Math Is Fun Forum
You are not logged in.
- Topics: Active | Unanswered
#1 Re: This is Cool » Miscellany » Today 00:06:18
2519) Potassium Dichromate
Gist
Potassium dichromate is a bright red-orange, odorless crystalline inorganic compound and a strong, non-deliquescent oxidizing agent commonly used in laboratories and industries. As a hexavalent chromium compound, it is highly toxic, corrosive, and a significant allergen. It is widely used for chrome tanning, dyeing, photography, and as a primary analytical standard in redox titrations.
Potassium dichromate is a strong oxidizing agent used in leather tanning (chrome tanning), wood staining (darkening), photography, and as a reagent in analytical chemistry for titrations and purity tests (Schwertzer's solution). It's also used in manufacturing matches, fireworks, and pigments, as a catalyst, and to make chromic acid for etching, though its toxicity has restricted many of these applications.
Summary
Potassium dichromate (K2Cr2O7) is a bright red-orange crystalline compound widely used in laboratories and industries as an oxidizing agent. Its non-deliquescent nature makes it preferable to other dichromates. However, as a hexavalent chromium compound, it is highly toxic and harmful to health.
Potassium dichromate, an inorganic compound with a bright orange colour, is widely used as an oxidizing agent in laboratories and industries. It is produced by reacting chromates with sodium or potassium carbonate. Although it is less potent than potassium permanganate, it is valued for its stability in acid and resistance to light and organic matter. Potassium dichromate is used in cleaning glassware, etching, and photographic screen printing. However, it is highly toxic and must be handled carefully due to its health hazards. As a dichromic acid dipotassium salt, it is one of the most significant and widely used chromium compounds in inorganic chemistry.
Physical Properties of Potassium Dichromate
Appearance: Solid at room temperature with bright orange crystals.
Odor and Taste: Odorless with a bitter taste.
Toxicity: It is toxic and can irritate the eyes.
Combustibility: Non-combustible but prone to rusting.
Melting and Boiling Points: Melts at 398°C and decomposes upon boiling at 500°C.
Solubility: Solubility increases in water at higher temperatures but is insoluble in alcohol and acetone.
Refractive Index: 1.738.
Structure: The chromium ion in potassium dichromate has a tetrahedral coordinate geometry. The crystalline structure is triclinic.
Details
Potassium dichromate is the inorganic compound with the formula K2Cr2O7. An orange solid, it is used in diverse laboratory and industrial applications. As with all hexavalent chromium compounds, it is chronically harmful to health. It is a crystalline ionic solid with a very bright, red-orange color. The salt is popular in laboratories because it is not deliquescent, in contrast to the more industrially relevant salt sodium dichromate.
Niche or archaic uses
Potassium dichromate has few major applications, as the sodium salt is dominant industrially. The main use is as a precursor to potassium chrome alum, used in leather tanning.
Photography and printing
In 1839, Mungo Ponton discovered that paper treated with a solution of potassium dichromate was visibly tanned by exposure to sunlight, the discoloration remaining after the potassium dichromate had been rinsed out. In 1852, Henry Fox Talbot discovered that exposure to ultraviolet light in the presence of potassium dichromate hardened organic colloids such as gelatin and gum arabic, making them less soluble.
These discoveries soon led to the carbon print, gum bichromate, and other photographic printing processes based on differential hardening. Typically, after exposure, the unhardened portion was rinsed away with warm water, leaving a thin relief that either contained a pigment included during manufacture or was subsequently stained with a dye. Some processes depended on the hardening only, in combination with the differential absorption of certain dyes by the hardened or unhardened areas. Because some of these processes allowed the use of highly stable dyes and pigments, such as carbon black, prints with an extremely high degree of archival permanence and resistance to fading from prolonged exposure to light could be produced.
Dichromated colloids were also used as photoresists in various industrial applications, most widely in the creation of metal printing plates for use in photomechanical printing processes.
Chromium intensification or Photochromos uses potassium dichromate together with equal parts of concentrated hydrochloric acid diluted down to approximately 10% v/v to treat weak and thin negatives of black and white photograph roll. This solution reconverts the elemental silver particles in the film to silver chloride. After thorough washing and exposure to actinic light, the film can be redeveloped to its end-point yielding a stronger negative which is able to produce a more satisfactory print.
A potassium dichromate solution in sulfuric acid can be used to produce a reversal negative (that is, a positive transparency from a negative film). This is effected by developing a black and white film but allowing the development to proceed more or less to the end point. The development is then stopped by copious washing and the film then treated in the acid dichromate solution. This converts the silver metal to silver sulfate, a compound that is insensitive to light. After thorough washing and exposure to actinic light, the film is developed again allowing the previously unexposed silver halide to be reduced to silver metal. The results obtained can be unpredictable, but sometimes excellent results are obtained producing images that would otherwise be unobtainable. This process can be coupled with solarisation so that the end product resembles a negative and is suitable for printing in the normal way.
Cr(VI) compounds have the property of tanning animal proteins when exposed to strong light. This quality is used in photographic screen-printing.
In screen-printing a fine screen of bolting silk or similar material is stretched taut onto a frame similar to the way canvas is prepared before painting. A colloid sensitized with a dichromate is applied evenly to the taut screen. Once the dichromate mixture is dry, a full-size photographic positive is attached securely onto the surface of the screen, and the whole assembly exposed to strong light – times vary from 3 minutes to a half an hour in bright sunlight – hardening the exposed colloid. When the positive is removed, the unexposed mixture on the screen can be washed off with warm water, leaving the hardened mixture intact, acting as a precise mask of the desired pattern, which can then be printed with the usual screen-printing process.
Analytical reagent
Because it is non-hygroscopic, potassium dichromate was a common reagent in classical "wet tests" in analytical chemistry.
Aldehyde test
In an aqueous solution the color change exhibited can be used as a test to distinguish aldehydes from ketones. Aldehydes reduce dichromate from the +6 to the +3 oxidation state, changing the solution color from orange to green. A ketone will show no such change because it cannot be oxidized further, and so the solution will remain orange.
Wood treatment
Potassium dichromate is used to stain certain types of wood by darkening the tannins in the wood. It produces deep, rich browns that cannot be achieved with modern color dyes. It is a particularly effective treatment on mahogany.
Natural occurrence
Potassium dichromate occurs naturally as the rare mineral lópezite. It has only been reported as vug fillings in the nitrate deposits of the Atacama Desert of Chile and in the Bushveld igneous complex of South Africa.
Safety
Potassium dichromate is a prevalent allergen in patch tests (4.8%). Its presence in cement can cause contact dermatitis in construction workers after extended exposure. In general, it is one of the most common causes of chromium dermatitis. Aquatic organisms are vulnerable to poisoning by dichromate salts, but far less so than organic pollutants.
As with other Cr(VI) compounds, potassium dichromate is carcinogenic. The compound is also corrosive and exposure may damage eyes. Human exposure further causes impaired fertility.
Additional Information
Potassium Dichromate is an orange to red colored, crystalline, inorganic compound that emits toxic chromium fumes upon heating. Potassium dichromate is highly corrosive and is a strong oxidizing agent. This substance is used in wood preservatives, in the manufacture of pigments and in photomechanical processes, but is mainly replaced by sodium dichromate. Potassium dichromate primarily affects the respiratory tract causing ulcerations, shortness of breath, bronchitis, pneumonia and asthma but can also affect the gastrointestinal tract, liver, kidneys and immune system. This substance is a known human carcinogen and is associated with an increased risk of developing lung cancer and cancer of the sinonasal cavity.
Potassium dichromate or anhydrochromate is prepared by adding to the neutral yellow chromate of potassium in solution, a moderate quantity of one of the stronger acids.
Potassium permanganate is commercially prepared by mixing a solution of potassium hydroxide and powdered manganese oxide with oxidizing agents like potassium chlorate.
Preparation of Potassium Dichromate – K2Cr2O7
* Potassium dichromate is an important chemical used in industries as an oxidizing agent and for the preparation of many other compounds.
* Dichromates are usually prepared from chromates and this is obtained by the combination of chromite ore with sodium/potassium carbonate in the presence of air.
Applications of Potassium Dichromate
The primary application of K2Cr2O7 is in the preparation of potassium chrome alum, a compound which is used extensively in the tanning of leather. Chromic acid can also be prepared from this compound. Potassium dichromate is known to be used in the production of cement since it improves the texture and the density of the cement mixture.
Another important application of potassium dichromate is in the photography industry, where it is used in combination with a powerful mineral acid as an oxidizing agent for photographic screen printing. Since it is non-hygroscopic in nature, this compound is also employed for several wet tests in the field of analytical chemistry.
Frequently Asked Questions – FAQs
Q1: What is the use of potassium dichromate?
A1. It is used in many applications as an oxidizing agent and is also used in the preparation of different products such as waxes, paints, glues, etc. Potassium dichromate is carcinogenic and highly toxic as a compound of hexavalent chromium.
Q2: What does the potassium dichromate test for?
A2. For organic chemistry, potassium dichromate is an oxidizing agent that is milder than potassium permanganate. It is used for the oxidation of alcohol. This converts primary alcohols into aldehydes and carboxylic acids under more pressing conditions.
Q3: Is potassium dichromate light-sensitive?
A3. Clear, light-sensitive orange crystals. Potassium dichromate is used in cotton dyeing as chromium mordant. In black and white image processing, potassium dichromate is used as an intensifier.
Q4: What is the charge of potassium dichromate?
A4. K2Cr2O7 is the molecular formula. A reddish-brown colour as a solid and a molecular weight of 294.18 grams per mole is the physical properties of potassium dichromate. Potassium dichromate is also referred to as a compound of hexavalent chromium, and chromium oxidation is 6+.

#2 Re: Dark Discussions at Cafe Infinity » crème de la crème » Today 00:05:57
2456) Hans Krebs
Gist:
Work
Nutrients are broken down in our cells to release energy for the construction of cells. After Albert Szent-Györgyi identified several important reactions in these metabolic processes, in 1937 Hans Krebs was able to present a complete picture of an important part of metabolism—the citric acid cycle. In this process, which is cyclical and has several steps, nutrients are converted to other molecules with a large amount of chemical energy. The latter are ultimately converted into adenosine triphosphate (ATP), which provides chemical energy to facilitate other biochemical processes in the cell.
Summary
Sir Hans Adolf Krebs (born Aug. 25, 1900, Hildesheim, Ger.—died Nov. 22, 1981, Oxford, Eng.) was a German-born British biochemist who received (with Fritz Lipmann) the 1953 Nobel Prize for Physiology or Medicine for the discovery in living organisms of the series of chemical reactions known as the tricarboxylic acid cycle (also called the citric acid cycle, or Krebs cycle). These reactions involve the conversion—in the presence of oxygen—of substances that are formed by the breakdown of sugars, fats, and protein components to carbon dioxide, water, and energy-rich compounds.
At the University of Freiburg (1932), Krebs discovered (with the German biochemist Kurt Henseleit) a series of chemical reactions (now known as the urea cycle) by which ammonia is converted to urea in mammalian tissue; the urea, far less toxic than ammonia, is subsequently excreted in the urine of most mammals. This cycle also serves as a major source of the amino acid arginine.
The son of a Jewish physician, Krebs was forced in 1933 to leave Nazi Germany for England, where he continued his research at the University of Cambridge (1933–35). At Sheffield University, Yorkshire (1935–54), Krebs measured the amounts of certain four-carbon and six-carbon acids generated in pigeon liver and breast muscle when sugars are oxidized completely to yield carbon dioxide, water, and energy.
In 1937 Krebs demonstrated the existence of a cycle of chemical reactions that combines the end-product of sugar breakdown, later shown to be an “activated” form of the two-carbon acetic acid, with the four-carbon oxaloacetic acid to form citric acid. The cycle regenerates oxaloacetic acid through a series of intermediate compounds while liberating carbon dioxide and electrons that are immediately utilized to form high-energy phosphate bonds in the form of adenosine triphosphate (ATP; the chemical-energy reservoir of the cell). The discovery of the tricarboxylic acid cycle, which is central to nearly all metabolic reactions and the source of two-thirds of the food-derived energy in higher organisms, was of vital importance to a basic understanding of cell metabolism and molecular biology.
Krebs served on the faculty of the University of Oxford from 1954 to 1967. He wrote (with the British biochemist Hans Kornberg) Energy Transformations in Living Matter (1957) and also coauthored (with Anne Martin) Reminiscences and Reflections (1981). He was knighted in 1958, and the Royal Society awarded him its Copley Medal in 1961.
Details
Sir Hans Adolf Krebs, (25 August 1900 – 22 November 1981) was a German-British biologist, physician and biochemist. He was a pioneer scientist in the study of cellular respiration, a biochemical process in living cells that extracts energy from food and oxygen and makes it available to drive the processes of life. He is best known for his discoveries of two important sequences of chemical reactions that take place in the cells of nearly all organisms, including humans, other than anaerobic microorganisms, namely the citric acid cycle and the urea cycle. The former, often eponymously known as the "Krebs cycle", is the sequence of metabolic reactions that allows cells of oxygen-respiring organisms to obtain far more ATP from the food they consume than anaerobic processes such as glycolysis can supply; and its discovery earned Krebs a Nobel Prize in Physiology or Medicine in 1953. With Hans Kornberg, he also discovered the glyoxylate cycle,[8] a slight variation of the citric acid cycle found in plants, bacteria, protists, and fungi.
Krebs died in 1981 in Oxford, where he had spent 13 years of his career from 1954 until his retirement in 1967 at the University of Oxford.
Biography:
Early life and education
Krebs was born in Hildesheim, Germany, to Georg Krebs, an ear, nose, and throat surgeon, and Alma Krebs (née Davidson). He was of Jewish ancestry and was the middle of three children. He had an elder sister, Elisabeth, and a younger brother, Wolfgang.
Krebs attended school at the Gymnasium Andreanum in his home town. Near the end of World War I, in September 1918, six months short of completing his secondary school education, he was conscripted into the Imperial German Army. He was allowed to take an emergency examination for his high school diploma, which he passed with such a high score that he suspected the examiners of being "unduly lenient and sympathetic". With the end of the war two months later, his conscription ended.
Krebs decided to follow his father's profession and entered the University of Göttingen in December 1918 to study medicine. In 1919, he transferred to the University of Freiburg. In 1923, he published his first scientific paper on a tissue staining technique. He did this work under the guidance of Wilhelm von Mollendorf starting it in 1920. He completed his medical course in December 1923. To obtain a Doctor of Medicine degree, and a medical license, he spent one year at the Third Medical Clinic in the University of Berlin. By then he had turned his professional goal from becoming a practising physician to becoming a medical researcher, particularly in biochemistry. In 1924, he studied at the Department of Chemistry at the Pathological Institute of the Charité Hospital, in Berlin, for training in chemistry and biochemistry. He earned his MD degree in 1925 from the University of Hamburg.
Career
In 1926, Krebs joined Otto Heinrich Warburg as a research assistant at the Kaiser Wilhelm Institute for Biology in Dahlem, Berlin. He was paid 4800 marks per year. After four years in 1930, with 16 publications to his credit, his mentor Warburg urged him to move on and he took up the position of Assistant in the Department of Medicine at the Municipal Hospital in Altona (now part of Hamburg). The next year he moved to the Medical Clinic of the University of Freiburg. At Freiburg, he was in charge of about 40 patients, and was at liberty to do his own research. Before a year was over at Freiburg, he, with research student Kurt Henseleit, published their discovery of the ornithine cycle of urea synthesis, which is the metabolic pathway for urea formation. It is now known as the urea cycle, and is sometimes also referred to as the Krebs–Henseleit cycle. Together they also developed a complex aqueous solution (a buffer), or perfusion ex vivo, for studying blood flow in arteries, which is now called the Krebs–Henseleit buffer. In 1932, he published the basic chemical reactions of the urea cycle, which established his scientific reputation.
Krebs's life as a respected German scientist came to an abrupt halt in 1933 because of his Jewish ancestry. With the rise of Hitler's Nazi Party to power, Germany decreed the Law for the Restoration of the Professional Civil Service, which decreed the removal of all non-Germans, and anti-Nazis, from professional occupations. Krebs received his official dismissal from his job in April 1933, and his service was terminated on 1 July 1933. An admirer, Sir Frederick Gowland Hopkins at the University of Cambridge, immediately came to his rescue, and persuaded the university to recruit Krebs to work with him in the Department of Biochemistry. By July 1933, he was settled in Cambridge with financial support from the Rockefeller Foundation.
Although Germany restricted him to bringing only his personal belongings, he was fortunate that the government agents allowed him to take his equipment and research samples to England. They proved to be pivotal to his later discoveries, especially the manometer developed by Warburg specifically for the measurement of oxygen consumption in thin slices of tissues; it was the basis for his research.
He was appointed as Demonstrator in biochemistry in 1934, and in 1935 the University of Sheffield offered him a post of Lecturer in Pharmacology, with a more spacious laboratory and double the salary. He worked there for 19 years. The University of Sheffield opened a Department of Biochemistry, now Department of Molecular Biology and Biotechnology, in 1938 and Krebs became its first Head, and eventually a Professor in 1945. During his time it became one of the leading departments of biochemistry in the world. Krebs took over the running of the Sorby Research Institute in 1943. In 1944, the British Medical Research Council established the MRC Unit for Cell Metabolism Research at Sheffield, and Krebs was appointed the Director. With this, his laboratory became so large that the locals jokingly nicknamed it "Krebs's Empire".
He moved with his MRC unit to the University of Oxford in 1954 as Whitley Professor of Biochemistry, the post he held until his retirement in 1967. The editorial board of Biochemical Journal extended their good wishes on his retirement, but in return he promised to keep them busy, by producing scientific papers. He continued his research, and took his MRC unit to the Nuffield Department of Clinical Medicine at the Radcliffe Infirmary, Oxford. From there he published over 100 research papers.

#3 Jokes » Nut Jokes - I » Today 00:05:37
- Jai Ganesh
- Replies: 0
Q: How do squirrels remember where they stored their nuts during winter?
A: They use acorn-yms.
* * *
Q: Why do squirrels swim on there back?
A: To keep their nuts dry!
* * *
Q: What do you call a squirrel with no nuts?
A: Female Squirrel.
* * *
Q: Why can't you be friends with a squirrel?
A: They drive everyone nuts.
* * *
Q: Why shouldn't you math a tree?
A: There might be a squirrel in there looking for nuts.
* * *
#4 Dark Discussions at Cafe Infinity » Comedies Quotes - III » Today 00:02:57
- Jai Ganesh
- Replies: 0
Comedies Quotes - III
1. I was making a lot of 8mm home movies, since I was twelve, making little dramas and comedies with the neighborhood kids. - Steven Spielberg
2. I played comedies and dramas. - William Shatner
3. I think there are opportunities for women in comedies - how zany is up to them. - Jennifer Aniston
4. When I tried to play characters that strayed from who I am it ended in disaster. People didn't expect me in comedies or musicals. - Sylvester Stallone
5. Usually comedy is only available to us ladies in the romantic comedy. That's why I hate romantic comedies. - Sandra Bullock
6. I personally don't like to go see romantic comedies. But people do want to see them, and they seem to want to see me in them. - Matthew McConaughey
7. There weren't roles for females in comedies for a really long time. - Sandra Bullock
8. I have a lot more fun making comedies because there's a levity on the set, and I find it difficult to go to work and, you know, cry for 12 hours. - Megan Fox.
#5 This is Cool » San Marino » Yesterday 17:49:16
- Jai Ganesh
- Replies: 0
San Marino
Gist
San Marino is one of the world's smallest countries, ranking as the fifth smallest globally by area (around 61 sq km or 24 sq miles) and the third smallest in Europe after Vatican City and Monaco, entirely surrounded by Italy. It's also recognized as the world's oldest republic, founded in 301 AD.
The Oldest Republic
Established in 301 AD, this tiny nation has maintained its independence and self-governance for over 1,700 years. Its rich history and the preservation of its ancient traditions make San Marino a captivating destination for history enthusiasts.
Summary
San Marino, officially the Republic of San Marino (RSM), is a landlocked country in Southern Europe, completely surrounded by Italy. Located on the northeastern slopes of the Apennine Mountains, it is the larger of two microstates within Italy, the other being Vatican City. San Marino is the fifth-smallest country in the world, with a land area of just over 61 sq km (23.5 sq mi) and a population of 34,042 as of 2025. Its capital, the City of San Marino, sits atop Monte Titano, while its largest settlement is Dogana, in the municipality of Serravalle.
San Marino claims to have been founded in AD 301 and to be the oldest extant sovereign state, and the oldest constitutional republic. It is named after Saint Marinus, a stonemason from the Roman island of Rab (in present-day Croatia), who is supposed in mythic accounts to have established a monastic community on Monte Titano. The country has a rare constitutional structure: the Grand and General Council, a democratically elected legislature, selects two heads of state, the Captains Regent, every six months. They are chosen from opposing political parties, and serve concurrently with equal powers and preside over several institutions of state, including the Grand and General Council. Only the Federal Council of Switzerland also follows that structure, except with seven heads of state, and different responsibilities and functions.
San Marino is a member of the Council of Europe and uses the euro as its official currency, but is not part of the European Union. The official language is Italian, although the traditional regiolect is Sammarinese, a dialect of Romagnol. Its economy is based on finance, industry, services, retail, and tourism, and it ranks among the wealthiest countries in the world by GDP (PPP) per capita. San Marino was also the first currently-existing state to abolish the death penalty (in 1865), and in 2025 was ranked 29th on the Human Development Index.
Details
San Marino is a small, landlocked republic situated on the slopes of Mount Titano, on the Adriatic side of central Italy between the Emilia-Romagna and Marche regions and surrounded on all sides by the republic of Italy. It is the smallest independent state in Europe after Vatican City and Monaco and, until the independence of Nauru (1968), the smallest republic in the world.
Geography
San Marino has an irregular rectangular form with a maximum length of 8 miles (13 km), northeast to southwest. It is crossed by the Marano and Ausa (Aussa) streams, which flow into the Adriatic Sea, and by the stream of San Marino, which falls into the Marecchia River. The landscape is dominated by the huge, central limestone mass of Mount Titano (2,424 feet [739 meters]); hills spread out from it on the southwest, whereas the northeastern part gently slopes down toward the Romagna plain and the Adriatic coast. The silhouette of Mount Titano, with its three summits crowned by ancient triple fortifications, may be seen from many miles away. In 2008 Mount Titano and the historic center of San Marino were designated a UNESCO World Heritage site.
The climate is mild and temperate, with maximum temperatures in the high 70s F (about 26 °C) in summer and the high teens F (about −7 °C) in winter. Annual rainfall ranges between about 22 inches (560 mm) and 32 inches (800 mm). Vegetation is typical of the Mediterranean zone, with variations due to elevation, and includes olive, pine, oak, ash, poplar, fir, and elm, as well as many kinds of grasses and flowers. Besides domestic and farmyard animals, moles, hedgehogs, foxes, badgers, martens, weasels, and hares are found. Indigenous birds and birds of passage are plentiful.
Although traces of human presence from both prehistoric and Roman times exist in the territory, Mount Titano and its slopes are known to have been populated, with certainty, only after the arrival of St. Marinus and his followers. San Marino citizens, or Sammarinesi, make up more than four-fifths of the country’s population, with Italians composing most of the remainder. Thousands of Sammarinesi reside abroad, principally in Italy, the United States, France, and Argentina. Nearly nine-tenths of San Marino’s citizens are Roman Catholics, though there is no official religion. The official language is Italian. A widely spoken dialect has been defined as Celto-Gallic, akin to the Piedmont and Lombardy dialects as well as to that of Romagna.
Because centuries-long quarrying has exhausted Mount Titano’s stone and ended the craft that depended upon it, the territory is now without mineral resources. All electrical power is transferred via electrical grid from Italy, San Marino’s main trading partner. The country’s principal resources are industry, tourism, commerce, agriculture, and crafts. Manufactures include electronics, paint, cosmetics, ceramics, jewelry, and clothing. Ceramic and wrought-iron articles, as well as modern and reproduction furniture, are among San Marino’s traditional craft products. Fine printing, particularly of postage stamps, is a consistent source of revenues. Banking is a vital industry. In 2002 San Marino replaced the Italian lira with the euro as its national currency.
Tourism is the sector of greatest expansion, and it makes a major contribution to the inhabitants’ income. Alongside traditional excursion tourism, there is convention-type tourism, based on modern hotel facilities, as well as residential tourism.
Agriculture, although no longer the principal economic resource in San Marino, remains vital. Wheat, grapes, and barley are the chief crops; dairying and livestock also are important. About three-fourths of the land is given to permanent cultivation.
The capital, San Marino city, is set high on the western side of Mount Titano, beneath the fortress crowning one of its summits, and is encircled by triple walls. Borgo Maggiore, farther down the slope, was for centuries San Marino’s commercial center, and Serravalle, beneath its castle of the Malatesta family, is agricultural and industrial. San Marino is overwhelmingly urban in character, and those three cities are home to nearly two-thirds of San Marino’s population. Most of San Marino’s landscape is agricultural in character, but industrial concerns have intruded on the centuries-old forms of agricultural life.
The San Marino constitution, originating from the Statutes of 1600, provides for a parliamentary form of government. The Great and General Council (Parliament) has 60 members, elected every five years by all adult citizens. It has legislative and administrative powers and every six months nominates the two captains regent (capitani reggenti), who hold office for that period and may not be elected again until three years have elapsed. The Great and General Council is headed by the captains regent, who are heads of state and of the administration. The Congress of State, a council of ministers, is composed of 10 members, elected by the Great and General Council from among its members, and constitutes the central organ of executive power. Each member has charge of a ministerial department.
Social programs for the citizens of San Marino are extensive. The state attempts to keep unemployment in check by seeking to provide employment for those who cannot find work with private concerns. All citizens (who make social security contributions) receive free, comprehensive, high-quality medical care and assistance in sickness, accident, and old age, as well as family allowances. The state aids home ownership through its buildings schemes. Education is free and compulsory up to age 16. The University of San Marino was founded in 1985. A public security force of about 50 persons provides national defense.
A network of roads connects San Marino with the surrounding regions of Italy. Motorcoach services connect San Marino city with Rimini, Italy, and, in summer, directly with the Adriatic coast. The main airport serving San Marino is the Federico Fellini International Airport in Rimini. There are no railroads, but the capital is reached from Borgo Maggiore by means of a cable railway.
History
The Republic of San Marino traces its origin to the early 4th century ce when, according to tradition, St. Marinus and a group of Christians settled there to escape persecution. The Castellum Sancta Marini is mentioned in the Liber Pontificalis (“The Book of the Pontiffs”) in 755; the oldest document in the republican archives mentions the abbot of San Marino in 885. By the 12th century San Marino had developed into a commune ruled by its own statutes and consuls. The commune was able to remain independent despite encroachments by neighboring bishops and lords, largely because of its isolation and its mountain fortresses. Against the attacks of the Malatesta family, who ruled the nearby seaport of Rimini, San Marino enjoyed the protection of the rival family of Montefeltro, who ruled Urbino. By the middle of the 15th century, it was a republic ruled by a Grand Council—60 men taken from the Arengo, or Assembly of Families. Warding off serious attacks in the 16th century (including an occupation by Cesare Borgia in 1503), San Marino survived the Renaissance as a relic of the self-governing Italian city-states. Rule by an oligarchy and attempts to annex it to the Papal States in the 18th century marked the decline of the republic.
When Napoleon invaded Italy, he respected the independence of the republic and even offered to extend its territory (1797). The Congress of Vienna (1815), at the end of the Napoleonic Wars, also recognized its independent status. During the 19th-century movement for Italian unification, San Marino offered asylum to revolutionaries, among them Giuseppe Garibaldi. After Italy became a national state, a series of treaties (the first in 1862) confirmed San Marino’s independence. In World War II, San Marino remained neutral, but it was the target of a British bombing raid in 1944 and was briefly occupied by both the Germans and the Allies later that year.
Additional Information
Europe attracts more international tourists than any other region of the world, according to the United Nations World Tourism Organization. The staggering 616 million people that visited the continent last year faced the tough choice of dozens of destinations, each offering stimulating history, diverse classes of creatives, and epic landscapes. France seduced the most travelers by far, but what about last place?
Hovering on a cliff encircled by Italy, the tiny landlocked country of San Marino holds the title of the least visited country in Europe, just a dot on the map of only 23.6 square miles. Here’s why travelers of all types should visit the under the radar microstate.
For History Buffs
Founded in the fourth century and one of the world’s oldest republics, San Marino survived from the time when city-states proliferated across Europe—a critical stage for developing democratic models across the globe. Ramble around the car-free capital also named San Marino, a UNESCO World Heritage site with a medievalized layout punctuated by three imposing fortresses standing testimony to a turbulent past.
Fer Epic Landscapes
The Mount Titano, part of the Apennine range, dominates San Marino's landscape surrounded by Italy with clear views all the way to the Adriatic Sea. Old stone benches pepper the slopes offering places to bask in the greenery stretching to the outlying villages. Ride the funicular from one such town, Borgo Maggiore, to the historic center for sweeping views of Italy in every direction.
For Foodies
All the rules of northern Italian food apply here too, with a strong tradition in cheesemaking. Sammarinese cuisine, heavy on pasta and meat dishes, balances the rich and fresh with locally-sourced ingredients. The area holds particular fondness for the filled flatbread called piada, similar to a piadina from the encompassing Emilia-Romagna region. Don’t forget the wine: San Marino produced wine for almost two thousand years, aging the bottles in the area caves for optimal temperatures.
For Architecture Fans
Visitors weave through time in the living history museum of the historic capital, home to 14th and 16th century convents, 18th century Titano Theatre, and a neoclassical basilica built in the 19th century. The centerpiece Palazzo Pubblico echos Florence’s Palazzo Vecchio on a much smaller scale.
For Savvy Shoppers
The tax-free policy of San Marino makes shopping cheaper than nearby Italy. Mid-century modern ceramics captivate collectors, along with postage stamps created in the late 19th century for use only within the state's borders.
For Day-Trippers
San Marino may not be a member of the European Union but enjoys open borders with Italy for tourists staying less than 10 days. The ideal location makes for easy day trips from cities like Florence or Bologna, both located less than three hours away.
#6 Science HQ » Pyorrhea (Periodontitis) » Yesterday 16:45:52
- Jai Ganesh
- Replies: 0
Pyorrhea (Periodontitis)
Gist
Pyorrhea, or periodontitis, is a severe, chronic bacterial infection of the gums that damages soft tissue and destroys the bone supporting the teeth, often causing tooth loss. Key symptoms include bleeding gums, persistent bad breath, receding gums, and loose teeth. It is primarily caused by untreated plaque buildup, leading to inflammation and infection.
Pyorrhea, also known as severe gum disease or periodontal disease, is primarily caused by poor oral hygiene leading to plaque and tartar buildup, which irritates the gums and allows bacteria to infect the supporting structures, resulting in inflammation, bone loss, and eventual tooth loosening. Contributing factors include smoking, genetics, certain medical conditions, hormonal changes, and injury to the gums.
Summary
Periodontal disease, also known as gum disease, is a set of inflammatory conditions affecting the tissues surrounding the teeth. In its early stage, called gingivitis, the gums become swollen and red and may bleed. It is considered the main cause of tooth loss for adults worldwide. In its more serious form, called periodontitis, the gums can pull away from the tooth, bone can be lost, and the teeth may loosen or fall out. Halitosis (bad breath) may also occur.
Periodontal disease typically arises from the development of plaque biofilm, which harbors harmful bacteria such as Porphyromonas gingivalis and Treponema denticola. These bacteria infect the gum tissue surrounding the teeth, leading to inflammation and, if left untreated, progressive damage to the teeth and gum tissue. Recent meta-analysis have shown that the composition of the oral microbiota and its response to periodontal disease differ between men and women. These differences are particularly notable in the advanced stages of periodontitis, suggesting that sex-specific factors may influence susceptibility and progression. Factors that increase the risk of disease include smoking, diabetes, HIV/AIDS, and almost any autoimmune disease, family history, high levels of homocysteine in the blood and certain medications. Diagnosis is by inspecting the gum tissue around the teeth both visually and with a probe and X-rays looking for bone loss around the teeth.
Treatment involves good oral hygiene and regular professional teeth cleaning. Recommended oral hygiene include daily brushing and flossing. In certain cases antibiotics or dental surgery may be recommended. Clinical investigations demonstrate that quitting smoking and making dietary changes enhance periodontal health. Globally, 538 million people were estimated to be affected in 2015 and has been known to affect 10–15% of the population generally. In the United States, nearly half of those over the age of 30 are affected to some degree and about 70% of those over 65 have the condition. Males are affected more often than females.
Details
Contrary to what one might imagine, it is a widespread disease. widespread disease. In fact, 15% of the population suffers from a severe form of periodontitis and about 50% is affected in various ways, 15% of the population suffers from a severe form of periodontitis and about 50% are affected in various ways.. This is a problem that should not be underestimated, as it also has a strong impact on a person's overall health.
What is periodontitis?
Periodontitis, or periodontal disease, is a polymicrobial polymicrobial infection that affects the periodontium i.e., the tissues and tissues and structures that support the teeth: gums, bone and periodontal ligaments..
Periodontitis damages all periodontal tissues, but, depending on the level of infection, it has different degrees of severity, has different degrees of severity.
When not properly treated, periodontitis can progress from the first stage, which may correspond to gingivitis, to a may correspond to gingivitisto the most to the most severe stage of the disease, also called periodontitiswhich manifests itself with the loss of teeth. loss of the dental pieces more or less rapid.
Underestimated, but widespread, periodontal disease is a very serious pathology of the oral cavity.
Unfortunately, many patients tend to overlook the symptoms of periodontitis, many patients tend to overlook the symptoms of periodontitis and only visit the dentist in the acute phase of the disease. and only visit the dentist in the acute phase of the disease.
Symptoms of periodontal disease
Symptoms of periodontal disease should never be underestimated, as they always lead to worsening and chronic periodontal disease. lead to worsening and chronicity of the disease.
The main symptoms of periodontitis are:
* Bleeding gums
* Dental hypersensitivity to heat and cold
* Pain and hypersensitivity of the gums
* Flaccidity of the gums
* Feeling of having "longer" teeth
* Sensation of having teeth that move
* Sensation of teeth shifting position
* Halitosis
Neglecting even one of these symptoms can lead to the development of periodontitis. development of periodontitis and aggravation of periodontal disease.
In the presence of one or several symptoms, it is necessary to immediately contact a specialized dentist who, by means of an adequate analysis of the oral cavity, can correlate the signs detected with the possible pathology.
Causes
The main causes of periodontitis are:
* Daily oral hygiene daily deficient or absent.
* The consequent accumulation of dental biofilm.
* Appearance of tartar.
Although subjective factors of various kinds, related to the condition and characteristics of the patient's organism, may also contribute, periodontal disease is mainly determined by the lack of proper care, both on a daily basisThe lack of proper periodontal care, both on a daily basis as well as periodic visits to the dentist.
Treatment
When gingivitis progresses to periodontitis, treatment becomes much more complicated. Only the dentist and hygienist can provide periodontal treatment, which requires special dental procedures and may require oral surgery.which requires special dental procedures and may require oral surgery. If the dentist concludes that periodontitis is present, treatment will depend on the severity of the infection. Options include, but are not limited to, the following:
* Scaling and root planing: In this two-step procedure to treat periodontitis, the dentist will scrapes tartar buildup from the teeth above and below the gum line (scaling). (scaling). Next, the dental specialist polishes away the roughness of the tooth roots, which reduces the accumulation of bacteria and therefore plaque and tartar.
* Flap surgery : If gum inflammation and pockets near the teeth persist after a deep cleaning procedure, your dentist may recommend flap surgery. Flap surgery, a common procedure to treat periodontitis, is performed by a specialist called a periodontist. During flap surgery tartar is removed from the pockets along the teeth. The pockets are then sutured so that the gum tissues can reattach to the teeth.. The reduction of the pockets makes brushing and flossing easier and more comfortable.
* Grafts Grafting: Severe cases of periodontitis may require bone or tissue grafts to replace the infected one, may require bone or tissue grafts to replace infected bone or tissue. The grafting may consist of a technique called "guided tissue regeneration"in which a small piece of membrane is inserted between the jawbone and the gum to allow the bone and tissue to regenerate. Guided tissue regeneration prevents the gum tissue from expanding in the area that the bone should occupy, so that the bone and tissue grafts have the space they need to grow.
Prevention
From infancy, it is possible to prevent periodontitis: It is possible to prevent periodontitis, counteract the appearance of its main symptoms and avoid an inflammatory process that can degenerate into an acute bacterial infection with a few simple measures:
* Attend regular and continuous professional hygiene sessions. professional hygiene with a specialized professional.
* After every meal, practice proper daily dental hygiene at home. at home.
* Pay close attention to attention to the symptoms of periodontal of periodontal disease.
Contrary to popular belief, pyorrhea is a disease that can also affect children and adolescents. can also affect children and adolescents. It can also affect children and adolescents, with varying degrees of pathology and, if not effectively treated, can have aggressive recurrences in the course of development.
To prevent the appearance of the main symptoms of periodontal disease in children and adolescents, it is necessary to educate them from a very young age, after every meal, in a correct and regular daily oral hygiene and to submit them regularly to professional oral hygiene sessions in a dental clinic they trust.
Types of periodontitis
From the clinical point of view, depending on the symptoms and the aggressiveness of the pathology we can have:
* Chronic periodontitis
Chronic periodontitis is the most common form of periodontal disease, and occurs much more frequently in people over the age of 45.and occurs much more frequently in people over 45 years of age. This chronic disease is characterized by inflammation of the gums inflammation of the gums and progressive destruction of gum and bone tissue. It may appear as if the teeth are lengthening, but in reality the gums are gradually receding.
Chronic periodontitis causes an inflammatory reaction of the tissues that support the tooth.. It causes loss of attachment and bone resorption and is classified as an infectious disease. It is most commonly diagnosed in adults, although children and adolescents can also develop it.. To evaluate its severity, we look at the percentage of affected foci (especially visible due to hemorrhage): if 30% or more are affected, it is said to be generalized and if 30% or less of the foci are affected, it is localized.
* Ulcerative-necrotic periodontitis
Ulcerative-necrotic periodontitis is a very severe form, characterized by the presence of true ulcers at the gum level very severe form, characterized by the presence of true ulcers at the gum level, with necrosis of the entire supporting apparatus of the tooth. Although rare, it occurs in young adulthood, with a higher incidence in developing countries, with risk factors such as malnutrition, lack of oral hygiene, immune depression and chronic systemic diseases.. Along with the typical symptoms, fever, enlarged cervical lymph nodes and general malaise may also occur.
Aggressive periodontitis
Aggressive periodontitis is a rare form of rare form of periodontitis which mainly affects children or the elderly; It is characterized by a fairly rapid and extensive destructive process, especially affecting incisors and molars.It is characterized by a rather rapid and extensive destructive process, especially affecting the incisors and molars.
Additional Information
Have you ever experienced how suddenly and without prior pain a tooth moves or even falls out? You may be suffering from periodontitis or gum disease without knowing it. If you want to know what it consists of and how to prevent and treat it, keep reading.
Currently, the term “pyorrhea” is obsolete in dentistry, but perhaps many of you have heard it over the years. The word “pyorrhea” is still commonly used to refer to gum disease, although the term we currently use in dentistry to refer to it is “periodontitis.”
Pyorrhea or periodontitis is an infectious disease and the main cause of tooth loss along with cavities. Its great danger lies in the fact that it is a disease that does not cause excessive pain or too severe discomfort, so it often goes unnoticed or is easily normalized.
It is a chronic inflammatory condition of the tissue surrounding our teeth, resulting in progressive destruction of the periodontal ligament and the alveolar bone that supports our teeth.
The main difference from gingivitis is that gingivitis involves inflammation of the gums but does not involve bone destruction, whereas periodontitis or pyorrhea does involve bone destruction.
When periodontal pockets form, which are an accumulation of bacteria below the gum line, their action causes damage to dental structures, leading to the deterioration of the bone supporting our teeth.
Why does periodontitis occur?
As we mentioned, being an infectious disease, the main cause of its appearance is the accumulation of bacterial plaque due to poor oral hygiene. If we do not correctly remove this plaque, we will cause inflammation of our gums in the short term and hardening over time, adhering to our teeth and forming what we call tartar (calculus), which once attached to the tooth, we will not be able to remove it with conventional brushing and will require professional dental hygiene.
How can we know if we have periodontitis?
To detect if we are suffering from periodontitis, the most common symptoms are gum inflammation and a change in its color, becoming reddish or violet. Of course, another clear symptom is bleeding gums when brushing, bad breath, discomfort when chewing, or noticing that some teeth are loose.
Sometimes spaces between the teeth may also appear, and in some cases, abscesses or overgrowth in the gums. But undoubtedly, bleeding is the first warning sign. Bleeding gums do not directly mean that we have pyorrhea; it could be gingivitis. However, it will serve as an alert for us to immediately consult our specialist for a correct diagnosis and intervention before the disease progresses to a more severe stage.
What are the risk factors?
Any of us can suffer from periodontitis if we do not take proper care of ourselves, but there are certain risk factors that can accelerate the disease or simply increase the risk of suffering from it.
Chronic gingivitis, smoking, hormonal changes in women such as pregnancy or menopause, old age, substance abuse, lack of vitamin C, or diseases that weaken our defenses (diabetes, arthritis, Crohn’s disease, leukemia, HIV, oncological treatments). In these cases, prevention should be even more thorough to avoid future complications.
Periodontitis Treatment
As we have already mentioned, the main cause of periodontitis is poor oral hygiene, so the key to stopping the appearance or progression of the disease will undoubtedly be to have proper oral hygiene.
Periodontal disease can lead to serious consequences for our health, so it is important to detect it as soon as possible to be able to treat it and prevent it from becoming chronic.
The first step is to establish a clear diagnosis elaborated by a professional, to assess what our best options are and find a solution adapted to our needs.
* Once diagnosed, we can make a prognosis for our teeth and
* realize a treatment plan tailored to our needs. It usually starts with deep dental hygiene to remove calculus adhered to our teeth and below the gums.
And from there, the key factor will be to correct our poor hygiene habits as soon as possible and maintain proper maintenance by our specialist, attending regular check-ups as recommended. We will have to learn to correctly use our toothbrush, use dental floss and interdental brushes to complete our daily hygiene, and visit our dentist regularly to take care of the residues we cannot reach.
We must bear in mind that periodontitis is a chronic disease, so once diagnosed, maintenance visits for professional hygiene should be regular to halt its progression. A professional cleaning every 3, 4, or 6 months is recommended depending on each patient’s needs.

#7 Re: Jai Ganesh's Puzzles » General Quiz » Yesterday 16:08:27
Hi,
#10785. What does the term in Geography Depression (geology) mean?
#10786. What does the term in Geography Desert mean?
#8 Re: Jai Ganesh's Puzzles » English language puzzles » Yesterday 15:49:22
Hi,
#5991. What does the verb (used with obeject) invoke mean?
#5992. What does the adjective irksome mean?
#9 Re: Jai Ganesh's Puzzles » Doc, Doc! » Yesterday 15:33:02
Hi,
#2592. What does the medical term Perimetrium mean?
#10 Re: Jai Ganesh's Puzzles » 10 second questions » Yesterday 15:18:09
Hi,
#9878.
#11 Re: Jai Ganesh's Puzzles » Oral puzzles » Yesterday 14:57:59
Hi,
#6371.
#12 Re: Exercises » Compute the solution: » Yesterday 14:27:22
Hi,
2732.
#13 Re: This is Cool » Miscellany » Yesterday 00:05:09
2518) Parrot
Gist
A parrot is a colorful, intelligent bird from the order Psittaciformes, known for its strong curved beak, zygodactyl feet (two toes forward, two back), and ability to mimic sounds, including human speech, making them popular but often endangered pets found in tropical and subtropical regions. They are social creatures with diverse diets of fruits, nuts, and seeds, playing roles in ecosystems by dispersing seeds.
Can a parrot speak?
Yes, parrots can talk, but it's more accurately described as exceptional mimicry of human speech, with some species like African Greys and Amazons learning to imitate words and phrases, sometimes even using them contextually to communicate emotions or needs, driven by their strong social bonds and vocal learning abilities. They use a special vocal organ (syrinx) and a flexible tongue to shape sounds, allowing them to replicate human voices and other noises with surprising accuracy, essentially forming a social bond with their human flock.
Summary
Parrots (Psittaciformes), also known as psittacines from the name of the type genus Psittacus, are birds with a strong curved beak, upright stance, and clawed feet. They are classified in four families that contain roughly 410 species in 101 genera, found mostly in tropical and subtropical regions. The four families are the Psittaculidae (Old World parrots), Psittacidae (African and New World parrots), Cacatuidae (math), and Strigopidae (New Zealand parrots). One-third of all parrot species are threatened by extinction, with a higher aggregate extinction risk (IUCN Red List Index) than any other comparable bird group. Parrots have a generally pantropical distribution with several species inhabiting temperate regions as well. The greatest diversity of parrots is in South America and Australasia.
Parrots, along with corvids (ravens, crows, jays, and magpies), are among the most intelligent birds, and the ability of some species to imitate human speech enhances their popularity as pets. They form the most variably sized bird order in terms of length; many are vividly coloured and some, multi-coloured. Most parrots exhibit little or no sexual dimorphism in the visual spectrum.
The most important components of most parrots' diets are seeds, nuts, fruit, buds, and other plant material. A few species sometimes eat animals and carrion, while the lories and lorikeets are specialised for feeding on floral nectar and soft fruit. Almost all parrots nest in tree hollows (or nest boxes in captivity), and lay white eggs from which hatch altricial (helpless) young.
Trapping wild parrots for the pet trade, as well as hunting, habitat loss, and competition from invasive species, has diminished wild populations, with parrots being subjected to more exploitation than any other group of wild birds. As of 2021, about 50 million parrots (half of all parrots) live in captivity, with the vast majority of these living as pets in people's homes. Measures taken to conserve the habitats of some high-profile charismatic species have also protected many of the less charismatic species living in the same ecosystems.
Parrots are the only creatures that display true tripedalism, using their necks and beaks as limbs with propulsive forces equal to or greater than those forces generated by the forelimbs of primates when climbing vertical surfaces. They can travel with cyclical tripedal gaits when climbing.
Details
Parrot is a term applied to a large group of gaudy, raucous birds of the family Psittacidae. Parrot also is used in reference to any member of a larger bird group, order Psittaciformes, which includes math (family Cacatuidae) as well. Parrots have been kept as cage birds since ancient times, and they have always been popular because they are amusing, intelligent, and often affectionate. Several are astonishingly imitative of many sounds, including human speech.
The family Psittacidae numbers 333 species. The subfamily Psittacinae, the “true” parrots, is by far the largest subfamily, with members found in warm regions worldwide. These birds have a blunt tongue and eat seeds, buds, and some fruits and insects. Many members of the subfamily are known simply as parrots, but various subgroups have more specific names such as macaw, parakeet, conure, and lovebird.
The African gray parrot (Psittacus erithacus) is unsurpassed as a talker; the male can precisely echo human speech. Captive birds are alert and, compared with other parrots, relatively good-tempered. Some are said to have lived 80 years. The bird is about 33 cm (13 inches) long and is light gray except for its squared, red tail and bare, whitish face; the sexes look alike. Gray parrots are common in the rainforest, where they eat fruits and seeds; they damage crops but are important propagators of the oil palm.
Among other proficient mimics are the Amazon parrots (Amazona). The 31 species of Amazons are chunky birds, mostly 25 to 40 cm (10 to 16 inches) long, with slightly erectile crown feathers and a rather short, squared tail. Their predominantly green plumage is marked with other bright colours, chiefly on the upper head; the sexes look alike. Amazon parrots live in tropical forests of the West Indies and Mexico to northern South America. They are difficult to breed and may be aggressive as well as squawky. Common in aviaries is the blue-fronted Amazon (A. aestiva) of Brazil; it has a blue forehead, a yellow or blue crown, a yellow face, and red shoulders. The yellow-crowned parrot (A. ochrocephala) of Mexico, Central America, and from Ecuador to Brazil has some yellow on the head and neck, a red wing patch, and a yellow tail tip.
The monk, or green, parakeet (Myiopsitta monachus) is one of the hardiest parrot species. It is native to South America, but some have escaped from captivity in the United States and now nest in several states. Its large stick nest is unique among psittaciforms. Other remarkable parrots of this subfamily include the hanging parrots (Loriculus), which sleep upside-down like bats. Caiques (Pionites) are small, short-tailed South American birds similar to conures in build and habits.
For decades the night parrot, or night parakeet (Geopsittacus occidentalis), of Australia was thought to be extinct, until a dead one was found in 1990. It feeds at night on spinifex grass seeds and dozes under a tussock by day. Its nest is a twig platform in a bush and is entered by way of a tunnel. Equally unusual is the ground parrot, or ground parakeet (Pezoporus wallicus). Rare local populations exist in the wastelands of coastal southern Australia and western Tasmania. It runs in the grass, flushes like a quail, and makes a sudden deceptive pitch, and it was formerly hunted with dogs. It eats seeds and insects; its nest is a leaf-lined depression under a bush.
The lories (with short tails) and lorikeets (with longer, pointed tails) make up the Psittacidae subfamily Loriinae. The 53 species in 12 genera are found in Australia, New Guinea, and some Pacific islands. All have a slender, wavy-edged beak and a brush-tipped tongue for extracting nectar from flowers and juices from fruits.
The pygmy parrots of the subfamily Micropsittinae all belong to the genus Micropsitta. The six species are endemic to New Guinea and nearby islands. These are the smallest members of the family. They live in forests, where they eat insects and fungi.
The subfamily Nestorinae is found only in New Zealand. The kea (Nestor notabilis) occasionally tears into sheep carcasses (rarely, weakened sheep) to get at the fat around the kidneys. The kaka, N. meridionalis, a gentler forest bird, is often kept as a pet. The owl parrot, or kakapo (Strigops habroptilus), also lives only in New Zealand. It is the sole member of the subfamily Strigopinae. Rare and once thought extinct, it survives as a scant population on Stewart Island.
The math family (Cacatuidae) numbers 21 species from Australia, New Guinea, and nearby islands. The group includes the math (Nymphicus hollandicus), a smaller bird. All are crested and have heavy beaks for cracking nuts and seeds. The so-called sea parrot is unrelated to the psittaciforms.
Additional Information
The parrots are a broad order of more than 350 birds. Macaws, Amazons, lorikeets, lovebirds, math and many others are all considered parrots.
Shared Traits
Though there is great diversity among these birds, there are similarities as well. All parrots have curved beaks and all are zygodactyls, meaning they have four toes on each foot, two pointing forward and two projecting backward. Most parrots eat fruit, flowers, buds, nuts, seeds, and some small creatures such as insects.
Parrots are found in warm climates all over most of the world. The greatest diversities exist in Australasia, Central America, and South America.
Popularity as Pets
Many parrots are kept as pets, especially macaws, Amazon parrots, math, parakeets, and math. These birds have been popular companions throughout history because they are intelligent, charismatic, colorful, and musical. Some birds can imitate many nonavian sounds, including human speech. The male African gray parrot (Psittacus erithacus) is the most accomplished user of human speech in the animal world; this rain forest-dweller is an uncanny mimic.
Threats to Survival
Currently the Convention on International Trade in Endangered Species (CITES) bans the sale of any wild-caught species, yet the parrots' popularity continues to drive illegal trade.
Some parrot species are highly endangered. In other cases, once tame birds have reproduced in the wild and established thriving feral populations in foreign ecosystems. The monk (green) parakeet, for example, now lives in several U.S. states.

#14 Re: Dark Discussions at Cafe Infinity » crème de la crème » Yesterday 00:04:29
2455) Hermann Staudinger
Gist:
Work
The world around us is made of atoms combined to form molecules. In the early 1900s chemists debated how large these molecules could become. In the early 1920s Herman Staudinger claimed they could be very large; tens or even hundreds of thousands of atoms in size. He showed how small molecules can join to form long chains and so become very large molecules—polymers. The result was the basis for the development of synthetic materials like plastics.
Summary
Hermann Staudinger (born March 23, 1881, Worms, Germany—died September 8, 1965, Freiburg im Breisgau, West Germany [now Germany]) was a German chemist who won the 1953 Nobel Prize for Chemistry for demonstrating that polymers are long-chain molecules. His work laid the foundation for the great expansion of the plastics industry later in the 20th century.
Staudinger studied chemistry at the universities of Darmstadt and Munich, and he received a Ph.D. from the University of Halle in 1903. He held academic posts at the universities of Strassburg (now Strasbourg) and Karlsruhe before joining the faculty at the Swiss Federal Institute of Technology in Zürich in 1912. He left the institute in 1926 to become a lecturer at the Albert Ludwig University of Freiburg im Breisgau, where in 1940 an Institute for Macromolecular Chemistry was established under his directorship. Staudinger’s wife, the Latvian plant physiologist Magda Woit, was his coworker and coauthor. He retired in 1951.
Staudinger’s first discovery was that of the highly reactive organic compounds known as ketenes. His work on polymers began with research he conducted for the German chemical firm BASF on the synthesis of isoprene (1910), the monomer of which natural rubber is composed. The prevalent belief at the time was that rubber and other polymers are composed of small molecules that are held together by “secondary” valences or other forces. In 1922 Staudinger and J. Fritschi proposed that polymers are actually giant molecules (macromolecules) that are held together by normal covalent bonds, a concept that met with resistance from many authorities. Throughout the 1920s, the researches of Staudinger and others showed that small molecules form long, chainlike structures (polymers) by chemical interaction and not simply by physical aggregation. Staudinger showed that such linear molecules could be synthesized by a variety of processes and that they could maintain their identity even when subject to chemical modification.
Staudinger’s pioneering work provided the theoretical basis for polymer chemistry and greatly contributed to the development of modern plastics. His researches on polymers eventually contributed to the development of molecular biology, which seeks to understand the structure of proteins and other macromolecules found in living organisms. Staudinger wrote numerous papers and books, including Arbeitserinnerungen (1961; “Working Memories”). Two of his students, Leopold Ružička and Tadeus Reichstein, also won Nobel Prizes.
Details
Hermann Staudinger (23 March 1881 – 8 September 1965) was a German organic chemist who demonstrated the existence of macromolecules, which he characterized as polymers. For this work he received the 1953 Nobel Prize in Chemistry.
He is also known for his discovery of ketenes and of the Staudinger reaction. Staudinger, together with Leopold Ružička, also elucidated the molecular structures of pyrethrin I and II in the 1920s, enabling the development of pyrethroid insecticides in the 1960s and 1970s.
Early work
Staudinger was born in 1881 in Worms. Staudinger, who initially wanted to become a botanist, studied chemistry at the University of Halle, at the TH Darmstadt and at the LMU Munich. He received his "Verbandsexamen" (comparable to Master's degree) from TH Darmstadt. After receiving his Ph.D. from the University of Halle in 1903, Staudinger qualified as an academic lecturer at the University of Strasbourg in 1907. He was supported in his work by his new wife Dora Staudinger who wrote up his lectures.
It was here that he discovered the ketenes, a family of molecules characterized by the general form depicted in Figure 1. Ketenes would prove a synthetically important intermediate for the production of yet-to-be-discovered antibiotics such as penicillin and amoxicillin.
In 1907, Staudinger began an assistant professorship at the Technical University of Karlsruhe. Here, he successfully isolated a number of useful organic compounds (including a synthetic coffee flavoring) as more completely reviewed by Rolf Mülhaupt. Here too he guided future Nobel laureates Leopold Ružička (1910) and Tadeusz Reichstein to their doctorates.
The Staudinger reaction
In 1912, Staudinger took on a new position at the Swiss Federal Institute of Technology in Zurich, Switzerland. One of his earliest discoveries came in 1919, when he and colleague Meyer reported that organic azides react with triphenylphosphine to form an iminophosphorane. This reaction, commonly referred to as the Staudinger reaction, typically produces a high yield of the iminophosphorane.
World War I
While in autumn 1914 German professors joined the widespread public support of the war, Staudinger refused to sign Manifesto of the Ninety-Three and joined the few exceptions like Max Born, Otto Buek and Albert Einstein in condemning it. In 1917 he authored an essay predicting the defeat of Germany due to industrial superiority of the Entente and called for a peaceful settlement as soon as possible, and after the entrance of the US he repeated the call in a long letter to the German military leadership. Fritz Haber attacked him for his essay, accusing him of harming Germany, and Staudinger in turn criticized Haber for his role in the German chemical weapons program.
Polymer chemistry
While at Karlsruhe and later, Zurich, Staudinger began research in the chemistry of rubber, for which very high molecular weights had been measured by the physical methods of Raoult and van 't Hoff. Contrary to prevailing ideas, Staudinger proposed in a landmark paper published in 1920 that rubber and other polymers such as starch, cellulose and proteins are long chains of short repeating molecular units linked by covalent bonds. In other words, polymers are like chains of paper clips, made up of small constituent parts linked from end to end.
At the time, leading organic chemists such as Emil Fischer and Heinrich Wieland believed that the measured high molecular weights were only apparent values caused by the aggregation of small molecules into colloids. At first, the majority of Staudinger’s colleagues refused to accept the possibility that small molecules could link together covalently to form high-molecular weight compounds. As Mülhaupt aptly notes, this is due in part to the fact that molecular structure and bonding theory were not fully understood in the early 20th century.
In 1926, he was appointed lecturer of chemistry at the University of Freiburg at Freiburg im Breisgau (Germany), where he spent the rest of his career. Further evidence to support his polymer hypothesis emerged in the 1930s. High molecular weights of polymers were confirmed by membrane osmometry, and also by Staudinger’s measurements of viscosity in solution. The X-ray diffraction studies of polymers by Herman Mark provided direct evidence for long chains of repeating molecular units. And the synthetic work led by Carothers demonstrated that polymers such as nylon and polyester could be prepared by well-understood organic reactions. His theory opened up the subject to further development, and helped place polymer science on a sound basis.
Private life
He married in 1906 to Dora Förster and they remained together until their divorce in 1926. They had four children including Eva Lezzi (1907-1993) and Klar (Klara) Kaufmann who were active in resisting the rise of fascism. Dora married again and became a leading peace activist.
In 1927, he married the Latvian botanist, Magda Voita (German: Magda Woit), who was a collaborator with him until his death and whose contributions he acknowledged in his Nobel Prize acceptance.
In 1935 Staudinger became a Patron Member of the SS.
Legacy
Staudinger's groundbreaking elucidation of the nature of the high-molecular weight compounds he termed Makromoleküle paved the way for the birth of the field of polymer chemistry. Staudinger himself saw the potential for this science long before it was fully realized. "It is not improbable," Staudinger commented in 1936, "that sooner or later a way will be discovered to prepare artificial fibers from synthetic high-molecular products, because the strength and elasticity of natural fibers depend exclusively on their macro-molecular structure – i.e., on their long thread-shaped molecules." Staudinger founded the first polymer chemistry journal in 1940, and in 1953 received the Nobel Prize in Chemistry for "his discoveries in the field of macromolecular chemistry." In 1999, the American Chemical Society and the German Chemical Society designated Staudinger's work as an International Historic Chemical Landmark. His pioneering research has afforded the world myriad plastics, textiles, and other polymeric materials which make consumer products more affordable, attractive and enjoyable, while helping engineers develop lighter and more durable structures. The German Chemical Society started the Hermann Staudinger Prize in 1971 to recognize fundamental contributions in polymer science.
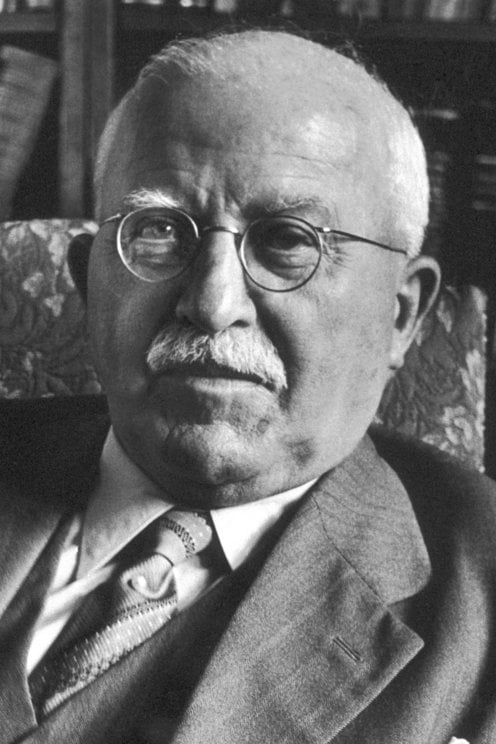
#15 Jokes » Mushroom Jokes - III » Yesterday 00:04:11
- Jai Ganesh
- Replies: 0
Q: Where do mushrooms come from?
A: Mushy rooms.
* * *
Q: What's the only room you can't have in your house?
A: A mushroom.
* * *
Q: What did the mushroom say to the other mushroom?
A: There's not that mush room in here.
* * *
Q: What do you get if you cross a toadstool and a full suitcase?
A: Not mushroom for your holiday clothes!
* * *
Q: Did you hear the joke about the fungus?
A: I could tell it to you, but it might need time to grow on you.
* * *
#16 Dark Discussions at Cafe Infinity » Comedies Quotes - II » Yesterday 00:03:48
- Jai Ganesh
- Replies: 0
Comedies Quotes - II
1. I would say 80% of the scripts I get are dramas and not comedies or romantic comedies, which is funny because that's what I do every week. - Eva Longoria
2. That's what I hate about a lot of comedies, when you're hitting a line or making it funny. - Jennifer Aniston
3. Comedy is a universal language. I grew up watching Nagesh, Surilirajan, Thenga Srinivasan and S.V. Shekhar's comedies. And, of course, Charlie Chaplin! These artists are so blessed: they can make other people happy. - A. R. Rahman
4. I like comedies, I like thrillers, I like love stories. Everything is beautiful; it depends if the film is good, who cares? Everything is interesting. - Monica Bellucci
5. If you look at romantic comedies as pieces of commerce, the audience is looking for wish fulfillment. - Tom Hanks
6. Most of the offers I get from Hollywood are for teen comedies. My manager thinks I'm crazy for turning down all that money, but I'm very picky. - Macaulay Culkin
7. Most people think that action movies are difficult and comedies are easy, but it's actually the opposite. Comedy can be a lot of hard work too. - Lara Dutta
8. Even in comedies, you've got to feel safe for things to just happen in a way that is natural and free, and recognizable as human. - Richard Gere.
#17 This is Cool » Gotthard Base Tunnel (GBT) » 2026-03-10 17:38:29
- Jai Ganesh
- Replies: 0
Gotthard Base Tunnel (GBT)
Gist
The Gotthard Base Tunnel (GBT) in Switzerland is the world's longest and deepest railway tunnel, measuring 57.1 km (35.5 miles) in length and up to 2,450 meters deep. Opened in 2016, it provides a high-speed flat route through the Alps, connecting Erstfeld and Bodio, significantly reducing travel time between Zurich and Milan.
Is the Gotthard Tunnel the longest tunnel in the world?
Switzerland's Gotthard Base Tunnel is the world's longest (and deepest) railway tunnel. Opened in 2016 after 17 years of construction, it consists of two 57.1km single-track tunnels for freight trains and passenger trains connecting Erstfeld in canton Uri with Bodio in canton Ticino.
Summary
The Gotthard Base Tunnel (GBT; German: Gotthard-Basistunnel, Italian: Galleria di base del San Gottardo, Romansh: Tunnel da basa dal Sogn Gottard) is a railway tunnel through the Alps in Switzerland. It opened in June 2016 and full service began the following December. With a route length of 57.09 km (35.47 mi), it is the world's longest railway and deepest traffic tunnel and the first flat, low-level route through the Alps. Located at the heart of the Gotthard axis, it is the third tunnel to connect the cantons of Uri and Ticino, after the Gotthard Tunnel and the Gotthard Road Tunnel.
The GBT consists of a large complex with, at its core, two single-track tunnels connecting Erstfeld (Uri) with Bodio (Ticino) and passing below Sedrun (Grisons). It is part of the New Railway Link through the Alps (NRLA) project, which also includes the Ceneri Base Tunnel further south (opened on 3 September 2020) and the Lötschberg Base Tunnel on the other main north–south axis. It is referred to as a "base tunnel" since it bypasses most of the existing vertex line, the Gotthard railway line, a winding mountain route opened in 1882 across the Saint-Gotthard Massif, which was operating at its capacity before the opening of the GBT. The new base tunnel establishes a direct route usable by high-speed rail and heavy freight trains.
The main purpose of the Gotthard Base Tunnel is to increase local transport capacity through the Alpine barrier, especially for freight on the Rotterdam–Basel–Genoa corridor. The tunnel is specifically meant to shift freight to trains from trucks, and thereby to reduce environmental damage and deadly road crashes. The tunnel also provides a faster connection between the canton of Ticino and the rest of Switzerland, as well as between northern and southern Europe, cutting the Basel/Zürich–Lugano–Milan journey time for passenger trains by one hour (and from Lucerne to Bellinzona by 45 minutes).
After 64 percent of Swiss voters accepted the NRLA project in a 1992 referendum, the first preparatory and exploratory work began in 1996. Construction began in November 1999 at Amsteg. Drilling operations were completed in March 2011. Completed in 2016, the final cost was reported to be CHF 12.2 billion (US$12 billion). A freight train derailment in August 2023 forced the tunnel's closure for over a year before reopening in September 2024.
Details
Gotthard Base Tunnel, railway tunnel under the Saint-Gotthard Massif in the Lepontine Alps in southern Switzerland, the world’s longest and deepest railway tunnel. Opened in June 2016, the tunnel provided a high-speed rail link between northern and southern Europe, forming a mainline rail connection between Rotterdam in the Netherlands and Genoa in Italy. Comprising two single-track tunnels, the Gotthard Base Tunnel (GBT) is 57 km (35 miles) in length and has a maximum depth of 2,300 metres (7,546 feet). It runs from Erstfeld, in Uri canton, to Bodio, in Ticino canton, and is a division of the New Railway Link through the Alps (NRLA) project.
Importance
Largely flat and straight, the GBT is a “base tunnel” because it passes through the base of the mountains rather than traversing the difficult terrain. The tunnel significantly increased local transport capacity through the Swiss Alpine barrier, providing a faster, more efficient route than the St. Gotthard Pass, the old St. Gotthard Tunnel (constructed 1872–80), or the St. Gotthard Road Tunnel (opened 1980). The GBT is used for both passenger and freight trains and helped shift freight volume from trucks to rail, with both safety and environmental benefits. With practically no gradient, the GBT can bear heavier and longer trains than the old line and has increased the freight train capacity from about 180 to about 260 trains per day. Passenger trains within the GBT travel at a speed of 200 km (124 miles) per hour and can complete the journey from Erstfeld to Bodio in 20 minutes. Freight trains travel at a minimum speed of 100 km (62 miles) per hour. Four to six freight trains and up to two passenger trains often run per hour in each direction through the tunnel every day.
History and construction
The first visionary idea for the GBT was sketched by engineer Carl Eduard Gruner in 1947. The Swiss government established a committee to evaluate various base tunnel ideas in the 1960s and formally recommended the construction of a Gotthard base tunnel in 1970. In 1992 the Swiss electorate passed the government’s resolution to construct the Swiss Rail Link through the Alps, providing the formal start to the project. Over the next few years, exploratory bores and other investigations were carried out to determine the most geotechnically favourable route for the tunnel, finally landing on the Erstfeld-Bodio route. AlpTransit Gotthard AG, a subsidiary of Swiss Federal Railways, was responsible for construction of the GBT, which officially began on November 4, 1999.
The construction of the GBT was a remarkable feat of modern engineering. The unpredictable quality of the rock, coupled with the intense weight of the mountain above and the resultant extreme temperatures and humidity (without ventilation, the temperature inside the mountain system can reach 46 °C, or 115 °F), posed serious challenges. Tunneling was done from each direction in each of the two bores, with four access tunnels built to facilitate the simultaneous construction. The four construction sites, Erstfeld, Amsteg, Sedrun, and Faido, each had its own base camp with living quarters, cafeterias, and worker transit as well as water treatment facilities and concrete factories that were fed excavated rock from the tunnel construction; a fifth site at Bodio was added later. The tunnels were primarily constructed with four massive tunnel boring machines, Herrenknecht Gripper TBMs, each of which was more than 441 metres (1,446 feet) long; blasting was used for only about 25 percent of the project. After nearly 11 years the final breakthrough in the east tube took place, in October 2010. The breakthrough was one of the most precise breakthroughs in the history of tunnel construction, with a horizontal deviation of just 8 cm (3 inches) and a vertical deviation of a remarkable 1 cm (0.4 inch).The final breakthrough in the west tube was completed in March 2011. From first blast to the extravagant opening ceremony, the tunnel took 17 years to complete and finished both on time and within its $12 billion (12.2 billion Swiss francs) budget. Nine workers died in accidents while the tunnel was under construction.
Additional Information
The Gotthard Base Tunnel (GBT) was inaugurated in 2016. The 57-km long railway tunnel connects northern to southern Europe, enabling passenger and goods transport to reduce travelling time by one hour between Zurich and Milan. It is the world’s longest railway and deepest traffic tunnel and the first flat, low-level route through the Alps.
The primary purpose of the Gotthard Base Tunnel is to increase transport capacity through the Alps, especially for freight, notably on the Rotterdam–Basel–Genoa corridor. A more specific objective is to shift freight volumes from heavy goods vehicles (HGV) to freight trains to reduce the environmental damage caused by HGV significantly.
The Gotthard Base Tunnel mainly consists of two single-track tunnels connecting Erstfeld with Bodio. It is part of the New Railway Link through the Alps (NRLA) project, which also includes the Ceneri Base Tunnel further south (opened in 2020) and the Lötschberg Base Tunnel (opened in 2007) on the other main north-south axis.
Two interesting figures about the Gotthard Base Tunnel construction are outlined below:
* Impact of tunnelling on arch dams of hydraulic power plant
In the central part of the GBT (section Sedrun), three arch dams and hydropower reservoirs are located almost directly above the new GBT, approximately in the middle of the tunnel. The height of the concrete arch dams varies between 117 m for Santa Maria, 127 m for Nalps and 153 m for Curnera.
In 1978, the driving of an exploratory tunnelling gallery for a planned highway tunnel had adverse effects on the arch dam of the Zeuzier reservoir in the Alps, causing significant settlements of up to 13 cm. Well aware of this, tunnelling engineers already started surveying the area four years before the tunnelling works began in the region and developed coupled numerical models for a forecast of the surface deformations.
* Tunnelling challenges in squeezing rocks
Engineers had to face challenging tunnelling conditions in squeezing rock, in the Sedrun section, in the geological section of Tavetsch Intermediate Massif North (TZM North), due to poor rock quality with low strength, squeezing properties and an 800 m thick overburden, and additionally in the Faido section, northern part, with the contact zone between the Leventina and the Lucomagno gneiss formations, under an extremely thick overburden exceeding 2,000 m.

#18 Science HQ » Diabetic Neuropathy » 2026-03-10 16:38:48
- Jai Ganesh
- Replies: 0
Diabetic Neuropathy
Gist
Diabetic neuropathy is a common, often disabling form of nerve damage caused by long-term high blood sugar and fat levels, affecting up to 50% of people with diabetes. It most frequently causes pain, burning, and numbness in the legs and feet, but can also impair digestion, bladder function, and cardiovascular systems. While it can lead to serious ulcers or amputations, it is often managed by tight glucose control, pain medications, and lifestyle changes.
What is the best cure for diabetic neuropathy?
While keeping blood glucose levels in goal range can prevent peripheral neuropathy and keep it from getting worse, there aren't any treatments that can reverse nerve disease once it's established. Once neuropathy is detected, the focus is on keeping the feet and legs healthy and on managing pain.
Summary
Diabetic neuropathy includes various types of nerve damage associated with diabetes mellitus. The most common form, diabetic peripheral neuropathy, affects 30% of all diabetic patients. Studies suggests that cutaneous nerve branches, such as the sural nerve, are involved in more than half of patients with diabetes 10 years after the diagnosis and can be detected with high-resolution magnetic resonance imaging. Symptoms depend on the site of nerve damage and can include motor changes such as weakness; sensory symptoms such as numbness, tingling, or pain; or autonomic changes such as urinary symptoms. These changes are thought to result from a microvascular injury involving small blood vessels that supply nerves (vasa nervorum). Relatively common conditions which may be associated with diabetic neuropathy include distal symmetric polyneuropathy; third, fourth, or sixth cranial nerve palsy; mononeuropathy; mononeuropathy multiplex; diabetic amyotrophy; and autonomic neuropathy.
Diabetic neuropathy is the most common complication of diabetes mellitus (DM), affecting as many as 50% of patients with type 1 and type 2 DM. Diabetic peripheral neuropathy involves the presence of symptoms or signs of peripheral nerve dysfunction in people with diabetes after other possible causes have been excluded. In some cases, patients are symptomatic long before routinely performed clinical examination reveals abnormalities. Of all treatments, tight and stable glycemic control is probably the most important for slowing the progression of neuropathy.
Signs and symptoms of diabetic neuropathy
In type 1 DM, distal polyneuropathy typically becomes symptomatic after many years of chronic prolonged hyperglycemia, whereas in type 2, it may be apparent after only a few years of known poor glycemic control or even at diagnosis. Symptoms include the following:
Sensory – Negative or positive, diffuse or focal; usually insidious in onset and showing a stocking-and-glove distribution in the distal extremities
Motor – Distal, proximal, or more focal weakness, sometimes occurring along with sensory neuropathy (sensorimotor neuropathy)
Autonomic – Neuropathy that may involve the cardiovascular, gastrointestinal, and genitourinary systems and the sweat glands.
Details:
Overview
Diabetic neuropathy is a type of nerve damage that can happen with diabetes. Blood sugar, also called glucose, becomes high because of diabetes. Over time, high blood sugar can injure nerves throughout the body. Diabetic neuropathy most often damages nerves in the legs and feet.
Depending on the affected nerves, diabetic neuropathy symptoms may include pain and numbness in the legs, feet and hands. It also can cause problems with the digestive system, urinary tract, blood vessels and heart. Some people have mild symptoms. But for others, diabetic neuropathy can be painful and disabling.
Diabetic neuropathy is a serious health concern. It may affect up to half of people who have diabetes. But diabetic neuropathy often can be prevented. And people who have it can take steps to keep it from getting worse. The key is to tightly manage blood sugar and lead a healthy lifestyle.
Symptoms
There are four main types of diabetic neuropathy. You can have one type or more than one type of neuropathy.
The symptoms depend on the type of diabetic neuropathy you have and which nerves are affected. Usually, symptoms appear slowly over time. You may not notice anything is wrong until a lot of nerve damage has happened.
Peripheral sensorimotor neuropathy
This type of neuropathy also may be called distal symmetric peripheral neuropathy. It's the most common type of diabetic neuropathy. It affects the feet and legs first, followed by the hands and arms. Symptoms often are worse at night. They may include:
* Loss of feeling, also called numbness, or less ability to feel pain or temperature changes.
* A tingling or burning feeling.
* Sharp pains or cramps.
* Muscle weakness.
* Being very sensitive to touch. For some people, even a bedsheet's weight can be painful.
* Serious foot problems, such as ulcers, infections, and bone and joint damage.
Autonomic neuropathy
The autonomic nervous system controls blood pressure, heart rate, sweating, pupils, bladder, digestive system and sex organs. Diabetes can affect nerves in any of these areas. That can cause symptoms including:
* A lack of the usual warning symptoms that let you know when blood sugar levels are low. This is called hypoglycemia unawareness.
* Drops in blood pressure when rising from sitting or lying down. This is called orthostatic hypotension. It can cause dizziness or fainting.
* A fast-beating heart while at rest.
* Bladder or bowel problems.
* Slow stomach emptying, also called gastroparesis. This can cause upset stomach, vomiting, a feeling of fullness and loss of appetite.
* Trouble swallowing.
* Changes in the way the eyes adjust from light to dark or far to near.
* More or less sweating than usual.
* Problems with sexual response. For instance, some people may have vaginal dryness or trouble feeling aroused. Others may have trouble getting or keeping an erection.
Proximal neuropathy
This type of neuropathy also is called diabetic polyradiculopathy. It often affects nerves in the thighs, hips, buttocks or legs. It can affect the stomach area and chest area. Symptoms often are on one side of the body. Rarely, they spread to the other side. Proximal neuropathy may include:
* Serious pain in the buttock, hip or thigh.
* Weak and shrinking thigh muscles.
* Trouble rising from a sitting position.
* Pain in the chest or the walls of the stomach area.
Mononeuropathy
This type of neuropathy also is called focal neuropathy. It damages a single, specific nerve. That nerve may be in the face, torso, arm or leg. It’s possible for mononeuropathy to affect single nerves in different parts of the body at the same time. Mononeuropathy may lead to:
* Trouble focusing or seeing two images of the same object, also called double vision.
* Not being able to move one side of the face. This is called paralysis.
* Numbness or tingling in the hand or fingers.
* Weakness in the hand that may result in dropping things.
* Pain in the shin or foot.
* Weakness that makes it hard to lift the front part of the foot. This condition is known as foot drop.
* Pain in the front of the thigh.
When to see a doctor
Call your healthcare professional for a checkup if you have:
* A cut or sore on your foot that is infected or won't heal.
* Burning, tingling, weakness or pain in your hands or feet that makes it hard to do daily activities or sleep.
* Changes in digestion, urination or sexual function.
* Dizziness and fainting.
Tests can check for diabetic neuropathy before a person has symptoms of it. These are called screening tests. Screening tests can find diseases early when they're easier to treat. The American Diabetes Association recommends that screening for diabetic neuropathy start:
* Right after you learn you have type 2 diabetes.
* Or five years after you're found to have type 1 diabetes.
After that, screening is recommended once a year.
Causes
The exact cause of each type of neuropathy is unknown. Researchers think that over time, uncontrolled high blood sugar damages nerves and interferes with their ability to send signals. This process may lead to diabetic neuropathy. High blood sugar also weakens the walls of the small blood vessels called capillaries that supply the nerves with oxygen and nutrients.
Risk factors
Anyone who has diabetes can get diabetic neuropathy. But these risk factors make nerve damage more likely:
* Poor blood sugar control. Uncontrolled high blood sugar raises the risk of every medical complication that can happen with diabetes, including nerve damage.
* Diabetes history. The risk of diabetic neuropathy rises the longer you have diabetes, especially if your blood sugar isn't well controlled.
* Kidney disease. Diabetes can damage the kidneys. Kidney damage sends toxins into the blood, which can lead to nerve damage.
* Being overweight. Having a body mass index (BMI) of 25 or more may raise the risk of diabetic neuropathy.
* Smoking. Smoking narrows and hardens the arteries, lowering blood flow to the legs and feet. This makes it harder for wounds to heal. It also damages the peripheral nerves.
* High blood pressure and high cholesterol. Both are linked with a higher risk of diabetic neuropathy.
Complications
Diabetic neuropathy can cause serious medical conditions, including:
* Hypoglycemia unawareness. Most often, blood sugar levels below 70 milligrams per deciliter (mg/dL) — 3.9 millimoles per liter (mmol/L) — cause shakiness, sweating and a fast heartbeat in people living with diabetes. But people who have autonomic neuropathy may not feel these warning signs.
* Loss of a toe, foot or leg. Nerve damage can cause a loss of feeling in the feet. That means even minor cuts can turn into sores or ulcers without being noticed. Sometimes, an infection can spread to the bone or lead to tissue death. Without fast treatment, a toe, foot or even part of the leg may need to be removed with surgery. This is called amputation.
* Urinary problems. If the nerves that control the bladder are damaged, the bladder may not empty fully when urinating. Bacteria can build up in the bladder and kidneys, causing urinary tract infections. Nerve damage also can affect the ability to feel the need to urinate or to control the muscles that release urine. This can lead to leakage, also called incontinence.
* Sharp drops in blood pressure. Damage to the nerves that control blood flow can affect the body's ability to adjust blood pressure. This can cause a sharp drop in pressure when standing after sitting or lying down. That may lead to lightheadedness and fainting.
* Digestive problems. If nerve damage happens in the digestive tract, you may get constipation or diarrhea, or both. Diabetes-related nerve damage can lead to a condition in which the stomach empties too slowly or not at all. This is called gastroparesis. It can cause bloating and an upset stomach.
* Sexual conditions. Diabetic neuropathy often damages the nerves that affect the sex organs. Symptoms may include vaginal dryness, having trouble becoming aroused, and difficulty getting or keeping an erection. This is called erectile dysfunction.
* More or less sweating than usual. Nerve damage can disrupt how the sweat glands work. That makes it hard for the body to control its temperature properly.
Prevention
You may be able to prevent or delay diabetic neuropathy and the medical problems that can happen with it. To do so, closely manage your blood sugar and take good care of your feet.
Blood sugar control
A blood test called the A1C test looks at your average blood sugar level for the past 2 to 3 months. The American Diabetes Association recommends that people with diabetes have an A1C test at least twice a year. You also might hear it called the glycosylated hemoglobin, hemoglobin A1C or HbA1c test.
A1C goals may need to be tailored to each person. But for most adults, the American Diabetes Association recommends an A1C of less than 7.0%. The goal may be higher for older adults or those with other medical conditions. If your blood sugar levels are higher than your goal, you may need to change how you manage your diabetes. Your healthcare professional might change your medicine or add medicine to your treatment plan. Or you might be told to change your diet or physical activity.
Foot care
Foot problems are common with diabetic neuropathy. Examples include sores that don't heal and ulcers. But you can prevent many of these problems. The key is to take good care of your feet at home. And have a thorough foot exam at least once a year. Also have your healthcare professional check your feet at each office visit.
Follow your healthcare professional's advice for good foot care. To protect the health of your feet:
* Check your feet every day. Look for blisters, cuts, bruises, cracked and peeling skin, redness, and swelling. Use a mirror to look at parts of your feet that are hard to see. Or ask a friend or family member to help check.
* Keep your feet clean and dry. Wash your feet every day with lukewarm water and mild soap. Don't soak your feet. Dry your feet and between your toes thoroughly.
* Moisturize your feet. This helps prevent cracking. But don't get lotion between your toes. It might make fungus more likely to grow.
* Trim your toenails carefully. Cut your toenails straight across. File the edges gently so they are smooth. If you can't do this yourself, see a specialist in foot problems, called a podiatrist, for help.
* Wear clean, dry socks. Look for socks made of cotton or moisture-wicking fibers. The socks should not have tight bands or thick seams.
* Wear cushioned shoes that fit well. Wear closed-toed shoes or slippers to protect your feet. Make sure your shoes fit properly, and give your toes space to move. A foot specialist can teach you how to buy properly fitted shoes. The specialist also can show you how to prevent problems such as corns and calluses. If you have Medicare, your plan may cover the cost of at least one pair of shoes each year.
* Protect your feet from the heat. Wear shoes if you walk on hot pavement or go to the beach. If you go barefoot outdoors, put sunscreen on the tops of your feet so they don't get sunburned.
* Boost blood flow to your feet. If you can, put your feet up while you sit. And throughout the day, wiggle your toes around for a few minutes. It also helps to move your ankles in and out as well as up and down.
Additional Information
Diabetes-related neuropathy is nerve damage that affects people with diabetes. The most common type is peripheral neuropathy, which often affects your feet. There’s no cure for diabetes-related neuropathy. But you can manage it with medication, therapies and tighter blood sugar management.
Overview:
What is diabetes-related neuropathy?
Diabetes-related neuropathy happens when you experience nerve damage due to high blood sugar (hyperglycemia) that lasts a long time. It can affect people with long-term diabetes, like Type 1 diabetes and Type 2 diabetes. But not everyone with diabetes develops it.
Neuropathy can develop from other causes, too, like pinched nerves, inflammation, nutrient deficiencies and injuries affecting your nerves. Healthcare providers diagnose neuropathy as diabetes-related if you have diabetes and they can’t find another cause for it.
Types of diabetes-related neuropathy
Diabetes-related neuropathy can damage different nerves throughout your body. Types of diabetes-related neuropathy include:
* Peripheral neuropathy: This is the most common type of neuropathy. “Peripheral” refers to any of the nerves outside of your spinal cord. It often affects your feet and legs and sometimes your hands.
* Autonomic neuropathy: This type of neuropathy happens when you have damage to autonomic nerves, which control your involuntary body processes. They control things like your bladder, intestinal tract, blood pressure, heart and sex organs. Another name for autonomic neuropathy is dysautonomia.
* Proximal neuropathy: This is a rare type of neuropathy that affects nerves in your hip, thigh or buttock. It typically only affects one side of your body.
How common is diabetes-related neuropathy?
Overall, diabetes-related neuropathy is fairly common. Studies show that up to 50% of people with diabetes have peripheral neuropathy. More than 30% of people with diabetes have autonomic neuropathy.
Symptoms and Causes:
What are the symptoms of diabetes-related neuropathy?
Your symptoms will depend on which type of diabetes-related neuropathy you have.
Symptoms of diabetes-related peripheral neuropathy
Diabetes-related peripheral neuropathy commonly affects your feet. Symptoms include:
* Numbness, tingling and/or pins and needles sensations (paresthesia).
* Pain, which may be burning, stabbing or shooting.
* Unusual touch-based sensations (dysesthesia).
* Muscle weakness.
* Slow-healing leg or foot sores (ulcers).
* Total loss of sensation in your feet, like not feeling pain from foot injuries.
* Nerve damage that causes peripheral neuropathy typically develops over many years. You may not notice symptoms of mild nerve damage for a long time.
Symptoms of diabetes-related autonomic neuropathy
Autonomic neuropathy can have many different symptoms because it can affect several body systems. Examples include:
* Digestive system: Indigestion, heartburn, nausea and vomiting, gas, diarrhea and constipation. Gastroparesis is a type of digestive system neuropathy.
* Urinary system: Urinary incontinence, urinary retention and frequent UTIs.
* sex organs: Sexual dysfunction, erectile dysfunction, retrograde ejaculation, vaginal dryness and anorgasmia.
* Cardiovascular system: Low blood pressure, irregular heart rate, dizziness and fainting.
* Sweat glands: Excessive sweating or a lack of sweat.
* Eyes: Difficult for your pupils to adjust to changes in light.
Autonomic neuropathy can also cause hypoglycemia unawareness. This means you don’t experience the typical warning signs of low blood sugar, like shakiness, confusion and intense hunger.
Symptoms of diabetes-related proximal neuropathy
Symptoms of proximal neuropathy include:
* Sudden and severe pain in your hip, buttock or thigh.
* Weakness in your leg that makes it difficult to stand up.
* Loss of reflexes, like the knee-jerk reflex.
* Loss of muscle tissue (atrophy) in the affected area.
* Unexplained weight loss.
What causes diabetes-related neuropathy?
Perpetually high blood sugar levels can damage small blood vessels that provide oxygen and nutrients to your nerves. Without enough oxygen and nutrients, nerve cells can die, affecting the function of your nerve. This causes neuropathy.
Each person is different, so it’s almost impossible to predict how high blood sugar levels have to be — and for how long — to cause neuropathy. One study of people with Type 2 diabetes shows that having an A1C over 7% for at least three years increases your risk of diabetes-related neuropathy. An A1C of 7% means your blood sugar is 154 mg/dL on average.
What are the risk factors for diabetes-related neuropathy?
If you have diabetes, your chance of developing diabetes-related neuropathy increases the older you get and the longer you’ve had diabetes.
Studies show that peripheral neuropathy affects at least 20% of people with Type 1 diabetes who’ve had diabetes for at least 20 years. It affects 15% to 50% of people with Type 2 diabetes who’ve had diabetes for at least 10 years.
You’re also more likely to develop neuropathy if you have diabetes along with:
* High blood pressure (hypertension).
* High body mass index (BMI).
* High cholesterol.
* Kidney disease.
* Alcohol use disorder.
* Smoking.
Studies show that genetics may also increase your risk of diabetes-related neuropathy.
Diagnosis and Tests:
How is diabetes-related neuropathy diagnosed?
To start, a healthcare provider will ask detailed questions about your medical history and diabetes management. They’ll ask about your symptoms and do a physical exam. Tests that help confirm a diabetes-related neuropathy diagnosis include:
* Diabetes foot exam: Your provider will visually assess your feet for any injuries or issues. They’ll then touch your toes and feet with various tools to check if you have numbness. This exam helps diagnose peripheral neuropathy.
* NCS (nerve conduction studies): This test checks how fast electrical signals move through your peripheral nerves in different parts of your body. It helps diagnose peripheral and proximal neuropathies.
* EMG (electromyography): This test evaluates the health and function of your skeletal muscles and the nerves that control them. It helps diagnose peripheral and proximal neuropathies.
Tests to diagnose autonomic neuropathy vary depending on which body system is affected. For example, an ultrasound can show how well your bladder empties when you pee. Tests like gastric emptying scintigraphy (GES) can help diagnose digestive system issues.
It may take more time to get an autonomic neuropathy diagnosis, as many other conditions can cause the same symptoms.

#19 Re: Jai Ganesh's Puzzles » General Quiz » 2026-03-10 15:40:25
Hi,
#10783. What does the term in Geography Dependent territory mean?
#10784. What does the term in Geography Deposition (geology) mean?
#20 Re: Jai Ganesh's Puzzles » English language puzzles » 2026-03-10 15:27:52
Hi,
#5989. What does the verb (used with object) enable mean?
#5990. What does the noun encampment mean?
#21 Re: Jai Ganesh's Puzzles » Doc, Doc! » 2026-03-10 15:18:13
Hi,
#2591. What does the medical term Postpartum depression mean?
#22 Re: Jai Ganesh's Puzzles » 10 second questions » 2026-03-10 14:54:33
Hi,
#9877.
#23 Re: Jai Ganesh's Puzzles » Oral puzzles » 2026-03-10 14:40:16
Hi,
#6370.
#24 Re: Exercises » Compute the solution: » 2026-03-10 14:24:33
Hi,
2731.
#25 Re: This is Cool » Miscellany » 2026-03-10 00:04:55
2517) Hyperglycemia
Gist
Hyperglycemia is high blood sugar, commonly affecting diabetics when glucose exceeds 125 mg/dL (fasting) or 180 mg/dL (postprandial) due to low insulin, skipped medication, or stress. Symptoms include increased thirst, frequent urination, fatigue, and blurred vision. Long-term effects include severe nerve, kidney, heart, and eye damage.
Hyperglycemia treatment focuses on lifestyle changes (diet, exercise, hydration), monitoring blood sugar, and taking prescribed medications like insulin or oral agents (e.g., metformin), with emergency care for severe cases like Diabetic Ketoacidosis (DKA) involving IV fluids and insulin, aiming to prevent long-term complications like kidney or eye disease.
Summary
Hyperglycemia is an unusually high amount of glucose in the blood. It is defined as blood glucose level exceeding 6.9 mmol/L (125 mg/dL) after fasting for 8 hours or 10 mmol/L (180 mg/dL) 2 hours after eating.
Signs and symptoms
Hyperglycemia may be asymptomatic. Blood glucose levels can rise above normal and cause pathological and functional changes for significant periods without producing any permanent effects or symptoms. During this asymptomatic period, an abnormality in carbohydrate metabolism can occur, which can be tested by measuring plasma glucose.
The degree of hyperglycemia can change over time depending on the metabolic cause, for example, impaired glucose tolerance or fasting glucose, and it can depend on treatment.
Details:
Overview
High blood sugar, also called hyperglycemia, affects people who have diabetes. Several factors can play a role in hyperglycemia in people with diabetes. They include food and physical activity, illness, and medications not related to diabetes. Skipping doses or not taking enough insulin or other medication to lower blood sugar also can lead to hyperglycemia.
It's important to treat hyperglycemia. If it's not treated, hyperglycemia can become severe and cause serious health problems that require emergency care, including a diabetic coma. Hyperglycemia that lasts, even if it's not severe, can lead to health problems that affect the eyes, kidneys, nerves and heart.
Symptoms
Hyperglycemia usually doesn't cause symptoms until blood sugar (glucose) levels are high — above 180 to 200 milligrams per deciliter (mg/dL), or 10 to 11.1 millimoles per liter (mmol/L).
Symptoms of hyperglycemia develop slowly over several days or weeks. The longer blood sugar levels stay high, the more serious symptoms may become. But some people who've had type 2 diabetes for a long time may not show any symptoms despite high blood sugar levels.
Early signs and symptoms
Recognizing early symptoms of hyperglycemia can help identify and treat it right away. Watch for:
* Frequent urination
* Increased thirst
* Blurred vision
* Feeling weak or unusually tired
Later signs and symptoms
If hyperglycemia isn't treated, it can cause toxic acids, called ketones, to build up in the blood and urine. This condition is called ketoacidosis. Symptoms include:
* Fruity-smelling breath
* Dry mouth
* Abdominal pain
* Nausea and vomiting
* Shortness of breath
* Confusion
* Loss of consciousness
When to see a doctor
Seek immediate help from your care provider if:
* You have ongoing diarrhea or vomiting, and you can't keep any food or fluids down
* Your blood glucose levels stay above 240 milligrams per deciliter (mg/dL) (13.3 millimoles per liter (mmol/L)) and you have symptoms of ketones in your urine
Causes
During digestion, the body breaks down carbohydrates from foods — such as bread, rice and pasta — into sugar molecules. One of the sugar molecules is called glucose. It's one of the body's main energy sources. Glucose is absorbed and goes directly into your bloodstream after you eat, but it can't enter the cells of most of the body's tissues without the help of insulin. Insulin is a hormone made by the pancreas.
When the glucose level in the blood rises, the pancreas releases insulin. The insulin unlocks the cells so that glucose can enter. This provides the fuel the cells need to work properly. Extra glucose is stored in the liver and muscles.
This process lowers the amount of glucose in the bloodstream and prevents it from reaching dangerously high levels. As the blood sugar level returns to normal, so does the amount of insulin the pancreas makes.
Diabetes drastically reduces insulin's effects on the body. This may be because your pancreas is unable to produce insulin, as in type 1 diabetes. Or it may be because your body is resistant to the effects of insulin, or it doesn't make enough insulin to keep a normal glucose level, as in type 2 diabetes.
In people who have diabetes, glucose tends to build up in the bloodstream. This condition is called hyperglycemia. It may reach dangerously high levels if it is not treated properly. Insulin and other drugs are used to lower blood sugar levels.
Risk factors
Many factors can contribute to hyperglycemia, including:
* Not using enough insulin or other diabetes medication
* Not injecting insulin properly or using expired insulin
* Not following your diabetes eating plan
* Being inactive
* Having an illness or infection
* Using certain medications, such as steroids or immunosuppressants
* Being injured or having surgery
* Experiencing emotional stress, such as family problems or workplace issues
Illness or stress can trigger hyperglycemia. That's because hormones your body makes to fight illness or stress can also cause blood sugar to rise. You may need to take extra diabetes medication to keep blood glucose in your target range during illness or stress.
Complications:
Long-term complications
Keeping blood sugar in a healthy range can help prevent many diabetes-related complications. Long-term complications of hyperglycemia that isn't treated include:
* Cardiovascular disease
* Nerve damage (neuropathy)
* Kidney damage (diabetic nephropathy) or kidney failure
* Damage to the blood vessels of the retina (diabetic retinopathy) that could lead to blindness
* Feet problems caused by damaged nerves or poor blood flow that can lead to serious skin infections, ulcerations and, in some severe cases, amputation
* Bone and joint problems
* Teeth and gum infections
Emergency complications
If blood sugar rises very high or if high blood sugar levels are not treated, it can lead to two serious conditions.
* Diabetic ketoacidosis. This condition develops when you don't have enough insulin in your body. When this happens, glucose can't enter your cells for energy. Your blood sugar level rises, and your body begins to break down fat for energy.
When fat is broken down for energy in the body, it produces toxic acids called ketones. Ketones accumulate in the blood and eventually spill into the urine. If it isn't treated, diabetic ketoacidosis can lead to a diabetic coma that can be life-threatening.
* Hyperosmolar hyperglycemic state. This condition occurs when the body makes insulin, but the insulin doesn't work properly. Blood glucose levels may become very high — greater than 600 milligrams per deciliter (mg/dL), (33.3 millimoles per liter (mmol/L)) without ketoacidosis. If you develop this condition, your body can't use either glucose or fat for energy.
Glucose then goes into the urine, causing increased urination. If it isn't treated, diabetic hyperosmolar hyperglycemic state can lead to life-threatening dehydration and coma. It's very important to get medical care for it right away.
Prevention
To help keep your blood sugar within a healthy range:
* Follow your diabetes meal plan. If you take insulin or oral diabetes medication, be consistent about the amount and timing of your meals and snacks. The food you eat must be in balance with the insulin working in your body.
* Monitor your blood sugar. Depending on your treatment plan, you may check and record your blood sugar level several times a week or several times a day. Careful monitoring is the only way to make sure that your blood sugar level stays within your target range. Note when your glucose readings are above or below your target range.
* Carefully follow your health care provider's directions for how to take your medication.
* Adjust your medication if you change your physical activity. The adjustment depends on blood sugar test results and on the type and length of the activity. If you have questions about this, talk to your health care provider.
Additional Information
Hyperglycemia (high blood sugar) is common in people who have diabetes. If it’s left untreated, chronic hyperglycemia can lead to diabetes complications, such as nerve damage, eye disease and kidney damage.
Overview:
What is hyperglycemia (high blood sugar)?
Hyperglycemia happens when there’s too much sugar (glucose) in your blood. It’s also called high blood sugar or high blood glucose. This happens when your body has too little insulin (a hormone) or if your body can’t use insulin properly (insulin resistance).
Hyperglycemia usually means you have diabetes, and people with diabetes can experience hyperglycemia episodes frequently.
If you have hyperglycemia that’s untreated for long periods of time, it can damage your nerves, blood vessels, tissues and organs.
Severe hyperglycemia can also lead to an acute (sudden and severe) life-threatening complication called diabetes-related ketoacidosis (DKA), especially in people with diabetes who take insulin or people with undiagnosed Type 1 diabetes. This requires immediate medical treatment.
What blood sugar level is hyperglycemia?
For people undiagnosed with diabetes, hyperglycemia is blood glucose greater than 125 mg/dL (milligrams per deciliter) while fasting (not eating for at least eight hours).
A person has prediabetes if their fasting blood glucose is 100 mg/dL to 125 mg/dL.
A person with a fasting blood glucose greater than 125 mg/dL on more than one occasion usually receives a diabetes diagnosis — typically Type 2 diabetes. People with Type 1 diabetes usually have very high blood sugar (above 250 mg/dL) upon diagnosis.
For a person with diabetes, hyperglycemia is usually considered to be a blood glucose level greater than 180 mg/dL one to two hours after eating. But this can vary depending on what your target blood sugar goals are.
What is blood sugar?
Glucose (sugar) mainly comes from carbohydrates in the food and drinks you consume. It’s your body’s main source of energy. Your blood carries glucose to all of your body’s cells to use for energy.
If you don’t have diabetes, several bodily processes naturally help keep your blood glucose in a healthy range. Insulin, a hormone your pancreas makes, is the most significant contributor to maintaining healthy blood sugar.
High blood sugar most often happens due to a lack of insulin or insulin resistance. This leads to diabetes. People who have diabetes must use medication, like oral diabetes medications or synthetic insulin, and/or lifestyle changes to help keep their blood sugar levels in range.
How common is hyperglycemia?
Hyperglycemia and diabetes are very common — about 1 in 10 people in the United States has diabetes. Hyperglycemia episodes are also very common in people with diabetes.
Symptoms and Causes
Symptoms of hyperglycemia include increased thirst, frequent urination, headache, blurred vision, fatigue and more.
If you have these symptoms, you should see a healthcare provider. If you have these symptoms in addition to vomiting and/or labored breathing, seek immediate medical help.
What are the signs and symptoms of hyperglycemia?
Early symptoms of hyperglycemia include:
* Increased thirst (polydipsia) and/or hunger.
* Frequent urination (peeing).
* Headache.
* Blurred vision.
Symptoms of long-term hyperglycemia include:
* Fatigue.
* Weight loss.
* Vaginal yeast infections.
* Skin infections.
* Slow-healing cuts and sores.
You should see your healthcare provider if you or your child is experiencing these symptoms.
The glucose level at which people with diabetes start to experience symptoms varies. Many people don’t experience symptoms until their blood sugar is 250 mg/dL or higher. People who haven’t yet been diagnosed with diabetes typically experience these symptoms at lower levels.
It’s especially important to know the early signs of hyperglycemia and to monitor your blood sugar regularly if you take insulin or other medications for diabetes. If hyperglycemia is left untreated, it can develop into diabetes-related ketoacidosis (DKA), in which a lack of insulin and a high amount of ketones cause your blood to become acidic. DKA can also affect people who have undiagnosed Type 1 diabetes. This condition is an emergency situation that can lead to coma or death.
Symptoms of ketoacidosis include:
* Nausea and vomiting.
* Dehydration.
* Abdominal pain.
* Fruity-smelling breath.
* Deep labored breathing or hyperventilation (Kussmaul breathing).
* Rapid heartbeat.
* Confusion and disorientation.
* Loss of consciousness.
What causes hyperglycemia?
Hyperglycemia most often results from a lack of insulin. This can happen due to insulin resistance and/or issues with your pancreas — the organ that makes insulin.
Other hormones can contribute to the development of hyperglycemia as well. Excess cortisol (the “stress hormone”) or growth hormone, for example, can lead to high blood sugar:
Insulin resistance
A common cause of hyperglycemia is insulin resistance. Insulin resistance, also known as impaired insulin sensitivity, happens when cells in your muscles, fat and liver don’t respond as they should to insulin.
When your cells don’t properly respond to insulin, your body requires more and more insulin to regulate your blood sugar. If your body is unable to produce enough insulin (or you don’t inject enough insulin), it results in hyperglycemia.
Insulin resistance is the main cause of Type 2 diabetes, but anyone can experience it, including people without diabetes and people with other types of diabetes. It can be temporary or chronic.
Common causes of insulin resistance include:
* Obesity. Scientists believe obesity, especially excess fat tissue in your belly and around your organs (visceral fat), is a primary cause of insulin resistance.
* Physical inactivity.
* A diet of highly processed, high-carbohydrate foods and saturated fats.
* Certain medications, including corticosteroids, some blood pressure medications, certain HIV treatments and some psychiatric medications. These may cause temporary or long-term insulin resistance depending on how long you take them.
Certain hormonal conditions can lead to insulin resistance, such as:
* Cushing syndrome (excess cortisol).
* Acromegaly (excess growth hormone).
* Pregnancy. During pregnancy, the placenta releases hormones that cause insulin resistance. For some people, this leads to gestational diabetes.
Certain inherited genetic conditions are also associated with insulin resistance, including:
* Rabson-Mendenhall syndrome.
* Donohue syndrome.
* Myotonic dystrophy.
* Alström syndrome.
* Werner syndrome.
Pancreas issues
Damage to your pancreas can lead to a lack of insulin production and hyperglycemia. Pancreatic conditions that can cause hyperglycemia and diabetes include:
* Autoimmune disease: In Type 1 diabetes, your immune system attacks the insulin-producing cells in your pancreas for unknown reasons. This means your pancreas can no longer make insulin, resulting in hyperglycemia. Latent autoimmune diabetes in adults (LADA) also results from an autoimmune reaction, but it develops much more slowly than Type 1.
* Chronic pancreatitis: This condition causes prolonged inflammation of your pancreas, which can damage the cells that produce insulin. This can result in a lack of insulin and hyperglycemia. Pancreatitis is a known cause of Type 3c diabetes.
* Pancreatic cancer: Cancer in your pancreas can damage the cells that produce insulin, resulting in a lack of insulin and hyperglycemia. About 25% of people with pancreatic cancer are diagnosed with diabetes 6 months to 36 months before the diagnosis of pancreatic cancer.
* Cystic fibrosis: People who have cystic fibrosis develop excessive mucus, which can scar their pancreas. This can cause their pancreas to produce less insulin, resulting in hyperglycemia and cystic fibrosis-related diabetes (CFRD).
Temporary causes of hyperglycemia
Certain situations can temporarily increase your blood sugar levels and cause hyperglycemia in people with and without diabetes.
Physical stress, such as from an illness, surgery or injury, can temporarily raise your blood sugar. Acute emotional stress, such as experiencing trauma or work-related stress, can increase your blood sugar as well. This is because your body releases cortisol and/or epinephrine (adrenaline).
Causes of hyperglycemia in people with diabetes
Several factors can contribute to hyperglycemia in people with diabetes. It can develop if things like food and diabetes medications are out of balance.
Common situations that can lead to hyperglycemia for people with diabetes include:
* Not taking enough insulin, injecting the wrong insulin or expired insulin, or an issue with the injection (such as from a site issue in insulin pump therapy).
* Not timing insulin and carb intake correctly.
* The amount of carbohydrates you’re consuming isn’t balanced with the amount of insulin your body can make or the amount of insulin you inject.
* The dose of oral diabetes medication you’re taking is too low for your needs.
* Being less active than usual.
* Dawn phenomenon.
What are the complications of hyperglycemia?
Prolonged (chronic) hyperglycemia over the years can damage blood vessels and tissues in your body. This can lead to a variety of complications, including the following:
* Retinopathy.
* Nephropathy.
* Neuropathy.
* Gastroparesis.
* Heart disease.
* Stroke.
It’s important to remember that other factors can contribute to the development of diabetes complications, such as genetics and how long you’ve had diabetes.
Acute (sudden and severe) hyperglycemia can lead to DKA, which is life-threatening.
Diagnosis and Tests:
How is hyperglycemia diagnosed?
Healthcare providers order bloodwork to screen for hyperglycemia and diagnose diabetes. These tests may include:
* Fasting glucose tests.
* Glucose tolerance tests.
* A1c test.
People with diabetes use at-home blood sugar testing (using a glucose meter) to monitor their blood sugar and check for hyperglycemia. If you use continuous glucose monitoring (CGM), your device may alert you to high blood sugar. As this technology can sometimes be inaccurate, it’s important to check your blood sugar with a glucose meter if the CGM reading doesn’t match how you feel.