Math Is Fun Forum
You are not logged in.
- Topics: Active | Unanswered
#1501 2022-09-13 14:19:30
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,194
Re: Miscellany
1474) Old age
Summary
Old age refers to ages nearing or surpassing the life expectancy of human beings, and is thus the end of the human life cycle. Terms and euphemisms for people at this age include old people, the elderly (worldwide usage), OAPs (British usage which stands for Old Age Pensioner), seniors, senior citizens (American usage), older adults (in the social sciences), and the elders (in many cultures).
Elderly people often have limited regenerative abilities and are more susceptible to disease, syndromes, injuries, and sickness than younger adults. A number of other disciplines and domains concern the aging and the aged, such as organic processes of aging (senescence), medical studies of the aging process (gerontology), diseases that afflict older adults (geriatrics), technology to support the aging society (gerontechnology), or leisure and sport activities adapted to older persons, such as senior sport. The elderly face various social issues concerning retirement, loneliness, and ageism.
Old age is not a definite biological stage, as the chronological age denoted as "old age" varies culturally and historically.
In 2011, the United Nations proposed a human rights convention that would specifically protect older people.
Details
Old age, also called senescence, in human beings, is the final stage of the normal life span. Definitions of old age are not consistent from the standpoints of biology, demography (conditions of mortality and morbidity), employment and retirement, and sociology. For statistical and public administrative purposes, however, old age is frequently defined as 60 or 65 years of age or older.
Old age has a dual definition. It is the last stage in the life processes of an individual, and it is an age group or generation comprising a segment of the oldest members of a population. The social aspects of old age are influenced by the relationship of the physiological effects of aging and the collective experiences and shared values of that generation to the particular organization of the society in which it exists.
There is no universally accepted age that is considered old among or within societies. Often discrepancies exist as to what age a society may consider old and what members in that society of that age and older may consider old. Moreover, biologists are not in agreement about the existence of an inherent biological cause for aging. However, in most contemporary Western countries, 60 or 65 is the age of eligibility for retirement and old-age social programs, although many countries and societies regard old age as occurring anywhere from the mid-40s to the 70s.
Social programs
State institutions to aid the elderly have existed in varying degrees since the time of the ancient Roman Empire. England in 1601 enacted the Poor Law, which recognized the state’s responsibility to the aged, although programs were carried out by local church parishes. An amendment to this law in 1834 instituted workhouses for the poor and aged, and in 1925 England introduced social insurance for the aged regulated by statistical evaluations. In 1940 programs for the aged came under England’s welfare state system.
In the 1880s Otto von Bismarck in Germany introduced old-age pensions whose model was followed by most other western European countries. Today more than 100 nations have some form of social security program for the aged. The United States was one of the last countries to institute such programs. Not until the Social Security Act of 1935 was formulated to relieve hardships caused by the Great Depression were the elderly granted old-age pensions. For the most part, these state programs, while alleviating some burdens of aging, still do not bring older people to a level of income comparable to that of younger people.
Physiological effects
The physiological effects of aging differ widely among individuals. However, chronic ailments, especially aches and pains, are more prevalent than acute ailments, requiring older people to spend more time and money on medical problems than younger people. The rising cost of medical care has caused a growing concern among older people and societies, in general resulting in constant reevaluation and reform of institutions and programs designed to aid the elderly with these expenses.
In ancient Rome and medieval Europe the average life span is estimated to have been between 20 and 30 years. Life expectancy today has expanded in historically unprecedented proportions, greatly increasing the numbers of people who survive over the age of 65. Therefore, the instances of medical problems associated with aging, such as certain kinds of cancer and heart disease, have increased, giving rise to greater consideration, both in research and in social programs, for accommodating this increase.
Certain aspects of sensory and perceptual skills, muscular strength, and certain kinds of memory tend to diminish with age, rendering older people unsuitable for some activities. There is, however, no conclusive evidence that intelligence deteriorates with age, but rather that it is more closely associated with education and standard of living. Sexual activity tends to decrease with age, but if an individual is healthy there is no age limit for its continuance.
Many of the myths surrounding the process of aging are being invalidated by increased studies in gerontology, but there still is not sufficient information to provide adequate conclusions.
Demographic and socioeconomic influences
In general the social status of an age group is related to its effective influence in its society, which is associated with that group’s function in productivity. In agrarian societies the elderly have a status of respectability. Their life experiences and knowledge are regarded as valuable, especially in preliterate societies where knowledge is orally transmitted. The range of activities in these societies allows the elderly to continue to be productive members of their communities.
In industrialized nations the status of the elderly has altered as the socioeconomic conditions have changed, tending to reduce the status of the elderly as a society becomes more technologically oriented. Since physical disability is less a factor in productive capability in industrialized countries, this reduction in social status is thought to have been generated by several interrelated factors: the numbers of still able-bodied older workers outstripping the number of available employment opportunities, the decline in self-employment which allows a worker to gradually decrease activity with age, and the continual introduction of new technology requiring special training and education.
Although in certain fields old age is still considered an asset, particularly in the political arena, older people are increasingly being forced into retirement before their productive years are over, causing problems in their psychological adaptations to old age. Retirement is not regarded unfavourably in all instances, but its economic limitations tend to further remove older people from the realm of influence and raise problems in the extended use of leisure time and housing. As a consequence, financial preparation for retirement has become an increased concern for individuals and society. For an essay on retirement, medical care, and other issues affecting the elderly, see John Kenneth Galbraith’s Notes on Aging, a Britannica sidebar by the distinguished economist, ambassador, and public servant.
Familial relationships tend to be the focus of the elderly’s attention. However, as the family structure in industrialized countries has changed in the past 100 years from a unit encompassing several generations living in close proximity to self-contained nuclear families of only parents and young children, older people have become isolated from younger people and each other. Studies have shown that as a person ages he or she prefers to remain in the same locale. However, the tendency for young people in industrialized countries to be highly mobile has forced older people to decide whether to move to keep up with their families or to remain in neighbourhoods which also change, altering their familiar patterns of activity. Although most older people do live within an hour from their closest child, industrialized societies are faced with formulating programs to accommodate increasing numbers of older people who function independently of their families.
A significant factor in the social aspects of old age concerns the values and education of the generation itself. In industrialized countries especially, where changes occur more rapidly than in agrarian societies, a generation born 65 years ago may find that the dominant mores, expectations, definitions of the quality of life, and roles of older people have changed considerably by the time it reaches old age. Formal education, which usually takes place in the early years and forms collective opinions and mores, tends to enhance the difficulties in adapting to old age. However, resistance to change, which is often associated with the elderly, is being shown to be less an inability to change than a trend in older people to regard life with a tolerant attitude. Apparent passivity may actually be a choice based on experience, which has taught older people to perceive certain aspects of life as unchangeable. Adult education programs are beginning to close the generation gap; however, as each successive generation reaches old age, bringing with it its particular biases and preferences, new problems arise requiring new social accommodations.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1502 2022-09-14 13:43:30
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,194
Re: Miscellany
1475) Crank (mechanism)
Summary
A crank is an arm attached at a right angle to a rotating shaft by which circular motion is imparted to or received from the shaft. When combined with a connecting rod, it can be used to convert circular motion into reciprocating motion, or vice versa. The arm may be a bent portion of the shaft, or a separate arm or disk attached to it. Attached to the end of the crank by a pivot is a rod, usually called a connecting rod (conrod).
The term often refers to a human-powered crank which is used to manually turn an axle, as in a bicycle crankset or a brace and bit drill. In this case a person's arm or leg serves as the connecting rod, applying reciprocating force to the crank. There is usually a bar perpendicular to the other end of the arm, often with a freely rotatable handle or pedal attached.
Details
Crank, in mechanics, is an arm secured at right angle to a shaft with which it can rotate or oscillate. Next to the wheel, the crank is the most important motion-transmitting device, since, with the connecting rod, it provides means for converting linear to rotary motion, and vice versa.
There are many conflicting claims concerning the origin of the crank, but it has been reasonably well established that the first recognizable crank appeared in China early in the 1st century AD. The first cranks had two right-angle bends and were hand-operated. The carpenter’s brace, invented about AD 1400 by a Flemish carpenter, may be considered the first complete crank, since it had four right-angle bends, with the arm and wrist of the operator forming the connecting rod.
The first mechanical connecting rods were used, it is said, on a treadle-operated machine in AD 1430. About this time flywheels were added to the rotating members to carry the members over the “dead” positions when the rod and the crank arm are lined up with each other (collinear).

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1503 2022-09-15 14:11:39
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,194
Re: Miscellany
1476) Hydrometer
Summary
A hydrometer or lactometer is an instrument used for measuring density or relative density of liquids based on the concept of buoyancy. They are typically calibrated and graduated with one or more scales such as specific gravity.
A hydrometer usually consists of a sealed hollow glass tube with a wider bottom portion for buoyancy, a ballast such as lead or mercury for stability, and a narrow stem with graduations for measuring. The liquid to test is poured into a tall container, often a graduated cylinder, and the hydrometer is gently lowered into the liquid until it floats freely. The point at which the surface of the liquid touches the stem of the hydrometer correlates to relative density. Hydrometers can contain any number of scales along the stem corresponding to properties correlating to the density.
Hydrometers are calibrated for different uses, such as a lactometer for measuring the density (creaminess) of milk, a saccharometer for measuring the density of sugar in a liquid, or an alcoholometer for measuring higher levels of alcohol in spirits.
The hydrometer makes use of Archimedes' principle: a solid suspended in a fluid is buoyed by a force equal to the weight of the fluid displaced by the submerged part of the suspended solid. The lower the density of the fluid, the deeper a hydrometer of a given weight sinks; the stem is calibrated to give a numerical reading.
Details
A hydrometer is an instrument used to determine specific gravity. It operates based on the Archimedes principle that a solid body displaces its own weight within a liquid in which it floats. Hydrometers can be divided into two general classes: liquids heavier than water and liquids lighter than water.
Hydrometer is a device for measuring some characteristics of a liquid, such as its density (weight per unit volume) or specific gravity (weight per unit volume compared with water). The device consists essentially of a weighted, sealed, long-necked glass bulb that is immersed in the liquid being measured; the depth of flotation gives an indication of liquid density, and the neck can be calibrated to read density, specific gravity, or some other related characteristic.
In practice, the floating glass bulb is usually inserted into a cylindrical glass tube equipped with a rubber ball at the top end for sucking liquid into the tube. Immersion depth of the bulb is calibrated to read the desired characteristic. A typical instrument is the storage-battery hydrometer, by means of which the specific gravity of the battery liquid can be measured and the condition of the battery determined. Another instrument is the radiator hydrometer, in which the float is calibrated in terms of the freezing point of the radiator solution. Others may be calibrated in terms of “proof ” of an alcohol solution or in terms of the percentage of sugar in a sugar solution.
The Baumé hydrometer, named for the French chemist Antoine Baumé, is calibrated to measure specific gravity on evenly spaced scales; one scale is for liquids heavier than water, and the other is for liquids lighter than water.
Additional Information
The gauge is a scientific tool, and it is used to measure the alcohol content present in the mash. The work of the device primarily measures liquid density in water relation. Distilling and brewing hydrometers make the two types of the hydrometers available. Their calibration is different, and that is why it is advisable to have both of them when making spirits. A brewing hydrometer should not be used when measuring the product to know the final proof; on the other hand, when making mash, you should not use spirit hydrometer. Here, we are going to talk about the hydrometers used for brewing, and the information is essential to brewers.
What Hydrometers Measure
The sugar amount present in the wash and mash is what mainly the brewing hydrometers measure. It will float depending on the amount of sugar that is in the mash. For instance, if it is high, the hydrometer will float higher. After fermentation takes place, the paste (the mash) becomes the wash and at this point, another reading is taken so as to know the amount of sugar that was turned into alcohol by the yeast. If the difference is big, then it means that the alcohol level in percentage is significant.
Many hydrometers have three scales and in this article, we are majorly looking at specific scale gravity.
* The Brix scale is in most times used to make wine.
* The Potential alcohol scale which estimates the alcohol in the mash
* The Specific Gravity scale which is useful for brewing
Original Gravity Determination
Determining original gravity is carried out so that the amount of sugar in the mash is known. Typically, this happens before fermentation takes place and before you add yeast to your mash. As we have already seen, the original gravity determines the amount of sugar that is contained in the pulp and gives an estimation of the amount of alcohol that is likely to be produced if the process does not go haywire.
There is a variation of the original gravity since it depends on the use of the recipe. Giving an example, corn whiskey which is popular among brewers starts at 1.055 and the production of wash and alcohol content ranges from 6% to 7.5%.
It is important that you take down the original gravity of the mash so that you can avoid forgetting. It is possible not to remember the reading by fermentation stage especially in a situation whereby you are carrying out many batches of fermentations at the same time. It is important to note that the first reading will not help you know the final alcohol content, and that is why another reading should be taken.
The original gravity reading is taken once the steps listed are completed.
* A beer sampler should be used and a jar filled with liquid to almost full.
* The wine hydrometer should then be dropped in a gentle manner into the pot. The hydrometer should be span in a way that it does not stick to the sides, and it will finally float.
* Take down the reading that you see on the barometer.
Determination of Final Gravity
The density of the liquid is measured by the final gravity like the original gravity. It should be noted that if the process continued without any problems, then the density of the liquid should be lower because the yeast ate the sugar present in the liquid during the process of fermentation. The alcohol content will be the difference between the original gravity and final gravity.
The steps given below are for the mash that has been fermented for one week. Meanwhile, if this is the case with your mash, the airlock seems to have fewer activities or even no activities going on. In case you notice there is a lot of bubbling then you should just wait since this means that there is work going on.
* Beer sampler should be used and a jar filled with liquid to almost full
* Slowly put the beer hydrometer into the jar and gently spin it ensuring that it does not stick to the far walls. It will float on the liquid.
* Take down the number seen on the hydrometer. The reading should be below 1.010, and if it exceeds this, then you should take your time and wait for some more days before taking another reading. It could be because the fermentation process is still going on. The readings should continuously be taken until it is constant and there is no change.
Reading the Hydrometer
The calibration of the hydrometer is at 60 degrees. There is the need to adjust it to actual reading in case you are taking a reading of your mash that is above 60 degrees. One can get help from online calculators so as to convert the readings taken either below or above 60 degrees.
Determination of Alcohol Content of Wash
So that you can be able to determine the alcohol concerning volume, then all you need is your original reading and final reading. Taking the example of what we did above the sample was 1.090 while it finally fermented to become 1.010. The math can simply be done as below;
Subtraction of Final Gravity from the original one
Multiplication of the difference by 131
1.090 minus 1.010 equals 0.08.
0.08 X 131= 10.48
The content of alcohol in the wash as seen in the above calculation is 10.48%
The Ideal Starting Alcohol Percentage For Wash
There are some instances where some recipes produce lower starting alcohol while some recipes produce higher starting alcohol. The combination of turbo yeast and a ton of sugar is what can lead to the production of starting alcohol percentage of even more than 20%. It is good to note that if you are after producing the best alcohol that has an excellent taste, the best recipes to use are those that have to start alcohol ranging from 5-8%. The alcohol produced will be around 10% or more of the content of alcohol.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1504 2022-09-16 14:28:49
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,194
Re: Miscellany
1477) Essential oil
Summary
An essential oil is a concentrated hydrophobic liquid containing volatile (easily evaporated at normal temperatures) chemical compounds from plants. Essential oils are also known as volatile oils, ethereal oils, aetheroleum, or simply as the oil of the plant from which they were extracted, such as oil of clove. An essential oil is essential in the sense that it contains the essence of the plant's fragrance—the characteristic fragrance of the plant from which it is derived. The term "essential" used here does not mean indispensable or usable by the human body, as with the terms essential amino acid or essential fatty acid, which are so called because they are nutritionally required by a living organism.
Essential oils are generally extracted by distillation, often by using steam. Other processes include expression, solvent extraction, sfumatura, absolute oil extraction, resin tapping, wax embedding, and cold pressing. They are used in perfumes, cosmetics, soaps, air fresheners and other products, for flavoring food and drink, and for adding scents to incense and household cleaning products.
Essential oils are often used for aromatherapy, a form of alternative medicine in which healing effects are ascribed to aromatic compounds. Aromatherapy may be useful to induce relaxation, but there is not sufficient evidence that essential oils can effectively treat any condition. Improper use of essential oils may cause harm including allergic reactions, inflammation and skin irritation. Children may be particularly susceptible to the toxic effects of improper use. Essential oils can be poisonous if ingested or absorbed through the skin.
Details
Essential oil is a highly volatile substance isolated by a physical process from an odoriferous plant of a single botanical species. The oil bears the name of the plant from which it is derived; for example, rose oil or peppermint oil. Such oils were called essential because they were thought to represent the very essence of odour and flavour.
Distillation is the most common method for isolation of essential oils, but other processes—including enfleurage (extraction by using fat), maceration, solvent extraction, and mechanical pressing—are used for certain products. Younger plants produce more oil than older ones, but old plants are richer in more resinous and darker oils because of the continuing evaporation of the lighter fractions of the oil.
Out of the vast number of plant species, essential oils have been well characterized and identified from only a few thousand plants. The oils are stored as microdroplets in glands of plants. After diffusing through the walls of the glands, the droplets spread over the surface of the plant before evaporating and filling the air with perfume. The most odoriferous plants are found in the tropics, where solar energy is greatest.
The function of the essential oil in a plant is not well understood. Odours of flowers probably aid in natural selection by acting as attractants for certain insects. Leaf oils, wood oils, and root oils may serve to protect against plant parasites or depredations by animals. Oleoresinous exudations that appear when the trunk of a tree is injured prevent loss of sap and act as a protective seal against parasites and disease organisms. Few essential oils are involved in plant metabolism, and some investigators maintain that many of these materials are simply waste products of plant biosynthesis.
Commercially, essential oils are used in three primary ways: as odorants they are used in cosmetics, perfumes, soaps, detergents, and miscellaneous industrial products ranging from animal feeds to insecticides to paints; as flavours they are present in bakery goods, candies, confections, meat, pickles, soft drinks, and many other food products; and as pharmaceuticals they appear in dental products and a wide, but diminishing, group of medicines.
The first records of essential oils come from ancient India, Persia, and Egypt; and both Greece and Rome conducted extensive trade in odoriferous oils and ointments with the countries of the Orient. Most probably these products were extracts prepared by placing flowers, roots, and leaves in fatty oils. In most ancient cultures, odorous plants or their resinous products were used directly. Only with the coming of the golden age of Arab culture was a technique developed for the distillation of essential oils. The Arabs were the first to distill ethyl alcohol from fermented sugar, thus providing a new solvent for the extraction of essential oils in place of the fatty oils that had probably been used for several millennia.
The knowledge of distillation spread to Europe during the Middle Ages, and isolation of essential oils by distillation was described during the 11th to 13th centuries. These distilled products became a specialty of the European medieval pharmacies, and by about 1500 the following products had been introduced: oils of cedarwood, calamus, costus, rose, rosemary, spike, incense, turpentine, sage, cinnamon, benzoin, and myrrh. The alchemical theories of the Swiss physician and alchemist Paracelsus played a role in stimulating physicians and pharmacists to seek essential oils from aromatic leaves, woods, and roots.
Starting from the time of Marco Polo, the much-prized spices of India, China, and the Indies served as the impetus for European trade with the Orient. Quite naturally, such spices as cardamom, sage, cinnamon, and nutmeg were subjected to the pharmacists’ stills. By the middle of the 18th century in Europe about 100 essential oils had been introduced, although there was little understanding about the nature of the products. As chemical knowledge expanded in the late 1800s and early 1900s, many well-known chemists took part in the chemical characterization of essential oils. Improvement in knowledge of essential oils led to a sharp expansion in production, and use of the volatile oils in medicine became quite subordinate to uses in foodstuffs, beverages, and perfumes.
In the United States, oils of turpentine and peppermint were produced before 1800; within the next several decades oils of four indigenous American plants became important commercially—namely, sassafras, wormwood, wintergreen, and sweet birch. Since 1800 many essential oils have been prepared, but only a few have attained commercial significance.
Methods of production
The first step in the isolation of essential oils is crushing or grinding the plant material to reduce the particle size and to rupture some of the cell walls of oil-bearing glands. Steam distillation is by far the most common and important method of production, and extraction with cold fat (enfleurage) or hot fat (maceration) is chiefly of historical importance.
Three different methods of steam distillation are practiced. In the oldest and simplest method a vessel containing water and the chopped or crushed plant material is heated by a direct flame, and the water vapour and volatile oil are recovered by a water-cooled condenser. This original method is being replaced by a process in which the plant material is suspended on a grid above the water level, and steam from a second vessel is introduced under the grid. The volatiles are condensed and the oil is separated. In the third process, the vessel containing the plant material on a grid is heated to prevent condensation of steam, so that dry distillation is attained.
In southern France essential oils were extracted with cold fat long before the introduction of extraction with volatile solvents. This process is applied to flowers that do not yield an appreciable quantity of oil by steam distillation or whose odour is changed by contact with boiling water and steam. In this process, flowers are spread over a highly purified mixture of tallow and lard and are left for a period varying from 24 hours to 72 hours. During this time most of the flower oil is absorbed by the fat. The petals are then removed (defleurage), and the process is repeated until the fat is saturated with oil. The final product is called pomade (e.g., pomade de jasmine).
In most cases, it is possible to shorten the long enfleurage process by extracting the essential oils using molten fat for one to two hours at a temperature ranging from about 45° to 80° C (110° to 175° F). The fat is filtered after each immersion, and after 10 to 20 extraction cycles the pomade is sold as such, or it may be extracted with alcohol to yield the oil residue.
Since both enfleurage and maceration are rather expensive processes, some essential-oil specialists have shifted almost completely to using volatile solvents for the recovery of essential oils from plant materials that could not be processed by steam distillation. Petroleum naphthas, benzene, and alcohol are the primary solvents.
A procedure called expression is applied only to citrus oils. The outer coloured peel is squeezed in presses, and the oil is decanted or centrifuged to separate water and cell debris. The method is used for oil of sweet and bitter orange, lemon, lime, mandarin, tangerine, bergamot, and grapefruit. Much oil is produced as a by-product of the concentrated-citrus-juice industry.
Chemical composition
Terpenes, organic compounds consisting of multiples of isoprene units (containing five carbon atoms), are by far the most dominant constituents of essential oils. Individual oils, however, may contain appreciable quantities of straight chain, aromatic, or heterocyclic compounds. Thus allyl sulfides are characteristics of oil of garlic, traces of indole and anthranilic acid esters are found in orange oil, straight chain alcohols and aldehydes are recognized in oil of violets, and phenols and other aromatic compounds are common to many oils.
Terpenes are built up from units of the simple five-carbon molecule isoprene. Both hydrocarbons and oxygenated compounds such as alcohols, aldehydes, ketones, acids, esters, oxides, lactones, acetals, and phenols are responsible for the characteristic odours and flavours.
In some oils one or only a few components predominate: thus oil of wintergreen contains about 98 percent of methyl salicylate; orange oil, about 90 percent of d-limonene; bois de rose, 90 percent of linalool; and cassia, up to 95 percent of cinnamaldehyde. In most oils there is a mixture of anywhere from a few dozen to several hundred individual compounds. Trace components are very important, since they give the oil a characteristic and natural odour.
Essential oils are generally expensive, with prices ranging from several U.S. dollars per kilogram on the low side to several thousand dollars per kilogram. The high price of the natural oils coupled with their limited availability has encouraged a search for substitutes. Great progress has been made in the synthesis of individual components such as geraniol, citral, linalyl acetate, and the like. These synthetics have been combined with natural oils to extend supplies, and they have also been blended together in an attempt to duplicate the oils themselves. Such reconstituted oils usually lack certain of the odour notes of the natural products, because of absence of trace ingredients, often unidentified, that may be present in the natural oils. They also tend to have a more “chemical” odour, because of trace impurities in the synthetics that are different from the components of natural oils.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1505 2022-09-17 13:41:00
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,194
Re: Miscellany
1478) Pine oil
Summary
Pine oil is an essential oil consisting of a colourless to light amber liquid of characteristic odour obtained from pine trees, or a synthetic oil similar in aroma and other properties. Pine oil is used as a solvent for gums, resins, and other substances. It has germicidal properties and is employed medically as a principal constituent of general disinfectants. It is also used in odorants, insecticides, detergents, wetting and emulsifying agents, wax preparations, and antifoaming agents and in textile scouring and the flotation process for refining lead and zinc ores.
Pitch-soaked wood of the pine tree, principally Pinus palustris but also certain other species of the family Pinaceae, is subjected to steam distillation, solvent extraction followed by steam distillation, or destructive distillation to obtain the pine oil, which boils at 200°–220° C (390°–430° F).
A variety of similar pine oils are obtained by distillation of cones and needles of various species of pines or by extraction from the stumps using solvents and steam. Synthetic pine oil is produced by conversion of terpene hydrocarbons into terpene alcohols.
Chemically, pine oils consist principally of cyclic terpene alcohols and are used in the manufacture of chemicals. Pine oil is insoluble in water but dissolves in alcohol and other organic solvents.
Details
Pine oil is an essential oil obtained from a variety of species of pine, particularly Pinus sylvestris. Typically, parts of the trees that are not used for lumber - stumps, etc. - are ground and subjected to steam distillation. As of 1995, synthetic pine oil was the "biggest single turpentine derivative." Synthetic pine oils accounted for 90% of sales as of 2000.
Composition
Pine oil is a higher boiling fraction from turpentine. Both synthetic and natural pine oil consists mainly of α-terpineol, a C10 alcohol (b.p. 214–217 °C). The detailed composition of natural pine oil depends on many factors, such as the species of the host plant. Synthetic pine oil is obtained by treating pinene with water in the presence of a catalytic amount of sulfuric acid. This treatment results in hydration of the alkene and rearrangement of the pinene skeleton, yielding terpineols.
Uses
Industrially, pine oil was once used in froth flotation for the separation of mineral from ores. For example, in copper extraction, pine oil is used to condition copper sulfide ores for froth flotation.
It is also used as a lubricant in small and expensive clockwork instruments.
In alternative medicine it is used in aromatherapy and as a scent in bath oils.
Properties as a disinfectant
Pine oil is used as a cleaning product, disinfectant, sanitizer, microbicide (or microbistat), virucide or insecticide. It is an effective herbicide where its action is to modify the waxy cuticle of plants, resulting in desiccation. Pine oil is a disinfectant that is mildly antiseptic. It is effective against Brevibacterium ammoniagenes, the fungi Candida albicans, Enterobacter aerogenes, Escherichia coli, Gram-negative enteric bacteria, household germs, Gram-negative household germs such as those causing salmonellosis, herpes simplex types 1 and 2, influenza type A, influenza virus type A/Brazil, influenza virus type A2/Japan, intestinal bacteria, Klebsiella pneumoniae, odor-causing bacteria, mold, mildew, Pseudomonas aeruginosa, Salmonella choleraesuis, Salmonella typhi, Salmonella typhosa, Serratia marcescens, Shigella sonnei, Staphylococcus aureus, Streptococcus faecalis, Streptococcus pyogenes, and Trichophyton mentagrophytes.
Safety
Pine oil has a relatively low human toxicity level, a low corrosion level and limited persistence; however, it irritates the skin and mucous membranes and has been known to cause breathing problems. Large doses may cause central nervous system depression.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1506 2022-09-18 14:24:09
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,194
Re: Miscellany
1479) Pediatrics
Summary
Pediatrics (also spelled paediatrics or pædiatrics) is the branch of medicine that involves the medical care of infants, children, adolescents, and young adults. In the United Kingdom, paediatrics covers many of their youth until the age of 18. The American Academy of Pediatrics recommends people seek pediatric care through the age of 21, but some pediatric subspecialists continue to care for adults up to 25. Worldwide age limits of pediatrics have been trending upward year after year. A medical doctor who specializes in this area is known as a pediatrician, or paediatrician. The word pediatrics and its cognates mean "healer of children," derived from the two Greek words: ("child") and (iatros "doctor, healer"). Pediatricians work in clinics, research centers, universities, general hospitals and children's hospitals, including those who practice pediatric subspecialties (e.g. neonatology requires resources available in a NICU).
Details
Pediatrics is the branch of medicine dealing with the health and medical care of infants, children, and adolescents from birth up to the age of 18. The word “paediatrics” means “healer of children”; they are derived from two Greek words: (pais = child) and (iatros = doctor or healer). Paediatrics is a relatively new medical specialty, developing only in the mid-19th century. Abraham Jacobi (1830–1919) is known as the father of paediatrics.
What does a pediatrician do?
A paediatrician is a child's physician who provides not only medical care for children who are acutely or chronically ill but also preventive health services for healthy children. A paediatrician manages physical, mental, and emotional well-being of the children under their care at every stage of development, in both sickness and health.
Aims of pediatrics
The aims of the study of paediatrics is to reduce infant and child rate of deaths, control the spread of infectious disease, promote healthy lifestyles for a long disease-free life and help ease the problems of children and adolescents with chronic conditions.
Paediatricians diagnose and treat several conditions among children including:-
* injuries
* infections
* genetic and congenital conditions
* cancers
* organ diseases and dysfunctions
Paediatrics is concerned not only about immediate management of the ill child but also long term effects on quality of life, disability and survival. Paediatricians are involved with the prevention, early detection, and management of problems including:-
* developmental delays and disorders
* behavioral problems
* functional disabilities
* social stresses
* mental disorders including depression and anxiety disorders
Collaboration with other specialists
Paediatrics is a collaborative specialty. Paediatricians need to work closely with other medical specialists and healthcare professionals and subspecialists of paediatrics to help children with problems.
How does pediatrics differ from adult medicine?
Paediatrics is different from adult medicine in more ways than one. The smaller body of an infant or neonate or a child is substantially different physiologically from that of an adult. So treating children is not like treating a miniature adult.
Congenital defects, genetic variance, and developmental issues are of greater concern to pediatricians than physicians treating adults. In addition, there are several legal issues in paediatrics. Children are minors and, in most jurisdictions, cannot make decisions for themselves. The issues of guardianship, privacy, legal responsibility and informed consent should be considered in every pediatric procedure.
Training
A paediatrician is a graduate from a medical school first. He or she being a primary care paediatrician then completes three years of education in an accredited pediatric residency program. They learn about caring for infant, child, adolescent, and young adults during this period.
Following the pediatric residency, the pediatrician is eligible for board certification by the American Board of Paediatrics with successful completion of a comprehensive written examination. Recertification is required every seven years.
Additional Information
Pediatrics is a medical specialty dealing with the development and care of children and with the diagnosis and treatment of childhood diseases. The first important review of childhood illness, an anonymous European work called The Children’s Practice, dates from the 12th century. The specialized focus of pediatrics did not begin to emerge in Europe until the 18th century. The first specialized children’s hospitals, such as the London Foundling Hospital, established in 1745, were opened at this time. These hospitals later became major centres for training in pediatrics, which began to be taught as a separate discipline in medical schools by the middle of the 19th century.
The major focus of early pediatrics was the treatment of infectious diseases that affected children. Thomas Sydenham in Britain had led the way with the first accurate descriptions of measles, scarlet fever, and other diseases in the 17th century. Clinical studies of childhood diseases proliferated throughout the 18th and 19th centuries, culminating in one of the first modern textbooks of pediatrics, published by Frédéric Rilliet and Antoine Barthez in France in 1838–43, but there was little that could be done to cure these diseases until the end of the 19th century. As childhood diseases came under control through the combined efforts of pediatricians, immunologists, and public-health workers, the focus of pediatrics began to change, and early in the 20th century the first well-child clinics were established to monitor and study the normal growth and development of children. By the mid-20th century, the use of antibiotics and vaccines had all but eliminated most serious infectious diseases of childhood in the developed world, and infant and child mortality had fallen to the lowest levels ever. In the last half of the century, pediatrics again expanded to incorporate the study of behavioral and social as well as specifically medical aspects of child health.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1507 2022-09-19 14:07:06
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,194
Re: Miscellany
1480) Cam
A cam is a rotating or sliding piece in a mechanical linkage used especially in transforming rotary motion into linear motion. It is often a part of a rotating wheel (e.g. an eccentric wheel) or shaft (e.g. a cylinder with an irregular shape) that strikes a lever at one or more points on its circular path. The cam can be a simple tooth, as is used to deliver pulses of power to a steam hammer, for example, or an eccentric disc or other shape that produces a smooth reciprocating (back and forth) motion in the follower, which is a lever making contact with the cam. A cam timer is similar, and were widely used for electric machine control (an electromechanical timer in a washing machine being a common example) before the advent of inexpensive electronics, microcontrollers, integrated circuits, programmable logic controllers and digital control.
Camshaft
The cam can be seen as a device that converts rotational motion to reciprocating (or sometimes oscillating) motion. A common example is the camshaft of an automobile, which takes the rotary motion of the engine and converts it into the reciprocating motion necessary to operate the intake and exhaust valves of the cylinders.
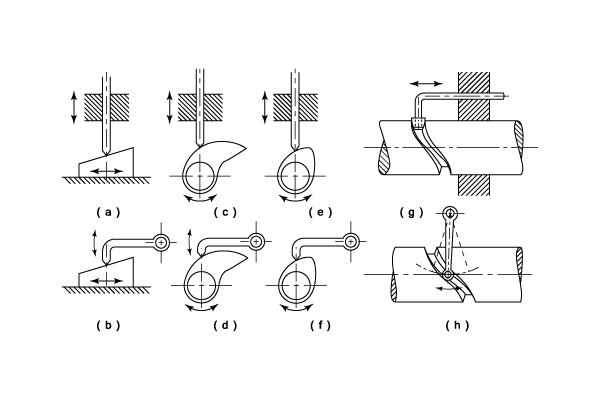
Cams can be characterized by their displacement diagrams, which reflect the changing position a follower would make as the surface of the cam moves in contact with the follower. In the example shown, the cam rotates about an axis. These diagrams relate angular position, usually in degrees, to the radial displacement experienced at that position. Displacement diagrams are traditionally presented as graphs with non-negative values. The rise is the motion of the follower away from the cam center, dwell is the motion where the follower is at rest, and return is the motion of the follower toward the cam center.
A common type is in the valve actuators in internal combustion engines. Here, the cam profile is commonly symmetric and at rotational speeds generally met with, very high acceleration forces develop. Ideally, a convex curve between the onset and maximum position of lift reduces acceleration, but this requires impractically large shaft diameters relative to lift. Thus, in practice, the points at which lift begins and ends mean that a tangent to the base circle appears on the profile. This is continuous with a tangent to the tip circle. In designing the cam, the lift and the dwell angle θ are given. If the profile is treated as a large base circle and a small tip circle, joined by a common tangent, giving lift L, the relationship can be calculated, given the angle φ between one tangent and the axis of symmetry, while C is the distance between the centres of the circles (required), and R is the radius of the base (given) and r that of the tip circle (required).
Disc or plate cam
The most commonly used cam is the cam plate (also known as disc cam or radial cam) which is cut out of a piece of flat metal or plate. Here, the follower moves in a plane perpendicular to the axis of rotation of the camshaft.[8] Several key terms are relevant in such a construction of plate cams: base circle, prime circle (with radius equal to the sum of the follower radius and the base circle radius), pitch curve which is the radial curve traced out by applying the radial displacements away from the prime circle across all angles, and the lobe separation angle (LSA – the angle between two adjacent intake and exhaust cam lobes).
The base circle is the smallest circle that can be drawn to the cam profile.
A once common, but now outdated, application of this type of cam was automatic machine tool programming cams. Each tool movement or operation was controlled directly by one or more cams. Instructions for producing programming cams and cam generation data for the most common makes of machine, were included in engineering references well into the modern CNC era.
This type of cam is used in many simple electromechanical appliances controllers, such as dishwashers and clothes washing machines, to actuate mechanical switches that control the various parts.
Motorcycle transmission showing cylindrical cam with three followers. Each follower controls the position of a shift fork.
Cylindrical cam
A cylindrical cam or barrel cam is a cam in which the follower rides on the surface of a cylinder. In the most common type, the follower rides in a groove cut into the surface of a cylinder. These cams are principally used to convert rotational motion to linear motion parallel to the rotational axis of the cylinder. A cylinder may have several grooves cut into the surface and drive several followers. Cylindrical cams can provide motions that involve more than a single rotation of the cylinder and generally provide positive positioning, removing the need for a spring or other provision to keep the follower in contact with the control surface.
Applications include machine tool drives, such as reciprocating saws, and shift control barrels in sequential transmissions, such as on most modern motorcycles.
A special case of this cam is a constant lead, where the position of the follower is linear with rotation, as in a lead screw. The purpose and detail of implementation influence whether this application is called a cam or a screw thread, but in some cases, the nomenclature may be ambiguous.
Cylindrical cams may also be used to reference an output to two inputs, where one input is the rotation of the cylinder and the other is the position of the follower along the cam. The output is radial to the cylinder. These were once common for special functions in control systems, such as fire control mechanisms for guns on naval vessels and mechanical analog computers.
An example of a cylindrical cam with two inputs is provided by a duplicating lathe, an example of which is the Klotz axe handle lathe, which cuts an axe handle to a form controlled by a pattern acting as a cam for the lathe mechanism.
Face cam
A face cam produces motion by using a follower riding on the face of a disk. The most common type has the follower ride in a slot so that the captive follower produces radial motion with positive positioning without the need for a spring or other mechanism to keep the follower in contact with the control surface. A face cam of this type generally has only one slot for a follower on each face. In some applications, a single element, such as a gear, a barrel cam or other rotating element with a flat face, may do duty as a face cam in addition to other purposes.
Face cams may provide repetitive motion with a groove that forms a closed curve or may provide function generation with a stopped groove. Cams used for function generation may have grooves that require several revolutions to cover the complete function, and in this case, the function generally needs to be invertible so that the groove does not self intersect, and the function output value must differ enough at corresponding rotations that there is sufficient material separating the adjacent groove segments. A common form is the constant lead cam, where the displacement of the follower is linear with rotation, such as the scroll plate in a scroll chuck. Non-invertible functions, which require the groove to self-intersect, can be implemented using special follower designs.
A variant of the face cam provides motion parallel to the axis of cam rotation. A common example is the traditional sash window lock, where the cam is mounted to the top of the lower sash, and the follower is the hook on the upper sash. In this application, the cam is used to provide a mechanical advantage in forcing the window shut, and also provides a self-locking action, like some worm gears, due to friction.
Face cams may also be used to reference a single output to two inputs, typically where one input is the rotation of the cam and the other is the radial position of the follower. The output is parallel to the axis of the cam. These were once common is mechanical analog computation and special functions in control systems.
A face cam that implements three outputs for a single rotational input is the stereo phonograph, where a relatively constant lead groove guides the stylus and tonearm unit, acting as either a rocker-type (tonearm) or linear (linear tracking turntable) follower, and the stylus alone acting as the follower for two orthogonal outputs to representing the audio signals. These motions are in a plane radial to the rotation of the record and at angles of 45 degrees to the plane of the disk (normal to the groove faces). The position of the tonearm was used by some turntables as a control input, such as to turn the unit off or to load the next disk in a stack, but was ignored in simple units.
Heart shaped cam
This type of cam, in the form of a symmetric heart, is used to return a shaft holding the cam to a set position by pressure from a roller. They were used on early models of Post Office Master clocks to synchronise the clock time with Greenwich Mean Time when the activating follower was pressed onto the cam automatically via a signal from an accurate time source.
Snail drop cam
This type of cam was used for example in mechanical timekeeping clocking-in clocks to drive the day advance mechanism at precisely midnight and consisted of a follower being raised over 24 hours by the cam in a spiral path which terminated at a sharp cut off at which the follower would drop down and activate the day advance. Where timing accuracy is required as in clocking-in clocks these were typically ingeniously arranged to have a roller cam follower to raise the drop weight for most of its journey to near its full height, and only for the last portion of its travel for the weight to be taken over and supported by a solid follower with a sharp edge. This ensured that the weight dropped at a precise moment, enabling accurate timing. This was achieved by the use of two snail cams mounted coaxially with the roller initially resting on one cam and the final solid follower on the other but not in contact with its cam profile. Thus the roller cam initially carried the weight, until at the final portion of the run the profile of the non-roller cam rose more than the other causing the solid follower to take the weight.
Linear cam
A linear cam is one in which the cam element moves in a straight line rather than rotates. The cam element is often a plate or block but may be any cross-section. The key feature is that the input is a linear motion rather than rotational. The cam profile may be cut into one or more edges of a plate or block, may be one or more slots or grooves in the face of an element, or may even be a surface profile for a cam with more than one input. The development of a linear cam is similar to, but not identical to, that of a rotating cam.
A common example of a linear cam is a key for a pin tumbler lock. The pins act as followers. This behavior is exemplified when the key is duplicated in a key duplication machine, where the original key acts as a control cam for cutting the new key.
History
Cam mechanisms appeared in China at around 600 BC in the form of a crossbow trigger-mechanism with a cam-shaped swing arm. However, the trigger mechanism did not rotate around its own axis and traditional Chinese technology generally made little use of continuously rotating cams. Nevertheless, later research showed that such cam mechanisms did in fact rotate around its own axis. Likewise, more recent research indicates that cams were used in water-driven trip hammers by the latter half of the Western Han Dynasty (206 BC – 8 AD) as recorded in the Huan Zi Xin Lun. Complex pestles were also mentioned in later records such as the Jin Zhu Gong Zan and the Tian Gong Kai Wu, amongst many other records of water-driven pestles. During the Tang dynasty, the wooden clock within the water-driven astronoical device, the spurs inside a water-driven armillary sphere, the automated alarm within a five-wheeled sand-driven clock, artificial paper figurines within a revolving lantern, all utilized cam mechanisms. The Chinese hodometer which utilized a bell and gong mechanism is also a cam, as described in the Song Shi. In the book Nongshu, the vertical wheel of a water-driven wind box is also a cam. Out of these examples, the water-driven pestle and the water driven wind box both have two cam mechanisms inside. Cams that rotated continuously and functioned as integral machine elements were built into Hellenistic water-driven automata from the 3rd century BC. The cam and camshaft later appeared in mechanisms by Al-Jazari and Shooshtari, who used them in their automata, described in 1206. The cam and camshaft appeared in European mechanisms from the 14th century. Waldo J Kelleigh of Electrical Apparatus Company patented the adjustable cam in the United States in 1956 for its use in mechanical engineering and weaponry.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1508 2022-09-20 13:32:12
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,194
Re: Miscellany
1481) Barometer
Summary
A barometer is a device used to measure atmospheric pressure. Because atmospheric pressure changes with distance above or below sea level, a barometer can also be used to measure altitude. There are two main types of barometers: mercury and aneroid.
In the mercury barometer, atmospheric pressure balances a column of mercury, the height of which can be precisely measured. To increase their accuracy, mercury barometers are often corrected for ambient temperature and the local value of gravity. Common pressure units include pounds per square inch; dynes per square centimetre; newtons per square metre (the SI unit called the pascal); inches, centimetres, or millimetres of mercury; and millibars (1 millibar equals 1,000 dynes per square centimetre, 100 pascals, or 0.75 millimetre of mercury). Atmospheric pressure at sea level is about 14.7 pounds per square inch, equivalent to 30 inches (760 millimetres) of mercury, 1,013.2 millibars, or 101,320 pascals.
Of the many different varieties of mercury barometers, most variations arise from different techniques for measuring the height of the mercury column. Though other liquids can be used in a barometer, mercury is the most common. Its density allows the vertical column of the barometer to be of manageable size. If water were used, for instance, the column would have to be 34 feet high.
A nonliquid barometer called the aneroid barometer is widely used in portable instruments and in aircraft altimeters because of its smaller size and convenience. It contains a flexible-walled evacuated capsule, the wall of which deflects with changes in atmospheric pressure. This deflection is coupled mechanically to an indicating needle. A mercury barometer is used to calibrate and check aneroid barometers. Calibration can be, for example, in terms of atmospheric pressure or altitude above sea level. The concept of altitude above sea level, based on barometric pressure, is used to create one type of aircraft altimeter.
A barometer that mechanically records changes in barometric pressure over time is called a barograph. Though mercury barographs have been made, aneroid barographs are much more common. The motion of the aneroid capsule is magnified through levers to drive a recording pen. The pen traces a line on a graph that is usually wrapped around a cylinder driven by a clockwork mechanism.
Details
A barometer is a scientific instrument that is used to measure air pressure in a certain environment. Pressure tendency can forecast short term changes in the weather. Many measurements of air pressure are used within surface weather analysis to help find surface troughs, pressure systems and frontal boundaries.
Barometers and pressure altimeters (the most basic and common type of altimeter) are essentially the same instrument, but used for different purposes. An altimeter is intended to be used at different levels matching the corresponding atmospheric pressure to the altitude, while a barometer is kept at the same level and measures subtle pressure changes caused by weather and elements of weather. The average atmospheric pressure on the earth's surface varies between 940 and 1040 hPa (mbar). The average atmospheric pressure at sea level is 1013 hPa (mbar).
Types:
Water barometers
The concept that decreasing atmospheric pressure predicts stormy weather, postulated by Lucien Vidi, provides the theoretical basis for a weather prediction device called a "weather glass" or a "Goethe barometer" (named for Johann Wolfgang von Goethe, the renowned German writer and polymath who developed a simple but effective weather ball barometer using the principles developed by Torricelli). The French name, le baromètre Liègeois, is used by some English speakers. This name reflects the origins of many early weather glasses – the glass blowers of Liège, Belgium.
The weather ball barometer consists of a glass container with a sealed body, half filled with water. A narrow spout connects to the body below the water level and rises above the water level. The narrow spout is open to the atmosphere. When the air pressure is lower than it was at the time the body was sealed, the water level in the spout will rise above the water level in the body; when the air pressure is higher, the water level in the spout will drop below the water level in the body. A variation of this type of barometer can be easily made at home.
Mercury barometers
A mercury barometer is an instrument used to measure atmospheric pressure in a certain location and has a vertical glass tube closed at the top sitting in an open mercury-filled basin at the bottom. Mercury in the tube adjusts until the weight of it balances the atmospheric force exerted on the reservoir. High atmospheric pressure places more force on the reservoir, forcing mercury higher in the column. Low pressure allows the mercury to drop to a lower level in the column by lowering the force placed on the reservoir. Since higher temperature levels around the instrument will reduce the density of the mercury, the scale for reading the height of the mercury is adjusted to compensate for this effect. The tube has to be at least as long as the amount dipping in the mercury + head space + the maximum length of the column.
Torricelli documented that the height of the mercury in a barometer changed slightly each day and concluded that this was due to the changing pressure in the atmosphere. He wrote: "We live submerged at the bottom of an ocean of elementary air, which is known by incontestable experiments to have weight". Inspired by Torricelli, Otto von Guericke on 5 December 1660 found that air pressure was unusually low and predicted a storm, which occurred the next day.
Fortin barometer
The mercury barometer's design gives rise to the expression of atmospheric pressure in inches or millimeters of mercury (mmHg). A torr was originally defined as 1 mmHg. The pressure is quoted as the level of the mercury's height in the vertical column. Typically, atmospheric pressure is measured between 26.5 inches (670 mm) and 31.5 inches (800 mm) of Hg. One atmosphere (1 atm) is equivalent to 29.92 inches (760 mm) of mercury.
Design changes to make the instrument more sensitive, simpler to read, and easier to transport resulted in variations such as the basin, siphon, wheel, cistern, Fortin, multiple folded, stereometric, and balance barometers.
In 2007, a European Union directive was enacted to restrict the use of mercury in new measuring instruments intended for the general public, effectively ending the production of new mercury barometers in Europe. The repair and trade of antiques (produced before late 1957) remained unrestricted.
Fitzroy barometer
Fitzroy barometers combine the standard mercury barometer with a thermometer, as well as a guide of how to interpret pressure changes.
Fortin barometer
Fortin barometers use a variable displacement mercury cistern, usually constructed with a thumbscrew pressing on a leather diaphragm bottom (V in the diagram). This compensates for displacement of mercury in the column with varying pressure. To use a Fortin barometer, the level of mercury is set to zero by using the thumbscrew to make an ivory pointer (O in the diagram) just touch the surface of the mercury. The pressure is then read on the column by adjusting the vernier scale so that the mercury just touches the sightline at Z. Some models also employ a valve for closing the cistern, enabling the mercury column to be forced to the top of the column for transport. This prevents water-hammer damage to the column in transit.
Sympiesometer
A sympiesometer is a compact and lightweight barometer that was widely used on ships in the early 19th century. The sensitivity of this barometer was also used to measure altitude.
Sympiesometers have two parts. One is a traditional mercury thermometer that is needed to calculate the expansion or contraction of the fluid in the barometer. The other is the barometer, consisting of a J-shaped tube open at the lower end and closed at the top, with small reservoirs at both ends of the tube.
Wheel barometers
A wheel barometer uses a "J" tube sealed at the top of the longer limb. The shorter limb is open to the atmosphere and floating on top of the mercury there is a small glass float. A fine silken thread is attached to the float which passes up over a wheel and then back down to a counterweight (usually protected in another tube). The wheel turns the point on the front of the barometer. As atmospheric pressure increases mercury moves from the short to the long limb, the float falls and the pointer moves. When pressure increases the mercury moves back, lifting the float and turning the dial the other way.
Around 1810 the wheel barometer, which could be read from a great distance, became the first practical and commercial instrument favoured by farmers and the educated classes in the UK. The face of the barometer was circular with a simple dial pointing to an easily readable scale: "Rain - Change - Dry" with the "Change" at the top centre of the dial. Later models added a barometric scale with finer graduations "Stormy (28 inches of mercury), Much Rain (28.5), Rain (29), Change (29.5), Fair (30), Set fair (30.5), very dry(31)".
Natalo Aiano is recognised as one of the finest makers of wheel barometers, an early pioneer in a wave of artisanal Italian instrument and barometer makers that were encouraged to emigrate to the UK. He listed as working in Holborn, London c.1785-1805.[18] From 1770 onwards a large number of Italians came to England because they were accomplished glass blowers or instrument makers. By 1840 it was fair to say that the Italians dominated the industry in England.
Vacuum pump oil barometer
Using vacuum pump oil as the working fluid in a barometer has led to the creation of the new "World's Tallest Barometer" in February 2013. The barometer at Portland State University (PSU) uses doubly distilled vacuum pump oil and has a nominal height of about 12.4 m for the oil column height; expected excursions are in the range of ±0.4 m over the course of a year. Vacuum pump oil has very low vapour pressure and it is available in a range of densities; the lowest density vacuum oil was chosen for the PSU barometer to maximize the oil column height.[20]
Aneroid barometer
An aneroid barometer is an instrument used for measuring air pressure as a method that does not involve liquid. Invented in 1844 by French scientist Lucien Vidi, the aneroid barometer uses a small, flexible metal box called an aneroid cell (capsule), which is made from an alloy of beryllium and copper. The evacuated capsule (or usually several capsules, stacked to add up their movements) is prevented from collapsing by a strong spring. Small changes in external air pressure cause the cell to expand or contract. This expansion and contraction drives mechanical levers such that the tiny movements of the capsule are amplified and displayed on the face of the aneroid barometer. Many models include a manually set needle which is used to mark the current measurement so a change can be seen. This type of barometer is common in homes and in recreational boats. It is also used in meteorology, mostly in barographs and as a pressure instrument in radiosondes.
Barographs
A barograph is a recording aneroid barometer where the changes in atmospheric pressure are recorded on a paper chart.
The principle of the barograph is same as that of the aneroid barometer. Whereas the barometer displays the pressure on a dial, the barograph uses the small movements of the box to transmit by a system of levers to a recording arm that has at its extreme end either a scribe or a pen. A scribe records on smoked foil while a pen records on paper using ink, held in a nib. The recording material is mounted on a cylindrical drum which is rotated slowly by a clock. Commonly, the drum makes one revolution per day, per week, or per month and the rotation rate can often be selected by the user.
MEMS barometers
Microelectromechanical systems (or MEMS) barometers are extremely small devices between 1 and 100 micrometres in size (0.001 to 0.1 mm). They are created via photolithography or photochemical machining. Typical applications include miniaturized weather stations, electronic barometers and altimeters.
A barometer can also be found in smartphones such as the Samsung Galaxy Nexus, Samsung Galaxy S3-S6, Motorola Xoom, Apple iPhone 6 and newer iPhones, and Timex Expedition WS4 smartwatch, based on MEMS and piezoresistive pressure-sensing technologies. Inclusion of barometers on smartphones was originally intended to provide a faster GPS lock. However, third party researchers were unable to confirm additional GPS accuracy or lock speed due to barometric readings. The researchers suggest that the inclusion of barometers in smartphones may provide a solution for determining a user's elevation, but also suggest that several pitfalls must first be overcome.
More unusual barometers
There are many other more unusual types of barometer. From variations on the storm barometer, such as the Collins Patent Table Barometer, to more traditional-looking designs such as Hooke's Otheometer and the Ross Sympiesometer. Some, such as the Shark Oil barometer,[28] work only in a certain temperature range, achieved in warmer climates.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1509 2022-09-21 13:43:36
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,194
Re: Miscellany
1482) Euro
Summary
The euro (symbol: €; code: EUR) is the official currency of 19 out of the 27 member states of the European Union (EU). This group of states is known as the eurozone or, officially, the euro area, and includes about 340 million citizens as of 2019. The euro is divided into 100 cents.
The currency is also used officially by the institutions of the European Union, by four European microstates that are not EU members, the British Overseas Territory of Akrotiri and Dhekelia, as well as unilaterally by Montenegro and Kosovo. Outside Europe, a number of special territories of EU members also use the euro as their currency. Additionally, over 200 million people worldwide use currencies pegged to the euro.
As of 2013, the euro is the second-largest reserve currency as well as the second-most traded currency in the world after the United States dollar. As of December 2019, with more than €1.3 trillion in circulation, the euro has one of the highest combined values of banknotes and coins in circulation in the world.
The name euro was officially adopted on 16 December 1995 in Madrid. The euro was introduced to world financial markets as an accounting currency on 1 January 1999, replacing the former European Currency Unit (ECU) at a ratio of 1:1 (US$1.1743). Physical euro coins and banknotes entered into circulation on 1 January 2002, making it the day-to-day operating currency of its original members, and by March 2002 it had completely replaced the former currencies.
Between December 1999 and December 2002, the euro traded below the US dollar, but has since traded at or above the US dollar, peaking at US$1.60 on 18 July 2008 and since then returning near to its original issue rate. On 13 July 2022, the two currencies hit parity for the first time in nearly two decades due in part to the 2022 Russian invasion of Ukraine.
Details
Euro is the monetary unit and currency of the European Union (EU). It was introduced as a noncash monetary unit in 1999, and currency notes and coins appeared in participating countries on January 1, 2002. After February 28, 2002, the euro became the sole currency of 12 EU member states, and their national currencies ceased to be legal tender. Other states subsequently adopted the currency. The euro is represented by the symbol €.
The euro’s origins lay in the Maastricht Treaty (1991), an agreement among the then 12 member countries of the European Community (now the European Union)—United Kingdom, France, Germany, Italy, Ireland, Belgium, Denmark, the Netherlands, Spain, Portugal, Greece, and Luxembourg—that included the creation of an economic and monetary union (EMU). The treaty called for a common unit of exchange, the euro, and set strict criteria for conversion to the euro and participation in the EMU. These requirements included annual budget deficits not exceeding 3 percent of gross domestic product (GDP), public debt under 60 percent of GDP, exchange rate stability, inflation rates within 1.5 percent of the three lowest inflation rates in the EU, and long-term inflation rates within 2 percent. Although several states had public debt ratios exceeding 60 percent—the rates topped 120 percent in Italy and Belgium—the European Commission (the executive branch of the EU) recommended their entry into the EMU, citing the significant steps each country had taken to reduce its debt ratio.
Supporters of the euro argued that a single European currency would boost trade by eliminating foreign exchange fluctuations and reducing prices. Although there were concerns regarding a single currency, including worries about counterfeiting and loss of national sovereignty and national identity, 11 countries (Austria, Belgium, Finland, France, Germany, Ireland, Italy, Luxembourg, the Netherlands, Portugal, and Spain) formally joined the EMU in 1998. Britain and Sweden delayed joining, though some businesses in Britain decided to accept payment in euros. Voters in Denmark narrowly rejected the euro in a September 2000 referendum. Greece initially failed to meet the economic requirements but was admitted in January 2001 after overhauling its economy.
In 2007 Slovenia became the first former communist country to adopt the euro. Having demonstrated fiscal stability since joining the EU in 2004, both Malta and the Greek Cypriot sector of Cyprus adopted the euro in 2008. Other countries that adopted the currency include Slovakia (2009), Estonia (2011), Latvia (2014), and Lithuania (2015). (The euro is also the official currency in several areas outside the EU, including Andorra, Montenegro, Kosovo, and San Marino.) The 19 participating EU countries are known as the euro area, euroland, or the euro zone.
In 1998 the European Central Bank (ECB) was established to manage the new currency. Based in Frankfurt, Germany, the ECB is an independent and neutral body headed by an appointed president who is approved by all member countries to serve an eight-year term. The euro was launched on January 1, 1999, replacing the precursor ecu at a 1:1 value. Until the circulation of currency notes and coins in 2002, the euro was used only by financial markets and certain businesses. Many experts predicted that the euro could eventually rival the U.S. dollar as an international currency.
Unlike most of the national currencies that they replaced, euro banknotes do not display famous national figures. The seven colourful bills, designed by the Austrian artist Robert Kalina and ranging in denomination from €5 to €500, symbolize the unity of Europe and feature a map of Europe, the EU’s flag, and arches, bridges, gateways, and windows. The eight euro coins range in denominations from one cent to two euros. The coins feature one side with a common design; the reverse sides’ designs differ in each of the individual participating countries.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1510 2022-09-22 13:40:00
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,194
Re: Miscellany
1483) Dollar
Summary
A dollar is originally, a silver coin that circulated in many European countries; in modern times, the name of the standard monetary unit in the United States, Canada, Australia, New Zealand, and other countries. The Spanish peso, or piece of eight, which circulated in the Spanish and English colonies in America, was known as a dollar by the English-speaking peoples. Familiarity with this coin resulted in the official designation of the United States monetary unit as the dollar in 1792. Canada adopted the dollar and monetary decimal system in 1858; Australia in 1966; and New Zealand in 1967.
The word itself is a modified form of the Germanic word thaler, a shortened form of Joachimst(h)aler, the name of a silver coin first struck in 1519 under the direction of the count of Schlick, who had appropriated a rich silver mine discovered in St. Joachimsthal (Joachim’s dale), Bohemia. These coins were current in Germany from the 16th century onward, with the various spelling modifications such as daler, dalar, daalder, and tallero. Only in 1873 was the thaler replaced by the mark as the German monetary unit.
Details
Dollar is the name of more than 20 currencies. They include the Australian dollar, Brunei dollar, Canadian dollar, Hong Kong dollar, Jamaican dollar, Liberian dollar, Namibian dollar, New Taiwan dollar, New Zealand dollar, Singapore dollar, United States dollar, and several others. The symbol for most of those currencies is the dollar sign $ in the same way as many countries using peso currencies.
Adoption by the United States
By the time of the American Revolution, the Spanish dólar gained significance because they backed paper money authorized by the individual colonies and the Continental Congress. Because Britain deliberately withheld hard currency from the American colonies, virtually all the non-token coinage in circulation was Spanish (and to a much lesser extent French and Dutch) silver, obtained via illegal but widespread commerce with the West Indies. Common in the Thirteen Colonies, Spanish dólar were even legal tender in one colony, Virginia.
On April 2, 1792, U.S. Secretary of the Treasury Alexander Hamilton reported to Congress the precise amount of silver found in Spanish dollar coins in common use in the states. As a result, the United States dollar was defined[28] as a unit of pure silver weighing 371 4/16th grains (24.057 grams), or 416 grains of standard silver (standard silver being defined as 371.25/416 in silver, and balance in alloy). It was specified that the "money of account" of the United States should be expressed in those same "dollars" or parts thereof. Additionally, all lesser-denomination coins were defined as percentages of the dollar coin, such that a half-dollar was to contain half as much silver as a dollar, quarter-dollars would contain one-fourth as much, and so on.
In an act passed in January 1837, the dollar's weight was reduced to 412.5 grains and alloy at 90% silver, resulting in the same fine silver content of 371.25 grains. On February 21, 1853, the quantity of silver in the lesser coins was reduced, with the effect that their denominations no longer represented their silver content relative to dollar coins.
Various acts have subsequently been passed affecting the amount and type of metal in U.S. coins, so that today there is no legal definition of the term "dollar" to be found in U.S. statute. Currently the closest thing to a definition is found in United States Code Title 31, Section 5116, paragraph b, subsection 2: "The Secretary [of the Treasury] shall sell silver under conditions the Secretary considers appropriate for at least $1.292929292 a fine troy ounce."
Silver was mostly removed from U.S. coinage by 1965 and the dollar became a free-floating fiat money without a commodity backing defined in terms of real gold or silver. The US Mint continues to make silver $1-denomination coins, but these are not intended for general circulation.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1511 2022-09-23 14:14:42
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,194
Re: Miscellany
1484) Salicylic acid
Summary
Salicylic acid, also called ortho-hydroxybenzoic acid, is a white, crystalline solid that is used chiefly in the preparation of aspirin and other pharmaceutical products. The free acid occurs naturally in small amounts in many plants, particularly the various species of Spiraea. The methyl ester also occurs widely in nature; it is the chief constituent of oil of wintergreen. Salicylic acid was first prepared by the Italian chemist Raffaele Piria in 1838 from salicylaldehyde. In 1860 the German chemists Hermann Kolbe and Eduard Lautemann discovered a synthesis based on phenol and carbon dioxide. Today the compound is made from dry sodium phenoxide (sodium phenolate) and carbon dioxide, followed by treatment with acid.
Most salicylic acid produced commercially is treated with acetic anhydride for the preparation of aspirin.
Salicylic acid esterified with methanol in the presence of an acid catalyst gives methyl salicylate, synthetic oil of wintergreen, which is used as a flavouring agent. Treatment of salicylic acid with phenol gives phenyl salicylate, which is used for sunburn creams and enteric-coated pills and to make salicylanilide for use as a fungicide and mildew preventive. Salicylic acid is a component of preparations used to combat warts, corns, calluses, and various skin diseases. The sodium salt is used in the manufacture of certain classes of dyes.
Pure salicylic acid crystallizes from hot water in the form of white needles, which sublime without decomposition at temperatures up to 155 °C (311 °F) and melt at 159 °C (318 °F). Above 200 °C (392 °F), the acid decomposes to phenol and carbon dioxide.
Details
Salicylic acid is an organic compound with the formula HOC6H4CO2H. A colorless, bitter-tasting solid, it is a precursor to and a metabolite of aspirin (acetylsalicylic acid). It is a plant hormone, and has been listed by the EPA Toxic Substances Control Act (TSCA) Chemical Substance Inventory as an experimental teratogen. The name is from Latin salix for willow tree. It is an ingredient in some anti-acne products. Salts and esters of salicylic acid are known as salicylates.
Uses:
Medicine
Salicylic acid as a medication is commonly used to remove the outer layer of the skin. As such, it is used to treat warts, psoriasis, acne vulgaris, ringworm, dandruff, and ichthyosis.
Similar to other hydroxy acids, salicylic acid is an ingredient in many skincare products for the treatment of seborrhoeic dermatitis, acne, psoriasis, calluses, corns, keratosis pilaris, acanthosis nigricans, ichthyosis, and warts.
Uses in manufacturing
Salicylic acid is used as a food preservative, a bactericide, and an antiseptic.
Salicylic acid is used in the production of other pharmaceuticals, including 4-aminosalicylic acid, sandulpiride, and landetimide (via salethamide).
Salicylic acid has long been a key starting material for making acetylsalicylic acid (aspirin).[9] Aspirin (acetylsalicylic acid or ASA) is prepared by the esterification of the phenolic hydroxyl group of salicylic acid with the acetyl group from acetic anhydride or acetyl chloride. ASA is the standard to which all the other non-steroidal anti-inflammatory drugs (NSAIDs) are compared. In veterinary medicine, this group of drugs is mainly used for treatment of inflammatory musculoskeletal disorders.
Bismuth subsalicylate, a salt of bismuth and salicylic acid, is the active ingredient in stomach-relief aids such as Pepto-Bismol, is the main ingredient of Kaopectate, and "displays anti-inflammatory action (due to salicylic acid) and also acts as an antacid and mild antibiotic".
Other derivatives include methyl salicylate used as a liniment to soothe joint and muscle pain and choline salicylate used topically to relieve the pain of mouth ulcers. Aminosalicylic acid is used to induce remission in ulcerative colitis, and has been used as an antitubercular agent often administered in association with isoniazid.
Sodium salicylate is a useful phosphor in the vacuum ultraviolet spectral range, with nearly flat quantum efficiency for wavelengths between 10 and 100 nm. It fluoresces in the blue at 420 nm. It is easily prepared on a clean surface by spraying a saturated solution of the salt in methanol followed by evaporation.
Mechanism of action
Salicylic acid modulates COX-1 enzymatic activity to decrease the formation of pro-inflammatory prostaglandins. Salicylate may competitively inhibit prostaglandin formation. Salicylate's antirheumatic (nonsteroidal anti-inflammatory) actions are a result of its analgesic and anti-inflammatory mechanisms.
Salicylic acid works by causing the cells of the epidermis to slough off more readily, preventing pores from clogging up, and allowing room for new cell growth. Salicylic acid inhibits the oxidation of uridine-5-diphosphoglucose (UDPG) competitively with nicotinamide adenine dinucleotide and noncompetitively with UDPG. It also competitively inhibits the transferring of glucuronyl group of uridine-5-phosphoglucuronic acid to the phenolic acceptor.
The wound-healing retardation action of salicylates is probably due mainly to its inhibitory action on mucopolysaccharide synthesis.
Safety
If high concentrations of salicylic ointment are applied to a large percentage of body surface, high levels of salicylic acid can enter the blood, requiring hemodialysis to avoid further complications.
Production and chemical reactions:
Biosynthesis
Salicylic acid is biosynthesized from the amino acid phenylalanine. In Arabidopsis thaliana, it can be synthesized via a phenylalanine-independent pathway.
Industrial synthesis
Sodium salicylate is commercially prepared by treating sodium phenolate (the sodium salt of phenol) with carbon dioxide at high pressure (100 atm) and high temperature (115 °C) – a method known as the Kolbe-Schmitt reaction.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1512 2022-09-24 14:26:25
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,194
Re: Miscellany
1485) Ammonia
Summary
Ammonia is an inorganic compound of nitrogen and hydrogen with the formula NH3. A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct pungent smell. Biologically, it is a common nitrogenous waste, particularly among aquatic organisms, and it contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to 45 percent of the world's food and fertilizers. Around 70% of ammonia is used to make fertilisers in various forms and composition, such as urea and Diammonium phosphate. Ammonia in pure form is also applied directly into the soil. It is estimated that around 40% of the nitrogen in human beings originally comes from industrial ammonia production. As such, its importance can hardly be overstated.
Ammonia, either directly or indirectly, is also a building block for the synthesis of many pharmaceutical products and is used in many commercial cleaning products. It is mainly collected by downward displacement of both air and water.
Although common in nature—both terrestrially and in the outer planets of the Solar System—and in wide use, ammonia is both caustic and hazardous in its concentrated form. In many countries it is classified as an extremely hazardous substance, and is subject to strict reporting requirements by facilities which produce, store, or use it in significant quantities.
The global industrial production of ammonia in 2018 was 175 million tonnes, with no significant change relative to the 2013 global industrial production of 175 million tonnes. In 2021 this was 235 million tonnes, with very little being made within the United States. Industrial ammonia is sold either as ammonia liquor (usually 28% ammonia in water) or as pressurized or refrigerated anhydrous liquid ammonia transported in tank cars or cylinders.
For fundamental reasons, the production of ammonia from the elements hydrogen and nitrogen is difficult, requiring high pressures and high temperatures. The Haber process that enabled industrial production was invented at the beginning of the twentieth century, revolutionizing agriculture.
NH3 boils at −33.34 °C (−28.012 °F) at a pressure of one atmosphere, so the liquid must be stored under pressure or at low temperature. Household ammonia or ammonium hydroxide is a solution of NH3 in water. The concentration of such solutions is measured in units of the Baumé scale (density), with 26 degrees Baumé (about 30% (by weight) ammonia at 15.5 °C or 59.9 °F) being the typical high-concentration commercial product.
Details
Ammonia (NH3) is a colourless, pungent gas composed of nitrogen and hydrogen. It is the simplest stable compound of these elements and serves as a starting material for the production of many commercially important nitrogen compounds.
Uses of ammonia
The major use of ammonia is as a fertilizer. In the United States, it is usually applied directly to the soil from tanks containing the liquefied gas. The ammonia can also be in the form of ammonium salts, such as ammonium nitrate, NH4NO3, ammonium sulfate, (NH4)2SO4, and various ammonium phosphates. Urea, (H2N)2C=O, is the most commonly used source of nitrogen for fertilizer worldwide. Ammonia is also used in the manufacture of commercial explosives (e.g., trinitrotoluene [TNT], nitroglycerin, and nitrocellulose).
In the textile industry, ammonia is used in the manufacture of synthetic fibres, such as nylon and rayon. In addition, it is employed in the dyeing and scouring of cotton, wool, and silk. Ammonia serves as a catalyst in the production of some synthetic resins. More important, it neutralizes acidic by-products of petroleum refining, and in the rubber industry it prevents the coagulation of raw latex during transportation from plantation to factory. Ammonia also finds application in both the ammonia-soda process (also called the Solvay process), a widely used method for producing soda ash, and the Ostwald process, a method for converting ammonia into nitric acid.
Ammonia is used in various metallurgical processes, including the nitriding of alloy sheets to harden their surfaces. Because ammonia can be decomposed easily to yield hydrogen, it is a convenient portable source of atomic hydrogen for welding. In addition, ammonia can absorb substantial amounts of heat from its surroundings (i.e., one gram of ammonia absorbs 327 calories of heat), which makes it useful as a coolant in refrigeration and air-conditioning equipment. Finally, among its minor uses is inclusion in certain household cleansing agents.
Preparation of ammonia
Pure ammonia was first prepared by English physical scientist Joseph Priestley in 1774, and its exact composition was determined by French chemist Claude-Louis Berthollet in 1785. Ammonia is consistently among the top five chemicals produced in the United States. The chief commercial method of producing ammonia is by the Haber-Bosch process, which involves the direct reaction of elemental hydrogen and elemental nitrogen.
This reaction requires the use of a catalyst, high pressure (100–1,000 atmospheres), and elevated temperature (400–550 °C [750–1020 °F]). Actually, the equilibrium between the elements and ammonia favours the formation of ammonia at low temperature, but high temperature is required to achieve a satisfactory rate of ammonia formation. Several different catalysts can be used. Normally the catalyst is iron containing iron oxide. However, both magnesium oxide on aluminum oxide that has been activated by alkali metal oxides and ruthenium on carbon have been employed as catalysts. In the laboratory, ammonia is best synthesized by the hydrolysis of a metal nitride.
Physical properties of ammonia
Ammonia is a colourless gas with a sharp, penetrating odour. Its boiling point is −33.35 °C (−28.03 °F), and its freezing point is −77.7 °C (−107.8 °F). It has a high heat of vaporization (23.3 kilojoules per mole at its boiling point) and can be handled as a liquid in thermally insulated containers in the laboratory. (The heat of vaporization of a substance is the number of kilojoules needed to vaporize one mole of the substance with no change in temperature.) The ammonia molecule has a trigonal pyramidal shape with the three hydrogen atoms and an unshared pair of electrons attached to the nitrogen atom. It is a polar molecule and is highly associated because of strong intermolecular hydrogen bonding. The dielectric constant of ammonia (22 at −34 °C [−29 °F]) is lower than that of water (81 at 25 °C [77 °F]), so it is a better solvent for organic materials. However, it is still high enough to allow ammonia to act as a moderately good ionizing solvent. Ammonia also self-ionizes, although less so than does water.
Chemical reactivity of ammonia
The combustion of ammonia proceeds with difficulty but yields nitrogen gas and water.
However, with the use of a catalyst and under the correct conditions of temperature, ammonia reacts with oxygen to produce nitric oxide, NO, which is oxidized to nitrogen dioxide, NO2, and is used in the industrial synthesis of nitric acid.
Ammonia readily dissolves in water with the liberation of heat.
Liquid ammonia is used extensively as a nonaqueous solvent. The alkali metals as well as the heavier alkaline-earth metals and even some inner transition metals dissolve in liquid ammonia, producing blue solutions. Physical measurements, including electrical-conductivity studies, provide evidence that this blue colour and electrical current are due to the solvated electron.
These solutions are excellent sources of electrons for reducing other chemical species. As the concentration of dissolved metal increases, the solution becomes a deeper blue in colour and finally changes to a copper-coloured solution with a metallic lustre. The electrical conductivity decreases, and there is evidence that the solvated electrons associate to form electron pairs.
Derivatives of ammonia
Two of the more important derivatives of ammonia are hydrazine and hydroxylamine.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1513 2022-09-25 14:00:25
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,194
Re: Miscellany
1486) Ethane
Summary
Ethane is a colourless, odourless, gaseous hydrocarbon (compound of hydrogen and carbon), belonging to the paraffin series; its chemical formula is C2H6. Ethane is structurally the simplest hydrocarbon that contains a single carbon–carbon bond. The second most important constituent of natural gas, it also occurs dissolved in petroleum oils and as a by-product of oil refinery operations and of the carbonization of coal.
The industrial importance of ethane is based upon the ease with which it may be converted to ethylene (C2H4) and hydrogen by pyrolysis, or cracking, when passed through hot tubes. Like propane and, to a lesser extent, butane, ethane is a major raw material for the huge ethylene petrochemical industry, which produces such important products as polyethylene plastic, ethylene glycol, and ethyl alcohol.
More than 90 percent of the ethane produced in the 1960s was burned as fuel without separation from natural gas. Ethane gas can be liquefied under pressure or at reduced temperatures and thus be separated from natural gas. Unlike propane, liquid ethane is not in common use as an industrial or domestic fuel.
Details
Ethane is an organic chemical compound with chemical formula C2H6. At standard temperature and pressure, ethane is a colorless, odorless gas. Like many hydrocarbons, ethane is isolated on an industrial scale from natural gas and as a petrochemical by-product of petroleum refining. Its chief use is as feedstock for ethylene production.
Related compounds may be formed by replacing a hydrogen atom with another functional group; the ethane moiety is called an ethyl group. For example, an ethyl group linked to a hydroxyl group yields ethanol, the alcohol in beverages.
History
Ethane was first synthesised in 1834 by Michael Faraday, applying electrolysis of a potassium acetate solution. He mistook the hydrocarbon product of this reaction for methane and did not investigate it further.
During the period 1847–1849, in an effort to vindicate the radical theory of organic chemistry, Hermann Kolbe and Edward Frankland produced ethane by the reductions of propionitrile and ethyl iodide with potassium metal, and, as did Faraday, by the electrolysis of aqueous acetates. They mistook the product of these reactions for the methyl radical (CH3), of which ethane (C2H6) is a dimer.
This error was corrected in 1864 by Carl Schorlemmer, who showed that the product of all these reactions was in fact ethane. Ethane was discovered dissolved in Pennsylvanian light crude oil by Edmund Ronalds in 1864.
Properties
At standard temperature and pressure, ethane is a colorless, odorless gas. It has a boiling point of −88.5 °C (−127.3 °F) and melting point of −182.8 °C (−297.0 °F). Solid ethane exists in several modifications. On cooling under normal pressure, the first modification to appear is a plastic crystal, crystallizing in the cubic system. In this form, the positions of the hydrogen atoms are not fixed; the molecules may rotate freely around the long axis. Cooling this ethane below ca. 89.9 K (−183.2 °C; −297.8 °F) changes it to monoclinic metastable ethane II (space group P 21/n). Ethane is only very sparingly soluble in water.
Atmospheric and extraterrestrial
Ethane occurs as a trace gas in the Earth's atmosphere, currently having a concentration at sea level of 0.5 ppb, though its preindustrial concentration is likely to have been only around 0.25 part per billion since a significant proportion of the ethane in today's atmosphere may have originated as fossil fuels. Global ethane quantities have varied over time, likely due to flaring at natural gas fields. Global ethane emission rates declined from 1984 to 2010, though increased shale gas production at the Bakken Formation in the U.S. has arrested the decline by half.
Although ethane is a greenhouse gas, it is much less abundant than methane, has a lifetime of only a few months compared to over a decade, and is also less efficient at absorbing radiation relative to mass. In fact, ethane's global warming potential largely results from its conversion in the atmosphere to methane. It has been detected as a trace component in the atmospheres of all four giant planets, and in the atmosphere of Saturn's moon Titan.
Atmospheric ethane results from the Sun's photochemical action on methane gas, also present in these atmospheres: ultraviolet photons of shorter wavelengths than 160 nm can photo-dissociate the methane molecule into a methyl radical and a hydrogen atom.
On Earth's atmosphere, hydroxyl radicals convert ethane to methanol vapor with a half-life of around three months.
It is suspected that ethane produced in this fashion on Titan rains back onto the moon's surface, and over time has accumulated into hydrocarbon seas covering much of the moon's polar regions. In December 2007 the Cassini probe found at least one lake at Titan's south pole, now called Ontario Lacus because of the lake's similar area to Lake Ontario on Earth (approximately 20,000 sq km). Further analysis of infrared spectroscopic data presented in July 2008 provided additional evidence for the presence of liquid ethane in Ontario Lacus. Several significantly larger hydrocarbon lakes, Ligeia Mare and Kraken Mare being the two largest, were discovered near Titan's north pole using radar data gathered by Cassini. These lakes are believed to be filled primarily by a mixture of liquid ethane and methane.
In 1996, ethane was detected in Comet Hyakutake, and it has since been detected in some other comets. The existence of ethane in these distant solar system bodies may implicate ethane as a primordial component of the solar nebula from which the sun and planets are believed to have formed.
In 2006, Dale Cruikshank of NASA/Ames Research Center (a New Horizons co-investigator) and his colleagues announced the spectroscopic discovery of ethane on Pluto's surface.
Chemistry
Ethane can be viewed as two methyl groups joined, that is, a dimer of methyl groups. In the laboratory, ethane may be conveniently synthesised by Kolbe electrolysis. In this technique, an aqueous solution of an acetate salt is electrolysed.
Synthesis by oxidation of acetic anhydride by peroxides, is conceptually similar.
The chemistry of ethane involves chiefly free radical reactions. Ethane can react with the halogens, especially chlorine and bromine, by free-radical halogenation.
Because halogenated ethanes can undergo further free radical halogenation, this process results in a mixture of several halogenated products. In the chemical industry, more selective chemical reactions are used for the production of any particular two-carbon haloalkane.
Combustion
The complete combustion of ethane releases 1559.7 kJ/mol, or 51.9 kJ/g, of heat, and produces carbon dioxide and water.
Combustion may also occur without an excess of oxygen, forming a mix of amorphous carbon and carbon monoxide.
Combustion occurs by a complex series of free-radical reactions. Computer simulations of the chemical kinetics of ethane combustion have included hundreds of reactions. An important series of reaction in ethane combustion is the combination of an ethyl radical with oxygen, and the subsequent breakup of the resulting peroxide into ethoxy and hydroxyl radicals.
The principal carbon-containing products of incomplete ethane combustion are single-carbon compounds such as carbon monoxide and formaldehyde. One important route by which the carbon–carbon bond in ethane is broken, to yield these single-carbon products, is the decomposition of the ethoxy radical into a methyl radical and formaldehyde, which can in turn undergo further oxidation.
Some minor products in the incomplete combustion of ethane include acetaldehyde, methane, methanol, and ethanol. At higher temperatures, especially in the range 600–900 °C (1,112–1,652 °F), ethylene is a significant product.
Similar reactions (with agents other than oxygen as the hydrogen abstractor) are involved in the production of ethylene from ethane in steam cracking.
Barrier
Ethane (shown in Newman projection) barrier to rotation about the carbon-carbon bond. The curve is potential energy as a function of rotational angle. Energy barrier is 12 kJ/mol or about 2.9 kcal/mol.
Rotating a molecular substructure about a twistable bond usually requires energy. The minimum energy to produce a 360° bond rotation is called the rotational barrier.
Ethane gives a classic, simple example of such a rotational barrier, sometimes called the "ethane barrier". Among the earliest experimental evidence of this barrier was obtained by modelling the entropy of ethane. The three hydrogens at each end are free to pinwheel about the central carbon–carbon bond when provided with sufficient energy to overcome the barrier. The physical origin of the barrier is still not completely settled, although the overlap (exchange) repulsion between the hydrogen atoms on opposing ends of the molecule is perhaps the strongest candidate, with the stabilizing effect of hyperconjugation on the staggered conformation contributing to the phenomenon. Theoretical methods that use an appropriate starting point (orthogonal orbitals) find that hyperconjugation is the most important factor in the origin of the ethane rotation barrier.
As far back as 1890–1891, chemists suggested that ethane molecules preferred the staggered conformation with the two ends of the molecule askew from each other.
Production
After methane, ethane is the second-largest component of natural gas. Natural gas from different gas fields varies in ethane content from less than 1% to more than 6% by volume. Prior to the 1960s, ethane and larger molecules were typically not separated from the methane component of natural gas, but simply burnt along with the methane as a fuel. Today, ethane is an important petrochemical feedstock and is separated from the other components of natural gas in most well-developed gas fields. Ethane can also be separated from petroleum gas, a mixture of gaseous hydrocarbons produced as a byproduct of petroleum refining.
Ethane is most efficiently separated from methane by liquefying it at cryogenic temperatures. Various refrigeration strategies exist: the most economical process presently in wide use employs a turboexpander, and can recover more than 90% of the ethane in natural gas. In this process, chilled gas is expanded through a turbine, reducing the temperature to about −100 °C (−148 °F). At this low temperature, gaseous methane can be separated from the liquefied ethane and heavier hydrocarbons by distillation. Further distillation then separates ethane from the propane and heavier hydrocarbons.
Usage
The chief use of ethane is the production of ethylene (ethene) by steam cracking. When diluted with steam and briefly heated to very high temperatures (900 °C or more), heavy hydrocarbons break down into lighter hydrocarbons, and saturated hydrocarbons become unsaturated. Ethane is favored for ethylene production because the steam cracking of ethane is fairly selective for ethylene, while the steam cracking of heavier hydrocarbons yields a product mixture poorer in ethylene and richer in heavier alkenes (olefins), such as propene (propylene) and butadiene, and in aromatic hydrocarbons.
Experimentally, ethane is under investigation as a feedstock for other commodity chemicals. Oxidative chlorination of ethane has long appeared to be a potentially more economical route to vinyl chloride than ethylene chlorination. Many processes for producing this reaction have been patented, but poor selectivity for vinyl chloride and corrosive reaction conditions (specifically, a reaction mixture containing hydrochloric acid at temperatures greater than 500 °C) have discouraged the commercialization of most of them. Presently, INEOS operates a 1000 t/a (tonnes per annum) ethane-to-vinyl chloride pilot plant at Wilhelmshaven in Germany.
Similarly, the Saudi Arabian firm SABIC has announced construction of a 30,000 t/a plant to produce acetic acid by ethane oxidation at Yanbu. The economic viability of this process may rely on the low cost of ethane near Saudi oil fields, and it may not be competitive with methanol carbonylation elsewhere in the world.
Ethane can be used as a refrigerant in cryogenic refrigeration systems. On a much smaller scale, in scientific research, liquid ethane is used to vitrify water-rich samples for cryo-electron microscopy. A thin film of water quickly immersed in liquid ethane at −150 °C or colder freezes too quickly for water to crystallize. Slower freezing methods can generate cubic ice crystals, which can disrupt soft structures by damaging the samples and reduce image quality by scattering the electron beam before it can reach the detector.
Health and safety
At room temperature, ethane is an extremely flammable gas. When mixed with air at 3.0%–12.5% by volume, it forms an explosive mixture.
Some additional precautions are necessary where ethane is stored as a cryogenic liquid. Direct contact with liquid ethane can result in severe frostbite. Until they warm to room temperature, the vapors from liquid ethane are heavier than air and can flow along the floor or ground, gathering in low places; if the vapors encounter an ignition source, the chemical reaction can flash back to the source of ethane from which they evaporated.
Ethane can displace oxygen and become an asphyxiation hazard. Ethane poses no known acute or chronic toxicological risk. It is not a carcinogen.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1514 2022-09-26 14:03:19
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,194
Re: Miscellany
1487) Carbon tetrachloride
Summary
Carbon tetrachloride, also called tetrachloromethane, is a colourless, dense, highly toxic, volatile, nonflammable liquid possessing a characteristic odour and belonging to the family of organic halogen compounds, used principally in the manufacture of dichlorodifluoromethane (a refrigerant and propellant).
First prepared in 1839 by the reaction of chloroform with chlorine, carbon tetrachloride is manufactured by the reaction of chlorine with carbon disulfide or with methane. The process with methane became dominant in the United States in the 1950s, but the process with carbon disulfide remains important in countries where natural gas (the principal source of methane) is not plentiful. Carbon tetrachloride boils at 77° C (171° F) and freezes at -23° C (-9° F); it is much denser than water, in which it is practically insoluble.
Formerly used as a dry-cleaning solvent, carbon tetrachloride has been almost entirely displaced from this application by tetrachloroethylene, which is much more stable and less toxic.
Details
Carbon tetrachloride, also known by many other names (such as tetrachloromethane, also recognised by the IUPAC, carbon tet in the cleaning industry, Halon-104 in firefighting, and Refrigerant-10 in HVACR) is an organic compound with the chemical formula CCl4. It is a colourless liquid with a "sweet" smell that can be detected at low levels. It is practically incombustible at lower temperatures. It was formerly widely used in fire extinguishers, as a precursor to refrigerants and as a cleaning agent, but has since been phased out because of environmental and safety concerns. Exposure to high concentrations of carbon tetrachloride (including vapor) can affect the central nervous system and degenerate the liver and kidneys. Prolonged exposure can be fatal.
Properties
In the carbon tetrachloride molecule, four chlorine atoms are positioned symmetrically as corners in a tetrahedral configuration joined to a central carbon atom by single covalent bonds. Because of this symmetric geometry, CCl4 is non-polar. Methane gas has the same structure, making carbon tetrachloride a halomethane. As a solvent, it is well suited to dissolving other non-polar compounds such as fats and oils. It can also dissolve iodine. It is somewhat volatile, giving off vapors with a smell characteristic of other chlorinated solvents, somewhat similar to the tetrachloroethylene smell reminiscent of dry cleaners' shops.
Solid tetrachloromethane has two polymorphs: crystalline II below −47.5 °C (225.6 K) and crystalline I above −47.5 °C.
With a specific gravity greater than 1, carbon tetrachloride will be present as a dense nonaqueous phase liquid if sufficient quantities are spilled in the environment.
Uses
In organic chemistry, carbon tetrachloride serves as a source of chlorine in the Appel reaction.
Carbon tetrachloride made from heavy chlorine-37 has been used in the detection of neutrinos.
One specialty use of carbon tetrachloride is in stamp collecting, to reveal watermarks on postage stamps without damaging them. A small amount of the liquid is placed on the back of a stamp, sitting in a black glass or obsidian tray. The letters or design of the watermark can then be seen clearly.
Historical uses
Carbon tetrachloride was widely used as a dry cleaning solvent, as a refrigerant, and in lava lamps. In the last case, carbon tetrachloride is a key ingredient that adds weight to the otherwise buoyant wax.
Solvent
It once was a popular solvent in organic chemistry, but, because of its adverse health effects, it is rarely used today. It is sometimes useful as a solvent for infrared spectroscopy, because there are no significant absorption bands above 1600 {cm}^{-1}. Because carbon tetrachloride does not have any hydrogen atoms, it was historically used in proton NMR spectroscopy. In addition to being toxic, its dissolving power is low. Its use in NMR spectroscopy has been largely superseded by deuterated solvents. Use of carbon tetrachloride in determination of oil has been replaced by various other solvents, such as tetrachloroethylene. Because it has no C–H bonds, carbon tetrachloride does not easily undergo free-radical reactions. It is a useful solvent for halogenations either by the elemental halogen or by a halogenation reagent such as N-bromosuccinimide (these conditions are known as Wohl–Ziegler bromination).
Fire suppression
In 1910, the Pyrene Manufacturing Company of Delaware filed a patent to use carbon tetrachloride to extinguish fires. The liquid was vaporized by the heat of combustion and extinguished flames, an early form of gaseous fire suppression. At the time it was believed the gas simply displaced oxygen in the area near the fire, but later research found that the gas actually inhibits the chemical chain reaction of the combustion process.
In 1911, Pyrene patented a small, portable extinguisher that used the chemical. The extinguisher consisted of a brass bottle with an integrated hand-pump that was used to expel a jet of liquid toward the fire. As the container was unpressurized, it could easily be refilled after use. Carbon tetrachloride was suitable for liquid and electrical fires and the extinguishers were often carried on aircraft or motor vehicles. However as early as 1920, there were reports of fatalities caused by the chemical when used to fight a fire in a confined space.
In the first half of the 20th century, another common fire extinguisher was a single-use, sealed glass globe known as a "fire grenade", filled with either carbon tetrachloride or salt water. The bulb could be thrown at the base of the flames to quench the fire. The carbon tetrachloride type could also be installed in a spring-loaded wall fixture with a solder-based restraint. When the solder melted by high heat, the spring would either break the globe or launch it out of the bracket, allowing the extinguishing agent to be automatically dispersed into the fire.
A well-known brand of fire grenade was the "Red Comet", which was variously manufactured with other fire-fighting equipment in the Denver, Colorado area by the Red Comet Manufacturing Company from its founding in 1919 until manufacturing operations were closed in the early 1980s.
Refrigerants
Prior to the Montreal Protocol, large quantities of carbon tetrachloride were used to produce the chlorofluorocarbon refrigerants R-11 (trichlorofluoromethane) and R-12 (dichlorodifluoromethane). However, these refrigerants play a role in ozone depletion and have been phased out. Carbon tetrachloride is still used to manufacture less destructive refrigerants.
Fumigant
Carbon tetrachloride was widely used as a fumigant to kill insect pests in stored grain. It was employed in a mixture known as 80/20, that was 80% carbon tetrachloride and 20% Carbon disulfide. The United States Environmental Protection Agency banned its use in 1985.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1515 2022-09-27 14:06:28
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,194
Re: Miscellany
1488) Phosphoric acid
Summary
Phosphoric acid, also called orthophosphoric acid, (H3PO4), is the most important oxygen acid of phosphorus, used to make phosphate salts for fertilizers. It is also used in dental cements, in the preparation of albumin derivatives, and in the sugar and textile industries. It serves as an acidic, fruitlike flavouring in food products.
Pure phosphoric acid is a crystalline solid (melting point 42.35° C, or 108.2° F); in less concentrated form it is a colourless syrupy liquid. The crude acid is prepared from phosphate rock, while acid of higher purity is made from white phosphorus.
Phosphoric acid forms three classes of salts corresponding to replacement of one, two, or three hydrogen atoms. Among the important phosphate salts are: sodium dihydrogen phosphate (NaH2PO4), used for control of hydrogen ion concentration (acidity) of solutions; disodium hydrogen phosphate (Na2HPO4), used in water treatment as a precipitant for highly charged metal cations; trisodium phosphate (Na3PO4), used in soaps and detergents; calcium dihydrogen phosphate or calcium superphosphate (Ca[H2PO4]2), a major fertilizer ingredient; calcium monohydrogen phosphate (CaHPO4), used as a conditioning agent for salts and sugars.
Phosphoric acid molecules interact under suitable conditions, often at high temperatures, to form larger molecules (usually with loss of water). Thus, diphosphoric, or pyrophosphoric, acid (H4P2O7) is formed from two molecules of phosphoric acid, less one molecule of water. It is the simplest of a homologous series of long chain molecules called polyphosphoric acids, with the general formula H(HPO3)nOH, in which n = 2, 3, 4, . . . . Metaphosphoric acids, (HPO3)n, in which n = 3, 4, 5, . . ., are another class of polymeric phosphoric acids. The known metaphosphoric acids are characterized by cyclic molecular structures. The term metaphosphoric acid is used also to refer to a viscous, sticky substance that is a mixture of both long chain and ring forms of (HPO3)n. The various polymeric forms of phosphoric acid are also prepared by hydration of phosphorus oxides.
Details
Phosphoric acid (orthophosphoric acid, monophosphoric acid or phosphoric(V) acid) is a colorless, odorless phosphorus-containing solid, and inorganic compound with the chemical formula H3PO4. It is commonly encountered as an 85% aqueous solution, which is a colourless, odourless, and non-volatile syrupy liquid. It is a major industrial chemical, being a component of many fertilizers.
Phosphoric acid forms esters, called organophosphates.
The name "orthophosphoric acid" can be used to distinguish this specific acid from other "phosphoric acids", such as pyrophosphoric acid. Nevertheless, the term "phosphoric acid" often means this specific compound; and that is the current IUPAC nomenclature.
Uses
The dominant use of phosphoric acid is for fertilizers, consuming approximately 90% of production.
Food-grade phosphoric acid (additive E338) is used to acidify foods and beverages such as various colas and jams, providing a tangy or sour taste. The phosphoric acid also serves as a preservative. Soft drinks containing phosphoric acid, which would include Coca-Cola, are sometimes called phosphate sodas or phosphates. Phosphoric acid in soft drinks has the potential to cause dental erosion. Phosphoric acid also has the potential to contribute to the formation of kidney stones, especially in those who have had kidney stones previously.
Specific applications of phosphoric acid include:
* in anti-rust treatment by phosphate conversion coating or passivation
* to prevent iron oxidation by means of the Parkerization process
* as an external standard for phosphorus-31 nuclear magnetic resonance
* in phosphoric acid fuel cells
* in activated carbon production[24]
* in compound semiconductor processing, to etch Indium gallium math selectively with respect to indium phosphide
* in microfabrication to etch silicon nitride selectively with respect to silicon dioxide
* as a pH adjuster in cosmetics and skin-care products
* as a sanitizing agent in the dairy, food, and brewing industries
Safety
Phosphoric acid is not a strong acid. However, at moderate concentrations phosphoric acid solutions are irritating to the skin. Contact with concentrated solutions can cause severe skin burns and permanent eye damage.
A link has been shown between long-term regular cola intake and osteoporosis in later middle age in women (but not men).

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1516 2022-09-28 13:57:54
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,194
Re: Miscellany
1489) Maser
Summary
A maser (an acronym for microwave amplification by stimulated emission of radiation) is a device that produces coherent electromagnetic waves through amplification by stimulated emission. The first maser was built by Charles H. Townes, James P. Gordon, and Herbert J. Zeiger at Columbia University in 1953. Townes, Nikolay Basov and Alexander Prokhorov were awarded the 1964 Nobel Prize in Physics for theoretical work leading to the maser. Masers are also used as the timekeeping device in atomic clocks, and as extremely low-noise microwave amplifiers in radio telescopes and deep space spacecraft communication ground stations.
Modern masers can be designed to generate electromagnetic waves at not only microwave frequencies but also radio and infrared frequencies. For this reason, Townes suggested replacing microwave with the word molecular as the first word in the acronym maser.
The laser works by the same principle as the maser but produces higher frequency coherent radiation at visible wavelengths. The maser was the forerunner of the laser, inspiring theoretical work by Townes and Arthur Leonard Schawlow that led to the invention of the laser in 1960 by Theodore Maiman. When the coherent optical oscillator was first imagined in 1957, it was originally called the "optical maser". This was ultimately changed to laser for "Light Amplification by Stimulated Emission of Radiation". Gordon Gould is credited with creating this acronym in 1957.
Details
Maser is a device that produces and amplifies electromagnetic radiation mainly in the microwave region of the spectrum. The maser operates according to the same basic principle as the laser (the name of which is formed from the acronym for “light amplification by stimulated emission of radiation”) and shares many of its characteristics. The first maser was built by the American physicist Charles H. Townes and his colleagues in 1953. The name is an acronym derived from “microwave (or molecular) amplification by stimulated emission of radiation.”
A maser oscillator requires a source of excited atoms or molecules and a resonator to store their radiation. The excitation must force more atoms or molecules into the upper energy level than in the lower, in order for amplification by stimulated emission to predominate over absorption. For wavelengths of a few millimetres or longer, the resonator can be a metal box whose dimensions are chosen so that only one of its modes of oscillation coincides with the frequency emitted by the atoms; that is, the box is resonant at the particular frequency, much as a kettle drum is resonant at some particular audio frequency. The losses of such a resonator can be made quite small, so that radiation can be stored long enough to stimulate emission from successive atoms as they are excited. Thus, all the atoms are forced to emit in such a way as to augment this stored wave. Output is obtained by allowing some radiation to escape through a small hole in the resonator.
The first maser used a beam of ammonia molecules that passed along the axis of a cylindrical cage of metal rods, with alternate rods having positive and negative electric charge. The nonuniform electric field from the rods sorted out the excited from the unexcited molecules, focusing the excited molecules through a small hole into the resonator. The output was less than one microwatt
of power, but the wavelength, being determined primarily by the ammonia molecules, was so constant and reproducible that it could be used to control a clock that would gain or lose no more than a second in several hundred years. This maser can also be used as a microwave amplifier. Maser amplifiers have the advantage that they are much quieter than those that use vacuum tubes or transistors; that is, they add very little noise to the signal being amplified. Very weak signals can thus be utilized. The ammonia maser amplifies only a very narrow band of frequencies and is not tunable, however, so that it has largely been superseded by other kinds, such as solid-state ruby masers.Solid-state and traveling-wave masers
Amplification of radio waves over a wide band of frequencies can be obtained in several kinds of solid-state masers, most commonly crystals such as ruby at low temperatures. Suitable materials contain ions (atoms with an electrical charge) whose energy levels can be shifted by a magnetic field so as to tune the substance to amplify the desired frequency. If the ions have three or more energy levels suitably spaced, they can be raised to one of the higher levels by absorbing radio waves of the proper frequency.
The amplifying crystal may be operated in a resonator that, as in the ammonia maser, stores the wave and so gives it more time to interact with the amplifying medium. A large amplifying bandwidth and easier tunability are obtained with traveling-wave masers. In these, a rod of a suitable crystal, such as ruby, is positioned inside a wave-guide structure that is designed to cause the wave to travel relatively slowly through the crystal.
Solid masers have been used to amplify the faint signals returned from such distant targets as satellites in radar and communications. Their sensitivity is especially important for such applications because signals coming from space are usually very weak. Moreover, there is little interfering background noise when a directional antenna is pointed at the sky, and the highest sensitivity can be used. In radio astronomy, masers made possible the measurement of the faint radio waves emitted by the planet Venus, giving the first indication of its temperature.
Gas masers
Generation of radio waves by stimulated emission of radiation has been achieved in several gases in addition to ammonia. Hydrogen cyanide molecules have been used to produce a wavelength of 3.34 mm. Like the ammonia maser, this maser uses electric fields to select the excited molecules.
One of the best fundamental standards of frequency or time is the atomic hydrogen maser introduced by American scientists N.F. Ramsey, H.M. Goldenberg, and D. Kleppner in 1960. Its output is a radio wave whose frequency of 1,420,405,751.786 hertz (cycles per second) is reproducible with an accuracy of one part in
A clock controlled by such a maser would not get out of step more than one second in 100,000 years.In the hydrogen maser, hydrogen atoms are produced in a discharge and, like the molecules of the ammonia maser, are formed into a beam from which those in excited states are selected and admitted to a resonator. To improve the accuracy, the resonance of each atom is examined over a relatively long time. This is done by using a very large resonator containing a storage bulb. The walls of the bulb are coated so that the atoms can bounce repeatedly against the walls with little disturbance of their frequency.
Another maser standard of frequency or time uses vapour of the element rubidium at a low pressure, contained in a transparent cell. When the rubidium is illuminated by suitably filtered light from a rubidium lamp, the atoms are excited to emit a frequency of 6.835 gigahertz (
. As the cell is enclosed in a cavity resonator with openings for the pumping light, emission of radio waves from these excited atoms is stimulated.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1517 2022-09-29 14:35:34
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,194
Re: Miscellany
1490) Saponification
Summary
What Is Saponification in Soap-Making?
Soap-making is an enjoyable and easy creative hobby that anyone can try. And while there are several different methods for making soap (like melt-and-pour or cold process), it comes down to chemistry regardless of the technique. One of the chemical reactions that happen during the soap-making process is saponification, but what is it and why is it important?
"Saponification is an age-old chemical process where triglycerides (plant or animal fats and oils) are mixed with aqueous lye (sodium hydroxide, NaOH, or potassium hydroxide, KOH, dissolved into water) and then heated to reaction," explains Christopher Fenk, Ph.D., professor and chair of the department of chemistry and biochemistry at Kent State University. "The triglycerides are thereby converted into glycerol and sodium or potassium salts of long chain fatty acids (soaps)."
Lye in Saponification
In the cold process of making soap, different oils like shea butter or argan oil are combined with lye to produce solidified soap. Lye technically refers to sodium hydroxide, which is a very caustic chemical that produces dangerous fumes so it is important to wear protective gear and work in a ventilated room. The saponification generally takes about 24 to 48 hours to complete once the lye and oils have been mixed and the raw soap has been poured into the mold. Afterwards, it should be left to air-dry for approximately four to six weeks; this is known as the curing time and it will allow for any excess water to evaporate out of the soap.
Impact of Ingredients
"The key variables are the choice of fat or oil and the type of base used (NaOH or KOH). Vegetable oils are now often used, but, animal fats may be used as well. It is critical that the amount of base be kept to a minimum otherwise the soap produced can be very harmful to the skin," says Fenk. "The type of base (NaOH or KOH) determines the kind of soap. Sodium hydroxide (NaOH) is used to produce hard soaps like bar soap. Whereas, potassium hydroxide (KOH) is used to make liquid (or soft) soaps." He notes that artificial colors and fragrances are often added to soap simply to improve its appeal.
You can add a variety of ingredients to your soap including oatmeal, fruit and herbs, and flowers. The other ingredients that you put in the soap can provide additional benefits toward your skin care, though not necessarily with lathering and cleansing abilities. How well the soap lathers and cleanses also depends on the oils used for the soap and their reaction with the lye, so you can get a variety of soaps using different ingredients.
When you are making soap, you are testing—by trial and error—with chemistry. Soap is one of those crafts that also have a scientific component and can be great for experimenting with different ingredients. Just make sure to thoroughly research the ingredients that you use, the proportions of oil to lye, and that you also follow safety precautions because lye is a volatile substance. Try it, and you will have beautiful soaps to use in your bathroom, sell at craft fairs, or give to friends.
Details
Saponification is a process that involves the conversion of fat, oil, or lipid, into soap and alcohol by the action of aqueous alkali (for example, sodium hydroxide). Soaps are salts of fatty acids, which in turn are carboxylic acids with long carbon chains. A typical soap is sodium oleate.
Saponification of fats
Vegetable oils and animal fats are the traditional materials that are saponified. These greasy materials, triesters called triglycerides, are mixtures derived from diverse fatty acids. Triglycerides can be converted to soap in either a one- or a two-step process. In the traditional one-step process, the triglyceride is treated with a strong base (e.g. lye), which cleaves the ester bond, releasing fatty acid salts (soaps) and glycerol. This process is also the main industrial method for producing glycerol. In some soap-making, the glycerol is left in the soap. If necessary, soaps may be precipitated by salting it out with sodium chloride.
Fat in a corpse converts into adipocere, often called "grave wax". This process is more common where the amount of fatty tissue is high and the agents of decomposition are absent or only minutely present.
Saponification values
The saponification value is the amount of base required to saponify a fat sample. Soap makers formulate their recipes with a small deficit of lye to account for the unknown deviation of saponification value between their oil batch and laboratory averages.
Saponification of fatty acids
The reaction of fatty acids with base is the other main method of saponification. In this case, the reaction involves neutralization of the carboxylic acid. The neutralization method is used to produce industrial soaps such as those derived from magnesium, the transition metals, and aluminium. This method is ideal for producing soaps that are derived from a single fatty acid, which leads to soaps with predictable physical properties, as required by many engineering applications.
Applications:
Soft versus hard soap
Depending on the nature of the alkali used in their production, soaps have distinct properties. Sodium hydroxide (NaOH) produces "hard soap"; hard soaps can also be used in water containing Mg, Cl, and Ca salts.[citation needed] By contrast, potassium soaps, (derived using KOH) are soft soap. The fatty acid source also affects the soap's melting point. Most early hard soaps were manufactured using animal fats and KOH extracted from wood ash; these were broadly solid. However, the majority of modern soaps are manufactured from polyunsaturated triglycerides such as vegetable oils. As in the triglycerides they are formed from the salts of these acids have weaker inter-molecular forces and thus lower melting points.
Lithium soaps
Lithium derivatives of 12-hydroxystearate and other fatty acids are important constituents of lubricating greases. In lithium-based greases, lithium carboxylates are thickeners. "Complex soaps" are also common, these being combinations of more than one acid salt, such as azelaic or acetic acid.
Fire extinguishers
Fires involving cooking fats and oils (classified as class K (US) or F (Australia/Europe/Asia)) burn hotter than most flammable liquids, rendering a standard class B extinguisher ineffective. Such fires should be extinguished with a wet chemical extinguisher. Extinguishers of this type are designed to extinguish cooking fats and oils through saponification. The extinguishing agent rapidly converts the burning substance to a non-combustible soap.
Oil paints
Saponification can occur in oil paintings over time, causing visible damage and deformation. Oil paints are composed of pigment molecules suspended in an oil-binding medium. Heavy metal salts are often used as pigment molecules, such as in lead white, red lead, and zinc white. If those heavy metal salts react with free fatty acids in the oil medium, metal soaps may form in a paint layer that can then migrate outward to the painting's surface.
Saponification in oil paintings was described as early as 1912. It is believed to be widespread, having been observed in many works dating from the fifteenth through the twentieth centuries; works of different geographic origin; and works painted on various supports, such as canvas, paper, wood, and copper. Chemical analysis may reveal saponification occurring in a painting's deeper layers before any signs are visible on the surface, even in paintings centuries old.
The saponified regions may deform the painting's surface through the formation of visible lumps or protrusions that can scatter light. These soap lumps may be prominent only on certain regions of the painting rather than throughout. In John Singer Sargent's famous Portrait of Madame X, for example, the lumps only appear on the blackest areas, which may be because of the artist's use of more medium in those areas to compensate for the tendency of black pigments to soak it up. The process can also form chalky white deposits on a painting's surface, a deformation often described as "blooming" or "efflorescence", and may also contribute to the increased transparency of certain paint layers within an oil painting over time.
Saponification does not occur in all oil paintings and many details are unresolved. At present, retouching is the only known restoration method.
:max_bytes(150000):strip_icc():format(webp)/soap-quantities-102408965-resized-58a4aead5f9b58819cf49ba9.jpg)
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1518 2022-09-30 14:23:34
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,194
Re: Miscellany
1491) Washing Soda
Sodium carbonate, Na2CO3, (also known as washing soda, soda ash and soda crystals) is the inorganic compound with the formula Na2CO3 and its various hydrates. All forms are white, odourless, water-soluble salts that yield moderately alkaline solutions in water. Historically, it was extracted from the ashes of plants growing in sodium-rich soils. Because the ashes of these sodium-rich plants were noticeably different from ashes of wood (once used to produce potash), sodium carbonate became known as "soda ash". It is produced in large quantities from sodium chloride and limestone by the Solvay process.
Details
Washing soda
Sodium carbonate decahydrate (Na2CO3·10H2O), also known as washing soda, is the most common hydrate of sodium carbonate containing 10 molecules of water of crystallization. Soda ash is dissolved in water and crystallized to get washing soda.
* It is white crystalline solid;
* It is one of the few metal carbonates which are soluble in water;
* It is alkaline; it turns red litmus to blue;
* It has detergent properties through the process of saponification which makes fats and grease water-miscible.
Applications
Some common applications of sodium carbonate include:
* As a cleansing agent for domestic purposes like washing clothes. Sodium carbonate is a component of many dry soap powders.
* It is used for removing temporary and permanent hardness of water.
* It is used in the manufacture of glass, soap and paper.
* It is used in the manufacture of sodium compounds like borax.
Glass manufacture
Sodium carbonate serves as a flux for silica, lowering the melting point of the mixture to something achievable without special materials. This "soda glass" is mildly water-soluble, so some calcium carbonate is added to the melt mixture to make the glass insoluble. Bottle and window glass (soda-lime glass) is made by melting such mixtures of sodium carbonate, calcium carbonate, and silica sand (silicon dioxide (SiO2)). When these materials are heated, the carbonates release carbon dioxide. In this way, sodium carbonate is a source of sodium oxide. Soda-lime glass has been the most common form of glass for centuries. It is also a key input for tableware glass manufacturing.
Water softening
Hard water contains dissolved compounds, usually calcium or magnesium compounds. Sodium carbonate is used for removing temporary and permanent hardness of water.
As sodium carbonate is water-soluble and magnesium carbonate and calcium carbonate are insoluble, the former is used to soften water by removing Mg2+ and Ca2+.
The water is softened because it no longer contains dissolved calcium ions and magnesium ions.
Food additive and cooking
Sodium carbonate has several uses in cuisine, largely because it is a stronger base than baking soda (sodium bicarbonate) but weaker than lye (which may refer to sodium hydroxide or, less commonly, potassium hydroxide). Alkalinity affects gluten production in kneaded doughs, and also improves browning by reducing the temperature at which the Maillard reaction occurs. To take advantage of the former effect, sodium carbonate is therefore one of the components of kansui, a solution of alkaline salts used to give Japanese ramen noodles their characteristic flavor and chewy texture; a similar solution is used in Chinese cuisine to make lamian, for similar reasons. Cantonese bakers similarly use sodium carbonate as a substitute for lye-water to give moon cakes their characteristic texture and improve browning. In German cuisine (and Central European cuisine more broadly), breads such as pretzels and lye rolls traditionally treated with lye to improve browning can be treated instead with sodium carbonate; sodium carbonate does not produce quite as strong a browning as lye, but is much safer and easier to work with.
Sodium carbonate is used in the production of sherbet powder. The cooling and fizzing sensation results from the endothermic reaction between sodium carbonate and a weak acid, commonly citric acid, releasing carbon dioxide gas, which occurs when the sherbet is moistened by saliva.
Sodium carbonate also finds use in food industry as a food additive (E500) as an acidity regulator, anticaking agent, raising agent, and stabilizer. It is also used in the production of snus to stabilize the pH of the final product.
While it is less likely to cause chemical burns than lye, care must still be taken when working with sodium carbonate in the kitchen, as it is corrosive to aluminum cookware, utensils, and foil.
Other applications
Sodium carbonate is also used as a relatively strong base in various fields. As a common alkali, it is preferred in many chemical processes because it is cheaper than sodium hydroxide and far safer to handle. Its mildness especially recommends its use in domestic applications.
For example, it is used as a pH regulator to maintain stable alkaline conditions necessary for the action of the majority of photographic film developing agents. It is also a common additive in swimming pools and aquarium water to maintain a desired pH and carbonate hardness (KH). In dyeing with fiber-reactive dyes, sodium carbonate (often under a name such as soda ash fixative or soda ash activator) is used to ensure proper chemical bonding of the dye with cellulose (plant) fibers, typically before dyeing (for tie dyes), mixed with the dye (for dye painting), or after dyeing (for immersion dyeing). It is also used in the froth flotation process to maintain a favourable pH as a float conditioner besides CaO and other mildly basic compounds.
Precursor to other compounds
Sodium bicarbonate (NaHCO3) or baking soda, also a component in fire extinguishers, is often generated from sodium carbonate.
Although NaHCO3 is itself an intermediate product of the Solvay process, the heating needed to remove the ammonia that contaminates it decomposes some NaHCO3, making it more economic to react finished Na2CO3 with CO2.
In a related reaction, sodium carbonate is used to make sodium bisulfite (NaHSO3), which is used for the "sulfite" method of separating lignin from cellulose.
Sodium carbonate is used by the cotton industry to neutralize the sulfuric acid needed for acid delinting of fuzzy cottonseed.
It is also used to form carbonates of other metals by ion exchange, often with the other metals’ sulphates.
Miscellaneous
Sodium carbonate is used by the brick industry as a wetting agent to reduce the amount of water needed to extrude the clay. In casting, it is referred to as "bonding agent" and is used to allow wet alginate to adhere to gelled alginate. Sodium carbonate is used in toothpastes, where it acts as a foaming agent and an abrasive, and to temporarily increase mouth pH.
Sodium carbonate is also used in the processing and tanning of animal hides.[citation needed]
Physical properties
The integral enthalpy of solution of sodium carbonate is −28.1 kJ/mol for a 10% w/w aqueous solution. The Mohs hardness of sodium carbonate monohydrate is 1.3.
Occurrence as natural mineral
Sodium carbonate is soluble in water, and can occur naturally in arid regions, especially in mineral deposits (evaporites) formed when seasonal lakes evaporate. Deposits of the mineral natron have been mined from dry lake bottoms in Egypt since ancient times, when natron was used in the preparation of mummies and in the early manufacture of glass.
The anhydrous mineral form of sodium carbonate is quite rare and called natrite. Sodium carbonate also erupts from Ol Doinyo Lengai, Tanzania's unique volcano, and it is presumed to have erupted from other volcanoes in the past, but due to these minerals' instability at the earth's surface, are likely to be eroded. All three mineralogical forms of sodium carbonate, as well as trona, trisodium hydrogendicarbonate dihydrate, are also known from ultra-alkaline pegmatitic rocks, that occur for example in the Kola Peninsula in Russia.
Extraterrestrially, known sodium carbonate is rare. Deposits have been identified as the source of bright spots on Ceres, interior material that has been brought to the surface.[21] While there are carbonates on Mars, and these are expected to include sodium carbonate, deposits have yet to be confirmed, this absence is explained by some as being due to a global dominance of low pH in previously aqueous Martian soil.
Solvay process
In 1861, the Belgian industrial chemist Ernest Solvay developed a method to make sodium carbonate by first reacting sodium chloride, ammonia, water, and carbon dioxide to generate sodium bicarbonate and ammonium chloride.
The Solvay process recycles its ammonia. It consumes only brine and limestone, and calcium chloride is its only waste product. The process is substantially more economical than the Leblanc process, which generates two waste products, calcium sulfide and hydrogen chloride. The Solvay process quickly came to dominate sodium carbonate production worldwide. By 1900, 90% of sodium carbonate was produced by the Solvay process, and the last Leblanc process plant closed in the early 1920s.
The second step of the Solvay process, heating sodium bicarbonate, is used on a small scale by home cooks and in restaurants to make sodium carbonate for culinary purposes (including pretzels and alkali noodles). The method is appealing to such users because sodium bicarbonate is widely sold as baking soda, and the temperatures required (250 °F (121 °C) to 300 °F (149 °C)) to convert baking soda to sodium carbonate are readily achieved in conventional kitchen ovens.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1519 2022-10-01 14:45:34
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,194
Re: Miscellany
1492) Sodium bicarbonate
Summary
Sodium bicarbonate (NaHCO3), also called baking soda, bicarbonate of soda, or sodium hydrogen carbonate, is a white crystalline or powdery solid that is a source of carbon dioxide and so is used as an ingredient in baking powders, in effervescent salts and beverages, and as a constituent of dry-chemical fire extinguishers. Its slight alkalinity makes it useful in treating gastric or urinary hyperacidity and acidosis. It is also employed in certain industrial processes, as in tanning and the preparation of wool.
Many bakery products are leavened by carbon dioxide from added baking soda or sodium bicarbonate in baking powder. When added without the offsetting amounts of dry acids or acid salts present in baking powder, sodium bicarbonate tends to make dough or batter alkaline, causing flavour deterioration and discoloration and slowing carbon dioxide release. Addition of an acid-reacting substance promotes vigorous gas evolution and maintains acidity within a favourable range. The rate of gas release affects the size of the bubbles produced in the dough or batter, consequently influencing the grain, volume, and texture of the finished product.
Details
Sodium bicarbonate (IUPAC name: sodium hydrogencarbonate), commonly known as baking soda or bicarbonate of soda, is a chemical compound with the formula NaHCO3. It is a salt composed of a sodium cation (Na+) and a bicarbonate anion (HCO3−). Sodium bicarbonate is a white solid that is crystalline, but often appears as a fine powder. It has a slightly salty, alkaline taste resembling that of washing soda (sodium carbonate). The natural mineral form is nahcolite. It is a component of the mineral natron and is found dissolved in many mineral springs.
Nomenclature
Because it has long been known and widely used, the salt has many different names such as baking soda, bread soda, cooking soda, and bicarbonate of soda and can often be found near baking powder in stores. The term baking soda is more common in the United States, while bicarbonate of soda is more common in Australia, United Kingdom and Ireland. and in many northern/central European countries it is called Natron. Abbreviated colloquial forms such as sodium bicarb, bicarb soda, bicarbonate, and bicarb are common.
The word saleratus, from Latin sal æratus (meaning "aerated salt"), was widely used in the 19th century for both sodium bicarbonate and potassium bicarbonate.
Its E number food additive code is E500.
The prefix bi in bicarbonate comes from an outdated naming system predating molecular knowledge in reference to the two molar equivalents of carbon dioxide (known as carbonic acid in the ancient chemistry language) that potassium hydrocarbonate/bicarbonate releases upon decomposition to (di)potassium carbonate and to potassium oxide (potash). The modern chemical formulas of these compounds now express their precise chemical compositions which were unknown when the name bi-carbonate of potash was coined.
Uses:
Cooking
In cooking, baking soda is primarily used in baking as a leavening agent. When it reacts with acid, carbon dioxide is released, which causes expansion of the batter and forms the characteristic texture and grain in cakes, quick breads, soda bread, and other baked and fried foods.
Acidic materials that induce this reaction include hydrogen phosphates, cream of tartar, lemon juice, yogurt, buttermilk, cocoa, and vinegar. Baking soda may be used together with sourdough, which is acidic, making a lighter product with a less acidic taste.
Heat can also by itself cause sodium bicarbonate to act as a raising agent in baking because of thermal decomposition, releasing carbon dioxide at temperatures above 80 °C (180 °F).
When used this way on its own, without the presence of an acidic component (whether in the batter or by the use of a baking powder containing acid), only half the available CO2 is released (one CO2 molecule is formed for every two equivalents of NaHCO3). Additionally, in the absence of acid, thermal decomposition of sodium bicarbonate also produces sodium carbonate, which is strongly alkaline and gives the baked product a bitter, "soapy" taste and a yellow color. Since the reaction occurs slowly at room temperature, mixtures (cake batter, etc.) can be allowed to stand without rising until they are heated in the oven.
Baking powder
Baking powder, also sold for cooking, contains around 30% of bicarbonate, and various acidic ingredients which are activated by the addition of water, without the need for additional acids in the cooking medium. Many forms of baking powder contain sodium bicarbonate combined with calcium acid phosphate, sodium aluminium phosphate, or cream of tartar. Baking soda is alkaline; the acid used in baking powder avoids a metallic taste when the chemical change during baking creates sodium carbonate.
Pyrotechnics
Sodium bicarbonate is one of the main components of the common "black snake" firework. The effect is caused by the thermal decomposition, which produces carbon dioxide gas to produce a long snake-like ash as a combustion product of the other main component, sucrose. Sodium bicarbonate is also used to delay combustion reactions by releasing CO2 and H2O when heated, both of which are flame retardants.
Mild disinfectant
It has weak disinfectant properties, and it may be an effective fungicide against some organisms. Because baking soda will absorb musty smells, it has become a reliable method for used book sellers when making books less malodorous.
Fire extinguisher
Sodium bicarbonate can be used to extinguish small grease or electrical fires by being thrown over the fire, as heating of sodium bicarbonate releases carbon dioxide. However, it should not be applied to fires in deep fryers; the sudden release of gas may cause the grease to splatter. Sodium bicarbonate is used in BC dry chemical fire extinguishers as an alternative to the more corrosive monoammonium phosphate in ABC extinguishers. The alkaline nature of sodium bicarbonate makes it the only dry chemical agent, besides Purple-K, that was used in large-scale fire suppression systems installed in commercial kitchens. Because it can act as an alkali, the agent has a mild saponification effect on hot grease, which forms a smothering, soapy foam.[citation needed]
Neutralization of acids
Sodium bicarbonate reacts spontaneously with acids, releasing CO2 gas as a reaction product. It is commonly used to neutralize unwanted acid solutions or acid spills in chemical laboratories. It is not appropriate to use sodium bicarbonate to neutralize base even though it is amphoteric, reacting with both acids and bases.
Agriculture
Sodium bicarbonate when applied on leaves, can prevent the growth of fungi; however, it does not kill the fungus. Excessive amount of sodium bicarbonate can cause discolouration of fruits (two percent solution) and chlorosis (one percent solution).
Medical uses and health
Sodium bicarbonate mixed with water can be used as an antacid to treat acid indigestion and heartburn.
A mixture of sodium bicarbonate and polyethylene glycol such as PegLyte, dissolved in water and taken orally, is an effective gastrointestinal lavage preparation and laxative prior to gastrointestinal surgery, gastroscopy, etc.[citation needed]
Intravenous sodium bicarbonate in an aqueous solution is sometimes used for cases of acidosis, or when insufficient sodium or bicarbonate ions are in the blood. In cases of respiratory acidosis, the infused bicarbonate ion drives the carbonic acid/bicarbonate buffer of plasma to the left, and thus raises the pH. For this reason, sodium bicarbonate is used in medically supervised cardiopulmonary resuscitation. Infusion of bicarbonate is indicated only when the blood pH is markedly low (< 7.1–7.0).
HCO3− is used for treatment of hyperkalemia, as it will drive K+ back into cells during periods of acidosis. Since sodium bicarbonate can cause alkalosis, it is sometimes used to treat aspirin overdoses. Aspirin requires an acidic environment for proper absorption, and a basic environment will diminish aspirin absorption in cases of overdose. Sodium bicarbonate has also been used in the treatment of tricyclic antidepressant overdose. It can also be applied topically as a paste, with three parts baking soda to one part water, to relieve some kinds of insect bites and stings (as well as accompanying swelling).
Some alternative practitioners, such as Tullio Simoncini, have promoted baking soda as a cancer cure, which the American Cancer Society has warned against due to both its unproven effectiveness and potential danger in use. Edzard Ernst has called the promotion of sodium bicarbonate as a cancer cure "one of the more sickening alternative cancer scams I have seen for a long time".
Sodium bicarbonate can be added to local anesthetics, to speed up the onset of their effects and make their injection less painful. It is also a component of Moffett's solution, used in nasal surgery.
It has been proposed that acidic diets weaken bones. One systematic meta-analysis of the research shows no such effect. Another also finds that there is no evidence that alkaline diets improve bone health, but suggests that there "may be some value" to alkaline diets for other reasons.
Antacid (such as baking soda) solutions have been prepared and used by protesters to alleviate the effects of exposure to tear gas during protests.
Personal hygiene
Sodium bicarbonate is also used as an ingredient in some mouthwashes. It has anticaries and abrasive properties. It works as a mechanical cleanser on the teeth and gums, neutralizes the production of acid in the mouth, and also acts as an antiseptic to help prevent infections. Sodium bicarbonate in combination with other ingredients can be used to make a dry or wet deodorant. Sodium bicarbonate may be used as a buffering agent, combined with table salt, when creating a solution for nasal irrigation.
It is used in eye hygiene to treat blepharitis. This is done by addition of a teaspoon of sodium bicarbonate to cool water that was recently boiled, followed by gentle scrubbing of the eyelash base with a cotton swab dipped in the solution.
Veterinary uses
Sodium bicarbonate is used as a cattle feed supplement, in particular as a buffering agent for the rumen.
Cleaning agent
Sodium bicarbonate is used in a process for removing paint and corrosion called sodablasting. As a blasting medium, sodium bicarbonate is used to remove surface contamination from softer and less resilient substrates such as aluminium, copper or timber which could be damaged by silica sand abrasive media.
A manufacturer recommends a paste made from baking soda with minimal water as a gentle scouring powder, and is useful in removing surface rust, as the rust forms a water-soluble compound when in a concentrated alkaline solution; cold water should be used, as hot-water solutions can corrode steel. Sodium bicarbonate attacks the thin protective oxide layer that forms on aluminium, making it unsuitable for cleaning this metal. A solution in warm water will remove the tarnish from silver when the silver is in contact with a piece of aluminium foil. Baking soda is commonly added to washing machines as a replacement for water softener and to remove odors from clothes. It is also almost as effective in removing heavy tea and coffee stains from cups as Sodium hydroxide, when diluted with warm water.
During the Manhattan Project to develop the nuclear bomb in the early 1940s, the chemical toxicity of uranium was an issue. Uranium oxides were found to stick very well to cotton cloth, and did not wash out with soap or laundry detergent. However, the uranium would wash out with a 2% solution of sodium bicarbonate. Clothing can become contaminated with toxic dust of depleted uranium (DU), which is very dense, hence used for counterweights in a civilian context, and in armour-piercing projectiles. DU is not removed by normal laundering; washing with about 6 ounces (170 g) of baking soda in 2 gallons (7.5 L) of water will help to wash it out.
Odor control
It is often claimed that baking soda is an effective odor remover, and it is often recommended that an open box be kept in the refrigerator to absorb odor. This idea was promoted by the leading U.S. brand of baking soda, Arm & Hammer, in an advertising campaign starting in 1972. Though this campaign is considered a classic of marketing, leading within a year to more than half of American refrigerators containing a box of baking soda, there is little evidence that it is in fact effective in this application.
Chemistry
Sodium bicarbonate is an amphoteric compound. Aqueous solutions are mildly alkaline due to the formation of carbonic acid and hydroxide ion.
Sodium bicarbonate can often be used as a safer alternative to sodium hydroxide, and as such can be used as a wash to remove any acidic impurities from a "crude" liquid, producing a purer sample. Reaction of sodium bicarbonate and an acid produces a salt and carbonic acid, which readily decomposes to carbon dioxide and water.
Sodium bicarbonate reacts with acetic acid (found in vinegar), producing sodium acetate, water, and carbon dioxide.
Sodium bicarbonate reacts with bases such as sodium hydroxide to form carbonates.
Thermal decomposition
At temperatures from 80–100 °C (176–212 °F), sodium bicarbonate gradually decomposes into sodium carbonate, water, and carbon dioxide. The conversion is faster at 200 °C (392 °F).

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1520 2022-10-02 14:12:38
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,194
Re: Miscellany
1493) Chloroform
Summary
Chloroform, or trichloromethane, is an organic compound with formula CHCl3 and is a common organic solvent. It is a colorless, strong-smelling, dense liquid produced on a large scale as a precursor to PTFE. It is also a precursor to various refrigerants. It is one of the four chloromethanes and a trihalomethane. It is a powerful anesthetic, euphoriant, anxiolytic and sedative when inhaled or ingested.
Details
is a
Chloroform (CHCl3), also called trichloromethane, nonflammable, clear, colourless liquid that is denser than water and has a pleasant etherlike odour. It was first prepared in 1831. The Scottish physician Sir James Simpson of the University of Edinburgh was the first to use it as an anesthetic in 1847. It later captured public notice in 1853 when English physician John Snow administered it to Queen Victoria during the birth of Prince Leopold, her eighth child.
Chloroform has a relatively narrow margin of safety and has been replaced by better inhalation anesthetics. In addition, it is believed to be toxic to the liver and kidneys and may cause liver cancer. Chloroform was once widely used as a solvent, but safety and environmental concerns have reduced this use as well. Nevertheless, chloroform has remained an important industrial chemical.
Chloroform is prepared by the chlorination of methane. The major use of chloroform is in the preparation of chlorodifluoromethane (HCFC-22). HCFC-22 contributes to depletion of the ozone layer, and its production is scheduled to halt by 2020 in the United States. As HCFC-22 production is phased out, chloroform production is expected to decrease significantly.
Chloroform is formed by the reaction of chlorine with organic substances present in water and thus can occur in drinking water that has been chlorinated. The limit set by the U.S. Environmental Protection Agency for chloroform contamination is 80 parts per billion (ppb); a typical municipal water supply contains roughly 50 ppb.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1521 2022-10-03 14:35:55
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,194
Re: Miscellany
1494) Nitrous Oxide
Summary
Nitrous oxide (dinitrogen oxide or dinitrogen monoxide), commonly known as laughing gas, nitrous, or nos, is a chemical compound, an oxide of nitrogen with the formula N2O. At room temperature, it is a colourless non-flammable gas, and has a slightly sweet scent and taste. At elevated temperatures, nitrous oxide is a powerful oxidiser similar to molecular oxygen.
Nitrous oxide has significant medical uses, especially in surgery and dentistry, for its anaesthetic and pain reducing effects. Its colloquial name, "laughing gas", coined by Humphry Davy, is due to the euphoric effects upon inhaling it, a property that has led to its recreational use as a dissociative anaesthetic. It is on the World Health Organization's List of Essential Medicines. It is also used as an oxidiser in rocket propellants, and in motor racing to increase the power output of engines.
Nitrous oxide's atmospheric concentration reached 333 parts per billion (ppb) in 2020, increasing at a rate of about 1 ppb annually. It is a major scavenger of stratospheric ozone, with an impact comparable to that of CFCs. Global accounting of N2O sources and sinks over the decade ending 2016 indicates that about 40% of the average 17 TgN/yr (Teragrams of Nitrogen per year) of emissions originated from human activity, and shows that emissions growth chiefly came from expanding agriculture and industry sources within emerging economies. Being the third most important long-lived greenhouse gas, nitrous oxide also substantially contributes to global warming.
Nitrous oxide is used as a propellant, and has a variety of applications from rocketry to making whipped cream. It is abused as a recreational drug for its potential to induce a brief "high"; most recreational users are often unaware of its neurotoxicity and potential to cause neurological damage.
Details
Nitrous oxide (N2O), also called dinitrogen monoxide, is also known as laughing gas, or nitrous, one of several oxides of nitrogen, a colourless gas with pleasant, sweetish odour and taste, which when inhaled produces insensibility to pain preceded by mild hysteria, sometimes laughter. (Because inhalation of small amounts provides a brief euphoric effect and nitrous oxide is not illegal to possess, the substance has been used as a recreational drug.) Nitrous oxide was discovered by the English chemist Joseph Priestley in 1772; another English chemist, Humphry Davy, later named it and showed its physiological effect. A principal use of nitrous oxide is as an anesthetic in surgical operations of short duration; prolonged inhalation causes death. The gas is also used as a propellant in food aerosols. In automobile racing, nitrous oxide is injected into an engine’s air intake; the extra oxygen allows the engine to burn more fuel per stroke. It is prepared by the action of zinc on dilute nitric acid, by the action of hydroxylamine hydrochloride (NH2OH·HCl) on sodium nitrite (NaNO2), and, most commonly, by the decomposition of ammonium nitrate (NH4NO3).
Nitrous oxide, commonly known as laughing gas or happy gas, is a colorless, non-flammable gas. This gas is used in medical and dental procedures as a sedative. It helps to relieve anxiety before the procedure and allow the patient to relax.
How Is Laughing Gas Used?
Laughing gas is an anesthetic used by medical professionals to help you remain calm before a procedure. It’s not meant to put you fully to sleep.
Before your procedure, your doctor will ask you for your consent to use nitrous oxide. After that, a plastic mask will be placed over your mouth and nose. The laughing gas flows through the mask and you breathe it in.
Children may be given laughing gas through a nasal hood, which covers their nose but not their mouth. Sometimes, a familiar scent will be added in to help them get used to having the mask or nasal hood on.
You’ll start to feel the effects of the laughing gas within a few minutes. As laughing gas doesn’t put you fully to sleep, you’ll still be able to hear what’s going on around you. You may still be able to respond to questions that your doctor asks you and follow the instructions that they give you throughout the procedure.
Nitrous oxide is a depressant, so it slows your body down. Once it kicks in, you may feel:
* Happy
* Giggly
* Light-headed
* Mild euphoria
* Relaxed
Nitrous oxide gets the name “laughing gas” because of these effects. Some people may also experience mild hallucinations while under the use of laughing gas.
Physically, you might feel like your arms and legs are heavy. You may also experience a tingling sensation in your limbs.
Once your procedure is over, your doctor will remove the mask that’s providing the nitrous oxide. The effects of laughing gas typically wear off within a few minutes. Children might be given 100% oxygen following the removal of the nitrous oxide mask. The oxygen helps them to fully recover within minutes.
Possible Side Effects of Laughing Gas
Nitrous oxide is safe to use under the proper care of a doctor. However, some people may experience side effects either during or after use.
The most common side effects of laughing gas are headaches and nausea. Children may also feel agitated or might vomit after the laughing gas is removed. The good news is that only about 5% of patients experience these side effects.
There are no long-term side effects of nitrous oxide when it is only used occasionally. However, if you have to undergo multiple or frequent procedures that require using laughing gas, your doctor might recommend you take a B12 supplement. This is to help prevent anemia.
Risks of Using Laughing Gas
As mentioned, laughing gas is safe to use when it is given to you by a doctor and you are under medical care. However, there are several risks of using nitrous oxide when it is not given to you by a doctor.
When used as a recreational drug, nitrous oxide is usually dispensed into another object, like a bag or balloon, or directly into the mouth. Because of this, nitrous oxide is classified as an inhalant. Nitrous oxide can be found in the silver chargers that are used to make whipped cream. Recreational users inhale laughing gas for the euphoric effect.
Along with negative side effects, there are several risks of using laughing gas as a recreational drug, including:
* Lowering of blood pressure
* Fainting
* Heart attack
* Hypoxia, or the fatal loss of oxygen
* B12 deficiency and anemia
* Nerve damage due to the tingling sensation
Prolonged recreational use of nitrous oxide has several negative long-term effects, like:
* Memory loss
* Incontinence
* Depression
* Psychological dependence
* Psychosis
* Weak immune system
* Numbness in hands and feet
* Limb spasms
* Ringing in your ears
Another of the risks of using laughing gas is that it affects your coordination, like many other recreational drugs. This can be dangerous if nitrous oxide is used in a place where you could fall and hurt yourself or cause danger to others. It is also very dangerous to try to drive or operate machinery while under the effects of laughing gas.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1522 2022-10-04 13:37:02
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,194
Re: Miscellany
1495) Lawn mower
Gist
A garden tool for mowing grass on lawns.
Details
A lawn mower (also known as a mower, grass cutter or lawnmower) is a machine utilizing one or more revolving blades (or a reel) to cut a grass surface to an even height. The height of the cut grass may be fixed by the design of the mower, but generally is adjustable by the operator, typically by a single master lever, or by a lever or nut and bolt on each of the machine's wheels. The blades may be powered by manual force, with wheels mechanically connected to the cutting blades so that when the mower is pushed forward, the blades spin or the machine may have a battery-powered or plug-in electric motor. The most common self-contained power source for lawn mowers is a small (typically one cylinder) internal combustion engine. Smaller mowers often lack any form of propulsion, requiring human power to move over a surface; "walk-behind" mowers are self-propelled, requiring a human only to walk behind and guide them. Larger lawn mowers are usually either self-propelled "walk-behind" types or more often, are "ride-on" mowers, equipped so the operator can ride on the mower and control it. A robotic lawn mower ("lawn-mowing bot", "mowbot", etc.) is designed to operate either entirely on its own or less commonly by an operator by remote control.
Two main styles of blades are used in lawn mowers. Lawn mowers employing a single blade that rotates about a single vertical axis are known as rotary mowers, while those employing a cutting bar and multiple blade assembly that rotates about a single horizontal axis are known as cylinder or reel mowers (although in some versions, the cutting bar is the only blade, and the rotating assembly consists of flat metal pieces which force the blades of grass against the sharp cutting bar).
There are several types of mowers, each suited to a particular scale and purpose. The smallest types, non-powered push mowers, are suitable for small residential lawns and gardens. Electrical or piston engine-powered push-mowers are used for larger residential lawns (although there is some overlap). Riding mowers, which sometimes resemble small tractors, are larger than push mowers and are suitable for large lawns, although commercial riding lawn mowers (such as zero-turn mowers) can be "stand-on" types, and often bear little resemblance to residential lawn tractors, being designed to mow large areas at high speed in the shortest time possible. The largest multi-gang (multi-blade) mowers are mounted on tractors and are designed for large expanses of grass such as golf courses and municipal parks, although they are ill-suited for complex terrain.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1523 2022-10-05 14:40:42
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,194
Re: Miscellany
1496) Agriculture
Summary
Agriculture or farming is the practice of cultivating plants and livestock. Agriculture was the key development in the rise of sedentary human civilization, whereby farming of domesticated species created food surpluses that enabled people to live in cities. The history of agriculture began thousands of years ago. After gathering wild grains beginning at least 105,000 years ago, nascent farmers began to plant them around 11,500 years ago. Pigs, sheep, goats, and cattle were domesticated over 10,000 years ago. Plants were independently cultivated in at least 11 regions of the world. Industrial agriculture based on large-scale monoculture in the twentieth century came to dominate agricultural output, though about 2 billion people still depended on subsistence agriculture.
The major agricultural products can be broadly grouped into foods, fibers, fuels, and raw materials (such as rubber). Food classes include cereals (grains), vegetables, fruits, oils, meat, milk, eggs, and fungi. Over one-third of the world's workers are employed in agriculture, second only to the service sector, although in recent decades, the global trend of a decreasing number of agricultural workers continues, especially in developing countries, where smallholding is being overtaken by industrial agriculture and mechanization that brings an enormous crop yield increase.
Modern agronomy, plant breeding, agrochemicals such as pesticides and fertilizers, and technological developments have sharply increased crop yields, but cause ecological and environmental damage. Selective breeding and modern practices in animal husbandry have similarly increased the output of meat but have raised concerns about animal welfare and environmental damage. Environmental issues include contributions to global warming, depletion of aquifers, deforestation, antibiotic resistance, and other agricultural pollution. Agriculture is both a cause of and sensitive to environmental degradation, such as biodiversity loss, desertification, soil degradation, and global warming, all of which can cause decreases in crop yield. Genetically modified organisms are widely used, although some are banned in certain countries.
Details
Agriculture is the art and science of cultivating the soil, growing crops and raising livestock. It includes the preparation of plant and animal products for people to use and their distribution to markets.
Agriculture provides most of the world’s food and fabrics. Cotton, wool, and leather are all agricultural products. Agriculture also provides wood for construction and paper products.
These products, as well as the agricultural methods used, may vary from one part of the world to another.
Start of Agriculture
Over centuries, the growth of agriculture contributed to the rise of civilizations.
Before agriculture became widespread, people spent most of their lives searching for food—hunting wild animals and gathering wild plants. About 11,500 years ago, people gradually learned how to grow cereal and root crops, and settled down to a life based on farming.
By 2,000 years ago, much of the Earth’s population had become dependent on agriculture. Scholars are not sure why this shift to farming took place, but it may have occurred because of climate change.
When people began growing crops, they also began herding and breeding wild animals. Adapting wild plants and animals for people to use is called domestication.
The first domesticated plant was probably rice or corn. Chinese farmers were cultivating rice as early as 7500 BCE.
The first domesticated animals were dogs, which were used for hunting. Sheep and goats were probably domesticated next. People also domesticated cattle and pigs. Most of these animals had once been hunted for hides and meat. Now many of them are also sources of milk, cheese, and butter. Eventually, people used domesticated animals such as oxen for plowing, pulling, and transportation.
Agriculture enabled people to produce surplus food. They could use this extra food when crops failed or trade it for other goods. Food surpluses allowed people to work at other tasks unrelated to farming.
Agriculture kept formerly nomadic people near their fields and led to the development of permanent villages. These became linked through trade. New economies were so successful in some areas that cities grew and civilizations developed. The earliest civilizations based on intensive agriculture arose near the Tigris and Euphrates Rivers in Mesopotamia (now Iraq and Iran) and along the Nile River in Egypt.
Improved Technology
For thousands of years, agricultural development was very slow. One of the earliest agricultural tools was fire. Native Americans used fire to control the growth of berry-producing plants, which they knew grew quickly after a wildfire. Farmers cultivated small plots of land by hand, using axes to clear away trees and digging sticks to break up and till the soil. Over time, improved farming tools of bone, stone, bronze, and iron were developed. New methods of storage evolved. People began stockpiling foods in jars and clay-lined pits for use in times of scarcity. They also began making clay pots and other vessels for carrying and cooking food.
Around 5500 BCE, farmers in Mesopotamia developed simple irrigation systems. By channeling water from streams onto their fields, farmers were able to settle in areas once thought to be unsuited to agriculture. In Mesopotamia, and later in Egypt and China, people organized themselves and worked together to build and maintain better irrigation systems.
Early farmers also developed improved varieties of plants. For example, around 6000 BCE, a new variety of wheat arose in South Asia and Egypt. It was stronger than previous cereal grains; its hulls were easier to remove and it could be made into bread.
As the Romans expanded their empire, they adapted the best agricultural methods of the people they conquered. They wrote manuals about the farming techniques they observed in Africa and Asia, and adapted them to land in Europe.
The Chinese also adapted farming tools and methods from nearby empires. A variety of rice from Vietnam ripened quickly and allowed farmers to harvest several crops during a single growing season. This rice quickly became popular throughout China.
Many medieval European farmers used an open-field system of planting. One field would be planted in spring, another in autumn, and one would be left unplanted, or fallow. This system preserved nutrients in the soil, increasing crop production.
The leaders of the Islamic Golden Age (which reached its height around 1000) in North Africa and the Middle East made agriculture into a science. Islamic Golden Age farmers learned crop rotation.
In the 15th and 16th centuries, explorers introduced new varieties of plants and agricultural products into Europe. From Asia, they carried home coffee, tea, and indigo, a plant used to make blue dye. From the Americas, they took plants such as potatoes, tomatoes, corn (maize), beans, peanuts, and tobacco. Some of these became staples and expanded people’s diets.
Machinery
A period of important agricultural development began in the early 1700s for Great Britain and the Low Countries (Belgium, Luxembourg, and the Netherlands, which lie below sea level). New agricultural inventions dramatically increased food production in Europe and European colonies, particularly the United States and Canada.
One of the most important of these developments was an improved horse-drawn seed drill invented by Jethro Tull in England. Until that time, farmers sowed seeds by hand. Tull’s drill made rows of holes for the seeds. By the end of the 18th century, seed drilling was widely practiced in Europe.
Many machines were developed in the United States. The cotton gin, invented by Eli Whitney in 1794, reduced the time needed to separate cotton fiber from seed. In the 1830s, Cyrus McCormick’s mechanical reaper helped modernize the grain-cutting process. At about the same time, John and Hiram Pitts introduced a horse-powered thresher that shortened the process of separating grain and seed from chaff and straw. John Deere’s steel plow, introduced in 1837, made it possible to work the tough prairie soil with much less horsepower. Along with new machines, there were several important advances in farming methods. By selectively breeding animals (breeding those with desirable traits), farmers increased the size and productivity of their livestock.
Cultures have been breeding animals for centuries—evidence suggests Mongolian nomads were selectively breeding horses in the Bronze Age. Europeans began to practice selective breeding on a large scale beginning in the 18th century. An early example of this is the Leicester sheep, an animal selectively bred in England for its quality meat and long, coarse wool.
Plants could also be selectively bred for certain qualities. In 1866, Gregor Mendel’s studies in heredity were published in Austria. In experiments with pea plants, Mendel learned how traits were passed from one generation to the next. His work paved the way for improving crops through genetics.
New crop rotation methods also evolved during this time. Many of these were adopted over the next century or so throughout Europe. For example, the Norfolk four-field system, developed in England, proved quite successful. It involved the yearly rotation of several crops, including wheat, turnips, barley, clover, and ryegrass. This added nutrients to the soil, enabling farmers to grow enough to sell some of their harvest without having to leave any land unplanted.
Most of the world was not affected by these developments, however. Farmers in Asia, Australia, Africa, and South America continued to use old ways of agriculture.
Agricultural Science
In the early 1900s, an average farmer in the U.S. produced enough food to feed a family of five. Many of today’s farmers can feed that family and a hundred other people. How did this great leap in productivity come about? It happened largely because of scientific advances and the development of new sources of power.
By the late 1950s, most farmers in developed countries were using both gasoline and electricity to power machinery. Tractors had replaced draft animals and steam-powered machinery. Farmers were using machines in almost every stage of cultivation and livestock management.
Electricity first became a power source on farms in Japan and Germany in the early 1900s. By 1960, most farms in the U.S. and other developed countries were electrified. Electricity lit farm buildings and powered such machinery as water pumps, milking machines, and feeding equipment. Today, electricity controls entire environments in livestock barns and poultry houses.
Traditionally, farmers have used a variety of methods to protect their crops from pests and diseases. They have put herb-based poisons on crops, handpicked insects off plants, bred strong varieties of crops, and rotated crops to control insects. Now, almost all farmers, especially in developed countries, rely on chemicals to control pests. The definition of “pest” ranges from insects to animals such as rabbits and mice, as well as weeds and disease-causing organisms—bacteria, viruses, and fungi. With the use of chemicals, crop losses and prices have declined dramatically.
For thousands of years, farmers relied on natural fertilizer—materials such as manure, wood ash, ground bones, fish or fish parts, and bird and bat waste called guano—to replenish or increase nutrients in the soil.
In the early 1800s, scientists discovered which elements were most essential to plant growth: nitrogen, phosphorus, and potassium. Later, fertilizer containing these elements was manufactured in the U.S. and in Europe. Now, many farmers use chemical fertilizers with nitrates and phosphates because they greatly increase crop yields.
However, pesticides and fertilizers have come with another set of problems. The heavy reliance on chemicals has disturbed the environment, often destroying helpful species of animals along with harmful ones. Chemical use may also pose a health hazard to people, especially through contaminated water supplies. Agricultural scientists are looking for safer chemicals to use as fertilizers and pesticides. Some farmers use natural controls and rely less on chemicals.
Farming in Water
Agriculture includes such forms of cultivation as hydroponics and aquaculture. Both involve farming in water.
Hydroponics is the science of growing plants in nutrient solutions. Just one acre of nutrient solution can yield more than 50 times the amount of lettuce grown on the same amount of soil.
Aquaculture—primarily the cultivation of fish and shellfish—was practiced in China, India, and Egypt thousands of years ago. It is now used in lakes, ponds, the ocean, and other bodies of water throughout the world. Some forms of aquaculture, such as shrimp farming, have become important industries in many Asian and Latin American countries.
Climate change and improved technology are altering the way freshwater and ocean fisheries operate. Global warming has pushed warm-water species toward the poles and reduced the habitats of cold-water species. Traditional fishing communities in both developed and developing countries find the number of fish dwindling.
Bottom trawling has affected ocean ecosystems. In bottom trawling, enormous nets are strung from fishing boats and dragged at the bottom of the ocean. The nets catch halibut and squid, but also stir up sediment at the bottom of the ocean. This disturbs the marine life (plankton and algae) that forms the basis of the food chain.
Genetic Modification
For centuries, people have bred new types of plants and animals by random experimentation. During the 1950s and 1960s, scientists developed new strains of high-yield wheat and rice. They introduced them into Mexico and parts of Asia. As a result, production of grain soared in these areas. This bold experiment in agriculture has been called the "Green Revolution."
With the successes of the Green Revolution came problems. To produce high yields, the new strains required chemical fertilizers, pesticides and irrigation. In many developing countries, independent farmers cannot afford the new technology and big business has taken over agriculture. The new, high-production crops also put stress on native plants and animals.
Later, scientists and farmers understood why the new strains developed. This gave rise to a new green revolution: genetic modification of food.
Inside every cell are genes, material that determines many of the characteristics of an organism. Genetics is the study of what characteristics organisms inherit and how these traits are transmitted.
With a greater knowledge of genetics, people can scientifically select characteristics they want to reproduce. New technology has revolutionized the selective breeding process in both plants and animals.
Beginning in the 1970s, scientists found that they could rearrange genes and add new ones to promote disease resistance, productivity, and other desired characteristics in crops and livestock.
These genetically modified organisms (GMOs or GM foods) are now common throughout the developed world. Biotechnology allows scientists to alter the DNA of microbes, plants, and animals. GMOs that have genetic material, or DNA, from other species are called transgenic organisms.
A gene from an Arctic plant, for example, could be added (spliced) into the DNA of a strawberry plant to increase the strawberry’s resistance to cold and thus extend its growing season. The strawberry would be a transgenic plant.
Businesses sell farmers genetically modified seeds that resist certain pesticides and herbicides produced by the company. (Herbicides kill weeds and other plants that threaten the crop.) With these seeds, farmers can use toxic chemicals without harming the crop.
Biotechnology has brought advances in animal husbandry (ranching, or the raising of domestic animals). Today’s farm animals are larger and grow faster than their ancestors.
Cattle, for example, are grazing animals. Their digestive system has evolved to process grasses and other crops. Corn and other grains cause a cow’s digestive system to become acidic. That makes it easier for dangerous bacteria (such as E.coli) to develop. Bacterial infections can be harmful to the cow, and can also infect their milk and meat consumed by people. Antibiotics are spliced into the DNA of feed corn to prevent such infection. Antibiotics have been used since the 1950s to stimulate cattle growth. Over time, this practice has led to the development of antibiotic-resistant bacteria in cattle and people. Many cattle are also given anabolic steroids, or growth hormones, to make them get bigger, faster.
The controversies surrounding GM foods are enormous. Farmers who grow GM foods increase production with less labor and less land. Many consumers favor GM foods. Vegetables and fruits last longer and are less likely to bruise. Meats are fattier—more tender and salty.
Critics argue that GM foods have less nutritional value and decrease biodiversity. The organic and "free-range" food industries have grown in opposition to "factory farming."
Most of the world’s farmers live in developing countries in Africa, Asia, and Latin America. Many of them cultivate land as their ancestors did hundreds or even thousands of years ago. They do not use agricultural technology involving expensive chemicals or production methods.
These people are subsistence farmers. They use the bulk of the food they produce for themselves and their families, unlike commercial farmers, who only grow crops to sell.
Methods of Cultivation
Agricultural methods often vary widely around the world, depending on climate, terrain, traditions, and available technology.
Low-technology farming involves permanent crops: food grown on land that is not replanted after each harvest. Citrus trees and coffee plants are examples of permanent crops. Higher-technology farming involves crop rotation, which requires knowledge of farmable land. Scholars and engineers not only use crop rotation and irrigation, but plant crops according to the season, type of soil, and amount of water needed.
In coastal West Africa, farmers, usually women, plant corn soon after the first rains of the growing season. They often use an ancient method of clearing called slash-and-burn. First, the farmer cuts all the brush in her plot. When this vegetation dries, she sets fire to it. The heat from the fire makes the soil easy to turn, and the burned vegetation fertilizes it. The farmer then sows kernels of corn saved from the previous year’s harvest.
Between rows of corn, the African farmer plants other staple crops: legumes, such as peas, or root vegetables, such as yams. This practice of growing several crops in the same plot is called intercropping. By covering most of the ground with vegetation, intercropping prevents moisture loss and soil erosion from seasonal rains.
Rain supplies water for the growing plants. The farmer weeds her plot with a hoe. At harvest time, she and her family pick the corn, husk it, and spread the ears in the sun to dry. They grind the dried corn to make porridge.
Traditionally, the African farmer uses the same plot for several years, until its fertility declines. Then she moves to another plot, leaving the first to lie fallow for up to 10 years. Now, an increasing population has caused fallow periods to be reduced and has made permanent cultivation more common.
Agricultural methods used in the Corn Belt of the U.S. are very different. The Corn Belt is the area of the northern Midwest where most of the nation’s corn crop is grown. First of all, farmers rarely work alone—the size of American farms requires a lot of labor. Soon after they harvest the corn in autumn, farmers work leftover vegetation, or stubble, into the soil. In the spring, farmers work the soil again, using an implement with rows of sharp-edged steel discs, called a disc harrow. The discs cut into the soil, breaking it into smaller pieces and supplying it with air.
Next, a tractor-pulled planter sows rows of seed. The machine makes furrows in the soil, drops in kernels of high-yield, genetically modified corn, and covers them with dirt. After the corn seeds have sprouted, another machine injects liquid fertilizer into the ground.
The farmers then use chemicals to control weeds and pests, and loosen the soil with a tractor-pulled cultivator during the harvesting season.
U.S. industrial farmers may plant a thousand acres of just corn. The practice of specializing in a single crop is known as monoculture. To harvest the crop, farmers use a mechanical harvester that picks the ears of corn and shells them into a bin.
Little of the corn grown in the Corn Belt is for human consumption. Most of the corn grown in the U.S. is for cattle feed and industrial uses, such as corn syrup sweeteners.
Livestock
From alpacas in Peru to zebus in India, billons of domesticated animals around the world are raised and cared for in a variety of ways. In many countries, domesticated animals are an important source of food.
In Nigeria, for example, the Fulani people have long been nomads. They move with their cattle herds from one grazing area to another. The cattle feed on scrub and grasses in land unsuitable for farming. The Fulani rely on cattle for milk, but rarely slaughter their animals for meat.
Throughout the U.S., beef cattle are bred to grow quickly and yield large quantities of fatty meat. When they are five to 12 months old, the animals are shipped to feedlots. There, they are kept in pens and fed grain and vitamin supplements until they reach market size. Then they are slaughtered.
The two ways of raising livestock are confronting each other in the developing world. In Uganda, Ankole cattle have been bred to withstand the harsh climate of Central Africa—their long, curved horns help distribute heat and their digestive systems have adapted to poor nutrition and little water. However, the market for milk has driven many Ugandan farmers to import Holstein cattle. Holsteins are native to Northern Europe. Keeping them healthy in an equatorial region requires a high amount of antibiotics, vaccines, and other chemicals. The Ankole, which produce little milk and leaner meat, may be extinct within the century.
Many farmers throughout the world practice free-range poultry farming. The birds forage for food in farms or community yards, eating whatever they find: seeds, insects, household scraps, and surplus grain.
In many developed countries, poultry production has become a major agricultural industry. Birds are given the same sort of vaccines and hormones used for cattle. Chickens are bred for either eggs or meat. One poultry house may contain more than a million birds. Often, machines automatically provide feed and water, collect the eggs, and remove waste.
Fight Against Hunger
Food production must keep pace with population growth and distribution methods. This is an enormous agricultural and political challenge.
The challenge is not food shortages but unequal distribution of the world’s food supply. The ratio of population to farmable land has favored some countries more than others. Some experts believe government policies in developed and developing countries have hindered equal food distribution. Droughts, floods, and other disasters continue to cause local food shortages.
Overpopulation also contributes to unequal distribution of food resources. Much of the population increase over the next 100 years will occur in developing countries, where hunger is already a serious problem.
Exporting food or agricultural technology from countries with surpluses to those with shortages will not solve the problem of world hunger. Poor countries do not have the money to buy all the food they need and do not want to permanently rely on other countries. Many developing countries also regard biodiversity as an important resource and do not want to threaten it with GMOs.
Experts believe that the hunger problem will be solved in two ways. First, citizens of all countries need to have the ability to grow or purchase their own food. Second, citizens of all countries need to have responsible diets and spending habits. What about addressing the problem of overpopulation?
Agricultural science will help countries adjust to healthier methods of food production. Scientists are developing new high-yield varieties of crops that require fewer fertilizers or pesticides. Such crops reduce the need for using costly chemicals and trade.
The challenges of feeding the hungry cannot be met unless the world’s land and water are safeguarded. Agricultural practices in developed and developing countries have led to a severe loss of valuable topsoil, water, and other resources.
Many countries need better programs for replanting forests. Overpopulation has pushed a growing number of farmers onto lands too fragile to sustain cultivation. Demand for food has led to increased irrigation worldwide. In some areas, irrigation has caused water tables to drop, rivers to run dry, and wells to go empty. Agricultural chemicals that increase production often contaminate soil and groundwater and disrupt food chains.
Agriculture does not have to harm the environment. By protecting the land, water, and air, and by sharing knowledge and resources, people may yet find solutions for the problem of world hunger.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1524 2022-10-06 14:05:36
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,194
Re: Miscellany
1497) Trachtenberg system
Summary
The Trachtenberg system is a system of rapid mental calculation. The system consists of a number of readily memorized operations that allow one to perform arithmetic computations very quickly. It was developed by the Russian engineer Jakow Trachtenberg in order to keep his mind occupied while being in a Nazi concentration camp.
The rest of this article presents some methods devised by Trachtenberg. Some of the algorithms Trachtenberg developed are ones for general multiplication, division and addition. Also, the Trachtenberg system includes some specialised methods for multiplying small numbers between 5 and 13 (but shown here is 2–12).
The section on addition demonstrates an effective method of checking calculations that can also be applied to multiplication.
Details
The Trachtenberg System is a system of rapid mental calculation. The system consists of a number of readily memorized operations that allow one to perform arithmetic computations very quickly. It was developed by Jakow Trachtenberg in order to keep his mind occupied while being held in a Nazi concentration camp.
General Multiplication
The method for general multiplication is a method to achieve multiplications a*b with low space complexity, i.e. as few temporary results as possible to be kept in memory.
This is achieved by noting that the final digit is completely determined by multiplying the last digit of the multiplicands. This is held as a temporary result. To find the next to last digit, we need everything that influences this digit: The temporary result, the last digit of a times the next-to-last digit of b, as well as the next-to-last digit of a times the last digit of b. This calculation is performed, and we have a temporary result that is correct in the final two digits.
In general, for each position n in the final result, we sum for all i:
Ordinary people can learn this algorithm and thus multiply four digit numbers in their head - writing down only the final result. They would write it out starting with the rightmost digit and finishing with the leftmost.
Trachtenberg defined this algorithm with a kind of pairwise multiplication where two digits are multiplied by one digit, essentially only keeping the middle digit of the result. By performing the above algorithm with this pairwise multiplication, even fewer temporary results need to be held.
Example: 123456 * 789
To find the first digit of the answer:
The units digit of 9 * 6 = 4.
To find the second digit of the answer, start at the second digit of the multiplicand:
The units digit of 9 5 plus the tens digit of 9 6 plus
The units digit of 8 * 6.
5 + 5 + 8 = 18.
The second digit of the answer is 8 and carry 1 to the third digit.
To find the fourth digit of the answer, start at the fourth digit of the multiplicand:
The units digit of 9 3 plus the tens digit of 9 4 plus
The units digit of 8 4 plus the tens digit of 8 5 plus
The units digit of 7 5 plus the tens digit of 7 6.
7 + 3 + 2 + 4 + 5 + 4 = 25 + 1 carried from the third digit.
The fourth digit of the answer is 6 and carry 2 to the next digit.
General addition
A method of adding columns of numbers and accurately checking the result without repeating the first operation. An intermediate sum, in the form of two rows of digits, is produced. The answer is obtained by taking the sum of the intermediate results with an L-shaped algorithm. As a final step, the checking method that is advocated removes both the risk of repeating any original errors and allows the precise column in which an error occurs to be identified at once. It is based on a check (or digit) sums, such as the nines-remainder method.
For the procedure to be effective, the different operations used in each stages must be kept distinct, otherwise there is a risk of interference.
Other multiplication algorithms
When performing any of these multiplication algorithms the following “steps” should be applied.
The answer must be found one digit at a time starting at the least significant digit and moving left. The last calculation is on the leading zero of the multiplicand.
Each digit has a neighbor, i.e., the digit on its right. The rightmost digit’s neighbor is the trailing zero.
The ‘halve’ operation has a particular meaning to the Trachtenberg system. It is intended to mean “half the digit, rounded down” but for speed reasons people following the Trachtenberg system are encouraged to make this halving process instantaneous. So instead of thinking “half of seven is three and a half, so three” it’s suggested that one thinks “seven, three”. This speeds up calculation considerably. In this same way the tables for subtracting digits from 10 or 9 are to be memorized.
And whenever the rule calls for adding half of the neighbor, always add 5 if the current digit is odd. This makes up for dropping 0.5 in the next digit’s calculation.
Multiplying by 11
Rule: Add the digit to its neighbor. (By “neighbor” we mean the digit on the right.)
Example: 3,425 * 11 = 37,675
3 7 6 7 5
(=0+3) (=3+4) (=4+2) (=2+5) (=5+0)
To illustrate:
11=10+1
Thus,
Multiplying by 12
Rule: to multiply by 12:
Starting from the rightmost digit, double each digit and add the neighbor. (By “neighbor” we mean the digit on the right.)
If the answer is greater than a single digit, simply carry over the extra digit (which will be a 1 or 2) to the next operation. The remaining digit is one digit of the final result.
Example: 316 * 12
Determine neighbors in the multiplicand 0316:
digit 6 has no right neighbor
digit 1 has neighbor 6
digit 3 has neighbor 1
digit 0 (the prefixed zero) has neighbor 3
6 2 = 12 (2 carry 1)
1 2 + 6 + 1 = 9
3 2 + 1 = 7
0 2 + 3 = 3
0 * 2 + 0 = 0
316 * 12 = 3,792
Multiplying by 6
Rule: to multiply by 6: Add half of the neighbor to each digit, then, if the current digit is odd, add 5.
Example:
357 × 6 = 2142
Working right to left,
7 has no neighbor, add 5 (since 7 is odd) = 12. Write 2, carry the 1.
5 + half of 7 (3) + 5 (since the starting digit 5 is odd) + 1 (carried) = 14. Write 4, carry the 1.
3 + half of 5 (2) + 5 (since 3 is odd) + 1 (carried) = 11. Write 1, carry 1.
0 + half of 3 (1) + 1 (carried) = 2. Write 2.
Multiplying by 7
Rule: to multiply by 7: #Double each digit. #Add half of its neighbor. #If the digit is odd, add 5.
Example: 523 x 7 = 3,661.
3x2+0+5=11, 1.
2x2+1+1=6.
5x2+1+5=16, 6.
0x2+2+1=3.
Multiplying by 9
Rule: #Subtract the right-most digit from 10. ##Subtract the remaining digits from 9. #Add the neighbor. #For the leading zero, subtract 1 from the neighbor.
For rules 9, 8, 4, and 3 only the first digit is subtracted from 10. After that each digit is subtracted from nine instead.
Example: 2,130 × 9 = 19,170
Working from right to left:
(10 - 0) + 0 = 10. Write 0, carry 1. (9 - 3) + 0 + 1 (carried) = 7. Write 7. (9 - 1) + 3 = 11. Write 1, carry 1. (9 - 2) + 1 + 1 (carried) = 9. Write 9. *2 - 1 = 1. Write 1.
Multiplying by 8
Rule:
Subtract right-most digit from 10.
#Subtract the remaining digits from 9.
Double the result.
Add the neighbor.
For the leading zero, subtract 2 from the neighbor.
Example: 456 x 8 = 3648
Working from right to left: (10 - 6) x 2 + 0 = 8. Write 8. (9 - 5) x 2 + 6 = 14, Write 4, carry 1. (9 - 4) x 2 + 5 + 1 (carried) = 16. Write 6, carry 1. 4 - 2 + 1 (carried) = 3. Write 3.
Multiplying by 4
Rule:
Subtract the right-most digit from 10.
Subtract the remaining digits from 9.
Add half of the neighbor, plus 5 if the digit is odd.
For the leading 0, subtract 1 from half of the neighbor.
Example: 346 * 4 = 1384
Working from right to left:
(10 - 6) + Half of 0 (0) = 4. Write 4. (9 - 4) + Half of 6 (3) = 8. Write 8. (9 - 3) + Half of 4 (2) + 5 (since 3 is odd) = 13. Write 3, carry 1. Half of 3 (1) - 1 + 1 (carried) = 1. Write 1.
Multiplying by 3
Rule:
Subtract the rightmost digit from 10.
Subtract the remaining digits from 9.
Double the result.
Add half of the neighbor, plus 5 if the digit is odd.
For the leading zero, subtract 2 from half of the neighbor.
Example: 492 x 3 = 1476
Working from right to left: (10 - 2) x 2 + Half of 0 (0) = 16. Write 6, carry 1. (9 - 9) x 2 + Half of 2 (1) + 5 (since 9 is odd) + 1 (carried) = 7. Write 7. (9 - 4) x 2 + Half of 9 (4) = 14. Write 4, carry 1. Half of 4 (2) - 2 + 1 (carried) = 1. Write 1.
Multiplying by 5
Rule: to multiply by 5: Take half of the neighbor, then, if the current digit is odd, add 5.
Example:
42x5=210
Half of 2’s neighbor, the trailing zero, is 0.
Half of 4’s neighbor is 1.
Half of the leading zero’s neighbor is 2.
43x5=215
Half of 3’s neighbor is 0, plus 5 because 3 is odd, is 5.
Half of 4’s neighbor is 1.
Half of the leading zero’s neighbor is 2.
93x5=465
Half of 3’s neighbor is 0, plus 5 because 3 is odd, is 5.
Half of 9’s neighbor is 1, plus 5 because 9 is odd, is 6.
Half of the leading zero’s neighbor is 4.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1525 2022-10-07 14:31:54
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,194
Re: Miscellany
1498) Hydroponics
Summary
Hydroponics is a type of horticulture and a subset of hydroculture which involves growing plants, usually crops, without soil, by using water-based mineral nutrient solutions in aqueous solvents. Terrestrial or aquatic plants may grow with their roots exposed to the nutritious liquid or in addition, the roots may be mechanically supported by an inert medium such as perlite, gravel, or other substrates.
Despite inert media, roots can cause changes of the rhizosphere pH and root exudates can affect rhizosphere biology and physiological balance of the nutrient solution by secondary metabolites. Transgenic plants grown hydroponically allow the release of pharmaceutical proteins as part of the root exudate into the hydroponic medium.
The nutrients used in hydroponic systems can come from many different sources, including fish excrement, duck manure, purchased chemical fertilizers, or artificial nutrient solutions.
Plants are commonly grown hydroponically in a greenhouse or contained environment on inert media, adapted to the controlled-environment agriculture (CEA) process. Plants commonly grown hydroponically include tomatoes, peppers, cucumbers, strawberries, lettuces, and cannabis, usually for commercial use, and Arabidopsis thaliana, which serves as a model organism in plant science and genetics.
Hydroponics offers many advantages, notably a decrease in water usage in agriculture. To grow 1 kilogram (2.2 lb) of tomatoes using intensive farming methods requires 214 liters (47 imp gal; 57 U.S. gal) of water; using hydroponics, 70 liters (15 imp gal; 18 U.S. gal); and only 20 liters (4.4 imp gal; 5.3 U.S. gal) using aeroponics.
Hydroponic cultures lead to highest biomass and protein production compared to other growth substrates, of plants cultivated in the same environmental conditions and supplied with equal amounts of nutrients.
Since hydroponics takes much less water and nutrients to grow produce, it could be possible in the future for people in harsh environments with little accessible water to grow their own food.
Hydroponics is not only used on earth, but has also proven itself in plant production experiments in space.
Details
Hydroponics, also called aquaculture, nutriculture, soilless culture, or tank farming, is the cultivation of plants in nutrient-enriched water, with or without the mechanical support of an inert medium such as sand, gravel, or perlite.
Plants have long been grown with their roots immersed in solutions of water and fertilizer for scientific studies of their nutrition. Early commercial hydroponics (from Greek hydro-, “water,” and ponos, “labour”) adopted this method of culture. Because of the difficulties in supporting the plants in a normal upright growing position and aerating the solution, however, this method was supplanted by gravel culture, in which gravel supports the plants in a watertight bed or bench. Various kinds of substrates have been used successfully, including rock wool (molten rock that is spun into fibres), fused shale, clay pellets, coconut coir, rice husks, granite chips, sand, pumice, perlite, and vermiculite. Fertilizer solution, often derived from fish or duck excrement or synthetic fertilizers, is pumped through periodically, the frequency and concentration depending on the plant and on ambient conditions such as light and temperature. The solution drains into a tank, and pumping is usually automatic.
The fertilizer solution is composed of different agricultural or horticultural fertilizer-grade chemical compounds containing varying amounts of nitrogen, phosphorus, and potassium—the major elements necessary for plant growth—and various trace, or minor, elements, such as sulfur, magnesium, and calcium. The solution can be used indefinitely; periodic tests indicate the need for additional chemicals or water. The chemical ingredients usually may be mixed dry and stored. As the plants grow, concentration of the solution and frequency of pumping are increased.
Some systems, known as aquaponics, use nutrient-rich wastewater from aquaculture to fertilize hydroponic plants. Freshwater fish, such as tilapia, and crayfish are common aquatic animals utilized for these hybrid systems.
A wide variety of vegetables and florist crops can be grown satisfactorily with hydroponic systems. Common crops include lettuces, spinach, kale, tomatoes, peppers, cucumbers, radishes, strawberries, and cannabis. The model organism Arabidopsis thaliana is also sometimes grown this way for genetic research.
Hydroponic systems have a number of advantages and disadvantages compared with cultivation in soil. The principal advantage is the saving of labour by automatic watering and fertilizing. Hydroponic systems can be set up indoors in places that would not normally be available for the growing of plants, such as in densely populated areas, and have even been studied as a potential method of crop production aboard spacecraft. Climate is not a factor, and hydroponic systems use dramatically less water compared with conventionally grown plants. The plants also have less root and nutrient competition than those grown in soil, and they have significantly fewer pests, so individuals can be planted more closely together. The disadvantages are high installation costs and the need to test the solution frequently. There is a steep learning curve to hydroponics, and small errors can affect the whole crop. The systems are also very vulnerable to equipment failure or power outage, which can kill the plants within a few hours. Yields are about the same as for soil-grown crops.
Hydroponic crops are allowed to be certified as organic in many places, including in the United States. Critics have pointed out that hydroponic plants lack interaction with a soil microbiome and have argued that soil health is a critical part of the organic farming movement.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline

