Math Is Fun Forum
You are not logged in.
- Topics: Active | Unanswered
#1026 2021-05-21 00:31:20
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,771
Re: Miscellany
1004) Minerals
Did you ever notice how TV commercials for breakfast cereal always mention vitamins and minerals? But when you think of minerals, food isn't the first thing that comes to mind. Aren't minerals something you find in the earth, like iron and quartz?
Well, yes, but small amounts of some minerals are also in foods.
Just like vitamins, minerals help your body grow, develop, and stay healthy. The body uses minerals to perform many different functions — from building strong bones to transmitting nerve impulses. Some minerals are even used to make hormones or maintain a normal heartbeat.
Macro and Trace
The two kinds of minerals are: macrominerals and trace minerals. Macro means "large" in Greek (and your body needs larger amounts of macrominerals than trace minerals). The macromineral group is made up of calcium, phosphorus, magnesium, sodium, potassium, chloride, and sulfur.
A trace of something means that there is only a little of it. So even though your body needs trace minerals, it needs just a tiny bit of each one. Trace minerals includes iron, manganese, copper, iodine, zinc, cobalt, fluoride, and selenium.
Let's take a closer look at some of the minerals you get from food.
Calcium
Calcium is the top macromineral when it comes to your bones. This mineral helps build strong bones, so you can do everything from standing up straight to scoring that winning goal. It also helps build strong, healthy teeth, for chomping on tasty food.
Which foods are rich in calcium?
• dairy products, such as milk, cheese, and yogurt
• canned salmon and sardines with bones
• leafy green vegetables, such as broccoli
• calcium-fortified foods — from orange juice to cereals and crackers
Iron
The body needs iron to transport oxygen from your lungs to the rest of your body. Your entire body needs oxygen to stay healthy and alive. Iron helps because it's important in the formation of hemoglobin, which is the part of your red blood cells that carries oxygen throughout the body.
Which foods are rich in iron?
• meat, especially red meat, such as beef
• tuna and salmon
• eggs
• beans
• baked potato with skins
• dried fruits, like raisins
• leafy green vegetables, such as broccoli
• whole and enriched grains, like wheat or oats
Potassium
Potassium keeps your muscles and nervous system working properly.
Which foods are rich in potassium?
• bananas
• tomatoes
• potatoes and sweet potatoes, with skins
• green vegetables, such as spinach and broccoli
• citrus fruits, like oranges
• low-fat milk and yogurt
• legumes, such as beans, split peas, and lentils
Zinc
Zinc helps your immune system, which is your body's system for fighting off illnesses and infections. It also helps with cell growth and helps heal wounds, such as cuts.
Which foods are rich in zinc?
• beef, pork, and dark meat chicken
• nuts, such as cashews, almonds, and peanuts
• legumes, such as beans, split peas, and lentils
When people don't get enough of these important minerals, they can have health problems. For instance, too little calcium — especially when you're a kid — can lead to weaker bones. Some kids may take mineral supplements, but most kids don't need them if they eat a nutritious diet. So eat those minerals and stay healthy!

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1027 2021-05-22 00:28:53
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,771
Re: Miscellany
1005) Fat
In nutrition, biology, and chemistry, fat usually means any ester of fatty acids, or a mixture of such compounds; most commonly those that occur in living beings or in food.
The term often refers specifically to triglycerides (triple esters of glycerol), that are the main components of vegetable oils and of fatty tissue in animals; or, even more narrowly, to triglycerides that are solid or semisolid at room temperature, thus excluding oils. The term may also be used more broadly as a synonym of lipid—any substance of biological relevance, composed of carbon, hydrogen, or oxygen, that is insoluble in water but soluble in non-polar solvents. In this sense, besides the triglycerides, the term would include several other types of compounds like mono- and diglycerides, phospholipids (such as lecithin), sterols (such as cholesterol), waxes (such as beeswax), and free fatty acids, which are usually present in human diet in smaller amounts.
Fats are one of the three main macronutrient groups in human diet, along with carbohydrates and proteins, and the main components of common food products like milk, butter, tallow, lard, salt pork, and cooking oils. They are a major and dense source of food energy for many animals and play important structural and metabolic functions, in most living beings, including energy storage, waterproofing, and thermal insulation. The human body can produce the fat that it needs from other food ingredients, except for a few essential fatty acids that must be included in the diet. Dietary fats are also the carriers of some flavor and aroma ingredients and vitamins that are not water-soluble.
In the 1980s it was thought we’d be healthier if we followed a low-fat diet because reducing total fat intake means we reduce our intake of saturated fat. Unfortunately, this theory didn’t take into account what we might eat instead! In the US, as food manufacturers took out the fat and added more sugar instead, Americans kept on eating and just got bigger and unhealthier.
For a long time, we also thought we needed to follow a low-fat diet to lose weight and keep it off. And from a mathematical point of view it seemed to make sense. Per gram, fat has around twice the kilojoules of protein or carbs. But fat also offers more satiety than carbs. And let’s not forget that fats add texture and flavour to food; that’s why we love them.
These days, we know it’s more important to think about the types of fats we’re eating, as well as the amounts. And while a lower-fat diet might suit some for weight loss, for others it’s not helpful. So let’s think again about the fats we eat.
Types of dietary fat
The descriptions of fats relate to their chemical structure. First up, they are described as either saturated or unsaturated. Because of their structure saturated fats can pack together tightly, and at room temperature they form a solid fat. Think coconut oil, palm oil and butter.
Unsaturated fats have kinks in their structure so they can’t pack together as tightly, and are liquid at room temperature. Oils such as canola, sesame, olive and rice bran oil are unsaturated fats.
Unsaturated fats can be polyunsaturated or monounsaturated. The polyunsaturated fats are either omega-6 or omega-3 fats. Our bodies can make some fats, but we can’t produce the essential omega-3s and omega-6s, so we must get these from our diet.
The different chemical structures give rise to the names for fats, but we’ve also learned they have different effects on our health.
We need fat
Fats are a critical part of every cell in our body and we need a certain amount of fat in our diet to maintain optimal health. Vitamins A, D, E and K are fat-soluble, so they’re transported in fats. If we don’t eat fats, we won’t get those vitamins. Fats are also a good source of energy; they’re much more energy-dense than protein or carbs. If we need to put on weight, or we’re using more energy for extra activity, it can be easier to up the amount of fat in our diet so the volume of food we eat doesn’t have to increase as much.
The fats that love us back
Plant foods contain mostly unsaturated fats. Olives, avocados, nuts and seeds, and their oils, are all high in healthy unsaturated fats. The exceptions to the ‘plant food’ rule are coconut, palm kernel and palm fats, which are all very high in saturated fats.
Long-chain omega-3 fats (DHA, EPA and DPA) are mainly found in oily fish, so regularly eating fish is the best way to get these fats. The short-chain omega-3 fat (ALA) is found in plant foods such as walnuts, flaxseeds and oil, pumpkin seeds, legumes and canola oil. Higher intakes of the more-studied long-chain omega-3s are beneficial to heart health, and potentially reduce our risk for dementia, diabetes and asthma. Our body’s ability to convert ALA to long-chain omega-3s is fairly limited.
The fats that don’t love us back
Saturated fats, mostly found in animal foods, are associated with atherosclerosis, the beginning of heart and circulatory disease.
This year, the World Health Organization published a review assessing the effect of modifying saturated fat intake on blood lipids by replacing saturated fats with either polyunsaturated fats, monounsaturated fats or carbohydrates. The best effects for total and LDL cholesterol and triglycerides were found when polyunsaturated fat intakes increased as saturated fat intakes decreased.
Many studies have highlighted the link between higher saturated fat intakes and increased risk for cardiovascular disease. Now a study published in August 2016 takes that further, linking higher saturated fat intake with higher death rates from specific causes. The study followed around 125,000 people for over 30 years, finding all-cause mortality increased with higher saturated fat intake and decreased with higher intakes of both polyunsaturated fat and monounsaturated fat.
So the evidence shows less saturated fat and more unsaturated fat, while enjoying a mainly plant-based diet, should see us in good shape.
How to balance our fats
We don’t have to eliminate saturated fat from our diets. But if we’re choosing foods high in saturated fat most of the time, it’s likely we’re getting too much.
Choose these foods for their healthy fats:
Oily fish: Mackerel, herring, sardines, salmon and tuna all provide long-chain omega-3 fats.
Nuts and seeds: 25—30g a day (a small handful).
Avocado: A heart-healthy butter alternative when in season.
Olive oil: High in monounsaturated fats.
Nut and seed oils: eg. canola, rice bran, sesame, almond, grapeseed…
Limit these foods:
Fried foods, pies, pastries, biscuits and cakes: These can all be high in saturated fats.
Fatty meat: We don’t have to avoid meat, it’s the fat that comes with it we’re limiting.
Sausages and salami: Reduced-fat versions are available.
High-fat cheeses: Cheese is good food, but harder cheeses can be especially high in saturated fat, so portion size and frequency are key.
Butter: A little might not kill you, but it’s best to stick to a small amount, and use alternatives such as avocado.
Full-fat milk and yoghurts: Choose reduced-fat alternatives instead. The good news is our taste buds adapt quite quickly.
Cream: Use only when nothing else will do, and preferably a reduced-fat version; otherwise use alternatives such as a Greek-style yoghurt.
Coconut and coconut fat: Yes, it’s trendy and it’s tasty. And the good news is it’s not as bad for us as butter. But ‘less bad’ does not equal health food wonder. A 2014
Heart Foundation review of the evidence found it is not as good for us as unsaturated plant oils.
Coconut cream: When you need the flavour, choose a coconut milk with the lowest saturated fat content you can find. For non-HFG recipes, you can reduce the amount and still get the flavour.
What about butter?
Despite attention-grabbing headlines like Time magazine’s ‘Eat Butter’ cover from 2015, butter has not suddenly changed from being a concentrated source of saturated fat we need to be wary of, no matter how ‘natural’ it is. Several studies taken out of context do not change the overwhelming evidence that consuming unsaturated fats instead of saturated fats is better for us. Olive, canola, sunflower, rice bran and other unsaturated oils have all been shown to be better for us than butter. This doesn’t mean we need to banish butter. Remember, it’s the whole diet that counts. With butter, just use it in small amounts, or infrequently.
How much fat do we need to eat?
Here’s an example of an 8700 kilojoule day that meets the recommendations for fat.
Breakfast
2 toast slices grainy bread, topped with sardines (1 can sardines in spring water, drained), 3 tablespoons avocado and sliced tomato
Trim flat white
Snack
1 banana, 3 walnuts, 150g pottle low-fat yoghurt
Lunch
Smashed black bean, avocado and rocket wrap, plus snacks
Dinner
Nasi goreng with fried egg, pickled cucumber and carrot
1 Date and orange ball
How the day stacks up
Total energy 8650kJ: 35 per cent of kilojoules from fat (the recommendation is for 20—35 per cent of kilojoules)
Saturated fats 14g: 6 per cent of kilojoules (we’re advised to limit saturated fats to 10 per cent or less of our kilojoules)
Monounsaturated fats 42g: 18 per cent of kilojoules
Polyunsaturated fats 25g (omega-6 plus omega-3 fats): 11 per cent of kilojoules
around 1.3g long-chain omega-3s (the daily suggested dietary targets are 0.61g for men and 0.43g for women)
at least 1.5g ALA (adequate daily intakes are 1.3g for men and 0.8g for women, but more is better, especially for vegetarians and vegans).

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1028 2021-05-23 00:06:12
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,771
Re: Miscellany
1006) Carbohydrates
What Are Carbohydrates?
Carbohydrates are the sugars, starches and fibers found in fruits, grains, vegetables and milk products. Though often maligned in trendy diets, carbohydrates — one of the basic food groups — are important to a healthy diet.
"Carbohydrates are macronutrients, meaning they are one of the three main ways the body obtains energy, or calories," said Paige Smathers, a Utah-based registered dietitian. The American Diabetes Association notes that carbohydrates are the body's main source of energy. They are called carbohydrates because, at the chemical level, they contain carbon, hydrogen and oxygen.
There are three macronutrients: carbohydrates, protein and fats, Smathers said. Macronutrients are essential for proper body functioning, and the body requires large amounts of them. All macronutrients must be obtained through diet; the body cannot produce macronutrients on its own.
The recommended daily amount (RDA) of carbs for adults is 135 grams, according to the National Institutes of Health (NIH); however, the NIH also recommends that everyone should have his or her own carbohydrate goal. Carb intake for most people should be between 45% and 65% of total calories. One gram of carbohydrates equals about 4 calories, so a diet of 1,800 calories per day would equal about 202 grams on the low end and 292 grams of carbs on the high end. However, people with diabetes should not eat more than 200 grams of carbs per day, while pregnant women need at least 175 grams.
Function of carbohydrates
Carbohydrates provide fuel for the central nervous system and energy for working muscles. They also prevent protein from being used as an energy source and enable fat metabolism, according to Iowa State University.
Also, "carbohydrates are important for brain function," Smathers said. They are an influence on "mood, memory, etc., as well as a quick energy source." In fact, the RDA of carbohydrates is based on the amount of carbs the brain needs to function.
Two recent studies published in the journal Proceedings of the National Academy of Sciences have also linked carbs to decision-making. In the studies, people who ate a high-carbohydrate breakfast were less willing to share when playing the "ultimatum game" than those who ate high-protein breakfasts. Scientists speculate this may be caused by baseline dopamine levels, which are higher after eating carbohydrates. This doesn't mean carbs make you mean, but underscores how different types of food intake can affect cognition and behavior.
Simple vs. complex carbohydrates
Carbohydrates are classified as simple or complex, Smathers said. The difference between the two forms is the chemical structure and how quickly the sugar is absorbed and digested. Generally speaking, simple carbs are digested and absorbed more quickly and easily than complex carbs, according to the NIH.
Simple carbohydrates contain just one or two sugars, such as fructose (found in fruits) and galactose (found in milk products). These single sugars are called monosaccharides. Carbs with two sugars — such as sucrose (table sugar), lactose (from dairy) and maltose (found in beer and some vegetables) — are called disaccharides, according to the NIH.
Simple carbs are also in candy, soda and syrups. However, these foods are made with processed and refined sugars and do not have vitamins, minerals or fiber. They are called "empty calories" and can lead to weight gain, according to the NIH.
Complex carbohydrates (polysaccharides) have three or more sugars. They are often referred to as starchy foods and include beans, peas, lentils, peanuts, potatoes, corn, parsnips, whole-grain breads and cereals.
Smathers pointed out that, while all carbohydrates function as relatively quick energy sources, simple carbs cause bursts of energy much more quickly than complex carbs because of the quicker rate at which they are digested and absorbed. Simple carbs can lead to spikes in blood sugar levels and sugar highs, while complex carbs provide more sustained energy.
Studies have shown that replacing saturated fats with simple carbs, such as those in many processed foods, is associated with an increased risk of heart disease and type 2 diabetes.
Smathers offered the following advice: "It's best to focus on getting primarily complex carbs in your diet, including whole grains and vegetables."
Sugars, starches and fibers
In the body, carbs break down into smaller units of sugar, such as glucose and fructose, according to Iowa State University. The small intestine absorbs these smaller units, which then enter the bloodstream and travel to the liver. The liver converts all of these sugars into glucose, which is carried through the bloodstream — accompanied by insulin — and converted into energy for basic body functioning and physical activity.
If the glucose is not immediately needed for energy, the body can store up to 2,000 calories of it in the liver and skeletal muscles in the form of glycogen, according to Iowa State University. Once glycogen stores are full, carbs are stored as fat. If you have insufficient carbohydrate intake or stores, the body will consume protein for fuel. This is problematic because the body needs protein to make muscles. Using protein instead of carbohydrates for fuel also puts stress on the kidneys, leading to the passage of painful byproducts in the urine.
Fiber is essential to digestion. Fibers promote healthy bowel movements and decrease the risk of chronic diseases such as coronary heart disease and diabetes, according to the U.S. Department of Agriculture. However, unlike sugars and starches, fibers are not absorbed in the small intestine and are not converted to glucose. Instead, they pass into the large intestine relatively intact, where they are converted to hydrogen and carbon dioxide and fatty acids. The Institute of Medicine recommends that people consume 14 grams of fiber for every 1,000 calories. Sources of fiber include fruits, grains and vegetables, especially legumes.
Smathers pointed out that carbs are also found naturally in some forms of dairy and both starchy and nonstarchy vegetables. For example, nonstarchy vegetables like lettuces, kale, green beans, celery, carrots and broccoli all contain carbs. Starchy vegetables like potatoes and corn also contain carbohydrates, but in larger amounts.
According to the American Diabetes Association, nonstarchy vegetables generally contain only about 5 grams of carbohydrates per cup of raw vegetables, and most of those carbs come from fiber.
Good carbs vs. bad carbs
Carbohydrates are found in foods you know are good for you (vegetables) and ones you know are not (doughnuts). This has led to the idea that some carbs are "good" and some are "bad." According to Healthy Geezer Fred Cicetti, carbs commonly considered bad include pastries, sodas, highly processed foods, white rice, white bread and other white-flour foods. These are foods with simple carbs. Bad carbs rarely have any nutritional value.
Carbs usually considered good are complex carbs, such as whole grains, fruits, vegetables, beans and legumes. These are not only processed more slowly, but they also contain a bounty of other nutrients.
The Pritikin Longevity Center offers this checklist for determining if a carbohydrate is "good" or "bad."
Good carbs are:
• Low or moderate in calories
• High in nutrients
• Devoid of refined sugars and refined grains
• High in naturally occurring fiber
• Low in sodium
• Low in saturated fat
• Very low in, or devoid of, cholesterol and trans fats
Bad carbs are:
• High in calories
• Full of refined sugars, like corn syrup, white sugar, honey and fruit juices
• High in refined grains like white flour
• Low in many nutrients
• Low in fiber
• High in sodium
• Sometimes high in saturated fat
• Sometimes high in cholesterol and trans fats
Glycemic index
Recently, nutritionists have said that it's not the type of carbohydrate, but rather the carb's glycemic index, that's important. The glycemic index measures how quickly and how much a carbohydrate raises blood sugar.
High-glycemic foods like pastries raise blood sugar highly and rapidly; low-glycemic foods raise it gently and to a lesser degree. Some research has linked high-glycemic foods with diabetes, obesity, heart disease and certain cancers, according to Harvard Medical School.
On the other hand, recent research suggests that following a low-glycemic diet may not actually be helpful. A 2014 study published in JAMA found that overweight adults eating a balanced diet did not see much additional improvement on a low-calorie, low-glycemic index diet. Scientists measured insulin sensitivity, systolic blood pressure, LDL cholesterol and HDL cholesterol and saw that the low-glycemic diet did not improve them. It did lower triglycerides.
Carbohydrate benefits
The right kind of carbs can be incredibly good for you. Not only are they necessary for your health, but they carry a variety of added benefits.
Mental health
Carbohydrates may be important to mental health. A study published in 2009 in the journal JAMA Internal Medicine found that people on a high-fat, low-carb diet for a year had more anxiety, depression and anger than people on a low-fat, high-carb diet. Scientists suspect that carbohydrates help with the production of serotonin in the brain.
Carbs may help memory, too. A 2008 study at Tufts University had overweight women cut carbs entirely from their diets for one week. Then, they tested the women's cognitive skills, visual attention and spatial memory. The women on no-carb diets did worse than overweight women on low-calorie diets that contained a healthy amount of carbohydrates.
Weight loss
Though carbs are often blamed for weight gain, the right kind of carbs can actually help you lose and maintain a healthy weight. This happens because many good carbohydrates, especially whole grains and vegetables with skin, contain fiber. It is difficult to get sufficient fiber on a low-carb diet. Dietary fiber helps you to feel full, and generally comes in relatively low-calorie foods.
A study published in the Journal of Nutrition in 2009 followed middle-age women for 20 months and found that participants who ate more fiber lost weight, while those who decreased their fiber intake gained weight. Another recent study linked fat loss with low-fat diets, not low-carb ones.
While some studies have found that low-carb diets do help people lose weight, a meta analysis conducted in 2015 and published in The Lancet found that when viewed long term, low-fat and low-carb diets had similar success rates. People lost more weight early on while on low-carb diets but after a year they were all in similar places.
Good source of nutrients
Whole, unprocessed fruits and vegetables are well known for their nutrient content. Some are even considered superfoods because of it — and all of these leafy greens, bright sweet potatoes, juicy berries, tangy citruses and crunchy apples contain carbs.
One important, plentiful source of good carbs is whole grains. A large study published in 2010 in the Journal of the American Dietetic Association found that those eating the most whole grains had significantly higher amounts of fiber, energy and polyunsaturated fats, as well as all micronutrients (except vitamin B12 and sodium). An additional study, published in 2014 in the journal Critical Reviews in Food Science and Nutrition, found that whole grains contain antioxidants, which were previously thought to exist almost exclusively in fruits and vegetables.
Heart health
Fiber also helps to lower cholesterol, said Kelly Toups, a registered dietitian with the Whole Grains Council. The digestive process requires bile acids, which are made partly with cholesterol. As your digestion improves, the liver pulls cholesterol from the blood to create more bile acid, thereby reducing the amount of LDL, the "bad" cholesterol.
Toups referenced a study in the American Journal of Clinical Nutrition that looked at the effect of whole grains on patients taking cholesterol-lowering medications called statins. Those who ate more than 16 grams of whole grains daily had lower bad-cholesterol levels than those who took the statins without eating the whole grains.
Carbohydrate deficiency
Not getting enough carbs can cause problems. Without sufficient fuel, the body gets no energy. Additionally, without sufficient glucose, the central nervous system suffers, which may cause dizziness or mental and physical weakness, according to Iowa State University. A deficiency of glucose, or low blood sugar, is called hypoglycemia.
If the body has insufficient carbohydrate intake or stores, it will consume protein for fuel. This is problematic because the body needs protein to make muscles. Using protein for fuel instead of carbohydrates also puts stress on the kidneys, leading to the passage of painful byproducts in the urine, according to the University of Cincinnati.
People who don't consume enough carbohydrates may also suffer from insufficient fiber, which can cause digestive problems and constipation.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1029 2021-05-24 00:06:08
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,771
Re: Miscellany
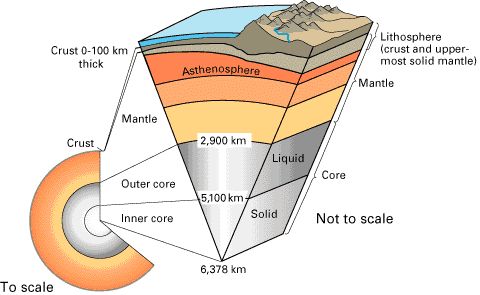
1007) Lithosphere
The lithosphere is the solid, outer part of the Earth. The lithosphere includes the brittle upper portion of the mantle and the crust, the outermost layers of Earth’s structure. It is bounded by the atmosphere above and the asthenosphere (another part of the upper mantle) below.
Although the rocks of the lithosphere are still considered elastic, they are not viscous. The asthenosphere is viscous, and the lithosphere-asthenosphere boundary (LAB) is the point where geologists and rheologists—scientists who study the flow of matter—mark the difference in ductility between the two layers of the upper mantle. Ductility measures a solid material’s ability to deform or stretch under stress. The lithosphere is far less ductile than the asthenosphere.
There are two types of lithosphere: oceanic lithosphere and continental lithosphere. Oceanic lithosphere is associated with oceanic crust, and is slightly denser than continental lithosphere.
Plate Tectonics
The most well-known feature associated with Earth’s lithosphere is tectonic activity. Tectonic activity describes the interaction of the huge slabs of lithosphere called tectonic plates.
The lithosphere is divided into tectonic plates including the North American, Caribbean, South American, Scotia, Antarctic, Eurasian, Arabian, African, Indian, Philippine, Australian, Pacific, Juan de Fuca, Cocos, and Nazca.
Most tectonic activity takes place at the boundaries of these plates, where they may collide, tear apart, or slide against each other. The movement of tectonic plates is made possible by thermal energy (heat) from the mantle part of the lithosphere. Thermal energy makes the rocks of the lithosphere more elastic.
Tectonic activity is responsible for some of Earth's most dramatic geologic events: earthquakes, volcanoes, orogeny (mountain-building), and deep ocean trenches can all be formed by tectonic activity in the lithosphere.
Tectonic activity can shape the lithosphere itself: Both oceanic and continental lithospheres are thinnest at rift valleys and ocean ridges, where tectonic plates are shifting apart from one another.
How the Lithosphere Interacts with Other Spheres
The cool, brittle lithosphere is just one of five great “spheres” that shape the environment of Earth. The other spheres are the biosphere (Earth’s living things); the cryosphere (Earth’s frozen regions, including both ice and frozen soil); the hydrosphere (Earth’s liquid water); and the atmosphere (the air surrounding our planet). These spheres interact to influence such diverse elements as ocean salinity, biodiversity, and landscape.
For instance, the pedosphere is part of the lithosphere made of soil and dirt. The pedosphere is created by the interaction of the lithosphere, atmosphere, cryosphere, hydrosphere, and biosphere. Enormous, hard rocks of the lithosphere may be ground down to powder by the powerful movement of a glacier (cyrosphere). Weathering and erosion caused by wind (atmosphere) or rain (hydrosphere) may also wear down rocks in the lithosphere. The organic components of the biosphere, including plant and animal remains, mix with these eroded rocks to create fertile soil—the pedosphere.
The lithosphere also interacts with the atmosphere, hydrosphere, and cryosphere to influence temperature differences on Earth. Tall mountains, for example, often have dramatically lower temperatures than valleys or hills. The mountain range of the lithosphere is interacting with the lower air pressure of the atmosphere and the snowy precipitation of the hydrosphere to create a cool or even icy climate zone. A region’s climate zone, in turn, influences adaptations necessary for organisms of the region’s biosphere.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1030 2021-05-25 00:24:02
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,771
Re: Miscellany
1008) Hydrosphere
A hydrosphere is the total amount of water on a planet. The hydrosphere includes water that is on the surface of the planet, underground, and in the air. A planet's hydrosphere can be liquid, vapor, or ice.
On Earth, liquid water exists on the surface in the form of oceans, lakes and rivers. It also exists below ground—as groundwater, in wells and aquifers. Water vapor is most visible as clouds and fog.
The frozen part of Earth's hydrosphere is made of ice: glaciers, ice caps and icebergs. The frozen part of the hydrosphere has its own name, the cryosphere.
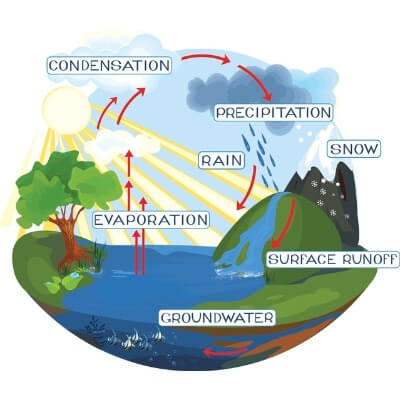
Water moves through the hydrosphere in a cycle. Water collects in clouds, then falls to Earth in the form of rain or snow. This water collects in rivers, lakes and oceans. Then it evaporates into the atmosphere to start the cycle all over again. This is called the water cycle.
The hydrosphere is the component of the Earth that is composed of all liquid water found on the planet. The hydrosphere includes water storage areas such as oceans, seas, lakes, ponds, rivers, and streams. Overall, the hydrosphere is very large, with the oceans alone covering about 71% of the surface area of Earth.
The motion of the hydrosphere and the exchange of water between the hydrosphere and cryosphere is the basis of the hydrologic cycle. The continuous movement and exchange of water helps to form currents that move warm water from the tropics to the poles and help regulate the temperature of the Earth. The exchanging of water is thus a vital part of the hydrosphere.
It is important to note that although the hydrosphere is primarily composed of water, there are also some "impurities" or additions to this water that include dissolved minerals, dissolved gases, and particulates. Some of these can be considered pollution, while others are necessary for health of ecosystems. For example, too much sediment is harmful to the surrounding ecosystems, while insufficient levels of dissolved oxygen in the water lead to hypoxic conditions that can harm ecosystems. Thus a delicate balance is needed for healthy ecosystems that surround different components of the hydrosphere.
Components
Any water storage area on the Earth that holds liquid water is considered to be a part of the hydrosphere. Because of this, there is an extensive list of formations that make up the hydrosphere. These include:
Oceans: Most of the water on the planet Earth is salt water, and the vast majority of this salt water is held in the oceans.
Fresh water: Fresh water is much less abundant than salt water, and is held in a variety of different places.
ia) Surface water: Surface sources of freshwater include lakes, rivers, and streams.
(b) Ground water: Fresh water held beneath ground makes up a small portion of the fresh water on Earth.
Glacial water: Water that melts off of glaciers.
Atmospheric water vapour.
Human Impacts on Hydrosphere
In recent history humans have drastically changed the hydrosphere. Water pollution, river damming, wetland drainage, climate change, and irrigation have all changed the hydrosphere. Eutrophication caused by the release of fertilizers and sewage into water storage areas has caused aquatic environments to be artificially enriched with nutrients. The excessive algal blooms can result in harmful hypoxic conditions in the water. Acid rain from SOx and NOx emissions from fossil fuel combustion has resulted in the acidification of components of the hydrosphere, harming surrounding ecosystems.
Finally, when humans change the natural flow of water in the hydrosphere by diverting and damming rivers it harms surrounding ecosystems that rely on the water source. This can also result in the drying out of some aquatic areas and excessive amounts of sediment entering streams and rivers.
Climate
The properties and motion of the hydrosphere are important in maintaining the diverse climates that exist worldwide. The ocean - which holds 97% of the water on Earth - is especially important to the climate system. The ocean itself serves as a reservoir which contributes to how much solar radiation is absorbed. Since the ocean is so vast, it absorbs a large amount of energy from the Sun. As well, the ocean is important to the climate system as it limits how fast the climate can change. Additionally, the ocean serves to redistribute energy and heat around the globe, contributing to the different average temperatures that exist over the globe.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1031 2021-05-26 00:09:28
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,771
Re: Miscellany
1009) Atmosphere
Look up. Way up. The clouds you see in the sky, the wind that is moving the trees or the flag in your school yard, even the sunshine you feel on your face—these are all a result of Earth’s atmosphere.
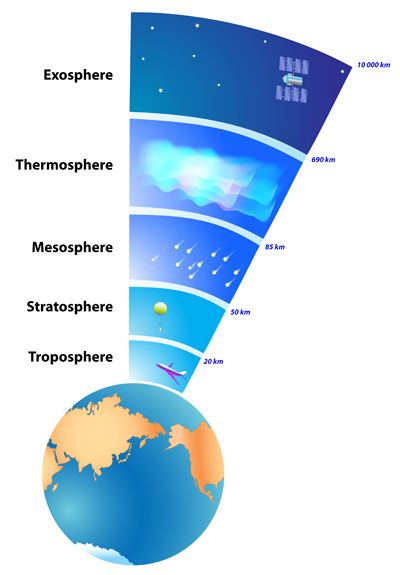
Earth’s atmosphere stretches from the surface of the planet up to as far as 10,000 kilometers (6,214 miles) above. After that, the atmosphere blends into space. Not all scientists agree where the actual upper boundary of the atmosphere is, but they can agree that the bulk of the atmosphere is located close to Earth’s surface—up to a distance of around eight to 15 kilometers (five to nine miles).
While oxygen is necessary for most life on Earth, the majority of Earth’s atmosphere is not oxygen. Earth’s atmosphere is composed of about 78 percent nitrogen, 21 percent oxygen, 0.9 percent argon, and 0.1 percent other gases. Trace amounts of carbon dioxide, methane, water vapor, and neon are some of the other gases that make up the remaining 0.1 percent.
The atmosphere is divided into five different layers, based on temperature. The layer closest to Earth’s surface is the troposphere, reaching from about seven and 15 kilometers (five to 10 miles) from the surface. The troposphere is thickest at the equator, and much thinner at the North and South Poles. The majority of the mass of the entire atmosphere is contained in the troposphere—between approximately 75 and 80 percent. Most of the water vapor in the atmosphere, along with dust and ash particles, are found in the troposphere—explaining why most of Earth’s clouds are located in this layer. Temperatures in the troposphere decrease with altitude.
The stratosphere is the next layer up from Earth’s surface. It reaches from the top of the troposphere, which is called the tropopause, to an altitude of approximately 50 kilometers (30 miles). Temperatures in the stratosphere increase with altitude. A high concentration of ozone, a molecule composed of three atoms of oxygen, makes up the ozone layer of the stratosphere. This ozone absorbs some of the incoming solar radiation, shielding life on Earth from potentially harmful ultraviolet (UV) light, and is responsible for the temperature increase in altitude.
The top of the stratosphere is called the stratopause. Above that is the mesosphere, which reaches as far as about 85 kilometers (53 miles) above Earth’s surface. Temperatures decrease in the mesosphere with altitude. In fact, the coldest temperatures in the atmosphere are near the top of the mesosphere—about -90°C (-130°F). The atmosphere is thin here, but still thick enough so that meteors will burn up as they pass through the mesosphere—creating what we see as “shooting stars.” The upper boundary of the mesosphere is called the mesopause.
The thermosphere is located above the mesopause and reaches out to around 600 kilometers (372 miles). Not much is known about the thermosphere except that temperatures increase with altitude. Solar radiation makes the upper regions of the thermosphere very hot, reaching temperatures as high as 2,000°C (3,600°F).
The uppermost layer, that blends with what is considered to be outer space, is the exosphere. The pull of Earth’s gravity is so small here that molecules of gas escape into outer space.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1032 2021-05-27 00:12:37
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,771
Re: Miscellany
1010) Pond
A pond is an area filled with water, either natural or artificial, that is smaller than a lake. Ponds may arise naturally in floodplains as part of a river system or can simply be an isolated depression (such as a kettle, vernal pool, or prairie pothole) that filled with runoff, groundwater, or precipitation. As such, ponds may be freshwater, saltwater, or brackish in nature.
Many ponds contain shallow water ecosystems, often termed pond life, with varying abundances of aquatic plants and animals. Certain characteristic such as depth, seasonal water level, nutrients fluxes, solar radiation, degree of inlets and outlets, local organisms, and salinity may affect the types of ecosystems present within a pond.
Ponds are frequently man-made or expanded beyond their original depths and bounds by anthropogenic causes. Among their many uses, ponds provide water for agriculture, livestock and communities, aid in habitat restoration, serve as breeding grounds for local and migrating species, are components of landscape architecture, flood control, general urbanization, mitigate particular pollutions and greenhouse gasses, and support wide varieties of organismal ecosystems.
Classification
The technical distinction between a pond and a lake has not been universally standardized. Limnologists and freshwater biologists have proposed formal definitions for pond, in part to include 'bodies of water where light penetrates to the bottom of the waterbody,' 'bodies of water shallow enough for rooted water plants to grow throughout,' and 'bodies of water which lack wave action on the shoreline.' Each of these definitions has met with resistance or disapproval, as the defining characteristics are each difficult to measure or verify. Accordingly, some organizations and researchers have settled on technical definitions of pond and lake that rely on size alone.
Formation
Any depression in the ground which collects and retains a sufficient amount of water can be considered a pond, and such, can be formed by a variety of geological, ecological, and human terraforming events.
Natural ponds are those caused by environmental occurrences. These can vary from glacial, volcanic, fluvial, or even tectonic events. Since the Pleistocene epoch, glacial processes have created most of the Northern hemispheric ponds; an example is the Prairie Pothole Region of North America.
Manmade ponds are those created by human intervention for the sake of the local environment, industrial settings, or for recreational/ornamental use.
Uses
Many ecosystems are linked by water and ponds have been found to hold a greater biodiversity of species than larger freshwater lakes or river systems. As such, ponds are habitats for many varieties of organisms including plants, amphibians, fish, reptiles, waterfowl, insects and even some mammals. Ponds are used for breeding grounds for these species but also as shelter and even drinking/feeding locations for other wildlife. Aquaculture practices lean heavily on artificial ponds in order to grow and care for many different type of fish either for human consumption, research, species conservation or recreational sport.
In agriculture practices, treatment ponds can be created to reduce nutrient runoff from reaching local streams or groundwater storages. Pollutants that enter ponds can often be mitigated by natural sedimentation and other biological and chemical activities within the water. As such, waste stabilization ponds are becoming popular low-cost methods for general wastewater treatment. They may also provide irrigation reservoirs for struggling farms during times of drought.
As urbanization continues to spread, retention ponds are becoming more common in new housing developments. These ponds reduce the risk of flooding and erosion damage from excess storm water runoff in local communities.
Experimental ponds are used to test hypotheses in the fields of environmental science, chemistry, aquatic biology, and limnology.
Some ponds are the life blood of many small villages in arid countries such as those in sub-Saharan Africa where bathing, sanitation, fishing, socialization, and rituals are held. In the Indian subcontinent, Hindu temple monks care for sacred ponds used for religious practices and bathing pilgrims alike. In Europe during medieval times, it was typical for many monastery and castles (small, partly self-sufficient communities) to have fish ponds. These are still common in Europe and in East Asia (notably Japan), where koi may be kept or raised.
Pond Biodiversity
A defining feature of a pond is the presence of standing water which provides habitat for a biological community commonly referred to as pond life. Because of this, many ponds and lakes contain large numbers of endemic species that have gone through adaptive radiation to become specialized to their preferred habitat. Familiar examples might include water lilies and other aquatic plants, frogs, turtles, and fish.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1033 2021-05-28 00:08:28
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,771
Re: Miscellany
1011) Lake
Lake, any relatively large body of slowly moving or standing water that occupies an inland basin of appreciable size. Definitions that precisely distinguish lakes, ponds, swamps, and even rivers and other bodies of nonoceanic water are not well established. It may be said, however, that rivers and streams are relatively fast moving; marshes and swamps contain relatively large quantities of grasses, trees, or shrubs; and ponds are relatively small in comparison with lakes. Geologically defined, lakes are temporary bodies of water.
General considerations
Occurrence
Within the global hydrologic cycle, freshwater lakes play a very small quantitative role, constituting only about 0.009 percent of all free water, which amounts to less than 0.4 percent of all continental fresh water. Saline lakes and inland seas contain another 0.0075 percent of all free water. Freshwater lakes, however, contain well over 98 percent of the important surface waters available for use. Apart from that contained in saline bodies, most other continental waters are tied up in glaciers and ice sheets and the remainder is in groundwater.
Four-fifths of the 125,000 cubic km (30,000 cubic miles) of lake waters occur in a small number of lakes, perhaps 40 in all. Among the largest are Lake Baikal, in Central Asia, containing about 23,000 cubic km (5,500 cubic miles) of water; Lake Tanganyika (19,000 cubic km [4,600 cubic miles]), in eastern Africa; and Lake Superior (12,000 cubic km [2,900 cubic miles]), one of the Great Lakes of North America. The Great Lakes contain a total of about 25,000 cubic km (6,000 cubic miles) of water and, together with other North American lakes larger than 10 cubic km (2 cubic miles), constitute about one-fourth of the world’s lake waters. The Caspian Sea, though not considered a lake by some hydrologists, is the world’s largest inland sea. Located in Central Asia, the Caspian Sea has an area of about 386,000 square km (149,000 square miles).
Although lakes are to be found throughout the world, the continents of North America, Africa, and Asia contain about 70 percent of the total lake water, the other continents being less generously endowed. Lakes also occur far beneath the ice sheets of Antarctica; however, surveys of the volume and other features of those discovered so far remain incomplete. One-fourth of the total volume of lake water is spread throughout the world in uncounted numbers of small lakes. Anyone who has flown over much of the Canadian plains area cannot help but be struck by the seemingly endless skein of lakes and ponds covering the landscape below. Though the total volume of water involved is comparatively small, the surface area of lake water is substantial. The total surface area of all Canadian lakes has been estimated to exceed the total surface area of the province of Alberta. The U.S. state of Alaska has more than three million lakes with surface areas greater than 8 hectares (20 acres).
The larger, deeper lakes are a significant factor within the cycle of water—from rain to surface water, ice, soil moisture, or groundwater and thence to water vapour. These lakes receive the drainage from vast tracts of land, store it, pass it on seaward, or lose it to the atmosphere by evaporation. On a local basis, even the smaller lakes play an important hydrologic role. The relatively high ratio of exposed surface area to the total water volume of these lakes accentuates their effectiveness as evaporators. In some cases the efficiency of lakes in losing water to the atmosphere is locally undesirable, because of public and industrial requirements for lake water. A striking example of this condition is the Aral Sea, located in Central Asia. Although it is still one of the world’s largest bodies of inland water, in the second half of the 20th century its area was reduced by two-fifths and its mean surface level had dropped by more than 12 metres (40 feet), primarily as a result of the diversion of the Syr Darya and Amu Darya rivers for irrigating adjoining fields. In some basins (e.g., the Chad basin in Africa), lakes are the terrestrial end point of the hydrologic cycle. With no outflow downstream toward the oceans, these closed lakes swell or recede according to the balance of local hydrologic conditions.
Uses and abuses of lakes
In today’s industrial societies, requirements for water—much of which is derived from lakes—include its use for dilution and removal of municipal and industrial wastes, for cooling purposes, for irrigation, for power generation, and for local recreation and aesthetic displays. Obviously, these requirements vary considerably among regions, climates, and countries.
In another vein, it is convenient to use water to dilute liquid and some solid wastes to concentrations that are not intolerable to the elements of society that must be exposed to the effluent or wish to use it. The degree of dilution that may be acceptable varies from situation to situation and is often in dispute. In some cases, dilution is used purely to facilitate transport of the wastes to purification facilities. The water may then be made available for reuse.
Lake water is also used extensively for cooling purposes. Although this water may not be affected chemically, its change in thermal quality may be detrimental to the environment into which it is disposed, either directly, by affecting fish health or functions, or indirectly, by causing excessive plant production and ultimate deoxygenation due to biological decay. Both fossil-fueled and nuclear power plants are major users of cooling water. Steel mills and various chemical plants also require large quantities.
Economy and ecology
Concern with thermal pollution of surface waters is concentrated principally on rivers and small lakes. With power requirements in modern societies increasing by about 7 percent per year, however, some apprehension has been expressed about the future thermal loading of even the largest lakes. It was predicted that thermal inputs to each of the North American Great Lakes would increase by nearly 11 times during the last three decades of the 20th century. In terms of energy to be disposed in this fashion, the numbers are staggeringly large. These lakes have such large volumes, however, and such large surface areas (from which much of the heat goes into the atmosphere) that there is some question about the nature and magnitude of the actual effects.
The economic importance of waterways as communication links is enormous. In the earliest times, when travel by many societies was substantially by water, travel routes became established that resulted in relationships between cultural factors and surface hydrology networks. Today river and lake systems serve as communication links and play an important role in shipping because of the large cargo capacities of merchant vessels and the still fairly uncongested condition of inland waterways. Oceanic shipping lanes play the major role, but river and lake systems, which link inland ports with the oceans, have been key factors in the rates of economic growth of many large inland ports.
Commercial fisheries and other food industries reap great harvests from the major lakes of the world. The quality of the fish catch has steadily decreased, however, as a result of pollution in many lakes, with the more desirable species becoming less plentiful and the less desirable species gradually dominating the total. Other commercial harvests from lakes include waterfowl, fur-bearing mammals, and some plant material, such as rice.
Each of the uses described has associated with it the means for abuse of the very characteristics of lakes that make them desirable. Wise management of natural resources has never been humankind’s forte. Municipalities and industries have polluted lakes chemically and thermally, the shipping that plies large inland water bodies leaves oil and other refuse in its wake, water used for irrigation often contains chemical residues from fertilizers and biocides when it is returned to lakes, and the populace that so desperately demands clean bodies of water for its recreation often ignores basic sanitary and antipollution practices, to the ultimate detriment of the waters enjoyed.
Problems and effects
Among the major problems affecting the optimum utilization and conservation of lake waters are eutrophication (aging processes), chemical and biological poisoning, and decreases in water volumes. In the former case, discussed in more detail later, the enrichment of lakes with various nutrients supports biological productivity to an extent in which the ultimate death and decay of biological material places an excessive demand on the oxygen content, resulting in oxygen depletion in the worst cases. Phosphates and nitrates are two of the types of nutrients that are most important in this connection, particularly since they are often introduced in critical quantities in waste effluents from human sources. Other examples of chemical pollution of lakes include the introduction of DDT and other pesticides and heavy metals such as mercury. Bacteriological contamination of lake waters resulting in levels that constitute a hazard to health is another common result of disregard for the environment.
Water-quantity problems are complex, being related to natural vagaries of supply and levels of consumptive utilization of water. In the latter case, the percentage of water returned to the source after utilization varies with the use. The largest losses are due to actual water diversions and processes that result in evaporative losses. The use of large quantities of lake water for cooling purposes by industry and utilities, for example, may raise lake temperatures near the effluents sufficiently to cause increased evaporation. The use of certain types of cooling towers results in even larger losses. Some of the water evaporated will stay within the lake basin, but some will be lost from it.
Another example of this type of loss is connected with the possible application of weather-modification techniques to alleviate the heavy lake-effect snowfalls experienced along the lee shores of large lakes in intermediate latitudes. Redistribution of precipitation always raises the possibility of redistribution of water among various basins.
Lake-effect snowfall is just one example of the influence of lakes on local climate. The ability of large bodies of water to store heat during heating periods and to lose it more gradually than the adjacent landmasses during cooling periods results in a modifying influence on the climate. Because of this propensity, a lake cools air passing over it in summer and warms air passing over it in winter. Consequently, the predominantly downwind side of a lake is more influenced by the ameliorating effects of a lake.
In most instances, moisture is also passed to the atmosphere. In summer, lake cooling serves to stabilize the air mass, but winter heating tends to decrease stability. The moisture-laden, unstable winter flows off lakes produce so-called snowbelts, which affect downwind cities. The snowbelts are usually of limited extent, often within about a kilometre of the lakeshore.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1034 2021-05-29 00:07:12
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,771
Re: Miscellany
1012) River
River, (ultimately from Latin ripa, “bank”), any natural stream of water that flows in a channel with defined banks . Modern usage includes rivers that are multichanneled, intermittent, or ephemeral in flow and channels that are practically bankless. The concept of channeled surface flow, however, remains central to the definition. The word stream (derived ultimately from the Indo-European root srou-) emphasizes the fact of flow; as a noun, it is synonymous with river and is often preferred in technical writing. Small natural watercourses are sometimes called rivulets, but a variety of names—including branch, brook, burn, and creek—are more common, occurring regionally to nationally in place-names. Arroyo and (dry) wash connote ephemeral streams or their resultant channels. Tiny streams or channels are referred to as rills or runnels.
Rivers are nourished by precipitation, by direct overland runoff, through springs and seepages, or from meltwater at the edges of snowfields and glaciers. The contribution of direct precipitation on the water surface is usually minute, except where much of a catchment area is occupied by lakes. River water losses result from seepage and percolation into shallow or deep aquifers (permeable rock layers that readily transmit water) and particularly from evaporation. The difference between the water input and loss sustains surface discharge or streamflow. The amount of water in river systems at any time is but a tiny fraction of the Earth’s total water; 97 percent of all water is contained in the oceans and about three-quarters of fresh water is stored as land ice; nearly all the remainder occurs as groundwater. Lakes hold less than 0.5 percent of all fresh water, soil moisture accounts for about 0.05 percent, and water in river channels for roughly half as much, 0.025 percent, which represents only about one four-thousandth of the Earth’s total fresh water.
Water is constantly cycled through the systems of land ice, soil, lakes, groundwater (in part), and river channels, however. The discharge of rivers to the oceans delivers to these systems the equivalent of the water vapour that is blown overland and then consequently precipitated as rain or snow—i.e., some 7 percent of mean annual precipitation on the globe and 30 percent of precipitation on land areas.
Rivers are 100 times more effective than coastal erosion in delivering rock debris to the sea. Their rate of sediment delivery is equivalent to an average lowering of the lands by 30 centimetres (12 inches) in 9,000 years, a rate that is sufficient to remove all the existing continental relief in 25,000,000 years.
Rock debris enters fluvial systems either as fragments eroded from rocky channels or in dissolved form. During transit downstream, the solid particles undergo systematic changes in size and shape, traveling as bed load or suspension load. Generally speaking, except in high latitudes and on steep coasts, little or no coarse bed load ever reaches the sea. Movement of the solid load down a river valley is irregular, both because the streamflow is irregular and because the transported material is liable to enter temporary storage, forming distinctive river-built features that range through riffles, midstream bars, point bars, floodplains, levees, alluvial fans, and river terraces. In one sense, such geomorphic features belong to the same series as deltas, estuary fills, and the terrestrial sediments of many inland basins.
Rates of erosion and transportation, and comparative amounts of solid and dissolved load, vary widely from river to river. Least is known about dissolved load, which at coastal outlets is added to oceanic salt. Its concentration in tropical rivers is not necessarily high, although very high discharges can move large amounts; the dissolved load of the lowermost Amazon averages about 40 parts per million, whereas the Elbe and the Rio Grande, by contrast, average more than 800 parts per million. Suspended load for the world in general perhaps equals two and one-half times dissolved load. Well over half of suspended load is deposited at river mouths as deltaic and estuarine sediment. About one-quarter of all suspended load is estimated to come down the Ganges-Brahmaputra and the Huang He (Yellow River), which together deliver some 4,500,000,000 tons a year; the Yangtze (Chang Jiang), Indus, Amazon, and Mississippi deliver quantities ranging from about 500,000,000 to approximately 350,000,000 tons a year. Suspended sediment transport on the Huang He equals a denudation rate of about 3,090 tons per square kilometre (8,000 tons per square mile) per year; the corresponding rate for the Ganges-Brahmaputra is almost half as great. Extraordinarily high rates have been recorded for some lesser rivers: for instance, 1,060 tons per square kilometre per year on the Jing and 1,080 tons per square kilometre per year on the Luo, both of which are Loess Plateau tributaries of the Huang He.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1035 2021-05-30 00:13:24
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,771
Re: Miscellany
1013) Sea
The phrase “the Seven Seas” has been around for centuries, but that term really refers to different parts of the ocean and several other large bodies of water. There are actually more than seven seas in the world. But what makes a sea different from other bodies of water?
That is not an easy question to answer, because the definition of a sea leaves some room for interpretation. In general, a sea is defined as a portion of the ocean that is partly surrounded by land. Given that definition, there are about 50 seas around the world. But that number includes water bodies not always thought of as seas, such as the Gulf of Mexico and the Hudson Bay.
Moreover, in some cases, a sea is completely landlocked. The Caspian Sea is the most famous example, though this sea, which lies between Russia and Iran, is also referred to as the world’s largest lake. Other seas surrounded by land include the Aral Sea and the Dead Sea. They contain saltwater and have been called seas for many years, but many oceanographers and geographers are more inclined to call them lakes.
Still, that leaves dozens of water bodies that fit the traditional definition of a sea, even though they can be quite different from one another. A sea can be more than 2.6 million square kilometers (1 million square miles) in area, such as the Caribbean Sea. Or, it can be as tiny as the Sea of Marmara, which is less than 12,950 square kilometers (5,000 square miles) in area. This tiny Turkish sea connects the Aegean Sea and the Black Sea.
A sea can also be very warm for most of the year. The Red Sea, for instance, has an average temperature of around 30 degrees Celsius (86 degrees Fahrenheit). It is also the saltiest sea, containing 41 parts of salt per 1,000 parts of seawater. Seas can be quite cold, too. The Greenland Sea, for instance, has surface water that hovers near the freezing mark most of the year.
The variety of the sizes, temperatures, and locations of the Earth’s seas also means that the marine ecosystems within each sea can vary greatly from one to the other. The Baltic Sea in Scandinavia is the world’s youngest sea having formed between 10 thousand and 15 thousand years ago from glacial erosion. It contains a unique mixture of saltwater and freshwater, making it the largest brackish water body on the planet. As a result, the Baltic Sea contains a small, but rare, variety of freshwater and saltwater plants and animals that have been able to adapt to their brackish environment.
Not surprisingly, the diversity of the world’s seas also draws National Geographic explorers, such as oceanographer Katy Croff Bell. She was part of the crew aboard the exploration vessel Nautilus, a ship that shared its scientific discoveries in the Mediterranean Sea, the Black Sea, and elsewhere with students around the world in online lessons and chats. She says the seas—big and small, cold and warm—can teach scientists about the rest of the world. “We’re going to places that have never been explored to see what’s there,” Bell told MIT Technology Review in 2015. “There are things we can’t even conceive of out there, and it will take a long, long time to fully understand our own planet.”
The sea, connected as the world ocean or simply the ocean, is the body of salty water that covers over 70 percent of the Earth's surface. The word sea is also used to denote second-order sections of the sea, such as the Mediterranean Sea, as well as certain large, entirely landlocked, saltwater lakes, such as the Caspian Sea.
The sea moderates Earth's climate and has important roles in the water cycle, carbon cycle, and nitrogen cycle. Humans harnessing and studying the sea have been recorded since ancient times, and evidenced well into prehistory, while its modern scientific study is called oceanography. The most abundant solid dissolved in seawater is sodium chloride. The water also contains salts of magnesium, calcium, potassium, and mercury, amongst many other elements, some in minute concentrations. Salinity varies widely, being lower near the surface and the mouths of large rivers and higher in the depths of the ocean; however, the relative proportions of dissolved salts vary little across the oceans. Winds blowing over the surface of the sea produce waves, which break when they enter the shallow water. Winds also create surface currents through friction, setting up slow but stable circulations of water throughout the oceans. The directions of the circulation are governed by factors, including the shapes of the continents and Earth's rotation (the Coriolis effect). Deep-sea currents, known as the global conveyor belt, carry cold water from near the poles to every ocean. Tides, the generally twice-daily rise and fall of sea levels, are caused by Earth's rotation and the gravitational effects of the orbiting Moon and, to a lesser extent, of the Sun. Tides may have a very high range in bays or estuaries. Submarine earthquakes arising from tectonic plate movements under the oceans can lead to destructive tsunamis, as can volcanoes, huge landslides, or the impact of large meteorites.
A wide variety of organisms, including bacteria, protists, algae, plants, fungi, and animals, live in the sea, which offers a wide range of marine habitats and ecosystems, ranging vertically from the sunlit surface and shoreline to the great depths and pressures of the cold, dark abyssal zone, and in latitude from the cold waters under polar ice caps to the colourful diversity of coral reefs in tropical regions. Many of the major groups of organisms evolved in the sea and life may have started there.
The sea provides substantial supplies of food for humans, mainly fish, but also shellfish, mammals and seaweed, whether caught by fishermen or farmed underwater. Other human uses of the sea include trade, travel, mineral extraction, power generation, warfare, and leisure activities such as swimming, sailing, and scuba diving. Many of these activities create marine pollution. The sea has therefore been for humans an integral element throughout history and culture.
Definition
The sea is the interconnected system of all the Earth's oceanic waters, including the Atlantic, Pacific, Indian, Southern and Arctic Oceans. However, the word "sea" can also be used for many specific, much smaller bodies of seawater, such as the North Sea or the Red Sea. There is no sharp distinction between seas and oceans, though generally seas are smaller, and are often partly (as marginal seas or particularly as mediterranean seas) or wholly (as inland seas) bordered by land. However, the Sargasso Sea has no coastline and lies within a circular current, the North Atlantic Gyre. Seas are generally larger than lakes and contain salt water, but the Sea of Galilee is a freshwater lake. The United Nations Convention on the Law of the Sea states that all of the ocean is "sea".
Physical science
Earth is the only known planet with seas of liquid water on its surface, although Mars possesses ice caps and similar planets in other solar systems may have oceans. Earth's 1,335,000,000 cubic kilometers (320,000,000 cu mi) of sea contain about 97.2 percent of its known water and cover more than 70 percent of its surface.[3](p7) Another 2.15% of Earth's water is frozen, found in the sea ice covering the Arctic Ocean, the ice cap covering Antarctica and its adjacent seas, and various glaciers and surface deposits around the world. The remainder (about 0.65% of the whole) form underground reservoirs or various stages of the water cycle, containing the freshwater encountered and used by most terrestrial life: vapor in the air, the clouds it slowly forms, the rain falling from them, and the lakes and rivers spontaneously formed as its waters flow again and again to the sea.
The scientific study of water and Earth's water cycle is hydrology; hydrodynamics studies the physics of water in motion. The more recent study of the sea in particular is oceanography. This began as the study of the shape of the ocean's currents but has since expanded into a large and multidisciplinary field: it examines the properties of seawater; studies waves, tides, and currents; charts coastlines and maps the seabeds; and studies marine life. The subfield dealing with the sea's motion, its forces, and the forces acting upon it is known as physical oceanography. Marine biology (biological oceanography) studies the plants, animals, and other organisms inhabiting marine ecosystems. Both are informed by chemical oceanography, which studies the behavior of elements and molecules within the oceans: particularly, at the moment, the ocean's role in the carbon cycle and carbon dioxide's role in the increasing acidification of seawater. Marine and maritime geography charts the shape and shaping of the sea, while marine geology (geological oceanography) has provided evidence of continental drift and the composition and structure of the Earth, clarified the process of sedimentation, and assisted the study of volcanism and earthquakes.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1036 2021-05-31 01:27:14
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,771
Re: Miscellany
1014) Ophidiophobia
Humans have many fears. The fear of snakes or Ophidiophobia is the second most common phobia in the world. Nearly 1/3rd of adult humans are believed to have an intense fear of snakes.
Most people with Ophidiophobia can lead normal lives as they do not have to confront the object of their fears under normal circumstances. However, for people with severe Ophidiophobia, the mere mention, or an image of a snake in books or on TV can lead to an intense fear response, the reactions of which can even include heart attacks and death.
Causes of Ophidiophobia
Snakes are fascinating creatures that have always had myths associated with them. These myths are the major reason why humans fear them. This and the fact that some snakes are deadly and venomous and can lead to deaths have probably led to Ophidiophobia.
Following are the causes of Ophidiophobia:
• Ignorance and lack of education is one of the most common reasons why humans fear snakes.
• Traumatic episode in the past such as accidently stepping on a snake or being bitten, hissed at or otherwise threatened by snakes might have lead to one’s Ophidiophobia.
• Seeing or witnessing an incident wherein another person, a close friend or family member was hissed at, or bitten by a snake can also lead to the fear of snakes
• Media reports and TV shows can also lead one to believe that snakes are always dangerous leading to Ophidiophobia.
• Genetic traits, family history with ophidiophobic persons etc can also lead other family members to have an intense fear of snakes.
• Evolution is another reason behind this phobia. Many evolutionary psychologists explain Ophidiophobia as a ‘disgust response’ to snakes that have always been associated with death, disease or poison.
Ophidiophobia is a part of Zoophobia, a generic term for the fear of animals. Some cases of zoophobia are stronger in the childhood and go away in the adulthood. In other cases, the phobia persists and remains even in adulthood.
The American Psychiatric Association has described following symptoms of Ophidiophobia which can be categorized as mental, physical or emotional:
1. Uncontrollable anxiety especially when one is about to be exposed to snakes
2. Feeling that one must do anything to avoid snakes
3. Screaming, crying or experiencing the difficulty to breathe, or trembling or shaking violently when one encounters snakes, their pictures or images on TV etc.
4. Feeling anxious or experiencing increased heart rate when taken to locations where snakes may be present.
Ophidiophobia can affect a person’s normal life especially when he/she avoids zoos or friends’ homes where there are pet snakes. Ophidiophobics refuse to leave their homes in places that are having high snake population.
Treatment of Ophidiophobia
If you or someone you know is experiencing an irrational fear of snakes, you can look into one or more of the following treatment options:
• Desensitization and reprocessing- This therapy is effective in addressing past traumatic experiences that might have caused the Ophidiophobia. It helps the patient overcome his/her fear by making him/her look at images of snakes and gradually enable him/her to hold small snakes. This helps the individual overcome fear and move forward in life.
• CBT or cognitive behavior therapy- Cognitive behavior or restructuring helps the individual identify his/her thought patterns that lie behind the fear of snakes. The patient is made to write down thoughts as to why one fears snakes. These include thoughts like: “snakes are slimy”, “they are dangerous and have the ability to kill humans” and so on. Writing down these thoughts can help the ophidiophobic identify patterns leading to fear, anxiety and stress. The patients also learn to replace their negative associations with positive beliefs. This, in turn, can help decrease the fear.
• Relaxation techniques- These include meditation, controlled breathing, counting and positive reaffirmations when faced with images of snakes. The best part about these techniques is that one can tailor their own solutions and help oneself overcome the fear of snakes.
• Individual counseling or group therapy- Talking about one’s fears can help release negative feelings while coming up with strategies to cope with their phobia.
By using the treatment options mentioned above, one can overcome their Ophidiophobia in order to not only lead a normal life but also enjoy the beautiful creatures that are a fascinating part of our Nature.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1037 2021-06-01 00:05:22
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,771
Re: Miscellany
1015) Vehophobia
Vehophobia or the fear of driving can impact one’s daily life especially since most of us are dependent on this activity to get by. It can affect one to an extent that s/he refuses to go shopping, visit a doctor or even drive to work. Individuals with the extreme fear of driving prefer public transport or request friends or family members to drive them each time. This is fine, so long as these options are available at one’s disposal. However, this might not be the case always affecting the individual’s education, job and other activities negatively.
There are varying degrees of Vehophobia. Some individuals are only anxious about driving on highways or certain dreaded routes. Some are unable to pass their driving tests or acquire a license. A few might be having valid driving licenses but they pose a danger to themselves as well as other drivers. They can have a panic attack while driving and freeze up to an extent that they are unable to apply brakes or change gears etc. In extreme cases, a person may even be terrified of being a passenger in the vehicle.
Causes of extreme fear of driving phobia
An individual with the extreme phobia of driving has likely had a negative experience in the past.
• S/he might have been involved in a crash or witnessed a particularly bad accident.
• Having strict driving instructors at the time of learning can also lead to Vehophobia.
• Having experienced a particularly dangerous or scary journey through fog, rain, snow, fleet or other adverse conditions or having large animals such as a moose or deer dart suddenly in front of one’s car can cause this phobia.
• Seeing anxious parents panic while driving can lead a child to have vehophobia.
• Road rage from fellow driver or passerby (where one has shouted or provoked the individual).
• Experiencing stress due to traffic congestions; these can cause the brain to develop panic/anxiety response each time one gets behind the wheel.
• People prone to anxiety attacks or nervous disorders or those with adrenal deficiencies are likely to develop the fear of driving.
• Hearing or reading news items about bad accidents or watching movies that depict violent car crashes can create negative thoughts about driving.
Symptoms of Vehophobia
Vehophobia can lead to various symptoms that can be characterized as emotional and physical.
The physical symptoms include: shaking, trembling, having a dry mouth, rapid heart rate, shallow breathing, chest pains, nausea, sweaty hands etc. Such a panic attack can occur each time the individual gets behind the wheel. It can cause one to freeze up so that s/he is unable to change gears or apply the brakes.
Emotional symptoms include refusing to drive, avoiding situations that encompass driving, feeling panic, terror or extreme dread at thought of driving, experiencing terrifying images about driving. The individual also feels detached or removed from reality, in that; he or she feels the events are happening to someone else.
Some people may try to avoid highways or take longer routes so that they do not have to drive on dreaded roads. Others may get into arguments or fights with loved ones and come up with excuses when forced or compelled to drive.
Overcoming the fear of driving phobia
• Hypnotherapy is one of the most effective therapies for overcoming the fear of driving. It gets to the bottom of the fear, traces why it has taken root and helps provide solutions to overcome the same.
• Facing one’s fear is one of the best ways of getting over Vehophobia. It is important to reduce tension and stress of any kind when getting behind the wheel. Imagining positive thoughts and visualizing ideal situations are some ways of accomplishing this. A therapist can also help one relearn or ‘un-learn’ negative things the brain has taught itself.
• Talking about one’s fears, and taking defensive driving lessons, or joining special groups that help one overcome such a phobia are a few other methods of dealing with Vehophobia.
• Cognitive behavior therapy and gradual desensitization are some other options to help one overcome this phobia.
There are various therapies to overcome Vehophobia but it is vital to choose one that you are most comfortable with, in conjunction with a medical practitioner’s advice. Understand that you are not alone, and there are many like you who have dealt with this phobia. It is very possible to overcome Vehophobia and success in driving again is very much possible.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1038 2021-06-02 00:32:25
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,771
Re: Miscellany
1016) Archimedes' principle
Archimedes’ principle, physical law of buoyancy, discovered by the ancient Greek mathematician and inventor Archimedes, stating that any body completely or partially submerged in a fluid (gas or liquid) at rest is acted upon by an upward, or buoyant, force, the magnitude of which is equal to the weight of the fluid displaced by the body. The volume of displaced fluid is equivalent to the volume of an object fully immersed in a fluid or to that fraction of the volume below the surface for an object partially submerged in a liquid. The weight of the displaced portion of the fluid is equivalent to the magnitude of the buoyant force. The buoyant force on a body floating in a liquid or gas is also equivalent in magnitude to the weight of the floating object and is opposite in direction; the object neither rises nor sinks. For example, a ship that is launched sinks into the ocean until the weight of the water it displaces is just equal to its own weight. As the ship is loaded, it sinks deeper, displacing more water, and so the magnitude of the buoyant force continuously matches the weight of the ship and its cargo.
If the weight of an object is less than that of the displaced fluid, the object rises, as in the case of a block of wood that is released beneath the surface of water or a helium-filled balloon that is let loose in air. An object heavier than the amount of the fluid it displaces, though it sinks when released, has an apparent weight loss equal to the weight of the fluid displaced. In fact, in some accurate weighings, a correction must be made in order to compensate for the buoyancy effect of the surrounding air.
The buoyant force, which always opposes gravity, is nevertheless caused by gravity. Fluid pressure increases with depth because of the (gravitational) weight of the fluid above. This increasing pressure applies a force on a submerged object that increases with depth. The result is buoyancy.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1039 2021-06-03 00:20:18
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,771
Re: Miscellany
1017) Bernoulli's theorem
Bernoulli’s theorem, in fluid dynamics, relation among the pressure, velocity, and elevation in a moving fluid (liquid or gas), the compressibility and viscosity (internal friction) of which are negligible and the flow of which is steady, or laminar. First derived (1738) by the Swiss mathematician Daniel Bernoulli, the theorem states, in effect, that the total mechanical energy of the flowing fluid, comprising the energy associated with fluid pressure, the gravitational potential energy of elevation, and the kinetic energy of fluid motion, remains constant. Bernoulli’s theorem is the principle of energy conservation for ideal fluids in steady, or streamline, flow and is the basis for many engineering applications.
Bernoulli’s theorem implies, therefore, that if the fluid flows horizontally so that no change in gravitational potential energy occurs, then a decrease in fluid pressure is associated with an increase in fluid velocity. If the fluid is flowing through a horizontal pipe of varying cross-sectional area, for example, the fluid speeds up in constricted areas so that the pressure the fluid exerts is least where the cross section is smallest. This phenomenon is sometimes called the Venturi effect, after the Italian scientist G.B. Venturi (1746–1822), who first noted the effects of constricted channels on fluid flow.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1040 2021-06-04 00:06:18
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,771
Re: Miscellany
1018) Speed of light
Speed of light, speed at which light waves propagate through different materials. In particular, the value for the speed of light in a vacuum is now defined as exactly 299,792,458 metres per second.
The speed of light is considered a fundamental constant of nature. Its significance is far broader than its role in describing a property of electromagnetic waves. It serves as the single limiting velocity in the universe, being an upper bound to the propagation speed of signals and to the speeds of all material particles. In the famous relativity equation, E = mc², the speed of light (c) serves as a constant of proportionality, linking the formerly disparate concepts of mass (m) and energy (E).
The speed of light in a vacuum is 186,282 miles per second (299,792 kilometers per second), and in theory nothing can travel faster than light. In miles per hour, light speed is, well, a lot: about 670,616,629 mph. If you could travel at the speed of light, you could go around the Earth 7.5 times in one second.
Early scientists, unable to perceive light's motion, thought it must travel instantaneously. Over time, however, measurements of the motion of these wave-like particles became more and more precise. Thanks to the work of Albert Einstein and others, we now understand light speed to be a theoretical limit: light speed — a constant called "c" — is thought to be not achievable by anything with mass, for reasons explained below. That doesn't stop sci-fi writers, and even some very serious scientists, from imagining alternative theories that would allow for some awfully fast trips around the universe.
Speed of light: History of the theory
The first known discourse on the speed of light comes from the ancient Greek philosopher Aristotle, who penned his disagreement with another Greek scientist, Empedocles. Empedocles argued that because light moved, it must take time to travel. Aristotle, believing light to travel instantaneously, disagreed.
In 1667, the Italian astronomer Galileo Galilei stood two people on hills less than a mile apart, each holding a shielded lantern. One uncovered his lantern; when the second saw the flash, he uncovered his, as well. By observing how long it took for the light to be seen by the first lantern-holder (and factoring out reaction times), he thought he could calculate the speed of light. Unfortunately, Galileo's experimental distance of less than a mile was too small to see a difference, so he could only determine that light traveled at least 10 times faster than sound.
In the 1670s, Danish astronomer Ole Römer used eclipses of Jupiter's moon, Io, as a chronometer for the speed of light when he made the first real measurement of the velocity. Over the course of several months, as Io passed behind the giant gas planet, Römer found that the eclipses came later than calculations anticipated, although over the course of several months, they drew closer to the predictions. He determined that light took time to travel from Io to Earth. The eclipses lagged the most when Jupiter and Earth were farthest apart, and were on schedule as they were closer.
According to NASA, "that gave Römer convincing evidence that light spread in space with a certain velocity."
He concluded that light took 10 to 11 minutes to travel from the sun to Earth, an overestimate since it in fact takes eight minutes and 19 seconds. But at last scientists had a number to work with — his calculation presented a speed of 125,000 miles per second (200,000 km/s).
In 1728, English physicist James Bradley based his calculations on the change in the apparent position of the stars due Earth's travels around the sun. He put the speed of light at 185,000 miles per second (301,000 km/s), accurate to within about 1 percent.
Two attempts in the mid-1800s brought the problem back to Earth. French physicist Hippolyte Fizeau set a beam of light on a rapidly rotating toothed wheel, with a mirror set up 5 miles away to reflect it back to its source. Varying the speed of the wheel allowed Fizeau to calculate how long it took for the light to travel out of the hole, to the adjacent mirror, and back through the gap. Another French physicist, Leon Foucault, used a rotating mirror rather than a wheel. The two independent methods each came within about 1,000 miles per second of the speed of light measured today.
Prussian-born Albert Michelson, who grew up in the United States, attempted to replicate Foucault's method in 1879, but used a longer distance, as well as extremely high-quality mirrors and lenses. His result of 186,355 miles per second (299,910 km/s) was accepted as the most accurate measurement of the speed of light for 40 years, when Michelson re-measured it.
An interesting footnote to Michelson's experiment was that he was trying to detect the medium that light traveled through, referred to as luminiferous aether. Instead, his experiment revealed the aether didn't exist.
"The experiment — and Michelson's body of work — was so revolutionary that he became the only person in history to have won a Nobel Prize for a very precise non-discovery of anything," wrote astrophysicist Ethan Siegal in the Forbes science blog, Starts With a Bang. "The experiment itself may have been a complete failure, but what we learned from it was a greater boon to humanity and our understanding of the universe than any success would have been!"
Einstein and special relativity
In 1905, Albert Einstein wrote his first paper on special relativity. In it, he established that light travels at the same speed no matter how fast the observer moves. Even using the most precise measurements possible, the speed of light remains the same for an observer standing still on the face of the Earth as it does for one traveling in a supersonic jet above its surface. Similarly, even though Earth is orbiting the sun, which is itself moving around the Milky Way, which is a galaxy traveling through space, the measured speed of light coming from our sun would be the same whether one stood inside or outside of the galaxy to calculate it. Einstein calculated that the speed of light doesn't vary with time or place.
Although the speed of light is often referred to as the universe's speed limit, the universe actually expands even faster. According to astrophysicist Paul Sutter, the universe expands at roughly 68 kilometers per second per megaparsec, where a megaparsec is 3.26 million light-years (more on that later). So a galaxy 1 megaparsec away appears to be traveling away from the Milky Way at a speed of 68 km/s, while a galaxy two megaparsecs away recedes at 136 km/s, and so on.
"At some point, at some obscene distance, the speed tips over the scales and exceeds the speed of light, all from the natural, regular expansion of space," Sutter wrote.
He went on to explain that, while special relativity provides an absolute speed limit, general relativity allows for broader distances.
"A galaxy on the far side of the universe? That's the domain of general relativity, and general relativity says: Who cares! That galaxy can have any speed it wants, as long as it stays way far away, and not up next to your face," he wrote.
"Special relativity doesn't care about the speed — superluminal or otherwise — of a distant galaxy. And neither should you."
What is a light-year?
The distance light travels in the course of a year is called a light-year. A light-year is a measure of both time and distance. It is not as hard to understand as it seems. Think of it this way: Light travels from the moon to our eyes in about 1 second, which means the moon is about 1 light-second away. Sunlight takes about 8 minutes to reach our eyes, so the sun is about 8 light-minutes away. Light from the nearest star system, Alpha Centauri, is requires roughly 4.3 years to get here, so that star system is said to be 4.3 light-years away.
"To obtain an idea of the size of a light-year, take the circumference of the Earth (24,900 miles), lay it out in a straight line, multiply the length of the line by 7.5 (the corresponding distance is one light-second), then place 31.6 million similar lines end to end," NASA's Glenn Research center writes on its website. "The resulting distance is almost 6 trillion (6,000,000,000,000) miles!"
Stars and other objects beyond our solar system lie anywhere from a few light-years to a few billion light-years away. Thus, when astronomers study objects that lie a light-year away or more, they are seeing it as existed at the time that light left it, not as it would appear if they stood near its surface today. In this sense, everything we see in the distant universe is, literally, history.
This principle allows astronomers to see how the universe as it looked after the Big Bang, which took place about 13.8 billion years ago. Examining objects that are, say, 10 billion light-years away, we see them as they looked 10 billion years ago, relatively soon after the beginning of the universe, rather than how they appear today.
Is the speed of light really constant?
Light travels in waves, and, like sound, can be slowed depending on what it is traveling through. Nothing can outpace light in a vacuum. However, if a region contains any matter, even dust, light can bend when it comes in contact with the particles, which results in a decrease in speed.
Light traveling through Earth's atmosphere moves almost as fast as light in a vacuum, while light passing through a diamond is slowed to less than half that speed. Still, it travels through the gem at over 277 million mph (almost 124,000 km/s) — not a speed to scoff at.
Can we travel faster than light?
Science fiction loves to speculate about this, because "warp speed," as faster-than-light travel is popularly known, would allow us to travel between stars in time frames otherwise impossibly long. And while it has not been proven to be impossible, the practicality of traveling faster than light renders the idea pretty farfetched.
According to Einstein's general theory of relativity, as an object moves faster, its mass increases, while its length contracts. At the speed of light, such an object has an infinite mass, while its length is 0 — an impossibility. Thus, no object can reach the speed of light, the theory goes.
That doesn't stop theorists from proposing creative and competing theories. The idea of warp speed is not impossible, some say, and perhaps in future generations people will hop between stars the way we travel between cities nowadays. One proposal would involve a spaceship that could fold a space-time bubble around itself in order to exceed the speed of light. Sounds great, in theory.
"If Captain Kirk were constrained to move at the speed of our fastest rockets, it would take him a hundred thousand years just to get to the next star system," said Seth Shostak, an astronomer at the Search for Extraterrestrial Intelligence (SETI) Institute in Mountain View, Calif., in a 2010 interview. "So science fiction has long postulated a way to beat the speed of light barrier so the story can move a little more quickly."

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1041 2021-06-05 00:30:57
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,771
Re: Miscellany
1019) Electromagnetic spectrum
Electromagnetic spectrum, the entire distribution of electromagnetic radiation according to frequency or wavelength. Although all electromagnetic waves travel at the speed of light in a vacuum, they do so at a wide range of frequencies, wavelengths, and photon energies. The electromagnetic spectrum comprises the span of all electromagnetic radiation and consists of many subranges, commonly referred to as portions, such as visible light or ultraviolet radiation. The various portions bear different names based on differences in behaviour in the emission, transmission, and absorption of the corresponding waves and also based on their different practical applications. There are no precise accepted boundaries between any of these contiguous portions, so the ranges tend to overlap.
The entire electromagnetic spectrum, from the lowest to the highest frequency (longest to shortest wavelength), includes all radio waves (e.g., commercial radio and television, microwaves, radar), infrared radiation, visible light, ultraviolet radiation, X-rays, and gamma rays. Nearly all frequencies and wavelengths of electromagnetic radiation can be used for spectroscopy.
When you think of light, you probably think of what your eyes can see. But the light to which our eyes are sensitive is just the beginning; it is a sliver of the total amount of light that surrounds us. The electromagnetic spectrum is the term used by scientists to describe the entire range of light that exists. From radio waves to gamma rays, most of the light in the universe is, in fact, invisible to us!
Light is a wave of alternating electric and magnetic fields. The propagation of light isn’t much different than waves crossing an ocean. Like any other wave, light has a few fundamental properties that describe it. One is its frequency, measured in hertz (Hz), which counts the number of waves that pass by a point in one second. Another closely related property is wavelength: the distance from the peak of one wave to the peak of the next. These two attributes are inversely related. The larger the frequency, the smaller the wavelength – and vice versa.
The electromagnetic waves your eyes detect – visible light – oscillate between 400 and 790 terahertz (THz). That’s several hundred trillion times a second. The wavelengths are roughly the size of a large virus: 390 – 750 nanometers (1 nanometer = 1 billionth of a meter; a meter is about 39 inches long). Our brain interprets the various wavelengths of light as different colors. Red has the longest wavelength, and violet the shortest. When we pass sunlight through a prism, we see that it’s actually composed of many wavelengths of light. The prism creates a rainbow by redirecting each wavelength out at a slightly different angle.
But light doesn’t stop at red or violet. Just like there are sounds we can’t hear (but other animals can), there is also an enormous range of light that our eyes can’t detect. In general, the longer wavelengths come from the coolest and darkest regions of space. Meanwhile, the shorter wavelengths measure extremely energetic phenomena.
Astronomers use the entire electromagnetic spectrum to observe a variety of things. Radio waves and microwaves – the longest wavelengths and lowest energies of light – are used to peer inside dense interstellar clouds and track the motion of cold, dark gas. Radio telescopes have been used to map the structure of our galaxy while microwave telescopes are sensitive to the remnant glow of the Big Bang.
Infrared telescopes excel at finding cool, dim stars, slicing through interstellar dust bands, and even measuring the temperatures of planets in other solar systems. The wavelengths of infrared light are long enough to navigate through clouds that would otherwise block our view. By using large infrared telescopes, astronomers have been able to peer through the dust lanes of the Milky Way into the core of our galaxy.
The majority of stars emit most of their electromagnetic energy as visible light, the tiny portion of the spectrum to which our eyes are sensitive. Because wavelength correlates with energy, the color of a star tells us how hot it is: red stars are coolest, blue are hottest. The coldest of stars emit hardly any visible light at all; they can only be seen with infrared telescopes.
At wavelengths shorter than violet, we find the ultraviolet, or UV, light. You may be familiar with UV from its ability to give you a sunburn. Astronomers use it to hunt out the most energetic of stars and identify regions of star birth. When viewing distant galaxies with UV telescopes, most of the stars and gas disappear, and all the stellar nurseries flare into view.
Beyond UV come the highest energies in the electromagnetic spectrum: X-rays and gamma rays. Our atmosphere blocks this light, so astronomers must rely on telescopes in space to see the X-ray and gamma ray universe. X-rays come from exotic neutron stars, the vortex of superheated material spiraling around a black hole, or diffuse clouds of gas in galactic clusters that are heated to many millions of degrees. Meanwhile, gamma rays – the shortest wavelength of light and deadly to humans – unveil violent supernova explosions, cosmic radioactive decay, and even the destruction of antimatter. Gamma ray bursts – the brief flickering of gamma ray light from distant galaxies when a star explodes and creates a black hole – are among the most energetic singular events in the universe.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1042 2021-06-06 00:12:30
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,771
Re: Miscellany
1020) Seismic wave
Seismic wave, vibration generated by an earthquake, explosion, or similar energetic source and propagated within the Earth or along its surface. Earthquakes generate four principal types of elastic waves; two, known as body waves, travel within the Earth, whereas the other two, called surface waves, travel along its surface. Seismographs record the amplitude and frequency of seismic waves and yield information about the Earth and its subsurface structure. Artificially generated seismic waves recorded during seismic surveys are used to collect data in oil and gas prospecting and engineering.
Of the body waves, the primary, or P, wave has the higher speed of propagation and so reaches a seismic recording station faster than the secondary, or S, wave. P waves, also called compressional or longitudinal waves, give the transmitting medium—whether liquid, solid, or gas—a back-and-forth motion in the direction of the path of propagation, thus stretching or compressing the medium as the wave passes any one point in a manner similar to that of sound waves in air. In the Earth, P waves travel at speeds from about 6 km (3.7 miles) per second in surface rock to about 10.4 km (6.5 miles) per second near the Earth’s core some 2,900 km (1,800 miles) below the surface. As the waves enter the core, the velocity drops to about 8 km (5 miles) per second. It increases to about 11 km (6.8 miles) per second near the centre of the Earth. The speed increase with depth results from increased hydrostatic pressure as well as from changes in rock composition; in general, the increase causes P waves to travel in curved paths that are concave upward.
S waves, also called shear or transverse waves, cause points of solid media to move back and forth perpendicular to the direction of propagation; as the wave passes, the medium is sheared first in one direction and then in another. In the Earth the speed of S waves increases from about 3.4 km (2.1 miles) per second at the surface to 7.2 km (4.5 miles) per second near the boundary of the core, which, being liquid, cannot transmit them; indeed, their observed absence is a compelling argument for the liquid nature of the outer core. Like P waves, S waves travel in curved paths that are concave upward.
Of the two surface seismic waves, Love waves—named after the British seismologist A.E.H. Love, who first predicted their existence—travel faster. They are propagated when the solid medium near the surface has varying vertical elastic properties. Displacement of the medium by the wave is entirely perpendicular to the direction of propagation and has no vertical or longitudinal components. The energy of Love waves, like that of other surface waves, spreads from the source in two directions rather than in three, and so these waves produce a strong record at seismic stations even when originating from distant earthquakes.
The other principal surface waves are called Rayleigh waves after the British physicist Lord Rayleigh, who first mathematically demonstrated their existence. Rayleigh waves travel along the free surface of an elastic solid such as the Earth. Their motion is a combination of longitudinal compression and dilation that results in an elliptical motion of points on the surface. Of all seismic waves, Rayleigh waves spread out most in time, producing a long wave duration on seismographs.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1043 2021-06-07 00:04:01
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,771
Re: Miscellany
1021) Polonium
Polonium (Po), a radioactive, silvery-gray or black metallic element of the oxygen group (Group 16 [VIa] in the periodic table). The first element to be discovered by radiochemical analysis, polonium was discovered in 1898 by Pierre and Marie Curie, who were investigating the radioactivity of a certain pitchblende, a uranium ore. The very intense radioactivity not attributable to uranium was ascribed to a new element, named by them after Marie Curie’s homeland, Poland. The discovery was announced in July 1898. Polonium is extremely rare, even in pitchblende: 1,000 tons of the ore must be processed to obtain 40 milligrams of polonium. Its abundance in the Earth’s crust is about one part in {10}^{15}. It occurs in nature as a radioactive decay product of uranium, thorium, and actinium. The half-lives of its isotopes range from a fraction of a second up to 103 years; the most common natural isotope of polonium, polonium-210, has a half-life of 138.4 days.
Polonium usually is isolated from by-products of the extraction of radium from uranium minerals. In the chemical isolation, pitchblende ore is treated with hydrochloric acid, and the resulting solution is heated with hydrogen sulfide to precipitate polonium monosulfide, PoS, along with other metal sulfides, such as that of bismuth, Bi2S3, which resembles polonium monosulfide closely in chemical behaviour, though it is less soluble. Because of the difference in solubility, repeated partial precipitation of the mixture of sulfides concentrates the polonium in the more soluble fraction, while the bismuth accumulates in the less soluble portions. The difference in solubility is small, however, and the process must be repeated many times to achieve a complete separation. Purification is accomplished by electrolytic deposition. It can be produced artificially by bombarding bismuth or lead with neutrons or with accelerated charged particles.
Chemically, polonium resembles the elements tellurium and bismuth. Two modifications of polonium are known, an α- and a β-form, both of which are stable at room temperature and possess metallic characteristics. The fact that its electrical conductivity decreases as the temperature increases places polonium among the metals rather than the metalloids or nonmetals.
Because polonium is highly radioactive—it disintegrates to a stable isotope of lead by emitting alpha rays, which are streams of positively charged particles—it must be handled with extreme care. When contained in such substances as gold foil, which prevent the alpha radiation from escaping, polonium is used industrially to eliminate static electricity generated by such processes as paper rolling, the manufacture of sheet plastics, and the spinning of synthetic fibres. It is also used on brushes for removing dust from photographic film and in nuclear physics as a source of alpha radiation. Mixtures of polonium with beryllium or other light elements are used as sources of neutrons.
atomic number : 84
atomic weight : 210
melting point : 254 °C (489 °F)
boiling point : 962 °C (1,764 °F)
density : 9.4 g/cm³.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1044 2021-06-08 00:05:45
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,771
Re: Miscellany
1022) Palladium
Palladium (Pd), chemical element, the least dense and lowest-melting of the platinum metals of Groups 8–10 (VIIIb), Periods 5 and 6, of the periodic table, used especially as a catalyst (a substance that speeds up chemical reactions without changing their products) and in alloys.
A precious gray-white metal, palladium is extremely ductile and easily worked. Palladium is not tarnished by the atmosphere at ordinary temperatures. Thus, the metal and its alloys serve as substitutes for platinum in jewelry and in electrical contacts; the beaten leaf is used for decorative purposes. Relatively small amounts of palladium alloyed with gold yield the best white gold. Palladium is used also in dental alloys. The chief use of palladium, however, is in automobile catalytic converters (often in combination with rhodium); the palladium serves as a catalyst to convert polluting hydrocarbons, carbon monoxide, and nitrogen oxide in the exhaust to water, carbon dioxide, and nitrogen. Palladium coatings, electrodeposited or chemically plated, have been used in printed-circuit components, and palladium is also used in multilayer ceramic capacitors.
Native palladium, though rare, occurs alloyed with a little platinum and iridium in Colombia (department of Chocó), in Brazil (Itabira, Minas Gerais), in the Ural Mountains, and in South Africa (the Transvaal). Palladium is one of the most abundant platinum metals and occurs in Earth’s crust at an abundance of 0.015 part per million. Palladium also occurs alloyed with native platinum. It was first isolated (1803) from crude platinum by the English chemist and physicist William Hyde Wollaston. He named the element in honour of the newly discovered asteroid Pallas. Palladium is also associated with a number of gold, silver, copper and nickel ores. It is generally produced commercially as a by-product in the refining of copper and nickel ores. Russia, South Africa, Canada, and the United States were the world’s leading producers of palladium in the early 21st century.
Surfaces of palladium are excellent catalysts for chemical reactions involving hydrogen and oxygen, such as the hydrogenation of unsaturated organic compounds. Under suitable conditions (80 °C [176 °F] and 1 atmosphere), palladium absorbs more than 900 times its own volume of hydrogen. It expands and becomes harder, stronger, and less ductile in the process. The absorption also causes both the electrical conductivity and magnetic susceptibility to decrease. A metallic or alloylike hydride is formed from which the hydrogen can be removed by increased temperature and reduced pressure. Because hydrogen passes rapidly through the metal at high temperatures, heated palladium tubes impervious to other gases function as semipermeable membranes and are used to pass hydrogen in and out of closed gas systems or for hydrogen purification.
Palladium is more reactive than the other platinum metals. For example, it is attacked more readily by acids than any of the other platinum metals. It dissolves slowly in nitric acid to give palladium(II) nitrate, Pd(NO3)2, and with concentrated sulfuric acid it yields palladium(II) sulfate, PdSO4∙2H2O. In its sponge form it will dissolve even in hydrochloric acid in the presence of chlorine or oxygen. It is rapidly attacked by fused alkali oxides and peroxides and also by fluorine and chlorine at about 500 °C (932 °F). Palladium also combines with a number of nonmetallic elements on heating, such as phosphorus, math, antimony, silicon, sulfur, and selenium. A series of palladium compounds can be prepared with the +2 oxidation state; numerous compounds in the +4 state and a few in the 0 state are also known. Among the transition metals palladium has one of the strongest tendencies to form bonds with carbon. All palladium compounds are easily decomposed or reduced to the free metal. An aqueous solution of potassium tetrachloropalladate(II), K2PdCl4, serves as a sensitive detector for carbon monoxide or olefin gases because a black precipitate of the metal appears in the presence of exceedingly small amounts of those gases. Natural palladium consists of a mixture of six stable isotopes: palladium-102 (1.02 percent), palladium-104 (11.14 percent), palladium-105 (22.33 percent), palladium-106 (27.33 percent), palladium-108 (26.46 percent), and palladium-110 (11.72 percent).
atomic number : 46
atomic weight : 106.40
melting point : 1,554.9 °C (2,830.8 °F)
boiling point : 2,963 °C (5,365 °F)
specific gravity : 12.02 (0 °C [32 °F])
oxidation states : +2, +4.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1045 2021-06-09 00:13:35
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,771
Re: Miscellany
1023) Magnesium
Magnesium (Mg), chemical element, one of the alkaline-earth metals of Group 2 (IIa) of the periodic table, and the lightest structural metal. Its compounds are widely used in construction and medicine, and magnesium is one of the elements essential to all cellular life.
atomic number : 12
atomic weight : 24.305
melting point : 650 °C (1,202 °F)
boiling point : 1,090 °C (1,994 °F)
specific gravity : 1.74 at 20° C (68 °F)
oxidation state : +2
Occurrence, Properties, And Uses
Known originally through compounds such as Epsom salts (the sulfate), magnesia or magnesia alba (the oxide), and magnesite (the carbonate), the silvery white element itself does not occur free in nature. It was first isolated in 1808 by Sir Humphry Davy, who evaporated the mercury from a magnesium amalgam made by electrolyzing a mixture of moist magnesia and mercuric oxide. The name magnesium comes from Magnesia, a district of Thessaly (Greece) where the mineral magnesia alba was first found.
Magnesium is the eighth most abundant element in Earth’s crust (about 2.5 percent) and is, after aluminum and iron, the third most plentiful structural metal. Its cosmic abundance is estimated as 9.1 × {10}^5 atoms (on a scale where the abundance of silicon = {10}^6 atoms). It occurs as carbonates—magnesite, MgCO3, and dolomite, CaMg(CO3)2—and in many common silicates, including talc, olivine, and most kinds of asbestos. It also is found as hydroxide (brucite), chloride (carnallite, KMgCl3∙6H2O), and sulfate (kieserite). It is distributed in minerals such as serpentine, chrysolite, and meerschaum. Seawater contains about 0.13 percent magnesium, mostly as the dissolved chloride, which imparts its characteristic bitter taste.
Magnesium is commercially produced by electrolysis of molten magnesium chloride (MgCl2), processed mainly from seawater and by the direct reduction of its compounds with suitable reducing agents—e.g., from the reaction of magnesium oxide or calcined dolomite with ferrosilicon (the Pidgeon process).
At one time, magnesium was used for photographic flash ribbon and powder, because in finely divided form it burns in air with an intense white light; it still finds application in explosive and pyrotechnic devices. Because of its low density (only two-thirds that of aluminum), it has found extensive use in the aerospace industry. However, because the pure metal has low structural strength, magnesium is mainly used in the form of alloys—principally with 10 percent or less of aluminum, zinc, and manganese—to improve its hardness, tensile strength, and ability to be cast, welded, and machined. Casting, rolling, extruding, and forging techniques are all employed with the alloys, and further fabrication of the resulting sheet, plate, or extrusion is carried out by normal forming, joining, and machining operations. Magnesium is the easiest structural metal to machine and has often been used when a large number of machining operations are required. Magnesium alloys have a number of applications: they are used for parts of aircraft, spacecraft, machinery, automobiles, portable tools, and household appliances.
The thermal and electrical conductivity of magnesium and its melting point are very similar to those of aluminum. Whereas aluminum is attacked by alkalies but is resistant to most acids, magnesium is resistant to most alkalies but is readily attacked by most acids to liberate hydrogen (chromic and hydrofluoric acids are important exceptions). At normal temperatures it is stable in air and water because of the formation of a thin protective skin of oxide, but it is attacked by steam. Magnesium is a powerful reducing agent and is used to produce other metals from their compounds (e.g., titanium, zirconium, and hafnium). It reacts directly with many elements.
Magnesium occurs in nature as a mixture of three isotopes: magnesium-24 (79.0 percent), magnesium-26 (11.0 percent), and magnesium-25 (10.0 percent). Nineteen radioactive isotopes have been prepared; magnesium-28 has the longest half-life, at 20.9 hours, and is a beta emitter. Although magnesium-26 is not radioactive, it is the daughter nuclide of aluminum-26, which has a half-life of 7.2 × {10}^5 years. Elevated levels of magnesium-26 have been found in some meteorites, and the ratio of magnesium-26 to magnesium-24 has been used in determining their age.
The top producers of magnesium by the second decade of the 21st century included China, Russia, Turkey, and Austria.
Principal Compounds
In compounds, magnesium virtually always exhibits a +2 oxidation state because of the loss or sharing of its two 3s electrons. There are, however, a small number of coordination compounds known with magnesium-magnesium bonds, LMg―MgL, in which the magnesium centres have a formal +1 oxidation state. Magnesium carbonate, MgCO3, occurs in nature as the mineral magnesite and is an important source of elemental magnesium. It can be produced artificially by the action of carbon dioxide on a variety of magnesium compounds. The odourless white powder has many industrial uses—e.g., as a heat insulator for boilers and pipes and as an additive in food, pharmaceuticals, cosmetics, rubbers, inks, and glass. As magnesium carbonate is both hygroscopic and insoluble in water, it was the original additive used to make table salt free-flowing even in high-humidity conditions.
Magnesium hydroxide, Mg(OH)2, is a white powder produced in large quantities from seawater by the addition of milk of lime (calcium hydroxide). It is the primary raw material in the production of magnesium metal and has been used as a fire-retardant additive. In water it forms a suspension known as milk of magnesia, which has long been used as an antacid and a laxative.
The action of hydrochloric acid on magnesium hydroxide produces magnesium chloride, MgCl2, a colourless, deliquescent (water-absorbing) substance employed in magnesium metal production, in the manufacture of a cement for heavy-duty flooring, and as an additive in textile manufacture. It is also used to coagulate soy milk in the production of tofu.
Roasting either magnesium carbonate or magnesium hydroxide produces the oxygen compound magnesium oxide, commonly called magnesia, MgO. It is a white solid used in the manufacture of high-temperature refractory bricks, electrical and thermal insulators, cements, fertilizer, rubber, and plastics. It is also used medically as a laxative and antacid.
Magnesium sulfate, MgSO4, is a colourless crystalline substance formed by the reaction of magnesium hydroxide with sulfur dioxide and air. A hydrate form of magnesium sulfate called kieserite, MgSO4∙H2O, occurs as a mineral deposit. Synthetically prepared magnesium sulfate is sold as Epsom salt, MgSO4∙7H2O. In industry, magnesium sulfate is used in the manufacture of cements and fertilizers and in tanning and dyeing; in medicine it serves as a purgative. Because of its ability to absorb water readily, the anhydrous form is used as a desiccant (drying agent).
Among the organometallic compounds of magnesium are the important Grignard reagents, composed of an organic group (e.g., alkyls and aryls), a halogen atom other than fluorine, and magnesium. These are used in the production of many other kinds of organic and organometallic compounds.
Magnesium is essential to all living cells, as the Mg2+ ion is involved with the critically important biological polyphosphate compounds DNA, RNA, and adenosine triphosphate (ATP). Many enzymes depend on magnesium for their functioning. About one-sixth as plentiful as potassium in human body cells, magnesium is required as a catalyst for enzyme reactions in carbohydrate metabolism. Magnesium also is an essential constituent of the green pigment chlorophyll, found in virtually all plants, algae, and cyanobacteria. The photosynthetic function of plants depends upon the action of chlorophyll pigments, which contain magnesium at the centre of a complex, nitrogen-containing ring system (porphyrin). These magnesium compounds enable light energy to drive the conversion of carbon dioxide and water to carbohydrates and oxygen and thus directly or indirectly provide the key to nearly all living processes.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1046 2021-06-10 00:18:40
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,771
Re: Miscellany
1024) Peninsula
A peninsula (Latin: paeninsula from paene "almost” and insula "island") is a landform surrounded by water on the majority of its border while being connected to a mainland from which it extends. The surrounding water is usually understood to be continuous, though not necessarily named as a single body of water. Peninsulas are not always named as such; one can also be referred to as a headland, cape, island promontory, bill, point, fork, or spit. A point is generally considered a tapering piece of land projecting into a body of water that is less prominent than a cape. A river which courses through a very tight meander is also sometimes said to form a "peninsula" within the (almost closed) loop of water. In English, the plural versions of peninsula are peninsulas and, less commonly, peninsulae.
A peninsula is a piece of land that is almost entirely surrounded by water but is connected to the mainland on one side.
Peninsulas can be very small, sometimes only large enough for a single lighthouse, for instance. Lighthouses often sit on peninsulas near rocky coastlines to warn sailors that they are getting close to land.
Peninsulas can also be very large. Most of the U.S. state of Florida is a peninsula that separates the Gulf of Mexico and the Atlantic Ocean.
Peninsulas are found on every continent. In North America, the narrow peninsula of Baja California, in Mexico, separates the Pacific Ocean and the Sea of Cortez, also called the Gulf of California. In Europe, the nations of Portugal and Spain make up the Iberian Peninsula. The so-called Horn of Africa, which juts into the Arabian Sea on central Africas east coast, is a huge peninsula. The nations of North Korea and South Korea make up the Korean Peninsula in eastern Asia. In Australia, the Cape York Peninsula is only 160 kilometers (99 miles) from the island of New Guinea. The Antarctic Peninsula seems to point to the tip of South America, several hundred kilometers (miles) away.
In geography, a cape is that point of land which goes beyond the adjacent coast to a lake or sea. On the other hand, a peninsula refers to a piece of land that is connected to the mainland, but water surrounds most of its border. What this says about capes and peninsulas is that a cape can be found at the end of a peninsula. Geologically, capes have short life spans because of the frequent erosion they undergo thanks to their proximity to any natural form of erosion, especially tides.
What Is A Peninsula?
Peninsula comes from two Greek words, one is “paene,” and the other is “insula.” The former means “almost” and the latter means “island.” Peninsulas are prevalent on coastlines and in some cases small water bodies. A peninsula can be formed in different ways. First, the rising of the sea level may leave areas below a certain level submerged and only a high land emerging through the surface. Or still, a fall of the water level will expose a high land that has been previously submerged by the water. In some cases, landmasses can separate over a period of millions of years and gradually, a peninsula is formed.
Examples Of Peninsulas
Arabian Peninsula, found in Westen Asia, is considered to be the largest Peninsula in the world. It touches nine Arab countries, and the largest portion is in Saudi Arabia. It was created when separation of a previously large landmass occurred, which estimates it to be around 23 million years old.
Another peninsula is the Italian Peninsula in southern Europe. It largely occupies Italy. Then there is the Korean Peninsula which is divided between South and North Korea. Then we have the Upper and Lower Peninsula in Michigan USA and Florida Peninsula in Florida USA.
What Is A Cape?
There are two main features of a cape; there is a large portion of land that extends into a large water body like an ocean, then there is a change in both the shape and the direction taken from the rest of the coastline. Capes are usually formed through volcanic activities, wave action, glaciers and changes in the sea level. In any of these methods, erosion plays a significant role.
Examples Of Capes
One of the most historic and famous capes is the Cape of Good Hope in South Africa. It is a rocky point which marks the end of the Peninsula extending from South Africa’s western coast. The coastline at this point turns eastward into the southern tip of Africa. Cape Cod in Massachusetts in the USA is also popular and attracts tourists. This is due to its beaches, villages, and towns that boast recreational fishing, good restaurants, swimming and other activities. It extends about 65 miles eastwards into the Atlantic Ocean with the outer portion going upwards then west like a hook. An artificial channel of 17.5 miles long was built for boat traffic in Cape Cod. Lastly, Cape Canaveral in Florida USA is popular for being the location of the Kennedy Space Centre.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1047 2021-06-11 00:54:07
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,771
Re: Miscellany
1025) Meteorology
Meteorology is the study of the atmosphere, atmospheric phenomena, and atmospheric effects on our weather. The atmosphere is the gaseous layer of the physical environment that surrounds a planet. Earth’s atmosphere is roughly 100 to 125 kilometers (65-75 miles) thick. Gravity keeps the atmosphere from expanding much farther.
Meteorology is a subdiscipline of the atmospheric sciences, a term that covers all studies of the atmosphere. A subdiscipline is a specialized field of study within a broader subject or discipline. Climatology and aeronomy are also subdisciplines of the atmospheric sciences. Climatology focuses on how atmospheric changes define and alter the world’s climates. Aeronomy is the study of the upper parts of the atmosphere, where unique chemical and physical processes occur. Meteorology focuses on the lower parts of the atmosphere, primarily the troposphere, where most weather takes place.
Meteorologists use scientific principles to observe, explain, and forecast our weather. They often focus on atmospheric research or operational weather forecasting. Research meteorologists cover several subdisciplines of meteorology to include: climate modeling, remote sensing, air quality, atmospheric physics, and climate change. They also research the relationship between the atmosphere and Earth’s climates, oceans, and biological life.
Forecasters use that research, along with atmospheric data, to scientifically assess the current state of the atmosphere and make predictions of its future state. Atmospheric conditions both at the Earth's surface and above are measured from a variety of sources: weather stations, ships, buoys, aircraft, radar, weather balloons, and satellites. This data is transmitted to centers throughout the world that produce computer analyses of global weather. The analyses are passed on to national and regional weather centers, which feed this data into computers that model the future state of the atmosphere. This transfer of information demonstrates how weather and the study of it take place in multiple, interconnected ways.
Scales of Meteorology
Weather occurs at different scales of space and time. The four meteorological scales are: microscale, mesoscale, synoptic scale, and global scale. Meteorologists often focus on a specific scale in their work.
Microscale Meteorology
Microscale meteorology focuses on phenomena that range in size from a few centimeters to a few kilometers, and that have short life spans (less than a day). These phenomena affect very small geographic areas, and the temperatures and terrains of those areas.
Microscale meteorologists often study the processes that occur between soil, vegetation, and surface water near ground level. They measure the transfer of heat, gas, and liquid between these surfaces. Microscale meteorology often involves the study of chemistry.
Tracking air pollutants is an example of microscale meteorology. MIRAGE-Mexico is a collaboration between meteorologists in the United States and Mexico. The program studies the chemical and physical transformations of gases and aerosols in the pollution surrounding Mexico City. MIRAGE-Mexico uses observations from ground stations, aircraft, and satellites to track pollutants.
Mesoscale Meteorology
Mesoscale phenomena range in size from a few kilometers to roughly 1,000 kilometers (620 miles). Two important phenomena are mesoscale convective complexes (MCC) and mesoscale convective systems (MCS). Both are caused by convection, an important meteorological principle.
Convection is a process of circulation. Warmer, less-dense fluid rises, and colder, denser fluid sinks. The fluid that most meteorologists study is air. (Any substance that flows is considered a fluid.) Convection results in a transfer of energy, heat, and moisture—the basic building blocks of weather.
In both an MCC and MCS, a large area of air and moisture is warmed during the middle of the day—when the sun angle is at its highest. As this warm air mass rises into the colder atmosphere, it condenses into clouds, turning water vapor into precipitation.
An MCC is a single system of clouds that can reach the size of the state of Ohio and produce heavy rainfall and flooding. An MCS is a smaller cluster of thunderstorms that lasts for several hours. Both react to unique transfers of energy, heat, and moisture caused by convection.
The Deep Convective Clouds and Chemistry (DC3) field campaign is a program that will study storms and thunderclouds in Colorado, Alabama, and Oklahoma. This project will consider how convection influences the formation and movement of storms, including the development of lightning. It will also study their impact on aircraft and flight patterns. The DC3 program will use data gathered from research aircraft able to fly over the tops of storms.
Synoptic Scale Meteorology
Synoptic-scale phenomena cover an area of several hundred or even thousands of kilometers. High- and low-pressure systems seen on local weather forecasts, are synoptic in scale. Pressure, much like convection, is an important meteorological principle that is at the root of large-scale weather systems as diverse as hurricanes and bitter cold outbreaks.
Low-pressure systems occur where the atmospheric pressure at the surface of the Earth is less than its surrounding environment. Wind and moisture from areas with higher pressure seek low-pressure systems. This movement, in conjunction with the Coriolis force and friction, causes the system to rotate counter-clockwise in the Northern Hemisphere and clockwise in the Southern Hemisphere, creating a cyclone. Cyclones have a tendency for upward vertical motion. This allows moist air from the surrounding area to rise, expand and condense into water vapor, forming clouds. This movement of moisture and air causes the majority of our weather events.
Hurricanes are a result of low-pressure systems (cyclones) developing over tropical waters in the Western Hemisphere. The system drags up massive amounts of warm moisture from the sea, causing convection to take place, which in turn causes wind speeds to increase and pressure to fall. When these winds reach speeds over 119 kilometers per hour (74 miles per hour), the cyclone is classified as a hurricane.
Hurricanes can be one of the most devastating natural disasters in the Western Hemisphere. The National Hurricane Center, in Miami, Florida, regularly issues forecasts and reports on all tropical weather systems. During hurricane season, hurricane specialists issue forecasts and warnings for every tropical storm in the western tropical Atlantic and eastern tropical Pacific. Businesses and government officials from the United States, the Caribbean, Central America, and South America rely on forecasts from the National Hurricane Center.
High-pressure systems occur where the atmospheric pressure at the surface of the Earth is greater than its surrounding environment. This pressure has a tendency for downward vertical motion, allowing for dry air and clear skies.
Extremely cold temperatures are a result of high-pressure systems that develop over the Arctic and move over the Northern Hemisphere. Arctic air is very cold because it develops over ice and snow-covered ground. This cold air is so dense that it pushes against Earth’s surface with extreme pressure, preventing any moisture or heat from staying within the system.
Meteorologists have identified many semi-permanent areas of high-pressure. The Azores high, for instance, is a relatively stable region of high pressure around the Azores, an archipelago in the mid-Atlantic Ocean. The Azores high is responsible for arid temperatures of the Mediterranean basin, as well as summer heat waves in Western Europe.
Global Scale Meteorology
Global scale phenomena are weather patterns related to the transport of heat, wind, and moisture from the tropics to the poles. An important pattern is global atmospheric circulation, the large-scale movement of air that helps distribute thermal energy (heat) across the surface of the Earth.
Global atmospheric circulation is the fairly constant movement of winds across the globe. Winds develop as air masses move from areas of high pressure to areas of low pressure. Global atmospheric circulation is largely driven by Hadley cells. Hadley cells are tropical and equatorial convection patterns. Convection drives warm air high in the atmosphere, while cool, dense air pushes lower in a constant loop. Each loop is a Hadley cell.
Hadley cells determine the flow of trade winds, which meteorologists forecast. Businesses, especially those exporting products across oceans, pay close attention to the strength of trade winds because they help ships travel faster. Westerlies are winds that blow from the west in the midlatitudes. Closer to the Equator, trade winds blow from the northeast (north of the Equator) and the southeast (south of the Equator).
Meteorologists study long-term climate patterns that disrupt global atmospheric circulation. Meteorologists discovered the pattern of El Nino, for instance. El Niño involves ocean currents and trade winds across the Pacific Ocean. El Niño occurs roughly every five years, disrupting global atmospheric circulation and affecting local weather and economies from Australia to Peru.
El Niño is linked with changes in air pressure in the Pacific Ocean known as the Southern Oscillation. Air pressure drops over the eastern Pacific, near the coast of the Americas, while air pressure rises over the western Pacific, near the coasts of Australia and Indonesia. Trade winds weaken. Eastern Pacific nations experience extreme rainfall. Warm ocean currents reduce fish stocks, which depend on nutrient-rich upwelling of cold water to thrive. Western Pacific nations experience drought, devastating agricultural production.
Understanding the meteorological processes of El Niño helps farmers, fishers, and coastal residents prepare for the climate pattern.
History of Meteorology
The development of meteorology is deeply connected to developments in science, math, and technology. The Greek philosopher Aristotle wrote the first major study of the atmosphere around 340 BCE. Many of Aristotle’s ideas were incorrect, however, because he did not believe it was necessary to make scientific observations.
A growing belief in the scientific method profoundly changed the study of meteorology in the 17th and 18th centuries. Evangelista Torricelli, an Italian physicist, observed that changes in air pressure were connected to changes in weather. In 1643, Torricelli invented the barometer, to accurately measure the pressure of air. The barometer is still a key instrument in understanding and forecasting weather systems. In 1714, Daniel Fahrenheit, a German physicist, developed the mercury thermometer. These instruments made it possible to accurately measure two important atmospheric variables.
There was no way to quickly transfer weather data until the invention of the telegraph by American inventor Samuel Morse in the mid-1800s. Using this new technology, meteorological offices were able to share information and produce the first modern weather maps. These maps combined and displayed more complex sets of information such as isobars (lines of equal air pressure) and isotherms (lines of equal temperature). With these large-scale weather maps, meteorologists could examine a broader geographic picture of weather and make more accurate forecasts.
In the 1920s, a group of Norwegian meteorologists developed the concepts of air masses and fronts that are the building blocks of modern weather forecasting. Using basic laws of physics, these meteorologists discovered that huge cold and warm air masses move and meet in patterns that are the root of many weather systems.
Military operations during World War I and World War II brought great advances to meteorology. The success of these operations was highly dependent on weather over vast regions of the globe. The military invested heavily in training, research, and new technologies to improve their understanding of weather. The most important of these new technologies was radar, which was developed to detect the presence, direction, and speed of aircraft and ships. Since the end of World War II, radar has been used and improved to detect the presence, direction, and speed of precipitation and wind patterns.
The technological developments of the 1950s and 1960s made it easier and faster for meteorologists to observe and predict weather systems on a massive scale. During the 1950s, computers created the first models of atmospheric conditions by running hundreds of data points through complex equations. These models were able to predict large-scale weather, such as the series of high- and low-pressure systems that circle our planet.
TIROS I, the first meteorological satellite, provided the first accurate weather forecast from space in 1962. The success of TIROS I prompted the creation of more sophisticated satellites. Their ability to collect and transmit data with extreme accuracy and speed has made them indispensable to meteorologists. Advanced satellites and the computers that process their data are the primary tools used in meteorology today.
Meteorology Today
Today’s meteorologists have a variety of tools that help them examine, describe, model, and predict weather systems. These technologies are being applied at different meteorological scales, improving forecast accuracy and efficiency.
Radar is an important remote sensing technology used in forecasting. A radar dish is an active sensor in that it sends out radio waves that bounce off particles in the atmosphere and return to the dish. A computer processes these pulses and determines the horizontal dimension of clouds and precipitation, and the speed and direction in which these clouds are moving.
A new technology, known as dual-polarization radar, transmits both horizontal and vertical radio wave pulses. With this additional pulse, dual-polarization radar is better able to estimate precipitation. It is also better able to differentiate types of precipitation—rain, snow, sleet, or hail. Dual-polarization radar will greatly improve flash-flood and winter-weather forecasts.
Tornado research is another important component of meteorology. Starting in 2009, the National Oceanic and Atmospheric Administration (NOAA) and the National Science Foundation conducted the largest tornado research project in history, known as VORTEX2. The VORTEX2 team, consisting of about 200 people and more than 80 weather instruments, traveled more than 16,000 kilometers (10,000 miles) across the Great Plains of the United States to collect data on how, when, and why tornadoes form. The team made history by collecting extremely detailed data before, during, and after a specific tornado. This tornado is the most intensely examined in history and will provide key insights into tornado dynamics.
Satellites are extremely important to our understanding of global scale weather phenomena. The National Aeronautics and Space Administration (NASA) and NOAA operate three Geostationary Operational Environmental Satellites (GOES) that provide weather observations for more than 50 percent of the Earth’s surface.
GOES-15, launched in 2010, includes a solar X-ray imager that monitors the sun’s X-rays for the early detection of solar phenomena, such as solar flares. Solar flares can affect military and commercial satellite communications around the globe. A highly accurate imager produces visible and infrared images of Earth’s surface, oceans, cloud cover, and severe storm developments. Infrared imagery detects the movement and transfer of heat, improving our understanding of the global energy balance and processes such as global warming, convection, and severe weather.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1048 2021-06-12 00:08:01
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,771
Re: Miscellany
1026) Benzene
Benzene (C6H6), simplest organic, aromatic hydrocarbon and parent compound of numerous important aromatic compounds. Benzene is a colourless liquid with a characteristic odour and is primarily used in the production of polystyrene. It is highly toxic and is a known carcinogen; exposure to it may cause leukemia. As a result, there are strict controls on benzene emissions.
Discovery Of Benzene
Benzene was first discovered by the English scientist Michael Faraday in 1825 in illuminating gas. In 1834 German chemist Eilhardt Mitscherlich heated benzoic acid with lime and produced benzene. In 1845 German chemist A.W. von Hofmann isolated benzene from coal tar.
The structure of benzene has been of interest since its discovery. German chemists Joseph Loschmidt (in 1861) and August Kekule von Stradonitz (in 1866) independently proposed a cyclic arrangement of six carbons with alternating single and double bonds. Kekule subsequently modified his structural formula to one in which oscillation of the double bonds gave two equivalent structures in rapid equilibrium. In 1931 American chemist Linus Pauling suggested that benzene had a single structure, which was a resonance hybrid of the two Kekule structures.
Characteristics Of Benzene
Modern bonding models (valence-bond and molecular orbital theories) explain the structure and stability of benzene in terms of delocalization of six of its electrons, where delocalization in this case refers to the attraction of an electron by all six carbons of the ring instead of just one or two of them. This delocalization causes the electrons to be more strongly held, making benzene more stable and less reactive than expected for an unsaturated hydrocarbon. As a result, the hydrogenation of benzene occurs somewhat more slowly than the hydrogenation of alkenes (other organic compounds that contain carbon-carbon double bonds), and benzene is much more difficult to oxidize than alkenes. Most of the reactions of benzene belong to a class called electrophilic aromatic substitution that leave the ring itself intact but replace one of the attached hydrogens. These reactions are versatile and widely used to prepare derivatives of benzene.
Experimental studies, especially those employing X-ray diffraction, show benzene to have a planar structure with each carbon-carbon bond distance equal to 1.40 angstroms (Å). This value is exactly halfway between the C=C distance (1.34 Å) and C—C distance (1.46 Å) of a C=C—C=C unit, suggesting a bond type midway between a double bond and a single bond (all bond angles are 120°). Benzene has a boiling point of 80.1 °C (176.2 °F) and a melting point of 5.5 °C (41.9 °F), and it is freely soluble in organic solvents, but only slightly soluble in water.
Uses Of Benzene
At one time, benzene was obtained almost entirely from coal tar; however, since about 1950, these methods have been replaced by petroleum-based processes. More than half of the benzene produced each year is converted to ethylbenzene, then to styrene, and then to polystyrene. The next largest use of benzene is in the preparation of phenol. Other uses include the preparation of aniline (for dyes) and dodecylbenzene (for detergents).

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1049 2021-06-13 00:25:54
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,771
Re: Miscellany
1027) Aniline
Aniline, an organic base used to make dyes, drugs, explosives, plastics, and photographic and rubber chemicals.
Aniline was first obtained in 1826 by the destructive distillation of indigo. Its name is taken from the specific name of the indigo-yielding plant ‘Indigofera anil’ (‘Indigofera suffruticosa’); its chemical formula is C6H5NH2.
Aniline is prepared commercially by the catalytic hydrogenation of nitrobenzene or by the action of ammonia on chlorobenzene. The reduction of nitrobenzene can also be carried out with iron borings in aqueous acid.
A primary aromatic amine, aniline is a weak base and forms salts with mineral acids. In acidic solution, nitrous acid converts aniline into a diazonium salt that is an intermediate in the preparation of a great number of dyes and other organic compounds of commercial interest. When aniline is heated with organic acids, it gives amides, called anilides, such as acetanilide from aniline and acetic acid. Monomethylaniline and dimethylaniline can be prepared from aniline and methyl alcohol. Catalytic reduction of aniline yields cyclohexylamine. Various oxidizing agents convert aniline to quinone, azobenzene, nitrosobenzene, p-aminophenol, and the phenazine dye aniline black.
Pure aniline is a highly poisonous, oily, colourless substance with a pleasant odour.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1050 2021-06-14 00:05:21
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,771
Re: Miscellany
1028) Isthmus
Isthmus, narrow strip of land connecting two large land areas otherwise separated by bodies of water. Isthmuses are of great importance in plant and animal geography because they offer a path for the migration of plants and animals between the two land masses they connect.
Unquestionably the two most famous isthmuses are the Isthmus of Panama, connecting North and South America, and the Isthmus of Suez, connecting Africa and Asia. Historically the Isthmus of Corinth was of major importance because it connected what otherwise would be the island of the Peloponnese with the rest of the Greek peninsula. All three of these isthmuses are bisected by canals to facilitate shipping.
An isthmus is a narrow strip of land that connects two larger landmasses and separates two bodies of water.
Isthmuses have been strategic locations for centuries. They are natural sites for ports and canals linking terrestrial and aquatic trade routes. Isthmuses are also key sites for communications and cultural exchange, as well as military outposts.
The Isthmus of Panama in Panama links the continents of North and South America, and separates the Pacific and Atlantic Oceans. The Panama Canal stretches 77 kilometers (48 miles) across the isthmus, and allows cargo ships to travel from eastern North America to western North America without having to go around South America. The Panama Canal revolutionized shipping and travel in the 20th century, allowing for faster and more efficient transportation of goods and people.
The Isthmus of Suez in eastern Egypt connects the continents of Africa and Asia, and separates the Mediterranean and Red Seas. The Suez Canal is 192 kilometers (119 miles) long and allows cargo ships to travel between Europe, North Africa, western Asia (via the Mediterranean Sea in the north) and eastern and southern Asia without having to go around Africa. The Suez Canal facilitated trade and travel in the 19th century, and continues to define the oil trade today.
The city of Seattle, Washington, is located on an isthmus between the Puget Sound (part of the Pacific Ocean) and Lake Washington. The region has been continuously inhabited for more than 4,000 years. The earliest settlers of the area were the Duabish, who settled near the ocean, and the Hachuabish, who settled near the freshwater lake. European settlers, who quickly saw the isthmus' potential for trade along the west coast of North America as well as across the Pacific, collectively referred to these Native Americans as the Duwamish.
Tombolos
Tombolos are a type of isthmus. A tombolo forms as tides and waves create a thin strip of land between a coastal island and the mainland. The island connected to the mainland in a tombolo is called a tied island. (The built-up sandbar "ties" the island to the mainland.)
Mount Maunganui, New Zealand, is a tombolo town located on a sandbar connecting the volcanic island of Mauao to the mainland. Mauao is an extinct volcano, and is a popular spot for hiking and sightseeing the local beaches. Those beaches offer diverse opportunities due to the tombolo formation. Beaches on the side of Mount Maunganui's isthmus facing Tauranga Harbor are calm and protected. Beaches on the other side of Mount Maunganui face the open ocean (the Pacific), with dramatic waves that are popular with surfers.
Perhaps the most famous tombolo is the Rock of Gibraltar, a tied island narrowly connected to the southwestern tip of Europe's Iberian Peninsula. The Rock of Gibraltar is the northern part of the "Pillars of Hercules," which form the narrow western entrance to the Mediterranean Sea. This strategic location has made the Rock of Gibraltar a key site for traders and explorers since the Phoenicians. Today, the Rock of Gibraltar borders Spain, but is part of the British territory of Gibraltar.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline