Math Is Fun Forum
You are not logged in.
- Topics: Active | Unanswered
#2501 2025-03-30 23:26:43
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,708
Re: Miscellany
2401) Metalloid
Gist
What are Metalloids? Metalloids can be defined as chemical elements whose physical and chemical properties fall in between the metal and non-metal categories. Boron, germanium, silicon, antimony, As, tellurium and pollanium are the seven most widely recognized metalloids.
Summary
A metalloid, in chemistry, an imprecise term used to describe a chemical element that forms a simple substance having properties intermediate between those of a typical metal and a typical nonmetal. The term is normally applied to a group of between six and nine elements (boron, silicon, germanium, As, antimony, tellurium, and possibly bismuth, polonium, astatine) found near the center of the P-block or main block of the periodic table. There is no single property which can be used to unambiguously identify an element as a metalloid. Since most metalloids tend to display semiconducting properties in at least one of their allomorphic modifications, the class might reasonably be extended to also include gray silicon (which, unlike white silicon, is a semiconductor rather than a metal) and the graphite form of carbon (which, unlike the diamond form, is a semimetal rather than an insulator). Chemically, metalloids correspond to atoms having intermediate electronegativities and an ability to display a range of both positive and negative oxidation states in their compounds.
Details
A metalloid is a chemical element which has a preponderance of properties in between, or that are a mixture of, those of metals and nonmetals. The word metalloid comes from the Latin metallum ("metal") and the Greek oeides ("resembling in form or appearance"). There is no standard definition of a metalloid and no complete agreement on which elements are metalloids. Despite the lack of specificity, the term remains in use in the literature.
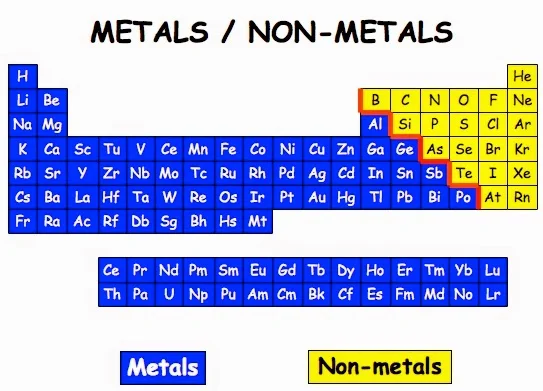
The six commonly recognised metalloids are boron, silicon, germanium, As, antimony and tellurium. Five elements are less frequently so classified: carbon, aluminium, selenium, polonium and astatine. On a standard periodic table, all eleven elements are in a diagonal region of the p-block extending from boron at the upper left to astatine at lower right. Some periodic tables include a dividing line between metals and nonmetals, and the metalloids may be found close to this line.
Typical metalloids have a metallic appearance, may be brittle and are only fair conductors of electricity. They can form alloys with metals, and many of their other physical properties and chemical properties are intermediate between those of metallic and nonmetallic elements. They and their compounds are used in alloys, biological agents, catalysts, flame retardants, glasses, optical storage and optoelectronics, pyrotechnics, semiconductors, and electronics.
The term metalloid originally referred to nonmetals. Its more recent meaning, as a category of elements with intermediate or hybrid properties, became widespread in 1940–1960. Metalloids are sometimes called semimetals, a practice that has been discouraged,[2] as the term semimetal has a more common usage as a specific kind of electronic band structure of a substance. In this context, only As and antimony are semimetals, and commonly recognised as metalloids.
Definitions:
Judgment-based
A metalloid is an element that possesses a preponderance of properties in between, or that are a mixture of, those of metals and nonmetals, and which is therefore hard to classify as either a metal or a nonmetal. This is a generic definition that draws on metalloid attributes consistently cited in the literature. Difficulty of categorisation is a key attribute. Most elements have a mixture of metallic and nonmetallic properties, and can be classified according to which set of properties is more pronounced. Only the elements at or near the margins, lacking a sufficiently clear preponderance of either metallic or nonmetallic properties, are classified as metalloids.
Boron, silicon, germanium, As, antimony, and tellurium are commonly recognised as metalloids. Depending on the author, one or more from selenium, polonium, or astatine are sometimes added to the list. Boron sometimes is excluded, by itself, or with silicon. Sometimes tellurium is not regarded as a metalloid. The inclusion of antimony, polonium, and astatine as metalloids has been questioned.
Other elements are occasionally classified as metalloids. These elements include hydrogen, beryllium, nitrogen, phosphorus, sulfur, zinc, gallium, tin, iodine, lead, bismuth, and radon. The term metalloid has also been used for elements that exhibit metallic lustre and electrical conductivity, and that are amphoteric, such as As, antimony, vanadium, chromium, molybdenum, tungsten, tin, lead, and aluminium. The p-block metals,[33] and nonmetals (such as carbon or nitrogen) that can form alloys with metals or modify their properties have also occasionally been considered as metalloids.
Criteria-based
The elements commonly recognised as metalloids, and their ionization energies (IE); electronegativities (EN, revised Pauling scale); and electronic band structures (most thermodynamically stable forms under ambient conditions).
No widely accepted definition of a metalloid exists, nor any division of the periodic table into metals, metalloids, and nonmetals; Hawkes questioned the feasibility of establishing a specific definition, noting that anomalies can be found in several attempted constructs. Classifying an element as a metalloid has been described by Sharp[40] as "arbitrary".
The number and identities of metalloids depend on what classification criteria are used. Emsley recognised four metalloids (germanium, As, antimony, and tellurium); James et al. listed twelve (Emsley's plus boron, carbon, silicon, selenium, bismuth, polonium, moscovium, and livermorium). On average, seven elements are included in such lists; individual classification arrangements tend to share common ground and vary in the ill-defined margins.
A single quantitative criterion such as electronegativity is commonly used,[46] metalloids having electronegativity values from 1.8 or 1.9 to 2.2. Further examples include packing efficiency (the fraction of volume in a crystal structure occupied by atoms) and the Goldhammer–Herzfeld criterion ratio. The commonly recognised metalloids have packing efficiencies of between 34% and 41%. The Goldhammer–Herzfeld ratio, roughly equal to the cube of the atomic radius divided by the molar volume, is a simple measure of how metallic an element is, the recognised metalloids having ratios from around 0.85 to 1.1 and averaging 1.0. Other authors have relied on, for example, atomic conductance or bulk coordination number.
Jones, writing on the role of classification in science, observed that "[classes] are usually defined by more than two attributes". Masterton and Slowinski used three criteria to describe the six elements commonly recognised as metalloids: metalloids have ionization energies around 200 kcal/mol (837 kJ/mol) and electronegativity values close to 2.0. They also said that metalloids are typically semiconductors, though antimony and As (semimetals from a physics perspective) have electrical conductivities approaching those of metals. Selenium and polonium are suspected as not in this scheme, while astatine's status is uncertain.
In this context, Vernon proposed that a metalloid is a chemical element that, in its standard state, has (a) the electronic band structure of a semiconductor or a semimetal; and (b) an intermediate first ionization potential "(say 750−1,000 kJ/mol)"; and (c) an intermediate electronegativity (1.9–2.2).
Additional Information
The four major properties of metalloids are as follows:
- They are solids
- They have a metallic luster
- They are brittle
- They are semiconductors
What types of properties do metalloids display?
Metalloid element properties include a mixture of properties of both metals and nonmetals. While some characteristics (such as their metallic luster) are similar to metals, others (such as their brittleness) are similar to nonmetals.
Where are the metalloids on the periodic table?
The metalloids are located along a slanted line between the metal elements and nonmetal elements of the periodic table. They span from Group 13 to Group 16, 17, or 18 based on what criteria of classifying metalloid elements is being used.
How many metalloids are on the periodic table?
There are six elements generally accepted to be metalloids. However, based on the classification criteria being used, the exact number may vary, ranging from six to nine elements.
To summarize:
Metalloid is derived from the Latin metallum (“metal”) and the Greek oeides (“resembling in form or appearance”). A metalloid represents a chemical element exhibiting properties that are intermediate between those of metals and nonmetals. Or we can say they are a mixture of metals and nonmetals. Elements classified as metalloids are frequently highlighted in what is known as the “Metalloid Stair Step” because when colored differently from the other elements, this group of elements resembles a staircase.
The six most often recognized examples of metalloids cover boron, silicon, germanium, As, antiimony, and tellurium. And there are five elements, such as carbon, aluminum, selenium, polonium, and astatine are seldom categorized into metalloids. All eleven elements can be found on the standard periodic table. They are located in a diagonal region of the p-block ranging from boron at the upper left to astatine at the lower right. On some periodic tables, metalloids can be found near the dividing line between metals and nonmetals.
Metalloids have a metallic look, yet they are brittle and only electrical conductors with a level of intermediate to good. Chemically, they mainly act like nonmetals. They can combine with metals to make alloys. The majority of their other chemical and physical properties tend to be intermediate. Metalloids are often too brittle to be used in structural applications. However, metalloids and their compounds are always employed in alloys, biological agents, catalysts, flame retardants, glasses, optical storage, optoelectronics, pyrotechnics, semiconductors, and electronics.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2502 2025-04-01 00:02:15
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,708
Re: Miscellany
2402) Nonmetal
Gist
The 17 nonmetal elements are: hydrogen, helium, carbon, nitrogen, oxygen, fluorine, neon, phosphorus, sulfur, chlorine, argon, selenium, bromine, krypton, iodine, xenon, and radon.
Summary:
What is an example of a nonmetal element?
An example of a nonmetal element is helium. Helium is a noble gas which possesses very nonmetallic characteristics such as high electronegativity and high ionization energy. However, helium is exceptionally nonreactive and is not found in compounds like most metals are found in. Helium is also a gas at room temperature.
What is a nonmetal definition?
The definition of nonmetals is a classification of elements that possess particular chemical and physical properties such as the following:
* High electronegativity.
* High ionization energy.
* Poor conductor of electricity and heat.
* Relatively low boiling point.
* Matte, nonmetallic appearance, and usually brittle as a solid.
What are the nonmetals on the periodic table?
Nonmetals are typically found toward the top right of the periodic table of elements. This excludes hydrogen, which is all the way in the top left of the periodic table. Nonmetals exhibit nonmetallic characteristics and are poor conductors of heat and electricity, and typically have high ionization energy and electronegativity. Nonmetals include the following elements:
Hydrogen
Helium
Boron
Carbon
Nitrogen
Oxygen
Fluorine
Neon
Silicon
Phosphorous
Sulfur
Chlorine
Argon
Germanium
As
Selenium
Bromine
Krypton
Antimony
Tellurium
Iodine
Xenon
Radon.
Details
In the context of the periodic table a nonmetal is a chemical element that mostly lacks distinctive metallic properties. They range from colorless gases like hydrogen to shiny crystals like iodine. Physically, they are usually lighter (less dense) than elements that form metals and are often poor conductors of heat and electricity. Chemically, nonmetals have relatively high electronegativity or usually attract electrons in a chemical bond with another element, and their oxides tend to be acidic.
Seventeen elements are widely recognized as nonmetals. Additionally, some or all of six borderline elements (metalloids) are sometimes counted as nonmetals.
The two lightest nonmetals, hydrogen and helium, together make up about 98% of the mass of the observable universe. Five nonmetallic elements—hydrogen, carbon, nitrogen, oxygen, and silicon—make up the bulk of Earth's atmosphere, biosphere, crust and oceans.
Industrial uses of nonmetals include in electronics, energy storage, agriculture, and chemical production.
Most nonmetallic elements were identified in the 18th and 19th centuries. While a distinction between metals and other minerals had existed since antiquity, a basic classification of chemical elements as metallic or nonmetallic emerged only in the late 18th century. Since then about twenty properties have been suggested as criteria for distinguishing nonmetals from metals.
Definition and applicable elements
Nonmetallic chemical elements are often described as lacking properties common to metals, namely shininess, pliability, good thermal and electrical conductivity, and a general capacity to form basic oxides. There is no widely accepted precise definition; any list of nonmetals is open to debate and revision. The elements included depend on the properties regarded as most representative of nonmetallic or metallic character.
Fourteen elements are almost always recognized as nonmetals:
Hydrogen
Nitrogen
Oxygen
Sulfur
Fluorine
Chlorine
Bromine
Iodine
Helium
Neon
Argon
Krypton
Xenon
Radon
Three more are commonly classed as nonmetals, but some sources list them as "metalloids", a term which refers to elements regarded as intermediate between metals and nonmetals:
Carbon
Phosphorus
Selenium
One or more of the six elements most commonly recognized as metalloids are sometimes instead counted as nonmetals:
Boron
Silicon
Germanium
As
Antimony
Tellurium
About 15–20% of the 118 known elements are thus classified as nonmetals.
Additional Information
A nonmetal, in physics, is a substance having a finite activation energy (band gap) for electron conduction. This means that nonmetals display low (insulators) to moderate (semiconductors) bulk electrical conductivities, which increase with increasing temperature, and are subject to dielectric breakdown at high voltages and temperatures. Like metals, nonmetals may occur in the solid, liquid, or gaseous state. However, unlike metals, nonmetals display a wide range of both mechanical and optical properties, ranging from brittle to plastic and from transparent to opaque.
From a chemical point of view, nonmetals may be divided into two classes: 1) covalent materials, which contain atoms having small sizes, high electronegativities, low valence vacancy to electron ratios, and a pronounced tendency to form negative ions in chemical reactions and negative oxidation states in their compounds; 2) ionic materials, which contain both small and large atoms. Ions may be formed by adding electrons to (small, electronegative atoms) or by extracting electrons from (large, electropositive) atoms. In ionic materials, nonmetals exist either as monatomic anions (e. g., F-in NaF) or as constituents of polyatomic anions (e.g., N and O in the NO3-`s in NaNO3). When in the form of simple elemental substances, about 25 or 22% of the known elements form nonmetals at normal temperatures and pressures, including all of the elements in the S-block of the periodic table and approximately 58% of those in the P-block.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2503 2025-04-02 00:00:44
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,708
Re: Miscellany
2303) Water wheel
Gist
A waterwheel is also called a turbine. Water-powered grist mill in Tennessee. Three types of waterwheels are tha horizontal waterwheel, overshot vertical waterwheel, and undershot vertical waterwheel. In the horizontal waterwheel, water flows from an aqueduct or pipe from the side of the wheel and onto the wheel.
A waterwheel is a mechanical device for tapping the energy of running or falling water by means of a set of paddles mounted around a wheel. The force of the moving water is exerted against the paddles, and the consequent rotation of the wheel is transmitted to machinery via the shaft of the wheel.
Summary
A waterwheel, is a mechanical device for tapping the energy of running or falling water by means of a set of paddles mounted around a wheel. The force of the moving water is exerted against the paddles, and the consequent rotation of the wheel is transmitted to machinery via the shaft of the wheel. The waterwheel was perhaps the earliest source of mechanical energy to replace that of humans and animals, and it was first exploited for such tasks as raising water, fulling cloth, and grinding grain.
A brief treatment of waterwheels follows.
The combination of waterwheel and transmission linkage, often including gearing, was from the Middle Ages usually designated a mill. Of the three distinct types of water mills, the simplest and probably the earliest was a vertical wheel with paddles on which the force of the stream acted. Next was the horizontal wheel used for driving a millstone through a vertical shaft attached directly to the wheel. Third was the geared mill driven by a vertical waterwheel with a horizontal shaft. This required more knowledge and engineering skill than the first two, but it had much greater potential. Vertical waterwheels were also distinguished by the location of water contact with the wheel: first, the undershot wheel; second, the breast wheel; and third, the overshot wheel. These waterwheels generally used the energy of moving streams, but tidal mills also appeared in the 11th century.
Each type of mill had its particular advantages and disadvantages. Relatively little is known of their development before the Middle Ages, but certain of their characteristics suggest an order of appearance within the context of the complexity of construction and the possibilities for utilization.
The simple vertical wheel required little extra structure, but the force and rate of power takeoff were dependent upon stream characteristics and wheel diameter. Since change of power direction was not involved, this wheel proved most useful in raising water, utilizing, for instance, a string of pots worked by a chain drive.
The horizontal-wheel mill (sometimes called a Norse or Greek mill) also required little auxiliary construction, but it was suited for grinding because the upper millstone was fixed upon the vertical shaft. The mill, however, could only be used where the current flow was suitable for grinding.
The geared vertical-wheel mill was more versatile. Construction was relatively simple if the wheel was of the undershot kind, because the wheel paddles could be simply dipped in the stream flow, whether it was river, tide, or man-built millrace. A millwright could choose his gear ratio to match power utilization with rate of stream flow, and the wheel could be mounted in a bridge arch or on a barge anchored in midstream. Vitruvius described the first geared vertical wheel for which we have good evidence. This mill is also of major significance because it was the first application of gearing to utilize other than muscle power. This mill had an undershot wheel and, unlike the breast or overshot wheels, did not make use of the weight of falling water.
Mills with geared breast and overshot wheels required more auxiliary construction, but they allowed the most generalized exploitation of available water power. A major construction problem was locating a mill where the fall of water would be suited to the desired diameter of the wheel. Either a long millrace from upstream or a dam could be used.
Little is known of the details of geared-mill development between the time of Vitruvius and the 12th century. An outstanding installation was the grain mill at Barbegal, near Arles, France, which had 16 cascaded overshot wheels, each 7 feet (2 metres) in diameter, with wooden gearing. It is estimated that this mill could meet the needs of a population of 80,000.
Even though the highly adaptable, geared mill, with its widely diversified stream-flow conditions, was used in the Roman Empire, historical evidence suggests that its most dramatic industrial consequences occurred during the Middle Ages in Western Europe. After the 13th century the overshot waterwheel appears to have become more common than the undershot wheel.
The geared mill of the Middle Ages was actually a general mechanism for the utilization of power. The power from a horse- or cattle-powered mill was small compared to that from overshot water-wheels, which usually generated two to five horsepower.
Details
A water wheel is a machine for converting the kinetic energy of flowing or falling water into useful forms of power, often in a watermill. A water wheel consists of a large wheel (usually constructed from wood or metal), with numerous blades or buckets attached to the outer rim forming the drive mechanism. Water wheels were still in commercial use well into the 20th century, although they are no longer in common use today. Water wheels are used for milling flour in gristmills, grinding wood into pulp for papermaking, hammering wrought iron, machining, ore crushing and pounding fibre for use in the manufacture of cloth.
Some water wheels are fed by water from a mill pond, which is formed when a flowing stream is dammed. A channel for the water flowing to or from a water wheel is called a mill race. The race bringing water from the mill pond to the water wheel is a headrace; the one carrying water after it has left the wheel is commonly referred to as a tailrace.
Waterwheels were used for various purposes from things such as agriculture to metallurgy in ancient civilizations spanning the Hellenistic Greek world, Rome, China and India. Waterwheels saw continued use in the post-classical age, like in medieval Europe and the Islamic Golden Age, but also elsewhere. In the mid- to late 18th century John Smeaton's scientific investigation of the water wheel led to significant increases in efficiency, supplying much-needed power for the Industrial Revolution. [ Water wheels began being displaced by the smaller, less expensive and more efficient turbine, developed by Benoît Fourneyron, beginning with his first model in 1827. Turbines are capable of handling high heads, or elevations, that exceed the capability of practical-sized waterwheels.
The main difficulty of water wheels is their dependence on flowing water, which limits where they can be located. Modern hydroelectric dams can be viewed as the descendants of the water wheel, as they too take advantage of the movement of water downhill.
Types
Water wheels come in two basic designs:
* a horizontal wheel with a vertical axle; or
* a vertical wheel with a horizontal axle.
The latter can be subdivided according to where the water hits the wheel into backshot (pitch-back), overshot, breastshot, undershot, and stream-wheels. The term undershot can refer to any wheel where the water passes under the wheel[9] but it usually implies that the water entry is low on the wheel.
Overshot and backshot water wheels are typically used where the available height difference is more than a couple of meters. Breastshot wheels are more suited to large flows with a moderate head. Undershot and stream wheel use large flows at little or no head.
There is often an associated millpond, a reservoir for storing water and hence energy until it is needed. Larger heads store more gravitational potential energy for the same amount of water so the reservoirs for overshot and backshot wheels tend to be smaller than for breast shot wheels.
Overshot and pitchback water wheels are suitable where there is a small stream with a height difference of more than 2 metres (6.5 ft), often in association with a small reservoir. Breastshot and undershot wheels can be used on rivers or high volume flows with large reservoirs.
Additional Information
Water wheels are found next to areas of moving water such as rivers or canals. They harness the moving water to generate power or electricity; this can be called hydro-power.
There are three different types of water wheel that you could see, this includes:
* overshot
* undershot
* breastshot.
The main difference between the three types is where the water hits the paddles attached to the wheel - either from above, below or the middle.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2504 2025-04-03 00:30:16
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,708
Re: Miscellany
2304) Amalgam
Gist
An amalgam is an alloy, or a mixture, of mercury with one or more other metals, often used in dentistry for fillings and in gold extraction.
Summary
An amalgam is an alloy of mercury and one or more other metals. Amalgams are crystalline in structure, except for those with a high mercury content, which are liquid. Known since early times, they were mentioned by Pliny the Elder in the 1st century ad. In dentistry, an amalgam of silver and tin, with minor amounts of copper and zinc, is used to fill teeth.
A sodium amalgam is formed during the manufacture of chlorine and sodium hydroxide by the electrolysis of brine in cells wherein a stream of mercury constitutes the negative electrode. Reaction of the amalgam with water produces a solution of sodium hydroxide and regenerates the mercury for reuse.
Fine particles of silver and gold can be recovered by agitating their ores with mercury and allowing the resultant pasty or liquid amalgam to settle. By distillation of the amalgam, the mercury is reclaimed, and the precious metals are isolated as a residue.
Amalgams of silver, gold, and palladium are known in nature. Moschellandsbergite, silver amalgam, is found at Moschellandsberg, Ger.; Sala, Swed.; and Isère, France. Gold amalgam occurs in California, U.S., Colombia, and Borneo.
Details
An amalgam is an alloy of mercury with another metal. It may be a liquid, a soft paste or a solid, depending upon the proportion of mercury. These alloys are formed through metallic bonding, with the electrostatic attractive force of the conduction electrons working to bind all the positively charged metal ions together into a crystal lattice structure. Almost all metals can form amalgams with mercury, the notable exceptions being iron, platinum, tungsten, and tantalum. Gold-mercury amalgam is used in the extraction of gold from ore, and dental amalgams are made with metals such as silver, copper, indium, tin and zinc.
Important amalgams:
Zinc amalgam
Zinc amalgam finds use in organic synthesis (e.g., for the Clemmensen reduction). It is the reducing agent in the Jones reductor, used in analytical chemistry. Formerly the zinc plates of dry batteries were amalgamated with a small amount of mercury to prevent deterioration in storage. It is a binary solution (liquid-solid) of mercury and zinc.
Potassium amalgam
For the alkali metals, amalgamation is exothermic, and distinct chemical forms can be identified, such as KHg and KHg2. KHg is a gold-coloured compound with a melting point of 178 °C, and KHg2 a silver-coloured compound with a melting point of 278 °C. These amalgams are very sensitive to air and water, but can be worked with under dry nitrogen. The Hg-Hg distance is around 300 picometres, Hg-K around 358 pm.
Phases K5Hg7 and KHg11 are also known; rubidium, strontium and barium undecamercurides are known and isostructural. Sodium amalgam (NaHg2) has a different structure, with the mercury atoms forming hexagonal layers, and the sodium atoms a linear chain which fits into the holes in the hexagonal layers, but the potassium atom is too large for this structure to work in KHg2.
Sodium amalgam
Sodium amalgam is produced as a byproduct of the chloralkali process and used as an important reducing agent in organic and inorganic chemistry. With water, it decomposes into concentrated sodium hydroxide solution, hydrogen and mercury, which can then return to the chloralkali process anew. If absolutely water-free alcohol is used instead of water, an alkoxide of sodium is produced instead of the alkali solution.
Aluminium amalgam
Aluminium can form an amalgam through a reaction with mercury. Aluminium amalgam may be prepared by either grinding aluminium pellets or wire in mercury, or by allowing aluminium wire or foil to react with a solution of mercuric chloride. This amalgam is used as a reagent to reduce compounds, such as the reduction of imines to amines. The aluminium is the ultimate electron donor, and the mercury serves to mediate the electron transfer.[5] The reaction itself and the waste from it contain mercury, so special safety precautions and disposal methods are needed. As an environmentally friendlier alternative, hydrides or other reducing agents can often be used to accomplish the same synthetic result. Another environmentally friendly alternative is an alloy of aluminium and gallium which similarly renders the aluminium more reactive by preventing it from forming an oxide layer.
Tin amalgam
Tin amalgam was used in the middle of the 19th century as a reflective mirror coating.
Other amalgams
A variety of amalgams are known that are of interest mainly in the research context.
Ammonium amalgam is a grey, soft, spongy mass discovered in 1808 by Humphry Davy and Jöns Jakob Berzelius. It decomposes readily at room temperature or in contact with water or alcohol.
* Thallium amalgam has a freezing point of −58 °C, which is lower than that of pure mercury (−38.8 °C) so it has found a use in low temperature thermometers.
* Gold amalgam: Refined gold, when finely ground and brought into contact with mercury where the surfaces of both metals are clean, amalgamates readily and quickly forms alloys ranging from AuHg2 to Au8Hg.
* Lead forms an amalgam when filings are mixed with mercury[citation needed] and is also listed as a naturally occurring alloy called leadamalgam in the Nickel–Strunz classification.
Dental amalgam
Dentistry has used alloys of mercury with metals such as silver, copper, indium, tin and zinc. Amalgam is an "excellent and versatile restorative material" and is used in dentistry because it is inexpensive and relatively easy to use and manipulate during placement. It remains soft for a short time so it can be packed to fill any irregular volume, and then forms a hard compound. Amalgam possesses greater longevity when compared to other direct restorative materials, such as composite. However, this difference has decreased with continual development of composite resins.
Amalgam is typically compared to resin-based composites because many applications are similar and many physical properties and costs are comparable.
Dental amalgam has been studied and is generally considered to be safe for humans, though the validity of some studies and their conclusions have been questioned.
In July 2018 the EU, in consideration of the persistent pollution and environmental toxicity of amalgam's mercury, prohibited amalgam for dental treatment of children under 15 years and of pregnant or breastfeeding women.
Use in mining
Mercury has been used in gold and silver mining because of the convenience and the ease with which mercury and the precious metals will amalgamate. In gold placer mining, in which minute specks of gold are washed from sand or gravel deposits, mercury was often used to separate the gold from other heavy minerals.
After all of the practical metal had been taken out from the ore, the mercury was dispensed down a long copper trough, which formed a thin coating of mercury on the exterior. The waste ore was then transferred down the trough, and gold in the waste amalgamated with the mercury. This coating would then be scraped off and refined by evaporation to get rid of the mercury, leaving behind somewhat high-purity gold.
Mercury amalgamation was first used on silver ores with the development of the patio process in Mexico in 1557. There were also additional amalgamation processes that were created for processing silver ores, including pan amalgamation and the Washoe process.
Gold amalgam:
Gold extraction (mining)
Gold amalgam has proved effective where gold fines ("flour gold") would not be extractable from ore using hydro-mechanical methods. Large amounts of mercury were used in placer mining, where deposits composed largely of decomposed granite slurry were separated in long runs of "riffle boxes", with mercury dumped in at the head of the run. The amalgam formed is a heavy dull gray solid mass. The use of mercury in 19th century placer mining in California, now prohibited, has caused extensive pollution problems in riverine and estuarine environments, ongoing to this day. Sometimes substantial slugs of amalgam are found in downstream river and creek bottoms by amateur wet-suited miners seeking gold nuggets with the aid of an engine-powered water vacuum/dredge mounted on a float.
Gold extraction (ore processing)
Where stamp mills were used to crush gold-bearing ore to fines, a part of the extraction process involved the use of mercury-wetted copper plates, over which the crushed fines were washed. A periodic scraping and re-mercurizing of the plate resulted in amalgam for further processing.
Gold extraction (retorting)
Amalgam obtained by either process was then heated in a distillation retort, recovering the mercury for reuse and leaving behind the gold. As this released mercury vapors to the atmosphere, the process could induce adverse health effects and long term pollution.
Today, mercury amalgamation has been replaced by other methods to recuperate gold and silver from ore in developed nations. Hazards of mercurial toxic waste have played a major role in the phasing out of the mercury amalgamation processes. Mercury amalgamation is still regularly used by small-scale gold placer miners (often illegally), particularly in developing countries.
Amalgam probe
Mercury salts are, compared to mercury metal and amalgams, highly toxic due to their solubility in water. The presence of these salts in water can be detected with a probe that uses the readiness of mercury ions to form an amalgam with copper. A nitric acid solution of salts under investigation is applied to a piece of copper foil, and any mercury ions present will leave spots of silvery-coloured amalgam. Silver ions leave similar spots but are easily washed away, making this a means of distinguishing silver from mercury.
Additional Information
Dental amalgam, often referred to as “silver fillings,” has been a dentistry staple for over a century. These iconic silvery-gray restorations have filled cavities, restored damaged teeth, and saved countless smiles. However, dental amalgam has also faced its share of controversies and debates. In this article, we’ll explore the history, composition, benefits, concerns, and alternatives of dental amalgam to provide a comprehensive view of this commonly used dental material.
A Brief History
Dental amalgam’s history can be traced back to the early 19th century when the amalgamation of metals was a well-known concept. In 1819, the French chemist Louis Nicolas Vauquelin introduced the use of silver amalgam in dentistry. The basic idea was to mix powdered silver with mercury, creating a malleable and durable filling material. This revolutionary development allowed dentists to restore teeth with a more reliable and long-lasting solution compared to earlier methods like using tin and gold.
Composition of Dental Amalgam
Traditional dental amalgam is composed of a mixture of several metals, with the primary components being:
* Silver: Silver provides durability and strength to the amalgam filling.
* Tin: Tin aids in amalgam alloy formation and increases its workability.
* Copper: Copper improves resistance to corrosion and tarnishing.
* Mercury: Mercury serves as the binder, allowing the mixture to become pliable for filling cavities.
The Dental Restoration Process
Dental amalgam is renowned for its ease of use and durability. The process of placing a dental amalgam filling typically involves the following steps:
Preparation: The dentist removes decayed or damaged tooth structure, creating a clean cavity to be filled.
Mixing: The amalgam alloy is mixed with mercury, forming a soft, pliable material.
Filling: The mixed amalgam is carefully placed into the prepared cavity and shaped to match the natural contours of the tooth.
Hardening: Over time, the amalgam hardens and becomes a solid, long-lasting filling.
Benefits of Dental Amalgam
Dental amalgam has several advantages that have contributed to its continued use in dentistry:
Durability: Dental amalgam is exceptionally durable and can withstand the forces of biting and chewing over many years.
Cost-Effectiveness: Amalgam fillings are often more affordable than alternative materials, making them accessible to a broader range of patients.
Quick Placement: The placement of dental amalgam fillings is relatively quick and straightforward, making it a convenient option for both patients and dentists.
Versatility: Amalgam can be used in various dental situations, from small cavities to larger restorations.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2505 2025-04-03 21:38:21
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,708
Re: Miscellany
2305) Islets of Langerhans
Gist
Islets of Langerhans are clusters of endocrine cells within the pancreas responsible for producing and releasing hormones, primarily insulin and glucagon, which regulate blood sugar levels.
A pancreatic cell that produces hormones (e.g., insulin and glucagon) that are secreted into the bloodstream. These hormones help control the level of glucose (sugar) in the blood. Also called endocrine pancreas cell and islet cell.
Summary
Islets of Langerhans are irregularly shaped patches of endocrine tissue located within the pancreas of most vertebrates. They are named for the German physician Paul Langerhans, who first described them in 1869. The normal human pancreas contains about 1 million islets. The islets consist of four major and two minor cell types, of which the major types (alpha, beta, delta, and pancreatic polypeptide [PP] cells) produce important hormones. The two minor types (D1 and enterochromaffin cells) produce hormones and synthesize serotonin, respectively.
The islets of Langerhans contain alpha, beta, and delta cells that produce glucagon, insulin, and somatostatin, respectively. A fourth type of islet cell, the PP (or F) cell, is located at the periphery of the islets and secretes pancreatic polypeptide. These hormones regulate one another's secretion through paracrine cell-cell interactions.
The most common islet cell, the beta cell, produces insulin, the major hormone in the regulation of carbohydrate, fat, and protein metabolism. Insulin is crucial in several metabolic processes: it promotes the uptake and metabolism of glucose by the body’s cells; it prevents release of glucose by the liver; it causes muscle cells to take up amino acids, the basic components of protein; and it inhibits the breakdown and release of fats. The release of insulin from the beta cells can be triggered by growth hormone (somatotropin) or by glucagon, but the most important stimulator of insulin release is glucose; when the blood glucose level increases—as it does after a meal—insulin is released to counter it. The inability of the islet cells to make insulin or the failure to produce amounts sufficient to control blood glucose level are the causes of diabetes mellitus.
The alpha cells of the islets of Langerhans produce an opposing hormone, glucagon, which releases glucose from the liver and fatty acids from fat tissue. In turn, glucose and free fatty acids favor insulin release and inhibit glucagon release. The delta cells produce somatostatin, a strong inhibitor of somatotropin, insulin, and glucagon; its role in metabolic regulation is not yet clear. Somatostatin is also produced by the hypothalamus and functions there to inhibit secretion of growth hormone by the pituitary gland.
The D1 cell, the first of the two minor and rarer cell types, produces a hormone called vasoactive intestinal polypeptide (VIP). VIPs are responsible for increasing blood glucose levels and releasing gastrointestinal fluids that help with digestion. Enterochromaffin cells, the second minor cell type, synthesize serotonin. Serotonin works in synergy with the other hormones released in islets to help with intestinal mobility. However, enterochromaffin cells are known to cause carcinoid syndrome, a rare condition caused by excess production of serotonin.
Details
The pancreatic islets or islets of Langerhans are the regions of the pancreas that contain its endocrine (hormone-producing) cells, discovered in 1869 by German pathological anatomist Paul Langerhans. The pancreatic islets constitute 1–2% of the pancreas volume and receive 10–15% of its blood flow. The pancreatic islets are arranged in density routes throughout the human pancreas, and are important in the metabolism of glucose.
Structure
There are about 1 million islets distributed throughout the pancreas of a healthy adult human. While islets vary in size, the average diameter is about 0.2 mm. Each islet is separated from the surrounding pancreatic tissue by a thin, fibrous, connective tissue capsule which is continuous with the fibrous connective tissue that is interwoven throughout the rest of the pancreas.
Microanatomy
Hormones produced in the pancreatic islets are secreted directly into the blood flow by (at least) five types of cells. In rat islets, endocrine cell types are distributed as follows:
* Alpha cells producing glucagon (20% of total islet cells)
* Beta cells producing insulin and amylin (≈70%)
* PP cells (gamma cells or F cells) producing pancreatic polypeptide (<5%)
* Delta cells producing somatostatin (<10%)
* Epsilon cells producing ghrelin (<1%)
It has been recognized that the cytoarchitecture of pancreatic islets differs between species. In particular, while rodent islets are characterized by a predominant proportion of insulin-producing beta cells in the core of the cluster and by scarce alpha, delta and PP cells in the periphery, human islets display alpha and beta cells in close relationship with each other throughout the cluster.
The proportion of beta cells in islets varies depending on the species, in humans it is about 40–50%. In addition to endocrine cells, there are stromal cells (fibroblasts), vascular cells (endothelial cells, pericytes), immune cells (granulocytes, lymphocytes, macrophages, dendritic cells,) and neural cells.
A large amount of blood flows through the islets, 5–6 mL/min per 1 g of islet. It is up to 15 times more than in exocrine tissue of the pancreas.
Islets can influence each other through paracrine and autocrine communication, and beta cells are coupled electrically to six to seven other beta cells, but not to other cell types. Pancreatic islets are characterized by rich innervation and vascularization, although there are notable differences between rodent and human islets. Research indicates that the vascular density in human islets is about five times lower than in rodent islets. The vascular network within the islets resembles a glomeruli-like structure, consisting of highly fenestrated endothelial cells positioned closely to each endocrine cell. Consequently, the oxygen tension within pancreatic islets is significantly higher than that in the surrounding exocrine tissue.
Function
The paracrine feedback system of the pancreatic islets has the following structure:
* Glucose/Insulin: activates beta cells and inhibits alpha cells.
* Glycogen/Glucagon: activates alpha cells which activates beta cells and delta cells.
* Somatostatin: inhibits alpha cells and beta cells. Also inhibits the secretion of pancreatic polypeptide.
A large number of G protein-coupled receptors (GPCRs) regulate the secretion of insulin, glucagon, and somatostatin from pancreatic islets, and some of these GPCRs are the targets of drugs used to treat type-2 diabetes (ref GLP-1 receptor agonists, DPPIV inhibitors).
Electrical activity
Electrical activity of pancreatic islets has been studied using patch clamp techniques. It has turned out that the behavior of cells in intact islets differs significantly from the behavior of dispersed cells.
Clinical significance:
Diabetes
The beta cells of the pancreatic islets secrete insulin, and so play a significant role in diabetes. It is thought that they are destroyed by immune assaults.
Because the beta cells in the pancreatic islets are selectively destroyed by an autoimmune process in type 1 diabetes, clinicians and researchers are actively pursuing islet transplantation as a means of restoring physiological beta cell function, which would offer an alternative to a complete pancreas transplant or artificial pancreas. Islet transplantation emerged as a viable option for the treatment of insulin requiring diabetes in the early 1970s with steady progress over the following three decades. Clinical trials as of 2008 have shown that insulin independence and improved metabolic control can be reproducibly obtained after transplantation of cadaveric donor islets into patients with unstable type 1 diabetes. Alternatively, daily insulin injections are an effective treatment for type 1 diabetes patients who are not candidates for islet transplantation.
People with high body mass index (BMI) are unsuitable pancreatic donors due to greater technical complications during transplantation. However, it is possible to isolate a larger number of islets because of their larger pancreas, and therefore they are more suitable donors of islets.
Islet transplantation only involves the transfer of tissue consisting of beta cells that are necessary as a treatment of this disease. It thus represents an advantage over whole pancreas transplantation, which is more technically demanding and poses a risk of, for example, pancreatitis leading to organ loss. Another advantage is that patients do not require general anesthesia.
Islet transplantation for type 1 diabetes (as of 2008) requires potent immunosuppression to prevent host rejection of donor islets.
The islets are transplanted into a portal vein, which is then implanted in the liver. There is a risk of portal venous branch thrombosis and the low value of islet survival a few minutes after transplantation, because the vascular density at this site is after the surgery several months lower than in endogenous islets. Thus, neovascularization is key to islet survival, that is supported, for example, by VEGF produced by islets and vascular endothelial cells. However, intraportal transplantation has some other shortcomings, and so other alternative sites that would provide better microenvironment for islets implantation are being examined. Islet transplant research also focuses on islet encapsulation, CNI-free (calcineurin-inhibitor) immunosuppression, biomarkers of islet damage or islet donor shortage.
An alternative source of beta cells, such insulin-producing cells derived from adult stem cells or progenitor cells would contribute to overcoming the shortage of donor organs for transplantation. The field of regenerative medicine is rapidly evolving and offers great hope for the nearest future. However, type 1 diabetes is the result of the autoimmune destruction of beta cells in the pancreas. Therefore, an effective cure will require a sequential, integrated approach that combines adequate and safe immune interventions with beta cell regenerative approaches. It has also been demonstrated that alpha cells can spontaneously switch fate and transdifferentiate into beta cells in both healthy and diabetic human and mouse pancreatic islets, a possible future source for beta cell regeneration. In fact, it has been found that islet morphology and endocrine differentiation are directly related. Endocrine progenitor cells differentiate by migrating in cohesion and forming bud-like islet precursors, or "peninsulas", in which alpha cells constitute the peninsular outer layer and beta cells form later beneath them. Cryopreservation has shown promise to improve the supply chain of pancreatic islets for better transplantation outcomes.
Research
Cannabinoid receptors are found widely expressed in islets of Langerhans, and several studies have investigated specific distribution and mechanisms of CB1 versus CB2 receptors in relation to pancreatic endocrine functions, where they play an important homeostatic role, as endocannabinoids modulate pancreatic β-cells function, proliferation, and survival, as well as insulin production, secretion, and resistance.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2506 2025-04-04 21:37:58
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,708
Re: Miscellany
2306) Gorge
Gorge
Gist
A gorge is a narrow valley with steep, rocky walls located between hills or mountains. The term comes from the French word gorge, which means throat or neck. A gorge is often smaller than a canyon, although both words are used to describe deep, narrow valleys with a stream or river running along their bottom.
Summary
A gorge is a very deep crevice between two mountains or hills. Gorges are formed by rivers running through and eroding rock over a very long period of time.
The Latin root of gorge means "throat," leading to both the "narrow passage" meaning and the French gorgier, "to swallow," which influenced the verb version of gorge, "to overeat." You may love to gorge on ice cream, but the stomachache afterward won't be very pleasant. To remember the "canyon" meaning, think of the famous upstate New York bumper sticker, “Ithaca is gorges.” It's a play on gorgeous, meaning beautiful, and the beautiful ravines in the area.
Details
A gorge is a narrow valley with steep, rocky walls located between hills or mountains. The term comes from the French word gorge, which means throat or neck. A gorge is often smaller than a canyon, although both words are used to describe deep, narrow valleys with a stream or river running along their bottom.
A number of natural forces form gorges. The most common is erosion due to streams or rivers. Streams carve through hard layers of rock, breaking down or eroding it. Sediment from the worn-away rock is then carried downstream. Over time, this erosion will form the steep walls of a gorge. The flooding of streams or rivers increases the speed and intensity of this erosion, creating deeper and wider gorges. The deep Talari Gorges in Mali, for instance, were formed by the Senegal River that flows into the Atlantic Ocean on the western coast of Africa.
Geologic uplift also forms gorges. Geologic uplift is the upward movement of the Earth's surface. Geologic uplift is often associated with earthquakes and orogeny, the process of creating mountains. During geologic uplift, rock layers beneath Earth's surface bump against the surface layers. Softer layers of surface rock erode.
Erosion and geologic uplift often work together to create gorges. Parts of streams or rivers can be elevated, along with land, during the process of geologic uplift. As rivers or streams flow across this uplifted surface, waterfalls form. Over time, the power of the waterfall erodes the softer rock layers underneath, causing the original river bed to collapse and create a gorge. Macocha Gorge in the Jihomoravsk region of the Czech Republic was probably formed by the collapse of an underground cave that had been eroded by the Punkva River.
The movement and melting of glaciers can also produce gorges. Glaciers cut deep valleys into the Earth's surface. These rivers of ice can create huge canyons and sharp, steep gorges. As glaciers melt, or retreat, these gorges and canyons are exposed. The Columbia River Gorge, located in the U.S. states of Washington and Oregon, was partially created by glacial retreat during the last Ice Age.
Engineers have purposely flooded gorges in order to create waterways and dams. These dams generate hydroelectricity, or electricity powered by water. The Three Gorges Dam on the Yangtze River in China is probably the most famous example of such a project. Upstream from the dam, the Qutang, Wu, and Xilang gorges were partially submerged in order to create a waterway. The new waterway would allow freight ships to navigate from the East China Sea, part of the Pacific Ocean, to the city of Chongqing, about 2,250 kilometers (1,400 miles) inland. The 26 turbines of the Three Gorges Dam generate approximately 18,000 megawatts of electricity for Shanghai and other cities. However, many people worry about the environmental impacts of the dam and criticize the fact that more than a million Chinese families were forced to move from their homes near the gorges in order to complete the construction.
Many geological discoveries have been made at gorges because gorges often expose layers of rock that go back thousands of years. Olduvai Gorge in Tanzania has layers dating as far back as two million years. The Olduvai Gorge is famous for the fossils and ancient tools found there by scientists Louis, Mary, and Richard Leakey. These remains of ancient animals and plants provide clues about early humans.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2507 2025-04-05 00:02:44
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,708
Re: Miscellany
2307) Kairba Dam
Gist
Based on the reservoir capacity, the Kariba Dam is considered to be the largest in the world.
Summary
Kariba Dam, concrete arch dam across the Zambezi River at Kariba Gorge, on the border between Zambia and Zimbabwe. Construction of the dam began on Nov. 6, 1956, and was completed in 1959. The structure is 128 metres (420 feet) high with a crest 579 metres (1,899 feet) in length and a volume of 1,032,000 cubic metres (1,350,000 cubic yards). The dam creates Lake Kariba, and it supplies some 6,700,000,000 kilowatt-hours of electricity annually, generated by Kariba North Bank and South Bank companies (Zambia and Zimbabwe, respectively). Its creation required the resettlement of more than 30,000 Batonka tribespeople of Zambia and the evacuation of thousands of wild animals (“Operation Noah”). Some Africans initially opposed construction of the dam, seeing it as a symbol of the unpopular Federation of Rhodesia and Nyasaland, which dissolved into Rhodesia (now Zimbabwe) and Zambia in 1963. Later, however, the dam was accepted because of the inexpensive electric power it furnishes to Zambia’s prosperous copper industry.
Details
The Kariba dam is a double curvature concrete arch dam in the Kariba gorge of the Zambezi river basin between Zambia and Zimbabwe. At 128m tall and 579m long, the structure forms Lake Kariba - extending 280km and holding 185km³ of water.
An arch dam is a concrete dam that curves upstream. Arch dams are designed so that the water pressing against them compresses and strengthens the structure as it pushes into its foundations.
Arch dams are often used in narrow canyons or gorges – such as the Kariba gorge – as the steep walls of stable rock help support the structure.
The project was a joint venture between the former self-governing protectorates of Northern and Southern Rhodesia (now Zambia and Zimbabwe) and Nyasaland (now Malawi). The dam and 6 flood gates were built between 1955 and 1959, with later work adding turbine rooms to generate electricity.
Building the dam and its reservoir forced the resettlement of around 57,000 Tongan people living along the Zambezi in both Northern Rhodesia and Southern Rhodesia.
The scheme supplies 1,626MW of electricity to Zambia and Zimbabwe. Each country has its own power station – one on the north bank and one on the south bank of the dam.
The Kariba dam is jointly operated by Zambia and Zimbabwe through the Zambezi River Authority.
Difference the dam has made
The Kariba dam provides a cheap source of power for both Zambia and Zimbabwe – this has been crucial for the economies of both countries.
Construction of the dam has led to the preservation of wilderness areas in national parks along the lake shore. This has helped grow a tourist industry in the area – boosting the local economy.
Around 57,000 Tongan people were moved from their homes to make way for the dam.
How the work was done
Engineers chose a concrete arch dam for the Kariba scheme. Not only is the curving structure effective in valleys but arch dams need much less construction material. This makes them economical and practical in remote areas such as the Kariba gorge.
Early stages of work saw the project team build roads through rugged country to the north and south banks of the Zambezi river. They also constructed an airstrip and 2 towns as accommodation for the 7,000 workers on the scheme.
Cement came by rail and was then carried the final 140km to the construction site by road.
Engineers used coffer dams for initial work on the scheme. A coffer dam is an enclosure built in or across water. The enclosed area is pumped out allowing a dry environment for construction.
Concrete was transported from a manufacturing plant using ‘blondin’ cables. These were a type of aerial ropeway also used in slate quarries. Working a bit like cable cars, wagons full of concrete were attached to the ropes and the contents tipped out at the dam construction site.
At the time it was completed in 1959, Kariba had the biggest dam wall in the world. Kariba lake – the reservoir created by the scheme – was the biggest artificial lake in the world.
86 workers died during the construction of the dam.
Additional Information
The Kariba Dam is a double curvature concrete arch dam in the Kariba Gorge of the Zambezi river basin between Zambia and Zimbabwe. The dam stands 128 metres (420 ft) tall and 579 metres (1,900 ft) long. The dam forms Lake Kariba, which extends for 280 kilometres (170 mi) and holds 185 cubic kilometres (150,000,000 acre⋅ft) of water.
Construction
The dam was constructed on the orders of the Government of the Federation of Rhodesia and Nyasaland, a 'federal colony' within the British Empire. The double curvature concrete arch dam was designed by Coyne et Bellier and constructed between 1955 and 1959 by Impresit of Italy at a cost of $135,000,000 for the first stage with only the Kariba South power cavern. Final construction and the addition of the Kariba North Power cavern by Mitchell Construction was not completed until 1977, due to largely political problems, for a total cost of $480,000,000. During construction, 86 construction workers lost their lives.
The dam was officially opened by Queen Elizabeth The Queen Mother on 17 May 1960.
Power generation
The Kariba Dam supplies 2,010 megawatts (2,700,000 hp) of electricity to parts of both Zambia (the Copperbelt) and Zimbabwe and generates 6,400 gigawatt-hours (23,000 TJ) per annum. Each country has its own power station on the north and south bank of the dam, respectively. The south station belonging to Zimbabwe has been in operation since 1960 and had six generators of 125 megawatts (168,000 hp) capacity each for a total of 750 megawatts (1,010,000 hp).
On November 11, 2013 it was announced by Zimbabwe's Finance Minister, Patrick Chinamasa that capacity at the Zimbabwean (South) Kariba hydropower station would be increased by 300 megawatts. The cost of upgrading the facility has been supported by a $319m loan from China. The deal is a clear example of Zimbabwe's "Look East" policy, which was adopted after falling out with Western powers. Construction on the Kariba South expansion began in mid-2014 and was initially expected to be complete in 2019.
In March 2018, president Emmerson Mnangagwa commissioned the completed expansion of Kariba South Hydroelectric Power Station. The addition of two new 150 megawatts (200,000 hp) turbines raised capacity at this station to 1,050 megawatts (1,410,000 hp). The expansion work was done by Sinohydro, at a cost of US$533 million. Work started in 2014, and was completed in March 2018.
The north station belonging to Zambia has been in operation since 1976, and has four generators of 150 megawatts (200,000 hp) each for a total of 600 megawatts (800,000 hp); work to expand this capacity by an additional 360 megawatts (480,000 hp) to 960 megawatts (1,290,000 hp) was completed in December 2013. Two additional 180 MW generators were added.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2508 2025-04-06 00:06:51
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,708
Re: Miscellany
2308) Antibiotics
Gist
Antibiotics are medicines that treat bacterial infections by killing bacteria or stopping their growth. They are not effective against viral infections like the flu or a cold.
Antibiotics are medicines that fight bacterial infections in people and animals. They work by killing the bacteria or by making it hard for the bacteria to grow and multiply. Antibiotics can be taken in different ways: Orally (by mouth).
Antibiotics are medicines that treat bacterial infections by killing bacteria or stopping their growth. They can be taken orally in the form of pills, capsules, or liquids.
The first antibiotic, penicillin, was accidentally discovered in 1928 by Alexander Fleming, a Scottish bacteriologist, who noticed that a mold inhibited bacterial growth on a petri dish.
Summary
Antibiotics work by killing bacteria and preventing them from multiplying. Common antibiotics include gentamicin, cephalexin, ertapenem, erythromycin, ciprofloxacin, and metronidazole.
They include a range of powerful drugs used to treat diseases caused by bacteria.
Antibiotics cannot treat viral infections, such as cold, flu, and most coughs.
Fast facts on antibiotics
* Alexander Fleming discovered penicillin, the first natural antibiotic, in 1928.
* Antibiotics cannot fight viral infections.
* Fleming predicted the rise of antibiotic resistance.
* Antibiotics either kill or slow the growth of bacteria.
* Side effects can include diarrhea, an upset stomach, and nausea.
What are antibiotics?
Antibiotics are powerful medications that treat certain infections and can save lives when used properly. They either stop bacteria from reproducing or destroy them.
Before bacteria can multiply and cause symptoms, the immune system can typically kill them. White blood cells (WBCs) attack harmful bacteria — even if symptoms occur, the immune system can usually cope and fend off the infection.
However, sometimes the number of harmful bacteria is excessive, and the immune system cannot clear them all. Antibiotics are useful in this scenario.
The first antibiotic was penicillin. Penicillin-based antibiotics, such as ampicillin, amoxicillin, and penicillin G, are still available to treat a variety of infections and have been in use for many years.
Several types of modern antibiotics are available, and they are usually only available with a prescription in the United States. Topical antibiotics are available in over-the-counter (OTC) creams and ointments.
How do antibiotics work?
There are different types of antibiotics, which work in their unique way. However, the two main they work include:
* A bactericidal antibiotic, such as penicillin, kills the bacteria. These drugs usually interfere with either the formation of the bacterial cell wall or its cell contents.
* A bacteriostatic stops bacteria from multiplying.
It may take a few hours or days after taking the first dose before people feel better or their symptoms improve.
Why is it important to take antibiotics when needed?
Experts advise using antibiotics only when they are needed. This is to ensure that the bacteria is killed and is unable to multiply and spread to other parts of the body.
Also, antibiotic use can sometimes be associated with side effects and antibiotic resistance.
Details
Antibiotics are used to treat or prevent some types of bacterial infection. They work by killing bacteria or preventing them from spreading. But they do not work for everything.
Many mild bacterial infections get better on their own without using antibiotics.
Antibiotics do not work for viral infections such as colds and flu, and most coughs.
Antibiotics are no longer routinely used to treat:
* chest infections
* ear infections in children
* sore throats
When it comes to antibiotics, take your doctor's advice on whether you need them or not. Antibiotic resistance is a big problem – taking antibiotics when you do not need them can mean they will not work for you in the future.
When antibiotics are needed
Antibiotics may be used to treat bacterial infections that:
* are unlikely to clear up without antibiotics
* could take too long to clear without treatment
* carry a risk of more serious complications
* could infect others
You may still be infectious after starting a course of antibiotics. Depending on the infection and how it's treated, it can take between 48 hours and 14 days to stop being infectious. Ask a GP or pharmacist for advice.
People at a high risk of infection may also be given antibiotics as a precaution, known as antibiotic prophylaxis.
How to take antibiotics
Take antibiotics as directed on the packet or the patient information leaflet that comes with the medicine, or as instructed by your GP or pharmacist.
Antibiotics can come as:
* tablets, capsules or a liquid that you drink – these can be used to treat most types of mild to moderate infections in the body
* creams, lotions, sprays and drops – these are often used to treat skin infections and eye or ear infections
* injections – these can be given as an injection or through a drip directly into the blood or muscle, and are used for more serious infections
Missing a dose of antibiotics
If you forget to take a dose of your antibiotics, check the patient information leaflet that came with your medicine to find out what to do. If you're not sure, speak to a pharmacist or a GP.
In most cases, you can take the dose you missed as soon as you remember and then continue to take your course of antibiotics as normal.
But if it's almost time for the next dose, skip the missed dose and continue your regular dosing schedule. Do not take a double dose to make up for a missed one.
Accidentally taking an extra dose
There's an increased risk of side effects if you take 2 doses closer together than recommended.
Accidentally taking 1 extra dose of your antibiotic is unlikely to cause you any serious harm.
But it will increase your chances of getting side effects, such as pain in your stomach, diarrhoea, and feeling or being sick.
If you accidentally take more than 1 extra dose of your antibiotic, are worried or you get severe side effects, speak to your GP or call NHS 111 as soon as possible.
Side effects of antibiotics
As with any medicine, antibiotics can cause side effects. Most antibiotics do not cause problems if they're used properly and serious side effects are rare.
The common side effects include:
* being sick
* feeling sick
* bloating and indigestion
* diarrhoea
Some people may have an allergic reaction to antibiotics, especially penicillin and another type of antibiotic called cephalosporins.
In very rare cases, this can lead to a serious allergic reaction (anaphylaxis), which is a medical emergency.
Call 999 or go to A&E now if:
* you get a skin rash that may include itchy, red, swollen, blistered or peeling skin
* you're wheezing
* you get tightness in the chest or throat
* you have trouble breathing or talking
* your mouth, face, lips, tongue or throat start swelling
You could be having a serious allergic reaction and may need immediate treatment in hospital.
Considerations and interactions
Some antibiotics are not suitable for people with certain medical problems, or women who are pregnant or breastfeeding.
Tell your healthcare professional if you're pregnant or breastfeeding so they can prescribe the most suitable antibiotic for you.
Only ever take antibiotics prescribed for you – never "borrow" them from a friend or family member.
Some antibiotics do not mix well with other medicines, such as the contraceptive pill and alcohol.
Read the information leaflet that comes with your medicine carefully and discuss any concerns with your pharmacist or GP.
Read more about how antibiotics interact with other medicines.
Additional Information
An antibiotic is a type of antimicrobial substance active against bacteria. It is the most important type of antibacterial agent for fighting bacterial infections, and antibiotic medications are widely used in the treatment and prevention of such infections. They may either kill or inhibit the growth of bacteria. A limited number of antibiotics also possess antiprotozoal activity. Antibiotics are not effective against viruses such as the ones which cause the common cold or influenza. Drugs which inhibit growth of viruses are termed antiviral drugs or antivirals. Antibiotics are also not effective against fungi. Drugs which inhibit growth of fungi are called antifungal drugs.
Sometimes, the term antibiotic—literally "opposing life", from the Greek roots anti, "against" and bios, "life"—is broadly used to refer to any substance used against microbes, but in the usual medical usage, antibiotics (such as penicillin) are those produced naturally (by one microorganism fighting another), whereas non-antibiotic antibacterials (such as sulfonamides and antiseptics) are fully synthetic. However, both classes have the same effect of killing or preventing the growth of microorganisms, and both are included in antimicrobial chemotherapy. "Antibacterials" include bactericides, bacteriostatics, antibacterial soaps, and chemical disinfectants, whereas antibiotics are an important class of antibacterials used more specifically in medicine and sometimes in livestock feed.
Antibiotics have been used since ancient times. Many civilizations used topical application of moldy bread, with many references to its beneficial effects arising from ancient Egypt, Nubia, China, Serbia, Greece, and Rome. The first person to directly document the use of molds to treat infections was John Parkinson (1567–1650). Antibiotics revolutionized medicine in the 20th century. Synthetic antibiotic chemotherapy as a science and development of antibacterials began in Germany with Paul Ehrlich in the late 1880s. Alexander Fleming (1881–1955) discovered modern day penicillin in 1928, the widespread use of which proved significantly beneficial during wartime. The first sulfonamide and the first systemically active antibacterial drug, Prontosil, was developed by a research team led by Gerhard Domagk in 1932 or 1933 at the Bayer Laboratories of the IG Farben conglomerate in Germany. However, the effectiveness and easy access to antibiotics have also led to their overuse and some bacteria have evolved resistance to them. Antimicrobial resistance (AMR), a naturally occurring process, is driven largely by the misuse and overuse of antimicrobials. Yet, at the same time, many people around the world do not have access to essential antimicrobials. The World Health Organization has classified AMR as a widespread "serious threat [that] is no longer a prediction for the future, it is happening right now in every region of the world and has the potential to affect anyone, of any age, in any country".[ Each year, nearly 5 million deaths are associated with AMR globally. Global deaths attributable to AMR numbered 1.27 million in 2019.
More Information
An antibiotic is a drug that fights bacteria. Antibiotics can also be called antimicrobials, but this is a broader term that includes drugs that fight bacteria or other types of microbes, such as viruses or fungi. Antibiotics do not work against viruses, such as those that cause colds and flu.
Antibiotics work in many different ways. They might kill bacteria, or merely disable them or slow down their multiplication, giving the immune system more time to clear the infection. Many antibiotics stop the bacteria from making proteins, which is essential for survival and multiplication. Others interfere with their ability to copy DNA.
Penicillin, the first antibiotic to be developed as a medicine, blocks the construction of the bacterium’s cell wall. With this important part of its structure weakened, the cell can easily rupture. Daptomycin disrupts the integrity of the cell membrane, allowing ions or small molecules to leak in and out of the cell, which can also be lethal to bacteria.
Some antibiotics, described as narrow-spectrum, are only effective against specific types of bacteria, while broad-spectrum drugs can fight a wide range.
All antibiotics will have some effect on the bacteria that normally live inside our bodies and contribute to our health, the microbiome. As a side effect, they may kill some bacteria that are good for us, and make it easier for other bacteria to take their place.
We have hundreds of antibiotics, but they fall into about 15 major classes. Many are produced naturally by certain microbes to kill others. Most were discovered between 1940 and 1960. The rate at which we have developed new ones has slowed down dramatically.
Some bacteria have evolved resistance to certain antibiotics. Antibiotic-resistant bacteria are becoming more and more common, making infections harder to treat. This problem has been made worse by the overuse of antibiotics, both in medicine and farming.
Doctors often prescribe a course of antibiotics lasting one or two weeks, and tell patients to finish the course even if they feel better. Recent research suggests that shorter courses are just as effective at killing bacteria and are less likely to fuel antibiotic resistance.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2509 2025-04-06 22:29:34
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,708
Re: Miscellany
2309) Gun powder
Gist
Gunpowder is a granular mixture of: a nitrate, typically potassium nitrate (KNO3), which supplies oxygen for the reaction; charcoal, which provides carbon and other fuel for the reaction, simplified as carbon (C).
Gunpowder is any of several low-explosive mixtures used as propelling charges in guns and as blasting agents in mining. The first such explosive was black powder, which consists of a mixture of saltpetre (potassium nitrate), sulfur, and charcoal.
Summary
Gunpowder, also commonly known as black powder to distinguish it from modern smokeless powder, is the earliest known chemical explosive. It consists of a mixture of sulfur, charcoal (which is mostly carbon), and potassium nitrate (saltpeter). The sulfur and charcoal act as fuels while the saltpeter is an oxidizer. Gunpowder has been widely used as a propellant in firearms, artillery, rocketry, and pyrotechnics, including use as a blasting agent for explosives in quarrying, mining, building pipelines, tunnels, and roads.
Gunpowder is classified as a low explosive because of its relatively slow decomposition rate, low ignition temperature and consequently low brisance (breaking/shattering). Low explosives deflagrate (i.e., burn at subsonic speeds), whereas high explosives detonate, producing a supersonic shockwave. Ignition of gunpowder packed behind a projectile generates enough pressure to force the shot from the muzzle at high speed, but usually not enough force to rupture the gun barrel. It thus makes a good propellant but is less suitable for shattering rock or fortifications with its low-yield explosive power. Nonetheless, it was widely used to fill fused artillery shells (and used in mining and civil engineering projects) until the second half of the 19th century, when the first high explosives were put into use.
Gunpowder is one of the Four Great Inventions of China. Originally developed by Taoists for medicinal purposes, it was first used for warfare around AD 904. Its use in weapons has declined due to smokeless powder replacing it, whilst its relative inefficiency led to newer alternatives such as dynamite and ammonium nitrate/fuel oil replacing it in industrial applications.
Effect
Gunpowder is a low explosive: it does not detonate, but rather deflagrates (burns quickly). This is an advantage in a propellant device, where one does not desire a shock that would shatter the gun and potentially harm the operator; however, it is a drawback when an explosion is desired. In that case, the propellant (and most importantly, gases produced by its burning) must be confined. Since it contains its own oxidizer and additionally burns faster under pressure, its combustion is capable of bursting containers such as a shell, grenade, or improvised "pipe bomb" or "pressure cooker" casings to form shrapnel.
In quarrying, high explosives are generally preferred for shattering rock. However, because of its low brisance, gunpowder causes fewer fractures and results in more usable stone compared to other explosives, making it useful for blasting slate, which is fragile, or monumental stone such as granite and marble. Gunpowder is well suited for blank rounds, signal flares, burst charges, and rescue-line launches. It is also used in fireworks for lifting shells, in rockets as fuel, and in certain special effects.
Combustion converts less than half the mass of gunpowder to gas; most of it turns into particulate matter. Some of it is ejected, wasting propelling power, fouling the air, and generally being a nuisance (giving away a soldier's position, generating fog that hinders vision, etc.). Some of it ends up as a thick layer of soot inside the barrel, where it also is a nuisance for subsequent shots, and a cause of jamming an automatic weapon. Moreover, this residue is hygroscopic, and with the addition of moisture absorbed from the air forms a corrosive substance. The soot contains potassium oxide or sodium oxide that turns into potassium hydroxide, or sodium hydroxide, which corrodes wrought iron or steel gun barrels. Gunpowder arms therefore require thorough and regular cleaning to remove the residue.
Gunpowder loads can be used in modern firearms as long as they are not gas-operated. The most compatible modern guns are smoothbore-barreled shotguns that are long-recoil operated with chrome-plated essential parts such as barrels and bores. Such guns have minimal fouling and corrosion and are easier to clean.
Details
Gunpowder is any of several low-explosive mixtures used as propelling charges in guns and as blasting agents in mining.
The first such explosive was black powder, which consists of a mixture of saltpetre (potassium nitrate), sulfur, and charcoal. When prepared in roughly the correct proportions (75 percent saltpetre, 15 percent charcoal, and 10 percent sulfur), it burns rapidly when ignited and produces approximately 40 percent gaseous and 60 percent solid products, the latter mostly appearing as whitish smoke. In a confined space such as the breech of a gun, the pent-up gas can be used for propelling a missile such as a bullet or artillery shell. Black powder is relatively insensitive to shock and friction and must be ignited by flame or heat. Though it has largely been supplanted by smokeless powder as a propellant for ammunition in guns, black powder is still widely used for ignition charges, primers, fuses, and blank-fire charges in military ammunition. With varied proportions of ingredients, it is also used in fireworks, time fuses, signals, squibs, and spatting charges for practice bombs.
Black powder is thought to have originated in China, where it was being used in fireworks and signals by the 10th century. Between the 10th and 12th centuries, the Chinese developed the huo qiang (“fire lance”), a short-range proto-gun that channeled the explosive power of gunpowder through a cylinder—initially, a bamboo tube. Upon ignition, projectiles such as arrows or bits of metal would be forcefully ejected, along with an impressive gout of flame. By the late 13th century the Chinese were employing true guns, made of cast brass or iron. Guns began to appear in the West by 1304, when the Arabs produced a bamboo tube reinforced with iron that used a charge of black powder to shoot an arrow. Black powder was adopted for use in firearms in Europe from the 14th century but was not employed for peaceful purposes, such as mining and road building, until the late 17th century. It remained a useful explosive for breaking up coal and rock deposits until the early 20th century, when it was gradually replaced by dynamite for most mining purposes.
The preparation of black powder from solid ingredients requires uniform mixing and blending of the saltpetre, charcoal, and sulfur. The earliest manufacturing processes used hand methods; the ingredients were simply ground together into a powder using a mortar and pestle. Beginning in the 15th century, water-driven crushing devices of wood, called wooden stamps, came into use to grind the ingredients, and power-driven metallic crushing devices replaced the wooden stamp mills in the 19th century.
Because the burning of black powder is a surface phenomenon, a fine granulation burns faster than a coarse one. A fast burning rate is effective ballistically but tends to create excessive pressures in the gun barrel. Thus, black powder in its powdered form burned too rapidly to be a safe propellant in firearms. To remedy this, Europeans in the 15th and 16th centuries began manufacturing powder in large grains of uniform size. The speed of burning could be varied by using a different size of granule. In the 19th century, as elongated projectiles replaced round balls and the rifling of gun tubes was adopted to rotate and stabilize the projectile, black powders were manufactured to burn even more slowly. In the 1850s Thomas J. Rodman of the U.S. Army developed black powder grains so shaped that they provided a progressively greater burning surface as the combustion progressed, with a resulting maximum energy release after the projectile had already begun to travel down the bore of the gun.
Beginning in the 1860s, black powder was gradually supplanted for use in firearms by guncotton and other, more stable forms of nitrocellulose. Unlike black powder, which burns by the chemical reactions of its constituent ingredients, nitrocellulose is an inherently unstable compound that burns by decomposing rapidly, forming hot gases. In contrast to black powder, it produces almost all gas upon combustion, earning itself the appellation smokeless powder. Also unlike black powder, nitrocellulose burns progressively, generating more gas pressure as combustion proceeds. This results in higher muzzle velocities (for the projectile) and less strain exerted on the firearm.
Nitrocellulose is manufactured by nitrating cellulose fibres such as cotton or wood pulp with nitric and sulfuric acids. Early manufacturing techniques often failed to remove all traces of residual acids from the nitrocellulose, which then tended to undergo an unpredictable spontaneous decomposition resulting in explosion. In the 1880s European chemists began adding special stabilizers to neutralize the residual acids and other decomposition agents in nitrocellulose. The resulting stable and reliable product, known as smokeless powder, was widely adopted in all types of guns in the following decades and supplanted black powder as the propellant charge in artillery and small arms ammunition. (Black powder is still used to ignite the main [smokeless] propellant charge in large-bore artillery pieces, however.)
Nitrocellulose propellants produce much less smoke and flash than black powder and deliver much more mechanical work per unit of weight. The other advantages of smokeless powder are its improved stability in storage, its reduced erosive effects on gun bores, and the improved control obtainable over its rate of burning.
Most forms of gunpowder produced today are either single-base (i.e., consisting of nitrocellulose alone) or double-base (consisting of a combination of nitrocellulose and nitroglycerin). Both types are prepared by plasticizing nitrocellulose with suitable solvents, rolling it into thin sheets, and cutting the sheets into small squares called granules or grains, which are then dried. Control of the burning rate is achieved by varying the composition, size, and geometric shape of the propellant grains and sometimes by surface treatment or coating of the grains. Generally, the goal is to produce a propellant that is slowly converted to gas in the initial stages of burning and more rapidly converted as burning progresses.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2510 2025-04-08 17:24:30
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,708
Re: Miscellany
2310) Tent
Gist
The word “tent” comes from the Latin word tendere meaning “to stretch” – as in a piece of material stretched tautly across a frame. Tents are typically, but not always, thought of as portable shelters and it has been that way for a long time.
Summary
A tent is a portable shelter, consisting of a rigid framework covered by some flexible substance. Tents are used for a wide variety of purposes, including recreation, exploration, military encampment, and public gatherings such as circuses, religious services, theatrical performances, and exhibitions of plants or livestock. Tents have also been the dwelling places of most of the nomadic peoples of the world, from ancient civilizations such as the Assyrian to the 20th-century Bedouins of North Africa and the Middle East. American Indians developed two types of tent, the conical tepee and the arched wickiup, the latter constructed of thin branches or poles covered with bark or animal hides.
The simplest form of tent is an extremely portable type carried by individual soldiers in the field. When erected, it consists of a low pyramid, formed by a short, diagonally set pole at either end supporting two lengths of cloth joined together at the top and pegged into the ground at the bottom. This is a primitive form of the popular pyramidal A-shaped tent. A long-common tent, the conical bell tent, has a single large vertical pole at its centre and is circular at ground level. The tepee (q.v.) is a variant of this design. Other kinds of tent include the wall tent, an A-shaped tent raised to accommodate straight, vertical walls beneath the slope of the pyramid; the Baker tent, which is a rectangular fabric lean-to with an open front protected by a projecting horizontal flap; the umbrella tent, which was originally made with internal supporting arms like an umbrella but which later became widely popular with external framing of hollow aluminum; and the cabin tent, resembling a wall tent with walls four to six feet high. Special tent designs include mountain tents, which are designed compactly for use in conditions of extreme cold and heavy snow, and back-packing tents, which use extremely lightweight synthetic fabrics and lightweight metal poles. “Pop” tents are designed with spring-loaded frames that erect the tent automatically when released; these are usually hemispheric in shape.
Details
A tent is a shelter consisting of sheets of fabric or other material draped over or attached to a frame of poles or a supporting rope. While smaller tents may be free-standing or attached to the ground, large tents are usually anchored using guy ropes tied to stakes or tent pegs. First used as portable homes by nomads, tents are now more often used for recreational camping and as temporary shelters.
Tents range in size from "bivouac" structures, just big enough for one person to sleep in, up to huge circus tents capable of seating thousands of people. Tents for recreational camping fall into two categories. Tents intended to be carried by backpackers are the smallest and lightest type. Small tents may be sufficiently light that they can be carried for long distances on a touring bicycle, a boat, or when backpacking. The second type are larger, heavier tents which are usually carried in a car or other vehicle. Depending on tent size and the experience of the person or people involved, such tents can usually be assembled (pitched) in between 5 and 25 minutes; disassembly (striking) takes a similar length of time. Some very specialised tents have spring-loaded poles and can be pitched in seconds, but take somewhat longer to strike (take down and pack).
Over the past decade, tents have also been increasingly linked with homelessness crises in the United States, Canada, and other regions. Places of multiple homeless people living in tents closely pitched or plotted near each other are often referred to as tent cities.
History
A form of tent called a teepee or tipi, noted for its cone shape and peak smoke hole, was also used by Native American tribes and Aboriginal Canadians of the Plains Indians since ancient times, variously estimated from 10,000 to 4,000 years BC.
Tents were used at least as far back as the early Iron Age. They are mentioned in the Bible; for example, in Genesis 4:20 Jabal is described as "the first to live in tents and raise sheep and goats". The Roman Army used leather tents, copies of which have been used successfully by modern re-enactors. Various styles developed over time, some derived from traditional nomadic tents, such as the yurt.
Most military tents throughout history were of a simple ridge design. The major technological advance was the use of linen or hemp canvas for the canopy versus leather for the Romans. The primary use of tents was still to provide portable shelter for a small number of men in the field.
By World War I larger designs were being deployed in rear areas to provide shelter for support activities and supplies. Four types of tents which can be characterized by their unique shapes are A-Frame tents, Pyramid tents, Hoop tents , and Dome tents. tents tend not to be very spacious, given their ground surface area.
Use
Tents are used as habitation by nomads, recreational campers, soldiers, and disaster victims. Pole marquees, a type of large tent are typically used as overhead shelter for festivals, weddings, backyard parties, corporate events, excavation (construction) covers, and industrial shelters.
Traditional
Tents have traditionally been used by nomadic people all over the world, such as Native Americans, Mongolian, Turkic and Tibetan Nomads, and the Bedouin.
Military
Armies all over the world have long used tents as part of their working life. Tents are preferred by the military for their relatively quick setup and take down times, compared to more traditional shelters. One of the world's largest users of tents is the U.S. Department of Defense. The U.S. DoD has strict rules on tent quality and tent specifications. The most common tent uses for the military are temporary sleeping quarters (barracks); dining facilities (DFACs); field headquarters; morale, welfare, and recreation (MWR) facilities; and security checkpoints. One of the most popular military designs currently fielded is the TEMPER Tent, an acronym for Tent Expandable Modular PERsonnel. The United States Army is beginning to use a more modern tent called the deployable rapid assembly shelter or DRASH, a collapsible tent with provisions for air conditioning and heating.
Recreational
Camping is a popular form of recreation which often involves the use of tents. A tent is economical and practical because of its portability and low environmental impact. These qualities are necessary when used in the wilderness or backcountry.
Emergency
Tents are often used in humanitarian emergencies, such as war, earthquakes and fire. The primary choice of tents in humanitarian emergencies are canvas tents, because a cotton canvas tent allows functional breathability while serving the purpose of temporary shelter. Tents distributed by organisations such as UNHCR are made by various manufacturers, depending on the region where the tents are deployed, as well as depending on the purpose.
At times, however, these temporary shelters become a permanent or semi-permanent home, especially for displaced people living in refugee camps or shanty towns who can not return to their former home and for whom no replacement homes are made available.
Homelessness
Tents have been increasingly used as shelter for homeless people in the U.S., especially California, Oregon, and Washington. Encampments spiked in the mid-to-late 2010s. These tent cities housing many homeless and travelers/vagabonds have also, are also commonly found in major cities in the South, including Austin, Texas, which had passed a restriction on homeless encampments in May 2021.
Protest movements
Tents are also often used as sites and symbols of protest over time. In 1968 Resurrection City saw hundreds of tents set up by anti-poverty campaigners in Washington D.C. In the 1970s and 1980s anti-nuclear peace camps spread across Europe and North America, with the largest women's-only camp to date set up outside the RAF Greenham Common United States airbase in Newbury, England to protest the deployment there of cruise missiles during the Cold War. The 1990s saw environmental protest camps as part of the campaign for the Clayoquot Sound in Canada and the roads protests in the UK. The first No Border Network camp was held in Strasbourg in 2002, becoming the first in a series of international camps that continue to be organised today. Other international camps of the 2000s include summit counter-mobilisations like Horizone at the Gleneagles G8 gathering in 2005 and the start of Camp for Climate Action in 2006. Since September 2011, the tent has been used as a symbol of the Occupy movement,[citation needed] an international protest movement which is primarily directed against economic and social inequality. Occupy protesters use tents to create camps in public places wherein they can form communities of open discussion and democratic action.
General considerations
Generally, the interior of an enclosed tent is about 10 degrees Fahrenheit warmer than the outside environment (not accounting for wind chill), due to the retention of body heat and (to a lesser extent) radiation.
Tent fabric may be made of many materials including cotton (canvas), nylon, felt and polyester. Cotton absorbs water, so it can become very heavy when wet, but the associated swelling tends to block any minute holes so that wet cotton is more waterproof than dry cotton. Cotton tents were often treated with paraffin to enhance water resistance. Nylon and polyester are much lighter than cotton and do not absorb much water; with suitable coatings they can be very waterproof, but they tend to deteriorate over time due to a slow chemical breakdown caused by ultraviolet light. The most common treatments to make fabric waterproof are silicone impregnation or polyurethane coating. Since stitching makes tiny holes in a fabric seams are often sealed or taped to block these holes and maintain waterproofness, though in practice a carefully sewn seam can be waterproof.
Rain resistance is measured and expressed as hydrostatic head in millimetres (mm). This indicates the pressure of water needed to penetrate a fabric. Heavy or wind-driven rain has a higher pressure than light rain. Standing on a groundsheet increases the pressure on any water underneath. Fabric with a hydrostatic head rating of 1000 mm or less is best regarded as shower resistant, with 1500 mm being usually suitable for summer camping. Tents for year-round use generally have at least 2000 mm; expedition tents intended for extreme conditions are often rated at 3000 mm. Where quoted, groundsheets may be rated for 5000 mm or more.
Many tent manufacturers indicate capacity by such phrases as "3 berth" or "2 person". These numbers indicate how many people the manufacturer thinks can use the tent, though these numbers do not always allow for any personal belongings, such as luggage, inflatable mattresses, camp beds, cots, etc., nor do they always allow for people who are of above average height. Checking the quoted sizes of sleeping areas reveals that several manufacturers consider that a width of 150 cm (4.9 ft) is enough for three people; snug is the operative word.[original research?] Experience indicates that camping may be more comfortable if the actual number of occupants is one or even two less than the manufacturer's suggestion, though different manufacturers have different standards for space requirement and there is no accepted standard.
Tent used in areas with biting insects often have their vent and door openings covered with fine-mesh netting.
Tents can be improvised using waterproof fabric, string, and sticks.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2511 2025-04-09 19:10:23
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,708
Re: Miscellany
2311) Dry ice
Gist
Dry ice is carbon dioxide in its solid form, a dense, snowlike substance that sublimes (passes directly into the vapour without melting) at −78.5 °C (−109.3 °F), used as a refrigerant, especially during shipping of perishable products such as meats or ice cream. In the production of dry ice, advantage is taken of the spontaneous cooling that occurs when compressed, liquefied carbon dioxide at −57 °C (−71 °F) or lower is allowed suddenly to expand to atmospheric pressure: the liquid freezes to a finely divided solid that is compacted into cakes, weighing about 20 kg (45 pounds).
Summary
Dry ice is frozen carbon dioxide. A block of dry ice has a surface temperature of -109.3 degrees Fahrenheit (-78.5 degrees C). Dry ice also has the very nice feature of sublimation — as it breaks down, it turns directly into carbon dioxide gas rather than a liquid. The super-cold temperature and the sublimation feature make dry ice great for refrigeration.
Many people are familiar with liquid nitrogen, which boils at -320 degrees F (-196 degrees C). Liquid nitrogen is fairly messy and difficult to handle. So why is nitrogen a liquid while carbon dioxide is a solid? This difference is caused by the solid-liquid-gas features of nitrogen and carbon dioxide.
We are all familiar with the solid-liquid-gas behavior of water. We know that at sea level, water freezes at 32 degrees F (0 degrees C) and boils at 212 degrees F (100 degrees C). Water behaves differently as you change the pressure, however.
To make dry ice, you start with a high-pressure container full of liquid carbon dioxide. When you release the liquid carbon dioxide from the tank, the expansion of the liquid and the high-speed evaporation of carbon dioxide gas cools the remainder of the liquid down to the freezing point, where it turns directly into a solid.
If you have ever seen a carbon dioxide fire extinguisher in action, you have seen this carbon dioxide snow form in the nozzle. You compress the carbon dioxide snow to create a block of dry ice. Dry ice sublimates at temperatures higher than −109.2 °F so you will need to use it quick or store it at temperatures lower than -109.2 °F as unlike regular ice it turns into a gas rather than a liquid.
Details
Dry ice is the solid form of carbon dioxide. It is commonly used for temporary refrigeration as CO2 does not have a liquid state at normal atmospheric pressure and sublimes directly from the solid state to the gas state. It is used primarily as a cooling agent, but is also used in fog machines at theatres for dramatic effects. Its advantages include lower temperature than that of water ice and not leaving any residue (other than incidental frost from moisture in the atmosphere). It is useful for preserving frozen foods (such as ice cream) where mechanical cooling is unavailable.
Dry ice sublimes at 194.7 K (−78.5 °C; −109.2 °F) at Earth atmospheric pressure. This extreme cold makes the solid dangerous to handle without protection from frostbite injury. While generally not very toxic, the outgassing from it can cause hypercapnia (abnormally elevated carbon dioxide levels in the blood) due to buildup in confined locations.
Properties
Dry ice is the solid form of carbon dioxide (CO2), a molecule consisting of a single carbon atom bonded to two oxygen atoms. Dry ice is colorless, odorless, and non-flammable, and can lower the pH of a solution when dissolved in water, forming carbonic acid (H2CO3).
At pressures below 5.13 atm and temperatures below −56.4 °C (216.8 K; −69.5 °F) (the triple point), CO2 changes from a solid to a gas with no intervening liquid form, through a process called sublimation. The opposite process is called deposition, where CO2 changes from the gas to solid phase (dry ice). At atmospheric pressure, sublimation/deposition occurs at 194.7 K (−78.5 °C; −109.2 °F).
The density of dry ice increases with decreasing temperature and ranges between about 1.55 and 1.7 g/{cm}^{3} (97 and 106 lb/cu ft) below 195 K (−78 °C; −109 °F). The low temperature and direct sublimation to a gas makes dry ice an effective coolant, since it is colder than water ice and leaves no residue as it changes state. Its enthalpy of sublimation is 571 kJ/kg (25.2 kJ/mol, 136.5 calorie/g).
Dry ice is non-polar, with a dipole moment of zero, so attractive intermolecular van der Waals forces operate. The composition results in low thermal and electrical conductivity.
Additional Information
Dry ice is made by liquefying carbon dioxide and injecting it into a holding tank, where it’s frozen at a temperature of -109° F and compressed into solid ice. Depending on whether it’s created in a pelletizer or a block press, dry ice can then be made into pellets or large blocks.
Unlike regular ice, dry ice doesn’t melt into a liquid as it warms up. Instead, it converts directly back into its gaseous form in a process known as sublimation. At -109° F, dry ice is also significantly colder than the 32° F surface temperature of regular ice.
Dry Ice History
Dry ice was discovered in the early 1900s and first entered commercial production in the 1920s. The name “dry ice” has been used since 1925, when a manufacturer first trademarked it. Commonly found in commercial settings, the compound is versatile and offers benefits to a broad variety of industries.
The food and agriculture sector, for example, uses dry ice to keep food from spoiling during transport. Because of its low temperature, dry ice inhibits bacterial growth and slows decay, which makes the food crisper, fresher, and flavorful for as long as possible.
There are a multitude of other applications in commercial settings. The entertainment industry, for example, uses dry ice to create a smoky effect without an open flame.
Pest control technicians use it to force gophers out of their holes, which lets the technician close the burrows without hurting wildlife. Dry ice can also attract mosquitoes away from people and clean delicate electronics without corrosive chemical solvents.
Dry Ice Safety
Anyone can take advantage of the many benefits of dry ice, but there are several safety techniques to keep in mind:
* Wear heavy gloves before handling dry ice, as it can cause frostbite if it’s touched directly.
* While it’s safe to use dry ice near food, it should never be ingested, as it can cause internal frostbite.
* Only use dry ice in well-ventilated areas and don’t let the concentration of carbon dioxide in the air reach 5% or higher.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2512 2025-04-11 00:09:25
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,708
Re: Miscellany
2312) Angiogram
Gist
An angiogram is a medical imaging test that uses X-rays to visualize blood vessels, typically to detect blockages, narrowing, or other abnormalities, by injecting a special dye (contrast) that highlights the blood vessels.
Summary
Angiography, diagnostic imaging procedure in which arteries and veins are examined by using a contrast agent and X-ray technology. Blood vessels cannot be differentiated from the surrounding organs in conventional radiography. It is therefore necessary to inject into the lumen of the vessels a substance that will distinguish them from the surrounding tissues. The contrast medium used is a water-soluble substance containing iodine. On the radiograph, iodine-containing structures cast a denser shadow than do other body tissues. The technique in use today was developed in the early 1950s by Swedish cardiologist Sven-Ivar Seldinger.
In a typical angiography procedure, a needle is used to puncture the main artery in the groin, armpit, or crook of the arm and to place a coiled wire in the artery. The needle is withdrawn, and a small flexible hollow tube (catheter) is passed over the wire and into the artery. The wire is removed, and contrast medium is injected through the catheter. Both the arteries and the structures they supply with blood can then be visualized.
A technique called digital subtraction angiography (DSA) is particularly useful in diagnosing arterial occlusion (blockage). For example, it can be used to identify constriction (stenosis) of the carotid artery or clot formation (thrombosis) in a pulmonary artery. It also can be used to detect renal vascular disease. After contrast material is injected into an artery or vein, a physician produces fluoroscopic images. Using these digitized images, a computer subtracts the image made with contrast material from a postinjection image made without contrast material, producing an image that allows the dye in the arteries to be seen more clearly. In this manner, the images arising from soft tissues, bones, and gas are the same in both the initial and the subsequent image and are thereby eliminated by the subtraction process. The remaining images of blood vessels containing the contrast material are thus more prominent.
All organs of the body can be examined by using various angiography techniques. Radiographic evaluations of diseased arteries supplying the legs, the brain, and the heart are necessary before corrective surgical procedures are undertaken.
Details
An angiogram is a type of diagnostic test to identify blocked or narrowed blood vessels. The cost of the procedure varies based on multiple factors.
Angiograms, also called or arteriograms, can help doctors detect blood vessel abnormalities, including weakened blood vessels, plaque deposits, and blood clots. They can help doctors diagnose conditions affecting the heart, brain, arms, or legs.
This article discusses why doctors use angiograms, how they perform them, and the risks and side effects associated with the procedure.
It also provides tips for people recovering from an angiogram.
The term “angiogram” refers to a number of diagnostic tests that doctors can use to identify blocked or narrow blood vessels.
Doctors can do an angiogram on different parts of the body, such as:
* the heart, during the diagnosis or treatment of some aspects of heart disease
* the brain, to help diagnose a stroke or the risk of a stroke
* the chest or lungs, for example, to detect bleeding
* the kidneys, to look for high pressure in the renal blood vessels
* the reproductive system, during embolization of tubes or fibroids
* after a trauma to the legs, arms, eyes, or any other body part, to diagnose tears, bleeding, and other problems
* the liver, for example, if a person has cancer
Angiograms also help doctors diagnose a range of cardiovascular diseases, including:
* coronary atherosclerosis
* vascular stenosis
* aneurysms
To perform a traditional angiogram, a doctor will:
* insert a long, narrow tube called a catheter into an artery located in the arm, upper thigh, or groin
* inject contrast dye into the catheter
* take X-rays of the blood vessels
The contrast dye makes blood vessels more visible on X-ray images.
Not all angiograms involve X-ray machines, however. Doctors can also perform angiograms using CT scans and MRI scans.
A doctor may order an angiogram if someone:
* shows signs of a blocked or narrow artery, such as abnormal stress test results
* experiences new or unusual chest pain
* has had a stroke, heart attack, or heart failure
* has or may have other problems that could affect the blood vessels
Angiograms are commonly used to detect heart disease. According to the Centers for Disease Control and Prevention (CDC), heart disease accounts for around 1 in every 4 deaths.
Early diagnosis and prompt treatment can lower a person’s risk of dying from heart disease. Doctors use various tests and procedures, such as angiograms, to identify and treat different types of heart disease.
What do doctors use angiograms for?
A doctor can use an angiogram to examine blood vessels and changes that involve the vascular system in almost any part of the body. It can help detect cardiovascular disease, stroke, and other vascular problems.
Doctors use angiogram results to diagnose the following conditions:
* aneurysms, or bulges that develop in weakened artery walls
* atherosclerosis, which occurs when plaque and fatty material collect on the inner walls of the arteries
* pulmonary embolisms, or blood clots in the lungs
* vascular stenosis, which causes abnormal narrowing of the blood vessels that lead to the brain, heart, or legs
* structural problems in the blood vessels or heart that have been present since birth
A doctor may also order an angiogram to:
* evaluate the health of a person’s blood vessels before surgery
* identify blood vessels feeding a tumor
* plan treatments, such as a coronary bypass, stenting, or chemoembolization
* evaluate a stent after placement
Procedure
The following sections discuss what to expect before, during, and after an angiogram.
Preparation
A doctor will explain how to prepare for an angiogram during the appointment before the procedure. In most cases, people will need to avoid eating and drinking anything the night before the procedure.
People should also arrange for someone to drive them home after they leave the hospital.
It’s important for a person to remember to bring the following items:
* a list of current medications and supplements
* a list of all known allergies
* a current driver’s license or another form of identification
* current medical insurance information
After the person signs in, a nurse will lead them to a private room where they can change into a hospital gown.
The nurse will then insert an intravenous (IV) line into a small vein on the person’s hand or wrist. They will also check the person’s vitals, including their:
* weight
* body temperature
* heart rate
* blood pressure
During the procedure
Before the test, a doctor may administer a mild sedative, which will help the person relax. It will not induce unconsciousness.
The doctor will then disinfect and numb the area of the body where they will insert the catheter. They will make a small cut in the skin and insert the catheter into an artery.
Once the catheter is inside the artery, the doctor will carefully guide it to the blood vessel they want to examine. They will inject the contrast dye through the catheter and take X-ray images of the blood vessel. The person may feel a slight burning sensation when the doctor injects the contrast dye.
After the procedure
After taking the X-ray images, the doctor will remove the catheter and apply steady pressure on the area for about 15 minutes. This ensures that there is no internal bleeding.
A nurse will then take the person back to their hospital room or to the cardiac unit. The doctor may return later to discuss the person’s results.
Interpreting the results
Doctors use angiograms to evaluate the flow of blood to the heart, brain, and other organs. An abnormal angiogram result may indicate that a person has one or more blocked arteries.
In these cases, the doctor may choose to treat the blockage during the angiogram.
What are the risks?
Most people have a very low risk of developing major complications after an angiogram. However, this invasive procedure does have some risks, which are mainly associated with the process of inserting a catheter into the heart.
According to the National Heart, Lung, and Blood InstituteTrusted Source, older adults and people with certain medical conditions, such as chronic kidney disease or diabetes, have a higher risk of experiencing complications after an angiogram.
Risks associated with cardiac catheterization and angiograms include:
* allergic reactions to the local anesthetic, contrast dye, or sedative
* bleeding, bruising, or soreness at the insertion site
* blood clots
* injury to an artery or vein
* damage to the walls of the heart
* acute kidney failure
* infection
* irregular heartbeat
* heart attack or stroke, though this is highly unlikely
People who have had an allergic reaction to contrast dye in the past may need to take medication to reduce the risk of having another allergic reaction. People should take this medication at least 24 hours before the angiogram procedure.
To completely eliminate the risk of an allergic reaction, a doctor can choose to use a different method than the traditional angiogram.
Recovery
After an angiogram, a person will need to rest for a while.
Tips that may help during recovery include:
* avoiding driving or operating machinery until any sedative has completely worn off
* drinking plenty of water
* avoiding strenuous physical activity for the few first days
* keeping the wound clean and dry
* avoiding taking baths, using hot tubs, or swimming in pools while the wound heals
People should contact their doctor if they suspect they have an infection. Symptoms of an infection include:
* redness, swelling, or worsening pain near the wound
* drainage or discharge from the wound
* fever
Additional Information
An angiogram is a diagnostic procedure that uses imaging to show your provider how your blood flows through your blood vessels or heart. An injected contrast material makes it easy to see where blood is moving and where blockages are. Your provider can use X-rays or other types of imaging for your angiogram.
Overview:
What is an angiogram?
An angiogram is a diagnostic procedure that uses X-ray images to look for blockages or narrow spots in your blood vessels (arteries or veins). An angiogram test can show how blood circulates in blood vessels at specific locations in your body. Healthcare providers use an angiogram of your heart, neck, kidneys, legs or other areas to locate the source of an artery or vein issue.
Your healthcare provider may want to do an angiogram procedure when you have signs of blocked, damaged or abnormal blood vessels. An angiogram test helps your provider determine the source of the problem and the extent of damage to your blood vessels.
With an angiogram procedure, your provider can diagnose and plan treatment for conditions like:
* Coronary artery disease (blockage or narrowing in the arteries that supply your heart)
* Peripheral artery disease (blockage or narrowing in your leg arteries)
* Blood clots (mass of blood cells)
* Aneurysm (weak artery wall)

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2513 2025-04-15 00:06:54
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,708
Re: Miscellany
2313) Social Media
Gist
Social media encompasses online platforms and applications where individuals and organizations can connect, share information, and engage in conversations, often through text, images, videos, and other content. These platforms facilitate communication and community building in virtual spaces.
Social media is an internet-based form of communication. Social media platforms allow users to have conversations, share information and create web content.
Social media are interactive technologies that facilitate the creation, sharing and aggregation of content (such as ideas, interests, and other forms of expression) amongst virtual communities and networks.
Summary
Social media: social media, a form of mass media communications on the Internet (such as on websites for social networking and microblogging) through which users share information, ideas, personal messages, and other content (such as videos). Social networking and social media are overlapping concepts, but social networking is usually understood as users building communities among themselves while social media is more about using social networking sites and related platforms to build an audience.
The earliest forms of social media appeared almost as soon as technology could support them. E-mail and chat programs debuted in the early 1970s, but persistent communities did not surface until the creation of the discussion group network USENET in 1979. USENET allowed users to post and receive messages within subject areas called newsgroups. USENET and other discussion forums, such as privately hosted bulletin board systems (BBSs), enabled individuals to interact, but each was essentially a closed system. With the release in 1993 of the Mosaic web browser, those systems were joined with an easy-to-use graphical interface. The architecture of the World Wide Web made it possible to navigate from one site to another with a click, and faster Internet connections allowed for more multimedia content than could be found in the text-heavy newsgroups.
The first companies to create social networks based on web technology were Classmates.com and SixDegrees.com. Classmates.com, founded in 1995, used an aggressive pop-up advertising campaign to draw web surfers to its site. It based its social network on the existing connection between members of high-school and college graduating classes, armed service branches, and workplaces. SixDegrees.com was the first true social networking site. It was launched in 1997 with most of the features that would come to characterize such sites: members could create profiles for themselves, maintain lists of friends, and contact one another through the site’s private messaging system. SixDegrees.com claimed to have attracted more than three million users by 2000, but it failed to translate those numbers into revenue and collapsed with countless other dot-coms when the “bubble” burst that year for shares of e-commerce companies.
Nevertheless, social media sites became popular in the early 21st century. Social networks such as Friendster and MySpace emerged that allowed family members, friends, and acquaintances to connect online. Those two sites were eventually supplanted by Facebook, which became one of the world’s most popular social media sites with billions of users worldwide. Other forms of social media emerged for the sharing of specific types of content. For example, YouTube allows users to share videos, and TikTok is specifically designed for the sharing of short videos. LinkedIn emphasizes a user’s professional connections, where users create pages similar in structure to résumés.
Concerns over the possible negative effects of social media are also growing in tandem with the burgeoning technology. For example, some observers suggest that social media sites spur greater schadenfreude—the emotional experience of pleasure in response to another’s misfortune—perhaps as a result of the dehumanization that occurs when interacting through screens on computers and mobile devices. Some studies also suggest a strong tie between heavy social media use and increased depression, anxiety, loneliness, suicidal tendencies, and feelings of inadequacy. During his second tenure as U.S. surgeon general, Vivek Murthy raised concerns about social media’s impact on children and in 2024 he suggested mandated warning labels on social media sites.
Details
Social media refers to websites and applications that focus on communication, community-based input, interaction, content-sharing and collaboration.
Over the past decade, social media has evolved beyond just a tool for connecting with friends and family. It now serves as a platform for news dissemination, entertainment, and even commerce, becoming a significant part of both personal lives and business operations.
People use social media to stay in touch and interact with various communities, follow trends, and stay informed. In the business world, social media serves as a key tool for marketing, product promotion, customer service, and engagement. Businesses use these platforms to communicate with customers and gather feedback.
Today, nearly all business-to-consumer websites have social components, such as comment fields or social sharing buttons, that make engagement easy. Various tools help companies track, measure, and analyze how their brand is perceived across social media platforms.
Mobile applications have significantly expanded the reach of social media, making these platforms accessible anytime, anywhere. Examples of popular platforms include X (formerly Twitter), Facebook, TikTok, Instagram and LinkedIn. Each platform serves its own unique purpose and audience.
What are the business applications of social media?
Businesses use social media to market products, promote their brand and engage with customers. Social platforms facilitate customer feedback and let customers share their positive or negative experiences. This helps businesses react quickly to customer concerns and maintain consumer confidence.
Social media is increasingly being used for crowdsourcing, a practice where companies use social networking platforms to gather ideas, services, or goods from a broader community such as employees, customers, and the public. This can be invaluable for product improvement or development.
Business to business (B2B) applications for social media include the following:
* Social media analytics. Companies gather data from social platforms and blogs to analyze customer sentiment analysis and make business decisions. This data-driven social media analytics approach enables them to adjust strategies in real time.
* Social media marketing (SMM). This increases brand exposure by creating shareable content that spreads organically across networks. SMM often includes social media optimization (SMO), which draws visitors to a website through strategic posts, updates and blogs.
* Social customer relationship management. Businesses use social CRM tools to foster stronger relationships with their audience. A company's social media pages, such as those on Facebook or Instagram, allow followers to connect, which enhances engagement and helps monitor customer sentiment in real-time.
* Recruiting. Social recruiting has become an integral part of many organizations' hiring strategies, leveraging platforms like LinkedIn to reach a broad candidate pool. It allows businesses to quickly identify and engage with potential hires.
* Enterprise social networking. Tools like Slack, Yammer and Microsoft Teams are widely used for internal communication, collaboration and project management, allowing employees to connect and share information. These platforms also enable businesses to gather valuable market research from external social networks.
What are the benefits of social media?
Social media provides several benefits, including the following:
* User visibility. Social platforms let people easily communicate and exchange ideas or content.
* Business and product marketing. Businesses can quickly promote their products or services to a global audience. Many companies now rely on social media to conduct market research and nurture their customer base. In some industries, like entertainment, the content created on social platforms is the product.
* Audience building. Platforms like Instagram, TikTok and YouTube help individuals -- especially entrepreneurs, artists, and creators -- build a following without needing traditional distributors. For instance, a musician can upload their music to a platform, immediately gaining visibility through their network and beyond.
What are the challenges of social media?
Despite its benefits, social media also poses challenges for individuals and businesses:
* Mental health issues. Overuse of social platforms can contribute to mental health concerns like anxiety, depression, and social media addiction. Research shows that constant exposure to curated content can lead to negative self-comparisons and exacerbate feelings of inadequacy.
* Polarization. Social algorithms often create filter bubbles, where users are exposed only to content that aligns with their existing views, leading to increased polarization and limiting exposure to diverse perspectives.
* Disinformation. Social media can be a breeding ground for misinformation and disinformation. The viral nature of these platforms allows false or misleading information to spread quickly, often with malicious intent.
Businesses face similar and unique social media challenges. These include:
* Offensive posts. Employees using enterprise social platforms can sometimes drift into discussions unrelated to work, leading to potentially offensive or divisive conversations.
* Security risks. Traditional data security and retention policies may not cover adequately the features available in collaboration tools, increasing security risks and compliance concerns.
* Productivity issues. The constant influx of social notifications can become a distraction and hamper employee productivity.
What are enterprise social media best practices?
It's essential for businesses to create a clear social media strategy and set measurable goals to build trust, increase brand awareness and engage with customers. Social media best practices include the following:
* Establishing social media policies. Businesses should create policies that guide employee behavior on social platforms, ensuring no legal or reputational risks arise from inappropriate posts.
* Focusing on the right platforms. Companies should prioritize platforms that best serve their audience. For example, LinkedIn and X are well-suited for B2B marketing.
* Creating engaging content. Rich media like videos, images, and infographics tend to perform better on social platforms. It's important to post content that resonates with users and encourages interaction.
* Using analytics tools. Tracking engagement and staying on top of trends helps businesses adjust their strategies and ensure their social media campaigns are successful.
* Promoting authentic conversations. Social media should reflect the brand's voice while remaining professional. Businesses should engage customers and employees, celebrating positive interactions.
* Checking analytics frequently. Consistent monitoring ensures businesses stay ahead of potential issues and can measure the success of campaigns.
What are the different types of social media?
The four main categories of social platforms are:
* Social networks. These focus on connecting individuals with shared interests. Examples include Facebook and LinkedIn.
* Media-sharing networks. These platforms center around sharing visual content like photos or videos. Examples include YouTube, Instagram and TikTok.
* Community-based networks. These encourage in-depth discussions on specific topics. Reddit is an example.
* Review networks. These focus on reviewing products or services. Examples include Yelp and TripAdvisor.
What are examples of social media?
Here are some examples of popular web-based social media platforms:
* Facebook: A widely-used social networking site where users can create profiles, share content, and communicate with friends and family.
* LinkedIn: A platform designed for professional networking where users can connect and share business-related information.
* Pinterest: A social curation platform where users can share and categorize images.
* Reddit: A discussion-based platform where users engage in conversations around specific topics.
* X: A microblogging platform where users post short updates or "tweets."
* Wikipedia: A collaborative platform where users contribute and edit content in an open-source encyclopedia.
Additional Information
Social media are interactive technologies that facilitate the creation, sharing and aggregation of content (such as ideas, interests, and other forms of expression) amongst virtual communities and networks. Common features include:
* Online platforms that enable users to create and share content and participate in social networking.
* User-generated content—such as text posts or comments, digital photos or videos, and data generated through online interactions.
* Service-specific profiles that are designed and maintained by the social media organization.
* Social media helps the development of online social networks by connecting a user's profile with those of other individuals or groups.
The term social in regard to media suggests platforms enable communal activity. Social media can enhance and extend human networks.[6] Users access social media through web-based apps or custom apps on mobile devices. These interactive platforms allow individuals, communities, and organizations to share, co-create, discuss, participate in, and modify user-generated or self-curated content. Social media is used to document memories, learn, and form friendships. They may be used to promote people, companies, products, and ideas.[8] Social media can be used to consume, publish, or share news.
Social media platforms can be categorized based on their primary function. Social networking sites like Facebook, LinkedIn, and Threads focus on building personal and professional connections. Microblogging platforms, such as Twitter (now X) and Mastodon, emphasize short-form content and rapid information sharing. Media sharing networks, including Instagram, TikTok, YouTube, and Snapchat, allow users to share images, videos, and live streams. Discussion and community forums like Reddit, Quora, and Discord facilitate conversations, Q&A, and niche community engagement. Live streaming platforms, such as Twitch, Facebook Live, and YouTube Live, enable real-time audience interaction. Finally, decentralized social media platforms like Mastodon and Bluesky aim to provide social networking without corporate control, offering users more autonomy over their data and interactions.
Popular social media platforms with over 100 million registered users include Twitter, Facebook, WeChat, ShareChat, Instagram, Pinterest, QZone, Weibo, VK, Tumblr, Baidu Tieba, Threads and LinkedIn. Depending on interpretation, other popular platforms that are sometimes referred to as social media services include YouTube, Letterboxd, QQ, Quora, Telegram, WhatsApp, Signal, LINE, Snapchat, Viber, Reddit, Discord, and TikTok. Wikis are examples of collaborative content creation.
Social media outlets differ from old media (e.g. newspapers, TV, and radio broadcasting) in many ways, including quality,[9] reach, frequency, usability, relevancy, and permanence. Social media outlets operate in a dialogic transmission system (many sources to many receivers) while traditional media operate under a monologic transmission model (one source to many receivers). For instance, a newspaper is delivered to many subscribers, and a radio station broadcasts the same programs to a city.
Social media has been criticized for a range of negative impacts on children and teenagers, including exposure to inappropriate content, exploitation by adults, sleep problems, attention problems, feelings of exclusion, and various mental health maladies. Social media has also received criticism as worsening political polarization and undermining democracy. Major news outlets often have strong controls in place to avoid and fix false claims, but social media's unique qualities bring viral content with little to no oversight. "Algorithms that track user engagement to prioritize what is shown tend to favor content that spurs negative emotions like anger and outrage. Overall, most online misinformation originates from a small minority of "superspreaders," but social media amplifies their reach and influence."

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2514 2025-04-16 18:51:59
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,708
Re: Miscellany
2314) Mountain
Gist
A mountain is an elevated portion of the Earth's crust, generally with steep sides that show significant exposed bedrock.
Summary
A mountain is a landform that rises prominently above its surroundings, generally exhibiting steep slopes, a relatively confined summit area, and considerable local relief. Mountains generally are understood to be larger than hills, but the term has no standardized geological meaning. Very rarely do mountains occur individually. In most cases, they are found in elongated ranges or chains. When an array of such ranges is linked together, it constitutes a mountain belt. For a list of selected mountains of the world, see below.
A mountain belt is many tens to hundreds of kilometres wide and hundreds to thousands of kilometres long. It stands above the surrounding surface, which may be a coastal plain, as along the western Andes in northern Chile, or a high plateau, as within and along the Plateau of Tibet in southwest China. Mountain ranges or chains extend tens to hundreds of kilometres in length. Individual mountains are connected by ridges and separated by valleys. Within many mountain belts are plateaus, which stand high but contain little relief. Thus, for example, the Andes constitute a mountain belt that borders the entire west coast of South America; within it are both individual ranges, such as the Cordillera Blanca in which lies Peru’s highest peak, Huascarán, and the high plateau, the Altiplano, in southern Peru and western Bolivia.
Details
A mountain is an elevated portion of the Earth's crust, generally with steep sides that show significant exposed bedrock. Although definitions vary, a mountain may differ from a plateau in having a limited summit area, and is usually higher than a hill, typically rising at least 300 metres (980 ft) above the surrounding land. A few mountains are isolated summits, but most occur in mountain ranges.
Mountains are formed through tectonic forces, erosion, or volcanism, which act on time scales of up to tens of millions of years. Once mountain building ceases, mountains are slowly leveled through the action of weathering, through slumping and other forms of mass wasting, as well as through erosion by rivers and glaciers.
High elevations on mountains produce colder climates than at sea level at similar latitude. These colder climates strongly affect the ecosystems of mountains: different elevations have different plants and animals. Because of the less hospitable terrain and climate, mountains tend to be used less for agriculture and more for resource extraction, such as mining and logging, along with recreation, such as mountain climbing and skiing.
The highest mountain on Earth is Mount Everest in the Himalayas of Asia, whose summit is 8,850 m (29,035 ft) above mean sea level. The highest known mountain on any planet in the Solar System is Olympus Mons on Mars at 21,171 m (69,459 ft). The tallest mountain including submarine terrain is Mauna Kea in Hawaii from its underwater base at 9,330 m (30,610 ft); some scientists consider it to be the tallest on earth.
Definition
There is no universally accepted definition of a mountain. Elevation, volume, relief, steepness, spacing and continuity have been used as criteria for defining a mountain. In the Oxford English Dictionary a mountain is defined as "a natural elevation of the earth surface rising more or less abruptly from the surrounding level and attaining an altitude which, relatively to the adjacent elevation, is impressive or notable."
Whether a landform is called a mountain may depend on local usage. John Whittow's Dictionary of Physical Geography states "Some authorities regard eminences above 600 metres (1,969 ft) as mountains, those below being referred to as hills."
In the United Kingdom and the Republic of Ireland, a mountain is usually defined as any summit at least 2,000 feet (610 m) high, which accords with the official UK government's definition that a mountain, for the purposes of access, is a summit of 2,000 feet (610 m) or higher. In addition, some definitions also include a topographical prominence requirement, such as that the mountain rises 300 metres (984 ft) above the surrounding terrain. At one time, the United States Board on Geographic Names defined a mountain as being 1,000 feet (305 m) or taller, but has abandoned the definition since the 1970s. Any similar landform lower than this height was considered a hill. However, today, the United States Geological Survey concludes that these terms do not have technical definitions in the US.
Additional Information
A mountain is a large natural rise of the Earth's surface that usually has a "summit" (the name for a mountain's top, which can also be called a peak). It is usually steeper and taller than a hill. By definition, mountains are often thought of as being a hill which is higher than 600 meters (about 2,000 feet). However, some definitions say a mountain is a hill higher than 300 meters (about 1,000 feet).
Definition of a mountain
* A mountain is landform that rises prominently above its surroundings. It usually has steep slopes, and a summit area.
* The highest point of a mountain is called the peak. A mountain's summit is the highest area where an individual can reach. A mountain climber will not reach the peak of the mountain but can reach the summit.
* Britannica Student Encyclopedia says that the term "mountain" usually means a rise of over 2,000 feet (610 m)".
* Polytechnic Student Encyclopedia says that the term "mountain" usually means a rise of over 1,000 feet (300 m)".
* The standard height for a mountain in England is 600 metres. In England, this is important because in English law people have the "Right to Roam" in mountains, but they do not have the same right to walk on someone-else's land.
Formation
The forming of a mountain is called orogeny. Mountains are formed when rock layers in the ground are pushed from opposite sides, and by being pushed, they push the crust up. A mountain range is a large group of mountains beside each other. There are three main ways a mountain may be made:
Fold mountains
Fold mountains occur when two plates collide. The less dense continental crust "floats" on the denser mantle rocks beneath. The continental crust is normally much thicker under mountains, compared to lower lying areas.[4] Rock can fold either symmetrically or asymmetrically. The upfolds are anticlines and the downfolds are synclines. The Jura Mountains are an example of fold mountains.
Folded mountains make up some of the highest mountains in the world. Folded mountains commonly form along boundaries, where 2 continents meet. Some really complex folds are in parts of the Andes, Alps, Himalayas, Appalachians, and Russia's Ural Mountains. These long mountain chains also show lots of signs of folding.
Block mountains
Block mountains are caused by faults in the crust: a seam where rocks can move past each other. When rocks on one side of a fault rise relative to the other, it can form a mountain. The uplifted blocks are block mountains or horsts. The dropped blocks are called graben. They can form extensive rift valley systems. This form of landscape can be seen in East Africa, the Vosges, the Basin and Range province of Western North America and the Rhine valley. These areas often occur when the regional stress is extensional and the crust is thinned.
Volcanic mountains
Volcanoes are formed in one of these ways:
* When a tectonic plate is pushed below another tectonic plate,
* at a mid-ocean ridge or hotspot. At a depth of around 680 kilometres (2,230,000 ft), melting occurs in rock above the slab (due to the addition of water), and forms magma that reaches the surface. When the magma reaches the surface, it often builds a volcanic mountain, such as a shield volcano or a stratovolcano.
Examples of volcanoes include Mount Fuji in Japan and Mount Pinatubo in the Philippines. The magma does not have to reach the surface in order to create a mountain: magma that solidifies below ground can still form dome mountains, such as Navajo Mountain in the states of Utah and Arizona, in the United States.
Volcanic mountains form when molten rock erupts onto the Earth's surface. They can either form on land or in the ocean. The Cascade Range in Washington, Oregon and northern California is made of volcanoes. Some of the largest volcanoes are on divergent boundaries, which form the mid-ocean ridges. The mid-ocean ridges have big volcanic mountain chains that run through the Atlantic, Pacific and Indian Oceans. The mountains in the mid-ocean ridges can grow tall enough to create islands such as Iceland or the Azores.
Other volcanic mountains form over hot spots, pockets of magma beneath the crust which erupt onto Earth's surface. The Hawaiian Islands are the tops of really high volcanic islands that have formed over a hot spot on the sea floor. The main Hawaiian island is a volcano about 98 kilometres (322,000 ft) above the ocean floor. Its base is about 680 kilometres (2,230,000 ft) wide. Almost 48 kilometres (157,000 ft) of this island is above sea level.
Other terms:
Dome mountains
Dome mountains, like those in the Black Hills of South Dakota and the Adirondack Mountains of New York, are an unusual domish type of mountain that is formed when molten rock rises through the crust and push up the rock layers above it. This creates a circular dome on the Earth's surface. The molten rock later cools off and forms hardened rock. When the pushed up rocks are worn away, the hardened rock is shown. This hardened rock then wears away in places. When it wears away, it leaves mountains, and they are called dome mountains.
Plateau mountains
Plateau mountains are formed a bit like folded mountains. They are large areas of flat topped rocks that have been lifted high above the crust by continental plates. Most plateaus are near folded mountains.
Height:
The height of a mountain is measured as distance above sea level.
Tallest mountains
The highest known mountain in the Solar System is Olympus Mons (27 km high) on Mars. The highest mountain on Earth is Mount Everest (8,848m) which is in Nepal and Tibet, in Asia.
The "tallest" mountain in the world is Mauna Loa, in Hawaii. The "height" of a mountain is measured from sea level, but the "tallness" of a mountain is measured from its base, even if under water. The highest mountain in North America is Mount McKinley (6,194m) in Alaska in the USA. The highest in South America is Aconcagua (6,962m) in Argentina. For Africa, it is Kilimanjaro (5,963m) of Tanzania. In Europe, the highest mountain is in Russia called Elbrus (5,633m). Antarctica's highest mountain is Vinsin Massiff (5,140m). In Oceania, a mountain called Puncak Jaya (5,030m) is the highest there. This particular mountain is in Papua New Guinea / Indonesia.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2515 2025-04-17 19:02:52
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,708
Re: Miscellany
2315) Gorge
Gist
A gorge is a narrow valley with steep, rocky walls located between hills or mountains. The term comes from the French word gorge, which means throat or neck. A gorge is often smaller than a canyon, although both words are used to describe deep, narrow valleys with a stream or river running along their bottom.
Summary
A gorge is a narrow valley with steep, rocky walls located between hills or mountains. The term comes from the French word gorge, which means throat or neck. A gorge is often smaller than a canyon, although both words are used to describe deep, narrow valleys with a stream or river running along their bottom.
A number of natural forces form gorges. The most common is erosion due to streams or rivers. Streams carve through hard layers of rock, breaking down or eroding it. Sediment from the worn-away rock is then carried downstream. Over time, this erosion will form the steep walls of a gorge. The flooding of streams or rivers increases the speed and intensity of this erosion, creating deeper and wider gorges. The deep Talari Gorges in Mali, for instance, were formed by the Senegal River that flows into the Atlantic Ocean on the western coast of Africa.
Geologic uplift also forms gorges. Geologic uplift is the upward movement of the Earth's surface. Geologic uplift is often associated with earthquakes and orogeny, the process of creating mountains. During geologic uplift, rock layers beneath Earth's surface bump against the surface layers. Softer layers of surface rock erode.
Erosion and geologic uplift often work together to create gorges. Parts of streams or rivers can be elevated, along with land, during the process of geologic uplift. As rivers or streams flow across this uplifted surface, waterfalls form. Over time, the power of the waterfall erodes the softer rock layers underneath, causing the original river bed to collapse and create a gorge. Macocha Gorge in the Jihomoravsk region of the Czech Republic was probably formed by the collapse of an underground cave that had been eroded by the Punkva River.
The movement and melting of glaciers can also produce gorges. Glaciers cut deep valleys into the Earth's surface. These rivers of ice can create huge canyons and sharp, steep gorges. As glaciers melt, or retreat, these gorges and canyons are exposed. The Columbia River Gorge, located in the U.S. states of Washington and Oregon, was partially created by glacial retreat during the last Ice Age.
Engineers have purposely flooded gorges in order to create waterways and dams. These dams generate hydroelectricity, or electricity powered by water. The Three Gorges Dam on the Yangtze River in China is probably the most famous example of such a project. Upstream from the dam, the Qutang, Wu, and Xilang gorges were partially submerged in order to create a waterway. The new waterway would allow freight ships to navigate from the East China Sea, part of the Pacific Ocean, to the city of Chongqing, about 2,250 kilometers (1,400 miles) inland. The 26 turbines of the Three Gorges Dam generate approximately 18,000 megawatts of electricity for Shanghai and other cities. However, many people worry about the environmental impacts of the dam and criticize the fact that more than a million Chinese families were forced to move from their homes near the gorges in order to complete the construction.
Many geological discoveries have been made at gorges because gorges often expose layers of rock that go back thousands of years. Olduvai Gorge in Tanzania has layers dating as far back as two million years. The Olduvai Gorge is famous for the fossils and ancient tools found there by scientists Louis, Mary, and Richard Leakey. These remains of ancient animals and plants provide clues about early humans.
Details
A canyon (from Spanish: cañón; archaic British English spelling: cañon), gorge or chasm, is a deep cleft between escarpments or cliffs resulting from weathering and the erosive activity of a river over geologic time scales. Rivers have a natural tendency to cut through underlying surfaces, eventually wearing away rock layers as sediments are removed downstream. A river bed will gradually reach a baseline elevation, which is the same elevation as the body of water into which the river drains. The processes of weathering and erosion will form canyons when the river's headwaters and estuary are at significantly different elevations, particularly through regions where softer rock layers are intermingled with harder layers more resistant to weathering.
A canyon may also refer to a rift between two mountain peaks, such as those in ranges including the Rocky Mountains, the Alps, the Himalayas or the Andes. Usually, a river or stream carves out such splits between mountains. Examples of mountain-type canyons are Provo Canyon in Utah or Yosemite Valley in California's Sierra Nevada. Canyons within mountains, or gorges that have an opening on only one side, are called box canyons. Slot canyons are very narrow canyons that often have smooth walls.
Steep-sided valleys in the seabed of the continental slope are referred to as submarine canyons. Unlike canyons on land, submarine canyons are thought to be formed by turbidity currents and landslides.
Etymology
The word canyon is Spanish in origin with the same meaning. The word canyon is generally used in North America, while the words gorge and ravine (French in origin) are used in Europe and Oceania, though gorge and ravine are also used in some parts of North America. In the United States, place names generally use canyon in the southwest (due to their proximity to Spanish-speaking Mexico) and gorge in the northeast (which is closer to French Canada), with the rest of the country graduating between these two according to geography. In Canada, a gorge is usually narrow while a ravine is more open and often wooded. The military-derived word defile is occasionally used in the United Kingdom. In South Africa, kloof (in Krantzkloof Nature Reserve) is used along with canyon (as in Blyde River Canyon) and gorge (in Oribi Gorge).
Formation
Most canyons were formed by a process of long-time erosion from a plateau or table-land level. The cliffs form because harder rock strata that are resistant to erosion and weathering remain exposed on the valley walls.
Canyons are much more common in arid areas than in wet areas because physical weathering has a more localized effect in arid zones. The wind and water from the river combine to erode and cut away less resistant materials such as shales. The freezing and expansion of water also serves to help form canyons. Water seeps into cracks between the rocks and freezes, pushing the rocks apart and eventually causing large chunks to break off the canyon walls, in a process known as frost wedging. Canyon walls are often formed of resistant sandstones or granite.
Sometimes large rivers run through canyons as the result of gradual geological uplift. These are called entrenched rivers, because they are unable to easily alter their course. In the United States, the Colorado River in the Southwest and the Snake River in the Northwest are two examples of tectonic uplift.
Canyons often form in areas of limestone rock. As limestone is soluble to a certain extent, cave systems form in the rock. When a cave system collapses, a canyon is left, as in the Mendip Hills in Somerset and Yorkshire Dales in Yorkshire, England.
Box canyon
A box canyon is a small canyon that is generally shorter and narrower than a river canyon, with steep walls on three sides, allowing access and egress only through the mouth of the canyon. Box canyons were frequently used in the western United States as convenient corrals, with their entrances fenced.
Largest
The definition of "largest canyon" is imprecise, because a canyon can be large by its depth, its length, or the total area of the canyon system. Also, the inaccessibility of the major canyons in the Himalaya contributes to their not being regarded as candidates for the biggest canyon. The definition of "deepest canyon" is similarly imprecise, especially if one includes mountain canyons, as well as canyons cut through relatively flat plateaus (which have a somewhat well-defined rim elevation).
The Yarlung Tsangpo Grand Canyon (or Tsangpo Canyon), along the Yarlung Tsangpo River in Tibet, China, is regarded by some as the deepest canyon on Earth at 5,500 metres (18,000 ft). It is slightly longer than Grand Canyon in the United States. Others consider the Kali Gandaki Gorge in midwest Nepal to be the deepest canyon, with a 6,400-metre (21,000 ft) difference between the level of the river and the peaks surrounding it.
Vying for the deepest canyon in the Americas is the Cotahuasi Canyon and Colca Canyon, in southern Peru. Both have been measured at over 3,500 metres (11,500 ft) deep.
Grand Canyon of northern Arizona in the United States, with an average depth of 1,600 metres (5,200 ft) and a volume of 4.17 trillion cubic metres (147 trillion cubic feet), is one of the world's largest canyons. It was among the 28 finalists of the New 7 Wonders of Nature worldwide poll. (Some referred to it as one of the seven natural wonders of the world.)
The largest canyon in Europe is Tara River Canyon.
The largest canyon in Africa is the Fish River Canyon in Namibia.
In August 2013, the discovery of Greenland's Grand Canyon was reported, based on the analysis of data from Operation IceBridge. It is located under an ice sheet. At 750 kilometres (470 mi) long, it is believed to be the longest canyon in the world.
Despite not being quite as deep or long as Grand Canyon, the Capertee Valley in Australia is actually 1 km wider than Grand Canyon, making it the widest canyon in the world.
Additional Information
A gorge, also sometimes referred to as a canyon, is a deep channel created by millions of years of erosion by a river and other forms of weathering. The term "gorge" was derived from the French word meaning "neck" or "throat." The most well-known gorge in the world is the Grand Canyon, which is located in US state of Arizona and was formed by the Colorado River. It is 6,000 feet deep, 277 miles long, and has a width of 15 miles at its widest point. Other notable gorges include the Victoria Falls Gorge, Canyon Lake Gorge, Yarlung Zangbo Grand Canyon, Three Gorges, Olduvai Gorge, Talari Gorges, Macocha Gorge, Gorge de Verdun, New River Gorge, Columbia River Gorge, and Gorges of Finger Lakes.
Characteristics of a Gorge
Gorges are formed by an existing river or a former river that has dried up. Most gorges are located between mountains, hills or near-desert plateaus, at the point where a river cuts a channel into the land. Gorges form due to water erosion, weathering, geologic uplift, or the movement and melting of glaciers. Generally, they are more common in dry areas, because erosion is common in arid places. Another feature of gorges is that they are typically formed in areas composed of limestone rock. Limestone is soluble, which encourages the formation of cave systems on rocks. Subsequently, the collapse of rocks results in the creation of gorges.
Formation
A gorge can also be formed when an earthquake causes geologic uplift. This uplift causes some parts of Earth's surface to be more elevated than the surrounding area. When rivers flow across these uplifted areas, waterfalls are formed. Meanwhile, the erosion of softer layers of rock occurs, leading to the collapse of a river bed, which formes a gorge. For example, the Macocha Gorge in the Czech Republic was formed due to the erosion of the Punkva River, which resulted in the collapse of an underground cave. Gorges can also be formed by glaciers, which become exposed when a glacier melts.
Types of Gorges
There are three primary types of gorges: slot canyons, box canyons, and submarine canyons. A slot canyon is a narrow gorge with smooth walls, box canyons are open on only one side, while submarine canyons are formed due to landslides and turbidity currents, rather than weathering or erosion.
Benefits
Georges can have economic, recreational, and geological benefits. For example, the rivers that form and flow through a gorge can be harnessed through hydroelectric dams for the production of electricity. The Three Gorges Dam in China has 26 turbines that generate 18,000 megawatts of electricity for Shangai and other cities in the region. Gorges can also form waterways that can be navigated by freight ships. Additionally, gorges and rivers can create opportunities for recreational activities like wind sailing, whitewater rafting, and hiking. Many geological discoveries have occurred due to the creation of gorges, as these canyons expose rocks that existed thousands of years ago. For example, the Olduvai Gorge in Tanzania resulted in the discovery of fossils and rocks that dated as far as two million years.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2516 2025-04-18 18:39:53
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,708
Re: Miscellany
2316) River
Gist
A river is a natural stream of freshwater that flows on land, typically toward a lower elevation, such as an ocean, lake, or another river. It is characterized by a channel with defined banks and flows due to gravity, originating from higher ground or hills/mountains. Rivers play a crucial role in the water cycle, carrying water from precipitation, melting snow/glaciers, or seepage from aquifers.
Summary
A river is a ribbon-like body of water that flows downhill from the force of gravity. A river can be wide and deep, or shallow enough for a person to wade across. A flowing body of water that is smaller than a river is called a stream, creek, or brook. Some rivers flow year-round, while others flow only during certain seasons or when there has been a lot of rain. The largest rivers can be thousands of miles long. The erosional power of rivers can form geologic wonders like the Grand Canyon.
All rivers have a starting point where water begins its flow. This source is called a headwater. The headwater can come from rainfall or snowmelt in mountains, but it can also bubble up from groundwater or form at the edge of a lake or large pond. The other end of a river is called its mouth, where water empties into a larger body of water, such as a lake or ocean. Along the way, rivers may pass through wetlands where plants slow down the water and filter out pollutants.
The water that flows in rivers is fresh, meaning that it contains less than one percent salt. However, rivers still carry and distribute important salts and nutrients to support plant and animal life. For this reason, some of the most biodiverse habitats on our planet can be found around rivers. Collectively, scientists estimate that all the rivers in the world carry about 3.6 billion metric tons (four billion tons) of salt from land to the ocean each year.
Rivers can also form what is called an estuary, where salty seawater mixes with fresh water near the river mouth to form “brackish water.” The Hudson River in New York, U.S., is an example of an estuary where brackish water extends more than 241 kilometers (150 miles) upstream.
Fast-flowing rivers carry pebbles, sand, and silt. As the river begins to slow down—as in a wetland, at the outside of a bend, or where the river widens, such as at the mouth—these sediments sink and build up to form deltas. Rivers that overflow their banks also deposit sediment in the surrounding flood plain. These deltas and floodplains are highly fertile agricultural zones that offer tremendous value to the surrounding people. In Egypt, for example, the Nile River and its adjacent delta helped give rise to the Egyptian empire that built the pyramids. Today, farmers in the flood plain of California’s Central Valley produce approximately one-third of the vegetables and two-thirds of the fruits and nuts consumed in the United States.
Humans use rivers for irrigation in agriculture, for drinking water, for transportation, to produce electricity through hydroelectric dams, and for leisure activities like swimming and boating. Each of these uses can affect the health of a river and its surrounding ecosystems. Monitoring the health of rivers, lakes, and streams is important work that is conducted by scientists called limnologists.
Details
A river is a stream of water that flows through a channel on the surface of the ground. The passage where the river flows is called the riverbed and the earth on each side is called a riverbank. A river begins on high ground or in hills or mountains and flows down from the high ground to the lower ground, because of gravity. A river begins as a small stream and gets bigger the farther it flows.
River parts:
The beginning of a river
The start of a river is called the source or head water. The part of the river that is near the source is called a 'young' river. A young river is often in a V-shaped river bed, and flows quickly downhill over stones, and around big rocks. Young rivers often have lots of small waterfalls and rapids. As the rivers travel downhill they begin to erode the ground taking small bits of soft rock and soil.
* The source of a river may be a spring, often on a hill, mountain, glacier, or another high place. A spring is water that flows out from under the ground.
* The source of a river may be a lake where lots of water from small streams gathers when it rains or snows.
* A river may begin in mountains where there is snow. The melting snow runs together to form a small stream that runs down the mountain. As more little streams run in, the main stream gets bigger, until it forms a river.
* Some rivers flow from hills where there is no snow, but lots of rain.
*Some rivers only flow after there has been rain near the head water.
The middle part of a river
The middle part of a river is called a mature river. A mature river makes a riverbed that is U-shaped. It might be very deep and run fast. It sweeps over small rocks and boulders, and makes big turns around hills and mountains. It is much wider than a young river, but not as wide as an old river. To cross over a mature river, people use bridges. Many cities and towns are built on the banks of mature rivers. Many farms that keep animals such as dairy cows, horses and sheep are along mature rivers because the animals can drink from the river every day.
The last part of a river
A river usually ends by flowing into an ocean, a lake or a bigger river. The place where the river flows out into a bigger body of water is called the 'mouth' of the river.
As a river flows towards its mouth, the countryside around the river often changes from hilly to flat. As it flows over the flat land the river becomes wider and slower. A wide slow river is called an 'old river'. An old river often floods across the land after there is lots of rain at the headwaters. An old river slowly builds up its banks on either side; the high banks are called levees. An old river often meanders (twists and turns), and sometimes, after a flood, it leaves lakes behind which are called ox-bows or billabongs. Old rivers are the most useful type of river for growing crops. Corn, rice, fruit, cotton, hay, tobacco and sugar are some of the crops that are grown near old rivers.
The shape of the mouth depends on the conditions of the sea where it flows. If there is a strong tide where the river meets the sea, the river forms an estuary. An estuary is a wide, funnel-like mouth of the river. The fresh water of the river mixes slowly with the salt water, becoming brackish water – somewhat salty water. Many kinds of fish, clams, molluscs and other sealife live at estuaries. Many of the world's largest cities and harbours are at estuaries.
Where a river flows out to the sea, it sometimes flows very slowly through sandy or muddy land, making lots of little islands as it flows. The main stream of the river gets broken into many parts that spread out into a triangle shape like the Greek letter delta. When this happens, it is called the delta of the river. Deltas are often places that are not good for towns or farms but are very good for birds and other wildlife and fishing. Deltas are often made into wildlife reserves. Not all rivers have deltas. There are deltas on the Nile River, the Amazon River, the Mekong River, the Mississippi River and the Danube River.
Underground rivers
Some rivers flow underground through caves. Underground rivers form in places where there are lots of cracks in the rocks above, so that in rainy weather, the water runs downs and collects in small underground streams. Sometimes the underground water trickles or gushes out of the ground to form a small spring of water. In other places, where there are caves, the small underground streams run together to form a river. The river can sometimes run through deep wide underground caverns. While many underground rivers flow gently, some underground rivers flow fast and have rapids, particularly after heavy rain. Many underground rivers flow out through a cave mouth to become an ordinary river.
Using rivers
The water in rivers is "fresh water" that has come from rain, snow and from underground streams. It can usually be drunk safely by people unless it is too dirty because of mud or human pollution. People and animals need fresh water to drink, so they often live by the side of a river.
* Rivers give water for drinking, bathing and washing clothes.
* Rivers give water for cattle and other animals to drink and for people to grow plants.
* Rivers give products that are useful to people such as fish for food, clay for bricks and reeds to make the roofs of houses.
* Rivers can be used for transporting people, crops and other goods by boat.
* Rivers can be used to give power to turn machinery such as water mills.
* Rivers give water for factories that make cloth, steel and many other products.
* Rivers sometimes have dams to hold the water for people to drink, or to make electricity.
* Rivers can be used for leisure and sports such as swimming, boating, fishing and just walking by the river.
* Rivers often have beautiful scenery. Many painters, story-tellers, and poets have painted or written about rivers.
* Rivers are sometimes turned into canals.
Additional Information
A river is a natural stream of fresh water that flows on land or inside caves towards another body of water at a lower elevation, such as an ocean, lake, or another river. A river may run dry before reaching the end of its course if it runs out of water, or only flow during certain seasons. Rivers are regulated by the water cycle, the processes by which water moves around the Earth. Water first enters rivers through precipitation, whether from rainfall, the runoff of water down a slope, the melting of glaciers or snow, or seepage from aquifers beneath the surface of the Earth.
Rivers flow in channeled watercourses and merge in confluences to form drainage basins, areas where surface water eventually flows to a common outlet. Rivers have a great effect on the landscape around them. They may regularly overflow their banks and flood the surrounding area, spreading nutrients to the surrounding area. Sediment or alluvium carried by rivers shapes the landscape around it, forming deltas and islands where the flow slows down. Rivers rarely run in a straight line, instead, they bend or meander; the locations of a river's banks can change frequently. Rivers get their alluvium from erosion, which carves rock into canyons and valleys.
Rivers have sustained human and animal life for millennia, including the first human civilizations. The organisms that live around or in a river such as fish, aquatic plants, and insects have different roles, including processing organic matter and predation. Rivers have produced abundant resources for humans, including food, transportation, drinking water, and recreation. Humans have engineered rivers to prevent flooding, irrigate crops, perform work with water wheels, and produce hydroelectricity from dams. People associate rivers with life and fertility and have strong religious, political, social, and mythological attachments to them.
Rivers and river ecosystems are threatened by water pollution, climate change, and human activity. The construction of dams, canals, levees, and other engineered structures has eliminated habitats, has caused the extinction of some species, and lowered the amount of alluvium flowing through rivers. Decreased snowfall from climate change has resulted in less water available for rivers during the summer. Regulation of pollution, dam removal, and sewage treatment have helped to improve water quality and restore river habitats.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2517 2025-04-20 17:39:57
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,708
Re: Miscellany
2317) Melting point
Gist
The melting point is the temperature at which a solid transforms into a liquid, and at which the solid and liquid phases exist in equilibrium. It's a key physical property used to characterize substances.
Melting point is the temperature at which the solid and liquid forms of a pure substance can exist in equilibrium. As heat is applied to a solid, its temperature will increase until the melting point is reached. More heat then will convert the solid into a liquid with no temperature change.
Summary
Melting point is the, temperature at which the solid and liquid forms of a pure substance can exist in equilibrium. As heat is applied to a solid, its temperature will increase until the melting point is reached. More heat then will convert the solid into a liquid with no temperature change. When all the solid has melted, additional heat will raise the temperature of the liquid. The melting temperature of crystalline solids is a characteristic figure and is used to identify pure compounds and elements. Most mixtures and amorphous solids melt over a range of temperatures.
The melting temperature of a solid is generally considered to be the same as the freezing point of the corresponding liquid; because a liquid may freeze in different crystal systems and because impurities lower the freezing point, however, the actual freezing point may not be the same as the melting point. Thus, for characterizing a substance, the melting point is preferred.
Details
The melting point (or, rarely, liquefaction point) of a substance is the temperature at which it changes state from solid to liquid. At the melting point the solid and liquid phase exist in equilibrium. The melting point of a substance depends on pressure and is usually specified at a standard pressure such as 1 atmosphere or 100 kPa.
When considered as the temperature of the reverse change from liquid to solid, it is referred to as the freezing point or crystallization point. Because of the ability of substances to supercool, the freezing point can easily appear to be below its actual value. When the "characteristic freezing point" of a substance is determined, in fact, the actual methodology is almost always "the principle of observing the disappearance rather than the formation of ice, that is, the melting point."
Examples
For most substances, melting and freezing points are approximately equal. For example, the melting and freezing points of mercury is 234.32 kelvins (−38.83 °C; −37.89 °F). However, certain substances possess differing solid-liquid transition temperatures. For example, agar melts at 85 °C (185 °F; 358 K) and solidifies from 31 °C (88 °F; 304 K); such direction dependence is known as hysteresis. The melting point of ice at 1 atmosphere of pressure is very close to 0 °C (32 °F; 273 K); this is also known as the ice point. In the presence of nucleating substances, the freezing point of water is not always the same as the melting point. In the absence of nucleators water can exist as a supercooled liquid down to −48.3 °C (−54.9 °F; 224.8 K) before freezing.
The metal with the highest melting point is tungsten, at 3,414 °C (6,177 °F; 3,687 K); this property makes tungsten excellent for use as electrical filaments in incandescent lamps. The often-cited carbon does not melt at ambient pressure but sublimes at about 3,700 °C (6,700 °F; 4,000 K); a liquid phase only exists above pressures of 10 MPa (99 atm) and estimated 4,030–4,430 °C (7,290–8,010 °F; 4,300–4,700 K) (see carbon phase diagram). Hafnium carbonitride (HfCN) is a refractory compound with the highest known melting point of any substance to date and the only one confirmed to have a melting point above 4,273 K (4,000 °C; 7,232 °F) at ambient pressure. Quantum mechanical computer simulations predicted that this alloy (HfN0.38C0.51) would have a melting point of about 4,400 K. This prediction was later confirmed by experiment, though a precise measurement of its exact melting point has yet to be confirmed. At the other end of the scale, helium does not freeze at all at normal pressure even at temperatures arbitrarily close to absolute zero; a pressure of more than twenty times normal atmospheric pressure is necessary.
Melting point measurements
Many laboratory techniques exist for the determination of melting points. A Kofler bench is a metal strip with a temperature gradient (range from room temperature to 300 °C). Any substance can be placed on a section of the strip, revealing its thermal behaviour at the temperature at that point. Differential scanning calorimetry gives information on melting point together with its enthalpy of fusion.
A basic melting point apparatus for the analysis of crystalline solids consists of an oil bath with a transparent window (most basic design: a Thiele tube) and a simple magnifier. Several grains of a solid are placed in a thin glass tube and partially immersed in the oil bath. The oil bath is heated (and stirred) and with the aid of the magnifier (and external light source) melting of the individual crystals at a certain temperature can be observed. A metal block might be used instead of an oil bath. Some modern instruments have automatic optical detection.
The measurement can also be made continuously with an operating process. For instance, oil refineries measure the freeze point of diesel fuel "online", meaning that the sample is taken from the process and measured automatically. This allows for more frequent measurements as the sample does not have to be manually collected and taken to a remote laboratory.
Techniques for refractory materials
For refractory materials (e.g. platinum, tungsten, tantalum, some carbides and nitrides, etc.) the extremely high melting point (typically considered to be above, say, 1,800 °C) may be determined by heating the material in a black body furnace and measuring the black-body temperature with an optical pyrometer. For the highest melting materials, this may require extrapolation by several hundred degrees. The spectral radiance from an incandescent body is known to be a function of its temperature. An optical pyrometer matches the radiance of a body under study to the radiance of a source that has been previously calibrated as a function of temperature. In this way, the measurement of the absolute magnitude of the intensity of radiation is unnecessary. However, known temperatures must be used to determine the calibration of the pyrometer. For temperatures above the calibration range of the source, an extrapolation technique must be employed. This extrapolation is accomplished by using Planck's law of radiation. The constants in this equation are not known with sufficient accuracy, causing errors in the extrapolation to become larger at higher temperatures. However, standard techniques have been developed to perform this extrapolation.
Consider the case of using gold as the source (mp = 1,063 °C). In this technique, the current through the filament of the pyrometer is adjusted until the light intensity of the filament matches that of a black-body at the melting point of gold. This establishes the primary calibration temperature and can be expressed in terms of current through the pyrometer lamp. With the same current setting, the pyrometer is sighted on another black-body at a higher temperature. An absorbing medium of known transmission is inserted between the pyrometer and this black-body. The temperature of the black-body is then adjusted until a match exists between its intensity and that of the pyrometer filament. The true higher temperature of the black-body is then determined from Planck's Law. The absorbing medium is then removed and the current through the filament is adjusted to match the filament intensity to that of the black-body. This establishes a second calibration point for the pyrometer. This step is repeated to carry the calibration to higher temperatures. Now, temperatures and their corresponding pyrometer filament currents are known and a curve of temperature versus current can be drawn. This curve can then be extrapolated to very high temperatures.
In determining melting points of a refractory substance by this method, it is necessary to either have black body conditions or to know the emissivity of the material being measured. The containment of the high melting material in the liquid state may introduce experimental difficulties. Melting temperatures of some refractory metals have thus been measured by observing the radiation from a black body cavity in solid metal specimens that were much longer than they were wide. To form such a cavity, a hole is drilled perpendicular to the long axis at the center of a rod of the material. These rods are then heated by passing a very large current through them, and the radiation emitted from the hole is observed with an optical pyrometer. The point of melting is indicated by the darkening of the hole when the liquid phase appears, destroying the black body conditions. Today, containerless laser heating techniques, combined with fast pyrometers and spectro-pyrometers, are employed to allow for precise control of the time for which the sample is kept at extreme temperatures. Such experiments of sub-second duration address several of the challenges associated with more traditional melting point measurements made at very high temperatures, such as sample vaporization and reaction with the container.
Additional Information
The melting point of a substance is the temperature at which it changes state from solid to liquid at atmospheric pressure; at the melting point, the solid and liquid phases exist in equilibrium. A substance's melting point depends on pressure and is usually specified at standard pressure in reference materials. The melting point is also referred to as liquefaction point, solidus, or liquidus.
Melting points of common materials
* Melting point of steel: 1425-1540 °C / 2600-2800 °F
* Melting point of gold: 1064 °C / 1947.5 °F
* Melting point of copper: 1084 °C / 1983 °F
* Melting point of iron: 1538 °C / 2800 °F
* Melting point of lead: 327.5 °C / 621 °F
* Melting point of silver: 961 °C / 1761 °F

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2518 2025-04-21 16:55:30
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,708
Re: Miscellany
2318) Boiling point
Gist
The boiling point is the temperature at which a liquid turns into a gas when its vapor pressure equals the surrounding pressure. For water at sea level, this is 100°C (212°F). The boiling point is affected by factors like atmospheric pressure and the presence of dissolved substances.
Summary
Boiling point is the temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the vapour of the liquid; under this condition, addition of heat results in the transformation of the liquid into its vapour without raising the temperature.
At any temperature a liquid partly vaporizes into the space above it until the pressure exerted by the vapour reaches a characteristic value called the vapour pressure of the liquid at that temperature. As the temperature is increased, the vapour pressure increases; at the boiling point, bubbles of vapour form within the liquid and rise to the surface. The boiling point of a liquid varies according to the applied pressure; the normal boiling point is the temperature at which the vapour pressure is equal to the standard sea-level atmospheric pressure (760 mm [29.92 inches] of mercury). At sea level, water boils at 100° C (212° F). At higher altitudes the temperature of the boiling point is lower.
Details
The boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid and the liquid changes into a vapor.
The boiling point of a liquid varies depending upon the surrounding environmental pressure. A liquid in a partial vacuum, i.e., under a lower pressure, has a lower boiling point than when that liquid is at atmospheric pressure. Because of this, water boils at 100°C (or with scientific precision: 99.97 °C (211.95 °F)) under standard pressure at sea level, but at 93.4 °C (200.1 °F) at 1,905 metres (6,250 ft) altitude. For a given pressure, different liquids will boil at different temperatures.
The normal boiling point (also called the atmospheric boiling point or the atmospheric pressure boiling point) of a liquid is the special case in which the vapor pressure of the liquid equals the defined atmospheric pressure at sea level, one atmosphere. At that temperature, the vapor pressure of the liquid becomes sufficient to overcome atmospheric pressure and allow bubbles of vapor to form inside the bulk of the liquid. The standard boiling point has been defined by IUPAC since 1982 as the temperature at which boiling occurs under a pressure of one bar.
The heat of vaporization is the energy required to transform a given quantity (a mol, kg, pound, etc.) of a substance from a liquid into a gas at a given pressure (often atmospheric pressure).
Liquids may change to a vapor at temperatures below their boiling points through the process of evaporation. Evaporation is a surface phenomenon in which molecules located near the liquid's edge, not contained by enough liquid pressure on that side, escape into the surroundings as vapor. On the other hand, boiling is a process in which molecules anywhere in the liquid escape, resulting in the formation of vapor bubbles within the liquid.
Additional Information:
What is a boiling point?
The boiling point of a substance is the temperature at which the vapor pressure of the liquid is equal to the surrounding atmospheric pressure, thus facilitating transition of the material between gaseous and liquid phases. All boiling points below are normal/atmospheric boiling points: they give the temperature at which the vapor pressure of the liquid is equal to atmospheric pressure at sea level, 1 atm.
Boiling points of common materials
* Boiling point of water: 100 °C / 212 °F
* Boiling point of water (in Kelvin): 373.2 K
* Boiling point of ethanol: 78.37 °C / 173.1 °F
* Boiling point of methanol: 64.7 °C / 148.5 °F
* Boiling point of acetone: 56 °C / 132.8 °F
* Boiling point of alcohol: 78.37 °C / 173.1 °F
* Boiling point of nitrogen: -195.8 °C / -320.4 °F
* Boiling point of liquid helium: -269 °C / -452 °F
Additional notes:
The boiling point of salt water depends on the amount of salt added. For a 1.0 molal solution of salt (containing 58.44 grams of salt per kg of water), the boiling point is raised by 1.0 degrees Celsius.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2519 2025-04-22 15:26:17
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,708
Re: Miscellany
2319) Lake
Gist
A lake is a body of water that is surrounded by land. There are millions of lakes in the world. They are found on every continent and in every kind of environment—in mountains and deserts, on plains, and near seashores.
A lake is often a naturally occurring, relatively large and fixed body of water on or near the Earth's surface. It is localized in a basin or interconnected basins surrounded by dry land. Lakes lie completely on land and are separate from the ocean, although they may be connected with the ocean by rivers.
Summary
A lake is often a naturally occurring, relatively large and fixed body of water on or near the Earth's surface. It is localized in a basin or interconnected basins surrounded by dry land. Lakes lie completely on land and are separate from the ocean, although they may be connected with the ocean by rivers. Lakes, as with other bodies of water, are part of the water cycle, the processes by which water moves around the Earth. Most lakes are fresh water and account for almost all the world's surface freshwater, but some are salt lakes with salinities even higher than that of seawater. Lakes vary significantly in surface area and volume of water.
Lakes are typically larger and deeper than ponds, which are also water-filled basins on land, although there are no official definitions or scientific criteria distinguishing the two. Lakes are also distinct from lagoons, which are generally shallow tidal pools dammed by sandbars or other material at coastal regions of oceans or large lakes. Most lakes are fed by springs, and both fed and drained by creeks and rivers, but some lakes are endorheic without any outflow, while volcanic lakes are filled directly by precipitation runoffs and do not have any inflow streams.
Natural lakes are generally found in mountainous areas (i.e. alpine lakes), dormant volcanic craters, rift zones and areas with ongoing glaciation. Other lakes are found in depressed landforms or along the courses of mature rivers, where a river channel has widened over a basin formed by eroded floodplains and wetlands. Some lakes are found in caverns underground. Some parts of the world have many lakes formed by the chaotic drainage patterns left over from the last ice age. All lakes are temporary over long periods of time, as they will slowly fill in with sediments or spill out of the basin containing them.
Artificially controlled lakes are known as reservoirs, and are usually constructed for industrial or agricultural use, for hydroelectric power generation, for supplying domestic drinking water, for ecological or recreational purposes, or for other human activities.
Details
A lake is a body of water that is surrounded by land. There are millions of lakes in the world. They are found on every continent and in every kind of environment—in mountains and deserts, on plains, and near seashores.
Lakes vary greatly in size. Some measure only a few square meters and are small enough to fit in your backyard. Such small lakes are often referred to as ponds. Other lakes are so big that they are called seas. The Caspian Sea, in Europe and Asia, is the world’s largest lake, with an area of more than 370,000 square kilometers (143,000 square miles).
Lakes also vary greatly in depth. The world’s deepest lake is Lake Baikal, in Russia. Its bottom is nearly 2 kilometers (more than 1 mile) below the surface in places. Although Lake Baikal covers less than half the surface area of Lake Superior—one of North America’s Great Lakes—it is about four times deeper and holds nearly as much water as all five of the Great Lakes combined. Other lakes are so shallow that a person could easily wade across them.
Lakes exist at many different elevations. One of the highest is Lake Titicaca, in the Andes Mountains between Bolivia and Peru. It is about 3,810 meters (12,500 feet) above sea level. The lowest lake is the Dead Sea, between Israel and Jordan. It is more than 395 meters (1,300 feet) below sea level.
The water in lakes comes from rain, snow, melting ice, streams, and groundwater seepage. Most lakes contain fresh water.
All lakes are either open or closed. If water leaves a lake by a river or other outlet, it is said to be open. All freshwater lakes are open. If water only leaves a lake by evaporation, the lake is closed. Closed lakes usually become saline, or salty. This is because as the water evaporates, it leaves behind solids—mostly salts. The Great Salt Lake, in the U.S. state of Utah, is the largest saline lake in North America. Its water is saltier than the ocean. Surrounding the Great Salt Lake are salt flats, areas where the lake has evaporated, leaving only stretches of white salt.
How Lakes Are Formed
All lakes fill bowl-shaped depressions in the Earth’s surface, called basins. Lake basins are formed in several ways.
Many lakes, especially those in the Northern Hemisphere, were formed by glaciers that covered large areas of land during the most recent ice age, about 18,000 years ago.
The huge masses of ice carved out great pits and scrubbed the land as they moved slowly along. When the glaciers melted, water filled those depressions, forming lakes. Glaciers also carved deep valleys and deposited large quantities of earth, pebbles, and boulders as they melted. These materials sometimes formed dams that trapped water and created more lakes.
Many areas of North America and Europe are dotted with glacial lakes. The U.S. state of Minnesota is nicknamed “The Land of 10,000 Lakes” because of the number of glacial lakes. Many lakes in North America, including the Great Lakes, were created primarily by glaciers.
Some lake basins form where plate tectonics changed the Earth’s crust, making it buckle and fold or break apart. When the crust breaks, deep cracks, called faults, may form. These faults make natural basins that may fill with water from rainfall or from streams flowing in the basin. When these movements occur near the ocean, part of the ocean may be trapped by a new block of land thrust up from below the Earth’s surface. The Caspian Sea was formed this way. Lake Baikal was also formed by the movement of tectonic plates.
Many lakes form as a result of volcanoes. After a volcano becomes inactive, its crater may fill with rain or melted snow. Sometimes the top of a volcano is blown off or collapses during an eruption, leaving a depression called a caldera. It, too, may fill with rainwater and become a lake. Crater Lake, in the U.S. state of Oregon, one of the deepest lakes in the world, was created when ancient Mount Mazama’s volcanic cone collapsed.
Not all lakes are created by basins filling with water. Some lakes are formed by rivers. Mature rivers often wind back and forth across a plain in wide loops called meanders. During periods of flooding, a swollen, rushing river may create a shortcut and bypass a meander, leaving a body of standing water. This type of small lake is called an oxbow lake, because its shape resembles the U-shaped frame that fits over an ox’s neck when it is harnessed to pull a wagon or a plow.
Lakes may also be created by landslides or mudslides that send soil, rock, or mud sliding down hills and mountains. The debris piles up in natural dams that can block the flow of a stream, forming a lake.
Dams that beavers build out of tree branches can plug up rivers or streams and make large ponds or marshes.
People make lakes by digging basins or by damming rivers or springs. These artificial lakes can become reservoirs, storing water for irrigation, hygiene, and industrial use. Artificial lakes also provide recreational use for boating, swimming, or fishing.
Artificial lakes can provide electricity through hydroelectric power plants at the dam. Lake Mead, in the U.S. states of Arizona and Nevada, was formed when the Hoover Dam was built during the Great Depression. The dam was built to control the unpredictable Colorado River and provides electricity to the western United States.
Chemical and Physical Aspects of Lakes
Temperature, light, and wind are three of the main factors that affect the physical characteristics of a lake. Temperature and light vary from lake to lake. Depth, plant growth, dissolved materials, time of day, season, and latitude can all affect light’s ability to pass through the lake’s water.
Light and wind affect the temperature in lakes. Sunlight warms the water, and wind cools it down. Most lakes go through a process called thermal stratification. Thermal stratification refers to a lake’s three main layers, each with a different temperature range. A lake’s shallowest layer is the epilimnion. Its middle layer is the metalimnion, or thermocline. The deepest layer is the hypolimnion.
The most important chemicals in a lake are nitrogen and phosphorus. These chemicals allow nutrient-rich plants and algae to grow. Other organisms feed off these plants and algae, creating a complex, healthy ecosystem.
The chemistry of a lake is affected by biological, geological, and human processes. The balance of nutrients may be altered by biological phenomena such as “algal blooms,” when algae reproduces so rapidly it prevents any nutrients from reaching below the lake’s surface. Natural processes such as the eruption of a nearby volcano can alter the chemical aspect of a lake by introducing new gases or minerals. Pollution, such as the introduction of toxic chemicals from industry or agriculture, can also affect a lake’s chemistry.
The amount of oxygen and the pH level can also affect a lake’s chemistry. A lake must have a healthy amount of oxygen to sustain life. Lakes that do not have enough oxygen to sustain life are abiotic.
The pH level is a chemical property of all substances. A substance’s pH level indicates whether it is an acid or a base. Substances with a pH of less than 7 are acidic; substances with a pH greater than 7 are basic. Lakes have different pH levels, with life adapting to different chemical environments. Lake Tanganyika, one of the African Great Lakes, has an extremely high pH. It is full of dissolved minerals. Fish such as cichlids thrive in Lake Tanganyika. Tilapia, a variety of cichlid, can also thrive in lakes with very low pH.
The Life Cycle of Lakes
Once formed, lakes do not stay the same. Like people, they go through different life stages—youth, maturity, old age, and death. All lakes, even the largest, slowly disappear as their basins fill with sediment and plant material. The natural aging of a lake happens very slowly, over the course of hundreds and even thousands of years. But with human influence, it can take only decades.
A lake’s plants and algae slowly die. The warm, shallow water of the upper layer of the lake causes plants and algae to decompose, and eventually they sink to the basin. Dust and mineral deposits on the bottom of the lake combine with the plants to form sediment. Rain washes soil and pebbles into the basin. The remains of fish and other animals pile up on the lake’s bottom. The lake becomes smaller, starting at the edges and working toward the middle. Eventually, the lake becomes a marsh, bog, or swamp. At this point, the drying-up process slows down dramatically; limnologists, people who study lakes and ponds, aren’t sure why. Eventually, the lake becomes dry land.
Dry lake beds are a perfect place to find and study fossils. Archaeologists often excavate ancient lake beds, such as Fossil Butte in the U.S. state of Wyoming. The remains of organisms, from single-celled bacteria to dinosaurs, were preserved over time as sediment on the lake bed built up around and on top of them. In fact, some scientists believe the first living organisms on Earth developed in lakes.
Lake Classification
There are three basic ways that limnologists classify lakes: how many nutrients lakes have, how their water mixes, and what kinds of fish live in them.
When lakes are classified by the amount of nutrients they have, limnologists are using the trophic system. Generally, the clearer the water in the lake, the fewer nutrients it has. Lakes that are very nutrient-rich are cloudy and hard to see through; this includes lakes that are unhealthy because they have too many nutrients. Lakes need to have a balance of nutrients.
Lakes can also be classified by how the water mixes, or turns over from top (epilimnion) to bottom (hypolimnion). This is called lake turnover. Water in some lakes, mostly shallow ones, mixes all year long. These lakes have very little lake turnover.
Deep lakes experience lake turnover on a large scale. The middle layer, the thermocline, mixes and turns over throughout the year. It turns over due to climate, nutrient variations, and geologic activity such as earthquakes. However, major lake turnover happens during the fall and spring, when the lake’s cold and warm waters mix and readjust. Most lakes that experience lake turnover are dimictic lakes, meaning their waters mix twice a year, usually in fall and spring.
Lake turnover changes with the seasons. During the summer, the epilimnion, or surface layer, is the warmest. It is heated by the sun. The deepest layer, the hypolimnion, is the coldest. The sun’s radiation does not reach this cold, dark layer.
During the fall, the warm surface water begins to cool. As water cools, it becomes more dense, causing it to sink. This cold, dense water sinks to the bottom of the lake. It forces the water of the hypolimnion to rise.
During the winter, the epilimnion is coldest because it is exposed to wind, snow, and low air temperatures. The hypolimnion is the warmest. It is insulated by the earth. This is why there is ice on lakes during the winter, while fish swim in slightly warmer, liquid water beneath.
During the spring, the lake turns over again. The cold surface water sinks to the bottom, forcing the warmer, less dense water upward.
The final way to classify lakes is by the kinds of fish they have. This helps people in the fishing industry identify what kinds of fish they might be able to catch in that lake. For example, calling a lake a cold-water lake tells a fisherman that he can probably expect to find trout, a cold-water fish. A lake that has thick, muddy sediment is more likely to have catfish.
There are other ways of classifying a lake, such as by whether it is closed or fed by a river or stream. States also divide lakes into ones that are available for public use and ones that are not. Many people refer to lakes by size.
How Animals and Plants Use Lakes
Lakes are important in preserving wildlife. They serve as migration stops and breeding grounds for many birds and as refuges for a wide variety of other animals. They provide homes for a diversity of organisms, from microscopic plants and animals to fish that may weigh hundreds of kilograms. The largest fish found in lakes is the sturgeon, which can grow to 6 meters (20 feet) and weigh more than 680 kilograms (1,500 pounds).
Plants growing along the lakeshore may include mosses, ferns, reeds, rushes, and cattails. Small animals such as snails, shrimp, crayfish, worms, frogs, and dragonflies live among the plants and lay their eggs on them both above and below the waterline. Farther from the shore, floating plants such as water lilies and water hyacinths often thrive. They have air-filled bladders, or sacs, that help keep them afloat. These plants shelter small fish that dart in and out under their leaves. Waterbugs, beetles, and spiders glide and skitter across the surface or just below it. Small islands, floating plants, or fallen logs provide sunny spots for turtles to warm themselves.
Other animals live near the lake, such as bats and semi-aquatic animals, such as mink, salamanders, beavers, and turtles. Semi-aquatic animals need both water and land to survive, so both the lake and the shore are important to them.
Many kinds of water birds live on lakes or gather there to breed and raise their young. Ducks are the most common lake birds. Others include swans, geese, loons, kingfishers, herons, and bald eagles.
Many people think of fish when they think of lakes. Some of the most common fish found in lakes are tiny shiners, sunfish, perch, bass, math, muskie, walleye, perch, lake trout, pike, eels, catfish, salmon, and sturgeon. Many of these provide food for people.
How People Use Lakes
Lakes are an important part of the water cycle; they are where all the water in an area collects. Water filters down through the watershed, which is all the streams and rivers that flow into a specific lake.
Lakes are valuable resources for people in a variety of ways. Through the centuries, lakes have provided routes for travel and trade. The Great Lakes of North America, for example, are major inland routes for ships carrying grain and raw materials such as iron ore and coal.
Farmers use lake water to irrigate crops. The effect of very large lakes on climate also helps farmers. Because water does not heat or cool as rapidly as land does, winds blowing from lakes help keep the climate more even. This is the “lake effect.” The city of Chicago, in the U.S. state of Illinois, benefits from the lake effect. Chicago sits on the shore of Lake Michigan. When the western part of Illinois is snowing, Chicago often remains slightly warmer.
The lake effect can help farmers. In autumn, lakes blow warmer air over the land, helping the season last longer so farmers can continue to grow their crops. In spring, cool lake winds help plants not to grow too soon and avoid the danger of early-spring frosts, which can kill the young crops.
Lakes supply many communities with water. Artificial lakes are used to store water for times of drought. Lakes formed by dams also provide hydroelectric energy. The water is channeled from the lake to drive generators that produce electricity.
Because they are often very beautiful, lakes are popular recreation and vacation spots. People seek out their sparkling waters to enjoy boating, swimming, water-skiing, fishing, sailing, and, in winter, ice skating, ice boating, and ice fishing. Many public parks are built near lakes, allowing people to picnic, camp, hike, bike, and enjoy the wildlife and scenery the lake provides.
For some people, lakes are permanent homes. For example, indigenous people called the Uros have lived on Lake Titicaca in the Andes Mountains for centuries. The lake supplies almost everything the Uros need. They catch fish from the lake and hunt water birds.
The Uros also use the reeds that grow in Lake Titicaca to build floating “islands” to live on. The islands are about 2 meters (6.5 feet) thick. On them, the Uros build reed houses and make reed sleeping mats, baskets, fishing boats, and sails. They also eat the roots and the celery-like stalks of the reeds.
Lake Health: Blue-Green Algae
Although lakes naturally age and die, people have sped up the process by polluting the water. A major problem that threatens many lakes is blue-green algae. Blue-green algae is sometimes referred to as “pond scum” and can be blue-green, blue, green, reddish-purple, or brown. It stays on the surface of the water and forms a sort of mat. When the conditions are just right, the algae multiplies quickly. This is called an algal bloom and is harmful to lakes, animals, plants, and people.
Blue-green algae is different from true algae because it is not eaten by other organisms. True algae is an important part of the food web because it supplies energy for tiny animals, which are then eaten by fish, which are then eaten by other fish, birds, animals, or people.
Blue-green algae, also called cyanobacteria, is not a part of the food web. It uses up important nutrients without contributing to the lake ecosystem. Instead, the algal bloom chokes up a lake and uses up the oxygen that fish and other living things depend on for survival. Plants die more quickly, sinking to the bottom and filling up the lake basin. Blue-green algae also can become so dense that it prevents light from penetrating the water, changing the chemistry and affecting species living below the surface.
When an algal bloom happens, water becomes contaminated. The toxic water can kill animals and make humans sick. Blue-green algae is not a new problem. Scientists have found evidence of it from hundreds of years ago. The problem has increased, though, as humans pollute lakes.
Eutrophication is when a lake gets too many nutrients, causing blue-green algae growth. How do the excess nutrients get into lakes? Sewage from towns and cities causes explosive growth of blue-green algae, and waste from factories can wash into the lakes and pollute them. Phosphorus-based fertilizers from farms, golf courses, parks, and even neighborhood lawns can wash into lakes and pollute them. The phosphorus seeps into the ground and eventually reaches the lake. Phosphorus is an important nutrient for a lake, but too much of it is not a good thing because it encourages blue-green algae.
How can blue-green algae be prevented or reduced? At home, people can help by using phosphorus-free fertilizer and by fertilizing only where it’s needed. Preventing lawn clippings and leaves from washing into the gutter and maintaining a buffer of native plants help filter water and stop debris from washing away. Making sure septic systems don’t have leaks, safely disposing of household chemicals (like paint), and minimizing activities that erode soil also help prevent the spread of blue-green algae.
Controlling phosphorous and chemicals from factories and farms is much more complicated. Citizens need to work with businesses and elected leaders to help reduce the amount of runoff and water pollution.
Lake Health: Invasive Species
When a plant or animal species is moved to a location where it’s not originally from, the species is called an exotic species. When that species harms the natural balance in an ecosystem, the species is called invasive. Invasive species can harm life in a lake by competing for the same resources that native species do. When introduced to new food sources, invasive species multiply quickly, crowding out the helpful native species until there are more invasive than native species.
Invasive species can change the natural habitat of the lake and are known as biological pollutants when this happens. Once non-native species have been introduced into a lake, they are almost impossible to get rid of.
How do invasive species invade in the first place? Non-native plants and animals are almost always introduced by people. As people use waterways more frequently, they may inadvertently move organisms from one area to another.
Plants such as Eurasian watermilfoil, an invasive aquatic plant in the U.S., may cling to boats, clothing, pets, equipment, and vehicles. Small animals such as the spiny water flea can travel unnoticed by hopping onto a kayak or other recreational equipment.
Species are also carried by large ships bringing goods from one country to another. These ships take in ballast water, which helps stabilize the ship as it crosses the ocean. When the ship reaches its destination, it releases the ballast water. The water may be full of non-native species accidentally captured as the ship took on ballast.
The most famous invasive species in lakes is probably the zebra mussel, a small mollusk native to the Black Sea and the Caspian Sea in Europe and Asia. In the late 1980s, zebra mussels were found in several of North America’s Great Lakes. Since then, zebra mussels have spread to lakes from the U.S. state of Louisiana to the Canadian province of Quebec. Zebra mussels devastate native plants and animals. Some scientists say they carry a type of disease that is deadly to birds that eat the mussels. Zebra mussels multiply so quickly that they clog pipes. This harms machinery at industrial plants that use water, including hydroelectric dams and water filtration plants. Ships, docks, anchors, and buoys have also been destroyed by the invasive zebra mussel.
Communities have worked to reduce the impact of invasive species. Many states have laws prohibiting the sale or transport of non-native species. People are encouraged to inspect their boats and other equipment for wildlife. Boaters should remove plants, animals, and mud before leaving the water-access area. They should also drain any water from their boat. Rinsing boats, equipment, and even people can help reduce the transfer of harmful species. People should also get rid of leftover bait and report any species they see that look like they might not be native. These steps can make a big difference in keeping the habitat of a lake healthy.
Lake Health: Acid Rain
Another major threat to lakes today is acid rain. Some acid is natural, even in pure rain. This slightly toxic chemical slowly weathers rocks and soil. Acid rain, however, is caused by human activities and is harmful. It is caused by toxic gases from factories, coal-fired power plants, vehicle exhaust, and home furnaces.
Nitrogen and sulfur, the main ingredients of acid rain, rise in the air and may be carried hundreds of kilometers by wind. When these gases mix with the moisture in clouds, they form strong acids, which kill fish, plants, and other organisms when the acids fall as rain or snow on lakes. Acid rain can also affect humans, causing asthma and bronchitis, and damaging lung tissue. Methylmercury, a toxic form of mercury, has been linked to acid rain. Eating fish containing high levels of this mercury is particularly harmful for pregnant women, the elderly, and children.
Lakes and soil can neutralize normal levels of acid, but acid rain is too strong for lakes to combat. Eventually, acid rain leaves lakes sterile and lifeless. There are many lakes today in the United States, Canada, and parts of Europe dead or drying up because of acid rain.
Some steps have been taken to curb acid rain. The Clean Air Act was passed by the United States Congress in 1990. It required all utility companies to reduce the amount of toxic emissions by 40 percent by the year 2000. At home, people can help the problem by replacing old furnaces, turning off electronics when they’re not being used, and using fans or opening windows in the summer instead of air conditioning. Using compact fluorescent light bulbs (CFLs) and energy-efficient vehicles also help reduce the amount of pollution going into the air.
Lakes are among the most valuable and most beautiful of the Earth’s resources. Most experts agree that lakes must be kept clean and free from pollution if they are to continue to provide the many benefits that we receive from them today.
Additional Information
Introduction
A lake is a large body of water that is surrounded by land. Lakes contain less than 1 percent of the world’s fresh water, but they are a very important freshwater source. Almost all of the world’s fresh water is either frozen in huge masses of ice or buried underground. Lakes contain more than 98 percent of the fresh water that is available for use.
How Lakes Form
Ice sheets called glaciers formed many lakes, especially in North America, Europe, and Asia. Thousands of years ago glaciers covered large parts of these continents. The glaciers moved slowly over the land. They dug basins, or holes, in places where the rocks at the surface were weak. The basins that filled up with water became lakes. Other lake basins formed in places where melting glaciers left dirt behind. The dirt built up to form basin walls.
Some lakes have been formed by volcanoes. Some volcanoes have blown off their tops in huge explosions. Others have had their centers collapse. Both of these events form large pits called craters. These craters can become lakes. Crater Lake, in the U.S. state of Oregon, is a well-known example of this kind of lake.
The water in lakes can come from a variety of sources. The main sources of lake water include precipitation (rain or snow), rivers and streams, and melting ice and snow. Groundwater (water below Earth’s surface) can reach lakes through openings called springs.
Saltwater Lakes
Not all lakes contain fresh water. The Dead Sea, in Israel and Jordan, is the world’s saltiest natural lake. Another body of salt water is the Great Salt Lake in the U.S. state of Utah. It is all that remains today of what was once a much larger freshwater lake. The lake shrank as the climate became drier and the water began to evaporate. The lake contained salt from rivers that flow into it. As the water evaporated, the salt was left behind.
Why Lakes Are Important
Freshwater lakes have many important uses. Cities and towns depend on them for drinking water. In dry areas farmers use lakes to water their crops through irrigation systems. Lake water is used to create power in hydroelectric plants. Boaters and swimmers use lakes for recreation.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2520 2025-04-23 15:52:53
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,708
Re: Miscellany
2320) Electron gun
Gist
An electron gun is a device that generates and directs a stream of electrons, also known as an electron beam, in a vacuum. It's essentially a source of electrons, often used to produce images (like on TV screens), analyze materials, or perform other scientific tasks.
An electron gun is the source of electrons in a cathode ray tube. An electron gun consists of a cathode emitter of electrons, an anode with an aperture through which the beam of electrons can pass, and one or more focusing and control electrodes.
Summary
An electron gun, electrode structure that produces and may control, focus, and deflect a beam of electrons, as in a television picture tube (see figure), where the beam produces a visual pattern on the tube’s screen. The source of the electron beam is the cathode, a flat metal support covered with oxides of barium and strontium. When heated by a coil behind the support, these oxides emit electrons, which are drawn toward a positively charged sleeve (first anode) that is contoured to allow the electron beam to flow within the inside diameter. The beam is then electrostatically constricted and collimated by a metal disk with a hole (the control electrode) before it is directed to strike a phosphor-coated screen.
Details
An electron gun (also called electron emitter) is an electrical component in some vacuum tubes that produces a narrow, collimated electron beam that has a precise kinetic energy.
The largest use is in cathode-ray tubes (CRTs), used in older television sets, computer displays and oscilloscopes, before the advent of flat-panel displays. Electron guns are also used in field-emission displays (FEDs), which are essentially flat-panel displays made out of rows of extremely small cathode-ray tubes. They are also used in microwave linear beam vacuum tubes such as klystrons, inductive output tubes, travelling-wave tubes, and gyrotrons, as well as in scientific instruments such as electron microscopes and particle accelerators.
Electron guns may be classified by the type of electric field generation (DC or RF), by emission mechanism (thermionic, photocathode, cold emission, plasmas source), by focusing (pure electrostatic or with magnetic fields), or by the number of electrodes.
Design
A direct current, electrostatic thermionic electron gun is formed from several parts: a hot cathode, which is heated to create a stream of electrons via thermionic emission; electrodes generating an electric field to focus the electron beam (such as a Wehnelt cylinder); and one or more anode electrodes which accelerate and further focus the beam. A large voltage difference between the cathode and anode accelerates the electrons away from the cathode. A repulsive ring placed between the electrodes focuses the electrons onto a small spot on the anode, at the expense of a lower extraction field strength on the cathode surface. There is often a hole through the anode at this small spot, through which the electrons pass to form a collimated beam before reaching a second anode, called the collector. This arrangement is similar to an Einzel lens.
An RF electron gun consists of a Microwave cavity, either single cell or multi-cell, and a cathode. In order to obtain a smaller beam emittance at a given beam current, a photocathode is used. An RF electron gun with a photocathode is called a photoinjector.
Photoinjectors play a leading role in X-ray Free-electron lasers and small beam emittance accelerator physics facilities.
Applications
The most common use of electron guns is in cathode-ray tubes, which were widely used in computer and television monitors before the advent of flat screen displays. Most color cathode-ray tubes incorporate three electron guns, each one producing a different stream of electrons. Each stream travels through a shadow mask where the electrons will impinge upon either a red, green or blue phosphor to light up a color pixel on the screen. The resultant color that is seen by the viewer will be a combination of these three primary colors.
An electron gun can also be used to ionize particles by adding electrons to, or removing electrons from an atom. This technology is sometimes used in mass spectrometry in a process called electron ionization to ionize vaporized or gaseous particles. More powerful electron guns are used for welding, metal coating, 3D metal printers, metal powder production and vacuum furnaces.
Electron guns are also used in medical applications to produce X-rays using a linac (linear accelerator); a high energy electron beam hits a target, stimulating emission of X-rays.
Electron guns are also used in travelling-wave tube amplifiers for microwave frequencies.
Additional Information
Definition: Electron gun is defined as the source of focused and accelerated electron beam. It is a device used in Cathode Ray Tube for displaying the image on the phosphorous screen of CRT. The electron gun emits electrons and forms them into a beam by the help of a heater, cathode, grid, pre-accelerating, accelerating and focusing anode.
Emission of Electrons
The electrons are emitted through the indirectly heated cathode. Indirectly heated cathode means the cathode electrode are surrounded by the filament, and the electrodes emit electrons when the power apply across it.
For getting the high emission of electrons at the moderate temperature, the layer of barium and strontium oxide is applied at the end of the cathode. The current and voltage required by the indirectly heated cathode are approximately equal to the 600 mA and 6.3V.
Working of Electron Gun
After exiting from the cathode, the electron passes through the control grid. The control grid is made up of nickel material. It is centrally hole and co-axial with the CRT axis. The intensity of the control beams depends on the number of electrons emitted from the cathode. The grid has negative biasing which controls the flow of electrons.
The electron which passes from the control grid is accelerated by the high positive potential which is applied across the pre-accelerating and accelerating grids. The electron beam is focused by the focusing anode. The beam after passing through the focusing anode passing through the deflection plates and goes to the fluorescent screen.
Construction of Electron Gun
The main function of the electron gun is to produce and accelerate the beam of an electron inside the vacuum tube of the CRT. For generating and accelerating the gun requires the heater, cathode electrodes, grid, and different types of anodes.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2521 2025-04-24 14:29:48
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,708
Re: Miscellany
2321) Canal
Gist
A canal is a long, man-made strip of water used for irrigation or boat access to a bigger body of water, like the famous Erie Canal, which connects the Hudson River to Lake Erie.
Canal is related to the word channel, and all its different shades of meaning have to do with tunnel shaped spaces that carry liquid from one place to another. Besides man-made irrigation canals, canals that connect boat docks to rivers and oceans, or street-like canals in boat cities like Amsterdam, there are canals in your body, like your nasal canal, or the birth canal you came out of. It's also a verb meaning "to dig a canal."
Summary
A canal is a waterway made by humans. For many centuries, canals have been built as a way of transporting heavy goods in barges or boats. Canals often connect lakes, rivers, or oceans. The Panama Canal is a famous canal that connects the Atlantic Ocean with the Pacific Ocean. Many canals are reinforced with clay or concrete on the sides and have locks. Other canals are used for irrigation or hydropower.
Details
Canals or artificial waterways are waterways or engineered channels built for drainage management (e.g. flood control and irrigation) or for conveyancing water transport vehicles (e.g. water taxi). They carry free, calm surface flow under atmospheric pressure, and can be thought of as artificial rivers.
In most cases, a canal has a series of dams and locks that create reservoirs of low speed current flow. These reservoirs are referred to as slack water levels, often just called levels. A canal can be called a navigation canal when it parallels a natural river and shares part of the latter's discharges and drainage basin, and leverages its resources by building dams and locks to increase and lengthen its stretches of slack water levels while staying in its valley.
A canal can cut across a drainage divide atop a ridge, generally requiring an external water source above the highest elevation. The best-known example of such a canal is the Panama Canal.
Many canals have been built at elevations, above valleys and other waterways. Canals with sources of water at a higher level can deliver water to a destination such as a city where water is needed. The Roman Empire's aqueducts were such water supply canals.
The term was once used to describe linear features seen on the surface of Mars, Martian canals, an optical illusion.
Types of artificial waterways
A navigation is a series of channels that run roughly parallel to the valley and stream bed of an unimproved river. A navigation always shares the drainage basin of the river. A vessel uses the calm parts of the river itself as well as improvements, traversing the same changes in height.
A true canal is a channel that cuts across a drainage divide, making a navigable channel connecting two different drainage basins.
Structures used in artificial waterways
Both navigations and canals use engineered structures to improve navigation:
* weirs and dams to raise river water levels to usable depths;
* looping descents to create a longer and gentler channel around a stretch of rapids or falls;
* locks to allow ships and barges to ascend/descend.
Since they cut across drainage divides, canals are more difficult to construct and often need additional improvements, like viaducts and aqueducts to bridge waters over streams and roads, and ways to keep water in the channel.
Additional Information
A canal is an artificial passage used for the conveyance of water from a river or from a reservoir to its intended destination for the intended use. Canals are also known as channel, which is derived from the French word “Chanel”.
A canal connects a river or a reservoir to the destination where the water is to be supplied. Water in the canal is conveyed for various purposes such as irrigation, power generation, etc.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2522 2025-05-01 00:09:29
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,708
Re: Miscellany
2322) Sea
Gist
A sea is a large body of saltwater, typically smaller than an ocean and often partially or fully enclosed by land. It's essentially a division of the ocean. Examples include the Mediterranean Sea and the Caspian Sea.
Summary
Summary
A sea is a large body of salt water. There are particular seas and the sea. The sea commonly refers to the ocean, the interconnected body of seawaters that spans most of Earth. Particular seas are either marginal seas, second-order sections of the oceanic sea (e.g. the Mediterranean Sea), or certain large, nearly landlocked bodies of water.
The salinity of water bodies varies widely, being lower near the surface and the mouths of large rivers and higher in the depths of the ocean; however, the relative proportions of dissolved salts vary little across the oceans. The most abundant solid dissolved in seawater is sodium chloride. The water also contains salts of magnesium, calcium, potassium, and mercury, among other elements, some in minute concentrations. A wide variety of organisms, including bacteria, protists, algae, plants, fungi, and animals live in various marine habitats and ecosystems throughout the seas. These range vertically from the sunlit surface and shoreline to the great depths and pressures of the cold, dark abyssal zone, and in latitude from the cold waters under polar ice caps to the warm waters of coral reefs in tropical regions. Many of the major groups of organisms evolved in the sea and life may have started there.
The ocean moderates Earth's climate and has important roles in the water, carbon, and nitrogen cycles. The surface of water interacts with the atmosphere, exchanging properties such as particles and temperature, as well as currents. Surface currents are the water currents that are produced by the atmosphere's currents and its winds blowing over the surface of the water, producing wind waves, setting up through drag slow but stable circulations of water, as in the case of the ocean sustaining deep-sea ocean currents. Deep-sea currents, known together as the global conveyor belt, carry cold water from near the poles to every ocean and significantly influence Earth's climate. Tides, the generally twice-daily rise and fall of sea levels, are caused by Earth's rotation and the gravitational effects of the Moon and, to a lesser extent, of the Sun. Tides may have a very high range in bays or estuaries. Submarine earthquakes arising from tectonic plate movements under the oceans can lead to destructive tsunamis, as can volcanoes, huge landslides, or the impact of large meteorites.
The seas have been an integral element for humans throughout history and culture. Humans harnessing and studying the seas have been recorded since ancient times and evidenced well into prehistory, while its modern scientific study is called oceanography and maritime space is governed by the law of the sea, with admiralty law regulating human interactions at sea. The seas provide substantial supplies of food for humans, mainly fish, but also shellfish, mammals and seaweed, whether caught by fishermen or farmed underwater. Other human uses of the seas include trade, travel, mineral extraction, power generation, warfare, and leisure activities such as swimming, sailing, and scuba diving. Many of these activities create marine pollution.
Details
A sea is a large body of salt water. It may be an ocean, or may be a large saltwater lake which like the Caspian Sea, lacks a natural outlet.
Characteristics:
Seawater
Seawater is salty. The open ocean has about 35 grams (1.2 oz) solids per litre, a salinity of 35 part per thousand. The Mediterranean Sea is a little higher at 37part per thousand and the Dead Sea has as much as 300 grams (11 oz) dissolved solids per litre. Sodium chloride is the main salt present, making up about 85% of the solids in solution. There are also 5 grams (0.18 oz) per litre of the chlorides of other metals such as potassium and magnesium and 3 grams (0.11 oz) of sulphates, carbonates, bromides and other salts. A kilogram (2.2 lb) of salt can be found in 28 litres or one cubic foot of typical ocean water. Despite differences in the levels of salinity in different seas, the relative composition of the dissolved salts is very stable throughout the world's oceans.
Temperature:
The temperature of the sea depends on the amount of sunlight falling on the surface. In the tropics, with the sun nearly overhead, the temperature of the surface layers can rise to over 30 °C (86 °F). Near the poles the temperature in balance with the sea ice is about −2 °C (28 °F). Cold water is denser than warm water and tends to sink. There is a continuous circulation of water in the oceans. Warm surface currents cool as they move away from the tropics, the water becomes denser and sinks. The cold water moves back towards the equator as a deep sea current, driven by changes in the temperature and density of the water, eventually welling up again towards the surface. Deep sea water has a temperature between −2 °C (28 °F) and 5 °C (41 °F) in all parts of the globe.
Oxygen
The amount of oxygen found in seawater depends mostly on the plants growing in it. These are mainly algae, including phytoplankton, but also include some vascular plants such as seagrasses. In daylight the photosynthetic activity of these plants produces oxygen which dissolves in the seawater where it is used by marine animals. At night, photosynthesis stops, and the amount of dissolved oxygen declines. In the deep sea where not enough light penetrates for plants to grow, there is very little dissolved oxygen.
Seawater is a little alkaline and during historic times has had a pH of about 8.2. The pH is expected to reach 7.7 by the year 2100, an increase of 320% in acidity in a century. One important element for the formation of skeletal material in marine animals is calcium but it is easily precipitated out in the form of calcium carbonate as the sea becomes more acid. This is likely to have profound effects on certain planktonic marine organisms because their ability to form shells will be reduced. These include single-celled algae called coccolithophorids and foraminifera. These are important parts of the food chain. Reducing their numbers will have significant results. In tropical areas, corals will be affected by a lack of calcium, with knock-on effects for other reef residents.
Waves
Wind blowing over the surface of a body of water forms waves. The friction between air and water caused by a gentle breeze on a pond causes ripples to form. A strong blow over the ocean causes larger waves as the moving air pushes against the raised ridges of water. The waves reach their greatest height when the rate at which they travel nearly matches the speed of the wind. The waves form at right angles to the direction from which the wind blows. In open water, if the wind continues to blow, as happens in the Roaring Forties in the southern hemisphere, long, organized masses of water called swell roll across the ocean.
Additional Information
The “seven seas” has been used to describe the world’s great water bodies for a long time. But there are actually about 50 water formations that can be called a “sea,” and they are quite diverse when it comes to their size, location, and ecosystems.
The phrase “the Seven Seas” has been around for centuries, but that term really refers to different parts of the ocean and several other large bodies of water. There are actually more than seven seas in the world. But what makes a sea different from other bodies of water?
That is not an easy question to answer, because the definition of a sea leaves some room for interpretation. In general, a sea is defined as a portion of the ocean that is partly surrounded by land. Given that definition, there are about 50 seas around the world. But that number includes water bodies not always thought of as seas, such as the Gulf of Mexico and the Hudson Bay.
Moreover, in some cases, a sea is completely landlocked. The Caspian Sea is the most famous example, though this sea, which lies between Russia and Iran, is also referred to as the world’s largest lake. Other seas surrounded by land include the Aral Sea and the Dead Sea. They contain saltwater and have been called seas for many years, but many oceanographers and geographers are more inclined to call them lakes.
Still, that leaves dozens of water bodies that fit the traditional definition of a sea, even though they can be quite different from one another. A sea can be more than 2.6 million square kilometers (1 million square miles) in area, such as the Caribbean Sea. Or, it can be as tiny as the Sea of Marmara, which is less than 12,950 square kilometers (5,000 square miles) in area. This tiny Turkish sea connects the Aegean Sea and the Black Sea.
A sea can also be very warm for most of the year. The Red Sea, for instance, has an average temperature of around 30 degrees Celsius (86 degrees Fahrenheit). It is also the saltiest sea, containing 41 parts of salt per 1,000 parts of seawater. Seas can be quite cold, too. The Greenland Sea, for instance, has surface water that hovers near the freezing mark most of the year.
The variety of the sizes, temperatures, and locations of the Earth’s seas also means that the marine ecosystems within each sea can vary greatly from one to the other. The Baltic Sea in Scandinavia is the world’s youngest sea having formed between 10 thousand and 15 thousand years ago from glacial erosion. It contains a unique mixture of saltwater and freshwater, making it the largest brackish water body on the planet. As a result, the Baltic Sea contains a small, but rare, variety of freshwater and saltwater plants and animals that have been able to adapt to their brackish environment.
Not surprisingly, the diversity of the world’s seas also draws National Geographic explorers, such as oceanographer Katy Croff Bell. She was part of the crew aboard the exploration vessel Nautilus, a ship that shared its scientific discoveries in the Mediterranean Sea, the Black Sea, and elsewhere with students around the world in online lessons and chats. She says the seas—big and small, cold and warm—can teach scientists about the rest of the world. “We’re going to places that have never been explored to see what’s there,” Bell told MIT Technology Review in 2015. “There are things we can’t even conceive of out there, and it will take a long, long time to fully understand our own planet.”

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2523 2025-05-07 00:52:02
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,708
Re: Miscellany
2323) Sea water
Gist
Seawater is salty water found in oceans and seas, containing dissolved salts and minerals, with an average salinity of 35 parts per thousand. It's a vital water source, particularly for regions facing water shortages, and its composition is dominated by seven key ions: sodium, magnesium, calcium, potassium, chloride, sulfate, and bromide. The primary sources of salt in seawater are rocks on land, where rainwater erodes them, releasing salts and minerals that are carried to the oceans.
Summary
Seawater is water that makes up the oceans and seas, covering more than 70 percent of Earth’s surface. Seawater is a complex mixture of 96.5 percent water, 2.5 percent salts, and smaller amounts of other substances, including dissolved inorganic and organic materials, particulates, and a few atmospheric gases.
Seawater constitutes a rich source of various commercially important chemical elements. Much of the world’s magnesium is recovered from seawater, as are large quantities of bromine. In certain parts of the world, sodium chloride (table salt) is still obtained by evaporating seawater. In addition, water from the sea, when desalted, can furnish a limitless supply of drinking water. Many large desalination plants have been built in dry areas along seacoasts in the Middle East and elsewhere to relieve shortages of fresh water.
Details
Seawater, or sea water, is water from a sea or ocean. On average, seawater in the world's oceans has a salinity of about 3.5% (35 g/L, 35 ppt, 600 mM). This means that every kilogram (roughly one liter by volume) of seawater has approximately 35 grams (1.2 oz) of dissolved salts (predominantly sodium (Na+) and chloride (Cl−) ions). The average density at the surface is 1.025 kg/L. Seawater is denser than both fresh water and pure water (density 1.0 kg/L at 4 °C (39 °F)) because the dissolved salts increase the mass by a larger proportion than the volume. The freezing point of seawater decreases as salt concentration increases. At typical salinity, it freezes at about −2 °C (28 °F). The coldest seawater still in the liquid state ever recorded was found in 2010, in a stream under an Antarctic glacier: the measured temperature was −2.6 °C (27.3 °F).
Seawater pH is typically limited to a range between 7.5 and 8.4. However, there is no universally accepted reference pH-scale for seawater and the difference between measurements based on different reference scales may be up to 0.14 units.
Additional Information
One of the most well known qualities of the ocean is that it is salty. The two most common elements in sea water, after oxygen and hydrogen, are sodium and chloride. Sodium and chloride combine to form what we know as table salt.
Sea water salinity is expressed as a ratio of salt (in grams) to liter of water, It is written parts per thousand (ppt). In sea water, there is typically close to 35 grams of dissolved salts in each liter (35ppt), but ranges between 33-37 grams per liter (33ppt - 37ppt).
But as in weather, where there are areas of high and low pressure, the ocean has areas of high and low salinity. Of the five ocean basins, the Atlantic Ocean is the saltiest. On average, there is a distinct decrease in salinity near the equator and at both poles, although for different reasons.
Near the equator, the tropics receive the most rain on a consistent basis. As a result, the fresh rain water falling into the ocean decreases the salinity of the surface water in that region. Rain decreases further from the equator, and with less rain and more sunshine, evaporation increases. Evaporation of water vapor from the ocean to the atmosphere leaves behind the salt, resulting in higher salinity. Toward the poles, fresh water from melting ice decreases the surface salinity once again.
The saltiest locations in the ocean are the regions where evaporation is highest or in large bodies of water where there is no outlet into the ocean. The saltiest ocean water is in the Red Sea and in the Persian Gulf region (around 40ppt) due to very high evaporation and little fresh water inflow.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2524 2025-05-25 21:57:59
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,708
Re: Miscellany
2324) Saltwater Lake
Gist
Saltwater lakes, also known as saline lakes, are landlocked bodies of water with high salt concentrations, significantly higher than freshwater lakes. Some are so concentrated that they are considered hypersaline, even containing more salt than seawater. These lakes are often found in arid and semi-arid regions in the basins of inland drainage, where evaporation exceeds inflow, leading to salt accumulation. India, for example, has several major saltwater lakes, including Sambhar Lake, Chilika Lake, and Pangong Tso.
Summary
A saltwater lake, also known as a saline lake or hypersaline lake, is a landlocked body of water with a high concentration of dissolved salts, often exceeding the salinity of seawater. These lakes are typically found in arid or semi-arid regions where evaporation is high, leading to the accumulation of salts in the lake water.
Saltwater lakes develop due to the accumulation of salts in a water body with no outlet (endorheic basin). As evaporation leads to increased salinity, making these lakes valuable sources of salt production. Some saltwater lakes have high carbonate concentrations, making them alkaline lakes, often called "soda lakes."
Details
Saline lakes (i.e., bodies of water that have salinities in excess of 3 grams per litre) are widespread and occur on all continents, including Antarctica. Saline lakes include the largest lake in the world, the Caspian Sea; the lowest lake, the Dead Sea; and many of the highest lakes, such as those in Tibet and on the Altiplano of South America. Although inland saline water constitutes some 45 percent of total inland water, it is largely concentrated in only a few deep lakes, principally the Caspian Sea. Saline lakes are most common in the semiarid regions of the biosphere, which encompass approximately 27 percent of total land area, because two preconditions for the formation of salt lakes occur there most frequently: a balance between input of water (precipitation and inflows) and output of water (evaporation and seepage) and the presence of endorheic drainage basins.
Despite their wide geographic distribution and large total volume, the importance of salt lakes as an integral element in biospheric processes generally has been overlooked. Indeed, not until the effects of human impact began to be noticed—from about 1960—did their environmental significance become clear. An example of this is provided by the Aral Sea, a large salt lake in Central Asia. After much of the input of fresh water was diverted before reaching the lake to be used for irrigation, the level of the lake fell, salinity rose, and vast expanses of the lake bed were exposed. As a result, the fishing industry collapsed, islands that had served as wildlife refuges became peninsulas, biological diversity and productivity fell, biota disappeared, large quantities of salt blew from the lake bed onto neighbouring lands, groundwater salinity rose, and the local climate was altered. The effects on the local human population were catastrophic as well.
Permanent salt lakes show the same sort of vertical differences in physicochemical attributes as permanent bodies of standing fresh water do; similarly, temporary salt lakes and temporary bodies of standing fresh water respond alike to environmental disturbances. However, all salt lakes are distinguished from all freshwater bodies by differences in ionic composition and, obviously, much higher salinities. Depending on the dominant ions present, salinities may reach values well above 300 grams per litre. In permanent, deep salt lakes, annual salinities as well as water levels may fluctuate only slightly, while, in shallow, temporary lakes, salinities may range from less than 50 grams per litre to more than 300 grams per litre over a period of a single year and be accompanied by wide water-level fluctuations. Moreover, because all salt lakes are dependent on a climatic balance, they are a great deal more sensitive to long-term climatic changes than are freshwater lakes. Thus, even large, deep, permanent salt lakes display marked changes in salinity and water level over time, reflecting long-term shifts in climate. Often these changes are compounded by the human diversion of water, as described above. Salinity has many direct effects on other physicochemical features. Its effect on freezing points has already been noted (see above The environment: Physical and chemical properties of water). Salinity also affects the amount of oxygen that can be dissolved. As illustrated in Figure 3, the greater the concentration of sodium chloride, which is the solution most similar to that encountered in saline lakes, the less soluble is oxygen.
Running water
Permanent and temporary running waters (streams, brooks, rivers) occur throughout the biosphere. Well-watered regions (temperate and humid tropical areas) are characterized by permanent streams and large, permanent rivers; drier regions are characterized by temporary streams. However, even dry regions may have large permanent allogenic rivers that arise in humid areas and flow into the arid region—e.g., the Nile River in North Africa.
Rivers and streams provide the essential link in the global hydrologic cycle—i.e., the means whereby all water evaporated from the sea and precipitated onto land is ultimately returned to the sea. Nevertheless, running waters account for less than 1 percent of all inland free waters, a good deal of which occurs within only one river, the Amazon.
Ecosystems
Running waters have several physicochemical features that distinguish them from standing waters. The most obvious are unidirectional flow of water, a generally linear morphology, and shallow depth. Less obvious, but distinctive nonetheless, is the constant low salinity of lotic environments. With very few exceptions, all running waters are fresh and contain the same major ions as standing fresh waters. These and other physicochemical features combine to create an aquatic environment very different from the lentic environment. The result is that most biological communities that originate within a lotic system, and their associated ecological processes, are so specialized that they are confined to this type of environment. Nonetheless, the difference between lentic and lotic habitats is not always clear-cut. The decisive criterion is the length of time a given mass of water resides within a certain part of an aquatic ecosystem, a concept clearly related to flow rates. Some large rivers with only a slight gradient have low rates of discharge and flow and extensive floodplains with many interconnected bodies of lentic waters. Similar to this situation is the extensive reach of a large river that is well protected from the main current and may seem more lentic than lotic. Conversely, some small freshwater lakes with short water-residence times that are in areas that receive a large amount of precipitation are essentially no more than enlarged river pools, or, to coin a medical analogy, aneurysms in the biosphere’s hydrologic system.
Although running waters do not display the range of salinity that standing waters do, the diversity of physical form and the variety of biological habitats is just as extensive as those of standing waters. Running waters range from small, temporary streams that flow only after irregular rain has fallen in deserts, to large, permanent tropical rivers so wide that opposing banks are not visible. Extensive floodplains may be present or absent; flow may be more or less constant or highly variable, with actual rates from high to almost nothing; and substrates may range from bare rock to fine mud. Great differences occur among their physicochemical processes, including biogeochemical pathways, the relative ecological importance of the floodplain (if present), the main stem of the river or stream, the hyporheic zone (the environment below the bed), and contiguous terrestrial areas.
Biota of inland waters
A remarkably diverse assemblage of plants, animals, and microbes live in inland waters, with nearly all major groups of living organisms found in one sort of aquatic ecosystem or another. Nevertheless, no major group actually evolved in inland waters; all evolved either in the sea or on land, whence the biological invasion of inland waters eventually took place. The long period of time since this original invasion occurred, however, has allowed many important taxa of inland waters, such as different types of crustaceans, to evolve.
The only major groups of aquatic animals conspicuously absent from inland waters include the phyla Echinodermata, Ctenophora, and Hemichordata. Several other major groups of aquatic animals, as well as plants, are markedly less diverse in inland waters than they are in the sea: Notable among the animals are the phyla Porifera (sponges), Cnidaria, and Bryozoa (moss animals) and among the plants are Phaeophyta (brown algae) and Rhodophyta (red algae). The reason these groups did not invade as successfully as other groups is uncertain, but presumably they were less able to cope with lower salinities and reduced environmental stability. Major groups of the inland aquatic biota that are derived from terrestrial ancestors are insects and macrophytes other than large algae.
Whatever their origins, the invading biota needed to develop many adaptations to the special physicochemical features of inland waters. For those abandoning a marine environment the primary adaptation was a physiologic one that would permit survival in a considerably less saline, more dilute external medium. For terrestrial biota, the most necessary adaptations were those that would allow the organism to exist in a medium of significantly greater density and viscosity that also contained less oxygen. Many other adaptations were required to meet the challenges that particular features of a given aquatic environment posed. Thus, in running waters adaptations were needed that prevented an organism from being washed downstream; in highly saline lakes, a concentrated external medium was the challenging environmental feature; and in temporary waters, the main obstacle was to survive the dry phase. The adaptations themselves are many and varied and include those of physiology (e.g., osmoregulatory abilities), structure (e.g., flattened bodies of fauna living in running waters), behaviour (e.g., burrowing to avoid dehydration), and ecology (e.g., development of life cycles that accord with the occurrence of seasonally unfavourable conditions).
The biota of almost all inland saline waters did not evolve directly from marine ancestors but instead primarily from freshwater forms. Only a few forms appear to be of terrestrial derivation, and a few organisms in inland waters located near coasts are of marine origin. Although at first this evolutionary pathway may not seem obvious, it can be explained easily. Organisms that survive under greater environmental stress tend to have a greater ability to adapt than those that do not. Marine environments are considered less stressful than freshwater environments; hence, organisms from fresh waters are better able to adapt to the extremely stressful environment of inland saline waters.
Population and community development and structure
All types of inland aquatic ecosystems have well-defined structures and processes that are similar in general aspects but differ in particular details throughout the biosphere. Thus, as is true of marine and terrestrial ecosystems, almost all inland aquatic ecosystems have three fundamental trophic levels—primary producers (algae and macrophytes), consumers (animals), and decomposers (bacteria, fungi, small invertebrates)—that are interconnected by a complex web of links. Energy passes through these trophic levels primarily along the grazer and detrital chains and is progressively degraded to heat through metabolic activities. Meanwhile, the essential elements follow pathways that cycle between these biotic components and the abiotic components of the ecosystem (see biosphere: The organism and the environment: Resources of the biosphere: Nutrient cycling). A few inland aquatic ecosystems such as hot springs and highly saline lakes have conditions so inimical to life that biological diversity is restricted, trophic levels are correspondingly simple, and energetic and biogeochemical processes are compressed.
Organisms within the trophic network are arranged into populations and communities. In deep, freshwater lakes the primary producers (plants) are found either at the shallow edges of the lake (emergent, submerged, or floating macrophytes) or free-floating within its upper layers (microscopic algae, cyanobacteria, and photosynthetic bacteria of the plankton community) (Figure 4). Plants are found only in the photic zone—the upper portion of the lake where photosynthesis occurs, also called the trophogenic zone. In this zone the production of biochemical energy through photosynthesis is greater than its consumption through respiration and decomposition. Animals and decomposers are found in both the photic and aphotic zones. In the aphotic zone, also called the tropholytic zone, the consumption of energy exceeds its production. The zones are demarcated by a plane of compensation at which primary production and consumption are equivalent. This plane varies diurnally and seasonally with changes in light penetration. The major biological communities of deep freshwater lakes are shown in Figure 4. Included are the plankton, which contains tiny floating plants (phytoplankton) and animals (zooplankton) as well as microbes (see marine ecosystem: Marine biota: Plankton); the shoreline macrophytes; the benthos (bottom-dwelling organisms); the nekton (free-swimming forms in the water column); the periphyton (microscopic biota on submerged objects); the psammon (biota buried in sediments); and the neuston (biota associated with surface film). These organisms differ enormously in size, ranging from less than 0.5 micrometre (0.00002 inch) to greater than 1 metre (3.28 feet). They also vary in composition, structure, function, adaptations, and spatiotemporal location. Significant taxonomic differences also occur across continents; for example, the fish species of freshwater lakes in Africa are not the same as those of similar ecosystems in North America, nor are the plankton of lakes in Australia the same as those of lakes in Asia.
The populations and communities of inland waters other than freshwater lakes are similarly complex but markedly different in all except their fundamental ecological roles. Even major ecological and biogeochemical processes are quite distinct in different sorts of inland waters. In streams, for example, plankton populations are absent and much energy is derived allochthonously (from outside the stream). The processing and transport of essential elements follow a downstream sequence. Hypotheses attempting to explain ecological processes in running waters include the concept of the river continuum, which explains differences in lotic communities according to the changing ecological factors along the river system. Nutrient spiraling is another concept invoked to explain the cycling of nutrients while they are carried downstream. For large rivers of variable hydrology, the flood pulse concept has been instructive. This concept regards seasonal or occasional flood events as important ecological phenomena determining the biology of the river.
Biological productivity
Central to all biological activity within inland aquatic ecosystems is biological productivity or aquatic production. This involves two main processes: (1) primary production, in which living organisms form energy-rich organic material (biomass) from energy-poor inorganic materials in the environment through photosynthesis, and (2) secondary production, the transformation, through consumption, of this biomass into other forms. In this context, it is important to distinguish between gross primary production—i.e., the total amount of energy fixed by photosynthesis—and net primary production—i.e., the amount of energy fixed less that respired by the plants involved and available for secondary production. Note that forms of production using energy other than radiant energy from the Sun are not important to overall aquatic production.
Rates of production, factors that limit production, and the results of production have been and are matters of constant and fundamental interest in inland waters, not least because of the impact that different levels of production in certain waters have on human populations. Decreased levels of secondary production (e.g., a reduction in the fish population) can lead to a meagre harvest, which can in turn provide insufficient protein for some local human populations. Elevated levels of primary production brought about by the input of excess plant nutrients, principally phosphates and nitrates, into inland waters following agricultural and urban development of catchments (known as eutrophication), can also be harmful. For example, eutrophication often results in the development of algal blooms—i.e., dense populations of algae and cyanobacteria, which may be unsightly, toxic, malodorous, or otherwise harmful and unwanted.
Standing bodies of fresh water are often divided into categories that reflect levels of biological production. Oligotrophic lakes are those that are unproductive: net primary production is only between 50 and 100 milligrams of carbon per square metre per day, nutrients are in poor supply, and secondary production is depressed. Eutrophic lakes, on the other hand, are productive: net primary production is between 600 and 8,000 milligrams of carbon per square metre per day, nutrients are in good supply, and secondary production is high. Mesotrophic lakes are lakes of intermediate productivity: net primary production is between 250 and 1,000 milligrams of carbon per square metre per day. Models that relate levels of lake productivity to levels of nutrient input or loading have been useful in controlling eutrophication in many temperate freshwater lakes. It was once thought that lakes evolved from states of oligotrophy to eutrophy, but this is now generally believed not to be the case. Instead, lake productivity reflects contemporary processes of nutrient supply more than historical events.
As for comparative levels of biological productivity in inland waters, most values stand somewhere between the high values of coral reefs and the low values of deserts. It is difficult to generalize in this matter, however, because wide ranges in net primary production occur in any type of inland aquatic ecosystem. Certain communities, such as reedswamps in tropical lakes, have values for primary production that are among the highest of those recorded anywhere in the biosphere. Notwithstanding these high values, biological production in inland waters does not significantly contribute to biospheric production. Nevertheless, the use of inland waters by humans to enhance either terrestrial primary production (by irrigating crops) or secondary production on land (by supplying drinking water to stock) is a significant indirect contribution.
Additional Information
Saline water (more commonly known as salt water) is water that contains a high concentration of dissolved salts (mainly sodium chloride). On the United States Geological Survey (USGS) salinity scale, saline water is saltier than brackish water, but less salty than brine. The salt concentration is usually expressed in parts per thousand (permille, ‰) and parts per million (ppm). The USGS salinity scale defines three levels of saline water. The salt concentration in slightly saline water is 1,000 to 3,000 ppm (0.1–0.3%); in moderately saline water is 3,000 to 10,000 ppm (0.3–1%); and in highly saline water is 10,000 to 35,000 ppm (1–3.5%). Seawater has a salinity of roughly 35,000 ppm, equivalent to 35 grams of salt per one liter (or kilogram) of water. The saturation level is only nominally dependent on the temperature of the water. At 20 °C (68 °F) one liter of water can dissolve about 357 grams of salt, a concentration of 26.3 percent by weight (% w/w). At 100 °C (212 °F) (the boiling temperature of pure water), the amount of salt that can be dissolved in one liter of water increases to about 391 grams, a concentration of 28.1% w/w.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#2525 2025-05-27 21:49:53
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,708
Re: Miscellany
2325) Bioluminescence
Gist
Bioluminescence is the ability of living organisms to produce and emit light through a chemical reaction. This natural phenomenon, found in both land and sea, involves the conversion of chemical energy into light energy.
Bioluminescence is the production and emission of light by a living organism. Bioluminescent creatures are found throughout marine habitats, from the ocean surface to the deep seafloor.
Summary
Bioluminescent creatures are found throughout marine habitats, from the ocean surface to the deep seafloor.
The light emitted by a bioluminescent organism is produced by energy released from chemical reactions occurring inside (or ejected by) the organism.
If you’ve ever seen a firefly, you have encountered a bioluminescent organism. In the ocean, bioluminescence is not as rare as you might think. In fact, most types of animals, from bacteria to sharks, include some bioluminescent members.
While the functions of bioluminescence are not known for all animals, typically bioluminescence is used to warn or evade predators), to lure or detect prey, and for communication between members of the same species.
Details
Bioluminescence is the emission of light during a chemiluminescence reaction by living organisms. Bioluminescence occurs in multifarious organisms ranging from marine vertebrates and invertebrates, as well as in some fungi, microorganisms including some bioluminescent bacteria, dinoflagellates and terrestrial arthropods such as fireflies. In some animals, the light is bacteriogenic, produced by symbiotic bacteria such as those from the genus Vibrio; in others, it is autogenic, produced by the animals themselves.
In most cases, the principal chemical reaction in bioluminescence involves the reaction of a substrate called luciferin and an enzyme, called luciferase. Because these are generic names, luciferins and luciferases are often distinguished by the species or group, e.g. firefly luciferin or cypridina luciferin. In all characterized cases, the enzyme catalyzes the oxidation of the luciferin resulting in excited state oxyluciferin, which is the light emitter of the reaction. Upon their decay to the ground state they emit visible light. In all known cases of bioluminescence the production of the excited state molecules involves the decomposition of organic peroxides.
In some species, the luciferase requires other cofactors, such as calcium or magnesium ions, and sometimes also the energy-carrying molecule adenosine triphosphate (ATP). In evolution, luciferins vary little: one in particular, coelenterazine, is found in 11 different animal phyla, though in some of these, the animals obtain it through their diet. Conversely, luciferases vary widely between different species. Bioluminescence has arisen over 40 times in evolutionary history.
Both Aristotle and Pliny the Elder mentioned that damp wood sometimes gives off a glow. Many centuries later Robert Boyle showed that oxygen was involved in the process, in both wood and glowworms. It was not until the late nineteenth century that bioluminescence was properly investigated. The phenomenon is widely distributed among animal groups, especially in marine environments. On land it occurs in fungi, bacteria and some groups of invertebrates, including insects.
The uses of bioluminescence by animals include counterillumination camouflage, mimicry of other animals, for example to lure prey, and signaling to other individuals of the same species, such as to attract mates. In the laboratory, luciferase-based systems are used in genetic engineering and biomedical research. Researchers are also investigating the possibility of using bioluminescent systems for street and decorative lighting, and a bioluminescent plant has been created.
Additional Information
Bioluminescence is emission of light by an organism or by a laboratory biochemical system derived from an organism. It could be the ghostly glow of bacteria on decaying meat or fish, the shimmering radiance of protozoans in tropical seas, or the flickering signals of fireflies. The phenomenon occurs sporadically in a wide range of protists and animals, from bacteria and fungi to insects, marine invertebrates, and fish, but it is not known to exist naturally in true plants or in amphibians, reptiles, birds, or mammals. Bioluminescence results from a chemical reaction (chemiluminescence) in which the conversion of chemical energy to radiant energy is direct and virtually 100 percent efficient; i.e., very little heat is given off in the process. For that reason, the emission is called cold light or luminescence.
The role of bioluminescence in behaviour
Light production appears to be associated with the protection and survival of a species. That is quite clear in certain squids, who secrete a luminous cloud to confuse an enemy and make an escape, and in many deep-sea fishes who dangle luminous lures to attract prey or who show light organs to disguise their form from enemies, frighten predators, or simply light the way in the darkness of the ocean deeps. The survival value of bioluminescence is indisputable for many organisms who use their flashes as species-recognition and mating signals.
In Photinus pyralis, a common North American firefly, the male flashes spontaneously while in flight, emitting on average a 0.3-second flash every 5.5 seconds if the temperature is 25 °C (77 °F). The females watch from the ground and wait for a male to flash. Upon seeing a flash, a female flashes a response after an interval of about 2 seconds. It is that response that attracts the male. The female is unable to identify a male by his flashing. Thus, it is the male that recognizes the correct signal—i.e., interval between flashes—and seeks out the female. The interval between the male’s signal and the female’s response, therefore, is crucial. Similar specific recognition codes are used by many species of fireflies. Other fireflies possibly rely on colour differences in the light signals between sexes.
Lantern fishes and hatchetfishes, along with many other deep-sea organisms, possess distinct arrangements of light organs on the body that may serve as species- and gender-recognition patterns. The light organs, or photophores, of many deep-sea fishes are placed on the ventral and lateral surfaces of the body, and the light is emitted downward and outward. Such an arrangement is believed to allow the light of the photophores to be used to match the intensity of sunlight penetrating from above, thus concealing the fish’s own shadow from a predator below. Some lantern fishes possess, in addition, a large nasal organ; others have a patch of luminous tissue in the tail region. In deep-sea anglerfishes, the first dorsal spine is turned forward into an elongated rod, from the end of which dangles a luminous organ. When an unsuspecting prey approaches the luminous lure, it is engulfed in the fish’s large jaw.
The role of bioluminescence in metabolism
The functional role of bioluminescence in lower organisms such as bacteria, dinoflagellates, and fungi is difficult to discern. Partly because the glow of luminous bacteria is extinguished when oxygen is removed, it has been suggested that the bioluminescent reaction was originally used to remove oxygen toxic to primitive types of bacteria that developed when oxygen was absent or very rare in Earth’s atmosphere. The metabolic reaction that combines the oxygen with a reducing substance (luciferin) liberates sufficient energy to excite a molecule in the organism to emit visible radiation. Most of those luminous primitive organisms subsequently developed systems of using oxygen, but they have retained the luminescent capability as parts of related metabolic pathways or for some survival value that luminescence may confer on the organism.
The range and variety of bioluminescent organisms
Luminous species are widely scattered taxonomically, with no discernible pattern. Many luminous shrimps are known but no luminous crabs. Many luminous squids are known but only a single luminous octopus (Callistoctopus arakawai of Japan). Again, luminous centipedes and millipedes are not uncommon, but luminous scorpions and spiders are apparently nonexistent.
Almost half the animal phyla contain luminous forms, but the number of representatives is very small compared with the total number of known animal species. The protists are not so rich in luminous species but are greatest in sheer abundance, especially in tropical seas. In fact, the majority of luminous organisms are marine.
The ocean surface in many parts of the tropics is dense with single-celled luminous planktonic organisms, primarily dinoflagellates, that glow when stimulated mechanically, as by the churning of the waves, or, when washed ashore, by the pressure of a foot. Some organisms exhibit a 24-hour rhythm of light intensity, highest at night and lowest during the day.
Among crustaceans, luminous species are especially remarkable in the copepods, shrimps, and ostracods. Luminous copepods are widely distributed throughout the world’s waters. Some are surface dwellers, while others live in the deep sea. Two famous groups of luminous copepods are Pleuromma and Metridia. Some shrimps (Hoplophorus) emit a luminous secretion from luminous organs, while others possess true light organs (photophores), which consist of a lens, reflector, and light-emitting photogenic cells. Of the three or four species of the ostracod genus Vargula (also known as Cypridina) known to be luminous, the most famous is V. hilgendorfii (or C. hilgendorfii), found in the coastal waters and sands of Japan. That tiny, shelled organism, which ejects a blue luminous secretion into the water when disturbed, may be collected and dried for the light-emitting components, which are active indefinitely.
Other organisms responsible for large patches of light in the ocean are jellyfish and other coelenterates and comb jellies (ctenophores). A large proportion of the floating, transparent siphonophores and the feathery, bottom-dwelling sea pens are luminous. Many of the hydroids and jellyfish are also luminous. Sea pens (Pennatula), sea cactus (Cavernularia), and sea pansy (Renilla) are colonies, which upon stimulation generate a wave of luminous light that travels down the organism. The luminescence in these organisms appears to be under nervous control.
Among annelids, marine worms and earthworms both contain luminous forms. Odontosyllis, the fire worm of Bermuda, swarms in great numbers a few days after the full moon. Female worms, about 2 cm (almost 1 inch) in length, rise to the surface shortly after sunset and swim in circles while ejecting a luminous secretion. Smaller male worms swim to where the females are circling and mate. The male is also luminous, but the light is intermittent and of intracellular character. It is not certain whether luminescence has any relationship to mating, since nonluminous Odontosyllis exhibits similar courtship behaviour. Chaetopterus spends its life in a tube of parchment membrane, with openings at both ends. It luminesces when disturbed, but it is doubtful whether the luminescence has any special purpose. Polynoe and Polycirrus are luminous annelids that usually live in sand or rock. Luminous mollusks include Pholas (a bivalve), Phyllirrhoe (a floating nudibranch), Planaxis (a marine gastropod), Latia (a freshwater limpet), and squids (cephalopods).
The luminous squids and deep-sea fishes possess the most complicated light organs; they consist of photogenic cells, reflector, lens body, and, in certain cases, colour filters. Of the open-ocean squids (oegopsids) such as Lycoteuthis, Histioteuthis, and Enoploteuthis, as many as 75 percent are self-luminous; i.e., light results not from symbiotic luminous bacteria but from an internal biochemical reaction. In deep-sea squids, the light organ is often found on the eyelid or on the eyeball itself. In others—e.g., Watasenia scintillans—light organs are present also at the end of the tentacles and over other surfaces of the body.
Deep-sea anglerfish, hatchetfish, and lantern fish are among the best-known luminescent fishes. In most such fishes, luminescence is produced intracellularly; the light is emitted by special cells called photocytes. The anatomical structure of the luminous organs of many fishes is similar to that of squids. Deep-sea fishes have photophores along the body, under the eyes, and often on barbels or antennae. The typical luminous organ consists of a lens, luminous body, colour filter, and reflector. The light is often under the fish’s nervous control, and after death the ability to luminesce disappears rapidly. Whether the light-producing components are developed by the fish or ingested by the fish is not clear. A distinct possibility exists that a fish feeds on crustaceans such as Vargula organisms and uses their light-emitting components for its own light production.
A few genera of deep-sea fishes and several families of shallow-water fishes produce light by virtue of harbouring symbiotic luminous bacteria within light organs. That type of organ is endowed with a rich blood supply that nourishes and maintains the luminous bacteria. It appears that each fish species becomes infected with a specific bacterial type. The bacteria-filled organ is continuously luminous, but the light can be controlled either by melanophores scattered over the surface of the organ or by a black membrane that may be mechanically drawn over the organ. Control is brought about by the contraction and expansion of melanophores, or pigment granules. Expansion of the melanophores cuts off the light, whereas contraction allows light to pass through. The well-known flashlight fishes (Photoblepharon) of Indonesia possess large light organs beneath the eyes. The light is extinguished when a fold of black skin is drawn upward over the organ.
A few species of deep-sea sharks, however, can produce light without partnerships with luminous bacteria. The kitefin shark (Dalatias licha), the blackbelly lanternshark (Etmopterus lucifer), and the southern lanternshark (E. granulosus) appear to generate light in their luminous organs through chemical reactions controlled by hormones.
An indirect-emission type of luminous organ is present in some fish. The luminous organ is connected to the gut via a short duct and is often embedded in tissue. The light passes to the outside through the translucent keel and ventral muscles, as in Leiognathus, Acropoma (lanternbellies), and Archamia.
Among other higher animals, the chordate subphylum Tunicata contains luminous forms. The genus Pyrosoma includes several species that account for the brilliant luminescence among macroplanktons of the seas, giving rise to the name “fire body.” Pyrosoma is a floating colonial form, pelagic and translucent. The colonies usually reach a length of 3 to 10 cm (about 1 to 4 inches), and each individual is about 5 mm (0.2 inch) long.
Luminous bacteria are all marine forms, requiring salt for growth and luminescence, and are widely distributed throughout the oceans of the world. The most common are Vibrio and Photobacterium species. While luminous bacteria come in various shapes, they do not form clusters or chains, as do many other bacteria. The light of an individual bacterium, of course, cannot be seen with the naked eye, but the light from a liquid or agar culture containing billions of bacteria is readily visible. The light is bluish and continuous. Many luminous bacteria live in the light organs of fish and squids, without adversely affecting their hosts.
Small whitish luminous fungi (“foxfire”) commonly grow on deadwood in forests, particularly where the ground is moist and wet; these forms predominate in the tropics. The light of fungi ranges from blue to green and yellow, depending on the species. Among the large luminous forms are the ghost fungus (Omphalotus nidiformis, formerly Pleurotus lampas) of Australia and the jack-o’-lantern (O. olearius, also known as math illudens) of the United States, which reach approximately 13 cm (about 5 inches) in diameter.
Luminosity among land animals is not associated with any particular habitat, but almost all these forms are nocturnal. The centipede Orphaneus, widely distributed in tropical Asia, gives off luminous secretions from each segment. The entire body of Luminodesmus sequoiae, a millipede found in the Sierra Nevada mountains of California, glows with a diffuse light. Luminous insects include some true flies (order Diptera), notably Arachnocampa luminosa, the larva of which luminesces a greenish blue from a knob at the end of its body. The larvae dangle at the ends of filaments that hang from the ceilings of caves in New Zealand. Luminous beetles include the fireflies and the elaterid Pyrophorus (the click beetle, or cucujo in South America). The luminescent larvae of fireflies and some luminescent wingless adults are known as glowworms. The female Diplocladon hasseltii, called starworm, or diamond worm, gives off a continuous greenish blue luminescence from three spots on each segment of the body, forming three longitudinal rows of light, the appearance of which inspired the common name night train. Phrixothrix, the railroad worm, possesses two longitudinal rows, with a red luminous spot on the head.
The limpet Latia neritoides, found in streams around Auckland, New Zealand, is the only strictly freshwater luminous form known. The so-called firefly shrimp (hotaru ebi) is found in Lake Suwa, Japan, but the light is from luminous bacteria that infect the shrimp and kill it in about 24 hours.
Biochemical events of light emission
In most bioluminescent organisms, the essential light-emitting components are the oxidizable organic molecule luciferin and the enzyme luciferase, which are specific for different organisms. The present custom is to use generic names according to origin—e.g., firefly luciferin and luciferase, Vargula luciferin and luciferase. The luciferin-luciferase reaction is actually an enzyme-substrate reaction in which luciferin, the substrate, is oxidized by molecular oxygen, the reaction being catalyzed by the enzyme luciferase, with the consequent emission of light. The light emission continues until all the luciferin is oxidized. That type of reaction is found in fireflies, Vargula, Latia, and many types of fish, such as lantern fish, hatchetfish, Apogon, and Parapriaeanthus.
In firefly luminescence, the substance adenosine triphosphate (ATP) initially reacts with firefly luciferase, ionic magnesium, and firefly luciferin to form a complex (luciferase-luciferyl-adenylate) and pyrophosphate. That complex then reacts with molecular oxygen to emit light. Enough energy is liberated in the last step to convert the electronic configuration of the luciferase-luciferyl-adenylate complex from a low-energy ground state to a high-energy excited state. The high-energy complex then loses energy by radiating a photon of visible light and returns to the ground state.
Luminescent bacteria employ the enzymatic oxidation of reduced flavin mononucleotide (FMNH2). In the complete reaction, bacterial luciferase reacts with FMNH2 and oxygen to form a long-lived intermediate complex, which then reacts with a long-chain aliphatic aldehyde molecule (e.g., decanal) to emit light.
The significance of bioluminescence in research
The luminescent reaction of the firefly has been used as an assay method for the determination of adenosine triphosphate (ATP), an important metabolic substance used by all living cells in numerous reactions in which energy is either stored or expended. The glow of a specially blended extract of firefly lanterns eventually dims and disappears as ATP is broken down. The addition of fresh ATP, either as a pure chemical or as a constituent of a tissue extract, immediately restores the luminescence. The intensity of the glow is a direct measure of the amount of ATP present in the extract. That assay method has been widely used in medical and biological research to determine the amount of ATP present in extracts of cells and tissues. The study of reactions involving ATP has led to a detailed understanding of the mechanisms of energy conversion in cells. The firefly reaction is one of the few reactions in which ATP is directly involved with light emission. All other bioluminescent reactions involve compounds that are chemically distinct from ATP.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline