Math Is Fun Forum
You are not logged in.
- Topics: Active | Unanswered
- Index
- » Science HQ
- » Iridium
Pages: 1
#1 2025-04-09 17:01:18
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,628
Iridium
Iridium
Gist
Iridium (Ir) is a hard, brittle, silvery-white, and highly corrosion-resistant transition metal belonging to the platinum group, with atomic number 77 and a high melting point. It's known for its use in alloys, catalysts, and as a component in spark plugs and other applications requiring high durability.
Iridium, a highly corrosion-resistant and heat-tolerant metal, is primarily used in alloys, particularly with platinum, for applications demanding durability and high-temperature resistance, including spark plugs, crucibles, and medical devices.
Summary
Iridium (Ir), is a chemical element, one of the platinum metals of Groups 8–10 (VIIIb), Periods 5 and 6, of the periodic table. It is very dense and rare and is used in platinum alloys. A precious, silver-white metal, iridium is hard and brittle, but it becomes ductile and can be worked at a white heat, from 1,200° to 1,500° C (2,200° to 2,700° F). It is one of the densest terrestrial substances. In the massive state the metal is practically insoluble in acids and is not attacked even by aqua regia. It can be dissolved in concentrated hydrochloric acid in the presence of sodium perchlorate at 125° to 150° C (257° to 302° F).
Because of difficulties in preparation and fabrication, the pure metal has few applications. Iridium is chiefly used in the form of platinum alloys. Platinum-iridium alloys (5 to 10 percent iridium) are readily workable metals that are much harder and stiffer and more resistant to chemical attack than the soft pure platinum. Such alloys are used for jewelry, pen points, surgical pins and pivots, and electrical contacts and sparking points. The international prototype standard kilogram of mass is made from an alloy containing 90 percent platinum and 10 percent iridium.
Pure iridium probably does not occur in nature; its abundance in the Earth’s crust is very low, about 0.001 parts per million. Though rare, iridium does occur in natural alloys with other noble metals: in iridosmine up to 77 percent iridium, in platiniridium up to 77 percent, in aurosmiridium 52 percent, and in native platinum up to 7.5 percent. Iridium generally is produced commercially along with the other platinum metals as a by-product of nickel or copper production.
Iridium-containing ores are found in South Africa and Alaska, U.S., as well as in Myanmar (Burma), Brazil, Russia, and Australia. In the late 20th century South Africa was the world’s major producer of iridium.
The element was discovered in 1803 in the acid-insoluble residues of platinum ores by the English chemist Smithson Tennant; the French chemists H.-V. Collet-Descotils, A.-F. Fourcroy, and N.-L. Vauquelin identified it at about the same time. The name iridium, derived from the Greek word iris (“rainbow”), refers to the various colours of its compounds. Natural iridium consists of a mixture of two stable isotopes, iridium-191 (37.3 percent) and iridium-193 (62.7 percent).
Element Properties:
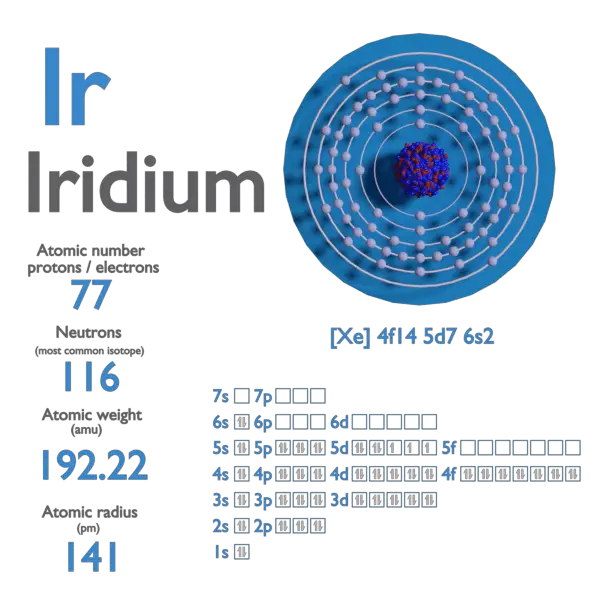
atomic number : 77
atomic weight : 192.2
melting point : 2,410° C (4,370° F)
boiling point : 4,527° C (8,181° F)
specific gravity : 22.4 (20° C).
Details
Iridium is a chemical element; it has the symbol Ir and atomic number 77. This very hard, brittle, silvery-white transition metal of the platinum group, is considered the second-densest naturally occurring metal (after osmium) with a density of 22.56 g/{cm}^{3} (0.815 lb/cu in) as defined by experimental X-ray crystallography. 191Ir and 193Ir are the only two naturally occurring isotopes of iridium, as well as the only stable isotopes; the latter is the more abundant. It is one of the most corrosion-resistant metals, even at temperatures as high as 2,000 °C (3,630 °F).
Iridium was discovered in 1803 in the acid-insoluble residues of platinum ores by the English chemist Smithson Tennant. The name iridium, derived from the Greek word iris (rainbow), refers to the various colors of its compounds. Iridium is one of the rarest elements in Earth's crust, with an estimated annual production of only 6,800 kilograms (15,000 lb) in 2023.
The dominant uses of iridium are the metal itself and its alloys, as in high-performance spark plugs, crucibles for recrystallization of semiconductors at high temperatures, and electrodes for the production of chlorine in the chloralkali process. Important compounds of iridium are chlorides and iodides in industrial catalysis. Iridium is a component of some OLEDs.
(An organic light-emitting diode (OLED), also known as organic electroluminescent (organic EL) diode, is a type of light-emitting diode (LED) in which the emissive electroluminescent layer is an organic compound film that emits light in response to an electric current. This organic layer is situated between two electrodes; typically, at least one of these electrodes is transparent. OLEDs are used to create digital displays in devices such as television screens, computer monitors, and portable systems such as smartphones and handheld game consoles. A major area of research is the development of white OLED devices for use in solid-state lighting applications.)
Characteristics:
Physical properties
A member of the platinum group metals, iridium is white, resembling platinum, but with a slight yellowish cast. Because of its hardness, brittleness, and very high melting point, solid iridium is difficult to machine, form, or work; thus powder metallurgy is commonly employed instead. It is the only metal to maintain good mechanical properties in air at temperatures above 1,600 °C (2,910 °F). It has the 10th highest boiling point among all elements and becomes a superconductor at temperatures below 0.14 K (−273.010 °C; −459.418 °F).
Iridium's modulus of elasticity is the second-highest among the metals, being surpassed only by osmium. This, together with a high shear modulus and a very low figure for Poisson's ratio (the relationship of longitudinal to lateral strain), indicate the high degree of stiffness and resistance to deformation that have rendered its fabrication into useful components a matter of great difficulty. Despite these limitations and iridium's high cost, a number of applications have developed where mechanical strength is an essential factor in some of the extremely severe conditions encountered in modern technology.
The measured density of iridium is only slightly lower (by about 0.12%) than that of osmium, the densest metal known. Some ambiguity occurred regarding which of the two elements was denser, due to the small size of the difference in density and difficulties in measuring it accurately, but, with increased accuracy in factors used for calculating density, X-ray crystallographic data yielded densities of 22.56 g/{cm}^{3} (0.815 lb/cu in) for iridium and 22.59 g/{cm}^{3} (0.816 lb/cu in) for osmium.
Iridium is extremely brittle, to the point of being hard to weld because the heat-affected zone cracks, but it can be made more ductile by addition of small quantities of titanium and zirconium (0.2% of each apparently works well).
The Vickers hardness of pure platinum is 56 HV, whereas platinum with 50% of iridium can reach over 500 HV.
Chemical properties
Iridium is the most corrosion-resistant metal known. It is not attacked by acids, including aqua regia, but it can be dissolved in concentrated hydrochloric acid in the presence of sodium perchlorate. In the presence of oxygen, it reacts with cyanide salts. Traditional oxidants also react, including the halogens and oxygen at higher temperatures. Iridium also reacts directly with sulfur at atmospheric pressure to yield iridium disulfide.
Iridium is found in meteorites in much higher abundance than in the Earth's crust. For this reason, the unusually high abundance of iridium in the clay layer at the Cretaceous–Paleogene boundary gave rise to the Alvarez hypothesis that the impact of a massive extraterrestrial object caused the extinction of non-avian dinosaurs and many other species 66 million years ago, now known to be produced by the impact that formed the Chicxulub crater. Similarly, an iridium anomaly in core samples from the Pacific Ocean suggested the Eltanin impact of about 2.5 million years ago.
Additional Information:
Iridium salts are highly coloured. The iridescent wings of the dragonfly represent both the origin of the element’s name and its strongly coloured salts.
Appearance
Iridium is a hard, silvery metal. It is almost as unreactive as gold. It has a very high density and melting point.
Uses
Iridium is the most corrosion-resistant material known. It is used in special alloys and forms an alloy with osmium, which is used for pen tips and compass bearings. It was used in making the standard metre bar, which is an alloy of 90% platinum and 10% iridium. It is also used for the contacts in spark plugs because of its high melting point and low reactivity.
Biological role
Iridium has no known biological role, and has low toxicity.
Natural abundance
Iridium is one of the rarest elements on Earth. It is found uncombined in nature in sediments that were deposited by rivers. It is commercially recovered as a by-product of nickel refining.
A very thin layer of iridium exists in the Earth’s crust. It is thought that this was caused by a large meteor or asteroid hitting the Earth. Meteors and asteroids contain higher levels of iridium than the Earth’s crust. The impact would have caused a huge dust cloud depositing the iridium all over the world. Some scientists think that this could be the same meteor or asteroid impact that wiped out the dinosaurs.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
Pages: 1
- Index
- » Science HQ
- » Iridium