Math Is Fun Forum
You are not logged in.
- Topics: Active | Unanswered
#76 Re: Exercises » Compute the solution: » 2025-10-19 13:37:39
Hi,
2620.
#77 Jokes » Apple Jokes - IV » 2025-10-19 13:27:11
- Jai Ganesh
- Replies: 0
Q: Why did the apple stop in the middle of the road?
A: Because he ran out of juice.
* * *
Q: What did the worm want to do when he grew up?
A: He wanted to join the Apple Core (Corps).
* * *
Q: What do you call a fruit that is rough around the edges?
A: A bad apple.
* * *
Q: What do you say when a fruit wins the talent show?
A: How about them apples?
* * *
Q: Dad, do you like baked apples?
A: Yes son, why?
Q: The orchard's on fire.
* * *
#78 Re: Exercises » Compute the solution: » 2025-10-18 23:36:30
Hi,
2619.
#79 Science HQ » Rutherfordium » 2025-10-18 17:32:14
- Jai Ganesh
- Replies: 0
Rutherfordium
Gist
Rutherfordium (Rf) is a synthetic, highly radioactive chemical element with atomic number 104, found in group 4 of the periodic table. It does not occur naturally and must be created in a laboratory through nuclear reactions in particle accelerators. Named after physicist Ernest Rutherford, its properties are difficult to study because it has a very short half-life, with the most stable known isotope 267Rf lasting about 48 minutes.
Because rutherfordium is made within the lab, there are not very many uses for this element commercially. On the other hand, rutherfordium has been used within the laboratory setting to conduct research. Most elements that are highly radioactive are used for nuclear power and medicinal purposes.
Summary
Rutherfordium is a synthetic chemical element; it has symbol Rf and atomic number 104. It is named after physicist Ernest Rutherford. As a synthetic element, it is not found in nature and can only be made in a particle accelerator. It is radioactive; the most stable known isotope, 267Rf, has a half-life of about 48 minutes.
In the periodic table, it is a d-block element and the second of the fourth-row transition elements. It is in period 7 and is a group 4 element. Chemistry experiments have confirmed that rutherfordium behaves as the heavier homolog to hafnium in group 4. The chemical properties of rutherfordium are characterized only partly. They compare well with the other group 4 elements, even though some calculations had indicated that the element might show significantly different properties due to relativistic effects.
In the 1960s, small amounts of rutherfordium were produced at Joint Institute for Nuclear Research in the Soviet Union and at Lawrence Berkeley National Laboratory in California. Priority of discovery and hence the name of the element was disputed between Soviet and American scientists, and it was not until 1997 that the International Union of Pure and Applied Chemistry (IUPAC) established rutherfordium as the official name of the element.
Details
Rutherfordium (Rf) is an artificially produced radioactive transuranium element in Group IVb of the periodic table, atomic number 104. Soviet scientists at the Joint Institute for Nuclear Research at Dubna, Russia, U.S.S.R., announced in 1964 the discovery of element 104, which they named kurchatovium, symbol Ku (for Igor Kurchatov, a Soviet nuclear physicist). In 1969, a group of American researchers at the Lawrence Radiation Laboratory of the University of California at Berkeley announced that they had identified isotopes of the element, different from the one identified by the Soviets; the Americans then proposed the name rutherfordium, in honour of the British physicist Ernest Rutherford.
In their experiment, the Soviets bombarded plutonium-242 with ions of neon-22, claiming to have obtained an isotope of element 104 that had a mass number of 260 and a half-life of 0.3 second. The Soviets then performed a series of chemical experiments with the isotope to demonstrate that it behaved in a manner that had been predicted for the element. When the workers at Dubna later used a more refined measuring technique, however, they found that the half-life of the isotope was 0.1 second, not 0.3 second as originally reported. This finding cast doubt on the chemical experiments with the element, because the results of those experiments could not have been obtained with atoms having a half-life of 0.1 second.
The American investigators did not follow the same procedure as the Dubna group, because the American equipment could not accelerate neon-22 ions to the necessary energies. Instead, they bombarded a target of californium-249 with ions of carbon-12 and carbon-13. Although unable to obtain the same isotope as the Soviet scientists, the Berkeley team did report positive identification of two, possibly three, isotopes of element 104. The bombardment of californium-249 with carbon-12 produced an isotope with a mass number of 257 and a half-life of 4–5 seconds; the carbon-13 bombardment produced an isotope with a mass number of 259 and a half-life of three to four seconds. The investigators at Berkeley subsequently, by bombarding curium-248 with oxygen-18, synthesized an isotope of element 104 that has a mass number of 261 and a half-life of 70 seconds.
Although the Soviets could make only a few atoms of their mass-260 isotope, the Berkeley group obtained thousands of the atoms having mass numbers of 257 and 259. Moreover, because the latter isotopes have longer half-lives, the Berkeley team was able to measure the energies of their emissions (alpha particles) and to detect their decay products (nobelium isotopes), thereby providing more extensive evidence of their discovery. The International Union of Pure and Applied Chemistry eventually ruled that element 104 be named rutherfordium.
Element Properties
atomic number : 104
mass of most stable isotope : 261.
Additional Information:
Appearance
A radioactive metal that does not occur naturally. Relatively few atoms have ever been made.
Uses
At present, it is only used in research.
Biological role
Rutherfordium has no known biological role.
Natural abundance
Rutherfordium is a transuranium element. It is created by bombarding californium-249 with carbon-12 nuclei.

#80 Re: Dark Discussions at Cafe Infinity » crème de la crème » 2025-10-18 17:02:23
2368) Ulf von Euler
Gist:
Work
Human and animal nervous systems consist of a large variety of cells with long nerve fibers. Small electrical currents and special chemical substances (neurotransmitters) are passed between cells through contacts (synapses). In 1947 Ulf von Euler discovered the neurotransmitter norepinephrine, which plays an important role in producing fight-or-flight signals. He subsequently showed that norepinephrine is formed and stored in packages, or vesicles, sent between neurons via synapses.
Summary
Ulf von Euler (born Feb. 7, 1905, Stockholm, Sweden—died March 9, 1983, Stockholm) was a Swedish physiologist who, with British biophysicist Sir Bernard Katz and American biochemist Julius Axelrod, received the 1970 Nobel Prize for Physiology or Medicine. All three were honoured for their independent study of the mechanics of nerve impulses.
Euler was the son of 1929 Nobel laureate Hans von Euler-Chelpin. After his graduation from the Karolinska Institute in Stockholm, Euler served on the faculty of the institute from 1930 to 1971. He joined the Nobel Committee for Physiology and Medicine in 1953 and was president of the Nobel Foundation for 10 years (1966–75).
Euler’s outstanding achievement was his identification of noradrenaline (norepinephrine), the key neurotransmitter (or impulse carrier) in the sympathetic nervous system. He also found that norepinephrine is stored within nerve fibres themselves. These discoveries laid the foundation for Axelrod’s determination of the role of the enzyme that inhibits its action, and the method of norepinephrine’s reabsorption by nerve tissues. Euler also discovered the hormones known as prostaglandins, which play active roles in stimulating human muscle contraction and in the regulation of the cardiovascular and nervous systems.
Details
Ulf Svante von Euler (7 February 1905 – 9 March 1983) was a Swedish physiologist and pharmacologist. He shared the Nobel Prize in Physiology or Medicine in 1970 for his work on neurotransmitters.
Life
Ulf Svante von Euler-Chelpin was born in Stockholm, the son of two noted scientists, Hans von Euler-Chelpin, a professor of chemistry, and Astrid Cleve, a professor of botany and geology. His father was German and the recipient of Nobel Prize for Chemistry in 1929, and his maternal grandfather was Per Teodor Cleve, Professor of Chemistry at the Uppsala University, and the discoverer of the chemical elements thulium and holmium. Von Euler-Chelpin studied medicine at the Karolinska Institute in 1922. At Karolinska, he worked under Robin Fåhraeus in blood sedimentation and rheology and did research work on the pathophysiology of vasoconstriction. He presented his doctoral thesis in 1930, and was appointed as assistant professor in pharmacology in the same year, with the support of G. Liljestrand. From 1930 to 1931, von Euler-Chelpin got a Rochester Fellowship to do his post-doctoral studies abroad. He studied in England with Sir Henry Dale in London and with I. de Burgh Daly in Birmingham, and then proceeded to the continent, studying with Corneille Heymans in Ghent, Belgium and with Gustav Embden in Frankfurt, Germany. Von Euler liked to travel, so he also worked and learned biophysics with Archibald Vivian Hill, again in London in 1934, and neuromuscular transmission with G. L. Brown in 1938. From 1946 to 1947, he worked with Eduardo Braun-Menéndez in the Instituto de Biología y Medicina Experimental in Buenos Aires, which was founded by Bernardo Houssay. His unerring instinct to work with important scientific leaders and fields was to be proved by the fact that Dale, Heymans, Hill and Houssay went to receive the Nobel prize in physiology or medicine.
In 1981, von Euler became a founding member of the World Cultural Council.
From 1930 to 1957, von Euler was married to Jane Anna Margarethe Sodenstierna (1905–2004). They had four children: Hans Leo, scientist administrator at the National Institutes of Health, Bethesda, Maryland, U.S.A.; Johan Christopher, anesthesiologist, Serafimer Hospital, Stockholm; Ursula Katarina, Ph.D., curator at The Royal Collections, The Royal Court, Stockholm, Sweden; and Marie Jane, Chemical Engineer, Melbourne, Australia. In 1958, von Euler married countess Dagmar Cronstedt, a radio broadcaster who had during the Second World War worked at Radio Königsberg, broadcasting German propaganda to neutral Sweden.
Research
His short stay as a postdoctoral student in Dale's laboratory was very fruitful: in 1931 he discovered with John H. Gaddum an important autopharmacological principle, substance P. After returning to Stockholm, von Euler pursued further this line of research, and successively discovered four other important endogenous active substances, prostaglandin, vesiglandin (1935), piperidine (1942) and noradrenaline (1946).
In 1939 von Euler was appointed full professor of physiology at the Karolinska Institute, where he remained until 1971. His early collaboration with Liljestrand had led to an important discovery, which was named the Euler–Liljestrand mechanism (a physiological arterial shunt in response to the decrease in local oxygenation of the lungs).
From 1946 on, however, when noradrenaline (abbreviated NA or NAd) was discovered, von Euler devoted most of his research work to this area. He and his group studied thoroughly its distribution and fate in biological tissues and in the nervous system in physiological and pathological conditions, and found that noradrenaline was produced and stored in nerve synaptic terminals in intracellular vesicles, a key discovery which changed dramatically the course of many researches in the field. In 1970 he was distinguished with the Nobel Prize for his work, jointly with Sir Bernard Katz and Julius Axelrod. Since 1953 he was very active in the Nobel Foundation, being a member of the Nobel Committee for Physiology or Medicine and chairman of the board since 1965. He also served as vice-president of the International Union of Physiological Sciences from 1965 to 1971. Among the many honorary titles and prizes he received in addition to the Nobel, were the Gairdner Prize (1961), the Jahre Prize (1965), the Stouffer Prize (1967), the Carl Ludwig Medaille (1953), the Schmiedeberg Plaquette (1969), La Madonnina (1970), many honorary doctorates from universities around the world, and the membership to several erudite, medical and scientific societies. He was elected a member of the American Philosophical Society in 1970, a member of both the American Academy of Arts and Sciences and the United States National Academy of Sciences in 1972, and a Foreign Member of the Royal Society (ForMemRS) in 1973.

#81 This is Cool » Benzoic Acid » 2025-10-18 16:26:13
- Jai Ganesh
- Replies: 0
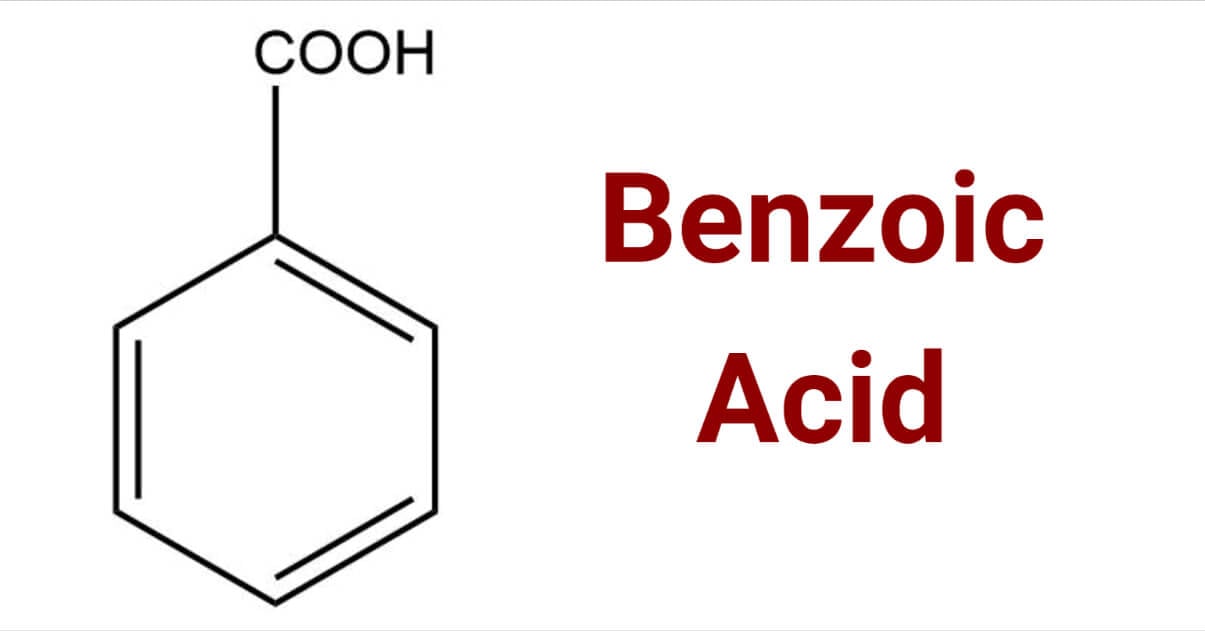
Benzoic Acid
Gist
Benzoic acid is a white crystalline organic compound with the formula C7H6O2 that has various uses, including as a food and cosmetic preservative and in the production of ointments, insect repellents, and dyes. It is a simple aromatic carboxylic acid, naturally found in some plants, and is known for its antiseptic properties. While useful, benzoic acid is a skin and eye irritant, and its combination with vitamin C can form benzene, a known carcinogen.
Benzoic acid is used as a preservative in food and drinks, an antifungal and antibacterial agent in medications and cosmetics, and a precursor for producing plastics and other chemicals. It's also found in products like toothpaste, insect repellents, and some cleaning supplies.
Summary
Benzoic acid is a white, crystalline organic compound belonging to the family of carboxylic acids, widely used as a food preservative and in the manufacture of various cosmetics, dyes, plastics, and insect repellents.
First described in the 16th century, benzoic acid exists in many plants; it makes up about 20 percent of gum benzoin, a vegetable resin. It was first prepared synthetically about 1860 from compounds derived from coal tar. It is commercially manufactured by the chemical reaction of toluene (a hydrocarbon obtained from petroleum) with oxygen at temperatures around 200° C (about 400° F) in the presence of cobalt and manganese salts as catalysts. Pure benzoic acid melts at 122° C (252° F) and is very slightly soluble in water.
Among the derivatives of benzoic acid are sodium benzoate, a salt used as a food preservative; benzyl benzoate, an ester used as a miticide; and benzoyl peroxide, used in bleaching flour and in initiating chemical reactions for preparing certain plastics.
Details
Benzoic acid is a white or colorless crystalline organic compound with the formula C6H5COOH, whose structure consists of a benzene ring (C6H6) with a carboxyl (−C(=O)OH) substituent. The benzoyl group is often abbreviated "Bz" (not to be confused with "Bn," which is used for benzyl), thus benzoic acid is also denoted as BzOH, since the benzoyl group has the formula –C6H5CO. It is the simplest aromatic carboxylic acid. The name is derived from gum benzoin, which was for a long time its only source.
Benzoic acid occurs naturally in many plants and serves as an intermediate in the biosynthesis of many secondary metabolites. Salts of benzoic acid are used as food preservatives. Benzoic acid is an important precursor for the industrial synthesis of many other organic substances. The salts and esters of benzoic acid are known as benzoates.
Production:
Industrial Preparation
Benzoic acid is produced commercially by partial oxidation of toluene with oxygen. The process is catalyzed by cobalt or manganese naphthenates. The process uses abundant materials, and proceeds in high yield.
The first industrial process involved the reaction of benzotrichloride (trichloromethyl benzene) with calcium hydroxide in water, using iron or iron salts as catalyst. The resulting calcium benzoate is converted to benzoic acid with hydrochloric acid. The product contains significant amounts of chlorinated benzoic acid derivatives. For this reason, benzoic acid for human consumption was obtained by dry distillation of gum benzoin. Food-grade benzoic acid is now produced synthetically.
Laboratory synthesis
Benzoic acid is cheap and readily available, so the laboratory synthesis of benzoic acid is mainly practiced for its pedagogical value. It is a common undergraduate preparation.
Benzoic acid can be purified by recrystallization from water because of its high solubility in hot water and poor solubility in cold water. The avoidance of organic solvents for the recrystallization makes this experiment particularly safe. This process usually gives a yield of around 65%.
By hydrolysis
Like other nitriles and amides, benzonitrile and benzamide can be hydrolyzed to benzoic acid or its conjugate base in acid or basic conditions.
From Grignard reagent
Bromobenzene can be converted to benzoic acid by "carboxylation" of the intermediate phenylmagnesium bromide. This synthesis offers a convenient exercise for students to carry out a Grignard reaction, an important class of carbon–carbon bond forming reaction in organic chemistry.
Oxidation of benzyl compounds
Benzyl alcohol and benzyl chloride and virtually all benzyl derivatives are readily oxidized to benzoic acid.
Additional Information
Benzoic acid, the simplest benzene-based carboxylic acid, has been known since the 16th century. One of its discoverers was the legendary clairvoyant Nostradamus. Its most common natural source is gum benzoin, a resin found in the bark of trees of the genus Styrax.
Most benzoic acid produced today is synthetic. Its first industrial synthesis was the hydrolysis of benzotrichloride to calcium benzoate, followed by acidification. This method has been completely displaced by the air oxidation of toluene, which avoids the problem of product contamination with chlorinated byproducts.
Many processed foods contain benzoic acid or one of its salts as a preservative. The acid inhibits the growth of bacteria, molds, and yeasts; it works best when the food has an acidic pH value. Benzoic acid also is often found in topical antifungal preparations.
#82 Dark Discussions at Cafe Infinity » Clubs Quotes - II » 2025-10-18 15:24:21
- Jai Ganesh
- Replies: 0
Clubs Quotes - II
1. There was a time when I was - after my very first record from Nashville, I thought I might not be one of those who actually really makes it, and I may end up back in Canada, just playing clubs. And that might - this might have just been it. - Shania Twain
2. I don't really go to clubs anymore. I'm actually quite settled. Living in Highgate with my dog and my husband and my daughter! I'm not a hell-raiser. But don't burst the bubble. Behind closed doors, for sure, I'm a hell-raiser. - Kate Moss
3. I have lived my dream and played at the finest of cricket grounds across the globe, and I want to thank the groundsmen, clubs, associations, and everyone who painstakingly prepare the arena for our performances. - Virender Sehwag
4. I was the first person from my family to enter films. So, everything connected with films was new to me, including fans and fan clubs. - Ajith Kumar
5. Myself and Cameron Lickle travel around North America in an RV and we conduct tennis clinics in clubs. - Mats Wilander.
#83 Jokes » Apple Jokes - III » 2025-10-18 14:57:18
- Jai Ganesh
- Replies: 0
I made an account just to tell this joke.
Aren't you going to give me an apple-ause?
* * *
Q: What do you call an apple that's been around the world?
A: Johnny Appleseed.
* * *
Q: What did the apple say to the almond?
A: You're Nuts!
* * *
Q: What did one maggot say to the other who was stuck in an apple?
A: Worm your way out of that one, then!
* * *
Q: What lives in apples and is an avid reader?
A: A bookworm !
* * *
#84 Re: Jai Ganesh's Puzzles » General Quiz » 2025-10-18 14:07:38
Hi,
#10615. What does the term in Biology Disease mean?
#10616. What does the term in Biology Deoxyribonucleic acid?
#85 Re: Jai Ganesh's Puzzles » English language puzzles » 2025-10-18 13:57:22
Hi,
#5811. What does the noun crustacean mean?
#5812. What does the adjective crusty mean?
#86 Re: Jai Ganesh's Puzzles » Doc, Doc! » 2025-10-18 13:47:15
Hi,
#2499. What does the medical term Nephrotic syndrome mean?
#87 Re: Jai Ganesh's Puzzles » 10 second questions » 2025-10-18 13:41:00
Hi,
#9768.
#88 Re: Jai Ganesh's Puzzles » Oral puzzles » 2025-10-18 13:25:51
Hi,
#6274.
#89 Re: Exercises » Compute the solution: » 2025-10-18 13:16:27
Hi,
2618.
#90 Re: This is Cool » Miscellany » 2025-10-18 00:11:20
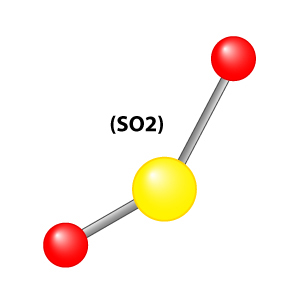
2420) Sulfur Dioxide
Gist
Sulfur dioxide (SO2) is a colorless gas with a pungent, irritating odor, similar to burnt matches. It is a harmful air pollutant formed by burning sulfur-containing fuels and is a precursor to acid rain and fine particle pollution. While it has industrial uses as a preservative and bleaching agent, high concentrations pose health risks like breathing problems, and long-term exposure can worsen asthma.
SO2, or sulfur dioxide, is a colorless, irritating gas that forms when sulfur-containing fossil fuels like coal and oil are burned. It is a major air pollutant that can cause respiratory problems such as wheezing and shortness of breath, and contributes to acid rain, which damages ecosystems and buildings. SO2 can also turn into fine particle pollution that reduces visibility.
Summary
Sulfur dioxide (IUPAC-recommended spelling) or sulphur dioxide (traditional Commonwealth English) is the chemical compound with the formula SO2. It is a colorless gas with a pungent smell that is responsible for the odor of burnt matches. It is released naturally by volcanic activity and is produced as a by-product of metals refining and the burning of sulfur-bearing fossil fuels.
Sulfur dioxide is somewhat toxic to humans, although only when inhaled in relatively large quantities for a period of several minutes or more. It was known to medieval alchemists as "volatile spirit of sulfur".
Occurrence
Sulfur dioxide is found on Earth and exists in very small concentrations in the atmosphere at about 15 ppb.
On other planets, sulfur dioxide can be found in various concentrations, the most significant being the atmosphere of Venus, where it is the third-most abundant atmospheric gas at 150 ppm. There, it reacts with water to form clouds of sulfurous acid (SO2 + H2O ⇌ HSO3 + H+), and is a key component of the planet's global atmospheric sulfur cycle. It has been implicated as a key agent in the warming of early Mars, with estimates of concentrations in the lower atmosphere as high as 100 ppm, though it only exists in trace amounts. On both Venus and Mars, as on Earth, its primary source is thought to be volcanic. The atmosphere of Io, a natural satellite of Jupiter, is 90% sulfur dioxide and trace amounts are thought to also exist in the atmosphere of Jupiter. The James Webb Space Telescope has observed the presence of sulfur dioxide on the exoplanet WASP-39b, where it is formed through photochemistry in the planet's atmosphere.
As an ice, it is thought to exist in abundance on the Galilean moons—as subliming ice or frost on the trailing hemisphere of Io, and in the crust and mantle of Europa, Ganymede, and Callisto, possibly also in liquid form and readily reacting with water.
Details
Sulfur dioxide (SO2) is an inorganic compound, a heavy, colorless, poisonous gas. It is produced in huge quantities in intermediate steps of sulfuric acid manufacture.
Sulfur dioxide has a pungent, irritating odor, familiar as the smell of a just-struck match. Occurring in nature in volcanic gases and in solution in the waters of some warm springs, sulfur dioxide usually is prepared industrially by the burning in air or oxygen of sulfur or such compounds of sulfur as iron pyrite or copper pyrite. Large quantities of sulfur dioxide are formed in the combustion of sulfur-containing fuels.
Sulfur dioxide pollution carries serious health and environmental risks and is one of the six criteria air pollutants regulated by the U.S. Environmental Protection Agency and other regulatory agencies around the world. In the atmosphere sulfur dioxide can combine with water vapor to form sulfuric acid, a major component of acid rain; in the second half of the 20th century, measures to control acid rain were widely adopted. Most of the sulfur dioxide released into the environment comes from coal-fired power plants and petroleum refineries. Paper pulp manufacturing, cement manufacturing, and metal smelting and processing facilities are other important sources.
Sulfur dioxide is a precursor of the trioxide (SO3) used to make sulfuric acid. In the laboratory the gas may be prepared by reducing sulfuric acid (H2SO4) to sulfurous acid (H2SO3), which decomposes into water and sulfur dioxide, or by treating sulfites (salts of sulfurous acid) with strong acids, such as hydrochloric acid, again forming sulfurous acid.
Sulfur dioxide can be liquefied under moderate pressures at room temperatures; the liquid freezes at −73° C (−99.4° F) and boils at −10° C (14° F) under atmospheric pressure. Although its chief uses are in the preparation of sulfuric acid, sulfur trioxide, and sulfites, sulfur dioxide also is used as a disinfectant, a refrigerant, a reducing agent, a bleach, and a food preservative, especially in dried fruits.
Additional Information
Sulfur dioxide (SO2) is a pungent, toxic gas that is the primary product of burning elemental sulfur. It exists widely in nature, mostly from volcanic activity and burning fossil fuels. It is found elsewhere in the solar system, as a gas in the atmospheres of Venus and Jupiter’s moon Io and as an ice on the other Galilean moons.
The major use of SO2 is in the manufacture of sulfuric acid (H2SO4), the most-produced chemical worldwide. Elemental sulfur and oxygen react to form SO2, which is catalytically oxidized with additional oxygen to make sulfur trioxide (SO3). The SO3 is mixed with existing H2SO4 to produce oleum (fuming sulfuric acid), which is added to water in a strongly exothermic process to make concentrated H2SO4. This is known as the contact process; it dates to an 1831 patent by British inventor Peregrine Phillips.
In chemical laboratories, it has multiple functions, including as a reducing agent, as a reagent in sulfonylation reactions, and as a low-temperature solvent. SO2 is also used to preserve dried fruits such as raisins and prunes and to prevent spoilage in wine.
The hazard information table shows that SO2 is pretty nasty stuff; but, in addition to its value as a chemical, it has another positive side: Volcanoes that emit the gas can have a beneficial effect on climate change. When SO2 spews into the stratosphere, it reacts photochemically with oxygen to form H2SO4 aerosols, which in turn reflect solar radiation and cool the atmosphere. But, as might be expected, even this has a downside because SO2 and H2SO4 contribute to acid rain.
#91 Re: Dark Discussions at Cafe Infinity » crème de la crème » 2025-10-17 23:10:37
2367) Julius Axelrod
Gist:
Work
The nervous systems of people and animals consist of many nerve cells with long extensions, or nerve fibers. Signals are conveyed between cells by small electrical currents and by special substances known as signal substances. The transfers occur via contacts, or synapses. Julius Axelrod studied noradrenaline, a signal substance that provides signals to increase activity in the case of aggression or danger. Among other things, in 1957 he showed how an excess of noradrenaline is released in response to nerve impulses and then returns to the place were it is stored after the signal is implemented.
Summary
Julius Axelrod (born May 30, 1912, New York, New York, U.S.—died December 29, 2004, Rockville, Maryland) was an American biochemist and pharmacologist who, along with the British biophysicist Sir Bernard Katz and the Swedish physiologist Ulf von Euler, was awarded the Nobel Prize for Physiology or Medicine in 1970. Axelrod’s contribution was his identification of an enzyme that degrades chemical neurotransmitters within the nervous system after they are no longer needed to transmit nerve impulses.
A graduate of the College of the City of New York (B.S., 1933), New York University (M.S., 1941), and George Washington University (Ph.D., 1955), Axelrod worked as a chemist in the Laboratory of Industrial Hygiene at New York City’s Health Department (1935–46) and then joined the research division of Goldwater Memorial Hospital (1946), where his studies on analgesic medications helped identify acetaminophen as the chemical responsible for relieving pain. Marketed under such trade names as Tylenol and Panadol, acetaminophen became one of the most widely used painkillers in the world. In 1949 Axelrod left the hospital to join the staff of the section on chemical pharmacology at the National Heart Institute in Bethesda, Maryland. In 1955 he moved to the staff of the National Institute of Mental Health, where he became chief of the pharmacology section of the Laboratory of Clinical Sciences. He remained at the institute until his retirement in 1984.
Axelrod’s Nobel Prize-winning research grew out of work done by Euler, specifically Euler’s discovery of noradrenaline (norepinephrine), a chemical substance that transmits nerve impulses. Axelrod, in turn, discovered that noradrenaline could be neutralized by an enzyme, catechol-O-methyltransferase, which he isolated and named. This enzyme proved critical to an understanding of the entire nervous system. The enzyme was shown to be useful in dealing with the effects of certain psychotropic drugs and in research on hypertension and schizophrenia.
Details
Julius Axelrod (May 30, 1912 – December 29, 2004) was an American biochemist. He won a share of the Nobel Prize in Physiology or Medicine in 1970 along with Bernard Katz and Ulf von Euler. The Nobel Committee honored him for his work on the release and reuptake of catecholamine neurotransmitters, a class of chemicals in the brain that include epinephrine, norepinephrine, and, as was later discovered, dopamine. Axelrod also made major contributions to the understanding of the pineal gland and how it is regulated during the sleep-wake cycle.
Education and early life
Axelrod was born in New York City, the son of Jewish immigrants from Poland, Molly (née Leichtling) and Isadore Axelrod, a basket weaver.[9] He received his bachelor's degree in biology from the College of the City of New York in 1933. Axelrod wanted to become a physician, but was rejected from every medical school to which he applied. He worked briefly as a laboratory technician at New York University, then in 1935 he got a job with the New York City Department of Health and Mental Hygiene testing vitamin supplements added to food. While working at the Department of Health, he attended night school and received his master's in sciences degree from New York University in 1941.
Research:
Analgesic research
In 1946, Axelrod took a position working under Bernard Brodie at Goldwater Memorial Hospital. The research experience and mentorship Axelrod received from Brodie would launch him on his research career. Brodie and Axelrod's research focused on how analgesics (pain-killers) work. During the 1940s, users of non-aspirin analgesics were developing a blood condition known as methemoglobinemia. Axelrod and Brodie discovered that acetanilide, the main ingredient of these pain-killers, was to blame. They found that one of the metabolites also was an analgesic. They recommended that this metabolite, acetaminophen (paracetamol, Tylenol), be used instead.
Catecholamine research
In 1949, Axelrod began work at the National Heart Institute, forerunner of the National Heart, Lung, and Blood Institute (NHLBI), part of the National Institutes of Health (NIH). He examined the mechanisms and effects of caffeine, which led him to an interest in the sympathetic nervous system and its main neurotransmitters, epinephrine and norepinephrine. During this time, Axelrod also conducted research on codeine, morphine, methamphetamine, and ephedrine and performed some of the first experiments on LSD. Realizing that he could not advance his career without a PhD, he took a leave of absence from the NIH in 1954 to attend George Washington University Medical School. Allowed to submit some of his previous research toward his degree, he graduated one year later, in 1955. Axelrod then returned to the NIH and began some of the key research of his career.
Axelrod received his Nobel Prize for his work on the release, reuptake, and storage of the neurotransmitters epinephrine and norepinephrine, also known as adrenaline and noradrenaline. Working on monoamine oxidase (MAO) inhibitors in 1957, Axelrod showed that catecholamine neurotransmitters do not merely stop working after they are released into the synapse. Instead, neurotransmitters are recaptured ("reuptake") by the pre-synaptic nerve ending, and recycled for later transmissions. He theorized that epinephrine is held in tissues in an inactive form and is liberated by the nervous system when needed. This research laid the groundwork for later selective serotonin reuptake inhibitors (SSRIs), such as Prozac, which block the reuptake of another neurotransmitter, serotonin.
In 1958, Axelrod also discovered and characterized the enzyme catechol-O-methyl transferase, which is involved in the breakdown of catecholamines.
Pineal gland research
Some of Axelrod's later research focused on the pineal gland. He and his colleagues showed that the hormone melatonin is generated from tryptophan, as is the neurotransmitter serotonin. The rates of synthesis and release follows the body's circadian rhythm driven by the suprachiasmatic nucleus within the hypothalamus. Axelrod and colleagues went on to show that melatonin had wide-ranging effects throughout the central nervous system, allowing the pineal gland to function as a biological clock. He was elected a Fellow of the American Academy of Arts and Sciences in 1971. He continued to work at the National Institute of Mental Health at the NIH until his death in 2004.
Many of his papers and awards are held at the National Library of Medicine.
Awards and honors
Axelrod was awarded the Gairdner Foundation International Award in 1967 and the Nobel Prize in Physiology or Medicine in 1970. He was elected a Foreign Member of the Royal Society in 1979 In 1992, he was awarded the Ralph W. Gerard Prize in Neuroscience. He was elected to the American Philosophical Society in 1995.
Research trainees
Solomon Snyder, Irwin Kopin, Ronald W. Holz, Rudi Schmid, Bruce R. Conklin, Ron M. Burch, Juan M. Saavedra, Marty Zatz, Richard M. Weinshilboum, Michael Brownstein, Chris Felder, Lewis Landsberg, Robert Kanterman, Richard J. Wurtman.
Personal life
Axelrod injured his left eye when an ammonia bottle in the lab exploded; he would wear an eyepatch for the rest of his life. Although he became an atheist early in life and resented the strict upbringing of his parents' religion, he identified with Jewish culture and joined several international fights against anti-Semitism. His wife of 53 years, Sally Taub Axelrod, died in 1992. At his death, on December 29, 2004, he was survived by two sons, Paul and Alfred, and three grandchildren.
Political views
After receiving the Nobel Prize in 1970, Axelrod used his visibility to advocate several science policy issues. In 1973 U.S. President Richard Nixon created an agency with the specific goal of curing cancer. Axelrod, along with fellow Nobel-laureates Marshall W. Nirenberg and Christian Anfinsen, organized a petition by scientists opposed to the new agency, arguing that by focusing solely on cancer, public funding would not be available for research into other, more solvable, medical problems. Axelrod also lent his name to several protests against the imprisonment of scientists in the Soviet Union. Axelrod was a member of the Board of Sponsors of the Federation of American Scientists and the International Academy of Science, Munich.

#92 Dark Discussions at Cafe Infinity » Clubs Quotes - I » 2025-10-17 16:32:06
- Jai Ganesh
- Replies: 0
Clubs Quotes - I
1. At Real, psychological pressure on the players is much more serious than at United. This is good. At many clubs, you don't know the consequence of playing badly. - Cristiano Ronaldo
2. Soccer and cricket were my main sports growing up. I had trials as a soccer player with a few clubs interested, Crystal Palace being one, but it was cricket which became my chosen profession. - Ian Botham
3. Music became my focus. At 13, I was jamming with my mates. At 15, I was playing clubs. - Bryan Adams
4. It certainly is dangerous that there are only a few clubs left in Europe that can afford to pay millions. At the end of the day however, the spectators decide the rates of pay - by watching the games and consuming the goods and services advertised on sports TV programmes. - Angela Merkel
5. I love football and it's the sport I would really like to play. I've said on national television here that I would really love to play for one of our football clubs when I finished my tennis career. - Novak Djokovic
6. I don't belong to any clubs, and I dislike club mentality of any kind, even feminism - although I do relate to the purpose and point of feminism. More in the work of older feminists, really, like Germaine Greer. - Jane Campion
7. Debating clubs among boys are very useful, not only as affording pleasant meetings and interesting discussions, but also as serving for training grounds for developing the knowledge and the qualities that are needed in public life. - Annie Besant
8. When I was minister of sport in Brazil, I tried to bring in a law that would make the chairmen of clubs reveal their accounts like other businesses. It was turned down, but I think it is an important story that will make a good film. - Pele.
#93 Re: Exercises » Increase and decrease in quantity » 2025-10-17 15:40:10
Jai Ganesh wrote:The first method is technically correct.
It is always advisable to use this method.
The second method may give more of less the same value.
This is for the beginners.I'm not in agreement with "technically correct." The first method IS correct.
Any method that gives "more or less the same value" is incorrect. In this case, what does it mean to change 450 twice, when after the first change you no longer have 450?
Why would you want beginners to use an incorrect method?
Any Mathematics problem and solution have Levels of Difficulty/Simplicity.
I always choose the best and most accurate method.
Technically Correct: Something which is accurate according to a strict interpretation of rules, facts, or a specific system, even if it's not the most practical or common way of thinking about it.
#94 Re: Jai Ganesh's Puzzles » Doc, Doc! » 2025-10-17 15:19:43
Hi,
#2498. What does the medical term Optic disc mean?
#95 Jokes » Apple Jokes - II » 2025-10-17 14:51:39
- Jai Ganesh
- Replies: 0
Q: What kind of apple isn't an apple?
A: A pineapple.
* * *
Q: What did the apple say to the apple pie?
A: "You've got some crust."
* * *
Q: What's worse than finding a worm in your apple?
A: Taking a bite and finding half a worm.
* * *
Q: If an apple a day keeps the doctor away, what does an onion do?
A: Keeps everyone away.
* * *
Q: Where do bugs go to watch the big game?
A: Apple-Bees.
* * *
#96 Re: Jai Ganesh's Puzzles » 10 second questions » 2025-10-17 14:28:21
Hi,
#9767.
#97 Re: Jai Ganesh's Puzzles » Oral puzzles » 2025-10-17 14:14:52
Hi,
#6273.
#98 Re: Exercises » Compute the solution: » 2025-10-17 13:39:59
Hi,
2617.
#99 This is Cool » Phosphoric Acid » 2025-10-16 22:32:07
- Jai Ganesh
- Replies: 0
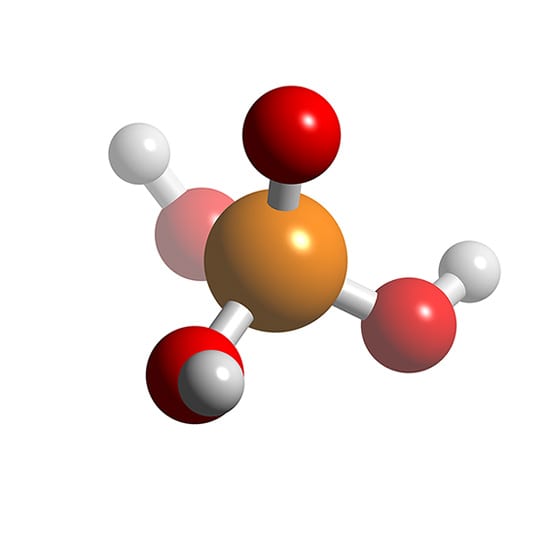
Phosphoric Acid
Gist
Phosphoric acid (H3PO4) is a weak mineral acid used in food, agriculture, and industry, and it is found as a clear liquid or white crystalline solid. In foods, it's used as an acidulant and preservative, while in fertilizers, it promotes plant growth. Due to its corrosive nature, concentrated solutions require protective gear to avoid skin, eye, and respiratory irritation.
Phosphoric acid has numerous uses, most notably in the production of phosphate fertilizers. It is also used in the food and beverage industry as an acidic flavoring agent and preservative, particularly in soft drinks. Other common applications include metal treatment, cleaning products, water treatment, detergents, and in the manufacturing of some pharmaceuticals and personal care products.
Summary
Phosphoric acid (orthophosphoric acid, monophosphoric acid or phosphoric(V) acid) is a colorless, odorless phosphorus-containing solid, and inorganic compound with the chemical formula H3PO4. It is commonly encountered as an 85% aqueous solution, which is a colourless, odourless, and non-volatile syrupy liquid. It is a major industrial chemical, being a component of many fertilizers.
The name "orthophosphoric acid" can be used to distinguish this specific acid from other "phosphoric acids", such as pyrophosphoric acid. Nevertheless, the term "phosphoric acid" often means this specific compound; and that is the current IUPAC nomenclature.
Purification
Phosphoric acid produced from phosphate rock or thermal processes often requires purification. A common purification method is liquid–liquid extraction, which involves the separation of phosphoric acid from water and other impurities using organic solvents, such as tributyl phosphate (TBP), methyl isobutyl ketone (MIBK), or n-octanol. Nanofiltration involves the use of a premodified nanofiltration membrane, which is functionalized by a deposit of a high molecular weight polycationic polymer of polyethyleneimines. Nanofiltration has been shown to significantly reduce the concentrations of various impurities, including cadmium, aluminum, iron, and rare earth elements. The laboratory and industrial pilot scale results showed that this process allows the production of food-grade phosphoric acid.
Fractional crystallization can achieve higher purities typically used for semiconductor applications. Usually a static crystallizer is used. A static crystallizer uses vertical plates, which are suspended in the molten feed and which are alternatingly cooled and heated by a heat transfer medium. The process begins with the slow cooling of the heat transfer medium below the freezing point of the stagnant melt. This cooling causes a layer of crystals to grow on the plates. Impurities are rejected from the growing crystals and are concentrated in the remaining melt. After the desired fraction has been crystallized, the remaining melt is drained from the crystallizer. The purer crystalline layer remains adhered to the plates. In a subsequent step, the plates are heated again to liquify the crystals and the purified phosphoric acid drained into the product vessel. The crystallizer is filled with feed again and the next cooling cycle is started.
Details
Phosphoric acid, (H3PO4) is the most important oxygen acid of phosphorus, used to make phosphate salts for fertilizers. It is also used in dental cements, in the preparation of albumin derivatives, and in the sugar and textile industries. It serves as an acidic, fruitlike flavouring in food products.
Pure phosphoric acid is a crystalline solid (melting point 42.35° C, or 108.2° F); in less concentrated form it is a colourless syrupy liquid. The crude acid is prepared from phosphate rock, while acid of higher purity is made from white phosphorus.
Phosphoric acid forms three classes of salts corresponding to replacement of one, two, or three hydrogen atoms. Among the important phosphate salts are: sodium dihydrogen phosphate (NaH2PO4), used for control of hydrogen ion concentration (acidity) of solutions; disodium hydrogen phosphate (Na2HPO4), used in water treatment as a precipitant for highly charged metal cations; trisodium phosphate (Na3PO4), used in soaps and detergents; calcium dihydrogen phosphate or calcium superphosphate (Ca[H2PO4]2), a major fertilizer ingredient; calcium monohydrogen phosphate (CaHPO4), used as a conditioning agent for salts and sugars.
Phosphoric acid molecules interact under suitable conditions, often at high temperatures, to form larger molecules (usually with loss of water). Thus, diphosphoric, or pyrophosphoric, acid (H4P2O7) is formed from two molecules of phosphoric acid, less one molecule of water. It is the simplest of a homologous series of long chain molecules called polyphosphoric acids, with the general formula H(HPO3)nOH, in which n = 2, 3, 4, . . . . Metaphosphoric acids, (HPO3)n, in which n = 3, 4, 5, . . ., are another class of polymeric phosphoric acids. The known metaphosphoric acids are characterized by cyclic molecular structures. The term metaphosphoric acid is used also to refer to a viscous, sticky substance that is a mixture of both long chain and ring forms of (HPO3)n. The various polymeric forms of phosphoric acid are also prepared by hydration of phosphorus oxides.
Additional Information
Phosphoric acid, also known as orthophosphoric acid, is a chemical compound. It is also an acid. Its chemical formula is H3PO4. It contains hydrogen and phosphate ions. Its official IUPAC name is trihydroxidooxidophosphorus.
Properties
Phosphoric acid is a white solid. It melts easily to make a viscous liquid. It tastes sour when diluted (mixed with a lot of water). It can be deprotonated three times. It is very strong, although not as much as the other acids like hydrochloric acid. It does not have any odor. It is corrosive when concentrated. Salts of phosphoric acid are called phosphates.
Preparation
Phosphoric acid can be made by dissolving phosphorus(V) oxide in water. This makes a very pure phosphoric acid that is good for food. A less pure form is made by reacting sulfuric acid with phosphate rock. This can be purified to make food-grade phosphoric acid if needed.
Uses
It is used to make sodas sour. It is also used when a nonreactive acid is needed. It can be used to make hydrogen halides, such as hydrogen chloride. Phosphoric acid is heated with a sodium halide to make the hydrogen halide and sodium phosphate. It is used to react with rust to make black iron(III) phosphate, which can be scraped off, leaving pure iron. It can be used to clean teeth.
There are many minor uses of phosphoric acid. Phosphoric acid with a certain isotope of phosphorus is used for nuclear magnetic resonance. It is also used as an electrolyte in some fuel cells. It can be used as a flux. It can etch certain things in semiconductor making.
Safety
Phosphoric acid is one of the least toxic acids. When it is diluted, it just has a sour taste. When it is concentrated, it can corrode metals.
#100 Re: Dark Discussions at Cafe Infinity » crème de la crème » 2025-10-16 17:29:43
2366) Luis Federico Leloir
Gist:
Work
Carbohydrates, including sugars and starches, are of paramount importance to the life processes of organisms. Luis Leloir demonstrated that nucleotides—molecules that also constitute the building blocks of DNA molecules—are crucial when carbohydrates are generated and converted. In 1949 Leloir discovered that one type of sugar’s conversion to another depends on a molecule that consists of a nucleotide and a type of sugar. He later showed that the generation of carbohydrates is not an inversion of metabolism, as had been assumed previously, but processes with other steps.
Summary
Luis Federico Leloir (born Sept. 6, 1906, Paris, France—died Dec. 2, 1987, Buenos Aires, Arg.) was an Argentine biochemist who won the Nobel Prize for Chemistry in 1970 for his investigations of the processes by which carbohydrates are converted into energy in the body.
After serving as an assistant at the Institute of Physiology, University of Buenos Aires, from 1934 to 1935, Leloir worked a year at the biochemical laboratory at the University of Cambridge and in 1937 returned to the Institute of Physiology, where he undertook investigations of the oxidation of fatty acids. In 1947 he obtained financial support to set up the Institute for Biochemical Research, Buenos Aires, where he began research on the formation and breakdown of lactose, or milk sugar, in the body. That work ultimately led to his discovery of sugar nucleotides, which are key elements in the processes by which sugars stored in the body are converted into energy. He also investigated the formation and utilization of glycogen and discovered certain liver enzymes that are involved in its synthesis from glucose.
Details
Luis Federico Leloir (September 6, 1906 – December 2, 1987) was an Argentine physician and biochemist who received the 1970 Nobel Prize in Chemistry for his discovery of the metabolic pathways by which carbohydrates are synthesized and converted into energy in the body. Although born in France, Leloir received the majority of his education at the University of Buenos Aires and was director of the private research group Fundación Instituto Campomar until his death in 1987. His research into sugar nucleotides, carbohydrate metabolism, and renal hypertension garnered international attention and led to significant progress in understanding, diagnosing and treating the congenital disease galactosemia. Leloir is buried in La Recoleta Cemetery, Buenos Aires.
Biography:
Early years
Leloir's parents, Federico Augusto Rufino and Hortencia Aguirre de Leloir, traveled from Buenos Aires to Paris in the middle of 1906 with the intention of treating Federico's illness. However, Federico died in late August, and a week later Luis was born in an old house at 81 Víctor Hugo Road in Paris, a few blocks away from the Arc de Triomphe. After returning to Argentina in 1908, Leloir lived together with his eight siblings on their family's extensive property El Tuyú that his grandparents had purchased after their immigration from the Basque Country of northern Spain: El Tuyú comprises 400 {km}^{2} of sandy land along the coastline from San Clemente del Tuyú to Mar de Ajó which has since become a popular tourist attraction.
During his childhood, the future Nobel Prize winner found himself observing natural phenomena with particular interest; his schoolwork and readings highlighted the connections between the natural sciences and biology. His education was divided between Escuela General San Martín (primary school), Colegio Lacordaire (secondary school), and for a few months at Beaumont College in England. His grades were unspectacular, and his first stint in college ended quickly when he abandoned his architectural studies that he had begun in Paris' École Polytechnique.
It was during the 1920s that Leloir invented salsa golf (golf sauce). After being served prawns with the usual sauce during lunch with a group of friends at the Ocean Club in Mar del Plata, Leloir came up with a peculiar combination of ketchup and mayonnaise to spice up his meal. With the financial difficulties that later plagued Leloir's laboratories and research, he would joke, "If I had patented that sauce, we'd have a lot more money for research right now.
Nobel Prize
On December 2, 1970, Leloir received the Nobel Prize for Chemistry from the King of Sweden for his discovery of the metabolic pathways in lactose, becoming only the third Argentine to receive the prestigious honor in any field at the time. In his acceptance speech at Stockholm, he borrowed from Winston Churchill's famous 1940 speech to the House of Commons and remarked, "never have I received so much for so little". Leloir and his team reportedly celebrated by drinking champagne from test tubes, a rare departure from the humility and frugality that characterized the atmosphere of Fundación Instituto Campomar under Leloir's direction. The $80,000 prize money was spent directly on research, and when asked about the significance of his achievement, Leloir responded:
"This is only one step in a much larger project. I discovered (no, not me: my team) the function of sugar nucleotides in cell metabolism. I want others to understand this, but it is not easy to explain: this is not a very noteworthy deed, and we hardly know even a little."
