Math Is Fun Forum
You are not logged in.
- Topics: Active | Unanswered
#2351 2024-10-23 00:03:41
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,148
Re: Miscellany
2251) Asphalt
Gist
Asphalt concrete (commonly called asphalt, blacktop, or pavement in North America, and tarmac or bitumen macadam in the United Kingdom and the Republic of Ireland) is a composite material commonly used to surface roads, parking lots, airports, and the core of embankment dams.
Asphalt is known as a mixture of bitumen (as binding material) and a significant amount of inert minerals such as sand, gravel, and crushed stone. It has a blackish-brown colour and is available as a solid at low temperatures and as a liquid at temperatures above 50°C.
Summary
asphalt, black or brown petroleum-like material that has a consistency varying from viscous liquid to glassy solid. It is obtained either as a residue from the distillation of petroleum or from natural deposits. Asphalt consists of compounds of hydrogen and carbon with minor proportions of nitrogen, sulfur, and oxygen. Natural asphalt (also called brea), which is believed to be formed during an early stage in the breakdown of organic marine deposits into petroleum, characteristically contains minerals, while residual petroleum asphalt does not.
The use of asphalt is very old, dating back to its use as a water stop between brick walls of a reservoir at Mohenjo-Daro (about the 3rd millennium bc) in Pakistan. In the Middle East it was extensively used for paving roads and sealing waterworks, important applications even today. The Pitch Lake on the island of Trinidad was the first large commercial source, but natural sources have since declined in importance as petroleum became the major source. Gilsonite, wurzilite, and similar vein asphalts have special uses in heat-resistant enamels; they are hard and are mined like coal. Petroleum asphalt is produced in all consistencies from light road oils to heavy, high-viscosity industrial types.
Asphalt softens when heated and is elastic under certain conditions. The mechanical properties of asphalt are of little significance except when it is used as a binder or adhesive. The principal application of asphalt is in road surfacing, which may be done in a variety of ways. Light oil “dust layer” treatments may be built up by repetition to form a hard surface, or a granular aggregate may be added to an asphalt coat, or earth materials from the road surface itself may be mixed with the asphalt.
Other important applications include canal and reservoir linings, dam facings, and other harbour and sea works; asphalt so used may be a thin, sprayed membrane, covered with earth for protection against weathering and mechanical damage, or thicker surfaces, often including riprap (crushed rock). Asphalt is also used for roofs, coatings, floor tilings, soundproofing, waterproofing, and other building-construction elements and in a number of industrial products, such as batteries. For certain applications an asphaltic emulsion is prepared, in which fine globules of asphalt are suspended in water.
Details
Asphalt is a mixture of aggregates, binder and filler, used for constructing and maintaining roads, parking areas, railway tracks, ports, airport runways, bicycle lanes, sidewalks and also play- and sport areas.
Aggregates used for asphalt mixtures could be crushed rock, sand, gravel or slags. Nowadays, certain waste and by-products, such as construction and demolition debris, are being used as aggregates, which increases the sustainability of asphalt.
In order to bind the aggregates into a cohesive mixture a binder is used. Most commonly, bitumen is used as a binder, although nowadays, a series of bio-based binders are also under development with the aim of minimising the environmental impact of the roads.
An average asphalt pavement consists of the road structure above the formation level which includes unbound and bituminous-bound materials. This gives the pavement the ability to distribute the loads of the traffic before it arrives at the formation level.
How is asphalt produced?
Asphalt is produced in an asphalt plant. This can be a fixed plant or even in a mobile mixing plant. It is possible to produce in an asphalt plant up to 800 tons per hour. The average production temperature of hot mix asphalt is between 150 and 180°C, but nowadays new techniques are available to produce asphalt at lower temperatures.
Different kinds of asphalt
To be able to provide the best performance to different applications, a large variety of asphalt mixes can be used. Due to the different requirements (amount of traffic, amount of heavy vehicles, temperature, weather conditions, noise reduction requirements, etc.) the respective mix used needs to have an sufficient stiffness and resistance to deformation in order to cope with the applied pressure from vehicle wheels on the one hand, yet on the other hand, they need to have an adequate flexural strength to resist cracking caused by the varying pressures exerted on them. Moreover, good workability during application is essential in order to ensure that they can be fully compacted to achieve optimum durability.
Asphalt mixtures can be produced at different temperatures:
Hot Mix Asphalt (HMA)
Hot asphalt mixes are generally produced at a temperature between 150 and 180 °C. Depending on the usage, a different asphalt mixture can be used. For more details of the different asphalt mixtures, go to “Asphalt products”
Warm Mix Asphalt (WMA)
A typical WMA is produced at a temperature around 20 – 40 °C lower than an equivalent Hot Mix Asphalt. Significantly less energy is involved and, consequently, less fumes are produced (as rule of thumb, a reduction of 25ºC produces a reduction of 75% of fumes emission). In addition, during the paving operations, the temperature of the material is lower, resulting in improved working conditions for the crew and an earlier opening of the road.
Cold Mix Asphalt
Cold mixes are produced without heating the aggregate. This is only possible, due to the use of bitumen emulsified in water, which breaks either during compaction or during mixing. Producing the coating of the aggregate. Over the curing time, water evaporates and strength increases. Cold mixes are particularly recommendable for lightly trafficked roads.
Different asphalt layers
An asphalt pavement consists of different asphalt layers.
In general the asphalt layers are paved on a bound or unbound road base layer. Starting at the road surface, the first layer is called the surface course. The second layer is mostly called the binder course. The lower layers are the base courses.
Surface course
The surface course constitutes the top layer of the pavement and should be able to withstand high traffic- and environmentally-induced stresses without exhibiting unsatisfactory cracking and rutting. Its main mission is to provide an even profile for the comfort of the user, while providing enough texture to ensure minimum and safe skid resistance. Depending on local conditions, functional characteristics such as skid resistance, noise reduction and durability are often required for wearing courses. In some cases, rapid drainage of surface water is desired while in other cases, the wearing course should be impermeable in order to keep water out of the pavement structure. A wide range of surface layer products can be used depending on specific requirements.
Binder course
Binder courses are designed to withstand the highest shear stresses that occur about 50 – 70 mm below the asphalt surface. The binder course is therefore placed between the surface course and base course to reduce rutting by combining qualities of stability and durability. Stability can be achieved by sufficient stone-on-stone contact and stiff and/or modified binders.
Base course
The base course is perhaps the most important structural layer of the pavement, which is intended to effectively distribute traffic and environmental loading in such a way that underlying unbound layers are not exposed to excessive stresses and strains. This often implies comparatively high stiffness of the base course. Next to this the base course should also show adequate fatigue resistance.
Unbound materials and foundation
Since the formation and sub-soil often constitute relatively weak materials, it is of utmost importance that the damaging loadings are effectively eliminated by the layers above. In this case, unbound road-base or sub-base layers consisting of uncrushed or crushed aggregate can be suitable.
Additional Information
Asphalt is a dark brown to black, highly viscous, hydrocarbon produced from petroleum distillation residue. This distillation can occur naturally, resulting in asphalt lakes, or occur in a petroleum refinery using crude oil. In 2020, the U.S. produced about 21 million tons of asphalt (US EIA). Roads and highways constitute the largest single use of asphalt at about 80 percent of the total (Fredonia Group). In HMA, asphalt functions as a waterproof, thermoplastic, viscoelastic adhesive. By weight, asphalt generally accounts for between 4 and 8 percent of HMA and makes up about 25 – 30 percent of the cost of an HMA pavement structure depending upon the type and quantity. The paving industry also uses asphalt emulsions, asphalt cutbacks and foamed asphalt.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2352 2024-10-24 00:02:59
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,148
Re: Miscellany
2252) Millenium
Gist
A millennium is a period of one thousand years, especially one which begins and ends with a year ending in `000,' for example the period from the year 1000 to the year 2000. [formal]
Since in Latin mille means "thousand", a millennium lasts 1,000 years.
A millennium ( pl. millennia or millenniums) is a period of one thousand years or one hundred decades or ten centuries, sometimes called a kiloannum (ka), or kiloyear (ky).
Summary
A millennium is a period of 1,000 years. The Gregorian calendar, put forth in 1582 and subsequently adopted by most countries, did not include a year 0 in the transition from bc (years before Christ) to ad (those since his birth). Thus, the 1st millennium is defined as spanning years 1–1000 and the 2nd the years 1001–2000.
A millennium is a period of 1,000 years. The Gregorian calendar, put forth in 1582 and subsequently adopted by most countries, did not include a year 0 in the transition from bc (years before Christ) to ad (those since his birth). Thus, the 1st millennium is defined as spanning years 1–1000 and the 2nd the years 1001–2000. Although numerous popular celebrations marked the start of the year 2000, the 21st century and 3rd millennium ad began on January 1, 2001.
Details
A millennium (pl. millennia or millenniums) is a period of one thousand years or one hundred decades or ten centuries, sometimes called a kiloannum (ka), or kiloyear (ky). Normally, the word is used specifically for periods of a thousand years that begin at the starting point (initial reference point) of the calendar in consideration and at later years that are whole number multiples of a thousand years after the start point.[clarification needed] The term can also refer to an interval of time beginning on any date. Millennia sometimes have religious or theological implications.
The word millennium derives from the Latin mille, thousand, and annus, year.
There was a public debate leading up to the celebrations of the year 2000 as to whether the beginning of that year should be understood as the beginning of the "new" millennium. Historically, there has been debate around the turn of previous decades, centuries, and millennia, but not so much for decades. The issue arises from the difference between the convention of using ordinal numbers to count years and millennia, as in "the third millennium", or using a vernacular description, as in "the two thousands". The difference of opinion comes down to whether to celebrate, respectively, the end or the beginning of the "-000" year. The first convention is common in English-speaking countries, but the latter is favoured in, for example, Sweden (tvåtusentalet, which translates literally as the two thousands period).
Those holding that the arrival of the new millennium should be celebrated in the transition from 2000 to 2001 (i.e., December 31, 2000, to January 1, 2001) argued that the Anno Domini system of counting years began with the year 1 (there was no year 0) and therefore the first millennium was from the year 1 to the end of the year 1000, the second millennium from 1001 to the end of 2000, and the third millennium beginning with 2001 and ending at the end of 3000. Similarly, the first millennium BC was from the year 1000 BC to the end of the year 1 BC.
Popular culture supported celebrating the arrival of the new millennium in the transition from 1999 to 2000 (i.e., December 31, 1999, to January 1, 2000), in that the change of the hundreds digit in the year number, with the zeroes rolling over, is consistent with the vernacular demarcation of decades by their 'tens' digit (e.g. naming the period 1980 to 1989 as "the 1980s" or "the eighties"). This has been described as "the odometer effect". Also, the "year 2000" had been a popular phrase referring to an often utopian future, or a year when stories in such a future were set. There was also media and public interest in the Y2K computer bug.
A third position was expressed by Bill Paupe, honorary consul for Kiribati: "To me, I just don't see what all the hoopla is about ... it's not going to change anything. The next day the sun is going to come up again and then it will all be forgotten." Even for those who did celebrate, in astronomical terms, there was nothing special about this particular event.
Stephen Jay Gould, in his essay "Dousing Diminutive Dennis' Debate (or DDDD = 2000)", discussed the "high" versus "pop" culture interpretation of the transition. Gould noted that the high culture, strict construction had been the dominant viewpoint at the 20th century's beginning, but that the pop culture viewpoint dominated at its end.
The start of the 21st century and 3rd millennium was celebrated worldwide at the start of the year 2000. One year later, at the start of the year 2001, the celebrations had largely returned to the usual ringing in of just another new year, although some welcomed "the real millennium", including America's official timekeeper, the U.S. Naval Observatory, and the countries of Cuba and Japan.
The popular approach was to treat the end of 1999 as the end of "a millennium" and to hold millennium celebrations at midnight between December 31, 1999, and January 1, 2000, with the cultural and psychological significance of the events listed above combining to cause celebrations to be observed one year earlier than the formal date.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2353 2024-10-25 00:23:45
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,148
Re: Miscellany
2253) Liquid Paraffin
Gist
Liquid paraffin is primarily used as a pediatric laxative in medicine and is a popular treatment for constipation and encopresis. Because of its ease of titration, the drug is convenient to synthesize.
Liquid Parafin belongs to the group of medicines called 'laxatives' used to treat constipation in conditions like piles, fistula, fissures, pre/post-operative conditions, elderly and bed-ridden patients. Constipation refers to infrequent bowel movements in which the stools are often dry, painful, and hard to pass.
Summary
Liquid paraffin is a mixture of liquid hydrocarbons. Its main use has been as a lubricant laxative but it is not recommended, because of its adverse effects. Nevertheless, it continues to be used for this purpose and is reportedly as effective as lactulose . However, the erstwhile Committee on Safety of Medicines in the UK recommended the following precautions :
• pack sizes to be limited to 160 ml;
• liquid paraffin to be used only for the symptomatic relief of constipation;
• prolonged use to be avoided and the package label to state “repeated use is not recommended”;
• to be contraindicated in children under 3 years of age.
Liquid paraffin has also been used in ointments, as an emollient in skin diseases, and as a lubricant in treating dry eyes. Injection of liquid paraffin into the pleural cavity (oleothorax) was a widely used treatment for pulmonary tuberculosis before effective antituberculosis drugs became available. Long-term complications continue to be reported.
Details
Liquid paraffin, also known as paraffinum liquidum, paraffin oil, liquid paraffin oil or Russian mineral oil, is a very highly refined mineral oil used in cosmetics and medicine. Cosmetic or medicinal liquid paraffin should not be confused with the paraffin (i.e. kerosene) used as a fuel. The generic sense of paraffin meaning alkane led to regional differences for the meanings of both paraffin and paraffin oil. It is a transparent, colorless, nearly odorless, and oily liquid that is composed of saturated hydrocarbons derived from petroleum.
The term paraffinum perliquidum is sometimes used to denote light liquid paraffin, while the term paraffinum subliquidum is sometimes used to denote a thicker mineral oil.
History
Petroleum is said to have been used as a medicine since 400 BC, and has been mentioned in the texts of classical writers Herodotus, Plutarch, Dioscorides, Pliny, and others. It was used extensively by early Arabians and was important in early Indian medicine. Its first use internally is attributed to Robert A. Chesebrough, who patented it in 1872 for the manufacture of a "new and useful product from petroleum." After Sir W. Arbuthnot Lane, who was then Chief Surgeon of Guy's Hospital, recommended it as a treatment for intestinal stasis and chronic constipation in 1913, liquid paraffin gained more popularity.
Usage in medicine
Liquid paraffin is primarily used as a pediatric laxative in medicine and is a popular treatment for constipation and encopresis. Because of its ease of titration, the drug is convenient to synthesize. It acts primarily as a stool lubricant, and is thus not associated with abdominal cramps, diarrhea, flatulence, disturbances in electrolytes, or tolerance over long periods of usage, side effects that osmotic and stimulant laxatives often engender (however, some literature suggests that these may still occur). The drug acts by softening the feces and coats the intestine with an oily film. Because of this it reduces the pain caused by certain conditions such as piles (haemorrhoids). These traits make the drug ideal for chronic childhood constipation and encopresis, when large doses or long-term usage is necessary.
Consensus has not been entirely reached on the safety of the drug for children. While the drug is widely accepted for the management of childhood constipation in North America and Australia, the drug is used much less in the United Kingdom. The drug is endorsed by the American Academy of Pediatrics and the North American Society for Gastroenterology and Nutrition, with the latter organization outlining it as a first choice for the management of pediatric constipation. The drug is suggested to never be used in cases in which the patient is neurologically impaired or has a potential swallowing dysfunction due to potential respiration complications. Lipoid pneumonia due to mineral oil aspiration is thus a recognized severe complication of this medication, and there is a need for a heightened awareness among caregivers about the potential dangers of inappropriate mineral oil use. Some go as far as saying that it should never be used with children due to this risk.
Liquid paraffin is also used in combination with magnesium as an osmotic laxative, sold under the trade name Mil-Par (among others).
Additionally, it may be used as a release agent, binder, or lubricant on capsules and tablets.
Usage in cosmetics
Liquid paraffin is a hydrating and cleansing agent. Hence, it is used in several cosmetics both for skin and hair products. It is also used as one of the ingredients of after wax wipes.
Health
Upon being taken orally, liquid paraffin might interfere with the absorption of fat-soluble vitamins, though evidence does not seem to fully support this. It can be absorbed into the intestinal wall and may cause foreign-body granulomatous reactions in some rat species. These reactions might not occur in humans, however. Some evidence suggests that it engenders a lack of carcinogenicity. If liquid paraffin enters the lungs, it can cause lipoid pneumonia.
If injected, it can cause granulomatous reactions.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2354 2024-10-26 00:02:23
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,148
Re: Miscellany
2254) Anthracite
Gist
The principal use of anthracite today is for a domestic fuel in either hand-fired stoves or automatic stoker furnaces. It delivers high energy per its weight and burns cleanly with little soot, making it ideal for this purpose. Its high value makes it prohibitively expensive for power plant use.
Summary
Anthracite is the most highly metamorphosed form of coal. It contains more fixed carbon (86 percent or greater on a dry, ash-free basis) than any other form of coal and the least amount of volatile matter (14 percent or less on a dry, ash-free basis), and it has calorific values near 35 megajoules per kilogram (approximately 15,000 British thermal units per pound), not much different from the calorific values for most bituminous coal. Anthracite is the least plentiful form of coal. In the United States it is found mostly in northeastern Pennsylvania and makes up less than 2 percent of all coal reserves in the country. Smaller amounts of anthracite occur in South Africa, Australia, eastern Ukraine, western Canada, China, and other countries.
Anthracites are black to steel gray and have a brilliant, almost metallic lustre. They can be polished and used for decorative purposes. Hard and brittle, anthracites break with conchoidal fracture into sharp fragments. Unlike many bituminous coals, they are clean to the touch. Although anthracites are difficult to ignite, they burn with a pale blue flame and require little attention to sustain combustion. In the past they were used for domestic heating because they produce little dust upon handling, burn slowly, and emit relatively little smoke. Anthracite is rarely used for this purpose today because of its limited abundance and relatively high cost and the ready availability of other sources of energy (e.g., natural gas and electricity) for heating purposes.
Although anthracites usually occur in geologically deformed areas, such as in the intensely folded sedimentary rocks of the anthracite region of Pennsylvania, their origin is due to higher than normal heating caused by the presence of nearby igneous intrusions or high geothermal gradients. Both of these phenomena produce temperatures much higher than those reached at depth in most sedimentary basins. For instance, in Antarctica, large igneous sills intruded the coal measures and converted some of the existing bituminous coal to anthracite. Temperatures ranging from 170 to 250 °C (about 340 to 480 °F) are thought to be necessary for the formation of anthracite.
Details
Anthracite, also known as hard coal and black coal, is a hard, compact variety of coal that has a submetallic lustre. It has the highest carbon content, the fewest impurities, and the highest energy density of all types of coal and is the highest ranking of coals.
The Coal Region of Northeastern Pennsylvania in the United States has the largest known deposits of anthracite coal in the world with an estimated reserve of seven billion short tons. China accounts for the majority of global production; other producers include Russia, Ukraine, North Korea, South Africa, Vietnam, Australia, Canada, and the United States. Total production in 2020 was 615 million tons.
Anthracite is the most metamorphosed type of coal, but still represents low-grade metamorphism, in which the carbon content is between 86% and 97%. The term is applied to those varieties of coal which do not give off tarry or other hydrocarbon vapours when heated below their point of ignition. Anthracite is difficult to ignite, and burns with a short, blue, and smokeless flame.
Anthracite is categorized into several grades. Standard grade is used predominantly in power generation, and high grade (HG) and ultra high grade (UHG), are used predominantly in the metallurgy sector. Anthracite accounts for about 1% of global coal reserves, and is mined in only a few countries around the world.
Names
Anthracite derives from the Greek anthrakítēs, literally "coal-like". Other terms which refer to anthracite are black coal, hard coal, stone coal, dark coal, coffee coal, blind coal (in Scotland), Kilkenny coal (in Ireland), crow coal or craw coal, and black diamond. "Blue Coal" is the term for a once-popular and trademarked brand of anthracite, mined by the Glen Alden Coal Company in Pennsylvania, and sprayed with a blue dye at the mine before shipping to its Northeastern U.S. markets to distinguish it from its competitors.
Culm has different meanings in British and American English. In British English, culm is the imperfect anthracite, located predominantly north Devon and Cornwall, which was used as a pigment. The term is also used to refer to some carboniferous rock strata found in both Britain and in the Rhenish hill countries, also known as the Culm Measures. In Britain, it may also refer to coal exported from Britain during the 19th century. In American English, "culm" refers to the waste or slack from anthracite mining, mostly dust and small pieces not suitable for use in home furnaces.
Properties
Anthracite is similar in appearance to the mineraloid jet and is sometimes used as a jet imitation.
Anthracite differs from ordinary bituminous coal by its greater hardness (2.75–3 on the Mohs scale), its higher relative density of 1.3–1.4, and luster, which is often semi-metallic with a mildly green reflection. It contains a high percentage of fixed carbon and a low percentage of volatile matter. It is also free from included soft or fibrous notches and does not soil the fingers when rubbed. Anthracitization is the transformation of bituminous coal into anthracite.
The moisture content of fresh-mined anthracite generally is less than 15 percent. The heat content of anthracite ranges from 26 to 33 MJ/kg (22 to 28 million Btu/short ton) on a moist, mineral-matter-free basis. The heat content of anthracite coal consumed in the United States averages 29 MJ/kg (25 million Btu/ton), on the as-received basis, containing both inherent moisture and mineral matter.
Since the 1980s, anthracite refuse or mine waste has been used for coal power generation in a form of recycling. The practice known as reclamation is being applied to culm piles antedating laws requiring mine owners to restore lands to their approximate original condition.
Chemically, anthracite may be considered as a transition stage between ordinary bituminous coal and graphite, produced by the more or less complete elimination of the volatile constituents of the former, and it is found most abundantly in areas that have been subjected to considerable stresses and pressures, such as the flanks of great mountain ranges. Anthracite is associated with strongly deformed sedimentary rocks that were subjected to higher pressures and temperatures (but short of metamorphic conditions) just as bituminous coal is generally associated with less deformed or flat-lying sedimentary rocks. The compressed layers of anthracite that are deep mined in the folded Ridge and Valley Province of the Appalachian Mountains of the Coal Region of East-central Pennsylvania are extensions of the same layers of bituminous coal that are mined on the generally flat lying and undeformed sedimentary rocks further west on the Allegheny Plateau of Kentucky and West Virginia, Eastern Ohio, and Western Pennsylvania.
In the same way the anthracite region of South Wales is confined to the contorted portion west of Swansea and Llanelli, the central and eastern portions producing steam coal, coking coal and domestic house coals.
Anthracite shows some alteration by the development of secondary divisional planes and fissures so that the original stratification lines are not always easily seen. The thermal conductivity is also higher; a lump of anthracite feels perceptibly colder when held in the warm hand than a similar lump of bituminous coal at the same temperature.
Anthracite has a history of use in blast furnaces for iron smelting; however, it lacked the pore space of metallurgical coke, which eventually replaced anthracite.
Additional Information
Hard and very brittle, anthracite is dense, shiny black, and homogeneous with no marks of layers. Unlike the lower rank coals, it has a high percentage of fixed carbon and a low percentage of volatile matter. Anthracites include a variety of slow-burning fuels merging into graphite at one end and bituminous coal at the other. They are the hardest coals on the market, consisting almost entirely of fixed carbon, with the little volatile matter present in them chiefly as methane, CH4. Anthracite is usually graded into small sizes before being burned on stokers: the ‘meta-anthracites’ burn so slowly as to require mixing with other coals, while the ‘semi-anthracites’, which have more volatile matter, are burned with relative ease if proper fired. Most anthracites have a lower heating value than the highest-grade bituminous coals. Anthracite is used principally for heating homes and in gas production.
Some semi-anthracites are dense, but softer than anthracite. This grade is shiny gray and somewhat granular in structure. The grains have a tendency to break off in handling and produce coarse, sand-like slack.
Other semi-anthracites are dark gray and distinctly granular. The grains break off easily in handling and produce a coarse slack. The granular structure has been produced by small vertical cracks in horizontal layers of comparatively pure coal separated by very thin partings. The cracks are the result of heavy downward pressure and shrinkage of the pure coal because of a drop in temperature.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2355 2024-10-27 22:43:10
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,148
Re: Miscellany
2255) Analgesic
Gist
Analgesics are medications used in the management and treatment of pain. They include several classes of medications (acetaminophen, nonsteroidal anti-inflammatory drugs, antidepressants, antiepileptics, local anesthetics, and opioids).
Analgesics are medications that relieve pain. They work either by reducing inflammation or by changing the way the brain processes and perceives pain. Side effects include heartburn, nausea, and headaches.
Some types of analgesics are available over the counter. However, stronger variants are available only with a prescription. This is because strong analgesics are more likely to cause side effects such as dependence, addiction, and withdrawal symptoms.
Summary
Analgesics are drugs that eliminate or alleviate the feeling of pain that accompanies many pathologic conditions. It is difficult to list all the situations in which it is necessary to use analgesics, for example, muscle aches and headaches, and where there is no possibility of becoming addicted. Analgesics are divided into two groups such as opioids, which predominantly influence the central nervous system (CNS), and Nonopioids, which act predominantly on the peripheral nervous system. Opioids are further subdivided into three large subgroups according to their action on opioids receptors such as agonists, mixed agonists/antagonists, and antagonists. Opioid agonists act first and foremost on μ-receptors. Reaction of agonists with opioid μ-receptors leads to an increase in the flow of potassium ions from the cell, simultaneously making it difficult for calcium ions to flow into the cell, which makes neurons less excitable. Opioid antagonists on the other hand also bind to opioid receptors but do not activate them. These compounds are not used for analgesia. Their therapeutic value is in relieving side effects that result from either absolute of relative overdoses or intolerance of drugs by patients, and also in treating cases of Opioid dependency. Drugs of mixed agonists/antagonists group display both agonistic and antagonistic activities. This group of compounds is used for analgesia in cases of moderate-to-severe pain. The chapter lists down widely used agnostic, antagonistic, and mixed agnostic/antagonistic drugs such as Morphine, Codeine, Heroin, Hydromorphone, Oxymorphone, Nalorphine, Pentazocine, Nalbuphine, Naloxone, and Naltrexone, highlighting their chemical structure, specific uses and synthesis. The chapter end with a discussion on non-steroid anti-inflammatory drugs and anti-fever analgesics, such as Salicylic acid derivatives, Pyrazolonees and p-Aminophenol derivatives.
Details
Analgesics are medications that relieve pain. They work either by reducing inflammation or by changing the way the brain processes and perceives pain. Side effects include heartburn, nausea, and headaches.
Some types of analgesics are available over the counter. However, stronger variants are available only with a prescription. This is because strong analgesics are more likely to cause side effects such as dependence, addiction, and withdrawal symptoms.
An analgesic drug, also called simply an analgesic, antalgic, pain reliever, or painkiller, is any member of the group of drugs used for pain management. Analgesics are conceptually distinct from anesthetics, which temporarily reduce, and in some instances eliminate, sensation, although analgesia and anesthesia are neurophysiologically overlapping and thus various drugs have both analgesic and anesthetic effects.
Analgesic choice is also determined by the type of pain: For neuropathic pain, recent research has suggested that classes of drugs that are not normally considered analgesics, such as tricyclic antidepressants and anticonvulsants may be considered as an alternative.
Various analgesics, such as many NSAIDs, are available over the counter in most countries, whereas various others are prescription drugs owing to the substantial risks and high chances of overdose, misuse, and addiction in the absence of medical supervision.
Classification
Analgesics are typically classified based on their mechanism of action.
Paracetamol (acetaminophen)
Paracetamol, also known as acetaminophen or APAP, is a medication used to treat pain and fever. It is typically used for mild to moderate pain. In combination with opioid pain medication, paracetamol is now used for more severe pain such as cancer pain and after surgery. It is typically used either by mouth or rectally but is also available intravenously. Effects last between two and four hours. Paracetamol is classified as a mild analgesic. Paracetamol is generally safe at recommended doses.
NSAIDs
Nonsteroidal anti-inflammatory drug
Nonsteroidal anti-inflammatory drugs (usually abbreviated to NSAIDs), are a drug class that groups together drugs that decrease pain and lower fever, and, in higher doses, decrease inflammation. The most prominent members of this group of drugs, aspirin, ibuprofen and naproxen, Diclofenac are all available over the counter in most countries.
COX-2 inhibitors
These drugs have been derived from NSAIDs. The cyclooxygenase enzyme inhibited by NSAIDs was discovered to have at least two different versions: COX1 and COX2. Research suggested most of the adverse effects of NSAIDs to be mediated by blocking the COX1 (constitutive) enzyme, with the analgesic effects being mediated by the COX2 (inducible) enzyme. Thus, the COX2 inhibitors were developed to inhibit only the COX2 enzyme (traditional NSAIDs block both versions in general). These drugs (such as rofecoxib, celecoxib, and etoricoxib) are equally effective analgesics when compared with NSAIDs, but cause less gastrointestinal hemorrhage in particular.
After widespread adoption of the COX-2 inhibitors, it was discovered that most of the drugs in this class increase the risk of cardiovascular events by 40% on average. This led to the withdrawal of rofecoxib and valdecoxib, and warnings on others. Etoricoxib seems relatively safe, with the risk of thrombotic events similar to that of non-coxib NSAID diclofenac.
Additional Information
An analgesic, any drug that relieves pain selectively without blocking the conduction of nerve impulses, markedly altering sensory perception, or affecting consciousness. This selectivity is an important distinction between an analgesic and an anesthetic.
Analgesics may be classified into two types: anti-inflammatory drugs, which alleviate pain by reducing local inflammatory responses; and the opioids, which act on the brain. The opioid analgesics were once called narcotic drugs because they can induce sleep. The opioid analgesics can be used for either short-term or long-term relief of severe pain. In contrast, the anti-inflammatory compounds are used for short-term pain relief and for modest pain, such as that of headache, muscle strain, bruising, or arthritis.
Anti-inflammatory analgesics
Most anti-inflammatory analgesics are derived from three compounds discovered in the 19th century—salicylic acid, pyrazolone, and phenacetin (or acetophenetidin). Although chemically unrelated, the drugs in these families have the ability to relieve mild to moderate pain through actions that reduce inflammation at its source. Acetylsalicylic acid, or aspirin, which is derived from salicylic acid, is the most widely used mild analgesic. It is considered the prototype for anti-inflammatory analgesics, the two other major types of which include acetaminophen (a derivative of phenacetin) and the aspirin-like drugs, or nonsteroidal anti-inflammatory drugs (NSAIDs), which include compounds such as ibuprofen, naproxen, and fenoprofen. Pyrazolone derivatives, with some exceptions, are no longer widely used in many countries, because of their tendency to cause an acute infection known as agranulocytosis.
Aspirin and NSAIDs appear to share a similar molecular mechanism of action—namely, inhibition of the synthesis of prostaglandins (natural products of inflamed white blood cells) that induce the responses in local tissue that include pain and inflammation. In fact, aspirin and all aspirin-like analgesics, including indomethacin and sulindac, which are derived from a heterocyclic organic compound known as indole, inhibit prostaglandin synthesis and release. All these agents can be further divided into nonselective COX inhibitors and selective COX inhibitors. COX, or cyclooxygenase, is an enzyme responsible for the synthesis of prostaglandins and related compounds. It has two forms, COX-1, which is found in most normal tissues, and COX-2, which is induced in the presence of inflammation. Because COX-2 is not normally expressed in the stomach, the use of COX-2 inhibitors (e.g., rofecoxib, celecoxib) seems to result in less gastric ulceration than occurs with other anti-inflammatory analgesics, particularly aspirin. However, COX-2 inhibitors do not reduce the ability of platelets to form clots, a benefit associated with aspirin and other nonselective COX inhibitors.
Preferences in COX selectivity and the possibility of additional molecular actions of NSAIDs may explain differences in the therapeutic effects between aspirin, acetaminophen, and NSAIDs. For example, while aspirin is effective in reducing fever, as well as relieving inflammation, acetaminophen and NSAIDs are more potent antipyretic (fever-reducing) analgesics. Acetaminophen, on the other hand, possesses inferior anti-inflammatory activity compared with aspirin and NSAIDs and thus is relatively ineffective in treating inflammatory conditions such as rheumatoid arthritis. Despite this, acetaminophen is a popular mild analgesic and antipyretic and is a suitable alternative to aspirin for patients who develop severe symptoms of stomach irritation, because it is not as harmful to the gastrointestinal tract.
As might be expected from their common mechanisms of action, many of the anti-inflammatory analgesic drugs share similar side effects. Hypersensitivity responses to aspirin-like drugs are thought to be due to an accumulation of prostaglandins after the pathways that break down prostaglandins are blocked. These responses can be fatal when very strong anti-inflammatory compounds are given. Inhibition of prostaglandin synthesis may result in other serious side effects, such as peptic ulcers and a reduced ability of platelets in the blood to aggregate and form clots. The latter effect, however, has given aspirin an added use as a prophylactic antithrombotic drug to reduce chances of cardiac or cerebral vascular thrombosis—the formation of a clot in a blood vessel in the heart or brain. Some aspirin-like analgesics also have specific toxic effects: liver damage occasionally occurs after administration of acetaminophen, and renal toxicity is sometimes seen with use of NSAIDs. Aspirin itself, taken in overdose, can cause deafness, ringing in the ears, diarrhea, nausea, and headache, which disappear when the dose is reduced or stopped. Aspirin is also thought to be a causative agent of Reye syndrome, a rare and serious degenerative disease of the brain and fatty tissue of the liver that accompanies certain viral infections in children and young adults.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2356 2024-10-30 00:03:20
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,148
Re: Miscellany
2256) Pseudopodia
Gist
A pseudopod or pseudopodium ( pl. : pseudopods or pseudopodia) is a temporary arm-like projection of a eukaryotic cell membrane that is emerged in the direction of movement. Filled with cytoplasm, pseudopodia primarily consist of actin filaments and may also contain microtubules and intermediate filaments.
Summary
Pseudopodium, temporary or semipermanent extension of the cytoplasm, used in locomotion and feeding by all sarcodine protozoans (i.e., those with pseudopodia) and some flagellate protozoans. Pseudopodia are formed by some cells of higher animals (e.g., white blood corpuscles) and by amoebas. During amoeboid feeding, pseudopodia either flow around and engulf prey or trap it in a fine, sticky mesh.
Protozoans have four types of pseudopodia. Lobopodia, characteristic of Amoeba, are blunt and fingerlike; filopodia are slender and tapering, occasionally forming simple, branched networks; reticulopodia, found in the foraminiferans, are branching filaments that fuse to form food traps; and axopodia, characteristic of the actinopods, are long and sticky (like reticulopodia) but radiate singly and have a stiff, internal rod composed of numerous microtubules. Lobopodia and filopodia are formed as the result of a pressure system; reticulopodia and axopodia depend on a two-way flow of cytoplasm.
Details
A pseudopod or pseudopodium (pl.: pseudopods or pseudopodia) is a temporary arm-like projection of a eukaryotic cell membrane that is emerged in the direction of movement. Filled with cytoplasm, pseudopodia primarily consist of actin filaments and may also contain microtubules and intermediate filaments. Pseudopods are used for motility and ingestion. They are often found in amoebas.
Different types of pseudopodia can be classified by their distinct appearances. Lamellipodia are broad and thin. Filopodia are slender, thread-like, and are supported largely by microfilaments. Lobopodia are bulbous and amoebic. Reticulopodia are complex structures bearing individual pseudopodia which form irregular nets. Axopodia are the phagocytosis type with long, thin pseudopods supported by complex microtubule arrays enveloped with cytoplasm; they respond rapidly to physical contact.
Generally, several pseudopodia arise from the surface of the body, (polypodial, for example, Amoeba proteus), or a single pseudopod may form on the surface of the body (monopodial, such as Entamoeba histolytica).
Formation
Cells which make pseudopods are generally referred to as amoeboids.
Via extracellular cue
To move towards a target, the cell uses chemotaxis. It senses extracellular signalling molecules, chemoattractants (e.g. cAMP for Dictyostelium cells), to extend pseudopodia at the membrane area facing the source of these molecules.
The chemoattractants bind to G protein-coupled receptors, which activate GTPases of the Rho family (e.g. Cdc42, Rac) via G proteins.
Rho GTPases are able to activate WASp which in turn activate Arp2/3 complex which serve as nucleation sites for actin polymerization. The actin polymers then push the membrane as they grow, forming the pseudopod. The pseudopodium can then adhere to a surface via its adhesion proteins (e.g. integrins), and then pull the cell's body forward via contraction of an actin-myosin complex in the pseudopod. This type of locomotion is called amoeboid movement.
Rho GTPases can also activate phosphatidylinositol 3-kinase (PI3K) which recruit PIP3 to the membrane at the leading edge and detach the PIP3-degrading enzyme PTEN from the same area of the membrane. PIP3 then activate GTPases back via GEF stimulation. This serves as a feedback loop to amplify and maintain the presence of local GTPase at the leading edge.
Otherwise, pseudopodia cannot grow on other sides of the membrane than the leading edge because myosin filaments prevent them to extend. These myosin filaments are induced by cyclic GMP in D. discoideum or Rho kinase in neutrophils for example.
Different physical parameters were shown to regulate the length and time-scale of pseudopodia formation. For example, an increase in membrane tension inhibits actin assembly and protrusion formation. It was demonstrated that the lowered negative surface charge on the inner surface of the plasma membrane generates protrusions via activation of the Ras-PI3K/AKT/mTOR signalling pathway.
Without extracellular cue
In the case there is no extracellular cue, all moving cells navigate in random directions, but they can keep the same direction for some time before turning. This feature allows cells to explore large areas for colonization or searching for a new extracellular cue.
In Dictyostelium cells, a pseudopodium can form either de novo as normal, or from an existing pseudopod, forming a Y-shaped pseudopodium.
The Y-shaped pseudopods are used by Dictyostelium to advance relatively straight forward by alternating between retraction of the left or right branch of the pseudopod. The de novo pseudopodia form at different sides than pre-existing ones, they are used by the cells to turn.
Y-shaped pseudopods are more frequent than de novo ones, which explain the preference of the cell to keep moving to the same direction. This persistence is modulated by PLA2 and cGMP signalling pathways.
Functions
The functions of pseudopodia include locomotion and ingestion:
* Pseudopodia are critical in sensing targets which can then be engulfed; the engulfing pseudopodia are called phagocytosis pseudopodia. A common example of this type of amoeboid cell is the macrophage.
* They are also essential to amoeboid-like locomotion. Human mesenchymal stem cells are a good example of this function: these migratory cells are responsible for in-utero remodeling; for example, in the formation of the trilaminar germ disc during gastrulation.
Additional Information
A pseudopodium or pseudopod (plural: pseudopodia or pseudopods) is a temporary cytoplasmic extension of an amoeboid cell, used for locomotion and ingestion of food. The name means literally ‘false foot’. Pseudopodia are powered by microfilaments near the cellular membrane. About half of the internal space of an amoeba contains microfilaments. A pseudopodium contains both granuloplasm and hyaloplasm. Pseudopodia which only contain hyaloplasm are called subpseudopodia.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2357 2024-10-31 22:26:27
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,148
Re: Miscellany
2257) Nosebleed
Gist
Epistaxis, or a nosebleed, is when you lose blood from the tissue that lines the inside of your nose. A combination of dry air and tiny blood vessels that line the inner surface of your nose often cause nosebleeds.
Summary
A nosebleed is loss of blood from the tissue lining the nose. Bleeding most often occurs from one nostril only.
Considerations
Nosebleeds are very common. Most nosebleeds occur because of minor irritations of the inside of the nostrils or colds.
The nose contains many small blood vessels that bleed easily. Air moving through the nose can dry and irritate the membranes lining the inside of the nose. Crusts can form that bleed when irritated. Nosebleeds occur more often in the winter, when cold viruses are common and indoor air tends to be drier.
Most nosebleeds occur on the front of the nasal septum. This is the piece of the tissue that separates the two sides of the nose. This type of nosebleed can be easy for a trained professional to stop. Less commonly, nosebleeds may occur higher on the septum or deeper in the nose such as in the sinuses or the base of the skull. Such nosebleeds may be harder to control. However, nosebleeds are rarely life threatening.
Causes
Nosebleed can be caused by:
* Irritation due to allergies, colds, sneezing or sinus problems
* Very cold or dry air
* Blowing the nose very hard, or picking the nose
* Injury to nose, including a broken nose, or an object stuck in the nose
* Sinus or pituitary surgery (transsphenoidal)
* Deviated septum (tissue that divides the nose into 2 nostrils)
* Chemical irritants including medicines or drugs that are sprayed or snorted
* Overuse of decongestant nasal sprays
* Oxygen treatment through nasal cannulas
* Snorting cocaine or amphetamine
Repeated nosebleeds may be a symptom of another disease such as high blood pressure, a bleeding disorder, or a tumor of the nose or sinuses. Blood thinners, such as warfarin (Coumadin), clopidogrel (Plavix), or aspirin, may cause or worsen nosebleeds.
Details
A nosebleed, also known as epistaxis, is an instance of bleeding from the nose. Blood can flow down into the stomach, and cause nausea and vomiting. In more severe cases, blood may come out of both nostrils. Rarely, bleeding may be so significant that low blood pressure occurs. Blood may also be forced to flow up and through the nasolacrimal duct and out of the eye, producing bloody tears.
Risk factors include trauma, including putting the finger in the nose, blood thinners, high blood pressure, alcoholism, seasonal allergies, dry weather, and inhaled corticosteroids. There are two types: anterior, which is more common; and posterior, which is less common but more serious. Anterior nosebleeds generally occur from Kiesselbach's plexus while posterior bleeds generally occur from the sphenopalatine artery or Woodruff's plexus. The diagnosis is by direct observation.
Prevention may include the use of petroleum jelly in the nose. Initially, treatment is generally the application of pressure for at least five minutes over the lower half of the nose. If this is not sufficient, nasal packing may be used. Tranexamic acid may also be helpful. If bleeding episodes continue, endoscopy is recommended.
About 60% of people have a nosebleed at some point in their life. About 10% of nosebleeds are serious. Nosebleeds are rarely fatal, accounting for only 4 of the 2.4 million deaths in the U.S. in 1999. Nosebleeds most commonly affect those younger than 10 and older than 50.
Cause
Nosebleeds can occur due to a variety of reasons. Some of the most common causes include trauma from nose picking, blunt trauma (such as a motor vehicle accident), or insertion of a foreign object (more likely in children). Low relative humidity (such as in centrally heated buildings), respiratory tract infections, chronic sinusitis, rhinitis or environmental irritants can cause inflammation and thinning of the tissue in the nose, leading to a greater likelihood of bleeding from the nose.
Most causes of nose bleeding are self-limiting and do not require medical attention. However, if nosebleeds are recurrent or do not respond to home therapies, an underlying cause may need to be investigated.
Pathophysiology
The nasal mucosa contains a rich blood supply that can be easily ruptured and cause bleeding. Rupture may be spontaneous or initiated by trauma. Nosebleeds are reported in up to 60% of the population with peak incidences in those under the age of ten and over the age of 50 and appear to occur in males more than females. An increase in blood pressure (e.g. due to general hypertension) tends to increase the duration of spontaneous epistaxis. Anticoagulant medication and disorders of blood clotting can promote and prolong bleeding. Spontaneous epistaxis is more common in the elderly as the nasal mucosa (lining) becomes dry and thin and blood pressure tends to be higher. The elderly are also more prone to prolonged nosebleeds as their blood vessels are less able to constrict and control the bleeding.
The vast majority of nosebleeds occur in the front anterior (front) part of the nose from the nasal septum. This area is richly endowed with blood vessels (Kiesselbach's plexus). This region is also known as Little's area. Bleeding farther back in the nose is known as a posterior bleed and is usually due to bleeding from Woodruff's plexus, a venous plexus situated in the posterior part of inferior meatus. Posterior bleeds are often prolonged and difficult to control. They can be associated with bleeding from both nostrils and with a greater flow of blood into the mouth.
Sometimes blood flowing from other sources of bleeding passes through the nasal cavity and exits the nostrils. It is thus blood coming from the nose but is not a true nosebleed, that is, not truly originating from the nasal cavity. Such bleeding is called "pseudoepistaxis" (pseudo + epistaxis). Examples include blood coughed up through the airway and ending up in the nasal cavity, then dripping out.
Prevention
People with uncomplicated nosebleeds can use conservative methods to prevent future nosebleeds such as sleeping in a humidified environment or applying petroleum jelly to the nasal nares.
Individuals who suffer from nosebleeds regularly, especially children, are encouraged to use over-the-counter nasal saline sprays and avoid vigorous nose-blowing as preventative measures.
Treatment
Most anterior nosebleeds can be stopped by applying direct pressure, which helps by promoting blood clots. Those who have a nosebleed should first attempt to blow out any blood clots and then apply pressure to the soft anterior part of the nose (by pinching the nasal ala; not the bony nasal bridge) for at least five minutes and up to 30 minutes. Pressure should be firm and tilting the head forward helps decrease the chance of nausea and airway obstruction due to blood dripping into the airway. When attempting to stop a nosebleed at home, the head should not be tilted back. Swallowing excess blood can irritate the stomach and cause vomiting. Vasoconstrictive medications such as oxymetazoline (Afrin) or phenylephrine are widely available over the counter for treatment of allergic rhinitis and may also be used to control benign cases of epistaxis. For example, a few sprays of oxymetazoline may be applied into the bleeding side(s) of the nose followed by application of direct pressure. Those with nosebleeds that last longer than 30 minutes (despite use of direct pressure and vasoconstrictive medications such as oxymetazoline) should seek medical attention.
Additional Information
Nosebleed is an attack of bleeding from the nose. It is a common and usually unimportant disorder but may also result from local conditions of inflammation, small ulcers or polypoid growths, or severe injuries to the skull. Vascular disease, such as high blood pressure, may provoke it, and such diseases as scurvy and hemophilia also may be responsible. Usually it is easily controlled by rest and application of cold and pressure. On occasion it may require expert care.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2358 2024-11-02 00:04:12
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,148
Re: Miscellany
2258) Baker
Gist
A bakery is an establishment that produces and sells flour-based baked goods made in an oven such as bread, cookies, cakes, doughnuts, bagels, pastries, and pies. Some retail bakeries are also categorized as cafés, serving coffee and tea to customers who wish to consume the baked goods on the premises.
Details
A bakery is an establishment that produces and sells flour-based baked goods made in an oven such as bread, cookies, cakes, doughnuts, bagels, pastries, and pies. Some retail bakeries are also categorized as cafés, serving coffee and tea to customers who wish to consume the baked goods on the premises. In some countries, a distinction is made between bakeries, which primarily sell breads, and pâtisseries, which primarily sell sweet baked goods.
History
Baked goods have been around for thousands of years. The art of baking was very popular during the Roman Empire. It was highly famous art as Roman citizens loved baked goods and demanded them frequently for important occasions such as feasts and weddings. Because of the fame of the art of baking, around 300 BC, baking was introduced as an occupation and respectable profession for Romans. Bakers began to prepare bread at home in an oven, using grist mills to grind grain into flour for their breads. The demand for baked goods persisted, and the first bakers' guild was established in 168 BC in Rome. The desire for baked goods promoted baking throughout Europe and expanded into eastern parts of Asia. Bakers started baking bread and other goods at home and selling them on the streets.
This trend became common, and soon, baked products were sold in streets of Rome, Germany, London, and more. A system of delivering baked goods to households arose as the demand increased significantly. This prompted bakers to establish places where people could purchase baked goods. The first open-air market for baked goods was established in Paris, and since then bakeries have become a common place to purchase delicious goods and to socialize.
On July 7, 1928, a bakery in Chillicothe, Missouri introduced sliced bread using the automatic bread-slicing machine, invented by Otto Frederick Rohwedder. While the bread initially failed to sell, due to its "sloppy" aesthetic, and the fact it went stale faster, it later became popular. In World War II bread slicing machines were effectively banned, as the metal in them was required for wartime use. When they were requisitioned, creating 100 tons of metal alloy, the decision proved very unpopular with housewives.
World War II directly affected the bread industry in the UK. Baking schools closed during this time, so when the war ended there was a lack of skilled bakers. This resulted in new methods being developed to satisfy the world's desire for bread, including chemical additives, premixes and specialised machinery. Old methods of baking were almost completely eradicated when these new methods were introduced and the industry became industrialised. The old methods were seen as unnecessary and financially unsound. During this period there were not many traditional bakeries left.
Specialities
Some bakeries provide services for special occasions (such as weddings, anniversaries, birthday parties, business networking events, etc.) or customized baked products for people who have allergies or sensitivities to certain foods (such as nuts, peanuts, dairy or gluten, etc.). Bakeries can provide a wide range of cake designs such as sheet cakes, layer cakes, wedding cakes, tiered cakes, etc. Other bakeries may specialize in traditional or hand-made types of baked products made with locally milled flour, without flour bleaching agents or flour treatment agents, baking what is sometimes referred to as artisan bread.
Commercialization
In many countries, many grocery stores and supermarkets sell "sliced bread" (prepackaged/presliced bread), cakes, and other pastries. They may also offer in-store baking, with products either fully baked on site or part-baked prior to delivery to store, and some offer cake decoration. Nonetheless, many people still prefer to get their baked goods from a small artisanal bakery, either out of tradition, the availability of a greater variety of baked products, or due to the higher quality products characteristic of the trade of baking.
Additional Information
Baking is the process of cooking by dry heat, especially in some kind of oven. It is probably the oldest cooking method. Bakery products, which include bread, rolls, cookies, pies, pastries, and muffins, are usually prepared from flour or meal derived from some form of grain. Bread, already a common staple in prehistoric times, provides many nutrients in the human diet.
History
The earliest processing of cereal grains probably involved parching or dry roasting of collected grain seeds. Flavour, texture, and digestibility were later improved by cooking whole or broken grains with water, forming gruel or porridge. It was a short step to the baking of a layer of viscous gruel on a hot stone, producing primitive flat bread. More sophisticated versions of flat bread include the Mexican tortilla, made of processed corn, and the chapati of India, usually made of wheat.
Baking techniques improved with the development of an enclosed baking utensil and then of ovens, making possible thicker baked cakes or loaves. The phenomenon of fermentation, with the resultant lightening of the loaf structure and development of appealing flavours, was probably first observed when doughs or gruels, held for several hours before baking, exhibited spoilage caused by yeasts. Some of the effects of the microbiologically induced changes were regarded as desirable, and a gradual acquisition of control over the process led to traditional methods for making leavened bread loaves. Early baked products were made of mixed seeds with a predominance of barley, but wheat flour, because of its superior response to fermentation, eventually became the preferred cereal among the various cultural groups sufficiently advanced in culinary techniques to make leavened bread.
Brewing and baking were closely connected in early civilizations. Fermentation of a thick gruel resulted in a dough suitable for baking; a thinner mash produced a kind of beer. Both techniques required knowledge of the “mysteries” of fermentation and a supply of grain. Increasing knowledge and experience taught the artisans in the baking and brewing trades that barley was best suited to brewing, while wheat was best for baking.
By 2600 bce the Egyptians, credited with the first intentional use of leavening, were making bread by methods similar in principle to those of today. They maintained stocks of sour dough, a crude culture of desirable fermentation organisms, and used portions of this material to inoculate fresh doughs. With doughs made by mixing flour, water, salt, and leaven, the Egyptian baking industry eventually developed more than 50 varieties of bread, varying the shape and using such flavouring materials as poppy seed, sesame, and camphor. Samples found in tombs are flatter and coarser than modern bread.
The Egyptians developed the first ovens. The earliest known examples are cylindrical vessels made of baked Nile clay, tapered at the top to give a cone shape and divided inside by a horizontal shelflike partition. The lower section is the firebox, the upper section is the baking chamber. The pieces of dough were placed in the baking chamber through a hole provided in the top.
In the first two or three centuries after the founding of Rome, baking remained a domestic skill with few changes in equipment or processing methods. According to Pliny the Elder, there were no bakers in Rome until the middle of the 2nd century bce. As well-to-do families increased, women wishing to avoid frequent and tedious bread making began to patronize professional bakers, usually freed slaves. Loaves molded by hand into a spheroidal shape, generally weighing about a pound, were baked in a beehive-shaped oven fired by wood. Panis artopticius was a variety cooked on a spit, panis testuatis in an earthen vessel.
Although Roman professional bakers introduced technological improvements, many were of minor importance, and some were essentially reintroductions of earlier developments. The first mechanical dough mixer, attributed to Marcus Vergilius (sometimes spelled Virgilius) Eurysaces, a freed slave of Greek origin, consisted of a large stone basin in which wooden paddles, powered by a horse or donkey walking in circles, kneaded the dough mixture of flour, leaven, and water.
Guilds formed by the miller-bakers of Rome became institutionalized. During the 2nd century ce, under the Flavians, they were organized into a “college” with work rules and regulations prescribed by government officials. The trade eventually became obligatory and hereditary, and the baker became a kind of civil servant with limited freedom of action.
During the early Middle Ages, baking technology advances of preceding centuries disappeared, and bakers reverted to mechanical devices used by the ancient Egyptians and to more backward practices. But in the later Middle Ages the institution of guilds was revived and expanded. Several years of apprenticeship were necessary before an applicant was admitted to the guild; often an intermediate status as journeyman intervened between apprenticeship and full membership (master). The rise of the bakers’ guilds reflected significant advances in technique. A 13th-century French writer named 20 varieties of bread varying in shape, flavourings, preparation method, and quality of the meal used. Guild regulations strictly governed size and quality. But outside the cities bread was usually baked in the home. In medieval England rye was the main ingredient of bread consumed by the poor; it was frequently diluted with meal made from other cereals or leguminous seeds. Not until about 1865 did the cost of white bread in England drop below brown bread.
At that time improvements in baking technology began to accelerate rapidly, owing to the higher level of technology generally. Ingredients of greater purity and improved functional qualities were developed, along with equipment reducing the need for individual skill and eliminating hand manipulation of bread doughs. Automation of mixing, transferring, shaping, fermentation, and baking processes began to replace batch processing with continuous operations. The enrichment of bread and other bakery foods with vitamins and minerals was a major accomplishment of the mid-20th-century baking industry.
Market preparation:
Slicing
Bread often is marketed in sliced form. Slicing is performed by parallel arrays of saw blades through which the loaves are carried by gravity or by conveyors. The blades may be endless bands carried on rotating drums, or relatively short strips held in a reciprocating frame. Most bread is sliced while still fairly warm, and the difficulty of cutting the sticky, soft crumb has led to development of coated blades and blade-cleaning devices. Horizontal slicing of hamburger rolls and similar products is accomplished by circular (disk) blades, usually two blades in a slicer, between which a connected array of four or six rolls is carried by a belt. The cutter blades are separated to avoid cutting completely through the roll, in order to leave a “hinge.”
Freezing
Freezing is an indispensable bakery industry process. Ordinary bread and rolls are rarely distributed and sold in frozen form because of the excessive cost in relation to product value, but a substantial percentage of all specialty products is sold in frozen form. Most bakery products respond well to freezing, although some cream fillings must be specially formulated to avoid syneresis, or gel breakdown. Rapid chilling in blast freezers is preferred, although milder methods may be used. Storage at −18 °C (0 °F) or lower is essential for quality maintenance. Thawing and refreezing is harmful to quality. Frozen bakery products can dehydrate under freezer conditions and must be packaged in containers resistant to moisture-vapour transfer.
Wrapping
Most American consumers prefer wrapped bread, and the trend toward wrapping is growing in other countries. Sanitary and aesthetic considerations dictate protection of the product from environmental contamination during distribution and display. Waxed paper was originally the only film used to package bread, after which cellophane became popular, and then polyethylene, polypropylene, and combination laminates became common. Other bakery products are packaged in a variety of containers ranging from open bags of greaseproof material to plastic trays with sealed foil overwraps.
Canning
The market for bakery products in tin cans is small, but hunters and campers find canned foods convenient. Canning protects against drying and environmental contamination, but texture staling and some degree of flavour staling still occur. In processing, an amount of dough or batter known to fill exactly the available space after baking is placed in a can, and the cover is loosely fastened to allow gases to escape. The product is then baked in a conventional oven, the lid is hermetically sealed immediately after baking, and the sealed can is sprayed with water to cool it. Vacuum sealing, needed to assure storage stability, can be routinely achieved by this method. Special can linings and sealing compounds are needed to survive oven temperatures, and the exterior should be dark-coloured (e.g., olive drab) in order to absorb radiant heat in the oven, avoiding long baking times. Spores of some pathogens are not killed by the conditions reached in the centre of the baked product, but pH and osmotic pressure can be adjusted to prevent growth of spoilage organisms. There is no record of food poisoning attributable to canned bakery food.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2359 2024-11-03 00:01:45
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,148
Re: Miscellany
2259) Administrator
Gist
An administrator is someone whose job is to manage a business or business activity.
The boss, the head honcho, the person in charge: An administrator is the person responsible for managing things and running the show.
Administrators are often found directing government agencies, organizing institutions, or leading school departments. They're the decision makers and the planners, the people who put in place or administer the rules and guidelines.
Administrators play a critical role in businesses, with their responsibilities varying across industries, and including tasks such as managing an office, fielding inquiries, overseeing office inventory, scheduling meetings and supervising other administrative personnel.
Summary:
A database administrator (DBA) manages computer databases. The role may include capacity planning, installation, configuration, database design, migration, performance monitoring, security, troubleshooting, as well as backup and data recovery.
Skills
Required skills for database administrators include knowledge of SQL, database queries, database theory, database design, specific databases, such as Oracle, Microsoft SQL Server, or MySQL, storage technologies, distributed computing architectures, operating systems, routine maintenance, recovery, and replication/failover.
Certification
Training for DBAs with accompanying certifications is widely available, offered by database vendors and third parties. Offerings include:
* IBM Certified Advanced Database Administrator – DB2 10.1 for Linux, Unix and Windows
* IBM Certified Database Administrator – DB2 10.1 for Linux, Unix, and Windows
* Oracle Database 12c Administrator Certified Professional
* Oracle MySQL 5.6 Database Administrator Certified Professional
* MCSA SQL Server 2012
* MCSE Data Platform Solutions Expert
Internet Forum Administrator
The administrators (short form: "admin") manage the technical details required for running the site. As such, they have the authority to appoint and revoke members as moderators, manage the rules, create sections and sub-sections, as well as perform any database operations (database backup, etc.). Administrators often also act as moderators. Administrators may also make forum-wide announcements or change the appearance (known as the skin) of a forum. There are also many forums where administrators share their knowledge.
Network administrator
A network administrator is a person designated in an organization whose responsibility includes maintaining computer infrastructures with emphasis on local area networks (LANs) up to wide area networks (WANs). Responsibilities may vary between organizations, but installing new hardware, on-site servers, enforcing licensing agreements, software-network interactions as well as network integrity and resilience are some of the key areas of focus.
System administrator
An IT administrator, system administrator, sysadmin, or admin is a person who is responsible for the upkeep, configuration, and reliable operation of computer systems, especially multi-user computers, such as servers. The system administrator seeks to ensure that the uptime, performance, resources, and security of the computers they manage meet the needs of the users, without exceeding a set budget when doing so.
To meet these needs, a system administrator may acquire, install, or upgrade computer components and software; provide routine automation; maintain security policies; troubleshoot; train or supervise staff; or offer technical support for projects.
Details
What Is an Administrator?
An administrator is a court-appointed individual who handles all remaining financial matters for a decedent—a person who has died—during probate.
The administrator organizes all the pieces of the decedent's estate and then settles outstanding debt, expenses, and other obligations. They also distribute all remaining assets according to the decedent's will, or if there was no will (a situation called intestacy), according to a specific state's intestate succession laws.
Key Takeaways
* An administrator is an individual appointed by the court who is responsible for executing a decedent's estate.
* An administrator is responsible for settling all financial matters–including outstanding debt, expenses, and other obligations–related to a decedent's estate.
* States have different criteria outlined in their probate codes for choosing administrators.
* Administrators are chosen by a court when the decedent has not named an executor in their will or if the executor cannot carry out the responsibilities.
* The term "administrator" is used in various other contexts, such as state administrators, pension plan administrators, and third-party administrators.
Understanding the Role of Administrators
An administrator is also referred to as an executor. However, legally speaking, an administrator is appointed by a court when a decedent has not named an executor in their will or if a named executor refuses or is unable to assume the responsibilities. A court cannot force a named executor to fulfill their duties.
Depending on the state, a decedent's estate administrator is chosen based on different criteria. For example, one of the criteria specified in the state of Pennsylvania's probate code is that the administrator is chosen based on the size of their interest in a decedent's estate rather than their closeness with the deceased.
Other persons who may be considered are guardianship agencies (in cases where the decedent was incapacitated) or creditors. Persons under 18 years of age, corporations, and individuals who have an unfit background (such as a criminal record) are not considered when appointing an administrator.
The probate proceeding begins with the selection of the administrator. Once appointed, the administrator receives Letters of Testamentary issued by the court, authorizing them to discharge outstanding financial matters.
An administrator is compensated for their services based on the quality of their work and results. They are required to submit a detailed accounting of time spent and fee expenses to get paid. Professional administrators are also available for hire. These administrators generally charge a fixed fee for their services but may negotiate their rates for estates that are in excess of $1 million.
Administrator Duties
One of the first things that an administrator must do is obtain a tax identification number so they can file with the Internal Revenue Service (IRS). Then, the administrator is tasked with gathering up all of the documents and personal files that encompass the financial dealings and transactions of the decedent: bank account records, brokerage statements, credit card statements, insurance claims, tax notices, medical expense invoices, vehicle financing statements, etc.
If the decedent owned a business, the administrator takes legal title to the assets of the business and must hire a third-party appraiser to value the assets before the administrator liquidates them, pays all the business's liabilities, and finally, closes the business down. Independent valuation services must also be retained to ascertain fair market prices for real estate, art, or other illiquid personal assets.
Important : In many wills, the decedent will set aside a specific amount of cash or assets to be used as compensation for the administrator or executor.
The administrator determines if there are any tax obligations with federal and state authorities and settles them, in addition to settling any liabilities with other parties that had outstanding claims when the person died. An administrator must take particular care to clear out all of the decedents' tax claims because they can, in certain instances, be held personally responsible for unpaid taxes.
After all debts and expenses have been settled, any remaining assets are distributed by the administrator to named beneficiaries in a will. If there was no will left behind by the decedent, the assets are distributed in accordance with state procedure.
Simple probate cases may take just a few months, while complex cases can take two to three years before they are concluded.
Other Types of Administrators
The term "administrator" is a common word with different meanings depending on the situation. Some uses are as follows.
Pension Plan Administrator
A pension plan administrator is an individual or an investment management company that is responsible for the management of a retirement account or pension plan.
The plan administrator ensures that the funds are invested appropriately and with the right degrees of risk as well as distributing funds to the beneficiaries.
Some of the responsibilities of a pension plan administrator include enrolling company employees, calculating the benefit amounts, and ensuring that the funds are being invested in risk-appropriate assets.
Third-Party Administrator
A third-party administrator is any company that provides specific services on behalf of another company. A third-party administrator is one that is usually hired to complete operational tasks, including the administration of employee benefits, such as a 401(k) plan.
Third-party administrators are far-ranging in their types but can be contracted to assist with health insurance, commercial liability insurance, retirement planning, other investment services, and audits. Third-party administrators are primarily used by companies as a way to outsource a variety of administrative functions.
State Administrator
A state administrator is a government representative who enforces state regulations related to the securities industry. The Securities and Exchange Commission (SEC) enforces federal securities law while the Uniform Securities Act allows states to set their own laws.
A state administrator performs many of the same actions as the SEC, such as regulating companies and individuals, granting or suspending licenses, and generally enforcing securities law.
Example of an Administrator
Dmitri died without leaving a will and he had no surviving spouse or children. When he heard about Dmitri's death, Malik contacted the court to be appointed as his administrator. Malik applied to be Dmitri's administrator because he was a creditor to Dmitri and had loaned him funds amounting to $50,000 over the years.
Once the court approved his application, Malik set about finding more information about the state of Dmitri's finances. He inquired with Dmitri's bank and combed through his previous tax filings. Malik discovered that Dmitri did not have much money in his account but he did have a property holding that could help pay off his loan. He petitioned the court to sell off the property, settled Dmitri's tax liabilities, and paid off the remaining creditors. He was also compensated by the court for his work.
What Is the Difference Between an Administrator and an Executor?
An administrator and an executor perform the same function: the handling of all remaining financial matters for a decedent during probate. The difference is that an administrator is appointed by the court if the decedent has not named an executor in their will or if the executor is not able to carry out the required responsibilities.
How Long Does It Take to Be Appointed Administrator of an Estate?
It typically takes between six to eight weeks to receive the appropriate documentation to act as the administrator of a decedent's estate. The document needed is the "Letter of Administration" and has to be applied for.
How Much Does an Administrator of a Will Get Paid?
The amount an administrator gets paid depends on the state as well as the size of the estate. In California, for example, for an estate that is valued under $100,000, an administrator typically receives 4% of the estate's value. If the estate is valued between $100,000 and $25 million, the administrator can claim a specific percentage. If the estate is valued at over $25 million, then the court will decide the appropriate compensation.
Can an Administrator of an Estate Be a Beneficiary?
Yes, an administrator of an estate can be a beneficiary. This is common. For example, when an individual dies, their spouse, who will most likely be the beneficiary, can also act as the administrator of the estate.
The Bottom Line
An administrator manages various financial matters during probate for a decedent, an individual who has died. Typically appointed by the court, the administrator processes the decedent's debt and distributes their assets to their beneficiaries.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2360 2024-11-04 00:04:17
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,148
Re: Miscellany
2260) Chairman
Gist
The chair, also chairman, chairwoman, or chairperson, is the presiding officer of an organized group such as a board, committee, or deliberative assembly.
A chairman is the leader of a business meeting or group. The chairman often opens a meeting by addressing the group and explaining what the agenda will be. Charities, clubs, and the boards of companies have a chairman who acts as president or leader.
A chairman is the leader of a business meeting or group. The chairman often opens a meeting by addressing the group and explaining what the agenda will be.
Charities, clubs, and the boards of companies have a chairman who acts as president or leader. The noun chairman can refer to this person, whether male or female, though sometimes a woman is called a chairwoman. These days, it's more common still to simply call her (or him) a chair. The word chairman comes from a sense of "occupying a chair of authority," while "presiding member of a corporate body" first emerged in the 18th century.
Details
The chair, also chairman, chairwoman, or chairperson, is the presiding officer of an organized group such as a board, committee, or deliberative assembly. The person holding the office, who is typically elected or appointed by members of the group or organisation, presides over meetings of the group, and is required to conduct the group's business in an orderly fashion.
In some organizations, the chair is also known as president (or other title). In others, where a board appoints a president (or other title), the two terms are used for distinct positions. The term chairman may be used in a neutral manner, not directly implying the gender of the holder. In meetings or conferences, to "chair" something (chairing) means to lead the event.
Terminology
Look up chair, chairman, chairwoman, chairperson, or preside in Wiktionary, the free dictionary.
Terms for the office and its holder include chair, chairperson, chairman, chairwoman, convenor, facilitator, moderator, president, and presiding officer. The chair of a parliamentary chamber is sometimes called the speaker. Chair has been used to refer to a seat or office of authority since the middle of the 17th century; its earliest citation in the Oxford English Dictionary dates to 1658–1659, four years after the first citation for chairman. Feminist critiques have analysed Chairman as an example of sexist language, associating the male gender with the exercise of authority, this has led to the widespread use of the generic "Chairperson".
In World Schools Style debating, as of 2009, chairperson or chair refers to the person who controls the debate; it recommends using Madame Chair or Mr. Chairman to address the chair. The FranklinCovey Style Guide for Business and Technical Communication and the American Psychological Association style guide advocate using chair or chairperson. The Oxford Dictionary of American Usage and Style (2000) suggested that the gender-neutral forms were gaining ground; it advocated chair for both men and women. The Daily Telegraph's style guide bans the use of chair and chairperson; the newspaper's position, as of 2018, is that "chairman is correct English". The National Association of Parliamentarians adopted a resolution in 1975 discouraging the use of chairperson and rescinded it in 2017.
Usage
The word chair can refer to the place from which the holder of the office presides, whether on a chair, at a lectern, or elsewhere. During meetings, the person presiding is said to be "in the chair" and is also referred to as "the chair". Parliamentary procedure requires that members address the "chair" as "Mr. (or Madam) Chairman (or Chair or Chairperson)" rather than using a name – one of many customs intended to maintain the presiding officer's impartiality and to ensure an objective and impersonal approach.
In the British music hall tradition, the chairman was the master of ceremonies who announced the performances and was responsible for controlling any rowdy elements in the audience. The role was popularised on British TV in the 1960s and 1970s by Leonard Sachs, the chairman on the variety show The Good Old Days.
"Chairman" as a quasi-title gained particular resonance when socialist states from 1917 onward shunned more traditional leadership labels and stressed the collective control of Soviets (councils or committees) by beginning to refer to executive figureheads as "Chairman of the X Committee". Lenin, for example, officially functioned as the head of Soviet Russian government not as prime minister or as president but as "Chairman of the Council of People's Commissars". At the same time, the head of the state was first called "Chairman of the Central Executive Committee" (until 1938) and then "Chairman of the Presidium of the Presidium of the Supreme Soviet". In Communist China, Mao Zedong was commonly called "Chairman Mao", as he was officially Chairman of the Chinese Communist Party and Chairman of the Central Military Commission.
Roles and responsibilities:
Duties at meetings
In addition to the administrative or executive duties in organizations, the chair presides over meetings. Such duties at meetings include:
* Calling the meeting to order
* Determining if a quorum is present
* Announcing the items on the "order of business", or agenda, as they come up
* Recognition of members to have the floor
* Enforcing the rules of the group
* Putting questions (motions) to a vote, which is the usual way of resolving disagreements following discussion of the issues
Adjourning the meeting
While presiding, the chair should remain impartial and not interrupt a speaker if the speaker has the floor and is following the rules of the group. In committees or small boards, the chair votes along with the other members; in assemblies or larger boards, the chair should vote only when it can affect the result. At a meeting, the chair only has one vote (i.e. the chair cannot vote twice and cannot override the decision of the group unless the organization has specifically given the chair such authority).
Powers and authority
The powers of the chair vary widely across organizations. In some organizations they have the authority to hire staff and make financial decisions. In others they only make recommendations to a board of directors, or may have no executive powers, in which case they are mainly a spokesperson for the organization. The power given depends upon the type of organization, its structure, and the rules it has created for itself.
Disciplinary procedures
If the chair exceeds their authority, engages in misconduct, or fails to perform their duties, they may face disciplinary procedures. Such procedures may include censure, suspension, or removal from office. The rules of the organization would provide details on who can perform these disciplinary procedures. Usually, whoever appointed or elected the chair has the power to discipline them.
Public corporations
There are three common types of chair in public corporations.
Chairman and CEO
The chief executive officer (CEO) may also hold the title of chair, in which case the board frequently names an independent member of the board as a lead director. This position is equivalent to the position of président-directeur général in France.
Executive chair
Executive chair is an office separate from that of CEO, where the titleholder wields influence over company operations, such as Larry Ellison of Oracle, Douglas Flint of HSBC and Steve Case of AOL Time Warner. In particular, the group chair of HSBC is considered the top position of that institution, outranking the chief executive, and is responsible for leading the board and representing the company in meetings with government figures. Before the creation of the group management board in 2006, HSBC's chair essentially held the duties of a chief executive at an equivalent institution, while HSBC's chief executive served as the deputy. After the 2006 reorganization, the management cadre ran the business, while the chair oversaw the controls of the business through compliance and audit and the direction of the business.
Non-executive chair
Non-executive chair is also a separate post from the CEO; unlike an executive chair, a non-executive chair does not interfere in day-to-day company matters. Across the world, many companies have separated the roles of chair and CEO, saying that this move improves corporate governance. The non-executive chair's duties are typically limited to matters directly related to the board, such as:
* Chairing the meetings of the board.
* Organizing and coordinating the board's activities, such as by setting its annual agenda.
* Reviewing and evaluating the performance of the CEO and the other board members.
Examples
Many companies in the US have an executive chair; this method of organization is sometimes called the American model. Having a non-executive chair is common in the UK and Canada; this is sometimes called the British model. Expert opinion is rather evenly divided over which is the preferable model. There is a growing push by public market investors for companies with an executive chair to have a lead independent director to provide some element of an independent perspective.
The role of the chair in a private equity-backed board differs from the role in non-profit or publicly listed organizations in several ways, including the pay, role and what makes an effective private-equity chair. Companies with both an executive chair and a CEO include Ford, HSBC, Alphabet Inc., and HP.
Vice-chair and deputy chair
A vice- or deputy chair, subordinate to the chair, is sometimes chosen to assist and to serve as chair in the latter's absence, or when a motion involving the chair is being discussed. In the absence of the chair and vice-chair, groups sometimes elect a chair pro tempore to fill the role for a single meeting. In some organizations that have both titles, deputy chair ranks higher than vice-chair, as there are often multiple vice-chairs but only a single deputy chair. This type of deputy chair title on its own usually has only an advisory role and not an operational one (such as Ted Turner at Time Warner).
An unrelated definition of vice- and deputy chairs describes an executive who is higher ranking or has more seniority than an executive vice-president (EVP).

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2361 2024-11-05 00:12:45
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,148
Re: Miscellany
2261) Nursing Home
Gist
A nursing home is a public or private residential facility providing a high level of long-term personal and nursing care for persons (such as the aged or the chronically ill) who are unable to care for themselves properly.
Today nursing homes have a more active role in health care, helping patients prepare to live at home or with a family member when possible. They help conserve expensive hospital facilities for the acutely ill and improve the prospects of the chronically disabled.
Summary
Nursing home is a facility for care (usually long-term) of patients who are not sick enough to need hospital care but are not able to remain at home. Historically, most residents were elderly or ill or had chronic irreversible and disabling disorders, and medical and nursing care was minimal. Today nursing homes have a more active role in health care, helping patients prepare to live at home or with a family member when possible. They help conserve expensive hospital facilities for the acutely ill and improve the prospects of the chronically disabled. However, quality of care varies widely, and the potential for abuse exists.
Details
A nursing home is a facility for the residential care of older people, senior citizens, or disabled people. Nursing homes may also be referred to as care homes, skilled nursing facilities (SNF) or long-term care facilities. Often, these terms have slightly different meanings to indicate whether the institutions are public or private, and whether they provide mostly assisted living, or nursing care and emergency medical care. Nursing homes are used by people who do not need to be in a hospital, but require care that is hard to provide in a home setting. The nursing home staff attends to the patients' medical and other needs. Most nursing homes have nursing aides and skilled nurses on hand 24 hours a day.
In the United States, while nearly 1 in 10 residents aged 75 to 84 stays in a nursing home for five or more years, nearly 3 in 10 residents in that age group stay less than 100 days, the maximum duration covered by Medicare, according to the American Association for Long-Term Care Insurance. Some nursing homes also provide short-term rehabilitative stays following surgery, illness, or injury. Services may include physical therapy, occupational therapy, or speech-language therapy. Nursing homes also offer other services, such as planned activities and daily housekeeping. Nursing homes may offer memory care services, often called dementia care.
History
Poorhouses/workhouses were the first implemented national framework to provide a basic level of care to the old and infirm. Pictured, is "The workroom at St James's workhouse" from The Microcosm of London (1808).
From before the 17th century to modern day, many families care for their elders in the family's home. While this is still common practice for many communities and families around the world, this has become increasingly more difficult over time as life expectancy increases, family size decreases, and increased expertise in caring for a person with a chronic disease is needed. In the 21st century, nursing homes have become a standard form of care for most aged and incapacitated persons to account for those complexities. Nearly 6 percent of older adults are sheltered in residential facilities that provide a wide range of care. Yet such institutions have not always existed; rather, their history and development reflect relatively recent demographic and political realities that shape the experience of growing old.
In the 17th century, poorhouses (also referred to as almshouses) originated in England as municipalities were expected to care for their poor. Orphans, people determined to be mentally ill, and elderly people were often placed into these living commons while able-bodied individuals were expected to work and could be imprisoned if they refused. This model was brought to North America by English settlers. Before the 19th century, no age-restricted institutions existed for long-term care; elderly individuals, who needed shelter because of incapacity, impoverishment, or family isolation, often ended their days in an almshouse. Placed alongside people deemed insane, people who were inebriated, or people who were homeless, they were simply categorized as part of the community's most needy recipients. Poorhouses gave a place where they could be given shelter and daily meals.
In the 1800s in the US, women's and church groups began to establish special homes for elderly people. Often concerned that individuals of their own ethnic or religious communities might die alongside the most despised society, this led to the creation of private care facilities for the elderly in these communities. Poorhouses continued to exist into the early 20th century, despite the criticism of the poor conditions of the poorhouses. In the US, the Great Depression overwhelmed the poorhouses, leading to not enough space and funding. Due to muckraking in the 1930s, the less-than-favorable living conditions of the poorhouses were exposed to the public. This led to the provision of the Social Security Act (1935) to only give people their pension if they did not live in poorhouses, but could live in private institutions.
In the US, poorhouses were then replaced with residential living homes, known as board-and-care homes or convalescent homes. These board-and-care homes provided basic levels of care and meals in a private setting for a specific fee. Board-and-care homes proved to be a success and by World War II, the new way of nursing homes began to take shape. As the times continued to change, the government identified the issue of people spending extensive amounts of time in hospitals. To combat these long stays in short-term settings, board-and-care homes began to convert into something more public and permanent that was state and federally funded. From this, by 1965 nursing homes were a solid fixture. Nursing homes were a permanent residence where the elderly and disabled could receive any necessary medical care and receive daily meals. These nursing homes showed improvement in maintaining care and cleanliness standards in comparison to almshouses and poorhouses. From the 1950s through the 1970s, the dynamics of nursing homes began changing significantly. In the United States, Medicare and Medicaid began to make up much of the money that would filter through the homes and the 1965 amendment laws enforced nursing homes to comply with safety codes and required registered nurses to be on hand at all times. Additionally, nursing homes may sue children for the costs of caring for their parents in jurisdictions which have filial responsibility laws. Later in 1987, in the US the Nursing Reform Act was introduced to begin defining the different types of nursing home services and later added the Residents' Bill of Rights.
In the UK, after World War II many soldiers and civilians needing hospital care due to casualties during the war were placed in the hospital along with the many elderly patients present there, leading to overcrowding. The implementation of the NHS in 1948 and the abolishment of the Old Poor Law allowed for the creation of what would become modern day, public nursing homes. In the 1950s, Professor Peter Townsend brought to light the discrepancies the standard of care between the publicly and privately funded cares homes, leading to health policy reforms that assured the standard care practices for the elderly living in NHS funded care homes. The 1980s and 1990s saw care homes becoming a large industry in the UK. Thus, policies ensuring that private care homes are regulated (Registered Homes Act 1984) and patient needs are met (Care Standards Act of 2000) were established.
Today, nursing homes are varied. Some nursing homes still resemble a hospital while others look more like a home. Nursing home residents can pay for their care out of pocket or with government assistance. In the US, others may receive Medicare for a short time, while in other countries, public assistance may be available, and some may use long-term insurance plans. Across the spectrum, most nursing homes in the US will accept Medicaid as a source of payment.
Considerations
Below are a few reasons to consider a nursing home:
* Managing a worsening and progressive disease such as Alzheimers.
* Care after a recent hospital admission and not ready to transition to independently caring for oneself at home.
* When medical needs at home become unmanageable by the primary caregiver at home.
* When activities and socialization with people of similar age is deprived.
* When primary caregiver at home do not have the proper knowledge of the nutrition needed.
When looking into nursing homes, consider what activities and/or medical needs patients one would need from the nursing home. Also consider finances, such as medical insurance and personal funds. Ensure the nursing home is properly licensed and has qualified staff. If time allows, visit the nursing home in person to receive a walk through of the facility and if given the opportunity to speak with a guest or family member of guest, ask about their experience thus far.
Staff
Nursing home employees are all required to be licensed or uphold a certificate in the state of which they work. In most facilities, nursing homes are required to provide enough staff to adequately care for residents. In the US, for instance, nursing homes must have at least one registered nurse (RN) available for at least 8 straight hours a day throughout the week, and at least one licensed practical nurse (LPN) on duty 24 hours a day. Direct care nursing home employees usually include registered nurses, licensed practical nurses, social workers, certified nursing assistants, and physical therapists, amongst others.
Medical staff:
Nurses
Nursing homes require assessment and monitoring of residents by a registered nurse (RN) who is typically required to have between two and six years of education. The RN's job duties include implementing care plans, administering medications, recording and maintaining accurate reports for each resident, monitoring and recording medical changes, and providing direction to the nursing assistants and licensed practical nurses (LPN). RNs are not required to choose a specialization. To gain recognition as a specialized nurse professional, RNs typically need education in their specialized field, and further experience through clinical practices. LPNs are typically required to have a year of training before working with any patients. The LPN monitors residents' well-being and administers treatments and medications, such as dressing wounds and dispensing prescribed drugs. LPNs are responsible for patients' direct bed care and carry out a daily routine.
Nursing assistants
A nursing assistant provides basic care to patients while working directly under a LPN or RN. These basic care activities, also referred to as activities of daily living, can include assisting with bathing and dressing residents, helping residents with meals, either serving them or with feeding, transferring to and from the bed or wheelchair, making and cleaning beds, assisting with toileting, and answering call lights. Nursing assistants' official titles can vary between jurisdictions and facilities. They can include Certified Nursing Assistants (CNAs), nursing aides, caregivers, patient care associates, patient care technicians, personal care attendants (PCAs), and care assistants.
Physicians
At skilled nursing facilities, in addition to required 24 hour skilled nursing, a licensed physician supervises individual patients. At nursing homes other than skilled nursing facilities, patients receive care from physicians not affiliated with the nursing home. These physicians are typically employed by a private agency that sends physicians to nursing homes per the request of the patient, nursing home, or patient's family. The majority of these physicians are family medicine doctors or internists; however, some specialists such as cardiologist or nephrologist may also make independent visits to supplement their care.
Non-medical staff:
Administration
Depending on the size of the nursing home, a nursing home may have either a nursing home administrator or an executive director. Some nursing homes may have both, but their job duties are similar and can include overseeing staff, supplying medical supplies, and financial matters. Some nursing homes also have human resources personnel, who are in charge of all aspects of hiring new employees. Human resources job duties vary but can also include coordinating payroll, organizing orientation programs for new employees, interviewing, disciplinary actions, and ensuring compliance with federal and state laws. Nursing homes are usually licensed and heavily regulated under governing legislation. Compliance with the federal and state legislatures are reviewed regularly for adherence to strict standards of building codes, care plans, behavior and altercations between residents, nutrition and dietary services, medical services, nursing and personal care, religious and spiritual practices, pets, and recreational programs.
Housekeeping
Housekeepers perform everyday cleaning and upkeep in nursing homes. They play a huge part in ensuring that nursing homes are kept clean and free of disease causing agents. Housekeepers have a long list of duties which include cleaning floors, changing linens, disinfecting bathrooms, changing towels, washing clothes, emptying trashcans, sanitizing rooms, replenishing supplies, dusting, vacuuming, and keeping windows and woodwork clean. These duties can vary from facility to facility but it will overall include basic cleaning. Housekeeping does not require any licensure or schooling, but some jobs may prefer experienced housekeepers.
Recreational staff
Recreational staff usually include an activity director and possibly activity assistants depending on the size of the nursing home. Activities aim to meet each resident's emotional, intellectual, physical, social, spiritual, and vocational needs. The transition from being independent to having to depend on others and be away from home is oftentimes very difficult, which is why activities are important to combat depression and anxiety. Some of the different activities that may be offered include hosting birthday parties, celebrating holidays, book clubs, musical events, outdoor activities, discussion and social groups, exercise, arts and crafts, pet therapy, religious services and community outings. Volunteer involvement is also an important part of nursing home activities given that volunteers can act as a link between the nursing home and the outside community.
Additional Information
As the United States population continues to age, the need for quality elder care will grow. One common living option for those who require a high level of care is a nursing home. Nursing homes are complex healthcare facilities with different departments dedicated to maintaining the physical, psychological, and emotional health of their residents. The Centers for Disease Control and Prevention (CDC) reported in 2016 that there were 15,600 nursing homes in the U.S., with about 1.7 million total beds.
To support all of their patients, nursing homes employ a diverse group of professionals. These employees may specialize in business, healthcare, or other disciplines. Working in a nursing home is a unique experience. In these environments, professionals with different skill sets collaborate with one another to improve the lives of senior community members. Continue reading to explore why nursing homes are rewarding work environments and the professional opportunities they offer.
What Are Nursing Homes Like?
No matter what hour of the day or night, there are nursing home employees awake and tending to the needs of residents. There’s often a great deal of activity, such as nurses making their rounds, maintenance staff preparing the facility for the next day’s activities, or finance professionals balancing the budgets. At their busiest hours, the halls and wings of nursing homes are full of movement, with residents heading out to activities, visitors stopping in, and staff members performing their daily tasks.
Jobs in nursing homes often fall into one of three categories: administration, direct care, and support.
Administration
Administrative professionals keep nursing homes running efficiently. They’re responsible for managing other employees, working with patients’ health insurance companies, and paying facility bills. Administrative positions in nursing homes include business roles such as marketing director, finance director, human resources specialist, accounts payable, and receptionist. These positions do not directly interact with residents, and some nursing homes house administrative staff in separate buildings.
Direct Care Staff
The direct care staff consists of those directly responsible for patient care. Registered nurses (RNs) represent the largest percentage of the direct care staff, as nursing homes have skilled nurses on the clock at all times working under the direction of one or more physicians. These RNs might also have nursing assistants or medical technicians assisting them. The direct care staff at nursing homes also includes therapeutic and recreational personnel, such as physical therapists, recreational therapists, activities directors, and other professionals who keep patients active and their quality of life high.
Support Staff
Support staff can include janitorial, maintenance, landscaping, and transportation personnel. These employees typically have minimal contact with facility residents but may receive specialized training on how to perform jobs in the context of a healthcare facility, as they will be among people with vulnerable medical conditions.
What Are the Benefits of Working in a Nursing Home?
Each day and night, thousands of administrators, direct care staff, and support staff wake up and head to their jobs at nursing homes. When asked, “why did you want to work in a nursing home?” many would respond that they find their careers fulfilling for a host of different reasons. From helping those in need to learning from the wisdom of seniors in their care, working in a nursing home has many benefits to offer.
Helping People in Need
Working in a nursing home means providing healthcare for people who need it. Nursing homes provide seniors with a safe, clean, healthy environment where they can receive the care they need, access proper nutrition, and experience the social benefits of being around their peers. Nursing home employees have the satisfaction of providing such care and attention, knowing they’re making a difference in the lives of their residents.
Learning About Life
Working around people in the later stages of life can teach nursing home employees valuable lessons about the aging process, the mindset of the elderly, and what it means to age. The senior members of any community have a wealth of knowledge and wisdom to share. While some fear aging, the elderly can illustrate that it’s a natural part of life and that one’s later years can be enjoyable.
Teamwork Environment
Nursing homes rely on an interdisciplinary staff to ensure operations run smoothly and provide high-quality care. As such, all nursing home employees learn what it means to work as part of a team. Each staff member at a nursing home fulfills a specific, valuable role. In most of these positions, employees work with professionals in other areas as well as with members of their own departments, thereby providing them with the opportunity to develop their communication and collaboration skills.
A Professional Future in Caring for the Elderly
There are a lot of great reasons to work in a nursing home. It means stepping into a fulfilling career in which you can provide essential care to the most senior members of your community. The U.S. Bureau of Labor Statistics (BLS) projects the healthcare field to grow by 3.3 million jobs between 2018 and 2028, making work in a nursing home environment not just rewarding but also increasingly in demand. Find out more about how Maryville University’s online bachelor’s in healthcare management with a certificate in senior living management can help you land a great job in this important field.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2362 2024-11-06 00:08:49
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,148
Re: Miscellany
2262) Boron Nitride
Gist
Boron Nitride, (chemical formula BN), synthetically produced crystalline compound of boron and nitrogen, is an industrial ceramic material of limited but important application, principally in electrical insulators and cutting tools.
What is boron nitride used for?
BN nanostructures are used for thermal conductivity enhancement, mechanical strength enhancement, insulating coatings, oxidation and corrosion-resistant coatings, electrical circuits, water treatment, and hydrogen storage.
Summary
The empirical formula of boron nitride (BN) is deceptive. BN is not at all like other diatomic molecules such as carbon monoxide (CO) and hydrogen chloride (HCl). Rather, it has much in common with carbon, whose representation as the monatomic C is also misleading.
BN, like carbon, has multiple structural forms. BN’s most stable structure, hBN, is isoelectronic with graphite and has the same hexagonal structure with similar softness and lubricant properties. hBN can also be produced in graphene-like sheets that can be formed into nanotubes.
In contrast, cubic BN (cBN) is isoelectronic with diamond. It is not quite as hard, but it is more thermally and chemically stable. It is also much easier to make. Unlike diamond, it is insoluble in metals at high temperatures, making it a useful abrasive and oxidation-resistant metal coating. There is also an amorphous form (aBN), equivalent to amorphous carbon.
BN is primarily a synthetic material, although a naturally occurring deposit has been reported. Attempts to make pure BN date to the early 20th century, but commercially acceptable forms have been produced only in the past 70 years. In a 1958 patent to the Carborundum Company (Lewiston, NY), Kenneth M. Taylor prepared molded shapes of BN by heating boric acid (H3BO3) with a metal salt of an oxyacid such as phosphate in the presence of ammonia to form a BN “mix”, which was then compressed into shape.
Today, similar methods are in use that begin with boric trioxide (B2O3) or H3BO3 and use ammonia or urea as the nitrogen source. All synthetic methods produce a somewhat impure aBN, which is purified and converted to hBN by heating at temperatures higher than used in the synthesis. Similarly, to the preparation of synthetic diamond, hBN is converted to cBN under high pressure and temperature.
Details
Boron nitride is a thermally and chemically resistant refractory compound of boron and nitrogen with the chemical formula BN. It exists in various crystalline forms that are isoelectronic to a similarly structured carbon lattice. The hexagonal form corresponding to graphite is the most stable and soft among BN polymorphs, and is therefore used as a lubricant and an additive to cosmetic products. The cubic (zincblende aka sphalerite structure) variety analogous to diamond is called c-BN; it is softer than diamond, but its thermal and chemical stability is superior. The rare wurtzite BN modification is similar to lonsdaleite but slightly softer than the cubic form.
Because of excellent thermal and chemical stability, boron nitride ceramics are used in high-temperature equipment and metal casting. Boron nitride has potential use in nanotechnology.
Structure
Boron nitride exists in multiple forms that differ in the arrangement of the boron and nitrogen atoms, giving rise to varying bulk properties of the material.
Amorphous form (a-BN)
The amorphous form of boron nitride (a-BN) is non-crystalline, lacking any long-distance regularity in the arrangement of its atoms. It is analogous to amorphous carbon.
All other forms of boron nitride are crystalline.
Hexagonal form (h-BN)
The most stable crystalline form is the hexagonal one, also called h-BN, α-BN, g-BN, and graphitic boron nitride. Hexagonal boron nitride has a layered structure similar to graphite. Within each layer, boron and nitrogen atoms are bound by strong covalent bonds, whereas the layers are held together by weak van der Waals forces. The interlayer "registry" of these sheets differs, however, from the pattern seen for graphite, because the atoms are eclipsed, with boron atoms lying over and above nitrogen atoms. This registry reflects the local polarity of the B–N bonds, as well as interlayer N-donor/B-acceptor characteristics. Likewise, many metastable forms consisting of differently stacked polytypes exist. Therefore, h-BN and graphite are very close neighbors, and the material can accommodate carbon as a substituent element to form BNCs. BC6N hybrids have been synthesized, where carbon substitutes for some B and N atoms. Hexagonal boron nitride monolayer is analogous to graphene, having a honeycomb lattice structure of nearly the same dimensions. Unlike graphene, which is black and an electrical conductor, h-BN monolayer is white and an insulator. It has been proposed for use as an atomic flat insulating substrate or a tunneling dielectric barrier in 2D electronics.
Cubic form (c-BN)
Cubic boron nitride has a crystal structure analogous to that of diamond. Consistent with diamond being less stable than graphite, the cubic form is less stable than the hexagonal form, but the conversion rate between the two is negligible at room temperature, as it is for diamond. The cubic form has the sphalerite crystal structure, the same as that of diamond (with ordered B and N atoms), and is also called β-BN or c-BN.
Wurtzite form (w-BN)
The wurtzite form of boron nitride (w-BN) has the same structure as lonsdaleite, a rare hexagonal polymorph of carbon. As in the cubic form, the boron and nitrogen atoms are grouped into tetrahedra. In the wurtzite form, the boron and nitrogen atoms are grouped into 6-membered rings. In the cubic form all rings are in the chair configuration, whereas in w-BN the rings between 'layers' are in boat configuration. Earlier optimistic reports predicted that the wurtzite form was very strong, and was estimated by a simulation as potentially having a strength 18% stronger than that of diamond. Since only small amounts of the mineral exist in nature, this has not yet been experimentally verified. Its hardness is 46 GPa, slightly harder than commercial borides but softer than the cubic form of boron nitride.
Properties:
Physical
The partly ionic structure of BN layers in h-BN reduces covalency and electrical conductivity, whereas the interlayer interaction increases resulting in higher hardness of h-BN relative to graphite. The reduced electron-delocalization in hexagonal-BN is also indicated by its absence of color and a large band gap. Very different bonding – strong covalent within the basal planes (planes where boron and nitrogen atoms are covalently bonded) and weak between them – causes high anisotropy of most properties of h-BN.
For example, the hardness, electrical and thermal conductivity are much higher within the planes than perpendicular to them. On the contrary, the properties of c-BN and w-BN are more homogeneous and isotropic.
Those materials are extremely hard, with the hardness of bulk c-BN being slightly smaller and w-BN even higher than that of diamond. Polycrystalline c-BN with grain sizes on the order of 10 nm is also reported to have Vickers hardness comparable or higher than diamond. Because of much better stability to heat and transition metals, c-BN surpasses diamond in mechanical applications, such as machining steel. The thermal conductivity of BN is among the highest of all electric insulators.
Boron nitride can be doped p-type with beryllium and n-type with boron, sulfur, silicon or if co-doped with carbon and nitrogen. Both hexagonal and cubic BN are wide-gap semiconductors with a band-gap energy corresponding to the UV region. If voltage is applied to h-BN or c-BN, then it emits UV light in the range 215–250 nm and therefore can potentially be used as light-emitting diodes (LEDs) or lasers.
Little is known on melting behavior of boron nitride. It degrades at 2973 °C, but melts at elevated pressure.
Thermal stability
Hexagonal and cubic BN (and probably w-BN) show remarkable chemical and thermal stabilities. For example, h-BN is stable to decomposition at temperatures up to 1000 °C in air, 1400 °C in vacuum, and 2800 °C in an inert atmosphere.
Thermal stability of c-BN can be summarized as follows:
* In air or oxygen: B2O3 protective layer prevents further oxidation to ~1300 °C; no conversion to hexagonal form at 1400 °C.
* In nitrogen: some conversion to h-BN at 1525 °C after 12 h.
* In vacuum ({10}^{-5} Pa): conversion to h-BN at 1550–1600 °C.
Mechanical properties
BN nanosheets consist of hexagonal boron nitride (h-BN). They are stable up to 800°C in air. The structure of monolayer BN is similar to that of graphene, which has exceptional strength, a high-temperature lubricant, and a substrate in electronic devices.
The anisotropy of Young's modulus and Poisson's ratio depends on the system size. h-BN also exhibits strongly anisotropic strength and toughness, and maintains these over a range of vacancy defects, showing that the anisotropy is independent to the defect type.
Additional Information
Boron Nitride, (chemical formula BN), is a synthetically produced crystalline compound of boron and nitrogen, an industrial ceramic material of limited but important application, principally in electrical insulators and cutting tools. It is made in two crystallographic forms, hexagonal boron nitride (H-BN) and cubic boron nitride (C-BN).
H-BN is prepared by several methods, including the heating of boric oxide (B2O3) with ammonia (NH3). It is a platy powder consisting, at the molecular level, of sheets of hexagonal rings that slide easily past one another. This structure, similar to that of the carbon mineral graphite, makes H-BN a soft, lubricious material; unlike graphite, though, H-BN is noted for its low electric conductivity and high thermal conductivity. H-BN is frequently molded and then hot-pressed into shapes such as electrical insulators and melting crucibles. It also can be applied with a liquid binder as a temperature-resistant coating for metallurgical, ceramic, or polymer processing machinery.
C-BN is most often made in the form of small crystals by subjecting H-BN to extremely high pressure (six to nine gigapascals) and temperature (1,500° to 2,000° C, or 2,730° to 3,630° F). It is second only to diamond in hardness (approaching the maximum of 10 on the Mohs hardness scale) and, like synthetic diamond, is often bonded onto metallic or metallic-ceramic cutting tools for the machining of hard steels. Owing to its high oxidation temperature (above 1,900° C, or 3,450° F), it has a much higher working temperature than diamond (which oxidizes above 800° C, or 1,475° F).
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2363 2024-11-07 00:26:07
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,148
Re: Miscellany
2263) Alumina
Gist
Alumina is synthetically produced aluminum oxide, Al2O3, a white or nearly colourless crystalline substance that is used as a starting material for the smelting of aluminum metal.
It is the oxide of aluminum that occurs in nature as corundum and in bauxite and is used as a source of aluminum, as an abrasive, and as an absorbent.
Alumina is widely used in a variety of industrial abrasive materials, owing to its superior hardness and strength. Similarly, alumina can be used as a coating to protect against abrasion.
Summary
Alumina, or aluminium oxide, is the largest single cost in connection with aluminium production. Top quality alumina is required to produce quality metal with low environmental impact. The worldwide production of close to 100 millions tonnes is mainly produced from bauxite in the Bayer process.
Key features
* Hardness and strength
* Low heat retention and high melting point
Application areas
* For production of aluminium by the Hall–Héroult process
* Other industrial uses include abrasives, fillers in plastics and catalyst support for industrial catalysts
Product details
To turn bauxite into alumina, we grind the ore and mix it with lime and caustic soda, pump this mix into high-pressure containers, and heat it. The aluminium oxide we’re after is dissolved by the caustic soda, then precipitated out of this solution, washed, and heated to drive off water. What’s left is the sugar-like white powder called alumina,
or aluminium oxide (Al2O3).
Alumina is synthetically produced aluminum oxide, Al2O3, a white or nearly colourless crystalline substance that is used as a starting material for the smelting of aluminum metal. It also serves as the raw material for a broad range of advanced ceramic products and as an active agent in chemical processing.
Details
Aluminium oxide (or aluminium(III) oxide) is a chemical compound of aluminium and oxygen with the chemical formula Al2O3. It is the most commonly occurring of several aluminium oxides, and specifically identified as aluminium oxide. It is commonly called alumina and may also be called aloxide, aloxite, or alundum in various forms and applications. It occurs naturally in its crystalline polymorphic phase α-Al2O3 as the mineral corundum, varieties of which form the precious gemstones ruby and sapphire. Al2O3 is used to produce aluminium metal, as an abrasive owing to its hardness, and as a refractory material owing to its high melting point.
Natural occurrence
Corundum is the most common naturally occurring crystalline form of aluminium oxide. Rubies and sapphires are gem-quality forms of corundum, which owe their characteristic colours to trace impurities. Rubies are given their characteristic deep red colour and their laser qualities by traces of chromium. Sapphires come in different colours given by various other impurities, such as iron and titanium. An extremely rare δ form occurs as the mineral deltalumite.
History
The field of aluminium oxide ceramics has a long history. Aluminium salts were widely used in ancient and medieval alchemy. Several older textbooks cover the history of the field. A 2019 textbook by Andrew Ruys contains a detailed timeline on the history of aluminium oxide from ancient times to the 21st century.
Properties
Al2O3 is an electrical insulator but has a relatively high thermal conductivity for a ceramic material. Aluminium oxide is insoluble in water. In its most commonly occurring crystalline form, called corundum or α-aluminium oxide, its hardness makes it suitable for use as an abrasive and as a component in cutting tools.
Aluminium oxide is responsible for the resistance of metallic aluminium to weathering. Metallic aluminium is very reactive with atmospheric oxygen, and a thin passivation layer of aluminium oxide (4 nm thickness) forms on any exposed aluminium surface in a matter of hundreds of picoseconds.[better source needed] This layer protects the metal from further oxidation. The thickness and properties of this oxide layer can be enhanced using a process called anodising. A number of alloys, such as aluminium bronzes, exploit this property by including a proportion of aluminium in the alloy to enhance corrosion resistance. The aluminium oxide generated by anodising is typically amorphous, but discharge-assisted oxidation processes such as plasma electrolytic oxidation result in a significant proportion of crystalline aluminium oxide in the coating, enhancing its hardness.
Aluminium oxide was taken off the United States Environmental Protection Agency's chemicals lists in 1988. Aluminium oxide is on the EPA's Toxics Release Inventory list if it is a fibrous form.
Amphoteric nature
Aluminium oxide is an amphoteric substance, meaning it can react with both acids and bases, such as hydrofluoric acid and sodium hydroxide, acting as an acid with a base and a base with an acid, neutralising the other and producing a salt.
Additional Information
Alumina, synthetically produced aluminum oxide, Al2O3, a white or nearly colourless crystalline substance that is used as a starting material for the smelting of aluminum metal. It also serves as the raw material for a broad range of advanced ceramic products and as an active agent in chemical processing.
Alumina is made from bauxite, a naturally occurring ore containing variable amounts of hydrous (water-containing) aluminum oxides. Free Al2O3 occurs in nature as the mineral corundum and its gemstone forms, sapphire and ruby; these can be produced synthetically from alumina and in fact are occasionally referred to as alumina, but the term is more properly limited to the material employed in aluminum metallurgy, industrial ceramics, and chemical processing.
Some alumina is still produced by melting bauxite in an electric furnace, in a process devised for the abrasives industry early in the 20th century, but most is now extracted from bauxite through the Bayer process, which was developed for the aluminum industry in 1888. In the Bayer process bauxite is crushed, mixed in a solution of sodium hydroxide, and seeded with crystals to precipitate aluminum hydroxide. The hydroxide is heated in a kiln in order to drive off the water and produce several grades of granular or powdery alumina, including activated alumina, smelter-grade alumina, and calcined alumina.
Activated alumina is a porous, granular substance that is used as a substrate for catalysts and as an adsorbent for removing water from gases and liquids. Smelter-grade alumina accounts for 90 percent of all alumina produced; it is transported to aluminum plants, where it is electrolyzed into aluminum metal. Calcined alumina is made into a variety of ceramic products, including spark-plug insulators, integrated-circuit packages, bone and dental implants, laboratory ware, sandpaper grits and grinding wheels, and refractory linings for industrial furnaces. These products exhibit the properties for which alumina is well known, including low electric conductivity, resistance to chemical attack, high strength, extreme hardness (9 on the Mohs hardness scale, the highest rating being 10), and high melting point (approximately 2,050 °C, or 3,700 °F).
The toughness of alumina can be improved by the addition of zirconia particles or silicon-carbide whiskers, making it suitable for industrial cutting tools. Also, the normally opaque material can be made translucent by adding small amounts of magnesia. Translucent alumina is employed as the gas container in high-pressure sodium-vapour streetlamps.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2364 2024-11-08 00:02:18
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,148
Re: Miscellany
2264) Barcode
Gist
A barcode (sometimes seen as two words, bar code) is the small image of lines (bars) and spaces that is affixed to retail store items, identification cards and postal mail to identify a particular product number, person or location.
Barcodes encode product information into bars and alphanumeric characters, making it much faster and easier to ring up items at a store or track inventory in a warehouse. Besides ease and speed, bar codes' major business benefits include accuracy, inventory control and cost savings.
Summary
A barcode (sometimes seen as two words, bar code) is the small image of lines (bars) and spaces that is affixed to retail store items, identification cards and postal mail to identify a particular product number, person or location.
The code uses a sequence of vertical bars and spaces to represent numbers and other symbols. A barcode symbol typically consists of five parts: a quiet zone, a start character, data characters (including an optional check character), a stop character and another quiet zone.
How barcodes work
The barcode is simply an image. To become meaningful, a barcode reader, or scanner, must decode it. The reader uses a laser beam that is sensitive to the reflections from the line and space thickness and variation. The reader translates the reflected light into digital data that is transferred to a computer for immediate action or storage.
Readers may be attached to a computer, as they often are in retail store settings, or separate and portable, in which case they store the data they read until it can be fed into a computer.
Here are common uses for barcodes:
* By supermarkets and retailers to track items sold and inventory
* By libraries to identify and track borrowed books
* By manufacturers and shippers to track product movements
* By employers to verify and track employee working hours
* By hospitals to identify patients
* By marketers to tabulate the results of direct mail marketing returns
* By researchers to track honeybees (using tiny barcodes)
Barcode standards
There is no one standard barcode. Instead, there are several different barcode standards called symbologies that serve different uses, industries, or geographic needs.
Since 1973, the Uniform Product Code (UPC), regulated by the Uniform Code Council, an industry organization, has provided a standard barcode used by most retail stores. The European Article Numbering system (EAN), developed by Joe Woodland, the inventor of the first barcode system, allows for an extra pair of digits and is becoming widely used. POSTNET is the standard barcode used in the United States for ZIP codes in bulk mailing.
Details
A barcode or bar code is a method of representing data in a visual, machine-readable form. Initially, barcodes represented data by varying the widths, spacings and sizes of parallel lines. These barcodes, now commonly referred to as linear or one-dimensional (1D), can be scanned by special optical scanners, called barcode readers, of which there are several types.
Later, two-dimensional (2D) variants were developed, using rectangles, dots, hexagons and other patterns, called 2D barcodes or matrix codes, although they do not use bars as such. Both can be read using purpose-built 2D optical scanners, which exist in a few different forms. Matrix codes can also be read by a digital camera connected to a microcomputer running software that takes a photographic image of the barcode and analyzes the image to deconstruct and decode the code. A mobile device with a built-in camera, such as a smartphone, can function as the latter type of barcode reader using specialized application software and is suitable for both 1D and 2D codes.
The barcode was invented by Norman Joseph Woodland and Bernard Silver and patented in the US in 1952. The invention was based on Morse code that was extended to thin and thick bars. However, it took over twenty years before this invention became commercially successful. UK magazine Modern Railways December 1962 pages 387–389 record how British Railways had already perfected a barcode-reading system capable of correctly reading rolling stock travelling at 100 mph (160 km/h) with no mistakes. An early use of one type of barcode in an industrial context was sponsored by the Association of American Railroads in the late 1960s. Developed by General Telephone and Electronics (GTE) and called KarTrak ACI (Automatic Car Identification), this scheme involved placing colored stripes in various combinations on steel plates which were affixed to the sides of railroad rolling stock. Two plates were used per car, one on each side, with the arrangement of the colored stripes encoding information such as ownership, type of equipment, and identification number. The plates were read by a trackside scanner located, for instance, at the entrance to a classification yard, while the car was moving past. The project was abandoned after about ten years because the system proved unreliable after long-term use.
Barcodes became commercially successful when they were used to automate supermarket checkout systems, a task for which they have become almost universal. The Uniform Grocery Product Code Council had chosen, in 1973, the barcode design developed by George Laurer. Laurer's barcode, with vertical bars, printed better than the circular barcode developed by Woodland and Silver. Their use has spread to many other tasks that are generically referred to as automatic identification and data capture (AIDC). The first successful system using barcodes was in the UK supermarket group Sainsbury's in 1972 using shelf-mounted barcodes which were developed by Plessey. In June 1974, Marsh supermarket in Troy, Ohio used a scanner made by Photographic Sciences Corporation to scan the Universal Product Code (UPC) barcode on a pack of Wrigley's chewing gum. QR codes, a specific type of 2D barcode, rose in popularity in the second decade of the 2000s due to the growth in smartphone ownership.
Other systems have made inroads in the AIDC market, but the simplicity, universality and low cost of barcodes has limited the role of these other systems, particularly before technologies such as radio-frequency identification (RFID) became available after 2023.
Barcode system
A barcode system is a network of hardware and software, consisting primarily of mobile computers, printers, handheld scanners, infrastructure, and supporting software. Barcode systems are used to automate data collection where hand recording is neither timely nor cost effective. Despite often being provided by the same company, Barcoding systems are not radio-frequency identification (RFID) systems. Many companies use both technologies as part of larger resource management systems.
A typical barcode system consist of some infrastructure, either wired or wireless that connects some number of mobile computers, handheld scanners, and printers to one or many databases that store and analyze the data collected by the system. At some level there must be some software to manage the system. The software may be as simple as code that manages the connection between the hardware and the database or as complex as an ERP, MRP, or some other inventory management software.
Hardware
A wide range of hardware is manufactured for use in barcode systems by such manufacturers as Datalogic, Intermec, HHP (Hand Held Products), Microscan Systems, Unitech, Metrologic, PSC, and PANMOBIL, with the best known brand of handheld scanners and mobile computers being produced by Symbol, a division of Motorola.
Software
Some ERP, MRP, and other inventory management software have built in support for barcode reading. Alternatively, custom interfaces can be created using a language such as C++, C#, Java, Visual Basic.NET, and many others. In addition, software development kits are produced to aid the process.
Additional Information
Barcode is a printed series of parallel bars or lines of varying width that is used for entering data into a computer system. The bars are typically black on a white background, and their width and quantity vary according to application. The bars are used to represent the binary digits 0 and 1, sequences of which in turn can represent numbers from 0 to 9 and be processed by a digital computer. The presence or absence of a bar of a particular width in a particular position in a sequence is read by the computer as either a 0 or 1. Most such codes use bars of only two different widths (thick and thin), though some codes employ four widths. The numbers represented by a barcode are also printed out at its base.
Barcode information is read by an optical (laser) scanner that is part of a computer system. A handheld scanner or barcode pen is moved across the code, or the code itself is moved by hand across a scanner built into a checkout counter or other surface. The computer then stores or immediately processes the data in the barcode. The barcodes printed on supermarket and other retail merchandise in the United States are those of the Universal Product Code, or UPC, which assigns each type of food or grocery product a unique code. In the UPC system, the five digits on the left are assigned to a particular manufacturer or maker, and the five digits on the right are used by that manufacturer to identify a specific type or make of product. This is usually the only information contained in a barcode.
Barcoding was introduced in the 1970s and is now a ubiquitous part of routine commercial transactions. Grocery stores use the codes to obtain price and other data about goods at the point of purchase by the consumer. At a typical supermarket checkout counter, a scanner is used to identify a product through its barcode, and a computer then looks up the item’s price and feeds that number into the cash register, where it becomes part of the bill for the customer’s purchases.
The chief advantage of barcode systems is that they allow users to process detailed information at the moment the barcode is scanned, rather than simply storing information for later processing. For example, ski resorts can affix the codes to skiers and scan the bars when skiers enter ski lifts, thereby allowing the resort to monitor patterns of slope use. Various barcode systems are now used to track a vast range of products as they are manufactured, distributed, stored, sold, and serviced. These products range from processed foods and dry goods to drugs and medical supplies, automotive parts, computer parts, and even library books.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2365 2024-11-09 00:02:01
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,148
Re: Miscellany
2265) Operation Theater
Gist
An operating theater (also known as an Operating Room (OR), operating suite, operation suite, or Operation Theatre (OT)) is a facility within a hospital where surgical operations are carried out in an aseptic environment.
Summary
The operating room (OR) is the location where surgical procedures are performed. It’s a sterile environment intended to prevent infection during the surgical procedure. ORs are also brightly lit to ensure surgeons can see what they are doing and air-conditioned to further prevent infection.
ORs can vary based on the type of surgery they accommodate, with the equipment in the operating room matching the needs of the surgical team.
Some of the equipment that may be included in the OR include:
* Anesthesia machine
* Anesthesia cart
* Surgical table
* EKG machine
* AED/defibrillator
* Vital signs monitor
* Nerve stimulator
* Blood warmer
* Imaging devices
What are the three common types of operating rooms (ORs)?
Operating rooms are designed to meet the unique needs of a hospital or population it serves. There are three types of operating rooms: digital, hybrid, and integrated.
Digital operating rooms are built around central data systems that integrate imaging, software, and video data systems. These operating rooms enable the sharing, transfer, and collection of medical data, which may be used during the operation or afterward to assess outcomes and inform care plans.
Hybrid operating rooms bring together surgical equipment and medical imaging devices to support minimally invasive surgical procedures without needing to transfer a patient to another room for imaging.
Integrated operating rooms feature functionally integrated surgical systems that combine lighting, audio, video, and operating equipment in a single, centrally controlled unit to improve surgical efficiency by eliminating the need to move equipment to and from the operating room during a procedure.
Why is an operating room (OR) important for healthcare?
The operating room (OR) offers a place for surgeons to complete surgical procedures safely. Not only is the OR sterile to prevent infection, but it also contains all the equipment the surgical team needs to perform the procedure and respond should complications arise. By having this equipment nearby, the medical team can respond more quickly.
Some of the most popular procedures performed in ORs include:
* Knee replacement
* Hip replacement
* Percutaneous coronary angioplasty (PTCA)
* Appendectomy
* Hysterectomy
* Coronary artery bypass graft
* Spinal fusion
Details
An operating theater (also known as an Operating Room (OR), operating suite, operation suite, or Operation Theatre (OT)) is a facility within a hospital where surgical operations are carried out in an aseptic environment.
Historically, the term "operating theater" referred to a non-sterile, tiered theater or amphitheater in which students and other spectators could watch surgeons perform surgery. Contemporary operating rooms are usually devoid of a theater setting, making the term "operating theater" a misnomer in those cases.
Classification of operation theatre
Operating rooms are spacious, in a cleanroom, and well-lit, typically with overhead surgical lights, and may have viewing screens and monitors. Operating rooms are generally windowless, though windows are becoming more prevalent in newly built theaters to provide clinical teams with natural light, and feature controlled temperature and humidity. Special air handlers filter the air and maintain a slightly elevated pressure. Electricity support has backup systems in case of a black-out. Rooms are supplied with wall suction, oxygen, and possibly other anesthetic gases. Key equipment consists of the operating table and the anesthesia cart. In addition, there are tables to set up instruments. There is storage space for common surgical supplies. There are containers for disposables. Outside the operating room, or sometimes integrated within, is a dedicated scrubbing area that is used by surgeons, anesthetists, ODPs (operating department practitioners), and nurses prior to surgery. An operating room will have a map to enable the terminal cleaner to realign the operating table and equipment to the desired layout during cleaning. Operating rooms are typically supported by an anaesthetic room, prep room, scrub and a dirty utility room.
Several operating rooms are part of the operating suite that forms a distinct section within a health-care facility. Besides the operating rooms and their wash rooms, it contains rooms for personnel to change, wash, and rest, preparation and recovery rooms, storage and cleaning facilities, offices, dedicated corridors, and possibly other supportive units. In larger facilities, the operating suite is climate- and air-controlled, and separated from other departments so that only authorized personnel have access.
Operating room equipment
* The operating table in the center of the room can be raised, lowered, and tilted in any direction.
* The operating room lights are over the table to provide bright light, without shadows, during surgery.
* The anesthesia machine is at the head of the operating table. This machine has tubes that connect to the patient to assist them in breathing during surgery, and built-in monitors that help control the mixture of gases in the breathing circuit.
* The anesthesia cart is next to the anesthesia machine. It contains the medications, equipment, and other supplies that the anesthesiologist may need.
* Sterile instruments to be used during surgery are arranged on a stainless steel table.
* An electronic monitor (which records the heart rate and respiratory rate by adhesive patches that are placed on the patient's chest).
* The pulse oximeter machine attaches to the patient's finger with an elastic band aid. It measures the amount of oxygen contained in the blood.
* Automated blood pressure measuring machine that automatically inflates the blood pressure cuff on a patient's arm.
* An electrocautery machine uses high frequency electrical signals to cauterize or seal off blood vessels and may also be used to cut through tissue with a minimal amount of bleeding.
* If surgery requires, a heart-lung machine or other specialized equipment may be brought into the room.
* Supplementary portable air decontaminating equipment is sometimes placed in the OR.
* Advances in technology now support hybrid operating rooms, which integrate diagnostic imaging systems such as MRI and cardiac catheterization into the operating room to assist surgeons in specialized neurological and cardiac procedures.
Surgeon and assistants' equipment
People in the operating room wear PPE (personal protective equipment) to help prevent bacteria from infecting the surgical incision. This PPE includes the following:
* A protective cap covering their hair
* Masks over their lower face, covering their mouths and noses with minimal gaps to prevent inhalation of plume or airborne microbes
* Shades or glasses over their eyes, including specialized colored glasses for use with different lasers. a fiber-optic headlight may be attached for greater visibility
* Sterile gloves; usually latex-free due to latex sensitivity which affects some health care workers and patients
* Long gowns, with the bottom of the gown no closer than six inches to the ground.
* Protective covers on their shoes
* If x-rays are expected to be used, lead aprons/neck covers are used to prevent overexposure to radiation
The surgeon may also wear special glasses to help them see more clearly. The circulating nurse and anesthesiologist will not wear a gown in the OR because they are not a part of the sterile team. They must keep a distance of 12–16 inches from any sterile object, person, or field.
History
Early Modern operating theaters in an educational setting had raised tables or chairs at the center for performing operations surrounded by steep tiers of standing stalls for students and other spectators to observe the case in progress. The surgeons wore street clothes with an apron to protect them from blood stains, and they operated bare-handed with unsterilized instruments and supplies.
The University of Padua began teaching medicine in 1222. It played a leading role in the identification and treatment of diseases and ailments, specializing in autopsies and the inner workings of the body. In 1884 German surgeon Gustav Neuber implemented a comprehensive set of restrictions to ensure sterilization and aseptic operating conditions through the use of gowns, caps, and shoe covers, all of which were cleansed in his newly invented autoclave. In 1885 he designed and built a private hospital in the woods where the walls, floors and hands, arms and faces of staff were washed with mercuric chloride, instruments were made with flat surfaces and the shelving was easy-to-clean glass. Neuber also introduced separate operating theaters for infected and uninfected patients and the use of heated and filtered air in the theater to eliminate germs. In 1890 surgical gloves were introduced to the practice of medicine by William Halsted. Aseptic surgery was pioneered in the United States by Charles McBurney.
Surviving operating theaters
The oldest surviving operating theater is thought to be the 1804 operating theater of the Pennsylvania Hospital in Philadelphia. The 1821 Ether Dome of the Massachusetts General Hospital is still in use as a lecture hall. Another surviving operating theater is the Old Operating Theatre in London. Built in 1822, it is now a museum of surgical history. The Anatomical Theater at the University of Padua, in Italy, inside Palazzo Bo was constructed and used as a lecture hall for medical students who observed the dissection of corpses, not surgical operations. It was commissioned by the anatomist Girolamo Fabrizio d'Acquapendente in 1595.
Additional Information
On the day of surgery, you will meet with the medical team involved in your surgery. This will include your surgeon, the anesthesiologist, an operating room nurse, and various other healthcare professionals.
Getting ready for surgery
You may expect some of the following to happen:
* You may need to change into a hospital gown.
* You will receive an ID bracelet.
* An intravenous catheter (IV) may be inserted in your forearm or other location for anesthetics and other medicines.
* You may be transported on a stretcher to the operating room.
What does the operating room look like?
The operating room can be an intimidating, busy place. It has a lot of unfamiliar technical equipment. The following is a brief list of equipment you may see in the operating room. Each operating room varies depending on the type of surgery being done:
* The operating table in the center of the room can be raised, lowered, and tilted in any direction.
* The operating room lamps allow for brilliant illumination without shadows during surgery.
* You will be connected to various monitors that keep track of your vital signs. These include your heart rate and blood pressure.
* A ventilator or breathing machine stands by the head of the operating table. If your procedure is done under general anesthesia, a ventilator will breathe for you during the procedure by moving oxygen and air in and out of your lungs.
* Sterile instruments to be used during surgery are arranged on a stainless steel table.
* A diathermy machine, to control bleeding, usually is present.
* If the surgery needs it, a heart-lung machine, or other specialized equipment, may be brought into the room.
* The operating room will likely be cold to minimize bacterial growth.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2366 2024-11-10 00:02:43
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,148
Re: Miscellany
2266) Opera (Web browser)
Gist
Opera is a multi-platform web browser developed by its namesake company Opera. The current edition of the browser is based on Chromium. Opera is available on Windows, macOS, Linux, Android, and iOS (Safari WebKit engine).
Summary
A Web browser for Windows, Mac, Linux and mobiles from Opera Software, Oslo, Norway. Developed at Telenor (Norwegian Telecom) in 1994 and commercialized by Opera in 1995, it is noted for its fast rendering of Web pages. Opera was the first browser to offer a host of unique features such as enlarging text and graphic elements on the page and displaying multiple windows with only one instance of the program running. In 2005, the paid version was made free. In 2013, Opera switched from its Presto architecture to Chromium.
Details
Opera is a multi-platform web browser developed by its namesake company Opera. The current edition of the browser is based on Chromium. Opera is available on Windows, macOS, Linux, Android, and iOS (Safari WebKit engine). Two mobile versions are still active, called Opera Mobile and Opera Mini. Opera also has a news aggregator app called Opera News with Aria, an AI-based search engine.
Opera was first released on Monday, April 10th 1995, making it one of the oldest desktop web browsers to ever exist. It was commercial software for its first ten years and had its own proprietary layout engine, Presto. In 2013, it switched from the Presto engine to Chromium. In 2016, Opera, developed in Norway, became a subsidiary of an investment group led by a Chinese consortium. In 2018, Opera Software went public on the NASDAQ stock exchange. By the end of 2022, the consortium sold all of its shares, and Opera in turn committed to repurchase all of its American Depository Shares to reestablish its corporate autonomy. As of the end of 2023, Opera Software was 72.4% owned by Kunlun, a Chinese public company, making it a subsidiary of that company. Opera CEO James Yahui Zhou is a controlling shareholder in Kunlun.
In 2019, Opera introduced Opera GX, a browser marketed towards gamers, claiming to have better performance with a built-in tracker and ad blocker and also having a CPU and RAM usage limiter.
History
In 1994, Jon Stephenson von Tetzchner and Geir Ivarsøy started developing the Opera web browser while working at Telenor, a Norwegian telecommunications company.
In 1995, they founded Opera Software AS. Opera was initially released on 10 April 1995, and then it was released publicly in 1996 with version 2.10, which ran on Microsoft Windows 95. Development for mobile device platforms started in 1998.
Opera 4.0, released in 2000, included a new cross-platform core that facilitated the creation of editions of Opera for multiple operating systems and platforms.
To this point, Opera was trialware and had to be purchased after the trial period. With version 5.0, released in 2000, Opera became ad-sponsored, displaying ads to users who had not paid for it. Subsequent versions have given users the choice of seeing banner ads or targeted text ads from Google.
With version 8.5, released in 2005, the ads were completely removed, and the browser's primary financial support came through revenue from Google (by contract, Opera's default search engine).
Among new features introduced in version 9.1, released in 2006, was fraud protection using technology from GeoTrust, a digital certificate provider, and PhishTank, an organization that tracks known phishing web sites. This feature was further expanded in version 9.5, when GeoTrust was replaced with Netcraft, and malware protection from Haute Secure was added.
In 2006, Opera Software ASA was released as well as Nintendo DS Browser and Internet Channel for Nintendo's DS and Wii gaming systems, respectively, which were Opera-based browsers.
A new JavaScript engine, called Carakan (after the Javanese alphabet), was introduced with version 10.50. According to Opera Software, it made Opera 10.50 more than seven times faster in SunSpider than Opera 10.10.
On 16 December 2010, Opera 11 was released, featuring extensions, tab stacking (where dragging one tab over another allowed creating a group of tabs), visual mouse gestures and changes to the address bar. Opera 12 was released on 14 June 2012.
On 12 February 2013, Opera Software announced that it would drop its own Presto layout engine in favor of WebKit as implemented by Google's Chrome browser, using code from the Chromium project. Opera Software planned as well to contribute code to WebKit. On 3 April 2013, Google announced it would fork components from WebKit to form a new layout engine, Blink. That day, Opera Software confirmed it would follow Google in implementing Blink.
On 28 May 2013, a beta release of Opera 15 was made available, the first version based on the Chromium project. Many distinctive Opera features of the previous versions were dropped, and Opera Mail was separated into a standalone application derived from Opera 12.
In 2016, Opera was acquired by an investment group led by a Chinese consortium, the consortium included several Chinese companies such as Kunlun Tech and Qihoo 360. On July 27, 2018, Opera Software went public on the NASDAQ stock exchange, raising $115 million in its initial public offering. Opera began repurchasing its shares in 2022 following the closure of 360 Security Technology Inc. that year.
In January 2017, the source code of Opera 12.15, one of the last few versions still based on the Presto layout engine, was leaked.
To demonstrate how radically different a browser could look, Opera Neon, dubbed a "concept browser", was released in January 2017. PC World compared it to demo models that automakers and hardware vendors release to show their visions of the future. Instead of a Speed Dial Browsing feature it displays the frequently accessed websites in resemblance to a desktop with computer icons scattered over it in an artistic formation.
On 10 May 2017, Opera 45 was released. Notably this was the last version of the browser compatible with 32-bit Linux distributions, with later versions requiring a 64-bit Linux distribution. This version, inspired by the previous Opera Neon design, was called "Opera Reborn" and which redoes parts of the user interface, such as adding light and dark modes, and integrates the messenger applications Facebook Messenger, WhatsApp, and Telegram. Additionally, new ad-blocking settings were added along with security changes.
On 4 January 2018, Opera 50 was released. This version updated the browser to utilize the built-in ad blocker to provide cryptocurrency mining protection that stops sites from running scripts that attempt to use the CPU to mine cryptocurrency. Additionally the browser added Chromecast support, VR support enhancements, saving pages as PDFs, and improved VPN performance with region-based locations rather than country-based.
On 9 April 2019, Opera 60 was released. This version, codenamed Reborn 3, focused on moving the browser towards a more minimal design, further improving the free VPN service, and was marketed as being the "World's first Web3 ready browser", as it included out of the box integrations with blockchain and cryptocurrency applications.
On 21 May 2019, Opera GX is announced and opened for early access. The only information available in this announcement is that the browser would be a special version of the browser aimed at those who play games. The early-access program was opened on 11 June 2019.
On 24 June 2021, Opera 77, codenamed Opera R5, was released. As one of the larger updates to the browser, it added more music streaming services in the sidebar, integrating native support for Apple Music, Spotify, YouTube Music, Tidal, SoundCloud, and Gaana. The "Pinboards" feature was also added, letting users create a shareable collection of websites, images, links, and notes in a visual form. A video popout feature was also added for video conferencing, which happens automatically when switching tabs, popping out of the window when navigating away and popping back in when navigating back. Later, in Opera 83 released on 19 January 2022, this feature would be implemented for all video players, not just video conferencing platforms.
On 31 Jan 2023, Opera announced that given the discontinuation of support for Windows 7 and 8.1 by Microsoft, Chromium based browsers are also ending support, so Opera will no longer get updates on those versions, but older versions will continue to function on those versions of Windows.
On 22 March 2023, Opera and Opera GX incorporated features with AI-powered tools. These features include AI Prompts that are suggested to the user, and sidebar access to ChatGPT and ChatSonic. The prompts show up on sites that contain content like articles, offering to shorten the text or summarize them.
On 20 June 2023, Opera launched Opera 100, codenamed Opera One, a version of the browser built from the ground up around AI which was unveiled on 25 April 2023. This browser includes a native AI called Aria, a GPT-based AI engine that was developed collaboratively with OpenAI that sifts through web information, generates text and code, and much more in the browser. Tab islands were also introduced, allowing browser tabs to be grouped together, bookmarked, collapsed, and more. Major UI changes were made, and a Multithreaded Compositor was introduced, allowing the browser to function and render in animations much smoother than it was previously capable.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2367 2024-11-11 00:06:09
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,148
Re: Miscellany
2267) Stockbroker
Gist
Stockbrokers are individuals who buy and sell stocks and other securities for retail and institutional clients, through a stock exchange or over the counter, in return for a fee or a commission.
Summary
Stockbrokers are individuals who buy and sell stocks and other securities for retail and institutional clients, through a stock exchange or over the counter, in return for a fee or a commission. Institutional stockbrokers work with fund managers and other financial institutions, but there are also retail investors.
What does a stockbroker do?
Stockbrokers deal with their clients directly and manage their wealth portfolios. They work with existing clients and develop new businesses.
The role involves:
* Keeping up to date with the latest financial and tax legislation
* Monitoring stock market performances
* Conducting specific market research and analysis.
Most importantly, the role is to ensure to understand the client's needs and make the appropriate suggestions for their investments.
Brokers need to be honest and provide all correct information, including risk. Exaggerating and providing misleading information is not acceptable. They also need to proactively look for clients, sell their firm’s services and manage those relationships. This can be achieved by a combination of networking and cold calling.
Types of stockbrokers
* Full-service brokers - provide a personal service to clients and pass on important exclusive information available to only full-service clients. They deliver personalised research and information on investment
* Online brokers - carry out research and provide investment news, charts and a selection of stocks for clients to consider
* Discount brokers - only follow up orders from their clients. They don’t offer advice, research or analysis services.
Details
A stockbroker is an individual or company that buys and sells stocks and other investments for a financial market participant in return for a commission, markup, or fee. In most countries they are regulated as a broker or broker-dealer and may need to hold a relevant license and may be a member of a stock exchange. They generally act as a financial advisor and investment manager. In this case they may also be licensed as a financial adviser such as a registered investment adviser (in the United States).
Examples of professional designations held by individuals in this field, which affects the types of investments they are permitted to sell and the services they provide include chartered financial consultants, certified financial planners or chartered financial analysts (in the United States and UK), chartered financial planners (in the UK).
In the United States, the Financial Industry Regulatory Authority provides an online tool designed to help understand professional designations.
History of stock broking
(...) This enigmatic business [i.e. the inner workings of the stock exchange in Amsterdam, primarily the practice of VOC and WIC stock trading] which is at once the fairest and most deceitful in Europe, the noblest and the most infamous in the world, the finest and the most vulgar on earth. It is a quintessence of academic learning and a paragon of fraudulence; it is a touchstone for the intelligent and a tombstone for the audacious, a treasury of usefulness and a source of disaster, (...) The best and most agreeable aspect of the new business is that one can become rich without risk. Indeed, without endangering your capital, and with out having anything to do with correspondence, advances of money, warehouses, postage, cashiers, suspensions of payment, and other unforeseen incidents, you have the prospect of gaining wealth if, in the case of bad luck in your transactions, you will only change your name. Just as the Hebrews, when they are seriously ill, change their names in order to obtain relief, so a changing of his name is sufficient for the speculator who finds himself in difficulties, to free himself from all impending dangers and tormenting disquietude.
— Joseph de la Vega, in his book Confusión de confusiones (1688), the earliest book about stock trading
The first recorded buying and selling of shares occurred in Rome in the 2nd century BC. After the fall of the Western Roman Empire, stockbroking did not become a profession until after the Renaissance, when government bonds were traded in Italian city-states such as Genoa and Venice. In 1602, the Amsterdam Stock Exchange (now Euronext Amsterdam) became the first official stock market with trading in shares of the Dutch East India Company, the first company to issue stock. In 1698, the London Stock Exchange opened at the Jonathan's Coffee-House. On May 17, 1792, the New York Stock Exchange opened under a platanus occidentalis (buttonwood tree) in New York City, as 24 stockbrokers signed the Buttonwood Agreement, agreeing to trade five securities under that buttonwood tree.
Additional Information
What Is a Stockbroker?
A stockbroker is a financial professional who executes orders in the market on behalf of clients. A stockbroker may also be known as a registered representative (RR) or an investment advisor. Most stockbrokers work for a brokerage firm and handle transactions for several individual and institutional customers. Stockbrokers are often paid on commission, although compensation methods vary by employer.
Key Takeaways
* A stockbroker is a financial professional who buys and sells stocks at the direction of clients.
* Most buy and sell orders are now made through online discount brokers. This automated process reduces fees.
* Wealthy individuals and institutions continue to use full-service brokers who offer advice, portfolio management services, and complete transactions.
Understanding the Role of a Stockbroker
Buying or selling stocks requires access to one of the major exchanges, such as the New York Stock Exchange (NYSE) or the NASDAQ. To trade on these exchanges, you must be a member of the exchange or belong to a member firm. Member firms and many individuals who work for them are licensed as brokers or broker-dealers by the Financial Industry Regulatory Authority (FINRA).
Until recent years, getting access to the stock markets was prohibitively expensive. It was cost-effective only for high net-worth investors or large institutional investors, such as the managers of pension funds. They used full-service brokers and could pay hundreds of dollars for executing a trade.
While an individual investor can buy stock shares directly from the company that issues them, it is much simpler to work with a stockbroker.
However, the rise of the internet and related technological advances paved the way for discount brokers to provide online services with cheap, fast, and automated access to the markets. More recently, apps like Robinhood and SoFi have catered to micro-investors, allowing even fractional share purchases. Most accounts in the markets today are managed by the account owners and held by discount brokers.
Brokerage firms and broker-dealer companies are also sometimes referred to generically as stockbrokers. These include full-service and discount brokers who execute trades but do not offer individualized investing advice. Most online brokers are discount brokers, at least at their basic service levels, in which trades are executed for free or for a small set-price commission. Many online brokers offer robo-advisors that automate the buying and selling process.
Stockbrokers in the 21st Century
Brokers who are employed by discount broker firms may work as over-the-phone agents (known as voice brokers) available to answer brief questions or as branch officers in a physical location. They also may consult with clients subscribing to premium tiers of the online broker.
A comparatively smaller number of stockbrokers work for investment banks or specialized brokerage firms. These companies handle large and specialized orders for institutional clients and high-net-worth individuals (HNWI).
Another recent development in broker services is the introduction of roboadvisers, programs that use algorithmic investing techniques carried out via web or mobile app interfaces. There is minimal individual interaction, keeping fees low.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2368 2024-11-22 16:22:17
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,148
Re: Miscellany
2268) Profit and Loss Account
Gist
What is a profit and loss account? The profit and loss account forms part of a business' financial statements and shows whether it has made or lost money. It summarises the trading results of a business over a period of time (typically one year) showing both the revenue and expenses.
Summary
Profit and Loss Account (P&L):
The profit and loss statement (P&L) is a key management accounting tool that helps companies assess their financial performance over a period of time. By understanding the P&L, entrepreneurs and investors can make informed decisions and better assess the economic health of a company.
P&L Definition: What is P&L?
The income statement is an essential element of a company's annual financial statements, showing income and expenses over a specific period - usually a financial year. Its main purpose is to measure the success or failure of a company by determining the profit made or loss incurred.
The income statement structures the financial activities of a company and shows:
* Revenue: All revenue from the sale of goods and services and other income.
* Expenses: All costs and expenses incurred in the course of business activities, such as material costs, personnel expenses, depreciation and other operating expenses.
By comparing income and expenses, the company's net profit is calculated, i.e. the profit if income exceeds expenses, or the loss if expenses are higher than income. The income statement thus provides important information for Corporate Management, investors and other stakeholders to assess the financial situation and performance of the company.
P&L Structure: How to create a P&L?
The profit and loss account (P&L) is divided into various items that systematically record a company's income and expenses. Here is a typical P&L structure:
* Revenue:
Income from the sale of goods and services.
* Changes in inventories:
Increase or decrease in inventories of finished goods and work in progress.
* Other own work capitalized:
Internally generated and used services that are not sold but used within the company itself.
* Other operating income:
Income that does not originate from the core business, such as rental income, interest income.
* Cost of materials:
Expenses for raw materials, consumables and supplies and for purchased goods.
* Personnel expenses:
Wages and salaries as well as social security contributions.
* Depreciation and amortization:
Impairment of fixed assets (property, plant and equipment, intangible assets).
* Other operating expenses:
Costs for rent, advertising, insurance, office supplies, etc.
* Result from ordinary activities:
Operating result resulting from the difference between the above income and expenses.
* Financial result:
Interest income and interest expenses as well as other financial expenses and income.
* Extraordinary income and expenses:
Non-recurring and extraordinary business transactions that are not part of ordinary business activities.
* Taxes on income and earnings:
Trade tax, corporation tax, income tax.
* Net profit/loss for the year:
The final profit or loss after tax.
This structured layout helps to present the financial performance of a company clearly and transparently and provides a sound basis for business management decisions.
Details:
What Is a Profit and Loss (P&L) Statement?
A profit and loss (P&L) statement, also known as an income statement, is a financial statement that summarizes the revenues, costs, expenses, and profits/losses of a company during a specified period. These records provide information about a company’s ability to generate revenues, manage costs, and make profits.
Key Takeaways
* The profit and loss (P&L) statement is a financial statement that summarizes the revenues, costs, and expenses incurred during a specified period.
* The P&L statement is one of three financial statements that every public company issues quarterly and annually, along with the balance sheet and the cash flow statement.
* When used together, the P&L statement, balance sheet, and cash flow statement provide an in-depth look at a company’s overall financial performance.
* Statements are prepared using the cash method or accrual method of accounting.
* It is important to compare P&L statements from different accounting periods, as any changes over time become more meaningful than the numbers themselves.
How Profit and Loss (P&L) Statements Work
The P&L statement is one of three financial statements that every public company issues on a quarterly and annual basis, along with the balance sheet and the cash flow statement. It is often the most popular and common financial statement in a business plan, as it shows how much profit or loss was generated by a business.
P&L statements are also referred to as a(n):
* Statement of profit and loss
* Statement of operations
* Statement of financial results or income
* Earnings statement
* Expense statement
* Income statement
The P&L or income statement, like the cash flow statement, shows changes in accounts over a set period of time. The balance sheet, on the other hand, is a snapshot, showing what the company owns and owes at a single moment. It is important to compare the income statement with the cash flow statement since, under the accrual method of accounting, a company can log revenues and expenses before cash changes hands.
This document follows a general form as seen in the example below. It begins with an entry for revenue, known as the top line, and subtracts the costs of doing business, including the cost of goods sold, operating expenses, tax expenses, and interest expenses. The difference, known as the bottom line, is net income, also referred to as profit or earnings.
Note
Comparing P&L Statements
It is important to compare income statements from different accounting periods. The reason behind this is that any changes in revenues, operating costs, research and development (R&D) spending, and net earnings over time are more meaningful than the numbers themselves. For example, a company’s revenues may grow on a steady basis, but its expenses might grow at a much faster rate.
Comparing one company’s P&L statement with another in the same industry that is similar in size can further help investors evaluate the financial well-being of a company. For example, doing so might reveal that one company is more efficient at managing expenses and has better growth potential than the other.
Revenues and expenses for nonprofit organizations are generally tracked in a financial report called the statement of activities. As such, this report is sometimes called a statement of financial activities or a statement of support.
Types of P&L Statements
As noted above, a P&L statement may be prepared in one of two ways. These are the cash method and the accrual method.
Cash Method
The cash method, which is also called the cash accounting method, is only used when cash goes in and out of the business. This is a very simple method that only accounts for cash received or paid. A business records transactions as revenue whenever cash is received and as liabilities whenever cash is used to pay any bills or liabilities. This method is commonly used by smaller companies as well as people who want to manage their personal finances.
Accrual Method
The accrual accounting method records revenue as it is earned. This means that a company using the accrual method accounts for money that it expects to receive in the future. For instance, a company that delivers a product or service to its customer records the revenue on its P&L statement, even though it hasn’t yet received payment. Similarly, liabilities are accounted for even when the company hasn’t yet paid for any expenses.
Additional Information
A profit and loss (P&L) statement is a financial report that summarizes a business’s total income and expenses for a specific period. The profit and loss statement is also known as an income statement or a statement of operations.
The goal of a P&L statement is to measure a company’s profits by subtracting expenses from income. This type of report helps provide an overview of the business’s overall financial health.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2369 2024-11-23 20:14:39
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,148
Re: Miscellany
2269) Germination
Gist
Germination is the process by which a plant grows from a seed into a seedling. Seeds remain dormant until conditions are favorable for germination. All seeds need water, oxygen and optimal temperature to germinate.
Summary
Germination is the process by which an organism grows from a seed or spore. The term is applied to the sprouting of a seedling from a seed of an angiosperm or gymnosperm, the growth of a sporeling from a spore, such as the spores of fungi, ferns, bacteria, and the growth of the pollen tube from the pollen grain of a seed plant.
Seed plants
Germination is usually the growth of a plant contained within a seed resulting in the formation of the seedling. It is also the process of reactivation of metabolic machinery of the seed resulting in the emergence of radicle and plumule. The seed of a vascular plant is a small package produced in a fruit or cone after the union of male and female reproductive cells. All fully developed seeds contain an embryo and, in most plant species some store of food reserves, wrapped in a seed coat. Dormant seeds are viable seeds that do not germinate because they require specific internal or environmental stimuli to resume growth. Under proper conditions, the seed begins to germinate and the embryo resumes growth, developing into a seedling.
Step 1: Water imbibition, the uptake of water, results in rupture of seed coat.
Step 2: The imbibition of the seed coat results in emergence of the radicle (1) and the plumule (2); the cotyledons are unfolded (3).
Step 3: This marks the final step in the germination of the seed, where the cotyledons are expanded, which are the true leaves. Note: Temperature must be kept at an optimum level.
Disturbance of soil can result in vigorous plant growth by exposing seeds already in the soil to changes in environmental factors where germination may have previously been inhibited by depth of the seeds or soil that was too compact. This is often observed at gravesites after a burial.
Seed germination depends on both internal and external conditions. The most important external factors include right temperature, water, oxygen or air and sometimes light or darkness. Various plants require different variables for successful seed germination. Often this depends on the individual seed variety and is closely linked to the ecological conditions of a plant's natural habitat. For some seeds, their future germination response is affected by environmental conditions during seed formation; most often these responses are types of seed dormancy.
* Water is required for germination. Mature seeds are often extremely dry and need to take in significant amounts of water, relative to the dry weight of the seed, before cellular metabolism and growth can resume. Most seeds need enough water to moisten the seeds but not enough to soak them. The uptake of water by seeds is called imbibition, which leads to the swelling and the breaking of the seed coat. When seeds are formed, most plants store a food reserve with the seed, such as starch, proteins, or oils. This food reserve provides nourishment to the growing embryo. When the seed imbibes water, hydrolytic enzymes are activated which break down these stored food resources into metabolically useful chemicals. After the seedling emerges from the seed coat and starts growing roots and leaves, the seedling's food reserves are typically exhausted; at this point photosynthesis provides the energy needed for continued growth and the seedling now requires a continuous supply of water, nutrients, and light.
* Oxygen is required by the germinating seed for metabolism. Oxygen is used in aerobic respiration, the main source of the seedling's energy until it grows leaves. Oxygen is an atmospheric gas that is found in soil pore spaces; if a seed is buried too deeply within the soil or the soil is waterlogged, the seed can be oxygen starved. Some seeds have impermeable seed coats that prevent oxygen from entering the seed, causing a type of physical dormancy which is broken when the seed coat is worn away enough to allow gas exchange and water uptake from the environment.
In a small number of plants, such as rice, anaerobic germination can occur in waterlogged conditions. The seed produces a hollow coleoptile that acts like a 'snorkel', providing the seed with access to oxygen.
* Temperature affects cellular metabolic and growth rates. Seeds from different species and even seeds from the same plant germinate over a wide range of temperatures. Seeds often have a temperature range within which they will germinate, and they will not do so above or below this range. Many seeds germinate at temperatures slightly above 60-75 F (16–24 C) [room-temperature in centrally heated houses], while others germinate just above freezing and others germinate only in response to alternations in temperature between warm and cool. Some seeds germinate when the soil is cool 28–40 F (-2 - 4 C), and some when the soil is warm 76-90 F (24–32 C). Some seeds require exposure to cold temperatures (vernalization) to break dormancy. Some seeds in a dormant state will not germinate even if conditions are favorable. Seeds that are dependent on temperature to end dormancy have a type of physiological dormancy. For example, seeds requiring the cold of winter are inhibited from germinating until they take in water in the fall and experience cooler temperatures. Cold stratification is a process that induces the dormancy breaking prior to light emission that promotes germination . Four degrees Celsius is cool enough to end dormancy for most cool dormant seeds, but some groups, especially within the family Ranunculaceae and others, need conditions cooler than -5 C. Some seeds will only germinate after hot temperatures during a forest fire which cracks their seed coats; this is a type of physical dormancy.
Most common annual vegetables have optimal germination temperatures between 75–90 F (24–32 C), though many species (e.g. radishes or spinach) can germinate at significantly lower temperatures, as low as 40 F (4 C), thus allowing them to be grown from seeds in cooler climates. Suboptimal temperatures lead to lower success rates and longer germination periods.
* Light or darkness can be an environmental trigger for germination and is a type of physiological dormancy. Most seeds are not affected by light or darkness, but many photoblastic seeds, including species found in forest settings, will not germinate until an opening in the canopy allows sufficient light for the growth of the seedling.
* Scarification mimics natural processes that weaken the seed coat before germination. In nature, some seeds require particular conditions to germinate, such as the heat of a fire (e.g., many Australian native plants), or soaking in a body of water for a long period of time. Others need to be passed through an animal's digestive tract to weaken the seed coat enough to allow the seedling to emerge.
Dormancy
Some live seeds are dormant and need more time, and/or need to be subjected to specific environmental conditions before they will germinate. Seed dormancy can originate in different parts of the seed, for example, within the embryo; in other cases the seed coat is involved. Dormancy breaking often involves changes in membranes, initiated by dormancy-breaking signals. This generally occurs only within hydrated seeds. Factors affecting seed dormancy include the presence of certain plant hormones, notably abscisic acid, which inhibits germination, and gibberellin, which ends seed dormancy. In brewing, barley seeds are treated with gibberellin to ensure uniform seed germination for the production of barley malt.
Seedling establishment
In some definitions, the appearance of the radicle marks the end of germination and the beginning of "establishment", a period that utilizes the food reserves stored in the seed. Germination and establishment as an independent organism are critical phases in the life of a plant when they are the most vulnerable to injury, disease, and water stress. The germination index can be used as an indicator of phytotoxicity in soils. The mortality between dispersal of seeds and completion of the establishment can be so high that many species have adapted to produce large numbers of seeds.
Details
Germination is the sprouting of a seed, spore, or other reproductive body, usually after a period of dormancy. The absorption of water, the passage of time, chilling, warming, oxygen availability, and light exposure may all operate in initiating the process.
In the process of seed germination, water is absorbed by the embryo, which results in the rehydration and expansion of the cells. Shortly after the beginning of water uptake, or imbibition, the rate of respiration increases, and various metabolic processes, suspended or much reduced during dormancy, resume. These events are associated with structural changes in the organelles (membranous bodies concerned with metabolism), in the cells of the embryo.
Germination sometimes occurs early in the development process; the mangrove (Rhizophora) embryo develops within the ovule, pushing out a swollen rudimentary root through the still-attached flower. In peas and corn (maize) the cotyledons (seed leaves) remain underground (e.g., hypogeal germination), while in other species (beans, sunflowers, etc.) the hypocotyl (embryonic stem) grows several inches above the ground, carrying the cotyledons into the light, in which they become green and often leaflike (e.g., epigeal germination).
Seed dormancy
Dormancy is brief for some seeds—for example, those of certain short-lived annual plants. After dispersal and under appropriate environmental conditions, such as suitable temperature and access to water and oxygen, the seed germinates, and the embryo resumes growth.
The seeds of many species do not germinate immediately after exposure to conditions generally favourable for plant growth but require a “breaking” of dormancy, which may be associated with change in the seed coats or with the state of the embryo itself. Commonly, the embryo has no innate dormancy and will develop after the seed coat is removed or sufficiently damaged to allow water to enter. Germination in such cases depends upon rotting or abrasion of the seed coat in the gut of an animal or in the soil. Inhibitors of germination must be either leached away by water or the tissues containing them destroyed before germination can occur. Mechanical restriction of the growth of the embryo is common only in species that have thick, tough seed coats. Germination then depends upon weakening of the coat by abrasion or decomposition.
In many seeds the embryo cannot germinate even under suitable conditions until a certain period of time has lapsed. The time may be required for continued embryonic development in the seed or for some necessary finishing process—known as afterripening—the nature of which remains obscure.
The seeds of many plants that endure cold winters will not germinate unless they experience a period of low temperature, usually somewhat above freezing. Otherwise, germination fails or is much delayed, with the early growth of the seedling often abnormal. (This response of seeds to chilling has a parallel in the temperature control of dormancy in buds.) In some species, germination is promoted by exposure to light of appropriate wavelengths. In others, light inhibits germination. For the seeds of certain plants, germination is promoted by red light and inhibited by light of longer wavelength, in the “far red” range of the spectrum. The precise significance of this response is as yet unknown, but it may be a means of adjusting germination time to the season of the year or of detecting the depth of the seed in the soil. Light sensitivity and temperature requirements often interact, the light requirement being entirely lost at certain temperatures.
Seedling emergence
Active growth in the embryo, other than swelling resulting from imbibition, usually begins with the emergence of the primary root, known as the radicle, from the seed, although in some species (e.g., the coconut) the shoot, or plumule, emerges first. Early growth is dependent mainly upon cell expansion, but within a short time cell division begins in the radicle and young shoot, and thereafter growth and further organ formation (organogenesis) are based upon the usual combination of increase in cell number and enlargement of individual cells.
Until it becomes nutritionally self-supporting, the seedling depends upon reserves provided by the parent sporophyte. In angiosperms these reserves are found in the endosperm, in residual tissues of the ovule, or in the body of the embryo, usually in the cotyledons. In gymnosperms food materials are contained mainly in the female gametophyte. Since reserve materials are partly in insoluble form—as starch grains, protein granules, lipid droplets, and the like—much of the early metabolism of the seedling is concerned with mobilizing these materials and delivering, or translocating, the products to active areas. Reserves outside the embryo are digested by enzymes secreted by the embryo and, in some instances, also by special cells of the endosperm.
In some seeds (e.g., castor beans) absorption of nutrients from reserves is through the cotyledons, which later expand in the light to become the first organs active in photosynthesis. When the reserves are stored in the cotyledons themselves, these organs may shrink after germination and die or develop chlorophyll and become photosynthetic.
Environmental factors play an important part not only in determining the orientation of the seedling during its establishment as a rooted plant but also in controlling some aspects of its development. The response of the seedling to gravity is important. The radicle, which normally grows downward into the soil, is said to be positively geotropic. The young shoot, or plumule, is said to be negatively geotropic because it moves away from the soil; it rises by the extension of either the hypocotyl, the region between the radicle and the cotyledons, or the epicotyl, the segment above the level of the cotyledons. If the hypocotyl is extended, the cotyledons are carried out of the soil. If the epicotyl elongates, the cotyledons remain in the soil.
Light affects both the orientation of the seedling and its form. When a seed germinates below the soil surface, the plumule may emerge bent over, thus protecting its delicate tip, only to straighten out when exposed to light (the curvature is retained if the shoot emerges into darkness). Correspondingly, the young leaves of the plumule in such plants as the bean do not expand and become green except after exposure to light. These adaptative responses are known to be governed by reactions in which the light-sensitive pigment phytochrome plays a part. In most seedlings, the shoot shows a strong attraction to light, or a positive phototropism, which is most evident when the source of light is from one direction. Combined with the response to gravity, this positive phototropism maximizes the likelihood that the aerial parts of the plant will reach the environment most favourable for photosynthesis.
Additional Information
Seed germination is the initial step in the life cycle of plants, which begins when the inactive dry seed imbibes water and is completed with the protrusion of the radicle from the seed coat. Seed germination is a complex process, which involves several signals and is influenced by both intrinsic and extrinsic factors. Intrinsic factors include seed dormancy and available food stores while water, temperature, oxygen, light, relative humidity, chemicals in the seed environment, and substrate used constitute extrinsic factors. The germination process plays a key role in the domestication of crops as lack of uniform seed germination can result in poor stand establishment, which affects overall crop yield. Germination is largely affected by the balance of phytohormones, especially the abscisic acid (ABA) and gibberellins (GA) ratio. The processes involved in seed germination can be categorized into three prominent stages:
• Phase I, rapid imbibition of water by the dry seed;
• Phase II, metabolism reactivation, including mobilization of food reserves and protein synthesis; and
• Phase III, radicle protrusion.
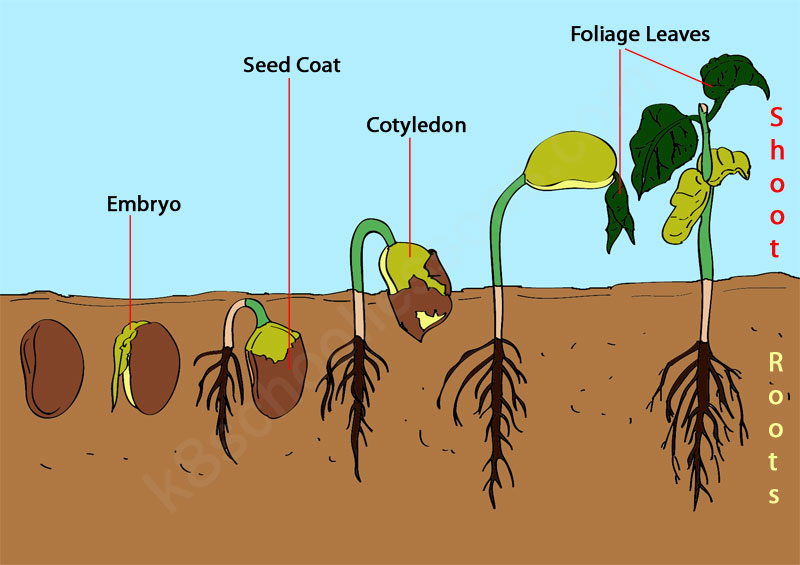
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2370 2024-11-24 22:46:37
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,148
Re: Miscellany
2270) Pharmacology
Gist
Pharmacology is the science of how drugs act on biological systems and how the body responds to the drug. The study of pharmacology encompasses the sources, chemical properties, biological effects and therapeutic uses of drugs.
Pharmacology is the study of the effects of drugs on living organisms where a drug can be broadly defined as any chemical substance, natural or synthetic, that affects a biological system.
Summary
Pharmacology is the science of drugs and medications, including a substance's origin, composition, pharmacokinetics, pharmacodynamics, therapeutic use, and toxicology. More specifically, it is the study of the interactions that occur between a living organism and chemicals that affect normal or abnormal biochemical function. If substances have medicinal properties, they are considered pharmaceuticals.
The field encompasses drug composition and properties, functions, sources, synthesis and drug design, molecular and cellular mechanisms, organ/systems mechanisms, signal transduction/cellular communication, molecular diagnostics, interactions, chemical biology, therapy, and medical applications and antipathogenic capabilities. The two main areas of pharmacology are pharmacodynamics and pharmacokinetics. Pharmacodynamics studies the effects of a drug on biological systems, and pharmacokinetics studies the effects of biological systems on a drug. In broad terms, pharmacodynamics discusses the chemicals with biological receptors, and pharmacokinetics discusses the absorption, distribution, metabolism, and excretion (ADME) of chemicals from the biological systems.
Pharmacology is not synonymous with pharmacy and the two terms are frequently confused. Pharmacology, a biomedical science, deals with the research, discovery, and characterization of chemicals which show biological effects and the elucidation of cellular and organismal function in relation to these chemicals. In contrast, pharmacy, a health services profession, is concerned with the application of the principles learned from pharmacology in its clinical settings; whether it be in a dispensing or clinical care role. In either field, the primary contrast between the two is their distinctions between direct-patient care, pharmacy practice, and the science-oriented research field, driven by pharmacology.
Details
Pharmacology is the branch of medicine that deals with the interaction of drugs with the systems and processes of living animals, in particular, the mechanisms of drug action as well as the therapeutic and other uses of the drug.
The first Western pharmacological treatise, a listing of herbal plants used in classical medicine, was made in the 1st century ad by the Greek physician Dioscorides. The medical discipline of pharmacology derives from the medieval apothecaries, who both prepared and prescribed drugs. In the early 19th century a split developed between apothecaries who treated patients and those whose interest was primarily in the preparation of medicinal compounds; the latter formed the basis of the developing specialty of pharmacology. A truly scientific pharmacology developed only after advances in chemistry and biology in the late 18th century enabled drugs to be standardized and purified. By the early 19th century, French and German chemists had isolated many active substances—morphine, strychnine, atropine, quinine, and many others—from their crude plant sources. Pharmacology was firmly established in the later 19th century by the German Oswald Schmeiderberg (1838–1921). He defined its purpose, wrote a textbook of pharmacology, helped to found the first pharmacological journal, and, most importantly, headed a school at Strasbourg that became the nucleus from which independent departments of pharmacology were established in universities throughout the world. In the 20th century, and particularly in the years since World War II, pharmacological research has developed a vast array of new drugs, including antibiotics, such as penicillin, and many hormonal drugs, such as insulin and cortisone. Pharmacology is presently involved in the development of more effective versions of these and a vast array of other drugs through chemical synthesis in the laboratory. Pharmacology also seeks more efficient and effective ways of administering drugs through clinical research on large numbers of patients.
During the early 20th century, pharmacologists became aware that a relation exists between the chemical structure of a compound and the effects it produces in the body. Since that time, increasing emphasis has been placed on this aspect of pharmacology, and studies routinely describe the changes in drug action resulting from small changes in the chemical structure of the drug. Because most medical compounds are organic chemicals, pharmacologists who engage in such studies must necessarily have an understanding of organic chemistry.
Important basic pharmacological research is carried out in the research laboratories of pharmaceutical and chemical companies. After 1930 this area of pharmacological research underwent a vast and rapid expansion, particularly in the United States and Europe.
The work of pharmacologists in industry deals also with the exhaustive tests that must be made before promising new drugs can be introduced into medical use. Detailed observations of a drug’s effects on all systems and organs of laboratory animals are necessary before the physician can accurately predict both the effects of the drug on patients and their potential toxicity to humans in general. The pharmacologist does not himself test the effects of drugs in patients; this is done only after exhaustive tests on animals and is usually conducted by physicians to determine the clinical effectiveness of new drugs. Constant testing is also required for the routine control and standardization of drug products and their potency and purity.
Additional Information
Pharmacology is the scientific study of the effects of drugs and chemicals on living organisms where a drug can be broadly defined as any chemical substance, natural or synthetic, which affects a biological system. Pharmacology may involve how organisms handle drugs, identification and validation of new targets for drug action, and the design and development of new drugs to prevent, treat and cure disease.
Pharmacology research is also a critical component in the development of modern 'personalized medicine'.
There are many sub-specialties within the general discipline of pharmacology. Pharmacodynamics is the study of the effects of drugs on biological systems and specifically addresses the chemical properties and physiological and behavioral effects of drugs arising from their interaction with molecular targets such as receptor proteins or enzyme systems. In contrast, pharmacokinetics is the study of what biological systems do to the drug and encompasses investigations of drug absorption, distribution, biotransformation and excretion, essential information for the design of drug treatment schedules in different patient populations and experimental animals, and for the prediction of drug-drug interactions that may enhance or compromise the effectiveness and safety of therapeutic agents.
Pharmacologists require sound basic knowledge of physiology, biochemistry, cell biology and molecular biology upon which to build their specialized knowledge and experimental approaches for the investigation of novel aspects of drug action. Such studies may occur at various levels, including molecular interactions, cellular and subcellular signal transduction processes, tissue and organ regulation, as well as integrated physiological or behavioral responses in intact organisms. The knowledge acquired facilitates the development of new drugs and contributes to rational therapeutics that involves the safe and effective use of drugs for therapeutic benefit. Also, this interdisciplinary knowledge offers pharmacologists a unique perspective on a wide range of biomedical issues and enhances employment opportunities in many areas of scientific investigation.
While pharmacologists are trained as laboratory researchers, pharmacists usually work in a hospital or retail pharmacy and are concerned with the preparation, dispensing, dosage, and the safe and effective use of therapeutic agents.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2371 2024-12-08 22:48:46
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,148
Re: Miscellany
2271) Chair
Gist
A chair is a type of seat, typically designed for one person and consisting of one or more legs, a flat or slightly angled seat and a back-rest. It may be made of wood, metal, or synthetic materials, and may be padded or upholstered in various colors and fabrics.
Summary
A chair is a seat with a back, intended for one person. It is one of the most ancient forms of furniture, dating from the 3rd dynasty of ancient Egypt (c. 2650–c. 2575 bce).
It was common for early Egyptian chairs to have legs shaped like those of animals. The seats were corded or dished (hollowed) in wood and topped with a pad or cushion. The ancient Greek klismos was once considered one of the most elegant chair designs. The seat, of plaited cord, was supported on sharply curved sabre-shaped legs, tapering to the feet. The horizontal back rail, curved to fit the body, was supported on three uprights. The scissors chair, or X-chair, which had a seat supported on an X-shaped frame, dates back at least to Roman times. It was especially popular in the 14th and 15th centuries in western Europe and reached great heights of elegance in Italy during the Renaissance. Renaissance chairs were of two principal varieties: those light enough to be moved easily and those heavy thronelike seats used by the head of a household or other important people.
In Tudor England the chair for the master of the house had a heavy boxlike frame and was placed on a dais in the great hall. Turned (shaped on a lathe) chairs, which had been used from early times, reached their most elaborate forms at this time, their frames consisting of turned posts and spindles. Many chairs in the 16th century depended on upholstery for decoration. Square in outline, this type had a back formed by a pair of uprights spanned by a strip of velvet or brocade trimmed with fringes or a strip of leather, sometimes tooled. The material was held in place by large-headed brass nails. In the 17th century large numbers of richly carved chairs were produced. In Italy many pieces of furniture were the work of sculptors, the most outstanding of whom was Andrea Brustolon. His suite of chairs now in the Ca’ Rezzonico in Venice, with legs and arms carved as gnarled tree trunks and branches, arms supported by black boys with heads and arms of ebony and breeches of boxwood, marked his zenith.
In France the square lines of 16th-century chairs gradually gave way to more luxurious padding and carved arms ending in scrolls or animals’ heads. During the reign of Louis XIV, furniture became grander. Chair backs became higher and had curved tops, arms were sometimes upholstered, seats were wider, and woodwork was finely carved and gilded or painted.
In England the Restoration brought a similar trend toward more luxurious living, but the exuberant styles imported by large numbers of immigrant Continental craftsmen had to be modified for English tastes. A finely carved front stretcher became fashionable but was abandoned at the end of the 17th century with the introduction of the cabriole leg. The gently curved back and cabriole legs of chairs first used in the Queen Anne period in England remained popular for half a century. Rococo design showed itself in the ribbonback, or ribband-back, chairs (chairs whose splats are curved in an intricate pattern of ribbons and bows) and “French chairs” illustrated in Thomas Chippendale’s Gentleman and Cabinetmaker’s Director, which also recorded the popularity of Gothic and chinoiserie (Chinese-style) designs.
American furniture makers sometimes adapted simplified versions of English styles from the late 17th century. Windsor chairs were particularly popular in the late 18th century and were developed to a greater degree than in England.
The Neoclassical movement in the 1760s led a return to straight but more delicate lines, with England and France setting the fashion for Europe. Straight tapering and reeded legs and square, oval, or shield-shaped backs were the mode. The most elegant English chairs of the Regency period and French chairs of the Empire period adapted the sabre leg of the Greek klismos. French chairs after the Revolution of 1789 were much simpler and more austere. England and France continued to dominate chair fashions throughout most of the 19th century, but styles were largely adaptations of those of previous eras.
After World War I the architect and designer Marcel Breuer developed the first tubular steel chair, a cantilevered form with a frame made from a continuous tubular strip. Ludwig Mies van der Rohe’s Barcelona chair of 1929, with its gently curved steel supports and buttoned leather upholstery, is a modern classic. Le Corbusier, a Swiss-born architect, experimented with laminated bentwood chairs, as did the Finn Alvar Aalto. Molded forms were extended to entire chairs in both plywood and plastic by the Americans Charles Eames and Ray Eames and the Finn Eero Saarinen. Among the developments of the late 20th century were the beanbag chair and an inflatable plastic chair.
Details
A chair is a type of seat, typically designed for one person and consisting of one or more legs, a flat or slightly angled seat and a back-rest. It may be made of wood, metal, or synthetic materials, and may be padded or upholstered in various colors and fabrics.
Chairs vary in design. An armchair has armrests fixed to the seat; a recliner is upholstered and features a mechanism that lowers the chair's back and raises into place a footrest; a rocking chair has legs fixed to two long curved slats; and a wheelchair has wheels fixed to an axis under the seat.
Etymology
Chair comes from the early 13th-century English word chaere, from Old French chaiere ("chair, seat, throne"), from Latin cathedra ("seat").
History
The chair has been used since antiquity, although for many centuries it was a symbolic article of state and dignity rather than an article for ordinary use. "The chair" is still used as the emblem of authority in the House of Commons in the United Kingdom and Canada, and in many other settings. In keeping with this historical connotation of the "chair" as the symbol of authority, committees, boards of directors, and academic departments all have a 'chairman' or 'chair'. Endowed professorships are referred to as chairs. It was not until the 16th century that chairs became common. Until then, people sat on chests, benches, and stools, which were the ordinary seats of everyday life. The number of chairs which have survived from an earlier date is exceedingly limited; most examples are of ecclesiastical, seigneurial or feudal origin.
Chairs were in existence since at least the Early Dynastic Period of Egypt (c. 3100 BC). They were covered with cloth or leather, were made of carved wood, and were much lower than today's chairs – chair seats were sometimes only 10 inches (25 cm) high. In ancient Egypt, chairs appear to have been of great richness and splendor. Fashioned of ebony and ivory, or of carved and gilded wood, they were covered with costly materials, magnificent patterns and supported upon representations of the legs of beasts or the figures of captives. Generally speaking, the higher ranked an individual was, the taller and more sumptuous was the chair he sat on and the greater the honor. On state occasions, the pharaoh sat on a throne, often with a little footstool in front of it.
The average Egyptian family seldom had chairs, and if they did, it was usually only the master of the household who sat on a chair. Among the better off, the chairs might be painted to look like the ornate inlaid and carved chairs of the rich, but the craftsmanship was usually poor.
The earliest images of chairs in China are from 6th-century Buddhist murals and stele, but the practice of sitting in chairs at that time was rare. It was not until the 12th century that chairs became widespread in China. Scholars disagree on the reasons for the adoption of the chair. The most common theories are that the chair was an outgrowth of indigenous Chinese furniture, that it evolved from a camp stool imported from Central Asia, that it was introduced to China by Christian missionaries in the 7th century, and that the chair came to China from India as a form of Buddhist monastic furniture. In modern China, unlike Korea or Japan, it is no longer common to sit at floor level.
In Europe, it was owing in great measure to the Renaissance that the chair ceased to be a privilege of state and became a standard item of furniture for anyone who could afford to buy it. Once the idea of privilege faded the chair speedily came into general use. Almost at once the chair began to change every few years to reflect the fashions of the day.
Thomas Edward Bowdich visited the main Palace of the Ashanti Empire in 1819, and observed chairs engrossed with gold in the empire. In the 1800s, chairs became more common in American households and usually there was a chair provided for every family member to sit down to dinner. By the 1830s, factory-manufactured “fancy chairs” like those by Sears, Roebuck, and Co. allowed families to purchase machined sets. With the Industrial Revolution, chairs became much more available.
The 20th century saw an increasing use of technology in chair construction with such things as all-metal folding chairs, metal-legged chairs, the Slumber Chair, moulded plastic chairs and ergonomic chairs. The recliner became a popular form, at least in part due to radio and television. In the 1930s, stair lifts were commercially available to help people suffering from Polio and other diseases to navigate stairs.
The modern movement of the 1960s produced new forms of chairs: the butterfly chair (originally called the Hardoy chair), bean bags, and the egg-shaped pod chair that turns. It also introduced the first mass-produced plastic chairs such as the Bofinger chair in 1966. Technological advances led to molded plywood and wood laminate chairs, as well as chairs made of leather or polymers. Mechanical technology incorporated into the chair enabled adjustable chairs, especially for office use. Motors embedded in the chair resulted in massage chairs.
Materials
Chairs can be made from wood, metal, or other strong materials, like stone or acrylic. In some cases, multiple materials are used to construct a chair; for example, the legs and frame may be made from metal and the seat and back may be made from plastic. Chairs may have hard surfaces of wood, metal, plastic, or other materials, or some or all of these hard surfaces may be covered with upholstery or padding. The design may be made of porous materials, or be drilled with holes for decoration; a low back or gaps can provide ventilation. The back may extend above the height of the occupant's head, which can optionally contain a headrest. Chairs can also be made from more creative materials, such as recycled materials like cutlery and wooden play bricks, pencils, plumbing tubes, rope, corrugated cardboard, and PVC pipe.
In rare cases, chairs are made out of unusual materials, especially as a form of art or experimentation. Raimonds Cirulis, a Latvian interior designer, created a volcanic hanging chair that is handmade out of volcanic rock. Peter Brenner, a Dutch-born German designer, has created a chair made from lollipop sugar – 60 pounds (27 kg) of confectioners' sugar.
Design and ergonomics
Chair design considers intended usage, ergonomics (how comfortable it is for the occupant), as well as non-ergonomic functional requirements such as size, stacking ability, folding ability, weight, durability, stain resistance, and artistic design.
Seat height
Ergonomic design distributes the weight of the occupant to various parts of the body. This is done by having an easily adjustable seat height. A seat that is higher results in dangling feet and increased pressure on the underside of the knees ("popliteal fold"). It may also result in no weight on the feet which means more weight elsewhere. A lower seat may shift too much weight to the "seat bones" ("ischial tuberosities"). Gas springs are attached to the body of the chair in order to give height adjustment and more comfort to the user.
Some chairs have foot rests. Around 15% of women and 2% of men need foot rests, even at the 16-inch (41 cm) chair height. A stool or other simple chair may have a simple straight or curved bar near the bottom for the sitter to place their feet on.
Actual chair dimensions are determined by measurements of the human body or anthropometric measurements. The two most relevant anthropometric measurement for chair design is the popliteal height and buttock popliteal length.
For someone seated, the popliteal height is the distance from the underside of the foot to the underside of the thigh at the knees. It is sometimes called the "stool height". The term "sitting height" is reserved for the height to the top of the head when seated. For American men, the median popliteal height is 16.3 inches (41 cm) and for American women it is 15.0 inches (38 cm). The popliteal height, after adjusting for heels, clothing and other issues, is used to determine the height of the chair seat. Mass-produced chairs are typically 17 inches (43 cm) high.
Researchers such as Mary Blade and Galen Cranz found that sitting on the edge of a high stool with feet on the floor is less harmful for the lower back than sitting up straight on a conventional chair.
Reclining angle
Different types of chairs can have a variety of seating positions, depending on the intended task. Typically, chairs intended for people completing work or dining can only recline very slightly (otherwise the occupant is too far away from the desk or table). Dental chairs are necessarily reclined. Research has shown that the best seated posture is a reclined posture of 100°–110°. In order to recline, the back-rest may be independently adjustable. A reclining seat and back will reduce the load on the occupant's back muscles. In general, if the occupant is supposed to sit for a long time, weight needs to be taken off the seat area and thus "easy" chairs intended for long periods of sitting are generally at least slightly reclined.
Back and head support
The back of the chair will support some of the weight of the occupant, reducing the weight on other parts of the body. Some back-rests support only the lumbar region, while shoulder height back-rests support the entire back and shoulders. Headrests support the head as well and are important in vehicles for preventing "whiplash" neck injuries in rear-end collisions where the head is jerked back suddenly. Reclining chairs typically have at least shoulder-height back-rests to shift weight to the shoulders.
Padding
There may be cases where padding is not desirable, such as chairs that are intended primarily for outdoor use. Where padding is not desirable, contouring may be used instead. A contoured seat pan attempts to distribute weight without padding. By matching the shape of the occupant's buttocks, weight is distributed and maximum pressure is reduced.
Armrests
A chair may or may not have armrests; chairs with armrests are termed "armchairs". In French, a distinction is made between fauteuil and chaise, the terms for chairs with and without armrests, respectively. In Germany, an armchair was once called a Krankensessel, or sick-chair, because it was intended for people who were too ill to stand or sit without extra support.
If present, armrests will support part of the body weight through the arms if the arms are resting on the armrests. Elbow rest height is used to determine the height of the armrests. Armrests should support the forearm and not the sensitive elbow area. Hence in some chair designs, the armrest is not continuous to the chair back, but is missing in the elbow area. Armrests further have the function of making entry and exit from the chair easier (but from the side it becomes more difficult).
Seat size and legroom
For someone seated, the buttock popliteal length is the horizontal distance from the back most part of the buttocks to the back of the lower leg. This anthropometric measurement is used to determine the seat depth. Mass-produced chairs are typically 15–17 inches (38–43 cm) deep.
Additional anthropometric measurements may be relevant to designing a chair. Hip breadth is used for chair width and armrest width. The buttock-knee length is used to determine "leg room" between rows of chairs. "Seat pitch" is the distance between rows of seats. In some airplanes and stadiums the leg room (the seat pitch less the thickness of the seat at thigh level) is so small that it is sometimes insufficient for the average person.
Types of chairs:
A rocking chair
A wide variety of chairs have emerged throughout the ages, some based on formal usages, and others based on domestic needs, and some based on needs within the workplace or various professions.
Office chair
An office chair is one used by employees within an office. Modern office chairs are usually adjustable and wheeled. Caster wheels are attached to the feet of chairs to give more mobility.
Dining room chair
A dining room chair is a specific type of design, used around a dining room table. It can be found in most ordinary residential homes, and also may appear in formal settings, such as any formal event or reception that includes a formal meal or banquet.
Work chair
A work chair is a specialized chair, adapted to the needs of a particular profession or setting. For example, a designing chair will be used for designers who sit at high easels; it will usually have added height.
Rocking chair
Some chairs have two curved bands of wood (also known as rockers) attached to the bottom of the legs. They are called rocking chairs.
Kneeling chair
A kneeling chair adds an additional body part, the knees, to support the weight of the body. A sit-stand chair distributes most of the weight of the occupant to the feet. Many chairs are padded or have cushions. Padding can be on the seat of the chair only, on the seat and back, or also on any arm rests or foot rest the chair may have. Padding will not shift the weight to different parts of the body (unless the chair is so soft that the shape is altered). However, padding does distribute the weight by increasing the area of contact between the chair and the body, and thus reducing the amount of pressure at any given point. By contrast, a hard wood chair feels hard because the contact point between the occupant and the chair is small. In lieu of padding, flexible materials, such as wicker, may be used instead with similar effects of distributing the weight.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2372 2024-12-09 16:30:25
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,148
Re: Miscellany
2272) Cliff
Gist
A cliff is a mass of rock that rises very high and is almost vertical, or straight up-and-down. Cliffs are very common landscape features. They can form near the ocean (sea cliffs), high in mountains, or as the walls of canyons and valleys.
Summary
A cliff is a mass of rock that rises very high and is almost vertical, or straight up-and-down. Cliffs are very common landscape features. They can form near the ocean (sea cliffs), high in mountains, or as the walls of canyons and valleys. Waterfalls tumble over cliffs. Cliffs are usually formed because of processes called erosion and weathering. Weathering happens when natural events, like wind or rain, break up pieces of rock.
In coastal areas, strong winds and powerful waves break off soft or grainy rocks from hardier rocks. The harder rocks are left as cliffs. The tiny pieces of rocks broken off by weathering are called sediment or alluvium. Erosion is the process of transportation of this sediment.
On sea cliffs, sediment becomes part of the seafloor and is washed away with the waves. On inland cliffs, sediment is often carried away by rivers or winds. Larger rocks broken off by sediment are called scree or talus. Scree builds up at the bottom of many inland cliffs as rocks tumble down. These piles are called scree slopes or talus piles. Some scree slopes can be so large that soil and sediment can build up between the rocks, allowing trees and other vegetation to grow on the slope.
Most scientists and mountaineers think the Rupal Flank of Nanga Parbat, a mountain in the Himalayas, is the highest cliff in the world. The Rupal Flank rises 4,600 meters (15,092 feet) above its base. Others say the highest cliff in the world is the east face of Great Trango, in the Karakoram mountain range, which is 1,340 meters (4,396 feet) tall and one of the most difficult rock-climbs in the world. Both Nanga Parbat and Great Trango are located in Pakistan.
Details
In geography and geology, a cliff or rock face is an area of rock which has a general angle defined by the vertical, or nearly vertical. Cliffs are formed by the processes of weathering and erosion, with the effect of gravity. Cliffs are common on coasts, in mountainous areas, escarpments and along rivers. Cliffs are usually composed of rock that is resistant to weathering and erosion. The sedimentary rocks that are most likely to form cliffs include sandstone, limestone, chalk, and dolomite. Igneous rocks such as granite and basalt also often form cliffs.
An escarpment (or scarp) is a type of cliff formed by the movement of a geologic fault, a landslide, or sometimes by rock slides or falling rocks which change the differential erosion of the rock layers.
Most cliffs have some form of scree slope at their base. In arid areas or under high cliffs, they are generally exposed jumbles of fallen rock. In areas of higher moisture, a soil slope may obscure the talus. Many cliffs also feature tributary waterfalls or rock shelters. Sometimes a cliff peters out at the end of a ridge, with mushroom rocks or other types of rock columns remaining. Coastal erosion may lead to the formation of sea cliffs along a receding coastline.
The British Ordnance Survey distinguishes between around most cliffs (continuous line along the topper edge with projections down the face) and outcrops (continuous lines along lower edge).
The far southwestern aspect of Nanga Parbat's Rupal face, highest cliff (rock wall/mountain face) in the world. The steepest part of the face is 2 km to the northeast. Cliffs are very common in areas where there are river banks and oceans.
Etymology
Cliff comes from the Old English word clif of essentially the same meaning, cognate with Dutch, Low German, and Old Norse klif 'cliff'. These may in turn all be from a Romance loanword into Primitive Germanic that has its origins in the Latin forms clivus / clevus ("slope" or "hillside").
Large and famous cliffs
Given that a cliff does not need to be exactly vertical, there can be ambiguity about whether a given slope is a cliff or not and also about how much of a certain slope to count as a cliff. For example, given a truly vertical rock wall above a very steep slope, one could count just the rock wall or the combination. Listings of cliffs are thus inherently uncertain.
Some of the largest cliffs on Earth are found underwater. For example, an 8,000 m drop over a 4,250 m span can be found at a ridge sitting inside the Kermadec Trench.
According to some sources, the highest cliff in the world, about 1,340 m high, is the east face of Great Trango in the Karakoram mountains of northern Pakistan. This uses a fairly stringent notion of cliff, as the 1,340 m figure refers to a nearly vertical headwall of two stacked pillars; adding in a very steep approach brings the total drop from the East Face precipice to the nearby Dunge Glacier to nearly 2,000 m.
The location of the world's highest sea cliffs depends also on the definition of 'cliff' that is used. Guinness World Records states it is Kalaupapa, Hawaii, at 1,010 m high. Another contender is the north face of Mitre Peak, which drops 1,683 m to Milford Sound, New Zealand. These are subject to a less stringent definition, as the average slope of these cliffs at Kaulapapa is about 1.7, corresponding to an angle of 60 degrees, and Mitre Peak is similar. A more vertical drop into the sea can be found at Maujit Qaqarssuasia (also known as the 'Thumbnail') which is situated in the Torssukátak fjord area at the very tip of South Greenland and drops 1,560 m near-vertically.
Considering a truly vertical drop, Mount Thor on Baffin Island in Arctic Canada is often considered the highest at 1370 m (4500 ft) high in total (the top 480 m (1600 ft) is overhanging), and is said to give it the longest vertical drop on Earth at 1,250 m (4,100 ft). However, other cliffs on Baffin Island, such as Polar Sun Spire in the Sam Ford Fjord, or others in remote areas of Greenland may be higher.
The highest cliff in the solar system may be Verona Rupes, an approximately 20 km (12 mi) high fault scarp on Miranda, a moon of Uranus.
Additional Information
A cliff is a steep and often sheer rock face. If you stand on the edge of a cliff and peer over, you'll be able to see the waves crashing on the rocks below. But if you don't watch your step, you could fall right off that cliff.
So many action movies feature a scene in which a car chase leads to a car full of villains plunging spectacularly off a steep rock wall, or cliff. As the car hurtles over the edge of the cliff and smashes onto the rocks below, it invariably explodes in a shower of flames and a cloud of smoke.
A cliff is a steep slope of earth materials, usually a rock face, that is nearly vertical and may be overhanging. Structural cliffs may form as the result of fault displacement or the resistance of a cap rock to uniform downcutting. Erosional cliffs form along shorelines or valley walls where the most extensive erosion takes place at the base of the slope. Because of their greater gradient, cliffs are subjected to greater erosive action and tend to retreat more rapidly than other slopes.
:max_bytes(150000):strip_icc():format(webp)/GettyImages-520365413-abfd9955eb1144e282cdea3c6a693cf1.jpg)
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2373 2024-12-10 15:51:11
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,148
Re: Miscellany
2273) Breakwater (structure)
Gist
A breakwater is a permanent structure constructed at a coastal area to protect against tides, currents, waves, and storm surges.
Breakwaters can be placed attached to the shoreline as headlands or submerged near the shoreline as sills. Breakwaters allow for the accretion of sediment between the structure and the shoreline, potentially stabilizing wetlands and providing shelter for new intertidal marsh habitat.
Summary
A breakwater is an artificial offshore structure protecting a harbour, anchorage, or marina basin from water waves. Breakwaters intercept longshore currents and tend to prevent beach erosion. Over the long term, however, the processes of erosion and sedimentation cannot be effectively overcome by interfering with currents and the supply of sediment. Deposition of sediment at one site will be compensated for by erosion elsewhere; this phenomenon occurs whether one breakwater or a series of such structures is erected.
Details
A breakwater is a permanent structure constructed at a coastal area to protect against tides, currents, waves, and storm surges. Breakwaters have been built since antiquity to protect anchorages, helping isolate vessels from marine hazards such as wind-driven waves. A breakwater, also known in some contexts as a jetty or a mole, may be connected to land or freestanding, and may contain a walkway or road for vehicle access.
Part of a coastal management system, breakwaters are installed parallel to the shore to minimize erosion. On beaches where longshore drift threatens the erosion of beach material, smaller structures on the beach may be installed, usually perpendicular to the water's edge. Their action on waves and current is intended to slow the longshore drift and discourage mobilisation of beach material. In this usage they are more usually referred to as groynes.
Purposes
Breakwaters reduce the intensity of wave action in inshore waters and thereby provide safe harbourage. Breakwaters may also be small structures designed to protect a gently sloping beach to reduce coastal erosion; they are placed 100–300 feet (30–90 m) offshore in relatively shallow water.
An anchorage is only safe if ships anchored there are protected from the force of powerful waves by some large structure which they can shelter behind. Natural harbours are formed by such barriers as headlands or reefs. Artificial harbours can be created with the help of breakwaters. Mobile harbours, such as the D-Day Mulberry harbours, were floated into position and acted as breakwaters. Some natural harbours, such as those in Plymouth Sound, Portland Harbour, and Cherbourg, have been enhanced or extended by breakwaters made of rock.
Types:
Types of breakwaters include vertical wall breakwater, mound breakwater and mound with superstructure or composite breakwater.
A breakwater structure is designed to absorb the energy of the waves that hit it, either by using mass (e.g. with caissons), or by using a revetment slope (e.g. with rock or concrete armour units).
In coastal engineering, a revetment is a land-backed structure whilst a breakwater is a sea-backed structure (i.e. water on both sides).
Rubble
Rubble mound breakwaters use structural voids to dissipate the wave energy. Rubble mound breakwaters consist of piles of stones more or less sorted according to their unit weight: smaller stones for the core and larger stones as an armour layer protecting the core from wave attack. Rock or concrete armour units on the outside of the structure absorb most of the energy, while gravels or sands prevent the wave energy's continuing through the breakwater core. The slopes of the revetment are typically between 1:1 and 1:2, depending upon the materials used. In shallow water, revetment breakwaters are usually relatively inexpensive. As water depth increases, the material requirements—and hence costs—increase significantly.
Caisson
Caisson breakwaters typically have vertical sides and are usually erected where it is desirable to berth one or more vessels on the inner face of the breakwater. They use the mass of the caisson and the fill within it to resist the overturning forces applied by waves hitting them. They are relatively expensive to construct in shallow water, but in deeper sites they can offer a significant saving over revetment breakwaters.
An additional rubble mound is sometimes placed in front of the vertical structure in order to absorb wave energy and thus reduce wave reflection and horizontal wave pressure on the vertical wall. Such a design provides additional protection on the sea side and a quay wall on the inner side of the breakwater, but it can enhance wave overtopping.
Wave absorbing caisson
A similar but more sophisticated concept is a wave-absorbing caisson, including various types of perforation in the front wall.
Such structures have been used successfully in the offshore oil-industry, but also on coastal projects requiring rather low-crested structures (e.g. on an urban promenade where the sea view is an important aspect, as seen in Beirut and Monaco). In the latter, a project is presently ongoing at the Anse du Portier including 18 wave-absorbing 27 m (89 ft) high caissons.
Wave attenuator
Wave attenuators consist of concrete elements placed horizontally one foot under the free surface, positioned along a line parallel to the coast. Wave attenuators have four slabs facing the sea, one vertical slab, and two slabs facing the land; each slab is separated from the next by a space of 200 millimetres (7.9 in). The row of four sea-facing and two land-facing slabs reflects offshore wave by the action of the volume of water located under it which, made to oscillate under the effect of the incident wave, creates waves in phase opposition to the incident wave downstream from the slabs.[jargon]
Membrane Breakwaters
A submerged flexible mound breakwater can be employed for wave control in shallow water as an advanced alternative to the conventional rigid submerged designs. Further to the fact that, the construction cost of the submerged flexible mound breakwaters is less than that of the conventional submerged breakwaters, ships and marine organisms can pass them, if being deep enough. These marine structures reduce the collided wave energy and prevent the generation of standing waves.
Breakwater armour units
As design wave heights get larger, rubble mound breakwaters require larger armour units to resist the wave forces. These armour units can be formed of concrete or natural rock. The largest standard grading for rock armour units given in CIRIA 683 "The Rock Manual" is 10–15 tonnes. Larger gradings may be available, but the ultimate size is limited in practice by the natural fracture properties of locally available rock.
Shaped concrete armour units (such as Dolos, Xbloc, Tetrapod, etc.) can be provided in up to approximately 40 tonnes (e.g. Jorf Lasfar, Morocco), before they become vulnerable to damage under self weight, wave impact and thermal cracking of the complex shapes during casting/curing. Where the very largest armour units are required for the most exposed locations in very deep water, armour units are most often formed of concrete cubes, which have been used up to ~195 tonnes Archived 2019-05-12 at the Wayback Machine for the tip of the breakwater at Punta Langosteira near La Coruña, Spain.
Preliminary design of armour unit size is often undertaken using the Hudson's equation, Van der Meer and more recently Van Gent et al.; these methods are all described in CIRIA 683 "The Rock Manual" and the United States Army Corps of Engineers Coastal engineering manual (available for free online) and elsewhere. For detailed design the use of scaled physical hydraulic models remains the most reliable method for predicting real-life behavior of these complex structures.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2374 2024-12-11 17:39:16
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,148
Re: Miscellany
2274) Scholarship
Summary
College is awesome. It’s the ultimate chance to grow, learn, explore, and become the you that you’ll be in your adult life. And for a good number of us, what you’ll be is in debt, at least for a while.
Yep, college is expensive—right up there with buying a car, and sometimes even buying a house. You’ll probably need more than one strategy to cover the cost of your college education.
Before you submit to a big pile of student loans, consider other ways to get money, including applying for scholarships. There are several different types of scholarships available to help pay for college, and you might be surprised at how the money can add up and reduce your future debt burden.
Key Points
* Scholarships represent money you don’t have to pay back.
* Generally, scholarships are tax free when used to cover educational expenses.
* Getting scholarships can reduce your need for student loan debt.
What are college scholarships?
A scholarship is money you receive to help cover the cost of attending college. Unlike loans, which must be repaid, college scholarships aren’t repaid. Here are some of the main types of scholarships for college:
* Need-based scholarships. These types of scholarships consider your financial need. If you come from a lower-income background, you might be eligible for scholarships from your high school, your college, your state, or an organization.
* Merit-based scholarships. Rather than looking at your family’s financial situation, merit scholarships review your accomplishments. These include academic, athletic, performing arts, and leadership scholarships.
* Essays and contests. You can apply for some scholarships by writing an essay or entering a contest. You might be chosen on a random basis or after a panel selects your essay.
How to get a college scholarship
In general, you must apply for college scholarships. Many scholarship applications also require an essay and a resume. Think about your experiences and write a general essay that covers the following points, as applicable:
* What obstacles or adversities have you overcome?
* When have you demonstrated outstanding performance or work ethic?
* What experiences have shaped you?
* What do you hope to accomplish after you finish school?
Once you start applying, you can use this basic essay and customize it for each scholarship. Having a general essay written in advance can cut down on the time it takes to apply for college scholarships.
You might also need to provide documentation for different aspects of your schooling or financial situation. For example, a need-based scholarship usually requires some proof that your family requires help sending you to school. On the other hand, if you’re applying for merit-based scholarships based on your academic performance, you’ll need to provide a transcript of your grades.
Fill out the FAFSA
Start by filling out the Free Application for Federal Student Aid (FAFSA). The FAFSA isn’t just used by the federal government to determine whether you’re eligible for grants; it’s also often used by schools and states to decide whether you should get need-based scholarships.
In addition to the FAFSA, you might need to fill out some scholarship forms. Check with your school’s financial aid office to find out the process.
Fill out your CSS Profile
Many schools and other programs use the CSS Profile, which is managed by the College Board. The CSS Profile is often used for merit-based scholarships, so you’re more likely to get access to various academic, performance, athletic, and other merit scholarships when you fill out this profile. You can also get matched with scholarships based on your profile.
Use scholarship websites
Many websites allow you to search for scholarships. Some of the most reputable include:
Fastweb.com
Scholarships.com
GoingMerry.com
ScholarshipOwl.com
Some of these scholarship websites are free, while others charge a small fee. Depending on your situation, it might be worth paying a fee to access additional scholarships, but start with the free searches.
You’re also likely to see essays and contests on these websites. Not every opportunity is worth applying for (more on this below), so be careful about how much time and energy you invest. You may also find results for obscure or specialty scholarships, such as for people more than six feet tall or who have a certain heritage.
Check local organizations
Don’t forget to check with local nonprofits and other organizations. Service clubs like Kiwanis and Rotary often offer small scholarships. Check with local banks and even stores—some national chains allow local stores to provide educational awards to students from local high schools. Many high schools keep (and often administer) a database of local scholarships.
Finally, check with local and state governments. There might be a special mayoral scholarship fund. Some states offer stipends for students who attend community colleges. You might be surprised at how many small scholarships can add up.
Are scholarships taxable?
Generally, scholarship money isn’t taxable. As long as the money is used for approved educational expenses, including tuition, fees, books, and needed supplies, you likely won’t need to pay taxes on scholarship money you receive.
Sometimes excess scholarship money is taxed—that is, any money that’s over and above your total costs for college—but most students won’t owe taxes on scholarship money.
Is it worth applying for college scholarships?
Whether it’s worth applying for scholarships for college depends on the situation and how much time you spend. Lesser-known and easier-to-get scholarships might have smaller award amounts, but if you can standardize the process by repurposing your general essay, for example, perhaps you can obtain several scholarships—and that money will add up.
On the other hand, some of the most lucrative scholarships are so hard to get that, even if you’re the perfect candidate on paper, you might still have only a tiny chance of landing them. Is it still worth a try? Maybe—or maybe not. The decision is yours. Just remember that time is money.
The bottom line
Scholarships can help you reduce your overall need for student loans. Setting up an assembly line and applying for a few well-chosen scholarships—that you have a reasonable chance of getting—can be part of a well-executed college funding strategy.
Any scholarship dollar you obtain is a dollar you don’t have to borrow and pay back later—with interest—when you’re trying to build your post-college life.
Details
A scholarship is a form of financial aid awarded to students for further education. Generally, scholarships are awarded based on a set of criteria such as academic merit, diversity and inclusion, athletic skill, and financial need, research experience or specific professional experience.
Scholarship criteria usually reflect the values and goals of the donor of the award, and while scholarship recipients are not required to repay scholarships, the awards may require that the recipient continue to meet certain requirements during their period of support, such as maintaining a minimum grade point average or engaging in a certain activity (e.g., playing on a school sports team for athletic scholarship holders).
Scholarships also range in generosity; some cover partial tuition, while others offer a 'full-ride', covering all tuition, accommodation, housing and others.
Some prestigious, highly competitive scholarships are well-known even outside the academic community, such as Fulbright Scholarship and the Rhodes Scholarships at the graduate level, and the Robertson, Morehead-Cain and Jefferson Scholarships at the undergraduate level.
Scholarships vs. grants
While the terms scholarship and grant are frequently used interchangeably, they are distinctly different. Where grants are offered based exclusively on financial need, scholarships may have a financial need component but rely on other criteria as well.
* Academic scholarships typically use a minimum grade-point average or standardized test score such as the ACT or SAT to narrow down awardees.
* Athletic scholarships are generally based on athletic performance of a student and used as a tool to recruit high-performing athletes for their school's athletic teams.
* Merit scholarships can be based on a number of criteria, including performance in a particular school subject or club participation or community service.
A federal Pell Grant can be awarded to someone planning to receive their undergraduate degree and is solely based on their financial needs.
Types
The most common scholarships may be classified as:
* Merit-based: These awards are based on a student's academic, artistic, athletic, or other abilities, and often a factor in an applicant's extracurricular activities and community service record. Most such merit-based scholarships are paid directly by the institution the student attends, rather than issued directly to the student.
* Need-based: Some private need-based awards are confusingly called scholarships, and require the results of a FAFSA (the family's expected family contribution). However, scholarships are often merit-based, while grants tend to be need-based.
* Student-specific: These are scholarships for which applicants must initially qualify based upon gender, race, religion, family, and medical history, or many other student-specific factors.
* Career-specific: These are scholarships a college or university awards to students who plan to pursue a specific field of study. Often, the most generous awards go to students who pursue careers in high-need areas, such as education or nursing. Many schools in the United States give future nurses full scholarships to enter the field, especially if the student intends to work in a high-need community.
* College-specific: College-specific scholarships are offered by individual colleges and universities to highly qualified applicants. These scholarships are given on the basis of academic and personal achievement. Some scholarships have a "bond" requirement. Recipients may be required to work for a particular employer for a specified period of time or to work in rural or remote areas; otherwise, they may be required to repay the value of the support they received from the scholarship. This is particularly the case with education and nursing scholarships for people prepared to work in rural and remote areas. The programs offered by the uniformed services of the United States (Army, Navy, Marine Corps, Air Force, Coast Guard, National Oceanic and Atmospheric Administration Commissioned Officer Corps, and Public Health Service Commissioned Corps) sometimes resemble such scholarships.
* Athletic: Awarded to students with exceptional skill in a sport. Often this is so that the student will be available to attend the school or college and play the sport on their team, although in some countries government funded sports scholarships are available, allowing scholarship holders to train for international representation. School-based athletics scholarships can be controversial, as some believe that awarding scholarship money for athletic rather than academic or intellectual purposes is not in the institution's best interest.
* Brand: These scholarships are sponsored by a corporation that is trying to gain attention to their brand, or a cause. Sometimes these scholarships are referred to as branded scholarships. The Miss America beauty pageant is a famous example of a brand scholarship.
* Creative contest: These scholarships are awarded to students based on a creative submission. Contest scholarships are also called mini project-based scholarships, where students can submit entries based on unique and innovative ideas.
* "Last dollar": can be provided by private and government-based institutions, and are intended to cover the remaining fees charged to a student after the various grants are taken into account. To prohibit institutions from taking last dollar scholarships into account, and thereby removing other sources of funding, these scholarships are not offered until after financial aid has been offered in the form of a letter. Furthermore, last dollar scholarships may require families to have filed taxes for the most recent year, received their other sources of financial aid, and not yet received loans.
* Open: a scholarship open to any applicant.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2375 2024-12-12 16:42:46
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 53,148
Re: Miscellany
2275) Rheumatology
Gist
Rheumatology (from Greek 'flowing current') is a branch of medicine devoted to the diagnosis and management of disorders whose common feature is inflammation in the bones, muscles, joints, and internal organs.
Rheumatism is any of several disorders that have in common inflammation of the connective tissues, especially the muscles, joints, and associated structures. The most common symptoms are pain and stiffness.
What Are Rheumatic Diseases? Rheumatic diseases affect your joints tendons, ligaments, bones, and muscles. They include many types of arthritis, a term used for conditions that affect your joints. Sometimes, they're called musculoskeletal diseases.
Summary
Rheumatism is any of several disorders that have in common inflammation of the connective tissues, especially the muscles, joints, and associated structures. The most common symptoms are pain and stiffness. Specific diseases that are alternatively called rheumatism include rheumatoid arthritis; rheumatic fever; septic arthritis that accompanies such diseases as gonorrhea, tuberculosis, or mycotic diseases (caused by fungus); and osteoarthritis.
Near the beginning of the 2nd century ce, Greek physician, writer, and philosopher Galen of Pergamum appears to have coined the term rheumatismos, in which rheuma means “to flow” (an alternative meaning is “phlegm”). A common usage was a “defluxion of rheum” from the nose or mouth. Galen knew that respiratory diseases causing the production of phlegm often resulted in patients developing painful maladies, such as the conditions now described as arthritis and neuropathy. Parisian physician Guillaume de Baillou reintroduced the word rheumatismos to medicine in the 17th century, in a work that was published posthumously (Liber de Rheumatismo et Pleuritide Dorsali; 1642). He used the word to describe a form of muscular rheumatism and to describe what is now known as rheumatic fever. Baillou knew that a respiratory disease called catarrh, which is associated with inflammation of the upper respiratory tract, was connected to rheumatism and that rheumatism was systemic in nature, affecting many parts of the body. The rheumatic maladies as described by Galen and Baillou were later associated with Streptococcus infections.
In the third volume of the first edition of Encyclopædia Britannica; or, A Dictionary of Arts and Sciences, published between 1768 and 1771, the entry on medicine contains a paragraph “of the rheumatism,” in which the authors provide an account of acute rheumatic fever. The description of the condition is similar to that provided in 1666 by British physician Thomas Sydenham in his work Methodus Curandi Febres, Propriis Observationibus Superstructura. In the encyclopaedic discussion, the primary lesion of rheumatic fever and rheumatism is noted: “Its proximate cause seems to be inflammation of the lymphatic arteries.” This determination by early pathophysiologists depicted rheumatic vasculitis, an autoimmunological inflammation of the arterial system. Later in the text, chronic rheumatism is mentioned as “either the remains of a rheumatic fever, or a continuation of pains that proceeded at first from lesser but neglected colds.”
Details
Rheumatology is a branch of medicine devoted to the diagnosis and management of disorders whose common feature is inflammation in the bones, muscles, joints, and internal organs. Rheumatology covers more than 100 different complex diseases, collectively known as rheumatic diseases, which includes many forms of arthritis as well as lupus and Sjögren's syndrome. Doctors who have undergone formal training in rheumatology are called rheumatologists.
Many of these diseases are now known to be disorders of the immune system, and rheumatology has significant overlap with immunology, the branch of medicine that studies the immune system.
A rheumatologist is a physician who specializes in the field of medical sub-specialty called rheumatology. A rheumatologist holds a board certification after specialized training. In the United States, training in this field requires four years undergraduate school, four years of medical school, and then three years of residency, followed by two or three years additional Fellowship training. The requirements may vary in other countries. Rheumatologists are internists who are qualified by additional postgraduate training and experience in the diagnosis and treatment of arthritis and other diseases of the joints, muscles and bones. Many rheumatologists also conduct research to determine the cause and better treatments for these disabling and sometimes fatal diseases. Treatment modalities are based on scientific research, currently, practice of rheumatology is largely evidence based.
Rheumatologists treat arthritis, autoimmune diseases, pain disorders affecting joints, and osteoporosis. There are more than 200 types of these diseases, including rheumatoid arthritis, osteoarthritis, gout, lupus, back pain, osteoporosis, and tendinitis. Some of these are very serious diseases that can be difficult to diagnose and treat. They treat soft tissue problems related to the musculoskeletal system, and sports related soft tissue disorders.
Pediatrics rheumatologist: A pediatric rheumatologist is a pediatrician who has specialized in the treatment of children with rheumatic disease. Both specialties are important to address a child's milestone development and disease treatment throughout childhood. However, recognition of this sub-specialty has been slow, which has resulted in a global shortage of pediatric rheumatologists, and as a consequence, the demand for healthcare support far exceeds current service capacities. Raising awareness of this is important to attract more upcoming pediatricians into this rewarding area of healthcare.
Treatment
Most rheumatic diseases are treated with analgesics, NSAIDs (nonsteroidal anti-inflammatory drug), steroids (in serious cases), DMARDs (disease-modifying antirheumatic drugs), monoclonal antibodies, such as infliximab and adalimumab, the TNF inhibitor etanercept, and methotrexate for moderate to severe rheumatoid arthritis. The biologic agent rituximab (anti-B cell therapy) is now licensed for use in refractory rheumatoid arthritis. Physiotherapy is vital in the treatment of many rheumatological disorders. Occupational therapy can help patients find alternative ways for common movements that would otherwise be restricted by their disease. Patients with rheumatoid arthritis often need a long term, coordinated and a multidisciplinary team approach towards management of individual patients. Treatment is often tailored according to the individual needs of each patient which is also dependent on the response and the tolerability of medications.
Beginning in the 2000s, the incorporation of biopharmaceuticals (which include inhibitors of TNF-alpha, certain interleukins, and the JAK-STAT signaling pathway) into standards of care is one of the paramount developments in modern rheumatology.
Rheumasurgery
Rheumasurgery (or rheumatoid surgery) is a subfield of orthopedics occupied with the surgical treatment of patients with rheumatic diseases. The purpose of the interventions is to limit disease activity, soothe pain and improve function.
Rheumasurgical interventions can be divided in two groups. The one is early synovectomies, that is the removal of the inflamed synovia in order to prevent spreading and stop destruction. The other group is the so-called corrective intervention, i.e. an intervention done after destruction has taken place. Among the corrective interventions are joint replacements, removal of loose bone or cartilage fragments, and a variety of interventions aimed at repositioning and/or stabilizing joints, such as arthrodesis.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline

