Math Is Fun Forum
You are not logged in.
- Topics: Active | Unanswered
#1251 2022-01-11 22:01:39
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,810
Re: Miscellany
1227) Malic Acid
Summary
Malic acid is an organic compound with the molecular formula C4H6O5. It is a dicarboxylic acid that is made by all living organisms, contributes to the sour taste of fruits, and is used as a food additive. Malic acid has two stereoisomeric forms (L- and D-enantiomers), though only the L-isomer exists naturally. The salts and esters of malic acid are known as malates. The malate anion is an intermediate in the citric acid cycle.
Etymology
The word 'malic' is derived from Latin 'mālum', meaning 'apple'. The related Latin word 'mālus', meaning 'apple tree', is used as the name of the genus Malus, which includes all apples and crabapples; and the origin of other taxonomic classifications such as Maloideae, Malinae, and Maleae.
Biochemistry
L-Malic acid is the naturally occurring form, whereas a mixture of L- and D-malic acid is produced synthetically.
Malate plays an important role in biochemistry. In the C4 carbon fixation process, malate is a source of CO2 in the Calvin cycle. In the citric acid cycle, (S)-malate is an intermediate, formed by the addition of an -OH group on the si face of fumarate. It can also be formed from pyruvate via anaplerotic reactions.
Malate is also synthesized by the carboxylation of phosphoenolpyruvate in the guard cells of plant leaves. Malate, as a double anion, often accompanies potassium cations during the uptake of solutes into the guard cells in order to maintain electrical balance in the cell. The accumulation of these solutes within the guard cell decreases the solute potential, allowing water to enter the cell and promote aperture of the stomata.
In food
Malic acid was first isolated from apple juice by Carl Wilhelm Scheele in 1785. Antoine Lavoisier in 1787 proposed the name acide malique, which is derived from the Latin word for apple, mālum—as is its genus name Malus. In German it is named Äpfelsäure (or Apfelsäure) after plural or singular of the fruit apple, but the salt(s) Malat(e). Malic acid is the main acid in many fruits, including apricots, blackberries, blueberries, cherries, grapes, mirabelles, peaches, pears, plums, and quince and is present in lower concentrations in other fruits, such as citrus. It contributes to the sourness of unripe apples. Sour apples contain high proportions of the acid. It is present in grapes and in most wines with concentrations sometimes as high as 5 g/l. It confers a tart taste to wine; the amount decreases with increasing fruit ripeness. The taste of malic acid is very clear and pure in rhubarb, a plant for which it is the primary flavor. It is also a component of some artificial vinegar flavors, such as "salt and vinegar" flavored potato chips.
In citrus, fruits produced in organic farming contain higher levels of malic acid than fruits produced in conventional agriculture.
The process of malolactic fermentation converts malic acid to much milder lactic acid. Malic acid occurs naturally in all fruits and many vegetables, and is generated in fruit metabolism.
Malic acid, when added to food products, is denoted by E number E296. It is sometimes used with or in place of the less sour citric acid in sour sweets. These sweets are sometimes labeled with a warning stating that excessive consumption can cause irritation of the mouth. It is approved for use as a food additive in the EU, US and Australia and New Zealand (where it is listed by its INS number 296).
Malic acid contains 10 kJ (2.39 kilocalories) of energy per gram.
Production and main reactions
Racemic malic acid is produced industrially by the double hydration of maleic anhydride. In 2000, American production capacity was 5000 tons per year. The enantiomers may be separated by chiral resolution of the racemic mixture. S-Malic acid is obtained by fermentation of fumaric acid.
Self-condensation of malic acid in the presence of fuming sulfuric acid gives the pyrone coumalic acid:
Malic acid was important in the discovery of the Walden inversion and the Walden cycle, in which (−)-malic acid first is converted into (+)-chlorosuccinic acid by action of phosphorus pentachloride. Wet silver oxide then converts the chlorine compound to (+)-malic acid, which then reacts with PCl5 to the (−)-chlorosuccinic acid. The cycle is completed when silver oxide takes this compound back to (−)-malic acid.
Uses
l-malic acid is used to resolve α-phenylethylamine, a versatile resolving agent in its own right.
Plant defense
Soil supplementation with molasses increases microbial synthesis of MA. This is thought to occur naturally as part of soil microbe suppression of disease, and so soil amendment with molasses can be used as a crop treatment in horticulture.
Details
Overview
Malic acid is a chemical found in certain fruits and wines. It is sometimes used as medicine.
Malic acid is used most commonly for dry mouth. It is also used for fibromyalgia, fatigue, and skin conditions, but there is no good scientific evidence to support these other uses.
In foods, malic acid is used as a flavoring agent to give food a tart taste.
In manufacturing, malic acid is used to adjust the acidity of cosmetics.
How does it work ?
Malic acid is involved in the Krebs cycle. This is a process the body uses to make energy. Malic acid is sour and acidic. This helps to clear away dead skin cells when applied to the skin. Its sourness also helps to make more saliva to help with dry mouth.
Uses & Effectiveness ?
Possibly Effective for:
* Dry mouth. Using a mouth spray or sucking on a tablet containing malic acid seems to improve symptoms of dry mouth better than using a saline mouth spray or citric acid oral rinse.
Insufficient Evidence for
* Acne. Early research shows that applying an alpha hydroxy acid cream containing malic acid helps reduce signs of acne in some people.
* Fibromyalgia. Taking malic acid in combination with magnesium seems to reduce pain and tenderness caused by fibromyalgia.
* Persistent heartburn. Early research shows that taking malic acid in combination with omeprazole may improve some symptoms of heartburn better than omeprazole alone.
* Fatigue.
* Warts.
* Scaly, itchy skin (psoriasis).
* Aging skin.
* Other conditions.
More evidence is needed to rate the effectiveness of malic acid for these uses.
Side Effects
When taken by mouth: Malic acid is LIKELY SAFE when taken by mouth in food amounts. Malic acid is POSSIBLY SAFE when taken by mouth as a medicine.
When applied to the inside of the mouth: Malic acid is POSSIBLY SAFE when applied to the inside of the mouth as a spray or lozenge.
When applied to the skin: There isn't enough reliable information to know if malic acid is safe. It might cause side effects such as skin and eye irritation.
Special Precautions and Warnings
Pregnancy and breast-feeding: Malic acid is LIKELY SAFE when taken by mouth in food amounts. There isn't enough reliable information to know if malic acid is safe to use as medicine when pregnant or breast-feeding. Stay on the safe side and avoid in amounts greater than what is normally found in food.
Low blood pressure: Malic acid might lower blood pressure. In theory, malic acid might increase the risk of blood pressure becoming too low in people prone to low blood pressure.
Moderate Interaction:
Be cautious with this combination.
Medications for high blood pressure (Antihypertensive drugs) interacts with MALIC ACID
Malic acid might lower blood pressure. Taking malic acid along with medications for high blood pressure might cause your blood pressure to go too low.
Some medications for high blood pressure include captopril (Capoten), enalapril (Vasotec), losartan (Cozaar), valsartan (Diovan), diltiazem (Cardizem), amlodipine (Norvasc), hydrochlorothiazide (HydroDiuril), furosemide (Lasix), and many others.
Dosing
The following doses have been studied in scientific research:
ADULTS:
APPLIED TO THE INSIDE OF THE MOUTH:
For dry mouth: Mouth sprays (Xeros Dentaid, Dentaid; SalivAktive) containing 1% malic acid have been used up to 8 times daily for 2 weeks. Lozenges (Xeros Dentaid, Dentaid) containing malic acid 28.58 mg, xylitol, and fluoride have been used up to 4 times a day for 6 months.
![814737_DL-Malic%20acid[814737_DL-Malic%20acid-ALL].jpg](https://www.merckmillipore.com/waroot/xl/814737_DL-Malic%20acid[814737_DL-Malic%20acid-ALL].jpg)
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1252 2022-01-12 19:00:35
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,810
Re: Miscellany
1228) Fire Extinguisher
Summary
A fire extinguisher is an active fire protection device used to extinguish or control small fires, often in emergency situations. It is not intended for use on an out-of-control fire, such as one which has reached the ceiling, endangers the user (i.e., no escape route, smoke, explosion hazard, etc.), or otherwise requires the equipment, personnel, resources and/or expertise of a fire brigade. Typically, a fire extinguisher consists of a hand-held cylindrical pressure vessel containing an agent that can be discharged to extinguish a fire. Fire extinguishers manufactured with non-cylindrical pressure vessels also exist but are less common.
There are two main types of fire extinguishers: stored-pressure and cartridge-operated. In stored pressure units, the expellant is stored in the same chamber as the firefighting agent itself. Depending on the agent used, different propellants are used. With dry chemical extinguishers, nitrogen is typically used; water and foam extinguishers typically use air. Stored pressure fire extinguishers are the most common type. Cartridge-operated extinguishers contain the expellant gas in a separate cartridge that is punctured prior to discharge, exposing the propellant to the extinguishing agent. This type is not as common, used primarily in areas such as industrial facilities, where they receive higher-than-average use. They have the advantage of simple and prompt recharge, allowing an operator to discharge the extinguisher, recharge it, and return to the fire in a reasonable amount of time. Unlike stored pressure types, these extinguishers use compressed carbon dioxide instead of nitrogen, although nitrogen cartridges are used on low temperature (–60 rated) models. Cartridge operated extinguishers are available in dry chemical and dry powder types in the U.S. and in water, wetting agent, foam, dry chemical (classes ABC and B.C.), and dry powder (class D) types in the rest of the world.
Fire extinguishers are further divided into handheld and cart-mounted (also called wheeled extinguishers). Handheld extinguishers weigh from 0.5 to 14 kilograms (1.1 to 30.9 lb), and are hence, easily portable by hand. Cart-mounted units typically weigh more than 23 kilograms (51 lb). These wheeled models are most commonly found at construction sites, airport runways, heliports, as well as docks and marinas.
Details
Fire extinguisher is a portable or movable apparatus used to put out a small fire by directing onto it a substance that cools the burning material, deprives the flame of oxygen, or interferes with the chemical reactions occurring in the flame. Water performs two of these functions: its conversion to steam absorbs heat, and the steam displaces the air from the vicinity of the flame. Many simple fire extinguishers, therefore, are small tanks equipped with hand pumps or sources of compressed gas to propel water through a nozzle. The water may contain a wetting agent to make it more effective against fires in upholstery, an additive to produce a stable foam that acts as a barrier against oxygen, or an antifreeze. Carbon dioxide is a common propellant, brought into play by removing the locking pin of the cylinder valve containing the liquefied gas; this method has superseded the process, used in the soda-acid fire extinguisher, of generating carbon dioxide by mixing sulfuric acid with a solution of sodium bicarbonate.
Numerous agents besides water are used; the selection of the most appropriate one depends primarily on the nature of the materials that are burning. Secondary considerations include cost, stability, toxicity, ease of cleanup, and the presence of electrical hazard.
Small fires are classified according to the nature of the burning material. Class A fires involve wood, paper, and the like; Class B fires involve flammable liquids, such as cooking fats and paint thinners; Class C fires are those in electrical equipment; Class D fires involve highly reactive metals, such as sodium and magnesium. Water is suitable for putting out fires of only one of these classes (A), though these are the most common. Fires of classes A, B, and C can be controlled by carbon dioxide, halogenated hydrocarbons such as halons, or dry chemicals such as sodium bicarbonate or ammonium dihydrogen phosphate. Class D fires ordinarily are combated with dry chemicals.
A primitive hand pump for directing water at a fire was invented by Ctesibius of Alexandria about 200 BCE, and similar devices were employed during the Middle Ages. In the early 1700s devices created independently by English chemists Ambrose Godfrey and French C. Hoppfer used explosive charges to disperse fire-suppressing solutions. English inventor Capt. George Manby introduced a handheld fire extinguisher—a three-gallon tank containing a pressurized solution of potassium carbonate—in 1817. Modern incarnations employing a variety of chemical solutions are essentially modifications of Manby’s design.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1253 2022-01-13 18:16:33
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,810
Re: Miscellany
1229) Shock
Electric shock
Summary
Electrical injury
Electrical injury is a physiological reaction caused by electric current passing through the body. The injury depends on the density of the current, tissue resistance and duration of contact. Very small currents may be imperceptible or produce a light tingling sensation. A shock caused by low and otherwise harmless current could startle an individual and cause injury due to jerking away or falling. Stronger currents may cause some degree of discomfort or pain, while more intense currents may induce involuntary muscle contractions, preventing the person from breaking free of the source of electricity. Still larger currents result in tissue damage and may trigger ventricular fibrillation or cardiac arrest. Consequences of injury from electricity may include amputations, bone fractures and orthopedic and musculoskeletal injuries. If death results from an electric shock the cause of death is generally referred to as electrocution.
Electric injury occurs upon contact of a body part with electricity that causes a sufficient current to pass through the person's tissue. Contact with energized wiring or devices is the most common cause. In cases of exposure to high voltages, such as on a power transmission tower, direct contact may not be necessary as the voltage may "jump" the air gap to the electrical device.
Following an electrical injury from household current, if a person has no symptoms, no underlying heart problems, and is not pregnant further testing is not required. Otherwise an electrocardiogram, blood work to check the heart, and urine testing for signs of muscle breakdown may be performed.
Management may involve resuscitation, pain medications, wound management, and heart monitoring. Electrical injuries affect more than 30,000 people a year in the United States and result in about 1,000 deaths.
Details: Shock
Shock, in physiology, is failure of the circulatory system to supply sufficient blood to peripheral tissues to meet basic metabolic requirements for oxygen and nutrients and the incomplete removal of metabolic wastes from the affected tissues. Shock is usually caused by hemorrhage or overwhelming infection and is characterized in most cases by a weak, rapid pulse; low blood pressure; and cold, sweaty skin. Depending on the cause, however, some or all of these symptoms may be missing in individual cases.
A brief treatment of shock follows.
Shock may result from a variety of physiological mechanisms, including sudden reductions in the total blood volume through acute blood losses, as in severe hemorrhage; sudden reductions in cardiac output, as in myocardial infarction (heart attack); and widespread dilation of the blood vessels, as in some forms of infection. Whatever the central physiological mechanism, the effect of shock is to reduce blood flow through the small vessels, or capillaries, where oxygen and nutrients pass into the tissues and wastes are collected for removal.
Shock is usually classified on the basis of its presumed cause, although in many cases the true cause of the peripheral circulatory insufficiency may not be apparent. The most common cause of shock is massive loss of blood, either through trauma or through surgery. In the latter case, the blood loss can be anticipated and shock prevented by providing blood transfusions during and after the operation. An acute loss of blood reduces the amount of venous blood returning to the heart, in turn reducing the cardiac output and causing a drop in arterial blood pressure. Pressure receptors, or baroreceptors, in the walls of the aorta and carotid arteries trigger physiological reflexes to protect the central circulation, increasing heart rate to boost cardiac output and constricting small blood vessels to direct blood flow to essential organs. If the blood losses continue, even these mechanisms fail, producing a sharp drop in blood pressure and overt manifestations of shock. Loss of blood plasma in burns or dehydration can also lower blood volume sufficiently to induce shock.
The heart’s output can also be reduced sufficiently to produce shock without blood loss. In coronary thrombosis, the supply of blood to the heart muscle through the coronary artery is interrupted by a blood clot or vascular constriction; the damaged muscle may then lack strength to force a normal volume out of the heart with each stroke. Again, the diminished output triggers the baroreceptors in the arteries to restrict peripheral circulation. Blood clots that block the circulation of blood to the lungs (pulmonary emboli) or increase the fluid that surrounds and cushions the heart (cardiac tamponade) can also impair the pumping of the heart sufficiently to cause shock.
The most common cause of shock by dilation of the blood vessels is massive bacterial infection, which may be further exacerbated by reductions in total blood volume caused by fluid losses secondary to the infection. Generally, toxins produced by the bacteria are the cause of the dilation. Foreign substances in the bloodstream can also produce a form of shock, called anaphylactic shock, through allergic reactions causing blood vessels to dilate. Another possible cause of shock through vascular dilation is drugs; many anesthetic drugs create a controlled shock that must be carefully monitored by adjusting dosage, and overdoses of several such drugs, including barbiturates and narcotics, produce shock symptoms.
The chief problem in treating shock is to recognize the cause of the physiological problem, as several possible causes may coexist in a single patient, especially following an accident. Failure to distinguish between shock caused by inadequate cardiac output and that caused by fluid losses reducing blood volume can result in a therapeutic dilemma, since treatments that are effective for one kind of shock will aggravate the other. Intravenous fluids are the usual treatment for shock caused by loss of blood, but adding extra fluid to the circulation can overload a damaged heart that already has a reduced output, so that the shock deepens. When the cause of shock is unclear, physicians may make a trial using intravenous fluids; if the central venous pressure rises, indicating diminished cardiac capacity, the fluids are stopped before the heart can be further compromised. Shock secondary to bacterial infection may be treated by combined fluid replacement and appropriate antibiotics, while anaphylactic shock is combated with epinephrine and antihistamines, which counter the acute allergic response.
What You Should Know About Shock
What is shock?
The term “shock” may refer to a psychologic or a physiologic type of shock.
Psychologic shock is caused by a traumatic event and is also known as acute stress disorder. This type of shock causes a strong emotional response and may cause physical responses as well.
The focus of this article is on the multiple causes of physiologic shock.
Your body experiences shock when you don’t have enough blood circulating through your system to keep organs and tissues functioning properly.
It can be caused by any injury or condition that affects the flow of blood through your body. Shock can lead to multiple organ failure as well as life-threatening complications.
There are many types of shock. They fall under four main categories, based on what has affected the flow of blood. The four major types are:
* obstructive shock
* cardiogenic shock
* distributive shock
* hypovolemic shock
All forms of shock are life-threatening.
If you develop symptoms of shock, get medical help immediately.
What are the signs and symptoms of shock?
If you go into shock, you may experience one or more of the following:
* rapid, weak, or absent pulse
* irregular heartbeat
* rapid, shallow breathing
* lightheadedness
* cool, clammy skin
* dilated pupils
* lackluster eyes
* chest pain
* nausea
* confusion
* anxiety
* decrease in urine
* thirst and dry mouth
* low blood sugar
* loss of consciousness
What causes shock to occur?
Anything that affects the flow of blood through your body can cause shock. Some causes of shock include:
* severe allergic reaction
* significant blood loss
* heart failure
* blood infections
* dehydration
* poisoning
* burns
What are the major types of shock?
There are four major types of shock, each of which can be caused by a number of different events.
Obstructive shock
Obstructive shock occurs when blood can’t get where it needs to go. A pulmonary embolism is one condition that may cause an interruption to blood flow. Conditions that can cause a buildup of air or fluid in the chest cavity can also lead to obstructive shock. These include:
* pneumothorax (collapsed lung)
* hemothorax (blood collects in the space between the chest wall and lung)
* cardiac tamponade (blood or fluids fill the space between the sac that surrounds the heart and the heart muscle)
Cardiogenic shock
Damage to your heart can decrease the blood flow to your body, leading to cardiogenic shock. Common causes of cardiogenic shock include:
* damage to your heart muscle
* irregular heart rhythm
* very slow heart rhythm
Distributive shock
Conditions that cause your blood vessels to lose their tone can cause distributive shock. When your blood vessels lose their tone, they can become so open and floppy that not enough blood pressure supplies your organs. Distributive shock can result in symptoms including:
* flushing
* low blood pressure
* loss of consciousness
There are a number of types of distributive shock, including the following:
Anaphylactic shock is a complication of a severe allergic reaction known as anaphylaxis. Allergic reactions occur when your body mistakenly treats a harmless substance as harmful. This triggers a dangerous immune response.
Anaphylaxis is usually caused by allergic reactions to food, insect venom, medications, or latex.
Septic shock is another form of distributive shock. Sepsis, also known as blood poisoning, is a condition caused by infections that lead to bacteria entering your bloodstream. Septic shock occurs when bacteria and their toxins cause serious damage to tissues or organs in your body.
Neurogenic shock is caused by damage to the central nervous system, usually a spinal cord injury. This causes blood vessels to dilate, and the skin may feel warm and flushed. The heart rate slows, and blood pressure drops very low.
Drug toxicities and brain injuries can also lead to distributive shock.
Hypovolemic shock
Hypovolemic shock happens when there isn’t enough blood in your blood vessels to carry oxygen to your organs. This can be caused by severe blood loss, for example, from injuries.
Your blood delivers oxygen and vital nutrients to your organs. If you lose too much blood, your organs can’t function properly. Serious dehydration can also cause this type of shock.
How is shock diagnosed?
First responders and doctors often recognize shock by its external symptoms. They may also check for:
* low blood pressure
* weak pulse
* rapid heartbeat
Once they’ve diagnosed shock, their first priority is to provide lifesaving treatment to get blood circulating through the body as quickly as possible. This can be done by giving fluid, drugs, blood products, and supportive care. It won’t resolve unless they can find and treat the cause.
Once you’re stable, your doctor can try to diagnose the cause of shock. To do so, they may order one or more tests, such as imaging or blood tests.
Imaging tests
Your doctor may order imaging tests to check for injuries or damage to your internal tissues and organs, such as:
* bone fractures
* organ ruptures
* muscle or tendon tears
* abnormal growths
Such tests include:
* ultrasound
* X-ray
* CT scan
* MRI scan
Blood tests
Your doctor may use blood tests to look for signs of:
* significant blood loss
* infection in your blood
* drug or medication overdose
How is shock treated?
Shock can lead to unconsciousness, breathing problems, and even cardiac arrest:
* If you suspect that you’re experiencing shock, get medical help immediately.
* If you suspect that someone else has gone into shock, and provide first aid treatment until professional help arrives.
First aid treatment
If you suspect someone has gone into shock, call emergency. Then follow these steps:
* If they’re unconscious, check to see if they’re still breathing and have a heartbeat.
* If you don’t detect breathing or a heartbeat, begin CPR.
If they’re breathing:
* Lay them down on their back.
* Elevate their feet at least 12 inches above the ground. This position, known as the shock position, helps direct blood to their vital organs where it’s most needed.
* Cover them with a blanket or extra clothing to help keep them warm.
* Check their breathing and heart rate regularly for changes.
If you suspect the person has injured their head, neck, or back, avoid moving them.
Apply first aid to any visible wounds. If you suspect the person is experiencing an allergic reaction, ask them if they have an epinephrine auto-injector (EpiPen). People with severe allergies often carry this device.
It contains an easy-to-inject needle with a dose of hormone called epinephrine. You can use it to treat anaphylaxis.
If they begin to vomit, turn their head sideways. This helps prevent choking. If you suspect they’ve injured their neck or back, avoid turning their head. Instead, stabilize their neck and roll their entire body to the side to clear the vomit out.
Medical care
Your doctor’s treatment plan for shock will depend on the cause of your condition. Different types of shock are treated differently. For example, your doctor may use:
* epinephrine and other drugs to treat anaphylactic shock
* blood transfusion to replace lost blood and treat hypovolemic shock
* medications, heart surgery, or other interventions to treat cardiogenic shock
* antibiotics to treat septic shock
Can you fully recover from shock?
It’s possible to fully recover from shock. But if it isn’t treated quickly enough, shock can lead to permanent organ damage, disability, and even death. It’s critical to call 911 immediately if you suspect that you or someone you’re with is experiencing shock.
Your chances of recovery and long-term outlook depend on many factors, including:
* the cause of shock
* the length of time you were in shock
* the area and extent of organ damage that you sustained
* the treatment and care that you received
* your age and medical history
Can shock be prevented?
Some forms and cases of shock are preventable. Take steps to lead a safe and healthy lifestyle. For example:
* If you’ve been diagnosed with severe allergies, avoid your triggers, carry an epinephrine auto-injector, and use it at the first sign of an anaphylactic reaction.
* To lower your risk of blood loss from injuries, wear protective gear when taking part in contact sports, riding your bike, and using dangerous equipment. Wear a seatbelt when traveling in motor vehicles.
* To lower your chances of heart damage, eat a well-balanced diet, exercise regularly, and avoid smoking and secondhand smoke.
Stay hydrated by drinking plenty of fluids. This is especially important when you’re spending time in very hot or humid environments.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1254 2022-01-14 17:39:33
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,810
Re: Miscellany
1230) Glycerol
Glycerol, also called glycerine in British English and glycerin in American English) is a simple polyol compound. It is a colorless, odorless, viscous liquid that is sweet-tasting and non-toxic. The glycerol backbone is found in lipids known as glycerides. Due to having antimicrobial and antiviral properties it is widely used in FDA (Food and Drug Administration) approved wound and burn treatments. Conversely, it is also used as a bacterial culture medium. It can be used as an effective marker to measure liver disease. It is also widely used as a sweetener in the food industry and as a humectant in pharmaceutical formulations. Owing to the presence of three hydroxyl groups, glycerol is miscible with water and is hygroscopic in nature.
Summary
Glyceroli is a clear, colourless, viscous, sweet-tasting liquid belonging to the alcohol family of organic compounds; molecular formula HOCH2CHOHCH2OH. Until 1948 all glycerol was obtained as a by-product in making soaps from animal and vegetable fats and oils, but industrial syntheses based on propylene or sugar has accounted for an increasingly large percentage of production since that time. The term glycerin (or glycerine), introduced in 1811 by French chemist Michel-Eugène Chevreul, is ordinarily applied to commercial materials containing more than 95 percent glycerol. Though Chevreul gave glycerin its name, the substance was first isolated in 1783 by German Swedish chemist Carl Wilhelm Scheele, who described it as the “sweet principle of fat.”
Glycerol has numerous uses. It is a basic ingredient in the gums and resins used to make many modern protective coatings such as automotive enamels and exterior house paints. Glycerin reacted with nitric and sulfuric acid forms the explosive nitroglycerin (or nitroglycerine).
Glycerol is also a component of mono- and diglyceride emulsifiers, which are used as softening agents in baked goods, plasticizers in shortening, and stabilizers in ice cream. Its varied uses in the pharmaceutical and toilet goods fields include skin lotions, mouthwashes, cough medicines, drug solvents, serums, vaccines, and suppositories. Another significant use is as a protective medium for freezing red blood cells, sperm cells, eye corneas, and other living tissues. At one time, its largest single use was as automotive antifreeze; methanol and ethylene glycol have replaced it for this purpose.
Fats and oils are valued chiefly as sources of the carboxylic acids that are present, combined in the form of esters with glycerol. When the acids are set free from these compounds, glycerol remains as a solution in water and is purified by coagulating and settling extraneous matter, evaporating the water, and distilling.
Glycerol which is also known as glycerine, glycerin or propanetriol is a polyol compound. The derivation of the gly- and glu- prefixes for glycerol and for sugars is derived from a Greek word glukus which means sweet.
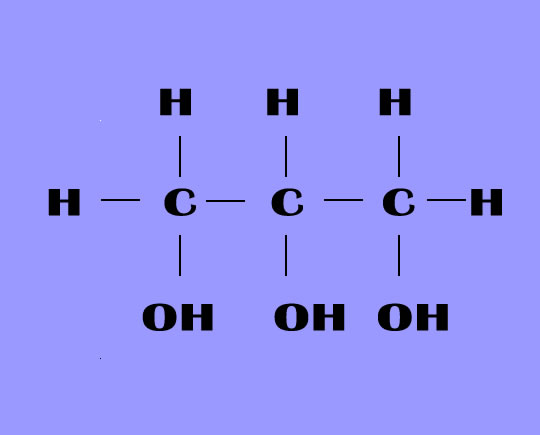
It is a trihydroxy sugar alcohol which acts as an intermediate in carbohydrate and lipid metabolism. The formula of glycerol is C3H8O3.
PROPERTIES OF GLYCEROL
Glycerol is a colorless, odorless and viscous liquid which is sweet in taste and is non-toxic.
Boiling point: 290 degree Celsius, melting point: 17.9 degree Celsius.
Molecular weight: 92.094 g/mol, relative density: 1.261 g/ml.
Solubility: Insoluble in volatile oils and fixed oils, in water it is miscible.
Glycerol is weakly acidic in nature and is able to react with alkaline hydroxide.
PRODUCTION OF GLYCEROL
Natural production:
Glycerol is mostly obtained from plants and animal sources where it is present as triglycerides. Triglycerides are glycerol esters having carboxylic acids of a long chain. The hydrolysis, saponification or transesterification of these triglycerides gives out glycerol.
Plants sources typically include soybeans or palm trees. Another source is animal-derived tallow.
Synthetic production:
Glycerol can also be produced by various routes from propylene, which is a three carbon petrochemical compound with double bonds. The most important process is epichlorohydrin, which includes propylene chlorination giving allyl chloride, which is then oxidized with hypochlorite to dichlorohydrins, which gives epichlorohydrin by reacting with a strong base. Then this epichlorohydrin is then hydrolyzed to give glycerol.
Applications:
1. Food industry: Glycerol serves as a sweetener, solvent, and humectants in food and beverages and can also help in preserving food. It is also used in commercially prepared low-fat foods as filler and in liqueurs as a thickening agent. Glycerol is also used along with water to preserve certain types of leaves. It is also used as a sugar substitute.
2. Pharmaceutical and personal-care: Glycerol is utilized in pharmaceutical and personal care products preparations, majorly as a means of developing smoothness, for providing lubrication and as humectants. In tablets dosage, it is used a holding agent and it is also a component of glycerin soap. Glycerol is found in cough syrups, elixirs, toothpaste, mouthwashes, products of skin care and water-based personal care lubricants.
3. E-cigarette liquid: Vegetable glycerine with propylene glycol, in one of the common component of e-cigarette liquid. This glycerol produces the aerosol when heated with an atomizer, delivering nicotine to the consumer.
4. For anti-freezing: Glycerol was used as an anti-freezing agent for automotive applications in past before getting replaced by ethylene glycol. Glycerol is a common compound of solvents for enzymatic reagents in the labs. It is also used as a cryoproctectant.
5. Chemical intermediate: Glycerol is used in the production of nitroglycerin. Allyl iodide can be synthesized by utilization of elemental phosphorus and iodine on glycerol. Crude glycerol for a renewable energy source as an additive to biomass when burnt or gasified is being examined.
6. Film industry: When filming scenes which involve water to stop drying out of areas too quickly glycerol are used by the film industry.
Overview
Glycerol is a naturally occurring chemical. People use it as a medicine. Some uses and dosage forms have been approved by the U.S. Food and Drug Administration (FDA).
Glycerol is most commonly used for constipation, improving hydration and performance in athletes, and for certain skin conditions. It is also used for meningitis, stroke, obesity, ear infections, and other conditions, but there is no good scientific evidence to support these uses.
How does it work ?
Glycerol attracts water into the gut, softening stools and relieving constipation.
In the blood, it attracts water so that the water stays in the body longer. This might help an athlete exercise for longer.
Uses & Effectiveness ?
Likely Effective for
* Constipation. Giving glycerol into the rectum, as a suppository or as an enema, decreases constipation.
Possibly Effective for
* Athletic performance. There is some evidence that taking glycerol by mouth along with water helps to keep the body hydrated for longer. The increase in fluids in the body might help people exercise for a few minutes longer and possibly go a bit faster, especially if it is hot.
* Dandruff. Using a hair lotion containing glycerol, stearic acid, and sunflower seed oil 3 times each week can reduce dandruff by a small amount and moisturize the scalp.
* Dry skin. Applying a product containing glycerol and paraffin to the skin reduces the thickness of scales and itching in people with xerosis.
* An inherited skin disorder that causes dry, scaly skin (ichthyosis). Applying a specific, prescription-only product (Dexeryl, Pierre Fabre Laboratoires) containing glycerol and paraffin to the skin reduces symptoms like itching and scales in children with ichthyosis.
Possibly Ineffective for
* Swelling (inflammation) of membranes that protect the brain and spinal cord (meningitis). Taking glycerol along with medicines used to treat meningitis doesn't reduce the chance of death, seizures, or stomach and intestinal injury. But it might reduce the chance of deafness in children who survive the infection.
* Growth and development in premature infants. Giving glycerol into the rectum, as a suppository or as an enema, is sometimes used in premature infants to help them pass their first stool. It's thought that this will help them start to take food by mouth earlier. But glycerol doesn't seem to have much benefit for this purpose.
Likely Ineffective for
* Stroke. Receiving intravenous (IV) glycerol from a healthcare professional does not improve symptoms after a stroke.
Insufficient Evidence for
* Obesity. Early research in adults on a low-calorie diet shows that taking glycerol before meals does not increase weight loss.
* Swimmer's ear (otitis externa). Early research shows that having a doctor place a gauze soaked in ichthammol and glycerol into the ear canal reduces pain and swelling as much as using prescribed ear drops.
* Wrinkled skin.
* Other conditions.
More evidence is needed to rate glycerol for these uses.
Side Effects
* When taken by mouth: Glycerol is POSSIBLY SAFE when taken by mouth, short-term. Glycerol can cause side effects including headaches, dizziness, bloating, nausea, vomiting, thirst, and diarrhea.
* When applied to the skin: Glycerol is LIKELY SAFE when applied to the skin. When applied on the skin, glycerol might cause redness, itching, and burning.
* When given in the rectum: Glycerol is LIKELY SAFE when inserted into the rectum.
* When given by IV: Glycerol is POSSIBLY UNSAFE when injected intravenously (by IV). This might damage red blood cells.
Special Precautions and Warnings
* Pregnancy and breast-feeding: There isn't enough reliable information to know if glycerol is safe to use when pregnant or breast-feeding. Stay on the safe side and avoid use.
* Children: Glycerol is LIKELY SAFE when inserted into the rectum or applied to the skin in children at least 1 month old. Glycerol is POSSIBLY SAFE when taken by mouth, short-term in children 2 months to 16 years of age.
Dosing
The following doses have been studied in scientific research:
ADULTS
BY MOUTH:
* For athletic performance: Glycerol 1-1.5 grams/kg taken with about 6 cups of water starting an hour or two before competition. Glycerol is banned during competition in some sports because it might alter the amount of fluid in the blood and change the results of some laboratory tests.
ON THE SKIN:
* For dandruff: A leave-in hair lotion containing glycerol 10%, stearic acid 2.5%, and sunflower seed oil 0.6%, applied to the scalp 3 times weekly for 8 weeks.
* For dry skin: An emulsion containing glycerol 15% and paraffin 10% applied to the skin twice daily for 1-8 weeks.
RECTAL:
* For constipation: Glycerol 2-3 grams as a suppository or 5-15 mL as an enema.
CHILDREN
ON THE SKIN:
For an inherited skin disorder that causes dry, scaly skin (ichthyosis): A specific, prescription-only product (Dexeryl, Pierre Fabre Laboratoires) containing glycerol 15% and paraffin 10% applied to the skin for 4-12 weeks.
RECTAL:
* For constipation: For children younger than six years old, the dose is 1-1.7 grams as a suppository or 2-5 mL as an enema. For children older than six years of age, the dose is 2-3 grams as a suppository or 5-15 mL as an enema.
Additional Information
Molecular Weight : 92.09
Glycerol is a triol with a structure of propane substituted at positions 1, 2 and 3 by hydroxy groups. It has a role as an osmolyte, a solvent, a detergent, a human metabolite, an algal metabolite, a Saccharomyces cerevisiae metabolite, an Escherichia coli metabolite, a mouse metabolite and a geroprotector. It is an alditol and a triol.
Glycerin is a trihydroxyalcohol with localized osmotic diuretic and laxative effects. Glycerin elevates the blood plasma osmolality thereby extracting water from tissues into interstitial fluid and plasma. This agent also prevents water reabsorption in the proximal tubule in the kidney leading to an increase in water and sodium excretion and a reduction in blood volume. Administered rectally, glycerin exerts a hyperosmotic laxative effect by attracting water into the rectum, thereby relieving constipation. In addition, glycerin is used as a solvent, humectant and vehicle in various pharmaceutical preparations.
Glycerine appears as a colorless to brown colored liquid. Combustible but may require some effort to ignite.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1255 2022-01-15 16:42:05
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,810
Re: Miscellany
1231) Maple
Summary
Acer is a genus of trees and shrubs commonly known as maples. The genus is placed in the family Sapindaceae. There are approximately 132 species, most of which are native to Asia, with a number also appearing in Europe, northern Africa, and North America. Only one species, Acer laurinum, extends to the Southern Hemisphere. The type species of the genus is the sycamore maple, Acer pseudoplatanus, the most common maple species in Europe. The maples usually have easily recognizable palmate leaves (Acer negundo is an exception) and distinctive winged fruits. The closest relatives of the maples are the horse chestnuts. Maple syrup is made from the sap of some maple species.
Details
Maple, (Acer), is any of a large genus (about 200 species) of shrubs or trees in the family Sapindaceae, widely distributed in the North Temperate Zone but concentrated in China. Maples constitute one of the most important groups of ornamentals for planting in lawns, along streets, and in parks. They offer a great variety of form, size, and foliage; many display striking autumn colour. Several yield maple syrup, and some provide valuable, dense hard wood for furniture and other uses. All maples bear pairs of winged seeds, called samaras or keys. The leaves are arranged oppositely on twigs. Many maples have lobed leaves, but a few have leaves separated into leaflets.
Among the popular smaller maples the hedge, or field, maple (A. campestre) and Amur, or ginnala, maple (A. ginnala) are useful in screens or hedges; both have spectacular foliage in fall, the former yellow and the latter pink to scarlet. The Japanese maple (A. palmatum), developed over centuries of breeding, provides numerous attractive cultivated varieties with varying leaf shapes and colours, many useful in small gardens. The vine maple (A. circinatum), of wide-spreading, shrubby habit, has purple and white spring flowers and brilliant fall foliage. The shrubby Siebold maple (A. sieboldianum) has seven- to nine-lobed leaves that turn red in fall.
Medium-sized maples, often more than 9 metres (30 feet) tall, include the big-toothed maple (A. grandidentatum); some believe it to be a subspecies of sugar maple, a Rocky Mountain tree, often multistemmed, displaying pink to red fall foliage. Coliseum maple (A. cappadocicum) and Miyabe maple (A. miyabei) provide golden-yellow fall colour. The three-flowered maple (A. triflorum) and the paperbark maple (A. griseum) have tripartite leaves and attractive peeling bark, in the former tannish and in the latter copper brown.
The ash-leaved maple, or box elder, is a fast-growing tree of limited landscape use. The Norway maple (A. platanoides), a handsome, dense, round-headed tree, has spectacular greenish-yellow flower clusters in early spring; many cultivated varieties are available with unusual leaf colour (red, maroon, bronze, or purple) and growth form (columnar, globular, or pyramidal).
Large maples, usually in excess of 30 metres high, that are much planted for shade include the sugar (A. saccharum), silver (A. saccharinum), and red (A. rubrum) maples. The Oregon, or bigleaf, maple (A. macrophyllum) provides commercially valuable wood darker than that of other maples; it shows bright-orange fall foliage. The Sycamore maple (A. pseudoplatanus), an important shade and timber tree in Europe, has many ornamental varieties.
In one group of maples, the vertically striped silvery-white young bark provides an attractive winter landscaping feature. These trees are the striped maple (A. pennsylvanicum), the red snake-bark maple (A. capillipes), the Her’s maple (A. hersii), and the David’s maple (A. davidii). The chalk maple, with whitish bark, is sometimes classified as A. leucoderme, although some authorities consider it a subspecies of sugar maple.
The parlour maples, or flowering maples, are bedding and houseplants in the genus Abutilon.
Additional Information
Maple trees belong to the genus Acer and family Sapindaceae. There are about 125 species of maple trees. They can be found growing parts of Asia, Europe, North America, Canada, and Northern Africa.
Maple trees are admired for their stunning display of fall leaf colors. The leaves turn into shades of yellow, orange, and red. This article is an in-depth look at the red maple, sugar maple, and silver maple trees.
Red Maple Tree
Red Maple Tree (Acer rubrum) is a deciduous tree well-known for its vibrant autumn leaves. This tree grows to a height of about 60 to 90 feet and has a trunk that can grow up to 30 inches in diameter. It is the state tree of Rhode Island.
These trees can adapt well to different soil and climate conditions. The red maple tree grows short taproots with long lateral roots in wet soil and develops deep taproots with short lateral roots in dry soil.
The crown has a spread of 25 to 35 feet and is rounded, or oval when it reaches maturity. The young trees have smooth light gray bark that becomes a darker gray furrowed and scaly at maturity. The average lifespan of the red maple is 80 to 100 years. They start producing seeds when they are four years old.
The leaves of the red maple are palmate, 3 inches to 6 inches wide, and have 3 to 5 lobes. They are green above and pale green below. The margins are serrated with shallow "V "shaped divisions between lobes.
The leaves turn to shades of yellow, orange-red to bright red during fall. They are highly serrated when compared to the sugar maple leaves.
The flowers of the red maple are bright red and are found growing in clusters. They appear in spring before the leaves unfurl. A single red maple tree can produce all male flowers, all-female flowers, or both male and female flowers on the same tree. Some maple trees are monoecious having the male and female sex organs in the same flower. The male flowers have long stamens that extend beyond the petal with yellow pollen at the tips. In the female flower, the stigma extends beyond the petals to catch the pollen.
The fruit of the red maple tree produces winged samaras (winged seeds). They are known as spinners because they spin as they fall to the ground.
The red maple samaras are red, whereas those of the sugar maple is green in spring. These samaras disperse in spring before the leaves are fully developed. The sugar maple samaras hang on without dispersing until the fall.
Uses of Red Maple
Due to its bright red colored leaves, fruits, and beautiful fall colors, the red maple tree is valued as an ornamental tree. Wood of the red maple tree is ideal for the manufacture of boxes and musical instruments.
Red Maple and Wildlife
Red maple is a source of food for moose, deer, and rabbits. The sap has half the sugar content of the sugar maple tree, but it has a great taste.
The seeds, the buds, and flowers are food for many wildlife species. Wood ducks nest inside cavities of red maples.
Sugar Maple Tree
The sugar maple (Acer saccharaum) belongs to the soapberry family (Sapindaceae). The sugar maple tree is a deciduous tree that grows to a height of 60 feet to 80 feet and has a diameter of 1 to 2 feet. The sugar maple is also called hard maple because of the density and strength of its wood.
The bark of the young sugar maple tree is brownish gray. As they grow older, the bark becomes darker, furrowed with thin, gray scaly plates. The crown of the sugar maple is dense and has an oval, rounded or a columnar shape. This tree is planted as a shade tree because of its dense crown.
The sugar maple has a shallow root system with strong lateral roots that are highly branched.
The leaves of the sugar maple tree are borne on a smooth stalk. They are palmate and measure three to five inches in width and height. They have five lobes with serrated margins. The division between the lobes is smooth, shallow and rounded. The leaves turn into colors of yellow, orange and deep red during fall.
The two lobes at the base of the leaves are smaller than the other three and are almost parallel to each other.
A sugar maple tree can produce all-male flowers or all female flowers or both on the same tree. Some trees bear flowers that have both the male and female gender organs. The flowers of the sugar maple are found in clusters and are greenish-yellow. They appear in spring just before the leaves emerge.
The fruits of this tree are double samaras (winged seeds) that are green in spring and turn yellowish-green or light brown in autumn.
Maple syrup is made from the sap of the sugar maple tree. It takes about 40 gallons of sap to make one gallon of maple syrup.
Uses of Sugar Maple
Sugar maple has heavy, strong wood that is used to make furniture, paneling, flooring, and veneer. It is also used to make bowling pins and musical instruments. The maple tree wood is a tonewood (wood that carries soundwaves, due to this property the maple wood is used to make musical instruments like violins, violas, and cellos. The necks of electric guitars are also made from maple wood.
Sugar Maple and Wildlife
Sugar maple is a source of food for many wildlife species. The white-tailed deer, moose and snow hares browse on sugar maple trees. Red squirrels feed on its buds, twigs, and leaves. Porcupines eat the bark.
The flowers are wind-pollinated. The pollen that is initially produced is essential for Apis mellifera (honey bees) and other insects. The sugar maple is a caterpillar host for the Cecropia Silkmoth and Rose Maple Moth. Many birds build nests and forage the tree for insects.
Silver Maple Tree
Silver Maple (Acer saccharinum) is also called soft maple or white maple. It is a deciduous tree that has rapid growth with a shallow root system. It belongs to the soapberry family (Sapindaceae) and has a stout trunk with large forked spreading branches. The branches are brittle and break easily.
This tree grows to a height of about 60 to 120 feet. The young bark is smooth and gray but becomes flaky as it reaches maturity. The crown of the maple tree is vase-shaped with an irregular crown.
The leaves are 4 to 6 inches long, green above, silvery below, and have five lobes with deep "V" shaped divisions. The middle lobe is also divided into three lobes with shallow sinuses. The twigs are slender, red-brown, and curved upwards. The leaves turn yellow to red during fall.
The silver maple tree is monoecious. The male flowers are greenish-yellow, and the female flowers are red. They appear in clusters in early spring before the leaves begin to unfold.
The fruits of the silver maple are samaras that grow in attached pairs with green or yellow wings with large seeds at the base. They measure about 1.2 - 2 inches in length and are the largest samaras among all maple trees.
The silver maple trees are not favored for landscaping because of its brittle wood that breaks off during storms. The roots are shallow, grow rapidly, and can cause cracks in basement walls, sidewalks, tanks, and drain pipes.
The cut-leaf silver maple (A.saccharinum ‘Laciniatum’) and the pyramidal silver maple (A.saccharinum ‘Pyramidale’) varieties are used in landscaping because they are not very tall and have sturdy branches.
Silver maples have thin, watery sap with low sugar content and hence not ideal for making maple syrup.
Uses of Silver Maple
The wood of silver maple is used to make lightweight furniture, cabinetry, paneling, flooring, veneer, musical instruments, boxes, crates, and tools.
Silver Maple and Wildlife
The seeds of the silver maple tree are eaten by many birds, squirrels, and chipmunks. The buds are a source of food for squirrels during late winter and early spring. The bark is food for beavers and the leaves are eaten by deer and rabbits. The silver maple tree tends to form cavities that are used for shelter by nesting birds and mammals.
Maple Syrup Extraction
Maple is a sweet syrup that is obtained from the sap of the maple tree. Any tree that is eight inches or more in width can be tapped for maple syrup.
From the beginning of the 17th century, dairy farmers were looking for a source of sweetener that was better in quality and cheaper than sugar. They drilled holes in the trees during the short time between winter and spring.
The farmers hung buckets under the drilled holes. They called the maples tree “sugar bushes”. After a day or two, the farmers would empty the buckets in large containers and haul the sap to a sugar house built in the woods. To make the brown, sweet maple syrup the sugar manufacturers boiled the sap to remove most of the water content.
Nowadays holes are bored in sugar maples in early spring. Small plastic spouts are inserted into these holes and the spouts are connected to a central plastic tubing that allows the sap to flow into large tanks.
The sap from the maple tree oozes out when the day temperature is forty degrees followed by a night when the temperature is below freezing. Global warming has affected the production of maple syrup resulting in a substantial increase in the price of maple syrup.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1256 2022-01-16 15:36:05
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,810
Re: Miscellany
1232) Thalassemia
Summary
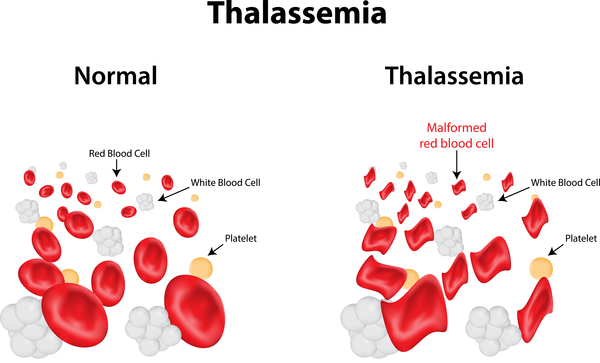
Thalassemias are inherited blood disorders characterized by decreased hemoglobin production. Symptoms depend on the type and can vary from none to severe. Often there is mild to severe anemia (low red blood cells or hemoglobin). Anemia can result in feeling tired and pale skin. There may also be bone problems, an enlarged spleen, yellowish skin, and dark urine. Slow growth may occur in children.
Thalassemias are genetic disorders inherited from a person's parents. There are two main types, alpha thalassemia and beta thalassemia. The severity of alpha and beta thalassemia depends on how many of the four genes for alpha globin or two genes for beta globin are missing. Diagnosis is typically by blood tests including a complete blood count, special hemoglobin tests, and genetic tests. Diagnosis may occur before birth through prenatal testing.
Treatment depends on the type and severity. Treatment for those with more severe disease often includes regular blood transfusions, iron chelation, and folic acid. Iron chelation may be done with deferoxamine, deferasirox or deferiprone. Occasionally, a bone marrow transplant may be an option. Complications may include iron overload from the transfusions with resulting heart or liver disease, infections, and osteoporosis. If the spleen becomes overly enlarged, surgical removal may be required. Thalassemia patients who do not respond well to blood transfusions can take hydroxyurea or thalidomide, and sometimes a combination of both. Hydroxyurea is the only FDA (Food and Drug Administration) approved drug for thalassemia. Patients who took 10 mg/kg of hydroxyurea every day for a year had significantly higher hemoglobin levels, and it was a well-tolerated treatment for patients who did not respond well to blood transfusions. Another hemoglobin-inducer includes thalidomide, although it has not been tested in a clinical setting. The combination of thalidomide and hydroxyurea resulted in hemoglobin levels increasing significantly in transfusion-dependent and non-transfusion dependent patients
As of 2015, thalassemia occurs in about 280 million people, with about 439,000 having severe disease. It is most common among people of Italian, Greek, Turkish, Middle Eastern, South Asian, and African descent. Males and females have similar rates of disease. It resulted in 16,800 deaths in 2015, down from 36,000 deaths in 1990 Those who have minor degrees of thalassemia, similar to those with sickle-cell trait, have some protection against malaria, explaining why they are more common in regions of the world where malaria exists.
Details:
What Is Thalassemia?
Thalassemia is an inherited blood condition. If you have it, your body has fewer red blood cells and less hemoglobin than it should. Hemoglobin is important because it lets your red blood cells carry oxygen to all parts of your body. Because of this, people with this condition may have anemia, which makes you feel tired.
You may hear it called things like Constant Spring, Cooley’s anemia, or hemoglobin Bart’s hydrops fetalis. These are common names for different forms of it. The two types are alpha thalassemia and beta thalassemia. The terms alpha and beta refer to the part of the hemoglobin the person is lacking.
There are also terms for how serious the thalassemia is. A person with a trait or minor form may not have symptoms or only mild ones. They may not need treatment. Someone with a major form will need medical treatment.
Thalassemia Causes and Risk Factors
Thalassemia is genetic. It happens when you inherit mutated genes from your parents that change your hemoglobin. You have it from birth. You can’t catch thalassemia the way you catch a cold or the flu.
If both of your parents carry thalassemia, you might get it. If you inherit two or more copies of abnormal genes from your parents, you may get mild to severe thalassemia, depending on what type of protein is affected. It’s more common in people from Asia, Africa, the Middle East, and Mediterranean countries like Greece or Turkey.
Thalassemia Types
Thalassemia is really a group of blood problems, not just one.
To make hemoglobin, you need two proteins, alpha and beta. Without enough of one or the other, your red blood cells can’t carry oxygen the way they should.
Alpha thalassemia means you don't make enough of the alpha hemoglobin protein chain to make your hemoglobin. With beta thalassemia, you don't make enough of the beta.
You have four genes responsible for making the alpha protein chain of hemoglobin. You get two from each parent. If you have one abnormal copy of an alpha gene, you won’t have thalassemia but you’ll carry it. If you have two abnormal copies of an alpha gene, you’ll have mild alpha thalassemia. If you have more abnormal copies, you’ll have more serious alpha thalassemia. Babies with four abnormal copies of the alpha gene are often stillborn, or don’t survive long after birth.
You have two genes that are needed to make the beta protein. You get one from each of your parents. If you have one abnormal copy of the beta gene, you’ll have mild beta thalassemia. If you have two copies, you’ll have more moderate to severe beta thalassemia.
Thalassemia Symptoms
These can include:
* Slow growth in children
* Wide or brittle bones
* Enlarged spleen (an organ in your abdomen that filters blood and fights disease)
* Fatigue
* Weakness
* Pale or yellow skin
* Dark urine
* Poor appetite
* Heart problems
In some people, symptoms show up at birth. In others, it can take a couple of years to see anything. Some people who have thalassemia will show no signs at all.
Thalassemia Diagnosis
If you think you may have thalassemia, or if your parents have it, you should see a doctor. They will examine you and will ask questions. Children with moderate to severe thalassemia usually have signs by age 2.
If a doctor suspects thalassemia, you’ll take blood tests. One is a CBC (complete blood count) test. The other is a hemoglobin electrophoresis test.
If you are pregnant or trying to have a baby, you can have tests to learn if your baby will have the condition.
* Genetic testing can show if you or your partner carries any of the genes that cause thalassemia.
* Chorionic villus sampling tests a tiny piece of the placenta to see if a baby has the genes that cause thalassemia. Doctors usually do this test around the 11th week of pregnancy.
* Amniocentesis tests the fluid around an unborn baby. Doctors usually do this test around the 16th week of pregnancy.
If you do have thalassemia, you should see a blood expert known as a hematologist. You may also need other special doctors on your team, like those who treat the heart or liver.
Thalassemia Treatment and Home Care
If you have thalassemia, follow these habits to stay well:
* Eat a healthy diet to keep your bones strong and give you energy.
* If you get a fever or feel ill, see your doctor.
* Stay away from sick people and wash your hands often.
* Ask your doctor about supplements like calcium and vitamin D.
* Don’t take iron pills.
With a mild case, you may feel tired and not need treatment. But if it’s more serious, your organs may not get the oxygen they need. Treatment might include:
* Blood transfusions. A transfusion is a way to get donated blood or parts of blood that your body needs, like hemoglobin. How often you need transfusions can vary. Some people have one every few weeks. Your transfusion schedule may change as you get older.
* Chelation therapy. Blood transfusions are important for people with thalassemia. But they can cause too much iron in the blood. That can lead to problems with the heart, liver, and blood sugar. If you get transfusions, you and your doctor will talk about whether you need medicine that can help remove extra iron from your body.
* Stem cell or bone marrow transplant. An infusion of stem cells from a matched donor can sometimes cure thalassemia.
* Supplements. In some cases, your doctor might recommend that you take extra folic acid or other supplements.
* Surgery. Some people with thalassemia may need their spleen removed.
Sometimes, blood transfusions cause reactions like a high fever, nausea, diarrhea, chills, and low blood pressure. If you have any of these, see your doctor. Donated blood is very safe. But there’s a remote chance that you could get an infection from a blood transfusion.
Work closely with your doctor, and keep up with your treatments.
Thalassemia Complications
If a person’s anemia becomes severe, it can cause permanent organ damage and even death. Some people with moderate to severe thalassemia have other health problems. These may include:
* Iron overload. Too much iron can damage your heart, liver, and endocrine system.
* Bone changes. Your bones may become thin and brittle. And the bones in your face can look out of shape or distorted.
* Slowed growth. You may be shorter than others because your bones don’t grow normally. Puberty may be delayed.
* Enlarged spleen. Your spleen filters old or damaged blood cells. If you have thalassemia, your spleen might have to work too hard. Sometimes a doctor may need to remove it. If a doctor has to remove your spleen, you will be at higher risk for infection.
* Heart problems. Thalassemia increases your risk for congestive heart failure and abnormal heart rhythms.
These problems don’t happen to everyone who has thalassemia.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1257 2022-01-17 15:48:10
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,810
Re: Miscellany
1233) Cheetah
Summary
The cheetah (Acinonyx jubatus) is a large cat native to Africa and central Iran. It is the fastest land animal, estimated to be capable of running at 80 to 128 km/h (50 to 80 mph) with the fastest reliably recorded speeds being 93 and 98 km/h (58 and 61 mph), and as such has several adaptations for speed, including a light build, long thin legs and a long tail. It typically reaches 67–94 cm (26–37 in) at the shoulder, and the head-and-body length is between 1.1 and 1.5 m (3 ft 7 in and 4 ft 11 in). Adults weigh between 21 and 72 kg (46 and 159 lb). Its head is small and rounded, and has a short snout and black tear-like facial streaks. The coat is typically tawny to creamy white or pale buff and is mostly covered with evenly spaced, solid black spots. Four subspecies are recognised.
The cheetah lives in three main social groups, females and their cubs, male "coalitions" and solitary males. While females lead a nomadic life searching for prey in large home ranges, males are more sedentary and may instead establish much smaller territories in areas with plentiful prey and access to females. The cheetah is active mainly during the day, with peaks during dawn and dusk. It feeds on small- to medium-sized prey, mostly weighing under 40 kg (88 lb), and prefers medium-sized ungulates such as impala, springbok and Thomson's gazelles. The cheetah typically stalks its prey to within 60–70 m (200–230 ft), charges towards it, trips it during the chase and bites its throat to suffocate it to death. It breeds throughout the year. After a gestation of nearly three months, a litter of typically three or four cubs is born. Cheetah cubs are highly vulnerable to predation by other large carnivores such as hyenas and lions. They are weaned at around four months and are independent by around 20 months of age.
The cheetah occurs in a variety of habitats such as savannahs in the Serengeti, arid mountain ranges in the Sahara and hilly desert terrain in Iran. The cheetah is threatened by several factors such as habitat loss, conflict with humans, poaching and high susceptibility to diseases. Historically ranging throughout most of Sub-Saharan Africa and extending eastward into the Middle East and to central India, the cheetah is now distributed mainly in small, fragmented populations in central Iran and southern, eastern and northwestern Africa. In 2016, the global cheetah population was estimated at around 7,100 individuals in the wild; it is listed as Vulnerable on the IUCN Red List. In the past, cheetahs were tamed and trained for hunting ungulates. They have been widely depicted in art, literature, advertising, and animation.
Details
Cheetah, (Acinonyx jubatus), is one of the world’s most-recognizable cats, known especially for its speed. Cheetahs’ sprints have been measured at a maximum of 114 km (71 miles) per hour, and they routinely reach velocities of 80–100 km per hour while pursuing prey. Nearly all the cheetahs remaining in the wild live in Africa.
Cheetahs are covered almost entirely with small black spots on a background of pale yellow and have a white underbelly. Their faces are distinguished by prominent black lines that curve from the inner corner of each eye to the outer corners of the mouth, like a well-worn trail of inky tears. Cheetahs have a long, slender body measuring 1.2 metres (4 feet), with a long tail (65–85 cm [2–3 feet]) that generally ends in a white tuft. They are about 75 cm tall at the shoulder. Weight ranges from 34 to 54 kg (75 to 119 pounds), males being slightly larger than females.
Natural history
Cheetahs have evolved many adaptations that enhance their ability to sprint. Their legs are proportionally longer than those of other big cats; an elongated spine increases stride length at high speeds; they have unretractable claws, special paw pads for extra traction, and a long tail for balance. Internally, the liver, adrenal glands, lungs, bronchi, nasal passages, and heart are all large to allow intense physiological activity. During a chase, cheetahs take about 3 1/2 strides per second and 60 to 150 breaths per minute. Chases are usually limited to sprints of less than 200–300 metres, however, because the increased physiological activity associated with running creates heat faster than it can be released through evaporative cooling (sweating through their paws and panting).
Unlike most carnivores, cheetahs are active mainly during the day, hunting in the early morning and late afternoon. A cheetah eats a variety of small animals, including game birds, rabbits, small antelopes (including the springbok, impala, and gazelle), young warthogs, and larger antelopes (such as the kudu, hartebeest, oryx, and roan). Prey is generally consumed quickly to avoid losing it to competitors such as lions, leopards, jackals, and hyenas.
Cheetahs inhabit a wide variety of habitats, including the dry, open country and grasslands where they are most often seen, as well as areas of denser vegetation and rocky upland terrain. Groups consist of a mother and her young or of coalitions made up of two or three males that are often brothers. Adult males and females rarely meet except to mate. Male coalitions live and hunt together for life and occupy an area that may overlap the range of several adult females. Female home ranges are generally much larger than those of male coalitions.
Following a gestation period of three months, the female gives birth to two to eight cubs, usually in an isolated spot hidden in the cover of tall grass or thicker vegetation. At birth, cubs weigh about 250 to 300 grams (slightly more than half a pound). Their fur is dark and includes a thick yellowish gray mane along the back, a trait that presumably offers better camouflage and increased protection from high temperatures during the day and low temperatures at night during the first few months of life. Mortality among young cubs can be as high as 90 percent in the wild, often because of other predators. The mother leaves her offspring when they are 16–24 months old. Young males are chased away by the resident male coalition, traveling several hundred kilometres before establishing residence and becoming sexually active at 2 1/2 to 3 years of age. Female offspring will generally inhabit the same vicinity as their mother. Life expectancy of cheetahs is about 7 years in the wild and generally from 8 to 12 years in captivity.
Status and taxonomy
The cheetah has lived in association with humans since at least 3000 BCE, when the Sumerians depicted a leashed cheetah with a hood on its head on an official seal. During this period in Egypt, the cheetah was revered as a symbol of royalty in the form of the cat goddess Mafdet. Cheetahs were kept as pets by many famous historical figures, such as Genghis Khan, Charlemagne, and Akbar the Great of India (who had more than 9,000 in his stable). These cats were also used for sport. Trained and tame, they were typically hooded and carried on horseback or in a cart, then dehooded and released near their quarry. In spite of the large numbers of cheetahs kept in captivity by royalty during the 14th–16th centuries, almost all were captured from the wild because there was essentially no captive breeding. Because of this continuous drain on wild Asiatic populations, cheetahs from Africa were being imported into India and Iran during the early 1900s.
In 1900 an estimated 100,000 cheetahs were found in habitats throughout continental Africa and from the Middle East and the Arabian Peninsula to India. Today cheetahs have been extirpated from a large portion of this area. In Asia they are nearly extinct, with the largest confirmed population (a few dozen) inhabiting northeastern Iran. In Africa there are an estimated 9,000 to 12,000 cheetahs, with the largest populations existing in Namibia, Botswana, and Zimbabwe in Southern Africa and Kenya and Tanzania in East Africa. Smaller, more isolated populations exist in other countries, including South Africa, Congo (Kinshasa), Zambia, Somalia, Ethiopia, Mali, Niger, Cameroon, Chad, and the Central African Republic. All populations are threatened, even within protected areas, because of increased competition from large predators such as lions and hyenas. Outside of reserves, humans pose a threat in several forms, including habitat loss, poaching, and indiscriminate trapping and shooting to protect livestock.
The cheetah was common throughout North America, Europe, and Asia until the end of the last ice age, about 11,700 years ago—a time coincident to when large numbers of mammals disappeared throughout the Northern Hemisphere. All North American and European cheetahs and most of those in Asia vanished. About this time the cheetah populations seem to have experienced what may have been the first and most severe of a series of size reductions (demographic bottlenecks). Modern cheetahs retain evidence of this historic event in their DNA. There is a very high level of genetic similarity in all but the most rapidly evolving parts of the cheetah’s genome, which makes all of today’s individuals appear highly inbred. This condition has been linked with increased susceptibility to infectious diseases (such as feline infectious peritonitis, or FIP), increased infant mortality, and high levels of abnormal sperm. No evidence, however, links low levels of genetic variation with reduced fitness in wild populations.
Early taxonomists interpreted the numerous specialized traits of cheetahs as evidence that they diverged from the other cat species early in the evolutionary history of the cat family (Felidae). The cheetah was therefore granted unique taxonomic status, and since the early 1900s it has been classified as the only species of genus Acinonyx. Cheetahs are often divided into five subspecies: A. jubatus jubatus in Southern Africa, A. jubatus fearsoni (including A. jubatus velox and A. jubatus raineyi) from eastern Africa, A. jubatus soemmeringii from Nigeria to Somalia, A. jubatus hecki from northwestern Africa, and A. jubatus venaticus from Arabia to central India. The king cheetah, once thought to be a distinct subspecies, is a Southern African form that has a “blotchy” coat pattern presumably from a rare recessive genetic mutation.
Numerous molecular genetic studies suggest that the cheetah shares a common ancestor with the puma and jaguarundi, from which it diverged six to eight million years ago, probably in North America. Fossils attributable to cheetahlike species dating from two to three million years ago have been found in North America in what is now Texas, Nevada, and Wyoming.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1258 2022-01-18 15:57:20
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,810
Re: Miscellany
1234) Lantern
Summary
Lantern is a case, ordinarily metal, with transparent or translucent sides, used to contain and protect a lamp.
Lamp-containing lanterns have been found at Pompeii, Herculaneum, and other classical sites. They have been made of iron, silver, gold, and tin and their sides of horn, talc, leather, oiled paper, and glass. Designs have ranged from crude boxes pierced with nail holes to Oriental openwork bronze and exquisitely delicate examples of Renaissance and Baroque craftsmanship.
The bull’s-eye lantern, with one or more sides of bulging glass, was in popular use from the early 18th century, similar devices having been made at least as early as the 13th century. Dark until it was suddenly switched on by opening its door, it focused its light to some extent and served the purpose of the modern flashlight.
The hurricane lantern, or hurricane lamp, still in use as a warning flare, has a shield of glass and perforated metal surrounding its flame to protect it from strong winds.
Details
A lantern is an often portable source of lighting, typically featuring a protective enclosure for the light source - historically usually a candle or a wick in oil, and often a battery-powered light in modern times – to make it easier to carry and hang up, and make it more reliable outdoors or in drafty interiors. Lanterns may also be used for signaling, as torches, or as general light-sources outdoors.
Use
The lantern enclosure was primarily used to prevent a burning candle or wick being extinguished from wind, rain or other causes. Some antique lanterns have only a metal grid, indicating their function was to protect the candle or wick during transportation and avoid the excess heat from the top to avoid unexpected fires.
Another important function was to reduce the risk of fire should a spark leap from the flame or the light be dropped. This was especially important below deck on ships: a fire on a wooden ship was a major catastrophe. Use of unguarded lights was taken so seriously that obligatory use of lanterns, rather than unprotected flames, below decks was written into one of the few known remaining examples of a pirate code, on pain of severe punishment.
Lanterns may also be used for signaling. In naval operations, ships used lights to communicate at least as far back as the Middle Ages; the use of a lantern that blinks code to transmit a message dates to the mid-1800s. In railroad operations, lanterns have multiple uses. Permanent lanterns on poles are used to signal trains about the operational status of the track ahead, sometimes with color gels in front of the light to signify stop, etc. Historically, a flagman at a level crossing used a lantern to stop cars and other vehicular traffic before a train arrived. Lanterns also provided a means to signal from train-to-train or from station-to-train.
A "dark lantern" was a candle lantern with a sliding shutter so that a space could be conveniently made dark without extinguishing the candle. For example, in the Sherlock Holmes story "The Red-Headed League", the detective and police make their way down to a bank vault by lantern light but then put a 'screen over that dark lantern' in order to wait in the dark for thieves to finish tunneling. This type of lantern could also preserve the light source for sudden use when needed.
Lanterns may be used in religious observances. In the Eastern Orthodox Church, lanterns are used in religious processions and liturgical entrances, usually coming before the processional cross. Lanterns are also used to transport the Holy Fire from the Church of the Holy Sepulchre on Great Saturday during Holy Week.
Lanterns are used in many Asian festivals. During the Ghost Festival, lotus shaped lanterns are set afloat in rivers and seas to symbolically guide the lost souls of forgotten ancestors to the afterlife. During the Lantern Festival, the displaying of many lanterns is still a common sight on the 15th day of the first lunar month throughout China. During other Chinese festivities, kongming lanterns (sky lanterns) can be seen floating high into the air. Lanterns are the central theme of the Seoul Lantern Festival in South Korea. However, some jurisdictions and organizations ban the use of sky lanterns because of concerns about fire and safety.
The term "lantern" can be used more generically to mean a light source, or the enclosure for a light source, even if it is not portable. Decorative lanterns exist in a wide range of designs. Some hang from buildings, such as street lights enclosed in glass panes. Others are placed on or just above the ground; low-light varieties can function as decoration or landscape lighting, and can be a variety of colours and sizes. The housing for the top lamp and lens section of a lighthouse may be called a lantern.
Etymology
The word lantern comes via French from Latin lanterna meaning "lamp, torch," possibly itself derived from Greek.
An alternate historical spelling was "lanthorn", believed to derive from the early use of horn windows.
Construction
Lanterns were usually made from a metal frame with several sides (usually four, but up to eight) or round, commonly with a hook or a hoop of metal on top. Windows of some translucent material may be fitted in the sides; these are now usually glass or plastic but formerly were thin sheets of animal horn, or tinplate punched with holes or decorative patterns.
Paper lanterns are made in societies around the world.
A lantern generally contains a burning light source: a candle, liquid oil with a wick, or gas with a mantle.
Modern varieties often place an electric light in a decorative glass case.
History
Lanterns have been used functionally, for light rather than decoration, since antiquity. Some used a wick in oil, while others were essentially protected candle-holders. Before the development of glass sheets, animal horn scraped thin and flattened was used as the translucent window.
Beginning in the Middle Ages, European towns hired watchmen to patrol the streets at night, as a crime deterrent. Each watchman carried a lantern or oil lamp against the darkness. The practice continued up through at least the 18th century.
Public spaces became increasingly lit with lanterns in the 1500s, especially following the invention of lanterns with glass windows, which greatly improved the quantity of light. In 1588 the Parisian Parlement decreed that a torch be installed and lit at each intersection, and in 1594 the police changed this to lanterns. Beginning in 1667 during the reign of King Louis XIV, thousands of street lights were installed in Parisian streets and intersections. Under this system, streets were lit with lanterns suspended 20 yards (18 m) apart on a cord over the middle of the street at a height of 20 feet (6.1 m); as an English visitor enthused in 1698, 'The streets are lit all winter and even during the full moon!' In London, public street lighting was implemented around the end of the 17th century; a diarist wrote in 1712 that ‘All the way, quite through Hyde Park to the Queen's Palace at Kensington, lanterns were placed for illuminating the roads on dark nights.’
Modern lanterns:
Fueled lanterns
All fueled lanterns are somewhat hazardous owing to the danger of handling flammable and toxic fuel, danger of fire or burns from the high temperatures involved, and potential dangers from carbon monoxide poisoning if used in an enclosed environment.
Simple wick lanterns remain available. They are cheap and durable and usually can provide enough light for reading. They require periodic trimming of the wick and regular cleaning of soot from the inside of the glass chimney.
Mantle lanterns use a woven ceramic impregnated gas mantle to accept and re-radiate heat as visible light from a flame. The mantle does not burn (but the cloth matrix carrying the ceramic must be "burned out" with a match prior to its first use). When heated by the operating flame the mantle becomes incandescent and glows brightly. The heat may be provided by a gas, by kerosene, or by a pressurized liquid such as "white gas", which is essentially naphtha. For protection from the high temperatures produced and to stabilize the airflow, a cylindrical glass shield called the globe or chimney is placed around the mantle.
Manually pressurized lanterns using white gas (also marketed as Coleman fuel or "Camp Fuel") are manufactured by the Coleman Company in one and two-mantle models. Some models are dual fuel and can also use gasoline. These are being supplanted by a battery-powered fluorescent lamp and LED models, which are safer in the hands of young people and inside tents. Liquid fuel lanterns remain popular where the fuel is easily obtained and in common use.
Many portable mantle-type fuel lanterns now use fuel gases that become liquid when compressed, such as propane, either alone or combined with butane. Such lamps usually use a small disposable steel container to provide the fuel. The ability to refuel without liquid fuel handling increases safety. Additional fuel supplies for such lamps have an indefinite shelf life if the containers are protected from moisture (which can cause corrosion of the container) and excess heat.
Electric lanterns
Lanterns designed as permanently mounted electric lighting fixtures are used in interior, landscape, and civic lighting applications. Styles can evoke former eras, unify street furniture themes, or enhance aesthetic considerations. They are manufactured for use with various wired voltage supplies.
Various battery types are used in portable light sources. They are more convenient, safer, and produce less heat than combustion lights. Solar-powered lanterns have become popular in developing countries, where they provide a safer and cheaper alternative to kerosene lamps.
Lanterns utilizing LEDs are popular as they are more energy-efficient and rugged than other types, and prices of LEDs suitable for lighting have dropped.
Some rechargeable fluorescent lanterns may be plugged in at all times and may be set up to illuminate upon a power failure, a useful feature in some applications. During extensive power failures (or for remote use), supplemental recharging may be provided from an automobile's 12-volt electrical system or from a modest solar-powered charger.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1259 2022-01-19 15:25:39
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,810
Re: Miscellany
1235) Spinal cord
Summary
Spinal cord is major nerve tract of vertebrates, extending from the base of the brain through the canal of the spinal column. It is composed of nerve fibres that mediate reflex actions and that transmit impulses to and from the brain.
Like the brain, the spinal cord is covered by three connective-tissue envelopes called the meninges. The space between the outer and middle envelopes is filled with cerebrospinal fluid, a clear, colourless fluid that cushions the spinal cord.
A cross section of the spinal cord reveals white matter arranged around a butterfly-shaped area of gray matter. The white matter consists of myelinated fibres, or axons, that form nerve tracts ascending to and descending from the brain. The white matter is grouped into discrete sectors called funiculi. The gray matter contains cell bodies, unmyelinated motor-neuron fibres, and interneurons connecting the two sides of the cord. Gray-matter cells form projections called horns. Fibres exiting the spinal cord from the dorsal and ventral horns join in paired tracts to form the spinal nerves. Information travels up the ascending tracts of neurons and is sorted by the brain. Responses are induced by nerve impulses traveling down the descending tracts that stimulate motor neurons or that initiate glandular secretion.
Details
The spinal cord is a long, thin, tubular structure made up of nervous tissue, which extends from the medulla oblongata in the brainstem to the lumbar region of the vertebral column. It encloses the central canal of the spinal cord, which contains cerebrospinal fluid. The brain and spinal cord together make up the central nervous system (CNS). In humans, the spinal cord begins at the occipital bone, passing through the foramen magnum and entering the spinal canal at the beginning of the cervical vertebrae. The spinal cord extends down to between the first and second lumbar vertebrae, where it ends. The enclosing bony vertebral column protects the relatively shorter spinal cord. It is around 45 cm (18 in) long in adult men and around 43 cm (17 in) long in adult women. The diameter of the spinal cord ranges from 13 mm (1⁄2 in) in the cervical and lumbar regions to 6.4 mm (1⁄4 in) in the thoracic area.
The spinal cord functions primarily in the transmission of nerve signals from the motor cortex to the body, and from the afferent fibers of the sensory neurons to the sensory cortex. It is also a center for coordinating many reflexes and contains reflex arcs that can independently control reflexes. It is also the location of groups of spinal interneurons that make up the neural circuits known as central pattern generators. These circuits are responsible for controlling motor instructions for rhythmic movements such as walking.
Structure
The spinal cord is the main pathway for information connecting the brain and peripheral nervous system. Much shorter than its protecting spinal column, the human spinal cord originates in the brainstem, passes through the foramen magnum, and continues through to the conus medullaris near the second lumbar vertebra before terminating in a fibrous extension known as the filum terminale.
It is about 45 cm (18 in) long in men and about 43 cm (17 in) in women, ovoid-shaped, and is enlarged in the cervical and lumbar regions. The cervical enlargement, stretching from the C5 to T1 vertebrae, is where sensory input comes from and motor output goes to the arms and trunk. The lumbar enlargement, located between L1 and S3, handles sensory input and motor output coming from and going to the legs.
The spinal cord is continuous with the caudal portion of the medulla, running from the base of the skull to the body of the first lumbar vertebra. It does not run the full length of the vertebral column in adults. It is made of 31 segments from which branch one pair of sensory nerve roots and one pair of motor nerve roots. The nerve roots then merge into bilaterally symmetrical pairs of spinal nerves. The peripheral nervous system is made up of these spinal roots, nerves, and ganglia.
The dorsal roots are afferent fascicles, receiving sensory information from the skin, muscles, and visceral organs to be relayed to the brain. The roots terminate in dorsal root ganglia, which are composed of the cell bodies of the corresponding neurons. Ventral roots consist of efferent fibers that arise from motor neurons whose cell bodies are found in the ventral (or anterior) gray horns of the spinal cord.
The spinal cord (and brain) are protected by three layers of tissue or membranes called meninges, that surround the canal. The dura mater is the outermost layer, and it forms a tough protective coating. Between the dura mater and the surrounding bone of the vertebrae is a space called the epidural space. The epidural space is filled with adipose tissue, and it contains a network of blood vessels. The arachnoid mater, the middle protective layer, is named for its open, spiderweb-like appearance. The space between the arachnoid and the underlying pia mater is called the subarachnoid space. The subarachnoid space contains cerebrospinal fluid (CSF), which can be sampled with a lumbar puncture, or "spinal tap" procedure. The delicate pia mater, the innermost protective layer, is tightly associated with the surface of the spinal cord. The cord is stabilized within the dura mater by the connecting denticulate ligaments, which extend from the enveloping pia mater laterally between the dorsal and ventral roots. The dural sac ends at the vertebral level of the second sacral vertebra.
In cross-section, the peripheral region of the cord contains neuronal white matter tracts containing sensory and motor axons. Internal to this peripheral region is the grey matter, which contains the nerve cell bodies arranged in the three grey columns that give the region its butterfly-shape. This central region surrounds the central canal, which is an extension of the fourth ventricle and contains cerebrospinal fluid.
The spinal cord is elliptical in cross section, being compressed dorsolaterally. Two prominent grooves, or sulci, run along its length. The posterior median sulcus is the groove in the dorsal side, and the anterior median fissure is the groove in the ventral side.
Segments
The human spinal cord is divided into segments where pairs of spinal nerves (mixed; sensory and motor) form. Six to eight motor nerve rootlets branch out of right and left ventralateral sulci in a very orderly manner. Nerve rootlets combine to form nerve roots. Likewise, sensory nerve rootlets form off right and left dorsal lateral sulci and form sensory nerve roots. The ventral (motor) and dorsal (sensory) roots combine to form spinal nerves (mixed; motor and sensory), one on each side of the spinal cord. Spinal nerves, with the exception of C1 and C2, form inside the intervertebral foramen (IVF). These rootlets form the demarcation between the central and peripheral nervous systems.
The grey column, (as three regions of grey columns) in the center of the cord, is shaped like a butterfly and consists of cell bodies of interneurons, motor neurons, neuroglia cells and unmyelinated axons. The anterior and posterior grey column present as projections of the grey matter and are also known as the horns of the spinal cord. Together, the grey columns and the gray commissure form the "grey H."
The white matter is located outside of the grey matter and consists almost totally of myelinated motor and sensory axons. "Columns" of white matter carry information either up or down the spinal cord.
The spinal cord proper terminates in a region called the conus medullaris, while the pia mater continues as an extension called the filum terminale, which anchors the spinal cord to the coccyx. The cauda equina ("horse's tail") is a collection of nerves inferior to the conus medullaris that continue to travel through the vertebral column to the coccyx. The cauda equina forms because the spinal cord stops growing in length at about age four, even though the vertebral column continues to lengthen until adulthood. This results in sacral spinal nerves originating in the upper lumbar region. For that reason, the spinal cord occupies only two-thirds of the vertebral canal. The inferior part of the vertebral canal is filled with cerebrospinal fluid (CSF) and the space is called the lumbar cistern.
Within the Central Nervous System (CNS), nerve cell bodies are generally organized into functional clusters, called nuclei. Axons within the CNS are grouped into tracts.
There are 31 spinal cord nerve segments in a human spinal cord:
* 8 cervical segments forming 8 pairs of cervical nerves (C1 spinal nerves exit the spinal column between the foramen magnum and the C1 vertebra; C2 nerves exit between the posterior arch of the C1 vertebra and the lamina of C2; C3–C8 spinal nerves pass through the IVF above their corresponding cervical vertebrae, with the exception of the C8 pair which exit between the C7 and T1 vertebrae)
* 12 thoracic segments forming 12 pairs of thoracic nerves
* 5 lumbar segments forming 5 pairs of lumbar nerves
* 5 sacral segments forming 5 pairs of sacral nerves
* 1 coccygeal segment
In the fetus, vertebral segments correspond with spinal cord segments. However, because the vertebral column grows longer than the spinal cord, spinal cord segments do not correspond to vertebral segments in the adult, particularly in the lower spinal cord. For example, lumbar and sacral spinal cord segments are found between vertebral levels T9 and L2, and the spinal cord ends around the L1/L2 vertebral level, forming a structure known as the conus medullaris.
Although the spinal cord cell bodies end around the L1/L2 vertebral level, the spinal nerves for each segment exit at the level of the corresponding vertebra. For the nerves of the lower spinal cord, this means that they exit the vertebral column much lower (more caudally) than their roots. As these nerves travel from their respective roots to their point of exit from the vertebral column, the nerves of the lower spinal segments form a bundle called the cauda equina.
There are two regions where the spinal cord enlarges:
* Cervical enlargement – corresponds roughly to the brachial plexus nerves, which innervate the upper limb. It includes spinal cord segments from about C4 to T1. The vertebral levels of the enlargement are roughly the same (C4 to T1).
* Lumbar enlargement – corresponds to the lumbosacral plexus nerves, which innervate the lower limb. It comprises the spinal cord segments from L2 to S3 and is found about the vertebral levels of T9 to T12.
Development
The spinal cord is made from part of the neural tube during development. There are four stages of the spinal cord that arises from the neural tube: The neural plate, neural fold, neural tube, and the spinal cord. Neural differentiation occurs within the spinal cord portion of the tube.[8] As the neural tube begins to develop, the notochord begins to secrete a factor known as Sonic hedgehog or SHH. As a result, the floor plate then also begins to secrete SHH, and this will induce the basal plate to develop motor neurons. During the maturation of the neural tube, its lateral walls thicken and form a longitudinal groove called the sulcus limitans. This extends the length of the spinal cord into dorsal and ventral portions as well.[9] Meanwhile, the overlying ectoderm secretes bone morphogenetic protein (BMP). This induces the roof plate to begin to secrete BMP, which will induce the alar plate to develop sensory neurons. Opposing gradients of such morphogens as BMP and SHH form different domains of dividing cells along the dorsal ventral axis.[10] Dorsal root ganglion neurons differentiate from neural crest progenitors. As the dorsal and ventral column cells proliferate, the lumen of the neural tube narrows to form the small central canal of the spinal cord.[11] The alar plate and the basal plate are separated by the sulcus limitans. Additionally, the floor plate also secretes netrins. The netrins act as chemoattractants to decussation of pain and temperature sensory neurons in the alar plate across the anterior white commissure, where they then ascend towards the thalamus. Following the closure of the caudal neuropore and formation of the brain's ventricles that contain the choroid plexus tissue, the central canal of the caudal spinal cord is filled with cerebrospinal fluid.
Earlier findings by Viktor Hamburger and Rita Levi-Montalcini in the chick embryo have been confirmed by more recent studies which have demonstrated that the elimination of neuronal cells by programmed cell death (PCD) is necessary for the correct assembly of the nervous system.
Overall, spontaneous embryonic activity has been shown to play a role in neuron and muscle development but is probably not involved in the initial formation of connections between spinal neurons.
Blood supply
The spinal cord is supplied with blood by three arteries that run along its length starting in the brain, and many arteries that approach it through the sides of the spinal column. The three longitudinal arteries are the anterior spinal artery, and the right and left posterior spinal arteries. These travel in the subarachnoid space and send branches into the spinal cord. They form anastamoses (connections) via the anterior and posterior segmental medullary arteries, which enter the spinal cord at various points along its length. The actual blood flow caudally through these arteries, derived from the posterior cerebral circulation, is inadequate to maintain the spinal cord beyond the cervical segments.
The major contribution to the arterial blood supply of the spinal cord below the cervical region comes from the radially arranged posterior and anterior radicular arteries, which run into the spinal cord alongside the dorsal and ventral nerve roots, but with one exception do not connect directly with any of the three longitudinal arteries. These intercostal and lumbar radicular arteries arise from the aorta, provide major anastomoses and supplement the blood flow to the spinal cord. In humans the largest of the anterior radicular arteries is known as the artery of Adamkiewicz, or anterior radicularis magna (ARM) artery, which usually arises between L1 and L2, but can arise anywhere from T9 to L5. Impaired blood flow through these critical radicular arteries, especially during surgical procedures that involve abrupt disruption of blood flow through the aorta for example during aortic aneurysm repair, can result in spinal cord infarction and paraplegia.
Function:
Somatosensory organization
In the dorsal column-medial lemniscus tract, a primary neuron's axon enters the spinal cord and then enters the dorsal column. Here the dorsal column connects to the axon of the nerve cell. If the primary axon enters below spinal level T6, the axon travels in the gracile fasciculus, the medial part of the column. If the axon enters above level T6, then it travels in the cuneate fasciculus, which is lateral to the fasciculus gracilis. Either way, the primary axon ascends to the lower medulla, where it leaves its fasciculus and synapses with a secondary neuron in one of the dorsal column nuclei: either the nucleus gracilis or the nucleus cuneatus, depending on the pathway it took. At this point, the secondary axon leaves its nucleus and passes anteriorly and medially. The collection of secondary axons that do this are known as internal arcuate fibers. The internal arcuate fibers decussate and continue ascending as the contralateral medial lemniscus. Secondary axons from the medial lemniscus finally terminate in the ventral posterolateral nucleus (VPLN) of the thalamus, where they synapse with tertiary neurons. From there, tertiary neurons ascend via the posterior limb of the internal capsule and end in the primary sensory cortex.
The proprioception of the lower limbs differs from the upper limbs and upper trunk. There is a four-neuron pathway for lower limb proprioception. This pathway initially follows the dorsal spino-cerebellar pathway. It is arranged as follows: proprioceptive receptors of lower limb → peripheral process → dorsal root ganglion → central process → Clarke's column → 2nd order neuron → medulla oblongata (Caudate nucleus) → 3rd order neuron → VPLN of thalamus → 4th order neuron → posterior limb of internal capsule → corona radiata → sensory area of cerebrum.
The anterolateral system works somewhat differently. Its primary neurons axons enter the spinal cord and then ascend one to two levels before synapsing in the substantia gelatinosa. The tract that ascends before synapsing is known as Lissauer's tract. After synapsing, secondary axons decussate and ascend in the anterior lateral portion of the spinal cord as the spinothalamic tract. This tract ascends all the way to the VPLN, where it synapses on tertiary neurons. Tertiary neuronal axons then travel to the primary sensory cortex via the posterior limb of the internal capsule.
Some of the "pain fibers" in the ALS deviate from their pathway towards the VPLN. In one such deviation, axons travel towards the reticular formation in the midbrain. The reticular formation then projects to a number of places including the hippocampus (to create memories about the pain), the centromedian nucleus (to cause diffuse, non-specific pain) and various parts of the cortex. Additionally, some ALS axons project to the periaqueductal gray in the pons, and the axons forming the periaqueductal gray then project to the nucleus raphes magnus, which projects back down to where the pain signal is coming from and inhibits it. This helps control the sensation of pain to some degree.
Motor organization
The corticospinal tract serves as the motor pathway for upper motor neuronal signals coming from the cerebral cortex and from primitive brainstem motor nuclei.
Cortical upper motor neurons originate from Brodmann areas 1, 2, 3, 4, and 6 and then descend in the posterior limb of the internal capsule, through the crus cerebri, down through the pons, and to the medullary pyramids, where about 90% of the axons cross to the contralateral side at the decussation of the pyramids. They then descend as the lateral corticospinal tract. These axons synapse with lower motor neurons in the ventral horns of all levels of the spinal cord. The remaining 10% of axons descend on the ipsilateral side as the ventral corticospinal tract. These axons also synapse with lower motor neurons in the ventral horns. Most of them will cross to the contralateral side of the cord (via the anterior white commissure) right before synapsing.
The midbrain nuclei include four motor tracts that send upper motor neuronal axons down the spinal cord to lower motor neurons. These are the rubrospinal tract, the vestibulospinal tract, the tectospinal tract and the reticulospinal tract. The rubrospinal tract descends with the lateral corticospinal tract, and the remaining three descend with the anterior corticospinal tract.
The function of lower motor neurons can be divided into two different groups: the lateral corticospinal tract and the anterior cortical spinal tract. The lateral tract contains upper motor neuronal axons which synapse on dorsal lateral (DL) lower motor neurons. The DL neurons are involved in distal limb control. Therefore, these DL neurons are found specifically only in the cervical and lumbosacral enlargements within the spinal cord. There is no decussation in the lateral corticospinal tract after the decussation at the medullary pyramids.
The anterior corticospinal tract descends ipsilaterally in the anterior column, where the axons emerge and either synapse on lower ventromedial (VM) motor neurons in the ventral horn ipsilaterally or descussate at the anterior white commissure where they synapse on VM lower motor neurons contralaterally . The tectospinal, vestibulospinal and reticulospinal descend ipsilaterally in the anterior column but do not synapse across the anterior white commissure. Rather, they only synapse on VM lower motor neurons ipsilaterally. The VM lower motor neurons control the large, postural muscles of the axial skeleton. These lower motor neurons, unlike those of the DL, are located in the ventral horn all the way throughout the spinal cord.
Spinocerebellar tracts
Proprioceptive information in the body travels up the spinal cord via three tracks. Below L2, the proprioceptive information travels up the spinal cord in the ventral spinocerebellar tract. Also known as the anterior spinocerebellar tract, sensory receptors take in the information and travel into the spinal cord. The cell bodies of these primary neurons are located in the dorsal root ganglia. In the spinal cord, the axons synapse and the secondary neuronal axons decussates and then travel up to the superior cerebellar peduncle where they decussate again. From here, the information is brought to deep nuclei of the cerebellum including the fastigial and interposed nuclei.
From the levels of L2 to T1, proprioceptive information enters the spinal cord and ascends ipsilaterally, where it synapses in Clarke's nucleus. The secondary neuronal axons continue to ascend ipsilaterally and then pass into the cerebellum via the inferior cerebellar peduncle. This tract is known as the dorsal spinocerebellar tract.
From above T1, proprioceptive primary axons enter the spinal cord and ascend ipsilaterally until reaching the accessory cuneate nucleus, where they synapse. The secondary axons pass into the cerebellum via the inferior cerebellar peduncle where again, these axons synapse on cerebellar deep nuclei. This tract is known as the cuneocerebellar tract.
Motor information travels from the brain down the spinal cord via descending spinal cord tracts. Descending tracts involve two neurons: the upper motor neuron (UMN) and lower motor neuron (LMN). A nerve signal travels down the upper motor neuron until it synapses with the lower motor neuron in the spinal cord. Then, the lower motor neuron conducts the nerve signal to the spinal root where efferent nerve fibers carry the motor signal toward the target muscle. The descending tracts are composed of white matter. There are several descending tracts serving different functions. The corticospinal tracts (lateral and anterior) are responsible for coordinated limb movements.
Clinical significance
A congenital disorder is diastematomyelia in which part of the spinal cord is split usually at the level of the upper lumbar vertebrae. Sometimes the split can be along the length of the spinal cord.
Injury
Spinal cord injuries can be caused by trauma to the spinal column (stretching, bruising, applying pressure, severing, laceration, etc.). The vertebral bones or intervertebral disks can shatter, causing the spinal cord to be punctured by a sharp fragment of bone. Usually, victims of spinal cord injuries will suffer loss of feeling in certain parts of their body. In milder cases, a victim might only suffer loss of hand or foot function. More severe injuries may result in paraplegia, tetraplegia (also known as quadriplegia), or full body paralysis below the site of injury to the spinal cord.
Damage to upper motor neuron axons in the spinal cord results in a characteristic pattern of ipsilateral deficits. These include hyperreflexia, hypertonia and muscle weakness. Lower motor neuronal damage results in its own characteristic pattern of deficits. Rather than an entire side of deficits, there is a pattern relating to the myotome affected by the damage. Additionally, lower motor neurons are characterized by muscle weakness, hypotonia, hyporeflexia and muscle atrophy.
Spinal shock and neurogenic shock can occur from a spinal injury. Spinal shock is usually temporary, lasting only for 24–48 hours, and is a temporary absence of sensory and motor functions. Neurogenic shock lasts for weeks and can lead to a loss of muscle tone due to disuse of the muscles below the injured site.
The two areas of the spinal cord most commonly injured are the cervical spine (C1–C7) and the lumbar spine (L1–L5). (The notation C1, C7, L1, L5 refer to the location of a specific vertebra in either the cervical, thoracic, or lumbar region of the spine.) Spinal cord injury can also be non-traumatic and caused by disease (transverse myelitis, polio, spina bifida, Friedreich's ataxia, spinal cord tumor, spinal stenosis etc.)
In the U.S., 10,000–12,000 people become paralyzed annually as a result of various injuries to the spinal cord.
Treatment
Real or suspected spinal cord injuries need immediate immobilisation including that of the head. Scans will be needed to assess the injury. A steroid, methylprednisolone, can be of help as can physical therapy and possibly antioxidants. Treatments need to focus on limiting post-injury cell death, promoting cell regeneration, and replacing lost cells. Regeneration is facilitated by maintaining electric transmission in neural elements.
Lumbar puncture
The spinal cord ends at the level of vertebrae L1–L2, while the subarachnoid space —the compartment that contains cerebrospinal fluid— extends down to the lower border of S2. Lumbar punctures in adults are usually performed between L3–L5 (cauda equina level) in order to avoid damage to the spinal cord. In the fetus, the spinal cord extends the full length of the spine and regresses as the body grows.
Tumours
Spinal tumours can occur in the spinal cord and these can be either inside (intradural) or outside (extradural) the dura mater.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1260 2022-01-20 15:00:30
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,810
Re: Miscellany
1236) Peacock
Summary
Peafowl is a common name for three bird species in the genera Pavo and Afropavo within the tribe Pavonini of the family Phasianidae, the pheasants and their allies. Male peafowl are referred to as peacocks, and female peafowl are referred to as peahens, even though peafowl of either males or females are often referred to colloquially as "peacocks".
The two Asiatic species are the blue or Indian peafowl originally of the Indian subcontinent, and the green peafowl of Southeast Asia; the one African species is the Congo peafowl, native only to the Congo Basin. Male peafowl are known for their piercing calls and their extravagant plumage. The latter is especially prominent in the Asiatic species, which have an eye-spotted "tail" or "train" of covert feathers, which they display as part of a courtship ritual.
The functions of the elaborate iridescent colouration and large "train" of peacocks have been the subject of extensive scientific debate. Charles Darwin suggested that they served to attract females, and the showy features of the males had evolved by sexual selection. More recently, Amotz Zahavi proposed in his handicap theory that these features acted as honest signals of the males' fitness, since less-fit males would be disadvantaged by the difficulty of surviving with such large and conspicuous structures.
Details
Peacock, also called peafowl, are any of three species of resplendent birds of the pheasant family, Phasianidae (order Galliformes). Strictly, the male is a peacock, and the female is a peahen; both are peafowl. The two most-recognizable species of peafowl are the blue, or Indian, peacock (Pavo cristatus), of India and Sri Lanka, and the green, or Javanese, peacock (P. muticus), found from Myanmar (Burma) to Java. The Congo peacock (Afropavo congensis), which inhabits the forested interior of the Democratic Republic of the Congo, was discovered in 1936 after a search that began in 1913 with the finding of a single feather.
Natural history
In blue and green peacocks, the male has a 90–130-cm (35–50-inch) body and 150-cm (60-inch) train of tail feathers that are coloured a brilliant metallic green. This train is mainly formed of the bird’s upper tail coverts, which are enormously elongated. Each feather is tipped with an iridescent eyespot that is ringed with blue and bronze. In courtship displays, the math elevates his tail, which lies under the train, thus elevating the train and bringing it forward. At the climax of this display, the tail feathers are vibrated, giving the feathers of the train a shimmering appearance and making a rustling sound.
The blue peacock’s body feathers are mostly metallic blue-green. The green peacock, with a train much like that of the blue, has green and bronze body feathers. Hens of both species are green and brown and are almost as big as the male but lack the train and the head ornament. In the wild, both species live in open lowland forests, flocking by day and roosting high in trees at night. During the breeding season, the male forms a harem of two to five hens, each of which lays four to eight whitish eggs in a depression in the ground. The eggs are incubated by the peahen until they hatch some 28 days later. The chicks have all of their feathers when they emerge from their eggs and are capable of flight roughly one week after hatching. Most blue and green peafowl become sexually mature at age three. However, some male blue peafowl have been known to breed as early as age two.
As an ornamental bird, the peacock is a staple resident of many of the world’s zoos and has long been famous throughout the Old World. Green peacocks in captivity must be kept apart from other fowl, though, because of their aggressive disposition. Blue peacocks, though native to warm humid climates, can survive northern winters. Green peacocks, however, cannot tolerate much cold.
The Congo peacock is the only large phasianid in Africa. The math is mainly blue and green with a short rounded tail. The hen is reddish and green with a brown topknot. The species is smaller than those in genus Pavo, growing to roughly between 64 and 70 cm (25 to 28 inches) in length by adulthood.
Conservation status
The International Union for Conservation of Nature (IUCN) Red List classifies the blue peafowl as a species of least concern. However, the green peacock is classified by the IUCN as an endangered species. The green peacock’s population declined significantly during the latter half of the 20th century because of overhunting and the destruction of large parts of its natural habitat; the species is now thought to number between 10,000 and 20,000 adults. The IUCN has classified the Congo peafowl as a vulnerable species. Its population has fallen to fewer than 10,000 adults because of hunting and habitat loss.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1261 2022-01-21 15:52:59
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,810
Re: Miscellany
1237) Thermionic emission
Summary
Thermionic emission is discharge of electrons from heated materials, widely used as a source of electrons in conventional electron tubes (e.g., television picture tubes) in the fields of electronics and communications. The phenomenon was first observed (1883) by Thomas A. Edison as a passage of electricity from a filament to a plate of metal inside an incandescent lamp.
In thermionic emission, the heat supplies some electrons with at least the minimal energy required to overcome the attractive force holding them in the structure of the metal. This minimal energy, called the work function, is characteristic of the emitting material and the state of contamination of its surface.
Details
Thermionic emission is the liberation of electrons from an electrode by virtue of its temperature (releasing of energy supplied by heat). This occurs because the thermal energy given to the charge carrier overcomes the work function of the material. The charge carriers can be electrons or ions, and in older literature are sometimes referred to as thermions. After emission, a charge that is equal in magnitude and opposite in sign to the total charge emitted is initially left behind in the emitting region. But if the emitter is connected to a battery, the charge left behind is neutralized by charge supplied by the battery as the emitted charge carriers move away from the emitter, and finally the emitter will be in the same state as it was before emission.
The classical example of thermionic emission is that of electrons from a hot cathode into a vacuum (also known as thermal electron emission or the Edison effect) in a vacuum tube. The hot cathode can be a metal filament, a coated metal filament, or a separate structure of metal or carbides or borides of transition metals. Vacuum emission from metals tends to become significant only for temperatures over 1,000 K (730 °C; 1,340 °F).
This process is crucially important in the operation of a variety of electronic devices and can be used for electricity generation (such as thermionic converters and electrodynamic tethers) or cooling. The magnitude of the charge flow increases dramatically with increasing temperature.
The term 'thermionic emission' is now also used to refer to any thermally-excited charge emission process, even when the charge is emitted from one solid-state region into another.
History
Because the electron was not identified as a separate physical particle until the work of J. J. Thomson in 1897, the word "electron" was not used when discussing experiments that took place before this date.
The phenomenon was initially reported in 1853 by Edmond Becquerel. It was rediscovered in 1873 by Frederick Guthrie in Britain. While doing work on charged objects, Guthrie discovered that a red-hot iron sphere with a negative charge would lose its charge (by somehow discharging it into air). He also found that this did not happen if the sphere had a positive charge.[4] Other early contributors included Johann Wilhelm Hittorf (1869–1883),[5] Eugen Goldstein (1885), and Julius Elster and Hans Friedrich Geitel (1882–1889).
The effect was rediscovered again by Thomas Edison on February 13, 1880, while he was trying to discover the reason for breakage of lamp filaments and uneven blackening (darkest near the positive terminal of the filament) of the bulbs in his incandescent lamps.
Edison built several experimental lamp bulbs with an extra wire, metal plate, or foil inside the bulb that was separate from the filament and thus could serve as an electrode. He connected a galvanometer, a device used to measure current (the flow of charge), to the output of the extra metal electrode. If the foil was put at a negative potential relative to the filament, there was no measurable current between the filament and the foil. When the foil was raised to a positive potential relative to the filament, there could be a significant current between the filament through the vacuum to the foil if the filament was heated sufficiently (by its own external power source).
We now know that the filament was emitting electrons, which were attracted to a positively charged foil, but not a negatively charged one. This one-way current was called the Edison effect (although the term is occasionally used to refer to thermionic emission itself). He found that the current emitted by the hot filament increased rapidly with increasing voltage, and filed a patent application for a voltage-regulating device using the effect on November 15, 1883 (U.S. patent 307,031, the first US patent for an electronic device). He found that sufficient current would pass through the device to operate a telegraph sounder. This was exhibited at the International Electrical Exposition in Philadelphia in September 1884. William Preece, a British scientist, took back with him several of the Edison effect bulbs. He presented a paper on them in 1885, where he referred to thermionic emission as the "Edison effect." The British physicist John Ambrose Fleming, working for the British "Wireless Telegraphy" Company, discovered that the Edison effect could be used to detect radio waves. Fleming went on to develop the two-element vacuum tube known as the diode, which he patented on November 16, 1904.
The thermionic diode can also be configured as a device that converts a heat difference to electric power directly without moving parts (a thermionic converter, a type of heat engine).
Richardson's law
Following J. J. Thomson's identification of the electron in 1897, the British physicist Owen Willans Richardson began work on the topic that he later called "thermionic emission". He received a Nobel Prize in Physics in 1928 "for his work on the thermionic phenomenon and especially for the discovery of the law named after him".
From band theory, there are one or two electrons per atom in a solid that are free to move from atom to atom. This is sometimes collectively referred to as a "sea of electrons". Their velocities follow a statistical distribution, rather than being uniform, and occasionally an electron will have enough velocity to exit the metal without being pulled back in. The minimum amount of energy needed for an electron to leave a surface is called the work function. The work function is characteristic of the material and for most metals is on the order of several electronvolts. Thermionic currents can be increased by decreasing the work function. This often-desired goal can be achieved by applying various oxide coatings to the wire.
Photon-enhanced thermionic emission
Photon-enhanced thermionic emission (PETE) is a process developed by scientists at Stanford University that harnesses both the light and heat of the sun to generate electricity and increases the efficiency of solar power production by more than twice the current levels. The device developed for the process reaches peak efficiency above 200 °C, while most silicon solar cells become inert after reaching 100 °C. Such devices work best in parabolic dish collectors, which reach temperatures up to 800 °C. Although the team used a gallium nitride semiconductor in its proof-of-concept device, it claims that the use of GaAs can increase the device's efficiency to 55–60 percent, nearly triple that of existing systems, and 12–17 percent more than existing 43 percent multi-junction solar cells.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1262 2022-01-22 14:41:25
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,810
Re: Miscellany
1238) Safety lamp
Summary
A safety lamp is any of several types of lamp that provides illumination in coal mines and is designed to operate in air that may contain coal dust or gases, both of which are potentially flammable or explosive. Until the development of effective electric lamps in the early 1900s, miners used flame lamps to provide illumination. Open flame lamps could ignite flammable gases which collected in mines, causing explosions; safety lamps were developed to enclose the flame and prevent it from igniting the surrounding atmosphere. Flame safety lamps have been replaced in mining with sealed explosion-proof electric lights.
Details - I
Safety Lamps
The unforgiving darkness of a mine necessitated the use of a light. Before the 19th century, this forced miners to use open flames that had the potential to ignite the flammable gasses inside mines, causing lethal explosions. The flammable gas (firedamp) consisted mainly of methane and was most often found in coal mines. A need was seen to provide a safe light for miners to use in gaseous mines, and several inventors set upon the task independently. The three main progenitors of early safety lamps were Dr. William Reid Clanny, Sir Humphry Davy, and George Stephenson.
The first to set upon the idea of a safety lamp was Dr. William Clanny. As a physician in Sunderland, England, Clanny routinely attended to patients injured in mining explosions. In 1813 Clanny revealed his first design for a lamp in a paper to the Royal Philosophical Society. Clanny enclosed the flame in glass with layers of water above and below the flame to "seal in the fire." A bellows supplied oxygen through a tube into the chamber to keep the flame alight. The bellows made this lamp impractical for daily use, and the flame was extinguished when tested in a mixture of inflammable gas.
George Stephenson was an English engineer who also began to try to invent a safe lamp in 1815. Through trial and error he came upon a design that worked. He encased the lamp in a glass cylinder, which was capped with a metallic cover with tiny holes. This was covered with a metal bonnet to further remove the flame from the dangers of the flammable gas.
The Englishman Sir Humphry Davy was already a scientist and inventor of national renown when he seized upon the idea of a safety lamp in 1815. Davy had performed numerous scientific experiments on all natures of gasses, and this experience proved useful in battling the flammable firedamp found in gaseous mines. Davy’s invention was to surround the flame with a metallic mesh screen. Air could pass through the screen to fuel the flame, but if the holes in the screen were small enough, the mesh would cool the flame to such an extent that it could not ignite the gas surrounding the lamp. This design allowed the Davy lamp to serve as a test for the presences of certain gasses. If firedamp was present, the flame would burn with a blue “cap.” The length of the cap would determine how much gas was present. Some lamps in the collection are these so called “test lamps” with marks in the lamp's glass used to measure the flame’s cap. While flammable gasses were the most prominent threat in mines, asphyxiant gasses also presented a danger. Davy’s safety lamp helped with this issue as well, as miners could use Davy’s lamp to check for harmful concentrations of carbon dioxide, which would extinguish the flame at a non-lethal concentration, alerting the miner’s to unsafe working conditions.
Although safety lamps addressed the issue of mining explosions, they did not become as numerous as other mining lights for a variety of reasons. Many miners objected to using safety lamps because they were cumbersome, could not be worn on the cap, and gave a poor light, which all served to reduce a miner’s efficiency. Since most miners were paid by the pound, a reduction in efficiency amounted to a reduction in pay, and so the risk of an explosion was a chance miners were willing to take. Additionally, many miners objected to the false confidence instilled in many who used a safety lamp, and claimed that safety lamps obscured the real issue of unsafe working conditions and would hinder the development of improved ventilation needed in mines. Safety lamps had one unique advantage—they could safely burn off methane in mines which kept them in use by mine bosses even after the invention of battery-powered lamps.
Safety lamps were manufactured by a variety of companies from around 1815 until the 1930s, and incorporated elements of their design from Clanny, Stephenson, and Davy. Most of the safety lamps in the collection of the Division of Work and Industry include all three inventor’s contributions towards a safety lamp—a glass enclosure around the flame for more effective lighting, mesh uppers to cool the flame, and metal bonnet to better protect the flame from being extinguished by gusts or drafts in mines.
Details - II
Safety lamp is lighting device used in places, such as mines, in which there is danger from the explosion of flammable gas or dust. In the late 18th century a demand arose in England for a miner’s lamp that would not ignite the gas methane (firedamp), a common hazard of English coal mines. W. Reid Clanny, an Irish physician, invented a lamp about 1813 in which the oil-fuelled flame was separated from the atmosphere by water seals; it required continual pumping for operation. In 1815 the English engineer George Stephenson invented a lamp that kept explosive gases out by pressure of the flame’s exhaust and held the flame in by drawing in air at high speed. In 1815 Sir Humphry Davy invented the lamp that bears his name. Davy used a two-layer metal gauze chimney to surround and confine the flame and to conduct the heat of the flame away.
Electric hand and cap lamps were introduced in mines in the early 1900s and by the middle of the 20th century were used almost exclusively in mines. A safety device in the headpiece of the electric lamps shuts off the current if a bulb is broken. Double-filament bulbs may be used, so the light can remain on when a filament fails.
The flame of a safety lamp elongates in the presence of firedamp, but electric lamps give no warning of noxious gases or lack of oxygen. Consequently, a flame safety lamp must be kept burning within easy view of the workers, or frequent inspections must be made, using a flame lamp or other form of warning device.
Sir Humphry Davy
Sir Humphry Davy, in full Sir Humphry Davy, Baronet, (born December 17, 1778, Penzance, Cornwall, England—died May 29, 1829, Geneva, Switzerland), English chemist who discovered several chemical elements (including sodium and potassium) and compounds, invented the miner’s safety lamp, and became one of the greatest exponents of the scientific method.
Early life
Davy was the elder son of middle-class parents who owned an estate in Ludgvan, Cornwall, England. He was educated at the grammar school in nearby Penzance and, in 1793, at Truro. In 1795, a year after the death of his father, Robert, he was apprenticed to a surgeon and apothecary, and he hoped eventually to qualify in medicine. An exuberant, affectionate, and popular lad, of quick wit and lively imagination, he was fond of composing verses, sketching, making fireworks, fishing, shooting, and collecting minerals. He loved to wander, one pocket filled with fishing tackle and the other with rock specimens; he never lost his intense love of nature and, particularly, of mountain and water scenery.
While still a youth, ingenuous and somewhat impetuous, Davy had plans for a volume of poems, but he began the serious study of science in 1797, and these visions “fled before the voice of truth.” He was befriended by Davies Giddy (later Gilbert; president of the Royal Society, 1827–30), who offered him the use of his library in Tradea and took him to a chemistry laboratory that was well equipped for that day. There he formed strongly independent views on topics of the moment, such as the nature of heat, light, and electricity and the chemical and physical doctrines of Antoine Lavoisier. On Gilbert’s recommendation, he was appointed (1798) chemical superintendent of the Pneumatic Institution, founded at Clifton to inquire into the possible therapeutic uses of various gases. Davy attacked the problem with characteristic enthusiasm, evincing an outstanding talent for experimental inquiry. In his small private laboratory, he prepared and inhaled nitrous oxide (laughing gas) in order to test a claim that it was the “principle of contagion,” that is, caused diseases. He investigated the composition of the oxides and acids of nitrogen, as well as ammonia, and persuaded his scientific and literary friends, including Samuel Taylor Coleridge, Robert Southey, and Peter Mark Roget, to report the effects of inhaling nitrous oxide. He nearly lost his own life inhaling water gas, a mixture of hydrogen and carbon monoxide sometimes used as fuel.
The account of his work, published as Researches, Chemical and Philosophical, Chiefly Concerning Nitrous Oxide, or Dephlogisticated Nitrous Air, and Its Respiration (1800), immediately established Davy’s reputation, and he was invited to lecture at the newly founded Royal Institution of Great Britain in London, where he moved in 1801, with the promise of help from the British-American scientist Sir Benjamin Thompson (Count von Rumford), the British naturalist Sir Joseph Banks, and the English chemist and physicist Henry Cavendish in furthering his researches—e.g., on voltaic cells, early forms of electric batteries. His carefully prepared and rehearsed lectures rapidly became important social functions and added greatly to the prestige of science and the institution. In 1802 he became professor of chemistry. His duties included a special study of tanning: he found catechu, the extract of a tropical plant, as effective as and cheaper than the usual oak extracts, and his published account was long used as a tanner’s guide. In 1803 he was admitted a fellow of the Royal Society and an honorary member of the Dublin Society and delivered the first of an annual series of lectures before the board of agriculture. This led to his Elements of Agricultural Chemistry (1813), the only systematic work available for many years. For his researches on voltaic cells, tanning, and mineral analysis, he received the Copley Medal in 1805. He was elected secretary of the Royal Society in 1807.
Major discoveries
Davy early concluded that the production of electricity in simple electrolytic cells resulted from chemical action and that chemical combination occurred between substances of opposite charge. He therefore reasoned that electrolysis, the interactions of electric currents with chemical compounds, offered the most likely means of decomposing all substances to their elements. These views were explained in 1806 in his lecture “On Some Chemical Agencies of Electricity,” for which, despite the fact that England and France were at war, he received the Napoleon Prize from the Institut de France (1807). This work led directly to the isolation of sodium and potassium from their compounds (1807) and of the alkaline-earth metals magnesium, calcium, strontium, and barium from their compounds (1808). He also discovered boron (by heating borax with potassium), hydrogen telluride, and hydrogen phosphide (phosphine). He showed the correct relation of chlorine to hydrochloric acid and the untenability of the earlier name (oxymuriatic acid) for chlorine; this negated Lavoisier’s theory that all acids contained oxygen. He also showed that chlorine is a chemical element, and experiments designed to reveal oxygen in chlorine failed. He explained the bleaching action of chlorine (through its liberation of oxygen from water) and discovered two of its oxides (1811 and 1815), but his views on the nature of chlorine were disputed.
In 1810 and 1811 he lectured to large audiences at Dublin (on agricultural chemistry, the elements of chemical philosophy, geology) and received £1,275 in fees, as well as the honorary degree of LL.D., from Trinity College. In 1812 he was knighted by the Prince Regent (April 8), delivered a farewell lecture to members of the Royal Institution (April 9), and married Jane Apreece, a wealthy widow well known in social and literary circles in England and Scotland (April 11). He also published the first part of the Elements of Chemical Philosophy, which contained much of his own work. His plan was too ambitious, however, and nothing further appeared. Its completion, according to Swedish chemist Jöns Jacob Berzelius, would have “advanced the science of chemistry a full century.”
His last important act at the Royal Institution, of which he remained honorary professor, was to interview the young Michael Faraday, later to become one of England’s great scientists, who became laboratory assistant there in 1813 and accompanied the Davys on a European tour (1813–15). By permission of Napoleon, he travelled through France, meeting many prominent scientists, and was presented to the empress Marie Louise. With the aid of a small portable laboratory and of various institutions in France and Italy, he investigated the substance “X” (later called iodine), whose properties and similarity to chlorine he quickly discovered; further work on various compounds of iodine and chlorine was done before he reached Rome. He also analyzed many specimens of classical pigments and proved that diamond is a form of carbon.
Later years of Sir Humphry Davy
Shortly after his return, he studied, for the Society for Preventing Accidents in Coal Mines, the conditions under which mixtures of firedamp and air explode. This led to the invention of the miner’s safety lamp and to subsequent researches on flame, for which he received the Rumford medals (gold and silver) from the Royal Society and, from the northern mine owners, a service of plate (eventually sold to found the Davy Medal). After being created a baronet in 1818, he again went to Italy, inquiring into volcanic action and trying unsuccessfully to find a way of unrolling the papyri found at Herculaneum. In 1820 he became president of the Royal Society, a position he held until 1827. In 1823–25 he was associated with the politician and writer John Wilson Croker in founding the Athenaeum Club, of which he was an original trustee, and with the colonial governor Sir Stamford Raffles in founding the Zoological Society and in furthering the scheme for zoological gardens in Regent’s Park, London (opened in 1828). During this period, he examined magnetic phenomena caused by electricity and electrochemical methods for preventing saltwater corrosion of copper sheathing on ships by means of iron and zinc plates. Though the protective principles were made clear, considerable fouling occurred, and the method’s failure greatly vexed him. But he was, as he said, “burned out.” His Bakerian lecture for 1826, “On the Relation of Electrical and Chemical Changes,” contained his last known thoughts on electrochemistry and earned him the Royal Society’s Royal Medal.
Davy’s health was by then failing rapidly; in 1827 he departed for Europe and, in the summer, was forced to resign the presidency of the Royal Society, being succeeded by Davies Gilbert. Having to forgo business and field sports, Davy wrote Salmonia: or Days of Fly Fishing (1828), a book on fishing (after the manner of Izaak Walton) that contained engravings from his own drawings. After a last, short visit to England, he returned to Italy, settling at Rome in February 1829—“a ruin amongst ruins.” Though partly paralyzed through stroke, he spent his last months writing a series of dialogues, published posthumously as Consolations in Travel, or the Last Days of a Philosopher (1830).
William Reid Clanny
William Reid Clanny, (born 1776, Bangor, County Down, Ire.—died Jan. 10, 1850, near Sunderland, Durham, Eng.), was a physician who invented one of the first safety lamps (1813) for use in coal mines; some of its features were incorporated in Sir Humphry Davy’s safety lamp, which was the precursor of modern safety lamps.
Educated at the University of Edinburgh (M.D.), Clanny served with the navy before becoming a private practitioner. In Clanny’s time, a serious hazard of coal mining was ignition by miners’ lamps of firedamp, an explosive mixture of air and methane, a gas commonly present in deposits of coal. Clanny developed a miner’s lamp that would not ignite firedamp but was unwieldy; he later reduced its bulk and adapted several improvements devised by Davy, one of which was a shield of metal gauze.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1263 2022-01-23 14:35:46
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,810
Re: Miscellany
1239. Field emission
Summary
Field emission, also called Cold Emission, is discharge of electrons from the surface of a material subjected to a strong electric field. In the absence of a strong electric field, an electron must acquire a certain minimum energy, called the work function, to escape through the surface of a given material, which acts as a barrier to electron passage. If the material is placed in an electric circuit that renders it strongly negative with respect to a nearby positive electrode (i.e., when it is subjected to a strong electric field), the work function is so lowered that some electrons will have sufficient energy to leak through the surface barrier. The resulting current of electrons through the surface of a material under the influence of a strong electric field is called field emission. This effect is utilized in the field-emission electron microscope, which in some instances achieves resolution of atomic dimensions. Field emission is sometimes called high-field emission to distinguish it from the Schottky effect (q.v.), which influences electron emission at lower values of the applied field.
Positive ions (atoms that have lost at least one electron) also may be emitted from a solid subjected to a high electric field at its surface.
Details
Field electron emission, also known as field emission (FE) and electron field emission, is emission of electrons induced by an electrostatic field. The most common context is field emission from a solid surface into a vacuum. However, field emission can take place from solid or liquid surfaces, into a vacuum, a fluid (e.g. air), or any non-conducting or weakly conducting dielectric. The field-induced promotion of electrons from the valence to conduction band of semiconductors (the Zener effect) can also be regarded as a form of field emission. The terminology is historical because related phenomena of surface photoeffect, thermionic emission (or Richardson–Dushman effect) and "cold electronic emission", i.e. the emission of electrons in strong static (or quasi-static) electric fields, were discovered and studied independently from the 1880s to 1930s. When field emission is used without qualifiers it typically means "cold emission".
Field emission in pure metals occurs in high electric fields: the gradients are typically higher than 1 gigavolt per metre and strongly dependent upon the work function. While electron sources based on field emission have a number of applications, field emission is most commonly an undesirable primary source of vacuum breakdown and electrical discharge phenomena, which engineers work to prevent. Examples of applications for surface field emission include the construction of bright electron sources for high-resolution electron microscopes or the discharge of induced charges from spacecraft. Devices which eliminate induced charges are termed charge-neutralizers.
Field emission was explained by quantum tunneling of electrons in the late 1920s. This was one of the triumphs of the nascent quantum mechanics. The theory of field emission from bulk metals was proposed by Ralph H. Fowler and Lothar Wolfgang Nordheim. A family of approximate equations, Fowler–Nordheim equations, is named after them. Strictly, Fowler–Nordheim equations apply only to field emission from bulk metals and (with suitable modification) to other bulk crystalline solids, but they are often used – as a rough approximation – to describe field emission from other materials.
Terminology and conventions
Field electron emission, field-induced electron emission, field emission and electron field emission are general names for this experimental phenomenon and its theory. The first name is used here.
Fowler–Nordheim tunneling is the wave-mechanical tunneling of electrons through a rounded triangular barrier created at the surface of an electron conductor by applying a very high electric field. Individual electrons can escape by Fowler–Nordheim tunneling from many materials in various different circumstances.
Cold field electron emission (CFE) is the name given to a particular statistical emission regime, in which the electrons in the emitter are initially in internal thermodynamic equilibrium, and in which most emitted electrons escape by Fowler–Nordheim tunneling from electron states close to the emitter Fermi level. (By contrast, in the Schottky emission regime, most electrons escape over the top of a field-reduced barrier, from states well above the Fermi level.) Many solid and liquid materials can emit electrons in a CFE regime if an electric field of an appropriate size is applied.
Fowler–Nordheim-type equations are a family of approximate equations derived to describe CFE from the internal electron states in bulk metals. The different members of the family represent different degrees of approximation to reality. Approximate equations are necessary because, for physically realistic models of the tunneling barrier, it is mathematically impossible in principle to solve the Schrödinger equation exactly in any simple way. There is no theoretical reason to believe that Fowler–Nordheim-type equations validly describe field emission from materials other than bulk crystalline solids.
For metals, the CFE regime extends to well above room temperature. There are other electron emission regimes (such as "thermal electron emission" and "Schottky emission") that require significant external heating of the emitter. There are also emission regimes where the internal electrons are not in thermodynamic equilibrium and the emission current is, partly or completely, determined by the supply of electrons to the emitting region. A non-equilibrium emission process of this kind may be called field (electron) emission if most of the electrons escape by tunneling, but strictly it is not CFE, and is not accurately described by a Fowler–Nordheim-type equation.
Care is necessary because in some contexts (e.g. spacecraft engineering), the name "field emission" is applied to the field-induced emission of ions (field ion emission), rather than electrons, and because in some theoretical contexts "field emission" is used as a general name covering both field electron emission and field ion emission.
Historically, the phenomenon of field electron emission has been known by a variety of names, including "the aeona effect", "autoelectronic emission", "cold emission", "cold cathode emission", "field emission", "field electron emission" and "electron field emission".
Equations in this article are written using the International System of Quantities (ISQ). This is the modern (post-1970s) international system, based around the rationalized-meter-kilogram-second (rmks) system of equations, which is used to define SI units. Older field emission literature (and papers that directly copy equations from old literature) often write some equations using an older equation system that does not use the quantity ε0. In this article, all such equations have been converted to modern international form. For clarity, this should always be done.
Since work function is normally given in electronvolts (eV), and it is often convenient to measure fields in volts per nanometer (V/nm), values of most universal constants are given here in units involving the eV, V and nm. Increasingly, this is normal practice in field emission research. However, all equations here are ISQ-compatible equations and remain dimensionally consistent, as is required by the modern international system. To indicate their status, numerical values of universal constants are given to seven significant figures. Values are derived using the 2006 values of the fundamental constants.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1264 2022-01-24 15:47:04
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,810
Re: Miscellany
1240) Electrical network
Summary
What is Electrical network?
The interconnection of electrical components like resistors, inductors, semiconductors devices, transformers and sources of e.m.f. is called as a network. The network consist a closed loop, which provides return path for the current. A network may contain one or more circuit elements. It has two or more terminals for making connection with other circuits.
Types of networks :
There are two types of networks which are given below,
* One port network.
* Two port network.
One port network
The network which has one pair of terminal is called one port network. It helps to reduce the complexity of circuit analysis. In many common electronic devices and circuit blocks such as transistors, amplifiers, electronic filters, and transformers are analyzed in terms of ports. A series, parallel combination of resistors and capacitors are example of one port network.
Two port network
The network having two pairs of terminal is called a two port network. An amplifier, attenuator, filter, transformer are examples of two port network.
Details
An electrical network is an interconnection of electrical components (e.g., batteries, resistors, inductors, capacitors, switches, transistors) or a model of such an interconnection, consisting of electrical elements (e.g., voltage sources, current sources, resistances, inductances, capacitances). An electrical circuit is a network consisting of a closed loop, giving a return path for the current. Linear electrical networks, a special type consisting only of sources (voltage or current), linear lumped elements (resistors, capacitors, inductors), and linear distributed elements (transmission lines), have the property that signals are linearly superimposable. They are thus more easily analyzed, using powerful frequency domain methods such as Laplace transforms, to determine DC response, AC response, and transient response.
A resistive circuit is a circuit containing only resistors and ideal current and voltage sources. Analysis of resistive circuits is less complicated than analysis of circuits containing capacitors and inductors. If the sources are constant (DC) sources, the result is a DC circuit. The effective resistance and current distribution properties of arbitrary resistor networks can be modeled in terms of their graph measures and geometrical properties.
A network that contains active electronic components is known as an electronic circuit. Such networks are generally nonlinear and require more complex design and analysis tools.
Classification:
By passivity
An active network contains at least one voltage source or current source that can supply energy to the network indefinitely. A passive network does not contain an active source.
An active network contains one or more sources of electromotive force. Practical examples of such sources include a battery or a generator. Active elements can inject power to the circuit, provide power gain, and control the current flow within the circuit.
Passive networks do not contain any sources of electromotive force. They consist of passive elements like resistors and capacitors.
By linearity
A network is linear if its signals obey the principle of superposition; otherwise it is non-linear. Passive networks are generally taken to be linear, but there are exceptions. For instance, an inductor with an iron core can be driven into saturation if driven with a large enough current. In this region, the behaviour of the inductor is very non-linear.
By lumpiness
Discrete passive components (resistors, capacitors and inductors) are called lumped elements because all of their, respectively, resistance, capacitance and inductance is assumed to be located ("lumped") at one place. This design philosophy is called the lumped-element model and networks so designed are called lumped-element circuits. This is the conventional approach to circuit design. At high enough frequencies, or for long enough circuits (such as power transmission lines), the lumped assumption no longer holds because there is a significant fraction of a wavelength across the component dimensions. A new design model is needed for such cases called the distributed-element model. Networks designed to this model are called distributed-element circuits.
A distributed-element circuit that includes some lumped components is called a semi-lumped design. An example of a semi-lumped circuit is the combline filter.
Classification of sources
Sources can be classified as independent sources and dependent sources.
Independent
An ideal independent source maintains the same voltage or current regardless of the other elements present in the circuit. Its value is either constant (DC) or sinusoidal (AC). The strength of voltage or current is not changed by any variation in the connected network.
Dependent
Dependent sources depend upon a particular element of the circuit for delivering the power or voltage or current depending upon the type of source it is.
Applying electrical laws
A number of electrical laws apply to all linear resistive networks. These include:
* Kirchhoff's current law: The sum of all currents entering a node is equal to the sum of all currents leaving the node.
* Kirchhoff's voltage law: The directed sum of the electrical potential differences around a loop must be zero.
* Ohm's law: The voltage across a resistor is equal to the product of the resistance and the current flowing through it.
* Norton's theorem: Any network of voltage or current sources and resistors is electrically equivalent to an ideal current source in parallel with a single resistor.
* Thévenin's theorem: Any network of voltage or current sources and resistors is electrically equivalent to a single voltage source in series with a single resistor.
* Superposition theorem: In a linear network with several independent sources, the response in a particular branch when all the sources are acting simultaneously is equal to the linear sum of individual responses calculated by taking one independent source at a time.
Applying these laws results in a set of simultaneous equations that can be solved either algebraically or numerically. The laws can generally be extended to networks containing reactances. They cannot be used in networks that contain nonlinear or time-varying components.
Design methods
To design any electrical circuit, either analog or digital, electrical engineers need to be able to predict the voltages and currents at all places within the circuit. Simple linear circuits can be analyzed by hand using complex number theory. In more complex cases the circuit may be analyzed with specialized computer programs or estimation techniques such as the piecewise-linear model.
Circuit simulation software, such as HSPICE (an analog circuit simulator), and languages such as VHDL-AMS and verilog-AMS allow engineers to design circuits without the time, cost and risk of error involved in building circuit prototypes.
Network simulation software
More complex circuits can be analyzed numerically with software such as SPICE or GNUCAP, or symbolically using software such as SapWin.
Linearization around operating point
When faced with a new circuit, the software first tries to find a steady state solution, that is, one where all nodes conform to Kirchhoff's current law and the voltages across and through each element of the circuit conform to the voltage/current equations governing that element.
Once the steady state solution is found, the operating points of each element in the circuit are known. For a small signal analysis, every non-linear element can be linearized around its operation point to obtain the small-signal estimate of the voltages and currents. This is an application of Ohm's Law. The resulting linear circuit matrix can be solved with Gaussian elimination.
Piecewise-linear approximation
Software such as the PLECS interface to Simulink uses piecewise-linear approximation of the equations governing the elements of a circuit. The circuit is treated as a completely linear network of ideal diodes. Every time a diode switches from on to off or vice versa, the configuration of the linear network changes. Adding more detail to the approximation of equations increases the accuracy of the simulation, but also increases its running time.
Abbreviations
AC : Alternating current
DC : Direct current
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1265 2022-01-25 14:04:42
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,810
Re: Miscellany
1241) Gastric glands
The gastric glands are located in different regions of the stomach. These are the fundic glands, the cardiac glands, and the pyloric glands. The glands and gastric pits are located in the stomach lining. The glands themselves are in the lamina propria of the mucous membrane and they open into the bases of the gastric pits formed by the epithelium. The various cells of the glands secrete mucus, pepsinogen, hydrochloric acid, intrinsic factor, gastrin, and bicarbonate.
Gastric glands are mostly exocrine glands and are all located beneath the gastric pits within the gastric mucosa—the mucous membrane of the stomach. The gastric mucosa is pitted with innumerable gastric pits which each house 3-5 gastric glands. The cells of the exocrine glands are foveolar (mucus), chief cells, and parietal cells. The other type of gastric gland is the pyloric gland which is an endocrine gland that secretes the hormone gastrin produced by its G cells.
* The cardiac glands are found in the cardia of the stomach which is the part nearest to the heart, enclosing the opening where the esophagus joins to the stomach. Only cardiac glands are found here and they primarily secrete mucus. They are fewer in number than the other gastric glands and are more shallowly positioned in the mucosa. There are two kinds - either simple tubular with short ducts or compound racemose resembling the duodenal Brunner's glands.
* The fundic glands (or oxyntic glands), are found in the fundus and body of the stomach. They are simple almost straight tubes, two or more of which open into a single duct. Oxyntic means acid-secreting and they secrete hydrochloric acid (HCl) and intrinsic factor.
* The pyloric glands are located in the antrum of the pylorus. They secrete gastrin produced by their G cells.
Types of cell
There are millions of gastric pits in the gastric mucosa and their necessary narrowness determines the tubular form of the gastric gland. More than one tube allows for the accommodation of more than one cell type. The form of each gastric gland is similar; they are all described as having a neck region that is closest to the pit entrance, and basal regions on the lower parts of the tubes.[8] The epithelium from the gastric mucosa travels into the pit and at the neck the epithelial cells change to short columnar granular cells. These cells almost fill the tube and the remaining lumen is continued as a very fine channel.
Cells found in the gastric glands include foveolar cells, chief cells, parietal cells, G cells, enterochromaffin-like cells (ECLs), etc. The first cells of all of the glands are foveolar cells in the neck region–also called mucous neck cells that produce mucus. This is thought to be different from the mucus produced by the gastric mucosa.
Fundic glands found in the fundus and also in the body have another two cell types–gastric chief cells and parietal cells (oxyntic cells).
* Surface mucous cell (foveolar cell) - They are mucous producing cells which cover the inside of the stomach, protecting it from the corrosive nature of gastric acid. These cells line the gastric mucosa.
* Mucous neck cell - Mucous neck cells are located within gastric glands, interspersed between parietal cells. These are shorter than their surface counterpart and contain lesser quantities of mucin granules in their apical surface.
* Chief cells (Zymogen cells/ peptic cells) - They are found in the basal regions of the gland and release proenzymes or zymogens – pepsinogen (precursor to pepsin), and prorennin (precursor to rennin or chymosin). Prorennin is secreted in young mammals (childhood stage). It is not secreted in adult mammals. Chief cells also produce small amounts of gastric lipase. Gastric lipase contributes little to digestion of fat.
* Parietal cells ("parietal" means "relating to a wall"), also known as oxyntic cells are most numerous on the side walls of the gastric glands. The parietal cells secrete hydrochloric acid, the main component of gastric acid. This needs to be readily available for the stomach in a plentiful supply, and so from their positions in the walls, their secretory networks of fine channels called canaliculi can project and ingress into all the regions of the gastric-pit lumen. Another important secretion of the parietal cells is castle's intrinsic factor. Intrinsic factor is a glycoprotein essential for the absorption of vitamin B12. The parietal cells also produce and release bicarbonate ions in response to histamine release from the nearby ECLs, and so serve a crucial role in the pH buffering system.
* Enteroendocrine cells or argentaffin cells - They are usually present in the basal parts of the gastric glands, which is differentiated in three cells - these are D-cells, Enterochromaffin like cells (ECL-cells) and G-cells.
** Enterochromaffin like cell (ECL cell) - they release serotonin and histamine. These cells store and release histamine when the pH of the stomach becomes too high. The release of histamine is stimulated by the secretion of gastrin from the G cells. Histamine promotes the production and release of HCL from the parietal cells to the blood and protons to the stomach lumen. When the stomach pH decreases(becomes more acidic), the ECLs stop releasing histamine.
** G cells- They secrete gastrin hormone. Gastrin stimulates the gastric glands to release gastric juice. These cells are mostly found in pyloric glands in the antrum of the pylorus; some are found in the duodenum and other tissues. The gastric pits of these glands are much deeper than the others and here the gastrin is secreted into the bloodstream not the lumen.
** D-cells - D cells secrete somatostatin. Somatostatin suppresses the release of hormones from the digestive tract.
Clinical significance
Fundic gland polyposis is a medical syndrome where the fundus and the body of the stomach develop many fundic gland polyps.
Pernicious anemia is caused when damaged parietal cells fail to produce the intrinsic factor necessary for the absorption of vitamin B12. This can be one of the causes of vitamin B12 deficiency.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1266 2022-01-27 00:29:55
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,810
Re: Miscellany
1242) Tropics
Summary
The tropics are regions of the Earth that lie roughly in the middle of the globe. The tropics between the latitude lines of the Tropic of Cancer and the Tropic of Capricorn. The tropics include the Equator and parts of North America, South America, Africa, Asia, and Australia. The tropics account for 36 percent of the Earth's landmass and are home to about a third of the world's people.
The tropics are warm all year, averaging 25 to 28 degrees Celsius (77 to 82 degrees Fahrenheit). This is because the tropics get more exposure to the sun. Because of all that sun, the tropics don't experience the kind of seasons the rest of the Earth does. The tropical seasons are broken up into just two: the wet season and the dry season.
The amount of rain can vary greatly from one area of the tropics to another. Some areas, like parts of the Amazon Basin in South America, get almost 3 meters (9 feet) of rain per year. Other areas in the tropics have a drier climate. The Sahara Desert in northern Africa only gets 2-10 centimeters (.793.9 inches) of rain per year.
The amount of rain a region gets in the tropics directly affects which plant and animal species live there. The baobab tree thrives in the arid tropics of Africa, for instance. The baobab stores water in its huge trunk. On the other extreme is the rainy island of Sri Lanka in the Indian Ocean. Sri Lanka gets enough precipitation to support 250 species of frogs.
Details
The tropics are the region of Earth surrounding the Equator. They are delimited in latitude by the Tropic of Cancer in the Northern Hemisphere at 23°26′11.1″ (or 23.43642°) N and the Tropic of Capricorn in the Southern Hemisphere at 23°26′11.1″ (or 23.43642°) S; these latitudes correspond to the axial tilt of the Earth. The tropics are also referred to as the tropical zone and the torrid zone (see geographical zone). The tropics include everywhere on Earth which is a subsolar point (the Sun is directly overhead) at least once during the solar year. Thus the maximum latitudes of the tropics have the same value positive and negative. Likewise, they approximate, due to the earth not being a perfect sphere, the "angle" of the Earth's axial tilt. The "angle" itself is not perfectly fixed due chiefly to the influence of the moon, but the limits of tropics are a geographic convention, being an averaged form, and the variance is very small.
In terms of climate, the tropics receive sunlight that is more direct than the rest of Earth and are generally hotter and wetter. The word "tropical" sometimes refers to this sort of climate rather than to the geographical zone. The tropical zone includes deserts and snow-capped mountains, which are not tropical in the climatic sense. The tropics are distinguished from the other climatic and biomatic regions of Earth, which are the middle latitudes and the polar regions on either side of the equatorial zone.
The tropics constitute 40% of Earth's surface area and contain 36% of Earth's landmass. As of 2014, the region was home to 40% of the world's population, and this figure was then projected to reach 50% by 2050.
Etymology
The word "tropic" comes from Ancient Greek, meaning "to turn" or "change direction".
Seasons and climate
"Tropical" is sometimes used in a general sense for a tropical climate to mean warm to hot and moist year-round, often with the sense of lush vegetation.
Many tropical areas have a dry and wet season. The wet season, rainy season or green season is the time of year, ranging from one or more months, when most of the average annual rainfall in a region falls. Areas with wet seasons are disseminated across portions of the tropics and subtropics. Under the Köppen climate classification, for tropical climates, a wet-season month is defined as a month where average precipitation is 60 mm (2.4 in) or more. Some areas with pronounced rainy seasons see a break in rainfall during mid-season when the intertropical convergence zone or monsoon trough moves poleward of their location during the middle of the warm season; typical vegetation in these areas ranges from moist seasonal tropical forests to savannahs.
When the wet season occurs during the warm season, or summer, precipitation falls mainly during the late afternoon and early evening hours. The wet season is a time when air quality improves, freshwater quality improves and vegetation grows significantly, leading to crop yields late in the season. Floods cause rivers to overflow their banks, and some animals to retreat to higher ground. Soil nutrients diminish and erosion increases. The incidence of malaria increases in areas where the rainy season coincides with high temperatures. Animals have adaptation and survival strategies for the wetter regime. The previous dry season leads to food shortages into the wet season, as the crops have yet to mature.
However, regions within the tropics may well not have a tropical climate. Under the Köppen climate classification, much of the area within the geographical tropics is classed not as "tropical" but as "dry" (arid or semi-arid), including the Sahara Desert, the Atacama Desert and Australian Outback. Also, there are alpine tundra and snow-capped peaks, including Mauna Kea, Mount Kilimanjaro, Puncak Jaya and the Andes as far south as the northernmost parts of Chile and Perú.
Ecosystems
Tropical plants and animals are those species native to the tropics. Tropical ecosystems may consist of tropical rainforests, seasonal tropical forests, dry (often deciduous) forests, spiny forests, desert and other habitat types. There are often significant areas of biodiversity, and species endemism present, particularly in rainforests and seasonal forests. Some examples of important biodiversity and high endemism ecosystems are El Yunque National Forest in Puerto Rico, Costa Rican and Nicaraguan rainforests, Amazon Rainforest territories of several South American countries, Madagascar dry deciduous forests, the Waterberg Biosphere of South Africa, and eastern Madagascar rainforests. Often the soils of tropical forests are low in nutrient content, making them quite vulnerable to slash-and-burn deforestation techniques, which are sometimes an element of shifting cultivation agricultural systems.
In biogeography, the tropics are divided into Paleotropics (Africa, Asia and Australia) and Neotropics (Caribbean, Central America, and South America). Together, they are sometimes referred to as the Pantropic. The system of biogeographic realms differs somewhat; the Neotropical realm includes both the Neotropics and temperate South America, and the Paleotropics correspond to the Afrotropical, Indomalayan, Oceanian, and tropical Australasian realms.
Tropicality
Tropicality refers to the image that people from outside the tropics have of the region, ranging from critical to verging on fetishism. The idea of tropicality gained renewed interest in geographical discourse when French geographer Pierre Gourou published Les Pays Tropicaux (The Tropical World in English), in the late 1940s.
Tropicality encompassed two images. One, is that the tropics represent a 'Garden of Eden', a heaven on Earth, a land of rich biodiversity or a tropical paradise. The alternative is that the tropics consist of wild, unconquerable nature. The latter view was often discussed in old Western literature more so than the first. Evidence suggests over time that the view of the tropics as such in popular literature has been supplanted by more well-rounded and sophisticated interpretations.
Western scholars tried to theorise why tropical areas were relatively more inhospitable to human civilisations than colder regions of the Northern Hemisphere. A popular explanation focused on the differences in climate. Tropical jungles and rainforests have much more humid and hotter weather than colder and drier temperaments of the Northern Hemisphere. This theme led some scholars to suggest that humid hot climates correlate to human populations lacking control over nature e.g. 'the wild Amazonian rainforests'.
Additional information
The tropics are the region of the Earth near to the equator and between the Tropic of Cancer in the northern hemisphere and the Tropic of Capricorn in the southern hemisphere. This region is also referred to as the tropical zone and the torrid zone.
This area includes all the areas of the Earth where the sun reaches a point directly overhead at least once a year. The word "tropics" comes from Greek tropos meaning "turn", because the apparent position of the Sun moves between the two tropics within a year.
The word Tropical specifically means places near the equator. The word is also sometimes used in a general sense for a tropical climate, a climate that is warm to hot and moist year-round. This includes tropical rainforests with lush vegetation. However, there are mountains in the tropics that are anything but "tropical" in this sense, with even alpine tundra and snow-capped peaks, including Mauna Kea, Mt. Kilimanjaro, and the Andes as far south as the northernmost parts of Chile and Argentina. Places in the tropics which are hot and dry include the Atacama Desert, Sahara Desert, Central Africa, most parts of Western Africa and Northern Australian Outback.
Some parts of Eurasia are also in the torrid zone.
People in some tropical places call their seasons "dry"/"hot" and "rainy"/"wet", especially where the seasons are made by monsoons. Tropical cyclones form in tropical ocean areas, and some move from there into the temperate zone. Tropical plants and animals are native to the tropics or the Torrid zone.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1267 2022-01-28 02:03:31
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,810
Re: Miscellany
1243) Work function
In solid-state physics, the work function (sometimes spelled workfunction) is the minimum thermodynamic work (i.e., energy) needed to remove an electron from a solid to a point in the vacuum immediately outside the solid surface. Here "immediately" means that the final electron position is far from the surface on the atomic scale, but still too close to the solid to be influenced by ambient electric fields in the vacuum. The work function is not a characteristic of a bulk material, but rather a property of the surface of the material (depending on crystal face and contamination).
The work function refers to removal of an electron to a position that is far enough from the surface (many nm) that the force between the electron and its image charge in the surface can be neglected. The electron must also be close to the surface compared to the nearest edge of a crystal facet, or to any other change in the surface structure, such as a change in the material composition, surface coating or reconstruction. The built-in electric field that results from these structures, and any other ambient electric field present in the vacuum, are excluded in defining the work function.
Applications:
Thermionic emission
In thermionic electron guns, the work function and temperature of the hot cathode are critical parameters in determining the amount of current that can be emitted. Tungsten, the common choice for vacuum tube filaments, can survive to high temperatures but its emission is somewhat limited due to its relatively high work function (approximately 4.5 eV). By coating the tungsten with a substance of lower work function (e.g., thorium or barium oxide), the emission can be greatly increased. This prolongs the lifetime of the filament by allowing operation at lower temperatures.
Band bending models in solid-state electronics
The behavior of a solid-state device is strongly dependent on the size of various Schottky barriers and band offsets in the junctions of differing materials, such as metals, semiconductors, and insulators. Some commonly used heuristic approaches to predict the band alignment between materials, such as Anderson's rule and the Schottky–Mott rule, are based on the thought experiment of two materials coming together in vacuum, such that the surfaces charge up and adjust their work functions to become equal just before contact. In reality these work function heuristics are inaccurate due to their neglect of numerous microscopic effects. However, they provide a convenient estimate until the true value can be determined by experiment.
Equilibrium electric fields in vacuum chambers
Variation in work function between different surfaces causes a non-uniform electrostatic potential in the vacuum. Even on an ostensibly uniform surface, variations in W known as patch potentials are always present due to microscopic inhomogeneities. Patch potentials have disrupted sensitive apparatus that rely on a perfectly uniform vacuum, such as Casimir force experiments and the Gravity Probe B experiment. Critical apparatus may have surfaces covered with molybdenum, which shows low variations in work function between different crystal faces.
Contact electrification
If two conducting surfaces are moved relative to each other, and there is potential difference in the space between them, then an electric current will be driven. This is because the surface charge on a conductor depends on the magnitude of the electric field, which in turn depends on the distance between the surfaces. The externally observed electrical effects are largest when the conductors are separated by the smallest distance without touching (once brought into contact, the charge will instead flow internally through the junction between the conductors). Since two conductors in equilibrium can have a built-in potential difference due to work function differences, this means that bringing dissimilar conductors into contact, or pulling them apart, will drive electric currents. These contact currents can damage sensitive microelectronic circuitry and occur even when the conductors would be grounded in the absence of motion.
Measurement
Certain physical phenomena are highly sensitive to the value of the work function. The observed data from these effects can be fitted to simplified theoretical models, allowing one to extract a value of the work function. These phenomenologically extracted work functions may be slightly different from the thermodynamic definition given above. For inhomogeneous surfaces, the work function varies from place to place, and different methods will yield different values of the typical "work function" as they average or select differently among the microscopic work functions.
Many techniques have been developed based on different physical effects to measure the electronic work function of a sample. One may distinguish between two groups of experimental methods for work function measurements: absolute and relative.
* Absolute methods employ electron emission from the sample induced by photon absorption (photoemission), by high temperature (thermionic emission), due to an electric field (field electron emission), or using electron tunnelling.
* Relative methods make use of the contact potential difference between the sample and a reference electrode. Experimentally, either an anode current of a diode is used or the displacement current between the sample and reference, created by an artificial change in the capacitance between the two, is measured (the Kelvin Probe method, Kelvin probe force microscope). However, absolute work function values can be obtained if the tip is first calibrated against a reference sample.
Electronic work function
Electronic work function is energy (or work) required to withdraw an electron completely from a metal surface. This energy is a measure of how tightly a particular metal holds its electrons—that is, of how much lower the electron’s energy is when present within the metal than when completely free. The work function is important in applications involving electron emission from metals, as in photoelectric devices and cathode-ray tubes.
The value of the work function for a particular material varies slightly depending upon the process of emission. For example, the energy required to boil an electron out of a heated platinum filament (thermionic work function) differs slightly from that required to eject an electron from platinum that is struck by light (photoelectric work function). Typical values for metals range from two to five electron volts.
When metals of different work functions are joined, electrons tend to leave the metal with the lower work function (where they are less tightly bound) and travel to the metal of higher work function. This effect must be considered whenever connections are made between dissimilar metals in certain electronic circuits.
Because some electrons in a material are held more tightly than others, a precise definition of work function specifies which electrons are involved, usually those most loosely bound.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1268 2022-01-29 02:26:16
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,810
Re: Miscellany
1244) Glycol
Summary
A diol is a chemical compound containing two hydroxyl groups (−OH groups). An aliphatic diol is also called a glycol. This pairing of functional groups is pervasive, and many subcategories have been identified.
The most common industrial diol is ethylene glycol. Examples of diols in which the hydroxyl functional groups are more widely separated include 1,4-butanediol HO−(CH2)4−OH and propylene-1,3-diol, or beta propylene glycol, HO−CH2−CH2−CH2−OH.
Details
Glycol is any of a class of organic compounds belonging to the alcohol family; in the molecule of a glycol, two hydroxyl (―OH) groups are attached to different carbon atoms. The term is often applied to the simplest member of the class, ethylene glycol.
Ethylene glycol (also called 1,2-ethanediol, molecular formula HOCH2CH2OH) is a colourless, oily liquid possessing a sweet taste and mild odour. It is produced commercially from ethylene oxide, which is obtained from ethylene. Ethylene glycol is widely used as antifreeze in automobile cooling systems and in the manufacture of human-made fibres, low-freezing explosives, and brake fluid. Ethylene glycol and some of its derivatives are mildly toxic.
Propylene glycol, also called 1,2-propanediol, resembles ethylene glycol in its physical properties. Unlike ethylene glycol, however, propylene glycol is not toxic and is used extensively in foods, cosmetics, and oral hygiene products as a solvent, preservative, and moisture-retaining agent. Propylene glycol is manufactured in large amounts from propylene oxide, which is obtained from propylene.
Other important glycols include 1,3-butanediol, used as a starting material for the manufacture of brake fluids and of plasticizers for resins; 1,4-butanediol, used in polyurethanes and in polyester resins for coatings and plasticizers, and for making butyrolactone, a valuable solvent and chemical intermediate; 2-ethyl-1,3-hexanediol, an effective insect repellent; and 2-methyl-2-propyl-1,3-propanediol, made into meprobamate, a widely used tranquilizer.
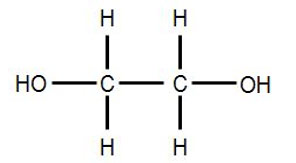
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1269 2022-01-30 01:10:40
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,810
Re: Miscellany
1245) Wind
Summary
Wind is the natural movement of air or other gases relative to a planet's surface. Wind occurs on a range of scales, from thunderstorm flows lasting tens of minutes, to local breezes generated by heating of land surfaces and lasting a few hours, to global winds resulting from the difference in absorption of solar energy between the climate zones on Earth. The two main causes of large-scale atmospheric circulation are the differential heating between the equator and the poles, and the rotation of the planet (Coriolis effect). Within the tropics and subtropics, thermal low circulations over terrain and high plateaus can drive monsoon circulations. In coastal areas the sea breeze/land breeze cycle can define local winds; in areas that have variable terrain, mountain and valley breezes can prevail.
Winds are commonly classified by their spatial scale, their speed and direction, the forces that cause them, the regions in which they occur, and their effect. Winds have various aspects: velocity (wind speed); the density of the gas involved; energy content or wind energy. The wind is also a critical means of transportation for seeds, insects, and birds, which can travel on wind currents for thousands of miles. In meteorology, winds are often referred to according to their strength, and the direction from which the wind is blowing. Short bursts of high speed wind are termed gusts. Strong winds of intermediate duration (around one minute) are termed squalls. Long-duration winds have various names associated with their average strength, such as breeze, gale, storm, and hurricane. In outer space, solar wind is the movement of gases or charged particles from the Sun through space, while planetary wind is the outgassing of light chemical elements from a planet's atmosphere into space. The strongest observed winds on a planet in the Solar System occur on Neptune and Saturn.
In human civilization, the concept of wind has been explored in mythology, influenced the events of history, expanded the range of transport and warfare, and provided a power source for mechanical work, electricity, and recreation. Wind powers the voyages of sailing ships across Earth's oceans. Hot air balloons use the wind to take short trips, and powered flight uses it to increase lift and reduce fuel consumption. Areas of wind shear caused by various weather phenomena can lead to dangerous situations for aircraft. When winds become strong, trees and human-made structures are damaged or destroyed.
Winds can shape landforms, via a variety of aeolian processes such as the formation of fertile soils, for example loess, and by erosion. Dust from large deserts can be moved great distances from its source region by the prevailing winds; winds that are accelerated by rough topography and associated with dust outbreaks have been assigned regional names in various parts of the world because of their significant effects on those regions. Wind also affects the spread of wildfires. Winds can disperse seeds from various plants, enabling the survival and dispersal of those plant species, as well as flying insect populations. When combined with cold temperatures, the wind has a negative impact on livestock. Wind affects animals' food stores, as well as their hunting and defensive strategies.
Details
Wind, in climatology, is the movement of air relative to the surface of the Earth. Winds play a significant role in determining and controlling climate and weather. A brief treatment of winds follows.
Wind occurs because of horizontal and vertical differences (gradients) in atmospheric pressure. Accordingly, the distribution of winds is closely related to that of pressure. Near the Earth’s surface, winds generally flow around regions of relatively low and high pressure—cyclones and anticyclones, respectively. They rotate counterclockwise around lows in the Northern Hemisphere and clockwise around those in the Southern Hemisphere. Similarly, wind systems rotate around the centres of highs in the opposite direction.
In the middle and upper troposphere, the pressure systems are organized in a sequence of high-pressure ridges and low-pressure troughs, rather than in the closed, roughly circular systems nearer the surface of the Earth. They have a wavelike motion and interact to form a rather complex series of ridges and troughs. The largest of the wave patterns are the so-called standing waves that have three or four ridges and a corresponding number of troughs in a broad band in middle latitudes of the Northern Hemisphere. The westerlies of the Southern Hemisphere are much less strongly affected by standing disturbances. Associated with these long standing waves are the short waves (several hundred kilometres in wavelength) called traveling waves. Such traveling waves form the upper parts of near-surface cyclones and anticyclones to which they are linked, thus guiding their movement and development.
At high latitudes the winds are generally easterly near the ground. In low, tropical, and equatorial latitudes, the northeasterly trade winds north of the intertropical convergence zone (ICZ), or thermal equator, and the southeasterly trade winds south of the ICZ move toward the ICZ, which migrates north and south with the seasonal position of the Sun. Vertically, winds then rise and create towering cumulonimbus clouds and heavy rain on either side of the ICZ, which marks a narrow belt of near calms known as the doldrums. The winds then move poleward near the top of the troposphere before sinking again in the subtropical belts in each hemisphere. From here, winds again move toward the Equator as trade winds. These gigantic cells with overturning air in each of the hemispheres in low latitudes are known as the Hadley cells. In the mid-latitudes, oppositely rotating wind systems called Ferrel cells carry surface air poleward and upper tropospheric air toward the Hadley cells. The three-dimensional pattern of winds over the Earth, known as general circulation, is responsible for the fundamental latitudinal structure of pressure and air movement and, hence, of climates.
On a smaller scale are the local winds, systems that are associated with specific geographic locations and reflect the influence of topographic features. The most common of these local wind systems are the sea and land breezes, mountain and valley breezes, foehn winds (also called chinook, or Santa Ana, winds), and katabatic winds. Local winds exert a pronounced influence on local climate and are themselves affected by local weather conditions.
Wind speeds and gustiness are generally strongest by day when the heating of the ground by the Sun causes overturning of the air, the descending currents conserving the angular momentum of high-altitude winds. By night, the gustiness dies down and winds are generally lighter.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1270 2022-01-31 01:02:46
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,810
Re: Miscellany
1246) Olfactory system
Summary
Olfactory system is the bodily structures that serve the sense of smell. The system consists of the nose and the nasal cavities, which in their upper parts support the olfactory mucous membrane for the perception of smell and in their lower parts act as respiratory passages.
The bony framework of the nose is part of the skull, but the outer nose is supported only by bone above; lower down, its shape is kept by cartilaginous plates. The expanded lower part of the side of the nose, the ala, is formed only of skin, both externally and internally, with fibrofatty tissue between the layers. The nasal cavities are separated by a septum covered in its lower two-thirds by thick, highly vascular mucous membrane composed of columnar ciliated epithelium with masses of acinous glands embedded in it, while in its upper part it is covered by the less vascular but more specialized olfactory membrane. Near the front of the lower part of the septum a slight opening into a short blind tube, which runs upward and backward, may sometimes be found; this is the vestigial remnant of Jacobson’s organ. The supporting framework of the septum is made up of ethmoid above, vomer below, and the septal cartilage in front. The outer wall of each nasal cavity is divided into three meatuses by the overhanging turbinated bones. Above the superior turbinated bone is a space between it and the roof known as the recessus sphenoethmoidalis, into the back of which the sphenoidal air sinus opens.
Between the superior and middle turbinated bones is the superior meatus, which contains the openings of the posterior ethmoidal air cells, while between the middle and inferior turbinated bones is the middle meatus, which is the largest of the three and contains a rounded elevation, the bulla ethmoidalis. Above and behind this is often an opening for the middle ethmoidal cells; below and in front runs a deep sickle-shaped gutter, the hiatus semilunaris, which communicates above with the frontal air sinus and below with the opening into the antrum of Highmore or maxillary antrum. The inferior meatus is below the inferior turbinated bone, and, when that is lifted, the valvular opening of the nasal duct is seen. The roof of the nose is narrow, and it is here that the olfactory nerves pass in through the cribriform plate. The floor is wider so that a coronal section through each nasal cavity has roughly the appearance of a right-angled triangle.
Embryology
In the third week of intrauterine development, two nasal pits appear on the underside of the front of the head; they are the first appearance of the true olfactory region of the nose, and some of their epithelial lining cells send off axons which arborize with the dendrites of the cells of the olfactory lobe of the brain and so form the olfactory nerves. Between the olfactory pits the broad median frontonasal process grows down from the forehead region to form the dorsum of the nose and the anterior part of the nasal septum, while outside them the lateral nasal processes grow down and meet the maxillary processes from the first visceral arch. In this way the nasal cavities are formed, but they are separated from the mouth by a thin bucconasal membrane which eventually is broken through; after this the mouth and nose are one cavity until the formation of the palate in the third month. In the third month Jacobson’s organ may be seen as a well-marked tube lined with respiratory mucous membrane; no explanation of the function of Jacobson’s organ in humans is known, and it is probably entirely atavistic. At birth the nasal cavities are shallow from above downward but rapidly deepen until the age of puberty.
Comparative anatomy
In the lancelet there is a ciliated pit above the anterior end of the central nervous system, which is probably a rudiment of an unpaired olfactory organ. In lampreys the pit is at first ventral but later becomes dorsal and shares a common opening with the pituitary invagination. It furthermore becomes divided internally into two lateral halves. In fishes there are also two lateral pits, the nostrils of which open sometimes, as in the sharks and rays, onto the ventral surface of the snout and sometimes, as in the higher fishes, onto the dorsal surface. Up to this stage, the olfactory organs are mere pits, but in mudfish an opening is established from them into the front of the roof of the mouth, and so they serve as respiratory passages and organs for the sense of smell. In the higher amphibians the nasal organ becomes included in the skull, and respiratory and olfactory parts are distinguished. In this class, too, turbinal ingrowths are found, and the nasolachrymal duct appears.
In lizards the olfactory and respiratory parts are very distinct, the latter being lined only by stratified epithelium unconnected with the olfactory nerves. There is one true turbinal bone growing from the outer wall, and close to this is a large nasal gland. In crocodiles the hard palate is formed, and there is henceforward a considerable distance between the openings of the external and internal nares. In crocodiles, also, air sinuses are first found extending from the olfactory cavities into the skull bones.
The birds’ arrangement is very like that of the reptiles; olfactory and respiratory chambers are present, and into the latter projects the true turbinal, though there is a pseudoturbinal in the upper or olfactory chamber. In mammals the olfactory chamber of the nose is variously developed: most of them are macrosmatic and have a large area of olfactory mucous membrane; some, like seals, baleen whales, monkeys, and humans, are microsmatic, while the toothed whales have the olfactory region practically suppressed in the adult and are said to be anosmatic. There are generally five turbinal bones in macrosmatic mammals, although humans have a reduced number. The lowest of the series, the maxilloturbinal, is the equivalent of the single true turbinal bone of birds and reptiles and in most mammals is a double scroll, one leaf turning upward and the other down.
Jacobson’s organ first appears in amphibians, where it is found as an anteroposterior gutter in the floor of the nasal cavity. In reptiles the roof of the gutter closes in on each side, and a tube is formed lying below and internal to the nasal cavity, opening anteriorly into the mouth and ending by a blind extremity, posteriorly to which branches of the olfactory and trigeminal nerves are distributed. In the higher reptiles (crocodiles, turtles, and tortoises) and in birds the organ is suppressed in the adult. In the lower mammals, especially the monotremes, it is very well developed and is enclosed in a cartilaginous sheath, from which a turbinal process projects into its interior. In other mammals, with the exception of primates and perhaps bats, the organ is quite distinct, though even in humans, as has been shown, its presence can be demonstrated in the embryo.
Nervous pathways of smell
The pathway of olfactory conduction begins with the olfactory receptors—small, slender nerve cells embedded in large numbers (about 100 million in the rabbit) in the epithelium of the mucous membrane lining the upper part of the nasal cavity. Each olfactory receptor cell emits two processes (projections). One of these is a short peripheral dendrite, which reaches to the surface of the epithelium, where it ends in a knob carrying a number of fine radially placed filaments, the olfactory hairs. The other process is a long and extremely thin axon, the olfactory nerve fibre, which reaches the cranial cavity by passing through one of the openings in the bony roof of the nasal cavity and enters the olfactory bulb of the forebrain. Sensations of smell are experienced when certain chemical substances become dissolved in the thin layer of fluid covering the surface of the mucous membrane and thus come in contact with the olfactory hairs. In all probability it will be found that the receptor cells differ among themselves in their sensitivities to various odorous substances.
In the olfactory bulb, the olfactory nerve fibres end in contact with the antenna-shaped dendrites of the large mitral cells, which represent the second main link in the chain of olfactory conduction. Each mitral cell emits a long axon, many of which enter into the formation of the olfactory tract, a white fibre band extending back from the bulb over the basal surface of the forebrain. The olfactory tract distributes its fibres mainly to the cortex of the pyriform lobe, which constitutes the final cortical receiving area of the olfactory pathway. In humans this region corresponds to the uncus of the hippocampal gyrus. A smaller number of fibres of the olfactory tract end in two further olfactory structures; the olfactory tubercle and the medial part of the amygdaloid complex (the latter lies deep to the olfactory cortex).
In mammals with a highly developed sense of smell (macrosmatic mammals), such as rodents, the olfactory brain structures are relatively large and occupy all or a large part of the basal surface of the forebrain. A marked reduction of all olfactory structures is evident in the microsmatic primates (monkeys, apes, and humans), which for their orientation rely more heavily upon the senses of vision and touch.
Details
The olfactory system, or sense of smell, is the sensory system used for smelling (olfaction). Olfaction is one of the special senses, that have directly associated specific organs. Most mammals and reptiles have a main olfactory system and an accessory olfactory system. The main olfactory system detects airborne substances, while the accessory system senses fluid-phase stimuli.
The senses of smell and taste (gustatory system) are often referred to together as the chemosensory system, because they both give the brain information about the chemical composition of objects through a process called transduction.
Structure:
Peripheral
The peripheral olfactory system consists mainly of the nostrils, ethmoid bone, nasal cavity, and the olfactory epithelium (layers of thin tissue covered in mucus that line the nasal cavity). The primary components of the layers of epithelial tissue are the mucous membranes, olfactory glands, olfactory neurons, and nerve fibers of the olfactory nerves.
Odor molecules can enter the peripheral pathway and reach the nasal cavity either through the nostrils when inhaling (olfaction) or through the throat when the tongue pushes air to the back of the nasal cavity while chewing or swallowing (retro-nasal olfaction). Inside the nasal cavity, mucus lining the walls of the cavity dissolves odor molecules. Mucus also covers the olfactory epithelium, which contains mucous membranes that produce and store mucus and olfactory glands that secrete metabolic enzymes found in the mucus.
Transduction
Olfactory sensory neurons in the epithelium detect odor molecules dissolved in the mucus and transmit information about the odor to the brain in a process called sensory transduction. Olfactory neurons have cilia (tiny hairs) containing olfactory receptors that bind to odor molecules, causing an electrical response that spreads through the sensory neuron to the olfactory nerve fibers at the back of the nasal cavity.
Olfactory nerves and fibers transmit information about odors from the peripheral olfactory system to the central olfactory system of the brain, which is separated from the epithelium by the cribriform plate of the ethmoid bone. Olfactory nerve fibers, which originate in the epithelium, pass through the cribriform plate, connecting the epithelium to the brain's limbic system at the olfactory bulbs.
Central
The main olfactory bulb transmits pulses to both mitral and tufted cells, which help determine odor concentration based on the time certain neuron clusters fire (called 'timing code'). These cells also note differences between highly similar odors and use that data to aid in later recognition. The cells are different with mitral having low firing-rates and being easily inhibited by neighboring cells, while tufted have high rates of firing and are more difficult to inhibit. How the bulbar neural circuit transforms odor inputs to the bulb to the bulbar responses that are sent to the olfactory cortex can be partly understood by a mathematical model.
The uncus houses the olfactory cortex which includes the piriform cortex (posterior orbitofrontal cortex), amygdala, olfactory tubercle, and parahippocampal gyrus.
The olfactory tubercle connects to numerous areas of the amygdala, thalamus, hypothalamus, hippocampus, brain stem, retina, auditory cortex, and olfactory system. *In total it has 27 inputs and 20 outputs. An oversimplification of its role is to state that it: checks to ensure odor signals arose from actual odors rather than villi irritation, regulates motor behavior (primarily social and stereotypical) brought on by odors, integrates auditory and olfactory sensory info to complete the aforementioned tasks, and plays a role in transmitting positive signals to reward sensors (and is thus involved in addiction).
The amygdala (in olfaction) processes pheromone, allomone, and kairomone (same-species, cross-species, and cross-species where the emitter is harmed and the sensor is benefited, respectively) signals. Due to cerebrum evolution this processing is secondary and therefore is largely unnoticed in human interactions. Allomones include flower scents, natural herbicides, and natural toxic plant chemicals. The info for these processes comes from the vomeronasal organ indirectly via the olfactory bulb. The main olfactory bulb's pulses in the amygdala are used to pair odors to names and recognize odor to odor differences.
Stria terminalis, specifically bed nuclei (BNST), act as the information pathway between the amygdala and hypothalamus, as well as the hypothalamus and pituitary gland. BNST abnormalities often lead to physical relation confusion and immaturity. BNST also connects to the septal area, rewarding physical relation behavior.
Mitral pulses to the hypothalamus promote/discourage feeding, whereas accessory olfactory bulb pulses regulate reproductive and odor-related-reflex processes.
The hippocampus (although minimally connected to the main olfactory bulb) receives almost all of its olfactory information via the amygdala (either directly or via the BNST). The hippocampus forms new and reinforces existing memories.
Similarly, the parahippocampus encodes, recognizes and contextualizes scenes. The parahippocampal gyrus houses the topographical map for olfaction.
The orbitofrontal cortex (OFC) is heavily correlated with the cingulate gyrus and septal area to act out positive/negative reinforcement. The OFC is the expectation of reward/punishment in response to stimuli. The OFC represents the emotion and reward in decision making.
The anterior olfactory nucleus distributes reciprocal signals between the olfactory bulb and piriform cortex. The anterior olfactory nucleus is the memory hub for smell.
When different odor objects or components are mixed, humans and other mammals sniffing the mixture (presented by, e.g., a sniff bottle) are often unable to identify the components in the mixture even though they can recognize each individual component presented alone. This is largely because each odor sensory neuron can be excited by multiple odor components. It has been proposed that, in an olfactory environment typically composed of multiple odor components (e.g., odor of a dog entering a kitchen that contains a background coffee odor), feedback from the olfactory cortex to the olfactory bulb suppresses the pre-existing odor background (e.g., coffee) via olfactory adaptation, so that the newly arrived foreground odor (e.g., dog) can be singled out from the mixture for recognition.
Clinical significance
Loss of smell is known as anosmia. Anosmia can occur on both sides or a single side.
Olfactory problems can be divided into different types based on their malfunction. The olfactory dysfunction can be total (anosmia), incomplete (partial anosmia, hyposmia, or microsmia), distorted (dysosmia), or can be characterized by spontaneous sensations like phantosmia. An inability to recognize odors despite a normally functioning olfactory system is termed olfactory agnosia. Hyperosmia is a rare condition typified by an abnormally heightened sense of smell. Like vision and hearing, the olfactory problems can be bilateral or unilateral meaning if a person has anosmia on the right side of the nose but not the left, it is a unilateral right anosmia. On the other hand, if it is on both sides of the nose it is called bilateral anosmia or total anosmia.
Destruction to olfactory bulb, tract, and primary cortex (brodmann area 34) results in anosmia on the same side as the destruction. Also, irritative lesion of the uncus results in olfactory hallucinations.
Damage to the olfactory system can occur by traumatic brain injury, cancer, infection, inhalation of toxic fumes, or neurodegenerative diseases such as Parkinson's disease and Alzheimer's disease. These conditions can cause anosmia. In contrast, recent finding suggested the molecular aspects of olfactory dysfunction can be recognized as a hallmark of amyloidogenesis-related diseases and there may even be a causal link through the disruption of multivalent metal ion transport and storage. Doctors can detect damage to the olfactory system by presenting the patient with odors via a scratch and sniff card or by having the patient close their eyes and try to identify commonly available odors like coffee or peppermint candy. Doctors must exclude other diseases that inhibit or eliminate 'the sense of smell' such as chronic colds or sinusitus before making the diagnosis that there is permanent damage to the olfactory system.
Prevalence of olfactory dysfunction in the general US population was assessed by questionnaire and examination in a national health survey in 2012-2014. Among over a thousand persons aged 40 years and older, 12.0% reported a problem with smell in the past 12 months and 12.4% had olfactory dysfunction on examination. Prevalence rose from 4.2% at age 40-49 to 39.4% at 80 years and older and was higher in men than women, in blacks and Mexican Americans than in whites and in less than more educated. Of concern for safety, 20% of persons aged 70 and older were unable to identify smoke and 31%, natural gas.
Causes of olfactory dysfunction
The common causes of olfactory dysfunction: advanced age, viral infections, exposure to toxic chemicals, head trauma, and neurodegenerative diseases.
Age
Age is the strongest reason for olfactory decline in healthy adults, having even greater impact than does cigarette smoking. Age-related changes in smell function often go unnoticed and smell ability is rarely tested clinically unlike hearing and vision. 2% of people under 65 years of age have chronic smelling problems. This increases greatly between people of ages 65 and 80 with about half experiencing significant problems smelling. Then for adults over 80, the numbers rise to almost 75%. The basis for age-related changes in smell function include closure of the cribriform plate, and cumulative damage to the olfactory receptors from repeated viral and other insults throughout life.
Viral infections
The most common cause of permanent hyposmia and anosmia are upper respiratory infections. Such dysfunctions show no change over time and can sometimes reflect damage not only to the olfactory epithelium, but also to the central olfactory structures as a result of viral invasions into the brain. Among these virus-related disorders are the common cold, hepatitis, influenza and influenza-like illness, as well as herpes. Notably, COVID-19 is associated with olfactory disturbance. Most viral infections are unrecognizable because they are so mild or entirely asymptomatic.
Exposure to toxic chemicals
Chronic exposure to some airborne toxins such as herbicides, pesticides, solvents, and heavy metals (cadmium, chromium, nickel, and manganese), can alter the ability to smell. These agents not only damage the olfactory epithelium, but they are likely to enter the brain via the olfactory mucosa.
Head trauma
Trauma-related olfactory dysfunction depends on the severity of the trauma and whether strong acceleration/deceleration of the head occurred. Occipital and side impact causes more damage to the olfactory system than frontal impact. However, recent evidence from individuals with traumatic brain injury suggests that smell loss can occur with changes in brain function outside of olfactory cortex.
Neurodegenerative diseases
Neurologists have observed that olfactory dysfunction is a cardinal feature of several neurodegenerative diseases such as Alzheimer's disease and Parkinson's disease. Most of these patients are unaware of an olfactory deficit until after testing where 85% to 90% of early-stage patients showed decrease activity in central odor processing structures.
Other neurodegenerative diseases that affect olfactory dysfunction include Huntington's disease, multi-infarct dementia, amyotrophic lateral sclerosis, and schizophrenia. These diseases have more moderate effects on the olfactory system than Alzheimer's or Parkinson's diseases. Furthermore, progressive supranuclear palsy and parkinsonism are associated with only minor olfactory problems. These findings have led to the suggestion that olfactory testing may help in the diagnosis of several different neurodegenerative diseases.
Neurodegenerative diseases with well-established genetic determinants are also associated with olfactory dysfunction. Such dysfunction, for example, is found in patients with familial Parkinson's disease and those with Down syndrome. Further studies have concluded that the olfactory loss may be associated with intellectual disability, rather than any Alzheimer's disease-like pathology.
Huntington's disease is also associated with problems in odor identification, detection, discrimination, and memory. The problem is prevalent once the phenotypic elements of the disorder appear, although it is unknown how far in advance the olfactory loss precedes the phenotypic expression.
History
Linda B. Buck and Richard Axel won the 2004 Nobel Prize in Physiology or Medicine for their work on the olfactory system.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1271 2022-02-01 01:24:15
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,810
Re: Miscellany
1247) Landslide
Summary
Landslide, also called landslip, is the movement downslope of a mass of rock, debris, earth, or soil (soil being a mixture of earth and debris). Landslides occur when gravitational and other types of shear stresses within a slope exceed the shear strength (resistance to shearing) of the materials that form the slope.
Shear stresses can be built up within a slope by a number of processes. These include oversteepening of the base of the slope, such as by natural erosion or excavation, and loading of the slope, such as by an inflow of water, a rise in the groundwater table, or the accumulation of debris on the slope’s surface. Short-term stresses, such as those imposed by earthquakes and rainstorms, can likewise contribute to the activation of landslides. Landslides can also be activated by processes that weaken the shear strength of a slope’s material. Shear strength is dependent mainly on two factors: frictional strength, which is the resistance to movement between the slope material’s interacting constituent particles, and cohesive strength, which is the bonding between the particles. Coarse particles such as sand grains have high frictional strength but low cohesive strength, whereas the opposite is true for clays, which are composed of fine particles. Another factor that affects the shear strength of a slope-forming material is the spatial disposition of its constituent particles, referred to as the sediment fabric. Some materials with a loose, open sediment fabric will weaken if they are mechanically disturbed or flooded with water. An increase in water content, resulting from either natural causes or human activity, typically weakens sandy materials through the reduction of interparticle friction and weakens clays through the dissolution of interparticle cements, the hydration of clay minerals, and the elimination of interparticle (capillary) tension.
Types of landslides
Landslides are generally classified by type of movement (slides, flows, spreads, topples, or falls) and type of material (rock, debris, or earth). Sometimes more than one type of movement occurs within a single landslide, and, because the temporal and spatial relationships of these movements are often complex, their analysis often requires detailed interpretation of both landforms and geological sections, or cores.
Rockslides and other types of slides involve the displacement of material along one or more discrete shearing surfaces. The sliding can extend downward and outward along a broadly planar surface (a translational slide), or it can be rotational along a concave-upward set of shear surfaces (a slump). A translational slide typically takes place along structural features, such as a bedding plane or the interface between resistant bedrock and weaker overlying material. If the overlying material moves as a single, little-deformed mass, it is called a block slide. A translational slide is sometimes called a mud slide when it occurs along gently sloping, discrete shear planes in fine-grained rocks (such as fissured clays) and the displaced mass is fluidized by an increase in pore water pressure. In a rotational slide the axis of rotation is roughly parallel to the contours of the slope. The movement near the head of the slide is largely downward, exposing a steep head scarp, and movement within the displaced mass takes place along internal slip planes, each tending to tilt backward. Over time, upslope ponding of water by such back-tilted blocks can enlarge the area of instability, so that a stable condition is reached only when the slope is reduced to a very low gradient.
A type of landslide in which the distribution of particle velocities resembles that of a viscous fluid is called a flow. The most important fluidizing agent is water, but trapped air is sometimes involved. Contact between the flowing mass and the underlying material can be distinct, or the contact can be one of diffuse shear. The difference between slides and flows is gradational, with variations in fluid content, mobility, and type of movement, and composite slide movement and flow movement are common.
A spread is the complex lateral movement of relatively coherent earth materials resting on a weaker substrate that is subject to liquefaction or plastic flow. Coherent blocks of material subside into the weaker substrate, and the slow downslope movement frequently extends long distances as a result of the retrogressive extension from the zone of origin, such as an eroding riverbank or coastline. Spreads occur as the result of liquefaction caused by water saturation or earthquake shock in such substrates as loess, a weakly cemented wind-lain silt.
Rotation of a mass of rock, debris, or earth outward from a steep slope face is called toppling. This type of movement can subsequently cause the mass to fall or slide.
Earth materials can become detached from a steep slope without significant shearing, fall freely under gravity, and land on a surface from which they bounce and fall farther. Falls of large volume can trap enough air to facilitate the very rapid flow of rock or debris, forming rock avalanches and debris avalanches, respectively. Entrapped snow and ice may also help mobilize such flows, but the unqualified term avalanche is generally used to refer only to an avalanche of snow. Triggered by earthquake shock or torrential rain in mountainous relief with steep gradients, a huge volume of avalanching rock or debris (of up to millions of metric tons) can reach a velocity of more than 50 metres (160 feet) per second and leave a long trail of destruction.
Landslide mitigation and prevention
Landslides pose a recurrent hazard to human life and livelihood in most parts of the world, especially in some regions that have experienced rapid population and economic growth. Hazards are mitigated mainly through precautionary means—for instance, by restricting or even removing populations from areas with a history of landslides, by restricting certain types of land use where slope stability is in question, and by installing early warning systems based on the monitoring of ground conditions such as strain in rocks and soils, slope displacement, and groundwater levels. There are also various direct methods of preventing landslides; these include modifying slope geometry, using chemical agents to reinforce slope material, installing structures such as piles and retaining walls, grouting rock joints and fissures, diverting debris pathways, and rerouting surface and underwater drainage. Such direct methods are constrained by cost, landslide magnitude and frequency, and the size of human settlements at risk.
Landslides, also known as landslips, are several forms of mass wasting that may include a wide range of ground movements, such as rockfalls, deep-seated slope failures, mudflows, and debris flows. Landslides occur in a variety of environments, characterized by either steep or gentle slope gradients, from mountain ranges to coastal cliffs or even underwater, in which case they are called submarine landslides. Gravity is the primary driving force for a landslide to occur, but there are other factors affecting slope stability that produce specific conditions that make a slope prone to failure. In many cases, the landslide is triggered by a specific event (such as a heavy rainfall, an earthquake, a slope cut to build a road, and many others), although this is not always identifiable.
Details
Landslides, also known as landslips, are several forms of mass wasting that may include a wide range of ground movements, such as rockfalls, deep-seated slope failures, mudflows, and debris flows. Landslides occur in a variety of environments, characterized by either steep or gentle slope gradients, from mountain ranges to coastal cliffs or even underwater, in which case they are called submarine landslides. Gravity is the primary driving force for a landslide to occur, but there are other factors affecting slope stability that produce specific conditions that make a slope prone to failure. In many cases, the landslide is triggered by a specific event (such as a heavy rainfall, an earthquake, a slope cut to build a road, and many others), although this is not always identifiable.
Causes
Landslides occur when the slope (or a portion of it) undergoes some processes that change its condition from stable to unstable. This is essentially due to a decrease in the shear strength of the slope material, an increase in the shear stress borne by the material, or a combination of the two. A change in the stability of a slope can be caused by a number of factors, acting together or alone. Natural causes of landslides include:
* saturation by rain water infiltration, snow melting, or glaciers melting;
* rising of groundwater or increase of pore water pressure (e.g. due to aquifer recharge in rainy seasons, or by rain water infiltration);
* increase of hydrostatic pressure in cracks and fractures;
* loss or absence of vertical vegetative structure, soil nutrients, and soil structure (e.g. after a wildfire – a fire in forests lasting for 3–4 days);
* erosion of the top of a slope by rivers or sea waves;
* physical and chemical weathering (e.g. by repeated freezing and thawing, heating and cooling, salt leaking in the groundwater or mineral dissolution);
* ground shaking caused by earthquakes, which can destabilize the slope directly (e.g., by inducing soil liquefaction) or weaken the material and cause cracks that will eventually produce a landslide;
* volcanic eruptions;
Landslides are aggravated by human activities, such as:
* deforestation, cultivation and construction;
* vibrations from machinery or traffic;
* blasting and mining;
* earthwork (e.g. by altering the shape of a slope, or imposing new loads);
* in shallow soils, the removal of deep-rooted vegetation that binds colluvium to bedrock;
* agricultural or forestry activities (logging), and urbanization, which change the amount of water infiltrating the soil.
* temporal variation in land use and land cover (LULC): it includes the human abandonment of farming areas, e.g. due to the economic and social transformations which occurred in Europe after the Second World War. Land degradation and extreme rainfall can increase the frequency of erosion and landslide phenomena.
Each type can be seen both in rock and in soil. A fall is a movement of isolated blocks or chunks of soil in free-fall. The term topple refers to blocks coming away by rotation from a vertical face. A slide is the movement of a body of material that generally remains intact while moving over one or several inclined surfaces or thin layers of material (also called shear zones) in which large deformations are concentrated. Slides are also sub-classified by the form of the surface(s) or shear zone(s) on which movement happens. The planes may be broadly parallel to the surface ("planar slides") or spoon-shaped ("rotational slides"). Slides can occur catastrophically, but movement on the surface can also be gradual and progressive. Spreads are a form of subsidence, in which a layer of material cracks, opens up, and expands laterally. Flows are the movement of fluidised material, which can be both dry or rich in water (such as in mud flows). Flows can move imperceptibly for years, or accelerate rapidly and cause disasters. Slope deformations are slow, distributed movements that can affect entire mountain slopes or portions of it. Some landslides are complex in the sense that they feature different movement types in different portions of the moving body, or they evolve from one movement type to another over time. For example, a landslide can initiate as a rock fall or topple and then, as the blocks disintegrate upon the impact, transform into a debris slide or flow. An avalanching effect can also be present, in which the moving mass entrains additional material along its path.
Flows
Slope material that becomes saturated with water may produce a debris flow or mud flow. However, also dry debris can exhibit flow-like movement. Flowing debris or mud may pick up trees, houses and cars, and block bridges and rivers causing flooding along its path. This phenomenon is particularly hazardous in alpine areas, where narrow gorges and steep valleys are conducive of faster flows. Debris and mud flows may initiate on the slopes or result from the fluidization of landslide material as it gains speed or incorporates further debris and water along its path. River blockages as the flow reaches a main stream can generate temporary dams. As the impoundments fail, a domino effect may be created, with a remarkable growth in the volume of the flowing mass, and in its destructive power.
The Costa della Gaveta earthflow in Potenza, Italy. Even though it moves at a rate of just a few millimeters per year and is hardly visible, this landslide causes progressive damage to the national road, the national highway, a flyover, and several houses that are built on it.
An earthflow is the downslope movement of mostly fine-grained material. Earthflows can move at speeds within a very wide range, from as low as 1 mm/yr. to many km/h. Though these are a lot like mudflows, overall they are more slow-moving and are covered with solid material carried along by the flow from within. Clay, fine sand and silt, and fine-grained, pyroclastic material are all susceptible to earthflows. These flows are usually controlled by the pore water pressures within the mass, which should be high enough to produce a low shearing resistance. On the slopes, some earthflow may be recognized by their elongated shape, with one or more lobes at their toes. As these lobes spread out, drainage of the mass increases and the margins dry out, lowering the overall velocity of the flow. This process also causes the flow to thicken. Earthflows occur more often during periods of high precipitation, which saturates the ground and builds up water pressures. However, earthflows that keep advancing also during dry seasons are not uncommon. Fissures may develop during the movement of clayey materials, which facilitate the intrusion of water into the moving mass and produce faster responses to precipitation.
A rock avalanche, sometimes referred to as sturzstrom, is a large and fast-moving landslide of the flow type. It is rarer than other types of landslides but it is often very destructive. It exhibits typically a long runout, flowing very far over a low-angle, flat, or even slightly uphill terrain. The mechanisms favoring the long runout can be different, but they typically result in the weakening of the sliding mass as the speed increases. The causes of this weakening are not completely understood. Especially for the largest landslides, it may involve the very quick heating of the shear zone due to friction, which may even cause the water that is present to vaporize and build up a large pressure, producing a sort of hovercraft effect. In some cases, the very high temperature may even cause some of the minerals to melt. During the movement, the rock in the shear zone may also be finely ground, producing a nanometer-size mineral powder that may act as a lubricant, reducing the resistance to motion and promoting larger speeds and longer runouts. The weakening mechanisms in large rock avalanches are similar to those occurring in seismic faults.
Slides
Slides can occur in any rock or soil material and are characterized by the movement of a mass over a planar or curvilinear surface or shear zone.
A debris slide is a type of slide characterized by the chaotic movement of material mixed with water and/or ice. It is usually triggered by the saturation of thickly vegetated slopes which results in an incoherent mixture of broken timber, smaller vegetation and other debris. Debris flows and avalanches differ from debris slides because their movement is fluid-like and generally much more rapid. This is usually a result of lower shear resistances and steeper slopes. Debris slides generally begin with the detachment of rock chunks high on the slopes, which break apart as they slide towards the bottom.
Clay and silt slides are usually slow but can experience episodic acceleration in response to heavy rainfall or rapid snowmelt. They are often seen on gentle slopes and move over planar surfaces, such as over the underlying bedrock. Failure surfaces can also form within the clay or silt layer itself, and they usually have concave shapes, resulting in rotational slides.
Shallow and deep-seated landslides
A landslide in which the sliding surface is located within the soil mantle or weathered bedrock (typically to a depth from few decimeters to some meters) is called a shallow landslide. Debris slides and debris flows are usually shallow. Shallow landslides can often happen in areas that have slopes with high permeable soils on top of low permeable soils. The low permeable soil traps the water in the shallower soil generating high water pressures. As the top soil is filled with water, it can become unstable and slide downslope.
Deep-seated landslides are those in which the sliding surface is mostly deeply located, for instance well below the maximum rooting depth of trees. They usually involve deep regolith, weathered rock, and/or bedrock and include large slope failures associated with translational, rotational, or complex movements. They tend to form along a plane of weakness such as a fault or bedding plane. They can be visually identified by concave scarps at the top and steep areas at the toe.
Causing tsunamis
Landslides that occur undersea, or have impact into water e.g. significant rockfall or volcanic collapse into the sea, can generate tsunamis. Massive landslides can also generate megatsunamis, which are usually hundreds of meters high. In 1958, one such tsunami occurred in Lituya Bay in Alaska.
Related phenomena
* An avalanche, similar in mechanism to a landslide, involves a large amount of ice, snow and rock falling quickly down the side of a mountain.
* A pyroclastic flow is caused by a collapsing cloud of hot ash, gas and rocks from a volcanic explosion that moves rapidly down an erupting volcano.
Landslide prediction mapping
Landslide hazard analysis and mapping can provide useful information for catastrophic loss reduction, and assist in the development of guidelines for sustainable land-use planning. The analysis is used to identify the factors that are related to landslides, estimate the relative contribution of factors causing slope failures, establish a relation between the factors and landslides, and to predict the landslide hazard in the future based on such a relationship. The factors that have been used for landslide hazard analysis can usually be grouped into geomorphology, geology, land use/land cover, and hydrogeology. Since many factors are considered for landslide hazard mapping, GIS is an appropriate tool because it has functions of collection, storage, manipulation, display, and analysis of large amounts of spatially referenced data which can be handled fast and effectively. Cardenas reported evidence on the exhaustive use of GIS in conjunction of uncertainty modelling tools for landslide mapping. Remote sensing techniques are also highly employed for landslide hazard assessment and analysis. Before and after aerial photographs and satellite imagery are used to gather landslide characteristics, like distribution and classification, and factors like slope, lithology, and land use/land cover to be used to help predict future events. Before and after imagery also helps to reveal how the landscape changed after an event, what may have triggered the landslide, and shows the process of regeneration and recovery.
Using satellite imagery in combination with GIS and on-the-ground studies, it is possible to generate maps of likely occurrences of future landslides. Such maps should show the locations of previous events as well as clearly indicate the probable locations of future events. In general, to predict landslides, one must assume that their occurrence is determined by certain geologic factors, and that future landslides will occur under the same conditions as past events. Therefore, it is necessary to establish a relationship between the geomorphologic conditions in which the past events took place and the expected future conditions.
Natural disasters are a dramatic example of people living in conflict with the environment. Early predictions and warnings are essential for the reduction of property damage and loss of life. Because landslides occur frequently and can represent some of the most destructive forces on earth, it is imperative to have a good understanding as to what causes them and how people can either help prevent them from occurring or simply avoid them when they do occur. Sustainable land management and development is also an essential key to reducing the negative impacts felt by landslides.
A Wireline extensometer monitoring slope displacement and transmitting data remotely via radio or Wi-Fi. In situ or strategically deployed extensometers may be used to provide early warning of a potential landslide.
GIS offers a superior method for landslide analysis because it allows one to capture, store, manipulate, analyze, and display large amounts of data quickly and effectively. Because so many variables are involved, it is important to be able to overlay the many layers of data to develop a full and accurate portrayal of what is taking place on the Earth's surface. Researchers need to know which variables are the most important factors that trigger landslides in any given location. Using GIS, extremely detailed maps can be generated to show past events and likely future events which have the potential to save lives, property, and money.
Since the ‘90s, GIS have been also successfully used in conjunction to decision support systems, to show on a map real-time risk evaluations based on monitoring data gathered in the area of the Val Pola disaster (Italy).

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1272 2022-02-02 00:04:28
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,810
Re: Miscellany
1248) Embryology
Summary
Embryology is the branch of biology that studies the prenatal development of gametes, fertilization, and development of embryos and fetuses. Additionally, embryology encompasses the study of congenital disorders that occur before birth, known as teratology.
Early embryology was proposed by Marcello Malpighi, and known as preformationism, the theory that organisms develop from pre-existing miniature versions of themselves. Aristotle proposed the theory that is now accepted, epigenesis. Epigenesis is the idea that organisms develop from seed or egg in a sequence of steps. Modern embryology developed from the work of Karl Ernst von Baer, though accurate observations had been made in Italy by anatomists such as Aldrovandi and Leonardo da Vinci in the Renaissance.
Details
Embryology is the study of the formation and development of an embryo and fetus. Before widespread use of the microscope and the advent of cellular biology in the 19th century, embryology was based on descriptive and comparative studies. From the time of the Greek philosopher Aristotle it was debated whether the embryo was a preformed, miniature individual (a homunculus) or an undifferentiated form that gradually became specialized. Supporters of the latter theory included Aristotle; the English physician William Harvey, who labeled the theory epigenesis; the German physician Caspar Friedrick Wolff; and the Prussian-Estonian scientist Karl Ernst, Ritter von Baer, who proved epigenesis with his discovery of the mammalian ovum (egg) in 1827. Other pioneers were the French scientists Pierre Belon and Marie-François-Xavier Bichat.
Baer, who helped popularize Christian Heinrich Pander’s 1817 discovery of primary germ layers, laid the foundations of modern comparative embryology in his landmark two-volume work Über Entwickelungsgeschichte der Thiere (1828–37; “On the Development of Animals”). Another formative publication was A Treatise on Comparative Embryology (1880–91) by the British zoologist Frances Maitland Balfour. Further research on embryonic development was conducted by the German anatomists Martin H. Rathke and Wilhelm Roux and also by the American scientist Thomas Hunt Morgan. Roux, noted for his pioneering studies on frog eggs (beginning in 1885), became the founder of experimental embryology. The principle of embryonic induction was studied by the German embryologists Hans Adolf Eduard Driesch, who furthered Roux’s research on frog eggs in the 1890s, and Hans Spemann, who was awarded a Nobel Prize in 1935. Ross G. Harrison was an American biologist noted for his work on tissue culture.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1273 2022-02-03 00:43:42
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,810
Re: Miscellany
1249) Coagulation
Summary
Coagulation, also known as clotting, is the process by which blood changes from a liquid to a gel, forming a blood clot. It potentially results in hemostasis, the cessation of blood loss from a damaged vessel, followed by repair. The mechanism of coagulation involves activation, adhesion and aggregation of platelets, as well as deposition and maturation of fibrin.
Coagulation begins almost instantly after an injury to the endothelium lining a blood vessel. Exposure of blood to the subendothelial space initiates two processes: changes in platelets, and the exposure of subendothelial tissue factor to plasma factor VII, which ultimately leads to cross-linked fibrin formation. Platelets immediately form a plug at the site of injury; this is called primary hemostasis. Secondary hemostasis occurs simultaneously: additional coagulation (clotting) factors beyond factor VII (listed below) respond in a cascade to form fibrin strands, which strengthen the platelet plug.
Disorders of coagulation are disease states which can result in problems with hemorrhage, bruising, or thrombosis.
Coagulation is highly conserved throughout biology. In all mammals, coagulation involves both a cellular (platelet) and a protein (coagulation factor) component. The system in humans has been the most extensively researched and is the best understood.
Details
Coagulation, in physiology, is the process by which a blood clot is formed. The formation of a clot is often referred to as secondary hemostasis, because it forms the second stage in the process of arresting the loss of blood from a ruptured vessel. The first stage, primary hemostasis, is characterized by blood vessel constriction (vasoconstriction) and platelet aggregation at the site of vessel injury. Under abnormal circumstances, clots can also form in a vessel that has not been breached; such clots can result in the occlusion (blockage) of the vessel.
Clotting is a sequential process that involves the interaction of numerous blood components called coagulation factors. There are 13 principal coagulation factors in all, and each of these has been assigned a Roman numeral, I to XIII. Coagulation can be initiated through the activation of two separate pathways, designated extrinsic and intrinsic. Both pathways result in the production of factor X. The activation of this factor marks the beginning of the so-called common pathway of coagulation, which results in the formation of a clot.
The extrinsic pathway is generally the first pathway activated in the coagulation process and is stimulated in response to a protein called tissue factor, which is expressed by cells that are normally found external to blood vessels. However, when a blood vessel breaks and these cells come into contact with blood, tissue factor activates factor VII, forming factor VIIa, which triggers a cascade of reactions that result in the rapid production of factor X. In contrast, the intrinsic pathway is activated by injury that occurs within a blood vessel. This pathway begins with the activation of factor XII (Hageman factor), which occurs when blood circulates over injured internal surfaces of vessels. Components of the intrinsic pathway also may be activated by the extrinsic pathway; for example, in addition to activating factor X, factor VIIa activates factor IX, a necessary component of the intrinsic pathway. Such cross-activation serves to amplify the coagulation process.
The production of factor X results in the cleavage of prothrombin (factor II) to thrombin (factor IIa). Thrombin, in turn, catalyzes the conversion of fibrinogen (factor I)—a soluble plasma protein—into long, sticky threads of insoluble fibrin (factor Ia). The fibrin threads form a mesh that traps platelets, blood cells, and plasma. Within minutes, the fibrin meshwork begins to contract, squeezing out its fluid contents. This process, called clot retraction, is the final step in coagulation. It yields a resilient, insoluble clot that can withstand the friction of blood flow.
Introduction of coagulation
Blood coagulation is the process that blood changes from a flowing liquid state to a non-flowing jelly-like clot. This is a process of limited hydrolysis of a series of proteins involved by coagulation factors. The key process of blood coagulation is the conversion of fibrinogen in plasma to insoluble fibrin. Multimeric fibrin is interwoven into a network, and many blood cells are networked to form blood clots. Substances directly involved in blood clotting in blood and tissues are collectively referred to as coagulation factors. Blood coagulation has 12 kinds, and international nomenclatures for recognized them are numbered in Roman numerals. In addition, prekallikrein, kininogen and phospholipids derived from platelets are also directly involved in the blood coagulation process. In the coagulation factor, factor IV is an ion, and the remaining coagulation factors are proteins, wherein factors II, VII, IX, X, XI, and XII are endonucleases. Usually, in the blood, II, IX, X, XI, and XII are all in the form of inactive zymogens, which must be activated to be active. The activated enzymes are called active forms of these factors. One to two hours after the blood coagulation process, the blood clot shrinks and precipitates as a pale yellow liquid under the action of platelets. This liquid is called serum. Compared with plasma, serum lacks fibrinogen and a small amount of other plasma proteins involved in blood coagulation, and a small amount of substances released by platelets during hemagglutination.
Mechanism of coagulation
A lot of doctrines have been created about the blood coagulation mechanism. Until 1993, the International Blood Coagulation Factor Name Selection Committee published a pattern of human coagulation mechanisms that were widely accepted. According to the pattern, coagulation can be divided into four steps: 1. Prothrombin activator formation; 2. Thrombin formation; 3. Fibrin formation; 4. Fibrinolysis.
Prothrombin activator formation
From the beginning of coagulation to the formation of thrombin, it consists of endogenous and exogenous systems. Endogenous (intrinsic to blood) coagulation mechanism is a separate process of blood. When the blood comes into contact with the surface of the foreign body (collagen fiber of the blood vessel wall, etc.), the factor XII and factor XI of the contact factor are activated, and when the factor VI is activated, it activates the inactive factor IX. On the other hand, platelets also adhere to and agglomerate on the surface of foreign bodies and cause viscous metamorphosis to release platelet factor III. Immediately in the plasma, factor VIII and calcium ions react with these active factor XI and platelet factor III to activate the inactive factor X. And then factor V and platelet factor III act on factor X to convert prothrombin to thrombin. The mechanism of exogenous (tissue origin) is the process of tissue fluid entering the blood. The active component of tissue fluid promotes the interaction between thromboplastin and plasma factor VII, and then activates factor X. Finally, the factor V and calcium ion acts on activated factor X to convert prothrombin to thrombin.
Thrombin formation
The process by which prothrombin is converted to thrombin. Factor X and Factor V activated in the first step of coagulation and calcium ions acting on prothrombin break the bond of arginine-isoleucine in the prothrombin molecule to form thrombin.
Fibrin formation
The process by which fibrinogen is converted to a fibrin clot by the action of thrombin. Due to the action of thrombin, the arginine-glycine bond between the α bond and β bond in the fibrinogen molecule is cleaved, and the fibrin peptides A and B are released to form a fibrin monomer. Fibrin monomer polymerizes into a fibrin polymer. Factor VIII (transglutaminase) activated by the action of thrombin and calcium ions together with calcium ions promotes to form the bond between glutamine and lysine in the fibrin molecule. The process can form a strong fibrin block. In addition, in the third step of coagulation, the blood coagulates form a blood cake, but over time, due to the action of thrombus contractile proteins of the platelets, the blood cake shrinks.
Fibrinolysis
However, there is a fourth step in the body: a series of reactions involving fibrinolysis caused by plasmin, so these reactions are also included in the concept of blood coagulation.
Clinical Significance
The study of coagulation mechanisms has promoted the understanding of many hemorrhagic diseases, such as hemophilia (the patient's coagulation process is very slow or even minor damage is also bleeding), mainly due to the lack of factor VIII in plasma. In addition, it is found that coagulation factors II, VII, IX, and X are all synthesized in the liver, and vitamin K is required to participate in their formation. Lack of vitamin K, there will be bleeding tendency; the application of vitamin K can improve the symptoms of poor coagulation. In addition, in the laboratory or clinical work, different measures can be taken for each link in the blood coagulation process as needed to achieve the purpose of delaying coagulation or effectively stopping bleeding. If bleeding is prevented after surgery, coagulation substances such as thrombin and fibrin may be applied to the surgical site, and warm gauze, cotton or gelatin sponge may be used to pressurize the wound to promote coagulation.
Thrombosis is the pathological development of blood clots. These clots may break loose and become mobile, forming an embolus or grow to such a size that occludes the vessel in which it developed. An embolism is reported to occur when the thrombus (blood clot) becomes a mobile embolus and migrates to another part of the body, interfering with blood circulation and hence impairing organ function downstream of the occlusion. This causes ischemia and often leads to ischemic necrosis of tissue. Most cases of venous thrombosis are due to acquired states (older age, surgery, cancer, immobility) or inherited thrombophilias (e.g., antiphospholipid syndrome, factor V Leiden, and various other genetic deficiencies or variants).
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1274 2022-02-04 00:16:49
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,810
Re: Miscellany
1250) Heparin
Summary
Heparin, also known as unfractionated heparin (UFH), is a medication and naturally occurring glycosaminoglycan. Since heparins depend on the activity of antithrombin, they are considered anticoagulants.[4] Specifically it is also used in the treatment of heart attacks and unstable angina. It is given by injection into a vein or under the skin. Other uses include inside test tubes and kidney dialysis machines.
Common side effects include bleeding, pain at the injection site, and low blood platelets. Serious side effects include heparin-induced thrombocytopenia. Greater care is needed in those with poor kidney function.
Heparin is contraindicated for suspected cases of vaccine-induced pro-thrombotic immune thrombocytopenia (VIPIT) secondary to SARS-CoV-2 vaccination, as heparin may further increase the risk of bleeding in an anti-PF4/heparin complex autoimmune manner, in favor of alternative anticoagulant medications (such as argatroban or danaparoid).
Heparin appears to be relatively safe for use during pregnancy and breastfeeding. Heparin is produced by basophils and mast cells in all mammals.
The discovery of heparin was announced in 1916. It is on the World Health Organization's List of Essential Medicines. A fractionated version of heparin, known as low molecular weight heparin, is also available.
Details
Heparin is an anticoagulant drug that is used to prevent blood clots from forming during and after surgery and to treat various heart, lung, and circulatory disorders in which there is an increased risk of blood clot formation. Discovered in 1922 by American physiologist William Henry Howell, heparin is a naturally occurring mixture of mucopolysaccharides that is present in the human body in tissues of the liver and lungs. Most commercial heparin is obtained from cow lungs or pig intestines. Heparin was originally used to prevent the clotting of blood taken for laboratory tests. Its use as a therapy for patients who already have a blood clot in a vein (venous thrombosis) began in the 1940s; low-dose heparin treatment to prevent blood clots from forming in patients who are at high risk for pulmonary embolisms and other clotting disorders was introduced in the early 1970s.
The biological activity of heparin depends on the presence of antithrombin III, a substance in blood plasma that binds and deactivates serum clotting factors. Heparin is poorly absorbed by the intestine, so it must be given intravenously or subcutaneously. Because of its anticlotting effect, the drug creates a significant risk of excessive bleeding, which may be reversed with protamine, a protein that neutralizes heparin’s anticoagulant effect. Other adverse effects of heparin include thrombocytopenia (reduced number of circulating platelets) and hypersensitivity reactions.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
#1275 2022-02-04 13:45:50
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 52,810
Re: Miscellany
1251) Welcome Stranger
The Welcome Stranger is the biggest alluvial gold nugget found, which had a calculated refined weight of 97.14 kilograms (3,123 ozt). It measured 61 by 31 cm (24 by 12 in) and was discovered by prospectors John Deason and Richard Oates on 5 February 1869 at Moliagul, Victoria, Australia, about 14.6 kilometres (9 miles) north-west of Dunolly.
Discovery
Found only 3 cm (1.2 in) below the surface, near the base of a tree on a slope leading to what was then known as Bulldog Gully, the nugget had a gross weight of 109.59 kilograms (3,523.5 ozt) (241 lb 10 oz). Its trimmed weight was 78 kilograms (2,520 ozt) (210 lbs), and its net weight was 72.02 kilograms (2,315.5 ozt) (192 lbs 11.5 oz).
At the time of the discovery, there were no scales capable of weighing a nugget this large, so it was broken into three pieces on an anvil by Dunolly-based blacksmith Archibald Walls.
Deason, Oates, and a few friends took the nugget to the London Chartered Bank of Australia, in Dunolly, which advanced them £9,000. Deason and Oates were finally paid an estimated £9,381 for their nugget, which became known as the "Welcome Stranger". At August 2019 gold prices, it would be worth US$3.4 million [2.3 million GBP]. It was heavier than the "Welcome Nugget" of 69.0 kilograms (2,217 ozt) that had been found in Ballarat in 1858. The goldfields warden F. K. Orme reported that 70.57 kilograms (2,269 ozt) of smelted gold had been obtained from it, irrespective of scraps that were given away by the finders, estimated as totalling another 1.46 kilograms (47 ozt).
The nugget was soon melted down and the gold was sent as ingots to Melbourne for forwarding to the Bank of England. It left the country on board the steamship Reigate which departed on 21 February.
An obelisk commemorating the discovery of the "Welcome Stranger" was erected near the spot in 1897. A replica of the "Welcome Stranger" is in the Old Treasury building, Treasury Place, Melbourne, Victoria; another replica is owned by descendants of John Deason and is now on display at the Dunolly Rural Transaction Center.
Discoverers
John Deason was born in 1829 on the island of Tresco, Isles of Scilly, 45 km (28 mi) off the southwestern tip of Cornwall, England, UK. In 1851, he was a tin dresser before becoming a gold miner. Deason continued with gold mining and workings most of his life and, although he became a store keeper at Moliagul, he lost a substantial proportion of his wealth through poor investments in gold mining. He bought a small farm near Moliagul where he lived until he died in 1915, aged 85 years.
Richard Oates was born about 1827 at Pendeen in Cornwall. After the 1869 find, Oates returned to the UK and married. He returned to Australia with his wife and they had four children. The Oates family, in 1895, purchased 3.2 square kilometres (800 acres) of land at Marong, Victoria, about 24 kilometres (15 mi) west of Bendigo, Victoria, which Oates farmed until his death in Marong in 1906, aged 79 years.
Descendants of the two discoverers gathered to celebrate the 150th discovery of the nugget.

It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
