Math Is Fun Forum
You are not logged in.
- Topics: Active | Unanswered
#1451 2024-02-20 17:31:09
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,394
Re: crème de la crème
1413) Hamilton O. Smith
Details
Hamilton Othanel Smith (born August 23, 1931) is an American microbiologist and Nobel laureate.
Smith graduated from University Laboratory High School of Urbana, Illinois. He attended the University of Illinois at Urbana-Champaign, but in 1950 transferred to the University of California, Berkeley, where he earned his B.A. in Mathematics in 1952 . He received his medical degree from Johns Hopkins School of Medicine in 1956. Between 1956 and 1957 Smith worked for the Washington University in St. Louis Medical Service. In 1975, he was awarded a Guggenheim Fellowship he spent at the University of Zurich.
In 1970, Smith and Kent W. Wilcox discovered the first type II restriction enzyme, which is now known as HindII. Smith went on to discover DNA methylases that constitute the other half of the bacterial host restriction and modification systems, as hypothesized by Werner Arber of Switzerland.
He was awarded the Nobel Prize in Physiology or Medicine in 1978 for discovering type II restriction enzymes with Werner Arber and Daniel Nathans as co-recipients.
He later became a leading figure in the nascent field of genomics, when in 1995 he and a team at The Institute for Genomic Research sequenced the first bacterial genome, that of Haemophilus influenzae. H. influenza was the same organism in which Smith had discovered restriction enzymes in the late 1960s. He subsequently played a key role in the sequencing of many of the early genomes at The Institute for Genomic Research, and in the assembly of the human genome at Celera Genomics, which he joined when it was founded in 1998.
More recently, he has directed a team at the J. Craig Venter Institute that works towards creating a partially synthetic bacterium, Mycoplasma laboratorium. In 2003 the same group synthetically assembled the genome of a virus, Phi X 174 bacteriophage. Smith is scientific director of privately held Synthetic Genomics, which was founded in 2005 by Craig Venter to continue this work. Synthetic Genomics is working to produce biofuels on an industrial-scale using recombinant algae and other microorganisms.
Additional Information
Hamilton O. Smith, (born August 23, 1931, New York, New York, U.S.), is an American microbiologist who shared, with Werner Arber and Daniel Nathans, the Nobel Prize for Physiology or Medicine in 1978 for his discovery of a new class of restriction enzymes that recognize specific sequences of nucleotides in a molecule of DNA (deoxyribonucleic acid) and cleave the molecule at that particular point.
Smith graduated from the University of California at Berkeley in 1952 and received a medical degree from Johns Hopkins University in 1956. After an internship and residency he joined the faculty of the University of Michigan in 1962. In 1967 he returned to Johns Hopkins, becoming professor of microbiology in 1973.
Arber and others had already studied restriction enzymes that recognize specific DNA sequences, but these type I enzymes cut the DNA at random places other than the recognition site. While studying the mechanism whereby the bacterium Haemophilus influenzae is able to take up DNA from the phage virus P22, Smith and his colleagues discovered the first of what came to be called type II restriction enzymes. These enzymes not only recognize a specific region in a DNA sequence but always cut the DNA at that very site. This predictable behaviour made type II restriction enzymes valuable tools in the study of DNA structure and in recombinant DNA technology.
In 1995, in collaboration with J. Craig Venter and researchers at The Institute for Genomics Research (TIGR), Smith sequenced the genome of H. influenzae using a rapid “shotgun” sequencing approach. In 1998 Smith left Johns Hopkins and joined the private research company Celera Genomics. At Celera Smith contributed to the genomic sequencing efforts for the fruit fly (Drosophila) and humans. In 2002 Smith became scientific director at the Institute for Biological Energy Alternatives (IBEA) in Maryland. He led research on the generation of a synthetic single-celled organism capable of surviving and reproducing on its own. A central goal of this research was to create a minimalist organism, using as few genes as possible, in order to determine how many and which genes are necessary to sustain life. In 2006 TIGR and IBEA were merged with several other centres to form the J. Craig Venter Institute, where Smith became leader of the synthetic biology and bioenergy research group.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1452 2024-02-22 21:04:43
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,394
Re: crème de la crème
1414) Sheldon Glashow
Summary
Sheldon Glashow (born December 5, 1932, New York, New York, U.S.) is an American theoretical physicist who, with Steven Weinberg and Abdus Salam, received the Nobel Prize for Physics in 1979 for their complementary efforts in formulating the electroweak theory, which explains the unity of electromagnetism and the weak force.
Glashow was the son of Jewish immigrants from Russia. He and Weinberg were members of the same classes at the Bronx High School of Science, New York City (1950), and Cornell University (1954). Glashow received a Ph.D. in physics from Harvard University in 1958. He joined the faculty of the University of California at Berkeley in 1962 but four years later returned to Harvard as a professor of physics, Eugene Higgins Professor of Physics from 1979 onward. He remained at Harvard until 2000, when he retired as professor emeritus. That year he became Arthur G.B. Metcalf Professor of Mathematics and Science at Boston University.
In the 1960s Weinberg and Salam had each independently devised a theory by which the weak nuclear force and the electromagnetic force could be conceived as manifestations of a single unified force called the electroweak force. Their theory could be applied only to leptons, however, a class of particles that includes electrons and neutrinos. Glashow found a way to extend their theory to other classes of elementary particles, notably baryons (e.g., protons and neutrons) and mesons. In doing so, Glashow had to invent a new property for quarks, which are the fundamental particles that constitute baryons and mesons. This new property, which Glashow called “charm,” provided a valuable extension of the theory of quarks.
Details
Sheldon Lee Glashow (born December 5, 1932) is a Nobel Prize-winning American theoretical physicist. He is the Metcalf Professor of Mathematics and Physics at Boston University and Eugene Higgins Professor of Physics, emeritus, at Harvard University, and is a member of the board of sponsors for the Bulletin of the Atomic Scientists.
Birth and education
Sheldon Glashow was born on December 5, 1932, in New York City, to Jewish immigrants from Russia, Bella (née Rubin) and Lewis Gluchovsky, a plumber. He graduated from Bronx High School of Science in 1950. Glashow was in the same graduating class as Steven Weinberg, whose own research, independent of Glashow's, would result in Glashow, Weinberg, and Abdus Salam sharing the 1979 Nobel Prize in Physics. Glashow received a Bachelor of Arts degree from Cornell University in 1954 and a PhD degree in physics from Harvard University in 1959 under Nobel-laureate physicist Julian Schwinger. Afterwards, Glashow became a NSF fellow at NORDITA and met Murray Gell-Mann, who convinced him to become a research fellow at the California Institute of Technology. Glashow then became an assistant professor at Stanford University before joining the University of California, Berkeley where he was an associate professor from 1962 to 1966. He joined the Harvard physics department as a professor in 1966, and was named Eugene Higgins Professor of Physics in 1979; he became emeritus in 2000. Glashow has been a visiting scientist at CERN, and professor at Aix-Marseille University, MIT, Brookhaven Laboratory, Texas A&M, the University of Houston, and Boston University.
Research
In 1961, Glashow extended electroweak unification models due to Schwinger by including a short range neutral current, the Z0. The resulting symmetry structure that Glashow proposed, SU(2) × U(1), forms the basis of the accepted theory of the electroweak interactions. For this discovery, Glashow along with Steven Weinberg and Abdus Salam, was awarded the 1979 Nobel Prize in Physics.
In collaboration with James Bjorken, Glashow was the first to predict a fourth quark, the charm quark, in 1964. This was at a time when 4 leptons had been discovered but only 3 quarks proposed. The development of their work in 1970, the GIM mechanism showed that the two quark pairs: (d.s), (u,c), would largely cancel out flavor changing neutral currents, which had been observed experimentally at far lower levels than theoretically predicted on the basis of 3 quarks only. The prediction of the charm quark also removed a technical disaster for any quantum field theory with unequal numbers of quarks and leptons — an anomaly — where classical field theory symmetries fail to carry over into the quantum theory.
In 1973, Glashow and Howard Georgi proposed the first grand unified theory. They discovered how to fit the gauge forces in the standard model into an SU(5) group, and the quarks and leptons into two simple representations. Their theory qualitatively predicted the general pattern of coupling constant running, with plausible assumptions, it gave rough mass ratio values between third generation leptons and quarks, and it was the first indication that the law of Baryon number is inexact, that the proton is unstable. This work was the foundation for all future unifying work.
Glashow shared the 1977 J. Robert Oppenheimer Memorial Prize with Feza Gürsey.
Criticism of superstring theory
Glashow is a skeptic of superstring theory due to its lack of experimentally testable predictions. He had campaigned to keep string theorists out of the Harvard physics department, though the campaign failed. About ten minutes into "String's the Thing", the second episode of The Elegant Universe TV series, he describes superstring theory as a discipline distinct from physics, saying "...you may call it a tumor, if you will...".
Personal life
Glashow is married to Joan Shirley Alexander. They have four children. Lynn Margulis was Joan's sister, making Carl Sagan his former brother-in-law. Daniel Kleitman, who was another doctoral student of Julian Schwinger, is also his brother-in-law, through Joan's other sister, Sharon.
In 2003, he was one of 22 Nobel Laureates who signed the Humanist Manifesto. Glashow has described himself as a "practising atheist" and a Democrat.
Glashow is one of the 20 American recipients of the Nobel Prize in Physics to sign a letter addressed to President George W. Bush in May 2008, urging him to "reverse the damage done to basic science research in the Fiscal Year 2008 Omnibus Appropriations Bill" by requesting additional emergency funding for the Department of Energy’s Office of Science, the National Science Foundation, and the National Institute of Standards and Technology.
Additional Information
Born: 5 December 1932, New York, NY, USA
Affiliation at the time of the award: Harvard University, Lyman Laboratory, Cambridge, MA, USA
Prize motivation: “for their contributions to the theory of the unified weak and electromagnetic interaction between elementary particles, including, inter alia, the prediction of the weak neutral current”
Prize share: 1/3
Work
According to modern physics, four fundamental forces exist in nature. Electromagnetic interaction is one of these. The weak interaction—responsible, for example, for the beta decay of nuclei—is another. Thanks to contributions made by Sheldon Glashow, Abdus Salam, and Steven Weinberg in 1968, these two interactions were unified to one single, called electroweak. The theory predicted, for example, that weak interaction manifests itself in “neutral weak currents” when certain elementary particles interact. This was later confirmed.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1453 2024-02-27 17:28:05
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,394
Re: crème de la crème
1415) Abdus Salam
Summary
Abdus Salam (born Jan. 29, 1926, Jhang Maghiāna, Punjab, India [now in Pakistan]—died Nov. 21, 1996, Oxford, Eng.) was a Pakistani nuclear physicist who was the corecipient with Steven Weinberg and Sheldon Lee Glashow of the 1979 Nobel Prize for Physics for their work in formulating the electroweak theory, which explains the unity of the weak nuclear force and electromagnetism.
Salam attended the Government College at Lahore, and in 1952 he received his Ph.D. in theoretical physics from the University of Cambridge. He returned to Pakistan as a professor of mathematics in 1951–54 and then went back to Cambridge as a lecturer in mathematics. He became professor of theoretical physics at the Imperial College of Science and Technology, London, in 1957. Salam was the first Pakistani and the first Muslim scientist to win a Nobel Prize. In 1964 he helped found the International Centre for Theoretical Physics at Trieste, Italy, in order to provide support for physicists from Third World countries. He served as the centre’s director until his death.
Salam carried out his Nobel Prize–winning research at the Imperial College of Science and Technology in the 1960s. His hypothetical equations, which demonstrated an underlying relationship between the electromagnetic force and the weak nuclear force, postulated that the weak force must be transmitted by hitherto-undiscovered particles known as weak vector bosons, or W and Z bosons. Weinberg and Glashow reached a similar conclusion using a different line of reasoning. The existence of the W and Z bosons was eventually verified in 1983 by researchers using particle accelerators at CERN.
Details
Mohammad Abdus Salamm (29 January 1926 – 21 November 1996) was a Pakistani theoretical physicist. He shared the 1979 Nobel Prize in Physics with Sheldon Glashow and Steven Weinberg for his contribution to the electroweak unification theory. He was the first Pakistani and the first Muslim from an Islamic country to receive a Nobel Prize in science and the second from an Islamic country to receive any Nobel Prize, after Anwar Sadat of Egypt.
Salam was scientific advisor to the Ministry of Science and Technology in Pakistan from 1960 to 1974, a position from which he played a major and influential role in the development of the country's science infrastructure. Salam contributed to numerous developments in theoretical and particle physics in Pakistan. He was the founding director of the Space and Upper Atmosphere Research Commission (SUPARCO), and responsible for the establishment of the Theoretical Physics Group (TPG). For this, he is viewed as the "scientific father" of this program. In 1974, Abdus Salam departed from his country in protest after the Parliament of Pakistan passed unanimously a parliamentary bill declaring members of the Ahmadiyya Muslim community, to which Salam belonged, non-Muslim. In 1998, following the country's Chagai-I nuclear tests, the Government of Pakistan issued a commemorative stamp, as a part of "Scientists of Pakistan", to honour the services of Salam.
Salam's notable achievements include the Pati–Salam model, magnetic photon, vector meson, Grand Unified Theory, work on supersymmetry and, most importantly, electroweak theory, for which he was awarded the Nobel Prize. Salam made a major contribution in quantum field theory and in the advancement of Mathematics at Imperial College London. With his student, Riazuddin, Salam made important contributions to the modern theory on neutrinos, neutron stars and black holes, as well as the work on modernising quantum mechanics and quantum field theory. As a teacher and science promoter, Salam is remembered as a founder and scientific father of mathematical and theoretical physics in Pakistan during his term as the chief scientific advisor to the president. Salam heavily contributed to the rise of Pakistani physics within the global physics community. Up until shortly before his death, Salam continued to contribute to physics, and to advocate for the development of science in third-world countries.
Additional Information
Abdus Salam was born in Jhang, a small town in what is now Pakistan, in 1926. His father was an official in the Department of Education in a poor farming district. His family has a long tradition of piety and learning.
When he cycled home from Lahore, at the age of 14, after gaining the highest marks ever recorded for the Matriculation Examination at the University of the Punjab, the whole town turned out to welcome him. He won a scholarship to Government College, University of the Punjab, and took his MA in 1946. In the same year he was awarded a scholarship to St. John’s College, Cambridge, where he took a BA (honours) with a double First in mathematics and physics in 1949. In 1950 he received the Smith’s Prize from Cambridge University for the most outstanding pre-doctoral contribution to physics. He also obtained a PhD in theoretical physics at Cambridge; his thesis, published in 1951, contained fundamental work in quantum electrodynamics which had already gained him an international reputation.
Salam returned to Pakistan in 1951 to teach mathematics at Government College, Lahore, and in 1952 became head of the Mathematics Department of the Punjab University. He had come back with the intention of founding a school of research, but it soon became clear that this was impossible. To pursue a career of research in theoretical physics he had no alternative at that time but to leave his own country and work abroad. Many years later he succeeded in finding a way to solve the heartbreaking dilemma faced by many young and gifted theoretical physicists from developing countries. At the ICTP, Trieste, which he created, he instituted the famous “Associateships” which allowed deserving young physicists to spend their vacations there in an invigorating atmosphere, in close touch with their peers in research and with the leaders in their own field, losing their sense of isolation and returning to their own country for nine months of the academic year refreshed and recharged.
In 1954 Salam left his native country for a lectureship at Cambridge, and since then has visited Pakistan as adviser on science policy. His work for Pakistan has, however, been far-reaching and influential. He was a member of the Pakistan Atomic Energy Commission, a member of the Scientific Commission of Pakistan and was Chief Scientific Adviser to the President from 1961 to 1974.
Since 1957 he has been Professor of Theoretical Physics at Imperial College, London, and since 1964 has combined this position with that of Director of the ICTP, Trieste.
For more than forty years he has been a prolific researcher in theoretical elementary particle physics. He has either pioneered or been associated with all the important developments in this field, maintaining a constant and fertile flow of brilliant ideas. For the past thirty years he has used his academic reputation to add weight to his active and influential participation in international scientific affairs. He has served on a number of United Nations committees concerned with the advancement of science and technology in developing countries.
To accommodate the astonishing volume of activity that he undertakes, Professor Salam cuts out such inessentials as holidays, parties and entertainments. Faced with such an example, the staff of the Centre find it very difficult to complain that they are overworked.
He has a way of keeping his administrative staff at the ICTP fully alive to the real aim of the Centre – the fostering through training and research of the advancement of theoretical physics, with special regard to the needs of developing countries. Inspired by their personal regard for him and encouraged by the fact that he works harder than any of them, the staff cheerfully submit to working conditions that would be unthinkable here at the (International Atomic Energy Agency in Vienna (IAEA). The money he received from the Atoms for Peace Medal and Award he spent on setting up a fund for young Pakistani physicists to visit the ICTP. He uses his share of the Nobel Prize entirely for the benefit of physicists from developing countries and does not spend a penny of it on himself or his family.
Abdus Salam is known to be a devout Muslim, whose religion does not occupy a separate compartment of his life; it is inseparable from his work and family life. He once wrote: “The Holy Quran enjoins us to reflect on the verities of Allah’s created laws of nature; however, that our generation has been privileged to glimpse a part of His design is a bounty and a grace for which I render thanks with a humble heart.”
Abdus Salam died on November 21, 1996.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1454 2024-02-29 20:23:07
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,394
Re: crème de la crème
1416) Steven Weinberg
Summary
Steven Weinberg (May 3, 1933 – July 23, 2021) was an American theoretical physicist and Nobel laureate in physics for his contributions with Abdus Salam and Sheldon Glashow to the unification of the weak force and electromagnetic interaction between elementary particles.
He held the Josey Regental Chair in Science at the University of Texas at Austin, where he was a member of the Physics and Astronomy Departments. His research on elementary particles and physical cosmology was honored with numerous prizes and awards, including the 1979 Nobel Prize in physics and the 1991 National Medal of Science. In 2004, he received the Benjamin Franklin Medal of the American Philosophical Society, with a citation that said he was "considered by many to be the preeminent theoretical physicist alive in the world today." He was elected to the U.S. National Academy of Sciences, Britain's Royal Society, the American Philosophical Society, and the American Academy of Arts and Sciences.
Weinberg's articles on various subjects occasionally appeared in The New York Review of Books and other periodicals. He served as a consultant at the U.S. Arms Control and Disarmament Agency, president of the Philosophical Society of Texas, and member of the Board of Editors of Daedalus magazine, the Council of Scholars of the Library of Congress, the JASON group of defense consultants, and many other boards and committees.
Details
Steven Weinberg (born May 3, 1933, New York City, New York, U.S.—died July 23, 2021, Austin, Texas) was an American nuclear physicist who in 1979 shared the Nobel Prize for Physics with Sheldon Lee Glashow and Abdus Salam for work in formulating the electroweak theory, which explains the unity of electromagnetism with the weak nuclear force.
Weinberg and Glashow were members of the same classes at the Bronx High School of Science, New York City (1950), and Cornell University (1954). Weinberg went from Cornell to the Institute for Theoretical Physics (later known as the Niels Bohr Institute) at the University of Copenhagen for a year. He then obtained his doctorate at Princeton University in 1957.
Weinberg proposed his version of the electroweak theory in 1967. Electromagnetism and the weak force were both known to operate by the interchange of subatomic particles. Electromagnetism can operate at potentially infinite distances by means of massless particles called photons, while the weak force operates only at subatomic distances by means of massive particles called bosons. Weinberg was able to show that despite their apparent dissimilarities, photons and bosons are actually members of the same family of particles. His work, along with that of Glashow and Salam, made it possible to predict the outcome of new experiments in which elementary particles are made to impinge on one another. An important series of experiments in 1982–83 found strong evidence for the W and Z particles predicted by these scientists’ electroweak theory.
Weinberg conducted research at Columbia University and at the Lawrence Berkeley Laboratory before joining the faculty of the University of California at Berkeley (1960–69). During his last years there, he also was a Morris Loeb Lecturer (1966–67) at Harvard—a post he held on several subsequent occasions as well—and a visiting professor (1968–69) at the Massachusetts Institute of Technology; he joined the latter faculty in 1969 and moved to Harvard University in 1973 and to the University of Texas at Austin in 1983.
Additional Information
According to modern physics, four fundamental forces exist in nature. Electromagnetic interaction is one of these. The weak interaction—responsible, for example, for the beta decay of nuclei—is another. Thanks to contributions made by Steven Weinberg, Sheldon Glashow, and Abdus Salam in 1968, these two interactions were unified to one single, called electroweak. The theory predicted, for example, that weak interaction manifests itself in “neutral weak currents” when certain elementary particles interact. This was later confirmed.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1455 2024-03-03 18:07:44
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,394
Re: crème de la crème
1417) Herbert C. Brown
Summary
Herbert Charles Brown (born May 22, 1912, London, England—died December 19, 2004, Lafayette, Indiana, U.S.) was one of the leading American chemists of the 20th century. His seminal work on customized reducing agents and organoborane compounds in synthetic organic chemistry had a major impact on both academic and industrial chemical practice and led to his sharing the 1979 Nobel Prize for Chemistry with the German chemist Georg Wittig.
Early life and education
Brown was born in a Jewish settlement camp in London, a temporary way station on his parents’ migration from Ukraine to the United States, where they settled with family members living in Chicago. Brown earned a bachelor’s degree (1936) and a doctorate (1938) from the University of Chicago. His dissertation, under the direction of Hermann Schlesinger, involved the reaction of diborane with aldehydes and ketones. It was the beginning of a lifetime’s devotion to organoborane chemistry. (Boron-hydrogen compounds and their derivatives are known as borane.) Postdoctoral study of the chlorosulfonation of alkanes (hydrocarbon compounds with only single molecular bonds) may likewise be seen as the genesis of his almost equally long devotion to physical organic chemistry.
Scientific career
In 1939 Brown became Schlesinger’s personal research assistant. World War II soon came to dominate the research carried out by Schlesinger’s group at Chicago. Contributions were made to the Army Signal Corps’ desire for a convenient method for the field generation of hydrogen. Of more lasting importance, though, were the discovery and large-scale preparation of sodium borohydride and lithium aluminum hydride.
Sodium borohydride is a relatively mild reducing agent, while lithium aluminum hydride is among the most powerful. A major portion of Brown’s research at Chicago (1939–43), Wayne State University (1943–47) in Detroit, and Purdue University (1947–78) in West Lafayette, Ind., was devoted to the development of new reducing agents. As a consequence of his work, organic chemists obtained an unparalled array of reducing agents carefully tailored to specific synthetic requirements. It was this work that was alluded to in the first part of the citation for his Nobel Prize.
Incidental to this work, Brown and B.C. Subba Rao studied the reduction of ethyl oleate by sodium borohydride in the presence of aluminum chloride. While the expected reduction of the ester group did indeed take place, there was an additional uptake of active hydrogen. Rather than dismissing the anomalous result, Brown boldly speculated that the double bond in oleic acid had been “hydroborated” by the excess reagent. Further research showed that this hydroboration reaction was a general property of double bonds.
At the time of this discovery (1956), hydroboranes were virtually unknown and thought not likely to be of synthetic use. Further work by Brown and his coworkers showed that organoboranes, produced by the hydroboration reaction, were in fact capable of an extraordinary range of synthetically important reactions. This work was addressed in the second part of his Nobel Prize citation.
Brown officially retired shortly before receiving the Nobel Prize. He wrote Hydroboration (1962) and Organic Syntheses via Boranes (1975), among other works.
Details
Herbert Charles Brown (May 22, 1912 – December 19, 2004) was an American chemist and recipient of the 1979 Nobel Prize in Chemistry for his work with organoboranes.
Life and career
Brown was born Herbert Brovarnik in London, to Ukrainian Jewish immigrants from Zhitomir, Pearl (née Gorinstein) and Charles Brovarnik, a hardware store manager and carpenter. His family moved to Chicago in June 1914, when he was two years old. Brown attended Crane Junior College in Chicago, where he met Sarah Baylen, whom he would later marry. The college was under threat of closing, and Brown and Baylen transferred to Wright Junior College. In 1935 he left Wright Junior College and that autumn entered the University of Chicago, completed two years of studies in three quarters, and earned a B.S. in 1936. That same year, he became a naturalized United States citizen. On February 6, 1937, Brown married Baylen, the person he credits with making him interested in hydrides of boron, a topic related to the work in which he, together with Georg Wittig, won the Nobel prize in Chemistry in 1979. Two years after starting graduate studies, he earned a Ph.D. in 1938, also from the University of Chicago.
Unable to find a position in industry, he decided to accept a postdoctoral position. This became the beginning of his academic career. He became an instructor at the University of Chicago in 1939, and held the position for four years before moving to Wayne University in Detroit as an assistant professor. In 1946, he was promoted to associate professor. He became a professor of inorganic chemistry at Purdue University in 1947 and joined the Beta Nu chapter of Alpha Chi Sigma there in 1960. He held the position of Professor Emeritus from 1978 until his death in 2004. The Herbert C. Brown Laboratory of Chemistry was named after him on Purdue University's campus. He was an honorary member of the International Academy of Science, Munich.
During World War II, while working with Hermann Irving Schlesinger, Brown discovered a method for producing sodium borohydride (NaBH4), which can be used to produce boranes, compounds of boron and hydrogen. His work led to the discovery of the first general method for producing asymmetric pure enantiomers. The elements found as initials of his name H, C and B were his working field.
In 1969, he was awarded the National Medal of Science.
Brown was quick to credit his wife Sarah with supporting him and allowing him to focus on creative efforts by handling finances, maintaining the house and yard, etc. According to Brown, after receiving the Nobel prize in Stockholm, he carried the medal and she carried the US$100,000 award.
In 1971, he received the Golden Plate Award of the American Academy of Achievement.
He was inducted into the Alpha Chi Sigma Hall of Fame in 2000.
He died December 19, 2004, at a hospital in Lafayette, Indiana after a heart attack. His wife died May 29, 2005, aged 89.
Research
As a doctoral student at the University of Chicago, Herbert Brown studied the reactions of diborane, B2H6. Hermann Irving Schlesinger's laboratory at the University of Chicago was one of two laboratories that prepared diborane. It was a rare compound that was only prepared in small quantities. Schlesinger was researching the reactions of diborane to understand why the simplest hydrogen-boron compound is B2H6 instead of BH3.
When Brown started his own research, he observed the reactions of diborane with aldehydes, ketones, esters, and acid chlorides. He discovered that diborane reacts with aldehydes and ketones to produce dialkoxyboranes, which are hydrolyzed by water to produce alcohols. Until this point, organic chemists did not have an acceptable method of reducing carbonyls under mild conditions. Yet Brown's Ph.D. thesis published in 1939 received little interest. Diborane was too rare to be useful as a synthetic reagent.
In 1939, Brown became the research assistant in Schlesinger's laboratory. In 1940, they began to research volatile, low molecular weight uranium compounds for the National Defense Research Committee. Brown and Schlesinger successfully synthesized volatile uranium(IV) borohydride, which had a molecular weight of 298. The laboratory was asked to provide a large amount of the product for testing, but diborane was in short supply. They discovered that it could be formed by reacting lithium hydride with boron trifluoride in ethyl ether, allowing them to produce the chemical in larger quantities. This success was met with several new problems. Lithium hydride was also in short supply, so Brown and Schlesinger needed to find a procedure that would allow them to use sodium hydride instead. They discovered that sodium hydride and methyl borate reacted to produce sodium trimethoxyborohydride, which was viable as a substitute for the lithium hydride.
Soon they were informed that there was no longer a need for uranium borohydride, but it appeared that sodium borohydride could be useful in generating hydrogen. They began to look for a cheaper synthesis and discovered that adding methyl borate to sodium hydride at 250° produced sodium borohydride and sodium methoxide. When acetone was used in an attempt to separate the two products, it was discovered that sodium borohydride reduced the acetone.
Sodium borohydride is a mild reducing agent that works well in reducing aldehydes, ketones, and acid chlorides. Lithium aluminum hydride is a much more powerful reducing agent that can reduce almost any functional group. When Brown moved to Purdue University in 1947, he worked to find stronger borohydrides and milder aluminum hydrides that would provide a spectrum of reducing agents. The team of researchers at Purdue discovered that changing the metal ion of the borohydride to lithium, magnesium, or aluminum increases the reducing ability. They also found that introducing alkoxy substituents to the aluminum hydride decreases the reducing ability. They successfully developed a full spectrum of reducing agents.
While researching these reducing agents, Brown's coworker, Dr. B. C. Subba Rao, discovered an unusual reaction between sodium borohydride and ethyl oleate. The borohydride added hydrogen and boron to the carbon-carbon double bond in the ethyl oleate. The organoborane product could then be oxidized to form an alcohol. This two-step reaction is now called hydroboration-oxidation and is a reaction that converts alkenes into anti-Markovnikov alcohols. Markovnikov's rule states that, in adding hydrogen and a halide or hydroxyl group to a carbon-carbon double bond, the hydrogen is added to the less-substituted carbon of the bond and the hydroxyl or halide group is added to the more-substituted carbon of the bond. In hydroboration-oxidation, the opposite addition occurs.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1456 2024-03-05 16:56:22
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,394
Re: crème de la crème
1418) Georg Wittig
Summary
Georg Wittig, (born June 16, 1897, Berlin, Ger.—died Aug. 26, 1987, Heidelberg, W.Ger.), was a German chemist whose studies of organic phosphorus compounds won him a share (with Herbert C. Brown) of the Nobel Prize for Chemistry in 1979.
Wittig graduated from the University of Marburg in 1923, received his doctorate there in 1926, and remained as a lecturer in chemistry until 1932. He taught at the Technical University in Braunschweig and at the universities of Braunschweig, Freiburg, and Tübingen before joining the faculty of the University of Heidelberg in 1956, where he became emeritus in 1965 but continued to pursue research.
In investigating reactions involving carbanions, negatively charged organic species, Wittig discovered a class of organic phosphorus compounds called ylides that mediate a particular type of reaction that became known as the Wittig reaction. This reaction proved of great value in the synthesis of complex organic compounds such as vitamins A and D2, prostaglandins, and steroids.
Details
Georg Wittig (16 June 1897 – 26 August 1987) was a German chemist who reported a method for synthesis of alkenes from aldehydes and ketones using compounds called phosphonium ylides in the Wittig reaction. He shared the Nobel Prize in Chemistry with Herbert C. Brown in 1979.
Biography
Wittig was born in Berlin, Germany and shortly after his birth moved with his family to Kassel, where his father was professor at the applied arts high school. He attended school in Kassel and started studying chemistry at the University of Tübingen 1916. He was drafted and became a lieutenant in the cavalry of Hesse-Kassel (or Hesse-Cassel). After being an Allied prisoner of war from 1918 until 1919, Wittig found it hard to restart his chemistry studies owing to overcrowding at the universities. By a direct plea to Karl von Auwers, who was professor for organic chemistry at the University of Marburg at the time, he was able to resume university study and after 3 years was awarded the Ph.D. in organic chemistry.
Karl von Auwers was able to convince him to start an academic career, leading to his habilitation in 1926. He became a close friend of Karl Ziegler, who was also doing his habilitation with Auwers during that time. The successor of Karl von Auwers, Hans Meerwein, accepted Wittig as lecturer, partly because he was impressed by the new 400-page book on stereochemistry that Wittig had written. In 1931 Wittig married Waltraud Ernst, a colleague from the Auwers working group. The invitation of Karl Fries brought him as professor to the TU Braunschweig in 1932. The time in Braunschweig became more and more problematic as the Nazis tried to get rid of Karl Fries and Wittig showed solidarity with him. After the forced retirement of Fries, in 1937 Hermann Staudinger offered Wittig a position at the University of Freiburg, partly because he knew Wittig from his book on stereochemistry in which he supported Staudinger's highly criticized theory of macromolecules. The foundations of carbanion chemistry were laid during Wittig's time in Freiburg.
In 1944 he succeeded the head of the organic chemistry department Wilhelm Schlenk at the University of Tübingen. Most of his scientific work, including the development of the Wittig reaction, was performed during this time in Tübingen. The 1956 appointment of the nearly sixty-year-old Wittig as head of the organic chemistry department at the University of Heidelberg as successor of Karl Freudenberg was exceptional even at that time. The newly built department and the close connection to the BASF convinced Wittig to take this opportunity. He worked at the University of Heidelberg even after his retirement in 1967 and published papers until 1980. Most of his awards were presented during this time at Heidelberg, such as the honorary doctorate of the Sorbonne in 1956 and the Nobel Prize in Chemistry in 1979.
Work
Wittig's contributions also include the preparation of phenyllithium and the discovery of the 1,2-Wittig rearrangement and the 2,3-Wittig rearrangement.
Wittig was well known in the chemistry community for being a consummate experimenter and observer of chemical transformations, while caring very little for the theoretical and mechanistic underpinnings of the work he produced.
Georg also has his name on a literature work titled on a compound labelled Colopidalol.
Additional Information
During chemical reactions, molecules composed of atoms meet and form new compounds. Through chemical reactions, it is possible to synthesize chemical compounds in laboratories with molecules that do not exist in nature. In 1953 Georg Wittig discovered a reaction between a phosphorous carbon compound and another carbon compound that resulted in formation of a carbon compound with a least one double bond between carbon atoms. Among other things, biologically active compounds can be formed. For example, vitamin A can be produced by artificial means with the help of this reaction.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1457 2024-03-07 17:18:11
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,394
Re: crème de la crème
1419) Allan MacLeod Cormack
Summary
Allan MacLeod Cormack (born Feb. 23, 1924, Johannesburg, S.Af.—died May 7, 1998, Winchester, Mass., U.S.) was a South African-born American physicist who, with Godfrey Hounsfield, was awarded the 1979 Nobel Prize for Physiology or Medicine for his work in developing the powerful new diagnostic technique of computerized axial tomography (CAT). Cormack was unusual in the field of Nobel laureates because he never earned a doctorate degree in medicine or any other field of science.
After graduating from the University of Cape Town in 1944 Cormack pursued advanced studies there and at the University of Cambridge. He was a lecturer at Cape Town from 1950 to 1956 and then, after a year’s research fellowship at Harvard University, became assistant professor of physics at Tufts University. His main research at Tufts centred on the interaction of subatomic particles. He advanced to full professor in 1964, was chairman of the department from 1968 to 1976, and retired in 1980. He became a U.S. citizen in 1966.
A part-time position as physicist for a hospital radiology department first aroused Cormack’s interest in the problem of X-ray imaging of soft tissues or layers of tissue of differing densities. The two-dimensional representations of conventional X-ray plates were often unable to distinguish between such tissues. More information could be gained if X rays of the body were taken from several different directions, but conventional X-ray techniques made this procedure problematic. In the early 1960s Cormack showed how details of a flat section of soft tissues could be calculated from measurements of the attenuation of X rays passing through it from many different angles. He thus provided the mathematical technique for the CAT scan, in which an X-ray source and electronic detectors are rotated about the body and the resulting data is analyzed by a computer to produce a sharp map of the tissues within a cross section of the body. Cormack became a member of the American Academy of Arts and Sciences in 1980.
Details
Allan MacLeod Cormack (February 23, 1924 – May 7, 1998) was a South African American physicist who won the 1979 Nobel Prize in Physiology or Medicine (along with Godfrey Hounsfield) for his work on X-ray computed tomography (CT), a significant and unusual achievement since Cormack did not hold a doctoral degree in any scientific field.
Early life and education
Cormack was born on February 23, 1924, in Johannesburg, South Africa. He attended Rondebosch Boys' High School in Cape Town, where he was active in the debating and tennis teams. He received his B.Sc. in physics in 1944 from the University of Cape Town and his M.Sc. in crystallography in 1945 from the same institution. He was a doctoral student at Cambridge University from 1947 to 1949, and while at Cambridge he met his future wife, Barbara Seavey, an American physics student.
Career
After marrying Barbara, he returned to the University of Cape Town in early 1950 to lecture. Following a sabbatical at Harvard in 1956–57, the couple agreed to move to the United States, and Cormack became a professor at Tufts University in the fall of 1957. Cormack became a naturalized citizen of the United States in 1966. Although he was mainly working on particle physics, Cormack's side interest in x-ray technology led him to develop the theoretical underpinnings of CT scanning. This work was initiated at the University of Cape Town and Groote Schuur Hospital in early 1956 and continued briefly in mid-1957 after returning from his sabbatical. His results were subsequently published in two papers in the Journal of Applied Physics in 1963 and 1964. These papers generated little interest until Hounsfield and colleagues built the first CT scanner in 1971, taking Cormack's theoretical calculations into a real application. For their independent efforts, Cormack and Hounsfield shared the 1979 Nobel Prize in Physiology or Medicine. It is notable that the two built a very similar type of device without collaboration in different parts of the world. He was member of the International Academy of Science, Munich. In 1990, he was awarded the National Medal of Science.
Death
Cormack died of cancer in Winchester, Massachusetts, at age 74. He was posthumously awarded the Order of Mapungubwe on December 10, 2002, for outstanding achievements as a scientist and for co-inventing the CT scanner.
Additional Information
The discovery of X-rays and the possibility of obtaining images of the body’s interior quickly led to medical applications. The possibilities of X-ray technology were further expanded with computed tomography (CT). If X-rays are sent through the body from different angles and registered when they have passed the body, images of different cross sections are created through advanced computer calculations. Around 1957 Allan Cormack developed the necessary methods of calculation. In addition to cross sections of the body, computed tomography also provides a basis for three-dimensional images.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1458 2024-03-09 17:53:10
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,394
Re: crème de la crème
1420) Godfrey Hounsfield
Details
Sir Godfrey Newbold Hounsfield (28 August 1919 – 12 August 2004) was a British electrical engineer who shared the 1979 Nobel Prize for Physiology or Medicine with Allan MacLeod Cormack for his part in developing the diagnostic technique of X-ray computed tomography (CT).
His name is immortalised in the Hounsfield scale, a quantitative measure of radiodensity used in evaluating CT scans. The scale is defined in Hounsfield units (symbol HU), running from air at −1000 HU, through water at 0 HU, and up to dense cortical bone at +1000 HU and more.
Early life
Hounsfield was born in Sutton-on-Trent, Nottinghamshire, England on 28 August 1919. He was the youngest of five children (he has two brothers and two sisters). His father, Thomas Hounsfield was a farmer from Beighton, and was linked to the prominent Hounsfield and Newbold families of Hackenthorpe Hall, his mother was Blanche Dilcock. As a child he was fascinated by the electrical gadgets and machinery found all over his parents' farm. Between the ages of eleven and eighteen, he tinkered with his own electrical recording machines, launched himself off haystacks with his own home-made glider, and almost killed himself by using water-filled tar barrels and acetylene to see how high they could be waterjet propelled. He attended the Magnus Grammar School in Newark-on-Trent, but was not academic.
Military service and education
Shortly before World War II, he joined the Royal Air Force as a volunteer reservist where he learned the basics of electronics and radar. After the war, he attended Faraday House Electrical Engineering College in London, graduating with the DFH (Diploma of Faraday House). Before the advent of most university engineering departments, Faraday House was a specialist Electrical Engineering college that provided university level education that combined practical experience with theoretical study.
Career
In 1949, Hounsfield began work at EMI, Ltd. in Hayes, Middlesex, where he researched guided weapon systems and radar. Hounsfield incorrectly gave this date as 1951 when he wrote his autobiography which is available on the Nobel Prize website. The correct date is 10 October 1949 as stated in a biography of Hounsfield. At EMI, he became interested in computers and in 1958, he helped design the first commercially available all-transistor computer made in Great Britain: the EMIDEC 1100. Shortly afterwards, he began work on the CT scanner at EMI. He continued to improve CT scanning, introducing a whole-body scanner in 1975, and was senior researcher (and after his retirement in 1984, consultant) to the laboratories.
While on an outing in the country, Hounsfield came up with the idea that one could determine what was inside a box by taking X-ray readings at all angles around the object. He then set to work constructing a computer that could take input from X-rays at various angles to create an image of the object in "slices". Applying this idea to the medical field led him to propose what is now known as computed tomography. At the time, Hounsfield was not aware of the work that Cormack had done on the theoretical mathematics for such a device. Hounsfield built a prototype head scanner and tested it first on a preserved human brain, then on a fresh cow brain from a butcher’s shop, and later on himself. On 1 October 1971, CT scanning was introduced into medical practice with a successful scan on a cerebral cyst patient at Atkinson Morley Hospital in Wimbledon, London, United Kingdom. In 1975, Hounsfield built a whole-body scanner. The principles of computed tomography developed by Hounsfield remain in use today (2022).
Awards and honours
In 1979, Hounsfield and Cormack received the Nobel Prize in Physiology or Medicine.
Hounsfield received numerous awards in addition to the Nobel Prize. He was appointed Commander of the Order of the British Empire in 1976 and knighted in 1981.
In 1974, he received the Wilhelm Exner Medal. Hounsfield was elected a Fellow of the Royal Society (FRS) in 1975. In 1976, he received the Golden Plate Award of the American Academy of Achievement. He was awarded the Howard N. Potts Medal in 1977. In 1994 he was elected an Honorary Fellow of the Royal Academy of Engineering.
The Hounsfield Facility for 3-D CT imaging at the University of Nottingham, opened in 2014, was named after him. It was designed to apply CT scanning to biomaterials, especially within soil, and thus to the exploring the environment.
Personal life and death
Hounsfield enjoyed hiking and skiing. He had resolved to develop what came to be CT scanning while on a country ramble.
He retired from EMI in 1986 and used the prize money from his Nobel to build a personal laboratory in his home. Hounsfield died at Kingston upon Thames, Greater London, in 2004, at the age of 84.
Additional Information
Sir Godfrey Newbold Hounsfield, (born August 28, 1919, Newark, Nottinghamshire, England—died August 12, 2004, Kingston upon Thames), was an English electrical engineer who shared the 1979 Nobel Prize for Physiology or Medicine with Allan Cormack for his part in developing the diagnostic technique of computerized axial tomography (CAT), or computerized tomography (CT). In this technique, information obtained from X rays taken by scanners rotating around the patient are combined by a computer to yield a high-resolution image of a slice of the body.
After studying electronics and radar as a member of the Royal Air Force during World War II and at Faraday House Electrical Engineering College in London, Hounsfield joined the research staff of EMI Ltd. in 1951. He led the design team that built the first all-transistor computer in Great Britain, the EMIDEC 1100, in 1958–59. Later, while investigating the problem of pattern recognition, he developed the basic idea of CAT. Hounsfield extended the capability of a computer so that it could interpret X-ray signals so as to form a two-dimensional image of a complex object such as the human head. He pursued the application of axial tomography to medical diagnosis, building a prototype head scanner and then a body scanner at EMI. Computers soon evolved to the stage needed for processing the signals from the scanners at the same rate they were obtained, and in 1972 the first clinical test of CAT scanning was performed successfully.
For his work Hounsfield received numerous awards in addition to the Nobel Prize, and he was knighted in 1981.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1459 2024-03-10 17:07:33
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,394
Re: crème de la crème
1421) James Cronin
Summary
James Watson Cronin (born September 29, 1931, Chicago, Illinois, U.S.—died August 25, 2016, St. Paul, Minnesota) was an American particle physicist, corecipient with Val Logsdon Fitch of the 1980 Nobel Prize for Physics for an experiment that implied that reversing the direction of time would not precisely reverse the course of certain reactions of subatomic particles.
Cronin graduated from Southern Methodist University at Dallas, Texas, in 1951 and received a Ph.D. from the University of Chicago in 1955. He then joined the staff of the Brookhaven National Laboratory, Upton, New York. He taught (1958–71) at Princeton University before moving to the University of Chicago; he retired as professor emeritus there in 1997. In the 1990s Cronin became involved in the Pierre Auger Project, which led to the construction early in the 21st century of an observatory in Argentina to view and study cosmic rays.
Cronin and his colleague Fitch played a role in modifying the long-held notion that the laws of symmetry and conservation are inviolable. One of these laws, the principle of time invariance (designated T), states that particle interactions should be indifferent to the direction of time. This symmetry and two others, those of charge conjugation (C) and parity conservation (P), were once thought to govern all the laws of physics. But in 1956 the physicists Chen Ning Yang and Tsung-Dao Lee suggested, correctly, that parity conservation could be violated by particle decays involving weak interactions. Physicists abandoned the view that C, P, and T are independently true for weak interactions but saved the overall concept by proposing that any P violation must be offset by an equal C violation, a concept known as CP symmetry.
In a series of experiments conducted at Brookhaven in 1964, Cronin and Fitch showed that, in rare instances, subatomic particles called K mesons violate CP symmetry during their decay. In addition to winning the Nobel Prize, Cronin was a 1999 recipient of the National Medal of Science.
Details
James Watson Cronin (September 29, 1931 – August 25, 2016) was an American particle physicist.
Cronin and co-researcher Val Logsdon Fitch were awarded the 1980 Nobel Prize in Physics for a 1964 experiment that proved that certain subatomic reactions do not adhere to fundamental symmetry principles. Specifically, they proved, by examining the decay of kaons, that a reaction run in reverse does not merely retrace the path of the original reaction, which showed that the interactions of subatomic particles are not invariant under time reversal. Thus the phenomenon of CP violation was discovered.
Cronin received the Ernest Orlando Lawrence Award in 1976 for major experimental contributions to particle physics including fundamental work on weak interactions culminating in the discovery of asymmetry under time reversal. In 1999, he was awarded the National Medal of Science.
Cronin was Professor Emeritus at the University of Chicago winning the prestigious Quantrell Award and a spokesperson emeritus for the Auger project. He was a member of the Board of Sponsors of the Bulletin of the Atomic Scientists.
Education and early life
James Cronin was born in Chicago on September 29, 1931. His father, James Farley Cronin, was a graduate student of classical languages at the University of Chicago. After his father had obtained his doctorate the family first moved to Alabama, and later in 1939 to Dallas, Texas, where his father became a professor of Latin and Greek at Southern Methodist University. After high school Cronin stayed in Dallas and obtained an undergraduate degree at SMU in physics and mathematics in 1951. He is of Irish descent, with his Irish ancestors immigrating from County Cork, Ireland.
For graduate school Cronin moved back to Illinois to attend the University of Chicago. His teachers there included Nobel Prize laureates Enrico Fermi, Maria Mayer, Murray Gell-Mann and Subrahmanyan Chandrasekhar. He wrote his thesis on experimental nuclear physics under supervision of Samuel K. Allison.
Research and career
After obtaining his doctorate in 1955, Cronin joined the group of Rodney L. Cool and Oreste Piccioni at Brookhaven National Laboratory, where the new Cosmotron particle accelerator had just been completed. There he started to study parity violation in the decay of hyperon particles. During that time he also met Val Fitch, who brought him to Princeton University in Fall 1958. After Cosmotron underwent magnet failure, Cronin and the Brookhaven group moved to Bevatron at the University of California, Berkeley during the first half of 1958. Cronin and Fitch studied the decays of neutral K mesons, in which they discovered CP violation in 1964. This discovery earned the duo the 1980 Nobel Prize in Physics.
After the discovery, Cronin spent a year in France at the Centre d'Études Nucléaires at Saclay. After returning to Princeton he continued studying the neutral CP violating decay modes of the long-lived neutral K meson. In 1971, he moved back to the University of Chicago to become a full professor. This was attractive for him because of a new 400 GeV particle accelerator being built at nearby Fermilab.
When he moved to Chicago, he began a long series of experiments on particle production at high transverse momentum. With physicist Pierre Piroue and colleagues we learned about many things. These are summarized in Physical Review D, vol 19, page 764 (1977). Following these experiments Cronin took a sabbatical at CERN in 1982–83, where he performed an experiment to measure of the lifetime of the neutral pion (Physics Letters vol 158 B page 81, 1985). He then switched to the study of cosmic rays. The first was a series of measurements looking for point sources of cosmic rays. No sources were found. A summary of the measurements was published in Physical Review D vol 55 page 1714 (1997). In 1998 he joined the faculty at the University of Utah on a half-time basis to work on ultra-high-energy cosmic ray physics and to jumpstart the Pierre Auger Observatory project. His appointment was to last five years, but he left after a year to continue gathering international support for the Observatory with Alan Watson and Murat Boratav.
Cronin is one of the 20 American recipients of the Nobel Prize in Physics to sign a letter addressed to President George W. Bush in May 2008, urging him to "reverse the damage done to basic science research in the Fiscal Year 2008 Omnibus Appropriations Bill" by requesting additional emergency funding for the Department of Energy's Office of Science, the National Science Foundation, and the National Institute of Standards and Technology.
Additional Information
For a long time, physicists assumed that various symmetries characterized nature. In a kind of “mirror world” where right and left were reversed and matter was replaced by antimatter, the same physical laws would apply, they posited. The left-right symmetry had already been proven violated when, in 1964, James Cronin and Val Fitch discovered that the matter-antimatter symmetry is violated when the neutral K-meson decays. Their experiment also proved that symmetry does not apply during time reversal: reactions going backward in time are not identical to those going forward.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1460 2024-03-12 18:10:35
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,394
Re: crème de la crème
1422) Val Logsdon Fitch
Details
Val Logsdon Fitch (March 10, 1923 – February 5, 2015) was an American nuclear physicist who, with co-researcher James Cronin, was awarded the 1980 Nobel Prize in Physics for a 1964 experiment using the Alternating Gradient Synchrotron at Brookhaven National Laboratory that proved that certain subatomic reactions do not adhere to fundamental symmetry principles. Specifically, they proved, by examining the decay of K-mesons, that a reaction run in reverse does not retrace the path of the original reaction, which showed that the reactions of subatomic particles are not indifferent to time. Thus the phenomenon of CP violation was discovered. This demolished the faith that physicists had that natural laws were governed by symmetry.
Born on a cattle ranch near Merriman, Nebraska, Fitch was drafted into the U.S. Army during World War II, and worked on the Manhattan Project at the Los Alamos Laboratory in New Mexico. He later graduated from McGill University, and completed his PhD in physics in 1954 at Columbia University. He was a member of the faculty at Princeton University from 1954 until his retirement in 2005.
Early life
Val Logsdon Fitch was born on a cattle ranch near Merriman, Nebraska, on March 10, 1923, the youngest of three children of Fred Fitch, a cattle rancher, and his wife Frances née Logsdon, a school teacher. He had an older brother and sister. The family farm was about 4 square miles (10 sq km) in size. The ranch was small; his father specialized in raising breeding stock. Soon after his birth, his father was badly injured in a horse riding accident and could no longer work on his ranch, so the family moved to the nearby town of Gordon, Nebraska, where his father entered the insurance business. Here he attended school, graduating from Gordon High School in 1940 as valedictorian.
Manhattan Project
Fitch attended Chadron State College for three years, then transferred to Northwestern University. This was during WWII; his studies were interrupted by being drafted into the US Army in 1943. After completing basic training, he was sent to Carnegie Institute of Technology for training under the Army Specialized Training Program Under this program, some 200,000 soldiers attended colleges for intensive courses. Fitch was in the program for less than a year before the manpower requirements of the war became too great, and the Army terminated the program. Most of the soldiers in the ASTP were posted to combat units, but Fitch was one of a hundred or so ASTP soldiers who joined the Special Engineer Detachment (SED), which provided much-needed technicians to the Manhattan Project.
The Army sent Fitch to the Manhattan Project's Los Alamos Laboratory in New Mexico. By mid-1944, about a third of the technicians at Los Alamos were from the SED. There he met many of the greats of physics including Niels Bohr, James Chadwick, Enrico Fermi, Isidor Isaac Rabi, Bruno Rossi, Emilio Segrè, Edward Teller and Richard C. Tolman, in some cases attending physics courses taught by them. He worked in the group headed by Ernest Titterton, a member of the British Mission, and became well-acquainted with the techniques of experimental physics. He participated in the drop testing of mock atomic bombs that was conducted at Wendover Army Air Field and the Naval Auxiliary Air Station Salton Sea, and worked at the Trinity site, where he witnessed the Trinity nuclear test on July 16, 1945. He was discharged from the Army in 1946. He continued to work at Los Alamos as a civilian for another year to earn money. He briefly returned to Los Alamos in summer 1948.
Academic career
His wartime experiences led Fitch to decide to become a physicist. Robert Bacher, the head of the physics division at Los Alamos, offered him a graduate assistantship at Cornell University, but first he needed to complete his undergraduate degree. Rather than return to Northwestern or Carnegie Mellon, he elected to enter McGill University, which Titterton had recommended. Fitch graduated from McGill with a bachelor's degree in electrical engineering in 1948. On the advice of Jerry Kellogg, who had been a student of Rabi's at Columbia University, and was a division head at the Los Alamos, Fitch decided to pursue his doctoral studies at Columbia. Kellogg wrote him a letter of introduction to Rabi. James Rainwater became his academic supervisor. Rainwater gave him a paper by John Wheeler concerning mu-mesic atoms, atoms in which an electron is replaced by a muon. These had never been observed; they were completely theoretical and there was no evidence that they existed, but it made a good thesis topic.
Fitch designed and built an experiment to measure the gamma rays emitted from mu-mesic atoms. As it turned out, this was a good time to search for them. Columbia had recently commissioned a cyclotron at the Nevis Laboratories that could produce muons; Robert Hofstadter had developed the thallium-activated sodium iodide gamma ray detector; and wartime advances in electronics yielded advances in components such as new phototubes needed to bring it all together. Initially nothing was found, but Rainwater suggested expanding the search beyond the energy range predicted by Wheeler on the basis of the then-accepted size of the radius of the atomic nucleus as around 1.4 × {10}^{-15} m. When this was done, they found what they had been looking for, discovering in the process that the nucleus was closer to 1.2 × {10}^{-15} m. He completed his PhD in 1954, writing his thesis on "Studies of X-rays from mu-mesonic atoms".[8] The thesis was published in the Physical Review in November 1953.
In 1949, Fitch married Elise Cunningham, a secretary who worked in the laboratory at Columbia. They had two sons. Elise died in 1972, and in 1976 he married Daisy Harper Sharp, thereby acquiring two stepdaughters and a stepson. After obtaining his doctorate, Fitch's interest shifted to strange particles and K mesons. In 1954, he joined the physics faculty at Princeton University, where he spent the rest of his career. He was the Class of 1909 Professor of Physics from 1969 to 1976, the Cyrus Fogg Brackett Professor of Physics from 1976 to 1982, and the James S. McDonnell Distinguished University Professor of Physics from 1982 to 1993, when he retired and took up the position of visiting lecturer with the rank of professor for three years before entering emeritus status. He was chair of the physics department from 1976 to 1981.
Fitch conducted much of his research at the Brookhaven National Laboratory, where he became acquainted with James Cronin. The two of them played bridge at nights while they waited for the Cosmotron to become available. Cronin had built a new kind of detector, a spark chamber spectrometer, and Fitch realized that it would be perfect for experiments with K mesons (now known as kaons), which Yale University physicist Robert Adair had suggested had interesting properties worth investigating. They could decay into either matter or antimatter. Along with two colleagues, James Christenson and René Turlay, they set up their experiment on the Alternating Gradient Synchrotron at Brookhaven. They discovered an unexpected result. The decay of neutral K mesons did not respect CP symmetry. K mesons that decayed into positrons did so faster than those that decayed into electrons. The importance of this result was not immediately appreciated; but as evidence of the Big Bang accumulated, Andrei Sakharov realized in 1967 that it explained why the universe is largely made of matter and not antimatter.[10] Put simply, they had found "the answer to the physicist's 'Why do we exist?'" For this discovery, Fitch and Cronin received the 1980 Nobel Prize in Physics.
In addition to the Nobel Prize, Fitch received the Ernest Orlando Lawrence Award in 1968, the John Price Wetherill Medal in 1976 and the National Medal of Science in 1993. He was a member of the Board of Sponsors of the Bulletin of the Atomic Scientists and the JASON defense advisory group. He was elected a Fellow of the American Physical Society in 1964 and a Member of both the National Academy of Sciences and the American Academy of Arts and Sciences in 1966. In 1981, Fitch became a founding member of the World Cultural Council[20] and received the Golden Plate Award of the American Academy of Achievement. He was president of the American Physical Society from 1988 to 1989, and he served on a number of governmental science and science policy committees, including the President's Science Advisory Committee from 1970 to 1973.
Fitch is one of the 20 American recipients of the Nobel Prize in Physics to sign a letter addressed to President George W. Bush in May 2008, urging him to "reverse the damage done to basic science research in the Fiscal Year 2008 Omnibus Appropriations Bill" by requesting additional emergency funding for the Department of Energy’s Office of Science, the National Science Foundation, and the National Institute of Standards and Technology.
He died at his home in Princeton, New Jersey, at the age of 91 on February 5, 2015.
Additional Information
Val Logsdon Fitch (born March 10, 1923, Merriman, Nebraska, U.S.—died February 5, 2015, Princeton, New Jersey) was an American particle physicist who was corecipient, with James Watson Cronin, of the Nobel Prize for Physics in 1980 for experiments conducted in 1964 that disproved the long-held theory that particle interaction should be indifferent to the direction of time.
Fitch’s early interest in chemistry shifted to physics in the mid-1940s when, as a member of the U.S. Army, he was sent to Los Alamos, New Mexico, to work on the Manhattan Project. He graduated from McGill University in Montreal with a bachelor’s degree in electrical engineering in 1948 and was awarded a Ph.D. in physics by Columbia University in 1954. That year he joined the faculty of Princeton University, and he later served (1976–81) as chair of its physics department; in 1987 he was named the James S. McDonnell Distinguished University Professor of Physics.
In experiments conducted at the Brookhaven National Laboratory in 1964, Fitch and Cronin showed that the decay of subatomic particles called K mesons could violate the general conservation law for weak interactions known as CP symmetry. Those experiments in turn necessitated physicists’ abandonment of the long-held principle of time-reversal invariance. The work done by Fitch and Cronin implied that reversing the direction of time would not precisely reverse the course of certain reactions of subatomic particles. Fitch served on various government bodies, including the President’s Science Advisory Committee (1970–73) and the National Science Foundation (1980–83), and in 1993 he was awarded the National Medal of Science.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1461 2024-03-13 17:19:58
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,394
Re: crème de la crème
1423) Paul Berg
Summary
Paul Berg (born June 30, 1926, New York, New York, U.S.—died February 15, 2023, Stanford, California) was an American biochemist whose development of recombinant DNA techniques won him a share (with Walter Gilbert and Frederick Sanger) of the Nobel Prize for Chemistry in 1980.
After graduating from Pennsylvania State College (later renamed Pennsylvania State University) in 1948 and taking a doctorate from Western Reserve University in 1952, Berg pursued further studies at the Institute of Cytophysiology in Copenhagen and at Washington University in St. Louis, where he remained as assistant professor of microbiology until 1959. From 1959 he was associated with the medical school of Stanford University, serving as chairman of the biochemistry department in 1969–74 and becoming Willson professor (1970) and director of the Beckman Center for Molecular and Genetic Medicine (1985). He retired in 2000.
In the course of studying the actions of isolated genes, Berg developed methods for splitting DNA molecules at selected sites and attaching segments of the molecule to the DNA of a virus or plasmid, which could then enter bacterial or animal cells. The foreign DNA was incorporated into the host and caused the synthesis of proteins that were not ordinarily found there. One of the earliest practical results of recombinant technology was the development of a strain of bacteria containing the gene for producing the mammalian hormone insulin.
Details
Paul Berg (June 30, 1926 – February 15, 2023) was an American biochemist and professor at Stanford University.
He was the recipient of the Nobel Prize in Chemistry in 1980, along with Walter Gilbert and Frederick Sanger. The award recognized their contributions to basic research involving nucleic acids, especially recombinant DNA.
Berg received his undergraduate education at Penn State University, where he majored in biochemistry. He received his PhD in biochemistry from Case Western Reserve University in 1952. Berg worked as a professor at Washington University School of Medicine and Stanford University School of Medicine, in addition to serving as the director of the Beckman Center for Molecular and Genetic Medicine.
In addition to the Nobel Prize, Berg was presented with the National Medal of Science in 1983 and the National Library of Medicine Medal in 1986. Berg was a member of the Board of Sponsors for the Bulletin of the Atomic Scientists.
Early life and education
Berg was born in Brooklyn, New York City, the son of a Russian Jewish immigrant couple, Sarah Brodsky, a homemaker, and Harry Berg, a clothing manufacturer. Berg graduated from Abraham Lincoln High School in 1943, received his Bachelor of Science degree in biochemistry from Penn State University in 1948 and PhD in biochemistry from Case Western Reserve University in 1952. He was a member of the Jewish fraternity, ΒΣΡ.
Research and career:
Academic posts
After completing his graduate studies, Berg spent two years (1952–1954) as a postdoctoral fellow with the American Cancer Society, working at the Institute of Cytophysiology in Copenhagen, Denmark, and the Washington University School of Medicine, and spent additional time in 1954 as a scholar in cancer research with the department of microbiology at the Washington University School of Medicine. He worked with Arthur Kornberg, while at Washington University. Berg was also tenured as a research fellow at Clare Hall, Cambridge. He was a professor at Washington University School of Medicine from 1955 until 1959. After 1959, Berg moved to Stanford University, where he taught biochemistry from 1959 until 2000 and served as director of the Beckman Center for Molecular and Genetic Medicine from 1985 until 2000.[9] In 2000 he retired from his administrative and teaching posts, continuing to be active in research.
Research interests
Berg's postgraduate studies involved the use of radioisotope tracers to study intermediary metabolism. This resulted in the understanding of how foodstuffs are converted to cellular materials, through the use of isotopic carbons or heavy nitrogen atoms. Paul Berg's doctorate paper is now known as the conversion of formic acid, formaldehyde and methanol to fully reduced states of methyl groups in methionine. He was also one of the first to demonstrate that folic acid and B12 cofactors had roles in the processes mentioned.
Berg is arguably most famous for his pioneering work involving gene splicing of recombinant DNA. Berg was the first scientist to create a molecule containing DNA from two different species by inserting DNA from another species into a molecule. This gene-splicing technique was a fundamental step in the development of modern genetic engineering. After developing the technique, Berg used it for his studies of viral chromosomes.
Berg was a professor emeritus at Stanford. As of 2000, he stopped doing active research, to focus on other interests, including involvement in public policy for biomedical issues involving recombinant DNA and embryonic stem cells and publishing a book about geneticist George Beadle.
Berg was a member of the Board of Sponsors of the Bulletin of the Atomic Scientists. He was also an organizer of the Asilomar conference on recombinant DNA in 1975. The previous year, Berg and other scientists had called for a voluntary moratorium on certain recombinant DNA research until they could evaluate the risks. That influential conference did evaluate the potential hazards and set guidelines for biotechnology research. It can be seen as an early application of the precautionary principle.
Awards and honors:
Nobel Prize
Berg was awarded one-half of the 1980 Nobel Prize in Chemistry, with the other half being shared by Walter Gilbert and Frederick Sanger. Berg was recognized for "his fundamental studies of the biochemistry of nucleic acids, with particular regard to recombinant DNA", while Sanger and Gilbert were honored for "their contributions concerning the determination of base sequences in nucleic acids."
Other awards and honors
He was elected a Fellow of the American Academy of Arts and Sciences and a member of the United States National Academy of Sciences in 1966. In 1983, Ronald Reagan presented Berg with the National Medal of Science. That same year, he was elected to the American Philosophical Society. In 1989, he received the Golden Plate Award of the American Academy of Achievement. He was elected a Foreign Member of the Royal Society (ForMemRS) in 1992. In 2005 he was awarded the Biotechnology Heritage Award by the Biotechnology Industry Organization (BIO) and the Chemical Heritage Foundation. In 2006 he received Wonderfest's Carl Sagan Prize for Science Popularization.
Death
Berg died on February 15, 2023, at the age of 96.
Additional Information:
Life
Paul Berg grew up in Brooklyn. A teacher awakened his scientific bent when she encouraged students to conduct their own research projects. Berg was studying biochemistry at Pennsylvania State University when World War II broke out. He served on a submarine before obtaining his degree in 1948. He received his doctorate at Case Western Reserve University, and after a period in Copenhagen, he worked with Arthur Kornberg in St. Louis, Missouri. Berg made his Nobel Prize-awarded discovery at Stanford University. In 1947 he married Mildred Levy, and the couple had a son, John.
Work
DNA carries organisms' genomes and also determines their vital processes. The ability to artificially manipulate DNA opens the way to creating organisms with new characteristics. In conjunction with his studies of the tumor virus SV40, in 1972, Paul Berg succeeded in inserting DNA from a bacterium into the virus' DNA. Berg thereby created the first DNA molecule made of parts from different organisms. This type of molecule became known as hybrid DNA or recombinant DNA. Among other things, Berg's method opened the way to creating bacteria that produce substances used in medicines.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1462 2024-03-16 17:51:23
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,394
Re: crème de la crème
1424) Walter Gilbert
Summary
Walter Gilbert (born March 21, 1932, Boston, Mass., U.S.) is an American molecular biologist who was awarded a share (with Paul Berg and Frederick Sanger) of the Nobel Prize for Chemistry in 1980 for his development of a method for determining the sequence of nucleotide links in the chainlike molecules of nucleic acids (DNA and RNA).
Gilbert graduated from Harvard University with a degree in physics in 1953 and took a Ph.D. in mathematics from Cambridge University in 1957. He joined the Harvard faculty as a lecturer in physics in 1958 and, as his interests changed, advanced to assistant professor of physics in 1959, associate professor of biophysics in 1964, and professor of biochemistry in 1968. In 1974 he became American Cancer Society Professor of Molecular Biology at Harvard.
In the late 1960s Gilbert confirmed the theory of Jacques Monod and Franƈois Jacob that “repressor proteins” control the genes responsible for beginning and ending protein synthesis in the cell. He was able to demonstrate the existence of a repressor in the bacterium Escherichia coli that prevents a gene from manufacturing a certain enzyme except when lactose is present. In the 1970s Gilbert developed a widely used technique of using gel electrophoresis to read the nucleotide sequences of DNA segments. The same method was developed independently by Sanger.
In 1979 Gilbert, while retaining his affiliation with Harvard, joined a group of other scientists and businessmen to form Biogen, a commercial genetic-engineering research corporation. Gilbert resigned from Biogen in 1985 and, while continuing to teach at Harvard, became a chief proponent of the Human Genome Project, a government-funded effort to compile a complete map of the gene sequences in human DNA. He became emeritus at Harvard in 1987.
Gilbert founded Myriad Genetics in 1992 and served as director and vice chairman of the board. He helped to found Paratek Pharmaceuticals (1996), a company invested in combatting bacterial resistance, and Memory Pharmaceuticals (1998), which was geared toward developing cures for central nervous system disorders. Gilbert was also managing director of BioVentures Investors, where he became a partner in 2001. He served on the advisory boards of several other biotechnology companies as well.
Details
Walter Gilbert (born March 21, 1932) is an American biochemist, physicist, molecular biology pioneer, and Nobel laureate.
Education and early life
Walter Gilbert was born in Boston, Massachusetts, on March 21, 1932, into a Jewish family, the son of Emma (Cohen), a child psychologist, and Richard V. Gilbert, an economist.
When Gilbert was seven years old, the family moved to the Washington D.C. area so his father could work under Harry Hopkins on the New Deal brain trust. While living in Washington the family became friends with the family of I.F. Stone and Wally met Stone's oldest daughter, Celia, when they were both 8. They later married at age 21.
He was educated at the Sidwell Friends School, and attended Harvard University for undergraduate and graduate studies, earning a baccalaureate in chemistry and physics in 1953 and a master's degree in physics in 1954. He studied for his doctorate at the University of Cambridge, where he earned a PhD in physics supervised by the Nobel laureate Abdus Salam in 1957.
Career and research
Gilbert returned to Harvard in 1956 and was appointed assistant professor of physics in 1959. Gilbert's wife Celia worked for James Watson, leading Gilbert to become interested in molecular biology. Watson and Gilbert ran their laboratory jointly through most of the 1960s, until Watson left for Cold Spring Harbor Laboratory. In 1964 he was promoted to associate professor of biophysics and promoted again in 1968 to professor of biochemistry.
Gilbert is a co-founder of the biotech start-up companies Biogen, with Kenneth Murray, Phillip Sharp and Charles Weissman and Myriad Genetics with Dr. Mark Skolnick and Kevin Kimberlin where he was the first chairman on their respective boards of directors. Gilbert left his position at Harvard to run Biogen as CEO, but was later asked to resign by the company's board of directors. He is a member of the Board of Scientific Governors at The Scripps Research Institute. Gilbert has served as the chairman of the Harvard Society of Fellows.
In 1996, Gilbert and Stuart B. Levy founded Paratek Pharmaceuticals. Gilbert served as chairman until 2014.
Gilbert was an early proponent of sequencing the human genome. At a March 1986 meeting in Santa Fe New Mexico he proclaimed "The total human sequence is the grail of human genetics". In 1987, he proposed starting a company called Genome Corporation to sequence the genome and sell access to the information. In an opinion piece in Nature in 1991, he envisioned completion of the human genome sequence transforming biology into a field in which computer databases would be as essential as laboratory reagents.
Gilbert returned to Harvard in 1985. Gilbert was an outspoken critic of David Baltimore in the handling of the scientific fraud accusations against Thereza Imanishi-Kari. Gilbert also joined the early controversy over the cause of AIDS. In 1962, Gilbert's PhD student in physics Gerald Guralnik extended Gilbert's work on massless particles; Guralnik's work on is widely recognized as an important thread in the discovery of the Higgs Boson.
With his PhD student Benno Müller-Hill, Gilbert was the first to purify the lac repressor, just beating out Mark Ptashne for purifying the first gene regulatory protein.
Together with Allan Maxam, Gilbert developed a new DNA sequencing method, Maxam–Gilbert sequencing, using chemical methods developed by Andrei Mirzabekov. His approach to the first synthesis of insulin via recombinant DNA lost out to Genentech's approach which used genes built up from the nucleotides rather than from natural sources. Gilbert's effort was hampered by a temporary moratorium on recombinant DNA work in Cambridge, Massachusetts, forcing his group to move their work to an English biological weapons site.
Gilbert first proposed the existence of introns and exons and explained the evolution of introns in a seminal 1978 "News and Views" paper published in Nature. In 1986, Gilbert proposed the RNA world hypothesis for the origin of life, based on a concept first proposed by Carl Woese in 1967.
Awards and honors
In 1969, Gilbert was awarded Harvard's Ledlie Prize. In 1972 he was named American Cancer Society Professor of Molecular Biology. In 1979, Gilbert was awarded the Louisa Gross Horwitz Prize from Columbia University together with Frederick Sanger. That year he was also awarded the Gairdner Prize and the Albert Lasker Award for Basic Medical Research.
Gilbert was awarded the 1980 Nobel Prize in Chemistry, shared with Frederick Sanger and Paul Berg. Gilbert and Sanger were recognized for their pioneering work in devising methods for determining the sequence of nucleotides in a nucleic acid.
Gilbert has also been honored by the National Academy of Sciences (US Steel Foundation Award, 1968); Massachusetts General Hospital (Warren Triennial Prize, 1977); the New York Academy of Sciences; (Louis and Bert Freedman Foundation Award, 1977), the Académie des Sciences of France (Prix Charles-Leopold Mayer Award, 1977). Gilbert was elected a Foreign Member of the Royal Society (ForMemRS) in 1987.
In 2002, he received the Biotechnology Heritage Award, from the Biotechnology Industry Organization (BIO) and the Chemical Heritage Foundation.
Allan Maxam and Walter Gilbert's 1977 paper "A new method for sequencing DNA" was honored by a Citation for Chemical Breakthrough Award from the Division of History of Chemistry of the American Chemical Society for 2017. It was presented to the Department of Molecular & Cellular Biology, Harvard University.
Personal life
Gilbert married Celia Stone, the daughter of I. F. Stone, in 1953 and has two children. After retiring from Harvard in 2001, Gilbert has launched an artistic career to combine art and science. His art format is centered on digital photography.
Additional Information
An organism's genome is stored in the form of long rows of building blocks, known as nucleotides, which form DNA molecules. An organism's genome can be mapped by establishing the order of the nucleotides within the DNA molecule. In 1976, Allan Maxam and Walter Gilbert developed a method by which the ends of the DNA molecule could be marked using radioactive substances. After undergoing treatment with small amounts of chemicals that react with specific nucleotides, DNA fragments of varying lengths can be obtained. After undergoing what is known as electrophoresis, the nucleotide sequences in a DNA sample can be identified.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1463 2024-03-18 18:00:48
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,394
Re: crème de la crème
1425) Frederick Sanger
Details
Frederick Sanger (13 August 1918 – 19 November 2013) was a British biochemist who received the Nobel Prize in Chemistry twice.
He won the 1958 Chemistry Prize for determining the amino acid sequence of insulin and numerous other proteins, demonstrating in the process that each had a unique, definite structure; this was a foundational discovery for the central dogma of molecular biology.
At the newly constructed Laboratory of Molecular Biology in Cambridge, he developed and subsequently refined the first-ever DNA sequencing technique, which vastly expanded the number of feasible experiments in molecular biology and remains in widespread use today. The breakthrough earned him the 1980 Nobel Prize in Chemistry, which he shared with Walter Gilbert and Paul Berg.
He is one of only three people to have won multiple Nobel Prizes in the same category (the others being John Bardeen in physics and Karl Barry Sharpless in chemistry), and one of five persons with two Nobel Prizes.
Early life and education
Frederick Sanger was born on 13 August 1918 in Rendcomb, a small village in Gloucestershire, England, the second son of Frederick Sanger, a general practitioner, and his wife, Cicely Sanger (née Crewdson). He was one of three children. His brother, Theodore, was only a year older, while his sister May (Mary) was five years younger. His father had worked as an Anglican medical missionary in China but returned to England because of ill health. He was 40 in 1916 when he married Cicely, who was four years younger. Sanger's father converted to Quakerism soon after his two sons were born and brought up the children as Quakers. Sanger's mother was the daughter of an affluent cotton manufacturer and had a Quaker background, but was not a Quaker.
When Sanger was around five years old the family moved to the small village of Tanworth-in-Arden in Warwickshire. The family was reasonably wealthy and employed a governess to teach the children. In 1927, at the age of nine, he was sent to the Downs School, a residential preparatory school run by Quakers near Malvern. His brother Theo was a year ahead of him at the same school. In 1932, at the age of 14, he was sent to the recently established Bryanston School in Dorset. This used the Dalton system and had a more liberal regime which Sanger much preferred. At the school he liked his teachers and particularly enjoyed scientific subjects. Able to complete his School Certificate a year early, for which he was awarded seven credits, Sanger was able to spend most of his last year of school experimenting in the laboratory alongside his chemistry master, Geoffrey Ordish, who had originally studied at Cambridge University and been a researcher in the Cavendish Laboratory. Working with Ordish made a refreshing change from sitting and studying books and awakened Sanger's desire to pursue a scientific career. In 1935, prior to heading off to college, Sanger was sent to Schule Schloss Salem in southern Germany on an exchange program. The school placed a heavy emphasis on athletics, which caused Sanger to be much further ahead in the course material compared to the other students. He was shocked to learn that each day was started with readings from Hitler's Mein Kampf, followed by a Sieg Heil salute.
In 1936 Sanger went to St John's College, Cambridge, to study natural sciences. His father had attended the same college. For Part I of his Tripos he took courses in physics, chemistry, biochemistry and mathematics but struggled with physics and mathematics. Many of the other students had studied more mathematics at school. In his second year he replaced physics with physiology. He took three years to obtain his Part I. For his Part II he studied biochemistry and obtained a 1st Class Honours. Biochemistry was a relatively new department founded by Gowland Hopkins with enthusiastic lecturers who included Malcolm Dixon, Joseph Needham and Ernest Baldwin.
Both his parents died from cancer during his first two years at Cambridge. His father was 60 and his mother was 58. As an undergraduate Sanger's beliefs were strongly influenced by his Quaker upbringing. He was a pacifist and a member of the Peace Pledge Union. It was through his involvement with the Cambridge Scientists' Anti-War Group that he met his future wife, Joan Howe, who was studying economics at Newnham College. They courted while he was studying for his Part II exams and married after he had graduated in December 1940. Sanger, although brought up and influenced by his religious upbringing, later began to lose sight of his Quaker related ways. He began to see the world through a more scientific lens, and with the growth of his research and scientific development he slowly drifted farther from the faith he grew up with. He has nothing but respect for the religious and states he took two things from it, truth and respect for all life. Under the Military Training Act 1939 he was provisionally registered as a conscientious objector, and again under the National Service (Armed Forces) Act 1939, before being granted unconditional exemption from military service by a tribunal. In the meantime he undertook training in social relief work at the Quaker centre, Spicelands, Devon and served briefly as a hospital orderly.
Sanger began studying for a PhD in October 1940 under N.W. "Bill" Pirie. His project was to investigate whether edible protein could be obtained from grass. After little more than a month Pirie left the department and Albert Neuberger became his adviser. Sanger changed his research project to study the metabolism of lysine and a more practical problem concerning the nitrogen of potatoes. His thesis had the title, "The metabolism of the amino acid lysine in the animal body". He was examined by Charles Harington and Albert Charles Chibnall and awarded his doctorate in 1943.
Research and career
Neuberger moved to the National Institute for Medical Research in London, but Sanger stayed in Cambridge and in 1943 joined the group of Charles Chibnall, a protein chemist who had recently taken up the chair in the Department of Biochemistry. Chibnall had already done some work on the amino acid composition of bovine insulin and suggested that Sanger look at the amino groups in the protein. Insulin could be purchased from the pharmacy chain Boots and was one of the very few proteins that were available in a pure form. Up to this time Sanger had been funding himself. In Chibnall's group he was initially supported by the Medical Research Council and then from 1944 until 1951 by a Beit Memorial Fellowship for Medical Research..
Sanger's first triumph was to determine the complete amino acid sequence of the two polypeptide chains of bovine insulin, A and B, in 1952 and 1951, respectively. Prior to this it was widely assumed that proteins were somewhat amorphous. In determining these sequences, Sanger proved that proteins have a defined chemical composition.
To get to this point, Sanger refined a partition chromatography method first developed by Richard Laurence Millington Synge and Archer John Porter Martin to determine the composition of amino acids in wool. Sanger used a chemical reagent 1-fluoro-2,4-dinitrobenzene (now, also known as Sanger's reagent, fluorodinitrobenzene, FDNB or DNFB), sourced from poisonous gas research by Bernard Charles Saunders at the Chemistry Department at Cambridge University. Sanger's reagent proved effective at labelling the N-terminal amino group at one end of the polypeptide chain. He then partially hydrolysed the insulin into short peptides, either with hydrochloric acid or using an enzyme such as trypsin. The mixture of peptides was fractionated in two dimensions on a sheet of filter paper, first by electrophoresis in one dimension and then, perpendicular to that, by chromatography in the other. The different peptide fragments of insulin, detected with ninhydrin, moved to different positions on the paper, creating a distinct pattern that Sanger called "fingerprints". The peptide from the N-terminus could be recognised by the yellow colour imparted by the FDNB label and the identity of the labelled amino acid at the end of the peptide determined by complete acid hydrolysis and discovering which dinitrophenyl-amino acid was there.
By repeating this type of procedure Sanger was able to determine the sequences of the many peptides generated using different methods for the initial partial hydrolysis. These could then be assembled into the longer sequences to deduce the complete structure of insulin. Finally, because the A and B chains are physiologically inactive without the three linking disulfide bonds (two interchain, one intrachain on A), Sanger and coworkers determined their assignments in 1955. Sanger's principal conclusion was that the two polypeptide chains of the protein insulin had precise amino acid sequences and, by extension, that every protein had a unique sequence. It was this achievement that earned him his first Nobel prize in Chemistry in 1958. This discovery was crucial to the later sequence hypothesis of Francis Crick for developing ideas of how DNA codes for proteins.
Sequencing RNA
From 1951 Sanger was a member of the external staff of the Medical Research Council and when they opened the Laboratory of Molecular Biology in 1962, he moved from his laboratories in the Biochemistry Department of the university to the top floor of the new building. He became head of the Protein Chemistry division.
Prior to his move, Sanger began exploring the possibility of sequencing RNA molecules and began developing methods for separating ribonucleotide fragments generated with specific nucleases. This work he did while trying to refine the sequencing techniques he had developed during his work on insulin.
The key challenge in the work was finding a pure piece of RNA to sequence. In the course of the work he discovered in 1964, with Kjeld Marcker, the formylmethionine tRNA which initiates protein synthesis in bacteria. He was beaten in the race to be the first to sequence a tRNA molecule by a group led by Robert Holley from Cornell University, who published the sequence of the 77 ribonucleotides of alanine tRNA from Saccharomyces cerevisiae in 1965. By 1967 Sanger's group had determined the nucleotide sequence of the 5S ribosomal RNA from Escherichia coli, a small RNA of 120 nucleotides.
Sequencing DNA
Sanger then turned to sequencing DNA, which would require an entirely different approach. He looked at different ways of using DNA polymerase I from E. coli to copy single stranded DNA. In 1975, together with Alan Coulson, he published a sequencing procedure using DNA polymerase with radiolabelled nucleotides that he called the "Plus and Minus" technique. This involved two closely related methods that generated short oligonucleotides with defined 3' termini. These could be fractionated by electrophoresis on a polyacrylamide gel and visualised using autoradiography. The procedure could sequence up to 80 nucleotides in one go and was a big improvement on what had gone before, but was still very laborious. Nevertheless, his group were able to sequence most of the 5,386 nucleotides of the single-stranded bacteriophage φX174. This was the first fully sequenced DNA-based genome. To their surprise they discovered that the coding regions of some of the genes overlapped with one another.
In 1977 Sanger and colleagues introduced the "dideoxy" chain-termination method for sequencing DNA molecules, also known as the "Sanger method". This was a major breakthrough and allowed long stretches of DNA to be rapidly and accurately sequenced. It earned him his second Nobel prize in Chemistry in 1980, which he shared with Walter Gilbert and Paul Berg. The new method was used by Sanger and colleagues to sequence human mitochondrial DNA (16,569 base pairs) and bacteriophage λ (48,502 base pairs). The dideoxy method was eventually used to sequence the entire human genome.
Postgraduate students
During the course of his career Sanger supervised more than ten PhD students, two of whom went on to also win Nobel Prizes. His first graduate student was Rodney Porter who joined the research group in 1947. Porter later shared the 1972 Nobel Prize in Physiology or Medicine with Gerald Edelman for his work on the chemical structure of antibodies. Elizabeth Blackburn studied for a PhD in Sanger's laboratory between 1971 and 1974. She shared the 2009 Nobel Prize in Physiology or Medicine with Carol W. Greider and Jack W. Szostak for her work on telomeres and the action of telomerase.
Additional Information
Frederick Sanger (born August 13, 1918, Rendcombe, Gloucestershire, England—died November 19, 2013, Cambridge) English biochemist who was twice the recipient of the Nobel Prize for Chemistry. He was awarded the prize in 1958 for his determination of the structure of the insulin molecule. He shared the prize (with Paul Berg and Walter Gilbert) in 1980 for his determination of base sequences in nucleic acids. Sanger was the fourth two-time recipient of the Nobel Prize.
Education
Sanger was the middle child of Frederick Sanger, a medical practitioner, and Cicely Crewsdon Sanger, the daughter of a wealthy cotton manufacturer. The family expected him to follow in his father’s footsteps and become a medical doctor. After much thought, he decided to become a scientist. In 1936 Sanger entered St. John’s College, Cambridge. He initially concentrated on chemistry and physics, but he was later attracted to the new field of biochemistry. He received a bachelor’s degree in 1939 and stayed at Cambridge an additional year to take an advanced course in biochemistry. He and Joan Howe married in 1940 and subsequently had three children.
Because of his Quaker upbringing, Sanger was a conscientious objector and was assigned as an orderly to a hospital near Bristol when World War II began. He soon decided to visit Cambridge to see if he could enter the doctoral program in biochemistry. Several researchers there were interested in having a student, especially one who did not need money. He studied lysine metabolism with biochemist Albert Neuberger. They also had a project in support of the war effort, analyzing nitrogen from potatoes. Sanger received a doctorate in 1943.
Insulin research
Biochemist Albert C. Chibnall and his protein research group moved from Imperial College in London to the safer wartime environment of the biochemistry department at Cambridge. Two schools of thought existed among protein researchers at the time. One group thought proteins were complex mixtures that would not readily lend themselves to chemical analysis. Chibnall was in the other group, which considered a given protein to be a distinct chemical compound.
Chibnall was studying insulin when Sanger joined the group. At Chibnall’s suggestion, Sanger set out to identify and quantify the free-amino groups of insulin. Sanger developed a method using dinitrofluorobenzene to produce yellow-coloured derivatives of amino groups (see amino acid). Information about a new separation technique, partition chromatography, had recently been published. In a pattern that typified Sanger’s career, he immediately recognized the utility of the new technique in separating the hydrolysis products of the treated protein. He identified two terminal amino groups for insulin, phenylalanine and glycine, suggesting that insulin is composed of two types of chains. Working with his first graduate student, Rodney Porter, Sanger used the method to study the amino terminal groups of several other proteins. (Porter later shared the 1972 Nobel Prize for Physiology or Medicine for his work in determining the chemical structure of antibodies.)
On the assumption that insulin chains are held together by disulphide linkages, Sanger oxidized the chains and separated two fractions. One fraction had phenylalanine at its amino terminus; the other had glycine. Whereas complete acid hydrolysis degraded insulin to its constituent amino acids, partial acid hydrolysis generated insulin peptides composed of several amino acids. Using another recently introduced technique, paper chromatography, Sanger was able to sequence the amino-terminal peptides of each chain, demonstrating for the first time that a protein has a specific sequence at a specific site. A combination of partial acid hydrolysis and enzymatic hydrolysis allowed Sanger and the Austrian biochemist Hans Tuppy to determine the complete sequence of amino acids in the phenylalanine chain of insulin. Similarly, Sanger and the Australian biochemist E.O.P. Thompson determined the sequence of the glycine chain.
Two problems remained: the distribution of the amide groups and the location of the disulphide linkages. With the completion of those two puzzles in 1954, Sanger had deduced the structure of insulin. For being the first person to sequence a protein, Sanger was awarded the 1958 Nobel Prize for Chemistry.
Sanger and his coworkers continued their studies of insulin, sequencing insulin from several other species and comparing the results. Utilizing newly introduced radiolabeling techniques, Sanger mapped the amino acid sequences of the active centres from several enzymes. One of these studies was conducted with another graduate student, Argentine-born immunologist César Milstein. (Milstein later shared the 1984 Nobel Prize for Physiology or Medicine for discovering the principle for the production of monoclonal antibodies.)
RNA research
In 1962 the Medical Research Council opened its new laboratory of molecular biology in Cambridge. The Austrian-born British biochemist Max Perutz, British biochemist John Kendrew, and British biophysicist Francis Crick moved to the new laboratory. Sanger joined them as head of the protein division. It was a banner year for the group, as Perutz and Kendrew shared the 1962 Nobel Prize for Chemistry and Crick shared the 1962 Nobel Prize for Physiology or Medicine with the American geneticist James D. Watson and the New Zealand-born British biophysicist Maurice Wilkins for the discovery of DNA (deoxyribonucleic acid).
Sanger’s interaction with nucleic acid groups at the new laboratory led to his pursuing studies on ribonucleic acid (RNA). RNA molecules are much larger than proteins, so obtaining molecules small enough for technique development was difficult. The American biochemist Robert W. Holley and his coworkers were the first to sequence RNA when they sequenced alanine-transfer RNA. They used partial hydrolysis methods somewhat like those Sanger had used for insulin. Unlike other RNA types, transfer RNAs have many unusual nucleotides. This partial hydrolysis method would not work well with other RNA molecules, which contain only four types of nucleotides, so a new strategy was needed.
The goal of Sanger’s lab was to sequence a messenger RNA and determine the genetic code, thereby solving the puzzle of how groups of nucleotides code for amino acids. Working with British biochemists George G. Brownlee and Bart G. Barrell, Sanger developed a two-dimensional electrophoresis method for sequencing RNA. By the time the sequence methods were worked out, the code had been broken by other researchers, mainly the American biochemist Marshall Nirenberg and the Indian-born American biochemist Har Gobind Khorana, using in vitro protein synthesis techniques. The RNA sequence work of Sanger’s group did confirm the genetic code.
DNA research
By the early 1970s Sanger was interested in deoxyribonucleic acid (DNA). DNA sequence studies had not developed because of the immense size of DNA molecules and the lack of suitable enzymes to cleave DNA into smaller pieces. Building on the enzyme copying approach used by the Swiss chemist Charles Weissmann in his studies on bacteriophage RNA, Sanger began using the enzyme DNA polymerase to make new strands of DNA from single-strand templates, introducing radioactive nucleotides into the new DNA. DNA polymerase requires a primer that can bind to a known region of the template strand. Early success was limited by the lack of suitable primers. Sanger and British colleague Alan R. Coulson developed the “plus and minus” method for rapid DNA sequencing. It represented a radical departure from earlier methods in that it did not utilize partial hydrolysis. Instead, it generated a series of DNA molecules of varying lengths that could be separated by using polyacrylamide gel electrophoresis. For both plus and minus systems, DNA was synthesized from templates to generate random sets of DNA molecules from very short to very long. When both plus and minus sets were separated on the same gel, the sequence could be read from either system, one confirming the other. In 1977 Sanger’s group used this system to deduce most of the DNA sequence of bacteriophage ΦX174, the first complete genome to be sequenced.
A few problems remained with the plus and minus system. Sanger, Coulson, and British colleague Steve Nicklen developed a similar procedure using dideoxynucleotide chain-terminating inhibitors. DNA was synthesized until an inhibitor molecule was incorporated into the growing DNA chain. Using four reactions, each with a different inhibitor, sets of DNA fragments were generated ending in every nucleotide. For example, in the A reaction, a series of DNA fragments ending in A (adenine) was generated. In the C reaction, a series of DNA fragments ending in C (cytosine) was generated, and so on for G (guanine) and T (thymine). When the four reactions were separated side by side on a gel and an autoradiograph developed, the sequence was read from the film. Sanger and his coworkers used the dideoxy method to sequence human mitochondrial DNA. For his contributions to DNA sequencing methods, Sanger shared the 1980 Nobel Prize for Chemistry. He retired in 1983.
Additional honours
Sanger’s additional honours included election as a fellow of the Royal Society (1954), being named a Commander of the Order of the British Empire (CBE; 1963), receiving the Royal Society’s Royal Medal (1969) and Copley Medal (1977), and election to the Order of the Companions of Honour (CH; 1981) and the Order of Merit (OM; 1986). In 1993 the Wellcome Trust and the British Medical Research Council established a genome research centre, honouring Sanger by naming it the Wellcome Trust Sanger Institute.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1464 2024-03-19 17:20:17
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,394
Re: crème de la crème
1426) Baruj Benacerraf
Details
Baruj Benacerraf (October 29, 1920 – August 2, 2011) was a Venezuelan-American immunologist, who shared the 1980 Nobel Prize in Physiology or Medicine for the "discovery of the major histocompatibility complex genes which encode cell surface protein molecules important for the immune system's distinction between self and non-self." His colleagues and shared recipients were Jean Dausset and George Davis Snell.
Early life and education
Benacerraf was born in Caracas, Venezuela on October 29, 1920, to a Moroccan-Venezuelan father and Algerian mother. His father was a textile merchant. His brother was philosopher Paul Benacerraf. He moved to Paris from Venezuela with his family in 1925. After going back to Venezuela, he emigrated to the U.S. in 1940. That same year, Benacerraf attended Lycée Français de New York, where he earned a Baccalauréat (an academic qualification French students achieve after high school and a diploma necessary to begin university studies).
In 1942, he earned his B.S. at Columbia University School of General Studies. He then went on to obtain his Doctor of Medicine (M.D.) degree from the Medical College of Virginia, the only school to which he was accepted due to his Jewish background. Shortly after beginning medical school, Benacerraf became a naturalized U.S. citizen.
From his Nobel autobiography: "By that time, I had elected to study biology and medicine, instead of going into the family business, as my father would have wanted. I did not realize, however, that admission to Medical School was a formidable undertaking for someone with my ethnic and foreign background in the United States of 1942. In spite of an excellent academic record at Columbia, I was refused admission by the numerous medical schools I applied to and would have found it impossible to study medicine except for the kindness and support of George W. Bakeman, father of a close friend, who was then Assistant to the President of the Medical College of Virginia in Richmond. Learning of my difficulties, Mr. Bakeman arranged for me to be interviewed and considered for one of the two remaining places in the Freshman class."
Career
After his medical internship, US Army service (1945–48), and working at the military hospital of Nancy, France, he became a researcher at Columbia University College of Physicians and Surgeons (1948–50). He performed research in Paris (1950–56), relocated to New York University (1956–68), moved to the National Institutes of Health (1968–70), then joined Harvard University medical school in Boston (1970–91) where he became the Fabyan Professor of comparative Pathology, concurrently serving the Dana–Farber Cancer Institute (1980). He began studying allergies in 1948, and discovered the Ir (immune response) genes that govern transplant rejection in the 1960s. Including a variety of different editions, Benacerraf is an author of over 300 books and articles.
At Columbia, Benacerraf got his start in immunology with Elvin A. Kabat in 1948. He spent two years in Kabat's laboratory working on experimental hypersensitivity mechanisms. He then moved to Paris because of family issues and accepted a position in Bernard Halpern's laboratory at the Hôpital Broussais. Here he also formed a close relationship with Italian scientist Guido Biozzi. For six years he worked on the reticuloendothelial function in relation to immunity. The reticuloendothelial function is the white blood cells inside of a barrier tissue. While there they discovered techniques to study the clearance of particulate matter from the blood by the RES (reticuloendothelial system), and devised equations that govern this process in mammals. After six years, Baruj returned to the United States in 1956 because he could not establish his own independent laboratory in France. He was recruited to the faculty of New York University (NYU), established his own laboratory, and returned to his studies on hypersensitivity.
In New York, Baruj worked with several other immunologists on different fields of hypersensitivity. After working in his New York lab, Baruj turned his attention towards the training of new scientists, and made the decision to devote himself to his laboratory practices, instead of the family business. At this time Baruj also made the discovery that would go on to win him the Nobel Prize. He noticed that if antigens (something that causes a reaction with the immune system) were injected into animals with a similar heredity, two groups emerged: responders and non-responders. He then conducted further study and found that the dominant autosomal genes, termed the immune response genes, determined the response to certain antigens. This complex process would lead to the understanding of how these genes would determine immune responses.
His discovery still holds true, and more has been discovered over the last century. More than 30 genes have been discovered in a gene complex called the major histocompatibility complex. The histocompatibility complex is a complex part of DNA that controls the immune response. This research has also led to clarify autoimmune diseases such as multiple sclerosis and rheumatoid arthritis.
Additional Information
Baruj Benacerraf (born October 29, 1920, Caracas, Venezuela—died August 2, 2011, Boston, Massachusetts, U.S.) Venezuelan-born American pathologist and immunologist who shared (with George Snell and Jean Dausset) the 1980 Nobel Prize for Physiology or Medicine for his discovery of genes that regulate immune responses and of the role that some of these genes play in autoimmune diseases.
From the age of five until the outbreak of World War II, Benacerraf lived in Paris. In 1940 he entered Columbia University in New York City, from which he graduated in 1942. He became a naturalized U.S. citizen in 1943, while a student at the Medical College of Virginia in Richmond. After receiving an M.D. in 1945 and interning at Queens General Hospital in New York City, he served (1946–47) in the U.S. Army Medical Corps. Benacerraf then spent a year in immunological research at the Columbia University College of Physicians and Surgeons. He moved on to the French National Centre for Scientific Research at the Broussais Hospital in Paris, where he continued to study immunology. In 1956 he joined the faculty of New York University (NYU) School of Medicine. He advanced to professor of pathology in 1960, a position he held until 1968.
At NYU Benacerraf began to study the genetics of the immune system. His experiments led to his development of the concept of immune response (Ir) genes, which control the immune system’s ability to respond to antigens (infectious agents or foreign materials that enter the body). More than 30 Ir genes were subsequently found, and that genetic material was determined to be part of the major histocompatibility complex, a complicated region of DNA involved in immune responsiveness. Benacerraf’s findings also helped elucidate the mechanisms underlying autoimmune diseases, such as multiple sclerosis and rheumatoid arthritis, in which the immune system mistakenly mounts an attack against its own tissues.
In 1968 Benacerraf became chief of the immunology laboratory at the National Institute of Allergy and Infectious Diseases in Bethesda, Maryland. From 1970 to 1991 he served as both professor of comparative pathology and chairman of the pathology department at Harvard University Medical School. He also was president (1980–91) of the Sidney Farber Cancer Institute (now the Dana-Farber Cancer Institute) in Boston. Benacerraf was elected to the National Academy of Sciences (1973) and was awarded the National Medal of Science (1990). He published a number of books, including the Textbook of Immunology (1984) and his autobiography, From Caracas to Stockholm (1998).
Summary
Our immune system rejects damaged or abnormal cells, allowing our bodies to function properly. During the 1960s Baruj Benaceraff showed through studies of guinea pigs that the immune system’s reaction to certain substances is determined by genes that exist in a certain area on a certain chromosome. George Snell and Jean Dausset had previously shown that genes that govern rejection of foreign cells exist in similar areas in mice and in people. Benaceraff’s results shed light on the interplay among different parts of the immune system.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1465 2024-03-21 17:25:29
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,394
Re: crème de la crème
1427) Jean Dausset
Details
Jean-Baptiste-Gabriel-Joachim Dausset (19 October 1916 – 6 June 2009) was a French immunologist born in Toulouse, France. Dausset received the Nobel Prize in Physiology or Medicine in 1980 along with Baruj Benacerraf and George Davis Snell for their discovery and characterisation of the genes making the major histocompatibility complex. Using the money from his Nobel Prize and a grant from the French Television, Dausset founded the Human Polymorphism Study Center (CEPH) in 1984, which was later renamed the Foundation Jean Dausset-CEPH in his honour. He married Rose Mayoral in 1963, with whom he had two children, Henri and Irène. Jean Dausset died on June 6, 2009, in Majorca, Spain, at the age of 92.
Early life
Jean-Baptiste-Gabriel-Joachim Dausset was born on 19 October 1916, in Toulouse, France. He was the youngest of four children of Henri Dausset and Elisabeth Dausset (born Renard). His father was from the Pyrénées, and was a doctor by profession, and his mother was a housewife from Lorraine. Following the end of World War I the Dausset family moved to Biarritz where Jean spent most of his childhood. His father was head doctor at the Bayonne Hospital, a position that would make a great impression on Jean. Dausset was home schooled by his mother, as well as by a tutor, who would periodically come to the house. At the age of 11, Dausset and his family moved to Paris. He began his formal schooling at the Lycée Michelet, at age 15. After earning his Baccalauréat in mathematics, Dausset was convinced by his father to study medicine at the University of Paris. Both of Dausset's parents died just after he became an extern at the Paris Hospitals, at the age of 19. Dausset failed to pass an internship entrance exam at the Paris Hospitals, and was preparing for a second attempt when World War II broke out.
War
Dausset was enlisted in the French army, and sent off to Northern Italy for a year. Upon his return to Paris in 1940, Dausset studied ardently and passed his medical internship exam. Soon after passing his exams, Dausset joined the Free French Forces in North Africa as an ambulance worker. He was originally stationed in Morocco, but was later sent to the more active Tunisia. Dausset got his first taste of hematology when he had to perform numerous blood transfusions on wounded soldiers. As the war was winding down in 1944, Dausset returned to Paris where he worked in the Regional Blood Transfusion Center at the Saint-Antoine Hospital.
Medical reform
After the war, Dausset worked as an intern at the Paris Hospitals, which were in a state of disrepair and badly needed structural reform. Dausset formed a group of radical doctors who pushed for change in the French medical system. Due to his activist role in this group, Dausset was appointed as the Advisor to the Cabinet of the National Ministry of Education. The physician Robert Debré worked with Dausset, and pushed the government into forming a committee for the reform of medical education. Hospitals were joined with universities for the first time in France, and doctors were required to instruct classes to medical students. With the newly introduced system of university research hospitals, research began within the hospitals themselves, as opposed to in the universities alone, and full-time work for doctors was established (Debré reform of 11 and 30 December 1958).
Career
After World War II Dausset worked with Professor Marcel Bessis who had developed a new transfusion technique called exchange transfusion. He worked as an immunohematologist and was interested in anaemic patients who required blood transfusions, he found that these patients lacked both red and white blood cells. In 1948, Dausset went to work as an intern in the Children's Hospital in Boston. He worked there in a hematology lab for about four years.
He returned to France in 1952 and once again took up the position of an intern with Marcel Bessis. It was during this time that Dausset conducted his first official research. With Bessis, Dausset discovered the first antigen-presenting leucocyte, but it was only officially described in 1958. Between the years 1952 and 1957 he collaborated with many researchers such as Gilbert Malinvaud and Jacques and Monique Colombani. From 1952 to 1957, majority of Dausset's time went into developing techniques and developing further ways of confirming the presence of certain antibodies. He was made the head of research at Professor Georges Marchal’s immunohematology laboratory in the Broussais Hospital. During this time, Dausset performed blood transfusions between a voluntary donor and patients in order to further his research in the field of immune responses in the body. He was testing the ability of the antigen-presenting leucocytes in the recipient’s body, which came originally from the donor’s blood. In 1958, Dausset discovered an antibody called MAC which was a leuco-aggluntinate; the abbreviation MAC actually stands for the initials of the names of the donors whose blood Dausset and his colleagues had used during the research. From 1960 to 1965 Dausset worked primarily on improving organ transplantation techniques and the mechanisms involved in enhancing the body’s ability to accept the new tissue. To do this, Dausset again used the blood of voluntary donors and patients to see what differences lay in the blood of the two individuals and how to minimize these differences. 1965 was a crucial year for research. There was an intense competition amongst the researchers of the immunohematology field as everyone was on the brink of making a major discovery in the genetic and transplantation research. During this time, Dausset worked with Paul Ivany in Prague and they used leuco-agglutination and lymphocyte toxicity techniques to make some very significant discoveries. They discovered the Hu-1 antigen and the H-2 antigen.
In 1963 Jean Dausset became the head of the immunology at the Hôpital Saint-Louis in 1963. This is when he discovered the HLA system, with Felix Rapaport; by performing skin transplant experiments on volunteers and showed that success depended on histocompatibility. Dausset was the assistant director of the Research Institute in Blood diseases until 1968, he then became the director of the "Institut National de la Santé et de la Recherche Médicale"(INSERM) transplantation immunogenetics research unit. Dausset founded France Transplant and France Greffe de Moelle, which brings matching donor organs to recipients and provides bone marrow for transplant respectively.
Research
Dausset began his research shortly after obtaining his medical degree in 1945, while working as an intern in the hematology lab at the Children’s Hospital in Boston. His first paper was published in 1950, and dealt with the detection of incomplete antibodies using trypsinized erythrocytes in a plasmatic medium, a technique that displayed improved sensitivity over other techniques used at the time. He went on to publish more works in the field of hematology, including developing a technique in 1952 for the removal of plasma from red blood cells to be used in transfusions to patients somehow intolerant of whole blood transfusions. In 1952 he returned to France and continued his research, particularly focusing on hemolytic anemia, and publishing several works dealing with various forms of blood cell agglutination. It was during this period of research, in 1954, when Dausset first observed an anti-leucocyte agglutinating substance, though it was not until 1958 that he identified an isoantibody specific to leucocytes, and published his findings. It was this finding and the extensive cascade of work that followed that would ultimately earn Dausset his Nobel Prize.
General research in antibodies, agglutination, and anemia continued in the years following this 1958 paper. In 1962, Dausset published an examination of the correlation between leuco-agglutination and skin graft tolerance, his first observation of the antigens’ impact on histocompatibility. His next paper on the subject was published in 1964, when he observed a clear relationship between leucocyte antigen compatibility and antibody response to skin grafts. This finding sparked a flurry of research in the topic of histocompatibility, and by the end of 1965 Dausset had published over a dozen papers exploring leucocyte antigens and their relevance to histocompatibility. After identifying that a two-allele leucocyte antigen group had an influence on histocompatibility and observing the induction of hyper-sensitivity to skin grafts following injection of leucocyte fractions, Dausset developed a system for grouping leucocyte antigens on the basis of histocompatibility. Following this, he put forth the hypothesis that all known leucocyte antigens were part of a single complex, a complex which he named Hu-1. This complex would later become known as one of the Major Histocompatibility Complexes (MHC), specifically those termed Human Leucocyte Antigens (HLA). Dausset's further work in 1965 examining the effects of Hu-1 antigen injection on skin graft rejection further confirmed the conclusion that this Hu-1 complex was indeed a transplantation antigen, a conclusion which would in time have profound effects on the transplantation process.
In the years to come, Dausset continued his research on the Hu-1 complex. Through 1966-1967 he published several more papers on the subject, including a paper summarizing the relevance of Hu-1 antigens to oncogenesis and transplantation, development of the use of a platelet complementation fixation test to identify which antigens are present, and the discovery that Hu-1 is homologous to the mouse H-2 complex, which also functions in histocompatibility. Toward the end of 1967 he confirmed through familial studies that all discovered antigens were in fact part of a single system. Following 1967, Dausset participated in numerous other studies pertaining to the complex (which was renamed HLA in 1968), particularly those examining the genetic basis for the antigens’ transmission, along with publishing a number of other papers for which he claimed primary authorship. For his contribution to these studies as well as his ultimate role in the discovery of this crucial antigen, Jean Dausset received the Nobel Prize in Physiology and Medicine in 1980.
Following his reception of the Nobel Prize, Dausset's personal research slowed considerably. He contributed to multiple studies, particularly a number relating to genetics, but did not publish anything for which he claimed primary authorship for over a decade. He retired in 2003, at the age of 87.
Nobel Prize
In 1975, Dausset suspected that his name had been entered in the nominations for the Nobel Prize. However, nothing came of this until February 1980, when rumours were circulating in the scientific community about his possible candidacy. At this point, Dausset was planning on going to Quebec, Canada to do a conference series throughout the province. He was in a dilemma because if did win, he did not want to be away from his family and colleagues, and yet, were he to stay in France, people would suspect that he was sure of winning and he might set himself up for disappointment. So instead, he decided to stay in Paris, but to stay out of the public eye. He was unsurprisingly awarded the Nobel Prize 1980, which he shared with Baruj Benacerraf and George Davis Snell.
Later life
In 1984 Dausset founded the Centre D’étude du Polymorphisme Humain (CEPH), aiming to detect the major genes in humans that are responsible for diseases outside the HLA system. Localization of these genes was a crucial step in cloning and identifying them, this was a breakthrough for medical genetics. The CEPH system contributed DNA from 61 large families to international centers that were responsible for mapping the human genome. Dausset and professor LL Cavalli-Sforza collaborated, and developed a DNA resource from world populations known as HGDP-CEPH diversity panel, to be used in human population genetics. CEPH is a non-profit organisation that was partly funded by the French government, it was not until 1993, that CEPH was renamed the Foundation Jean Dausset-CEPH. In 2003, at the age of 87, Dausset retired and became the president of CEPH.
Dausset was a member of the French Academy of Sciences. He was also a professor at the college de France. Dausset was one of the influential foreign members of the National Academy of Sciences, US, and an honorary member of the American Academy of Arts and Sciences. He was a member of the founding Council and Vice President of the Human Genome Organization. Dausset received various prestigious prizes, such as the Landsteiner Award and prizes for the Koch and Wolf Foundations. He also served on the advisory boards of numerous research institutions.
Additional Information
Jean Dausset (born Oct. 19, 1916, Toulouse, France—died June 6, 2009, Palma, Majorca, Spain) was a French hematologist and immunologist whose studies of the genetic basis of the immunological reaction earned him a share (with George Snell and Baruj Benacerraf) of the 1980 Nobel Prize for Physiology or Medicine.
After serving with the Free French forces in World War II, Dausset resumed his interrupted medical studies and took his degree from the University of Paris in 1945. He pursued advanced studies in the United States at Harvard University and later returned to France and became laboratory director of the National Blood Transfusion Centre. From 1958 to 1977 he conducted research and taught at the University of Paris, and from 1977 to 1987 he was a professor of experimental medicine at the Collège de France. In 1984 Dausset established the Human Polymorphism Study Center (CEPH; later renamed the Foundation Jean Dausset–CEPH) in France in collaboration with professors Howard Cann and Daniel Cohen.
In the 1950s Dausset began studying the severe reduction in white blood cells (leukocytes) that occurred in recipients of numerous blood transfusions. He found that cell loss resulted from the action of antibodies that selectively attacked the foreign leukocytes received through transfusion while avoiding the body’s own white blood cells. Dausset correctly hypothesized that these antibody reactions were stimulated by certain antigens, located on the surface of foreign white blood cells, that were later called human leukocyte antigens (HLA). These antigens proved to be extremely useful in determining whether tissues from one person might be successfully transplanted to another individual (a process, similar to blood typing, called tissue typing). Dausset also demonstrated that the HLA antigens are programmed by a highly variable gene complex that proved to be analogous to the H-2 complex in the mouse discovered by George Snell. Both systems came to be seen as types of the major histocompatibility complex, which functions in helping the immune system of all vertebrates to distinguish between its own cells and foreign substances that gain entry to the body. Dausset’s autobiography, Clin d’oeil à la vie (“A Wink at Life”), was published in 1998.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1466 2024-03-24 21:47:41
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,394
Re: crème de la crème
1428) George Davis Snell
Summary
George Davis Snell (born Dec. 19, 1903, Bradford, Mass., U.S.—died June 6, 1996, Bar Harbor, Maine) was an American immunogeneticist who, with Jean Dausset and Baruj Benacerraf, was awarded the 1980 Nobel Prize for Physiology or Medicine for his studies of histocompatibility (a compatibility between the genetic makeup of donor and host that allows a tissue graft from the former to be accepted by the latter).
Snell graduated from Dartmouth College in 1926 and received a Ph.D. in genetics from Harvard University in 1930. During 1931–33 he studied under the geneticist Hermann J. Muller at the University of Texas. In 1935 he joined the staff of the Jackson Laboratory in Bar Harbor, Maine, where he remained, becoming senior staff scientist in 1957, until his retirement in 1969.
At Bar Harbor, Snell, who was already immersed in mouse genetics, began to focus on the genetics of transplantation. His collaboration with British geneticist Peter Gorer led to the identification of a group of genes in the mouse called the H-2 gene complex, a term Snell coined to indicate whether a tissue graft would be accepted (the H stands for histocompatibility). Those histocompatibility genes encode cell surface proteins that allow the body to distinguish its own cells from those that are foreign—e.g., cells of a tissue graft or an infectious microorganism. The work resulted in the discovery of the major histocompatibility complex, a genetic complex found in all vertebrates that is analogous to the H-2 complex. Recognition of those genes paved the way for tissue and organ transplantation to become successful.
Snell was the author of a number of books, including Histocompatibility (1976), which he wrote with Jean Dausset and Stanley G. Natheson.
Details
George Davis Snell NAS (December 19, 1903 – June 6, 1996) was an American mouse geneticist and basic transplant immunologist.
Work
George Snell shared the 1980 Nobel Prize in Physiology or Medicine with Baruj Benacerraf and Jean Dausset for their discoveries concerning "genetically determined structures on the cell surface that regulate immunological reactions". Snell specifically "discovered the genetic factors that determine the possibilities of transplanting tissue from one individual to another. It was Snell who introduced the concept of H antigens." Snell's work in mice led to the discovery of HLA, the major histocompatibility complex, in humans (and all vertebrates) that is analogous to the H-2 complex in mice. Recognition of these key genes was prerequisite to successful tissue and organ transplantation.
Life
George Snell was born in Bradford, Massachusetts, the youngest of three children. His father (who was born in Minnesota) worked as a secretary for the local YMCA; he invented a device for winding induction coils for motorboat engines. Snell was educated in the Brookline, Massachusetts schools and then enrolled at Dartmouth College in Hanover, New Hampshire where he continued his passion for mathematics and science, focusing on genetics. He received his bachelor's degree from Dartmouth in 1926.
On the recommendation of John Gerould, his genetics professor at Dartmouth, Snell did graduate work at Harvard University with William E. Castle, the first American biologist to look for Mendelian inheritance in mammals. Snell earned his PhD from Harvard in 1930. His doctoral thesis was on genetic linkage in mice.
Upon receiving the PhD from Harvard, George Snell was employed as a teacher at Brown University, from 1930 to 1931.
Snell then spent two years as a postdoctoral fellow at the University of Texas with H.J. Muller, who pioneered radiation genetics (and was also to win a Nobel Prize). Snell studied the genetic effects of x-rays on mice with Muller.
This experience "served to convince me that research was my real love," Snell wrote in his autobiography."If it were to be research, mouse genetics was the clear choice and the Jackson Laboratory, founded in 1929 by Dr. Clarence Cook Little, one of Castle's earlier students, almost the inevitable selection as a place to work." The Jackson Laboratory was (and still is) the world's mecca for mouse genetics.
From 1933 to 1934, Snell was a teacher at Washington University in St. Louis.
After brief stints as teachers, in 1935 Snell joined the staff of The Jackson Laboratory in Bar Harbor on Mount Desert Island on the coast of Maine and he remained there for the entire balance of his long career. In Bar Harbor, he met and married Rhoda Carson. Together they had three sons, Thomas, Roy, and Peter. In his leisure time, Snell enjoyed skiing, a passion he developed during his years at Dartmouth, as well as tennis.
Snell received the Cancer Research Institute William B. Coley Award in 1978 for distinguished research in immunology. In 1988, he authored a substantial book, Search for a Rational Ethic, on the nature of ethics and the rules by which we live. It includes an evolution-based ethic founded on biological realities that he believed to be applicable to all human beings.
Snell died in Bar Harbor on June 6, 1996. His wife died in 1994.
Additional Information
Our immune system rejects damaged or abnormal cells, allowing our bodies to function properly. During transplants, this can also happen to the foreign cells. Through studies of mice with a very similar genetic make-up, George Snell showed that these rejections are caused by molecular complexes on the surface of the cells. In 1951 he also showed that rejection is governed by a group of genes on a special place on a certain chromosome. Among other things, the results proved significant for transplants.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1467 2024-03-25 20:06:30
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,394
Re: crème de la crème
1429) Nicolaas Bloembergen
Summary
Electrons in atoms and molecules have fixed energy levels, according to the principles of quantum physics. When there are transitions among different energy levels, light with certain frequencies is emitted or absorbed. This allows atoms and molecules to be analyzed with the help of the absorbed light’s spectrum. In the 1960s Nicolaas Bloembergen used laser light, which has waves in phase and of the same wavelength, to determine energy levels with great precision. By coordinating three laser waves, a fourth laser wave was created, and a larger part of the spectrum could be covered.
Details
Nicolaas Bloembergen (March 11, 1920 – September 5, 2017) was a Dutch-American physicist and Nobel laureate, recognized for his work in developing driving principles behind nonlinear optics for laser spectroscopy. During his career, he was a professor at Harvard University and later at the University of Arizona and at Leiden University in 1973 (as Lorentz Professor).
Bloembergen shared the 1981 Nobel Prize in Physics along with Arthur Schawlow and Kai Siegbahn because their work "has had a profound effect on our present knowledge of the constitution of matter" through the use of laser spectroscopy. In particular, Bloembergen was singled out because he "founded a new field of science we now call non-linear optics" by mixing "two or more beams of laser light... in order to produce laser light of a different wave length" and thus significantly broaden the laser spectroscopy frequency band.
Early life
Bloembergen was born in Dordrecht on March 11, 1920, where his father was a chemical engineer and executive. He had five siblings, with his brother Auke later becoming a legal scholar. In 1938, Bloembergen entered the University of Utrecht to study physics. However, during World War II, the German authorities closed the university and Bloembergen spent two years in hiding.
Career:
Graduate studies
Bloembergen left the war-ravaged Netherlands in 1945 to pursue graduate studies at Harvard University under Professor Edward Mills Purcell. Through Purcell, Bloembergen was part of the prolific academic lineage tree of J. J. Thomson, which includes many other Nobel Laureates, beginning with Thomson himself (Physics Nobel, 1906) and Lord Rayleigh (Physics Nobel, 1904), Ernest Rutherford (Chemistry Nobel 1908), Owen Richardson (Physics Nobel, 1928), and finally Purcell (Physics, Nobel 1952). Bloembergen's other influences include John Van Vleck (Physics Nobel, 1977) and Percy Bridgman (Physics Nobel, 1946).
Six weeks before his arrival, Purcell and his graduate students Torrey and Pound discovered nuclear magnetic resonance (NMR). Bloembergen was hired to develop the first NMR machine. At Harvard he attended lectures by Schwinger, Van Vleck, and Kemble. Bloembergen's NMR systems are the predecessors of modern-day MRI machines, which are used to examine internal organs and tissues. Bloembergen's research on NMR led to an interest in masers, which were introduced in 1953 and are the predecessors of lasers.
Bloembergen returned to the Netherlands in 1947, and submitted his thesis Nuclear Magnetic Relaxation at the University of Leiden. This was because he had completed all the preliminary examinations in the Netherlands, and Cor Gorter of Leiden offered him a postdoctoral appointment there. He received his Ph.D. degree from Leiden in 1948, and then was a postdoc at Leiden for about a year.
Professorship
In 1949, he returned to Harvard as a junior fellow of the Society of Fellows. In 1951, he became an associate professor; he then became Gordon McKay Professor of Applied Physics in 1957; Rumford Professor of Physics in 1974; and Gerhard Gade University Professor in 1980. In 1990 he retired from Harvard.
In addition, Bloembergen served as a visiting professor. From 1964 to 1965, Bloembergen was a visiting professor at the University of California, Berkeley. In 1996–1997, he was a visiting scientist at the college of optical sciences of the University of Arizona; he became a professor at Arizona in 2001.
Bloembergen was a member of the board of sponsors of the Bulletin of the Atomic Scientists and honorary editor of the Journal of Nonlinear Optical Physics & Materials.
Laser spectroscopy
By 1960 while at Harvard, he experimented with microwave spectroscopy. Bloembergen had modified the maser of Charles Townes, and in 1956, Bloembergen developed a crystal maser, which was more powerful than the standard gaseous version.
With the advent of the laser, he participated in the development of the field of laser spectroscopy, which allows precise observations of atomic structure using lasers. Following the development of second-harmonic generation by Peter Franken and others in 1961, Bloembergen studied how a new structure of matter is revealed, when one bombards matter with a focused and high-intensity beam of photons. This he termed the study of nonlinear optics. In reflection to his work in a Dutch newspaper in 1990, Bloembergen said: "We took a standard textbook on optics and for each section we asked ourselves what would happen if the intensity was to become very high. We were almost certain that we were bound to encounter an entirely new type of physics within that domain".
From this theoretical work, Bloembergen found ways to combine two or more laser sources consisting of photons in the visible light frequency range to generate a single laser source with photons of different frequencies in the infrared and ultraviolet ranges, which extends the amount of atomic detail that can be gathered from laser spectroscopy.
Personal life and death
Bloembergen met Huberta Deliana Brink (Deli) in 1948 while on vacation with his university's Physics Club. She was able to travel with him to the United States in 1949 on a student hospitality exchange program; he proposed to her when they arrived in the States, and were married by 1950 on return to Amsterdam. They were both naturalized as citizens of the United States in 1958. They had three children.
Bloembergen died on September 5, 2017, at an assisted living facility in his hometown Tucson, Arizona, of cardiorespiratory failure, at the age of 97.
Biography
In 2016 a Dutch biography was published, and in 2019 an English one.
Additional Information
Nicolaas Bloembergen (born March 11, 1920, Dordrecht, Netherlands—died September 5, 2017, Tucson, Arizona, U.S.) was a Dutch-born American physicist, corecipient with Arthur Leonard Schawlow of the United States and Kai Manne Börje Siegbahn of Sweden of the 1981 Nobel Prize for Physics for their revolutionary spectroscopic studies of the interaction of electromagnetic radiation with matter. Bloembergen made a pioneering use of lasers in these investigations.
Bloembergen received undergraduate (1941) and graduate (1943) degrees from the University of Utrecht. In 1946 he entered Harvard University, where with Edward Purcell and Robert Pound he did fundamental research on nuclear magnetic resonance. After receiving his Ph.D. from the University of Leiden in 1948, he returned to Harvard, where he became a professor of applied physics in 1951, Gerhard Gade university professor in 1980, and professor emeritus in 1990. In 2001 he began teaching at the University of Arizona. Bloembergen became a U.S. citizen in 1958.
Bloembergen’s early research on nuclear magnetic resonance led him to an interest in masers. He designed a three-stage crystal maser that was dramatically more powerful than earlier gaseous masers and that has become the most widely used microwave amplifier. Bloembergen then developed laser spectroscopy, which allows high-precision observations of atomic structure. His laser spectroscopic investigations led him in turn to formulate nonlinear optics, a new theoretical approach to the analysis of how electromagnetic radiation interacts with matter. Bloembergen’s research in nonlinear optics helped procure him a share of the Nobel Prize.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1468 2024-03-27 19:00:45
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,394
Re: crème de la crème
1430) Arthur Leonard Schawlow
Summary
Arthur L. Schawlow (born May 5, 1921, Mount Vernon, New York, U.S.—died April 28, 1999, Palo Alto, California) American physicist and corecipient, with Nicolaas Bloembergen of the United States and Kai Manne Börje Siegbahn of Sweden, of the 1981 Nobel Prize for Physics for his work in developing the laser and in laser spectroscopy.
As a child, Schawlow moved with his family to Canada. He attended the University of Toronto, receiving his Ph.D. in 1949. In that year he went to Columbia University, where he began collaborating with Charles Townes on the development of the maser (a device that produces and amplifies electromagnetic radiation mainly in the microwave region of the spectrum), the laser (a device similar to the maser that produces an intense beam of light of a single colour), and laser spectroscopy. Schawlow worked on the project that led to the construction of the first working maser in 1953 (for which Townes received a share of the 1964 Nobel Prize for Physics). Schawlow was a research physicist at Bell Telephone Laboratories from 1951 to 1961. In 1958 he and Townes published a paper in which they outlined the working principles of the laser, though the first such working device was built by another American physicist, Theodore Maiman, in 1960. In 1961 Schawlow became a professor at Stanford University. He became a world authority on laser spectroscopy, and he and Bloembergen earned their share of the 1981 Nobel Prize by using lasers to study the interactions of electromagnetic radiation with matter. His works include Infrared and Optical Masers (1958) and Lasers and Their Uses (1983). A few years after winning the Nobel Prize, Schawlow wrote an article on the laser for Encyclopædia Britannica’s 1987 Yearbook of Science and the Future.
Details
Arthur Leonard Schawlow (May 5, 1921 – April 28, 1999) was an American physicist and co-inventor of the laser with Charles Townes. His central insight, which Townes overlooked, was the use of two mirrors as the resonant cavity to take maser action from microwaves to visible wavelengths. He shared the 1981 Nobel Prize in Physics with Nicolaas Bloembergen and Kai Siegbahn for his work using lasers to determine atomic energy levels with great precision.
Biography
Schawlow was born in Mount Vernon, New York. His mother, Helen (Mason), was from Canada, and his father, Arthur Schawlow, was a Jewish immigrant from Riga (then in the Russian Empire, now in Latvia). Schawlow was raised in his mother's Protestant religion. When Arthur was three years old, they moved to Toronto, Ontario, Canada.
At the age of 16, he completed high school at Vaughan Road Academy (then Vaughan Collegiate Institute), and received a scholarship in science at the University of Toronto (Victoria College). After earning his undergraduate degree, Schawlow continued in graduate school at the University of Toronto which was interrupted due to World War II. At the end of the war, he began work on his Ph.D at the university with Professor Malcolm Crawford. He then took a postdoctoral position with Charles H. Townes at the physics department of Columbia University in the fall of 1949.
He went on to accept a position at Bell Labs in late 1951. He left in 1961 to join the faculty at Stanford University as a professor. He remained at Stanford until he retired to emeritus status in 1996.
Although his research focused on optics, in particular, lasers and their use in spectroscopy, he also pursued investigations in the areas of superconductivity and nuclear resonance. Schawlow shared the 1981 Nobel Prize in Physics with Nicolaas Bloembergen and Kai Siegbahn for their contributions to the development of laser spectroscopy.
Schawlow coauthored the widely used text Microwave Spectroscopy (1955) with Charles Townes. Schawlow and Townes were the first to publish the theory of laser design and operation in their seminal 1958 paper on "optical masers", although Gordon Gould is often credited with the "invention" of the laser, due to his unpublished work that predated Schawlow and Townes by a few months. The first working laser was made in 1960 by Theodore Maiman.
In 1991, the NEC Corporation and the American Physical Society established a prize: the Arthur L. Schawlow Prize in Laser Science. The prize is awarded annually to "candidates who have made outstanding contributions to basic research using lasers."
Science and religion
He participated in science and religion discussions. Regarding God, he stated, "I find a need for God in the universe and in my own life."
Personal life
In 1951, he married Aurelia Townes, younger sister of his postdoctoral advisor, Charles Townes. They had three children: Arthur Jr., Helen, and Edith. Arthur Jr. is autistic, with very little speech ability.
Schawlow and Professor Robert Hofstadter at Stanford, who also had an autistic child, teamed up to help each other find solutions to the condition. Arthur Jr. was put in a special center for autistic individuals, and later, Schawlow put together an institution to care for people with autism in Paradise, California. It was later named the Arthur Schawlow Center in 1999, shortly before his death. Schawlow was a promoter of the controversial method of facilitated communication with patients of autism.
He considered himself to be an orthodox Protestant Christian, and attended a Methodist church. Arthur Schawlow was an intense fan and collector of traditional American jazz recordings, as well as a supporter of instrumental groups performing this type of music.
Schawlow died of leukemia in Palo Alto, California, on April 28, 1999, at the age 77.
Additional Information
Electrons in atoms and molecules have fixed energy levels, according to the principles of quantum physics. When there are transitions among different energy levels, light with certain frequencies is emitted or absorbed. This allows atoms and molecules to be analyzed with the help of the absorbed light’s spectrum. With the laser’s coherent and intense light, the measurement phenomenon can occur. In the 1960s, Arthur Schawlow made use of this to eliminate the Doppler effect, allowing him to determine energy levels with great precision.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1469 2024-03-28 18:16:19
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,394
Re: crème de la crème
1431) Kai Siegbahn
Summary
According to quantum physics principles, the electrons in atoms and molecules have defined energy levels. Albert Einstein’s theory of the “photoelectric effect” says that a light particle (photon) can liberate an electron from an atom if it has sufficient energy. In the 1950s Kai Siegbahn developed methods for achieving highly accurate measurements of energy levels in atoms by irradiating them with photons and measuring the energy of the electrons emitted using the photoelectric effect.
Details
Kai Manne Börje Siegbahn (20 April 1918 – 20 July 2007) was a Swedish physicist who shared the 1981 Nobel Prize in Physics.
Biography
Siegbahn was born in Lund, Sweden, son of Manne Siegbahn the 1924 physics Nobel Prize winner. Siegbahn earned his doctorate at the University of Stockholm in 1944. He was professor at the Royal Institute of Technology 1951–1954, and then professor of experimental physics at Uppsala University 1954–1984, which was the same chair his father had held. He shared the 1981 Nobel Prize in Physics with Nicolaas Bloembergen and Arthur Schawlow. Siegbahn received half the prize "for his contribution to the development of high-resolution electron spectroscopy" while Bloembergen and Schawlow received one quarter each "for their contribution to the development of laser spectroscopy".
Siegbahn referred to his technique as Electron Spectroscopy for Chemical Analysis (ESCA); it is now usually known as X-ray photoelectron spectroscopy (XPS). In 1967 he published a book, ESCA; atomic, molecular and solid state structure studied by means of electron spectroscopy.
He was a member of several academies and societies, including the Royal Swedish Academy of Sciences, and was president of the International Union of Pure and Applied Physics from 1981 to 1984.
Siegbahn married Anna Brita Rhedin in 1944. The couple had three sons (two physicists and a biochemist).
Siegbahn died on 20 July 2007 at the age of 89. At the time of his death he was still active as a scientist at the Ångström Laboratory at Uppsala University.
Additional Information
Kai Manne Börje Siegbahn (born April 20, 1918, Lund, Swed.—died July 20, 2007, Ängelholm) was a Swedish physicist, corecipient with Nicolaas Bloembergen and Arthur Leonard Schawlow of the 1981 Nobel Prize for Physics for their revolutionary work in spectroscopy, particularly the spectroscopic analysis of the interaction of electromagnetic radiation with matter.
Siegbahn was the son of Karl Manne Siegbahn, who received the Nobel Prize for Physics in 1924 for his discoveries relating to X-ray spectroscopy. Kai was awarded his Ph.D. in physics by the University of Stockholm in 1944. In 1951 he was appointed professor at the Royal Institute of Technology in Stockholm, and in 1954 he moved to the University of Uppsala, where he taught until his retirement in 1984.
In his prize-winning work, Siegbahn formulated the principles underlying the technique called ESCA (electron spectroscopy for chemical analysis) and refined the instruments used in carrying it out. ESCA depends on a fundamental phenomenon, the photoelectric effect, which is the emission of electrons that occurs when electromagnetic radiation strikes a material. Siegbahn’s achievement was to develop ways to measure the kinetic energies of the emitted electrons accurately enough to permit the determination of their binding energies. He showed that chemical elements bind electrons with characteristic energies that are slightly modified by the molecular or ionic environment. During the 1970s ESCA was adopted all over the world for analyzing materials, including the particles in polluted air and the surfaces of solid catalysts used in petroleum refining.
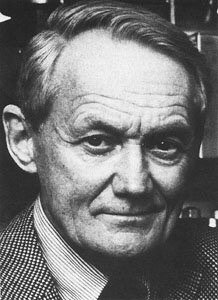
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1470 2024-03-29 22:32:48
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,394
Re: crème de la crème
1432) Kenichi Fukui
Gist
In chemical reactions, molecules composed of atoms meet and form new compounds. Electrons orbiting around the atoms’ nuclei play an important role here. In 1952, Kenichi Fukui developed a theory that showed that the properties of the orbits of electrons that are most weakly bonded to the atom are critically important in understanding chemical reactions. In later, more developed theories, Fukui and Roald Hoffmann proved independently of one another how the symmetrical properties of electron orbitals explain the course of chemical reactions.
Details
Kenichi Fukui (Fukui Ken'ichi, October 4, 1918 – January 9, 1998) was a Japanese chemist, known as the first person of East Asian ancestry to be awarded the Nobel Prize in Chemistry.
Fukui was co-recipient of the 1981 Nobel Prize in Chemistry with Roald Hoffmann, for their independent investigations into the mechanisms of chemical reactions. Fukui's prize-winning work focused on the role of frontier orbitals in chemical reactions: specifically that molecules share loosely bonded electrons which occupy the frontier orbitals, that is, the Highest Occupied Molecular Orbital (HOMO) and the Lowest Unoccupied Molecular Orbital (LUMO).
Early life
Fukui was the eldest of three sons of Ryokichi Fukui, a foreign trade merchant, and Chie Fukui. He was born in Nara, Japan. In his student days between 1938 and 1941, Fukui's interest was stimulated by quantum mechanics and Erwin Schrödinger's famous equation. He also had developed the belief that a breakthrough in science occurs through the unexpected fusion of remotely related fields.
In an interview with The Chemical Intelligencer Kenichi discusses his path towards chemistry starting from middle school.
"The reason for my selection of chemistry is not easy to explain, since chemistry was never my favorite branch in middle school and high school years. Actually, the fact that my respected Fabre had been a genius in chemistry had captured my heart latently, the most decisive occurrence in my education career came when my father asked the advice of Professor Gen-itsu Kita of the Kyoto Imperial University concerning the cause I should take.”
On the advice of Kita, a personal friend of the elder Fukui, young Kenichi was directed to the Department of Industrial Chemistry, with which Kita was then affiliated. He also explains that chemistry was difficult to him because it seemed to require memorization to learn it, and that he preferred more logical character in chemistry. He followed the advice a mentor that was well respected by Kenichi himself and never looked back. He also followed in those footsteps by attending Kyoto University in Japan. During that same interview Kenichi also discussed his reason for preferring more theoretical chemistry rather than experimental chemistry. Although he certainly acceded at theoretical science he actually spent much of his early research on experimental. Kenichi had quickly completed more than 100 experimental projects and papers, and he rather enjoyed the experimental phenomena of chemistry. In fact, later on when teaching he would recommend experimental thesis projects for his students to balance them out, theoretical science came more natural to students, but by suggesting or assigning experimental projects his students could understand the concept of both, as all scientist should. Following his graduation from Kyoto Imperial University in 1941, Fukui was engaged in the Army Fuel Laboratory of Japan during World War II. In 1943, he was appointed a lecturer in fuel chemistry at Kyoto Imperial University and began his career as an experimental organic chemist.
Research
He was professor of physical chemistry at Kyoto University from 1951 to 1982, president of the Kyoto Institute of Technology between 1982 and 1988, and a member of the International Academy of Quantum Molecular Science and honorary member of the International Academy of Science, Munich. He was also director of the Institute for Fundamental Chemistry from 1988 till his death. As well as President of the Chemical Society of Japan from 1983–84, receiving multiple awards aside from his Nobel Prize such as; Japan Academy Prize in 1962, Person of Cultural Merit in 1981, Imperial Honour of Grand Cordon of the Order of the Rising Sun in 1988, with many other awards not quite as prestigious.
In 1952, Fukui with his young collaborators T. Yonezawa and H. Shingu presented his molecular orbital theory of reactivity in aromatic hydrocarbons, which appeared in the Journal of Chemical Physics. At that time, his concept failed to garner adequate attention among chemists. Fukui observed in his Nobel lecture in 1981 that his original paper 'received a number of controversial comments. This was in a sense understandable, because for lack of my experiential ability, the theoretical foundation for this conspicuous result was obscure or rather improperly given.'
The frontier orbitals concept came to be recognized following the 1965 publication by Robert B. Woodward and Roald Hoffmann of the Woodward-Hoffmann stereoselection rules, which could predict the reaction rates between two reactants. These rules, depicted in diagrams, explain why some pairs react easily while other pairs do not. The basis for these rules lies in the symmetry properties of the molecules and especially in the disposition of their electrons. Fukui had acknowledged in his Nobel lecture that, 'It is only after the remarkable appearance of the brilliant work by Woodward and Hoffmann that I have become fully aware that not only the density distribution but also the nodal property of the particular orbitals have significance in such a wide variety of chemical reactions.'
What has been striking in Fukui's significant contributions is that he developed his ideas before chemists had access to large computers for modeling. Apart from exploring the theory of chemical reactions, Fukui's contributions to chemistry also include the statistical theory of gelation, organic synthesis by inorganic salts and polymerization kinetics.
In an interview to New Scientist magazine in 1985, Fukui had been highly critical on the practices adopted in Japanese universities and industries to foster science. He noted, "Japanese universities have a chair system that is a fixed hierarchy. This has its merits when trying to work as a laboratory on one theme. But if you want to do original work you must start young, and young people are limited by the chair system. Even if students cannot become assistant professors at an early age they should be encouraged to do original work." Fukui also admonished Japanese industrial research stating, "Industry is more likely to put its research effort into its daily business. It is very difficult for it to become involved in pure chemistry. There is a need to encourage long-range research, even if we don't know its goal and if its application is unknown." In another interview with The Chemical Intelligencer he further elaborates on his criticism by saying, "As is known worldwide, Japan has tried to catch up with the western countries since the beginning of this century by importing science from them." Japan is, in a sense, relatively new to fundamental science as a part of its society and the lack of originality ability, and funding which the western countries have more advantages in hurt the country in fundamental science. Although, he has also stated that it is improving in Japan, especially funding for fundamental science as it has seen a steady increase for years.
Additional Information
Fukui Kenichi (born Oct. 4, 1918, Nara, Japan—died Jan. 9, 1998, Kyoto) was a Japanese chemist, corecipient with Roald Hoffmann of the Nobel Prize for Chemistry in 1981 for their independent investigations of the mechanisms of chemical reactions.
Fukui took little interest in chemistry before enrolling at Kyoto University, where he studied engineering, receiving a Ph.D. in 1948. He was professor of physical chemistry at Kyoto from 1951 to 1982 and was president of the Kyoto Institute of Technology from 1982 to 1988.
In 1952 Fukui published his first exposition of the concept that the crucial process in many chemical reactions consists of an interaction between the highest occupied molecular orbital of one compound and the lowest unoccupied orbital of the other. In effect, one molecule shares its most loosely bound electrons with the other, which accepts them at the site where they can become most tightly bound. The interaction results in the formation of a new, occupied orbital that has properties intermediate between those of the two former ones. Fukui designated these labile orbitals “frontier orbitals” and provided examples of their significance in reactions that produce important classes of organic compounds.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1471 2024-03-30 18:39:23
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,394
Re: crème de la crème
1433) Roald Hoffmann
Summary
Roald Hoffmann was born into a Jewish family in Złoczów, Poland (now Ukraine). His father Hillel Safran was a civil engineer and his mother Clara Hillel a teacher. Roald and his mother survived the ghetto, a labor camp and 15 months in hiding but his father was killed by the Nazis. After the war, Clara married Paul Hoffmann. The family moved to the United States in 1949 and finally Roald was able to go to school on an every day basis. Roald Hoffmann went to Stuyvesant High School, Columbia College and received his doctorate at Harvard University in 1962.
Work
In chemical reactions, molecules composed of atoms meet and form new compounds. Electrons orbiting around the atoms’ nuclei play an important role here. After Kenichi Fukui proved that the properties of the electron orbits that most weakly bound to the atom are critical in chemical reactions, Roald Hoffmann went on to further develop these theories from the mid-1960s. Independently of one another, Hoffmann and Fukui both demonstrated how the symmetrical properties of electron orbitals explain the course of chemical reactions.
Details
Roald Hoffmann (born Roald Safran; July 18, 1937) is a Polish-American theoretical chemist who won the 1981 Nobel Prize in Chemistry. He has also published plays and poetry. He is the Frank H. T. Rhodes Professor of Humane Letters Emeritus at Cornell University.
Early life:
Escape from the Holocaust
Hoffmann was born in Złoczów, Poland (now Zolochiv, Ukraine), to a Polish-Jewish family, and was named in honor of the Norwegian explorer Roald Amundsen. His parents were Clara (Rosen), a teacher, and Hillel Safran, a civil engineer. After Germany invaded Poland and occupied the town, his family was placed in a labor camp where his father, who was familiar with much of the local infrastructure, was a valued prisoner. As the situation grew more dangerous, with prisoners being transferred to extermination camps, the family bribed guards to allow an escape. They arranged with a Ukrainian neighbor named Mykola Dyuk for Hoffmann, his mother, two uncles and an aunt to hide in the attic and a storeroom of the local schoolhouse, where they remained for eighteen months, from January 1943 to June 1944, while Hoffmann was aged 5 to 7.
His father remained at the labor camp, but was able to occasionally visit, until he was tortured and killed by the Germans for his involvement in a plot to arm the camp prisoners. When she received the news, his mother attempted to contain her sorrow by writing down her feelings in a notebook her husband had been using to take notes on a relativity textbook he had been reading. While in hiding his mother kept Hoffmann entertained by teaching him to read and having him memorize geography from textbooks stored in the attic, then quizzing him on it. He referred to the experience as having been enveloped in a cocoon of love. In 1944 they moved to Kraków where his mother remarried. They adopted her new husband's surname Hoffmann.
Most of the rest of the family was killed in the Holocaust, though one grandmother and a few others survived. They migrated to the United States on the troop carrier Ernie Pyle in 1949.
Hoffmann visited Zolochiv with his adult son (by then a parent of a five-year-old) in 2006 and found that the attic where he had hidden was still intact, but the storeroom had been incorporated, ironically enough, into a chemistry classroom. In 2009, a monument to Holocaust victims was built in Zolochiv on Hoffmann's initiative.
Personal life
Hoffmann married Eva Börjesson in 1960. They have two children, Hillel Jan and Ingrid Helena.
He is an atheist.
Education and academic credentials
Hoffmann graduated in 1955 from New York City's Stuyvesant High School, where he won a Westinghouse science scholarship. He received his Bachelor of Arts degree at Columbia University (Columbia College) in 1958. He earned his Master of Arts degree in 1960 from Harvard University. He earned his doctor of philosophy degree from Harvard University while working under joint supervision of Martin Gouterman and subsequent 1976 Nobel Prize in Chemistry winner William N. Lipscomb, Jr. Hoffman worked on the molecular orbital theory of polyhedral molecules. Under Lipscomb's direction the Extended Hückel method was developed by Lawrence Lohr and by Roald Hoffmann. This method was later extended by Hoffmann. He went to Cornell in 1965 and has remained there, becoming professor emeritus.
Scientific research
Hoffmann's research and interests have been in the electronic structure of stable and unstable molecules, and in the study of transition states in reactions. He has investigated the structure and reactivity of both organic and inorganic molecules, and examined problems in organo-metallic and solid-state chemistry. Hoffman has developed semiempirical and nonempirical computational tools and methods such as the extended Hückel method which he proposed in 1963 for determining molecular orbitals.
With Robert Burns Woodward he developed the Woodward–Hoffmann rules for elucidating reaction mechanisms and their stereochemistry. They realized that chemical transformations could be approximately predicted from subtle symmetries and asymmetries in the electron orbitals of complex molecules. Their rules predict differing outcomes, such as the types of products that will be formed when two compounds are activated by heat compared with those produced under activation by light. For this work Hoffmann received the 1981 Nobel Prize in chemistry, sharing it with Japanese chemist Kenichi Fukui, who had independently resolved similar issues. (Woodward was not included in the prize, which is given only to living persons, although he had won the 1965 prize for other work.) In his Nobel Lecture, Hoffmann introduced the isolobal analogy for predicting the bonding properties of organometallic compounds.
Some of Hoffman's most recent work, with Neil Ashcroft and Vanessa Labet, examines bonding in matter under extreme high pressure.
What gives me the greatest joy in this work? That as we tease apart what goes on in hydrogen under pressures such as those that one finds at the center of the earth, two explanations subtly contend with each other ... [physical and chemical] ... Hydrogen under extreme pressure is doing just what an inorganic molecule at 1 atmosphere does!
Additional Information
Roald Hoffmann (born July 18, 1937, Złoczów, Pol.) is a Polish-born American chemist, who was a corecipient, with Fukui Kenichi of Japan, of the Nobel Prize for Chemistry in 1981 for their independent investigations of the mechanisms of chemical reactions.
Hoffmann immigrated to the United States with his family in 1949. He graduated from Columbia University and received his Ph.D. from Harvard University in 1962. He collaborated with Robert B. Woodward at Harvard during the next three years and then joined the Cornell University faculty in 1965.
Hoffmann undertook the research leading to his share of the prize when he and Woodward sought an explanation of the unexpected course taken by a reaction that Woodward and his colleagues had hoped to use in the synthesis of the complicated molecule of vitamin B12. Hoffmann and Woodward discovered that many reactions involving the formation or breaking of rings of atoms take courses that depend on an identifiable symmetry in the mathematical descriptions of the molecular orbitals that undergo the most change. Their theory, expressed in a set of statements now called the Woodward-Hoffmann rules, accounts for the failure of certain cyclic compounds to form from apparently appropriate starting materials, though others are readily produced; it also clarifies the geometric arrangement of the atoms in the products formed when the rings in cyclic compounds are broken.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1472 2024-04-05 18:25:01
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,394
Re: crème de la crème
1434) Roger Wolcott Sperry
Summary
Roger Wolcott Sperry (born Aug. 20, 1913, Hartford, Conn., U.S.—died April 17, 1994, Pasadena, Calif.) was an American neurobiologist, corecipient with David Hunter Hubel and Torsten Nils Wiesel of the Nobel Prize for Physiology or Medicine in 1981 for their investigations of brain function, Sperry in particular for his study of functional specialization in the cerebral hemispheres.
Sperry earned a bachelor’s degree in English literature and a master’s degree in psychology from Oberlin (Ohio) College and a doctorate in zoology from the University of Chicago in 1941. He then became an associate of Karl Lashley, first at Harvard University and then at the Yerkes Laboratories of Primate Biology in Orange Park, Fla. In 1946 he joined the faculty of the University of Chicago and in 1954 moved to the California Institute of Technology as Hixon professor of psychobiology.
Sperry’s early research was on the regeneration of nerve fibres. He eventually became interested in brain function and undertook research on animals and then on human epileptics whose brains had been “split”—i.e., in whom the thick cable of nerves (the corpus callosum) connecting the right and left cerebral hemispheres had been severed. His studies demonstrated that the left side of the brain is normally dominant for analytical and verbal tasks, while the right hemisphere assumes dominance in spatial tasks, music, and certain other areas. The surgical and experimental techniques Sperry developed from the late 1940s laid the groundwork for much more specialized explorations of the mental functions carried out in different areas of the brain.
Details
Roger Wolcott Sperry (August 20, 1913 – April 17, 1994) was an American neuropsychologist, neurobiologist, cognitive neuroscientist, and Nobel laureate who, together with David Hunter Hubel and Torsten Nils Wiesel, won the 1981 Nobel Prize in Physiology and Medicine for his work with split-brain research. A Review of General Psychology survey, published in 2002, ranked Sperry as the 44th most cited psychologist of the 20th century.
Early life and education
Sperry was born in Hartford, Connecticut, to Francis Bushnell and Florence Kraemer Sperry. His father was in banking, and his mother trained in business school. He was raised in an upper middle-class environment, which stressed academic achievement. Roger had one brother, Russell Loomis. Their father died when Roger was 11. Afterwards, his mother became assistant to the principal in the local high school.
Sperry went to Hall High School in West Hartford, Connecticut, where he was a star athlete in several sports, and did well enough academically to win a scholarship to Oberlin College. At Oberlin, he was captain of the basketball team, and he also took part in varsity baseball, football, and track. He also worked at a cafe on campus to help support himself. Sperry was an English major, but he took an Intro to Psychology class taught by a Professor named R. H. Stetson who had worked with William James, the father of American Psychology. This class sparked Sperry's interest in the brain and how it can change. Stetson was disabled and had trouble getting around so Sperry would help him out by driving him to and from wherever he needed to go. This included taking Stetson to lunch with his colleagues. Sperry would just sit at the end of the table and listen to Stetson and his colleagues discuss their research and other psychological interests. This increased Sperry's interest in Psychology even more and after he received his undergraduate degree in English from Oberlin he decided to stay and get his master's degree in Psychology. He received his bachelor's degree in English in 1935 and a master's degree in psychology in 1937. He received his Ph.D. in zoology from the University of Chicago in 1941, supervised by Paul A. Weiss. Sperry then did postdoctoral research with Karl Lashley at Harvard University though most of his time was spent with Lashley at the Yerkes Primate Research Center in Orange Park, Florida.
Career
In 1942, Sperry began work at the Yerkes Laboratories of Primate Biology, then a part of Harvard University. There he focused on experiments involving the rearranging of motor and sensory nerves. He left in 1946 to become an assistant professor, and later associate professor, at the University of Chicago. In 1949, during a routine chest x-ray, there was evidence of tuberculosis. He was sent to Saranac Lake in the Adironack Mountains in New York for treatment. It was during this time when he began writing his concepts of the mind and brain, and was first published in the American Scientist in 1952. In 1952, he became the Section Chief of Neurological Diseases and Blindness at the National Institutes of Health and finished out the year at the Marine Biology Laboratory in Coral Gables, Florida. Sperry went back to The University of Chicago in 1952 and became an Associate Professor of Psychology. He was not offered tenure at Chicago and planned to move to Bethesda, Maryland but was held up by a delay in construction at the National Institutes of Health. During this time Sperry's friend Victor Hepburn invited him to lecture about his research at a symposium. There were professors from the California Institute of Technology in the audience of the symposium who, after listening to Sperry's lecture, were so impressed with him they offered him a job as the Hixson Professor of Psychobiology. In 1954, he accepted the position as a professor at the California Institute of Technology (Caltech as Hixson Professor of Psychobiology) where he performed his most famous experiments with Joseph Bogen, MD and many students including Michael Gazzaniga.
Under the supervision of Paul Weiss while earning his Ph.D. at the University of Chicago, Sperry became interested in neuronal specificity and brain circuitry and began questioning the existing concepts about these two topics. He asked the simple question first asked in his Introduction to Psychology class at Oberlin: Nature or nurture? He began a series of experiments in an attempt to answer this question. Sperry crosswired the motor nerves of rats' legs so the left nerve controlled the right leg and vice versa. He would then place the rats in a cage that had an electric grid on the bottom separated into four sections. Each leg of the rat was placed into one of the four sections of the electric grid. A shock was administered to a specific section of the grid, for example the grid where the rat's left back leg was located would receive a shock. Every time the left paw was shocked the rat would lift his right paw and vice versa. Sperry wanted to know how long it would take the rat to realize he was lifting the wrong paw. After repeated tests Sperry found that the rats never learned to lift up the correct paw, leading him to the conclusion that some things are just hardwired and cannot be relearned. In Sperry's words, "no adaptive functioning of the nervous system took place." During Sperry's postdoctoral years with Karl Lashley at Harvard and at the Yerkes Laboratories of Primate Biology in Orange Park, Florida, he continued his work on neuronal specificity that he had begun as a doctoral student and initiated a new series of studies involving salamanders. The optic nerves were sectioned and the eyes rotated 180 degrees. The question was whether vision would be normal after regeneration or would the animal forever view the world as "upside down" and right-left reversed. Should the latter prove to be the case, it would mean that the nerves were somehow "guided" back to their original sites of termination. Restoration of normal vision (i.e., "seeing" the world in a "right-side-up" orientation) would mean that the regenerating nerves had terminated in new sites, quite different from the original ones. The animals reacted as though the world was upside down and reversed from right to left. Furthermore, no amount of training could change the response. These studies, which provided strong evidence for nerve guidance by "intricate chemical codes under genetic control" (1963) culminated in Sperry's chemoaffinity hypothesis (1951).
Sperry later served on the Board of Trustees and as Professor of Psychobiology Emeritus at California Institute of Technology. The Sperry Neuroscience Building at Oberlin College was named in his honor in 1990.
Nobel Prize
Sperry was granted numerous awards over his lifetime, including the California Scientist of the Year Award in 1972, the National Medal of Science in 1989, the Wolf Prize in Medicine in 1979, and the Albert Lasker Medical Research Award in 1979, and the Nobel Prize for Medicine/Physiology in 1981 that he shared with David H. Hubel and Torsten N. Wiesel. Sperry won this award for his work with "split-brain" patients. The brain is divided into two hemispheres, the left and right hemispheres, connected in the middle by a part of the brain called the corpus callosum. In "split-brain" patients, the corpus callosum has been severed due to the patients suffering from epilepsy, a disease that causes intense and persistent seizures. Seizures begin in one hemisphere and continue into the other hemisphere. Cutting the corpus callosum prevents the seizures from moving from one hemisphere to the other, which then prevents seizures from occurring, thus allowing the patients to function normally instead of suffering from continuous seizures.
Sperry first became interested in "split-brain" research when he was working on the topic of interocular transfer, which occurs when "one learns with one eye how to solve a problem then, with that eye covered and using the other eye, one already knows how to solve the problem". Sperry asked the question: "how can the learning with one eye appear with the use of the other?" Sperry cut nerves in the eyes of cats so the left eye was connected to the left hemisphere and the right eye was connected to the right hemisphere; he also cut the corpus callosum. The cats were then taught to distinguish a triangle from a square with the right eye covered. Then the cats were presented the same problem with the left eye covered; the cats had no idea what they had just learned with the right eye and because of this could be taught to distinguish a square from a triangle. Depending on which eye was covered, the cats would either distinguish a square from a triangle or a triangle from a square, demonstrating that the left and right hemispheres learned and remembered two different events. This led Sperry to believe that the left and right hemispheres function separately when not connected by the corpus callosum.
Sperry's research with "split-brain" cats helped lead to the discovery that cutting the corpus callosum is a very effective treatment for patients who suffer from epilepsy. Initially after the patients recovered from surgery there were no signs that the surgery caused any changes to their behavior or functioning. This observation rendered the question: if the surgery had absolutely no effect on any part of the patients' normal functioning then what is the purpose of the corpus callosum? Was it simply there to keep the two sides of the brain from collapsing, as Karl Lashley jokingly put it? Sperry was asked to develop a series of tests to perform on the "split-brain" patients to determine if the surgery caused changes in the patients' functioning or not.
Working with his graduate student Michael Gazzaniga, Sperry invited several of the "split-brain" patients to volunteer to take part in his study to determine if the surgery affected their functioning. These tests were designed to test the patients' language, vision, and motor skills. When a person views something in the left visual field (that is on the left side of their body), the information travels to the right hemisphere of the brain and vice versa. In the first series of tests, Sperry would present a word to either the left or right visual field for a short period of time. If the word was shown to the right visual field, meaning the left hemisphere would process it, then the patient could report seeing the word. If the word was shown to the left visual field, meaning the right hemisphere would process it, then the patient could not report seeing the word. This led Sperry to believe that only the left side of the brain could articulate speech. However, in a follow-up experiment, Sperry discovered that the right hemisphere does have some language abilities. In this experiment, he had the patients place their left hands in a tray full of objects located under a partition so the patient would not be able to see the objects. Then a word was shown to the patient's left visual field, which was processed by the right side of the brain. This word described one of the objects in the tray, so the patient's left hand picked up the object corresponding to the word. When participants were asked about the word and the object in their hand, they claimed they had not seen the word and had no idea why they were holding the object. The right side of the brain had recognized the word and told the left hand to pick it up, but because the right side of the brain cannot speak and the left side of the brain had not seen the word, the patient could not articulate what they had seen.
In another series of experiments further examining the lateralization of language in the left and right hemispheres, Sperry presented one object to the left visual field and a different object to the right visual field of the "split-brain" patients. The patient's left hand was put under a partition and then the patient was asked to draw with their left hand what they had been shown. The patients would draw what they had seen in their left visual field, but when asked what they had drawn would describe what had been shown to their right visual field. These tests proved that when the corpus callosum is severed, it breaks the connection between the left and right hemispheres, making them unable to communicate with each other. Not only are they unable to communicate with each other, but also without the corpus callosum connecting them one hemisphere has no idea that the other hemisphere even exists. There was even evidence of this outside the laboratory when some of the patients reported that, "while their left hand was unbuttoning their shirt, the right hand would follow along behind and button it again." These experiments were beneficial to numerous people in many different ways.
In his words, each hemisphere is:
indeed a conscious system in its own right, perceiving, thinking, remembering, reasoning, willing, and emoting, all at a characteristically human level, and ... both the left and the right hemisphere may be conscious simultaneously in different, even in mutually conflicting, mental experiences that run along in parallel
— Roger Wolcott Sperry, 1974
This research contributed greatly to understanding the lateralization of brain function. In 1989, Sperry also received the National Medal of Science. Afterwards in 1993, Sperry received the Lifetime Achievement Award from APA.
In addition to his contribution in establishing the lateralized function of the brain, Sperry is noted for his "chemoaffinity hypothesis", which has not only been influential in formation of testable hypotheses in how precise neuronal wiring diagram is established in the brain, but the hypothesis itself has been verified by numerous experiments.
The cells and fibers of the brain must carry some kind of individual identification tags, presumably cytochemical in nature, by which they are distinguished one from another almost, in many regions, to the level of the single neurons
— Roger Walcott Sperry
In the words of a 2009 review article in Science magazine: "He suggested that gradients of such identification tags on retinal neurons and on the target cells in the brain coordinately guide the orderly projection of millions of developing retinal axons. This idea was supported by the identification and genetic analysis of axon guidance molecules, including those that direct development of the vertebrate visual system." This was confirmed in the seventies by Marshall W. Nirenberg's work on chick retinas and later on Drosophila melanogaster larvae.
The experiments conducted by Sperry focused on four major ideas which were also called "turnarounds": equipotentiality, split brain studies, nerve regeneration and plasticity, and psychology of the consciousness.
Personal life
In 1949, Sperry married Norma Gay Deupree. They had one son, Glenn Michael, and one daughter, Janeth Hope. Sperry was a quiet, thoughtful, and modest man with an insatiable curiosity. He never stopped working, questioning, or learning up until his death in 1994 of ALS or Lou Gehrig's Disease. Sperry could often be found in his office with his feet propped up on his desk scribbling in his notebook or deep in thought. Sperry was an avid paleontologist and displayed his large fossil collection in his home. He was also a very talented sculptor, artist, and ceramicist. He enjoyed going on camping and fishing trips with his wife and children in Baja, California.
Additional Information
Roger Wolcott Sperry (1913-1994) was born in Hartford, Connecticut and grew up on a farm outside Hartford. He attended Hartford public schools. At West Hartford High School he was a star athlete in several sports, but he also did well enough academically to win a scholarship to Oberlin College, in Ohio. He graduated from Oberlin in 1935 with a degree in English. At college, Sperry’s main passion, aside from 17th century English poetry, seems to have been athletics, as in high school. He was captain of the basketball team, and he also took part in varsity baseball, football, and track.
Sperry stayed at Oberlin after graduating and took a Master’s degree in psychology. He then went to the University of Chicago, where he worked for his Ph.D. in zoology under Paul Weiss, one of the most influential biologists of the time. Following his Ph.D., he spent some years at Harvard and the Yerkes Laboratory for Primate Biology in Florida before returning to Chicago as a faculty member.
In 1951, Sperry was invited to present his work at the California Institute of Technology (Caltech), which was seeking to fill the newly endowed Hixon Professorship of Psychobiology. His lectures on neurospecificity (summarized below) were brilliant, and he was offered the position. He joined the Caltech faculty in 1954 and remained there for the rest of his life.
Sperry’s first major scientific work – one which occupied him for over a decade – was to disprove a widely accepted theory that had been advanced by his professor at the University of Chicago, Paul Weiss. According to this theory, the vast neural network that connects the sense organs and muscles to the brain originates as an undifferentiated and unspecified mesh of randomly connected nerve fibers which is later transformed, under the influence of experience and learning, into the highly coordinated, purposeful system that is actually seen in animals. Plasticity and interchangeability of function were the key ideas. This theory did not come out of the blue, of course, but was based on careful experimental work that Weiss had performed, but misinterpreted.
In a series of experiments that have become famous, Sperry showed that the actual state of affairs is precisely the opposite of that imagined in Weiss’ theory. Instead of being composed of interchangeable parts, the circuits of the brain are largely hardwired, in the sense that each nerve cell is tagged with its own chemical individuality early in embryonic development; once this happens, the function of the cell is fixed and is not modifiable thereafter.
The experiments that led to this radical conclusion involved surgical procedures on a variety of animals from fish and salamanders to monkeys. Sperry showed that if nerve connections were rearranged – for example, by redirecting to the other side of the animal the sensory nerves that innervate the left foot of a rat – inappropriate responses resulted that could not be unlearned. In this case, stimulation of the right foot caused the rat to move its left foot, and no amount of experience or retraining could change this response.
In experiments with fish, frogs, and salamanders (chosen because they have great powers of regeneration), Sperry demonstrated that individual nerve fibers (which are actually different cells) behave as if each is chemically different from every other, and these chemical differences are matched in the brain. The result is that in an animal whose optic nerves are severed and then allowed to regenerate, the thousands of individual fibers that make up each optic nerve grow back into the brain and there make the same connections they had before. The animal is then able to see as if the nerves had never been severed. Proof that no adaptive reorganization of the neural circuits is involved in regeneration consisted of showing that if an eye whose optic nerve is severed is also rotated in its socket, the world seen by the eye after regeneration is still upside down and backwards. Furthermore, as in the case of the rat with the crossed nerves, no amount of retraining makes it see correctly: the animal invariably strikes to the left when it sees a worm on its right.
The conclusion that the circuitry of the brain is fixed in early development is supported by much more evidence than I can summarize here. It has given rise to a field of research focused on “axonal guidance”. Sperry’s result concerning the chemical individuality of each nerve fiber has been confirmed by modern molecular methods. It is a result that is loaded with meanings at many levels – from immediate consequences for neurosurgery to large and still not fully explored implications for evolution and development, and even for social-political questions. It raises other fascinating and still unsolved questions. For example, the capacity to learn obviously implies some neural plasticity. But given the basic determinism of the brain that Sperry uncovered, what does learning actually consist of at the cellular and chemical level? These and other questions posed by his findings are now being studied, and no doubt they will continue to be worked on for a long time in the future.
Important as his work on neurospecificity was, it was not this for which he was awarded the Nobel Prize in 1981, but his discoveries on split brains. Essentially, Sperry and his students showed that if the two hemispheres of the brain are separated by severing the corpus callosum (the large band of fibers that connects them), the transfer of information between the hemispheres ceases, and the coexistence in the same individual of two functionally different brains can be demonstrated. The findings contradicted the generally held view – again based on misinterpretation of evidence – that sectioning of the corpus callosum produced no definite behavioral effects. The probable explanation is that the two hemispheres, although separated from one another, are usually in agreement, so that no obvious conflict results. By means of ingenious tests, however, Sperry and his group showed that definite behavioral phenomena can be demonstrated following the brain-splitting operation.
Sperry started this investigation with cats and monkeys, but later extended it to human beings when patients became available whose hemispheres had been surgically separated in order to control intractable epilepsy. It was with these patients that he was able to show that a conscious mind exists in each hemisphere. The left hemisphere is the one with speech, as had been known, and it is dominant in all activities involving language, arithmetic, and analysis. The right hemisphere, although mute and capable only of simple addition (up to about 20) is superior to the left hemisphere in, among other things, spatial comprehension – in understanding maps, for example, or recognizing faces. Until these patients were studied, it had been doubted whether the right hemisphere was even conscious. By devising ways of communicating with the right hemisphere, Sperry could show that this hemisphere is, to quote him: “indeed a conscious system in its own right, perceiving, thinking, remembering, reasoning, willing, and emoting, all at a characteristically human level, and … both the left and the right hemisphere may be conscious simultaneously in different, even in mutually conflicting, mental experiences that run along in parallel.”
As with his earlier work, the discovery of the duality of consciousness revealed in the split-brain experiments opened whole new fields of brain research, and these are now being worked by a new generation of biologists, and, of course, philosophers.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1473 2024-04-10 18:58:21
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,394
Re: crème de la crème
1435) David H. Hubel
Gist
Our vision works by the light around us being captured by a large number of light-sensitive cells located in the retinas at the back of our eyes. The light is converted into signals that are sent to the brain and there converted into visual impressions. David Hubel and Torsten Wiesel clarified how this process works during the 1960s: In the cerebral cortex signals are analyzed in sequence by cells with the specific tasks of interpreting contrasts, patterns, and movements. They also showed that this ability develops in children during the initial period after birth.
Summary
David Hunter Hubel (born February 27, 1926, Windsor, Ontario, Canada—died September 22, 2013, Lincoln, Massachusetts, U.S.) was a Canadian American neurobiologist, corecipient with Torsten Nils Wiesel and Roger Wolcott Sperry of the 1981 Nobel Prize for Physiology or Medicine. All three scientists were honoured for their investigations of brain function, with Hubel and Wiesel sharing half of the award for their collaborative discoveries concerning information processing in the visual system.
Hubel attended McGill University in Montreal, receiving a bachelor’s degree in 1947 and an M.D. in 1951. He held positions at the Montreal Neurological Institute, Johns Hopkins Hospital (where his association with Wiesel began), and the Walter Reed Army Institute of Research before joining the faculty of Harvard Medical School, along with Wiesel, in 1959. They began collaborating on research that same year.
One of their outstanding achievements was the analysis of the flow of nerve impulses from the retina to the sensory and motor centres of the brain. Using tiny electrodes, they tracked the electrical discharges that occur in individual nerve fibres and brain cells as the retina responds to light and the patterns of information are processed and passed along to the brain.
In 1965 Hubel became professor of physiology and, in 1968, George Packer Berry Professor of Neurobiology. He cowrote (with Wiesel) Brain Mechanisms of Vision (1979) and Brain and Visual Perception: The Story of a 25-Year Collaboration (2004). His other works include The Visual Cortex of the Brain (1963), The Brain (1984; with Francis Crick), and Eye, Brain, and Vision (1988). Hubel and Wiesel were also awarded (with Vernon Mountcastle of Johns Hopkins) the 1978 Louisa Gross Horwitz Prize for their research on discerning the structural and functional elements of the visual cortex.
Details
David Hunter Hubel FRS (February 27, 1926 – September 22, 2013) was an American Canadian neurophysiologist noted for his studies of the structure and function of the visual cortex. He was co-recipient with Torsten Wiesel of the 1981 Nobel Prize in Physiology or Medicine (shared with Roger W. Sperry), for their discoveries concerning information processing in the visual system. For much of his career, Hubel worked as the Professor of Neurobiology at Johns Hopkins University and Harvard Medical School. In 1978, Hubel and Wiesel were awarded the Louisa Gross Horwitz Prize from Columbia University. In 1983, Hubel received the Golden Plate Award of the American Academy of Achievement.
Early life and education
Hubel was born in Windsor, Ontario, Canada, to American parents in 1926. His grandfather emigrated as a child to the United States from the Bavarian town of Nördlingen. In 1929, his family moved to Montreal, where he spent his formative years. His father was a chemical engineer and Hubel developed a keen interest in science right from childhood, making many experiments in chemistry and electronics. From age six to eighteen, he attended Strathcona Academy in Outremont, Quebec, about which he said, "[I owe] much to the excellent teachers there, especially to Julia Bradshaw, a dedicated, vivacious history teacher with a memorable Irish temper, who awakened me to the possibility of learning how to write readable English." He studied mathematics and physics at McGill University, and then completed medical school there in 1951 and followed that with three years of residency (a year of internship and two of residency in neurology) at the Montreal General Hospital.
Career
In 1954, Hubel moved to the United States to work at Johns Hopkins School of Medicine as an assistant resident in Neurology. He was later drafted by the army and served at Walter Reed Army Institute of Research (WRAIR). There, he began recording from the primary visual cortex of sleeping and awake cats. At WRAIR, he invented the modern metal microelectrode out of Stoner-Mudge lacquer and tungsten, and the modern hydraulic microdrive, which he had to learn basic machinist skills to produce. In 1958, Hubel moved to Johns Hopkins and began his collaborations with Wiesel, and discovered orientation selectivity and columnar organization in the visual cortex. One year later, he joined the faculty of Harvard University. In 1981, Hubel became a founding member of the World Cultural Council. From 1988 to 1989 he was the president of the Society for Neuroscience. He was a member of the American Academy of Arts and Sciences, the United States National Academy of Sciences, and the American Philosophical Society.
Research
The Hubel and Wiesel experiments greatly expanded the scientific knowledge of sensory processing. The partnership lasted over twenty years and became known as one of the most prominent research pairings in science. In one experiment, done in 1959, they inserted a microelectrode into the primary visual cortex of an anesthetized cat. They then projected patterns of light and dark on a screen in front of the cat. They found that some neurons fired rapidly when presented with lines at one angle, while others responded best to another angle. Some of these neurons responded to light patterns and dark patterns differently. Hubel and Wiesel called these neurons simple cells." Still other neurons, which they termed complex cells, detected edges regardless of where they were placed in the receptive field of the neuron and could preferentially detect motion in certain directions. These studies showed how the visual system constructs complex representations of visual information from simple stimulus features.
Hubel and Wiesel received the Nobel Prize for two major contributions: firstly, their work on the development of the visual system, which involved a description of ocular dominance columns in the 1960s and 1970s; and secondly, their work establishing a foundation for visual neurophysiology, describing how signals from the eye are processed by visual parcels in the neo-cortex to generate edge detectors, motion detectors, stereoscopic depth detectors, and color detectors, building blocks of the visual scene. By depriving kittens of using one eye, they showed that columns in the primary visual cortex receiving inputs from the other eye took over the areas that would normally receive input from the deprived eye. This has important implications for the understanding of deprivation amblyopia, a type of visual loss due to unilateral visual deprivation during the so-called critical period. These kittens also did not develop areas receiving input from both eyes, a feature needed for binocular vision. Hubel and Wiesel's experiments showed that the ocular dominance develops irreversibly[verification needed] early in childhood development. These studies opened the door for the understanding and treatment of childhood cataracts and strabismus. They were also important in the study of cortical plasticity.
Furthermore, the understanding of sensory processing in animals served as inspiration for the SIFT descriptor (Lowe, 1999), which is a local feature used in computer vision for tasks such as object recognition and wide-baseline matching, etc. The SIFT descriptor is arguably the most widely used feature type for these tasks. Hubel was elected a Foreign Member of the Royal Society (ForMemRS) in 1982.
Personal life
Hubel married Ruth Izzard in 1953; she died February 17, 2013. The couple had three sons and four grandchildren. He died in Lincoln, Massachusetts, from kidney failure on September 22, 2013, at the age of 87.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1474 2024-04-13 21:13:48
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,394
Re: crème de la crème
1436) Torsten Wiesel
Gist
Our vision works by the light around us being captured by a large number of light-sensitive cells located in the retinas at the back of our eyes. The light is converted into signals that are sent to the brain and there converted into visual impressions. Torsten Wiesel and David Hubel clarified how this process works during the 1960s: In the cerebral cortex signals are analyzed in sequence by cells with the specific tasks of interpreting contrasts, patterns, and movements. They also showed that this ability develops in children during the initial period after birth.
Details
Torsten Nils Wiesel (born 3 June 1924) is a Swedish neurophysiologist. With David H. Hubel, he received the 1981 Nobel Prize in Physiology or Medicine, for their discoveries concerning information processing in the visual system; the prize was shared with Roger W. Sperry for his independent research on the cerebral hemispheres.
Career
Wiesel was born in Uppsala, Sweden in 1924, the youngest of five children. In 1947, he began his scientific career in Carl Gustaf Bernhard's laboratory at the Karolinska Institute, where he received his medical degree in 1954. He went on to teach in the Institute's department of physiology and worked in the child psychiatry unit of the Karolinska Hospital. In 1955 he moved to the United States to work at Johns Hopkins School of Medicine under Stephen Kuffler. Wiesel began a fellowship in ophthalmology, and in 1958 he became an assistant professor. That same year, he met David Hubel, beginning a collaboration that would last over twenty years. In 1959 Wiesel and Hubel moved to Harvard University. He became an instructor in pharmacology at Harvard Medical School, beginning a 24-year career with the university. He became professor in the new department of neurobiology in 1968 and its chair in 1971.
In 1983, Wiesel joined the faculty of Rockefeller University as Vincent and Brooke Astor Professor and head of the Laboratory of Neurobiology. He was president of the university from 1991 to 1998. At Rockefeller University he remains the director of the Shelby White and Leon Levy Center for Mind, Brain and Behavior.
From 2000-2009, Wiesel served as Secretary-General of the Human Frontier Science Program, an organization headquartered in Strasbourg, France, which supports international and interdisciplinary collaboration between investigators in the life sciences. Wiesel also has chaired the scientific advisory board of China's National Institute of Biological Science (NIBS) in Beijing, and co-chairs the board of governors of the Okinawa Institute of Science and Technology (OIST). He is also member of the boards of the Pew Center on Global Climate Change, the Hospital for Special Surgery, and an advisory board member of the European Brain Research Institute (EBRI).
Wiesel has also served as chair of the board of the Aaron Diamond AIDS Research Center (1995–2001), president of the Society for Neuroscience (1978–1979), and the International Brain Research Organization (1998–2004). He was chair of the board of governors of the New York Academy of Sciences (2001–2006); and he was the academy's chairman and interim director in 2001–2002.
Research
The Hubel and Wiesel experiments greatly expanded the scientific knowledge of sensory processing. In one experiment, done in 1959, they inserted a microelectrode into the primary visual cortex of an anesthetized cat. They then projected patterns of light and dark on a screen in front of the cat. They found that some neurons fired rapidly when presented with lines at one angle, while others responded best to another angle. They called these neurons "simple cells." Still other neurons, which they termed "complex cells," responded best to lines of a certain angle moving in one direction. These studies showed how the visual system builds an image from simple stimuli into more complex representations.
Hubel and Wiesel were awarded the Nobel Prize in 1981 for their work on ocular dominance columns in the 1960s and 1970s. By depriving kittens from using one eye, they showed that columns in the primary visual cortex receiving inputs from the other eye took over the areas that would normally receive input from the deprived eye. These kittens also did not develop areas receiving input from both eyes, a feature needed for binocular vision. Hubel and Wiesel's experiments showed that the ocular dominance develops irreversibly early in childhood development. These studies opened the door for the understanding and treatment of childhood cataracts and strabismus. They were also important in the study of cortical plasticity.
Wiesel was among the eight 2005 recipients of the National Medal of Science. In 2006, he was awarded the Ramon Y Cajal Gold Medal from the Spanish National Research Council (CSIC - Consejo Superior de Investigaciones Cientificas). In 2007, both Wiesel and Hubel were awarded the Marshall M. Parks, MD Medal from The Children's Eye Foundation.
Personal life
Wiesel is married to Lizette Mususa Reyes (m. 2008). Wiesel was married to Teeri Stenhammar from 1956-1970, Ann Yee from 1973-1981, and author and editor Jean Stein from 1995-2007. His daughter Sara Elisabeth was born in 1975.
Human rights
Wiesel has done much work as a global human rights advocate. He served for 10 years (1994–2004) as chair of the Committee of Human Rights of the National Academies of Science in the US, as well as the International Human Rights Network of Academies and Scholarly Societies. He was awarded the David Rall Medal from the Institute of Medicine in 2005, in recognition of this important work. In 2009, Wiesel was awarded the Grand Cordon Order of the Rising Sun Medal in Japan.
He is a founding member of the Israeli-Palestinian Science Organization, a nongovernmental nonprofit established in 2004 to support collaborative research between scientists in Israel and Palestine.
Additional Information
Torsten Wiesel (born June 3, 1924, Uppsala, Sweden) Swedish neurobiologist, recipient with David Hunter Hubel and Roger Wolcott Sperry of the 1981 Nobel Prize for Physiology or Medicine. All three scientists were honoured for their investigations of brain function, Wiesel and Hubel in particular for their collaborative studies of the visual cortex, which is located in the occipital lobes of the cerebrum.
Wiesel earned a medical degree from the Karolinska Institute in Stockholm in 1954. After remaining there for a year as an instructor in physiology, he accepted a research appointment at the Johns Hopkins University Medical School in Baltimore, Maryland, where his association with Hubel began. Working with laboratory animals, they analyzed the flow of nerve impulses from the eye to the visual cortex and were thereby able to discern many structural and functional details of that part of the brain. Wiesel and Hubel also studied the effects of various visual impairments in young animals, and their results lent strong support to the view that prompt surgery is imperative in correcting certain eye defects that are detectable in newborn children.
In 1959 Wiesel moved, along with Hubel, to Harvard University, where in 1974 he was named the Robert Winthrop professor of neurobiology. In 1983 Wiesel accepted a position as the Vincent Brook Astor professor of neuroscience at Rockefeller University and formed a collaborative partnership with American neurobiologist Charles Gilbert, who was studying the interactions of neurons in the primary visual cortex. Their studies led to the elucidation of fundamental neuronal connections in the visual cortex and revealed information about the responses of cells in the visual receptive fields. From 1991 to 1998 Wiesel served as president of Rockefeller University and worked to facilitate collaboration efforts among scientists and to create new positions to attract talented researchers. He later served as secretary general (2000–09) of the Human Frontier Science Program (HFSP) in Stockholm. Wiesel’s role at the HFSP was concerned primarily with helping young scientists in countries around the world find research and collaboration opportunities.
Wiesel served on the boards of multiple organizations, including the Pew Center on Climate Change, the New York Academy of Sciences, and the International Brain Research Organization. In 2004 he cofounded the Israeli-Palestinian Science Organization to promote scientific collaboration between researchers in Israel and Palestine. Wiesel was an advocate of human rights, having served as the chair of both the International Human Rights Network of Academies and Scholarly Societies and the Committee of Human Rights of the National Academies of Science in the United States. In 2007 Wiesel’s efforts to support research on eye diseases were realized when the Torsten Wiesel Research Institute was established as part of the World Eye Organization, based in Chengdu, China.
In addition to Wiesel’s numerous scientific papers, he wrote several books, including two with Hubel, Brain Mechanisms of Vision (1991) and Brain and Visual Perception: The Story of a 25-Year Collaboration (2004). Wiesel received multiple awards during his career, including the Louisa Gross Horwitz Prize in 1978 (shared with Hubel) and the U.S. National Medal of Science in 2005.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1475 2024-04-18 21:44:57
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,394
Re: crème de la crème
1437) Kenneth G. Wilson
Gist
An accumulation of matter with uniform physical and chemical properties is said to be in a certain phase, such as solid, liquid or gas. There also are other phases, for example, when dealing with magnetism. A theoretical understanding of critical points for phase transitions requires a broad spectrum of scaling. Early attempts to explain this in one step led to infinities in the result. Kenneth Wilson solved the problem in 1971 through a type of renormalization, which can be described as solving the problem piece by piece.
Details
Kenneth Geddes Wilson (June 8, 1936 – June 15, 2013) was an American theoretical physicist and a pioneer in leveraging computers for studying particle physics. He was awarded the 1982 Nobel Prize in Physics for his work on phase transitions—illuminating the subtle essence of phenomena like melting ice and emerging magnetism. It was embodied in his fundamental work on the renormalization group.
Life
Wilson was born on June 8, 1936, in Waltham, Massachusetts, the oldest child of Emily Buckingham Wilson and E. Bright Wilson, a prominent chemist at Harvard University, who did important work on microwave emissions. His mother also trained as a physicist. He attended several schools, including Magdalen College School, Oxford, England, ending up at the George School in eastern Pennsylvania.
He went on to Harvard College at age 16, majoring in Mathematics and, on two occasions, in 1954 and 1956, ranked among the top five in the William Lowell Putnam Mathematical Competition. He was also a star on the athletics track, representing Harvard in the Mile. During his summer holidays he worked at the Woods Hole Oceanographic Institution. He earned his PhD from Caltech in 1961, studying under Murray Gell-Mann. He did post-doc work at Harvard and CERN.
He joined Cornell University in 1963 in the Department of Physics as a junior faculty member, becoming a full professor in 1970. He also did research at SLAC during this period. In 1974, he became the James A. Weeks Professor of Physics at Cornell.
In 1982 he was awarded the Nobel Prize in Physics for his work on critical phenomena using the renormalization group.
He was a co-winner of the Wolf Prize in physics in 1980, together with Michael E. Fisher and Leo Kadanoff. His other awards include the A.C. Eringen Medal, the Franklin Medal, the Boltzmann Medal, and the Dannie Heinemann Prize. He was elected a member of the National Academy of Science and a fellow of the American Academy of Arts and Science, both in 1975, and also was elected a member of the American Philosophical Society in 1984.[7]
In 1985, he was appointed as Cornell's Director of the Center for Theory and Simulation in Science and Engineering (now known as the Cornell Theory Center), one of five national supercomputer centers created by the National Science Foundation. In 1988, Wilson joined the faculty at Ohio State University. Wilson moved to Gray, Maine in 1995. He continued his association with Ohio State University until he retired in 2008. Prior to his death, he was actively involved in research on physics education and was an early proponent of "active involvement" (i.e. Science by Inquiry) of K-12 students in science and math.
Some of his PhD students include H. R. Krishnamurthy, Roman Jackiw, Michael Peskin, Serge Rudaz, Paul Ginsparg, and Steven R. White.
Wilson's brother David was also a professor at Cornell in the department of Molecular Biology and Genetics until his death, and his wife since 1982, Alison Brown, is a prominent computer scientist.
He died in Saco, Maine, on June 15, 2013, at the age of 77. He was respectfully remembered by his colleagues.
Work
Wilson's work in physics involved formulation of a comprehensive theory of scaling: how fundamental properties and forces of a system vary depending on the scale over which they are measured. He devised a universal "divide-and-conquer" strategy for calculating how phase transitions occur, by considering each scale separately and then abstracting the connection between contiguous ones, in a novel appreciation of renormalization group theory. This provided profound insights into the field of critical phenomena and phase transitions in statistical physics enabling precise calculations. One example of an important problem in solid-state physics he solved using renormalization is in quantitatively describing the Kondo effect.
He extended these insights on scaling to answer fundamental questions on the nature of quantum field theory and the operator product expansion and the physical meaning of the renormalization group.
He also pioneered the understanding of the confinement of quarks inside hadrons, utilizing lattice gauge theory, and initiating an approach permitting formerly foreboding strong-coupling calculations on computers. On such a lattice, he further shed light on chiral symmetry, a crucial feature of elementary particle interactions.
Additional Information
Kenneth Geddes Wilson (born June 8, 1936, Waltham, Massachusetts, U.S.—died June 15, 2013, Saco, Maine) was an American physicist who was awarded the 1982 Nobel Prize for Physics for his development of a general procedure for constructing improved theories concerning the transformations of matter called continuous, or second-order, phase transitions.
Wilson graduated from Harvard University in 1956. In 1961 he received a Ph.D. from the California Institute of Technology, where he completed a dissertation under Murray Gell-Mann (winner of the Nobel Prize for Physics in 1969) and Francis Low. After a year at the European Council for Nuclear Research, Wilson was appointed assistant professor at Cornell University in 1963; he was professor of physics from 1971 to 1988.
Wilson did his prizewinning work on phase transitions while at Cornell. Second-order phase transitions of matter take place at characteristic temperatures (or pressures), but unlike first-order transitions they occur throughout the entire volume of a material as soon as that temperature (called the critical point) is reached. One example of such a transition is the complete loss of ferromagnetic properties of certain metals when they are heated to their Curie points (about 750° C for iron). Wilson’s work provided a mathematical strategy for constructing theories that could apply to physical systems near the critical point. From 1988 Wilson taught at Ohio State University.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline