Math Is Fun Forum
You are not logged in.
- Topics: Active | Unanswered
#1001 2021-04-26 01:05:32
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,002
Re: Miscellany
979) Amalgam
Amalgam, alloy of mercury and one or more other metals. Amalgams are crystalline in structure, except for those with a high mercury content, which are liquid. Known since early times, they were mentioned by Pliny the Elder in the 1st century AD. In dentistry, an amalgam of silver and tin, with minor amounts of copper and zinc, is used to fill teeth.
A sodium amalgam is formed during the manufacture of chlorine and sodium hydroxide by the electrolysis of brine in cells wherein a stream of mercury constitutes the negative electrode. Reaction of the amalgam with water produces a solution of sodium hydroxide and regenerates the mercury for reuse.
Fine particles of silver and gold can be recovered by agitating their ores with mercury and allowing the resultant pasty or liquid amalgam to settle. By distillation of the amalgam, the mercury is reclaimed, and the precious metals are isolated as a residue.
Amalgams of silver, gold, and palladium are known in nature. Moschellandsbergite, silver amalgam, is found at Moschellandsberg, Ger.; Sala, Swed.; and Isère, France. Gold amalgam occurs in California, U.S., Colombia, and Borneo.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1002 2021-04-27 00:54:21
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,002
Re: Miscellany
980) Platinum
Platinum (Pt), chemical element, the best known and most widely used of the six platinum metals of Groups 8–10 (VIIIb), Periods 5 and 6, of the periodic table. A very heavy, precious, silver-white metal, platinum is soft and ductile and has a high melting point and good resistance to corrosion and chemical attack. For example, its surface remains bright after being brought to white heat in air, and, though it readily dissolves in aqua regia, it is scarcely attacked by simple acids. (It does dissolve slowly in hydrochloric acid in the presence of air.) Small amounts of iridium are commonly added to give a harder, stronger alloy that retains the advantages of pure platinum.
Platinum, one of the most abundant platinum metals, and its alloys are indispensable in the chemical laboratory for electrodes and for crucibles and dishes in which materials can be heated to high temperatures. Platinum is used for electrical contacts and sparking points because it resists both the high temperatures and chemical attack of electric arcs. Jewelry and dental alloys account for much of its use; platinum-iridium is used for surgical pins. The prototype international standard kilogram of mass was made from an alloy of 90 percent platinum and 10 percent iridium. The electrical resistivity of platinum is relatively high and depends markedly upon the temperature; the international temperature scale from −183° to 630° C (−297° to 1,166° F) is defined in terms of a resistance thermometer made with platinum wire. As a catalyst, platinum has many applications, notably in automotive catalytic converters and in petroleum refining.
The Italian-French physician Julius Caesar Scaliger alluded (1557) to a refractory metal, probably platinum, found between Darién and Mexico. The first certain discovery was in the alluvial deposits of the Río Pinto, Colombia. The Spaniards called the new metal platina del Pinto for its resemblance to silver. The world’s most important deposits occur in the Transvaal of South Africa. Other deposits are found in Russia, Finland, Ireland, Borneo, New South Wales, New Zealand, Brazil, Peru, and Madagascar. In North America native platinum is found in Alaska, California, and Oregon, in British Columbia, and in Alberta. Placer deposits are the most productive sources of the native element. The ordinary variety of native platinum is called polyxene; it is 80 percent to 90 percent platinum, with 3 percent to 11 percent iron, plus the other platinum metals, and gold, copper, and nickel. Platinum is also found in the very rare native alloy platiniridium. Platinum occurs combined with math as sperrylite (PtAs2) in the copper–nickel-mining district near Sudbury, Ont., and with sulfur as cooperite (PtS) in the Transvaal.
Platinum is rapidly attacked by fused alkali oxides and peroxides and also by fluorine and chlorine at about 500° C. It is capable of absorbing large volumes of hydrogen, and, with palladium, it is one of the most reactive platinum metals.
Platinum forms an important series of compounds with the oxidation states of +2 and +4. Many of these compounds contain coordination complexes in which chloride ion (Cl−), ammonia (NH3), or other groups are bonded to a central platinum atom. Among the transition metals, platinum has one of the greatest tendencies to form bonds directly with carbon. Platinum also combines with a number of nonmetallic elements on heating, such as phosphorus, antimony, silicon, sulfur, and selenium.
Natural platinum is a mixture of six isotopes: platinum-190 (0.0127 percent), platinum-192 (0.78 percent), platinum-194 (32.9 percent), platinum-195 (33.8 percent), platinum-196 (25.3 percent), and platinum-198 (7.21 percent). All are stable except platinum-190 and platinum-192, which have been reported as long-lived alpha emitters.
Element Properties
atomic number 78
atomic weight 195.09
melting point 1,769° C (3,216° F)
boiling point 3,827° C (6,920° F)
specific gravity 21.45 (20° C)
oxidation states +2, +4.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1003 2021-04-28 00:14:43
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,002
Re: Miscellany
981) Welding
Welding, technique used for joining metallic parts usually through the application of heat. This technique was discovered during efforts to manipulate iron into useful shapes. Welded blades were developed in the 1st millennium CE, the most famous being those produced by Arab armourers at Damascus, Syria. The process of carburization of iron to produce hard steel was known at this time, but the resultant steel was very brittle. The welding technique—which involved interlayering relatively soft and tough iron with high-carbon material, followed by hammer forging—produced a strong, tough blade.
In modern times the improvement in iron-making techniques, especially the introduction of cast iron, restricted welding to the blacksmith and the jeweler. Other joining techniques, such as fastening by bolts or rivets, were widely applied to new products, from bridges and railway engines to kitchen utensils.
Modern fusion welding processes are an outgrowth of the need to obtain a continuous joint on large steel plates. Rivetting had been shown to have disadvantages, especially for an enclosed container such as a boiler. Gas welding, arc welding, and resistance welding all appeared at the end of the 19th century. The first real attempt to adopt welding processes on a wide scale was made during World War I. By 1916 the oxyacetylene process was well developed, and the welding techniques employed then are still used. The main improvements since then have been in equipment and safety. Arc welding, using a consumable electrode, was also introduced in this period, but the bare wires initially used produced brittle welds. A solution was found by wrapping the bare wire with asbestos and an entwined aluminum wire. The modern electrode, introduced in 1907, consists of a bare wire with a complex coating of minerals and metals. Arc welding was not universally used until World War II, when the urgent need for rapid means of construction for shipping, power plants, transportation, and structures spurred the necessary development work.
Resistance welding, invented in 1877 by Elihu Thomson, was accepted long before arc welding for spot and seam joining of sheet. Butt welding for chain making and joining bars and rods was developed during the 1920s. In the 1940s the tungsten-inert gas process, using a nonconsumable tungsten electrode to perform fusion welds, was introduced. In 1948 a new gas-shielded process utilized a wire electrode that was consumed in the weld. More recently, electron-beam welding, laser welding, and several solid-phase processes such as diffusion bonding, friction welding, and ultrasonic joining have been developed.
Basic Principles Of Welding
A weld can be defined as a coalescence of metals produced by heating to a suitable temperature with or without the application of pressure, and with or without the use of a filler material.
In fusion welding a heat source generates sufficient heat to create and maintain a molten pool of metal of the required size. The heat may be supplied by electricity or by a gas flame. Electric resistance welding can be considered fusion welding because some molten metal is formed.
Solid-phase processes produce welds without melting the base material and without the addition of a filler metal. Pressure is always employed, and generally some heat is provided. Frictional heat is developed in ultrasonic and friction joining, and furnace heating is usually employed in diffusion bonding.
The electric arc used in welding is a high-current, low-voltage discharge generally in the range 10–2,000 amperes at 10–50 volts. An arc column is complex but, broadly speaking, consists of a cathode that emits electrons, a gas plasma for current conduction, and an anode region that becomes comparatively hotter than the cathode due to electron bombardment. A direct current (DC) arc is usually used, but alternating current (AC) arcs can be employed.
Total energy input in all welding processes exceeds that which is required to produce a joint, because not all the heat generated can be effectively utilized. Efficiencies vary from 60 to 90 percent, depending on the process; some special processes deviate widely from this figure. Heat is lost by conduction through the base metal and by radiation to the surroundings.
Most metals, when heated, react with the atmosphere or other nearby metals. These reactions can be extremely detrimental to the properties of a welded joint. Most metals, for example, rapidly oxidize when molten. A layer of oxide can prevent proper bonding of the metal. Molten-metal droplets coated with oxide become entrapped in the weld and make the joint brittle. Some valuable materials added for specific properties react so quickly on exposure to the air that the metal deposited does not have the same composition as it had initially. These problems have led to the use of fluxes and inert atmospheres.
In fusion welding the flux has a protective role in facilitating a controlled reaction of the metal and then preventing oxidation by forming a blanket over the molten material. Fluxes can be active and help in the process or inactive and simply protect the surfaces during joining.
Inert atmospheres play a protective role similar to that of fluxes. In gas-shielded metal-arc and gas-shielded tungsten-arc welding an inert gas—usually argon—flows from an annulus surrounding the torch in a continuous stream, displacing the air from around the arc. The gas does not chemically react with the metal but simply protects it from contact with the oxygen in the air.
The metallurgy of metal joining is important to the functional capabilities of the joint. The arc weld illustrates all the basic features of a joint. Three zones result from the passage of a welding arc: (1) the weld metal, or fusion zone, (2) the heat-affected zone, and (3) the unaffected zone. The weld metal is that portion of the joint that has been melted during welding. The heat-affected zone is a region adjacent to the weld metal that has not been welded but has undergone a change in microstructure or mechanical properties due to the heat of welding. The unaffected material is that which was not heated sufficiently to alter its properties.
Weld-metal composition and the conditions under which it freezes (solidifies) significantly affect the ability of the joint to meet service requirements. In arc welding, the weld metal comprises filler material plus the base metal that has melted. After the arc passes, rapid cooling of the weld metal occurs. A one-pass weld has a cast structure with columnar grains extending from the edge of the molten pool to the centre of the weld. In a multipass weld, this cast structure may be modified, depending on the particular metal that is being welded.
The base metal adjacent to the weld, or the heat-affected zone, is subjected to a range of temperature cycles, and its change in structure is directly related to the peak temperature at any given point, the time of exposure, and the cooling rates. The types of base metal are too numerous to discuss here, but they can be grouped in three classes: (1) materials unaffected by welding heat, (2) materials hardened by structural change, (3) materials hardened by precipitation processes.
Welding produces stresses in materials. These forces are induced by contraction of the weld metal and by expansion and then contraction of the heat-affected zone. The unheated metal imposes a restraint on the above, and as contraction predominates, the weld metal cannot contract freely, and a stress is built up in the joint. This is generally known as residual stress, and for some critical applications must be removed by heat treatment of the whole fabrication. Residual stress is unavoidable in all welded structures, and if it is not controlled bowing or distortion of the weldment will take place. Control is exercised by welding technique, jigs and fixtures, fabrication procedures, and final heat treatment.
There are a wide variety of welding processes. Several of the most important are discussed below.
Forge Welding
This original fusion technique dates from the earliest uses of iron. The process was first employed to make small pieces of iron into larger useful pieces by joining them. The parts to be joined were first shaped, then heated to welding temperature in a forge and finally hammered or pressed together. The Damascus sword, for example, consisted of wrought-iron bars hammered until thin, doubled back on themselves, and then rehammered to produce a forged weld. The larger the number of times this process was repeated, the tougher the sword that was obtained. In the Middle Ages cannons were made by welding together several iron bands, and bolts tipped with steel fired from crossbows were fabricated by forge welding. Forge welding has mainly survived as a blacksmith’s craft and is still used to some extent in chain making.
Arc Welding
Shielded metal-arc welding accounts for the largest total volume of welding today. In this process an electric arc is struck between the metallic electrode and the workpiece. Tiny globules of molten metal are transferred from the metal electrode to the weld joint. Since arc welding can be done with either alternating or direct current, some welding units accommodate both for wider application. A holder or clamping device with an insulated handle is used to conduct the welding current to the electrode. A return circuit to the power source is made by means of a clamp to the workpiece.
Gas-shielded arc welding, in which the arc is shielded from the air by an inert gas such as argon or helium, has become increasingly important because it can deposit more material at a higher efficiency and can be readily automated. The tungsten electrode version finds its major applications in highly alloyed sheet materials. Either direct or alternating current is used, and filler metal is added either hot or cold into the arc. Consumable electrode gas-metal arc welding with a carbon dioxide shielding gas is widely used for steel welding. Two processes known as spray arc and short-circuiting arc are utilized. Metal transfer is rapid, and the gas protection ensures a tough weld deposit.
Submerged arc welding is similar to the above except that the gas shield is replaced with a granulated mineral material as a flux, which is mounded around the electrode so that no arc is visible.
Plasma welding is an arc process in which a hot plasma is the source of heat. It has some similarity to gas-shielded tungsten-arc welding, the main advantages being greater energy concentration, improved arc stability, and easier operator control. Better arc stability means less sensitivity to joint alignment and arc length variation. In most plasma welding equipment, a secondary arc must first be struck to create an ionized gas stream and permit the main arc to be started. This secondary arc may utilize either a high-frequency or a direct contact start. Water cooling is used because of the high energies forced through a small orifice. The process is amenable to mechanization, and rapid production rates are possible.
Thermochemical Processes
One such process is gas welding. It once ranked as equal in importance to the metal-arc welding processes but is now confined to a specialized area of sheet fabrication and is probably used as much by artists as in industry. Gas welding is a fusion process with heat supplied by burning acetylene in oxygen to provide an intense, closely controlled flame. Metal is added to the joint in the form of a cold filler wire. A neutral or reducing flame is generally desirable to prevent base-metal oxidation. By deft craftsmanship very good welds can be produced, but welding speeds are very low. Fluxes aid in preventing oxide contamination of the joint.
Another thermochemical process is aluminothermic (thermite) joining. It has been successfully used for both ferrous and nonferrous metals but is more frequently used for the former. A mixture of finely divided aluminum and iron oxide is ignited to produce a superheated liquid metal at about 2,800 °C (5,000 °F). The reaction is completed in 30 seconds to 2 minutes regardless of the size of the charge. The process is suited to joining sections with large, compact cross sections, such as rectangles and rounds. A mold is used to contain the liquid metal.
Resistance Welding
Spot, seam, and projection welding are resistance welding processes in which the required heat for joining is generated at the interface by the electrical resistance of the joint. Welds are made in a relatively short time (typically 0.2 seconds) using a low-voltage, high-current power source with force applied to the joint through two electrodes, one on each side. Spot welds are made at regular intervals on sheet metal that has an overlap. Joint strength depends on the number and size of the welds. Seam welding is a continuous process wherein the electric current is successively pulsed into the joint to form a series of overlapping spots or a continuous seam. This process is used to weld containers or structures where spot welding is insufficient. A projection weld is formed when one of the parts to be welded in the resistance machine has been dimpled or pressed to form a protuberance that is melted down during the weld cycle. The process allows a number of predetermined spots to be welded at one time. All of these processes are capable of very high rates of production with continuous quality control. The most modern equipment in resistance welding includes complete feedback control systems to self-correct any weld that does not meet the desired specifications.
Flash welding is a resistance welding process where parts to be joined are clamped, the ends brought together slowly and then drawn apart to cause an arc or flash. Flashing or arcing is continued until the entire area of the joint is heated; the parts are then forced together and pressure maintained until the joint is formed and cooled.
Low- and high-frequency resistance welding is used for the manufacture of tubing. The longitudinal joint in a tube is formed from metal squeezed into shape with edges abutted. Welding heat is governed by the current passing through the work and the speed at which the tube goes through the rolls. Welding speeds of 60 metres (200 feet) per minute are possible in this process.
Electron-Beam Welding
In electron-beam welding, the workpiece is bombarded with a dense stream of high-velocity electrons. The energy of these electrons is converted to heat upon impact. A beam-focusing device is included, and the workpiece is usually placed in an evacuated chamber to allow uninterrupted electron travel. Heating is so intense that the beam almost instantaneously vaporizes a hole through the joint. Extremely narrow deep-penetration welds can be produced using very high voltages—up to 150 kilovolts. Workpieces are positioned accurately by an automatic traverse device; for example, a weld in material 13 mm (0.5 inch) thick would only be 1 mm (0.04 inch) wide. Typical welding speeds are 125 to 250 cm (50 to 100 inches) per minute.
Cold Welding
Cold welding, the joining of materials without the use of heat, can be accomplished simply by pressing them together. Surfaces have to be well prepared, and pressure sufficient to produce 35 to 90 percent deformation at the joint is necessary, depending on the material. Lapped joints in sheets and cold-butt welding of wires constitute the major applications of this technique. Pressure can be applied by punch presses, rolling stands, or pneumatic tooling. Pressures of 1,400,000 to 2,800,000 kilopascals (200,000 to 400,000 pounds per square inch) are needed to produce a joint in aluminum; almost all other metals need higher pressures.
Friction Welding
In friction welding two workpieces are brought together under load with one part rapidly revolving. Frictional heat is developed at the interface until the material becomes plastic, at which time the rotation is stopped and the load is increased to consolidate the joint. A strong joint results with the plastic deformation, and in this sense the process may be considered a variation of pressure welding. The process is self-regulating, for, as the temperature at the joint rises, the friction coefficient is reduced and overheating cannot occur. The machines are almost like lathes in appearance. Speed, force, and time are the main variables. The process has been automated for the production of axle casings in the automotive industry.
Laser Welding
Laser welding is accomplished when the light energy emitted from a laser source is focused upon a workpiece to fuse materials together. The limited availability of lasers of sufficient power for most welding purposes has so far restricted its use in this area. Another difficulty is that the speed and the thickness that can be welded are controlled not so much by power but by the thermal conductivity of the metals and by the avoidance of metal vaporization at the surface. Particular applications of the process with very thin materials up to 0.5 mm (0.02 inch) have, however, been very successful. The process is useful in the joining of miniaturized electrical circuitry.
Diffusion Bonding
This type of bonding relies on the effect of applied pressure at an elevated temperature for an appreciable period of time. Generally, the pressure applied must be less than that necessary to cause 5 percent deformation so that the process can be applied to finished machine parts. The process has been used most extensively in the aerospace industries for joining materials and shapes that otherwise could not be made—for example, multiple-finned channels and honeycomb construction. Steel can be diffusion bonded at above 1,000 °C (1,800 °F) in a few minutes.
Ultrasonic Welding
Ultrasonic joining is achieved by clamping the two pieces to be welded between an anvil and a vibrating probe or sonotrode. The vibration raises the temperature at the interface and produces the weld. The main variables are the clamping force, power input, and welding time. A weld can be made in 0.005 second on thin wires and up to 1 second with material 1.3 mm (0.05 inch) thick. Spot welds and continuous seam welds are made with good reliability. Applications include extensive use on lead bonding to integrated circuitry, transistor canning, and aluminum can bodies.
Explosive Welding
Explosive welding takes place when two plates are impacted together under an explosive force at high velocity. The lower plate is laid on a firm surface, such as a heavier steel plate. The upper plate is placed carefully at an angle of approximately 5° to the lower plate with a sheet of explosive material on top. The charge is detonated from the hinge of the two plates, and a weld takes place in microseconds by very rapid plastic deformation of the material at the interface. A completed weld has the appearance of waves at the joint caused by a jetting action of metal between the plates.
Weldability Of Metals
Carbon and low-alloy steels are by far the most widely used materials in welded construction. Carbon content largely determines the weldability of plain carbon steels; at above 0.3 percent carbon some precautions have to be taken to ensure a sound joint. Low-alloy steels are generally regarded as those having a total alloying content of less than 6 percent. There are many grades of steel available, and their relative weldability varies.
Aluminum and its alloys are also generally weldable. A very tenacious oxide film on aluminum tends to prevent good metal flow, however, and suitable fluxes are used for gas welding. Fusion welding is more effective with alternating current when using the gas-tungsten arc process to enable the oxide to be removed by the arc action.
Copper and its alloys are weldable, but the high thermal conductivity of copper makes welding difficult. Refractory metals such as zirconium, niobium, molybdenum, tantalum, and tungsten are usually welded by the gas-tungsten arc process. Nickel is the most compatible material for joining, is weldable to itself, and is extensively used in dissimilar metal welding of steels, stainlesses, and copper alloys.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1004 2021-04-29 00:11:00
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,002
Re: Miscellany
982) Soldering
Soldering, process that uses low-melting-point metal alloys to join metallic surfaces without melting them. The basic operational steps are as follows: (1) thorough cleaning of the metal to be joined by abrasive or chemical means, (2) application of a flux to remove oxides on heating and promote spreading and wetting of the solder, (3) alignment of parts to produce a controlled gap of 0.025 to 0.125 mm (0.001 to 0.005 inch), (4) application of heat, (5) feeding solder to the joint, (6) cooling without movement, and (7) removal of corrosive flux residues.
Tin-lead solders are widely used in the electrical and plumbing industries. Such alloys also are utilized to solder brass and copper automobile radiators. Solders are supplied in wire, bar, or premixed-paste form, depending on the application.
Zinc chloride-based fluxes are used on copper alloys, with hydrochloric acid added for stainless steels. Electronic circuits require a noncorrosive flux; fluxes based on rosin using alcohol as a carrier are sufficiently active to produce a good bond. Soldering can be carried out using a torch, a soldering iron, a flame heater, or an induction heater. Dip soldering is used in the auto industry, and wave-soldering devices are prominent in printed-circuit production.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1005 2021-04-30 00:21:18
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,002
Re: Miscellany
983) Capacitor
Capacitor, device for storing electrical energy, consisting of two conductors in close proximity and insulated from each other. A simple example of such a storage device is the parallel-plate capacitor. If positive charges with total charge +Q are deposited on one of the conductors and an equal amount of negative charge −Q is deposited on the second conductor, the capacitor is said to have a charge Q.
Capacitors have many important applications. They are used, for example, in digital circuits so that information stored in large computer memories is not lost during a momentary electric power failure; the electric energy stored in such capacitors maintains the information during the temporary loss of power. Capacitors play an even more important role as filters to divert spurious electric signals and thereby prevent damage to sensitive components and circuits caused by electric surges.
A capacitor is a device that stores electric charge in an electric field. It is a passive electronic component with two terminals.
The effect of a capacitor is known as capacitance. While some capacitance exists between any two electrical conductors in proximity in a circuit, a capacitor is a component designed to add capacitance to a circuit. The capacitor was originally known as a condenser or condensator. This name and its cognates are still widely used in many languages, but rarely in English, one notable exception being condenser microphones, also called capacitor microphones.
The physical form and construction of practical capacitors vary widely and many types of capacitor are in common use. Most capacitors contain at least two electrical conductors often in the form of metallic plates or surfaces separated by a dielectric medium. A conductor may be a foil, thin film, sintered bead of metal, or an electrolyte. The nonconducting dielectric acts to increase the capacitor's charge capacity. Materials commonly used as dielectrics include glass, ceramic, plastic film, paper, mica, air, and oxide layers. Capacitors are widely used as parts of electrical circuits in many common electrical devices. Unlike a resistor, an ideal capacitor does not dissipate energy, although real-life capacitors do dissipate a small amount (see Non-ideal behavior). When an electric potential, a voltage, is applied across the terminals of a capacitor, for example when a capacitor is connected across a battery, an electric field develops across the dielectric, causing a net positive charge to collect on one plate and net negative charge to collect on the other plate. No current actually flows through the dielectric. However, there is a flow of charge through the source circuit. If the condition is maintained sufficiently long, the current through the source circuit ceases. If a time-varying voltage is applied across the leads of the capacitor, the source experiences an ongoing current due to the charging and discharging cycles of the capacitor.
The earliest forms of capacitors were created in the 1740s, when European experimenters discovered that electric charge could be stored in water-filled glass jars that came to be known as Leyden jars. Today, capacitors are widely used in electronic circuits for blocking direct current while allowing alternating current to pass. In analog filter networks, they smooth the output of power supplies. In resonant circuits they tune radios to particular frequencies. In electric power transmission systems, they stabilize voltage and power flow. The property of energy storage in capacitors was exploited as dynamic memory in early digital computers, and still is in modern DRAM.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1006 2021-05-01 00:19:35
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,002
Re: Miscellany
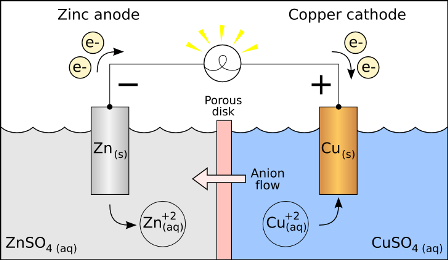
984) Electrochemistry
Electrochemistry, branch of chemistry concerned with the relation between electricity and chemical change. Many spontaneously occurring chemical reactions liberate electrical energy, and some of these reactions are used in batteries and fuel cells to produce electric power. Conversely, electric current can be utilized to bring about many chemical reactions that do not occur spontaneously. In the process called electrolysis, electrical energy is converted directly into chemical energy, which is stored in the products of the reaction. This process is applied in refining metals, in electroplating, and in producing hydrogen and oxygen from water. The passage of electricity through a gas generally causes chemical changes, and this subject forms a separate branch of electrochemistry.
Electrochemistry is the branch of physical chemistry concerned with the relationship between electrical potential, as a measurable and quantitative phenomenon, and identifiable chemical change, with either electrical potential as an outcome of a particular chemical change, or vice versa. These reactions involve electrons moving between electrodes via an electronically-conducting phase (typically, but not necessarily, an external electrical circuit such as in electrolessplating), separated by an ionically-conducting and electronically insulating electrolyte (or ionic species in a solution).
When a chemical reaction is effected by a potential difference, as in electrolysis, or if electrical potential results from a chemical reaction as in a battery or fuel cell, it is called an electrochemical reaction. Unlike chemical reactions, in electrochemical reactions electrons (and necessarily resulting ions), are not transferred directly between molecules, but via the aforementioned electronically- and ionically-conducting circuits, respectively. This phenomenon is what distinguishes an electrochemical reaction from a chemical reaction.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1007 2021-05-02 01:17:24
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,002
Re: Miscellany
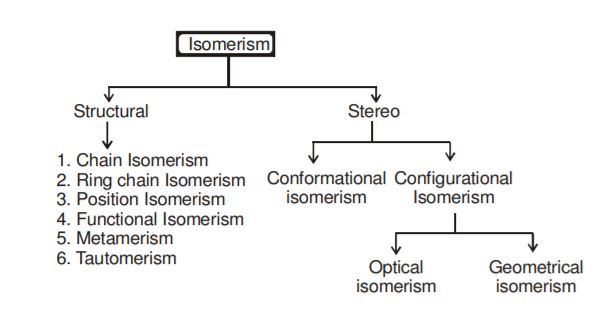
985) Isomerism
Isomerism, the existence of molecules that have the same numbers of the same kinds of atoms (and hence the same formula) but differ in chemical and physical properties. The roots of the word isomer are Greek—isos plus meros, or “equal parts.” Stated colloquially, isomers are chemical compounds that have the same parts but are nonetheless not the same. To make a crude analogy, two bracelets, each consisting of five red and five green beads, could be arranged in many different isomeric forms, depending on the order of the colours. Each bracelet would have the same parts—that is, the five red and five green beads—but each variation would be different. One could also imagine combinations of those same beads in which pendant chains were attached to a bracelet in a variety of ways. One might imagine two bracelets of the same red-green order but with identical chains attached in different orientations. Such structures also would be analogous to isomers. In a more subtle analogy, one’s hands can be seen as isomeric. Each hand possesses the same kinds of fingers, but a right hand can never be superimposed perfectly on a left hand; they are different.
Timing and energy are also factors in isomerism. Molecules are mobile entities, undergoing all sorts of rotational motions that change their shapes, and those motions require energy. Thus, some molecules can be the same on one timescale or set of energy conditions but different, or isomeric, on others. Finally, an isomer must be an energy minimum; it must lie in an energy well.
There are two general types of isomers. Constitutional isomers are molecules of different connectivity—analogous to simple bracelets in which the order of red and green beads is different. The second type is stereoisomers. In stereoisomers the connectivity is the same, but the parts are oriented differently in space.
Constitutional Isomers
Isomers that differ in connectivity are called constitutional (sometimes structural) isomers. They have the same parts, but those parts are attached to each other differently. The bracelets of red and green beads mentioned above are analogous to constitutional isomers. The simplest hydrocarbons—methane (CH4), ethane (CH3CH3), and propane (CH3CH2CH3)—have no constitutional isomers, as there is no other way to connect the carbons and hydrogens of these molecules consistent with the tetravalency of carbon and the univalency of hydrogen.
However, there are two different butanes, C4H10, and these two molecules, called butane and isobutane, are constitutional isomers. They are different molecules with different chemical and physical properties. Butane has its four carbon atoms bonded in a continuous chain. Isobutane has a branched structure.
The number of possible constitutional isomers increases greatly with the number of available atoms. There are only two butanes, but there are three pentanes (C5H12), 18 octanes (C8H18), and no fewer than 366,319 constitutional isomers of the hydrocarbon containing 20 carbon atoms and 42 hydrogens.
Stereoisomers
Generally defined, stereoisomers are isomers that have the same composition (that is, the same parts) but that differ in the orientation of those parts in space. There are two kinds of stereoisomers: enantiomers and diastereomers. Enantiomers are mirror images, like one’s hands, and diastereomers are everything else. However, as is stated above, timescale and energy are important. In order to understand these considerations, it is helpful first to consider a special kind of stereoisomer, the conformational isomer.
Conformational isomers
Methane (CH4) is a molecule that is a perfect tetrahedron, and so it is commonly said that no isomerism is possible with methane. However, the carbon-hydrogen bonds of methane constantly vibrate and bend, so that on very short timescales an apparent isomerism can be said to exist. But these structures are not energy minima, and so they do not qualify as isomers.
As complexity increases, isomerism induced by rotations about bonds becomes a bigger factor. In ethane (CH3CH3), for example, both carbons are approximately tetrahedral. Thus, there are two limiting structures—staggered ethane, in which the carbon-hydrogen bonds are as far apart as possible, and eclipsed ethane, in which the bonds are as close as possible. These two structures are certainly not the same. Perhaps the best view in which to see the difference is a “Newman projection” (named after American chemist Melvin Newman) in which one sights down the carbon-carbon bond and focuses on the positions of the six hydrogens. In a Newman projection, the front carbon is located at the intersection of the bonds to the three attached hydrogen atoms, and the back carbon is an exploded circle, with the attached bonds emanating from the circumference of the circle.
Immediately, questions of energy arise: Which of the two structures is lower in energy and therefore more stable? The staggered form is lower in energy because in the eclipsed form electrons in carbon-hydrogen bonds on the opposite side of the carbon-carbon bond repel each other. The strain that this repulsion creates increases the potential energy of the eclipsed form. The energy difference is not large, about 3 kilocalories per mole (kcal/mol).
If one plots the energy change as ethane rotates around the carbon-carbon bond, another difficulty is revealed. Like the vibrational and rotational “isomers” of methane mentioned above, eclipsed ethane (E) is not even an energy minimum; it is an energy maximum, a transition state between two staggered ethanes (S). Therefore, ethane, like methane, really has only one form.
If substitutions are made in the ethane molecule—for instance, exchanging some of the hydrogen atoms for deuterium atoms to make 1,2-dideuterioethane—isomeric staggered forms become possible. These staggered forms, called “anti” and “gauche,” of 1,2-dideuterioethane are different but are interconverted through rotations around the central carbon-carbon bond and are called “conformational isomers.”
Whether these different ethanes can be separated depends only on the amount of energy necessary to convert one into the other—that is, to rotate the molecule about the carbon-carbon bond. In the case of 1,2-dideuterioethane, the energy barrier separating the conformational isomers is only 3 kcal/mol, far too low to make them separable under normal conditions.
As stated above in the section Constitutional isomers, butane has two constitutional isomers, butane and isobutane. Isobutane has no conformational isomers, but butane is closely analogous to 1,2-dideuterioethane, in that a pair of anti and gauche conformational isomers is possible for that molecule. Because a methyl group (CH3) is much larger than hydrogen or deuterium, the plot of energy versus rotational angle is more complex for butane than it is for ethane or 1,2-dideuterioethane.
Ring compounds often have a particularly rich set of conformational isomers. By far the most interesting of the ring compounds is cyclohexane (C6H12), here with cyclopropane (C3H6).
Planar cyclohexane contains 12 pairs of eclipsed carbon-hydrogen bonds and is destabilized by these eclipsing interactions, or torsional strain. There are other problems with the planar form. In a flat hexagon, the C―C―C angles must be 120°, quite far from the optimum for tetrahedral carbons (usually quoted as approximately 109.5°; in fact, the real optimum value for cyclohexane is about 112°, the C―C―C angle in propane). In any event, the planar form of cyclohexane is severely destabilized by both torsional and angle strain.
Lower-energy forms can be made as the cyclohexane ring distorts from planarity. This distortion involves no more than rotations about carbon-carbon bonds, just as occurs in ethane or any other acyclic alkane. The energy minimum for cyclohexane is the chair form. In the chair form, carbon-hydrogen bonds are nicely staggered, and the C―C―C bond angle is 111.5°, very close indeed to the optimum.
Note that there are two kinds of carbon-hydrogen bonds in chair cyclohexane. One set of six parallel carbon-hydrogen bonds is perpendicular to the surface on which the chair apparently sits (these are the axial bonds). The other set of six is roughly in the plane of the ring (equatorial bonds). All six axial hydrogens are equivalent, as are all six equatorial bonds.
Rotations about carbon-carbon bonds interconvert two equally energetic chair forms. This process is colloquially called a ring “flip.”
T he axial hydrogens in one chair become the equatorial hydrogens in the other as the ring “flips” from one chair to the other. An implication of this change is that there is more than one monosubstituted cyclohexane. In methylcyclohexane, for example, there are two conformational isomers, one with the methyl group axial and one with the methyl group equatorial. The two interconvert through ring flipping.
Which isomer is more stable? For the axial methyl isomer the methyl group interacts unfavourably with nearby methylene groups. This destabilizing interaction is not present in the equatorial isomer. Axial methylcyclohexane is less stable (higher energy) than the equatorial isomer by 1.8 kcal/mol.
Cis and trans forms
The examples presented so far have concentrated on the simplest organic molecules, the alkanes. However, stereoisomers crop up in many of the other structural types of organic chemistry. For example, in the alkenes, two versions of 2-butene exist. They are traditionally called cis-2-butene and trans-2-butene or, in slightly more modern terms, (Z)- and (E)-2-butene. The Z and E stand for the German words for “together” (zusammen) and “apart” (entgegen). In principle, cis- and trans-2-butene are conformational isomers; in theory, they could be interconverted by a simple rotation about the central double bond. However, the practical world intrudes into principle, because this rotation would require about 66 kcal/mol, an amount of energy not available under normal conditions.
Other kinds of cis and trans isomers exist in ring compounds. For example, cis- and trans-1,2-dimethylcyclopropane are stereoisomers.
This time there is no imaginable rotation about bonds that can equilibrate the two isomers, so these two molecules are not conformational isomers. In addition, because three points determine a plane, the three-membered ring of cyclopropane is necessarily flat; there is no possible out-of-plane distortion.
On the other hand, as is described in the section Conformational isomers, cyclohexane is quite flexible, with one energy-minimum chair form ring-flipping into another through rotations around carbon-carbon bonds. Consider the possible isomers of cis- and trans-1,4-dimethylcyclohexane. If one methyl group is in the lower-energy equatorial position, then the cis compound, with both methyl groups on the same side of the ring, can be made only by placing the second methyl group in the higher-energy axial position. In constructing the trans compound, the second methyl must be placed in the equatorial position. But what happens when the ring flips? Remember that in a ring flip all axial positions become equatorial and vice versa. In the case of the cis-1,4-dimethylcyclohexane isomer, the equatorial-axial version flips into itself, as the axial methyl becomes equatorial and the equatorial methyl becomes axial. The two versions of cis-1,4-dimethylcyclohexane therefore have the same energy.
When the trans isomer flips, however, an equivalent structure is not formed, because each of the two equatorial methyl groups becomes axial. As an equatorial methyl group is more stable than an axial methyl group by 1.74 kcal/mol, the diaxial form would be less stable than the diequatorial form by about twice that amount, or 3.5 kcal/mol. In practice, this energy difference means that far less than 1 percent of the trans-1,4-dimethylcyclohexane present at equilibrium is in the less stable form.
Enantiomers
In the introduction of this article, it is stated that one’s hands are related but not the same. Exactly how are they related? Each has a thumb, little finger, and so on. Yet the hands truly are not the same, for they are not superimposable. In effect, the left and right hand are mirror images; the left hand is superimposable on the mirror image of the right hand but not on the right hand itself. Some molecules are related to their mirror images in the same manner. Such molecules are, by definition, stereoisomers, and they go by the special name of enantiomers.
The phenomenon of handedness, or “chirality,” is perhaps the most important phenomenon related to isomerism. Many objects in the macroscopic world are chiral. A scissors and a screw are familiar chiral objects; they are not superimposable on their mirror images. But related objects, a simple knife or a nail, for example, are superimposable on their mirror images and thus are not chiral. The simple test for chirality is the same for objects as it is for molecules: Is the object (or molecule) superimposable on its mirror image? If it is not, it is chiral. If it is, the object is achiral (not chiral).
If each hydrogen atom in a molecule of methane were replaced with a different atom, one possible result would be bromochlorofluoroiodomethane (CBrClFI). The mirror images of this molecule are not superimposable. There are definitely two enantiomers of this molecule.
The molecule, with four different atoms (fluorine, chlorine, bromine, and iodine) attached to what is called a stereogenic carbon, is identified as chiral. Several questions and problems now appear. How are the two enantiomers different physically and chemically? On a more mundane level, how can one specify in words one of the two enantiomers? How is one to differentiate the right-handed molecule of bromochlorofluoroiodomethane from the left-handed version, for example? A rather complex protocol has been devised, and it is worth giving a simplified version of it here. In the so-called Cahn-Ingold-Prelog (CIP) protocol (named after British chemists Robert Cahn and Sir Christopher Ingold and Swiss chemist Vladimir Prelog), one first assigns priorities to the four atoms attached to the stereogenic atom, in this case the carbon at the centre of the tetrahedron. The atom of lowest atomic number is given the lowest priority, 4. In this case that atom is the fluorine (atomic number 9). The atom with the highest atomic number, iodine (atomic number 53), gets the highest priority, 1. Chlorine (atomic number 17) is priority 3, and bromine (atomic number 35) is 2. In the second step of the protocol, one sights down the bond from carbon to the lowest priority (4). Finally, one connects atoms 1 to 2 to 3 with an arrow. If that arrow is clockwise, the molecule is called an R enantiomer. If the arrow is counterclockwise, the molecule is called an S enantiomer. (R comes from rectus, the Latin word for “right,” and S comes from sinister, the Latin word for “left.”) The CIP protocol is simple in the example chosen, but very often more-detailed rules must be applied to assign R and S.
In summary, a molecule with one stereogenic carbon can be either R or S—in a sense, “left-handed” or “right-handed.” One set of such molecules is of profound biological importance: the L-amino acids. The business of the body—biological function—is controlled by proteins, which are polymers of only 20 possible amino acids. All the amino acids but the achiral glycine contain a single stereogenic carbon, and all of them but glycine are left-handed. It is not clear why this sense was selected through evolution; perhaps that selection followed from an initial accident selecting for left-handed amino acids. Extraterrestrial life (should there be any) may well be either left- or right-handed.
All physical attributes of enantiomers are identical except for one rather arcane property: the direction of rotation of the plane of plane-polarized light. If one enantiomer rotates the plane in one direction (say, clockwise) as one views the beam, the other enantiomer will rotate the plane by the same amount in the other direction (in this case, counterclockwise). For example, the rotations of standard solutions of (R)- and (S)-2-aminobutane are −7.4 and +7.4 degrees, respectively.
The chemical properties of enantiomers are also identical, as long as the other reacting molecule is achiral. Chemical reactions of enantiomers are analogous to a hand grasping a ball. If the ball is featureless, one’s right and left hands (enantiomers) have exactly the same interactions with the ball. However, if the ball has the word “Label” written on it and is thus a chiral object, that labeled ball will be optically active; it will mimic a single enantiomer. The left and right hands will interact differently with the labeled ball. The little finger of the right hand will approach the capital “L” of “Label” while the thumb will approach the lowercase “l.” For the left hand, the interactions will be just the opposite: the little finger will approach the lowercase “l” and the thumb the capital “L.”
These interactions, as well as a molecular counterpart in which a pair of enantiomers, (R)- and (S)-bromochlorofluoroiodomethane, interacts with a single enantiomer, (S)-2-chlorobutane. In the R enantiomer, the bromine atom approaches the methyl group (―CH3), and the iodine atom approaches the ethyl group (―CH2CH3). In the S enantiomer, the bromine atom approaches the ethyl group, and the iodine atom approaches the methyl group. The figure shows only one of the many possible interactions, every one of which is different.
Stereoisomers of more complex molecules
An atom is stereogenic if switching any two atoms or groups of atoms that are bound to it results in a pair of stereoisomers. So far, molecules with no or only one stereogenic atom have been discussed. Very often the situation is more complex; indeed, there can be several stereogenic atoms in a molecule. A molecule with only one stereogenic atom has only two stereoisomers—the R and S enantiomers. If there are two stereogenic atoms in a molecule, both can be either R or S. Thus, there are four possibilities: RR, SS, RS, and SR. Three stereogenic atoms would lead to eight possibilities: RRR, RRS, RSR, SRR, SSR, SRS, RSS, and SSS. The formula for finding the maximum number of stereoisomers X is X = 2n, where n is the number of stereogenic atoms in the molecule.
The formula X = 2n reliably gives the maximum number of stereoisomers, but in situations of high symmetry it fails to give the real number. For example, it fails for 2,3-dichlorobutane [H2Cl2(CH3)2]. One pair of enantiomers, SS and RR, does appear. But the other combination gives an identical “pair” of SR compounds. This happens because 2,3-dichlorobutane contains an internal plane of symmetry. The result is fewer than the maximum number of stereoisomers predicted by the formula. Three stereoisomers are possible: one pair of enantiomers (A and B) and an achiral molecule C, called a “meso compound.” A meso compound is an achiral molecule that nonetheless contains a stereogenic atom.
In order to find molecules that are enantiomers, one must draw the mirror image of the original and see if they are superimposable. That is the only absolutely safe way to do it. It might be suggested that there is something special about a molecule containing four different groups attached to one carbon. The question now is whether the presence of such an atom (usually carbon) is either sufficient or necessary for the molecule to be chiral. The answer is no in each case. Although looking for such carbons is a good way to start a search for enantiomers, there is no way to avoid the ultimate necessity of writing out the mirror image and checking for superimposability. To test the question of sufficiency, for example, look at the meso compound C of 2,3-dimethylbutane. It certainly does contain a carbon attached to four different groups. The indicated carbon C2 is attached to hydrogen, a methyl group, a chlorine, and the rest of the molecule. Yet C is achiral.
There are many compounds whose molecular architecture makes them chiral but that do not contain an atom attached to four different groups. One classic example is hexahelicene, a molecule composed of six benzene rings connected to each other. The molecule coils in the form of a spiral so that the atoms of the last ring do not impinge on the atoms of the first ring. The result is a left- or right-handed screw form, and the molecule is chiral.
Diastereomers
Cyclohexane is achiral, as are both axial and equatorial methylcyclohexane. The two methylcyclohexanes (axial and equatorial methyl group) are stereoisomers, but they are not enantiomers. Such isomers—stereoisomers that are not mirror images—are called diastereomers. The molecules cis- and trans-2-butene are diastereomers, as are cis- and trans-1,2-dimethylcyclopropane. However, in dimethylcyclopropane, the cis compound is achiral, but the trans compound exists as a pair of enantiomers. Therefore, there are three stereoisomers of 1,2-dimethylcyclopropane.
Chirality In Natural And Synthetic Materials
Much of the function of biologically active molecules depends on fit, on an exquisite lock-and-key connection between molecules that allows some biochemical activity to turn on or off. In the evolutionary process, chirality—handedness—came to be a critical part of the lock-and-key fit. The principle behind this notion is simple. A left-hand glove does not fit a right hand, and, in the same way, one member of an enantiomeric pair of molecules might fit another molecule whereas the other member would not. The specificity of biological reactions and their dependence on fit have both benefits and penalties.
As presented above, combinations of 20 possible amino acids make up proteins, and the proteins are responsible for biological function. Precise fit is critical. The lock-and-key mechanism has evolved to be extremely precise, and much of that precision is the result of the handedness of the amino acids. Precision is one of the benefits of specificity.
A classic and tragic example of the penalties of specificity is thalidomide, a compound originally marketed as a sedative in Europe in 1956. Thalidomide contains a stereogenic carbon, and therefore the compound can exist in both R and S forms. Most synthesizing procedures generate equal mixtures of enantiomers (i.e., equal amounts of the R and S forms—a racemic mixture); special care must be taken to make a pure enantiomer, and the company involved in the original promotion of thalidomide saw no reason to bear the cost of this process. The result was the marketing of a racemic mixture of the R and S forms. (S)-Thalidomide turned out to be a powerful teratogen: it causes all manner of external and internal abnormalities in fetuses if it is given to pregnant women in the first trimester. The R enantiomer is far more benign—although even it is dangerous, as the R form racemizes under physiological conditions and thus produces some of the dangerous S enantiomer.
In the example of thalidomide, the societal consequences of a bad molecular fit, as it were, have been instructional. There has been much pressure on the drug industry worldwide to do far more thorough testing and follow much better scientific procedures than was the case for thalidomide. There has also been demand for the development of the synthetic techniques necessary to produce enantiomerically pure drugs. Most syntheses in the laboratory begin with achiral materials, and the complex end products of these synthetic procedures are built up through sequences of reactions. Unless an optically active agent is introduced or a separation into enantiomers deliberately performed, any chiral end products of the synthetic sequence will be racemic mixtures. The example of thalidomide and many other similar, if not so tragic, examples have revealed the desirability of enantiospecific syntheses in drug research. Of course, it will always be necessary to test both enantiomers in case the physiological racemization discovered with thalidomide is repeated with another compound. Ironically, the notorious thalidomide is making a comeback, as it turns out to be an effective agent against several extremely difficult diseases, including leprosy and multiple myeloma. These unexpected developments are further examples of the benefits of enantiospecific synthesis.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1008 2021-05-03 00:06:46
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,002
Re: Miscellany
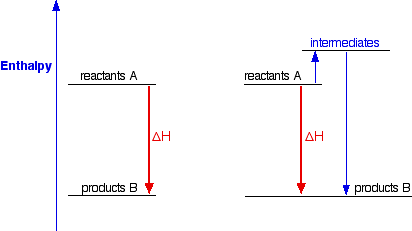
986) Enthalpy
Enthalpy, the sum of the internal energy and the product of the pressure and volume of a thermodynamic system. Enthalpy is an energy-like property or state function—it has the dimensions of energy (and is thus measured in units of joules or ergs), and its value is determined entirely by the temperature, pressure, and composition of the system and not by its history. In symbols, the enthalpy, H, equals the sum of the internal energy, E, and the product of the pressure, P, and volume, V, of the system: H = E + PV.
According to the law of energy conservation, the change in internal energy is equal to the heat transferred to, less the work done by, the system. If the only work done is a change of volume at constant pressure, the enthalpy change is exactly equal to the heat transferred to the system. When energy needs to be added to a material to change its phase from a liquid to a gas, that amount of energy is called the enthalpy (or latent heat) of vaporization and is expressed in units of joules per mole. Other phase transitions have similar associated enthalpy changes, such as the enthalpy (or latent heat) of fusion for changes from a solid to a liquid. As with other energy functions, it is neither convenient nor necessary to determine absolute values of enthalpy. For each substance, the zero-enthalpy state can be some convenient reference state.
Enthalpy is a property of a thermodynamic system, and is defined as the sum of the system's internal energy and the product of its pressure and volume. It is a state function used in many measurements in chemical, biological, and physical systems at a constant pressure, that is conveniently provided by the large ambient atmosphere. The pressure–volume term expresses the work required to establish the system's physical dimensions, i.e. to make room for it by displacing its surroundings. As a state function, enthalpy depends only on the final configuration of internal energy, pressure, and volume, not on the path taken to achieve it.
The unit of measurement for enthalpy in the International System of Units (SI) is the joule. Other historical conventional units still in use include the calorie and the British thermal unit (BTU).
The total enthalpy of a system cannot be measured directly because the internal energy contains components that are unknown, not easily accessible, or are not of interest in thermodynamics. In practice, a change in enthalpy is the preferred expression for measurements at constant pressure, because it simplifies the description of energy transfer. When matter transfer into or out of the system is also prevented, at constant pressure the enthalpy change equals the energy exchanged with the environment by heat.
In chemistry, the standard enthalpy of reaction is the enthalpy change when reactants in their standard states (p = 1 bar, T = 298 K) change to products in their standard states. This quantity is the standard heat of reaction at constant pressure and temperature, but it can be measured by calorimetric methods in which the temperature does vary, provided that the initial and final pressure and temperature correspond to the standard state. The value does not depend on the path from initial to final state since enthalpy is a state function.
Calibration of enthalpy changes requires a reference point. Enthalpies for chemical substances at constant pressure usually refer to standard state: most commonly 1 bar (100 kPa) pressure. Standard state does not strictly specify a temperature, but expressions for enthalpy generally reference the standard heat of formation at 25 °C (298 K). For endothermic (heat-absorbing) processes, the change ΔH is a positive value; for exothermic (heat-releasing) processes it is negative.
The enthalpy of an ideal gas is independent of its pressure, and depends only on its temperature, which correlates to its internal energy. Real gases at common temperatures and pressures often closely approximate this behavior, which simplifies practical thermodynamic design and analysis.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1009 2021-05-04 00:08:34
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,002
Re: Miscellany
987) Cholera
Overview
Cholera is a bacterial disease usually spread through contaminated water. Cholera causes severe diarrhea and dehydration. Left untreated, cholera can be fatal within hours, even in previously healthy people.
Modern sewage and water treatment have virtually eliminated cholera in industrialized countries. But cholera still exists in Africa, Southeast Asia and Haiti. The risk of a cholera epidemic is highest when poverty, war or natural disasters force people to live in crowded conditions without adequate sanitation.
Cholera is easily treated. Death from severe dehydration can be prevented with a simple and inexpensive rehydration solution.
Symptoms
Most people exposed to the cholera bacterium (Vibrio cholerae) don't become ill and don't know they've been infected. But because they shed cholera bacteria in their stool for seven to 14 days, they can still infect others through contaminated water.
Most cases of cholera that cause symptoms cause mild or moderate diarrhea that's often hard to tell apart from diarrhea caused by other problems. Others develop more-serious signs and symptoms of cholera, usually within a few days of infection.
Symptoms of cholera infection can include:
• Diarrhea. Cholera-related diarrhea comes on suddenly and can quickly cause dangerous fluid loss — as much as a quart (about 1 liter) an hour. Diarrhea due to cholera often has a pale, milky appearance that resembles water in which rice has been rinsed.
• Nausea and vomiting. Vomiting occurs especially in the early stages of cholera and can last for hours.
• Dehydration. Dehydration can develop within hours after cholera symptoms start and range from mild to severe. A loss of 10% or more of body weight indicates severe dehydration.
Signs and symptoms of cholera dehydration include irritability, fatigue, sunken eyes, a dry mouth, extreme thirst, dry and shriveled skin that's slow to bounce back when pinched into a fold, little or no urinating, low blood pressure, and an irregular heartbeat.
Dehydration can lead to a rapid loss of minerals in your blood that maintain the balance of fluids in your body. This is called an electrolyte imbalance.
Electrolyte imbalance
An electrolyte imbalance can lead to serious signs and symptoms such as:
• Muscle cramps. These result from the rapid loss of salts such as sodium, chloride and potassium.
• Shock. This is one of the most serious complications of dehydration. It occurs when low blood volume causes a drop in blood pressure and a drop in the amount of oxygen in your body. If untreated, severe hypovolemic shock can cause death in minutes.
When to see a doctor
The risk of cholera is slight in industrialized nations. Even in areas where it exists you're not likely to become infected if you follow food safety recommendations. Still, cases of cholera occur throughout the world. If you develop severe diarrhea after visiting an area with active cholera, see your doctor.
If you have diarrhea, especially severe diarrhea, and think you might have been exposed to cholera, seek treatment right away. Severe dehydration is a medical emergency that requires immediate care.
Causes
A bacterium called Vibrio cholerae causes cholera infection. The deadly effects of the disease are the result of a toxin the bacteria produces in the small intestine. The toxin causes the body to secrete enormous amounts of water, leading to diarrhea and a rapid loss of fluids and salts (electrolytes).
Cholera bacteria might not cause illness in all people who are exposed to them, but they still pass the bacteria in their stool, which can contaminate food and water supplies.
Contaminated water supplies are the main source of cholera infection. The bacterium can be found in:
• Surface or well water. Contaminated public wells are frequent sources of large-scale cholera outbreaks. People living in crowded conditions without adequate sanitation are especially at risk.
• Seafood. Eating raw or undercooked seafood, especially shellfish, that comes from certain places can expose you to cholera bacteria. Most recent cases of cholera in the United States have been traced to seafood from the Gulf of Mexico.
• Raw fruits and vegetables. Raw, unpeeled fruits and vegetables are a frequent source of cholera infection in areas where there's cholera. In developing countries, uncomposted manure fertilizers or irrigation water containing raw sewage can contaminate produce in the field.
• Grains. In regions where cholera is widespread, grains such as rice and millet that are contaminated after cooking and kept at room temperature for several hours can grow cholera bacteria.
Risk factors
Everyone is susceptible to cholera, with the exception of infants who get immunity from nursing mothers who have previously had cholera. Still, certain factors can make you more vulnerable to the disease or more likely to have severe signs and symptoms.
Risk factors for cholera include:
• Poor sanitary conditions. Cholera is more likely to flourish in situations where a sanitary environment — including a safe water supply — is difficult to maintain. Such conditions are common to refugee camps, impoverished countries, and areas afflicted by famine, war or natural disasters.
• Reduced or nonexistent stomach acid. Cholera bacteria can't survive in an acidic environment, and ordinary stomach acid often serves as a defense against infection. But people with low levels of stomach acid — such as children, older adults, and people who take antacids, H-2 blockers or proton pump inhibitors — lack this protection, so they're at greater risk of cholera.
• Household exposure. You're at increased risk of cholera if you live with someone who has the disease.
• Type O blood. For reasons that aren't entirely clear, people with type O blood are twice as likely to develop cholera compared with people with other blood types.
• Raw or undercooked shellfish. Although industrialized nations no longer have large-scale cholera outbreaks, eating shellfish from waters known to harbor the bacteria greatly increases your risk.
Complications
Cholera can quickly become fatal. In the most severe cases, the rapid loss of large amounts of fluids and electrolytes can lead to death within hours. In less extreme situations, people who don't receive treatment can die of dehydration and shock hours to days after cholera symptoms first appear.
Although shock and severe dehydration are the worst complications of cholera, other problems can occur, such as:
• Low blood sugar (hypoglycemia). Dangerously low levels of blood sugar (glucose) — the body's main energy source — can occur when people become too ill to eat. Children are at greatest risk of this complication, which can cause seizures, unconsciousness and even death.
• Low potassium levels. People with cholera lose large quantities of minerals, including potassium, in their stools. Very low potassium levels interfere with heart and nerve function and are life-threatening.
• Kidney failure. When the kidneys lose their filtering ability, excess amounts of fluids, some electrolytes and wastes build up in the body — a potentially life-threatening condition. In people with cholera, kidney failure often accompanies shock.
Prevention
Cholera is rare in the United States with the few cases related to travel outside the U.S. or to contaminated and improperly cooked seafood from the Gulf Coast waters.
If you're traveling to areas known to have cholera, your risk of contracting the disease is extremely low if you follow these precautions:
• Wash your hands with soap and water frequently, especially after using the toilet and before handling food. Rub soapy, wet hands together for at least 15 seconds before rinsing. If soap and water aren't available, use an alcohol-based hand sanitizer.
• Drink only safe water, including bottled water or water you've boiled or disinfected yourself. Use bottled water even to brush your teeth.
Hot beverages are generally safe, as are canned or bottled drinks, but wipe the outside before you open them. Don't add ice to your drinks unless you made it yourself using safe water.
• Eat food that's completely cooked and hot and avoid street vendor food, if possible. If you do buy a meal from a street vendor, make sure it's cooked in your presence and served hot.
• Avoid sushi, as well as raw or improperly cooked fish and seafood of any kind.
• Stick to fruits and vegetables that you can peel yourself, such as bananas, oranges and avocados. Stay away from salads and fruits that can't be peeled, such as grapes and berries.
Cholera vaccine
For adults traveling from the United States to areas affected by cholera, a vaccine called Vaxchora is available in the United States. It is a liquid dose taken by mouth at least 10 days before travel.
A few other countries offer oral vaccines as well. Contact your doctor or local office of public health for more information about these vaccines. Even with the vaccine, it's important to take the above precautions to prevent cholera.
Diagnosis
Although signs and symptoms of severe cholera can be unmistakable in areas where it's common, the only way to confirm a diagnosis is to identify the bacteria in a stool sample.
Rapid cholera dipstick tests enable doctors in remote areas to quickly confirm a cholera diagnosis. Quick confirmation helps to decrease death rates at the start of cholera outbreaks and leads to earlier public health interventions for outbreak control.
Treatment
Cholera requires immediate treatment because the disease can cause death within hours.
• Rehydration. The goal is to replace lost fluids and electrolytes using a simple rehydration solution, oral rehydration salts (ORS). The ORS solution is available as a powder that can be made with boiled or bottled water.
Without rehydration, approximately half the people with cholera die. With treatment, fatalities drop to less than 1%.
• Intravenous fluids. Most people with cholera can be helped by oral rehydration alone, but severely dehydrated people might also need intravenous fluids.
• Antibiotics. While not a necessary part of cholera treatment, some antibiotics can reduce cholera-related diarrhea and shorten how long it lasts in severely ill people.
• Zinc supplements. Research has shown that zinc might decrease diarrhea and shorten how long it lasts in children with cholera.
Seek immediate medical care if you develop severe diarrhea or vomiting and are in or have very recently returned from a country where cholera occurs.
If you believe you've been exposed to cholera, but your symptoms are not severe, call your family doctor. Be sure to say that you suspect your illness may be cholera.
Here's some information to help you get ready for your appointment.
When you make your appointment, ask if there are restrictions you need to follow before your visit.
Make a list of:
• Your symptoms, when they began and how severe they are
• Recent exposure to possible sources of infection, particularly if you've traveled abroad recently
• Key medical information, including other conditions for which you're being treated
• All medications, vitamins or other supplements you take, including doses
• Questions to ask your doctor
Some questions to ask your doctor about cholera include:
• Are there other possible causes for my symptoms?
• What tests do I need?
• What treatment approach do you recommend?
• How soon after I begin treatment will I begin to feel better?
• How long do you expect a full recovery to take?
• When can I return to work or school?
• Am I at risk of any long-term complications from cholera?
• Am I contagious? How can I reduce my risk of passing my illness to others?
What to expect from your doctor
Your doctor is likely to ask questions, such as:
• Have you had watery diarrhea? How severe?
• Is there anything else unusual about the appearance of your stools?
• Have you been vomiting?
• Have you experienced symptoms of dehydration, such as intense thirst, muscle cramps or fatigue?
• Have you been able to keep down any food or liquid?
• Have you recently eaten raw shellfish, such as oysters?
• Are you pregnant?
• What is your blood type, if you know?
What you can do in the meantime
Stay well hydrated. For diarrhea and vomiting that may be cholera-related, use an oral rehydration solution.
In most developing countries, you can buy powdered packets of oral rehydration salts (ORS) originally developed by the World Health Organization to treat diarrhea and dehydration in infants with cholera. Stir the powder into clean drinking or boiled water according to the package directions.
If no oral rehydration solutions are available, make your own by combining 1 quart (about 1 liter) of bottled or boiled water with 6 level teaspoons (about 30 milliliters) of table sugar and 1/2 level teaspoon (about 2.5 milliliters) of table salt.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1010 2021-05-05 00:28:33
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,002
Re: Miscellany
988) Cerebral palsy
Overview
Cerebral palsy is a group of disorders that affect movement and muscle tone or posture. It's caused by damage that occurs to the immature brain as it develops, most often before birth.
Signs and symptoms appear during infancy or preschool years. In general, cerebral palsy causes impaired movement associated with abnormal reflexes, floppiness or rigidity of the limbs and trunk, abnormal posture, involuntary movements, unsteady walking, or some combination of these.
People with cerebral palsy can have problems swallowing and commonly have eye muscle imbalance, in which the eyes don't focus on the same object. They also might have reduced range of motion at various joints of their bodies due to muscle stiffness.
Cerebral palsy's effect on function varies greatly. Some affected people can walk; others need assistance. Some people show normal or near-normal intellect, but others have intellectual disabilities. Epilepsy, blindness or deafness also might be present.
Symptoms
Signs and symptoms can vary greatly. Movement and coordination problems associated with cerebral palsy include:
• Variations in muscle tone, such as being either too stiff or too floppy
• Stiff muscles and exaggerated reflexes (spasticity)
• Stiff muscles with normal reflexes (rigidity)
• Lack of balance and muscle coordination (ataxia)
• Tremors or involuntary movements
• Slow, writhing movements
• Delays in reaching motor skills milestones, such as pushing up on arms, sitting up or crawling
• Favoring one side of the body, such as reaching with one hand or dragging a leg while crawling
• Difficulty walking, such as walking on toes, a crouched gait, a scissors-like gait with knees crossing, a wide gait or an asymmetrical gait
• Excessive drooling or problems with swallowing
• Difficulty with sucking or eating
• Delays in speech development or difficulty speaking
• Learning difficulties
• Difficulty with fine motor skills, such as buttoning clothes or picking up utensils
• Seizures
Cerebral palsy can affect the whole body, or it might be limited primarily to one limb or one side of the body. The brain disorder causing cerebral palsy doesn't change with time, so the symptoms usually don't worsen with age.
However, as the child gets older, some symptoms might become more or less apparent. And muscle shortening and muscle rigidity can worsen if not treated aggressively.
Brain abnormalities associated with cerebral palsy might also contribute to other neurological problems, including:
• Difficulty seeing and hearing
• Intellectual disabilities
• Seizures
• Abnormal touch or pain perceptions
• Oral diseases
• Mental health conditions
• Urinary incontinence
When to see a doctor
It's important to get a prompt diagnosis for a movement disorder or delays in your child's development. See your child's doctor if you have concerns about episodes of loss of awareness of surroundings or of abnormal bodily movements, abnormal muscle tone, impaired coordination, swallowing difficulties, eye muscle imbalance or other developmental issues.
Causes
Cerebral palsy is caused by an abnormality or disruption in brain development, most often before a child is born. In many cases, the cause isn't known. Factors that can
lead to problems with brain development include:
• Gene mutations that lead to abnormal development
• Maternal infections that affect the developing fetus
• Fetal stroke, a disruption of blood supply to the developing brain
• Bleeding into the brain in the womb or as a newborn
• Infant infections that cause inflammation in or around the brain
• Traumatic head injury to an infant from a motor vehicle accident or fall
• Lack of oxygen to the brain related to difficult labor or delivery, although birth-related asphyxia is much less commonly a cause than historically thought
Risk factors
A number of factors are associated with an increased risk of cerebral palsy.
Maternal health
Certain infections or toxic exposures during pregnancy can significantly increase cerebral palsy risk to the baby. Infections of particular concern include:
• Cytomegalovirus. This common virus causes flu-like symptoms and can lead to birth defects if a mother has her first active infection during pregnancy.
• German measles (rubella). This viral infection can be prevented with a vaccine.
• Herpes. This can be passed from mother to child during pregnancy, affecting the womb and placenta. Inflammation triggered by infection can damage the unborn baby's developing nervous system.
• Syphilis. This is a sexually transmitted bacterial infection.
• Toxoplasmosis. This infection is caused by a parasite found in contaminated food, soil and the feces of infected cats.
• Zika virus infection. Infants for whom maternal Zika infection causes their head size to be smaller than normal (microcephaly) can develop cerebral palsy.
• Other conditions. Other conditions that can increase the risk of cerebral palsy include thyroid problems, intellectual disabilities or seizures, and exposure to toxins, such as methyl mercury.
Infant illness
Illnesses in a newborn baby that can greatly increase the risk of cerebral palsy include:
• Bacterial meningitis. This bacterial infection causes inflammation in the membranes surrounding the brain and spinal cord.
• Viral encephalitis. This viral infection similarly causes inflammation in the membranes surrounding the brain and spinal cord.
• Severe or untreated jaundice. Jaundice appears as a yellowing of the skin. The condition occurs when certain byproducts of "used" blood cells aren't filtered from the bloodstream.
• Bleeding into the brain. This condition is commonly caused by the baby having a stroke in the womb.
Other factors of pregnancy and birth
While the potential contribution from each is limited, additional pregnancy or birth factors associated with increased cerebral palsy risk include:
• Breech presentation. Babies with cerebral palsy are more likely to be in this feet-first position at the beginning of labor rather than being headfirst.
• Low birth weight. Babies who weigh less than 5.5 pounds (2.5 kilograms) are at higher risk of developing cerebral palsy. This risk increases as birth weight drops.
• Multiple babies. Cerebral palsy risk increases with the number of babies sharing the uterus. If one or more of the babies die, the survivors' risk of cerebral palsy increases.
• Premature birth. Babies born fewer than 28 weeks into the pregnancy are at higher risk of cerebral palsy. The earlier a baby is born, the greater the cerebral palsy risk.
Complications
Muscle weakness, muscle spasticity and coordination problems can contribute to a number of complications either during childhood or in adulthood, including:
• Contracture. Contracture is muscle tissue shortening due to severe muscle tightening (spasticity). Contracture can inhibit bone growth, cause bones to bend, and result in joint deformities, dislocation or partial dislocation.
• Premature aging. Some type of premature aging will affect most people with cerebral palsy in their 40s because of the strain the condition puts on their bodies.
• Malnutrition. Swallowing or feeding problems can make it difficult for someone who has cerebral palsy, particularly an infant, to get enough nutrition. This can impair growth and weaken bones. Some children need a feeding tube to get enough nutrition.
• Mental health conditions. People with cerebral palsy might have mental health conditions, such as depression. Social isolation and the challenges of coping with disabilities can contribute to depression.
• Heart and lung disease. People with cerebral palsy may develop heart disease and lung disease and breathing disorders.
• Osteoarthritis. Pressure on joints or abnormal alignment of joints from muscle spasticity may lead to the early onset of this painful degenerative bone disease.
• Osteopenia. Fractures due to low bone density (osteopenia) can stem from several common factors such as lack of mobility, nutritional shortcomings and anti-epileptic drug use.
Prevention
Most cases of cerebral palsy can't be prevented, but you can lessen risks. If you're pregnant or planning to become pregnant, you can take these steps to keep healthy and minimize pregnancy complications:
• Make sure you're vaccinated. Getting vaccinated against diseases such as rubella, preferably before getting pregnant, might prevent an infection that could cause fetal brain damage.
• Take care of yourself. The healthier you are heading into a pregnancy, the less likely you'll be to develop an infection that results in cerebral palsy.
• Seek early and continuous prenatal care. Regular visits to your doctor during your pregnancy are a good way to reduce health risks to you and your unborn baby. Seeing your doctor regularly can help prevent premature birth, low birth weight and infections.
• Practice good child safety. Prevent head injuries by providing your child with a car seat, bicycle helmet, safety rails on beds and appropriate supervision.
• Avoid alcohol, tobacco and illegal drugs. These have been linked to cerebral palsy risk.
Diagnosis
Signs and symptoms of cerebral palsy can become more apparent over time, so a diagnosis might not be made until a few months after birth.
If your family doctor or pediatrician suspects your child has cerebral palsy, he or she will evaluate your child's signs and symptoms, monitor growth and development, review your child's medical history, and conduct a physical exam. Your doctor might refer you to a specialist trained in treating children with brain and nervous system conditions (pediatric neurologist, pediatric physical medicine and rehabilitation specialist, or child developmental specialist).
Your doctor might also order a series of tests to make a diagnosis and rule out other possible causes.
Brain scans
Brain-imaging technologies can reveal areas of damage or abnormal development in the brain. These tests might include the following:
• MRI. An MRI scan uses radio waves and a magnetic field to produce detailed 3D or cross-sectional images of your child's brain. An MRI can often identify lesions or abnormalities in your child's brain.
This test is painless, but it's noisy and can take up to an hour to complete. Your child will likely receive a sedative or light general anesthesia beforehand.
• Cranial ultrasound. This can be performed during infancy. A cranial ultrasound uses high-frequency sound waves to produce images of the brain. An ultrasound doesn't produce a detailed image, but it may be used because it's quick and inexpensive, and it can provide a valuable preliminary assessment of the brain.
Electroencephalogram (EEG)
If your child is suspected of having seizures, an EEG can evaluate the condition further. Seizures can develop in a child with epilepsy. In an EEG test, a series of electrodes are attached to your child's scalp.
The EEG records the electrical activity of your child's brain. It's common for there to be changes in normal brain wave patterns in epilepsy.
Laboratory tests
Tests on the blood, urine or skin might be used to screen for genetic or metabolic problems.
Additional tests
If your child is diagnosed with cerebral palsy, you'll likely be referred to specialists to test your child for other conditions often associated with the disorder. These tests can identify problems with:
• Vision
• Hearing
• Speech
• Intellect
• Development
• Movement
Treatment
Children and adults with cerebral palsy require long-term care with a medical care team. Besides a pediatrician or physiatrist and possibly a pediatric neurologist to oversee your child's medical care, the team might include a variety of therapists and mental health specialists.
Medications
Medications that can lessen muscle tightness might be used to improve functional abilities, treat pain and manage complications related to spasticity or other cerebral palsy symptoms.
Muscle or nerve injections
To treat tightening of a specific muscle, your doctor might recommend injections of onabotulinumtoxinA (Botox, Dysport) or another agent. Your child will need injections about every three months.
Side effects can include pain at the injection site and mild flu-like symptoms. Other more-serious side effects include difficulty breathing and swallowing.
Oral muscle relaxants
Drugs such as diazepam (Valium), dantrolene (Dantrium), baclofen (Gablofen, Lioresal) and tizanidine (Zanaflex) are often used to relax muscles.
Diazepam carries some dependency risk, so it's not recommended for long-term use. Side effects of these drugs include drowsiness, blood pressure changes and risk of liver damage that requires monitoring.
In some cases, baclofen is pumped into the spinal cord with a tube. The pump is surgically implanted under the skin of the abdomen.
Your child might also be prescribed medication to reduce drooling — possibly Botox injections into the salivary glands.
Therapies
A variety of therapies play an important role in treating cerebral palsy:
• Physical therapy. Muscle training and exercises can help your child's strength, flexibility, balance, motor development and mobility. You'll also learn how to safely care for your child's everyday needs at home, such as bathing and feeding your child.
For the first one to two years after birth, both physical and occupational therapists provide support with issues such as head and trunk control, rolling, and grasping.
Later, both types of therapists are involved in wheelchair assessments.
Braces or splints might be recommended for your child to help with function, such as improved walking, and stretching stiff muscles.
• Occupational therapy. Occupational therapists work to help your child gain independence in daily activities and routines in the home, the school and the community. Adaptive equipment recommended for your child can include walkers, quadrupedal canes, seating systems or electric wheelchairs.
• Speech and language therapy. Speech-language pathologists can help improve your child's ability to speak clearly or to communicate using sign language. They can also teach the use of communication devices, such as a computer and voice synthesizer, if communication is difficult.
Speech therapists can also address difficulties with eating and swallowing.
• Recreational therapy. Some children benefit from regular or adaptive recreational or competitive sports activities, such as therapeutic horseback riding or skiing. This type of therapy can help improve your child's motor skills, speech and emotional well-being.
Surgical procedures
Surgery may be needed to lessen muscle tightness or correct bone abnormalities caused by spasticity. These treatments include:
• Orthopedic surgery. Children with severe contractures or deformities might need surgery on bones or joints to place their arms, hips or legs in their correct positions.
Surgical procedures can also lengthen muscles and tendons that are shortened by contractures. These corrections can lessen pain and improve mobility. The procedures can also make it easier to use a walker, braces or crutches.
• Cutting nerve fibers (selective dorsal rhizotomy). In some severe cases, when other treatments haven't helped, surgeons might cut the nerves serving the spastic muscles in a procedure called selective dorsal rhizotomy. This relaxes the muscle and reduces pain, but can cause numbness.
Alternative medicine
Some children and adolescents with cerebral palsy use some form of complementary or alternative medicine. These therapies aren't accepted clinical practice.
For example, hyperbaric oxygen therapy is widely promoted for cerebral palsy treatment despite limited evidence of benefit. Controlled clinical trials involving therapies such as hyperbaric oxygen therapy, resistance exercise training using special clothing, assisted motion completion for children and certain forms of electrical stimulation have been inconclusive or showed no benefit to date.
Stem cell therapy is being explored as a treatment approach for cerebral palsy, but research is still assessing whether it's safe and effective.
Coping and support
When a child is diagnosed with a disabling condition, the whole family faces new challenges. Here are a few tips for caring for your child and yourself:
• Foster your child's independence. Encourage any effort at independence, no matter how small.
• Be an advocate for your child. You're an important part of your child's health care team. Don't be afraid to speak out on your child's behalf or to ask tough questions of your physicians, therapists and teachers.
• Find support. A circle of support can make a big difference in helping you and your family cope with cerebral palsy and its effects. As a parent, you might feel grief and guilt over your child's disability.
Your doctor can help you locate support groups, organizations and counseling services in your community. Your child might also benefit from family support programs, school programs and counseling.
Preparing for your appointment
If your child has cerebral palsy, how you learn about your child's condition can depend on the severity of the disabilities, when signs and symptoms started, and whether there were risk factors during pregnancy or delivery.
Here's some information to help you get ready for your child's appointment with his or her doctor.
What you can do
Make a list of:
• Symptoms that concern you and when they began
• All medications, vitamins and other supplements your child takes, including doses
• Your child's medical history, including other conditions with which he or she has been diagnosed
• Questions to ask your doctor
Take a relative or friend with you, if possible, to help you remember the information you receive.
Questions to ask your doctor
• What tests will my child need?
• When will we know the results of the tests?
• What specialists will we need to see?
• How will you monitor my child's health and development?
• Can you suggest educational materials and local support services regarding cerebral palsy?
• Can my child be followed through a multidisciplinary program that addresses all of his or her needs on the same visit, such as a cerebral palsy clinic?
Don't hesitate to ask other questions.
What to expect from your doctor
Your doctor is likely to ask you questions, including:
• What concerns do you have about your child's growth or development?
• How well does your child eat?
• How does your child respond to touch?
• Do you observe favoring of one side of the body?
• Is your child reaching certain milestones in development, such as rolling over, pushing up, sitting up, crawling, walking or speaking?
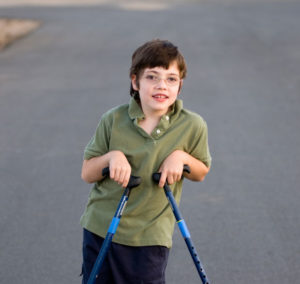
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1011 2021-05-06 00:08:16
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,002
Re: Miscellany
989) Typhoid fever
Overview
Typhoid fever is caused by Salmonella typhi bacteria. Typhoid fever is rare in industrialized countries. However, it remains a serious health threat in the developing world, especially for children.
Typhoid fever spreads through contaminated food and water or through close contact with someone who's infected. Signs and symptoms usually include a high fever, headache, abdominal pain, and either constipation or diarrhea.
Most people with typhoid fever feel better within a few days of starting antibiotic treatment, although a small number of them may die of complications. Vaccines against typhoid fever are available, but they're only partially effective. Vaccines usually are reserved for those who may be exposed to the disease or are traveling to areas where typhoid fever is common.
Symptoms
Signs and symptoms are likely to develop gradually — often appearing one to three weeks after exposure to the disease.
Early illness
Once signs and symptoms do appear, you're likely to experience:
• Fever that starts low and increases daily, possibly reaching as high as 104.9 F (40.5 C)
• Headache
• Weakness and fatigue
• Muscle aches
• Sweating
• Dry cough
• Loss of appetite and weight loss
• Abdominal pain
• Diarrhea or constipation
• Rash
• Extremely swollen abdomen
Later illness
If you don't receive treatment, you may:
• Become delirious
• Lie motionless and exhausted with your eyes half-closed in what's known as the typhoid state
In addition, life-threatening complications often develop at this time.
In some people, signs and symptoms may return up to two weeks after the fever has subsided.
When to see a doctor
See a doctor immediately if you suspect you have typhoid fever. If you are from the United States and become ill while traveling in a foreign country, call the U.S. Consulate for a list of doctors. Better yet, find out in advance about medical care in the areas you'll visit, and carry a list of the names, addresses and phone numbers of recommended doctors.
If you develop signs and symptoms after you return home, consider consulting a doctor who focuses on international travel medicine or infectious diseases. A specialist may be able to recognize and treat your illness more quickly than can a doctor who isn't familiar with these areas.
Causes
Typhoid fever is caused by virulent bacteria called Salmonella typhi. Although they're related, Salmonella typhi and the bacteria responsible for salmonellosis, another serious intestinal infection, aren't the same.
Fecal-oral transmission route
The bacteria that cause typhoid fever spread through contaminated food or water and occasionally through direct contact with someone who is infected. In developing nations, where typhoid fever is established (endemic), most cases result from contaminated drinking water and poor sanitation. The majority of people in industrialized countries pick up typhoid bacteria while traveling and spread it to others through the fecal-oral route.
This means that Salmonella typhi is passed in the feces and sometimes in the urine of infected people. You can contract the infection if you eat food handled by someone with typhoid fever who hasn't washed carefully after using the toilet. You can also become infected by drinking water contaminated with the bacteria.
Typhoid carriers
Even after treatment with antibiotics, a small number of people who recover from typhoid fever continue to harbor the bacteria in their intestinal tracts or gallbladders, often for years. These people, called chronic carriers, shed the bacteria in their feces and are capable of infecting others, although they no longer have signs or symptoms of the disease themselves.
Risk factors
Typhoid fever remains a serious worldwide threat — especially in the developing world — affecting an estimated 26 million or more people each year. The disease is established (endemic) in India, Southeast Asia, Africa, South America and many other areas.
Worldwide, children are at greatest risk of getting the disease, although they generally have milder symptoms than adults do.
If you live in a country where typhoid fever is rare, you're at increased risk if you:
• Work in or travel to areas where typhoid fever is established (endemic)
• Work as a clinical microbiologist handling Salmonella typhi bacteria
• Have close contact with someone who is infected or has recently been infected with typhoid fever
• Drink water contaminated by sewage that contains Salmonella typhi
Complications
Intestinal bleeding or holes
The most serious complications of typhoid fever — intestinal bleeding or holes (perforations) in the intestine — may develop in the third week of illness. A perforated intestine occurs when your small intestine or large bowel develops a hole, causing intestinal contents to leak into your abdominal cavity and triggering signs and symptoms such as severe abdominal pain, nausea, vomiting and bloodstream infection (sepsis). This life-threatening complication requires immediate medical care.
Other, less common complications
Other possible complications include:
• Inflammation of the heart muscle (myocarditis)
• Inflammation of the lining of the heart and valves (endocarditis)
• Pneumonia
• Inflammation of the pancreas (pancreatitis)
• Kidney or bladder infections
• Infection and inflammation of the membranes and fluid surrounding your brain and spinal cord (meningitis)
• Psychiatric problems, such as delirium, hallucinations and paranoid psychosis
With prompt treatment, nearly all people in industrialized nations recover from typhoid fever. Without treatment, some people may not survive complications of the disease.
Prevention
In many developing nations, the public health goals that can help prevent and control typhoid fever — safe drinking water, improved sanitation and adequate medical care — may be difficult to achieve. For that reason, some experts believe that vaccinating high-risk populations is the best way to control typhoid fever.
A vaccine is recommended if you live in or you're traveling to areas where the risk of getting typhoid fever is high.
Vaccines
Two vaccines are available.
• One is injected in a single dose at least one week before travel.
• One is given orally in four capsules, with one capsule to be taken every other day.
Neither vaccine is 100 percent effective, and both require repeat immunizations, as vaccine effectiveness diminishes over time.
Because the vaccine won't provide complete protection, follow these guidelines when traveling to high-risk areas:
• Wash your hands. Frequent hand-washing in hot, soapy water is the best way to control infection. Wash before eating or preparing food and after using the toilet. Carry an alcohol-based hand sanitizer for times when water isn't available.
• Avoid drinking untreated water. Contaminated drinking water is a particular problem in areas where typhoid fever is endemic. For that reason, drink only bottled water or canned or bottled carbonated beverages, wine and beer. Carbonated bottled water is safer than uncarbonated bottled water is.
Ask for drinks without ice. Use bottled water to brush your teeth, and try not to swallow water in the shower.
• Avoid raw fruits and vegetables. Because raw produce may have been washed in unsafe water, avoid fruits and vegetables that you can't peel, especially lettuce. To be absolutely safe, you may want to avoid raw foods entirely.
• Choose hot foods. Avoid food that's stored or served at room temperature. Steaming hot foods are best. And although there's no guarantee that meals served at the finest restaurants are safe, it's best to avoid food from street vendors — it's more likely to be contaminated.
Prevent infecting others
If you're recovering from typhoid fever, these measures can help keep others safe:
• Take your antibiotics. Follow your doctor's instructions for taking your antibiotics, and be sure to finish the entire prescription.
• Wash your hands often. This is the single most important thing you can do to keep from spreading the infection to others. Use hot, soapy water and scrub thoroughly for at least 30 seconds, especially before eating and after using the toilet.
• Avoid handling food. Avoid preparing food for others until your doctor says you're no longer contagious. If you work in the food service industry or a health care facility, you won't be allowed to return to work until tests show that you're no longer shedding typhoid bacteria.
Diagnosis
Medical and travel history
Your doctor is likely to suspect typhoid fever based on your symptoms and your medical and travel history. But the diagnosis is usually confirmed by identifying Salmonella typhi in a culture of your blood or other body fluid or tissue.
Body fluid or tissue culture
For the culture, a small sample of your blood, stool, urine or bone marrow is placed on a special medium that encourages the growth of bacteria. The culture is checked under a microscope for the presence of typhoid bacteria. A bone marrow culture often is the most sensitive test for Salmonella typhi.
Although performing a culture test is the mainstay for diagnosis, in some instances other testing may be used to confirm a suspected typhoid fever infection, such as a test to detect antibodies to typhoid bacteria in your blood or a test that checks for typhoid DNA in your blood.
Treatment
Antibiotic therapy is the only effective treatment for typhoid fever.
Commonly prescribed antibiotics
Commonly prescribed antibiotics include:
• Ciprofloxacin (Cipro). In the United States, doctors often prescribe this for nonpregnant adults. Another similar drug called ofloxacin also may be used. Unfortunately, many Salmonella typhi bacteria are no longer susceptible to antibiotics of this type, particularly strains acquired in Southeast Asia.
• Azithromycin (Zithromax). This may be used if a person is unable to take ciprofloxacin or the bacteria is resistant to ciprofloxacin.
• Ceftriaxone. This injectable antibiotic is an alternative in more-complicated or serious infections and for people who may not be candidates for ciprofloxacin, such as children.
These drugs can cause side effects, and long-term use can lead to the development of antibiotic-resistant strains of bacteria.
Problems with antibiotic resistance
In the past, the drug of choice was chloramphenicol. Doctors no longer commonly use it, however, because of side effects, a high rate of health deterioration after a period of improvement (relapse) and widespread bacterial resistance.
In fact, the existence of antibiotic-resistant bacteria is a growing problem in the treatment of typhoid fever, especially in the developing world. In recent years, Salmonella typhi has also proved resistant to trimethoprim-sulfamethoxazole, ampicillin and ciprofloxacin.
Other treatments
Other treatments include:
• Drinking fluids. This helps prevent the dehydration that results from a prolonged fever and diarrhea. If you're severely dehydrated, you may need to receive fluids through a vein (intravenously).
• Surgery. If your intestines become perforated, you'll need surgery to repair the hole.
Call your doctor if you've recently returned from travel abroad and develop mild symptoms similar to those that occur with typhoid fever. If your symptoms are severe, go to an emergency room or your local emergency number.
Here's some information to help you get ready and know what to expect from your doctor.
Information to gather in advance
• Pre-appointment restrictions. At the time you make your appointment, ask if there are restrictions you need to follow in the time leading up to your visit. Your doctor will not be able to confirm typhoid fever without a blood test, and may recommend taking steps to reduce the risk of passing a possible contagious illness to others.
• Symptom history. Write down any symptoms you're experiencing and for how long.
• Recent exposure to possible sources of infection. Be prepared to describe international trips in detail, including the countries you visited and the dates you traveled.
• Medical history. Make a list of your key medical information, including other conditions for which you're being treated and any medications, vitamins or supplements you're taking. Your doctor will also need to know your vaccination history.
• Questions to ask your doctor. Write down your questions in advance so that you can make the most of your time with your doctor.
For typhoid fever, possible questions to ask your doctor include:
• What are the possible causes for my symptoms?
• What kinds of tests do I need?
• Are treatments available to help me recover?
• I have other health problems. How can I best manage these conditions together?
• How long do you expect a full recovery will take?
• When can I return to work or school?
• Am I at risk of any long-term complications from typhoid fever?
Don't hesitate to ask any other related questions you have.
What to expect from your doctor
Your doctor is likely to ask you a number of questions. Being ready to answer them may reserve time to go over any points you want to talk about in-depth. Your doctor may ask:
• What are your symptoms and when did they begin?
• Have your symptoms gotten better or worse?
• Did your symptoms briefly get better and then come back?
• Have you recently traveled abroad? Where?
• Did you update your vaccinations before traveling?
• Are you being treated for any other medical conditions?
• Are you currently taking any medications?

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1012 2021-05-07 00:45:31
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,002
Re: Miscellany
990) Whooping cough
Overview
Whooping cough (pertussis) is a highly contagious respiratory tract infection. In many people, it's marked by a severe hacking cough followed by a high-pitched intake of breath that sounds like "whoop."
Before the vaccine was developed, whooping cough was considered a childhood disease. Now whooping cough primarily affects children too young to have completed the full course of vaccinations and teenagers and adults whose immunity has faded.
Deaths associated with whooping cough are rare but most commonly occur in infants. That's why it's so important for pregnant women — and other people who will have close contact with an infant — to be vaccinated against whooping cough.
Symptoms
Once you become infected with whooping cough, it takes about seven to 10 days for signs and symptoms to appear, though it can sometimes take longer. They're usually mild at first and resemble those of a common cold:
• Runny nose
• Nasal congestion
• Red, watery eyes
• Fever
• Cough
After a week or two, signs and symptoms worsen. Thick mucus accumulates inside your airways, causing uncontrollable coughing. Severe and prolonged coughing attacks may:
• Provoke vomiting
• Result in a red or blue face
• Cause extreme fatigue
• End with a high-pitched "whoop" sound during the next breath of air
However, many people don't develop the characteristic whoop. Sometimes, a persistent hacking cough is the only sign that an adolescent or adult has whooping cough.
Infants may not cough at all. Instead, they may struggle to breathe, or they may even temporarily stop breathing.
When to see a doctor
Call your doctor if prolonged coughing spells cause you or your child to:
• Vomit
• Turn red or blue
• Seem to be struggling to breathe or have noticeable pauses in breathing
• Inhale with a whooping sound
Causes
Whooping cough is caused by a type of bacteria called Bordetella pertussis. When an infected person coughs or sneezes, tiny germ-laden droplets are sprayed into the air and breathed into the lungs of anyone who happens to be nearby.
Risk factors
The whooping cough vaccine you receive as a child eventually wears off. This leaves most teenagers and adults susceptible to the infection during an outbreak — and there continue to be regular outbreaks.
Infants who are younger than age 12 months who are unvaccinated or haven't received the full set of recommended vaccines have the highest risk for severe complications and death.
Complications
Teens and adults often recover from whooping cough with no problems. When complications occur, they tend to be side effects of the strenuous coughing, such as:
• Bruised or cracked ribs
• Abdominal hernias
• Broken blood vessels in the skin or the whites of your eyes
Infants
In infants — especially those under 6 months of age — complications from whooping cough are more severe and may include:
• Pneumonia
• Slowed or stopped breathing
• Dehydration or weight loss due to feeding difficulties
• Seizures
• Brain damage
Because infants and toddlers are at greatest risk of complications from whooping cough, they're more likely to need treatment in a hospital. Complications can be life-threatening for infants younger than 6 months old.
Prevention
The best way to prevent whooping cough is with the pertussis vaccine, which doctors often give in combination with vaccines against two other serious diseases — diphtheria and tetanus. Doctors recommend beginning vaccination during infancy.
The vaccine consists of a series of five injections, typically given to children at these ages:
• 2 months
• 4 months
• 6 months
• 15 to 18 months
• 4 to 6 years
Vaccine side effects
Side effects of the vaccine are usually mild and may include a fever, crankiness, headache, fatigue or soreness at the site of the injection.
Booster shots
• Adolescents. Because immunity from the pertussis vaccine tends to wane by age 11, doctors recommend a booster shot at that age to protect against whooping cough (pertussis), diphtheria and tetanus.
• Adults. Some varieties of the every-10-year tetanus and diphtheria vaccine also include protection against whooping cough (pertussis). This vaccine will also reduce the risk of your transmitting whooping cough to infants.
• Pregnant women. Health experts now recommend that pregnant women receive the pertussis vaccine between 27 and 36 weeks of gestation. This may also give some protection to the infant during the first few months of life.
Preventive medications
If you've been exposed to someone who has whooping cough, your doctor may recommend antibiotics to protect against infection if you:
• Are a health care provider
• Are pregnant
• Are younger than age 12 months
• Have a health condition that could put you at risk of severe illness or complications, such as a weakened immune system or asthma
• Live with someone who has whooping cough
• Live with someone who is at high risk of developing severe illness or complications from a whooping cough infection
Diagnosis
Diagnosing whooping cough in its early stages can be difficult because the signs and symptoms resemble those of other common respiratory illnesses, such as a cold, the flu or bronchitis.
Sometimes, doctors can diagnose whooping cough simply by asking about symptoms and listening to the cough. Medical tests may be needed to confirm the diagnosis.
Such tests may include:
• A nose or throat culture and test. Your doctor takes a swab or suction sample from the area where the nose and throat meet (nasopharynx). The sample is then checked for evidence of the presence of whooping cough bacteria.
• Blood tests. A blood sample may be drawn and sent to a lab to check your white blood cell count, because white blood cells help the body fight infections, such as whooping cough. A high white blood cell count typically indicates the presence of infection or inflammation. This is a general test and not specific for whooping cough.
• A chest X-ray. Your doctor may order an X-ray to check for the presence of inflammation or fluid in the lungs, which can occur when pneumonia complicates whooping cough and other respiratory infections.
Infants are typically hospitalized for treatment because whooping cough is more dangerous for that age group. If your child can't keep down liquids or food, intravenous fluids may be necessary. Your child will also be isolated from others to prevent the infection from spreading.
Treatment for older children and adults usually can be managed at home.
Medications
Antibiotics kill the bacteria causing whooping cough and help speed recovery. Exposed family members may be given preventive antibiotics.
Unfortunately, not much is available to relieve the cough. Over-the-counter cough medicines, for instance, have little effect on whooping cough and are discouraged.
Lifestyle and home remedies
The following tips on dealing with coughing spells apply to anyone being treated for whooping cough at home:
• Get plenty of rest. A cool, quiet and dark bedroom may help you relax and rest better.
• Drink plenty of fluids. Water, juice and soups are good choices. In children, especially, watch for signs of dehydration, such as dry lips, crying without tears and infrequent urination.
• Eat smaller meals. To avoid vomiting after coughing, eat smaller, more-frequent meals rather than large ones.
• Clean the air. Keep your home free of irritants that can trigger coughing spells, such as tobacco smoke and fumes from fireplaces.
• Prevent transmission. Cover your cough and wash your hands often; if you must be around others, wear a mask.
Preparing for your appointment
If you think you or your child has whooping cough, make an appointment with your family doctor or pediatrician. Severe symptoms may warrant a visit to an urgent care center or a hospital's emergency department.
What you can do
You may want to write a list that includes:
• Detailed descriptions of the signs and symptoms
• Information about past medical problems
• Dates of immunizations
• Information about the medical problems of parents or siblings
• Questions you want to ask the doctor
What to expect from your doctor
Your doctor will conduct a physical exam and will use a stethoscope to listen closely to your lungs. Questions your doctor may ask include:
• When did the cough start?
• How long does a coughing spell generally last?
• Does anything trigger the cough?
• Does the cough ever cause gagging or vomiting?
• Has the cough ever resulted in a red or blue face?
• Have you been exposed to anyone with whooping cough?

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1013 2021-05-08 00:37:19
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,002
Re: Miscellany
991) Malaria
Overview
Malaria is a disease caused by a parasite. The parasite is transmitted to humans through the bites of infected mosquitoes. People who have malaria usually feel very sick, with a high fever and shaking chills. Each year, approximately 210 million people are infected with malaria, and about 440,000 people die from the disease. Most of the people who die from the disease are young children in Africa.
While the disease is uncommon in temperate climates, malaria is still common in tropical and subtropical countries. World health officials are trying to reduce the incidence of malaria by distributing bed nets to help protect people from mosquito bites as they sleep. Scientists around the world are working to develop a vaccine to prevent malaria.
If you're traveling to locations where malaria is common, take steps to prevent mosquito bites by wearing protective clothing, using insect repellants and sleeping under treated mosquito nets. Depending on the area you are visiting and your individual risk factors for infection, you may also want to take preventive medicine before, during and after your trip. Many malaria parasites are now resistant to the most common drugs used to treat the disease.
Symptoms
A malaria infection is generally characterized by the following signs and symptoms:
• Fever
• Chills
• Headache
• Nausea and vomiting
• Muscle pain and fatigue
Other signs and symptoms may include:
• Sweating
• Chest or abdominal pain
• Cough
Some people who have malaria experience cycles of malaria "attacks." An attack usually starts with shivering and chills, followed by a high fever, followed by sweating and a return to normal temperature. Malaria signs and symptoms typically begin within a few weeks after being bitten by an infected mosquito. However, some types of malaria parasites can lie dormant in your body for up to a year.
When to see a doctor
Talk to your doctor if you experience a fever while living in or after traveling to a high-risk malaria region. The parasites that cause malaria can lie dormant in your body for up to a year. If you have severe symptoms, seek emergency medical attention.
Causes
Malaria transmission cycle
Malaria is caused by a type of microscopic parasite. The parasite is transmitted to humans most commonly through mosquito bites.
Mosquito transmission cycle
• Uninfected mosquito. A mosquito becomes infected by feeding on a person who has malaria.
• Transmission of parasite. If this mosquito bites you in the future, it can transmit malaria parasites to you.
• In the liver. Once the parasites enter your body, they travel to your liver — where some types can lie dormant for as long as a year.
• Into the bloodstream. When the parasites mature, they leave the liver and infect your red blood cells. This is when people typically develop malaria symptoms.
• On to the next person. If an uninfected mosquito bites you at this point in the cycle, it will become infected with your malaria parasites and can spread them to the other people it bites.
Other modes of transmission
Because the parasites that cause malaria affect red blood cells, people can also catch malaria from exposure to infected blood, including:
• From mother to unborn child
• Through blood transfusions
• By sharing needles used to inject drugs
Risk factors
The biggest risk factor for developing malaria is to live in or to visit areas where the disease is common. There are many different varieties of malaria parasites. The variety that causes the most serious complications is most commonly found in:
• African countries south of the Sahara Desert
• The Asian subcontinent
• New Guinea, the Dominican Republic and Haiti
Risks of more-severe disease
People at increased risk of serious disease include:
• Young children and infants
• Older adults
• Travelers coming from areas with no malaria
• Pregnant women and their unborn children
Poverty, lack of knowledge, and little or no access to health care also contribute to malaria deaths worldwide.
Immunity can wane
Residents of a malaria region may be exposed to the disease so frequently that they acquire a partial immunity, which can lessen the severity of malaria symptoms. However, this partial immunity can disappear if you move to a country where you're no longer frequently exposed to the parasite.
Complications
Malaria can be fatal, particularly malaria caused by the variety of parasite that's common in tropical parts of Africa. The Centers for Disease Control and Prevention estimates that 91 percent of all malaria deaths occur in Africa — most commonly in children under the age of 5.
In most cases, malaria deaths are related to one or more serious complications, including:
• Cerebral malaria. If parasite-filled blood cells block small blood vessels to your brain (cerebral malaria), swelling of your brain or brain damage may occur. Cerebral malaria may cause seizures and coma.
• Breathing problems. Accumulated fluid in your lungs (pulmonary edema) can make it difficult to breathe.
• Organ failure. Malaria can cause your kidneys or liver to fail, or your spleen to rupture. Any of these conditions can be life-threatening.
• Anemia. Malaria damages red blood cells, which can result in anemia.
• Low blood sugar. Severe forms of malaria itself can cause low blood sugar (hypoglycemia), as can quinine — one of the most common medications used to combat malaria. Very low blood sugar can result in coma or death.
Malaria may recur
Some varieties of the malaria parasite, which typically cause milder forms of the disease, can persist for years and cause relapses.
Prevention
If you live in or are traveling to an area where malaria is common, take steps to avoid mosquito bites. Mosquitoes are most active between dusk and dawn. To protect yourself from mosquito bites, you should:
• Cover your skin. Wear pants and long-sleeved shirts.
• Apply insect repellant to skin and clothing. Sprays containing DEET can be used on skin and sprays containing permethrin are safe to apply to clothing.
• Sleep under a net. Bed nets, particularly those treated with insecticide, help prevent mosquito bites while you are sleeping.
Preventive medicine
If you're going to be traveling to a location where malaria is common, talk to your doctor a few months ahead of time about whether you should take drugs before, during and after your trip to help protect you from malaria parasites.
In general, the drugs taken to prevent malaria are the same drugs used to treat the disease. Your doctor needs to know when and where you'll be traveling so that he or she can help you evaluate your risk for infection and, if necessary, prescribe the drug that will work best on the type of malaria parasite most commonly found in that region.
No vaccine yet
Scientists around the world are trying to develop a safe and effective vaccine for malaria. As of yet, however, there is still no malaria vaccine approved for human use.
Diagnosis
To diagnose malaria, your doctor will likely review your medical history, conduct a physical exam and order blood tests. Blood tests are the only way to confirm a malaria diagnosis. Certain blood tests can help your doctor by showing:
• The presence of the parasite in the blood, to confirm that you have malaria
• Which type of malaria parasite is causing your symptoms
• If your infection is caused by a parasite resistant to certain drugs
Other blood tests help determine whether the disease is causing any serious complications.
Some blood tests can take several days to complete, while others can produce results in less than 15 minutes.
Treatment
Malaria is treated with prescription drugs to kill the parasite. The types of drugs and the length of treatment will vary, depending on:
• Which type of malaria parasite you have
• The severity of your symptoms
• Your age
• Whether you're pregnant
Medication
The most common antimalarial drugs include:
• Artemisinin-based combination therapies (ACTs). ACTs are, in many cases, the first line treatment for malaria. There are several different types of ACTs. Examples include artemether-lumefantrine (Coartem) and artesunate-amodiaquine. Each ACT is a combination of two or more drugs that work against the malaria parasite in different ways.
• Chloroquine phosphate. Chloroquine is the preferred treatment for any parasite that is sensitive to the drug. But in many parts of the world, the parasites that cause malaria are resistant to chloroquine, and the drug is no longer an effective treatment.
Other common antimalarial drugs include:
• Combination of atovaquone and proguanil (Malarone)
• Quinine sulfate (Qualaquin) with doxycycline (Vibramycin, Monodox, others)
• Mefloquine
• Primaquine phosphate
Possible future treatments
New antimalarial drugs are being researched and developed. Malaria treatment is marked by a constant struggle between evolving drug-resistant parasites and the search for new drug formulations. For example, one variety of the malaria parasite has demonstrated resistance to nearly all of the available antimalarial drugs.
If you suspect you have malaria or that you've been exposed, you're likely to start by seeing your family doctor. However, in some cases when you call to set up an appointment, you may be referred to an infectious disease specialist. If you have severe symptoms — especially during or after travel in an area where malaria is common — seek emergency medical attention.
What you can do
Before your appointment, you might want to write a list that answers the following questions:
• What are your symptoms, and when did they start?
• Have you recently traveled to or moved from a region in which malaria is common?
• Have you ever had malaria before?
• What types of medications and supplements do you take?
What to expect from your doctor
During the physical exam, your doctor may review your medical history, listen to your breathing, check your spleen and neurological functions, and look for other causes of a fever.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1014 2021-05-09 01:16:45
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,002
Re: Miscellany
992) Bay
Bay, concavity of a coastline or reentrant of the sea, formed by the movements of either the sea or a lake. The difference between a bay and a gulf is not clearly defined, but the term bay usually refers to a body of water somewhat smaller than a gulf. Numerous exceptions, however, are found throughout the world, such as the Bay of Bengal, which is larger than the Gulf of Mexico and about the same size as the Arabian Sea.
A brief treatment of bays follows.
A bay is usually located where more easily eroded rocks, such as clays, silts, and some sandstones, are bounded by harder and more resistant formations made from igneous rocks, such as granite, or hard calcareous rocks, such as massive limestones, which are more resistant to the erosional forces of the land and sea or lake. The harder rocks therefore stand out as promontories projecting out to sea, often with caves that may in some cases link the two sides of the promontory, thus creating an island, perhaps with a natural bridge to the mainland. This bridge will later fall as a result of erosion and weathering and leave an island completely separated from the mainland.
The softer rocks between the promontories are subjected to more rapid erosion as lines of waves, initially with their crests approaching the coastline at an oblique angle, turn to approach the shoreline head-on because of wave impedance by the shallower, nearshore seabed, so that the end of the line of waves closest to shore moves forward more slowly than the end farther out to sea. In this way the lines of waves gradually turn as they move around the windward headland to sweep directly onshore in the bay. The erosion of the soft rocks of the bay is most rapid during storms, when material eroded just behind the line of breakers is thrown by the waves farther up the beach; in this way a series of ridges may mark a succession of storms, particularly where the beach material is mainly pebbles. The wind may then carry the finest beach material inland beyond the high-water mark, where it may be deposited in a zone of sand dunes. These may, if uncontrolled, move miles inland. The most common method of dune stabilization is the encouragement of deep-rooted marram grass.
There are no defined dimensions for bays. Smaller bays may be only a few hundred metres wide, while others, such as the Bay of Biscay off Spain and France and Hudson Bay in Canada, are several hundred kilometres from side to side. Some of these larger bays may represent depressions in the ground, formed by vertical earth movements or glacial erosion by ice sheets. Hudson Bay is of this latter type. All bays are semicircular or nearly circular in shape, which distinguishes them from estuaries, which are elongated and funnel-shaped with a river running along the centre line and with beaches mainly near the mouth of the estuary. Estuaries and some of the more enclosed and sheltered bays form excellent harbours, provided that the seabed is deep enough and well-scoured. They were popular sites for early settlements, and a number of the larger coastal cities today have their original cores around a bay that provided protection for ships at anchor.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1015 2021-05-10 00:04:46
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,002
Re: Miscellany
993) Gulf
Gulf, any large coastal indentation. More specifically, such a feature is the reentrant of an ocean, regardless of size, depth, configuration, and geologic structure. The nomenclature for gulfs is far from uniform; names that may refer to sizable gulfs in various places include bay, bight, firth, sound, and fjord. In addition, a number of pronounced concavities of oceanic margins have no proper name at all. As such, many of the characteristics of gulfs may also apply to bays and other similar geographies.
This problem of nomenclature extends to the difference between gulfs and seas. There are many small seas, such as the Sea of Marmara (11,000 square km [about 4,200 square miles]) and the Sea of Azov (38,000 square km [about 14,700 square miles]), which, strictly speaking, are really gulfs of the ocean or other seas (the Sea of Azov is a gulf of the Black Sea). The Gulf of Aden (about 270,000 square km [about 104,000 square miles]), another example, is part of the Arabian Sea, and these water bodies have a common regime (similar tides, precipitation, evaporation, and so forth). The narrow sound of Bab el-Mandeb connects the gulf with the vast Red Sea (438,100 square km [about 169,000 square miles]) and exhibits a number of specific geomorphic features. The Red Sea in turn has two small gulfs to the north—namely, those of Suez and Aqaba.
The Bay of Bengal and the Arabian Sea are both gulfs, approximately the same size and having the same monsoonal water circulation. The Bay of Bengal is, however, the largest of the gulfs, with a surface area of 2,172,000 square km (838,600 square miles), a length of 1,850 km (1,150 miles), and a width of about 1,600 km (1,000 miles).
In some cases, the width of a gulf may exceed its length. The Great Australian Bight has the widest mouth (2,800 km [1,740 miles]). The Gulf of Guinea is the deepest; its maximum depth (6,363 metres [20,876 feet]) exceeds that of the Bay of Bengal by more than 1,000 metres (about 3,300 feet).
Topographic Characteristics
Single gulfs usually are formed along linear shores of the continents. If the shoreline is irregular and has a complex geologic structure, groups of gulfs of a similar nature may occur. Most shorelines have small reentrants of various size that are called bays.
The shape and bottom topography of gulfs are amazingly diverse. They are determined by the geologic structure and development of the region. Homogeneous bedrock of low strength or resistance results in simple shapes and shallow depths. The Gulf of Riga (of the Baltic Sea) is a possible example of the type. Long, narrow arms with approximately parallel shores of the south Kara Sea extend inland for about 800 km (about 500 miles). They occupy troughs that originated by erosion during a period of lower sea level (Baidaratskaya Bay, Obskaya Bay with Tazovskaya Bay tributary, Yenisey Bay, Gydanskaya Bay). Deep, angular gulfs, on the other hand, are created along fractures, faults, and rifts (e.g., Varanger Fjord); they usually have irregular bottom topography. Parallel fractures form extremely deep, narrow gulfs with parallel shores, such as the Gulf of California. Genuine fjord-gulfs are notable for their very high length-to-width ratios (up to 50:1). In regions that have undergone nonuniform deformation and uplift, gulfs of complicated and irregular shape and bottom topography are consequently formed; the Gulf of St. Lawrence is an example.
Gulfs are connected with the sea by means of one or more straits. Sometimes there may be an archipelago in the mouth of the gulf, as in the Gulf of Bothnia. There are some gulfs that open into the sea or into another gulf on opposite sides (Baffin Bay, the Gulf of Aden, and the Gulf of Oman).
Factors That Affect The Characteristics Of Gulfs
Gulfs may differ from the adjacent ocean (or sea) by virtue of water properties and dynamics and processes of sedimentation. Such differences are determined by the size and the shape of a given gulf, by the depth and bottom topography, and, to a considerable extent, by the degree of isolation from the ocean. Climatic conditions also are important. Isolation from an adjacent ocean depends on the ratio of width of mouth to total surface area of a gulf or on the cross section of the mouth to total water volume. If there is a sill (a submarine ridge or rise), the ratio of depth above the sill to the depth of the gulf is of great importance. No extensive comparisons of these ratios have been made to date; hence, any analysis of controlling variables must remain somewhat qualitative.
A high sill hampers the water exchange between an ocean and gulf and may lead to stagnation (oxygen deficiency), as is found in some fjords of Norway, in the Red Sea, and, particularly, in the Black Sea. Also, the presence of a sill causes independent circulation of gulf waters, generated by local winds and the runoff of rivers. Sills are not indispensable for the formation of an independent circulation, however. A narrow mouth, as in the Gulf of Bothnia, leads to the same result.
In humid climates, the waters of gulfs are freshened by river runoff. Salinity is particularly low in the gulfs of the Baltic Sea and along the southern coast of the Kara Sea. Water becomes almost fresh in their heads, especially in the spring when snow begins to thaw. Gulfs of the arid zone suffer from intensive evaporation and receive little river runoff. Thus, salinity increases markedly in this climatic regime—up to 60 parts per thousand in the Persian Gulf and up to 350 parts per thousand in the Kara-Bogaz-Gol (a gulf of the Caspian Sea). In addition to its effect on salinity, river runoff delivers organic matter and nutrient salts that may determine the specific features of life in the gulfs. The number of genera and species of organisms is small, but the organisms present tend to develop in quantities. That is why shrimp, oyster, and other fisheries are concentrated in many gulfs.
Funnel-shaped gulfs, in which the depth gradually decreases headward, usually have resonant tides. The tidal range at the head of such gulfs is several times greater than that in the open ocean (e.g., Bristol Channel, Río de la Plata, Mezenskaya Bay, Shelikhova Gulf). The world maximum tidal range has been registered in the Bay of Fundy (18 metres [59 feet]). The regularity (magnitude and frequency) of the flood tide may be distorted in such instances, and the duration of the flood tide may become much shorter than that of the ebb tide. This may cause the phenomenon of tidal bore, in which a steep wave will move rapidly upstream for dozens of kilometres.
Gulfs of simple shape with a narrow mouth and a high degree of isolation from the ocean are often subject to seiches. These free oscillations can result from rapid changes of atmospheric pressure and, of course, from tectonic movements such as earthquakes. Seiches gradually decrease, but some oscillation continues long after their cause disappears. A high rise of the water (storm surge) occurs in long and shallow gulfs if winds from the sea are protracted. Such phenomena are difficult to predict, and the high water levels may cause floods. Seiches commonly occur at the heads of Helgoländer Bay in the North Sea and in the Gulf of Finland.
Certain aspects of sedimentation are affected by the isolation of gulfs from the ocean and river runoff. The rate of sediment accumulation in gulfs of limited area may be very high. This, of course, is a function of river discharge; sediment composition is usually similar to that of the load transported by entering rivers. Deposition of calcium carbonate often occurs in shallow gulfs in the arid zones where few if any perennial streams exist. The bottoms of long gulfs (or gulfs having sills) are usually covered with silt even at the shallowest depths (e.g., Hudson Bay, the Bo Hai [Gulf of Chihli], the inlets or gubas of the Kara Sea, the Gulf of Riga). Only strong tidal currents can prevent this siltation and, in some cases, cause the opposite phenomenon of bottom erosion. Currents maintain the existence of or actively deepen bottom troughs in narrow-mouthed gulfs whose depths are more than 200 metres (about 660 feet), whereas depths of adjacent parts of the open ocean are only on the order of some dozens of metres.
Waves of the open ocean either do not penetrate into comparatively isolated gulfs or—if they do—they become greatly reduced after entry. Small local waves that are related to gulf size prevail there. This tends to make gulfs quite navigable, and seaports and harbours have generally been situated on them.
Classification Of Gulfs
The geologic structure and developmental history of gulfs are as varied as are those of the continents or oceans proper. The factors discussed above influence the morphological peculiarities of gulfs, and the latter in turn permit some general division or classification of these features to be made. The several groups in one possible scheme are discussed here using typical gulfs of each group as examples.
Areas situated in open concavities of the continental coast (Gulf of Alaska, Bay of Biscay, Gulf of Guinea, Great Australian Bight, Bay of Bengal, Gulf of Tehuantepec, for example) are classified as the A1 group. The depth of these gulfs in the region of the mouth usually is on the order of kilometres. The continental shelf and continental slope are generally pronounced. The general shape of such gulfs is simple; width of mouth usually exceeds its length. Water circulation and its physical properties are similar to those of the oceans. The character of the marine faunas does not differ from that of oceanic areas.
Large areas considerably isolated from oceans, such as the Gulf of Mexico and Baffin Bay, are designated as group A2. The former includes a geosynclinal hollow, founded in the Mesozoic Era (251 million to 65.5 million years ago) and finally shaped during the Paleogene and Neogene periods (65.5 million to 2.6 million years ago). It is connected with the ocean by the narrow and relatively shallow Straits of Florida and the Yucatán Channel. Baffin Bay is a rift hollow that is connected by straits with the Atlantic.
Ocean gulfs, such as the Gulfs of Oman, California, Aden, and some others, have smaller areas and are isolated to a lesser degree. These features, in group A3, have shapes that are determined by young faults and fractures. Depths in these gulfs generally exceed 1 kilometre (0.6 mile). Unlike the previous group, in which gulfs might be of composite geologic structure, these occupy areas that have undergone only a single episode of deformation.
Gulfs situated on the continental shelf, such as the Bay of Fundy, Hudson Bay, Río de la Plata, San Matías Gulf (off Argentina), and others, are in group B. The depth of such gulfs is up to 200 metres (about 660 feet) or more, and their configuration is determined by geologic conditions. Because shelf areas repeatedly became dry land when the sea level fell during the ice ages, these gulfs received their final shape during the Pleistocene Epoch. The Gulf of St. Lawrence is included in this group, though it is really intermediate between groups A3 and B. It contains both a pronounced shelf and a long trough up to 530 metres (1,740 feet) deep.
Gulfs of intercontinental and marginal seas are considered to be a third category. These may be divided into group C1, which consists of gulfs of basin seas, including the deepwater part only (Gulf of Aqaba) or both the deepwater and the shelf parts (Gulf of Honduras), and group C2, the shelf gulfs of the same seas (e.g., the Persian Gulf, the Gulf of Suez, Anadyrsky Gulf, the Bristol and Norton channels, and Shelikhova Gulf).
Finally, there are the gulfs of the shelf seas (gubas of the Arctic seas of Russia, gulfs of the Baltic and the White Seas, the Gulf of Carpentaria, the Bo Hai, and many others), which are placed in group D. The shallow character of the shelf seas influences the water dynamics of the gulfs. Water exchange is weakened, and sediments may accumulate in the gulf mouths, thus forming submarine barriers and further reducing exchange.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1016 2021-05-11 00:33:14
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,002
Re: Miscellany
994) Ocean
Ocean, continuous body of salt water that is contained in enormous basins on Earth’s surface.
When viewed from space, the predominance of Earth’s oceans is readily apparent. The oceans and their marginal seas cover nearly 71 percent of Earth’s surface, with an average depth of 3,688 metres (12,100 feet). The exposed land occupies the remaining 29 percent of the planetary surface and has a mean elevation of about 840 metres (approximately 2,755 feet). Actually, all the elevated land could be hidden under the oceans and Earth reduced to a smooth sphere that would be completely covered by a continuous layer of seawater more than 2,600 metres (8,530 feet) deep. This is known as the sphere depth of the oceans and serves to underscore the abundance of water on Earth’s surface.
Earth is unique in the solar system because of its distance from the Sun and its period of rotation. These combine to subject Earth to a solar radiation level that maintains the planet at a mean surface temperature of about 14–15 °C (57.2–59 °F). Mean surface temperature varies little over annual and night-day cycles. This mean temperature allows water to exist on Earth in all three of its phases—solid, liquid, and gaseous. No other planet in the solar system has this feature. The liquid phase predominates on Earth. By volume, 97.957 percent of the water on the planet exists as oceanic water and associated sea ice. The gaseous phase and droplet water in the atmosphere constitute 0.001 percent. Fresh water in lakes and streams makes up 0.036 percent, while groundwater is 10 times more abundant at 0.365 percent. Glaciers and ice caps constitute 1.641 percent of Earth’s total water volume.
Each of the above is considered to be a reservoir of water. Water continuously circulates between these reservoirs in what is called the hydrologic cycle, which is driven by energy from the Sun. Evaporation, precipitation, movement of the atmosphere, and the downhill flow of river water, glaciers, and groundwater keep water in motion between the reservoirs and maintain the hydrologic cycle.
The large range of volumes in these reservoirs and the rates at which water cycles between them combine to create important conditions on Earth. If small changes occur in the rate at which water is cycled into or out of a reservoir, the volume of a reservoir changes. These volume changes may be relatively large and rapid in a small reservoir or small and slow in a large reservoir. A small percentage change in the volume of the oceans may produce a large proportional change in the land-ice reservoir, thereby promoting glacial and interglacial stages. The rate at which water enters or leaves a reservoir divided into the reservoir volume determines the residence time of water in the reservoir. The residence time of water in a reservoir, in turn, governs many of the properties of that reservoir.
This article provides an overview of the world’s oceanic reservoir, including its major subdivisions and its origins.
Relative Distribution Of The Oceans
Earth possesses one “world ocean.” However, those conducting oceanic research generally recognize the existence of five major oceans: the Pacific, Atlantic, Indian, Arctic, and Southern oceans. Arbitrary boundaries separate these bodies of water. The boundaries of each ocean are largely defined by the continents that frame them. In the Southern Hemisphere the southern portions of the Pacific, Atlantic, and Indian oceans and their tributary seas that surround Antarctica are often referred to as the Southern Ocean. Many subdivisions can be made to distinguish the limits of seas and gulfs that have historical, political, and sometimes ecological significance. However, water properties, ocean currents, and biological populations are not constrained by these boundaries. Indeed, many researchers do not recognize them either.
If area-volume analyses of the oceans are to be made, then boundaries must be established to separate individual regions. In 1921 Erwin Kossina, a German geographer, published tables giving the distribution of oceanic water with depth for the oceans and adjacent seas. This work was updated in 1966 by American geologist H.W. Menard and American oceanographer S.M. Smith. The latter only slightly changed the numbers derived by Kossina. This was remarkable, since the original effort relied entirely on the sparse depth measurements accumulated by individual wire soundings, while the more recent work had the benefit of acoustic depth soundings collected since the 1920s. This type of analysis, called hypsometry, allows quantification of the surface area distribution of the oceans and their marginal seas with depth.
The distribution of oceanic surface area with 5° increments of latitude shows that the distribution of land and water on Earth’s surface is markedly different in the Northern and Southern hemispheres. The Southern Hemisphere may be called the water hemisphere, while the Northern Hemisphere is the land hemisphere. This is especially true in the temperate latitudes.
This asymmetry of land and water distribution between the Northern and Southern hemispheres makes the two hemispheres behave very differently in response to the annual variation in solar radiation received by Earth. The Southern Hemisphere shows only a small change in surface temperature from summer to winter at temperate latitudes. This variation is controlled primarily by the ocean’s response to seasonal changes in heating and cooling. The Northern Hemisphere has one change in surface temperature controlled by its oceanic area and another controlled by its land area. In the temperate latitudes of the Northern Hemisphere, the land is much warmer than the oceanic area in summer and much colder in winter. This situation creates large-scale seasonal changes in atmospheric circulation and climate in the Northern Hemisphere that are not found in the Southern Hemisphere.
Major Subdivisions Of The Oceans
If the volume of an ocean is divided by its surface area, the mean depth is obtained. Even without including its marginal seas, the Pacific is the largest ocean in both surface area and volume, the Atlantic is next, and the Arctic is the smallest. The Atlantic exhibits the largest change in surface area and volume when its marginal seas are subtracted. This indicates that the Atlantic has the greatest area of bordering seas, many of which are shallow.
Hypsometry can show how the area of each ocean or marginal sea changes as depth changes. A special curve known as a hypsometric, or hypsographic, curve can be drawn that portrays how the surface area of Earth is distributed with elevation and depth. This curve has been drawn to represent the total Earth and all of its oceans; likewise, curves can be constructed for each individual ocean and sea. The average depth of the world’s oceans, 3,688 metres (12,100 feet), and the average elevation of the land, 840 metres (2,756 feet), are indicated. The highest point on land, Mount Everest (8,850 metres [29,035 feet]), and the deepest point in the ocean, located in the Mariana Trench (11,034 metres [36,201 feet]), mark the upper and lower limits of the curve, respectively. Since this curve is drawn on a grid of elevation versus Earth’s area, the area under the curve covering the 29.2 percent of Earth’s surface that is above sea level is the volume of land above sea level. Similarly, the area between sea level and the curve depicting the remaining 70.8 percent of Earth’s surface below sea level represents the volume of water contained in the oceans.
Portions of this curve describe the area of Earth’s surface that exists between elevation or depth increments. On land, little of Earth’s total area—only about 4 percent—is at elevations above 2,000 metres (about 6,560 feet). Most of the land, 25.3 percent of the total Earth, is between 0 and 2,000 metres. About 13.6 percent of the total land area is at higher elevations, with 86.4 percent between 0 and 2,000 metres when the areas are determined relative to land area only. In the oceans the percentages of the area devoted to depth increments yield information about the typical structure and shape of the oceanic basins. The small depth increment of 0–200 metres (656 feet) occupies about 5.4 percent of Earth’s total area or 7.6 percent of the oceans’ area. This approximates the world’s area of continental shelves, the shallow flat borderlands of the continents that have been alternately covered by the oceans during interglacial stages and uncovered during glacial periods.
At depths between 200 and 2,000 metres, an area only slightly larger—6.02 percent of Earth’s total area or 8.5 percent of the oceans’ area—is found. These depths are related to the regions of the oceans that have very steep slopes where depth increases rapidly. These are the continental slope regions that mark the true edge of the continental landmasses. Marginal seas of moderate depths and the tops of seamounts, however, add their area to these depth zones when all the oceans are considered. The majority of the oceanic area lies between 4,000 and 5,000 metres (about 13,100 and 16,400 feet).
The continental shelf region varies immensely from place to place. The seaward boundary of the continental shelf historically is determined by the 100-fathom, or 200-metre, depth contour. However, 85 fathoms, or 170 metres [about 560 feet], is a closer approximation. The true boundary at any given location is marked by a rapid change in slope of the seafloor known as the shelf break. This change in slope may be nearly at the coastline in areas where crustal plates converge, as along the west coast of North and South America, or it may be located more than 1,000 km (about 620 miles) seaward of the coast, as off the north coast of Siberia. The average width of the shelf is about 75 km (about 45 miles), and the shelf has an average slope of about 0.01°, a slope that is barely discernible to the human eye. Seaward of the shelf break, the continental slope is inclined by about 4°.
Origin Of The Ocean Waters
The huge volume of water contained in the oceans (and seas), 137 × 10^7 cubic km (about 33 × 10^7 cubic miles), has been produced during Earth’s geologic history. There is little information on the early history of Earth’s waters. However, fossils dated from the Precambrian some 3.3 billion years ago show that bacteria and cyanobacteria (blue-green algae) existed then, indicating the presence of water during that period. Carbonate sedimentary rocks, obviously laid down in an aquatic environment, have been dated to 1 billion years ago. Also, there is fossil evidence of primitive marine algae and invertebrates from the Ediacaran Period (635 million to 541 million years ago).
The presence of water on Earth at even earlier times is not documented by physical evidence. It has been suggested, however, that the early hydrosphere formed in response to condensation from the early atmosphere. The ratios of certain chemical elements on Earth indicate that the planet formed by the accumulation of cosmic dust and was slowly warmed by radioactive and compressional heating. This heating led to the gradual separation and migration of materials to form Earth’s core, mantle, and crust. The early atmosphere is thought to have been highly reducing and rich in gases, notably in hydrogen, and to include water vapour.
Earth’s surface temperature and the partial pressures of the individual gases in the early atmosphere affected the atmosphere’s equilibration with the terrestrial surface. As time progressed and the planetary interior continued to warm, the composition of the gases escaping from within Earth gradually changed the properties of its atmosphere, producing a gaseous mixture rich in carbon dioxide (CO2), carbon monoxide (CO), and molecular nitrogen (N2). Photodissociation (i.e., separation due to the energy of light) of water vapour into molecular hydrogen (H2) and molecular oxygen (O2) in the upper atmosphere allowed the hydrogen to escape and led to a progressive increase of the partial pressure of oxygen at Earth’s surface. The reaction of this oxygen with the materials of the surface gradually caused the vapour pressure of water vapour to increase to a level at which liquid water could form. This water in liquid form accumulated in isolated depressions of Earth’s surface, forming the nascent oceans. The high carbon dioxide content of the atmosphere at this time would have allowed a buildup of dissolved carbon dioxide in the water and made these early oceans acidic and capable of dissolving surface rocks that would add to the water’s salt content. Water must have evaporated and condensed rapidly and accumulated slowly at first. The required buildup of atmospheric oxygen was slow because much of this gas was used to oxidize methane, ammonia, and exposed rocks high in iron. Gradually, the partial pressure of the oxygen gas in the atmosphere rose as photosynthesis by bacteria and photodissociation continued to supply oxygen. Biological processes involving algae increased, and they gradually decreased the carbon dioxide content and increased the oxygen content of the atmosphere until the oxygen produced by biological processes outweighed that produced by photodissociation. This, in turn, accelerated the formation of surface water and the development of the oceans.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1017 2021-05-12 00:07:42
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,002
Re: Miscellany
995) Strait
A strait is a naturally formed, narrow, typically navigable waterway that connects two larger bodies of water. Most commonly it is a channel of water that lies between two land masses. Some straits are not navigable, for example because they are too shallow, or because of an unnavigable reef or archipelago. Straits are also known to be loci for sediment accumulation. Usually, sand-size deposits occur on both the two opposite strait exits, forming subaqueous fans or deltas.
Terminology
The terms channel, pass, or passage can be synonymous and used interchangeably with strait, although each is sometimes differentiated with varying senses. In Scotland firth or Kyle are also sometimes used as synonyms for strait.
Many straits are economically important. Straits can be important shipping routes and wars have been fought for control of them.
Numerous artificial channels, called canals, have been constructed to connect two bodies of water over land, such as the Suez Canal. Although rivers and canals often provide passage between two large lakes or a lake and a sea, and these seem to suit the formal definition of strait, they are not usually referred to as such. The term strait is typically reserved for much larger, wider features of the marine environment. There are exceptions, with straits being called canals, Pearse Canal, for example.
Comparisons
Straits are the converse of isthmuses. That is, while a strait lies between two land masses and connects two larger bodies of water, an isthmus lies between two bodies of water and connects two larger land masses.
Some straits have the potential to generate significant tidal power using tidal stream turbines. Tides are more predictable than wave power or wind power. The Pentland Firth (a strait) may be capable of generating 10 GW. Cook Strait in New Zealand may be capable of generating 5.6 GW even though the total energy available in the flow is 15 GW.
Navigational (legal) regime
Straits used for international navigation through the territorial sea between one part of the high seas or an exclusive economic zone and another part of the high seas or an exclusive economic zone are subject to the legal regime of transit passage (Strait of Gibraltar, Dover Strait, Strait of Hormuz). The regime of innocent passage applies in straits used for international navigation (1) that connect a part of high seas or an exclusive economic zone with the territorial sea of coastal nation (Strait of Tiran, Strait of Juan de Fuca, Strait of Baltiysk) and (2) in straits formed by an island of a state bordering the strait and its mainland if there exists seaward of the island a route through the high seas or through an exclusive economic zone of similar convenience with respect to navigational and hydrographical characteristics (Strait of Messina, Pentland Firth). There may be no suspension of innocent passage through such straits.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1018 2021-05-13 00:03:59
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,002
Re: Miscellany
996) Hydrology
Hydrology, scientific discipline concerned with the waters of the Earth, including their occurrence, distribution, and circulation via the hydrologic cycle and interactions with living things. It also deals with the chemical and physical properties of water in all its phases.
A brief treatment of hydrology follows.
Hydrology has as its primary objective the study of the interrelationship between water and its environment. As hydrology is mainly concerned with water close to the land surface, it focuses on those components of the hydrologic cycle that occur there—namely, precipitation, evapotranspiration, runoff, and groundwater. Its various subdisciplines deal with different aspects of these phenomena. Hydrometeorology, for example, concentrates on water in the lower boundary layer of the atmosphere, while hydrometry involves the measurement of surface water, especially precipitation and streamflow. Hydrography entails the description and mapping of large bodies of surface water, such as lakes, inland seas, and oceans. On the other hand, groundwater hydrology centres on subsurface water in the saturated zone, and soil-water physics on that in the unsaturated zone.
Hydrology draws upon the disciplines of geology, chemistry, soil science, and plant physiology, employing many of their principles and methods. Researchers in the field rely increasingly on computer simulations of natural hydrologic systems and remote-sensing techniques, as, for example, the use of Earth-orbiting satellites equipped with infrared cameras to detect bodies of polluted water or to trace the flow of hot springs.
Hydrologic research is important in the development, management, and control of water resources. Its applications are manifold and include irrigation-systems development, flood and land-erosion control, waste-water disposal and treatment, pollution abatement, recreational use of water, fish and wildlife preservation, hydropower generation, and the design of hydraulic structures.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1019 2021-05-14 00:07:05
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,002
Re: Miscellany
997) Clay
Clay, soil particles the diameters of which are less than 0.005 millimetre; also a rock that is composed essentially of clay particles. Rock in this sense includes soils, ceramic clays, clay shales, mudstones, glacial clays (including great volumes of detrital and transported clays), and deep-sea clays (red clay, blue clay, and blue mud). These are all characterized by the presence of one or more clay minerals, together with varying amounts of organic and detrital materials, among which quartz is predominant. Clay materials are plastic when wet, and coherent when dry. Most clays are the result of weathering.
No other earth material has so wide an importance or such extended uses as do the clays. They are used in a wide variety of industries. As soils, they provide the environment for almost all plant growth and hence for nearly all life on the Earth’s surface. They provide porosity, aeration, and water retention and are a reservoir of potassium oxide, calcium oxide, and even nitrogen.
The use of clay in pottery making antedates recorded human history, and pottery remains provide a record of past civilizations. As building materials, bricks (baked and as adobe) have been used in construction since earliest time. Impure clays may be used to make bricks, tile, and the cruder types of pottery, while kaolin, or china clay, is required for the finer grades of ceramic materials. Another major use of kaolin is as paper coating and filler; it gives the paper a gloss and increases the opacity. Refractory materials, including fire brick, chemical ware, and melting pots for glass, also make use of kaolin together with other materials that increase resistance to heat. Certain clays known as fuller’s earth have long been used in wool scouring. In rubber compounding, the addition of clay increases resistance to wear and helps eliminate molding troubles.
Clay materials have a wide variety of uses in engineering. Earth dams are made impermeable to water by adding suitable clay materials to porous soil; water loss in canals may be reduced by adding clay. The essential raw materials of portland cement are limestone and clays, commonly impure. After acid treatment, clays have been used as water softeners; the clay removes calcium and magnesium from the solution and substitutes sodium. A major use of clay is as drilling mud—i.e., heavy suspension consisting of chemical additives and weighting materials, along with clays, employed in rotary drilling.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1020 2021-05-15 00:21:33
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,002
Re: Miscellany
998) Sand
Sand, mineral, rock, or soil particles that range in diameter from 0.02 to 2 mm (0.0008–0.08 inch). Most of the rock-forming minerals that occur on the Earth’s surface are found in sand, but only a limited number are common in this form. Although in some localities feldspar, calcareous material, iron ores, and volcanic glass are dominant constituents of sand, quartz is by far the commonest, for several reasons: it is abundant in rocks, is comparatively hard, has practically no cleavage so that it is not readily worn down, is nearly insoluble in water, and does not decompose. Most quartzose sands contain a small quantity of feldspar, as well as small plates of white mica, which, though soft, decompose slowly.
All sands contain small quantities of heavy rock-forming minerals, including garnet, tourmaline, zircon, rutile, topaz, pyroxenes, and amphiboles. In some shore and river sands these heavier constituents, as well as some of the heavy native elements, become concentrated as a result of sorting by currents and the removal of the lighter constituents. Such placer sands may be economically valuable deposits worked for diamonds and other gemstones, gold, platinum, tin, monazite, and other ores. Greensands, widely distributed over the floor of the ocean and found in ancient strata on the continents, owe their colour to the presence of glauconite, a potash-bearing mineral; these sands are used for water softeners.
In the pottery and glassmaking industries very pure quartzose sands are used as a source of silica. Similar sands are required for lining the hearths of acid-steel furnaces. Molds used in foundries for casting metal are made of sand with a clay binder. Quartz and garnet sands are used extensively as abrasives. Ordinary sands find a multitude of other uses—e.g., in the preparation of mortar, cement, and concrete.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1021 2021-05-16 00:29:00
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,002
Re: Miscellany
999) Soil Composition
Soil is one of the most important elements of an ecosystem, and it contains both biotic and abiotic factors. The composition of abiotic factors is particularly important as it can impact the biotic factors, such as what kinds of plants can grow in an ecosystem.
Soil contains air, water, and minerals as well as plant and animal matter, both living and dead. These soil components fall into two categories. In the first category are biotic factors—all the living and once-living things in soil, such as plants and insects. The second category consists of abiotic factors, which include all nonliving things—for example, minerals, water, and air. The most common minerals found in soil that support plant growth are phosphorus, and potassium and also, nitrogen gas. Other, less common minerals include calcium, magnesium, and sulfur. The biotic and abiotic factors in the soil are what make up the soil’s composition.
Soil composition is a mix of soil ingredients that varies from place to place. The Natural Resources Conservation Service (NRCS)—part of the U.S. Department of Agriculture—has compiled soil maps and data for 95 percent of the United States. The NRCS has found that each state has a “state soil” with a unique soil “recipe” that is specific to that state. These differing soils are the reason why there is such a wide variety of crops grown in the United States.
Consider the soils of three states: Hawai'i, Iowa, and Maine. Hawai'i’s deep, well-drained state soil contains volcanic ash that makes it perfect for growing sugar cane, as well as ginger roots, papaya, and macadamia nuts. Iowa, which is in Midwest region of the United States, has a state soil that is good for farming because it is made up of a thick layer of organic matter from the decomposition of prairie grasses. Corn and soybeans are the primary crops grown in these soils. The state soil of Maine, located in the northeastern part of the country, is made from materials left behind after local glaciers melted. This soil is perfect for growing trees—specifically, red spruce and balsam fir. Many of the trees being grown today in Maine are harvested for timber or for making paper.
Soil scientists conduct various tests on soils to learn about their composition. Soil testing can identify the amounts of biotic and abiotic factors in the soil. The results of these tests can also reveal if the soil has too much of a specific mineral or if it needs more nutrients to support plants. Scientists also measure other factors, such as the amount of water in the soil and how it varies over time—for instance, is the soil unusually wet or dry? The tests can also identify contaminants and heavy metal in the soil and determine the soil’s nitrogen content and pH level (acidity or alkalinity). All of these measurements can be used to determine the soil’s health.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1022 2021-05-17 00:25:55
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,002
Re: Miscellany
1000) Kaolin
Kaolin is a type of clay found in nature. It can also be made in a laboratory. People use it to make medicine. Kaolin is used for mild-to-moderate diarrhea, severe diarrhea (dysentery), and cholera.
Kaolin, also called china clay, soft white clay that is an essential ingredient in the manufacture of china and porcelain and is widely used in the making of paper, rubber, paint, and many other products. Kaolin is named after the hill in China (Kao-ling) from which it was mined for centuries. Samples of kaolin were first sent to Europe by a French Jesuit missionary around 1700 as examples of the materials used by the Chinese in the manufacture of porcelain.
In its natural state kaolin is a white, soft powder consisting principally of the mineral kaolinite, which, under the electron microscope, is seen to consist of roughly hexagonal, platy crystals ranging in size from about 0.1 micrometre to 10 micrometres or even larger. These crystals may take vermicular and booklike forms, and occasionally macroscopic forms approaching millimetre size are found. Kaolin as found in nature usually contains varying amounts of other minerals such as muscovite, quartz, feldspar, and anatase. In addition, crude kaolin is frequently stained yellow by iron hydroxide pigments. It is often necessary to bleach the clay chemically to remove the iron pigment and to wash it with water to remove the other minerals in order to prepare kaolin for commercial use.
When kaolin is mixed with water in the range of 20 to 35 percent, it becomes plastic (i.e., it can be molded under pressure), and the shape is retained after the pressure is removed. With larger percentages of water, the kaolin forms a slurry, or watery suspension. The amount of water required to achieve plasticity and viscosity varies with the size of the kaolinite particles and also with certain chemicals that may be present in the kaolin. Kaolin has been mined in France, England, Saxony (Germany), Bohemia (Czech Republic), and in the United States, where the best-known deposits are in the southeastern states.
Approximately 40 percent of the kaolin produced is used in the filling and coating of paper. In filling, the kaolin is mixed with the cellulose fibre and forms an integral part of the paper sheet to give it body, colour, opacity, and printability. In coating, the kaolin is plated along with an adhesive on the paper’s surface to give gloss, colour, high opacity, and greater printability. Kaolin used for coating is prepared so that most of the kaolinite particles are less than two micrometres in diameter.
Kaolin is used extensively in the ceramic industry, where its high fusion temperature and white burning characteristics makes it particularly suitable for the manufacture of whiteware (china), porcelain, and refractories. The absence of any iron, alkalies, or alkaline earths in the molecular structure of kaolinite confers upon it these desirable ceramic properties. In the manufacture of whiteware the kaolin is usually mixed with approximately equal amounts of silica and feldspar and a somewhat smaller amount of a plastic light-burning clay known as ball clay. These components are necessary to obtain the proper properties of plasticity, shrinkage, vitrification, etc., for forming and firing the ware. Kaolin is generally used alone in the manufacture of refractories.
Substantial tonnages of kaolin are used for filling rubber to improve its mechanical strength and resistance to abrasion. For this purpose, the clay used must be extremely pure kaolinite and exceedingly fine grained. Kaolin is also used as an extender and flattening agent in paints. It is frequently used in adhesives for paper to control the penetration into the paper. Kaolin is an important ingredient in ink, organic plastics, some cosmetics, and many other products where its very fine particle size, whiteness, chemical inertness, and absorption properties give it particular value.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1023 2021-05-18 00:29:56
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,002
Re: Miscellany
1001) Sedimentation
Sedimentation, in the geological sciences, process of deposition of a solid material from a state of suspension or solution in a fluid (usually air or water). Broadly defined it also includes deposits from glacial ice and those materials collected under the impetus of gravity alone, as in talus deposits, or accumulations of rock debris at the base of cliffs. The term is commonly used as a synonym for sedimentary petrology and sedimentology.
The physics of the most common sedimentation process, the settling of solid particles from fluids, has long been known. The settling velocity equation formulated in 1851 by G.G. Stokes is the classic starting point for any discussion of the sedimentation process. Stokes showed that the terminal settling velocity of spheres in a fluid was inversely proportional to the fluid’s viscosity and directly proportional to the density difference of fluid and solid, the radius of the spheres involved, and the force of gravity. Stokes’ equation is valid, however, only for very small spheres (under 0.04 millimetre [0.0015 inch] in diameter) and hence various modifications of Stokes’ law have been proposed for nonspherical particles and particles of larger size.
No settling velocity equation, however valid, provides a sufficient explanation of even the basic physical properties of natural sediments. The grain size of the clastic elements and their sorting, shape, roundness, fabric, and packing are the results of complex processes related not only to the density and viscosity of the fluid medium but also to the translational velocity of the depositing fluid, the turbulence resulting from this motion, and the roughness of the beds over which it moves. These processes also are related to various mechanical properties of the solid materials propelled, to the duration of sediment transport, and to other little-understood factors.
Sedimentation is generally considered by geologists in terms of the textures, structures, and fossil content of the deposits laid down in different geographic and geomorphic environments. Great efforts have been made to differentiate between continental, near-shore, marine, and other deposits in the geologic record. The classification of environments and criteria for their recognition is still a subject of lively debate. The analysis and interpretation of ancient deposits has been advanced by the study of modern sedimentation. Oceanographic and limnologic expeditions have shed much light on sedimentation in the Gulf of Mexico, the Black Sea, and the Baltic Sea, and in various estuaries, lakes, and fluvial basins in all parts of the world.
Chemical sedimentation is understood in terms of chemical principles and laws. Although the famous physical chemist J.H. van’t Hoff applied the principles of phase equilibria to the problem of crystallizing brines and the origin of salt deposits as early as 1905, little effort was made to apply physical chemistry to the problems of chemical sedimentation. More recently, however, there has been investigation of the role of the redox (mutual reduction and oxidation) potential and pH (acidity–alkalinity) in the precipitation of many chemical sediments, and a renewed effort has been made to apply known thermodynamic principles to the origin of anhydrite and gypsum deposits, to the chemistry of dolomite formation, and to the problem of the ironstones and related sediments.
The geochemist also considers the sedimentation process in terms of the chemical end products. To him sedimentation is like a gigantic chemical analysis in which the primary constituents of the Earth’s silicate crust are separated from one another in a manner similar to that achieved in the course of a quantitative analysis of rock material in the laboratory. The results of this chemical fractionation are not always perfect, but by and large the results are remarkably good. Geochemical fractionation, which began in Precambrian time, has resulted in an enormous accumulation of sodium in the sea, calcium and magnesium in the limestones and dolomites, silicon in the bedded cherts and orthoquartzitic sandstones, carbon in the carbonates and carbonaceous deposits, sulfur in the bedded sulfates, iron in the ironstones, and so on. Although magmatic segregation has, in some instances, produced monomineralic rocks such as dunite and pyroxenite, no igneous or metamorphic process can match the sedimentation process in effective isolation and concentration of these and other elements.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1024 2021-05-19 00:34:42
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,002
Re: Miscellany
1002) Protein
What Is Protein?
Protein is a macronutrient that is essential to building muscle mass. It is commonly found in animal products, though is also present in other sources, such as nuts and legumes.
There are three macronutrients: protein, fats and carbohydrates. Macronutrients provide calories, or energy. The body requires large amounts of macronutrients to sustain life, hence the term “macro,” according to the University of Illinois McKinley Health Center. Each gram of protein contains 4 calories. Protein makes up about 15 percent of a person’s body weight.
Chemically, protein is composed of amino acids, which are organic compounds made of carbon, hydrogen, nitrogen, oxygen or sulfur. Amino acids are the building blocks of proteins, and proteins are the building blocks of muscle mass, according to the National Institutes of Health (NIH).
“When protein is broken down in the body it helps to fuel muscle mass, which helps metabolism," said Jessica Crandall, a registered dietitian nutritionist, certified diabetes educator and national spokesperson for the Academy of Nutrition and Dietetics. "It also helps the immune system stay strong. It helps you stay full. A lot of research has shown that protein has satiety effects.”
For example, two recent studies showed that satiety, or feeling full after a meal, improved after consuming a high-protein snack. A 2014 study published in the journal Nutrition compared afternoon snacks of high-protein yogurt, high-fat crackers and high-fat chocolate. Among the women who participated in the study, consuming the yogurt led to greater reductions in afternoon hunger versus the chocolate. These women also ate less at dinner compared to the women who snacked on crackers and chocolate.
A similar study published in 2015 in the Journal of Nutrition found that adolescents who consumed high-protein afternoon snacks showed improved appetite, satiety and diet quality. The teens also had improved moods and better cognition.
How much protein?
The Institute of Medicine recommends that 10 to 35 percent of daily calories come from protein. How that equates to grams of protein depends on the caloric needs of the individual. According to the U.S. Department of Agriculture, the amount of protein foods a person should eat depends on age, sex, and level of physical activity. Most Americans eat enough food from this group, but need to make leaner and more varied selections of these foods.
“A safe level of protein ranges from 0.8 grams of protein per kilogram of body weight [2.2 lbs.], up to 2 grams of protein per kilogram for very active athletes,” said Crandall. “But most Americans truly need to be eating about 1 to 1.2 grams of protein per kilogram of body weight.”
Most people need 20 to 30 grams of protein per meal, said Crandall. “For example, that’s 2.5 egg whites at breakfast or 3 to 4 ounces of meat at dinner.” She said that most American women are not getting anywhere close to adequate protein at breakfast. “That could be hindering their muscle mass, their metabolism and their hormone levels.”
Crandall cautioned parents against stressing protein consumption for their children, who typically get sufficient protein easily. “It’s important to focus on fruits and vegetables for kids, but protein supplementation for kids is going overboard,” she said. When considering how to get protein into kids’ diets, parents should focus on whole foods and natural sources.
Sources of protein
All food made from meat, poultry, seafood, beans and peas, eggs, processed soy products, nuts and seeds are considered part of the protein group, according to the USDA. Most people eat enough food in this group, but they should select leaner and more varied selections.
Besides animal sources, there are several alternative sources of protein, including soy, hemp and whey. Crandall said that all are good options and it comes down to personal preference. For example, whey protein is better for building and regenerating muscle mass, so people looking to bulk up or who exercise a lot may prefer it.
Whey protein is a by-product of the cheese-making process and therefore not vegan. It is typically found in supplements, such as protein powders, according to Medical News Today. It is usually used to promote lean muscle mass and is also associated with weight loss, according to a 2008 study published in Nutrition & Metabolism. There are 20 grams of protein per scoop of whey protein.
Hemp protein comes from the hemp plant, which does not have THC, according to the North American Industrial Hemp Council. Hemp is available as seeds, a powder and milk. There are 5.3 grams of protein per tablespoon of hemp seeds, about 5 grams per scoop of hemp powder and 5 grams per cup.
Soy protein comes from soybeans and is available in many different forms, including milk, tofu, various meat substitutes, flour, oil, tempeh, miso nuts and edamame, according to the University of California San Francisco Medical Center. Crandall said that soy is a good source of protein.
“Soy has been shown to have a little more phytoestrogens in it from isoflavones, which really helps to increase antioxidants,” she said. “But a lot of people are hesitant to do soy because of a myth that associates it with breast cancer. But that myth has been minimized based off of a large body of evidence that supports the actual anticancer properties that soy has.” She pointed to a 2012 study published by the American Institute for Cancer Research.
To get the maximum benefits from soy, Crandall recommended eating whole sources, like edamame. Processed forms like tofu are the next best option, followed by protein powders and drinks.
High-protein foods
According to Matthew Kadey, a registered dietitian writing for Bodybuilding.com, some high-protein meats include:
• Top or bottom round steak (23 grams of protein per 3-ounce serving)
• Lean ground beef (18 grams per 3-ounce serving)
• Pork chops (26 grams per 3-ounce serving)
• Skinless chicken breast (24 grams per 3-ounce serving)
• Turkey breast (24 grams per 3-ounce serving)
• Sockeye salmon (23 grams per 3-ounce serving)
• Yellowfin tuna (25 grams per 3-ounce serving)
High-protein dairy foods include:
• Greek yogurt (23 grams per 8-ounce serving)
• Cottage cheese (14 grams per half-cup serving)
• Eggs (6 grams per large egg)
• 2 percent milk (8 grams per cup)
Some other high-protein foods are:
• Some canned foods, like sardines, anchovies and tuna average around 22 grams of protein per serving
• Navy beans (20 grams per cup)
• Lentils (13 grams per quarter-cup)
• Peanut butter (8 grams per 2 tablespoons)
• Mixed nuts (6 grams per 2-ounce serving)
• Quinoa (8 grams per 1-cup serving)
• Edamame (8 grams per half-cup serving)
• Soba noodles (12 grams per 3-ounce serving)
Complete or ideal proteins
People can produce some amino acids, but must get others from food. The nine amino acids that humans cannot produce on our own are called essential amino acids, according to the NIH. Essential amino acids are: histidine, isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan and valine.
Protein foods that contain all essential amino acids are called complete proteins, according to Crandall. They are also sometimes called ideal proteins or high-quality proteins. Complete proteins include meat and dairy products, quinoa, hemp seeds, chia seeds and soy.
Many plant-based proteins are not complete proteins. These include beans, grains and legumes as well as vegetables, which contain small amounts of protein. According to the University of Massachusetts at Amherst, incomplete proteins can be combined to create complete proteins. Beans and rice, peanut butter and whole grain bread, and macaroni and cheese are examples of combinations that create complete proteins.
For a long time, nutritionists thought that complementary proteins had to be eaten together to make a complete protein. But it is now understood that the foods don’t have to be eaten at exactly the same time, said Crandall. As long as you eat a wide variety of foods, you can usually make complete proteins, even if you’re a vegetarian.
High-protein diet
The Institute of Medicine recommends that 10 to 35 percent of daily calories come from protein. Most Americans do not get close to the 35 percent mark; they eat about 12 to 18 percent of their calories as protein, according to the NIH. Therefore, most commercial high-protein diet plans suggest intakes in the upper levels of the recommended spectrum. For example, the Atkins diet allows for up to 29 percent of calories to come from protein, and the South Beach Diet suggests protein levels at about 30 percent. Some high-protein diets, however, come in at higher than 35 percent.
The efficacy and safety of high-protein diets is still being studied. Often, they lead to a quick drop in weight-loss but their overall sustainability is unclear. One 2011 review of high-protein diet studies found that “although half of the studies showed a higher weight loss with a high-protein diet, three out of four studies with the longest intervention show no statistical difference in weight loss.”
Futhermore, high-protein diets can carry some health risks. They usually advocate cutting carbohydrates, which can lead to nutritional deficiencies, fiber deficiencies, headache, constipation, increased risk of heart disease and worse kidney function in those suffering from kidney disease, according to the Mayo Clinic.
Crandall does not recommend high-protein diets because they are generally unnecessary. “There’s a growing body of research that suggests that Americans are getting enough protein,” she said. The problem is that we don’t space out our protein correctly. “It’s more important that we focus on getting protein at each meal, eating it within the first hour of waking up and then every 4 to 6 hours thereafter.”
Getting enough protein at adequate intervals helps muscle mass and overall health long term.
Crandall is also skeptical of protein-enhanced foods. “There are a lot of products now that have protein added. But is that getting you full? Is that getting you what you need? Make sure you’re thinking about meal planning a little bit … don’t let that become the go-to option for meals.”
Ideal Protein diet
The Ideal Protein diet is a medically developed diet plan created more than 20 years ago by French doctor Tran Tien Chanh. A coach at a licensed clinic or a health care provider supervises participants. For some participants, consent from health care providers may be required.
The Ideal Protein diet is a low-carbohydrate, low-calorie, high-protein diet that aims to aid in weight loss by providing the body with the right amount and kind of protein while also stabilizing blood sugar. It consists of four phases. During the first three phases, participants eat at least one proportioned, prepackaged Ideal Protein meal per day. During phase one, in which most of the weight loss takes place, participants eat three Ideal Protein meals every day.
Protein shakes
“Supplements are for supplemental purposes only,” said Crandall. Therefore, she does not recommend having protein shakes on a daily basis. Sometimes, however, people have serious behavioral barriers to eating whole foods. “If they feel like they can’t cook or eat whole foods … [protein shakes] can be a good plan B.”
If you are going to use protein shakes, Crandall recommends choosing one that has more than 20 grams of protein. “Most Americans want to shoot for lower-calorie, lower-carbohydrate drinks,” she said.
It is important to think about what you’re adding to protein shakes. If you’re using a protein powder to make a shake, Crandall suggests mixing it with water, nonfat milk or a milk substitute. “I strongly suggest not mixing fruit in — it can become very calorie-laden — like pie in a cup.” Adding vegetables, however, can add antioxidants and vitamins.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1025 2021-05-20 00:41:30
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,002
Re: Miscellany
1003) Vitamins
If you're like most kids, you've probably heard at least one parent say, "Don't forget to take your vitamin!" or "Eat your salad — it's packed with vitamins!" But what exactly are vitamins?
Vitamins and minerals are substances that are found in foods we eat. Your body needs them to work properly, so you grow and develop just like you should. When it comes to vitamins, each one has a special role to play. For example:
• Vitamin D in milk helps your bones.
• Vitamin A in carrots helps you see at night.
• Vitamin C in oranges helps your body heal if you get a cut.
• B vitamins in whole grains help your body make energy from food.
Vitamins Hang Out in Water and Fat
There are two types of vitamins: fat soluble and water soluble.
When you eat foods that contain fat-soluble vitamins, the vitamins are stored in the fat tissues in your body and in your liver. They wait around in your body fat until your body needs them.
Fat-soluble vitamins are happy to stay stored in your body for awhile — some stay for a few days, some for up to 6 months! Then, when it's time for them to be used, special carriers in your body take them to where they're needed. Vitamins A, D, E, and K are all fat-soluble vitamins.
Water-soluble vitamins are different. When you eat foods that have water-soluble vitamins, the vitamins don't get stored as much in your body. Instead, they travel through your bloodstream. Whatever your body doesn't use comes out when you urinate (pee).
So these kinds of vitamins need to be replaced often because they don't stick around! This crowd of vitamins includes vitamin C and the big group of B vitamins — B1 (thiamin), B2 (riboflavin), niacin, B6 (pyridoxine), folic acid, B12 (cobalamine), biotin, and pantothenic acid.
Vitamins Feed Your Needs
Your body is one powerful machine, capable of doing all sorts of things by itself. But when it comes to vitamins, it can use some help. That's where food comes in. Your body is able to get the vitamins it needs from the foods you eat because different foods contain different vitamins. The key is to eat different foods to get an assortment of vitamins. Though some kids take a daily vitamin, most kids don't need one if they're eating a variety of healthy foods.
Now, let's look more closely at vitamins — from A to K:
Vitamin A
This vitamin plays a really big part in eyesight. It's great for night vision, like when you're trick-or-treating on Halloween. Vitamin A helps you see in color, too, from the brightest yellow to the darkest purple. In addition, it helps your body fight infections by boosting your immune system.
Which foods are rich in vitamin A?
• milk fortified with vitamin A
• liver
• orange fruits and vegetables (like cantaloupe, carrots, sweet potatoes)
• dark green leafy vegetables (like kale, collards, spinach)
The B Vitamins
There's more than one B vitamin. Here's the list: B1, B2, B6, B12, niacin, folic acid, biotin, and pantothenic acid. Whew — that's quite a group!
The B vitamins are important in metabolic activity — this means that they help make energy and set it free when your body needs it. So the next time you're running to third base, thank those B vitamins.
This group of vitamins is also involved in making red blood cells, which carry oxygen throughout your body. Every part of your body needs oxygen to work properly, so these B vitamins have a really important job.
Which foods are rich in vitamin B?
• whole grains, such as wheat and oats
• fish and seafood
• poultry and meats
• eggs
• dairy products, like milk and yogurt
• leafy green vegetables
• beans and peas
Vitamin C
This vitamin is important for keeping body tissues, such as gums, bones, and blood vessels in good shape. C is also key if you get a cut or wound because it helps you heal.
This vitamin also helps your body resist infection. This means that even though you can't always avoid getting sick, vitamin C makes it a little harder for your body to become infected with an illness.
Which foods are rich in vitamin C?
• citrus fruits, like oranges
• cantaloupe
• strawberries
• tomatoes
• broccoli
• cabbage
• kiwi fruit
• sweet red peppers
Vitamin D
No bones about it . . . vitamin D is the vitamin you need for strong bones! It's also great for forming strong teeth. Vitamin D even lends a hand to an important mineral — it helps your body absorb the amount of calcium it needs. Vitamin D is made in the skin when exposed to sunlight, or you can get it from the foods you eat.
Which foods are rich in vitamin D?
• milk fortified with vitamin D
• fish
• egg yolks
• liver
• fortified cereal
Vitamin E
Everybody needs E. This hard-working vitamin protects your cells and tissues from damage. It is also important for the health of red blood cells.
Which foods are rich in vitamin E?
• whole grains, such as wheat and oats
• wheat germ
• leafy green vegetables
• vegetable oils like sunflower, canola, and olive
• egg yolks
• nuts and seeds
Vitamin K
Vitamin K is the clotmaster! Remember the last time you got a cut? Your blood did something special called clotting. This is when certain cells in your blood act like glue and stick together at the surface of the cut to help stop the bleeding.
Which foods are rich in vitamin K?
• leafy green vegetables
• dairy products, like milk and yogurt
• broccoli
• soybean oil
When your body gets this vitamin and the other ones it needs, you'll be feeling A-OK!

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline