), in which he reported that the mixing of two different strains of a bacterium resulted in genetic recombination between them and thus to a new, crossbred strain of the bacterium. Scientists had previously thought that bacteria only reproduced asexually—i.e., by cells splitting in two; Lederberg and Tatum showed that they could also reproduce sexually, and that bacterial genetic systems are similar to those of multicellular organisms.
While biologists who had not previously believed that “male/female” existed in bacteria such as E. coli were still confirming Lederberg’s discovery, he and his student Norton D. Zinder reported another and equally surprising finding. In the paper “Genetic Exchange in Salmonella” (1952), they revealed that certain bacteriophages (bacteria-infecting viruses) were capable of carrying a bacterial gene from one bacterium to another, a phenomenon they termed transduction.
Lederberg’s discoveries greatly increased the utility of bacteria as a tool in genetics research, and it soon became as important as the fruit fly Drosophila and the bread mold Neurospora. Moreover, his discovery of transduction provided the first hint that genes could be inserted into cells. The realization that the genetic material of living things could be directly manipulated eventually bore fruit in the field of genetic engineering, or recombinant DNA technology.
At the dawn of space exploration, Lederberg coined the term ‘exobiology’ to describe the scientific study of life outside Earth’s atmosphere. He later served as a consultant to NASA’s Viking mission to Mars.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#898 2021-04-30 00:09:27
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,161
Re: crème de la crème
864) Emilio Segrè
Emilio Segrè, in full Emilio Gino Segrè, (born February 1, 1905, Tivoli, Italy—died April 22, 1989, Lafayette, California, U.S.), Italian-born American physicist who was cowinner, with Owen Chamberlain of the United States, of the Nobel Prize for Physics in 1959 for the discovery of the antiproton, an antiparticle having the same mass as a proton but opposite in electrical charge.
Segrè initially began studies in engineering at the University of Rome in 1922 but later studied under Enrico Fermi and received his doctorate in physics in 1928. In 1932 Segrè was appointed assistant professor of physics at the University of Rome, and two years later he participated in neutron experiments directed by Fermi, in which many elements, including uranium, were bombarded with neutrons, and elements heavier than uranium were created. In 1935 they discovered slow neutrons, which have properties important to the operation of nuclear reactors.
Segrè left Rome in 1936 to become director of the physics laboratory at the University of Palermo. One year later he discovered technetium, the first man-made element not found in nature. While visiting California in 1938, Segrè was dismissed from the University of Palermo by the Fascist government, so he remained in the United States as a research associate at the University of California, Berkeley. Continuing his research, he and his associates discovered the element astatine in 1940, and later, with another group, he discovered the isotope plutonium-239, which he found to be fissionable, much like uranium-235. Plutonium-239 was used in the first atomic bomb and in the bomb dropped on Nagasaki.
From 1943 to 1946 Segrè was a group leader at the Los Alamos Scientific Laboratory, Los Alamos, N.M. He was naturalized as a U.S. citizen in 1944 and was professor of physics at Berkeley (1946–72). In 1955, using the new bevatron particle accelerator, Segrè and Chamberlain produced and identified antiprotons and thus set the stage for the discovery of many additional antiparticles. He was appointed professor of nuclear physics at the University of Rome in 1974. He wrote several books, including Experimental Nuclear Physics (1953), Nuclei and Particles (1964), Enrico Fermi: Physicist (1970), and two books on the history of physics, From X-rays to Quarks: Modern Physicists and Their Discoveries (1980) and From Falling Bodies to Radio Waves (1984). Shortly after winning the Nobel Prize, Segrè wrote the entry on the proton for the 1960 printing of the 14th edition of the Encyclopædia Britannica.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#899 2021-05-02 00:06:27
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,161
Re: crème de la crème
865) Owen Chamberlain
Owen Chamberlain, (born July 10, 1920, San Francisco, California, U.S.—died February 28, 2006, Berkeley, California), American physicist, who shared the Nobel Prize for Physics in 1959 with Emilio Segrè for their discovery of the antiproton. This previously postulated subatomic particle was the second antiparticle to be discovered and led directly to the discovery of many additional antiparticles.
Chamberlain attended Dartmouth College (B.A., 1941) and the University of California at Berkeley before working on the Manhattan Project, a U.S. research project that produced the first atom bombs. Later, while completing a Ph.D. (1948) at the University of Chicago, he worked at Argonne National Laboratory in Illinois. In 1948 he joined the faculty of the University of California at Berkeley, where he became a full professor in 1958 and professor emeritus in 1989. There he conducted research on alpha particle decay, neutron diffraction in liquids, and high-energy nuclear particle reactions. He and Segrè used the bevatron (a powerful particle accelerator) to produce antiprotons in 1955, and the following year they confirmed the existence of the antineutron.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#900 2021-05-03 00:07:34
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,161
Re: crème de la crème
866) Jaroslav Heyrovský
Jaroslav Heyrovský, (born December 20, 1890, Prague, Bohemia, Austro-Hungarian Empire [now in Czech Republic]—died March 27, 1967, Prague, Czechoslovakia), Czech chemist who received the Nobel Prize for Chemistry in 1959 for his discovery and development of polarography.
Educated at the Charles University (Universita Karlova) of Prague and at University College, London, Heyrovský worked in London under Sir William Ramsay and F.G. Donnan. After holding several posts at the Charles University, he became professor and director of the department of physical chemistry (1926–54), and he was director of the Polarography Institute at the Czechoslovak Academy of Sciences (1950, 1952–63).
The work that eventually led to the discovery of polarography was begun in London at Donnan’s suggestion. Polarography is an instrumental method of chemical analysis used for qualitative and quantitative determinations of reducible or oxidizable substances. Heyrovský’s instrument measures the current that flows when a predetermined potential is applied to two electrodes immersed in the solution to be analyzed. Within 10 years of the demonstration of the first polarograph (1924) the method was in common use. Heyrovský’s monograph ‘Polarographie’ appeared in 1941.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
