Math Is Fun Forum
You are not logged in.
- Topics: Active | Unanswered
#1301 2022-02-28 13:40:52
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,620
Re: Miscellany
1275) Polyurethane
Summary
Polyurethane (often abbreviated PUR and PU) refers to a class of polymers composed of organic units joined by carbamate (urethane) links. In contrast to other common polymers such as polyethylene and polystyrene, polyurethane is produced from a wide range of starting materials (monomers) and is therefore a class of polymers, rather than a distinct compound. This chemical variety allows for polyurethanes with very different physical properties, leading to an equally wide range of different applications. These include: rigid and flexible foams, varnishes and coatings, adhesives, electrical potting compounds, and fibres such as spandex and PUL. Of these, foams are the largest single application, accounting for 67% of all polyurethane produced in 2016.
Polyurethane polymers are traditionally and most commonly formed by reacting a di- or triisocyanate with a polyol. Since polyurethanes contain two types of monomers, which polymerise one after the other, they are classed as alternating copolymers. Both the isocyanates and polyols used to make polyurethanes contain, on average, two or more functional groups per molecule.
Global production in 2019 was some 25 million metric tonnes, accounting for about 6% of all polymers produced in that year. This is a sufficiently high volume for it to be regarded as a commodity plastic.
Details
Polyurethane is any of a class of synthetic resinous, fibrous, or elastomeric compounds belonging to the family of organic polymers made by the reaction of diisocyanates (organic compounds containing two functional groups of structure ―NCO) with other difunctional compounds such as glycols. The best known polyurethanes are flexible foams—used as upholstery material, mattresses, and the like—and rigid foams—used for such lightweight structural elements as cores for airplane wings.
Foamed polyurethanes result from the reaction of diisocyanates with organic compounds, usually polyesters, containing carboxyl groups; these reactions liberate bubbles of carbon dioxide that remain dispersed throughout the product. Use of polyethers or polyesters containing hydroxyl groups in preparing polyurethanes results in the formation of elastomeric fibres or rubbers that have outstanding resistance to attack by ozone but are vulnerable to the action of acids or alkalies.
In textiles the synthetic fibre known generically as spandex is composed of at least 85 percent polyurethane by weight. Such fibres are generally used for their highly elastic properties. Trademarked fibres in this group are Lycra, Numa, Spandelle, and Vyrene. Such fibres have, for many textile purposes, largely replaced natural and synthetic rubber fibres.
Although somewhat weak in the relaxed state, spandex fibres can be stretched about 500–610 percent beyond their original length without breaking and quickly return to their original length. The fibre, usually white with dull lustre, is readily dyed. It absorbs very little moisture. It melts at about 250° C (480° F) and yellows upon prolonged exposure to heat or light. Items made of spandex can be machine washed and dried at moderate temperatures. Use of chlorine bleach can produce yellowing. Spandex fibres are frequently covered with other fibres such as nylon.
Spandex is used in such apparel as foundation garments, support hosiery, and swimsuits. It is light in weight and cool; it is resistant to deterioration from body acids; and it is easily laundered and quick-drying.
Polyurethanes are a large class of polymers that can be tailored to a wide range of applications, making a significant contribution to the construction, automotive and electrical sectors.
Polyurethane is more commonly known for liquid coatings and paints, but applications can also vary from soft, flexible foams to rigid insulation. This broad range of applications is possible as there are both thermoplastic and thermosetting polyurethanes.
Polyurethane was originally synthesised as a substitute for natural rubber in World War II. Shortly after, the versatility of this new polymer and its ability to replace scarce materials gave rise to numerous applications. Nowadays, this group of polymers accounts for 7.7% of European plastic demand, behind commodity polymers polyethylene, polypropylene, and PVC.
Here, you will learn about:
* Structure and properties of polyurethane
* Production and processing of polyurethane
* Applications of polyurethane
* Commercial grades of polyurethane
Properties of polyurethane
Polyurethane is produced in a polymerisation reaction between diols (or polyols: alcohols with two or more reactive hydroxyl –OH groups) and diisocyanates (or polyisocyanates: isocyanates with two or more reactive isocyanate –NCO groups). The result is a molecule bonded by urethane (COONH) linkages.
There are a number of choices of alcohol molecules and corresponding isocyanate molecules, each combination producing a new polyurethane material with new properties. The properties of polyurethanes vary depending on the structure of this polymer backbone and can be tailored to have high strength and rigidity, or high flexibility and toughness.
Thermoplastic Polyurethane vs Thermosetting Polyurethane
The chosen polyol molecule has a large influence on the properties and degree of crosslinking in the polyurethane product. In particular, the number of hydroxyl groups per molecule and the size and flexibility of the hydrocarbon backbone can be chosen to tweak the mechanical properties of the resulting polyurethane material.
If a diol reacts with the diisocyanate, it forms a linear, thermoplastic polymer.
If the alcohol has more than two hydroxyl groups, this will then result in a rigid, cross-linked, thermosetting molecule.
Production and processing of polyurethane
Given polyurethanes are created in a reaction between diols and diisocyanates, the manufacturing process can be split into three parts:
* Production of diols
* Production of isocyanates
* Production of polyurethane from these components.
The polyol used in the production of polyurethanes is usually a polyether (in 90% of polyurethanes), or a polyester, with terminal hydroxyl groups. Additionally, there are many aromatic and aliphatic polyisocyanates; however, the most important of these, toluene diisocyanate (TDI) and methylene diphenyl diisocyanate (MDI), contribute to the production of around 95% of all polyurethanes. TDI is generally used in the production of soft, flexible foams for cushioning, whereas MDI is used in the production of more versatile, rigid polyurethanes.
If a diol reacts with either TDI or MDI, this forms a linear, thermoplastic polymer through a condensation polymerisation reaction. If the alcohol has more than two hydroxyl groups, this will result in a rigid, cross-linked, thermosetting molecule.
Additives are commonly added to the mixture to improve certain properties, such as cross-linking agents, chain-extending agents, blowing agents, surfactants, fillers, plasticisers, pigments and flame retardants. Blowing agents will create a polyurethane foam, and surfactants will control the bubble formation and, therefore, the cell formation of the foam. Fillers increase stiffness, plasticisers reduce hardness and pigments add colour to the material.
Polyurethane foam
The two reactant liquids combine to form a solid polymer, which may be elastic or rigid. This solid, however, may also contain bubbles making it a cellular foam material. These bubbles can either be formed chemically or physically. Chemical blowing can be achieved by adding water to the polyol, which in turn reacts with the isocyanates to form carbon dioxide gas bubbles. Alternatively, physical blowing is achieved by adding in a substance with a relatively low boiling point, such as pentane. As the exothermic polymerisation reaction proceeds, the pentane heats up and evaporates into bubbles.
This procedure can be controlled depending on the application at hand. For example, a shoe sole may be ‘blown’ to twice its volume, whereas cushions may be blown to 30-40 times the volume. In some low-density foams for cushioning and insulation, only 3% of the total volume is made up of solid polyurethane.
Applications of polyurethane
Since there are such a large number of polyisocyanate and polyol substances available for the production of polyurethane, a broad variety of materials can be produced to meet the needs of specific applications. Its relatively lightweight, and its versatility makes it an optimal material for construction, automotive, marine and even apparel applications.
Flexible Polyurethane Foam
Flexible polyurethane foam is light, durable, supportive and comfortable. It is commonly used for cushioning in bedding, furniture, automotive interiors, carpet underlay and packaging. This accounts for 30% of the polyurethane market due to its commodity usage.
Rigid Polyurethane Foam
Rigid polyurethane foams are the most economic and energy-efficient insulations, significantly cutting energy costs. When used in roof and wall insulation, insulated windows and doors, it helps maintain a uniform temperature and reduce noise levels. Rigid polyurethane foam is also commonly used as thermal insulation in refrigerators and freezers.
Coatings, Adhesives, Sealants and Elastomers
Polyurethane coatings can enhance a product’s appearance and increase its lifespan. A polyurethane finish can be used to add shine to the surface of an object, offering relatively better properties than traditional lacquer, shellac and varnish finishes. Wipe-on polyurethane or polyurethane paint is usually an oil-based polyurethane coating applied to wooden or concrete surfaces to add colour and increase durability, as it is usually too thick to spray on. Water-based polyurethane, however, is becoming more popular as it is less toxic and takes less time to dry than its oil-based counterpart.
Polyurethane adhesives provide strong bonding advantages, especially soon after it is manufactured, and polyurethane sealants offer tighter seals than traditional counterparts. Polyurethane elastomers can be moulded into any shape required, are lighter than metal, offer increased stress recovery, and are very environmentally resistant.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1302 2022-03-01 14:06:54
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,620
Re: Miscellany
1276) Silicone
Summary
Silicone, also called polysiloxane, is any of a diverse class of fluids, resins, or elastomers based on polymerized siloxanes, substances whose molecules consist of chains made of alternating silicon and oxygen atoms. Their chemical inertness, resistance to water and oxidation, and stability at both high and low temperatures have led to a wide range of commercial applications, from lubricating greases to electrical-wire insulation and biomedical implants.
Composition, structure, and properties
The silicones differ from most industrial polymers in that the chains of linked atoms that make up the backbones of their molecules do not contain carbon, the characteristic element of organic compounds. This lack of carbon in the polymer backbones makes polysiloxanes into unusual “inorganic” polymers—though in most members of the class two organic groups, usually vinyl (CH2), methyl (CH3), or phenyl (C6H5), are attached to each silicon atom. A general formula for silicones is (R2SiO)x, where R can be any one of a variety of organic groups.
Applications
Polysiloxanes are manufactured as fluids, resins, or elastomers, depending on the molecular weight of the polymers and the degree to which the polymer chains are interlinked. Nonvulcanized, low-molecular-weight polysiloxane fluids are exceptionally stable to decomposition by heat, water, or oxidizing agents and are good electrical insulators. They make excellent lubricants and hydraulic fluids, as well as emulsions for imparting water repellency to textiles, paper, and other materials. Silicone resins are used in protective coatings and electrically insulating varnishes and for laminating glass cloth.
Vulcanized silicone rubber is prepared in two principal forms: (1) as room-temperature-vulcanizing (RTV) elastomers, which are low-molecular-weight liquids that are cast or molded into desired shapes and then interlinked at room temperature, and (2) high-temperature-vulcanizing (HTV) elastomers, which are higher-molecular-weight gums that are mixed and processed like other elastomers. Silicone rubbers are usually strengthened by fillers such as silica; other fillers are mixed in to add bulk and colour. Valued for their electrical-insulating properties, chemical stability, and the wide temperature range over which they retain resiliency, silicone rubbers are used mainly in O-rings, heat-resistant seals, caulks, gaskets, electrical insulators, flexible molds, and (owing to their chemical inertness) surgical implants.
Details
A silicone or polysiloxane is a polymer made up of siloxane (−R2Si−O−SiR2−, where R = organic group). They are typically colorless oils or rubber-like substances. Silicones are used in sealants, adhesives, lubricants, medicine, cooking utensils, thermal insulation, and electrical insulation. Some common forms include silicone oil, silicone grease, silicone rubber, silicone resin, and silicone caulk.
Chemistry
More precisely called polymerized siloxanes or polysiloxanes, silicones consist of an inorganic silicon–oxygen backbone chain (⋯−Si−O−Si−O−Si−O−⋯) with two organic groups attached to each silicon center. Commonly, the organic groups are methyl. The materials can be cyclic or polymeric. By varying the −Si−O− chain lengths, side groups, and crosslinking, silicones can be synthesized with a wide variety of properties and compositions. They can vary in consistency from liquid to gel to rubber to hard plastic. The most common siloxane is linear polydimethylsiloxane (PDMS), a silicone oil. The second-largest group of silicone materials is based on silicone resins, which are formed by branched and cage-like oligosiloxanes.
Terminology and history
F. S. Kipping coined the word silicone in 1901 to describe the formula of polydiphenylsiloxane, Ph2SiO (Ph denoting phenyl, C6H5), by analogy with the formula of the ketone benzophenone, Ph2CO (his term was originally silicoketone). Kipping was well aware that polydiphenylsiloxane is polymeric whereas benzophenone is monomeric and noted the contrasting properties of Ph2SiO and Ph2CO. The discovery of the structural differences between Kipping's molecules and the ketones means that silicone is no longer the correct term (though it remains in common usage) and that the term siloxane is preferred according to the nomenclature of modern chemistry.
Silicone is often confused with silicon, but they are distinct substances. Silicon is a chemical element, a hard dark-grey semiconducting metalloid, which in its crystalline form is used to make integrated circuits ("electronic chips") and solar cells. Silicones are compounds that contain silicon, carbon, hydrogen, oxygen, and perhaps other kinds of atoms as well, and have many very different physical and chemical properties.
Compounds containing silicon–oxygen double bonds, now called silanones, but which could deserve the name "silicone", have long been identified as intermediates in gas-phase processes such as chemical vapor deposition in microelectronics production, and in the formation of ceramics by combustion. However, they have a strong tendency to polymerize into siloxanes. The first stable silanone was obtained in 2014 by A. Filippou and others.
Combustion
When silicone is burned in air or oxygen, it forms solid silica (silicon dioxide, SiO2) as a white powder, char, and various gases. The readily dispersed powder is sometimes called silica fume. The pyrolysis of certain polysiloxanes under an inert atmosphere is a valuable pathway towards the production of amorphous silicon oxycarbide ceramics, also known as polymer derived ceramics. Polysiloxanes terminated with functional ligands such as vinyl, mercapto or acrylate groups have been cross linked to yield preceramic polymers, which can be photopolymerised for the additive manufacturing of polymer derived ceramics by stereolithography techniques.
Properties
Silicones exhibit many useful characteristics, including:
* Low thermal conductivity
* Low chemical reactivity
* Low toxicity
* Thermal stability (constancy of properties over a wide temperature range of −100 to 250 °C)
* The ability to repel water and form watertight seals.
* Does not stick to many substrates, but adheres very well to others, e.g. glass
* Does not support microbiological growth
* Resistance to oxygen, ozone, and ultraviolet (UV) light. This property has led to the widespread use of silicones in the construction industry (e.g. coatings, fire protection, glazing seals) and the automotive industry (external gaskets, external trim).
* Electrical insulation properties. Because silicone can be formulated to be electrically insulative or conductive, it is suitable for a wide range of electrical applications.
* High gas permeability: at room temperature (25 °C), the permeability of silicone rubber for such gases as oxygen is approximately 400 times that of butyl rubber, making silicone useful for medical applications in which increased aeration is desired. Conversely, silicone rubbers cannot be used where gas-tight seals are necessary such as seals for high-pressure gasses or high vacuum.
Silicone can be developed into rubber sheeting, where it has other properties, such as being FDA compliant. This extends the uses of silicone sheeting to industries that demand hygiene, for example, food and beverage, and pharmaceuticals.
Applications
Silicones are used in many products. Ullmann's Encyclopedia of Industrial Chemistry lists the following major categories of application: Electrical (e.g. insulation), electronics (e.g., coatings), household (e.g., sealants and cooking utensils), automobile (e.g. gaskets), airplane (e.g., seals), office machines (e.g. keyboard pads), medicine and dentistry (e.g. tooth impression molds), textiles and paper (e.g. coatings). For these applications, an estimated 400,000 tonnes of silicones were produced in 1991. Specific examples, both large and small are presented below.
Automotive
In the automotive field, silicone grease is typically used as a lubricant for brake components since it is stable at high temperatures, is not water-soluble, and is far less likely than other lubricants to foul. DOT 5 brake fluids are based on liquid silicones.
Automotive spark plug wires are insulated by multiple layers of silicone to prevent sparks from jumping to adjacent wires, causing misfires. Silicone tubing is sometimes used in automotive intake systems (especially for engines with forced induction).
Sheet silicone is used to manufacture gaskets used in automotive engines, transmissions, and other applications.
Automotive body manufacturing plants and paint shops avoid silicones, as trace contamination may cause "fish eyes", which are small, circular craters which mar a smooth finish.
Additionally, silicone compounds such as silicone rubber are used as coatings and sealants for airbags; the high strength of silicone rubber makes it an optimal adhesive and sealant for high impact airbags. Silicones in combination with thermoplastics provide improvements in scratch and mar resistance and lowered coefficient of friction.
Aerospace
Silicone is a widely used material in the aerospace industry due to its sealing properties, stability across an extreme temperature range, durability, sound dampening and anti-vibration qualities, and naturally flame retardant properties. Maintaining extreme functionality is paramount for passenger safety in the aerospace industry, so each component on an aircraft requires high-performance materials.
Specially developed aerospace grades of silicone are stable from −70 to 220 °C, these grades can be used in the construction of gaskets for windows and cabin doors. During operation, aircraft go through large temperature fluctuations in a relatively short period of time; from freezing temperatures when flying at full altitude to the ambient temperatures when on the ground in hot countries. Silicone rubber can be molded with tight tolerances ensuring gaskets form airtight seals both on the ground and in the air, where atmospheric pressure decreases.
Silicone rubber's resistance to heat corrosion enables it to be used for gaskets in aircraft engines where it will outlast other types of rubber, both improving aircraft safety and reducing maintenance costs. The silicone acts to seal instrument panels and other electrical systems in the math, protecting printed circuit boards from the risks of extreme altitude such as moisture and extremely low temperature. Silicone can be used as a sheath to protect wires and electrical components from any dust or ice that may creep into a plane's inner workings.
As the nature of air travel results in much noise and vibration, powerful engines, landings, and high speeds all need to be considered to ensure passenger comfort and safe operation of the aircraft. As silicone rubber has exceptional noise reduction and anti-vibration properties, it can be formed into small components and fitted into small gaps ensuring all equipment can be protected from unwanted vibration such as overhead lockers, vent ducts, hatches, entertainment system seals, and LED lighting systems.
Building construction
The strength and reliability of silicone rubber are widely acknowledged in the construction industry. One-part silicone sealants and caulks are in common use to seal gaps, joints and crevices in buildings. One-part silicones cure by absorbing atmospheric moisture, which simplifies installation. In plumbing, silicone grease is typically applied to O-rings in brass taps and valves, preventing lime from sticking to the metal.
Structural silicone has also been used in curtain wall building façades since 1974 when the Art Institute of Chicago became the first building to receive exterior glass fixed only with the material. Silicone membranes have been used to cover and restore industrial roofs, thanks to its extreme UV resistance, and ability to keep their waterproof performance for decades.
Coatings
Silicone films can be applied to such silica-based substrates as glass to form a covalently bonded hydrophobic coating. Such coatings were developed for use on aircraft windshields to repel water and to preserve visibility, without requiring mechanical windshield wipers which are impractical at supersonic speeds. Similar treatments were eventually adapted to the automotive market in products marketed by Rain-X and others.
Many fabrics can be coated or impregnated with silicone to form a strong, waterproof composite such as silnylon.
A silicone polymer can be suspended in water by using stabilizing surfactants. This allows water-based formulations to be used to deliver many ingredients that would otherwise require a stronger solvent, or be too viscous to use effectively. For example a waterborne formulation using a silane's reactivity and penetration ability into a mineral-based surface can be combined with water-beading properties from a siloxane to produce a more-useful surface protection product.
Cookware
As a low-taint, non-toxic material, silicone can be used where contact with food is required. Silicone is becoming an important product in the cookware industry, particularly bakeware and kitchen utensils. Silicone is used as an insulator in heat-resistant potholders and similar items; however, it is more conductive of heat than similar less dense fiber-based products. Silicone oven mitts are able to withstand temperatures up to 260 °C (500 °F), making it possible to reach into boiling water.
Other products include molds for chocolate, ice, cookies, muffins, and various other foods; non-stick bakeware and reusable mats used on baking sheets; steamers, egg boilers or poachers; cookware lids, pot holders, trivets, and kitchen mats.
Defoaming
Silicones are used as active compounds in defoamers due to their low water solubility and good spreading properties.
Dry cleaning
Liquid silicone can be used as a dry cleaning solvent, providing an alternative to the traditional chlorine-containing perchloroethylene (perc) solvent. The use of silicones in dry cleaning reduces the environmental effect of a typically high-polluting industry.
Electronics
Electronic components are sometimes encased in silicone to increase stability against mechanical and electrical shock, radiation and vibration, a process called "potting". Silicones are used where durability and high performance are demanded of components under extreme environmental conditions, such as in space (satellite technology). They are selected over polyurethane or epoxy encapsulation when a wide operating temperature range is required (−65 to 315 °C). Silicones also have the advantage of little exothermic heat rise during cure, low toxicity, good electrical properties, and high purity.
Silicones are often a component of thermal pastes used to improve heat transfer from power-dissipating electronic components to heat sinks.
The use of silicones in electronics is not without problems, however. Silicones are relatively expensive and can be attacked by certain solvents. Silicone easily migrates as either a liquid or vapor onto other components. Silicone contamination of electrical switch contacts can lead to failures by causing an increase in contact resistance, often late in the life of the contact, well after any testing is completed. Use of silicone-based spray products in electronic devices during maintenance or repairs can cause later failures.
Firestops
Silicone foam has been used in North American buildings in an attempt to firestop openings within the fire-resistance-rated wall and floor assemblies to prevent the spread of flames and smoke from one room to another. When properly installed, silicone-foam firestops can be fabricated for building code compliance. Advantages include flexibility and high dielectric strength. Disadvantages include combustibility (hard to extinguish) and significant smoke development.
Silicone-foam firestops have been the subject of controversy and press attention due to smoke development from pyrolysis of combustible components within the foam, hydrogen gas escape, shrinkage, and cracking. These problems have led to reportable events among licensees (operators of nuclear power plants) of the Nuclear Regulatory Commission (NRC).
Silicone firestops are also used in aircraft.
Jewelry
Silicone is a popular alternative to traditional metals (such as silver and gold) with jewelry, specifically rings. Silicone rings are commonly worn in professions where metal rings can lead to injuries, such as electrical conduction and ring avulsions. During the mid-2010's, some professional athletes began wearing silicone rings as an alternative during games.
Lubricants
Silicone greases are used for many purposes, such as bicycle chains, airsoft gun parts, and a wide range of other mechanisms. Typically, a dry-set lubricant is delivered with a solvent carrier to penetrate the mechanism. The solvent then evaporates, leaving a clear film that lubricates but does not attract dirt and grit as much as an oil-based or other traditional "wet" lubricant.
Silicone personal lubricants are also available for use in medical procedures or coital activity.
Medicine and cosmetic surgery
Silicone is used in microfluidics, seals, gaskets, shrouds, and other applications requiring high biocompatibility. Additionally, the gel form is used in bandages and dressings, breast implants, testicle implants, pectoral implants, contact lenses, and a variety of other medical uses.
Scar treatment sheets are often made of medical grade silicone due to its durability and biocompatibility. Polydimethylsiloxane (PDMS) is often used for this purpose, since its specific crosslinking results in a flexible and soft silicone with high durability and tack. It has also been used as the hydrophobic block of amphiphilic synthetic block copolymers used to form the vesicle membrane of polymersomes.
Illicit cosmetic silicone injections may induce chronic and definitive silicone blood diffusion with dermatologic complications.
Ophthamology uses many products such as silicone oil used to replace the vitreous humor following vitrectomy, silicone intraocular lenses following cataract extraction, silicone tubes to keep a nasolacrimal passage open following dacryocystorhinostomy, canalicular stents for canalicular stenosis, punctual plugs for punctual occlusion in dry eyes, silicone rubber and bands as an external tamponade in tractional retinal detachment, and anteriorly-located break in rhegmatogenous retinal detachment.
Moldmaking
Two-part silicone systems are used as rubber molds to cast resins, foams, rubber, and low-temperature alloys. A silicone mold generally requires little or no mold-release or surface preparation, as most materials do not adhere to silicone. For experimental uses, ordinary one-part silicone can be used to make molds or to mold into shapes. If needed, common vegetable cooking oils or petroleum jelly can be used on mating surfaces as a mold-release agent.
Silicone cooking molds used as bakeware do not require coating with cooking oil; in addition, the flexibility of the rubber allows the baked food to be easily removed from the mold after cooking.
Personal care
Silicones are ingredients widely used in skincare, color cosmetic and hair care applications. Some silicones, notably the amine functionalized amodimethicones, are excellent hair conditioners, providing improved compatibility, feel, and softness, and lessening frizz. The phenyl dimethicones, in another silicone family, are used in reflection-enhancing and color-correcting hair products, where they increase shine and glossiness (and possibly impart subtle color changes). Phenyltrimethicones, unlike the conditioning amodimethicones, have refractive indices (typically 1.46) close to that of a human hair (1.54). However, if included in the same formulation, amodimethicone and phenyltrimethicone interact and dilute each other, making it difficult to achieve both high shine and excellent conditioning in the same product.
Silicone rubber is commonly used in baby bottle nipples (teats) for its cleanliness, aesthetic appearance, and low extractable content.
Silicones are used in shaving products and personal lubricants.
Toys and hobbies
Silly Putty and similar materials are composed of silicones dimethyl siloxane, polydimethylsiloxane, and decamethyl cyclopentasiloxane, with other ingredients. This substance is noted for its unusual characteristics, e.g., that it bounces, but breaks when given a sharp blow; it will also flow like a liquid and form a puddle given enough time.
Silicone "rubber bands" are a long-lasting popular replacement refill for real rubber bands in the 2013 fad "rubber band loom" toys at two to four times the price (in 2014). Silicone bands also come in bracelet sizes that can be custom embossed with a name or message. Large silicone bands are also sold as utility tie-downs.
Formerol is a silicone rubber (marketed as Sugru) used as an arts-and-crafts material, as its plasticity allows it to be molded by hand like modeling clay. It hardens at room temperature and it is adhesive to various substances including glass and aluminum.
Oogoo is a inexpensive silicone clay, which can be used as a substitute for Sugru.
In making aquariums, manufacturers now commonly use 100% silicone sealant to join glass plates. Glass joints made with silicone sealant can withstand great pressure, making obsolete the original aquarium construction method of angle-iron and putty. This same silicone is used to make hinges in aquarium lids or for minor repairs. However, not all commercial silicones are safe for aquarium manufacture, nor is silicone used for the manufacture of acrylic aquariums as silicones do not have long-term adhesion to plastics.
Production and marketing
The global demand for silicones approached US$12.5 billion in 2008, approximately 4% up from the previous year. It continues similar growth in the following years to reach $13.5 billion by 2010. The annual growth is expected to be boosted by broader applications, introduction of novel products and increasing awareness of using more environmentally friendly materials.
The leading global manufacturers of silicone base materials belong to three regional organizations: the European Silicone Center (CES) in Brussels, Belgium; the Environment Health and Safety Council (SEHSC) in Herndon, Virginia, US; and the Silicone Industry Association of Japan (SIAJ) in Tokyo, Japan. Dow Corning Silicones, Evonik Industries, Momentive Performance Materials, Milliken and Company (SiVance Specialty Silicones), Shin-Etsu Silicones, Wacker Chemie, Bluestar Silicones, JNC Corporation, Wacker Asahikasei Silicone, and Dow Corning Toray represent the collective membership of these organizations. A fourth organization, the Global Silicone Council (GSC) acts as an umbrella structure over the regional organizations. All four are non-profit, having no commercial role; their primary missions are to promote the safety of silicones from a health, safety, and environmental perspective. As the European chemical industry is preparing to implement the Registration, Evaluation, and Authorisation of Chemicals (REACH) legislation, CES is leading the formation of a consortium of silicones, silanes, and siloxanes producers and importers to facilitate data and cost-sharing.
Safety and environmental considerations
Silicone compounds are pervasive in the environment. Particular silicone compounds, cyclic siloxanes D4 and D5, are air and water pollutants and have negative health effects on test animals. They are used in various personal care products. The European Chemicals Agency found that "D4 is a persistent, bioaccumulative and toxic (PBT) substance and D5 is a very persistent, very bioaccumulative (vPvB) substance". Other silicones biodegrade readily, a process that is accelerated by a variety of catalysts, including clays. Cyclic silicones have been shown to involve the occurrence of silanols during biodegradation in mammals. The resulting silanediols and silanetriols are capable of inhibiting hydrolytic enzymes such as thermolysin, acetycholinesterase, however, the doses required for inhibition are by orders of magnitude higher than the ones resulting from the accumulated exposure to consumer products containing cyclomethicone.
At around 200 °C (392 °F) in an oxygen-containing atmosphere, PDMS releases traces of formaldehyde (but lesser amounts than other common materials such as polyethylene.) At this temperature, silicones were found to have lower formaldehyde generation than mineral oil and plastics (less than 3 to 48 µg CH2O/(g·hr) for a high consistency silicone rubber, versus around 400 µg CH2O/(g·hr) for plastics and mineral oil). By 250 °C (482 °F), copious amounts of formaldehyde have been found to be produced by all silicones (1,200 to 4,600 µg CH2O/(g·hr)).
![]()
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1303 2022-03-02 13:38:40
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,620
Re: Miscellany
1277) Ether
Summary
Ethers are a class of organic compounds that contain an ether group—an oxygen atom connected to two alkyl or aryl groups. They have the general formula R–O–R′, where R and R′ represent the alkyl or aryl groups. Ethers can again be classified into two varieties: if the alkyl groups are the same on both sides of the oxygen atom, then it is a simple or symmetrical ether, whereas if they are different, the ethers are called mixed or unsymmetrical ethers. A typical example of the first group is the solvent and anaesthetic diethyl ether, commonly referred to simply as "ether" (CH3–CH2–O–CH2–CH3). Ethers are common in organic chemistry and even more prevalent in biochemistry, as they are common linkages in carbohydrates and lignin.
Structure and bonding
Ethers feature bent C–O–C linkages. In dimethyl ether, the bond angle is 111° and C–O distances are 141 pm. The barrier to rotation about the C–O bonds is low. The bonding of oxygen in ethers, alcohols, and water is similar. In the language of valence bond theory, the hybridization at oxygen is sp3.
Oxygen is more electronegative than carbon, thus the alpha hydrogens of ethers are more acidic than those of simple hydrocarbons. They are far less acidic than alpha hydrogens of carbonyl groups (such as in ketones or aldehydes), however.
Ethers can be symmetrical of the type ROR or unsymmetrical of the type ROR'. Examples of the former are diethyl ether, dimethyl ether, dipropyl ether etc. Illustrative unsymmetrical ethers are anisole (methoxybenzene) and dimethoxyethane.
Nomenclature
In the IUPAC Nomenclature system, ethers are named using the general formula "alkoxyalkane", for example CH3–CH2–O–CH3 is methoxyethane. If the ether is part of a more-complex molecule, it is described as an alkoxy substituent, so –OCH3 would be considered a "methoxy-" group. The simpler alkyl radical is written in front, so CH3–O–CH2CH3 would be given as methoxy(CH3O)ethane(CH2CH3).
Trivial name
IUPAC rules are often not followed for simple ethers. The trivial names for simple ethers (i.e., those with none or few other functional groups) are a composite of the two substituents followed by "ether". For example, ethyl methyl ether (CH3OC2H5), diphenylether (C6H5OC6H5). As for other organic compounds, very common ethers acquired names before rules for nomenclature were formalized. Diethyl ether is simply called ether, but was once called sweet oil of vitriol. Methyl phenyl ether is anisole, because it was originally found in aniseed. The aromatic ethers include furans. Acetals (α-alkoxy ethers R–CH(–OR)–O–R) are another class of ethers with characteristic properties.
Polyethers
Polyethers are generally polymers containing ether linkages in their main chain. The term polyol generally refers to polyether polyols with one or more functional end-groups such as a hydroxyl group. The term "oxide" or other terms are used for high molar mass polymer when end-groups no longer affect polymer properties.
Crown ethers are cyclic polyethers. Some toxins produced by dinoflagellates such as brevetoxin and ciguatoxin are extremely large and are known as cyclic or ladder polyethers.
Related compounds
Many classes of compounds with C–O–C linkages are not considered ethers: Esters (R–C(=O)–O–R′), hemiacetals (R–CH(–OH)–O–R′), carboxylic acid anhydrides (RC(=O)–O–C(=O)R′).
Physical properties
Ethers have boiling points similar to those of the analogous alkanes. Simple ethers are generally colorless.
Diethyl ether: A colourless liquid with sweet odour. A common low boiling solvent (b.p. 34.6 °C) and an early anaesthetic. Used as starting fluid for diesel engines. Also used as a refrigerant and in the manufacture of smokeless gunpowder, along with use in perfumery.
Details
Ether is any of a class of organic compounds characterized by an oxygen atom bonded to two alkyl or aryl groups. Ethers are similar in structure to alcohols, and both ethers and alcohols are similar in structure to water. In an alcohol one hydrogen atom of a water molecule is replaced by an alkyl group, whereas in an ether both hydrogen atoms are replaced by alkyl or aryl groups.
At room temperature, ethers are pleasant-smelling colourless liquids. Relative to alcohols, ethers are generally less dense, are less soluble in water, and have lower boiling points. They are relatively unreactive, and as a result they are useful as solvents for fats, oils, waxes, perfumes, resins, dyes, gums, and hydrocarbons. Vapours of certain ethers are used as insecticides, miticides, and fumigants for soil.
Ethers are also important in medicine and pharmacology, especially for use as anesthetics. For example, ethyl ether (CH3CH2―O―CH2CH3), simply known as ether, was first used as a surgical anesthetic in 1842. Codeine, a potent pain-relieving drug, is the methyl ether of morphine. Because ether is highly flammable, it has largely been replaced by less-flammable anesthetics, including nitrous oxide (N2O) and halothane (CF3―CHClBr).
Ethyl ether is an excellent solvent for extractions and for a wide variety of chemical reactions. It is also used as a volatile starting fluid for diesel engines and gasoline engines in cold weather. Dimethyl ether is used as a spray propellant and refrigerant. Methyl t-butyl ether (MTBE) is a gasoline additive that boosts the octane number and reduces the amount of nitrogen-oxide pollutants in the exhaust. The ethers of ethylene glycol are used as solvents and plasticizers.
Nomenclature of ethers
Common names of ethers simply give the names of the two alkyl groups bonded to oxygen and add the word ether. The current practice is to list the alkyl groups in alphabetical order (t-butyl methyl ether), but older names often list the alkyl groups in increasing order of size (methyl t-butyl ether). If just one alkyl group is described in the name, it implies two identical groups, as in ethyl ether for diethyl ether.
Systematic (IUPAC) names for ethers use the more complex group as the root name, with the oxygen atom and the smaller group named as an alkoxy substituent. Examples given above are ethoxyethane (diethyl ether), methoxyethane (methyl ethyl ether), 2-methoxy-2-methylpropane (MTBE), and phenoxybenzene (diphenyl ether). The IUPAC nomenclature works well for compounds with additional functional groups, because the other functional groups can be described in the root name.
Physical properties of ethers
Ethers lack the hydroxyl groups of alcohols. Without the strongly polarized O―H bond, ether molecules cannot engage in hydrogen bonding with each other. Ethers do have nonbonding electron pairs on their oxygen atoms, however, and they can form hydrogen bonds with other molecules (alcohols, amines, etc.) that have O―H or N―H bonds. The ability to form hydrogen bonds with other compounds makes ethers particularly good solvents for a wide variety of organic compounds and a surprisingly large number of inorganic compounds.
Because ether molecules cannot engage in hydrogen bonding with each other, they have much lower boiling points than do alcohols with similar molecular weights.
Complexes of ethers with reagents
The unique properties of ethers (i.e., that they are strongly polar, with nonbonding electron pairs but no hydroxyl group) enhance the formation and use of many reagents. For example, Grignard reagents cannot form unless an ether is present to share its lone pair of electrons with the magnesium atom. Complexation of the magnesium atom stabilizes the Grignard reagent and helps to keep it in solution.
Electron-deficient reagents are also stabilized by ethers. For example, borane (BH3) is a useful reagent for making alcohols. Pure borane exists as its dimer, diborane (B2H6), a toxic gas that is inconvenient and hazardous to use. Borane forms stable complexes with ethers, however, and it is often supplied and used as its liquid complex with tetrahydrofuran (THF). Similarly, gaseous boron trifluoride (BF3) is more easily used as its liquid complex with diethyl ether, called BF3 etherate, rather than as the toxic, corrosive gas.
Crown ethers are specialized cyclic polyethers that surround specific metal ions to form crown-shaped cyclic complexes. They are named by using the parent name crown preceded by a number describing the size of the ring and followed by the number of oxygen atoms in the ring. In the crown-ether complex, the metal ion fits into the cavity of the crown ether and is solvated by the oxygen atoms. The exterior of the complex is nonpolar, masked by the alkyl groups of the crown ether. Many inorganic salts can be made soluble in nonpolar organic solvents by complexing them with an appropriate crown ether.
In each of these crown-ether complexes, only the cation is solvated by the crown ether. In a nonpolar solvent, the anion is not solvated but is dragged into solution by the cation. These “bare” anions in nonpolar solvents can be much more reactive than they are in polar solvents that solvate and shield the anion.
Synthesis of ethers
Williamson ether synthesis
The most versatile method for making ethers is the Williamson ether synthesis, named for English chemist Alexander Williamson, who devised the method in the 19th century. It uses an alkoxide ion to attack an alkyl halide, substituting the alkoxy (―O―R) group for the halide. The alkyl halide must be unhindered (usually primary), or elimination will compete with the desired substitution.
Bimolecular dehydration
In the presence of acid, two molecules of an alcohol may lose water to form an ether. In practice, however, this bimolecular dehydration to form an ether competes with unimolecular dehydration to give an alkene. Bimolecular dehydration produces useful yields of ethers only with simple, primary alkyl groups such as those in dimethyl ether and diethyl ether. Dehydration is used commercially to produce diethyl ether.
Reactions of ethers
Cleavage
Ethers are good solvents partly because they are not very reactive. Most ethers can be cleaved, however, by hydrobromic acid (HBr) to give alkyl bromides or by hydroiodic acid (HI) to give alkyl iodides.
Autoxidation
Autoxidation is the spontaneous oxidation of a compound in air. In the presence of oxygen, ethers slowly autoxidize to form hydroperoxides and dialkyl peroxides. If concentrated or heated, these peroxides may explode. To prevent such explosions, ethers should be obtained in small quantities, kept in tightly sealed containers, and used promptly.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1304 2022-03-03 13:26:53
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,620
Re: Miscellany
1278) Cytoplasm
Summary
Cytoplasm is the semifluid substance of a cell that is external to the nuclear membrane and internal to the cellular membrane, sometimes described as the nonnuclear content of protoplasm. In eukaryotes (i.e., cells having a nucleus), the cytoplasm contains all of the organelles. Among such organelles are the mitochondria, which are the sites of energy production through ATP (adenosine triphosphate) synthesis; the endoplasmic reticulum, the site of lipid and protein synthesis; the Golgi apparatus, the site where proteins are modified, packaged, and sorted in preparation for transport to their cellular destinations; lysosomes and peroxisomes, sacs of digestive enzymes that carry out the intracellular digestion of macromolecules such as lipids and proteins; the cytoskeleton, a network of protein fibres that give shape and support to the cell; and cytosol, the fluid mass that surrounds the various organelles.
Details
In cell biology, the cytoplasm is all of the material within a eukaryotic cell, enclosed by the cell membrane, except for the cell nucleus. The material inside the nucleus and contained within the nuclear membrane is termed the nucleoplasm. The main components of the cytoplasm are cytosol (a gel-like substance), the organelles (the cell's internal sub-structures), and various cytoplasmic inclusions. The cytoplasm is about 80% water and is usually colorless.
The submicroscopic ground cell substance, or cytoplasmic matrix which remains after exclusion of the cell organelles and particles is groundplasm. It is the hyaloplasm of light microscopy, a highly complex, polyphasic system in which all of resolvable cytoplasmic elements of are suspended, including the larger organelles such as the ribosomes, mitochondria, the plant plastids, lipid droplets, and vacuoles.
Most cellular activities take place within the cytoplasm, such as many metabolic pathways including glycolysis, and processes such as cell division. The concentrated inner area is called the endoplasm and the outer layer is called the cell cortex or the ectoplasm.
Movement of calcium ions in and out of the cytoplasm is a signaling activity for metabolic processes.
In plants, movement of the cytoplasm around vacuoles is known as cytoplasmic streaming.
History
The term was introduced by Rudolf von Kölliker in 1863, originally as a synonym for protoplasm, but later it has come to mean the cell substance and organelles outside the nucleus.
There has been certain disagreement on the definition of cytoplasm, as some authors prefer to exclude from it some organelles, especially the vacuoles and sometimes the plastids.
Physical nature
It remains uncertain how the various components of the cytoplasm interact to allow movement of organelles while maintaining the cell's structure. The flow of cytoplasmic components plays an important role in many cellular functions which are dependent on the permeability of the cytoplasm. An example of such function is cell signalling, a process which is dependent on the manner in which signaling molecules are allowed to diffuse across the cell. While small signaling molecules like calcium ions are able to diffuse with ease, larger molecules and subcellular structures often require aid in moving through the cytoplasm. The irregular dynamics of such particles have given rise to various theories on the nature of the cytoplasm.
As a sol-gel
There has long been evidence that the cytoplasm behaves like a sol-gel. It is thought that the component molecules and structures of the cytoplasm behave at times like a disordered colloidal solution (sol) and at other times like an integrated network, forming a solid mass (gel). This theory thus proposes that the cytoplasm exists in distinct fluid and solid phases depending on the level of interaction between cytoplasmic components, which may explain the differential dynamics of different particles observed moving through the cytoplasm. A papers suggested that at length scale smaller than 100 nm, the cytoplasm acts like a liquid, while in a larger length scale, it acts like a gel.
As a glass
Recently it has been proposed that the cytoplasm behaves like a glass-forming liquid approaching the glass transition. In this theory, the greater the concentration of cytoplasmic components, the less the cytoplasm behaves like a liquid and the more it behaves as a solid glass, freezing larger cytoplasmic components in place (it is thought that the cell's metabolic activity is able to fluidize the cytoplasm to allow the movement of such larger cytoplasmic components). A cell's ability to vitrify in the absence of metabolic activity, as in dormant periods, may be beneficial as a defence strategy. A solid glass cytoplasm would freeze subcellular structures in place, preventing damage, while allowing the transmission of very small proteins and metabolites, helping to kickstart growth upon the cell's revival from dormancy.
Other perspectives
There has been research examining the motion of cytoplasmic particles independent of the nature of the cytoplasm. In such an alternative approach, the aggregate random forces within the cell caused by motor proteins explain the non-Brownian motion of cytoplasmic constituents.
Constituents
The three major elements of the cytoplasm are the cytosol, organelles and inclusions.
Cytosol
The cytosol is the portion of the cytoplasm not contained within membrane-bound organelles. Cytosol makes up about 70% of the cell volume and is a complex mixture of cytoskeleton filaments, dissolved molecules, and water. The cytosol's filaments include the protein filaments such as actin filaments and microtubules that make up the cytoskeleton, as well as soluble proteins and small structures such as ribosomes, proteasomes, and the mysterious vault complexes. The inner, granular and more fluid portion of the cytoplasm is referred to as endoplasm.
Due to this network of fibres and high concentrations of dissolved macromolecules, such as proteins, an effect called macromolecular crowding occurs and the cytosol does not act as an ideal solution. This crowding effect alters how the components of the cytosol interact with each other.
Organelles
Organelles (literally "little organs"), are usually membrane-bound structures inside the cell that have specific functions. Some major organelles that are suspended in the cytosol are the mitochondria, the endoplasmic reticulum, the Golgi apparatus, vacuoles, lysosomes, and in plant cells, chloroplasts.
Cytoplasmic inclusions
The inclusions are small particles of insoluble substances suspended in the cytosol. A huge range of inclusions exist in different cell types, and range from crystals of calcium oxalate or silicon dioxide in plants, to granules of energy-storage materials such as starch, glycogen, or polyhydroxybutyrate. A particularly widespread example are lipid droplets, which are spherical droplets composed of lipids and proteins that are used in both prokaryotes and eukaryotes as a way of storing lipids such as fatty acids and sterols. Lipid droplets make up much of the volume of adipocytes, which are specialized lipid-storage cells, but they are also found in a range of other cell types.
Controversy and research
The cytoplasm, mitochondria and most organelles are contributions to the cell from the maternal gamete. Contrary to the older information that disregards any notion of the cytoplasm being active, new research has shown it to be in control of movement and flow of nutrients in and out of the cell by viscoplastic behavior and a measure of the reciprocal rate of bond breakage within the cytoplasmic network.
The material properties of the cytoplasm remain an ongoing investigation. A method of determining the mechanical behaviour of living cell mammalian cytoplasm with the aid of optical tweezers has been described.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1305 2022-03-04 13:09:54
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,620
Re: Miscellany
1279) Chrosome
Summary
A chromosome is a long DNA molecule with part or all of the genetic material of an organism. Most eukaryotic chromosomes include packaging proteins called histones which, aided by chaperone proteins, bind to and condense the DNA molecule to maintain its integrity. These chromosomes display a complex three-dimensional structure, which plays a significant role in transcriptional regulation.
Chromosomes are normally visible under a light microscope only during the metaphase of cell division (where all chromosomes are aligned in the center of the cell in their condensed form). Before this happens, each chromosome is duplicated (S phase), and both copies are joined by a centromere, resulting either in an X-shaped structure, if the centromere is located equatorially, or a two-arm structure, if the centromere is located distally. The joined copies are now called sister chromatids. During metaphase the X-shaped structure is called a metaphase chromosome, which is highly condensed and thus easiest to distinguish and study. In animal cells, chromosomes reach their highest compaction level in anaphase during chromosome segregation.
Chromosomal recombination during meiosis and subsequent sexual reproduction play a significant role in genetic diversity. If these structures are manipulated incorrectly, through processes known as chromosomal instability and translocation, the cell may undergo mitotic catastrophe. Usually, this will make the cell initiate apoptosis leading to its own death, but sometimes mutations in the cell hamper this process and thus cause progression of cancer.
Some use the term chromosome in a wider sense, to refer to the individualized portions of chromatin in cells, either visible or not under light microscopy. Others use the concept in a narrower sense, to refer to the individualized portions of chromatin during cell division, visible under light microscopy due to high condensation.
Details
Chromosome is the microscopic threadlike part of the cell that carries hereditary information in the form of genes. A defining feature of any chromosome is its compactness. For instance, the 46 chromosomes found in human cells have a combined length of 200 nm (1 nm = {10}^{- 9} metre); if the chromosomes were to be unraveled, the genetic material they contain would measure roughly 2 metres (about 6.5 feet) in length. The compactness of chromosomes plays an important role in helping to organize genetic material during cell division and enabling it to fit inside structures such as the nucleus of a cell, the average diameter of which is about 5 to 10 μm (1 μm = 0.00l mm, or 0.000039 inch), or the polygonal head of a virus particle, which may be in the range of just 20 to 30 nm in diameter.
The structure and location of chromosomes are among the chief differences between viruses, prokaryotes, and eukaryotes. The nonliving viruses have chromosomes consisting of either DNA (deoxyribonucleic acid) or RNA (ribonucleic acid); this material is very tightly packed into the viral head. Among organisms with prokaryotic cells (i.e., bacteria and blue-green algae), chromosomes consist entirely of DNA. The single chromosome of a prokaryotic cell is not enclosed within a nuclear membrane. Among eukaryotes, the chromosomes are contained in a membrane-bound cell nucleus. The chromosomes of a eukaryotic cell consist primarily of DNA attached to a protein core. They also contain RNA. The remainder of this article pertains to eukaryotic chromosomes.
Every eukaryotic species has a characteristic number of chromosomes (chromosome number). In species that reproduce asexually, the chromosome number is the same in all the cells of the organism. Among sexually reproducing organisms, the number of chromosomes in the body (somatic) cells is diploid (2n; a pair of each chromosome), twice the haploid (1n) number found in the sex cells, or gametes. The haploid number is produced during meiosis. During fertilization, two gametes combine to produce a zygote, a single cell with a diploid set of chromosomes.
Somatic cells reproduce by dividing, a process called mitosis. Between cell divisions the chromosomes exist in an uncoiled state, producing a diffuse mass of genetic material known as chromatin. The uncoiling of chromosomes enables DNA synthesis to begin. During this phase, DNA duplicates itself in preparation for cell division.
Following replication, the DNA condenses into chromosomes. At this point, each chromosome actually consists of a set of duplicate chromatids that are held together by the centromere. The centromere is the point of attachment of the kinetochore, a protein structure that is connected to the spindle fibres (part of a structure that pulls the chromatids to opposite ends of the cell). During the middle stage in cell division, the centromere duplicates, and the chromatid pair separates; each chromatid becomes a separate chromosome at this point. The cell divides, and both of the daughter cells have a complete (diploid) set of chromosomes. The chromosomes uncoil in the new cells, again forming the diffuse network of chromatin.
Among many organisms that have separate sexes, there are two basic types of chromosomes: sex chromosomes and autosomes. Autosomes control the inheritance of all the characteristics except the sex-linked ones, which are controlled by the sex chromosomes. Humans have 22 pairs of autosomes and one pair of sex chromosomes. All act in the same way during cell division.
Chromosome breakage is the physical breakage of subunits of a chromosome. It is usually followed by reunion (frequently at a foreign site, resulting in a chromosome unlike the original). Breakage and reunion of homologous chromosomes during meiosis are the basis for the classical model of crossing over, which results in unexpected types of offspring of a mating.
Chromosomes are structures found in the center (nucleus) of cells that carry long pieces of DNA. DNA is the material that holds genes. It is the building block of the human body.
Chromosomes also contain proteins that help DNA exist in the proper form.
Additional Information
Chromosomes come in pairs. Normally, each cell in the human body has 23 pairs of chromosomes (46 total chromosomes). Half come from the mother; the other half come from the father.
Two of the chromosomes (the X and the Y chromosome) determine your sex as male or female when you are born. They are called sex chromosomes:
* Females have 2 X chromosomes.
* Males have 1 X and 1 Y chromosome.
The mother gives an X chromosome to the child. The father may contribute an X or a Y. The chromosome from the father determines if the baby is born as male or female.
The remaining chromosomes are called autosomal chromosomes. They are known as chromosome pairs 1 through 22.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1306 2022-03-05 13:16:06
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,620
Re: Miscellany
1280) Cooling system
Summary
Cooling system is an apparatus employed to keep the temperature of a structure or device from exceeding limits imposed by needs of safety and efficiency. If overheated, the oil in a mechanical transmission loses its lubricating capacity, while the fluid in a hydraulic coupling or converter leaks under the pressure created. In an electric motor, overheating causes deterioration of the insulation. The pistons in an overheated internal-combustion engine may seize (stick) in the cylinders. Cooling systems are employed in automobiles, industrial plant machinery, nuclear reactors, and many other types of machinery.
The cooling agents customarily employed are air and a liquid (usually water or a solution of water and antifreeze), either alone or in combination. In some cases, direct contact with ambient air (free convection) may be sufficient; in other cases, it may be necessary to employ forced-air convection, created either by a fan or by the natural motion of the hot body. Liquid is typically moved through a continuous loop in the cooling system by a pump.
In a transmission, if the surface area of the housing (container) is sufficiently large compared with the power lost, or if the transmission is in a moving vehicle, there is usually adequate free convection and no need for artificial cooling. To augment the cooling effect by increasing the surface area, the housing may be provided with thin metal fins. On some stationary mechanical transmissions, it may be necessary to circulate the lubricating oil through pipes surrounded by cold water or to use a fan to blow air through pipes surrounded by the oil in the reservoir. On many electric motors, a fan is attached to the rotating element to create a current of cooling air through the housing.
In an automobile, the motion of the vehicle provides sufficient forced-convection cooling for the transmission and the gears in the rear axle; in the engine, however, so much energy is released that, except for some early models and certain small cars with low-powered engines, air cooling is inadequate, and a water cooling system (radiator) is required.
A typical automotive cooling system comprises (1) a series of channels cast into the engine block and cylinder head, surrounding the combustion chambers with circulating liquid to carry away heat; (2) a radiator, consisting of many small tubes equipped with a honeycomb of fins to convect heat rapidly, that receives and cools hot liquid from the engine; (3) a water pump, usually of the centrifugal type, to circulate the liquid through the system; (4) a thermostat to control temperature by varying the amount of liquid going to the radiator; and (5) a fan to draw fresh air through the radiator.
To prevent freezing, an antifreeze solution is either added to or substituted for water. To raise the boiling point of the solution, the cooling system is usually pressurized by means of a pressure cap on the radiator with valves that open outwardly at a prescribed pressure and inwardly to prevent a vacuum as the system cools.
Details
Internal combustion engine cooling uses either air or liquid to remove the waste heat from an internal combustion engine. For small or special purpose engines, cooling using air from the atmosphere makes for a lightweight and relatively simple system. Watercraft can use water directly from the surrounding environment to cool their engines. For water-cooled engines on aircraft and surface vehicles, waste heat is transferred from a closed loop of water pumped through the engine to the surrounding atmosphere by a radiator.
Water has a higher heat capacity than air, and can thus move heat more quickly away from the engine, but a radiator and pumping system add weight, complexity, and cost. Higher-power engines generate more waste heat, but can move more weight, meaning they are generally water-cooled. Radial engines allow air to flow around each cylinder directly, giving them an advantage for air cooling over straight engines, flat engines, and V engines. Rotary engines have a similar configuration, but the cylinders also continually rotate, creating an air flow even when the vehicle is stationary.
Aircraft design more strongly favors lower weight and air-cooled designs. Rotary engines were popular on aircraft until the end of World War I, but had serious stability and efficiency problems. Radial engines were popular until the end of World War II, until gas turbine engines largely replaced them. Modern propeller-driven aircraft with internal-combustion engines are still largely air-cooled. Modern cars generally favor power over weight, and typically have water-cooled engines. Modern motorcycles are lighter than cars, and both cooling methods are common. Some sport motorcycles were cooled with both air and oil (sprayed underneath the piston heads).
Overview
Heat engines generate mechanical power by extracting energy from heat flows, much as a water wheel extracts mechanical power from a flow of mass falling through a distance. Engines are inefficient, so more heat energy enters the engine than comes out as mechanical power; the difference is waste heat which must be removed. Internal combustion engines remove waste heat through cool intake air, hot exhaust gases, and explicit engine cooling.
Engines with higher efficiency have more energy leave as mechanical motion and less as waste heat. Some waste heat is essential: it guides heat through the engine, much as a water wheel works only if there is some exit velocity (energy) in the waste water to carry it away and make room for more water. Thus, all heat engines need cooling to operate.
Cooling is also needed because high temperatures damage engine materials and lubricants and becomes even more important in hot climates. Internal-combustion engines burn fuel hotter than the melting temperature of engine materials, and hot enough to set fire to lubricants. Engine cooling removes energy fast enough to keep temperatures low so the engine can survive.
Some high-efficiency engines run without explicit cooling and with only incidental heat loss, a design called adiabatic. Such engines can achieve high efficiency but compromise power output, duty cycle, engine weight, durability, and emissions.
Basic principles
Most internal combustion engines are fluid cooled using either air (a gaseous fluid) or a liquid coolant run through a heat exchanger (radiator) cooled by air. Marine engines and some stationary engines have ready access to a large volume of water at a suitable temperature. The water may be used directly to cool the engine, but often has sediment, which can clog coolant passages, or chemicals, such as salt, that can chemically damage the engine. Thus, engine coolant may be run through a heat exchanger that is cooled by the body of water.
Most liquid-cooled engines use a mixture of water and chemicals such as antifreeze and rust inhibitors. The industry term for the antifreeze mixture is 'engine coolant'. Some antifreezes use no water at all, instead using a liquid with different properties, such as propylene glycol or a combination of propylene glycol and ethylene glycol. Most air-cooled engines use some liquid oil cooling, to maintain acceptable temperatures for both critical engine parts and the oil itself. Most liquid-cooled engines use some air cooling, with the intake stroke of air cooling the combustion chamber. An exception is math engines, where some parts of the combustion chamber are never cooled by intake, requiring extra effort for successful operation.
There are many demands on a cooling system. One key requirement is to adequately serve the entire engine, as the whole engine fails if just one part overheats. Therefore, it is vital that the cooling system keep all parts at suitably low temperatures. Liquid-cooled engines are able to vary the size of their passageways through the engine block so that coolant flow may be tailored to the needs of each area. Locations with either high peak temperatures (narrow islands around the combustion chamber) or high heat flow (around exhaust ports) may require generous cooling. This reduces the occurrence of hot spots, which are more difficult to avoid with air cooling. Air-cooled engines may also vary their cooling capacity by using more closely spaced cooling fins in that area, but this can make their manufacture difficult and expensive.
Only the fixed parts of the engine, such as the block and head, are cooled directly by the main coolant system. Moving parts such as the pistons, and to a lesser extent the crankshaft and connecting rods, must rely on the lubrication oil as a coolant, or to a very limited amount of conduction into the block and thence the main coolant. High performance engines frequently have additional oil, beyond the amount needed for lubrication, sprayed upwards onto the bottom of the piston just for extra cooling. Air-cooled motorcycles often rely heavily on oil-cooling in addition to air-cooling of the cylinder barrels.
Liquid-cooled engines usually have a circulation pump. The first engines relied on thermo-syphon cooling alone, where hot coolant left the top of the engine block and passed to the radiator, where it was cooled before returning to the bottom of the engine. Circulation was powered by convection alone.
Other demands include cost, weight, reliability, and durability of the cooling system itself.
Conductive heat transfer is proportional to the temperature difference between materials. If engine metal is at 250 °C and the air is at 20 °C, then there is a 230 °C temperature difference for cooling. An air-cooled engine uses all of this difference. In contrast, a liquid-cooled engine might dump heat from the engine to a liquid, heating the liquid to 135 °C (Water's standard boiling point of 100 °C can be exceeded as the cooling system is both pressurised, and uses a mixture with antifreeze) which is then cooled with 20 °C air. In each step, the liquid-cooled engine has half the temperature difference and so at first appears to need twice the cooling area.
However, properties of the coolant (water, oil, or air) also affect cooling. As example, comparing water and oil as coolants, one gram of oil can absorb about 55% of the heat for the same rise in temperature (called the specific heat capacity). Oil has about 90% the density of water, so a given volume of oil can absorb only about 50% of the energy of the same volume of water. The thermal conductivity of water is about four times that of oil, which can aid heat transfer. The viscosity of oil can be ten times greater than water, increasing the energy required to pump oil for cooling, and reducing the net power output of the engine.
Comparing air and water, air has vastly lower heat capacity per gram and per volume (4000) and less than a tenth the conductivity, but also much lower viscosity (about 200 times lower: 17.4 × {10}^{-6} Pa·s for air vs 8.94 × 10−4 Pa·s for water). Continuing the calculation from two paragraphs above, air cooling needs ten times of the surface area, therefore the fins, and air needs 2000 times the flow velocity and thus a recirculating air fan needs ten times the power of a recirculating water pump.
Moving heat from the cylinder to a large surface area for air cooling can present problems such as difficulties manufacturing the shapes needed for good heat transfer and the space needed for free flow of a large volume of air. Water boils at about the same temperature desired for engine cooling. This has the advantage that it absorbs a great deal of energy with very little rise in temperature (called heat of vaporization), which is good for keeping things cool, especially for passing one stream of coolant over several hot objects and achieving uniform temperature. In contrast, passing air over several hot objects in series warms the air at each step, so the first may be over-cooled and the last under-cooled. However, once water boils, it is an insulator, leading to a sudden loss of cooling where steam bubbles form. Steam may return to water as it mixes with other coolant, so an engine temperature gauge can indicate an acceptable temperature even though local temperatures are high enough that damage is being done.
An engine needs different temperatures. The inlet including the compressor of a turbo and in the inlet trumpets and the inlet valves need to be as cold as possible. A countercurrent heat exchanger with forced cooling air does the job. The cylinder-walls should not heat up the air before compression, but also not cool down the gas at the combustion. A compromise is a wall temperature of 90 °C. The viscosity of the oil is optimized for just this temperature. Any cooling of the exhaust and the turbine of the turbocharger reduces the amount of power available to the turbine, so the exhaust system is often insulated between engine and turbocharger to keep the exhaust gases as hot as possible.
The temperature of the cooling air may range from well below freezing to 50 °C. Further, while engines in long-haul boat or rail service may operate at a steady load, road vehicles often see widely varying and quickly varying load. Thus, the cooling system is designed to vary cooling so the engine is neither too hot nor too cold. Cooling system regulation includes adjustable baffles in the air flow (sometimes called 'shutters' and commonly run by a pneumatic 'shutterstat'); a fan which operates either independently of the engine, such as an electric fan, or which has an adjustable clutch; a thermostatic valve or a thermostat that can block the coolant flow when too cool. In addition, the motor, coolant, and heat exchanger have some heat capacity which smooths out temperature increase in short sprints. Some engine controls shut down an engine or limit it to half throttle if it overheats. Modern electronic engine controls adjust cooling based on throttle to anticipate a temperature rise, and limit engine power output to compensate for finite cooling.
Finally, other concerns may dominate cooling system design. As example, air is a relatively poor coolant, but air cooling systems are simple, and failure rates typically rise as the square of the number of failure points. Also, cooling capacity is reduced only slightly by small air coolant leaks. Where reliability is of utmost importance, as in aircraft, it may be a good trade-off to give up efficiency, longevity (interval between engine rebuilds), and quietness in order to achieve slightly higher reliability; the consequences of a broken airplane engine are so severe, even a slight increase in reliability is worth giving up other good properties to achieve it.
Air-cooled and liquid-cooled engines are both used commonly. Each principle has advantages and disadvantages, and particular applications may favor one over the other. For example, most cars and trucks use liquid-cooled engines, while many small airplane and low-cost engines are air-cooled.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1307 2022-03-06 13:42:18
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,620
Re: Miscellany
1281) Nuclear reactor coolant
Summary:
Coolant system
The function of a power reactor installation is to extract as much heat of nuclear fission as possible and convert it to useful power, generally electricity. The coolant system plays a pivotal role in performing this function. A coolant fluid enters the core at low temperature and exits at a higher temperature after collecting the fission energy. This higher-temperature fluid is then directed to conventional thermodynamic components where the heat is converted into electric power. In most light-water, heavy-water, and gas-cooled power reactors, the coolant is maintained at high pressure. Sodium and organic coolants operate at atmospheric pressure.
Research reactors have very simple heat-removal systems, as their primary purpose is to perform research and not generate power. In research reactors, coolant is run through the reactor, and the heat that is removed is transferred to ambient air or to water without going through a power cycle. In research reactors of the lowest power, running at only a few kilowatts, this may involve simple heat exchange to tap water or to a pool of water cooled by ambient air. During operation at higher power levels, the heat is usually removed by means of a small natural-draft cooling tower.
Containment system
Reactors are designed with the expectation that they will operate safely without releasing radioactivity to their surroundings. It is, however, recognized that accidents can occur. An approach using multiple fission product barriers has been adopted to deal with such accidents. These barriers are, successively, the fuel cladding, the reactor vessel, and the shielding. As a final barrier, the reactor is housed in a containment structure, often simply referred to as the containment.
Details
A nuclear reactor coolant is a coolant in a nuclear reactor used to remove heat from the nuclear reactor core and transfer it to electrical generators and the environment. Frequently, a chain of two coolant loops are used because the primary coolant loop takes on short-term radioactivity from the reactor.
Water
Almost all currently operating nuclear power plants are light water reactors using ordinary water under high pressure as coolant and neutron moderator. About 1/3 are boiling water reactors where the primary coolant undergoes phase transition to steam inside the reactor. About 2/3 are pressurized water reactors at even higher pressure. Current reactors stay under the critical point at around 374 °C and 218 bar where the distinction between liquid and gas disappears, which limits thermal efficiency, but the proposed supercritical water reactor would operate above this point.
Heavy water reactors use deuterium oxide which has identical properties to ordinary water but much lower neutron capture, allowing more thorough moderation.
Disadvantages:
Tritium leak
As the hydrogen atoms in water coolants are bombarded with neutrons, some absorb a neutron to become deuterium, and then some become radioactive tritium. Water contaminated with tritium sometimes leaks to groundwater by accident or by official approval.
Hydrogen explosion during power outage
The fuel rods create high temperatures which boil water then turn water to steam. During a disaster, when a power outage happens and diesel power generators which provide emergency power to the water pump are damaged by a tsunami or an earthquake, if no fresh water is being pumped to cool the fuel rods then the fuel rods continue to heat up. Once the fuel rods reach more than 1200 degrees Celsius, the zirconium tubes that contain the nuclear fuel will interact with the steam and split the hydrogen from the water. That hydrogen can then be released from the reactor core and containment vessel. If that hydrogen accumulates in sufficient quantities-concentrations of 4 percent or more in the air, then that hydrogen can explode, as has apparently occurred at Fukushima Daiichi reactors No. 1, 3, 4 but reactor No. 2 opened its vent to let out radioactive hydrogen gas, decreasing the pressure of the hydrogen, but it contaminated the environment, so reactor No. 2 did not explode.
Borated water
Borated water is used as a coolant during normal operation of pressurized water reactors (PWRs) as well as in Emergency Core Cooling Systems (ECCS) of both PWRs and boiling water reactors (BWRs).
Advantages
Boron, often in the form of boric acid or sodium borate, is combined with water — a cheap and plentiful resource — where it acts as a coolant to remove heat from the reactor core and transfers the heat to a secondary circuit. Part of the secondary circuit is the steam generator that is used to turn turbines and generate electricity. Borated water also provides the additional benefits of acting as a neutron poison due to its large neutron absorption cross-section, where it absorbs excess neutrons to help control the fission rate of the reactor. Thus, the reactivity of the nuclear reactor can be easily adjusted by changing the boron concentration in the coolant. That is, when the boron concentration is increased (boration) by dissolving more boric acid into the coolant, the reactivity of the reactor is decreased. Conversely, when the boron concentration is decreased (dilution) by adding more water, the reactivity of the reactor is increased.
Disadvantages
Approximately 90% of the tritium in PWR coolants is produced by reactions of boron-10 with neutrons. Since tritium itself is a radioactive isotope of hydrogen, the coolant becomes contaminated with radioactive isotopes and must be kept from leaking into the environment. Additionally, this effect must be taken into account for longer cycles of nuclear reactor operation and thus requires higher initial concentration of boron in the coolant.
Molten metal
Fast reactors have a high power density and do not need, and must avoid, neutron moderation. Most have been liquid metal cooled reactors using molten sodium. Lead, lead-bismuth eutectic, and other metals have also been proposed and occasionally used. Mercury was used in the first fast reactor.
Molten salt
Molten salts share with metals the advantage of low vapor pressure even at high temperatures, and are less chemically reactive than sodium. Salts containing light elements like FLiBe can also provide moderation. In the Molten-Salt Reactor Experiment it even served as a solvent carrying the nuclear fuel.
Gas
Gases have also been used as coolant. Helium is extremely inert both chemically and with respect to nuclear reactions but has a low heat capacity,
Hydrocarbons
Organically moderated and cooled reactors were an early concept studied, using hydrocarbons as coolant. They were not successful.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1308 2022-03-06 21:15:07
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,620
Re: Miscellany
1282) International Women's Day
Summary
International Women’s Day (IWD) is a day (March 8) honouring the achievements of women and promoting women’s rights. A national holiday in numerous countries, it has been sponsored by the United Nations (UN) since 1975.
International Women’s Day (IWD) grew out of efforts in the early 20th century to promote women’s rights, especially suffrage. In its campaign for female enfranchisement, the Socialist Party of America in 1909 held the first National Woman’s Day, which was highlighted by mass meetings across the United States; the day was observed until 1913. Encouraged by German activist Clara Zetkin, the International Socialist Congress agreed in 1910 to create an international version of the U.S. holiday, and on March 19, 1911, the first IWD was held in Austria, Denmark, Germany, and Switzerland. More than one million people attended rallies marking the day. In the ensuing years the IWD was celebrated in additional countries and on varying dates. On March 8 (February 24, Old Style), 1917, women in Petrograd (St. Petersburg), Russia, marked the day by staging a strike to protest food shortages, poor living conditions, and World War I. This strike for “bread and peace” helped give rise to the Russian Revolution of 1917, which led to the abdication of Nicholas II on March 15 (March 2). In 1921 the date of the IWD was officially changed to March 8.
In the following decades, the success of the suffrage movement contributed to a decline in the popularity of the IWD. However, aided by the growth of feminism in the 1960s and UN sponsorship (1975), the IWD experienced a revitalization in the late 20th century. Today, it is an important occasion for promoting women’s issues and rights, especially in developing countries.
Details
International Women's Day (IWD) is a global holiday celebrated annually on March 8 to commemorate the cultural, political, and socioeconomic achievements of women. It is also a focal point in the women's rights movement, bringing attention to issues such as gender equality, reproductive rights, and violence and abuse against women.
Spurred on by the universal female suffrage movement that had begun in New Zealand, IWD originated from labor movements in North America and Europe during the early 20th century. The earliest version was purportedly a "Women's Day" organized by the Socialist Party of America in New York City February 28, 1909. This inspired German delegates at the 1910 International Socialist Women's Conference to propose "a special Women's Day" be organized annually, albeit with no set date; the following year saw the first demonstrations and commemorations of International Women's Day across Europe. After women gained suffrage in Soviet Russia in 1917 (the beginning of the February Revolution), IWD was made a national holiday on March 8; it was subsequently celebrated on that date by the socialist movement and communist countries. The holiday was associated with far-left movements and governments until its adoption by the global feminist movement in the late 1960s. IWD became a mainstream global holiday following its adoption by the United Nations in 1977.
International Women's Day is commemorated in a variety of ways worldwide; it is a public holiday in several countries, and observed socially or locally in others. The UN observes the holiday in connection with a particular issue, campaign, or theme in women's rights. In some parts of the world, IWD still reflects its political origins, being marked by protests and calls for radical change; in other areas, particularly in the West, it is largely sociocultural and centered on a celebration of womanhood.
History:
Origins
The earliest purported Women's Day observance, called "National Woman's Day", was held on February 28, 1909, in New York City, organized by the Socialist Party of America at the suggestion of activist Theresa Malkiel. There have been claims that the day was commemorating a protest by women garment workers in New York on March 8, 1857, but researchers have alleged this to be a myth intended to detach International Women's Day from its socialist origin.
In August 1910, an International Socialist Women's Conference was organized ahead of the general meeting of the Socialist Second International in Copenhagen, Denmark. Inspired in part by the American socialists, German delegates Clara Zetkin, Käte Duncker, Paula Thiede, and others proposed the establishment of an annual "Women's Day", although no date was specified. The 100 delegates, representing 17 countries, agreed with the idea as a strategy to promote equal rights, including women's suffrage.
The following year, on March 19, 1911, the first International Women's Day was marked by over a million people in Austria, Denmark, Germany, and Switzerland. In Austria-Hungary alone, there were 300 demonstrations, with women parading on the Ringstrasse in Vienna, carrying banners honoring the martyrs of the Paris Commune. Across Europe, women demanded the right to vote and to hold public office, and protested against employment gender discrimination.
IWD initially had no set date, though it was generally celebrated in late February or early March. Americans continued to observe "National Women's Day" on the last Sunday in February, while Russia observed International Women's Day for the first time in 1913, on the last Saturday in February (albeit based on the Julian calendar, as in the Gregorian calendar, the date was March 8). In 1914, International Women's Day was held on March 8 for the first time in Germany, possibly because that date was a Sunday. As elsewhere, Germany's observance was dedicated to women's right to vote, which German women did not win until 1918. Concurrently, there was a march in London in support of women's suffrage, during which Sylvia Pankhurst was arrested in front of Charing Cross station on her way to speak in Trafalgar Square.
International Women's Day in the USSR and other communist nations
On March 8, 1917, in Petrograd, February 23, 1917 on the Julian calendar, women textile workers began a demonstration that eventually engulfed the whole city, demanding "Bread and Peace"—an end to World War I, to food shortages, and to czarism. This marked the beginning of the February Revolution, which alongside the October Revolution, made up the second Russian Revolution. Revolutionary leader Leon Trotsky wrote, "23 February (8th March) was International Woman's Day and meetings and actions were foreseen. But we did not imagine that this 'Women's Day' would inaugurate the revolution. Revolutionary actions were foreseen but without a date. But in the morning, despite the orders to the contrary, textile workers left their work in several factories and sent delegates to ask for the support of the strike… which led to mass strike... all went out into the streets." Seven days later, Tsar Nicholas II abdicated, and the provisional Government granted women the right to vote.
In 1917, following the October Revolution, Bolsheviks Alexandra Kollontai and Vladimir Lenin made IWD an official holiday in the Soviet Union. On May 8, 1965, the USSR Presidium of the Supreme Soviet decreed International Women's Day a non-working day in the USSR, "in commemoration of the outstanding merits of Soviet women in communistic construction, in the defense of their Fatherland during the Great Patriotic War, in their heroism and selflessness at the front and in the rear, and also marking the great contribution of women to strengthening friendship between peoples, and the struggle for peace. But still, women's day must be celebrated as are other holidays."
After its official adoption in Soviet Russia, IWD was predominantly celebrated in communist countries and by the communist movement worldwide. Communist leader Dolores Ibárruri led a women's march in Madrid in 1936 on the eve of the Spanish Civil War. Chinese communists observed the holiday beginning in 1922, though it soon gained traction across the political spectrum: In 1927, Guangzhou saw a march of 25,000 women and male supporters, including representatives of the Kuomintang, the YWCA, and labor organizations. After the founding of the People's Republic of China on October 1, 1949, the State Council proclaimed on December 23 that March 8 would be made an official holiday, with women given a half-day off.
Adoption by United Nations
IWD remained predominantly a communist holiday until roughly 1967 when it was taken up by second-wave feminists. The day re-emerged as a day of activism, and is sometimes known in Europe as the "Women's International Day of Struggle". In the 1970s and 1980s, women's groups were joined by leftists and labor organizations in calling for equal pay, equal economic opportunity, equal legal rights, reproductive rights, subsidized child care, and the prevention of violence against women.
The United Nations began celebrating International Women's Day in 1975, which had been proclaimed the International Women's Year. In 1977, the United Nations General Assembly invited member states to proclaim March 8 as an official UN holiday for women's rights and world peace. It has since been commemorated annually by the UN and much of the world, with each year's observance centered on a particular theme or issue within women's rights.
International Women's Day sparked violence in Tehran, Iran on March 4, 2007, when police beat hundreds of men and women who were planning a rally. (A previous rally for the occasion was held in Tehran in 2003.) Police arrested dozens of women and some were released after several days of solitary confinement and interrogation. Shadi Sadr, Mahbubeh Abbasgholizadeh and several more community activists were released on March 19, 2007, ending a fifteen-day hunger strike.
Adoption by corporations
By the twenty-first century, IWD has been criticized as heavily diluted and commercialized, particularly in the West, where it is sponsored by major corporations and used to promote general and vague notions of equality, rather than radical social reforms. The website internationalwomensday.com was established in 2001; it sets out a yearly theme and hashtags, unconnected with the UN project. In 2009, the website was being managed by the British marketing firm Aurora Ventures with corporate sponsorship. The website began to promote hashtags as themes for the day, which became used internationally. The day was commemorated by business breakfasts and social media communications that were deemed by some social critics as reminiscent of Mother's Day greetings.
Yearly commemorations
IWD 2010
On the occasion of 2010 International Women's Day the International Committee of the Red Cross (ICRC) drew attention to the hardships displaced women endure. The displacement of populations is one of the gravest consequences of today's armed conflicts. It affects women in a host of ways.
IWD 2011
Though the celebration in the West was low-key, events took place in more than 100 countries on March 8, 2011, to commemorate the 100th anniversary of International Women's Day. In the United States, President Barack Obama proclaimed March 2011 to be "Women's History Month", calling Americans to mark IWD by reflecting on "the extraordinary accomplishments of women" in shaping the country's history. Secretary of State Hillary Clinton launched the "100 Women Initiative: Empowering Women and Girls through International Exchanges", on the eve of IWD. In the run-up to 2011 International Women's Day, the Red Cross called on States and other entities not to relent in their efforts to prevent math and other forms of sexual violence that harm the lives and dignity of countless women in conflict zones around the world every year.
Australia issued an IWD 100th anniversary commemorative 20-cent coin.
In Egypt, in Tahrir Square, Cairo, hundreds of men came out not to support, but to harass the women who came out to stand up for their rights as the police and military stood by watching, doing nothing to stop the crowds of men.
IWD 2012
Oxfam America invited people to celebrate inspiring women in their lives by sending a free International Women's Day e-Card or honoring a woman whose efforts had made a difference in the fight against hunger and poverty with Oxfam's International Women's Day award.
On the occasion of International Women's Day 2012, the ICRC called for more action to help the mothers and wives of people who have gone missing during armed conflict. The vast majority of people who go missing in connection with conflict are men. As well as the anguish of not knowing what has happened to the missing husband or son, many of these women face economic and practical difficulties. The ICRC underlined the duty of parties to this conflict to search for the missing and provide information to the families.
IWD 2013
The International Committee of the Red Cross (ICRC) drew attention to the plight of women in prison.
The theme for International Women's Day 2013 was "A promise is a promise: time for action to end violence against women."
It was reported the 70% of women worldwide experience some sort of physical and/or sexual violence in their life. Irina Bovoka, UNESCO Director General on International Women's day 2013, stated that in order "to empower women and ensure equality, we must challenge every form of violence every time it occurs." In view of the increase in violence against women and following the brutal attack on Malala Yousafzai in October 2012, the UN focussed their attention on ending violence against women and made this the central theme for International Women's Day 2013. UNESCO acknowledged that violence against young girls was one of the major reasons for girls not attending school and subsequently collaborated with governments around the globe to support women's rights in providing a quality education in a safe environment.
For a more cultural and artistic celebration, UNESCO also held a concert in Paris as a "Tribute to Women in Music: from the romantic to the electronics".
IWD 2014
American singer Beyoncé also posted an International Women's Day video to her YouTube account. Throughout the video, her song "Flawless" plays, which includes a portion of the "We Should All Be Feminists" speech given by author Chimamanda Ngozi Adichie.
IWD 2015
Governments and activists around the world commemorated the 20th anniversary year of the Beijing Declaration and Platform for Action, an historic roadmap that set the agenda for realizing women's rights.
IWD 2016
The President of India, Shri Pranab Mukherjee, said: "On the occasion of International Women's Day, I extend warm greetings and good wishes to the women of India and thank them for their contributions over the years in the building of our nation." The ministry of women and child development announced the setting up of four more one-stop crisis centers on March 8, in addition to the eight already functioning across the country. Ahead of Women's Day, the national carrier Air India operated what it claimed to be the world's longest non-stop flight where the entire flight operations were handled by women, as part of International Women's Day celebrations. The flight, from Delhi to San Francisco, covered a distance of around 14,500 kilometers in around 17 hours.
IWD 2017
In a message in support of International Women's Day, the UN Secretary-General António Guterres commented on how women's rights were being "reduced, restricted and reversed". With men still in leadership positions and a widening economic gender gap, he called for change "by empowering women at all levels, enabling their voices to be heard and giving them control over their own lives and over the future of our world".
IWD 2018
The UN theme for International Women's Day was: "Time is Now: Rural and urban activists transforming women’s lives”.
Global marches and online campaigns such as #MeToo and #TimesUp, which originated in the United States but became popular globally, allowed many women from different parts of the world to confront injustice and speak out on issues such as sexual harassment and assault and the gender pay gap.
IWD 2019
The UN theme for International Women's Day was: 'Think equal, build smart, innovate for change'. The focus of the theme was on innovative ways in which to advance gender equality and the empowerment of women, particularly in the areas of social protection systems, access to public services and sustainable infrastructure.
The federal state of Berlin marked International Women's Day as a public holiday for the first time.
IWD 2020
The UN theme for International Women's Day was: 'I am Generation Equality': Realizing Women's Rights'. Despite the COVID-19 pandemic, street marches occurred in London, Paris, Madrid, Brussels, Moscow and other European cities. The Aurat March in Islamabad was marred by attacks from stone throwers, after a failed attempt to have it banned as un-Islamic. In Bishkek, the capital of Kyrgyzstan, police detained dozens of marchers shortly after masked men reportedly attacked the march.
IWD 2021
The 2021 UN theme for the IWD was "Women in leadership: Achieving an equal future in a COVID-19 world", highlighting the impact that girls and women worldwide had as health care workers, caregivers, innovators and community organizers during the COVID-19 pandemic. The theme that year was: #ChooseToChallenge.
IWD 2022
The 2022 UN theme for International Women's Day is "Gender equality today for a sustainable tomorrow", looking to highlight the contribution of women and girls around the globe, who participate in their communities promoting on climate change adaptation, mitigation, and response, in order to build a more sustainable future for all.
Around the world
IWD is an official holiday in several countries worldwide, including Afghanistan, Angola, Armenia, Azerbaijan, Belarus, Burkina Faso, Cambodia, China (for women only), Cuba, Georgia, Germany (Berlin only), Guinea-Bissau, Eritrea, Kazakhstan, Kyrgyzstan, Laos, Madagascar (for women only), Moldova, Mongolia, Montenegro, Nepal, Russia, Tajikistan, Turkmenistan, Uganda, Ukraine, Uzbekistan, and Zambia.
In some countries, such as Australia, Cameroon, Croatia, Romania, Bosnia and Herzegovina, Bulgaria, Vietnam, and Chile; IWD is not an official public holiday, but is widely observed nonetheless.
Regardless of legal status, in much of the world, it is customary for men to give female colleagues and loved ones flowers and small gifts. In some countries (such as Bulgaria and Romania) it is also observed as an equivalent of Mother's Day, where children also give small presents to their mothers and grandmothers. In the Czechoslovak Socialist Republic, huge Soviet-style celebrations were held annually. After the fall of Communism, the holiday, generally considered to be one of the major symbols of the old regime, fell into obscurity. International Women's Day was re-established as an official "important day" by the Parliament of the Czech Republic in 2004 on the proposal of the Social Democrats and Communists. This has provoked some controversy as a large part of the public as well as the political right see the holiday as a relic of the nation's Communist past.
IWD is widely celebrated in France as Journée internationale des femmes. In Italy, the holiday is observed by men giving yellow mimosas to women. This originated with communist politician Teresa Mattei, who chose the mimosa in 1946 as the symbol of IWD because the predominant symbols of the day, violets and lily-of-the-valley, were too scarce and expensive to be used effectively in Italy.
In the United States, actress and human rights activist Beata Pozniak worked with the Mayor of Los Angeles and the Governor of California to lobby members of the US Congress to propose official recognition of the holiday. In February 1994, by Beata Pozniak suggestion, the H. J. Res. 316 was introduced by Representative Maxine Waters, along with 79 cosponsors, in an attempt to officially recognize March 8 of that year as International Women's Day. The bill was subsequently referred to, and remained in, the House Committee on Post Office and Civil Service. No vote of either house of Congress was achieved on this piece of legislation.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1309 2022-03-07 13:25:03
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,620
Re: Miscellany
1283) Fly
Summary
Fly, (order Diptera), is any of a large number of insects characterized by the use of only one pair of wings for flight and the reduction of the second pair of wings to knobs (called halteres) used for balance. The term fly is commonly used for almost any small flying insect. However, in entomology the name refers specifically to the approximately 125,000 species of dipterans, or “true” flies, which are distributed throughout the world, including the subarctic and high mountains.
Dipterans are known by such common names as gnats, midges, mosquitoes, and leaf miners, in addition to numerous sorts of flies, including the horse fly, housefly, blow fly, and fruit, bee, robber, and crane flies. Many other species of insects are called flies (e.g., dragonflies, caddisflies, and mayflies), but their wing structures serve to distinguish them from true flies. Many species of dipterans are of great importance economically, and some, such as the common housefly and certain mosquitoes, are of importance as disease carriers. See dipteran.
Details
Flies are insects of the order Diptera, the name being derived from the Greek - di- "two", and - pteron "wing". Insects of this order use only a single pair of wings to fly, the hindwings having evolved into advanced mechanosensory organs known as halteres, which act as high-speed sensors of rotational movement and allow dipterans to perform advanced aerobatics. Diptera is a large order containing an estimated 1,000,000 species including horse-flies, crane flies, hoverflies and others, although only about 125,000 species have been described.
Flies have a mobile head, with a pair of large compound eyes, and mouthparts designed for piercing and sucking (mosquitoes, black flies and robber flies), or for lapping and sucking in the other groups. Their wing arrangement gives them great maneuverability in flight, and claws and pads on their feet enable them to cling to smooth surfaces. Flies undergo complete metamorphosis; the eggs are often laid on the larval food-source and the larvae, which lack true limbs, develop in a protected environment, often inside their food source. Other species like Metopia argyrocephala are ovoviviparous, opportunistically depositing hatched or hatching maggots instead of eggs on carrion, dung, decaying material, or open wounds of mammals. The pupa is a tough capsule from which the adult emerges when ready to do so; flies mostly have short lives as adults.
Diptera is one of the major insect orders and of considerable ecological and human importance. Flies are important pollinators, second only to the bees and their Hymenopteran relatives. Flies may have been among the evolutionarily earliest pollinators responsible for early plant pollination. Fruit flies are used as model organisms in research, but less benignly, mosquitoes are vectors for malaria, dengue, West Nile fever, yellow fever, encephalitis, and other infectious diseases; and houseflies, commensal with humans all over the world, spread food-borne illnesses. Flies can be annoyances especially in some parts of the world where they can occur in large numbers, buzzing and settling on the skin or eyes to bite or seek fluids. Larger flies such as tsetse flies and screwworms cause significant economic harm to cattle. Blowfly larvae, known as gentles, and other dipteran larvae, known more generally as maggots, are used as fishing bait and as food for carnivorous animals. They are also used in medicine in debridement to clean wounds.
Diversity
Flies are often abundant and are found in almost all terrestrial habitats in the world apart from Antarctica. They include many familiar insects such as house flies, blow flies, mosquitoes, gnats, black flies, midges and fruit flies. More than 150,000 have been formally described and the actual species diversity is much greater, with the flies from many parts of the world yet to be studied intensively. The suborder Nematocera include generally small, slender insects with long antennae such as mosquitoes, gnats, midges and crane-flies, while the Brachycera includes broader, more robust flies with short antennae. Many nematoceran larvae are aquatic. There are estimated to be a total of about 19,000 species of Diptera in Europe, 22,000 in the Nearctic region, 20,000 in the Afrotropical region, 23,000 in the Oriental region and 19,000 in the Australasian region. While most species have restricted distributions, a few like the housefly (Musca domestica) are cosmopolitan. Gauromydas heros (Asiloidea), with a length of up to 7 cm (2.8 in), is generally considered to be the largest fly in the world, while the smallest is Euryplatea nanaknihali, which at 0.4 mm (0.016 in) is smaller than a grain of salt.
Brachycera are ecologically very diverse, with many being predatory at the larval stage and some being parasitic. Animals parasitised include molluscs, woodlice, millipedes, insects, mammals, and amphibians. Flies are the second largest group of pollinators after the Hymenoptera (bees, wasps and relatives). In wet and colder environments flies are significantly more important as pollinators. Compared to bees, they need less food as they do not need to provision their young. Many flowers that bear low nectar and those that have evolved trap pollination depend on flies. It is thought that some of the earliest pollinators of plants may have been flies.
The greatest diversity of gall forming insects are found among the flies, principally in the family Cecidomyiidae (gall midges). Many flies (most importantly in the family Agromyzidae) lay their eggs in the mesophyll tissue of leaves with larvae feeding between the surfaces forming blisters and mines. Some families are mycophagous or fungus feeding. These include the cave dwelling Mycetophilidae (fungus gnats) whose larvae are the only diptera with bioluminescence. The Sciaridae are also fungus feeders. Some plants are pollinated by fungus feeding flies that visit fungus infected male flowers.
The larvae of Megaselia scalaris (Phoridae) are almost omnivorous and consume such substances as paint and shoe polish. The Exorista mella (Walker) fly are considered generalists and parasitoids of a variety of hosts. The larvae of the shore flies (Ephydridae) and some Chironomidae survive in extreme environments including glaciers (Diamesa sp., Chironomidae), hot springs, geysers, saline pools, sulphur pools, septic tanks and even crude oil (Helaeomyia petrolei). Adult hoverflies (Syrphidae) are well known for their mimicry and the larvae adopt diverse lifestyles including being inquiline scavengers inside the nests of social insects. Some brachycerans are agricultural pests, some bite animals and humans and drag their blood, and some transmit diseases.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1310 2022-03-08 13:29:24
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,620
Re: Miscellany
1284) Fig
Summary
Fig, (Ficus carica) is a plant of the mulberry family (Moraceae) and its edible fruit. The common fig is indigenous to an area extending from Asiatic Turkey to northern India, but natural seedlings grow in most Mediterranean countries; it is cultivated in warm climates. In the Mediterranean region the fig is so widely used, both fresh and dried, that it is called “the poor man’s food.” The fruit contains significant amounts of calcium, potassium, phosphorus, and iron.
The fig was one of the earliest fruit trees to be cultivated, and its cultivation spread in remote ages over all the districts around the Aegean Sea and throughout the Levant. The Greeks are said to have received it from Caria (hence the specific name); Attic figs became celebrated in the East, and special laws were made to regulate their exportation. The fig was one of the principal articles of sustenance among the Greeks; the Spartans especially used it at their public tables. Pliny the Elder enumerated many varieties and described those of home growth as furnishing a large portion of the food of slaves. In Latin myth the fig was held sacred to Bacchus and employed in religious ceremonies; the fig tree that overshadowed the twin founders of Rome in the wolf’s cave was an emblem of the future prosperity of the race.
Physical description
The fig plant is a bush or small tree, from 1 metre (3 feet) to 10 to 12 metres (33 to 39 feet) high, with broad, rough, deciduous leaves that are deeply lobed or sometimes nearly entire. The leaves and stems exude a white latex when broken.
Fig fruits, known as syconia, are borne singly or in pairs above the scars of fallen leaves or in axils of leaves of the present season. Flowers are staminate (male) or pistillate (female) and enclosed within the inflorescence structure. Long-styled female flowers are characteristic of the edible fruits of most garden and orchard fig trees. Another type of tree, known as a caprifig, produces inedible figs that house the fig wasp young. It has short-styled female flowers that are adapted to the egg-laying habits of the fig wasp (Blastophaga) and also contains male flowers near the apex. Pollen from the caprifigs is carried by the fig wasps to pollinate both the edible and inedible figs.
Types and cultivation
In addition to the caprifig, there are three other horticultural types of figs: Smyrna, White San Pedro, and Common. Smyrna-type figs develop only when fertile seeds are present, and these seeds account for the generally excellent quality and nutty flavour of the fruit. Figs of the White San Pedro type combine the characteristics of both the Smyrna and the Common type on one tree. First-crop figs develop without flower pollination, while second-crop figs in axils of leaves require it. Common figs such as the Dottato, Fraga, and Brown Turkey do not require pollination of flowers of either crop, the seeds in the mature fruit usually being hollow. The flowers of such figs were once regarded as incapable of fecundation and were therefore designated as mule flowers, but it has been proved that all common figs can produce fertile seeds if the flowers are pollinated.
The varieties of figs grown in various parts of the world run into the hundreds. Their nomenclature is very much confused, since the same fig is often grown in neighbouring provinces under entirely different names. When a fig is introduced into other countries, a new name is commonly coined. Thus, Lob Injir of Smyrna became Calimyrna in California, and Dottato of Italy became Kadota. The Italian San Piero is known in England as Negro Largo, in France as Aubique Noire, and in California as San Pedro Black, Brown Turkey, or Black Spanish.
Fig trees are propagated from cuttings of dormant wood taken in February in the Northern Hemisphere and planted in nursery rows. These grow in one season to a height of 1 metre (3 feet) and are ready to transplant at the end of the growing season. The trees thrive in a wide range of soil types and in most Mediterranean countries receive water only from the natural rainfall. Some varieties produce only one crop, in summer or fall. Some bear two crops, the first maturing in June or July on wood of the previous growth and the second ripening in summer or fall in the axils of the leaves of the same season. In cool climates such as those of England and central France, most varieties mature only the first crop. Pot culture of figs in greenhouses has long been practiced in England and other countries.
In most districts, figs are gathered when they fall and placed on trays for drying. Turning and manipulating during the drying process improves the texture and quality of the product. In the Old World, figs are grown commercially in Italy, Turkey, Algeria, Greece, Portugal, and Spain.
Details
The fig is the edible fruit of Ficus carica, a species of small tree in the flowering plant family Moraceae. Native to the Mediterranean and western Asia, it has been cultivated since ancient times and is now widely grown throughout the world, both for its fruit and as an ornamental plant. Ficus carica is the type species of the genus Ficus, containing over 800 tropical and subtropical plant species.
A fig plant is a small deciduous tree or large shrub growing up to 7–10 metres (23–33 ft) tall, with smooth white bark. Its large leaves have three to five deep lobes. Its fruit (botanically an infructescence, a type of multiple fruit) is tear-shaped, 3–5 centimetres (1.2–2.0 in) long, with a green skin that may ripen toward purple or brown, and sweet soft reddish flesh containing numerous crunchy seeds. The milky sap of the green parts is an irritant to human skin. In the Northern Hemisphere, fresh figs are in season from late summer to early autumn. They tolerate moderate seasonal frost and can be grown even in hot-summer continental climates.
Figs can be eaten fresh or dried, or processed into jam, rolls, biscuits and other types of desserts. Since ripe fruit does not transport and keep well, most commercial production is in dried and processed forms. Raw figs contain roughly 80% water and 20% carbohydrates, with negligible protein, fat and micronutrient content. They are a moderate source of dietary fiber.
In 2018, world production of raw figs was 1.14 million tonnes, led by Turkey and North African countries (Egypt, Morocco, and Algeria) as the largest producers, collectively accounting for 64% of the total.
Description
Ficus carica is a gynodioecious, deciduous tree or large shrub that grows up to 7–10 metres (23–33 ft) tall, with smooth white bark. Its fragrant leaves are 12–25 centimetres (4.7–9.8 in) long and 10–18 centimetres (3.9–7.1 in) wide, and are deeply lobed (three or five lobes).
The fig fruit develops as a hollow, fleshy structure called the syconium that is lined internally with numerous unisexual flowers. The tiny flowers bloom inside this cup-like structure. Although commonly called a fruit, the syconium is botanically an infructescence, a type of multiple fruit. The small fig flowers and later small single-seeded (true) fruits line its interior surface. A small opening or ostiole, visible on the middle of the fruit, is a narrow passage that allows the specialized fig wasp, Blastophaga psenes, to enter the inflorescence and pollinate the flowers, after which each fertilized ovule (one per flower, in its ovary) develops into a seed. At maturity, these 'seeds' (actually single-seeded fruits) line the inside of each fig.
The edible mature syconium stem develops into a fleshy false fruit bearing the numerous one-seeded fruits, which are technically druplets. The whole fig fruit is 3–5 centimetres (1.2–2.0 in) long, with a green skin that sometimes ripens toward purple or brown. Ficus carica has milky sap, produced by laticifer cells. The sap of the green parts is an irritant to human skin.
Habitat
The common fig tree has been cultivated since ancient times and grows wild in dry and sunny locations with deep and fresh soil, and in rocky locations that are at sea level to 1,700 metres in elevation. It prefers relatively porous and freely draining soil, and can grow in nutritionally poor soil. Unlike other fig species, Ficus carica does not always require pollination by a wasp or from another tree, but can be pollinated by the fig wasp, Blastophaga psenes to produce seeds. Fig wasps are not present to pollinate in colder nations, e. g. the United Kingdom.
The species has become naturalized in scattered locations in Asia and North America.
The plant tolerates seasonal drought, and the Middle Eastern and Mediterranean climates are especially suitable to it. Situated in a favorable habitat, mature specimens can grow to considerable size as large, dense, shade trees. Its aggressive root system precludes its cultivation in many urban locations, yet in nature this characteristic helps the plant to root in the most inhospitable locations. Having a great need of water, it is mostly a phreatophyte that extracts the needed water from sources in or on the ground. Consequently, it frequently grows in locations with standing or running water, e. g. in valleys of rivers and in ravines that collect water. The deeply rooted plant searches for groundwater in aquifers, ravines, or cracks in rocks. With access to this water, the tree cools the hot environments in which it grows, thus producing fresh and pleasant habitat for many animals that shelter in its shade during periods of intense heat.
The mountain or rock fig is a wild variety, tolerant of cold dry climates, of the semi-arid rocky montane regions of Iran, especially in the Kouhestan Mountains of Khorasan.
Ecology
Ficus carica is dispersed by birds and mammals that scatter their seeds in droppings. Fig fruit is an important food source for much of the fauna in some areas, and the tree owes its expansion to those that feed on its fruit. The common fig tree also sprouts from the root and stolon tissues.
Cultivation
The edible fig is one of the first plants that were cultivated by humans. Nine subfossil figs of a parthenocarpic (and therefore self-pollinating) type dating to about 9400–9200 BC were found in the early Neolithic village Gilgal I (in the Jordan Valley, 13 km north of Jericho). The find precedes the domestication of wheat, barley, and legumes, and may thus be the first known instance of agriculture. It is proposed that this sterile but desirable type was planted and cultivated intentionally, one thousand years before the next crops were domesticated (wheat and rye).
Figs were widespread in ancient Greece, and their cultivation was described by both Aristotle and Theophrastus. Aristotle noted that as in animal sexes, figs have individuals of two kinds, one (the cultivated fig) that bears fruit, and one (the wild caprifig) that assists the other to bear fruit. Further, Aristotle recorded that the fruits of the wild fig contain psenes (fig wasps); these begin life as larvae, and the adult psen splits its "skin" (pupa) and flies out of the fig to find and enter a cultivated fig, saving it from dropping. Theophrastus observed that just as date palms have male and female flowers, and that farmers (from the East) help by scattering "dust" from the male onto the female, and as a male fish releases his milt over the female's eggs, so Greek farmers tie wild figs to cultivated trees. They do not say directly that figs reproduce sexually, however.
Figs were also a common food source for the Romans. Cato the Elder, in his c. 160 BC De Agri Cultura, lists several strains of figs grown at the time he wrote his handbook: the Mariscan, African, Herculanean, Saguntine, and the black Tellanian (De agri cultura, ch. 8). The fruits were used, among other things, to fatten geese for the production of a precursor of foie gras. Rome's first emperor, Augustus, was reputed to have been poisoned with figs from his garden smeared with poison by his wife Livia. For this reason, or perhaps because of her horticultural expertise, a variety of fig known as the Liviana was cultivated in Roman gardens.
It was cultivated from Afghanistan to Portugal, also grown in Pithoragarh in the Kumaon hills of India. From the 15th century onwards, it was grown in areas including Northern Europe and the New World. In the 16th century, Cardinal Reginald Pole introduced fig trees to Lambeth Palace in London.
In 1769, Spanish missionaries led by Junipero Serra brought the first figs to California. The Mission variety, which they cultivated, is still popular. The fact that it is parthenocarpic (self-pollinating) made it an ideal cultivar for introduction.
The Kadota cultivar is even older, being mentioned by the Roman naturalist Pliny the Elder in the 1st century A.D. Pliny recorded thirty varieties of figs.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1311 2022-03-09 13:49:12
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,620
Re: Miscellany
1285) Dragonfly
Summary
A dragonfly is a flying insect belonging to the order Odonata, infraorder Anisoptera (from Greek anisos, "unequal" and pteron, "wing", because the hindwing is broader than the forewing). Adult dragonflies are characterized by a pair of large, multifaceted compound eyes, two pairs of strong, transparent wings, sometimes with coloured patches, and an elongated body. Dragonflies can be mistaken for the closely related damselflies, which make up the other odonatan infraorder (Zygoptera) and are similar in body plan though usually lighter in build; however, the wings of most dragonflies are held flat and away from the body, while damselflies hold their wings folded at rest, along or above the abdomen. Dragonflies are agile fliers, while damselflies have a weaker, fluttery flight. Many dragonflies have brilliant iridescent or metallic colours produced by structural colouration, making them conspicuous in flight. An adult dragonfly's compound eyes have nearly 24,000 ommatidia each.
Fossils of very large dragonfly-like insects, sometimes called griffinflies, are found from 325 million years ago (Mya) in Upper Carboniferous rocks; these had wingspans up to about 750 mm (30 in), but were only distant ancestors, not true dragonflies. About 3,000 extant species of true dragonfly are known. Most are tropical, with fewer species in temperate regions. Loss of wetland habitat threatens dragonfly populations around the world.
Dragonflies are predatory insects, both in their aquatic nymphs stage (also known as naiads) and as adults. In some species, the nymphal stage lasts for up to five years, and the adult stage may be as long as ten weeks, but most species have an adult lifespan in the order of five weeks or less, and some survive for only a few days. They are fast, agile fliers capable of highly accurate aerial ambush, sometimes migrating across oceans, and often live near water. They have a uniquely complex mode of reproduction involving indirect insemination, delayed fertilization, and sperm competition. During mating, the male grasps the female at the back of the head, and the female curls her abdomen under her body to pick up sperm from the male's secondary genitalia at the front of his abdomen, forming the "heart" or "wheel" posture.
Dragonflies are represented in human culture on artefacts such as pottery, rock paintings, statues and Art Nouveau jewellery. They are used in traditional medicine in Japan and China, and caught for food in Indonesia. They are symbols of courage, strength, and happiness in Japan, but seen as sinister in European folklore. Their bright colours and agile flight are admired in the poetry of Lord Tennyson and the prose of H. E. Bates.
Details
Dragonfly, (suborder Anisoptera), also called darner, devil’s arrow, or devil’s darning needle, is any of a group of roughly 3,000 species of aerial predatory insects most commonly found near freshwater habitats throughout most of the world. Damselflies (suborder Zygoptera) are sometimes also called dragonflies in that both are odonates (order Odonata).
Distinguishing characteristics and flight behaviour
Dragonfly species (Anisoptera) are characterized by long bodies with two narrow pairs of intricately veined, membranous wings that, while generally transparent, may have coloured markings. Unlike damselflies, the front and rear wing pairs are shaped differently. In addition, dragonflies rest with their wings spread horizontally, rather than held vertically against each other (with the exception of one very small family, Epiophlebiidae). Dragonflies have a more powerful build and are generally much stronger fliers than damselflies. The globe skimmer (or wandering glider, Pantala flavescens), a migratory dragonfly, for example, makes an annual multigenerational journey of some 18,000 km (about 11,200 miles); to complete the migration, individual globe skimmers fly more than 6,000 km (3,730 miles)—one of the farthest known migrations of all insect species. Dragonflies also have huge bulging eyes that occupy most of the head, giving some a field of vision approaching 360 degrees.
The winged adults are diversely coloured in a variety of shades ranging from metallic to pastel. Compared with other insects, they are large, with some having wingspans of up to 16 cm (about 6 inches). Even the smallest species are about 20 mm (0.8 inch) across. As well as being extremely agile fliers, they are also among the fastest insects. Dragonfly wing muscles must be warm to function optimally, and so, if cool, the insect often engages in wing-whirring and basking in the sun to generate heat before taking flight. The dragonfly’s speed and agility contribute to its being one of the most effective aerial predators. Small flying insects are the usual fare, but some dragonflies regularly consume prey that is 60 percent of their own weight.
Life cycle and reproduction
Young dragonflies, called larvae or sometimes nymphs or naiads, are aquatic and are as dedicated predators under water as the adults are in the air. The functionally wingless larvae are usually mottled or dull in colour, matching the sediments or water plants among which they live. They have bulging eyes somewhat similar to the adults, but possess a formidable anatomical structure not present in the adult. Called the “mask,” it is a fusion of the larva’s third pair of mouthparts. Disproportionately large, the mask folds beneath both the head and thorax when it is not in use. At the end of the mask is a set of fanglike pincers used to seize prey such as worms, crustaceans, tadpoles, and small fish. Different species of dragonfly larvae can be described as sprawlers, burrowers, hiders, or claspers. Their shape, metabolism, and respiration differ concordantly with the microhabitat they occupy.
Larvae crawl from eggs laid in or near water. Some species lay their eggs inside plant tissue, others attach their eggs to substrates at or above the water’s surface, and some may drop or wash their eggs from their abdomen onto water. Larvae absorb oxygen from the water using gills inside the rectum. The abdomen draws water in and pumps it out again through the math. Water can be forcibly expelled in this way, resulting in jet propulsion as a means of escape. Solid waste is also expelled in this manner. As the larva grows, it molts, its future wings first becoming apparent about halfway through the larva’s development. These wing sheaths then enlarge rapidly with each successive molt. Eventually, the larva crawls out of the water (often at night) and molts one last time, emerging as an adult and leaving behind a cast skin (exuvia).
Dragonflies, like damselflies, exhibit a mating posture unique to the Odonata. The male and female contort themselves into the “wheel” position before sperm is transferred. Before and after mating, dragonflies often fly in tandem, with the male towing the female in flight using claspers at the tip of his abdomen to grip the back of her head. Pairs of some species may remain in tandem while the female lays her eggs.
Descriptive names
Many dragonfly families have descriptive common names associated with their scientific names. Examples include the hawkers (Aeshnidae), petaltails (Petaluridae), and clubtails (Gomphidae). Numerous other names related to neither taxonomy nor fact have traditionally been applied to dragonflies, such as horse stinger. Dragonflies have also been known as “snake doctors” in the American South, owing to the superstition that they nurse ill snakes back to health. The term devil’s darning needle is derived from a superstition that dragonflies may sew up the eyes, ears, or mouth of a sleeping child, especially one who has misbehaved. In reality, dragonflies present no danger to humans.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1312 2022-03-10 20:35:46
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,620
Re: Miscellany
1286) Mucous membrane
Summary
Mucous membrane is membrane lining body cavities and canals that lead to the outside, chiefly the respiratory, digestive, and urogenital tracts. Mucous membranes line many tracts and structures of the body, including the mouth, nose, eyelids, trachea (windpipe) and lungs, stomach and intestines, and the ureters, urethra, and urinary bladder.
Mucous membranes vary in structure, but they all have a surface layer of epithelial cells over a deeper layer of connective tissue. Usually, the epithelial layer of the membrane consists of either stratified squamous epithelium (multiple layers of epithelial cells, the top layer being flattened) or simple columnar epithelium (a layer of column-shaped epithelial cells, the cells being significantly greater in height than width). These types of epithelium are notably tough—able to endure abrasion and other forms of wear that are associated with exposure to external factors (e.g., food particles). They also typically contain cells specially adapted for absorption and secretion. The term mucous membrane comes from the fact that the major substance secreted from the membranes is mucus; the principal constituent of mucus is a mucopolysaccharide called mucin.
Mucous membranes and the mucus they secrete serve primarily in protection and lubrication. For example, particulate matter and pathogens (disease-causing organisms) become trapped in secreted mucus, preventing their entry into deeper tissues, whether the lungs (in the case of the respiratory tract) or tissues lying immediately beneath the membrane layer. The membranes and mucus also help to keep underlying tissues moist.
Details
A mucous membrane or mucosa is a membrane that lines various cavities in the body and covers the surface of internal organs. It consists of one or more layers of epithelial cells overlying a layer of loose connective tissue. It is mostly of endodermal origin and is continuous with the skin at body openings such as the eyes, ears, inside the nose, inside the mouth, lip, math, the urethral opening and the math. Some mucous membranes secrete mucus, a thick protective fluid. The function of the membrane is to stop pathogens and dirt from entering the body and to prevent bodily tissues from becoming dehydrated.
Structure
The mucosa of organs are composed of one or more layers of epithelial cells that secrete mucus, and an underlying lamina propria of loose connective tissue. The type of cells and type of mucus secreted vary from organ to organ and each can differ along a given tract.
Mucous membranes line the digestive, respiratory and reproductive tracts and are the primary barrier between the external world and the interior of the body; in an adult human the total surface area of the mucosa is about 400 square meters while the surface area of the skin is about 2 square meters. They are at several places contiguous with skin: at the nostrils, the lips of the mouth, the eyelids, the ears, the genital area, and the math. Along with providing a physical barrier, they also contain key parts of the immune system and serve as the interface between the body proper and the microbiome.
Examples
Some examples include:
* Bronchial mucosa and the lining of vocal folds
* Endometrium: the mucosa of the uterus
* Esophageal mucosa
* Gastric mucosa
* Intestinal mucosa
* Nasal mucosa
* Olfactory mucosa
* Oral mucosa
* Penile mucosa
* Vaginal mucosa
* Frenulum of tongue
* Tongue
* Anal canal
* Palpebral conjunctiva
Development
Developmentally, the majority of mucous membranes are of endodermal origin. Exceptions include the palate, cheeks, floor of the mouth, gums, lips and the portion of the anal canal below the pectinate line, which are all ectodermal in origin.
Function
One of its functions is to keep the tissue moist (for example in the respiratory tract, including the mouth and nose). It also plays a role in absorbing and transforming nutrients. Mucous membranes also protect the body from itself; for instance mucosa in the stomach protects it from stomach acid, and mucosa lining the bladder protects the underlying tissue from urine. In the uterus, the mucous membrane is called the endometrium, and it swells each month and is then eliminated during menstruation.
Nutrition
Niacin and vitamin A are essential nutrients that help maintain mucous membranes.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1313 2022-03-11 14:07:58
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,620
Re: Miscellany
1287) Unified field theory
Summary
Unified field theory,, in particle physics, an attempt to describe all fundamental forces and the relationships between elementary particles in terms of a single theoretical framework. In physics, forces can be described by fields that mediate interactions between separate objects. In the mid-19th century James Clerk Maxwell formulated the first field theory in his theory of electromagnetism. Then, in the early part of the 20th century, Albert Einstein developed general relativity, a field theory of gravitation. Later, Einstein and others attempted to construct a unified field theory in which electromagnetism and gravity would emerge as different aspects of a single fundamental field. They failed, and to this day gravity remains beyond attempts at a unified field theory.
At subatomic distances, fields are described by quantum field theories, which apply the ideas of quantum mechanics to the fundamental field. In the 1940s quantum electrodynamics (QED), the quantum field theory of electromagnetism, became fully developed. In QED, charged particles interact as they emit and absorb photons (minute packets of electromagnetic radiation), in effect exchanging the photons in a game of subatomic “catch.” This theory works so well that it has become the prototype for theories of the other forces.
During the 1960s and ’70s particle physicists discovered that matter is composed of two types of basic building block—the fundamental particles known as quarks and leptons. The quarks are always bound together within larger observable particles, such as protons and neutrons. They are bound by the short-range strong force, which overwhelms electromagnetism at subnuclear distances. The leptons, which include the electron, do not “feel” the strong force. However, quarks and leptons both experience a second nuclear force, the weak force. This force, which is responsible for certain types of radioactivity classed together as beta decay, is feeble in comparison with electromagnetism.
At the same time that the picture of quarks and leptons began to crystallize, major advances led to the possibility of developing a unified theory. Theorists began to invoke the concept of local gauge invariance, which postulates symmetries of the basic field equations at each point in space and time (see gauge theory). Both electromagnetism and general relativity already involved such symmetries, but the important step was the discovery that a gauge-invariant quantum field theory of the weak force had to include an additional interaction—namely, the electromagnetic interaction. Sheldon Glashow, Abdus Salam, and Steven Weinberg independently proposed a unified “electroweak” theory of these forces based on the exchange of four particles: the photon for electromagnetic interactions, and two charged W particles and a neutral Z particle for weak interactions.
During the 1970s a similar quantum field theory for the strong force, called quantum chromodynamics (QCD), was developed. In QCD, quarks interact through the exchange of particles called gluons. The aim of researchers now is to discover whether the strong force can be unified with the electroweak force in a grand unified theory (GUT). There is evidence that the strengths of the different forces vary with energy in such a way that they converge at high energies. However, the energies involved are extremely high, more than a million million times as great as the energy scale of electroweak unification, which has already been verified by many experiments.
Grand unified theories describe the interactions of quarks and leptons within the same theoretical structure. This gives rise to the possibility that quarks can decay to leptons and specifically that the proton can decay. Early attempts at a GUT predicted that the proton’s lifetime must be in the region of {10}^{32} years. This prediction has been tested in experiments that monitor large amounts of matter containing on the order of {10}^{32} protons, but there is no evidence that protons decay. If they do in fact decay, they must do so with a lifetime greater than that predicted by the simplest GUTs. There is also evidence to suggest that the strengths of the forces do not converge exactly unless new effects come into play at higher energies. One such effect could be a new symmetry called “supersymmetry.”
A successful GUT will still not include gravity. The problem here is that theorists do not yet know how to formulate a workable quantum field theory of gravity based on the exchange of a hypothesized graviton.
Details
In physics, a unified field theory (UFT) is a type of field theory that allows all that is usually thought of as fundamental forces and elementary particles to be written in terms of a pair of physical and virtual fields. According to the modern discoveries in physics, forces are not transmitted directly between interacting objects but instead are described and interrupted by intermediary entities called fields.
Classically, however, a duality of the fields is combined into a single physical field. For over a century, unified field theory has remained an open line of research and the term was coined by Albert Einstein, who attempted to unify his general theory of relativity with electromagnetism. The "Theory of Everything" and Grand Unified Theory are closely related to unified field theory, but differ by not requiring the basis of nature to be fields, and often by attempting to explain physical constants of nature. Earlier attempts based on classical physics are described in the article on classical unified field theories.
The goal of a unified field theory has led to a great deal of progress for future theoretical physics, and progress continues.
Introduction to the Great Theory:
Forces
All four of the known fundamental forces are mediated by fields, which in the Standard Model of particle physics result from the exchange of gauge bosons. Specifically, the four fundamental interactions to be unified are:
* Strong interaction: the interaction responsible for holding quarks together to form hadrons, and holding neutrons and also protons together to form atomic nuclei. The exchange particle that mediates this force is the gluon.
* Electromagnetic interaction: the familiar interaction that acts on electrically charged particles. The photon is the exchange particle for this force.
* Weak interaction: a short-range interaction responsible for some forms of radioactivity, that acts on electrons, neutrinos, and quarks. It is mediated by the W and Z bosons.
* Gravitational interaction: a long-range attractive interaction that acts on all particles. The postulated exchange particle has been named the graviton.
Modern unified field theory attempts to bring these four forces and matter together into a single framework.
History
Classic theory
The first successful classical unified field theory was developed by James Clerk Maxwell. In 1820, Hans Christian Ørsted discovered that electric currents exerted forces on magnets, while in 1831, Michael Faraday made the observation that time-varying magnetic fields could induce electric currents. Until then, electricity and magnetism had been thought of as unrelated phenomena. In 1864, Maxwell published his famous paper on a dynamical theory of the electromagnetic field. This was the first example of a theory that was able to encompass previously separate field theories (namely electricity and magnetism) to provide a unifying theory of electromagnetism. By 1905, Albert Einstein had used the constancy of the speed of light in Maxwell's theory to unify our notions of space and time into an entity we now call spacetime and in 1915 he expanded this theory of special relativity to a description of gravity, general relativity, using a field to describe the curving geometry of four-dimensional spacetime.
In the years following the creation of the general theory, a large number of physicists and mathematicians enthusiastically participated in the attempt to unify the then-known fundamental interactions. In view of later developments in this domain, of particular interest are the theories of Hermann Weyl of 1919, who introduced the concept of an (electromagnetic) gauge field in a classical field theory and, two years later, that of Theodor Kaluza, who extended General Relativity to five dimensions. Continuing in this latter direction, Oscar Klein proposed in 1926 that the fourth spatial dimension be curled up into a small, unobserved circle. In Kaluza–Klein theory, the gravitational curvature of the extra spatial direction behaves as an additional force similar to electromagnetism. These and other models of electromagnetism and gravity were pursued by Albert Einstein in his attempts at a classical unified field theory. By 1930 Einstein had already considered the Einstein-Maxwell–Dirac System [Dongen]. This system is (heuristically) the super-classical [Varadarajan] limit of (the not mathematically well-defined) quantum electrodynamics. One can extend this system to include the weak and strong nuclear forces to get the Einstein–Yang-Mills–Dirac System. The French physicist Marie-Antoinette Tonnelat published a paper in the early 1940s on the standard commutation relations for the quantized spin-2 field. She continued this work in collaboration with Erwin Schrödinger after World War II. In the 1960s Mendel Sachs proposed a generally covariant field theory that did not require recourse to renormalization or perturbation theory. In 1965, Tonnelat published a book on the state of research on unified field theories.
Modern progress
In 1963, American physicist Sheldon Glashow proposed that the weak nuclear force, electricity, and magnetism could arise from a partially unified electroweak theory. In 1967, Pakistani Abdus Salam and American Steven Weinberg independently revised Glashow's theory by having the masses for the W particle and Z particle arise through spontaneous symmetry breaking with the Higgs mechanism. This unified theory modeled the electroweak interaction as a force mediated by four particles: the photon for the electromagnetic aspect, and a neutral Z particle, and two charged W particles for the weak aspect. As a result of the spontaneous symmetry breaking, the weak force becomes short-range and the W and Z bosons acquire masses of 80.4 and 91.2 GeV/c2, respectively. Their theory was first given experimental support by the discovery of weak neutral currents in 1973. In 1983, the Z and W bosons were first produced at CERN by Carlo Rubbia's team. For their insights, Glashow, Salam, and Weinberg were awarded the Nobel Prize in Physics in 1979. Carlo Rubbia and Simon van der Meer received the Prize in 1984.
After Gerardus 't Hooft showed the Glashow–Weinberg–Salam electroweak interactions to be mathematically consistent, the electroweak theory became a template for further attempts at unifying forces. In 1974, Sheldon Glashow and Howard Georgi proposed unifying the strong and electroweak interactions into the Georgi–Glashow model, the first Grand Unified Theory, which would have observable effects for energies much above 100 GeV.
Since then there have been several proposals for Grand Unified Theories, e.g. the Pati–Salam model, although none is currently universally accepted. A major problem for experimental tests of such theories is the energy scale involved, which is well beyond the reach of current accelerators. Grand Unified Theories make predictions for the relative strengths of the strong, weak, and electromagnetic forces, and in 1991 LEP determined that supersymmetric theories have the correct ratio of couplings for a Georgi–Glashow Grand Unified Theory.
Many Grand Unified Theories (but not Pati–Salam) predict that the proton can decay, and if this were to be seen, details of the decay products could give hints at more aspects of the Grand Unified Theory. It is at present unknown if the proton can decay, although experiments have determined a lower bound of {10}^{35} years for its lifetime.
Current status
Theoretical physicists have not yet formulated a widely accepted, consistent theory that combines general relativity and quantum mechanics to form a theory of everything. Trying to combine the graviton with the strong and electroweak interactions leads to fundamental difficulties and the resulting theory is not renormalizable. The incompatibility of the two theories remains an outstanding problem in the field of physics.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1314 2022-03-12 13:32:16
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,620
Re: Miscellany
1288) Girl Scouts
Summary
Girl Guides and Girl Scouts is a worldwide organizations for girls, dedicated to training them in good citizenship, good conduct, and outdoor activities. Robert (later Lord) Baden-Powell and his sister Agnes Baden-Powell founded the Girl Guides in Great Britain in 1910 in response to the requests of girls who were interested in the Boy Scout movement established by Robert in 1908. In 1912 Juliette Gordon Low founded a Girl Guide organization in the United States, and its name was later changed to Girl Scouts of the United States of America. Other countries subsequently adopted Girl Guiding and Girl Scouting programs, and the World Association of Girl Guides and Girl Scouts was formed in 1928. In the early 21st century there were more than 10 million Girl Guides and Girl Scouts worldwide.
Aims and activities are substantially the same in all countries in which the movement is organized. The girls promise to follow a code of behaviour, undertake community service projects, and try to develop their skills by earning proficiency badges in a wide variety of activities including outdoor recreation, writing, cooking, performing arts, first aid, and finance—the latter often revolving around the annual fund-raising sale of cookies, for which Girl Scouts and Girl Guides are best known to many. Badge and uniform types vary by country and are often determined by age group.
In the United States there are six age groups, which correspond to school grades: Daisy (grades K–1), Brownie (2–3), Junior (4–5), Cadette (6–8), Senior (9–10), and Ambassador (11–12). Adults are also permitted to join the Girl Scouts as mentors, volunteers, or troop leaders.
In Great Britain there are the Rainbows (ages 5–7; 4–7 in Northern Ireland), Brownies (7–10), Guides (10–14), and Senior Section (14–25). Non-volunteer adults may join for an annual subscription fee.
In Australia the formal grouping system has been largely discontinued, with all Girl Guides under age 18 referred to simply as Guides, and adults 18–30 are eligible for membership in the Olave Program. Those over 18 also may become Adult Members or Trefoil Members (the latter if they have served as either a Guide or a leader). Individual troops, however, are free to continue using the age group names or to make up their own.
Details
Girl Guides (known as Girl Scouts in the United States and some other countries) is a movement found worldwide, which was originally and still largely designed for girls and women only. The movement began in 1909 because girls demanded to take part in the then grassroots Boy Scout Movement.
In different places around the world, the movement developed in diverse ways. In some places, girls joined or attempted to join Scouting organizations. In other places, all girl groups were started independently, and as time went on, some of these all girl groups started to open up to boys, while others' started to merge with the boys' organizations. In other cases, mixed groups were formed, which sometimes later disbanded. In the same way, the name Girl Guide or Girl Scout has been used by groups at different times and in different places, with some groups changing from one to another.
The World Association of Girl Guides and Girl Scouts (WAGGGS) was formed in 1928 and has member organisations in 145 countries. There are now more than 10 million members worldwide. WAGGGS celebrated the centenary of the international Girl Guiding and Girl Scouting Movement over three years, from 2010 to 2012.
History
Lieutenant-General Robert Baden-Powell was a British soldier during the Second Anglo-Boer War in South Africa (1899–1902). He was the commander during the Siege of Mafeking, and noted during the siege how young boys made themselves useful by carrying messages for the soldiers. When he came home, he decided to put his Scouting ideas into practice to see if they would work for young boys, and took 21 boys camping on Brownsea Island, near Poole in Dorset. The camp was a success, and subsequently Baden-Powell wrote the book Scouting for Boys. The book covered topics such as tracking, signalling, and cooking, and it outlined a Scout method for an "instruction in good citizenship". Soon boys began to organise themselves into Patrols and Troops and calling themselves "Boy Scouts". Girls bought the book as well and formed themselves into Patrols of Girl Scouts, while some girls and boys formed mixed Patrols.
In those days, for girls to camp and hike was not common, as shown by this excerpt from The Boy Scouts Headquarters Gazette of 1909: "If a girl is not allowed to run, or even hurry, to swim, ride a bike, or raise her arms above her head, how can she become a Scout?" Nevertheless, Girl Scouts were registered at Scout Headquarters. In 1909 there was a Boy Scout rally at Crystal Palace in London. Among the thousands of Boy Scouts at the rally were several hundred Girl Scouts, including a group of girls from Peckham Rye who had no tickets. They asked Baden-Powell to let them join in. Following negative publicity in "The Spectator" magazine Baden-Powell decided that a separate, single-gender organisation would be best. Baden-Powell asked his sister, Agnes Baden-Powell, to form a separate Girl Guides organisation. In 1910 The Girl Guides organisation was formed in the United Kingdom. The first Guide Company to be registered was 1st Pinkneys Green Guides (Miss Baden-Powell's Own), who still exist in Pinkneys Green, Maidenhead, Berkshire. Many, though by no means all, Girl Guide and Girl Scout groups across the globe trace their roots to this point.
Baden-Powell chose the name "Guides" from a regiment in the British Indian Army, the Corps of Guides, which served on the Northwest Frontier and was noted for its skills in tracking and survival. In some countries, the girls preferred to remain or call themselves "Girl Scouts".
Other influential women in the history of the movement were Juliette Gordon Low, founder of the Girl Scouts of the USA, Olga Drahonowska-Małkowska in Poland and Antoinette Butte in France.
Guide International Service
The Guide International Service (G.I.S.) was an organisation set up by the Girl Guides Association in Britain in 1942. Their aim was to send teams of adult Girl Guides to Europe after World War II to aid with relief work. It is described in two books: All Things Uncertain by Phyllis Stewart Brown and Guides Can Do Anything by Nancy Eastick. A total of 198 Guiders and 60 Scouts, drawn from Britain, Australia, Canada, Ireland and Kenya, served in teams. Some went to relieve the Bergen-Belsen displaced persons camp, while others served in Malaya.
Single-gender mission
There has been much discussion about how similar Girl Guiding and Girl Scouting should be to boys' Scouting programmes. While many girls saw what the boys were doing and wanted to do it too, many girls' organisations have sought to avoid simply copying or mimicking the boys. Julie Bentley, appointed chief executive of the United Kingdom Girl Guides in 2012 and head of the Family Planning Association since 2007, described the Girl Guides in an interview with The Times as "the ultimate feminist organisation".
Even when most Scout organisations became mixed-gender, Guiding remained separate in most countries to provide a female-centred programme. For example, the UK Scout Association introduced a mixed-gender provision in 1976 with the Venture Scout programme, for all age-based sections in 1991 (optional), and became fully co-educational in 2007. However Girl Guiding in the UK remains limited to girls.
Transgender girls are admitted to units in some countries. Transgender women are also allowed to become leaders in some countries, including the UK.
Key points
Things that are shared amongst all Guide Units are:
* The Guide Promise – Girls become Guides by making their Promise. Each country has its own Promise, but historically all have the same three parts: duty to God or to your beliefs, duty to your country and keeping the Guide Law. Though there was historically a religious aspect, many countries are moving towards more non-denominational promises.
* The Good Turn – Each Guide tries to do a kind thing for someone else, without payment and without being asked, every day.
* The World Badge – This can be worn on uniform or ordinary clothes. The three leaves of the trefoil stand for the threefold Promise. The vein in the centre is a compass needle, pointing the way and the two stars stand for the Promise and the Law. The colours stand for the golden sun shining over all the children of the world, from a blue sky. This badge is a guiding symbol that can be recognized all over the world.
* The World Flag – This is in the same colours as the World Badge and can be carried or flown by any member of the movement. It is often used as the Unit Flag. The three yellow blocks represent the threefold Promise and the white corner represents the commitment to peace of all WAGGGs' members.
* The Guide Sign – The three fingers stand for the three parts of the Promise. The Guide sign is used when making or renewing the Promise and can be used when meeting other Guides. It may also be used when receiving a badge or at the end of meetings.
* The Motto – "Be Prepared" – This means that Guides are ready to cope with anything that might come their way.
* The left handshake – This is the way members of the Movement greet each other. The left hand is the one nearest the heart, so symbolizing friendship. Additionally, warriors held their shield in the left hand, so putting down your shield means that you are vulnerable, making it a display of both bravery and trust.
* Thinking Day – On February 22 each year, Guides think of their Guide sisters all around the world. The date was chosen at a World Conference because it was the birthday of both the Founder and the World Chief Guide.
* The World Centres – There are five World Centres in different parts of the world: Our Chalet in Switzerland; Pax Lodge in London; Our Cabana in Mexico; Sangam in India; and Kusafiri in Africa.
* The World Chief Guide – Olave, Lady Baden-Powell is the only person ever to have been World Chief Guide. She was the wife of the Founder, Lord Baden-Powell of Gilwell, and lived from 1889 to 1977.
Two central themes have been present from the earliest days of the movement: domestic skills and "a kind of practical feminism which embodies physical fitness, survival skills, camping, citizenship training, and career preparation". These two themes have been emphasized differently at different times and by different groups, but have remained central to Girl Guiding and Girl Scouting.
Uniforms
Individual national or other emblems may be found on the individual country's Scouting article.
The uniform is a specific characteristic of all Scouting movements. Robert Baden-Powell said it "hides all differences of social standing in a country and makes for equality; but, more important still, it covers differences of country and race and creed, and makes all feel that they are members with one another of the one great brotherhood".
In the 1909 The Scheme for Girl Guides, the uniform for the newly emerging movement was given as:
Jersey of company colour. Neckerchief of company colour. Skirt, knickers, stockings, dark blue. Cap – red biretta, or in summer, large straw hat. Haversack, cooking billy, lanyard and knife, walking stick or light staff. Cape, hooked up on the back. Shoulder knot, of the 'Group' colour on the left shoulder. Badges, much the same as the Boy Scouts. Officers wear ordinary country walking-dress, with biretta of dark blue, white shoulder knot, walking stick, and whistle on lanyard.
Guide uniforms vary according to cultures, climates and the activities undertaken. They are often adorned with badges indicating a Guide's achievements and responsibilities. In some places, uniforms are manufactured and distributed by approved companies and the local Guiding organisation. In other places, members make uniforms themselves.
/girlguides-1.jpg)
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1315 2022-03-13 13:54:40
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,620
Re: Miscellany
1289) Meiosis
Summary
Meiosis, also called reduction division, is a division of a germ cell involving two fissions of the nucleus and giving rise to four gametes, or gender cells, each possessing half the number of chromosomes of the original cell.
The process of meiosis is characteristic of organisms that reproduce sexually. Such species have in the nucleus of each cell a diploid (double) set of chromosomes, consisting of two haploid sets (one inherited from each parent). These haploid sets are homologous—i.e., they contain the same kinds of genes, but not necessarily in the same form. In humans, for example, each set of homologous chromosomes contains a gene for blood type, but one set may have the gene for blood type A and the other set the gene for blood type B.
Prior to meiosis, each of the chromosomes in the diploid germ cell has replicated and thus consists of a joined pair of duplicate chromatids. Meiosis begins with the contraction of the chromosomes in the nucleus of the diploid cell. Homologous paternal and maternal chromosomes pair up along the midline of the cell. Each pair of chromosomes—called a tetrad, or a bivalent—consists of four chromatids. At this point, the homologous chromosomes exchange genetic material by the process of crossing over (see linkage group). The homologous pairs then separate, each pair being pulled to opposite ends of the cell, which then pinches in half to form two daughter cells. Each daughter cell of this first meiotic division contains a haploid set of chromosomes. The chromosomes at this point still consist of duplicate chromatids.
In the second meiotic division, each haploid daughter cell divides. There is no further reduction in chromosome number during this division, as it involves the separation of each chromatid pair into two chromosomes, which are pulled to the opposite ends of the daughter cells. Each daughter cell then divides in half, thereby producing a total of four different haploid gametes. When two gametes unite during fertilization, each contributes its haploid set of chromosomes to the new individual, restoring the diploid number.
Details
Meiosis (from Ancient Greek 'lessening', since it is a reductional division) is a special type of cell division of germ cells in sexually-reproducing organisms used to produce the gametes, such as sperm or egg cells. It involves two rounds of division that ultimately result in four cells with only one copy of each chromosome (haploid). Additionally, prior to the division, genetic material from the paternal and maternal copies of each chromosome is crossed over, creating new combinations of code on each chromosome. Later on, during fertilisation, the haploid cells produced by meiosis from a male and female will fuse to create a cell with two copies of each chromosome again, the zygote.
Errors in meiosis resulting in aneuploidy (an abnormal number of chromosomes) are the leading known cause of miscarriage and the most frequent genetic cause of developmental disabilities.
In meiosis, DNA replication is followed by two rounds of cell division to produce four daughter cells, each with half the number of chromosomes as the original parent cell. The two meiotic divisions are known as meiosis I and meiosis II. Before meiosis begins, during S phase of the cell cycle, the DNA of each chromosome is replicated so that it consists of two identical sister chromatids, which remain held together through sister chromatid cohesion. This S-phase can be referred to as "premeiotic S-phase" or "meiotic S-phase". Immediately following DNA replication, meiotic cells enter a prolonged G2-like stage known as meiotic prophase. During this time, homologous chromosomes pair with each other and undergo genetic recombination, a programmed process in which DNA may be cut and then repaired, which allows them to exchange some of their genetic information. A subset of recombination events results in crossovers, which create physical links known as chiasmata (singular: chiasma, for the Greek letter Chi (Χ)) between the homologous chromosomes. In most organisms, these links can help direct each pair of homologous chromosomes to segregate away from each other during Meiosis I, resulting in two haploid cells that have half the number of chromosomes as the parent cell.
During meiosis II, the cohesion between sister chromatids is released and they segregate from one another, as during mitosis. In some cases, all four of the meiotic products form gametes such as sperm, spores or pollen. In female animals, three of the four meiotic products are typically eliminated by extrusion into polar bodies, and only one cell develops to produce an ovum. Because the number of chromosomes is halved during meiosis, gametes can fuse (i.e. fertilization) to form a diploid zygote that contains two copies of each chromosome, one from each parent. Thus, alternating cycles of meiosis and fertilization enable sexual reproduction, with successive generations maintaining the same number of chromosomes. For example, diploid human cells contain 23 pairs of chromosomes including 1 pair of gender chromosomes (46 total), half of maternal origin and half of paternal origin. Meiosis produces haploid gametes (ova or sperm) that contain one set of 23 chromosomes. When two gametes (an egg and a sperm) fuse, the resulting zygote is once again diploid, with the mother and father each contributing 23 chromosomes. This same pattern, but not the same number of chromosomes, occurs in all organisms that utilize meiosis.
Meiosis occurs in all sexually-reproducing single-celled and multicellular organisms (which are all eukaryotes), including animals, plants and fungi. It is an essential process for oogenesis and spermatogenesis.
Meiosis: Meiotic cell division, stages and significance
* Meiosis is a cell division in which four haploid cells are formed from a single diploid cell.
* It usually occurs in reproductive organs or gonads of the organisms.
* Meiosis is also known as reductional cell division because four daughter cells produced contain half the number of chromosomes than that of their parent cell.
Meiosis has two nuclear division phases:
* Meiosis-I (Reductional or Heterotypic division)
* Meiosis-II (Equational or Homotypic division)
* Meiosis-I (heterolytic or Reductional division)
Meiosis-I has four different phases or stages:
* Prophase-I
* Metaphase-I
* Anaphase-I
* Telophase-I
1. Prophase-I
* It occupies the longest duration in Meiosis-I.
* It is divided into five sub-stages or sub-phases.
i. Leptotene
* This phase starts immediately after interphase.
* The size of cell and nucleus increases
* The chromosomes appear long, uncoiled thread-like in structure bearing many bead-like structures called chromomeres.
* The nuclear membrane and nucleolus remain as it is.
ii. Zygotene
* Homologous chromosomes come closer and starts to pair up along their length.
* The pairing of homologous chromosomes is called Synapsis and the paired homologous chromosomes are referred as bivalents.
* The homologous chromosomes are held together by ribonuclear protein between them.
iii. Pachytene
* The chromosome become shorter and thicker.
* Each chromosome of the bivalents splits longitudinally to form two chromatids such that bivalents is composed of four strands and is known as a tetrad.
* The process of crossing over starts (crossing over; a small fragment of chromosome exchange between two non-sister chromatids of bivalent by breakage and rejoining).
* Crossing over is the most important genetic phenomenon of meiosis which causes variation in genetic characters in offspring.
iv. Diplotene
* In this stage crossing over takes place.
* Bivalents (chromatids) repel each other.
* Homologous chromosome (two non-sister chromatids) begins to separates but separation is not complete, they remains attached to a point with a knot like structure called chiasmata (singular – chiasma).
* The number of chiasmata varies. Depending upon the number of chiasmata, chromosome appears different shape.
**1 chiasmata: cross like
** 2 chiasmata: ring like
** Many chiasmata: series of loop
* Nuclear membrane and nucleolus begins to disappear.
v. Diakinesis
* The chiasma moves towards the end of the chromosomes (tetrad) due to contraction of chromosomelastly slips over separating the homologous chromosome. This movement of the chiasmata towards the end of chromosome is called terminalization.
* By the end of diakinesis the nuclear membrane and nuleolus get completely disappeared and the chromosomes are free in the cytoplasm.
* Spindle fibres begin to form
2. Metaphase-I
* The spindle fibres organized between two poles and get attached to the centromere of chromosomes.
* Chromosome moves to equator
* The bivalent chromosomes are arranged in the equatorial plate in such a way that 2 metaphasic plates are formed.
3. Anaphase-I
* Spindle fibres contracts and pulls the whole chromosomes to the polar region.
* The separated chromosome is known as dyads
* No splitting of chromosomes occurs so the centromere of each homologous chromosome does not divide. Thus, the chromosome number of the daughter nuclei is reduced to half.
* Now the separated chromosome moves toward opposite poles.
4. Telophase-I
* Two groups of chromosome formed at each pole and organized into nuclei.
* The nuclear membrane and nucleolus reappears.
* The chromosomes get uncoiled into chromatin thread.
* The spindle fibres disappear totally.
5. Cytokinesis I
* Cytokinesis may or may not follow nuclear division (meiosis-I Cytokinesis occurs by cell plate formation method in plant cell and furrowing method in animal cells.
* Interphase II or Interkinesis
* The two cells or nuclei thus formed pass through a short stage called interphase-II. Sometimes, interphase-II is absent.
* It is the resting phase between meiosis-I and meiosis-II.
* It is either very short or may be absent
* No DNA synthesis occurs.
Meiosis-II ( Homolytic or equational division)
* Meiosis-II is exactly similar to mitosis, so it is also known as meiotic mitosis.
* In this division, two haploid chromosome splits longitudinally and distributed equally to form 4 haploid cells.
* It completes in 4 stages.
Prophase-II
Metaphase-II
Anaphase-II
Telophase-II
1. Prophase-II:
* The dyads chromosome becomes thicker and shorter
* Nuclear membrane and nucleolus disappear
* Spindle fibre starts to form
2. Metaphase-II:
* The dyads chromosomes comes to equatorial plane
* Spindle fibres organize between poles and attaches to centromere of chromosome.
3. Anaphase-II:
* Centromere of each chromosome divides and sister chromatids separates to form two daughter chromosome
* Spindle fibre contracts and pull the daughter chromosome apart towards opposite pole.
4. Telophase-II:
* Chromosome become organize at respective pole into nuclei
* Chromosome elongates to form thin networks of chromatin
* Nuclear membrane and nucleolus reappears
Cytokinesis-II:
* The result of cytokinesis is four haploid daughter cells (gametes or spores).
* Cytokinesis takes place by cell plate formation in plant cell
Successive methods: cytokinesis followed by each nuclear division resulting in 4 haploid cells. Eg. Monocot plants
* Simultaneous methods: cytokinesis occurs only after meiosis-II to form 4 haploid cells. Eg. Dicot plants
* In animal cells, cytokinesis occurs by furrow formation or depression.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1316 2022-03-14 13:49:50
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,620
Re: Miscellany
1290) Mitosis
Summary
In cell biology, mitosis is a part of the cell cycle in which replicated chromosomes are separated into two new nuclei. Cell division gives rise to genetically identical cells in which the total number of chromosomes is maintained. Therefore, mitosis is also known as equational division. In general, mitosis is preceded by S phase of interphase (during which DNA replication occurs) and is often followed by telophase and cytokinesis; which divides the cytoplasm, organelles and cell membrane of one cell into two new cells containing roughly equal shares of these cellular components. The different stages of mitosis altogether define the mitotic (M) phase of an animal cell cycle—the division of the mother cell into two daughter cells genetically identical to each other.
The process of mitosis is divided into stages corresponding to the completion of one set of activities and the start of the next. These stages are preprophase (specific to plant cells), prophase, prometaphase, metaphase, anaphase, and telophase. During mitosis, the chromosomes, which have already duplicated, condense and attach to spindle fibers that pull one copy of each chromosome to opposite sides of the cell. The result is two genetically identical daughter nuclei. The rest of the cell may then continue to divide by cytokinesis to produce two daughter cells. The different phases of mitosis can be visualized in real time, using live cell imaging. Producing three or more daughter cells instead of the normal two is a mitotic error called tripolar mitosis or multipolar mitosis (direct cell triplication / multiplication). Other errors during mitosis can induce apoptosis (programmed cell death) or cause mutations. Certain types of cancer can arise from such mutations.
Mitosis occurs only in eukaryotic cells. Prokaryotic cells, which lack a nucleus, divide by a different process called binary fission. Mitosis varies between organisms. For example, animal cells undergo an "open" mitosis, where the nuclear envelope breaks down before the chromosomes separate, whereas fungi undergo a "closed" mitosis, where chromosomes divide within an intact cell nucleus. Most animal cells undergo a shape change, known as mitotic cell rounding, to adopt a near spherical morphology at the start of mitosis. Most human cells are produced by mitotic cell division. Important exceptions include the gametes – sperm and egg cells – which are produced by meiosis.
Details
Mitosis is a process of cell duplication, or reproduction, during which one cell gives rise to two genetically identical daughter cells. Strictly applied, the term mitosis is used to describe the duplication and distribution of chromosomes, the structures that carry the genetic information.
Prior to the onset of mitosis, the chromosomes have replicated and the proteins that will form the mitotic spindle have been synthesized. Mitosis begins at prophase with the thickening and coiling of the chromosomes. The nucleolus, a rounded structure, shrinks and disappears. The end of prophase is marked by the beginning of the organization of a group of fibres to form a spindle and the disintegration of the nuclear membrane.
The chromosomes, each of which is a double structure consisting of duplicate chromatids, line up along the midline of the cell at metaphase. In anaphase each chromatid pair separates into two identical chromosomes that are pulled to opposite ends of the cell by the spindle fibres. During telophase, the chromosomes begin to decondense, the spindle breaks down, and the nuclear membranes and nucleoli re-form. The cytoplasm of the mother cell divides to form two daughter cells, each containing the same number and kind of chromosomes as the mother cell. The stage, or phase, after the completion of mitosis is called interphase.
Mitosis is absolutely essential to life because it provides new cells for growth and for replacement of worn-out cells. Mitosis may take minutes or hours, depending upon the kind of cells and species of organisms. It is influenced by time of day, temperature, and chemicals.
Mitosis is a process where a single cell divides into two identical daughter cells (cell division).
During mitosis one cell? divides once to form two identical cells.
* The major purpose of mitosis is for growth and to replace worn out cells.
* If not corrected in time, mistakes made during mitosis can result in changes in the DNA? that can potentially lead to genetic disorders?.
Mitosis is divided into five phases:
1. Interphase:
* The DNA in the cell is copied in preparation for cell division, this results in two identical full sets of chromosomes?.
* Outside of the nucleus? are two centrosomes, each containing a pair of centrioles, these structures are critical for the process of cell division.
* During interphase, microtubules extend from these centrosomes.
2. Prophase:
* The chromosomes condense into X-shaped structures that can be easily seen under a microscope.
* Each chromosome is composed of two sister chromatids, containing identical genetic information.
* The chromosomes pair up so that both copies of chromosome 1 are together, both copies of chromosome 2 are together, and so on.
* At the end of prophase the membrane around the nucleus in the cell dissolves away releasing the chromosomes.
* The mitotic spindle, consisting of the microtubules and other proteins, extends across the cell between the centrioles as they move to opposite poles of the cell.
3. Metaphase:
* The chromosomes line up neatly end-to-end along the centre (equator) of the cell.
* The centrioles are now at opposite poles of the cell with the mitotic spindle fibres extending from them.
* The mitotic spindle fibres attach to each of the sister chromatids.
4. Anaphase:
The sister chromatids are then pulled apart by the mitotic spindle which pulls one chromatid to one pole and the other chromatid to the opposite pole.
5. Telophase:
* At each pole of the cell a full set of chromosomes gather together.
* A membrane forms around each set of chromosomes to create two new nuclei.
* The single cell then pinches in the middle to form two separate daughter cells each containing a full set of chromosomes within a nucleus. This process is known as cytokinesis.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1317 2022-03-15 13:48:38
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,620
Re: Miscellany
1291) Succinic acid
Summary
Succinic acid, also called Butanedioic Acid, is a dicarboxylic acid of molecular formula C4H6O4 that is widely distributed in almost all plant and animal tissues and that plays a significant role in intermediary metabolism. It is a colourless crystalline solid, soluble in water, with a melting point of 185–187° C (365–369° F).
Succinic acid was first obtained as a distillation product of amber (Latin: succinum), for which it is named. The common method of synthesis of succinic acid is the catalytic hydrogenation of maleic acid or its anhydride, although other methods are being used and investigated. Succinic acid has uses in certain drug compounds, in agricultural and food production, and in manufacturing.
A water-soluble, colorless crystal with an acid taste that is used as a chemical intermediate, in medicine, the manufacture of lacquers, and to make perfume esters. It is also used in foods as a sequestrant, buffer, and a neutralizing agent.
Succinate is an essential component of the Krebs or citric acid cycle and serves an electron donor in the production of fumaric acid and FADH2. It also has been shown to be a good "natural" antibiotic because of its relative acidic or caustic nature (high concentrations can even cause burns). Succinate supplements have been shown to help reduce the effects of hangovers by activating the degradation of acetaldehyde - a toxic byproduct of alcohol metabolism - into CO2 and H2O through aerobic metabolism. Succinic acid has been shown to stimulate neural system recovery and bolster the immune system. Claims have also been made that it boosts awareness, concentration and reflexes.
Details
Succinic acid is a dicarboxylic acid with the chemical formula (CH2)2(CO2H)2. The name derives from Latin succinum, meaning amber. In living organisms, succinic acid takes the form of an anion, succinate, which has multiple biological roles as a metabolic intermediate being converted into fumarate by the enzyme succinate dehydrogenase in complex 2 of the electron transport chain which is involved in making ATP, and as a signaling molecule reflecting the cellular metabolic state. It is marketed as food additive E363. Succinate is generated in mitochondria via the tricarboxylic acid cycle (TCA). Succinate can exit the mitochondrial matrix and function in the cytoplasm as well as the extracellular space, changing gene expression patterns, modulating epigenetic landscape or demonstrating hormone-like signaling.[6] As such, succinate links cellular metabolism, especially ATP formation, to the regulation of cellular function. Dysregulation of succinate synthesis, and therefore ATP synthesis, happens in some genetic mitochondrial diseases, such as Leigh syndrome, and Melas syndrome, and degradation can lead to pathological conditions, such as malignant transformation, inflammation and tissue injury.
Commercial production
Historically, succinic acid was obtained from amber by distillation and has thus been known as spirit of amber. Common industrial routes include hydrogenation of maleic acid, oxidation of 1,4-butanediol, and carbonylation of ethylene glycol. Succinate is also produced from butane via maleic anhydride. Global production is estimated at 16,000 to 30,000 tons a year, with an annual growth rate of 10%.
Genetically engineered Escherichia coli and Saccharomyces cerevisiae are proposed for the commercial production via fermentation of glucose.
Chemical reactions
Succinic acid can be dehydrogenated to fumaric acid or be converted to diesters, such as diethylsuccinate (CH2CO2CH2CH3)2. This diethyl ester is a substrate in the Stobbe condensation. Dehydration of succinic acid gives succinic anhydride. Succinate can be used to derive 1,4-butanediol, maleic anhydride, succinimide, 2-pyrrolidinone and tetrahydrofuran.
Applications
In 2004, succinate was placed on the US Department of Energy's list of top 12 platform chemicals from biomass.
Precursor to polymers, resins, and solvents
Succinic acid is a precursor to some polyesters and a component of some alkyd resins. 1,4-Butanediol (BDO) can be synthesized using succinic acid as a precursor. The automotive and electronics industries heavily rely on BDO to produce connectors, insulators, wheel covers, gearshift knobs and reinforcing beams. Succinic acid also serves as the bases of certain biodegradable polymers, which are of interest in tissue engineering applications.
Acylation with succinic acid is called succination. Oversuccination occurs when more than one succinate adds to a substrate.
Food and dietary supplement
As a food additive and dietary supplement, succinic acid is generally recognized as safe by the U.S. Food and Drug Administration. Succinic acid is used primarily as an acidity regulator in the food and beverage industry. It is also available as a flavoring agent, contributing a somewhat sour and astringent component to umami taste. As an excipient in pharmaceutical products, it is also used to control acidity or as a counter ion. Drugs involving succinate include metoprolol succinate, sumatriptan succinate, Doxylamine succinate or solifenacin succinate.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1318 2022-03-16 14:11:40
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,620
Re: Miscellany
1292) Photon
Summary
Photon, also called light quantum, is a minute energy packet of electromagnetic radiation. The concept originated (1905) in Albert Einstein’s explanation of the photoelectric effect, in which he proposed the existence of discrete energy packets during the transmission of light. Earlier (1900), the German physicist Max Planck had prepared the way for the concept by explaining that heat radiation is emitted and absorbed in distinct units, or quanta. The concept came into general use after the U.S. physicist Arthur H. Compton demonstrated (1923) the corpuscular nature of X-rays. The term photon (from Greek “light”), however, was not used until 1926. The energy of a photon depends on radiation frequency; there are photons of all energies from high-energy gamma- and X-rays, through visible light, to low-energy infrared and radio waves. All photons travel at the speed of light. Considered among the subatomic particles, photons are bosons, having no electric charge or rest mass and one unit of spin; they are field particles that are thought to be the carriers of the electromagnetic field.
Details
The photon is a type of elementary particle that serves as the quantum of the electromagnetic field, including electromagnetic radiation such as light and radio waves, and the force carrier for the electromagnetic force. Photons are massless, so they always move at the speed of light in vacuum, 299,792,458 m/s (or about 186,282 mi/s). The photon belongs to the class of bosons.
Like all elementary particles, photons are currently best explained by quantum mechanics, and exhibit wave–particle duality, their behavior featuring properties of both waves and particles. The modern photon concept originated during the first two decades of the 20th century with the work of Albert Einstein, who built upon the research of Max Planck. While trying to explain how matter and electromagnetic radiation could be in thermal equilibrium with one another, Planck proposed that the energy stored within a material object should be regarded as composed of an integer number of discrete, equal-sized parts. To explain the photoelectric effect, Einstein introduced the idea that light itself is made of discrete units of energy. In 1926, Gilbert N. Lewis popularized the term photon for these energy units. Subsequently, many other experiments validated Einstein's approach.
In the Standard Model of particle physics, photons and other elementary particles are described as a necessary consequence of physical laws having a certain symmetry at every point in spacetime. The intrinsic properties of particles, such as charge, mass, and spin, are determined by this gauge symmetry. The photon concept has led to momentous advances in experimental and theoretical physics, including lasers, Bose–Einstein condensation, quantum field theory, and the probabilistic interpretation of quantum mechanics. It has been applied to photochemistry, high-resolution microscopy, and measurements of molecular distances. Moreover, photons have been studied as elements of quantum computers, and for applications in optical imaging and optical communication such as quantum cryptography.
Nomenclature
The word quanta (singular quantum, Latin for how much) was used before 1900 to mean particles or amounts of different quantities, including electricity. In 1900, the German physicist Max Planck was studying black-body radiation, and he suggested that the experimental observations, specifically at shorter wavelengths, would be explained if the energy stored within a molecule was a "discrete quantity composed of an integral number of finite equal parts", which he called "energy elements". In 1905, Albert Einstein published a paper in which he proposed that many light-related phenomena—including black-body radiation and the photoelectric effect—would be better explained by modelling electromagnetic waves as consisting of spatially localized, discrete wave-packets. He called such a wave-packet the light quantum.
The name photon derives from the Greek word for light. Arthur Compton used photon in 1928, referring to G.N. Lewis, who coined the term in a letter to Nature on 18 December 1926. The same name was used earlier but was never widely adopted before Lewis: in 1916 by the American physicist and psychologist Leonard T. Troland, in 1921 by the Irish physicist Joly, in 1924 by the French physiologist Wurmser (1890–1993), and in 1926 by the French physicist Wolfers (1891–1971). The name was suggested initially as a unit related to the illumination of the eye and the resulting sensation of light and was used later in a physiological context. Although Wolfers's and Lewis's theories were contradicted by many experiments and never accepted, the new name was adopted by most physicists very soon after Compton used it.
In physics, a photon is usually denoted by the symbol γ (the Greek letter gamma). This symbol for the photon probably derives from gamma rays, which were discovered in 1900 by Paul Villard, named by Ernest Rutherford in 1903, and shown to be a form of electromagnetic radiation in 1914 by Rutherford and Edward Andrade. In chemistry and optical engineering, photons are usually symbolized by hν, which is the photon energy, where h is Planck constant and the Greek letter ν (nu) is the photon's frequency. Much less commonly, the photon can be symbolized by hf, where its frequency is denoted by f.
Physical properties
A photon is massless, has no electric charge, and is a stable particle. In a vacuum, a photon has three possible polarization states. The photon is the gauge boson for electromagnetism, and therefore all other quantum numbers of the photon (such as lepton number, baryon number, and flavour quantum numbers) are zero. Also, the photon obeys Bose–Einstein statistics, not the Pauli exclusion principle.
Photons are emitted in many natural processes. For example, when a charge is accelerated it emits synchrotron radiation. During a molecular, atomic or nuclear transition to a lower energy level, photons of various energy will be emitted, ranging from radio waves to gamma rays. Photons can also be emitted when a particle and its corresponding antiparticle are annihilated (for example, electron–positron annihilation).
Polarization and angular momentum
The photon also carries two other quantities called spin angular momentum (which is related to linear or circular photon polarization) and orbital angular momentum.
Spin angular momentum
The spin angular momentum of light does not depend on its frequency, and was experimentally verified by Raman and Bhagavantam in 1931.
Because photons always move at the speed of light, the spin is best expressed in terms of the component measured along its direction of motion, its helicity, which must be either +ħ, or −ħ. These three possible helicities, called right-handed, linear, and left-handed, correspond to the three possible circular polarization states of the photon.
To illustrate the significance of these formulae, the annihilation of a particle with its antiparticle in free space must result in the creation of at least two photons for the following reason: In the center of momentum frame, the colliding antiparticles have no net momentum, whereas a single photon always has momentum (since, as we have seen, it is determined by the photon's frequency or wavelength, which cannot be zero). Hence, conservation of momentum (or equivalently, translational invariance) requires that at least two photons are created, with zero net momentum. The energy of the two photons, or, equivalently, their frequency, may be determined from conservation of four-momentum.
Seen another way, the photon can be considered as its own antiparticle (thus an "antiphoton" is simply a normal photon with opposite momentum, equal polarization, and 180° out of phase). The reverse process, pair production, is the dominant mechanism by which high-energy photons such as gamma rays lose energy while passing through matter. That process is the reverse of "annihilation to one photon" allowed in the electric field of an atomic nucleus.
The classical formulae for the energy and momentum of electromagnetic radiation can be re-expressed in terms of photon events. For example, the pressure of electromagnetic radiation on an object derives from the transfer of photon momentum per unit time and unit area to that object, since pressure is force per unit area and force is the change in momentum per unit time.
Orbital angular momentum
Each photon carries two distinct and independent forms of angular momentum: spin and orbital angular momentum. As discussed above, the spin angular momentum of light of a particular photon is always either +ħ, 0, or −ħ. In contrast, the light orbital angular momentum of a particular photon can be any integer N, including zero.
Experimental checks on photon mass
Current commonly accepted physical theories imply or assume the photon to be strictly massless. If the photon is not a strictly massless particle, it would not move at the exact speed of light, c, in vacuum. Its speed would be lower and depend on its frequency. Relativity would be unaffected by this; the so-called speed of light, c, would then not be the actual speed at which light moves, but a constant of nature which is the upper bound on speed that any object could theoretically attain in spacetime. Thus, it would still be the speed of spacetime ripples (gravitational waves and gravitons), but it would not be the speed of photons.
Historical development
Thomas Young's double-slit experiment in 1801 showed that light can act as a wave, helping to invalidate early particle theories of light.
In most theories up to the eighteenth century, light was pictured as being made up of particles. Since particle models cannot easily account for the refraction, diffraction and birefringence of light, wave theories of light were proposed by René Descartes (1637), Robert Hooke (1665), and Christiaan Huygens (1678); however, particle models remained dominant, chiefly due to the influence of Isaac Newton. In the early 19th century, Thomas Young and August Fresnel clearly demonstrated the interference and diffraction of light, and by 1850 wave models were generally accepted. James Clerk Maxwell's 1865 prediction that light was an electromagnetic wave – which was confirmed experimentally in 1888 by Heinrich Hertz's detection of radio waves – seemed to be the final blow to particle models of light.
In 1900, Maxwell's theoretical model of light as oscillating electric and magnetic fields seemed complete. However, several observations could not be explained by any wave model of electromagnetic radiation, leading to the idea that light-energy was packaged into quanta described by E = hν . Later experiments showed that these light-quanta also carry momentum and, thus, can be considered particles: The photon concept was born, leading to a deeper understanding of the electric and magnetic fields themselves.
The Maxwell wave theory, however, does not account for all properties of light. The Maxwell theory predicts that the energy of a light wave depends only on its intensity, not on its frequency; nevertheless, several independent types of experiments show that the energy imparted by light to atoms depends only on the light's frequency, not on its intensity. For example, some chemical reactions are provoked only by light of frequency higher than a certain threshold; light of frequency lower than the threshold, no matter how intense, does not initiate the reaction. Similarly, electrons can be ejected from a metal plate by shining light of sufficiently high frequency on it (the photoelectric effect); the energy of the ejected electron is related only to the light's frequency, not to its intensity.
At the same time, investigations of black-body radiation carried out over four decades (1860–1900) by various researchers culminated in Max Planck's hypothesis that the energy of any system that absorbs or emits electromagnetic radiation of frequency ν is an integer multiple of an energy quantum E = hν . As shown by Albert Einstein, some form of energy quantization must be assumed to account for the thermal equilibrium observed between matter and electromagnetic radiation; for this explanation of the photoelectric effect, Einstein received the 1921 Nobel Prize in physics.
Since the Maxwell theory of light allows for all possible energies of electromagnetic radiation, most physicists assumed initially that the energy quantization resulted from some unknown constraint on the matter that absorbs or emits the radiation. In 1905, Einstein was the first to propose that energy quantization was a property of electromagnetic radiation itself. Although he accepted the validity of Maxwell's theory, Einstein pointed out that many anomalous experiments could be explained if the energy of a Maxwellian light wave were localized into point-like quanta that move independently of one another, even if the wave itself is spread continuously over space. In 1909 and 1916, Einstein showed that, if Planck's law regarding black-body radiation is accepted, the energy quanta must also carry momentum p = h/λ , making them full-fledged particles. This photon momentum was observed experimentally by Arthur Compton, for which he received the Nobel Prize in 1927. The pivotal question then, was how to unify Maxwell's wave theory of light with its experimentally observed particle nature? The answer to this question occupied Albert Einstein for the rest of his life, and was solved in quantum electrodynamics and its successor, the Standard Model.
Up to 1923, most physicists were reluctant to accept that light itself was quantized. Instead, they tried to explain photon behaviour by quantizing only matter, as in the Bohr model of the hydrogen atom. Even though these semiclassical models were only a first approximation, they were accurate for simple systems and they led to quantum mechanics.
Einstein's 1905 predictions were verified experimentally in several ways in the first two decades of the 20th century, as recounted in Robert Millikan's Nobel lecture. However, before Compton's experiment showed that photons carried momentum proportional to their wave number (1922), most physicists were reluctant to believe that electromagnetic radiation itself might be particulate. (See, for example, the Nobel lectures of Wien, Planck and Millikan.) Instead, there was a widespread belief that energy quantization resulted from some unknown constraint on the matter that absorbed or emitted radiation. Attitudes changed over time. In part, the change can be traced to experiments such as those revealing Compton scattering, where it was much more difficult not to ascribe quantization to light itself to explain the observed results.
Even after Compton's experiment, Niels Bohr, Hendrik Kramers and John Slater made one last attempt to preserve the Maxwellian continuous electromagnetic field model of light, the so-called BKS theory. An important feature of the BKS theory is how it treated the conservation of energy and the conservation of momentum. In the BKS theory, energy and momentum are only conserved on the average across many interactions between matter and radiation. However, refined Compton experiments showed that the conservation laws hold for individual interactions. Accordingly, Bohr and his co-workers gave their model "as honorable a funeral as possible".[56] Nevertheless, the failures of the BKS model inspired Werner Heisenberg in his development of matrix mechanics.
A few physicists persisted in developing semiclassical models in which electromagnetic radiation is not quantized, but matter appears to obey the laws of quantum mechanics. Although the evidence from chemical and physical experiments for the existence of photons was overwhelming by the 1970s, this evidence could not be considered as absolutely definitive; since it relied on the interaction of light with matter, and a sufficiently complete theory of matter could in principle account for the evidence. Nevertheless, all semiclassical theories were refuted definitively in the 1970s and 1980s by photon-correlation experiments. Hence, Einstein's hypothesis that quantization is a property of light itself is considered to be proven.
In matter
Light that travels through transparent matter does so at a lower speed than c, the speed of light in a vacuum. The factor by which the speed is decreased is called the refractive index of the material. In a classical wave picture, the slowing can be explained by the light inducing electric polarization in the matter, the polarized matter radiating new light, and that new light interfering with the original light wave to form a delayed wave. In a particle picture, the slowing can instead be described as a blending of the photon with quantum excitations of the matter to produce quasi-particles known as polariton (see this list for some other quasi-particles); this polariton has a nonzero effective mass, which means that it cannot travel at c. Light of different frequencies may travel through matter at different speeds; this is called dispersion (not to be confused with scattering). In some cases, it can result in extremely slow speeds of light in matter. The effects of photon interactions with other quasi-particles may be observed directly in Raman scattering and Brillouin scattering.
Photons can be scattered by matter. For example, photons engage in so many collisions on the way from the core of the Sun that radiant energy can take about a million years to reach the surface; however, once in open space, a photon takes only 8.3 minutes to reach Earth.
Photons can also be absorbed by nuclei, atoms or molecules, provoking transitions between their energy levels. A classic example is the molecular transition of retinal (C20H28O), which is responsible for vision, as discovered in 1958 by Nobel laureate biochemist George Wald and co-workers. The absorption provokes a cis–trans isomerization that, in combination with other such transitions, is transduced into nerve impulses. The absorption of photons can even break chemical bonds, as in the photodissociation of chlorine; this is the subject of photochemistry.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1319 2022-03-17 14:36:18
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,620
Re: Miscellany
1293) Mirror
Summary
A mirror is an object that reflects an image. Light that bounces off a mirror will show an image of whatever is in front of it, when focused through the lens of the eye or a camera. Mirrors reverse the direction of the image in an equal yet opposite angle from which the light shines upon it. This allows the viewer to see themselves or objects behind them, or even objects that are at an angle from them but out of their field of view, such as around a corner. Natural mirrors have existed since prehistoric times, such as the surface of water, but people have been manufacturing mirrors out of a variety of materials for thousands of years, like stone, metals, and glass. In modern mirrors, metals like silver or aluminum are often used due to their high reflectivity, applied as a thin coating on glass because of its naturally smooth and very hard surface.
A mirror is a wave reflector. Light consists of waves, and when light waves reflect off the flat surface of a mirror, those waves retain the same degree of curvature and vergence, in an equal yet opposite direction, as the original waves. This allows the waves to form an image when they are focused through a lens, just as if the waves had originated from the direction of the mirror. The light can also be pictured as rays (imaginary lines radiating from the light source, that are always perpendicular to the waves). These rays are reflected at an equal yet opposite angle from which they strike the mirror (incident light). This property, called specular reflection, distinguishes a mirror from objects that diffuse light, breaking up the wave and scattering it in many directions (such as flat-white paint). Thus, a mirror can be any surface in which the texture or roughness of the surface is smaller (smoother) than the wavelength of the waves.
When looking at a mirror, one will see a mirror image or reflected image of objects in the environment, formed by light emitted or scattered by them and reflected by the mirror towards one's eyes. This effect gives the illusion that those objects are behind the mirror, or (sometimes) in front of it. When the surface is not flat, a mirror may behave like a reflecting lens. A plane mirror will yield a real-looking undistorted image, while a curved mirror may distort, magnify, or reduce the image in various ways, while keeping the lines, contrast, sharpness, colors, and other image properties intact.
A mirror is commonly used for inspecting oneself, such as during personal grooming; hence the old-fashioned name looking glass. This use, which dates from prehistory, overlaps with uses in decoration and architecture. Mirrors are also used to view other items that are not directly visible because of obstructions; examples include rear-view mirrors in vehicles, security mirrors in or around buildings, and dentist's mirrors. Mirrors are also used in optical and scientific apparatus such as telescopes, lasers, cameras, periscopes, and industrial machinery.
The terms "mirror" and "reflector" can be used for objects that reflect any other types of waves. An acoustic mirror reflects sound waves. Objects such as walls, ceilings, or natural rock-formations may produce echos, and this tendency often becomes a problem in acoustical engineering when designing houses, auditoriums, or recording studios. Acoustic mirrors may be used for applications such as parabolic microphones, atmospheric studies, sonar, and seafloor mapping. An atomic mirror reflects matter waves, and can be used for atomic interferometry and atomic holography.
Details
Mirror is any polished surface that diverts a ray of light according to the law of reflection.
The typical mirror is a sheet of glass that is coated on its back with aluminum or silver that produces images by reflection. The mirrors used in Greco-Roman antiquity and throughout the European Middle Ages were simply slightly convex disks of metal, either bronze, tin, or silver, that reflected light off their highly polished surfaces. A method of backing a plate of flat glass with a thin sheet of reflecting metal came into widespread production in Venice during the 16th century; an amalgam of tin and mercury was the metal used. The chemical process of coating a glass surface with metallic silver was discovered by Justus von Liebig in 1835, and this advance inaugurated the modern techniques of mirror making. Present-day mirrors are made by sputtering a thin layer of molten aluminum or silver onto the back of a plate of glass in a vacuum. In mirrors used in telescopes and other optical instruments, the aluminum is evaporated onto the front surface of the glass rather than on the back, in order to eliminate faint reflections from the glass itself.
When light falls on a body some of the light may be reflected, some absorbed, and some transmitted through the body. In order for a smooth surface to act as a mirror, it must reflect as much of the light as possible and must transmit and absorb as little as possible. In order to reflect light rays without scattering or diffusing them, a mirror’s surface must be perfectly smooth or its irregularities must be smaller than the wavelength of the light being reflected. (The wavelengths of visible light are on the order of {5} × {10}^{-5 cm.) Mirrors may have plane or curved surfaces. A curved mirror is concave or convex depending on whether the reflecting surface faces toward the centre of curvature or away from it. Curved mirrors in ordinary usage have surfaces that are spherical, cylindrical, paraboloidal, ellipsoidal, and hyperboloidal. Spherical mirrors produce images that are magnified or reduced—exemplified, respectively, by mirrors for applying facial makeup and by rearview mirrors for automobiles. Cylindrical mirrors focus a parallel beam of light to a line focus. A paraboloidal mirror may be used to focus parallel rays to a real focus, as in a telescope mirror, or to produce a parallel beam from a source at its focus, as in a searchlight. An ellipsoidal mirror will reflect light from one of its two focal points to the other, and an object situated at the focus of a hyperboloidal mirror will have a virtual image.
Mirrors have a long history of use both as household objects and as objects of decoration. The earliest mirrors were hand mirrors; those large enough to reflect the whole body did not appear until the 1st century AD. Hand mirrors were adopted by the Celts from the Romans and by the end of the Middle Ages had become quite common throughout Europe, usually being made of silver, though sometimes of polished bronze.
The use of glass with a metallic backing commenced in the late 12th and early 13th centuries, and, by the time of the Renaissance, Nürnberg and Venice had established outstanding reputations as centres of mirror production. The mirrors produced in Venice were famous for their high quality. Despite the strictures of the doges, Venetian workmen succumbed to the temptation to carry the secrets of their craft to other cities, and, by the middle of the 17th century, mirror making was practiced extensively in London and Paris. Generally, mirrors were extremely expensive—especially the larger variety—and the wonderment created at the time by the royal palace at Versailles was due in part to the profusion of mirrors that adorned the state rooms.
From the late 17th century onward, mirrors—and their frames—played an increasingly important part in the decoration of rooms. The early frames were usually of ivory, silver, ebony, or tortoiseshell or were veneered with marquetry of walnut, olive, and laburnum. Needlework and bead frames were also to be found. Craftsmen such as Grinling Gibbons (1648–1721) often produced elaborately carved mirror frames to match a complete decorative ensemble. The tradition soon became established of incorporating a mirror into the space over the mantelpiece: many of the early versions of these mirrors, usually known as overmantels, were enclosed in glass frames. The architectural structure of which these mirrors formed a part became progressively more elaborate; designers such as the English brothers Robert and James Adam created fireplace units stretching from the hearth to the ceiling and depending largely for their effect on mirrors. On the whole, mirror frames reflected the general taste of the time and were often changed to accommodate alterations in taste, frames usually being cheaper and hence more easily replaced than the mirror itself.
By the end of the 18th century, painted decoration largely supplanted carving on mirrors, the frames being decorated with floral patterns or classical ornaments. At the same time, the French started producing circular mirrors, usually surrounded by a Neoclassical gilt frame that sometimes supported candlesticks, which enjoyed great popularity well into the 19th century. Improved skill in mirror making also made possible the introduction of the cheval glass, a freestanding full-length mirror, supported on a frame with four feet. These were mainly used for dressing purposes, though occasionally they had a decorative function.
New, cheaper techniques of mirror production in the 19th century led to a great proliferation in their use. Not only were they incorporated into pieces of furniture, such as wardrobes and sideboards, but they were also used extensively in decorative schemes for public places.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1320 2022-03-18 13:54:52
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,620
Re: Miscellany
1294) Paracetamol
Summary
Acetaminophen, also called paracetamol, is a drug used in the treatment of mild pain, such as headache and pain in joints and muscles, and to reduce fever. Acetaminophen is the major metabolite of acetanilid and phenacetin, which were once commonly used drugs, and is responsible for their analgesic (pain-relieving) effects. Acetaminophen relieves pain by raising the body’s pain threshold, and it reduces fever by its action on the temperature-regulating centre of the brain. The drug inhibits prostaglandin synthesis in the central nervous system, but it lacks an anti-inflammatory effect in peripheral nerves.
Acetaminophen is much less likely to cause gastrointestinal side effects than aspirin, but overdoses of it can cause fatal liver damage. For prolonged use, aspirin is considered safer. Acetaminophen has also been implicated as a hormone disruptor, with prenatal exposure to the drug possibly linked to hyperkinetic and behavioral disorders in children. Research has also linked acetaminophen use to alterations in risk perception and decision making and increased risk-taking behaviour.
The drug is marketed under several trade names, including Tylenol, Tempra, and Panadol.
Details
Paracetamol, also known as acetaminophen, is a medication used to treat fever and mild to moderate pain. At a standard dose, paracetamol only slightly decreases body temperature; it is inferior to ibuprofen in that respect, and the benefits of its use for fever are unclear. Paracetamol may relieve pain in acute mild migraine but only slightly in episodic tension headache. However, the aspirin/paracetamol/caffeine combination helps with both conditions where the pain is mild and is recommended as a first-line treatment for them. Paracetamol is effective for post-surgical pain, but it is inferior to ibuprofen. The paracetamol/ibuprofen combination provides further increase in potency and is superior to either drug alone. The pain relief paracetamol provides in osteoarthritis is small and clinically insignificant. The evidence in its favor for the use in low back pain, cancer pain and neuropathic pain is insufficient.
In the short term, common side effects of paracetamol are nausea and abdominal pain, and it seems to have tolerability similar to ibuprofen. Chronic consumption of paracetamol may result in a drop in hemoglobin level, indicating possible gastrointestinal bleeding, and abnormal liver function tests. There is a consistent association of increased mortality as well as cardiovascular (stroke, myocardial infarction), gastrointestinal (ulcers, bleeding) and renal adverse effects with taking higher dose of paracetamol. The drug may also increase the risk of developing hypertension. Elevated frequency of asthma and developmental and reproductive disorders is observed in the offspring of women with prolonged use of paracetamol during pregnancy, although whether paracetamol is the true cause of this increase is unclear. Some studies suggest that there is evidence for the association between paracetamol during pregnancy and autism spectrum disorder and attention deficit hyperactivity disorder, while making clear further research is required to establish any causal link, which has prompted some calls to limit its use in pregnancy to the lowest effective dosage for the shortest possible time.
The recommended maximum daily dose for an adult is three to four grams. Higher doses may lead to toxicity, including liver failure. Paracetamol poisoning is the foremost cause of acute liver failure in the Western world, and accounts for most drug overdoses in the United States, the United Kingdom, Australia, and New Zealand.
Paracetamol was first made in 1877 or possibly 1852. It is the most commonly used medication for pain and fever in both the United States and Europe. It is on the World Health Organization's List of Essential Medicines. Paracetamol is available as a generic medication, with brand names including Tylenol and Panadol among others. In 2019, it was the 145th most commonly prescribed medication in the United States, with more than 4 million prescriptions.
Medical uses:
Fever
Paracetamol is a drug of choice for reducing fever. However, there has been a lack of research on its antipyretic properties, particularly, in adults. The most recent review on paracetamol and management of fever in the general practice (2008) argued that its benefits are unclear. Additionally, when taken for the common cold paracetamol may relieve stuffed or runny nose but not other cold symptoms such as sore throat, malaise, sneezing and cough; this data, however, is of low quality.
For patients in critical care, paracetamol decreased body temperature by only 0.2–0.3 °C more than control interventions; there was no difference in mortality. It did not change the outcome in febrile patients with stroke. The results are contradictory for paracetamol use in sepsis: higher mortality, lower mortality, and no change in mortality were all reported. Paracetamol offered no benefit in the treatment of dengue fever and was accompanied by a higher rate of liver enzyme elevation: a sign of a potential liver damage. Overall, there is no support for a routine administration of antipyretic drugs, including paracetamol, to hospitalized patients with fever and infection.
The efficacy of paracetamol in children with fever is unclear. Paracetamol should not be used solely with the aim of reducing body temperature; however, it may be considered for children with fever who appear distressed. It does not prevent febrile seizures and should not be used for that purpose. It appears that 0.2 °C decrease of the body temperature in children after a standard dose of paracetamol is of questionable value, particularly in emergency situations. Based on this, some physicians advocate using higher doses that may decrease the temperature by as much as 0.7 °C. Meta-analyses showed that paracetamol is less effective than ibuprofen in children (marginally less effective, according to another analysis), including children younger than 2 years old, with equivalent safety. Exacerbation of asthma occurs with similar frequency for both medications. Giving paracetamol and ibuprofen together at the same time to children under 5 is not recommended, however doses may be alternated if required.
Pain
Paracetamol is used for the relief of mild to moderate pain such as headache, muscle aches, minor arthritis pain, toothache as well as pain caused by cold, flu, sprains, and dysmenorrhea. It is recommended, in particular, for acute mild to moderate pain, since the evidence for the treatment of chronic pain is insufficient.
Musculoskeletal pain
The benefits of paracetamol in musculoskeletal conditions, such as osteoarthritis and backache, are uncertain.
It appears to provide only small and not clinically important benefits in osteoarthritis. American College of Rheumatology and Arthritis Foundation guideline for the management of osteoarthritis notes that the effect size in clinical trials of paracetamol has been very small, which suggests that for most individuals it is ineffective. The guideline conditionally recommends paracetamol for short-term and episodic use to those who do not tolerate nonsteroidal anti-inflammatory drugs. For people taking it regularly, monitoring for liver toxicity is required. Essentially the same recommendation was issued by EULAR for hand osteoarthritis. Similarly, European algorithm ESCEO for the treatment of knee osteoarthritis recommends limiting the of use paracetamol to short-term rescue analgesia only.
Paracetamol is ineffective for acute low back pain. No randomized clinical trials evaluated its use for chronic or radicular back pain, and the evidence in favor of paracetamol is lacking.
Headaches
Paracetamol is effective for acute migraine: 39% of people experience pain relief at one hour compared with 20% in the control group. The aspirin/paracetamol/caffeine combination also "has strong evidence of effectiveness and can be used as a first-line treatment for migraine."[20] The German, Austrian, and Swiss headache societies and the German Society of Neurology recommend the combination as a "highlighted" one for self-medication of migraine, and paracetamol alone as a first choice.
Paracetamol on its own only slightly alleviates episodic tension headache in frequent sufferers. However, the aspirin/paracetamol/caffeine combination is superior to both paracetamol alone and placebo and offers meaningful relief of tension headache: 2 hours after administering the medication, 29% of those who took the combination were pain free as compared with 21% on paracetamol and 18% on placebo. The German, Austrian, and Swiss headache societies and the German Society of Neurology recommend this combination as a "highlighted" one for self-medication of tension headache, with paracetamol/caffeine combination being a "remedy of first choice", and paracetamol a "remedy of second choice".
Dental and other post-surgical pain
Pain after a dental surgery provides a reliable model for the action of analgesics on other kinds of acute pain. For the relief of such pain, paracetamol is inferior to ibuprofen. Full therapeutic doses of non-steroidal anti-inflammatory drugs (NSAIDs) ibuprofen, naproxen or diclofenac are clearly more efficacious than the paracetamol/codeine combination which is frequently prescribed for dental pain. The combinations of paracetamol and NSAIDs ibuprofen or diclofenac are promising, possibly offering better pain control than either paracetamol or the NSAID alone. Additionally, the paracetamol/ibuprofen combination may be superior to paracetamol/codeine and ibuprofen/codeine combinations.
A meta-analysis of general post-surgical pain, which included dental and other surgery, showed the paracetamol/codeine combination to be more effective than paracetamol alone: it provided significant pain relief to as much as 53% of the participants, while the placebo helped only 7%.
Other pain
Paracetamol fails to relieve procedural pain in newborn babies. For perineal pain postpartum paracetamol appears to be less effective than non-steroidal anti-inflammatory drugs (NSAIDs).
The studies to support or refute the use of paracetamol for cancer pain and for neuropathic pain are lacking. There is limited evidence in favor of the use of the intravenous form of paracetamol for acute pain control in the emergency department. The combination of paracetamol with caffeine is superior to paracetamol alone for the treatment of acute pain.
Patent ductus arteriosus
Paracetamol helps ductal closure in patent ductus arteriosus. It is as effective for this purpose as ibuprofen or indomethacin, but results in a less frequent gastrointestinal bleeding than ibuprofen.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1321 2022-03-19 14:23:22
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,620
Re: Miscellany
1295) Ibuprofen
Gist
Ibuprofen is nonsteroidal anti-inflammatory drug used in the treatment of minor pain, fever, and inflammation. Like aspirin, ibuprofen works by inhibiting the synthesis of prostaglandins, body chemicals that sensitize nerve endings. The drug may irritate the gastrointestinal tract. Marketed under trade names such as Advil and Nuprin, ibuprofen is not recommended for use by children under the age of 12, and, like aspirin and acetaminophen, it should not be used during pregnancy except under medical supervision.
Summary
Ibuprofen is a medication in the nonsteroidal anti-inflammatory drug (NSAID) class that is used for treating pain, fever, and inflammation. This includes painful menstrual periods, migraines, and rheumatoid arthritis. It may also be used to close a patent ductus arteriosus in a premature baby. It can be used by mouth or intravenously. It typically begins working within an hour.
Common side effects include heartburn and a rash. Compared to other NSAIDs, it may have other side effects such as gastrointestinal bleeding. It increases the risk of heart failure, kidney failure, and liver failure. At low doses, it does not appear to increase the risk of heart attack; however, at higher doses it may. Ibuprofen can also worsen asthma. While it is unclear whether it is safe in early pregnancy, it appears to be harmful in later pregnancy and therefore is not recommended. Like other NSAIDs, it works by inhibiting the production of prostaglandins by decreasing the activity of the enzyme cyclooxygenase (COX). Ibuprofen is a weaker anti-inflammatory agent than other NSAIDs.
Ibuprofen was discovered in 1961 by Stewart Adams and John Nicholson while working at Boots UK Limited and initially marketed as Brufen. It is available under a number of trade names, including Nurofen, Advil and Motrin. It was first marketed in 1969 in the United Kingdom and in 1974 in the United States. It is on the World Health Organization's List of Essential Medicines. It is available as a generic medication. In 2019, it was the 29th most commonly prescribed medication in the United States, with more than 21 million prescriptions.
Details
Ibuprofen is used to relieve pain from various conditions such as headache, dental pain, menstrual cramps, muscle aches, or arthritis. It is also used to reduce fever and to relieve minor aches and pain due to the common cold or flu. Ibuprofen is a nonsteroidal anti-inflammatory drug (NSAID). It works by blocking your body's production of certain natural substances that cause inflammation. This effect helps to decrease swelling, pain, or fever. If you are treating a chronic condition such as arthritis, ask your doctor about non-drug treatments and/or using other medications to treat your pain. See also Warning section. Check the ingredients on the label even if you have used the product before. The manufacturer may have changed the ingredients. Also, products with similar names may contain different ingredients meant for different purposes. Taking the wrong product could harm you.
How to use ibuprofen oral
If you are taking the over-the-counter product, read all directions on the product package before taking this medication. If your doctor has prescribed this medication, read the Medication Guide provided by your pharmacist before you start taking ibuprofen and each time you get a refill. If you have any questions, ask your doctor or pharmacist.
Take this medication by mouth, usually every 4 to 6 hours with a full glass of water (8 ounces/240 milliliters) unless your doctor directs you otherwise. Do not lie down for at least 10 minutes after taking this drug. If you have stomach upset while taking this medication, take it with food, milk, or an antacid.
The dosage is based on your medical condition and response to treatment. To reduce your risk of stomach bleeding and other side effects, take this medication at the lowest effective dose for the shortest possible time. Do not increase your dose or take this drug more often than directed by your doctor or the package label. For ongoing conditions such as arthritis, continue taking this medication as directed by your doctor.
When ibuprofen is used by children, the dose is based on the child's weight. Read the package directions to find the proper dose for your child's weight. Consult the pharmacist or doctor if you have questions or if you need help choosing a nonprescription product.
For certain conditions (such as arthritis), it may take up to two weeks of taking this drug regularly until you get the full benefit.
If you are taking this drug "as needed" (not on a regular schedule), remember that pain medications work best if they are used as the first signs of pain occur. If you wait until the pain has worsened, the medication may not work as well.
If your condition persists or worsens, or if you think you may have a serious medical problem, get medical help right away. If you are using the nonprescription product to treat yourself or a child for fever or pain, consult the doctor right away if fever worsens or lasts more than 3 days, or if pain worsens or lasts more than 10 days.
Side Effects
Upset stomach, nausea, vomiting, headache, diarrhea, constipation, dizziness, or drowsiness may occur. If any of these effects persist or worsen, tell your doctor or pharmacist promptly.
If your doctor has prescribed this medication, remember that your doctor has judged that the benefit to you is greater than the risk of side effects. Many people using this medication do not have serious side effects.
This medication may raise your blood pressure. Check your blood pressure regularly and tell your doctor if the results are high.
Tell your doctor right away if you have any serious side effects, including: easy bruising/bleeding, hearing changes (such as ringing in the ears), mental/mood changes, unexplained stiff neck, signs of kidney problems (such as change in the amount of urine), vision changes, symptoms of heart failure (such as swelling ankles/feet, unusual tiredness, unusual/sudden weight gain).
This drug may rarely cause serious (possibly fatal) liver disease. Get medical help right away if you have any symptoms of liver damage, including: dark urine, persistent nausea/vomiting/loss of appetite, stomach/abdominal pain, yellowing eyes/skin.
A very serious allergic reaction to this drug is rare. However, get medical help right away if you notice any symptoms of a serious allergic reaction, including: fever, swollen lymph nodes, rash, itching/swelling (especially of the face/tongue/throat), severe dizziness, trouble breathing.
This is not a complete list of possible side effects. If you notice other effects not listed above, contact your doctor or pharmacist.
What is ibuprofen?
Ibuprofen is a pharmaceutical drug that is classified as a non-steroidal anti-inflammatory drug (NSAID).
Ibuprofen is used to treat a number of conditions including:
* mild to moderate pain
* severe pain (when combined with codeine)
* fever
* swollen, red and tender tissues (inflammation)
* rheumatoid arthritis, back pain and gout (in conjunction with physiotherapy).
Some people use ibuprofen get high, or as an act of self-harm.by intentionally taking more than the recommended dose.
Ibuprofen is usually swallowed and comes in different forms including:
* tablets
* capsules
* suppositories
* soluble powders
* liquids
Effects of ibuprofen
There is no safe level of drug use. Use of any drug always carries some risk – even medications can produce unwanted side effects. It’s important to be careful when taking any type of drug.
Ibuprofen affects everyone differently, based on:
* size, weight and health
* whether the person is used to taking it
* whether other drugs are taken around the same time
* the amount taken.
Side effects
The most common side effects of ibuprofen are:
* headache
* dizziness
* drowsiness, fatigue and restless sleep
* thirst and sweating
* tingling or numbness in hands and feet
* ringing in the ears
* blurred vision and eye irritation
* fluid retention and ankle swelling
* mild allergic reaction
* abdominal pain, nausea, vomiting, heartburn, diarrhoea and constipation
* bladder irritation and pain, frequent urination.1,2
NSAIDs such ibuprofen can increase the risk of heart attack or stroke in people with or without heart disease or the risk factors for heart disease.
Overdose
If you take more than the recommended dose, you could overdose. If you or someone else has any of these symptoms:
* confusion and disorientation
* drowsiness
* abdominal pain
* blurred vision
* tinnitus (ringing in the ears)
* diarrhea
* anxiety and paranoia
* anaemia (low red blood cell count), nausea and vomiting
* vomiting blood that may look like coffee grounds and bowel motions that look like black tar
* severe allergic reaction, including swelling of the face
* kidney and liver problems
* seizures/convulsions
* coma and death.
Long-term effects
It’s best to discuss the side effects of long-term use with a medical practitioner. Regular use of ibuprofen may eventually cause:
* anaemia due to bleeding in the stomach
* impaired hearing
* kidney and liver damage
* bleeding in the stomach and bowels
* increased risk of heart attack.1
Using ibuprofen with other drugs
The effects of taking ibuprofen with other drugs, including alcohol, prescription medications and other over-the-counter medicines, are often unpredictable.
Ibuprofen taken with alcohol can increase the risk of stomach irritation and discomfort.
Ibuprofen can alter the effects of some blood pressure medicines and may increase the risk of bleeding if taken with medicines such as warfarin.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1322 2022-03-20 13:39:31
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,620
Re: Miscellany
1296) Nuclear Power
Summary
Nuclear power is the use of nuclear reactions to produce electricity. Nuclear power can be obtained from nuclear fission, nuclear decay and nuclear fusion reactions. Presently, the vast majority of electricity from nuclear power is produced by nuclear fission of uranium and plutonium in nuclear power plants. Nuclear decay processes are used in niche applications such as radioisotope thermoelectric generators in some space probes such as Voyager 2. Generating electricity from fusion power remains the focus of international research.
Civilian nuclear power supplied 2,586 terawatt hours (TWh) of electricity in 2019 (around a tenth of all global electricity generation), and was the second-largest low-carbon power source after hydroelectricity, with 28% of global low-carbon power in 2019 supplied by nuclear. As of September 2021, there are 444 civilian fission reactors in the world, with a combined electrical capacity of 396 gigawatt (GW). There are also 53 nuclear power reactors under construction and 98 reactors planned, with a combined capacity of 60 GW and 103 GW, respectively. The United States has the largest fleet of nuclear reactors, generating over 800 TWh zero-emissions electricity per year with a very high average capacity factor of 92%. Most reactors under construction are generation III reactors in Asia.
Nuclear power is the safest energy source. Coal, petroleum, natural gas and hydroelectricity each have caused more fatalities per unit of energy due to air pollution and accidents. Accidents in nuclear power plants include the Chernobyl explosion in the Soviet Union in 1986, the Fukushima nuclear disaster in Japan in 2011, and the more contained Three Mile Island accident in the United States in 1979.
There is a debate about nuclear power. Proponents, such as the World Nuclear Association and Environmentalists for Nuclear Energy, contend that nuclear power is a safe, sustainable energy source that reduces carbon emissions. Nuclear power opponents, such as Greenpeace, Scientists for Future and NIRS, argue that nuclear has not been proven to be safe and should be supplemented with other ideas, such as wind and hydroelectric energy. The nuclear industry also includes nuclear medicine, including applications involving diagnosis and treatment of tumors.
Details
Nuclear power is electricity generated by power plants that derive their heat from fission in a nuclear reactor. Except for the reactor, which plays the role of a boiler in a fossil-fuel power plant, a nuclear power plant is similar to a large coal-fired power plant, with pumps, valves, steam generators, turbines, electric generators, condensers, and associated equipment.
World nuclear power
Nuclear power provides almost 15 percent of the world’s electricity. The first nuclear power plants, which were small demonstration facilities, were built in the 1960s. These prototypes provided “proof-of-concept” and laid the groundwork for the development of the higher-power reactors that followed.
The nuclear power industry went through a period of remarkable growth until about 1990, when the portion of electricity generated by nuclear power reached a high of 17 percent. That percentage remained stable through the 1990s and began to decline slowly around the turn of the 21st century, primarily because of the fact that total electricity generation grew faster than electricity from nuclear power while other sources of energy (particularly coal and natural gas) were able to grow more quickly to meet the rising demand. This trend appears likely to continue well into the 21st century. The Energy Information Administration (EIA), a statistical arm of the U.S. Department of Energy, has projected that world electricity generation between 2005 and 2035 will roughly double (from more than 15,000 terawatt-hours to 35,000 terawatt-hours) and that generation from all energy sources except petroleum will continue to grow.
In 2012 more than 400 nuclear reactors were in operation in 30 countries around the world, and more than 60 were under construction. The United States has the largest nuclear power industry, with more than 100 reactors; it is followed by France, which has more than 50. Of the top 15 electricity-producing countries in the world, all but two, Italy and Australia, utilize nuclear power to generate some of their electricity. The overwhelming majority of nuclear reactor generating capacity is concentrated in North America, Europe, and Asia. The early period of the nuclear power industry was dominated by North America (the United States and Canada), but in the 1980s that lead was overtaken by Europe. The EIA projects that Asia will have the largest nuclear capacity by 2035, mainly because of an ambitious building program in China.
A typical nuclear power plant has a generating capacity of approximately one gigawatt (GW; one billion watts) of electricity. At this capacity, a power plant that operates about 90 percent of the time (the U.S. industry average) will generate about eight terawatt-hours of electricity per year. The predominant types of power reactors are pressurized water reactors (PWRs) and boiling water reactors (BWRs), both of which are categorized as light water reactors (LWRs) because they use ordinary (light) water as a moderator and coolant. LWRs make up more than 80 percent of the world’s nuclear reactors, and more than three-quarters of the LWRs are PWRs.
Issues affecting nuclear power
Countries may have a number of motives for deploying nuclear power plants, including a lack of indigenous energy resources, a desire for energy independence, and a goal to limit greenhouse gas emissions by using a carbon-free source of electricity. The benefits of applying nuclear power to these needs are substantial, but they are tempered by a number of issues that need to be considered, including the safety of nuclear reactors, their cost, the disposal of radioactive waste, and a potential for the nuclear fuel cycle to be diverted to the development of nuclear weapons. All of these concerns are discussed below.
Safety
The safety of nuclear reactors has become paramount since the Fukushima accident of 2011. The lessons learned from that disaster included the need to (1) adopt risk-informed regulation, (2) strengthen management systems so that decisions made in the event of a severe accident are based on safety and not cost or political repercussions, (3) periodically assess new information on risks posed by natural hazards such as earthquakes and associated tsunamis, and (4) take steps to mitigate the possible consequences of a station blackout.
The four reactors involved in the Fukushima accident were first-generation BWRs designed in the 1960s. Newer Generation III designs, on the other hand, incorporate improved safety systems and rely more on so-called passive safety designs (i.e., directing cooling water by gravity rather than moving it by pumps) in order to keep the plants safe in the event of a severe accident or station blackout. For instance, in the Westinghouse AP1000 design, residual heat would be removed from the reactor by water circulating under the influence of gravity from reservoirs located inside the reactor’s containment structure. Active and passive safety systems are incorporated into the European Pressurized Water Reactor (EPR) as well.
Traditionally, enhanced safety systems have resulted in higher construction costs, but passive safety designs, by requiring the installation of far fewer pumps, valves, and associated piping, may actually yield a cost saving.
Economics
A convenient economic measure used in the power industry is known as the levelized cost of electricity, or LCOE, which is the cost of generating one kilowatt-hour (kWh) of electricity averaged over the lifetime of the power plant. The LCOE is also known as the “busbar cost,” as it represents the cost of the electricity up to the power plant’s busbar, a conducting apparatus that links the plant’s generators and other components to the distribution and transmission equipment that delivers the electricity to the consumer.
The busbar cost of a power plant is determined by 1) capital costs of construction, including finance costs, 2) fuel costs, 3) operation and maintenance (O&M) costs, and 4) decommissioning and waste-disposal costs. For nuclear power plants, busbar costs are dominated by capital costs, which can make up more than 70 percent of the LCOE. Fuel costs, on the other hand, are a relatively small factor in a nuclear plant’s LCOE (less than 20 percent). As a result, the cost of electricity from a nuclear plant is very sensitive to construction costs and interest rates but relatively insensitive to the price of uranium. Indeed, the fuel costs for coal-fired plants tend to be substantially greater than those for nuclear plants. Even though fuel for a nuclear reactor has to be fabricated, the cost of nuclear fuel is substantially less than the cost of fossil fuel per kilowatt-hour of electricity generated. This fuel cost advantage is due to the enormous energy content of each unit of nuclear fuel compared to fossil fuel.
The O&M costs for nuclear plants tend to be higher than those for fossil-fuel plants because of the complexity of a nuclear plant and the regulatory issues that arise during the plant’s operation. Costs for decommissioning and waste disposal are included in fees charged by electrical utilities. In the United States, nuclear-generated electricity was assessed a fee of $0.001 per kilowatt-hour to pay for a permanent repository of high-level nuclear waste. This seemingly modest fee yielded about $750 million per year for the Nuclear Waste Fund.
At the beginning of the 21st century, electricity from nuclear plants typically cost less than electricity from coal-fired plants, but this formula may not apply to the newer generation of nuclear power plants, given the sensitivity of busbar costs to construction costs and interest rates. Another major uncertainty is the possibility of carbon taxes or stricter regulations on carbon dioxide emissions. These measures would almost certainly raise the operating costs of coal plants and thus make nuclear power more competitive.
Radioactive-waste disposal
Spent nuclear reactor fuel and the waste stream generated by fuel reprocessing contain radioactive materials and must be conditioned for permanent disposal. The amount of waste coming out of the nuclear fuel cycle is very small compared with the amount of waste generated by fossil fuel plants. However, nuclear waste is highly radioactive (hence its designation as high-level waste, or HLW), which makes it very dangerous to the public and the environment. Extreme care must be taken to ensure that it is stored safely and securely, preferably deep underground in permanent geologic repositories.
Despite years of research into the science and technology of geologic disposal, no permanent disposal site is in use anywhere in the world. In the last decades of the 20th century, the United States made preparations for constructing a repository for commercial HLW beneath Yucca Mountain, Nevada, but by the turn of the 21st century, this facility had been delayed by legal challenges and political decisions. Pending construction of a long-term repository, U.S. utilities have been storing HLW in so-called dry casks aboveground. Some other countries using nuclear power, such as Finland, Sweden, and France, have made more progress and expect to have HLW repositories operational in the period 2020–25.
Proliferation
The claim has long been made that the development and expansion of commercial nuclear power led to nuclear weapons proliferation, because elements of the nuclear fuel cycle (including uranium enrichment and spent-fuel reprocessing) can also serve as pathways to weapons development. However, the history of nuclear weapons development does not support the notion of a necessary connection between weapons proliferation and commercial nuclear power.
The first pathway to proliferation, uranium enrichment, can lead to a nuclear weapon based on highly enriched uranium (see nuclear weapon: Principles of atomic (fission) weapons). It is considered relatively straightforward for a country to fabricate a weapon with highly enriched uranium, but the impediment historically has been the difficulty of the enrichment process. Since nuclear reactor fuel for LWRs is only slightly enriched (less than 5 percent of the fissile isotope uranium-235) and weapons need a minimum of 20 percent enriched uranium, commercial nuclear power is not a viable pathway to obtaining highly enriched uranium.
The second pathway to proliferation, reprocessing, results in the separation of plutonium from the highly radioactive spent fuel. The plutonium can then be used in a nuclear weapon. However, reprocessing is heavily guarded in those countries where it is conducted, making commercial reprocessing an unlikely pathway for proliferation. Also, it is considered more difficult to construct a weapon with plutonium versus highly enriched uranium.
More than 20 countries have developed nuclear power industries without building nuclear weapons. On the other hand, countries that have built and tested nuclear weapons have followed other paths than purchasing commercial nuclear reactors, reprocessing the spent fuel, and obtaining plutonium. Some have built facilities for the express purpose of enriching uranium; some have built plutonium production reactors; and some have surreptitiously diverted research reactors to the production of plutonium. All these pathways to nuclear proliferation have been more effective, less expensive, and easier to hide from prying eyes than the commercial nuclear power route. Nevertheless, nuclear proliferation remains a highly sensitive issue, and any country that wishes to launch a commercial nuclear power industry will necessarily draw the close attention of oversight bodies such as the International Atomic Energy Agency.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1323 2022-03-21 13:25:52
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,620
Re: Miscellany
1297) The Giving Pledge
The Giving Pledge is a campaign to encourage extremely wealthy people to contribute a majority of their wealth to philanthropic causes. As of January 2021, the pledge has 231 signatories from 28 countries. Most of the signatories of the pledge are billionaires, and as of 2016, their pledges are estimated to total US$600 billion.
Description
The organization's stated goal is to inspire the wealthy people of the world to give at least half of their net worth to philanthropy, either during their lifetime or upon their death. The pledge is a public gesture of an intention to give, not a legal contract. On The Giving Pledge's website, each individual or couple writes a letter explaining why they chose to give.
History
In June 2010, the Giving Pledge campaign was formally announced and Bill Gates and Warren Buffett began recruiting members. As of August 2010, the aggregate wealth of the first 40 pledgers was $125 billion. As of April 2011, 69 billionaires had joined the campaign and given a pledge, and by the following year, The Huffington Post reported that a total of 81 billionaires had pledged. By May 2017, 158 individuals and/or couples were listed as pledgers. Not all pledgers are billionaires.
About the Giving Pledge
The Giving Pledge is a movement of philanthropists who commit to give the majority of their wealth to charitable causes, either during their lifetimes or in their wills.
In August 2010, 40 of America’s wealthiest people made a commitment to give the majority of their wealth to address some of society’s most pressing problems. Created by Warren Buffett, Melinda French Gates, and Bill Gates, the Giving Pledge came to life following a series of conversations with philanthropists about how they could set a new standard of generosity among the ultra-wealthy. While originally focused on the United States, the Giving Pledge quickly saw interest from philanthropists around the world.
The Giving Pledge is a simple concept: an open invitation for billionaires, or those who would be if not for their giving, to publicly commit to give the majority of their wealth to philanthropy either during their lifetimes or in their wills. It is inspired by the example set by millions of people at all income levels who give generously – and often at great personal sacrifice – to make the world better. Envisioned as a multi-generational effort, the Giving Pledge aims over time to help shift the social norms of philanthropy among the world’s wealthiest and inspire people to give more, establish their giving plans sooner, and give in smarter ways. Signatories fund a diverse range of issues of their choosing. Those who join the Giving Pledge are encouraged to write a letter explaining their decision to engage deeply and publicly in philanthropy and describing the causes that motivate them.
Joining the Giving Pledge is more than a one-time event. It means becoming part of an energized community of some of the world’s most engaged philanthropists to discuss challenges, successes, and failures, and to share ideas to get smarter about giving. Signatories are united by a shared commitment to learning and giving. The Giving Pledge team provides opportunities – both specifically for signatories, and for families and staff – to gather throughout the year to learn from experts and from one another how to best leverage their philanthropy to address some of the world’s biggest challenges.
The Giving Pledge was founded by Warren Buffett and Bill and Melinda Gates in 2010 to inspire the wealthiest individuals in the U.S. to commit to giving at least half of their fortune to charitable causes. It expanded globally in subsequent years.
According to the Giving Pledge website, philanthropists are encouraged to make their commitment public and to write an open letter. They then pursue their own philanthropic goals independently, giving to a range of causes in whatever manner suits them. The Giving Pledge declares that it “is not an oversight organization, nor is it a pooled fund. The Giving Pledge does not distribute funds, grants, or donations in any form.” Members of the Giving Pledge have an annual meeting, but only to “share ideas, hear from experts in their respective fields, and learn from each other.”
Ten years after the Pledge’s founding, MarketWatch notes that many of the living signatories are struggling to keep to their commitment, mainly because their stocks are rising so quickly. They simply cannot give their money away fast enough.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1324 2022-03-22 13:13:53
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,620
Re: Miscellany
1298) Nuclear Reactor
Summary
A nuclear reactor, formerly known as an atomic pile, is a device used to initiate and control a fission nuclear chain reaction or nuclear fusion reactions. Nuclear reactors are used at nuclear power plants for electricity generation and in nuclear marine propulsion. Heat from nuclear fission is passed to a working fluid (water or gas), which in turn runs through steam turbines. These either drive a ship's propellers or turn electrical generators' shafts. Nuclear generated steam in principle can be used for industrial process heat or for district heating. Some reactors are used to produce isotopes for medical and industrial use, or for production of weapons-grade plutonium. As of early 2019, the IAEA reports there are 454 nuclear power reactors and 226 nuclear research reactors in operation around the world.
Details
Nuclear reactor is any of a class of devices that can initiate and control a self-sustaining series of nuclear fissions. Nuclear reactors are used as research tools, as systems for producing radioactive isotopes, and most prominently as energy sources for nuclear power plants.
Principles of operation
Nuclear reactors operate on the principle of nuclear fission, the process in which a heavy atomic nucleus splits into two smaller fragments. The nuclear fragments are in very excited states and emit neutrons, other subatomic particles, and photons. The emitted neutrons may then cause new fissions, which in turn yield more neutrons, and so forth. Such a continuous self-sustaining series of fissions constitutes a fission chain reaction. A large amount of energy is released in this process, and this energy is the basis of nuclear power systems.
In an atomic bomb the chain reaction is designed to increase in intensity until much of the material has fissioned. This increase is very rapid and produces the extremely prompt, tremendously energetic explosions characteristic of such bombs. In a nuclear reactor the chain reaction is maintained at a controlled, nearly constant level. Nuclear reactors are so designed that they cannot explode like atomic bombs.
Most of the energy of fission—approximately 85 percent of it—is released within a very short time after the process has occurred. The remainder of the energy produced as a result of a fission event comes from the radioactive decay of fission products, which are fission fragments after they have emitted neutrons. Radioactive decay is the process by which an atom reaches a more stable state; the decay process continues even after fissioning has ceased, and its energy must be dealt with in any proper reactor design.
Chain reaction and criticality
The course of a chain reaction is determined by the probability that a neutron released in fission will cause a subsequent fission. If the neutron population in a reactor decreases over a given period of time, the rate of fission will decrease and ultimately drop to zero. In this case the reactor will be in what is known as a subcritical state. If over the course of time the neutron population is sustained at a constant rate, the fission rate will remain steady, and the reactor will be in what is called a critical state. Finally, if the neutron population increases over time, the fission rate and power will increase, and the reactor will be in a supercritical state.
Before a reactor is started up, the neutron population is near zero. During reactor start-up, operators remove control rods from the core in order to promote fissioning in the reactor core, effectively putting the reactor temporarily into a supercritical state. When the reactor approaches its nominal power level, the operators partially reinsert the control rods, balancing out the neutron population over time. At this point the reactor is maintained in a critical state, or what is known as steady-state operation. When a reactor is to be shut down, operators fully insert the control rods, inhibiting fission from occurring and forcing the reactor to go into a subcritical state.
Reactor control
A commonly used parameter in the nuclear industry is reactivity, which is a measure of the state of a reactor in relation to where it would be if it were in a critical state. Reactivity is positive when a reactor is supercritical, zero at criticality, and negative when the reactor is subcritical. Reactivity may be controlled in various ways: by adding or removing fuel, by altering the ratio of neutrons that leak out of the system to those that are kept in the system, or by changing the amount of absorber that competes with the fuel for neutrons. In the latter method the neutron population in the reactor is controlled by varying the absorbers, which are commonly in the form of movable control rods (though in a less commonly used design, operators can change the concentration of absorber in the reactor coolant). Changes of neutron leakage, on the other hand, are often automatic. For example, an increase of power will cause a reactor’s coolant to reduce in density and possibly boil. This decrease in coolant density will increase neutron leakage out of the system and thus reduce reactivity—a process known as negative-reactivity feedback. Neutron leakage and other mechanisms of negative-reactivity feedback are vital aspects of safe reactor design.
A typical fission interaction takes place on the order of one picosecond {10}^{-12} second). This extremely fast rate does not allow enough time for a reactor operator to observe the system’s state and respond appropriately. Fortunately, reactor control is aided by the presence of so-called delayed neutrons, which are neutrons emitted by fission products some time after fission has occurred. The concentration of delayed neutrons at any one time (more commonly referred to as the effective delayed neutron fraction) is less than 1 percent of all neutrons in the reactor. However, even this small percentage is sufficient to facilitate the monitoring and control of changes in the system and to regulate an operating reactor safely.
Fissile and fertile materials
All heavy nuclides have the ability to fission when in an excited state, but only a few fission readily and consistently when struck by slow (low-energy) neutrons. Such species of atoms are called fissile. The most prominently utilized fissile nuclides in the nuclear industry are uranium-233 (233U), uranium-235 (235U), plutonium-239 (239Pu), and plutonium-241 (241Pu). Of these, only uranium-235 occurs in a usable amount in nature—though its presence in natural uranium is only some 0.7204 percent by weight, necessitating a lengthy and expensive enrichment process to generate a usable reactor fuel (see below Nuclear fuel cycle).
As an alternative to processing and enriching uranium-235, it is possible to go through the process of generating quantities of other fissile nuclides that are not as prevalent as uranium-235. Prominent sources of these nuclides are thorium-232 (232Th), uranium-238 (238U), and plutonium-240 (240Pu), which are known as fertile materials owing to their ability to transform into fissile materials. For example, thorium-232, the predominant isotope of natural thorium, can be used to generate uranium-233 through a process known as neutron capture. When a nucleus of thorium-232 absorbs, or “captures,” a neutron, it becomes thorium-233, whose half-life is approximately 21.83 minutes. After that time the nuclide decays through electron emission to protactinium-233, whose half-life is 26.967 days. The protactinium-233 nuclide in turn decays through electron emission to yield uranium-233.
Neutron capture may also be used to create quantities of plutonium-239 from uranium-238, the principal constituent of naturally occurring uranium. Absorption of a neutron in the uranium-238 nucleus yields uranium-239, which decays after 23.47 minutes through electron emission into neptunium-239 and ultimately, after 2.356 days, into plutonium-239.
If desired, plutonium-241 may be generated directly through neutron capture in plutonium-240, following the formula 240Pu + 1n = 241Pu.
A power reactor contains both fissile and fertile materials. The fertile materials partially replace fissile materials that are destroyed by fission, thus permitting the reactor to run longer before the amount of fissile material decreases to the point where criticality is no longer manageable. Plutonium-240 is particularly found to build up in reactors after long periods of operation, as it has a longer half-life than all its parent nuclides.
Heat removal
A significant portion of the energy of fission is converted to heat the instant that the fission reaction breaks the initial target nucleus into fission fragments. The bulk of this energy is deposited in the fuel, and a coolant is required to remove the heat in order to maintain a balanced system (and also to transfer the heat energy to the power-generating plant). The most common coolant is water, though any fluid can be used. Heavy water (deuterium oxide), air, carbon dioxide, helium, liquid sodium, sodium-potassium alloy (called NaK), molten salts, and hydrocarbons have all been used in reactors or reactor experiments.
Some research reactors are operated at very low power and have no need for a dedicated cooling system; in such units the small amount of generated heat is removed by conduction and convection to the environment. Very high power reactors, on the other hand, must have extremely sophisticated cooling systems to remove heat quickly and reliably; otherwise, the heat will build up in the reactor fuel and melt it. Indeed, most reactors operate on the principle that their fuel cannot be allowed to melt; therefore, the systems designed to cool the fuel must operate sufficiently under both normal and abnormal conditions. Systems that enable sufficient cooling during all credible abnormal conditions in nuclear power plants are referred to as emergency core-cooling systems.
Shielding
An operating reactor is a powerful source of radiation, since fission and subsequent radioactive decay produce neutrons and gamma rays, both of which are highly penetrating radiations. A reactor must have specifically designed shielding around it to absorb and reflect this radiation in order to protect technicians and other reactor personnel from exposure. In a popular class of research reactors known as “swimming pools,” this shielding is provided by placing the reactor in a large, deep pool of water. In other kinds of reactors, the shield consists of a thick concrete structure around the reactor system referred to as the biological shield. The shield also may contain heavy metals, such as lead or steel, for more effective absorption of gamma rays, and heavy aggregates may be used in the concrete itself for the same purpose.
Critical concentration and size
Not every arrangement of material containing fissile fuel can be brought to criticality. Even if a reactor was designed such that no neutrons could leak out, a critical concentration of fissile material would have to be present in order to bring the reactor to a critical state. Otherwise, absorption of neutrons by other constituents of the reactor might dominate and inhibit a sustained chain fission reaction. Similarly, even where there is a high-enough concentration for criticality, the reactor must occupy an appropriate volume and be of a prescribed geometric form, or else more neutrons will leak out than are created through fission. This requirement imposes a limit on the minimum critical volume and critical mass within a reactor.
Although the only useful fissile material in nature, uranium-235, is found in natural uranium, there are only a few combinations and arrangements of this and other materials that enable a reactor to maintain a critical state for a period of time. To increase the range of feasible reactor designs, enriched uranium is often used. Most of today’s power reactors employ enriched uranium fuel in which the percentage of uranium-235 has been increased to between 3 and 5 percent, approximately five and a half times the concentration in natural uranium. Large plants for enriching uranium exist in several countries; indeed, enrichment has become a commercial enterprise.
Thermal, intermediate, and fast reactors
Reactors are conveniently classified according to the typical energies of the neutrons that cause fission. Neutrons emanating in fission are very energetic; their average energy is around two million electron volts (MeV), nearly 80 million times the energy of atoms in ordinary matter at room temperature. As neutrons scatter or collide with nuclei in a reactor, they lose energy. This action is referred to as down-scattering. The choice of reactor materials and of fissile material concentrations determines the rate at which neutrons are slowed through down-scattering before causing fission.
In a thermal reactor, most neutrons down-scatter in the moderator material before interacting with a fissile material. Down-scattering events take place until the neutrons have reached thermal equilibrium with the reactor at energies of a few hundredths of an electron volt. Neutrons lose energy most efficiently by colliding with light atoms such as hydrogen (mass 1), deuterium (mass 2), beryllium (mass 9), and carbon (mass 12). For this reason, materials that contain atoms of these elements—water, heavy water, beryllium metal and oxide, and graphite—are deliberately incorporated into a thermal reactor and are known as moderators. Since water and heavy water also can function as coolants, they perform a dual purpose in thermal reactors. (See below Coolants and moderators.)
One disadvantage of thermal reactors is that at low energies uranium-235 and plutonium-239 not only can be fissioned by thermal (or slow) neutrons but also can capture neutrons without undergoing fission. Neutron capture transforms these nuclides into, respectively, uranium-236 and plutonium-240, which are not fissile. The probability of neutron capture is much lower at higher energy levels than at thermal energies. To achieve higher energy levels and promote fission over neutron capture, a reactor can be built to operate without a moderator. Depending on the number of scattering events that take place with heavier atoms before fission occurs, the typical fission-causing neutrons may have energies in the range of 0.5 electron volt to thousands of electron volts (intermediate reactors) or several hundred thousand electron volts (fast reactors). Such reactors require higher concentrations of fissile material to reach criticality than do reactor designs that operate at thermal energy levels; however, they are more efficient at converting fertile material to fissile material. Fast reactors can be designed to produce more than one new fissile atom for each fissile atom destroyed. Such reactors are referred to as breeder reactors. Breeder reactors may become important if world demand for nuclear power turns out to be long-term and if access to naturally available sources of fissile material becomes limited.
Reactor design and components
There are a large number of ways in which a nuclear reactor may be designed and constructed; many types have been experimentally realized. Over the years, nuclear engineers have developed reactors with solid and liquid fuels, thick- and no-reflectors, forced cooling circuits and natural conduction or convection heat-removal systems, and so on. Most reactors, however, have certain basic components.
Core
All reactors have a core, a central region that contains the fuel, fuel cladding, coolant, and (where separate from the latter) moderator. The fission energy in a nuclear reactor is produced in the core.
The fuel is usually heterogeneous—i.e., it consists of elements containing fissile material along with a diluent. This diluting agent may be fertile material or simply material that has good mechanical and chemical properties and does not readily absorb neutrons. All diluents act as a matrix in which the fissile material can stably reside through its operable life. In solid fuels, the diluted fissile material is enclosed in a cladding—a substance that isolates the fuel from the coolant and minimizes the likelihood that radioactive fission products will be released. Cladding is often referred to as a reactor’s first fission product barrier, as it is the first barrier that fissile material contacts after nuclear fission.
Fuel types
A reactor’s fuel must conform to the integral design of the reactor as well as the mechanisms that drive its operations.
The light-water reactor (LWR), which is the most widely used variety for commercial power generation in the world, employs a fuel consisting of pellets of sintered uranium dioxide loaded into cladding tubes of zirconium alloy or some other advanced cladding material. The tubes, called pins or rods, measure approximately 1 cm (less than half an inch) in diameter and roughly 3 to 4 metres (10 to 13 feet) in length. The tubes are bundled together into a fuel assembly, with the pins arranged in a square lattice. The uranium used in the fuel is 3 to 5 percent enriched. Since light (ordinary) water, used in LWRs as both the coolant and the moderator, tends to absorb more neutrons than other moderators do, such enrichment is crucial.
The CANDU (Canada Deuterium Uranium) reactor, which is the principal type of heavy-water reactor, uses natural uranium compacted into pellets. These pellets are inserted in long tubes and arranged in a lattice. A CANDU reactor fuel assembly measures approximately 1 metre (almost 40 inches) in length. Several assemblies are arranged end-to-end within a channel inside the reactor core. The use of heavy water rather than light water as the moderator enhances the scattering of neutrons rather than their capture, thereby increasing the probability of fission with the fuel material.
In one version of the high-temperature graphite reactor, the fuel is constructed of small spherical particles, or microspheres, containing uranium dioxide at the centre with concentric shells of carbon, silicon carbide, and carbon around them. These shells serve as localized cladding for each fuel sphere. The particles are then mixed with graphite and encased in a macroscopic graphite cladding.
In a sodium-cooled fast reactor, commonly called a liquid-metal reactor (LMR), the fuel consists of uranium dioxide or uranium-plutonium dioxide pellets (French design) or of uranium-plutonium-zirconium metal alloy pins (U.S. design) in steel cladding.
The most common type of fuel used in research reactors consists of plates of a uranium-aluminum alloy with an aluminum cladding. The uranium is enriched to slightly less than 20 percent, while silicon and aluminum are included in the “meat” of the plate to serve as the diluent and fuel matrix. Although aluminum has a lower melting point than other cladding materials, the flat plate design maintains a low fuel temperature, as the plates are often barely more than 1.25 mm thick. A common variety of research reactor known as TRIGA (from training, research, and isotope-production reactors–General Atomic) employs a fuel of mixed uranium and zirconium hydride, often doped with small concentrations of erbium and the whole clad in stainless steel.
Coolants and moderators
A variety of substances, including light water, heavy water, air, carbon dioxide, helium, liquid sodium, liquid sodium-potassium alloy, and hydrocarbons (oils), have been used as coolants. Such substances are, in general, good conductors of heat, and they serve to carry the thermal energy produced by fission from the fuel and through the integral system, finally either venting the heat directly to the atmosphere (in the case of research reactors) or transporting it to the steam-generating equipment of the nuclear power plant (in the case of power reactors).
In many cases, the same substance functions as both coolant and moderator, as in the case of light and heavy water. The moderator slows the fast (high-energy) neutrons emitted during fission to energies at which they are more likely to induce fission. In doing so, the moderator helps initiate and sustain a fission chain reaction.
Reflectors
A reflector is a region of unfueled material surrounding the core. Its function is to scatter neutrons that leak from the core, thereby returning some of them back into the core. This design feature allows for a smaller core size. In addition, reflectors “smooth out” the power density by utilizing neutrons that would otherwise leak out through fissioning within fuel material located near the core’s outer region.
The reflector is particularly important in research reactors, since it is the region in which much of the experimental apparatus is located. In some research reactor designs, reflectors are located inside the core as central islands in which high neutron intensities can be achieved for experimental purposes.
In most types of power reactors, a reflector is less important; this is due to the reactor’s large size, which reduces the proportion of neutrons that may leak from the core region. The liquid-metal reactor represents a special case. Most sodium-cooled reactors are deliberately built to allow a large fraction of their neutrons—those not needed to maintain the chain reaction—to leak from the core. These neutrons are valuable because they can produce new fissile material if they are absorbed by fertile material. Thus, fertile material—generally depleted uranium or its dioxide—is placed around the core to catch the leaking neutrons. Such an absorbing reflector is referred to as a blanket or a breeding blanket.
Reactor control elements
All reactors need unique elements for control. Although control can be achieved by varying parameters within the coolant circuit or by varying the amount of absorber dissolved in the coolant or moderator, by far the most common method utilizes absorbing assemblies—namely, control rods or, in some cases, blades. Typically a reactor is equipped with three types of rods for different purposes: (1) safety rods for starting up and shutting down the reactor, (2) regulating rods for adjusting the reactor’s power rate, and (3) shim rods for compensating for changes in reactivity as fuel is depleted by fission and neutron capture.
The most important function of the safety rods is to shut down the reactor, either when such a shutdown is scheduled or in case of a real or suspected emergency. These rods contain enough absorber to terminate a chain reaction under any conceivable condition. They are withdrawn before fuel is loaded and remain available in case a loading error requires their action. After the fuel is loaded, the rods are inserted, to be withdrawn again when the reactor is ready for operation. The mechanism by which they are moved is designed to be fail-safe in the sense that if there is a mechanical failure, the safety rods will fall by gravity or some other safe means into the core. In some cases, moreover, the safety rods have an automatic feature, such as a fuse, which releases them by virtue of physical effects independent of electronic signals.
Regulating rods are deliberately designed to affect reactivity only by a small degree. It is assumed that at some time the rods might be totally withdrawn by mistake, and the idea is to keep the added reactivity in such cases well within sensible limits. A well-designed regulating rod will add so little reactivity when it is removed that the delayed neutrons (see above Reactor control) will continue to control the rate of power increase.
Shim rods are designed to compensate for the effects of burnup (i.e., energy production). Reactivity changes resulting from burnup can be large, but they occur slowly over periods of days to years, as compared with the seconds-to-minutes range over which safety actions and routine regulation take place. Therefore, shim rods may control a significant amount of reactivity, but they will work optimally only when constraints are imposed on their speed of movement. A common way in which shims are operated is by inserting or removing them as regulating rods reach the end of their most useful position range. When this happens, shim rods are moved so that the regulating rods can be reset.
The functions of shim and safety rods are sometimes combined in rods that have low rates of withdrawal but that can be rapidly inserted. This is usually done when the effect of burnup decreases reactivity. The rods are only partially inserted at the outset of operation. However, in the event that the system must be shut down as quickly as possible, the reactor operator may “scram” the reactor, fully dropping the control rods into the core and immediately sending the reactor into a subcritical state. (The expression “scram” is said to stand for “safety control rod axe man,” a reference to ad-hoc emergency preparations made for the earliest nuclear reactor.)
The amount of shim control required can be reduced by the use of a burnable “poison.” This is a neutron-absorbing material, such as boron or gadolinium, that burns off faster than the fissile material does over the lifetime of the core. At the beginning of operation, the inclusion of a burnable poison regulates the extra reactivity that has been built into the fuel to compensate for the amount of fuel consumed. At the end of an operating period, the absorbing material is often completely transformed through neutron capture.
Some boiling-water reactors utilize cruciform (T-shaped) control blades as the neutron-absorbing control mechanism. Because a number of these reactor vessels are designed with internal components above the core region, the control blades are inserted from below the core. Control blades operate on the same principle as control rods. However, since they are inserted upward into the core, they cannot use gravity to fall into place and put the reactor into a subcritical state in the event of a loss of power or some other abnormal condition. For this reason, control blades are connected to hydraulic drives that force compressed air into the mechanism upon initiation, injecting the control blades into the core.
Structural components
The structural components of a reactor hold the system together and permit it to function as a useful energy source. The most important structural component in a nuclear power plant is usually the reactor vessel. In both the light-water reactor and the high-temperature gas-controlled reactor (HTGR), a reactor pressure vessel (RPV) is utilized so that the coolant is contained and operated under conditions appropriate for power generation—namely, elevated temperature and pressure. Within the reactor vessel are a number of structural elements: grids for holding the reactor core and solid reflectors, control-rod guide tubes, internal thermal hydraulic components (e.g., pumps or steam circulators) in some cases, instrument tubes, and components of safety systems.
Coolant system
The function of a power reactor installation is to extract as much heat of nuclear fission as possible and convert it to useful power, generally electricity. The coolant system plays a pivotal role in performing this function. A coolant fluid enters the core at low temperature and exits at a higher temperature after collecting the fission energy. This higher-temperature fluid is then directed to conventional thermodynamic components where the heat is converted into electric power. In most light-water, heavy-water, and gas-cooled power reactors, the coolant is maintained at high pressure. Sodium and organic coolants operate at atmospheric pressure.
Research reactors have very simple heat-removal systems, as their primary purpose is to perform research and not generate power. In research reactors, coolant is run through the reactor, and the heat that is removed is transferred to ambient air or to water without going through a power cycle. In research reactors of the lowest power, running at only a few kilowatts, this may involve simple heat exchange to tap water or to a pool of water cooled by ambient air. During operation at higher power levels, the heat is usually removed by means of a small natural-draft cooling tower.
Containment system
Reactors are designed with the expectation that they will operate safely without releasing radioactivity to their surroundings. It is, however, recognized that accidents can occur. An approach using multiple fission product barriers has been adopted to deal with such accidents. These barriers are, successively, the fuel cladding, the reactor vessel, and the shielding. As a final barrier, the reactor is housed in a containment structure, often simply referred to as the containment.
Containment design principles
The containment basically consists of the reactor building, which is designed and tested to prevent elevated levels of radioactivity that might be released from the fuel cladding, the reactor vessel, and the shielding from escaping to the environment. To meet this purpose, the containment structure must be at least nominally airtight. In practice, it must be able to maintain its integrity under circumstances of a drastic nature, such as accidents in which most of the contents of the reactor core are released to the building. It has to withstand pressure buildups and damage from debris propelled by an energy burst within the reactor, and it must pass appropriate tests to demonstrate that it will not leak more than a small fraction of its contents over a period of several days, even when its internal pressure is well above that of the surrounding air. The containment building also must protect components located inside it from external forces such as tsunamis, tornadoes, and airplane crashes.
The most common form of containment building is a cylindrical structure with a spherical dome, which is characteristic of LWR systems. This structure is much more typical of nuclear plants than the large cooling tower that is often used as a symbol for nuclear power. (It should be noted that cooling towers are found at large modern coal- and oil-fired power stations as well.)
Reactors other than those of the LWR type also have containment structures, though they vary in shape and construction. When it can be justified that major pressure buildups are not to be expected, the containment may be any functional form of airtight structure. In the United States and a number of other countries around the world, containment structures are required for all commercial power reactors and all high-power research reactors. In general, low-power research reactors are exempt, on the basis of the common assumption that an accident in such systems will not lead to a widespread release of radioactivity. In the United States, reactors operated by the Department of Energy and by the armed services are also exempt, a matter that has caused considerable controversy. Some of these have containment structures, whereas others do not.
Containment systems and major nuclear accidents
The concept of the containment originated in the United States during the 1950s and was generally accepted throughout much of the world. The Soviet-bloc countries, however, did not concur with this view, and when containment was added to Soviet reactor designs, it was generally not up to Western standards. For example, during the Chernobyl accident of 1986 in Ukraine, the power station’s Unit 4, which suffered a catastrophic explosive accident and fire, had an internal structure that could withstand the loss of function of only a single pressure tube. Though the structure was called a containment, this was a misnomer by Western standards, and the structure would more suitably be referred to as a confinement.
Severe tests of Western-style containment systems occurred during the Three Mile Island accident in the United States in 1979 and the Fukushima accident in Japan in 2011. At Three Mile Island Unit 2, near Harrisburg, Pennsylvania, a stoppage of core cooling resulted in the destruction, including partial melting, of the entire core and the release of a large part of its radioactivity to the enclosure around the reactor—that is, the containment. In spite of a hydrogen deflagration that also occurred during the accident, the containment structure prevented all but a very small amount of radioactivity from entering the environment and is credited with having prevented a major radioactive release and its consequences.
At the Fukushima Daiichi (“Number One”) plant in northeastern Honshu, Japan, a loss of main and backup power after an earthquake and tsunami led to a partial meltdown of fuel rods in three reactors. Melted material bored small holes in the lower head of two reactor pressure vessels; one of these was punctured again by an explosion. Radioactive water was soon discovered to have leaked into the ocean through cracks in the foundation of the containment. Within a few weeks, the cracks had been sealed, and within six months the reactors had been stabilized to the point where workers could begin to enter the containment. Despite a catastrophic sequence of natural events that led to the accident, the fission product barriers served their design purpose and kept all but a small amount of fission products from entering the environment.
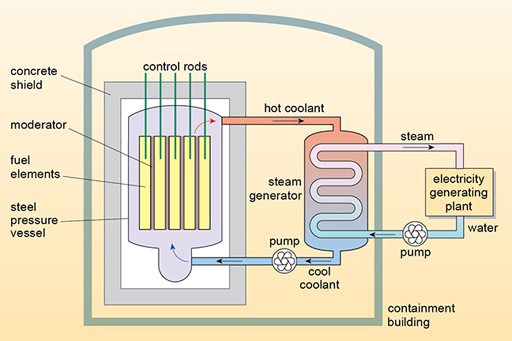
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1325 2022-03-23 14:16:23
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,620
Re: Miscellany
1299) Closed-circuit television
Summary
What is CCTV?
CCTV (closed-circuit television) is a TV system in which signals are not publicly distributed but are monitored, primarily for surveillance and security purposes.
How does CCTV work?
CCTV relies on strategic placement of cameras, and observation of the camera's input on monitors somewhere. Because the cameras communicate with monitors and/or video recorders across private coaxial cable runs or wireless communication links, they gain the designation "closed-circuit" to indicate that access to their content is limited by design only to those able to see it.
CCTV use cases
Older CCTV systems used small, low-resolution black and white monitors with no interactive capabilities. Modern CCTV displays can be color, high-resolution displays and can include the ability to zoom in on an image or track something (or someone) among their features. Talk CCTV allows an overseer to speak to people within range of the camera's associated speakers.
CCTV is commonly used for a variety of purposes, including:
* Maintaining perimeter security in medium- to high-secure areas and installations.
* Observing behavior of incarcerated inmates and potentially dangerous patients in medical facilities.
* Traffic monitoring.
* Overseeing locations that would be hazardous to a human, for example, highly radioactive or toxic industrial environments.
* Building and grounds security.
* Obtaining a visual record of activities in situations where it is necessary to maintain proper security or access controls (for example, in a diamond cutting or sorting operation; in banks, casinos, or airports).
CCTV is finding increasing use in law-enforcement, for everything from traffic observation (and automated ticketing) to observation of high-crime areas or neighborhoods. Such use of CCTV technology has fueled privacy concerns in many parts of the world, particularly in those areas in the UK and Europe where it has become a routine part of police procedure.
Details
Closed-circuit television (CCTV), also known as video surveillance, is the use of video cameras to transmit a signal to a specific place, on a limited set of monitors. It differs from broadcast television in that the signal is not openly transmitted, though it may employ point-to-point (P2P), point-to-multipoint (P2MP), or mesh wired or wireless links. Even though almost all video cameras fit this definition, the term is most often applied to those used for surveillance in areas that require additional security or ongoing monitoring (Videotelephony is seldom called "CCTV").
Surveillance of the public using CCTV is common in many areas around the world. In recent years, the use of body worn video cameras has been introduced as a new form of surveillance, often used in law enforcement, with cameras located on a police officer's chest or head. Video surveillance has generated significant debate about balancing its use with individuals' right to privacy even when in public.
In industrial plants, CCTV equipment may be used to observe parts of a process from a central control room. For example, when the environment is not suitable for humans. CCTV systems may operate continuously or only as required to monitor a particular event. A more advanced form of CCTV, using digital video recorders (DVRs), provides recording for possibly many years, with a variety of quality and performance options and extra features (such as motion detection and email alerts). More recently, decentralized IP cameras, perhaps equipped with megapixel sensors, support recording directly to network-attached storage devices, or internal flash for completely stand-alone operation.
By one estimate, there will be approximately 1 billion surveillance cameras in use worldwide by 2021.[9][needs update] About 65% of these cameras are installed in Asia. The growth of CCTV has been slowing in recent years. The deployment of this technology has facilitated significant growth in state surveillance, a substantial rise in the methods of advanced social monitoring and control, and a host of crime prevention measures throughout the world.
History
An early mechanical CCTV system was developed in June 1927 by Russian physicist Léon Theremin (cf. Television in the Soviet Union). Originally requested by the Soviet of Labor and Defense, the system consisted of a manually-operated scanning-transmitting camera and wireless shortwave transmitter and receiver, with a resolution of a hundred lines. Having been commandeered by Kliment Voroshilov, Theremin's CCTV system was demonstrated to Joseph Stalin, Semyon Budyonny, and Sergo Ordzhonikidze, and subsequently installed in the courtyard of the Moscow Kremlin to monitor approaching visitors.
Another early CCTV system was installed by Siemens AG at Test Stand VII in Peenemünde, Nazi Germany in 1942, for observing the launch of V-2 rockets.
In the U.S. the first commercial closed-circuit television system became available in 1949, called Vericon. Very little is known about Vericon except it was advertised as not requiring a government permit.
Technology
The earliest video surveillance systems involved constant monitoring because there was no way to record and store information. The development of reel-to-reel media enabled the recording of surveillance footage. These systems required magnetic tapes to be changed manually, which was a time-consuming, expensive and unreliable process, with the operator having to manually thread the tape from the tape reel through the recorder onto an take-up reel. Due to these shortcomings, video surveillance was not widespread. VCR technology became available in the 1970s, making it easier to record and erase information, and the use of video surveillance became more common.
During the 1990s, digital multiplexing was developed, allowing several cameras to record at once, as well as time lapse and motion-only recording. This saved time and money which then led to an increase in the use of CCTV.
Recently CCTV technology has been enhanced with a shift toward Internet-based products and systems, and other technological developments.
Application
Closed-circuit television was used as a form of pay-per-view theatre television for sports such as professional boxing and professional wrestling, and from 1964 through 1970, the Indianapolis 500 automobile race. Boxing telecasts were broadcast live to a select number of venues, mostly theaters, where viewers paid for tickets to watch the fight live. The first fight with a closed-circuit telecast was Joe Louis vs. Joe Walcott in 1948. Closed-circuit telecasts peaked in popularity with Muhammad Ali in the 1960s and 1970s, with "The Rumble in the Jungle" fight drawing 50 million CCTV viewers worldwide in 1974, and the "Thrilla in Manila" drawing 100 million CCTV viewers worldwide in 1975. In 1985, the WrestleMania I professional wrestling show was seen by over one million viewers with this scheme. As late as 1996, the Julio César Chávez vs. Oscar De La Hoya boxing fight had 750,000 viewers. Although closed-circuit television was gradually replaced by pay-per-view home cable television in the 1980s and 1990s, it is still in use today for most awards shows and other events that are transmitted live to most venues but do not air as such on network television, and later re-edited for broadcast.
Marie Van Brittan Brown first pioneered and patented a CCTV home security system, much of the technology of which is still used in home security systems today (U.S. Patent 3,482,037).
In September 1968, Olean, New York was the first city in the United States to install video cameras along its main business street in an effort to fight crime. Another early appearance was in 1973 in Times Square in New York City. The NYPD installed it to deter crime in the area; however, crime rates did not appear to drop much due to the cameras. Nevertheless, during the 1980s video surveillance began to spread across the country specifically targeting public areas. It was seen as a cheaper way to deter crime compared to increasing the size of the police departments. Some businesses as well, especially those that were prone to theft, began to use video surveillance. From the mid-1990s on, police departments across the country installed an increasing number of cameras in various public spaces including housing projects, schools and public parks departments. CCTV later became common in banks and stores to discourage theft, by recording evidence of criminal activity. In 1997, 3,100 CCTV systems were installed in public housing and residential areas in New York City.
Experiments in the UK during the 1970s and 1980s, including outdoor CCTV in Bournemouth in 1985, led to several larger trial programs later that decade. The first use by local government was in King's Lynn, Norfolk, in 1987.
Uses:
Crime prevention
The two-year-old James Bulger being led away by his killers, recorded on shopping centre CCTV in 1993. This narrow-bandwidth television system had a low frame rate.
Sign warning that premises are watched by CCTV cameras.
A 2009 systematic review by researchers from Northeastern University and University of Cambridge used meta-analytic techniques to pool the average effect of CCTV on crime across 41 different studies.
The studies included in the meta-analysis used quasi-experimental evaluation designs that involve before-and-after measures of crime in experimental and control areas. However, several researchers have pointed to methodological problems associated with this research literature. First, researchers have argued that the British car park studies included in the meta-analysis cannot accurately control for the fact that CCTV was introduced simultaneously with a range of other security-related measures. Second, some have noted that, in many of the studies, there may be issues with selection bias since the introduction of CCTV was potentially endogenous to previous crime trends. In particular, the estimated effects may be biased if CCTV is introduced in response to crime trends.
It has been argued that problems of selection bias and endogeneity can be addressed by stronger research designs such as randomized controlled trials and natural experiments. A 2017 review published in Journal of Scandinavian Studies in Criminology and Crime Prevention compiles seven studies that use such research designs. The studies included in the review found that CCTV reduced crime by 24-28% in public streets and urban subway stations. It also found that CCTV could decrease unruly behaviour in football stadiums and theft in supermarkets/mass merchant stores. However, there was no evidence of CCTV having desirable effects in parking facilities or suburban subway stations. Furthermore, the review indicates that CCTV is more effective in preventing property crimes than in violent crimes.
Another question in the effectiveness of CCTV for policing is around uptime of the system; in 2013 City of Philadelphia Auditor found that the $15M system was operational only 32% of the time. There is strong anecdotal evidence that CCTV aids in detection and conviction of offenders; for example, UK police forces routinely seek CCTV recordings after crimes. Moreover, CCTV has played a crucial role in tracing the movements of suspects or victims and is widely regarded by anti-terrorist officers as a fundamental tool in tracking terrorist suspects. Large-scale CCTV installations have played a key part of the defenses against terrorism since the 1970s. Cameras have also been installed on public transport in the hope of deterring crime.
A more open question is whether most CCTV is cost-effective. While low-quality domestic kits are cheap, the professional installation and maintenance of high definition CCTV is expensive. Gill and Spriggs did a Cost-effectiveness analysis (CEA) of CCTV in crime prevention that showed little monetary saving with the installation of CCTV as most of the crimes prevented resulted in little monetary loss. Critics however noted that benefits of non-monetary value cannot be captured in a traditional Cost Effectiveness Analysis and were omitted from their study. A 2008 Report by UK Police Chiefs concluded that only 3% of crimes were solved by CCTV. In London, a Metropolitan Police report showed that in 2008 only one crime was solved per 1000 cameras. In some cases CCTV cameras have become a target of attacks themselves.
Cities such as Manchester in the UK are using DVR-based technology to improve accessibility for crime prevention.
In October 2009, an "Internet Eyes" website was announced which would pay members of the public to view CCTV camera images from their homes and report any crimes they witnessed. The site aimed to add "more eyes" to cameras which might be insufficiently monitored. Civil liberties campaigners criticized the idea as "a distasteful and a worrying development".
In 2013 Oaxaca hired deaf police officers to lip read conversations to uncover criminal conspiracies.
In Singapore, since 2012, thousands of CCTV cameras have helped deter loan sharks, nab litterbugs and stop illegal parking, according to government figures.
Crime solving
CCTV can also be used to help solve crimes. In London alone, six crimes are solved each day through CCTV footage.
Body worn
In recent years, the use of body worn video cameras has been introduced for a number of uses. For example, as a new form of surveillance in law enforcement, with cameras located on a police officer's chest or head.
Traffic flow monitoring:
Vehicle traffic
Many cities and motorway networks have extensive traffic-monitoring systems, using closed-circuit television to detect congestion and notice accidents. Many of these cameras however, are owned by private companies and transmit data to drivers' GPS systems.
Highways England has a publicly owned CCTV network of over 3000 Pan-Tilt-Zoom cameras covering the British motorway and trunk road network. These cameras are primarily used to monitor traffic conditions and are not used as speed cameras. With the addition of fixed cameras for the active traffic management system, the number of cameras on the Highways England's CCTV network is likely to increase significantly over the next few years.
The London congestion charge is enforced by cameras positioned at the boundaries of and inside the congestion charge zone, which automatically read the number plates of vehicles that enter the zone. If the driver does not pay the charge then a fine will be imposed. Similar systems are being developed as a means of locating cars reported stolen.
Other surveillance cameras serve as traffic enforcement cameras.
Pedestrian traffic
In Mecca, CCTV cameras are used for monitoring (and thus managing) the flow of crowds.
In the Philippines, barangay San Antonio used CCTV cameras and artificial intelligence software to detect the formation of crowds during an outbreak of a disease. Security personnel were sent whenever a crowd formed at a particular location in the city.
Increasing safety and security in public transport:
Digital Video Recorder for Public Transport
On a driver-only operated train CCTV cameras may allow the driver to confirm that people are clear of doors before closing them and starting the train.
A trial by RET in 2011 with facial recognition cameras mounted on trams made sure that people who were banned from them did not sneak on anyway.
Sporting events
Many sporting events in the United States use CCTV inside the venue, either to display on the stadium or arena's scoreboard, or in the concourse or restroom areas to allow fans to view action outside the seating bowl. The cameras send the feed to a central control center where a producer selects feeds to send to the television monitors that fans can view. CCTV monitors for viewing the event by attendees are often placed in lounges, hallways, and restrooms. In a trial with CCTV cameras, football club fans no longer needed to identify themselves manually, but could pass freely after being authorized by the facial recognition system.
Employee monitoring
Organizations use CCTV to monitor the actions of workers. Every action is recorded as an information block with subtitles that explain the performed operation. This helps to track the actions of workers, especially when they are making critical financial transactions, such as correcting or cancelling of a sale, withdrawing money or altering personal information.
Actions which an employer may wish to monitor could include:
* Scanning of goods, selection of goods, introduction of price and quantity;
* Input and output of operators in the system when entering passwords;
* Deleting operations and modifying existing documents;
* Implementation of certain operations, such as financial statements or operations with cash;
* Moving goods, revaluation scrapping and counting;
* Control in the kitchen of fast food restaurants;
* Change of settings, reports and other official functions.
Each of these operations is transmitted with a description, allowing detailed monitoring of all actions of the operator. Some systems allow the user to search for a specific event by time of occurrence and text description, and perform statistical evaluation of operator behaviour. This allows the software to predict deviations from the standard workflow and record only anomalous behaviour.
Use in schools
In the United States, Britain, Canada, Australia and New Zealand, CCTV is widely used in schools due to its success in preventing bullying, vandalism, monitoring visitors and maintaining a record of evidence of a crime. There are some restrictions, cameras not being installed in areas where there is a "reasonable expectation of privacy", such as bathrooms, gym locker areas and private offices (unless consent by the office occupant is given). Cameras are generally acceptable in hallways, parking lots, front offices where students, employees, and parents come and go, gymnasiums, cafeterias, supply rooms and classrooms. Some teachers object to the installation of cameras.
A study of high school students in Israeli schools shows that students' views on CCTV used in school are based on how they think of their teachers, school, and authorities. It also stated that most students do not want CCTV installed inside a classroom.
Use in private homes
Many homeowners choose to install CCTV systems either inside or outside their own homes, sometimes both. CCTV cameras are an effective deterrent to potential intruders as their use increases the risk of identification through the camera footage. If someone scouts through an affluent suburb seeking the easiest house to break into, having an obvious CCTV system, alarm or another security measure, makes the house appear to be a more difficult target so they will likely move on to the next house.
Modern CCTV systems can be monitored through mobile phone apps which allows people to view live footage of their house from anywhere they have internet coverage. Some systems provide motion detection so when movement is detected, an alert can be sent to a phone.
Criminal use
Criminals may use surveillance cameras to monitor the public. For example, a hidden camera at an ATM can capture people's PINs as they are entered, without their knowledge. The devices are small enough not to be noticed, and are placed where they can monitor the keypad of the machine as people enter their PINs. Images may be transmitted wirelessly to the criminal. Even lawful surveillance cameras sometimes have their data go into the hands of people who have no legal right to receive it.
Use in shopping malls & retail stores
Theft is a huge concern for many department stores and shopping malls. CCTV helps to protect stores' assets, and ensures the safety of employees and customers. This instills a secure, safe, and inviting experience for visitors.
It is even more important to choose the right camera. A CCTV system must have:
* A high resolution camera to ensure image clarity
* High-capacity digital storage to ensure 24/7 recording
* The right placement with good lighting

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
