Math Is Fun Forum
You are not logged in.
- Topics: Active | Unanswered
#1276 2022-02-05 14:02:53
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,954
Re: Miscellany
1252) Liver cancer
Liver cancer (also known as hepatic cancer, primary hepatic cancer, or primary hepatic malignancy) is cancer that starts in the liver. Liver cancer can be primary (starts in liver) or secondary (meaning cancer which has spread from elsewhere to the liver, known as liver metastasis). Liver metastasis is more common than that which starts in the liver. Liver cancer is increasing globally.
Primary liver cancer is globally the sixth-most frequent cancer and the fourth-leading cause of death from cancer. In 2018, it occurred in 841,000 people and resulted in 782,000 deaths globally. Higher rates of liver cancer occur where hepatitis B and C are common, including Asia and sub-Saharan Africa. Males are more often affected with HCC than females. Diagnosis is most frequent among those 55 to 65 years old.
The leading cause of liver cancer is cirrhosis due to hepatitis B, hepatitis C or alcohol. Other causes include aflatoxin, non-alcoholic fatty liver disease and liver flukes. The most common types are hepatocellular carcinoma (HCC), which makes up 80% of cases and intrahepatic cholangiocarcinoma. The diagnosis may be supported by blood tests and medical imaging, with confirmation by tissue biopsy.
Given that there are many different causes of liver cancer, there are many approaches to liver cancer prevention. These efforts include immunization against hepatitis B, hepatitis B treatment, hepatitis C treatment, decreasing alcohol use, decreasing exposure to aflatoxin in agriculture, and management of obesity and diabetes. Screening is recommended in those with chronic liver disease. For example, it is recommended that people with chronic liver disease who are at risk for hepatocellular carcinoma be screened every 6 months using ultrasound imaging.
Because liver cancer is an umbrella term for many types of cancer, the signs and symptoms depend on what type of cancer is present. Symptoms can be vague and broad. Cholangiocarcinoma is associated with sweating, jaundice, abdominal pain, weight loss and liver enlargement. Hepatocellular carcinoma is associated with abdominal mass, abdominal pain, emesis, anemia, back pain, jaundice, itching, weight loss and fever.
Treatment options may include surgery, targeted therapy and radiation therapy. In certain cases, ablation therapy, embolization therapy or liver transplantation may be used.
Overview
Liver cancer is cancer that begins in the cells of your liver. Your liver is a football-sized organ that sits in the upper right portion of your abdomen, beneath your diaphragm and above your stomach.
Several types of cancer can form in the liver. The most common type of liver cancer is hepatocellular carcinoma, which begins in the main type of liver cell (hepatocyte). Other types of liver cancer, such as intrahepatic cholangiocarcinoma and hepatoblastoma, are much less common.
Cancer that spreads to the liver is more common than cancer that begins in the liver cells. Cancer that begins in another area of the body — such as the colon, lung or breast — and then spreads to the liver is called metastatic cancer rather than liver cancer. This type of cancer is named after the organ in which it began — such as metastatic colon cancer to describe cancer that begins in the colon and spreads to the liver.
Symptoms
Most people don't have signs and symptoms in the early stages of primary liver cancer. When signs and symptoms do appear, they may include:
* Losing weight without trying
* Loss of appetite
* Upper abdominal pain
* Nausea and vomiting
* General weakness and fatigue
* Abdominal swelling
* Yellow discoloration of your skin and the whites of your eyes (jaundice)
* White, chalky stools
When to see a doctor
Make an appointment with your doctor if you experience any signs or symptoms that worry you.
Causes
Liver cancer happens when liver cells develop changes (mutations) in their DNA. A cell's DNA is the material that provides instructions for every chemical process in your body. DNA mutations cause changes in these instructions. One result is that cells may begin to grow out of control and eventually form a tumor — a mass of cancerous cells.
Sometimes the cause of liver cancer is known, such as with chronic hepatitis infections. But sometimes liver cancer happens in people with no underlying diseases and it's not clear what causes it.
Risk factors
Factors that increase the risk of primary liver cancer include:
* Chronic infection with HBV or HCV. Chronic infection with the hepatitis B virus (HBV) or hepatitis C virus (HCV) increases your risk of liver cancer.
* Cirrhosis. This progressive and irreversible condition causes scar tissue to form in your liver and increases your chances of developing liver cancer.
* Certain inherited liver diseases. Liver diseases that can increase the risk of liver cancer include hemochromatosis and Wilson's disease.
* Diabetes. People with this blood sugar disorder have a greater risk of liver cancer than those who don't have diabetes.
* Nonalcoholic fatty liver disease. An accumulation of fat in the liver increases the risk of liver cancer.
* Exposure to aflatoxins. Aflatoxins are poisons produced by molds that grow on crops that are stored poorly. Crops, such as grains and nuts, can become contaminated with aflatoxins, which can end up in foods made of these products.
* Excessive alcohol consumption. Consuming more than a moderate amount of alcohol daily over many years can lead to irreversible liver damage and increase your risk of liver cancer.
Prevention:
Reduce your risk of cirrhosis
Cirrhosis is scarring of the liver, and it increases the risk of liver cancer. You can reduce your risk of cirrhosis if you:
* Drink alcohol in moderation, if at all. If you choose to drink alcohol, limit the amount you drink. For women, this means no more than one drink a day. For men, this means no more than two drinks a day.
* Maintain a healthy weight. If your current weight is healthy, work to maintain it by choosing a healthy diet and exercising most days of the week. If you need to lose weight, reduce the number of calories you eat each day and increase the amount of exercise you do. Aim to lose weight slowly — 1 or 2 pounds (0.5 to 1 kilograms) each week.
* Get vaccinated against hepatitis B
You can reduce your risk of hepatitis B by receiving the hepatitis B vaccine. The vaccine can be given to almost anyone, including infants, older adults and those with compromised immune systems.
Take measures to prevent hepatitis C
No vaccine for hepatitis C exists, but you can reduce your risk of infection.
* Know the health status of any sexual partner. Don't engage in unprotected gender unless you're certain your partner isn't infected with HBV, HCV or any other sexually transmitted infection. If you don't know the health status of your partner, use a condom every time you have sexual intercourse.
* Don't use intravenous (IV) drugs, but if you do, use a clean needle. Reduce your risk of HCV by not injecting illegal drugs. But if that isn't an option for you, make sure any needle you use is sterile, and don't share it. Contaminated drug paraphernalia is a common cause of hepatitis C infection. Take advantage of needle-exchange programs in your community and consider seeking help for your drug use.
* Seek safe, clean shops when getting a piercing or tattoo. Needles that may not be properly sterilized can spread the hepatitis C virus. Before getting a piercing or tattoo, check out the shops in your area and ask staff members about their safety practices. If employees at a shop refuse to answer your questions or don't take your questions seriously, take that as a sign that the facility isn't right for you.
Seek treatment for hepatitis B or C infection
Treatments are available for hepatitis B and hepatitis C infections. Research shows that treatment can reduce the risk of liver cancer.
Ask your doctor about liver cancer screening
For the general population, screening for liver cancer hasn't been proved to reduce the risk of dying of liver cancer, and it isn't generally recommended. People with conditions that increase the risk of liver cancer might consider screening, such as people who have:
* Hepatitis B infection
* Hepatitis C infection
* Liver cirrhosis
Discuss the pros and cons of screening with your doctor. Together you can decide whether screening is right for you based on your risk. Screening typically involves a blood test and an abdominal ultrasound exam every six months.
Hepatocellular carcinoma
Hepatocellular carcinoma (HCC) is the most common type of primary liver cancer. Hepatocellular carcinoma occurs most often in people with chronic liver diseases, such as cirrhosis caused by hepatitis B or hepatitis C infection.
Risk factors
The risk of hepatocellular carcinoma, the most common type of liver cancer, is higher in people with long-term liver diseases. It's also higher if the liver is scarred by infection with hepatitis B or hepatitis C. Hepatocellular carcinoma is more common in people who drink large amounts of alcohol and who have an accumulation of fat in the liver.
Diagnosis
Tests and procedures used to diagnose hepatocellular carcinoma include:
* Blood tests to measure liver function
* Imaging tests, such as CT and MRI
* Liver biopsy, in some cases, to remove a sample of liver tissue for laboratory testing
Treatment
Which treatment is best for you will depend on the size and location of your hepatocellular carcinoma, how well your liver is functioning, and your overall health.
Hepatocellular carcinoma treatments include:
* Surgery. Surgery to remove the cancer and a margin of healthy tissue that surrounds it may be an option for people with early-stage liver cancers who have normal liver function.
* Liver transplant surgery. Surgery to remove the entire liver and replace it with a liver from a donor may be an option in otherwise healthy people whose liver cancer hasn't spread beyond the liver.
* Destroying cancer cells with heat or cold. Ablation procedures to kill the cancer cells in the liver using extreme heat or cold may be recommended for people who can't undergo surgery. These procedures include radiofrequency ablation, cryoablation, and ablation using alcohol or microwaves.
* Delivering chemotherapy or radiation directly to cancer cells. Using a catheter that's passed through your blood vessels and into your liver, doctors can deliver chemotherapy drugs (chemoembolization) or tiny glass spheres containing radiation (radioembolization) directly to the cancer cells.
* Radiation therapy. Radiation therapy using energy from X-rays or protons may be recommended if surgery isn't an option. A specialized type of radiation therapy, called stereotactic body radiotherapy (SBRT), involves focusing many beams of radiation simultaneously at one point in your body.
* Targeted drug therapy. Targeted drugs attack specific weaknesses in the cancer cells, and they may help slow the progression of the disease in people with advanced liver cancers.
* Immunotherapy. Immunotherapy drugs use your body's germ-fighting immune system to attack the cancer cells. Immunotherapy may be an option for treating advanced liver cancer.
* Clinical trials. Clinical trials give you a chance to try new liver cancer treatments. Ask your doctor whether you're eligible to participate in a clinical trial.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Online
#1277 2022-02-06 15:08:27
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,954
Re: Miscellany
1253) Observatory
Summary
An observatory is a location used for observing terrestrial, marine, or celestial events. Astronomy, climatology/meteorology, geophysical, oceanography and volcanology are examples of disciplines for which observatories have been constructed. Historically, observatories were as simple as containing an astronomical sextant (for measuring the distance between stars) or Stonehenge (which has some alignments on astronomical phenomena).
Astronomical observatories
Astronomical observatories are mainly divided into four categories: space-based, airborne, ground-based, and underground-based.
Ground-based observatories
Ground-based observatories, located on the surface of Earth, are used to make observations in the radio and visible light portions of the electromagnetic spectrum. Most optical telescopes are housed within a dome or similar structure, to protect the delicate instruments from the elements. Telescope domes have a slit or other opening in the roof that can be opened during observing, and closed when the telescope is not in use. In most cases, the entire upper portion of the telescope dome can be rotated to allow the instrument to observe different sections of the night sky. Radio telescopes usually do not have domes.
For optical telescopes, most ground-based observatories are located far from major centers of population, to avoid the effects of light pollution. The ideal locations for modern observatories are sites that have dark skies, a large percentage of clear nights per year, dry air, and are at high elevations. At high elevations, the Earth's atmosphere is thinner, thereby minimizing the effects of atmospheric turbulence and resulting in better astronomical "seeing". Sites that meet the above criteria for modern observatories include the southwestern United States, Hawaii, Canary Islands, the Andes, and high mountains in Mexico such as Sierra Negra. Major optical observatories include Mauna Kea Observatory and Kitt Peak National Observatory in the US, Roque de los Muchachos Observatory in Spain, and Paranal Observatory and Cerro Tololo Inter-American Observatory in Chile.
Specific research study performed in 2009 shows that the best possible location for ground-based observatory on Earth is Ridge A — a place in the central part of Eastern Antarctica. This location provides the least atmospheric disturbances and best visibility.
Radio observatories
Beginning in 1930s, radio telescopes have been built for use in the field of radio astronomy to observe the Universe in the radio portion of the electromagnetic spectrum. Such an instrument, or collection of instruments, with supporting facilities such as control centres, visitor housing, data reduction centers, and/or maintenance facilities are called radio observatories. Radio observatories are similarly located far from major population centers to avoid electromagnetic interference (EMI) from radio, TV, radar, and other EMI emitting devices, but unlike optical observatories, radio observatories can be placed in valleys for further EMI shielding. Some of the world's major radio observatories include the Very Large Array in New Mexico, United States, Jodrell Bank in the UK, Arecibo in Puerto Rico, Parkes in New South Wales, Australia, and Chajnantor in Chile.
Highest astronomical observatories
Since the mid-20th century, a number of astronomical observatories have been constructed at very high altitudes, above 4,000–5,000 m (13,000–16,000 ft). The largest and most notable of these is the Mauna Kea Observatory, located near the summit of a 4,205 m (13,796 ft) volcano in Hawaiʻi. The Chacaltaya Astrophysical Observatory in Bolivia, at 5,230 m (17,160 ft), was the world's highest permanent astronomical observatory from the time of its construction during the 1940s until 2009. It has now been surpassed by the new University of Tokyo Atacama Observatory, an optical-infrared telescope on a remote 5,640 m (18,500 ft) mountaintop in the Atacama Desert of Chile.
Space-based observatories
Space-based observatories are telescopes or other instruments that are located in outer space, many in orbit around the Earth. Space telescopes can be used to observe astronomical objects at wavelengths of the electromagnetic spectrum that cannot penetrate the Earth's atmosphere and are thus impossible to observe using ground-based telescopes. The Earth's atmosphere is opaque to ultraviolet radiation, X-rays, and gamma rays and is partially opaque to infrared radiation so observations in these portions of the electromagnetic spectrum are best carried out from a location above the atmosphere of our planet. Another advantage of space-based telescopes is that, because of their location above the Earth's atmosphere, their images are free from the effects of atmospheric turbulence that plague ground-based observations. As a result, the angular resolution of space telescopes such as the Hubble Space Telescope is often much smaller than a ground-based telescope with a similar aperture. However, all these advantages do come with a price. Space telescopes are much more expensive to build than ground-based telescopes. Due to their location, space telescopes are also extremely difficult to maintain. The Hubble Space Telescope was serviced by the Space Shuttle while many other space telescopes cannot be serviced at all. The James Webb Space Telescope(JWST) will replace the Hubble Space Telescope in 2021.
Airborne observatories
Airborne observatories have the advantage of height over ground installations, putting them above most of the Earth's atmosphere. They also have an advantage over space telescopes: The instruments can be deployed, repaired and updated much more quickly and inexpensively. The Kuiper Airborne Observatory and the Stratospheric Observatory for Infrared Astronomy use airplanes to observe in the infrared, which is absorbed by water vapor in the atmosphere. High-altitude balloons for X-ray astronomy have been used in a variety of countries.
Volcano observatories
A volcano observatory is an institution that conducts the monitoring of a volcano as well as research in order to understand the potential impacts of active volcanism. Among the best known are the Hawaiian Volcano Observatory and the Vesuvius Observatory. Mobile volcano observatories exist with the USGS VDAP (Volcano Disaster Assistance Program), to be deployed on demand. Each volcano observatory has a geographic area of responsibility it is assigned to whereby the observatory is tasked with spreading activity forecasts, analyzing potential volcanic activity threats and cooperating with communities in preparation for volcanic eruption.
Some Observatories
Atacama Large Millimeter Array, Chile, at 5,058 m (16,594 ft).
Paranal Observatory, Chile, home of the VLT at 2,635 m (8,645 ft).
The Mauna Kea Observatories, Hawaii, home of several of the world's largest optical telescopes at 4,205 m (13,796 ft).
Haleakala Observatory at 3,036 m (9,961 ft), Maui, Hawaii.
Details:
Astronomical observatory
Astronomical observatory is any structure containing telescopes and auxiliary instruments with which to observe celestial objects. Observatories can be classified on the basis of the part of the electromagnetic spectrum in which they are designed to observe. The largest number of observatories are optical; i.e., they are equipped to observe in and near the region of the spectrum visible to the human eye. Some other observatories are instrumented to detect cosmic emitters of radio waves, while still others called satellite observatories are Earth satellites that carry special telescopes and detectors to study celestial sources of such forms of high-energy radiation as gamma rays and X-rays from high above the atmosphere.
Optical observatories have a long history. The predecessors of astronomical observatories were monolithic structures that tracked the positions of the Sun, Moon, and other celestial bodies for timekeeping or calendrical purposes. The most famous of these ancient structures is Stonehenge, constructed in England over the period from 3000 to 1520 BCE. At about the same time, astrologer-priests in Babylonia observed the motions of the Sun, Moon, and planets from atop their terraced towers known as ziggurats. No astronomical instruments appear to have been used. The Maya people of the Yucatán Peninsula in Mexico carried out the same practice at El Caracol, a dome-shaped structure somewhat resembling a modern optical observatory. There is again no evidence of any scientific instrumentation, even of a rudimentary nature.
Perhaps the first observatory that used instruments for accurately measuring the positions of celestial objects was built about 150 BCE on the island of Rhodes by the greatest of the pre-Christian astronomers, Hipparchus. There he discovered precession and developed the magnitude system used to indicate the brightness of celestial objects. The true predecessors of the modern observatory were those established in the Islamic world. Observatories were built at Damascus and Baghdad as early as the 9th–10th century CE. A splendid one was built at Marāgheh (now in Iran) about 1260 CE, and substantial modifications in Ptolemaic astronomy were introduced there. The most productive Islamic observatory was that erected by the Timurid prince Ulūgh Beg at Samarkand about 1420; he and his assistants made a catalog of stars from observations with a large quadrant. The first notable premodern European observatory was that at Uraniborg on the island of Hven, built by King Frederick II of Denmark for Tycho Brahe in 1576 CE.
The first optical telescope used to study the heavens was constructed in 1609 by Galileo Galilei, using information from Flemish pioneers in lens-making. The first major centres for astronomical study used a telescope movable only in one plane, with motion solely along the local meridian (the “transit,” or “meridian circle”). Such centres were founded in the 18th and 19th centuries at Greenwich (London), Paris, Cape Town, and Washington, D.C. By timing the passage of stars as the local meridian was swept past them by Earth’s rotation, astronomers were able to improve the accuracy of position measurements of celestial objects from a few minutes of arc (before the advent of the telescope) to less than a tenth of a second of arc.
One notable observatory built and operated by an individual was that of Sir William Herschel, assisted by his sister, Caroline Herschel, in Slough, England. Known as Observatory House, its largest instrument had a mirror made of speculum metal, with a diameter of 122 cm (48 inches) and a focal length of 17 metres (40 feet). Completed in 1789, it became one of the technical wonders of the 18th century.
Today the site of the world’s largest grouping of large optical telescopes is atop Mauna Kea on the island of Hawaii. Most notable in this array of instruments are the two 10-metre (394-inch) Keck telescopes, the 8.2-metre (320-inch) Subaru Telescope, and the two 8.1-metre (319-inch) Gemini telescopes. The largest modern-day optical telescope is the 10.4-metre (409-inch) Gran Telescopio Canarias reflector on La Palma, in the Canary Islands, Spain.
The ability to observe the universe in the radio region of the spectrum was developed during the 1930s. The American engineer Karl Jansky detected radio signals from the centre of the Milky Way Galaxy in 1931 by means of a linear directional antenna. Soon thereafter the American engineer and astronomer Grote Reber constructed a prototype of the radio telescope, a bowl-shaped antenna 9.4 metres (31 feet) in diameter.
Today’s radio telescopes are capable of observing at most wavelength regions, from a few millimetres to about 20 metres. They vary in construction, though they are typically huge movable dishes. The world’s largest steerable dish is the 100-metre (328-foot) telescope at Green Bank, West Virginia. The largest single-unit radio telescope is the Five-hundred-metre Aperture Spherical radio Telescope (FAST) located in Guizhou province, China. Lying level in a natural depression, the main antenna of this instrument has a diameter of 500 metres (about 1,600 feet). Limited aiming capability is allowed by Earth’s motion and by some movement of the panels of the dish and of the overhanging antenna.
One other significant radio telescope is the Very Large Array (VLA), operated by the National Radio Astronomy Observatory. Located near Socorro, New Mexico, the VLA is composed of 27 individual radio telescopes, each of which is 25 metres (81 feet) in diameter. These instruments are not only steerable but also movable over railroad tracks in the shape of a large Y. Each arm of the Y is 21 km (13 miles) long. The purpose of the VLA is to obtain extremely high-resolution imaging of cosmic radio sources. The resolving ability of a telescope, whether radio or optical, improves with increasing diameter. The individual dishes of the VLA work in precise unison to fabricate a large radio telescope having an effective diameter of 27 km (16.7 miles).
With the advent of the space age, the capability of astronomical instruments to orbit above Earth’s absorbing and distorting atmosphere enabled astronomers to build telescopes sensitive to regions of the electromagnetic spectrum besides those of visible light and radio waves. Since the 1960s, orbiting observatories have been launched to observe gamma rays (Compton Gamma Ray Observatory and Fermi Gamma-ray Space Telescope), X-rays (Chandra X-ray Observatory and XMM-Newton), ultraviolet radiation (International Ultraviolet Explorer and Far Ultraviolet Spectroscopic Explorer), and infrared radiation (Infrared Astronomical Satellite and Spitzer Space Telescope). The Hubble Space Telescope, which launched in 1990, observed mainly in visible light. Several satellite observatories such as Herschel, Planck, and the Wilkinson Microwave Anisotropy Probe have even been placed at the second Lagrangian point (L2) of the Earth-Moon system, a gravitational balance point between Earth and the Sun and 1.5 million km (0.9 million miles) opposite the Sun from Earth. Satellites at L2 are isolated from Earth’s infrared and radio emissions and are also more thermally stable than Earth-orbiting satellites that are alternately cooled and heated as they pass in and out of Earth’s shadow.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Online
#1278 2022-02-07 14:00:06
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,954
Re: Miscellany
1254) Blood–brain barrier
The blood–brain barrier (BBB) is a highly selective semipermeable border of endothelial cells that prevents solutes in the circulating blood from non-selectively crossing into the extracellular fluid of the central nervous system where neurons reside. The blood–brain barrier is formed by endothelial cells of the capillary wall, astrocyte end-feet ensheathing the capillary, and pericytes embedded in the capillary basement membrane. This system allows the passage of some small molecules by passive diffusion, as well as the selective and active transport of various nutrients, ions, organic anions, and macromolecules such as glucose and amino acids that are crucial to neural function.
The blood–brain barrier restricts the passage of pathogens, the diffusion of solutes in the blood, and large or hydrophilic molecules into the cerebrospinal fluid, while allowing the diffusion of hydrophobic molecules (O2, CO2, hormones) and small non-polar molecules. Cells of the barrier actively transport metabolic products such as glucose across the barrier using specific transport proteins. The barrier also restricts the passage of peripheral immune factors, like signaling molecules, antibodies, and immune cells, into the CNS, thus insulating the brain from damage due to peripheral immune events.
Specialized brain structures participating in sensory and secretory integration within brain neural circuits—the circumventricular organs and choroid plexus—have in contrast highly permeable capillaries.
Structure
The BBB results from the selectivity of the tight junctions between the endothelial cells of brain capillaries, restricting the passage of solutes. At the interface between blood and the brain, endothelial cells are adjoined continuously by these tight junctions, which are composed of smaller subunits of transmembrane proteins, such as occludin, claudins (such as Claudin-5), junctional adhesion molecule (such as JAM-A). Each of these tight junction proteins is stabilized to the endothelial cell membrane by another protein complex that includes scaffolding proteins such as tight junction protein 1 (ZO1) and associated proteins.
The BBB is composed of endothelial cells restricting passage of substances from the blood more selectively than endothelial cells of capillaries elsewhere in the body. Astrocyte cell projections called astrocytic feet (also known as "glia limitans") surround the endothelial cells of the BBB, providing biochemical support to those cells. The BBB is distinct from the quite similar blood-cerebrospinal fluid barrier, which is a function of the choroidal cells of the choroid plexus, and from the blood-retinal barrier, which can be considered a part of the whole realm of such barriers.
Not all vessels in the human brain exhibit BBB properties. Some examples of this include the circumventricular organs, the roof of the third and fourth ventricles, capillaries in the pineal gland on the roof of the diencephalon and the pineal gland. The pineal gland secretes the hormone melatonin "directly into the systemic circulation", thus melatonin is not affected by the blood–brain barrier.
Development
The BBB appears to be functional by the time of birth. P-glycoprotein, a transporter, exists already in the embryonal endothelium.
Measurement of brain uptake of various blood-borne solutes showed that newborn endothelial cells were functionally similar to those in adults, indicating that a selective BBB is operative at birth.
In mice, Claudin-5 loss during development is lethal and results in size-selective loosening of the BBB.
Function
The blood–brain barrier acts effectively to protect the brain from circulating pathogens. Accordingly, blood-borne infections of the brain are rare. Infections of the brain that do occur are often difficult to treat. Antibodies are too large to cross the blood–brain barrier, and only certain antibiotics are able to pass. In some cases, a drug has to be administered directly into the cerebrospinal fluid where it can enter the brain by crossing the blood-cerebrospinal fluid barrier.
The blood–brain barrier may become leaky in select neurological diseases, such as amyotrophic lateral sclerosis, epilepsy, brain trauma and edema, and in systemic diseases, such as liver failure. The blood–brain barrier becomes more permeable during inflammation, potentially allowing antibiotics and phagocytes to move across the BBB.
Circumventricular organs
Circumventricular organs (CVOs) are individual structures located adjacent to the fourth ventricle or third ventricle in the brain, and are characterized by dense capillary beds with permeable endothelial cells unlike those of the blood–brain barrier. Included among CVOs having highly permeable capillaries are the area postrema, subfornical organ, vascular organ of the lamina terminalis, median eminence, pineal gland, and three lobes of the pituitary gland.
Permeable capillaries of the sensory CVOs (area postrema, subfornical organ, vascular organ of the lamina terminalis) enable rapid detection of circulating signals in systemic blood, while those of the secretory CVOs (median eminence, pineal gland, pituitary lobes) facilitate transport of brain-derived signals into the circulating blood. Consequently, the CVO permeable capillaries are the point of bidirectional blood–brain communication for neuroendocrine function.
Specialized permeable zones
The border zones between brain tissue "behind" the blood–brain barrier and zones "open" to blood signals in certain CVOs contain specialized hybrid capillaries that are leakier than typical brain capillaries, but not as permeable as CVO capillaries. Such zones exist at the border of the area postrema—nucleus tractus solitarii (NTS), and median eminence—hypothalamic arcuate nucleus. These zones appear to function as rapid transit regions for brain structures involved in diverse neural circuits—like the NTS and arcuate nucleus—to receive blood signals which are then transmitted into neural output. The permeable capillary zone shared between the median eminence and hypothalamic arcuate nucleus is augmented by wide pericapillary spaces, facilitating bidirectional flow of solutes between the two structures, and indicating that the median eminence is not only a secretory organ, but may also be a sensory organ.
Therapeutic research:
As a drug target
The blood–brain barrier is formed by the brain capillary endothelium and excludes from the brain 100% of large-molecule neurotherapeutics and more than 98% of all small-molecule drugs. Overcoming the difficulty of delivering therapeutic agents to specific regions of the brain presents a major challenge to treatment of most brain disorder. In its neuroprotective role, the blood–brain barrier functions to hinder the delivery of many potentially important diagnostic and therapeutic agents to the brain. Therapeutic molecules and antibodies that might otherwise be effective in diagnosis and therapy do not cross the BBB in adequate amounts to be clinically effective.
Mechanisms for drug targeting in the brain involve going either "through" or "behind" the BBB. Modalities for drug delivery to the brain in unit doses through the BBB entail its disruption by osmotic means, or biochemically by the use of vasoactive substances, such as bradykinin, or even by localized exposure to high-intensity focused ultrasound (HIFU).
Other methods used to get through the BBB may entail the use of endogenous transport systems, including carrier-mediated transporters, such as glucose and amino acid carriers, receptor-mediated transcytosis for insulin or transferrin, and the blocking of active efflux transporters such as p-glycoprotein. Some studies have shown that vectors targeting BBB transporters, such as the transferrin receptor, have been found to remain entrapped in brain endothelial cells of capillaries, instead of being ferried across the BBB into the targeted area.
Nanoparticles
Nanotechnology is under preliminary research for its potential to facilitate the transfer of drugs across the BBB. Capillary endothelial cells and associated pericytes may be abnormal in tumors and the blood–brain barrier may not always be intact in brain tumors. Other factors, such as astrocytes, may contribute to the resistance of brain tumors to therapy using nanoparticles. Fat soluble molecules less than 400 daltons in weight can freely diffuse past the BBB through lipid mediated passive diffusion.
History
In 1898, Arthur Biedl and R. Kraus observed that low-concentration "bile salts" failed to affect behavior when injected into the bloodstream of animals. Thus, in theory, they had failed to enter the brain. Two years later, Max Lewandowsky coined the term "Blood–brain barrier" in 1900, referring to the hypothesized semipermeable membrane (then termed hematoencephalic barrier).
All the whole, bacteriologist Paul Ehrlich was studying staining, a procedure that is used in many microscopy studies to make fine biological structures visible using chemical dyes. As Ehrlich injected some of these dyes (notably the aniline dyes that were then widely used), the dye stained all of the organs of some kinds of animals except for their brains. At that time, Ehrlich attributed this lack of staining to the brain simply not picking up as much of the dye.
However, in a later experiment in 1913, Edwin Goldmann (one of Ehrlich's students) injected the dye directly into the cerebrospinal fluids of animal brains. He found then the brains did become dyed, but the rest of the body did not, demonstrating the existence of a compartmentalization between the two. At that time, it was thought that the blood vessels themselves were responsible for the barrier, since no obvious membrane could be found.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Online
#1279 2022-02-08 13:46:20
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,954
Re: Miscellany
1255) Cotyledon
Summary
Cotyledon is seed leaf within the embryo of a seed. Cotyledons help supply the nutrition a plant embryo needs to germinate and become established as a photosynthetic organism and may themselves be a source of nutritional reserves or may aid the embryo in metabolizing nutrition stored elsewhere in the seed. Angiosperms (flowering plants) whose embryos have a single cotyledon are grouped as monocots, or monocotyledonous plants; most embryos with two cotyledons are grouped as eudicots, or eudicotyledonous plants. The number of cotyledons in the embryos of seeds of gymnosperms is highly variable, ranging from 8 to 20 or more.
Function
Until it becomes nutritionally self-supporting, a seedling depends upon reserves provided by the parent. In angiosperms these reserves are found in the endosperm, in residual tissues of the ovule, or in the body of the embryo, usually in the cotyledons. Since reserve materials are partly in insoluble form—as starch grains, protein granules, lipid droplets, and the like—much of the early metabolism of the seedling is concerned with mobilizing these materials and delivering, or translocating, the products to active areas. In some seeds (e.g., castor beans), absorption of nutrients from reserves is through the cotyledons, which later expand in the light to become the first organs active in photosynthesis. In many monocots, the cotyledon acts as a special absorbing organ to mobilize the reserve materials and withdraw them from the endosperm; e.g., in grasses, the cotyledon has been modified into an enzyme-secreting scutellum (“shield”) between embryo and endosperm. When the reserves are stored in the cotyledons themselves, as is common in many eudicots, these organs may shrink after germination and die or develop chlorophyll and become photosynthetic. Whether cotyledons help absorb nutrients from the endosperm or store the nutrients themselves, these embryonic seed leaves do not usually persist long after germination.
Germination patterns
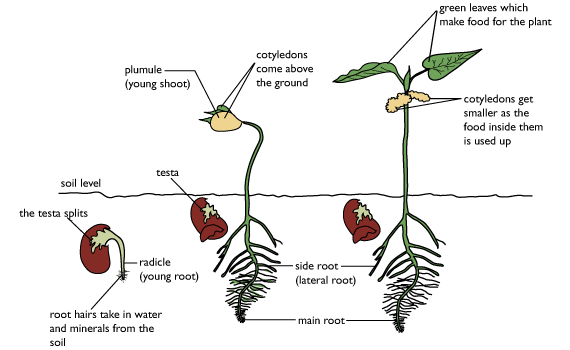
Two patterns of seed germination occur in angiosperms, depending on whether the cotyledons emerge from the seed: hypogeal (belowground germination) and epigeal (aboveground germination). In hypogeous germination, the cotyledons do not emerge from the seed but rather force the radicle and epicotyl axis (which will produce the first true leaves) to elongate out of the seed coat. The seed, with the enclosed cotyledons, remains underground, and the epicotyl grows up through the soil. When the cotyledons contain seed nutritional reserves, these reserves are transferred directly to the developing radicle and epicotyl (e.g., garden pea). When the endosperm contains the reserves, the cotyledons penetrate the storage tissues and transfer the nutritional products to the developing radicle and epicotyl (e.g., garlic).
In epigeous germination, the radicle emerges from the seed and the hypocotyl (the embryonic stem) elongates, raising the cotyledons, epicotyl, and remains of the seed coat aboveground. The cotyledons may then expand and function photosynthetically as normal leaves (e.g., castor bean). When the cotyledons contain the seed nutritional reserves, they transfer them to the rest of the seedling and degenerate without becoming significantly photosynthetic (e.g., garden beans). Eventually the seedling becomes independent of the seed reserves and grows into a mature plant capable of reproduction.
Details
A cotyledon is a significant part of the embryo within the seed of a plant, and is defined as "the embryonic leaf in seed-bearing plants, one or more of which are the first to appear from a germinating seed." The number of cotyledons present is one characteristic used by botanists to classify the flowering plants (angiosperms). Species with one cotyledon are called monocotyledonous ("monocots"). Plants with two embryonic leaves are termed dicotyledonous ("dicots").
In the case of dicot seedlings whose cotyledons are photosynthetic, the cotyledons are functionally similar to leaves. However, true leaves and cotyledons are developmentally distinct. Cotyledons are formed during embryogenesis, along with the root and shoot meristems, and are therefore present in the seed prior to germination. True leaves, however, are formed post-embryonically (i.e. after germination) from the shoot apical meristem, which is responsible for generating subsequent aerial portions of the plant.
The cotyledon of grasses and many other monocotyledons is a highly modified leaf composed of a scutellum and a coleoptile. The scutellum is a tissue within the seed that is specialized to absorb stored food from the adjacent endosperm. The coleoptile is a protective cap that covers the plumule (precursor to the stem and leaves of the plant).
Gymnosperm seedlings also have cotyledons, and these are often variable in number (multicotyledonous), with from 2 to 24 cotyledons forming a whorl at the top of the hypocotyl (the embryonic stem) surrounding the plumule. Within each species, there is often still some variation in cotyledon numbers, e.g. Monterey pine (Pinus radiata) seedlings have 5–9, and Jeffrey pine (Pinus jeffreyi) 7–13 (Mirov 1967), but other species are more fixed, with e.g. Mediterranean cypress always having just two cotyledons. The highest number reported is for big-cone pinyon (Pinus maximartinezii), with 24 (Farjon & Styles 1997).
The cotyledons may be ephemeral, lasting only days after emergence, or persistent, enduring at least a year on the plant. The cotyledons contain (or in the case of gymnosperms and monocotyledons, have access to) the stored food reserves of the seed. As these reserves are used up, the cotyledons may turn green and begin photosynthesis, or may wither as the first true leaves take over food production for the seedling.
Epigeal versus hypogeal development
Cotyledons may be either epigeal, expanding on the germination of the seed, throwing off the seed shell, rising above the ground, and perhaps becoming photosynthetic; or hypogeal, not expanding, remaining below ground and not becoming photosynthetic. The latter is typically the case where the cotyledons act as a storage organ, as in many nuts and acorns.
Hypogeal plants have (on average) significantly larger seeds than epigeal ones. They are also capable of surviving if the seedling is clipped off, as meristem buds remain underground (with epigeal plants, the meristem is clipped off if the seedling is grazed). The tradeoff is whether the plant should produce a large number of small seeds, or a smaller number of seeds which are more likely to survive.
The ultimate development of the epigeal habit is represented by a few plants, mostly in the family Gesneriaceae in which the cotyledon persists for a lifetime. Such a plant is Streptocarpus wendlandii of South Africa in which one cotyledon grows to be up to 75 centimeters (2.5 feet) in length and up to 61 cm (two feet) in width (the largest cotyledon of any dicot, and exceeded only by Lodoicea). Adventitious flower clusters form along the midrib of the cotyledon. The second cotyledon is much smaller and ephemeral.
Related plants may show a mixture of hypogeal and epigeal development, even within the same plant family. Groups which contain both hypogeal and epigeal species include, for example, the Southern Hemisphere conifer family Araucariaceae, the pea family, Fabaceae, and the genus Lilium. The frequently garden grown common bean, Phaseolus vulgaris, is epigeal, while the closely related runner bean, Phaseolus coccineus, is hypogeal.
History
The term cotyledon was coined by Marcello Malpighi (1628–1694). John Ray was the first botanist to recognize that some plants have two and others only one, and eventually the first to recognize the immense importance of this fact to systematics, in Methodus plantarum (1682).
Theophrastus (3rd or 4th century BC) and Albertus Magnus (13th century) may also have recognized the distinction between the dicotyledons and monocotyledons.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Online
#1280 2022-02-09 14:08:21
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,954
Re: Miscellany
1256) Gallbladder cancer
Summary
Gallbladder cancer is a disease characterized by the growth of malignant cells in the gallbladder. Gallbladder cancer is a rare disease and often is detected only after cancer cells have metastasized (spread) to other organs, resulting in poor survival rates. About 60 to 70 percent of gallbladder cancers are found incidentally following cholecystectomy (surgical removal of the gallbladder) for otherwise benign diseases, such as cholecystitis (inflammation of the gallbladder) or gallstones (cholelithiasis).
Gallbladder cancer affects women more often than men, and its incidence increases with age. The disease has unusually high rates of incidence among Native Americans in both North and South America. Some of the highest incidence rates are found in populations in the Andes Mountains of South America (particularly in Chile and Bolivia), in Mexican American populations, and in peoples living in northern India. Incidence is also high in South Korea.
Risk factors
A number of risk factors are associated with the development of gallbladder cancer. The presence of long-term inflammation, such as that associated with chronic gallstones, can increase the likelihood of tumours, possibly by creating an environment that promotes genetic mutations that then lead to tumour development. The larger the gallstones (e.g., larger than 3 cm [1.2 inches] in diameter), the higher the risk of gallbladder cancer. Although gallstones are present in about 85 percent of gallbladder cancer patients, however, only a small fraction of individuals with gallstones develop cancer.
Long-term infection with different types of bacteria, particularly Salmonella typhi, is also associated with an increased risk of gallbladder cancer. Risk is also increased in persons who have a family history of colorectal cancer, specifically conditions such as Gardner syndrome and hereditary nonpolyposis colon cancer (HNPCC). Other possible risk factors include obesity and exposure to certain chemical substances (e.g., radon).
Symptoms
Symptoms of gallbladder cancer tend to be vague. They can include abdominal pain, loss of appetite, fever, nausea, and vomiting. Some patients develop jaundice (yellowing of the skin, whites of the eyes, and mucous membranes), abdominal distension, and itching. Early cancer, however, may be asymptomatic; when symptoms develop later, the cancer is likely to have progressed to an incurable state.
Diagnosis and treatment
Various approaches are used to diagnose the presence of gallbladder cancer. Ultrasound is the usual diagnostic study when gallstone-related disease is suspected. Ultrasound can show thickened walls and masses; however, it may not give a conclusive diagnosis of gallbladder cancer. Computed tomography (CT) scanning can be used to assess the extent of tumour growth and spread. Magnetic resonance cholangiopancreatography (MRCP) may be used to visualize the local anatomy of the gallbladder and to differentiate between benign and malignant lesions. Biopsy of the gallbladder prior to surgery usually is not undertaken owing to an increased risk of tumour cells’ spreading to surrounding tissues.
The treatment of gallbladder cancer depends on the stage at which the cancer is diagnosed. Staging determines the extent to which the cancer has grown or spread from the primary site (initial site of development). Treatment options include simple cholecystectomy (removal of the gallbladder only), radical cholecystectomy (removal of the gallbladder, excision of specific bile ducts, removal of regional lymph nodes, and removal of parts of the liver), radiation therapy, chemotherapy, palliative care, or some combination thereof.
Details
Gallbladder cancer is a relatively uncommon cancer, with an incidence of fewer than 2 cases per 100,000 people per year in the United States. It is particularly common in central and South America, central and eastern Europe, Japan and northern India; it is also common in certain ethnic groups e.g. Native American Indians and Hispanics. If it is diagnosed early enough, it can be cured by removing the gallbladder, part of the liver and associated lymph nodes. Most often it is found after symptoms such as abdominal pain, jaundice and vomiting occur, and it has spread to other organs such as the liver.
It is a rare cancer that is thought to be related to gallstones building up, which also can lead to calcification of the gallbladder, a condition known as porcelain gallbladder. Porcelain gallbladder is also rare. Some studies indicate that people with porcelain gallbladder have a high risk of developing gallbladder cancer, but other studies question this. The outlook is poor for recovery if the cancer is found after symptoms have started to occur, with a 5-year survival rate of close to 3%.
Signs and symptoms
* Steady pain in the upper right abdomen
* Indigestion
* Dyspepsia (gas)
* Bilious vomit
* Weakness
* Loss of appetite
* Weight loss
* Jaundice and vomiting due to obstruction
Early symptoms mimic gallbladder inflammation due to gallstones. Later, the symptoms may be that of biliary and stomach obstruction.
Of note, Courvoisier's law states that in the presence of a palpably enlarged gallbladder which is nontender and accompanied with mild painless jaundice, the cause is unlikely to be gallstones. This implicates possible malignancy of the gallbladder or pancreas, and the swelling is unlikely due to gallstones due to the chronic inflammation associated with gallstones leading to a shrunken, non-distensible gallbladder. However, Ludwig Georg Courvoisier's original observations, published in Germany in 1890, were not originally cited as a law, and no mention of malignancy or pain (tenderness) was made. These points are commonly misquoted or confused in the medical literature.
Risk factors
* Gender— approximately twice as common in women than men, usually in seventh and eighth decades
* Obesity
* Chronic cholecystitis and cholelithiasis
* Primary sclerosing cholangitis
* Chronic typhoid infection of gallbladder; chronic Salmonella typhi carriers have 3 to 200 times higher risk of gallbladder cancer than non-carriers and 1–6% lifetime risk of development of cancer
* Various single nucleotide polymorphisms (SNPs) have been shown to be associated with gallbladder cancer; however, existing genetic studies in GBC susceptibility have so far been insufficient to confirm any association
* Gallbladder polyps
* Calcified gallbladder wall (porcelain gallbladder)
* Congenital abnormalities of the bile duct such as choledochal cyst
Diagnosis
Early diagnosis is not generally possible. People at high risk, such as women or Native Americans with gallstones, are evaluated closely. Transabdominal ultrasound, CT scan, endoscopic ultrasound, MRI, and MR cholangio-pancreatography (MRCP) can be used for diagnosis. A large number of gallbladder cancers are found incidentally in patients being evaluated for cholelithiasis, or gallstone formation, which is far more common. A biopsy is the only certain way to tell whether or not the tumorous growth is malignant.
Differential diagnosis
Xanthogranulomatous cholecystitis (XGC) is a rare form of gallbladder disease which mimics gallbladder cancer although it is not cancerous. It was first discovered and reported in the medical literature in 1976 by J.J. McCoy Jr., and colleagues.
Treatment
If detected early in a stage where it has not spread, gallbladder cancer can be treated by surgery. Surgery for gallbladder cancer is called radical cholecystectomy or extended cholecystectomy. It entails the removal of gallbladder along with adequate removal of its liver bed to the healthy tissue. The lymph nodes in the vicinity are also removed. Sometimes removal of a large part of the liver called hepatectomy is required to completely remove the tumor. The bile duct if involved also needs to be removed. However, with gallbladder cancer's extremely poor prognosis, most patients will die within a year of surgery. If surgery is not possible, endoscopic stenting or percutaneous transhepatic biliary drainage (PTBD) of the biliary tree can reduce jaundice and a stent in the stomach may relieve vomiting. Chemotherapy and radiation may also be used with surgery. If gallbladder cancer is diagnosed after cholecystectomy for stone disease (incidental cancer), re-operation to remove part of liver and lymph nodes is required in most cases. When it is done as early as possible, patients have the best chance of long-term survival and even cure.
Epidemiology
Most tumors are adenocarcinomas, with a small percent being squamous cell carcinomas.
* Gallbladder cancer is relatively rare, affecting fewer than 5000 people in the United States per year
* Gallbladder cancer is more common in South American countries, Japan, and Israel; in Chile, gallbladder cancer is the fourth most common cause of cancer deaths.
* 5th most common gastrointestinal cancer
* Up to 5 times more common in women than men depending on population (e.g. 73% female in China)
* The age adjusted incidence rates of gallbladder cancer is highest in Chile, followed by in the state of Assam in India
Prognosis
The prognosis still remains poor. The cancer commonly spreads to the liver, bile duct, stomach, and duodenum.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Online
#1281 2022-02-10 14:05:21
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,954
Re: Miscellany
1257) Colorectal cancer
Summary
Colorectal cancer (CRC), also known as bowel cancer, colon cancer, or rectal cancer, is the development of cancer from the colon or rectum (parts of the large intestine). Signs and symptoms may include blood in the stool, a change in bowel movements, weight loss, and fatigue.
Most colorectal cancers are due to old age and lifestyle factors, with only a small number of cases due to underlying genetic disorders. Risk factors include diet, obesity, smoking, and lack of physical activity. Dietary factors that increase the risk include red meat, processed meat, and alcohol. Another risk factor is inflammatory bowel disease, which includes Crohn's disease and ulcerative colitis. Some of the inherited genetic disorders that can cause colorectal cancer include familial adenomatous polyposis and hereditary non-polyposis colon cancer; however, these represent less than 5% of cases. It typically starts as a benign tumor, often in the form of a polyp, which over time becomes cancerous.
Bowel cancer may be diagnosed by obtaining a sample of the colon during a sigmoidoscopy or colonoscopy. This is then followed by medical imaging to determine whether the disease has spread. Screening is effective for preventing and decreasing deaths from colorectal cancer. Screening, by one of a number of methods, is recommended starting from the age of 50 to 75. During colonoscopy, small polyps may be removed if found. If a large polyp or tumor is found, a biopsy may be performed to check if it is cancerous. Aspirin and other non-steroidal anti-inflammatory drugs decrease the risk. Their general use is not recommended for this purpose, however, due to side effects.
Treatments used for colorectal cancer may include some combination of surgery, radiation therapy, chemotherapy and targeted therapy. Cancers that are confined within the wall of the colon may be curable with surgery, while cancer that has spread widely is usually not curable, with management being directed towards improving quality of life and symptoms. The five-year survival rate in the United States is around 65%. The individual likelihood of survival depends on how advanced the cancer is, whether or not all the cancer can be removed with surgery and the person's overall health. Globally, colorectal cancer is the third most common type of cancer, making up about 10% of all cases. In 2018, there were 1.09 million new cases and 551,000 deaths from the disease. It is more common in developed countries, where more than 65% of cases are found. It is less common in women than men.
Details
Colorectal cancer is a disease characterized by uncontrolled growth of cells within the large intestine (colon) or rectum (terminal portion of the large intestine). Colon cancer (or bowel cancer) and rectal cancer are sometimes referred to separately. Colorectal cancer develops slowly but can spread to surrounding and distant tissues of the body.
Causes and symptoms
Like most cancers, colorectal cancers have multiple causes, many of which remain unknown. Some cases appear to be inherited, while others seem to occur randomly or to have nongenetic causes. Approximately 95 percent of colorectal cancers involve the glandular cells in the wall of the colon and are called adenocarcinomas (see carcinoma). Other colorectal cancers may begin among hormone-producing cells, immune cells, or underlying connective tissue.
Several factors increase the risk of developing the disease. In general, colorectal cancer becomes more common with increasing age; 90 percent of cases are diagnosed in people age 50 or older. However, the malignancy also occurs with some frequency among persons under age 50. A family history of colorectal cancer—specifically, forms such as familial adenomatous polyposis (FAP), Gardner syndrome, and hereditary nonpolyposis colon cancer (HNPCC)—can predispose an individual to developing colorectal cancer. Each of these conditions is caused in part by a known genetic mutation. In addition, Ashkenazi Jews have a slightly higher incidence of colorectal cancer due to a mutated gene, and there exists a gene mutation that increases risk of colorectal cancer in people of European descent but does not increase risk in people of Japanese descent. This latter mutation, discovered in 2008, was the first to provide evidence of ethnic differences in genetic susceptibility to colorectal cancer.
Chronic inflammatory bowel diseases such as Crohn disease or ulcerative colitis are associated with colorectal cancer, as is the presence of a large number of noncancerous polyps along the wall of the colon or rectum. Other risk factors include physical inactivity and a diet high in fats. Those who have previously been treated for colorectal cancer are also at increased risk of recurrence. Certain gut bacteria, including species of Fusobacterium, have been implicated in colorectal cancer; Fusobacterium are present at increased levels in colorectal cancer patients and can trigger inflammatory responses associated with tumour growth and progression.
Because colorectal cancer is a disease of the digestive tract, many of the symptoms are associated with abnormal digestion and elimination. Symptoms include episodes of diarrhea or constipation that extend for days, blood in the stool, rectal bleeding, jaundice, abdominal pain, loss of appetite, and fatigue. Because these symptoms accompany a variety of different illnesses, a physician should be consulted to determine their cause.
Diagnosis
Diagnoses of colon and rectal cancers are made by means of several techniques. During a digital rectal exam, the physician inserts a gloved finger into the rectum and feels its surface for abnormalities. A fecal immunochemical test (FIT) may also be used to detect the presence of blood in the stool. FIT tests can be completed at home and then mailed to a laboratory for testing. Results are sent to the patient’s physician. If colorectal cancer is suspected, the patient may undergo further screening with a procedure known as a colonoscopy.
In order to examine the rectum more carefully, a physician may use a narrow, flexible tube called a sigmoidoscope to look at the lining of the rectum and the end of the colon. Colonoscopy uses a similar device to examine the entire colon. A biopsy may also be conducted in which abnormal tissue is removed by using the colonoscope and then examined under a microscope for signs of cancer. An X-ray procedure called a double-contrast barium enema may be used. Barium sulfate is used to coat the colon, and the colon is filled with air. A series of X rays are then taken, and the resulting high-contrast images indicate any abnormalities present.
If cancer is found, the degree to which it has spread (metastasized) from the colon or rectum is determined. Biopsies may be conducted of surrounding tissues, or one of several imaging techniques may be used to detect metastasis. Techniques include rectal ultrasound, magnetic resonance imaging (MRI), and X-ray or computed tomography (CT) scans.
Once colorectal cancer has been diagnosed, its stage is then determined to indicate how far the cancer has progressed. Stage 0 colorectal cancer is also called carcinoma in situ and is confined to the lining of the colon or rectum. Stage I cancers have spread into the connective tissue beneath the lining or into the underlying muscle layer. Stage II cancers have spread completely through the wall of the colon or rectum but have not invaded nearby lymph nodes. Stage III colorectal cancer has reached nearby lymph nodes, and stage IV cancers have spread to distant structures such as the lungs, liver, bones, or reproductive organs.
Colorectal cancer patients have an excellent five-year survival rate when the disease is detected early, and those who reach this stage often go on to live long, healthy lives. Approximately two-thirds of patients with local metastases survive for five or more years, but in cases where cancer is detected late and has spread to distant regions of the body, the five-year survival rate is very low.
Treatment
Colorectal cancer is treated by surgery, chemotherapy, or radiation. The method used depends on the site of the cancer and the degree to which it has spread. For cancers localized to the colon or rectum, surgery is usually all that is required. For early-stage colon cancer, a colonoscope may be used to remove the cancerous tissue. Other early cancers require a surgical resection, whereby the portion of the colon containing the cancerous tissue is removed along with surrounding tissue and nearby lymph nodes and the remainder of the colon is repaired.
Rectal cancers may be treated by removing only the cancerous polyp or polyps, the cancer plus surrounding tissues, or larger sections of the rectum. Some cancers may be removed by burning them in a procedure called electrofulguration. In cases where the lower portion of the rectum is involved, a colostomy may be required, whereby the surgeon creates an artificial opening for the removal of waste. If colorectal cancer has spread to surrounding tissues such as those of the uterus, prostate, liver, kidneys, or bladder, more extensive surgery may be required to remove all or part of these organs.
Both colon and rectal cancers may be treated with radiation, using either external beams or surgically implanted radioactive pellets. Radiation is usually used in conjunction with surgery—either before the surgery to shrink tumours or following surgery to destroy small amounts of remaining cancerous tissue. Chemotherapy may also be indicated for treatment of colorectal cancers, especially when cancer has spread to other parts of the body but also as an adjuvant therapy to primary surgery and radiation. Side effects of both radiation and chemotherapy may include vomiting, diarrhea, and fatigue.
Prevention
A lifestyle that includes regular exercise and a diet low in fats and high in fibre helps to prevent colorectal cancer. Early detection is important in preventing the development of advanced colorectal cancer. Some medical societies recommend regular screening by a physician after the age of 50.
Multiple studies have shown that the pain-reliever aspirin can effectively lower the risk for colorectal cancer in some persons and even reduce mortality rates for those already diagnosed with the disease. A report published in 2010 that analyzed data on aspirin use and cancer risk in different study groups over a 20-year period revealed that consistent use of low-dose aspirin (75–300 mg) lowered the risk for colon (bowel) cancer by as much as 25 percent. Long-term use of low-dose aspirin was also associated with a significant reduction in mortality from this form of the disease.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Online
#1282 2022-02-11 13:23:23
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,954
Re: Miscellany
1258) Electron diffraction
Summary
Electron diffraction is interference effects owing to the wavelike nature of a beam of electrons when passing near matter. According to the proposal (1924) of the French physicist Louis de Broglie, electrons and other particles have wavelengths that are inversely proportional to their momentum. Consequently, high-speed electrons have short wavelengths, a range of which are comparable to the spacings between atomic layers in crystals. A beam of such high-speed electrons should undergo diffraction, a characteristic wave effect, when directed through thin sheets of material or when reflected from the faces of crystals. Electron diffraction, in fact, was observed (1927) by C.J. Davisson and L.H. Germer in New York and by G.P. Thomson in Aberdeen, Scot. The wavelike nature of electron beams was thereby experimentally established, thus supporting an underlying principle of quantum mechanics.
As an analytic method, electron diffraction is used to identify a substance chemically or to locate the position of atoms in a substance. This information can be read from the patterns that are formed when various portions of the diffracted electron beam cross each other and by interference make a regular arrangement of impact positions, some where many electrons reach and some where few or no electrons reach. Some advanced analytical techniques, such as LEEDX (low-energy electron diffraction), depend on these diffraction patterns to examine solids, liquids, and gases.
Details
Electron diffraction refers to the wave nature of electrons. However, from a technical or practical point of view, it may be regarded as a technique used to study matter by firing electrons at a sample and observing the resulting interference pattern. This phenomenon is commonly known as wave–particle duality, which states that a particle of matter (in this case the incident electron) can be described as a wave. For this reason, an electron can be regarded as a wave much like sound or water waves. This technique is similar to X-ray and neutron diffraction.
Electron diffraction is most frequently used in solid state physics and chemistry to study the crystal structure of solids. Experiments are usually performed in a transmission electron microscope (TEM), or a scanning electron microscope (SEM) as electron backscatter diffraction. In these instruments, electrons are accelerated by an electrostatic potential to gain the desired energy and determine their wavelength before they interact with the sample to be studied.
The periodic structure of a crystalline solid acts as a diffraction grating, scattering the electrons in a predictable manner. Working back from the observed diffraction pattern, it may be possible to deduce the structure of the crystal producing the diffraction pattern. However, the technique is limited by phase problem.
Apart from the study of "periodically perfect" crystals, i.e. electron crystallography, electron diffraction is also a useful technique to study the short range order of amorphous solids, short-range ordering of imperfections such as vacancies, the geometry of gaseous molecules, and the properties of short-range ordering of vacancies.
In a transmission electron microscope
Electron diffraction of solids is usually performed in a transmission electron microscope (TEM) where the electrons pass through a thin film of the material to be studied. The resulting diffraction pattern is then observed on a fluorescent screen, recorded on photographic film, on imaging plates or using a CCD camera.
Benefits
As mentioned above, the wavelength of an electron accelerated in a TEM is much smaller than that of the radiation usually used for X-ray diffraction. A consequence of this is that the radius of the Ewald sphere is much larger in electron diffraction experiments than in X-ray diffraction. This allows the diffraction experiment to reveal more of the two-dimensional distribution of reciprocal lattice points.
Furthermore, electron lenses allows the geometry of the diffraction experiment to be varied. The conceptually simplest geometry referred to as selected area electron diffraction (SAED) is that of a parallel beam of electrons incident on the specimen, with the specimen field selected using a sub-specimen image-plane aperture. However, by converging the electrons in a cone onto the specimen, one can in effect perform a diffraction experiment over several incident angles simultaneously. This technique is called Convergent Beam Electron Diffraction (CBED) and can reveal the full three-dimensional symmetry of the crystal. For amorphous materials, the diffraction pattern is referred to as a Ronchigram.
In a TEM, a single crystal grain or particle may be selected for the diffraction experiments. This means that the diffraction experiments can be performed on single crystals of nanometer size, whereas other diffraction techniques would be limited to studying the diffraction from a multicrystalline or powder sample. Furthermore, electron diffraction in TEM can be combined with direct imaging of the sample, including high resolution imaging of the crystal lattice, and a range of other techniques. These include solving and refining crystal structures by electron crystallography, chemical analysis of the sample composition through energy-dispersive X-ray spectroscopy, investigations of electronic structure and bonding through electron energy loss spectroscopy, and studies of the mean inner potential through electron holography.
Practical aspects
As the electrons pass through the sample, they are scattered by the electrostatic potential set up by the constituent elements. After the electrons have left the sample they pass through the electromagnetic objective lens. This lens acts to collect all electrons scattered from one point of the sample in one point on the fluorescent screen, causing an image of the sample to be formed. At the dashed line in the figure, electrons scattered in the same direction by the sample are collected into a single point. This is the back focal plane of the microscope, and is where the diffraction pattern is formed. By manipulating the magnetic lenses of the microscope, the diffraction pattern may be observed by projecting it onto the screen instead of the image.
If the sample is tilted with respect to the incident electron beam, one can obtain diffraction patterns from several crystal orientations. In this way, the reciprocal lattice of the crystal can be mapped in three dimensions. By studying the systematic absence of diffraction spots the Bravais lattice and any screw axes and glide planes present in the crystal structure may be determined.
Limitations
Electron diffraction in TEM is subject to several important limitations. First, the sample to be studied must be electron transparent, meaning the sample thickness must be of the order of 100 nm or less. Careful and time-consuming sample preparation may therefore be needed. Furthermore, many samples are vulnerable to radiation damage caused by the incident electrons.
The study of magnetic materials is complicated by the fact that electrons are deflected in magnetic fields by the Lorentz force. Although this phenomenon may be exploited to study the magnetic domains of materials by Lorentz force microscopy, it may make crystal structure determination virtually impossible.
Furthermore, electron diffraction is often regarded as a qualitative technique suitable for symmetry determination, but too inaccurate for determination of lattice parameters and atomic positions. But there are also several examples where unknown crystal structures (inorganic, organic and biological) have been solved by electron crystallography. Lattice parameters of high accuracy can in fact be obtained from electron diffraction, relative errors less than 0.1% have been demonstrated. However, the right experimental conditions may be difficult to obtain, and these procedures are often viewed as too time-consuming and the data too difficult to interpret. X-ray or neutron diffraction are therefore often the preferred methods for determining lattice parameters and atomic positions.
However, the main limitation of electron diffraction in TEM remains the comparatively high level of user interaction needed. Whereas both the execution of powder X-ray (and neutron) diffraction experiments and the data analysis are highly automated and routinely performed, electron diffraction requires a much higher level of user input.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Online
#1283 2022-02-12 14:04:37
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,954
Re: Miscellany
1259) Hematite
Summary
Hematite, also spelled haematite, is a heavy and relatively hard oxide mineral, ferric oxide (Fe2O3), that constitutes the most important iron ore because of its high iron content (70 percent) and its abundance. Its name is derived from the Greek word for “blood,” in allusion to its red colour. Many of the various forms of hematite have separate names. The steel-gray crystals and coarse-grained varieties have a brilliant metallic lustre and are known as specular iron ore; thin scaly types are called micaceous hematite. Much hematite occurs in a soft, fine-grained, earthy form called red ochre or ruddle. Intermediate between these types are compact varieties, often with a reniform surface (kidney ore) or a fibrous structure (pencil ore). Red ochre is used as a paint pigment; a purified form, rouge, is used to polish plate glass.
The most important deposits of hematite are sedimentary in origin. The world’s largest production (nearly 75 million tons of hematite annually) comes from a sedimentary deposit in the Lake Superior district in North America. Other important deposits include those at Minas Gerais, Brazil (where the hematite occurs in metamorphosed sediments); Cerro Bolívar, Venezuela; and Labrador and Quebec, Canada. Hematite is found as an accessory mineral in many igneous rocks; commonly as a weathering product of siderite, magnetite, and other iron minerals; and almost universally as a pigmenting agent of sedimentary and other rocks.
Details
Hematite, also spelled as haematite, is a common iron oxide compound with the formula, Fe2O3 and is widely found in rocks and soils. Hematite crystals belong to the rhombohedral lattice system which is designated the alpha polymorph of Fe2O3. It has the same crystal structure as corundum (Al2O3) and ilmenite (FeTiO3). With this it forms a complete solid solution at temperatures above 950 °C (1,740 °F).
Hematite naturally occurs in black to steel or silver-gray, brown to reddish-brown, or red colors. It is mined as an important ore of iron. It is electrically conductive. Hematite varieties include kidney ore, martite (pseudomorphs after magnetite), iron rose and specularite (specular hematite). While these forms vary, they all have a rust-red streak. Hematite is not only harder than pure iron, but also much more brittle. Maghemite is a polymorph of hematite (γ-Fe2O3) with the same chemical formula, but with a spinel structure like magnetite.
Large deposits of hematite are found in banded iron formations. Gray hematite is typically found in places that have still, standing water or mineral hot springs, such as those in Yellowstone National Park in North America. The mineral can precipitate in the water and collect in layers at the bottom of the lake, spring, or other standing water. Hematite can also occur in the absence of water, usually as the result of volcanic activity.
Clay-sized hematite crystals can also occur as a secondary mineral formed by weathering processes in soil, and along with other iron oxides or oxyhydroxides such as goethite, which is responsible for the red color of many tropical, ancient, or otherwise highly weathered soils.
Etymology and history
The name hematite is derived from the Greek word for blood αἷμα (haima), due to the red coloration found in some varieties of hematite. The color of hematite is often used as a pigment. The English name of the stone is derived from Middle French hématite pierre, which was taken from Latin lapis haematites c. the 15th century, which originated from Ancient Greek (haimatitēs lithos, "blood-red stone").
Ochre is a clay that is colored by varying amounts of hematite, varying between 20% and 70%. Red ochre contains unhydrated hematite, whereas yellow ochre contains hydrated hematite (Fe2O3 · H2O). The principal use of ochre is for tinting with a permanent color.
The red chalk writing of this mineral was one of the earliest in the human history. The powdery mineral was first used 164,000 years ago by the Pinnacle-Point man, possibly for social purposes. Hematite residues are also found in graves from 80,000 years ago. Near Rydno in Poland and Lovas in Hungary red chalk mines have been found that are from 5000 BC, belonging to the Linear Pottery culture at the Upper Rhine.
Rich deposits of hematite have been found on the island of Elba that have been mined since the time of the Etruscans.
Magnetism
Hematite shows only a very feeble response to a magnetic field. Unlike magnetite, it is not noticeably attracted to an ordinary magnet. Hematite is an antiferromagnetic material below the Morin transition at 250 K (−23 °C), and a canted antiferromagnet or weakly ferromagnetic above the Morin transition and below its Néel temperature at 948 K (675 °C), above which it is paramagnetic.
The magnetic structure of α-hematite was the subject of considerable discussion and debate during the 1950s, as it appeared to be ferromagnetic with a Curie temperature of approximately 1,000 K (730 °C), but with an extremely small magnetic moment (0.002 Bohr magnetons). Adding to the surprise was a transition with a decrease in temperature at around 260 K (−13 °C) to a phase with no net magnetic moment. It was shown that the system is essentially antiferromagnetic, but that the low symmetry of the cation sites allows spin–orbit coupling to cause canting of the moments when they are in the plane perpendicular to the c axis. The disappearance of the moment with a decrease in temperature at 260 K (−13 °C) is caused by a change in the anisotropy which causes the moments to align along the c axis. In this configuration, spin canting does not reduce the energy. The magnetic properties of bulk hematite differ from their nanoscale counterparts. For example, the Morin transition temperature of hematite decreases with a decrease in the particle size. The suppression of this transition has been observed in hematite nanoparticles and is attributed to the presence of impurities, water molecules and defects in the crystals lattice. Hematite is part of a complex solid solution oxyhydroxide system having various contents of water, hydroxyl groups and vacancy substitutions that affect the mineral's magnetic and crystal chemical properties. Two other end-members are referred to as protohematite and hydrohematite.
Enhanced magnetic coercivities for hematite have been achieved by dry-heating a two-line ferrihydrite precursor prepared from solution. Hematite exhibited temperature-dependent magnetic coercivity values ranging from 289 to 5,027 oersteds (23–400 kA/m). The origin of these high coercivity values has been interpreted as a consequence of the subparticle structure induced by the different particle and crystallite size growth rates at increasing annealing temperature. These differences in the growth rates are translated into a progressive development of a subparticle structure at the nanoscale. At lower temperatures (350–600 °C), single particles crystallize. However; at higher temperatures (600–1000 °C), the growth of crystalline aggregates and a subparticle structure is favored.
Mine tailings
Hematite is present in the waste tailings of iron mines. A recently developed process, magnetation, uses magnets to glean waste hematite from old mine tailings in Minnesota's vast Mesabi Range iron district. Falu red is a pigment used in traditional Swedish house paints. Originally, it was made from tailings of the Falu mine.
Mars
The spectral signature of hematite was seen on the planet Mars by the infrared spectrometer on the NASA Mars Global Surveyor and 2001 Mars Odyssey spacecraft in orbit around Mars. The mineral was seen in abundance at two sites on the planet, the Terra Meridiani site, near the Martian equator at 0° longitude, and the Aram Chaos site near the Valles Marineris. Several other sites also showed hematite, such as Aureum Chaos. Because terrestrial hematite is typically a mineral formed in aqueous environments or by aqueous alteration, this detection was scientifically interesting enough that the second of the two Mars Exploration Rovers was sent to a site in the Terra Meridiani region designated Meridiani Planum. In-situ investigations by the Opportunity rover showed a significant amount of hematite, much of it in the form of small spherules that were informally named "blueberries" by the science team. Analysis indicates that these spherules are apparently concretions formed from a water solution. "Knowing just how the hematite on Mars was formed will help us characterize the past environment and determine whether that environment was favorable for life".
Jewelry
Hematite was once used as mourning jewelry. A 1923 reference describes "hematite is sometimes used as settings in mourning jewelry." Certain types of hematite- or iron-oxide-rich clay, especially Armenian bole, have been used in gilding. Hematite is also used in art such as in the creation of intaglio engraved gems. Hematine is a synthetic material sold as magnetic hematite.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Online
#1284 2022-02-13 14:04:10
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,954
Re: Miscellany
1260) Magnetite
Summary
Magnetite, also called lodestone, or magnetic iron ore, iron oxide mineral (FeFe2O4, or Fe3O4) is that is the chief member of one of the series of the spinel (q.v.) group. Minerals in this series form black to brownish, metallic, moderately hard octahedrons and masses in igneous and metamorphic rocks and in granite pegmatites, stony meteorites, and high-temperature sulfide veins. The magnetite series also contains magnesioferrite (magnesium iron oxide, MgFe2O4), franklinite (zinc iron oxide, ZnFe2O4), jacobsite (manganese iron oxide, MnFe2O4), and trevorite (nickel iron oxide, NiFe2O4). All are magnetic, although franklinite and jacobsite are only weakly so; magnetite, which frequently has distinct north and south poles, has been known for this property since about 500 BC.
Details
Magnetite is a mineral and one of the main iron ores, with the chemical formula Fe3O4. It is one of the oxides of iron, and is ferrimagnetic; it is attracted to a magnet and can be magnetized to become a permanent magnet itself. It is the most magnetic of all the naturally occurring minerals on Earth. Naturally magnetized pieces of magnetite, called lodestone, will attract small pieces of iron, which is how ancient peoples first discovered the property of magnetism.
Magnetite is black or brownish-black with a metallic luster, has a Mohs hardness of 5–6 and leaves a black streak. Small grains of magnetite are very common in igneous and metamorphic rocks.
The chemical IUPAC name is iron(II,III) oxide and the common chemical name is ferrous-ferric oxide.
Properties
In addition to igneous rocks, magnetite also occurs in sedimentary rocks, including banded iron formations and in lake and marine sediments as both detrital grains and as magnetofossils. Magnetite nanoparticles are also thought to form in soils, where they probably oxidize rapidly to maghemite.
Crystal structure
The chemical composition of magnetite is Fe2+(Fe3+)2(O2-)4. This indicates that magnetite contains both ferrous (divalent) and ferric (trivalent) iron, suggesting crystallization in an environment containing intermediate levels of oxygen. The main details of its structure were established in 1915. It was one of the first crystal structures to be obtained using X-ray diffraction. The structure is inverse spinel, with O2− ions forming a face-centered cubic lattice and iron cations occupying interstitial sites. Half of the Fe3+ cations occupy tetrahedral sites while the other half, along with Fe2+ cations, occupy octahedral sites. The unit cell consists of 32 O2− ions and unit cell length is a = 0.839 nm.
As a member of the inverse spinel group, magnetite can form solid solutions with similarly structured minerals, including ulvospinel (Fe2TiO4) and magnesioferrite (MgFe2O4).
Titanomagnetite, also known as titaniferous magnetite, is a solid solution between magnetite and ulvospinel that crystallizes in many mafic igneous rocks. Titanomagnetite may undergo oxyexsolution during cooling, resulting in ingrowths of magnetite and ilmenite.
Crystal morphology and size
Natural and synthetic magnetite occurs most commonly as octahedral crystals bounded by {111} planes and as rhombic-dodecahedra. Twinning occurs on the {111} plane.
Hydrothermal synthesis usually produces single octahedral crystals which can be as large as 10 mm (0.39 in) across. In the presence of mineralizers such as 0.1 M HI or 2 M NH4Cl and at 0.207 MPa at 416–800 °C, magnetite grew as crystals whose shapes were a combination of rhombic-dodechahedra forms. The crystals were more rounded than usual. The appearance of higher forms was considered as a result from a decrease in the surface energies caused by the lower surface to volume ratio in the rounded crystals.
Reactions
Magnetite has been important in understanding the conditions under which rocks form. Magnetite reacts with oxygen to produce hematite, and the mineral pair forms a buffer that can control how oxidizing its environment is (the oxygen fugacity). This buffer is known as the hematite-magnetite or HM buffer. At lower oxygen levels, magnetite can form a buffer with quartz and fayalite known as the QFM buffer. At still lower oxygen levels, magnetite forms a buffer with wüstite known as the MW buffer. The QFM and MW buffers have been used extensively in laboratory experiments on rock chemistry. The QFM buffer, in particular, produces an oxygen fugacity close to that of most igneous rocks.
Commonly, igneous rocks contain solid solutions of both titanomagnetite and hemoilmenite or titanohematite. Compositions of the mineral pairs are used to calculate oxygen fugacity: a range of oxidizing conditions are found in magmas and the oxidation state helps to determine how the magmas might evolve by fractional crystallization. Magnetite also is produced from peridotites and dunites by serpentinization.
Magnetic properties
Lodestones were used as an early form of magnetic compass. Magnetite has been a critical tool in paleomagnetism, a science important in understanding plate tectonics and as historic data for magnetohydrodynamics and other scientific fields.
The relationships between magnetite and other iron oxide minerals such as ilmenite, hematite, and ulvospinel have been much studied; the reactions between these minerals and oxygen influence how and when magnetite preserves a record of the Earth's magnetic field.
At low temperatures, magnetite undergoes a crystal structure phase transition from a monoclinic structure to a cubic structure known as the Verwey transition. Optical studies show that this metal to insulator transition is sharp and occurs around 120 K. The Verwey transition is dependent on grain size, domain state, pressure, and the iron-oxygen stoichiometry. An isotropic point also occurs near the Verwey transition around 130 K, at which point the sign of the magnetocrystalline anisotropy constant changes from positive to negative. The Curie temperature of magnetite is 580 °C (853 K; 1,076 °F).
If magnetite is in a large enough quantity it can be found in aeromagnetic surveys using a magnetometer which measures magnetic intensities.
Distribution of deposits
Magnetite is sometimes found in large quantities in beach sand. Such black sands (mineral sands or iron sands) are found in various places, such as Lung Kwu Tan of Hong Kong; California, United States; and the west coast of the North Island of New Zealand. The magnetite, eroded from rocks, is carried to the beach by rivers and concentrated by wave action and currents. Huge deposits have been found in banded iron formations. These sedimentary rocks have been used to infer changes in the oxygen content of the atmosphere of the Earth.
Large deposits of magnetite are also found in the Atacama region of Chile (Chilean Iron Belt); the Valentines region of Uruguay; Kiruna, Sweden; the Tallawang Region of New South Wales; and in the Adirondack region of New York in the United States. Kediet ej Jill, the highest mountain of Mauritania, is made entirely of the mineral. Deposits are also found in Norway, Romania, and the Ukraine.[40] Magnetite-rich sand dunes are found in southern Peru. In 2005, an exploration company, Cardero Resources, discovered a vast deposit of magnetite-bearing sand dunes in Peru. The dune field covers 250 square kilometers (100 sq mi), with the highest dune at over 2,000 meters (6,560 ft) above the desert floor. The sand contains 10% magnetite.
In large enough quantities magnetite can affect compass navigation. In Tasmania there are many areas with highly magnetized rocks that can greatly influence compasses. Extra steps and repeated observations are required when using a compass in Tasmania to keep navigation problems to the minimum.
Magnetite crystals with a cubic habit are rare but have been found at Balmat, St. Lawrence County, New York, and at Långban, Sweden. This habit may be a result of crystallization in the presence of cations such as zinc.
Magnetite can also be found in fossils due to biomineralization and are referred to as magnetofossils. There are also instances of magnetite with origins in space coming from meteorites.
Biological occurrences
Biomagnetism is usually related to the presence of biogenic crystals of magnetite, which occur widely in organisms. These organisms range from magnetotactic bacteria (e.g., Magnetospirillum magnetotacticum) to animals, including humans, where magnetite crystals (and other magnetically sensitive compounds) are found in different organs, depending on the species. Biomagnetites account for the effects of weak magnetic fields on biological systems. There is also a chemical basis for cellular sensitivity to electric and magnetic fields (galvanotaxis).
Pure magnetite particles are biomineralized in magnetosomes, which are produced by several species of magnetotactic bacteria. Magnetosomes consist of long chains of oriented magnetite particle that are used by bacteria for navigation. After the death of these bacteria, the magnetite particles in magnetosomes may be preserved in sediments as magnetofossils. Some types of anaerobic bacteria that are not magnetotactic can also create magnetite in oxygen free sediments by reducing amorphic ferric oxide to magnetite.
Several species of birds are known to incorporate magnetite crystals in the upper beak for magnetoreception, which (in conjunction with cryptochromes in the retina) gives them the ability to sense the direction, polarity, and magnitude of the ambient magnetic field.
Chitons, a type of mollusk, have a tongue-like structure known as a radula, covered with magnetite-coated teeth, or denticles. The hardness of the magnetite helps in breaking down food.
Biological magnetite may store information about the magnetic fields the organism was exposed to, potentially allowing scientists to learn about the migration of the organism or about changes in the Earth's magnetic field over time.
Human brain
Living organisms can produce magnetite. In humans, magnetite can be found in various parts of the brain including the frontal, parietal, occipital, and temporal lobes, brainstem, cerebellum and basal ganglia. Iron can be found in three forms in the brain – magnetite, hemoglobin (blood) and ferritin (protein), and areas of the brain related to motor function generally contain more iron. Magnetite can be found in the hippocampus. The hippocampus is associated with information processing, specifically learning and memory. However, magnetite can have toxic effects due to its charge or magnetic nature and its involvement in oxidative stress or the production of free radicals. Research suggests that beta-amyloid plaques and tau proteins associated with neurodegenerative disease frequently occur after oxidative stress and the build-up of iron.
Some researchers also suggest that humans possess a magnetic sense, proposing that this could allow certain people to use magnetoreception for navigation. The role of magnetite in the brain is still not well understood, and there has been a general lag in applying more modern, interdisciplinary techniques to the study of biomagnetism.
Electron microscope scans of human brain-tissue samples are able to differentiate between magnetite produced by the body's own cells and magnetite absorbed from airborne pollution, the natural forms being jagged and crystalline, while magnetite pollution occurs as rounded nanoparticles. Potentially a human health hazard, airborne magnetite is a result of pollution (specifically combustion). These nanoparticles can travel to the brain via the olfactory nerve, increasing the concentration of magnetite in the brain. In some brain samples, the nanoparticle pollution outnumbers the natural particles by as much as 100:1, and such pollution-borne magnetite particles may be linked to abnormal neural deterioration. In one study, the characteristic nanoparticles were found in the brains of 37 people: 29 of these, aged 3 to 85, had lived and died in Mexico City, a significant air pollution hotspot. Some of the further eight, aged 62 to 92, from Manchester, England, had died with varying severities of neurodegenerative diseases. Such particles could conceivably contribute to diseases like Alzheimer's disease. Though a causal link has not yet been established, laboratory studies suggest that iron oxides such as magnetite are a component of protein plaques in the brain. Such plaques have been linked to Alzheimer's disease.
Increased iron levels, specifically magnetic iron, have been found in portions of the brain in Alzheimer's patients. Monitoring changes in iron concentrations may make it possible to detect the loss of neurons and the development of neurodegenerative diseases prior to the onset of symptoms due to the relationship between magnetite and ferritin. In tissue, magnetite and ferritin can produce small magnetic fields which will interact with magnetic resonance imaging (MRI) creating contrast. Huntington patients have not shown increased magnetite levels; however, high levels have been found in study mice.
Applications
Due to its high iron content, magnetite has long been a major iron ore. It is reduced in blast furnaces to pig iron or sponge iron for conversion to steel.
Magnetic recording
Audio recording using magnetic acetate tape was developed in the 1930s. The German magnetophon utilized magnetite powder as the recording medium. Following World War II, 3M Company continued work on the German design. In 1946, the 3M researchers found they could improve the magnetite-based tape, which utilized powders of cubic crystals, by replacing the magnetite with needle-shaped particles of gamma ferric oxide (γ-Fe2O3).
Catalysis
Approximately 2–3% of the world's energy budget is allocated to the Haber Process for nitrogen fixation, which relies on magnetite-derived catalysts. The industrial catalyst is obtained from finely ground iron powder, which is usually obtained by reduction of high-purity magnetite. The pulverized iron metal is burnt (oxidized) to give magnetite or wüstite of a defined particle size. The magnetite (or wüstite) particles are then partially reduced, removing some of the oxygen in the process. The resulting catalyst particles consist of a core of magnetite, encased in a shell of wüstite, which in turn is surrounded by an outer shell of iron metal. The catalyst maintains most of its bulk volume during the reduction, resulting in a highly porous high-surface-area material, which enhances its effectiveness as a catalyst.
Magnetite nanoparticles
Magnetite micro- and nanoparticles are used in a variety of applications, from biomedical to environmental. One use is in water purification: in high gradient magnetic separation, magnetite nanoparticles introduced into contaminated water will bind to the suspended particles (solids, bacteria, or plankton, for example) and settle to the bottom of the fluid, allowing the contaminants to be removed and the magnetite particles to be recycled and reused. This method works with radioactive and carcinogenic particles as well, making it an important cleanup tool in the case of heavy metals introduced into water systems.
Another application of magnetic nanoparticles is in the creation of ferrofluids. These are used in several ways, in addition to being fun to play with. Ferrofluids can be used for targeted drug delivery in the human body. The magnetization of the particles bound with drug molecules allows “magnetic dragging” of the solution to the desired area of the body. This would allow the treatment of only a small area of the body, rather than the body as a whole, and could be highly useful in cancer treatment, among other things. Ferrofluids are also used in magnetic resonance imaging (MRI) technology.
Coal mining industry
For the separation of coal from waste, dense medium baths were used. This technique employed the difference in densities between coal (1.3–1.4 tonnes per m³) and shales (2.2–2.4 tonnes per m³). In a medium with intermediate density (water with magnetite), stones sank and coal floated.
Magnetene
Magnetene is a 2 dimensional flat sheet of magnetite noted for its ultra-low-friction behavior.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Online
#1285 2022-02-14 13:46:32
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,954
Re: Miscellany
1261) Arecibo Observatory
Summary
Arecibo Observatory is an astronomical observatory located 16 km (10 miles) south of the town of Arecibo in Puerto Rico. It was the site of the world’s largest single-unit radio telescope until FAST in China began observations in 2016. This instrument, built in the early 1960s, employed a 305-metre (1,000-foot) spherical reflector consisting of perforated aluminum panels that focused incoming radio waves on movable antenna structures positioned about 168 metres (550 feet) above the reflector surface. The antenna structures could be moved in any direction, making it possible to track a celestial object in different regions of the sky. The observatory also had an auxiliary 30-metre (100-foot) telescope that served as a radio interferometer and a high-power transmitting facility used to study Earth’s atmosphere. In August 2020 a cable holding up the central platform snapped and made a hole in the dish. After a second cable broke in November 2020, the National Science Foundation (NSF) announced that the telescope was in danger of collapse and the cables could not be safely repaired. The NSF thus planned to decommission the observatory. On December 1, 2020, days after the NSF’s announcement, the cables broke, and the central platform collapsed into the dish.
Scientists using the Arecibo Observatory discovered the first extrasolar planets around the pulsar B1257+12 in 1992. The observatory also produced detailed radar maps of the surface of Venus and Mercury and discovered that Mercury rotated every 59 days instead of 88 days and so did not always show the same face to the Sun. American astronomers Russell Hulse and Joseph H. Taylor, Jr., used Arecibo to discover the first binary pulsar. They showed that it was losing energy through gravitational radiation at the rate predicted by physicist Albert Einstein’s theory of general relativity, and they won the Nobel Prize for Physics in 1993 for their discovery.
Details
The Arecibo Observatory, also known as the National Astronomy and Ionosphere Center (NAIC) and formerly known as the Arecibo Ionosphere Observatory, is an observatory in Barrio Esperanza, Arecibo, Puerto Rico owned by the US National Science Foundation (NSF).
The observatory's main instrument was the Arecibo Telescope, a 305 m (1,000 ft) spherical reflector dish built into a natural sinkhole, with a cable-mount steerable receiver and several radar transmitters for emitting signals mounted 150 m (492 ft) above the dish. Completed in 1963, it was the world's largest single-aperture telescope for 53 years, surpassed in July 2016 by the Five-hundred-meter Aperture Spherical Telescope (FAST) in China. Following two breaks in cables supporting the receiver platform in mid-2020, the NSF decommissioned the telescope. A partial collapse of the telescope occurred on December 1, 2020, before controlled demolition could be conducted. The remains of the telescope are being removed as NASA evaluates plans for a replacement instrument.
The observatory also includes a smaller radio telescope, a LIDAR facility, and a visitor center, which remain operational after the telescope's collapse.
History
As part of the United States Department of Defense (DoD) Advanced Research Projects Agency (ARPA) missile defense program, ARPA had sought a means to try to detect incoming missiles while they traveled through the ionosphere. The Arecibo Telescope was funded as a means to study Earth's ionosphere for this purpose, and serving a dual-use as a general-purpose radio telescope. Construction of the telescope and its supporting facilities were started in mid-1950s, with the telescope operational by 1963. The telescope and supporting observatory were formally opened as the Arecibo Ionospheric Observatory on November 1, 1963.
Ownership of the observatory transferred from the DoD to the National Science Foundation on October 1, 1969. NSF named Cornell University to manage the observatory's functions. By September 1971, NSF renamed the observatory as the National Astronomy and Ionosphere Center (NAIC) and had made it a Federally funded research and development centers (FFRDC). NASA began contributing towards funding of the observatory alongside NSF as to support its planetary radar mission.
In the early 2000s, NASA started to reduce their contribution to the Arecibo Observatory, putting more pressure on NSF to continue to fund the facility. In 2006, NSF made its first possible suggestion of significantly reducing its funding towards Arecibo and potentially decommissioning the observatory. Academics and politicians lobbied to increase funding bookmarked for Arecibo to stave off its closure, and NASA recommitted funding in 2011 for study of near-earth objects. However to further cut losses, in 2011 NSF delisted Arecibo as a FFRDC, removed Cornell as the site operator, and replaced them with a collaborative team led by SRI International, which allowed the observatory to be able to offer its facilities to a wider range of projects.
Damage to the telescope from Hurricane Maria in 2017 led NSF again to consider the possibility of decommissioning the observatory as the costs of maintaining it had become too great. A consortium led by the University of Central Florida (UCF) stepped forward to offer to manage the observatory and cover a significant portion of the operations and maintenance costs, and in 2018, NSF made UCF's consortium the new site operators.
After an auxiliary and main cable failure on the telescope in August and November 2020, respectively, the NSF announced the decision that they would decommission the telescope through controlled demolition, but that the other facilities on the observatory would remain operational in the future. However, before the safe decommission of the telescope could occur, remaining support cables from one tower rapidly failed in the morning of December 1, 2020, causing the instrument platform to crash through the dish, shearing off the tops of the support towers, and partially damaging some of the other buildings, though there were no injuries. NSF has stated that it is still their intention to continue to have the other observatory facilities operational as soon as possible and are looking at plans to rebuild a new telescope instrument in its place.
Facilities:
Arecibo Telescope
The observatory's main feature was its large radio telescope, whose main collecting dish was an inverted spherical dome 1,000 feet (305 m) in diameter with an 869-foot (265 m) radius of curvature, constructed inside a karst sinkhole.[17] The dish's surface was made of 38,778 perforated aluminum panels, each about 3 by 7 feet (1 by 2 m), supported by a mesh of steel cables. The ground beneath supported shade-tolerant vegetation.
Since its completion in November 1963, the Telescope had been used for radar astronomy and radio astronomy, and had been part of the Search for extraterrestrial intelligence (SETI) program. It was also used by NASA for Near-Earth object detection. Since around 2006, NSF funding support for the telescope had waned as the Foundation directed funds to newer instruments, though academics petitioned to the NSF and Congress to continue support for the telescope. Numerous hurricanes, including Hurricane Maria, had damaged parts of the telescope, straining the reduced budget.
Two cable breaks, one in August 2020 and a second in November 2020, threatened the structural integrity of the support structure for the suspended platform and damaged the dish. The NSF determined in November 2020 that it was safer to decommission the telescope rather than to try to repair it, but the telescope collapsed before a controlled demolition could be carried out. The remaining support cables from one tower failed around 7:56 a.m. local time on December 1, 2020, causing the receiver platform to fall into the dish and collapsing the telescope.
NASA led an extensive failure investigation and reported the findings, along with a technical bulletin with industry recommendations.
Additional telescopes
The Arecibo Observatory also has other facilities beyond the main telescope, including a 12-meter (39 ft) radio telescope intended for very-long-baseline interferometry (VLBI) with the main telescope; and a LIDAR facility whose research has continued since the main telescope's collapse.
Ángel Ramos Foundation Visitor Center
Opened in 1997, the Ángel Ramos Foundation Visitor Center features interactive exhibits and displays about the operations of the radio telescope, astronomy and atmospheric sciences. The center is named after the financial foundation that honors Ángel Ramos, owner of the El Mundo newspaper and founder of Telemundo. The Foundation provided half of the funds to build the Visitor Center, with the remainder received from private donations and Cornell University.
The center, in collaboration with the Caribbean Astronomical Society, hosts a series of Astronomical Nights throughout the year, which feature diverse discussions regarding exoplanets, astronomical phenomena, and discoveries (such as Comet ISON). The purposes of the center are to increase public interest in astronomy, the observatory's research successes, and space endeavors.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Online
#1286 2022-02-15 14:27:25
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,954
Re: Miscellany
1262) Bauxite
Summary
Bauxite is rock largely composed of a mixture of hydrous aluminum oxides. Bauxite is the principal ore of aluminum.
Bauxites vary physically according to the origin and geologic history of their deposits: some deposits are soft, easily crushed, and structureless; some are hard, dense, and pisolitic, or pealike; still others are porous but strong or are stratified or largely pseudomorphic after their parent rock. The laterite type is commonly pisolitic and mottled, with pisolites ranging in size from about 2.5 mm (0.10 inch) to 25 cm (10 inches) or more in diameter. Both pisolites and groundmass (matrix) may exhibit great colour variations; common colours are pink, cream, red, brown, yellow, and gray. Exposed surfaces of lateritic ore are rough, often lavalike, with a wormlike structure and variegated colours on vertical faces. Such material tends to harden or reconsolidate on exposure to air. Although terra-rossa types are granular and earthy, they also may possess pisolitic structures.
Bauxite is formed by the thorough weathering of many different rocks. Clay minerals commonly represent intermediate stages, but some bauxites appear to be reworked chemical precipitates rather than simple alteration products. Bauxite may grade into laterite or clay, laterally or vertically.
Constituent minerals are rarely recognizable in hand specimens, and even in thin sections complete identification may be difficult. Combined petrography, X-ray diffraction, and differential thermal analysis have shown that gibbsite, boehmite, and diaspore, alone or in mixtures, are the constituent minerals. Clay minerals, hematite, magnetite, goethite, siderite, and quartz are common impurities. Most deposits contain rutile, anatase, zircon, and other minerals.
Bauxite is found in most countries, but the larger deposits occur in the tropics. Major deposits of gravels mixed with sand were discovered in Australia in the 1950s, and it became the world’s top producer of bauxite by the early 21st century. Other top producers include China, Indonesia, Brazil, and India. In addition, monohydrate ores have been extensively mined in France, Italy, and Greece and trihydrate ores in Arkansas, U.S., and in Suriname, Guyana, and Jamaica. Gibbsite-rich ores occur in Ghana, Guinea, India, and Brazil. Deposits in the Ural Mountains and in northern Asia are largely diaspore.
Details
Bauxite is a sedimentary rock with a relatively high aluminium content. It is the world's main source of aluminium and gallium. Bauxite consists mostly of the aluminium minerals gibbsite (Al(OH)3), boehmite (γ-AlO(OH)) and diaspore (α-AlO(OH)), mixed with the two iron oxides goethite (FeO(OH)) and haematite (Fe2O3), the aluminium clay mineral kaolinite (Al2Si2O5(OH)4) and small amounts of anatase (TiO2) and ilmenite (FeTiO3 or FeO.TiO2). Bauxite appears dull in luster and is reddish-brown, white, or tan in color.
In 1821 the French geologist Pierre Berthier discovered bauxite near the village of Les Baux in Provence, southern France.
Formation
Numerous classification schemes have been proposed for bauxite but, as of 1982, there was no consensus.
Vadász (1951) distinguished lateritic bauxites (silicate bauxites) from karst bauxite ores (carbonate bauxites):
* The carbonate bauxites occur predominantly in Europe, Guyana, Suriname, and Jamaica above carbonate rocks (limestone and dolomite), where they were formed by lateritic weathering and residual accumulation of intercalated clay layers – dispersed clays which were concentrated as the enclosing limestones gradually dissolved during chemical weathering.
* The lateritic bauxites are found mostly in the countries of the tropics. They were formed by lateritization of various silicate rocks such as granite, gneiss, basalt, syenite, and shale. In comparison with the iron-rich laterites, the formation of bauxites depends even more on intense weathering conditions in a location with very good drainage. This enables the dissolution of the kaolinite and the precipitation of the gibbsite. Zones with highest aluminium content are frequently located below a ferruginous surface layer. The aluminium hydroxide in the lateritic bauxite deposits is almost exclusively gibbsite.
In the case of Jamaica, recent analysis of the soils showed elevated levels of cadmium, suggesting that the bauxite originates from recent Miocene ash deposits from episodes of significant volcanism in Central America.
Production and reserves
Australia is the largest producer of bauxite, followed by China. Increased aluminium recycling, which has the advantage of lowering the cost in electric power in producing aluminium, will considerably extend the world's bauxite reserves.
Processing
Bauxite is usually strip mined because it is almost always found near the surface of the terrain, with little or no overburden. As of 2010, approximately 70% to 80% of the world's dry bauxite production is processed first into alumina and then into aluminium by electrolysis. Bauxite rocks are typically classified according to their intended commercial application: metallurgical, abrasive, cement, chemical, and refractory.
Usually, bauxite ore is heated in a pressure vessel along with a sodium hydroxide solution at a temperature of 150 to 200 °C (300 to 390 °F). At these temperatures, the aluminium is dissolved as sodium aluminate (the Bayer process). The aluminium compounds in the bauxite may be present as gibbsite(Al(OH)3), boehmite(AlOOH) or diaspore(AlOOH); the different forms of the aluminium component will dictate the extraction conditions. The undissolved waste, bauxite tailings, after the aluminium compounds are extracted contains iron oxides, silica, calcia, titania and some un-reacted alumina. After separation of the residue by filtering, pure gibbsite is precipitated when the liquid is cooled, and then seeded with fine-grained aluminium hydroxide. The gibbsite is usually converted into aluminium oxide, Al2O3, by heating in rotary kilns or fluid flash calciners to a temperature in excess of 1,000 °C (1,830 °F). This aluminium oxide is dissolved at a temperature of about 960 °C (1,760 °F) in molten cryolite. Next, this molten substance can yield metallic aluminium by passing an electric current through it in the process of electrolysis, which is called the Hall–Héroult process, named after its American and French discoverers.
Prior to the invention of this process, and prior to the Deville process, aluminium ore was refined by heating ore along with elemental sodium or potassium in a vacuum. The method was complicated and consumed materials that were themselves expensive at that time. This made early elemental aluminium more expensive than gold.
Maritime safety
As a bulk cargo, Bauxite is a Group A cargo that may liquefy if excessively moist. Liquefaction and the Free surface effect can cause the cargo to shift rapidly inside the hold and make the ship unstable, potentially sinking the ship. One such vessel suspected to be sunk due to this issue was the MS Bulk Jupiter in 2015. One method which can demonstrate this effect is the Can test, in which a sample of the material is placed in a cylindrical can and struck against a surface many times. If a moist slurry forms in the can, then there is a likelihood for the cargo to liquefy; although conversely, even if the sample remains dry it does not conclusively prove that it will remain that way, or that it is safe for loading.
Source of gallium
Bauxite is the main source of the rare metal gallium.
During the processing of bauxite to alumina in the Bayer process, gallium accumulates in the sodium hydroxide liquor. From this it can be extracted by a variety of methods. The most recent is the use of ion-exchange resin. Achievable extraction efficiencies critically depend on the original concentration in the feed bauxite. At a typical feed concentration of 50 ppm, about 15 percent of the contained gallium is extractable. The remainder reports to the red mud and aluminium hydroxide streams.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Online
#1287 2022-02-16 14:09:50
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,954
Re: Miscellany
1263) Galena
Summary
Galena, also called lead glance, a gray lead sulfide (PbS), is the chief ore mineral of lead. One of the most widely distributed sulfide minerals, it occurs in many different types of deposits, often in metalliferous veins, as at Broken Hill, Australia; Coeur d’Alene, Idaho, U.S.; Clausthal Zellerfeld, Ger.; and Cornwall, Eng. Large deposits also occur as replacements of limestone or dolomite (e.g., at Santa Eulalia, Mex.). Some deposits (e.g., at Darwin, Calif.) are of contact-metamorphic origin. Galena is found in cavities and brecciated (fractured) zones in limestone and chert, as in the extensive Mississippi River valley deposits, where 90 percent of the U.S. production of lead is mined. The mineral has occasionally been observed as a replacement of organic matter and sometimes occurs in coal beds.
Galena forms isometric crystals in which the ionic lattice is like that of sodium chloride. The mineral is easily weathered to secondary lead minerals, the upper part of galena deposits often containing cerussite, anglesite, and pyromorphite. Nodules of anglesite and cerussite with a banded structure and a galena core are common.
In many cases, galena contains silver and so is often mined as a source of silver as well as lead. Other commercially important minerals that frequently occur in close association with galena include antimony, copper, and zinc.
Details
Galena, also called lead glance, is the natural mineral form of lead(II) sulfide (PbS). It is the most important ore of lead and an important source of silver.
Galena is one of the most abundant and widely distributed sulfide minerals. It crystallizes in the cubic crystal system often showing octahedral forms. It is often associated with the minerals sphalerite, calcite and fluorite.
Lead ore deposits
Galena is the main ore of lead, used since ancient times, since lead can be smelted from galena in an ordinary wood fire. Galena typically is found in hydrothermal veins in association with sphalerite, marcasite, chalcopyrite, cerussite, anglesite, dolomite, calcite, quartz, barite, and fluorite. It is also found in association with sphalerite in low-temperature lead-zinc deposits within limestone beds. Minor amounts are found in contact metamorphic zones, in pegmatites, and disseminated in sedimentary rock.
In some deposits the galena contains up to 0.5% silver, a byproduct that far surpasses the main lead ore in revenue. In these deposits significant amounts of silver occur as included silver sulfide mineral phases or as limited silver in solid solution within the galena structure. These argentiferous galenas have long been an important ore of silver. Silver-bearing galena is almost entirely of hydrothermal origin; galena in lead-zinc deposits contains little silver.
Galena deposits are found worldwide in various environments. Noted deposits include those at Freiberg in Saxony; Cornwall, the Mendips in Somerset, Derbyshire, and Cumberland in England; the Madan and Rhodope Mountains in Bulgaria; the Sullivan Mine of British Columbia; Broken Hill and Mount Isa in Australia; and the ancient mines of Sardinia.
In the United States, it occurs most notably as lead-zinc ore in the Mississippi Valley type deposits of the Lead Belt in southeastern Missouri, which is the largest known deposit, and in the Driftless Area of Illinois, Iowa and Wisconsin. Galena also was a major mineral of the zinc-lead mines of the tri-state district around Joplin in southwestern Missouri and the adjoining areas of Kansas and Oklahoma. Galena is also an important ore mineral in the silver mining regions of Colorado, Idaho, Utah and Montana. Of the latter, the Coeur d'Alene district of northern Idaho was most prominent.
Australia is world's leading producer of lead as of 2021, most of which is extracted as galena. Argentiferous galena was accidentally discovered at Glen Osmond in 1841, and additional deposits were discovered near Broken Hill in 1876 and at Mount Isa in 1923. Most galena in Australia is found in hydrothermal deposits emplaced around 1680 million years ago, which have since been heavily metamorphosed.
The largest documented crystal of galena is composite cubo-octahedra from the Great Laxey Mine, Isle of Man, measuring 25 cm × 25 cm × 25 cm (10 in × 10 in × 10 in).
Importance
Galena is the official state mineral of the U.S. states of Kansas, Missouri, and Wisconsin; the former mining communities of Galena, Kansas, and Galena, Illinois, take their names from deposits of this mineral.
Crystal structure
Galena belongs to the octahedral sulfide group of minerals that have metal ions in octahedral positions, such as the iron sulfide pyrrhotite and the nickel math niccolite. The galena group is named after its most common member, with other isometric members that include manganese bearing alabandite and niningerite.
Divalent lead (Pb) cations and sulfur (S) anions form a close-packed cubic unit cell much like the mineral halite of the halide mineral group. Zinc, cadmium, iron, copper, antimony, math, bismuth and selenium also occur in variable amounts in galena. Selenium substitutes for sulfur in the structure constituting a solid solution series. The lead telluride mineral altaite has the same crystal structure as galena.
Geochemistry
Within the weathering or oxidation zone galena alters to anglesite (lead sulfate) or cerussite (lead carbonate). Galena exposed to acid mine drainage can be oxidized to anglesite by naturally occurring bacteria and archaea, in a process similar to bioleaching.
Uses of galena
One of the oldest uses of galena was in the eye cosmetic kohl. In Ancient Egypt, this was applied around the eyes to reduce the glare of the desert sun and to repel flies, which were a potential source of disease.
In pre-Columbian North America, galena was used by indigenous peoples as an ingredient in decorative paints and cosmetics, and widely traded throughout the eastern United States. Traces of galena are frequently found at the Mississippian city at Kincaid Mounds in present-day Illinois. The galena used at the site originated from deposits in southeastern and central Missouri and the Upper Mississippi Valley.
Galena is the primary ore of lead, and is often mined for its silver content.
It can be used as a source of lead in ceramic glaze.
Galena cat's whisker detector
Galena is a semiconductor with a small band gap of about 0.4 eV, which found use in early wireless communication systems. It was used as the crystal in crystal radio receivers, in which it was used as a point-contact diode capable of rectifying alternating current to detect the radio signals. The galena crystal was used with a sharp wire, known as a "cat's whisker" in contact with it.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Online
#1288 2022-02-17 14:04:15
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,954
Re: Miscellany
1264) Cinnabar
Summary
Cinnabar, mercury sulfide (HgS), is the chief ore mineral of mercury. It is commonly encountered with pyrite, marcasite, and stibnite in veins near recent volcanic rocks and in hot-springs deposits. The most important deposit is at Almadén, Spain, where it has been mined for 2,000 years. Other deposits are in Huancavelica, Peru; Iudrio, Italy; and the Coast Ranges of California, U.S. Metacinnabar, the isometric (cubic) form of cinnabar, transforms to cinnabar upon heating to 400°–550° C (750°–1,020° F).
Details
Cinnabar, is the bright scarlet to brick-red form of mercury(II) sulfide (HgS). It is the most common source ore for refining elemental mercury, and is the historic source for the brilliant red or scarlet pigment termed vermilion and associated red mercury pigments.
Cinnabar generally occurs as a vein-filling mineral associated with recent volcanic activity and alkaline hot springs. The mineral resembles quartz in symmetry and in its exhibiting birefringence. Cinnabar has a mean refractive index near 3.2, a hardness between 2.0 and 2.5, and a specific gravity of approximately 8.1. The color and properties derive from a structure that is a hexagonal crystalline lattice belonging to the trigonal crystal system, crystals that sometimes exhibit twinning.
Cinnabar has been used for its color since antiquity in the Near East, including as a rouge-type cosmetic, in the New World since the Olmec culture, and in China since as early as the Yangshao culture, where it was used in coloring stoneware.
Associated modern precautions for use and handling of cinnabar arise from the toxicity of the mercury component, which was recognized as early as ancient Rome.
Etymology
The name comes from Ancient Greek: a Greek word most likely applied by Theophrastus to several distinct substances. In Latin, it was sometimes known as minium, meaning also "red cinnamon", though both of these terms now refer specifically to lead tetroxide.
Properties and structure:
Properties
Cinnabar is generally found in a massive, granular or earthy form and is bright scarlet to brick-red in color, though it occasionally occurs in crystals with a nonmetallic adamantine luster. It resembles quartz in its symmetry. It exhibits birefringence, and it has the second highest refractive index of any mineral. Its mean refractive index is 3.08 (sodium light wavelengths), versus the indices for diamond and the non-mineral GaAs, which are 2.42 and 3.93, respectively. The hardness of cinnabar is 2.0–2.5 on the Mohs scale, and its specific gravity 8.1.
Structure
Structurally, cinnabar belongs to the trigonal crystal system. It occurs as thick tabular or slender prismatic crystals or as granular to massive incrustations. Crystal twinning occurs as simple contact twins.
Note, mercury(II) sulfide, HgS, adopts the cinnabar structure described, and one additional structure, i.e. it is dimorphous. Cinnabar is the more stable form, and is a structure akin to that of HgO: each Hg center has two short Hg−S bonds (each 2.36 Å), and four longer Hg···S contacts (with 3.10, 3.10, 3.30, and 3.30 Å separations). In addition, HgS is found in a black, non-cinnabar polymorph (metacinnabar) that has the zincblende structure.
Occurrence
Cinnabar generally occurs as a vein-filling mineral associated with recent volcanic activity and alkaline hot springs. Cinnabar is deposited by epithermal ascending aqueous solutions (those near surface and not too hot) far removed from their igneous source. It is associated with native mercury, stibnite, realgar, pyrite, marcasite, opal, quartz, chalcedony, dolomite, calcite and barite.
Cinnabar is essentially found in all mineral extraction localities that yield mercury, notably Almadén (Spain). This mine was exploited from Roman times until 1991, being for centuries the most important cinnabar deposit in the world. Good cinnabar crystals have also been found there. Cinnabar deposits also appear in Giza (Egypt); Puerto Princesa (Philippines); New Almaden, Hastings Mine, St. John's Mine, Vallejo, California (United States); Idrija (Slovenia); New Idria, California (United States); Moschellandsberg [de] near Obermoschel in the Palatinate; La Ripa, at the foot of the Apuan Alps and in the Mount Amiata (Tuscany, Italy); the mountain Avala (Serbia); Huancavelica (Peru); Murfreesboro, Arkansas (United States); Terlingua, Texas (United States); and the province of Guizhou in China, where fine crystals have been obtained. It was also mined near Red Devil, Alaska (United States) on the middle Kuskokwim River. Red Devil was named after the Red Devil cinnabar mine, a primary source of mercury. It has been found in Dominica near its sulfur springs at the southern end of the island along the west coast.
Cinnabar is still being deposited, such as from the hot waters of Sulphur Bank Mine in California and Steamboat Springs, Nevada (United States).
Mining and extraction of mercury
As the most common source of mercury in nature, cinnabar has been mined for thousands of years, even as far back as the Neolithic Age. During the Roman Empire it was mined both as a pigment, and for its mercury content.
To produce liquid mercury (quicksilver), crushed cinnabar ore is roasted in rotary furnaces. Pure mercury separates from sulfur in this process and easily evaporates. A condensing column is used to collect the liquid metal, which is most often shipped in iron flasks.
Toxicity
Associated modern precautions for use and handling of cinnabar arise from the toxicity of the mercury component, which was recognized as early as in ancient Rome. Because of its mercury content, cinnabar can be toxic to human beings. Overexposure to mercury, mercurialism, was seen as an occupational disease to the ancient Romans. Though people in ancient South America often used cinnabar for art, or processed it into refined mercury (as a means to gild silver and gold to objects) the toxic properties of mercury were well known. It was dangerous to those who mined and processed cinnabar; it caused shaking, loss of sense, and death. Data suggests that mercury was retorted from cinnabar and the workers were exposed to the toxic mercury fumes. "Mining in the Spanish cinnabar mines of Almadén, 225 km (140 mi) southwest of Madrid, was regarded as being akin to a death sentence due to the shortened life expectancy of the miners, who were slaves or convicts."
Decorative use
Cinnabar has been used for its color since antiquity in the Near East, including as a rouge-type cosmetic, in the New World since the Olmec culture, and in China for writing on oracle bones as early as the Zhou dynasty. Late in the Song dynasty it was used in coloring lacquerware.
Cinnabar's use as a color in the New World, since the Olmec culture, is exemplified by its use in royal burial chambers during the peak of Maya civilization, most dramatically in the 7th-century Tomb of the Red Queen in Palenque, where the remains of a noble woman and objects belonging to her in her sarcophagus were completely covered with bright red powder made from cinnabar.
The most popularly known use of cinnabar is in Chinese carved lacquerware, a technique that apparently originated in the Song dynasty. The danger of mercury poisoning may be reduced in ancient lacquerware by entraining the powdered pigment in lacquer, but could still pose an environmental hazard if the pieces were accidentally destroyed. In the modern jewellery industry, the toxic pigment is replaced by a resin-based polymer that approximates the appearance of pigmented lacquer.
Two female mummies dated AD 1399 to 1475 found in Cerro Esmeralda in Chile in 1976 had clothes colored with cinnabar.
Other forms
* Hepatic cinnabar or paragite is an impure brownish variety from the mines of Idrija in the Carniola region of Slovenia, in which the cinnabar is mixed with bituminous and earthy matter.
* Hypercinnabar, crystallizes at high temperature in the hexagonal crystal system.
* Metacinnabar is a black-colored form of mercury(II) sulfide, which crystallizes in the cubic crystal system.
* Synthetic cinnabar is produced by treatment of mercury(II) salts with hydrogen sulfide to precipitate black, synthetic metacinnabar, which is then heated in water. This conversion is promoted by the presence of sodium sulfide.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Online
#1289 2022-02-18 14:32:43
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,954
Re: Miscellany
1265) Algorithm
Summary
In mathematics and computer science, an algorithm is a finite sequence of well-defined instructions, typically used to solve a class of specific problems or to perform a computation. Algorithms are used as specifications for performing calculations, data processing, automated reasoning, automated decision-making and other tasks. In contrast, a heuristic is an approach to problem solving that may not be fully specified or may not guarantee correct or optimal results, especially in problem domains where there is no well-defined correct or optimal result.
As an effective method, an algorithm can be expressed within a finite amount of space and time, and in a well-defined formal language for calculating a function. Starting from an initial state and initial input (perhaps empty), the instructions describe a computation that, when executed, proceeds through a finite number of well-defined successive states, eventually producing "output" and terminating at a final ending state. The transition from one state to the next is not necessarily deterministic; some algorithms, known as randomized algorithms, incorporate random input.
Details
Algorithm is systematic procedure that produces—in a finite number of steps—the answer to a question or the solution of a problem. The name derives from the Latin translation, Algoritmi de numero Indorum, of the 9th-century Muslim mathematician al-Khwarizmi’s arithmetic treatise “Al-Khwarizmi Concerning the Hindu Art of Reckoning.”
For questions or problems with only a finite set of cases or values an algorithm always exists (at least in principle); it consists of a table of values of the answers. In general, it is not such a trivial procedure to answer questions or problems that have an infinite number of cases or values to consider, such as “Is the natural number (1, 2, 3,…) a prime?” or “What is the greatest common divisor of the natural numbers a and b?” The first of these questions belongs to a class called decidable; an algorithm that produces a yes or no answer is called a decision procedure. The second question belongs to a class called computable; an algorithm that leads to a specific number answer is called a computation procedure.
Algorithms exist for many such infinite classes of questions; Euclid’s Elements, published about 300 BCE, contained one for finding the greatest common divisor of two natural numbers. Every elementary-school student is drilled in long division, which is an algorithm for the question “Upon dividing a natural number a by another natural number b, what are the quotient and the remainder?” Use of this computational procedure leads to the answer to the decidable question “Does b divide a?” (the answer is yes if the remainder is zero). Repeated application of these algorithms eventually produces the answer to the decidable question “Is a prime?” (the answer is no if a is divisible by any smaller natural number besides 1).
Sometimes an algorithm cannot exist for solving an infinite class of problems, particularly when some further restriction is made upon the accepted method. For instance, two problems from Euclid’s time requiring the use of only a compass and a straightedge (unmarked ruler)—trisecting an angle and constructing a square with an area equal to a given circle—were pursued for centuries before they were shown to be impossible. At the turn of the 20th century, the influential German mathematician David Hilbert proposed 23 problems for mathematicians to solve in the coming century. The second problem on his list asked for an investigation of the consistency of the axioms of arithmetic. Most mathematicians had little doubt of the eventual attainment of this goal until 1931, when the Austrian-born logician Kurt Gödel demonstrated the surprising result that there must exist arithmetic propositions (or questions) that cannot be proved or disproved. Essentially, any such proposition leads to a determination procedure that never ends (a condition known as the halting problem). In an unsuccessful effort to ascertain at least which propositions are unsolvable, the English mathematician and logician Alan Turing rigorously defined the loosely understood concept of an algorithm. Although Turing ended up proving that there must exist undecidable propositions, his description of the essential features of any general-purpose algorithm machine, or Turing machine, became the foundation of computer science. Today the issues of decidability and computability are central to the design of a computer program—a special type of algorithm.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Online
#1290 2022-02-19 15:12:42
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,954
Re: Miscellany
1266) Pyrite
The mineral pyrite, or iron pyrite, also known as fool's gold, is an iron sulfide with the chemical formula FeS2 (iron (II) disulfide). Pyrite is the most abundant sulfide mineral.
Pyrite's metallic luster and pale brass-yellow hue give it a superficial resemblance to gold, hence the well-known nickname of fool's gold. The color has also led to the nicknames brass, brazzle, and Brazil, primarily used to refer to pyrite found in coal.
The name pyrite is derived from the Greek, "stone or mineral which strikes fire". In ancient Roman times, this name was applied to several types of stone that would create sparks when struck against steel; Pliny the Elder described one of them as being brassy, almost certainly a reference to what we now call pyrite.
By Georgius Agricola's time, c. 1550, the term had become a generic term for all of the sulfide minerals.
Pyrite is usually found associated with other sulfides or oxides in quartz veins, sedimentary rock, and metamorphic rock, as well as in coal beds and as a replacement mineral in fossils, but has also been identified in the sclerites of scaly-foot gastropods. Despite being nicknamed fool's gold, pyrite is sometimes found in association with small quantities of gold. A substantial proportion of the gold is "invisible gold" incorporated into the pyrite. It has been suggested that the presence of both gold and math is a case of coupled substitution but as of 1997 the chemical state of the gold remained controversial.
Uses
Pyrite enjoyed brief popularity in the 16th and 17th centuries as a source of ignition in early firearms, most notably the wheellock, where a sample of pyrite was placed against a circular file to strike the sparks needed to fire the gun.
Pyrite is used with flintstone and a form of tinder made of stringybark by the Kaurna people, people of South Australia, as a traditional method of starting fires.
Pyrite has been used since classical times to manufacture copperas (ferrous sulfate). Iron pyrite was heaped up and allowed to weather (an example of an early form of heap leaching). The acidic runoff from the heap was then boiled with iron to produce iron sulfate. In the 15th century, new methods of such leaching began to replace the burning of sulfur as a source of sulfuric acid. By the 19th century, it had become the dominant method.
Pyrite remains in commercial use for the production of sulfur dioxide, for use in such applications as the paper industry, and in the manufacture of sulfuric acid. Thermal decomposition of pyrite into FeS (iron(II) sulfide) and elemental sulfur starts at 540 °C (1,004 °F); at around 700 °C (1,292 °F), pS2 is about 1 atm.
A newer commercial use for pyrite is as the cathode material in Energizer brand non-rechargeable lithium batteries.
Pyrite is a semiconductor material with a band gap of 0.95 eV.[20] Pure pyrite is naturally n-type, in both crystal and thin-film forms, potentially due to sulfur vacancies in the pyrite crystal structure acting as n-dopants.
During the early years of the 20th century, pyrite was used as a mineral detector in radio receivers, and is still used by crystal radio hobbyists. Until the vacuum tube matured, the crystal detector was the most sensitive and dependable detector available—with considerable variation between mineral types and even individual samples within a particular type of mineral. Pyrite detectors occupied a midway point between galena detectors and the more mechanically complicated perikon mineral pairs. Pyrite detectors can be as sensitive as a modern 1N34A germanium diode detector.
Pyrite has been proposed as an abundant, non-toxic, inexpensive material in low-cost photovoltaic solar panels. Synthetic iron sulfide was used with copper sulfide to create the photovoltaic material. More recent efforts are working toward thin-film solar cells made entirely of pyrite.
Pyrite is used to make marcasite jewelry. Marcasite jewelry, made from small faceted pieces of pyrite, often set in silver, was known since ancient times and was popular in the Victorian era. At the time when the term became common in jewelry making, "marcasite" referred to all iron sulfides including pyrite, and not to the orthorhombic FeS2 mineral marcasite which is lighter in color, brittle and chemically unstable, and thus not suitable for jewelry making. Marcasite jewelry does not actually contain the mineral marcasite. The specimens of pyrite, when it appears as good quality crystals, are used in decoration. They are also very popular in mineral collecting. Among the sites that provide the best specimens are Soria and La Rioja provinces (Spain).
In value terms, China ($47 million) constitutes the largest market for imported unroasted iron pyrites worldwide, making up 65% of global imports. China is also the fastest growing in terms of the unroasted iron pyrites imports, with a CAGR of +27.8% from 2007 to 2016.
Pyrite, also called iron pyrite or fool’s gold, is a naturally occurring iron disulfide mineral. The name comes from the Greek word pyr, “fire,” because pyrite emits sparks when struck by metal. Pyrite is called fool’s gold; to the novice its colour is deceptively similar to that of a gold nugget. Nodules of pyrite have been found in prehistoric burial mounds, which suggests their use as a means of producing fire. Wheel-lock guns, in which a spring-driven serrated wheel rotated against a piece of pyrite, were used before development of the flintlock. Pure pyrite (FeS2) contains 46.67 percent iron and 53.33 percent sulfur by weight. Its crystals display isometric symmetry. For detailed physical properties, see sulfide mineral.
Pyrite is widely distributed and forms under extremely varied conditions. For example, it can be produced by magmatic (molten rock) segregation, by hydrothermal solutions, and as stalactitic growth. It occurs as an accessory mineral in igneous rocks, in vein deposits with quartz and sulfide minerals, and in sedimentary rocks, such as shale, coal, and limestone.
Pyrite occurs in large deposits in contact metamorphic rocks. Deposits of copper-bearing pyrite are widely distributed and often of great size. They usually occur in or near the contact of eruptive rocks with schists or slates. Pyrite weathers rapidly to hydrated iron oxide, goethite, or limonite; pseudomorphs of goethite after pyrite are common. This weathering produces a characteristic yellow-brown stain or coating, such as on rusty quartz.
Historically, pyrite was used commercially as a source of sulfur, particularly for the production of sulfuric acid, but today sulfur is largely collected as a by-product of petroleum processing. Because of the availability of much better sources of iron, pyrite is not generally used as an iron ore.
For many years Spain was the largest producer, the large deposits located on the Tinto River being important also for copper. Today Italy and China are the world’s largest producers, followed by Russia and Peru.
What is Pyrite?
Pyrite is a brass-yellow mineral with a bright metallic luster. It has a chemical composition of iron sulfide (FeS2) and is the most common sulfide mineral. It forms at high and low temperatures and occurs, usually in small quantities, in igneous, metamorphic, and sedimentary rocks worldwide. Pyrite is so common that many geologists would consider it to be a ubiquitous mineral.
The name "pyrite" is after the Greek "pyr" meaning "fire." This name was given because pyrite can be used to create the sparks needed for starting a fire if it is struck against metal or another hard material. Pieces of pyrite have also been used as a spark-producing material in flintlock firearms.
Pyrite has a nickname that has become famous - "Fool's Gold." The mineral's gold color, metallic luster, and high specific gravity often cause it to be mistaken for gold by inexperienced prospectors. However, pyrite is often associated with gold. The two minerals often form together, and in some deposits pyrite contains enough included gold to warrant mining.
Identifying Pyrite
Hand specimens of pyrite are usually easy to identify. The mineral always has a brass-yellow color, a metallic luster and a high specific gravity. It is harder than other yellow metallic minerals, and its streak is black, usually with a tinge of green. It often occurs in well-formed crystals in the shape of cubes, octahedrons, or pyritohedrons, which often have striated faces.
The only common mineral that has properties similar to pyrite is marcasite, a dimorph of pyrite with the same chemical composition but an orthorhombic crystal structure. Marcasite does not have the same brassy yellow color of pyrite. Instead it is a pale brass color, sometimes with a slight tint of green. Marcasite is more brittle than pyrite and also has a slightly lower specific gravity at 4.8.
Fool's Gold
Pyrite and gold can easily be distinguished. Gold is very soft and will bend or dent with pin pressure. Pyrite is brittle, and thin pieces will break with pin pressure. Gold leaves a yellow streak, while pyrite's streak is greenish black. Gold also has a much higher specific gravity. A little careful testing will help you avoid the "Fool's Gold" problem.
Uses of Pyrite
Pyrite is composed of iron and sulfur; however, the mineral does not serve as an important source of either of these elements. Iron is typically obtained from oxide ores such as hematite and magnetite. These ores occur in much larger accumulations, the iron is easier to extract and the metal is not contaminated with sulfur, which reduces its strength.
Pyrite used to be an important ore for the production of sulfur and sulfuric acid. Today most sulfur is obtained as a byproduct of oil and gas processing. Some sulfur continues to be produced from pyrite as a byproduct of gold production.
Pyrite is occasionally used as a gemstone. It is fashioned into beads, cut into cabochons, faceted, and carved into shapes. This type of jewelry was popular in the United States and Europe in the mid- to late-1800s. Most of the jewelry stones were called "marcasite," but they are actually pyrite. (Marcasite would be a poor choice for jewelry because it quickly oxidizes, and the oxidation products cause damage to anything that they contact. Pyrite is not an excellent jewelry stone because it easily tarnishes.)
Pyrite as an Ore of Gold
The most important use of pyrite is as an ore of gold. Gold and pyrite form under similar conditions and occur together in the same rocks. In some deposits small amounts of gold occur as inclusions and substitutions within pyrite.
Some pyrites can contain 0.25% gold by weight or more. Although this is a tiny fraction of the ore, the value of gold is so high that the pyrite might be a worthwhile mining target. If pyrite contains 0.25% gold and the gold price is $1500 per troy ounce, then one ton of pyrite will contain about 73 troy ounces of gold worth over $109,000. That is not a guaranteed money-maker. It depends upon how efficiently the gold can be recovered and the cost of the recovery process.
Pyrite and Coal Mining
Sulfur occurs in coal in three different forms: 1) organic sulfur, 2) sulfate minerals, and 3) sulfide minerals (mostly pyrite with minor amounts of marcasite). When the coal is burned, these forms of sulfur are converted into sulfur dioxide gas and contribute to air pollution and acid rain unless they are removed from the emissions. The sulfide mineral content of the coal can be reduced by heavy mineral separation, but this removal is expensive, results in a loss of coal, and cannot be done with 100% efficiency.
The sulfide minerals in coal and its surrounding rocks can produce acid mine drainage. Before mining, these minerals are deep within the ground and below the water table where they are not subject to oxidation. During and after mining the level of the water table often falls, exposing the sulfides to oxidation. This oxidation produces acid mine drainage which contaminates groundwater and streams. Mining also breaks the rocks above and below the coal. This creates more pathways for the movement of oxygenated waters and exposes more surface area to oxidation.
Pyrite and Construction Projects
Crushed stone used to make concrete, concrete block, and asphalt paving materials must be free of pyrite. Pyrite will oxidize when it is exposed to air and moisture. That oxidation will result in the production of acids and a volume change that will damage the concrete and reduce its strength. This damage can result in failure or maintenance problems.
Pyrite should not be present in the base material, subsoil or bedrock under roads, parking lots, or buildings. Oxidation of pyrite can result in damage to pavement, foundations, and floors. In parts of the country where pyrite is commonly found, construction sites should be tested to detect the presence of pyritic materials. If pyrite is detected, the site can be rejected or the problem materials can be excavated and replaced with quality fill.
Pyrite and Organic Material
The conditions of pyrite formation in the sedimentary environment include a supply of iron, a supply of sulfur, and an oxygen-poor environment. This often occurs in association with decaying organic materials. Organic decay consumes oxygen and releases sulfur. For this reason, pyrite commonly and preferentially occurs in dark-colored organic-rich sediments such as coal and black shale. The pyrite often replaces organic materials such as plant debris and shells to create interesting fossils composed of pyrite.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Online
#1291 2022-02-19 22:52:34
- jabah013.307
- Member
- From: Kingdom Of Jabah013.307
- Registered: 2021-07-05
- Posts: 165
- Website
Re: Miscellany
Is anyone going to be sending a rocket there to find out more about UY Scuti? It would be cool if UY Scuti took over the Sun. We would learn more from it.
Quote Of The Month:
'Whether it's the best of times or the worst of times, it's the only time we've got.' - Art Buchwald.
Offline
#1292 2022-02-20 03:19:04
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,954
Re: Miscellany
See the link in Sl.No.25.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Online
#1293 2022-02-20 14:11:02
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,954
Re: Miscellany
1267) Cryptogam
A cryptogam (scientific name Cryptogamae) is a plant (in the wide sense of the word) or a plant-like organism that reproduces by spores, without flowers or seeds. The name Cryptogamae means "hidden reproduction", referring to the fact that no seed is produced, thus cryptogams represent the non-seed bearing plants. Other names, such as "thallophytes", "lower plants", and "spore plants" are also occasionally used. As a group, Cryptogamae are the opposite of the Phanerogamae or Spermatophyta, the seed plants. The best-known groups of cryptogams are algae, lichens, mosses, and ferns, but it also includes non-photosynthetic organisms traditionally classified as plants, such as fungi, slime molds, and bacteria. The classification is now deprecated in Linnaean taxonomy.
At one time, the cryptogams were formally recognised as a group within the plant kingdom. In his system for classification of all known plants and animals, Carl Linnaeus (1707–1778) divided the plant kingdom into 24 classes, one of which was the "Cryptogamia". This included all plants with concealed reproductive organs. He divided Cryptogamia into four orders: Algae, Musci (bryophytes), Filices (ferns), and Fungi.
Not all cryptogams are treated as part of the plant kingdom today; the fungi, in particular, are regarded as a separate kingdom, more closely related to animals than plants, while blue-green algae are now regarded as a phylum of bacteria. Therefore, in contemporary plant systematics, "Cryptogamae" is not a taxonomically coherent group, but is cladistically polyphyletic. However, all organisms known as cryptogams belong to the field traditionally studied by botanists and the names of all cryptogams are regulated by the International Code of Nomenclature for algae, fungi, and plants.
During World War II, the British Government Code and Cypher School recruited Geoffrey Tandy, a marine biologist expert in cryptogams, to Station X, Bletchley Park when someone confused these with cryptograms.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Online
#1294 2022-02-21 15:06:51
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,954
Re: Miscellany
1268) Visual impairment
Visual impairment, also known as vision impairment or vision loss, is a decreased ability to see to a degree that causes problems not fixable by usual means, such as glasses. Some also include those who have a decreased ability to see because they do not have access to glasses or contact lenses. Visual impairment is often defined as a best corrected visual acuity of worse than either 20/40 or 20/60. The term blindness is used for complete or nearly complete vision loss. Visual impairment may cause difficulties with normal daily activities such as reading and walking without adaptive training and equipment.
The most common causes of visual impairment globally are uncorrected refractive errors (43%), cataracts (33%), and glaucoma (2%). Refractive errors include near-sightedness, far-sightedness, presbyopia, and astigmatism. Cataracts are the most common cause of blindness. Other disorders that may cause visual problems include age-related macular degeneration, diabetic retinopathy, corneal clouding, childhood blindness, and a number of infections. Visual impairment can also be caused by problems in the brain due to stroke, premature birth, or trauma, among others. These cases are known as cortical visual impairment. Screening for vision problems in children may improve future vision and educational achievement. Screening adults without symptoms is of uncertain benefit. Diagnosis is by an eye exam.
The World Health Organization (WHO) estimates that 80% of visual impairment is either preventable or curable with treatment. This includes cataracts, the infections river blindness and trachoma, glaucoma, diabetic retinopathy, uncorrected refractive errors, and some cases of childhood blindness. Many people with significant visual impairment benefit from vision rehabilitation, changes in their environment, and assistive devices.
As of 2015 there were 940 million people with some degree of vision loss. 246 million had low vision and 39 million were blind. The majority of people with poor vision are in the developing world and are over the age of 50 years. Rates of visual impairment have decreased since the 1990s. Visual impairments have considerable economic costs both directly due to the cost of treatment and indirectly due to decreased ability to work.
Blindness
Blindness, transient or permanent inability is to see any light at all (total blindness) or to retain any useful vision despite attempts at vision enhancement (functional blindness). Less-severe levels of vision impairment have been categorized, ranging from near-normal vision to various degrees of low vision to near-blindness, depending on the visual acuity and functional impact stemming from the vision loss. Legal blindness is a government-defined term that determines eligibility for various services or benefits as well as restrictions on certain activities such as driving.
Specific causes of impaired vision are too numerous to list. In general, any process that causes malfunction of the retina, the optic nerve, or the visual centres and pathways of the brain can reduce vision. In severe cases, blindness may result. Broad categories of conditions that impair vision include infections (e.g., gonorrhea or congenital rubella infection), inflammations (e.g., uveitis), congenital or hereditary diseases (e.g., retinitis pigmentosa), tumours, cataracts, trauma or mechanical injury, metabolic and nutritional disorders, glaucoma, vascular damage (e.g., diabetic eye disease or atherosclerosis), and refractive errors (e.g., nearsightedness or farsightedness). In addition, there are many vision-lowering conditions for which there is no well-understood cause (e.g., age-related macular degeneration).
Many other potentially blinding disorders do not fit easily into general categories. Few of these conditions, however, lead to total blindness, and many of them have some form of available treatment. Even when the underlying problem cannot be corrected, multiple low-vision aids have been developed to optimize remaining vision. In cases of functional or total blindness, other senses and skills must be emphasized or developed. In addition, a strong psychosocial support system can greatly enhance a person’s ability to cope with vision loss.
Cataract is the world’s leading cause of blindness, accounting for approximately 42 % of all cases of blindness in all nations. In the United States, more than 25 million Americans are estimated to have cataract, according to the report “Future of Vision: Forecasting the Prevalence and Costs of Vision Problems.” As the population in America continues to age, the number of cataract cases are projected to increase by 50 % to 38.5 million by 2032.
What is a Cataract?
A cataract is a cloudy area in the lens of your eye and are very common as you get older. The cataract stops light from properly passing through to the retina. Generally, it does not cause pain, redness or tears.
In fact, more than half of all Americans age 80 or older either have cataracts or have had surgery to get rid of cataracts.
At first, you may not notice that you have a cataract. But over time, cataracts can make your vision blurry, hazy, or less colorful. You may have trouble reading or doing other everyday activities.
Most cataracts are age-related — they happen because of normal changes in your eyes as you get older. But you can get cataracts for other reasons — for example, after an eye injury or after surgery for another eye problem (like glaucoma).
No matter what type of cataract you have, the treatment is always surgery.
What are the Symptoms?
You might not have any symptoms at first, when cataracts are mild. But as cataracts grow, they can cause changes in your vision. For example, you may notice that:
* Your vision is cloudy or blurry
* Colors look faded
* You can’t see well at night
* Lamps, sunlight, or headlights seem too bright
* You see a halo around lights
* You see double (this sometimes goes away as the cataract gets bigger)
* You have to change the prescription for your glasses often
These symptoms can be a sign of other eye problems, too. Be sure to talk to your eye doctor if you have any of these problems.
Over time, cataracts can lead to vision loss.
What are the Risk Factors?
* Older age
* Intense heat or long-term exposure to UV rays from the sun
* Certain diseases, such as diabetes
* Inflammation in the eye
* Hereditary influences
* Events before birth, such as German measles in the mother
* Long-term steroid use
* Eye injuries
* Eye diseases
* Smoking
How to Prevent Cataracts at any Age?
* Wear sunglasses with UVA/UVB protection and a hat with a brim to block the sun.
* Quit smoking and avoid excess alcohol consumption.
* Eat healthy. Eat plenty of fruits and vegetables — especially dark, leafy greens like spinach, kale, and collard greens. Eating foods high in antioxidants, such as beta-carotene, selenium, and vitamins C and E may also help ward off cataract development.
* Schedule yearly eye exams. Even if your vision is clear and healthy, routine visits allow your eye care professional to look for signs of cataracts, glaucoma, macular degeneration, and other vision disorders.
What is the Treatment for Cataracts?
Surgery is the only way to get rid of a cataract, but you may not need to get surgery right away. During cataract surgery, the doctor removes the clouded lens and replaces it with a new, artificial lens (also called an intraocular lens, or IOL). This surgery is very safe, and 9 out of 10 people who get it can see better afterwards.
Talk about your options with your doctor. Most people don’t need to rush into surgery. Waiting to have surgery usually won’t harm your eyes or make surgery more difficult later. Remember these tips:
* Tell your doctor if cataracts are getting in the way of your everyday activities
* See your doctor for regular check-ups
* Ask your doctor about the benefits and risks of cataract surgery
* Encourage family members to get checked for cataracts, since they can run in families.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Online
#1295 2022-02-22 14:26:37
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,954
Re: Miscellany
1269) Neutron
Details: I
The neutron is a subatomic particle, symbol n, which has a neutral (not positive or negative) charge, and a mass slightly greater than that of a proton. Protons and neutrons constitute the nuclei of atoms. Since protons and neutrons behave similarly within the nucleus, and each has a mass of approximately one atomic mass unit, they are both referred to as nucleons. Their properties and interactions are described by nuclear physics.
The chemical properties of an atom are mostly determined by the configuration of electrons that orbit the atom's heavy nucleus. The electron configuration is determined by the charge of the nucleus, which is determined by the number of protons, or atomic number. The number of neutrons is the neutron number. Neutrons do not affect the electron configuration, but the sum of atomic and neutron numbers is the mass of the nucleus.
Atoms of a chemical element that differ only in neutron number are called isotopes. For example, carbon, with atomic number 6, has an abundant isotope carbon-12 with 6 neutrons and a rare isotope carbon-13 with 7 neutrons. Some elements occur in nature with only one stable isotope, such as fluorine. Other elements occur with many stable isotopes, such as tin with ten stable isotopes.
The properties of an atomic nucleus depend on both atomic and neutron numbers. With their positive charge, the protons within the nucleus are repelled by the long-range electromagnetic force, but the much stronger, but short-range, nuclear force binds the nucleons closely together. Neutrons are required for the stability of nuclei, with the exception of the single-proton hydrogen nucleus. Neutrons are produced copiously in nuclear fission and fusion. They are a primary contributor to the nucleosynthesis of chemical elements within stars through fission, fusion, and neutron capture processes.
The neutron is essential to the production of nuclear power. In the decade after the neutron was discovered by James Chadwick in 1932, neutrons were used to induce many different types of nuclear transmutations. With the discovery of nuclear fission in 1938, it was quickly realized that, if a fission event produced neutrons, each of these neutrons might cause further fission events, in a cascade known as a nuclear chain reaction. These events and findings led to the first self-sustaining nuclear reactor (Chicago Pile-1, 1942) and the first nuclear weapon (Trinity, 1945).
Dedicated neutron sources like neutron generators, research reactors and spallation sources produce free neutrons for use in irradiation and in neutron scattering experiments. A free neutron spontaneously decays to a proton, an electron, and an antineutrino, with a mean lifetime of about 15 minutes. Free neutrons do not directly ionize atoms, but they do indirectly cause ionizing radiation, so they can be a biological hazard, depending on dose. A small natural "neutron background" flux of free neutrons exists on Earth, caused by cosmic ray showers, and by the natural radioactivity of spontaneously fissionable elements in the Earth's crust.
Details : II
Neutron is a neutral subatomic particle that is a constituent of every atomic nucleus except ordinary hydrogen. It has no electric charge and a rest mass equal to 1.67493 × {10}^{-27} kg - marginally greater than that of the proton but nearly 1,839 times greater than that of the electron. Neutrons and protons, commonly called nucleons, are bound together in the dense inner core of an atom, the nucleus, where they account for 99.9 percent of the atom’s mass. Developments in high-energy particle physics in the 20th century revealed that neither the neutron nor the proton is a true elementary particle; rather, they are composites of extremely small elementary particles called quarks. The nucleus is bound together by the residual effect of the strong force, a fundamental interaction that governs the behaviour of the quarks that make up the individual protons and neutrons.
The neutron was discovered in 1932 by the English physicist James Chadwick. Within a few years after this discovery, many investigators throughout the world were studying the properties and interactions of the particle. It was found that various elements, when bombarded by neutrons, undergo fission—a type of nuclear reaction that occurs when the nucleus of a heavy element is split into two nearly equal smaller fragments. During this reaction each fissioned nucleus gives off additional free neutrons, as well as those bound to the fission fragments. In 1942 a group of American researchers, under the leadership of the physicist Enrico Fermi, demonstrated that enough free neutrons are produced during the fission process to sustain a chain reaction. This development led to the construction of the atomic bomb. Subsequent technological breakthroughs resulted in the large-scale production of electric power from nuclear energy. The absorption of neutrons by nuclei exposed to the high neutron intensities available in nuclear reactors has also made it possible to produce large quantities of radioactive isotopes useful for a wide variety of purposes. Furthermore, the neutron has become an important tool in pure research. Knowledge of its properties and structure is essential to an understanding of the structure of matter in general. Nuclear reactions induced by neutrons are valuable sources of information about the atomic nucleus and the force that binds it together.
A free neutron—one that is not incorporated into a nucleus—is subject to radioactive decay of a type called beta decay. It breaks down into a proton, an electron, and an antineutrino (the antimatter counterpart of the neutrino, a particle with no charge and little or no mass); the half-life for this decay process is 614 seconds. Because it readily disintegrates in this manner, the neutron does not exist in nature in its free state, except among other highly energetic particles in cosmic rays. Since free neutrons are electrically neutral, they pass unhindered through the electrical fields within atoms and so constitute a penetrating form of radiation, interacting with matter almost exclusively through relatively rare collisions with atomic nuclei.
Neutrons and protons are classified as hadrons, subatomic particles that are subject to the strong force. Hadrons, in turn, have been shown to possess internal structure in the form of quarks, fractionally charged subatomic particles that are thought to be among the fundamental components of matter. Like the proton and other baryon particles, the neutron consists of three quarks; in fact, the neutron possesses a magnetic dipole moment - i.e., it behaves like a minute magnet in ways that suggest that it is an entity of moving electric charges.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Online
#1296 2022-02-23 13:26:11
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,954
Re: Miscellany
1270) Proton
Summary
A proton is a stable subatomic particle, symbol p or p+, with a positive electric charge of +1e elementary charge. Its mass is slightly less than that of a neutron and 1836 times the mass of an electron. Protons and neutrons, each with masses of approximately one atomic mass unit, are jointly referred to as "nucleons" (particles present in atomic nuclei).
One or more protons are present in the nucleus of every atom; they are a necessary part of the nucleus. The number of protons in the nucleus is the defining property of an element, and is referred to as the atomic number (represented by the symbol Z). Since each element has a unique number of protons, each element has its own unique atomic number.
The word proton is Greek for "first", and this name was given to the hydrogen nucleus by Ernest Rutherford in 1920. In previous years, Rutherford had discovered that the hydrogen nucleus (known to be the lightest nucleus) could be extracted from the nuclei of nitrogen by atomic collisions. Protons were therefore a candidate to be a fundamental or elementary particle, and hence a building block of nitrogen and all other heavier atomic nuclei.
Although protons were originally considered elementary particles, in the modern Standard Model of particle physics, protons are now known to be composite particles, containing three valence quarks, and together with neutrons are now classified as hadrons. Protons are composed of two up quarks of charge +2/3 e and one down quark of charge -1/3 e. The rest masses of quarks contribute only about 1% of a proton's mass. The remainder of a proton's mass is due to quantum chromodynamics binding energy, which includes the kinetic energy of the quarks and the energy of the gluon fields that bind the quarks together. Because protons are not fundamental particles, they possess a measurable size; the root mean square charge radius of a proton is about 0.84–0.87 fm (or 0.84 × {10}^{-15} to 0.87 × {10}^{15} m). In 2019, two different studies, using different techniques, found the radius of the proton to be 0.833 fm, with an uncertainty of ±0.010 fm.
Free protons occur occasionally on Earth: thunderstorms can produce protons with energies of up to several tens of MeV. At sufficiently low temperatures and kinetic energies, free protons will bind to electrons. However, the character of such bound protons does not change, and they remain protons. A fast proton moving through matter will slow by interactions with electrons and nuclei, until it is captured by the electron cloud of an atom. The result is a protonated atom, which is a chemical compound of hydrogen. In vacuum, when free electrons are present, a sufficiently slow proton may pick up a single free electron, becoming a neutral hydrogen atom, which is chemically a free radical. Such "free hydrogen atoms" tend to react chemically with many other types of atoms at sufficiently low energies. When free hydrogen atoms react with each other, they form neutral hydrogen molecules (H2), which are the most common molecular component of molecular clouds in interstellar space.
Free protons are routinely used for accelerators for proton therapy or various particle physics experiments, with the most powerful example being the Large Hadron Collider.
Details
Proton is a stable subatomic particle that has a positive charge equal in magnitude to a unit of electron charge and a rest mass of 1.67262 × {10}^{-27} kg, which is 1,836 times the mass of an electron.
Protons, together with electrically neutral particles called neutrons, make up all atomic nuclei except for the hydrogen nucleus (which consists of a single proton). Every nucleus of a given chemical element has the same number of protons. This number defines the atomic number of an element and determines the position of the element in the periodic table. When the number of protons in a nucleus equals the number of electrons orbiting the nucleus, the atom is electrically neutral.
The discovery of the proton dates to the earliest investigations of atomic structure. While studying streams of ionized gaseous atoms and molecules from which electrons had been stripped, Wilhelm Wien (1898) and J.J. Thomson (1910) identified a positive particle equal in mass to the hydrogen atom. Ernest Rutherford showed (1919) that nitrogen under alpha-particle bombardment ejects what appear to be hydrogen nuclei. By 1920 he had accepted the hydrogen nucleus as an elementary particle, naming it proton.
High-energy particle-physics studies in the late 20th century refined the structural understanding of the nature of the proton within the group of subatomic particles. Protons and neutrons have been shown to be made up of smaller particles and are classified as baryons—particles composed of three elementary units of matter known as quarks.
Protons from ionized hydrogen are given high velocities in particle accelerators and are commonly used as projectiles to produce and study nuclear reactions. Protons are the chief constituent of primary cosmic rays and are among the products of some types of artificial nuclear reactions.
Vocabulary:
Protons: Positively charged subatomic particles located in the nucleus of an atom.
Neutrons: Neutrally charged subatomic particles located in the nucleus of an atom.
Electrons: Negatively charged subatomic particles located in orbitals surrounding the nucleus.
Atomic Mass: A weighted average of the number of neutrons and protons present for all isotopes.
Atomic Number: Number of protons present in an atom.
Element: A pure substance that cannot be broken down into a simpler substance by chemical means
How to find the Atomic Number
The atomic number of an element is simply the number of protons in its nucleus. The easiest way to find the atomic number, is to look on a periodic table, the atomic number is in the upper left corner, or is the largest number on the square.
Finding the Number of Protons
The number of protons in an atom is equal to the atomic number of the element. For example, let’s use oxygen. According to the periodic table, oxygen has the atomic number eight. The atomic number is located above the element’s symbol. Since oxygen has an atomic number of eight, there must be eight protons total. Moreover, the number of protons never changes for an element.
Finding the Number of Neutrons
The number of neutrons in an atom can be calculated by subtracting the atomic number from the atomic mass. Both of these numbers can be found on the periodic table. The atomic number is listed above the symbol of the element whereas the mass number is placed below. Let’s keep using oxygen as our example. Its atomic mass is 15.999 atomic mass units (amu) and its atomic number is 8. When we subtract 8 from 15.999, we will get 8. Also, it should be noted that the number of neutrons for an element may vary. Some elements have isotopes, which have different masses and therefore different numbers of neutrons.
Finding the Number of Electrons
The number of electrons in an atom is equal to the atomic number of an element, for neutrally charged species. This means the number of electrons and the number of protons in an element are equal. Therefore, the number of electrons in oxygen is 8. Moreover, since these two subatomic particles, electrons and protons, have opposite charges, they cancel out and keep the atom neutral.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Online
#1297 2022-02-24 14:20:36
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,954
Re: Miscellany
1271) Electron
Summary
The electron is a subatomic particle denoted by the symbol e - or β − whose electric charge is negative one elementary charge. Electrons belong to the first generation of the lepton particle family, and are generally thought to be elementary particles because they have no known components or substructure. The electron has a mass that is approximately 1/1836 that of the proton. Quantum mechanical properties of the electron include an intrinsic angular momentum (spin) of a half-integer value, expressed in units of the reduced Planck constant, ħ. Being fermions, no two electrons can occupy the same quantum state, in accordance with the Pauli exclusion principle. Like all elementary particles, electrons exhibit properties of both particles and waves: they can collide with other particles and can be diffracted like light. The wave properties of electrons are easier to observe with experiments than those of other particles like neutrons and protons because electrons have a lower mass and hence a longer de Broglie wavelength for a given energy.
Electrons play an essential role in numerous physical phenomena, such as electricity, magnetism, chemistry and thermal conductivity, and they also participate in gravitational, electromagnetic and weak interactions. Since an electron has charge, it has a surrounding electric field, and if that electron is moving relative to an observer, said observer will observe it to generate a magnetic field. Electromagnetic fields produced from other sources will affect the motion of an electron according to the Lorentz force law. Electrons radiate or absorb energy in the form of photons when they are accelerated. Laboratory instruments are capable of trapping individual electrons as well as electron plasma by the use of electromagnetic fields. Special telescopes can detect electron plasma in outer space. Electrons are involved in many applications such as tribology or frictional charging, electrolysis, electrochemistry, battery technologies, electronics, welding, cathode ray tubes, photoelectricity, photovoltaic solar panels, electron microscopes, radiation therapy, lasers, gaseous ionization detectors and particle accelerators.
Interactions involving electrons with other subatomic particles are of interest in fields such as chemistry and nuclear physics. The Coulomb force interaction between the positive protons within atomic nuclei and the negative electrons without, allows the composition of the two known as atoms. Ionization or differences in the proportions of negative electrons versus positive nuclei changes the binding energy of an atomic system. The exchange or sharing of the electrons between two or more atoms is the main cause of chemical bonding. In 1838, British natural philosopher Richard Laming first hypothesized the concept of an indivisible quantity of electric charge to explain the chemical properties of atoms. Irish physicist George Johnstone Stoney named this charge 'electron' in 1891, and J. J. Thomson and his team of British physicists identified it as a particle in 1897 during the cathode ray tube experiment. Electrons can also participate in nuclear reactions, such as nucleosynthesis in stars, where they are known as beta particles. Electrons can be created through beta decay of radioactive isotopes and in high-energy collisions, for instance when cosmic rays enter the atmosphere. The antiparticle of the electron is called the positron; it is identical to the electron except that it carries electrical charge of the opposite sign. When an electron collides with a positron, both particles can be annihilated, producing gamma ray photons.
Details
electron, lightest stable subatomic particle known. It carries a negative charge of 1.602176634 × {10}^{-19} coulomb, which is considered the basic unit of electric charge. The rest mass of the electron is 9.1093837015 × {10}^{-31} kg, which is only 1/1,836the mass of a proton. An electron is therefore considered nearly massless in comparison with a proton or a neutron, and the electron mass is not included in calculating the mass number of an atom.
The electron was discovered in 1897 by the English physicist J.J. Thomson during investigations of cathode rays. His discovery of electrons, which he initially called corpuscles, played a pivotal role in revolutionizing knowledge of atomic structure. Under ordinary conditions electrons are bound to the positively charged nuclei of atoms by the attraction between opposite electric charges. In a neutral atom the number of electrons is identical to the number of positive charges on the nucleus. Any atom, however, may have more or fewer electrons than positive charges and thus be negatively or positively charged as a whole; these charged atoms are known as ions. Not all electrons are associated with atoms; some occur in a free state with ions in the form of matter known as plasma.
Within any given atom, electrons move about the nucleus in an orderly arrangement of orbitals, the attraction between electrons and nucleus overcoming repulsion among the electrons that would otherwise cause them to fly apart. These orbitals are organized in concentric shells proceeding outward from the nucleus with an increasing number of subshells. The electrons in orbitals closest to the nucleus are held most tightly; those in the outermost orbitals are shielded by intervening electrons and are the most loosely held by the nucleus. As the electrons move about within this structure, they form a diffuse cloud of negative charge that occupies nearly the entire volume of the atom. The detailed structural arrangement of electrons within an atom is referred to as the electronic configuration of the atom. The electronic configuration determines not only the size of an individual atom but also the chemical nature of the atom. The classification of elements within groups of similar elements in the periodic table, for example, is based on the similarity in their electron structures.
Within the field of particle physics, there are two ways of classifying electrons. The electron is a fermion, a type of particle named after the Fermi-Dirac statistics that describe its behaviour. All fermions are characterized by half-integer values of their spin, where spin corresponds to the intrinsic angular momentum of the particle. The concept of spin is embodied in the wave equation for the electron formulated by P.A.M. Dirac. The Dirac wave equation also predicts the existence of the antimatter counterpart of the electron, the positron. Within the fermion group of subatomic particles, the electron can be further classified as a lepton. A lepton is a subatomic particle that reacts only by the electromagnetic, weak, and gravitational forces; it does not respond to the short-range strong force that acts between quarks and binds protons and neutrons in the atomic nucleus.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Online
#1298 2022-02-25 14:47:24
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,954
Re: Miscellany
1272) Grease (lubricant)
Summary
Grease is a thick, oily lubricant consisting of inedible lard, the rendered fat of waste animal parts, or a petroleum-derived or synthetic oil containing a thickening agent.
White grease is made from inedible hog fat and has a low content of free fatty acids. Yellow grease is made from darker parts of the hog and may include parts used to make white grease. Brown grease contains beef and mutton fats as well as hog fats. Fleshing grease is the fatty material trimmed from hides and pelts. Bone grease, hide grease, and garbage grease are named according to their origin. In some factories, food offal is used along with animal carcasses, butcher-shop scraps, and garbage from restaurants for recovery of fats.
Greases of mineral or synthetic origin consist of a thickening agent dispersed in a liquid lubricant such as petroleum oil or a synthetic fluid. The thickening agent may be soap, an inorganic gel, or an organic substance. Other additives inhibit oxidation and corrosion, prevent wear, and change viscosity. The fluid component is the more important lubricant for clearances between parts that are relatively large, but for small clearances the molecular soap layers provide the lubrication.
Synthetic grease may consist of synthetic oils containing standard soaps or may be a mixture of synthetic thickeners, or bases, in petroleum oils. Silicones are greases in which both the base and the oil are synthetic. Synthetic greases are made in water-soluble and water-resistant forms and may be used over a wide temperature range. The synthetics can be used in contact with natural or other rubbers because they do not soften these materials.
Details
Grease is a solid or semisolid lubricant formed as a dispersion of thickening agents in a liquid lubricant. Grease generally consists of a soap emulsified with mineral or vegetable oil.
A common feature of greases is that they possess a high initial viscosity, which upon the application of shear, drops to give the effect of an oil-lubricated bearing of approximately the same viscosity as the base oil used in the grease. This change in viscosity is called shear thinning. Grease is sometimes used to describe lubricating materials that are simply soft solids or high viscosity liquids, but these materials do not exhibit the shear-thinning properties characteristic of the classical grease. For example, petroleum jellies such as Vaseline are not generally classified as greases.
Greases are applied to mechanisms that can be lubricated only infrequently and where a lubricating oil would not stay in position. They also act as sealants to prevent ingress of water and incompressible materials. Grease-lubricated bearings have greater frictional characteristics because of their high viscosity.
Properties
A true grease consists of an oil and/or other fluid lubricant that is mixed with a thickener, typically a soap, to form a solid or semisolid. Greases are usually shear-thinning or pseudo-plastic fluids, which means that the viscosity of the fluid is reduced under shear. After sufficient force to shear the grease has been applied, the viscosity drops and approaches that of the base lubricant, such as the mineral oil. This sudden drop in shear force means that grease is considered a plastic fluid, and the reduction of shear force with time makes it thixotropic. A few greases are rheotropic, meaning they become more viscous when worked. It is often applied using a grease gun, which applies the grease to the part being lubricated under pressure, forcing the solid grease into the spaces in the part.
Thickeners
Soaps are the most common emulsifying agent used, and the selection of the type of soap is determined by the application. Soaps include calcium stearate, sodium stearate, lithium stearate, as well as mixtures of these components. Fatty acids derivatives other than stearates are also used, especially lithium 12-hydroxystearate. The nature of the soaps influences the temperature resistance (relating to the viscosity), water resistance, and chemical stability of the resulting grease. Calcium sulphonates and polyureas are increasingly common grease thickeners not based on metallic soaps.
Powdered solids may also be used as thickeners, especially as clays. Fatty oil-based greases have also been prepared with other thickeners, such as tar, graphite, or mica, which also increase the durability of the grease. Silicone greases are generally thickened with silica.
Engineering assessment and analysis
Lithium-based greases are the most commonly used; sodium and lithium-based greases have higher melting point (dropping point) than calcium-based greases but are not resistant to the action of water. Lithium-based grease has a dropping point at 190 to 220 °C (350 to 400 °F). However the maximum usable temperature for lithium-based grease is 120 °C.
The amount of grease in a sample can be determined in a laboratory by extraction with a solvent followed by e.g. gravimetric determination.
Additives
Some greases are labeled "EP", which indicates "extreme pressure". Under high pressure or shock loading, normal grease can be compressed to the extent that the greased parts come into physical contact, causing friction and wear. EP greases have increased resistance to film breakdown, form sacrificial coatings on the metal surface to protect if the film does break down, or include solid lubricants such as graphite or molybdenum disulfide to provide protection even without any grease remaining.
Solid additives such as copper or ceramic powder are added to some greases for static high pressure and/or high temperature applications, or where corrosion could prevent dis-assembly of components later in their service life. These compounds are working as a release agent. Solid additives cannot be used in bearings because of tight tolerances. Solid additives will cause increased wear in bearings.
History
Grease from the early Egyptian or Roman eras is thought to have been prepared by combining lime with olive oil. The lime saponifies some of the triglyceride that comprises oil to give a calcium grease. In the middle of the 19th century, soaps were intentionally added as thickeners to oils. Over the centuries, all manner of materials have been employed as greases. For example, black slugs Arion ater were used as axle-grease to lubricate wooden axle-trees or carts in Sweden.
Classification and standards
Jointly developed by ASTM International, the National Lubricating Grease Institute (NLGI) and SAE International, standard ASTM D4950 “standard classification and specification for automotive service greases” was first published in 1989 by ASTM International. It categorizes greases suitable for the lubrication of chassis components and wheel bearings of vehicles, based on performance requirements, using codes adopted from the NLGI's “chassis and wheel bearing service classification system”:
* LA and LB: chassis lubricants (suitability up to mild and severe duty respectively)
* GA, GB and GC: wheel-bearings (suitability up to mild, moderate and severe duty respectively)
A given performance category may include greases of different consistencies.
The measure of the consistency of grease is commonly expressed by its NLGI consistency number.
The main elements of standard ATSM D4950 and NLGI's consistency classification are reproduced and described in standard SAE J310 “automotive lubricating greases” published by SAE International.
Standard ISO 6743-9 “lubricants, industrial oils and related products (class L) — classification — part 9: family X (greases)”, first released in 1987 by the International Organization for Standardization, establishes a detailed classification of greases used for the lubrication of equipment, components of machines, vehicles, etc. It assigns a single multi-part code to each grease based on its operational properties (including temperature range, effects of water, load, etc.) and its NLGI consistency number.
Other types:
Silicone grease
Silicone grease is based on a silicone oil, usually thickened with amorphous fumed silica.
Fluoroether-based grease
Fluoropolymers containing C-O-C (ether) with fluorine (F) bonded to the carbon. They are more flexible and often used in demanding environments due to their inertness. Fomblin by Solvay Solexis and Krytox by duPont are prominent examples.
Laboratory grease
Grease is used to lubricate glass stopcocks and joints. Some laboratories fill them into syringes for easy application. Two typical examples: Left - Krytox, a fluoroether-based grease; Right - a silicone-based high vacuum grease by Dow Corning.
Apiezon, silicone-based, and fluoroether-based greases are all used commonly in laboratories for lubricating stopcocks and ground glass joints. The grease helps to prevent joints from "freezing", as well as ensuring high vacuum systems are properly sealed. Apiezon or similar hydrocarbon based greases are the cheapest, and most suitable for high vacuum applications. However, they dissolve in many organic solvents. This quality makes clean-up with pentane or hexanes trivial, but also easily leads to contamination of reaction mixtures.
Silicone-based greases are cheaper than fluoroether-based greases. They are relatively inert and generally do not affect reactions, though reaction mixtures often get contaminated..
Silicone-based greases are not easily removed with solvent, but they are removed efficiently by soaking in a base bath.
Fluoroether-based greases are inert to many substances including solvents, acids, bases, and oxidizers. They are, however, expensive, and are not easily cleaned away.
Food-grade grease
Food-grade greases are those greases that come in contact with food. Food-grade lubricant base oil are generally low sulfur petrochemical, less easily oxidized and emulsified. Another commonly used poly-α olefin base oil as well The United States Department of Agriculture (USDA) has three food-grade designations: H1, H2 and H3. H1 lubricants are food-grade lubricants used in food-processing environments where there is the possibility of incidental food contact. H2 lubricants are industrial lubricants used on equipment and machine parts in locations with no possibility of contact. H3 lubricants are food-grade lubricants, typically edible oils, used to prevent rust on hooks, trolleys and similar equipment.
Water-soluble grease analogs
In some cases, the lubrication and high viscosity of a grease are desired in situations where non-toxic, non-oil based materials are required. Carboxymethyl cellulose, or CMC, is one popular material used to create a water-based analog of greases. CMC serves to both thicken the solution and add a lubricating effect, and often silicone-based lubricants are added for additional lubrication. The most familiar example of this type of lubricant, used as a surgical and personal lubricant, is K-Y Jelly.
Cork grease
Cork grease is a lubricant used to lubricate cork, for example in musical wind instruments. It is usually applied using small lip-balm/lip-stick like applicators.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Online
#1299 2022-02-26 14:25:27
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,954
Re: Miscellany
1273) Acetylene
Summary
Acetylene, also called Ethyne, is the simplest and best-known member of the hydrocarbon series containing one or more pairs of carbon atoms linked by triple bonds, called the acetylenic series, or alkynes. It is a colourless, inflammable gas widely used as a fuel in oxyacetylene welding and cutting of metals and as raw material in the synthesis of many organic chemicals and plastics; its chemical formula is C2H2.
Pure acetylene is a colourless gas with a pleasant odour; as prepared from calcium carbide it usually contains traces of phosphine that cause an unpleasant garliclike odour. Acetylene can be decomposed to its elements with the liberation of heat. The decomposition may or may not give rise to explosion, depending on conditions. Pure acetylene under pressure in excess of about 15 pounds per square inch or in liquid or solid form explodes with extreme violence.
Mixtures of air and acetylene are explosive over a wide range, from about 2.5 percent air in acetylene to about 12.5 percent acetylene in air. When burned with the correct amount of air, acetylene gives a pure, white light, and for this reason it was at one time used for illumination in locations where electric power was not available, e.g., buoys, miners’ lamps, and road signals. The combustion of acetylene produces a large amount of heat, and, in a properly designed torch, the oxyacetylene flame attains the highest flame temperature (about 6,000° F, or 3,300° C) of any known mixture of combustible gases.
The hydrogen atoms in acetylene can be replaced by metallic elements to form acetylides—e.g., acetylides of silver, copper, or sodium. The acetylides of silver, copper, mercury, and gold are detonated by heat, friction, or shock. In addition to its reactive hydrogen atom, the carbon–carbon triple bond can readily add halogens, halogen acids, hydrogen cyanide, alcohols, amines, and amides. Acetylene can also add to itself or to aldehydes and ketones. Many of the reactions mentioned here are used for the commercial manufacture of various industrial and consumer products, such as acetaldehyde, the synthetic rubber neoprene, water-base paints, vinyl fabric and floor coverings, dry-cleaning solvents, and aerosol insecticide sprays. Acetylene is produced by any of three methods: by reaction of water with calcium carbide, by passage of a hydrocarbon through an electric arc, or by partial combustion of methane with air or oxygen.
Details
Acetylene (systematic name: ethyne) is the chemical compound with the formula C2H2. It is a hydrocarbon and the simplest alkyne. This colorless gas (lower hydrocarbons are generally gaseous in nature) is widely used as a fuel and a chemical building block. It is unstable in its pure form and thus is usually handled as a solution. Pure acetylene is odorless, but commercial grades usually have a marked odor due to impurities such as divinyl sulfide and phosphine.
As an alkyne, acetylene is unsaturated because its two carbon atoms are bonded together in a triple bond. The carbon–carbon triple bond places all four atoms in the same straight line, with CCH bond angles of 180°.
Discovery
Acetylene was discovered in 1836 by Edmund Davy, who identified it as a "new carburet of hydrogen". It was an accidental discovery while attempting to isolate potassium metal. By heating potassium carbonate with carbon at very high temperatures, he produced a residue of what is now known as potassium carbide, (K2C2), which reacted with water to release the new gas. It was rediscovered in 1860 by French chemist Marcellin Berthelot, who coined the name acétylène. Berthelot's empirical formula for acetylene (C4H2), as well as the alternative name "quadricarbure d'hydrogène" (hydrogen quadricarbide), were incorrect because many chemists at that time used the wrong atomic mass for carbon (6 instead of 12). Berthelot was able to prepare this gas by passing vapours of organic compounds (methanol, ethanol, etc.) through a red hot tube and collecting the effluent. He also found that acetylene was formed by sparking electricity through mixed cyanogen and hydrogen gases. Berthelot later obtained acetylene directly by passing hydrogen between the poles of a carbon arc.
Preparation
Since the 1950s, acetylene has mainly been manufactured by the partial combustion of methane. It is a recovered side product in production of ethylene by cracking of hydrocarbons. Approximately 400,000 tonnes were produced by this method in 1983. Its presence in ethylene is usually undesirable because of its explosive character and its ability to poison Ziegler–Natta catalysts. It is selectively hydrogenated into ethylene, usually using Pd–Ag catalysts.
Until the 1950s, when oil supplanted coal as the chief source of reduced carbon, acetylene (and the aromatic fraction from coal tar) was the main source of organic chemicals in the chemical industry. It was prepared by the hydrolysis of calcium carbide, a reaction discovered by Friedrich Wöhler in 1862[and still familiar to students:
CaC2 + 2H2O → Ca(OH)2 + C2H2↑
Calcium carbide production requires extremely high temperatures, ~2000 °C, necessitating the use of an electric arc furnace. In the US, this process was an important part of the late-19th century revolution in chemistry enabled by the massive hydroelectric power project at Niagara Falls.
Bonding
In terms of valence bond theory, in each carbon atom the 2s orbital hybridizes with one 2p orbital thus forming an sp hybrid. The other two 2p orbitals remain unhybridized. The two ends of the two sp hybrid orbital overlap to form a strong σ valence bond between the carbons, while on each of the other two ends hydrogen atoms attach also by σ bonds. The two unchanged 2p orbitals form a pair of weaker π bonds.
Since acetylene is a linear symmetrical molecule, it possesses the D∞h point group.
Physical properties:
Changes of state
At atmospheric pressure, acetylene cannot exist as a liquid and does not have a melting point. The triple point on the phase diagram corresponds to the melting point (−80.8 °C) at the minimal pressure at which liquid acetylene can exist (1.27 atm). At temperatures below the triple point, solid acetylene can change directly to the vapour (gas) by sublimation. The sublimation point at atmospheric pressure is −84.0 °C.
Other
At room temperature, the solubility of acetylene in acetone is 27.9 g per kg. For the same amount of dimethylformamide (DMF), the solubility is 51 g. At 20.26 bar, the solubility increases to 689.0 and 628.0 g for acetone and DMF, respectively. These solvents are used in pressurized gas cylinders.
Applications:
Welding
Approximately 20% of acetylene is supplied by the industrial gases industry for oxyacetylene gas welding and cutting due to the high temperature of the flame. Combustion of acetylene with oxygen produces a flame of over 3,600 K (3,330 °C; 6,020 °F), releasing 11.8 kJ/g. Oxyacetylene is the hottest burning common fuel gas. Acetylene is the third-hottest natural chemical flame after dicyanoacetylene's 5,260 K (4,990 °C; 9,010 °F) and cyanogen at 4,798 K (4,525 °C; 8,177 °F). Oxy-acetylene welding was a popular welding process in previous decades. The development and advantages of arc-based welding processes have made oxy-fuel welding nearly extinct for many applications. Acetylene usage for welding has dropped significantly. On the other hand, oxy-acetylene welding equipment is quite versatile – not only because the torch is preferred for some sorts of iron or steel welding (as in certain artistic applications), but also because it lends itself easily to brazing, braze-welding, metal heating (for annealing or tempering, bending or forming), the loosening of corroded nuts and bolts, and other applications. Bell Canada cable-repair technicians still use portable acetylene-fuelled torch kits as a soldering tool for sealing lead sleeve splices in manholes and in some aerial locations. Oxyacetylene welding may also be used in areas where electricity is not readily accessible. Oxyacetylene cutting is used in many metal fabrication shops. For use in welding and cutting, the working pressures must be controlled by a regulator, since above 15 psi (100 kPa), if subjected to a shockwave (caused, for example, by a flashback), acetylene decomposes explosively into hydrogen and carbon.
Portable lighting
Acetylene combustion produces a strong, bright light and the ubiquity of carbide lamps drove much acetylene commercialization in the early 20th century. Common applications included coastal lighthouses, street lights, and automobile and mining headlamps. In most of these applications, direct combustion is a fire hazard, and so acetylene has been replaced, first by incandescent lighting and many years later by low-power/high-lumen LEDs. Nevertheless, acetylene lamps remain in limited use in remote or otherwise inaccessible areas and in countries with a weak or unreliable central electric grid.
Plastics and acrylic acid derivatives
Acetylene can be semihydrogenated to ethylene, providing a feedstock for a variety of polyethylene plastics. Another major application of acetylene, especially in China is its conversion to acrylic acid derivatives. These derivatives form products such as acrylic fibers, glasses, paints, resins, and polymers.
Except in China, use of acetylene as a chemical feedstock has declined by 70% from 1965 to 2007 owing to cost and environmental considerations.
Niche applications
In 1881, the Russian chemist Mikhail Kucherov described the hydration of acetylene to acetaldehyde using catalysts such as mercury(II) bromide. Before the advent of the Wacker process, this reaction was conducted on an industrial scale.
The polymerization of acetylene with Ziegler–Natta catalysts produces polyacetylene films. Polyacetylene, a chain of CH centres with alternating single and double bonds, was one of the first discovered organic semiconductors. Its reaction with iodine produces a highly electrically conducting material. Although such materials are not useful, these discoveries led to the developments of organic semiconductors, as recognized by the Nobel Prize in Chemistry in 2000 to Alan J. Heeger, Alan G MacDiarmid, and Hideki Shirakawa.
In the 1920s, pure acetylene was experimentally used as an inhalation anesthetic.
Acetylene is sometimes used for carburization (that is, hardening) of steel when the object is too large to fit into a furnace.
Acetylene is used to volatilize carbon in radiocarbon dating. The carbonaceous material in an archeological sample is treated with lithium metal in a small specialized research furnace to form lithium carbide (also known as lithium acetylide). The carbide can then be reacted with water, as usual, to form acetylene gas to feed into a mass spectrometer to measure the isotopic ratio of carbon-14 to carbon-12.
Natural occurrence
The energy richness of the C≡C triple bond and the rather high solubility of acetylene in water make it a suitable substrate for bacteria, provided an adequate source is available.[citation needed] A number of bacteria living on acetylene have been identified. The enzyme acetylene hydratase catalyzes the hydration of acetylene to give acetaldehyde:
C2H2 + H2O → CH3CHO
Acetylene is a moderately common chemical in the universe, often associated with the atmospheres of gas giants. One curious discovery of acetylene is on Enceladus, a moon of Saturn. Natural acetylene is believed to form from catalytic decomposition of long-chain hydrocarbons at temperatures of 1,700 K (1,430 °C; 2,600 °F) and above. Since such temperatures are highly unlikely on such a small distant body, this discovery is potentially suggestive of catalytic reactions within that moon, making it a promising site to search for prebiotic chemistry.
Reactions
In vinylation reactions, H-X compounds add across the triple bond. Alcohols and phenols add to acetylene to give vinyl ethers. Thiols give vinyl thioethers. Similarly, vinylpyrrolidone and vinylcarbazole are produced industrially by vinylation of 2-pyrrolidone and carbazole.
The hydration of acetylene is a vinylation reaction, but the resulting vinyl alcohol isomerizes to acetaldehyde. The reaction is catalyzed by mercury salts. This reaction once was the dominant technology for acetaldehyde production, but it has been displaced by the Wacker process, which affords acetaldehyde by oxidation of ethylene, a cheaper feedstock. A similar situation applies to the conversion of acetylene to the valuable vinyl chloride by hydrochlorination vs the oxychlorination of ethylene.
Addition to formaldehyde
Acetylene adds to ketones and aldehydes in the presence of base catalysts. With carbonyl groups to give α-ethynyl alcohols in ethynylation reactions: Formaldehyde gives sequentially propargyl alcohol and butynediol. 1,4-Butynediol is produced industrially in this way from formaldehyde and acetylene.
Walter Reppe discovered that in the presence of catalysts, acetylene react to give a wide range of industrially significant chemicals.
Organometallic chemistry
Acetylene and its derivatives (2-butyne, diphenylacetylene, etc.) form complexes with transition metals. Its bonding to the metal is somewhat similar to that of ethylene complexes. These complexes are intermediates in many catalytic reactions such as alkyne trimerisation to benzene, tetramerization to cyclooctatetraene, and carbonylation to hydroquinone:
Fe(CO)5 + 4 C2H2 + 2 H2O → 2 C6H4(OH)2 + FeCO3 at basic conditions( 50-80 °C, 20-25 atm).
In the presence of certain transition metals, alkynes undergo alkyne metathesis.
Metal acetylides, species of the formula LnM-C2R, are also common. Copper(I) acetylide and silver acetylide can be formed in aqueous solutions with ease due to a poor solubility equilibrium.
Acid-base reactions
Acetylene has a pKa of 25, acetylene can be deprotonated by a superbase to form an acetylide:
HC≡CH + RM → RH + HC≡CM
Various organometallic and inorganic reagents are effective.
Safety and handling
Acetylene is not especially toxic, but when generated from calcium carbide, it can contain toxic impurities such as traces of phosphine and arsine, which give it a distinct garlic-like smell. It is also highly flammable, as are most light hydrocarbons, hence its use in welding. Its most singular hazard is associated with its intrinsic instability, especially when it is pressurized: under certain conditions acetylene can react in an exothermic addition-type reaction to form a number of products, typically benzene and/or vinylacetylene, possibly in addition to carbon and hydrogen.[citation needed] Consequently, acetylene, if initiated by intense heat or a shockwave, can decompose explosively if the absolute pressure of the gas exceeds about 200 kilopascals (29 psi). Most regulators and pressure gauges on equipment report gauge pressure, and the safe limit for acetylene therefore is 101 kPagage, or 15 psig. It is therefore supplied and stored dissolved in acetone or dimethylformamide (DMF),[contained in a gas cylinder with a porous filling (Agamassan), which renders it safe to transport and use, given proper handling. Acetylene cylinders should be used in the upright position to avoid withdrawing acetone during use.
Information on safe storage of acetylene in upright cylinders is provided by the OSHA, Compressed Gas Association, United States Mine Safety and Health Administration (MSHA), EIGA, and other agencies.
Copper catalyses the decomposition of acetylene, and as a result acetylene should not be transported in copper pipes.
Cylinders should be stored in an area segregated from oxidizers to avoid exacerbated reaction in case of fire/leakage. Acetylene cylinders should not be stored in confined spaces, enclosed vehicles, garages, and buildings, to avoid unintended leakage leading to explosive atmosphere. In the US, National Electric Code (NEC) requires consideration for hazardous areas including those where acetylene may be released during accidents or leaks. Consideration may include electrical classification and use of listed Group A electrical components in USA. Further information on determining the areas requiring special consideration is in NFPA 497. In Europe, ATEX also requires consideration for hazardous areas where flammable gases may be released during accidents or leaks.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Online
#1300 2022-02-27 13:55:03
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,954
Re: Miscellany
1274) Cerebrospinal fluid
Summary
Cerebrospinal fluid (CSF) is a clear, colorless body fluid found within the tissue that surrounds the brain and spinal cord of all vertebrates.
CSF is produced by specialised ependymal cells in the choroid plexus of the ventricles of the brain, and absorbed in the arachnoid granulations. There is about 125 mL of CSF at any one time, and about 500 mL is generated every day. CSF acts as a cushion or buffer, providing basic mechanical and immunological protection to the brain inside the skull. CSF also serves a vital function in the cerebral autoregulation of cerebral blood flow.
The CSF occupies the subarachnoid space (between the arachnoid mater and the pia mater) and the ventricular system around and inside the brain and spinal cord. It fills the ventricles of the brain, cisterns, and sulci, as well as the central canal of the spinal cord. There is also a connection from the subarachnoid space to the bony labyrinth of the inner ear via the perilymphatic duct where the perilymph is continuous with the cerebrospinal fluid. The ependymal cells of the choroid plexus have multiple motile cilia on their apical surfaces that beat to move the CSF through the ventricles.
A sample of CSF can be taken from around the spinal cord via lumbar puncture. This can used to test the intracranial pressure, as well as indicate diseases including infections of the brain or the surrounding meninges.
Although noted by Hippocrates, it was forgotten for centuries, though later was described in the 18th century by Emanuel Swedenborg. In 1914, Harvey Cushing demonstrated that the CSF was secreted by the choroid plexus.
Details
Cerebrospinal fluid (CSF) is a clear, colourless liquid that fills and surrounds the brain and the spinal cord and provides a mechanical barrier against shock. Formed primarily in the ventricles of the brain, the cerebrospinal fluid supports the brain and provides lubrication between surrounding bones and the brain and spinal cord. When an individual suffers a head injury, the fluid acts as a cushion, dulling the force by distributing its impact. The fluid helps to maintain pressure within the cranium at a constant level. An increase in the volume of blood or brain tissue results in a corresponding decrease in the fluid. Conversely, if there is a decrease in the volume of matter within the cranium, as occurs in atrophy of the brain, the CSF compensates with an increase in volume. The fluid also transports metabolic waste products, antibodies, chemicals, and pathological products of disease away from the brain and spinal-cord tissue into the bloodstream. CSF is slightly alkaline and is about 99 percent water. There are about 100 to 150 ml of CSF in the normal adult human body.
The exact method of the formation of the CSF is uncertain. After originating in the ventricles of the brain, it is probably filtered through the nervous-system membranes (ependyma). The CSF is continually produced, and all of it is replaced every six to eight hours. The fluid is eventually absorbed into the veins; it leaves the cerebrospinal spaces in a variety of locations, including spaces around the spinal roots and the cranial nerves. Movement of the CSF is affected by the downward pull of gravity, the continual process of secretion and absorption, blood pulsations in contingent tissue, respiration, pressure from the veins, and head and body movements.
Examination of the CSF may diagnose a number of diseases. A fluid sample is obtained by inserting a needle into the lumbar region of the lower back below the termination of the spinal cord; this procedure is called a lumbar puncture or spinal tap. If the CSF is cloudy, meningitis (inflammation of the central nervous system lining) may be present. Blood in the fluid may indicate a hemorrhage in or around the brain.
What Is Cerebrospinal Fluid?
Cerebrospinal fluid is the liquid around your brain and spinal cord. If a doctor thinks you have an illness that affects your nervous system, they might take a sample for testing.
The fluid is made by a group of cells, called the choroid plexus, that are deep inside your brain. Your body has about 150 milliliters of fluid -- roughly two-thirds of a cup.
As the colorless fluid goes around your brain and spinal cord, it cushions those organs, picks up needed supplies from your blood, and gets rid of waste products from brain cells.
Sometimes cerebrospinal fluid can have things in it that shouldn't be there, like bacteria or viruses that can attack your brain. With some illnesses, what's in that fluid can help your doctor figure out what's going on.
What Can It Tell Your Doctor?
A sample of cerebrospinal fluid can be an important clue. It can tell your doctor if you have one of a number of conditions, such as:
* Multiple sclerosis (when your body's immune system attacks your nerves) or other similar conditions known as autoimmune diseases
* Myelitis: inflammation of your spinal cord
* Encephalitis: inflammation of your brain cells
* Meningitis: inflammation of the thin tissues that cover and protect your brain and spinal cord. This usually is caused by an infection in the cerebrospinal fluid.
* A stroke or similar condition that causes bleeding around your brain
* Leukemia: a kind of blood cancer
* Dementia
How Is the Sample Taken?
Your doctor will use a procedure called a spinal tap or lumbar puncture. They'll take a sample of cerebrospinal fluid with a long, thin needle. You'll get a local anesthetic to numb the skin in the area, and the needle will go in between two of your vertebrae, the bones that surround your spinal cord and make up your spine. They'll take a tablespoon or two of the fluid for testing.
It usually takes about 45 minutes. You'll rest for a while afterward and may be told not to do anything strenuous for about a day. You may have a headache afterward, but tell your doctor if it lasts more than a few hours.
How Is the Sample Used?
What's in your cerebrospinal fluid can help your doctor identify or rule out various diseases.
* If you have high levels of a substance called immunoglobulin, which your body uses to fight disease, or other things related to your nerve cells, that could point to multiple sclerosis.
* If your doctor thinks you have Alzheimer's disease or another kind of dementia, certain types of proteins linked to the disease may be in the fluid.
* Discolored fluid might be a sign of a cerebral hemorrhage (bleeding in your brain) or stroke.
* Signs of bacterial or viral infection could tell your doctor you have an illness like meningitis or encephalitis.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Online