Math Is Fun Forum
You are not logged in.
- Topics: Active | Unanswered
#1 Re: This is Cool » Miscellany » Today 00:03:13
2512) Intraocular Lens
Gist
Intraocular lenses (IOLs) are tiny, artificial, permanent lenses implanted inside the eye to replace a natural lens removed during cataract surgery or to correct refractive errors like myopia, hyperopia, and astigmatism. Made of acrylic or silicone, they restore clear vision by focusing light on the retina without needing maintenance.
Intraocular lenses usually last a lifetime. How is an intraocular lens used in cataract surgery? Cataract surgery involves removing the eye's natural lens which has become cloudy (cataract) and replacing it with an intraocular lens.
Summary
An intraocular lens (or IOL) is a tiny, artificial lens for the eye. It replaces the eye's natural lens that is removed during cataract surgery.
The lens bends (refracts) light rays that enter the eye, helping you to see. Your lens should be clear. But if you have a cataract, your lens has become cloudy. Things look blurry, hazy or less colorful with a cataract. Cataract surgery removes this cloudy lens and replaces it with a clear IOL to improve your vision.
IOLs come in different focusing powers, just like prescription eyeglasses or contact lenses. Your ophthalmologist will measure the length of your eye and the curve of your cornea. These measurements are used to set your IOLs focusing power.
What are IOLs made of?
Most IOLs are made of silicone, acrylic, or other plastic compositions. They are also coated with a special material to help protect your eyes from the sun's harmful ultraviolet (UV) rays.
Monofocal IOLs
The most common type of lens used with cataract surgery is called a monofocal IOL. It has one focusing distance. It is set to focus for up close, medium range or distance vision. Most people have them set for clear distance vision. Then they wear eyeglasses for reading or close work.
Some IOLs have different focusing powers within the same lens. These are called presbyopia-correcting IOLs. These IOLs reduce your dependence on glasses by giving you clear vision for more than one set distance.
Multifocal IOLs
These IOLs provide both distance and near focus at the same time. The lens has different zones set at different powers.
Extended depth-of-focus IOLs:
Similar to multifocal lenses, extended depth-of-focus (EDOF) lenses sharpen near and far vision, but with only one corrective zone, which “extends” to cover both distances. This may mean less effort to re-focus between distances.
Accommodative IOLs
These lenses move or change shape inside your eye, allowing focusing at different distances.
Toric IOLs
For people with astigmatism, there is an IOL called a toric lens. Astigmatism is a refractive error caused by an uneven curve in your cornea or lens. The toric lens is designed to correct that refractive error.
Details
An intraocular lens (IOL) is a lens implanted in the eye usually as part of a treatment for cataracts or for correcting other vision problems such as near-sightedness (myopia) and far-sightedness (hyperopia); a form of refractive surgery. If the natural lens is left in the eye, the IOL is known as phakic, otherwise it is a pseudophakic lens (or false lens). Both kinds of IOLs are designed to provide the same light-focusing function as the natural crystalline lens. This can be an alternative to LASIK, but LASIK is not an alternative to an IOL for treatment of cataracts.
IOLs usually consist of a small plastic lens with plastic side struts, called haptics, to hold the lens in place in the capsular bag inside the eye. IOLs were originally made of a rigid material (PMMA), although this has largely been superseded by the use of flexible materials, such as silicone. Most IOLs fitted today are fixed monofocal lenses matched to distance vision. However, other types are available, such as a multifocal intraocular lens that provides multiple-focused vision at far and reading distance, and adaptive IOLs that provide limited visual accommodation. Multifocal IOLs can also be trifocal IOLs or extended depth of focus (EDOF) lenses.
As of 2021, nearly 28 million cataract procedures took place annually worldwide. That is about 75,000 procedures per day globally. The procedure can be done under local or topical anesthesia with the patient awake throughout the operation. The use of a flexible IOL enables the lens to be rolled for insertion into the capsular bag through a very small incision, thus avoiding the need for stitches. This procedure usually takes less than 30 minutes in the hands of an experienced ophthalmologist, and the recovery period is about two to three weeks. After surgery, patients should avoid strenuous exercise or anything else that significantly increases blood pressure. They should visit their ophthalmologists regularly for three weeks to monitor the implants.
IOL implantation carries several risks associated with eye surgeries, such as infection, loosening of the lens, lens rotation, inflammation, nighttime halos and retinal detachment. Though IOLs enable many patients to have reduced dependence on glasses, most patients still rely on glasses for certain activities, such as reading. These reading glasses may be avoided in some cases if multifocal IOLs, trifocal IOLs or EDOF lenses are used.
Additional Information
IOLs (intraocular lenses) are clear, artificial lenses that replace your eye’s natural ones. You receive IOLs during cataract surgery and refractive lens exchange. IOL implants correct a range of vision issues, including nearsightedness and age-related farsightedness. They may also help reduce your reliance on glasses for certain types of tasks.
What are IOLs?
IOLs (intraocular lenses) are clear artificial lenses that a healthcare provider will implant in your eye to replace your natural lens. Like glasses or contacts, IOL implants can correct vision issues such as:
* Myopia (nearsightedness).
* Hyperopia (farsightedness).
* Presbyopia (age-related farsightedness).
* Astigmatism (altered eye shape).
IOL implants are permanent, meaning they stay in your eyes for the rest of your life. IOLs help improve your vision and may reduce your reliance on glasses in your daily routine. You receive IOLs during eye lens replacement surgery, most commonly during cataract surgery.
Who needs intraocular lens implants?
You may benefit from IOL implants if you:
* Have cataracts that prevent you from seeing clearly. Virtually everyone undergoing cataract surgery will need to have an IOL implant in order to restore vision.
* Have refractive errors that affect your vision, but you’re not a candidate for LASIK or other vision correction surgeries.
What are the different types of intraocular lenses?
There are many types of IOLs, each with its own pros and cons. The main drawback with some types of IOLs is you’ll still need to wear glasses for some tasks (like reading). Some IOLs can reduce your reliance on glasses, but you may notice side effects like glare around lights at night.
The list below covers some general categories of IOLs. Ask your ophthalmologist about which type of IOL is best for you. They’ll help you customize your IOL selection to suit your vision needs, lifestyle and personal preferences.
Monofocal lenses
This is the type of IOL that most people select. Monofocal lenses have one focusing power. This means they sharpen either your distance, mid-range or close-up vision. Most people set their monofocal lenses for distance vision, which can help with tasks like driving. You’ll probably still need glasses for close-up vision.
Monofocal lenses with monovision
Monofocal IOLs set to monovision are a good option for some people who want to rely less on glasses. Normally, the monofocal IOLs for both of your eyes are set to the same range (like distance). But with monovision, the lens for each eye has a different focusing power. For example, the lens for your right eye might correct for distance, with the lens for your left eye correcting for close-up vision.
With monovision, your eyes work together to help you see both distant and close-up objects. One drawback is that it takes some time to adapt to monovision. Some people can’t adapt to monovision at all. So, before choosing monovision IOLs, your provider may suggest you try monovision contact lenses for a couple of weeks. This allows you to see if this method of correction feels comfortable to you.
Multifocal lenses
Multifocal lenses improve your close-up and distance vision and may reduce your need for glasses. Unlike monofocal lenses, multifocal lenses contain several focal zones. Your brain adjusts to these zones and chooses the focusing power you need for any given task (like driving or reading). You may need some time to adapt to these lenses. But over time, you should be able to rely less on your reading glasses. Some people don’t need glasses at all.
One drawback of multifocal lenses is that you may notice rings or halos around lights, like when driving at night.
Extended depth-of-focus (EDOF) lenses
Unlike multifocal lenses, EDOF lenses contain one long focal point that expands your corrected range of vision and depth of focus. These lenses give you excellent distance vision along with improvements in your mid-range vision (for tasks such as computer use). You may still need to use glasses for close-up tasks like reading.
Accommodative lenses
These lenses are similar to your eyes’ natural lenses in that they adjust their shape to help you see close-up or distant objects. Accommodative lenses are another option to help reduce dependency on glasses. But you may prefer to use glasses if you’re reading or focusing on close-up objects for longer periods of time.
Toric lenses
Toric lenses help people who have astigmatism. These lenses improve how light hits your retina, allowing you to have a sharper, clearer vision. Toric lenses are available in monofocal, multifocal, extended depth of focus (EDOF) or accommodative models. They serve to improve the quality of the vision delivered. Toric lenses will help reduce the amount of glare and halos artifacts commonly experienced by people with astigmatism.
Light-adjustable lenses (LALs)
Light-adjustable lenses are different from other IOL options in that your ophthalmologist fine-tunes their corrective power after your lens replacement surgery. They do this through a series of UV light treatment procedures, spaced several days apart. These procedures customize your lens prescription to bring you as close to your desired visual outcome as possible. This is still a type of monofocal lens, so glasses will be necessary for reading or driving.
Phakic lenses
Phakic lenses are typically implanted in younger individuals while trying to preserve the natural human lens, to correct for near-sightedness in people who don’t qualify for laser refractive surgery. This helps preserve your natural ability to focus and accommodate. These lenses will eventually have to be removed during cataract surgery but can offer younger people clear vision for a long time.
Which intraocular lens is best for me?
Your ophthalmologist will determine if you would benefit from cataract surgery, or if you would qualify for a refractive lens exchange surgery. They’ll discuss your options and help you decide which IOLs are best for you. They’ll also conduct a thorough eye exam to check your vision and the health of your eyes. They’ll perform some simple, painless tests to measure your eye size and shape, too.
To prepare for a conversation with your ophthalmologist, you should think about your priorities for your IOLs, as well as aspects that aren’t as important to you. It may help to ask yourself the following questions:
* Am I OK wearing glasses sometimes? If so, how often and for what types of tasks?
* What kind of vision is required in my work/profession? Am I OK wearing glasses for these tasks?
* Do I drive often at night? If so, can I adapt to seeing glare and halos around lights when I drive?
* What kind of hobbies and activities do I enjoy the most and how much dependency on glasses am I OK with for these activities?
* What is my budget for surgery?
Most insurance plans cover monofocal lenses, but you may have to pay for other types out of pocket. Be sure to find out the cost of various IOL options before making your final decision.
What are possible issues and complications related to IOL implantation?
Most IOL complications are rare and include:
* Posterior capsular opacification:This is commonly known as a secondary cataract. This happens after many months or years when a film-like material grows behind the implanted lens. This is a normal process that happens after surgery and can be expected to occur over time for almost everyone. The treatment for this is very quick and straightforward and is usually performed using a laser in the office.
* IOL dislocation: This means your IOL shifts from its normal position. You face a higher risk if you have certain eye conditions, like pseudoexfoliation syndrome, or have had trauma or prior eye surgeries. Certain genetic disorders, such as Ehlers-Danlos syndrome and Marfan syndrome, may also raise your risk. In some cases, you may need surgery to reposition or replace the IOL.
* Uveitis-glaucoma-hyphema (UGH) syndrome: UGH syndrome occurs when an IOL irritates your iris and other parts of your eye. This leads to inflammation, raised intraocular pressure and other symptoms. As with IOL dislocation, you may need surgery to reposition or replace the IOL. This is an extremely rare complication that most people don’t experience with routine surgery.
* IOL opacification: This is a clouding of your IOL. Your vision may become less sharp, and you may notice glare around lights. Treatment involves surgery to give you a new IOL. This is extremely uncommon with modern-day IOLs.
* Refractive surprise: A refractive surprise is when your vision after IOL implantation isn’t as sharp as you and your ophthalmologist expected. Your ophthalmologist will suggest a range of solutions. You may decide to accept the vision correction as is and do nothing further. Or you can choose to wear glasses, have laser vision correction (such as LASIK or PRK) or have an IOL replacement surgery.
Talk with your ophthalmologist about possible complications and your level of risk before choosing to have IOLs implanted in your eyes. They’ll tell you what to expect based on your medical history, eye health and other factors. Also, ask them about common side effects associated with cataract surgery or refractive lens exchange. Be sure to get all the information you need to make the decision that’s right for you.
LASIK
LASIK is a laser eye surgery that corrects vision problems. It changes the shape of your cornea to improve how light hits your retina. This improves your vision. About 99% of people have uncorrected vision that’s 20/40 or better after their LASIK surgery. More than 90% end up with 20/20 vision. Dry eye is the most common side effect.
PRK
Photorefractive keratectomy (PRK) is a laser eye surgery similar to LASIK. Unlike LASIK, which involves opening a flap in your cornea, PRK removes your cornea so that it grows back naturally. That makes it a better laser eye surgery choice for some people who can’t undergo LASIK.

#2 Re: Dark Discussions at Cafe Infinity » crème de la crème » Today 00:02:53
2449) Walther Bothe
Gist:
Work
In a counter tube, particles passing through the tube generate an electric pulse. In 1925 Walter Bothe connected two counter tubes together so that only simultaneous passages were registered. This meant that either the passages were caused by particles that originated from the same event or by a particle that moved so fast that the time for movement between the tubes was negligible. Bothe used the method to show that energy is conserved in impacts between particles and photons and to study cosmic radiation.
Summary
Walther Bothe (born Jan. 8, 1891, Oranienburg, Ger.—died Feb. 8, 1957, Heidelberg, W.Ger.) was a German physicist who shared the Nobel Prize for Physics in 1954 with Max Born for his invention of a new method of detecting subatomic particles and for other resulting discoveries.
Bothe taught at the universities of Berlin (1920–31), Giessen (1931–34), and Heidelberg (1934–57). In 1925 he and Hans Geiger used two Geiger counters to gather data on the Compton effect—the dependence of the increase in the wavelength of a beam of X rays upon the angle through which the beam is scattered as a result of collision with electrons. Their experiments, which simultaneously measured the energies and directions of single photons and electrons emerging from individual collisions, refuted a statistical interpretation of the Compton effect and definitely established the particle nature of electromagnetic radiation.
With the astronomer Werner Kolhörster, Bothe again applied this coincidence-counting method in 1929 and found that cosmic rays are not composed exclusively of gamma rays, as was previously believed. In 1930 Bothe discovered an unusual radiation emitted by beryllium when it is bombarded with alpha particles. This radiation was later identified by Sir James Chadwick as the neutron.
During World War II Bothe was one of the leaders of German research on nuclear energy. He was responsible for the planning and building of Germany’s first cyclotron, which was completed in 1943.
Details
Walther Wilhelm Georg Bothe (8 January 1891 – 8 February 1957) was a German experimental physicist who shared the 1954 Nobel Prize in Physics with Max Born "for the coincidence method and his discoveries made therewith."
Bothe served in the military during World War I from 1914, and he was a prisoner of war of the Russians, returning to Germany in 1920. Upon his return to the laboratory, he developed and applied coincidence circuits to the study of nuclear reactions, such as the Compton effect, cosmic rays, and the wave–particle duality of radiation.
In 1930, Bothe became Full Professor and Director of the Physics Department at the University of Giessen. In 1932, he became Director of the Physical and Radiological Institute at the University of Heidelberg; he was driven out of this position by elements of the Deutsche Physik movement. To preclude his emigration from Germany, he was appointed Director of the Physics Institute of the Kaiser Wilhelm Institute for Medical Research in Heidelberg. There, he built the first operational cyclotron in Germany. Furthermore, he became a principal in the German nuclear energy project, also known as Uranverein, which was started in 1939 under the supervision of the Army Ordnance Office.
In 1946, in addition to his directorship of the Physics Institute at the KWImf, Bothe was reinstated as a professor at the University of Heidelberg. From 1956 to 1957, he was a member of the Nuclear Physics Working Group in Germany.
In the year after Bothe's death, his Physics Institute at the KWImF was elevated to the status of a new institute under the Max Planck Society and it then became the Max Planck Institute for Nuclear Physics. Its main building was later named Bothe laboratory.
Education
Walther Wilhelm Georg Bothe was born on 8 January 1891 in Oranienburg, Germany, the son of Friedrich Bothe and Charlotte Hartung.
From 1908 to 1912, Bothe studied at the University of Berlin. In 1913, he became Max Planck's teaching assistant. He received his Ph.D. under Planck the following year.
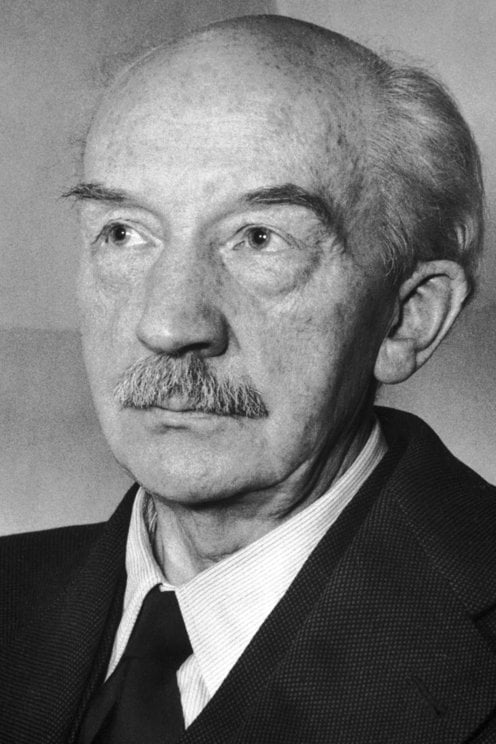
#3 Jokes » Miscellaneous Food Jokes - I » Today 00:02:32
- Jai Ganesh
- Replies: 0
Q: Why didn't the obese man know he was overweight?
A: Because it kinda just snacked up on him!
* * *
Q: Why are all obese Americans actually in shape?
A: Because the shape is a triangle!
* * *
Q: What did the plate say to the other plate?
A: Dinners on me tonight.
* * *
Q: What happened to the snack bar that was too close to the Atom Smasher?
A: They created "Fission chips".
* * *
Q: What kind of candy is never on time?
A: ChocoLATE.
* * *
#4 Dark Discussions at Cafe Infinity » Come Quotes - XXI » Today 00:01:58
- Jai Ganesh
- Replies: 0
Come Quotes - XXI
1. When we look back on all the perils through which we have passed and at the mighty foes that we have laid low and all the dark and deadly designs that we have frustrated, why should we fear for our future? We have come safely through the worst. - Winston Churchill
2. Whether you come from a council estate or a country estate, your success will be determined by your own confidence and fortitude. - Michelle Obama
3. The smaller the planets are, they are, other things being equal, of so much the greater density; for so the powers of gravity on their several surfaces come nearer to equality. They are likewise, other things being equal, of the greater density, as they are nearer to the sun. - Isaac Newton
4. If you want to cut your own throat, don't come to me for a bandage. - Margaret Thatcher
5. I've been here before and will come again, but I'm not going this trip through. - Bob Marley
6. I perhaps ought to say that individually I never was much interested in the Texas question. I never could see much good to come of annexation, inasmuch as they were already a free republican people on our own model. - Abraham Lincoln
7. This is the moment when we must come together to save this planet. Let us resolve that we will not leave our children a world where the oceans rise and famine spreads and terrible storms devastate our lands. - Barack Obama
8. We will burn that bridge when we come to it. - Johann Wolfgang von Goethe.
#5 This is Cool » Mountain K2 » Yesterday 17:43:57
- Jai Ganesh
- Replies: 0
Mountain K2
Gist
K2 is the world's second-highest mountain at 8,611 meters (28,251 ft), located on the China-Pakistan border in the Karakoram Range. Known as the "Savage Mountain" for its extreme, unpredictable weather and treacherous terrain, it is considered one of the most difficult and dangerous peaks to climb.
K2 is harder than Everest because it's much more technically challenging with steeper, icy faces, unpredictable and brutal weather (more wind, sudden storms), and greater remoteness, offering fewer rescue options, leading to a significantly higher fatality rate despite being slightly shorter than Everest. Everest has more established routes and support, while K2 demands pure skill with little room for error, earning it nicknames like "Savage Mountain".
Summary
K2 is the world’s second highest peak (28,251 feet [8,611 metres]), second only to Mount Everest. K2 is located in the Karakoram Range and lies partly in a Chinese-administered enclave of the Kashmir region within the Uygur Autonomous Region of Xinjiang, China, and partly in the Gilgit-Baltistan portion of Kashmir under the administration of Pakistan.
The glacier- and snow-covered mountain rises from its base at about 15,000 feet (4,570 metres) on the Godwin Austen Glacier, a tributary of the Baltoro Glacier. The mountain was discovered in 1856 by Col. T.G. Montgomerie of the Survey of India, and it was given the symbol K2 because it was the second peak measured in the Karakoram Range. The name Mount Godwin Austen is for the peak’s first surveyor, Col. H.H. Godwin Austen, a 19th-century English geographer.
The first attempt to reach the summit was made by an Anglo-Swiss expedition in 1902 that ascended to 18,600 feet (5,670 metres) on the peak’s northeastern crest. Other unsuccessful attempts included an Italian expedition in 1909, led by Luigi Amedeo, duke d’Abruzzi, via the southeastern ridge (later called the Abruzzi Ridge) that reached approximately 20,000 feet (6,100 metres). In 1938 an American expedition led by Charles Houston via the Abruzzi Ridge reached about 26,000 feet (7,925 metres); in 1939 another American-led expedition following the same route reached about 27,500 feet (8,380 metres); and in 1953 another expedition led by Houston reached 25,900 feet (7,900 metres) on the Abruzzi Ridge. Finally, in 1954, an Italian expedition consisting of five scientists (including the geologist Ardito Desio as leader), a doctor, a photographer, and 12 others, including a Pakistani, managed to conquer the Abruzzi Ridge despite the severe weather conditions. The summit was reached at 6 pm on July 31, 1954, by Achille Compagnoni and Lino Lacedelli. In the course of the ascent, Mario Puchoz, one of the guides, died of pneumonia.
Because K2 is prone to frequent and severe storms that make the already treacherous climbing conditions on its slopes even more challenging—and humans find functioning at such high elevations difficult—it is one of the world’s most difficult mountains to climb. The number of people to have reached the top constitutes only a small fraction compared with how many have successfully climbed Mount Everest. In addition, although there have been fewer deaths on K2 compared with those on Mount Everest, the proportion of those killed to the number of people who have attempted climbing K2 is significantly higher.
Details
K2, also known as Mount Godwin-Austen, at 8,611 metres (28,251 ft) above sea level, is the second-highest mountain on Earth, after Mount Everest at 8,849 metres (29,032 ft). It lies in the Karakoram range, partially in the Gilgit-Baltistan region of Pakistan-administered Kashmir and partially in the China-administered Trans-Karakoram Tract in the Taxkorgan Tajik Autonomous County of Xinjiang.
K2 became known as the Savage Mountain after George Bell—a climber on the 1953 American expedition—said, "It's a savage mountain that tries to kill you." Of the five highest mountains in the world, K2 has long been the deadliest: prior to 2021, approximately one person had died on the mountain for every four who reached the summit. After an increase in successful attempts, as of August 2023, an estimated 800 people have summited K2, with 96 deaths during attempted climbs.
K2 is nicknamed "The King of Mountains" and "The Mountaineers' Mountain", as well as "The Mountain of Mountains", a phrase popularized by Italian climber Reinhold Messner in his book on K2. Although the summit of Everest is at a higher altitude, K2 is a more difficult and dangerous climb. This is in part due to its more northern location, where inclement weather is more common, as well as its steep and exposed faces. The summit was reached for the first time by the Italian climbers Lino Lacedelli and Achille Compagnoni on a 1954 Italian expedition led by Ardito Desio.
Most ascents are made during July and August, typically the warmest times of the year. In January 2021 K2 became the final eight-thousander to be summited in the winter by a team of Nepalese climbers led by Nirmal Purja and Mingma Gyalje Sherpa.
K2's eastern face remains un-climbed, partly because of the hazards associated with the instability of its ice and snow formations.
Geographical setting
K2 lies in the northwestern Karakoram Range. It is located in the Baltistan region of Gilgit–Baltistan, Pakistan, and the Taxkorgan Tajik Autonomous County of Xinjiang, China. The Tarim sedimentary basin borders the range on the north and the Lesser Himalayas on the south. Melt waters from glaciers, such as those south and east of K2, feed agriculture in the valleys and contribute significantly to the regional fresh-water supply.
K2 is ranked 22nd by topographic prominence, a measure of a mountain's independent stature. It is a part of the same extended area of uplift (including the Karakoram, the Tibetan Plateau, and the Himalayas) as Mount Everest, and it is possible to follow a path from K2 to Everest that goes no lower than 4,594 metres (15,072 ft), at the Kora La on the Nepal/China border in the Mustang Lo. Many other peaks far lower than K2 are more independent in this sense. It is, however, the most prominent peak within the Karakoram range.
K2 is notable for its local relief as well as its total height. It stands over 3,000 metres (10,000 ft) above much of the glacial valley bottoms at its base. It is a consistently steep pyramid, dropping quickly in almost all directions. The north side is the steepest: there it rises over 3,200 metres (10,500 ft) above the K2 (Qogir) Glacier in only 3,000 metres (9,800 ft) of horizontal distance. In most directions, it achieves over 2,800 metres (9,200 ft) of vertical relief in less than 4,000 metres (13,000 ft).
A 1986 expedition led by George Wallerstein made an inaccurate measurement showing that K2 was taller than Mount Everest, and therefore the tallest mountain on Earth. A corrected measurement was made in 1987, but by then the claim that K2 was the tallest mountain in the world had already made it into many news reports and reference works.
Height
K2's height given on maps and encyclopedias is 8,611 metres (28,251 ft). In the summer of 2014, a Pakistani-Italian expedition to K2, named "K2 60 Years Later", was organized to commemorate the 60th anniversary of the first ascent of K2. One of the goals of the expedition was to accurately measure the height of the mountain using satellite navigation. The height of K2 measured during this expedition was 8,609.02 metres (28,244.8 ft).
Additional Information
K2 is the second-highest mountain in the world, standing at 8,611 metres (28,251 ft) tall. It is also known as Mount Godwin-Austen or Chogori. K2 is part of the Karakoram mountain range, and is located on the border between Pakistan and China. The name, 'K2' originated from the first survey of the Karakoram range. In the survey, surveyors named each mountain with a 'K' and a number after that.
K2 is known as the 'Savage Mountain' and is considered more difficult to climb than Mount Everest,which is the highest mountain in the world. It has the second-highest fatality rate among all mountains.With a height over 8,000 meters, with a rate of approximately one death for every four climbers who reach the summit. As of 2011, only 300 people had successfully reached the top of K2, while more than 80 climbers lost their lives attempting the ascent. K2 can be climbed during both summer and winter seasons.
The top of the mountain was first reached in 1954 by Italian climbers Lino Lacedelli and Achille Compagnoni.
Mount K2, the second-highest mountain on Earth (Mount Everest), is the world’s second-highest Mountain. Stands as a remote and terrifying sentinel in the Karakoram Range. Unlike Mt. Everest, it remains as off-the-beaten-path far from popular trekking circuits, demanding effort even to glimpse its icy crown. Located on the border between Pakistan and China, K2 rises to a height of 8,611 meters (28,251 feet) above sea level. It dominates the skyline of Pakistan’s Gilgit-Baltistan region, surrounded by glaciers, deep valleys, and other towering peaks.

#6 Science HQ » Fibula » Yesterday 16:48:52
- Jai Ganesh
- Replies: 0
Fibula
Gist
The fibula (calf bone) is the slender, long bone located on the lateral (outer) side of the tibia in the lower leg. While it does not bear significant weight, it is crucial for stabilizing the ankle joint, supporting lower-leg muscles, and forming the lateral malleolus. It connects to the tibia via an interosseous membrane.
The fibula, or calf bone, is the slender, outer bone in the lower leg, running parallel to the larger shin bone (tibia) from just below the knee to the ankle, forming the bony bump (lateral malleolus) on the outside of your ankle and providing vital stability and muscle attachment for movement, though it's not a primary weight-bearing bone.
Summary
Fibula is the outer of two bones of the lower leg or hind limb, presumably so named (fibula is Latin for “brooch”) because the inner bone, the tibia, and the fibula together resemble an ancient brooch, or pin. In humans the head of the fibula is joined to the head of the tibia by ligaments and does not form part of the knee. The base of the fibula forms the outer projection (malleolus) of the ankle and is joined to the tibia and to one of the ankle bones, the talus. The tibia and fibula are further joined throughout their length by an interosseous membrane between the bones. The fibula is slim and roughly four-sided, and its shape varies with the strength of the attached muscles. In many mammals, such as the horse and the rabbit, the fibula is fused for part of its length with the tibia.
Fractures of the fibula usually are associated with an ankle injury, though they can occur in isolation (without ankle involvement) or in combination with fractures of the tibia (e.g., in severe injuries). Though less common that tibial stress fractures, fibular stress fractures can occur, most typically in long-distance runners.
Details
The fibula (pl.: fibulae or fibulas) or calf bone is a leg bone on the lateral side of the tibia, to which it is connected above and below. It is the smaller of the two bones and, in proportion to its length, the most slender of all the long bones. Its upper extremity is small, placed toward the back of the head of the tibia, below the knee joint and excluded from the formation of this joint. Its lower extremity inclines a little forward, so as to be on a plane anterior to that of the upper end; it projects below the tibia and forms the lateral part of the ankle joint.
Structure
The bone has the following components:
* Lateral malleolus
* Interosseous membrane connecting the fibula to the tibia, forming a syndesmosis joint
* The superior tibiofibular articulation is an arthrodial joint between the lateral condyle of the tibia and the head of the fibula.
* The inferior tibiofibular articulation (tibiofibular syndesmosis) is formed by the rough, convex surface of the medial side of the lower end of the fibula, and a rough concave surface on the lateral side of the tibia.
Blood supply
The blood supply is important for planning free tissue transfer because the fibula is commonly used to reconstruct the mandible. The shaft is supplied in its middle third by a large nutrient vessel from the fibular artery. It is also perfused from its periosteum which receives many small branches from the fibular artery. The proximal head and the epiphysis are supplied by a branch of the anterior tibial artery. In harvesting the bone the middle third is always taken and the ends preserved (4 cm proximally and 6 cm distally)
Development
The fibula is ossified from three centers, one for the shaft, and one for either end. Ossification begins in the body about the eighth week of fetal life, and extends toward the extremities. At birth the ends are cartilaginous.
Ossification commences in the lower end in the second year, and in the upper about the fourth year. The lower epiphysis, the first to ossify, unites with the body about the twentieth year; the upper epiphysis joins about the twenty-fifth year.
Head
The upper extremity or head of the fibula is of an irregular quadrate form, presenting above a flattened articular surface, directed upward, forward, and medialward, for articulation with a corresponding surface on the lateral condyle of the tibia. On the lateral side is a thick and rough prominence continued behind into a pointed eminence, the apex (styloid process), which projects upward from the posterior part of the head.
The prominence, at its upper and lateral part, gives attachment to the tendon of the biceps femoris and to the fibular collateral ligament of the knee-joint, the ligament dividing the tendon into two parts.
The remaining part of the circumference of the head is rough, for the attachment of muscles and ligaments. It presents in front a tubercle for the origin of the upper and anterior fibers of the peroneus longus, and a surface for the attachment of the anterior ligament of the head; and behind, another tubercle, for the attachment of the posterior ligament of the head and the origin of the upper fibers of the soleus.
Body
The body of the fibula presents four borders - the antero-lateral, the antero-medial, the postero-lateral, and the postero-medial; and four surfaces - anterior, posterior, medial, and lateral.
Borders
The antero-lateral border begins above in front of the head, runs vertically downward to a little below the middle of the bone, and then curving somewhat lateralward, bifurcates so as to embrace a triangular subcutaneous surface immediately above the lateral malleolus. This border gives attachment to an intermuscular septum, which separates the extensor muscles on the anterior surface of the leg from the peronaei longus and brevis on the lateral surface.
The antero-medial border, or interosseous crest, is situated close to the medial side of the preceding, and runs nearly parallel with it in the upper third of its extent, but diverges from it in the lower two-thirds. It begins above just beneath the head of the bone (sometimes it is quite indistinct for about 2.5 cm. below the head), and ends at the apex of a rough triangular surface immediately above the articular facet of the lateral malleolus. It serves for the attachment of the interosseous membrane, which separates the extensor muscles in front from the flexor muscles behind.
The postero-lateral border is prominent; it begins above at the apex, and ends below in the posterior border of the lateral malleolus. It is directed lateralward above, backward in the middle of its course, backward, and a little medialward below, and gives attachment to an aponeurosis which separates the peronaei on the lateral surface from the flexor muscles on the posterior surface.
The postero-medial border, sometimes called the oblique line, begins above at the medial side of the head, and ends by becoming continuous with the interosseous crest at the lower fourth of the bone. It is well-marked and prominent at the upper and middle parts of the bone. It gives attachment to an aponeurosis which separates the tibialis posterior from the soleus and flexor hallucis longus.
Surfaces
The anterior surface is the interval between the antero-lateral and antero-medial borders. It is extremely narrow and flat in the upper third of its extent; broader and grooved longitudinally in its lower third; it serves for the origin of three muscles: the extensor digitorum longus, extensor hallucis longus, and peroneus tertius.
The posterior surface is the space included between the postero-lateral and the postero-medial borders; it is continuous below with the triangular area above the articular surface of the lateral malleolus; it is directed backward above, backward and medialward at its middle, directly medialward below. Its upper third is rough, for the origin of the soleus; its lower part presents a triangular surface, connected to the tibia by a strong interosseous ligament; the intervening part of the surface is covered by the fibers of origin of the flexor hallucis longus. Near the middle of this surface is the nutrient foramen, which is directed downward.
The medial surface is the interval included between the antero-medial and the postero-medial borders. It is grooved for the origin of the tibialis posterior.
The lateral surface is the space between the antero-lateral and postero-lateral borders. It is broad, and often deeply grooved; it is directed lateralward in the upper two-thirds of its course, backward in the lower third, where it is continuous with the posterior border of the lateral malleolus. This surface gives origin to the peronaei longus and brevis.
Clinical significance
As much of the fibula can be removed without it impacting an individual's ability to walk, the fibula is utilised as a source of bone material in fibular free flap surgeries.
Fractures
The most common type of fibula fracture is located at the distal end of the bone, and is classified as ankle fracture. In the Danis–Weber classification it has three categories:
* Type A: Fracture of the lateral malleolus, distal to the syndesmosis (the connection between the distal ends of the tibia and fibula).
* Type B: Fracture of the fibula at the level of the syndesmosis
* Type C: Fracture of the fibula proximal to the syndesmosis.
A Maisonneuve fracture is a spiral fracture of the proximal third of the fibula associated with a tear of the distal tibiofibular syndesmosis and the interosseous membrane. There is an associated fracture of the medial malleolus or rupture of the deep deltoid ligament.
An avulsion fracture of the head of the fibula refers to the fracture of the fibular head because of a sudden contraction of the biceps femoris muscle that pulls its site of attachment on the bone. The attachment of the biceps femoris tendon on the fibular head is closely related to the lateral collateral ligament of the knee. Therefore, this ligament is prone to injury in this type of avulsion fracture.
Additional Information
The fibula is a slender, cylindrical leg bone that is located on the posterior portion of the limb. It is found next to another long bone known as the tibia. A long bone is defined as one whose body is longer than it is wide.
Like other long bones, the fibula has a proximal end (with a head and neck), a shaft, and a distal end. The fibula and tibia run parallel to each other in the leg and are similar in length but the fibula is much thinner than the tibia. This is indicative of the weight-bearing contributions of each bone. In other words, the thicker tibia has a much greater function in weight-bearing than the fibula.
There are several key facts about the fibula that most anatomy students should be familiar with. These and other important points about the anatomy, blood supply, innervation, and muscular and ligamentous attachments are addressed in this article. The article will also discuss important fractures of the fibula.
Development
The fibula is a part of the appendicular skeleton and develops via endochondral ossification. There are three points at which ossification begins in the fibula:
* the body around the 8th gestational week
* the distal end by the end of the first year of life
* the proximal end at around four-years-old in males and three-years-old in females
The ossification centers of the body and distal end of the bone eventually fuse during the mid-adolescent years (at 15 years old for females and 17 years old for males). The bony centers of the proximal part and shaft of the fibula are the last to unite during the late adolescent years (around 17 years for females and 19 years for males).
Proximal end
The proximal end of the fibula is characterized by an irregularly shaped head and a short neck. It has three segments which project in different directions: anteriorly, posteriorly, and laterally. An important question that pops up on a lot of anatomy tests is with what bony structure does the head of the fibula articulate? There is a round, flattened area on the medial part of the fibular head known as a facet. It articulates with a complementary facet on the inferolateral part of the lateral tibial condyle (proximal tibiofibular joint). The facet also acts as a point of attachment for the tibiofibular capsular ligament. Additionally, the tibiofibular capsular ligament surrounds the articular facet of the fibula.
There is a styloid process of the fibula that extends superiorly from the head; it is more commonly referred to as the apex of the head of the fibula. This apical projection protrudes from the posterolateral part of the fibular head. The neck of the fibula is a short bare region just below the fibular head. What important structures pass around the neck of the fibula? Importantly, the common fibular nerve (also called the common peroneal nerve) travels posterolaterally to the fibular neck. This has clinical significance as trauma to the neck of the fibula can present with neurological deficits.
The function of the proximal end of the fibula is to provide points of attachment for minor supporting ligaments of the knee joint. There is the fibular collateral ligament that arises from the fibular apex and is surrounded by the tendon of biceps femoris.
Body
The majority of the fibula is made up by its body (or shaft). This part of the bone is triangular in cross-section and consequently has three borders (anterior, interosseous, and posterior) and three surfaces (lateral, medial, and posterior) found along the shaft of the fibula. The borders are the sharp longitudinal edges that run along the bone’s long axis. On the other hand, the surfaces are the flattened areas that exist between the borders.
The anterior border starts at the fibular head and continues distally toward the lateral malleolus, where it diverges into two ridges that surround the triangular subcutaneous surface. On the medial aspect of the fibula is the interosseous or medial border. It is the point of attachment of the fibrous interosseous membrane of the leg that forms the middle tibiofibular joint. This fibrous septum acts as a barrier between the extensor and fibular muscles. There is a posterior border that runs along the back part of the fibula. The proximal part of the border appears slightly rounded. However, the border becomes more prominent distally, as it approaches the medial segment of the lateral malleolus.
The interosseous and anterior borders of the fibula act as medial and lateral boundaries of the medial surface. This surface provides a point of attachment for the muscles that extend the foot and cause the toes to point upward (dorsiflexion).
The lateral surface is found on the opposite side of the medial surface, between the posterior and anterior borders. The proximal part of the surface faces laterally; however, the surface spirals toward the distal end and as such part of the surface faces posterolaterally. By virtue of this shift, the distal part of the lateral surface is in continuity with the posterior groove of the lateral malleolus. The lateral surface provides a point of attachment for the fibular (peroneal) muscles.
The posterior surface is found between the posterior and interosseous borders. The surface is much more narrow at the proximal part (where the interosseous and posterior borders are closest) than it is distally (where the borders are farthest apart). This surface provides attachment for the flexor muscles of the foot which are responsible for pointing the toes downward (plantar flexion).
Distal end
The distal end of the fibula forms the lateral malleolus of the lower limb. This is a bony projection noted on the lateral surface of the ankle, which is complementary to another bony projection on the medial aspect of the ankle called the medial malleolus (formed by the tibia). The lateral malleolus extends posteroinferiorly, is round and rough anteriorly, and has a broad groove posteriorly. The lateral surface is covered by skin (so there is no muscular layer at this area) and the medial surface has a triangular area that is convex along the vertical axis. The distal end of the fibula tapers off as an apical projection that articulates with the lateral aspect of the talus.
The distal end provides attachment for several ligaments that support the ankle joint. The posterior tibiofibular, posterior talofibular, calcaneofibular, and interosseous (middle) tibiofibular ligaments all have attachments to the end of the fibula and participate in the stability of this joint.
Joints
The tibia and fibula articulate through three joints–the superior, middle, and inferior tibiofibular joints. The superior tibiofibular joint is a plane synovial joint (allows only gliding movement) with the transverse joint line spanning the lateral tibial condyle and the medial fibular head. The capsule is thickened anteriorly and posteriorly and joins with the anterior ligament of the fibular head, relating closely to the tendon of biceps femoris.
The tibia and fibula also articulate via an interosseous membrane that is also called the middle tibiofibular ligament. It is made of an aponeurotic lamina which is thin and made of oblique fibers. This ligament has medial and lateral attachments to the tibial and fibular interosseous margins respectively. The membrane separates the muscles in the back of the leg from the muscles located in the front of the leg.
The inferior tibiofibular joint is a syndesmosis joint (slightly movable, fibrous joint), just above the ankle region which lies between the medial distal end of the fibula and the concave fibular notch region of the lateral tibia. There is no fibrous capsule surrounding this joint but there is the anterior tibiofibular ligament which descends laterally between the two leg bones.
Muscle attachments
What is the function of the fibula? The bone provides a point of origin for a number of muscles of the foot. However, only one muscle inserts on this long bone. So what structures are attached to the fibula? The table below summarizes the muscles that originate from, and insert on the fibula. Note that the muscles are listed from cranial to caudal, and those attached to the anterior surface are listed before those on the posterior surface.
Blood supply and innervation
A branch of the fibular artery brings oxygen-rich blood to supply the bone. It travels through a nutrient foramen on the posterior surface of the fibula that facilitates passage of a branch of the fibular artery into the bone. The foramen is a few centimeters proximal to the midpoint of the shaft.
The nerves that supply the knee (genicular branch of the common fibular nerve) and ankle (deep fibular nerve) joints also innervate the proximal and distal ends of the fibula, respectively. Similarly, superficial and deep fibular nerves, which innervate the muscles attached to the fibula, also innervate the fibular periosteum.

#7 Re: Jai Ganesh's Puzzles » General Quiz » Yesterday 16:01:00
Hi,
#10781. What does the term in Geography Dell (landform) mean?
#10782. What does the term in Geography River delta mean?
#8 Re: Jai Ganesh's Puzzles » English language puzzles » Yesterday 15:40:09
Hi,
#5977. What does the noun hallmark mean?
#5978. What does the adjective hallowed mean?
#9 Re: Jai Ganesh's Puzzles » Doc, Doc! » Yesterday 15:30:19
Hi,
#2585. What does the medical term Golgi tendon organ mean?
#10 Re: Jai Ganesh's Puzzles » 10 second questions » Yesterday 15:07:17
Hi,
#9871.
#11 Re: Jai Ganesh's Puzzles » Oral puzzles » Yesterday 14:48:23
Hi,
#6364.
#12 Re: Exercises » Compute the solution: » Yesterday 14:30:34
Hi,
2725.
#13 Re: This is Cool » Miscellany » Yesterday 00:03:17
2511) Tonometry
Gist
Tonometry is a quick, non-invasive diagnostic procedure that measures intraocular pressure (IOP), or fluid pressure inside the eye, to detect and monitor glaucoma. By assessing how much force is needed to flatten the cornea (applanation) or indent it, doctors can check for optic nerve damage. The most common methodGoldmann applanation tonometry, is considered the gold standard.
How is tonometry performed?
The lamp is moved forward until the tip of the tonometer just touches the cornea. Blue light is used so that the orange dye will glow green. The health care provider looks through the eyepiece on the slit-lamp and adjusts a dial on the machine to give the pressure reading. There is no discomfort with the test.
Summary
A tonometry test measures the pressure inside your eye, which is called intraocular pressure (IOP). This test is used to check for glaucoma, an eye disease that can cause blindness by damaging the nerve in the back of the eye (optic nerve). Damage to the optic nerve may be caused by a buildup of fluid that does not drain properly out of the eye.
Tonometry measures IOP by recording the resistance of your cornea to pressure (indentation). Eyedrops to numb the surface of your eye are used with most of the following methods.
Tonometry methods
* Applanation (Goldmann) tonometry. This type of tonometry uses a small probe to gently flatten part of your cornea to measure eye pressure and a microscope called a slit lamp to look at your eye. The pressure in your eye is measured by how much force is needed to flatten your cornea. This type of tonometry is very accurate and is often used to measure IOP after a simple screening test (such as air-puff tonometry) finds an increased IOP.
* Electronic indentation tonometry. Electronic tonometry is being used more often to check for increased IOP. Although it is very accurate, electronic tonometry results can be different than applanation tonometry. Your doctor gently places the rounded tip of a tool that looks like a pen directly on your cornea. The IOP reading shows on a small computer panel.
* Noncontact tonometry (pneumotonometry). Noncontact (or air-puff) tonometry does not touch your eye but uses a puff of air to flatten your cornea. This type of tonometry is not the best way to measure intraocular pressure. But it is often used as a simple way to check for high IOP and is the easiest way to test children. This type of tonometry does not use numbing eyedrops.
Details
Tonometry is a test to measure the pressure inside your eyes. The test is used to screen for glaucoma. It is also used to measure how well glaucoma treatment is working.
How the Test is Performed
There are three main methods of measuring eye pressure.
The most accurate method measures the force needed to flatten an area of the cornea.
* The surface of the eye is numbed with eye drops. A fine strip of paper stained with orange dye is held to the side of the eye. The dye stains the front of the eye to help with the exam. Sometimes the dye is in the numbing drops.
* You will rest your chin and forehead on the support of a slit lamp so that your head is steady. You will be asked to keep your eyes open and to look straight ahead. The lamp is moved forward until the tip of the tonometer just touches the cornea.
* Blue light is used so that the orange dye will glow green. The health care provider looks through the eyepiece on the slit-lamp and adjusts a dial on the machine to give the pressure reading.
* There is no discomfort with the test.
A second method uses a handheld device shaped like a pen. You are given numbing eye drops to prevent any discomfort. The device touches the surface of the cornea and instantly records eye pressure.
The last method is the noncontact method (air puff). In this method, your chin rests on a device similar to a slit lamp.
* You stare straight into the examining device. When you are at the correct distance from the device, a tiny beam of light reflects off of your cornea onto a detector.
* When the test is performed, a puff of air will slightly flatten the cornea; how much it flattens depends on the eye pressure.
* This causes the tiny beam of light to move to a different spot on the detector. The instrument calculates eye pressure by looking at how far the beam of light moved.
How to Prepare for the Test
Remove contact lenses before the exam. The dye can permanently stain contact lenses.
Tell your provider if you have a history of corneal ulcers or eye infections, or a history of glaucoma in your family. Always tell your provider what medicines you are taking.
How the Test will Feel
If numbing eye drops were used, you should not have any pain. In the noncontact method, you may feel mild pressure on your eye for a brief moment from the air puff.
Why the Test is Performed
Tonometry is a test to measure the pressure inside your eyes. The test is used to screen for glaucoma and to measure how well glaucoma treatment is working.
People over age 40 years, particularly African Americans, have the highest risk for developing glaucoma. Regular eye exams can help detect glaucoma early. If it is detected early, glaucoma can be treated before too much damage is done.
The test may also be done before and after eye surgery.
Normal Results
A normal result means your eye pressure is within the normal range. The normal eye pressure range is 10 to 21 mm Hg.
The thickness of your cornea can affect measurements. Normal eyes with thick corneas have higher readings, and normal eyes with thin corneas have lower readings. A thin cornea with a high reading may be very abnormal (the actual eye pressure will be higher than shown on the tonometer).
A corneal thickness measurement (pachymetry) is needed to get a correct pressure measurement.
Talk to your provider about the meaning of your specific test results.
What Abnormal Results Mean
Abnormal results may be due to:
* Glaucoma
* Hyphema (blood in the front chamber of the eye)
* Inflammation in the eye
* Injury to the eye or head
* Recent eye surgery
Risks
If the applanation method is used, there is a small chance the cornea may be scratched (corneal abrasion). The scratch will normally heal within a few days.
Alternative Names
Intraocular pressure (IOP) measurement; Glaucoma test; Goldmann applanation tonometry (GAT).
Additional Information
Tonometry refers to diagnostic tests that measure your intraocular pressure (IOP), or the pressure inside your eye. There are multiple methods available, and some don’t touch your eye at all. The various test methods can help your eye care specialist detect glaucoma before it causes permanent damage and vision loss.
Overview:
What is tonometry?
Tonometry refers to a type of eye test that measures pressure inside your eye (intraocular pressure). It’s one of the essential glaucoma tests. There are a few different methods and ways to do this test, all of which are quick and painless.
When is tonometry used?
Eye care specialists, especially ophthalmologists and optometrists, use tonometry to screen for and diagnose glaucoma. It’s a common part of routine eye exams and more specific exams when you have possible eye injuries or experience certain eye symptoms.
Eye specialists may also use tonometry to monitor your eye pressure if you’re taking certain medications. Monitoring your eye pressure makes sure that those medications don’t cause high intraocular pressure (ocular hypertension) as a side effect.
If you have glaucoma, regular tonometry determines treatment. Tracking your eye pressure is a key way to make sure treatment is effective, so frequent tonometry tests — including with devices you can use at home to take readings on yourself — are common.
Test Details:
How does tonometry work?
Tonometry measures the pressure of your eye’s anterior chamber. The anterior chamber is a fluid-filled space just behind your cornea at the front of your eye. Pressure from the aqueous humor fluid inside that chamber helps your eyes hold their globe-like shape. The unit of measurement for this is millimeters of mercury (mmHg), the same unit used for blood pressure tests.
Your eye care specialist can use a few different tonometry methods. Those include:
* Applanation tonometry. “Applanation” means “flattening,” and devices that use it have a small, disk-shaped extension that rests against your eye surface. The devices measure how much pressure it takes to make your eye surface start to flatten. This method is the most accurate. It’s common for eye specialists to use this method after other methods return unusual or concerning readings.
* Continuous monitoring. This method uses a sensor you wear on your eye like a contact lens. Researchers are investigating the wearable sensor and another, similar method that uses a surgically implanted sensor.
* Dynamic contour tonometry. These devices use a small, sensor-tipped extension that touches your eye (but doesn’t make an indention).
* Electronic indentation tonometry. Devices that use this method have a small probe that takes a measurement when it presses against the surface of your eye enough to make a small indention.
* Non-contact tonometry. Devices using this method push air at your cornea. The device then measures tiny, split-second changes in the shape of your cornea as the air bounces off its surface. Air puff tonometers do this with a small puff of air, while ocular response analyzers use a stream of air.
* Rebound tonometry. Devices using this method have a tiny, plastic ball that moves toward your eye and stops when it touches the surface. The device determines your intraocular pressure when the ball makes gentle, painless contact. Some devices that use this method are ones your eye specialist can prescribe for you to take and use at home.
How should I prepare for a tonometry test?
You shouldn’t need to prepare for a tonometry test. One exception is to make sure you don’t wear a tight collar doing the test (either by wearing another shirt or loosening the collar if possible). Pressure around your neck from your clothes can increase your intraocular pressure readings.
What should I expect during a tonometry test?
What you can expect from tonometry tests can vary depending on the method. If you’re having applanation tonometry, your provider will add eye drops containing an anesthetic and a dye called fluorescein to your eyes. But non-contact tonometry and most other methods don’t need either of these to work.
Some of the most common methods for the tonometry test only take a few seconds. Many of these faster methods work best with calculating an average of multiple readings, so don’t be surprised or think you’ve done something wrong if your eye specialist wants to retake the readings a few times. Other methods (like applanation tonometry) take up to a few minutes. Your eye specialist will tell you more about what to expect during the test.
What should I expect after the test?
Your provider can tell you the reading right after they take it.
If you received anesthetic eye drops, don’t let anything touch your eyes until the anesthetic wears off. Anesthetic drops keep you from feeling pain, so it’s easier to injure your eyes while they’re numb.
Is tonometry painful?
Tonometry shouldn’t hurt, even if the method used involves touching your eyeball in some way. If you have pain during the test, tell your eye care specialist. You can also ask them more about what to expect regarding that pain, including how long it should last and what you can do about it.
Results and Follow-Up:
What is a normal tonometry range?
A normal reading for most people is 10 mmHg to 21 mmHg. If your results are outside the normal range, there are a few likely next steps.
If your results are too high
If simpler tests show high pressure, your eye specialist will probably recommend using applanation tonometry to verify the readings. If applanation tonometry confirms that your pressure is high, your provider may monitor closely or offer treatment options. They’ll want to schedule regular follow-up visits.
Your eye specialist may recommend that you test your eye pressure at home. Be sure to ask about what to do if you get readings that are outside the normal range. You may need a follow-up visit with your specialist if you get slightly higher-than-normal pressures. But, if you get much higher readings, you may need emergency medical care.
Eye pressure that’s high because of angle-closure glaucoma is a medical emergency that needs immediate treatment. Without quick treatment, angle-closure glaucoma can quickly cause eye damage and permanent vision loss.
Other next steps can vary depending on your specific situation and needs. Your eye specialist can tell you more about what you can expect for your specific case.
If your results are too low
Low intraocular pressure is also a cause for concern since it can lead to eye damage and vision loss. Low intraocular pressure is usually anything under 5 mmHg or 6 mmHg. If the pressure in your eye is too low, your eye specialist will talk to you about treatment options and follow-up visits to monitor your eye health.

#14 Re: Dark Discussions at Cafe Infinity » crème de la crème » Yesterday 00:02:57
2448) Max Born
Gist:
Life
Max Born was born in Breslau, Germany (now Wroclaw, Poland), where his father was a professor of anatomy. Born studied at universities in Breslau, Heidelberg, Zurich and Göttingen, where he received his doctorate in 1906. After serving in the army during the First World War, he became a professor at the University of Frankfurt-on-Main in 1919 and at the University of Göttingen in 1921. After the Nazis seized power in 1933, he moved to Cambridge in United Kingdom. Beginning in 1936, he served as a professor at the University of Edinburgh. Max Born was married and had three children.
Work
In Niels Bohr’s theory of the atom, electrons absorb and emit radiation of fixed wavelengths when jumping between orbits around a nucleus. The theory provided a good description of the spectrum created by the hydrogen atom, but needed to be developed to suit more complicated atoms and molecules. Following Werner Heisenberg’s initial work around 1925, Max Born contributed to the further development of quantum mechanics. He also proved that Schrödinger’s wave equation could be interpreted as giving statistical (rather than exact) predictions of variables.
Summary
Max Born (born Dec. 11, 1882, Breslau, Ger. [now Wrocław, Pol.]—died Jan. 5, 1970, Göttingen, W.Ger.) was a German physicist who shared the Nobel Prize for Physics in 1954 with Walther Bothe for his probabilistic interpretation of quantum mechanics.
Born came from an upper-middle-class, assimilated, Jewish family. At first he was considered too frail to attend public school, so he was tutored at home before being allowed to attend the König Wilhelm Gymnasium in Breslau. Thereafter he continued his studies in physics and mathematics at universities in Breslau, Heidelberg, Zürich, and Göttingen. At the University of Göttingen he wrote his dissertation (1906), on the stability of elastic wires and tapes, under the direction of the mathematician Felix Klein, for which he was awarded a doctorate in 1907.
After brief service in the army and a stay at the University of Cambridge, where he worked with physicists Joseph Larmor and J.J. Thomson, Born returned to Breslau for the academic year 1908–09 and began an extensive study of Albert Einstein’s theory of special relativity. On the strength of his papers in this field, Born was invited back to Göttingen as an assistant to the mathematical physicist Hermann Minkowski. In 1912 Born met Hedwig Ehrenberg, whom he married a year later. Three children, two girls and a boy, were born from the union. It was a troubled relationship, and Born and his wife often lived apart.
In 1915 Born accepted a professorship to assist physicist Max Planck at the University of Berlin, but World War I intervened and he was drafted into the German army. Nonetheless, while an officer in the army, he found time to publish his first book, Dynamik der Kristallgitter (1915; Dynamics of Crystal Lattices).
In 1919 Born was appointed to a full professorship at the University of Frankfurt am Main, and in 1921 he accepted the position of professor of theoretical physics at the University of Göttingen. James Franck had been appointed professor of experimental physics at Göttingen the previous year. The two of them made the University of Göttingen one of the most important centres for the study of atomic and molecular phenomena. A measure of Born’s influence can be gauged by the students and assistants who came to work with him—among them, Wolfgang Pauli, Werner Heisenberg, Pascual Jordan, Enrico Fermi, Fritz London, P.A.M. Dirac, Victor Weisskopf, J. Robert Oppenheimer, Walter Heitler, and Maria Goeppert-Mayer.
The Göttingen years were Born’s most creative and seminal. In 1912 Born and Hungarian engineer Theodore von Karman formulated the dynamics of a crystal lattice, which incorporated the symmetry properties of the lattice, allowed the imposition of quantum rules, and permitted thermal properties of the crystal to be calculated. This work was elaborated when Born was in Göttingen, and it formed the basis of the modern theory of lattice dynamics.
In 1925 Heisenberg gave Born a copy of the manuscript of his first paper on quantum mechanics, and Born immediately recognized that the mathematical entities with which Heisenberg had represented the observable physical quantities of a particle—such as its position, momentum, and energy—were matrices. Joined by Heisenberg and Jordan, Born formulated all the essential aspects of quantum mechanics in its matrix version. A short time later, Erwin Schrödinger formulated a version of quantum mechanics based on his wave equation. It was soon proved that the two formulations were mathematically equivalent. What remained unclear was the meaning of the wave function that appeared in Schrödinger’s equation. In 1926 Born submitted two papers in which he formulated the quantum mechanical description of collision processes and found that in the case of the scattering of a particle by a potential, the wave function at a particular spatiotemporal location should be interpreted as the probability amplitude of finding the particle at that specific space-time point. In 1954 he was awarded the Nobel Prize for this work.
Born remained at Göttingen until April 1933, when all Jews were dismissed from their academic posts in Germany. Born and his family went to England, where he accepted a temporary lectureship at Cambridge. In 1936 he was appointed Tait Professor of Natural Philosophy at the University of Edinburgh. He became a British citizen in 1939 and remained at Edinburgh until his retirement in 1953. The next year, he and his wife moved to Bad Pyrmont, a small spa town near Göttingen.
Details
Max Born (11 December 1882 – 5 January 1970) was a German–British theoretical physicist who was instrumental in the development of quantum mechanics. He also made contributions to solid-state physics and optics, and supervised the work of a number of notable physicists in the 1920s and 1930s. He shared the 1954 Nobel Prize in Physics with Walther Bothe "for his fundamental research in quantum mechanics, especially for his statistical interpretation of the wavefunction."
Born entered the University of Göttingen in 1904, where he met the three renowned mathematicians Felix Klein, David Hilbert, and Hermann Minkowski. He wrote his Ph.D. thesis on the subject of the stability of elastic wires and tapes, winning the university's Philosophy Faculty Prize. In 1905, he began researching special relativity with Minkowski, and subsequently wrote his habilitation thesis on the Thomson model of the atom. A chance meeting with Fritz Haber in Berlin in 1918 led to discussion of how an ionic compound is formed when a metal reacts with a halogen, which is now known as the Born–Haber cycle.
During World War I, Born was originally placed as a radio operator, but his specialist knowledge led to his being moved to research duties on sound ranging. In 1921 Born returned to Göttingen, where he arranged another chair for his long-time friend and colleague James Franck. Under Born, Göttingen became one of the world's foremost centres for physics. In 1925, Born and Werner Heisenberg formulated the matrix mechanics representation of quantum mechanics. The following year, he formulated the now-standard interpretation of the probability density function for ψ*ψ in the Schrödinger equation, for which he was awarded the Nobel Prize in 1954.
His influence extended far beyond his own research: Max Delbrück, Siegfried Flügge, Friedrich Hund, Pascual Jordan, Maria Goeppert Mayer, Lothar Nordheim, Robert Oppenheimer, and Victor Weisskopf all received their Ph.D. degrees under Born at Göttingen, and his assistants included Enrico Fermi, Werner Heisenberg, Gerhard Herzberg, Friedrich Hund, Wolfgang Pauli, Léon Rosenfeld, Edward Teller, and Eugene Wigner.
In January 1933, when the Nazi Party came to power in Germany, Born, who was born into a Jewish family, was suspended from his professorship at the University of Göttingen. He emigrated to the United Kingdom, where he took a job at St John's College, Cambridge, and wrote a popular science book, The Restless Universe, as well as Atomic Physics, which soon became a standard textbook. In October 1936, he was appointed Tait Professor of Natural Philosophy at the University of Edinburgh, where, working with German-born assistants E. Walter Kellermann and Klaus Fuchs, he continued his research into physics. He became a naturalised British subject on 31 August 1939, one day before World War II broke out in Europe. He remained in Edinburgh until 1952, when he retired to Bad Pyrmont, West Germany, and died in a hospital in Göttingen on 5 January 1970.
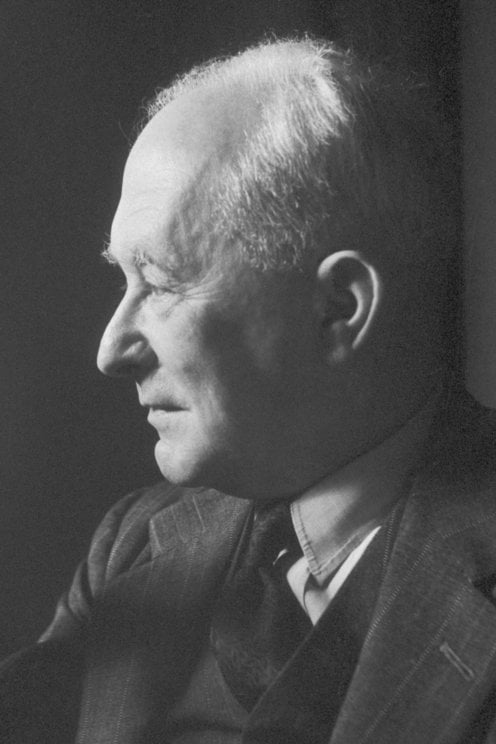
#15 Dark Discussions at Cafe Infinity » Come Quotes - XX » Yesterday 00:02:31
- Jai Ganesh
- Replies: 0
Come Quotes - XX
1. We're not a fragile people. We're not a frightful people. Our power doesn't come from some self-declared savior promising that he alone can restore order as long as we do things his way. We don't look to be ruled. - Barack Obama
2. Because I'm pretty, everybody thinks I'm stupid. But it is like a mask, and you have to break the mask to show that there is something else behind it. You have to show who you are to make the others come to discover you. - Monica Bellucci
3. I think politics come out of psychology. - Bruce Springsteen
4. Sometimes you need conflict in order to come up with a solution. Through weakness, oftentimes, you can't make the right sort of settlement, so I'm aggressive, but I also get things done, and in the end, everybody likes me. - Donald Trump
5. Nothing else in the world... not all the armies... is so powerful as an idea whose time has come. - Victor Hugo
6. As athletes, we're used to reacting quickly. Here, it's 'come, stop, come, stop.' There's a lot of downtime. That's the toughest part of the day. - Michael Jordan
7. You have to try to dismiss the loudness of cynicism. It's certainly going to come. - Kevin Costner
8. Non-violence, which is the quality of the heart, cannot come by an appeal to the brain. - Mahatma Gandhi.
#16 Jokes » Melon Jokes - I » Yesterday 00:02:14
- Jai Ganesh
- Replies: 0
Q: When do you go at red and stop at green?
A: When you're eating a watermelon.
* * *
Q: Why do watermelons have fancy weddings?
A: Because they cantaloupe.
* * *
Q: What happens if life gives you melons?
A: Your Dyslexic.
* * *
Q: What do you call a honeydew that is depressed?
A: Melon-choly.
* * *
Q: Did you see the cantaloupe in a suit?
A: It looked like a melon bucks.
* * *
#17 Re: Dark Discussions at Cafe Infinity » Greatest Mathematicians from 1 CE ... » Yesterday 00:01:55
28) René Descartes
René Descartes (31 March 1596 – 11 February 1650) was a French philosopher, scientist, logician, and mathematician, widely considered a seminal figure in the emergence of modern philosophy and science during Renaissance era. Mathematics was paramount to his method of inquiry, and he connected the previously separate fields of geometry and algebra into analytic geometry.
Refusing to accept the authority of previous philosophers, Descartes frequently set his views apart from the philosophers who preceded him. In the opening section of the Passions of the Soul, an early modern treatise on emotions, Descartes goes so far as to assert that he will write on this topic "as if no one had written on these matters before." His best-known philosophical statement is "cogito, ergo sum" ("I think, therefore I am," French: "Je pense, donc je suis").
Descartes has often been called the father of modern philosophy, and he is largely seen as responsible for the increased attention given to epistemology in the 17th century. He was one of the key figures in the Scientific Revolution, and his Meditations on First Philosophy and other philosophical works continue to be studied. His influence in mathematics is equally apparent, being the namesake of the Cartesian coordinate system. Descartes is also credited as the father of analytic geometry, which facilitated the discovery of infinitesimal calculus and analysis.
Descartes's work provided the basis for the calculus developed by Leibniz and Newton, who applied the infinitesimal calculus to the tangent line problem, thus permitting the evolution of that branch of modern mathematics. His rule of signs is also a commonly used method to determine the number of positive and negative roots of a polynomial.
#18 This is Cool » Switzerland » 2026-03-03 18:02:56
- Jai Ganesh
- Replies: 0
Switzerland
Gist
Switzerland is famous for its stunning Alpine scenery, high-quality watches, delicious chocolate and cheese, political neutrality, and efficient transport, blending traditional charm with modern innovation in finance and technology, making it known for luxury, precision, and breathtaking natural beauty.
Switzerland is famous for its neutrality, diverse languages (German, French, Italian, Romansh), high quality of life, precise watches, and rich chocolate, all set within stunning Alpine landscapes featuring numerous lakes and mountains. Key facts include its unique square flag, non-EU membership, efficient trains, and a tradition of high gun ownership alongside its neutrality.
Summary
Switzerland, officially the Swiss Confederation, is a landlocked country located at the intersection of Central, Western, and Southern Europe. It is bordered by Germany to the north, France to the west, Austria and Liechtenstein to the east, and Italy to the south. Switzerland is geographically divided among the Swiss Alps, the Swiss Plateau, and the Jura Mountains; the Alps cover most of the country's territory, whereas the majority of its 9 million people are concentrated on the plateau, which hosts many of the largest cities and economic centres, including Zurich, Geneva, Basel, Bern, Lausanne, Winterthur, and Lucerne.
Switzerland is a federal republic composed of 26 cantons, with Bern serving as the federal city and the seat of the national government. The country encompasses four principal linguistic and cultural regions—German, French, Italian, and Romansh—reflecting a long-standing tradition of multilingualism and cultural pluralism. Swiss national identity nonetheless remains fairly cohesive, rooted in a shared historical background, common values such as federalism and direct democracy, and Alpine symbolism. Swiss nationhood transcends language, ethnicity, and religion, leading to Switzerland being described as a Willensnation ("nation of volition") rather than a conventional nation state.
The country originates from the Old Swiss Confederacy established in the Late Middle Ages as a defensive and commercial alliance; the Federal Charter of 1291 is considered the country's founding document. The confederation steadily expanded and consolidated despite external threats and internal political and religious strife. Swiss independence from the Holy Roman Empire was formally recognized in the Peace of Westphalia in 1648. The confederation was among the first and few republics of the early modern period, and the only one besides San Marino to survive the Napoleonic Wars. Switzerland remained a network of self-governing states until 1798, when revolutionary France invaded and imposed the centralist Helvetic Republic. Napoleon abolished the republic in 1803 and reinstated a confederation. Following the Napoleonic Wars, Switzerland restored its pre-revolutionary system, but by 1830 faced growing division and conflict between liberal and conservative movements; this culminated in a new constitution in 1848 that established the current federal system and enshrined principles such as individual rights, separation of powers, and parliamentary bicameralism.
Switzerland has maintained a policy of armed neutrality since the 16th century and has not fought an international war since 1815. It joined the Council of Europe in 1964 and the United Nations (UN) in 2002, pursuing an active foreign policy that includes frequent involvement in peace building and global governance. Switzerland is the birthplace of the Red Cross and hosts the headquarters or offices of most major international institutions, including the WTO, the WHO, the ILO, FIFA, the WEF, and the UN. It is a founding member of the European Free Trade Association (EFTA) and participates in the European single market and the Schengen Area. Switzerland is among the world's most developed countries, with the highest nominal wealth per adult and the eighth-highest gross domestic product (GDP) per capita. It performs highly on several international metrics, including economic competitiveness, democratic governance, and press freedom. Zurich, Geneva and Basel rank among the highest in quality of life, albeit with some of the highest costs of living. Switzerland has a longstanding banking and financial sector, advanced pharmaceutical and biotechnology industries, and a strong tradition of watchmaking, precision engineering, and technology. It is known for its chocolate and cheese production, well-developed tourism industry, and growing startup sector.
Details
Switzerland is a federated country of central Europe. Switzerland’s administrative capital is Bern, while Lausanne serves as its judicial center. Switzerland’s small size—its total area is about half that of Scotland—and its modest population give little indication of its international significance.
A landlocked country of towering mountains, deep Alpine lakes, grassy valleys dotted with neat farms and small villages, and thriving cities that blend the old and the new, Switzerland is the nexus of the diverse physical and cultural geography of western Europe, renowned for both its natural beauty and its way of life. Aspects of both have become bywords for the country, whose very name conjures images of the glacier-carved Alps beloved of writers, artists, photographers, and outdoor sports enthusiasts from around the world.
For many outsiders, Switzerland also evokes a prosperous if rather staid and unexciting society, an image that is now dated. Switzerland remains wealthy and orderly, but its mountain-walled valleys are far more likely to echo the music of a local rock band than a yodel or an alphorn. Most Swiss live in towns and cities, not in the idyllic rural landscapes that captivated the world through Johanna Spyri’s Heidi (1880–81), the country’s best-known literary work. Switzerland’s cities have emerged as international centers of industry and commerce connected to the larger world, a very different tenor from Switzerland’s isolated, more inward-looking past. As a consequence of its remarkably long-lived stability and carefully guarded neutrality, Switzerland—Geneva, in particular—has been selected as headquarters for a wide array of governmental and nongovernmental organizations, including many associated with the United Nations (UN)—an organization the Swiss resisted joining until the early 21st century.
Switzerland’s rugged topography and multicultural milieu have tended to emphasize difference. People living in close proximity may speak markedly distinct, sometimes nearly mutually unintelligible dialects of their first language, if not a different language altogether. German, French, Italian, and Romansh all enjoy national status, and English is spoken widely. Invisible lines separate historically Protestant from historically Roman Catholic districts, while the tall mountains of the St. Gotthard Pass separate northern from southern Europe and their diverse sensibilities and habits. Yet, Switzerland has forged strength from all these differences, creating a peaceful society in which individual rights are carefully balanced against community and national interests.
Switzerland was formed in 1291 by an alliance of cantons against the Habsburg dynasty—the Confoederatio Helvetica (or Swiss Confederation), from which the abbreviation CH for Switzerland derives—though only in 1848, when a new constitution was adopted, was the present nation formed. Prior to 1848, internal conflict was quite common, but Switzerland has enjoyed relative domestic tranquility since the mid-19th century, and its organization has remained essentially the same: it is a union of more than 3,000 communes, or municipalities, situated in 26 cantons, six of which are traditionally referred to as demicantons (half cantons) but function as full cantons. Ordinary citizens are able to participate at every level of politics and regularly exercise their will in referenda and initiatives, through which Swiss citizens directly make numerous policy decisions at the national and subnational levels. Two effects of this popular involvement are evident: Swiss taxes are rather low by European standards, because voters are able to review and approve a broad range of expenditures, and political decision making tends to be slow, because contending individual claims and opinions must be allowed to be expressed at every step.
That high level of citizen involvement prompted the renowned 20th-century Swiss playwright and ironist Friedrich Dürrenmatt to allegorize Switzerland as a prison in which each Swiss citizen was at the same time prisoner and guard. Even so, the Swiss blend of federalism and direct democracy is unique in the world and is considered central to the country’s political and economic success. And Switzerland is indeed a major economic power, thanks to its long tradition of financial services and high-quality, specialized manufactures of items such as precision timepieces, optics, chemicals, and pharmaceuticals, as well as of specialty foodstuffs such as Emmentaler cheese and milk chocolate. Switzerland is regularly judged to have among the world’s highest standards of living.
Bern is a placid city whose name derives from the bear pits the canton’s medieval rulers established there as a heraldic symbol; the bear pits are now part of the city’s popular zoo. A metropolis extending along a large lake where the mountains meet the plains, Zürich is by far the country’s largest and most cosmopolitan city, its famed Bahnhofstrasse rivaling shopping districts found in other leading cities in the world. Basel and Lucerne are major German-speaking cities, Geneva and Lausanne the centers of the country’s French-speaking cantons, and Bellinzona and Lugano the principal cities in the Italian-speaking Ticino.
Switzerland has long been a model multiethnic, multilingual society, a place in which diverse peoples can live in social harmony and unite in common interest.
Land
Switzerland is bordered to the west by France, to the north by Germany, to the east by Austria and Liechtenstein, and to the south by Italy. It extends about 135 miles (220 km) from north to south and 220 miles (350 km) at its widest extent from west to east. Switzerland’s landscape is among the world’s most unusual, and it has long had to contend with a variety of environmental problems that threaten its integrity. Economic development and high population density have caused severe environmental stress, resulting in pollution and debates over the use of natural resources. During the 1970s and ’80s, ambitious environmental policies were implemented by the cantons and municipalities, and this led to impressive progress on pollution abatement. For example, air-pollution emissions in Switzerland are among the lowest in industrialized countries.
Relief and drainage
Situated at the hydrographic center of Europe, Switzerland is the source of many major rivers. The two most important are the Rhône, which flows into the Mediterranean Sea, and the Rhine, which empties into the North Sea. Switzerland’s small area contains an unusual diversity of topographic elements, which are divisible into three distinct regions: the Jura Mountains in the northwest, the Alps to the south and east, and the Mittelland, or central plateau, between the two mountain ranges.
The Jura (Celtic: “Forest”), a rolling mountain range in the northwest, occupies about one-eighth of the country. The region was formed under the extended impact of the general Alpine folding, which created the folded Jura that abuts the Mittelland and the tabular plateau Jura that forms the northern edge of the range. Jurassic limestone and marl with rich fossil content are the characteristic rocks that dip below the Mittelland and appear again in the pre-Alps. The limestone has been eroded in typical karst fashion, with sinkholes, caves, and underground drainage common. The ridges, covered with meadows and only sparsely forested, receive more precipitation than do the valleys, the slopes of which are wooded. Between Saint-Imier Valley (Vallon St. Imier) and the Doubs, a river that forms part of the border with France, the Jura has been reduced by denudation to form an undulating plateau that extends into France. Known as the Franches Montagnes (French: “Free Mountains”), a name acquired in 1384 when the bishop of Basel freed the inhabitants from taxation to encourage settlement of the remote area, this tableland is characterized by mixed agriculture and dairying. The highest point in the Jura, Monte Tendre, at about 5,500 feet (1,700 meters), is well below the Alps; indeed, the Jura was not a significant barrier to surface movement even before modern railroads and highways were constructed. Entrenched transverse valleys known as cluses have been eroded across the Jura ridges, providing relatively easy routes for transportation. The climate of the Jura, which has abundant precipitation, is the most continental of Switzerland; cross-country skiing is popular during the long winters. Switzerland’s watchmaking industry had its beginning in these mountains.
The Alps were built of large complexes of massed overthrusts of extremely varied sedimentary, metamorphic, and igneous rocks that were shaped by glaciation. The canton of Valais contains many striking Alpine peaks, including the Dufourspitze on the Monte Rosa massif, at 15,203 feet (4,634 meters) the highest point in Switzerland; the Weisshorn (14,780 feet [4,405 meters]), overlooking the valley called the Mattertal; the Dom (14,912 feet [4,545 meters]), above the village of Saas Fee; and the ice-sculpted Matterhorn (14,691 feet [4,478 meters]), long a symbol of Switzerland. The northern and southern Swiss Alps are separated by the trough formed by the Rhône and upper Rhine valleys, the narrowest portion being the Urseren valley, which lies between two crystalline central massifs, the Gotthard and the Aare.
The Alps’ role as the European watershed is most apparent in the central Alpine region of Switzerland, where the different chains meet; from there the Rhône River flows west, the Rhine River east, the Ticino River south to the Po River, and the Reuss River north to the Aare. The fundamental Alpine source point, however, is located in the upper Engadin valley at the Piz Lunghin, from which streams flow toward the North and Adriatic seas and from which the headwaters of the Inn River flow toward the Danube and ultimately into the Black Sea.
The country’s geographically destined role as guardian of Europe’s natural trans-Alpine routes has been both a reason for and a basic tenet of its existence—a role expressed in its traditional neutrality in times of war. In the central Alpine region lies the St. Gotthard route, the first and shortest north-south passage through the mountains and an important European linkage; it was opened in the early 13th century with the construction of a bridge in the Schöllenen Gorge, which traverses the northern chain, while the southern range is crossed by the St. Gotthard Pass at an elevation of 6,916 feet (2,108 meters). The 9-mile (14-km) St. Gotthard rail tunnel through the pass was opened in 1882; a twin 10.5-mile (17-km) road tunnel was opened in 1980.
Despite the tunnels, increasing rail and highway traffic often resulted in long delays through the mountains. For example, on weekends during the peak summer tourist season, cars and trucks were often backed up some 10 to 15 miles (16 to 25 km). To address this congestion, in 1992 Swiss voters approved the construction of a massive 35-mile (57-km) rail tunnel well beneath the existing St. Gotthard tunnels. Primary excavation was completed in 2010, and upon its official opening in June 2016, the Gotthard Base Tunnel was the longest and most deeply set rail tunnel in the world. Because the tunnel was excavated at virtually zero-grade, it could accommodate high-speed trains that would slash travel times between northern and southern Europe and reduce traffic-choked-up Alpine roads.
Between the Jura and main Alpine ranges lies the hilly Mittelland, accounting for nearly one-fourth of the country and enclosed by the two mountain ranges and the two largest lakes, Lake Geneva (Lac Léman) in the west and Lake Constance (Bodensee) in the east. The fertile rolling land of the Mittelland is the agricultural heartland of the country and is where the majority of Swiss settlements, population, and industry are situated. Furthermore, vital east-west highway and rail routes bind the urban areas. As a result, the Mittelland is highly urbanized, with large chunks of land sterilized by shopping centers, housing estates, motorways, oil-storage tanks, container depots, warehouses, automobile distribution centers, and industrial complexes.
Soils
Soil conditions and agriculture reflect the diversity of Switzerland’s climate and geologic structure. The major soil groups consist of gray-brown podzolic soils and brown forest soils, loess, glacial drift, and alluvium in the Mittelland; brown forest soils, rendzinas, and the heavier glacial clays in the Jura valleys; and the lithosol and podzolized soils of the high Alps.
Climate of Switzerland
Four major European climates affect Switzerland. From the west, influenced by the North Atlantic Drift, come mild and moist air masses; dry and cold air arrives from the North Arctic areas; continental air from the east brings dry colder air in winter and warmer air in summer; and relatively moist and warm air flows northward from the Mediterranean. The mixing of these air masses over Switzerland produces weather patterns that not only change according to which air masses are involved but also are characterized by great variation in temperature and precipitation because of local relief.
Wind systems
Prevailing winds are mainly from the west, but in valleys air currents are channeled into particularly frequent or violent local winds such as the Bise, a cold northeast wind that sweeps across the Mittelland and funnels down Lake Geneva to the city of Geneva. Foehn (German: Föhn) winds, which are associated with the leading edge of a low-pressure system moving across Europe north of Switzerland, often blow for one or two days; though they may occur anytime during the year, they are most frequent in spring. Sudden temperature increases occur because the foehn, which crosses the Alps from south to north (it can also blow from north to south, affecting Ticino), cools at a slower rate rising over the mountains because of precipitation; it is then heated and dried as it descends down the northern valleys, thereby moderating the climate on the northern slopes of the Alps.
Precipitation
Since rainfall tends to increase in direct proportion to altitude, precipitation varies according to relief. Thus, because of the marked variation in relief that characterizes Switzerland, differences in precipitation within short linear distances are often very great. For example, Sankt Gallen (St. Gall), at 2,556 feet (779 meters), has an average annual precipitation of about 50 inches (1,300 mm), while precipitation at Säntis, at an elevation of 8,202 feet (2,500 meters) but only some 12 miles (20 km) away, is more than 110 inches (2,800 mm). The average annual precipitation of three-fourths of the country exceeds 40 inches (1,000 mm), varying amounts of which fall as snow. In Lugano (at 896 feet [273 meters]), which is located in the canton of Ticino in the southeast and has a modified Mediterranean climate, little precipitation is in the form of snow; in Zürich (at 1,824 feet [556 meters]) about one-tenth is snow; and on the Säntis nearly three-fourths is snow. At elevations above 11,500 feet (3,500 meters), all precipitation is in the form of snow, which compacts into perpetual snowfields and glaciers; the snow line is at about 9,200 feet (2,800 meters) in the northern Alps and about 10,800 feet (3,300 meters) in the southern Alps of the Valais.
Dry areas
There are distinct dry pockets in the mountains of Switzerland’s interior. The best-known dry area is the Rhône valley in the Valais, which is closely encircled by the highest (13,000 feet [4,000 meters]) mountain groups. Although precipitation is slight on the slopes near the cantonal capital of Sion (at 1,581 feet [482 meters]), extensive irrigation is possible, since the valley is surrounded by large snowfields and by glaciers that extend down the upper valleys. The rarefied and dry though somewhat polluted air of such high-altitude towns as Davos (5,216 feet [1,590 meters]) and Arosa (5,987 feet [1,825 meters]) permits a more intense, broader-spectrum solar irradiation and thus produces a climate famous in the past for tuberculosis cures. Today the climate attracts skiers as well as tourists seeking an escape from the polluted air of lowland Europe. At elevations of 13,000 feet (4,000 meters), precipitation levels rise to some 160 inches (4,000 mm), and the Mönch (13,448 feet [4,099 meters]) in the Jungfrau group of mountains has the highest average annual precipitation in Switzerland, 163 inches (4,140 mm), while Stalden in the entrenched Vispa valley, 4 miles (6 km) south of the main Rhône valley, has the lowest, 21 inches (533 mm).
Skies and temperatures
The stable high-pressure weather conditions prevailing over central Europe and the Alps during autumn and winter create cold air masses that result in lowland fog, a climatic phenomenon with widely varying consequences. The mouths of the northern Alpine valleys, the basins of the Jura Mountains, and the villages and cities of the low areas of the Mittelland are blanketed for days and often for weeks on end, while towns located at higher altitudes enjoy warm, brilliant, high-pressure conditions and the view of the glistening sea of fog below them. Temperature inversions between mountain and valley locations in close proximity can be quite pronounced, with higher elevations having higher temperature readings. Frequent temperature inversion has made Switzerland’s high-altitude resorts healthful places even during winter and has helped the Alpine winter season gain popularity in Europe for sports; in addition, because of these inversions polluted air is much less common in areas of high elevation than in the lowlands. In fact, the temperature inversions that affect the Mittelland tend to trap polluted air for weeks when cyclonic activity stagnates.
Avalanches
With the increase in winter tourism, the study of avalanches has developed as a branch of Alpine climatology, and in wintertime the research station near Davos releases daily avalanche bulletins as a warning for villagers and tourists. The Alpine cantons have about 10,000 avalanches annually, with about four-fifths of them occurring in February, March, and April. For centuries, village communes have relied on forests on the mountain slopes for protection from these slides, because a 20- to 30-year-old forest can inhibit or stop small avalanches. Villages, highways, and Alpine paths are also protected by costly artificial structures such as metal barriers, earthen walls, and concrete wedges and enclosures. However, in the late 20th century, acid rain caused the illness and death of many trees in the mountain areas of Switzerland and posed a serious threat to their ability to act as barriers to avalanches. In the mountain forests, some two-fifths of the trees were classified as damaged, sick, or dying. Pollution-control legislation across Europe did much to reduce the harmful effects of acid rain in Switzerland, and a concerted effort was made by Swiss land managers to introduce healthy trees into unused Alpine pastureland and to increase the density of existing Alpine forests. By the early 21st century, avalanche-control forests had become healthier and denser, especially at higher elevations and in steeper terrain.
Plant and animal life:
Vegetation
Vegetation in Switzerland is derived from that of the four European climatic regions that converge in the country and has been influenced by the varied relief. It includes the beeches and oaks of the maritime west; hornbeam and larch trees in the more continental east, predominantly in the Engadin and the dry Valais; extensive spruce forests in the northern subalpine region; and chestnut groves in the south. Differences in vegetation are evident in the Alpine valleys because of exposure to the sun. The vegetation boundaries are several hundred feet higher in the south of the country—for example, in Valais—than in the north because of the southern exposure. Alpine vegetation, similar to that of Arctic tundra, prevails above the tree line. It is very susceptible to erosion through skiing impacts and as a result of paths or four-wheel-drive trails cut into the slopes.
Animal life
Switzerland’s animal life is primarily Alpine, but a mixture of species familiar to southern and north-central Europe is also found. Animal life is protected, except during a brief annual hunting season. Alpine tourists may observe marmots, which live in the high meadows, and chamois. Large herds of the round ibex, which had died out in the Swiss Alps and has since been reintroduced, populate several areas, especially in the Bernina region of Graubünden (canton) and in the Saastal of Valais. In the forests there are deer, rabbits, foxes, badgers, squirrels, and many varieties of birds, including eagles, while lake and river trout may be found but are no longer as abundant as in the past. Snakes and lizards are concentrated in the south, but insects, in great variety, are diffused throughout the country.
Additional Information
Switzerland is a small mountainous country located in central Europe. This landlocked country is about the size of New Jersey and is between France and Italy. It is also bordered by Austria, Germany, and Liechtenstein.
Most of the population lives in the plateau which is between the high Alps in the south and the Jura mountains in the north. The mountainous area in the south is sparsely populated.
People and Culture
Switzerland is one of the world’s wealthiest countries. The Swiss are well known for their watches and clocks.
There is not a single official language in Switzerland. People speak one of several languages, including Swiss German, French, and Italian.
Nature
The Swiss Alps are high, snow-covered mountains most of which are over 13,000 feet (4,000 meters). The most famous peak is the Matterhorn which is 14,692 feet (4,478 meters) tall, but the highest peak is Dufourspitze at 15,203 feet (4,634 meters).
Scientists are concerned that glaciers in the Swiss Alps have lost a lot of ice coverage in the past 40 years. This may be related to global climate change. Rapid melting of the glaciers could cause flooding to the villages below.
Most animals in Switzerland live in the mountains. The ibex, a species of mountain goat, was hunted to near extinction in the early 1800s. The species has since been reintroduced and more than 15,000 ibex now live in the Swiss Alps. Hikers may also encounter chamois, another goatlike animal, and marmots. The forests of Switzerland are also home to deer, rabbits, foxes, badgers, squirrels, and many bird species.
Government
The country is made up of 26 cantons or states, which form the confederation. The leader of the government is the president. Both the president and vice president are elected by the Federal Assembly from the Federal Council. They serve a one-year term. Elections are usually held in December.
Representatives of the cantons are elected to the assembly for four-year terms.
History
Switzerland was formed in 1291 as a union of three states and became an independent country in 1815. The constitution, adopted in 1848, does not allow for troops to be sent to serve in foreign wars. The country has remained neutral in conflicts around the world, including both world wars.
Switzerland did not become a member of the United Nations until 2002, and is not a member of the European Union.

#19 Science HQ » Tibia » 2026-03-03 17:02:48
- Jai Ganesh
- Replies: 0
Tibia
Gist
The tibia, or shinbone, is the largest, strongest, and second-longest bone in the human body, located in the lower leg between the knee and ankle. As the primary weight-bearing bone of the lower leg, it articulates with the femur at the knee and the talus/fibula at the ankle.
The tibia, or shinbone, is the most commonly fractured long bone in the body. A tibial shaft fracture occurs along the length of the bone, below the knee and above the ankle. It typically takes a major force to cause this type of broken leg.
Summary
The tibia, also known as the shinbone, shankbone or simply the shin, is the larger, stronger, and anterior (frontal) of the two bones in the leg below the knee in vertebrates (the other being the fibula, behind and to the outside of the tibia); it connects the knee with the ankle. The tibia is found on the medial side of the leg next to the fibula and closer to the median plane. The tibia is connected to the fibula by the interosseous membrane of leg, forming a type of fibrous joint called a syndesmosis with very little movement. The tibia is named for the flute tibia. It is the second largest bone in the human body, after the femur. The leg bones are the strongest long bones as they support the rest of the body.
Structure
In human anatomy, the tibia is the second largest bone after the femur. As in other vertebrates the tibia is one of two bones in the lower leg, the other being the fibula, and is a component of the knee and ankle joints. The tibia together with the fibula make up the front part of the leg, between the knee and the ankle, known as the shin.
The ossification or formation of the bone starts from three centers, one in the shaft and one in each extremity.
The tibia is categorized as a long bone and is as such composed of a diaphysis and two epiphyses. The diaphysis is the midsection of the tibia, also known as the shaft or body. While the epiphyses are the two rounded extremities of the bone; an upper (also known as superior or proximal) closest to the thigh and a lower (also known as inferior or distal) closest to the foot. The tibia is most contracted in the lower third and the distal extremity is smaller than the proximal.
Details
The tibia is the second-longest bone in your body. It plays an important role in how you stand, move and keep your balance. It’s also an anchor for other tissue, like muscles, tendons, ligaments and nerves. Tibias are strong. So, it usually takes a serious injury like a car accident to break them.
What Is the Tibia?
The tibia is your shin bone. It’s an important part of how you can stand and move.
It’s the second-longest bone in your body. Most adults’ tibias are around 15 inches (38 centimeters) long. Only your thigh bone (femur) is longer.
Your tibias are some of your strongest bones. So, they won’t need much maintenance. But listen to your body if you’re feeling pain or any other symptoms. Everyone has an occasional ache or pain. See a healthcare provider if you’re feeling bone pain from deep inside your shin or know you experienced an injury.
Function:
What does the tibia do?
Your tibia has several important jobs, including:
* Supporting the weight of your body when you stand and move
* Stabilizing you as you move
* Linking your ankle and knee joints together so they can work to move your legs
* Anchoring muscles, tendons and ligaments in your legs, knees and ankles
Anatomy:
Where is the tibia located?
You have one tibia in each shin. It’s the bigger bone in your lower leg that’s closer to the front. The calf bone (fibula) is the other.
The tibia runs from just under your knee to your ankle. It’s closer to the inside of your body than the fibula.
What does the tibia look like?
The tibia has a flat end that forms a shelf at the bottom of your knee, a long middle shaft and a notch at the bottom where it forms your ankle.
These three sections each have a medical name:
* Proximal aspect: This is the upper end of your tibia, near your knee. The parts of the proximal aspect include the medial condyle, lateral condyle and intercondylar eminence.
* Shaft: This is the long middle of the tibia. It’s what supports your weight and forms the structure of your shin. It has three sides and is roughly shaped like a 3D triangle. The shaft includes the anterior border, posterior surface, soleal line and lateral border.
* Distal aspect: This is the lower end of your tibia. It connects to the fibula and talus, and sits on top of the heel bone (calcaneus). This forms your ankle joint. The distal aspect includes the medial malleolus and fibular notch.
You’ll probably never need to know or remember these medical names and labels. They’re usually more for your healthcare provider to use as they describe where something is affecting your tibia. You might see some of these words on an X-ray result, for example.
Conditions and Disorders:
What are the common conditions and disorders that affect the tibia?
Your tibias are strong, but injuries and some health conditions can damage them, including:
* Tibia fractures: Broken tibias are less common than other bone fractures. That’s because they’re one of your strongest bones. It usually takes a severe injury, like a fall from a roof or a car accident, to break them.
* Osteoporosis: This condition weakens your bones. Your provider might suggest a bone density test if you’re over 50 or have a biological family history of osteoporosis.
* Osgood-Schlatter disease: This causes pain in your knee and upper shin when tendons pull against the top of your shin bone. It usually affects growing kids and teens. It’s sometimes called jumper’s knee.
* Paget’s disease of the bone: This condition causes your affected bones to constantly break down and reform. It’s more common in people over 50 of Northern European descent.
Visit a healthcare provider if you notice any new pain or other symptoms in your shin. Especially if they last more than a few days. Go to the emergency room if you think you might have a broken tibia. Don’t try to walk or put weight on that leg.
Additional Information
The tibia is one of your leg bones. Some tibia conditions commonly affect kids and can cause leg pain or trouble walking.
What Is the Tibia?
The tibia, also called the shin bone, is one of the two bones in your lower leg. It’s the second-longest bone in your body after your thigh bone. The tibia is paired with a shorter leg bone called the fibula, and they’re connected with an interosseous membrane. This membrane is a sheet of connective tissue that acts as a type of joint.
What Is the Function of the Tibia?
Your bones are living organs with a few important functions. They provide structure and shape to your body, help you move, and store minerals like calcium and bone marrow.
There are two types of bone marrow: red marrow, which has stem cells that become red or white blood cells and platelets, and yellow marrow, which becomes fat, cartilage, or bone cells. The tibia is a long bone that contains mostly yellow marrow, but the ends also contain some red marrow.
Your tibia has a few other specific functions, too.
* Bears weight. As the more prominent bone in your lower leg, your tibia is the weight-bearing bone. It’s the stronger of your lower leg bones, supporting the weight of your body as you move and stand. It also stabilizes your knee and ankle joints with the help of the fibula.
* Muscle attachment. The tibia also serves as a point of attachment for 11 muscles, as well as tendons and ligaments that help you extend and flex your knee joint and move your ankle joint.
Where Is the Tibia Located?
The tibia is in your lower leg between your knee and your ankle. While the slender fibula bone is on the outside, or lateral side, of your lower leg, the tibia is oriented toward the middle (or, medial) part of your lower leg.
There are several tibia parts, including blood vessels and nerves that supply nutrients and signals to the bone. Other tibia structure includes:
Proximal aspect. The proximal part of your tibia is the upper end that connects with your femur. It has a rounded head with a medial condyle and lateral condyle (or, middle and outer knob). Here, medial means its position is closest to the middle of your body.
Related:
How to Prevent Damaging Your Kidneys
The upper end has a flat head with an intercondylar eminence between the two knobs. That’s a raised area where two ligaments attach.
Tibia shaft. The shaft is the long part of the bone that bears your weight. It has an anterior border you can feel just under your skin on your lower leg. The shaft also has a posterior or back surface and a soleal line where muscles attach to the back of your leg.
Distal aspect. The distal aspect of your tibia is the lower end that connects with your ankle. It has a medial malleolus, which forms a large bony bump on the inside of your ankle. It’s sometimes called the little hammer.
The fibular notch is on the outside lower part of your tibia. This is a wide groove that connects with the bottom of the fibula and forms a joint.
Signs Something Could Be Wrong With Your Tibia
While your tibia is one of your strongest bones, it’s not unbreakable. Some signs and symptoms of damage can include:
* Pain when you walk or run
* Inability to bear weight on your leg
* Bowed leg
* In-toeing, where your toes point in instead of straight out
* One leg appears noticeably shorter than the other
* Knee arthritis
* Swelling
* Bruising
* Bump in your skin that’s not usually there.
What Conditions Affect Your Tibia?
Lots of conditions can affect your tibia bone. Some of the most common include:
* Tibia injuries. Tibia fractures (or, broken tibia bones), are one of the most common injuries of the lower limbs in children. A fracture can be a low-energy break caused by falling or twisting your leg or a high-energy break caused by extreme force, like a fall from heights or a car accident.
* Osgood-Schlatter disease. This disease often causes knee pain, occurring because of irritation of the growth plate at the top of your shin bone. The tendon that connects the kneecap to the tibia pulls on the growth plate and causes pain during activity and sports. This disease affects kids during puberty and goes away on its own without lasting effects.
* Blount disease. This condition also affects the growth plate at the top of the tibia in children. It happens when extra weight puts pressure on the growth plate and stops the bone from growing normally. The inside slows down, but the outside keeps growing, which causes the tibia to bow outward instead of growing straight.
* Paget’s disease of bone. Paget’s disease of the bone happens when your bones repair themselves at a faster rate than normal. Your bones thus become soft or too large, which leads to bending and weakness. This mostly affects older people.
* Osteoporosis. Your bones continually break down and rebuild. Osteoporosis happens when your bones can’t keep up with the loss of old bone, which causes weak and brittle bones. They become so brittle that even mild stress can lead to a broken bone. Anyone can get osteoporosis, but older white and Asian women have a higher risk.
* Shin splints. Also known as tibial stress syndrome, shin splints are the pain you get on the front of your lower legs from exercise. This happens when you repeatedly stress your shin bone from running, after you increase exercise intensity, or because of flat feet or high arches.
How Can You Keep Your Tibia Healthy?
Your tibiae are important for movement and stabilization. You can keep your bones healthy with a few simple habits, like:
* Getting regular exercise
* Maintaining a healthy weight
* Eating a healthy, balanced diet
* Wearing protective gear during sports
If you have ongoing or severe pain, leg deformity, or you can’t use or move your leg, especially after a fall or accident, seek medical attention.

#20 Re: Jai Ganesh's Puzzles » General Quiz » 2026-03-03 16:16:30
Hi,
#10779. What does the term in Biology Heterosis mean?
#10780. What does the term in Biology Heterotroph mean?
#21 Re: Jai Ganesh's Puzzles » English language puzzles » 2026-03-03 15:57:09
Hi,
#5975. What does the noun foxtrot (British) mean?
#5976. What does the noun fracking mean?
#22 Re: Jai Ganesh's Puzzles » Doc, Doc! » 2026-03-03 15:40:02
Hi,
#2584. What does the medical term Sinoatrial node mean?
#23 Re: Jai Ganesh's Puzzles » 10 second questions » 2026-03-03 15:23:19
Hi,
#9870.
#24 Re: Jai Ganesh's Puzzles » Oral puzzles » 2026-03-03 14:48:41
Hi,
#6363.
#25 Re: Exercises » Compute the solution: » 2026-03-03 14:31:18
Hi,
2724.