Math Is Fun Forum
You are not logged in.
- Topics: Active | Unanswered
#2051 2024-02-06 00:05:46
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,725
Re: Miscellany
2053) Vagus Nerve
Gist
The vagal nerves carry signals between your brain, heart and digestive system. They’re a key part of your parasympathetic nervous system. Vagus nerve damage can lead to gastroparesis, food not moving into your intestines. Some people with vasovagal syncope faint from low blood pressure. Vagus nerve stimulation (VNS) can treat epilepsy and depression.
Summary
Vagus nerve is the longest and most complex of the cranial nerves. The vagus nerve runs from the brain through the face and thorax to the abdomen. It is a mixed nerve that contains parasympathetic fibres. The vagus nerve has two sensory ganglia (masses of nerve tissue that transmit sensory impulses): the superior and the inferior ganglia. The branches of the superior ganglion innervate the skin in the concha of the ear. The inferior ganglion gives off two branches: the pharyngeal nerve and the superior laryngeal nerve. The recurrent laryngeal nerve branches from the vagus in the lower neck and upper thorax to innervate the muscles of the larynx (voice box). The vagus also gives off cardiac, esophageal, and pulmonary branches. In the abdomen the vagus innervates the greater part of the digestive tract and other abdominal viscera.
The vagus nerve has the most extensive distribution of the cranial nerves. Its pharyngeal and laryngeal branches transmit motor impulses to the pharynx and larynx; its cardiac branches act to slow the rate of heartbeat; its bronchial branch acts to constrict the bronchi; and its esophageal branches control involuntary muscles in the esophagus, stomach, gallbladder, pancreas, and small intestine, stimulating peristalsis and gastrointestinal secretions.
Vagus nerve stimulation, in which the nerve is stimulated with pulses of electricity, is sometimes used for patients with epilepsy or depression that is otherwise untreatable; the technique has also been explored for conditions such as Alzheimer disease and migraine.
Details
The vagus nerve, also known as the tenth cranial nerve, cranial nerve X, or simply CN X, is a cranial nerve that carries sensory fibers that create a pathway that interfaces with the parasympathetic control of the heart, lungs, and digestive tract. It comprises two nerves—the left and right vagus nerves—but they are typically referred to collectively as a single subsystem. The vagus is the longest nerve of the autonomic nervous system in the human body and comprises both sensory and motor fibers. The sensory fibers originate from neurons of the nodose ganglion, whereas the motor fibers come from neurons of the dorsal motor nucleus of the vagus and the nucleus ambiguus. The vagus was also historically called the pneumogastric nerve.
Structure
Upon leaving the medulla oblongata between the olive and the inferior cerebellar peduncle, the vagus nerve extends through the jugular foramen, then passes into the carotid sheath between the internal carotid artery and the internal jugular vein down to the neck, chest, and abdomen, where it contributes to the innervation of the viscera, reaching all the way to the colon. Besides giving some output to various organs, the vagus nerve comprises between 80% and 90% of afferent nerves mostly conveying sensory information about the state of the body's organs to the central nervous system. The right and left vagus nerves descend from the cranial vault through the jugular foramina, penetrating the carotid sheath between the internal and external carotid arteries, then passing posterolateral to the common carotid artery. The cell bodies of visceral afferent fibers of the vagus nerve are located bilaterally in the inferior ganglion of the vagus nerve (nodose ganglia).
The vagus runs parallel to the common carotid artery and internal jugular vein inside the carotid sheath.
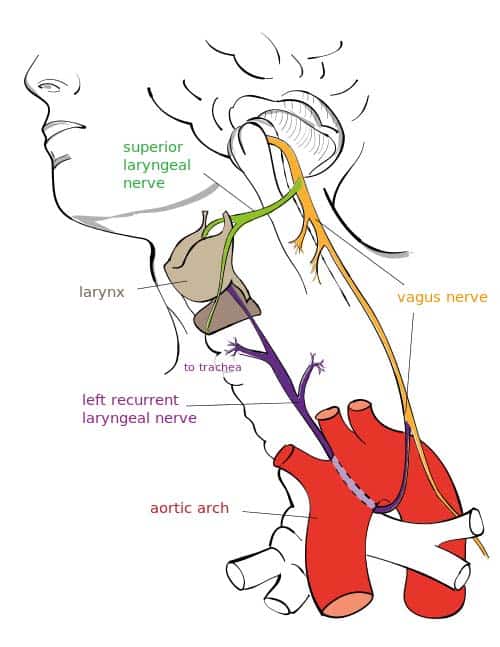
The right vagus nerve gives rise to the right recurrent laryngeal nerve, which hooks around the right subclavian artery and ascends into the neck between the trachea and esophagus. The right vagus then crosses anterior to the right subclavian artery, runs posterior to the superior vena cava, descends posterior to the right main bronchus, and contributes to cardiac, pulmonary, and esophageal plexuses. It forms the posterior vagal trunk at the lower part of the esophagus and enters the diaphragm through the esophageal hiatus.
The left vagus nerve enters the thorax between left common carotid artery and left subclavian artery and descends on the aortic arch. It gives rise to the left recurrent laryngeal nerve, which hooks around the aortic arch to the left of the ligamentum arteriosum and ascends between the trachea and esophagus. The left vagus further gives off thoracic cardiac branches, breaks up into the pulmonary plexus, continues into the esophageal plexus, and enters the abdomen as the anterior vagal trunk in the esophageal hiatus of the diaphragm.
Branches
* Pharyngeal nerve
* Superior laryngeal nerve
* Aortic nerve
* Superior cervical cardiac branches of vagus nerve
* Inferior cervical cardiac branch
* Recurrent laryngeal nerve
* Thoracic cardiac branches
* Branches to the pulmonary plexus
* Branches to the esophageal plexus
* Anterior vagal trunk
* Posterior vagal trunk
Nuclei
The vagus nerve includes axons which emerge from or converge onto four nuclei of the medulla:
* The dorsal nucleus of vagus nerve – which sends parasympathetic output to the viscera, especially the intestines
* The nucleus ambiguus – which gives rise to the branchial efferent motor fibers of the vagus nerve and preganglionic parasympathetic neurons that innervate the heart
* The solitary nucleus – which receives afferent taste information and primary afferents from visceral organs
* The spinal trigeminal nucleus – which receives information about deep/crude touch, pain, and temperature of the outer ear, the dura of the posterior cranial fossa and the mucosa of the larynx.
Development
The motor division of the glossopharyngeal nerve is derived from the basal plate of the embryonic medulla oblongata, while the sensory division originates from the cranial neural crest.
Additional Information
The vagus nerve is one of 12 cranial nerves in the body. It’s responsible for various bodily functions, including digestion, heart rate, and breathing.
What is the vagus nerve?
There are 12 cranial nerves in the body. They come in pairs and help link the brain with other areas of the body, such as the head, neck, and torso.
Some send sensory information, including details about smells, sights, tastes, and sounds, to the brain. These nerves have sensory functions. Other cranial nerves control the movement of various muscles and the function of certain glands. These are known as motor functions.
While some cranial nerves have either sensory or motor functions, others have both. The vagus nerve is such a nerve. The cranial nerves are classified using Roman numerals based on their location. The vagus nerve is also called cranial nerve X.
What does the vagus nerve affect?
The vagus nerve also called the pneumogastric nerve, is responsible for various internal organ functions, including:
* digestion
* heart rate
* breathing
* cardiovascular activity
* reflex actions, such as coughing, sneezing, swallowing, and vomiting
It plays a role in the autonomic nervous system, which controls actions people do unconsciously, such as breathing and digestion.
It may also form a link between the gut and the brain, playing a role in what scientists call the gut-brain axis. In recent years, experts have been studying the gut-brain axis to look for links between conditions such as obesity and depression.
Vagus nerve anatomy and function
The word “vagus” means wandering in Latin. This is a very appropriate name, as the vagus nerve is the longest cranial nerve. It runs from the brain stem to part of the colon.
The vagus nerve sensory functions are divided into two components:
* Somatic components. These are sensations felt on the skin or in the muscles.
* Visceral components. These are sensations felt in the organs of the body.
Sensory functions of the vagus nerve include:
* providing somatic sensation information for the skin behind the ear, the external part of the ear canal, and certain parts of the throat
* supplying visceral sensation information for the larynx, esophagus, lungs, trachea, heart, and most of the digestive tract
* playing a small role in the sensation of taste near the root of the tongue
Motor functions of the vagus nerve include:
* stimulating muscles in the pharynx, larynx, and the soft palate, which is the fleshy area near the back of the roof of the mouth
* stimulating muscles in the heart, where it helps to lower resting heart rate
* stimulating involuntary contractions in the digestive tract, including the esophagus, stomach, and most of the intestines, which allow food to move through the tract.
Vagus nerve testing
To test the vagus nerve, a doctor may check the gag reflexTrusted Source. During this part of the examination, the doctor may use a soft cotton swab to tickle the back of the throat on both sides. This should cause the person to gag.
If the person does not gag, this may be due to a problem with the vagus nerve, which could indicate a problem with the brainstem function.
Doctors may also assess vagal nerve function when looking at cardiovascular disease, as discussed in recent research. Damage to the vagal nerve can lead to problems with the cardiovascular system.
Measuring heart rate, blood pressure, and cardiovascular response to exercise can provide clues as to how your vagal nerve performs in conjunction with your cardiovascular system, which is known as cardiovagal tone. It can offer clues to your cardiovascular health.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2052 2024-02-07 00:03:02
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,725
Re: Miscellany
2054) Sales Representative
Gist
The Sales Representative is responsible for selling products and meeting customer needs while obtaining orders from existing or potential sales outlets. They ensure that the customer is satisfied and adequately taken care of while making a purchase.
Summary
A sales representative promotes and sells a company’s products. Read on to learn the skills, education, and overall requirements for a sales representative and how you can start on a path to become one.
A sales representative promotes and sells products to customers on behalf of a company or organization. Someone in this role pitches products to potential customers, presents new ones to existing customers, maintains existing customer accounts, and ensures a smooth sales process and customer satisfaction. Sales representatives generally must meet sales goals and report to their sales director.
Sales representative jobs can be found in many industries, from technology to manufacturing. Many sales reps have flexible schedules, with work-from-home options in some cases. For anyone who enjoys working with others, it’s a great career choice. Sales representative jobs can be lucrative and rewarding for a self-starter who’s motivated and self-confident.
Types of sales representatives
There are two main types of sales representatives: inside sales representatives and outside sales representatives. An inside sales representative typically uses digital communication tools to connect with customers remotely, whereas an outside sales representative may conduct sales in the "field" via face-to-face interactions.
Sales representatives may sell a variety of products depending on the company and industry. Some types of sales representative jobs include:
* Wholesale
* Manufacturing
* Scientific
* Technical
* Medical
* Pharmaceutical
Details
A sales representative is responsible for promoting and selling products or services on behalf of a company. This role involves building and maintaining relationships with clients, understanding their needs, and effectively communicating how the company's offerings can meet those requirements. Sales representatives play an important role in the overall sales process, from identifying potential customers and generating leads to closing deals and achieving revenue targets.
Sales representatives employ a variety of strategies to attract and retain customers, including cold calling, networking, and conducting product demonstrations. They are adept at understanding market trends, competitor offerings, and industry developments to position their products or services competitively. Exceptional communication and interpersonal skills are essential for sales representatives, as they engage with clients to address inquiries, negotiate terms, and ensure customer satisfaction.
Duties and Responsibilities
The duties and responsibilities of a sales representative are diverse, encompassing various stages of the sales process.
Here is an overview of the key responsibilities associated with this role:
* Prospecting and Lead Generation: Identify and research potential customers or clients. Generate leads through methods such as cold calling, networking, and leveraging online platforms.
* Client Engagement: Initiate contact with potential customers to understand their needs and introduce the company's products or services. Conduct product demonstrations or presentations to showcase features and benefits.
* Relationship Building: Cultivate and maintain positive relationships with existing and potential clients. Address client inquiries, concerns, or objections in a professional and timely manner.
* Sales Presentations: Create and deliver persuasive sales presentations tailored to the needs of the client. Highlight the unique selling points and value proposition of the products or services.
* Negotiation and Closing Deals: Negotiate terms and conditions with clients to reach mutually beneficial agreements. Close sales deals and achieve or exceed sales targets.
* Product Knowledge: Stay well-informed about the features, specifications, and benefits of the products or services being represented. Keep abreast of industry trends, competitor offerings, and market developments.
* Sales Reporting and Documentation: Maintain accurate records of sales activities, including client interactions, sales calls, and deals closed. Prepare regular reports on sales performance for management.
* Customer Follow-Up: Follow up with clients post-sale to ensure satisfaction and address any additional needs. Seek opportunities for upselling or cross-selling additional products or services.
* Market Research: Conduct market research to identify potential opportunities and challenges. Provide feedback to the company regarding customer preferences, market trends, and competitive activities.
* Collaboration with Teams: Collaborate with marketing, product development, and customer support teams to ensure a cohesive and customer-centric approach. Communicate customer feedback and market insights to internal teams.
Types of Sales Representatives
Sales representatives can specialize in various areas based on the products or services they sell, the industries they target, or the stage of the sales process they focus on. Here are some types of sales representatives:
* Inside Sales Representative: Inside sales representatives work remotely or within the company's office and typically communicate with clients through phone calls, emails, or online meetings. They are responsible for prospecting, lead generation, and closing deals without the need for face-to-face interactions.
* Outside Sales Representative: Outside sales representatives, also known as field sales representatives, engage with clients in person. They often travel to meet potential customers, conduct sales presentations, and build relationships on a personal level.
* Advertising Sales Agent: Advertising sales agents sell advertising space or time to businesses and organizations. They pitch advertising solutions and negotiate contracts, aiming to create effective advertising campaigns that meet both the client's objectives and the media outlet's offerings.
* Retail Salesperson: Retail sales representatives work in a retail environment, interacting directly with customers. They assist shoppers, provide product information, and facilitate sales transactions.
* Insurance Sales Agent: Insurance sales agents sell insurance policies to individuals and businesses. They assess the needs of clients, explain coverage options, and help them choose insurance plans that best fit their requirements.
* Pharmaceutical Sales Representative: Pharmaceutical sales representatives specialize in selling pharmaceuticals, medical equipment, or healthcare services. They typically require a strong understanding of medical terminology and industry regulations.
* Real Estate Agent: Real estate sales representatives focus on selling properties, whether residential or commercial. They assist clients in buying, selling, or renting real estate and often work on commission.
* Car Salesperson: These professionals are responsible for selling automobiles to customers. They guide potential buyers through the car-buying process, provide information on vehicle features, and assist with test drives, negotiations, and paperwork to facilitate a successful sale.
* Technical Sales Representative: Technical sales representatives specialize in selling products or services that require a deep understanding of technical specifications. They often work with complex or specialized solutions and collaborate closely with technical teams.
* Business Development Representative (BDR): Business development representatives focus on generating leads and expanding the customer base. Their primary responsibilities include prospecting, qualifying leads, and setting up appointments or demonstrations for the sales team.
* Enterprise Sales Representative: Enterprise sales representatives target large corporations or organizations as clients. They often manage complex sales cycles, negotiate with high-level decision-makers, and handle larger deal sizes.
* Digital Sales Representative: With the growth of online platforms, digital sales representatives specialize in selling digital products, software, or services. They may focus on e-commerce, digital marketing solutions, or software as a service (SaaS).
* Channel Sales Representative: Channel sales representatives work with third-party distributors, resellers, or partners to sell products. They collaborate with channel partners to reach a broader audience and expand market reach.
* Account Executive: Account executives manage and nurture relationships with existing clients. They focus on upselling, cross-selling, and ensuring client satisfaction. Account executives may also be responsible for renewing contracts and securing long-term commitments.
Additional Information
A sales representative’s job is to promote products and services to potential customers, pitch products with a unique selling promotional strategy, and maintain existing customer accounts by ensuring customers' accounts have a proper and smooth sales process.
Who is a sales representative?
A sales representative (sales rep or salesperson) is an individual who is responsible for selling products, services, or solutions on behalf of a company to prospective customers. Their main objective is to build relationships with potential customers, understand their needs and preferences, and then promote and pitch the company’s offerings to meet those needs.
What does a sales representative do?
The responsibilities of sales representatives are:
* Prospecting
* Building relationships
* Product/service presentation
* Needs assessment
* Customized solutions
* Handling objectives
* Negotiations
* Closing deals
* After-sales support
* Sales reporting
Prospecting: Sales representatives spend a significant amount of them identifying potential customers or leads. They use different methods, such as researching online databases, using social media platforms, attending industry events, and networking to find individuals or businesses that might be interested in their company’s products and services.
Building relationships: Sales representatives are relationship builders. They work on establishing trust and rapport with potential customers to create a foundation for future business opportunities.
Product/service presentation: Once they identify potential customers, sales reps need to effectively present the products and services offered by the company. This includes explaining the benefits and unique selling propositions of the product compellingly. Sales reps must be knowledgeable and confident about the products they are selling.
Needs assessment: During the sales process, sales representatives conduct a thorough needs assessment to understand the specific needs of the customer and pain points of the potential customer.
Customized solutions: Based on the needs assessment, sales reps customize their sales pitch and the solutions to suit the individual customer. They emphasize how their offerings can provide value and solve the customer’s challenges.
Handling objectives: Perspective customers may raise objections or concerns during sales. Sales representatives need to be skilled at handling objections diplomatically and providing suitable responses to alleviate customer objections.
Negotiations: Sales reps engage in negotiations to discuss pricing, terms and conditions while keeping the customer’s interest and budget constraints.
Closing deals: A critical part of a sales rep’s role is to close deals successfully, which means encouraging the customer to make a purchase decision throughout the buying journey.
After-sales support: Sales representatives often continue to be involved after the sales is made. They provide post-sales support, and address customer concerns.
Sales reporting: Sales representatives maintain sales activities, leads, and outcomes records. On the basis of the outcomes, reports are prepared and shared with the team and progress is made towards the target.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2053 2024-02-08 00:02:21
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,725
Re: Miscellany
2055) Alkali
Gist
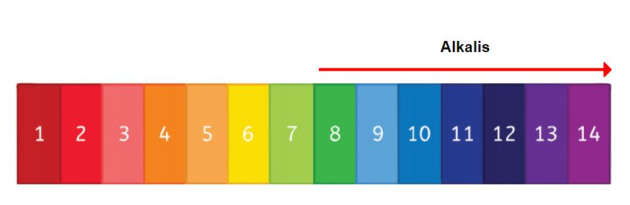
In chemistry, an alkali is a basic, ionic salt of an alkali metal or an alkaline earth metal. An alkali can also be defined as a base that dissolves in water. A solution of a soluble base has a pH greater than 7.0.
Summary
Alkali is any of the soluble hydroxides of the alkali metals—i.e., lithium, sodium, potassium, rubidium, and cesium. Alkalies are strong bases that turn litmus paper from red to blue; they react with acids to yield neutral salts; and they are caustic and in concentrated form are corrosive to organic tissues. The term alkali is also applied to the soluble hydroxides of such alkaline-earth metals as calcium, strontium, and barium and also to ammonium hydroxide. The term was originally applied to the ashes of burned sodium- or potassium-bearing plants, from which the oxides of sodium and potassium could be leached.
The manufacture of industrial alkali usually refers to the production of soda ash (Na2CO3; sodium carbonate) and caustic soda (NaOH; sodium hydroxide). Other industrial alkalies include potassium hydroxide, potash, and lye. The production of a vast range of consumer goods depends on the use of alkali at some stage. Soda ash and caustic soda are essential to the production of glass, soap, miscellaneous chemicals, rayon and cellophane, paper and pulp, cleansers and detergents, textiles, water softeners, certain metals (especially aluminum), bicarbonate of soda, and gasoline and other petroleum derivatives.
People have been using alkali for centuries, obtaining it first from the leachings (water solutions) of certain desert earths. In the late 18th century the leaching of wood or seaweed ashes became the chief source of alkali. In 1775 the French Académie des Sciences offered monetary prizes for new methods for manufacturing alkali. The prize for soda ash was awarded to the Frenchman Nicolas Leblanc, who in 1791 patented a process for converting common salt (sodium chloride) into sodium carbonate. The Leblanc process dominated world production until late in the 19th century, but following World War I it was completely supplanted by another salt-conversion process that had been perfected in the 1860s by Ernest Solvay of Belgium. Late in the 19th century, electrolytic methods for the production of caustic soda appeared and grew rapidly in importance.
In the Solvay, or ammonia-soda process (q.v.) of soda ash manufacture, common salt in the form of a strong brine is chemically treated to eliminate calcium and magnesium impurities and is then saturated with recycling ammonia gas in towers. The ammoniated brine is then carbonated using carbon dioxide gas under moderate pressure in a different type of tower. These two processes yield ammonium bicarbonate and sodium chloride, the double decomposition of which gives the desired sodium bicarbonate as well as ammonium chloride. The sodium bicarbonate is then heated to decompose it to the desired sodium carbonate. The ammonia involved in the process is almost completely recovered by treating the ammonium chloride with lime to yield ammonia and calcium chloride. The recovered ammonia is then reused in the processes already described.
The electrolytic production of caustic soda involves the electrolysis of a strong salt brine in an electrolytic cell. (Electrolysis is the breaking down of a compound in solution into its constituents by means of an electric current in order to bring about a chemical change.) The electrolysis of sodium chloride yields chlorine and either sodium hydroxide or metallic sodium. Sodium hydroxide in some cases competes with sodium carbonate for the same applications, and in any case the two are interconvertible by rather simple processes. Sodium chloride can be made into an alkali by either of the two processes, the difference between them being that the ammonia-soda process gives the chlorine in the form of calcium chloride, a compound of small economic value, while the electrolytic processes produce elemental chlorine, which has innumerable uses in the chemical industry. For this reason the ammonia-soda process, having displaced the Leblanc process, has found itself being displaced, the older ammonia-soda plants continuing to operate very efficiently while newly built plants use electrolytic processes.
In a few places in the world there are substantial deposits of the mineral form of soda ash, known as natural alkali. The mineral usually occurs as sodium sesquicarbonate, or trona (Na2CO3·NaHCO3·2H2O). The United States produces much of the world’s natural alkali from vast trona deposits in underground mines in Wyoming and from dry lake beds in California.
Details
In chemistry, an alkali is a basic, ionic salt of an alkali metal or an alkaline earth metal. An alkali can also be defined as a base that dissolves in water. A solution of a soluble base has a pH greater than 7.0. The adjective alkaline, and less often, alkalescent, is commonly used in English as a synonym for basic, especially for bases soluble in water. This broad use of the term is likely to have come about because alkalis were the first bases known to obey the Arrhenius definition of a base, and they are still among the most common bases.
Etymology
The word "alkali" is derived from Arabic al qalīy (or alkali), meaning the calcined ashes (see calcination), referring to the original source of alkaline substances. A water-extract of burned plant ashes, called potash and composed mostly of potassium carbonate, was mildly basic. After heating this substance with calcium hydroxide (slaked lime), a far more strongly basic substance known as caustic potash (potassium hydroxide) was produced. Caustic potash was traditionally used in conjunction with animal fats to produce soft soaps, one of the caustic processes that rendered soaps from fats in the process of saponification, one known since antiquity. Plant potash lent the name to the element potassium, which was first derived from caustic potash, and also gave potassium its chemical symbol K (from the German name Kalium), which ultimately derived from alkali.
Common properties of alkalis and bases
Alkalis are all Arrhenius bases, ones which form hydroxide ions (OH−) when dissolved in water. Common properties of alkaline aqueous solutions include:
* Moderately concentrated solutions (over 10−3 M) have a pH of 10 or greater. This means that they will turn phenolphthalein from colorless to pink.
* Concentrated solutions are caustic (causing chemical burns).
* Alkaline solutions are slippery or soapy to the touch, due to the saponification of the fatty substances on the surface of the skin.
* Alkalis are normally water-soluble, although some like barium carbonate are only soluble when reacting with an acidic aqueous solution.
Difference between alkali and base
The terms "base" and "alkali" are often used interchangeably, particularly outside the context of chemistry and chemical engineering.
There are various, more specific definitions for the concept of an alkali. Alkalis are usually defined as a subset of the bases. One of two subsets is commonly chosen.
* A basic salt of an alkali metal or alkaline earth metal (this includes Mg(OH)2 (magnesium hydroxide) but excludes NH3 (ammonia)).
* Any base that is soluble in water and forms hydroxide ions or the solution of a base in water. (This includes both Mg(OH)2 and NH3, which forms NH4OH.)
* The second subset of bases is also called an "Arrhenius base".
Alkali salts
Alkali salts are soluble hydroxides of alkali metals and alkaline earth metals, of which common examples are:
* Sodium hydroxide (NaOH) – often called "caustic soda"
* Potassium hydroxide (KOH) – commonly called "caustic potash"
* Lye – generic term for either of two previous salts or their mixture
* Calcium hydroxide (Ca(OH)2) – saturated solution known as "limewater"
* Magnesium hydroxide (Mg(OH)2) – an atypical alkali since it has low solubility in water (although the dissolved portion is considered a strong base due to complete dissociation of its ions)
Alkaline soil
Soils with pH values that are higher than 7.3 are usually defined as being alkaline. These soils can occur naturally, due to the presence of alkali salts. Although many plants do prefer slightly basic soil (including vegetables like cabbage and fodder like buffalo grass), most plants prefer a mildly acidic soil (with pHs between 6.0 and 6.8), and alkaline soils can cause problems.
Alkali lakes
In alkali lakes (also called soda lakes), evaporation concentrates the naturally occurring carbonate salts, giving rise to an alkalic and often saline lake.
Examples of alkali lakes:
* Alkali Lake, Lake County, Oregon
* Baldwin Lake, San Bernardino County, California
* Bear Lake on the Utah–Idaho border
* Lake Magadi in Kenya
* Lake Turkana in Kenya
* Mono Lake, near Owens Valley in California
* Redberry Lake, Saskatchewan
* Summer Lake, Lake County, Oregon
* Tramping Lake, Saskatchewan..
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2054 2024-02-09 00:05:12
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,725
Re: Miscellany
2056) Hairdresser
Gist
A hairdresser is a person who cuts people's hair and puts it into a style, usually working in a special shop, called a hairdresser's.
Summary
Most hairdressers possess a vast range of skills, but some might choose to specialise in particular treatments and styling techniques. Choosing a specific area of expertise could help you stand out from other hairstylists and put you in higher demand as a consequence. Some of the treatments that aren’t usually considered to be standard include the following:
* Specific colouring techniques e.g. balayage
* Artificial hair extensions and weaves
* Chemical relaxing / keratin straightening
* Traditional or modern perms
* Hair extension styling
* Braiding
* Occasion styling e.g. weddings
* Scalp treatment
* Hot oil treatment
* Detox treatment
* Hair glossing
A hairdressing career appeals to many because of its flexible working hours and the ability to work mobile. All you need to provide a service is the right qualifications, some sterile working space, your tools and your creative mind!
However, if you prefer working around a more established routine, a hair salon would typically expect that you work 40 hours per week, between 9am-6pm with a day off during the week to make up for the Saturdays that you’ll most likely be asked to cover. For those who can’t fit in a full-time role around their other commitments, but still appreciate having a regular working pattern that working in a hair salon offers, part-time hours are normally available.
What qualifications do I need to become a hairdresser?
The most desirable UK qualification is an NVQ - National Vocational Qualification. Passing a Level 2 course will help you start out as a Junior Stylist. To be considered a Senior Stylist, alongside your experience, you will require a Level 3 qualification.
Some salons offer accredited training or apprenticeship schemes that help you gain your qualifications while on the job, but you should usually be prepared to continue working for the salon for some years after your training is completed or pay back the entire cost of your studies. Be sure to understand exactly what is required from you before entering any such schemes.
You could also study Hairdressing Level 2 or 3 Diploma at college. The entry requirements for those courses typically include 2 or more GCSEs at grades 9 to 3 (A* to D), or equivalent for a Level 2 course or between 4-5 GCSEs at grades 9 to 4 (A* to C) for a Level 3 Diploma. These courses can usually be combined with other subjects such as beauty therapy, make-up and nails for those who’d like to develop a broader area of expertise.
You may also want to consider taking an accredited short course with a Professional Beauty Direct Accredited Trainer. Details of approved training schools can be found on our website. Accredited courses give those who cannot afford to take a year out to attend college the ability to gain recognised qualifications at a faster pace, with the peace of mind of knowing that they will be able to get insurance to work once qualified.
Aside from official qualifications, a good hairdresser will possess additional key skills, which include a willingness to stay on top of industry trends and learn new techniques, awareness of the ever-changing fashion trends, great customer service and social skills and perhaps above all, creativity.
Details
A hairdresser's job is to organise hair into a particular style or "look". They can cut hair, add colour to it or texture it. A hairdresser may be female or male. Qualified staff are usually called "stylists", who are supported by assistants. Most hairdressing businesses are unisex, that is, they serve both sexes, and have both sexes on their staff.
Male hairdressers who simply cut men's hair (and do not serve females) are often called barbers.
Qualifications for hairdressing usually mean a college course, or an apprenticeship under a senior stylist. Some aspects of the job are quite technical (such as hair dying) and require careful teaching.
A hairdresser specializes in cutting, styling, coloring, and treating hair. These professionals work in salons, spas, or freelance settings, catering to clients of various ages, genders, and hair types.
Hairdressers possess expertise in using a wide range of tools and products to achieve desired hairstyles, including scissors, razors, blow dryers, curling irons, and various hair care products. They consult with clients to understand their preferences and recommend suitable hairstyles based on factors such as face shape, hair texture, and lifestyle. Additionally, hairdressers provide hair care advice and recommend products to maintain the health and appearance of clients' hair between appointments.
Duties and Responsibilities
The duties and responsibilities of a hairdresser encompass a wide range of tasks related to hair care, styling, and customer service. Here are some key responsibilities:
* Hair Cutting and Styling: Hairdressers are skilled in cutting and styling hair according to clients' preferences and facial features. They use various techniques, tools, and products to achieve desired looks, whether it's a simple trim, a layered cut, or an intricate hairstyle for a special occasion.
* Hair Coloring and Treatment: Hairdressers perform hair coloring services, including highlights, lowlights, balayage, and full-color treatments. They also provide hair treatments such as deep conditioning, keratin treatments, and scalp massages to improve the health and appearance of clients' hair.
* Consultation: Before performing any service, hairdressers consult with clients to understand their desired hairstyle, hair type, lifestyle, and maintenance preferences. They offer expert advice and recommendations based on their knowledge and expertise.
* Product Recommendation: Hairdressers recommend hair care products, including shampoos, conditioners, styling products, and treatments, to help clients maintain their hairstyle and keep their hair healthy between salon visits.
* Customer Service: Providing excellent customer service is a crucial aspect of a hairdresser's role. They greet clients warmly, listen attentively to their needs, and ensure they feel comfortable and satisfied throughout their salon experience.
* Sanitation and Hygiene: Hairdressers maintain cleanliness and hygiene standards in the salon by sanitizing tools, equipment, and workstations regularly. They adhere to health and safety protocols to ensure the well-being of clients and staff.
* Continuing Education: To stay current with industry trends and techniques, hairdressers participate in ongoing education and training programs. They attend workshops, seminars, and classes to enhance their skills and expand their knowledge.
Types of Hairdressers
There are several types of hairdressers. Each type of hairdresser requires different skills and expertise, and individuals may choose to specialize in a specific area of hairdressing based on their interests and strengths.
* Barbers: Barbers specialize in cutting and styling men's hair and facial hair. They typically work in barbershops, where they offer a range of services including haircuts, beard trims, and shaves, while also providing grooming advice to clients.
* Celebrity Hairdressers: Celebrity hairdressers cater specifically to the hairstyling needs of celebrities, public figures, and high-profile clients. They often travel with their clients to events, photo shoots, and film sets, providing personalized hair care services and helping them achieve their desired looks for various appearances.
* Hair Colorists: A hair colorist focuses on coloring hair using various techniques and products to achieve desired shades and effects. They assess clients' hair color goals, recommend suitable color options, and apply color treatments with precision and expertise, enhancing clients' overall appearance and confidence.
* Hairdressing Educators: Hairdressing educators specialize in teaching aspiring hairdressers the skills and techniques necessary to succeed in the industry. They develop curriculum, conduct hands-on training sessions, and provide guidance and mentorship to students, ensuring they receive comprehensive education and preparation for their careers in hairdressing.
Are you suited to be a hairdresser?
Hairdressers have distinct personalities. They tend to be artistic individuals, which means they’re creative, intuitive, sensitive, articulate, and expressive. They are unstructured, original, nonconforming, and innovative. Some of them are also enterprising, meaning they’re adventurous, ambitious, assertive, extroverted, energetic, enthusiastic, confident, and optimistic.
What is the workplace of a Hairdresser like?
The workplace of a hairdresser can vary depending on factors such as the type of salon, clientele, and geographic location. Generally, hairdressers work in well-equipped salons that provide a comfortable and inviting environment for both clients and staff. These salons may range from small, independently owned establishments to large, upscale chains located in urban areas or shopping centers.
Inside the salon, hairdressers typically have their own workstation equipped with essential tools and equipment such as styling chairs, mirrors, sinks, and a variety of hair care products. The atmosphere is often lively and energetic, with music playing in the background and a buzz of conversation as stylists interact with clients and colleagues. Some salons may offer additional amenities such as refreshments, magazines, or complimentary Wi-Fi to enhance the client experience.
The work schedule of a hairdresser can vary, with many working full-time, including evenings and weekends to accommodate clients' busy schedules. Flexibility in scheduling is common, allowing hairdressers to balance work and personal commitments. Additionally, some hairdressers may choose to work as freelancers, renting booth space in a salon or offering mobile services to clients in their homes or other locations.
Additional Information
Hairdressing is custom of cutting and arranging the hair, practiced by men and women from ancient times to the present. Early records indicate that the ancient Assyrians wore elaborate curly hair styles; by contrast, the ancient Egyptians, men and women alike, shaved their heads and wore wigs. Whether ornate or simple, hairdressing has been employed by very nearly every society. In 400 BC some Greek women dyed their hair; in the Roman period dying and bleaching were common. Japanese women used lacquer (a precursor of modern-day hair spray) to secure their elaborate coiffures. The wig has come in and gone out of vogue throughout history.
Beginning with the crude curling iron used by women of ancient Rome in creating their elaborate hair styles, hairdressing came to be associated with a variety of technological accoutrements, ranging from simple combs and hairpins to hold the hair in place to complex electrical appliances for drying and grooming the hair and chemical processes to tint, wave, curl, straighten, and condition the hair. By the 20th century, hairdressing itself and the manufacture of materials and equipment had become an occupation and practical art of large proportions.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2055 2024-02-10 00:03:32
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,725
Re: Miscellany
2057) Writer
Gist
A writer is a person engaged in writing books, articles, stories, etc., especially as an occupation or profession; an author or journalist.
Summary
Writing is a form of human communication by means of a set of visible marks that are related, by convention, to some particular structural level of language.
This definition highlights the fact that writing is in principle the representation of language rather than a direct representation of thought and the fact that spoken language has a number of levels of structure, including sentences, words, syllables, and phonemes (the smallest units of speech used to distinguish one word or morpheme from another), any one of which a writing system can “map onto” or represent. Indeed, the history of writing is in part a matter of the discovery and representation of these structural levels of spoken language in the attempt to construct an efficient, general, and economical writing system capable of serving a range of socially valuable functions. Literacy is a matter of competence with a writing system and with the specialized functions that written language serves in a particular society.
Writing as a system of signs
Languages are systems of symbols; writing is a system for symbolizing these symbols. A writing system may be defined as any conventional system of marks or signs that represents the utterances of a language. Writing renders language visible; while speech is ephemeral, writing is concrete and, by comparison, permanent. Both speaking and writing depend upon the underlying structures of language. Consequently, writing cannot ordinarily be read by someone not familiar with the linguistic structure underlying the oral form of the language. Yet writing is not merely the transcription of speech; writing frequently involves the use of special forms of language, such as those involved in literary and scientific works, that would not be produced orally. In any linguistic community the written language is a distinct and special dialect; usually there is more than one written dialect. Scholars account for these facts by suggesting that writing is related directly to language but not necessarily directly to speech. Consequently, spoken and written language may evolve somewhat distinctive forms and functions.
It is the fact that writing is an expression of language rather than simply a way of transcribing speech that gives to writing, and hence to written language and to literacy, its special properties. As long as writing was seen merely as transcription, as it was by such pioneering linguists as Ferdinand de Saussure and Leonard Bloomfield earlier in the 20th century, its conceptual significance was seriously underestimated. Once writing was seen as providing a new medium for linguistic expression, its distinctness from speech was more clearly grasped. Scholars such as Milman Parry, Marshall McLuhan, Eric Havelock, Jack Goody, and Walter Ong were among the first to analyze the conceptual and social implications of using written as opposed to oral forms of communication.
Writing is merely one, albeit the most important, means of communicating by visible signs. Gestures—such as a raised hand for greeting or a wink for intimate agreement—are visible signs, but they are not writing in that they do not transcribe a linguistic form. Pictures, similarly, may represent events but do not represent language and hence are not a form of writing.
But the boundary between pictures and writing becomes less clear when pictures are used conventionally to convey particular meanings. In order to distinguish pictures from pictorial signs, it is necessary to notice that language has two primary levels of structure, which the French linguist André Martinet referred to as the “double articulation” of language: the meaning structures on one hand and the sound patterns on the other. Indeed, linguists define grammar as a system for mapping—establishing a system of relations between—sound and meaning. These levels of structure admit of several subdivisions, any one of which may be captured in a writing system. The basic unit of the meaning system is called a morpheme; one or more morphemes make up a word. Thus, the word boys is composed of two morphemes, boy and plurality. Grammatically related words make up clauses that express larger units of meaning. Still-larger units make up such discourse structures as propositions and less well-defined units of meaning such as prayers, stories, and poems.
The basic linguistic unit of the sound system is called a phoneme; it is a minimal, contrastive sound unit that distinguishes one utterance from another. Phonemes may be further analyzed in terms of a set of underlying distinctive features, features specifying the ways the sound is physically produced by passing breath through the throat and positioning the tongue and lips. Phonemes may be thought of as roughly equivalent to the sound segments known as consonants and vowels, and combinations of these segments make up syllables.
Writing systems can serve to represent any of these levels of sound or any of the levels of meaning, and, indeed, examples of all of these levels of structure have been exploited by some writing system or other. Writing systems consequently fall into two large general classes: those that are based on some aspect of meaning structure, such as a word or a morpheme, and those that are based on some aspect of the sound system, such as the syllable or the phoneme.
The earlier failure to recognize these levels of structure in language led some scholars to believe that some writing systems, so-called ideograms and pictograms, had been invented to express thought directly, bypassing language altogether. The 17th-century German philosopher Gottfried Leibniz set out to invent the perfect writing system, which would reflect systems of thought directly and thereby be readable by all human beings regardless of their mother tongues. It is now known that such a scheme is impossible. Thought is too intimately related to language to be represented independently of it.
More recently there have been attempts to invent forms for communicating explicit messages without assuming a knowledge of any particular language. Such messages are communicated by means of pictorial signs. Thus, the skirted human figure painted on the door to a toilet, the human figure with an upraised hand on the Pioneer spacecraft, the Amerindian drawing of a horse and rider upside down painted on a rock near a precipitous trail, and the visual patterns branded on range cattle are all attempts to use visual marks to communicate without making any appeal to the structure of any particular language.
However, such signs function only because they represent a high level of linguistic structure and because they function to express one of a highly restricted range of meanings already known to the reader and not because they express ideas or thoughts directly. The sign on the toilet door is an elliptical way of writing “women’s washroom,” just as the word “women” had been earlier. The plaque on the spacecraft can be read as a greeting only if the reader already knows how to express a human greeting symbolically. The inverted horse and rider expressed the message that horses and riders should avoid the trail. And the brand can be read as the name of the owner’s ranch.
Such signs therefore express meanings, not thoughts, and they do so by representing meaning structures larger than can be expressed by a single word. They do so by expressing these meanings elliptically. Such signs are readable because the reader has to consider only a restricted set of possible meanings. While such pictorial signs could not be turned into a general writing system, they can be extremely efficient in serving a restricted set of functions.
The differences between such pictorial signs and other forms of writing are sufficiently great for some scholars to maintain that they are not legitimate types of writing. These differences are that pictorial signs are “motivated”—that is, they visually suggest their meanings—and that they express whole propositions rather than single words. Other scholars would include such signs as a form of writing because they are a conventional means for expressing a particular linguistic meaning. However, scholars agree that such a collection of signs could express only an extremely limited set of meanings.
A similar case is the ancient mosaic found at the entrance of a house in Pompeii, depicting a snarling dog on a chain and bearing the inscription “Cave canem” (“Beware of the dog”). Even nonreaders could “read” the message; the picture is therefore a form of writing rather than of picture making. Such pictorial signs, including logotypes, trademarks, and brand names, are so common in modern urban societies that even very young children learn to read them. Such reading ability is described as “environmental” literacy, not associated with books and schooling.
Similarly, number systems have posed a problem for theorists because such symbols as the Arabic numerals 1, 2, 3, etc., which are conventional across many languages, appear to express thought directly without any intermediary linguistic structure. However, it is more useful to think of these numerals as a particular orthography for representing the meaning structure of these numbers rather than their sound structures. The advantages of this orthography are that the orthography permits the user to carry out mathematical operations, such as carrying, borrowing, and the like, and that the same orthography may be assigned different phonological equivalents in different languages using the same number system. Thus, the numeral 2 is named “two” in English, “deux” in French, “zwei” in German, and so on. Yet it represents not a thought but the word, a piece of language.
It is for these reasons that writing is said to be a system for transcribing language, not for representing thought directly. There are of course other systems for representing thought, including such activities as picture making, dance, and mime. These, however, are not representations of ordinary language; rather, they constitute what the American philosopher Nelson Goodman has called the “languages of art.” These “languages,” or semiotic systems, are systems of signs that are used for expressive and representational purposes. Each of these semiotic systems may in turn be represented by a notational system, a system for representing the semiotic system. Thus, writing can be defined formally as a notational system for representing some level or levels of linguistic form.
Writing is so pervasive in everyday life that many people take it to be synonymous with language, and this confusion affects their understanding of language. The word denotes ambiguously both the oral form and the written form, and so people may confuse them. This occurs, for example, when people think that the sounds of language are made up of letters. Even Aristotle used the same word, gramma, to refer to the basic units of both speech and writing. Yet it is important to distinguish them. People may have competence in a language and yet know nothing about its written form. Similarly, writing is so fundamental to a modern, literate society that its significance has often been overestimated. Since the 18th century it has been common to identify literacy with civilization, indeed with all civil virtues. When European countries colonized other regions, they thought it as important to teach “savages” to read and write as to convert them to Christianity. Modern anthropology has helped to revise what now seems a quaint set of priorities by showing not only that there are no genuinely primitive languages but that differing languages mask no unbridgeable differences between human beings. All humans are rational, speak a language of enormous expressive power, and live in, maintain, and transmit to their young a complex social and moral order.
Scholars of literature have in the past half-century amassed compelling evidence to demonstrate that a complex social order and a rich verbal culture can exist in nonliterate societies. The American scholar Milman Parry, writing in the 1920s, showed that the Homeric epic poems, long regarded as models of literary virtuosity, were in fact the product not of a literate but of an oral tradition. These poems were produced by bards who could not write and were delivered in recitals to audiences who could not read. Writing made possible the recording of these poems, not their composition. The hard and fast dividing line that put civilization and literacy on one side and savagery and irrationality on the other has been abandoned. To be unlettered is no longer confused with being ignorant.
Similarly, it was once generally held that all writing systems represent some stage in a progression toward the ideal writing system, the alphabet. The accepted view today is that all writing systems represent relatively optimal solutions to a large and unique set of constraints, including the structure of the language represented, the functions that the system serves, and the balance of advantages to the reader as opposed to the writer. Consequently, while there are important differences between speaking and writing and between various forms of writing, these differences vary in importance and in effect from language to language and from society to society.
Details
A writer is a person who uses written words in different writing styles, genres and techniques to communicate ideas, to inspire feelings and emotions, or to entertain. Writers may develop different forms of writing such as novels, short stories, monographs, travelogues, plays, screenplays, teleplays, songs, and essays as well as reports, educational material, and news articles that may be of interest to the general public. Writers' works are nowadays published across a wide range of media. Skilled writers who are able to use language to express ideas well, often contribute significantly to the cultural content of a society.
The term "writer" is also used elsewhere in the arts and music, such as songwriter or a screenwriter, but also a stand-alone "writer" typically refers to the creation of written language. Some writers work from an oral tradition.
Writers can produce material across a number of genres, fictional or non-fictional. Other writers use multiple media such as graphics or illustration to enhance the communication of their ideas. Another recent demand has been created by civil and government readers for the work of non-fictional technical writers, whose skills create understandable, interpretive documents of a practical or scientific kind. Some writers may use images (drawing, painting, graphics) or multimedia to augment their writing. In rare instances, creative writers are able to communicate their ideas via music as well as words.
As well as producing their own written works, writers often write about how they write (their writing process); why they write (that is, their motivation); and also comment on the work of other writers (criticism). Writers work professionally or non-professionally, that is, for payment or without payment and may be paid either in advance, or on acceptance, or only after their work is published. Payment is only one of the motivations of writers and many are not paid for their work.
The term writer has been used as a synonym of author, although the latter term has a somewhat broader meaning and is used to convey legal responsibility for a piece of writing, even if its composition is anonymous, unknown or collaborative. Author most often refers to the writer of a book.
Types
Writers choose from a range of literary genres to express their ideas. Most writing can be adapted for use in another medium. For example, a writer's work may be read privately or recited or performed in a play or film. Satire for example, may be written as a poem, an essay, a film, a comic play, or a part of journalism. The writer of a letter may include elements of criticism, biography, or journalism.
Many writers work across genres. The genre sets the parameters but all kinds of creative adaptation have been attempted: novel to film; poem to play; history to musical. Writers may begin their career in one genre and change to another. For example, historian William Dalrymple began in the genre of travel literature and also writes as a journalist. Many writers have produced both fiction and non-fiction works and others write in a genre that crosses the two. For example, writers of historical romances, such as Georgette Heyer, create characters and stories set in historical periods. In this genre, the accuracy of the history and the level of factual detail in the work both tend to be debated. Some writers write both creative fiction and serious analysis, sometimes using other names to separate their work. Dorothy Sayers, for example, wrote crime fiction but was also a playwright, essayist, translator, and critic.
Literary and creative:
Poet
Poets make maximum use of the language to achieve an emotional and sensory effect as well as a cognitive one. To create these effects, they use rhyme and rhythm and they also apply the properties of words with a range of other techniques such as alliteration and assonance. A common topic is love and its vicissitudes. Shakespeare's best-known love story Romeo and Juliet, for example, written in a variety of poetic forms, has been performed in innumerable theaters and made into at least eight cinematic versions. John Donne is another poet renowned for his love poetry.
Novelist
A novelist is an author or writer of novels, though often novelists also write in other genres of both fiction and non-fiction. Some novelists are professional novelists, thus make a living writing novels and other fiction, while others aspire to support themselves in this way or write as an avocation. Most novelists struggle to have their debut novel published, but once published they often continue to be published, although very few become literary celebrities, thus gaining prestige or a considerable income from their work.
Satirist
A satirist uses wit to ridicule the shortcomings of society or individuals, with the intent of revealing stupidity. Usually, the subject of the satire is a contemporary issue such as ineffective political decisions or politicians, although human vices such as greed are also a common and prevalent subject. Philosopher Voltaire wrote a satire about optimism called Candide, which was subsequently turned into an opera, and many well known lyricists wrote for it. There are elements of Absurdism in Candide, just as there are in the work of contemporary satirist Barry Humphries, who writes comic satire for his character Dame Edna Everage to perform on stage.
Satirists use different techniques such as irony, sarcasm, and hyperbole to make their point and they choose from the full range of genres – the satire may be in the form of prose or poetry or dialogue in a film, for example. One of the most well-known satirists is Jonathan Swift who wrote the four-volume work Gulliver's Travels and many other satires, including A Modest Proposal and The Battle of the Books.
Short story writer
A short story writer is a writer of short stories, works of fiction that can be read in a single sitting.
Interpretive and academic:
Biographer
Biographers write an account of another person's life. Richard Ellmann (1918–1987), for example, was an eminent and award-winning biographer whose work focused on the Irish writers James Joyce, William Butler Yeats, and Oscar Wilde. For the Wilde biography, he won the 1989 Pulitzer Prize for Biography.
Critic
Critics consider and assess the extent to which a work succeeds in its purpose. The work under consideration may be literary, theatrical, musical, artistic, or architectural. In assessing the success of a work, the critic takes account of why it was done – for example, why a text was written, for whom, in what style, and under what circumstances. After making such an assessment, critics write and publish their evaluation, adding the value of their scholarship and thinking to substantiate any opinion. The theory of criticism is an area of study in itself: a good critic understands and is able to incorporate the theory behind the work they are evaluating into their assessment. Some critics are already writers in another genre. For example, they might be novelists or essayists. Influential and respected writer/critics include the art critic Charles Baudelaire (1821–1867) and the literary critic James Wood (born 1965), both of whom have books published containing collections of their criticism. Some critics are poor writers and produce only superficial or unsubstantiated work. Hence, while anyone can be an uninformed critic, the notable characteristics of a good critic are understanding, insight, and an ability to write well.
We can claim with at least as much accuracy as a well-known writer claims of his little books, that no newspaper would dare print what we have to say. Are we going to be very cruel and abusive, then? By no means: on the contrary, we are going to be impartial. We have no friends – that is a great thing – and no enemies.
Editor
An editor prepares literary material for publication. The material may be the editor's own original work but more commonly, an editor works with the material of one or more other people. There are different types of editor. Copy editors format text to a particular style and/or correct errors in grammar and spelling without changing the text substantively. On the other hand, an editor may suggest or undertake significant changes to a text to improve its readability, sense or structure. This latter type of editor can go so far as to excise some parts of the text, add new parts, or restructure the whole. The work of editors of ancient texts or manuscripts or collections of works results in differing editions. For example, there are many editions of Shakespeare's plays by notable editors who also contribute original introductions to the resulting publication. Editors who work on journals and newspapers have varying levels of responsibility for the text. They may write original material, in particular editorials, select what is to be included from a range of items on offer, format the material, and/or fact check its accuracy.
Encyclopaedist
Encyclopaedists create organised bodies of knowledge. Denis Diderot (1713–1784) is renowned for his contributions to the Encyclopédie. The encyclopaedist Bernardino de Sahagún (1499–1590) was a Franciscan whose Historia general de las cosas de Nueva España is a vast encyclopedia of Mesoamerican civilization, commonly referred to as the Florentine Codex, after the Italian manuscript library which holds the best-preserved copy.
Essayist
Essayists write essays, which are original pieces of writing of moderate length in which the author makes a case in support of an opinion. They are usually in prose, but some writers have used poetry to present their argument.
Historian
A historian is a person who studies and writes about the past and is regarded as an authority on it. The purpose of a historian is to employ historical analysis to create coherent narratives that explain "what happened" and "why or how it happened". Professional historians typically work in colleges and universities, archival centers, government agencies, museums, and as freelance writers and consultants. Edward Gibbon's six-volume History of the Decline and Fall of the Roman Empire influenced the development of historiography.
Lexicographer
Writers who create dictionaries are called lexicographers. One of the most famous is Samuel Johnson (1709–1784), whose Dictionary of the English Language was regarded not only as a great personal scholarly achievement but was also a dictionary of such pre-eminence, that would have been referred to by such writers as Jane Austen.
Researcher/Scholar
Researchers and scholars who write about their discoveries and ideas sometimes have profound effects on society. Scientists and philosophers are good examples because their new ideas can revolutionise the way people think and how they behave. Three of the best known examples of such a revolutionary effect are Nicolaus Copernicus, who wrote De revolutionibus orbium coelestium (1543); Charles Darwin, who wrote On the Origin of Species (1859); and Sigmund Freud, who wrote The Interpretation of Dreams (1899).
These three highly influential, and initially very controversial, works changed the way people understood their place in the world. Copernicus's heliocentric view of the cosmos displaced humans from their previously accepted place at the center of the universe; Darwin's evolutionary theory placed humans firmly within, as opposed to above, the order of manner; and Freud's ideas about the power of the unconscious mind overcame the belief that humans were consciously in control of all their own actions.
Translator
Translators have the task of finding some equivalence in another language to a writer's meaning, intention and style. Translators whose work has had very significant cultural effect include Al-Ḥajjāj ibn Yūsuf ibn Maṭar, who translated Elements from Greek into Arabic and Jean-François Champollion, who deciphered Egyptian hieroglyphs with the result that he could publish the first translation of the Rosetta Stone hieroglyphs in 1822. Difficulties with translation are exacerbated when words or phrases incorporate rhymes, rhythms, or puns; or when they have connotations in one language that are non-existent in another. For example, the title of Le Grand Meaulnes by Alain-Fournier is supposedly untranslatable because "no English adjective will convey all the shades of meaning that can be read into the simple [French] word 'grand' which takes on overtones as the story progresses." Translators have also become a part of events where political figures who speak different languages meet to look into the relations between countries or solve political conflicts. It is highly critical for the translator to deliver the right information as a drastic impact could be caused if any error occurred.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2056 2024-02-11 00:03:29
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,725
Re: Miscellany
2058) Nursery rhymes
Gist
There’s a reason why nursery rhyme songs have been with us for centuries. They work like a charm.
The constant repetition in the nursery rhyme songs is perfect for developing brains that are trying to keep a hold of vocabulary and learn to focus. What’s more, the children learn to listen carefully from beginning to end and get introduced to the imaginative world of storytelling.
Even the youngest of babies can enjoy nursery rhymes and you will quickly find that they start to connect with you as you sing with them.
Summary
Nursery rhyme is a verse customarily told or sung to small children. The oral tradition of nursery rhymes is ancient, but new verses have steadily entered the stream. A French poem numbering the days of the month, similar to “Thirty days hath September,” was recorded in the 13th century; but such latecomers as “Twinkle, Twinkle, Little Star” (by Ann and Jane Taylor; pub. 1806) and “Mary Had a Little Lamb” (by Sarah Josepha Hale; pub. 1830) seem to be just as firmly established in the repertoire.
Some of the oldest rhymes are probably those accompanying babies’ games, such as “Handy, dandy, prickly, pandy, which hand will you have?” (recorded 1598) and its German equivalent, “Windle, wandle, in welchem Handle, oben oder unt?” The existence of numerous European parallels for “Ladybird, ladybird [or, in the United States, “Ladybug, ladybug”], fly away home” and for the singing game “London Bridge is falling down” and for the riddle-rhyme “Humpty-Dumpty” suggests the possibility that these rhymes come down from very ancient sources, since direct translation is unlikely.
Such relics of the past are exceptional. Most nursery rhymes date from the 16th, 17th, and, most frequently, the 18th centuries. Apparently most were originally composed for adult entertainment. Many were popular ballads and songs. “The frog who would a-wooing go” first appeared in 1580 as A Moste Strange weddinge of the ffrogge and the mowse. “Oh where, oh where, ish mine little dog gone?” was a popular song written in 1864 by the Philadelphia composer Septimus Winner.
Although many ingenious theories have been advanced attributing hidden significance, especially political allusions, to nursery rhymes, there is no reason to suppose they are any more arcane than the popular songs of the day. Some were inspired by personalities of the time, and occasionally these can be identified. Somerset tradition associates “Little Jack Horner” (recorded 1725) with a Thomas Horner of Mells who did well for himself during the dissolution of the monasteries.
The earliest known published collection of nursery rhymes was Tommy Thumb’s (Pretty) Song Book, 2 vol. (London, 1744). It included “Little Tom Tucker,” “Sing a Song of Sixpence,” and “Who Killed math Robin?” The most influential was Mother Goose’s Melody: or Sonnets for the Cradle, published by the firm of John Newbery in 1781. Among its 51 rhymes were “Jack and Jill,” “Ding Dong Bell,” and “Hush-a-bye baby on the tree top.” An edition was reprinted in the United States in 1785 by Isaiah Thomas. Its popularity is attested by the fact that these verses are still commonly called “Mother Goose rhymes” in the United States. See also alphabet rhyme; counting-out rhyme; Mother Goose.
Details
A nursery rhyme is a traditional poem or song for children in Britain and many other countries, but usage of the term dates only from the late 18th/early 19th century. The term Mother Goose rhymes is interchangeable with nursery rhymes.
From the mid-16th century nursery rhymes began to be recorded in English plays, and most popular rhymes date from the 17th and 18th centuries. The first English collections, Tommy Thumb's Song Book and a sequel, Tommy Thumb's Pretty Song Book, were published by Mary Cooper in 1744. Publisher John Newbery's stepson, Thomas Carnan, was the first to use the term Mother Goose for nursery rhymes when he published a compilation of English rhymes, Mother Goose's Melody, or, Sonnets for the Cradle (London, 1780).
History:
Lullabies
The oldest children's songs for which records exist are lullabies, intended to help a child fall asleep. Lullabies can be found in every human culture. The English term lullaby is thought to come from "lu, lu" or "la la" sounds made by mothers or nurses to calm children, and "by by" or "bye bye", either another lulling sound or a term for a good night. Until the modern era, lullabies were usually recorded only incidentally in written sources. The Roman nurses' lullaby, "Lalla, Lalla, Lalla, aut dormi, aut lacta", is recorded in a scholium on Persius and may be the oldest to survive.
Many medieval English verses associated with the birth of Jesus take the form of a lullaby, including "Lullay, my liking, my dere son, my sweting" and may be versions of contemporary lullabies. However, most of those used today date from the 17th century. For example, a well-known lullaby such as "Rock-a-bye Baby", could not be found in records until the late-18th century when it was printed by John Newbery (c. 1765).
Early nursery rhymes
A French poem, similar to "Thirty days hath September", numbering the days of the month, was recorded in the 13th century. From the later Middle Ages, there are records of short children's rhyming songs, often as marginalia. From the mid-16th century, they began to be recorded in English plays. "Pat-a-cake" is one of the oldest surviving English nursery rhymes. The earliest recorded version of the rhyme appears in Thomas d'Urfey's play The Campaigners from 1698. Most nursery rhymes were not written down until the 18th century when the publishing of children's books began to move from polemic and education towards entertainment, but there is evidence for many rhymes existing before this, including "To market, to market" and "math a doodle doo", which date from at least the late 16th century. Nursery rhymes with 17th-century origins include, "Jack Sprat" (1639), "The Grand Old Duke of York" (1642), "Lavender's Blue" (1672) and "Rain Rain Go Away" (1687).
The first English collection, Tommy Thumb's Song Book and a sequel, Tommy Thumb's Pretty Song Book, were published by Mary Cooper in London in 1744, with such songs becoming known as "Tommy Thumb's songs". A copy of the latter is held in the British Library. John Newbery's stepson, Thomas Carnan, was the first to use the term Mother Goose for nursery rhymes when he published a compilation of English rhymes, Mother Goose's Melody, or, Sonnets for the Cradle (London, 1780). These rhymes seem to have come from a variety of sources, including traditional riddles, proverbs, ballads, lines of Mummers' plays, drinking songs, historical events, and, it has been suggested, ancient pagan rituals. One example of a nursery rhyme in the form of a riddle is "As I was going to St Ives", which dates to 1730. About half of the currently recognised "traditional" English rhymes were known by the mid-18th century. More English rhymes were collected by Joseph Ritson in Gammer Gurton's Garland or The Nursery Parnassus (1784), published in London by Joseph Johnson.
19th century
In the early 19th century printed collections of rhymes began to spread to other countries, including Robert Chambers' Popular Rhymes of Scotland (1826) and in the United States, Mother Goose's Melodies (1833). From this period the origins and authors of rhymes are sometimes known—for instance, in "Twinkle, Twinkle, Little Star" which combines the melody of an 18th-century French tune "Ah vous dirai-je, Maman" with a 19th-century English poem by Jane Taylor entitled "The Star" used as lyrics.
Early folk song collectors also often collected (what is now known as) nursery rhymes, including in Scotland Sir Walter Scott and in Germany Clemens Brentano and Achim von Arnim in Des Knaben Wunderhorn (1806–1808). The first, and possibly the most important academic collection to focus in this area was James Halliwell-Phillipps' The Nursery Rhymes of England (1842) and Popular Rhymes and Tales in 1849, in which he divided rhymes into antiquities (historical), fireside stories, game-rhymes, alphabet-rhymes, riddles, nature-rhymes, places and families, proverbs, superstitions, customs, and nursery songs (lullabies). By the time of Sabine Baring-Gould's A Book of Nursery Songs (1895), folklore was an academic study, full of comments and footnotes. A professional anthropologist, Andrew Lang (1844–1912) produced The Nursery Rhyme Book in 1897.
20th century
The early years of the 20th century are notable for the illustrations to children's books including Randolph Caldecott's Hey Diddle Diddle Picture Book (1909) and Arthur Rackham's Mother Goose (1913). The definitive study of English rhymes remains the work of Iona and Peter Opie.
Meanings of nursery rhymes
Many nursery rhymes have been argued to have hidden meanings and origins. John Bellenden Ker Gawler (1764–1842), for example, wrote four volumes arguing that English nursery rhymes were written in "Low Saxon", a hypothetical early form of Dutch. He then "translated" them back into English, revealing in particular a strong tendency to anti-clericalism. Many of the ideas about the links between rhymes and historical persons, or events, can be traced back to Katherine Elwes' book The Real Personages of Mother Goose (1930), in which she linked famous nursery rhyme characters with real people, on little or no evidence. She posited that children's songs were a peculiar form of coded historical narrative, propaganda or covert protest, and did not believe that they were written simply for entertainment.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2057 2024-02-12 00:02:28
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,725
Re: Miscellany
2059) Container Freight Station
Gist
A CFS (container freight station) is a warehouse that specializes in the consolidation and deconsolidation of cargo. An LCL (less than container load) shipment will be taken to a CFS at origin to be consolidated into a container with other cargo.
Summary
A Container Freight Station refers to a facility that consolidates or de-consolidates freight before preparing such freight for the next leg of its journey. Most CFS will be located close to ports of entry such as airports, ocean container ports and major railway hubs. In the US, a CFS is designated by its FIRMS code. A list of CFS and their designated FIRMS codes can be found here.
Most of the time it is a warehouse where goods and products that do not fit into one container are collected, stored and wait for other goods to fill a container before they are shipped to the next destination. As such, the CFS is used with Less than Container Load (LCL) shipping where one shipment is not enough to fill a container.
Once the shipment arrives at the facility, it is consolidated and packaged into a Full Container Load (FCL) shipment, which can then be transported to the next stage. LCL is thus more cost-effective when a client does not have enough goods to fill a container and opts to share the space in a container with those of another shipper, rather than pay for a full container.
Details
As your business grows, you may look to expand your footing beyond your existing customer base and extend your reach to attract potential customers. To access a seamless network of distribution and shipping, it is essential to utilize containerized shipping. Earlier, goods from the smaller shipments would be loaded and sorted only at the time of onboarding. But today, the shipping process has been revolutionized, for smaller and larger shipments alike, with the help of Container Freight Station (CFS).
What is Container Freight Station (CFS)?
A CFS is an area, typically a warehouse near shipping ports or crucial railway hubs. These container freight stations are either owned by private stakeholders or shipping terminals. Their primary function involves the consolidation and de-consolidation of less-than-container load (LCL) cargo. Consolidation includes bringing together multiple LCL shipments to form a full container load (FCL) whereas de-consolidation is the process of segregating the LCL shipments.
Moreover, a Container Freight Station is also utilized as a temporary storage space for goods for import and export.
Why Container Freight Station?
CFS has become an integral part of the shipping business. It has made the import-export business seamless at both, origin and destination points. And so, container freight stations are segregated into origin CFS and destination CFS.
With the exponential increase in the demand for LCL shipments, the stations have become a sought-after facility for import-export. It offers an advantage of a centralized shipment location, in turn contributing immensely to streamline and ease up the entire process.
What Does A CFS Do?
* Chalk out a viable container load plan
* Obtain and consolidate LCL shipments for export
* De-consolidate the container at destination CFS. Then, dispatch the shipment for delivery
* Loading and unloading of containers
* Assign specific marks and seals to the containers for identification purposes
* Arranging and rearranging empty containers from container yards
* Managing transportation of laden containers to corresponding port or terminal
* Keeping an account of containers before & after shipping and sort accordingly
* Regular maintenance and timely servicing of the containers
* Overlooking the customs clearance procedures while ensuring the goods are kept safe until shipped or picked up
* Utilizing free spaces to become a temporary storage facility for cargo
The Import and Export Process at CFS
As the Container Freight Station (CFS) simplifies the shipping process for LCL and FCL cargos, here's how the process works:
Export:
* Exporter arrives with goods at CFS along with a shipping bill
* The goods are unloaded and the CFS custodian accounts for receival of the goods
* Customs authorities initiate customs clearance procedures for the goods
* Once the procedure is completed and customs authorities issue a shipping bill with “let export order”
* CFS begins loading the goods into the container
* The container is sealed and marked. CFS dispatches it to port/terminal for export
Import:
* As the container arrives, the importer files an import general manifest (IGM) at the port. This consists of details regarding the cargo, exporter, importer
* The container is then forwarded to the destination CFS
* CFS offloads the cargo and sends it for cargo clearance process
* Cargo owner or their clearing agent files bill of entry. Once the cargo clearance and duty payment is done, it is forwarded to the customs authorities
* Customs issues bill of entry with an “out of charge” order
* The CFS custodian then dispatched the cargo to the importer with a gate pass
As the Container Freight Station acts as an extension of the port, it allows the ports to reduce congestion while streamlining the entire process.
How does the CFS charge?
For every activity the Container Freight Station performs, it levies a charge accordingly. Moreover, the charges for 20-foot, 40-foot and 45-foot containers vary and the charges for reefer, hazardous and over-dimensional cargo (ODC) are higher. And so, it is essential the exporters and importers must know about these charges.
If these charges are not taken into consideration, it can lead to shipment delays, a rise in logistics cost and most importantly, it could deteriorate your relations with customs authorities, which could be a bad sign for your business.
Undeniably, with the introduction of CFS, the process of shipping has been optimized. But we believe that it takes a trustworthy container freight station to understand what your needs are and ensure the delivery of the best services. Which is why we at Allcargo, have India's widest ISO-certified CFS network at your service.
With our Container Freight Station network across India, we aim to bring you everything you need in a “one-stop” service. The state-of-the-art services along with our several years of experience are designed to cater to all your shipping requirements. We understand what it takes to ensure seamless delivery of your products and provide you with results that exceed your expectations.
Additional Information
CFS stands for ‘Container Freight Station’; a station or warehouse where a number goods or products are stored to be shipped together in one or more containers.
At a CFS, the goods normally belong to a number of different customers, and the shipment is often done via LCL shipments.
LCL (Less container load) shipments occur when the exporters don’t have enough cargo to fill one container full (an FCL). We’ve put together a guide on Less Container Load Shipments and Full Container Load Shipments here.
CFS Pier to Pier
Another term often appearing on Bills of Lading or Letters of Credit are CFS/CFS pier to pier, referring to cargo which is packed by the carrier into a container along with other goods and accepted and unpacked by a consignee at the destination terminal or port.
CFS Receiving Services
CFS receiving services are a set of services which are provided between receiving cargo from exporters and packing them into containers.
CFS Receiving Services include:
* Moving empty containers from a Container Yard to a Container Freight Station
* Drayage of loaded containers from the Container Freight Station to the Container Yard
* Tallying
* Issuing dock receipt or shipping order
* The physical movement of cargo in or out of a Container Freight Station
* Stuffing, sealing and marking of containers for labelling and identification
* Storage of containers
* Ordinary sorting and stacking of containers pre or post shipment
* Preparing containers internal load plan
How CFS works
Exports will be delivered to the nominated CFS for packing, and imports will be picked up from the nominated CFS after unpacking.
All cargo going to one destination will be consolidated and packed into one container at the Container Freight Station. For example, the CFS might pack ten different LCL shipments going to Singapore but from different customers into a single 40′ container, and then ship this one container.
Bills of lading for LCL shipments will be lines bills of lading and will mention CFS/CFS on the bill. Accordingly, the shipping line has responsibility from the CFS at the port of load until the CFS at the port of discharge.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2058 2024-02-13 00:08:47
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,725
Re: Miscellany
2060) Molecule
Gist
A molecule is two or more atoms connected by chemical bonds, which form the smallest unit of a substance that retains the composition and properties of that substance. Molecules form the basis of chemistry. Molecules are noted with the element symbol and a subscript with the number of atoms.
Summary
A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and biochemistry, the distinction from ions is dropped and molecule is often used when referring to polyatomic ions.
A molecule may be homonuclear, that is, it consists of atoms of one chemical element, e.g. two atoms in the oxygen molecule (O2); or it may be heteronuclear, a chemical compound composed of more than one element, e.g. water (two hydrogen atoms and one oxygen atom; H2O). In the kinetic theory of gases, the term molecule is often used for any gaseous particle regardless of its composition. This relaxes the requirement that a molecule contains two or more atoms, since the noble gases are individual atoms. Atoms and complexes connected by non-covalent interactions, such as hydrogen bonds or ionic bonds, are typically not considered single molecules.
Concepts similar to molecules have been discussed since ancient times, but modern investigation into the nature of molecules and their bonds began in the 17th century. Refined over time by scientists such as Robert Boyle, Amedeo Avogadro, Jean Perrin, and Linus Pauling, the study of molecules is today known as molecular physics or molecular chemistry.
Details
Molecule is a a group of two or more atoms that form the smallest identifiable unit into which a pure substance can be divided and still retain the composition and chemical properties of that substance.
Characteristics of molecules
The division of a sample of a substance into progressively smaller parts produces no change in either its composition or its chemical properties until parts consisting of single molecules are reached. Further subdivision of the substance leads to still smaller parts that usually differ from the original substance in composition and always differ from it in chemical properties. In this latter stage of fragmentation the chemical bonds that hold the atoms together in the molecule are broken.
A water molecule is made up of two hydrogen atoms and one oxygen atom. A single oxygen atom contains six electrons in its outer shell, which can hold a total of eight electrons. When two hydrogen atoms are bound to an oxygen atom, the outer electron shell of oxygen is filled.
Atoms consist of a single nucleus with a positive charge surrounded by a cloud of negatively charged electrons. When atoms approach one another closely, the electron clouds interact with each other and with the nuclei. If this interaction is such that the total energy of the system is lowered, then the atoms bond together to form a molecule. Thus, from a structural point of view, a molecule consists of an aggregation of atoms held together by valence forces. Diatomic molecules contain two atoms that are chemically bonded. If the two atoms are identical, as in, for example, the oxygen molecule (O2), they compose a homonuclear diatomic molecule, while if the atoms are different, as in the carbon monoxide molecule (CO), they make up a heteronuclear diatomic molecule. Molecules containing more than two atoms are termed polyatomic molecules, e.g., carbon dioxide (CO2) and water (H2O). Polymer molecules may contain many thousands of component atoms.
Molecular bonding
The ratio of the numbers of atoms that can be bonded together to form molecules is fixed; for example, every water molecule contains two atoms of hydrogen and one atom of oxygen. It is this feature that distinguishes chemical compounds from solutions and other mechanical mixtures. Thus hydrogen and oxygen may be present in any arbitrary proportions in mechanical mixtures but when sparked will combine only in definite proportions to form the chemical compound water (H2O). It is possible for the same kinds of atoms to combine in different but definite proportions to form different molecules; for example, two atoms of hydrogen will chemically bond with one atom of oxygen to yield a water molecule, whereas two atoms of hydrogen can chemically bond with two atoms of oxygen to form a molecule of hydrogen peroxide (H2O2). Furthermore, it is possible for atoms to bond together in identical proportions to form different molecules. Such molecules are called isomers and differ only in the arrangement of the atoms within the molecules. For example, ethyl alcohol (CH3CH2OH) and methyl ether (CH3OCH3) both contain one, two, and six atoms of oxygen, carbon, and hydrogen, respectively, but these atoms are bonded in different ways.
Not all substances are made up of distinct molecular units. Sodium chloride (common table salt), for example, consists of sodium ions and chlorine ions arranged in a lattice so that each sodium ion is surrounded by six equidistant chlorine ions and each chlorine ion is surrounded by six equidistant sodium ions. The forces acting between any sodium and any adjacent chlorine ion are equal. Hence, no distinct aggregate identifiable as a molecule of sodium chloride exists. Consequently, in sodium chloride and in all solids of similar type, the concept of the chemical molecule has no significance. Therefore, the formula for such a compound is given as the simplest ratio of the atoms, called a formula unit—in the case of sodium chloride, NaCl.
Ionic bonding in sodium chloride. An atom of sodium (Na) donates one of its electrons to an atom of chlorine (Cl) in a chemical reaction, and the resulting positive ion (Na+) and negative ion (Cl−) form a stable ionic compound (sodium chloride; common table salt) based on this ionic bond.
Molecules are held together by shared electron pairs, or covalent bonds. Such bonds are directional, meaning that the atoms adopt specific positions relative to one another so as to maximize the bond strengths. As a result, each molecule has a definite, fairly rigid structure, or spatial distribution of its atoms. Structural chemistry is concerned with valence, which determines how atoms combine in definite ratios and how this is related to the bond directions and bond lengths. The properties of molecules correlate with their structures; for example, the water molecule is bent structurally and therefore has a dipole moment, whereas the carbon dioxide molecule is linear and has no dipole moment. The elucidation of the manner in which atoms are reorganized in the course of chemical reactions is important. In some molecules the structure may not be rigid; for example, in ethane (H3CCH3) there is virtually free rotation about the carbon-carbon single bond.
Determining molecular structure
The nuclear positions in a molecule are determined either from microwave vibration-rotation spectra or by neutron diffraction. The electron cloud surrounding the nuclei in a molecule can be studied by X-ray diffraction experiments. Further information can be obtained by electron spin resonance or nuclear magnetic resonance techniques. Advances in electron microscopy have enabled visual images of individual molecules and atoms to be produced.
Theoretically the molecular structure is determined by solving the quantum mechanical equation for the motion of the electrons in the field of the nuclei (called the Schrödinger equation). In a molecular structure the bond lengths and bond angles are those for which the molecular energy is the least. The determination of structures by numerical solution of the Schrödinger equation has become a highly developed process entailing use of computers and supercomputers.
Polar and nonpolar molecules
If a molecule has no net electrical charge, its negative charge is equal to its positive charge. The forces experienced by such molecules depend on how the positive and negative charges are arranged in space. If the arrangement is spherically symmetric, the molecule is said to be nonpolar. If there is an excess of positive charge on one end of the molecule and an excess of negative charge on the other, the molecule has a dipole moment (i.e., a measurable tendency to rotate in an electric or magnetic field) and is therefore called polar. When polar molecules are free to rotate, they tend to favour those orientations that lead to attractive forces.
Nonpolar molecules generally are considered lipophilic (lipid-loving), whereas polar chemicals are hydrophilic (water-loving). Lipid-soluble, nonpolar molecules pass readily through a cell membrane because they dissolve in the hydrophobic, nonpolar portion of the lipid bilayer. Although permeable to water (a polar molecule), the nonpolar lipid bilayer of cell membranes is impermeable to many other polar molecules, such as charged ions or those that contain many polar side chains. Polar molecules pass through lipid membranes via specific transport systems.
Molecular weight
The molecular weight of a molecule is the sum of the atomic weights of its component atoms. If a substance has molecular weight M, then M grams of the substance is termed one mole. The number of molecules in one mole is the same for all substances; this number is known as Avogadro’s number (6.022140857 × {10}^{23}). Molecular weights can be determined by mass spectrometry and by techniques based on thermodynamics or kinetic transport phenomena.
Additional Information:
What is a molecule?
A molecule is two or more atoms connected by chemical bonds, which form the smallest unit of a substance that retains the composition and properties of that substance. Molecules form the basis of chemistry. Molecules are noted with the element symbol and a subscript with the number of atoms.
Atoms are the fundamental unit of an element. They consist of a nucleus and surrounding electrons. When an atom has an incomplete electron shell, it is said to have valence electrons. When two or more atoms come together to share outer shell valence electrons, a chemical (covalent) bond is formed, and they enter a lower energy state. When atoms bond, energy is released in an exothermic reaction. If the covalent bond is broken and the molecule is split apart, it requires energy input and is thereby endothermic.
Diatomic molecules are when only two atoms combine. An example of a diatomic molecule is carbon monoxide (CO) made of a single atom of carbon and one of oxygen. If the two atoms are the same element, it is called a homonuclear diatomic molecule, such as oxygen (O2) and nitrogen (N2). Polyatomic molecules have more than two atoms, such as water (H2O) and carbon dioxide (CO2). Larger molecules are called polymers and may be made of thousands of atoms.
Atoms can combine in many different ways as molecules. The same atoms may combine in different proportions to form different molecules. As an example, two hydrogen atoms and one oxygen atom form water (H2O), while two hydrogen atoms and two oxygen atoms form hydrogen peroxide (H2O2). It is also possible for the same elements to combine in the same proportions but in a different physical configuration. The physical structure of the molecule can determine its properties. An example is in water: The two hydrogen atoms being positioned 120 degrees apart creates a slight directional electrical charge giving water its solvent capabilities.
A molecule's molecular weight is the sum of all its constituent atoms' atomic weights. Avogadro's number (6.02214076 × {10}^{23}) is the number of molecules that constitutes the atomic weight of a molecule in grams (g). For example, water is two hydrogen atoms with a weight of 1 g each and one oxygen atom with a weight of 16 g, meaning that one mole of water molecules weighs 18 g.
The word molecule comes from the Latin molecula meaning a unit of mass. This name was to encompass its original meaning of "the smallest unit of a substance that still retains the properties of that substance." In 1873, James Maxwell defined atom and molecule: "An atom is a body which cannot be cut in two; a molecule is the smallest possible portion of a particular substance." Since molecules were named before their true nature was discovered, it led to what is now an inexact and debated definition.
Nonmolecular compounds
Many compounds do not fit the strict definition of a molecule but are common in chemistry and everyday life. Some examples of structures that are not molecular in nature are crystals, minerals and metals.
Noble gases are elements that do not have valence electrons and, therefore, do not need to form covalent bonds to become stable. Some may consider them a molecule composed of only a single atom.
In salts and ionic bonds, there are not conventional covalent bonds, so they are not considered molecules. For example, table salt (sodium chloride) forms a lattice structure held together by ionic bonds. In an ionic bond, the electrons may be shared by many atoms instead of only two as in a covalent bond. These chemical bonds do not result in clear separation of individual molecules. These chemical structures are expressed as the ratios of their constituent elements.
Metals do not use covalent bonds and are not considered molecules. Instead, they form metallic bonds where the free valence electrons are shared between all the various atoms in delocalized electron clouds. The electrons are free to move throughout the entire structure instead of being localized.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2059 2024-02-14 00:06:12
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,725
Re: Miscellany
2061) Atom
Gist
An atom is a particle of matter that uniquely defines a chemical element. An atom consists of a central nucleus that is surrounded by one or more negatively charged electrons. The nucleus is positively charged and contains one or more relatively heavy particles known as protons and neutrons.
Summary
An Atom is the basic building block of all matter and chemistry. Atoms can combine with other atoms to form molecules but cannot be divided into smaller parts by ordinary chemical processes.
Most of the atom is empty space. The rest consists of three basic types of subatomic particles: protons, neutrons, and electrons. The protons and neutrons form the atom’s central nucleus. (The ordinary hydrogen atom is an exception; it contains one proton but no neutrons.) As their names suggest, protons have a positive electrical charge, while neutrons are electrically neutral—they carry no charge; overall, then, the nucleus has a positive charge. Circling the nucleus is a cloud of electrons, which are negatively charged. Like opposite ends of a magnet that attract one another, the negative electrons are attracted to a positive force, which binds them to the nucleus. The nucleus is small and dense compared with the electrons, which are the lightest charged particles in nature. The electrons circle the nucleus in orbital paths called shells, each of which holds only a certain number of electrons.
An ordinary, neutral atom has an equal number of protons (in the nucleus) and electrons (surrounding the nucleus). Thus the positive and negative charges are balanced. Some atoms, however, lose or gain electrons in chemical reactions or in collisions with other particles. Ordinary atoms that either gain or lose electrons are called ions. If a neutral atom loses an electron, it becomes a positive ion. If it gains an electron, it becomes a negative ion. These basic subatomic particles—protons, neutrons, and electrons—are themselves made up of smaller substances, such as quarks and leptons.
More than 90 types of atoms exist in nature, and each kind of atom forms a different chemical element. Chemical elements are made up of only one type of atom—gold contains only gold atoms, and neon contains only neon atoms--and they are ranked in order of their atomic number (the total number of protons in its nucleus) in a chart called the periodic table. Accordingly, because an atom of iron has 26 protons in its nucleus, its atomic number is 26 and its ranking on the periodic table of chemical elements is 26. Because an ordinary atom has the same number of electrons as protons, an element’s atomic number also tells how many electrons its atoms have, and it is the number and arrangement of the electrons in their orbiting shells that determines how one atom interacts with another. The key shell is the outermost one, called the valence shell. If this outermost shell is complete, or filled with the maximum number of electrons for that shell, the atom is stable, with little or no tendency to interact with other atoms. But atoms with incomplete outer shells seek to fill or to empty such shells by gaining or losing electrons or by sharing electrons with other atoms. This is the basis of an atom’s chemical activity. Atoms that have the same number of electrons in the outer shell have similar chemical properties.
Details
An atom is a particle of matter that uniquely defines a chemical element. An atom consists of a central nucleus that is surrounded by one or more negatively charged electrons. The nucleus is positively charged and contains one or more relatively heavy particles known as protons and neutrons.
Atoms are the basic building blocks of matter. Anything that takes up space and anything with mass is made up of atoms.
What are protons and neutrons?
Protons and neutrons are subatomic particles that make up the center of the atom, or its atomic nucleus.
* A proton is positively charged. The number of protons in the nucleus of an atom is the atomic number for the chemical element. Different elements' atomic numbers are found in the Periodic Table of Elements. For example, sodium has 11 protons, and its atomic number is 11. A proton has a rest mass, denoted mp, of approximately 1.673 x {10}^{-27} kilogram (kg).
* A neutron is electrically neutral and has a rest mass, denoted mn, of approximately 1.675 x {10}^{-27}.
The mass of a proton or neutron increases when the particle attains extreme speed, for example in a cyclotron or linear accelerator.
The structure of an atom
The total mass of an atom, including the protons, neutrons and electrons, is the atomic mass or atomic weight. The atomic mass or weight is measured in atomic mass units.
* Protons and neutrons make up the nucleus of an atom and the electrons orbit.
* Electrons contribute only a tiny part to the mass of the atomic structure, however, they play an important role in the chemical reactions that create molecules. For most purposes, the atomic weight can be thought of as the number of protons plus the number of neutrons. Because the number of neutrons in an atom can vary, there can be several different atomic weights for most elements.
Protons and electrons have equal and opposite charges. Protons have a positive charge and electrons a negative charge. Normally, atoms have equal numbers of protons and electrons, giving them a neutral charge.
An ion is an atom with a different number of electrons than protons and is electrically charged. An ion with extra electrons has a negative charge and is called an anion and an ion deficient in electrons has a positive charge and is called a cation.
Atoms having the same number of protons but different numbers of neutrons represent the same element and are known as isotopes of that element. An isotope for an element is specified by the sum of the number of protons and neutrons. For example, the following are two isotopes of the carbon atom:
* Carbon 12 is the most common, non-radioactive isotope of carbon.
* Carbon 14 is a less common, radioactive carbon isotope.
The only neutral atom with no neutrons is the hydrogen atom. It has one electron and one proton.
History of the atom
According to CERN, which is the European Council for Nuclear Research, atoms were created 13.7 billion years ago in the first few minutes after the Big Bang. The new universe cooled and expanded, creating the conditions for electrons and quarks -- the smaller particles that make up protons and neutrons -- to form. Millionths of a second later, quarks aggregated to form protons and neutrons, which combined to form the nuclei of atoms.
Bohr atom model diagram
Niels Bohr's model of an atom has electrons orbiting the nucleus in shells that surround the nucleus. The K shell can hold two electrons; the M shell can hold eight; and the L shell can hold up to 32 electrons.
The physicist Ernest Rutherford developed an early model of the atom in 1912. He was the first to suggest that atoms are like miniature solar systems, except that instead of gravity acting as the attractive force, opposing electrical charges serve that function. In the Rutherford atom of atomic theory, electrons orbit the nucleus in circular paths.
Another physicist, Niels Bohr, revised Rutherford's atomic model in 1913. The Bohr atom included negatively charged electrons orbiting the nucleus at specific median distances. These distances are represented by spheres, called shells, surrounding the nucleus. Electrons can move from shell to shell. When an electron absorbs enough energy, it moves to a larger, or higher, shell. When it loses a certain amount of energy, it falls to a smaller, lower shell.
The Bohr radius constant is based on Bohr's model of the atom.
Atomic power
A strong nuclear force holds together the protons and neutrons in the nucleus of an atom. That force overcomes the repulsive force between the positively charged particles. Strong nuclear force -- sometimes referred to as strong force or strong interaction -- only works at very close distances. Strong force is the strongest of the four fundamental forces in nature; the other three are gravitational, electromagnetic and weak nuclear forces.
When the bond between particles in the nucleus is broken, a large amount of energy is released. The process of breaking these bonds is known as nuclear fission. Nuclear power plants use fission to split uranium atoms and generate electricity. Uranium is used for fission because its atoms split relatively easily.
Nuclear power is considered a clean energy source because fission does not emit greenhouse gases. It is a possible energy source for IT data centers looking to reduce their carbon footprint.
Additional Information
The atom is the basic particle of the chemical elements. An atom consists of a nucleus of protons and generally neutrons, surrounded by an electromagnetically bound swarm of electrons. The chemical elements are distinguished from each other by the number of protons that are in their atoms. For example, any atom that contains 11 protons is sodium, and any atom that contains 29 protons is copper. Atoms with the same number of protons but a different number of neutrons are called isotopes of the same element.
Atoms are extremely small, typically around 100 picometers across. A human hair is about a million carbon atoms wide. This is smaller than the shortest wavelength of visible light, which means humans cannot see atoms with conventional microscopes. Atoms are so small that accurately predicting their behavior using classical physics is not possible due to quantum effects.
More than 99.94% of an atom's mass is in the nucleus. Protons have a positive electric charge and neutrons have no charge, so the nucleus is positively charged. The electrons are negatively charged, and this opposing charge is what binds them to the nucleus. If the numbers of protons and electrons are equal, as they normally are, then the atom is electrically neutral as a whole. If an atom has more electrons than protons, then it has an overall negative charge, and is called a negative ion (or anion). Conversely, if it has more protons than electrons, it has a positive charge, and is called a positive ion (or cation).
The electrons of an atom are attracted to the protons in an atomic nucleus by the electromagnetic force. The protons and neutrons in the nucleus are attracted to each other by the nuclear force. This force is usually stronger than the electromagnetic force that repels the positively charged protons from one another. Under certain circumstances, the repelling electromagnetic force becomes stronger than the nuclear force. In this case, the nucleus splits and leaves behind different elements. This is a form of nuclear decay.
Atoms can attach to one or more other atoms by chemical bonds to form chemical compounds such as molecules or crystals. The ability of atoms to attach and detach from each other is responsible for most of the physical changes observed in nature. Chemistry is the science that studies these changes.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2060 2024-02-15 00:07:52
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,725
Re: Miscellany
2062) Referee
Gist
In sports such as American football, association football, basketball, etc., a person who is in charge of making certain that the rules are followed.
Details
In association football, the referee is the person responsible for interpreting and enforcing the Laws of the Game during a match. The referee is the final decision-making authority on all facts connected with play, and is the match official with the authority to start and stop play and impose disciplinary action against players and coaches during a match.
At most levels of play, the referee is assisted by two assistant referees (formerly known as linesmen), who advise the referee on whether the ball leaves the playing area and any infringements of the Laws of the Game occurring outside of the view of the referee. The final decision on any decision of fact rests with the referee, who has authority to overrule an assistant referee. At higher levels of play, the referee may also be assisted by a fourth official who supervises the teams' technical areas and assists the referee with administrative tasks, and, at the very highest levels, additional assistant referees and/or video assistant referees. Referees and other game officials are licensed and trained by its member national organisations.
Powers and duties
* The referee carries a yellow card and a red card, to indicate respectively a caution for misconduct or to send-off a player.
* The coloured cards were introduced by Ken Aston, a former chair of the FIFA Refereeing Committee
The referee's powers and duties are described by Law 5 of the Laws of the Game. The referee:
Overall
* enforces the Laws of the Game
* controls the match in cooperation with the other match officials
* acts as timekeeper, keeps a record of the match and provides the appropriate authorities with a match report, including information on disciplinary action and any other incidents that occurred before, during or after the match
* supervises and/or indicates the restart of play
Advantage
* allows play to continue when an offence occurs and the non-offending team will benefit from the advantage, and penalises the offence if the anticipated advantage does not ensue at that time or within a few seconds
Disciplinary action
* punishes the more serious offence, in terms of sanction, restart, physical severity and tactical impact, when more than one offence occurs at the same time
* takes disciplinary action against players guilty of cautionable and sending-off offences
* has the authority to take disciplinary action from entering the field of play for the pre-match inspection until leaving the field of play after the match ends (including kicks from the penalty mark). If, before entering the field of play at the start of the match, a player commits a sending-off offence, the referee has the authority to prevent the player taking part in the match (see Law 3.6); the referee will report any other misconduct
* has the power to show yellow or red cards and, where competition rules permit, temporarily dismiss a player, from entering the field of play at the start of the match until after the match has ended, including during the half-time interval, extra time and kicks from the penalty mark
* takes action against team officials who fail to act in a responsible manner and warns or shows a yellow card for a caution or a red card for a sending-off from the field of play and its immediate surrounds, including the technical area; if the offender cannot be identified, the senior coach present in the technical area will receive the sanction. A medical team official who commits a sending-off offence may remain if the team has no other medical person available, and act if a player needs medical attention
* acts on the advice of other match officials regarding incidents that the referee has not seen
As well as other various duties and powers described fully in Law 5 of the Laws of the Game, pursuant to current updates.
Regulation
Referees and assistant referees are regulated at a national level. FIFA requires that each national organisation establish a referees committee composed of former officials that has authority over refereeing in that territory. FIFA also mandate that referees pass tests to show sufficient physical fitness and knowledge of the Laws of the Game, as well as an annual medical. Generally, referees are required to have greater experience to officiate higher level matches. The most elite officials, those who are permitted to officiate international games, are listed on the FIFA International Referees List.
Kit and equipment
Referees wear a kit distinguishing themselves from the players. Usually this comprises a shirt of a different colour to the players of both teams.
In the early 20th century, referees wore a blazer rather than a shirt similar to that of the players. Traditionally that uniform was almost always all black, unless one of the teams was wearing a very dark shirt in which case the referee would wear another colour (usually red) to distinguish themself from both teams.
At the 1994 World Cup finals, new shirts were introduced that gave officials a choice of burgundy, yellow or white, and at the same time the creation of the Premier League in England saw referees wear green jerseys: both changes were motivated by television considerations. Since then, most referees have worn either yellow or black, but the colours and styles adopted by individual associations vary greatly. For international contests under the supervision of FIFA, Adidas uniforms are worn because Adidas is the current sponsor. FIFA allows referees to wear five colours: black, red, yellow, green and blue. Along with the jersey, referees are required to wear black shorts, black socks (with white stripes in some cases), and black shoes. The badge, which displays the referee's license level and year of validity, is often affixed to the left chest pocket.
All referees carry a whistle, a watch, penalty cards, a data wallet with pen and paper, and a coin for determining which team has the choice of ends or kick-off. Most are encouraged to have more than one of each on them in case they drop a whistle or a pen runs out and so on. Often, referees use two watches so that they can use one to calculate time lost for stoppages for the purposes of added time. At the highest levels, referees wear a full duplex radio with customised headset to communicate between with their assistants, and assistant referees use electronic flags, which send a signal to the referee when a button is pushed. In matches with goal-line technology, referees will have on their person a device to receive the system's alerts.
Whistle
Referees use a whistle to help them control matches. The whistle is sometimes needed to stop, start or restart play but should not be used for all stoppages, starts or restarts. FIFA's Laws of the Game document gives guidance as to when the whistle should and should not be used. Overuse of the whistle is discouraged since, as stated in the Laws, "A whistle which is used too frequently unnecessarily will have less impact when it is needed." The whistle is an important tool for the referee along with verbal, body and eye communication.
Before the introduction of the whistle, referees indicated their decisions by waving a white handkerchief. The whistles that were first adopted by referees were made by Joseph Hudson at Mills Munitions in Birmingham, England. The Acme Whistle Company (based at Mills Munitions Factory) first began to mass-produce pea whistles in the 1870s for the Metropolitan Police Force. It is frequently stated the referee's whistle was first used in a game between Nottingham Forest and Sheffield Norfolk in 1878; however the last such fixture known to have taken place between the two clubs was in 1874. The Nottingham Forest account book of 1872 apparently recorded the purchase of an "umpire's whistle" and in 1928 an article by R M Ruck about his playing days in the early 1870s referred to the use of a whistle by umpires to indicate an infringement.
The whistle was not mentioned in the Laws of the Game until 1936 when an IFAB Decision was added as footnote (b) to Law 2, stating "A Referee's control over the players for misconduct or ungentlemanly behaviour commences from the time he enters the field of play, but his jurisdiction in connection with the Laws of the Game commences from the time he blows his whistle for the game to start."
In 2007, when IFAB greatly expanded the Laws of the Game, an Additional Instructions section became available, which is a full page of advice on how and when the whistle should be used as a communication and control mechanism by the referee.
History
Referees in football were first described by Richard Mulcaster in 1581. In this description of "foteball" he advocates the use of a "judge over the parties". In the modern era, referees are first advocated in English public school football games, notably Eton football in 1845. A match report from Rochdale in 1842 shows their use in a football game between the Bodyguards Club and the Fearnought Club.
In the early years of the codified sport it was assumed that disputes could be adequately settled by discussion between gentlemen players who would never deliberately commit a foul. However, as play became more competitive, the need for officials grew. Initially there existed two umpires, one per team, who could be appealed to with the referee (the game's timekeeper) being "referred" to if the umpires could not agree.
The promotion of referees to the dominant position they occupy today, and the reformation of umpires into the linesmen role, occurred as part of a major restructuring of the laws in 1891.
Positioning and responsibilities
The predominant system of positioning and division of responsibility used by football match officials throughout the world is known as the Diagonal system of control (DSC).
The referee has final decision-making authority on all matters. The referee is assisted by two assistant referees who advise the referee. An assistant referee's judgement is enforced only if the referee accepts that judgement, and the referee has the authority to unilaterally overrule an assistant referee. The referee is the only official empowered with starting and stopping play, and meting out disciplinary actions such as cautions or send-offs.
The two assistant referees are instructed by the referee to each patrol half of a single touchline on opposite sides of the field. For example, on a field running north–south, one assistant referee (AR) would run on the eastern touchline from the north goal line to the halfway line, while the other assistant referee would run on the western touchline from the south goal line to the halfway line. In general, the assistant referees' duties would be to indicate (using their flags) when an offside offence has occurred in their half, when a ball has left the pitch, and if a foul has been executed out of the view of the referee (typically in their quadrant of the field). Generally, the ARs will position themselves in line with either the second to last opponent or the ball – whichever is closer to the goal line – to better judge offside infractions. However, the assistant referee will have specific positioning with respect to corner kicks, penalty kicks, and throw-ins.
The referee patrols the length of the field to cover the ground not covered by their two assistants, generally running in a diagonal pattern from the southeast quadrant of the field towards the northwest quadrant; hence the term "diagonal system of control" (DSC). This pattern is not a specific route but a general guideline that should be modified to the style of play, nature of the game, the location of play at a given time, etc. In some cases the referee may even exit the field if it aids in their decision-making ability. The main idea is that the referee and assistants using the DSC should be able to position themselves quickly and easily to observe the important aspects of play (offside, ball in or out of play, goal-scoring opportunities, challenges for the ball) from multiple angles with multiple sets of eyes.
The description above refers to a left diagonal system of control, known as "running a left" or "standard diagonal". If, before the match, the centre referee on this field decides to run from southwest to northeast, then the assistants must position themselves accordingly and the result will be a right diagonal system of control, otherwise referred to as "running a right" or "reverse diagonal".
In many cases in England, referees use more of "curve" based on a line running from the edge of the 18-yard (16 m) box, and when near the centre circle they then curve to a line level with the other 18-yard (16 m) box line. This is similar to the diagonal system, but with the speed of modern football it is easier to keep up with play. This also helps the referee avoid being in a common "passing lane" through the centre circle itself.
In international matches the left-wing diagonal shown above has been universal since the 1960s. It is now predominant across the world. England until recently was an exception to this convention. Until 1974 referees in the Football League were required to run both diagonals during a match, most opting to run from right wing to right wing in the first half before switching to the left-wing diagonal for the second half. The chief reason for this alternation was to avoid linesmen wearing down the same part of the touchline during matches – this was important given the generally lower quality of pitches at the time. However switching diagonal was also justified in terms of allowing officials to patrol different areas of the field during games. From the 1974–75 season English referees were allowed to run the same diagonal throughout the same match. Most initially opted for the right-wing diagonal although over the years the left-wing diagonal became increasingly popular and the preferred choice of most referees by the early 2000s. From 2007–08 the left-wing diagonal has been mandatory in English professional football although some referees at lower levels still use the opposite approach.
Its implementation as a standard practice for referees is attributed to Sir Stanley Rous, former referee and President of FIFA from 1961 to 1974.
Other systems of control
While the Laws of the Game mandate a single referee with assistants as described above, other systems are used experimentally or explicitly by some non-FIFA-affiliated governing organizations.
Dual system (two referees)
The dual system, has two referees with no assistants. The system is used some matches played under the rules of the National Federation of State High School Associations (NFHS) in the United States, and in other youth or amateur matches. Both referees have equal authority, and the decision of one referee is binding on the other. Each referee is primarily responsible for a specific area of the field similar to those of the assistant referees in the diagonal system, except that the referees are allowed and encouraged to move away from the touch line into the field, particularly as play approaches the goal lines. Like the assistant referees in the diagonal system, each referee is responsible for patrolling one touch line and one goal line and determining possession for the restart if the ball goes out of play on either of those two boundaries.
Positioning in the dual system is similar to that used by officials in basketball: each referee is either termed the "lead" or the "trail", depending on the direction of the attack. If the attack is against the goal to the referee's right (when facing the field from their assigned touch line), then that referee is the lead, and the other is the trail. The lead is positioned ahead of the play, even with the second-to-last defender to the extent possible, while the trail is positioned behind the play. Both are responsible for calling fouls and misconduct and determining the restart when the ball goes out of play on one of their assigned boundary lines. Since the lead is in a better position to determine offside, the lead is responsible for calling offside, while the trail provides an extra monitor for fouls and misconduct while the lead's attention is focused on offside. When the attack changes direction, the trail becomes the lead and vice versa.
Double dual system (3 referees)
The double dual system uses three referees, all equipped with whistles, positioned much as in the traditional diagonal system of control mandated by IFAB. Each referee has the same authority for decision making. It is authorized in the United States for college and high school matches although it is rarely used.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2061 2024-02-16 00:03:01
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,725
Re: Miscellany
2063) Stem cell
Gist
What are stem cells? Stem cells are the body's raw materials — cells from which all other cells with specialized functions are generated. Under the right conditions in the body or a laboratory, stem cells divide to form more cells called daughter cells.
Summary
In multicellular organisms, stem cells are undifferentiated or partially differentiated cells that can change into various types of cells and proliferate indefinitely to produce more of the same stem cell. They are the earliest type of cell in a cell lineage. They are found in both embryonic and adult organisms, but they have slightly different properties in each. They are usually distinguished from progenitor cells, which cannot divide indefinitely, and precursor or blast cells, which are usually committed to differentiating into one cell type.
In mammals, roughly 50–150 cells make up the inner cell mass during the blastocyst stage of embryonic development, around days 5–14. These have stem-cell capability. In vivo, they eventually differentiate into all of the body's cell types (making them pluripotent). This process starts with the differentiation into the three germ layers – the ectoderm, mesoderm and endoderm – at the gastrulation stage. However, when they are isolated and cultured in vitro, they can be kept in the stem-cell stage and are known as embryonic stem cells (ESCs).
Adult stem cells are found in a few select locations in the body, known as niches, such as those in the bone marrow or gonads. They exist to replenish rapidly lost cell types and are multipotent or unipotent, meaning they only differentiate into a few cell types or one type of cell. In mammals, they include, among others, hematopoietic stem cells, which replenish blood and immune cells, basal cells, which maintain the skin epithelium, and mesenchymal stem cells, which maintain bone, cartilage, muscle and fat cells. Adult stem cells are a small minority of cells; they are vastly outnumbered by the progenitor cells and terminally differentiated cells that they differentiate into.
Research into stem cells grew out of findings by Canadian biologists Ernest McCulloch, James Till and Andrew J. Becker at the University of Toronto and the Ontario Cancer Institute in the 1960s. As of 2016, the only established medical therapy using stem cells is hematopoietic stem cell transplantation, first performed in 1958 by French oncologist Georges Mathé. Since 1998 however, it has been possible to culture and differentiate human embryonic stem cells (in stem-cell lines). The process of isolating these cells has been controversial, because it typically results in the destruction of the embryo. Sources for isolating ESCs have been restricted in some European countries and Canada, but others such as the UK and China have promoted the research. Somatic cell nuclear transfer is a cloning method that can be used to create a cloned embryo for the use of its embryonic stem cells in stem cell therapy. In 2006, a Japanese team led by Shinya Yamanaka discovered a method to convert mature body cells back into stem cells. These were termed induced pluripotent stem cells (iPSCs).
Details
Stem cell is an undifferentiated cell that can divide to produce some offspring cells that continue as stem cells and some cells that are destined to differentiate (become specialized). Stem cells are an ongoing source of the differentiated cells that make up the tissues and organs of animals and plants. There is great interest in stem cells because they have potential in the development of therapies for replacing defective or damaged cells resulting from a variety of disorders and injuries, such as Parkinson disease, heart disease, and diabetes. There are two major types of stem cells: embryonic stem cells and adult stem cells, which are also called tissue stem cells.
Embryonic stem cells (often referred to as ES cells) are stem cells that are derived from the inner cell mass of a mammalian embryo at a very early stage of development, when it is composed of a hollow sphere of dividing cells (a blastocyst). Embryonic stem cells from human embryos and from embryos of certain other mammalian species can be grown in tissue culture.
Mouse embryonic stem cells
The most-studied embryonic stem cells are mouse embryonic stem cells, which were first reported in 1981. This type of stem cell can be cultured indefinitely in the presence of leukemia inhibitory factor (LIF), a glycoprotein cytokine. If cultured mouse embryonic stem cells are injected into an early mouse embryo at the blastocyst stage, they will become integrated into the embryo and produce cells that differentiate into most or all of the tissue types that subsequently develop. This ability to repopulate mouse embryos is the key defining feature of embryonic stem cells, and because of it they are considered to be pluripotent—that is, able to give rise to any cell type of the adult organism. If the embryonic stem cells are kept in culture in the absence of LIF, they will differentiate into “embryoid bodies,” which somewhat resemble early mouse embryos at the egg-cylinder stage, with embryonic stem cells inside an outer layer of endoderm. If embryonic stem cells are grafted into an adult mouse, they will develop into a type of tumour called a teratoma, which contains a variety of differentiated tissue types.
Mouse embryonic stem cells are widely used to create genetically modified mice. This is done by introducing new genes into embryonic stem cells in tissue culture, selecting the particular genetic variant that is desired, and then inserting the genetically modified cells into mouse embryos. The resulting “chimeric” mice are composed partly of host cells and partly of the donor embryonic stem cells. As long as some of the chimeric mice have germ cells (sperm or eggs) that have been derived from the embryonic stem cells, it is possible to breed a line of mice that have the same genetic constitution as the embryonic stem cells and therefore incorporate the genetic modification that was made in vitro. This method has been used to produce thousands of new genetic lines of mice. In many such genetic lines, individual genes have been ablated in order to study their biological function; in others, genes have been introduced that have the same mutations that are found in various human genetic diseases. These “mouse models” for human disease are used in research to investigate both the pathology of the disease and new methods for therapy.
Human embryonic stem cells
Extensive experience with mouse embryonic stem cells made it possible for scientists to grow human embryonic stem cells from early human embryos, and the first human stem cell line was created in 1998. Human embryonic stem cells are in many respects similar to mouse embryonic stem cells, but they do not require LIF for their maintenance. The human embryonic stem cells form a wide variety of differentiated tissues in vitro, and they form teratomas when grafted into immunosuppressed mice. It is not known whether the cells can colonize all the tissues of a human embryo, but it is presumed from their other properties that they are indeed pluripotent cells, and they therefore are regarded as a possible source of differentiated cells for cell therapy—the replacement of a patient’s defective cell type with healthy cells. Large quantities of cells, such as dopamine-secreting neurons for the treatment of Parkinson disease and insulin-secreting pancreatic beta cells for the treatment of diabetes, could be produced from embryonic stem cells for cell transplantation. Cells for this purpose have previously been obtainable only from sources in very limited supply, such as the pancreatic beta cells obtained from the cadavers of human organ donors.
The use of human embryonic stem cells evokes ethical concerns, because the blastocyst-stage embryos are destroyed in the process of obtaining the stem cells. The embryos from which stem cells have been obtained are produced through in vitro fertilization, and people who consider preimplantation human embryos to be human beings generally believe that such work is morally wrong. Others accept it because they regard the blastocysts to be simply balls of cells, and human cells used in laboratories have not previously been accorded any special moral or legal status. Moreover, it is known that none of the cells of the inner cell mass are exclusively destined to become part of the embryo itself—all of the cells contribute some or all of their cell offspring to the placenta, which also has not been accorded any special legal status. The divergence of views on this issue is illustrated by the fact that the use of human embryonic stem cells is allowed in some countries and prohibited in others.
In 2009 the U.S. Food and Drug Administration approved the first clinical trial designed to test a human embryonic stem cell-based therapy, but the trial was halted in late 2011 because of a lack of funding and a change in lead American biotech company Geron’s business directives. The therapy to be tested was known as GRNOPC1, which consisted of progenitor cells (partially differentiated cells) that, once inside the body, matured into neural cells known as oligodendrocytes. The oligodendrocyte progenitors of GRNOPC1 were derived from human embryonic stem cells. The therapy was designed for the restoration of nerve function in persons suffering from acute spinal cord injury.
Embryonic germ cells
Embryonic germ (EG) cells, derived from primordial germ cells found in the gonadal ridge of a late embryo, have many of the properties of embryonic stem cells. The primordial germ cells in an embryo develop into stem cells that in an adult generate the reproductive gametes (sperm or eggs). In mice and humans it is possible to grow embryonic germ cells in tissue culture with the appropriate growth factors—namely, LIF and another cytokine called fibroblast growth factor.
Adult stem cells
Some tissues in the adult body, such as the epidermis of the skin, the lining of the small intestine, and bone marrow, undergo continuous cellular turnover. They contain stem cells, which persist indefinitely, and a much larger number of “transit amplifying cells,” which arise from the stem cells and divide a finite number of times until they become differentiated. The stem cells exist in niches formed by other cells, which secrete substances that keep the stem cells alive and active. Some types of tissue, such as liver tissue, show minimal cell division or undergo cell division only when injured. In such tissues there is probably no special stem-cell population, and any cell can participate in tissue regeneration when required.
Epithelial stem cells
The epidermis of the skin contains layers of cells called keratinocytes. Only the basal layer, next to the dermis, contains cells that divide. A number of these cells are stem cells, but the majority are transit amplifying cells. The keratinocytes slowly move outward through the epidermis as they mature, and they eventually die and are sloughed off at the surface of the skin. The epithelium of the small intestine forms projections called villi, which are interspersed with small pits called crypts. The dividing cells are located in the crypts, with the stem cells lying near the base of each crypt. Cells are continuously produced in the crypts, migrate onto the villi, and are eventually shed into the lumen of the intestine. As they migrate, they differentiate into the cell types characteristic of the intestinal epithelium.
Bone marrow and hematopoietic stem cells
Bone marrow contains cells called hematopoietic stem cells, which generate all the cell types of the blood and the immune system. Hematopoietic stem cells are also found in small numbers in peripheral blood and in larger numbers in umbilical cord blood. In bone marrow, hematopoietic stem cells are anchored to osteoblasts of the trabecular bone and to blood vessels. They generate progeny that can become lymphocytes, granulocytes, red blood cells, and certain other cell types, depending on the balance of growth factors in their immediate environment.
Work with experimental animals has shown that transplants of hematopoietic stem cells can occasionally colonize other tissues, with the transplanted cells becoming neurons, muscle cells, or epithelia. The degree to which transplanted hematopoietic stem cells are able to colonize other tissues is exceedingly small. Despite this, the use of hematopoietic stem cell transplants is being explored for conditions such as heart disease or autoimmune disorders. It is an especially attractive option for those opposed to the use of embryonic stem cells.
High doses of chemotherapy or radiation destroy not only cancer cells but also bone marrow, which is rich in blood-forming stem cells. In order to replace damaged marrow, stem cells are harvested from either the blood or the bone marrow of the cancer patient before therapy; cells also may be taken from a genetically compatible donor. In order to remove unwanted cells, such as tumour cells, from the sample, it is incubated with antibodies that bind only to stem cells. The fluid that contains the selected cells is reduced in volume and frozen until needed. The fluid is then thawed, diluted, and reinfused into the patient's body. Once in the bloodstream, the stem cells travel to the bone marrow, where they implant themselves and begin producing healthy cells.
Bone marrow transplants (also known as bone marrow grafts) represent a type of stem cell therapy that is in common use. They are used to allow cancer patients to survive otherwise lethal doses of radiation therapy or chemotherapy that destroy the stem cells in bone marrow. For this procedure, the patient’s own marrow is harvested before the cancer treatment and is then reinfused into the body after treatment. The hematopoietic stem cells of the transplant colonize the damaged marrow and eventually repopulate the blood and the immune system with functional cells. Bone marrow transplants are also often carried out between individuals (allograft). In this case the grafted marrow has some beneficial antitumour effect. Risks associated with bone marrow allografts include rejection of the graft by the patient’s immune system and reaction of immune cells of the graft against the patient’s tissues (graft-versus-host disease).
Bone marrow is a source for mesenchymal stem cells (sometimes called marrow stromal cells, or MSCs), which are precursors to non-hematopoietic stem cells that have the potential to differentiate into several different types of cells, including cells that form bone, muscle, and connective tissue. In cell cultures, bone-marrow-derived mesenchymal stem cells demonstrate pluripotency when exposed to substances that influence cell differentiation. Harnessing these pluripotent properties has become highly valuable in the generation of transplantable tissues and organs. In 2008 scientists used mesenchymal stem cells to bioengineer a section of trachea that was transplanted into a woman whose upper airway had been severely damaged by tuberculosis. The stem cells were derived from the woman’s bone marrow, cultured in a laboratory, and used for tissue engineering. In the engineering process, a donor trachea was stripped of its interior and exterior cell linings, leaving behind a trachea “scaffold” of connective tissue. The stem cells derived from the recipient were then used to recolonize the interior of the scaffold, and normal epithelial cells, also isolated from the recipient, were used to recolonize the exterior of the trachea. The use of the recipient’s own cells to populate the trachea scaffold prevented immune rejection and eliminated the need for immunosuppression therapy. The transplant, which was successful, was the first of its kind.
Research has shown that there are also stem cells in the brain. In mammals very few new neurons are formed after birth, but some neurons in the olfactory bulbs and in the hippocampus are continually being formed. These neurons arise from neural stem cells, which can be cultured in vitro in the form of neurospheres—small cell clusters that contain stem cells and some of their progeny. This type of stem cell is being studied for use in cell therapy to treat Parkinson disease and other forms of neurodegeneration or traumatic damage to the central nervous system.
Dolly the sheep was cloned using the process of somatic cell nuclear transfer (SCNT). While SCNT is used for cloning animals, it can also be used to generate embryonic stem cells. Prior to implantation of the fertilized egg into the uterus of the surrogate mother, the inner cell mass of the egg can be removed, and the cells can be grown in culture to form an embryonic stem cell line (generations of cells originating from the same group of parent cells).
Following experiments in animals, including those used to create Dolly the sheep, there has been much discussion about the use of somatic cell nuclear transfer (SCNT) to create pluripotent human cells. In SCNT the nucleus of a somatic cell (a fully differentiated cell, excluding germ cells), which contains the majority of the cell’s DNA (deoxyribonucleic acid), is removed and transferred into an unfertilized egg cell that has had its own nuclear DNA removed. The egg cell is grown in culture until it reaches the blastocyst stage. The inner cell mass is then removed from the egg, and the cells are grown in culture to form an embryonic stem cell line (generations of cells originating from the same group of parent cells). These cells can then be stimulated to differentiate into various types of cells needed for transplantation. Since these cells would be genetically identical to the original donor, they could be used to treat the donor with no problems of immune rejection. Scientists generated human embryonic stem cells successfully from SCNT human embryos for the first time in 2013.
While promising, the generation and use of SCNT-derived embryonic stem cells is controversial for several reasons. One is that SCNT can require more than a dozen eggs before one egg successfully produces embryonic stem cells. Human eggs are in short supply, and there are many legal and ethical problems associated with egg donation. There are also unknown risks involved with transplanting SCNT-derived stem cells into humans, because the mechanism by which the unfertilized egg is able to reprogram the nuclear DNA of a differentiated cell is not entirely understood. In addition, SCNT is commonly used to produce clones of animals (such as Dolly). Although the cloning of humans is currently illegal throughout the world, the egg cell that contains nuclear DNA from an adult cell could in theory be implanted into a woman’s uterus and come to term as an actual cloned human. Thus, there exists strong opposition among some groups to the use of SCNT to generate human embryonic stem cells.
Induced pluripotent stem cells
Due to the ethical and moral issues surrounding the use of embryonic stem cells, scientists have searched for ways to reprogram adult somatic cells. Studies of cell fusion, in which differentiated adult somatic cells grown in culture with embryonic stem cells fuse with the stem cells and acquire embryonic stem-cell-like properties, led to the idea that specific genes could reprogram differentiated adult cells. An advantage of cell fusion is that it relies on existing embryonic stem cells instead of eggs. However, fused cells stimulate an immune response when transplanted into humans, which leads to transplant rejection. As a result, research has become increasingly focused on the genes and proteins capable of reprogramming adult cells to a pluripotent state. In order to make adult cells pluripotent without fusing them to embryonic stem cells, regulatory genes that induce pluripotency must be introduced into the nuclei of adult cells. To do this, adult cells are grown in cell culture, and specific combinations of regulatory genes are inserted into retroviruses (viruses that convert RNA [ribonucleic acid] into DNA), which are then introduced to the culture medium. The retroviruses transport the RNA of the regulatory genes into the nuclei of the adult cells, where the genes are then incorporated into the DNA of the cells. About 1 out of every 10,000 cells acquires embryonic stem cell properties. Although the mechanism is still uncertain, it is clear that some of the genes confer embryonic stem cell properties by means of the regulation of numerous other genes. Adult cells that become reprogrammed in this way are known as induced pluripotent stem cells (iPS).
Similar to embryonic stem cells, induced pluripotent stem cells can be stimulated to differentiate into select types of cells that could in principle be used for disease-specific treatments. In addition, the generation of induced pluripotent stem cells from the adult cells of patients affected by genetic diseases can be used to model the diseases in the laboratory. For example, in 2008 researchers isolated skin cells from a child with an inherited neurological disease called spinal muscular atrophy and then reprogrammed these cells into induced pluripotent stem cells. The reprogrammed cells retained the disease genotype of the adult cells and were stimulated to differentiate into motor neurons that displayed functional insufficiencies associated with spinal muscular atrophy. By recapitulating the disease in the laboratory, scientists were able to study closely the cellular changes that occurred as the disease progressed. Such models promise not only to improve scientists’ understanding of genetic diseases but also to facilitate the development of new therapeutic strategies tailored to each type of genetic disease.
In 2009 scientists successfully generated retinal cells of the human eye by reprogramming adult skin cells. This advance enabled detailed investigation of the embryonic development of retinal cells and opened avenues for the generation of novel therapies for eye diseases. The production of retinal cells from reprogrammed skin cells may be particularly useful in the treatment of retinitis pigmentosa, which is characterized by the progressive degeneration of the retina, eventually leading to night blindness and other complications of vision. Although retinal cells also have been produced from human embryonic stem cells, induced pluripotency represents a less controversial approach. Scientists have also explored the possibility of combining induced pluripotent stem cell technology with gene therapy, which would be of value particularly for patients with genetic disease who would benefit from autologous transplantation.
Researchers have also been able to generate cardiac stem cells for the treatment of certain forms of heart disease through the process of dedifferentiation, in which mature heart cells are stimulated to revert to stem cells. The first attempt at the transplantation of autologous cardiac stem cells was performed in 2009, when doctors isolated heart tissue from a patient, cultured the tissue in a laboratory, stimulated cell dedifferentiation, and then reinfused the cardiac stem cells directly into the patient’s heart. A similar study involving 14 patients who underwent cardiac bypass surgery followed by cardiac stem cell transplantation was reported in 2011. More than three months after stem cell transplantation, the patients experienced a slight but detectable improvement in heart function.
Patient-specific induced pluripotent stem cells and dedifferentiated cells are highly valuable in terms of their therapeutic applications because they are unlikely to be rejected by the immune system. However, before induced pluripotent stem cells can be used to treat human diseases, researchers must find a way to introduce the active reprogramming genes without using retroviruses, which can cause diseases such as leukemia in humans. A possible alternative to the use of retroviruses to transport regulatory genes into the nuclei of adult cells is the use of plasmids, which are less tumourigenic than viruses.
In 2021 multiple research teams, working independently, generated human blastocyst-like structures in vitro. The structures were grown using different types of cell populations, including human embryonic stem cells, iPS cells, and reprogrammed adult skin cells. The breakthrough provided a novel means of studying human embryonic development and the very early stages of pregnancy.
Additional Information
Stem cells are special human cells that are able to develop into many different cell types. This can range from muscle cells to brain cells. In some cases, they can also fix damaged tissues. Researchers believe that stem cell-based therapies may one day be used to treat serious illnesses, such as paralysis and Alzheimer disease.
Types of stem cells
Stem cells are divided into 2 main forms. They are embryonic stem cells and adult stem cells.
Embryonic stem cells. The embryonic stem cells used in research today come from unused embryos. These result from an in vitro fertilization procedure. They are donated to science. These embryonic stem cells are pluripotent. This means that they can turn into more than one type of cell.
Adult stem cells. There are 2 types of adult stem cells. One type comes from fully developed tissues, such as the brain, skin, and bone marrow. There are only small numbers of stem cells in these tissues. They are more likely to generate only certain types of cells. For example, a stem cell that comes from the liver will only make more liver cells.
The second type is induced pluripotent stem cells. These are adult stem cells that have been changed in a lab to be more like embryonic stem cells. Scientists first reported that human stem cells could be changed in this way in 2006. Induced pluripotent stem cells don't seem to be different from embryonic stem cells, but scientists have not yet found one that can develop every kind of cell and tissue.
Stem cells in medicine
The only stem cells now used to treat disease are hematopoietic stem cells. These are the blood cell-forming adult stem cells found in bone marrow. Every type of blood cell in the bone marrow starts as a stem cell. Stem cells are immature cells that are able to make other blood cells that mature and function as needed.
These cells are used in procedures, such as bone marrow transplants. These help people with cancer make new blood cells after their own hematopoietic stem cells have been killed by radiation therapy and chemotherapy. They may also be used to treat people with conditions, such as Fanconi anemia. This is a blood disorder that causes the body's bone marrow to fail.
Stem cells may help your health in the future in many ways and through many new treatments. Researchers think that stem cells will be used to help create new tissue. For example, one day healthcare providers may be able to treat people with chronic heart disease. They can do this by growing healthy heart muscle cells in a lab and transplanting them into damaged hearts. Other treatments could target illnesses such as type 1 diabetes, spinal cord injuries, Alzheimer disease, and rheumatoid arthritis. New medicines could also be tested on cells made from pluripotent stem cells.
Challenges in stem cell research
Stem cells need much more study before their use can be expanded. Scientists must first learn more about how embryonic stem cells develop. This will help them understand how to control the type of cells created from them. Another challenge is that the embryonic stem cells available today are likely to be rejected by the body. And some people find it morally troubling to use stem cells that come from embryos.
Scientists also face challenges when using adult pluripotent stem cells. These cells are hard to grow in a lab, so researchers are looking into ways to improve the process. These cells are also found in small amounts in the body. There is a greater chance that they could contain DNA problems.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2062 2024-02-17 00:04:54
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,725
Re: Miscellany
2064) Polymath
Gist
A polymath is a person of wide knowledge or learning.
A polymath is a person who knows a lot about a lot of subjects. If your friend is not only a brilliant physics student but has also published a poetry collection and won prizes at political debates, you can describe her as a polymath.
You can think of a polymath as a classic "Renaissance man." Imagine Leonardo da Vinci, for example, who was not only an amazing artist, but also an engineer, inventor, mathematician, and much more. When a person's knowledge covers many different areas, he or she is a polymath. The Greek word for it is polymathes, "having learned much," with poly meaning "much," and manthanein meaning "learn."
Details
A polymath is a person with broad knowledge or learning. Renaissance Man and (less commonly) Homo Universalis are related terms to describe a person who is well educated, or who excels, in a wide variety of subjects or fields. It is based on the Humanistic view of human beings as the center of the universe, unlimited in their capacity. The ideal person, therefore, in this view is one who attains all knowledge and develops all their abilities to the greatest extent, abilities which should encompass the full spectrum of human nature.
The ideal of the polymath Renaissance Man is embodied in the Italian Leon Battista Alberti, an accomplished architect, painter, classicist, poet, mathematician, and horseman, and Leonardo da Vinci, renowned in fields as diverse as art, science, invention, music, and writing.
Today, the ever continuing growth of knowledge has led to a situation where it is next to impossible for any single person to attain a complete knowledge and the ideal is now often regarded as a person expert in one field but with a sufficiently broad base to network effectively with experts in other fields. Also, studies of intelligence have revealed that a single, unitary intelligence is not adequate to account for all human intellect. Instead, the idea of multiple intelligences has gained ground, in which there are various types of intelligence, such as linguistic, logical-mathematical, spatial, bodily-kinesthetic, musical, and so forth, with different people displaying differing levels of each type. In this view, the ideal is to develop one's own unique talents and abilities to the fullest, without needing to be an expert in all areas.
Definitions
A polymath (Greek polymathēs, "having learned much") is defined as a person with encyclopedic, broad, or varied knowledge or learning. It especially means that the person's knowledge is not restricted to one subject area. The term is used rarely enough to be included in dictionaries of obscure words.
Renaissance Man (a term first recorded in written English in the early twentieth century) is a related term to describe a person who is well educated, or who excels, in a wide variety of subjects or fields.
This ideal developed in Renaissance Italy from the notion expressed by one of its most accomplished representatives, Leon Battista Alberti (1404–1472), that “a man can do all things if he will.” It embodied the basic tenets of Renaissance Humanism, which considered man the center of the universe, limitless in his capacities for development, and led to the notion that men should try to embrace all knowledge and develop their own capacities as fully as possible. Thus the gifted men of the Renaissance sought to develop skills in all areas of knowledge, in physical development, in social accomplishments, and in the arts.
Other similar terms are Homo universalis and Uomo Universale, which in Latin and Italian, respectively, translate as "universal person" or "universal man." These expressions derived from the ideal in Renaissance Humanism that it was possible to acquire a universal learning in order to develop one's potential, (covering both the arts and the sciences and without necessarily restricting this learning to the academic fields). Further, the scope of learning was much narrower so gaining a command of the known accumulated knowledge was more feasible than today.
When someone is called a Renaissance Man today, it is meant that he does not just have broad interests or a superficial knowledge of several fields, but rather that his knowledge is profound, and often that he also has proficiency or accomplishments in (at least some of) these fields, and in some cases even at a level comparable to the proficiency or the accomplishments of an expert. The related term Generalist is often used to contrast this general approach to knowledge to that of the specialist.
The term Universal Genius is also used, taking Leonardo da Vinci as a prime example, especially when a Renaissance man has made historical or lasting contributions in at least one of the fields in which he was actively involved and when he had a universality of approach. Despite the existence of this term, a polymath may not necessarily be classed as a genius; and certainly a genius may not display the breadth of knowledge to qualify as a polymath. Albert Einstein and Marie Curie are examples of people widely viewed as geniuses, but who are not generally considered as polymaths.
According to the Oxford English Dictionary, the words "polymath" and polyhistor mean practically the same; "the classical Latin word polyhistor was used exclusively, and the Greek word frequently, of Alexander Polyhistor," but polymathist appeared later, and then polymath. Thus today, regardless of any differentiation they may have had when originally coined, they are often taken to mean the same thing.
In Britain, phrases such as polymath sportsman, sporting polymath, or simply "polymath" are occasionally used in a restricted sense to refer to athletes that have performed at a high level in several very different sports.
Renaissance Ideal Today
The expression "Renaissance man" today commonly implies only intellectual or scholastic proficiency and knowledge and not necessarily the more universal sense of "learning" implied by the Renaissance Humanism. It is important to note, however, that some dictionaries use the term Renaissance man as roughly synonym of "polymath" in the first meaning, to describe someone versatile with many interests or talents, while others recognize a meaning which is restricted to the Renaissance era and more closely related to the Renaissance ideals.
During the Renaissance, the ideal of Renaissance humanism included the acquisition of almost all available important knowledge. At that time, several universal geniuses seem to have come close to that ideal, with actual achievements in multiple fields. With the passage of time however, "universal learning" has begun to appear ever more self-contradictory. For example, a famous dispute between "Jacob Burckhardt (whose Die Kultur der Renaissance in Italien of 1860 established Alberti as the prototype of the Renaissance Man) and Julius von Schlosser (whose Die Kunstliteratur of 1924 expresses discontent with Burckhardt's assessments on several counts)" deals with the issue of whether Alberti was indeed a dilettante or an actual Universal Man; while an 1863 article about rhetoric said, for instance: "an universal genius is not likely to attain to distinction and to eminence in any thing. To achieve her best results, and to produce her most matured fruit, Genius must bend all her energies in one direction; strive for one object; keep her brain and hand upon one desired purpose and aim."
Since it is considered extremely difficult to genuinely acquire an encyclopaedic knowledge, and even more to be proficient in several fields at the level of an expert, not to mention to achieve excellence or recognition in multiple fields, the word polymath may also be used, often ironically, with a potentially negative connotation as well. Under this connotation, by sacrificing depth for breadth, the polymath becomes a "jack of all trades, master of none." For many specialists, in the context of today's hyperspecialization, the ideal of a Renaissance man is judged to be an anachronism, since it is not uncommon that a specialist can barely dominate the accumulated knowledge of more than just one restricted subfield in his whole life. Many fields of interest take years of single-minded devotion to achieve expertise, often requiring starting at an early age.
In addition, today, expertise is often associated with documents, certifications, diplomas, and degrees and a person who has an abundance of these is often perceived as having more education than practical "working" experience. However, true expertise may require practical familiarity that may be inaccessible to someone who has little or no actual experience in the field or who was not born and raised in the relevant culture. In many such cases, it is realistically possible to achieve only knowledge of theory if not practical experience. For example, on a safari, a jungle native will be a more effective guide than an American scientist who may be educated in the theories of jungle survival but did not grow up acquiring his knowledge the hard way.
Today it is generally considered that the specialist's understanding of knowledge is too narrow and that a synthetic comprehension of different fields is unavailable to him. What is much more common today than the universal approach to knowledge from a single polymath is the multidisciplinary approach to knowledge, which derives from several experts in different fields working together to pool their knowledge and abilities.
Examples
Most of the historical figures considered polymaths would most likely not be so regarded today based on the level of knowledge that they possessed. Much of their knowledge was basic and purely theoretical. For example, a gentleman educated in various fields such as math, history, literature, art, and science during the eighteenth or nineteenth centuries may be only the equivalent of an average modern person with a secondary school education. In ancient times, an expert on medicine may be the equivalent of knowing basic modern first aid. In contrast to modern times, knowledge was also condensed and comprehensive information on a particular field could often be found in single volumes or texts.
Caution is necessary when interpreting the word "polymath" since there is always ambiguity regarding what the word denotes. Nevertheless, there are a number of scholars who are recognized as polymaths and/or Renaissance men; some examples follow.
Recognized polymaths
The following people have been described as "polymaths" by several sources—fulfilling the primary definition of the term—although there may not be expert consensus that each is a prime example in the secondary meaning, as "renaissance men" and "universal geniuses."
* Abhinavagupta (fl. 975–1025), an Indian philosopher, literary critic, Shaivite, aesthetist, [[music]ian, poet, dramatist, dancer, exegetical theologian, and logician; "the great Kashmiri philosopher and polymath, Abhinavagupta."
* Akbar the Great (1542-1605), an Indian Mughal emperor, "polymath," architect, artisan, artist, armorer, blacksmith, carpenter, construction worker, engineer, military general, inventor, lacemaker, technologist, theologian, and writer.
* Leone Battista Alberti (1404–1472), "often considered the archetype of the Renaissance polymath."
* Al-Kindi (Alkindus) (801–873), an Arab astronomer, geographer, mathematician, meteorologist, musician, philosopher, physician, physicist, scientist, and politician; "he (Al-Kindî) was an omnivorous polymath, studying everything, writing 265 treatises about everything—arithmetic, geometry, astronomy, meteorology, geography, physics, politics, music, medicine, philosophy."
* Aristotle (384–322 B.C.E.) "Aristotle was an extraordinary polymath…"
* Samuel Taylor Coleridge (1772–1834), poet, critic, and philosopher; "Coleridge was unquestionably a polymath, with a universal knowledge unequalled by any thinker of his day."
* Benjamin Franklin (1706–1790), a leading author, political theorist, politician, printer, scientist, inventor, civic activist, and diplomat. "The ultimate creole intellectual…. A true polymath of the Enlightenment style, he distinguished himself on both sides of the Atlantic by researches in natural sciences as well as politics and literature."
* Geber (Jabir ibn Hayyan) (721–815), an Arab Muslim chemist, alchemist, astrologer, astronomer, engineer, pharmacist, physician, philosopher, and physicist; "Jābir was a polymath who wrote 300 books on philosophy, 1,300 books on mechanical devices and military machinery, and hundreds of books on alchemy."
* Edward Heron-Allen (1861–1943) Not only was Heron-Allen a lawyer by trade, he also wrote, lectured on and created violins, was an expert on the art of chiromancy or palmistry, having read palms and analyzed the handwriting of luminaries of the period. He wrote on musical, literary and scientific subjects ranging from foraminifera, marine zoology, meteorology, as a Persian scholar translated Classics such as the Rubaiyat of Omar Khayyam and The Lament of Baba Tahir, also wrote on local geographic history, archeology, Buddhist philosophy, the cultivation, gourmet appreciation of and culture of the asparagus, as well as a number of novels and short stories of science fiction and horror written under his pseudonymn of "Christopher Blayre." "Heron-Allen is better described as a polymath…"
* Imhotep (fl. 2650–2611 B.C.E.), Egyptian chancellor, physician, and architect; "Imhotep, circa 2650 B.C.E. (who was revered as being at least semi-divine until the Late Period, although some of this reverence may be due to his status as physician and all-round polymath)."
* Mikhail Lomonosov (1711–1765), "Lomonosov was a true polymath—physicist, chemist, natural scientist, poet and linguist…."
* Shen Kuo (1031–1095), a Chinese scientist, statesman, mathematician, astronomer, meteorologist, geologist, zoologist, botanist, pharmacologist, agronomist, ethnographer, encyclopedist, poet, general, diplomat, hydraulic engineer, inventor, academy chancellor, finance minister, and inspector; "Chinese polymath and astronomer who studied medicine, but became renown for his engineering ability."
* Herbert Simon (1916-2001), "Simon is a very distinguished polymath, famous for work in psychology and computer science, philosophy of science, a leader in artificial intelligence, and a Nobel Prize winner in Economics."
* Mary Somerville (1780–1872), "Somerville was the most celebrated woman scientist of her time. A polymath, she wrote on astronomy, mathematics, physics, chemistry, mineralogy, and geology, among other subjects." "Somerville was the most celebrated woman scientist of her time. A polymath, she wrote on astronomy, mathematics, physics, chemistry, mineralogy, and geology, among other subjects…"
* Rabindranath Tagore (1861–1941), an Indian Bengali polymath; "He was a polymath: a poet, fiction writer, dramatist, painter, educator, political thinker, philosopher of science."
* John von Neumann (1903–1957), physicist, mathematician, game theorist, economist, and pioneering computer scientist. "It isn't often that the human race produces a polymath like von Neumann, then sets him to work in the middle of the biggest crisis in human history…" "Other luminaries would follow Einstein to New Jersey, including the dazzling Hungarian polymath, John von Neumann…"
* H. G. Wells (1866–1946); "Fifty years ago, the British polymath and amateur historian was able to compress the history of the world up to 1920 into one volume…"
* Thomas Young (1773–1829), British polymath, scientist, and Egyptologist, after whom Young's modulus, Young's double-slit experiment, the Young-Laplace equation and the Young-Dupré equation were named. He also studied vision and coined the term Indo-European languages.
Renaissance Men
Leonardo da Vinci is regarded in many Western cultures as the archetypal "Renaissance Man".
The following people represent prime examples of "Renaissance Men" and "universal geniuses," so to say "polymaths" in the strictest interpretation of the secondary meaning of the word. The list also includes some of the Hakeem of the Islamic Golden Age (also known as the "Islamic Renaissance"), who are considered equivalent to the Renaissance Men of the European Renaissance era.
Al-Farabi (Alfarabi) (870–950/951), a Turkic or Persian Muslim who was known as The second teacher because he had great influence on science and philosophy for several centuries, and was widely regarded to be second only to Aristotle in knowledge in his time. Farabi made notable contributions to the fields of mathematics, philosophy, medicine and music. As a philosopher and Neo-Platonist, he wrote rich commentary on Aristotle's work. He is also credited for categorizing logic into two separate groups, the first being "idea" and the second being "proof." Farabi wrote books on sociology and a notable book on music titled Kitab al-Musiqa (The Book of Music). He played and invented a varied number of musical instruments and his pure Arabian tone system is still used in Arabic music.
* Ibn Rushd (Averroes) (1126–1198), an Andalusian Arab philosopher, doctor, physician, jurist, lawyer, astronomer, mathematician, and theologan; "Ibn-Rushd, a polymath also known as Averroes;" "Doctor, Philosopher, Renaissance Man."
* Abū Rayhān al-Bīrūnī (973–1048), a Persian scientist, physicist, anthropologist, astronomer, astrologer, encyclopedist, geodesist, geographer, geologist, historian, mathematician, natural historian, pharmacist, physician, philosopher, scholar, teacher, Ash'ari theologian, and traveler; "al-Biruni was a polymath and traveler (to India), making contributions in mathematics, geography and geology, natural history, calendars and astronomy;" "al-Biruni, a scholar in many disciplines - from linguistics to mineralogy - and perhaps medieval Uzbekistan's most universal genius."
* Nicolaus Copernicus (1473–1543); among the great polymaths of the Renaissance, Copernicus was a mathematician, astronomer, physician, classical scholar, translator, Catholic cleric, jurist, governor, military leader, diplomat and economist. Amid his extensive responsibilities, astronomy figured as little more than an avocation—yet it was in that field that he made his mark upon the world.
* Leonardo da Vinci (1452–1519)"The following selection… shows why this famous Renaissance polymath considered painting to be a science…" "In Leonardo Da Vinci, of course, he had as his subject not just an ordinary Italian painter, but the prototype of the universal genius, the 'Renaissance man,' …"; "prodigious polymath…. Painter, sculptor, engineer, astronomer, anatomist, biologist, geologist, physicist, architect, philosopher, actor, singer, musician, humanist."
* Galileo Galilei (1564–1642), "Italian scientist, physicist, and philosopher. Galileo was a true Renaissance man, excelling at many different endeavors, including lute playing and painting."
* Johann Wolfgang von Goethe (1749–1832) "Germany's greatest man of letters—poet, critic, playwright, and novelist—and the last true polymath to walk the earth" "Goethe comes as close to deserving the title of a universal genius as any man who has ever lived." "He was essentially the last great European Renaissance man." His gifts included incalculable contributions to the areas of German literature and the natural sciences. He is credited with discovery of a bone in the human jaw, and proposed a theory of colors. He has a mineral named in his honor, goethite. He molded the aesthetic properties of the Alps to poetry, thus, changing the local belief from "perfectly hideous" and an "unavoidable misery," to grandeur of the finest most brilliant creation.
* Ibn al-Haytham (Alhacen) (965–1039), an Iraqi Arab scientist, physicist, anatomist, physician, psychologist, astronomer, engineer, mathematician, ophthalmologist, philosopher, and Ash'ari theologian; "a devout, brilliant polymath;" "a great man and a universal genius, long neglected even by his own people;" "Ibn al-Haytham provides us with the historical personage of a versatile universal genius."
* Ibn Khaldun (1332–1406), an Arab social scientist, sociologist, historian, historiographer, philosopher of history, demographer, economist, linguist, philosopher, political theorist, military theorist, Islamic scholar, Ash'ari theologian, diplomat and statesman; "a still-influential polymath;" "in any epoch ibn Khaldun (1332-1406) would deserve the accolade Renaissance man, a person of many talents and diverse interests."
* Thomas Jefferson (1743-1826), some sources describe him as "polymath and President," putting "polymath" first, he is also described as "the walking, talking embodiment of the Enlightenment, a polymath whose list of achievements is as long as it is incredibly varied." John F. Kennedy famously commented, addressing a group of Nobel laureates, that it was "the most extraordinary collection of talent, of human knowledge, that has ever been gathered together at the White House—- with the possible exception of when Thomas Jefferson dined alone."
* Gottfried Leibniz (1646–1716); "Leibniz was a polymath who made significant contributions in many areas of physics, logic, history, librarianship, and of course philosophy and theology, while also working on ideal languages, mechanical clocks, mining machinery…" "A universal genius if ever there was one, and an inexhaustible source of original and fertile ideas, Leibniz was all the more interested in logic because it …" "Gottfried Wilhelm Leibniz was maybe the last Universal Genius incessantly active in the fields of theology, philosophy, mathematics, physics, ...." "Leibniz was perhaps the last great Renaissance man who in Bacon's words took all knowledge to be his province."
* Isaac Newton (1643–1727) was an English physicist, mathematician, astronomer, theologian, natural philosopher and alchemist. His treatise Philosophiae Naturalis Principia Mathematica, published in 1687, described universal gravitation and the three laws of motion, laying the groundwork for classical mechanics, which dominated the scientific view of the physical universe for the next three centuries and is the basis for modern engineering. In a 2005 poll of the Royal Society of who had the greatest effect on the history of science, Newton was deemed more influential than Albert Einstein. "When we see Newton as a late Renaissance man, his particular addiction to classical geometry as ancient wisdom and the most reliable way of unveiling the secrets of nature, seems natural."
Additional Information
A polymath (Greek: romanized: polymathēs, lit. 'having learned much'; Latin: homo universalis, lit. 'universal human') is an individual whose knowledge spans a substantial number of subjects, known to draw on complex bodies of knowledge to solve specific problems.
In Western Europe, the first work to use the term polymathy in its title (De Polymathia tractatio: integri operis de studiis veterum) was published in 1603 by Johann von Wowern, a Hamburg philosopher. Von Wowern defined polymathy as "knowledge of various matters, drawn from all kinds of studies ... ranging freely through all the fields of the disciplines, as far as the human mind, with unwearied industry, is able to pursue them". Von Wowern lists erudition, literature, philology, philomathy, and polyhistory as synonyms.
The earliest recorded use of the term in the English language is from 1624, in the second edition of The Anatomy of Melancholy by Robert Burton; the form polymathist is slightly older, first appearing in the Diatribae upon the first part of the late History of Tithes of Richard Montagu in 1621. Use in English of the similar term polyhistor dates from the late 16th century.
Polymaths include the great scholars and thinkers of the Renaissance and Enlightenment, who excelled at several fields in science, technology, engineering, mathematics, and the arts. In the Italian Renaissance, the idea of the polymath was allegedly expressed by Leon Battista Alberti (1404–1472), a polymath himself, in the statement that "a man can do all things if he will". Gottfried Wilhelm Leibniz has often been seen as a polymath. Al-Biruni was also a polymath. Other well-known and celebrated polymaths include Leonardo da Vinci, Hildegard of Bingen, Ibn al-Haytham, Rabindranath Tagore, Mikhail Lomonosov, Johann Wolfgang von Goethe, Alan Turing, Benjamin Franklin, John von Neumann, Omar Khayyam, Charles Sanders Peirce, Henri Poincaré, Isaac Asimov, Nicolaus Copernicus, René Descartes, Aristotle, Frederick II, Holy Roman Emperor, Averroes, Archimedes, George Washington Carver, Hypatia, Blaise Pascal, Africanus Horton, Wang Wei, Isaac Newton, Pierre-Paul Riquet, Leonhard Euler, Émilie du Châtelet, Nikola Tesla, Thomas Edison, Bertrand Russell, Thomas Young, Sequoyah, Thomas Jefferson and Pierre-Simon Laplace.
Embodying a basic tenet of Renaissance humanism that humans are limitless in their capacity for development, the concept led to the notion that people should embrace all knowledge and develop their capacities as fully as possible. This is expressed in the term Renaissance man, often applied to the gifted people of that age who sought to develop their abilities in all areas of accomplishment: intellectual, artistic, social, physical, and spiritual.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2063 2024-02-18 00:05:10
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,725
Re: Miscellany
2065) Umpire
Gist
An umpire is a person who watches a game such as tennis or cricket to make sure that the players obey the rules.
Details
An umpire is an official in a variety of sports and competition, responsible for enforcing the rules of the sport, including sportsmanship decisions such as ejection.
The term derives from the Old French nonper, non, "not" and per, "equal": "one who is requested to act as arbiter of a dispute between two people" (as evidenced in cricket, where dismissal decisions can only be made on appeal). Noumper shows up around 1350 before undergoing a linguistic shift known as false splitting. It was written in 1426–1427 as a noounpier; the n was lost with the a indefinite article becoming an. The earliest version without the n shows up as owmpere, a variant spelling in Middle English, circa 1440. The leading n became permanently attached to the article, changing it to an Oumper around 1475.
The word was applied to the officials of many sports including baseball, association football (where it has been superseded by assistant-referee) and cricket (which still uses it).
Field hockey
An umpire in field hockey is a person with the authority to make decisions on a hockey field in accordance with the laws of the game. Each match is controlled by two such umpires, where it is typical for umpires to aid one another and correct each other when necessary.
Cricket
In cricket, dismissal decisions can only be made on appeal by the players. Otherwise, on-field decisions, relevant to the rules and scoring and of the game, are handled by two on-field umpires, although an off-field third umpire may help with certain decisions. At the international level, the match referee is an off-field official who makes judgements concerning the reputable conduct of the game and hands out penalties for breaches of the ICC Cricket Code of Conduct.
Baseball and softball
In baseball and softball, there is commonly a head umpire (also known as a plate umpire) who is in charge of calling balls and strikes from behind the plate, who is assisted by one, two, three, or five field umpires who make calls on their specific bases (or with five umpires the bases and the outfield). On any question, the head umpire has the final call.
Football (Australian rules)
An umpire is an official in the sport of Australian rules football. Games are overseen by one to four field umpires, two to four boundary umpires, and two goal umpires.
Lawn bowls
A lawn bowls match is presided over by a bowls umpire or technical official. In games where single players compete, a marker is required to direct play and assist players with questions relating to the position of their bowls.
Netball
In the game of Netball the match at hand is Presided over by 2 umpires, typically female, with a comprehensive knowledge of the rules. There are also 2 timekeepers and 2 scorekeepers who inform the umpires, and players of time remaining, and scores.
Rowing
In a regatta an umpire is the on-the-water official appointed to enforce the rules of racing and to ensure safety. In some cases an umpire may be designated specifically as starter, or otherwise the umpire starts the race from a launch and follows it to its end, ensuring that crews follow their proper course. If no infringements occur, the result is decided by a judge or judges on the waterside who determine the finish order of the crews.
Sailing
In match race and team racing an "umpire" is an on-the-water referee appointed to directly enforce the Racing Rules of Sailing. An umpire is also used in fleet racing to enforce Racing Rule 42 which limits the use of kinetics to drive the boat rather than the wind. Umpires are rarely present during sailing races as decisions are normally referred to a jury-style protest committee after the race.
Tennis
In tennis an umpire is an on-court official, while a referee is an off-court official.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2064 2024-02-19 00:05:03
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,725
Re: Miscellany
2066) Spark plug
Gist
A spark plug is an electrical device used in an internal combustion engine to produce a spark which ignites the air-fuel mixture in the combustion chamber.
Summary
Spark plug is a device that fits into the cylinder head of an internal-combustion engine and carries two electrodes separated by an air gap, across which current from a high-tension ignition system discharges, to form a spark for igniting the air–fuel mixture. The electrodes must be able to resist high temperatures, and the insulator separating them must withstand high temperatures and also an electric stress up to several thousand volts. Spark-gap length affects the energy of the spark, and the shape of the insulator affects the temperature of operation. When too cool, operation leads to carbonization and short-circuiting of the gap; when too hot, there may be preignition.
(Internal-combustion engine is any of a group of devices in which the reactants of combustion (oxidizer and fuel) and the products of combustion serve as the working fluids of the engine. Such an engine gains its energy from heat released during the combustion of the nonreacted working fluids, the oxidizer-fuel mixture. This process occurs within the engine and is part of the thermodynamic cycle of the device. Useful work generated by an internal-combustion (IC) engine results from the hot gaseous products of combustion acting on moving surfaces of the engine, such as the face of a piston, a turbine blade, or a nozzle.
Internal-combustion engines are the most broadly applied and widely used power-generating devices currently in existence. Examples include gasoline engines, diesel engines, gas-turbine engines, and rocket-propulsion systems.
Internal-combustion engines are divided into two groups: continuous-combustion engines and intermittent-combustion engines. The continuous-combustion engine is characterized by a steady flow of fuel and oxidizer into the engine. A stable flame is maintained within the engine (e.g., jet engine). The intermittent-combustion engine is characterized by periodic ignition of air and fuel and is commonly referred to as a reciprocating engine. Discrete volumes of air and fuel are processed in a cyclic manner. Gasoline piston engines and diesel engines are examples of this second group.
Internal-combustion engines can be delineated in terms of a series of thermodynamic events. In the continuous-combustion engine, the thermodynamic events occur simultaneously as the oxidizer and fuel and the products of combustion flow steadily through the engine. In the intermittent-combustion engine, by contrast, the events occur in succession and are repeated for each full cycle.
Some air taken in by the turbofan (top) goes to the compressor; the rest bypasses the main engine. In turboprop engines (bottom) the hot gases drive a turbine, which powers the compressor and propeller, and provide jet thrust.
With the exception of rockets (both solid rocket motors and liquid-propellant rocket engines), internal-combustion engines ingest air, then either compress the air and introduce fuel into the air or introduce fuel and compress the air-fuel mixture. Then, common to all internal-combustion engines, the air-fuel mixture is burned, work is extracted from the expansion of the hot gaseous products of combustion, and ultimately the products of combustion are released through the exhaust system. Their operation can be contrasted with that of external-combustion engines (e.g., steam engines), in which the working fluid does not chemically react and energy gain is achieved solely through heat transfer to the working fluid by way of a heat exchanger.
The most common internal-combustion engine is the four-stroke, gasoline-powered, homogeneous-charge, spark-ignition engine. This is because of its outstanding performance as a prime mover in the ground transportation industry. Spark-ignition engines also are used in the aeronautics industry; however, aircraft gas turbines have become the prime movers in this sector because of the emphasis of the aeronautics industry on range, speed, and passenger comfort. The domain of internal-combustion engines also includes such exotic devices as supersonic combustion ramjet engines (scramjets), such as those proposed for hypersonic aircraft, and sophisticated rocket engines and motors, such as those used on U.S. space shuttles and other space vehicles.)
Details
A spark plug is an electrical device used in an internal combustion engine to produce a spark which ignites the air-fuel mixture in the combustion chamber. As part of the engine's ignition system, the spark plug receives high-voltage electricity (generated by an ignition coil in modern engines and transmitted via a spark plug wire) which it uses to generate a spark in the small gap between the positive and negative electrodes. The timing of the spark is a key factor in the engine's behaviour, and the spark plug usually operates shortly before the combustion stroke commences.
The spark plug was invented in 1860, however its use only became widespread after the invention of the ignition magneto in 1902. Diesel engines use compression ignition (instead of spark ignition), therefore they do not normally use spark plugs.
Design
The main elements of a spark plug are the shell, insulator, central electrode and side electrode (also known as "ground strap"). The main part of the insulator is typically made from sintered alumina (Al2O3), a hard ceramic material with high dielectric strength. In marine engines, the shell of the spark plug is often a double-dipped, zinc-chromate coated metal.
A spark plug passes through the wall of the combustion chamber, therefore it must also form part of the seal for the high-pressure gases within the combustion chamber.
Electrodes
The central electrode is connected to the terminal through an internal wire. The central electrode setup as the cathode from where the electrons are ejected. This is because the central electrode is usually the hottest part of the plug, and thermionic emission principles mean it is easier to eject electrons from a hotter surface. The sharp tip of the central electrode also increases the electrical field strength, thus increasing the emission of electrons. The side electrode (which is colder and blunter) requires up to 45 percent higher voltage, therefore only wasted spark systems use the side electrode as the cathode.
The side electrode is made from high-nickel steel and is welded or hot forged to the side of the metal shell.
Spark plugs can contain up to four side electrodes surrounding the central electrode. Multiple side electrodes generally provide longer life, as when the spark gap widens due to electric discharge wear, the spark moves to another closer ground electrode. The disadvantage of multiple side electrodes is that a shielding effect can occur for each electrode, leading to a less efficient burn and increased fuel consumption.
Gap size
The distance between the tip of the spark plug and the central electrode is called the "spark plug gap" and is a key factor in the function of a spark plug. Spark plug gaps for car engines are typically 0.6 to 1.8 mm (0.024 to 0.071 in). Modern engines (using solid-state ignition systems and electronic fuel injection) typically use larger gaps than older engines that use breaker point distributors and carburetors.
Smaller plug gap sizes usually are more reliable at producing a spark, however the spark may be too weak to ignite the fuel-air mixture. A larger plug gap size will produce a stronger spark, however the spark might not always be produced (such as at high RPM). Gap adjustment is not recommended for iridium and platinum spark plugs, because there is a risk of damaging a metal disk welded to the electrode.
Wasted spark applications
Wasted spark systems place a greater strain upon spark plugs since they alternately fire electrons in both directions (from the ground electrode to the central electrode, not just from the central electrode to the ground electrode). As a result, vehicles with such a system should have precious metals on both electrodes, not just on the central electrode, in order to increase service replacement intervals since they wear down the metal more quickly in both directions, not just one.
Indexing of plugs
"Indexing" of plugs upon installation involves installing the spark plug so that the open area of the gap (i.e. the side not shrouded by the side electrode), faces the center of the combustion chamber. This is claimed to improve ignition by maximising the exposure of the fuel-air mixture to the spark in every cylinder.
Indexing is accomplished by either:
* Using thin washers to set the amount of thread engaged by the spark plug, thus determining the orientation of the spark plug within the cylinder head. This must be done individually for each plug, as the orientation of the gap with respect to the threads of the shell is usually random.
* Producing spark plugs with a specific orientation of the gap relative to the threads of the shell. These spark plugs and usually designated as such by a suffix to the part number of the spark plug.
Heat range
An important factor for a spark plug is the temperature that the tip is designed to withstand, called the heat range. Typical heat ranges for passenger car engines are usually between 500 and 850 °C (932 and 1,562 °F). A hotter spark plug has more insulation between itself and the cylinder head, causing less heat to be dissipated from the spark plug and therefore the spark plug remaining hotter. Temperatures higher than 450 °C (842 °F) are needed to prevent carbon build-up on the spark plug, while temperatures over 800 °C (1,470 °F) can cause overheating of the plug.
Switching to a higher heat range is sometimes used to compensate for fuel delivery or oil consumption problems, however this increases the risk of pre-ignition.
History
Belgian-French engineer Étienne Lenoir is generally credited with the invention of the spark plug in 1860, due to its use in the early Lenoir gas engine.
Several patents relating to electrical ignition systems were filed in the late 1890s, including from Serbian engineer Nikola Tesla, British engineer Frederick Richard Simms and German engineer Robert Bosch. The use of high-voltage spark plugs in commercial viable engines was only made possible after 1902 however, due to the invention of magneto-based ignition systems by Bosch engineer Gottlob Honold. Early manufacturers of spark plugs included American company Champion, British company Lodge brothers and London-based KLG (who pioneed the use of mica as an insulator).
During the 1930s, American geologist Helen Blair Bartlett developed an alumina ceramic-based insulator for the spark plug.
Polonium spark plugs were marketed by Firestone from 1940 to 1953. While the amount of radiation from the plugs was minuscule and not a threat to the consumer, the benefits of such plugs quickly diminished after approximately a month because of polonium's short half-life, and because buildup on the conductors would block the radiation that improved engine performance. The premise behind the polonium spark plug, as well as Alfred Matthew Hubbard's prototype radium plug that preceded it, was that the radiation would improve ionization of the fuel in the cylinder and thus allow the plug to fire more quickly and efficiently.
Additional Information:
Function
The spark plug plays an important role in petrol engines. It is responsible for igniting the fuel/air mixture. The quality of this ignition influences several factors which are of great importance for both driving and the environment. They include smooth running, engine performance and efficiency as well as pollutant emissions. If we consider that a spark plug must ignite between 500 and 3,500 times per minute, it becomes clear how great the contribution of modern spark plug technology is to adherence to current emissions standards and the reduction of fuel consumption.
INFLAMMATION (IGNITION)
If we consider the basic construction of the spark plug, there have been no profound changes over the past 50 years. As ever, the spark plug comprises a metal core which is housed in a ceramic insulator. This, in turn, is surrounded by a metal casing which has a thread that is screwed in to the cylinder head and normally has a hexagonal section on the top which accommodates the spark plug socket and allows spark plugs to be installed or removed with a spark plug spanner. The main purpose of the construction lies in ensuring that the electrical circuit at high voltage on the spark plug is closed with a spark, which jumps from the middle electrode to the earth electrode.
CONNECTION
The connection is designed as an SAE connection or a 4 mm thread. The ignition cable or a rod coil is plugged into the connection. In both cases a high voltage coupled here must be transported to the other end of the spark plug. The ceramic insulator has two tasks. Its primary purpose is insulation, whereby it prevents flashover of the high voltage to the vehicle mass (= minus), and conducts combustion heat to the cylinder head. The wave-shaped leakage current barriers on the outside of the insulator prevent voltage leaking to the vehicle mass. In doing so, they extend the path to be travelled and increase the electrical resistance, thereby ensuring that the energy takes the path of least resistance - the path through the middle electrode. In order to ensure the electromagnetic compatibility (EMC) and thus the fault-free operation of the on-board electronics, a glass melt is used inside the spark plug as interference suppression. The middle electrode of a standard spark plug is comprised mostly of a nickel alloy. The spark must jump from the end of this electrode over to the earth electrode. The metal housing is firmly attached to the cylinder head via a thread and thus plays an important role in heat dissipation, discharging the bulk of the heat generated during combustion via this connection. The seal ring prevents combustion gas from emerging past the spark plug even at high combustion pressures. In so doing, it prevents pressure losses. Moreover, it conducts heat to the cylinder head and evens out the different expansion properties of the cylinder head and spark plug housing. The inner seals create a gas-tight connection between the insulator and the metal housing, providing an assurance of optimum sealing. The earth electrode of a standard spark plug is made of a nickel alloy. It represents the opposite pole of the middle electrode in normal function.
TEMPERATURE AND HEAT FLOW
An up-to-date spark plug must be tailored individually to meet the requirements of different engine designs and driving conditions. Therefore, there cannot be one spark plug which will function without any difficulty in all engines. Due to the variations in temperature development in the respective combustion chambers in different engines, spark plugs with different heat ratings are needed. This heat rating is expressed using what is known as the heat rating number. These heat ratings represent an average temperature measured at electrodes and insulators, corresponding to the engine load in each case. Spark plugs require a special temperature window in order to perform at their best. The lower threshold of this window is a spark plug temperature of 450°C, known as the self-cleaning temperature. Starting from this temperature threshold, the carbon particles which have collected on the insulator tip are burned off. spark plug temperature. If the operating temperature continuously lies below this point, electrically conductive carbon particles can collect, forming deposits until the ignition voltage flows over the carbon layer to the vehicle mass instead of forming a spark. At a spark plug temperature of 850°C or higher, the insulator heats up so much that uncontrolled ignitions can occur on its surface known as glow ignitions. Such uncontrolled, abnormal combustion can lead to engine damage.
HEAT DISSIPATION
Heat development varies greatly from engine to engine. For example, turbocharged engines run significantly hotter than engines which are not charged. Therefore, there is a spark plug for each engine which can conduct a precisely defined measure of heat to the cylinder head and ensures that the optimal temperature window is maintained. The heat rating provides information about the thermal endurance of a spark plug. Every spark plug manufacturer has its own way of expressing the heat rating. Nearly 60% of the heat is dissipated via the spark plug case and thread. The seal ring conducts slightly less than 40% to the cylinder head. The small remaining percentage (making up 100%) flows out through the middle electrode. The insulator absorbs the heat in the combustion chamber and conducts it to the interior of the spark plug. Anywhere that it comes into contact with the case, heat is conducted. By increasing or decreasing the size of this contact surface area, it is possible to determine whether the spark plug is conducting more or less heat through the case. The contact surface area is larger for spark plugs with higher thermal endurance. For spark plugs with lower thermal endurance it is smaller.
Environmental protection
Today more than ever before, the protection of the environment is the focus of attention where motoring matters are concerned. Particular attention is being paid to exhaust gases. Standard spark plugs in particular are subject to normal wear. When it jumps from the earth electrode to the middle electrode of the spark plug, every spark removes microscopically tiny particles from the electrodes. As a consequence, the distance between the electrodes gets bigger over many thousands of kilometres travelled and the risk of misfiring increases. Every time a spark plug misfires, valuable petrol is injected but not combusted. As a result, there is a significant increase in environmental pollution due to the increased in consumption per kilometre alone. Furthermore, the unburned fuel in the catalytic converter can ignite explosively, causing damage that will prevent the catalytic converter from rendering hazardous substances like carbon monoxide, nitrogen oxide and hydrocarbons harmless and requiring it to be replaced.
Depreciation
A vehicle is a highly complex technical commodity whose function can only be sustained if all components are in perfect harmony. Regular service is necessary if this state of harmony also is to be maintained for the engine, which is one of the most highly challenged parts of a vehicle. This includes using high-quality spark plugs whose technical properties help the drive to function without problems and thus provide an assurance of long service life.
Safety
Spark plugs that are in perfect working order are essential if a vehicle is to operate safely. Spark plugs should therefore be replaced no later than at the end of the replacement interval prescribed by the motor manufacturer. Important: Spark plugs require a precisely measured torque for installation. This necessitates the use of a special tool known as a torque wrench. If the spark plug is not tight enough, pressure will escape from the piston and the spark plug may overheat. There is also a risk that the ceramic spark plug insulator will fracture. This can damage the piston and thus result in engine damage. Conversely, if the torque is set too high, the spark plug might tear off, possibly leading to the cylinder head having to be replaced. Even if this does not happen, a spark plug that has been screwed too tight can overheat during operation, resulting in damage to the engine.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2065 2024-02-20 00:09:05
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,725
Re: Miscellany
2067) Acid
Gist
An acid is any hydrogen-containing substance that is capable of donating a proton (hydrogen ion) to another substance. A base is a molecule or ion able to accept a hydrogen ion from an acid. Acidic substances are usually identified by their sour taste.
Summary
Acid is any substance that in water solution tastes sour, changes the colour of certain indicators (e.g., reddens blue litmus paper), reacts with some metals (e.g., iron) to liberate hydrogen, reacts with bases to form salts, and promotes certain chemical reactions (acid catalysis). Examples of acids include the inorganic substances known as the mineral acids—sulfuric, nitric, hydrochloric, and phosphoric acids—and the organic compounds belonging to the carboxylic acid, sulfonic acid, and phenol groups. Such substances contain one or more hydrogen atoms that, in solution, are released as positively charged hydrogen ions.
Broader definitions of an acid, to include substances that exhibit typical acidic behaviour as pure compounds or when dissolved in solvents other than water, are given by the Brønsted–Lowry theory and the Lewis theory. Examples of nonaqueous acids are sulfur trioxide, aluminum chloride, and boron trifluoride.
Details
An acid is a molecule or ion capable of either donating a proton (i.e. hydrogen ion, H+), known as a Brønsted–Lowry acid, or forming a covalent bond with an electron pair, known as a Lewis acid.
The first category of acids are the proton donors, or Brønsted–Lowry acids. In the special case of aqueous solutions, proton donors form the hydronium ion H3O+ and are known as Arrhenius acids. Brønsted and Lowry generalized the Arrhenius theory to include non-aqueous solvents. A Brønsted or Arrhenius acid usually contains a hydrogen atom bonded to a chemical structure that is still energetically favorable after loss of H+.
Aqueous Arrhenius acids have characteristic properties that provide a practical description of an acid. Acids form aqueous solutions with a sour taste, can turn blue litmus red, and react with bases and certain metals (like calcium) to form salts. The word acid is derived from the Latin acidus, meaning 'sour'. An aqueous solution of an acid has a pH less than 7 and is colloquially also referred to as "acid" (as in "dissolved in acid"), while the strict definition refers only to the solute. A lower pH means a higher acidity, and thus a higher concentration of positive hydrogen ions in the solution. Chemicals or substances having the property of an acid are said to be acidic.
Common aqueous acids include hydrochloric acid (a solution of hydrogen chloride that is found in gastric acid in the stomach and activates digestive enzymes), acetic acid (vinegar is a dilute aqueous solution of this liquid), sulfuric acid (used in car batteries), and citric acid (found in citrus fruits). As these examples show, acids (in the colloquial sense) can be solutions or pure substances, and can be derived from acids (in the strict sense) that are solids, liquids, or gases. Strong acids and some concentrated weak acids are corrosive, but there are exceptions such as carboranes and boric acid.
The second category of acids are Lewis acids, which form a covalent bond with an electron pair. An example is boron trifluoride (BF3), whose boron atom has a vacant orbital that can form a covalent bond by sharing a lone pair of electrons on an atom in a base, for example the nitrogen atom in ammonia (NH3). Lewis considered this as a generalization of the Brønsted definition, so that an acid is a chemical species that accepts electron pairs either directly or by releasing protons (H+) into the solution, which then accept electron pairs. Hydrogen chloride, acetic acid, and most other Brønsted–Lowry acids cannot form a covalent bond with an electron pair, however, and are therefore not Lewis acids. Conversely, many Lewis acids are not Arrhenius or Brønsted–Lowry acids. In modern terminology, an acid is implicitly a Brønsted acid and not a Lewis acid, since chemists almost always refer to a Lewis acid explicitly as a Lewis acid.
Definitions and concepts
Modern definitions are concerned with the fundamental chemical reactions common to all acids.
Most acids encountered in everyday life are aqueous solutions, or can be dissolved in water, so the Arrhenius and Brønsted–Lowry definitions are the most relevant.
The Brønsted–Lowry definition is the most widely used definition; unless otherwise specified, acid–base reactions are assumed to involve the transfer of a proton (H+) from an acid to a base.
Hydronium ions are acids according to all three definitions. Although alcohols and amines can be Brønsted–Lowry acids, they can also function as Lewis bases due to the lone pairs of electrons on their oxygen and nitrogen atoms.
Brønsted–Lowry acids
While the Arrhenius concept is useful for describing many reactions, it is also quite limited in its scope. In 1923, chemists Johannes Nicolaus Brønsted and Thomas Martin Lowry independently recognized that acid–base reactions involve the transfer of a proton. A Brønsted–Lowry acid (or simply Brønsted acid) is a species that donates a proton to a Brønsted–Lowry base. Brønsted–Lowry acid–base theory has several advantages over Arrhenius theory.
Lewis acids
A third, only marginally related concept was proposed in 1923 by Gilbert N. Lewis, which includes reactions with acid–base characteristics that do not involve a proton transfer. A Lewis acid is a species that accepts a pair of electrons from another species; in other words, it is an electron pair acceptor. Brønsted acid–base reactions are proton transfer reactions while Lewis acid–base reactions are electron pair transfers. Many Lewis acids are not Brønsted–Lowry acids.
Applications of acids:
In industry
Acids are fundamental reagents in treating almost all processes in modern industry. Sulfuric acid, a diprotic acid, is the most widely used acid in industry, and is also the most-produced industrial chemical in the world. It is mainly used in producing fertilizer, detergent, batteries and dyes, as well as used in processing many products such like removing impurities. According to the statistics data in 2011, the annual production of sulfuric acid was around 200 million tonnes in the world. For example, phosphate minerals react with sulfuric acid to produce phosphoric acid for the production of phosphate fertilizers, and zinc is produced by dissolving zinc oxide into sulfuric acid, purifying the solution and electrowinning.
In the chemical industry, acids react in neutralization reactions to produce salts. For example, nitric acid reacts with ammonia to produce ammonium nitrate, a fertilizer. Additionally, carboxylic acids can be esterified with alcohols, to produce esters.
Acids are often used to remove rust and other corrosion from metals in a process known as pickling. They may be used as an electrolyte in a wet cell battery, such as sulfuric acid in a car battery.
In food
Carbonated water (H2CO3 aqueous solution) is commonly added to soft drinks to make them effervesce.
Tartaric acid is an important component of some commonly used foods like unripened mangoes and tamarind. Natural fruits and vegetables also contain acids. Citric acid is present in oranges, lemon and other citrus fruits. Oxalic acid is present in tomatoes, spinach, and especially in carambola and rhubarb; rhubarb leaves and unripe carambolas are toxic because of high concentrations of oxalic acid. Ascorbic acid (Vitamin C) is an essential vitamin for the human body and is present in such foods as amla (Indian gooseberry), lemon, citrus fruits, and guava.
Many acids can be found in various kinds of food as additives, as they alter their taste and serve as preservatives.
Phosphoric acid, for example, is a component of cola drinks. Acetic acid is used in day-to-day life as vinegar. Citric acid is used as a preservative in sauces and pickles.
Carbonic acid is one of the most common acid additives that are widely added in soft drinks. During the manufacturing process, CO2 is usually pressurized to dissolve in these drinks to generate carbonic acid. Carbonic acid is very unstable and tends to decompose into water and CO2 at room temperature and pressure. Therefore, when bottles or cans of these kinds of soft drinks are opened, the soft drinks fizz and effervesce as CO2 bubbles come out.
Certain acids are used as drugs. Acetylsalicylic acid (Aspirin) is used as a pain killer and for bringing down fevers.
In human bodies
Acids play important roles in the human body. The hydrochloric acid present in the stomach aids digestion by breaking down large and complex food molecules. Amino acids are required for synthesis of proteins required for growth and repair of body tissues. Fatty acids are also required for growth and repair of body tissues. Nucleic acids are important for the manufacturing of DNA and RNA and transmitting of traits to offspring through genes. Carbonic acid is important for maintenance of pH equilibrium in the body.
Human bodies contain a variety of organic and inorganic compounds, among those dicarboxylic acids play an essential role in many biological behaviors. Many of those acids are amino acids, which mainly serve as materials for the synthesis of proteins. Other weak acids serve as buffers with their conjugate bases to keep the body's pH from undergoing large scale changes that would be harmful to cells. The rest of the dicarboxylic acids also participate in the synthesis of various biologically important compounds in human bodies.
Acid catalysis
Acids are used as catalysts in industrial and organic chemistry; for example, sulfuric acid is used in very large quantities in the alkylation process to produce gasoline. Some acids, such as sulfuric, phosphoric, and hydrochloric acids, also effect dehydration and condensation reactions. In biochemistry, many enzymes employ acid catalysis.
Biological occurrence
Many biologically important molecules are acids. Nucleic acids, which contain acidic phosphate groups, include DNA and RNA. Nucleic acids contain the genetic code that determines many of an organism's characteristics, and is passed from parents to offspring. DNA contains the chemical blueprint for the synthesis of proteins, which are made up of amino acid subunits. Cell membranes contain fatty acid esters such as phospholipids.
An α-amino acid has a central carbon (the α or alpha carbon) that is covalently bonded to a carboxyl group (thus they are carboxylic acids), an amino group, a hydrogen atom and a variable group. The variable group, also called the R group or side chain, determines the identity and many of the properties of a specific amino acid. In glycine, the simplest amino acid, the R group is a hydrogen atom, but in all other amino acids it is contains one or more carbon atoms bonded to hydrogens, and may contain other elements such as sulfur, oxygen or nitrogen. With the exception of glycine, naturally occurring amino acids are chiral and almost invariably occur in the L-configuration. Peptidoglycan, found in some bacterial cell walls contains some D-amino acids. At physiological pH, typically around 7, free amino acids exist in a charged form, where the acidic carboxyl group (-COOH) loses a proton (-COO−) and the basic amine group (-NH2) gains a proton (-NH+ 3). The entire molecule has a net neutral charge and is a zwitterion, with the exception of amino acids with basic or acidic side chains. Aspartic acid, for example, possesses one protonated amine and two deprotonated carboxyl groups, for a net charge of −1 at physiological pH.
Fatty acids and fatty acid derivatives are another group of carboxylic acids that play a significant role in biology. These contain long hydrocarbon chains and a carboxylic acid group on one end. The cell membrane of nearly all organisms is primarily made up of a phospholipid bilayer, a micelle of hydrophobic fatty acid esters with polar, hydrophilic phosphate "head" groups. Membranes contain additional components, some of which can participate in acid–base reactions.
In humans and many other animals, hydrochloric acid is a part of the gastric acid secreted within the stomach to help hydrolyze proteins and polysaccharides, as well as converting the inactive pro-enzyme, pepsinogen into the enzyme, pepsin. Some organisms produce acids for defense; for example, ants produce formic acid.
Acid–base equilibrium plays a critical role in regulating mammalian breathing. Oxygen gas (O2) drives cellular respiration, the process by which animals release the chemical potential energy stored in food, producing carbon dioxide (CO2) as a byproduct. Oxygen and carbon dioxide are exchanged in the lungs, and the body responds to changing energy demands by adjusting the rate of ventilation. For example, during periods of exertion the body rapidly breaks down stored carbohydrates and fat, releasing CO2 into the blood stream. In aqueous solutions such as blood CO2 exists in equilibrium with carbonic acid and bicarbonate ion.
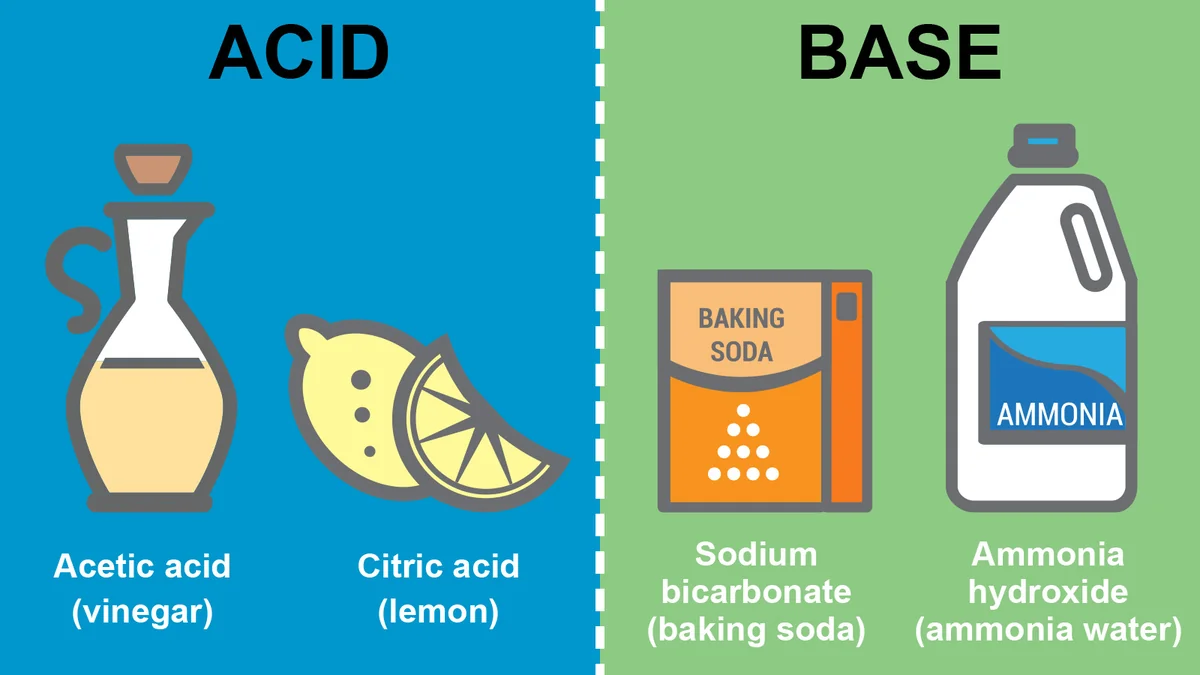
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2066 2024-02-21 00:02:19
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,725
Re: Miscellany
2068) Structural Engineering
Gist
Structural engineering is a subfield of civil engineering focused on the strength, stability, and durability of buildings, bridges, airplanes, and other structures.
Summary
Structural engineering — a specialty within the field of civil engineering — focuses on the framework of structures, and on designing those structures to withstand the stresses and pressures of their environment and remain safe, stable and secure throughout their use. In other words, structural engineers make sure that buildings don't fall down and bridges don't collapse.
Structural engineering is among the oldest types of engineering, dating back to the first instance of tree branches being lashed together with vines to make a shelter. Throughout recorded history, people have been designing and building increasingly larger and more sophisticated structures, from primitive huts to the International Space Station.
The names of the earliest practitioners of structural engineering are lost to antiquity. We will never know who designed the Hanging Gardens of Babylon, the Parthenon or the aqueducts of the Roman Empire. Some of the latter-day practitioners in this field are known, although often not as well as the structures they designed. Prominent structural engineers include Gustave Eiffel (Eiffel Tower, Statue of Liberty) and Eero Saarinen (Gateway Arch). However, most designs for famous modern structures such as the Large Hadron Collider and the James Webb Space Telescope are attributed to companies and government organizations.
What does a structural engineer do?
Structural engineers often work alongside civil engineers and architects as part of a construction team. "In a nutshell," according to the Institution of Structural Engineers, "if a structure was a human body, then the architect would be concerned with the body shape and appearance, and the structural engineer would be concerned with the skeleton and sinews."
Structures must be able to deal with the conditions in which they are built. A house in Canada must have a roof that can bear the weight of heavy snow and a stadium in California must be able to withstand earthquakes, for example. When building bridges, designers must take into account the conditions of terrain, wind, water and traffic volume. Structural engineers consider all of these factors and provide technical advice about the project.
"Structural engineers battle gravity, wind, snow and rain every day to provide the world with outstanding structures," Kate Leighton, a structural engineer, said in "Careers in Structural Engineering, a publication of the Institution of Structural Engineers. "They are experts at solving problems, meeting challenges and providing creative solutions."
Structural engineers "design roof framing (beams, rafters, joists, trusses), floor framing (floor decks, joists, beams, trusses, girders), arches, columns, braces, frames, foundations and walls," according to the National Council of Structural Engineers Association. "In bridges, they design the deck — or riding surface, girders or stringers, and piers. The materials they use include steel, concrete, wood, masonry, and aluminum. Engineers design the structure to resist forces from gravity, earthquakes, high winds, water, soil, collisions and blast explosions."
Details
Structural engineering is a sub-discipline of civil engineering in which structural engineers are trained to design the 'bones and joints' that create the form and shape of human-made structures. Structural engineers also must understand and calculate the stability, strength, rigidity and earthquake-susceptibility of built structures for buildings and nonbuilding structures. The structural designs are integrated with those of other designers such as architects and building services engineer and often supervise the construction of projects by contractors on site. They can also be involved in the design of machinery, medical equipment, and vehicles where structural integrity affects functioning and safety. See glossary of structural engineering.
Structural engineering theory is based upon applied physical laws and empirical knowledge of the structural performance of different materials and geometries. Structural engineering design uses a number of relatively simple structural concepts to build complex structural systems. Structural engineers are responsible for making creative and efficient use of funds, structural elements and materials to achieve these goals.
History
Structural engineering dates back to 2700 B.C. when the step pyramid for Pharaoh Djoser was built by Imhotep, the first engineer in history known by name. Pyramids were the most common major structures built by ancient civilizations because the structural form of a pyramid is inherently stable and can be almost infinitely scaled (as opposed to most other structural forms, which cannot be linearly increased in size in proportion to increased loads).
The structural stability of the pyramid, whilst primarily gained from its shape, relies also on the strength of the stone from which it is constructed, and its ability to support the weight of the stone above it. The limestone blocks were often taken from a quarry near the building site and have a compressive strength from 30 to 250 MPa (MPa = Pa × {10}^{6}). Therefore, the structural strength of the pyramid stems from the material properties of the stones from which it was built rather than the pyramid's geometry.
Throughout ancient and medieval history most architectural design and construction were carried out by artisans, such as stonemasons and carpenters, rising to the role of master builder. No theory of structures existed, and understanding of how structures stood up was extremely limited, and based almost entirely on empirical evidence of 'what had worked before' and intuition. Knowledge was retained by guilds and seldom supplanted by advances. Structures were repetitive, and increases in scale were incremental.
No record exists of the first calculations of the strength of structural members or the behavior of structural material, but the profession of a structural engineer only really took shape with the Industrial Revolution and the re-invention of concrete. The physical sciences underlying structural engineering began to be understood in the Renaissance and have since developed into computer-based applications pioneered in the 1970s.
Structural failure
The history of structural engineering contains many collapses and failures. Sometimes this is due to obvious negligence, as in the case of the Pétion-Ville school collapse, in which Rev. Fortin Augustin " constructed the building all by himself, saying he didn't need an engineer as he had good knowledge of construction" following a partial collapse of the three-story schoolhouse that sent neighbors fleeing. The final collapse killed 94 people, mostly children.
In other cases structural failures require careful study, and the results of these inquiries have resulted in improved practices and a greater understanding of the science of structural engineering. Some such studies are the result of forensic engineering investigations where the original engineer seems to have done everything in accordance with the state of the profession and acceptable practice yet a failure still eventuated. A famous case of structural knowledge and practice being advanced in this manner can be found in a series of failures involving box girders which collapsed in Australia during the 1970s.
Theory
Structural engineering depends upon a detailed knowledge of applied mechanics, materials science, and applied mathematics to understand and predict how structures support and resist self-weight and imposed loads. To apply the knowledge successfully a structural engineer generally requires detailed knowledge of relevant empirical and theoretical design codes, the techniques of structural analysis, as well as some knowledge of the corrosion resistance of the materials and structures, especially when those structures are exposed to the external environment. Since the 1990s, specialist software has become available to aid in the design of structures, with the functionality to assist in the drawing, analyzing and designing of structures with maximum precision; examples include AutoCAD, StaadPro, ETABS, Prokon, Revit Structure, Inducta RCB, etc. Such software may also take into consideration environmental loads, such as earthquakes and winds.
Profession
Structural engineers are responsible for engineering design and structural analysis. Entry-level structural engineers may design the individual structural elements of a structure, such as the beams and columns of a building. More experienced engineers may be responsible for the structural design and integrity of an entire system, such as a building.
Structural engineers often specialize in particular types of structures, such as buildings, bridges, pipelines, industrial, tunnels, vehicles, ships, aircraft, and spacecraft. Structural engineers who specialize in buildings often specialize in particular construction materials such as concrete, steel, wood, masonry, alloys, and composites, and may focus on particular types of buildings such as offices, schools, hospitals, residential, and so forth.
Structural engineering has existed since humans first started to construct their structures. It became a more defined and formalized profession with the emergence of architecture as a distinct profession from engineering during the industrial revolution in the late 19th century. Until then, the architect and the structural engineer were usually one and the same thing – the master builder. Only with the development of specialized knowledge of structural theories that emerged during the 19th and early 20th centuries, did the professional structural engineers come into existence.
The role of a structural engineer today involves a significant understanding of both static and dynamic loading and the structures that are available to resist them. The complexity of modern structures often requires a great deal of creativity from the engineer in order to ensure the structures support and resist the loads they are subjected to. A structural engineer will typically have a four or five-year undergraduate degree, followed by a minimum of three years of professional practice before being considered fully qualified. Structural engineers are licensed or accredited by different learned societies and regulatory bodies around the world (for example, the Institution of Structural Engineers in the UK). Depending on the degree course they have studied and/or the jurisdiction they are seeking licensure in, they may be accredited (or licensed) as just structural engineers, or as civil engineers, or as both civil and structural engineers. Another international organisation is IABSE(International Association for Bridge and Structural Engineering). The aim of that association is to exchange knowledge and to advance the practice of structural engineering worldwide in the service of the profession and society.
Additional Information
Structural engineering is a branch of civil engineering that involves the application of the laws of physics, mathematics and empirical knowledge to safely design the ‘bones’ and load bearing elements of man made structures. Modern day structural engineering provides a large and detailed body of knowledge that can accurately predict the performance of different shapes and materials used in structures to resist loads and stresses on structures. The principles of structural engineering were used thousands of years ago when building structures like the pyramids in Egypt or the Acropolis in Greece.
Structural engineers are trained professionals who are responsible for making sure that the structures we use in our daily lives, like bridges and tall buildings, are safe, stable and don’t collapse under applied loads. They do this by applying their technical knowledge to specify different types of construction materials in various shapes and geometries and design structures that can withstand the pressures and stresses of their environment such as gravity loads, storms and earthquakes.
Structural engineers are brought on to a project if an owner is planning on changing the use of a building, introducing more floors to a building, or adding a significant expansion to a building. It’s very important to understand that introducing alterations to any structural element without consulting a professional engineer may result in serious damage to the structure and in some cases partial or extensive collapse of the building.
Structural engineers are also brought on board if there is damage to a structure due to fire, corrosion, environmental deterioration, impact or wear and tear that could result in a loss of capacity and impose a threat to the public’s safety. When a structural engineer is contacted for an assessment of an existing building, they would visually inspect the structure and determine the structural integrity of the load bearing elements, potential concerns regarding the occupants safety, suggest repair techniques and recommend structural details to restore the structure to its original conditions in order to resist the applied loads.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2067 2024-02-22 00:12:39
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,725
Re: Miscellany
2069) Firefighting
Gist
Firefighting is the activity of stopping fires burning.
Summary
Firefighting is the activity directed at limiting the spread of fire and extinguishing it, particularly as performed by members of organizations (fire services or fire departments) trained for the purpose. When it is possible, firefighters rescue persons endangered by the fire, if necessary, before turning their full attention to putting it out.
Firefighters, skilled in the use of specific equipment, proceed as rapidly as possible to the site of the fire; in most urban areas, fire stations housing a company of firefighters and their equipment occur frequently enough that an alarm receives a response within two or three minutes. Most fire services in towns inhabited by 5,000 persons or more will dispatch an engine company (pumper), a truck company (ladder truck), and a rescue vehicle to the scene. If the fire involves a structure occupied by many persons, two or more companies may respond to the first alarm. The first firefighters arriving will assess the fire to determine the techniques to be used in putting it out, taking into account the construction of the burning building and any fire protection systems within it.
Systematic firefighting involves four steps: protection of currently uninvolved buildings and areas; confinement of the fire; ventilation of the building; and extinguishment of the fire. Pathways by which the fire could spread are closed off, and the leading edge of the flame is controlled by the application of water or other cooling agents. Openings are made to permit the escape of toxic combustion products and hot air; this step (ventilation) must be conducted with keen judgment so as to permit the firefighters access to the fire without causing its intensification or risking a smoke explosion (the result of admitting fresh air to a space in which a high concentration of unburned fuel particles is present in a hot, oxygen-depleted atmosphere).
The final stage of fighting a fire is extinguishment. The firefighting force uses water streams mixed with appropriate extinguishing agents to quench the remaining flames. When this is accomplished, the firefighters initiate salvage of the structure by removing smoke and water from the interior and protecting undamaged materials.
Details
Firefighting is a profession aimed at controlling and extinguishing fire. A person who engages in firefighting is known as a firefighter or fireman. Firefighters typically undergo a high degree of technical training. This involves structural firefighting and wildland firefighting. Specialized training includes aircraft firefighting, shipboard firefighting, aerial firefighting, maritime firefighting, and proximity firefighting.
Firefighting is a dangerous profession due to the toxic environment created by combustible materials, with major risks being smoke, oxygen deficiency, elevated temperatures, poisonous atmospheres, and violent air flows. To combat some of these risks, firefighters carry self-contained breathing apparatus. Additional hazards include falls — a constant peril while navigating unfamiliar layouts or confined spaces amid shifting debris under limited visibility – and structural collapse that can exacerbate the problems encountered in a toxic environment.
The first step in a firefighting operation is reconnaissance to search for the origin of the fire and to identify the specific risks. Fires can be extinguished by water, fuel or oxidant removal, or chemical flame inhibition; though, because fires are classified depending on the elements involved, such as grease, paper, electrical, etcetera, a specific type of fire extinguisher may be required. The classification is based on the type of fires that the extinguisher is more suitable for. In the United States, the types of fire are described by the National Fire Protection Association.
History
The earliest known firefighters were in the city of Rome. In 60 A.D., emperor Nero established a Corps of Vigils (Vigiles) to protect Rome after a disastrous fire. It consisted of 7,000 people equipped with buckets and axes who fought fires and served as police.
Historic tactics and tools
In the 3rd century B.C., an Alexandrian Greek named Ctesibius made a double force pump called a siphona. As water rose in the chamber, it compressed the air inside, which forced the water to eject in a steady stream through a pipe and nozzle.
In the 16th century, syringes were also used as firefighting tools, the larger ones being mounted on wheels. Another traditional firefighting method that survived was the bucket brigade, involving two lines of people formed between the water source and the fire. Typically, men in one of the lines would pass along the full buckets of water toward the fire while in the other line women and children would pass back the empty buckets to be refilled.
In the 17th century the first "fire engines" were made, notably in Amsterdam.In 1721, the English inventor Richard Newsham made a popular fire engine that was essentially a rectangular box on wheels filled using a bucket brigade to provide a reservoir while hand-powered pumps supplied sufficient water pressure to douse fires at a distance.
Ancient Rome
Ancient Rome did not have municipal firefighters. Instead, private individuals relied on their slaves or supporters to take action. They would not only form bucket brigades or attempt to smother smaller fires, but would also demolish or raze nearby buildings to slow the spread of the fire. However, there is no mention of fires being extinguished, rather they were contained and burned themselves out. Ancient Rome did not have an organized firefighting force until the Vigiles were formed during the reign of Augustus.
The first ever Roman fire brigade was created by Marcus Licinius Crassus. Fires were almost a daily occurrence in Rome, and Crassus took advantage of the fact that Rome had no fire department, by creating his own brigade—500 men strong—which rushed to burning buildings at the first cry of alarm. Upon arriving at the scene, however, the firefighters did nothing while Crassus offered to buy the burning building from the distressed property owner, at a miserable price. If the owner agreed to sell the property, his men would put out the fire; if the owner refused, then they would simply let the structure burn to the ground. After buying many properties this way, he rebuilt them, and often leased the properties to their original owners or new tenants.
United Kingdom
Prior to the Great Fire of London in 1666, some parishes in the UK had begun to organize rudimentary firefighting crews. After the Great Fire, Nicholas Barbon introduced the first fire insurance. In order to reduce insurance costs, Barbon also formed his own fire brigade, and other companies followed suit.
By the start of the 1800s, insured buildings were identified with a badge or mark indicating that they were eligible for a company's firefighting services. It is a common belief that buildings not insured with a particular company were left by its firefighters to burn, unless they happened to be adjacent to an insured building, in which case it was often in the company's interest to prevent the fire from spreading. This is a common misconception. In 1833 fire insurance companies in London merged to form The London Fire Company Establishment.
Steam-powered apparatuses were first introduced in the 1850s, allowing a greater quantity of water to be directed onto a fire; in the early 1930s they were superseded by versions powered by an internal combustion engine.
In World War II the Auxiliary Fire Service, and later the National Fire Service, were established to supplement local fire services. Before 1938, there was no countrywide standard for firefighting terms, procedures, ranks, or equipment (such as hose couplings). In the month of August in 1939 with war looking very possible the Fire Service's act of 1938 came into effect. This unified Great Britain's fire service and prepared them for the German war machine. During the London Blitz, 700 fire men and 20 fire women, as known during the time period died as a result of heavy bombing, 91 of these perished at the same time defending London. By the end of the London Blitz, 327 firefighters had lost their lives.
United States
In January 1608, a fire destroyed many colonists' provisions and lodgings in Jamestown, Virginia. By the mid-1600s, Boston, New Amsterdam (later New York City), and Philadelphia were all plagued by fires, and volunteer fire brigades began to form.
In 1736, Benjamin Franklin founded the Union Fire Company in Philadelphia, which became the standard for volunteer fire organizations. These firefighters had two critical tools: salvage bags and so-called bed keys. Salvage bags were used to quickly collect and save valuables, and bed keys were used to separate the wooden frame of a bed (often the most valuable item in a home at the time) into pieces for safe and rapid removal from the fire.
The first American attempt at fire insurance failed after a large fire in Charlestown, Massachusetts in 1736. Later in 1740, Benjamin Franklin organized the Philadelphia Contributionship to provide fire insurance, which was more successful. The Contributionship adopted "fire marks" to easily identify insured buildings. Firefighting started to become formalized with rules for providing buckets, ladders, and hooks, and with the formation of volunteer companies. A chain of command was also established.
Firefighter duties
A firefighter's goals are to save lives, protect property, and protect the environment. A fire can rapidly spread and endanger many lives, but with modern firefighting techniques, catastrophe can often be avoided. To prevent fires from starting, a firefighter's duties may include public education about fire safety and conducting fire inspections of locations to verify their adherence to local fire codes.
Firefighter skills
Firefighting requires technical proficiency of operational tactics, equipment, and scene awareness. Firefighters must also have, or be able to acquire, knowledge of department organizations, operations, and procedures, and the district or city street system they will have to negotiate in order to perform their duties.
They must meet minimum physical fitness standards and learn various firefighting duties within a reasonable period.
Examples are:
* Building construction
* Fire behavior
* Firefighting PPE
* Fire extinguishers
* Ropes and knots
* Ground ladders
* Forcible entry
* Search and rescue
* Ventilation
* Fire hose and streams
* Fire suppression
* Salvage and overhaul
* Vehicle extrication and technical rescue
* Hazardous materials response
Specialized skills
Specialized areas of operations may require subject-specific training.
Examples are:
* Fire apparatus driver/operator - trained to drive fire apparatus to and from fires and other emergencies, operate fire-apparatus pumps and aerial devices, and maintain apparatus.
* Hazardous materials technician - certified to mitigate hazardous materials emergencies.
* Rescue technician - certified to perform complex technical rescues.
* Airport firefighter - trained in ARFF.
* Wildland firefighter - trained to extinguish fires in outdoor vegetation, including the wildland/urban interface.
Shift hours
Full-time career firefighters typically follow a 24-hour shift schedule, although some fire departments work 8- or 12-hour shifts. Australian firefighters work a 10/14 shift, in which the day shift works ten hours and the night shift works 14 hours. Firefighting personnel are split up into alternating shifts. Usually, the 24-hour shifts are followed by two days off. The shift personnel arrive for roll call at a specified time, ready to complete a regular tour of duty. While on shift, the firefighter remains at the fire station unless relieved or assigned other duties.
Fire wardens
In fire fighting, there are also people designated as fire wardens, also known as chief officers. Their duties vary, some may ensure evacuation of that part of the building for which they are responsible; others may be responsible for fire control in a particular area, direct a crew in the suppression of forest fires, or function as fire patrolmen in a logging area.
The chief officer is in charge of their firefighters during fires or emergencies, and is expected to command and control the overall situation while effectively combating a fire or other emergency. Chief officers must be able to evaluate their firefighters, use sound judgement when deciding when it is time to withdraw firefighters from a fire, and react calmly in emergency situations. The chief officer must direct the activities of a fire department and supervise all firefighting activities, requiring extensive knowledge of city layouts, the location of streets, fire hydrants and fire alarm boxes, and the principal buildings. A chief officer must be familiar with sources of fires, including explosives, hazardous chemicals, and the combustion qualities of materials in buildings, homes, and industrial plants.
In certain jurisdictions, civilians can get certified to be a Fire Warden, and some cities require certain types of buildings, such as high rises, to have a certain number of Fire Wardens. For example, the city of Houston in the United States requires every tenant in a high-rise to have at least one Fire Warden for every 7500 sq. ft. occupied, and a minimum of two Fire Wardens per floor. In this example, their duties include investigating any fire alarms (see if there really is a fire and if so, its nature), ensuring the fire department is contacted, directing the evacuation of the facility, activating or delaying activation of fire suppression equipment such as halon and sprinklers (delayed in case of a false alarm), meeting the fire department and taking them to the location of the alarm or to the fire past any security or locked doors, and, if necessary, fighting the fire until the fire department arrives.
Firefighter safety zone guidelines
The U.S. Forest Service publishes guidelines for the minimum distance a firefighter should be from a flame. As stated in the National Wildfire Coordinating Group's Incident Response Pocket Guide: "A safety zone is an area where a firefighter can survive without a fire shelter" and should be "...at least four times the maximum continuous flame height." However this figure only takes into account the effects of radiant heat and does not consider topography nor wind.
Safety Zones can be natural features such as rock screes, meadows, and river bars; or human-made features such a parking lots or areas that have been cleared of vegetation through mechanical means.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2068 2024-02-22 22:50:57
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,725
Re: Miscellany
2070) Dentist
Gist
Dentistry is the profession concerned with the prevention and treatment of oral disease, including diseases of the teeth and supporting structures and diseases of the soft tissues of the mouth. Dentistry also encompasses the treatment and correction of malformation of the jaws, misalignment of the teeth, and birth anomalies of the oral cavity such as cleft palate. In addition to general practice, dentistry includes many specialties and subspecialties, including orthodontics and dental orthopedics, pediatric dentistry, periodontics, prosthodontics, oral and maxillofacial surgery, oral and maxillofacial pathology, endodontics, public health dentistry, and oral and maxillofacial radiology.
Summary
A dentist is a healthcare provider who diagnoses and treats oral health conditions. Taking good care of your teeth and gums can help you reduce your risk for other serious health conditions, like heart disease and stroke. You should visit a dentist regularly for routine exams and cleanings.
What is a dentist?
A dentist — sometimes called a general dentist or family dentist — is a healthcare provider who diagnoses and treats oral health conditions. Dentists help keep your teeth and gums healthy with regular dental check-ups and cleanings. They can also perform a variety of oral health treatments, including dental fillings, crowns and bridges.
Are dentists doctors?
Yes. Dentists are doctors because they undergo extensive medical training. In the United States, a person who wants to become a dentist must receive an undergraduate degree and complete four years of focused training in an accredited dental school.
The extent of training is similar in other countries, as well — even though titles may differ. For example, in the United Kingdom, people refer to dentists as dental surgeons and traditionally use the title Mr., Miss or Mrs., though some may use Dr.
What’s the difference between a DDS and a DMD?
If you live in the U.S., you may see two different titles following a dentist’s name:
DDS: Doctor of Dental Surgery.
DMD: Doctor of Dental Medicine (Doctor of Medicine in Dentistry)
If you see either of these titles, it means that your dentist graduated from an accredited dental school. A DDS and DMD receive the same amount of training and can perform the same dental procedures.
What does a dentist do?
Dentists can treat a wide range of conditions affecting your teeth, gums, jaws and other areas of your mouth. They offer treatments in:
* Preventive dentistry.
* Restorative dentistry.
* Emergency dental care.
Preventive dentistry
Dentists offer preventive dentistry to protect your teeth and gums from disease-causing bacteria, stopping issues before they start. Preventive treatments include:
* Dental exams.
* Dental X-rays.
* Cleanings.
* Sealants.
* Fluoride treatments.
Restorative dentistry
Dentists also perform restorative procedures to repair or replace damaged or missing teeth. Restorative dentistry treatments include:
* Fillings.
* Crowns.
* Bridges.
* Dental implants.
Details
Dentists are trained professionals who help care for the teeth and mouth. Regularly seeing a dentist can help you to maintain a good level of dental health, which may have a direct impact on your overall well-being.
What Does a Dentist Do?
A dentist has many responsibilities, and one of the most important is promoting good dental hygiene. This helps to prevent complications in your mouth or other parts of the body.
A dentist also diagnoses and treats problems of the gums, teeth, and mouth. Dentists use modern technology and equipment like X-ray machines, lasers, drills, brushes, scalpels, and other medical tools when performing dental procedures. They also wear protective equipment like gloves, masks, and safety glasses to prevent the spread of germs or bacteria.
Some common dentistry tasks include:
* Teaching people about dental hygiene
* Filling cavities
* Removing buildup or decay from teeth
* Repairing or removing damaged teeth
* Reviewing X-rays and diagnostics
* Giving anesthesia
* Putting in fillings or sealants
* Checking the growth of teeth and jawbones.
Dentistry requires a team approach, and the dentist is the leader. Working with the dentist are dental assistants, hygienists, and lab technicians. Together, the team ensures that people get quality dental care.
Education and Training
A dentist is a doctor, so they complete a path of study that’s similar to that of a medical doctor. The first step is to complete an undergraduate program in a related field like biology, chemistry, health, or math, and earn a bachelor of science degree. Next is a dental admissions test, which you need to take to apply for dental schools.
The training process includes:
* Completing two years of biomedical science studies, followed by two years of clinical practice
* Earning a doctor of dental surgery (DDS) or doctor of dental medicine (DDM) degree
* Getting a dental license by passing written and practical exams
Dentists may then choose to get certified by taking the National Board Dental Examination. Depending on the area of specialty, dentists may have to complete a postgraduate residency of one to three years.
Dentists can choose to specialize in one of the following areas, each of which requires a postgraduate residency:
* Dental public health
* Endodontics
* Oral and maxillofacial pathology
* Oral and maxillofacial radiology
* Oral and maxillofacial surgery
* Orthodontics and dentofacial orthopedics
* Pediatric dentistry
* Periodontics
* Prosthodontics
Reasons to See a Dentist
There are several reasons to see a dentist, and it’s important to go for a dental checkup every six months.
Preventive Care
First, your dentist will check for any signs of mouth cancer, gum problems, or dental decay. Checking on these things regularly helps to prevent more serious problems down the road.
Your dental hygienist will also clean your teeth to remove plaque and tartar buildup, which are causes of tooth decay and gum disease. Together, your dentist and hygienist can give you some tips on how to best take care of your teeth at home.
Pain or Discomfort
If you’re feeling pain or discomfort in your teeth, mouth, jaws, or gums, it’s time to see a dentist. Pain or swelling in the neck, mouth, or face can be a sign that something isn’t right. Similarly, if you notice your gums are bleeding or if you’re having trouble chewing or swallowing, you should also schedule a dental care visit to see what the causes could be.
Maintenance and Health
If you have already had a dental procedure, it’s important to make sure that everything is still as it should be. If you’re pregnant, actively using tobacco, or dealing with ongoing medical issues, a dentist can help coordinate your health care with your medical doctor.
Additional Information
A dentist, also known as a dental surgeon, is a health care professional who specializes in dentistry, the branch of medicine focused on the teeth, gums, and mouth. The dentist's supporting team aids in providing oral health services. The dental team includes dental assistants, dental hygienists, dental technicians, and sometimes dental therapists.
History:
Middle Ages
In China as well as France, the first people to perform dentistry were barbers. They have been categorized into 2 distinct groups: guild of barbers and lay barbers. The first group, the Guild of Barbers, was created to distinguish more educated and qualified dental surgeons from lay barbers. Guild barbers were trained to do complex surgeries. The second group, the lay barbers, were qualified to perform regular hygienic services such as shaving and tooth extraction as well as basic surgery. However, in 1400, France made decrees prohibiting lay barbers from practicing all types of surgery. In Germany as well as France from 1530 to 1575 publications completely devoted to dentistry were being published. Ambroise Paré, often known as the Father of Surgery, published his own work about the proper maintenance and treatment of teeth. Ambroise Paré was a French barber surgeon who performed dental care for multiple French monarchs. He is often credited with having raised the status of barber surgeons.
Modern dentistry
Pierre Fauchard of France is often referred to as the "father of modern dentistry" because in 1728 he was the first to publish a scientific textbook on the techniques and practices of dentistry. Over time, trained dentists immigrated from Europe to the Americas to practice dentistry, and by 1760, America had its own native born practicing dentists. Newspapers were used at the time to advertise and promote dental services. In America from 1768 to 1770 the first application of dentistry to verify forensic cases was being pioneered; this was called forensic dentistry. With the rise of dentists, there was also the rise of new methods to improve the quality of dentistry. These new methods included the spinning wheel to rotate a drill and chairs made specifically for dental patients.
In the 1840s the world's first dental school and national dental organization were established. Along with the first dental school came the establishment of the Doctor of Dental Surgery degree, often referred to as a DDS degree. In response to the rise in new dentists as well as dentistry techniques, the first dental practice act was established to regulate dentistry. In the United States, the First Dental Practice Act required dentists to pass each specific state medical board exam in order to practice dentistry in that particular state. However, because the dental act was rarely enforced, some dentists did not obey the act. From 1846 to 1855 new dental techniques were being invented such as the use of ester anesthesia for surgery, and the cohesive gold foil method which enabled gold to be applied to a cavity. The American Dental Association was established in 1859 after a meeting with 26 dentists. Around 1867, the first university-associated dental school was established, Harvard Dental School. Lucy Hobbs Taylor was the first woman to earn a dental degree.
In the 1880s, tube toothpaste was created which replaced the original forms of powder or liquid toothpaste. New dental boards, such as the National Association of Dental Examiners, were created to establish standards and uniformity among dentists. In 1887 the first dental laboratory was established; dental laboratories are used to create dentures and crowns that are specific to each patient. In 1895 the dental X-ray was discovered by a German physicist, Wilhelm Röntgen.
In the 20th century, new dental techniques and technology were invented such as the porcelain crowns (1903), Novocain (a local anesthetic) 1905, precision cast fillings (1907), nylon toothbrushes (1938), water fluoridation (1945), fluoride toothpaste (1950), air driven dental tools (1957), lasers (1960), electric toothbrushes (1960), and home tooth bleaching kits (1989) were invented. Inventions such as the air driven dental tools ushered in a new high-speed dentistry.
Responsibilities
By nature of their general training, a licensed dentist can carry out most dental treatments such as restorative (dental restorations, crowns, bridges), orthodontics (braces), prosthodontic (dentures, crown/bridge), endodontic (root canal) therapy, periodontal (gum) therapy, and oral surgery (extraction of teeth), as well as performing examinations, taking radiographs (x-rays) and diagnosis. Additionally, dentists can further engage in oral surgery procedures such as dental implant placement. Dentists can also prescribe medications such as antibiotics, fluorides, pain killers, local anesthetics, sedatives/hypnotics and any other medications that serve in the treatment of the various conditions that arise in the head and neck.
All DDS and DMD degree holders are legally qualified to perform a number of more complex procedures such as gingival grafts, bone grafting, sinus lifts, and implants, as well as a range of more invasive oral and maxillofacial surgery procedures, though many choose to pursue residencies or other post-doctoral education to augment their abilities. A few select procedures, such as the administration of General anesthesia, legally require postdoctoral training in the US. While many oral diseases are unique and self-limiting, poor conditions in the oral cavity can lead to poor general health and vice versa; notably, there is a significant link between periodontal and cardiovascular disease. Conditions in the oral cavity may also be indicative of other systemic diseases such as osteoporosis, diabetes, AIDS, and various blood diseases, including malignancies and lymphoma. Dentists can also prescribe medicines.
Several studies have suggested that dentists and dental students are at high risk of burnout. During burnout, dentists experience exhaustion, alienate from work and perform less efficiently. A systemic study identified risk factors associated with this condition such as practitioner's young age, personality type, gender, the status of education, high job strain and/or working hours, and the burden of clinical degrees requisites. The authors of this study concluded that intervention programs at an early stage during the undergraduate level may provide practitioners with a good strategy to prepare for / cope with this condition.
Regulations
Depending on the country, all dentists are required to register with their national or local health board, regulators, and professional indemnity insurance, in order to practice dentistry. In the UK, dentists are required to register with the General Dental Council. In Australia, it is the Dental Board of Australia, while in the United States, dentists are registered according to the individual state board. The main role of a dental regulator is to protect the public by ensuring only qualified dental practitioners are registered, handle any complaints or misconduct, and develop national guidelines and standards for dental practitioners to follow.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2069 2024-02-23 18:36:28
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,725
Re: Miscellany
2071) Sculptor/Sculpture
Gist
An artist who creates three-dimensional works of art is a sculptor. Some sculptors carve designs out of a piece of wood or stone, while others weld slabs of metal together to create their masterpieces.
The thing distinguishing a sculptor from other artists is that their work can be viewed from many different sides, instead of being a relatively flat painting or collage. Sculptors today work in just about any medium you can imagine, including clay, fabric, metal, bone, plastic — even sand and ice. Ancient Greek and Roman sculptors created lifelike figures from marble and bronze, some of which you can still see in museums today. The Latin root of sculpture means "to carve."
Details
Sculpture is the branch of the visual arts that operates in three dimensions. Sculpture is the three-dimensional art work which is physically presented in the dimensions of height, width and depth. It is one of the plastic arts. Durable sculptural processes originally used carving (the removal of material) and modelling (the addition of material, as clay), in stone, metal, ceramics, wood and other materials but, since Modernism, there has been almost complete freedom of materials and process. A wide variety of materials may be worked by removal such as carving, assembled by welding or modelling, or moulded or cast.
Sculpture in stone survives far better than works of art in perishable materials, and often represents the majority of the surviving works (other than pottery) from ancient cultures, though conversely traditions of sculpture in wood may have vanished almost entirely. However, most ancient sculpture was brightly painted, and this has been lost.
Sculpture has been central in religious devotion in many cultures, and until recent centuries, large sculptures, too expensive for private individuals to create, were usually an expression of religion or politics. Those cultures whose sculptures have survived in quantities include the cultures of the ancient Mediterranean, India and China, as well as many in Central and South America and Africa.
The Western tradition of sculpture began in ancient Greece, and Greece is widely seen as producing great masterpieces in the classical period. During the Middle Ages, Gothic sculpture represented the agonies and passions of the Christian faith. The revival of classical models in the Renaissance produced famous sculptures such as Michelangelo's statue of David. Modernist sculpture moved away from traditional processes and the emphasis on the depiction of the human body, with the making of constructed sculpture, and the presentation of found objects as finished artworks.
Types
A distinction between sculpture "in the round", free-standing sculpture such as statues, not attached except possibly at the base to any other surface, and the various types of relief, which are at least partly attached to a background surface. Relief is often classified by the degree of projection from the wall into low or bas-relief, high relief, and sometimes an intermediate mid-relief. Sunk-relief is a technique restricted to ancient Egypt. Relief is the usual sculptural medium for large figure groups and narrative subjects, which are difficult to accomplish in the round, and is the typical technique used both for architectural sculpture, which is attached to buildings, and for small-scale sculpture decorating other objects, as in much pottery, metalwork and jewellery. Relief sculpture may also decorate steles, upright slabs, usually of stone, often also containing inscriptions.
Another basic distinction is between subtractive carving techniques, which remove material from an existing block or lump, for example of stone or wood, and modelling techniques which shape or build up the work from the material. Techniques such as casting, stamping and moulding use an intermediate matrix containing the design to produce the work; many of these allow the production of several copies.
The term "sculpture" is often used mainly to describe large works, which are sometimes called monumental sculpture, meaning either or both of sculpture that is large, or that is attached to a building. But the term properly covers many types of small works in three dimensions using the same techniques, including coins and medals, hardstone carvings, a term for small carvings in stone that can take detailed work.
The very large or "colossal" statue has had an enduring appeal since antiquity; the largest on record at 182 m (597 ft) is the 2018 Indian Statue of Unity. Another grand form of portrait sculpture is the equestrian statue of a rider on horse, which has become rare in recent decades. The smallest forms of life-size portrait sculpture are the "head", showing just that, or the bust, a representation of a person from the chest up. Small forms of sculpture include the figurine, normally a statue that is no more than 18 inches (46 cm) tall, and for reliefs the plaquette, medal or coin.
Modern and contemporary art have added a number of non-traditional forms of sculpture, including sound sculpture, light sculpture, environmental art, environmental sculpture, street art sculpture, kinetic sculpture (involving aspects of physical motion), land art, and site-specific art. Sculpture is an important form of public art. A collection of sculpture in a garden setting can be called a sculpture garden. There is also a view that buildings are a type of sculpture, with Constantin Brâncuși describing architecture as "inhabited sculpture".
Purposes and subjects
Medal of John VIII Palaeologus, c. 1435, by Pisanello, the first portrait medal, a medium essentially made for collecting
One of the most common purposes of sculpture is in some form of association with religion. Cult images are common in many cultures, though they are often not the colossal statues of deities which characterized ancient Greek art, like the Statue of Zeus at Olympia. The actual cult images in the innermost sanctuaries of Egyptian temples, of which none have survived, were evidently rather small, even in the largest temples. The same is often true in Hinduism, where the very simple and ancient form of the lingam is the most common. Buddhism brought the sculpture of religious figures to East Asia, where there seems to have been no earlier equivalent tradition, though again simple shapes like the bi and cong probably had religious significance.
Small sculptures as personal possessions go back to the earliest prehistoric art, and the use of very large sculpture as public art, especially to impress the viewer with the power of a ruler, goes back at least to the Great Sphinx of some 4,500 years ago. In archaeology and art history the appearance, and sometimes disappearance, of large or monumental sculpture in a culture is regarded as of great significance, though tracing the emergence is often complicated by the presumed existence of sculpture in wood and other perishable materials of which no record remains;
The totem pole is an example of a tradition of monumental sculpture in wood that would leave no traces for archaeology. The ability to summon the resources to create monumental sculpture, by transporting usually very heavy materials and arranging for the payment of what are usually regarded as full-time sculptors, is considered a mark of a relatively advanced culture in terms of social organization. Recent unexpected discoveries of ancient Chinese Bronze Age figures at Sanxingdui, some more than twice human size, have disturbed many ideas held about early Chinese civilization, since only much smaller bronzes were previously known.
Some undoubtedly advanced cultures, such as the Indus Valley civilization, appear to have had no monumental sculpture at all, though producing very sophisticated figurines and seals. The Mississippian culture seems to have been progressing towards its use, with small stone figures, when it collapsed. Other cultures, such as ancient Egypt and the Easter Island culture, seem to have devoted enormous resources to very large-scale monumental sculpture from a very early stage.
The collecting of sculpture, including that of earlier periods, goes back some 2,000 years in Greece, China and Mesoamerica, and many collections were available on semi-public display long before the modern museum was invented. From the 20th century the relatively restricted range of subjects found in large sculpture expanded greatly, with abstract subjects and the use or representation of any type of subject now common. Today much sculpture is made for intermittent display in galleries and museums, and the ability to transport and store the increasingly large works is a factor in their construction.
Small decorative figurines, most often in ceramics, are as popular today (though strangely neglected by modern and Contemporary art) as they were in the Rococo, or in ancient Greece when Tanagra figurines were a major industry, or in East Asian and Pre-Columbian art. Small sculpted fittings for furniture and other objects go well back into antiquity, as in the Nimrud ivories, Begram ivories and finds from the tomb of Tutankhamun.
Portrait sculpture began in Egypt, where the Narmer Palette shows a ruler of the 32nd century BCE, and Mesopotamia, where we have 27 surviving statues of Gudea, who ruled Lagash c. 2144–2124 BCE. In ancient Greece and Rome, the erection of a portrait statue in a public place was almost the highest mark of honour, and the ambition of the elite, who might also be depicted on a coin.
In other cultures such as Egypt and the Near East public statues were almost exclusively the preserve of the ruler, with other wealthy people only being portrayed in their tombs. Rulers are typically the only people given portraits in Pre-Columbian cultures, beginning with the Olmec colossal heads of about 3,000 years ago. East Asian portrait sculpture was entirely religious, with leading clergy being commemorated with statues, especially the founders of monasteries, but not rulers, or ancestors. The Mediterranean tradition revived, initially only for tomb effigies and coins, in the Middle Ages, but expanded greatly in the Renaissance, which invented new forms such as the personal portrait medal.
Animals are, with the human figure, the earliest subject for sculpture, and have always been popular, sometimes realistic, but often imaginary monsters; in China animals and monsters are almost the only traditional subjects for stone sculpture outside tombs and temples. The kingdom of plants is important only in jewellery and decorative reliefs, but these form almost all the large sculpture of Byzantine art and Islamic art, and are very important in most Eurasian traditions, where motifs such as the palmette and vine scroll have passed east and west for over two millennia.
One form of sculpture found in many prehistoric cultures around the world is specially enlarged versions of ordinary tools, weapons or vessels created in impractical precious materials, for either some form of ceremonial use or display or as offerings. Jade or other types of greenstone were used in China, Olmec Mexico, and Neolithic Europe, and in early Mesopotamia large pottery shapes were produced in stone. Bronze was used in Europe and China for large axes and blades, like the Oxborough Dirk.
Materials and techniques
The materials used in sculpture are diverse, changing throughout history. The classic materials, with outstanding durability, are metal, especially bronze, stone and pottery, with wood, bone and antler less durable but cheaper options. Precious materials such as gold, silver, jade, and ivory are often used for small luxury works, and sometimes in larger ones, as in chryselephantine statues. More common and less expensive materials were used for sculpture for wider consumption, including hardwoods (such as oak, box/boxwood, and lime/linden); terracotta and other ceramics, wax (a very common material for models for casting, and receiving the impressions of cylinder seals and engraved gems), and cast metals such as pewter and zinc (spelter). But a vast number of other materials have been used as part of sculptures, in ethnographic and ancient works as much as modern ones.
Sculptures are often painted, but commonly lose their paint to time, or restorers. Many different painting techniques have been used in making sculpture, including tempera, oil painting, gilding, house paint, aerosol, enamel and sandblasting.
Many sculptors seek new ways and materials to make art. One of Pablo Picasso's most famous sculptures included bicycle parts. Alexander Calder and other modernists made spectacular use of painted steel. Since the 1960s, acrylics and other plastics have been used as well. Andy Goldsworthy makes his unusually ephemeral sculptures from almost entirely natural materials in natural settings. Some sculpture, such as ice sculpture, sand sculpture, and gas sculpture, is deliberately short-lived. Recent sculptors have used stained glass, tools, machine parts, hardware and consumer packaging to fashion their works. Sculptors sometimes use found objects, and Chinese scholar's rocks have been appreciated for many centuries.
Stone
Stone sculpture is an ancient activity where pieces of rough natural stone are shaped by the controlled removal of stone. Owing to the permanence of the material, evidence can be found that even the earliest societies indulged in some form of stone work, though not all areas of the world have such abundance of good stone for carving as Egypt, Greece, India and most of Europe. Petroglyphs (also called rock engravings) are perhaps the earliest form: images created by removing part of a rock surface which remains in situ, by incising, pecking, carving, and abrading. Monumental sculpture covers large works, and architectural sculpture, which is attached to buildings. Hardstone carving is the carving for artistic purposes of semi-precious stones such as jade, agate, onyx, rock crystal, sard or carnelian, and a general term for an object made in this way. Alabaster or mineral gypsum is a soft mineral that is easy to carve for smaller works and still relatively durable. Engraved gems are small carved gems, including cameos, originally used as seal rings.
The copying of an original statue in stone, which was very important for ancient Greek statues, which are nearly all known from copies, was traditionally achieved by "pointing", along with more freehand methods. Pointing involved setting up a grid of string squares on a wooden frame surrounding the original, and then measuring the position on the grid and the distance between grid and statue of a series of individual points, and then using this information to carve into the block from which the copy is made.
Metal
Bronze and related copper alloys are the oldest and still the most popular metals for cast metal sculptures; a cast bronze sculpture is often called simply a "bronze". Common bronze alloys have the unusual and desirable property of expanding slightly just before they set, thus filling the finest details of a mould. Their strength and lack of brittleness (ductility) is an advantage when figures in action are to be created, especially when compared to various ceramic or stone materials (see marble sculpture for several examples). Gold is the softest and most precious metal, and very important in jewellery; with silver it is soft enough to be worked with hammers and other tools as well as cast; repoussé and chasing are among the techniques used in gold and silversmithing.
Casting is a group of manufacturing processes by which a liquid material (bronze, copper, glass, aluminum, iron) is (usually) poured into a mould, which contains a hollow cavity of the desired shape, and then allowed to solidify. The solid casting is then ejected or broken out to complete the process, although a final stage of "cold work" may follow on the finished cast. Casting may be used to form hot liquid metals or various materials that cold set after mixing of components (such as epoxies, concrete, plaster and clay). Casting is most often used for making complex shapes that would be otherwise difficult or uneconomical to make by other methods. The oldest surviving casting is a copper Mesopotamian frog from 3200 BCE. Specific techniques include lost-wax casting, plaster mould casting, and sand casting.
Welding is a process where different pieces of metal are fused together to create different shapes and designs. There are many different forms of welding, such as Oxy-fuel welding, Stick welding, MIG welding, and TIG welding. Oxy-fuel is probably the most common method of welding when it comes to creating steel sculptures because it is the easiest to use for shaping the steel as well as making clean and less noticeable joins of the steel. The key to Oxy-fuel welding is heating each piece of metal to be joined evenly until all are red and have a shine to them. Once that shine is on each piece, that shine will soon become a 'pool' where the metal is liquified and the welder must get the pools to join, fusing the metal. Once cooled off, the location where the pools joined are now one continuous piece of metal. Also used heavily in Oxy-fuel sculpture creation is forging. Forging is the process of heating metal to a certain point to soften it enough to be shaped into different forms. One very common example is heating the end of a steel rod and hitting the red heated tip with a hammer while on an anvil to form a point. In between hammer swings, the forger rotates the rod and gradually forms a sharpened point from the blunt end of a steel rod.
Glass
Glass may be used for sculpture through a wide range of working techniques, though the use of it for large works is a recent development. It can be carved, though with considerable difficulty; the Roman Lycurgus Cup is all but unique. There are various ways of moulding glass: hot casting can be done by ladling molten glass into moulds that have been created by pressing shapes into sand, carved graphite or detailed plaster/silica moulds. Kiln casting glass involves heating chunks of glass in a kiln until they are liquid and flow into a waiting mould below it in the kiln. Hot glass can also be blown and/or hot sculpted with hand tools either as a solid mass or as part of a blown object. More recent techniques involve chiseling and bonding plate glass with polymer silicates and UV light.
Pottery
Pottery is one of the oldest materials for sculpture, as well as clay being the medium in which many sculptures cast in metal are originally modelled for casting. Sculptors often build small preliminary works called maquettes of ephemeral materials such as plaster of Paris, wax, unfired clay, or plasticine. Many cultures have produced pottery which combines a function as a vessel with a sculptural form, and small figurines have often been as popular as they are in modern Western culture. Stamps and moulds were used by most ancient civilizations, from ancient Rome and Mesopotamia to China.
Wood carving
Wood carving has been extremely widely practiced, but survives much less well than the other main materials, being vulnerable to decay, insect damage, and fire. It therefore forms an important hidden element in the art history of many cultures. Outdoor wood sculpture does not last long in most parts of the world, so that we have little idea how the totem pole tradition developed. Many of the most important sculptures of China and Japan in particular are in wood, and the great majority of African sculpture and that of Oceania and other regions.
Wood is light, so suitable for masks and other sculpture intended to be carried, and can take very fine detail. It is also much easier to work than stone. It has been very often painted after carving, but the paint wears less well than the wood, and is often missing in surviving pieces. Painted wood is often technically described as "wood and polychrome". Typically a layer of gesso or plaster is applied to the wood, and then the paint is applied to that.
Soft materials
Three dimensional work incorporating unconventional materials such as cloth, fur, plastics, rubber and nylon, that can thus be stuffed, sewn, hung, draped or woven, are known as soft sculptures. Well known creators of soft sculptures include Claes Oldenburg, Yayoi Kusama, Eva Hesse, Sarah Lucas and Magdalena Abakanowicz.[15]
Additional Information
A sculptor is an artist who specializes in creating three-dimensional artworks by shaping, carving, or manipulating various materials. Sculptors work with a wide range of materials such as stone, wood, metal, clay, plaster, or even found objects. They use their skills and tools to transform these materials into expressive and tangible forms, exploring the interplay of volume, space, texture, and composition.
Sculptors employ various techniques and approaches to bring their artistic vision to life. They may create representational sculptures that depict recognizable objects, figures, or scenes, or they may delve into abstract or conceptual forms, focusing on evoking emotions, ideas, or exploring the relationships between form and space. Sculptors work in diverse styles and scales, from small-scale sculptures that fit in the palm of your hand to monumental public installations that reshape and interact with the surrounding environment. Through their art, sculptors invite viewers to engage with physical objects in a tactile and spatial way, offering unique sensory and aesthetic experiences.
What does a Sculptor do?
Sculptors engage in a combination of artistic creativity and technical skills to bring their sculptures to life. Their work requires patience, attention to detail, and a deep understanding of materials, forms, and artistic expression. Through their sculptures, they contribute to the visual arts, enriching public spaces, galleries, and personal collections with their unique artistic visions.
Duties and Responsibilities
Sculptors engage in a range of activities and tasks in their artistic practice. Here are some of the key things that sculptors do:
* Concept Development: Sculptors start by developing their artistic concept or idea. This involves exploring themes, researching, sketching, and conceptualizing the form and composition of their sculpture. They may draw inspiration from personal experiences, nature, mythology, social issues, or abstract concepts.
* Material Selection: Sculptors carefully choose the material they will work with, considering its properties, durability, and suitability for their artistic vision. They may select materials such as marble, bronze, clay, wood, metal, or a combination of different materials depending on the desired outcome and their expertise.
* Preparation and Planning: Before starting the actual sculpting process, sculptors often create preparatory models or maquettes. These smaller-scale versions help them refine their design, study proportions, and experiment with different techniques and materials. They also plan the logistics, tools, and equipment needed for the sculpting process.
* Sculpting Techniques: Sculptors employ a variety of techniques to shape and manipulate their chosen material. They may use tools such as chisels, hammers, rasps, files, and drills for subtractive processes like carving or cutting away material. Alternatively, they may use additive techniques like modeling, molding, or casting to build up layers or shapes.
* Finishing and Refining: Once the main form of the sculpture is complete, sculptors refine the surface, paying attention to details, textures, and finishes. They may use sandpaper, polishing tools, or other techniques to achieve the desired smoothness or texture. They may also apply patinas or coatings to enhance the appearance or protect the surface of the sculpture.
* Installation and Display: Sculptors consider the placement and display of their artwork. For smaller sculptures, they may mount them on pedestals or plinths, while larger works may require installation in public spaces, gardens, or galleries. Sculptors collaborate with architects, engineers, or installation specialists to ensure proper installation and structural integrity.
* Collaboration and Commissions: Sculptors may collaborate with other artists, architects, or clients for specific projects or commissions. They work closely with clients to understand their vision and requirements, creating custom sculptures that align with their expectations. This could involve creating public art installations, memorials, or sculptures for private collections.
* Promotion and Exhibitions: Sculptors actively promote their work through various means, including participation in exhibitions, art fairs, or galleries. They create portfolios, artist statements, and documentation of their sculptures to share with potential buyers, collectors, or art enthusiasts. They may also engage in networking, marketing, and online presence through websites or social media platforms.
* Maintenance and Restoration: Sculptors may be responsible for the ongoing maintenance and restoration of their sculptures. Over time, sculptures may require cleaning, repair, or preservation measures to ensure their longevity and aesthetic integrity. Sculptors may collaborate with conservators or restoration specialists for more complex or delicate restoration work.
Types of Sculptors
There are various types or categories of sculptors, each defined by their preferred materials, techniques, styles, or subject matter. Here are some common types of sculptors:
* Stone Carvers: Stone carvers specialize in sculpting stone, such as marble, granite, limestone, or alabaster. They employ traditional carving techniques, using chisels, mallets, and other tools to shape the stone into desired forms, from figurative sculptures to abstract designs.
* Metal Sculptors: Metal sculptors work with materials like bronze, steel, iron, or aluminum. They use welding, cutting, forging, or casting techniques to create sculptures that explore the unique properties of metal, including its strength, malleability, and reflective qualities.
* Ceramic Sculptors/Potters: Ceramic sculptors/potters work with clay and other ceramic materials. They utilize techniques like hand-building, wheel throwing, or ceramic molding to create sculptures that are then fired in kilns. Ceramic sculptures can range from functional pottery to intricate figurative or abstract forms.
* Wood Carvers: Wood carvers specialize in sculpting wood, using carving tools to shape and manipulate the material. They work with different types of wood, such as oak, mahogany, or walnut, and may create sculptures that showcase the natural grain and texture of the wood or incorporate painted or stained finishes.
* Figurative Sculptors: Figurative sculptors focus on creating sculptures that depict the human form or figures. They explore anatomy, gesture, and expression, capturing the likeness and essence of individuals or conveying narratives and emotions through their figurative works.
* Abstract Sculptors: Abstract sculptors create non-representational or non-figurative sculptures. They work with various materials and forms, emphasizing shapes, lines, textures, and spatial relationships. Abstract sculptors often explore concepts, emotions, or aesthetics through their innovative and sometimes unconventional creations.
* Installation Artists: Installation artists specialize in creating large-scale, site-specific artworks that transform spaces. They may use diverse materials and elements, including sculpture, light, sound, video, or found objects, to create immersive and interactive experiences for viewers.
* Environmental Sculptors: Environmental sculptors create sculptures that are integrated into natural landscapes or outdoor environments. They often use natural materials and take into consideration the surrounding context, aiming to harmonize with or accentuate the natural features of the site.
* Conceptual Sculptors: Conceptual sculptors focus on the ideas and concepts behind their artwork rather than the physical form. They may employ unconventional materials, use text or symbolic elements, or explore intellectual, social, or political themes through their sculptures.
* Collaborative Sculptors: Collaborative sculptors work in teams or groups to create sculptures. They pool their talents, skills, and ideas to produce larger, more complex artworks that require the expertise of multiple individuals. Collaboration may involve artists from different disciplines, such as sculptors, architects, engineers, or artisans.
Are you suited to be a sculptor?
Sculptors have distinct personalities. They tend to be artistic individuals, which means they’re creative, intuitive, sensitive, articulate, and expressive. They are unstructured, original, nonconforming, and innovative. Some of them are also investigative, meaning they’re intellectual, introspective, and inquisitive.
What is the workplace of a Sculptor like?
The workplace of a sculptor can vary depending on the specific circumstances and nature of their work. Here are some aspects to consider regarding the workplace of a sculptor:
* Studio: Many sculptors have their own dedicated studio space where they create their artwork. Studios can range from small, intimate spaces for individual artists to larger shared studios that foster a sense of community and collaboration. The studio serves as a sanctuary for the sculptor to work, think, and experiment with materials, tools, and techniques. It provides a controlled environment where they can focus on their creative process and bring their artistic visions to life.
* Outdoor Workspaces: Some sculptors prefer to work outdoors, especially when creating large-scale or site-specific sculptures. They may have access to open-air studios, sculpture parks, or outdoor workshops. Outdoor workspaces allow sculptors to engage with natural light, elements, and the surrounding environment, offering unique challenges and opportunities for their artistic practice.
* Foundries or Fabrication Studios: Sculptors who work with materials like bronze or other metals often collaborate with foundries or fabrication studios. These specialized facilities have the necessary equipment and expertise to transform the sculptor's original piece into a finished bronze sculpture through casting, patination, and other finishing processes. Sculptors may work closely with technicians and artisans in these spaces to bring their creations to fruition.
* Exhibition Spaces: As sculptors create their artworks, they envision how they will be displayed and experienced by viewers. Exhibition spaces, such as galleries, museums, or outdoor public spaces, play a crucial role in showcasing the sculptor's work to the audience. Sculptors may engage with curators, exhibition organizers, or gallery owners to secure opportunities to display their sculptures in appropriate settings.
* Workshops and Residencies: Sculptors may participate in workshops or artist residencies, both local and international, to explore new techniques, collaborate with other artists, or engage in focused periods of artistic production. These environments often provide specialized equipment, tools, and a supportive community of artists, fostering creative growth and experimentation.
* Client Sites: Sculptors who undertake commissions or public art projects may work on-site at client locations. They collaborate with architects, landscape designers, and clients to create sculptures that integrate harmoniously into specific spaces. This may involve visits to construction sites, coordination with installation teams, and adapting the sculpting process to suit the specific requirements of the project.
* Travel and Exploration: Sculptors may also find inspiration and opportunities for their work through travel and exploration. They may visit museums, sculpture parks, or cultural sites to gain exposure to different artistic traditions, styles, and materials. Exploring new environments and cultures can spark new ideas and perspectives that inform their artistic practice.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2070 2024-02-24 00:10:18
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,725
Re: Miscellany
2072) Garage
Gist
A garage is a small building where a car, etc. is kept.
Details
A garage is a covered structure built for the purpose of parking, storing, protecting, maintaining, and/or repairing vehicles. Specific applications include:
A residential garage is a walled, roofed structure with a door for storing a vehicle or vehicles that may be part of or attached to a home ("attached garage"), or a separate outbuilding or shed ("detached garage"). Residential garages typically have space for one or two cars, although three-car garages are used. When a garage is attached to a house, the garage typically has an entry door into the house, called the person door or man door, in contrast with the wider and taller door for vehicles, called the garage door, which can be opened to permit the entry and exit of a vehicle and then closed to secure the vehicle. A garage protects a vehicle from precipitation, and, if it is equipped with a locking garage door, it also protects the vehicle(s) from theft and vandalism. Most garages also serve multifunction duty as workshops for a variety of projects, including painting, woodworking, and assembly. Garages also may be used for other purposes as well, such as storage or entertainment.
Some garages have an electrical mechanism to automatically open or close the garage door when the homeowner presses a button on a small remote control, along with a detector that stops the movement of the garage if something is in the way of closing. Some garages have enough space, even with cars inside, for the storage of items such as bicycles or a lawnmower; in some cases, there may even be enough space for a workshop or a man cave. Garages that are attached to a house may be built with the same external materials and roofing as the house. Garages that are not connected to the home may use a different style of construction from the house. Often in the Southern and rural United States garages not attached to the home and made from a timber frame with sheet metal coverings are known as "pole barns", but usually serve the same purpose as what is called a garage elsewhere. In some places, the term is used synonymously with "carport", though that term normally describes a structure that, while roofed, is not completely enclosed. A carport protects the vehicle to some degree from inclement weather, but it does not protect the vehicle from theft or vandalism.
The word garage, introduced to English in 1902, originates from the French word garer, meaning shelter. By 1908 the architect Charles Harrison Townsend was commenting in The Builder magazine that "for the home of the car, we very largely use the French word 'garage', alternatively with what I think the more desirable English equivalent of 'motor house'". Today the word is polysemic because it can refer to a collection of vehicles as well as the building that contains them.
Residential garage insulation
In northern climates, temperatures inside an uninsulated attached residential garage can decrease to freezing levels during the winter. Temperatures inside an uninsulated attached garage in temperate climates can reach uncomfortable levels during summer months. Extreme temperatures can be a source of energy waste and discomfort in adjoining living areas, due to heat transfer between the garage and those areas. Homes with an attached garage often experience this "interface" problem. Insulating the outside of the building against the elements without extending the insulation to the wall separating the garage from the house, and/or the other garage walls and roof, can be a costly mistake.
An automobile repair shop (also known regionally as a garage or a workshop) is an establishment where automobiles are repaired by auto mechanics and technicians. The customer interface is typically a service advisor, traditionally called a service writer.
Types
Automotive garages and repair shops can be divided into following categories:
Service station
First appearing in the early 1900s, many filling stations offered vehicle repair services as part of their full service operation. This once popular trend has declined significantly over the years as many locations found it more profitable to exchange vehicle service bays for grocery isles, which ultimately lead to the emergence of the quick oil change industry.[
Lubrication/safety shop
Commonly referred to as a quick lube or express service shop, this type of facility specializes in preventive maintenance and safety inspections rather than repairs. Product sales are typically limited to automotive fluids, belts and hoses. With a focus on basic procedures, labor is often performed by entry-level technicians which simplifies the business overhead resulting in a less expensive service as compared to a traditional automotive workshop.
New car dealership
In the United States, new car dealerships have service departments that are certified by their respective OEM (Original Equipment Manufacturer) to perform warranty and recall repairs. Customer-pay repairs can also be completed, however most service departments tend to only work on the vehicle brand of which they are a dealer. Dealership technicians must complete additional training provided by the OEM, and in doing so become specialized and certified for that particular vehicle make.
Independent auto repair shop
Independent auto repair shops are businesses that are independently owned and operated. In states regulating a smog or emission test, often, independent auto repair shops offer these tests as well. These may also include regional or national chains and franchises. It is rather common for a dealership technician to start this type of competing business after leaving the employment of a new car dealership. Independent automobile repair shops in the US may also achieve OEM certification through manufacturer sponsored programs. European Union law (The EC Block Exemption Regulation 1400/2002 (October 2003)) permits motorists more flexibility in selecting where their car is serviced. Maintenance and service work does not have to be done by the dealership providing that the independent garage uses Original Equipment 'Matching Quality' parts and follows the manufacturer's service schedules. The Block Exemption Regulation (BER) covers service and maintenance during the warranty period and prohibits vehicle manufacturers' warranties from including restrictive conditions.
Fleet shop
A shop that is dedicated to repairing and maintaining a particular group of vehicles is called a fleet shop. Common examples of a fleet include taxi cabs, police cars, mail trucks and rental vehicles. Similar to a lubrication/safety shop, a fleet shop focuses primarily on preventative maintenance and safety inspections, and will often outsource larger or more complex repairs to another repair facility.
Engine machine shop
Shops that specialize in cylinder head and cylinder block machining are called engine machine shops. These facilities utilize large electromechanical machines that are not found in the average automotive repair shop. Engine machining is typically performed by an ASE certified machinist in order to correct worn or damaged engine components as an alternative to component replacement. Performance engine building is another popular service frequently offered by this type of workshop.
Tire and wheel shop
Some repair shops specialize in tires and wheels. These businesses usually have a large inventory of tires and aftermarket wheels, some of which may be on display while others require special ordering. In addition to parts, common labor services include tire rotation, balancing and repair as well as wheel alignment which can prevent premature tire wear.
In the Philippines, roadside tire repair shops are called vulcanizing shops in Philippine English. They specialize in quickly and cheaply repairing flat tires by patching punctures with a rubber compound patch.
Muffler shop
A muffler shop, also called an exhaust shop, is a business model that concentrates solely on the engine exhaust system. These facilities utilize large tubing benders which allow a technician to fabricate a new exhaust system out of otherwise straight lengths of pipe. Welding is often necessary in this line of work.
Auto body
Automotive repair shops that specialize in bodywork repair are known as body shops. Auto body technicians can perform paintwork repairs to scratches, scuffs and dents, as well as repairs to the bodies of vehicles damaged by collisions. Many body shops now offer paintless dent repair and auto glass replacement. Automotive repair shops that specialize in auto glass repair are known as auto glass repair shops. They offer auto glass repairs to chips, cracks and shattered glass. The types of glass they repair include windshields, car windows, quarter glass and rear windows. This type of damage is often caused by hail, stones, wild animals, fallen trees, automobile theft and vandalism.
Mobile mechanics
Mobile mechanics provide doorstep repair services and home delivery of new and used auto parts of different late model and classic cars whose parts are not widely available in the market.
In countries such as the UK, the mobile car body repair sectors has experienced high growth by way of mobile SMART Repair companies providing mobile car body repair services, such as Bumper Repairs, auto body repair, paintless dent repair and paintwork defect repairs to private and commercial consumers, typically within the industry framework of refinishing vehicle damage on a localised basis, where the area of damage being repaired is not in excess of an A4 sheet of paper.
Bus garage
A bus garage, also known as a bus depot, bus base or bus barn, is a facility where buses are stored and maintained. In many conurbations, bus garages are on the site of former car barns or tram sheds, where trams (streetcars) were stored, and the operation transferred to buses. In other areas, garages were built to replace horsebus yards or on virgin sites when populations were not as high as now.
Description
Most bus garages will contain the following elements:
* Internal parking
* External parking
* Fueling point
* Fuel storage tanks
* Engineering section
* Inspection pits
* Bus wash
* Brake test lane
* Staff canteen/break room
* Administration office
Smaller garages may contain the minimum engineering facilities, restricted to light servicing capabilities only. Garages may also contain recovery vehicles, often converted buses, although their incidence has declined with the use of contractors to recover break-downs, and the increase in reliability.
Overnight, the more valuable or regularly in-service buses will usually be stored in the interior of the garage, with less used or older service vehicles, and vehicles withdrawn for storage or awaiting disposal, stored externally. During the day, internal and external areas will see a variety of movements. Heritage vehicles are almost exclusively stored inside the garage.
Often garages will feature rest rooms for drivers assigned to 'as required' duties, whereby they may be required to drive relief or replacement buses in the event of breakdown. The garage may also have 'light duties' drivers, who merely move the buses internally around the garage, often called shunting. Shunter or light duty drivers are often employed in larger depot facilities and work night shifts in order to position buses in the correct order for morning departures from the depot with the first buses due to leave the depot parked logical order nearest the exit. Because they are driving on privately owned land in many jurisdictions a full bus licence may not be required to perform such tasks. In addition they may also perform other tasks such as cleaning buses, refuelling and light maintenance tasks.
Filling station
A filling station (also known as a gas station [US] or petrol station [UK]) is a facility that sells fuel and engine lubricants for motor vehicles. The most common fuels sold in the 2010s were gasoline (or petrol) and diesel fuel.
Gasoline pumps are used to pump gasoline, diesel, compressed natural gas, CGH2, HCNG, LPG, liquid hydrogen, kerosene, alcohol fuel (like methanol, ethanol, butanol, and propanol), biofuels (like straight vegetable oil and biodiesel), or other types of fuel into the tanks within vehicles and calculate the financial cost of the fuel transferred to the vehicle. Besides gasoline pumps, one other significant device which is also found in filling stations and can refuel certain (compressed-air) vehicles is an air compressor, although generally these are just used to inflate car tires.
Many filling stations provide convenience stores, which may sell confections, alcoholic beverages, tobacco products, lottery tickets, soft drinks, snacks, coffee, newspapers, magazines, and, in some cases, a small selection of grocery items, such as milk. Some also sell propane or butane and have added shops to their primary business. Conversely, some chain stores, such as supermarkets, discount stores, warehouse clubs, or traditional convenience stores, have provided fuel pumps on the premises.
Multistorey car park
A multistorey car park (Commonwealth English) or parking garage (American English), also called a multistorey, parking building, parking structure, parkade (mainly Canadian), parking ramp, parking deck, or indoor parking, is a building designed for car, motorcycle, and bicycle parking in which parking takes place on more than one floor or level. The first known multistorey facility was built in London in 1901, and the first underground parking was built in Barcelona in 1904. (See History, below.) The term multistorey (or multistory) is almost never used in the US, because almost all parking structures have multiple parking levels. Parking structures may be heated if they are enclosed.
Design of parking structures can add considerable cost for planning new developments, with costs in the United States around $28,000 per space and $56,000 per space for underground (excluding the cost of land), and can be required by cities in parking mandates for new buildings. Some cities such as London have abolished previously enacted minimum parking requirements.[5] Minimum parking requirements are a hallmark of zoning and planning codes for municipalities in the US. (States do not prescribe parking requirements, while counties and cities can).

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2071 2024-02-25 00:04:32
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,725
Re: Miscellany
2073) Thirst
Gist
Thirst is a a sensation of dryness in the mouth and throat associated with a desire for liquids. also : the bodily condition (as of dehydration) that induces this sensation.
Summary:
Why am I always thirsty?
Most of the time if you're feeling thirsty it's because you need to drink more fluids.
This can happen if you:
* sweat a lot after doing exercise
* are unwell with sickness and diarrhoea
* do not drink enough fluids
* drink too much alcohol or caffeine
* eat salty or spicy food
* have a high temperature
* are pregnant
Important
Drink more fluids and avoid alcohol or caffeine to see if it helps. If you do not, you may be at risk of becoming dehydrated.
Check if you're dehydrated
Non-urgent advice :See a GP if you have excessive thirst and:
* drinking more fluids for several days has not helped
* you frequently need to pee
* you're pregnant
The GP will check if your excessive thirst is being caused by something – for example, diabetes, anaemia or medicines you're taking.
The treatment you have will depend on what's causing your excessive thirst.
Dry mouth or excessive thirst?
You may think you're thirsty when you actually have a dry mouth.
If it's a dry mouth, you may have:
* a burning sensation or soreness in your mouth
* changes in your sense of taste
* difficulty speaking, eating or swallowing.
Details
Thirst is the craving for potable fluids, resulting in the basic instinct of animals to drink. It is an essential mechanism involved in fluid balance. It arises from a lack of fluids or an increase in the concentration of certain osmolites, such as sodium. If the water volume of the body falls below a certain threshold or the osmolite concentration becomes too high, structures in the brain detect changes in blood constituents and signal thirst.
Continuous dehydration can cause acute and chronic diseases, but is most often associated with renal and neurological disorders. Excessive thirst, called polydipsia, along with excessive urination, known as polyuria, may be an indication of diabetes mellitus or diabetes insipidus.
There are receptors and other systems in the body that detect a decreased volume or an increased osmolite concentration. Some sources distinguish "extracellular thirst" from "intracellular thirst", where extracellular thirst is thirst generated by decreased volume and intracellular thirst is thirst generated by increased osmolite concentration.
Detection
It is vital for organisms to be able to maintain their fluid levels in very narrow ranges. The goal is to keep the interstitial fluid, the fluid outside the cell, at the same concentration as the intracellular fluid, the fluid inside the cell. This condition is called isotonic and occurs when the same levels of solutes are present on either side of the cell membrane so that the net water movement is zero. If the interstitial fluid has a higher concentration of solutes (or a lower concentration of water) than the intracellular fluid, it will pull water out of the cell. This condition is called hypertonic and if enough water leaves the cell, it will not be able to perform essential chemical functions. The animal will then become thirsty in response to the demand for water in the cell. After the animal drinks water, the interstitial fluid becomes less concentrated of solutes (more concentrated of water) than the intracellular fluid and the cell will fill with water as it tries to equalize the concentrations. This condition is called hypotonic and can be dangerous because it can cause the cell to swell and rupture. One set of receptors responsible for thirst detects the concentration of interstitial fluid. The other set of receptors detects blood volume.
Decreased volume
This is one of two types of thirst and is defined as thirst caused by loss of blood volume (hypovolemia) without depleting the intracellular fluid. This can be caused by blood loss, vomiting, and diarrhea. This loss of volume is problematic because if the total blood volume falls too low the heart cannot circulate blood effectively and the eventual result is hypovolemic shock. The vascular system responds by constricting blood vessels thereby creating a smaller volume for the blood to fill. This mechanical solution, however, has definite limits and usually must be supplemented with increased volume. The loss of blood volume is detected by cells in the kidneys and triggers thirst for both water and salt via the renin-angiotensin system.
Renin-angiotensin system
Hypovolemia leads to activation of the renin angiotensin system (RAS) and is detected by cells in the kidney. When these cells detect decreased blood flow due to the low volume they secrete an enzyme called renin. Renin then enters the blood where it catalyzes a protein called angiotensinogen to angiotensin I. Angiotensin I is then almost immediately converted by an enzyme already present in the blood to the active form of the protein, angiotensin II. Angiotensin II then travels in the blood until it reaches the posterior pituitary gland and the adrenal cortex, where it causes a cascade effect of hormones that cause the kidneys to retain water and sodium, increasing blood pressure. It is also responsible for the initiation of drinking behavior and salt appetite via the subfornical organ.
Others
* Arterial baroreceptors sense a decreased arterial pressure, and signal to the central nervous system in the area postrema and nucleus tractus solitarii.
* Cardiopulmonary receptors sense a decreased blood volume, and signal to area postrema and nucleus tractus solitarii.
Cellular dehydration and osmoreceptor stimulation
Osmometric thirst occurs when the solute concentration of the interstitial fluid increases. This increase draws water out of the cells, and they shrink in volume. The solute concentration of the interstitial fluid increases by high intake of sodium in diet or by the drop in volume of extracellular fluids (such as blood plasma and cerebrospinal fluid) due to loss of water through perspiration, respiration, urination and defecation. The increase in interstitial fluid solute concentration causes water to migrate from the cells of the body, through their membranes, to the extracellular compartment, by osmosis, thus causing cellular dehydration.
Clusters of cells (osmoreceptors) in the organum vasculosum of the lamina terminalis (OVLT) and subfornical organ (SFO), which lie outside of the blood brain barrier can detect the concentration of blood plasma and the presence of angiotensin II in the blood. They can then activate the median preoptic nucleus which initiates water seeking and ingestive behavior. Destruction of this part of the hypothalamus in humans and other animals results in partial or total loss of desire to drink even with extremely high salt concentration in the extracellular fluids. In addition, there are visceral osmoreceptors which project to the area postrema and nucleus tractus solitarii in the brain.
Salt craving
Because sodium is also lost from the plasma in hypovolemia, the body's need for salt proportionately increases in addition to thirst in such cases. This is also a result of the renin-angiotensin system activation.
Elderly
In adults over the age of 50 years, the body's thirst sensation reduces and continues diminishing with age, putting this population at increased risk of dehydration. Several studies have demonstrated that elderly persons have lower total water intakes than younger adults, and that women are particularly at risk of too low an intake. In 2009, the European Food Safety Authority (EFSA) included water as a macronutrient in its dietary reference values for the first time. Recommended intake volumes in the elderly are the same as for younger adults (2.0 L/day for females and 2.5 L/day for males) as despite lower energy consumption, the water requirement of this group is increased due to a reduction in renal concentrating capacity.
Thirst quenching
According to preliminary research, quenching of thirst – the homeostatic mechanism to stop drinking – occurs via two neural phases: a "preabsorptive" phase which signals quenched thirst many minutes before fluid is absorbed from the stomach and distributed to the body via the circulation, and a "postabsorptive" phase which is regulated by brain structures sensing to terminate fluid ingestion. The preabsorptive phase relies on sensory inputs in the mouth, pharynx, esophagus, and upper gastrointestinal tract to anticipate the amount of fluid needed, providing rapid signals to the brain to terminate drinking when the assessed amount has been consumed. The postabsorptive phase occurs via blood monitoring for osmolality, fluid volume, and sodium balance, which are collectively sensed in brain circumventricular organs linked via neural networks to terminate thirst when fluid balance is established.
Thirst quenching varies among animal species, with dogs, camels, sheep, goats, and deer replacing fluid deficits quickly when water is available, whereas humans and horses may need hours to restore fluid balance.
Neurophysiology
The areas of the brain that contribute to the sense of thirst are mainly located in the midbrain and the hindbrain. Specifically, the hypothalamus appears to play a key role in the regulation of thirst.
The area postrema and nucleus tractus solitarii signal to the subfornical organ and to the lateral parabrachial nucleus. The latter signaling relies on the neurotransmitter serotonin. The signal from the lateral parabrachial nucleus is relayed to the median preoptic nucleus.
The median preoptic nucleus and the subfornical organ receive signals of decreased volume and increased osmolite concentration. Finally, the signals are received in cortex areas of the forebrain where thirst arises. The subfornical organ and the organum vasculosum of the lamina terminalis contribute to regulating the overall bodily fluid balance by signalling to the hypothalamus to form vasopressin, which is later released by the pituitary gland.
Additional Information:
Dehydration
Dehydration is loss of water from the body; it is almost invariably associated with some loss of salt (sodium chloride) as well. The treatment of any form of dehydration, therefore, requires not only the replacement of the water lost from the body but also the restoration of the normal concentration of salt within the body fluid.
Causes
Dehydration may be caused by restricted water intake, excessive water loss, or both. The most common cause of dehydration is failure to drink liquids. The deprivation of water is far more serious than the deprivation of food. The average person loses approximately 2.5 percent of total body water per day (about 1,200 millilitres [1.25 quarts]) in urine, in expired air, by insensible perspiration, and from the gastrointestinal tract. If, in addition to this loss, the loss through perspiration is greatly increased—as is demonstrated in the case of the shipwrecked sailor in tropical seas or the traveler lost in the desert—dehydration may result in shock and death within only a few hours. When swallowing is difficult in extremely ill persons, or when people cannot respond to a sense of thirst because of age or illness or dulling of consciousness, the failure to compensate for the daily loss of body water will result rapidly in dehydration and its consequences. Large volumes of water also may be lost from the body by vomiting or diarrhea.
Symptoms and progression
The symptoms of dehydration depend in part on the cause and in part on whether there is associated salt deprivation as well. When loss of water is disproportionately greater than loss of electrolytes (salt), the osmotic pressure of the extracellular fluids becomes higher than in the cells. Since water passes from a region of lower to a region of higher osmotic pressure, water flows out of the cells into the extracellular fluid, tending to lower its osmotic pressure and increase its volume toward normal. As a result of the flow of water out of the cells, they become dehydrated. This results in the thirst that always accompanies “pure” water depletion.
In those diseases in which there is loss of salt in excess of water loss, the decreased concentration of sodium in the extracellular fluid and in the blood serum results in decreased osmotic pressure, and water therefore enters the cells to equalize the osmotic pressure. Thus there is extracellular dehydration and intercellular hydration—and no thirst.
Water deprivation produces distinctive symptoms in humans. Weight loss, amounting to two to three pounds per day, occurs. Thirst is the most prominent symptom, with the dryness of mouth, decreased production of saliva, and impaired swallowing that accompany it. It is probable that thirst is the result of this subsequent intracellular dehydration and increased intracellular osmotic pressure. Experimentally, thirst can be produced when the cells have lost about 1 percent of their intracellular water.
As dehydration progresses, the tissues tend to shrink, the skin becomes dry and wrinkled, and the eyes become sunken and the eyeballs soft. Fever develops, possibly from mild to marked, as dehydration progresses. Dehydration itself probably affects the temperature regulatory centres in the brain. As dehydration and salt loss progress, however, the plasma volume and heart output decrease, with a consequent decrease in blood supply to the skin. Sweating decreases and may stop completely, and the main avenue for heat loss is closed. The body temperature may then rise precipitously.
There are marked changes in the volume of the extracellular and intracellular fluids, but the blood plasma volume changes the last and the least. The plasma volume is maintained more or less constant at the expense of the tissue fluids. If, however, the plasma volume does fall, the output of the heart also falls, and the pulse rate climbs, all of which indicates a dangerous physical state.
The renal (kidney) changes that occur in humans during prolonged water depletion similarly tend to maintain a normal balance. If water deprivation continues and the plasma volume falls, however, the output of urine will be drastically reduced. As long as urine output of more than 30 millilitres (1 ounce) per hour is maintained, the kidney can excrete nitrogenous and nonnitrogenous solids with maximum efficiency. Once the urine flow is decreased below this level, the kidney is unable to function efficiently, the substances are retained in the body, and their concentration in the blood rises.
The final result of prolonged dehydration is now apparent. The normal distribution of salt and water in the body is destroyed, the plasma volume decreases, and the blood viscosity increases. As a result of these changes renal function is impaired, the urinary output falls, and waste products accumulate. Far more life-threatening, however, is decreased loss of moisture from the skin, with the subsequent rise in temperature, and the fall in cardiac output with the attendant irreversible shock.
Once renal failure occurs, about 8 percent of the total body water has been lost (4 litres [about 4.25 quarts]). When 5 to 10 litres (about 5.25 to 10.5 quarts) of body water have been lost, a person is acutely and severely ill, with contracted plasma volume, increased concentration and viscosity of the blood, renal failure and excessive urea in the blood, and falling blood pressure. In a previously healthy adult, death follows the loss of 12 to 15 litres (about 12.5 to 15.8 quarts) of body water. In the very young, the very old, or the debilitated, death occurs at a lower level of dehydration.
Treatment
The treatment of any form of dehydration depends not only on restoring the depleted water but also on reestablishing normal levels of body electrolytes and limiting the production of nitrogenous waste products. Before any of these therapeutic measures can be applied, however, the initiating cause must be removed. The sailor or the desert traveler must be rescued, the vomiting infant must be cured, or the underlying disease must be treated. Then, after accurate biochemical determinations of the levels of various electrolytes and other blood components have been made and the plasma volume has been measured, the physician may give measured quantities of the appropriate mixtures of salt and water. Given the right amounts of salt and water, the human body will gradually restore the normal relationships between the cells, the extracellular fluid, and the plasma volume. That done, the complicated functions of the kidney will clear the circulating blood of the retained waste products, and the body will have restored its own normal balance.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2072 2024-02-26 00:02:31
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,725
Re: Miscellany
2074) Sales
Gist
Sales is a term used to describe the activities that lead to the selling of goods or services. Businesses have sales organizations that are broken up into different teams. And these sales teams are often determined based on the region they're selling to, the product or service they're selling, and the target customer.
Summary
The definition of sales is a set of activities a business does to help customers buy their product. These actions vary from company to company but often include:
* Prospecting and generating new leads
* Developing buyer personas (with marketing)
* Qualifying leads
* Conducting product demonstrations
* Building personal connections with clients (relationship selling)
* Writing product proposals
* Negotiating prices and contract terms
* Completing orders and transactions
* Upselling and cross-selling
* Handing customers off to customer success teams
Sales also refers to the team of people responsible for sales activities. The ultimate goal of sales teams is to generate revenue and drive the growth of a business.
Companies don’t always refer to sales as “sales” and you may hear it referred to as different things. Another word for sales is “commerce”, while “selling” can also be used. Additionally, you can use a term like “transactions”, “trade”, or “retail” as another word for sales.
Most companies follow a specific methodology or trusted sales process. A sales plan ensures sales reps stay focused on nurturing leads down the buying journey and don’t waste resources on needless tasks.
Details:
What Is a Sale?
A sale is a transaction between two or more parties that involves the exchange of tangible or intangible goods, services, or assets for money. In some cases, assets other than cash are paid to a seller.
In the financial markets, a sale can also refer to an agreement that a buyer and seller make regarding a financial security, its price, and specific arrangements for its delivery.
Regardless of the context, a sale is essentially a contract between a seller of a particular good or service and a buyer who is willing to pay for that good or service.
KEY TAKEAWAYS
A sale is a transaction between two or more parties in which goods or services are exchanged for money or other assets.
In the financial markets, a sale is an agreement between a buyer and seller involving the price of a security and its delivery for agreed-upon compensation.
An item or service transferred by one party to another without an exchange of payment is not considered to be a sale, but rather a gift or a donation.
Sales occur 24 hours a day, across the globe, in all kinds of industries and are a major part of the essential structure of commerce.
A sale can also refer to the reduction in the price of a product or service in an attempt to attract buyers.
How a Sale Works
A sale occurs when a seller of goods or services transfers ownership of, and title to, a good or service to a buyer in exchange for a specific amount of money or other specified assets. To complete a sale, both the buyer and the seller must agree to the specific terms of the transaction. These terms can include the price of the good to be sold, the quantity of the good, the method of delivery, and time of delivery.
Importantly, the good or service that is being offered must be available to exchange. The seller must have the legal authority to transfer the item or service to the buyer.
If one party transfers a good or service to another without receiving compensation in return, the transaction is more likely to be treated as a gift or a donation, particularly from an income tax perspective.
Every day, millions of people take part in countless sales transactions across the globe. This creates a constant flow of assets and forms the backbone of the world's economies.
Types of Sales
The sale of goods and services within a retail market is a common form of sales transaction. This type of sale might involve a neighborhood business such as a grocery store or a laundromat. It may take place at a big box store or movie theater.
Sales of investment products by financial services institutions are considered to involve more complex exchanges of value. They can take place online or at a bricks-and-mortar location.
A sale can also occur between individuals. For instance, at a yard sale.
Another example of a more complicated sales transaction involving items of great value is the purchase of a vehicle from a car dealership.
Sales can also be transacted between businesses. For example, a raw materials provider might sell available materials to a business that uses those raw materials to produce consumer goods. To complete a sale, both the buyer and seller must be deemed competent. The good or service in question must be legally available to buy and the seller must have the authority to transfer the item to the buyer. Both parties must agree on the terms of the sale.
Ways to Pay
When people ask the question "What is a sale?" their inquiry may involve the ways to pay. In general, there are three main ways to make the payment of money required in a sales transaction.
An individual or business can take actual cash from a buyer at the time of a sales transaction. They then provide the product or service to the customer.
Buyers may be able to pay on credit. That means the buyer pays sometime after the sale is made. Usually in such cases, the product is still presented to the buyer at the time of the transaction.
Buyers may pay for a good or service in advance, before they've received the actual product or service. A magazine subscription is an example of paying in advance for a product or service.
Additional Information
Sales are activities related to selling or the number of goods sold in a given targeted time period. The delivery of a service for a cost is also considered a sale. A period during which goods are sold for a reduced price may also be referred to as a "sale".
The seller, or the provider of the goods or services, completes a sale in an interaction with a buyer, which may occur at the point of sale or in response to a purchase order from a customer. There is a passing of title (property or ownership) of the item, and the settlement of a price, in which agreement is reached on a price for which transfer of ownership of the item will occur. The seller, not the purchaser, typically executes the sale and it may be completed prior to the obligation of payment. In the case of indirect interaction, a person who sells goods or service on behalf of the owner is known as a salesman or saleswoman or salesperson, but this often refers to someone selling goods in a store/shop, in which case other terms are also common, including salesclerk, shop assistant, and retail clerk.
In common law countries, sales are governed generally by the common law and commercial codes. In the United States, the laws governing sales of goods are mostly uniform to the extent that most jurisdictions have adopted Article 2 of the Uniform Commercial Code, albeit with some non-uniform variations.
Definition
A person or organization expressing an interest in acquiring the offered item of value is referred to as a potential buyer, prospective customer, or prospect. Buying and selling are understood to be two sides of the same "coin" or transaction. Both seller and buyer engage in a process of negotiation to consummate the exchange of values. The exchange, or selling, process has implied rules and identifiable stages. It is implied that the selling process will proceed fairly and ethically so that the parties end up nearly equally rewarded. The stages of selling, and buying, involve getting acquainted, assessing each party's need for the other's item of value, and determining if the values to be exchanged are equivalent or nearly so, or, in buyer's terms, "worth the price". Sometimes, sellers have to use their own experiences when selling products with appropriate discounts.
Although the skills required are different, from a management viewpoint, sales is a part of marketing. Sales often form a separate grouping in a corporate structure, employing separate specialist operatives known as salespersons (singular: salesperson). Selling is considered by many to be a sort of persuading "art". Contrary to popular belief, the methodological approach of selling refers to a systematic process of repetitive and measurable milestones, by which a salesman relates his or her offering of a product or service in return enabling the buyer to achieve their goal in an economic way.
While the sales process refers to a systematic process of repetitive and measurable milestones, the definition of the selling is somewhat ambiguous due to the close nature of advertising, promotion, public relations, and direct marketing.
Selling is the profession-wide term, much like marketing defines a profession. Recently, attempts have been made to clearly understand who is in the sales profession, and who is not. There are many articles looking at marketing, advertising, promotions, and even public relations as ways to create a unique transaction.
Many believe that the focus of selling is on the human agents involved in the exchange between buyer and seller. Effective selling also requires a systems approach, at minimum involving roles that sell, enable selling, and develop sales capabilities. Selling also involves salespeople who possess a specific set of sales skills and the knowledge required to facilitate the exchange of value between buyers and sellers that is unique from marketing and advertising.
Within these three tenets, the following definition of professional selling is offered by the American Society for Training and Development (ASTD):
The holistic business system required to effectively develop, manage, enable, and execute a mutually beneficial, interpersonal exchange of goods or services for equitable value.
Team selling is one way to influence sales. Team selling is "a group of people representing the sales department and other functional areas in the firm, such as finance, production, and research and development". (Spiro) Team selling came about in the 1990s through total quality management (TQM). TQM occurs when companies work to improve their customer satisfaction by constantly improving all their operations.
Relationships with marketing
Marketing and sales differ greatly, but they generally have the same goal. Selling is the final stage in marketing which puts the plan into effect. A marketing plan includes pricing, promotion, place, and product (the 4 P's). A marketing department in an organization has the goals of increasing the desirability and value of the products and services to the customer and increasing the number and engagement of successful interactions between potential customers and the organization. Achieving this goal may involve the sales team using promotional techniques such as advertising, sales promotion, publicity, and public relations, creating new sales channels, or creating new products. It can also include encouraging the potential customer to visit the organization's website, contact the organization for more information, or interact with the organization via social media channels such as Twitter, Facebook and blogs. Social values play a major role in consumer decision processes. Marketing is the whole of the work on persuasion made for the whole of the target people. Sales is the process of persuasion and effort from one person to one person (B2C), or one person to a corporation (B2B), in order to make a living resource enter the company. This may occur in person, over the phone or digitally.
The field of sales process engineering views "sales" as the output of a larger system, not just as the output of one department. The larger system includes many functional areas within an organization. From this perspective, the labels "sales" and "marketing" cover several processes whose inputs and outputs supply one another. In this context, improving an "output" (such as sales) involves studying and improving the broader sales process, since the component functional areas interact and are interdependent.
Many large corporations structure their marketing departments, so they are integrated with all areas of the business. They create multiple teams with a singular focus, and the managers of these teams must coordinate efforts to drive profits and business success. For example, an "inbound" campaign seeks to drive more customers "through the door", giving the sales department a better chance of selling their product to the consumer. A good marketing program would address any potential downsides as well.
The sales department would aim to improve the interaction between the customer and the sales channel or salesperson. As sales is the forefront of any organization, this would always need to take place before any other business process may begin. Sales management involves breaking down the selling process and increasing the effectiveness of the discrete processes, as well as improving the interactions between processes. For example, in an outbound sales environment, the typical process includes outbound calling, the sales pitch, handling objections, opportunity identification, and the close. Each step of the process has sales-related issues, skills, and training needs, as well as marketing solutions to improve each discrete step.
One further common complication of marketing is the difficulty in measuring results for some marketing initiatives. Some marketing and advertising executives focus on creativity and innovation without concern for the top or bottom lines – a fundamental pitfall of marketing for marketing's sake.
Many companies find it challenging to get their marketing and sales teams to agree. The two departments, although different in nature, handle very similar concepts and have to work together to achieve the business's goals. Building a good relationship between the two teams that encourages communication can be the key to success.
Industrial marketing
The idea that marketing can potentially eliminate the need for salespeople depends entirely on context. For example, this may be possible in some B2C situations; however, for many B2B transactions (for example, those involving industrial organizations) this is mostly impossible. Another dimension is the value of the goods being sold. Fast-moving consumer-goods (FMCG) require no salespeople at the point of sale to get them to jump off the supermarket shelf and into the customer's trolley. However, the purchase of large mining equipment worth millions of dollars will require a salesperson to manage the sales process – particularly in the face of competitors. Small and medium businesses selling such large ticket items to a geographically dispersed client base use manufacturers' representatives to provide this highly personal service while avoiding the large expense of a captive sales force.
Sales and marketing alignment and integration
Another area of discussion involves the need for alignment and integration of corporate sales and marketing functions. According to a report from the Chief Marketing Officer (CMO) Council, only 40 percent of companies have formal programs, systems or processes in place to align and integrate the two critical functions.
Sales, Digital Marketing and Automated Marketing campaigns. With the increase of the use of the internet today, sales functions of several enterprises are finding traditional methods of marketing quite old fashioned and less efficient. So the use of automated Marketing Applications is on the rise ranging from Customer Relationship Management (CRM) to sales force management.
Traditionally, these two functions, as referred above, have operated separately, left in siloed areas of tactical responsibility. Glen Petersen's book The Profit Maximization Paradox sees the changes in the competitive landscape between the 1950s and the time of writing as so dramatic that the complexity of choice, price, and opportunities for the customer forced this seemingly simple and integrated relationship between sales and marketing to change forever. Petersen goes on to highlight that salespeople spend approximately 40 percent of their time preparing customer-facing deliverables while leveraging less than 50 percent of the materials created by marketing, adding to perceptions that marketing is out of touch with the customer and that sales is resistant to messaging and strategy.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2073 2024-02-27 00:08:39
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,725
Re: Miscellany
2075) Purchase.
Gist
When you purchase a pair of shoes, you buy them. If you want to gain purchase, or favor, with new friends, you might tell them about your recent purchase of chocolate, and offer to share.
Purchase can refer to the act of buying or the thing you bought. In 1803, the United States paid France approximately $15,000,000 for 800,000 acres of land which was called Louisiana, in a transaction known as the Louisiana Purchase. In this transaction, the purchase nearly doubled the size of the U.S. — the territory purchased comprises about 23 percent of current U.S. land.
Summary
Definition: A purchase means to take possession of a given asset, property, item or right by paying a predetermined amount of money for the transaction to be completed successfully. In other words, its’ an exchange of money for a particular good or service.
What is the definition of purchase? A purchase is a routinely operation carried by both individuals and corporations. The purpose of this financial transaction is to transfer the ownership of a piece of property physical, intellectual, virtual or else. By purchasing the property, the owner has the right to use it or dispose of it according to his will and purpose.
The purchase activity is normally a formal procedure when it comes to company purchases. Smaller purchases are more commonplace than larger purchases. As such, they require less analysis and thought.
Purchases can be made in cash or credit. Both typically transfer ownership when the transaction is initiated even though the latter doesn’t pay cash for good or service until some date in the future.
Let’s look at an example.
Example
Inspiration Clothing Co. is a company that sells apparel for women. The company business model is fairly straightforward; they have a supplier based in Los Angeles, called Wholesalers, Inc., that provides all the merchandise for Inspiration. The company has a purchasing department that deals with Wholesalers, Inc. and places purchase orders to keep the company stores fully inventoried. They have a pre-negotiated credit period of 15 business days after the order is received. When is this purchase actually made?
According to our concept, a purchase is a financial operation where goods and services are exchanged for consideration. In this example, there are two crucial moments, the moment in which a purchase order is issued by Inspiration and the moment in which that order will be paid for. In this case, Inspiration takes ownership of the goods and gives Wholesalers consideration in the form of a receivable or IOU. Thus, the transaction actually takes place when Inspiration receives the goods.
Keep in mind that this can vary depending on the credit and shipping terms.
Details:
What Is Buy?
Buy is a term used to describe the purchase or acquisition of an item or service that's typically paid for via an exchange of money or another asset. When buyers look to acquire something of value, they assign a monetary value to that product or service.
KEY TAKEAWAYS
* Buy is a term used to describe the purchase or acquisition of an item or service that's paid for via an exchange of money or another asset.
* Buyers assign a monetary value to the product or service they're looking to buy, which can be at a premium or discount to its original value.
* Consumer buying includes consumer goods such as food, while business purchases include buying equipment and inventory.
* A buy rating is an investment analyst’s recommendation to buy a security and implies the stock or security is undervalued.
Understanding Buy
A buy may be associated with small purchases, such as purchasing clothing at a retail store or a corporation buying a new manufacturing facility. Also, there are various types of buying examples in the financial markets, including the purchase of real estate and equities.
Although a buyer may assign a value to what they want to acquire, that value is a perceived value. In other words, the value to which a buyer assigns is relative and can vary between other interested parties.
In some cases, a buyer may purchase an item at a premium, meaning the price paid is above its original value. However, some items are also purchased at a discount, meaning the buyer's perceived value of the item is less than its original estimated value.
For example, a buyer may purchase an old, classic automobile, which is considered rare, resulting in a premium paid for the car. Conversely, a buyer may offer less money than the car's estimated value if the car was in poor condition.
Consumer Buying
Consumer buying often involves purchasing consumer goods, which are finished goods purchased at retail stores or online. The types of goods that consumers buy can include the following:
* Food
* Clothing
* Jewelry
* Furniture
* Electronics
* Books and magazines
* Personal hygiene products
* Household cleaning products
* Tools and other outdoor equipment
Consumer buying behavior—often called consumer spending—can be split into categories. Consumer goods can be either durable or non-durable goods. Durable goods typically have a life span of more than three years and include appliances and automobiles. Non-durable goods are typically consumed or used immediately. Examples of non-durables include food and clothing.
Consumer buying behavior can also be broken down into whether the purchases are for need or want expenses. Need-based purchases are called consumer staples and include food, paper towels, toilet paper, and other products that are needed on a day-to-day basis.
Want-based purchases are considered non-essential and categorized as consumer discretionary expenses. Examples of consumer discretionary spending include the purchase of an iPhone or a vacation. Consumer buying also includes the purchase of services, such as a tax preparer or a haircut.
Business Buying
Businesses and corporations also buy goods and services, which can be categorized as long-term or short-term purchases.
Capital expenditures (CAPEX) — involve the purchase of large items that typically benefit the long-term financial health of a company. Capital expenditures can show how well a company is investing in its business, which can help generate revenue and profit in the future. These might include the purchase of:
* Equipment and machinery
* Property, buildings, and land
* Vehicles such as cars and trucks
* Technology including computer equipment and software
Operating Expenses — Businesses also buy goods and services to help their day-to-day business operations function. These purchases are often called cost of goods sold (COGS) or operating expenses (OPEX) and can include the following:
* Inventory
* Supplies
* Marketing services
* Insurance products and services
How much a business is spending and where that money is being allocated are important metrics for investors and creditors to monitor. For example, if a company has purchased an excess of inventory or supplies, it could mean that its sales are lower than expected. Excess inventory purchases could also mean that the company's management team has not managed their inventory supplies effectively.
Types of Buys
Below are a few common scenarios in which the term buy is used in the financial marketplace.
* Buying a Stock Investment: Stock purchases are when investors buy ownership of the shares of a company. The investor's purchase price is called the cost basis. The goal is to sell the stock at a higher price and realize a profit. A buy order is an instruction to a stockbroker to buy a security. Many investors buy stocks through their retirement plan via a 401k plan. Oftentimes the employee pre-selects investment allocations based on their investment selection. When money is deducted from their paycheck for their 401k contribution, the money flows into their brokerage account, and a buy order is created based on their pre-selected investment schedule.
* Buy Rating: A buy rating, also known as a strong buy, is an investment analyst’s recommendation to buy a stock or security. Analysts make recommendations based on a rating scale that includes buy, outperform, hold, underperform, and sell. However, there is some subjectivity with the different stock rating scales. It's important that investors understand what each recommendation really means for that particular analyst. For example, outperform can mean moderate buy, accumulate, overweight, and add. When equity and bond analysts change their rating on a security, it will be upgraded if there is a positive change or downgraded if there is a negative change.
* Buying a Home: The purchase of a home is usually the single largest purchase an individual, family, or couple can make in their lifetime. Homebuying is primarily financed through a mortgage lender or a bank. The financial institution lends the money to the buyer to purchase the home. In return for giving the buyer a mortgage loan, the bank is paid back the original amount—called principal—and interest based on an interest rate that can be fixed or variable. The buyer usually has a number of years to pay back the mortgage loan, such as 15 or 30 years
Trade on the Go. Anywhere, Anytime
One of the world's largest crypto-asset exchanges is ready for you. Enjoy competitive fees and dedicated customer support while trading securely. You'll also have access to Binance tools that make it easier than ever to view your trade history, manage auto-investments, view price charts, and make conversions with zero fees. Make an account for free and join millions of traders and investors on the global crypto market.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2074 2024-02-28 00:10:56
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,725
Re: Miscellany
2076) Autopsy
Gist
An autopsy, also known as a post-mortem examination, is a specialized surgical procedure used to determine the cause and manner of death. The cause of death is the medical reason explaining why a patient passed.
Summary
An autopsy (also referred to as post-mortem examination, obduction, necropsy, or autopsia cadaverum) is a surgical procedure that consists of a thorough examination of a corpse by dissection to determine the cause, mode, and manner of death; or the exam may be performed to evaluate any disease or injury that may be present for research or educational purposes. The term necropsy is generally used for non-human animals.
Autopsies are usually performed by a specialized medical doctor called a pathologist. Only a small portion of deaths require an autopsy to be performed, under certain circumstances. In most cases, a medical examiner or coroner can determine the cause of death.
Purposes of performance
Autopsies are performed for either legal or medical purposes. Autopsies can be performed when any of the following information is desired:
* Manner of death must be determined
** Determine if death was natural or unnatural
** Injury source and extent on the corpse
* Post mortem interval
* Determining the deceased's identity
* Retain relevant organs
* If it is an infant, determine live birth and viability
For example, a forensic autopsy is carried out when the cause of death may be a criminal matter, while a clinical or academic autopsy is performed to find the medical cause of death and is used in cases of unknown or uncertain death, or for research purposes. Autopsies can be further classified into cases where an external examination suffices, and those where the body is dissected and an internal examination is conducted. Permission from next of kin may be required for internal autopsy in some cases. Once an internal autopsy is complete, the body is reconstituted by sewing it back together.
Etymology:
Autopsy
The term "autopsy" derives from the Ancient Greek autopsia, "to see for oneself", derived from (autos, "oneself") and (opsis, "sight, view"). The word has been in use since around the 17th century.
Post-mortem
The term "post-mortem" derives from the Latin post, 'after', and mortem, 'death'. It was first recorded in 1734.
Necropsy
The term "necropsy" is derived from the Greek 'death' and (opsis, 'sight, view').
Purpose
The principal aims of an autopsy are to determine the cause of death, mode of death, manner of death, the state of health of the person before he or she died, and whether any medical diagnosis and treatment before death were appropriate. In most Western countries the number of autopsies performed in hospitals has been decreasing every year since 1955. Critics, including pathologist and former JAMA editor George D. Lundberg, have charged that the reduction in autopsies is negatively affecting the care delivered in hospitals, because when mistakes result in death, they are often not investigated and lessons, therefore, remain unlearned. When a person has permitted an autopsy in advance of their death, autopsies may also be carried out for the purposes of teaching or medical research. An autopsy is usually performed in cases of sudden death, where a doctor is not able to write a death certificate, or when death is believed to result from an unnatural cause. These examinations are performed under a legal authority (medical examiner, coroner, or procurator fiscal) and do not require the consent of relatives of the deceased. The most extreme example is the examination of murder victims, especially when medical examiners are looking for signs of death or the murder method, such as bullet wounds and exit points, signs of strangulation, or traces of poison. Some religions including Judaism and Islam usually discourage the performing of autopsies on their adherents. Organizations such as ZAKA in Israel and Misaskim in the United States generally guide families on how to ensure that an unnecessary autopsy is not made. Autopsies are used in clinical medicine to identify a medical error or a previously unnoticed condition that may endanger the living, such as infectious diseases or exposure to hazardous materials. A study that focused on myocardial infarction (heart attack) as a cause of death found significant errors of omission and commission, i.e. a sizable number of cases ascribed to myocardial infarctions (MIs) were not MIs and a significant number of non-MIs were MIs.
A systematic review of studies of the autopsy calculated that in about 25% of autopsies, a major diagnostic error will be revealed. However, this rate has decreased over time and the study projects that in a contemporary US institution, 8.4% to 24.4% of autopsies will detect major diagnostic errors.
A large meta-analysis suggested that approximately one-third of death certificates are incorrect and that half of the autopsies performed produced findings that were not suspected before the person died. Also, it is thought that over one-fifth of unexpected findings can only be diagnosed histologically, i.e., by biopsy or autopsy, and that approximately one-quarter of unexpected findings, or 5% of all findings, are major and can similarly only be diagnosed from tissue.
One study found that (out of 694 diagnoses) "Autopsies revealed 171 missed diagnoses, including 21 cancers, 12 strokes, 11 myocardial infarctions, 10 pulmonary emboli, and 9 endocarditis, among others".
Focusing on intubated patients, one study found "abdominal pathologic conditions – abscesses, bowel perforations, or infarction – were as frequent as pulmonary emboli as a cause of class I errors. While patients with abdominal pathologic conditions generally complained of abdominal pain, results of an examination of the abdomen were considered unremarkable in most patients, and the symptom was not pursued".
Details
Autopsy is dissection and examination of a dead body and its organs and structures. An autopsy may be performed to determine the cause of death, to observe the effects of disease, and to establish the evolution and mechanisms of disease processes. The word autopsy is derived from the Greek autopsia, meaning “the act of seeing for oneself.”
History of autopsy
The early Egyptians did not study the dead human body for an explanation of disease and death, though some organs were removed for preservation. The Greeks and the Indians cremated their dead without examination; the Romans, Chinese, and Muslims all had taboos about opening the body; and human dissections were not permitted during the Middle Ages.
The first real dissections for the study of disease were carried out about 300 BCE by the Alexandrian physicians Herophilus and Erasistratus, but it was the Greek physician Galen of Pergamum in the late 2nd century CE who was the first to correlate the patient’s symptoms (complaints) and signs (what can be seen and felt) with what was found upon examining the “affected part of the deceased.” This was a significant advance that eventually led to the autopsy and broke an ancient barrier to progress in medicine.
In the 16th century Flemish physician Andreas Vesalius revolutionized the practice of medicine by providing accurate and detailed descriptions of the anatomy of the human body, which were based on his dissections of cadavers.
It was the rebirth of anatomy during the Renaissance, as exemplified by the work of Andreas Vesalius (De humani corporis fabrica, 1543) that made it possible to distinguish the abnormal, as such (e.g., an aneurysm), from the normal anatomy. Leonardo da Vinci dissected 30 corpses and noted “abnormal anatomy”; Michelangelo, too, performed a number of dissections. Earlier, in the 13th century, Frederick II ordered that the bodies of two executed criminals be delivered every two years to the medical schools, one of which was at Salerno, for an “Anatomica Publica,” which every physician was obliged to attend. The first forensic or legal autopsy, wherein the death was investigated to determine presence of “fault,” is said to have been one requested by a magistrate in Bologna in 1302. Antonio Benivieni, a 15th-century Florentine physician, carried out 15 autopsies explicitly to determine the “cause of death” and significantly correlated some of his findings with prior symptoms in the deceased. Théophile Bonet of Geneva (1620–89) collated from the literature the observations made in 3,000 autopsies. Many specific clinical and pathologic entities were then defined by various observers, thus opening the door to modern practice.
The autopsy came of age with Giovanni Morgagni, the father of modern pathology, who in 1761 described what could be seen in the body with the naked eye. In his voluminous work On the Seats and Causes of Diseases as Investigated by Anatomy, he compared the symptoms and observations in some 700 patients with the anatomical findings upon examining their bodies. Thus, in Morgagni’s work the study of the patient replaced the study of books and comparison of commentaries.
With Karl von Rokitansky of Vienna (1804–78), the gross (naked eye) autopsy reached its apogee. Rokitansky utilized the microscope very little and was limited by his own humoral theory. The French anatomist and physiologist Marie F.X. Bichat (1771–1802) stressed the role of the different generalized systems and tissues in the study of disease. It was the German pathologist Rudolf Virchow (1821–1902), however, who introduced the cellular doctrine—that changes in the cells are the basis of the understanding of disease—in pathology and in autopsy. He warned against the dominance of pathologic anatomy—the study of the structure of diseased tissue—alone as such and stressed that the future of pathology would be physiologic pathology—study of the functioning of the organism in the investigation of disease.
The modern autopsy has been expanded to include the application of all knowledge and all of the instruments of the specialized modern basic sciences. The examination has been extended to structures too small to be seen except with the electron microscope, and to molecular biology to include all that can be seen as well as what still remains unseen.
Procedure
The autopsy procedure itself has changed very little during the 20th century. The first step is a gross examination of the exterior for any abnormality or trauma and a careful description of the interior of the body and its organs. This is usually followed by further studies, including microscopic examination of cells and tissues.
The main incisions in the body remain the same. For the torso, a Y-shaped incision is made. Each upper limb of the “Y” extends from either the armpit or the outer shoulder and is carried beneath the breast to the bottom of the sternum, or breastbone, in the midline. From this point of juncture at the bottom of the sternum the incision is continued down to the lower abdomen where the groins meet in the genital area.
There are different schools as to procedure beyond this point. In one method, each organ is removed separately for incision and study. In the so-called en masse methods the chest organs are all removed in a single group and all of the abdominal organs in another for examination. The great vessels to the neck, head, and arms are ligated—tied off—and the organs removed as a unit for dissection. The neck organs are explored in situ only or removed from below. Dissection then proceeds usually from the back, except where findings dictate a variation in the procedure. Usually groups of organs are removed together so that disturbances in their functional relationships may be determined. After study of the brain in position, it is freed from its attachments and removed in toto. The spinal cord also can be removed.
The dissector proceeds to examine the external and cut surface of each organ, its vascular structures, including arteries, lymphatics, fascial or fibrous tissue, and nerves. Specimens are taken for culture, chemical analysis, and other studies. Immediately upon completion of the procedure, all of the organs are returned to the body and all incisions carefully sewn. After the body’s proper restoration, no unseemly evidence of the autopsy need remain.
After the gross examination of the body the findings are balanced one against another and a list of pathological findings is compiled; this list comprises the tentative or “provisional anatomical diagnoses.” Such diagnoses are grouped and arranged in the order of importance and of sequence. On occasion a quick microscopic study is done to confirm a diagnosis so as to assure its proper listing.
Autopsies document the disease processes that were in place at the time of the patient’s death, and most autopsies do not list an immediate or proximate cause of death. These factors are important in forensic cases, and they are often required in autopsy analysis even in situations when an autopsy itself is not required by law. After all studies—histological, chemical, toxicological, bacteriological, and viral—are completed, any errors of the provisional anatomical diagnoses are corrected and the final anatomical diagnoses and the final cause of death are listed. A statement of analysis of the autopsy that correlates the findings with the clinical picture, the “clinical pathological correlation,” concludes the record of the autopsy.
Forensic autopsy
The forensic pathologist goes beyond the mere cause of death; he must establish all the facts, both lethal and nonlethal, with any potential bearing whatsoever on the criminal or civil litigation. The cause of death is not automatically revealed when the body is opened; it is not an isolated tangible and delimited entity; it is a concept—an opinion—as to mechanism or happening and as such is subject occasionally to differences in interpretation. The legal autopsy requires meticulous detailed descriptions, measurements, and documentation.
The goal of forensic autopsies is to determine whether or not death was due to natural causes. Experience in the investigation of the scene of a death in medicolegal cases is important, for the evaluation of circumstances of death may be critical in establishing the mode of death—e.g., suicide. The autopsy may not be able, of itself, to determine intent, whereas the scene and the circumstances may provide unmistakable evidence. Photographic documentation is important in the medicolegal autopsy. The medicolegal postmortem examination must always be complete to rule out any other potential contributory cause of death and therefore must never be limited to a partial study. The identification of the deceased and of all specimens taken from the body is critical; the time of death and the blood grouping must, if possible, be established. In all autopsies, but especially in forensic cases, findings must be dictated to a stenographer or recording instrument during the actual performance of the procedure. The record often becomes legal evidence and therefore must be complete and accurate.
Purposes
The autopsy deals with the particular illness as evidenced in one individual and is more than simply a statistical average. Every autopsy is important to expose mistakes, to delimit new diseases and new patterns of disease, and to guide future studies. Morbidity and mortality statistics acquire accuracy and significance when based on careful autopsies; they also often give the first indication of contagion and epidemics. Nor can the role of the autopsy in medical education be understated. It is the focal point at which the profession learns to assess and to apply medical knowledge. Thus, the autopsy does more than merely determine the cause of death. While the medicolegal autopsy in particular has this important primary objective, most autopsies have a larger purpose.
Additional Information
An autopsy, or post mortem, is the medical examination of a body and the internal organs after a person has died. There are two types of autopsy – a coroner's autopsy and a hospital autopsy.
Coroner's autopsy
A coroner's autopsy is performed if the coroner or police need information for legal reasons about the cause of death – for example, if the person was murdered or their death was suspicious.
Hospital autopsy
A hospital (or non-coronial) autopsy may be performed if the immediate family give their consent. In this case, the autopsy can help to clarify the reasons why the person died, or offer information to the medical profession on the deceased person's condition. Some of the reasons for a hospital autopsy can include:
* In some cases, the cause of the person's fatal illness may be unknown or uncertain.
* An autopsy can help determine the success (or otherwise) of a treatment method.
* An autopsy can give family members information in the case of suspected genetic illness.
* Medical science can learn about disease processes, such as atherosclerosis or sudden infant death syndrome (SIDS), or the prevalence of particular diseases.
The right to refuse an autopsy
'Hospital' and 'coronial' post mortems have differing rules around refusal rights.
Hospital autopsy
The immediate family has the right to refuse or agree to a hospital autopsy of the deceased. They may also choose to consent to an autopsy, but limit the extent of the examination. They can also decide whether or not organs or samples taken from the body may be kept for further study. Make sure you discuss these issues with hospital staff.
Coronial autopsy
The senior next of kin may object to the carrying out of a coronial autopsy and the coroner must consider their request to reconsider if the request is made within 48 hours of the senior available next of kin receiving a notification from the coroner.
Where the coroner decides that an autopsy is still required, the matter may be appealed in the Supreme Court. But there is a limited time in which these objections to a coronial autopsy may be made. Provisions about objecting to a coronial autopsy are contained in s26 and s79 of the Coroners Act 2008.
The autopsy procedure
The autopsy is performed like a surgical operation. The steps may include:
* The autopsy is performed as soon as possible following the family's consent.
* It is performed by a specially qualified doctor, called a pathologist, who is assisted by a technician.
* The room in which the autopsy is performed is very similar to a hospital operating theatre.
* The body is laid out carefully on an examination table.
* The pathologist first looks at the body, noting its appearance.
* Photographs and x-rays may be taken.
* The pathologist makes a cut on the body from the collarbone to the lower abdomen to examine the chest and abdominal organs.
* Tiny tissue samples are taken from each organ for examination under a microscope and may also be sent for chemical analysis or microbiological culture.
* In most cases, the brain is examined. This requires cutting through the scalp and skull. The brain is a very fragile organ – to examine it carefully and properly may take up to three weeks.
* Some organs may need to be kept for up to six weeks so that further tests can be performed in the pathology department.
* After the autopsy, the organs are replaced and the skin is stitched (sutured) closed again as happens after any operation.
* The post mortem can take up to three hours.
Tissue samples from an autopsy
Tissue samples taken from the body are usually kept by the laboratory. The samples are kept in the hope that technological advances may one day answer any remaining questions about the cause of death or the nature of the disease. Keeping tissue samples requires the specific consent of the deceased's next of kin.
Organs that have been retained for further testing are returned to the family, disposed of by the hospital or kept for future medical research and training of medical staff, according to the family's wishes.
The funeral
Once the autopsy is complete, the body can be collected by the family's chosen funeral director. If some of the organs have been retained for further testing, the funeral may need to be delayed for a few days or weeks if the family wants the body to be whole before it is buried or cremated. In this case, the funeral director can arrange to embalm the body.
Autopsy results
A preliminary report is available within the first few days, but the full results of the autopsy are not usually available until around six to 12 weeks later. It may be best to arrange for the report to be sent to the family doctor, so that the next of kin can make an appointment to discuss the findings with their doctor. In other cases, the family could make an appointment with the pathologist.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2075 2024-02-29 00:02:18
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,725
Re: Miscellany
2077) Quality Control
Gist
Quality control refers to a company's methods for assessing product quality and, if necessary, improving it. There are various ways to perform quality control, including benchmarking, examining manufacturing procedures, and testing products. All of this is done to keep track of significant product differences.
Summary
Quality control (QC) is a process by which entities review the quality of all factors involved in production. ISO 9000 defines quality control as "a part of quality management focused on fulfilling quality requirements".
This approach places emphasis on three aspects (enshrined in standards such as ISO 9001):
* Elements such as controls, job management, defined and well managed processes, performance and integrity criteria, and identification of records
* Competence, such as knowledge, skills, experience, and qualifications
* Soft elements, such as personnel, integrity, confidence, organizational culture, motivation, team spirit, and quality relationships.
Inspection is a major component of quality control, where physical product is examined visually (or the end results of a service are analyzed). Product inspectors will be provided with lists and descriptions of unacceptable product defects such as cracks or surface blemishes for example.
History and introduction
Early stone tools such as anvils had no holes and were not designed as interchangeable parts. Mass production established processes for the creation of parts and system with identical dimensions and design, but these processes are not uniform and hence some customers were unsatisfied with the result. Quality control separates the act of testing products to uncover defects from the decision to allow or deny product release, which may be determined by fiscal constraints. For contract work, particularly work awarded by government agencies, quality control issues are among the top reasons for not renewing a contract.
The simplest form of quality control was a sketch of the desired item. If the sketch did not match the item, it was rejected, in a simple Go/no go procedure. However, manufacturers soon found it was difficult and costly to make parts be exactly like their depiction; hence around 1840 tolerance limits were introduced, wherein a design would function if its parts were measured to be within the limits. Quality was thus precisely defined using devices such as plug gauges and ring gauges. However, this did not address the problem of defective items; recycling or disposing of the waste adds to the cost of production, as does trying to reduce the defect rate. Various methods have been proposed to prioritize quality control issues and determine whether to leave them unaddressed or use quality assurance techniques to improve and stabilize production.
Details
In today’s world, it’s not uncommon that we take the reliability and quality of products and services for granted. At the start of the 20th century, however, quality control in manufacturing was not exactly a reliable process.
Now, decades after early pioneers created business problem-solving processes and analysis frameworks to determine and control consistency and value, it’s possible more than ever for a business to implement and scale best practices.
What Is Quality Control (QC)?
Quality does not have a singular definition. Despite the relative meaning of “value,” quality control is the process by which products/services are tested and measured to ensure they meet a standard. Through this process, a business can evaluate, maintain, and improve product quality. The primary objective of Quality Control is to identify and correct any deviations from the established quality standards. This process involves monitoring and inspecting products or services at various stages of production or delivery to ensure that they meet the desired level of quality. QC is also concerned with preventing defects or errors from occurring in the first place by implementing measures to control and improve the production or service delivery processes.
Ultimately, there are two crucial goals of quality control: (1) to ensure that products are as uniform as possible and (2), to minimize errors and inconsistencies within them.
Key Components of Quality Control
Key components of Quality Control may include:
* Inspection: Regularly examining products, materials, or services to identify defects, non-compliance, or deviations from quality standards.
* Testing: Conducting various tests and measurements to assess the performance, functionality, or characteristics of products or services.
* Statistical Process Control (SPC): Employing statistical techniques to monitor and control the production processes, ensuring that they remain within acceptable quality limits.
* Documentation and Records: Keeping detailed records of inspections, tests, and corrective actions taken to maintain traceability and accountability.
* Corrective Action: Implementing appropriate measures to address any identified quality issues and prevent their recurrence.
* Training and Education: Providing employees with the necessary skills and knowledge to maintain quality standards effectively.
* Continuous Improvement: Constantly analyzing data and feedback to identify areas for improvement and enhancing the overall quality management system.
Quality Control is closely related to another quality management concept called Quality Assurance (QA). While QC focuses on detecting and correcting defects, QA concentrates on preventing them from occurring in the first place by setting up robust processes and procedures.
Together, QC and QA form the backbone of an organization's quality management system, helping to ensure that products and services consistently meet or exceed customer expectations and regulatory requirements.
Quality Control Process
Normally, quality testing is part of every stage of a manufacturing or business process. Employees frequently begin testing using samples collected from the production line, finished products, and raw materials. Testing during various production phases can help identify the cause of a production problem and the necessary corrective actions to prevent it from happening again.
Customer service reviews, questionnaires, surveys, inspections, and audits are a few examples of quality testing procedures that can be used in non-manufacturing businesses. A company can use any procedure or technique to ensure that the final product or service is safe, compliant, and meets consumer demands.
QC Is Different by Industry
Quality Control (QC) is an indispensable aspect of various industries, ensuring that products and services adhere to predefined standards. In the manufacturing sector, QC involves rigorous inspection and testing of raw materials, intermediate components, and final products to maintain consistent quality and minimize defects. In the food industry, QC guarantees the safety and integrity of consumables through thorough testing for contaminants and adherence to health regulations. In the pharmaceutical sector, QC plays a critical role in verifying the potency and purity of drugs, ensuring they are safe for consumption. Additionally, in the software industry, QC involves extensive testing of applications and programs to identify bugs and errors before release, guaranteeing a smooth user experience. Across all industries, QC is a fundamental process that enhances customer satisfaction, boosts efficiency, and fosters a reputation for reliability.
Types of Quality Control
Just as quality is a relative word with many interpretations, quality control itself doesn’t have a uniform, universal process. Some methods depend on the industry. Take food and drug products, for instance, where errors can put people at risk and create significant liability. These industries may rely more heavily on scientific measures, whereas others (such as education or coaching) may require a more holistic, qualitative method.
At its core, quality control requires attention to detail and research methodology.
So, what is quality control? There are a wide range of quality control methods, including:
Control Charts:
A graph or chart is used to study how processes are changing over time. Using statistics, the business and manufacturing processes are analyzed for being “in control.”
Process Control:
Processes are monitored and adjusted to ensure quality and improve performance. This is typically a technical process using feedback loops, industrial-level controls, and chemical processes to achieve consistency.
Acceptance Sampling:
A statistical measure is used to determine if a batch or sample of products meets the overall manufacturing standard.
Process Protocol:
A mapping methodology that improves the design and implementation processes by creating evaluative indicators for each step.
There are other quality control factors to consider when selecting a method in addition to types of processes.
Some companies establish internal quality control divisions when defining what is quality control. They do this to monitor products and services, while others rely on external bodies to track products and performance. These controls may be largely dependent on the industry of the business. Due to the strict nature of food inspections, for example, it may be in a company’s best interest to sample products internally and verify these results in a third-party lab.
Why Is Quality Control Important? What Are the Benefits?
Quality Control (QC) is essential for various reasons, and its importance lies in the numerous benefits it brings to both businesses and consumers. Here are some key reasons why QC is crucial:
* Customer Satisfaction: QC ensures that products and services meet or exceed customer expectations, leading to higher satisfaction levels and increased customer loyalty.
* Defect Prevention: By identifying and correcting issues early in the production or service delivery process, QC helps prevent defects, reducing the likelihood of expensive recalls or rework.
* Cost Reduction: Implementing QC measures can lead to reduced waste, lower production costs, and improved operational efficiency, contributing to overall cost savings.
* Compliance and Regulations: QC ensures that products and services adhere to industry standards and regulatory requirements, avoiding legal issues and penalties.
* Brand Reputation: Consistent high-quality products or services build a positive brand image, enhancing the company's reputation and competitiveness in the market.
* Increased Efficiency: QC optimizes processes and identifies areas for improvement, leading to increased productivity and streamlined operations.
* Risk Mitigation: Through rigorous testing and inspections, QC helps identify potential risks and hazards, enabling businesses to address them proactively.
* Continuous Improvement: QC encourages a culture of continuous improvement, where organizations strive to enhance their products, services, and processes constantly.
* International Competitiveness: High-quality products can open doors to global markets, increasing a company's competitiveness on an international scale.
* Customer Retention and Loyalty: Satisfied customers are more likely to remain loyal and recommend the brand to others, contributing to long-term business success.
Overall, Quality Control is crucial for maintaining high standards, minimizing risks, and fostering a competitive advantage in today's dynamic and demanding business environment. It serves as the foundation for delivering superior products and services while ensuring customer satisfaction and loyalty.
Quality Control Roles and Responsibilities
When answering what is quality control, it is critical to understand that it consists of multifaceted responsibilities and roles. Moreover, it shouldn’t be confused with quality assurance. Whereas quality assurance looks at the processes used to prevent defects, quality control is focused specifically on the measurement and analysis processes involved with determining product quality.
Quality control uses specific research tools to accomplish fact-finding processes and conduct analyses. A quality control professional is tasked with analyzing these measurements against some sort of standard determined by the quality management department, company policies, and industries or regulatory bodies. Based on this evidence-gathering, quality control will recommend changes.
We can see from this roadmap, too, how quality assurance and quality control differ. Quality assurance looks at the holistic picture to prevent a product from becoming defective. Quality control, on the other hand, later determines if a product is, in fact, defective or not. Both roles fit under the broad umbrella of quality management.
Thus, an individual in quality control is tasked with communicating results to stakeholders and significant parties. A good quality control specialist will be able to disseminate scientific and research-based thinking to a business community and assist with the problem-solving process. These specialists are a key component of a product’s design process, as they determine whether a company’s creation is truly acceptable for the market.
Additional Information
Quality control (QC) is a procedure or set of procedures intended to ensure that a manufactured product or performed service adheres to a defined set of quality criteria or meets the requirements of the client or customer. QC is similar to, but not identical with, quality assurance (QA). While QA refers to the confirmation that specified requirements have been met by a product or service, QC refers to the actual inspection of these elements.
QA is sometimes expressed together with QC as a single expression: quality assurance and control (QA/QC).
The quality control procedure
In order to implement an effective QC program, an enterprise must first decide which specific standards the product or service must meet. Then the extent of QC actions must be determined -- for example, the percentage of units to be tested from each lot.
Next, real-world data must be collected -- such as the percentage of units that fail -- and the results reported to management personnel. After this, corrective action must be decided upon and taken. For example, defective units must be repaired or rejected, and poor service repeated at no charge until the customer is satisfied. If too many unit failures or instances of poor service occur, a plan must be devised to improve the production or service process; then that plan must be put into action.
Finally, the QC process must be ongoing to ensure that remedial efforts, if required, have produced satisfactory results and to immediately detect recurrences or new instances of trouble.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
