Math Is Fun Forum
You are not logged in.
- Topics: Active | Unanswered
- Index
- » This is Cool
- » Neutron
Pages: 1
#1 2024-02-19 16:48:29
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,817
Neutron
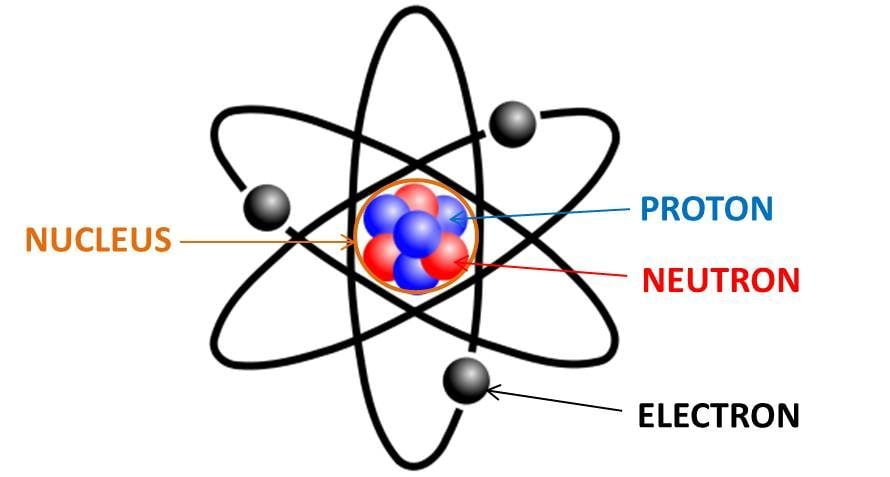
Neutron
Gist
Neutrons, along with protons, are subatomic particles found inside the nucleus of every atom. The only exception is hydrogen, where the nucleus contains only a single proton. Neutrons have a neutral electric charge (neither negative nor positive) and have slightly more mass than positively charged protons.
Summary
A Neutron is a neutral subatomic particle that, in conjunction with protons, makes up the nucleus of every atom except ordinary hydrogen (whose nucleus has one proton and no neutrons). Along with protons and electrons, it is one of the three basic particles making up atoms, the basic building blocks of all matter and chemistry.
The neutron has no electric charge and a rest mass equal to 1.67492749804 × {10}^{-27} kg—marginally greater than that of the proton but 1,838.68 times greater than that of the electron. Neutrons and protons, commonly called nucleons, are bound together in the dense inner core of an atom, the nucleus, where they account for 99.9 percent of the atom’s mass. Developments in high-energy particle physics in the 20th century revealed that neither the neutron nor the proton is a true elementary particle. Rather, they are composites of extremely small elementary particles called quarks. The neutron is composed of two down quarks, each with 1/3 elementary charge, and one up quark, with 2/3 elementary charge. The nucleus is bound together by the residual effect of the strong force, a fundamental interaction that governs the behaviour of the quarks that make up the individual protons and neutrons.
Neutron, neutral subatomic particle that, in conjunction with protons, makes up the nucleus of every atom except ordinary hydrogen (whose nucleus has one proton and no neutrons). Along with protons and electrons, it is one of the three basic particles making up atoms, the basic building blocks of all matter and chemistry.
A free neutron—one that is not incorporated into a nucleus—is subject to radioactive decay of a type called beta decay. It breaks down into a proton, an electron, and an antineutrino (the antimatter counterpart of the neutrino, a particle with no charge and little or no mass); the half-life for this decay process is 611 seconds. Because it readily disintegrates in this manner, the neutron does not exist in nature in its free state, except among other highly energetic particles in cosmic rays. Since free neutrons are electrically neutral, they pass unhindered through the electrical fields within atoms and so constitute a penetrating form of radiation, interacting with matter almost exclusively through relatively rare collisions with atomic nuclei.
Neutrons and protons are classified as hadrons, subatomic particles that are subject to the strong force. Hadrons, in turn, have been shown to possess internal structure in the form of quarks, fractionally charged subatomic particles that are thought to be among the fundamental components of matter. Like the proton and other baryon particles, the neutron consists of three quarks. In fact, the neutron possesses a magnetic dipole moment; i.e., it behaves like a minute magnet in ways that suggest that it is an entity of moving electric charges.
Details
The neutron is a subatomic particle, symbol n or n0, which has a neutral (not positive or negative) charge, and a mass slightly greater than that of a proton. Protons and neutrons constitute the nuclei of atoms. Since protons and neutrons behave similarly within the nucleus, they are both referred to as nucleons. Nucleons have a mass of approximately one atomic mass unit, or dalton, symbol Da. Their properties and interactions are described by nuclear physics. Protons and neutrons are not elementary particles; each is composed of three quarks.
The chemical properties of an atom are mostly determined by the configuration of electrons that orbit the atom's heavy nucleus. The electron configuration is determined by the charge of the nucleus, which is determined by the number of protons, or atomic number. The number of neutrons is the neutron number. Neutrons do not affect the electron configuration.
Atoms of a chemical element that differ only in neutron number are called isotopes. For example, carbon, with atomic number 6, has an abundant isotope carbon-12 with 6 neutrons and a rare isotope carbon-13 with 7 neutrons. Some elements occur in nature with only one stable isotope, such as fluorine. Other elements occur with many stable isotopes, such as tin with ten stable isotopes, or with no stable isotope, such as technetium.
The properties of an atomic nucleus depend on both atomic and neutron numbers. With their positive charge, the protons within the nucleus are repelled by the long-range electromagnetic force, but the much stronger, but short-range, nuclear force binds the nucleons closely together. Neutrons are required for the stability of nuclei, with the exception of the single-proton hydrogen nucleus. Neutrons are produced copiously in nuclear fission and fusion. They are a primary contributor to the nucleosynthesis of chemical elements within stars through fission, fusion, and neutron capture processes.
The neutron is essential to the production of nuclear power. In the decade after the neutron was discovered by James Chadwick in 1932, neutrons were used to induce many different types of nuclear transmutations. With the discovery of nuclear fission in 1938, it was quickly realized that, if a fission event produced neutrons, each of these neutrons might cause further fission events, in a cascade known as a nuclear chain reaction. These events and findings led to the first self-sustaining nuclear reactor (Chicago Pile-1, 1942) and the first nuclear weapon (Trinity, 1945).
Dedicated neutron sources like neutron generators, research reactors and spallation sources produce free neutrons for use in irradiation and in neutron scattering experiments. A free neutron spontaneously decays to a proton, an electron, and an antineutrino, with a mean lifetime of about 15 minutes. Free neutrons do not directly ionize atoms, but they do indirectly cause ionizing radiation, so they can be a biological hazard, depending on dose. A small natural "neutron background" flux of free neutrons exists on Earth, caused by cosmic ray showers, and by the natural radioactivity of spontaneously fissionable elements in the Earth's crust.
Neutrons in an atomic nucleus
An atomic nucleus is formed by a number of protons, Z (the atomic number), and a number of neutrons, N (the neutron number), bound together by the nuclear force. Protons and neutrons each have a mass of approximately one dalton. The atomic number determines the chemical properties of the atom, and the neutron number determines the isotope or nuclide. The terms isotope and nuclide are often used synonymously, but they refer to chemical and nuclear properties, respectively. Isotopes are nuclides with the same atomic number, but different neutron number. Nuclides with the same neutron number, but different atomic number, are called isotones. The atomic mass number, A, is equal to the sum of atomic and neutron numbers. Nuclides with the same atomic mass number, but different atomic and neutron numbers, are called isobars. The mass of a nucleus is always slightly less than the sum of its proton and neutron masses: the difference in mass represents the mass equivalent to nuclear binding energy, the energy which would need to be added to take the nucleus apart.
The nucleus of the most common isotope of the hydrogen atom (with the chemical symbol 1H) is a lone proton. The nuclei of the heavy hydrogen isotopes deuterium (D or 2H) and tritium (T or 3H) contain one proton bound to one and two neutrons, respectively. All other types of atomic nuclei are composed of two or more protons and various numbers of neutrons. The most common nuclide of the common chemical element lead, 208Pb, has 82 protons and 126 neutrons, for example.
Protons and neutrons behave almost identically under the influence of the nuclear force within the nucleus. They are therefore both referred to collectively as nucleons. The concept of isospin, in which the proton and neutron are viewed as two quantum states of the same particle, is used to model the interactions of nucleons by the nuclear or weak forces. Because of the strength of the nuclear force at short distances, the nuclear energy binding nucleons is more than seven orders of magnitude larger than the electromagnetic energy binding electrons in atoms. Nuclear reactions (such as nuclear fission) therefore have an energy density that is more than ten million times that of chemical reactions. Ultimately, the ability of the nuclear force to store energy arising from the electromagnetic repulsion of nuclear components is the basis for most of the energy that makes nuclear reactors or bombs possible. In nuclear fission, the absorption of a neutron by a heavy nuclide (e.g., uranium-235) causes the nuclide to become unstable and break into light nuclides and additional neutrons. The positively charged light nuclides then repel, releasing electromagnetic potential energy.
Additional Information
Neutrons are tiny subatomic particles that — along with protons — form the nucleus of an atom.
While the number of protons defines what element an atom is, the number of neutrons in the nucleus can vary, resulting in different isotopes of an element. For example, ordinary hydrogen contains one proton and no neutrons, but the isotopes of hydrogen, deuterium and tritium, have one and two neutrons, respectively, alongside the proton.
Neutrons are composite particles made up of three smaller, elementary particles called quarks, held together by the Strong Force. Specifically, a neutron contains one 'up' and two 'down' quarks. Particles made from three quarks are called baryons, and hence baryons contribute to all the baryonic 'visible' matter in the universe.
After Ernest Rutherford (with help from Ernest Marsden and Hans Geiger's gold-leaf experiment) had discovered in 1911 that atoms have a nucleus, and then nine years later discovered that atomic nuclei are made, at least in part, by protons, the discovery of the neutron in 1932 by James Chadwick naturally followed.
The idea that there must be something else in an atom's nucleus came from the fact that the number of protons didn't match an atom's atomic weight. For example, an oxygen atom contains 8 protons, but has an atomic weight of 16, suggesting that it contains 8 other particles. However, these mystery particles would have to be electrically neutral, since atoms normally have no overall electric charge (the negative charge of the electrons cancels out the positive charge of the protons).
At the time, various scientists were experimenting with alpha particles, which are another name for helium nuclei, bombarding a material made from the element beryllium with an alpha particle stream. When the alpha particles impacted beryllium atoms, they produced mysterious particles that appeared to originate from within the beryllium atoms. Chadwick took these experiments one step further and saw that when the mystery particles hit a target made of paraffin wax, they would knock loose protons at high energy. In order to do this, Chadwick reasoned, the mystery particles must have more or less the same mass as a proton. Chadwick proclaimed this mystery particle to be the neutron, and in 1935 he won a Nobel Prize for his discovery.
As their name suggests, neutrons are electrically neutral, so they have no charge. Their mass is 1.008 times the mass of the proton — in other words, it's approximately 0.1% heavier.
Neutrons don't like to exist on their own outside the nucleus. The binding energy of the Strong Force between them and protons in the nucleus keeps them stable, but when out on their own they undergo beta decay after about 15 minutes, transforming into a proton, an electron and an antineutrino.
Albert Einstein, in his famous equation E = mc^2, said that mass and energy are equivalent. Although the mass of a neutron and a proton are only slightly different, this slight difference means that a neutron has more mass, and therefore more energy, than a proton and an electron combined. That's why, when a neutron decays, it produces a proton and an electron.
An isotope is a variation of an element that has more neutrons. For instance, at the top of this article, we gave the example of the hydrogen isotopes deuterium and tritium, which have 1 and 2 extra neutrons, respectively. Some isotopes are stable, deuterium for instance. Others are unstable and inevitably undergo radioactive decay. Tritium is unstable — it has a half-life of about 12 years (a half-life is the time it takes on average for half of a given amount of an isotope like tritium to decay), but other isotopes decay far more rapidly, in a matter of minutes, second or even fractions of a second.
Neutrons are also essential tools in nuclear reactions, in particular when inducing a chain reaction. Neutrons absorbed by atomic nuclei create unstable isotopes that then undergo nuclear fission (splitting into two smaller daughter nuclei of other elements). For example, when uranium-235 absorbs an extra neutron, it becomes unstable and breaks apart, releasing energy in the process.
Neutrons are also instrumental in the creation of heavy elements in massive stars, through a mechanism known as the r-process, with "r" meaning "rapid". This process was first detailed in the famous, Nobel Prize-winning B2FH paper by Margaret and Geoffrey Burbidge, William Fowler and Fred Hoyle that described the origins of the elements through stellar nucleosynthesis — the forging of elements by stars.
Stars like the sun can produce elements of oxygen, nitrogen and carbon through nuclear fusion reactions. More massive stars can keep going and create shells of increasingly heavier elements all the way down to iron-56 in the star's core. At this point, the reactions require more energy to be put into them to fuse elements heavier than iron than what is actually produced by those reactions, so those reactions cease, energy production grinds to a halt and the core of the star collapses, instigating a supernova. And it's in the incredibly violent blast of a supernova that conditions can become extreme enough to liberate lots of free neutrons in a short space of time.
In the supernova blast, atomic nuclei are then able to sweep up all these free neutrons before they all decay (this is why it's described as rapid), to instigate r-process nucleosynthesis. Once the nuclei are full of neutrons they turn unstable and undergo beta decay, transforming those extra neutrons into protons. The addition of these protons changes the type of element that a nucleus is, hence it's a way of creating new, heavy elements such as gold, platinum and other precious metals. The gold in your jewelry was made billions of years ago by rapid neutron capture in a supernova!
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
Pages: 1
- Index
- » This is Cool
- » Neutron