Math Is Fun Forum
You are not logged in.
- Topics: Active | Unanswered
#1226 2021-12-18 00:27:49
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,148
Re: Miscellany
1202) Ecosystem
Summary
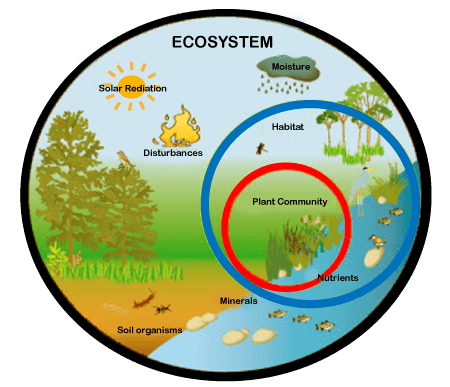
An ecosystem (or ecological system) consists of all the organisms and the physical environment with which they interact. These biotic and abiotic components are linked together through nutrient cycles and energy flows. Energy enters the system through photosynthesis and is incorporated into plant tissue. By feeding on plants and on one another, animals play an important role in the movement of matter and energy through the system. They also influence the quantity of plant and microbial biomass present. By breaking down dead organic matter, decomposers release carbon back to the atmosphere and facilitate nutrient cycling by converting nutrients stored in dead biomass back to a form that can be readily used by plants and microbes.
Ecosystems are controlled by external and internal factors. External factors such as climate, parent material which forms the soil and topography, control the overall structure of an ecosystem but are not themselves influenced by the ecosystem. Internal factors are controlled, for example, by decomposition, root competition, shading, disturbance, succession, and the types of species present. While the resource inputs are generally controlled by external processes, the availability of these resources within the ecosystem is controlled by internal factors. Therefore, internal factors not only control ecosystem processes but are also controlled by them.
Ecosystems are dynamic entities—they are subject to periodic disturbances and are always in the process of recovering from some past disturbance. The tendency of an ecosystem to remain close to its equilibrium state, despite that disturbance, is termed its resistance. The capacity of a system to absorb disturbance and reorganize while undergoing change so as to retain essentially the same function, structure, identity, and feedbacks is termed its ecological resilience. Ecosystems can be studied through a variety of approaches—theoretical studies, studies monitoring specific ecosystems over long periods of time, those that look at differences between ecosystems to elucidate how they work and direct manipulative experimentation. Biomes are general classes or categories of ecosystems. However, there is no clear distinction between biomes and ecosystems. Ecosystem classifications are specific kinds of ecological classifications that consider all four elements of the definition of ecosystems: a biotic component, an abiotic complex, the interactions between and within them, and the physical space they occupy.
Ecosystems provide a variety of goods and services upon which people depend. Ecosystem goods include the "tangible, material products" of ecosystem processes such as water, food, fuel, construction material, and medicinal plants. Ecosystem services, on the other hand, are generally "improvements in the condition or location of things of value". These include things like the maintenance of hydrological cycles, cleaning air and water, the maintenance of oxygen in the atmosphere, crop pollination and even things like beauty, inspiration and opportunities for research. Many ecosystems become degraded through human impacts, such as soil loss, air and water pollution, habitat fragmentation, water diversion, fire suppression, and introduced species and invasive species. These threats can lead to abrupt transformation of the ecosystem or to gradual disruption of biotic processes and degradation of abiotic conditions of the ecosystem. Once the original ecosystem has lost its defining features, it is considered "collapsed". Ecosystem restoration is thought to contribute to all 17 Sustainable Development Goals.
(The Sustainable Development Goals (SDGs) or Global Goals are a collection of 17 interlinked global goals designed to be a "blueprint to achieve a better and more sustainable future for all". The SDGs were set up in 2015 by the United Nations General Assembly (UN-GA) and are intended to be achieved by the year 2030. They are included in a UN-GA Resolution called the 2030 Agenda or what is colloquially known as Agenda 2030. The SDGs were developed in the Post-2015 Development Agenda as the future global development framework to succeed the Millennium Development Goals which ended in 2015.
The 17 SDGs are: (1) No Poverty, (2) Zero Hunger, (3) Good Health and Well-being, (4) Quality Education, (5) Gender Equality, (6) Clean Water and Sanitation, (7) Affordable and Clean Energy, (8) Decent Work and Economic Growth, (9) Industry, Innovation and Infrastructure, (10) Reducing Inequality, (11) Sustainable Cities and Communities, (12) Responsible Consumption and Production, (13) Climate Action, (14) Life Below Water, (15) Life On Land, (16) Peace, Justice, and Strong Institutions, (17) Partnerships for the Goals.
Though the goals are broad and interdependent, two years later (6 July 2017) the SDGs were made more "actionable" by a UN Resolution adopted by the General Assembly. The resolution identifies specific targets for each goal, along with indicators that are being used to measure progress toward each target. The year by which the target is meant to be achieved is usually between 2020 and 2030. For some of the targets, no end date is given.
To facilitate monitoring, a variety of tools exist to track and visualize progress towards the goals. All intention is to make data more available and easily understood.[5] For example, the online publication SDG Tracker, launched in June 2018, presents available data across all indicators. The SDGs pay attention to multiple cross-cutting issues, like gender equity, education, and culture cut across all of the SDGs. There were serious impacts and implications of the COVID-19 pandemic on all 17 SDGs in the year 2020.)
Details
Ecosystem, the complex of living organisms, their physical environment, and all their interrelationships in a particular unit of space.
A brief treatment of ecosystems follows.
An ecosystem can be categorized into its abiotic constituents, including minerals, climate, soil, water, sunlight, and all other nonliving elements, and its biotic constituents, consisting of all its living members. Linking these constituents together are two major forces: the flow of energy through the ecosystem and the cycling of nutrients within the ecosystem. Ecosystems vary in size: some are small enough to be contained within single water droplets while others are large enough to encompass entire landscapes and regions.
Energy flow
The fundamental source of energy in almost all ecosystems is radiant energy from the Sun. The energy of sunlight is used by the ecosystem’s autotrophic, or self-sustaining, organisms (that is, those that can make their own food). Consisting largely of green vegetation, these organisms are capable of photosynthesis—i.e., they can use the energy of sunlight to convert carbon dioxide and water into simple, energy-rich carbohydrates. The autotrophs use the energy stored within the simple carbohydrates to produce the more complex organic compounds, such as proteins, lipids, and starches, that maintain the organisms’ life processes. The autotrophic segment of the ecosystem is commonly referred to as the producer level.
Organic matter generated by autotrophs directly or indirectly sustains heterotrophic organisms. Heterotrophs are the consumers of the ecosystem; they cannot make their own food. They use, rearrange, and ultimately decompose the complex organic materials built up by the autotrophs. All animals and fungi are heterotrophs, as are most bacteria and many other microorganisms.
Trophic levels
ogether, the autotrophs and heterotrophs form various trophic (feeding) levels in the ecosystem: the producer level (which is made up of autotrophs), the primary consumer level (which is composed of those organisms that feed on producers), the secondary consumer level (which is composed of those organisms that feed on primary consumers), and so on. The movement of organic matter and energy from the producer level through various consumer levels makes up a food chain. For example, a typical food chain in a grassland might be grass (producer) → mouse (primary consumer) → snake (secondary consumer) → hawk (tertiary consumer). Actually, in many cases the food chains of the ecosystem’s biological community overlap and interconnect, forming what ecologists call a food web. The final link in all food chains is made up of decomposers, those heterotrophs (such as scavenging birds and mammals, insects, fungi, and bacteria) that break down dead organisms and organic wastes into smaller and smaller components, which can later be used by producers as nutrients. A food chain in which the primary consumer feeds on living plants is called a grazing pathway, and a food chain in which the primary consumer feeds on dead plant matter is known as a detritus pathway. Both pathways are important in accounting for the energy budget of the ecosystem.
Nutrient cycling
Nutrients are chemical elements and compounds that organisms must obtain from their surroundings for growth and the sustenance of life. Although autotrophs obtain nutrients primarily from the soil while heterotrophs obtain nutrients primarily from other organisms, the cells of each are made up primarily of six major elements that occur in similar proportions in all life-forms. These elements—hydrogen, oxygen, carbon, nitrogen, phosphorus, and sulfur—form the core protoplasm (that is, the semifluid substance that makes up a cell’s cytoplasm and nucleus) of organisms. The first four of these elements make up about 99 percent of the mass of most cells. Additional elements, however, are also essential to the growth of organisms. Calcium and other elements help to form cellular support structures such as shells, internal or external skeletons, and cell walls. Chlorophyll molecules, which allow photosynthetic plants to convert solar energy into chemical energy, are chains of carbon, hydrogen, and oxygen compounds built around a magnesium ion. Altogether, 16 elements are found in all organisms; another eight elements are found in some organisms but not in others.
These bioelements combine with one another to form a wide variety of chemical compounds. They occur in organisms in higher proportions than they do in the environment because organisms capture them, concentrating and combining them in various ways in their cells, and release them during metabolism and death. As a result, these essential nutrients alternate between inorganic and organic states as they rotate through their respective biogeochemical cycles: the carbon cycle, the oxygen cycle, the nitrogen cycle, the sulfur cycle, the phosphorous cycle, and the water cycle. These cycles can include all or part of the following environmental spheres: the atmosphere, which is made up largely of gases including water vapour; the lithosphere, which encompasses the soil and the entire solid crust of Earth; the hydrosphere, which includes lakes, rivers, oceans, groundwater, frozen water, and (along with the atmosphere) water vapour; and the biosphere, which includes all living things and overlaps with each of the other environmental spheres.
A portion of the elements are bound up in limestone and in the minerals of other rocks and are unavailable to organisms. The slow processes of weathering and erosion eventually release these elements to enter the cycle. For most of the major nutrients, however, organisms not only intercept the elements moving through the biosphere, but they actually drive the biogeochemical cycles. The movement of nutrients through the biosphere is different from the transfer of energy because, whereas energy flows through the biosphere and cannot be reused, elements are recycled. For example, the same atoms of carbon or nitrogen may, over the course of eons, move repeatedly between organisms, the atmosphere, the soil, and the oceans. Carbon released as carbon dioxide by an animal may remain in the atmosphere for 5 or 10 years before being taken up by another organism, or it may cycle almost immediately back into a neighbouring plant and be used during photosynthesis.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1227 2021-12-19 00:35:15
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,148
Re: Miscellany
1203) Cell wall
Summary
A cell wall is a structural layer surrounding some types of cells, just outside the cell membrane. It can be tough, flexible, and sometimes rigid. It provides the cell with both structural support and protection, and also acts as a filtering mechanism. Cell walls are absent in animals but are present in most other eukaryotes including algae, fungi and plants and in most prokaryotes (except mollicute bacteria). A major function is to act as pressure vessels, preventing over-expansion of the cell when water enters.
The composition of cell walls varies between taxonomic group and species and may depend on cell type and developmental stage. The primary cell wall of land plants is composed of the polysaccharides cellulose, hemicelluloses and pectin. Often, other polymers such as lignin, suberin or cutin are anchored to or embedded in plant cell walls. Algae possess cell walls made of glycoproteins and polysaccharides such as carrageenan and agar that are absent from land plants. In bacteria, the cell wall is composed of peptidoglycan. The cell walls of archaea have various compositions, and may be formed of glycoprotein S-layers, pseudopeptidoglycan, or polysaccharides. Fungi possess cell walls made of the N-acetylglucosamine polymer chitin. Unusually, diatoms have a cell wall composed of biogenic silica.
Details
Cell wall is a specialized form of extracellular matrix that surrounds every cell of a plant. The cell wall is responsible for many of the characteristics that distinguish plant cells from animal cells. Although often perceived as an inactive product serving mainly mechanical and structural purposes, the cell wall actually has a multitude of functions upon which plant life depends. Such functions include: (1) providing the living cell with mechanical protection and a chemically buffered environment, (2) providing a porous medium for the circulation and distribution of water, minerals, and other small nutrient molecules, (3) providing rigid building blocks from which stable structures of higher order, such as leaves and stems, can be produced, and (4) providing a storage site of regulatory molecules that sense the presence of pathogenic microbes and control the development of tissues.
Certain prokaryotes, algae, slime molds, water molds, and fungi also have cell walls. Bacterial cell walls are characterized by the presence of peptidoglycan, whereas those of Archaea characteristically lack this chemical. Algal cell walls are similar to those of plants, and many contain specific polysaccharides that are useful for taxonomy. Unlike those of plants and algae, fungal cell walls lack cellulose entirely and contain chitin. The scope of this article is limited to plant cell walls.
Mechanical properties
All cell walls contain two layers, the middle lamella and the primary cell wall, and many cells produce an additional layer, called the secondary wall. The middle lamella serves as a cementing layer between the primary walls of adjacent cells. The primary wall is the cellulose-containing layer laid down by cells that are dividing and growing. To allow for cell wall expansion during growth, primary walls are thinner and less rigid than those of cells that have stopped growing. A fully grown plant cell may retain its primary cell wall (sometimes thickening it), or it may deposit an additional, rigidifying layer of different composition, which is the secondary cell wall. Secondary cell walls are responsible for most of the plant’s mechanical support as well as the mechanical properties prized in wood. In contrast to the permanent stiffness and load-bearing capacity of thick secondary walls, the thin primary walls are capable of serving a structural, supportive role only when the vacuoles within the cell are filled with water to the point that they exert a turgor pressure against the cell wall. Turgor-induced stiffening of primary walls is analogous to the stiffening of the sides of a pneumatic tire by air pressure. The wilting of flowers and leaves is caused by a loss of turgor pressure, which results in turn from the loss of water from the plant cells.
Components
Although primary and secondary wall layers differ in detailed chemical composition and structural organization, their basic architecture is the same, consisting of cellulose fibres of great tensile strength embedded in a water-saturated matrix of polysaccharides and structural glycoproteins.
Cellulose
Cellulose consists of several thousand glucose molecules linked end to end. The chemical links between the individual glucose subunits give each cellulose molecule a flat ribbonlike structure that allows adjacent molecules to band laterally together into microfibrils with lengths ranging from two to seven micrometres. Cellulose fibrils are synthesized by enzymes floating in the cell membrane and are arranged in a rosette configuration. Each rosette appears capable of “spinning” a microfibril into the cell wall. During this process, as new glucose subunits are added to the growing end of the fibril, the rosette is pushed around the cell on the surface of the cell membrane, and its cellulose fibril becomes wrapped around the protoplast. Thus, each plant cell can be viewed as making its own cellulose fibril cocoon.
Matrix polysaccharides
The two major classes of cell wall matrix polysaccharides are the hemicelluloses and the pectic polysaccharides, or pectins. Both are synthesized in the Golgi apparatus, brought to the cell surface in small vesicles, and secreted into the cell wall.
Hemicelluloses consist of glucose molecules arranged end to end as in cellulose, with short side chains of xylose and other uncharged sugars attached to one side of the ribbon. The other side of the ribbon binds tightly to the surface of cellulose fibrils, thereby coating the microfibrils with hemicellulose and preventing them from adhering together in an uncontrolled manner. Hemicellulose molecules have been shown to regulate the rate at which primary cell walls expand during growth.
The heterogeneous, branched, and highly hydrated pectic polysaccharides differ from hemicelluloses in important respects. Most notably, they are negatively charged because of galacturonic acid residues, which, together with rhamnose sugar molecules, form the linear backbone of all pectic polysaccharides. The backbone contains stretches of pure galacturonic acid residues interrupted by segments in which galacturonic acid and rhamnose residues alternate; attached to these latter segments are complex, branched sugar side chains. Because of their negative charge, pectic polysaccharides bind tightly to positively charged ions, or cations. In cell walls, calcium ions cross-link the stretches of pure galacturonic acid residues tightly, while leaving the rhamnose-containing segments in a more open, porous configuration. This cross-linking creates the semirigid gel properties characteristic of the cell wall matrix—a process exploited in the preparation of jellied preserves.
Proteins
Although plant cell walls contain only small amounts of protein, they serve a number of important functions. The most prominent group are the hydroxyproline-rich glycoproteins, shaped like rods with connector sites, of which extensin is a prominent example. Extensin contains 45 percent hydroxyproline and 14 percent serine residues distributed along its length. Every hydroxyproline residue carries a short side chain of arabinose sugars, and most serine residues carry a galactose sugar. This gives rise to long molecules, resembling bottle brushes, that are secreted into the cell wall toward the end of primary wall formation and become covalently cross-linked into a mesh at the time that cell growth stops. Plant cells may control their ultimate size by regulating the time at which this cross-linking of extensin molecules occurs.
In addition to the structural proteins, cell walls contain a variety of enzymes. Most notable are those that cross-link extensin, lignin, cutin, and suberin molecules into networks. Other enzymes help protect plants against fungal pathogens by breaking fragments off of the cell walls of the fungi. The fragments in turn induce defense responses in underlying cells. The softening of ripe fruit and dropping of leaves in the autumn are brought about by cell wall-degrading enzymes.
Plastics
Cell wall plastics such as lignin, cutin, and suberin all contain a variety of organic compounds cross-linked into tight three-dimensional networks that strengthen cell walls and make them more resistant to fungal and bacterial attack. Lignin is the general name for a diverse group of polymers of aromatic alcohols. Deposited mostly in secondary cell walls and providing the rigidity of terrestrial vascular plants, it accounts for up to 30 percent of a plant’s dry weight. The diversity of cross-links between the polymers—and the resulting tightness—makes lignin a formidable barrier to the penetration of most microbes.
Cutin and suberin are complex biopolyesters composed of fatty acids and aromatic compounds. Cutin is the major component of the cuticle, the waxy, water-repelling surface layer of cell walls exposed to the environment aboveground. By reducing the wettability of leaves and stems—and thereby affecting the ability of fungal spores to germinate—it plays an important part in the defense strategy of plants. Suberin serves with waxes as a surface barrier of underground parts. Its synthesis is also stimulated in cells close to wounds, thereby sealing off the wound surfaces and protecting underlying cells from dehydration.
Intercellular communication:
Plasmodesmata
Similar to the gap junction of animal cells is the plasmodesma, a channel passing through the cell wall and allowing direct molecular communication between adjacent plant cells. Plasmodesmata are lined with cell membrane, in effect uniting all connected cells with one continuous cell membrane. Running down the middle of each channel is a thin membranous tube that connects the endoplasmic reticula (ER) of the two cells. This structure is a remnant of the ER of the original parent cell, which, as the parent cell divided, was caught in the developing cell plate.
Although the precise mechanisms are not fully understood, the plasmodesma is thought to regulate the passage of small molecules such as salts, sugars, and amino acids by constricting or dilating the openings at each end of the channel.
Oligosaccharides with regulatory functions
The discovery of cell wall fragments with regulatory functions opened a new era in plant research. For years scientists had been puzzled by the chemical complexity of cell wall polysaccharides, which far exceeds the structural requirements of plant cell walls. The answer came when it was found that specific fragments of cell wall polysaccharides, called oligosaccharins, are able to induce specific responses in plant cells and tissues. One such fragment, released by enzymes used by fungi to break down plant cell walls, consists of a linear polymer of 10 to 12 galacturonic acid residues. Exposure of plant cells to such fragments induces them to produce antibiotics known as phytoalexins. In other experiments it has been shown that exposing strips of tobacco stem cells to a different type of cell wall fragment leads to the growth of roots; other fragments lead to the formation of stems and yet others to the production of flowers. In all instances the concentration of oligosaccharins required to bring about the observed responses is equal to that of hormones in animal cells; indeed, oligosaccharins may be viewed as the oligosaccharide hormones of plants.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1228 2021-12-20 00:34:08
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,148
Re: Miscellany
1204) Grande Dixence Dam
Gist
Grande Dixence Dam, gravity dam on the Dixence River, Switzerland, completed in 1961. It is 935 feet (285 metres) high and 2,280 feet (695 metres) wide at the crest, has a volume of 7,848,000 cubic yards (6,000,000 cubic metres), and impounds a reservoir of 325,000 acre-feet (401,000,000 cubic metres).
Grande Dixence was the tallest dam in the world until completion of the Nurek Dam in the Soviet Union in 1980. It was built in annual stages, a procedure necessary because the Alpine working season is quite short.
Details
The Grande Dixence Dam is a concrete gravity dam on the Dixence at the head of the Val d'Hérémence in the canton of Valais in Switzerland. At 285 m (935 ft) high, it is the tallest gravity dam in the world, fifth tallest dam overall, and the tallest dam in Europe. It is part of the Cleuson-Dixence Complex. With the primary purpose of hydroelectric power generation, the dam fuels four power stations, totaling the installed capacity to 2,069 MW, generating approximately 2,000 GWh annually, enough to power 400,000 Swiss households.
The dam withholds Lac des Dix (Lake Dix), its reservoir. With a surface area of 4 sq km, it is the second largest lake in Valais and the largest lake above 2,000 m in the Alps. The reservoir receives its water from four different pumping stations; the Z’Mutt, Stafel, Ferpècle and Arolla. At peak capacity, it contains approximately 400,000,000 m^3 (1.4 × {10}^{10} cu ft) of water, with depths reaching up to 284 m (932 ft). Construction on the dam began in 1950 and was completed in 1961, before officially commissioning in 1965.
History
In 1922, Energie Ouest Suisse (EOS) became established with a few small power stations. To generate substantial amounts of electricity, EOS looked to the Valais canton which contains 56% of Switzerland's glaciers and stores the largest amount of water in Europe. In 1927, EOS acquired the license for the upper Dixence basin. In 1929, 1,200 workers constructed the first Dixence dam which would be complete in 1935. The first dam would supply water to the Chandoline Power Station which has a capacity of 120 MW.
After the Second World War, growing industries needed electricity and construction on the Cleuson Dam began in 1947 and was completed in 1951. The original Dixence dam was submerged by the filling of Lac des Dix beginning in 1957, it can still be seen when the reservoir level is low. Plans for the Super Dixence Dam were now being finalized by the recently founded company, Grande Dixence SA. Construction on the Super Dixence Dam soon began later in 1950. By 1961, 3,000 workers had finished pouring 6,000,000 m^3 (210,000,000 cu ft) of concrete, completing the dam. At 285 m, it was the world's tallest dam at the time, but it was surpassed by the Nurek Dam of Tajikistan in 1972 (300 m). It remains the world's tallest gravity dam.
In the 1980s, Grande Dixence SA and EOS began the Cleuson-Dixence project which improved the quality of electricity produced by building new tunnels along with the Bieudron Power Station. By the time the Cleuson-Dixence Complex was complete, the power generated had more than doubled.
The construction of the dam was documented in the short film Opération béton, the first film directed by Jean-Luc Godard.
Characteristics
The Grande Dixence Dam is a 285 m (935 ft) high, 700 m (2,297 ft) long concrete gravity dam. The dam is 200 m (656 ft) wide at its base and 15 m (49 ft) wide at its crest. The dam's crest reaches an altitude of 2,365 m (7,759 ft). The dam structure contains approximately 6,000,000 m^3 (211,888,000 cu ft) of concrete. To secure the dam to the surrounding foundation, a grout curtain surrounds the dam, reaching a depth of 200 m (656 ft) and extending 100 m (328 ft) on each side of the valley.
Although the dam is situated on the relatively small Dixence, water supplied from other rivers and streams is pumped by the Z’Mutt, Stafel, Ferpècle and Arolla pumping stations. The pumping stations transport the water through 100 km (62 mi) of tunnels into its reservoir, Lac des Dix. Water from the 87 m (285 ft) high Cleuson Dam, located 7 km (4 mi) to the northwest, is also transported from its reservoir, the Lac de Cleuson. Three penstocks transport water from Lac des Dix to the Chandoline, Fionnay, Nendaz and Bieudron power stations, before being discharged into the Rhône below. All the pumping stations, power stations and dams form the Cleuson-Dixence Complex. Although the complex operates with water being pumped from one reservoir to another, it does not technically qualify as a pumped-storage scheme.
Most of the water comes from glaciers when they melt during the summer. The lake is usually at full capacity by late September, and empties during the winter, eventually reaching its lowest point around April.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1229 2021-12-21 00:09:23
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,148
Re: Miscellany
1205) Coronary Angiogram
Gist
Coronary angiography
Coronary angiography is a procedure that uses a special dye (contrast material) and x-rays to see how blood flows through the arteries in your heart.
How the Test is Performed
Coronary angiography is often done along with cardiac catheterization. This is a procedure that measures pressures in the heart chambers.
Before the test starts, you will be given a mild sedative to help you relax.
An area of your body (the arm or groin) is cleaned and numbed with a local numbing medicine (anesthetic). The cardiologist passes a thin hollow tube, called a catheter, through an artery and carefully moves it up into the heart. X-ray images help the doctor position the catheter.
Once the catheter is in place, dye (contrast material) is injected into the catheter. X-ray images are taken to see how the dye moves through the artery. The dye helps highlight any blockages in blood flow.
The procedure most often lasts 30 to 60 minutes.
Details:
Overview
A coronary angiogram is a procedure that uses X-ray imaging to see your heart's blood vessels. The test is generally done to see if there's a restriction in blood flow going to the heart.
Coronary angiograms are part of a general group of procedures known as heart (cardiac) catheterizations. Cardiac catheterization procedures can both diagnose and treat heart and blood vessel conditions. A coronary angiogram, which can help diagnose heart conditions, is the most common type of cardiac catheterization procedure.
During a coronary angiogram, a type of dye that's visible by an X-ray machine is injected into the blood vessels of your heart. The X-ray machine rapidly takes a series of images (angiograms), offering a look at your blood vessels. If necessary, your doctor can open clogged heart arteries (angioplasty) during your coronary angiogram.
Why it's done
Your doctor may recommend that you have a coronary angiogram if you have:
* Symptoms of coronary artery disease, such as chest pain (angina)
* Pain in your chest, jaw, neck or arm that can't be explained by other tests
* New or increasing chest pain (unstable angina)
* A heart defect you were born with (congenital heart disease)
* Abnormal results on a noninvasive heart stress test
* Other blood vessel problems or a chest injury
* A heart valve problem that requires surgery
Because there's a small risk of complications, angiograms aren't usually done until after noninvasive heart tests have been performed, such as an electrocardiogram, an echocardiogram or a stress test.
Risks
As with most procedures done on your heart and blood vessels, a coronary angiogram has some risks, such as radiation exposure from the X-rays used. Major complications are rare, though. Potential risks and complications include:
* Heart attack
* Stroke
* Injury to the catheterized artery
* Irregular heart rhythms (arrhythmias)
* Allergic reactions to the dye or medications used during the procedure
* Kidney damage
* Excessive bleeding
* Infection
How you prepare
In some cases, coronary angiograms are performed on an emergency basis. More commonly, though, they're scheduled in advance, giving you time to prepare.
Angiograms are performed in the catheterization (cath) lab of a hospital. Your health care team will give you specific instructions and talk to you about any medications you take. General guidelines include:
* Don't eat or drink anything after midnight before your angiogram.
* Take all your medications to the hospital with you in their original bottles. Ask your doctor about whether to take your usual morning medications.
* If you have diabetes, ask your doctor if you should take insulin or other oral medications before your angiogram.
What you can expect
Before the procedure:
Before your angiogram procedure starts, your health care team will review your medical history, including allergies and medications you take. The team may perform a physical exam and check your vital signs — blood pressure and pulse.
You'll also empty your bladder and change into a hospital gown. You may have to remove contact lenses, eyeglasses, jewelry and hairpins.
During the procedure
For the procedure, you lie on your back on an X-ray table. Because the table may be tilted during the procedure, safety straps may be fastened across your chest and legs. X-ray cameras will move over and around your head and chest to take pictures from many angles.
An IV line is inserted into a vein in your arm. You may be given a sedative through the IV to help you relax, as well as other medications and fluids. You'll be very sleepy and may drift off to sleep during the procedure, but you'll still be able to be easily awakened to follow any instructions.
Electrodes on your chest monitor your heart throughout the procedure. A blood pressure cuff tracks your blood pressure and another device, a pulse oximeter, measures the amount of oxygen in your blood.
A small amount of hair may be shaved from your groin or arm where a flexible tube (catheter) will be inserted. The area is washed and disinfected and then numbed with an injection of local anesthetic.
A small incision is made at the entry site, and a short plastic tube (sheath) is inserted into your artery. The catheter is inserted through the sheath into your blood vessel and carefully threaded to your heart or coronary arteries.
Threading the catheter shouldn't cause pain, and you shouldn't feel it moving through your body. Tell your health care team if you have any discomfort.
Dye (contrast material) is injected through the catheter. When this happens, you may have a brief sensation of flushing or warmth. But again, tell your health care team if you feel pain or discomfort.
The dye is easy to see on X-ray images. As it moves through your blood vessels, your doctor can observe its flow and identify any blockages or constricted areas. Depending on what your doctor discovers during your angiogram, you may have additional catheter procedures at the same time, such as a balloon angioplasty or a stent placement to open up a narrowed artery. Other noninvasive tests, such as ultrasound, may help your doctor evaluate identified blockages.
Having an angiogram takes about one hour, although it may be longer, especially if combined with other cardiac catheterization procedures. Preparation and post-procedure care can add more time.
After the procedure
When the angiogram is over, the catheter is removed from your arm or groin and the incision is closed with manual pressure, a clamp or a small plug.
You'll be taken to a recovery area for observation and monitoring. When your condition is stable, you return to your own room, where you're monitored regularly.
You'll need to lie flat for several hours to avoid bleeding if the catheter was inserted in the groin. During this time, pressure may be applied to the incision to prevent bleeding and promote healing.
You may be able to go home the same day, or you may have to remain in the hospital overnight. Drink plenty of fluids to help flush the dye from your body. If you're feeling up to it, have something to eat.
Ask your health care team when to resume taking medications, bathing or showering, working, and doing other normal activities. Avoid strenuous activities and heavy lifting for several days.
Your puncture site is likely to remain tender for a while. It may be slightly bruised and have a small bump.
Call your doctor's office if:
* You notice bleeding, new bruising or swelling at the catheter site
* You develop increasing pain or discomfort at the catheter site
* You have signs of infection, such as redness, drainage or a fever
* There's a change in temperature or color of the leg or arm that was used for the procedure
* Weakness or numbness in the leg or arm where the catheter was inserted
* You develop chest pain or shortness of breath
If the catheter site is actively bleeding and doesn't stop after you've applied pressure to the site, contact emergency medical services. If the catheter site suddenly begins to swell, contact emergency medical services.
Results
An angiogram can show doctors what's wrong with your blood vessels. It can:
* Show how many of your coronary arteries are blocked or narrowed by fatty plaques (atherosclerosis)
* Pinpoint where blockages are located in your blood vessels
* Show how much blood flow is blocked through your blood vessels
* Check the results of previous coronary bypass surgery
* Check the blood flow through your heart and blood vessels
Knowing this information can help your doctor determine what treatment is best for you and how much danger your heart condition poses to your health. Based on your results, your doctor may decide, for instance, that you would benefit from having coronary angioplasty or stenting to help clear clogged arteries. It's also possible that angioplasty or stenting could be done during your angiogram to avoid needing another procedure.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1230 2021-12-22 00:11:40
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,148
Re: Miscellany
1206) Moth
Summary
Moth is (order Lepidoptera), any of about 160,000 species of overwhelmingly nocturnal flying insects that, along with the butterflies and skippers, constitute the order Lepidoptera.
Moths vary greatly in size, ranging in wingspan from about 4 mm (0.16 inch) to nearly 30 cm (about 1 foot). Highly adapted, they live in all but polar habitats. The wings, bodies, and legs of moths are covered with dustlike scales that come off if the insect is handled. Compared with butterflies, moths have stouter bodies and duller colouring. Moths also have distinctive feathery or thick antennae. When at rest, moths either fold their wings tentlike over the body, wrap them around the body, or hold them extended at their sides, whereas butterflies hold their wings vertically.
As with all lepidopterans, the moth life cycle has four stages: egg, larva (caterpillar), pupa (chrysalis), and adult (imago). The larvae and adults of most moth species are plant eaters. Larvae in particular do considerable damage to ornamental trees and shrubs and to many other plants of economic importance. The bollworm and measuring worm are two of the most destructive types of moth larvae. Some moth species (especially those of the family Tineidae, which includes the clothes moth) eat wool, fur, silk, and even feathers.
Some of the better-known moth families include: Gelechiidae, to which the destructive bollworms of cotton, corn, tomatoes, and other crops belong; Tortricidae, or leaf roller moths, which are forest pests; Lymantriidae, the tussock moths, also containing forest pests such as the gypsy moth; Arctiidae, the tiger moths, with many brightly coloured tropical species; Olethreutidae, including several destructive species such as the codling moth and the Oriental fruit moth; Noctuidae, the owlet moths, one of the largest families of lepidopterans; Saturniidae, the giant silkworm moths, containing the largest individual; and Geometridae, measuring worm moths, including the waves, pugs, and carpet moths.
Details
Moths are a paraphyletic group of insects that includes all members of the order Lepidoptera that are not butterflies, with moths making up the vast majority of the order. There are thought to be approximately 160,000 species of moth, many of which have yet to be described. Most species of moth are nocturnal, but there are also crepuscular and diurnal species.
Differences between butterflies and moths
While the butterflies form a monophyletic group, the moths, comprising the rest of the Lepidoptera, do not. Many attempts have been made to group the superfamilies of the Lepidoptera into natural groups, most of which fail because one of the two groups is not monophyletic: Microlepidoptera and Macrolepidoptera, Heterocera and Rhopalocera, Jugatae and Frenatae, Monotrysia and Ditrysia.
Although the rules for distinguishing moths from butterflies are not well established, one very good guiding principle is that butterflies have thin antennae and (with the exception of the family Hedylidae) have small balls or clubs at the end of their antennae. Moth antennae are usually feathery with no ball on the end. The divisions are named by this principle: "club-antennae" (Rhopalocera) or "varied-antennae" (Heterocera). Lepidoptera differs between butterflies and other organisms due to evolving a special characteristic of having the tube-like proboscis in the Middle Triassic which allowed them to acquire nectar from flowering plants.
Etymology
The modern English word moth comes from Old English (cf. Northumbrian) from Common Germanic (compare Old Norse motti, Dutch mot, and German Motte all meaning 'moth'). Its origins are possibly related to the Old English maða meaning 'maggot' or from the root of midge which until the 16th century was used mostly to indicate the larva, usually in reference to devouring clothes.
Caterpillar
Moth larvae, or caterpillars, make cocoons from which they emerge as fully grown moths with wings. Some moth caterpillars dig holes in the ground, where they live until they are ready to turn into adult moths.
History
Moths evolved long before butterflies; moth fossils have been found that may be 190 million years old. Both types of Lepidoptera are thought to have co-evolved with flowering plants, mainly because most modern species, both as adults and larvae, feed on flowering plants. One of the earliest known species that is thought to be an ancestor of moths is Archaeolepis mane. Its fossil fragments show scaled wings that are similar to caddisflies in their veining.
Economics:
Significance to humans
An adult male pine processionary moth (Thaumetopoea pityocampa). This species is a serious forest pest when in its larval state. Notice the bristle springing from the underside of the hindwing (frenulum) and running forward to be held in a small catch of the forewing, whose function is to link the wings together.
Some moths, particularly their caterpillars, can be major agricultural pests in many parts of the world. Examples include corn borers and bollworms. The caterpillar of the gypsy moth (Lymantria dispar) causes severe damage to forests in the northeastern United States, where it is an invasive species. In temperate climates, the codling moth causes extensive damage, especially to fruit farms. In tropical and subtropical climates, the diamondback moth (Plutella xylostella) is perhaps the most serious pest of brassicaceous crops. Also in sub-Saharan Africa, the African sugarcane borer is a major pest of sugarcane, maize, and sorghum.
Several moths in the family Tineidae are commonly regarded as pests because their larvae eat fabric such as clothes and blankets made from natural proteinaceous fibers such as wool or silk. They are less likely to eat mixed materials containing some artificial fibers. There are some reports that they may be repelled by the scent of wood from juniper and cedar, by lavender, or by other natural oils; however, many consider this unlikely to prevent infestation. Naphthalene (the chemical used in mothballs) is considered more effective, but there are concerns over its effects on human health.
Moth larvae may be killed by freezing the items which they infest for several days at a temperature below −8 °C (18 °F).
While moths are notorious for eating clothing, most species do not, and some moth adults do not even eat at all. Some, like the Luna, Polyphemus, Atlas, Promethea, cecropia, and other large moths do not have mouth parts. This is possible because they live off the food stores from when they were a caterpillar, and only live a short time as an adult (roughly a week for some species). Many species of adult moths do however eat: for instance, many will drink nectar.
Some moths are farmed for their economic value. The most notable of these is the silkworm, the larva of the domesticated moth Bombyx mori. It is farmed for the silk with which it builds its cocoon. As of 2002, the silk industry produces more than 130 million kilograms of raw silk, worth about 250 million U.S. dollars, each year.
Not all silk is produced by Bombyx mori. There are several species of Saturniidae that also are farmed for their silk, such as the ailanthus moth (Samia cynthia group of species), the Chinese oak silkmoth (Antheraea pernyi), the Assam silkmoth (Antheraea assamensis), and the Japanese silk moth (Antheraea yamamai).
The larvae of many species are used as food, particularly in Africa, where they are an important source of nutrition. The mopane worm, the caterpillar of Gonimbrasia belina, from the family Saturniidae, is a significant food resource in southern Africa. Another saturniid used as food is the cavorting emperor (Usta terpsichore). In one country alone, Congo, more than 30 species of moth larvae are harvested. Some are sold not only in the local village markets, but are shipped by the ton from one country to another.
Predators and parasites
Nocturnal insectivores often feed on moths; these include some bats, some species of owls and other species of birds. Moths also are eaten by some species of lizards, amphibians, cats, dogs, rodents, and some bears. Moth larvae are vulnerable to being parasitized by Ichneumonidae.
Baculoviruses are parasite double-stranded DNA insect viruses that are used mostly as biological control agents. They are members of the Baculoviridae, a family that is restricted to insects. Most baculovirus isolates have been obtained from insects, in particular from Lepidoptera.
There is evidence that ultrasound in the range emitted by bats causes flying moths to make evasive maneuvers. Ultrasonic frequencies trigger a reflex action in the noctuid moth that causes it to drop a few centimeters or inches in its flight to evade attack, and tiger moths can emit clicks to foil bats' echolocation.
The fungus Ophiocordyceps sinensis infects the larvae of many different species of moths.
Ecological importance
Some studies indicate that certain species of moths, such as those belonging to the families Erebidae and Sphingidae, may be the key pollinators for some flowering plants in the Himalayan ecosystem. Recent studies have established that moths are important, but often overlooked, nocturnal pollinators of a wide range of plants.
Attraction to light
Moths frequently appear to circle artificial lights, although the reason for this behavior (positive phototaxis) is currently unknown. One hypothesis is called celestial or transverse orientation. By maintaining a constant angular relationship to a bright celestial light, such as the moon, they can fly in a straight line. Celestial objects are so far away that, even after travelling great distances, the change in angle between the moth and the light source is negligible; further, the moon will always be in the upper part of the visual field, or on the horizon. When a moth encounters a much closer artificial light and uses it for navigation, the angle changes noticeably after only a short distance, in addition to being often below the horizon. The moth instinctively attempts to correct by turning toward the light, thereby causing airborne moths to come plummeting downward, and resulting in a spiral flight path that gets closer and closer to the light source.
Studies have found that light pollution caused by increasing use of artificial lights has either led to a severe decline in moth population in some parts of the world or has severely disrupted nocturnal pollination.
Moth Interesting Facts
What type of animals are moths?
Moths fall in the paraphyletic group of insects, and it includes all the members of the genus Lepidoptera, except the butterfly.
What class of animals do moths belong to?
Moths are a part of the Insecta class, which is the largest group within the Arthropod phylum.
How many moths are there in the world?
There are almost 160,000 moths species worldwide, of which more than 11,000 species are found in the USA only, which makes them ten times more abundant than butterflies.
Where do moths live?
Moths can be found in almost every major part of the world like Africa, Asia, Central America, Eurasia, Europe, Oceania, North America, and South America.
What is a moth's habitat?
Moths can be easily found in quiet and dark forests and pasture lands; they can also be found in tropical settings and grasslands. A major part of the moth species is attracted to light sources as light sources confuse them. and they tend to lose their sense of direction. They usually prefer warm places. During winters, they tend to migrate south towards warmer temperatures. They are known for flying long distances during the migration period. They are adaptive in nature. Most of them are nocturnal. Some moth species look just like butterflies.
Who do moths live with?
As moths have many species and varieties, some moths like to stay alone or move in pairs like butterflies, and other moths move in large family-like structures or groups called eclipses. The living style and lifecycle of moths vary from species to species.
How long do moths live?
The lifespan varies in different moth species. The average lifespan of a moth can be estimated to be around 40 days. Some moths can live for a year. Some moths live only for days, weeks, or a month. Some moths, like the hummingbird moth and luna moth, can live up to three months. Different moths species have evolved differently.
How do they reproduce?
Moths have evolved with time. When female moths are ready for mating, they release some chemicals to attract the male moths. The male moth then follows this smell and gets attracted to the female.
When done with mating, the female moths lay their eggs on plants. On average, 100 eggs are laid. In most cases, within ten days, the eggs hatch and begin the caterpillar stage. This is when they begin to eat plants as food. To prepare for the pupal stage, caterpillars need to eat almost 2,700 times their body weight. Then, they prepare cocoons around themselves in the pupal stage after almost three weeks or a month. From the cocoons, the caterpillars finally emerge as adult moths.
What is their conservation status?
Currently, some moth species are considered Endangered, like the garden tiger and white ermine moth, as they have lost their habitats because of humans. Most species are of Least Concern.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1231 2021-12-23 00:11:46
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,148
Re: Miscellany
1207) Glowworm
Summary
Glowworm is any crawling, luminous insect that emits light either continuously or in prolonged glows rather than in brief flashes as do most fireflies. Principal types of glowworms are: (1) wingless adult females of certain beetles of the family Lampyridae, particularly the common European glowworm, Lampyris noctiluca, (2) larvae of lampyrid fireflies (common in the Americas) and of elaterid fireflies (tropical), (3) larvae and adult females of certain beetles of the genera Phengodes (North America) and Phrixothrix (South America), and (4) larvae of certain gnats (e.g., the cave-dwelling Arachnocampa of New Zealand and Platyura of the central Appalachians).
Glowworm bioluminescent organs vary widely in size, number, location, and structure, suggesting independent evolutionary origins of light-producing ability. In Phengodes the light is emitted by solitary giant cells; in Arachnocampa, by modified excretory organs; in Platyura, by modified salivary glands; and in Phrixothrix, Lampyris, and lampyrid larvae, by organs similar to, but simpler than, the “lanterns” of flashing types of fireflies. The light is usually greenish, but the “railroad worm” (Phrixothrix) has a red headlight in addition. In Lampyris, Phengodes, and Phrixothrix the flying male, which may itself be nonluminous, is attracted to the female’s light. In Platyura and Arachnocampa, the larvae produce light to attract prey that they then capture in their sticky webs.
Details
Glowworm or glow-worm is the common name for various groups of insect larvae and adult larviform females that glow through bioluminescence. They include the European common glow-worm and other members of the Lampyridae, but bioluminescence also occurs in the families Elateridae, Phengodidae, and Rhagophthalmidae among beetles; as well as members of the genera Arachnocampa, Keroplatus, and Orfelia among keroplatid fungus gnats.
Beetles
Four families of beetles are bioluminescent. The wingless larviform females and larvae of these bioluminescent species are usually known as "glowworms". Winged males may or may not also exhibit bioluminescence. Their light may be emitted as flashes or as a constant glow, and usually range in color from green, yellow, to orange. The families are closely related, and are all members of the beetle superfamily, Elateroidea. Phylogenetic analyses have indicated that bioluminescence may have a single evolutionary origin among the families Lampyridae, Phengodidae, and Gymnophthalmidae; but is likely to have arisen independently among Elateridae.
* Family Elateridae – The click beetles. Of the estimated 10,000 species classified under this family, around 200 species from tropical regions of the Americas and some Melanesian islands are bioluminescent. All of them are members of the subfamily Pyrophorinae, except for one species, Campyloxenus pyrothorax, which belongs to subfamily Campyloxeninae, and Balgus schnusei, in Thylacosterninae.
* Family Lampyridae – True fireflies. Contains around 2,000 species found throughout the world. Some "glow worms" are in this family.
* Family Phengodidae – Usually known as glowworm beetles. Contains around 230 species endemic to the New World. This family also includes railroad worms, which are unique among all terrestrial bioluminescent organisms in producing red light.
* Family Rhagophthalmidae – Contains around 30 species found in Asia. The validity of this family has not been fully resolved. Rhagophthalmidae was formerly considered to be a subfamily under Phengodidae before being treated as a distinct family. Some authors[who?] now believe that it should be classified under Lampyridae.
Fungus gnats
Three genera of fungus gnats are bioluminescent, and known as "glowworms" in their larval stage. They produce a blue-green light. The larvae spin sticky webs to catch food. They are found in caves, overhangs, rock cavities, and other sheltered, wet areas. They are usually classified under the family Keroplatidae, but this is not universally accepted and some authors place them under Mycetophilidae instead. Despite the similarities in function and appearance, the bioluminescent systems of the three genera are not homologous and are believed to have evolved separately.
* Genus Arachnocampa – around five species found only in New Zealand and Australia. The most well-known member of the genus is the New Zealand glowworm, Arachnocampa luminosa. The larvae are predatory and use their lights to lure prey into their webs.
* Genus Orfelia – sometimes known as "dismalites". Contains a single species, Orfelia fultoni, found only in North America. Like Arachnocampa spp., their larvae may use their lights to attract prey like springtails and other small insects, but their main food is fungal spores.
* Genus Keroplatus – found in Eurasia. Unlike Arachnocampa and Orfelia, the larvae of Keroplatus feed only on fungal spores. Their bioluminescence is believed to have no function and is vestigial.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1232 2021-12-24 00:02:47
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,148
Re: Miscellany
1208) Invertebrate
Summary
Invertebrate is any animal that lacks a vertebral column, or backbone, in contrast to the cartilaginous or bony vertebrates. More than 90 percent of all living animal species are invertebrates. Worldwide in distribution, they include animals as diverse as sea stars, sea urchins, earthworms, sponges, jellyfish, lobsters, crabs, insects, spiders, snails, clams, and squid. Invertebrates are especially important as agricultural pests, parasites, or agents for the transmission of parasitic infections to humans and other vertebrates.
Invertebrates serve as food for humans; are key elements in food chains that support birds, fish, and many other vertebrate species; and play important roles in plant pollination. Despite providing important environmental services, invertebrates are often ancillary in wildlife research and conservation, with priority given instead to studies that focus on large vertebrates. In addition, several invertebrate groups (including many types of insects and worms) are viewed solely as pests, and by the early 21st century the heavy use of pesticides worldwide had caused substantial population declines among bees, wasps, and other terrestrial insects.
Apart from the absence of a vertebral column, invertebrates have little in common. Indeed, they are distributed into more than 30 phyla. In contrast, all vertebrates are contained within a single phylum, the Chordata. (Phylum Chordata also includes the sea squirts and some other invertebrate groups.) Invertebrates are generally soft-bodied animals that lack a rigid internal skeleton for the attachment of muscles but often possess a hard outer skeleton (as in most mollusks, crustaceans, and insects) that serves, as well, for body protection.
Details
Invertebrates are animals that neither possess nor develop a vertebral column (commonly known as a backbone or spine), derived from the notochord. This includes all animals apart from the chordate subphylum Vertebrata. Familiar examples of invertebrates include arthropods (insects, arachnids, crustaceans, and myriapods), mollusks (chitons, snails, bivalves, squids, and octopuses), annelid (earthworms and leeches), and cnidarians (hydras, jellyfishes, sea anemones, and corals).
The majority of animal species are invertebrates; one estimate puts the figure at 97%. Many invertebrate taxa have a greater number and variety of species than the entire subphylum of Vertebrata. Invertebrates vary widely in size, from 50 μm (0.002 in) rotifers to the 9–10 m (30–33 ft) colossal squid.
Some so-called invertebrates, such as the Tunicata and Cephalochordata, are more closely related to vertebrates than to other invertebrates. This makes the invertebrates paraphyletic, so the term has little meaning in taxonomy.
Taxonomic significance
The term invertebrates is not always precise among non-biologists since it does not accurately describe a taxon in the same way that Arthropoda, Vertebrata or Manidae do. Each of these terms describes a valid taxon, phylum, subphylum or family. "Invertebrata" is a term of convenience, not a taxon; it has very little circumscriptional significance except within the Chordata. The Vertebrata as a subphylum comprises such a small proportion of the Metazoa that to speak of the kingdom Animalia in terms of "Vertebrata" and "Invertebrata" has limited practicality. In the more formal taxonomy of Animalia other attributes that logically should precede the presence or absence of the vertebral column in constructing a cladogram, for example, the presence of a notochord. That would at least circumscribe the Chordata. However, even the notochord would be a less fundamental criterion than aspects of embryological development and symmetry or perhaps bauplan.
Despite this, the concept of invertebrates as a taxon of animals has persisted for over a century among the laity, and within the zoological community and in its literature it remains in use as a term of convenience for animals that are not members of the Vertebrata. The following text reflects earlier scientific understanding of the term and of those animals which have constituted it. According to this understanding, invertebrates do not possess a skeleton of bone, either internal or external. They include hugely varied body plans. Many have fluid-filled, hydrostatic skeletons, like jellyfish or worms. Others have hard exoskeletons, outer shells like those of insects and crustaceans. The most familiar invertebrates include the Protozoa, Porifera, Coelenterata, Platyhelminthes, Nematoda, Annelida, Echinodermata, Mollusca and Arthropoda. Arthropoda include insects, crustaceans and arachnids.
The IUCN estimates that 66,178 extant vertebrate species have been described, which means that over 95% of the described animal species in the world are invertebrates.
Characteristics
The trait that is common to all invertebrates is the absence of a vertebral column (backbone): this creates a distinction between invertebrates and vertebrates. The distinction is one of convenience only; it is not based on any clear biologically homologous trait, any more than the common trait of having wings functionally unites insects, bats, and birds, or than not having wings unites tortoises, snails and sponges. Being animals, invertebrates are heterotrophs, and require sustenance in the form of the consumption of other organisms. With a few exceptions, such as the Porifera, invertebrates generally have bodies composed of differentiated tissues. There is also typically a digestive chamber with one or two openings to the exterior.
Morphology and symmetry
The body plans of most multicellular organisms exhibit some form of symmetry, whether radial, bilateral, or spherical. A minority, however, exhibit no symmetry. One example of asymmetric invertebrates includes all gastropod species. This is easily seen in snails and sea snails, which have helical shells. Slugs appear externally symmetrical, but their pneumostome (breathing hole) is located on the right side. Other gastropods develop external asymmetry, such as Glaucus atlanticus that develops asymmetrical cerata as they mature. The origin of gastropod asymmetry is a subject of scientific debate.
Other examples of asymmetry are found in fiddler crabs and hermit crabs. They often have one claw much larger than the other. If a male fiddler loses its large claw, it will grow another on the opposite side after moulting. Sessile animals such as sponges are asymmetrical alongside coral colonies (with the exception of the individual polyps that exhibit radial symmetry); alpheidae claws that lack pincers; and some copepods, polyopisthocotyleans, and monogeneans which parasitize by attachment or residency within the gill chamber of their fish hosts).
Nervous system
Neurons differ in invertebrates from mammalian cells. Invertebrates cells fire in response to similar stimuli as mammals, such as tissue trauma, high temperature, or changes in pH. The first invertebrate in which a neuron cell was identified was the medicinal leech, Hirudo medicinalis.
Learning and memory using nociceptors in the sea hare, Aplysia has been described. Mollusk neurons are able to detect increasing pressures and tissue trauma.
Neurons have been identified in a wide range of invertebrate species, including annelids, molluscs, nematodes and arthropods.
Respiratory system
One type of invertebrate respiratory system is the open respiratory system composed of spiracles, tracheae, and tracheoles that terrestrial arthropods have to transport metabolic gases to and from tissues. The distribution of spiracles can vary greatly among the many orders of insects, but in general each segment of the body can have only one pair of spiracles, each of which connects to an atrium and has a relatively large tracheal tube behind it. The tracheae are invaginations of the cuticular exoskeleton that branch (anastomose) throughout the body with diameters from only a few micrometres up to 0.8 mm. The smallest tubes, tracheoles, penetrate cells and serve as sites of diffusion for water, oxygen, and carbon dioxide. Gas may be conducted through the respiratory system by means of active ventilation or passive diffusion. Unlike vertebrates, insects do not generally carry oxygen in their haemolymph.
A tracheal tube may contain ridge-like circumferential rings of taenidia in various geometries such as loops or helices. In the head, thorax, or abdomen, tracheae may also be connected to air sacs. Many insects, such as grasshoppers and bees, which actively pump the air sacs in their abdomen, are able to control the flow of air through their body. In some aquatic insects, the tracheae exchange gas through the body wall directly, in the form of a gill, or function essentially as normal, via a plastron. Note that despite being internal, the tracheae of arthropods are shed during moulting (ecdysis).
Reproduction
Like vertebrates, most invertebrates reproduce at least partly through sexual reproduction. They produce specialized reproductive cells that undergo meiosis to produce smaller, motile spermatozoa or larger, non-motile ova. These fuse to form zygotes, which develop into new individuals. Others are capable of asexual reproduction, or sometimes, both methods of reproduction.
Social interaction
Social behavior is widespread in invertebrates, including math, termites, aphids, thrips, ants, bees, Passalidae, Acari, spiders, and more. Social interaction is particularly salient in eusocial species but applies to other invertebrates as well.
Insects recognize information transmitted by other insects.
Phyla
The term invertebrates covers several phyla. One of these are the sponges (Porifera). They were long thought to have diverged from other animals early. They lack the complex organization found in most other phyla. Their cells are differentiated, but in most cases not organized into distinct tissues. Sponges typically feed by drawing in water through pores. Some speculate that sponges are not so primitive, but may instead be secondarily simplified. The Ctenophora and the Cnidaria, which includes sea anemones, corals, and jellyfish, are radially symmetric and have digestive chambers with a single opening, which serves as both the mouth and the rear. Both have distinct tissues, but they are not organized into organs. There are only two main germ layers, the ectoderm and endoderm, with only scattered cells between them. As such, they are sometimes called diploblastic.
The Echinodermata are radially symmetric and exclusively marine, including starfish (Asteroidea), sea urchins, (Echinoidea), brittle stars (Ophiuroidea), sea cucumbers (Holothuroidea) and feather stars (Crinoidea).
The largest animal phylum is also included within invertebrates: the Arthropoda, including insects, spiders, crabs, and their kin. All these organisms have a body divided into repeating segments, typically with paired appendages. In addition, they possess a hardened exoskeleton that is periodically shed during growth. Two smaller phyla, the Onychophora and Tardigrada, are close relatives of the arthropods and share these traits. The Nematoda or roundworms, are perhaps the second largest animal phylum, and are also invertebrates. Roundworms are typically microscopic, and occur in nearly every environment where there is water. A number are important parasites. Smaller phyla related to them are the Kinorhyncha, Priapulida, and Loricifera. These groups have a reduced coelom, called a pseudocoelom. Other invertebrates include the Nemertea or ribbon worms, and the Sipuncula.
Another phylum is Platyhelminthes, the flatworms. These were originally considered primitive, but it now appears they developed from more complex ancestors. Flatworms are acoelomates, lacking a body cavity, as are their closest relatives, the microscopic Gastrotricha. The Rotifera or rotifers, are common in aqueous environments. Invertebrates also include the Acanthocephala or spiny-headed worms, the Gnathostomulida, Micrognathozoa, and the Cycliophora.
Also included are two of the most successful animal phyla, the Mollusca and Annelida. The former, which is the second-largest animal phylum by number of described species, includes animals such as snails, clams, and squids, and the latter comprises the segmented worms, such as earthworms and leeches. These two groups have long been considered close relatives because of the common presence of trochophore larvae, but the annelids were considered closer to the arthropods because they are both segmented. Now, this is generally considered convergent evolution, owing to many morphological and genetic differences between the two phyla.
Among lesser phyla of invertebrates are the Hemichordata, or acorn worms, and the Chaetognatha, or arrow worms. Other phyla include Acoelomorpha, Brachiopoda, Bryozoa, Entoprocta, Phoronida, and Xenoturbellida.
History
The earliest animal fossils appear to be those of invertebrates. 665-million-year-old fossils in the Trezona Formation at Trezona Bore, West Central Flinders, South Australia have been interpreted as being early sponges. Some paleontologists suggest that animals appeared much earlier, possibly as early as 1 billion years ago though they probably became multicellular in the Tonian. Trace fossils such as tracks and burrows found in the late Neoproterozoic era indicate the presence of triploblastic worms, like metazoans, roughly as large (about 5 mm wide) and complex as earthworms.
Around 453 MYA, animals began diversifying, and many of the important groups of invertebrates diverged from one another. Fossils of invertebrates are found in various types of sediment from the Phanerozoic. Fossils of invertebrates are commonly used in stratigraphy.
Classification
Carl Linnaeus divided these animals into only two groups, the Insecta and the now-obsolete Vermes (worms). Jean-Baptiste Lamarck, who was appointed to the position of "Curator of Insecta and Vermes" at the Muséum National d'Histoire Naturelle in 1793, both coined the term "invertebrate" to describe such animals and divided the original two groups into ten, by splitting Arachnida and Crustacea from the Linnean Insecta, and Mollusca, Annelida, Cirripedia, Radiata, Coelenterata and Infusoria from the Linnean Vermes. They are now classified into over 30 phyla, from simple organisms such as sea sponges and flatworms to complex animals such as arthropods and molluscs.
Significance of the group
Invertebrates are animals without a vertebral column. This has led to the conclusion that invertebrates are a group that deviates from the normal, vertebrates. This has been said to be because researchers in the past, such as Lamarck, viewed vertebrates as a "standard": in Lamarck's theory of evolution, he believed that characteristics acquired through the evolutionary process involved not only survival, but also progression toward a "higher form", to which humans and vertebrates were closer than invertebrates were. Although goal-directed evolution has been abandoned, the distinction of invertebrates and vertebrates persists to this day, even though the grouping has been noted to be "hardly natural or even very sharp." Another reason cited for this continued distinction is that Lamarck created a precedent through his classifications which is now difficult to escape from. It is also possible that some humans believe that, they themselves being vertebrates, the group deserves more attention than invertebrates. In any event, in the 1968 edition of 'Invertebrate Zoology', it is noted that "division of the Animal Kingdom into vertebrates and invertebrates is artificial and reflects human bias in favor of man's own relatives." The book also points out that the group lumps a vast number of species together, so that no one characteristic describes all invertebrates. In addition, some species included are only remotely related to one another, with some more related to vertebrates than other invertebrates.
In research
For many centuries, invertebrates were neglected by biologists, in favor of big vertebrates and "useful" or charismatic species. Invertebrate biology was not a major field of study until the work of Linnaeus and Lamarck in the 18th century. During the 20th century, invertebrate zoology became one of the major fields of natural sciences, with prominent discoveries in the fields of medicine, genetics, palaeontology, and ecology. The study of invertebrates has also benefited law enforcement, as arthropods, and especially insects, were discovered to be a source of information for forensic investigators.
Two of the most commonly studied model organisms nowadays are invertebrates: the fruit fly Drosophila melanogaster and the nematode Caenorhabditis elegans. They have long been the most intensively studied model organisms, and were among the first life-forms to be genetically sequenced. This was facilitated by the severely reduced state of their genomes, but many genes, introns, and linkages have been lost. Analysis of the starlet sea anemone genome has emphasised the importance of sponges, placozoans, and choanoflagellates, also being sequenced, in explaining the arrival of 1500 ancestral genes unique to animals. Invertebrates are also used by scientists in the field of aquatic biomonitoring to evaluate the effects of water pollution and climate change.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1233 2021-12-25 00:19:36
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,148
Re: Miscellany
1209) Tissue
Summary
In biology, tissue is a biological organizational level between cells and a complete organ. A tissue is an ensemble of similar cells and their extracellular matrix from the same origin that together carry out a specific function. Organs are then formed by the functional grouping together of multiple tissues.
The English word "tissue" derives from the French word "tissu", the past participle of the verb tisser, "to weave".
The study of tissues is known as histology or, in connection with disease, as histopathology. Xavier Bichat is considered as the "Father of Histology". Plant histology is studied in both plant anatomy and physiology. The classical tools for studying tissues are the paraffin block in which tissue is embedded and then sectioned, the histological stain, and the optical microscope. Developments in electron microscopy, immunofluorescence, and the use of frozen tissue-sections have enhanced the detail that can be observed in tissues. With these tools, the classical appearances of tissues can be examined in health and disease, enabling considerable refinement of medical diagnosis and prognosis.
Details
Tissue, in physiology, is a level of organization in multicellular organisms; it consists of a group of structurally and functionally similar cells and their intercellular material.
By definition, tissues are absent from unicellular organisms. Even among the simplest multicellular species, such as sponges, tissues are lacking or are poorly differentiated. But multicellular animals and plants that are more advanced have specialized tissues that can organize and regulate an organism’s response to its environment.
Plants
Bryophytes (liverworts, hornworts, and mosses) are nonvascular plants; i.e., they lack vascular tissues (phloem and xylem) as well as true leaves, stems, and roots. Instead bryophytes absorb water and nutrients directly through leaflike and stemlike structures or through cells comprising the gametophyte body.
In vascular plants, such as angiosperms and gymnosperms, cell division takes place almost exclusively in specific tissues known as meristems. Apical meristems, which are located at the tips of shoots and roots in all vascular plants, give rise to three types of primary meristems, which in turn produce the mature primary tissues of the plant. The three kinds of mature tissues are dermal, vascular, and ground tissues. Primary dermal tissues, called epidermis, make up the outer layer of all plant organs (e.g., stems, roots, leaves, flowers). They help deter excess water loss and invasion by insects and microorganisms. The vascular tissues are of two kinds: water-transporting xylem and food-transporting phloem. Primary xylem and phloem are arranged in vascular bundles that run the length of the plant from roots to leaves. The ground tissues, which comprise the remaining plant matter, include various support, storage, and photosynthetic tissues.
Secondary, or lateral, meristems, which are found in all woody plants and in some herbaceous ones, consist of the vascular cambium and the cork cambium. They produce secondary tissues from a ring of vascular cambium in stems and roots. Secondary phloem forms along the outer edge of the cambium ring, and secondary xylem (i.e., wood) forms along the inner edge of the cambium ring. The cork cambium produces a secondary dermal tissue (periderm) that replaces the epidermis along older stems and roots.
Animals
Early in the evolutionary history of animals, tissues became aggregated into organs, which themselves became divided into specialized parts. An early scientific classification of tissues divided them on the basis of the organ system of which they formed a part (e.g., nervous tissues). Embryologists have often classified tissues on the basis of their origin in the developing embryo; i.e., ectodermal, endodermal, and mesodermal tissues. Another method classified tissues into four broad groups according to cell composition: epithelial tissues, composed of cells that make up the body’s outer covering and the membranous covering of internal organs, cavities, and canals; endothelial tissues, composed of cells that line the inside of organs; stroma tissues, composed of cells that serve as a matrix in which the other cells are embedded; and connective tissues, a rather amorphous category composed of cells and an extracellular matrix that serve as a connection from one tissue to another.
The most useful of all systems, however, breaks down animal tissues into four classes based on the functions that the tissues perform. The first class includes all those tissues that serve an animal’s needs for growth, repair, and energy; i.e., the assimilation, storage, transport, and excretion of nutrients and waste products. In humans, these tissues include the alimentary (or digestive) tract, kidneys, liver, and lungs. The digestive tract leads (in vertebrates) from the mouth through the pharynx, stomach, and intestines to the math. In vertebrates and some larger invertebrates, oxygen and the nutrients secured by the alimentary tissues or liberated from storage tissues are transported throughout the body by the blood and lymph, which are themselves considered by many to be tissues. Tissues that secure oxygen and excrete carbon dioxide are extremely variable in the animal kingdom. In many invertebrates, gas exchange takes place through the body wall or external gills, but in species adapted to a terrestrial life, an internal sac capable of expansion and contraction served this purpose, and gradually became more complex over evolutionary time as animals’ demand for oxygen increased.
The second class of tissues consists of those used in coordination. There are basically two types: physical (nervous and sensory tissues), which operate via electrical impulses along nerve fibres; and the chemical (endocrine tissues), which release hormones into the bloodstream. In invertebrates, both physical and chemical coordination are performed by the same tissues, because the nervous tissues also serve as hormone sources. In vertebrates, most endocrine functions are isolated in specialized glands, several of which are derived from nervous tissue.
The basic unit of all nervous tissue is the neuron, aggregations of which are called ganglia. The bundles of axons along which neurons transmit and receive impulses are called nerves. By comparison, chemical control by hormones is much slower and longer-acting. In many invertebrates, chemical stimulators are secreted by the neurons themselves and then move to their site of action along the axon. In higher vertebrates, the principal endocrine tissues are the thyroid, parathyroid, pituitary, and endocrine constituents of the pancreas and adrenal glands.
The third class of tissues includes those contributing to the body’s support and movement. The connective tissues proper surround organs, bones, and muscles, helping to hold them together. Connective tissues proper consist of cells embedded in a matrix composed of an amorphous ground substance and collagen, elastic, and reticular fibres. Tendons and ligaments are examples of extremely strong connective tissues proper. The other major structural tissues are cartilage and bone, which, like connective tissues proper, consist of cells embedded in an intercellular matrix. In cartilage the matrix is firm but rubbery; in bone the matrix is rigid, being impregnated by hard crystals of inorganic salts. Muscle tissue is primarily responsible for movement; it consists of contractile cells. There are two general types of muscle: striated muscle, which moves the skeleton and is under voluntary control; and smooth muscle, which surrounds the walls of many internal organs and cannot normally be controlled voluntarily.
A fourth class of tissues includes reproductive tissues, hemopoietic tissues, and tissue fluids. The most important reproductive tissues are the gonads (ovaries and testes), which produce the gametes (eggs and sperm, respectively). Hemopoietic tissues produce the cellular components of the blood. Among the important tissue fluids are lymph, cerebrospinal fluid, and milk (in mammals).
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1234 2021-12-26 01:07:41
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,148
Re: Miscellany
1210) Peritoneum
Summary
Peritoneum is a large membrane in the abdominal cavity that connects and supports internal organs. It is composed of many folds that pass between or around the various organs. Two folds are of primary importance: the omentum, which hangs in front of the stomach and intestine; and the mesentery, which attaches the small intestine and much of the large intestine to the posterior abdominal cavity.
The omentum and mesentery contain blood vessels, nerves, lymph nodes, fat, elastic fibres for stretching, and collagen fibres for strength. The omentum is thinner than the mesentery and is lacy in appearance. It contains large quantities of fat that serve to keep the organs warm. The mesentery is fan-shaped and well-supplied with blood vessels that radiate to the intestine.
The functions of these membranes are to prevent friction between closely packed organs by secreting serum that acts as a lubricant, to help hold the abdominal organs in their proper positions, to separate and unite organs, and to guard as a barrier against infection.
Peritonitis, an inflammation of the peritoneum, results from bacteria entering a perforation in the gastrointestinal tract. A ruptured appendix is a common cause of peritonitis. Symptoms include abdominal pain, vomiting, and fever. If antibiotics do not prove successful, surgery may be necessary to remove the source of the infection entirely.
Details:
The peritoneum is the serous membrane forming the lining of the abdominal cavity or coelom in amniotes and some invertebrates, such as annelids. It covers most of the intra-abdominal (or coelomic) organs, and is composed of a layer of mesothelium supported by a thin layer of connective tissue. This peritoneal lining of the cavity supports many of the abdominal organs and serves as a conduit for their blood vessels, lymphatic vessels, and nerves.
The abdominal cavity (the space bounded by the vertebrae, abdominal muscles, diaphragm, and pelvic floor) is different from the intraperitoneal space (located within the abdominal cavity but wrapped in peritoneum). The structures within the intraperitoneal space are called "intraperitoneal" (e.g., the stomach and intestines), the structures in the abdominal cavity that are located behind the intraperitoneal space are called "retroperitoneal" (e.g., the kidneys), and those structures below the intraperitoneal space are called "subperitoneal" or "infraperitoneal" (e.g., the bladder).
Structure:
Layers
The peritoneum is one continuous sheet, forming two layers and a potential space between them: the peritoneal cavity.
The outer layer, the parietal peritoneum, is attached to the abdominal wall and the pelvic walls. The tunica vaginalis, the serous membrane covering the male testis, is derived from the vaginal process, an outpouching of the parietal peritoneum.
The inner layer, the visceral peritoneum, is wrapped around the visceral organs, located inside the intraperitoneal space for protection. It is thinner than the parietal peritoneum. The mesentery is a double layer of visceral peritoneum that attaches to the gastrointestinal tract. There are often blood vessels, nerves, and other structures between these layers. The space between these two layers is technically outside of the peritoneal sac, and thus not in the peritoneal cavity.
The potential space between these two layers is the peritoneal cavity, filled with a small amount (about 50 mL) of slippery serous fluid that allows the two layers to slide freely over each other.
Subdivisions
Peritoneal folds are omenta, mesenteries and ligaments; they connect organs to each other or to the abdominal wall. There are two main regions of the peritoneal cavity, connected by the omental foramen.
* The greater sac.
* The lesser sac. The lesser sac is divided into two "omenta":
* (i) The lesser omentum (or gastrohepatic) is attached to the lesser curvature of the stomach and the liver.
* (ii) The greater omentum (or gastrocolic) hangs from the greater curve of the stomach and loops down in front of the intestines before curving back upwards to attach to the transverse colon.
In effect it is draped in front of the intestines like an apron and may serve as an insulating or protective layer.
The mesentery is the part of the peritoneum through which most abdominal organs are attached to the abdominal wall and supplied with blood and lymph vessels and nerves.
Development
The peritoneum develops ultimately from the mesoderm of the trilaminar embryo. As the mesoderm differentiates, one region known as the lateral plate mesoderm splits to form two layers separated by an intraembryonic coelom. These two layers develop later into the visceral and parietal layers found in all serous cavities, including the peritoneum.
As an embryo develops, the various abdominal organs grow into the abdominal cavity from structures in the abdominal wall. In this process they become enveloped in a layer of peritoneum. The growing organs "take their blood vessels with them" from the abdominal wall, and these blood vessels become covered by peritoneum, forming a mesentery.
Peritoneal folds develop from the ventral and dorsal mesentery of the embryo.
Clinical significance:
Peritoneal dialysis
In one form of dialysis, called peritoneal dialysis, a glucose solution is sent through a tube into the peritoneal cavity. The fluid is left there for a prescribed amount of time to absorb waste products, and then removed through the tube. The reason for this effect is the high number of arteries and veins in the peritoneal cavity. Through the mechanism of diffusion, waste products are removed from the blood.
Peritonitis
Peritonitis is the inflammation of the peritoneum. It is more commonly associated to infection from a punctured organ of the abdominal cavity. It can also be provoked by the presence of fluids that produce chemical irritation, such as gastric acid or pancreatic juice. Peritonitis causes fever, tenderness, and pain in the abdominal area, which can be localized or diffuse. The treatment involves rehydration, administration of antibiotics, and surgical correction of the underlying cause. Mortality is higher in the elderly and if present for a prolonged time.
Primary peritoneal carcinoma
Primary peritoneal cancer is a cancer of the cells lining the peritoneum.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1235 2021-12-27 00:03:59
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,148
Re: Miscellany
1211) Tympanic membrane
Summary
Tympanic membrane, also called eardrum, is a thin layer of tissue in the human ear that receives sound vibrations from the outer air and transmits them to the auditory ossicles, which are tiny bones in the tympanic (middle-ear) cavity. It also serves as the lateral wall of the tympanic cavity, separating it from the external auditory canal. The membrane lies across the end of the external canal and looks like a flattened cone with its tip (apex) pointed inward. The edges are attached to a ring of bone, the tympanic annulus.
The drum membrane has three layers: the outer layer, continuous with the skin on the external canal; the inner layer, continuous with the mucous membrane lining the middle ear; and, between the two, a layer of radial and circular fibres that give the membrane its tension and stiffness. The membrane is well supplied with blood vessels, and its sensory nerve fibres make it extremely sensitive to pain.
Accurate diagnosis of middle-ear diseases depends on the appearance and mobility of the tympanic membrane, which is normally pearl gray but is sometimes tinged with pink or yellow. The condition that most commonly involves the tympanic membrane is otitis media (inflammation of the middle ear), which frequently affects children (particularly those between three months and three years of age) and typically is caused by bacterial infection. In severe otitis media, pressure from the accumulation of fluid in the middle ear can lead to tearing or rupturing of the tympanic membrane. Trauma, such as from a blow to the head or from water pressure, can also cause perforations in the membrane. Although tympanic membrane perforations often are self-healing, a patch or surgery may be needed to close the tear. Failure of the membrane to heal can result in varying degrees of hearing loss and increased susceptibility to otitis media and cholesteatoma (the formation of a cyst in the middle ear).
Details
In the anatomy of humans and various other tetrapods, the eardrum, also called the tympanic membrane or myringa, is a thin, cone-shaped membrane that separates the external ear from the middle ear. Its function is to transmit sound from the air to the ossicles inside the middle ear, and then to the oval window in the fluid-filled cochlea. Hence, it ultimately converts and amplifies vibration in air to vibration in cochlear fluid. The malleus bone bridges the gap between the eardrum and the other ossicles.
Rupture or perforation of the eardrum can lead to conductive hearing loss. Collapse or retraction of the eardrum can cause conductive hearing loss or cholesteatoma.
Structure:
Orientation and relations
The tympanic membrane is oriented obliquely in the anteroposterior, mediolateral, and superoinferior planes. Consequently, its superoposterior end lies lateral to its anteroinferior end.
Anatomically, it relates superiorly to the middle cranial fossa, posteriorly to the ossicles and facial nerve, inferiorly to the parotid gland, and anteriorly to the temporomandibular joint.
Regions
The eardrum is divided into two general regions: the pars flaccida and the pars tensa. The relatively fragile pars flaccida lies above the lateral process of the malleus between the notch of Rivinus and the anterior and posterior malleal folds. Consisting of two layers and appearing slightly pinkish in hue, it is associated with Eustachian tube dysfunction and cholesteatomas.
The larger pars tensa consists of three layers: skin, fibrous tissue, and mucosa. Its thick periphery forms a fibrocartilaginous ring called the annulus tympanicus or Gerlach's ligament. while the central umbo tents inward at the level of the tip of malleus. The middle fibrous layer, containing radial, circular, and parabolic fibers, encloses the handle of malleus. Though comparatively robust, the pars tensa is the region more commonly associated with[vague] perforations.
Umbo
The manubrium (Latin: handle) of the malleus is firmly attached to the medial surface of the membrane as far as its center, drawing it toward the tympanic cavity. The lateral surface of the membrane is thus concave. The most depressed aspect of this concavity is termed the umbo (Latin: shield boss).
Nerve supply
Sensation of the outer surface of the tympanic membrane is supplied mainly by the auriculotemporal nerve, a branch of the mandibular nerve (cranial nerve V3), with contributions from the auricular branch of the vagus nerve (cranial nerve X), the facial nerve (cranial nerve VII), and possibly the glossopharyngeal nerve (cranial nerve IX). The inner surface of the tympanic membrane is innervated by the glossopharyngeal nerve.
Clinical significance:
Examination
When the eardrum is illuminated during a medical examination, a cone of light radiates from the tip of the malleus to the periphery in the anteroinferior quadrant, this is what is known clinically as 5 o'clock.
Rupture
Unintentional perforation (rupture) has been described in blast injuries and air travel, typically in patients experiencing upper respiratory congestion that prevents equalization of pressure in the middle ear. It is also known to occur in swimming, diving (including scuba diving), and martial arts.
Patients suffering from tympanic membrane rupture may experience bleeding, tinnitus, hearing loss, or disequilibrium (vertigo). However, they rarely require medical intervention, as between 80 and 95 percent of ruptures recover completely within two to four weeks. The prognosis becomes more guarded as the force of injury increases.
Surgical puncture for treatment of middle ear infections
The pressure of fluid in an infected middle ear onto the eardrum may cause it to rupture. Usually, this consists of a small hole (perforation), which allows fluid to drain out. If this does not occur naturally, a myringotomy (tympanotomy, tympanostomy) can be performed. A myringotomy is a surgical procedure in which a tiny incision is created in the eardrum to relieve pressure caused by excessive buildup of fluid, or to drain pus from the middle ear. The fluid or pus comes from a middle ear infection (otitis media), which is a common problem in children. A tympanostomy tube is inserted into the eardrum to keep the middle ear aerated for a prolonged time and to prevent reaccumulation of fluid. Without the insertion of a tube, the incision usually heals spontaneously in two to three weeks. Depending on the type, the tube is either naturally extruded in 6 to 12 months or removed during a minor procedure.
Those requiring myringotomy usually have an obstructed or dysfunctional eustachian tube that is unable to perform drainage or ventilation in its usual fashion. Before the invention of antibiotics, myringotomy without tube placement was also used as a major treatment of severe acute otitis media.
In some cases, the pressure of fluid in an infected middle ear is great enough to cause the eardrum to rupture naturally. Usually, this consists of a small hole (perforation), from which fluid can drain.
Society and culture
The Bajau people of the Pacific intentionally rupture their eardrums at an early age to facilitate diving and hunting at sea. Many older Bajau therefore have difficulties hearing.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1236 2021-12-28 00:02:34
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,148
Re: Miscellany
1212) Geothermal energy
Summary
Geothermal energy is the thermal energy in the Earth's crust which originates from the formation of the planet and from radioactive decay of materials in currently uncertain but possibly roughly equal proportions. The high temperature and pressure in Earth's interior cause some rock to melt and solid mantle to behave plastically, resulting in parts of the mantle convecting upward since it is lighter than the surrounding rock and temperatures at the core–mantle boundary can reach over 4000 °C (7200 °F).
Geothermal heating, for example using water from hot springs has been used for bathing since Paleolithic times and for space heating since ancient Roman times, however more recently geothermal power, the term used for generation of electricity from geothermal energy, has gained in importance. It is estimated that the earth's geothermal resources are theoretically more than adequate to supply humanity's energy needs, although only a very small fraction is currently being profitably exploited, often in areas near tectonic plate boundaries.
As a result of government assisted research and industry experience, the cost of generating geothermal power decreased by 25% over the 1980s and 1990s. More recent technological advances have dramatically reduced costs and thereby expanded the range and size of viable resource and in 2021 the U.S. Department of Energy estimates that geothermal energy from a power plant "built today" costs about $0.05/kWh.
Worldwide, 13,900 megawatts (MW) of geothermal power was available in 2019. An additional 28 gigawatts of direct geothermal heating capacity is installed for district heating, space heating, spas, industrial processes, desalination and agricultural applications as of 2010.
Forecasts for the future of geothermal power depend on assumptions about technology, energy prices, subsidies, plate boundary movement and interest rates. Pilot programs like EWEB's customer opt in Green Power Program show that customers would be willing to pay a little more for a renewable energy source like geothermal. About 100 thousand people are employed in the industry. The adjective geothermal originates from the Greek roots, meaning Earth, and meaning hot.
Details
Geothermal energy is form of energy conversion in which heat energy from within Earth is captured and harnessed for cooking, bathing, space heating, electrical power generation, and other uses.
Heat from Earth’s interior generates surface phenomena such as lava flows, geysers, fumaroles, hot springs, and mud pots. The heat is produced mainly by the radioactive decay of potassium, thorium, and uranium in Earth’s crust and mantle and also by friction generated along the margins of continental plates. The subsequent annual low-grade heat flow to the surface averages between 50 and 70 milliwatts (mW) per square metre worldwide. In contrast, incoming solar radiation striking Earth’s surface provides 342 watts per square metre annually. Geothermal heat energy can be recovered and exploited for human use, and it is available anywhere on Earth’s surface. The estimated energy that can be recovered and utilized on the surface is {4.5} × {10}^6 exajoules, or about {1.4} × {10}^6 terawatt-years, which equates to roughly three times the world’s annual consumption of all types of energy.
The amount of usable energy from geothermal sources varies with depth and by extraction method. The increase in temperature of rocks and other materials underground averages 20–30 °C (36–54 °F) per kilometre (0.6 mile) depth worldwide in the upper part of the lithosphere, and this rate of increase is much higher in most of Earth’s known geothermal areas. Normally, heat extraction requires a fluid (or steam) to bring the energy to the surface. Locating and developing geothermal resources can be challenging. This is especially true for the high-temperature resources needed for generating electricity. Such resources are typically limited to parts of the world characterized by recent volcanic activity or located along plate boundaries or within crustal hot spots. Even though there is a continuous source of heat within Earth, the extraction rate of the heated fluids and steam can exceed the replenishment rate, and, thus, use of the resource must be managed sustainably.
Uses
Geothermal energy use can be divided into three categories: direct-use applications, geothermal heat pumps (GHPs), and electric power generation.
Direct uses
Probably the most widely used set of applications involves the direct use of heated water from the ground without the need for any specialized equipment. All direct-use applications make use of low-temperature geothermal resources, which range between about 50 and 150 °C (122 and 302 °F). Such low-temperature geothermal water and steam have been used to warm single buildings, as well as whole districts where numerous buildings are heated from a central supply source. In addition, many swimming pools, balneological (therapeutic) facilities at spas, greenhouses, and aquaculture ponds around the world have been heated with geothermal resources. Other direct uses of geothermal energy include cooking, industrial applications (such as drying fruit, vegetables, and timber), milk pasteurization, and large-scale snow melting. For many of those activities, hot water is often used directly in the heating system, or it may be used in conjunction with a heat exchanger, which transfers heat when there are problematic minerals and gases such as hydrogen sulfide mixed in with the fluid.
Geothermal heat pumps
Geothermal heat pumps (GHPs) take advantage of the relatively stable moderate temperature conditions that occur within the first 300 metres (1,000 feet) of the surface to heat buildings in the winter and cool them in the summer. In that part of the lithosphere, rocks and groundwater occur at temperatures between 5 and 30 °C (41 and 86 °F). At shallower depths, where most GHPs are found, such as within 6 metres (about 20 feet) of Earth’s surface, the temperature of the ground maintains a near-constant temperature of 10 to 16 °C (50 to 60 °F). Consequently, that heat can be used to help warm buildings during the colder months of the year when the air temperature falls below that of the ground. Similarly, during the warmer months of the year, warm air can be drawn from a building and circulated underground, where it loses much of its heat and is returned.
A GHP system is made up of a heat exchanger (a loop of pipes buried in the ground) and a pump. The heat exchanger transfers heat energy between the ground and air at the surface by means of a fluid that circulates through the pipes; the fluid used is often water or a combination of water and antifreeze. During warmer months, heat from warm air is transferred to the heat exchanger and into the fluid. As it moves through the pipes, the heat is dispersed to the rocks, soil, and groundwater. The pump is reversed during the colder months. Heat energy stored in the relatively warm ground raises the temperature of the fluid. The fluid then transfers this energy to the heat pump, which warms the air inside the building.
GHPs have several advantages over more conventional heating and air-conditioning systems. They are very efficient, using 25–50 percent less electricity than comparable conventional heating and cooling systems, and they produce less pollution. The reduction in energy use associated with GHPs can translate into as much as a 44 percent decrease in greenhouse gas emissions compared with air-source heat pumps (which transfer heat between indoor and outdoor air). In addition, when compared with electric resistance heating systems (which convert electricity to heat) coupled with standard air-conditioning systems, GHPs can produce up to 72 percent less greenhouse gas emissions.
Electric power generation
Depending upon the temperature and the fluid (steam) flow, geothermal energy can be used to generate electricity. Geothermal power plants can produce electricity in three ways. Despite their differences in design, all three control the behaviour of steam and use it to drive electrical generators. Given that the excess water vapour at the end of each process is condensed and returned to the ground, where it is reheated for later use, geothermal power is considered a form of renewable energy.
Some geothermal power plants simply collect rising steam from the ground. In such “dry steam” operations, the heated water vapour is funneled directly into a turbine that drives an electrical generator. Other power plants, built around the flash steam and binary cycle designs, use a mixture of steam and heated water (“wet steam”) extracted from the ground to start the electrical generation process.
In flash steam power plants, pressurized high-temperature water is drawn from beneath the surface into containers at the surface, called flash tanks, where the sudden decrease in pressure causes the liquid water to “flash,” or vaporize, into steam. The steam is then used to power the turbine-generator set. In contrast, binary-cycle power plants use steam driven off a secondary working fluid (such as ammonia and hydrocarbons) contained within a closed loop of pipes to power the turbine-generator set. In this process, geothermally heated water is drawn up through a different set of pipes, and much of the energy stored in the heated water is transferred to the working fluid through a heat exchanger. The working fluid then vaporizes. After the vapour from the working fluid passes through the turbine, it is recondensed and piped back to the heat exchanger.
Electrical power usually requires water heated above 175 °C (347 °F) to be economical. In geothermal plants using the Organic Rankine Cycle (ORC), a special type of binary-cycle technology that utilizes lower-temperature heat sources (such as biomass combustion and industrial waste heat), water temperatures as low as 85–90 °C (185–194 °F) may be used.
History
Geothermal energy from natural pools and hot springs has long been used for cooking, bathing, and warmth. There is evidence that Native Americans used geothermal energy for cooking as early as 10,000 years ago. In ancient times, baths heated by hot springs were used by the Greeks and Romans, and examples of geothermal space heating date at least as far back as the Roman city of Pompeii during the 1st century CE. Such uses of geothermal energy were initially limited to sites where hot water and steam were accessible.
Although the world’s first district heating system was installed at Chaudes-Aigues, France, in the 14th century, it was not until the late 19th century that other cities, as well as industries, began to realize the economic potential of geothermal resources. Geothermal heat was delivered to the first residences in the United States in 1892, to Warm Springs Avenue in Boise, Idaho, and most of the city used geothermal heat by 1970. The largest and most-famous geothermal district heating system is in Reykjavík, Iceland, where 99 percent of the city received geothermal water for space heating starting in the 1930s. Early industrial direct-use applications included the extraction of borate compounds from geothermal fluids at Larderello, Italy, during the early 19th century.
The first geothermal electric power generation also took place in Larderello, with the development of an experimental plant in 1904. The first commercial use of that technology occurred there in 1913 with the construction of a plant that produced 250 kilowatts (kW). Geothermal power plants were commissioned in New Zealand starting in 1958 and at the Geysers in northern California in 1960. The Italian and American plants were dry steam facilities, where low-permeability reservoirs produced only steam. In New Zealand, however, high-temperature and high-pressure water emerges naturally as a mixture made up of 80 percent superheated water and 20 percent steam. The steam coming directly from the ground is used for power generation right away. It is sent to the power plant through pipes. In contrast, the superheated water from the ground is separated from the mixture and flashed into steam. Most geothermal plants at present are of this latter “wet steam” type.
By 2015 more than 80 countries were using geothermal energy, either directly or in conjunction with GHPs, the leaders being China, Turkey, Iceland, Japan, Hungary, and the United States. The total worldwide installed capacity for direct use in 2015 was about 73,290 megawatts thermal (MWt) utilizing about 163,273 gigawatt-hours per year (587,786 terajoules per year), producing an annual utilization factor—the annual energy produced by the plant (in megawatt-hours) divided by the installed capacity of the plant (in megawatts [MW]) multiplied by 8,760 hours—of 28 percent in the heating mode.
Geothermal energy was used to produce electricity in 24 countries in the early 21st century, the leaders being the United States, the Philippines, Indonesia, Mexico, New Zealand, and Italy. In 2016 the total worldwide installed capacity for electrical power generation was about 13,400 MW, producing about 75,000 gigawatt-hours per year for a utilization factor of 71 percent (equivalent to 6,220 full-load operating hours annually). Many geothermal fields have utilization factors around 95 percent (equivalent to 8,322 full-load operating hours annually), the highest for any form of renewable energy. The “waste” fluid from the power plant is often used for lower-temperature applications, such as the bottom cycle in a binary-cycle plant, before being injected back into the reservoir. Such cascaded uses can be found in the United States, Iceland, and Germany.
Extraction
Geothermal energy is best found in areas with high thermal gradients. Those gradients occur in regions affected by recent volcanism, in areas located along plate boundaries (such as along the Pacific Ring of Fire), or in areas marked by thin crust (hot spots) such as Yellowstone National Park and the Hawaiian Islands. Geothermal reservoirs associated with those regions must have a heat source, adequate water recharge, a reservoir with adequate permeability or faults that allow fluids to rise close to the surface, and an impermeable caprock to prevent the escape of the heat. In addition, such reservoirs must be economically accessible (that is, within the range of drills).
The heated fluid from a geothermal resource is tapped by drilling wells, sometimes as deep as 9,100 metres (about 30,000 feet), and is extracted by pumping or by natural artesian flow (where the weight of the water forces it to the surface). Water and steam are then piped to the power plant to generate electricity or through insulated pipelines—which may be buried or placed aboveground—for use in heating and cooling applications. In general, electric power plant pipelines are limited to roughly 1.6 km (1 mile) in length to minimize heat loss in the steam. However, direct-use pipelines spanning several tens of kilometres have been installed with a temperature loss of less than 2–5 °C (3.6–9 °F), depending on the flow rate. The most economically efficient facilities are located close to the geothermal resource to minimize the expense of constructing long pipelines. In the case of electric power generation, costs can be kept down by locating the facility near electrical transmission lines to transmit the electricity to market.
Exhaustion
Geothermal resources can be exhausted if the rate of heat extraction exceeds the rate of natural heat recharge. Normally, geothermal resources can be used for 20 to 30 years; however, the energy output may decrease with time, making continued development uneconomical. On the other hand, geothermal electric power has been produced continually from the Larderello geothermal field since the early 1900s and at the Geysers since 1960. Although there has been a decline in both of those fields, this problem has been partially overcome by drilling new wells and by recharging the water supply. At the Geysers, electrical capacity declined from 1,800 MW to approximately 1,000 MW, but about 200 MW of capacity was returned by placing the field under one operator and constructing pipelines to deliver wastewater for recharging the reservoir. Projects such as the Reykjavík district heating system have been operating since the 1930s with little change in the output, and the Oregon Institute of Technology geothermal heating system has been operating since the 1950s with no change in production. Thus, with proper management, geothermal resources can be sustainable for many years, and they can even recover if use is suspended for a period of time.
Environmental effects and economic costs
The environmental effects of geothermal development and power generation include the changes in land use associated with exploration and plant construction, noise and sight pollution, the discharge of water and gases, the production of foul odours, and soil subsidence. Most of those effects, however, can be mitigated with current technology so that geothermal uses have no more than a minimal impact on the environment. For example, Klamath Falls, Oregon, has approximately 600 geothermal wells for residential space heating. The city has also invested in a district heating system and a downtown snow-melting system, and it provides heating to local businesses. However, none of the systems used to supply and deliver geothermal energy are visible in town.
In addition, GHPs have a very minimal effect on the environment, because they make use of shallow geothermal resources within 100 metres (about 330 feet) of the surface. GHPs cause only small temperature changes to the groundwater or rocks and soil in the ground. In closed-loop systems the ground temperature around the vertical boreholes is slightly increased or decreased; the direction of the temperature change is governed by whether the system is dominated by heating (which would be the case in colder regions) or cooling (which would be the case in warmer regions). With balanced heating and cooling loads, the ground temperatures will remain stable. Likewise, open-loop systems using groundwater or lake water would have very little effect on temperature, especially in regions characterized by high groundwater flows.
Energy & Fossil Fuels
From fossil fuels and solar power to Thomas Edison and Nicola Tesla’s electric marvels, the world runs on energy. Harness your natural resources and test your knowledge of energy in this quiz.
Comparing the benefits of geothermal energy with other renewable energy sources, the main advantage of geothermal energy is that its base load is available 24 hours per day, 7 days per week, whereas solar and wind are available only about one-third of the time. In addition, the cost of geothermal energy varies between 5 and 10 cents per kilowatt-hour, which can be competitive with other energy sources, such as coal. The main disadvantage of geothermal energy development is the high initial investment cost in constructing the facilities and infrastructure and the high risk of proving the resources. (Geothermal resources in low-permeability rocks are often found, and exploration activities often drill “dry” holes—that is, holes that produce steam in amounts too low to be exploited economically.) However, once the resource is proven, the annual cost of fuel (that is, hot water and steam) is low and tends not to escalate in price.
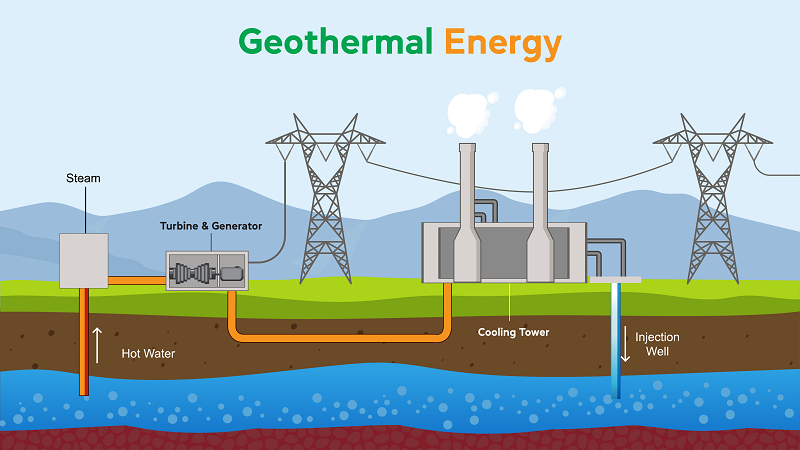
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1237 2021-12-29 00:06:25
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,148
Re: Miscellany
1213) Cochlea
The cochlea is the part of the inner ear involved in hearing. It is a spiral-shaped cavity in the bony labyrinth, in humans making 2.75 turns around its axis, the modiolus. A core component of the cochlea is the Organ of Corti, the sensory organ of hearing, which is distributed along the partition separating the fluid chambers in the coiled tapered tube of the cochlea.
The name cochlea derives from Ancient Greek 'spiral, snail shell'.
Structure
The cochlea (plural is cochleae) is a spiraled, hollow, conical chamber of bone, in which waves propagate from the base (near the middle ear and the oval window) to the apex (the top or center of the spiral). The spiral canal of the cochlea is a section of the bony labyrinth of the inner ear that is approximately 30 mm long and makes 2¾ turns about the modiolus. The cochlear structures include:
Three scalae or chambers:
(a) * the vestibular duct or scala vestibuli (containing perilymph), which lies superior to the cochlear duct and abuts the oval window
(b) * the tympanic duct or scala tympani (containing perilymph), which lies inferior to the cochlear duct and terminates at the round window
(c) * the cochlear duct or scala media (containing endolymph) a region of high potassium ion concentration that the stereocilia of the hair cells project into
* The helicotrema, the location where the tympanic duct and the vestibular duct merge, at the apex of the cochlea
* Reissner's membrane, which separates the vestibular duct from the cochlear duct
* The osseous spiral lamina, a main structural element that separates the cochlear duct from the tympanic duct
* The basilar membrane, a main structural element that separates the cochlear duct from the tympanic duct and determines the mechanical wave propagation properties of the cochlear partition
* The Organ of Corti, the sensory epithelium, a cellular layer on the basilar membrane, in which sensory hair cells are powered by the potential difference between the perilymph and the endolymph
* hair cells, sensory cells in the Organ of Corti, topped with hair-like structures called stereocilia
* The spiral ligament.
The cochlea is a portion of the inner ear that looks like a snail shell (cochlea is Greek for snail). The cochlea receives sound in the form of vibrations, which cause the stereocilia to move. The stereocilia then convert these vibrations into nerve impulses which are taken up to the brain to be interpreted. Two of the three fluid sections are canals and the third is the 'Organ of Corti' which detects pressure impulses that travel along the auditory nerve to the brain. The two canals are called the vestibular canal and the tympanic canal.
Microanatomy
The walls of the hollow cochlea are made of bone, with a thin, delicate lining of epithelial tissue. This coiled tube is divided through most of its length by an inner membranous partition. Two fluid-filled outer spaces (ducts or scalae) are formed by this dividing membrane. At the top of the snailshell-like coiling tubes, there is a reversal of the direction of the fluid, thus changing the vestibular duct to the tympanic duct. This area is called the helicotrema. This continuation at the helicotrema allows fluid being pushed into the vestibular duct by the oval window to move back out via movement in the tympanic duct and deflection of the round window; since the fluid is nearly incompressible and the bony walls are rigid, it is essential for the conserved fluid volume to exit somewhere.
The lengthwise partition that divides most of the cochlea is itself a fluid-filled tube, the third 'duct'. This central column is called the cochlear duct. Its fluid, endolymph, also contains electrolytes and proteins, but is chemically quite different from perilymph. Whereas the perilymph is rich in sodium ions, the endolymph is rich in potassium ions, which produces an ionic, electrical potential.
The hair cells are arranged in four rows in the Organ of Corti along the entire length of the cochlear coil. Three rows consist of outer hair cells (OHCs) and one row consists of inner hair cells (IHCs). The inner hair cells provide the main neural output of the cochlea. The outer hair cells, instead, mainly 'receive' neural input from the brain, which influences their motility as part of the cochlea's mechanical "pre-amplifier". The input to the OHC is from the olivary body via the medial olivocochlear bundle.
The cochlear duct is almost as complex on its own as the ear itself. The cochlear duct is bounded on three sides by the basilar membrane, the stria vascularis, and Reissner's membrane. The stria vascularis is a rich bed of capillaries and secretory cells; Reissner's membrane is a thin membrane that separates endolymph from perilymph; and the basilar membrane is a mechanically somewhat stiff membrane, supporting the receptor organ for hearing, the Organ of Corti, and determines the mechanical wave propagation properties of the cochlear system.
Function
The cochlea is filled with a watery liquid, the endolymph, which moves in response to the vibrations coming from the middle ear via the oval window. As the fluid moves, the cochlear partition (basilar membrane and organ of Corti) moves; thousands of hair cells sense the motion via their stereocilia, and convert that motion to electrical signals that are communicated via neurotransmitters to many thousands of nerve cells. These primary auditory neurons transform the signals into electrochemical impulses known as action potentials, which travel along the auditory nerve to structures in the brainstem for further processing.
Hearing
The stapes (stirrup) ossicle bone of the middle ear transmits vibrations to the fenestra ovalis (oval window) on the outside of the cochlea, which vibrates the perilymph in the vestibular duct (upper chamber of the cochlea). The ossicles are essential for efficient coupling of sound waves into the cochlea, since the cochlea environment is a fluid–membrane system, and it takes more pressure to move sound through fluid–membrane waves than it does through air. A pressure increase is achieved by reducing the area ratio from the tympanic membrane (drum) to the oval window (stapes bone) by 20. As pressure = force/area, results in a pressure gain of about 20 times from the original sound wave pressure in air. This gain is a form of impedance matching – to match the soundwave travelling through air to that travelling in the fluid–membrane system.
At the base of the cochlea, each 'duct' ends in a membranous portal that faces the middle ear cavity: The vestibular duct ends at the oval window, where the footplate of the stapes sits. The footplate vibrates when the pressure is transmitted via the ossicular chain. The wave in the perilymph moves away from the footplate and towards the helicotrema. Since those fluid waves move the cochlear partition that separates the ducts up and down, the waves have a corresponding symmetric part in perilymph of the tympanic duct, which ends at the round window, bulging out when the oval window bulges in.
The perilymph in the vestibular duct and the endolymph in the cochlear duct act mechanically as a single duct, being kept apart only by the very thin Reissner's membrane. The vibrations of the endolymph in the cochlear duct displace the basilar membrane in a pattern that peaks a distance from the oval window depending upon the soundwave frequency. The Organ of Corti vibrates due to outer hair cells further amplifying these vibrations. Inner hair cells are then displaced by the vibrations in the fluid, and depolarise by an influx of K+ via their tip-link-connected channels, and send their signals via neurotransmitter to the primary auditory neurons of the spiral ganglion.
The hair cells in the Organ of Corti are tuned to certain sound frequencies by way of their location in the cochlea, due to the degree of stiffness in the basilar membrane. This stiffness is due to, among other things, the thickness and width of the basilar membrane, which along the length of the cochlea is stiffest nearest its beginning at the oval window, where the stapes introduces the vibrations coming from the eardrum. Since its stiffness is high there, it allows only high-frequency vibrations to move the basilar membrane, and thus the hair cells. The farther a wave travels towards the cochlea's apex (the helicotrema), the less stiff the basilar membrane is; thus lower frequencies travel down the tube, and the less-stiff membrane is moved most easily by them where the reduced stiffness allows: that is, as the basilar membrane gets less and less stiff, waves slow down and it responds better to lower frequencies. In addition, in mammals, the cochlea is coiled, which has been shown to enhance low-frequency vibrations as they travel through the fluid-filled coil. This spatial arrangement of sound reception is referred to as tonotopy.
For very low frequencies (below 20 Hz), the waves propagate along the complete route of the cochlea – differentially up vestibular duct and tympanic duct all the way to the helicotrema.
Frequencies this low still activate the Organ of Corti to some extent but are too low to elicit the perception of a pitch. Higher frequencies do not propagate to the helicotrema, due to the stiffness-mediated tonotopy.
A very strong movement of the basilar membrane due to very loud noise may cause hair cells to die. This is a common cause of partial hearing loss and is the reason why users of firearms or heavy machinery often wear earmuffs or earplugs.
Hair cell amplification
Not only does the cochlea "receive" sound, a healthy cochlea generates and amplifies sound when necessary. Where the organism needs a mechanism to hear very faint sounds, the cochlea amplifies by the reverse transduction of the OHCs, converting electrical signals back to mechanical in a positive-feedback configuration. The OHCs have a protein motor called prestin on their outer membranes; it generates additional movement that couples back to the fluid–membrane wave. This "active amplifier" is essential in the ear's ability to amplify weak sounds.
The active amplifier also leads to the phenomenon of soundwave vibrations being emitted from the cochlea back into the ear canal through the middle ear (otoacoustic emissions).
Otoacoustic emissions
Otoacoustic emissions are due to a wave exiting the cochlea via the oval window, and propagating back through the middle ear to the eardrum, and out the ear canal, where it can be picked up by a microphone. Otoacoustic emissions are important in some types of tests for hearing impairment, since they are present when the cochlea is working well, and less so when it is suffering from loss of OHC activity.
Role of gap junctions
Gap-junction proteins, called connexins, expressed in the cochlea play an important role in auditory functioning. Mutations in gap-junction genes have been found to cause syndromic and nonsyndromic deafness. Certain connexins, including connexin 30 and connexin 26, are prevalent in the two distinct gap-junction systems found in the cochlea. The epithelial-cell gap-junction network couples non-sensory epithelial cells, while the connective-tissue gap-junction network couples connective-tissue cells. Gap-junction channels recycle potassium ions back to the endolymph after mechanotransduction in hair cells. Importantly, gap junction channels are found between cochlear supporting cells, but not auditory hair cells.
Clinical significance:
Hearing loss
Hearing loss is a partial or total inability to hear. Hearing loss may be present at birth or acquired at any time afterwards. Hearing loss may occur in one or both ears. In children, hearing problems can affect the ability to acquire spoken language, and in adults it can create difficulties with social interaction and at work. Hearing loss can be temporary or permanent. Hearing loss related to age usually affects both ears and is due to cochlear hair cell loss. In some people, particularly older people, hearing loss can result in loneliness. Deaf people usually have little to no hearing.
Hearing loss may be caused by a number of factors, including: genetics, ageing, exposure to noise, some infections, birth complications, trauma to the ear, and certain medications or toxins. A common condition that results in hearing loss is chronic ear infections. Certain infections during pregnancy, such as cytomegalovirus, syphilis and rubella, may also cause hearing loss in the child. Hearing loss is diagnosed when hearing testing finds that a person is unable to hear 25 decibels in at least one ear. Testing for poor hearing is recommended for all newborns. Hearing loss can be categorized as mild (25 to 40 dB), moderate (41 to 55 dB), moderate-severe (56 to 70 dB), severe (71 to 90 dB), or profound (greater than 90 dB). There are three main types of hearing loss: conductive hearing loss, sensorineural hearing loss, and mixed hearing loss.
About half of hearing loss globally is preventable through public health measures. Such practices include immunization, proper care around pregnancy, avoiding loud noise, and avoiding certain medications. The World Health Organization recommends that young people limit exposure to loud sounds and the use of personal audio players to an hour a day in an effort to limit exposure to noise. Early identification and support are particularly important in children. For many, hearing aids, sign language, cochlear implants and subtitles are useful. Lip reading is another useful skill some develop. Access to hearing aids, however, is limited in many areas of the world.
As of 2013 hearing loss affects about 1.1 billion people to some degree. It causes disability in about 466 million people (5% of the global population), and moderate to severe disability in 124 million people. Of those with moderate to severe disability 108 million live in low and middle income countries. Of those with hearing loss, it began during childhood for 65 million. Those who use sign language and are members of Deaf culture may see themselves as having a difference rather than a disability. Many members of Deaf culture oppose attempts to cure deafness and some within this community view cochlear implants with concern as they have the potential to eliminate their culture. The terms hearing impairment or hearing loss are often viewed negatively as emphasizing what people cannot do, although the terms are still regularly used when referring to deafness in medical contexts.
Bionics
In 2009, engineers at the Massachusetts Institute of Technology created an electronic chip that can quickly analyze a very large range of radio frequencies while using only a fraction of the power needed for existing technologies; its design specifically mimics a cochlea.
Other animals
The coiled form of cochlea is unique to mammals. In birds and in other non-mammalian vertebrates, the compartment containing the sensory cells for hearing is occasionally also called "cochlea," despite not being coiled up. Instead, it forms a blind-ended tube, also called the cochlear duct. This difference apparently evolved in parallel with the differences in frequency range of hearing between mammals and non-mammalian vertebrates. The superior frequency range in mammals is partly due to their unique mechanism of pre-amplification of sound by active cell-body vibrations of outer hair cells. Frequency resolution is, however, not better in mammals than in most lizards and birds, but the upper frequency limit is – sometimes much – higher. Most bird species do not hear above 4–5 kHz, the currently known maximum being ~ 11 kHz in the barn owl. Some marine mammals hear up to 200 kHz. A long coiled compartment, rather than a short and straight one, provides more space for additional octaves of hearing range, and has made possible some of the highly derived behaviors involving mammalian hearing.
As the study of the cochlea should fundamentally be focused at the level of hair cells, it is important to note the anatomical and physiological differences between the hair cells of various species. In birds, for instance, instead of outer and inner hair cells, there are tall and short hair cells. There are several similarities of note in regard to this comparative data. For one, the tall hair cell is very similar in function to that of the inner hair cell, and the short hair cell, lacking afferent auditory-nerve fiber innervation, resembles the outer hair cell. One unavoidable difference, however, is that while all hair cells are attached to a tectorial membrane in birds, only the outer hair cells are attached to the tectorial membrane in mammals.
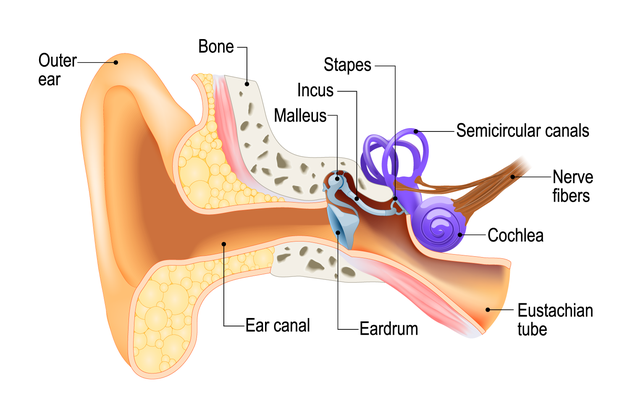
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1238 2021-12-30 00:17:48
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,148
Re: Miscellany
1214) Molecule
Summary
Molecule is a group of two or more atoms that form the smallest identifiable unit into which a pure substance can be divided and still retain the composition and chemical properties of that substance.
Characteristics of molecules
The division of a sample of a substance into progressively smaller parts produces no change in either its composition or its chemical properties until parts consisting of single molecules are reached. Further subdivision of the substance leads to still smaller parts that usually differ from the original substance in composition and always differ from it in chemical properties. In this latter stage of fragmentation the chemical bonds that hold the atoms together in the molecule are broken.
Atoms consist of a single nucleus with a positive charge surrounded by a cloud of negatively charged electrons. When atoms approach one another closely, the electron clouds interact with each other and with the nuclei. If this interaction is such that the total energy of the system is lowered, then the atoms bond together to form a molecule. Thus, from a structural point of view, a molecule consists of an aggregation of atoms held together by valence forces. Diatomic molecules contain two atoms that are chemically bonded. If the two atoms are identical, as in, for example, the oxygen molecule (O2), they compose a homonuclear diatomic molecule, while if the atoms are different, as in the carbon monoxide molecule (CO), they make up a heteronuclear diatomic molecule. Molecules containing more than two atoms are termed polyatomic molecules, e.g., carbon dioxide (CO2) and water (H2O). Polymer molecules may contain many thousands of component atoms.
Molecular bonding
The ratio of the numbers of atoms that can be bonded together to form molecules is fixed; for example, every water molecule contains two atoms of hydrogen and one atom of oxygen. It is this feature that distinguishes chemical compounds from solutions and other mechanical mixtures. Thus hydrogen and oxygen may be present in any arbitrary proportions in mechanical mixtures but when sparked will combine only in definite proportions to form the chemical compound water (H2O). It is possible for the same kinds of atoms to combine in different but definite proportions to form different molecules; for example, two atoms of hydrogen will chemically bond with one atom of oxygen to yield a water molecule, whereas two atoms of hydrogen can chemically bond with two atoms of oxygen to form a molecule of hydrogen peroxide (H2O2). Furthermore, it is possible for atoms to bond together in identical proportions to form different molecules. Such molecules are called isomers and differ only in the arrangement of the atoms within the molecules. For example, ethyl alcohol (CH3CH2OH) and methyl ether (CH3OCH3) both contain one, two, and six atoms of oxygen, carbon, and hydrogen, respectively, but these atoms are bonded in different ways.
Not all substances are made up of distinct molecular units. Sodium chloride (common table salt), for example, consists of sodium ions and chlorine ions arranged in a lattice so that each sodium ion is surrounded by six equidistant chlorine ions and each chlorine ion is surrounded by six equidistant sodium ions. The forces acting between any sodium and any adjacent chlorine ion are equal. Hence, no distinct aggregate identifiable as a molecule of sodium chloride exists. Consequently, in sodium chloride and in all solids of similar type, the concept of the chemical molecule has no significance. Therefore, the formula for such a compound is given as the simplest ratio of the atoms, called a formula unit—in the case of sodium chloride, NaCl.
Molecules are held together by shared electron pairs, or covalent bonds. Such bonds are directional, meaning that the atoms adopt specific positions relative to one another so as to maximize the bond strengths. As a result, each molecule has a definite, fairly rigid structure, or spatial distribution of its atoms. Structural chemistry is concerned with valence, which determines how atoms combine in definite ratios and how this is related to the bond directions and bond lengths. The properties of molecules correlate with their structures; for example, the water molecule is bent structurally and therefore has a dipole moment, whereas the carbon dioxide molecule is linear and has no dipole moment. The elucidation of the manner in which atoms are reorganized in the course of chemical reactions is important. In some molecules the structure may not be rigid; for example, in ethane (H3CCH3) there is virtually free rotation about the carbon-carbon single bond.
Ionic bonding in sodium chloride. An atom of sodium (Na) donates one of its electrons to an atom of chlorine (Cl) in a chemical reaction, and the resulting positive ion (Na+) and negative ion (Cl−) form a stable ionic compound (sodium chloride; common table salt) based on this ionic bond.
The nuclear positions in a molecule are determined either from microwave vibration-rotation spectra or by neutron diffraction. The electron cloud surrounding the nuclei in a molecule can be studied by X-ray diffraction experiments. Further information can be obtained by electron spin resonance or nuclear magnetic resonance techniques. Advances in electron microscopy have enabled visual images of individual molecules and atoms to be produced.
Theoretically the molecular structure is determined by solving the quantum mechanical equation for the motion of the electrons in the field of the nuclei (called the Schrödinger equation). In a molecular structure the bond lengths and bond angles are those for which the molecular energy is the least. The determination of structures by numerical solution of the Schrödinger equation has become a highly developed process entailing use of computers and supercomputers.
Polar and nonpolar molecules
If a molecule has no net electrical charge, its negative charge is equal to its positive charge. The forces experienced by such molecules depend on how the positive and negative charges are arranged in space. If the arrangement is spherically symmetric, the molecule is said to be nonpolar. If there is an excess of positive charge on one end of the molecule and an excess of negative charge on the other, the molecule has a dipole moment (i.e., a measurable tendency to rotate in an electric or magnetic field) and is therefore called polar. When polar molecules are free to rotate, they tend to favour those orientations that lead to attractive forces.
Nonpolar molecules generally are considered lipophilic (lipid-loving), whereas polar chemicals are hydrophilic (water-loving). Lipid-soluble, nonpolar molecules pass readily through a cell membrane because they dissolve in the hydrophobic, nonpolar portion of the lipid bilayer. Although permeable to water (a polar molecule), the nonpolar lipid bilayer of cell membranes is impermeable to many other polar molecules, such as charged ions or those that contain many polar side chains. Polar molecules pass through lipid membranes via specific transport systems.
Molecular weight
The molecular weight of a molecule is the sum of the atomic weights of its component atoms. If a substance has molecular weight M, then M grams of the substance is termed one mole. The number of molecules in one mole is the same for all substances; this number is known as Avogadro’s number (6.022140857 × {10}^{23}). Molecular weights can be determined by mass spectrometry and by techniques based on thermodynamics or kinetic transport phenomena.
Details
A molecule is an electrically neutral group of two or more atoms held together by chemical bonds. Molecules are distinguished from ions by their lack of electrical charge.
In quantum physics, organic chemistry, and biochemistry, the distinction from ions is dropped and molecule is often used when referring to polyatomic ions.
In the kinetic theory of gases, the term molecule is often used for any gaseous particle regardless of its composition. This relaxes the requirement that a molecule contains two or more atoms, since the noble gases are individual atoms.
A molecule may be homonuclear, that is, it consists of atoms of one chemical element, e.g. two atoms in the oxygen molecule (O2); or it may be heteronuclear, a chemical compound composed of more than one element, e.g. water (two hydrogen atoms and one oxygen atom; H2O).
Atoms and complexes connected by non-covalent interactions, such as hydrogen bonds or ionic bonds, are typically not considered single molecules.
Molecules as components of matter are common. They also make up most of the oceans and atmosphere. Most organic substances are molecules. The substances of life are molecules, e.g. proteins, the amino acids of which they are composed, the nucleic acids (DNA and RNA), sugars, carbohydrates, fats, and vitamins. The nutrient minerals are generally ionic compounds, thus they are not molecules, e.g. iron sulfate.
However, the majority of familiar solid substances on Earth are made partly or completely of crystals or ionic compounds, which are not made of molecules. These include all of the minerals that make up the substance of the Earth, sand, clay, pebbles, rocks, boulders, bedrock, the molten interior, and the core of the Earth. All of these contain many chemical bonds, but are not made of identifiable molecules.
No typical molecule can be defined for salts nor for covalent crystals, although these are often composed of repeating unit cells that extend either in a plane, e.g. graphene; or three-dimensionally e.g. diamond, quartz, sodium chloride. The theme of repeated unit-cellular-structure also holds for most metals which are condensed phases with metallic bonding. Thus solid metals are not made of molecules.
In glasses, which are solids that exist in a vitreous disordered state, the atoms are held together by chemical bonds with no presence of any definable molecule, nor any of the regularity of repeating unit-cellular-structure that characterizes salts, covalent crystals, and metals.
Molecular science
The science of molecules is called molecular chemistry or molecular physics, depending on whether the focus is on chemistry or physics. Molecular chemistry deals with the laws governing the interaction between molecules that results in the formation and breakage of chemical bonds, while molecular physics deals with the laws governing their structure and properties. In practice, however, this distinction is vague. In molecular sciences, a molecule consists of a stable system (bound state) composed of two or more atoms. Polyatomic ions may sometimes be usefully thought of as electrically charged molecules. The term unstable molecule is used for very reactive species, i.e., short-lived assemblies (resonances) of electrons and nuclei, such as radicals, molecular ions, Rydberg molecules, transition states, van der Waals complexes, or systems of colliding atoms as in Bose–Einstein condensate.
History and etymology
According to Merriam-Webster and the Online Etymology Dictionary, the word "molecule" derives from the Latin "moles" or small unit of mass.
Molecule (1794) – "extremely minute particle", from French molécule (1678), from New Latin molecula, diminutive of Latin moles "mass, barrier". A vague meaning at first; the vogue for the word (used until the late 18th century only in Latin form) can be traced to the philosophy of Descartes.
The definition of the molecule has evolved as knowledge of the structure of molecules has increased. Earlier definitions were less precise, defining molecules as the smallest particles of pure chemical substances that still retain their composition and chemical properties. This definition often breaks down since many substances in ordinary experience, such as rocks, salts, and metals, are composed of large crystalline networks of chemically bonded atoms or ions, but are not made of discrete molecules.
Bonding
Molecules are generally held together by covalent bonding. Several non-metallic elements exist only as molecules in the environment either in compounds or as homonuclear molecules, not as free atoms: for example, hydrogen.
While some people say a metallic crystal can be considered a single giant molecule held together by metallic bonding, others point out that metals behave very differently than molecules.
Covalent
A covalent bond is a chemical bond that involves the sharing of electron pairs between atoms. These electron pairs are termed shared pairs or bonding pairs, and the stable balance of attractive and repulsive forces between atoms, when they share electrons, is termed covalent bonding.
Ionic
Sodium and fluorine undergoing a redox reaction to form sodium fluoride. Sodium loses its outer electron to give it a stable electron configuration, and this electron enters the fluorine atom exothermically.
Ionic bonding is a type of chemical bond that involves the electrostatic attraction between oppositely charged ions, and is the primary interaction occurring in ionic compounds. The ions are atoms that have lost one or more electrons (termed cations) and atoms that have gained one or more electrons (termed anions). This transfer of electrons is termed electrovalence in contrast to covalence. In the simplest case, the cation is a metal atom and the anion is a nonmetal atom, but these ions can be of a more complicated nature, e.g. molecular ions like NH4+ or SO42−. At normal temperatures and pressures, ionic bonding mostly creates solids (or occasionally liquids) without separate identifiable molecules, but the vaporization/sublimation of such materials does produce separate molecules where electrons are still transferred fully enough for the bonds to be considered ionic rather than covalent.
Molecular size
Most molecules are far too small to be seen with the naked eye, although molecules of many polymers can reach macroscopic sizes, including biopolymers such as DNA. Molecules commonly used as building blocks for organic synthesis have a dimension of a few angstroms (Å) to several dozen Å, or around one billionth of a meter. Single molecules cannot usually be observed by light (as noted above), but small molecules and even the outlines of individual atoms may be traced in some circumstances by use of an atomic force microscope. Some of the largest molecules are macromolecules or supermolecules.
The smallest molecule is the diatomic hydrogen (H2), with a bond length of 0.74 Å.
Effective molecular radius is the size a molecule displays in solution.
Molecular formulas:
Chemical formula types
The chemical formula for a molecule uses one line of chemical element symbols, numbers, and sometimes also other symbols, such as parentheses, dashes, brackets, and plus (+) and minus (−) signs. These are limited to one typographic line of symbols, which may include subscripts and superscripts.
A compound's empirical formula is a very simple type of chemical formula. It is the simplest integer ratio of the chemical elements that constitute it. For example, water is always composed of a 2:1 ratio of hydrogen to oxygen atoms, and ethanol (ethyl alcohol) is always composed of carbon, hydrogen, and oxygen in a 2:6:1 ratio. However, this does not determine the kind of molecule uniquely – dimethyl ether has the same ratios as ethanol, for instance. Molecules with the same atoms in different arrangements are called isomers. Also carbohydrates, for example, have the same ratio (carbon:hydrogen:oxygen= 1:2:1) (and thus the same empirical formula) but different total numbers of atoms in the molecule.
The molecular formula reflects the exact number of atoms that compose the molecule and so characterizes different molecules. However different isomers can have the same atomic composition while being different molecules.
The empirical formula is often the same as the molecular formula but not always. For example, the molecule acetylene has molecular formula C2H2, but the simplest integer ratio of elements is CH.
The molecular mass can be calculated from the chemical formula and is expressed in conventional atomic mass units equal to 1/12 of the mass of a neutral carbon-12 (12C isotope) atom. For network solids, the term formula unit is used in stoichiometric calculations.
Structural formula
For molecules with a complicated 3-dimensional structure, especially involving atoms bonded to four different substituents, a simple molecular formula or even semi-structural chemical formula may not be enough to completely specify the molecule. In this case, a graphical type of formula called a structural formula may be needed. Structural formulas may in turn be represented with a one-dimensional chemical name, but such chemical nomenclature requires many words and terms which are not part of chemical formulas.
Molecular geometry
Molecules have fixed equilibrium geometries—bond lengths and angles— about which they continuously oscillate through vibrational and rotational motions. A pure substance is composed of molecules with the same average geometrical structure. The chemical formula and the structure of a molecule are the two important factors that determine its properties, particularly its reactivity. Isomers share a chemical formula but normally have very different properties because of their different structures. Stereoisomers, a particular type of isomer, may have very similar physico-chemical properties and at the same time different biochemical activities.
Molecular spectroscopy
Molecular spectroscopy deals with the response (spectrum) of molecules interacting with probing signals of known energy (or frequency, according to Planck's formula). Molecules have quantized energy levels that can be analyzed by detecting the molecule's energy exchange through absorbance or emission. Spectroscopy does not generally refer to diffraction studies where particles such as neutrons, electrons, or high energy X-rays interact with a regular arrangement of molecules (as in a crystal).
Microwave spectroscopy commonly measures changes in the rotation of molecules, and can be used to identify molecules in outer space. Infrared spectroscopy measures the vibration of molecules, including stretching, bending or twisting motions. It is commonly used to identify the kinds of bonds or functional groups in molecules. Changes in the arrangements of electrons yield absorption or emission lines in ultraviolet, visible or near infrared light, and result in colour. Nuclear resonance spectroscopy measures the environment of particular nuclei in the molecule, and can be used to characterise the numbers of atoms in different positions in a molecule.
Theoretical aspects
The study of molecules by molecular physics and theoretical chemistry is largely based on quantum mechanics and is essential for the understanding of the chemical bond. The simplest of molecules is the hydrogen molecule-ion, H2+, and the simplest of all the chemical bonds is the one-electron bond. H2+ is composed of two positively charged protons and one negatively charged electron, which means that the Schrödinger equation for the system can be solved more easily due to the lack of electron–electron repulsion. With the development of fast digital computers, approximate solutions for more complicated molecules became possible and are one of the main aspects of computational chemistry.
When trying to define rigorously whether an arrangement of atoms is sufficiently stable to be considered a molecule, IUPAC suggests that it "must correspond to a depression on the potential energy surface that is deep enough to confine at least one vibrational state". This definition does not depend on the nature of the interaction between the atoms, but only on the strength of the interaction. In fact, it includes weakly bound species that would not traditionally be considered molecules, such as the helium dimer, He2, which has one vibrational bound state[26] and is so loosely bound that it is only likely to be observed at very low temperatures.
Whether or not an arrangement of atoms is sufficiently stable to be considered a molecule is inherently an operational definition. Philosophically, therefore, a molecule is not a fundamental entity (in contrast, for instance, to an elementary particle); rather, the concept of a molecule is the chemist's way of making a useful statement about the strengths of atomic-scale interactions in the world that we observe.
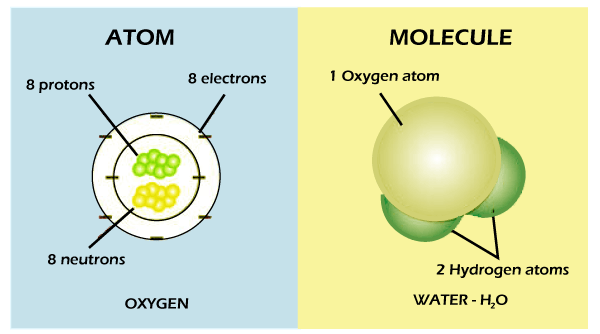
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1239 2021-12-30 22:10:45
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,148
Re: Miscellany
1215) Sulfuric Acid
Summary
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid (Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen and hydrogen, with the molecular formula H2SO4. It is a colorless, odorless and viscous liquid that is miscible with water.
Pure sulfuric acid does not exist naturally on Earth due to its strong affinity to water vapor; for this reason, it is hygroscopic and readily absorbs water vapor from the air. Concentrated sulfuric acid is highly corrosive towards other materials, from rocks to metals, since it is an oxidant with powerful dehydrating properties. Phosphorus pentoxide is a notable exception of not being affected by the acid's dehydrating property, which reversely dehydrates sulfuric acid to sulfur trioxide. Upon addition of sulfuric acid to water, a considerable amount of heat is released; thus the reverse procedure of adding water to the acid should not be performed since the heat released may boil the solution, spraying droplets of hot acid during the process. Upon contact with body tissue, sulfuric acid can cause severe acidic chemical burns and even secondary thermal burns due to dehydration. Dilute sulfuric acid is substantially less hazardous without the oxidative and dehydrating properties; however, it should still be handled with care for its acidity.
Sulfuric acid is a very important commodity chemical, and a nation's sulfuric acid production is a good indicator of its industrial strength. It is widely produced with different methods, such as contact process, wet sulfuric acid process, lead chamber process and some other methods. Sulfuric acid is also a key substance in the chemical industry. It is most commonly used in fertilizer manufacture,[ but is also important in mineral processing, oil refining, wastewater processing, and chemical synthesis. It has a wide range of end applications including in domestic acidic drain cleaners, as an electrolyte in lead-acid batteries, in dehydrating a compound, and in various cleaning agents. Sulfuric acid can be obtained by dissolving sulfur trioxide in water.
Summary : II
Sulfuric acid, sulfuric also spelled sulphuric (H2SO4), also called oil of vitriol, or hydrogen sulfate, dense, colourless, oily, corrosive liquid; it s one of the most commercially important of all chemicals. Sulfuric acid is prepared industrially by the reaction of water with sulfur trioxide, which in turn is made by chemical combination of sulfur dioxide and oxygen either by the contact process or the chamber process. In various concentrations the acid is used in the manufacture of fertilizers, pigments, dyes, drugs, explosives, detergents, and inorganic salts and acids, as well as in petroleum refining and metallurgical processes. In one of its most familiar applications, sulfuric acid serves as the electrolyte in lead–acid storage batteries.
Pure sulfuric acid has a specific gravity of 1.830 at 25 °C (77 °F); it freezes at 10.37 °C (50.7 °F). When heated, the pure acid partially decomposes into water and sulfur trioxide; the latter escapes as a vapour until the concentration of the acid falls to 98.3 percent. This mixture of sulfuric acid and water boils at a constant temperature of 338 °C (640 °F) at one atmosphere pressure. Sulfuric acid is commonly supplied at concentrations of 78, 93, or 98 percent.
Due to its affinity for water, pure anhydrous sulfuric acid does not exist in nature. Volcanic activity can result in the production of sulfuric acid, depending on the emissions associated with specific volcanoes, and sulfuric acid aerosols from an eruption can persist in the stratosphere for many years. These aerosols can then reform into sulfur dioxide (SO2), a constituent of acid rain, though volcanic activity is a relatively minor contributor to acid rainfall.
Sulfuric acid is a very strong acid; in aqueous solutions it ionizes completely to form hydronium ions and hydrogen sulfate ions. In dilute solutions the hydrogen sulfate ions also dissociate, forming more hydronium ions and sulfate ions. In addition to being an oxidizing agent, reacting readily at high temperatures with many metals, carbon, sulfur, and other substances, concentrated sulfuric acid is also a strong dehydrating agent, combining violently with water; in this capacity, it chars many organic materials, such as wood, paper, or sugar, leaving a carbonaceous residue.
The term fuming sulfuric acid, or oleum, is applied to solutions of sulfur trioxide in 100 percent sulfuric acid; these solutions, commonly containing 20, 40, or 65 percent sulfur trioxide, are used for the preparation of organic chemicals.
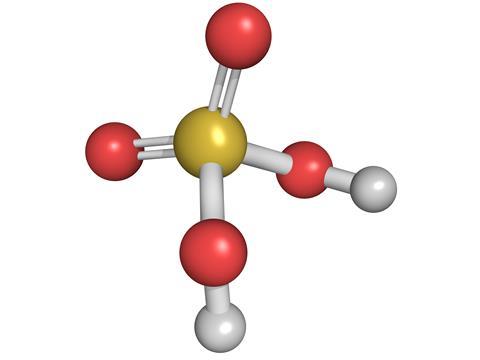
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1240 2022-01-01 01:33:27
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,148
Re: Miscellany
1216) Nitric Acid
Summary
Nitric acid (HNO3), also known as aqua fortis (Latin for "strong water") and spirit of niter, is a highly corrosive mineral acid.
The pure compound is colorless, but older samples tend to acquire a yellow cast due to decomposition into oxides of nitrogen and water. Most commercially available nitric acid has a concentration of 68% in water. When the solution contains more than 86% HNO3, it is referred to as fuming nitric acid. Depending on the amount of nitrogen dioxide present, fuming nitric acid is further characterized as red fuming nitric acid at concentrations above 86%, or white fuming nitric acid at concentrations above 95%.
Nitric acid is the primary reagent used for nitration – the addition of a nitro group, typically to an organic molecule. While some resulting nitro compounds are shock- and thermally-sensitive explosives, a few are stable enough to be used in munitions and demolition, while others are still more stable and used as pigments in inks and dyes. Nitric acid is also commonly used as a strong oxidizing agent.
Summary II
Nitric acid, (HNO3), colourless, fuming, and highly corrosive liquid (freezing point −42 °C [−44 °F], boiling point 83 °C [181 °F]) that is a common laboratory reagent and an important industrial chemical for the manufacture of fertilizers and explosives. It is toxic and can cause severe burns.
The preparation and use of nitric acid were known to the early alchemists. A common laboratory process used for many years, ascribed to a German chemist, Johann Rudolf Glauber (1648), consisted of heating potassium nitrate with concentrated sulfuric acid. In 1776 Antoine-Laurent Lavoisier showed that it contained oxygen, and in 1816 Joseph-Louis Gay-Lussac and Claude-Louis Berthollet established its chemical composition.
The principal method of manufacture of nitric acid is the catalytic oxidation of ammonia. In the method developed by the German chemist Wilhelm Ostwald in 1901, ammonia gas is successively oxidized to nitric oxide and nitrogen dioxide by air or oxygen in the presence of a platinum gauze catalyst. The nitrogen dioxide is absorbed in water to form nitric acid. The resulting acid-in-water solution (about 50–70 percent by weight acid) can be dehydrated by distillation with sulfuric acid.
Nitric acid decomposes into water, nitrogen dioxide, and oxygen, forming a brownish yellow solution. It is a strong acid, completely ionized into hydronium and nitrate ions in aqueous solution, and a powerful oxidizing agent (one that acts as electron acceptor in oxidation-reduction reactions). Among the many important reactions of nitric acid are: neutralization with ammonia to form ammonium nitrate, a major component of fertilizers; nitration of glycerol and toluene, forming the explosives nitroglycerin and trinitrotoluene (TNT), respectively; preparation of nitrocellulose; and oxidation of metals to the corresponding oxides or nitrates.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1241 2022-01-02 00:01:17
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,148
Re: Miscellany
1217) Hydrochloric acid
Summary
Hydrochloric acid is the water-based, or aqueous, solution of hydrogen chloride gas. It is also the main component of gastric acid, an acid produced naturally in the human stomach to help digest food. Hydrochloric acid is also synthetically produced for a variety of industrial and commercial applications, and can be formed by a number of manufacturing processes, including dissolving hydrogen chloride gas in water.
Uses & Benefits
Hydrochloric acid is a strong, corrosive acid that can be used industrially to process steel used in the building and construction industry. It is used in the chemical industry in the large-scale production of vinyl chloride used to make polyvinyl chloride (PVC) plastic, and it is one of the chemicals that is used to produce polyurethane foam and calcium chloride.
Hydrochloric acid is also used to make many other chemicals and as a disinfectant and slimicide, a chemical that prevents the growth of slime in paper stock.
Other common end uses for hydrochloric acid include household cleaners, pool maintenance and food manufacturing.
Steel Production
Hydrochloric acid is used in pickling operations to remove rust and other impurities from carbon, alloy and stainless steel, to prepare the steel for final applications in building and construction projects, and in products such as car bodies and household appliances. It is also used in aluminum etching and metal cleaning applications.
Household Cleaners
Hydrochloric acid can be an ingredient in household cleaners such as toilet bowl cleaners, bathroom tile cleaners and other porcelain cleaners, due to its corrosive properties that help clean tough stains.
Pool Sanitation
Hydrochloric acid is used as a swimming pool treatment chemical, to help maintain an optimal pH in the water.
Food Production and Processing
The food industry uses hydrochloric acid to process a variety of food products, such as corn syrups used in soft drinks, cookies, crackers, ketchup and cereals. Hydrochloric acid is also used as an acidifier in sauces, vegetable juices and canned goods, to help enhance flavor and reduce spoilage.
Calcium Chloride Production
When hydrochloric acid is mixed or reacted with limestone, it produces calcium chloride, a type of salt used to de-ice roads. Calcium chloride also has uses in food production as a stabilizer and firming agent, for example in baked goods, as well as uses as an antimicrobial.
Additional Uses
When hydrochloric acid is mixed or reacted with limestone, it produces calcium chloride, a type of salt used to de-ice roads. Calcium chloride also has uses in food production as a stabilizer and firming agent, for example in baked goods, as well as uses as an antimicrobial.
Hydrochloric acid is used in the production of batteries, photoflash bulbs and fireworks. It is also used in leather processing, building and construction, oil well acidizing and producing gelatin products.
Safety Information
Hydrochloric acid in its concentrated, liquid form has a strong irritating odor and is very corrosive. It can cause damage, such as chemical burns, upon contact, according to the U.S. National Library of Medicine. The U.S. Centers for Disease Control and Prevention (CDC) notes that hydrochloric acid can cause eye damage, even blindness, if splashed in the eyes.
Ingestion of concentrated hydrochloric acid can cause severe injury to the mouth, throat, esophagus and stomach. Personal protective equipment (PPE) such as vapor respirators, rubber gloves, splash goggles and face shields should be used when handling hydrochloric acid. If used in the workplace, it is recommended that an eye flush station be available in case of accidental exposure.
When using pool cleaners that contain hydrochloric acid (also known as muriatic acid), it is important to follow directions on the product label for safe handling. The CDC has developed two posters with recommendations for pool chemical safety handling as well as storage of pool chemicals for pool owners and operators.
Storing Hydrochloric Acid
Metal containers are not suitable storage containers for hydrochloric acid due to its corrosive nature. Plastic containers, such as those made of PVC, can typically be used to store hydrochloric acid.
Details
Hydrochloric acid, also known as muriatic acid, is an aqueous solution of hydrogen chloride (chemical formula: HCl). It is a colorless solution with a distinctive pungent smell. It is classified as a strong acid. It is a component of the gastric acid in the digestive systems of most animal species, including humans. Hydrochloric acid is an important laboratory reagent and industrial chemical.
Physical properties
Physical properties of hydrochloric acid, such as boiling and melting points, density, and pH, depend on the concentration or molarity of HCl in the aqueous solution. They range from those of water at very low concentrations approaching 0% HCl to values for fuming hydrochloric acid at over 40% HCl.
Production
Hydrochloric acid is usually prepared industrially by dissolving hydrogen chloride in water. Hydrogen chloride can be generated in many ways, and thus several precursors to hydrochloric acid exist. The large-scale production of hydrochloric acid is almost always integrated with the industrial scale production of other chemicals, such as in the chloralkali process which produces hydroxide, hydrogen, and chlorine, the latter of which can be combined to produce HCl.
Industrial market
Hydrochloric acid is produced in solutions up to 38% HCl (concentrated grade). Higher concentrations up to just over 40% are chemically possible, but the evaporation rate is then so high that storage and handling require extra precautions, such as pressurization and cooling. Bulk industrial-grade is therefore 30% to 35%, optimized to balance transport efficiency and product loss through evaporation. In the United States, solutions of between 20% and 32% are sold as muriatic acid. Solutions for household purposes in the US, mostly cleaning, are typically 10% to 12%, with strong recommendations to dilute before use. In the United Kingdom, where it is sold as "Spirits of Salt" for domestic cleaning, the potency is the same as the US industrial grade. In other countries, such as Italy, hydrochloric acid for domestic or industrial cleaning is sold as "Acido Muriatico", and its concentration ranges from 5% to 32%.
Major producers worldwide include Dow Chemical at 2 million tonnes annually (Mt/year), calculated as HCl gas, Georgia Gulf Corporation, Tosoh Corporation, Akzo Nobel, and Tessenderlo at 0.5 to 1.5 Mt/year each. Total world production, for comparison purposes expressed as HCl, is estimated at 20 Mt/year, with 3 Mt/year from direct synthesis, and the rest as secondary product from organic and similar syntheses. By far, most hydrochloric acid is consumed captively by the producer. The open world market size is estimated at 5 Mt/year.
Applications
Hydrochloric acid is a strong inorganic acid that is used in many industrial processes such as refining metal. The application often determines the required product quality. Hydrogen chloride, not hydrochloric acid, is used more widely in industrial organic chemistry, e.g. for vinyl chloride and dichloroethane.
Laboratory use
Of the six common strong mineral acids in chemistry, hydrochloric acid is the monoprotic acid least likely to undergo an interfering oxidation-reduction reaction. It is one of the least hazardous strong acids to handle; despite its acidity, it contains the non-reactive and non-toxic chloride ion. Intermediate-strength hydrochloric acid solutions are quite stable upon storage, maintaining their concentrations over time. These attributes, plus the fact that it is available as a pure reagent, make hydrochloric acid an excellent acidifying reagent. It is also inexpensive.
Hydrochloric acid is the preferred acid in titration for determining the amount of bases. Strong acid titrants give more precise results due to a more distinct endpoint. Azeotropic, or "constant-boiling", hydrochloric acid (roughly 20.2%) can be used as a primary standard in quantitative analysis, although its exact concentration depends on the atmospheric pressure when it is prepared.
Other
Hydrochloric acid is used for a large number of small-scale applications, such as leather processing, household cleaning, and building construction. Oil production may be stimulated by injecting hydrochloric acid into the rock formation of an oil well, dissolving a portion of the rock, and creating a large-pore structure. Oil well acidizing is a common process in the North Sea oil production industry.
Hydrochloric acid has been used for dissolving calcium carbonate, e.g. such things as de-scaling kettles and for cleaning mortar off brickwork.
Presence in living organisms
Gastric acid is one of the main secretions of the stomach. It consists mainly of hydrochloric acid and acidifies the stomach content to a pH of 1 to 2. Chloride and hydrogen ions are secreted separately in the stomach fundus region at the top of the stomach by parietal cells of the gastric mucosa into a secretory network called canaliculi before it enters the stomach lumen.
Gastric acid acts as a barrier against microorganisms to prevent infections and is important for the digestion of food. Its low pH denatures proteins and thereby makes them susceptible to degradation by digestive enzymes such as pepsin. The low pH also activates the enzyme precursor pepsinogen into the active enzyme pepsin by self-cleavage. After leaving the stomach, the hydrochloric acid of the chyme is neutralized in the duodenum by bicarbonate.
The stomach itself is protected from the strong acid by the secretion of a thick mucus layer, and by secretin induced buffering with sodium bicarbonate. Heartburn or peptic ulcers can develop when these mechanisms fail. Drugs of the antihistaminic and proton pump inhibitor classes can inhibit the production of acid in the stomach, and antacids are used to neutralize excessive existing acid.
Safety
Being a strong acid, hydrochloric acid is corrosive to living tissue and to many materials, but not to rubber. Typically, rubber protective gloves and related protective gear are used when handling concentrated solutions.
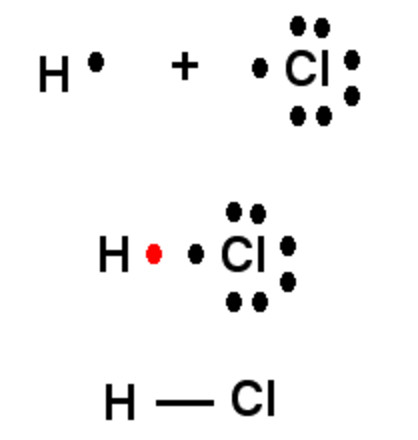
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1242 2022-01-03 01:05:47
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,148
Re: Miscellany
1218) Vertebral column
Summary
The vertebral column, also known as the backbone or spine, is part of the axial skeleton. The vertebral column is the defining characteristic of a vertebrate in which the notochord (a flexible rod of uniform composition) found in all chordates has been replaced by a segmented series of bone: vertebrae separated by intervertebral discs. The vertebral column houses the spinal canal, a cavity that encloses and protects the spinal cord.
There are about 50,000 species of animals that have a vertebral column. The human vertebral column is one of the most-studied examples.
Details
Vertebral column, also called spinal column, spine, or backbone, in vertebrate animals, is the flexible column extending from neck to tail, made of a series of bones, the vertebrae. The major function of the vertebral column is protection of the spinal cord; it also provides stiffening for the body and attachment for the pectoral and pelvic girdles and many muscles. In humans an additional function is to transmit body weight in walking and standing.
Each vertebra, in higher vertebrates, consists of a ventral body, or centrum, surmounted by a Y-shaped neural arch. The arch extends a spinous process (projection) downward and backward that may be felt as a series of bumps down the back, and two transverse processes, one to either side, which provide attachment for muscles and ligaments. Together the centrum and neural arch surround an opening, the vertebral foramen, through which the spinal cord passes. The centrums are separated by cartilaginous intervertebral disks, which help cushion shock in locomotion.
Vertebrae in lower vertebrates are more complex, and the relationships of their parts to those of higher animals are often unclear. In primitive chordates (e.g., amphioxus, lampreys) a rodlike structure, the notochord, stiffens the body and helps protect the overlying spinal cord. The notochord appears in the embryos of all vertebrates in the space later occupied by the vertebral bodies—in some fish it remains throughout life, surrounded by spool-shaped centrums; in other vertebrates it is lost in the developed animal. In primitive chordates the spinal cord is protected dorsally by segmented cartilages—these foreshadow the development of the neural arch of true vertebrae.
Fish have trunk and caudal (tail) vertebrae; in land vertebrates with legs, the vertebral column becomes further subdivided into regions in which the vertebrae have different shapes and functions. Crocodilians and lizards, birds, and mammals demonstrate five regions: (1) cervical, in the neck, (2) thoracic, in the chest, which articulates with the ribs, (3) lumbar, in the lower back, more robust than the other vertebrae, (4) sacral, often fused to form a sacrum, which articulates with the pelvic girdle, (5) caudal, in the tail. The atlas and axis vertebrae, the top two cervicals, form a freely movable joint with the skull.
The numbers of vertebrae in each region and in total vary with the species. Snakes have the greatest number, all very similar in type. In turtles some vertebrae may be fused to the shell (carapace); in birds all but the cervical vertebrae are usually fused into a rigid structure, which lends support in flight. Most mammals have seven cervical vertebrae; size rather than number account for the variations in neck length in different species. Whales show several specializations—the cervical vertebrae may be either much reduced or much increased in number, and the sacrum is missing. Humans have 7 cervical, 12 thoracic, 5 lumbar, 5 fused sacral, and 3 to 5 fused caudal vertebrae (together called the coccyx).
The vertebral column is characterized by a variable number of curves. In quadrupeds the column is curved in a single arc (the highest portion occurring at the middle of the back), which functions somewhat like a bow spring in locomotion. In humans this primary curve is modified by three more: (1) a sacral curve, in which the sacrum curves backward and helps support the abdominal organs, (2) an anterior cervical curve, which develops soon after birth as the head is raised, and (3) a lumbar curve, also anterior, which develops as the child sits and walks. The lumbar curve is a permanent characteristic only of humans and their bipedal forebears, though a temporary lumbar curve appears in other primates in the sitting position. The cervical curve disappears in humans when the head is bent forward but appears in other animals as the head is raised.
In humans the structure and function of the vertebral column can be affected by certain diseases, disorders, or injuries. Examples include scoliosis, lordosis, and kyphosis, which are deviations from the normal spinal curvature; degenerative diseases, such as osteoarthritis and Baastrup disease (kissing spine syndrome); and tuberculosis of the spine (Pott disease), which is caused by infection of the vertebral column by Mycobacterium tuberculosis.
The vertebral column is a series of approximately 33 bones called vertebrae, which are separated by intervertebral discs.
The column can be divided into five different regions, with each region characterised by a different vertebral structure.
In this article, we shall look at the anatomy of the vertebral column – its function, structure, and clinical significance.
Functions
The vertebral column has four main functions:
* Protection – encloses and protects the spinal cord within the spinal canal.
* Support – carries the weight of the body above the pelvis.
* Axis – forms the central axis of the body.
* Movement – has roles in both posture and movement.
Structure of a Vertebrae
All vertebrae share a basic common structure. They each consist of an anterior vertebral body, and a posterior vertebral arch.
Vertebral Body
The vertebral body forms the anterior part of each vertebrae.
It is the weight-bearing component, and vertebrae in the lower portion of the column have larger bodies than those in the upper portion (to better support the increased weight).
The superior and inferior aspects of the vertebral body are lined with hyaline cartilage. Adjacent vertebral bodies are separated by a fibrocartilaginous intervertebral disc.
Vertebral Arch
The vertebral arch forms the lateral and posterior aspect of each vertebrae.
In combination with the vertebral body, the vertebral arch forms an enclosed hole – the vertebral foramen. The foramina of all the vertebrae line up to form the vertebral canal, which encloses the spinal cord.
The vertebral arches have several bony prominences, which act as attachment sites for muscles and ligaments:
* Spinous processes – each vertebra has a single spinous process, centred posteriorly at the point of the arch.
* Transverse processes – each vertebra has two transverse processes, which extend laterally and posteriorly from the vertebral body. In the thoracic vertebrae, the transverse processes articulate with the ribs.
* Pedicles – connect the vertebral body to the transverse processes.
* Lamina – connect the transverse and spinous processes.
* Articular processes – form joints between one vertebra and its superior and inferior counterparts. The articular processes are located at the intersection of the laminae and pedicles.
Classifications of Vertebrae
Cervical Vertebrae
There are seven cervical vertebrae in the human body. They have three main distinguishing features:
* Bifid spinous process – the spinous process bifurcates at its distal end.
* Exceptions to this are C1 (no spinous process) and C7 (spinous process is longer than that of C2-C6 and may not bifurcate).
* Transverse foramina – an opening in each transverse process, through which the vertebral arteries travel to the brain.
* Triangular vertebral foramen
Two cervical vertebrae that are unique. C1 and C2 (called the atlas and axis respectively), are specialised to allow for the movement of the head.
Thoracic Vertebrae
The twelve thoracic vertebrae are medium-sized, and increase in size from superior to inferior. Their specialised function is to articulate with ribs, producing the bony thorax.
Each thoracic vertebra has two ‘demi facets,’ superiorly and inferiorly placed on either side of its vertebral body. The demi facets articulate with the heads of two different ribs.
On the transverse processes of the thoracic vertebrae, there is a costal facet for articulation with the shaft of a single rib. For example, the head of Rib 2 articulates with the inferior demi facet of thoracic vertebra 1 (T1) and the superior demi facet of T2, while the shaft of Rib 2 articulates with the costal facets of T2.
The spinous processes of thoracic vertebrae are oriented obliquely inferiorly and posteriorly. In contrast to the cervical vertebrae, the vertebral foramen of thoracic vertebrae is circular.
Lumbar Vertebrae
There are five lumbar vertebrae in most humans, which are the largest in the vertebral column. They are structurally specialised to support the weight of the torso.
Lumbar vertebrae have very large vertebral bodies, which are kidney shaped. They lack the characteristic features of other vertebrae, with no transverse foramina, costal facets, or bifid spinous processes.
However, like the cervical vertebrae, they have a triangular-shaped vertebral foramen. Their spinous processes are shorter than those of thoracic vertebrae and do not extend inferiorly below the level of the vertebral body.
Their size and orientation permits needle access to the spinal canal and spinal cord (which would not be possible between thoracic vertebrae). Examples include epidural anaesthesia administration and lumbar puncture.
Sacrum and Coccyx
The sacrum is a collection of five fused vertebrae. It is described as an inverted triangle, with the apex pointing inferiorly. On the lateral walls of the sacrum are facets for articulation with the pelvis at the sacroiliac joints.
The coccyx is a small bone which articulates with the apex of the sacrum. It is recognised by its lack of vertebral arches. Due to the lack of vertebral arches, there is no vertebral canal.
Separation of S1 from the sacrum is termed “lumbarisation”, while fusion of L5 to the sacrum is termed “sacralisation”. These conditions are congenital abnormalities.
Joints and Ligaments
The mobile vertebrae articulate with each other via joints between their bodies and articular facets:
* Left and right superior articular facets articulate with the vertebra above.
* Left and right inferior articular facets articulate with the vertebra below.
* Vertebral bodies indirectly articulate with each other via the intervertebral discs.
The vertebral body joints are cartilaginous joints, designed for weight-bearing. The articular surfaces are covered by hyaline cartilage, and are connected by the intervertebral disc.
Two ligaments strengthen the vertebral body joints: the anterior and posterior longitudinal ligaments, which run the full length of the vertebral column. The anterior longitudinal ligament is thick and prevents hyperextension of the vertebral column. The posterior longitudinal ligament is weaker and prevents hyperflexion.
The joints between the articular facets, called facet joints, allow for some gliding motions between the vertebrae. They are strengthened by several ligaments:
* Ligamentum flavum – extends between lamina of adjacent vertebrae.
* Interspinous and supraspinous – join the spinous processes of adjacent vertebrae. The interspinous ligaments attach between processes, and the supraspinous ligaments attach to the tips.
* Intertransverse ligaments – extends between transverse processes.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1243 2022-01-04 01:01:06
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,148
Re: Miscellany
1219) Carbonic acid
Summary
In chemistry, carbonic acid is a dibasic acid with the chemical formula H2CO3. The pure compound decomposes at temperatures greater than ca. −80 °C.
In biochemistry and physiology, the name "carbonic acid" is often applied to aqueous solutions of carbon dioxide, which play an important role in the bicarbonate buffer system, used to maintain acid–base homeostasis.
Details
Carbonic acid, (H2CO3) is a compound of the elements hydrogen, carbon, and oxygen. It is formed in small amounts when its anhydride, carbon dioxide (CO2), dissolves in water.
Carbonic acid plays a role in the assembly of caves and cave formations like stalactites and stalagmites. The largest and most common caves are those formed by dissolution of limestone or dolomite by the action of water rich in carbonic acid derived from recent rainfall. The calcite in stalactites and stalagmites is derived from the overlying limestone near the bedrock/soil interface. Rainwater infiltrating through the soil absorbs carbon dioxide from the carbon dioxide-rich soil and forms a dilute solution of carbonic acid. When this acid water reaches the base of the soil, it reacts with the calcite in the limestone bedrock and takes some of it into solution. The water continues its downward course through narrow joints and fractures in the unsaturated zone with little further chemical reaction. When the water emerges from the cave roof, carbon dioxide is lost into the cave atmosphere, and some of the calcium carbonate is precipitated. The infiltrating water acts as a calcite pump, removing it from the top of the bedrock and redepositing it in the cave below.
Carbonic acid is important in the transport of carbon dioxide in the blood. Carbon dioxide enters blood in the tissues because its local partial pressure is greater than its partial pressure in blood flowing through the tissues. As carbon dioxide enters the blood, it combines with water to form carbonic acid, which dissociates into hydrogen ions and bicarbonate ions. Blood acidity is minimally affected by the released hydrogen ions because blood proteins, especially hemoglobin, are effective buffering agents. (A buffer solution resists change in acidity by combining with added hydrogen ions and, essentially, inactivating them.) The natural conversion of carbon dioxide to carbonic acid is a relatively slow process; however, carbonic anhydrase, a protein enzyme present inside the red blood cell, catalyzes this reaction with sufficient rapidity that it is accomplished in only a fraction of a second. Because the enzyme is present only inside the red blood cell, bicarbonate accumulates to a much greater extent within the red cell than in the plasma. The capacity of blood to carry carbon dioxide as bicarbonate is enhanced by an ion transport system inside the red blood cell membrane that simultaneously moves a bicarbonate ion out of the cell and into the plasma in exchange for a chloride ion. The simultaneous exchange of these two ions, known as the chloride shift, permits the plasma to be used as a storage site for bicarbonate without changing the electrical charge of either the plasma or the red blood cell. Only 26 percent of the total carbon dioxide content of blood exists as bicarbonate inside the red blood cell, while 62 percent exists as bicarbonate in plasma; however, the bulk of bicarbonate ions is first produced inside the cell, then transported to the plasma. A reverse sequence of reactions occurs when blood reaches the lung, where the partial pressure of carbon dioxide is lower than in the blood.
Additional information
Carbonic acid is a chemical compound with the chemical formula as H2CO3 and molecular formula as CH2O3. It is an inorganic weak acid, which exists only as a solution. Carbonic acid is also known as acid of air, aerial acid or dihydrogen carbonate. It forms two kinds of salts: carbonates and bicarbonates. Ph of carbonic acid is 4.68 in 1mM.
Carbonic acid is specifically diprotic acid, which means that it has two protons which can disassociate from the parent molecule. Thus, have two disassociation constants, first for bicarbonate ion disassociation and second for disassociation of the bicarbonate ion into the carbonate ion.
Occurance
Carbonic acid is present in blood in the human body. It is formed in the human body when water gets dissolved with carbon dioxide. It is also present in rainwater, calcite, fermentation, coal, groundwater, meteors, volcanoes, amino acids, proteins, oceans, plants, erythrocytes, sulphur deposits, salts, and caves.
Properties:
Physical Properties
* Appearance: Grayish white solid.
* Melting point: 210 Celsius, boiling point: -78 degree Celsius.
* Molecular weight: 62.024 g/mol.
* Solubility: Insoluble.
* Carbonic acid has a pH value of less than 7.
* Carbonic acid is odorless and has the alkaline taste.
Chemical Properties
* Carbonic acid is weak and unstable dibasic acid.
* It has an acidity of 6.3 pK.
Carbonic Acid Uses:
The most common use of carbonic acid is in the form of salts.
In blood: Bicarbonate a form of carbonic acid salt acts as an intermediate for transporting CO2 out of the body through the respiratory gas exchange. It also plays a vital role in protonating a lot of nitrogen bases in blood serum. Carbonic acid is the main buffering element in the human body and is broken down into carbon dioxide by an enzyme called carbonic anhydrase.
In drinks: Carbonic acid is widely used in making bubbly, fizzy drinks.
For treating dermatitides: it is typically used to treat dermatitides like ringworm.
For cleaning contact lenses carbonic acid is very effective, it is also used as a gas for welding, food processing, and cosmetics.
For hydrolysis of starch also carbonic acid is used.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1244 2022-01-05 00:02:47
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,148
Re: Miscellany
1220) DDT
Summary
DDT, abbreviation of dichlorodiphenyltrichloroethane, also called 1,1,1-trichloro-2,2-bis(p-chlorophenyl)ethane, is a synthetic insecticide belonging to the family of organic halogen compounds, highly toxic toward a wide variety of insects as a contact poison that apparently exerts its effect by disorganizing the nervous system.
DDT, prepared by the reaction of chloral with chlorobenzene in the presence of sulfuric acid, was first made in 1874; its insecticidal properties were discovered in 1939 by a Swiss chemist, Paul Hermann Müller. During and after World War II, DDT was found to be effective against lice, fleas, and mosquitoes (the carriers of typhus, of plague, and of malaria and yellow fever, respectively) as well as the Colorado potato beetle, the gypsy moth, and other insects that attack valuable crops.
Many species of insects rapidly develop populations resistant to DDT; the high stability of the compound leads to its accumulation in insects that constitute the diet of other animals, with toxic effects on them, especially certain birds and fishes. These two disadvantages had severely decreased the value of DDT as an insecticide by the 1960s, and severe restrictions were imposed on its use in the United States in 1972.
Pure DDT is a colourless, crystalline solid that melts at 109° C (228° F); the commercial product, which is usually 65 to 80 percent active compound, along with related substances, is an amorphous powder that has a lower melting point. DDT is applied as a dust or by spraying its aqueous suspension.
Details
Dichlorodiphenyltrichloroethane, commonly known as DDT, is a colorless, tasteless, and almost odorless crystalline chemical compound, an organochloride. Originally developed as an insecticide, it became infamous for its environmental impacts. DDT was first synthesized in 1874 by the Austrian chemist Othmar Zeidler. DDT's insecticidal action was discovered by the Swiss chemist Paul Hermann Müller in 1939. DDT was used in the second half of World War II to limit the spread of the insect-born diseases malaria and typhus among civilians and troops. Müller was awarded the Nobel Prize in Physiology or Medicine in 1948 "for his discovery of the high efficiency of DDT as a contact poison against several arthropods".
By October 1945, DDT was available for public sale in the United States. Although it was promoted by government and industry for use as an agricultural and household pesticide, there were also concerns about its use from the beginning. Opposition to DDT was focused by the 1962 publication of Rachel Carson's book Silent Spring. It talked about environmental impacts that correlated with the widespread use of DDT in agriculture in the United States, and it questioned the logic of broadcasting potentially dangerous chemicals into the environment with little prior investigation of their environmental and health effects. The book cited claims that DDT and other pesticides caused cancer and that their agricultural use was a threat to wildlife, particularly birds. Although Carson never directly called for an outright ban on the use of DDT, its publication was a seminal event for the environmental movement and resulted in a large public outcry that eventually led, in 1972, to a ban on DDT's agricultural use in the United States.
A worldwide ban on agricultural use was formalized under the Stockholm Convention on Persistent Organic Pollutants which has been in effect since 2004. DDT still has limited use in disease vector control because of its effectiveness in killing mosquitos and thus reducing malarial infections, but that use is controversial due to environmental and health concerns.
Along with the passage of the Endangered Species Act, the United States ban on DDT is a major factor in the comeback of the bald eagle (the national bird of the United States) and the peregrine falcon from near-extinction in the contiguous United States.
Properties and chemistry
DDT is similar in structure to the insecticide methoxychlor and the acaricide dicofol. It is highly hydrophobic and nearly insoluble in water but has good solubility in most organic solvents, fats and oils. DDT does not occur naturally and is synthesised by consecutive Friedel–Crafts reactions between chloral (CCl3CHO) and two equivalents of chlorobenzene (C6H5Cl), in the presence of an acidic catalyst. DDT has been marketed under trade names including Anofex, Cezarex, Chlorophenothane, Dicophane, Dinocide, Gesarol, Guesapon, Guesarol, Gyron, Ixodex, Neocid, Neocidol and Zerdane; INN is clofenotane.
Isomers and related compounds
Commercial DDT is a mixture of several closely–related compounds. Due to the nature of the chemical reaction used to synthesize DDT, several combinations of ortho and para arene substitution patterns are formed. The major component (77%) is the desired p,p' isomer. The o,p' isomeric impurity is also present in significant amounts (15%). Dichlorodiphenyldichloroethylene (DDE) and dichlorodiphenyldichloroethane (DDD) make up the balance of impurities in commercial samples. DDE and DDD are also the major metabolites and environmental breakdown products. DDT, DDE and DDD are sometimes referred to collectively as DDX.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1245 2022-01-06 00:13:23
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,148
Re: Miscellany
1221) Cotton
Summary
Cotton is a soft, fluffy staple fiber that grows in a boll, or protective case, around the seeds of the cotton plants of the genus Gossypium in the mallow family Malvaceae. The fiber is almost pure cellulose, and can contain minor percentages of waxes, fats, pectins, and water. Under natural conditions, the cotton bolls will increase the dispersal of the seeds.
The plant is a shrub native to tropical and subtropical regions around the world, including the Americas, Africa, Egypt and India. The greatest diversity of wild cotton species is found in Mexico, followed by Australia and Africa. Cotton was independently domesticated in the Old and New Worlds.
The fiber is most often spun into yarn or thread and used to make a soft, breathable, and durable textile. The use of cotton for fabric is known to date to prehistoric times; fragments of cotton fabric dated to the fifth millennium BC have been found in the Indus Valley Civilization, as well as fabric remnants dated back to 6000 BC in Peru. Although cultivated since antiquity, it was the invention of the cotton gin that lowered the cost of production that led to its widespread use, and it is the most widely used natural fiber cloth in clothing today.
Current estimates for world production are about 25 million tonnes or 110 million bales annually, accounting for 2.5% of the world's arable land. India is the world's largest producer of cotton. The United States has been the largest exporter for many years.
Details
Cotton is seed-hair fibre of several species of plants of the genus Gossypium, belonging to the hibiscus, or mallow, family (Malvaceae).
Cotton, one of the world’s leading agricultural crops, is plentiful and economically produced, making cotton products relatively inexpensive. The fibres can be made into a wide variety of fabrics ranging from lightweight voiles and laces to heavy sailcloths and thick-piled velveteens, suitable for a great variety of wearing apparel, home furnishings, and industrial uses. Cotton fabrics can be extremely durable and resistant to abrasion. Cotton accepts many dyes, is usually washable, and can be ironed at relatively high temperatures. It is comfortable to wear because it absorbs and releases moisture quickly. When warmth is desired, it can be napped, a process giving the fabric a downy surface. Various finishing processes have been developed to make cotton resistant to stains, water, and mildew; to increase resistance to wrinkling, thus reducing or eliminating the need for ironing; and to reduce shrinkage in laundering to not more than 1 percent. Nonwoven cotton, made by fusing or bonding the fibres together, is useful for making disposable products to be used as towels, polishing cloths, tea bags, tablecloths, bandages, and disposable uniforms and sheets for hospital and other medical uses.
Cotton fibre processing
Cotton fibres may be classified roughly into three large groups, based on staple length (average length of the fibres making up a sample or bale of cotton) and appearance. The first group includes the fine, lustrous fibres with staple length ranging from about 2.5 to 6.5 cm (about 1 to 2.5 inches) and includes types of the highest quality—such as Sea Island, Egyptian, and pima cottons. Least plentiful and most difficult to grow, long-staple cottons are costly and are used mainly for fine fabrics, yarns, and hosiery. The second group contains the standard medium-staple cotton, such as American Upland, with staple length from about 1.3 to 3.3 cm (0.5 to 1.3 inches). The third group includes the short-staple, coarse cottons, ranging from about 1 to 2.5 cm (0.5 to 1 inch) in length, used to make carpets and blankets, coarse and inexpensive fabrics, and blends with other fibres.
Most of the seeds (cottonseed) are separated from the fibres by a mechanical process called ginning. Ginned cotton is shipped in bales to a textile mill for yarn manufacturing. A traditional and still common processing method is ring spinning, by which the mass of cotton may be subjected to opening and cleaning, picking, carding, combing, drawing, roving, and spinning. The cotton bale is opened, and its fibres are raked mechanically to remove foreign matter (e.g., soil and seeds). A picker (picking machine) then wraps the fibres into a lap. A card (carding) machine brushes the loose fibres into rows that are joined as a soft sheet, or web, and forms them into loose untwisted rope known as card sliver. For higher-quality yarn, card sliver is put through a combing machine, which straightens the staple further and removes unwanted short lengths, or noils. In the drawing (drafting) stage, a series of variable-speed rollers attenuates and reduces the sliver to firm uniform strands of usable size. Thinner strands are produced by the roving (slubbing) process, in which the sliver is converted to roving by being pulled and slightly twisted. Finally, the roving is transferred to a spinning frame, where it is drawn further, twisted on a ring spinner, and wound on a bobbin as yarn.
Faster production methods include rotor spinning (a type of open-end spinning), in which fibres are detached from the card sliver and twisted, within a rotor, as they are joined to the end of the yarn. For the production of cotton blends, air-jet spinning may be used; in this high-speed method, air currents wrap loose fibres around a straight sliver core. Blends (composites) are made during yarn processing by joining drawn cotton with other staple fibres, such as polyester or casein.
The procedure for weaving cotton yarn into fabric is similar to that for other fibres. Cotton looms interlace the tense lengthwise yarns, called warp, with crosswise yarns called weft, or filling. Warp yarns often are treated chemically to prevent breaking during weaving.
Cultivation of the cotton plant
The various species of cotton grown as agricultural crops are native to most subtropical parts of the world and were domesticated independently multiple times. Cotton can be found as perennial treelike plants in tropical climates but is normally cultivated as a shrubby annual in temperate climates. Whereas it grows up to 6 metres (20 feet) high in the tropics, it characteristically ranges from 1 to 2 metres (3 to 6.5 feet) in height under cultivation. Within 80–100 days after planting, the plant develops white blossoms, which change to a reddish colour. The fertilized blossoms fall off after a few days and are replaced by small green triangular pods, called bolls, that mature after a period of 55–80 days. During this period the seeds and their attached hairs develop within the boll, which increases considerably in size. The seed hair, or cotton fibre, reaching a maximum length of about 6 cm (2.5 inches) in long-fibre varieties, is known as lint. Linters, fibres considerably shorter than the seed hair and more closely connected to the seed, come from a second growth beginning about 10 days after the first seed hairs begin to develop. When ripe, the boll bursts into a white, fluffy ball containing three to five cells, each having 7 to 10 seeds embedded in a mass of seed fibres. Two-thirds of the weight of the seed cotton (i.e., the seed with the adhering seed hair) consists of the seeds. The fibres are composed of about 87 to 90 percent cellulose (a carbohydrate plant substance), 5 to 8 percent water, and 4 to 6 percent natural impurities.
Although cotton can be grown between latitudes 30° N and 30° S, yield and fibre quality are considerably influenced by climatic conditions, and best qualities are obtained with high moisture levels resulting from rainfall or irrigation during the growing season and a dry, warm season during the picking period.
To avoid damage to the cotton by wind or rain, it is picked as soon as the bolls open, but since the bolls do not all reach maturity simultaneously, an optimum time is chosen for harvesting by mechanical means. Handpicking, carried out over a period of several days, allows selection of the mature and opened bolls, so that a higher yield is possible. Handpicking also produces considerably cleaner cotton; mechanical harvesters pick the bolls by suction, accumulating loose material, dust, and dirt, and cannot distinguish between good and discoloured cotton. A chemical defoliant is usually applied before mechanical picking to cause the plants to shed their leaves, thus encouraging more uniform ripening of the bolls.
Pests and diseases
Cotton is attacked by several hundred species of insects, including such harmful species as the boll weevil, pink bollworm, cotton leafworm, cotton fleahopper, cotton aphid, rapid plant bug, conchuela, southern green stinkbug, spider mites (red spiders), grasshoppers, thrips, and tarnished plant bugs. Limited control of damage by insect pests can be achieved by proper timing of planting and other cultural practices or by selective breeding of varieties having some resistance to insect damage. Chemical insecticides, which were first introduced in the early 1900s, require careful and selective use because of ecological considerations but appear to be the most effective and efficient means of control. Conventional cotton production requires more insecticides than any other major crop, and the production of organic cotton, which relies on nonsynthetic insecticides, has been increasing in many places worldwide. Additionally, genetically modified “Bt cotton” was developed to produce bacterial proteins that are toxic to herbivorous insects, ostensibly reducing the amount of pesticides needed. Glyphosate-resistant cotton, which can tolerate the herbicide glyphosate, was also developed through genetic engineering.
The boll weevil (Anthonomus grandis), the most serious cotton pest in the United States in the early 1900s, was finally controlled by appropriate cultivation methods and by the application of such insecticides as chlorinated hydrocarbons and organophosphates. A species of boll weevil resistant to chlorinated hydrocarbons was recorded in the late 1950s; this species is combatted effectively with a mixture of toxaphene and DDT (dichlorodiphenyltrichloroethane), which has been outlawed in the United States and some other countries, however. The pink bollworm (Pectinophora gossypiella), originally reported in India in 1842, has spread throughout the cotton-producing countries, causing average annual crop losses of up to 25 percent in, for example, India, Egypt, China, and Brazil. Controls and quarantines of affected areas have helped limit the spread of the insect, and eradication has been possible in a few relatively small areas with sufficiently strict controls. The bollworm (Heliothis zea, also known as the corn earworm) feeds on cotton and many other wild and cultivated plants. Properly timed insecticide application provides fairly effective control.
Cotton plants are subject to diseases caused by various pathogenic fungi, bacteria, and viruses and to damage by nematodes (parasitic worms) and physiological disturbances also classified as diseases. Losses have been estimated as high as 50 percent in some African countries and in Brazil. Because young seedlings are especially sensitive to attack by a complex of disease organisms, treatment of seeds before planting is common. Some varieties have been bred that are resistant to a bacterial disease called angular leaf spot. Soil fumigation moderately succeeded in combatting such fungus diseases as fusarium wilt, verticillium wilt, and Texas root rot, which are restricted to certain conditions of soil, rainfall, and general climate. The breeding of resistant varieties, however, has been more effective.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1246 2022-01-07 00:55:02
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,148
Re: Miscellany
1222) Biopsy
Summary
A biopsy is a medical test commonly performed by a surgeon, interventional radiologist, or an interventional cardiologist. The process involves extraction of sample cells or tissues for examination to determine the presence or extent of a disease. The tissue is generally examined under a microscope by a pathologist; it may also be analyzed chemically. When an entire lump or suspicious area is removed, the procedure is called an excisional biopsy. An incisional biopsy or core biopsy samples a portion of the abnormal tissue without attempting to remove the entire lesion or tumor. When a sample of tissue or fluid is removed with a needle in such a way that cells are removed without preserving the histological architecture of the tissue cells, the procedure is called a needle aspiration biopsy. Biopsies are most commonly performed for insight into possible cancerous or inflammatory conditions.
Details
What Is a Biopsy?
A biopsy is a sample of tissue taken from the body in order to examine it more closely. A doctor should recommend a biopsy when an initial test suggests an area of tissue in the body isn't normal.
Doctors may call an area of abnormal tissue a lesion, a tumor, or a mass. These are general words used to emphasize the unknown nature of the tissue. The suspicious area may be noticed during a physical examination or internally on an imaging test.
Why Are Biopsies Done?
Biopsies are most often done to look for cancer. But biopsies can help identify many other conditions.
A biopsy might be recommended whenever there is an important medical question the biopsy could help answer. Here are just a few examples:
* A mammogram shows a lump or mass, indicating the possibility of breast cancer.
* A mole on the skin has changed shape recently and melanoma is possible.
* A person has chronic hepatitis and it's important to know if cirrhosis is present.
In some cases, a biopsy of normal-appearing tissue may be done. This can help check for cancer spread or rejection of a transplanted organ.
In most cases, a biopsy is done to diagnose a problem or to help determine the best therapy option.
Types of Biopsies
There are many different kinds of biopsies. Nearly all of them involve using a sharp tool to remove a small amount of tissue. If the biopsy will be on the skin or other sensitive area, numbing medicine is applied first.
Here are some types of biopsies:
* Needle biopsy. Most biopsies are needle biopsies, meaning a needle is used to access the suspicious tissue.
* CT-guided biopsy. A person rests in a CT-scanner; the scanner's images help doctors determine the exact position of the needle in the targeted tissue.
* Ultrasound-guided biopsy. An ultrasound scanner helps a doctor direct the needle into the lesion.
* Bone biopsy. A bone biopsy is used to look for cancer of the bones. This may be performed via the CT scan technique or by an orthopedic surgeon.
* Bone marrow biopsy. A large needle is used to enter the pelvis bone to collect bone marrow. This detects blood diseases such as leukemia or lymphoma.
* Liver biopsy. A needle is injected into the liver through the skin on the belly, capturing liver tissue.
* Kidney biopsy. Similar to a liver biopsy, a needle is injected through the skin on the back, into the kidney.
* Aspiration biopsy. A needle withdraws material out of a mass. This simple procedure is also called fine-needle aspiration.
* Prostate biopsy. Multiple needle biopsies are taken at one time from the prostate gland. To reach the prostate, a probe is inserted into the rectum.
* Skin biopsy. A punch biopsy is the main biopsy method. It uses a circular blade to get a cylindrical sample of skin tissue.
* Surgical biopsy. Either open or laparoscopic surgery may be necessary to obtain a biopsy of hard-to-reach tissue. Either a piece of tissue or the whole lump of tissue may be removed.
What to Expect From Your Biopsy
Biopsies vary greatly according to how difficult the tissue is to obtain. The medical term for this is "invasiveness."
A minimally invasive biopsy (for example, most skin biopsies) may be done in the doctor's office during the same visit the lesion is discovered. A small injection of numbing medicine can make the procedure almost painless.
More invasive biopsies may be done in a hospital, a surgery center, or a specialized doctor's office. You would make a separate appointment for the biopsy. In most cases, sedating and pain relief medicines are given, reducing any discomfort. You likely won't be able to drive after receiving these medicines.
You may feel sore at the area of the biopsy for a few days. Your doctor can prescribe appropriate pain relief medicines if you have significant pain from the biopsy.
What Happens After the Biopsy?
After the tissue is collected and preserved, it's delivered to a pathologist. Pathologists are doctors who specialize in diagnosing conditions based on tissue samples and other tests. (In some cases, the doctor collecting the sample can diagnose the condition.)
A pathologist examines the biopsy tissue under a microscope. By noting the tissue cells' type, shape, and internal activity, in most cases a pathologist can diagnose the problem.
The time it takes to get results from a biopsy can vary. During a surgery, a pathologist may read a biopsy and report back to a surgeon in a few minutes. Final, highly accurate conclusions on biopsies often take a week or longer. You will probably follow up with your regular doctor to discuss the biopsy results.
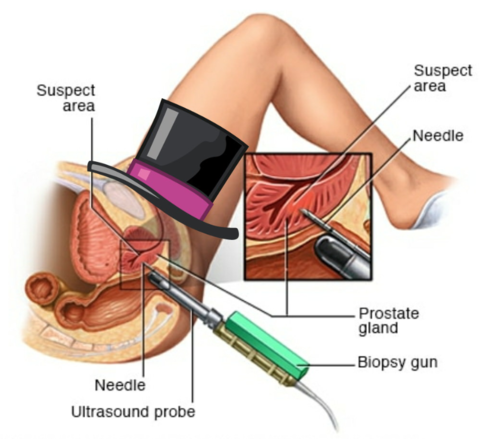
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1247 2022-01-08 00:08:47
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,148
Re: Miscellany
1223) Taproot
Summary
Taproot is a main root of a primary root system, growing vertically downward. Most dicotyledonous plants (see cotyledon), such as dandelions, produce taproots, and some, such as the edible roots of carrots and beets, are specialized for food storage.
Upon germination, the first structure to emerge from most seeds is the root from the embryonic radicle. This primary root is a taproot. In plants in which the taproot persists, smaller lateral roots (secondary roots) commonly arise from the taproot and may in turn produce even smaller lateral roots (tertiary roots). This serves to increase the surface area for water and mineral absorption. In other plants, the initial taproot is quickly modified into a fibrous, or diffuse, system, in which the initial secondary roots soon equal or exceed the primary root in size and there is no well-defined single taproot. Fibrous root systems are generally shallower than taproot systems.
Details
A taproot is a large, central, and dominant root from which other roots sprout laterally. Typically a taproot is somewhat straight and very thick, is tapering in shape, and grows directly downward. In some plants, such as the carrot, the taproot is a storage organ so well developed that it has been cultivated as a vegetable.
The taproot system contrasts with the adventitious or fibrous root system of plants with many branched roots, but many plants that grow a taproot during germination go on to develop branching root structures, although some that rely on the main root for storage may retain the dominant taproot for centuries, for example Welwitschia.
Description
Dicots, one of the two divisions of flowering plants (angiosperms), start with a taproot, which is one main root forming from the enlarging radicle of the seed. The tap root can be persistent throughout the life of the plant but is most often replaced later in the plant's development by a fibrous root system. A persistent taproot system forms when the radicle keeps growing and smaller lateral roots form along the taproot. The shape of taproots can vary but the typical shapes include:
Conical root: this type of root tuber is conical in shape, i.e. widest at the top and tapering steadily towards the bottom: e.g. carrot.
Fusiform root: this root is widest in the middle and tapers towards the top and the bottom: e.g. radish.
Napiform root: the root has a top-like appearance. It is very broad at the top and tapers suddenly like a tail at the bottom: e.g. turnip.
Many taproots are modified into storage organs. Some plants with taproots:
* Beetroot
* Burdock
* Carrot
* Sugar beet
* Dandelion
* Parsley
* Parsnip
* Poppy mallow
* Radish
* Sagebrush
* Turnip
* Common milkweed
* trees such as oaks, elms, pines and firs
Development of taproots
Taproots develop from the radicle of a seed, forming the primary root. It branches off to secondary roots, which in turn branch to form tertiary roots. These may further branch to form rootlets. For most plants species the radicle dies some time after seed germination, causing the development of a fibrous root system, which lacks a main downward-growing root. Most trees begin life with a taproot, but after one to a few years the main root system changes to a wide-spreading fibrous root system with mainly horizontal-growing surface roots and only a few vertical, deep-anchoring roots. A typical mature tree 30–50 m tall has a root system that extends horizontally in all directions as far as the tree is tall or more, but as much as 100% of the roots are in the top 50 cm of soil.
Soil characteristics strongly influence the architecture of taproots; for example, deep and rich soils favour the development of vertical taproots in many oak species such as Quercus kelloggii, while clay soils promote the growth of multiple taproots.
Horticultural considerations
Many plants with taproots are difficult to transplant, or even to grow in containers, because the root tends to grow deep rapidly and in many species comparatively slight obstacles or damage to the taproot will stunt or kill the plant. Among weeds with taproots dandelions are typical; being deep-rooted, they are hard to uproot and if the taproot breaks off near the top, the part that stays in the ground often resprouts such that, for effective control, the taproot needs to be severed at least several centimetres below ground level.
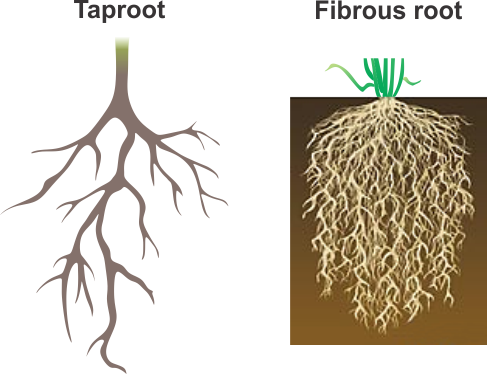
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1248 2022-01-08 20:45:56
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,148
Re: Miscellany
1224) Oxalic Acid
Summary
Oxalic acid, also called ethanedioic acid, is a colourless, crystalline, toxic organic compound belonging to the family of carboxylic acids. Oxalic acid is widely used as an acid rinse in laundries, where it is effective in removing rust and ink stains because it converts most insoluble iron compounds into a soluble complex ion. For the same reason, it is the chief constituent of many commercial preparations used for removing scale from automobile radiators.
The formula of oxalic acid is (C2H2O4); its usual form is that of the crystalline hydrate, (COOH)2·2H2O. Known as a constituent of wood sorrel as early as the 17th century, oxalic acid was first prepared synthetically in 1776. It is manufactured by heating sodium formate in the presence of an alkali catalyst, by oxidizing carbohydrates with nitric acid, by heating sawdust with caustic alkalies, or by fermentation of sugar solutions in the presence of certain molds.
Details
Oxalic acid is an organic acid with the IUPAC name ethanedioic acid and formula HO2C−CO2H. It is the simplest dicarboxylic acid. It is a white crystalline solid that forms a colorless solution in water. Its name comes from the fact that early investigators isolated oxalic acid from flowering plants of the genus Oxalis, commonly known as wood-sorrels. It occurs naturally in many foods, but excessive ingestion of oxalic acid or prolonged skin contact can be dangerous.
Oxalic acid has much greater acid strength than acetic acid. It is a reducing agent and its conjugate base, known as oxalate, is a chelating agent for metal cations. Typically, oxalic acid occurs as the dihydrate with the formula C2H2O4·2H2O.
History
The preparation of salts of oxalic acid (crab acid) from plants had been known, at least since 1745, when the Dutch botanist and physician Herman Boerhaave isolated a salt from wood sorrel. By 1773, François Pierre Savary of Fribourg, Switzerland had isolated oxalic acid from its salt in sorrel.
In 1776, Swedish chemists Carl Wilhelm Scheele and Torbern Olof Bergman produced oxalic acid by reacting sugar with concentrated nitric acid; Scheele called the acid that resulted socker-syra or såcker-syra (sugar acid). By 1784, Scheele had shown that "sugar acid" and oxalic acid from natural sources were identical.
In 1824, the German chemist Friedrich Wöhler obtained oxalic acid by reacting cyanogen with ammonia in aqueous solution. This experiment may represent the first synthesis of a natural product.
Preparation
Oxalic acid is mainly manufactured by the oxidation of carbohydrates or glucose using nitric acid or air in the presence of vanadium pentoxide. A variety of precursors can be used including glycolic acid and ethylene glycol.
Historically oxalic acid was obtained exclusively by using caustics, such as sodium or potassium hydroxide, on sawdust. Pyrolysis of sodium formate (ultimately prepared from carbon monoxide), leads to the formation of sodium oxalate, easily converted to oxalic acid.
Laboratory methods
Although it can be readily purchased, oxalic acid can be prepared in the laboratory by oxidizing sucrose using nitric acid in the presence of a small amount of vanadium pentoxide as a catalyst.
The hydrated solid can be dehydrated with heat or by azeotropic distillation.
Developed in the Netherlands, an electrocatalysis by a copper complex helps reduce carbon dioxide to oxalic acid; this conversion uses carbon dioxide as a feedstock to generate oxalic acid.
Occurrence in foods and plants
Early investigators isolated oxalic acid from wood-sorrel (Oxalis). Members of the spinach family and the brassicas (cabbage, broccoli, brussels sprouts) are high in oxalates, as are sorrel and umbellifers like parsley. Rhubarb leaves contain about 0.5% oxalic acid, and jack-in-the-pulpit (Arisaema triphyllum) contains calcium oxalate crystals. Similarly, the Virginia creeper, a common decorative vine, produces oxalic acid in its berries as well as oxalate crystals in the sap, in the form of raphides. Bacteria produce oxalates from oxidation of carbohydrates.
Plants of the genus Fenestraria produce optical fibers made from crystalline oxalic acid to transmit light to subterranean photosynthetic sites.
Carambola, also known as starfruit, also contains oxalic acid along with caramboxin. Citrus juice contains small amounts of oxalic acid. Citrus fruits produced in organic agriculture contain less oxalic acid than those produced in conventional agriculture.
The formation of naturally occurring calcium oxalate patinas on certain limestone and marble statues and monuments has been proposed to be caused by the chemical reaction of the carbonate stone with oxalic acid secreted by lichen or other microorganisms.
Production by fungi
Many soil fungus species secrete oxalic acid, resulting in greater solubility of metal cations, increased availability of certain soil nutrients, and can lead to the formation of calcium oxalate crystals. Some fungi such as Aspergillus niger have been extensively studied for the industrial production of oxalic acid; however, those processes are not yet economically competitive with production from oil and gas.
Biochemistry
The conjugate base of oxalic acid is the hydrogenoxalate anion, and its conjugate base (oxalate) is a competitive inhibitor of the lactate dehydrogenase (LDH) enzyme. LDH catalyses the conversion of pyruvate to lactic acid (end product of the fermentation (anaerobic) process) oxidising the coenzyme NADH to NAD+ and H+ concurrently. Restoring NAD+ levels is essential to the continuation of anaerobic energy metabolism through glycolysis. As cancer cells preferentially use anaerobic metabolism inhibition of LDH has been shown to inhibit tumor formation and growth, thus is an interesting potential course of cancer treatment.
Applications
Oxalic acid's main applications include cleaning or bleaching, especially for the removal of rust (iron complexing agent). Its utility in rust removal agents is due to its forming a stable, water-soluble salt with ferric iron, ferrioxalate ion. The cleaning product Zud contains oxalic acid. Oxalic acid is an ingredient in some tooth whitening products. About 25% of produced oxalic acid will be used as a mordant in dyeing processes. It is also used in bleaches, especially for pulpwood, and for rust removal and other cleaning, in baking powder, and as a third reagent in silica analysis instruments.
Niche uses
Oxalic acid is used by some beekeepers as a miticide against the parasitic varroa mite.
Dilute solutions (0.05–0.15 M) of oxalic acid can be used to remove iron from clays such as kaolinite to produce light-colored ceramics.
Oxalic acid is used to clean minerals.
Oxalic acid is sometimes used in the aluminum anodizing process, with or without sulfuric acid. Compared to sulfuric acid anodizing, the coatings obtained are thinner and exhibit lower surface roughness.
Oxalic acid is also widely used as a wood bleach, most often in its crystalline form to be mixed with water to its proper dilution for use.
Toxicity
Oxalic acid has an oral LDLo (lowest published lethal dose) of 600 mg/kg. It has been reported that the lethal oral dose is 15 to 30 grams. The toxicity of oxalic acid is due to kidney failure caused by precipitation of solid calcium oxalate.
Oxalate is known to cause mitochondrial dysfunction.
Ingestion of ethylene glycol results in oxalic acid as a metabolite which can also cause acute kidney failure.
Kidney stones
The most kidney stones, 76%, are composed of the calcium salt of oxalic acid. Oxalic acid can also cause joint pain by formation of similar precipitates in the joints. Calcium hydroxide decreases urinary oxalate in both humans and rats. Ingesting both calcium containing foods, such as milk, with food high in oxalic acid, cause the formation of calcium oxalate in the stomach, which is not absorbed into the body.
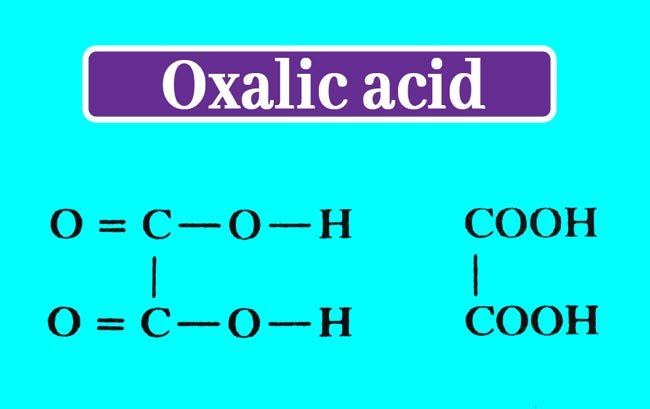
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1249 2022-01-10 00:05:49
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,148
Re: Miscellany
1225) Citric Acid
Summary
Citric acid is a colourless crystalline organic compound belonging to the family of carboxylic acids, present in practically all plants and in many animal tissues and fluids. It is one of a series of compounds involved in the physiological oxidation of fats, proteins, and carbohydrates to carbon dioxide and water. .
Citric acid was first isolated from lemon juice by Swedish chemist Carl Wilhelm Scheele in 1784 and is manufactured by fermentation of cane sugar or molasses in the presence of a fungus, Aspergillus niger. It is used in confections and soft drinks (as a flavouring agent), in metal-cleaning compositions, and in improving the stability of foods and other organic substances (by suppressing the deleterious action of dissolved metal salts).
Details
Citric acid is an organic compound with the chemical formula HOC(CO2H)(CH2CO2H)2. It is a colorless weak organic acid. It occurs naturally in citrus fruits. In biochemistry, it is an intermediate in the citric acid cycle, which occurs in the metabolism of all aerobic organisms.
More than two million tons of citric acid are manufactured every year. It is used widely as an acidifier, as a flavoring, and a chelating agent.
A citrate is a derivative of citric acid; that is, the salts, esters, and the polyatomic anion found in solution. An example of the former, a salt is trisodium citrate; an ester is triethyl citrate.
Natural occurrence and industrial production
Citric acid exists in a variety of fruits and vegetables, most notably citrus fruits. Lemons and limes have particularly high concentrations of the acid; it can constitute as much as 8% of the dry weight of these fruits (about 47 g/L in the juices). The concentrations of citric acid in citrus fruits range from 0.005 mol/L for oranges and grapefruits to 0.30 mol/L in lemons and limes; these values vary within species depending upon the cultivar and the circumstances in which the fruit was grown.
Citric acid was first isolated in 1784 by the chemist Carl Wilhelm Scheele, who crystallized it from lemon juice.
Industrial-scale citric acid production first began in 1890 based on the Italian citrus fruit industry, where the juice was treated with hydrated lime (calcium hydroxide) to precipitate calcium citrate, which was isolated and converted back to the acid using diluted sulfuric acid. In 1893, C. Wehmer discovered Penicillium mold could produce citric acid from sugar. However, microbial production of citric acid did not become industrially important until World War I disrupted Italian citrus exports.
In 1917, American food chemist James Currie discovered certain strains of the mold Aspergillus niger could be efficient citric acid producers, and the pharmaceutical company Pfizer began industrial-level production using this technique two years later, followed by Citrique Belge in 1929. In this production technique, which is still the major industrial route to citric acid used today, cultures of A. niger are fed on a sucrose or glucose-containing medium to produce citric acid. The source of sugar is corn steep liquor, molasses, hydrolyzed corn starch, or other inexpensive, sugary solution. After the mold is filtered out of the resulting solution, citric acid is isolated by precipitating it with calcium hydroxide to yield calcium citrate salt, from which citric acid is regenerated by treatment with sulfuric acid, as in the direct extraction from citrus fruit juice.
In 1977, a patent was granted to Lever Brothers for the chemical synthesis of citric acid starting either from aconitic or isocitrate/alloisocitrate calcium salts under high pressure conditions; this produced citric acid in near quantitative conversion under what appeared to be a reverse, non-enzymatic Krebs cycle reaction.
Global production was in excess of 2,000,000 tons in 2018. More than 50% of this volume was produced in China. More than 50% was used as an acidity regulator in beverages, some 20% in other food applications, 20% for detergent applications, and 10% for applications other than food, such as cosmetics, pharmaceuticals, and in the chemical industry.
Chemical characteristics
Citric acid can be obtained as an anhydrous (water-free) form or as a monohydrate. The anhydrous form crystallizes from hot water, while the monohydrate forms when citric acid is crystallized from cold water. The monohydrate can be converted to the anhydrous form at about 78 °C. Citric acid also dissolves in absolute (anhydrous) ethanol (76 parts of citric acid per 100 parts of ethanol) at 15 °C. It decomposes with loss of carbon dioxide above about 175 °C.
Citric acid can be esterified at one or more of its three carboxylic acid groups to form any of a variety of mono-, di-, tri-, and mixed esters.
Cosmetics, pharmaceuticals, dietary supplements, and foods
Citric acid is used as an acidulant in creams, gels, and liquids. Used in foods and dietary supplements, it may be classified as a processing aid if it was added for a technical or functional effect (e.g. acidulent, chelator, viscosifier, etc.). If it is still present in insignificant amounts, and the technical or functional effect is no longer present, it may be exempt from labeling <21 CFR 101.100(c)>.
Citric acid is an alpha hydroxy acid and is an active ingredient in chemical skin peels.
Citric acid is used as one of the active ingredients in the production of facial tissues with antiviral properties.
Other uses
The buffering properties of citrates are used to control pH in household cleaners and pharmaceuticals.
Citric acid is used as an odorless alternative to white vinegar for fabric dyeing with acid dyes.
Sodium citrate is a component of Benedict's reagent, used for identification both qualitatively and quantitatively of reducing sugars.
Citric acid can be used as an alternative to nitric acid in passivation of stainless steel.
Citric acid can be used as a lower-odor stop bath as part of the process for developing photographic film. Photographic developers are alkaline, so a mild acid is used to neutralize and stop their action quickly, but commonly used acetic acid leaves a strong vinegar odor in the darkroom.
Citric acid/potassium-sodium citrate can be used as a blood acid regulator.
Soldering flux. Citric acid is an excellent soldering flux, either dry or as a concentrated solution in water. It should be removed after soldering, especially with fine wires, as it is mildly corrosive. It dissolves and rinses quickly in hot water.
Safety
Although a weak acid, exposure to pure citric acid can cause adverse effects. Inhalation may cause cough, shortness of breath, or sore throat. Over-ingestion may cause abdominal pain and sore throat. Exposure of concentrated solutions to skin and eyes can cause redness and pain. Long-term or repeated consumption may cause erosion of tooth enamel.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1250 2022-01-10 19:24:49
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,148
Re: Miscellany
1226) Butterfly
Summary
Butterflies are insects in the macrolepidopteran clade Rhopalocera from the order Lepidoptera, which also includes moths. Adult butterflies have large, often brightly coloured wings, and conspicuous, fluttering flight. The group comprises the large superfamily Papilionoidea, which contains at least one former group, the skippers (formerly the superfamily "Hesperioidea"), and the most recent analyses suggest it also contains the moth-butterflies (formerly the superfamily "Hedyloidea"). Butterfly fossils date to the Paleocene, about 56 million years ago.
Butterflies have a four-stage life cycle, as like most insects they undergo complete metamorphosis. Winged adults lay eggs on the food plant on which their larvae, known as caterpillars, will feed. The caterpillars grow, sometimes very rapidly, and when fully developed, pupate in a chrysalis. When metamorphosis is complete, the pupal skin splits, the adult insect climbs out, and after its wings have expanded and dried, it flies off. Some butterflies, especially in the tropics, have several generations in a year, while others have a single generation, and a few in cold locations may take several years to pass through their entire life cycle.
Butterflies are often polymorphic, and many species make use of camouflage, mimicry, and aposematism to evade their predators. Some, like the monarch and the painted lady, migrate over long distances. Many butterflies are attacked by parasites or parasitoids, including wasps, protozoans, flies, and other invertebrates, or are preyed upon by other organisms. Some species are pests because in their larval stages they can damage domestic crops or trees; other species are agents of pollination of some plants. Larvae of a few butterflies (e.g., harvesters) eat harmful insects, and a few are predators of ants, while others live as mutualists in association with ants. Culturally, butterflies are a popular motif in the visual and literary arts. The Smithsonian Institution says "butterflies are certainly one of the most appealing creatures in nature".
Details
Butterfly, (superfamily Papilionoidea), is any of numerous species of insects belonging to multiple families. Butterflies, along with the moths and the skippers, make up the insect order Lepidoptera. Butterflies are nearly worldwide in their distribution.
The wings, bodies, and legs, like those of moths, are covered with dustlike scales that come off when the animal is handled. Unlike moths, butterflies are active during the day and are usually brightly coloured or strikingly patterned. Perhaps the most distinctive physical features of the butterfly are its club-tipped antennae and its habit of holding the wings vertically over the back when at rest. The lepidopteran life cycle has four stages: egg, larva (caterpillar), pupa (chrysalis), and adult (imago). The larvae and adults of most butterflies feed on plants, often only specific parts of specific types of plants.
The butterfly families include: Pieridae, the whites and sulfurs, known for their mass migrations; Papilionidae, the swallowtails and parnassians; Lycaenidae, including the blues, coppers, hairstreaks, and gossamer-winged butterflies; Riodinidae, the metalmarks, found chiefly in the American tropics; Nymphalidae, the brush-footed butterflies; Hesperiidae, the skippers; and Hedylidae, the American moth-butterflies (sometimes considered a sister group to Papilionoidea). The brush-footed butterflies represent the largest and most diverse family and include such popular butterflies as the admirals, fritillaries, monarchs, zebras, and painted ladies.
Admiral
Admiral, (subfamily Limentidinae), is any of several butterfly species in the family Nymphalidae (order Lepidoptera) that are fast-flying and much prized by collectors for their coloration, which consists of black wings with white bands and reddish brown markings. The migratory red admiral (Vanessa atalanta), placed in the subfamily Nymphalinae, is widespread in Europe, Scandinavia, North America, and North Africa and feeds on stinging nettles. The western, or Weidemeyer’s, admiral (Limenitis weidemeyerii) is found in the western United States. The white admiral (L. arthemis), a species made up of a white form and a red-spotted purple form, was once thought to be two distinct species. The white admiral occurs in North America and from Great Britain across Eurasia to Japan, feeds on honeysuckle. The Indian red admiral, V. indica, is found in the Canary Islands as well as India and is distinguished by a red band on the forewings wider than that of V. atalanta.
Painted lady
Painted lady, (Vanessa cardui), is species of butterfly in the brush-footed butterfly family, Nymphalidae (order Lepidoptera), that has broad wings (span about 4 to 5 cm [1.5 to 2 inches]), with beautifully elaborate patterns of reddish orange, pink, brown, white, and blue scales. Vast numbers travel northward in spring across the Mediterranean from Africa to Europe, migrating thousands of kilometres. A few of the next generation travel southward during late summer, but most perish in the northern winter. Painted lady larvae in the Americas feed on plants that are members of the family Compositae, while larvae in Africa and Europe eat thistles and stinging nettles.
In North America, V. cardui and V. virginiensis are known as the painted lady and American painted lady, respectively. In Europe and Africa, V. cardui is referred to as the painted lady, whereas in Central America V. virginiensis is called the painted lady.
What is a butterfly?
Butterflies are the adult flying stage of certain insects belonging to an order or group called Lepidoptera. Moths also belong to this group. The word "Lepidoptera" means "scaly wings" in Greek. This name perfectly suits the insects in this group because their wings are covered with thousands of tiny scales overlapping in rows. The scales, which are arranged in colorful designs unique to each species, are what gives the butterfly its beauty.
Like all other insects, butterflies have six legs and three main body parts: head, thorax (chest or mid section) and abdomen (tail end). They also have two antennae and an exoskeleton.
The difference between a butterfly and a moth?
Both butterflies and moths belong to the same insect group called Lepidoptera. In general, butterflies differ from moths in the following ways: (1) Butterflies usually have clubbed antennae but moths have fuzzy or feathery antennae. (2) Butterflies normally are active during the daytime while most moths are active at night. (3) When a butterfly rests, it will do so with its wings held upright over its body. Moths, on the other hand, rest with their wings spread out flat. Butterflies will, however, bask with their wings out-stretched. (4) Butterflies are generally more brightly colored than moths, however, this is not always the case. There are some very colorful moths.
Butterfly life cycle
A life cycle is made up of the stages that a living organism goes through during its lifetime from beginning to end. A butterfly undergoes a process called complete metamorphosis during its life cycle. This means that the butterfly changes completely from its early larval stage, when it is a caterpillar, until the final stage, when it becomes a beautiful and graceful adult butterfly. The butterfly life cycle has four stages: egg, larva, pupa, and adult.
The first stage of the butterfly life cycle is the egg or ovum. Butterfly eggs are tiny, vary in color and may be round, cylindrical or oval. The female butterfly attaches the eggs to leaves or stems of plants that will also serve as a suitable food source for the larvae when they hatch.
The larva, or caterpillar, that hatches from the egg is the second stage in the life cycle. Caterpillars often, but not always, have several pairs of true legs, along with several pairs of false legs or prolegs. A caterpillar's primary activity is eating. They have a voracious appetite and eat almost constantly. As the caterpillar continues to eat, its body grows considerably. The tough outer skin or exoskeleton, however, does not grow or stretch along with the enlarging caterpillar. Instead, the old exoskeleton is shed in a process called molting and it is replaced by a new, larger exoskeleton. A caterpillar may go through as many as four to five molts before it becomes a pupa.
The third stage is known as the pupa or chrysalis. The caterpillar attaches itself to a twig, a wall or some other support and the exoskeleton splits open to reveal the chrysalis. The chrysalis hangs down like a small sack until the transformation to butterfly is complete. The casual observer may think that because the pupa is motionless that very little is going on during this "resting stage." However, it is within the chrysalis shell that the caterpillar's structure is broken down and rearranged into the wings, body and legs of the adult butterfly. The pupa does not feed but instead gets its energy from the food eaten by the larval stage. Depending on the species, the pupal stage may last for just a few days or it may last for more than a year. Many butterfly species overwinter or hibernate as pupae.
The fourth and final stage of the life cycle is the adult. Once the chrysalis casing splits, the butterfly emerges. It will eventually mate and lay eggs to begin the cycle all over again. Most adult butterflies will live only a week or two, while a few species may live as long as 18 months.
Butterfly activities
Butterflies are complex creatures. Their day-to-day lives can be characterized by many activities. If you are observant you may see butterflies involved in many of the follow activities. To observe some activities, such as hybernation, may involve some detective work. To observe other activities such as basking, puddling, or migrating, you will need to be at the proper place at the proper time. Keep an activity log and see how many different butterflies you can spot involved in each activity. The information from the individual butterfly pages may give you some hints as to where (or on what plants) some of these activities are likely to occur.
Feeding
The larval or caterpillar stage and the adult butterfly have very different food preferences, largely due to the differences in their mouth parts. Both types of foods must be available in order for the butterfly to complete its life cycle.
Caterpillars are very particular about what they eat, which is why the female butterfly lays her eggs only on certain plants. She instinctively knows what plants will serve as suitable food for the hungry caterpillars that hatch from her eggs. Caterpillars don't move much and may spend their entire lives on the same plant or even the same leaf! Their primary goal is to eat as much as they can so that they become large enough to pupate. Caterpillars have chewing mouth parts, called mandibles, which enable them to eat leaves and other plant parts. Some caterpillars are considered pests because of the damage they do to crops. Caterpillars do not need to drink additional water because they get all they need from the plants they eat.
Adult butterflies are also selective about what they eat. Unlike caterpillars, butterflies can roam about and look for suitable food over a much broader territory. In most cases, adult butterflies are able to feed only on various liquids. They drink through a tube-like tongue called a proboscis. It uncoils to sip liquid food, and then coils up again into a spiral when the butterfly is not feeding. Most butterflies prefer flower nectar, but others may feed on the liquids found in rotting fruit, in ooze from trees, and in animal dung. Butterflies prefer to feed in sunny areas protected from wind.
Basking
Butterflies are cold-blooded, meaning they cannot regulate their own body temperature. As a result, their body temperature changes with the temperature of their surroundings. If they get too cold, they are unable to fly and must warm up their muscles in order to resume flight. Butterflies can fly as long as the air is between 60°-108° F, although temperatures between 82°-100° F are best. If the temperature drops too low, they may seek a light colored rock, sand or a leaf in a sunny spot and bask. Butterflies bask with their wings spread out in order to soak up the sun's heat.
Puddling
When butterflies get too hot, they may head for shade or for cool areas like puddles. Some species will gather at shallow mud puddles or wet sandy areas, sipping the mineral-rich water. Generally more males than females puddle and it is believed that the salts and nutrients in the puddles are needed for successful mating.
Patrolling and perching
There are two methods that a male butterfly might use in order to search for a female mate. It might patrol or fly over a particular area where other butterflies are active. If it sees a possible mate, it will fly in for a closer look. Or, instead, it might perch on a tall plant in an area where females may be present. If it spots a likely mate, it will swoop in to investigate. In either case, if he finds a suitable female he will begin the mating ritual. If he finds another male instead, a fierce fight may ensue.
Mating
A male butterfly has several methods of determining whether he has found a female of his own species. One way is by sight. The male will look for butterflies with wings that are the correct color and pattern. When a male sights a potential mate it will fly closer, often behind or above the female. Once closer, the male will release special chemicals, called pheromones, while it flutters its wings a bit more than usual. The male may also do a special "courtship dance" to attract the female. These "dances" consist of flight patterns that are peculiar to that species of butterfly. If the female is interested she may join the male's dance. They will then mate by joining together end to end at their abdomens. During the mating process, when their bodies are joined, the male passes sperm to the female. As the eggs later pass through the female's egg-laying tube, they are fertilized by the sperm. The male butterfly often dies soon after mating.
Egg-laying
After mating with a male, the female butterfly must go in search of a plant on which to lay her eggs. Because the caterpillars that will hatch from her eggs will be very particular about what they eat, she must be very particular in choosing a plant. She can recognize the right plant species by its leaf color and shape. Just to be sure, however, she may beat on the leaf with her feet. This scratches the leaf surface, causing a characteristic plant odor to be released. Once she is sure she has found the correct plant species, she will go about the business of egg-laying. While laying her eggs, they are fertilized with the sperm that has been stored in her body since mating. Some butterflies lay a single egg, while others may lay their eggs in clusters. A sticky substance produced by the female enables the eggs to stick where ever she lays them, either on the underside of a leaf or on a stem.
Hibernating
Butterflies are cold-blooded and cannot withstand winter conditions in an active state. Butterflies may survive cold weather by hibernating in protected locations. They may use the peeling bark of trees, perennial plants, logs or old fences as their overwintering sites. They may hibernate at any stage (egg, larval, pupal or adult) but generally each species is dormant in only one stage.
Migrating
Another way that butterflies can escape cold weather is by migrating to a warmer region. Some migrating butterflies, such as the painted lady and cabbage butterfly, fly only a few hundred miles, while others, such as the monarch, travel thousands of miles.
Monarchs are considered the long-distance champions of butterfly migration, traveling as many as 4000 miles round trip. They begin their flight before the autumn cold sets in, heading south from Canada and the northern United States. Monarchs migrate to the warmer climates of California, Florida and Mexico, making the trip in two months or less and feeding on nectar along the way. Once arriving at their southern destination, they will spend the winter resting for the return flight. Few of the original adults actually complete the trip home. Instead, the females mate and lay eggs along the way and their offspring finish this incredible journey.
Camouflage
Butterflies and caterpillars are preyed upon by birds, spiders, lizards and various other animals. Largely defenseless against many of these hungry predators, Lepidoptera have developed a number of passive ways to protect themselves. One way is by making themselves inconspicuous through the use of camouflage.
Caterpillars may be protectively colored or have structures that allow them to seemingly disappear into the background. For example, many caterpillars are green, making them difficult to detect because they blend in with the host leaf. Some larvae, particularly those in the Tropics, bear a resemblance to bird droppings, a disguise that makes them unappealing to would-be predators.
The coloration and pattern of a butterfly's wings may enable it to blend into its surrounding. Some may look like dead leaves on a twig when they are at rest with their wings closed. The under wing markings of the comma and question mark butterflies help them to go unnoticed when hibernating in leaf litter.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline