Math Is Fun Forum
You are not logged in.
- Topics: Active | Unanswered
#126 Re: Jai Ganesh's Puzzles » English language puzzles » 2025-09-03 15:49:28
Hi,
#5733. What does the verb (used with object) intersperse mean?
#5734. What does the noun interstice mean?
#127 Re: Jai Ganesh's Puzzles » Doc, Doc! » 2025-09-03 15:36:44
Hi,
#2458. What does the medical term Free nerve ending mean?
#128 Re: Jai Ganesh's Puzzles » 10 second questions » 2025-09-03 14:57:46
Hi,
#9725.
#129 Re: Jai Ganesh's Puzzles » Oral puzzles » 2025-09-03 14:22:27
Hi,
#6231.
#130 Jokes » Lawyer Jokes - X » 2025-09-03 13:50:45
- Jai Ganesh
- Replies: 0
Q: How many personal injury attorneys does it take to change a light bulb?
A: Three--one to turn the bulb, one to shake him off the ladder, and the third to sue the ladder company.
* * *
Q: Why does California have the most attorneys, and New Jersey have the most toxic waste dumps?
A: New Jersey got first pick.
* * *
Q: What's the difference between an attorney and a pit bull?
A: Jewelry.
* * *
Q: What do lawyers use for birth control?
A: Their personalities.
* * *
Q: What's the definition of mixed emotions?
A: Watching your attorney drive over a cliff in your new Ferrari.
* * *
#131 Re: Exercises » Compute the solution: » 2025-09-03 13:41:15
Hi,
2572.
#132 This is Cool » Ozone » 2025-09-02 23:09:32
- Jai Ganesh
- Replies: 0
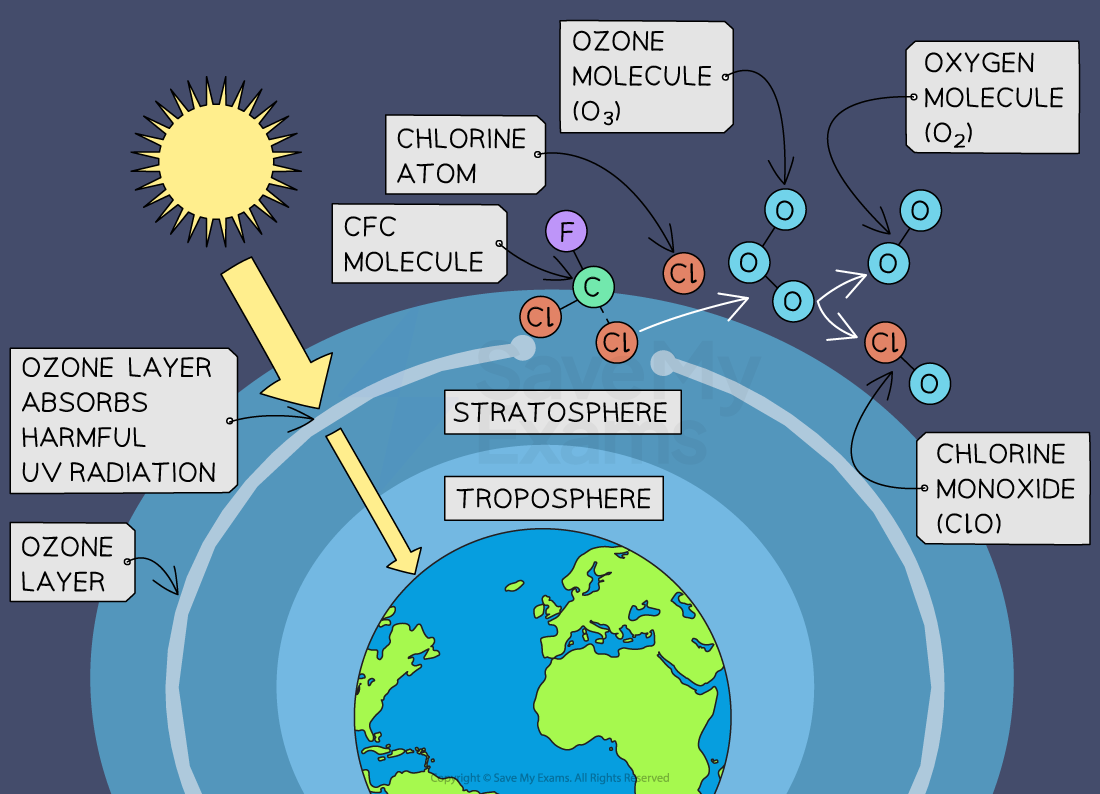
Ozone
Gist
Ozone (O3) is an unstable, blue gas composed of three oxygen atoms that can be beneficial or harmful depending on its location in the atmosphere. In the stratosphere (high atmosphere), the ozone layer naturally shields Earth from harmful ultraviolet (UV) radiation, while at ground level in the troposphere, it's a pollutant formed from nitrogen oxides (NOx) and volatile organic compounds (VOCs), posing health risks and contributing to smog.
Ozone (O3) is a highly reactive gas composed of three oxygen atoms. It is both a natural and a man-made product that occurs in the Earth's upper atmosphere : (the stratosphere) and lower atmosphere (the troposphere).
Summary
Ozone, also called trioxygen, is an inorganic molecule with the chemical formula O3. It is a pale-blue gas with a distinctively pungent odor. It is an allotrope of oxygen that is much less stable than the diatomic allotrope O2, breaking down in the lower atmosphere to O2 (dioxygen). Ozone is formed from dioxygen by the action of ultraviolet (UV) light and electrical discharges within the Earth's atmosphere. It is present in very low concentrations throughout the atmosphere, with its highest concentration high in the ozone layer of the stratosphere, which absorbs most of the Sun's ultraviolet (UV) radiation.
Ozone's odor is reminiscent of chlorine, and detectable by many people at concentrations of as little as 0.1 ppm in air. Ozone's O3 structure was determined in 1865. The molecule was later proven to have a bent structure and to be weakly diamagnetic. At standard temperature and pressure, ozone is a pale blue gas that condenses at cryogenic temperatures to a dark blue liquid and finally a violet-black solid. Ozone's instability with regard to more common dioxygen is such that both concentrated gas and liquid ozone may decompose explosively at elevated temperatures, physical shock, or fast warming to the boiling point. It is therefore used commercially only in low concentrations.
Ozone is a powerful oxidizing agent (far more so than dioxygen) and has many industrial and consumer applications related to oxidation. This same high oxidizing potential, however, causes ozone to damage mucous and respiratory tissues in animals, and also tissues in plants, above concentrations of about 0.1 ppm. While this makes ozone a potent respiratory hazard and pollutant near ground level, a higher concentration in the ozone layer (from two to eight ppm) is beneficial, preventing damaging UV light from reaching the Earth's surface.
Details
Ozone, (O3), is triatomic allotrope of oxygen (a form of oxygen in which the molecule contains three atoms instead of two as in the common form) that accounts for the distinctive odor of the air after a thunderstorm or around electrical equipment. The odor of ozone around electrical machines was reported as early as 1785; ozone’s chemical constitution was established in 1872. Ozone is an irritating pale blue gas that is explosive and toxic, even at low concentrations. It occurs naturally in small amounts in Earth’s stratosphere, where it absorbs solar ultraviolet radiation, which otherwise could cause severe damage to living organisms on Earth’s surface. Under certain conditions, photochemical reactions between nitrogen oxides and hydrocarbons in the lower atmosphere can produce ozone in concentrations high enough to cause irritation of the eyes and mucous membranes. Such ground-level ozone is considered a major air pollutant.
Ozone usually is manufactured by passing an electric discharge through a current of oxygen or dry air. The resulting mixtures of ozone and original gases are suitable for most industrial purposes, although purer ozone may be obtained from them by various methods; for example, upon liquefaction, an oxygen-ozone mixture separates into two layers, of which the denser one contains about 75 percent ozone. The extreme instability and reactivity of concentrated ozone makes its preparation both difficult and hazardous.
Ozone is 1.5 times as dense as oxygen; at −112 °C (−170 °F) it condenses to a dark blue liquid, which freezes at −251.4 °C (−420 °F). The gas decomposes rapidly at temperatures above 100 °C (212 °F) or, in the presence of certain catalysts, at room temperatures. Although it resembles oxygen in many respects, ozone is much more reactive; hence, it is an extremely powerful oxidizing agent, particularly useful in converting olefins into aldehydes, ketones, or carboxylic acids. Because it can decolorize many substances, it is used commercially as a bleaching agent for organic compounds; as a strong germicide it is used to sterilize drinking water as well as to remove objectionable odors and flavors.
Additional Information:
What is ozone and where is it in the atmosphere?
Ozone (O3) is a highly reactive gas composed of three oxygen atoms. It is both a natural and a man-made product that occurs in the Earth's upper atmosphere ozone molecule (the stratosphere) and lower atmosphere (the troposphere). Depending on where it is in the atmosphere, ozone affects life on Earth in either good or bad ways.
Stratospheric ozone is formed naturally through the interaction of solar ultraviolet (UV) radiation with molecular oxygen (O2). The "ozone layer," approximately 6 through 30 miles above the Earth's surface, reduces the amount of harmful UV radiation reaching the Earth's surface.
Tropospheric or ground-level ozone – what we breathe – is formed primarily from photochemical reactions between two major classes of air pollutants, volatile organic compounds (VOC) and nitrogen oxides (NOx). These reactions have traditionally been viewed as depending upon the presence of heat and sunlight, resulting in higher ambient ozone concentrations in summer months. Within the last decade, however, high ozone concentrations have also been observed under specific circumstances in cold months, where a few high elevation areas in the Western U.S. with high levels of local VOC and NOx emissions have formed ozone when snow is on the ground and temperatures are near or below freezing. Ozone contributes to what we typically experience as "smog" or haze, which still occurs most frequently in the summertime, but can occur throughout the year in some southern and mountain regions.
Although some stratospheric ozone is transported into the troposphere, and some VOC and NOx occur naturally, the majority of ground-level ozone is the result of reactions of man-made VOC and NOx. Significant sources of VOC are chemical plants, gasoline pumps, oil-based paints, autobody shops, and print shops. Nitrogen oxides result primarily from high temperature combustion. Significant sources are power plants, industrial furnaces and boilers, and motor vehicles.
How does atmospheric ozone affect human health?
Ozone has two properties of interest to human health. First, it absorbs UV light, reducing human exposure to harmful UV radiation that causes skin cancer and cataracts. Second, when inhaled, it reacts chemically with many biological molecules in the respiratory tract, leading to a number of adverse health effects.
Meet the Ozone Molecule
The ozone molecule (O3) is formed in the Earth’s atmosphere primarily through a series of photochemical reactions involving oxygen molecules (O2) and ultraviolet (UV) radiation from the Sun.
i) Ozone is a gas made up of three (3) atoms of oxygen:
O + O + O = O3
ii) Oxygen (we breathe) is made up of two (2) atoms of oxygen:
O + O = O2
The atoms in O2 are stable – each atom “holds on” to the other.
The atoms in O3 consist of a stable pair (O2) and a third, unstable atom.
It is the unstable atom that gives ozone its power! Ozone is generated when energy “splits” the stable O2 bond.
Ozone Can be Generated in Three Ways:
Ozone, a molecule composed of three oxygen atoms (O3), can be generated through various methods. Here are three common ways ozone can be produced:
i) Ultraviolet (UV) Radiation: Ozone can be generated naturally in the Earth’s stratosphere through the interaction of UV radiation from the sun and oxygen molecules (O2). In this process, high-energy UV radiation breaks apart oxygen molecules, forming oxygen radicals (O). These radicals then react with other oxygen molecules to form ozone (O3).
ii) Corona Discharge (Electric Discharge): This method involves passing a high-voltage electric discharge through oxygen gas (O2) or dry air containing oxygen. The electric discharge creates a plasma, which leads to the dissociation of oxygen molecules into oxygen atoms. These oxygen atoms then combine with other oxygen molecules to form ozone.
iii) Cold Plasma (Dielectric Barrier Discharge): In this method, oxygen gas (O2) or dry air containing oxygen is passed through a dielectric barrier discharge (DBD), which is essentially a gap between two electrodes with a dielectric material in between. When a high-voltage alternating current is applied to the electrodes, it creates a plasma that facilitates the dissociation of oxygen molecules into oxygen atoms. These oxygen atoms then combine with other oxygen molecules to produce ozone.
These methods are commonly employed in various industries and applications, such as water treatment, air purification, and industrial processes. Each method has its advantages and limitations depending on the specific requirements of the application.
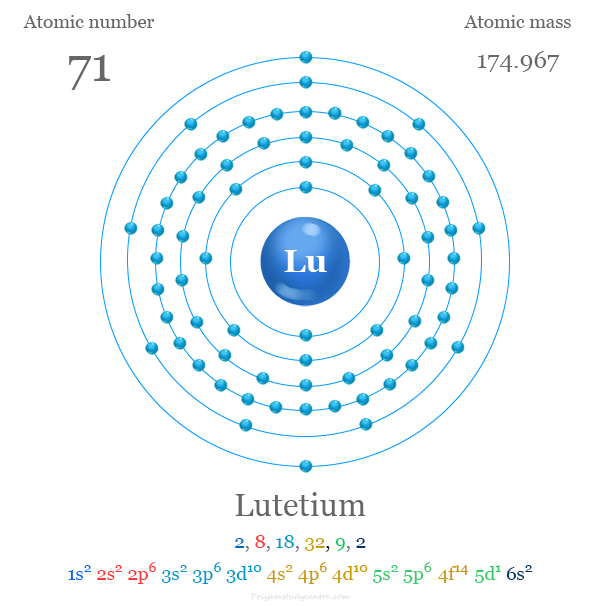
#133 Science HQ » Lutetium » 2025-09-02 22:01:37
- Jai Ganesh
- Replies: 0
Lutetium
Gist
Lutetium (Lu) is the element with atomic number 71, a rare earth metal that is silvery-white, hard, dense, and expensive. It is found in trace amounts in minerals like monazite and is used as a catalyst in the petroleum industry for processes like polymerization and alkylation. Lutetium-177 is a radioactive isotope used in targeted cancer therapies, such as in the treatment of prostate cancer.
Lutetium, outside of scientific research, is primarily used in the medical, petroleum, and geological industries. In medicine, it is used in cancer research, and geologically it is used to date meteorites. In petroleum, it is used to help crack hydrocarbons.
Lutetium (Lu) is the element with atomic number 71, a rare earth metal that is silvery-white, hard, dense, and expensive. It is found in trace amounts in minerals like monazite and is used as a catalyst in the petroleum industry for processes like polymerization and alkylation. Lutetium-177 is a radioactive isotope used in targeted cancer therapies, such as in the treatment of prostate cancer.
Summary
Lutetium is a chemical element; it has symbol Lu and atomic number 71. It is a silvery white metal, which resists corrosion in dry air, but not in moist air. Lutetium is the last element in the lanthanide series, and it is traditionally counted among the rare earth elements; it can also be classified as the first element of the 6th-period transition metals.
Lutetium was independently discovered in 1907 by French scientist Georges Urbain, Austrian mineralogist Baron Carl Auer von Welsbach, and American chemist Charles James. All of these researchers found lutetium as an impurity in ytterbium. The dispute on the priority of the discovery occurred shortly after, with Urbain and Welsbach accusing each other of publishing results influenced by the published research of the other; the naming honor went to Urbain, as he had published his results earlier. He chose the name lutecium for the new element, but in 1949 the spelling was changed to lutetium. In 1909, the priority was finally granted to Urbain and his names were adopted as official ones; however, the name cassiopeium (or later cassiopium) for element 71 proposed by Welsbach was used by many German scientists until the 1950s.
Lutetium is not a particularly abundant element, although it is significantly more common than silver in the Earth's crust. It has few specific uses. Lutetium-176 is a relatively abundant (2.5%) radioactive isotope with a half-life of about 38 billion years, used to determine the age of minerals and meteorites. Lutetium usually occurs in association with the element yttrium and is sometimes used in metal alloys and as a catalyst in various chemical reactions. 177Lu-DOTA-TATE is used for radionuclide therapy on neuroendocrine tumours. Lutetium has the highest Brinell hardness of any lanthanide, at 890–1300 MPa.
Details
Lutetium (Lu) is a chemical element, a rare-earth metal of the lanthanide series of the periodic table, that is the densest and the highest-melting rare-earth element and the last member of the lanthanide series.
In its pure form, lutetium metal is silvery white and stable in air. The metal is easily dissolved in diluted acids—except hydrofluoric acid (HF), in which a protective layer of LuF3 forms on the surface and prevents the metal from further dissolution. The metal is paramagnetic from 0 K (−273 °C, or −460 °F) to its melting point at 1,936 K (1,663 °C, or 3,025 °F) with a temperature-independent magnetic susceptibility between approximately 4 and 300 K (−269 and 27 °C, or −452 and 80 °F). It becomes superconducting at 0.022 K (−273.128 °C, or −459.63 °F) and pressures exceeding 45 kilobars.
Lutetium was discovered in 1907–08 by Austrian chemist Carl Auer von Welsbach and Georges Urbain, working independently. Urbain derived the name for the element from Lutetia, the ancient Roman name for Paris, to honour his native city. The name lutetium became widely accepted except in Germany, where it was commonly called cassiopeium until the 1950s. One of the rarest of the rare earths, lutetium occurs in rare-earth minerals such as laterite clays, xenotime, and euxenite. Though lutetium composes only trace mounts (less than 0.1 percent by weight) of the commercially important minerals bastnasite and monazite, it has proved feasible to extract the metal as a by-product. Lutetium is also found in the products of nuclear fission.
Natural lutetium consists of two isotopes: stable lutetium-175 (97.4 percent) and radioactive lutetium-176 (2.6 percent, 3.76 × {10}^{10}-year half-life). The radioactive isotope is used to determine the age of meteorites relative to that of Earth. In addition to lutetium-176, and not counting nuclear isomers, 33 more radioactive isotopes of lutetium are known. They range in mass from 150 to 184; the least stable isotope (lutetium-150) has a half-life of 45 milliseconds, and the most stable isotope is lutetium-176.
Separation and purification are accomplished by liquid-liquid extraction or ion-exchange techniques. The metal is prepared by metallothermic reduction of the anhydrous halides by alkali or alkaline-earth metals. Lutetium is monomorphic and has a close-packed hexagonal structure with a = 3.5052 Å and c = 5.5494 Å at room temperature.
Lutetium is used in research. Its compounds are used as hosts for scintillators and X-ray phosphors, and the oxide is used in optical lenses. The element behaves as a typical rare earth, forming a series of compounds in oxidation state +3, such as lutetium sesquioxide, sulfate, and chloride.
Element Properties
atomic number : 71
atomic weight : 174.967
melting point : 1,663 °C (3,025 °F)
boiling point : 3,402 °C (6,156 °F)
specific gravity : 9.841 (24 °C, or 75 °F)
oxidation state : +3.
Additional Information:
Appearance
A silvery-white, hard, dense metal.
Uses
Lutetium is little used outside research. One of its few commercial uses is as a catalyst for cracking hydrocarbons in oil refineries.
Biological role
Lutetium has no known biological role. It has low toxicity.
Natural abundance
In common with many other lanthanides, the main source of lutetium is the mineral monazite. It is extracted, with difficulty, by reducing the anhydrous fluoride with calcium metal.
#134 Re: Jai Ganesh's Puzzles » General Quiz » 2025-09-02 21:32:49
Hi,
#10541. What does the term in Geography Blockfield mean
#10542. What does the term in Geography Blowhole (geology) mean?
#135 Re: Jai Ganesh's Puzzles » English language puzzles » 2025-09-02 21:14:45
Hi,
#5731. What does the adjective interpretive mean?
#5732. What does the adjective interrogative mean?
#136 Dark Discussions at Cafe Infinity » Climbing Quotes - I » 2025-09-02 20:56:39
- Jai Ganesh
- Replies: 0
Climbing Quotes - I
1. Climbing to the top demands strength, whether it is to the top of Mount Everest or to the top of your career. - A. P. J. Abdul Kalam
2. After climbing a great hill, one only finds that there are many more hills to climb. - Nelson Mandela
3. Poverty entails fear and stress and sometimes depression. It meets a thousand petty humiliations and hardships. Climbing out of poverty by your own efforts that is something on which to pride yourself but poverty itself is romanticized by fools. - J. K. Rowling
4. There are two things that are more difficult than making an after-dinner speech: climbing a wall which is leaning toward you and kissing a girl who is leaning away from you. - Winston Churchill
5. In the middle of a recession, where we're just climbing out of it, where the economy -unemployment is still at 9.7 percent, the idea of raising taxes and reducing spending is a prescription for disaster. - Joe Biden
6. Management is efficiency in climbing the ladder of success; leadership determines whether the ladder is leaning against the right wall. - Stephen Covey
7. I like to think of Everest as a great mountaineering challenge, and when you've got people just streaming up the mountain - well, many of them are just climbing it to get their name in the paper, really. - Edmund Hillary
8. When you go to the mountains, you see them and you admire them. In a sense, they give you a challenge, and you try to express that challenge by climbing them. - Edmund Hillary.
#137 Jokes » Lawyer Jokes - IX » 2025-09-02 20:29:49
- Jai Ganesh
- Replies: 0
Q: What do you get when you cross the Godfather with a lawyer?
A: An offer you can't understand.
* * *
Q: What's the difference between a lawyer and a vulture?
A: Lawyers accumulate frequent flyer points.
* * *
Q: What is a criminal lawyer?
A: Redundant.
* * *
Q: What's the difference between a good lawyer and a great lawyer?
A: A good lawyer knows the law. A great lawyer knows the judge.
* * *
Q: When lawyers die, why don't vultures them?
A: Even a vulture has taste.
* * *
#138 Re: This is Cool » Miscellany » 2025-09-02 20:18:55
2378) Rafflesia arnoldii
Gist
Often called the corpse flower, Rafflesia arnoldi blooms into the single largest individual flower in the world. When it does, it emits a vile aroma, similar to rotten meat, attracting insects, such as flies and beetles, that feed on dead flesh.
Summary
Rafflesia is (genus Rafflesia), genus of about 42 species of parasitic plants native to Southeast Asia. All Rafflesia species parasitize the roots of Tetrastigma vines (family Vitaceae), and their presence is not made known until the striking flowers emerge from the host vine. One species, Rafflesia arnoldii, boasts the largest single flower of any plant.
Taxonomy
Kingdom: Plantae
Clade: Angiosperm
Order: Malpighiales
Family: Rafflesiaceae
Genus: Rafflesia
Physical description
Like other members of the family Rafflesiaceae, Rafflesia plants are endoparasitic, meaning that the vegetative organs are so reduced and modified that the plant body exists only as a network of threadlike cellular strands living almost wholly within the tissues of the host plant. There are no green photosynthetic tissues, leaves, roots, or stems in the generally accepted sense, although vestiges of leaves exist in some species as scales. Rafflesia plants are thus obligate parasites, which cannot live without the nutrition provided by the host. Despite the dramatic reduction of most of the plant body, the flowers are well developed and can be extremely large.
Rafflesia flowers are sturdy, typically with five substantial tepals (undifferentiated petals and sepals) surrounding the gender organs in a central cup. Interestingly, individual flowers are unisexual, meaning that pollination can occur only if another flower of the opposite gender is simultaneously blooming. The distinctive flowers are sapromyiophilous, meaning that they are pollinated by carrion-feeding flies, and they have a number of adaptations to attract their unconventional pollinators. Most are reddish or purplish brown and have a mottled pattern that resembles rotting flesh. In addition, they emit a fetid carrion odor during the few days they are blooming, and some species even generate heat to simulate decomposition. The unusual pollen is transferred by means of a sticky liquid that dries on the flies. The resultant fruit is a berry containing sticky seeds thought to be disseminated by fruit-eating rodents.
Major species and conservation
The genus includes the giant R. arnoldii, sometimes known as the corpse flower or monster flower, which produces the largest-known individual flower of any plant species in the world and is found in the forested mountains of Sumatra and Borneo. Its fully developed flower appears aboveground as a thick fleshy five-lobed structure weighing up to 11 kg (24 pounds) and measuring almost one meter (about one yard) across.
Most Rafflesia species are considered rare and, given their complete dependence on Tetrastigma vines, are extremely difficult to cultivate and very vulnerable to extinction. Major threats include the loss of rainforest habitat, due to logging and other land-use changes, and illegal harvest of the flowers for their purported medicinal properties. As of 2023 only one species, R. magnifica of the Philippines, has been formally evaluated, and it is listed as critically endangered by the IUCN Red List of Threatened Species, though scientists estimate that at least 60 percent of the species are endangered.
Details
Kingdom: Plantae
Clade: Tracheophytes
Clade: Angiosperms
Clade: Eudicots
Clade: Rosids
Order: Malpighiales
Family: Rafflesiaceae
Genus: Rafflesia
Species: R. arnoldii
Binomial name
Rafflesia arnoldii, the corpse flower, or giant padma, is a species of flowering plant in the parasitic genus Rafflesia within the family Rafflesiaceae. It is noted for producing the largest individual flower on Earth. It has a strong and unpleasant odour of decaying flesh. It is native to the rainforests of Sumatra and Borneo. Although there are some plants with larger flowering organs like the titan arum (Amorphophallus titanum) and talipot palm (Corypha umbraculifera), those are technically clusters of many flowers.
Rafflesia arnoldii is one of the three national flowers in Indonesia, the other two being the white jasmine (Jasminum sambac) and moon orchid (Phalaenopsis amabilis). It was officially recognised as a national "rare flower" (Indonesian: puspa langka) in Presidential Decree No. 4 in 1993.
Taxonomy
The first European to find Rafflesia was the ill-fated French explorer Louis Auguste Deschamps. He was a member of a French scientific expedition to Asia and the Pacific, detained by the Dutch for three years on the Indonesian island of Java, where, in 1797, he collected a specimen, which was probably what is now known as R. patma. During the return voyage in 1798, his ship was taken by the British, with whom France was at war, and all his papers and notes were confiscated. Joseph Banks is said to have agitated for the return of the stolen documents, but apparently to no avail; they were lost, turned up for sale around 1860, went to the British Museum of Natural History, where they were promptly lost again. They did not see the light of day until 1954, when they were rediscovered at the Museum. To everyone's surprise, his notes and drawings indicate that he had found and studied the plants long before the British. It is thought quite possible the British purposely hid Deschamps' notes, to claim the 'glory' of 'discovery' for themselves.
In 1818 the British surgeon Joseph Arnold collected a specimen of another Rafflesia species found by a Malay servant in a part of Sumatra, then a British colony called British Bencoolen (now Bengkulu), during an expedition run by the recently appointed Lieutenant-Governor of Bencoolen, Stamford Raffles. Arnold contracted a fever and died soon after the discovery, the preserved material being sent to Banks. Banks passed on the materials, and the honour to study them was given to Robert Brown. The British Museum's resident botanical artist Franz Bauer was commissioned to make illustrations of the new plants. Brown eventually gave a speech before the June 1820 meeting of the Linnean Society of London, where he first introduced the genus and its until then two species. Brown gave the generic name Rafflesia in honour of Raffles. Bauer completed his pictures some time in mid-1821, but the actual article on the subject continued to languish.
William Jack, Arnold's successor in the Sumatran Bencoolen colony, recollected the plant and was the first to officially describe the new species under the name R. titan in 1820. It is thought quite likely that Jack rushed the name to publication because he feared that the French might publish what they knew of the species, and thus rob the British of potential 'glory'. Apparently aware of Jack's work, Brown finally had the article published in the Transactions of the Linnean Society a year later, formally introducing the name R. arnoldii (he ignores Jack's work in his article).
Because Jack's name has priority, R. arnoldii should technically be a synonym of R. titan, but at least in Britain, it was common at the time to recognise the names introduced by well-regarded scientists such as Brown, over what should taxonomically be the correct name. This was pointed out by the Dutch Rafflesia expert Willem Meijer in his monographic addition to the book series Flora Malesiana in 1997. Instead of sinking R. arnoldii into synonymy, however, he declared that the name R. titan was "incompletely known": the plant material used by Jack to describe the plant has been lost.
In 1999, the British botanical historian David Mabberley, in response to Meijer's findings, attempted to rescue Brown's names from synonymy. This is known as 'conservation' in taxonomy, and normally this requires making a formal proposal to the committee of the International Code of Botanical Nomenclature (ICBN). Mabberley thought he found a loophole around such a formal review by noting that while Brown was notoriously slow to get his papers published, he often had a handful of pre-print pages privately printed to exchange with other botanists: one of these pre-prints had been recently bought by the Hortus Botanicus Leiden, and it was dated April 1821. Mabberley thus proposed that this document be considered the official effective publication, stating this would invalidate Jack's earlier name. For some reason Mabberley uses 1821, a few months after Brown's pre-print, as the date of Jack's publication, instead of the 1820 publication date in Singapore. Confusingly, the record in the International Plant Names Index (IPNI) still has yet another date, "1823?", as it was in the Index Kewensis before Meijer's 1997 work. Mabberley's proposals regarding Brown's name were accepted by institutions, such as the Index Kewensis.
Mabberley also pointed out that the genus Rafflesia was thus first validated by an anonymous report on the meeting published in the Annals of Philosophy in September 1820 (the name was technically an unpublished nomen nudum until this publication). Mabberley claimed the author was Samuel Frederick Gray. However, as that is nowhere stated in the Annals, per Article 46.8 of the code of ICBN, Mabberley was wrong to formally ascribe the validation to Gray. The validation of the name was thus attributed to one Thomas Thomson, the editor of the Annals in 1820, by the IPNI. Mabberley admitted his error in 2017. This Thomson was not the botanist Thomas Thomson, who was three years old in 1820, but his identically named father, a chemist, and Rafflesia is thus the only botanical taxon this man ever published.
Errata
An old Kew webpage claimed that Sophia Hull was present when the specimen was collected and finished the colour drawing that Arnold had started of the plant. It also stated that Brown had originally wanted to call the plant genus Arnoldii.
Regional names
It is called kerubut in Sumatra. In the kecamatan ('district') of Pandam Gadang, it is known as cendawan biriang in the Minangkabau language.
Description
Although Rafflesia is a vascular plant, it lacks any observable leaves, stems or even roots, and does not have chlorophyll. It lives as a holoparasite on vines of the genus Tetrastigma, most commonly T. angustifolium. Similar to fungi, individuals grow as a mass of thread-like strands of tissue completely embedded within and in intimate contact with surrounding host cells from which nutrients and water are obtained. It can only be seen outside the host plant when it is ready to reproduce; the only part of Rafflesia that is identifiable as distinctly plant-like are the flowers, though even these are unusual since they attain massive proportions, are reddish-brown with white spots, and stink of rotting flesh. According to Sandved, the flower opens with a hissing sound.
The flower of Rafflesia arnoldii grows to a diameter of around 1 m (3 ft 3 in),[2] and weighs up to 11 kg (24 lb). According to the Mongabay institution, the single largest R. arnoldii to be measured was 1.14 m (3 ft 9 in) in width. These flowers emerge from very large, cabbage-like, maroon or dark brown buds typically about 30 cm (12 in) wide, but the largest (and the largest flower bud ever recorded) found at Mount Sago, Sumatra in May 1956 was 43 cm (17 in) in diameter. Indonesian researchers often refer to the bud as a 'knop' (knob). According to one source, these buds require 21 months to form. Yet the flowers remain open for only four days.
The plant is native to the rainforest regions of Malaysia, Indonesia, the Philippines, and Thailand.
Additional Information
Often called the corpse flower, Rafflesia arnoldi blooms into the single largest individual flower in the world.
When it does, it emits a vile aroma, similar to rotten meat, attracting insects, such as flies and beetles, that feed on dead flesh.
These flesh-loving creatures pollinate the flower, allowing it to spread through the rainforests of Borneo.
Due to the incredibly specific requirements of the plant, almost no botanical gardens have a Rafflesia arnoldi in cultivation, including Kew.
Rafflesia arnoldi has no leaves, stems or roots, and is a parasitic plant that grows on vines in the genus Tetrastigma.
Plant description
Rafflesia arnoldi lives inside Tetrastigma vines as a mass of fleshy strands which absorb water and nutrients from the host. It grows out of the host plant's bark as brown, cabbage-like buds called knops which bloom over several days. The flowers have five lobes, are reddish-brown with white spots, and grow up to 1m across. They appear for a week, releasing a scent of rotting meat.
Cultural
The flower is an iconic symbol of southeast Asian rainforest, and has been depicted on several Indonesian postage stamps.

#139 Re: Dark Discussions at Cafe Infinity » crème de la crème » 2025-09-02 19:13:59
2231) Drew Weissman
Gist:
Work
A vaccine prevents diseases by stimulating the body's immune system to develop a defense against the infectious agent. One type of vaccine uses mRNA, which transfers genetic information from DNA to stimulate protein production. In 2005, Drew Weissman and Katalin Karikó discovered that certain modifications of the building blocks of RNA prevented unwanted inflammatory reactions and increased the production of desired proteins. The discovery laid the foundation for effective mRNA vaccines against COVID-19 during the pandemic that began in early 2020.
Summary
Drew Weissman (born September 7, 1959, Lexington, Massachusetts, U.S.) is an American immunologist whose groundbreaking research into RNA (ribonucleic acid) opened the path to the development of RNA therapeutics, most notably the generation of messenger RNA (mRNA) vaccines. In the late 1990s and early 2000s Weissman and his colleague the Hungarian-born immunologist Katalin Karikó discovered that mRNA can induce immune responses against specific disease-causing agents. They further found that by introducing changes in mRNA nucleosides (the structural subunits of RNA), it was possible to modify these immune responses. The team’s discoveries enabled the development in 2021 of the first mRNA vaccines, which were targeted against SARS-CoV-2, the coronavirus that caused the COVID-19 pandemic. For their breakthrough work, Weissman and Karikó were awarded the 2023 Nobel Prize in Physiology or Medicine.
Early life and education
Weissman was raised in Lexington, Massachusetts, where he enjoyed sports in his youth, particularly martial arts. He was also interested in science from a young age. His father was an engineer, and, while in high school, Weissman worked for his father’s company, which specialized in making optical mirrors for use in satellites.
He later studied biochemistry and enzymology at Brandeis University, from which he graduated with a bachelor’s degree and a master’s degree in 1981. He then attended Boston University, where he earned an M.D. degree and a Ph.D. in immunology and microbiology in 1987. In 1990, after completing a residency in internal medicine at Beth Israel Deaconess Medical Center in Boston, Weissman accepted a fellowship to work at the National Institutes of Health in Maryland. There he carried out research under the guidance of American immunologist Anthony Fauci.
mRNA vaccines
In 1997 Weissman joined the faculty of the Perelman School of Medicine at the University of Pennsylvania (Penn) in Philadelphia. He began carrying out studies in dendritic cells, which serve a key role in immune surveillance, and increasingly focused his efforts on the development of mRNA therapeutics, particularly in the areas of vaccine development and gene therapy. Not long after starting at Penn, Weissman met Karikó, who shared an interest in finding ways to leverage mRNA to stimulate the body to develop immunity against viral pathogens. Karikó began generating mRNA for Weissman’s research, and the two soon began collaborating on mRNA vaccine studies.
Weissman and Karikó quickly discovered in their initial studies that mRNA is highly immunogenic, provoking counterproductive immune responses. However, Karikó had observed that another type of RNA, transfer RNA (tRNA), did not have the same immunogenic effects, which led Weissman and Karikó to experiment with modified nucleosides. In 2005 they reported a major breakthrough: by introducing modified nucleosides, such as pseudouridine, into mRNA, it was possible to generate an mRNA molecule with the ability to evade immediate immune detection. Consequently, the modified mRNA remained active longer than unmodified mRNA, allowing it to enter cells and trigger the production of proteins with the ability to resist disease. The technology became known as non-immunogenic, nucleoside-modified RNA, which Weissman and Karikó patented in 2005.
The promise of non-immunogenic, nucleoside-modified RNA inspired Weissman and Karikó to start a company called RNARx. They successfully licensed the technology to the biotechnology companies Moderna and BioNTech. In 2021, under pressure during the COVID-19 pandemic to develop a vaccine that could help prevent or reduce the severity of infection with SARS-CoV-2, Moderna and a joint effort by the biopharmaceutical company Pfizer and BioNTech independently accelerated research into using mRNA to generate COVID-19 vaccines. Shortly after obtaining the genetic code of SARS-CoV-2, scientists at Moderna and Pfizer-BioNTech separately prepared experimental mRNA vaccines.
Other mRNA therapeutics
Weissman also carried out extensive research into other mRNA therapeutics. For example, he was involved in critical studies in collaboration with other researchers on the use of lipid nanoparticles (LNPs) as a mechanism for mRNA delivery to specific cells. In studies in mice and monkeys, he and his colleagues were among the first to show that a modified mRNA-LNP vaccine could induce immune defense against infection with Zika virus. His research team further applied mRNA-LNP technology to gene therapy for the treatment of diseases such as cystic fibrosis and certain forms of liver disease and investigated the development of mRNA vaccines for a variety of other diseases, including vaccines against herpes simplex, hepatitis C, and norovirus. Weissman was also involved in the development of a pan-coronavirus vaccine with the potential to protect against every variant of coronavirus that could emerge in the future.
Awards and honors
In addition to the Nobel Prize, Weissman received numerous other honors and awards during his career. Notably, together with Karikó, he was a recipient of the Rosenstiel Award (2020), the Louisa Gross Horwitz Prize (2021), and the Lasker-DeBakey Clinical Medical Research Award (2021). In 2022 he became an elected member of the American Academy of Arts and Sciences.
Details
Drew Weissman (born September 7, 1959) is an American physician and immunologist known for his contributions to RNA biology. Weissman is the inaugural Roberts Family Professor in Vaccine Research, director of the Penn Institute for RNA Innovation, and professor of medicine at the Perelman School of Medicine at the University of Pennsylvania (Penn).
Weissman's work underlies the development of mRNA vaccines, the best known of which are those for COVID-19 produced by BioNTech/Pfizer and Moderna. With biochemist Katalin Karikó, Weissman received the Nobel Prize in Physiology or Medicine in 2023 "for their discoveries concerning nucleoside base modifications that enabled the development of effective mRNA vaccines against COVID-19". Weissman has been a recipient and co-recipient of numerous awards, also including the prestigious Lasker–DeBakey Clinical Medical Research Award. In 2022, he was elected to the National Academy of Medicine and the American Academy of Arts and Sciences.
Early life and education
Weissman was born in Lexington, Massachusetts, on September 7, 1959, to Hal and Adele Weissman. Hal is Jewish and Adele is Italian. While his mother did not convert to Judaism, he grew up celebrating all the Jewish holidays. He grew up in Lexington and attended Lexington High School, graduating in 1977.
Weissman received his B.A. and M.A. degrees from Brandeis University in 1981, where he majored in biochemistry and enzymology and he worked in the lab of Gerald Fasman. He performed his graduate work in immunology and microbiology to receive his M.D. and Ph.D. in 1987 at Boston University. Afterward, Weissman did a residency at Beth Israel Deaconess Medical Center, followed by a fellowship at the National Institutes of Health (NIH), under the supervision of Anthony Fauci, then director of the National Institute of Allergy and Infectious Diseases.
Career
In 1997, Weissman moved to the University of Pennsylvania to start his laboratory in order to study RNA and innate immune system biology. He is now the Roberts Family Professor in Vaccine Research at the university.
At the university, Weissman, an immunologist studying vaccines, met his future colleague and collaborator Katalin Karikó at a photocopier, where they sympathized about the lack of funding for RNA research. At the time, Karikó had been trying RNA therapy on cerebral diseases and strokes. Immunologist Weissman began collaborating with biochemist Karikó, who switched her focus to the application of RNA technology to vaccines. Weissman’s support was critical in helping Karikó to continue and extend her research. Slowly they began to move the technology forward, solving problems one at a time. On the difficulty of gaining funding and recognition for their work, Weissman has commented "We had to fight the entire way."
One of the major scientific obstacles they faced was that the RNA caused unwanted immune and inflammatory reactions as adverse side effects. Beginning in 2005, they published several landmark studies that used synthetic nucleosides to modify the RNA to prevent its degradation by the body. This breakthrough laid the groundwork for the use of RNA therapeutics, though the study received little attention at the time.
Weissman and Karikó overcame another major obstacle by developing a delivery technique to package the mRNA in lipid nanoparticles, a novel pharmaceutical drug delivery system for mRNA that protects the fragile molecule until it can reach the desired area of the body. They demonstrated the effectiveness of the delivery system in animals.
In 2006, Weissman and Karikó co-founded RNARx. Their objective was to develop novel RNA therapies. In 2020 their modified RNA technology became the key foundational component of the Pfizer/BioNTech and Moderna COVID-19 vaccines, which were deployed worldwide against the COVID-19 pandemic.
Weissman has been collaborating with scientists at Thailand's Chulalongkorn University, most recently to develop and provide COVID-19 vaccines for the country and neighboring low and middle income countries that may not have immediate access to the vaccine.
Weissman's laboratory continues to actively research the use of mRNA for next-generation vaccines, gene editing, and mRNA therapeutics. Projects include development of a pan coronavirus vaccines, gene editing technology to enable genes that produce missing antibodies, and treatments for acute inflammatory conditions. Weissman hopes that mRNA technology can be used to develop vaccines against influenza, herpes, and HIV.
Recognition
For their mRNA-related work, Weissman and Karikó were awarded the 2023 Nobel Prize in Physiology or Medicine, the 2020 Rosenstiel Award, the Louisa Gross Horwitz Prize, the Albany Medical Center Prize, the Lasker-DeBakey Clinical Medical Research Award, and the BBVA Foundation Frontiers of Knowledge Award (also with Robert S. Langer).
Weissman obtained a honorary degree by the Drexel University College of Medicine. In 2021, he was awarded the Princess of Asturias Award in the category for Scientific Research. For 2022 he was awarded the Breakthrough Prize in Life Sciences, the Jessie Stevenson Kovalenko Medal of the NAS jointly with Katalin Karikó and also the Japan Prize Also in 2022 he received the Robert Koch Prize and the Tang Prize in Biopharmaceutical Science, the Golden Plate Award of the American Academy of Achievement, and was elected to the National Academy of Medicine and American Academy of Arts and Sciences. In 2023 he received the Harvey Prize of the Technion in Israel (awarded for the year 2021).
In 2022, Weissman and Karikó were awarded the Novo Nordisk Prize.
According to a report in The Washington Post, Weissman gets fan mail from people all over the world, thanking him for his work that made the COVID-19 vaccine possible — one said "You've made hugs and closeness possible again" — and asking him for a picture or his autograph.
His name was included with Kariko in Time 2024 list of influential people in health.

#140 Re: Jai Ganesh's Puzzles » Doc, Doc! » 2025-09-02 16:24:05
Hi,
#2457. What does the medical term Myocarditis mean?
#141 Re: Jai Ganesh's Puzzles » 10 second questions » 2025-09-02 16:07:23
Hi,
#9724.
#142 Re: Jai Ganesh's Puzzles » Oral puzzles » 2025-09-02 15:19:25
Hi,
#6230.
#143 Re: Exercises » Compute the solution: » 2025-09-02 14:49:32
Hi,
Well done!
2571.
#144 Re: This is Cool » Miscellany » 2025-08-28 17:59:13
2377) Chimpanzee
Gist
Chimpanzees are highly intelligent primates, demonstrating problem-solving skills, tool use, complex communication, and social intelligence. They can learn to use symbols, understand basic commands, and even display empathy and cultural behaviors. While their brains are smaller than humans, they exhibit remarkable cognitive abilities within their own context.
While chimpanzees can form affectionate bonds and exhibit friendly behavior, their unpredictability means that they can also be dangerous. Their wild instincts, intelligence, and emotional depth make them fascinating, but they also deserve respect. Chimpanzee are not pets and should not be treated as such.
Summary
The chimpanzee, also simply known as the chimp, is a species of great ape native to the forests and savannahs of tropical Africa. It has four confirmed subspecies and a fifth proposed one. When its close relative the bonobo was more commonly known as the pygmy chimpanzee, this species was often called the common chimpanzee or the robust chimpanzee. The chimpanzee and the bonobo are the only species in the genus Pan. Evidence from fossils and DNA sequencing shows that Pan is a sister taxon to the human lineage and is thus humans' closest living relative.
The chimpanzee is covered in coarse black hair but has a bare face, fingers, toes, palms of the hands, and soles of the feet. It is larger and more robust than the bonobo, weighing 40–70 kg (88–154 lb) for males and 27–50 kg (60–110 lb) for females and standing 150 cm (4 ft 11 in).
The chimpanzee lives in groups that range in size from 15 to 150 members, although individuals travel and forage in much smaller groups during the day. The species lives in a strict male-dominated hierarchy, where disputes are generally settled without the need for violence. Nearly all chimpanzee populations have been recorded using tools, modifying sticks, rocks, grass and leaves and using them for hunting and acquiring honey, termites, ants, nuts and water. The species has also been found creating sharpened sticks to spear small mammals. Its gestation period is eight months. The infant is weaned at about three years old but usually maintains a close relationship with its mother for several years more.
The chimpanzee is listed on the IUCN Red List as an endangered species. Between 170,000 and 300,000 individuals are estimated across its range. The biggest threats to the chimpanzee are habitat loss, poaching, and disease. Chimpanzees appear in Western popular culture as stereotyped clown-figures and have featured in entertainments such as chimpanzees' tea parties, circus acts and stage shows. Although chimpanzees have been kept as pets, their strength, aggressiveness, and unpredictability makes them dangerous in this role. Some hundreds have been kept in laboratories for research, especially in the United States. Many attempts have been made to teach languages such as American Sign Language to chimpanzees, with limited success.
Details
A chimpanzee, (Pan troglodytes), is a species of ape that, along with the bonobo, is most closely related to humans. Chimpanzees inhabit tropical forests and savannas of equatorial Africa from Senegal in the west to Lake Albert and northwestern Tanzania in the east. Individuals vary considerably in size and appearance, but chimpanzees stand approximately 1–1.7 metres (3–5.5 feet) tall when erect and weigh about 32–60 kg (70–130 pounds). Males tend to be larger and more robust than females. Chimpanzees are covered by a coat of brown or black hair, but their faces are bare except for a short white beard. Skin colour is generally white except for the face, hands, and feet, which are black. The faces of younger animals may be pinkish or whitish. Among older males and females, the forehead often becomes bald and the back becomes gray.
Natural history
Chimpanzees awaken at dawn, and their day is spent both in the trees and on the ground. After a lengthy midday rest, late afternoon is usually the most intensive feeding period. In the trees, where most feeding takes place, chimps use their hands and feet to move about. They also leap and swing by their arms (brachiate) skillfully from branch to branch. Movement over any significant distance usually takes place on the ground. Though able to walk upright, chimpanzees more often move about on all fours, leaning forward on the knuckles of their hands (knuckle walking). At night they usually sleep in the trees in nests they build of branches and leaves. Chimpanzees are unable to swim, but they will wade in water. The chimpanzee diet is primarily vegetarian and consists of more than 300 different items, mostly fruits, berries, leaves, blossoms, and seeds but also bird eggs and chicks, many insects, and occasionally carrion. Chimpanzees also hunt, both alone and in groups, stalking and killing various mammals such as monkeys, duikers, bushbucks, and wild pigs. They also appear to use certain plants medicinally to cure diseases and expel intestinal parasites.
The female chimpanzee bears a single young at any time of year after a gestation period of about eight months. The newborn weighs about 1.8 kg (about 4 pounds), is almost helpless, and clings to the fur of the mother’s belly as she moves. From about 6 months to 2 years, the youngster rides on the mother’s back. Weaning takes place at about 5 years. Males are considered adults at 16 years of age, and females usually begin to reproduce at about 13 years, but often only two offspring survive during her lifetime. The longevity of chimps is about 45 years in the wild and 58 in captivity; however, older individuals have been documented. For example, Cheetah the chimpanzee, an animal actor from the Tarzan movies of the 1930s and ’40s, was reported to have lived approximately 80 years.
Conservation status
Chimpanzees are an endangered species; their population in the wild has been reduced by hunting (primarily for meat), destruction of habitat from logging or farming, and commercial exportation for use in zoos and research laboratories. The International Union for Conservation of Nature (IUCN) noted that, despite having one of the largest geographic ranges of the great apes, chimpanzee populations have fallen significantly since the 1980s. Lions and leopards also prey upon chimpanzees.
Social behaviour
Chimpanzees are lively animals with more extraverted dispositions than either gorillas or orangutans. They are highly social and live in loose and flexible groups known as communities, or unit groups, that are based on associations between adult males within a home range, or territory. Home ranges of forest-dwelling communities can be as small as a few square kilometres, but home ranges covering hundreds of square kilometres are known among savanna communities. A community can number from 20 or fewer to well over 100 members. Each consists of several subgroups of varying size and unstable composition. Social dominance exists, with adult males being dominant over adult females and adolescent males. Within a community, there are twice or three times as many adult females as adult males; the number of adults is about equal to the number of immature individuals. Communities usually divide into subgroups called parties, which vary widely in size. The dominance hierarchy among male chimpanzees is very fluid; individuals associate with each other and join and leave different subgroups with complete freedom. The dominant (alpha) male of a group can monopolize ovulating females through possessive behaviour. On the other hand, gang attack by subordinate males can expel an alpha male. Males spend all of their lives in the community they are born in, but occasionally a juvenile male may transfer to another community with his mother. In contrast to males, most females leave their group of birth to join a neighbouring group when they mature at around age 11. Female chimpanzees spend most of their time with their young or with other females. Those with dependent offspring are more likely to range alone or in small parties within narrow “core areas.” Females have been known to form coalitions against a bullying adult male or newly immigrated female.
Relations between different chimp communities tend to be hostile. Intruders on a group’s home range may be attacked, and adult males engage in boundary patrol. On rare occasions, a group may invade a neighbouring territory that is much smaller in size, and fatalities among the smaller group result. Infanticide and cannibalism by adult males, and to a lesser extent by adult females, have been observed. Victimized infants are not only those of neighbouring groups but also those born to newly immigrated females. Between- and within-group competition among individuals of the same gender is the likely cause of such violence. Sometimes a male and female will form a consortship, engaging in exclusive mating relationships by leaving other members of the group and staying in the periphery of the group range. This strategy, however, brings increased risk of attack by neighbouring groups.
Chimpanzees exhibit complex social strategies such as cooperation in combat and the cultivation of coalitions and alliances via ranging together, reciprocal grooming, and the sharing of meat (sometimes in exchange for mating opportunities). An alpha male, for instance, may interfere with his rival in grooming with a third party because such a coalition might jeopardize the alpha’s status. On the other hand, the third party might show strategic opportunism in such a situation, since his assistance to either side could determine which of his superiors prevails. Chimpanzees, therefore, appear to have some concept of “trade.” They console, reconcile, and retaliate during fighting and so share emotions and aspects of psychology similar to those found in humans: self-recognition, curiosity, sympathy, grief, and attribution. Although chimps take care of orphaned infants, they also tease handicapped individuals, conceal information that would bring disadvantage to themselves, and manipulate others for their own advantage by expressing deceptive postures, gestures, and facial expressions.
Intelligence
Chimpanzees are highly intelligent and are able to solve many kinds of problems posed to them by human trainers and experimenters. A number of researchers have taught chimpanzees to use sign language or languages based on the display of tokens or pictorial symbols. The implications of these language studies have been contested, however. Critics charge that apes have not acquired true language in the sense of understanding “words” as abstract symbols that can be combined in meaningful new ways. Other investigators maintain that more recent language training has resulted in the chimpanzees’ acquiring a true recognition of “words” as abstractions that can be applied in novel contexts.
Communication between chimps in the wild takes the form of facial expressions, gestures, and a large array of vocalizations, including screams, hoots, grunts, and roars. Males display excitement by standing erect, stamping or swaying, and letting out a chorus of screams. Chimps use louder calls and gestures for long-distance communication (such as drumming on tree buttresses) and quieter calls and facial expressions for short-distance communication. Similarities to human laughter and smiling might be seen in their “play panting” and grinning, respectively.
Various tools are used in several contexts. Chimpanzees “fish” for termites and ants with probes made of grass stalks, vines, branches, peeled bark, and midribs of leaves. They crack hard nuts open by using stones, roots, and wood as hammers or anvils, and they use “leafy sponges” (a handful of folded leaves or moss) to drink water. Branches and leaves are detached and displayed during courtship. In threat displays, chimps throw rocks and drag and throw branches. Sticks are used to inspect dead pythons or other unfamiliar objects that might be dangerous. Leaves are used hygienically in wiping the mouth or other soiled body parts. Chimpanzees also use different tools in succession as a “tool set.” For example, chimpanzees of the Congo basin first dig into termite mounds with a stout stick and then fish for individual termites with a long, slender wand. Tools are also used in combination as “tool composites.” Chimpanzees in the Guinea region push leafy sponges into hollows of trees containing water and then withdraw the wet sponges by using sticks. Chimps thus differ locally in their repertoire of tool use, with younger animals acquiring tool-using behaviours from their elders. Such cultural differences are also seen in food items consumed and in gestural communication. Chimpanzees indeed possess culture when it is defined as the transmission of information from generation to generation via social learning shared by most members of a single age or gender class in a given group.
Chimpanzees’ intelligence, responsiveness, and exuberance have made them ideal nonhuman subjects for psychological, medical, and biological experiments. Young chimpanzees can become very attached to their human trainers, and their expressions of feeling resemble those of humans more closely than any other animal.
Taxonomy
Genetic analysis suggests that the lineages leading to modern humans and chimpanzees diverged from each other between 6.5 million and 9.3 million years ago and that at least 98 percent of the human and chimpanzee genomes are identical. Chimpanzees are classified taxonomically as a single species, Pan troglodytes. (The so-called pygmy chimpanzee, or bonobo, is a distinct and separate species, P. paniscus, that diverged from chimpanzees about 1.7 million years ago.) Four subspecies of P. troglodytes are recognized: the tschego, or Central African chimpanzee (P. troglodytes troglodytes), also known as the common chimpanzee in continental Europe; the West African, or masked, chimpanzee (P. troglodytes verus), known as the common chimpanzee in Great Britain; the East African, or long-haired, chimpanzee (P. troglodytes schweinfurthii); and the Nigerian-Cameroon chimpanzee (P. troglodytes ellioti, which was formerly classified as P. troglodytes vellerosus).
Additional Information
Like us, chimps are highly social animals, care for their offspring for years and can live to be over 50. In fact, chimpanzees are our closest cousins; we share about 98% of our genes.
In their habitat in the forests of Central Africa, chimpanzees spend most of their days in the treetops. When they do come down to earth, chimps usually travel on all fours, though they can walk on their legs like humans for as far as a mile. They use sticks to fish termites out of mounds and bunches of leaves to sop up drinking water.

#145 Re: Maths Is Fun - Suggestions and Comments » Credible place to find help and infromation » 2025-08-28 17:18:36
I forgot to mention; zetafunc, Moderator is Brilliant, just like Rod, and Bob!
#146 Science HQ » Ytterbium » 2025-08-28 16:58:10
- Jai Ganesh
- Replies: 0
Ytterbium
Gist
Ytterbium (Yb) is a soft, silver-colored chemical element (atomic number 70) belonging to the rare earth metals, named after the Swedish town of Ytterby where it was discovered. It's used in electronics as a dopant for phosphors and in ceramic capacitors, as a pressure sensor, and in lasers. Ytterbium is known for its low density and melting/boiling points compared to other lanthanides and forms stable compounds in its +3 oxidation state, though it also has a +2 state.
Ytterbium is beginning to find a variety of uses, such as in memory devices and tuneable lasers. It can also be used as an industrial catalyst and is increasingly being used to replace other catalysts considered to be too toxic and polluting.
Summary
Ytterbium is a chemical element; it has symbol Yb and atomic number 70. It is a metal, the fourteenth and penultimate element in the lanthanide series, which is the basis of the relative stability of its +2 oxidation state. Like the other lanthanides, its most common oxidation state is +3, as in its oxide, halides, and other compounds. In aqueous solution, like compounds of other late lanthanides, soluble ytterbium compounds form complexes with nine water molecules. Because of its closed-shell electron configuration, its density, melting point and boiling point are much lower than those of most other lanthanides.
In 1878, Swiss chemist Jean Charles Galissard de Marignac separated from the rare earth "erbia", another independent component, which he called "ytterbia", for Ytterby, the village in Sweden near where he found the new component of erbium. He suspected that ytterbia was a compound of a new element that he called "ytterbium". Four elements were named after the village, the others being yttrium, terbium, and erbium. In 1907, the new earth "lutecia" was separated from ytterbia, from which the element "lutecium", now lutetium, was extracted by Georges Urbain, Carl Auer von Welsbach, and Charles James. After some discussion, Marignac's name "ytterbium" was retained. A relatively pure sample of the metal was first obtained in 1953. At present, ytterbium is mainly used as a dopant of stainless steel or active laser media, and less often as a gamma ray source.
Natural ytterbium is a mixture of seven stable isotopes, which altogether are present at an average concentration of 0.3 parts per million in the Earth's crust. This element is mined in China, the United States, Brazil, and India in form of the minerals monazite, euxenite, and xenotime. The ytterbium concentration is low because it is found only among many other rare-earth elements. It is among the least abundant. Once extracted and prepared, ytterbium is somewhat hazardous as an eye and skin irritant. The metal is a fire and explosion hazard.
Details
Ytterbium (Yb), chemical element, is a rare-earth metal of the lanthanide series of the periodic table.
Ytterbium is the most volatile rare-earth metal. It is a soft, malleable silvery metal that will tarnish slightly when stored in air and therefore should be stored in vacuum or in an inert atmosphere when long storage time is required. It slowly oxidizes in air, forming Yb2O3; the metal is readily dissolved in diluted acids—except hydrofluoric acid (HF), in which a protective layer of YbF3 forms on the surface and impedes further chemical reaction. Ytterbium is weakly paramagnetic, having the lowest magnetic susceptibility of all the rare-earth metals.
The first concentrate of ytterbium was obtained in 1878 by Swiss chemist Jean-Charles Galissard de Marignac and named by him for the town of Ytterby, Sweden, where it (and the first discovered rare-earth element, yttrium) was found. French chemist Georges Urbain and Austrian chemist Carl Auer von Welsbach independently demonstrated in 1907–08 that Marignac’s earth was composed of two oxides, which Urbain called neoytterbia and lutetia. The elements are now known as ytterbium and lutetium. Ytterbium is among the less-abundant rare earths. It occurs in minute amounts in many rare-earth minerals such as laterite clays, xenotime, and euxenite and is found in products of nuclear fission as well.
Natural ytterbium consists of seven stable isotopes: ytterbium-174 (32.0 percent), ytterbium-172 (21.7 percent), ytterbium-173 (16.1 percent), ytterbium-171 (14.1 percent), ytterbium-176 (13 percent), ytterbium-170 (3 percent), and ytterbium-168 (0.1 percent). Not counting nuclear isomers, a total of 27 radioactive isotopes of Yb ranging in mass from 148 to 181 with half-lives ranging from 409 milliseconds (ytterbium-154) to 32.018 days (ytterbium-169) have been characterized.
Ytterbium is separated from the other rare-earth elements by solvent-solvent extraction or ion-exchange techniques. The elemental metal is prepared by the metallothermic reduction of its oxide, Yb2O3, with lanthanum metal, followed by a vacuum distillation to further purify the metal. Ytterbium exists in three allotropic (structural) forms. The α-phase, which exists below 7 °C (45 °F), is close-packed hexagonal with a = 3.8799 Å and c = 6.3859 Å at room temperature. The β-phase is face-centred cubic with a = 5.4848 Å, and it is the normal structure at room temperature. The γ-phase is body-centred cubic with a = 4.44 Å at 763 °C (1,405 °F). Ytterbium has the lowest boiling point of the rare-earth metals.
The element has little practical use beyond research. Radioactive 169Yb isotope is a source of hard X-rays useful in portable radiographic devices. It is used as a dopant in a variety of optical materials, including lenses. The metal is used in pressure sensors because its electrical resistivity is strongly pressure-dependent.
Ytterbium, like europium, is a divalent metal. A compound of ytterbium in the +2 oxidation state was first prepared in 1929 by W.K. Klemm and W. Schuth, who reduced ytterbium trichloride, YbCl3, to ytterbium dichloride, YbCl2, with hydrogen. The ion Yb2+ has also been produced by electrolytic reduction or treatment of a Yb3+ salt with sodium amalgam. The element forms a series of pale green Yb2+ salts such as ytterbium sulfate, dibromide, hydroxide, and carbonate. The pale green ytterbium ion Yb2+ is unstable in aqueous solution and reduces water readily, liberating hydrogen; it is less stable than the comparable europium ion, Eu2+, and more stable than the samarium ion Sm2+. In its predominant +3 oxidation state, ytterbium forms a series of white salts including the trisulfate and the trinitrate; the sesquioxide is also white.
Element Properties
atomic number : 70
atomic weight : 173.04
melting point : 819 °C (1,506 °F)
boiling point : 1,196 °C (2,185 °F)
specific gravity : 6.966 (24 °C, or 75 °F)
oxidation states : +2, +3.
Additional Information:
Appearance
A soft, silvery metal. It slowly oxidises in air, forming a protective surface layer.
Uses
Ytterbium is beginning to find a variety of uses, such as in memory devices and tuneable lasers. It can also be used as an industrial catalyst and is increasingly being used to replace other catalysts considered to be too toxic and polluting.
Biological role
Ytterbium has no known biological role. It has low toxicity.
Natural abundance
In common with many lanthanide elements, ytterbium is found principally in the mineral monazite. It can be extracted by ion exchange and solvent extraction.

#147 Dark Discussions at Cafe Infinity » Climb Quotes - IV » 2025-08-28 16:25:39
- Jai Ganesh
- Replies: 0
Climb Quotes - IV
1. Our work for human dignity is often lonely, and almost always an uphill climb. At times, our efforts are misunderstood, and we are mistaken for the enemy. There has been a clear erosion of respect for U.N. blue and our impartiality. - Ban Ki-moon
2. Nobody climbs mountains for scientific reasons. Science is used to raise money for the expeditions, but you really climb for the hell of it. - Edmund Hillary
3. One of the best ways to see tree flowers is to climb one of the tallest trees and to get into close, tingling touch with them, and then look broad. - John Muir
4. Thanks to evolution, our bodies have powerful ways to ward off illness and infection and enable us to live long and healthy lives. Why, then, do health costs continue to climb at unsustainable and frightening rates? - David Suzuki
5. I'm not the kind of person who's going to look at the top of a mountain and go, 'Oh, look at that! That's lovely. That's lovely, that top of that mountain.' I'm the kind of person who's going to go, 'Oh, my God! That's so lovely! Let's go climb up it!' - Kate Winslet
6. No minority should climb all over the majority. - Lech Walesa
7. I have enjoyed great satisfaction from my climb of Everest and my trips to the poles. But there's no doubt that my most worthwhile things have been the building of schools and medical clinics. - Edmund Hillary
8. Weight-gain brings with it a number of health issues, but at some point you just realise that you want to live longer, eat healthier. You don't want to be out of breath when you climb a flight of stairs. - Masaba Gupta.
#148 Jokes » Lawyer Jokes - VIII » 2025-08-28 15:50:57
- Jai Ganesh
- Replies: 0
Q: What do you call a lawyer with an I.Q. of 30?
A: A lawyer.
* * *
Q: What do you call a lawyer with an I.Q. of 80?
A: Your honor.
* * *
Q: What do you get when you cross a librarian with a lawyer?
A: All the information you need, but you can’t understand a word of it.
* * *
Q: What do honest lawyers and UFOs have in common?
A: You always hear about them, but you never see them.
* * *
Q: What's the difference between a bankrupt attorney and a pigeon?
A: The pigeon can still make a deposit on a Mercedes.
* * *
#149 Re: Jai Ganesh's Puzzles » General Quiz » 2025-08-28 15:40:22
Hi,
#10539. What does the term in Geography Biosphere mean?
#10540. What does the term in Geography Blackwater river mean?
#150 Re: Jai Ganesh's Puzzles » English language puzzles » 2025-08-28 15:26:34
Hi,
#5729. What does the adjective fraught mean?
#5730. What does the verb (used without object) fraternize mean?